Patents
Literature
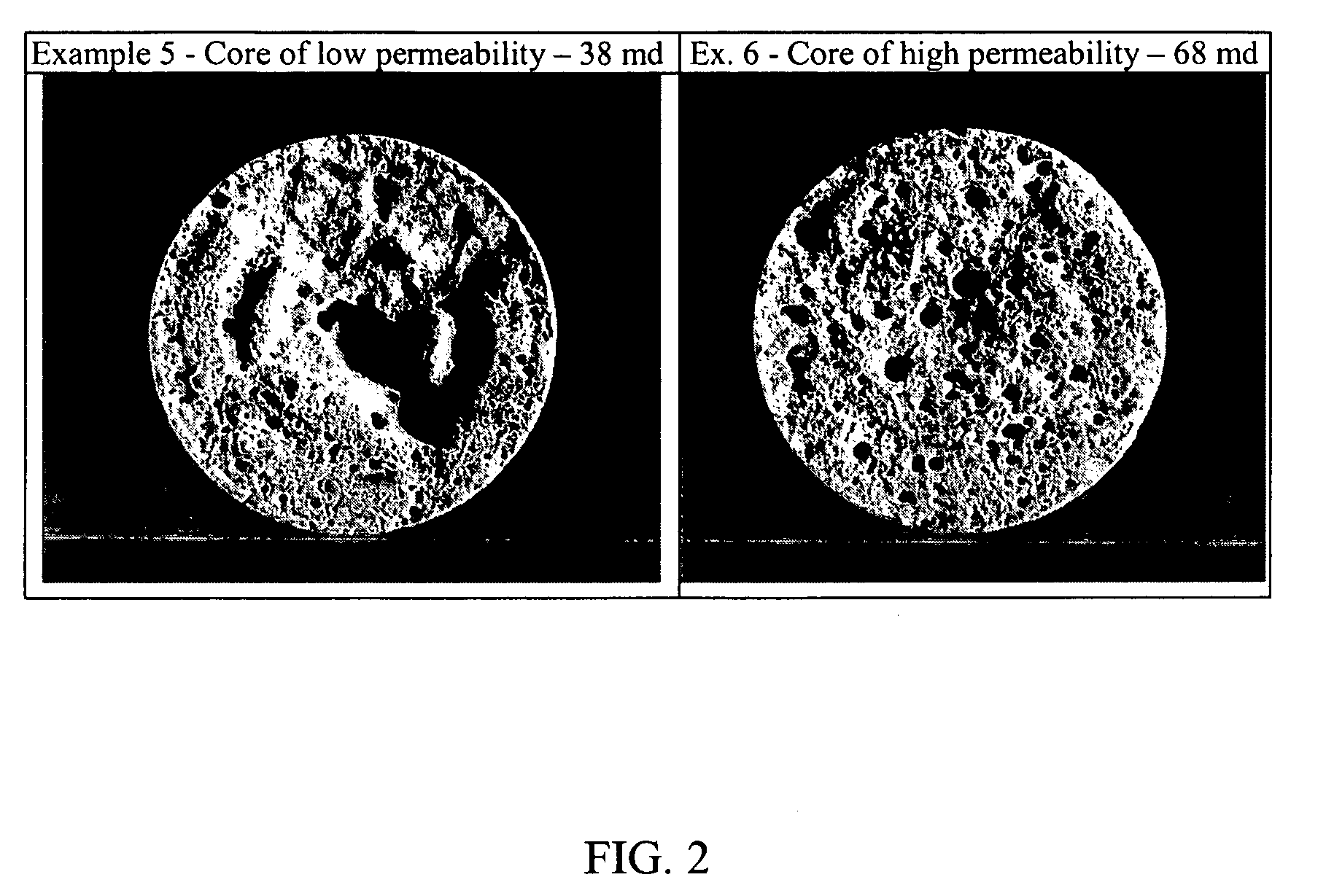
6732 results about "Saline water" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
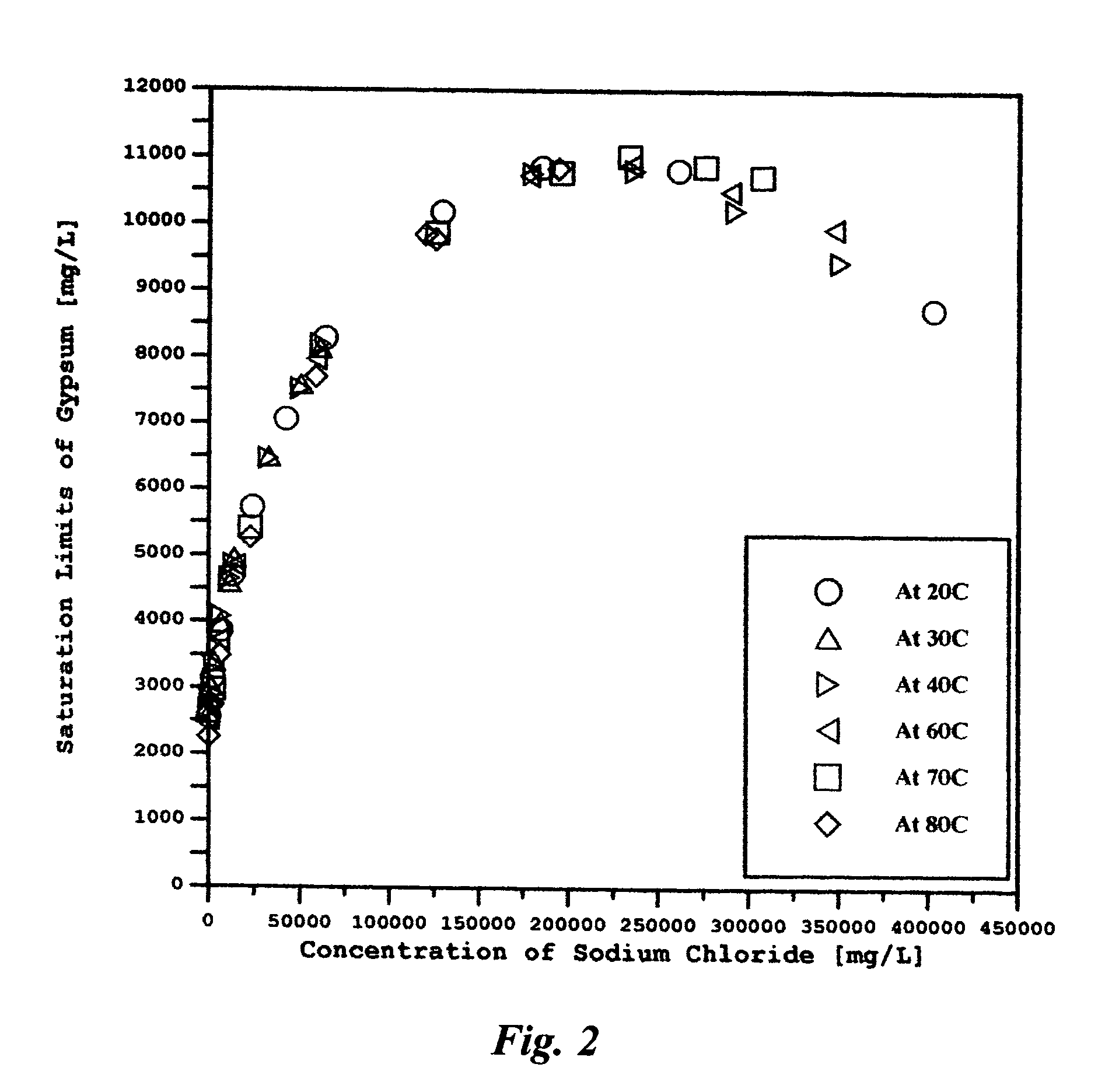
Saline water (more commonly known as salt water) is water that contains a high concentration of dissolved salts (mainly NaCl). The salt concentration is usually expressed in parts per thousand (permille, ‰) or parts per million (ppm). The United States Geological Survey classifies saline water in three salinity categories. Salt concentration in slightly saline water is around 1,000 to 3,000 ppm (0.1–0.3%), in moderately saline water 3,000 to 10,000 ppm (0.3–1%) and in highly saline water 10,000 to 35,000 ppm (1–3.5%). Seawater has a salinity of roughly 35,000 ppm, equivalent to 35 grams of salt per one liter (or kilogram) of water. The saturation level is dependent on the temperature of the water. At 20 °C one liter of water can dissolve about 357 grams of salt, a concentration of 26.3%. At boiling (100 °C) the amount that can be dissolved in one liter of water increases to about 391 grams, a concentration of 28.1%.
