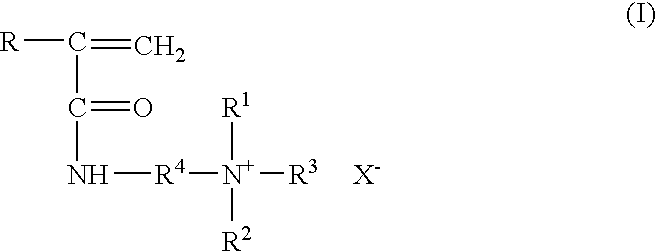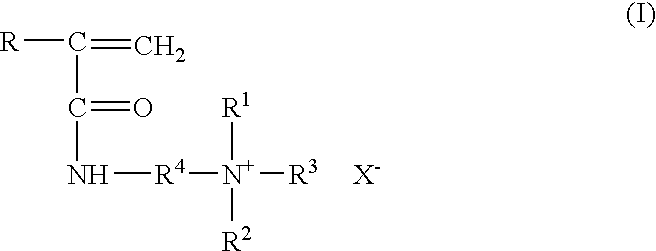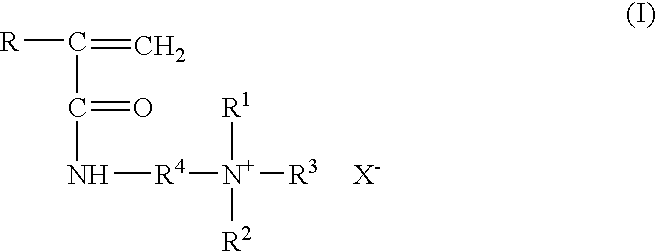Patents
Literature
194 results about "Zinc bromide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
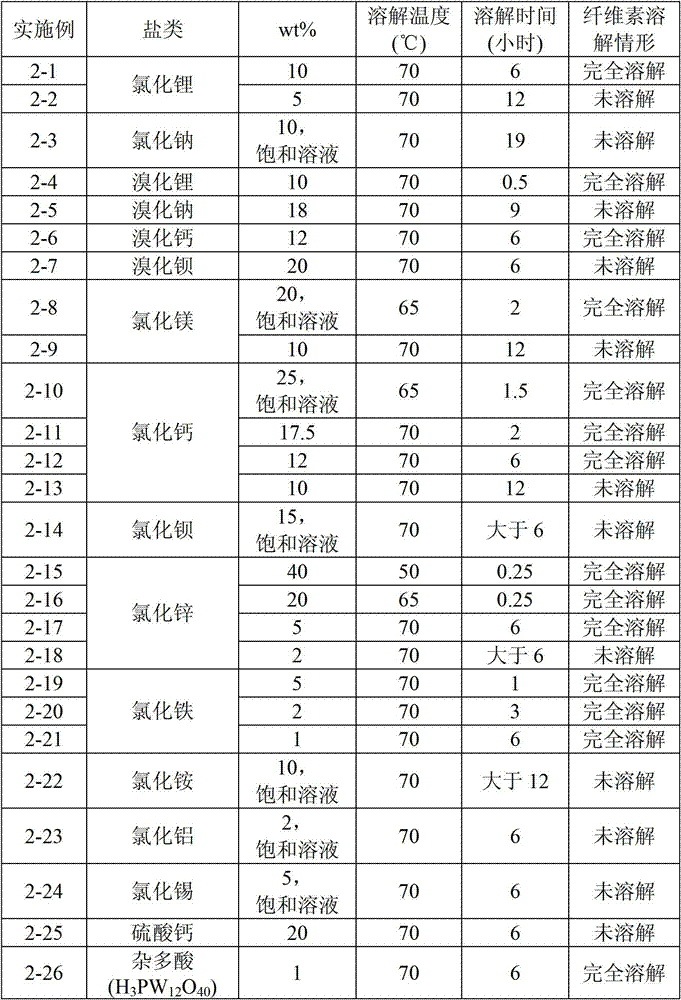
Zinc bromide (ZnBr₂) is an inorganic compound with the chemical formula ZnBr₂. It is a colourless salt that shares many properties with zinc chloride (ZnCl₂), namely a high solubility in water forming acidic solutions, and solubility in organic solvents. It is hygroscopic and forms a dihydrate ZnBr₂ · 2H₂O.
Scaling inhibitors and method for using the same in high density brines
InactiveUS20050067164A1Promote resultsParticular in controlCleaning apparatusFluid removalSolubilityZinc bromide
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. The formulation has particular application in the removal of zinc sulfide and iron sulfide scales formed when zinc bromide brines are used as completion fluids. The copolymer exhibit high solubility in high-density brines, such as zinc bromide brines. The polymers may be introduced into an oil or gas well as a portion of a carrier fluid or with brine. The preferred copolymer for use in the invention contains an acrylamide unit and a diallyldimethylammonium salt and, optionally, an acrylic acid or a salt thereof. The weight average molecular weight of such inhibitor copolymers is generally between from about 500,000 to about 5,000,000.
Owner:BAKER HUGHES INC
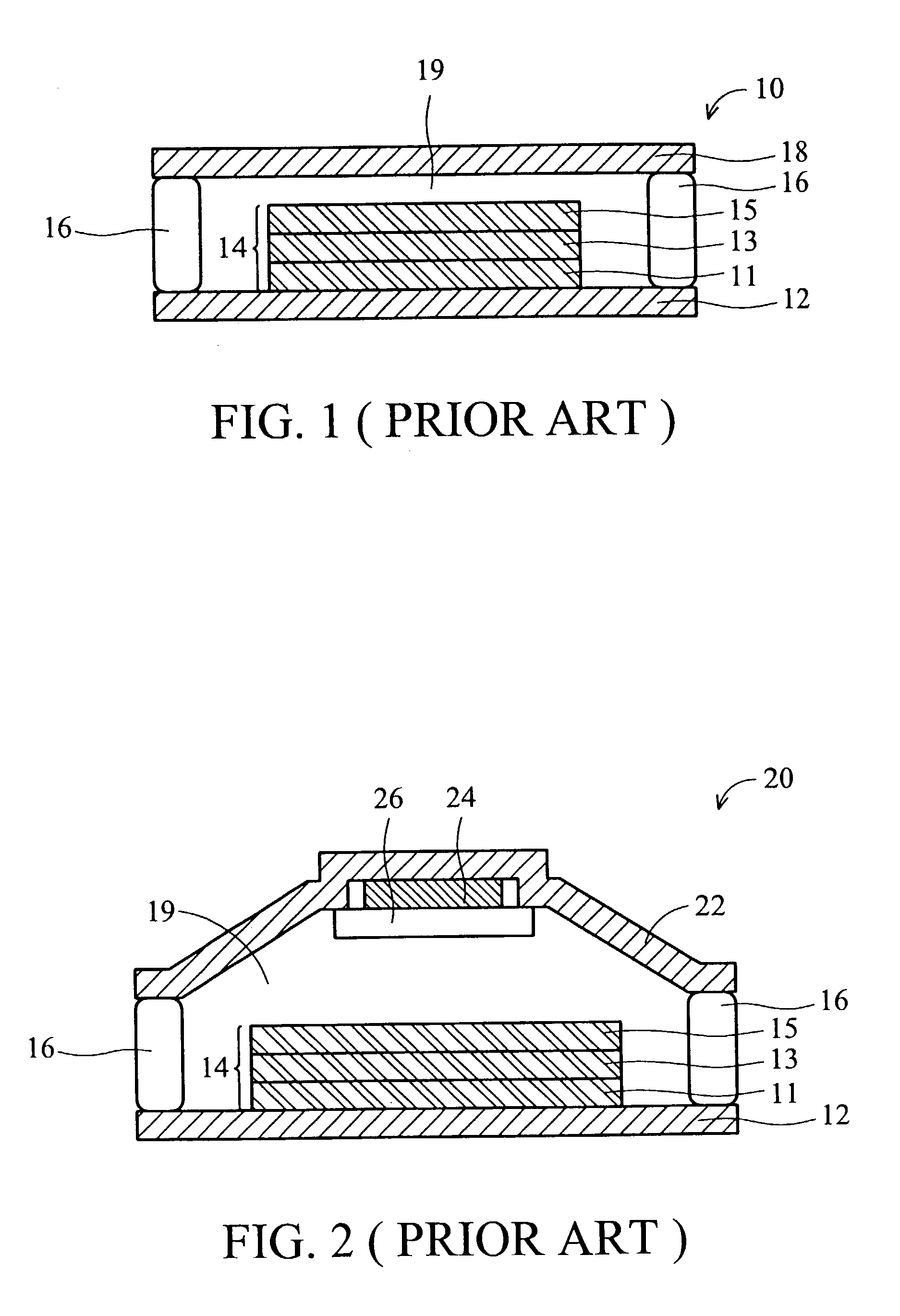
Packaging material used for a display device and method of forming thereof
InactiveUS20040084686A1Non-macromolecular adhesive additivesSemiconductor/solid-state device detailsZinc bromideAlkaline earth metal
A packaging material used for a display device which is a desiccative-containing adhesive agent. The desiccative-containing adhesive agent is composed of a liquid-state organic material selected from a group including epoxy resin, polyurethane, bakelite, polyamide, acrylic resin and polysiloxane, and a solid-state desiccative selected from a group including alkaline metal oxide, alkaline-earth metal oxide, metallic halide, barium oxide, calcium oxide, calcium sulfate, calcium chloride, lithium chloride, calcium bromide, potassium Carbonate, aluminum oxide, magnesium oxide, copper sulfate, zinc chloride, zinc bromide, cobalt chloride, silica gel, zeolite and molecular sieve.
