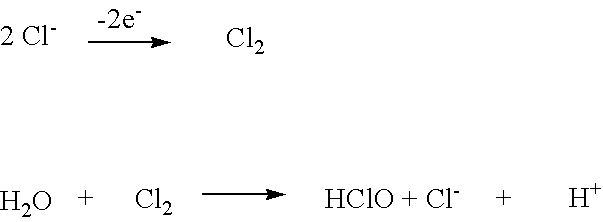
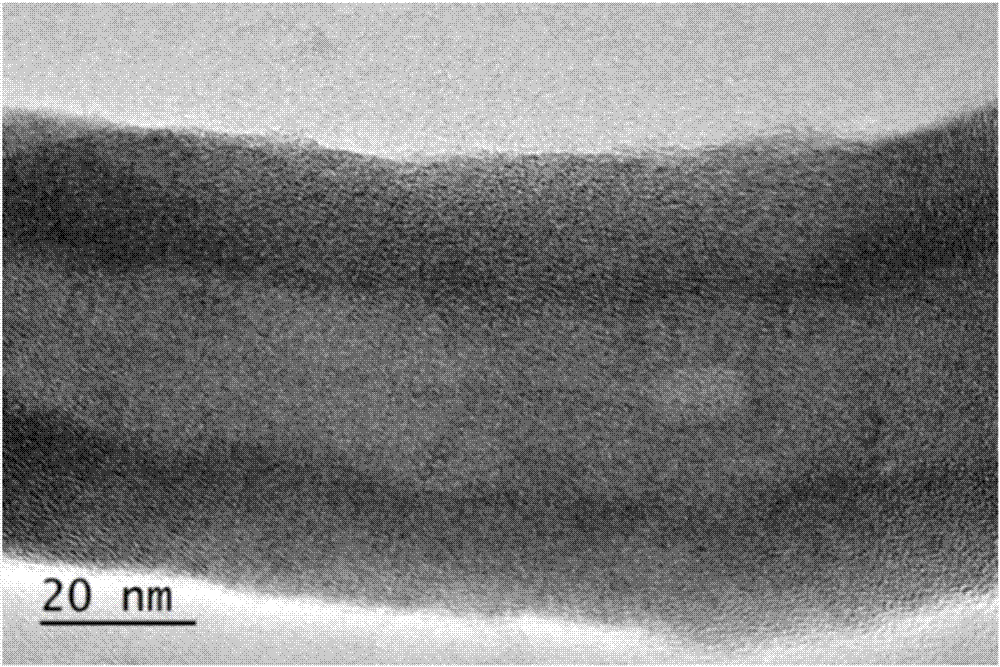
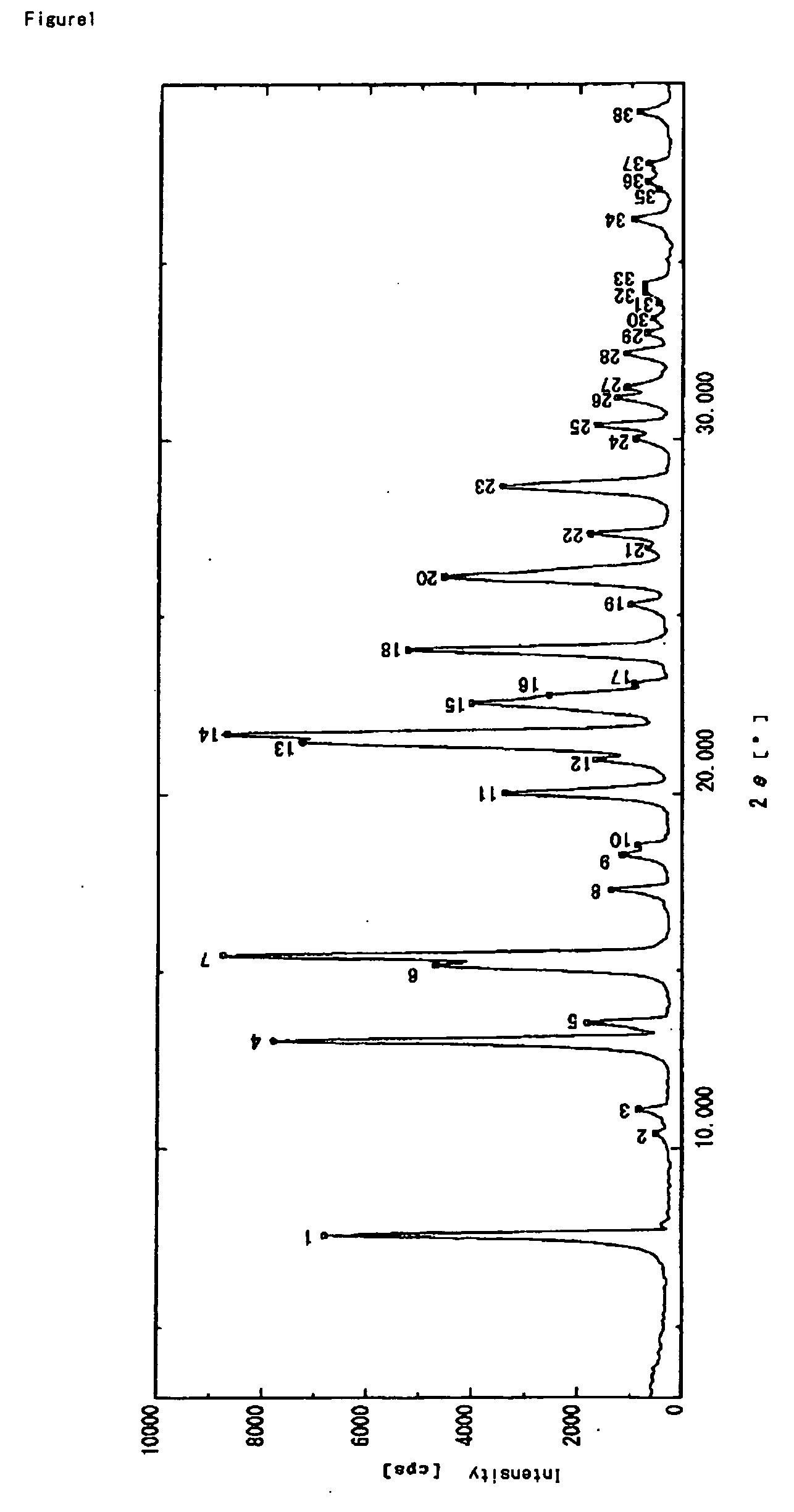
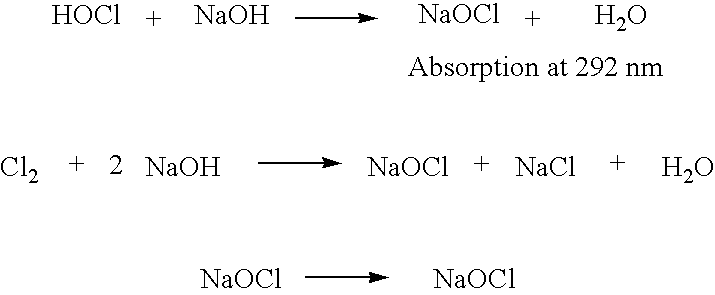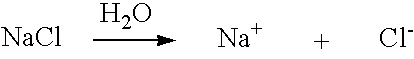Patents
Literature
12467 results about "Inorganic salts" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Porous polymeric matrices made of natural polymers and synthetic polymers and optionally at least one cation and methods of making
A porous polymeric matrix containing at least one natural polymer and at least one synthetic polymer and optionally at least one cation. Furthermore, a method of making a porous polymeric matrix involving mixing at least one natural polymer and inorganic salts with a solution comprising at least one solvent and at least one synthetic polymer to form a slurry, casting the slurry in a mold and removing the solvent to form solid matrices, immersing the solid matrices in deionized water to allow natural polymer cross-linking and pore creation to occur simultaneously, and drying the matrices to create a porous polymeric matrix; wherein the matrix contains a cation. Also, a method of making a porous polymeric matrix, involving mixing at least one natural polymer in an aqueous solvent and mixing at least one synthetic polymer in an organic solvent, combining the mixtures and casting in a mold, and separately removing said aqueous solvent and said organic solvent to form a porous polymeric matrix; wherein the porous polymeric matrix does not contain a cation.
