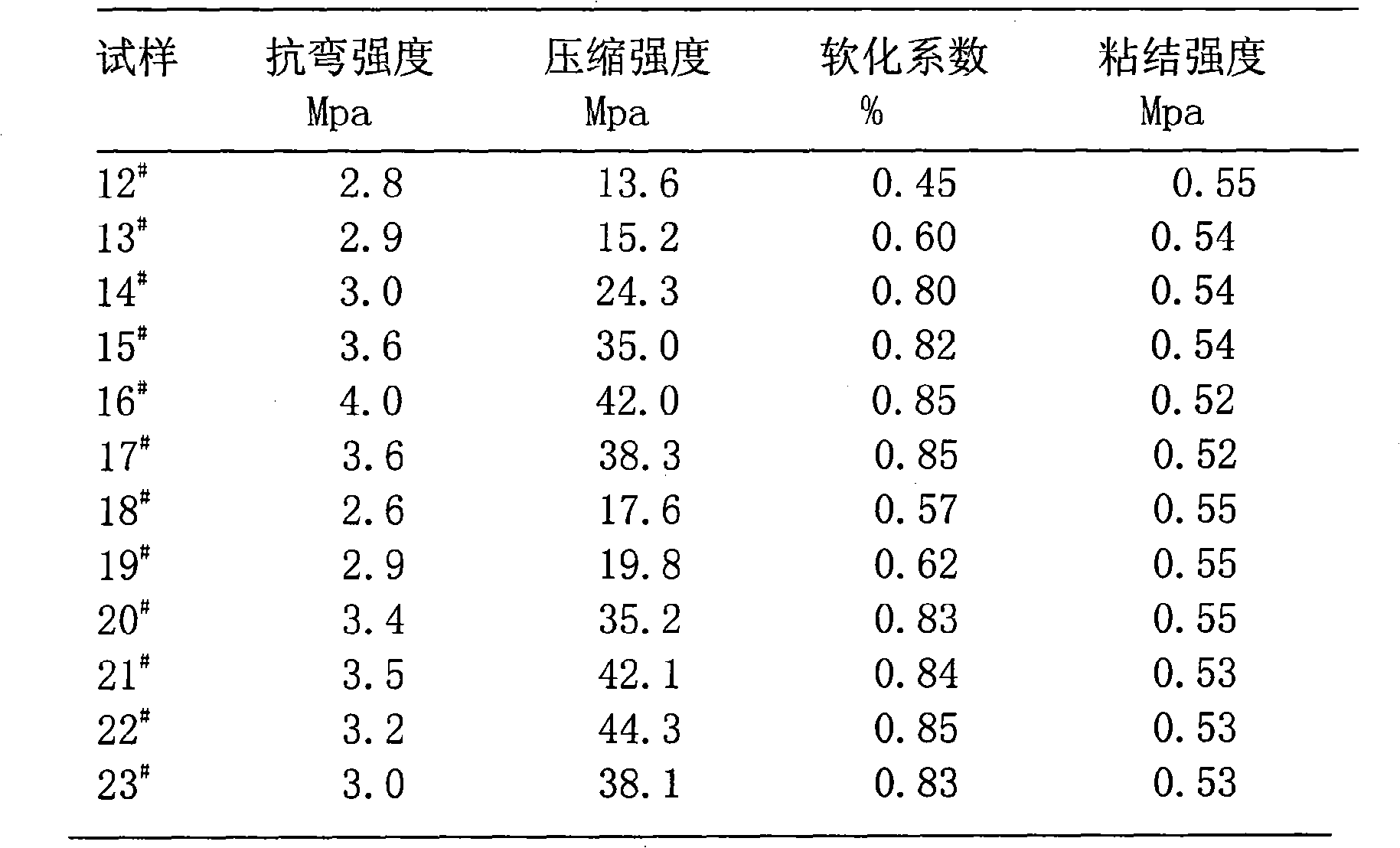
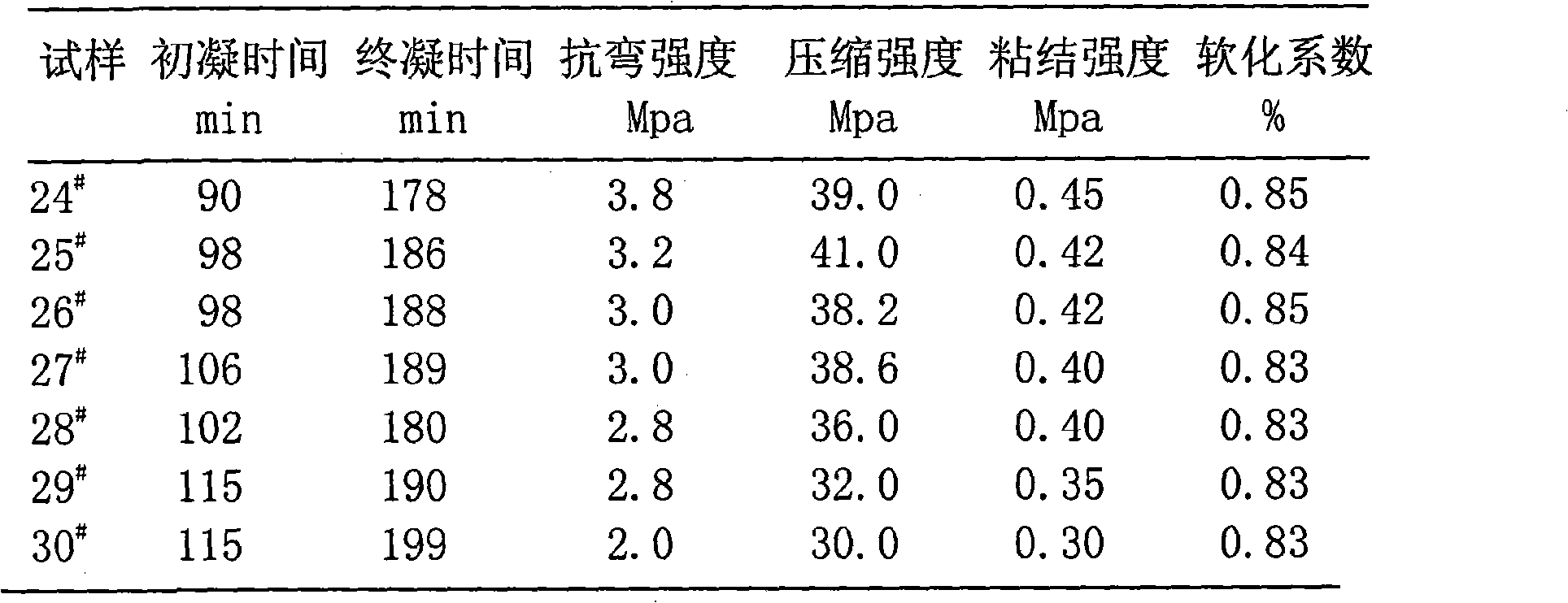
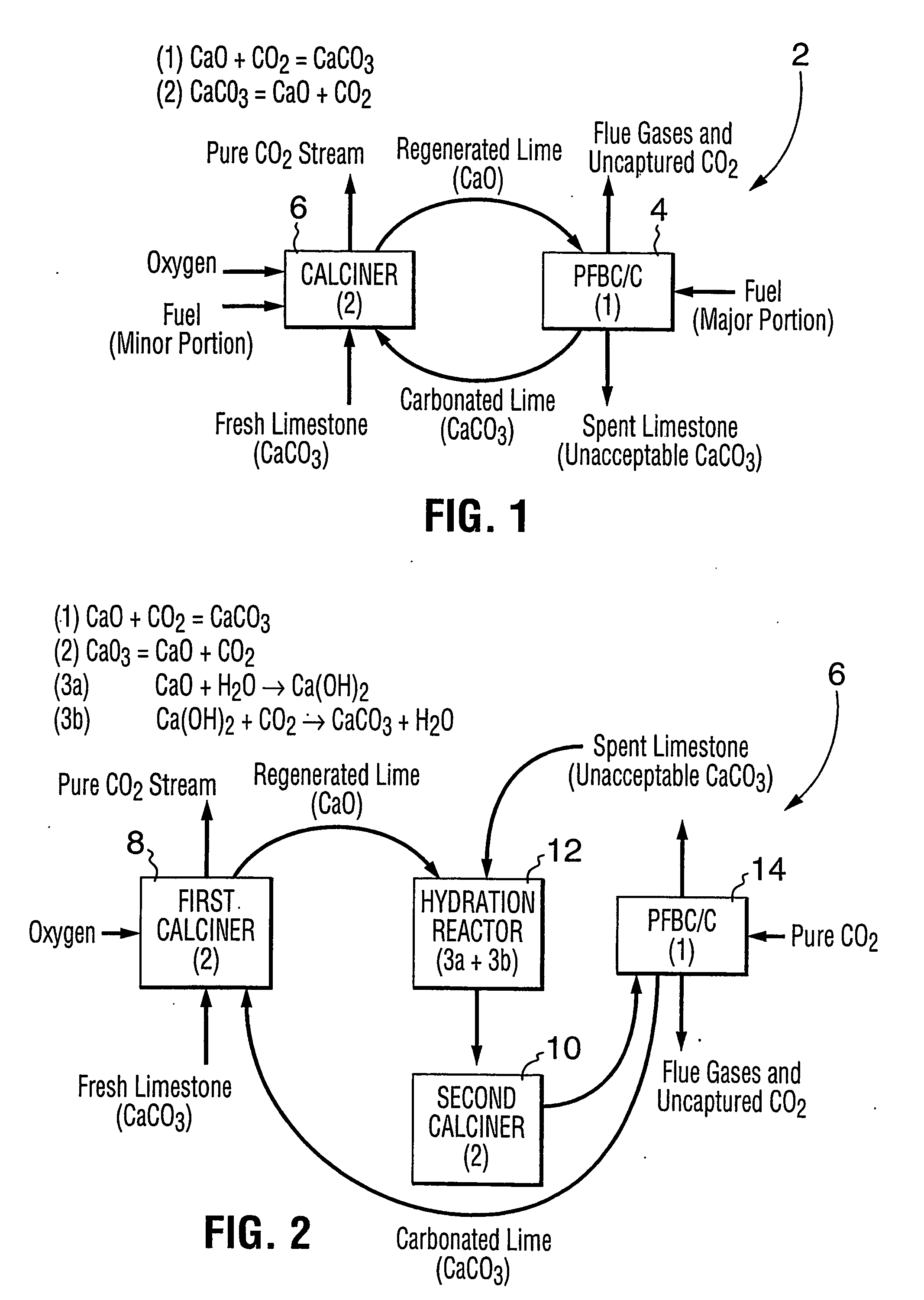
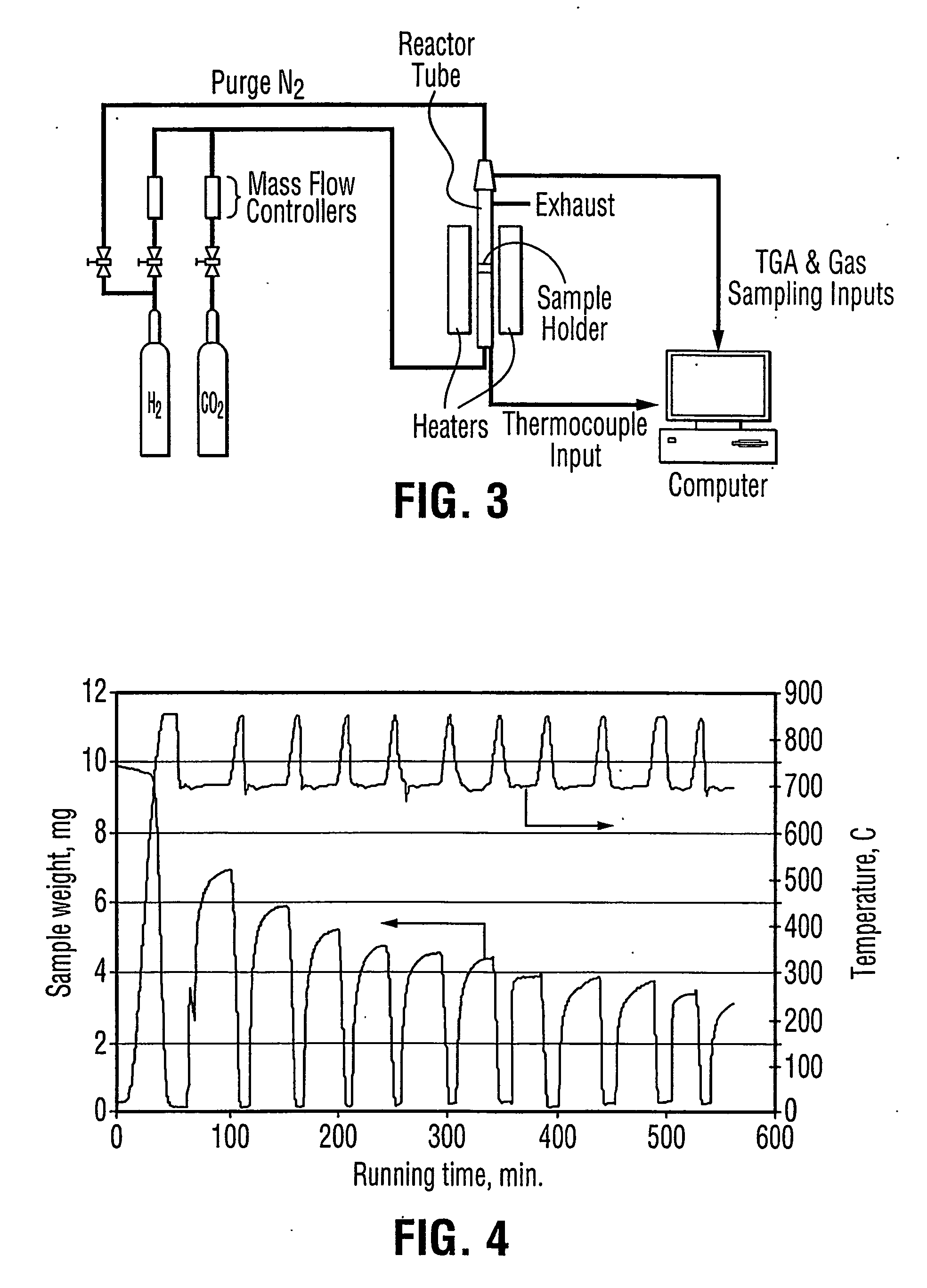
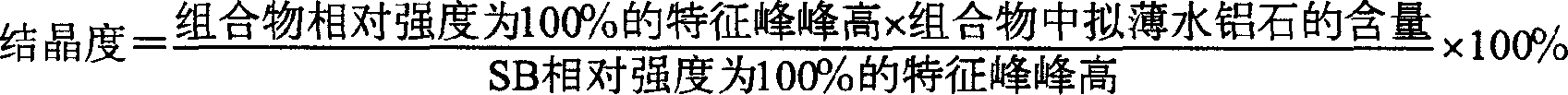Patents
Literature
14750 results about "Calcination" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
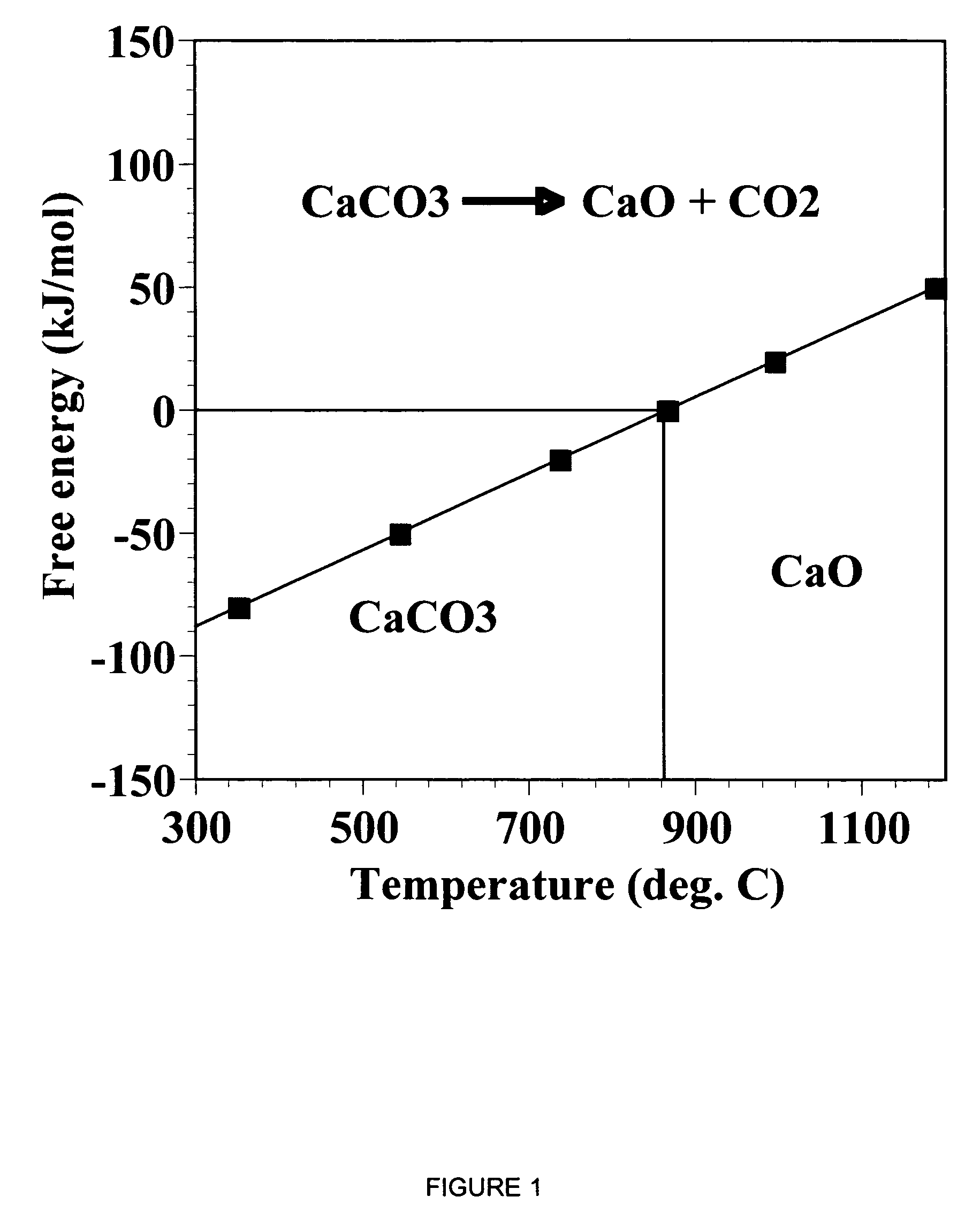
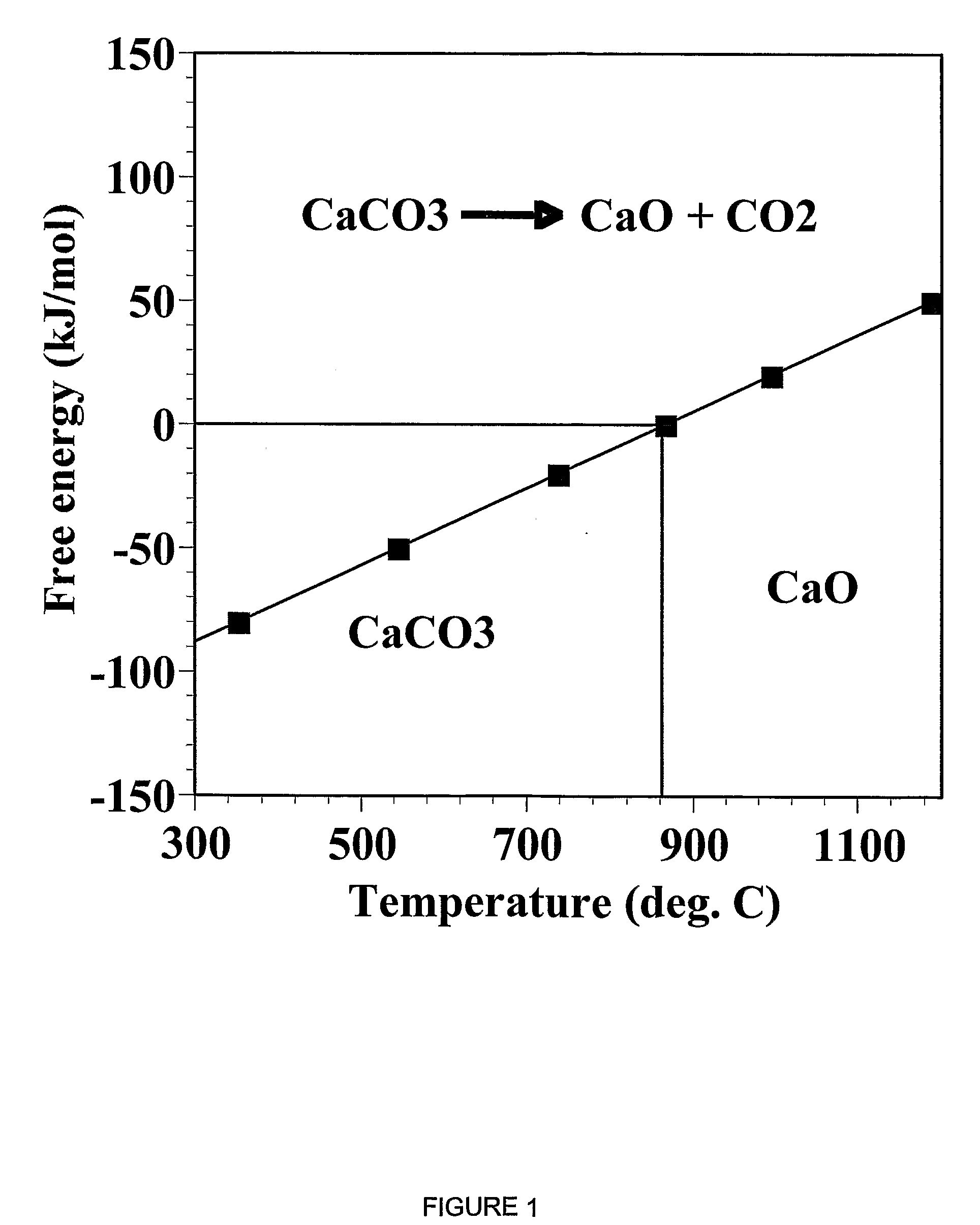
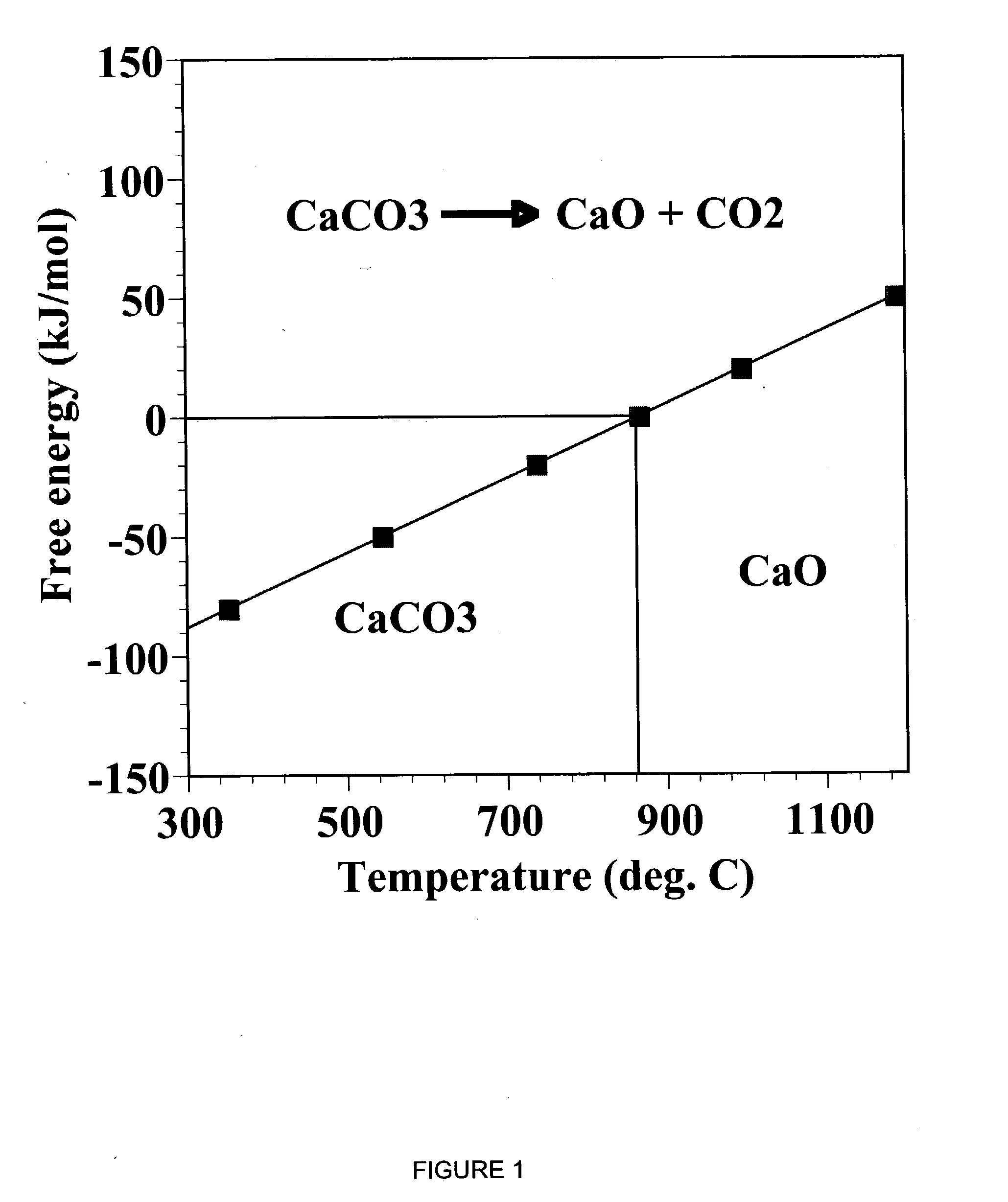
The IUPAC defines calcination as "heating to high temperatures in air or oxygen". However, calcination is also used to mean a thermal treatment process in the absence or limited supply of air or oxygen applied to ores and other solid materials to bring about a thermal decomposition. A calciner is a steel cylinder that rotates inside a heated furnace and performs indirect high-temperature processing (550–1150 °C, or 1000–2100 °F) within a controlled atmosphere.
