Application of shikimic acid in preparing drug for preventing or treating demyelinating diseases
A demyelinating disease and shikimic acid technology, applied in the fields of bioengineering and medicine, can solve problems such as unreported application of shikimic acid, and achieve the effects of treating demyelinating diseases, promoting differentiation and maturation, and solving the failure of remyelination
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
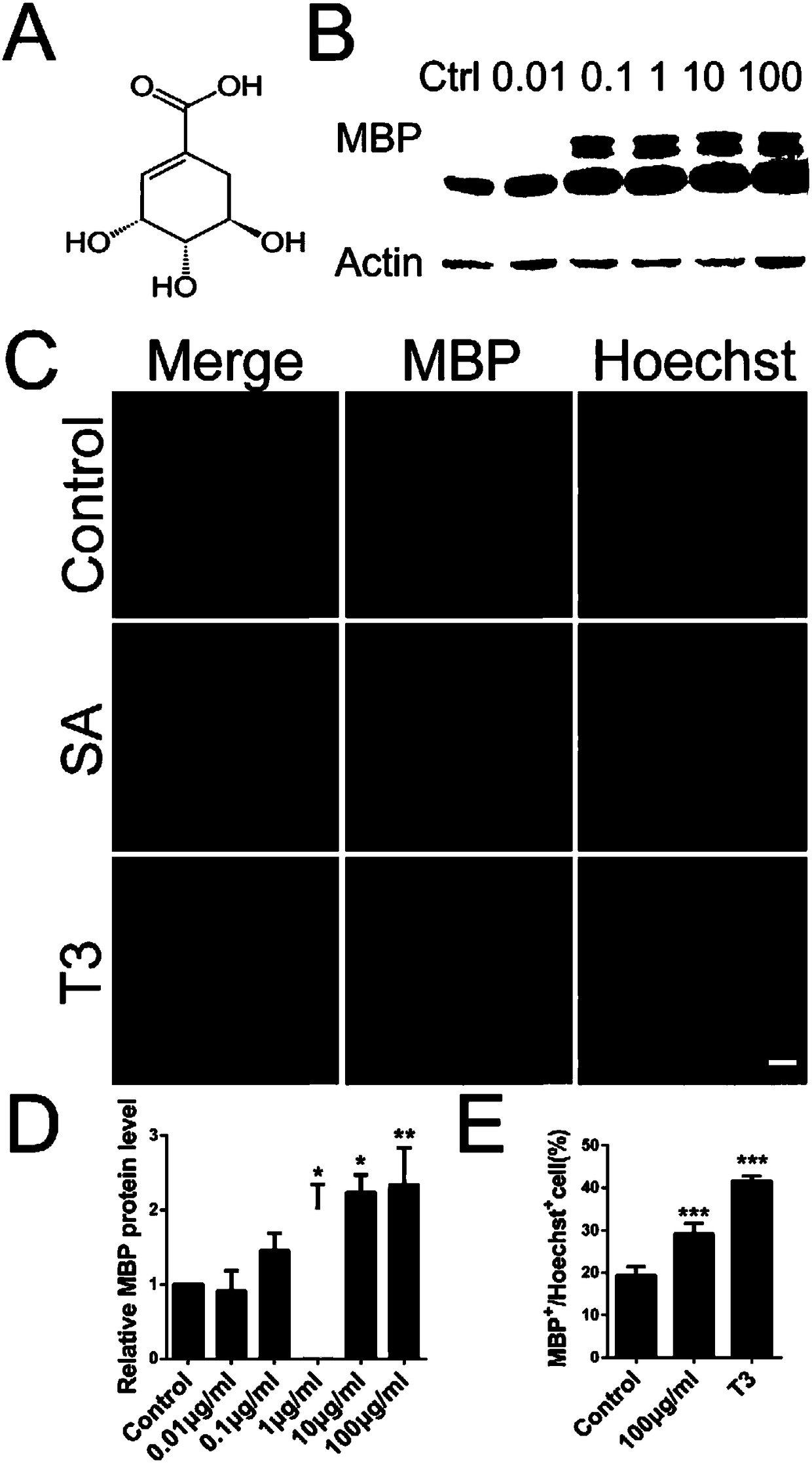
[0032] Promoting effect of shikimic acid (SA) on the differentiation of oligodendrocyte precursor cells (OPCs).
[0033] 1. Experimental method:
[0034] In order to study and confirm the effect of shikimic acid on the differentiation of OPCs, qRT-PCR, Western blot, and immunocytochemistry were used to confirm that shikimic acid can promote the differentiation and maturation of OPCs from the mRNA level, protein level, and morphological level.
[0035] 1. Experimental cells: primary cultured oligodendrocyte precursor cells (OPCs).
[0036] 2. Experimental animals: Sprague-Dawley rats and C57BL / 6J mice used in this experiment were all purchased from Shanghai Xipuerbichem Laboratory Animal Co., Ltd.
