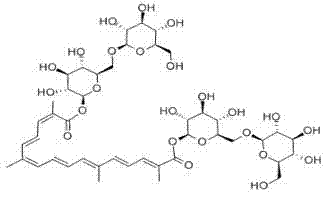
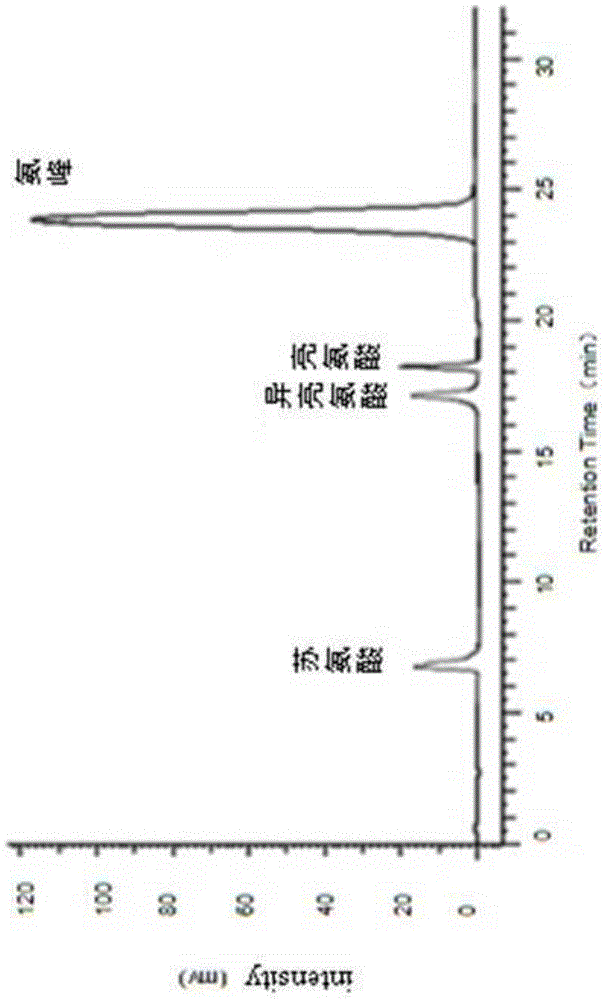
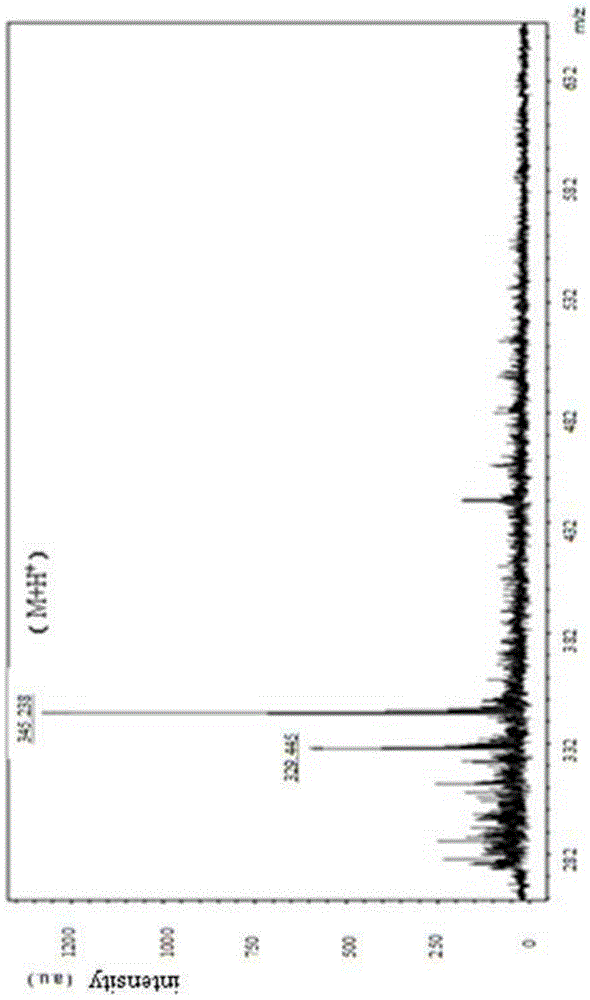
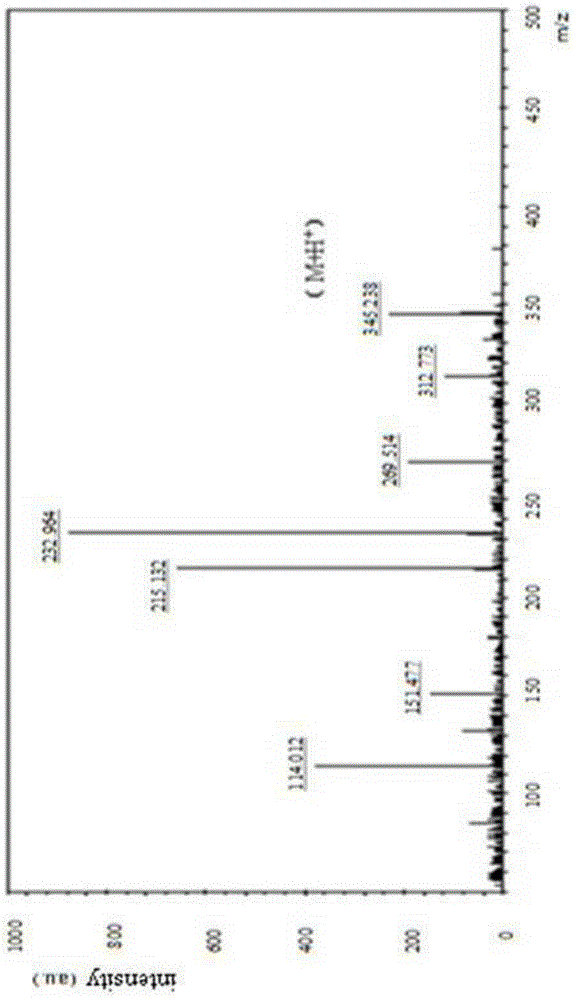
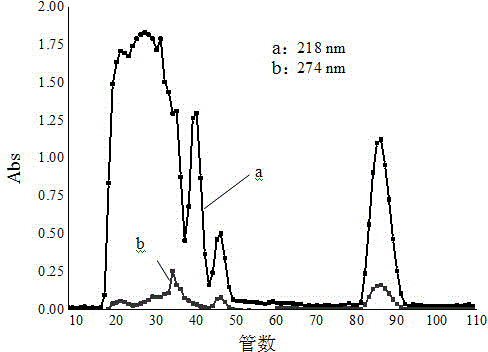
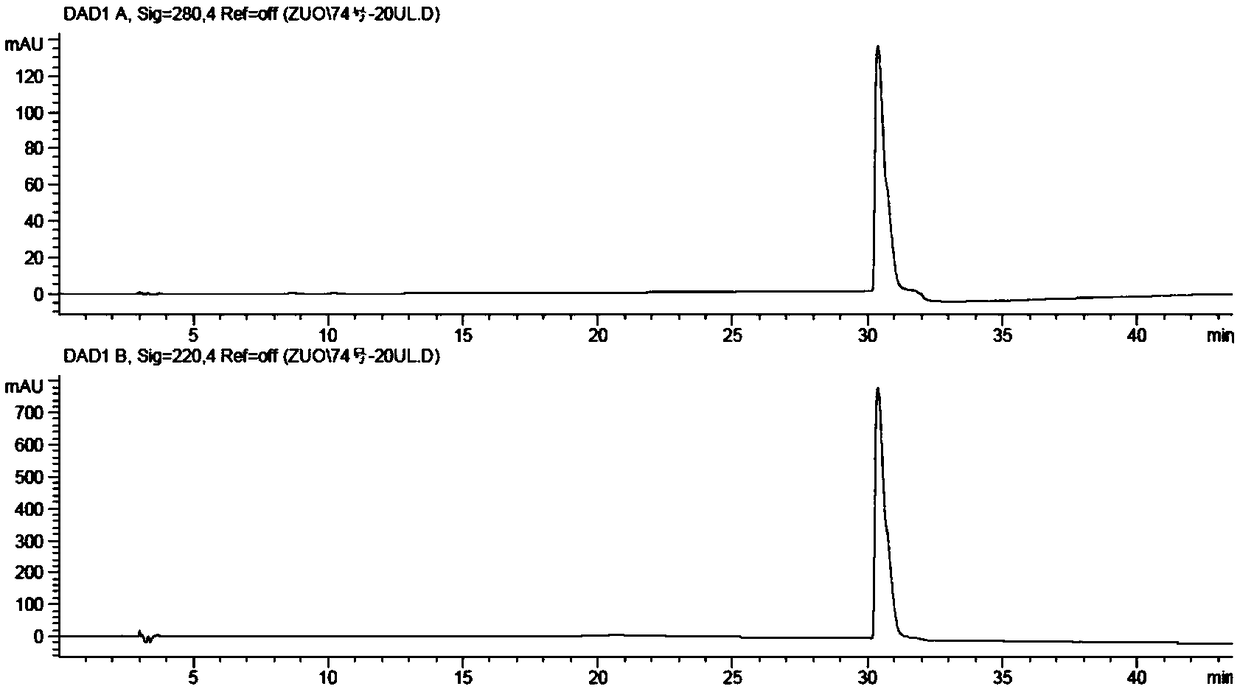
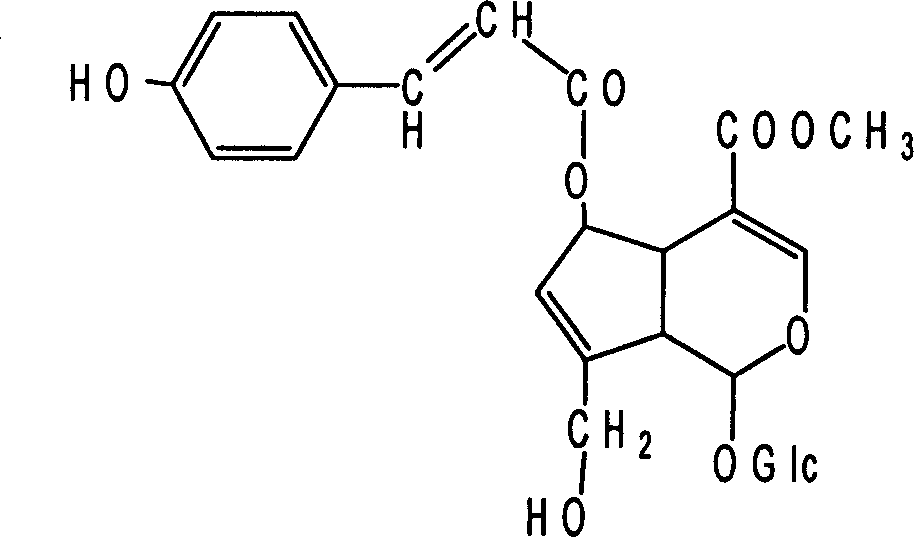
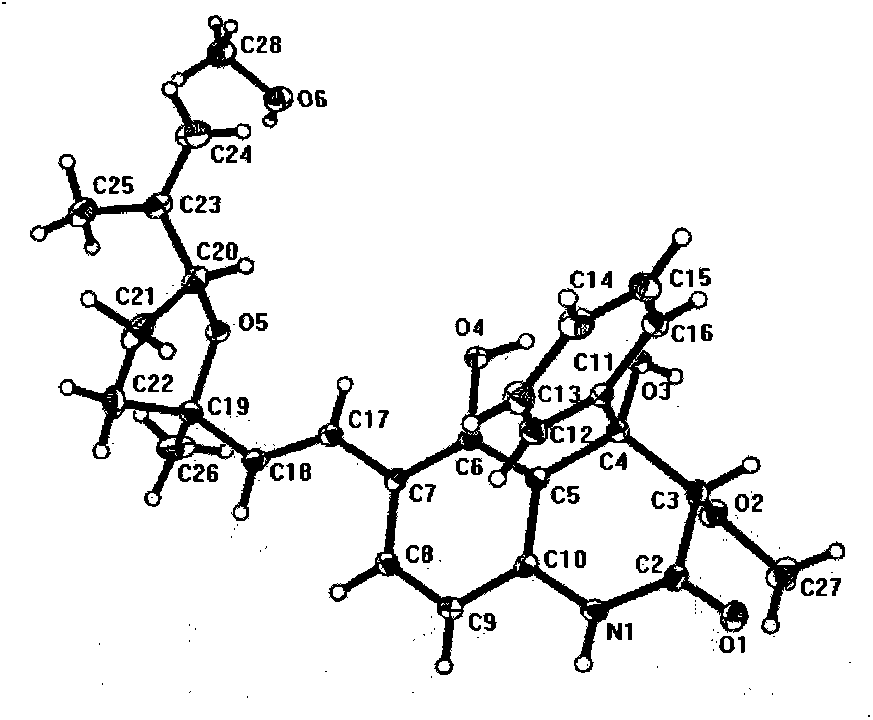
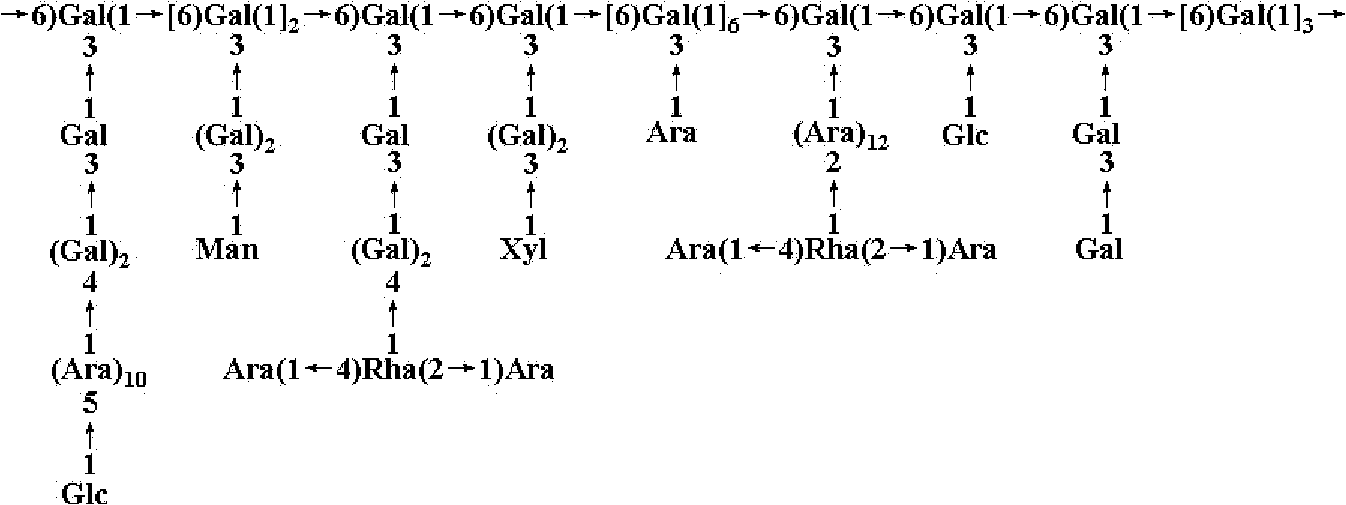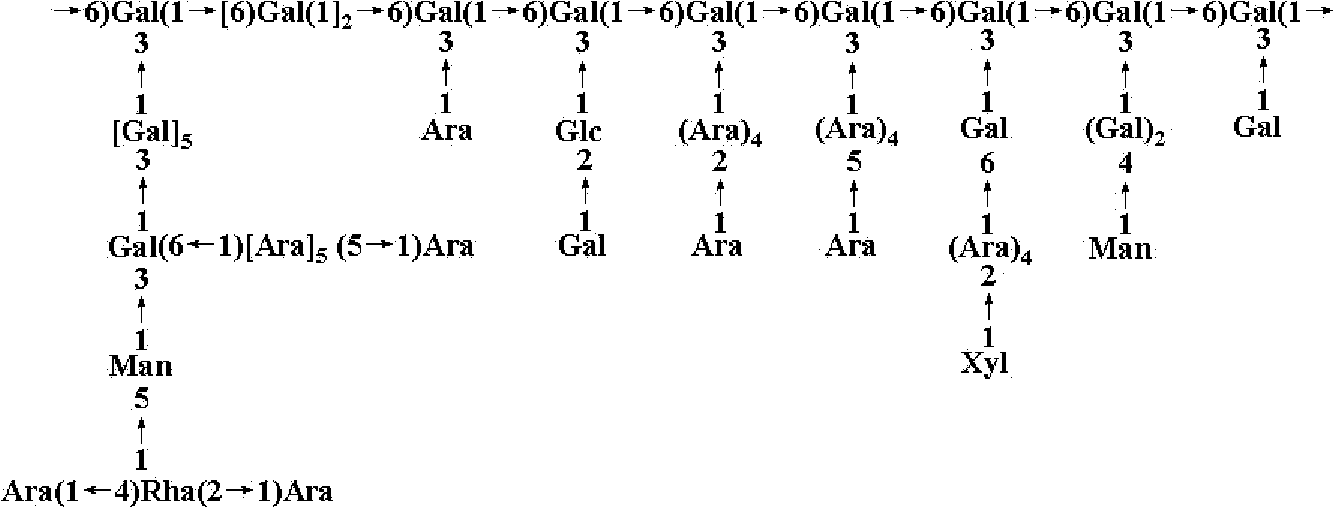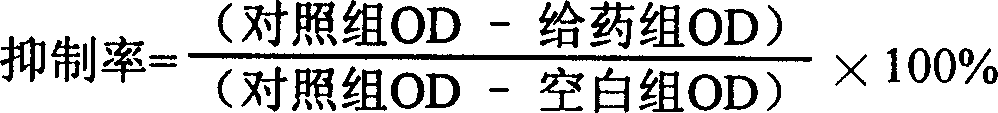Patents
Literature
884 results about "Sephadex" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
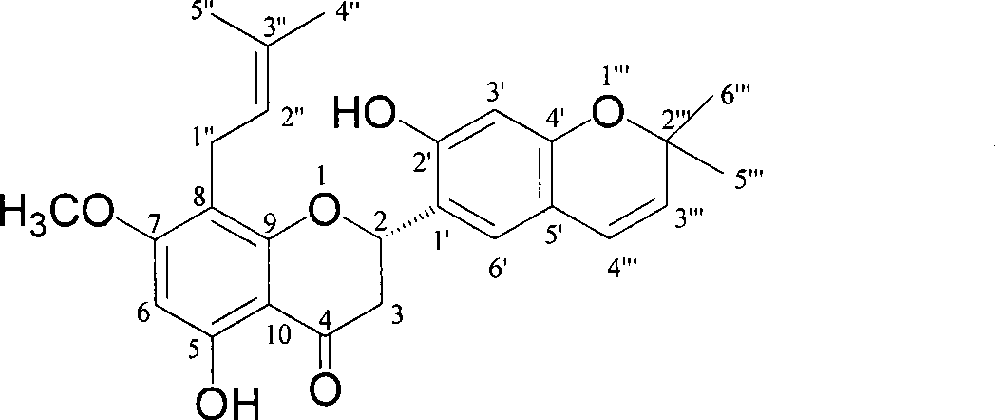
Sephadex is a trademark for cross-linked dextran gel used for gel filtration. It was launched by Pharmacia in 1959, after development work by Jerker Porath and Per Flodin. The name is derived from separation Pharmacia dextran. It is normally manufactured in a bead form and most commonly used for gel filtration columns. By varying the degree of cross-linking, the fractionation properties of the gel can be altered.
Sea cucumber polypeptide, preparation method and application thereof
The invention discloses a sea cucumber polypeptide, wherein a preparation method thereof comprises the following steps: (1) getting fresh stichopus japonicus, removing entrails, cleaning and crushing the stichopus japonicus into small blocks, and adding water to form homogenate; after the enzymolysis of the homogenate liquid, centrifugating and getting the supernatant; adding ethanol, standing, centrifugating and getting the supernatant, concentrating the supernatant at reducing pressure and drying the concentrate to form a crude product of the sea cucumber polypeptide; (2) dissolving the crude product of the sea cucumber polypeptide by using distilled water, carrying out gel filtration chromatography by Sephadex LH-20, eluting the sea cucumber polypeptide by double-distilled water, getting a second peak, collecting active components, and condensing, freezing and freeze-drying the active components; and (3) dissolving the active components by double-distilled water, carrying out ion exchange chromatography by Q Sepharose Fast Flow, linearly eluting the active components by NaCl solution, desalting, getting the second peak, collecting the active components, and condensing, freeze-drying the active components to form the sea cucumber polypeptide. The sea cucumber polypeptide can be used for preparing medicaments or health-care products for increasing leukocytes and also can be used for preparing medicaments or health-care products for multiplying marrow cells after chemotherapy.
Owner:SHANDONG UNIV
Method for extracting sulforaphen
The invention discloses a method of extracting sulforaphen. The smashed seeds of west orchids are added with two to three times as much water, the pH value is regulated to 3.8 to 4.2, ascorbic acid is added, and hydrolysis lasts seven to nine hours under the temperature of 20 to 30 DEG C; after being refrigerated and dried, the raw solution is added with fifteen to twenty five times as much propanone, supersonic extraction lasts forty to eighty minutes, and pumping filtration or double-gauze filtration follows; under the temperature of 30 to 50 DEG C, filtrate undergoes vacuum concentration in order to obtain crude sulforaphen; the crude