Hydrogen production from carbonaceous material
InactiveUS6790430B1Avoid the needCalcium/strontium/barium carbonatesGas turbine plantsCalcinationExothermic reaction
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE +1
Comprehensive recovering method of waste lithium iron phosphate battery
InactiveCN101847763AImprove performanceLow priceWaste accumulators reclaimingProcess efficiency improvementAdhesiveCalcination
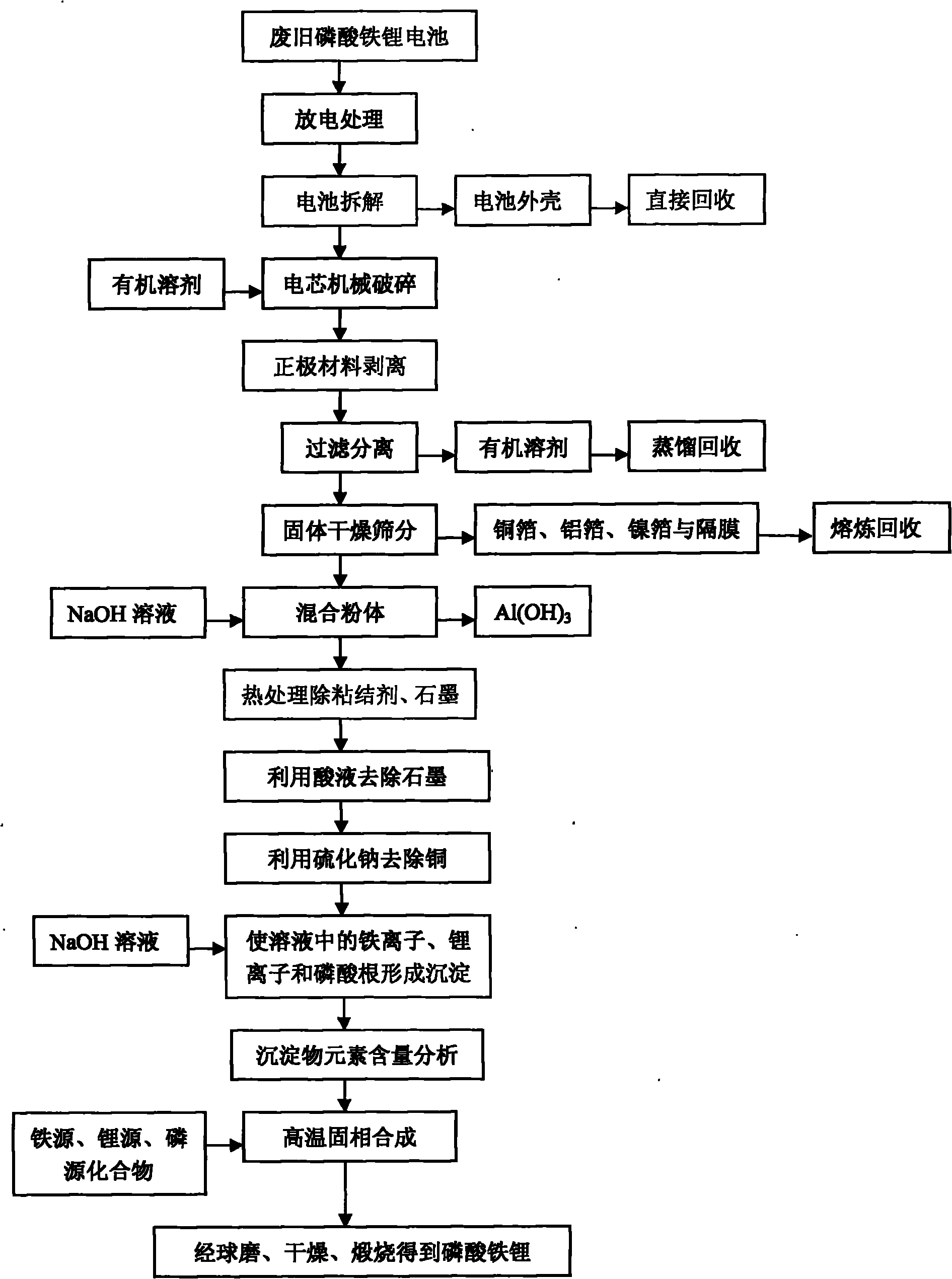
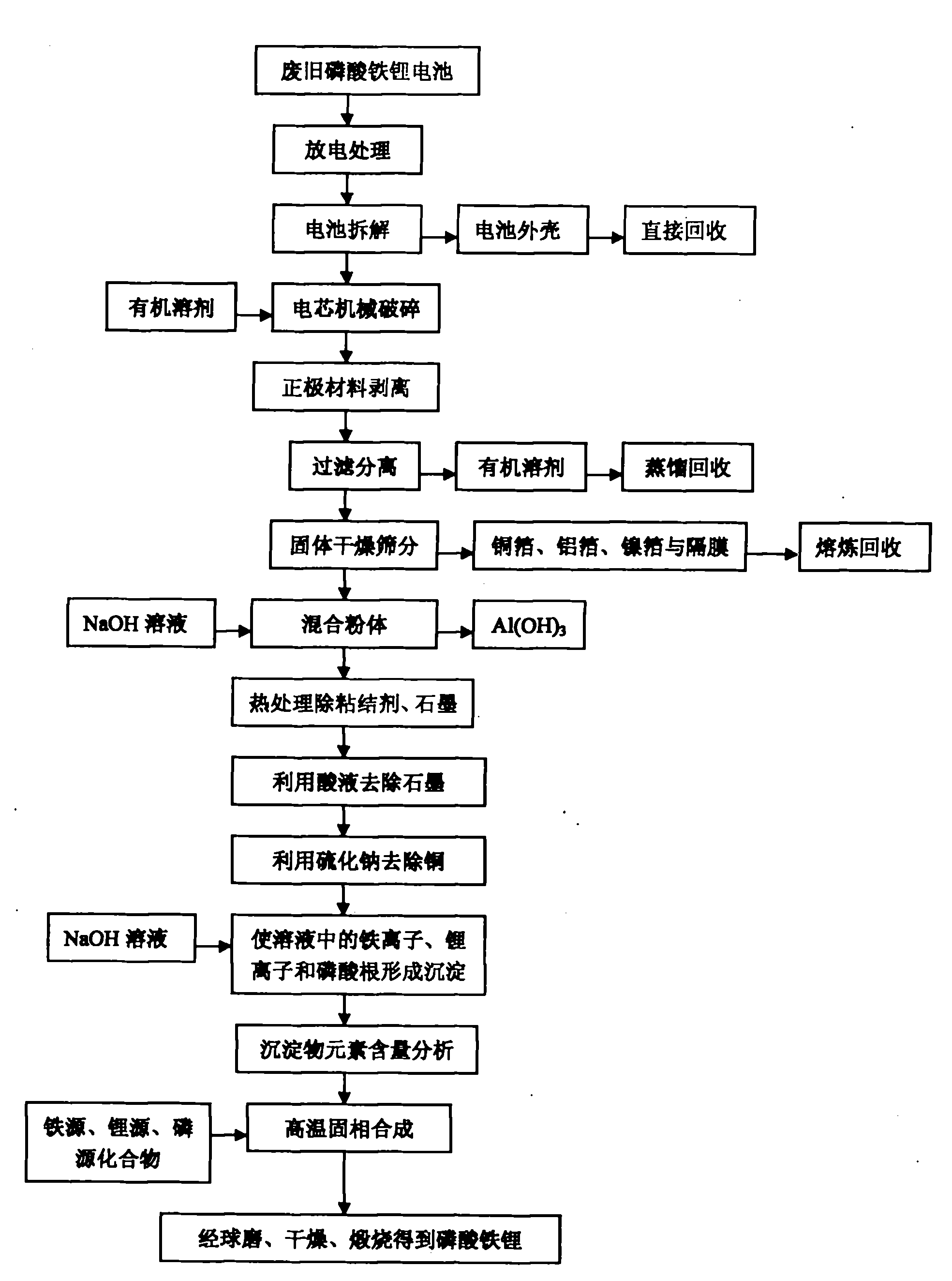
The invention provides a comprehensive recovering method of waste lithium iron phosphate batteries, which has simple and reasonable process, low recovering cost and high added value. The method comprises the following steps: utilizing an organic solvent to dissolve an adhesive on battery cell fragments, and realizing the separation of lithium iron phosphate material and clean aluminum and copper foils through screening, wherein the aluminum and copper foils are recovered by smelting; utilizing a NaOH solution to remove residual aluminum foil scraps in the lithium iron phosphate material, and removing graphite and remaining adhesive by heat treatment; after dissolving the lithium iron phosphate with acid, utilizing sodium sulphide to remove copper ions, and utilizing the NaOH solution or ammonia solution to allow iron, lithium and phosphorus ions in the solution to generate sediments; adding iron source, lithium source or phosphorus source compounds to adjust the molar ratio of iron, lithium and phosphorus; and finally adding a carbon source, and obtaining a lithium iron phosphate cathode material through ball milling and calcination in inert atmosphere. After the treatment of the steps, the recovery rate of valuable metals in the batteries is more than 95%, and the comprehensive recovery rate of the lithium iron phosphate cathode material is more than 90%.
Owner:CHERY AUTOMOBILE CO LTD
Separation of carbon dioxide (CO2) from gas mixtures
ActiveUS7618606B2Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentCo2 removalSorbent
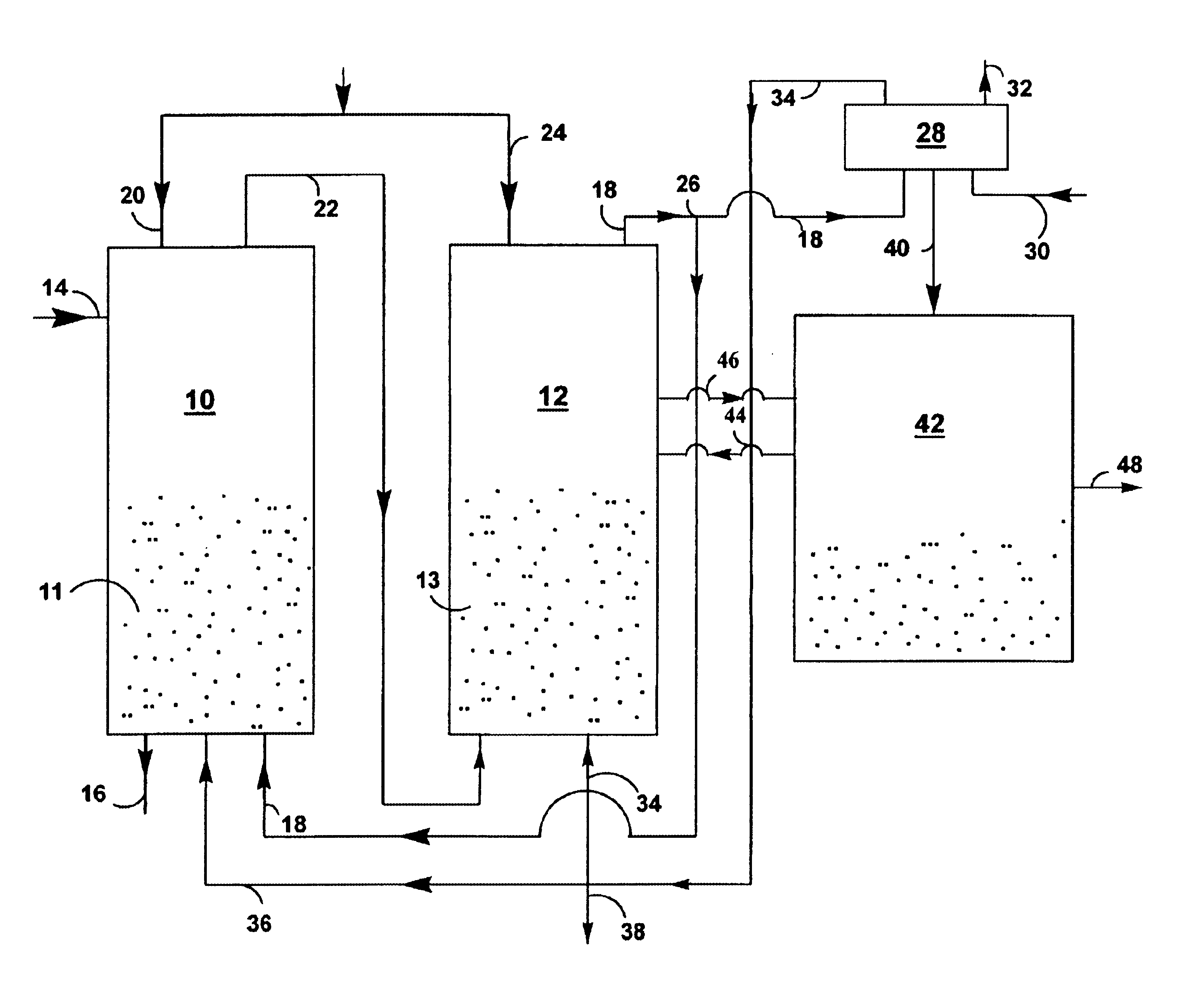
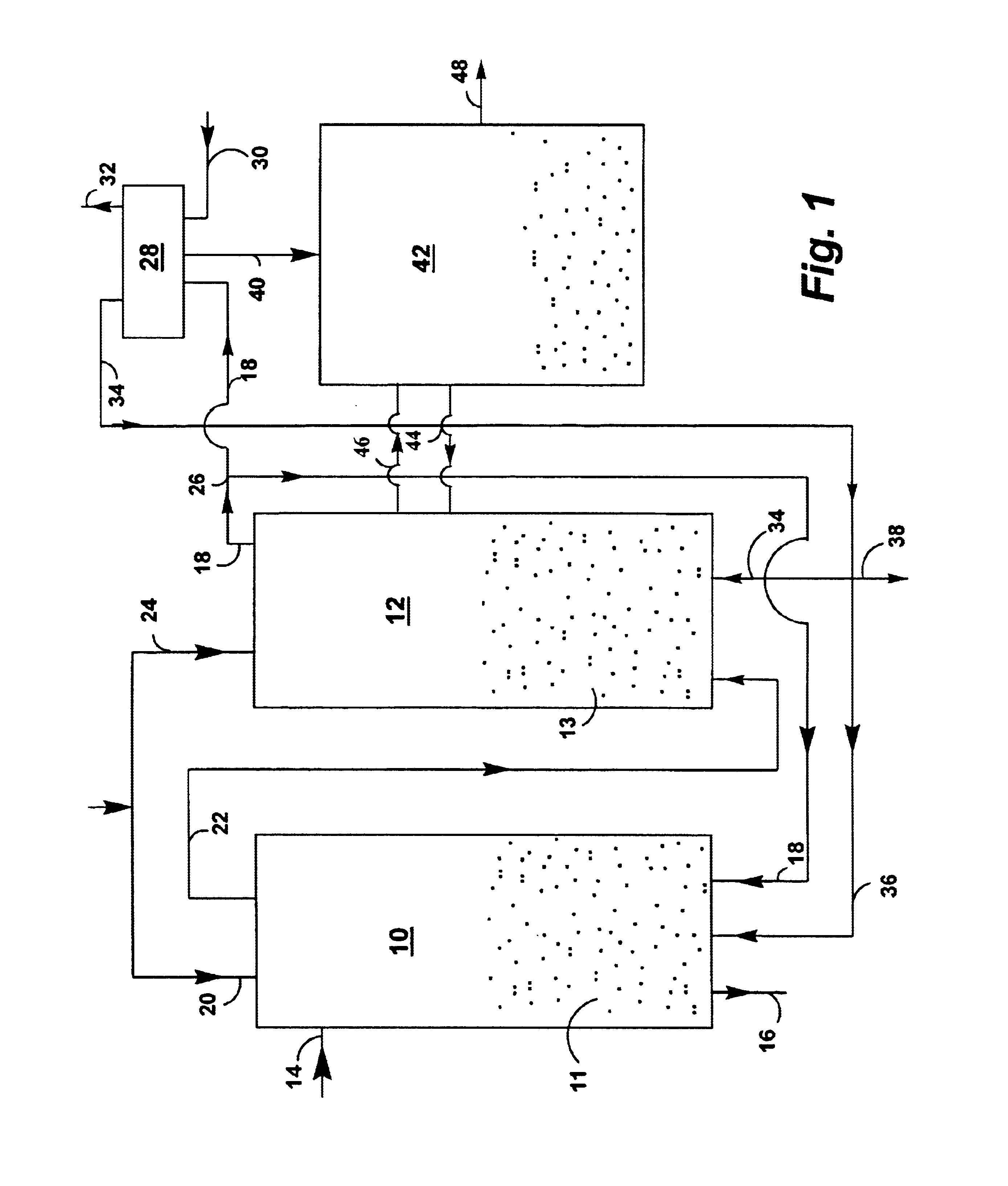
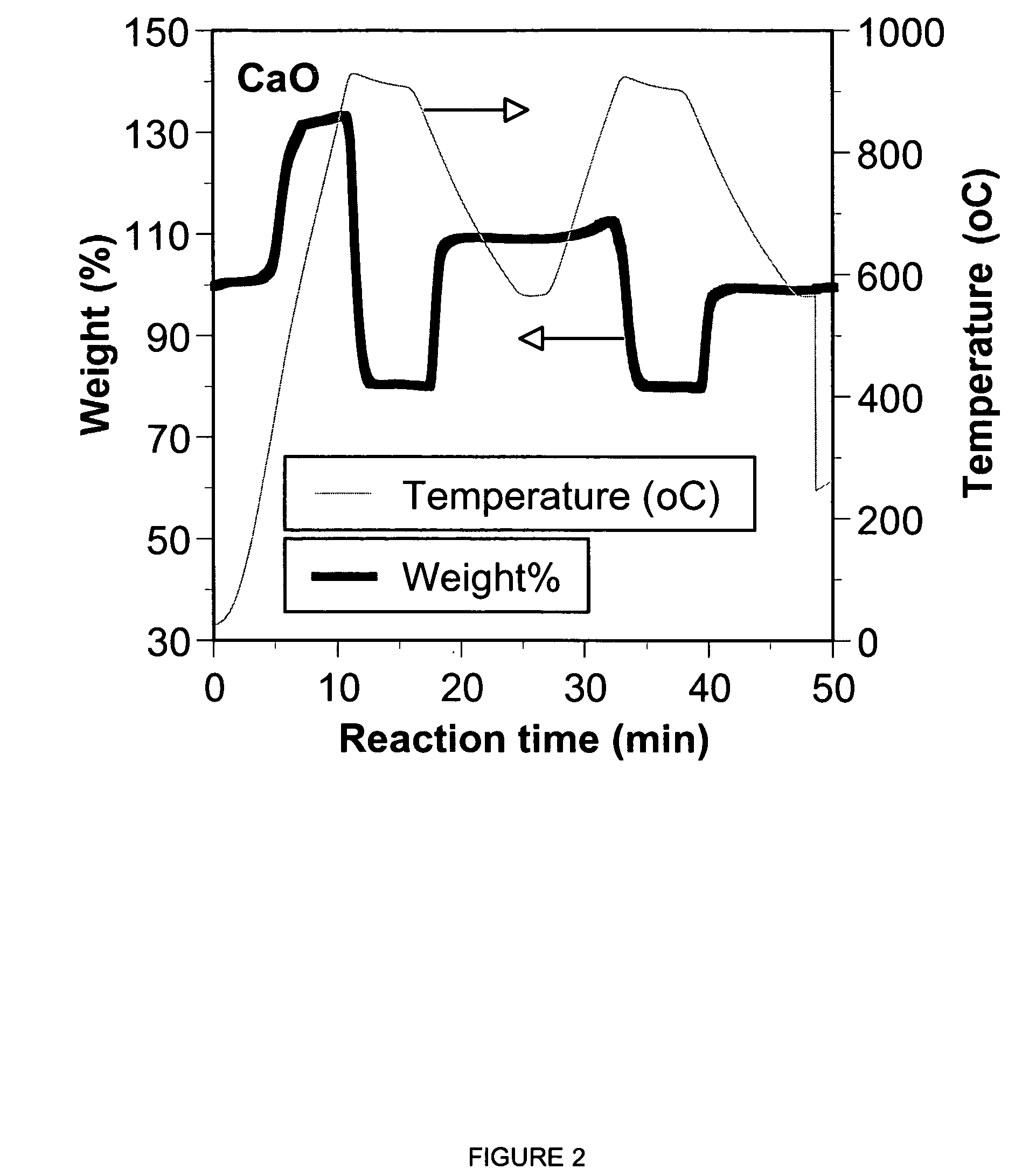
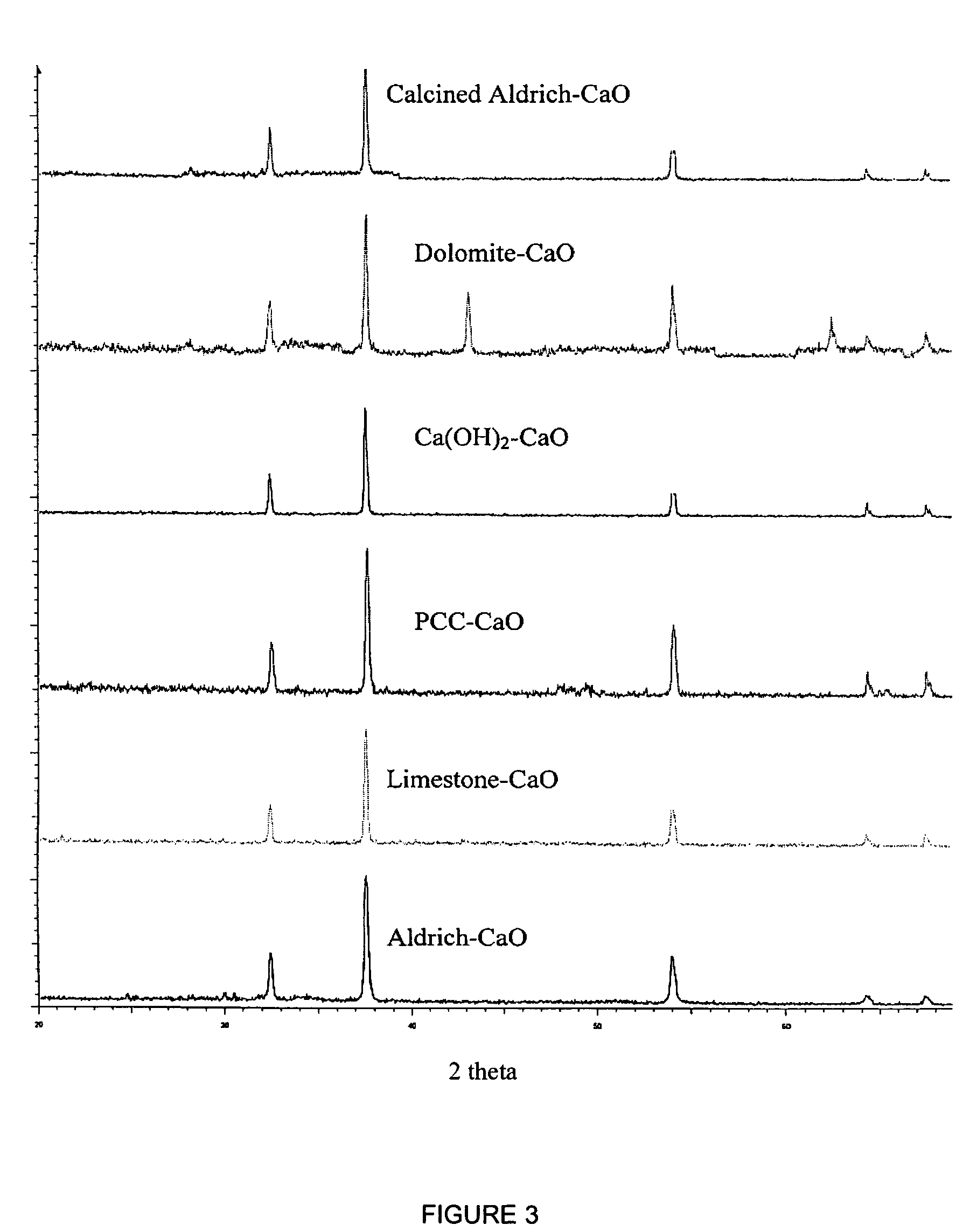
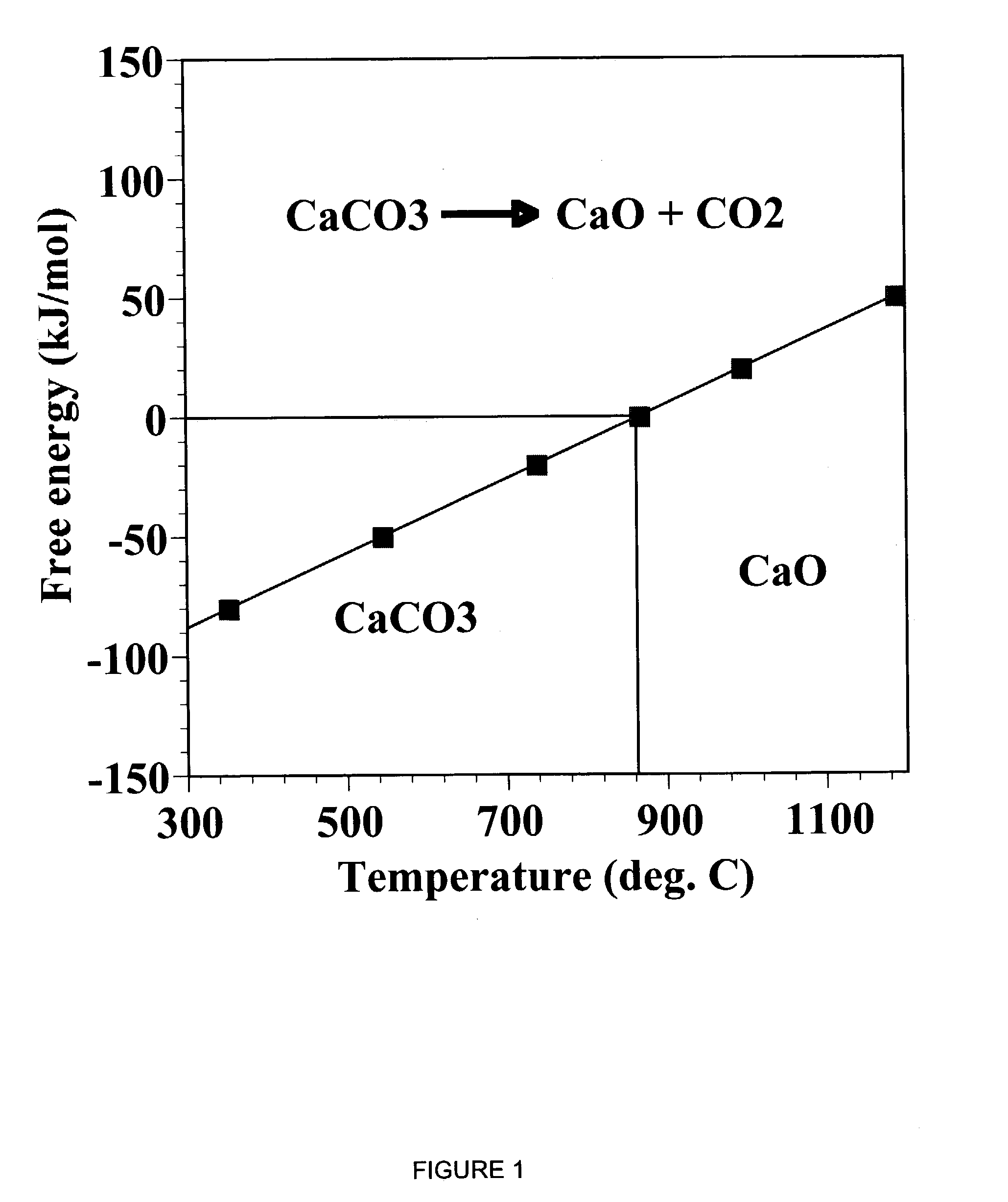
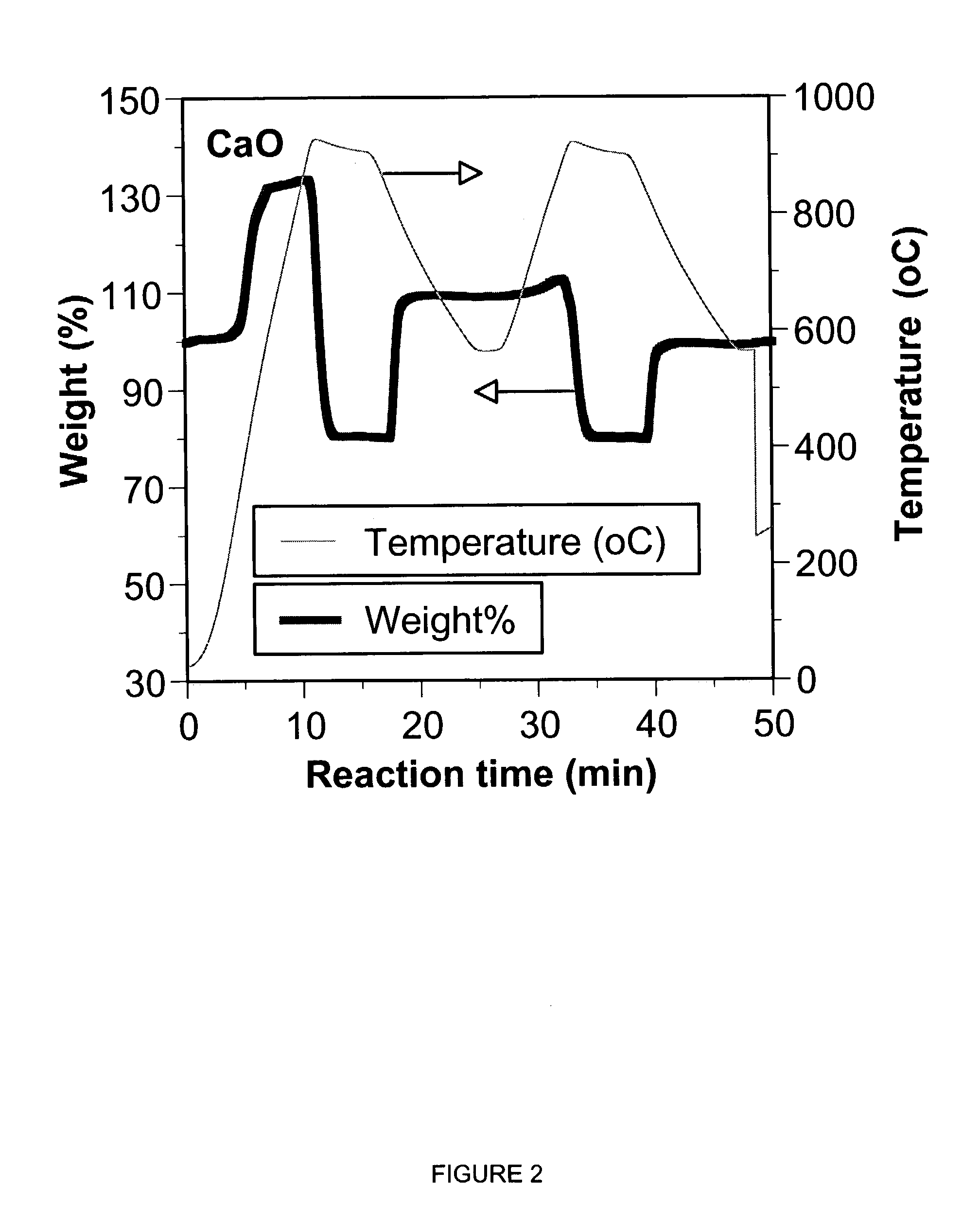
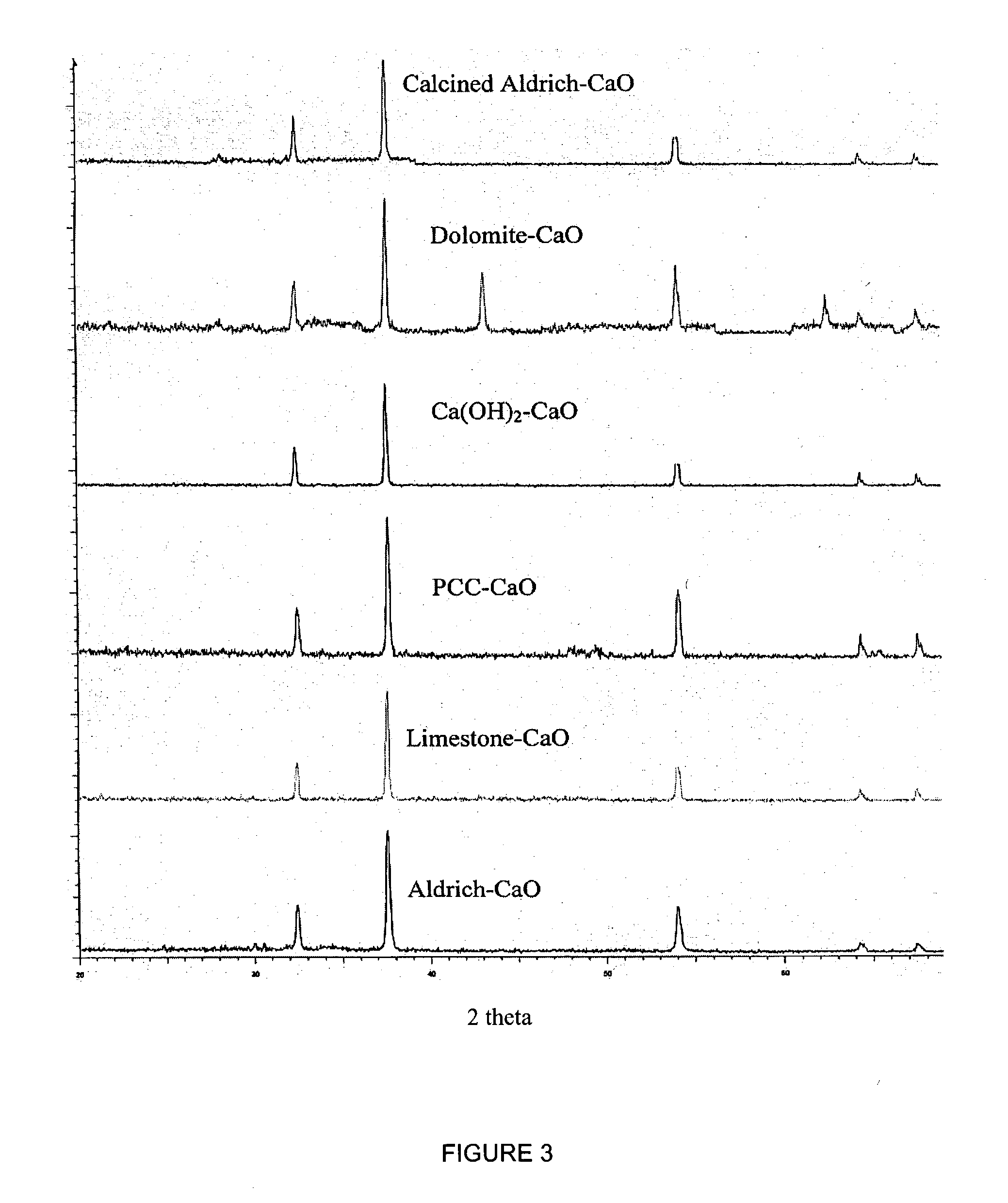
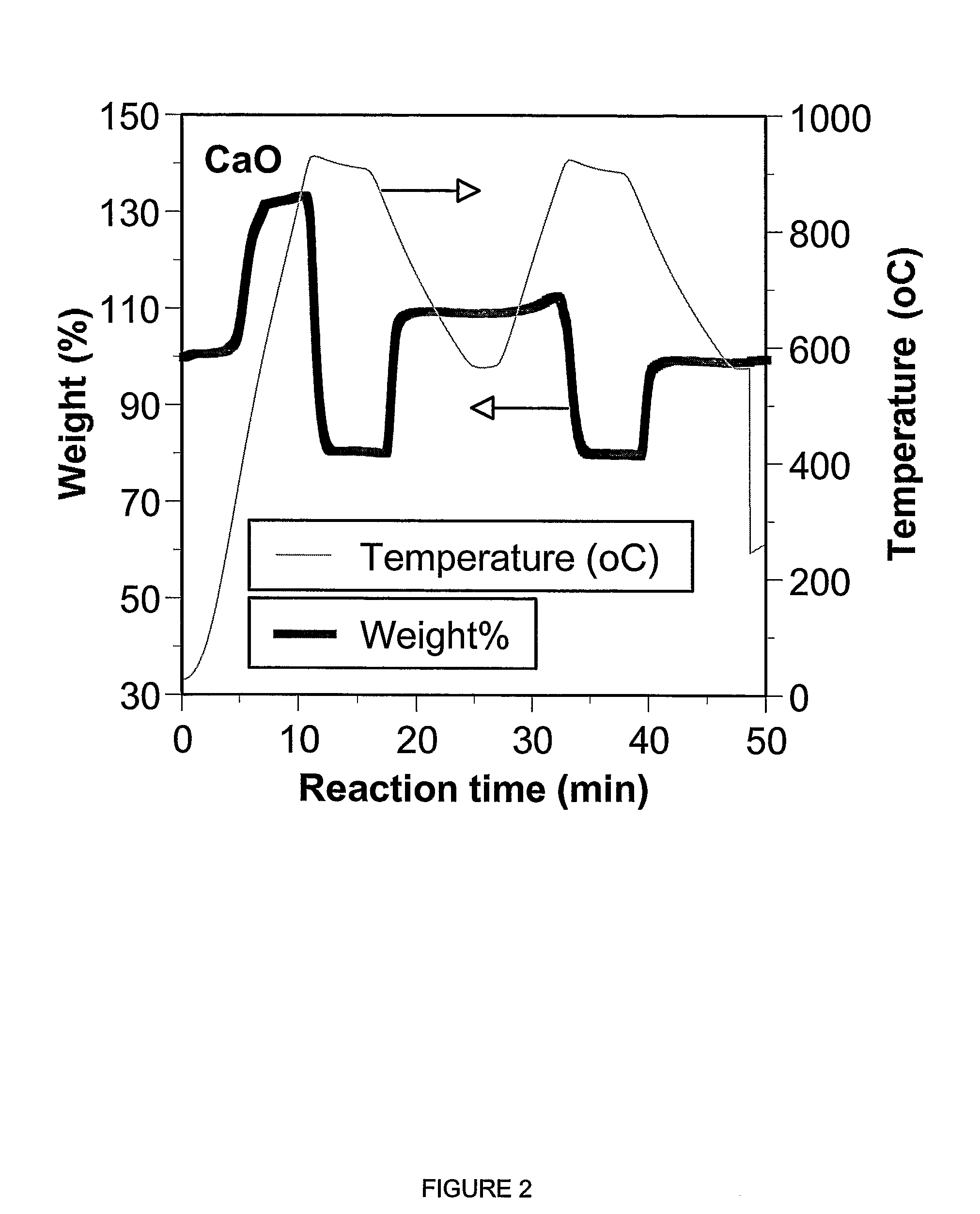
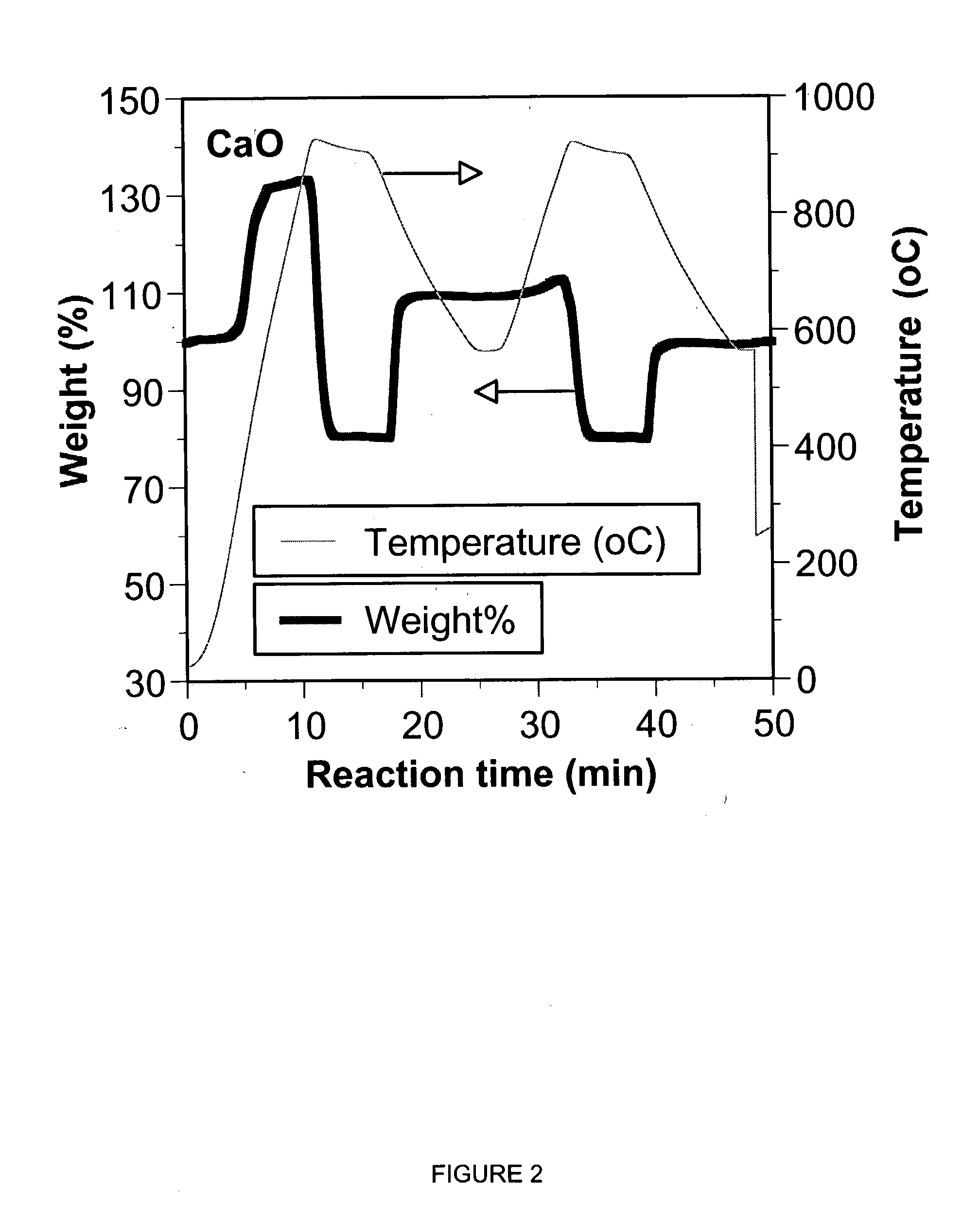
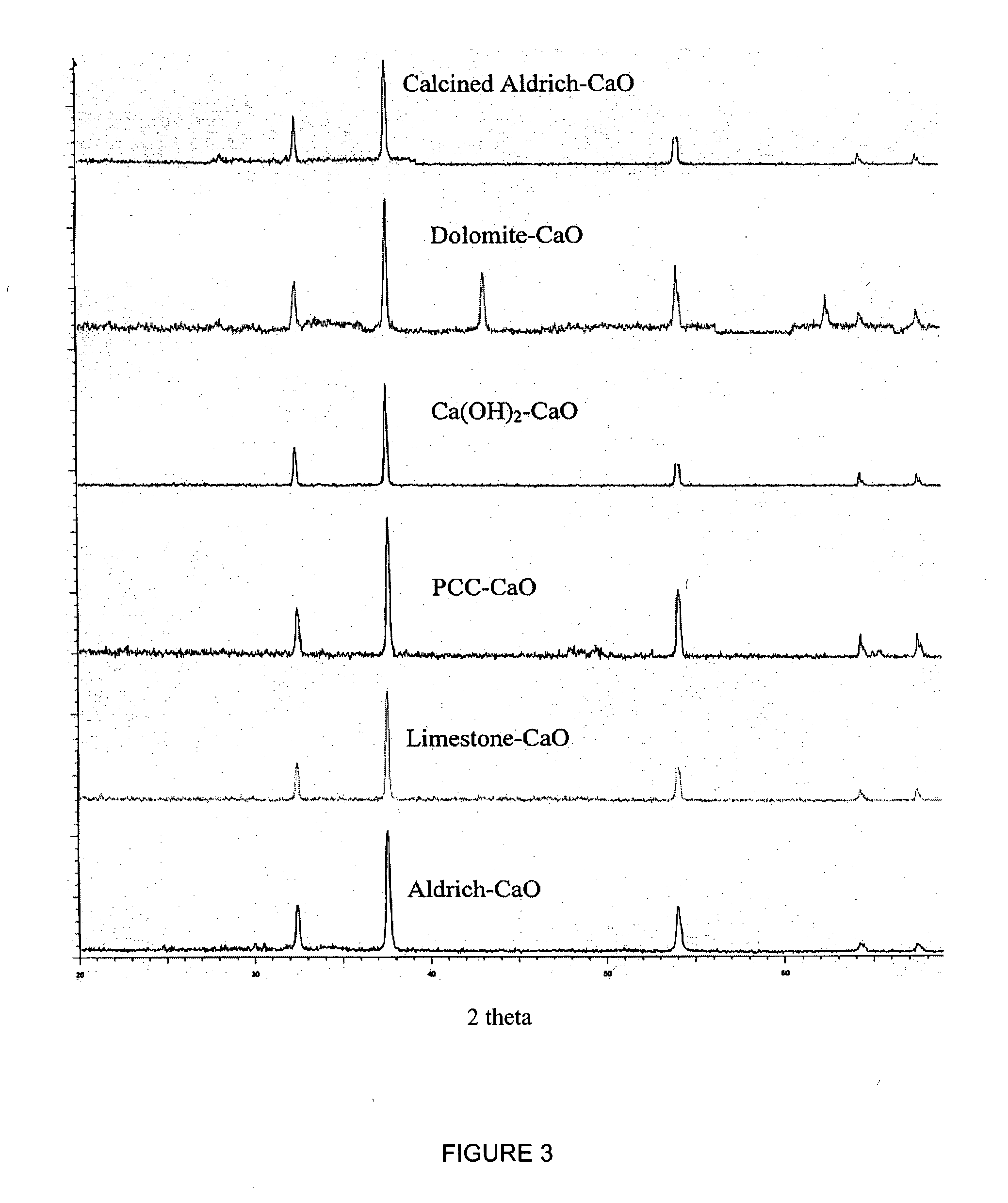
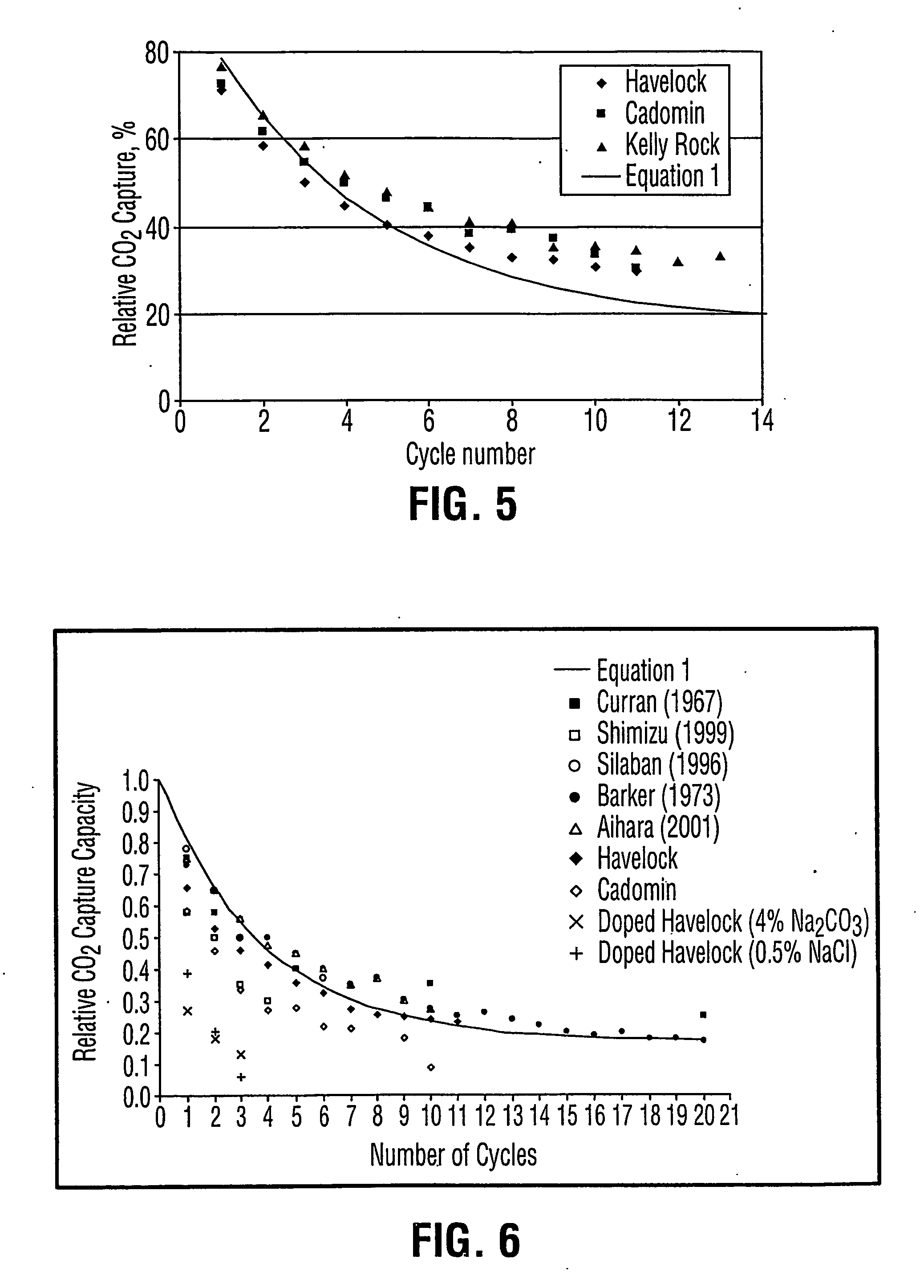
