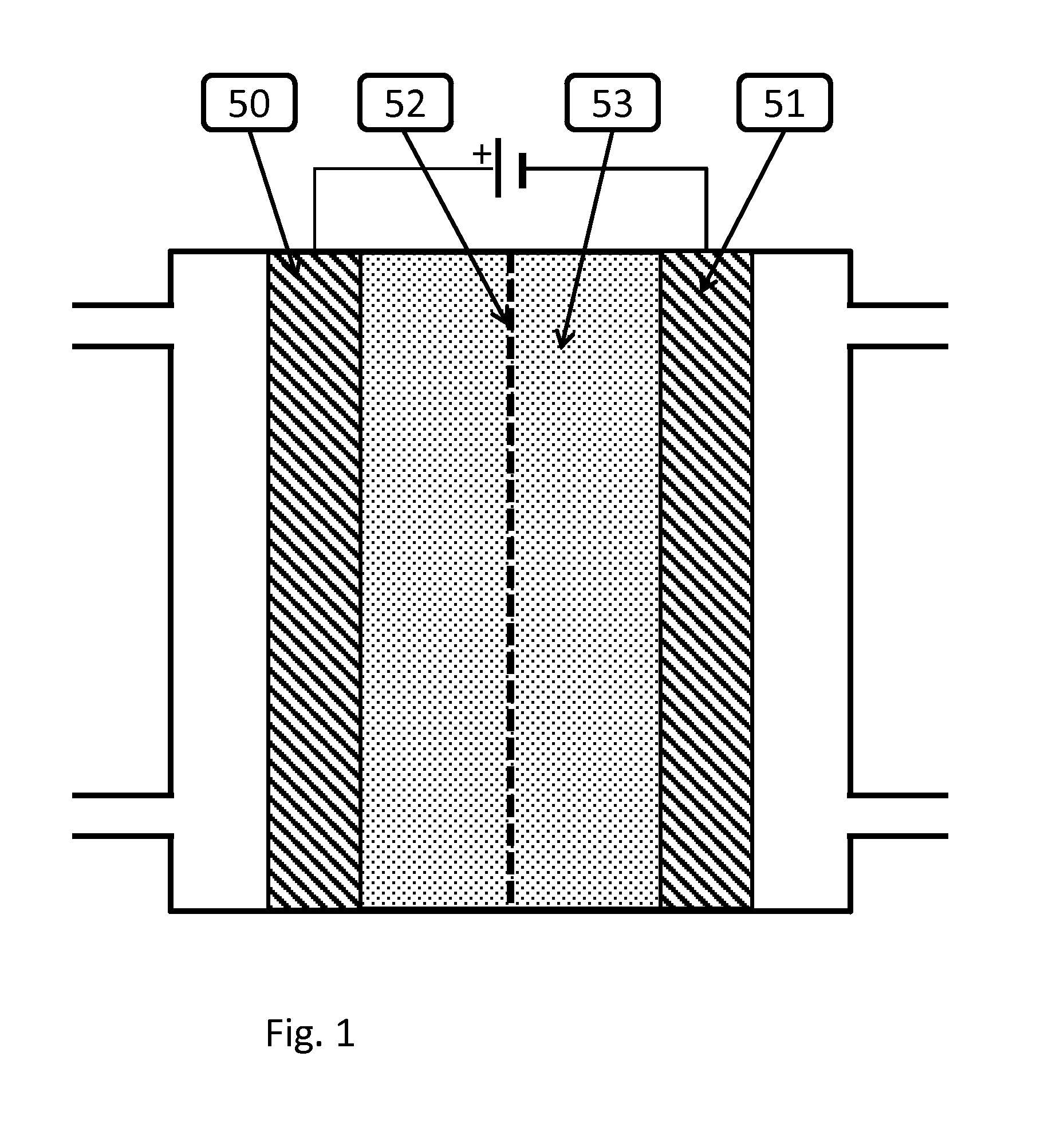
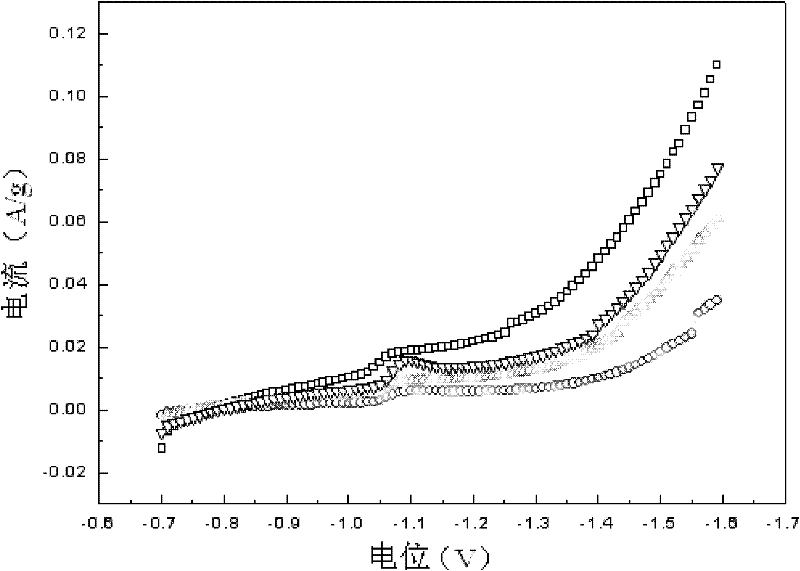
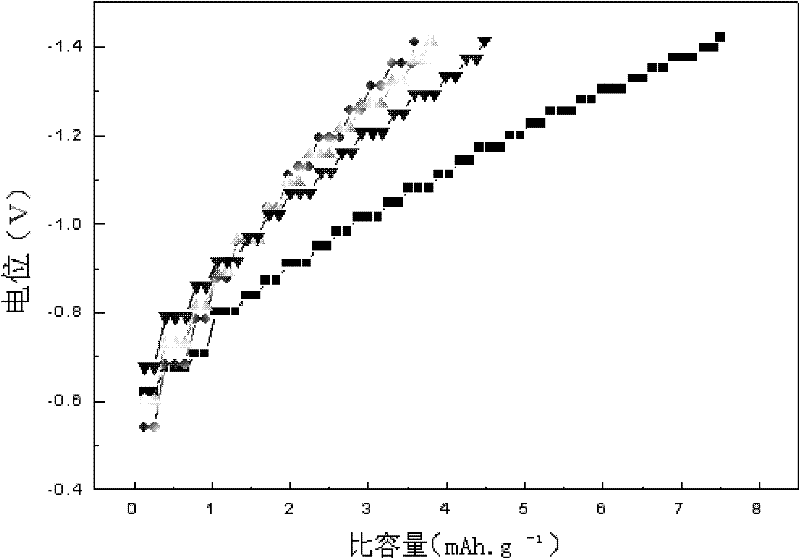
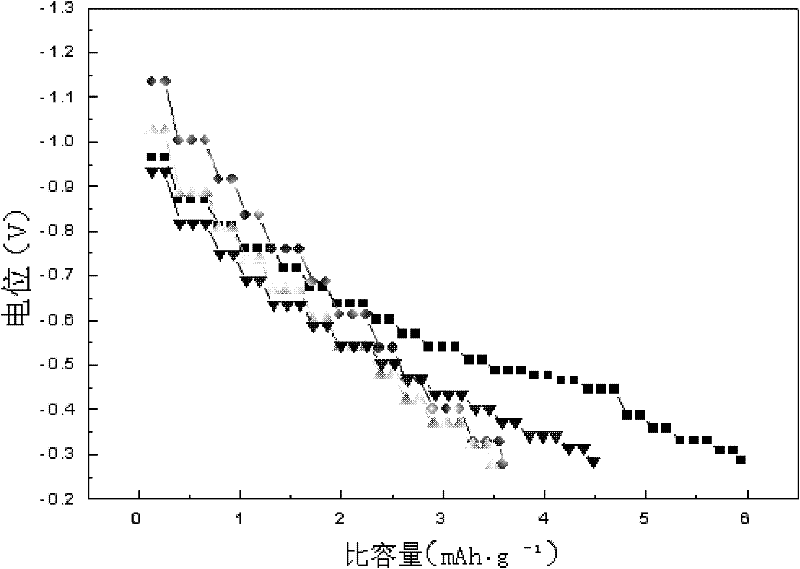
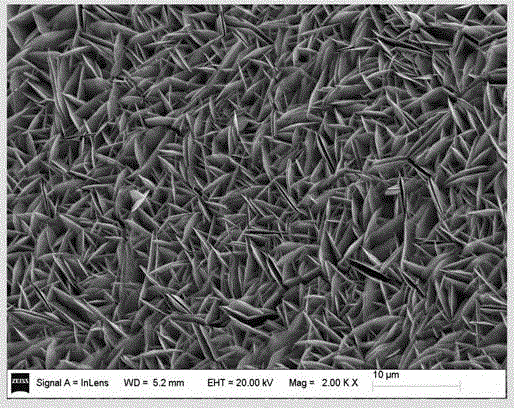
Patents
Literature
576 results about "Overpotential" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
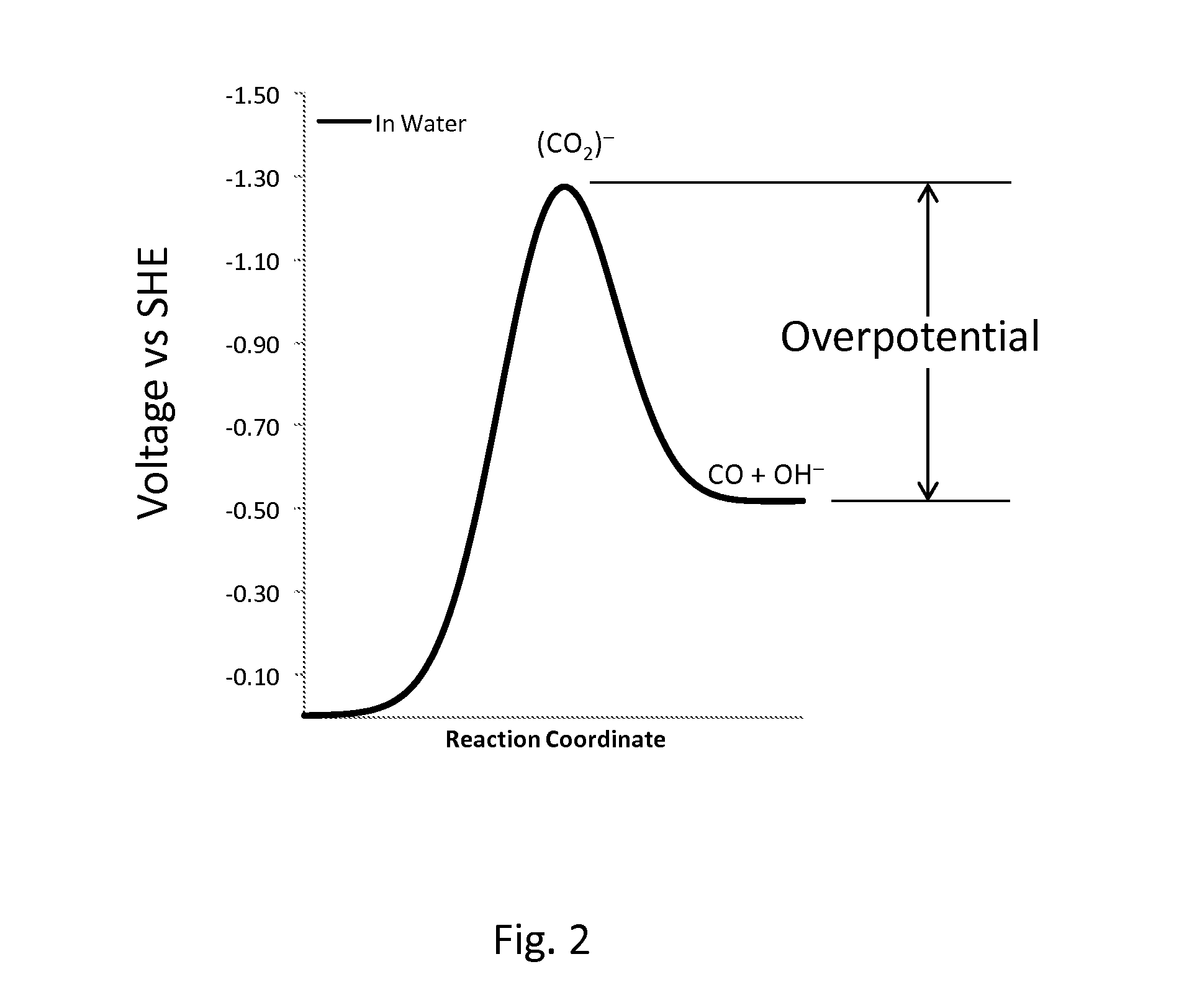
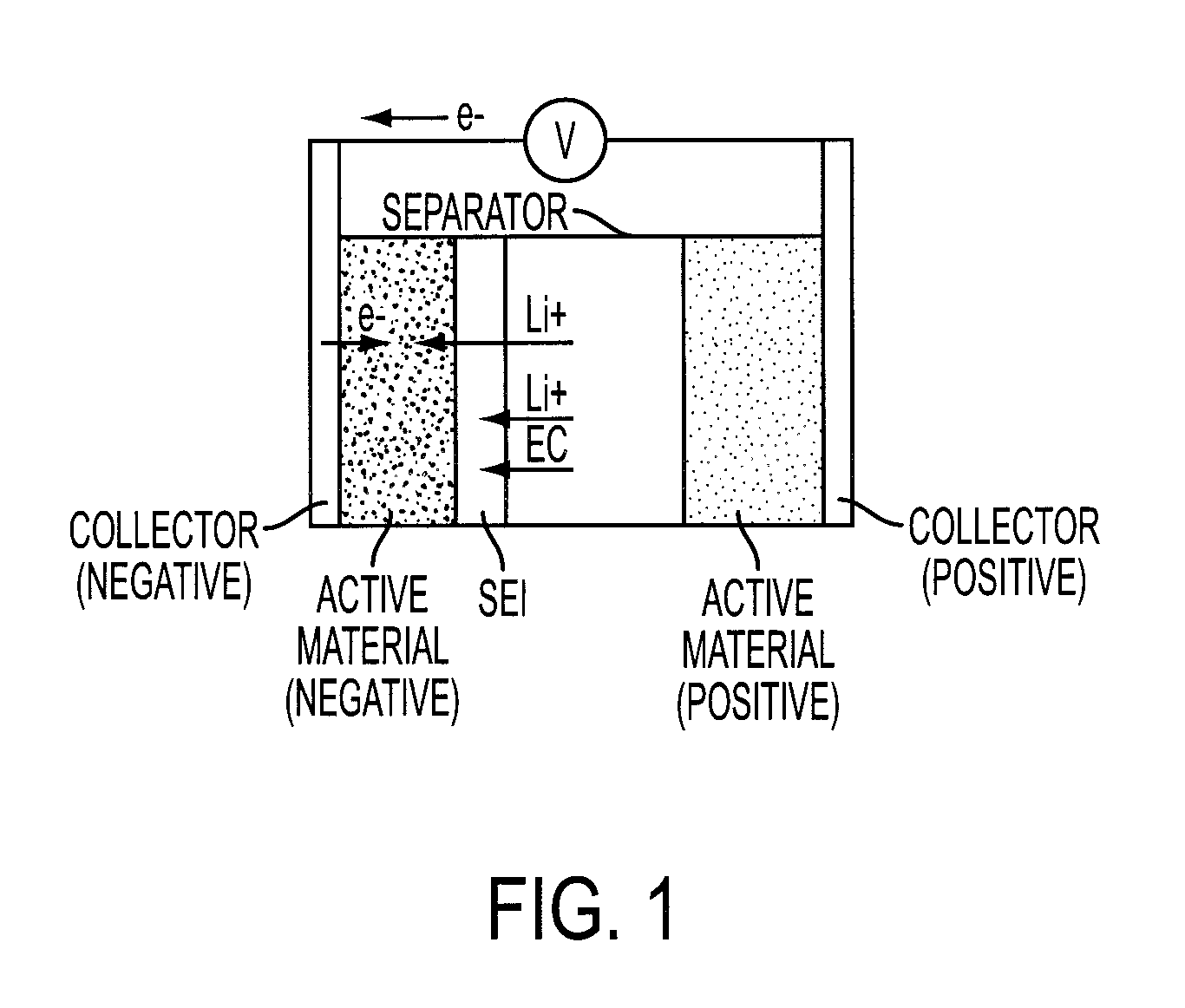
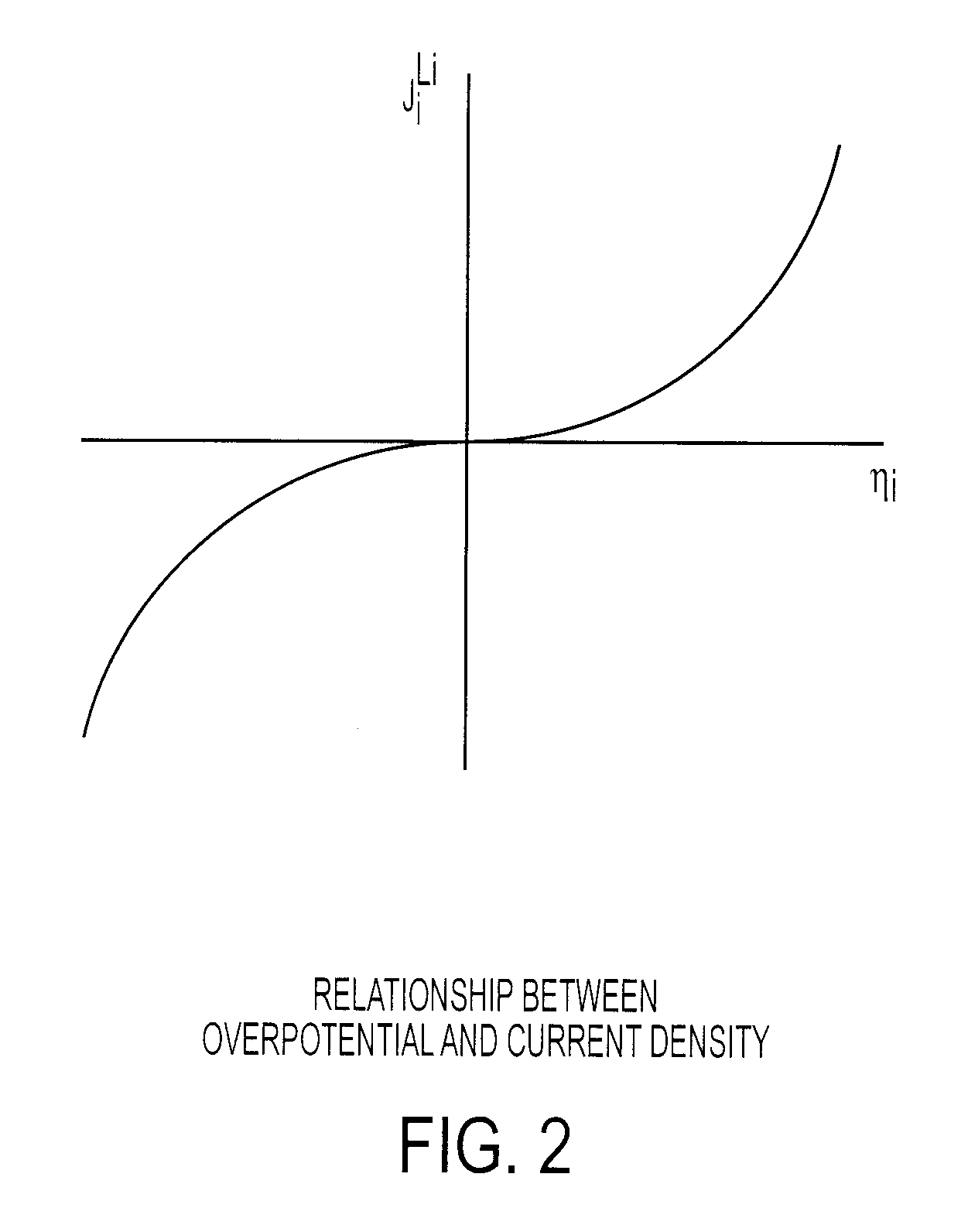
In electrochemistry, overpotential is the potential difference (voltage) between a half-reaction's thermodynamically determined reduction potential and the potential at which the redox event is experimentally observed. The term is directly related to a cell's voltage efficiency. In an electrolytic cell the existence of overpotential implies the cell requires more energy than thermodynamically expected to drive a reaction. In a galvanic cell the existence of overpotential means less energy is recovered than thermodynamics predicts. In each case the extra/missing energy is lost as heat. The quantity of overpotential is specific to each cell design and varies across cells and operational conditions, even for the same reaction. Overpotential is experimentally determined by measuring the potential at which a given current density (typically small) is achieved.
