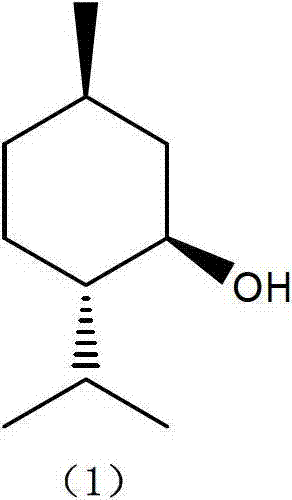
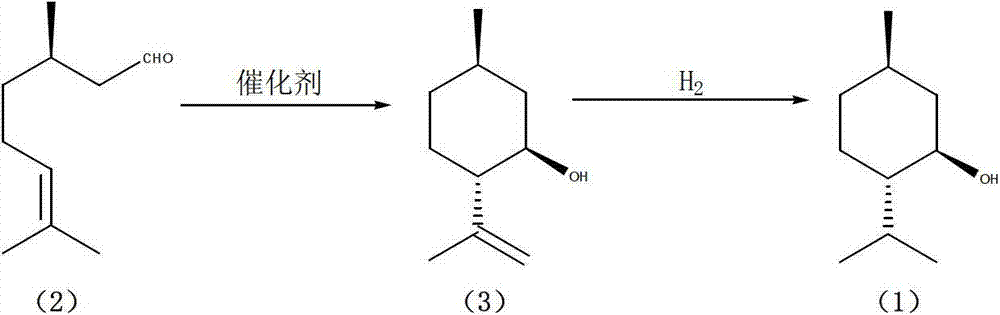
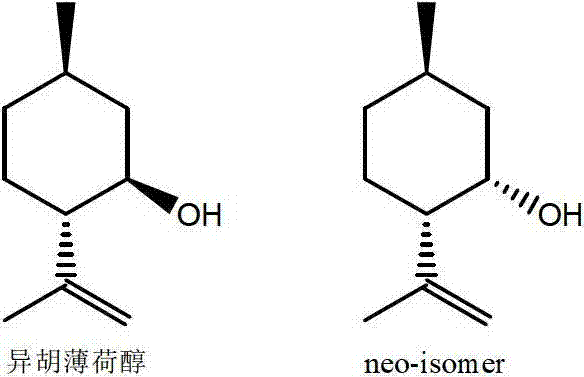
Method for preparing L-menthol
A technology for menthol and isopulegol, applied in the field of preparing L-menthol, can solve problems such as catalyst instability, lack of industrial value, and inability to reuse, achieve significant innovation, reduce production costs, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 500 grams of zinc bromide aqueous solution with a weight concentration of 5% in a 1000 mL dry three-port reactor equipped with a thermometer and magnetic stirring, then add 20 grams of NaY type molecular sieves to the aqueous solution, and then the system is sealed at 100 °C for 2 hours. After completion of the reaction, water was concentrated and removed under reduced pressure, and dried at 200°C, followed by calcination at 400°C for 2 hours to obtain the catalyst.
Embodiment 2
[0036] Adding 250 grams of zinc bromide aqueous solution with a weight concentration of 10% in a 1000 mL dry three-port reactor equipped with a thermometer and magnetic stirring, followed by adding 25 grams of NaY type molecular sieves to the aqueous solution, and then the system was sealed at 50 °C for 4 hours. After the reaction, the water was concentrated and removed under reduced pressure, and dried at 100° C., followed by roasting at 600° C. for 0.5 hour to obtain the catalyst.
Embodiment 3
[0038] Add the catalyst 50g of embodiment 1, 200mL of anhydrous toluene in 500mL equipped with thermometer, dropping funnel, magnetic stirring dry three-port reactor full of nitrogen, 50g of citronellal (ee value 99%) is transferred to constant Then drop it into the system in the pressure dropping funnel, and keep stirring vigorously, and the temperature of the system is controlled between -5-0°C. Stirring was continued at this temperature for 2 hours after the dropwise addition was complete. After the reaction was completed, the mixture was filtered to obtain a colorless clear liquid, and the filter cake was recovered for use. Then the organic phase was washed with 2*20 mL of deionized water, dried with 20 mL of saturated sodium chloride aqueous solution, and then dried over anhydrous sodium sulfate, filtered to remove inorganic salts, and the solvent was concentrated. Subsequently, the product was fractionated under reduced pressure (20 cm long to the thorn distillation col...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com