sulforaphen is chromatographed by a silica gel column and gradiently eluted by n-hexane acetone solution, the thin-layer chromatography or HPLC tests and determines fractions containing the sulforaphen, and the crude sulforaphen undergoes vacuum concentration under the temperature of 30 to 50 DEG C; after being resolved in a small amount of acetone, the crude sulforaphen is chromatographed by a Sephadex LH-20 column, the acetone is used as eluent, the thin-layer chromatography or HPLC tests, determines and collects liquor containing the sulforaphen, and by vacuum concentration under the temperature of 30 to 50 DEG C, a sulforaphen product with eighty percent purity is produced. The method of the invention can also be used to extract the sulforaphen from the seeds of radishes, cabbages and mustards.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Giant salamander active peptide and application
ActiveCN106047968AImprove immunityPromote proliferationPeptide preparation methodsFermentationFiltration membraneHydrolysate
The invention discloses a giant salamander active peptide and an application. The giant salamander active peptide is prepared by the following steps: conducting enzymatic hydrolysis on giant salamander meat which serves as a raw material by virtue of a complex enzyme of marine alkaline protease and papain; separating an enzymatic hydrolysate by virtue of a trypsin immobilized ultra-filtration membrane separator; and then conducting separation and purification by virtue of Sephadex LH-20 molecular sieve chromatography and high performance liquid chromatography, so that the giant salamander active peptide is obtained. The giant salamander active peptide is capable of effectively scavenging free radicals and boosting body immunity, and meanwhile, the active peptide can also promote proliferation of skin fibroblasts; therefore, the giant salamander active peptide has a broad application prospect in the fields of food, medicines and cosmetics.
Owner:ZHANGJIAJIE JINCHI ANDRIAS DAVIDIANUS BIOLOGICAL SCI
Method for preparing antibody-maytansine alkaloid medicine conjugate
ActiveCN103254311AReduce hidden dangersImprove efficiencyImmunoglobulins against cell receptors/antigens/surface-determinantsPeptide preparation methodsAnion-exchange chromatographyAlkaloid
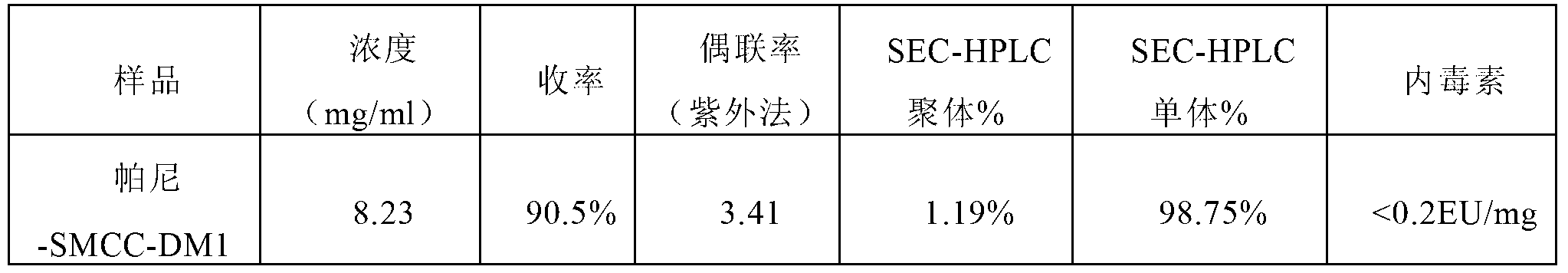
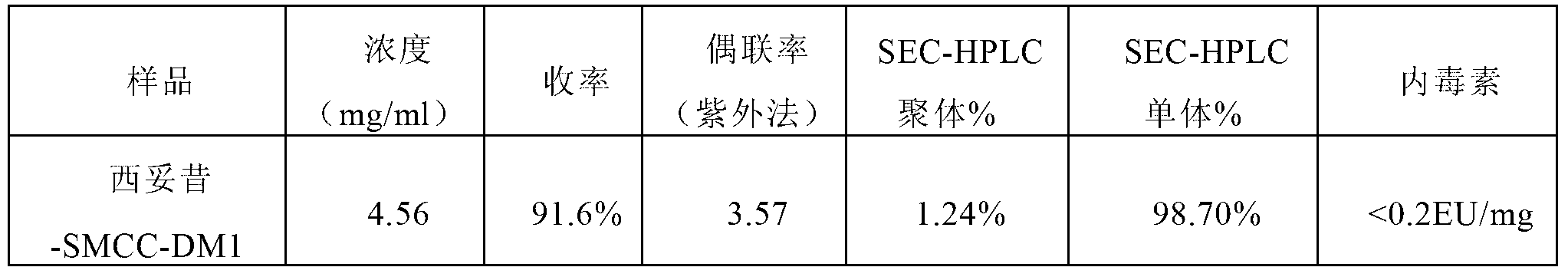
The invention relates to a method for preparing antibody-maytansine alkaloid medicine conjugate. The method comprises the following steps of replacing the antibody into a reaction buffer solution; dissolving a dual-function bridging agent-maytansine alkaloid with an organic solvent so as to prepare the mother liquor of maytansine alkaloid medicine; mixing the replaced antibody with the mother liquor of maytansine alkaloid medicine for coupled reaction for 1-4 hours at 20-30 DEG C; and carrying out anion exchange chromatography on the reaction liquid, conducting Sephadex TMG25 desalination chromatographic column purification on collected flow liquid, and collecting a first peak sample as the prepared antibody-maytansine alkaloid medicine conjugate. The prepared antibody-maytansine alkaloid medicine conjugate is proper in coupling rate, high in purity and low in endotoxin content.
