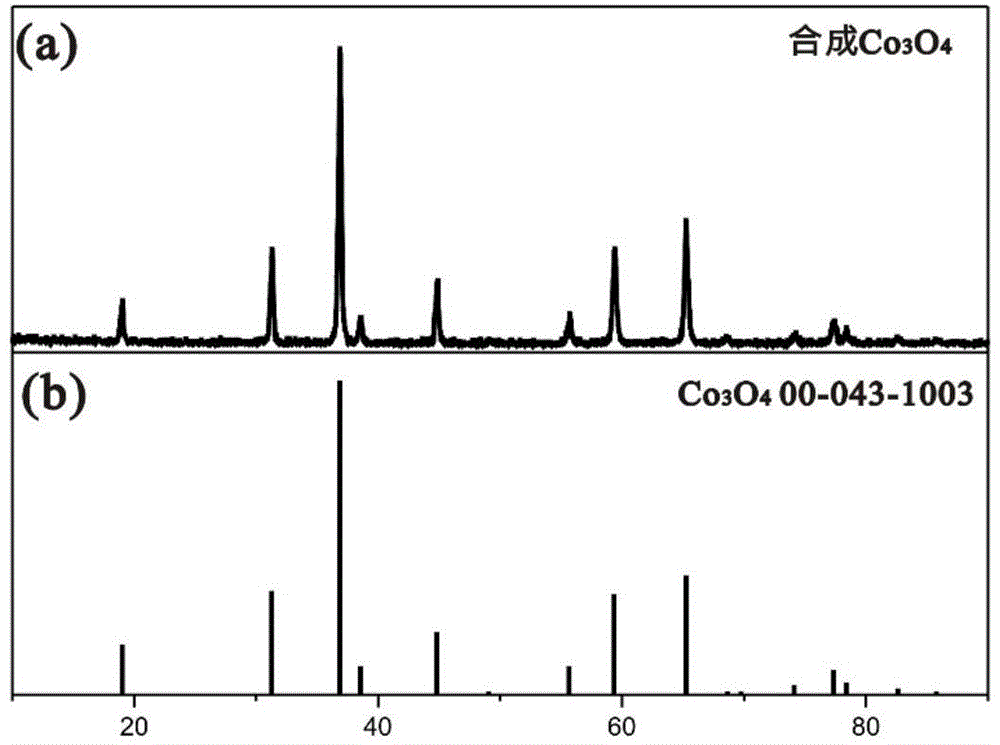
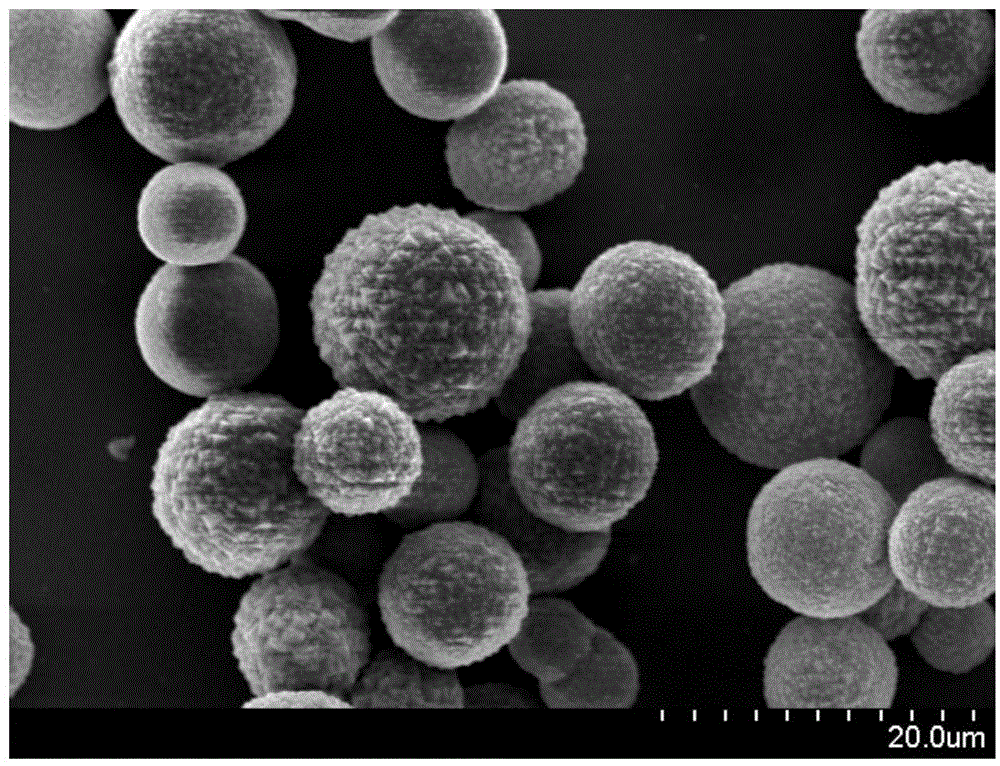
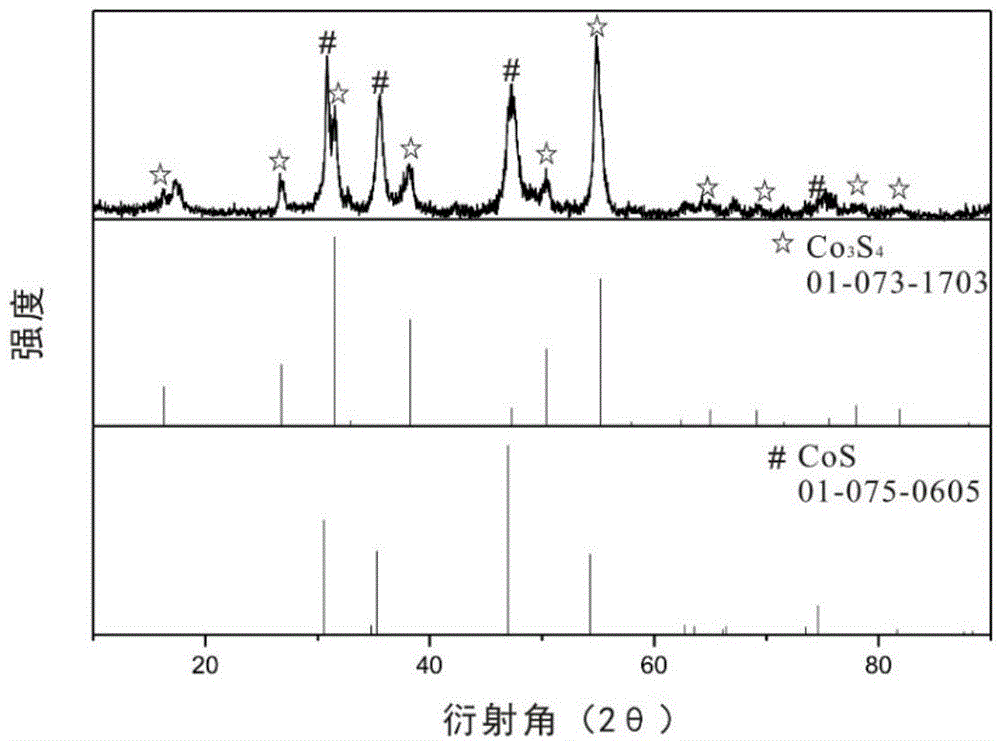
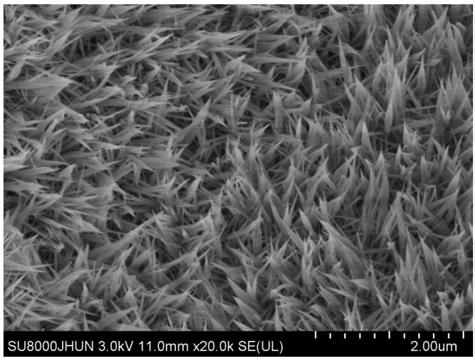
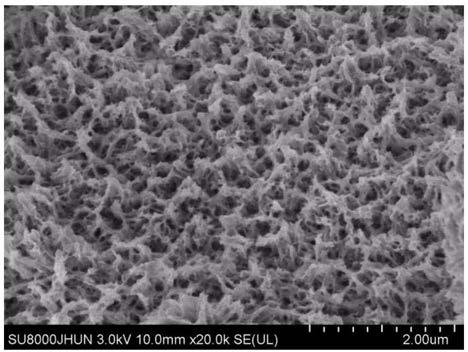
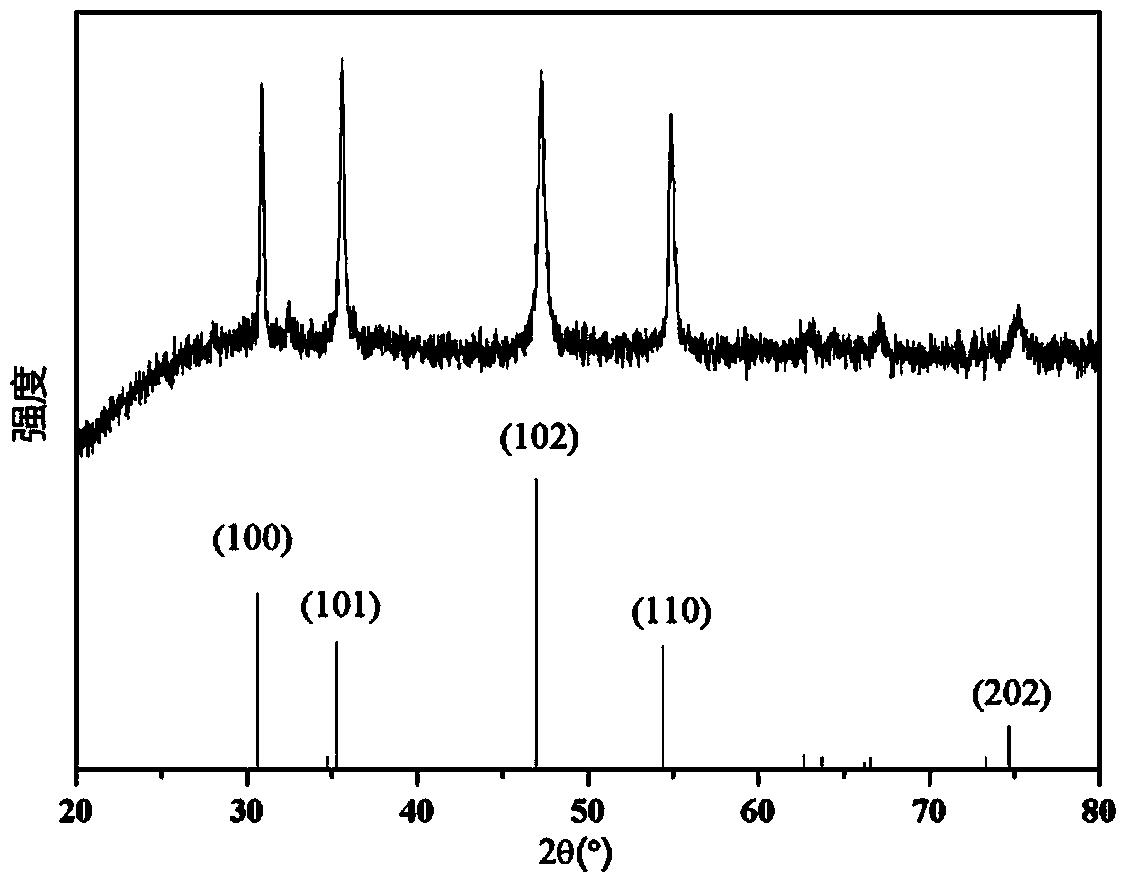
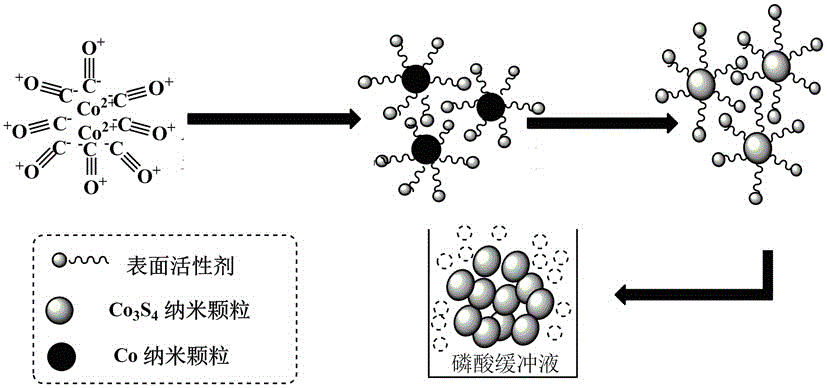
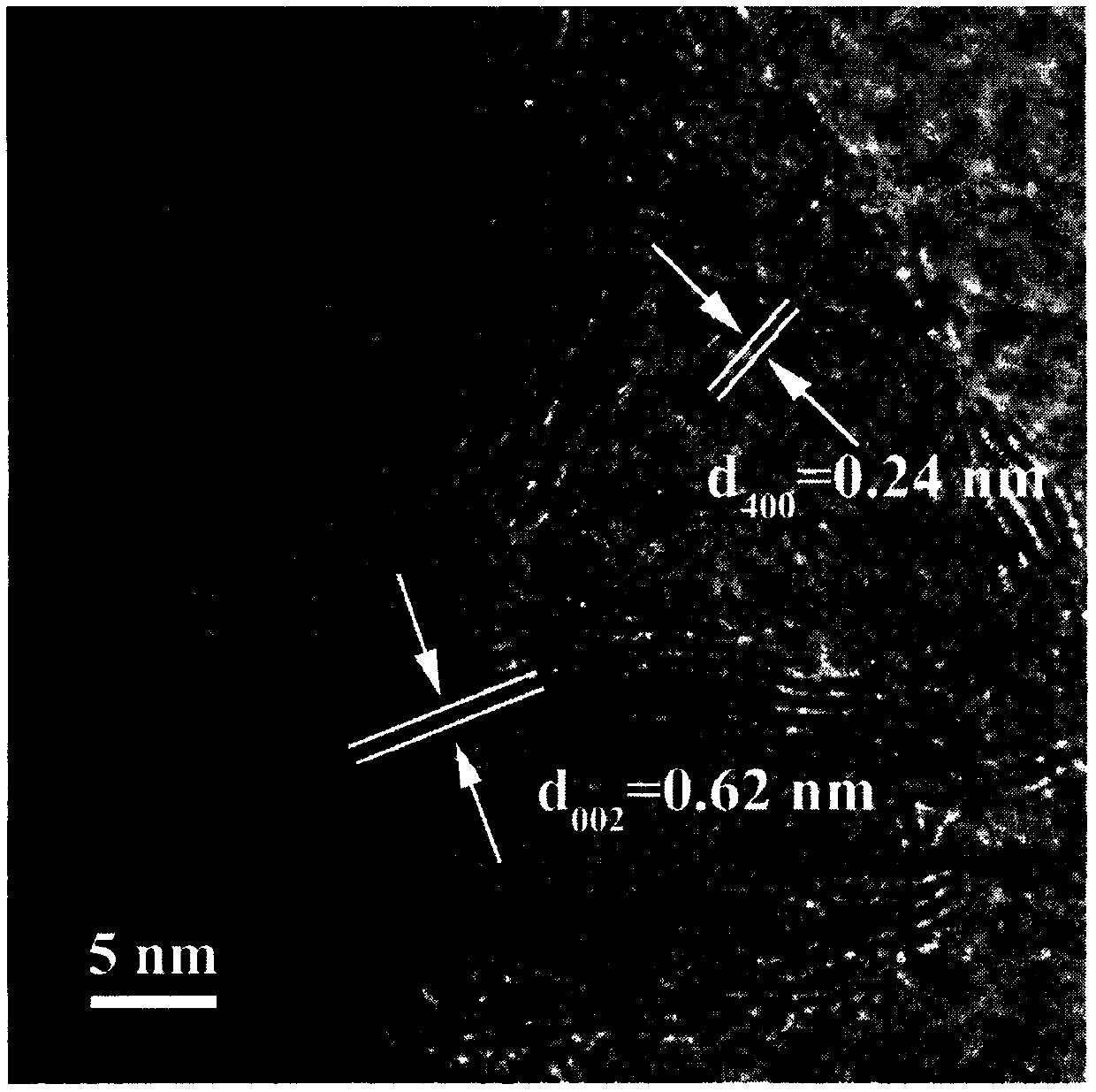
Patents
Literature
185results about "Cobalt sulfides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
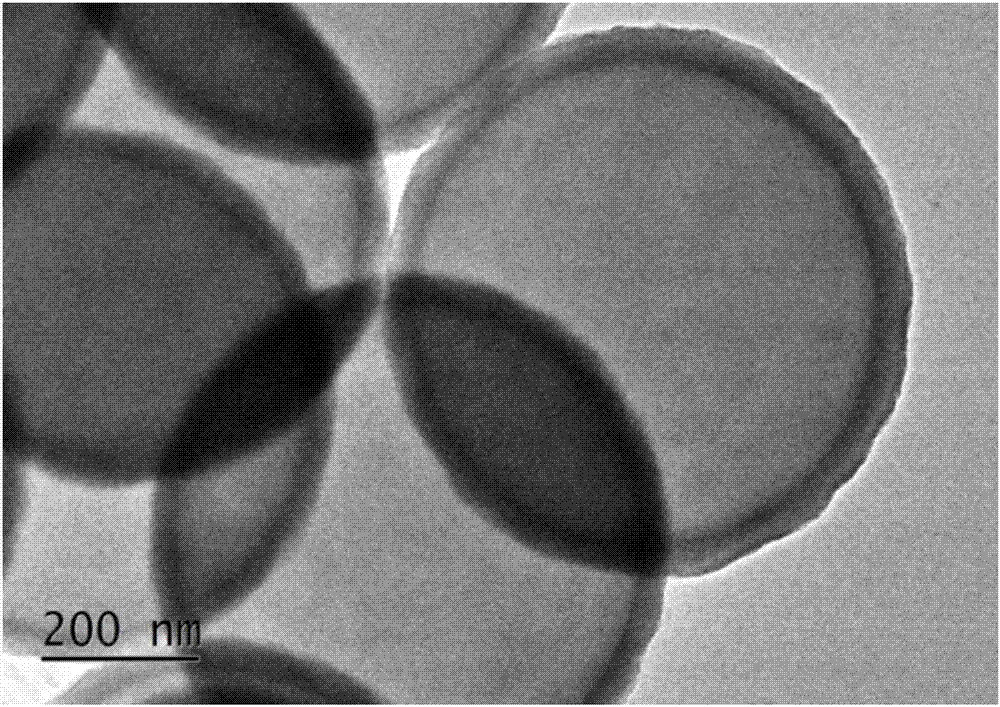
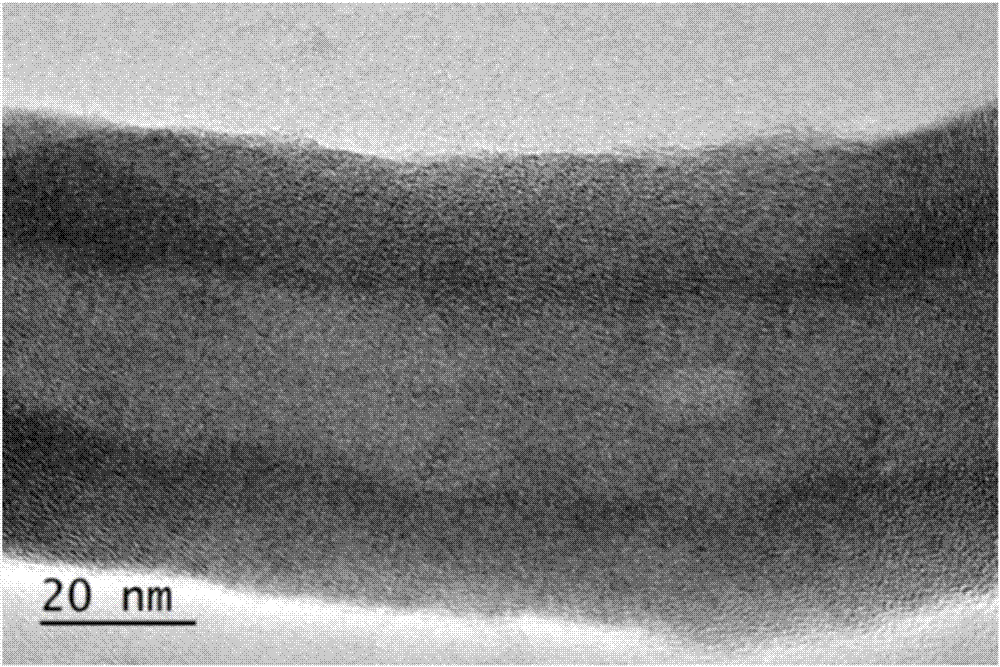
Sulfide coated particle as well as preparation method and application thereof
InactiveCN107983272AUniform thicknessThickness is easy to controlCobalt sulfidesZinc sulfidesMetallic sulfideLithium-ion battery
The invention discloses a sulfide coated particle as well as a preparation method and an application thereof. The sulfide coated particle comprises a core and a shell coating the core, wherein the core is prepared from at least one of metal, oxide, metal hydroxide, metal inorganic salt, elemental carbon or oxysome thereof, carbide, nitride, semiconductor and organic matter; the shell is prepared from metal sulfide. The to-be-coated core is mixed with the metal salt, a reducing agent and a sulfur source with a liquid phase method, metal sulfide is precipitated to the particle surface through in-situ reduction, and uniform, continuous and controllable coating of the core surface with metal sulfide is realized. The coating method is simple, reaction conditions are mild, universality is high,a coating layer is controllable in thickness, complete and uniform, and the sulfide coated particle has quite broad practical application prospect in the fields of electrocatalysis, lithium ion batteries, biomedicine and the like.
