Pertussis vaccine preparation and combined vaccine thereof
A combined vaccine and pertussis technology, which can be used in drug combinations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as insufficient direct evidence.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
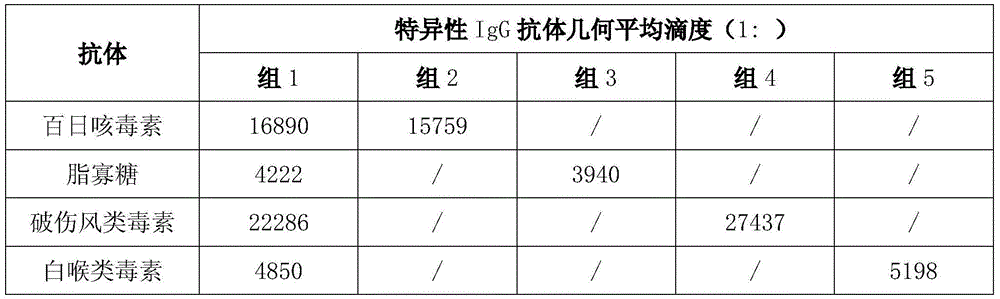
Image
Examples
Embodiment 1
[0037] Fermentation culture of pertussis bacteria
[0038] Open the strain of Bacillus pertussis, inoculate it on the modified bag-ginger medium (Bordet-Gengou), cultivate it at 35-37°C for no more than 72 hours, and transfer it to S-S medium (Stainer-Scholte) for no more than 48 hours hour to prepare the seed solution. Inoculate the seed solution in a fermenter containing liquid medium, the initial seed concentration OD 600 0.1-0.4, cultured at 35-37°C to bacterial concentration OD 600 Stop fermentation at 5.0-10.0, take samples for Gram staining microscopic examination and pure bacteria experiment. Add thimerosal to the non-polluting culture to sterilize it, and separate the bacteria and culture solution by a continuous flow centrifuge for future use.
Embodiment 2
[0040] Pertussis toxin extraction and detoxification
[0041] Example 1 The culture solution harvested by centrifugation was concentrated by ultrafiltration and replaced with a buffer solution (pH7.6) of 0.2M PB and 0.5M NaCl. After filtration and clarification, the sample was loaded on Butyl Sepharose (Butyl) hydrophobic chromatography After the column was fully washed with buffer, it was eluted with 0.2M PB (pH 6.2), and the eluate was collected. After diluting the eluate with water for injection, load it onto a Trisacryl Blue column with 0.05M PB (pH 6.2), wash it thoroughly, and elute linearly with 0.1M PB (pH 7.6) with a NaCl concentration of 0–500mM to collect the target peak . After the eluate was concentrated by ultrafiltration, it was loaded on a Sephacryl S-200 chromatographic column, and the elution peak before Kd 0.1 was collected to obtain the target protein pertussis toxin.
[0042] Add glutaraldehyde to the pertussis toxin to a final concentration of 0.5±0.1%,...
Embodiment 3
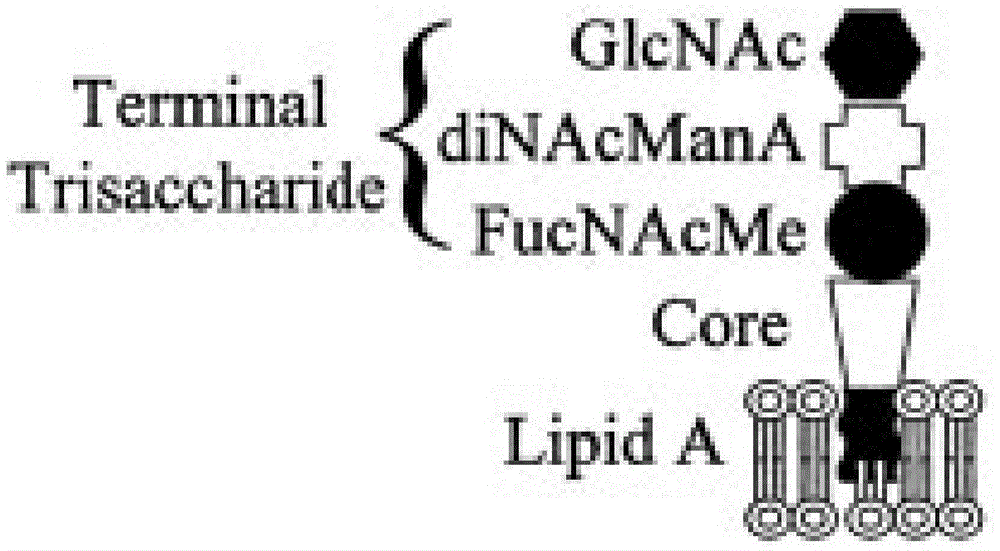
[0044] Lipooligosaccharide extraction and conjugate preparation
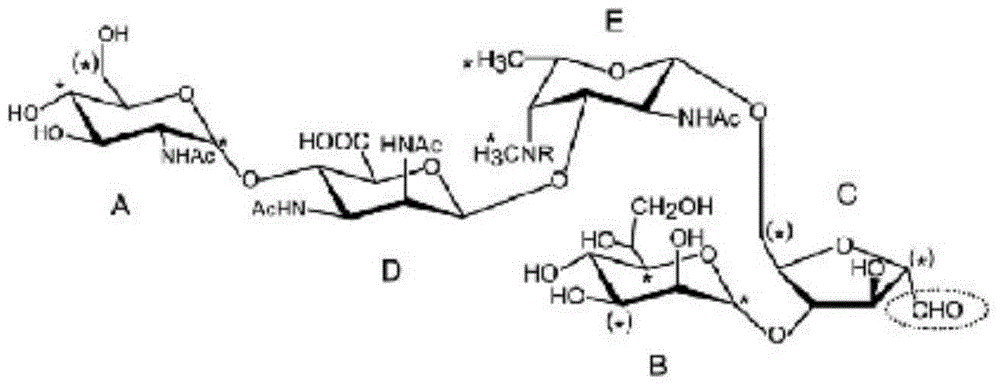
[0045] Extract lipooligosaccharides by conventional hot phenol method, collect them by ultracentrifugation, dissolve them into water for injection / 5% sodium nitrite / 30% acetic acid solution mixed at the same ratio at a concentration of 2-3mg / ml, incubate at 25°C for 4 hours, 200,000g After ultracentrifugation for 2 hours, the supernatant was collected, concentrated and purified by Bio-Gel P-2 chromatography to obtain detoxified lipooligosaccharides.
[0046] Mix and dissolve detoxified lipooligosaccharide and tetanus toxoid with 0.5M dipotassium hydrogen phosphate solution (pH 9.0), wherein the dissolved concentration of detoxified lipooligosaccharide is 10 mg / ml, and the dissolved concentration of tetanus toxoid is 2.5 mg / ml, after adding sodium borocyanide equivalent to sugar, airtight and react at 37°C for 7 days, the reaction solution was purified by Sephadex G-100 to obtain lipooligosaccharide conjugates. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com