Owner:QILU PHARMA CO LTD
Process for preparing glucose-reducing sand sagebrush polysaccharide and its use
InactiveCN1654481AReduce contentAvoid damageOrganic active ingredientsMetabolism disorderChromatographic separationLiver and kidney
The present invention is sand sage polysaccharide, prepared with sand sage seed deoiling dreg as material and through extraction, separation, enzymolysis and chromatographic separation, and its preparation process and application. The preparation process features 50-80 concentration alcohol solution extraction, extraction with mixed chloroform and normal butyl alcohol liquid, enzymolysis with papain, and chromatographic separation in sephadex G-20 column. Sand sage polysaccharide is used in treating alloxan diabetes, reducing the damage of liver and kidney caused by diabetes, lowering the content of glutamic-oxaloacetic transminase, glutamic-pyruvic transminase and urea nitrogen in blood, and inhibiting diabetes caused weight loss.
Owner:NORTHWEST NORMAL UNIVERSITY
Oyster polysaccharide, preparing method and its application in preparing cosmetics
ActiveCN101012285AImprove smoothnessIncrease moist feelingCosmetic preparationsToilet preparationsSephadexMolecular sieve
The invention discloses an oyster polysaccharide (alpha-1, 4; 1, 6-gluglucosan and beta-1, 6-gluglucosan with relative molecular weight at 4000-6500Da) and making method and application in the cosmetics, which comprises the following steps: washing Dalian bay oyster or Atlantic oyster through water at 0-10 deg.c; boiling oyster at 70-80 deg.c under 0.3-0.5 Mpa for 20-45min; filtering; collecting supernatant; centrifuging to remove sediment; adjusting pH value of supernatant to 5-6 through acetic acid; acidolyzing for 1-3h; intercepting material with molecular weight less than 30000Da through film separator; adjusting pH value of filtrate over 30000Da to 6-8 through NaOH; centrifuging to remove sediment; dialyzing supernatant in the distilled water; passing tomographic column of Sephadex G-100 molecular sieve; collecting material at adsorbing peak with OD at 280 nm; freezing; drying.
Owner:源美生物技术开发(大连)有限公司
Triple-enzyme hydrolysis preparation method of anti-tumor polypeptides of spirulina
ActiveCN104561208APromote development and utilizationHas inhibitory effectPeptide preparation methodsFermentationHydrolysateChymotrypsin
The invention provides a triple-enzyme hydrolysis preparation method of anti-tumor polypeptides of spirulina. The method comprises the following steps: preparing a solution having a concentration of 5 percent from spirulina powder with ultrapure water; repeatedly freezing and thawing, homogenizing and performing ultrasonic treatment, centrifuging to obtain supernate, freezing and drying for later use; adding the prepared protein fluid into pepsin for enzyme hydrolysis, controlling the pH value, adding trypsin for enzyme hydrolysis, adding chymotrypsin for enzyme hydrolysis, controlling the pH value, deactivating enzyme, cooling, and centrifuging to obtain supernate; sequentially filtering with ultrafiltration membranes having molecular weight cut-off of 10KD, 5KD and 3KD respectively to obtain three kinds of spirulina protein enzymatic hydrolysates; carrying out sephadex G15 column chromatography on 0-3K of enzymatic hydrolysate, eluting with water, and collecting four polypeptide ingredients, namely anti-tumor polypeptides of spirulina. The obtained spirulina polypeptide and monopeptide ingredients can be used for establishing a theoretical basis for development and utilization of anti-tumor medicines and health foods.
Owner:SOUTH CHINA UNIV OF TECH
Mytilus edulis enzymolysis polypeptide and preparation method and application thereof
ActiveCN102558296AStrong inhibitory effect on proliferationSimple processHydrolysed protein ingredientsPeptide preparation methodsProstate cancer cellChromatographic separation
The invention discloses a mytilus edulis enzymolysis polypeptide. The mytilus edulis enzymolysis polypeptide is characterized by containing the following amino acid sequence: Asp Leu Tyr. The mytilus edulis enzymolysis polypeptide is prepared by adopting the following steps of: (1) preparing homogenate from mytilus edulis meat, adding alkaline protease, deactivating the protease, centrifuging, and taking clear solution of the upper layer; (2) performing ultra-filtration on the clear solution, collecting hydrolysate with the molecular weight of below 3K, concentrating, and performing freeze drying; (3) performing chromatographic separation by adopting a DEAE-SepharoseFF ion exchange column; (4) performing chromatographic separation by adopting a Sephadex G-25 gel column; and (5) performing high performance liquid chromatography purification. The invention also discloses application of the mytilus edulis enzymolysis polypeptide prepared by the steps in prostatic cancer resistance. Compared with the prior art, the invention has the advantages that: the mytilus edulis is subjected to enzymolysis and purification by adopting an optimal protease and an optimal technology, a strong cell proliferation inhibiting effect is achieved when the obtained target peptide is applied to prostatic cancer resistant cells, and a feasible research path is provided for resisting prostatic cancer.
Owner:ZHEJIANG OCEAN UNIV
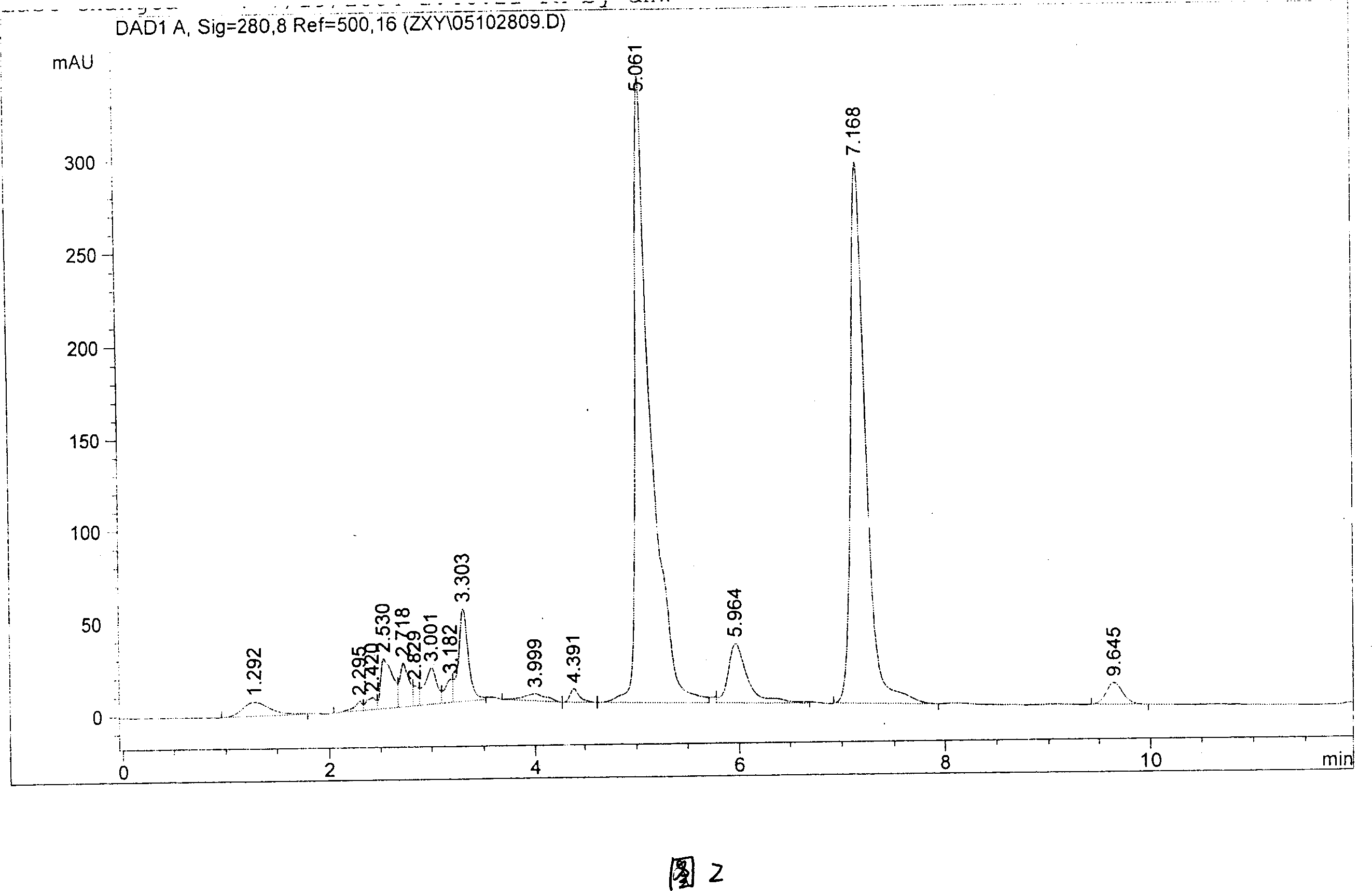
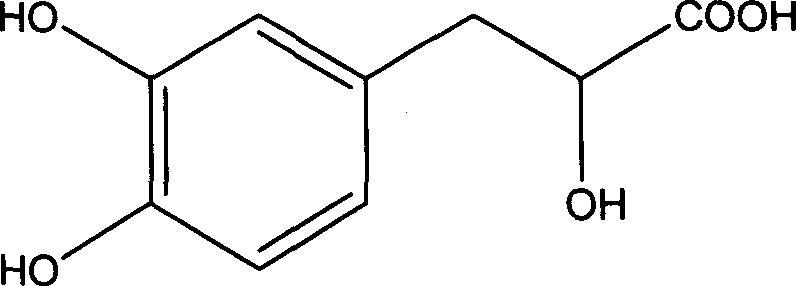
Method of preparing high purity Danshensu
InactiveCN101012163ASimple processThe process is easy to controlCarboxylic compound separation/purificationPlant ingredientsSephadexOrganic solvent
The invention discloses a making method of high-purity tanshinone, which comprises the following steps: (1) heating and refluxing to extract salvia miltiorrhizae root and stalk through 0.1-6.0mol / L acid water / alcohol solution; (2) adsorbing extract through adsorbing column with large-hole resin adsorbent; (3) eluting through water until pH value is 7 first and hydrophilic solvent then; collecting 3-5 column bulks to elute tanshinone completely; (4) evaporating; adding organic solvent; heating; dissolving; crystallizing; or adopting polyamide, gluglucosan gel Sephadex LH-20, MCI GEL CHP20P, C4, C8 or C18 bond phase; obtaining the product.
Owner:上海朗萨医药科技有限公司
Treatment method for enhancing stability of blueberry cyanidin
InactiveCN103435589AImprove featuresImprove function and effectOrganic chemistryGallic acid esterSephadex gels
The invention relates to a treatment method for enhancing stability of blueberry cyanidin. The treatment method comprises the following steps: extracting with a 0.5% trifluoroacetic acid (TFA) methanol solution, and sequentially purifying through an ion exchange resin column Amberlite XAD-7 and a Sephadex column Sephadex LH-20 to obtain a blueberry cyanidin refined substance; carrying out nutgall acylation reaction on the blueberry cyanidin refined substance and prepared triacetyl nutgall acyl chloride to introduce galloyl group with ortho-triphenolhydroxy group into the molecular structure, thereby obtaining the modified product nutgall acylated cyanidin. When high-performance liquid chromatography is used for determining the gallic acid amount generated after hydrolyzing the modified product, the nutgall acylation degree is 55-63%. The experiment proves that the stability of the modified product is obviously enhanced. The method for treating blueberry cyanidin is simple to operate, and has the advantages low cost, low pollution and high acylation degree. The molecular modification can enhance the primary characteristics of the blueberry cyanidin, so that the effects are enhanced; and multiple functional groups are introduced to endow the blueberry cyanidin with new physiological activity, thereby widening the application range.
