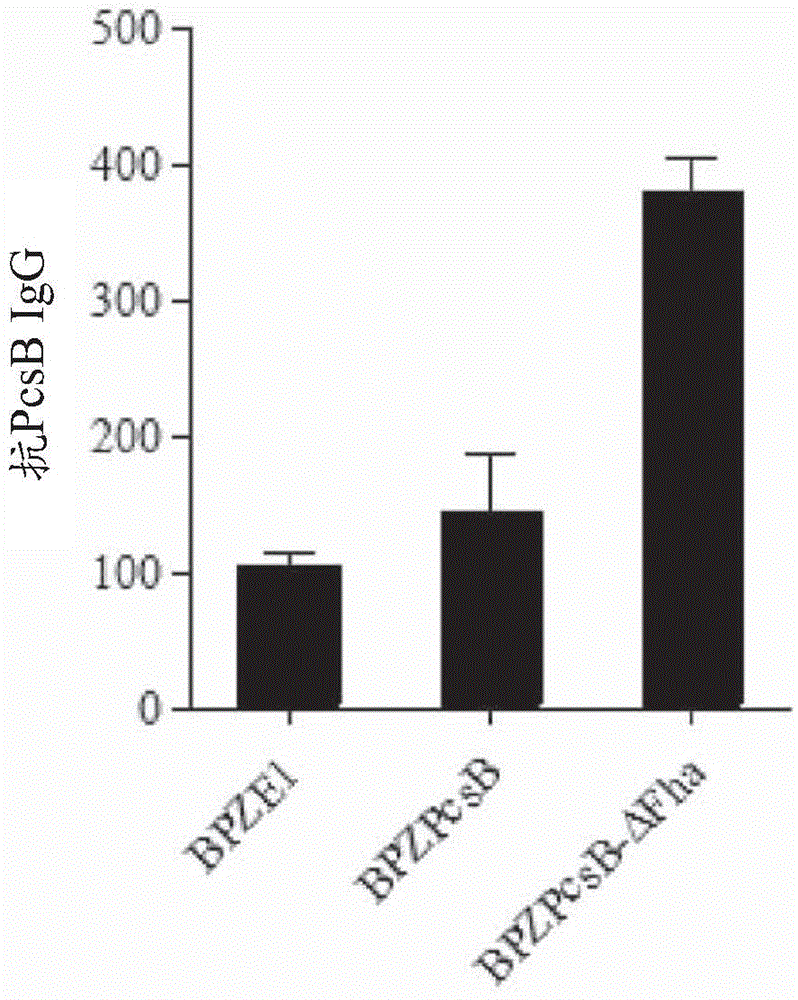
Patents
Literature
40 results about "Pertussis toxin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pertussis toxin (PT) is a protein-based AB₅-type exotoxin produced by the bacterium Bordetella pertussis, which causes whooping cough. PT is involved in the colonization of the respiratory tract and the establishment of infection. Research suggests PT may have a therapeutic role in treating a number of common human ailments, including hypertension, viral infection, and autoimmunity.
High level expression of recombinant toxin proteins
ActiveUS20110287443A1High yieldQuality improvementAntibody mimetics/scaffoldsBacteria peptidesBacteroidesHigh level expression
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PFENEX
High level expression of recombinant toxin proteins
The present invention relates to the field of recombinant toxin protein production in bacterial hosts. In particular, the present invention relates to production processes for obtaining high levels of a recombinant CRM197, Diphtheria Toxin, Pertussis Toxin, Tetanus Toxoid Fragment C, Cholera Toxin B, Cholera holotoxin, and Pseudomonas Exotoxin A, from a bacterial host.
Owner:PELICAN TECH HLDG INC
Separated and purified acellular pertussis-diphtheria-tetanus, b-type haemophilus influenzae and A-group and C-group meningococcus combined vaccine and preparation method thereof
InactiveCN104689309ARelieve painReduce the burden onAntibacterial agentsBacterial antigen ingredientsHemagglutininAluminium hydroxide
The invention discloses a combined vaccine and a preparation method thereof. The combined vaccine is formed by a component A and a component B, wherein the component A is a liquid preparation and composed of pertussis toxin, filamentous hemagglutinin, pertussis adhesion, diphtheria toxoid, tetanus toxoid, aluminium hydroxide and sodium chloride; the component B is a freeze-drying preparation and composed of A-group meningitis polysaccharide conjugate, C-group meningitis polysaccharide conjugate, b-type haemophilus influenzae polysaccharide conjugate and lactose. The preparation method of the vaccine includes the steps of preparing of acellular pertussis-diphtheria-tetanus vaccine semi-finished products, A-group and C-group meningococcocci and b-type haemophilus influenzae combined vaccine semi-finished products, split charging and packaging. The vaccine has the characteristics of being safe, effective, controllable and capable of preventing diseases through an injection, the preparation method is easy to operate, preparation is facilitated, cost is low, and the combined vaccine is suitable for industrialized mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Modification of pertussis toxin
InactiveUS6018022ABeneficially modifyingBacterial antigen ingredientsBacteriaBiological propertyPertussis toxin
The three-dimensional structure of crystalline pertussis holotoxin (PT) has been determined by X-ray crystallography. Crystal structures have also been determined for complexes of pertussis toxin with molecules relevant to the biological activity of PT. These three-dimensional structures were analyzed to identify functional amino acids appropriate for modification to alter the biological properties of PT. Similar procedures may be used to predict amino acids which contribute to the toxicity of the holotoxin, to produce immunoprotective, genetically-detoxified analogs of pertussis toxin.