Owner:US SEC AGRI
Light emitting element
InactiveUS20130105787A1Improve light emission efficiencySufficient durability lifeOrganic chemistrySolid-state devicesSilyleneAlkaline earth metal
Provided is an organic thin film light emitting element which has achieved all of improved luminous efficiency, improved driving voltage and improved durability life. Specifically provided is a light emitting element which comprises a hole transport layer and an electron transport layer between a positive electrode and a negative electrode and emits light by means of electrical energy. The light emitting element is characterized in that: the hole transport layer of the light emitting element contains a compound represented by general formula (1); the electron transport layer contains a donor compound; and the donor compound is an alkali metal, an inorganic salt containing an alkali metal, a complex of an alkali metal and an organic substance, an alkaline earth metal, an inorganic salt containing an alkaline earth metal, or a complex of an alkaline earth metal and an organic substance. (In the formula, R1-R20 each represents one group selected from the group consisting of hydrogen, deuterium, an alkyl group, a cycloalkyl group, an amino group, an aryl group, a heterocyclic group, a heteroaryl group, an alkenyl group, a cycloalkenyl group, an alkynyl group, analkoxy group, an alkylthio group, an arylether group, an arylthioether group, a halogen, a cyano group, a —P(═O)R24R25 group and a silyl group; R24 and R25 each represents an aryl group or a heteroaryl group; and these substituents may be further substituted, or adjacent two substituents may combine together to form a ring. Meanwhile, R21-R23 may be the same or different and each represents one group selected from the group consisting of an alkyl group, a cycloalkyl group, an aryl group and a heteroaryl group; and these substituents maybe further substituted.)
Owner:TORAY IND INC
Method for fracturing subterranean formations
InactiveUS6875728B2Reduce speedReduce the amount of waterFluid removalFlushingInorganic saltsFracturing fluid
A method of fracturing a formation with a fracturing fluid wherein the formation has particulate material that swells or migrates upon exposure to the fracturing fluid comprises preparing a fracturing fluid comprising (1) a thickening compound comprising a first surfactant selected from the group consisting of a cationic surfactant having only a single cationic group, an amphoteric surfactant and a mixture thereof; and, an anionic surfactant; and, (2) water, wherein no or essentially no inorganic salt is added to the fracturing fluid; and using the fracturing fluid to fracture the formation. A method for recycling a fracturing fluid is also provided.
Owner:BAKER HUGHES INC
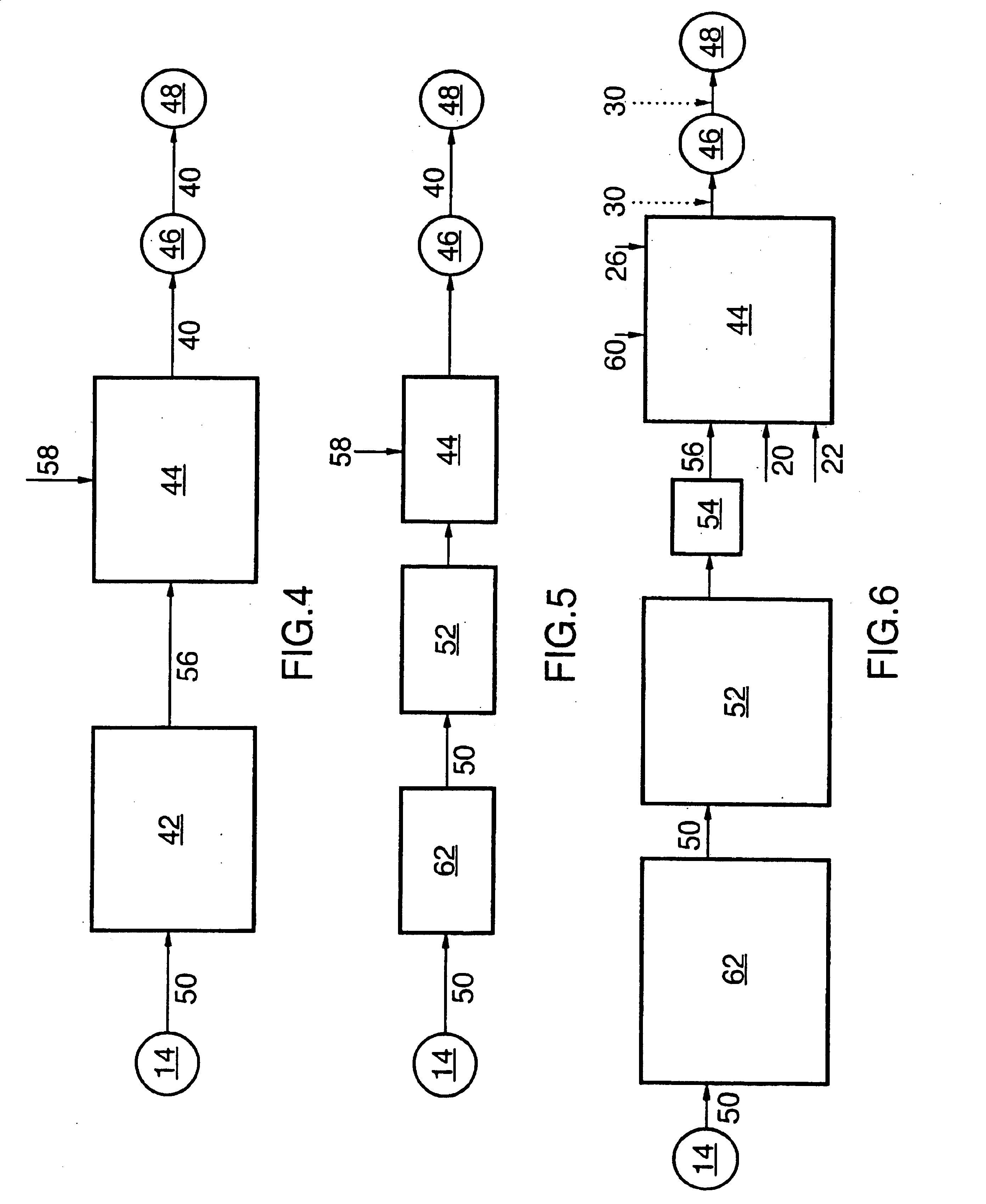
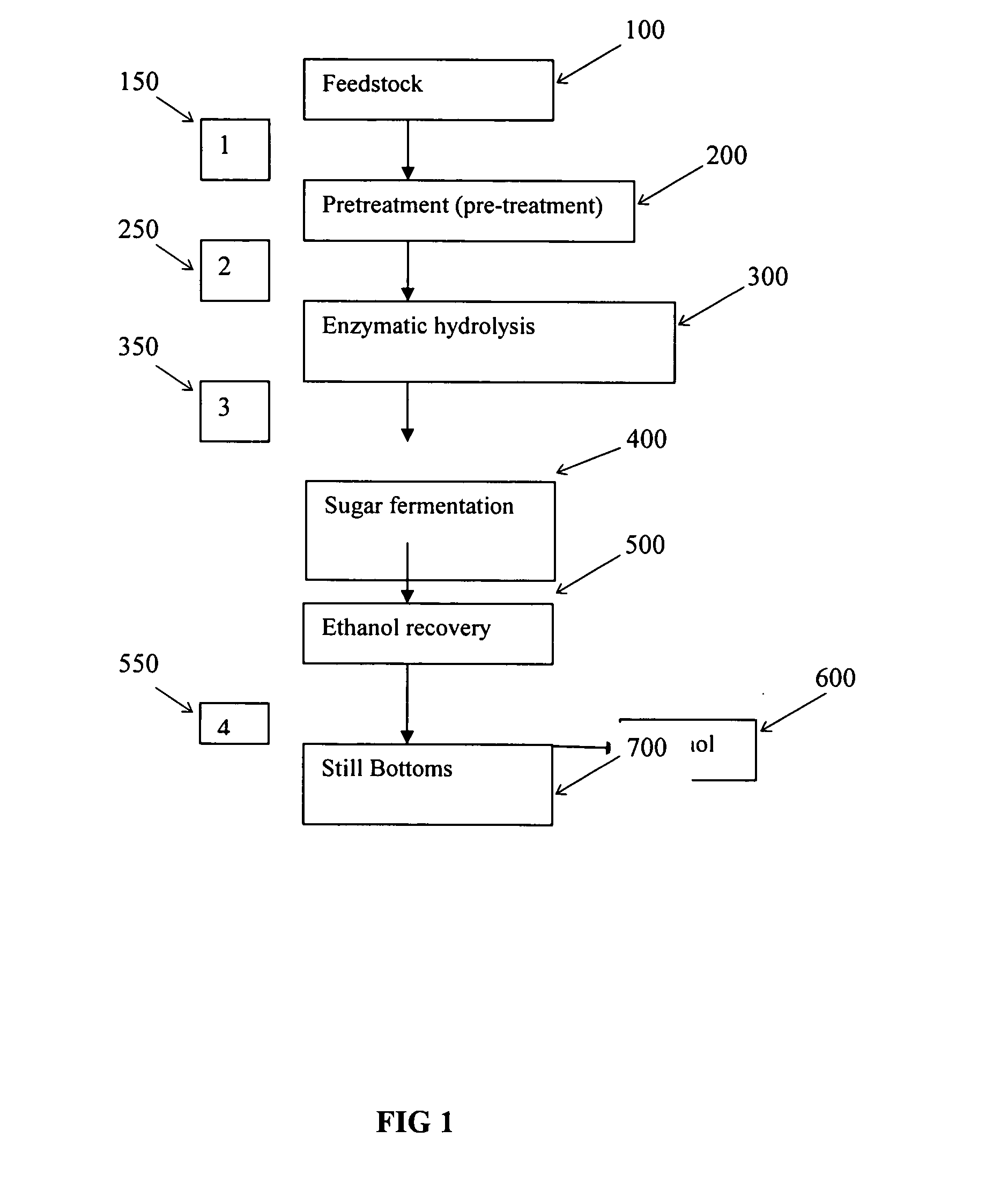
Inorganic salt recovery during processing of lignocellulosic feedstocks
InactiveUS20080102502A1Reduce inhibitionOvercome disadvantagesBiofuelsFermentationInorganic saltsCellulose
A method for recovering inorganic salt during processing of a lignocellulosic feedstock is provided. The method comprises pretreating the lignocellulosic feedstock by adding an acid or a base to the feedstock to produce a pretreated lignocellulosic feedstock. A soluble base or acid is then added to the pretreated lignocellulosic feedstock to adjust the pH and produce a neutralized feedstock. The neutralized feedstock is then hydrolyzed to produce an hydrolyzed feedstock and a sugar stream. Inorganic salt is recovered from a wash stream obtained from the pretreated lignocellulosic feedstock, a stream obtained from the neutralized feedstock, a stream obtained from the sugar stream, or a combination of these streams. The inorganic salt may be concentrated, clarified, recovered and purified by crystallization, electrodialysis, drying, or agglomeration and granulation, and then used as desired, for example, as a fertilizer.