Owner:BEIJING FORESTRY UNIVERSITY
Separation and purification method of crocin I monomer and crocin II monomer
ActiveCN103665060AAvoid wastingEasy to separateEsterified saccharide compoundsSugar derivativesChromatographic separationChinese traditional
The invention relates to a method capable of synchronously separating and purifying a crocin I monomer and a crocin II monomer, and belongs to the technical field of separation and purification for active ingredients of Chinese traditional medicines. The method comprises the following steps: taking dried stigmas of Crocus sativus as raw materials, performing 90-95% ethanol extraction, concentration, filter, efficient preparative liquid chromatography separation, product recovery and sephadex gel column chromatography to obtain the crocin I monomer and the crocin II monomer. By virtue of the optimum technique and parameter conditions, the content of the crocin I monomer and the crocin II monomer in the product is up to 99% above respectively; the method is stable in the whole technical process, convenient to operate, high in separation efficiency and low in cost, and can separate out and prepare the crocin I monomer and the crocin II monomer with high purity and large scale.
Owner:CHENGDU PUSH BIOLOGICAL TECH
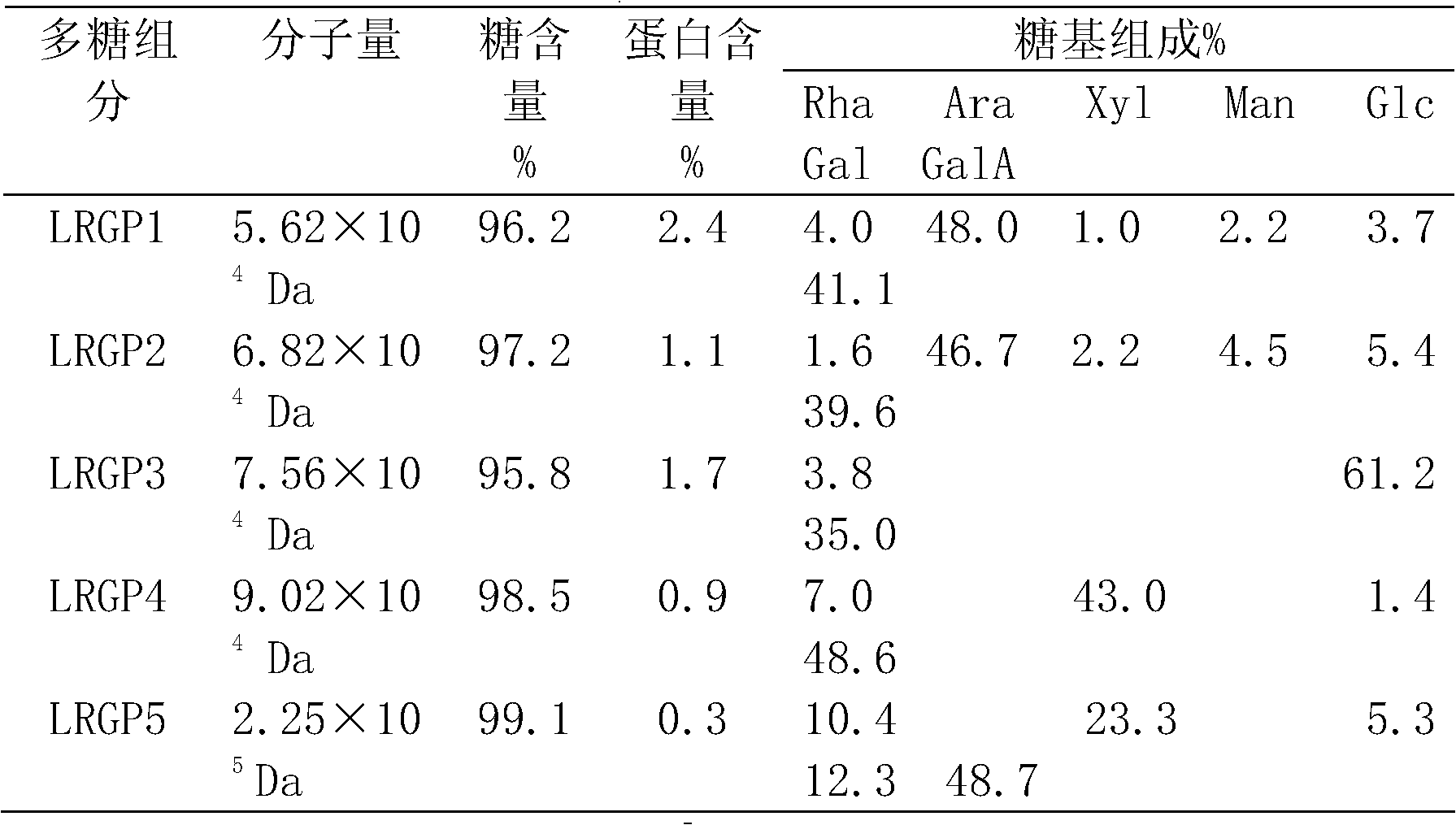
Separation and purification method of lycium ruthenicum polysaccharide and five polysaccharides obtained through separation
The invention relates to a separation and purification method of a lycium ruthenicum polysaccharide and five polysaccharides obtained through separation. Lycium ruthenicum fruits serve as raw materials and a polysaccharide purified product is obtained through extraction by means of ultrasound heating, depoteinization by trichloroacetic acid, decoloration by hydrogen peroxide, diethyl-aminoethanol (DEAE) cellulose column chromatography, Sephadex G-100 column chromatography and the like. According to structure analysis, the lycium ruthenicum polysaccharide is arabinogalactan in multiple branches. The extraction process is performed under a relatively moderate condition and a carbohydrate chain portion on polysaccharide molecules is conserved completely. After purification, five different polysaccharide components are obtained. The five polysaccharides obtained through separation are clear in structure, controllable in quality and significant in the fields of food, cosmetic, health care products, medicines and the like.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for extracting polygahatous polysaccharides from traditional Chinese medicine rhizoma polygonati
The invention discloses a method for extracting polygahatous polysaccharides from traditional Chinese medicine rhizoma polygonati. The method comprises the steps of cleaning, drying and grinding the rhizoma polygonati; performing secondary microwave auxiliary extraction on rhizoma polygonati powder through hexanol serving as a solvent, filtering and combining secondary extraction liquid; recycling the hexanol from the combined extraction liquid, then concentrating the extraction liquid, adding ethyl alcohol, performing uniform stirring, putting into an ultrasonic oscillator for ultrasonic oscillation, standing, performing centrifugal filtration to obtain precipitates, and drying the precipitates to obtain coarse polygahatous polysaccharides; removing proteins from the coarse polygahatous polysaccharides through an ethanol solution, recycling ethanol, concentrating to concentrated paste, dissolving the concentrated paste into a Tris-HCl buffering solution, and purifying the polygahatous polysaccharides twice through DE-52 cellulose column chromatography; performing third-time purification through distilled water by SephadexG-100 sephadex gel filtration chromatography, concentrating and carrying out freeze drying to obtain a refined polygahatous polysaccharides product. The extraction method disclosed by the invention is simple and feasible; the production efficiency is high; the quality of the prepared product is high; the yield is over 90 percent, and the purity is over 99 percent; the method is suitable for industrial large-scale production.
Owner:XUANCHENG BAICAO PLANT IND & TRADE CO LTD
Preparation of salvianic acid A sodium pure product
InactiveCN101434534AReduce pollutionLow costCarboxylic compound separation/purificationPlant ingredientsSephadexOrganic solvent
The invention discloses a preparation method of a pure product of Salvianic acid A sodium, which comprises processing steps: 1. salvia miltiorrhizae is extracted by a hydrothermal method, and an extracting solution is condensed, deposited in alcohol and filtered; 2. a filtrate is condensed, deposited in alcohol and filtered; 3. the filtrate is condensed, added with water and filtered, and the filtrate is put into a macroporous absorption resin column and washed by water; 4. after a water lotion is condensed, the step is carried out in accordance with step (5); or the water lotion is put into a polyamide column and washed by water, and after the water lotion is condensed, the step is carried out according to step (5); 5. the water lotion after condensation is mixed with silicon gel, dried, column-packed and eluted by an organic solvent, and then an eluent is collected, recovered to be dry to obtain dry paste; and 6. the dry paste is taken and dissolved by the organic solvent, and PH value is adjusted, and then the material is stood and crystallized; or the dry paste is taken and dissolved by a solvent, and then put into a Sephadex LH-20 column or reversed-phase silica gel column. The PH value is adjusted by the eluent, and standing and crystallizing are carried out. The Salvianic acid A sodium prepared by the method has purity of more than or equal to 98 percent (standardized by NICPBP in respect of standard substances). Furthermore, the method has low cost, less pollution to environment and easily industrialized production.
Owner:HARBIN PHARMA GROUP SANJING NUOJIE PRARMACEUTICAL
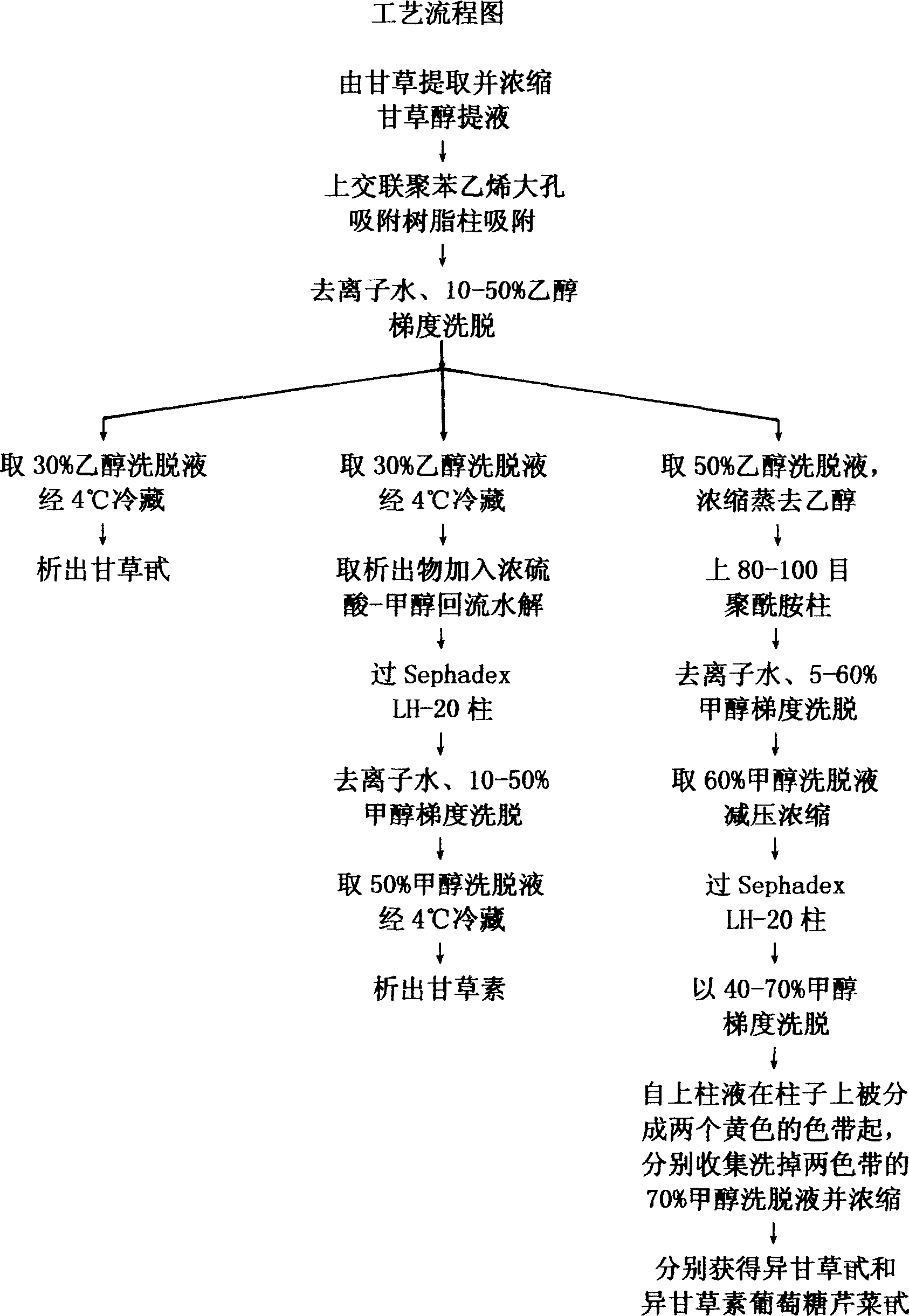
Method for extracting multiple liquorice flavone form liquorice
InactiveCN1865273AFree from pollutionReduce manufacturing costSugar derivativesSugar derivatives preparationPolyamideGLYCYRRHIZA EXTRACT
The invention discloses a multi-flavone component extracting and purifying method from liquorice, which comprises the following steps: 1. extracting and condensing glycyrol liquid; 2. adsorbing through large-hole resin; eluting the water and alcohol gradient; 3. remaining eluent of different density alcohol to continue different technologies( freezing, adding concentrated sulfuric acid-carbinol to do reflux hydrolysis; adding 80-100 order polyamide column; eluting water and carbinol; passing Sephadex LH-20 column; eluting again to produce multi-flavone component within iquiritin, liquiritigenin, isoliquiritin and isoliquiritin glucose apiin).