Owner:CONNAUGHT LAB +1
Modification of pertussis toxin
InactiveUS6168928B1Beneficially modifyingHydrolasesMicrobiological testing/measurementBiological propertyPertussis toxin
The three-dimensional structure of crystalline pertussis holotoxin (PT) has been determined by X-ray crystallography. Crystal structures have also been determined for complexes of pertussis toxin with molecules relevant to the biological activity of PT. These three-dimensional structures were analyzed to identify functional amino acids appropriate for modification to alter the biological properties of PT. Similar procedures may be used to predict amino acids which contribute to the toxicity of the holotoxin, to produce immunoprotective, genetically-detoxified analogs of pertussis toxin.
Owner:CONNAUGHT LAB
Proteinaceous adjuvants
Owner:AVENTIS PASTEUR LTD
Application of PITPNM3 gene in preparing medicine for inhibiting breast cancer invasion and transfer
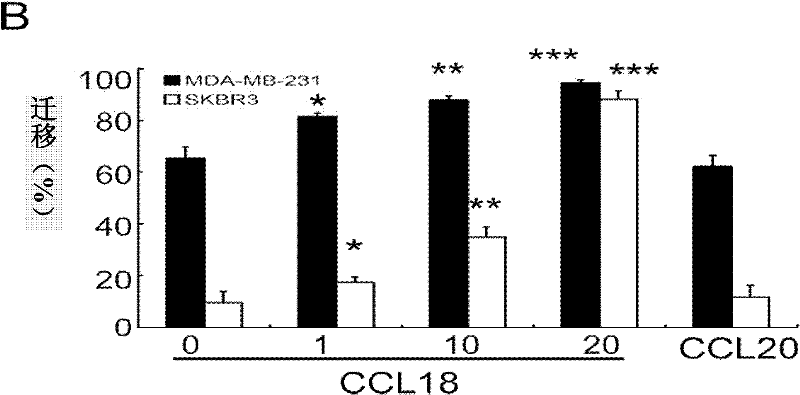
InactiveCN102160895APeptide/protein ingredientsGenetic material ingredientsG protein-coupled receptorPertussis toxin
The invention provides application of PITPNM3 gene in preparing medicines for inhibiting breast cancer invasion and transfer. CCL18 promotes breast cancer invasion and transfer, and the PITPNM3 is identified as a CCL18 receptor and is a new target for inhibiting breast cancer invasion and transfer. The medicine for inhibiting breast cancer invasion and transfer takes a medicine for inhibiting the PITPNM3 gene in a targeted way as an active ingredient. The medicine for inhibiting the PITPNM3 gene in the targeted way takes siRNA expressed by PITPNM3 or / and a G protein coupling receptor inhibitor of pertussis toxin as active ingredients to inhibit the biological effect caused by combination of the CCL18 and the PITPNM3. The invention provides the CCL18 to promote the breast cancer invasion and transfer through the PITPNM3 for the first time, the PITPNM3 is the receptor of the CCL18, and the PITPNM3 receptor can be inhibited in the targeted way so as to inhibit the breast cancer invasion and transfer.