Owner:INST OF CHEM CHINESE ACAD OF SCI +1
Electrocatalyst with cobalt-based multi-stage nano-composite structure for oxygen production by electrolysis of water and preparation method of electrocatalyst
InactiveCN106011926ALow costEasy to operateCobalt sulfidesElectrolytic inorganic material coatingFiberCarbon fibers
The invention provides an electrocatalyst with a cobalt-based multi-stage nano-composite structure for oxygen production by electrolysis of water and a preparation method of the electrocatalyst. The preparation method comprises the following steps: dissolving cobalt nitrate hexahydrate, urea and ammonium fluoride in deionized water to obtain a precursor solution; transferring the precursor solution into a hydrothermal reactor; adding carbon fiber paper; enabling basic cobalt carbonate nanowires to grow on the carbon fiber paper through solvothermal reaction; after finish of reaction, naturally cooling; then taking out a product; washing and drying to obtain a carbon fiver paper loaded basic cobalt carbonate nanowire composite structure; by taking powdered sulfur as the raw material, preparing a carbon fiber paper loaded cobalt sulfide nanowire composite structure through low-temperature sulfuration reaction under the condition of an inert gas; and finally, electroplating the surface of the carbon fiber paper loaded cobalt sulfide nanowire composite structure with a layer of cobalt hydroxide nanosheets by use of the electrochemical deposition method so as to obtain the electrocatalyst with the cobalt-based multi-stage nano-composite structure for oxygen production by electrolysis of water. As the sulfide and the hydroxide of transition metal cobalt are adopted as the catalyst, in comparison with noble metals, the cost of the catalyst is lowered.
Owner:JIANGSU UNIV
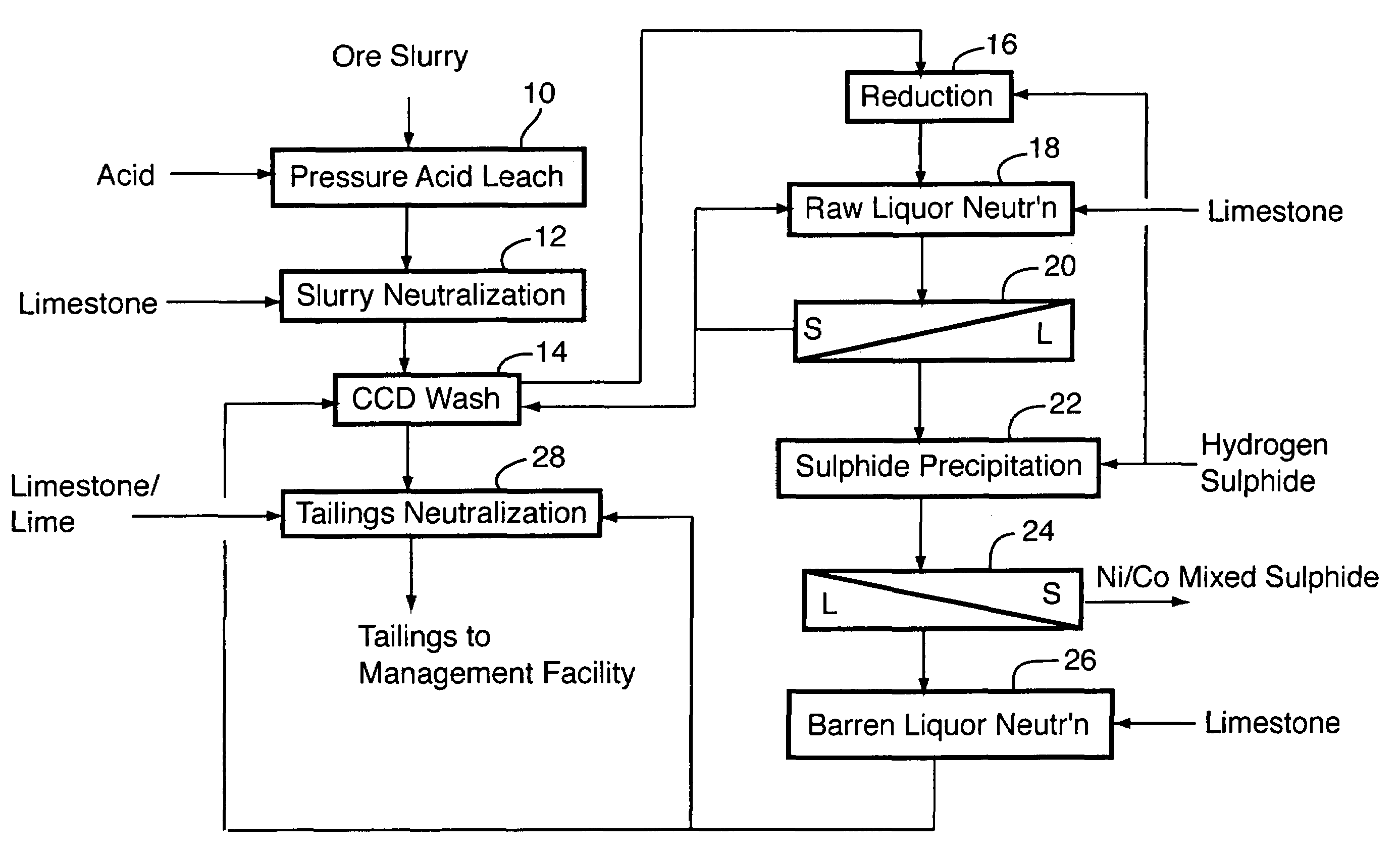
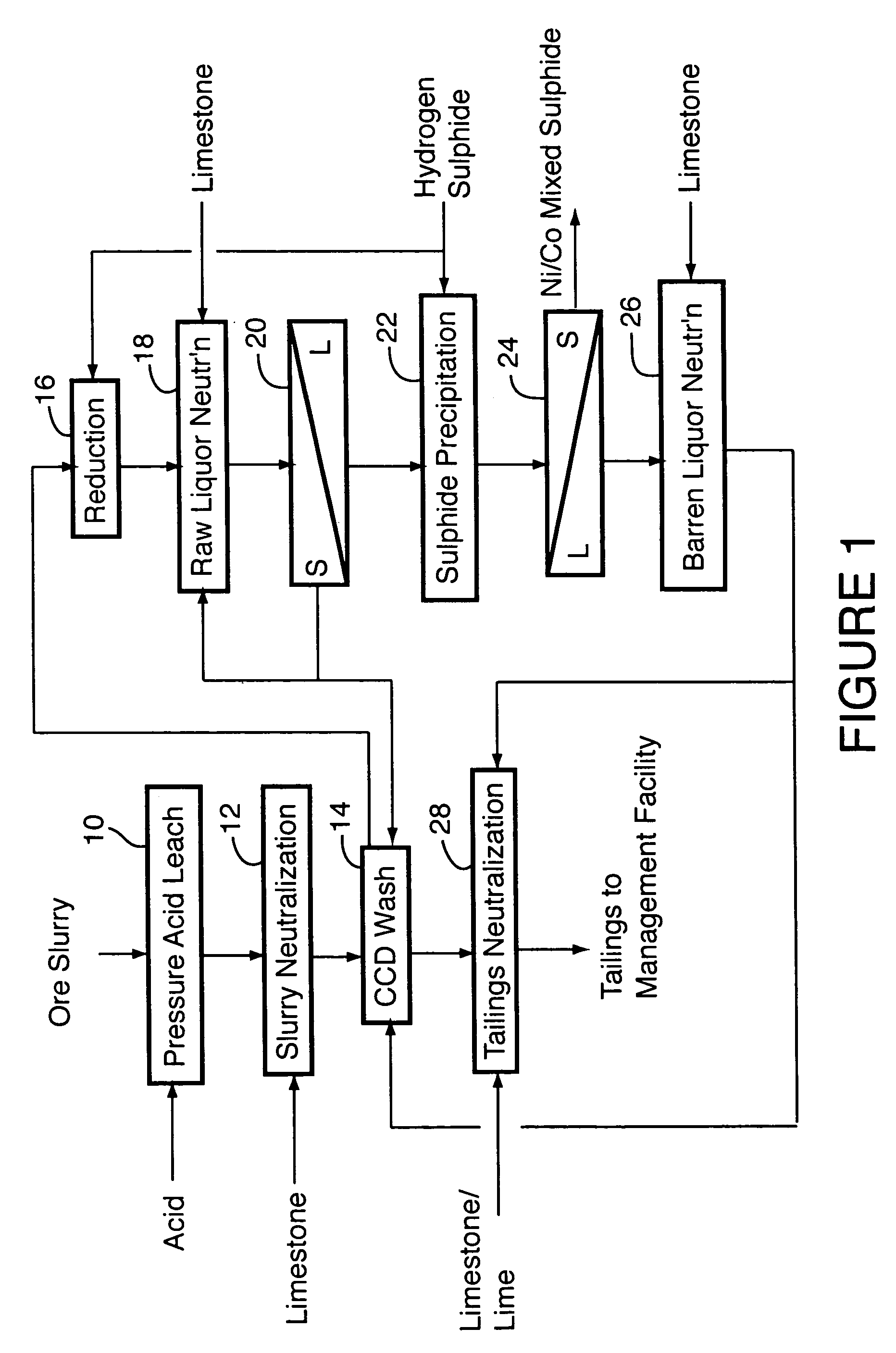
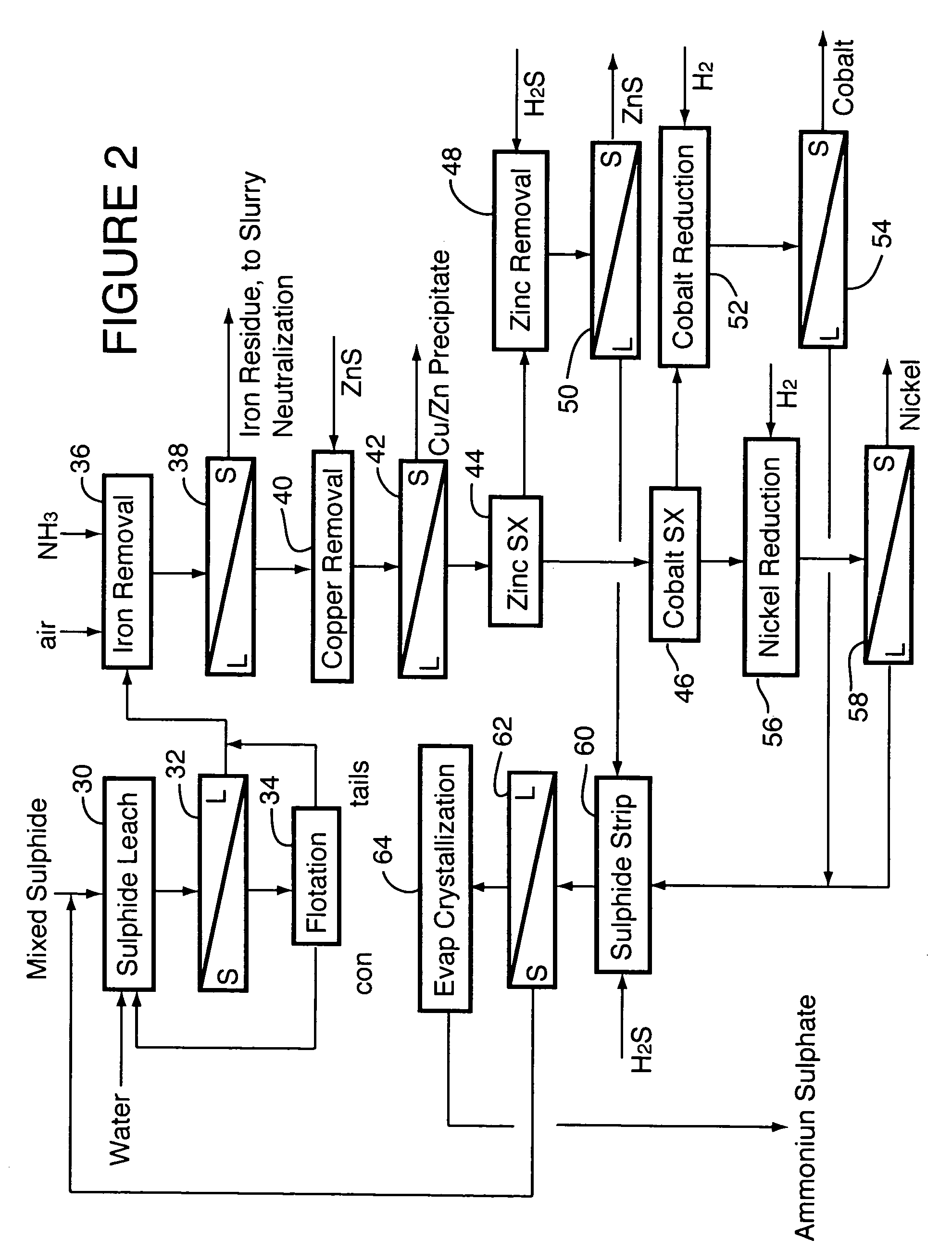
Process for recovery of nickel and cobalt from laterite ore
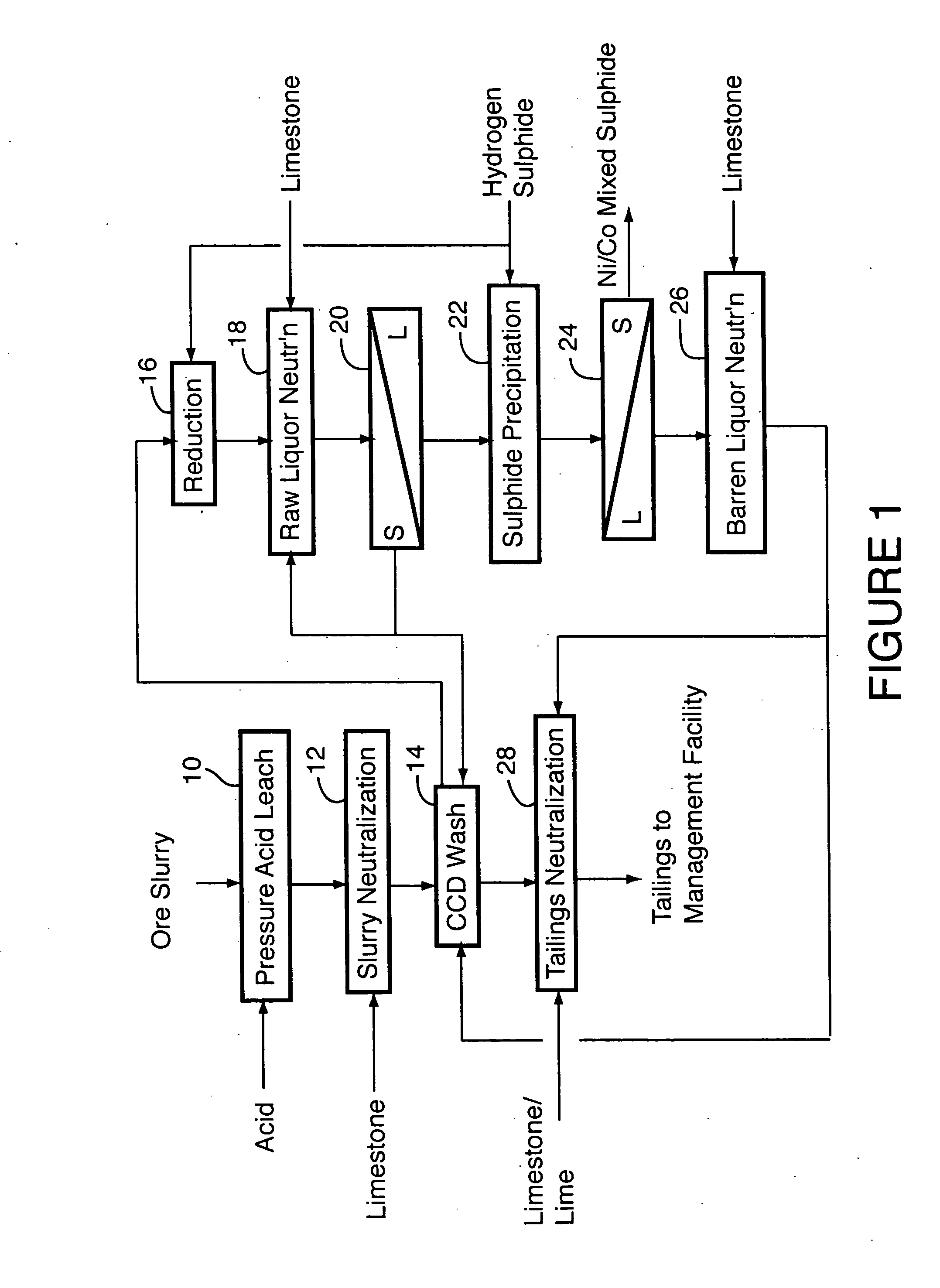
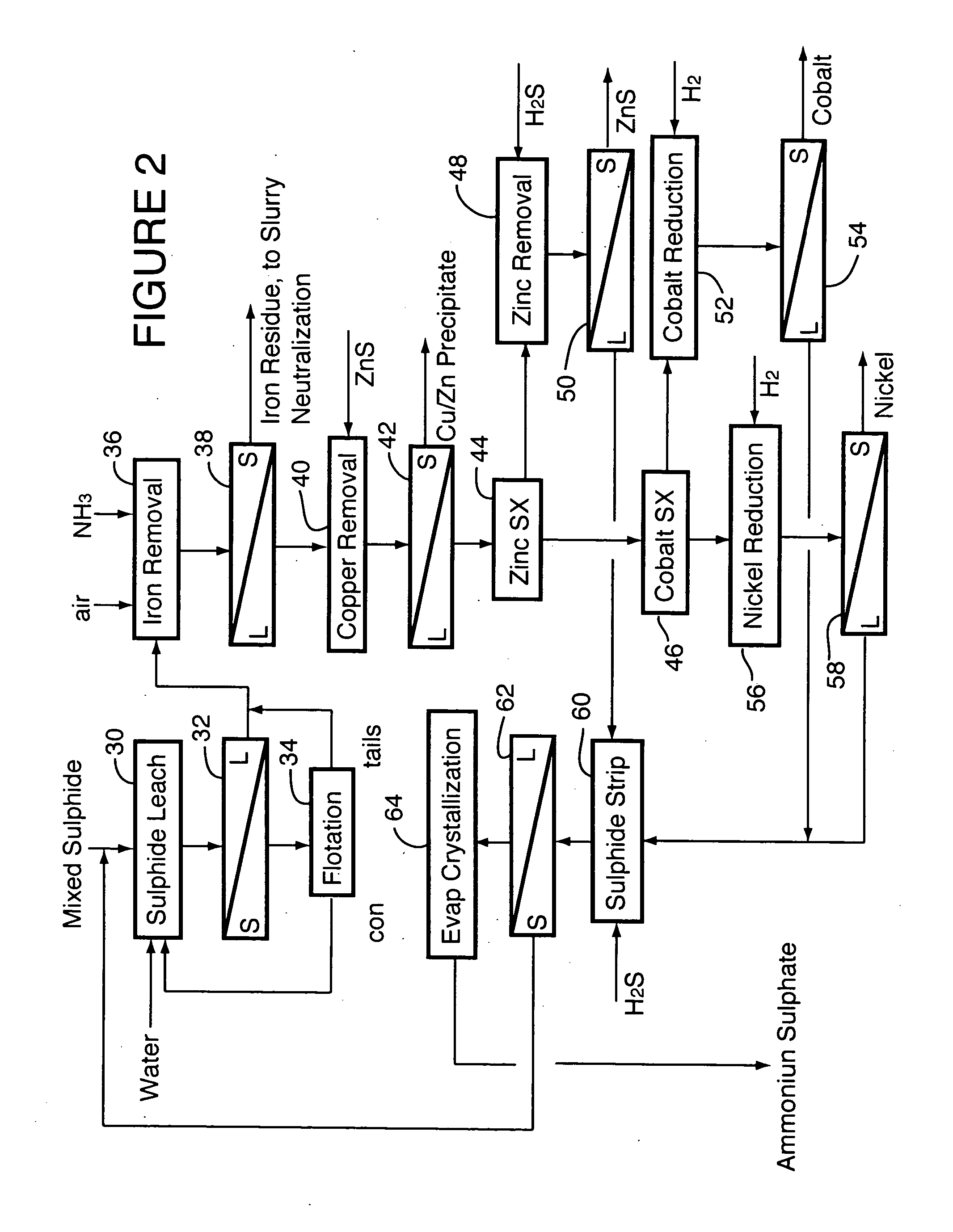
ActiveUS20060228279A1Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INTERNATIONAL
Preparation method and application of cobalt sulfur compound
ActiveCN104993132AWide range of sourcesHigh specific capacityCobalt sulfidesCell electrodesSemiconductor materialsSolar cell
Relating to cobalt sulfur compounds, the invention provides a preparation method and application of a cobalt sulfur compound. The method includes: dissolving a water soluble cobalt source and urea in a mixed solvent to form a solution, carrying out reaction to obtain cobalt carbonate, performing calcinations to obtain a cobalt oxide, and reacting the cobalt oxide with a sulfur source in a reducing atmosphere to obtain a micrometer-scale cobalt sulfur compound. The micrometer-scale cobalt sulfur compound can be spherical cobalt sulfur compound or lamellar square-like cobalt sulfur compound, and the obtained cobalt sulfur compound can be Co9S8, CoS, Co3S4 and CoS2, etc. The cobalt sulfur compound prepared by the preparation method provided by the invention can be applied as an electrode active material in preparation of secondary battery electrodes. According to the invention, specific shape cobalt sulfide can be prepared, is low in synthesis cost and has high tap density, and can be applied to secondary battery electrode materials, optical parametric oscillators, semiconductor materials, solar cells and other aspects.