A reaction-based process has been developed for the selective removal of carbon dioxide from a multicomponent gas mixture. The proposed process effects the separation of CO2 from a mixture of gases by its reaction with metal oxides. The Calcium based Reaction Separation for CO2 process consists of contacting a CO2 laden gas with calcium oxide in a reactor such that CaO captures the CO2 by the formation of calcium carbonate. Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent. The “regenerated” CaO is then recycled for the further capture of more CO2. This process also identifies the application of a mesoporous CaCO3 structure, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Sorbent for separation of carbon dioxide (CO2) from gas mixtures
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
Separation of Carbon Dioxide (Co2) From Gas Mixtures By Calcium Based Reaction Separation (Cars-Co2) Process
InactiveUS20080233029A1Good repeatabilityMaterial nanotechnologyCombustible gas catalytic treatmentSorbentTransformation ratio
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS—CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCOa). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS—CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
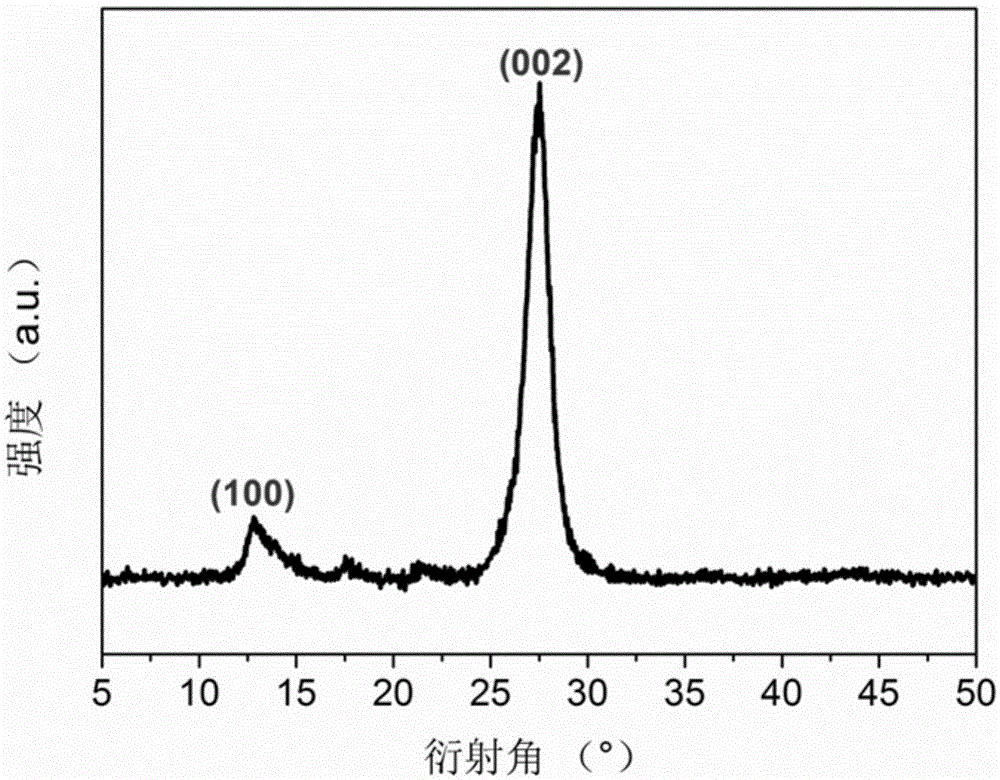
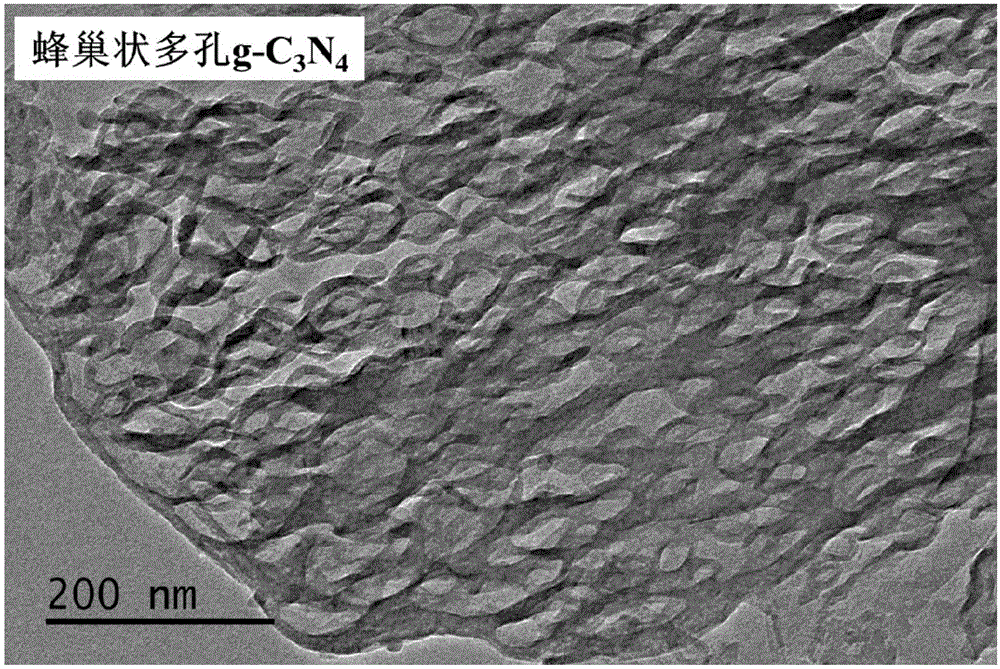
Graphite-phase carbon nitride (g-C3N4) material and preparation method and application thereof
ActiveCN105126893APlay a pore-forming roleAppropriate speedPhysical/chemical process catalystsWater/sewage treatment by irradiationCalcinationMaterials science
The invention relates to a method for preparing a graphite-phase carbon nitride (g-C3N4) material. The method includes the steps that a carbon nitride precursor and ammonium salt are evenly mixed, and then calcination is conducted so that the porous g-C3N4 material can be obtained. The ammonium salt is any one of ammonium base salts capable of generating ammonia gas through thermal decomposition or is the combination of at least two of the ammonium base salts. In the preparation process of the g-C3N4 material, the ammonium salt is added into the carbon nitride precursor to be mixed. In the high-temperature calcining process, the ammonium salt is subjected to pyrogenic decomposition to generate gas, a pore-forming effect on the g-C3N4 material is achieved, and the cellular porous g-C3N4 material is obtained. In the preparation process of the g-C3N4 material, template agents are not used, and thus the method is simple, efficient and environmentally friendly; the prepared g-C3N4 material is high in photocatalytic activity and can be used in the pollution control processes such as exhaust gas and wastewater treatment.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
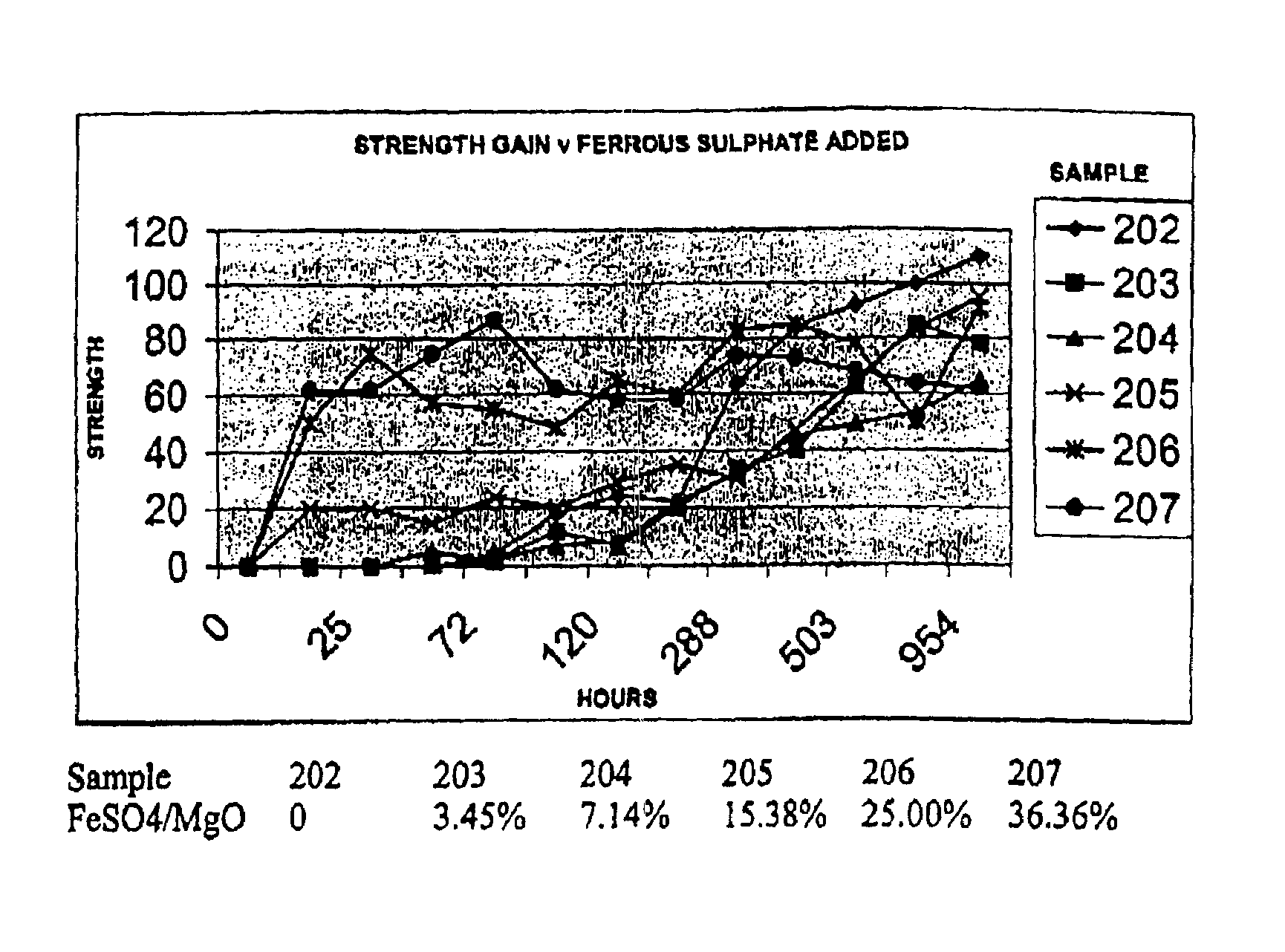
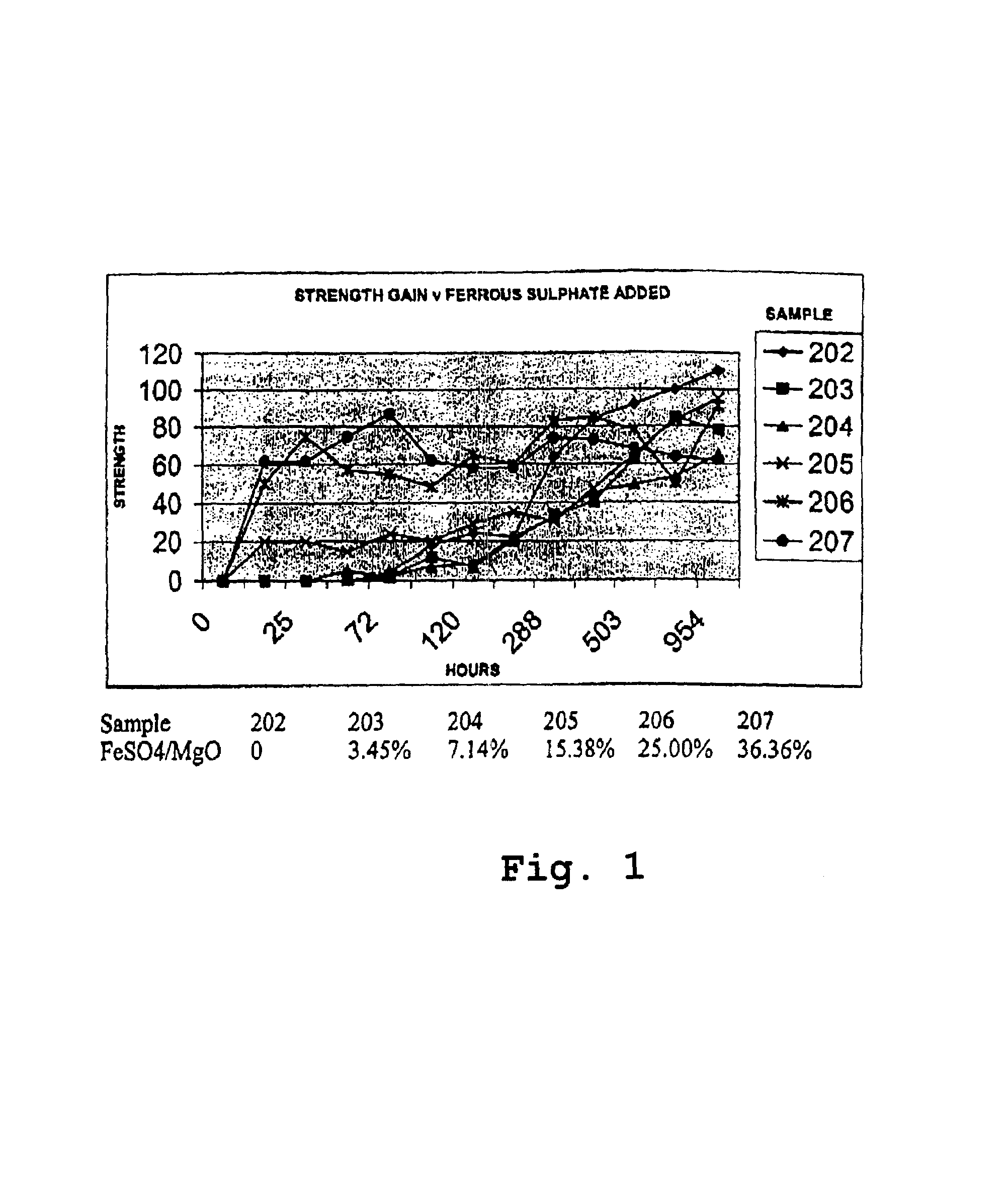
Reactive magnesium oxide cements