Owner:IOGEN ENERGY CORP
Alumina-coated granules, as well as preparation method and application thereof
InactiveCN103606660AThickness adjustment and controlUniform thicknessCell electrodesLithium-ion batteryLithium electrode
The invention discloses alumina-coated granules, as well as a preparation method and application thereof. The alumina-coated granules consist of cores and shells which coat the cores. The cores are made of at least one of materials of metals, oxides, metal hydroxides, metal inorganic salts, non-metals, carbides, nitrides, lithium salts, semiconductors and organic compounds; the shell is made of Al2O3. By adopting a liquid phase method, the cores to be coated are mixed with aluminum salts, metal aluminum is precipitated by producing an alkaline environment in situ or externally adding alkaline, so that uniform, continuous and controllable coating can be formed on the surfaces of the cores. The coating method provided by the invention is simple, and has mild conditions and high universality; the coating layer has controllable thickness, completeness and uniformity; the alumina-coated granules has high practicability and a great application prospect in the field of catalysis, lithium ion batteries, surface-enhanced Raman, biomedicine and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Methods for producing a total product in the presence of sulfur
Methods of producing a total product are described. A method includes continuously contacting a feed with a hydrogen source in the presence of one or more inorganic salt catalysts and steam to produce a total product, wherein the feed has at least 0.02 grams of sulfur, per gram of feed; and producing a total product that includes coke and the crude product. The crude product has a sulfur content of at most 90% of the sulfur content of the feed.
Owner:SHELL OIL CO
Addition of zwitterionic surfactant to water soluble polymer to increase the stability of the polymers in aqueous solutions containing salt and/or surfactants
ActiveUS20090111716A1Improvement of electrolytic stabilityImprove performanceFlushingDrilling compositionInorganic saltsWater soluble
An aqueous fluid composition useful for the recovery of hydrocarbons from a subterranean formation, including a mixture of water, a water soluble polymer, an inorganic salt and at least one zwitterionic surfactant and methods of using same.
Owner:RHODIA OPERATIONS SAS
Water-based drilling fluids
InactiveUS20060019834A1Reduce molecular weightFlushingDrilling compositionWater basedInorganic salts
A water-based drilling fluid composition includes water and at least one rheology modifier and / or fluid loss control agent, and at least one other ingredient of polymeric additive, inorganic salts, dispersants, shale stabilizers, weighting agents, or finely divided clay particles, depending upon the desired attributes, wherein the rheology modifier and / or the fluid loss control agent comprises carboxymethylated raw cotton linters (CM-RCL) made from the baled raw cotton linters or comminuted raw cotton linters with increased bulk density.
Owner:HERCULES LLC
Oxygen scavenging films
InactiveUS20100255231A1Metal-working apparatusGlass/slag layered productsParticulatesAlkaline earth metal
A well dispersed oxygen scavenging particulate compounded in a polymer matrix. The oxygen scavenging formulation consists of iron powder with a mean particle sizes within 1-25 um and pre-coated with at least one or more activating and acidifying powdered compounds, usually in the form of solid organic and inorganic salts of alkaline and alkaline earth metals such as sodium chloride and sodium bisulfate. The pre-coated iron particulate is dispersed into a polymer resin by using a conventional melt processing method such as twin-screw extrusion. The oxygen scavenging compound is mixed with polymer pellets in the solid state prior to melting. The polymer resin pellets and the coated iron powder are preferably treated with a surfactant in the dry state to help dispersing the iron / salt powder with the resin pellets. The melt extruded compounds are pelletized and kept in the dry state to prevent premature activation.
Owner:MULTISORB TECH INC
Methods for producing a total product in the presence of sulfur
Methods of producing a total product are described. A method includes continuously contacting a feed with a hydrogen source in the presence of one or more inorganic salt catalysts and steam to produce a total product, wherein the feed has at least 0.02 grams of sulfur, per gram of feed; and producing a total product that includes coke and the crude product. The crude product has a sulfur content of at most 90% of the sulfur content of the feed.
Owner:SHELL OIL CO
Method for preparing suaeda salsa biogenetic salt
InactiveCN102551028AFull of nutritionRich in constant saltFood preparationNatural organic matterAmino acid
The invention discloses a method for preparing a suaeda salsa biogenetic salt. The method comprises the following steps of: taking parts, such as stems and leaves, above roots of fresh suaedasalsa; juicing; adding water into residual solid parts and extracting once; mixing the two liquids; and performing enzymolysis, boiling, decoloration, heavy metal removal, concentration and crystallization onthe mixed liquid, thus obtaining a finished product. The yield of the product is 1.2-2.1%. The product comprises inorganic salts serving as major ingredients and natural organic matters serving as minor ingredients. The inorganic salts mainly comprise sodium chloride, potassium chloride and magnesium sulfate as well as a trace amount of trace elements (Ca, P, Fe, Zn, Se, I, Cu and Mn) and the organic matters in the product mainly comprises vitamins, amino acid and polysaccharide. During preparation, any chemical substances are not required; furthermore, the raw materials are abundant, readilyavailable and low in cost, and have extremely high economic additional value; and moreover, the preparation method is simple and feasible, is easy to produce and operate, and is wide in market prospect.