Owner:XIAMEN BERSI BIOLOGICAL TECH
Lactobacillus casei bacteriocin and use thereof in feed
The invention discloses lactobacillus casei bacteriocin and use thereof in feed. The lactobacillus casei provided by the invention is separated from Tibet plateau traditional fermented yak yogurt, and the produced bacteriocin is obtained by ammonium sulfate precipitation, Sephadex G-100 gel filtration and reversed-phase high-performance liquid chromatography (RP-HPLC). The bacteriocin has thermal and acid stability, can be degraded by protease and has a wide antibacterial spectrum. When the bacteriocin is added into feed, and the growth of bacteria can be inhibited. The bacteriocin has a bright prospect in use as a feed additive.
Owner:SICHUAN UNIV
Preparation method of rice bran protein peptide with ACE inhibitory activity
ActiveCN104450839AIncrease added valueEase the morbidityPeptide preparation methodsFermentationHigh morbidityHydrolysis
The invention discloses a preparation method of rice bran protein peptide with ACE inhibitory activity. The method is characterized in that rice bran is adopted as a raw material, and comprises the following steps: firstly extracting rice bran protein, then preparing rice bran protein hydrolysate by adopting an enzyme hydrolysis method, and separating and purifying the rice bran ACE inhibitory peptide by adopting a hyperfiltration method, sephadex G-15 and a RP-HPLC method. According to the preparation method, the rich and cheap rice bran resource is effectively utilized to prepare the rice bran protein peptide with the high ACE inhibitory activity. The decompression function of the rice bran ACE inhibitory peptide can be maximally utilized, so that on one hand, the additional value of the rice bran is increased, and the good economic benefit and market value can be created; on the other hand, the morbidity of the hypertension can be effectively reduced, the high morbidity can be controlled, the human beings can be protected from the harm of the hypertension, the application value and social benefit are good, and the theoretical foundation and the experiment evidence can be provided for researching the structure and decompression mechanism of the rice bran protein ACE inhibitory peptide.
Owner:黑龙江省北大荒米业集团有限公司
Preparation method of small water turtle anti-tumor polypeptide
ActiveCN104630318APromote development and utilizationHas inhibitory effectHydrolysed protein ingredientsPeptide preparation methodsMeat pasteSephadex
The invention provides a preparation method of a small water turtle anti-tumor polypeptide. The method comprises the following steps: mixing 3 to 20g of meat paste of small water turtle and ultrapure water; agitating to obtain a mixed solution at concentration of 10 to 50% (w / v); adding trypsin to perform enzymolysis; deactivating enzyme after enzymolysis; cooling until reaching room temperature; centrifuging enzymatic hydrolysate; collecting supernate; sequentially filtering through ultrafiltration membranes which respectively have molecular cut off of 10KD, 5KD and 3KD, so as to obtain three enzymatic hydrolysates; performing sephadex G-15 column chromatography for 0-3K enzymatic hydrolysate; eluting with water; collecting four polypeptide components under a certain detection wavelength so as to obtain small water turtle anti-tumor polypeptide. The prepared small water turtle anti-tumor polypeptide is beneficial for the development and utilization of anti-tumor drugs and health foods.
Owner:广东省几何细胞生物科技有限公司
Anti-oxidation and DPP-IV inhibition active peptide derived from apostichopus japonicus
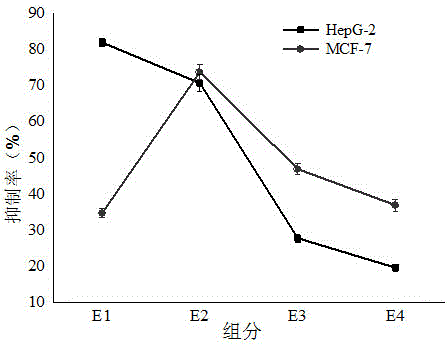
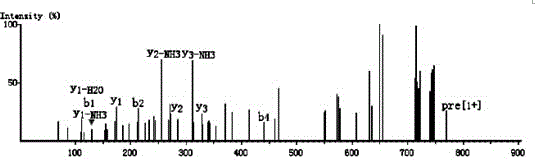
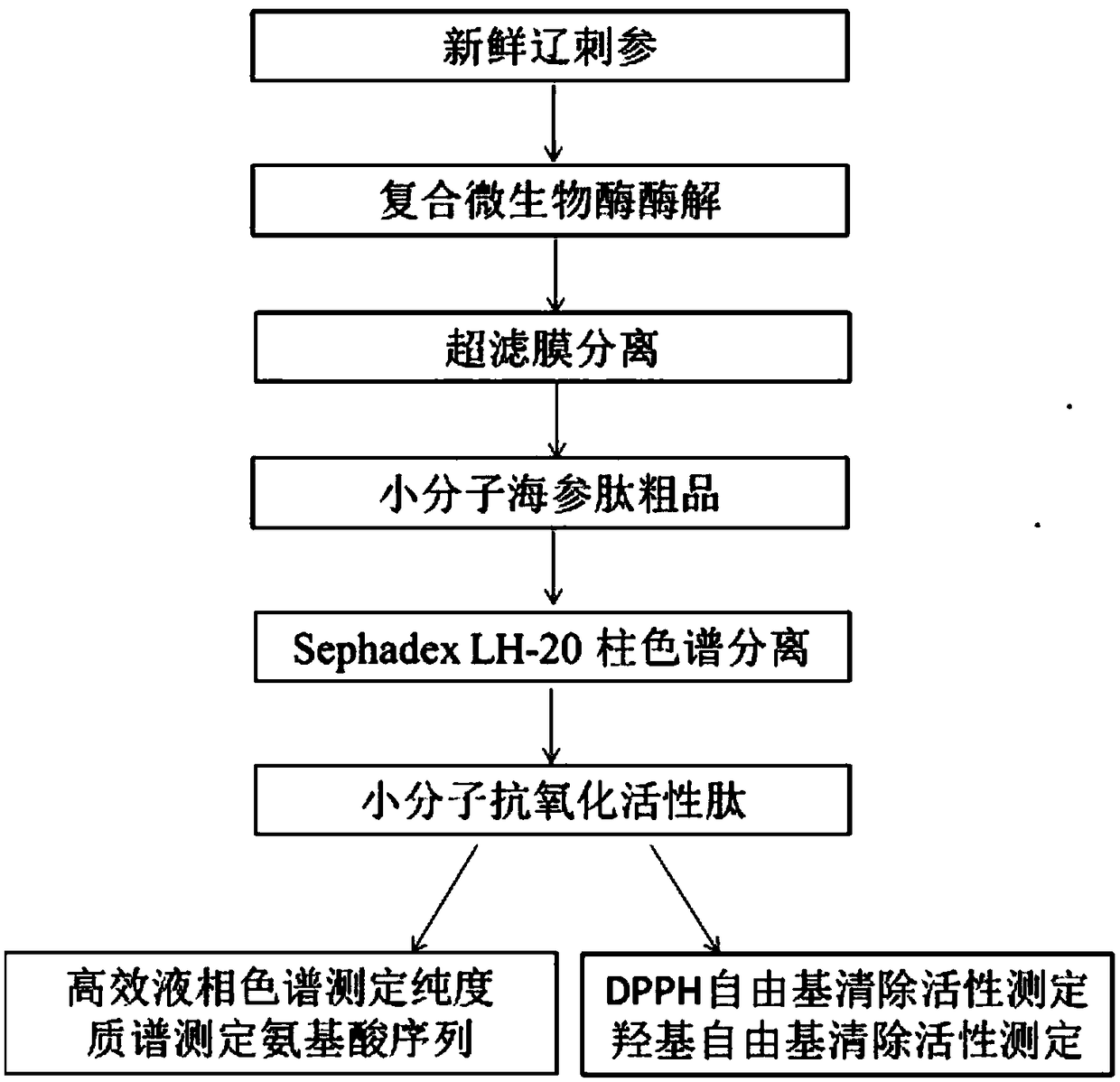
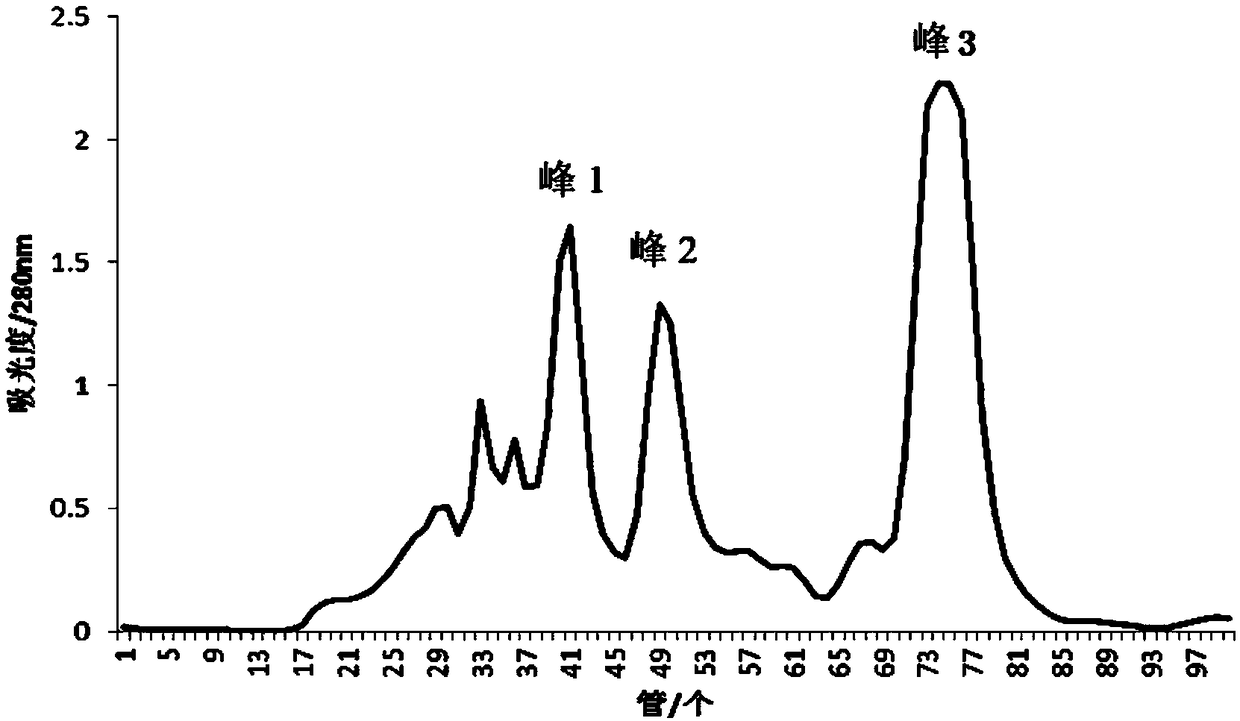
ActiveCN109400678AHigh puritySmall molecular weightPeptide preparation methodsFermentationDiseaseSeparation technology
The invention belongs to the field of marine organism small-molecular active peptides, and particularly relates to an anti-oxidation and DPP-IV inhibition active peptide derived from apostichopus japonicus by enzymolysis. The amino acid sequence of the anti-oxidation and DPP-IV inhibition active peptide is Ser-Arg-Pro-Gln-Tyr-Pro-Gln-Tyr-Pro-Ser. The anti-oxidation and DPP-IV inhibition active peptide is prepared by the following steps: adding water into fresh apostichopus japonicus for homogenizing, and placing the homogenate in an enzymolysis tank; performing enzymolysis with compound protease to obtain enzymatic hydrolysate; treating the enzymatic hydrolysate with a membrane separation technology to obtain a small-molecular active peptide crude product; separating the crude product through Sephadex LH-20 to obtain a small-molecular active peptide. The purity of the small-molecular active peptide as measured by adopting RP-HPLC (Reversed-Phase High Performance Liquid Chromatography)is greater than 95 percent, and the amino acid composition of the active peptide is measured by high performance liquid chromatography / mass spectrometry, so that the amino acid sequence of the small-molecular peptide is determined finally. The active small-molecular peptide derived from the apostichopus japonicus has the advantages of small molecular weight, simple separating-purifying steps, easiness in preparation, high purity and higher oxidization resistance and DPP-IV inhibition activity, has the characteristics of naturalness, safety and high efficiency, can be applied to the preventionand treatment of relevant diseases such as diabetes mellitus as an antioxidant as well as a DPP-IV inhibitor, and has a wide application prospect in the fields of foods, health care products and medicines.