Novel hydraulic cements are disclosed that include reactive magnesium oxide prepared by low temperature calcination. The cements can be formulated to suit a large number of applications with various setting times, strength and levels of sustainability either by adding iron salts such as ferrous sulfate or blending with other compatible faster setting hydraulic cements such as Portland cement or by using both methods.The compositions are able to incorporate relatively large amounts of low cost pozzolans such as fly ash to advantage as well as wastes. Many excellent properties are exhibited and in particular good comprehensive strength and resistance to sulfates is able to be achieved.
Owner:TECECO
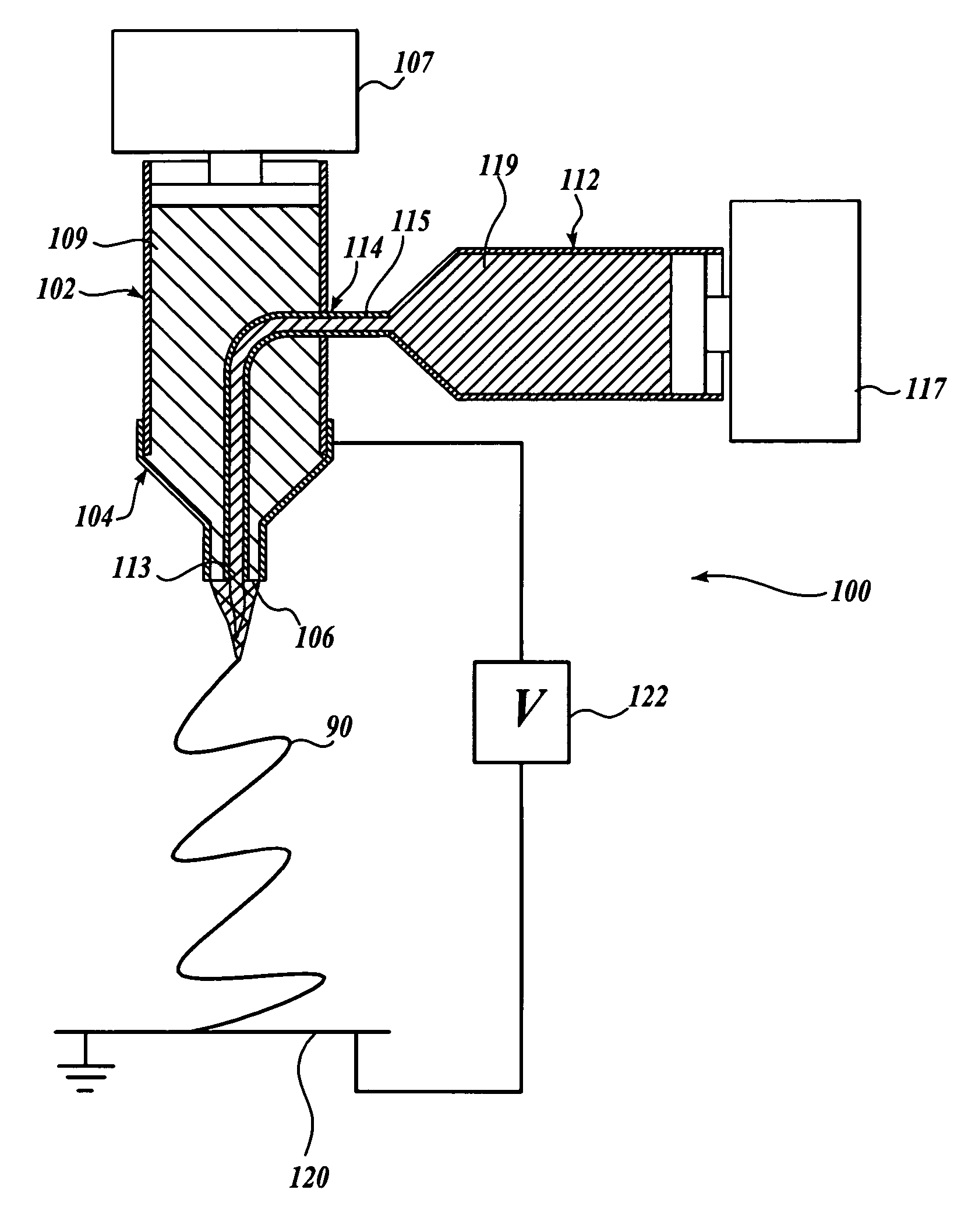
Electrospinning of fine hollow fibers
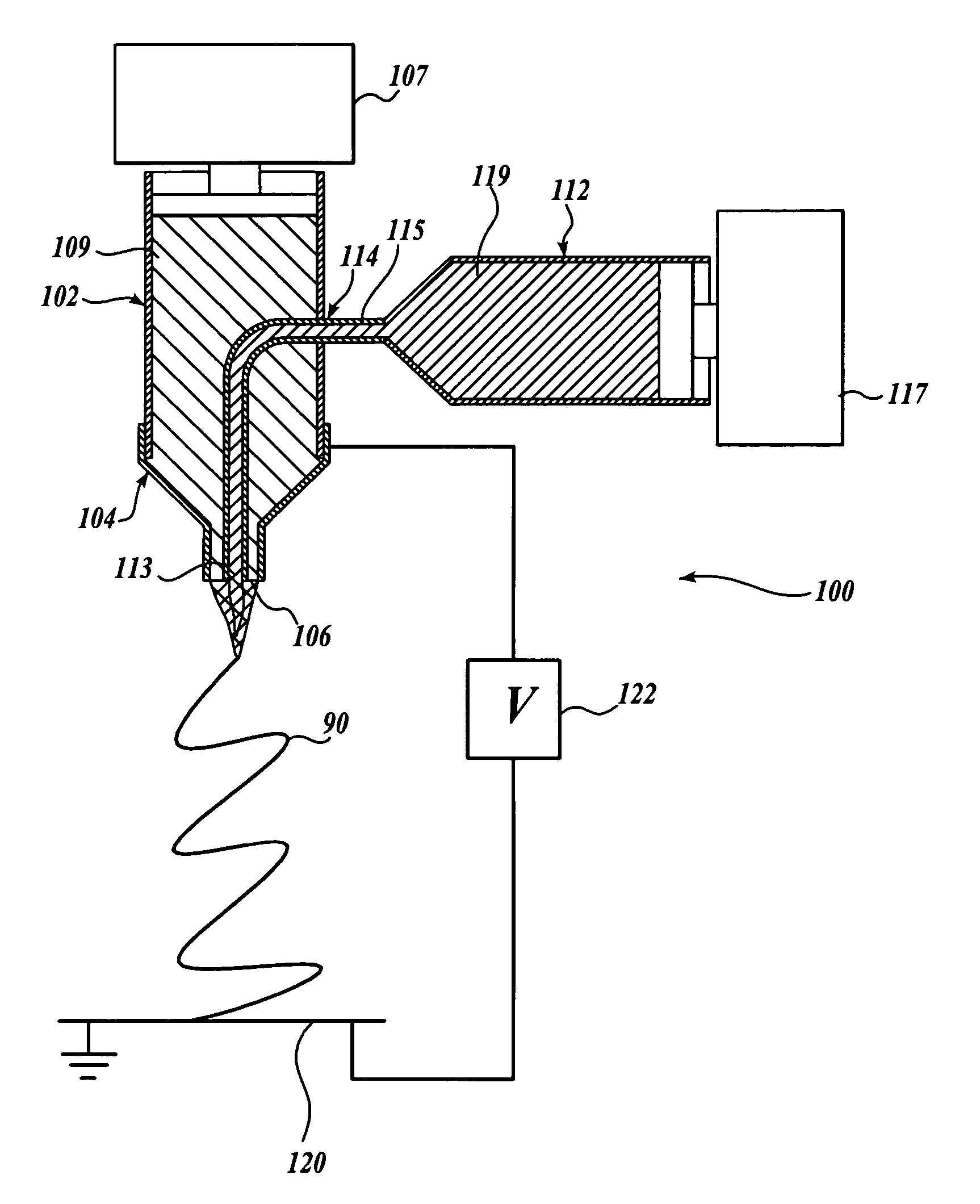
A method for electrospinning nanofibers having a core-sheath, tubular, or composite structure is disclosed. The process uses a spinneret having first and second capillaries that channel first and second fluids in the spinneret, the second capillary surrounding the first. A high voltage is applied between the spinneret and a spaced conductive collector. In one embodiment, the first fluid is a mineral oil and the second fluid is a polymeric solution that may include a polymer, a catalyst, a solvent, and a sol-gel precursor. The as-spun nanofiber includes an oil core and a composite sheath. The oil may be removed to produce a composite tubular fiber or the polymer and oil may be removed by calcination to produce a ceramic tubular fiber. In other embodiments, miscible fluids are used to produce porous nanofibers, selected additives functionalize the surfaces of the nanofibers and / or conjugated polymers are used.
Owner:UNIV OF WASHINGTON
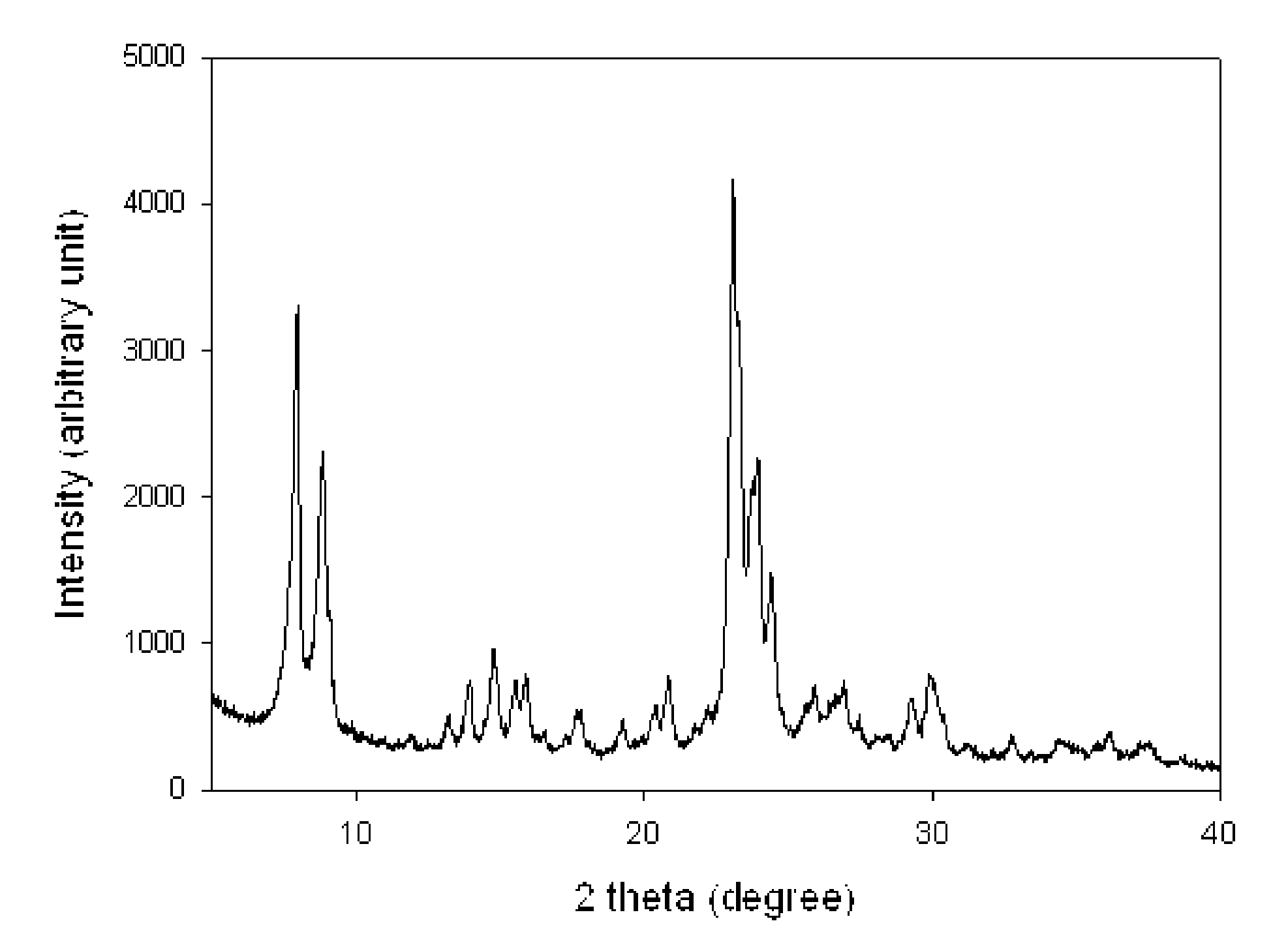
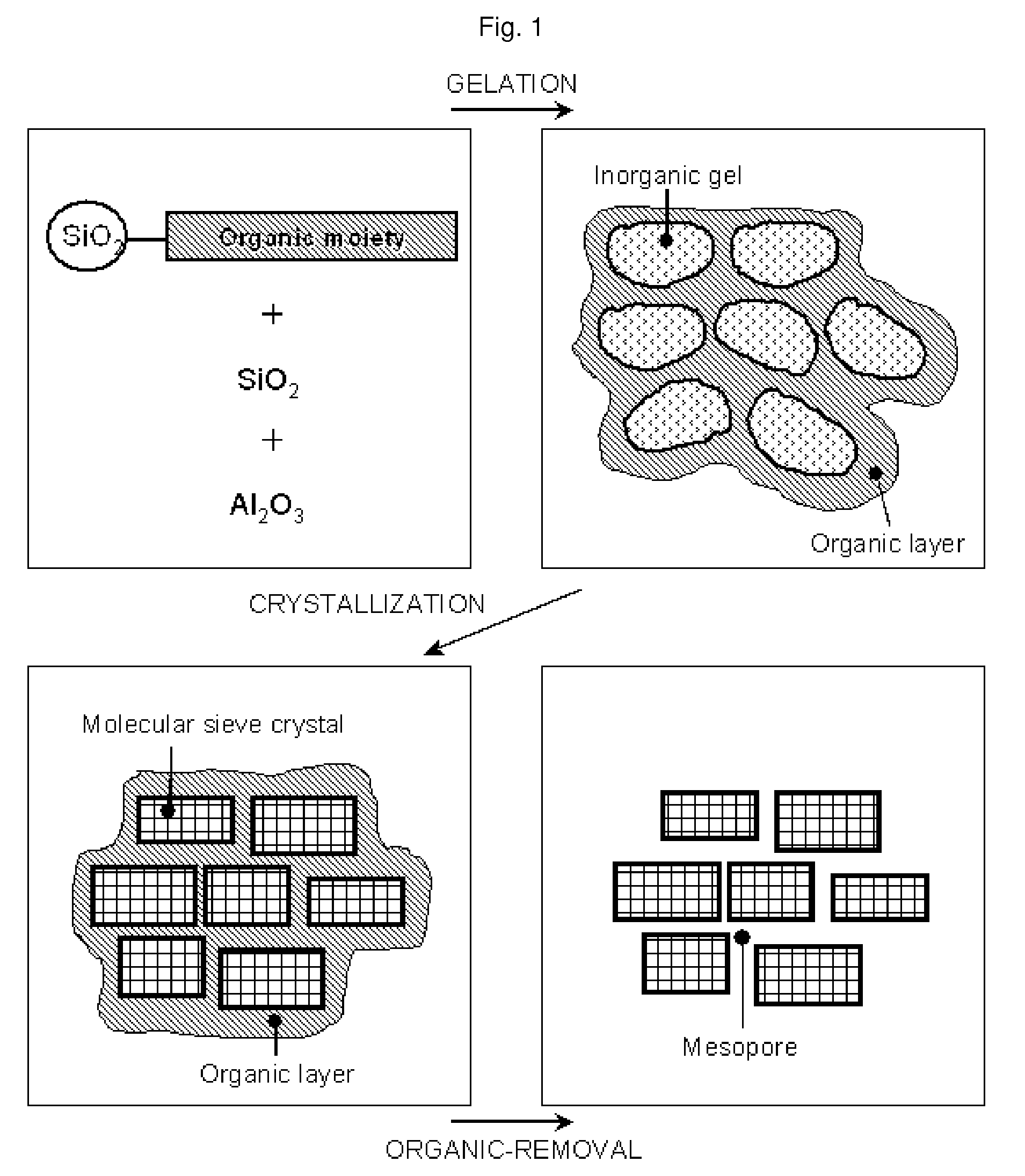
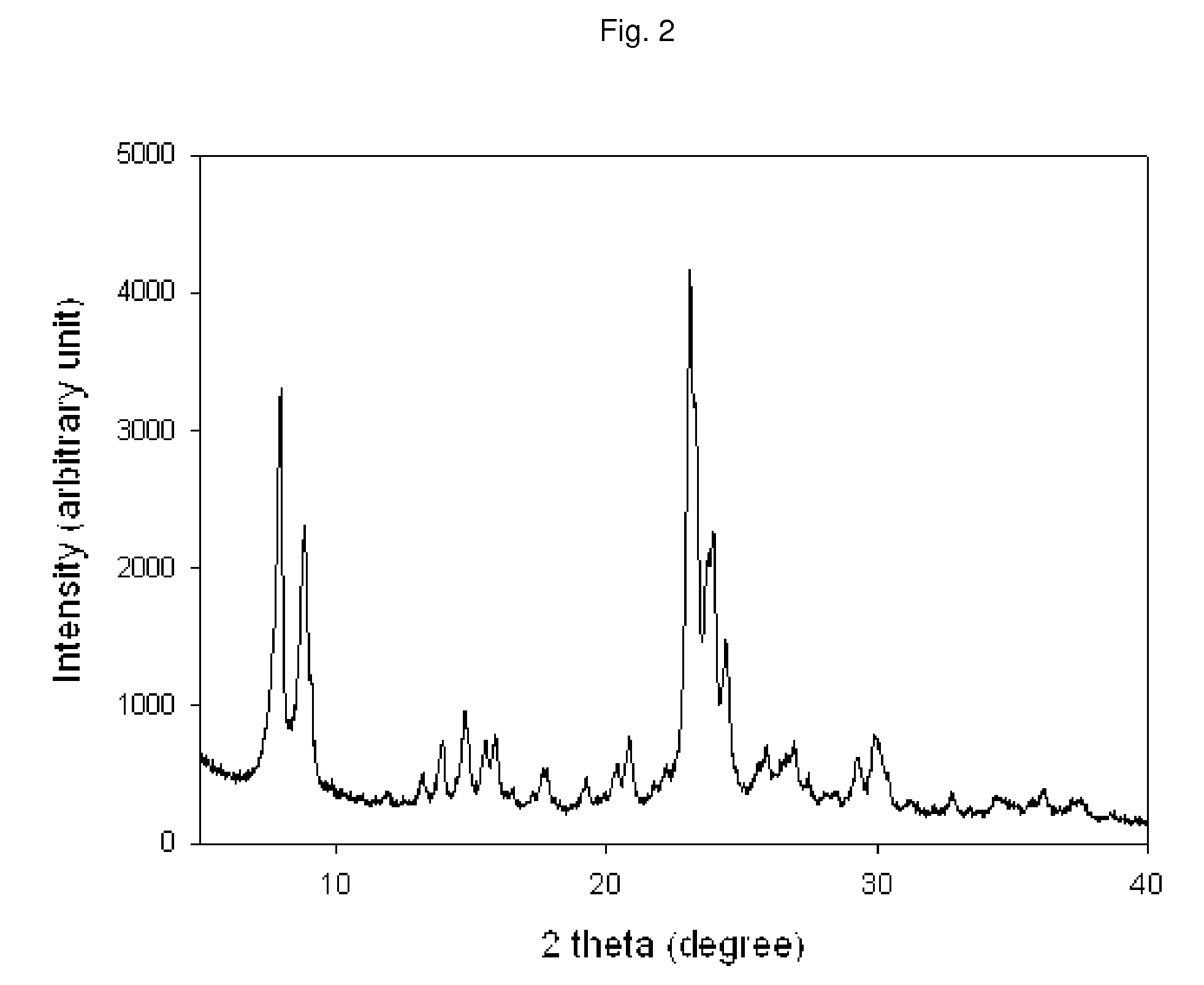
Method of the preparation of microporous crystalline molecular sieve possessing mesoporous frameworks
InactiveUS7785563B2Facilitate diffusion and adsorptionImprove overall utilizationAluminium compoundsSilicaMolecular sieveChemical treatment
The present invention relates to a method of preparing a microporous crystalline molecular sieve having mesoporous skeleton, comprising following steps: (a) adding a meso-SDA (meso-Structure Directing Agent) into a gel composition of synthesizing molecular sieve, (b) subjecting the mixture obtained in the above step (a) to crystallization by a hydrothermal reaction, a microwave reaction, a dry-gel synthesis, etc., and (c) removing selectively organic materials from the resulted material obtained in the above step (b) by a calcination or a chemical treatment. Molecular sieve having mesoporous skeleton synthesized by the present invention exhibits, as compared with conventional zeolite, a good molecule diffusion ability and a greatly improved catalytic activity.