Owner:DELTA OPTOELECTRONICS +1
Zinc / polyhalide energy storage cell
InactiveCN102479968AEasy to moveIncrease energy densityRegenerative fuel cellsZinc bromideHigh energy
The invention discloses a zinc / polyhalide energy storage cell. An electrode of conductive inert material and an electrolyte solution of zinc chloride solution and zinc bromide solution are used to form a zinc / polyhalide energy storage cell. The zinc / polyhalide energy storage cell employs zinc and polyhalide with small electrochemical equivalent and high hydrogen evolution overpotential are used as active substances of the cell. Anode and cathode electric pair has high potential difference, and the two together decide that the cell technology has high energy density and power density. Compared with a zinc bromine liquid flow cell, a new system energy storage cell has energy density increased by 30%, power density increased by 10%, obviously enhanced removability and reduced cost of the cell system. The anode and the cathode of the cell employ electrolytes with a same element composition to avoid negative influence on cell performance and life caused by crossed contamination of electrolytes; the cell of the invention has advantages of long cycling life, low cost and good mobility and can be widely applied to fields of energy electric power, transportation and information communication, etc.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI +1
Method for inhibiting or controlling inorganic scale formations with copolymers of acrylamide and quaternary ammonium salts
InactiveUS7398824B1Improve efficiencyImprove thermal stabilityCleaning apparatusFluid removalZinc bromideMethyl group
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. Suitable copolymers include those comprising an acrylamide unit and a quaternary ammonium salt group, and optionally an acrylate and / or nitrogen heterocyclic monomer including those wherein the quaternary ammonium salt is a unit of the formula:wherein R is methyl or hydrogen; R4 is a C1 to C6 alkyl group, optionally substituted with halogen, hydroxyl and alkoxy groups, X is halogen; and R1, R2 and R3 are independently selected from the group consisting of alkyl and alkoxy groups. Suitable nitrogen heterocyclic compounds include N-vinylpyrrolidone, N-vinylformamide, N-vinylacetamide, N-vinylcaprolactam, N-vinylimidazole and N-vinylpyridine. The copolymers have particular applicability in the control and inhibition of zinc sulfide or iron sulfide scales, typically formed when zinc bromide brines are used as fluids in the treatment of a gas or oil well, such as a completion fluid.
Owner:BAKER HUGHES INC
Catalyst used in synthesis of propylene carbonate and preparation method and application thereof
ActiveCN102049303AHigh selectivityLow costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsZinc bromideHalogen
The invention relates to a catalyst used in synthesis of propylene carbonate from carbon dioxide and propylene oxide, and a preparation method and application thereof. The catalyst comprises the following components in percentage by weight: 5 to 15 percent of zinc salt, 15 to 25 percent of organic amine and 70 percent of silicon dioxide, wherein the zinc salt, halogen and the organic amine are used as active ingredients of the catalyst; SiO2 is used as a carrier; the zinc salt is zinc chloride or zinc bromide; the organic amine is aminopropyltriethoxysilane; the SiO2 carrier is prepared from tetraethoxysilane under an alkaline condition by hydrolysis; the catalyst has specific surface of between 160 to 250m<2> / g and pore volume of between 0.7 and 0.9cm<3> / g; the conversion rate of the propylene oxide can reach 90.2 percent, and selectivity of the propylene carbonate reaches 97.5 percent; and after the catalyst is repeatedly used for 4 times, the conversion rate of the propylene oxide is 89.1 percent, and the selectivity of the propylene carbonate is 97.6 percent.
Owner:PETROCHINA CO LTD
Method for preparing hexachlorobutadiene
InactiveCN101525267ASimple processLow equipment requirementsHalogenated hydrocarbon preparationZinc bromideHexachlorobutadiene
The invention provides a method for preparing hexachlorobutadiene by using 1, 1- dibromo-tetrafluoroethane, comprising: zinc powder and aprotic polar solvent are added into a reactor I protected by inert gas to lead the temperature to reach 50-90 DEG C; the 1, 1- dibromo-tetrafluoroethane is added into the reactor I within 0.5-3.5h and then reacts for 1.5-5h at the temperature of 50-120 DEG C; after that, the zinc powder reacts with the 1, 1- dibromo-tetrafluoroethane to produce trifluoro-ethylene zinc bromide; the reactor I is cooled to be room temperature, and the solution in the reactor I is added into a reactor II containing catalyst and solvent at the temperature below 1-40 DEG C within 0.5-3h and then reacts for 1-7h at the temperature of 0-60 DEG C; after the reaction, the obtained product is collected by a liquid nitrogen cold trap. The invention has the advantages of short synthetic route, simple technique, high raw material conversion rate, high yield of product and high purity of crude product, so that the product is simple to separate and purify, and the environmental pollution is reduced.
Owner:HENAN UNIVERSITY OF TECHNOLOGY
Method for inhibiting or controlling inorganic scale formations
InactiveUS7159655B2Favorable result inhibition and controlParticular in controlCleaning apparatusFluid removalZinc bromideSolubility
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. The formulation has particular application in the removal of zinc sulfide and iron sulfide scales formed when zinc bromide brines are used as completion fluids. The copolymer exhibit high solubility in high-density brines, such as zinc bromide brines. The polymers may be introduced into an oil or gas well as a portion of a carrier fluid or with brine. The preferred copolymer for use in the invention contains an acrylamide unit and a diallyldimethylammonium salt and, optionally, an acrylic acid or a salt thereof. The weight average molecular weight of such inhibitor copolymers is generally between from about 500,000 to about 5,000,000.