Real-time self-calibrating sensor system and method
ActiveUS20070163894A1Immobilised enzymesBioreactor/fermenter combinationsVoltage referenceLinear regression
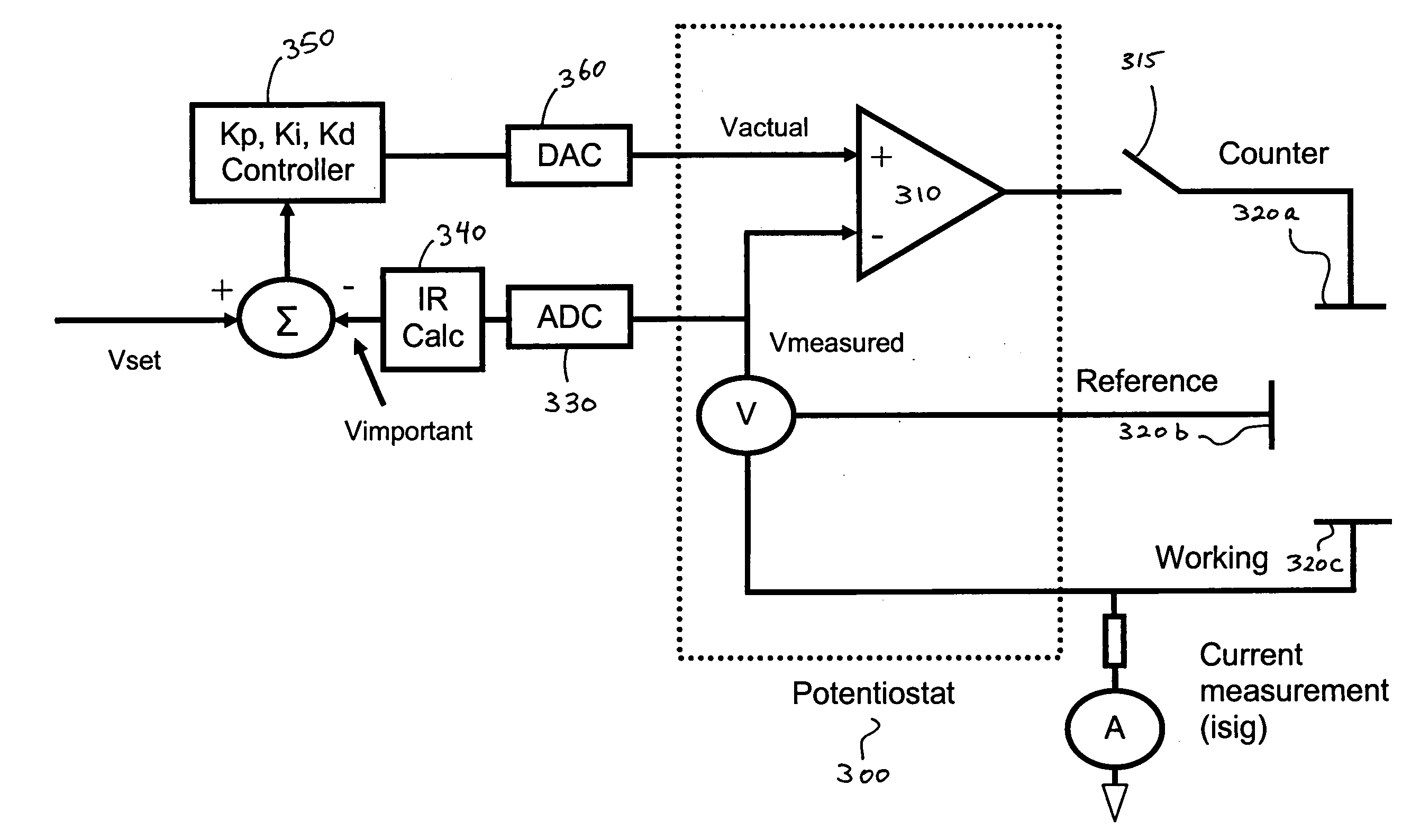
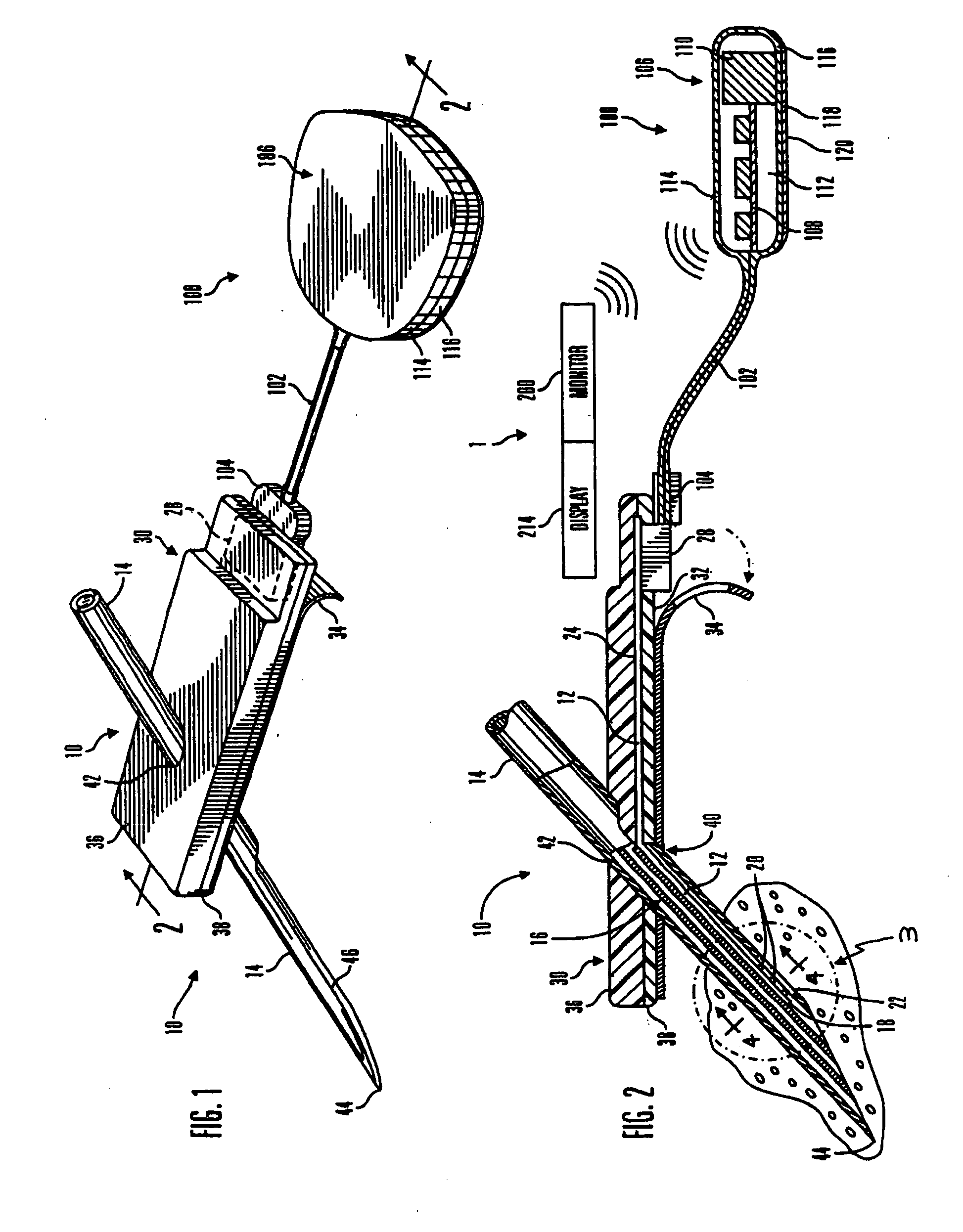
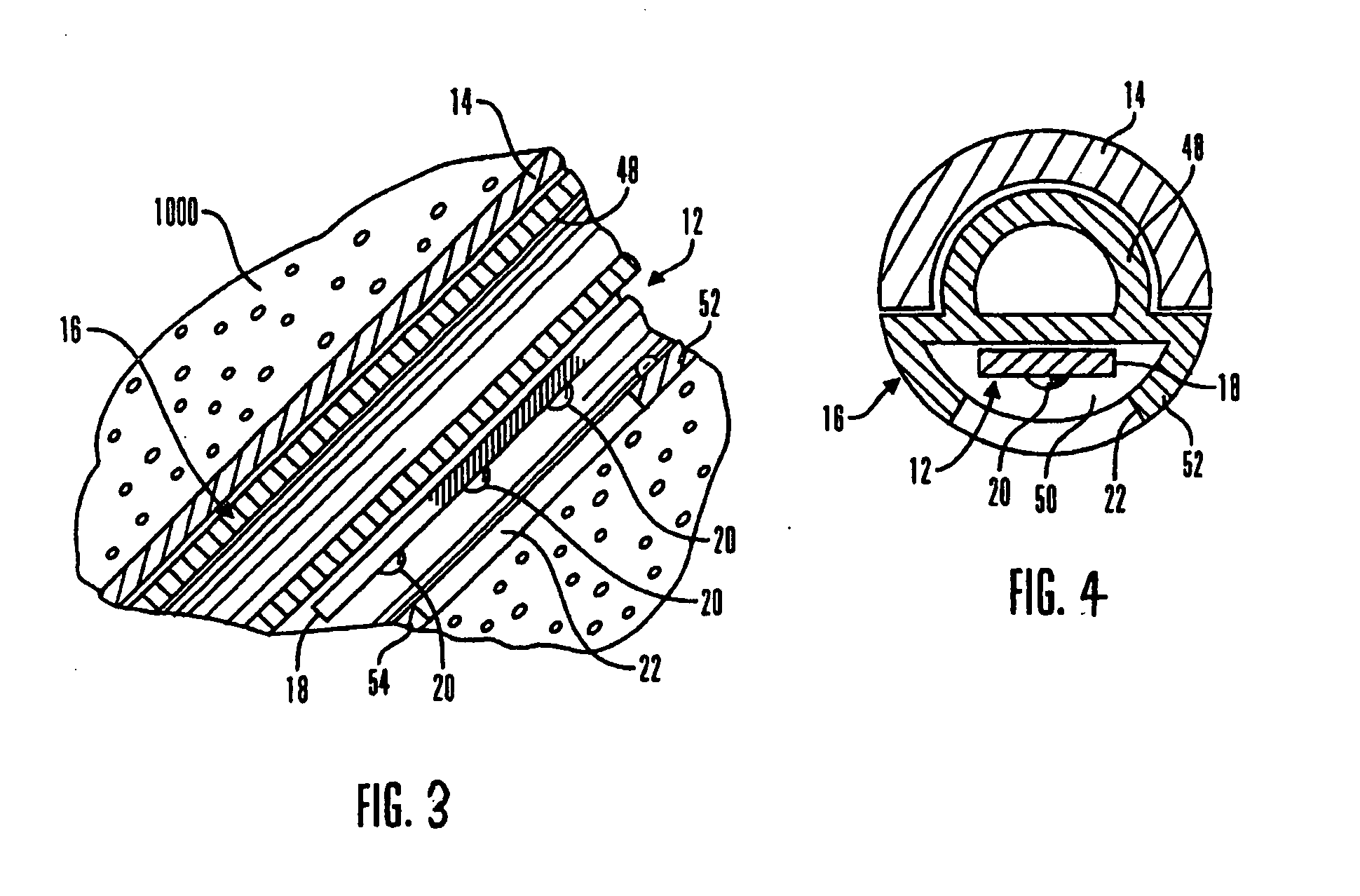
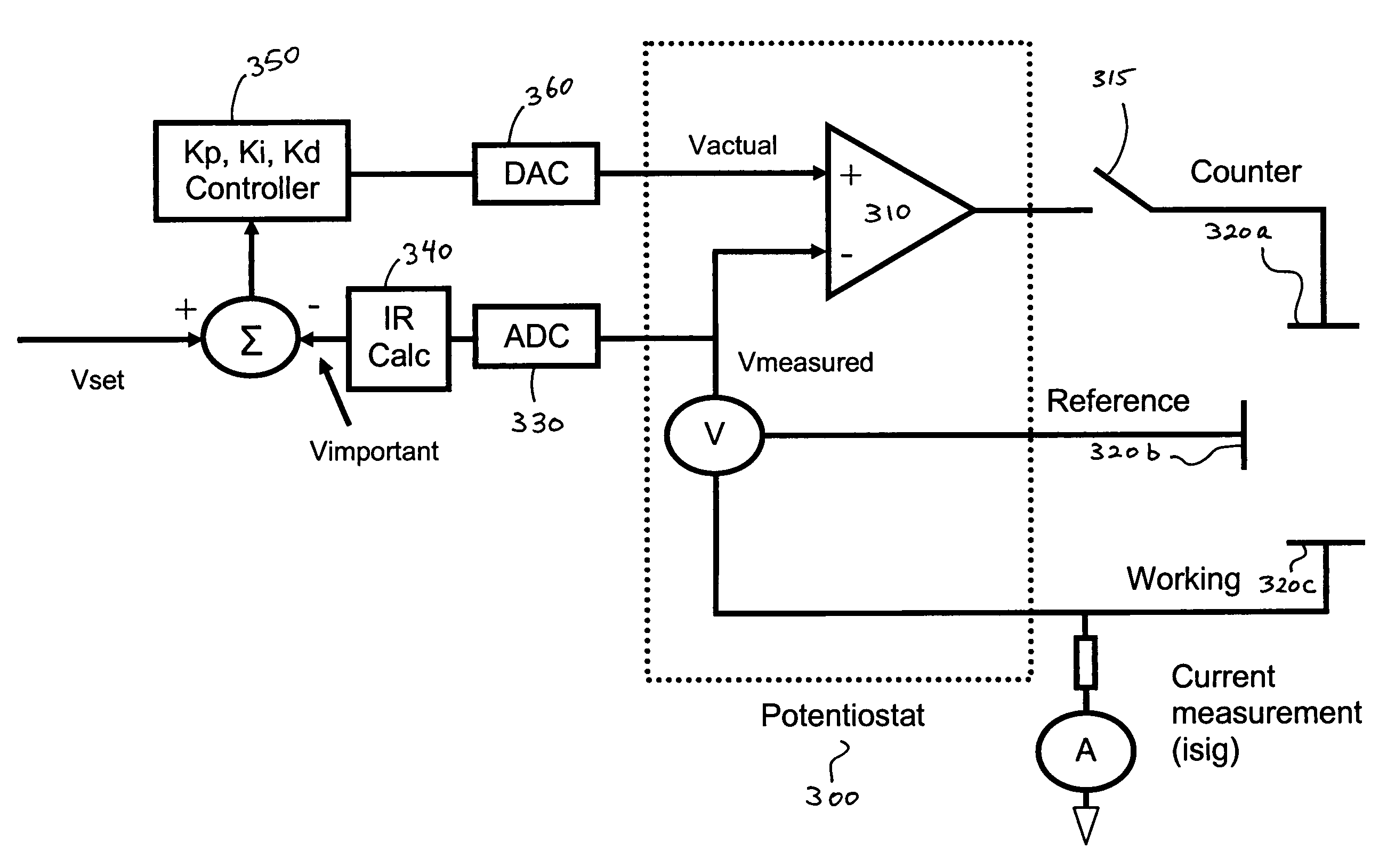
A system and method for calibrating a sensor of a characteristic monitoring system in real time utilizes a self-calibration module for periodic determination of, and compensation for, the IR drop across unwanted resistances in a cell. A current-interrupt switch is used to open the self-calibration module circuit and either measure the IR drop using a high-frequency (MHz) ADC module, or estimate it through linear regression of acquired samples of the voltage across the sensor's working and reference electrodes (Vmeasured) over time. The IR drop is then subtracted from the closed-circuit value of Vmeasured to calculate the overpotential that exists in the cell (Vimportant). Vimportant may be further optimized by subtracting the value of the open-circuit voltage (Voc) across the sensor's working and reference electrodes. The values of Vmeasured and Vimportant are then controlled by respective first and second control units to compensate for the IR drop.