Owner:SUN YAT SEN MEMORIAL HOSPITAL SUN YAT SEN UNIV
Immunogenic compositions for use in vaccination against bordetella
InactiveUS20180071380A1Efficient combinationAntibacterial agentsBacterial antigen ingredientsVaccinationVirulent characteristics
The present application relates to immunogenic compositions comprising a mixture of Bordetella (e.g., B. pertussis) antigens and an oil in water nanoemulsion. In particular, the invention provides immunogenic compositions comprising nanoemulsion and a combination of Bordetella (e.g., B. pertussis) antigens that have different functions, for example, combinations including B. pertussis adherence factors (adhesins), B. pertussis toxins or B. pertussis virulence factors. Vaccines, methods of treatment, uses of and processes to make a pertussis or whooping cough vaccine are also described. Compositions and methods of the present invention find use in, among other things, clinical (e.g. therapeutic and preventative medicine (e.g., vaccination)) and research applications.
Owner:NANOBIO CORP +1
Separation and purification process for acellular whooping cough antigen albumen
InactiveCN1504482ALow costGood effectAntibacterial agentsBacterial antigen ingredientsPertussis toxinElution
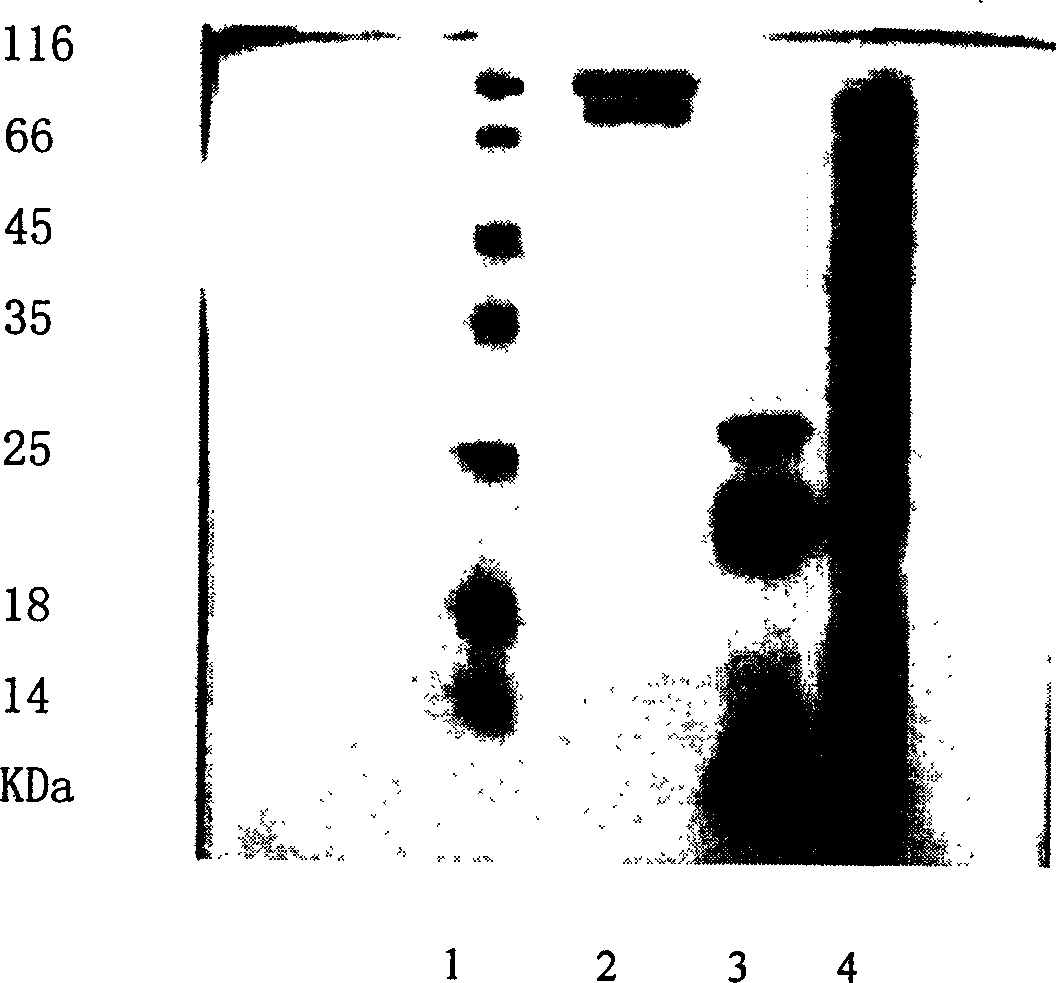
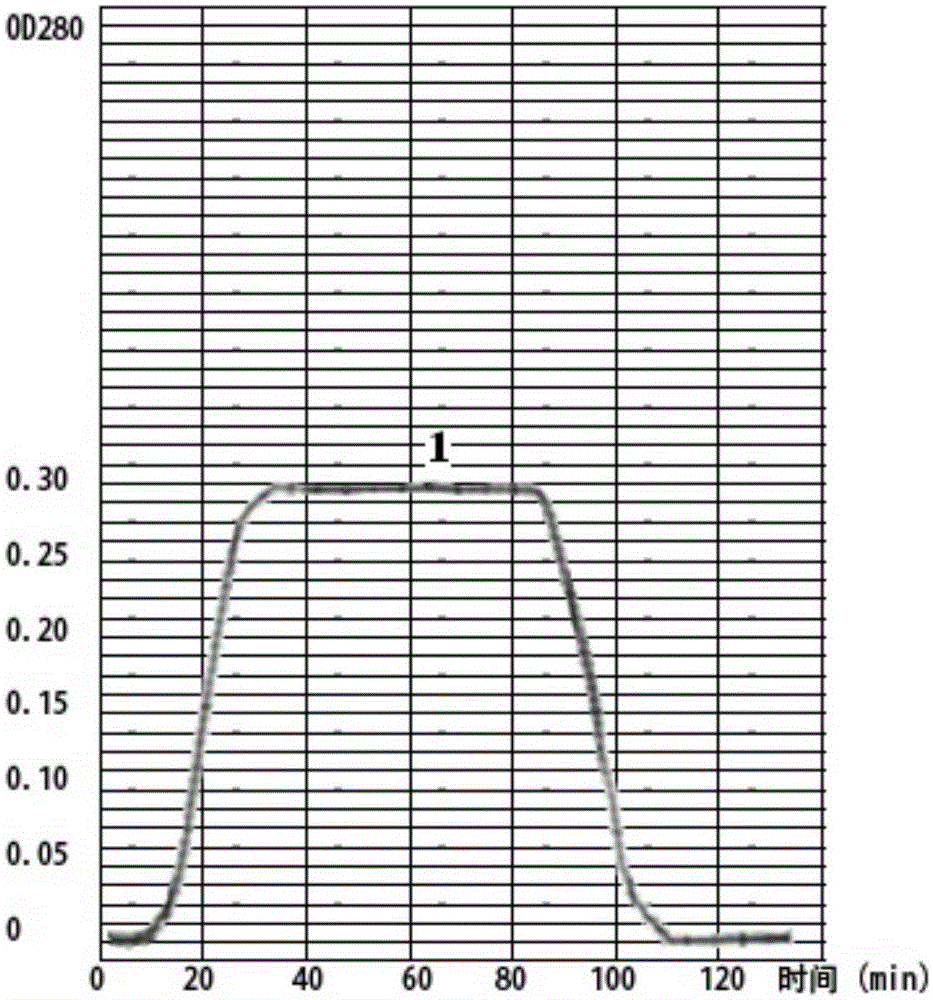
The invention relates to a method for further separating and purifying pertussis antigen for the supernatant fluid of pertussis bacilliculture liquid, wherein the screen selected domestic pearlstone is used as column chromatography material in the process, the sample is concentrated for conductance adjustment processing and elution condition optimized for further segregation and purification, to obtain the Filamentous hamagglutmin (FHA) and pertassis toxin (PT), the polyacrylamide gel electrophoresis (SDS-PAGE) evaluation has shown that its purity is as high as 85-95%.