Owner:DALIAN SHENLAN PEPTIDE TECH R & D CO LTD
Method for abstracting and separating iridoid compound monomer from oldenlandia diffusa
The invention relates to a method for extracting and separating more than 90% iridoid glycoside compound monomer from efflorescence snake tongue grass, wherein the iridoid glycoside compound monomer of efflorescence snake tongue grass is E-6-0-coumaricacetate paederoside methyl ester. The method comprises the following steps: (1)putting the disintegrated efflorescence snake tongue grass in the primary alcohol aqueous solution in order to heat, reflux and extract; filtering; getting the concrete (I)by concentrating; adding the water in order to dissolve; filtering; getting the concrete (II)by concentrating; (2)dissolving the concrete (II)with the secondary alcohol aqueous solution; separating with macroreticular resin column; eluting with the tertiary alcohol aqueous solution; concentrating the eluent; getting the primary product; (3)proceeding with chromatography with Sephadex LH-20 column after dissolving the primary product with the fourth alcohol aqueous solution; proceeding with gradient elution with the fifth alcohol solution; concentrating; crystallizing; getting the product E-6-0-coumaricacetate paederoside methyl ester. The method can recycle the column material and solvent, which doesn't pollute the environment, has the low cost, the high product efficient and the easy operation, and is fit for the small-scale extraction separation of the laboratory and the industry extraction separation.
Owner:SHANGPHARMA INNOVATION JIANGXI CO LTD
Terpenoid dihydroquinolone alkaloid compound as well as crystal, preparation method and application thereof as marine anti-fouling agent
The invention provides a terpenoid dihydroquinolone alkaloid compound as well as a crystal, a preparation method and an application thereof as a marine anti-fouling agent. The preparation method is characterized by firstly carrying out strain culture on a fungus Scopulariopsis sp.(TA01-33), then carrying out fermentation culture on the fungus, removing the thallus through filtration and using ethyl acetate for extraction after filtrate concentration; carrying out normal phase silica-gel column chromatography, Sephadex LH-20 gel column chromatography and high performance liquid chromatography (HPLC) in sequence, thus obtaining the compound in the formula I in the specification. The compound in the formula I and pharmaceutically acceptable salts or crystal thereof can be used for preparing a high-efficiency and low-toxicity marine anti-fouling agent.
Owner:OCEAN UNIV OF CHINA
Novel flavonoid extracted from Maackia amurensis
InactiveCN101434592AHas anti-tumor cell proliferation effectOrganic active ingredientsSugar derivativesSephadexChemical composition
The invention pertains to the technical field of medicaments, and relates to a new flavonoid compound extracted from Maackia amurensis. The invention uses various separation methods including silica gel column chromatography, macroporous adsorptive resins, sephadex LH-20 column chromatography, semi-preparation type high-efficiency liquid chromatography, and the like. 54 compounds and 14 flavonoid compounds are seperated from the maackia amurensis, wherein, 7 new chemical components are included, which are respectively Maackiaflavanone A, Maackiaflavanone B, Maackiapentone, Maackiapterocarpan A, Maackiapterocarpan B, Maackiaisoflavonoside and 8-3, 3-dimethylallyl-5-hydroxy-7-methoxy chromone. A preliminary activity study indicates that the compound has certain effect of inhibiting the proliferation of tumor cells, thereby providing a basis for the development and research in the future.
Owner:SHENYANG PHARMA UNIVERSITY
Anoectochilus roxburghiiv flavone extraction process and application thereof in skin care products
ActiveCN106420521AProtect active ingredientsHigh extraction rateCosmetic preparationsToilet preparationsSephadexVacuum pressure
The invention relates to an anoectochilus roxburghii flavone extraction process and application thereof in skin care products. The extraction process comprises the following steps of S1, conducting pulping, freeze drying and smashing on the anoectochilus roxburghii in sequence, and conducting extraction on the smashed anoectochilus roxburghii powder to obtain an anoectochilus roxburghii flavone crude extract liquid; S2, filtering the crude extract liquid obtained in the S1, reducing pressure and concentrating to obtain an anoectochilus roxburghii flavone crude extract; S3, conducting purification on the anoectochilus roxburghii flavone crude extract by adopting a Sephadex LH-20 gel column, so that an anoectochilus roxburghii flavone extract is obtained, wherein technological conditions in the S1 for freeze drying is a freezing temperature of 70-80 DEG C, freezing time of 40-60 hours and vacuum pressure of 15-25Pa. The provided extraction technology has high an extraction rate, and the anoectochilus roxburghii flavone extract obtained by extraction has high content of effective constituents, less impurities, and good antioxidant activity and tyrosinase activity inhibition.
Owner:GUANGDONG MARUBI BIOLOGICAL TECH CO LTD
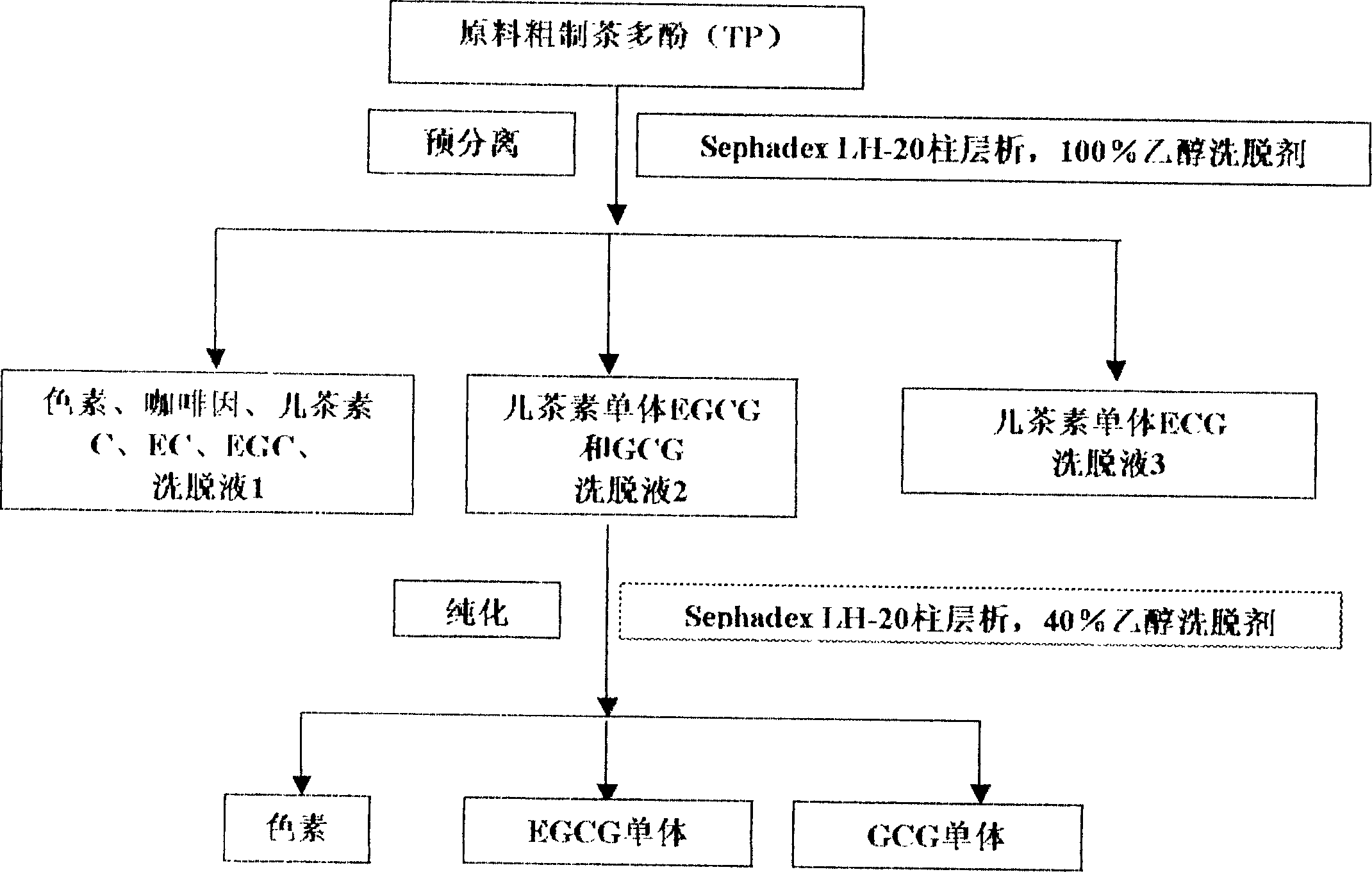
Separation purification method of catechin monomer
InactiveCN1603319AEasy to separateImprove separation efficiencyOrganic chemistrySephadexPurification methods
This invention relates to separation and purification method of catechin monomer EGCG and ECG. The features are that dextrane gel Sephadex LH-20 is column filling and eluant is absolute ethyl alcohol. Then column chromatography is made by dextrane gel Sephadex LH-20 and 40% ethanol aqueous solution be eluant, that is chromatography column non- gradient expendable separation is adopted. Comparing to original method, equipments are facilitated greatly and the method is simple, cost is low. The solvent is nontoxic and separation period is short, and monomer extraction rate and product purity are both high.
Owner:HEFEI UNIV OF TECH
Preparation technology of stilbene glucoside in polygonum multiflorum
The invention discloses a preparation technology of a stilbene glucoside extract product in polygonum multiflorum and a production technology of a stilbene glucoside fine product by using a stilbene glucoside crude product as a raw material. The preparation technology of the stilbene glucoside extract product comprises the following steps of: extracting the medicinal material polygonum multiflorum by a dilute ethanol heating reflux method, condensing the extract to a certain volume, cooling and standing, filtering, extracting by the adding ethyl acetate into a filtrate, merging the extracts, condensing to a stiff paste, followed by vacuum drying of the stiff paste and crushing, redissolving by the use of water or an ethanol aqueous solution, performing vacuum drying and crushing to obtain the stilbene glucoside extract product. The technology preparation of the stilbene glucoside fine product comprises the following steps of: using the stilbene glucoside crude product as a raw material, separating by silica gel column chromatography, and purifying by Sephadex LH-20 column chromatography to obtain the stilbene glucoside fine product. The technology provided by the invention has advantages of simple operation, high recovery rate and good stability, and is suitable for industrial production.