Owner:XIAMEN UNIV
Recovery of nickel, cobalt, iron, silica, zinc and copper from laterite ore by sulfuric acid leaching
ActiveUS7387767B2Efficient separation and recoveryHigh purityCobalt sulfidesSolvent extractionFree solutionSlurry
A process for recovering nickel and cobalt values from nickel- and cobalt-containing laterite ores as an enriched mixed nickel and cobalt sulphide intermediate and for producing nickel and cobalt metal from the nickel and cobalt sulphide intermediate. The laterite ore is leached as a slurry in a pressure acid leach containing an excess of aqueous sulphuric acid at high pressure and temperature, excess free acid in the leach slurry is partially neutralized to a range of 5 to 10 g / L residual free H2SO4 and washed to yield a nickel- and cobalt-containing product liquor, the product liquor is subjected to a reductant to reduce any Cr(VI) in solution to Cr(III), the reduced product liquor is neutralized to precipitate ferric iron and silicon at a pH of about 3.5 to 4.0, and the neutralized and reduced product liquor is contacted with hydrogen sulphide gas to precipitate nickel and cobalt sulphides. The precipitated nickel and cobalt sulphides can be leached in a water slurry in a pressure oxidation leach, the leach solution subjected to iron hydrolysis and precipitation, the iron-free solution contacted with zinc sulphide to precipitate copper, the iron- and copper-free solution subjected to zinc and cobalt extraction by solvent extraction to produce a nickel raffinate, the nickel raffinate contacted with hydrogen gas to produce nickel powder and the cobalt strip solution from the solvent extraction step contacted with hydrogen gas to produce cobalt powder.
Owner:SHERRITT INC
Preparation method of transition metal oxide/sulfide nano composite material
InactiveCN106966443ARealization reservationUniform sizeCobalt sulfidesNickel oxides/hydroxidesVulcanizationPotassium ferricyanide
The invention discloses a preparation method of a transition metal oxide / sulfide nano composite material. The preparation method includes: adopting a room-temperature standing method to combine cobalt and nickel transition metal ions with potassium ferricyanide organic ligand to prepare a Prussian blue derivative nanocube; adding sodium sulfide into a water solution for vulcanization reaction to obtain a Prussian blue derivative nanocube with a vulcanized shell; calcining to obtain a MOF-based transition metal oxide / sulfide nanocube composite material with a core-shell structure. The transition metal oxide / sulfide nanocube composite material prepared by the method breaks through limitation that most structures of conventional MOF-based composite materials are simple spherical hollow structures, and structure of the composite material is a nanocube with the core-shell structure, so that size is uniform; the composite material has wide applicability in preparation methods of MOF-based transition metal compounds and has great application prospect in the field of supercapacitors and lithium ion batteries.