Owner:KOREA ADVANCED INST OF SCI & TECH
Core-shell synthesis of carbon-supported alloy nanoparticle catalysts
InactiveUS7053021B1Good monodispersityEfficient activationMaterial nanotechnologyTransportation and packagingFuel cellsBiological activation
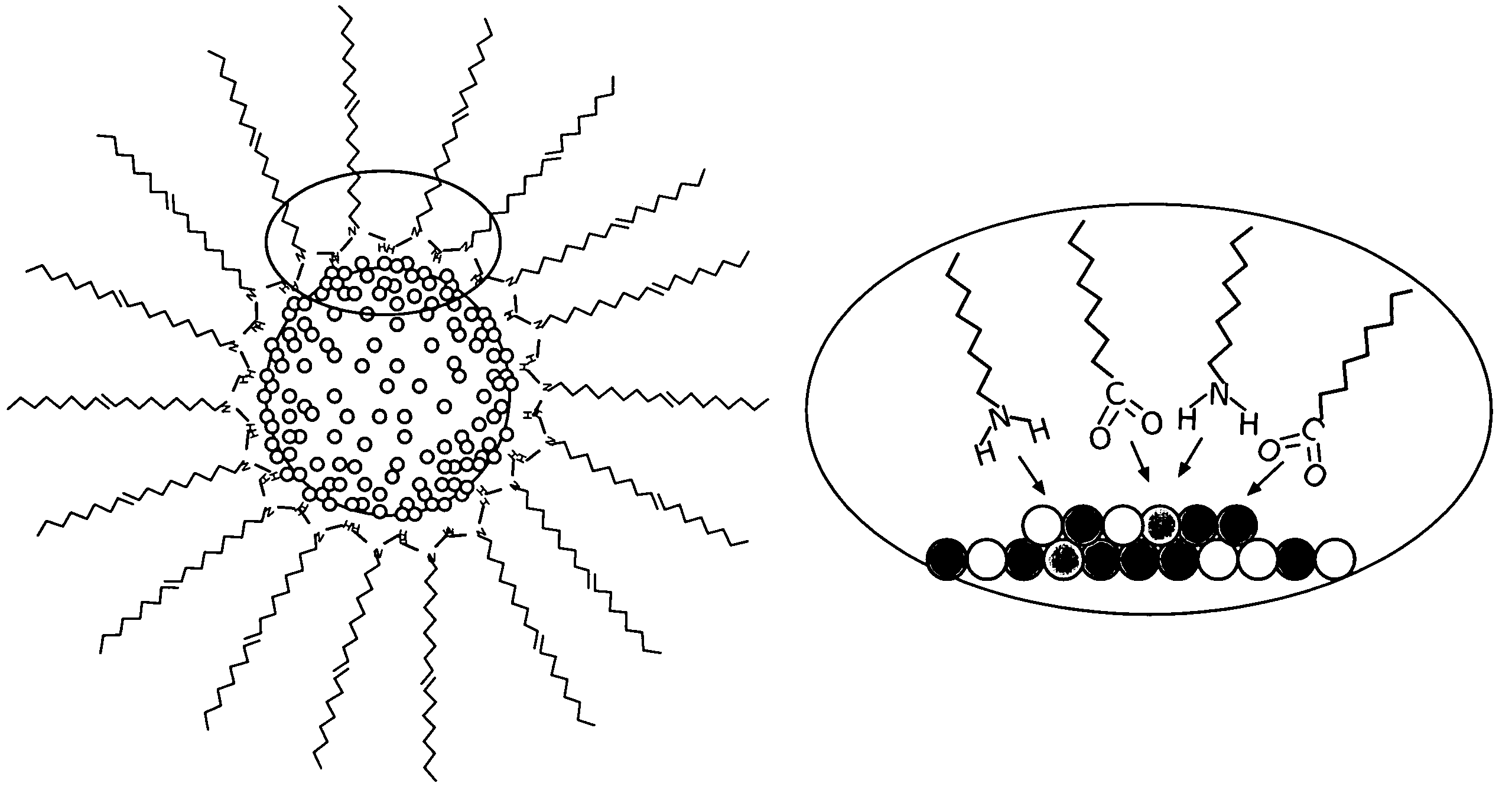
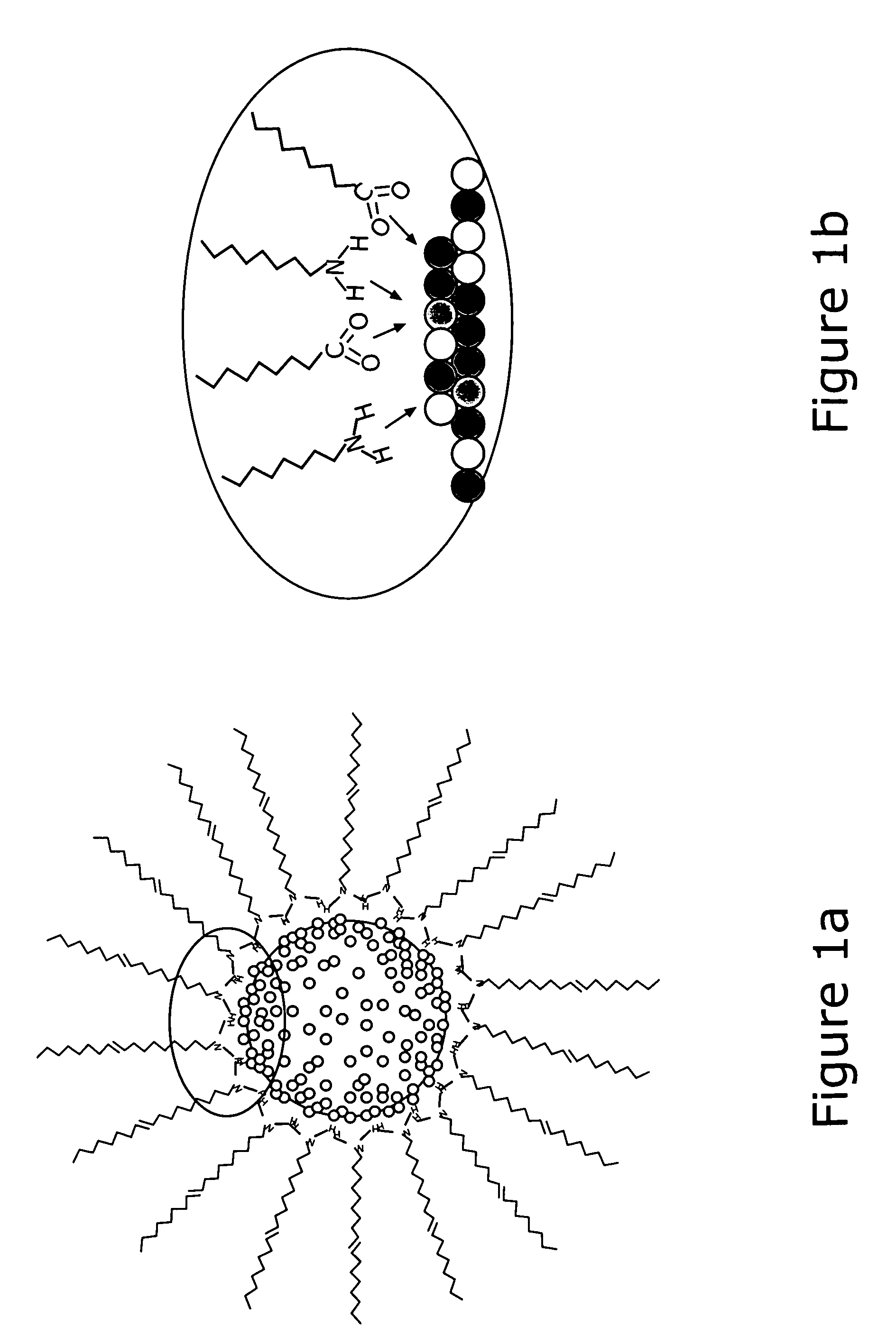
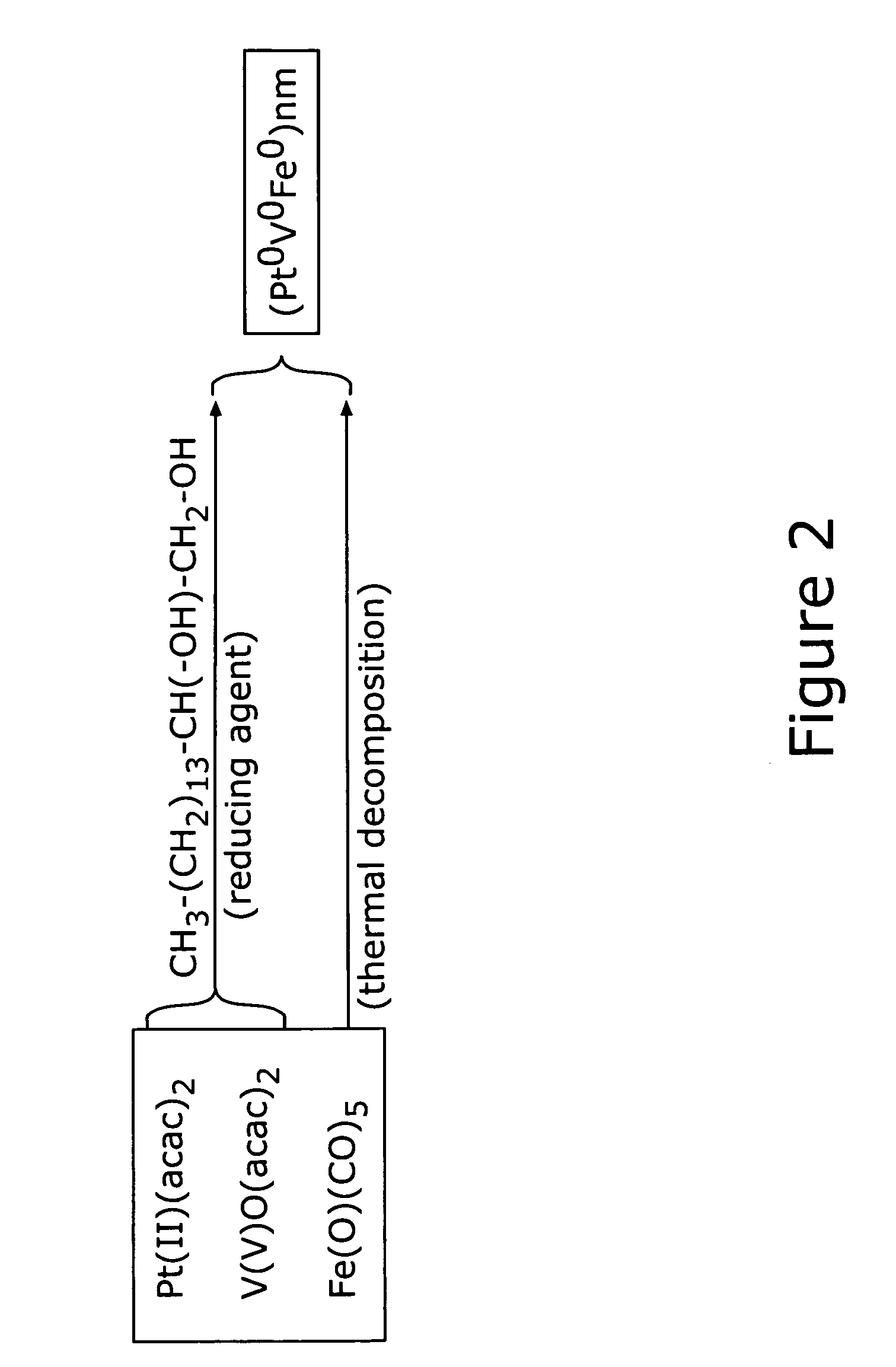
There is provided a method of preparing carbon supported, ternary alloy composition core-shell PtVFe nanoparticles for use as fuel cell electrocatalysts. These catalysts have been found particularly useful for oxygen reduction reactions. The alloy nanoparticles can be assembled on carbon supports which then may undergo subsequent activation and / or calcination treatments. The method, combined with new synthetic feed and processing conditions, provides core-shell PtVFe alloy nanoparticles of 1–3 nm size. The catalyst-produced high monodispersity, controlled composition are highly dispersed, and have a uniform distribution. Finally, the correlation of the preparation and treatment parameters to the ORR catalytic activities of the prepared nanoparticles is described. The catalysts exhibit ORR in the range of 2 to 4 times more than a standard Pt / carbon catalyst.
Owner:HONDA MOTOR CO LTD +1
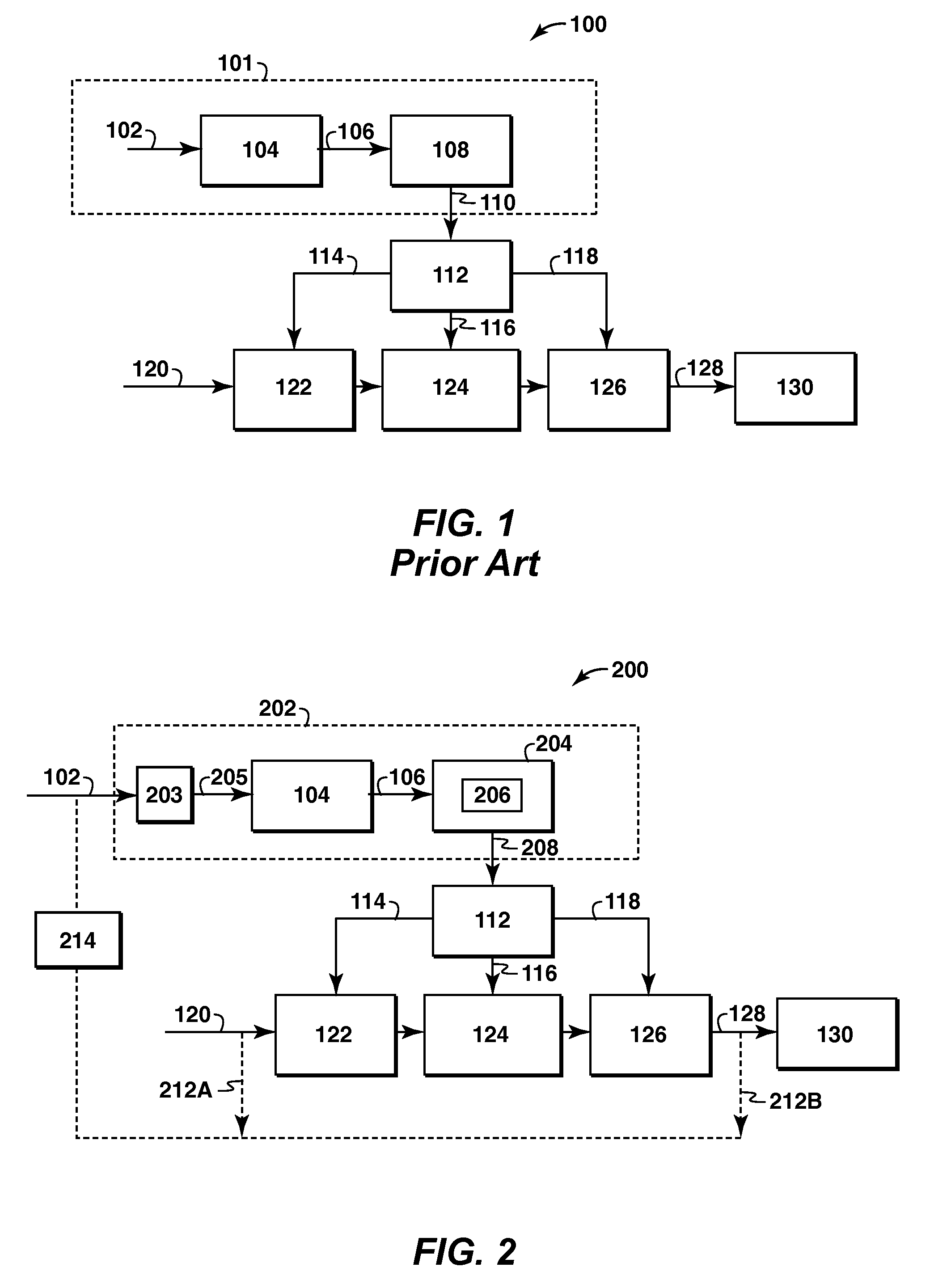
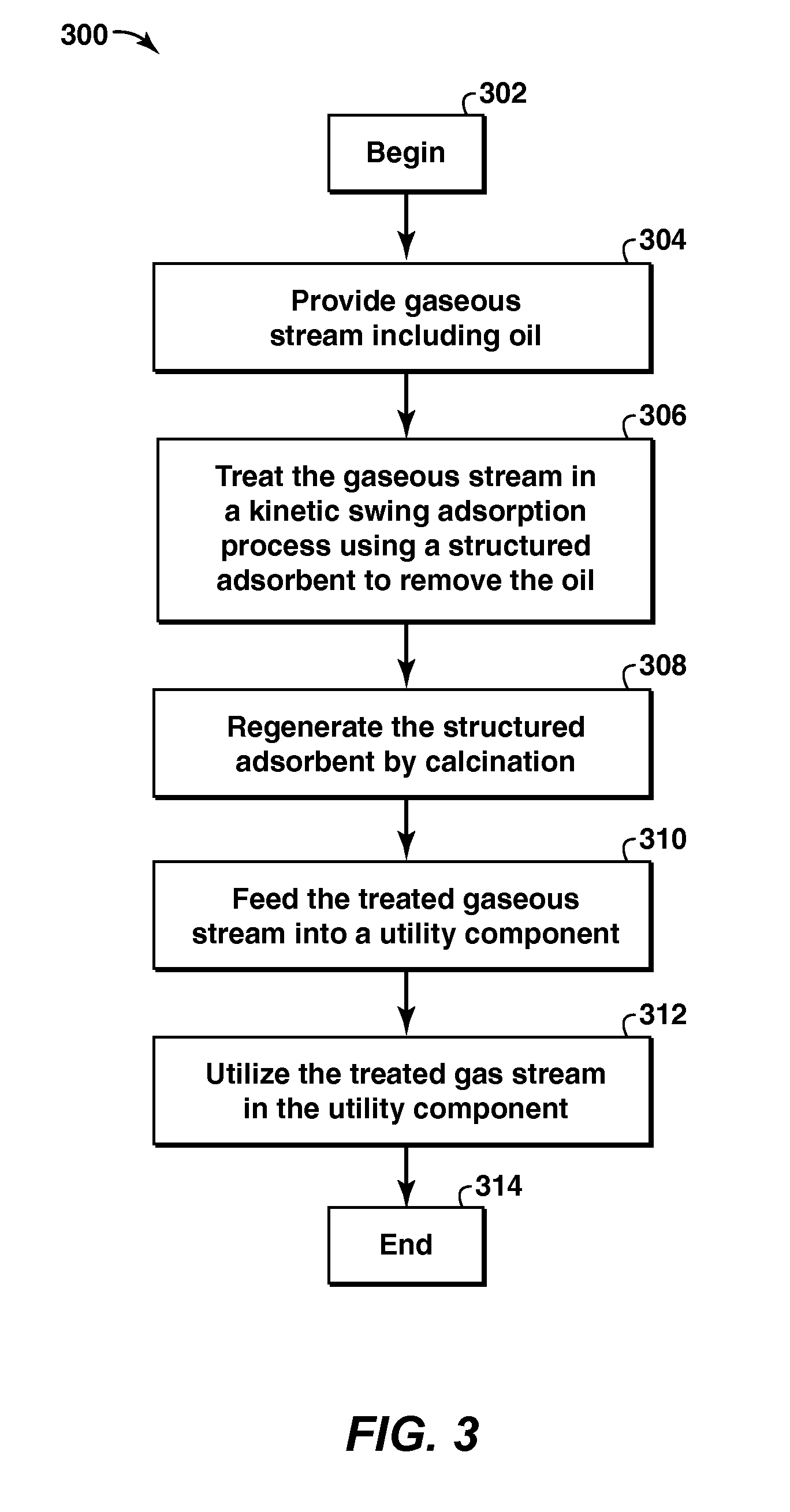
Method and Apparatus For Removal Of Oil From Utility Gas Stream
The present application is directed to a method and system for preparing gaseous utility streams from gaseous process streams, particularly, removing oil contamination from such streams prior to use in a dry gas seal. The methods and systems may include at least one kinetic swing adsorption process including pressure swing adsorption, temperature swing adsorption, calcination, and inert purge processes to treat gaseous streams for use in dry gas seals of rotating equipment such as compressors, turbines and pumps and other utilities. The adsorbent materials used include a high surface area solid structured microporous and mesoporous materials.