Owner:YOULIKAI BIOLOGICAL TECH BEIJING
Pertussis vaccine preparation and combined vaccine thereof
InactiveCN104906569AAntibacterial agentsBacterial antigen ingredientsChemical synthesisCarrier protein
The invention relates to a pertussis vaccine preparation and combined vaccine thereof. Pertussis lipooligosaccharide is obtained through bacterial culture purification or chemical synthesis, wherein the pertussis lipooligosaccharide obtained through purification is hydrolyzed to remove the endotoxin activity, the pertussis lipooligosaccharide obtained through chemical synthesis comprises a pertussis lipooligosaccharide tail end trisaccharide structure, and the lipooligosaccharide and carrier protein are coupled to prepare conjugate, namely the pertussis lipooligosaccharide conjugate with immunogenicity. The pertussis toxin is detoxicated pertussis toxin obtained through chemical detoxication or genetic engineering detoxication, and the weight proportion of the pertussis lipooligosaccharide conjugate to the pertussis toxin is 5-20 [mu]g:15-30 [mu]g.
Owner:BEIJING ZHIFEI LVZHU BIOPHARM +2
Non-toxic double mutant forms of pertussis toxin as adjuvants
InactiveUS7169399B2Good immunogenic activityExcellent adjuvantAntibacterial agentsBiocideAntigenGlycine
The invention relates to the use of an antigen which is a non-toxic double mutant form of pertussis toxin for the manufacture of a vaccine composition for intranasal administration to induce an immune response against B. pertussis infection. The invention also relates to the use of a non-toxic double mutant form of pertussis toxin for the manufacture of an adjuvant composition for stimulating or enhancing a protective immune response of an antigen co-administered therewith. The non-toxic double mutant is preferably one in which the glutamic acid 129 amino acid in the S1 sub-unit has been substituted by glycine and the arginine 9 amino acid has been substituted by lysine.
Owner:UCB PHARMA LTD SLOUGH
Acellular pertussis combined vaccine and preparation method thereof
InactiveCN104707134AClear ingredientsLittle side effectsAntibacterial agentsBacterial antigen ingredientsHemagglutininSide effect
The invention discloses an acellular pertussis combined vaccine and a preparation method thereof and belongs to the technical field of production and preparation of vaccines. The acellular pertussis combined vaccine is prepared from the following raw material components: 5-40Mu g of pertussis toxin, 5-40Mu g of filamentous hemagglutinin, 2-10Mu g of pertussis adhesin, 10-25lf of diphtheria toxoid, 4-10lf of tetanus toxoid, 1.0-2.0mg / ml of aluminium hydroxide and 7.5-9.5g / L of sodium chloride. The preparation method of the acellular pertussis combined vaccine comprises the following steps: preparing monovalent vaccine original fluids, mixing and diluting. The acellular pertussis combined vaccine is clear and definite in ingredients, quality control can be easily realized, the side effect is small, and the safety is high; and the preparation method of the acellular pertussis combined vaccine is simple to operate, convenient in preparation and low in cost, so that the acellular pertussis combined vaccine is applicable to industrial mass production.
Owner:CHENGDU OLYMVAX BIOPHARM
Pertussis toxin gene: cloning and expression of protective antigen
A cloned gene encoding the expression of an antigenic mutant pertussis toxin with substantially reduced enzymatic activity has been described.
Owner:UNITED STATES OF AMERICA
Modified pertussis toxin
The development of subunits and subunit analogs of the Bordetella exotoxin by recombinant DNA techniques provides vaccine products that retain their biological activity, are highly immunogenic, and can confer protection against disease challenge. Genetically-engineered modifications of the subunits can result in products that retain immunogenicity, yet are free of enzymatic activity associated with toxin of reactogenicity.
Owner:AMGEN INC
Whooping cough genetic engineering blending second unit vaccine and preparing method thereof
InactiveCN101199843AEfficient expressionOvercome technical difficulties that are difficult to expressAntibacterial agentsBacterial antigen ingredientsSide effectPertussis toxin
The invention relates to a pertussis genetic-engineering fused subunit vaccine, which is fused protein produced by connecting pertussis toxin S1 subunit mutant with a peptide Fs. The invention also relates to a preparation method of bordetella pertussis double-subunit genetic engineering vaccine. The invention, which overcomes a variety of deficiencies of the traditional vaccine and the traditional preparation method, has the advantages of mass production potential, easy operation, high security, small side effect and no reverse mutation.