Owner:MEDTRONIC MIMIMED INC
Novel catalyst mixtures
InactiveUS20110237830A1Low rateRaise the overpotentialOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsChemical reactionCompound (substance)
Catalysts comprised of at least one catalytically active element and at least one helper catalyst are disclosed. The catalysts may be used to increase the rate, the selectivity or lower the overpotential of chemical reactions. These catalysts may be useful for a variety of chemical reactions including in particular the electrochemical conversion of carbon dioxide to formic acid.
Owner:DIOXIDE MATERIALS
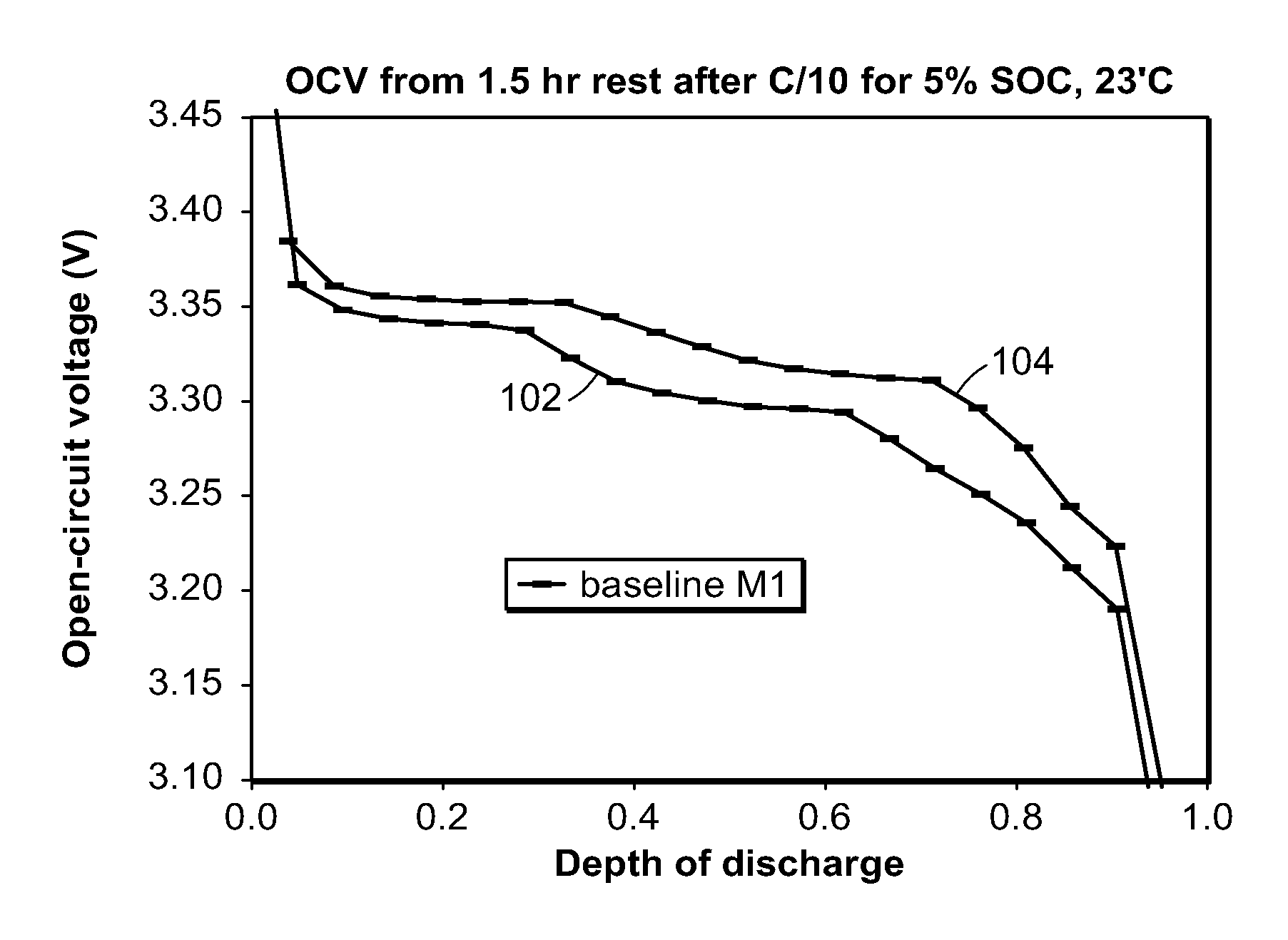
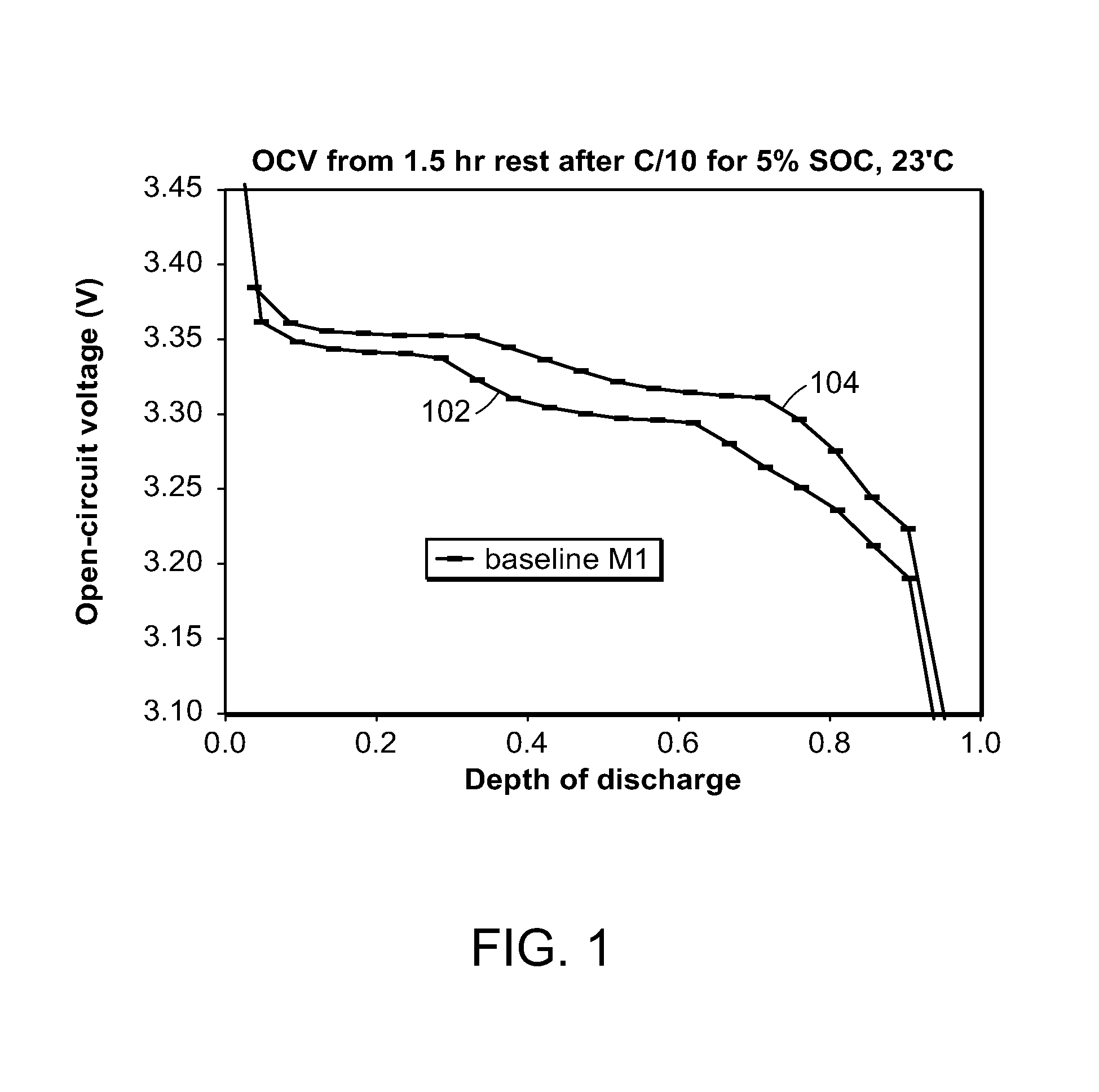
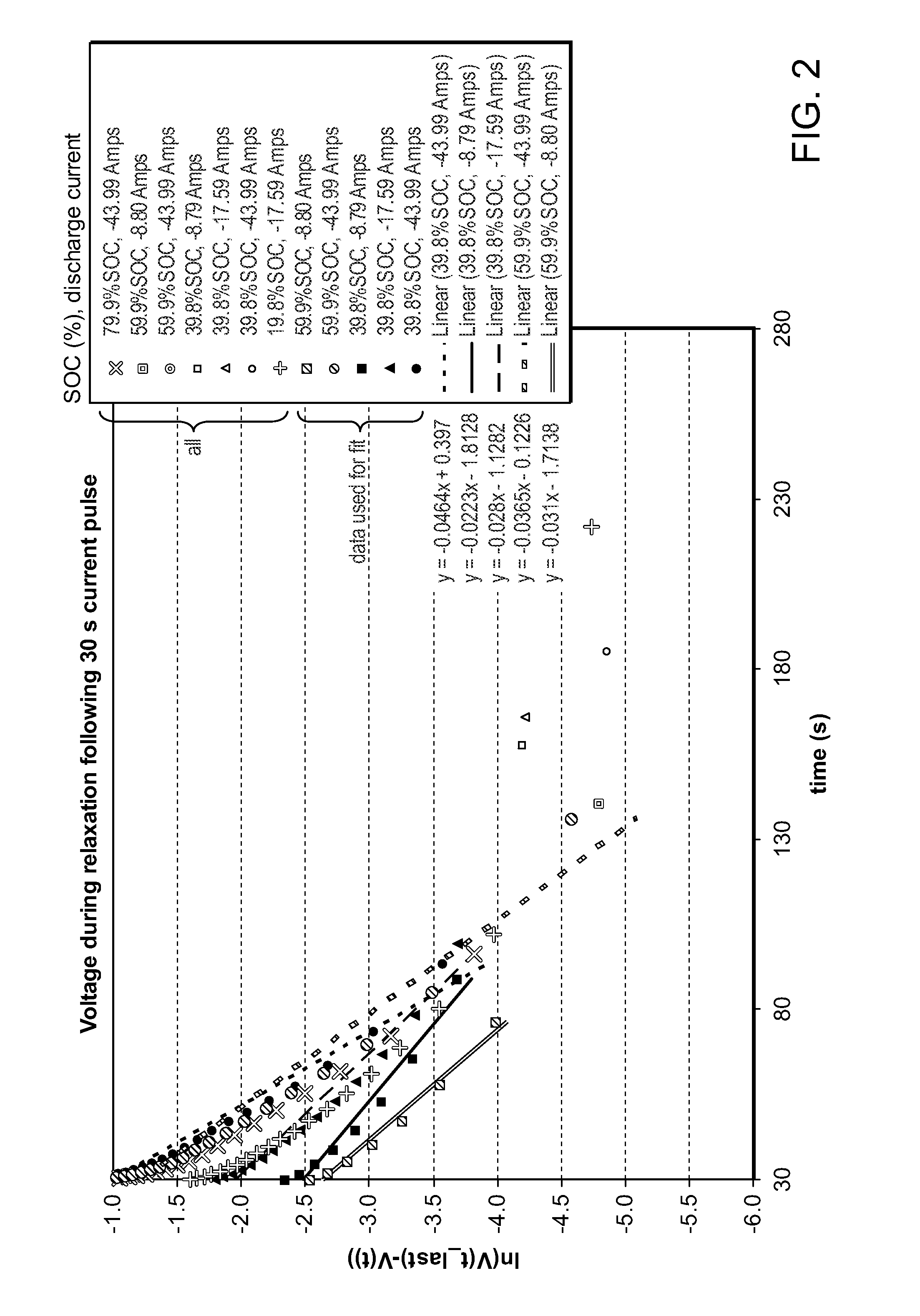
Method and system for determining state of charge of an energy delivery device
A method of determining state of charge of an energy delivery device includes sampling voltage values of the energy delivery device during relaxation of the device. The method further includes regressing an open circuit voltage value and the total overpotential being relaxed. The regression includes a predetermined time constant of relaxation associated with the energy delivery device. One embodiment uses the equation V(t)=OCV−α exp(−t / tau), where V(t) represents the sampled voltage values, t represents times at which each of the voltage values are sampled, OCV represents the open circuit voltage value of the energy delivery device, α represents the overpotential value, and tau represents the time constant of relaxation. The method uses a predetermined profile that relates open circuit voltage of the energy delivery device to state of charge of the device, to determine a particular state of charge corresponding to the regressed open circuit voltage value.