Owner:EXXONMOBIL UPSTREAM RES CO
Method for cooperative activation of fly ash and decomposition of gypsum for recovery of sulfur resource
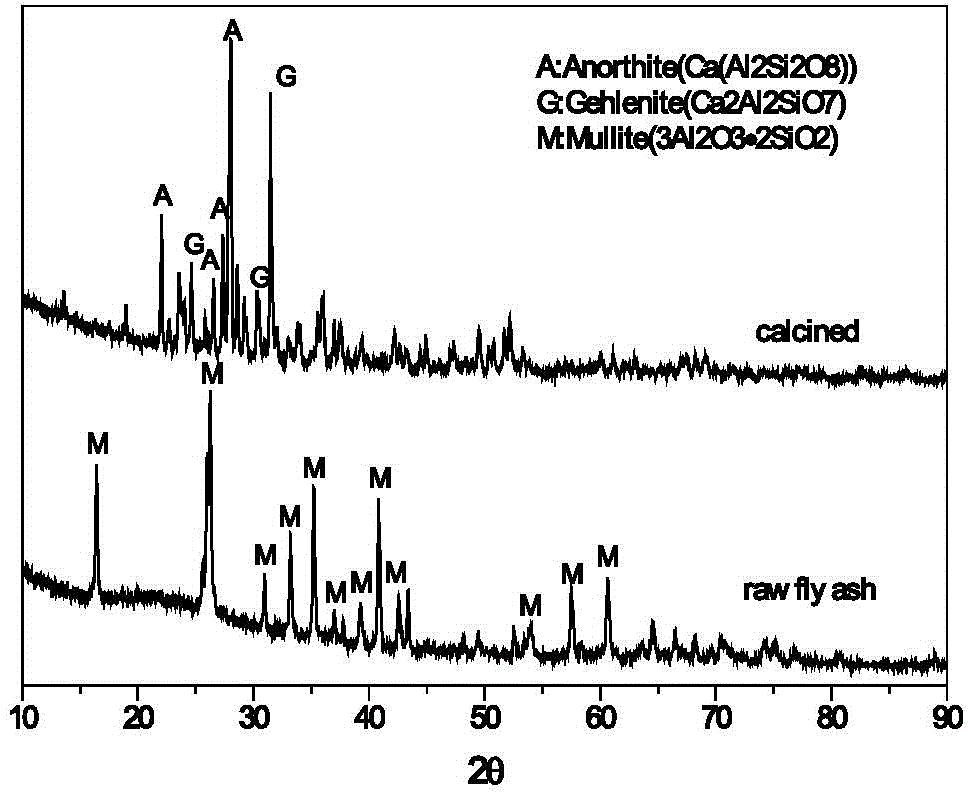
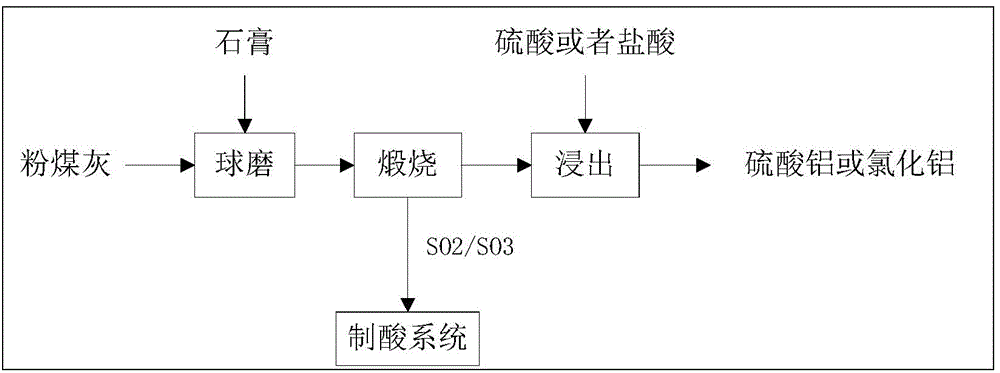
The invention provides a method for cooperative activation of fly ash and decomposition of gypsum for recovery of a sulfur resource. According to the method, solid waste, i.e., fly ash, discharged by a coal-fired power plant or coal-fired boiler is used as a raw material, a certain proportion of desulfurized gypsum discharged by the coal-fired power plant or waste phosphogypsum produced in the phosphorus chemical industry is added and mixed with the fly ash, then the obtained mixture is subjected to ball milling, and activation and calcination at a temperature of 950 to 1450 DEG C are carried out for 5 to 180 min; calcium sulfate in the gypsum are almost totally decomposed after calcination, and produced gas contains sulfur dioxide or sulfur trioxide which can be used as feed gas for preparation of sulfuric acid; and calcination enables solid fly ash to be activated, leaching with a sulfuric acid or hydrochloric acid solution is carried out at a temperature of 50 to 100 DEG C, and the leaching rate of alumina is greater than 80%. The method provided by the invention has the advantages that since all the raw materials are solid waste, the purpose of treating the waste by using the waste is achieved; elemental sulphur in the gypsum can be recovered; and the fly ash can be activated and activity of the fly ash can be improved, so a high alumina recovery rate at a low temperature can be realized. With the method, high-efficiency extraction of alumina in the fly ash is realized; the sulfur resource in the gypsum is recovered; shortage in industrial sulphur in the sulfuric acid industry in China is compensated; and the method has good economic benefits and wide industrial application prospects.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method of producing an aromatics alkylation catalyst
This invention provides a method of producing an aromatics alkylation catalyst comprising the steps of: (a) synthesizing a layered oxide material MCM-56 in the presence of alkali and / or alkaline earth metal cations; (b) prior to any calcination of the MCM-56, subjecting the MCM-56 produced in step (a) to ammonium ion exchange so as to at least partially replace the alkaline or alkaline earth metal cations associated with the MCM-56 with ammonium ions; then (c) heating the ammonium-exchanged MCM-56 to decompose the ammonium cations and convert the MCM-56 into the hydrogen form; and (d) after step (b), forming the MCM-56 into catalyst particles. The resultant catalyst exhibits enhanced activity in the alkylation of benzene with ethylene and propylene.
Owner:MOBIL OIL CORP
Monolithic honeycomb structure made of porous ceramic and use as a particle filter
InactiveUS6582796B1Prevent the evaporation of the waterEasy curingInternal combustion piston enginesSilencing apparatusSodium BentoniteOxygen
A monolithic honeycomb-type structure useful in particular as a particle filter for exhaust gases from diesel engines has a number of passages that empty into the end faces of said monolith, but are alternately open and sealed. The monolith consists of a porous refractory material that comprises: 70 to 97% by mass of alpha and / or beta crystallographic-type silicon carbide that has at least one particle size and preferably at least two particle sizes, and 3 to 30% by mass of at least one bonding ceramic phase in the form of a micronic powder or particles that are obtained by atomization, comprising at least one simple oxide, for example, B2O3, Al2O3, SiO2, MgO, K2O, Li2O, Na2O, CaO, BaO, TiO, ZrO2 and Fe2O3 and / or at least one mixed oxide, for example, the alkaline aluminosilicates (of Li, Na, or K) or alkaline-earth aluminosilicates (of Mg, Ca, Sr or Ba), clays, bentonite, feldspars or other natural silico-aluminous materials. The production of the monolith comprises a calcination stage under an oxygen-containing atmosphere at a temperature up to 1650° C., but less than 1550° C.
Owner:INST FR DU PETROLE
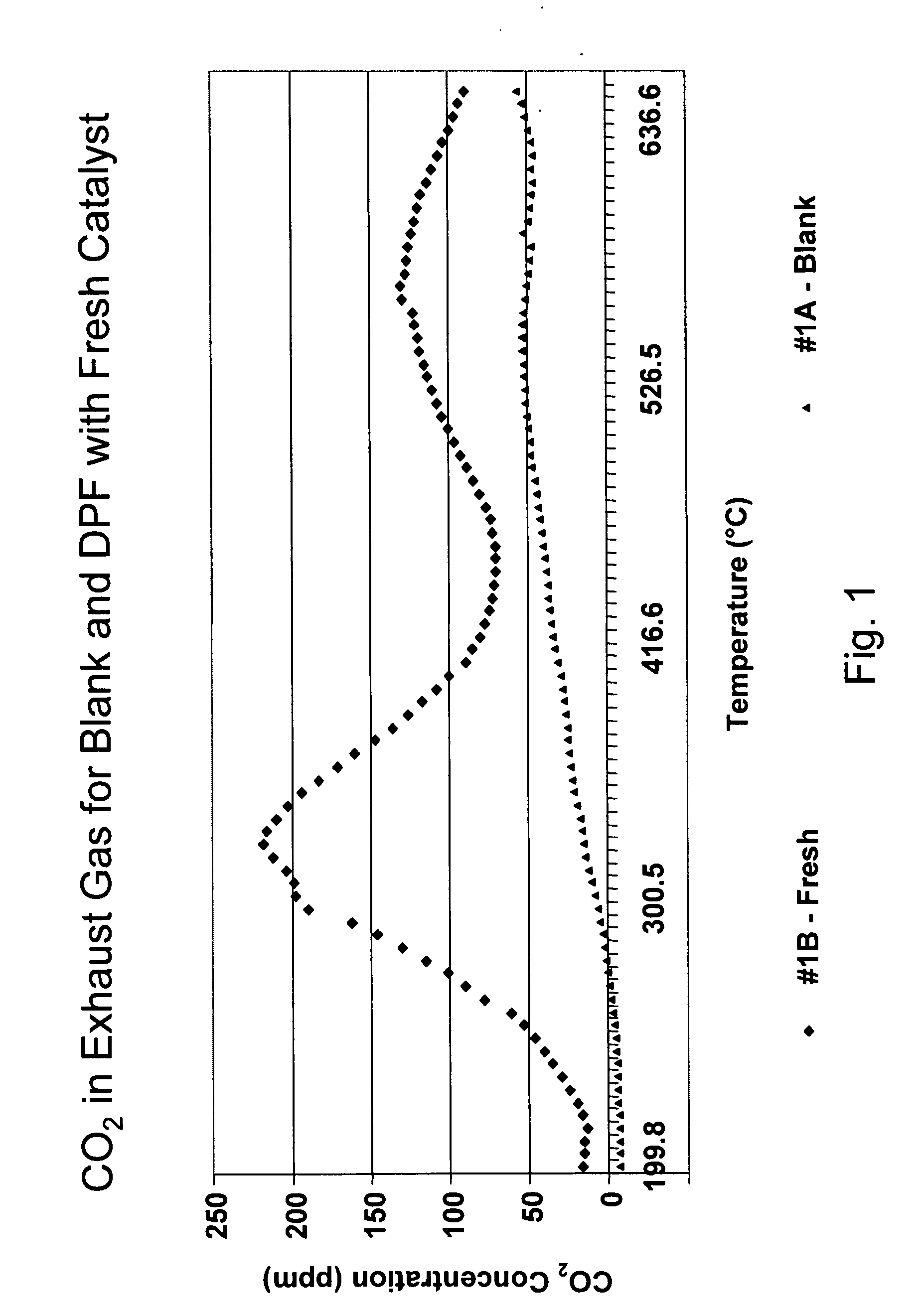
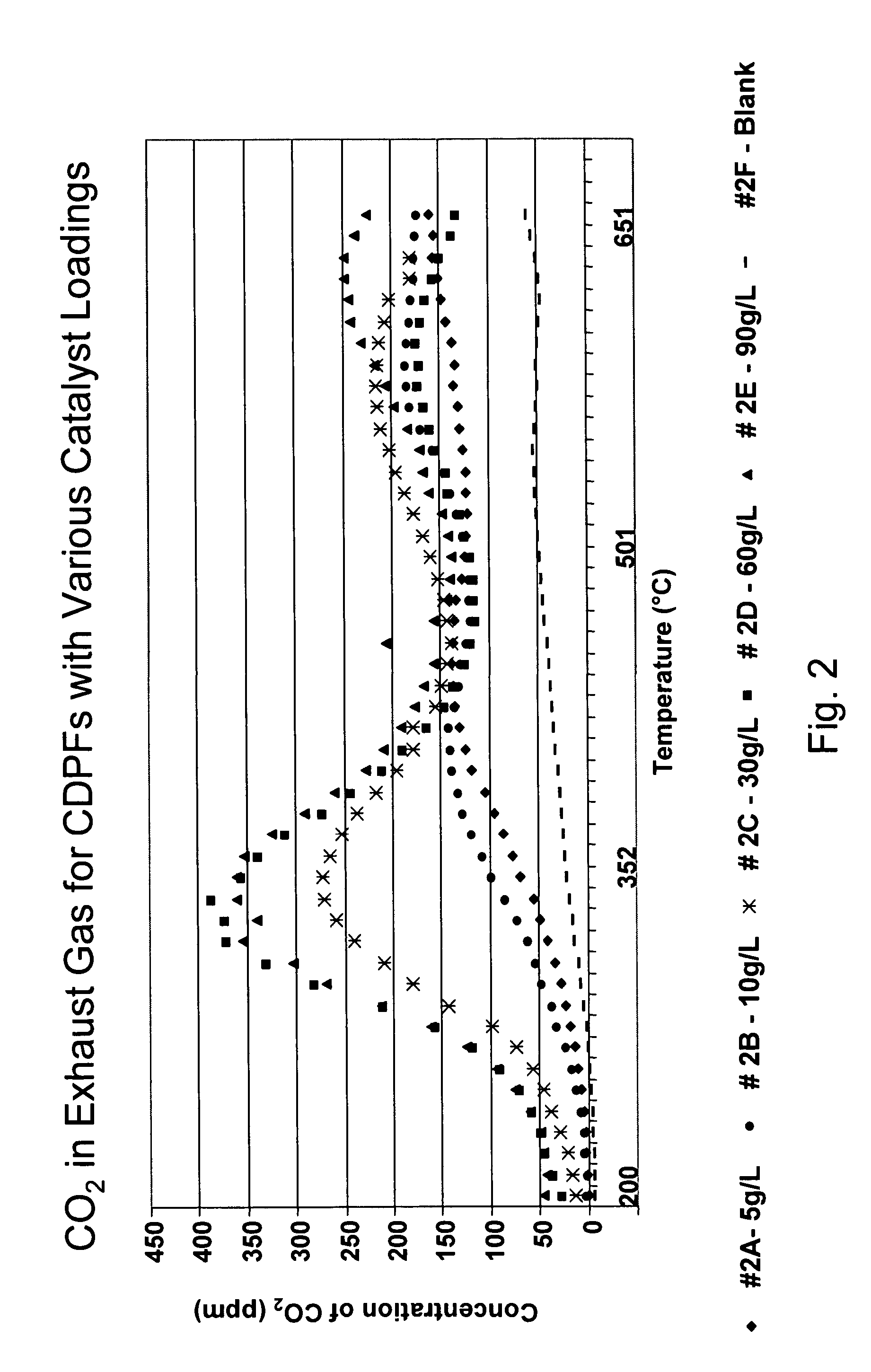
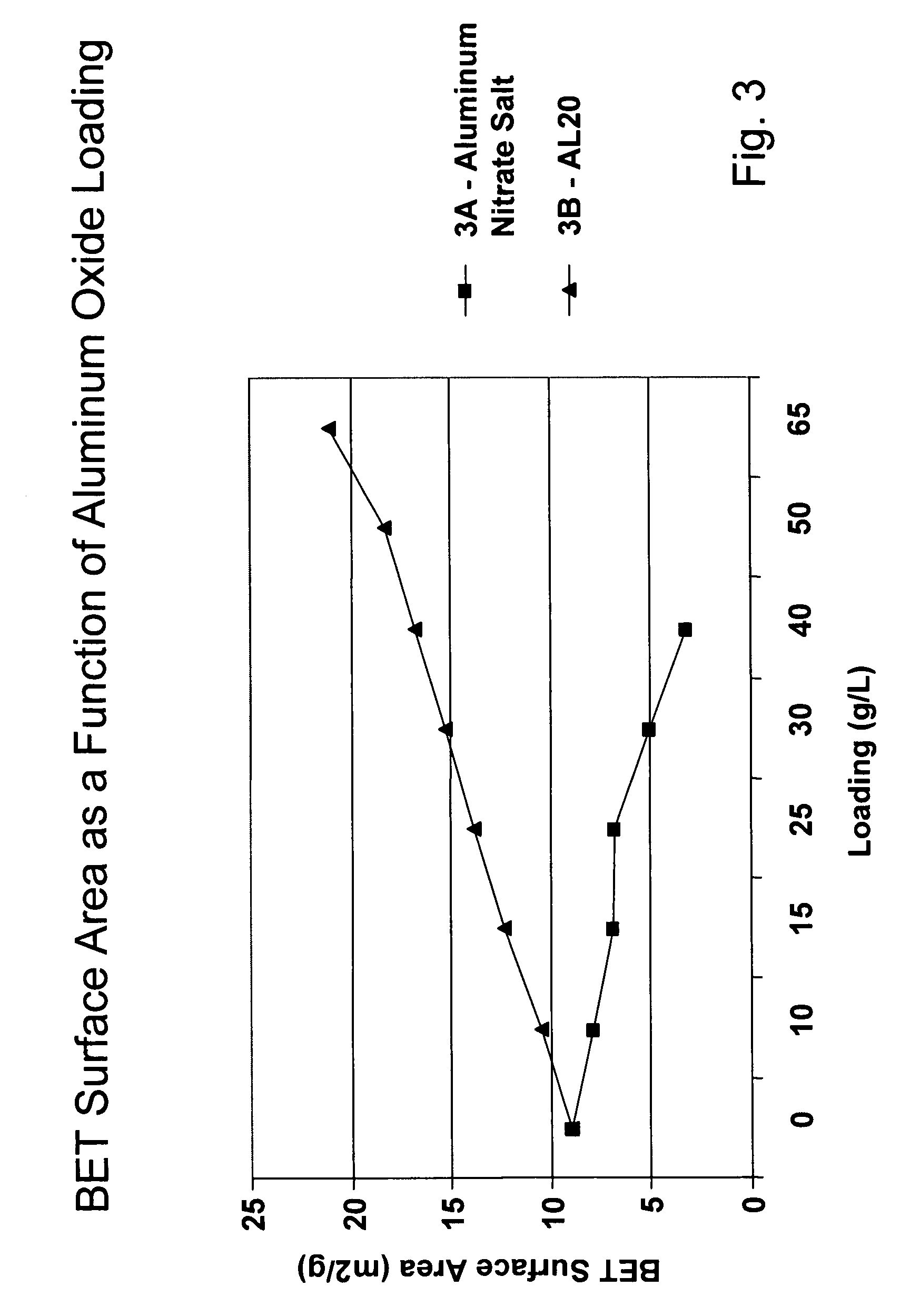
Platinum group metal-free catalysts for reducing the ignition temperature of particulates on a diesel particulate filter
A catalyzed diesel particulate filter (CDPF) and a method for filtering particulates from diesel engine exhaust are provided, where the catalyzed diesel particulate filter includes a substrate and a catalyst composition, where the catalyst composition contains at least one first component, at least one second component, and at least one third component, where the first component is at least one first component selected from the group consisting of cerium and a lanthanide and mixtures thereof, the at least one second component is selected from the group consisting of cobalt, copper, manganese and mixtures thereof; and the third component comprises strontium, where the first component, the second component, and the third component are in an oxide form after calcination. The catalyst on the catalyzed diesel particulate filter lowers the temperature at which particulates are removed from the CDPF by oxidizing the particulates on the filter. The catalyzed diesel particulate filter may also include a washcoat. Washcoats prepared from colloidal aluminum oxide may have higher surface areas and pore volumes loadings than washcoats containing aluminum oxide prepared from aluminum nitrate.
Owner:CATALYTIC SOLUTIONS INC
Quasi-thin empholite composition containing organic reaming agent
ActiveCN1611300AReduce crystallinityLarge hole volumeCatalyst activation/preparationRefining to eliminate hetero atomsDiasporeResidual oil
The present invention relates to a pseudothin diaspore composition containing organic pore-expanding agent. Said composition contains 92-99.5 wt% of pseudothin diaspore and 0.5-8 wt% of organic pore-expanding agent, in which the crystallinity of pseudothin diaspore is 10-70%. In the pseudothin diaspore composition provided by said invention the organic pore-expanding agent content is low, after high-temperature calcination the alumina with large pore capacity and large pore diameter can be obtained. Said alumina can be used as carrier material for preparing hydrodemetalization catalyst for heavy oil, residual oil and short residuum specially.
Owner:CHINA PETROLEUM & CHEM CORP +1
Recovery of common salt and marine chemicals from brine
InactiveUS6776972B2High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Method for preparing graphene-like two-dimensional laminar titanium carbide nanoplate
InactiveCN104016345AHigh purityHigh crystallinityMaterial nanotechnologyTitanium carbideSem micrographsCrystallinity
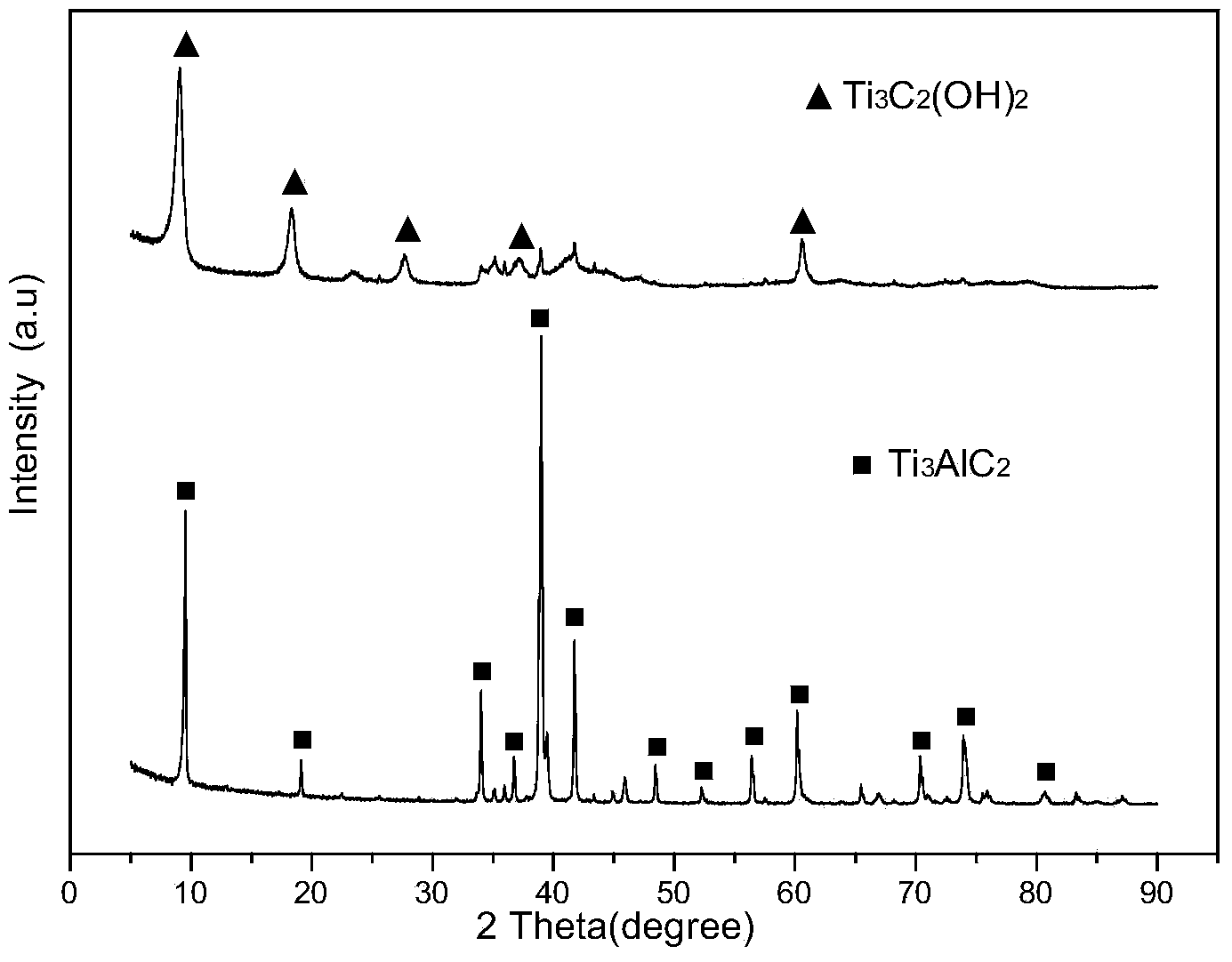
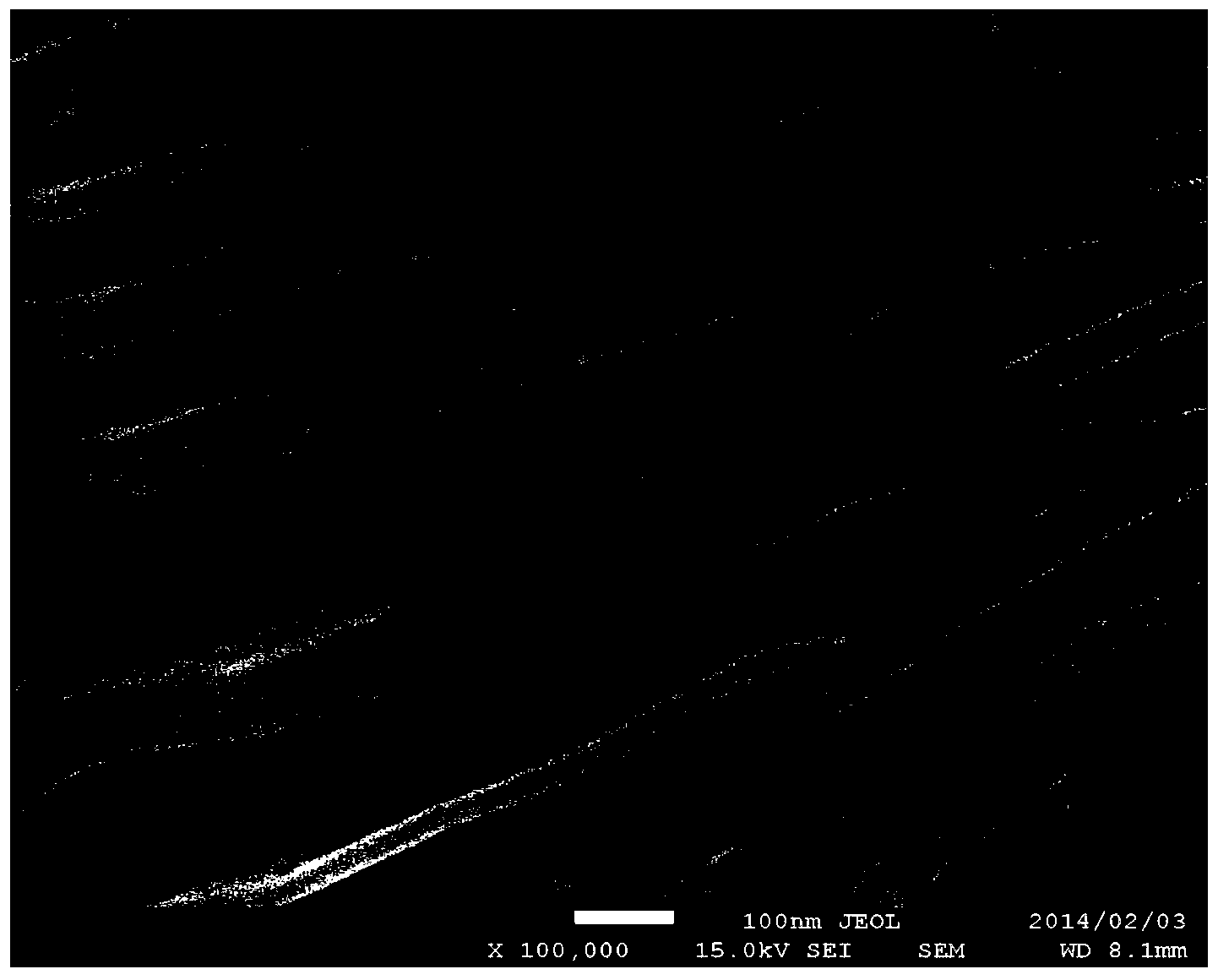
The invention discloses a method for preparing a graphene-like two-dimensional laminar titanium carbide nanoplate. The method comprises the following steps: preparing Ti3AlC2 powder by an in-situ hot-pressing solid-liquid reaction; preparing two-dimensional titanium carbide by a chemical liquid phase reaction; performing vacuum calcination post-treatment, and the like. The method can be used for preparing a Ti3AlC2 precursor with excellent crystallinity and high purity under simple process flow, stable process parameters, controllable process, high efficiency, low cost, short time and low pressure; the information that the transverse size of the two dimensional Ti3AlC2 nanoplate prepared by the method can be 5-10 microns, the average thickness of a single layer is about 10-20 nanometers can be found from an SEM picture, the inter-laminar spacing is remarkably enlarged after calcination treatment, and the laminar surface is regular and smooth.