Owner:SHANDONG KAIER MARINE BIOLOGICAL TECH
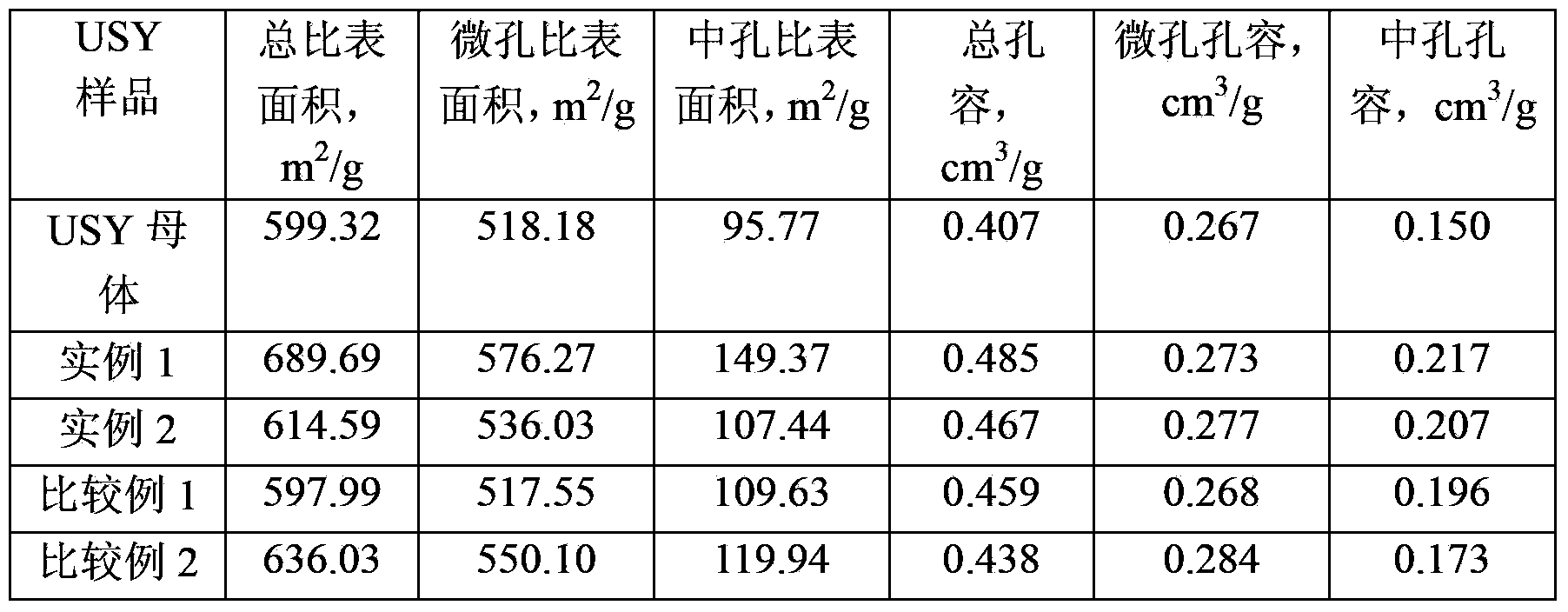
Method for modifying mesoporous-rich USY (Ultra-Stable Y) molecular sieve in combined manner
InactiveCN104229823ASmall cell constantIncreased secondary pore contentFaujasite aluminosilicate zeoliteInorganic saltsMolecular sieve
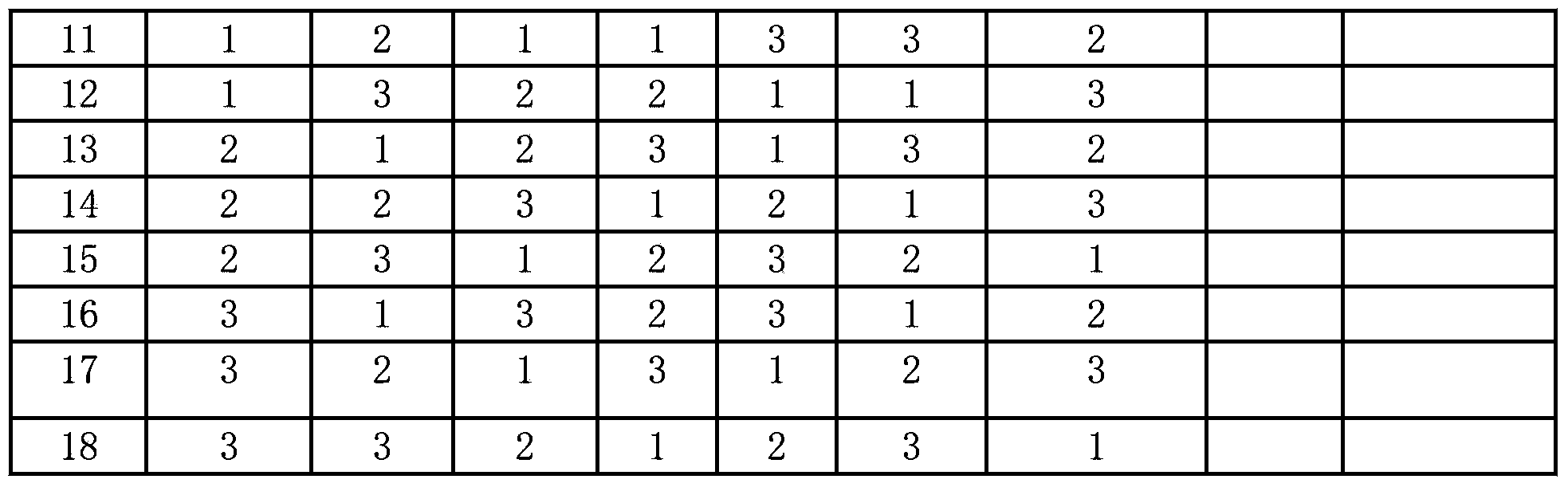
The invention relates to a method for modifying a USY (Ultra-Stable Y) molecular sieve. The method is characterized in that organic acid and an inorganic salt dealuminizing reagent are simultaneously added in a modifying process for organic acid-inorganic salt combined modification, and optimum process conditions, namely optimum concentration, volume ratio, reaction time, reaction temperature and the like, of an organic acid and an inorganic salt solution are determined by virtue of an orthogonal test. Compared with an industrial USY molecular sieve, the USY molecular sieve obtained by adopting the method disclosed by the invention is obviously increased in secondary pore content, can be kept at higher crystallinity and is enhanced in silica-alumina ratio, reduced in lattice constant and suitable for high-medium oil type hydrocracking catalyst carriers.
Owner:PETROCHINA CO LTD +1
Composition and process for lubricated plastic working of metals
InactiveUS6455476B1Work treatment devicesMetallic material coating processesInorganic saltsPhosphate
A lubricant composition for the plastic working of metals that does not require a phosphate undercoating, is waterborne, requires only a simple application process of immersion or spraying followed by drying, and provides an excellent lubricating performance comprises synthetic resin, water-soluble inorganic salt, and water. The weight ratio of the content of salt to that of synthetic resin is from 0.25:1 to 9: 1. This composition can also contain liquid and / or solid lubricating agent(s) and an extreme pressure additive.
Owner:HENKEL KGAA
Process for producing sodium sulfate and sodium chloride in Na2SO4-NaCl-H2O system
InactiveCN1944256AIncrease profitReduce energy consumptionAlkali metal sulfite/sulfate purificationAlkali metal halide purificationInorganic saltsSeparation technology
The process of producing sodium sulfate and sodium chloride in a Na2SO4-NaCl-H2O system belongs to the field of mixed inorganic solution evaporating separation technology. Mixed solution of sodium sulfate and sodium chloride as material is first evaporated and then separated to obtain sodium sulfate, sodium chloride and evaporated mother liquor; the evaporated mother liquor is low temperature evaporated and separated to obtain sodium chloride and salt-making mother liquor; and the salt-making mother liquor is evaporated and separated to obtain sodium sulfate and saltpeter-making mother liquor. The present invention has the features of high main and side product quality, high material adaptability, low cost, low cost, no waste draining, etc.
Owner:CHINA LIGHT IND INT ENG CO LTD +1
Apparatus and process for mediated electrochemical oxidation of materials
A unique apparatus unique apparatus and process that uses mediated electrochemical oxidation (MEO) for: (1) Destruction of: a) nearly all organic solid, liquid, and gases materials, except fluorinated hydrocarbons; b) all biological solid, liquid, and gases materials; c) and / or dissolution and decontamination (such as cleaning equipment and containers, etc.) of nearly all inorganic solid, liquid, or gas where higher oxidation states exist which includes, but is not limited to, halogenated inorganic compounds (except fluorinated), inorganic pesticides and herbicides, inorganic fertilizers, carbon residues, inorganic carbon compounds, mineral formations, mining tailings, inorganic salts, metals and metal compounds, etc.); and d) combined materials (e.g. a mixture of any of the foregoing with each other); henceforth collectively referred to as materials. (2) Sterilization / disinfection of equipment, glassware, etc., by destroying all existing infectious materials. (3) Dissolution of transuranic / actinide materials and / or destruction of the oxidizable components in the hazardous waste portion of mixed waste. (4) Generation of hydrogen and oxygen from MEO of materials. (5) Alteration of organic, biological, and inorganic materials by MEO to produce other compounds from these materials. The materials are introduced into an apparatus for contacting the materials with an electrolyte containing the oxidized form of one or more reversible redox couples, at least one of which is produced electrochemically by anodic oxidation at the anode of an electrochemical cell. The oxidized forms of any other redox couples present are produced either by similar anodic oxidation or reaction with the oxidized form of other redox couples present and capable of affecting the required redox reaction. The oxidized species of the redox couples oxidize the materials molecules and are themselves converted to their reduced form, whereupon they are reoxidized by either of the aforementioned mechanisms and the redox cycle continues until all oxidizable material species, including intermediate reaction products, have undergone the desired degree of oxidation. The entire process takes place at temperatures between ambient and approximately 100° C. The oxidation process may be enhanced by the addition of reaction enhancements, such as: ultrasonic energy and / or ultraviolet radiation.