Owner:FUZHOU UNIV
Cobalt sulfide-based composite material as well as preparation method and application thereof
ActiveCN111370707AUniform particle sizeGood lookingMaterial nanotechnologyCobalt sulfidesElectrical batteryLithium–air battery
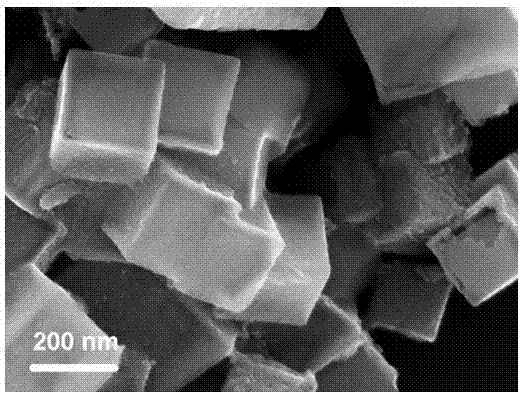
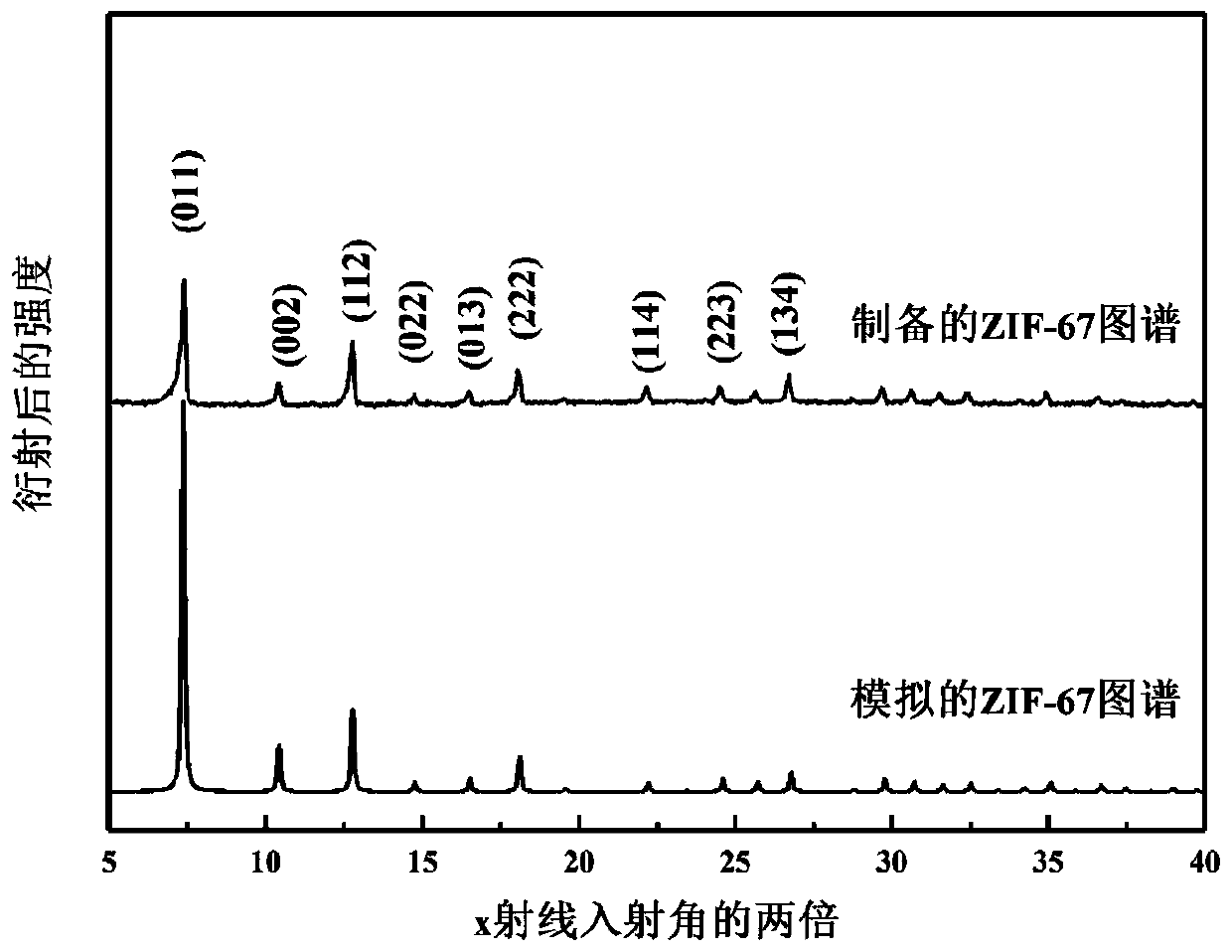
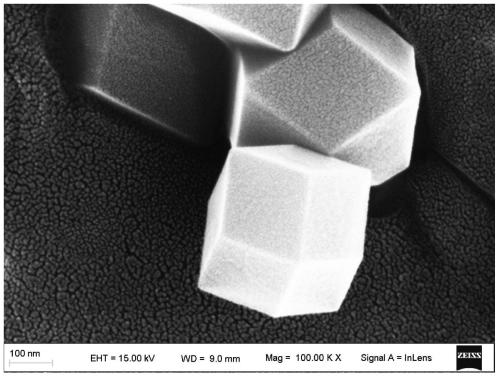
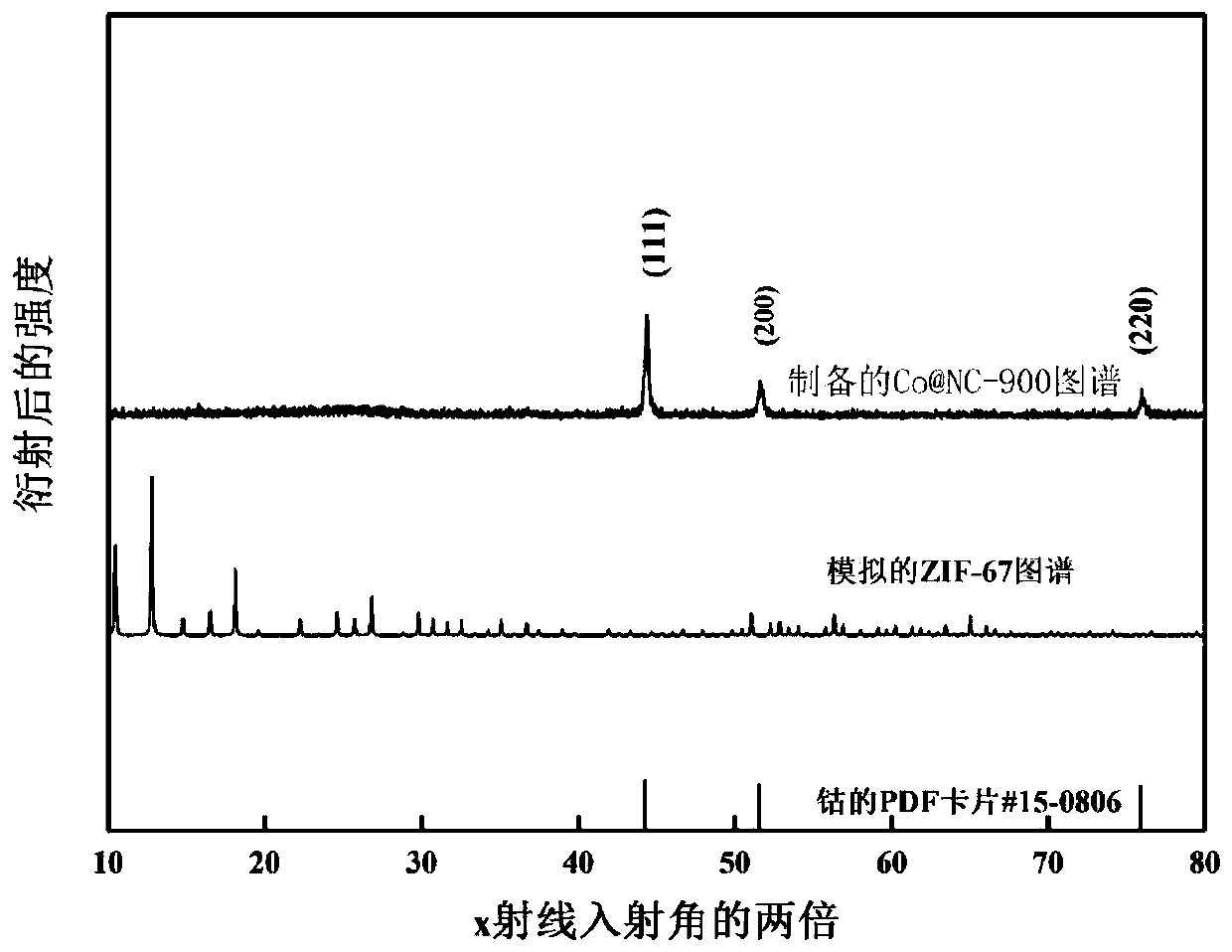
The invention provides a cobalt sulfide-based composite material as well as a preparation method and application thereof. The cobalt sulfide-based composite material is CoS2NC, the preparation methodof the cobalt sulfide-based composite material comprises the following steps: (1) synthesizing a ZIF-67 precursor by using a precipitation method; (2) transferring the ZIF-67 precursor into a tubularfurnace, and calcining the ZIF-67 precursor in an inert gas environment to obtain a nitrogen-doped carbon-coated cobalt-based nano material CoNC; and (3) taking the nitrogen-doped carbon-coated cobalt-based nano material CoNC as a Co source, taking sulfur powder as a sulfur source, fully mixing the Co source and the sulfur source, and carrying out secondary calcination under the protection of inert gas to obtain the cobalt sulfide-based composite material. The cobalt sulfide-based composite material prepared by the method is good in electrochemical performance, high in specific surface area and good in crystallinity; when applied to the lithium-air battery, the material has high specific capacity and cycle performance, and the preparation method is simple and suitable for large-scale production.
Owner:东北大学秦皇岛分校
Nanoparticles and systems and methods for synthesizing nanoparticles through thermal shock
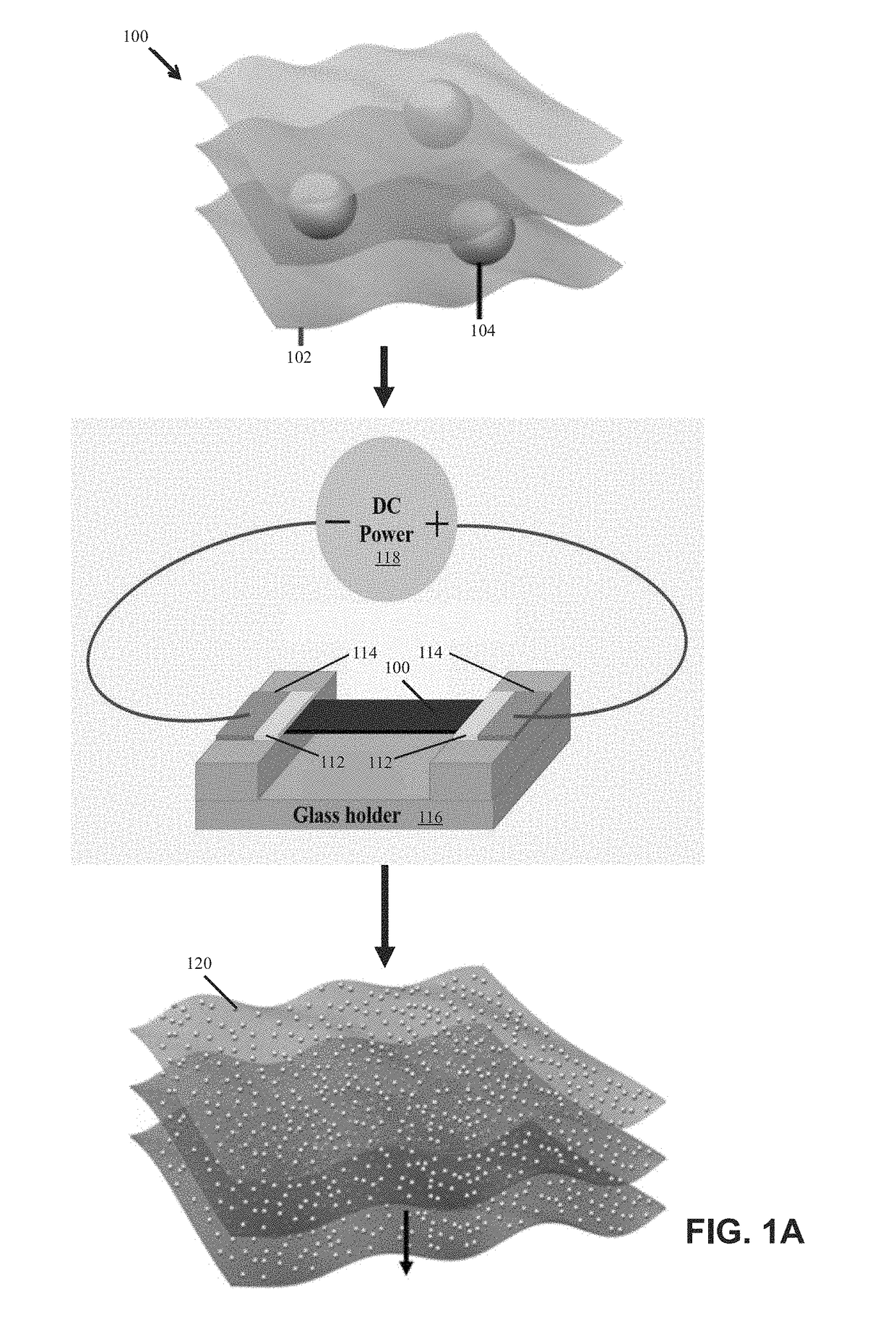
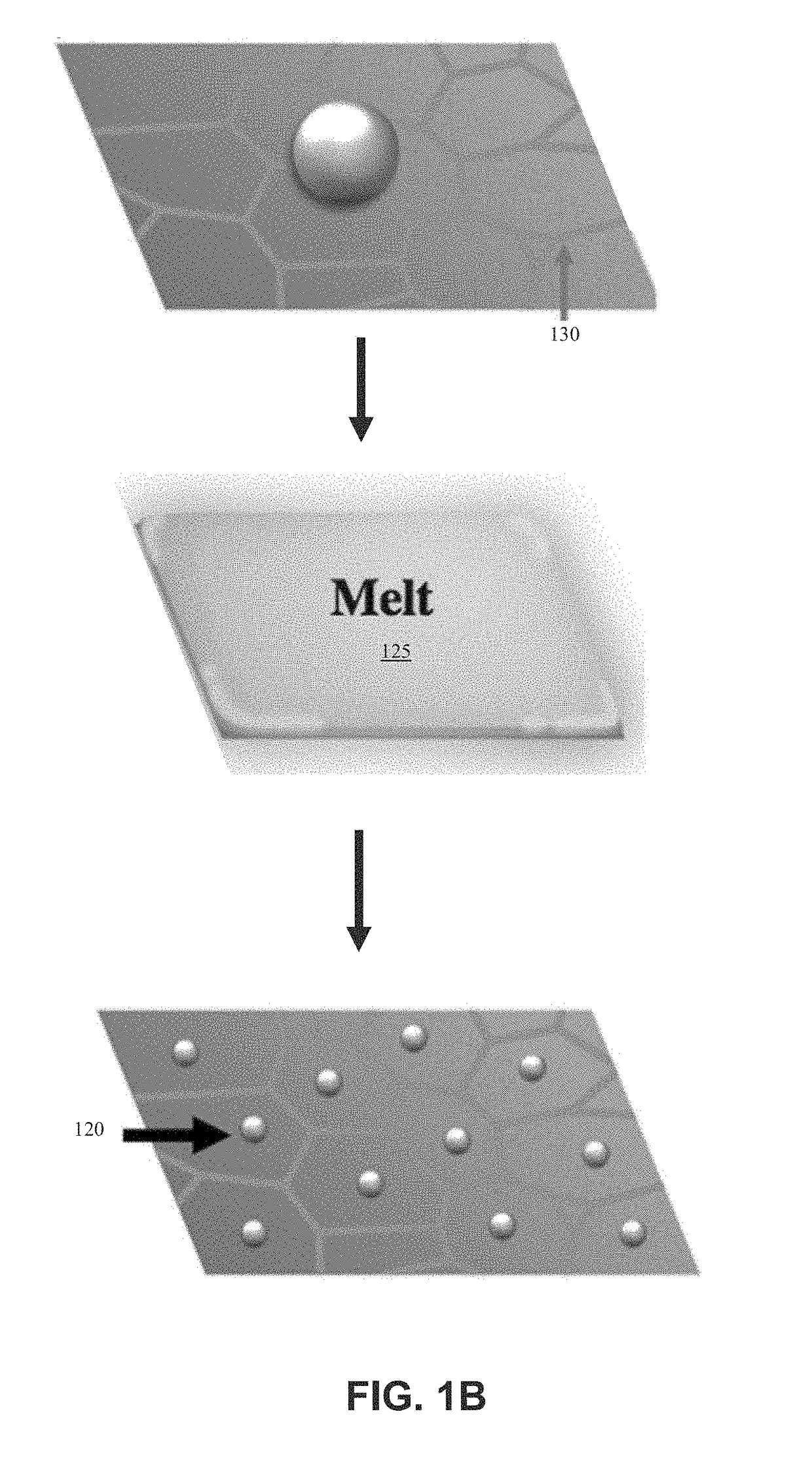
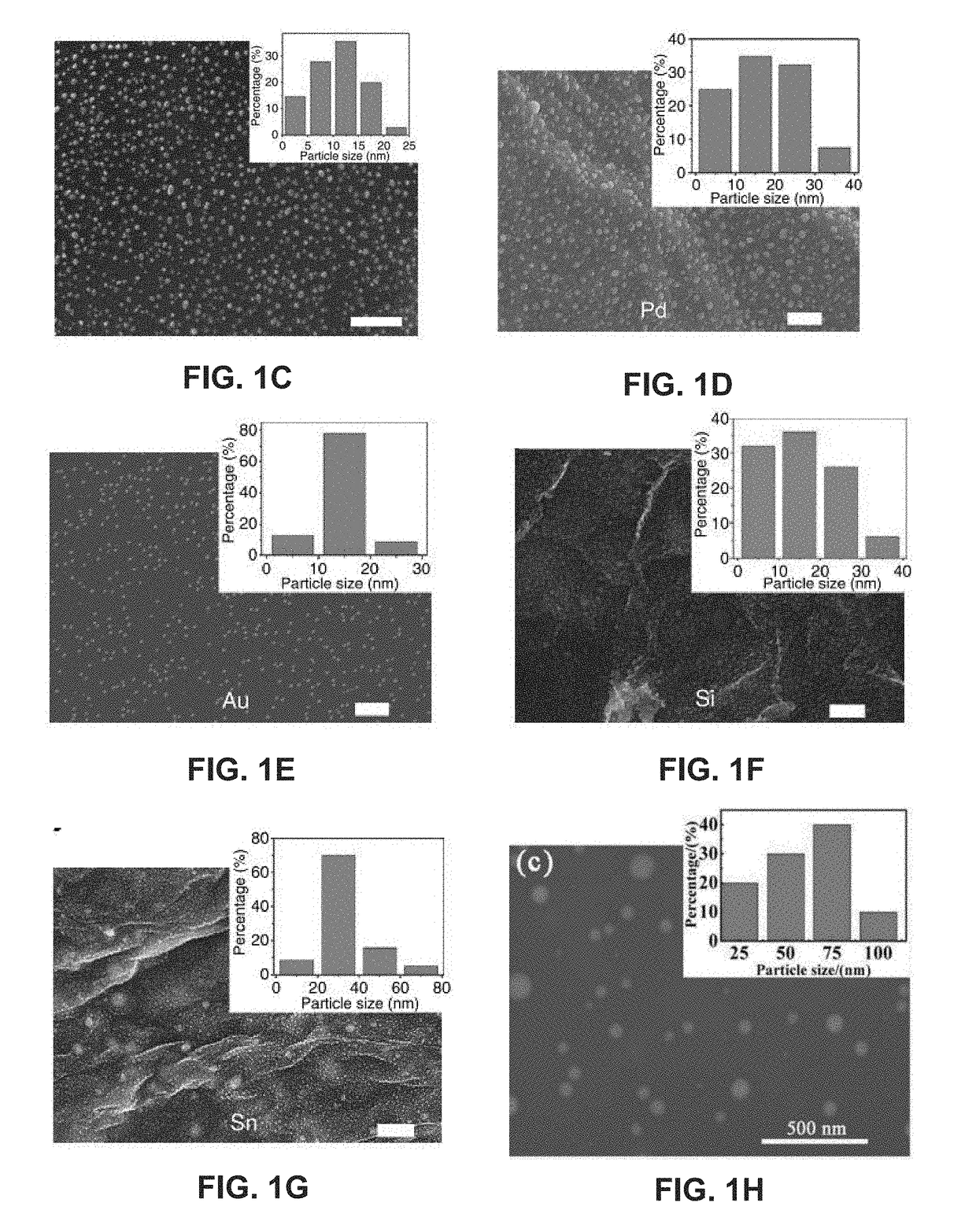
ActiveUS20180369771A1Increase temperatureHeat treatmentsMaterial nanotechnologyThermal energyThermal shock
Systems and methods of synthesizing nanoparticles on substrates using rapid, high temperature thermal shock. A method involves depositing micro-sized particles or salt precursors on a substrate, and applying a rapid, high temperature thermal pulse or shock to the micro-sized particles or the salt precursors and the substrate to cause the micro-sized particles or the salt precursors to become nanoparticles on the substrate. A system may include a rotatable member that receives a roll of a substrate sheet having micro-sized particles or salt precursors; a motor that rotates the rotatable member so as to unroll consecutive portions of the substrate sheet from the roll; and a thermal energy source that applies a short, high temperature thermal shock to consecutive portions of the substrate sheet that are unrolled from the roll by rotating the first rotatable member. Some systems and methods produce nanoparticles on existing substrate. The nanoparticles may be metallic, ceramic, inorganic, semiconductor, or compound nanoparticles. The substrate may be a carbon-based substrate, a conducting substrate, or a non-conducting substrate. The high temperature thermal shock process may be enabled by electrical Joule heating, microwave heating, thermal radiative heating, plasma heating, or laser heating.