Owner:BEIJING SL PHARMA +2
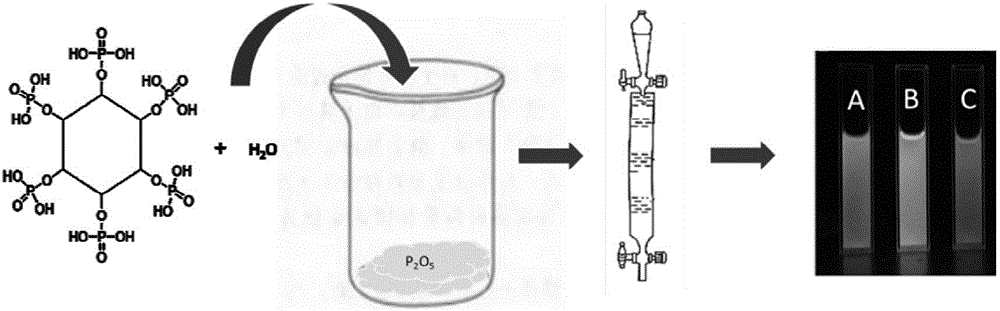
Preparation method and application of phosphorus-doped fluorescent carbon quantum dots
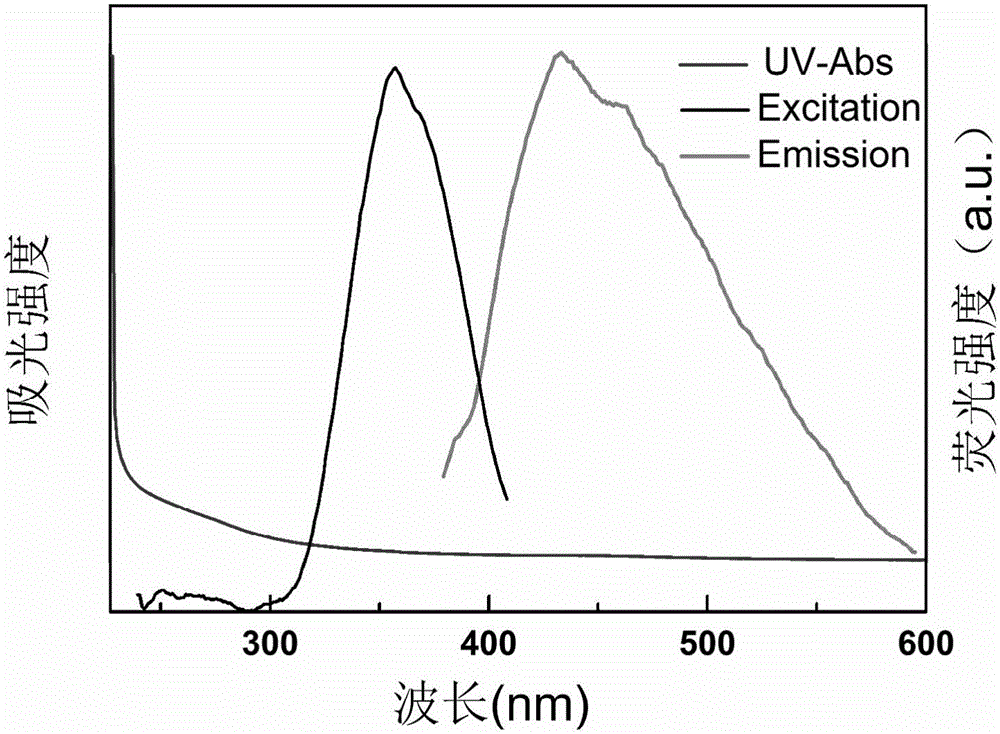
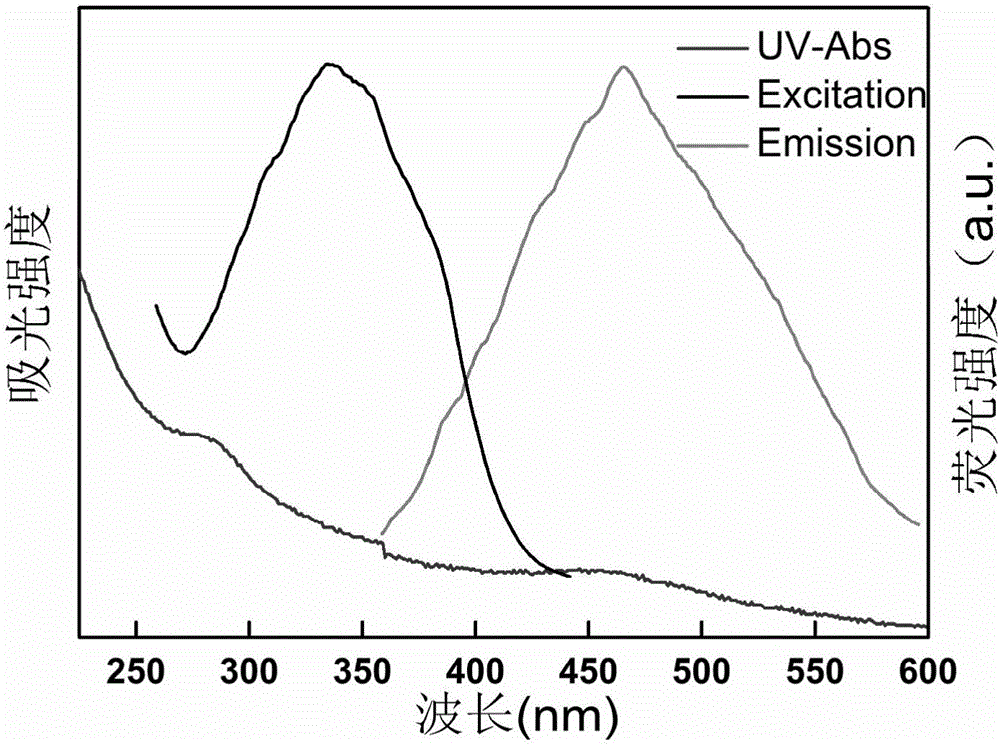
InactiveCN105950145AEasy to operateWide variety of sourcesFluorescence/phosphorescenceLuminescent compositionsFreeze-dryingPhytic acid
The invention relates to a preparation method of phosphorus-doped fluorescent carbon quantum dots. The preparation method comprises the following steps: (1) adding phytic acid into a glass container, then adding secondary water, fully stirring, and carrying out ultrasonic treatment to obtain a clear solution; then, rapidly adding the clear solution into phosphorus pentoxide to obtain a dark brown solution; (2) after the glass container is naturally cooled, filtering the dark brown solution by using filter paper, and removing undissolved substance to obtain a clear dark brown solution; (3) separating by means of exclusion chromatography, wherein sephadex G-25 is taken as filler, and water is taken as a mobile phase, and separating according to time order to obtain three carbon quantum dot aqueous solutions; (4) respectively carrying out freeze drying on the three carbon quantum dot aqueous solutions to obtain three target products. The method is simple in operation technology, wide in source of raw materials, low in price of the raw materials, low in requirement for separation conditions and free from energy consumption; the obtained carbon quantum dots are stable in optical properties. The prepared phosphorus-doped fluorescent carbon quantum dots can be used for Fe<3+> ion detection, tetracycline detection and cell imaging.
Owner:SHANXI UNIV
Natural refrigerated fresh meat film antistaling agent and application
InactiveCN102461642AImprove use valueSave resourcesMeat/fish preservation by coatingMeat/fish preservation using chemicalsWater bathsFreeze-drying
The invention belongs to the field of natural product preparation, and particularly relates to a polymethoxylated flavonoids extract which is prepared from orange peels and application thereof. An antistaling agent provided by the invention is prepared from the following steps of: drying, grinding, screening and placing the orange peels into ethanol solution, adding cellulose, using a water bath for digestion and enzymolysis, and obtaining flavonoids ethanol extracting solution; then concentrating and removing the ethanol of the extracting solution, extracting with diethyl ether, merging a ether layer, and washing the ether layer until a water phase part is colorless; evaporating the diethyl ether, refrigerating, drying, and obtaining a polymethoxylated flavonoids crude; dissolving the polymethoxylated flavonoids crude in methyl alcohol, letting the solution to pass through a Sephadex LH-20 gel column, using the methyl alcohol-water to carry out gradient elution, adopting an ultraviolet detector to detect, collecting the maximum absorption peak, concentrating, refrigerating, drying and obtaining the polymethoxylated flavonoids extract; using absolute ethyl alcohol to redissolve, adding activated carbon to decolorize, concentrating, refrigerating, drying, and then obtaining polymethoxylated flavonoids extract powder; and compounding the polymethoxylated flavonoids extract with polysorbate and edible alcohol, then obtaining the antistaling agent. The antistaling agent can be used for keeping refrigerated fresh meat fresh.
Owner:HUAZHONG AGRI UNIV
Preparation of wood louse powder extract and uses thereof
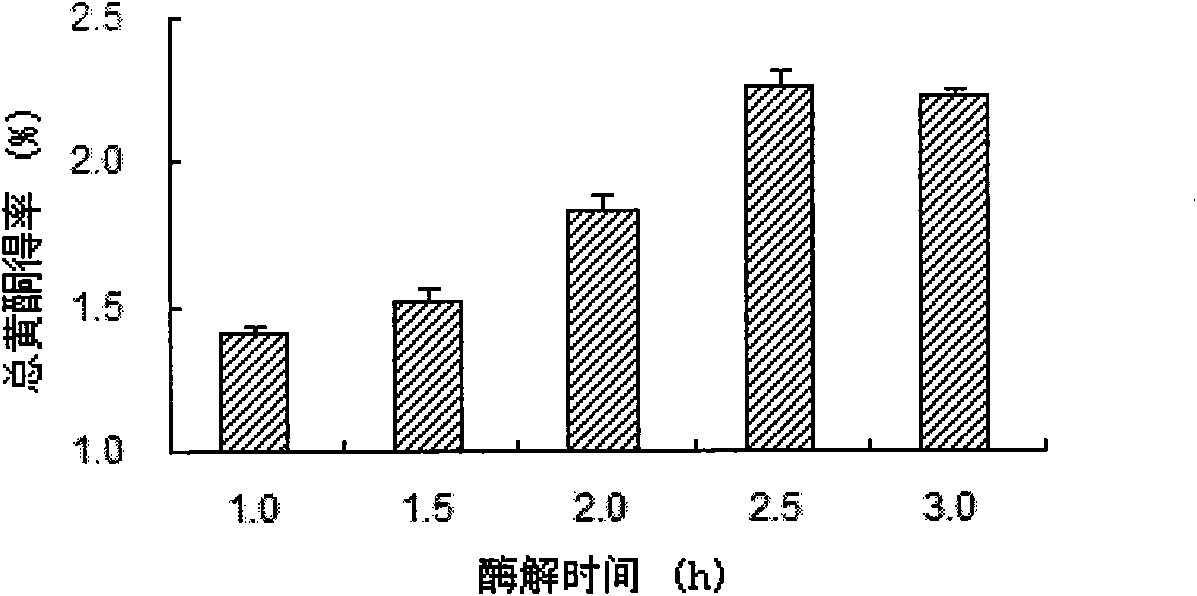
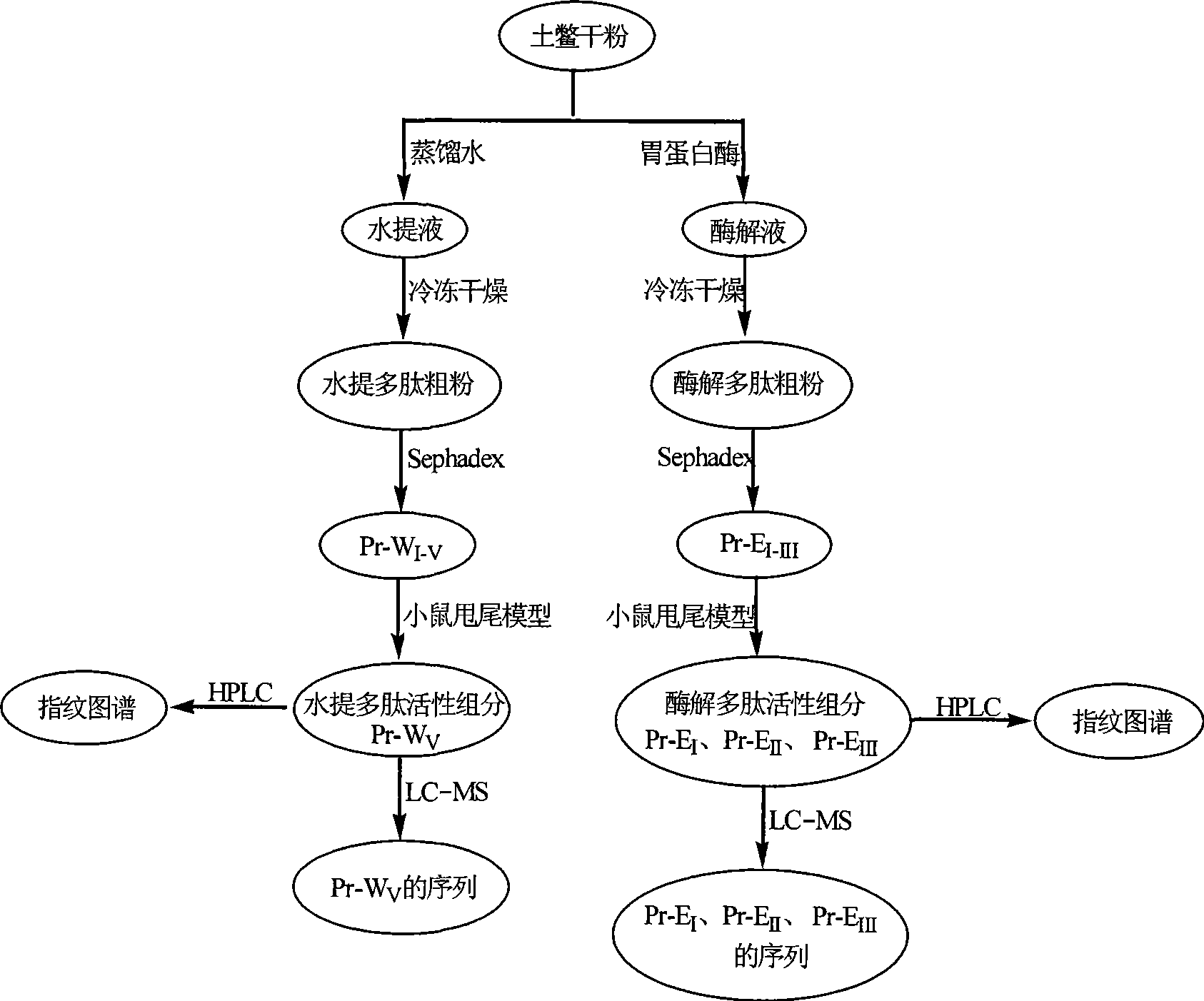
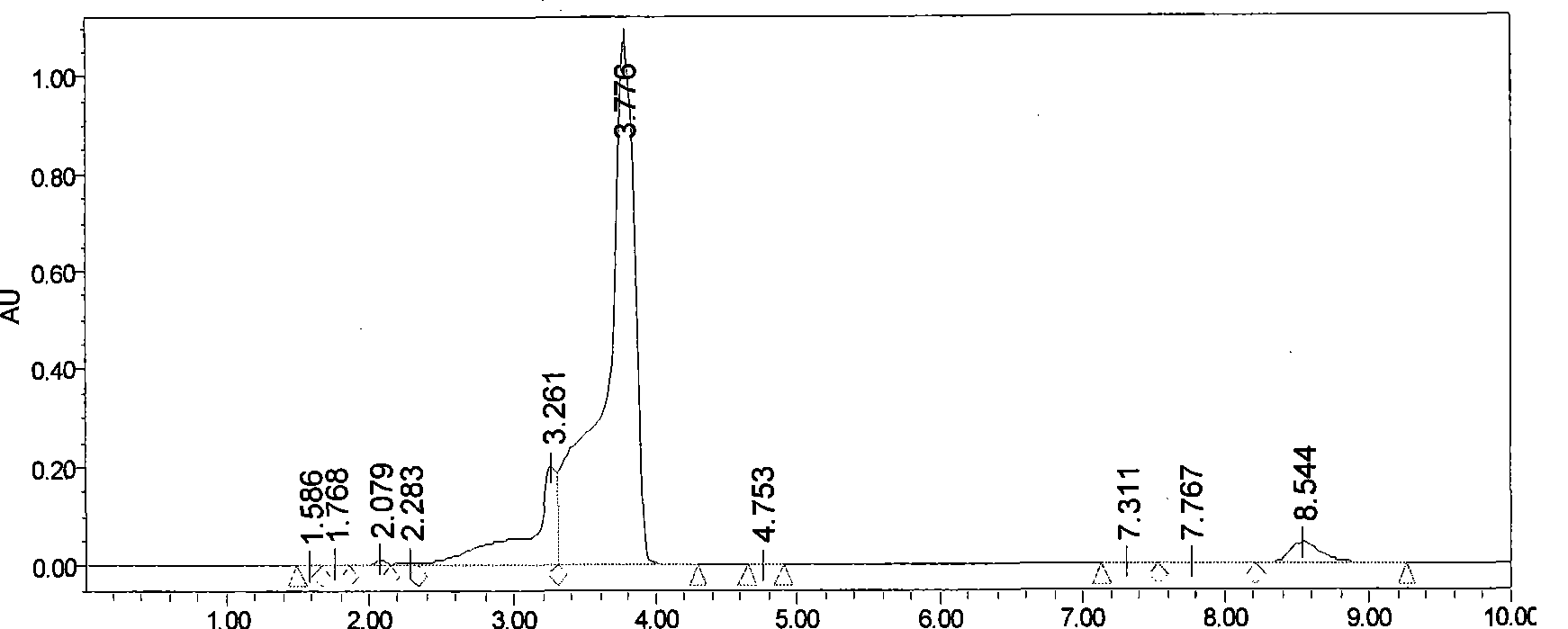
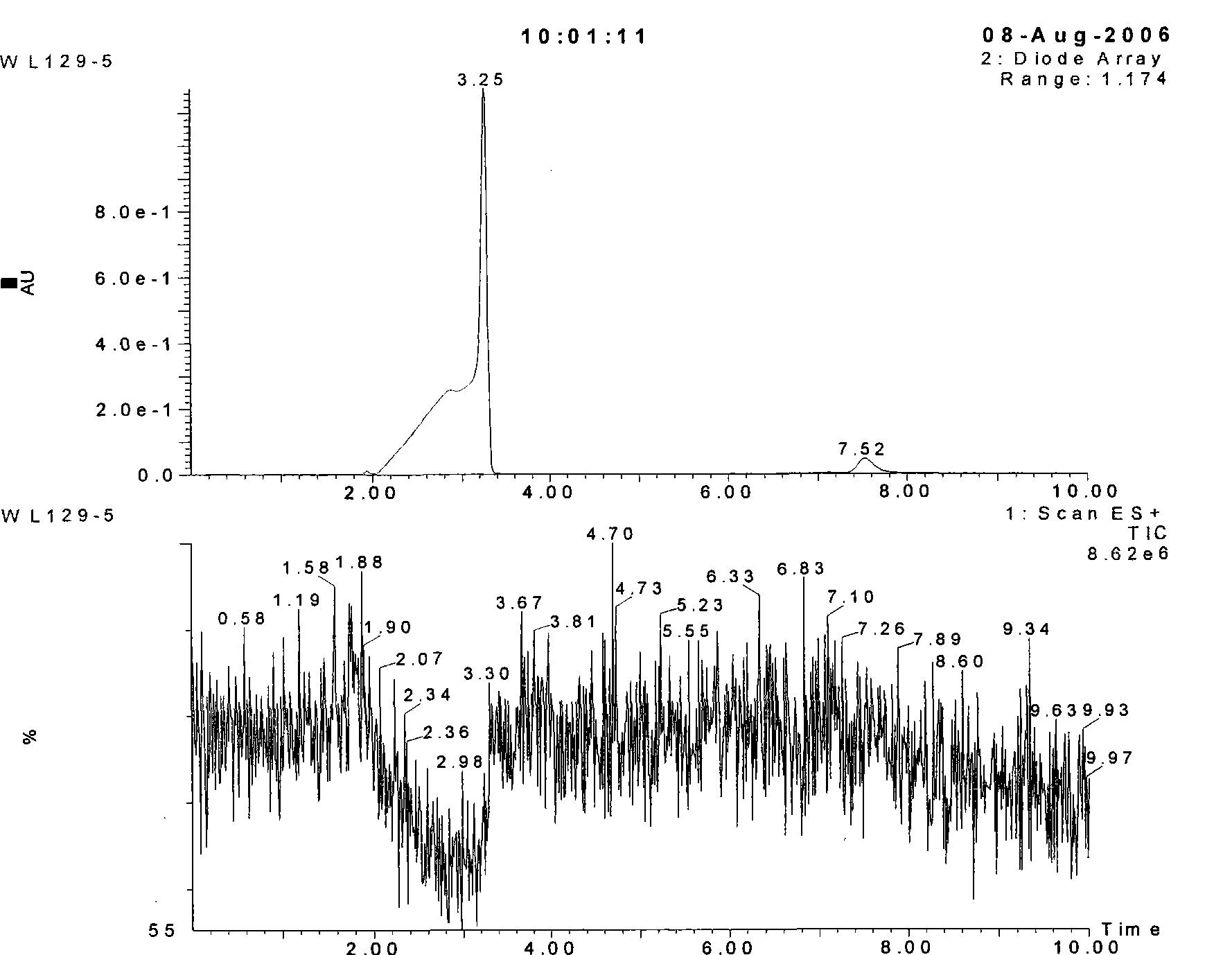