Owner:SCIMIST LNC
Physiologically balanced, ionized, acidic solution and methodology for use in wound healing
Described herein is a physiologically-balanced, acidic solution. Typically the solution is prepared by a chemical reactions or by the electrolysis of a solution comprising a mixture of an inorganic salt to form a physiologically balanced solution. This invention also relates to methods for use of the solutions, including a specialized bandage which may be used in combination with the solutions, or optionally with other topically applied materials. A mixture of inorganic salts and, optionally minerals, is used in order to mimic the electrolyte concentration and mixture of body fluid in an isotonic state. The solution typically comprises of one halide salt of lithium, sodium, potassium, calcium, and other cations. Typically the halide is fluoride, chloride, bromide, or iodide, and most typically chloride. A typical electrolyzed solution of the present invention has a pH within the range of about 2 to about 5, an oxidation reduction potential within the range of about +600 mV to about +1200 mV, and hypohalous acid concentration in the range of about 10 ppm to about 200 ppm. The solution has bactericidal, fungicidal, and sporicidal properties. The composition of the invention is nontoxic and has antibacterial properties, and is useful in any application in which antimicrobial properties are desirable.
Owner:NOVABAY PHARM INC
Chromium-free passivation liquid for galvanized sheet and manufacture method thereof
InactiveCN101250699AImprove bindingImprove corrosion resistanceMetallic material coating processesChromium freeSealant
The invention in particular relates to passivation solution without chrome which is used for galvanized sheets and a method thereof. The technical scheme thereof comprises: firstly, dissolving inorganic salt corrosion inhibitor, then, adding the inorganic salt corrosion inhibitor into a stirred tank, adding dispersant, organic acid, sealant, silicone-acrylate emulsion and water while stirring, then, using inorganic acid or alkali to regulate the pH value to be 2.0-5.0, and then stirring for 1-2 hours under the condition that the temperature is 20-30 DEG C, the content of the components of each liter is: the inorganic salt corrosion inhibitor 10-55g, additive 4-10g, the organic acid 5-20g, the sealant 5-30g, the silicone-acrylate emulsion 150-300g, and the rest is the water. The method of the invention can additionally form a layer of organic resin separate layer on the basis of forming an inorganic metal compound precipitation film, additionally, since the silicon compound is added, not only the binding force between a passivation layer and zinc coating can be increased, but also the corrosion resistance, the scrubbing resistance and the wear resistance of the passivation layer can be increased, and the coating treatment after passivation can not be affected.
Owner:WUHAN UNIV OF SCI & TECH
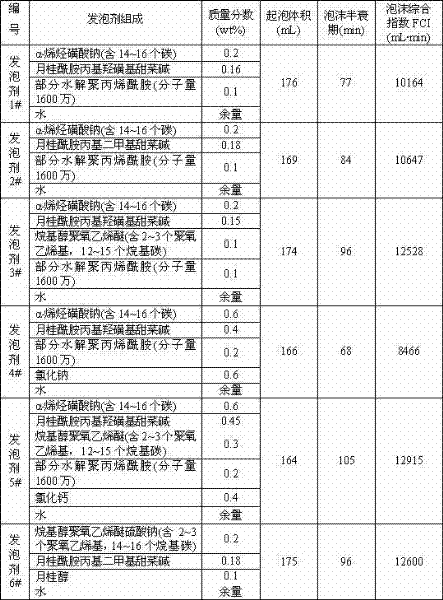
Foaming agent suitable for oil-field development
ActiveCN102504788AHigh production costReduce manufacturing costDrilling compositionActive agentEngineering
The invention provides a foaming agent suitable for oil-field development. The foaming agent suitable for oil-field development comprises the following components by weight percent: 0.05-1.0% of anionic surfactant, 0.05-1.0% of amphoteric surfactant, 0-1.0% of nonionic surfactant, 0.01-1.0% of foam stabilizer, 0-0.8% of inorganic salt and the balance water. The composition and proportioning of the foaming agent can be adjusted at any time according to the actual situations of different application fields of oil-field development, the foaming agent is convenient in production, has wide applicability and remarkable economic benefit; and the foaming agent has good biodegradability and can not pollute the environment or damage the formation.
Owner:PETROCHINA CO LTD +1
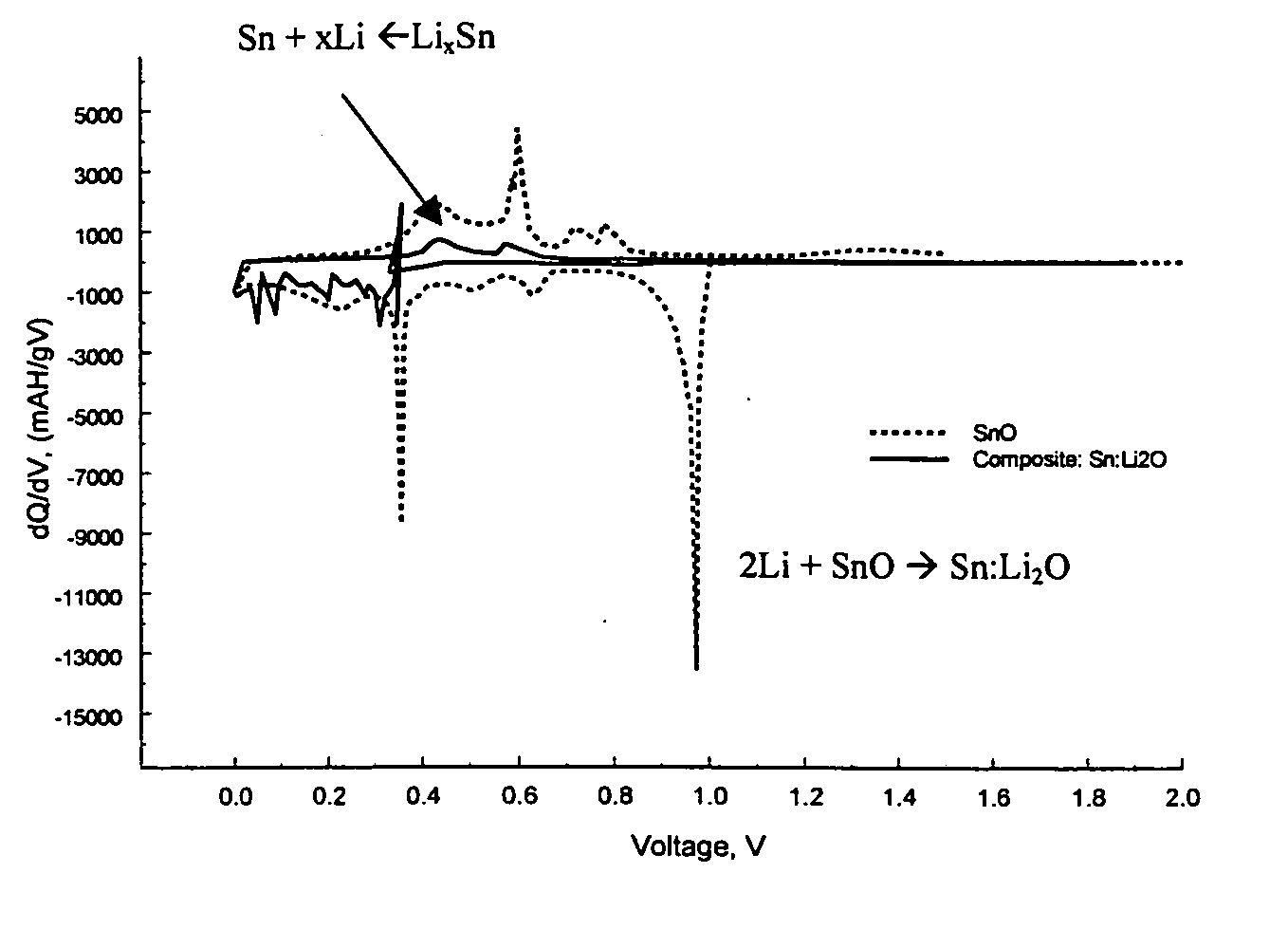
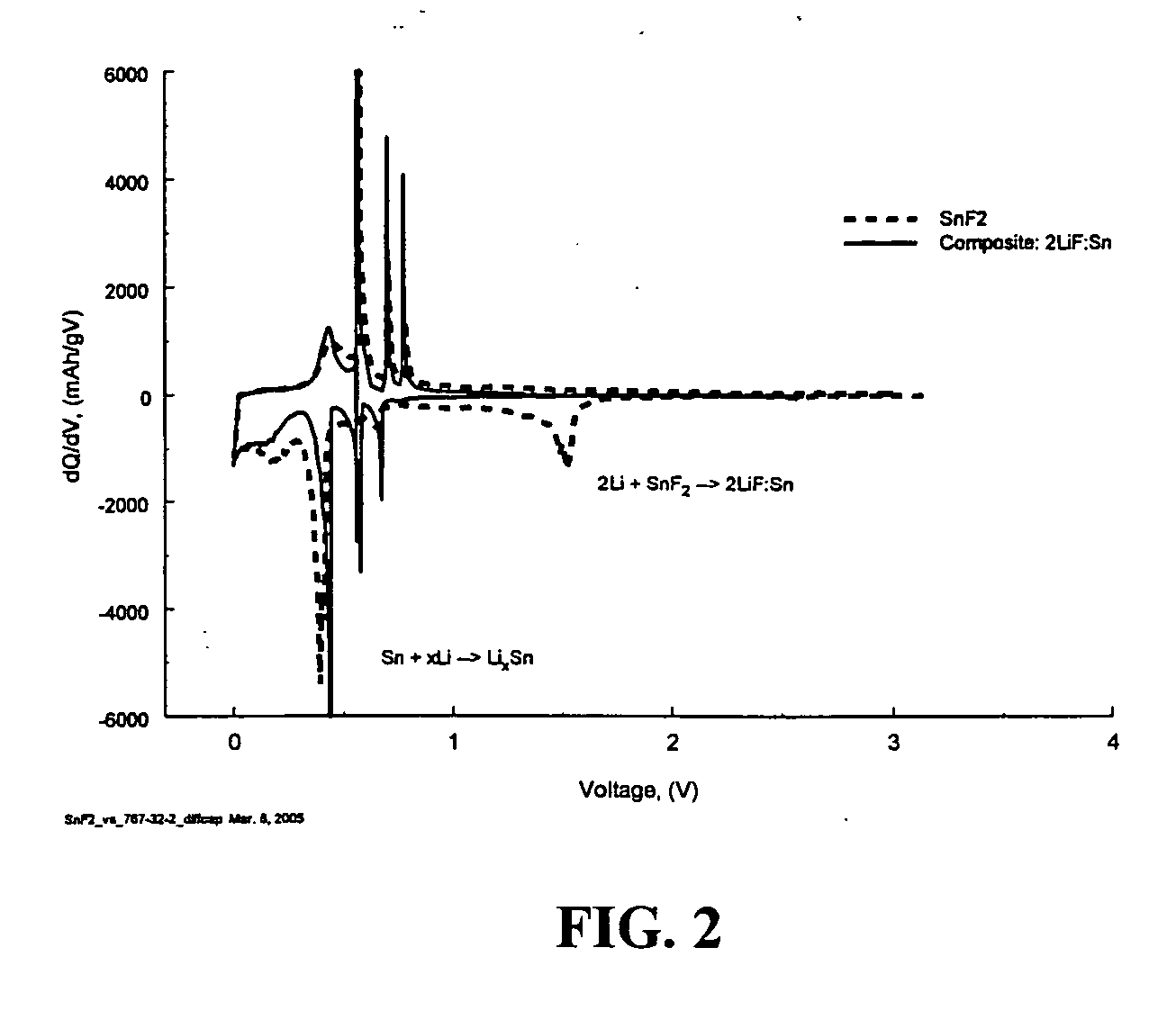
Composite materials of nano-dispersed silicon and tin and methods of making the same
Composite compounds of tin and lithium, silicon and lithium, or tin, silicon, and lithium having tin and silicon nano-dispersed in a lithium-containing matrix may be used as electrode materials and particularly anode materials for use with rechargeable batteries. Methods of making the composite compounds include the oxidation of alloys, the reaction of stabilized lithium metal powder with tin and silicon oxides, and the reaction of inorganic salts of lithium with tin and silicon containing compounds.
Owner:LIVENT USA CORP
Formaldehyde gas oxidation catalyst under room temperature