[0037] 3. Reagents and consumables commonly used in the experiment: DMEM cell culture medium and Neurobasal / B27 culture medium were purchased from Gibco Company of the United States; fetal bovine serum FBS was purchased from PAA Company of Australia; 0.25% Trypsin trypsin, L-polyly...
Embodiment 2
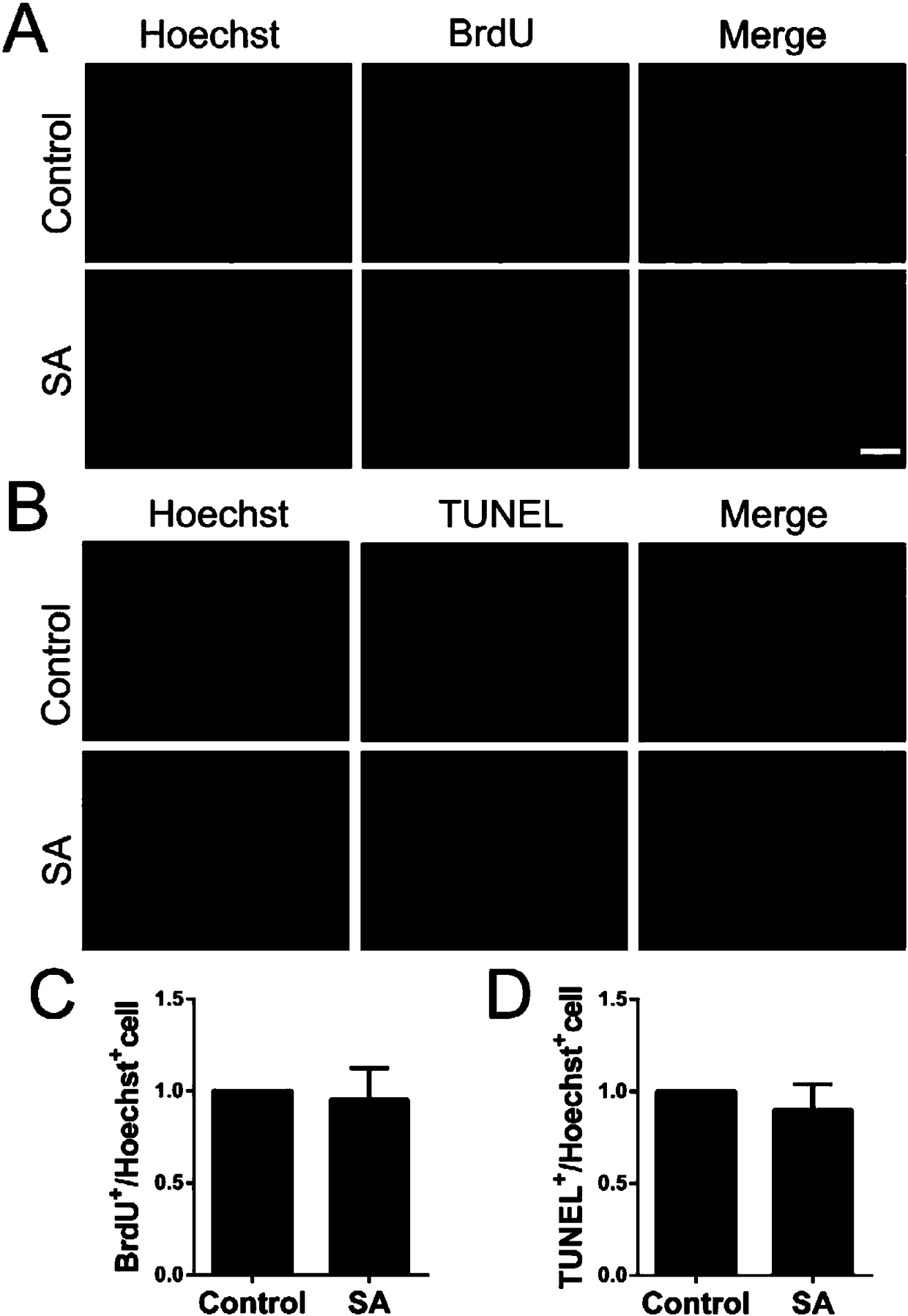
[0069] Shikimic acid does not affect the proliferation and apoptosis of OPCs cultured in vitro
[0070] 1. Experimental method:
[0071] In order to verify that shikimic acid does not affect other changes in cells while promoting OPCs differentiation, BrdU binding assay and TUNEL assay were used to study cell proliferation and apoptosis, reflecting the effects of drugs on cells from two indicators.
[0072] 1. Experimental cells: primary cultured oligodendrocyte precursor cells (OPCs).
[0073]2. Experimental reagents, consumables and instruments: DMEM culture medium, Neurobasal / B27 were purchased from Gibco, USA; fetal bovine serum FBS was purchased from PAA, Australia; 0.25% Trypsin, L-polylysine (poly-L -lysine, PLL) was purchased from Sigma, USA. Other routine reagent materials in the laboratory were purchased from Shanghai Sinopharm Group, and cell culture dishes and plates were purchased from Corning Incorporated.