Owner:A123 SYSTEMS LLC
Real-time self-calibrating sensor system and method
ActiveUS7774038B2Immobilised enzymesBioreactor/fermenter combinationsElectrical batteryVoltage reference
A system and method for calibrating a sensor of a characteristic monitoring system in real time utilizes a self-calibration module for periodic determination of, and compensation for, the IR drop across unwanted resistances in a cell. A current-interrupt switch is used to open the self-calibration module circuit and either measure the IR drop using a high-frequency (MHz) ADC module, or estimate it through linear regression of acquired samples of the voltage across the sensor's working and reference electrodes (Vmeasured) over time. The IR drop is then subtracted from the closed-circuit value of Vmeasured to calculate the overpotential that exists in the cell (Vimportant). Vimportant may be further optimized by subtracting the value of the open-circuit voltage (Voc) across the sensor's working and reference electrodes. The values of Vmeasured and Vimportant are then controlled by respective first and second control units to compensate for the IR drop.
Owner:MEDTRONIC MIMIMED INC
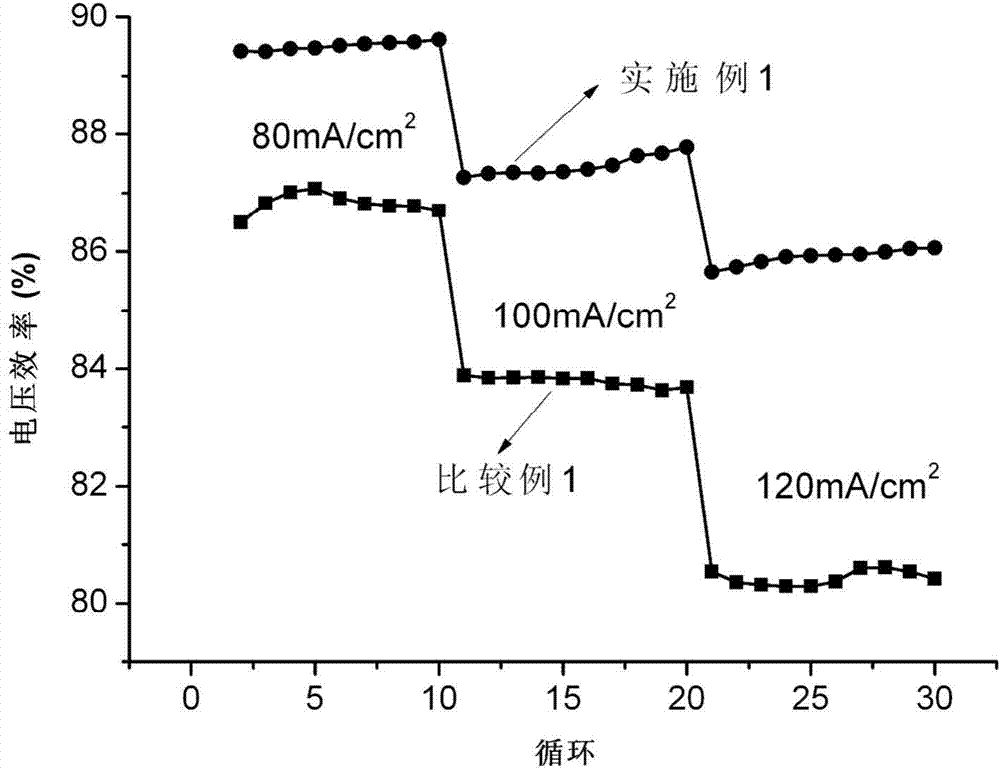
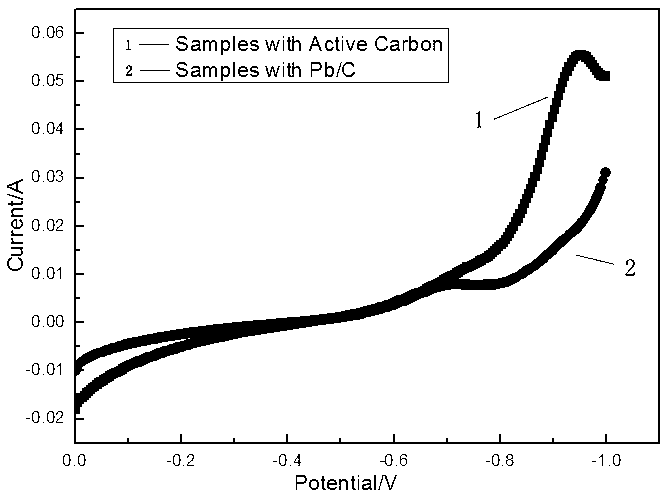
Modified activated carbon with high hydrogen evolution potential and preparation method thereof as well as lead-acid battery negative lead paste containing modified activated carbon
InactiveCN102306784AHigh discharge specific capacityImprove cycle performanceCell electrodesActivated carbonElectrode potential
The invention relates to a modified activated carbon with high hydrogen evolution potential and a preparation method thereof as well as a lead-acid battery negative lead paste containing the modified activated carbon and relates to a modified activated carbon and a preparation method thereof as well as a lead-acid battery negative lead paste containing the modified activated carbon. According to the invention, the problem of the poor cycle life of the lead-acid battery caused by the unmatching between the added activated carbon and the lead electrode potential, the large hydrogen-evolution speed and the serious hydrogen evolution in the existing lead-acid battery negative electrode is solved. In the modified activated carbon provided by the invention, a hydrogen evolution inhibitor is loaded on the activated carbon, wherein the hydrogen evolution inhibitor is one or a mixture of more than one selected from In2O3, Ga2O3 and Bi2O3, or the hydrogen evolution inhibitor is In(OH)3, Ga(OH)3 or Bi(OH)3. The modified activated carbon is prepared by a ball-milling method or a solvent precipitation method. The hydrogen evolution overpotential of the modified activated carbon is improved obviously, the hydrogen evolution speed of the modified activated carbon is reduced and the hydrogen evolution potential of the modified activated carbon is matched with a Pb electrode potential; and meanwhile, the discharge specific capacity in a cyclic process can be obviously improved, the cycle performance can be obviously improved, and the specific capacity can still reach 90mAh.g<-1> after being cycled for 500 times.
Owner:HARBIN INST OF TECH
Ultrathin nanosheet array electro-catalytic material with nano-porous structure and oxygen vacancies
The invention relates to an ultrathin nanosheet array electro-catalytic material with a nano-porous structure and oxygen vacancies. The material is a cobaltosic oxide primary nanosheet array which grows vertically on a conductive substrate and is doped with a metal; an ultrathin nanosheet with oxygen vacancies and nanopores is obtained on each primary nanosheet; the conductive substrate is a titanium sheet or a foamed nickel sheet, and the doped metal is zinc, nickel or manganese; and the thickness of each cobaltosic oxide ultrathin nanosheet doped with the metal is 1.22 nm, nanosheets are in a three-dimensional porous structure, and the nano-pore diameter is 3-6 nm. The ultrathin nanosheet array electro-catalytic material with the nano-porous structure and oxygen vacancies has the following advantages: the material can effectively reduce the overpotential and the spike potential of an oxygen evolution reaction, increase the conversion rate of a single cobalt atom and work continuously and stably in an alkali environment; the steps of a preparation method of the material are simple, the operation is convenient, the cost is low, and the material is environmental-friendly; and new ideas and strategies are provided for the function-oriented design and the performance optimization of an oxygen evolution catalyst of a water electrolysis system.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
Preparation method and electroplating method of normal-temperature environment-friendly sulfate trivalent chromium electroplating liquid
The invention provides a preparation method and an electroplating method of normal-temperature environment-friendly sulfate trivalent chromium electroplating liquid, which relates to electroplating liquid and electroplating technology. The invention solves the problems of poor stability of the traditional trivalent chromium electroplating liquid, low precipitating and depositing rate of harmful gas in an electroplating process, more components of the electroplating liquid, sensitivity for impurities, poor corrosion resistance of an electroplating layer, and the like. The normal-temperature environment-friendly sulfate trivalent chromium electroplating liquid mainly comprises the following components of main salt, a complexation stabilizing agent, a combined additive, a buffering agent and a conducting salt. The electroplating liquid can work at normal temperature, thereby saving energy resources and having simple technology and high deposition rate, wherein the deposition rate can reach above 0.22micron.min<-1> under 6A / dm<2>. An anode of the invention is a Ti-based rare-metal tantalum-iridium-titanium anode, the anode oxygen-evolution overpotential is low, and harmful hexavalent chrome can not be generated. The normal-temperature environment-friendly sulfate trivalent chromium electroplating liquid has good corrosion resistance and high stability.