ActiveCN1714930ARaw materials are readily availableSimple conditions of useDispersed particle separationMetal/metal-oxides/metal-hydroxide catalystsCatalytic oxidationSilicon dioxide
The present invention provides a kind of formaldehyde gas oxidizing catalyst for room temperature use. Metal oxide is first obtained with soluble metal nitrate, carbonate or other inorganic salt and through oxidation and precipitation; and then loaded with small amount of metal to form the catalyst with room temperature formaldehyde oxidizing activity. The catalyst has metal oxide as main body and supported noble metal as active component, and features that the metal oxide may be oxide of Al, Ni, Mn, Si or Fe, and the noble may be Pt, Ru, Au, Rh or Pd. The catalyst of the present invention has high catalytic activity, and long sustaining time. The catalyst has formaldehyde converting rate up to 100 %, and results in low power consumption.
Owner:RES CENT FOR ECO ENVIRONMENTAL SCI THE CHINESE ACAD OF SCI
Method for preparing mesoporous silica molecular sieve fiber
InactiveCN101387019AIncrease the areaIncrease the apertureInorganic material artificial filamentsMolecular-sieve and base-exchange compoundsFiberNanowire
The present invention discloses a method for preparing a wide aperture mesoporous silica molecular sieve fiber. An industrialized nonionics or cationic surfactant is adopted as a template. An organic or inorganic silicon source is adopted as a precursor. In the state where various auxiliary reagents, such as inorganic salt, alcohol and the like, are added, the fiber is synthesized through the cooperative assembly between a surface active agent and inorganic species and a hydrothermal treatment process. The mesoporous silica molecular sieve fiber is between 3 and 20 nm in aperture, between 0.3 and 2.5 cm^3 / g in pore volume, and between 600 and 1200 cm^2 / g in specific area. The material is easy to obtain, the technical requirements are comparatively simple, and the operation is feasible. The method has a very wide application range in terms of the preparation of composite fortifying fiber and semiconductor porous nanometer tube and nanometer wire in the fields of nanometer microelectrode and aviation material.
Owner:SHANGHAI INST OF TECH
Sulfide coated particle as well as preparation method and application thereof
InactiveCN107983272AUniform thicknessThickness is easy to controlCobalt sulfidesZinc sulfidesMetallic sulfideLithium-ion battery
The invention discloses a sulfide coated particle as well as a preparation method and an application thereof. The sulfide coated particle comprises a core and a shell coating the core, wherein the core is prepared from at least one of metal, oxide, metal hydroxide, metal inorganic salt, elemental carbon or oxysome thereof, carbide, nitride, semiconductor and organic matter; the shell is prepared from metal sulfide. The to-be-coated core is mixed with the metal salt, a reducing agent and a sulfur source with a liquid phase method, metal sulfide is precipitated to the particle surface through in-situ reduction, and uniform, continuous and controllable coating of the core surface with metal sulfide is realized. The coating method is simple, reaction conditions are mild, universality is high,a coating layer is controllable in thickness, complete and uniform, and the sulfide coated particle has quite broad practical application prospect in the fields of electrocatalysis, lithium ion batteries, biomedicine and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Lignin carbon nanofiber and preparation method thereof
ActiveCN101768799AImprove spinnabilityGuaranteed reliabilityFilament/thread formingFibre chemical featuresFiberCarbon fibers
The invention discloses a lignin carbon nanofiber and a preparation method thereof, belonging to the field of materials. The preparation method comprises 5 steps of lignin pretreatment, preparation of spinning solution, electrostatic spinning, pre-oxidation and carbonization. The preparation method is characterized in that the lignin pretreatment means that lignin with the relative molecular mass within 5,000 to 50,000 is selected, and then the treatment of removing carbohydrate and inorganic salt is respectively carried out on alkali lignin and acetic acid lignin (or formic acid lignin). The diameter of the obtained carbon nanofiber is within 50 to 300nm and the length is within 1 to 10mum. As the obtained lignin has good spinnability, the nanofiber can be spun and further processed into the carbon nanofiber without synthetic macromolecules. In addition, the method adopts simple electrospinning equipment for electrospinning, the diameter of a spinning nozzle is large, the blockage cannot easily occur, and the working reliability of the spinning equipment is ensured.