Owner:ARMY MEDICAL UNIV
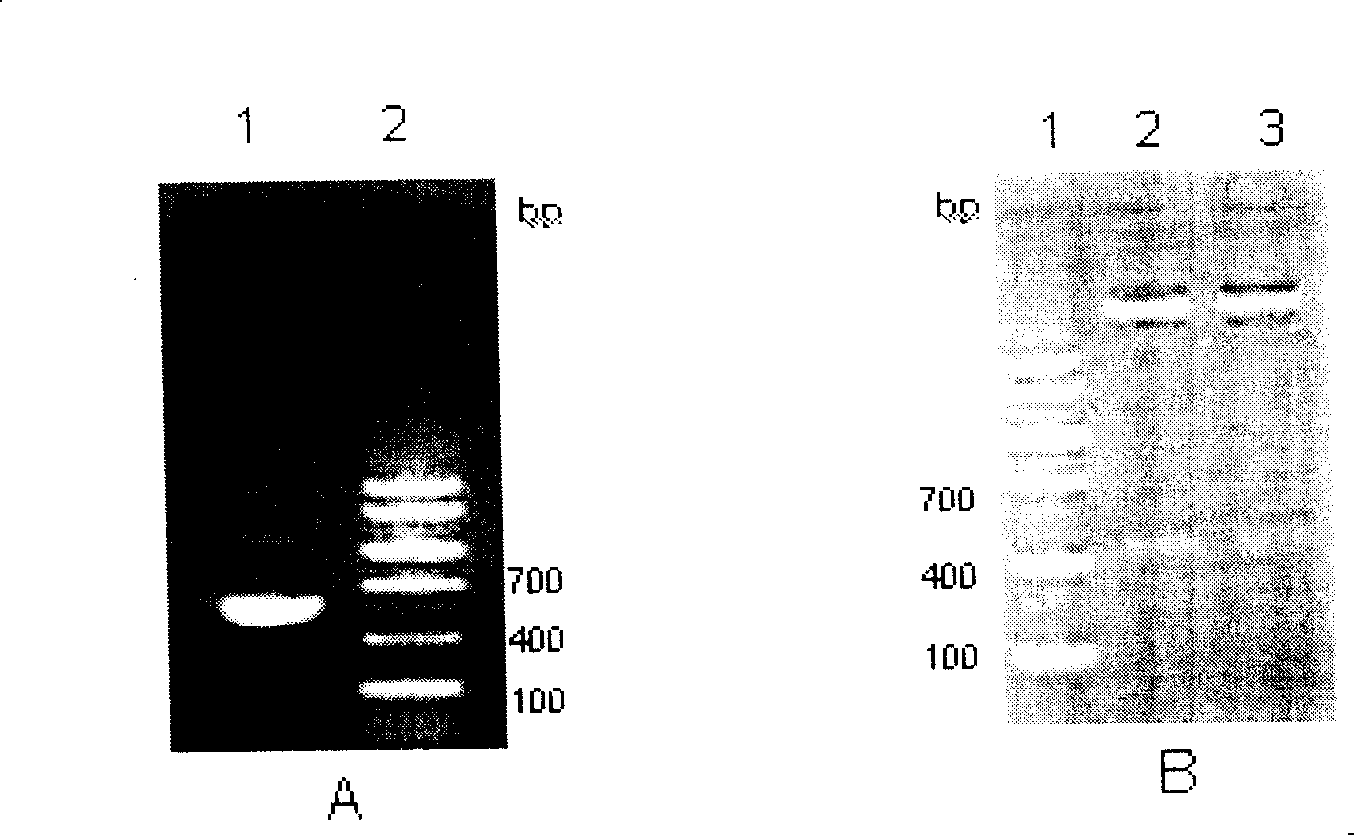
Bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof
InactiveCN105039259AIntegrity guaranteedImproving immunogenicityAntibacterial agentsImmunoglobulins against bacteriaAntigenImmune monitoring
The invention relates to a bordetella pertussis PT (pertussis toxin) antigen monoclonal antibody and application thereof, and belongs to the field of preparation of biological products. The bordetella pertussis PT antigen monoclonal antibody is secreted by hybridoma cell strains capable of stably secreting bordetella pertussis FT antigen monoclonal antibodies, and a preservation number of the hybridoma cell strains is CGMCC No.10588. The bordetella pertussis PT antigen monoclonal antibody and the application have the advantages that the monoclonal antibody is high in potency and good in specificity, and can be applied to monitoring the quality in bordetella pertussis vaccine production procedures, carrying out enzyme-linked immunosorbent assay on contents of PT components in finished vaccine and preparing bordetella pertussis PT antigen enzyme-linked immune monitoring reagents or enzyme-linked immune monitoring reagent kits.
Owner:SINOVAC RES & DEV
Proteinaceous adjuvants
InactiveUS20030072774A1Avoid problemsRegulate immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantSide effect
A modulated immune response to an antigen is achieved by coadministering the antigen and a genetically-detoxified pertussis holotoxin, particularly one retaining its immunogenicity, to a host. The modulated immune response enables immunogenic compositions, including multivalent pediatric vaccines, such as DTP, to be provided which produce a modulated immune response in the absence of extrinsic adjuvants, such as alum. The adjuvanting effect achieved by the genetically-detoxified pertussis holotoxin enables at least the same level of adjuvanting effect to be achieved as previously attained by alum, without the undesirable side effects thereof.
Owner:AVENTIS PASTUER LTD
Separation and purification method for pertussis antigen
InactiveCN105175517AThe separation and purification method is simpleRapid separation and purification methodAntibacterial agentsBacterial antigen ingredientsHemagglutininElectrophoreses
The invention provides a separation and purification method for a pertussis antigen. The separation and purification method comprises the steps of with diatomite and hydroxylapatite as column chromatography materials, after concentrating, regulating and conducting a bordetella pertussis fermentation solution, enriching the pertussis antigen by using the diatomite, and then, purifying by using the hydroxylapatite to obtain pertussis toxin (PT) and filamentous hemagglutinin (FHA). Proven through SDS-PAGE electrophoresis detection, the purities of both a PT protein and an FHA protein are higher than 95%. The method provided by the invention is simple in operation and capable of obtaining a high-purity antigen, not only greatly reducing the production cost, but also remarkably improving the quality of a product and increasing the yield of the product so as to be the simple and rapid separation and purification method for the pertussis antigen; in addition, the economic benefit is high, and the market application prospect is wide.