Owner:HARBIN INST OF TECH
Preparation method of foam electrode for water electrolysis
InactiveCN101748426AReduce precipitation overpotentialReduce energy consumptionElectrode shape/formsHigh activityEnergy consumption
The invention discloses a preparation method of a foam electrode for water electrolysis, which belongs to the technical field of water electrolysis. Foam nickel is selected as the base material of the electrode material; oil stains on the surface of the foam nickel are removed so that the surface is clean; the foam nickel is soaked in diluted acid solution, and the surface of the foam nickel is activated; pure water is used to flush the activated foam nickel, and the foam nickel is quickly soaked into an electrodeposition tank as a cathode for electrodeposition; the active surface is prepared; the foam electrode with the active surface is thoroughly cleaned, dried and thermally treated; and the thermally treated foam material is cut to prepare the electrode material. The high-activity foam electrode material prepared through the method provided by the invention can be used for the electrode material in the electrolysis hydrogen production industry, can reduce hydrogen precipitation overpotential in the electrolysis process, reduce energy consumption, and improve the production efficiency.
Owner:GRIPM ADVANCED MATERIALS CO LTD
Detection of biological molecules
InactiveUS20060096870A1Immobilised enzymesBioreactor/fermenter combinationsHigh concentrationCorrelation coefficient
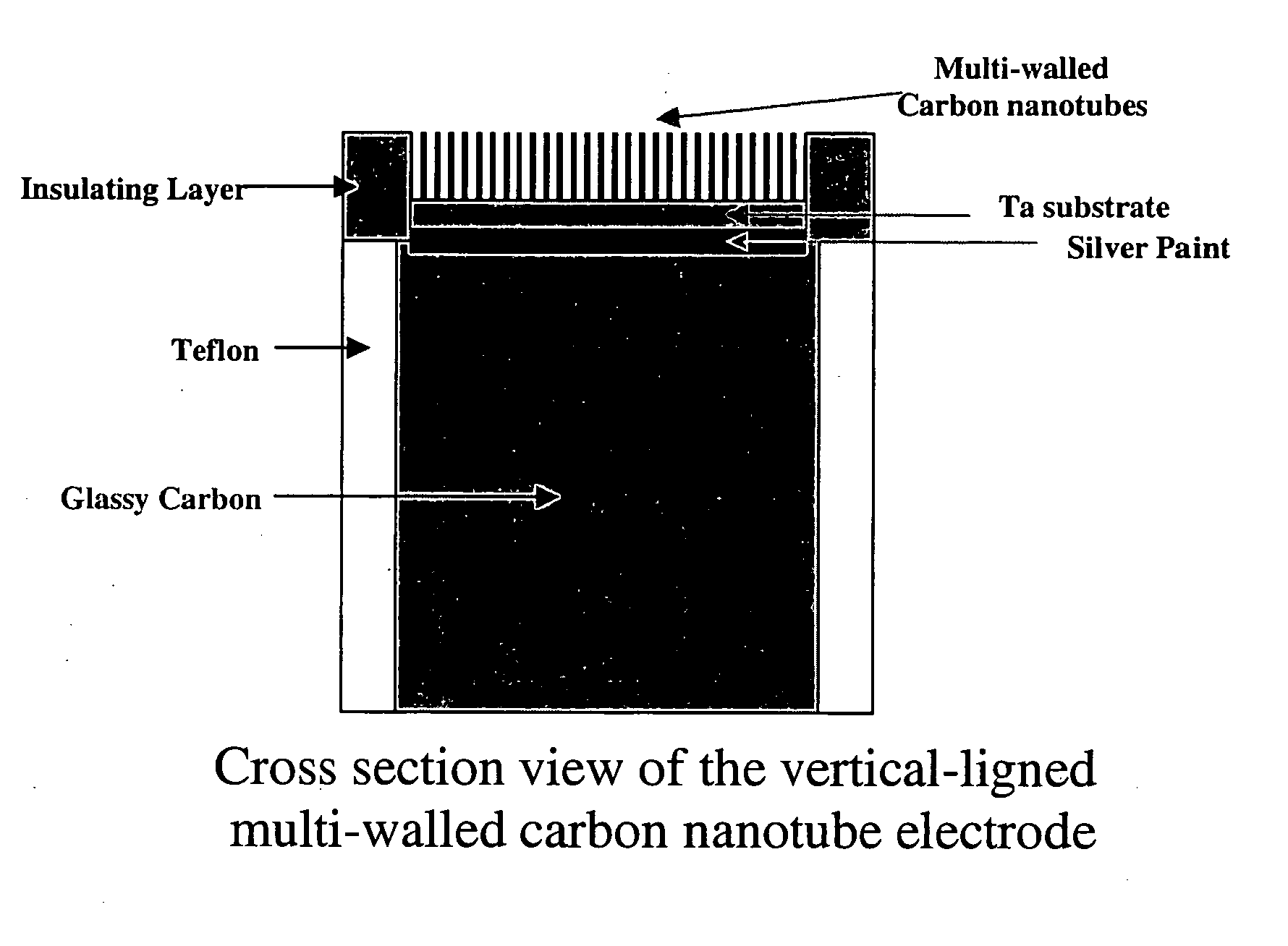
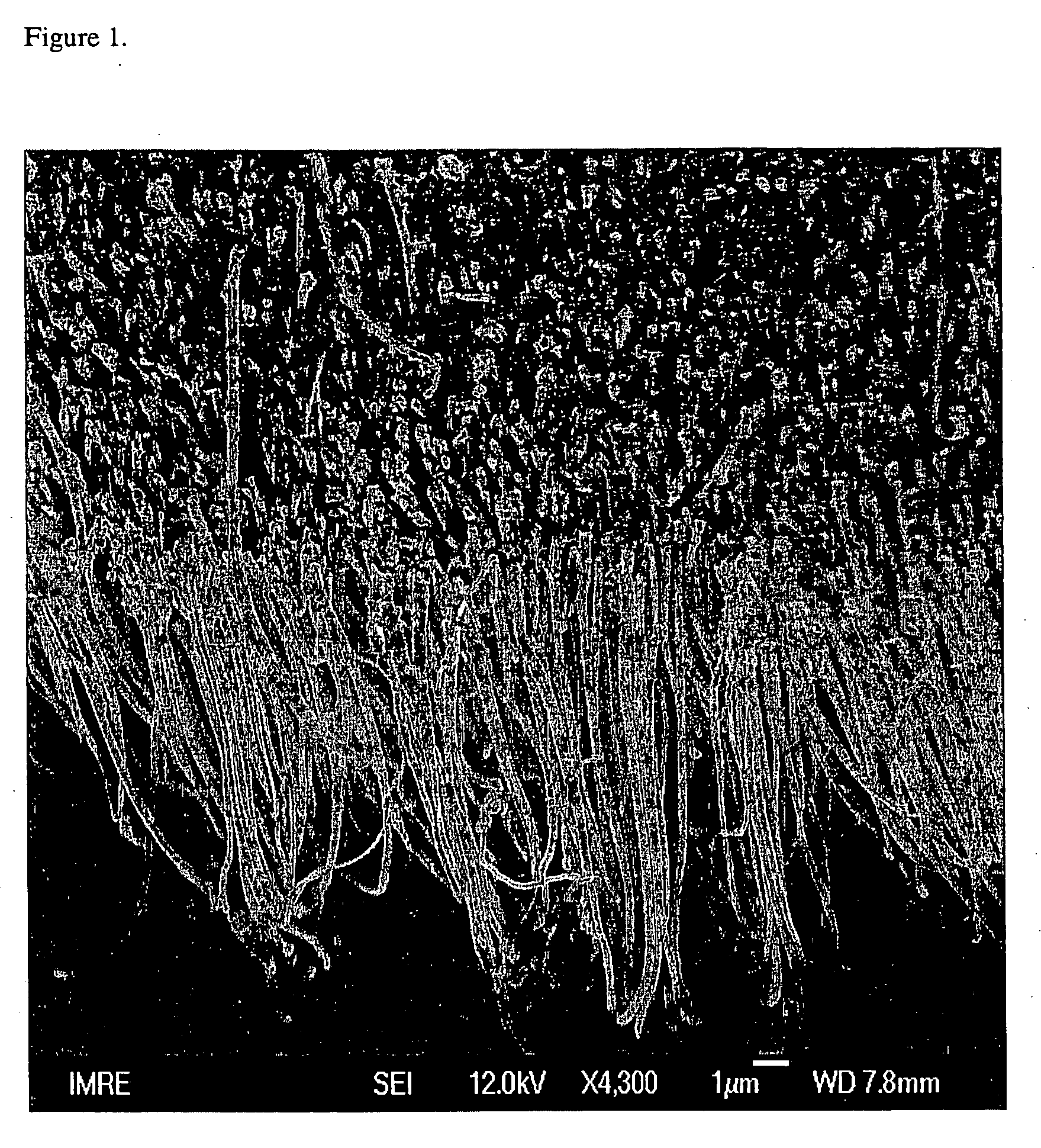
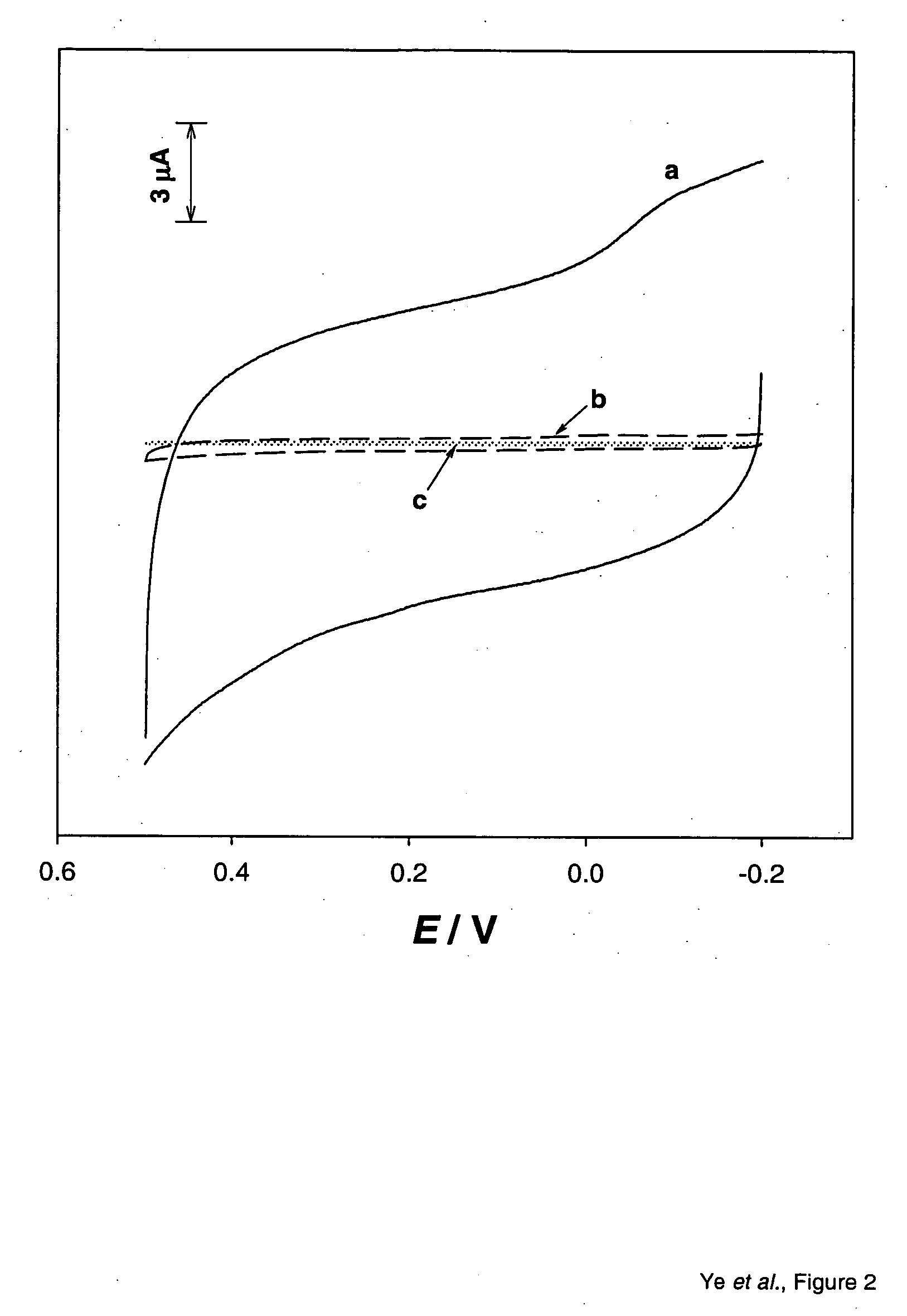
An apparatus uses carbon nanotubes for electrochemical analysis of biological molecules of interest such as Uric Acid, illustrating how the voltammetric behaviors of uric acid (UA) and L-ascorbic acid (L-AA) at a well-aligned, carbon nanotube, electrode may be used in a biochemical assay. Compared to glassy carbon, a carbon nanotube electrode reduces troublesome overpotentials. Based on its differential catalytic function toward the oxidation of UA and L-AA, the carbon nanotube electrode can be used for a selective determination of UA in the presence of L-AA. The peak current obtained from DPV was linearly dependent on the UA concentration in the range of 0.2 μM to 80 μM with a correlation coefficient of 0.997. The detection limit (3δ) for UA was found to be 0.1 μM. The device allows for detection of UA in a human urine sample, even in the presence of high concentrations of L-AA, using only simple dilution.
Owner:NAT UNIV OF SINGAPORE
Zinc / polyhalide energy storage cell
InactiveCN102479968AEasy to moveIncrease energy densityRegenerative fuel cellsZinc bromideHigh energy
The invention discloses a zinc / polyhalide energy storage cell. An electrode of conductive inert material and an electrolyte solution of zinc chloride solution and zinc bromide solution are used to form a zinc / polyhalide energy storage cell. The zinc / polyhalide energy storage cell employs zinc and polyhalide with small electrochemical equivalent and high hydrogen evolution overpotential are used as active substances of the cell. Anode and cathode electric pair has high potential difference, and the two together decide that the cell technology has high energy density and power density. Compared with a zinc bromine liquid flow cell, a new system energy storage cell has energy density increased by 30%, power density increased by 10%, obviously enhanced removability and reduced cost of the cell system. The anode and the cathode of the cell employ electrolytes with a same element composition to avoid negative influence on cell performance and life caused by crossed contamination of electrolytes; the cell of the invention has advantages of long cycling life, low cost and good mobility and can be widely applied to fields of energy electric power, transportation and information communication, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
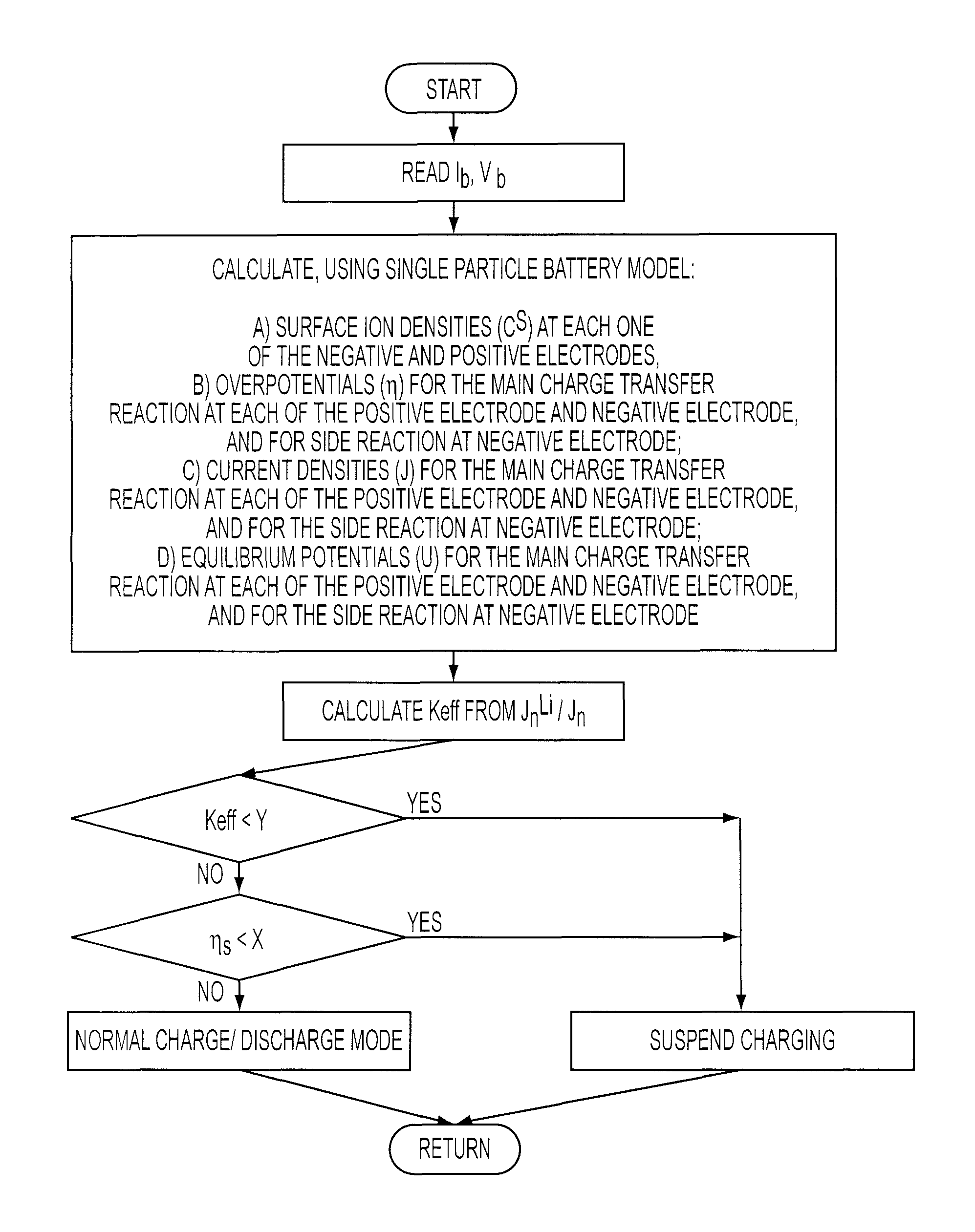
Method of controlling battery charging
InactiveUS8040106B2Lighter and more inexpensive energy storage devicesHybrid vehiclesBatteries circuit arrangementsBattery chargePresent method
The present teachings are directed toward methods of controlling the charging of a battery. The method includes the steps of receiving current and voltage output information for the battery during a charging / discharging cycle at a certain time interval, and using a model to determine both charging efficiency of the battery and the overpotential for a side reaction. These values for the charging efficiency and the overpotential of the side reaction are then compared to respective first and second given values. If either the charging efficiency or the overpotential is less than their respective given values, then the charging of the battery is suspended. The present method is particularly applicable to Li-ion batteries.
Owner:HONDA MOTOR CO LTD
Oxygen ion conducting materials
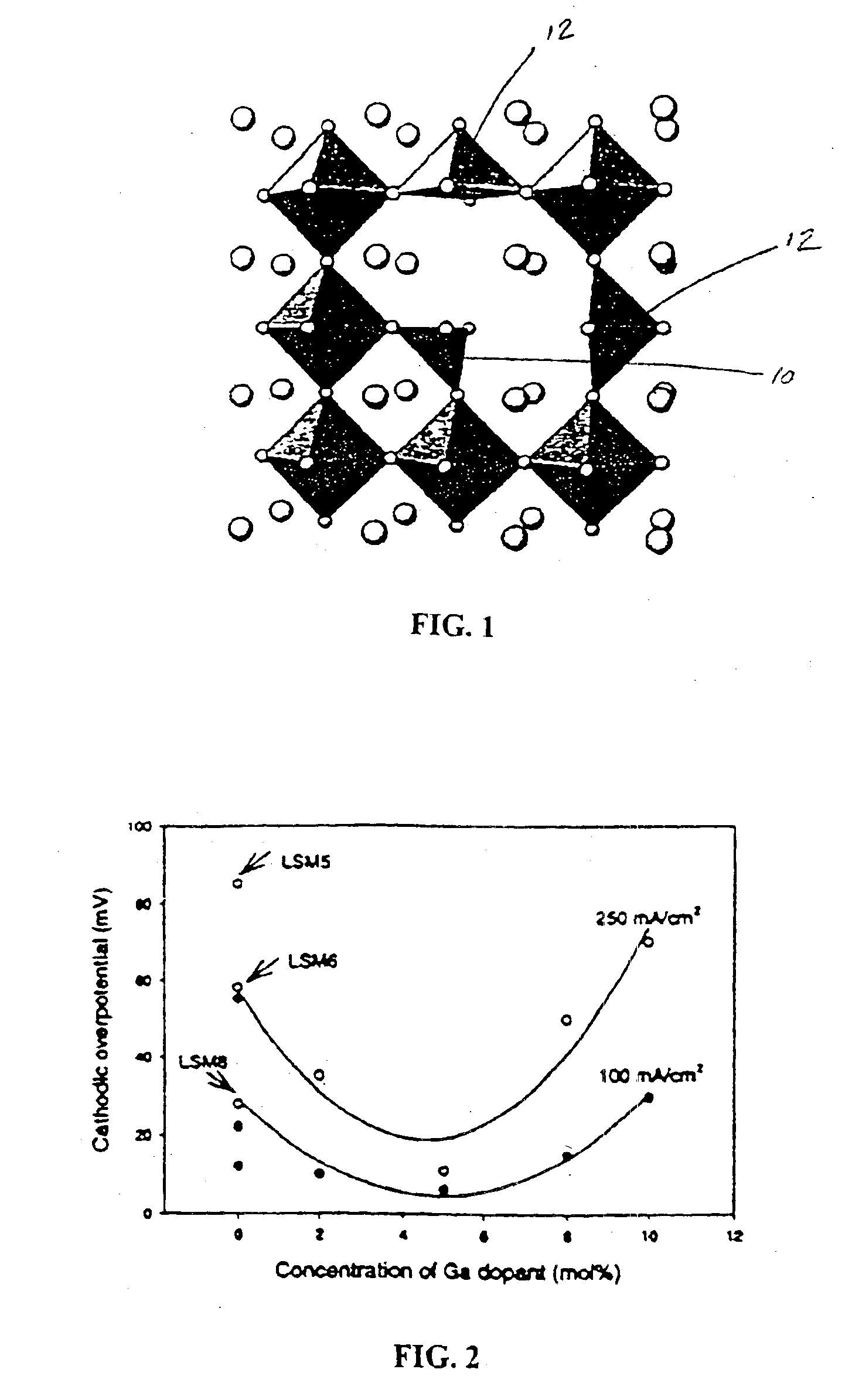
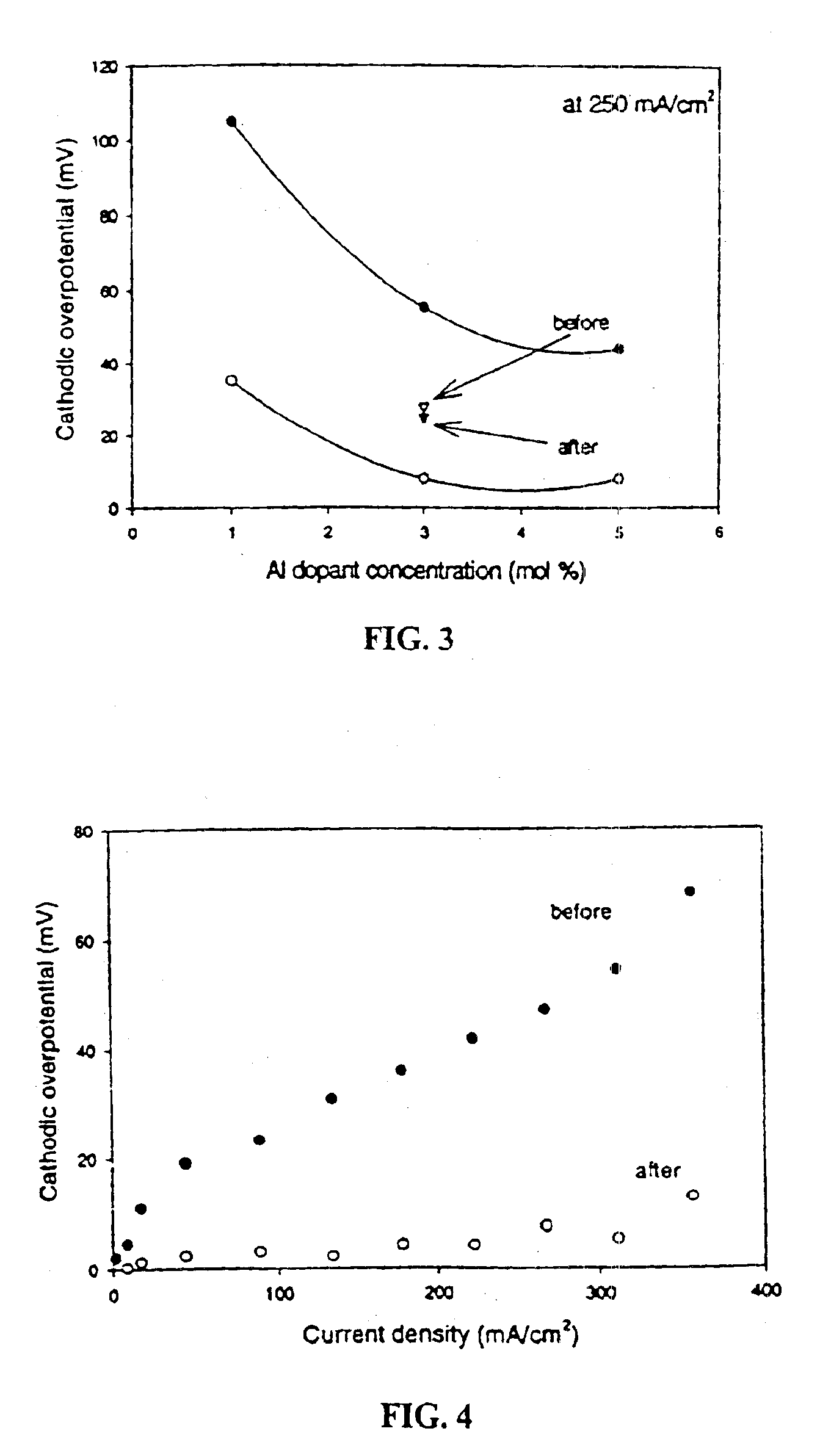
InactiveUS6916570B2Improve conductivityHigh activityHeterogenous catalyst chemical elementsNon-aqueous electrolyte accumulator electrodesDopantFuel cells
An oxygen ion conducting ceramic oxide that has applications in industry including fuel cells, oxygen pumps, oxygen sensors, and separation membranes. The material is based on the idea that substituting a dopant into the host perovskite lattice of (La,Sr)MnO3 that prefers a coordination number lower than 6 will induce oxygen ion vacancies to form in the lattice. Because the oxygen ion conductivity of (La,Sr)MnO3 is low over a very large temperature range, the material exhibits a high overpotential when used. The inclusion of oxygen vacancies into the lattice by doping the material has been found to maintain the desirable properties of (La,Sr)MnO3, while significantly decreasing the experimentally observed overpotential.
