Preparation method of cobalt sulfide, product of preparation method and application
A technology of sulfide and vulcanizing agent, applied in the direction of cobalt compounds, chemical instruments and methods, cobalt sulfide, etc., to achieve the effects of uniform deposition, reduced energy consumption, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
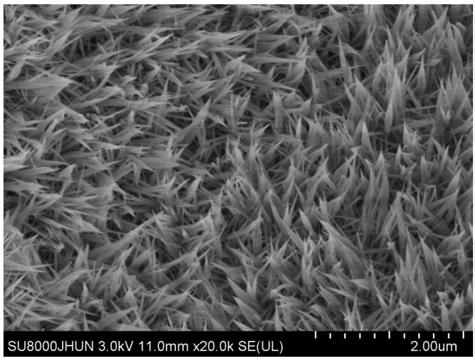
[0042] Weigh 3mmol of cobalt nitrate and 15mmol of urea and dissolve them in 60mL of deionized water. After the dissolution is complete, a purple-red solution is obtained. Pour the solution into a 100mL hydrothermal reaction kettle, then tighten the reaction kettle and put it in a blast oven at 90°C. Heating for 6 hours, after the reaction, a nano-precipitation of cobalt basic carbonate was obtained, which was washed with deionized water and absolute ethanol several times and dried by centrifugation to obtain a purple-red basic cobalt carbonate nano-particle powder, and then weighed 0.1g of the powder Pour into 30mL sodium sulfide solution (0.27mol / L), stir and react at 25°C for 0.01h, then pour the solution into a centrifuge tube and centrifuge several times to separate the precipitate, and finally dry the precipitate at low temperature to obtain amorphous cobalt sulfide nanoparticle powder.
Embodiment 2
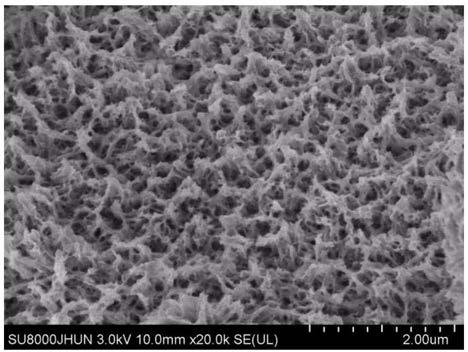
[0044] Weigh 3mmol of cobalt nitrate, 15mmol of urea and 6mmol of ammonium fluoride and dissolve in 60mL of deionized water. After the dissolution is complete, a purple solution is obtained. In an oven, heat at 90°C for 6 hours. After the reaction, a nano-precipitate of cobalt basic carbonate is obtained. The precipitate is washed with deionized water and absolute ethanol several times and dried by centrifugation to obtain a purple-red basic cobalt carbonate nano-particle powder. Weigh 0.1g powder and pour it into 30mL sodium sulfide solution (0.27mol / L), stir and react at 0°C for 24 hours, then pour the solution into a centrifuge tube and centrifuge several times to separate the precipitate, and finally dry the precipitate at low temperature to obtain Amorphous cobalt sulfide nanoparticle powder.
Embodiment 3
[0046]Weigh 3mmol of cobalt nitrate and 15mmol of urea and dissolve them in 60mL of deionized water. After the dissolution is complete, a purple-red solution is obtained. At the same time, cut out 2.5cm*1cm of nickel foam and clean it with acetone, 3mol / L hydrochloric acid and deionized water. , then pour the mixed solution of cobalt nitrate and urea into a 100mL hydrothermal reaction kettle, and immerse the cleaned foamed nickel into the solution, then put the reaction kettle into a blast oven, heat at 90°C for 6h, and take out the foamed nickel After drying at low temperature, the basic cobalt carbonate supported by nickel foam can be obtained, and then the dried nickel foam-supported basic cobalt carbonate is immersed in 30mL sodium sulfide solution (0.27mol / L), reacted at 25°C for 24h, and then The foamed nickel is taken out and dried at a low temperature to obtain the amorphous cobalt sulfide nanomaterial supported by the foamed nickel.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com