Owner:SOUTH CHINA UNIV OF TECH
Low density cements for use in cementing operations
A cement mix suitable for blocking or plugging an abandoned pipeline or back filling a mine shaft, tunnel or excavations contains Portland cement or a cement blend of two components selected from Portland cement, fly ash, pozzolan, slag, silica fume and gypsum; diatomaceous earth; zeolite and an inorganic salt accelerator. The cement mix may further contain an alkali metasilicate and / or alkali silicate. A cementitious slurry, formulated from the cement mix, may have a density less than or equal to 1500 kg / m3, and exhibits good compressive strength.
Owner:BAKER HUGHES INC
Stable solid preparations
It is intended to provide a process for producing unstable amorphous benzimidazole compounds having a proton pump inhibitor function, and stable solid preparations for medicinal use containing these compounds which are produced by blending such an amorphous benzimidazole compound with a nontoxic base such as a basic inorganic salt, forming an intermediate coating layer on the layer containing the active ingredient and further forming an enteric coating layer or a release-controlling coating layer.
Owner:TAKEDA PHARMA CO LTD
Medium-high temperature composite structural heat storage material, preparation method and application thereof
InactiveCN102888209AHigh phase change enthalpyGood chemical compatibilityHeat-exchange elementsMicro nanoMass ratio
The invention relates to a medium-high temperature (120-1000 DEG C or higher) composite structural heat storage material. The medium-high temperature composite structural heat storage material comprises an inorganic salt phase change latent heat material, a sensitive heat storage material and a heat conduction reinforcing material, wherein the mass ratio of the inorganic salt phase change latent heat material to the sensitive heat storage material is of 1: (0.1-10); and the heat conduction reinforcing material is of 0.0001-1kg / (kg heat storage material) based on mass ratio. The preparation method comprises steps as follows: uniformly mixing the inorganic salt phase change latent heat material with the sensitive heat storage material and the heat conduction reinforcing material; pressurizing to form green blank; and then heating and sintering, so as to obtain the medium-high temperature composite structural heat storage material. The medium-high temperature composite structural heat storage material provided by the invention is capable of obviously reducing the corrosion resistance of the sensitive heat storage material; meanwhile, the thermal conductivity of the composite heat storage material is markedly improved by virtue of the micro-nano doping of the heat conduction reinforcing material; and moreover, high heat storage density is achieved, and wide application prospect is provided.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Pulverized coal carriability improver
InactiveUS6083289AGreat varietyImprove liquiditySolid fuel pretreatmentSolid fuelsInorganic saltsCombustion