Owner:UCHICAGO ARGONNE LLC
Graphene oxide-modified electrode material for energy storage flow battery
ActiveCN105529473AOmission of processingOmit timeCell electrodesRegenerative fuel cellsElectrochemical responseHigh current density
The invention relates to a graphene oxide-modified electrode material for an energy storage flow battery and a preparation method of the graphene oxide-modified electrode material. The electrode material comprises a carbon material matrix and a graphene oxide compound on the surface of the carbon material matrix. According to the method, graphene oxide is loaded on the surface of a carbon electrode by a hydrothermal process; the specific surface area of the modified electrode material is greatly increased; the contact area of an electrolyte and the electrode is increased; reactivity sites of the electrode and the electrolyte are increased; meanwhile, oxygen-containing functional groups of the modified electrode are rapidly increased; the internal resistance of electrochemical reaction of an all-vanadium redox flow battery electrode and the transport resistance of electrons are reduced; and overpotential of the battery under high current density is reduced, so that the voltage efficiency and the energy efficiency of an all-vanadium redox flow battery under the high current density are greatly improved.
Owner:INST OF CHEM CHINESE ACAD OF SCI
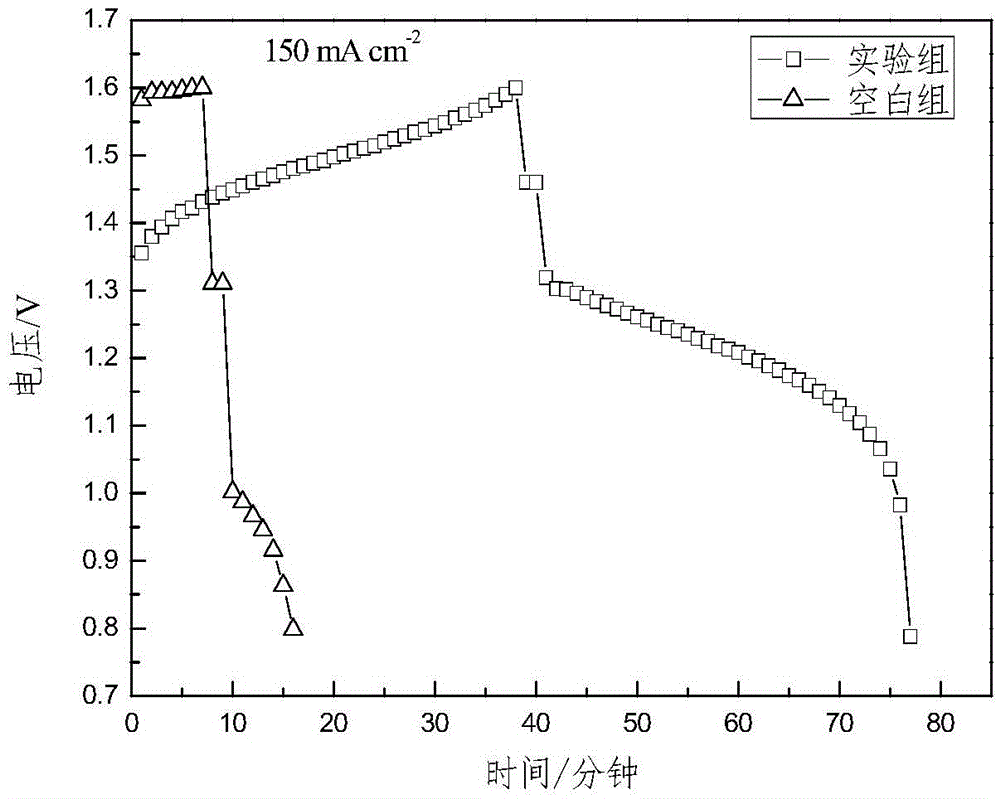
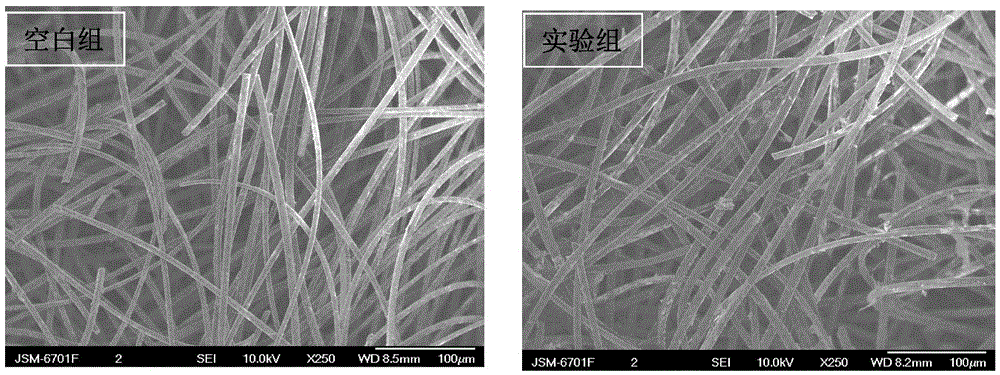
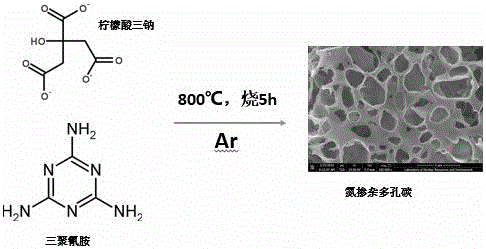
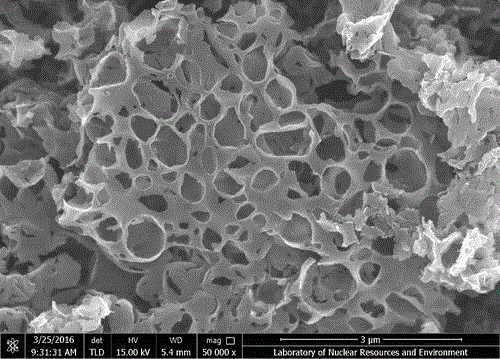
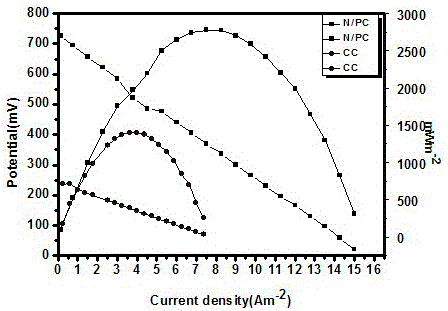
Synthetic method for nitrogen-doped porous carbon, and application of nitrogen-doped porous carbon in positive electrode of microbial fuel cell
InactiveCN106207239AGood biocompatibilityLarge specific surface areaCell electrodesBiochemical fuel cellsPorous carbonOverpotential
The invention discloses a synthetic method for nitrogen-doped porous carbon N-C, and an application of the nitrogen-doped porous carbon in a positive electrode of a microbial fuel cell. The synthetic method comprises the following steps of taking melamine as a nitrogen source, and taking sodium citrate as a carbon source, mixing and grinding the melamine and the sodium citrate based on a certain proportion; and calcining the mixture in inert gas Ar at a temperature of 800 DEG C for 5h to successfully prepare the nitrogen-doped porous carbon N-C which is used as the microbial positive electrode material. The synthetic method and the application have the advantages as follows: on one hand, when the nitrogen-doped porous carbon N-C is used as the positive electrode of the microbial fuel cell, the attachment of electrogenesis microbes in the positive electrode can be facilitated; and on the other hand, the positive electrode activation overpotential is lowered, so that the electrogenesis power density of the microbial fuel cell is remarkably improved.
Owner:NANCHANG HANGKONG UNIVERSITY
Nano-high-entropy alloy electrocatalyst and preparation method thereof
ActiveCN110280255AHigh activityImprove conductivityCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsSpace groupPorous carbon
The invention relates to a nano-high-entropy alloy electrocatalyst and a preparation method thereof, and belongs to the technical field of new material preparation. The material is composed of a three-dimensional porous carbon substrate and FeCoNiCrCu high-entropy alloy nanoparticles loaded on the three-dimensional porous carbon substrate; the nanoparticles are an FeNi alloy structure monoclinic system, and the space group is Pm6; the molar ratio of Fe to Co to Ni to Cr to Cu is 1:1:1:1:1. The preparation method comprises the following steps: 1) dissolving a template agent-sodium chloride, a carbon source, and urea with deionized water, adding a doping source, magnetically stirring and freezing until totally solid, and then performing vacuum drying; 2) performing heat treatment and then cooling to room temperature to obtain powder; 3) washing, filtering and drying the powder to obtain the nano-high-entropy alloy electrocatalyst; 4) producing the nano-high-entropy alloy electrocatalyst into a working electrode and performing electrochemical performance test. The diameter of the nano-high-entropy alloy nanoparticles is 10 to 100 nm; according to a reaction of oxygen evolution through catalysis of the high-entropy alloy electrocatalyst, the initial potential is 1.50 to 1.63 V, the overpotential is 360 to 460 mV when the current density is 10 mA cm<-2>, and the Tafel slope is 70 to 120 mV dec<-1>.
Owner:东北大学秦皇岛分校
Real-time self-calibrating sensor system and method
A system and method for calibrating a sensor of a characteristic monitoring system in real time utilizes a self-calibration module for periodic determination of, and compensation for, the IR drop across unwanted resistances in a cell. A current-interrupt switch is used to open the self-calibration module circuit and either measure the IR drop using a high-frequency (MHz) ADC module, or estimate it through linear regression of acquired samples of the voltage across the sensor's working and reference electrodes (Vmeasured) over time. The IR drop is then subtracted from the closed-circuit value of Vmeasured to calculate the overpotential that exists in the cell (Vimportant). Vimportant may be further optimized by subtracting the value of the open-circuit voltage (Voc) across the sensor's working and reference electrodes. The values of Vmeasured and Vimportant are then controlled by respective first and second control units to compensate for the IR drop.
Owner:MEDTRONIC MIMIMED INC
Preparation method of carbon-coated transition metal phosphide composite material and application of carbon-coated transition metal phosphide composite material to oxygen evolution reaction
ActiveCN106552654AImprove performanceLow onset potentialPhysical/chemical process catalystsElectrodesVolumetric Mass DensityOxygen
The invention provides a preparation method of a carbon-coated transition metal phosphide composite material and application of the carbon-coated transition metal phosphide composite material to the oxygen evolution reaction. Firstly, a pointed transition metal hydroxide precursor containing anion intercalations of a phosphorus source and a carbon source between layers is prepared through synthesis, and then the podiform carbon-coated transition metal phosphide composite material is obtained through high-temperature roasting. The composite material is applied to the positive pole reaction-oxygen evolution reaction of electrolysis of water, can effectively improve the performance of a catalyst, namely lowering the take-off potential and improving catalytic activity, and is long in service life and good in stability. Meanwhile, raw materials of the composite material are low in cost and have abundant reserves, the preparation method is simple, and environment friendliness is achieved. Under the alkaline condition of 0.1-1 M KOH, the overpotential required by the electric current density of 10 mA / cm<2> is 280-340 mV, and the Tafel slope is 60-80 mV / dec; and under the constant voltage of 1.65 V, the circulation time is as long as 1-24 hours or longer.
Owner:BEIJING UNIV OF CHEM TECH
Ternary nickeliron phosphidenanosheet, preparation method thereof and application of electrolytic water
ActiveCN108083242ASimple preparation processEasy to operateMaterial nanotechnologyElectrolysis componentsElectrolysisElectrolytic capacitor
The invention provides a ternary nickeliron phosphidenanosheet.The nanosheet is a Ni<x>Fe<1-x>P<2> nanosheet grown on a substrate, x is greater than 0 and smaller than 1, the size of the nanosheet is500-1000nm, and the thickness is 10-30nm. The invention further provides a preparation method and application of the ternary nickeliron phosphidenanosheet. The nanosheethas good crystallinity, stablechemical property and high electrochemical activity. The nanosheet can be applied to electrochemical hydrogen evolution, and the overpotential is as low as 151mV when the current density reaches 10mA / cm<2>. The nanosheet can be applied to electrochemical oxygenevolution, and the overpotential is as low as 200mV when the current density reaches 10mA / cm<2>. The nanosheet has very good performance when being applied to electrochemical full-dissolved water, and the required impressed voltageis only 1.51V when the current density reaches 10mA / cm<2>.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Double-function negative electrode and applications of double-function negative electrode as all-vanadium flow battery negative electrode
ActiveCN104518221AReduces electrochemical polarizationIncrease working current densityMaterial nanotechnologyCell electrodesFormateCharge transfer resistance
The invention relates to an all-vanadium flow battery double-function negative electrode, wherein a carbon material is adopted as a substrate, a Bi-containing electro-catalyst is modified on the surface of the substrate, the Bi-containing electro-catalyst is one or more than two selected from a Bi elementary substance, Bi2O3, a Bi halide and a Bi metal salt, the Bi halide is bismuth fluoride, bismuth trichloride, bismuth bromide, or bismuth iodide, and the Bi metal salt is bismuth sulfate, bismuth nitrate, bismuth phosphate, bismuth formate or bismuth acetate. According to the present invention, the electrode is suitable for the negative electrode of the all-vanadium flow battery, the electrocatalysis activity and the electrochemical reversibility of the electrode material on the V<2+> / V<3+> oxidation reduction reaction can be substantially improved, and the charge transfer resistance can be reduced; and the high hydrogen evolution overpotential is provided so as to inhibit the occurrence of the hydrogen evolution reaction and prolong the service life of the battery.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Lead/carbon composite for storage battery and preparation method of lead/carbon composite material
ActiveCN103137955AIncrease the proportionAvoid uneven mixingCell electrodesCarbon compositesCharge current
The invention discloses a lead / carbon composite for a storage battery. The lead / carbon composite is prepared from the following raw materials in percent by weight: 85-99.9 percent of lead-salt solution and 0.1-15 percent of conductive carbon material, wherein the lead salt solution is prepared from a soluble lead salt and deionized water, and the mol concentration of the lead salt solution is 0.1-0.8M. The invention also discloses a preparation method of the lead / carbon composite for the storage battery. The lead / carbon composite is substantially a conductive carbon material modified by lead oxide. Lead has the characteristics of low resistivity, high plasticity and high hydrogen evolution overpotential. The characteristics of the lead ensure that the hydrogen evolution overpotential of the carbon material after modified by PbO in a lead-acid battery is remarkably increased; and meanwhile the charging current occupied by partial hydrogen evolution reaction in a charging process is reduced, and the charging efficiency is increased.