Owner:HOHAI UNIV
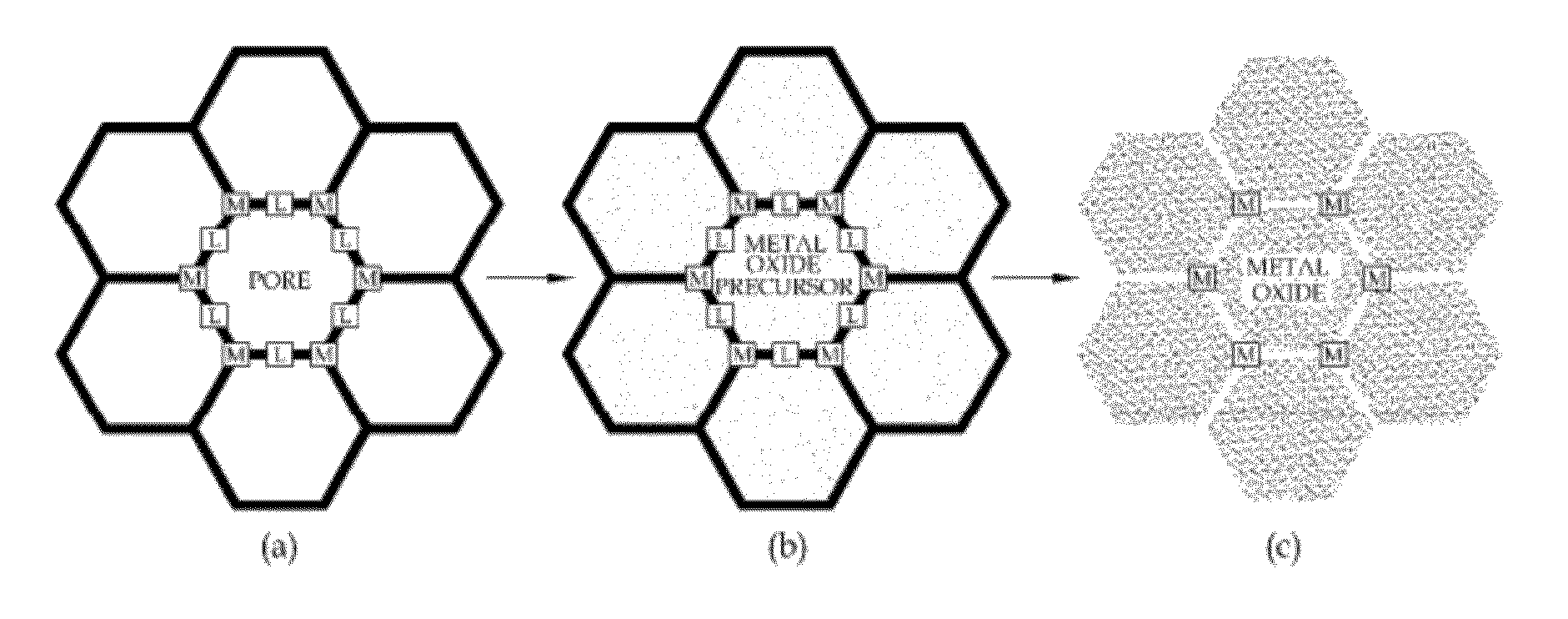
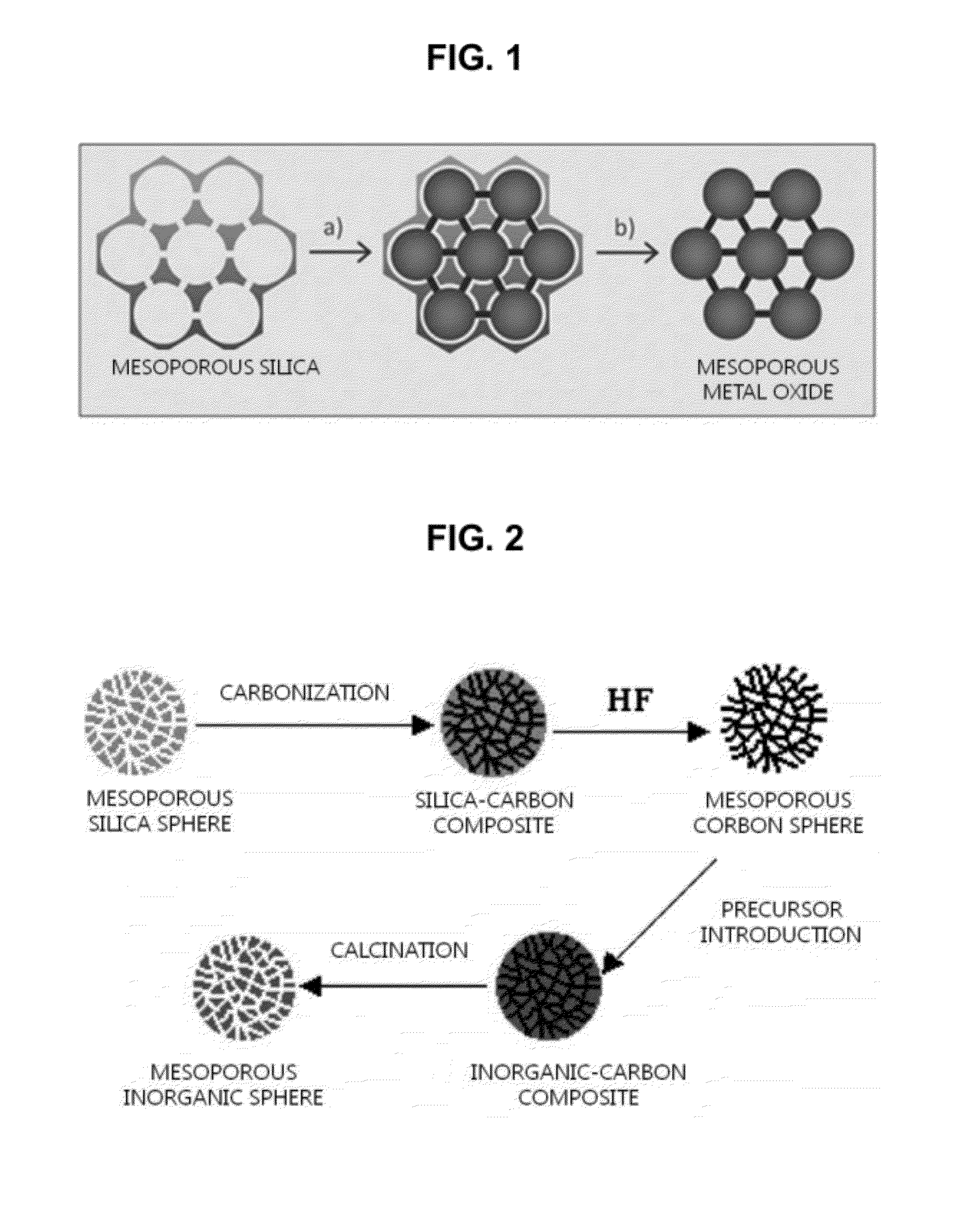
Method of manufacturing porous metal oxide
InactiveUS20120149560A1Increase surface areaEasy to useCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsMetal-organic frameworkThermal treatment
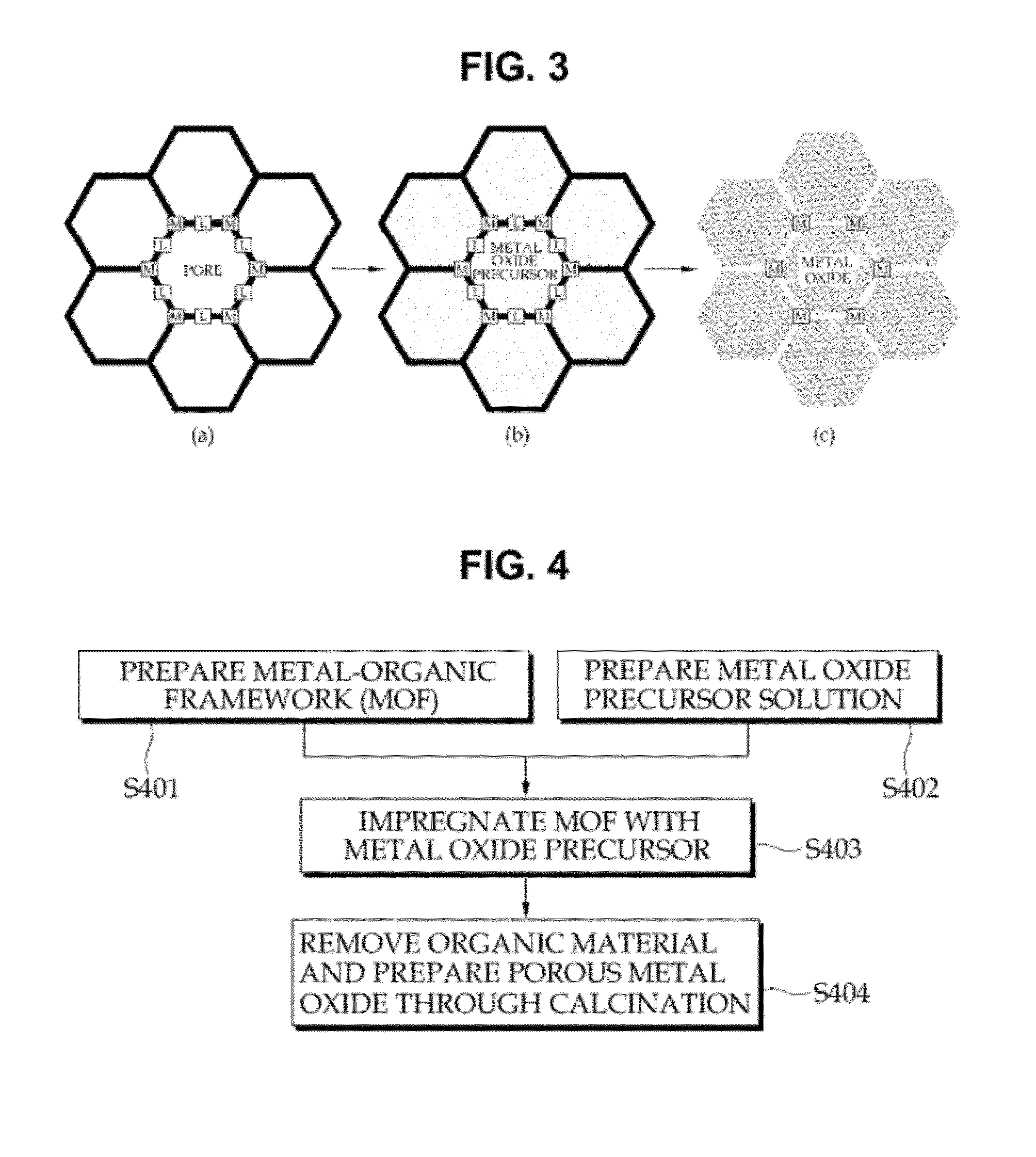
Provided is a method of manufacturing porous metal oxide, the method including: preparing a metal-organic framework (MOF) wherein an ion of a metal to be used as a catalyst is linked to an organic ligand; impregnating the MOF with a precursor solution of metal oxide to be manufactured; and thermally treating the metal oxide precursor solution-impregnated MOF to remove the organic ligand. The inventive method of manufacturing porous metal oxide involves the impregnation of a metal oxide precursor solution in a MOF wherein metal ions are uniformly linked to organic ligands and the thermal treatment (calcination) of the metal oxide precursor solution-impregnated MOF to remove the organic ligands.
Owner:ELECTRONICS & TELECOMM RES INST
Method for preparing fewer-layer graphene on basis of biomass waste
ActiveCN105060289AReduce pollutionAbundant and easy-to-obtain raw materialsCarbon layerArgon atmosphere
The invention discloses a method for preparing fewer-layer graphene on the basis of biomass waste, which comprises the following steps: carrying out hydrothermal treatment on the biomass waste, and carrying out carbonization by heating and calcination, thereby obtaining a carbonization material; immersing the carbonization material in an acid solution to remove impurities, thereby obtaining biomass carbon; and quickly heating the biomass carbon in an argon atmosphere, and carrying out high-temperature graphitization to obtain the biomass fewer-layer graphene. The hydrothermal process is combined with the high-temperature graphitization to directly strip the biomass waste, and the carbonization and high-temperature graphitization are carried out. Thus, the prepared biomass fewer-layer graphene has the advantages of fewer layers (2-10 layers), fewer defects, fewer oxy groups, high electric conductivity and small carbon layer interval. The method is simple to operate, has the advantages of low cost and high graphene yield, and can easily implement industrialized large-scale production. The prepared biomass fewer-layer graphene can be used in the fields of lithium ion batteries, supercapacitors and the like, is beneficial to green production of battery industry, and has important practical value and favorable application prospects.
Owner:湖南宸宇富基新能源科技有限公司
Preparation method of polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis
ActiveCN105107536AHigh crystallinityIncrease the areaElectrolysis componentsPhysical/chemical process catalystsAir atmosphereElectrolysis
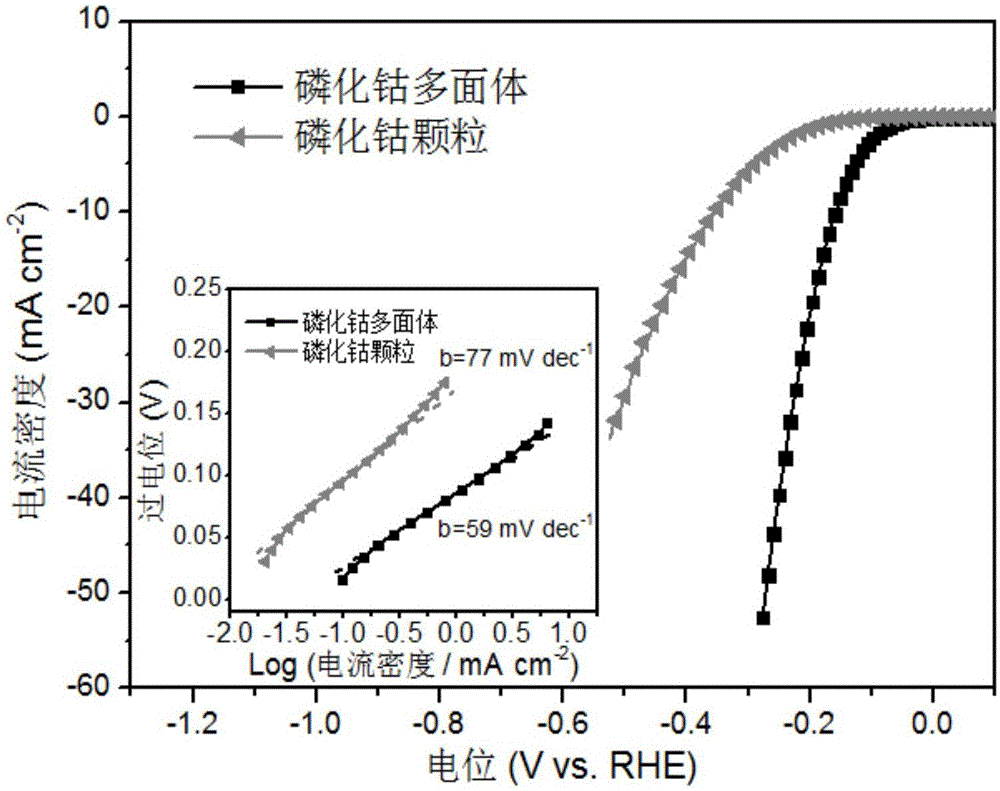
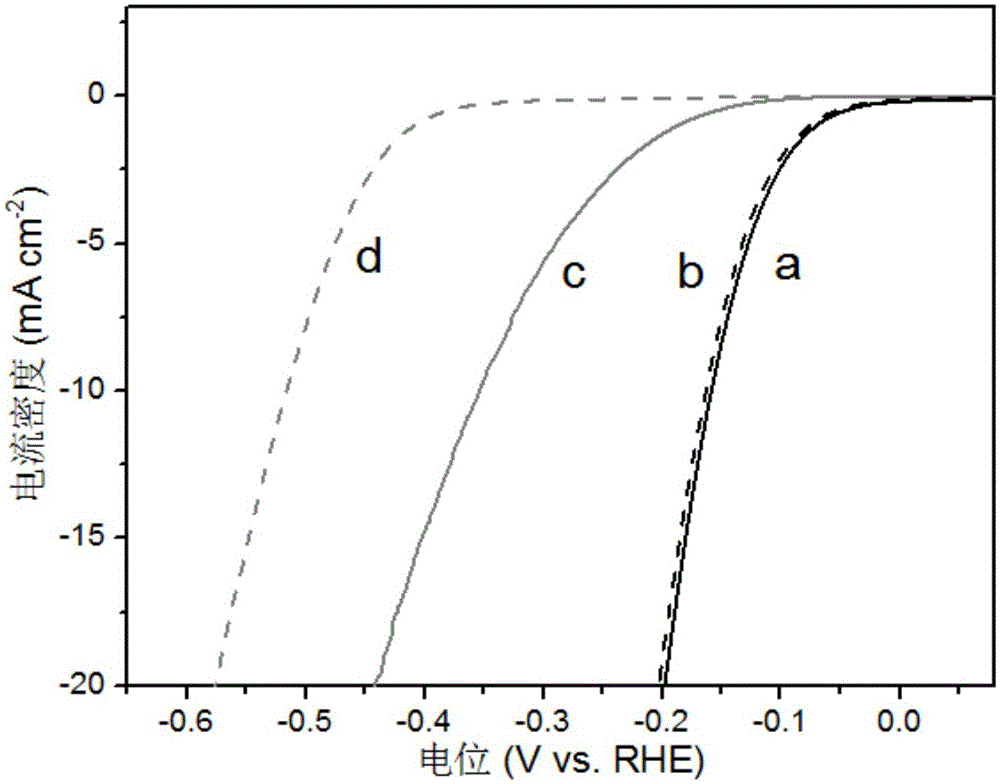
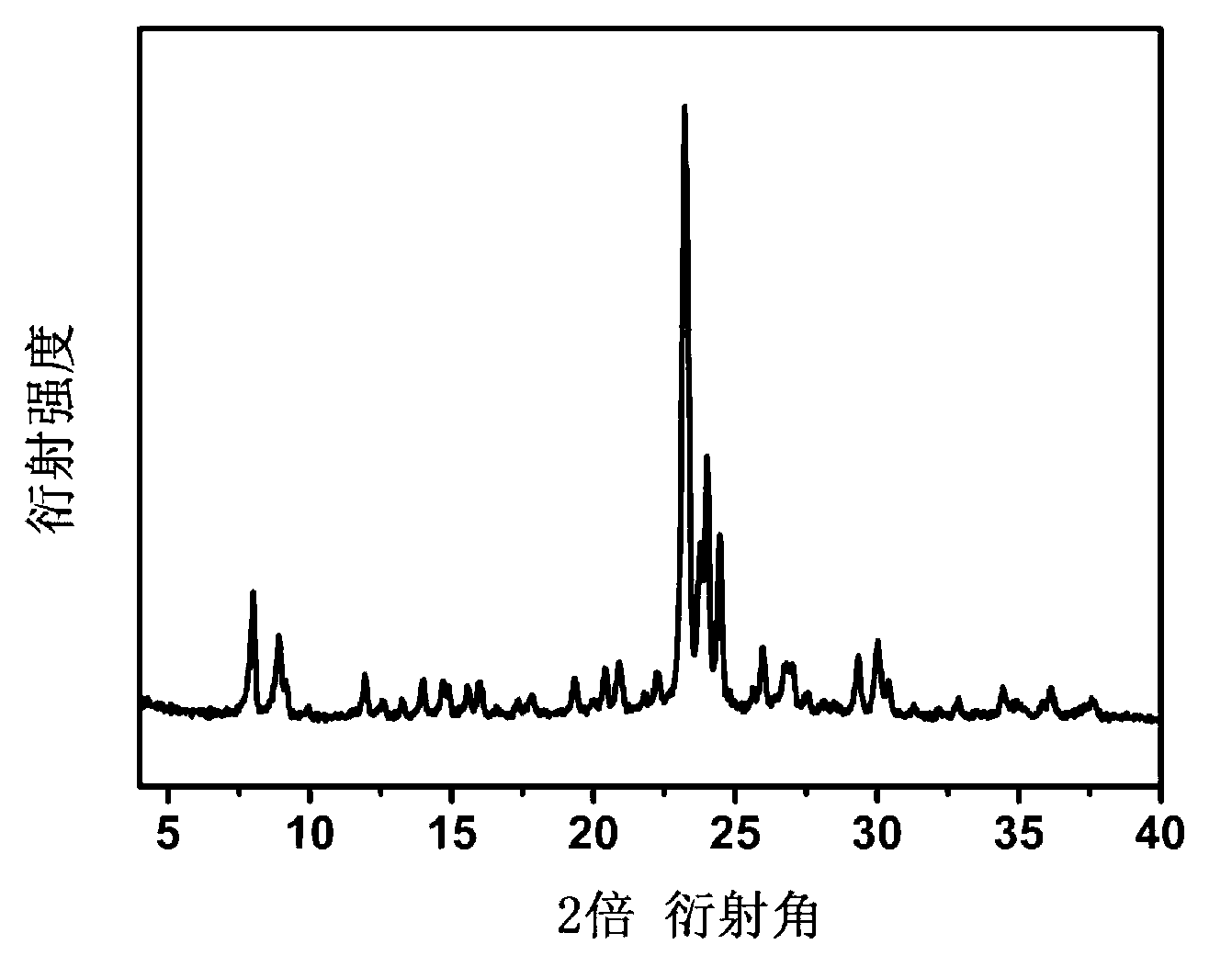
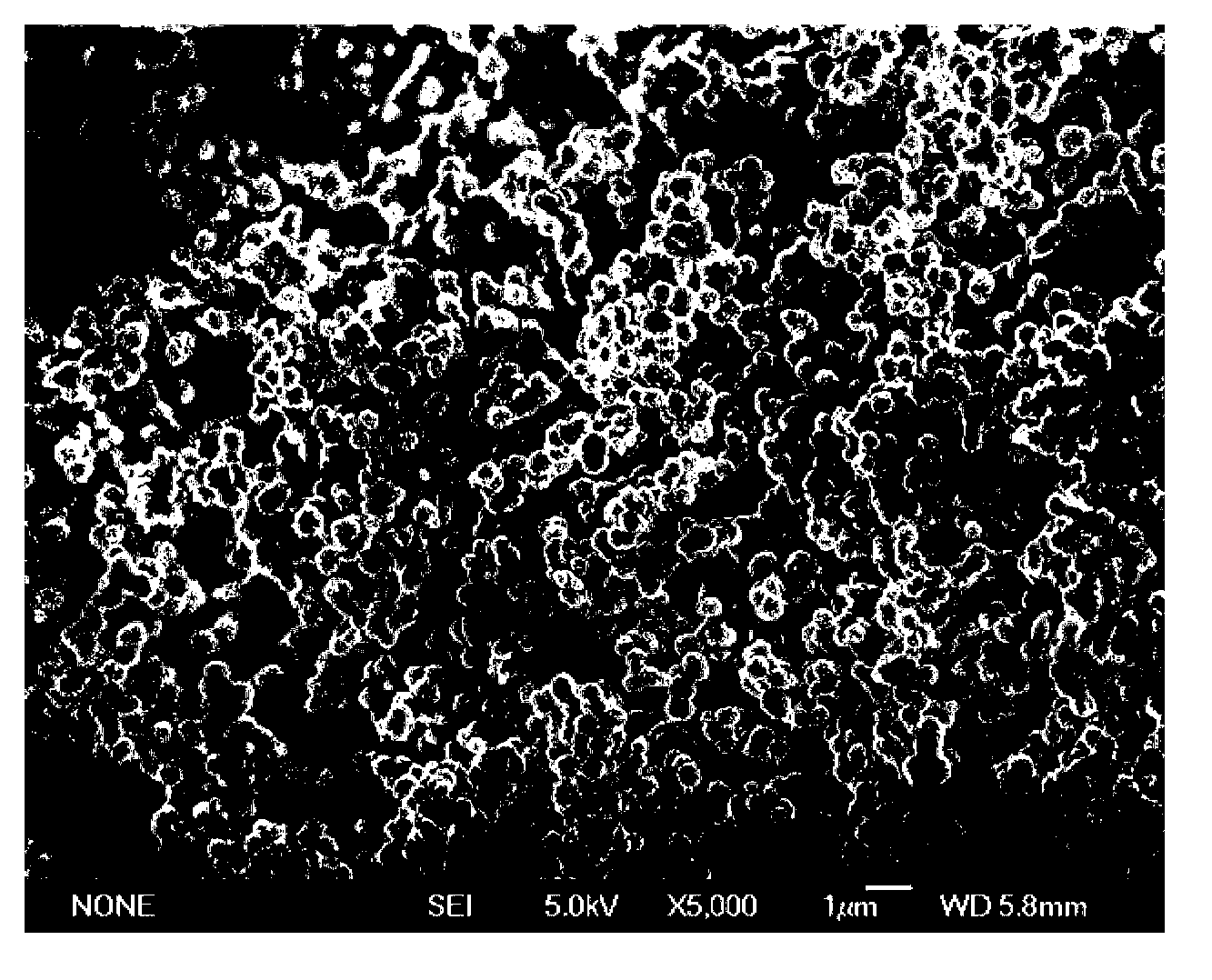
A preparation method of a polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis comprises steps as follows: Co(NO3)2*6H2O and 2-methylimidazole are dissolved in methanol respectively, a 2-methylimidazole solution is poured into a Co(NO3)2 solution, the mixture is stirred and then aged at the room temperature, a product is centrifugally separated, vacuum drying is performed after washing with methanol, and a polyhedral metal organic frame ZIF-67 is obtained; then the polyhedral metal organic frame ZIF-67 is placed in a tube furnace, cobaltosic oxide is obtained through calcination in the air atmosphere, then the cobaltosic oxide and NaH2PO2*H2O are placed at two ends of a porcelain boat respectively, the NaH2PO2*H2O is located in the windward position of the tube furnace, and the polyhedral cobalt phosphide catalyst for hydrogen production through water electrolysis is obtained through calcination in the inert atmosphere. The crystallinity of the prepared cobalt phosphide catalyst material is high, the polyhedral morphology of a metal organic frame template is kept, the catalyst shows excellent properties in an electrocatalytic hydrogen evolution reaction, and the preparation technology is simple in process.