Owner:BAKER HUGHES INC
New synthesis process of canagliflozin
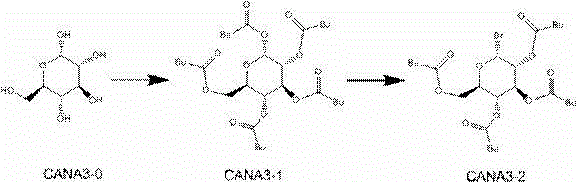
The present invention discloses a new synthesis process of canagliflozin. The new synthesis process comprises: adopting 2-methyl benzoic acid as a starting raw material, and adopting a self-made catalyst, iodic acid and iodine to carry out a reaction to produce an intermediate 1, or adopting 2-methyl benzoic acid as a starting raw material, and adding liquid bromine under effects of a metal reagent and a catalyst to synthesize an intermediate 2; optionally selecting the intermediate 1 or 2 to carry out an acylation reaction with thionyl chloride, and then carrying out a Friedel-Crafts reaction to produce an intermediate 3; adopting ALPHA-D-glucose as a raw material, carrying out a reaction with pivaloyl chloride to protect all hydroxyl, and carrying out a reaction with zinc bromide and bromotrimethylsilane to produce an intermediate 4; linking the intermediate 3 and the intermediate 4 to produce an intermediate 5; and finally under an acid condition, removing the pivaloyl to produce the target compound. The new synthesis process has characteristics of high yield, mild condition, safety, reliability, cheap and easily available raw material, and easy production cost control, and is suitable for industrial production.
Owner:HAIMEN RUIYI MEDICAL TECH
Preparation method of graphene-ZnO nanoparticle composite material
ActiveCN103482614ASmall grain sizeSimple methodMaterial nanotechnologyZinc oxides/hydroxidesZinc bromideFiltration
The invention discloses a preparation method of a graphene-ZnO nanoparticle composite material. The preparation method comprises the following steps: step 1): dissolving raw materials, namely graphene oxide and zinc salt, in water according to a certain mass ratio and uniformly mixing; step 2): separating the graphene oxide from the well mixed liquid, obtained in the step 1, by centrifugation or suction filtration and washing with water or alcohol; and step 3) performing heat treatment on the product, obtained in the step 2, in air or oxygen to get the graphene-ZnO nanoparticle composite material, wherein the zinc salt is one of zinc fluoride, zinc chloride, zinc bromide, zinc sulfate, zinc nitrate, zinc acetate and zinc phosphate, the heat treatment temperature is 150-1000 DEG C, and the heat treatment time is 5s-10h. The preparation method disclosed by the invention is simple, green and environment-friendly, and can effectively reduce the use of chemical reagents.
Owner:SOUTHEAST UNIV
Electrolyte for rechargeable electrochemical cell
ActiveUS20170194666A1Increased durabilityImprove stabilityLarge-sized flat cells/batteriesFinal product manufacturePolymer chemistryElectrolytic agent
The present invention provides an aqueous electrolyte for use in rechargeable zinc-halide storage batteries that possesses improved stability and durability and improves zinc-halide battery performance. One aspect of the present invention provides an electrolyte for use in a secondary zinc bromine electrochemical cell comprising from about 30 wt % to about 40 wt % of ZnBr2 by weight of the electrolyte; from about 5 wt % to about 15 wt % of KBr; from about 5 wt % to about 15 wt % of KCl; and one or more quaternary ammonium agents, wherein the electrolyte comprises from about 0.5 wt % to about 10 wt % of the one or more quaternary ammonium agents.
Owner:EOS ENERGY TECH HLDG LLC
Method for preparing L-menthol
InactiveCN103086845AAvoid purification difficultiesAvoid Yield ProblemsPreparation by isomerisationMolecular sieve catalystsZinc bromideMolecular sieve
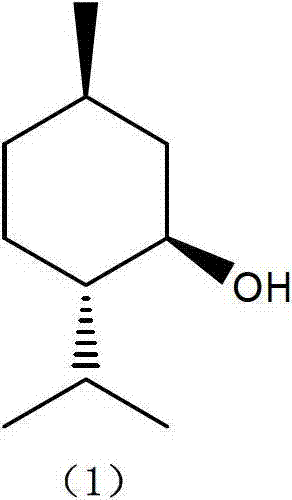
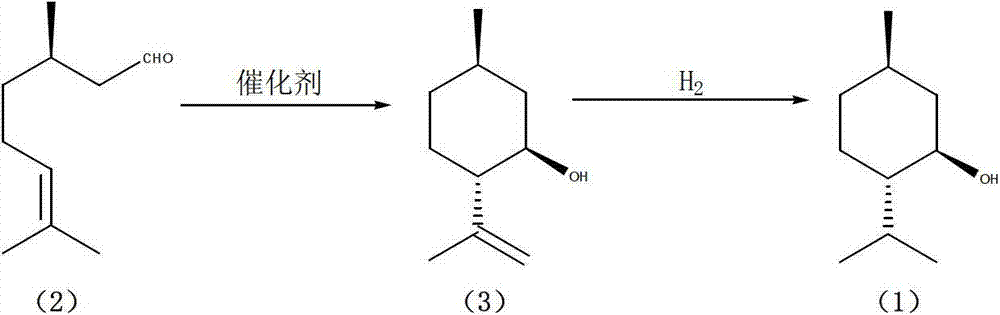
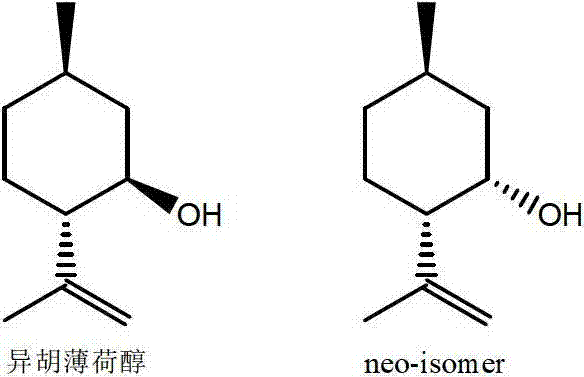
The invention discloses a method for preparing L-menthol, which comprises the following steps:(1) reacting d-citronellal in a solvent in the presence of a catalyst, and collecting isopulegol from the reaction product, wherein the catalyst is a zinc-bromide-modified NaY molecular sieve; and carrying out high-pressure hydrogenation on the obtained isopulegol to obtain the target product L-menthol. The obtained isopulegol can be subjected to high-pressure hydrogenation to obtain the target product L-menthol without purification. The invention has the characteristics of mild reaction conditions, high stereoselectivity, high yield and the like, and is simple to operate; and the catalyst is simple to recover, and can be used repeatedly. The method disclosed by the invention avoids the problems of difficulty in product purification, low yield and the like in the traditional synthesis technique of the compounds, and greatly lowers the production cost.
Owner:上海统益生物科技有限公司
Method for preparing cyclic carbonate by taking NHC/ZnBr2 system as catalyst
InactiveCN102702165AOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsZinc bromideSynthesis methods
The invention discloses a method for preparing a cyclic carbonate by taking a NHC / ZnBr2 system as a catalyst, which comprises the step of catalyzing epoxide and carbon dioxide to synthesize the corresponding cyclic carbonate by cycloaddition by taking the NHC / ZnBr2 system consisting of N, N'-(2,6-diisopropylphenyl)-imidazoline hydrochloride, potassium carbonate and zinc bromide as the catalyst, wherein the reaction pressure is 0.1 MPa, the reaction temperature is 80 DEG C, the reaction time is 24 h and the reaction solvent is DMSO. The synthesis method has the characteristics of mild reaction condition, high catalytic activity, single product, simplicity in purification operation and the like.