Owner:SHENZHEN CENT POWER TECH
Three-dimensional multi-layered structure cobalt-nickel-aluminum ternary metal electrocatalyst for oxygen evolution reaction as well as preparation and application methods thereof
ActiveCN108531938ALarge electrochemical active areaEasy to desorbElectrode shape/formsMetal-organic frameworkVolumetric Mass Density
The invention provides a three-dimensional multi-layered structure cobalt-nickel-aluminum ternary metal electrocatalyst for oxygen evolution reaction as well as a preparation and application method thereof. The electrocatalyst is based on layered double hydroxides (LDHs) and zeolite imidazole metal-organic frameworks (ZIF-67), nickel foam (NF) serves as a substrate, and a ZIF-67 / CoNiAl-LDH / NF three-dimensional multi-layed structure catalyst is synthesized through a two-step hydrothermal method. The electrocatalyst as well as the preparation and application method thereof have the advantages that through the introduction of the two-dimensional ZIF-67, the electrochemical active area of the catalyst can be improved, the formed unique three-dimensional multi-layed porous structure and a carrier with high specific surface area and abundant pore channels are beneficial for reducing the potential barrier of the oxygen evolution reaction, in addition, a super-hydrophobic interface is formed between a solid phase and a liquid phase, so that oxygen can be easily desorbed, the occurrence of the oxygen evolution reaction can be promoted, the electrocatalyst is applied to the oxygen evolutionreaction, the electric current density is 10 mA / cm<2>, the reaction overpotential is 0.3-0.41V, the Tafel slope is 88-245 mV / dec<-1>, in addition, the stability is good, and a good application prospect can be achieved.
Owner:BEIJING UNIV OF CHEM TECH
Transition metal nitride/carbon electrocatalyst and preparation method and application of transition metal nitride/carbon electrocatalyst
ActiveCN108671953AImprove electrocatalytic performanceImprove HER performancePhysical/chemical process catalystsElectrodesElectrolysisHydrogen
The invention relates to electrochemical power sources and the field of electrochemical catalysis, and particularly relates to a transition metal nitride / carbon electrocatalyst. The transition metal nitride / carbon electrocatalyst comprises a substrate and a shell, the substrate is coated by the shell, and the shell comprises copper nitride and further comprises a carbon material or a nitrogen doped carbon material. The invention further provides a preparation method of the electrocatalyst, and the electrocatalyst is prepared by forming MOF of copper in advance, then further aminating and calcining through one step, and preparing the electrocatalyst. The processes of oxygen precipitation and hydrogen precipitation show the low overpotential in the alkaline environment, and the electrocatalyst can be used as a catalyst material of alkaline efficient electrolysed water.
Owner:CENT SOUTH UNIV
Cobalt-carbon porous nanocomposite oxygen reduction electrocatalyst and its preparation method and application
The invention discloses a metal organic skeleton compound-based cobalt-carbon porous nanocomposite oxygen reduction electrocatalyst and its preparation method and application. The cobalt-carbon porous nanocomposite oxygen reduction electrocatalyst is prepared from a metal organic skeleton compound [Co(O-BDC)(bbp)] as a precursor through high-temperature calcination. The carbon material has a porous structure. A cobalt element is uniformly supported on the carbon material. The oxygen reduction electrocatalyst has high electrical conductivity and a large specific surface area and effectively reduces the overpotential of oxygen reduction. Through a rotating disk electrode and a rotating ring electrode, it is proved that the oxygen reduction process is an ideal 4 electron oxygen reduction catalytic process. The electrocatalyst fully performs synergism of cobalt and a carbon material in electrocatalysis and has great theoretical and practical significance for the development of novel electrochemical catalysts and energy conversion and storage devices.
Owner:安徽伏碳科技有限公司
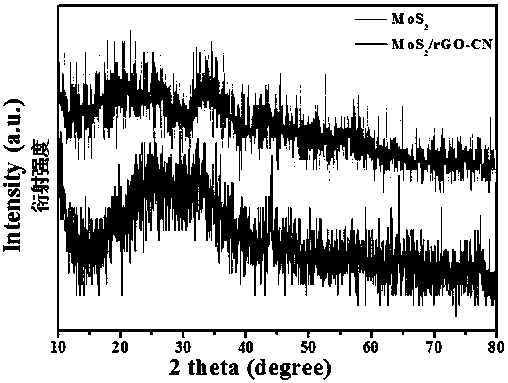
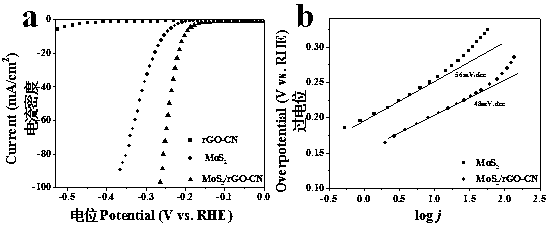
Preparation method and application of MoS2/rGO-CN composite material
ActiveCN107670679AReduce accumulationImprove conductivityPhysical/chemical process catalystsElectrodesN dimethylformamideDissolution
The invention belongs to the technical field of electrocatalytic hydrogen evolution, and relates to a preparation method and application of a molybdenum disulfide / reduced graphene oxide-nitrogen carbide (MoS2 / rGO-CN) composite material. The preparation method comprises the steps of: firstly adding graphite oxide into deionized water, adding melamine into the obtained mixture, then performing ultrasound dissolution so as to form a colloidal solution, adopting a hydrothermal method to prepare aerogel of reduced graphene oxide-nitrogen carbide, and then performing a solvothermal reaction to obtain the target product by adopting ammonium tetrathiomolybdate as a molybdenum source and a sulfur source and N,N-dimethylformamide as a solvent. The preparation method of the aerogel of the reduced graphene oxide-nitrogen carbide is simple and high in yield, and since the MoS2 / rGO-CN is prepared by using the one-step solvothermal method, the preparation method has low cost and high repeatability and facilitates large-scale synthesis; by means of the prepared MoS2 / rGO-CN composite material, the accumulation of the molybdenum disulfide is reduced, and the quantity of active sites is increased; the conductivity and the active area of the MoS2 can be improved through the combination of the MoS2 with the rGO-CN, and when the prepared MoS2 / rGO-CN composite material is applied to an electrocatalytic hydrogen evolution reaction, excellent catalytic performance can be exhibited, and when the current density is 10 mA.cm<-2>, the overpotential is 203 mV, and the Tafel slope is 48 mV.dec<-1>.
Owner:JIANGSU UNIV
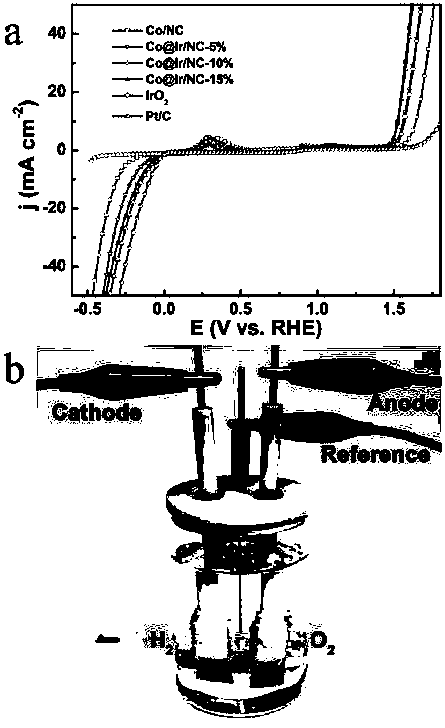
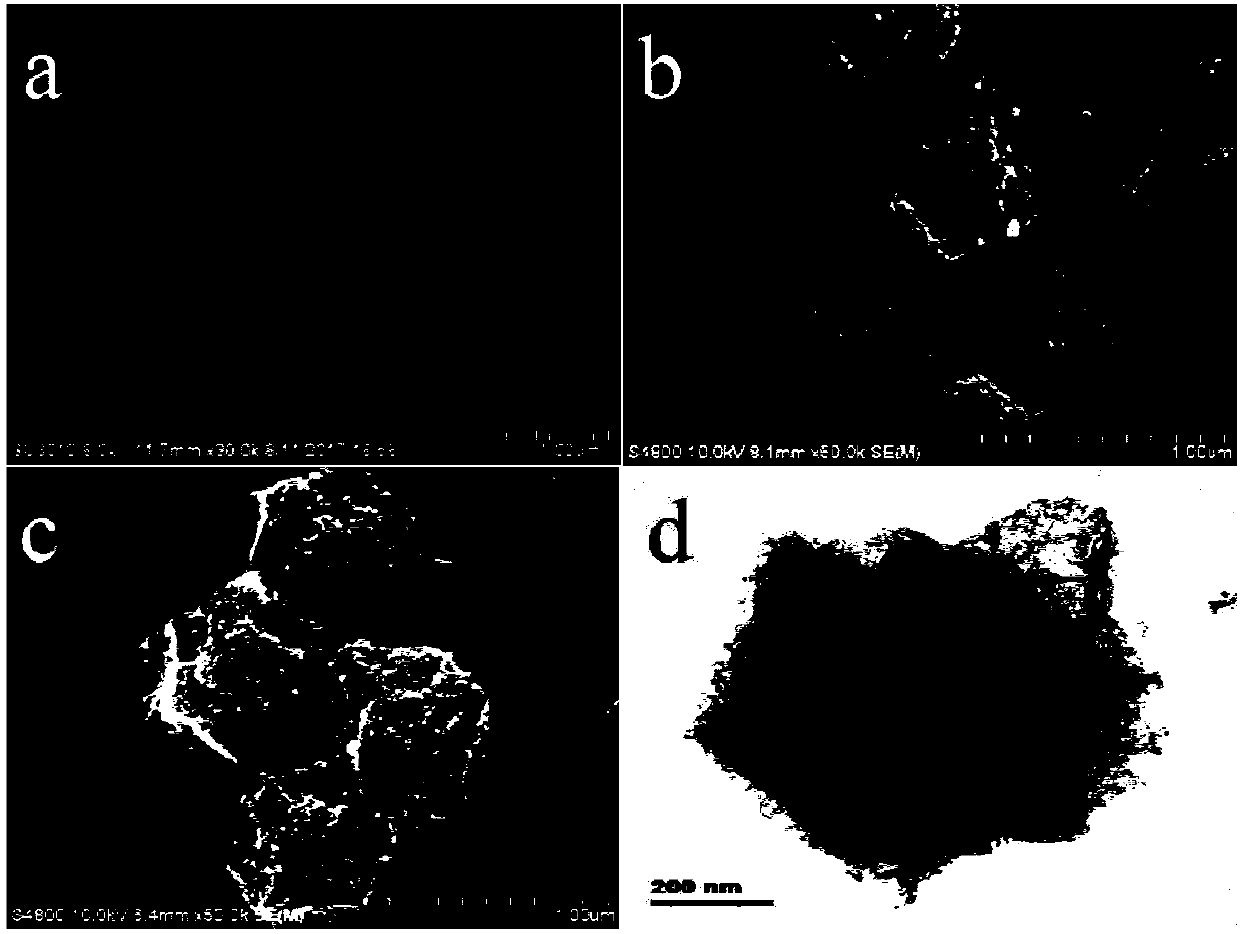
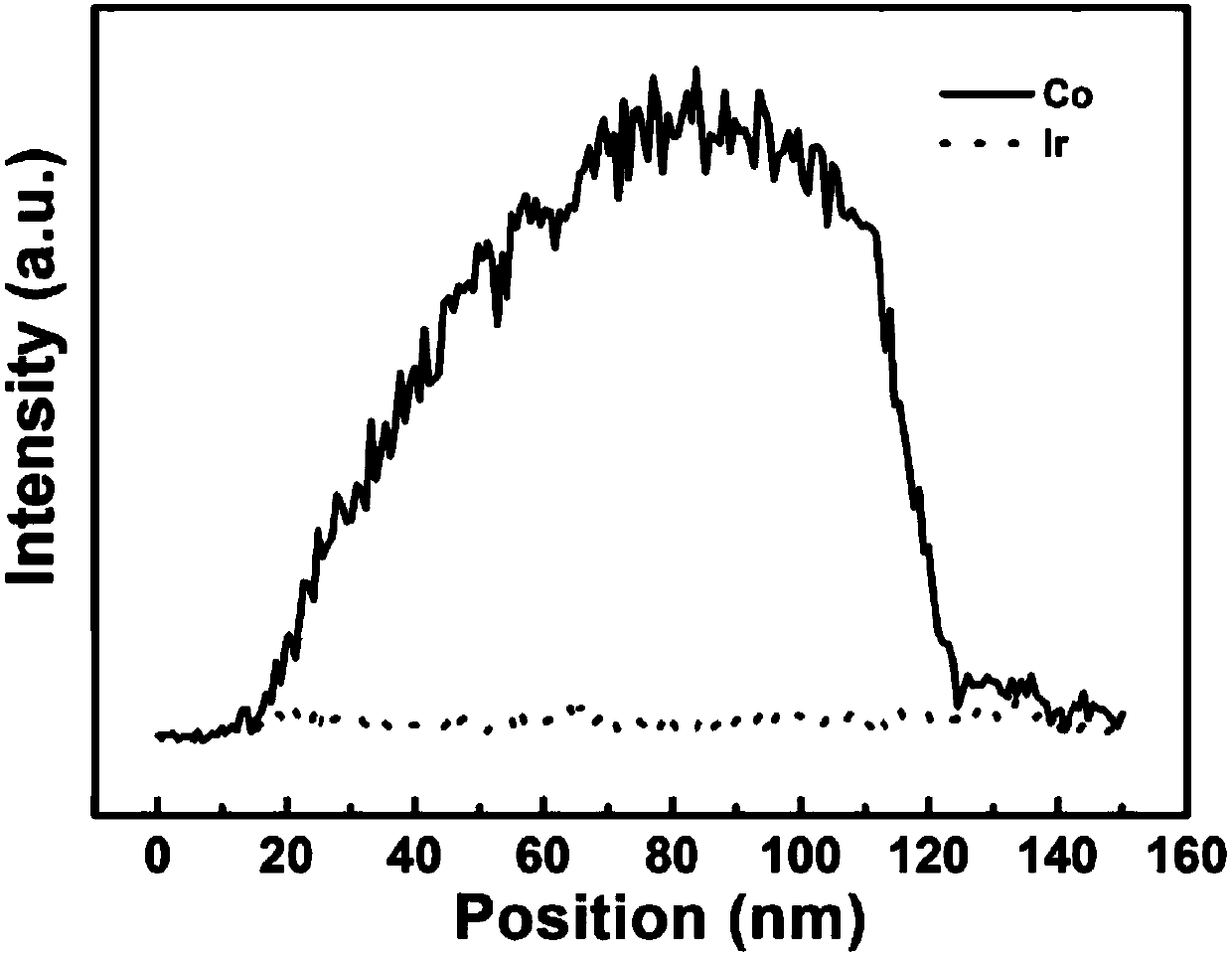
Preparation method of N-doped porous carbon coated nano-particles of Co-Ir core-shell structure and application of N-doped porous carbon coated nano-particles of Co-Ir core-shell structure to catalytic water splitting
InactiveCN108048866AThe preparation process is simple and straightforwardReduce energy consumptionTransportation and packagingMetal-working apparatusIridiumPorous carbon
The invention discloses a preparation method of N-doped porous carbon coated nano-particles (Co@Ir / NC-x, and x is the quality ratio of Ir) of a Co-Ir core-shell structure and application of the N-doped porous carbon coated nano-particles of the Co-Ir core-shell structure to catalytic water splitting. The preparation method has the advantages that (1) the preparation process is simple and direct, and energy consumption is low, specifically, Co / NC obtained after a zeolite imidazole framework material (ZIF-67 for short) is calcined is directly subjected to Galvanic replacement with Ir<3+> at theroom temperature, high temperature and high pressure are not needed, and accordingly the energy consumption is low; (2) the catalytic performance is good, and the stability is high, specifically, a Co@Ir / NC-10% sample is in a 1M KOH solution, in an oxygen producing test, the current density is 10 mA cm<-2>, the overpotential is 280 mV, and the performance is higher than IrO2; in a hydrogen producing test, the current density is 10 mA cm<-2>, the overpotential is -121 mV; besides, after a stability test of 12 h, the oxygen producing activity of IrO2 is attenuated by 55.8% while the oxygen producing activity of Co@Ir / NC-10% is attenuated only by 20.6%, and the hydrogen producing stability of Co@Ir / NC-10% is far higher than that of commercial Pt / C under the same conditions; and (3) the catalyst cost is low and the Co source is wide, specifically, the nano-particles are of the core-shell structure with Co as a core and Ir as a shell, the amount of Ir is reduced on the basis of more exposedcatalytic activity sites, a core metal precursor Co is wide in source and low in cost, the catalyst cost is greatly reduced, and great commercial application prospects are achieved.