Owner:UNIV OF MARYLAND
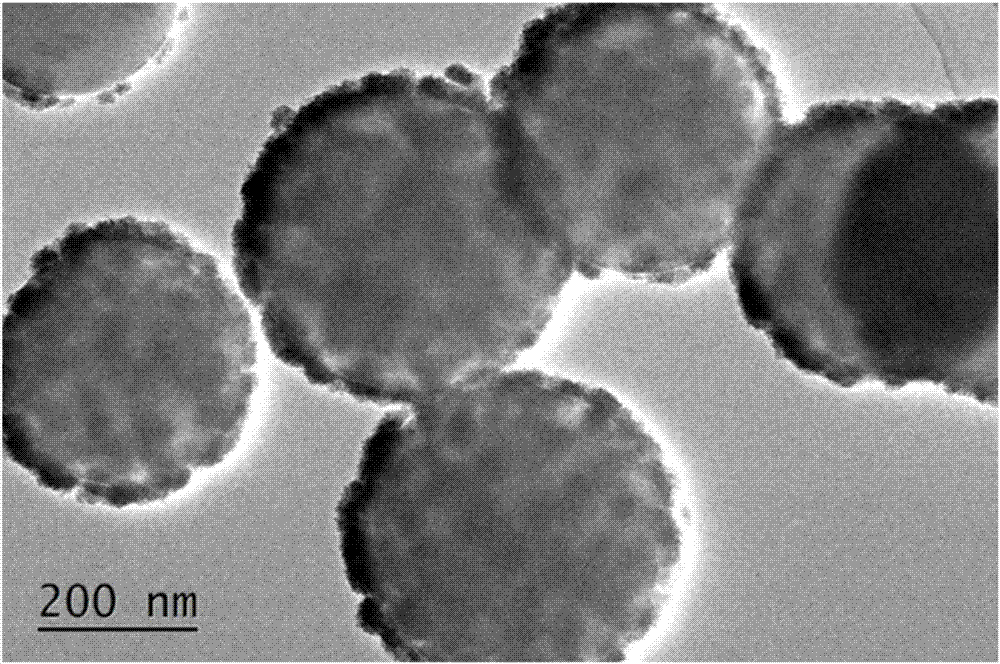
Preparation method of hollow cobalt sulfide microsphere catalyst
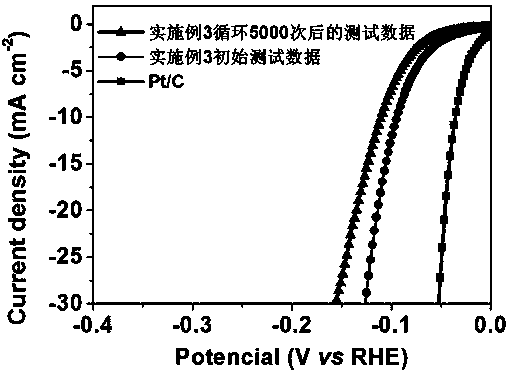
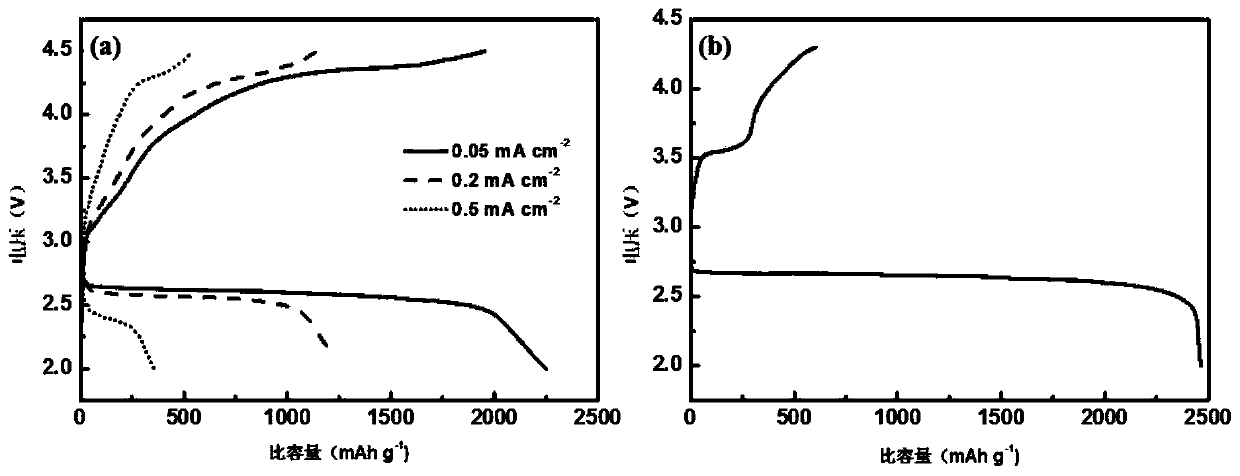
The invention discloses a preparation method of a hollow cobalt sulfide microsphere catalyst. The method comprises the following steps: dissolving a cobalt source and a sulfur source into a solvent and adding a certain amount of ethylene diamine; sealing the solution into a stainless steel high-pressure reactor with the volume of 70mL, which has a polytetrafluoroethylene lining; cooling the solution to room temperature after reaction and taking out precipitate; washing and drying to obtain a cobalt sulfide material. The method disclosed by the invention has the advantages of simplicity, controllability, short reaction time, low cost and easiness in mass production. The prepared cobalt sulfide material has a hollow microsphere structure consisting of nanometer acicular crystals, and has theadvantages of higher specific surface area, higher porosity and good electrochemical hydrogen production performance. Under the condition of strong base, the cobalt sulfide material still has lower catalytic starting point potential of 27.9mV (when the electric current density is 1mA cm<-2>); electric potential is only 89.7mV when the current density is 10mA cm<-2>; the preparation method has a good prospect of wide application in producing hydrogen by using brine electrolysis under the alkaline conditions.
Owner:FUZHOU UNIV
Hollow nanocrystals and method of making
Owner:RGT UNIV OF CALIFORNIA
Nanoreactors and Method of Making
Described herein are nanoreactors having various shapes that can be produced by a simple chemical process. The nanoreactors described herein may have a shell as thin as 0.5 nm and outside diameters that can be controlled by the process of making and have a nanoparticle enclosed therein. The nanoreactors have catalytic activity and may be used to catalyze a variety of chemical reactions.
Owner:RGT UNIV OF CALIFORNIA
Method for preparing sulfide composite nano film
InactiveCN108305792ALarge specific surface areaUniform and stable electrical propertiesMaterial nanotechnologyCobalt sulfidesThioureaSulfide
The invention relates to a composite material for a capacitor, especially to a method for preparing a sulfide composite nano film. A Co9S8 nano-needle array is prepared; a Co9S8-CuS three-dimensionalnano composite structure is prepared; a Co9S8-MoS2 three-dimensional nano composite structure is prepared; and a Co9S8-NiS2 three-dimensional nano composite structure is prepared. Matching of metal salt and thiourea with different concentrations and proportions is carried out; the reaction time and temperature are controlled by using a simple hydrothermal synthesis method; the three-dimensional nano composite structures of Co9S8-CuS and the like are prepared on carbon cloth; and thus a three-dimensional composite structure of a Co9S8 nano-needle array coated with several kinds of metal sulfidenano structures is prepared. The structure having a regular shape, a large specific surface area, and uniform and stable electrical performance can be applied to a super capacitor as a working electrode material and the excellent electrical performance is presented. The method has advantages of high repeatability and simple operation can be produced in large scale.
Owner:XIAMEN UNIV
Nanosheet sulfide hollow sphere and preparation method and application thereof
InactiveCN108502934AImprove electrochemical performanceMaterial nanotechnologyCobalt sulfidesNickel sulfideInorganic sulfide
The invention discloses a nanosheet sulfide hollow sphere and a preparation method and application thereof. The nanosheet sulfide hollow sphere comprises a hollow sphere and a plurality of nanosheets,wherein the multiple nanosheets are distributed at the surface of the hollow sphere, and the average diameter of the hollow sphere is 300 to 400nm; the average length of each nanosheet is 25 to 40nm,and the average thickness of each nanosheet is 0.5 to 1nm; the nanosheet sulfide hollow sphere is prepared from at least one of components of cobalt sulfide, nickel sulfide and cobalt-nickel sulfide.The nanosheet sulfide hollow sphere has the advantages that the electrochemical property is excellent; the nanosheet sulfide hollow sphere can be used as an electrode material for a lithium battery.The preparation method has the advantages that the procedures are simple, and the raw materials are easy to obtain.
Owner:ANHUI NORMAL UNIV
Preparation method and application of hollow porous transition metal chalcogenide nanosheets
InactiveCN106698365AAdjustable microscopic morphologyComposition is easy to controlMaterial nanotechnologyCobalt sulfidesSulfurIon exchange
The invention discloses a preparation method and application of hollow porous transition metal chalcogenide nanosheets. The preparation method comprises the following steps: performing a reaction on an alkaline solution of transition metal salt and water to obtain solid nanosheets of transition metal hydroxide, using the transition metal hydroxide as a precursor, performing a reaction on the transition metal hydroxide and chalcogenide anion X<2->, and performing anion exchange on the X<2-> and OH<-> to obtain the hollow porous nanosheets. The preparation method provided by the invention is an anion exchange and hydrothermal synthesis method, and is simple in process, low in cost and environment-friendly; and through adoption of different transition metal elements, the preparation method can be used for preparing a variety of transition metal chalcogen compound hollow porous nano-tablets, so that the preparation method is high in universality.
Owner:TIANJIN UNIV
Cobalt sulfide photocatalyst, and preparation method and application thereof
ActiveCN106238072AImprove degradation efficiencyFix bugs that don't respondMaterial nanotechnologyPhysical/chemical process catalystsDyeing wastewaterEthylene glycol
The invention discloses a cobalt sulfide photocatalyst. The chemical formula of the cobalt sulfide photocatalyst is Co2.67S4. The preparation method of the photocatalyst comprises the following steps: adding cobalt chloride and sodium sulfide to ethylene glycol, and uniformly mixing cobalt chloride, sodium sulfide and ethylene glycol to obtain a mixed solution; and carrying out a heating reaction on the mixed solution to obtain the cobalt sulfide photocatalyst. The cobalt sulfide photocatalyst has the advantages of full-spectrum response and high photocatalysis efficiency, and can be applied to catalytic degradation of dye wastewater.
Owner:HUNAN UNIV
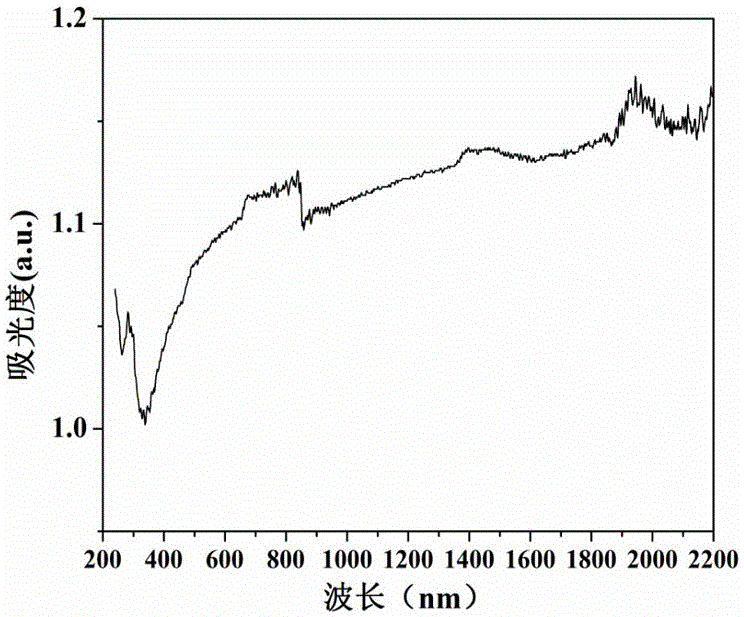
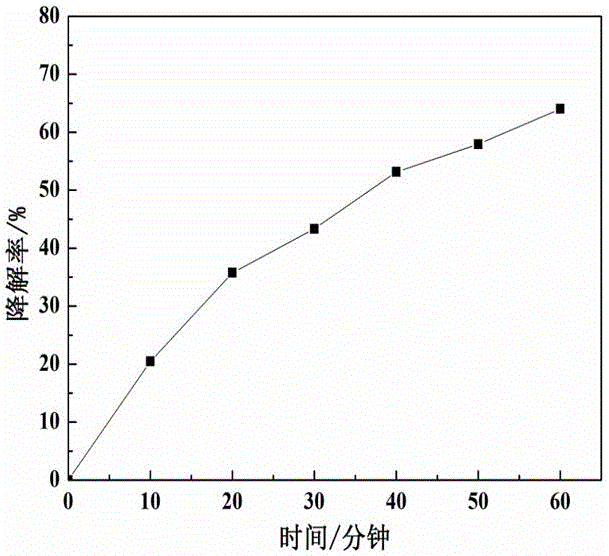
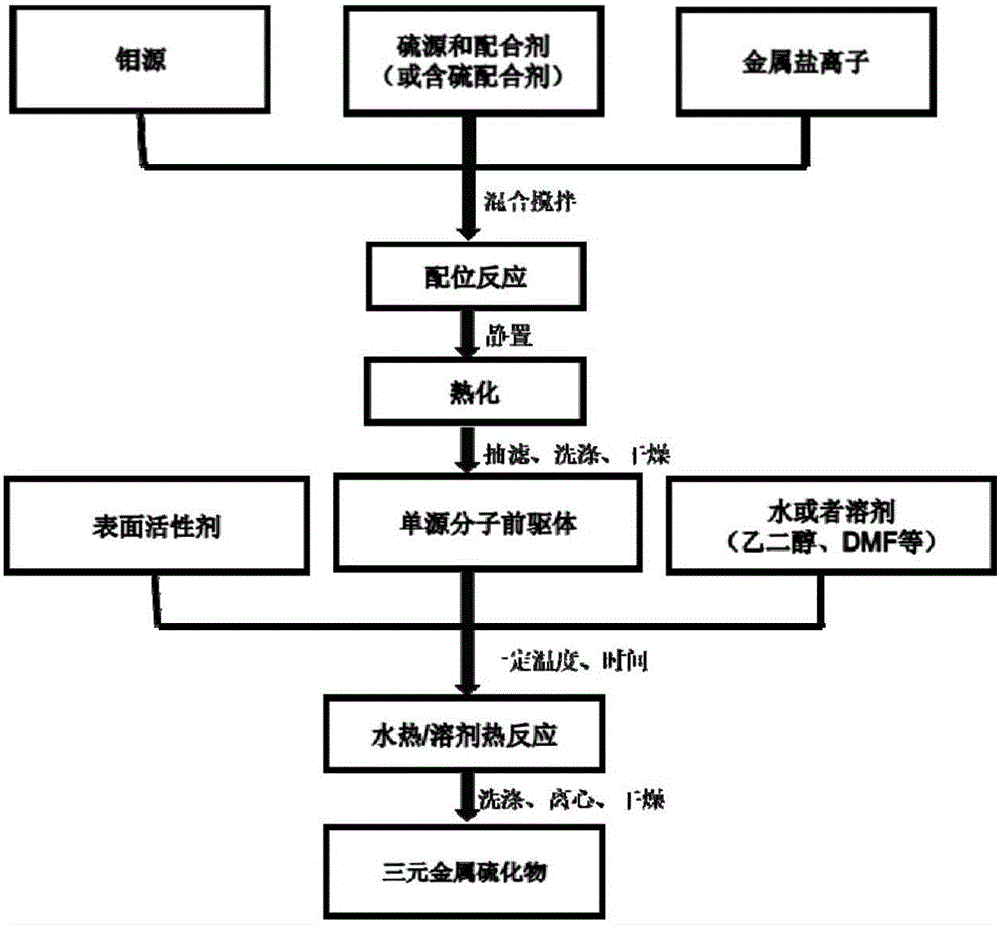
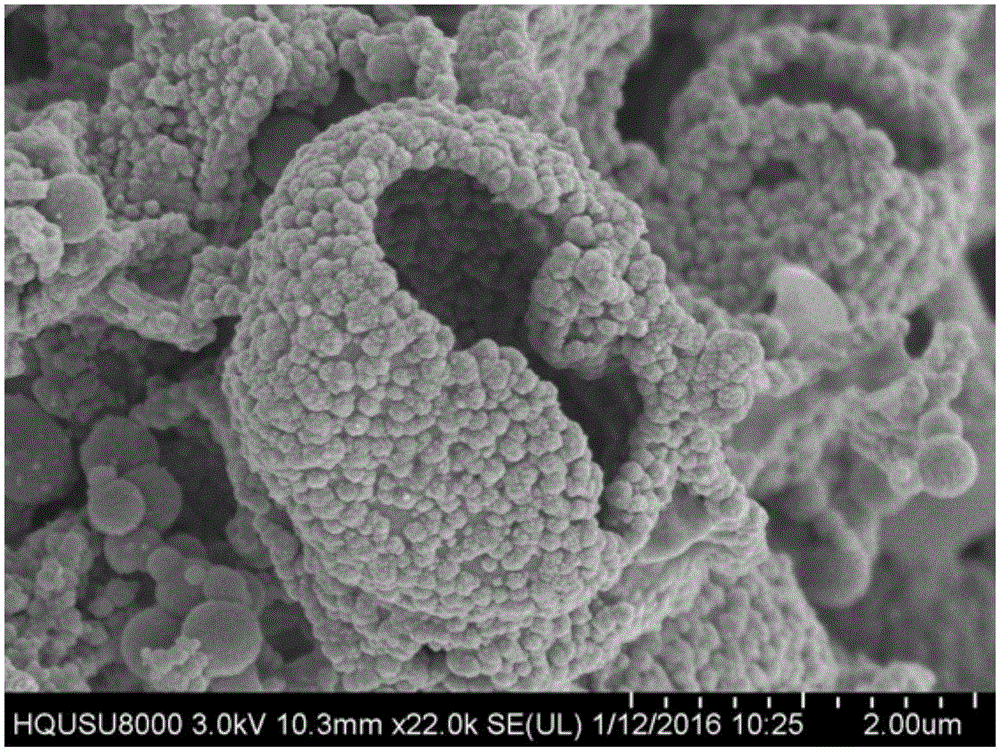
Controllable preparation method of Co-Mo-S (cobalt-molybdenum-sulfur) ternary metal sulfide
InactiveCN105753070ANothing producedSynthetic conditions are mildCobalt sulfidesOperabilityMetallic sulfide
The invention relates to a controllable preparation method of Co-Mo-S (cobalt-molybdenum-sulfur) ternary metal sulfide. The controllable preparation method comprises the following steps of firstly, preparing a single-source molecule precursor, combining with a hydrothermal / solvent thermal method, and utilizing the advantages of the two methods to prepare the Co-Mo-S ternary metal sulfide in a controllable way. The controllable preparation method has the advantages that the synthesizing condition is moderate, the preparation method is simple, the operability is high, the generation of harmful gases in the reaction process is avoided, the environmentally-friendly effect is realized, and the controllable preparation of shape, size and the like of the Co-Mo-S ternary metal sulfide can be realized.