Owner:BEIJING MINHAI BIOTECH
Method for separating and purifying whooping cough toxins and filamentous hemagglutinin
InactiveCN104311647AQuality is easy to controlEasy to operateDepsipeptidesPeptide preparation methodsHemagglutininUltrafiltration
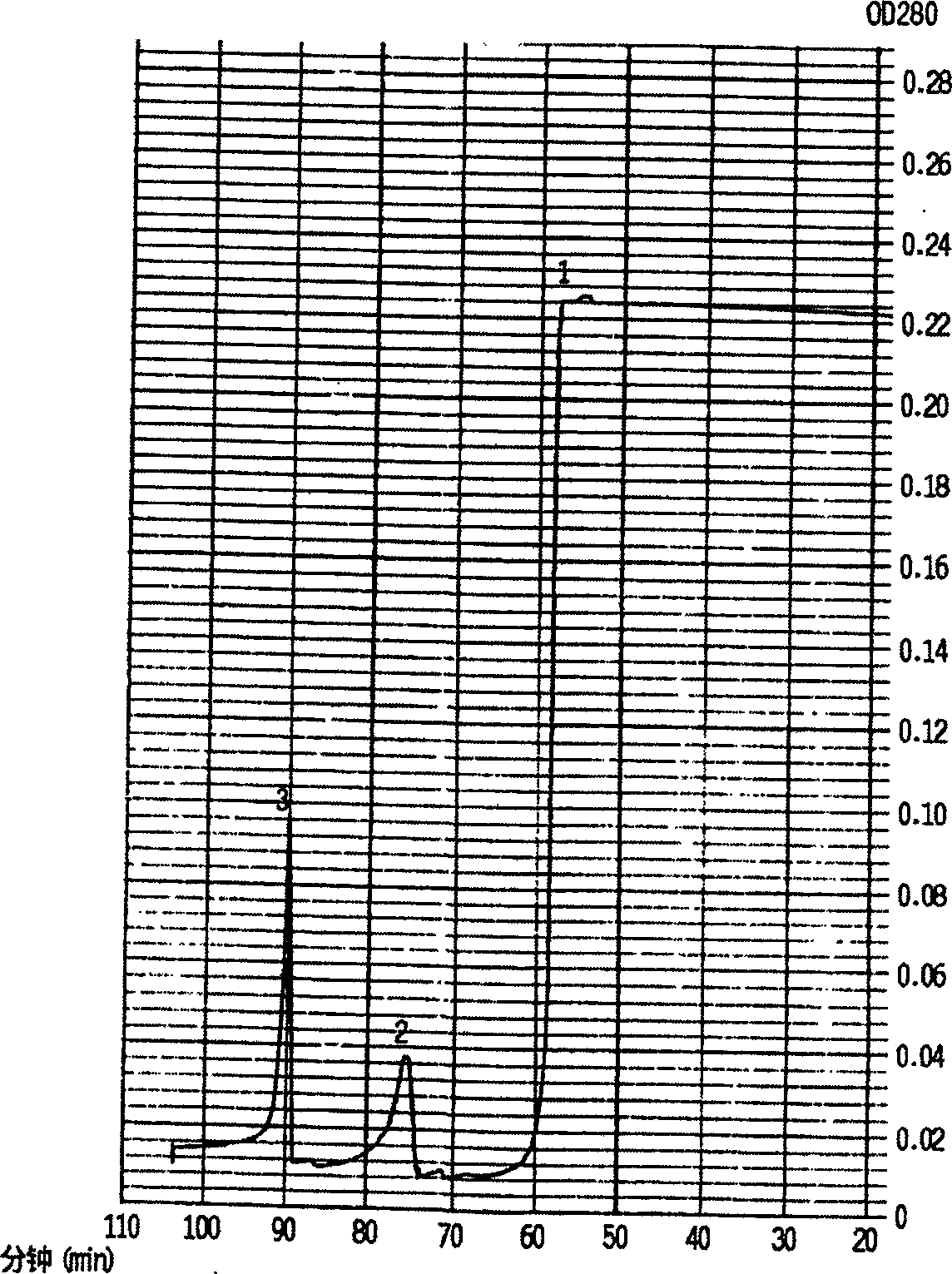
The invention discloses a method for separating and purifying whooping cough toxins and filamentous hemagglutinin. The method comprises the following steps: S1, performing sample treatment, namely ending culture of bordetella pertussis, removing the thallus, collecting the supernatant, and concentrating the supernatant by using an ultrafiltration membrane; S2, performing primary chromatography comprising the sub-steps of eluting hybrid proteins, separating the whooping cough toxins and separating and purifying the filamentous hemagglutinin; and S3, performing secondary chromatography, namely loading a coarse product of the separated whooping cough toxins onto a CaptoMMC chromatographic column, eluting the buffer solution, collecting the eluant at the OD280 elution peak, thereby obtaining the purified whooping cough toxins. With the adoption of the CaptoSPImpRes chromatographic column and the CaptoMMC chromatographic column, the supernatant of the bordetella pertussis culture solution is separated and purified, so that PT and FHA can be accurately obtained, the quality is controllable, the purity of the separated and purified whooping cough antigen proteins is over 95 percent, and the method has the advantages of simplicity in operation, high purification speed and low cost.
Owner:CHENGDU OLYMVAX BIOPHARM
Use of pertussis toxin as a therapeutic agent
The present application relates to the use of a pertussis toxin, and its derivatives, analogs, salts and pharmaceutical equivalents. In one embodiment, the invention provides a method of treating an autoimmune disease by administering pertussis toxin to the individual. In another embodiment, the autoimmune disease is multiple sclerosis. In another embodiment, the invention provides a method of treating a neurodegenerative disease such as Alzheimer's disease or Parkinson's disease by administering pertussis toxin, or a derivative, analog, salt or pharmaceutical equivalent.