[0074] 3. Experimental drugs:
[0075] Medicine of ...
Embodiment 3
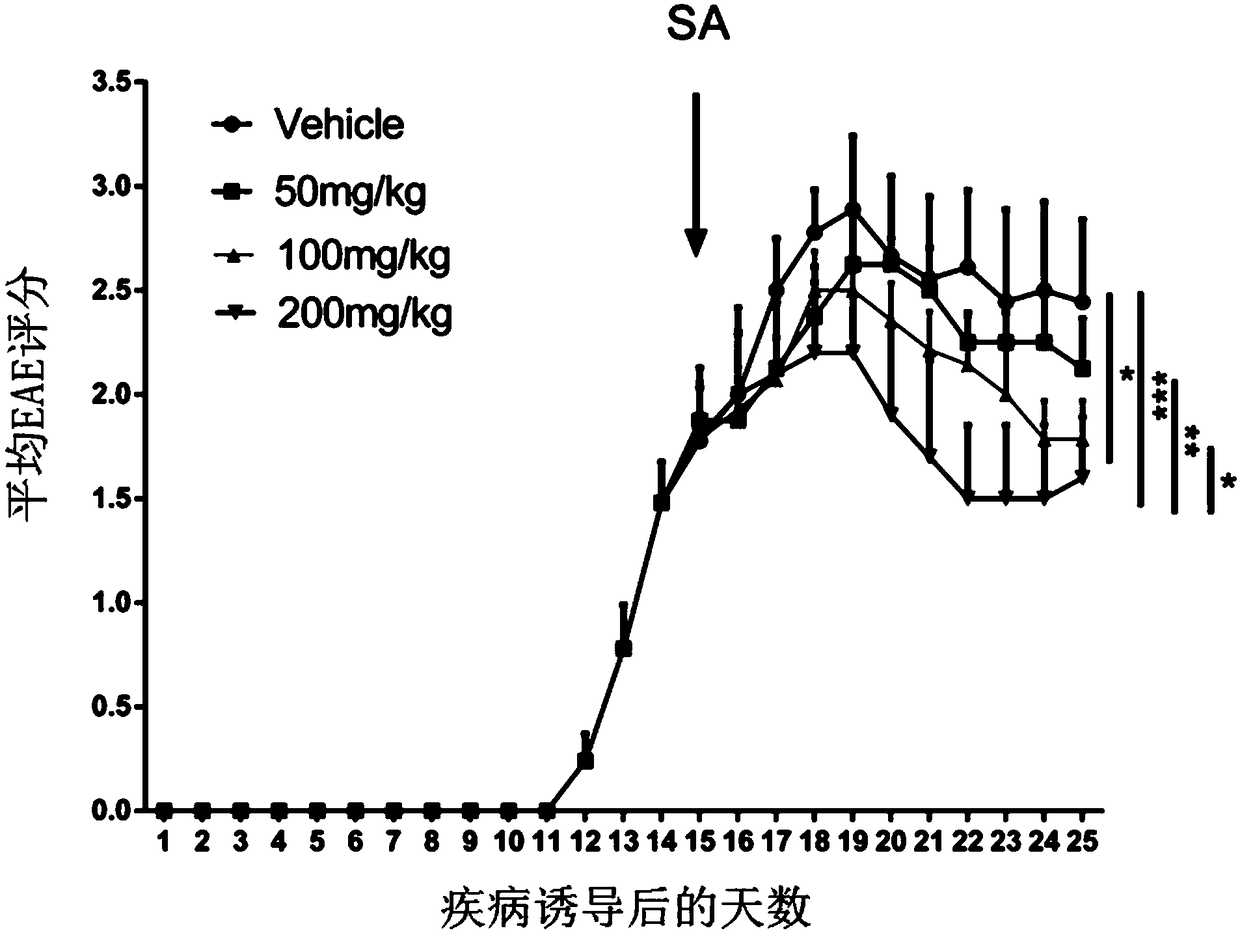
[0084] Shikimic acid alleviates disease state in EAE mice
[0085] 1. Experimental method:
[0086] In order to further verify the therapeutic effect of shikimic acid on demyelinating diseases, MOG 33-55 The EAE mouse model was induced, and the therapeutic administration was adopted, and the intraperitoneal administration was started on the 15th day after the model was established, once a day. After the modeling, the mice were observed every day until 25 days after the modeling.
[0087] 1. Experimental animals: C57BL / 6J adult female mice were purchased from Shanghai Slack Company. All animal experiments were performed in accordance with the animal management regulations and ethical requirements of the Animal Management Committee of the Naval Medical University of the Chinese People's Liberation Army.
[0088] 2. Experimental reagent: MOG 35-55 (GL Biochem), MTB (H37Ra strain; Difco), IFA (Incomplete Freund's adjuvant; Sigma-Aldrich), PTX (pertussis toxin; Difcolaboratorie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com