Owner:HUAQIAO UNIVERSITY
Preparation method of cobalt sulfide, product of preparation method and application
ActiveCN109516505AExcellent electrochemical hydrogen evolution catalytic activityVulcanization reaction temperature is lowPhysical/chemical process catalystsCobalt sulfidesVulcanizationReaction temperature
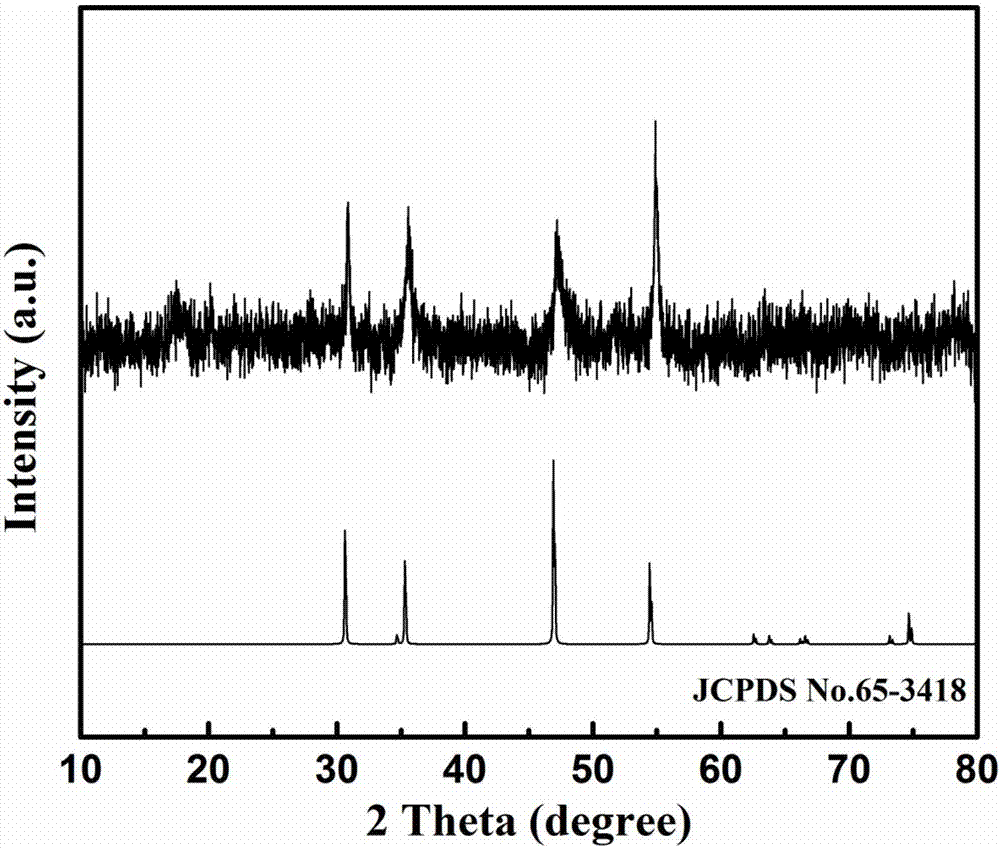
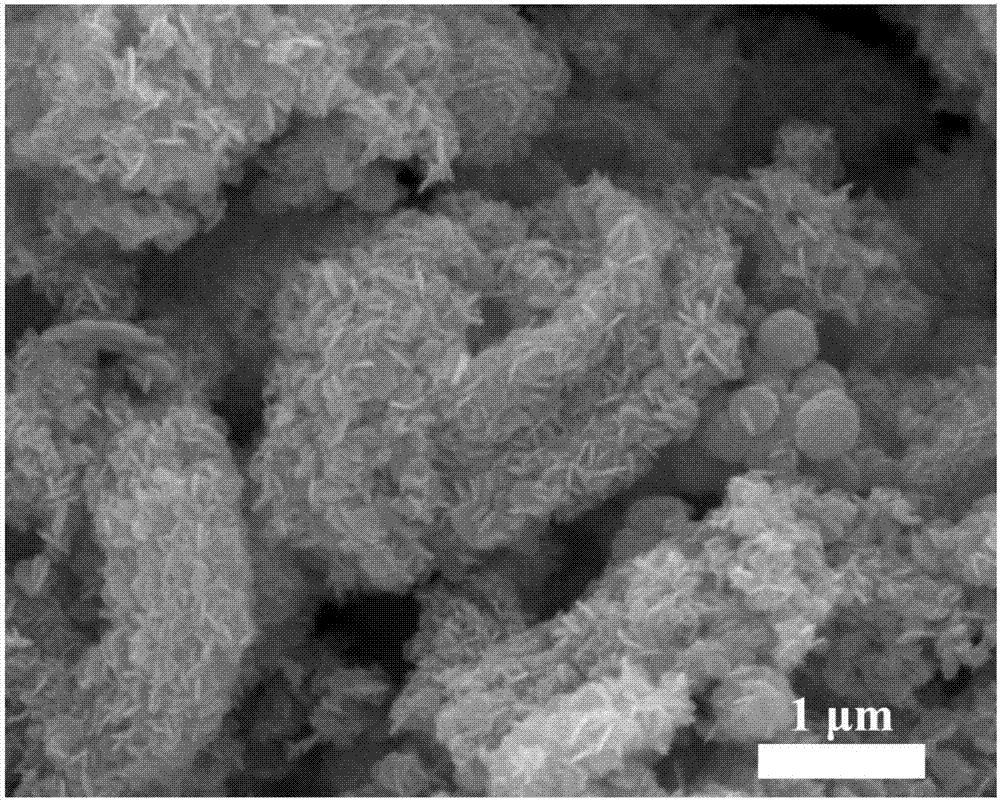
The invention discloses a preparation method of cobalt sulfide, a product of the preparation method and application. The preparation method comprises the following steps: (1) weighing cobalt salt andurea and dissolving into water; stirring to obtain a uniformly-mixed solution; (2) carrying out hydrothermal reaction on the solution to obtain basic cobalt carbonate sediment; (3) centrifuging and drying the basic cobalt carbonate sediment; adding a water solution containing a vulcanizing agent and carrying out low-temperature vulcanization to obtain amorphous cobalt sulfide nanoparticles. According to the preparation method provided by the invention, a cobalt sulfide nano-material with a regular shape and an amorphous structure is rapidly prepared through the low-temperature vulcanization; the vulcanization reaction temperature is low, the reaction time is short, the energy consumption is remarkably reduced and the large-scale production is facilitated; the prepared cobalt sulfide nanoparticles have the amorphous structure; and compared with cobalt sulfide with a crystal structure, the cobalt sulfide provided by the invention has more excellent electrochemical hydrogen evolution catalytic activity.
Owner:JIANGHAN UNIVERSITY
Rechargeable magnesium battery positive electrode material with nano porous metal sulfide and application method thereof
ActiveCN103872322AHigh crystallinitySmall particlesMaterial nanotechnologyCobalt sulfidesElectrical batteryMagnesium ion
The invention discloses a rechargeable magnesium battery positive electrode material with nano porous metal sulfide and an application method thereof. The positive electrode material is represented by a chemical formula MyCoS(1+x), wherein M is one transition metal element; the x is more than 0 and less than or equal to 1 and the y is more than or equal to 0 and less than or equal to 1. The positive electrode material has a good crystallization property and a regular shape; the reversible embedding / releasing of magnesium ions can be realized. A positive plate is prepared from the positive electrode material and further is prepared into a battery. A rechargeable magnesium battery has an obvious charging / discharging voltage platform and is high in stable specific discharge capacity; after the rechargeable magnesium battery is recycled for 60 times, the specific discharge capacity is also more than 80% of an initial capacity. The rechargeable magnesium battery positive electrode material provided by the invention has excellent electrochemical performance, and is simple in preparation process and low in price, the raw materials are readily available, and the application prospect is very wide.
Owner:SHANGHAI JIAO TONG UNIV
Process for producing metal sulfide
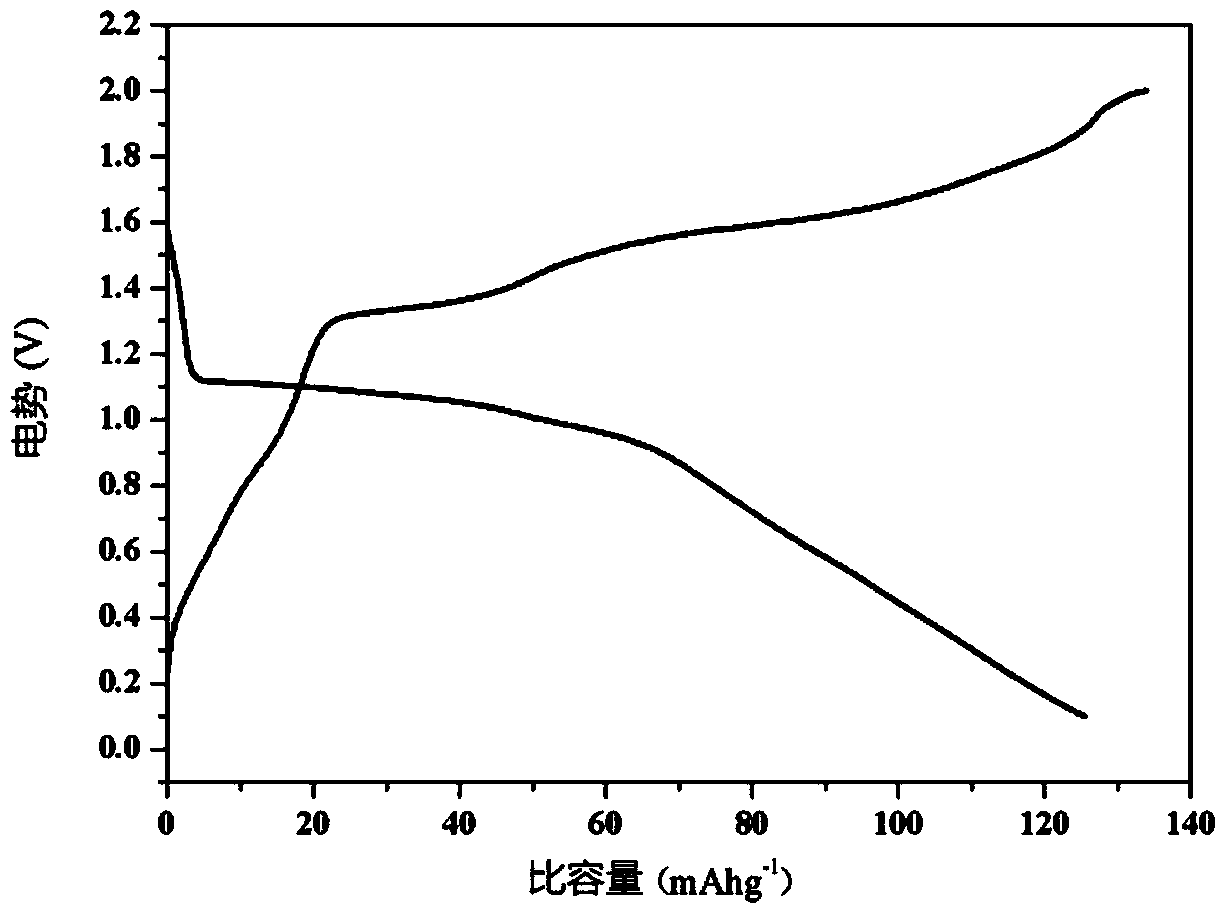
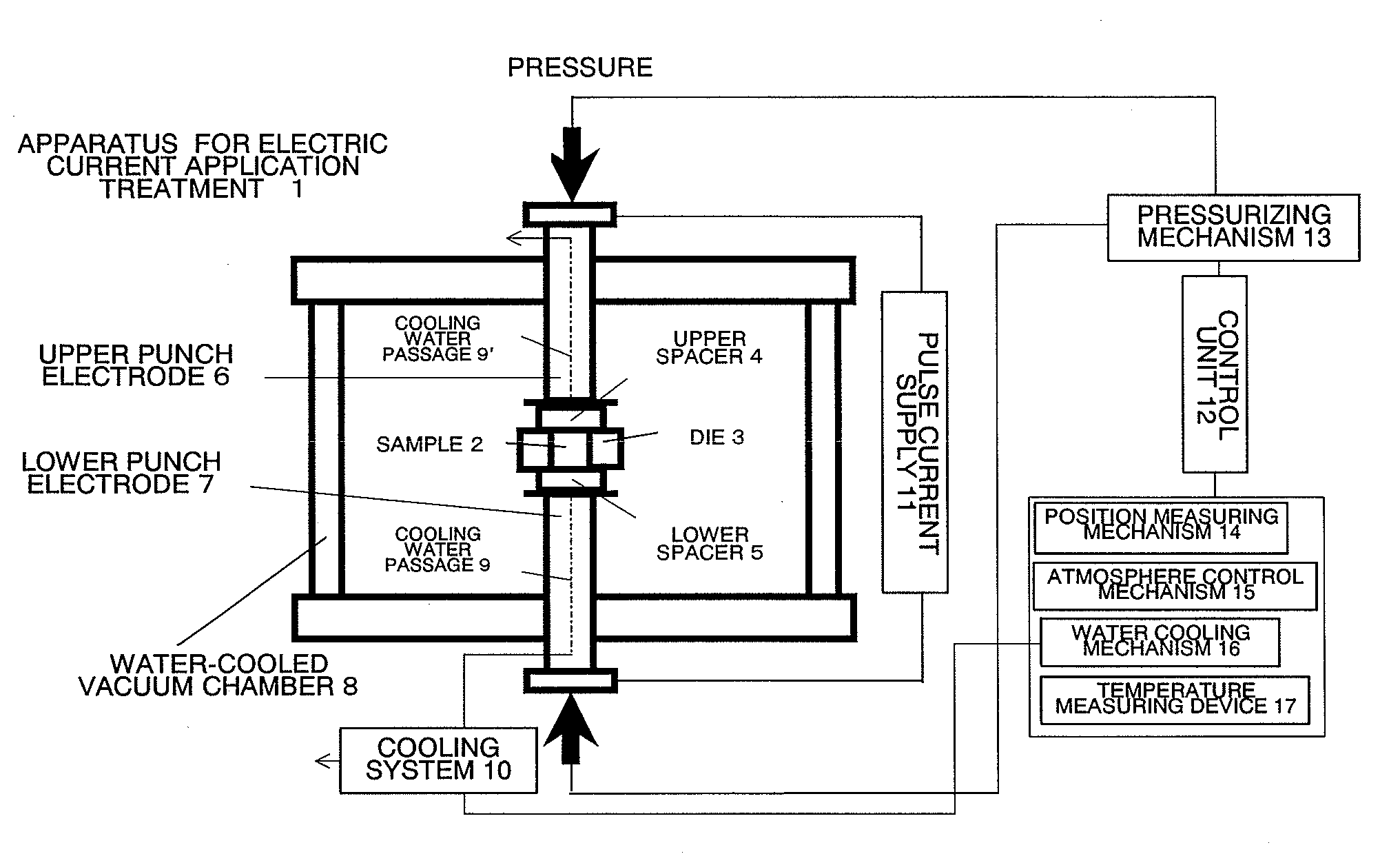
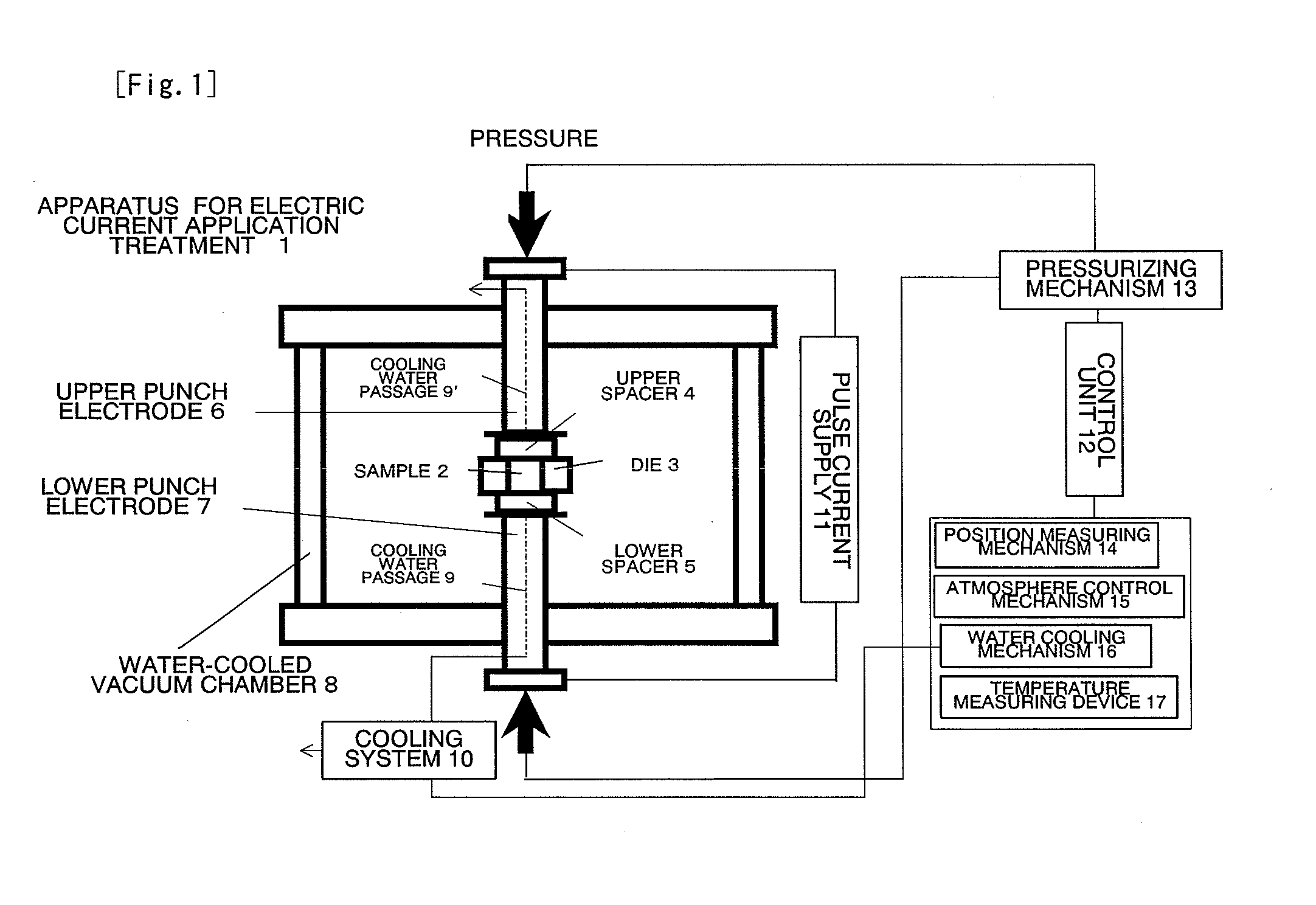
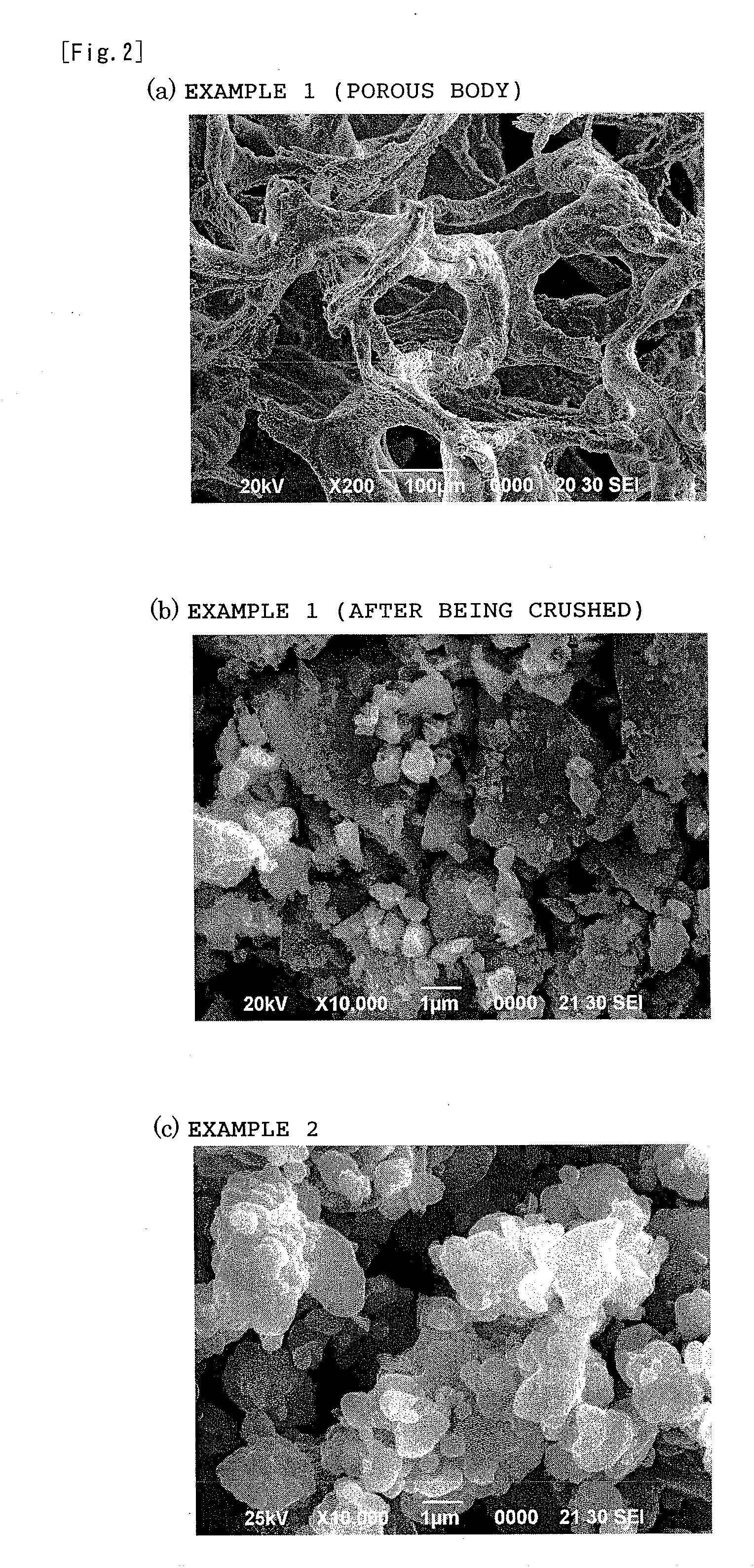
InactiveUS20110223481A1Efficient productionShort timeCobalt sulfidesElectrolysis componentsLithiumPulsed DC
The present invention provides a production process of a metal sulfide, which includes placing a metal component and sulfur in a conductive container, and applying a pulsed direct current to the container in a non-oxidizing atmosphere to cause the metal component to react with sulfur, and also provides a metal sulfide obtained by the process and represented by a composition formula: MSx, wherein M is at least one member selected from the group consisting of Ni, Cu, Fe, and Co, and 1<x≦2. The present invention is capable of easily producing a metal sulfide with a high proportion of sulfur atoms, which is expected to exhibit excellent properties as a positive-electrode active material for a high capacity lithium secondary battery.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Preparation method of cobalt sulfide nanomaterial and method for detecting hydrogen peroxide with cobalt sulfide nanomaterial