Owner:XUZHOU NORMAL UNIVERSITY
Selective liquid phase air oxidation of toluene catalysed by composite catalytic system
InactiveUS6743952B2Preparation by oxidation reactionsOrganic compound preparationRecyclable catalystZinc bromide
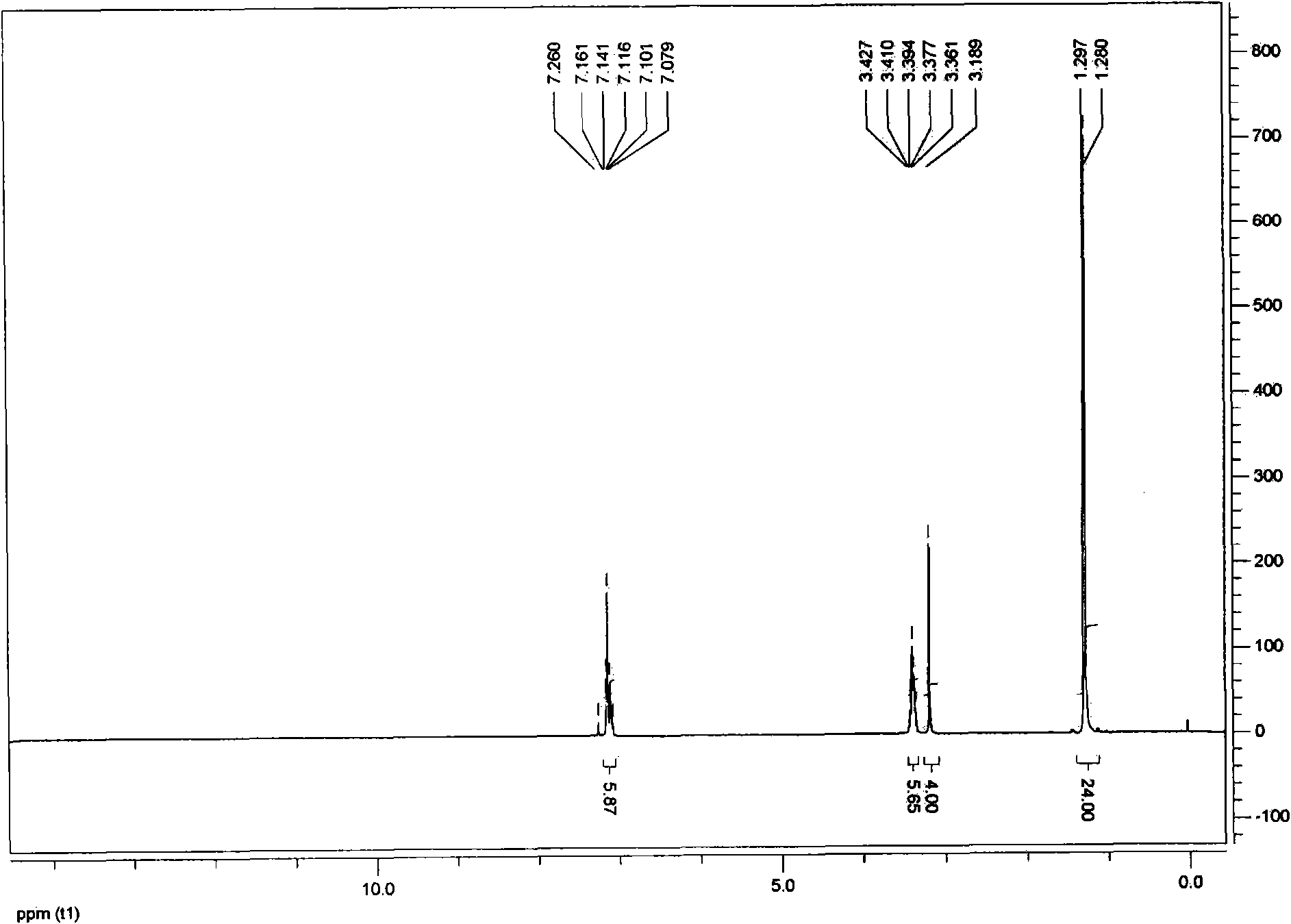
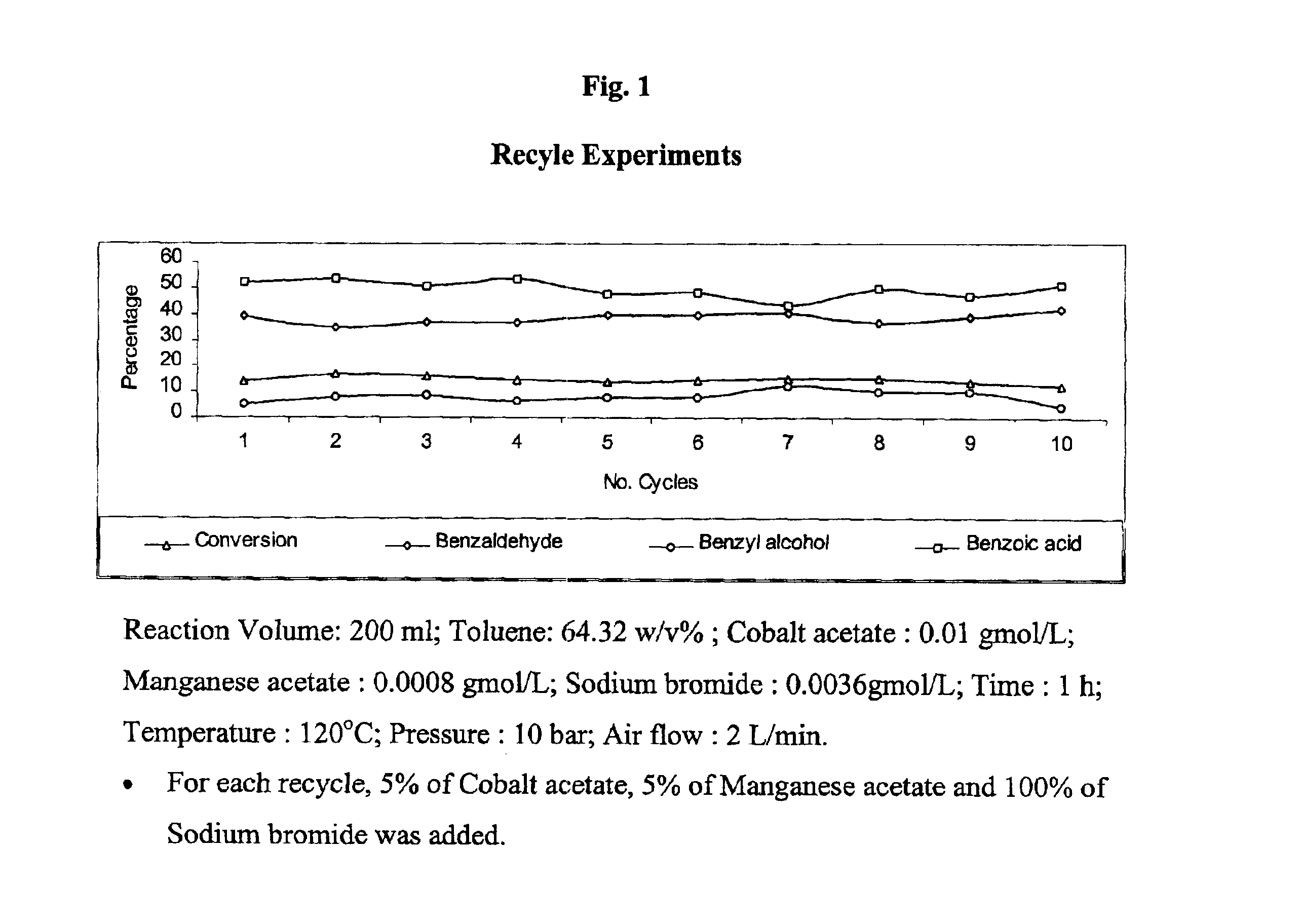
The present invention provides an improved process for the production of benzaldehyde with 40-50% selectivity by the catalytic liquid phase air oxidation of toluene using a composite catalytic system comprising salts of iron, cobalt, manganese, molybdenum or nickel as recyclable catalyst, salts of manganese or copper as recyclable co-catalyst and cobalt bromide, sodium bromide, sodium chloride and zinc bromide as promoter.