Owner:DIGNITY HEALTH
Application of pomalidomide to preparation of medicine for relieving multiple sclerosis
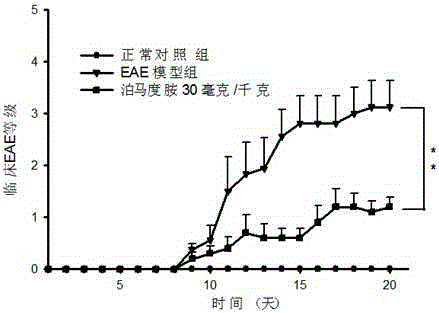
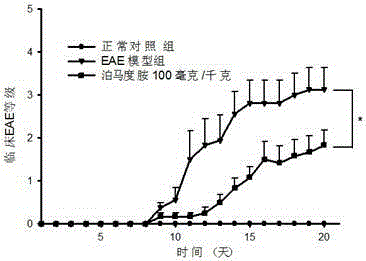
InactiveCN105213392AImprove repair functionImprove protectionOrganic active ingredientsNervous disorderRefractory Multiple MyelomaPertussis toxin
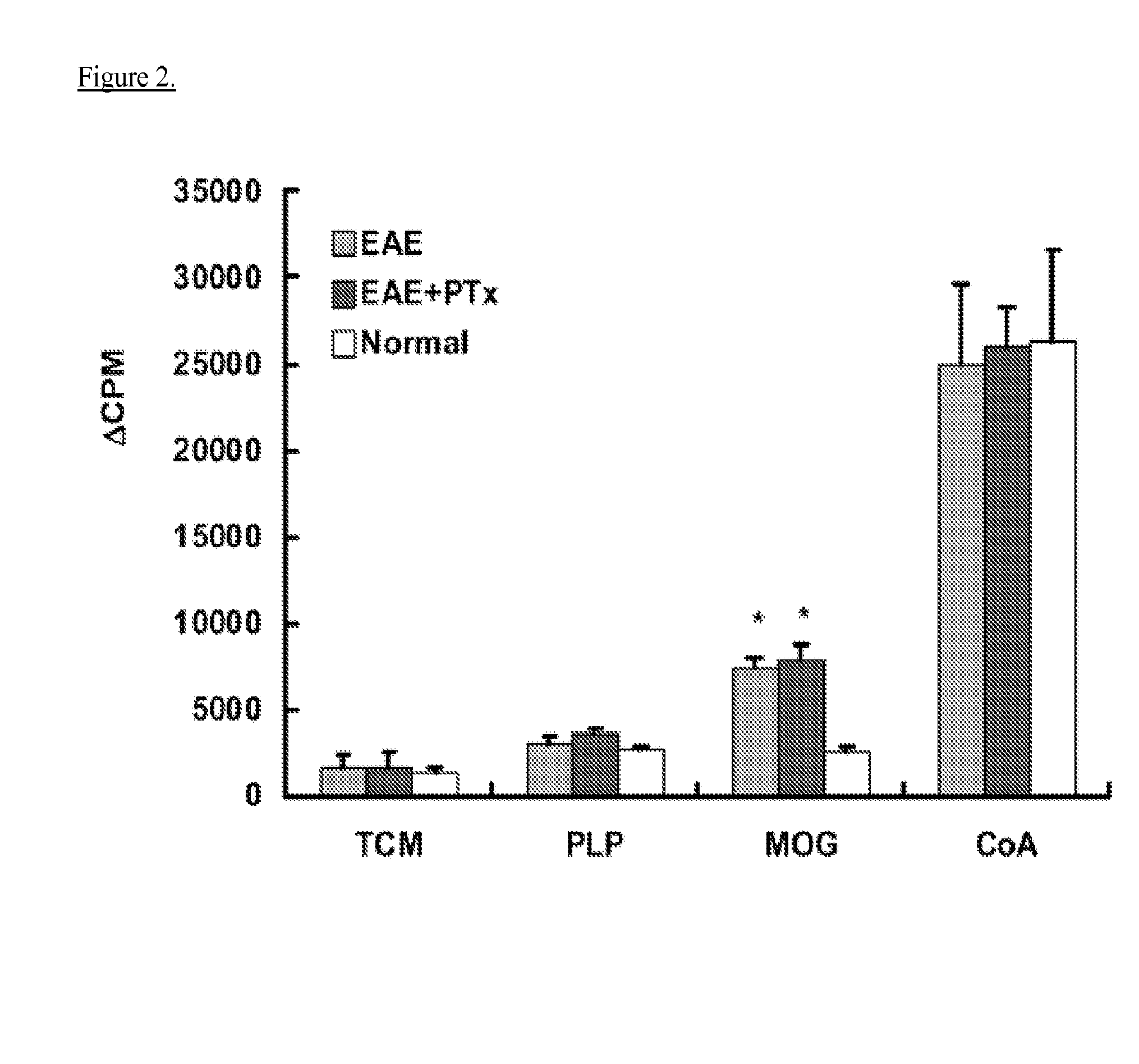
The invention relates to a medicine for treating multiple sclerosis, which is a derivative (pomalidomide) of thalidomide and is mainly applied to recurrent refractory multiple myeloma at present. Non-clinical study testifies that pomalidomide has a remarkable protection effect on EAE (Experiment Allergic Encephalomyelitis) mice with inflammatory demyelination induced by M.Tuberculosis (4 mg / ml), MOG 35-55 (2 mg / ml) and Pertussis toxin (200 ng per mouse), and can be used for treating multiple sclerosis.
Owner:ZHEJIANG UNIV
Use of pertussis toxin as a therapeutic agent
ActiveUS20140113865A1Mitigating clinical motor symptomMinimizing T cell infiltrationNervous disorderPeptide/protein ingredientsPertussis toxinAutoimmune responses
The present application relates to the use of a pertussis toxin, and its derivatives, analogs, salts and pharmaceutical equivalents. In one embodiment, the invention provides a method of treating an autoimmune disease by administering pertussis toxin to the individual. In another embodiment, the autoimmune disease is multiple sclerosis. In another embodiment, the invention provides a method of treating a neurodegenerative disease such as Alzheimer's disease or Parkinson's disease by administering pertussis toxin, or a derivative, analog, salt or pharmaceutical equivalent.