Owner:SOUTH CHINA UNIV OF TECH
Positive grid alloy of lead-acid battery and manufacturing method of alloy
ActiveCN103199263AReduce severe dehydrationLower reduction potentialElectrode carriers/collectorsAlloyOverpotential
The invention discloses a positive grid alloy of a lead-acid battery and a manufacturing method of the alloy. The alloy includes lead, calcium, tin, aluminum and silver. The positive grid alloy containing silver of the lead-acid battery is helpful to the reduction of reduction potentials of Pb and PbSO4, thereby enabling Pb2+ to be easily reduced into metal Pb. The silver has an inhibiting effect on the formation of PbO when a constant potential is polarized, the intensity and the creeping strength of the positive grid alloy are improved, and an over-aging effect of the positive grid alloy in a use process is slowed down; and the addition of the sliver can inhibit the increase of a corrosive layer of the positive grid alloy, thereby facilitating the deep discharge cycle of the lead-acid battery, and preventing capacity fading phenomena in the early stage. Meanwhile, through the addition of the sliver, hydrogen evolution overpotentials are enhanced, the separation of hydrogen on a cathode of the alloy is inhibited, the problem of serious water loss of the battery is reduced, and the cycle life of the battery is prolonged. Moreover, the manufacturing process of the alloy is simple.
Owner:CHERY AUTOMOBILE CO LTD
Device and method for producing hydrogen and generating electricity
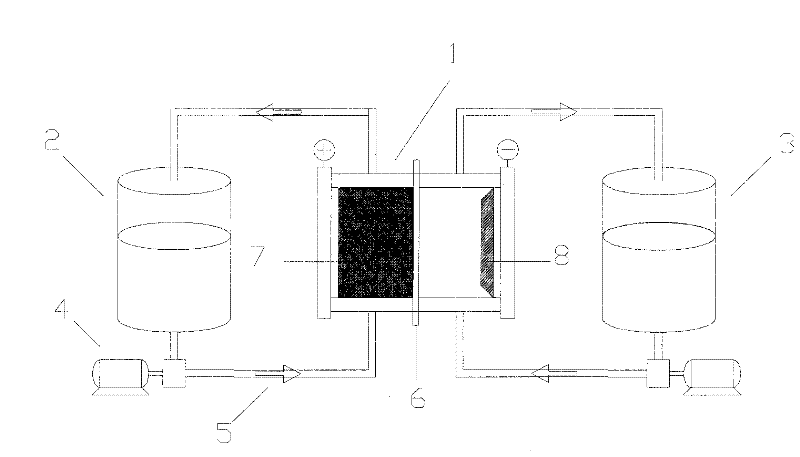
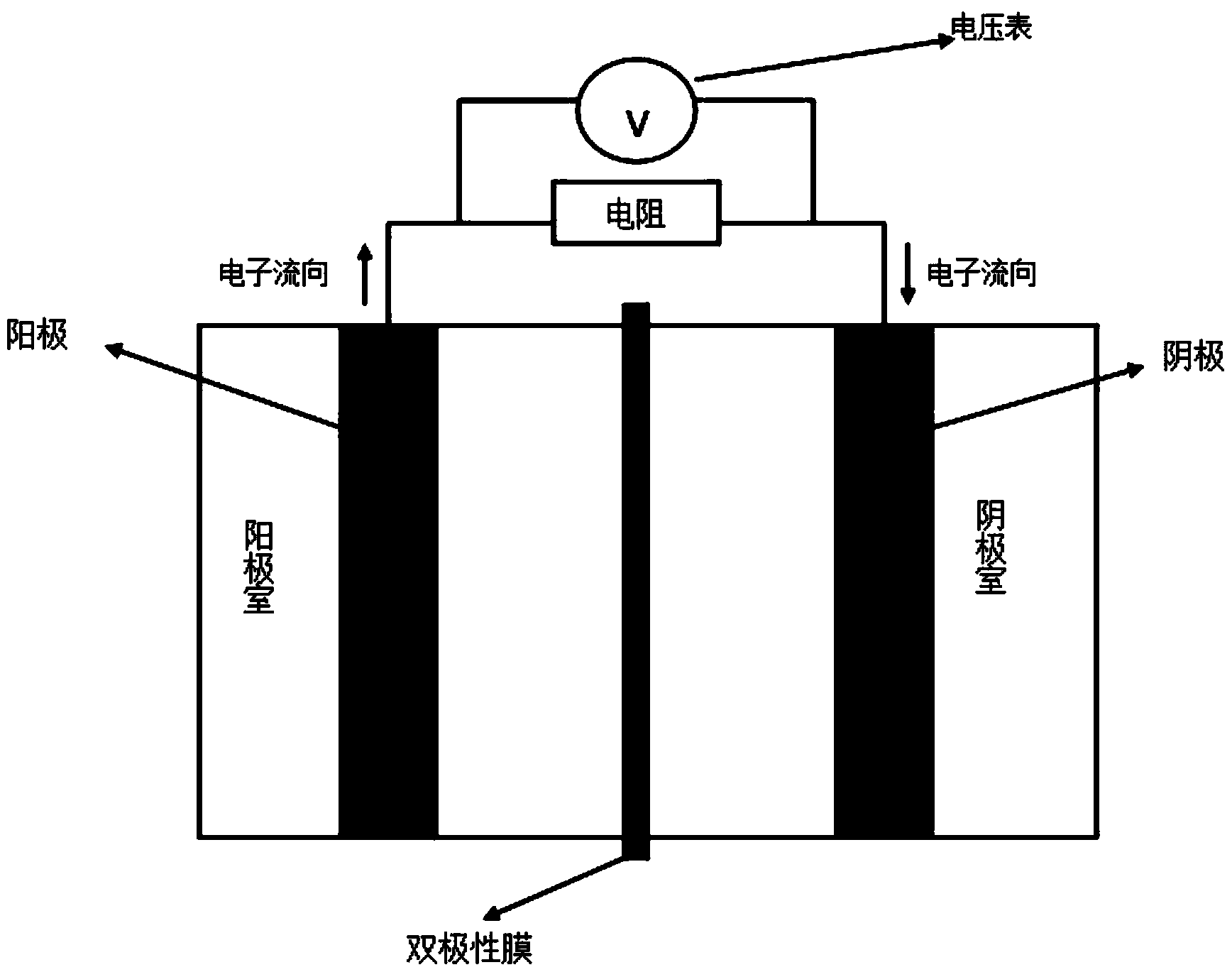
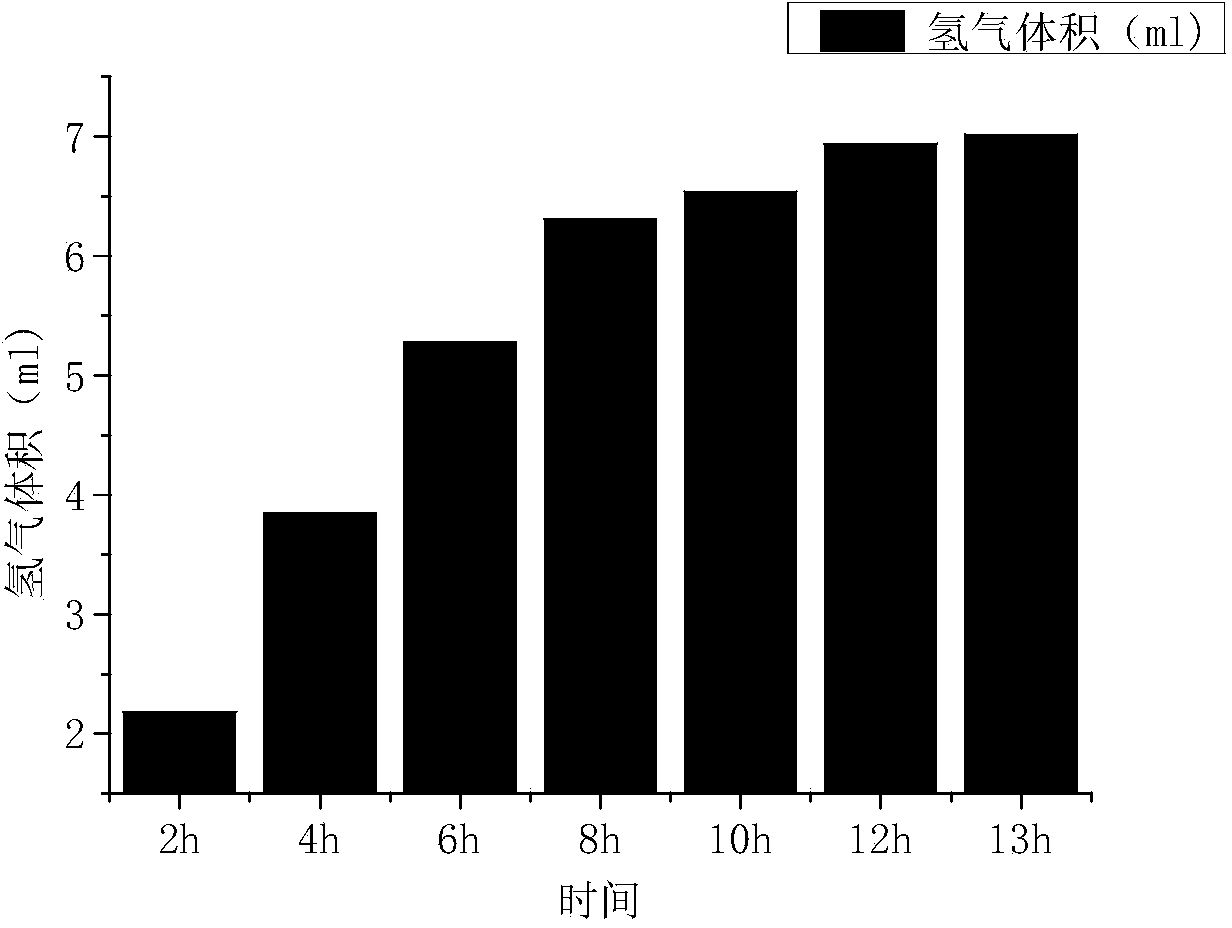
ActiveCN103820807ASave energyRealize external power supplyElectrolysis componentsChemical industryElectricityHydrogen
The invention discloses a device and a method for producing hydrogen and generating electricity. The device comprises an electrochemical tank and an ion exchange membrane arranged inside the electrochemical tank, wherein the electrochemical tank is divided into an anode chamber and a cathode chamber by the ion exchange membrane; an anode and a cathode are arranged in the anode chamber and the cathode chamber respectively, and are connected with a resistance through a wire. The method and the device are characterized in that the anode comprises a catalyzer which can electro-oxidizing corresponding substrates with high efficiency, and an anode base; the cathode is made of a material with lower hydrogen evolution overpotential; the substrates and a neutral or alkaline electrolyte solution are accommodated in the anode chamber, a neutral or acidic electrolyte solution is accommodated in the cathode chamber, and the substrates are catalyzed and oxidized by the catalyzer, and then produce hydrogen under the action of the cathode chamber. The device and the method for producing hydrogen and generating electricity can produce hydrogen in the case that direct current is not provided, and can generate electricity; in addition, the device and the method can be used as a storage technology for hydrogen, and have favorable application prospects.
Owner:DONGGUAN WOJIESEN WATER TREATMENT
3D lithium-philic porous metal current collector, negative electrode, and preparation and application thereof
ActiveCN110828829AImprove cycle lifeTake advantage ofElectrode carriers/collectorsLi-accumulatorsLithium metalOverpotential
The invention belongs to the field of a lithium battery electrode material and particularly discloses a 3D lithium-philic porous metal current collector. The current collector comprises a 3D porous metal current collector and at least one of gold, silver and platinum compounded on a framework of the 3D porous metal current collector. The invention further discloses a preparation method and an application of the metal current collector. A 3D lithium-philic porous lithium ion negative electrode is particularly prepared. The current collector is advantaged in that at least one metal of gold, silver and platinum on the porous metal framework reduces the overpotential in the lithium metal nucleation and deposition process, uniform deposition and dissolution of lithium metal in the continuous cycle process are realized, growth of dendrites is effectively avoided, and the cycle life of a lithium metal battery is greatly prolonged.
Owner:CENT SOUTH UNIV
Porous-carbon loaded metal composite material and preparing method and application thereof
ActiveCN105642326AExcellent Catalytic Electrochemical Hydrogen and Oxygen ProductionExcellent total water splitting performancePhysical/chemical process catalystsElectrodesPorous carbonDecomposition
The invention provides a porous-carbon loaded metal composite material and a preparing method and application thereof. The method includes the steps that NiMoO4 nanometer rods and carbon-source monomers are reacted in Tris reagent solutions to obtain NiMoO4 / carbon source precursors; the NioMO4 / carbon source precursors are calcined to obtain the porous-carbon loaded metal composite material. The porous-carbon loaded metal composite material comprises porous-carbon carriers, nickel and molybdenum carbide, wherein the nickel and the molybdenum carbide are loaded to the porous-carbon carriers. The method is simple in step and easy to operate, and has the advantages of being convenient, rapid and the like. The porous-carbon loaded nickel and molybdenum carbide composite material has the excellent catalyzing electrochemistry hydrogen-producing and oxygen-producing performance and the excellent full water decomposition performance. An experiment shows that when the composite material serves as a catalyst in a hydrogen producing reaction, and when the overpotential is 0.25 V, the electric current density can be 52 mA / cm<2>; in a full hydrolysis reaction, when the electric potential is 1.68 V, the electric current density can be 10 mA / cm<2>, the performance is excellent, and the porous-carbon loaded metal composite material has the good application prospect in the field of electro-catalysis hydrogen producing and oxygen producing and the field of full water decomposition.
Owner:UNIV OF SCI & TECH OF CHINA
Flexible three-dimensional porous nitrogen-doped carbon nanotube/cobalt phosphide composite material and preparation method and application thereof
InactiveCN109055962ALow costThe synthesis process is simpleElectrode shape/formsElectrolysisGas phase
The invention discloses a flexible three-dimensional porous nitrogen-doped carbon nanotube / cobalt phosphide composite material and a preparation method and application thereof. According to the flexible three-dimensional porous nitrogen-doped carbon nanotube / cobalt phosphide composite material and the preparation method and application thereof, low-cost commercial melamine sponges are used as a three-dimensional carbon material skeleton template, a zeolite imidazole structure metal organic framework is used as a cobalt source, phosphorization is carried out by a low-temperature vapor deposition method, and the flexible three-dimensional porous nitrogen-doped carbon nanotube / cobalt phosphide composite material with high conductivity and a high specific surface area is obtained. The preparation process of the flexible three-dimensional porous nitrogen-doped carbon nanotube / cobalt phosphide composite material is relatively simple, the cost is low, and the preparation process is suitable for industrial application. The flexible three-dimensional porous nitrogen-doped carbon nanotube / cobalt phosphide composite material is used as an electrode material for hydrogen evolution catalysis ofelectrolyzed water, and has lower hydrogen evolution catalysis overpotential, a larger electrocatalytic active area and excellent cycle stability.
Owner:ZHONGBEI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com