Owner:COUNCIL OF SCI & IND RES
Scaling inhibitors and method for using the same in high density brines
InactiveUS20070175635A1Promote resultsParticular in controlCleaning apparatusFluid removalSolubilityZinc bromide
A formulation containing a copolymer derived from a cationic monomer effectively inhibits and controls the formation of inorganic scales. The formulation has particular application in the removal of zinc sulfide and iron sulfide scales formed when zinc bromide brines are used as completion fluids. The copolymer exhibit high solubility in high-density brines, such as zinc bromide brines. The polymers may be introduced into an oil or gas well as a portion of a carrier fluid or with brine. The preferred copolymer for use in the invention contains an acrylamide unit and a diallyldimethylammonium salt and, optionally, an acrylic acid or a salt thereof. The weight average molecular weight of such inhibitor copolymers is generally between from about 500,000 to about 5,000,000.
Owner:BAKER HUGHES HLDG LLC
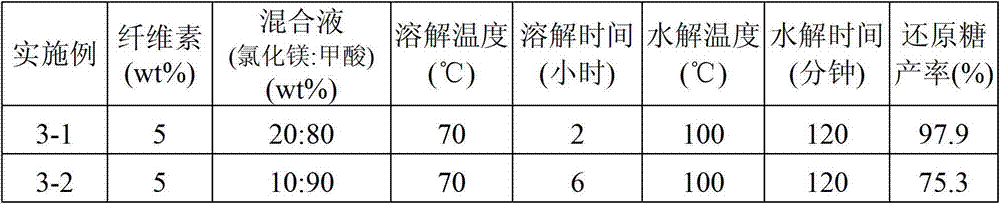
Sugar products and fabrication method thereof
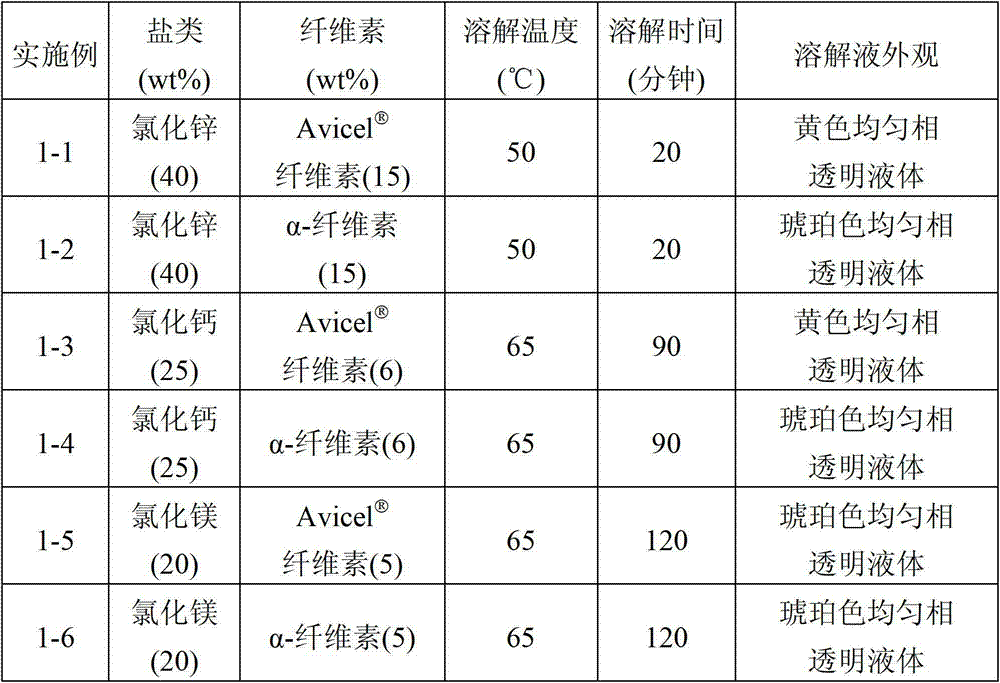
In an embodiment of the present disclosure, a method for fabricating a sugar product is provided. The method includes mixing formic acid and lithium chloride, magnesium chloride, calcium chloride, zinc chloride or iron chloride or lithium bromide, magnesium bromide, calcium bromide, zinc bromide or iron bromide or heteropoly acid to form a mixing solution, adding a cellulosic biomass to the mixing solution for a dissolution reaction, and adding water to the mixing solution for a hydrolysis reaction to obtain a sugar product. The present disclosure also provides a sugar product fabricated from the method.
Owner:IND TECH RES INST
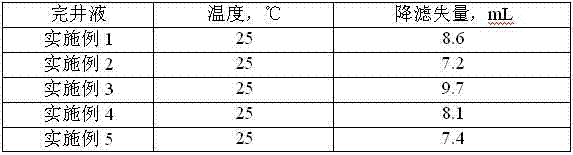
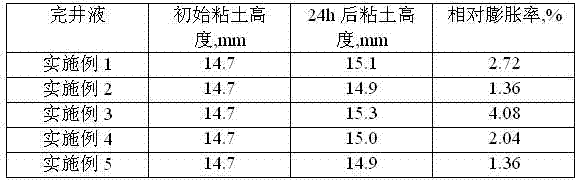
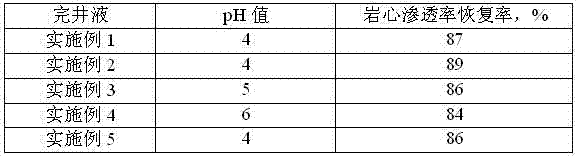
Low-permeability solid-free completion fluid
Owner:DAQING HUAYING CHEM IND
Method of Increasing pH of High-Density Brines
It has been discovered that carbonate powders and bicarbonate powders are useful to increase the pH and corrosion resistance of high-density brines, such as zinc bromide brines, without significantly reducing their densities. The carbonates and / or bicarbonates should be water-soluble and may be sodium, potassium, magnesium and / or ammonium carbonates and / or bicarbonates and the like. The carbonates and / or bicarbonates are easily added in powder or other finely divided solid form and are completely dissolved in the brine prior to pumping the brine into a subterranean formation.