Owner:DIGNITY HEALTH
A method for separation and purification of multiple antigenic components of pertussis
ActiveCN108570098BGuaranteed stabilityHigh recovery rateDepsipeptidesPeptide preparation methodsPertussis toxinCulture fluid
The present invention relates to a method for separating and purifying multiple antigenic components of pertussis, wherein the various antigens are pertussis toxin (PT) antigen, filamentous hemagglutinin (FHA) antigen and pertussis adhesin (PRN) antigen, and the The method comprises the following steps: (1) fermenting and culturing the pertussis strain, and harvesting the culture supernatant and bacterial precipitate respectively; (2) separating and purifying PT antigen from the culture supernatant, and separating and purifying PRN antigen and FHA antigen from the bacterial precipitate.
Owner:WUHAN INST OF BIOLOGICAL PROD CO LTD
Pertussis vaccine micro needle array and preparation method thereof
ActiveCN108379573AAccurate doseOperational securityAntibacterial agentsBacterial antigen ingredientsAdjuvantPertussis toxin
The invention discloses a preparation method of a pertussis vaccine micro needle array. The method comprises the following steps that (1) a micro needle array mold is used; the micro needle array moldcomprises a base plate; a rectangular groove is formed in the upper surface of the base plate; the bottom wall of the rectangular groove is provided with a conical groove with the downward array sharp end; (2) a pertussis toxin water solution and an adjuvant water solution are uniformly mixed to obtain a first solution; the first solution is added into the rectangular groove; still standing is performed under the vacuum conditions, so that the first solution is fully filled in the conical groove; the redundant first solution outside the conical groove is absorbed; drying is performed; (3) a high-molecular polymer solution is smeared in the rectangular groove; drying is performed; a mold with a conical bulge is removed; vacuum drying is performed. The pertussis vaccine micro needle array obtained by the method has the advantages that the dosage is accurate; the product specification is realized; the operation is safe.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI +1
Pertussis toxin gene: cloning and expression of protective antigen
A cloned gene encoding the expression of an antigenic mutant pertussis toxin with substantially reduced enzymatic activity has been described.
Owner:CIEPLAK WITOLD
Use of pertussis toxin as a therapeutic agent
ActiveUS10596233B2Increased blood vessel densityHigh expressionPeptide/protein ingredientsPharmaceutical delivery mechanismDiseasePertussis toxin
The present application relates to the use of pertussis toxin, and its derivatives, analogs, salts and pharmaceutical equivalents. In one embodiment, the invention provides a method of treating or preventing a neurological disease or injury by administering pertussis toxin to the individual.
Owner:DIGNITY HEALTH
Compositions exhibiting ADP-ribosyltransferase activity and methods for the preparation and use thereof
InactiveUS7341733B2Promoting prophylacticPromoting therapeutic responseOrganic active ingredientsPeptide/protein ingredientsPertussis toxinMedicine
Compositions characterized by ADP-ribosyltransferase activity are useful in promoting prophylactic and / or therapeutic responses as are promoted by, e.g., pertussis toxin but directed against another target antigen (e.g., a cancer-related antigen) in a mammalian patient.
Owner:KASLOW HARVEY R
A pertussis vaccine microneedle array and preparation method thereof
ActiveCN108379573BAccurate doseOperational securityAntibacterial agentsBacterial antigen ingredientsAdjuvantPertussis toxin
The invention discloses a preparation method of a pertussis vaccine micro needle array. The method comprises the following steps that (1) a micro needle array mold is used; the micro needle array moldcomprises a base plate; a rectangular groove is formed in the upper surface of the base plate; the bottom wall of the rectangular groove is provided with a conical groove with the downward array sharp end; (2) a pertussis toxin water solution and an adjuvant water solution are uniformly mixed to obtain a first solution; the first solution is added into the rectangular groove; still standing is performed under the vacuum conditions, so that the first solution is fully filled in the conical groove; the redundant first solution outside the conical groove is absorbed; drying is performed; (3) a high-molecular polymer solution is smeared in the rectangular groove; drying is performed; a mold with a conical bulge is removed; vacuum drying is performed. The pertussis vaccine micro needle array obtained by the method has the advantages that the dosage is accurate; the product specification is realized; the operation is safe.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI +1
Novel recombinant bordetella strains
ActiveCN104936976AAntibacterial agentsSsRNA viruses negative-senseHeterologousBordetella pertussis DNA
The present invention relates to a recombinant genetically attenuated Bordetella strain expressing a hybrid protein comprising the N-terminal fragment of filamentous haemagglutinin (FHA) and a heterologous epitope or antigenic protein or protein fragment, different from FHA, wherein the gene coding for the native FHA protein is inactivated. The Bordetella strain is preferably a Bordetella pertussis strain, but may also be another Bordetella species, such as Bordetella bronchiseptica, Bordetella parapertussis or Bordetella avium. The invention further provides a life attenuated vaccine for the treatment of a mucosal or systemic infectious disease comprising a Bordetella strain as defined above intended to elicit a immune response against pathogens responsible for systemic or mucosal infections, including of the upper or lower respiratory tract. The present invention also relates to a method for prophylaxis of an infectious disease in a mammal, comprising administering to said mammal an effective amount of a vaccine comprising in a suitable vehicle a recombinant attenuated Bordetella strain expressing a fusion protein comprising the N-terminal fragment of filamentous haemagglutinin (FHA) and a heterologous epitome or antigenic protein or protein fragment, different from FHA, wherein the gene coding for the native FHA protein is inactivated.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com