PCT No. PCT / JP97 / 00668 Sec. 371 Date Sep. 25, 1998 Sec. 102(e) Date Sep. 25, 1998 PCT Filed Mar. 5, 1997 PCT Pub. No. WO97 / 36009 PCT Pub. Date Oct. 2, 1997The use of pulverized coal as the fuel to be injected into metallurgical or combustion furnace becomes possible enabled by improving the transportability thereof. Further, a pulverized coal is provided, which is inhibiting from bridging or channeling in a hopper, or piping choking. A water-soluble inorganic salt having a polar group is made to adhere to pulverized coal which is prepared from raw coal having an average HGI of 30 or above and which is in a dry state at the injection port of a metallurgical or combustion furnace, The inorganic salt is selected from among BaCl2, CaCl2, Ca(NO2)2, Ca(NO3)2, Ca(ClO)2, K2CO3, KCl, MgCl2, MgSO4, NH4BF4, NH4Cl, (NH4)2SO4, Na2CO3, NaCl, NaClO3, NaNO2, NaNO3, NaOH, Na2S2O3, Na2S2O5, HNO3, H2SO4, H2CO3, and HCl.
Owner:KAO CORP
Concrete super instant coagulant
The invention discloses a concrete early-strength agent, which is characterized in comprising the following components according to weight percentage: inorganic salt early-strength component 35-55 percent, organic early-strength component 5-10 percent, water reducing component 15-25 percent, wherein the inorganic salt early-strength component is prepared by at least two among sulfate, carbonate, nitrate, and nitrite; the organic early-strength component selects any one among calcium formate, sodium acetate, calcium oxalate, triethanolamine, tri-iso-propanolamine and carbamide; the water reducing component selects one among naphthalenesulfuric acid type, melamine type and polycarboxylate type. The invention makes the concrete be coagulated and hardened rapidly under the condition of low temperature, so that the early strength of the concrete is greatly improved as well as later strength is ensured.
Owner:ZHONGYIFENG CONSTR GRP +1
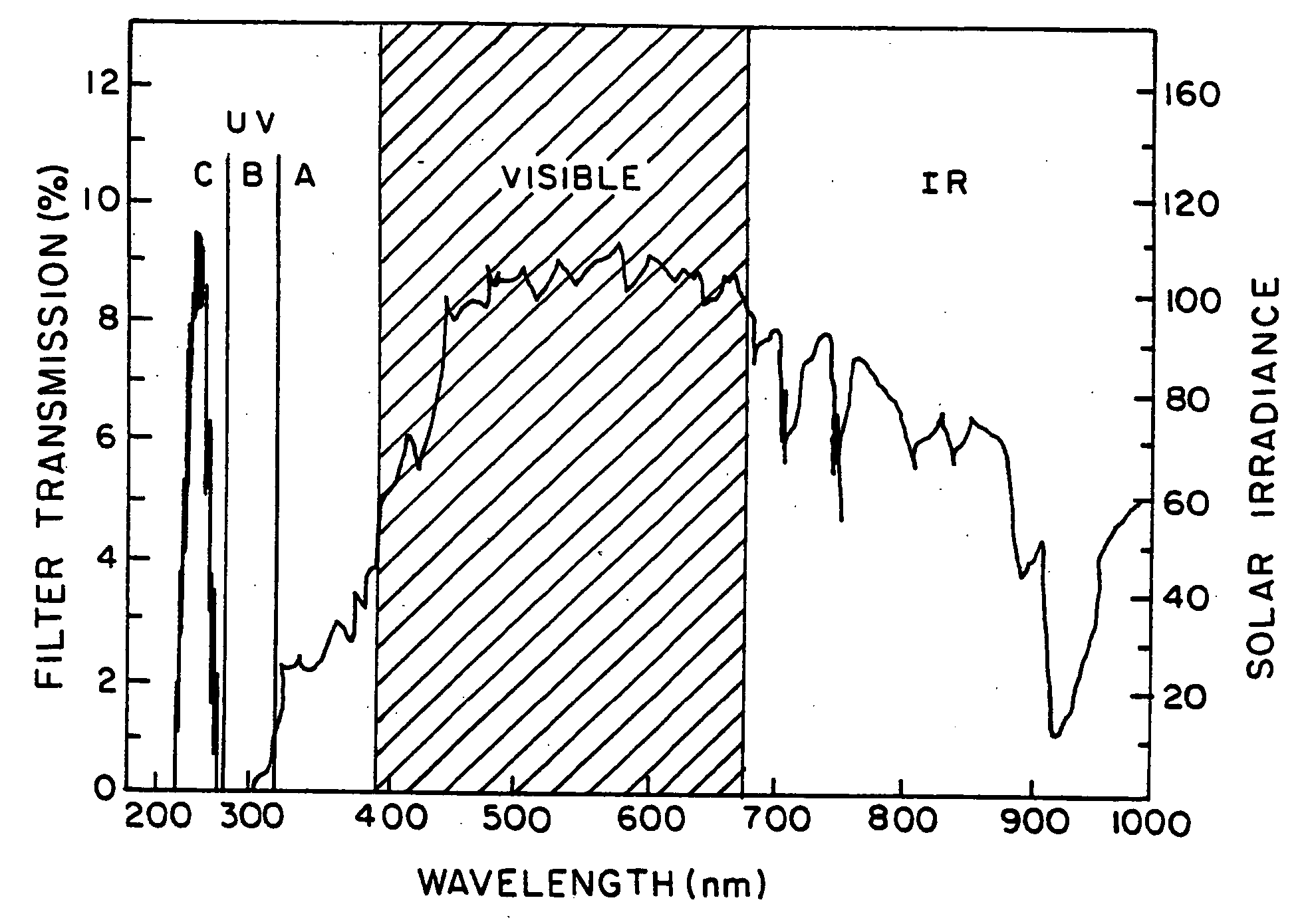
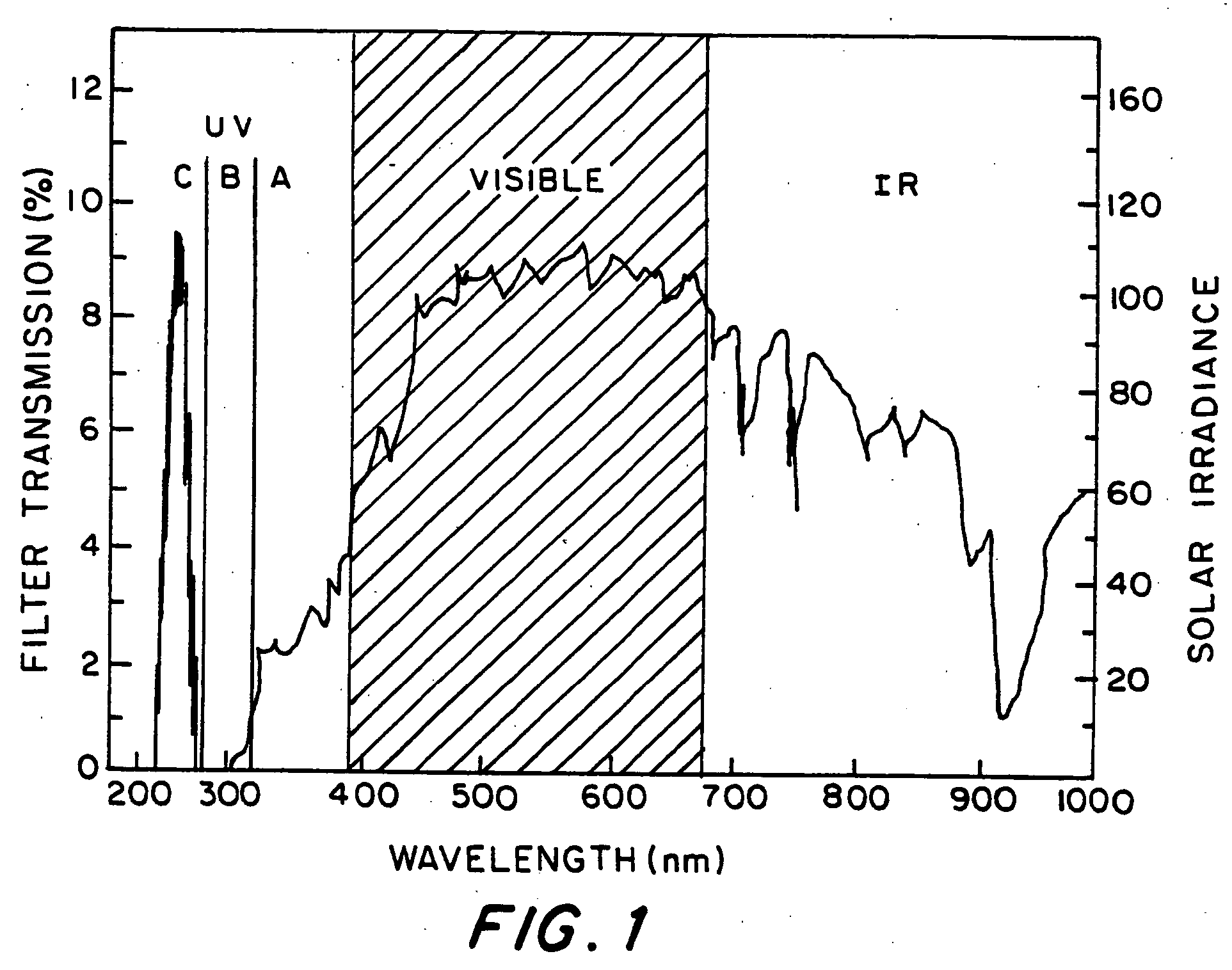
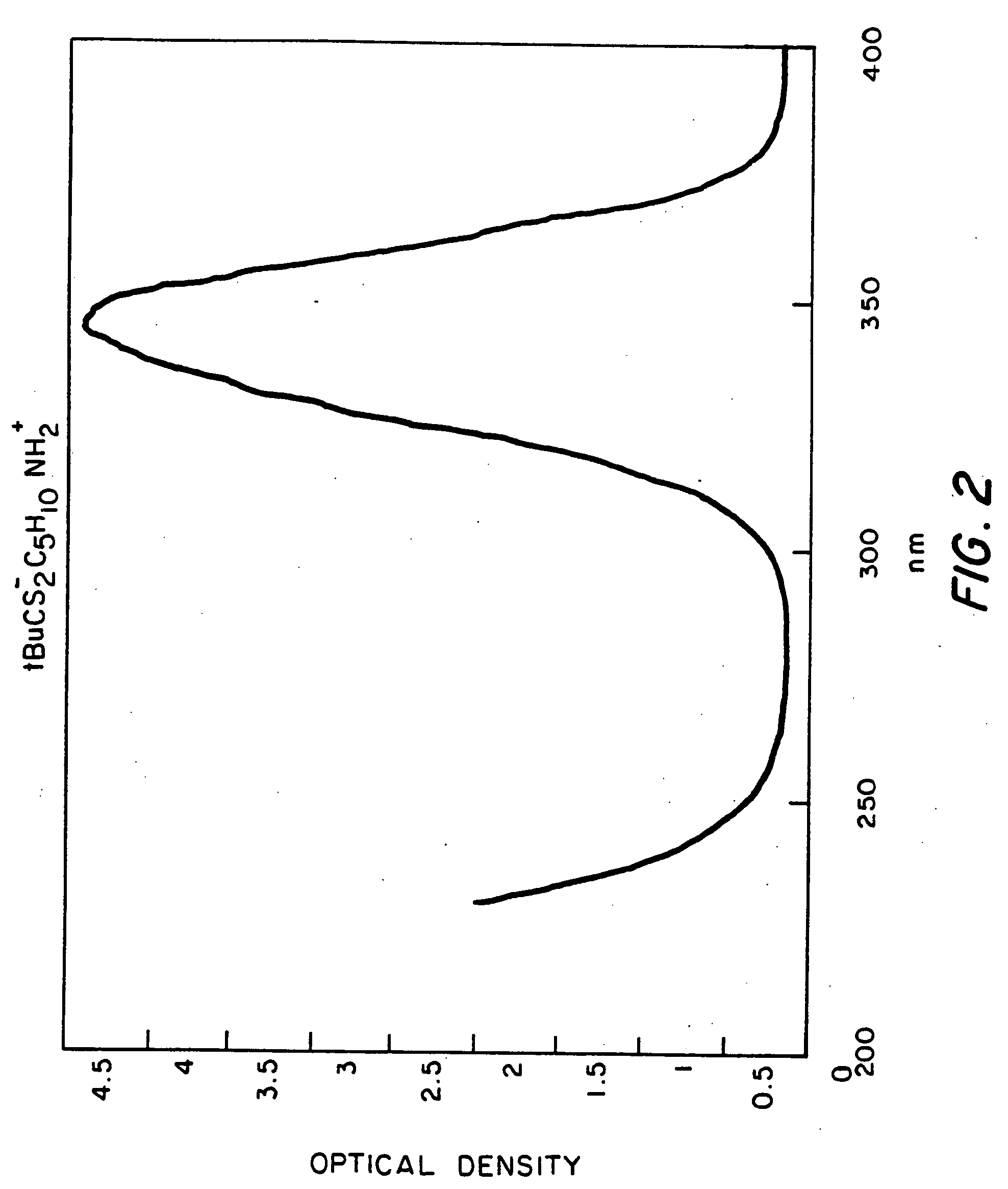
Optical filter comprising solar blind dyes and UV-transparent substrates
The invention provides an optical filter comprising: a. an organic, solar blind filter dye; and b. a UV-transparent, non-scattering and chemically stable substrate. The substrate may be a UV-transparent nanoporous silica glass solid having pores that are substantially filled with a UV-transparent solvent, which has been selected to dissolve said dye and also to match the refractive index of the nanoporous silica glass solid. Alternatively, the substrate may be a UV-transparent inorganic salt compressed to form a solid body. The invention also provides for methods of making these embodiments and an optical device comprising such an optical filter. The filter provides an efficient solar blind filter that is chemically and dimensionally stable.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
Multifunctional scrubbing cleanser
InactiveCN1524939AGood removal effectEasy to cleanSurface-active detergent compositionsInorganic saltsCleansers skin
A multifunctional scouring detergent with constituents (by weight percentage) of, mixed solvent 2-70, surface active agent 3-30, inorganic salt 0-10, water for the rest. The multifunctional cleaning agent can effectively remove the stains of chewing gum, scotch tape colloid residual and paint film coating.
Owner:李伟光
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com