Owner:BAKER HUGHES INC
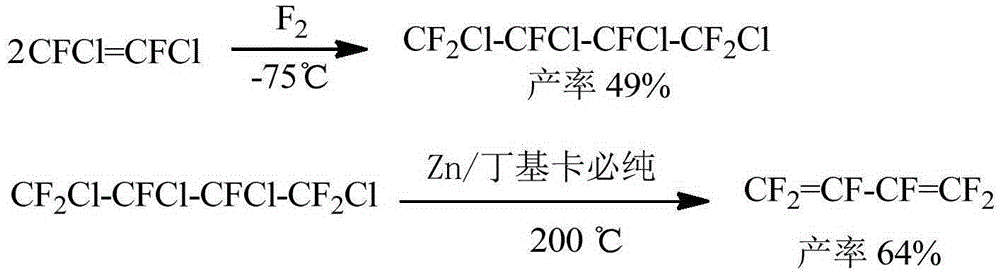
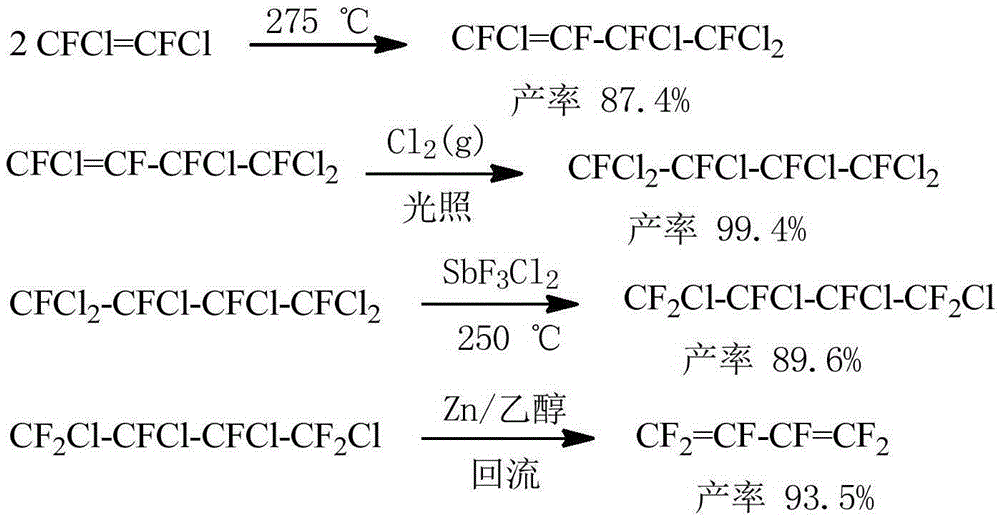
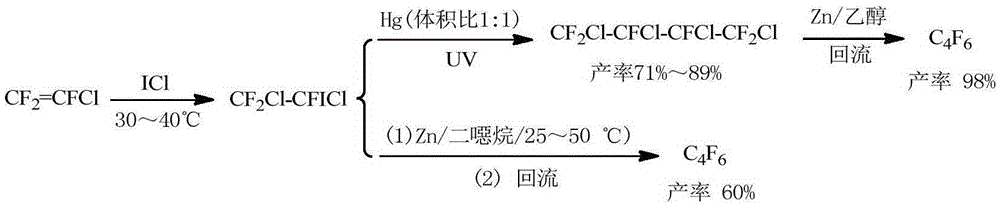
Preparation method of hexafluorobutadiene
PendingCN110590495ALower reaction costQuick responsePreparation by hydrogen halide split-offPreparation by halogen additionZinc bromideHydrogenation reaction
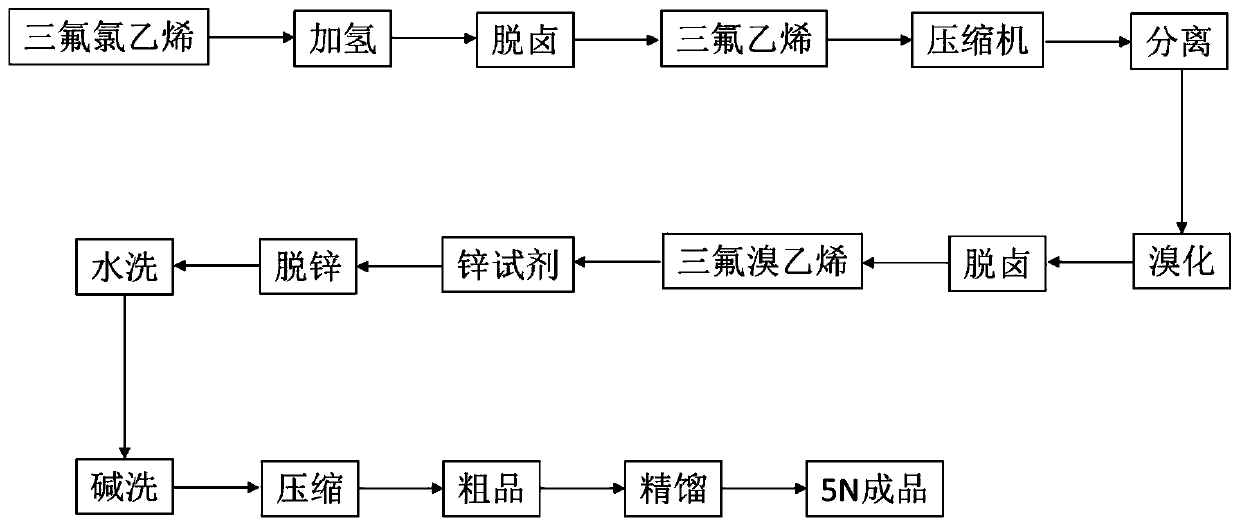
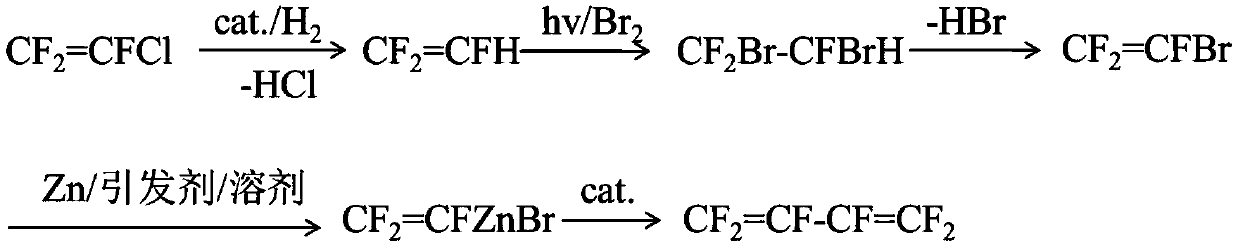
The invention discloses a preparation method of hexafluorobutadiene. The preparation method includes the following steps that (1) a hydrogenation reaction is carried out with trifluorochlor oethyleneas raw materials under the action of a first catalyst to obtain trifluoroethylene; (2) the trifluoroethylene is introduced into a bromination reactor for conducting a bromination reaction with brominein the reactor, 1,2-dibromo-1,1,2-trifluoroethane is obtained, and then trifluorobromoethylene is obtained by dehydrobromination; (3) the trifluorobromoethylene reacts in a reactor containing a solvent, an initiator and zinc powder to generate zinc reagent trifluorovinyl zinc bromide; and (4) the zinc reagent trifluorovinyl zinc bromide is coupled under the action of a second catalyst to obtain the hexafluorobutadiene. The preparation method has the advantages of low energy consumption, high yield, high reaction efficiency and the like.
Owner:FUJIAN DEER TECH CORP
New method for preparing hexafluorobutadiene
ActiveCN105272818ACheap and easy to getLow costHalogenated hydrocarbon preparationZinc bromideGas phase
Owner:泉州宇极新材料科技有限公司
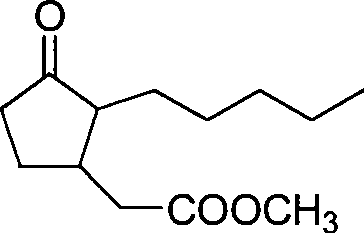
Method for preparing methyl dihydrojasmonate
InactiveCN101519355AShort synthetic routeShort synthesis cycleOrganic compound preparationCarboxylic acid esters preparationZinc bromideMethyl dihydrojasmonate
The invention provides a method for synthesizing methyl dihydrojasmonate. 2-pentyl cyclopentenone is adopted as a raw material; the raw material and allyl zinc bromide are subjected to 1,4-Michael addition to form 2-pentyl-3-allyl cyclopentanone; the 2-pentyl-3-allyl cyclopentanone is oxidized by sodium periodate in the presence of a ruthenium trichloride hexahydrate catalyst to synthesize dihydrojasmonic acid; and then the dihydrojasmonic acid is esterified to form the methyl dihydrojasmonate. The raw material of the method is cheap and low in cost; the process is simple and convenient in operation; the reaction conditions are mild and easy to control; the synthesis route is short and the synthesis period is reduced greatly; and the reaction product is single, high in yield (up to 65 to 85 percent), less in environmental pollution, and environmentally-friendly.