InactiveCN105692719AUniform particle size distributionImprove accuracyCobalt sulfidesMaterial analysis by observing effect on chemical indicatorColor reactionOleic Acid Triglyceride
The invention relates to the field of food detection, in particular to a preparation method of a cobalt sulfide nanomaterial and a method for detecting hydrogen peroxide with the cobalt sulfide nanomaterial. O-dichlorobenzene, oleic acid and trioctyl phosphine oxide react with an o-dichlorobenzene solution containing carbonyl cobalt, then reaction products react with an o-dichlorobenzene solution containing sulfur, the reaction products are sequentially washed and centrifuged with methanol and distilled water repeatedly, and cobalt sulfide nanomaterial powder is obtained. According to the method for detecting hydrogen peroxide with the cobalt sulfide nanomaterial, to be specific, a phosphate buffer solution of cobalt sulfide is added to a Tirs-Hcl buffer solution, then a to-be-detected solution and a TMB color developing agent are added, and whether the to-be-detected solution contains hydrogen peroxide or not is judged through observation of color reaction. The method is high in detection speed and accuracy and has better specificity to detection of hydrogen peroxide.
Owner:TAIYUAN UNIV OF TECH
Preparation method and application of Co3S4 @ MoS2 core-shell structure nano-sheet array material
InactiveCN110697782AImprove permeabilityImprove collection efficiencyMaterial nanotechnologyCobalt sulfidesCapacitanceNickel substrate
The invention provides a preparation method and application of a Co3S4 @ MoS2 core-shell structure nano-sheet array material. Compared with the prior art, a core-shell array structure of Co3S4 nanosheets @ MoS2 nanosheets is synthesized on a foam nickel substrate by a simple one-step hydrothermal synthesis method. The product obtained by the preparation method has the advantages of crosslinked three-dimensional network structure, uniform size, adjustable appearance, low production cost and high repeatability. The prepared Co3S4 @ MoS2 core-shell structure nano-sheet array material is used as an electrode material of a supercapacitor, has high specific capacitance and excellent cycle stability, and has potential application value in the field of energy storage.
Owner:NINGBO UNIV
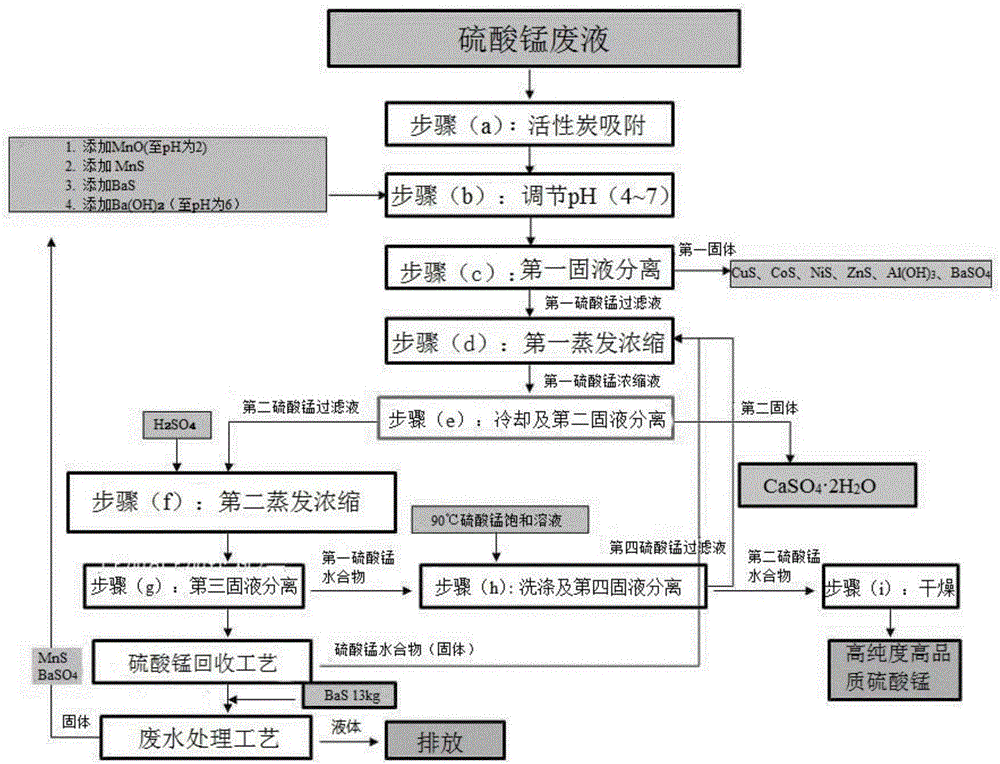
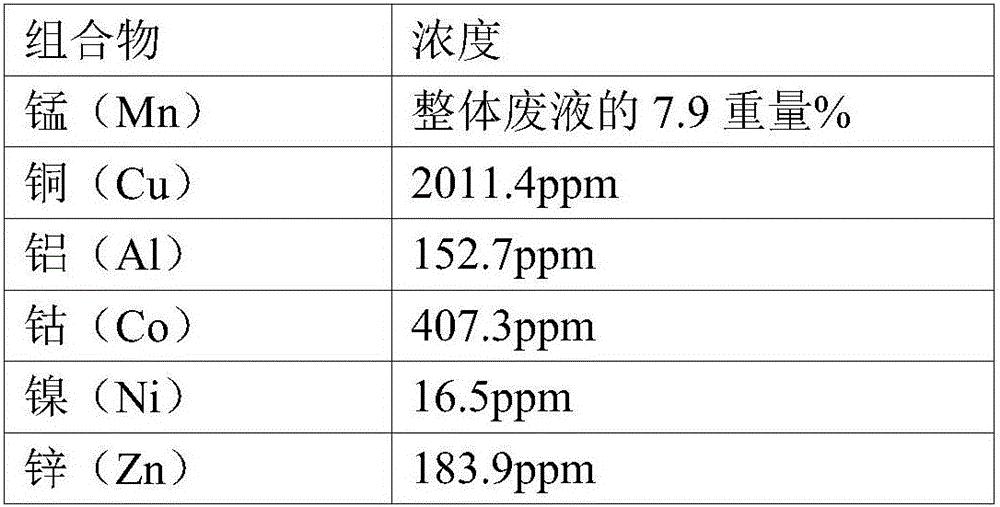
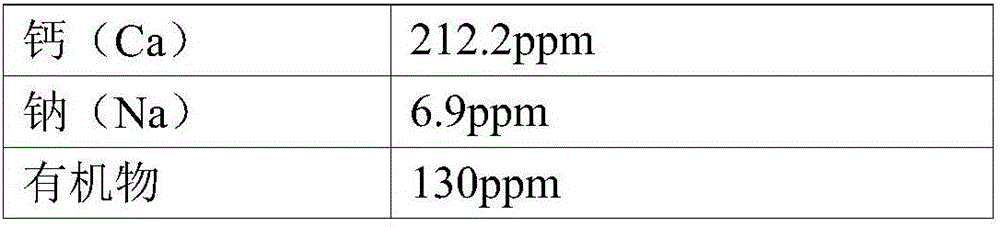
Manufacturing method of high purity manganese sulphate from manganese sulphate waste liquid
InactiveCN106542583AGuaranteed SolubilityAvoid secondary pollutionCobalt sulfidesWaste accumulators reclaimingManganese sulphateElectrical battery
Provided is a method of manufacturing high-purity, high-quality manganese sulfate which can be immediately used for manufacturing a lithium ion secondary battery from manganese sulfate waste liquid of a wasted battery. Since impurities are removed from the manganese sulfate waste liquid by using sulfides causing no secondary contamination in the manganese sulfate waste liquid and the manganese sulfate is manufactured by performing evaporation concentration through heating, the manufacturing method is very environment-friendly and economical. Since the manganese recovering process improving the manufacturing yield of the manganese sulfate and the waste water treatment process capable of recycling the source materials and discharging waste water are integrated, the manufacturing method is very efficient and environment-friendly. The manufacturing method is applied to the recycling industry, and thus, it is possible to obtain effects of preventing environmental pollution and facilitating recycling the resources.