Owner:TSINGHUA UNIV
Microporous-mesoporous molecular sieve containing noble metal, preparation method and application to catalytic reduction of p-nitrophenol
InactiveCN103011189AReduce pollutionLow cost of industrializationMolecular sieve catalystsOrganic compound preparationWater bathsDispersity
The invention belongs to the technical field of molecular sieve preparation, and particularly relates to an in-situ preparation method for a microporous-mesoporous molecular sieve containing noble metal. The in-situ preparation method comprises the steps as follows: adding a coupling pore-forming agent, a silicon source, an aluminum source or a titanium source, and an alkali source into a water solution of noble metal nano particles in sequence under the water bath condition; and ageing, drying, crystallizing, drying and carrying out high-temperature calcination to obtain the microporous-mesoporous molecular sieve containing the noble metal. The prepared microporous-mesoporous molecular sieve is provided with a hierarchical pore structure; the noble metal nano particles with high dispersity are covered in situ while a mesoporous structure is generated; and a synthetic method is convenient and simple, saves energy and reduces emission. A multifunctional catalyst prepared with the method integrates the advantages of the microporous channels of the molecular sieve, the transgranular meso pores and the intergranular meso pores of the molecular sieve and the noble metal nano particles, and is more suitable for catalytic reactions of sulfur-containing large molecules such as hydrogen desulfurization and the like.
Owner:JILIN UNIV
Method for repair and regeneration of waste lithium iron phosphate battery cathode material
ActiveCN102208707AReduce pollutionSave resourcesSolid waste disposalWaste accumulators reclaimingLithium iron phosphateEngineering
The invention discloses a method for repair and regeneration of waste lithium iron phosphate battery cathode materials, which allows a lithium-source solution or a suspension to react with a recovered waste lithium iron phosphate battery material by a hydrothermal reaction or a solvent-thermal reaction, or allows the recovered waste lithium iron phosphate battery material to be processed by solid-phase ball-milling and calcination with the lithium source, performs liquid-phase or solid-phase direct lithium-supplementing repair of delithiated waste lithium iron phosphate, and then performs pertinent repair and regeneration by coating conductive agents or coating conductive agents and doping metal ions. The invention adopts a direct repair and regeneration method; the repaired waste lithium iron phosphate battery cathode material has excellent performance, and the specific capacity can reach above 90% of the specific capacity before discard; the method not only can effectively reduce environmental pollution of waste batteries, but also can make full use of waste resources and changes waste into valuables.
Owner:HEFEI UNIV OF TECH
Separation of carbon dioxide (CO2) from gas mixtures by calcium based reaction separation (CaRS-CO2) process
A reaction-based process has been developed for the selective removal of carbon dioxide (CO2) from a multicomponent gas mixture to provide a gaseous stream depleted in CO2 compared to the inlet CO2 concentration in the stream. The proposed process effects the separation of CO2 from a mixture of gases (such as flue gas / fuel gas) by its reaction with metal oxides (such as calcium oxide). The Calcium based Reaction Separation for CO2 (CaRS-CO2) process consists of contacting a CO2 laden gas with calcium oxide (CaO) in a reactor such that CaO captures the CO2 by the formation of calcium carbonate (CaCO3). Once “spent”, CaCO3 is regenerated by its calcination leading to the formation of fresh CaO sorbent and the evolution of a concentrated stream of CO2. The “regenerated” CaO is then recycled for the further capture of more CO2. This carbonation-calcination cycle forms the basis of the CaRS-CO2 process. This process also identifies the application of a mesoporous CaCO3 structure, developed by a process detailed elsewhere, that attains >90% conversion over multiple carbonation and calcination cycles. Lastly, thermal regeneration (calcination) under vacuum provided a better sorbent structure that maintained reproducible reactivity levels over multiple cycles.
Owner:THE OHIO STATES UNIV
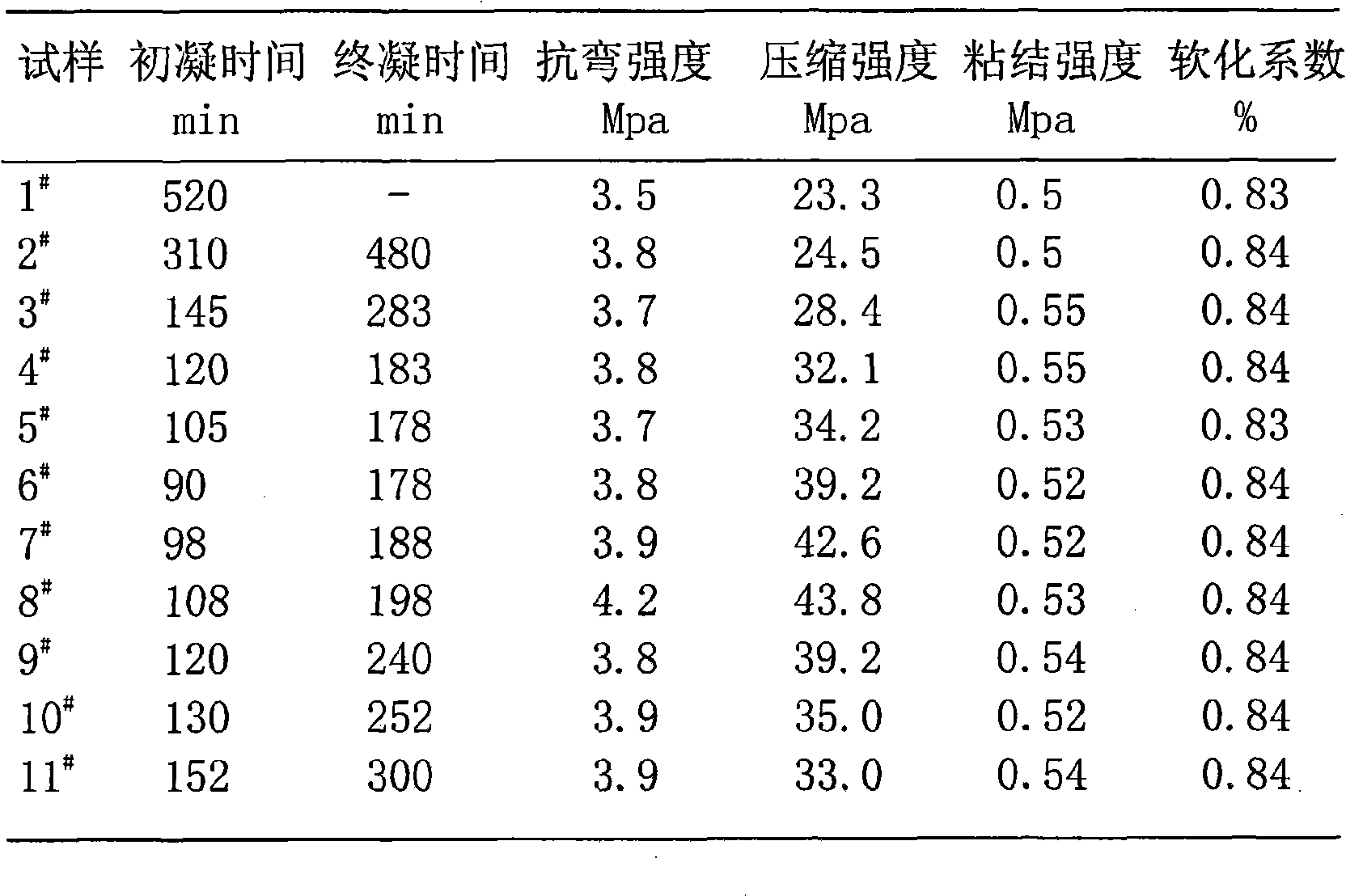
High-strength water-resistant plastering gypsum and producing method thereof
The invention relates to a high-strength water-proof gypsum plaster and a production method thereof. The inventive high-strength water-proof gypsum plaster is made from the following materials in weight proportions: 10-99% of gypsum-based composite binding agent, 0-90% of aggregates, 0.08-4% of additives and 0-16.1% of adsorbent. The production method comprises the following steps: weighing raw materials at the given ratio, drying the aggregates, mixing all raw materials, and packaging. The inventive high-strength water-proof gypsum plaster has high strength and good water resistance; can be made from chemical gypsum materials without needing calcination, such as desulfurized gypsum, phosphogypsum, fluorgypsum and citric acid gypsum; and has the advantages of low production cost, energy conservation, discharge reduction and environmental friendliness.
Owner:长沙归一新材料科技股份有限公司
Pre-treatment of lime-based sorbents using hydration
InactiveUS20070092427A1Increase capacityIncrease carbon dioxide capture capacityCalcium/strontium/barium carbonatesOther chemical processesCombustionSorbent
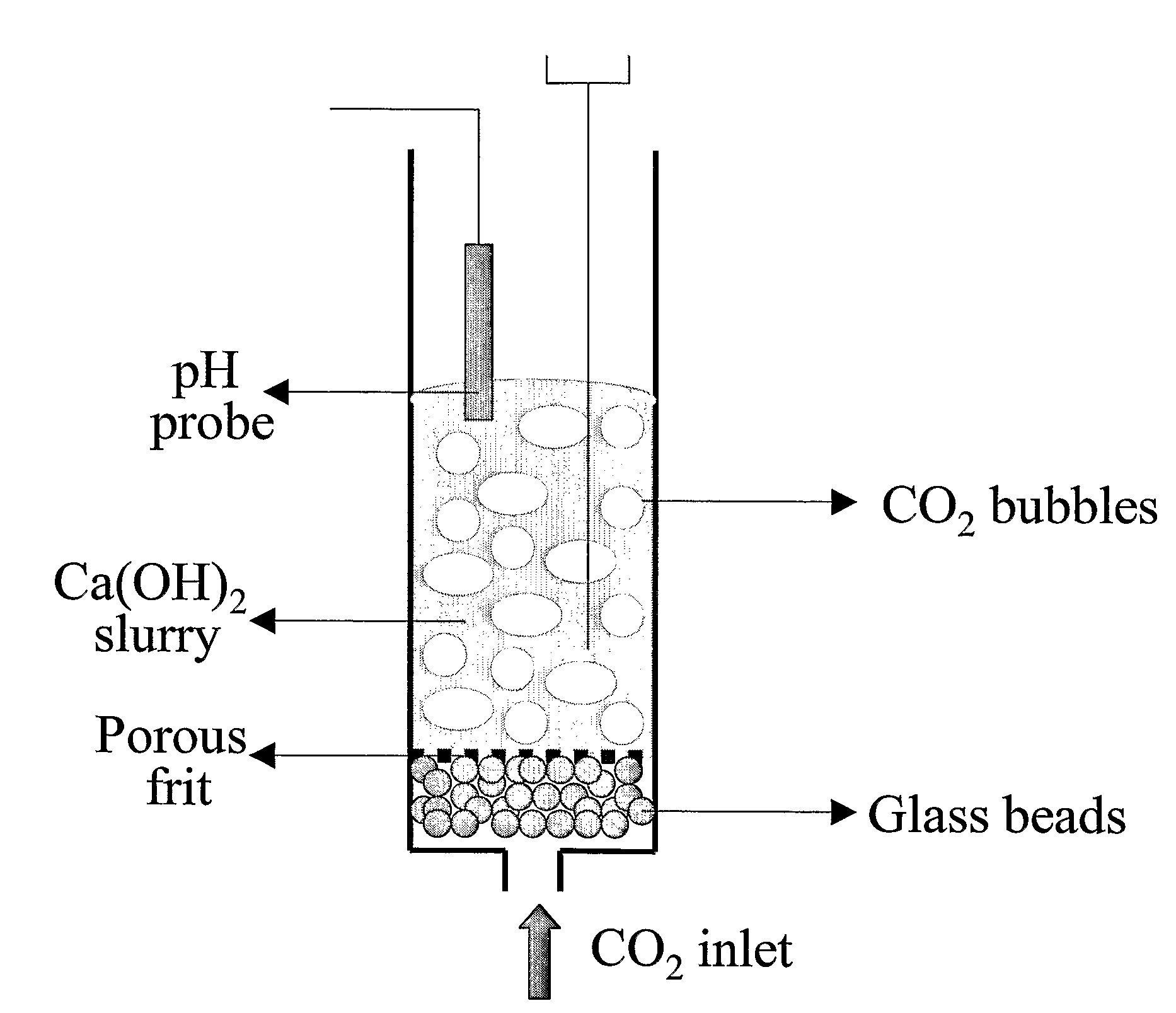
The present invention discloses a method and an apparatus for reactivating lime-based sorbents and increasing the carbon dioxide-capture capacity of the sorbent in the combustion of carbon-containing fuels. The present invention teaches the pretreatment of the lime-based sorbent using a hydration process after each process of carbon dioxide separation. The invention is useful in reducing the need to add additional sorbent to maintain the carbonation / calcination cycle. The regenerative potential of the sorbent as manifested by the present invention leads to increased carbon dioxide-capture capacity of the sorbent.
Owner:HER MAJESTY THE QUEEN & RIGHT OF CANADA REPRESENTED BY THE MIN OF NATURAL RESOURCES
Desulfurization process and novel bimetallic sorbent systems for same
Novel sorbent systems for the desulfurization of cracked-gasoline and diesel fuels are provided which are comprised of a bimetallic promotor on a particulate support such as that formed of zinc oxide and an inorganic or organic carrier. Such bimetallic promotors are formed of at least two metals of the group consisting of nickel, cobalt, iron, manganese, copper, zinc, molybdenum, tungsten, silver, tin, antimony and vanadium with the valence of same being reduced, preferably to zero. Processes for the production of such sorbents are provided wherein the sorbent is prepared from impregnated particulate supports or admixed to the support composite prior to particulation, drying, and calcination. Further disclosed is the use of such novel sorbents in the desulfurization of cracked-gasoline and diesel fuels whereby there is achieved not only removal of sulfur but also an increase in the olefin retention in the desulfurized product. Such sorbents can also be utilized for the treatment of other sulfur-containing streams such as diesel fuels.
Owner:CHINA PETROCHEMICAL CORP
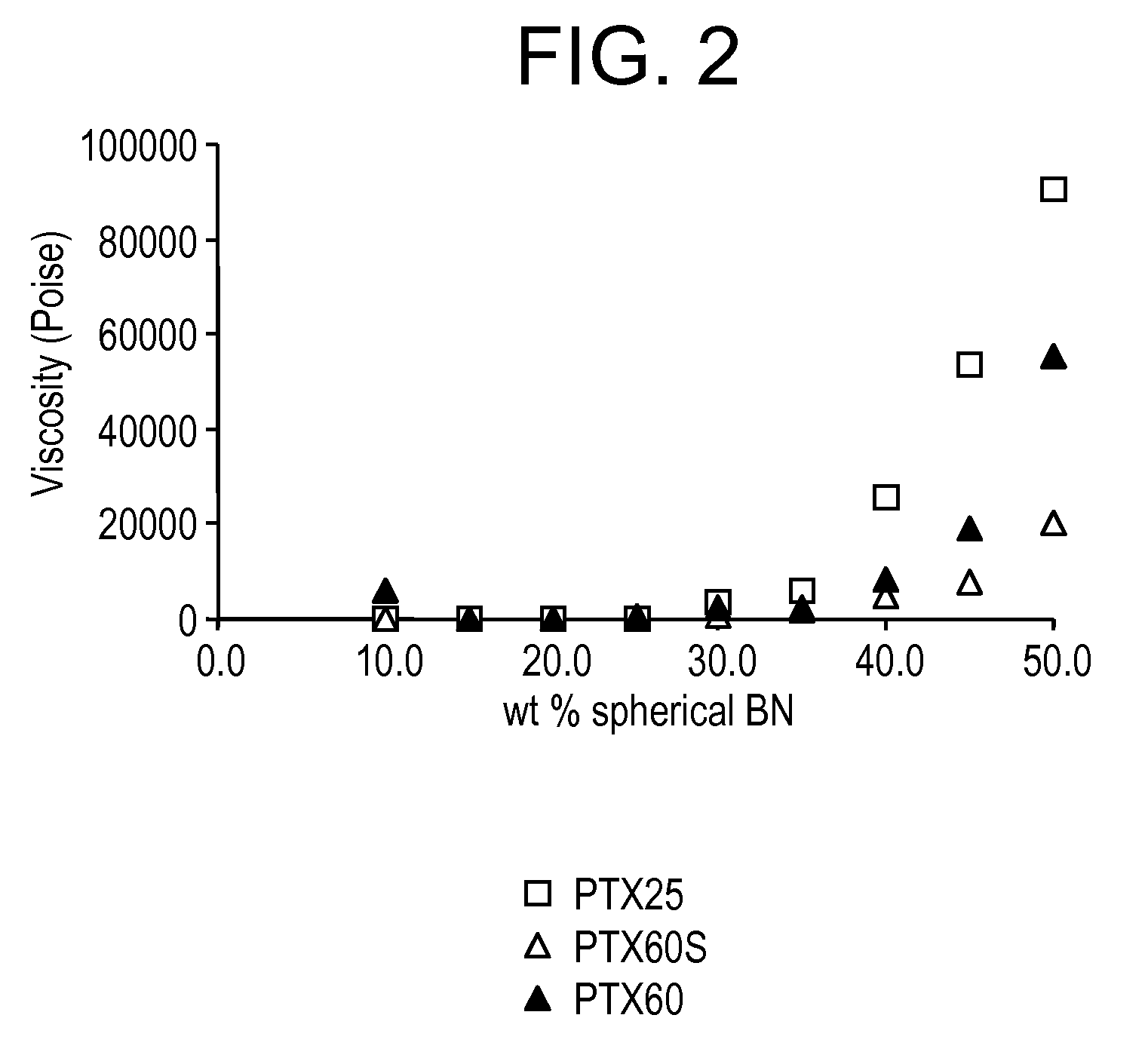
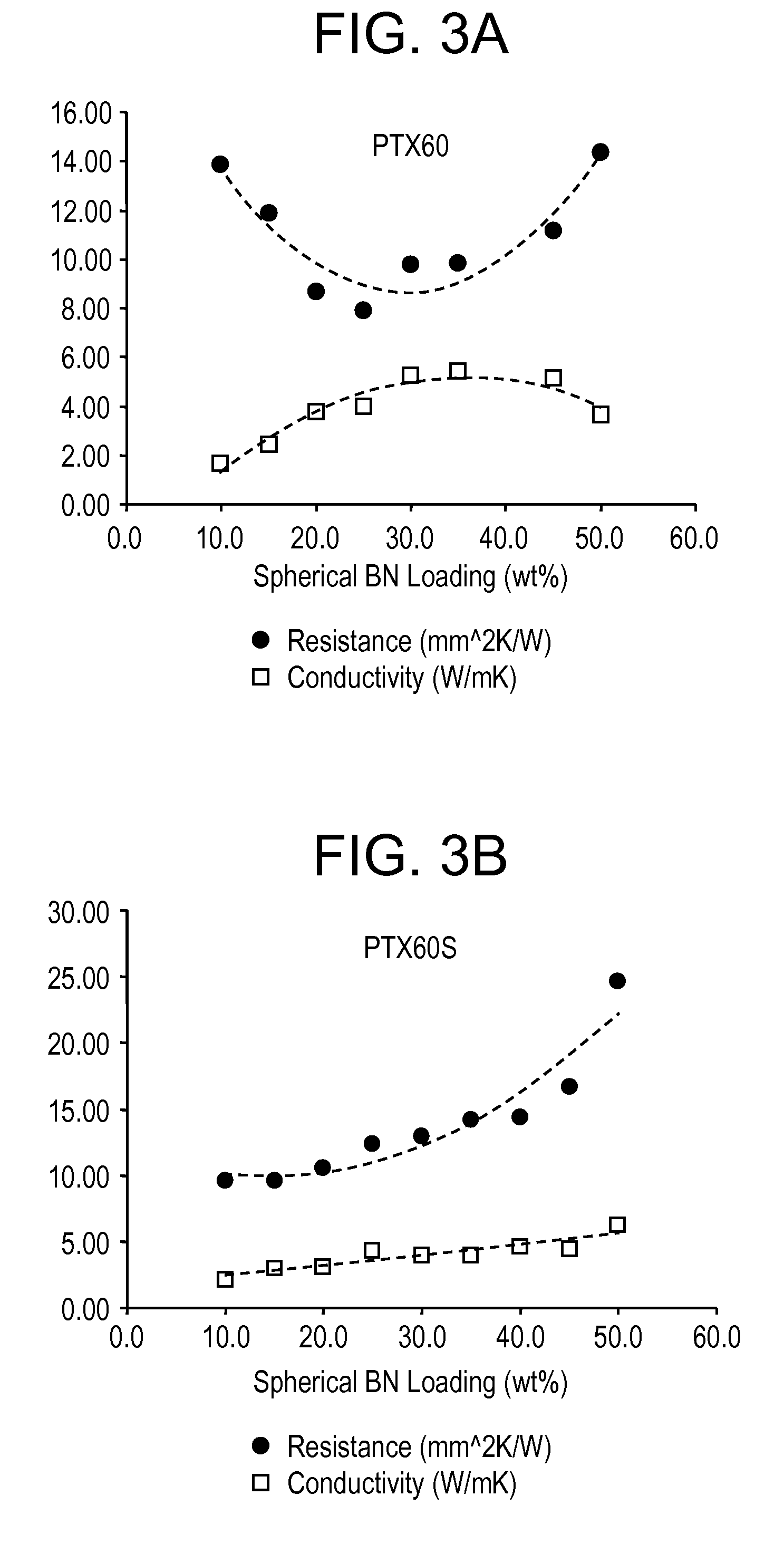
Enhanced boron nitride composition and compositions made therewith
A boron nitride composition having its surface treated with a coating layer comprising at least one of a silane, a siloxane, a carboxylic derivative, and mixtures thereof, wherein the coating layer adheres to at least 10% of the surface of the boron nitride. The boron nitride powder surface is first treated by either a calcination process, or by coating with at least an inorganic compound for the surface to have a plurality of reactive sites containing at least a functional group that is reactive to at least one functional group of the final coating layer.
Owner:MOMENTIVE PERFORMANCE MATERIALS QUARTZ INC
Process for the coating of the flow channels of a honeycomb form catalytic converter carrier with a dispersion coating
A process for the coating of the flow channels of a cylindrical, honeycomb form catalyst carrier with a dispersion coating through filling of the vertically oriented flow channels with a fill quantity of the coating dispersion through the bottom face of the catalyst carrier and subsequent downward emptying and clearance extraction of the flow channels, as well as drying and calcination of the catalyst carrier, by the following steps: a) filling of the flow channels with a fill quantity that is about 10% greater than the empty volume of the flow channels, so that the coating dispersion goes over the upper face of the catalyst carrier after completion of the filling cycle, b) removal of the excess coating dispersion at the top before emptying of the flow channels and c) emptying and clearance extraction of the flow channels through an extraction impulse, which is generated by connection of a vacuum tank with the bottom face of the catalyst carrier, whereby the time between the beginning of the fill cycle and the end of the emptying and clearance extraction amounts to no more than 5 seconds.
Owner:DMC2 DEGUSSA METALS +1
Preparation method of ozone oxidation catalyst loaded with polymetallic oxide
InactiveCN105363465AImprove the removal rate of chemical oxygen demand (COD)High removal rateMolecular sieve catalystsMetal/metal-oxides/metal-hydroxide catalystsChemical oxygen demandPretreatment method
The embodiment of the invention discloses a preparation method of an ozone oxidation catalyst loaded with polymetallic oxide. The method comprises the following steps that an ozone oxidation catalyst carrier is pretreated, wherein the pretreatment method comprises the steps that the ozone oxidation catalyst carrier is soaked through an acid solution after being washed through water, and then the soaked carrier is washed to be neutral and dried; immersion treatment is conducted on the pretreated ozone oxidation catalyst carrier at least once, and then calcination is conducted; the immersion treatment comprises the steps that the pretreated ozone oxidation catalyst carrier is immersed in an immersion solution to be immersed for 6-48 h at the temperature ranging from 20 DEG C to 100 DEG C, and then drying treatment is conducted, wherein the immersion solution is a mixed solution of nitrate, sulfate and acetate or chloride of three metal elements of manganese, nickel, iron, cerium, cobalt and copper, and the concentration of each metal element in the immersion solution ranges from 0.01 mol / L to 1.00 mol / L. By means of the preparation method of the ozone oxidation catalyst loaded with the polymetallic oxide, the removal rate of chemical oxygen demand (COD) in sewage can be significantly increased.
Owner:POTEN ENVIRONMENT GRP +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com