Owner:NORTHWEST NORMAL UNIVERSITY
Supported catalyst, preparation method and applications thereof
ActiveCN106140262AHigh selectivityEasy to prepareMolecular sieve catalystsHydrocarbon from halogen organic compoundsZinc bromideHydrogen
The invention discloses a supported catalyst, its preparation method and application. The supported catalyst is composed of zinc oxide, zinc bromide, additive oxide and magnesium-modified hydrogen-type ZSM-5 molecular sieve carrier. According to the weight content of the catalyst, the content of zinc oxide is 0.5%-20%. The zinc content is 10%-50%, and the weight content of the auxiliary oxide in the catalyst is 0.1%-10% in terms of metal elements. The preparation method of the catalyst comprises the following process: (1) treating the hydrogen ZSM-5 molecular sieve with a magnesium salt solution to obtain a magnesium-modified ZSM-5 molecular sieve carrier; (2) introducing zinc oxide into the magnesium-modified hydrogen Type ZSM-5 molecular sieve carrier; (3) carry out bromination treatment on the carrier after introducing zinc oxide; (4) prepare auxiliary oxide; (5) introduce auxiliary oxide into step (3) brominated in the material. The catalyst can convert methyl bromide into isobutene with high selectivity.
Owner:CHINA PETROLEUM & CHEM CORP +1
Preparation method of silicon-based material by taking alloy as reducing agent
InactiveCN107195895ASimple processEasy to operateCell electrodesSecondary cellsZinc bromidePotassium
The invention relates to a preparation method of a silicon-based material by taking alloy as a reducing agent. The method comprises the steps of taking silicon dioxide as a precursor, mechanically mixing an alloy reducing agent and a low-melting-point salt, pressing a block, and performing sintering in a vacuum or inertia atmosphere at 150-600 DEG C for 2-10 hours; and washing the product with hydrochloric acid and water, and drying the product to obtain the silicon-based material, wherein the silicon dioxide is one of MCM-41, MCM-48, MCM-50 and SBA-15, the alloy reducing agent is one of magnesium alloy and aluminum alloy, the magnesium alloy is one of magnesium aluminum, magnesium lithium, magnesium calcium and magnesium sodium, the aluminum alloy is one of aluminum sodium, aluminum lithium, aluminum calcium and aluminum potassium, the low-melting-point salt is one of aluminum chloride, aluminum bromide , zinc chloride and zinc bromide, the mole ratio of alloy and the silicon dioxide is 2-4, and the mole ratio of the low-melting-point and the silicon dioxide is 3-10. The silicon-based material prepared by the method is small in particle size, is of a porous structure, can be used for a lithium ion battery negative electrode material or a portable hydrogen preparation material, and has very good application prospect.
Owner:CHINA JILIANG UNIV
Preparing method used for flow microsphere zinc electrode of secondary zinc battery
InactiveCN102646816AIncrease the areaInhibit sheddingAlkaline accumulator electrodesZinc bromideMicrosphere
The invention discloses a preparing method used for a flow microsphere zinc electrode of a secondary zinc battery. Zinc-plated conducting microspheres are highly distributed in flow electrolyte consisting of zinc salt solution of zinc bromide and zinc chloride, quaternary ammonium salt and hydrobromic acid solution, and the zinc-plated conducting microspheres and a three-dimensional mesh current-collection body commonly constitute the flowable microsphere zinc electrode, wherein the zinc-plated conducting microspheres are prepared by the following steps that: 50-200mum-diameter conducting microspheres are placed in a cathode region of an electrolytic tank to be electroplated and are stirred at the same time so that plating layers of the microspheres are uniform, the microspheres with the moderate thickness of the plating layers can be obtained through time control, zinc-plated solution consists of Zn<2+> with the concentration being 3-25g / L and NaOH with the concentration being 15-180g / L, the cathode-current density is 0.5-4.0A / dm<2>, and the temperature is 10-40 DEG C. According to the preparing method, the deformation and the pine-tree crystal of a solid zinc polar plate in the charging and discharging processes of the zinc electrode and the dropping of active substances caused by the deformation and the pine-tree crystal can be effectively avoided, thereby improving the performance of the zinc electrode of the zinc battery obviously and realizing large-current charging and discharging.
Owner:CENT SOUTH UNIV
Method of increasing pH of high-density brines
InactiveUS6894008B2Improve corrosion resistanceRaise the pHDrilling rodsOther chemical processesZinc bromidePotassium
It has been discovered that carbonate powders and bicarbonate powders are useful to increase the pH and corrosion resistance of high-density brines, such as zinc bromide brines, without significantly reducing their densities. The carbonates and / or bicarbonates should be water-soluble and may be sodium, potassium, magnesium and / or ammonium carbonates and / or bicarbonates and the like. The carbonates and / or bicarbonates are easily added in powder or other finely divided solid form.
Owner:BAKER HUGHES INC
Synthesis of gamma-undecalactone
The invention discloses a method for synthesizing gamma-undecalactone, which belongs to a synthesis technology of the gamma-undecalactone. The method takes n-octanol as a solvent, boric acid or zinc bromide as a catalyst, acrylic acid as a raw material and di-t-butyl peroxide or dibenzoyl peroxide as an initiator, and comprises the following steps: firstly, adding partial n-octanol and the catalyst into a reaction kettle to be mixed and heated; secondly, mixing the remaining n-octanol, the initiator and the acrylic acid and dipping the mixture into the reaction kettle; thirdly, making the mixture react for a period; and finally obtaining reaction liquid containing the gamma-undecalactone. The method has the advantages that the reaction is carried out at normal pressure, byproducts of tert-butyl alcohol are easy to discharge from a system during the reaction process, and simultaneously the equipment used by the method is simple, the synthesis cost is low and the yield of the gamma-undecalactone is high.
Owner:TIANJIN UNIV
Ultralow-mercury catalyst used for acetylene hydrochlorination
ActiveCN105032455AHigh activityImprove stabilityMolecular sieve catalystsPreparation by halogen halide additionZinc bromideCupric bromide
The invention discloses an ultralow-mercury catalyst, and particularly relates to a catalyst with the ultralow content of mercuric chloride and suitable for acetylene hydrochlorination to synthesize chloroethylene. The catalyst comprises a main activity component, auxiliary component A metal bromide, auxiliary component B metal bromide and carriers, wherein the main activity component is mercuric chloride, and the content of the mercuric chloride accounts for 0.5-4% of the total mass of the catalyst carriers; the auxiliary component A metal bromide includes one or more of cupric bromide, cobaltous bromide, manganous bromide, nickel bromide and zinc bromide, and the metal element content of the auxiliary component A metal bromide accounts for 0.1-15% of the total mass of the catalyst carriers; the auxiliary component B metal bromide includes one or more of potassium bromide, barium bromide, sodium bromide and lithium bromide, and the metal element content of the auxiliary component B metal bromide accounts for 0.01-10% of the total mass of the catalyst carriers; the carriers include activated carbon with the specific surface area of 300-2500 m<2> / g and molecular sieve, or silica gel, or zeolite or kieselguhr. While activity and stability of the ultralow-mercury catalyst are ensured, the mercury resource in the catalyst is brought into full play to reach the maximum use efficiency, and the novel ultralow-mercury catalyst is environmentally friendly.