Owner:KNU IND COOPERATION FOUND
Metal-phase molybdenum disulfide, self-supporting electrode, preparation methods and application
ActiveCN111847514AIncrease contentSimple and safe operationMaterial nanotechnologyElectrode manufacturing processesLithiumHydrothermal synthesis
The invention relates to the field of materials, and particularly discloses metal-phase molybdenum disulfide, a self-supporting electrode, preparation methods and application. The metal-phase molybdenum disulfide comprises the following raw materials: molybdenum trioxide (serving as a molybdenum source), a sulfur source, a proper amount of a reducing agent and a proper amount of water, wherein a ratio of the number of molybdenum atoms in the molybdenum trioxide to the number of sulfur atoms in the sulfur source is 1: (5-40). According to the metal-phase molybdenum disulfide provided by an embodiment of the invention, molybdenum trioxide is adopted as the molybdenum source, hydrothermal synthesis from a proper amount of the sulfur source, the reducing agent and the water is carried out to prepare the metal-phase molybdenum disulfide, and the content of the metal-phase molybdenum disulfide in the product is high; and the preparation method of the metal-phase molybdenum disulfide providedby the invention has the advantages of simplicity and safety in operation, short preparation period and suitability for large-scale production, solves the problems of long preparation period and lowsafety due to the fact that most of conventional metal-phase molybdenum disulfide is synthesized through lithium ion delamination, and has wide market prospects.
Owner:JILIN UNIV
Lithium air battery cathode material and preparation method thereof, and lithium air battery
InactiveCN110010915AHas electrochemical catalytic activityGood dispersionFuel and secondary cellsCobalt sulfidesVulcanizationDodecahedron
The invention discloses a lithium air battery cathode material and a preparation method thereof, and a lithium air battery. The cathode material is a cobalt sulfide which is a hollow core-shell dodecahedron structure; ZIF-67 and a sulphur source are dissolved in a solvent to form a mixed solution; the mixed solution is subjected to heating, heat preservation, cooling and drying by employing a hydrothermal method to take out a precipitate, namely the lithium air battery cathode material. The hydrothermal method is employed to perform vulcanization of the ZIF-67 to obtain the cathode material, the preparation method is simple, the cathode material has the electrochemical catalytic activity, the lithium air battery prepared by employing the cathode material is excellent, has the coulombic efficiency up to 86.6%, can show better reversibility, and can effectively improve the cycle life of the battery: when the specific discharge capacity is controlled to 500 mAh g<-1>, the lithium air battery can stably cycle for 32 loops until the voltage is stabilized above 2.0V.
Owner:NANJING UNIV OF POSTS & TELECOMM
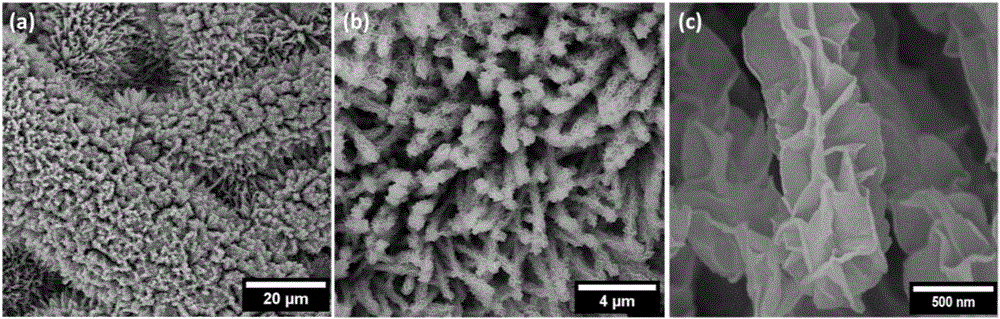
Preparation method and application of cobalt sulfide having nano-lamella assembled three-dimensional annular micro-nano structure
ActiveCN107244699AImprove electrochemical performanceImprove sodium storage performanceMaterial nanotechnologyCobalt sulfidesMicro nanoPolymer science
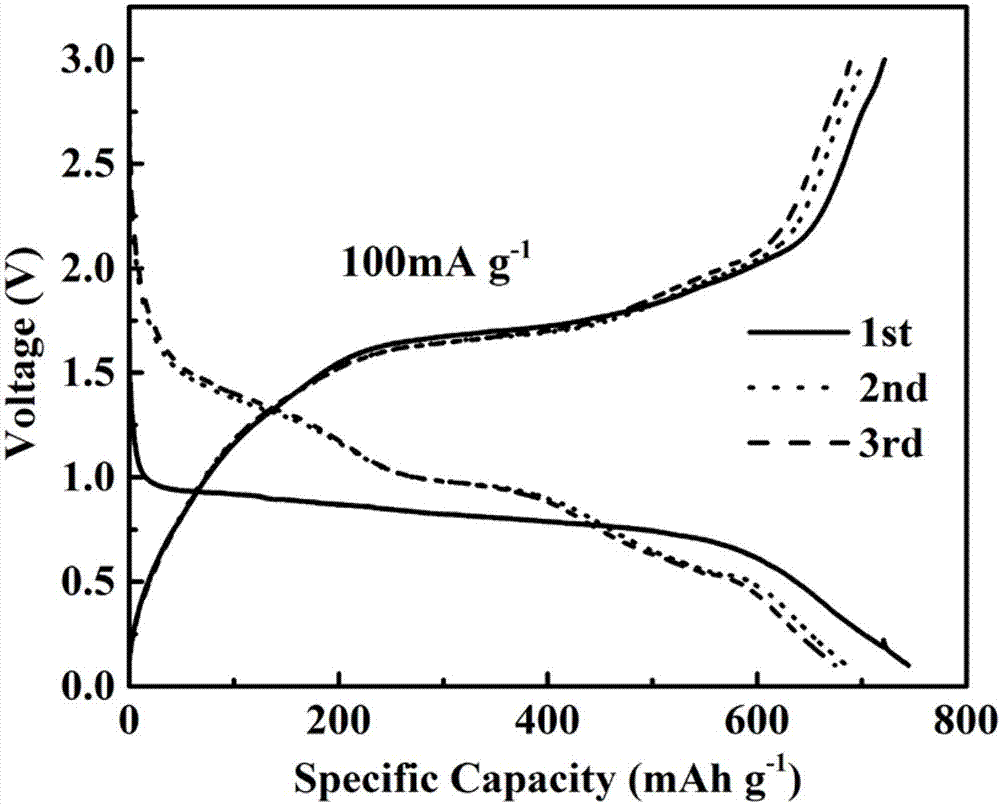
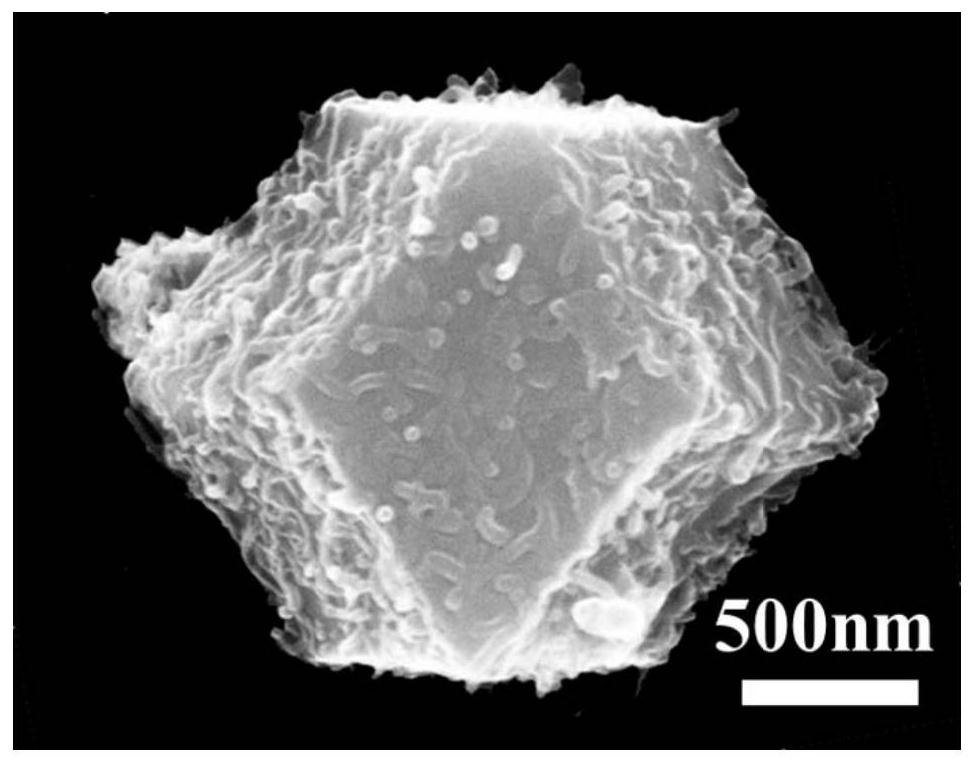
The invention belongs to the technical field of sulfide nano-materials, and discloses a preparation method and an application of cobalt sulfide having a nano-lamella assembled three-dimensional annular micro-nano structure. The preparation method comprises the following steps: dissolving CoCl2.6H2O in ethylene diamine, adding thioacetamide, transferring the above obtained solution into a reaction kettle, and washing and drying the obtained product to obtain the cobalt sulfide having a nano-lamella assembled three-dimensional annular micro-nano structure. The method has the advantages of simplicity in operation, easiness in preparation, high controllability, cheap synthesis raw materials and high yield. The cobalt sulfide having a nano-lamella assembled three-dimensional annular micro-nano structure has good electrochemical performances. The initial discharge specific capacity of the cobalt sulfide is 745 mAh / g when the current density is 100 mA / g, and the capacity of the cobalt sulfide still reaches up to 392 mAh / g after 200 cycles when the current density is 1 A / g, so the cobalt sulfide has a good cycle stability, is a sodium ion battery negative electrode material having excellent performances, and has a wide application prospect in the energy storage field.
Owner:WUHAN TEXTILE UNIV
Preparation method of micron cobalt disulfide composite material
ActiveCN111924887AReduce pyrolysisPromote growthCobalt sulfidesCell electrodesCarbon nanotubeCapacitance
The invention provides a preparation method of a micron cobalt disulfide composite material and application of the micron cobalt disulfide composite material as an electrode, belonging to the technical field of energy storage and conversion materials. The preparation method comprises the following steps: firstly, synthesizing a carbon nanotube reinforced metal organic framework ZIF-67 in situ, then carrying out a low-temperature controllable confinement reaction, and successively conducting carbonizing and vulcanizing to prepare the micron cobalt disulfide composite material with surface functional group modification and a porous structure. The cobalt disulfide synthesized by the method is uniformly packaged in the porous carbon skeleton, has a large specific surface area and abundant pores with proper pore diameters, and inherits surface functional group structures of a metal organic framework and a carbon nanotube. The micron cobalt disulfide composite material synthesized by the method is used as the electrode; the phenomena of side reactions, volume expansion, intermediate product dissolution and the like of the material in the charging and discharging process are effectively inhibited, the synergistic effect of embedding-conversion-pseudocapacitance hybrid energy storage is promoted, and high specific capacity, high volume energy density and excellent cycling stability areshown.
Owner:UNIV OF SCI & TECH BEIJING
Production process of sulfide containing nickel and cobalt
InactiveUS20100135878A1High yieldImprove utilization efficiencyCobalt sulfidesLiquid solutions solvent extractionGas phaseSodium hydrosulfide
Owner:SUMITOMO METAL MINING CO LTD
Method of preparing dye-sensitized solar cell XS (X=Co, Ni) counter electrode
The invention discloses a method of preparing a dye-sensitized solar cell XS (X=Co, Ni) counter electrode. The method comprises the following steps: X(CH3COO)2.4H2O (X=Co, Ni) and thiourea are dissolved in a mixed solution of ammonia and ethanol; FTO conductive glass is cleaned and is placed, at an angle of 50 DEG, in a stainless steel reactor with a polytetrafluoroethylene ling, and the conductive surface faces downwardly; and the mixed solution is transferred to the reactor for solvent thermal reaction. After the reaction, the conductive glass is taken out, ethanol is used for cleaning and drying, and thus, the XS (X=Co, Ni) counter electrode is obtained. According to the XS (X=Co, Ni) counter electrode preparation method disclosed by the invention, the method is simple, the preparation period is short, and quick and large-area preparation can be carried out; and besides, the prepared counter electrode has small interface charge transfer resistance and high in catalytic activity, the thin film has light transmittance, and the photoelectric conversion efficiency of the dye-sensitized solar cell can be effectively enhanced.
Owner:CHINA THREE GORGES UNIV
Preparation method of Co9S8/MoS2 multilevel structure composite material
InactiveCN110790318AEasy to manufactureLow equipment requirementsCobalt sulfidesMolybdenum sulfidesOrganic solventAqueous solution
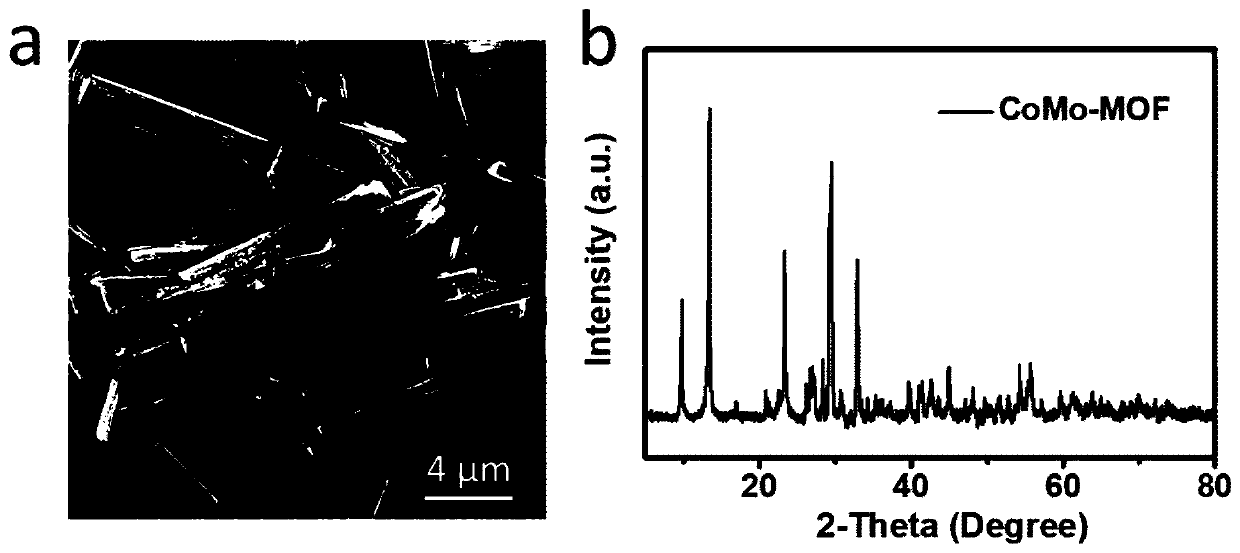
The invention relates to the technical field of nano-materials, in particular to a preparation method of a Co9S8 / MoS2 multilevel structure composite material. The preparation method includes: 1) dissolving an organic ligand in an aqueous solution containing molybdenum and cobalt to prepare a precursor solution, then carrying out oil bath heating reaction on the precursor solution, and then performing filtering to obtain a CoMo-MOF material; 2) dispersing the obtained CoMo-MOF material in an organic solvent to prepare a dispersion liquid, then carrying out hydrothermal reaction on the dispersion liquid, then performing filtering, and conducting cleaning and drying to obtain CoMo-S powder; and 3) placing the CoMo-S powder in a reducing atmosphere for high-temperature calcination to obtain the Co9S8 / MoS2 multilevel structure composite material. The preparation method provided by the invention is concise and efficient, has low equipment requirements, and is easy to realize industrial production; the preparation stability is high, the size and the morphology of the product can be effectively controlled in each step, and the structural stability and the size uniformity of the product aremaintained.
Owner:ZHEJIANG UNIV OF TECH
Graphene-based hollow cobaltous sulfide nanocrystal capable of efficiently activating persulfate and preparation method thereof
ActiveCN108706573APromote degradationFast transferMaterial nanotechnologyPhysical/chemical process catalystsSulfideSolvent
The invention belongs to the field of environmental catalyst synthesis and discloses a graphene-based hollow cobaltous sulfide nanocrystal capable of efficiently activating persulfate and a preparation method thereof. The preparation method comprises the following steps: growing a zeolite-type imidazate frame 67 on the surface of graphene oxide by a precipitation method; taking the imidazate frame67 as a self-template, taking thioacetamide as a sulfur source, and preparing cobaltosic sulfide of a hollow structure by virtue of a solvothermal reaction; and finally, calcining in an inert atmosphere, converting the cobaltosic sulfide into hollow cobaltous sulfide by virtue of desulfurization reaction, and reducing graphene oxide into graphene, thereby obtaining the graphene-based hollow cobaltous sulfide nanocrystal. The hollow cobaltous sulfide nanocrystal prepared by the method disclosed by the invention has the advantages of high catalytic activity, high free radical yield, simple recycling and the like, can achieve the effects of enhancing the conventional oxidation method, obviously shortening the reaction time and greatly reducing the usage amount of the catalyst and oxidizing agent, and has obvious technical and economic advantages.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com