The invention discloses a preparation method of eupolyphaga seu steleophagadry dry powder extract. The method adopted by the invention includes water extraction method or enzymolysis method. The eupolyphaga seu steleophagadry dry powder is firstly processed with water extraction or enzymolysis; the water extraction liquid or enzymolysis liquid is chromatographically separated through Sephadex G25column to obtain the polypeptide component series, and then the polypeptide component is frozen and dried to prepare frozen dry powder. The model evaluation of mouse ear swelling indicates that the polypeptide component series of eupolyphaga seu steleophagadry dry powder extract has obvious inhibition effect on pain. The experiment of feeding mice for a month indicates that the polypeptide component has no toxicity and no dependence. The invention provides the application of polypeptide component series of eupolyphaga seu steleophagadry dry powder extract in the preparation of analgesic medicines.
Owner:ZHEJIANG MEDICINE CO LTD XINCHANG PHAMACEUTICAL FACTORY
Method for preparing epigallocatechin-3-gallate by resin adsorption method
InactiveCN101386614AIon-exchange process apparatusOrganic chemistryCross-linkChromatographic separation
The invention discloses a method for preparing EGCG by resin absorption method. The method uses tea extract as a raw material and comprises the following steps of: using 35 to 40 percent of ethanol water solution to dissolve the tea extract, applying ultra-high cross-linked resin absorption column on the collection liquid for chromatographic separation so as to remove other catechin components, applying a polar macroporous resin absorption column on the collection liquid for decoloration, performing nanofiltration and concentration, and using a Sephadex LH-20 column for chromatography and further purification, freezing and drying the collection liquid so as to obtain EGCG monomer. The purity of the product is more than 90 percent and the yield rate is over 30 percent. The method has simple process, makes the concentration at a normal temperature, avoids high-temperature oxygenation of the catechin, has nontoxic solvent and high-quality product, and meets the demands of food and pharmacy industries.
Owner:JIMEI UNIV
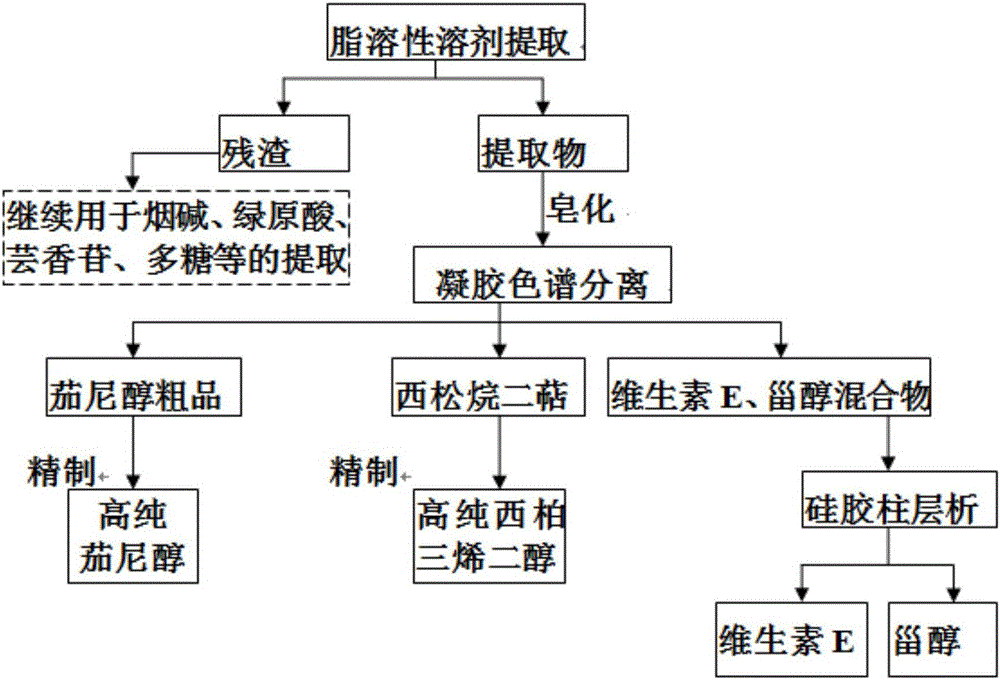
Method for extracting solanesol, cembrane diterpene, vitamin E and phytosterol from tobaccos simultaneously
ActiveCN106008444AImprove utilization efficiencyRealize cascade utilizationTobacco preparationOrganic compound preparationNicotiana tabacumChlorogenic acid
The invention discloses a method for extracting solanesol, cembrane diterpene, vitamin E and phytosterol from tobaccos simultaneously. The method comprises the steps that 1, extraction is carried out; 2, saponification is carried out, wherein saponified solanesol and other fat-soluble active ingredient extracts are obtained; 3, sephadex gel column separation is carried out, wherein solanesol, vitamin E and phytosterol (mixture) and cembrane diterpene crude extract are obtained respectively; 4, silica-gel column chromatography is carried out, wherein after silica-gel column chromatography, high-purity solanesol, vitamin E and phytosterol are obtained respectively; the obtained cembrane diterpene is subjected to silica-gel column chromatography, and alpha-4,8,13-cembratriene-1,3-diol and beta-4,8,13-cembratriene-1,3-diol are obtained respectively. Comprehensive extraction of tobacco fat-soluble active ingredients is achieved, tobacco residue obtained after fat-soluble active ingredients are extracted can be used for extraction of tobacco protein, chlorogenic acid, rutin, polysaccharide and nicotine, gradient utilization of tobacco active ingredients is achieved, and the utilization value of tobacco resources, particularly waste tobaccos is improved.
Owner:TOBACCO RES INST CHIN AGRI SCI ACAD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com