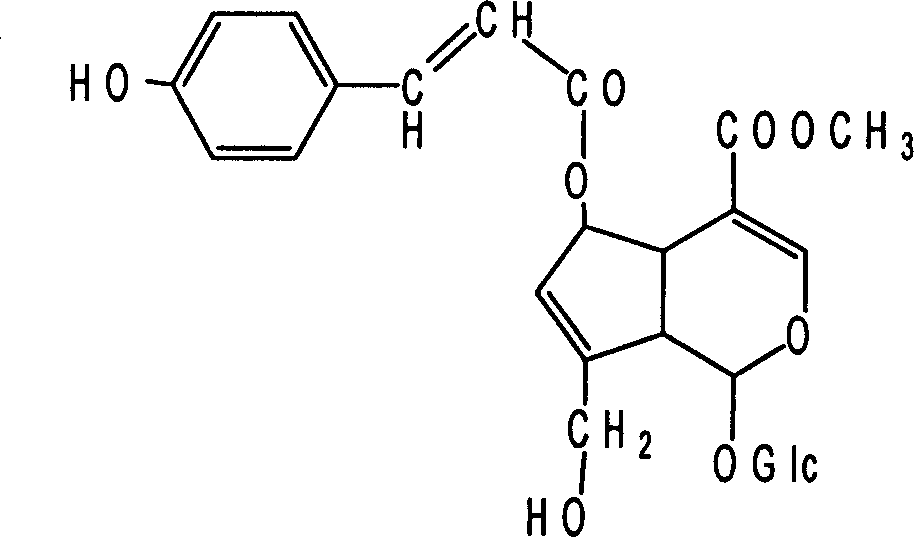
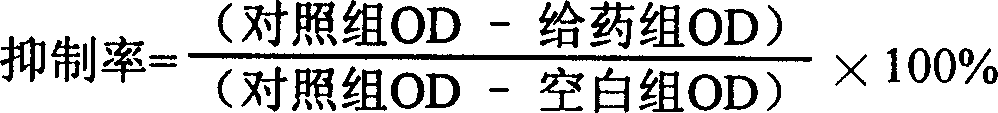Method for abstracting and separating iridoid compound monomer from oldenlandia diffusa
A technology of Hedyotis diffusa and iridoids is applied in the field of phytochemistry, can solve the problems of high cost, low product yield, complicated methods and the like, and achieves low cost, high yield, easy control and operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1Kg of pulverized Hedyotis diffusa is heated and refluxed with ethanol aqueous solution of 95% ethanol to extract twice, each time for 2 hours, the weight ratio of said Hedyotis diffusa to ethanol aqueous solution is 1: 1.5, filter and concentrated to obtain extract (I) (relative density 1.197, 60°C), dissolved in water, filtered, and the filtrate was concentrated under reduced pressure to obtain extract (II) (relative density 1.156, 60°C). The extract (II) is dissolved in an aqueous ethanol solution with a volume concentration of ethanol of 20%, and the weight ratio of the extract (II) to the aqueous ethanol solution is 1:5; on MCI gel CHP 20P (styrene) macroporous resin column chromatography, The weight ratio of extract (II) and macroporous resin MCI gel CHP 20P is 1: 1; With 20%, 30%, 40%, 50%, 60% ethanol gradient elution, thin-layer chromatography tracking detection, at 50 There is a large spot in the eluting part of % ethanol, after collecting the spot and concent...
Embodiment 2-6
[0035] Embodiment 2-6 Extraction, separation steps are referring to embodiment 1, what adopted in the embodiment is pulverized Hedyotis diffusa 1kg, the extraction of each example, separation conditions see table 1 below
[0036] Table 1
[0037] Example
[0038] 4
[0039] 6
Embodiment 7
[0040] Example 7 Inhibitory Action Test on Cancer Cell Lines
[0041] Test substance: E-6-O-coumaroyl gallinosin methyl ester
[0042] Positive control: 5-fluorouracil (5-FU)
[0043] Cell lines: lung cancer A549 and liver cancer SMMC-7721 (purchased from Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences)
[0044] experimental method:
[0045] E-6-O-coumaroyl gallinacein methyl ester is made into mother liquor with culture medium (10 -4 mol / L), and then diluted with culture medium to different concentrations before use.
[0046] 8 MTT (thiazole blue) test:
[0047] A549 and SMMC-7721 cells were inoculated into 96-well cell culture plates at 3,000 and 5,000 cells per well, respectively. After 24 hours of culture, the supernatant was discarded, and 100 μL of culture solution containing different concentrations of drugs was added to each well, with concentrations of 10 -4 , 10 -5 , 10 -6 , 10 -7 , 10 -8 mol / L, the same concentration of 5-fluorourac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com