Owner:XINJIANG CORPS MODERN GREEN CHLOR ALKALI CHEM ENG RES CENT LTD
Composite anti-bacteria agent, antibiotic paint and antibiotic aluminum composite board
InactiveCN101411337AMeet the needs of antimicrobial performanceBiocideAntifouling/underwater paintsZinc bromideSodium Pyrithione
The invention discloses a complex antibacterial agent, an antibacterial coating and an antibacterial aluminum composite plate, and relates to an antibacterial agent, a coating composition containing the antibacterial agent and a laminar product consisting of flat thin layers. The complex antibacterial agent comprises silver-zinc titanium dioxide, zinc bromide and zinc pyrithione, and the mass ratio of the silver-zinc titanium dioxide to the zinc bromide to the zinc pyrithione is 7 to 1 to 1.5; the antibacterial coating contains the complex antibacterial agent with the mass percentage of between 0.24 and 18.9 percent; and the antibacterial aluminum composite plate comprises an aluminum panel and an aluminum bottom board, and the outer surface of the aluminum panel is coated with the antibacterial coating. The antibacterial performance of the antibacterial aluminum composite plate can reach the technical requirement of the national building material antibacterial standard JC / T 897-2002, and the antibacterial aluminum composite plate can satisfy the demand of the antibacterial performance of national building decoration materials and can also remedy the disadvantages of foreign sources of goods.
Owner:GUANGZHOU WELLSTRONG NEW MATERIAL CO LTD
Electrolyte for rechargeable electrochemical cell
InactiveUS20180013185A1Increased durabilityImprove stabilityFuel and secondary cellsLarge-sized flat cells/batteriesElectrolytic agentZinc bromide
The present invention provides an aqueous electrolyte for use in rechargeable zinc-halide storage batteries that possesses improved stability and durability and improves zinc-halide battery performance. One aspect of the present invention provides an electrolyte for use in a secondary zinc bromine electrochemical cell comprising from about 30 wt % to about 40 wt % of ZnBr2 by weight of the electrolyte; from about 5 wt % to about 15 wt % of KBr; from about 5 wt % to about 15 wt % of KCl; and one or more quaternary ammonium agents, wherein the electrolyte comprises from about 0.5 wt % to about 10 wt % of the one or more quaternary ammonium agents.
Owner:EOS ENERGY TECH HLDG LLC
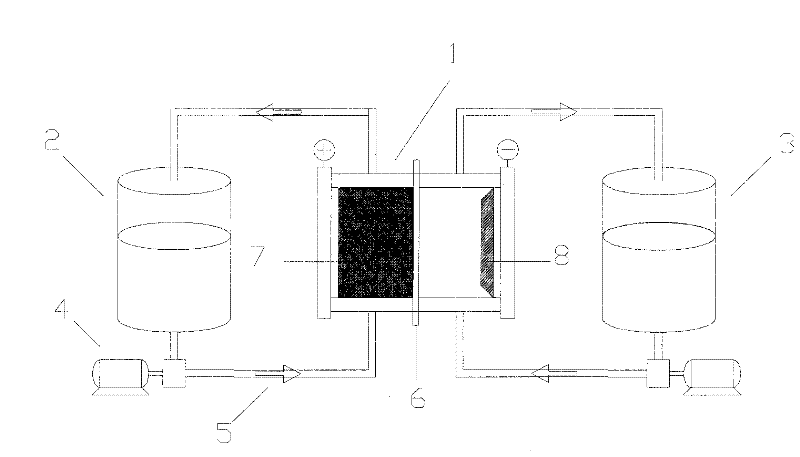
Electrolyte used for zinc bromine flow battery
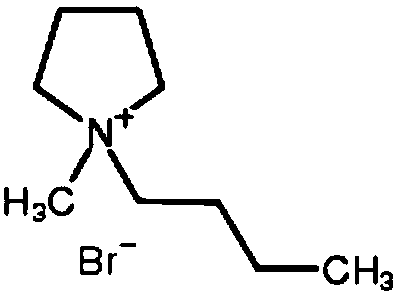
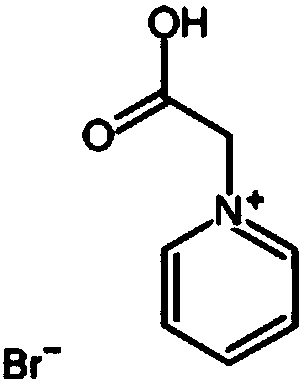
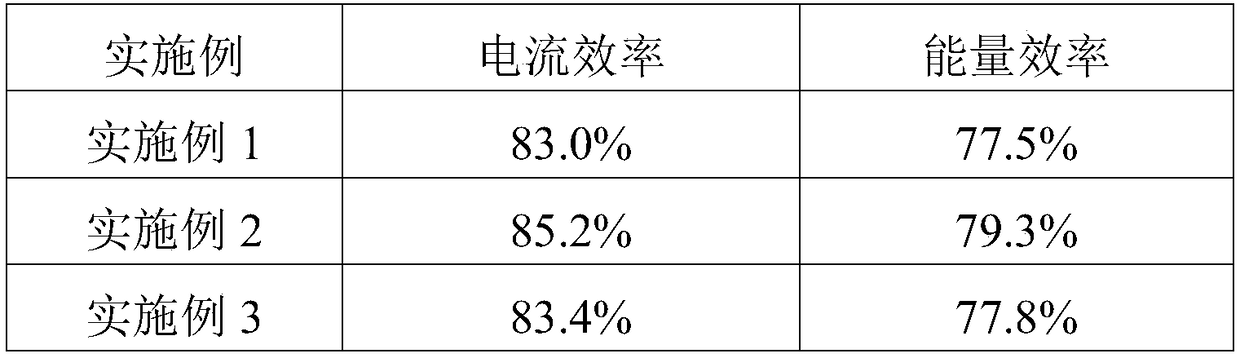
ActiveCN108711633APromote growthReduce short circuitRegenerative fuel cellsFuel cell detailsZinc bromideBromine
The invention discloses an electrolyte used for a zinc bromine flow battery. The electrolyte used for the zinc bromine flow battery disclosed by the invention comprises an anode electrolyte and a cathode electrolyte. Each of the anode electrolyte and the cathode electrolyte consists of the following components: zinc bromide, zinc chloride, a complexing agent, a dendritic crystal resistant agent, awetting agent and water. Compared with the prior art, the complexing agent, the dendritic crystal resistant agent and the wetting agent are added into the electrolyte used for the zinc bromine flow battery, so that the electrolyte used for the zinc bromine flow battery can improve the current efficiency and energy efficiency of the battery.
Owner:衡阳市大宇锌业有限公司
Solder paste flux composition
InactiveUS7052558B1Easy to weldExcellent solventWelding/cutting media/materialsSoldering mediaZinc bromideNonylphenol ethoxylate
A soldering paste flux for use in soldering copper and copper alloy piping and the like is formed of 40–70% nonylphenol ethoxylate (preferably Tergitol NO-10® made by Dow Chemical), 10–30% glyceryl monostearate, 3–10% acid activator, 3–10% water, and 4–15% mineral salt. The acid activator is preferably a mineral acid and, most preferably, hydrobromic acid. The mineral salt is preferably zinc bromide.
Owner:CHEM & METALS TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com