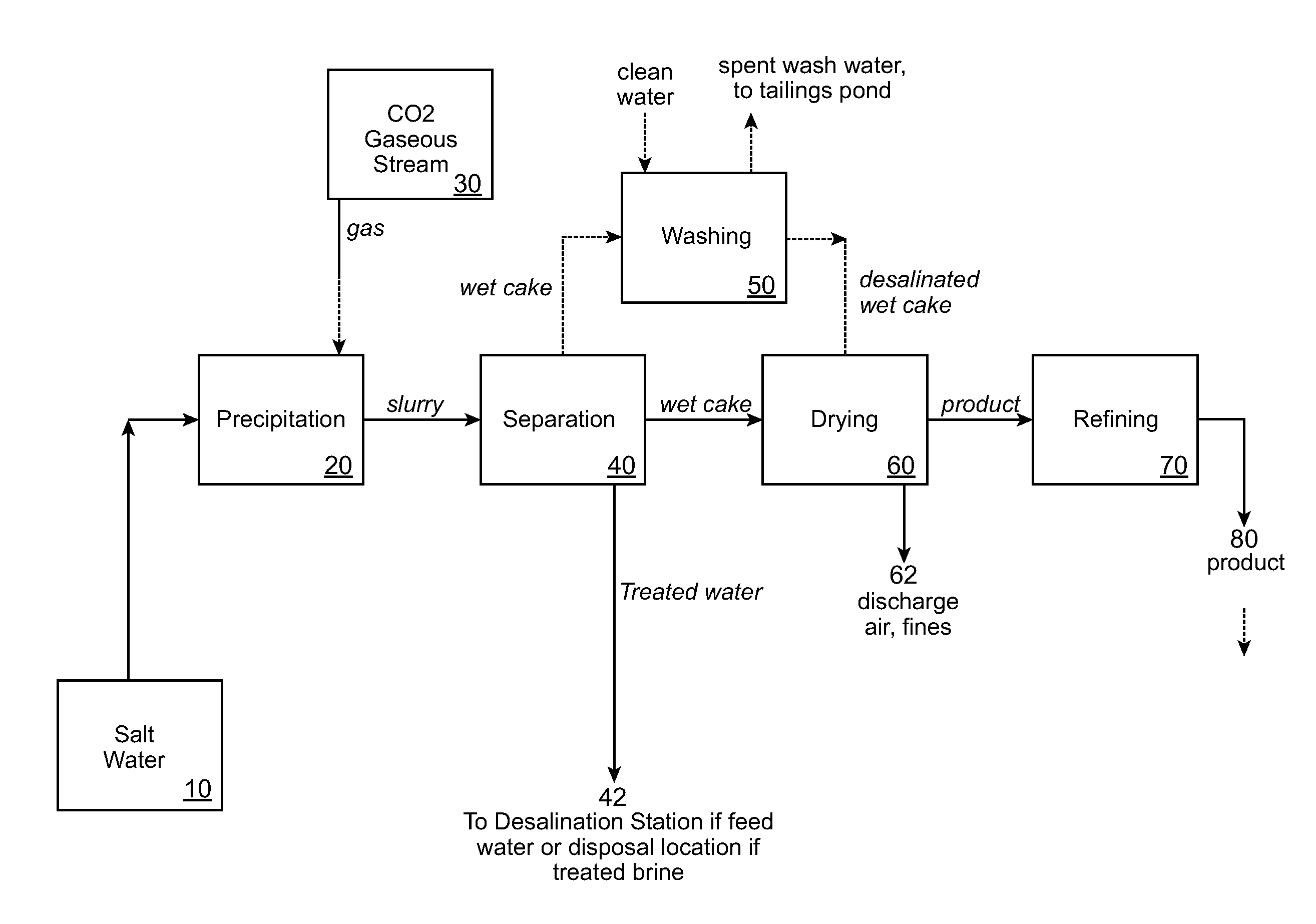
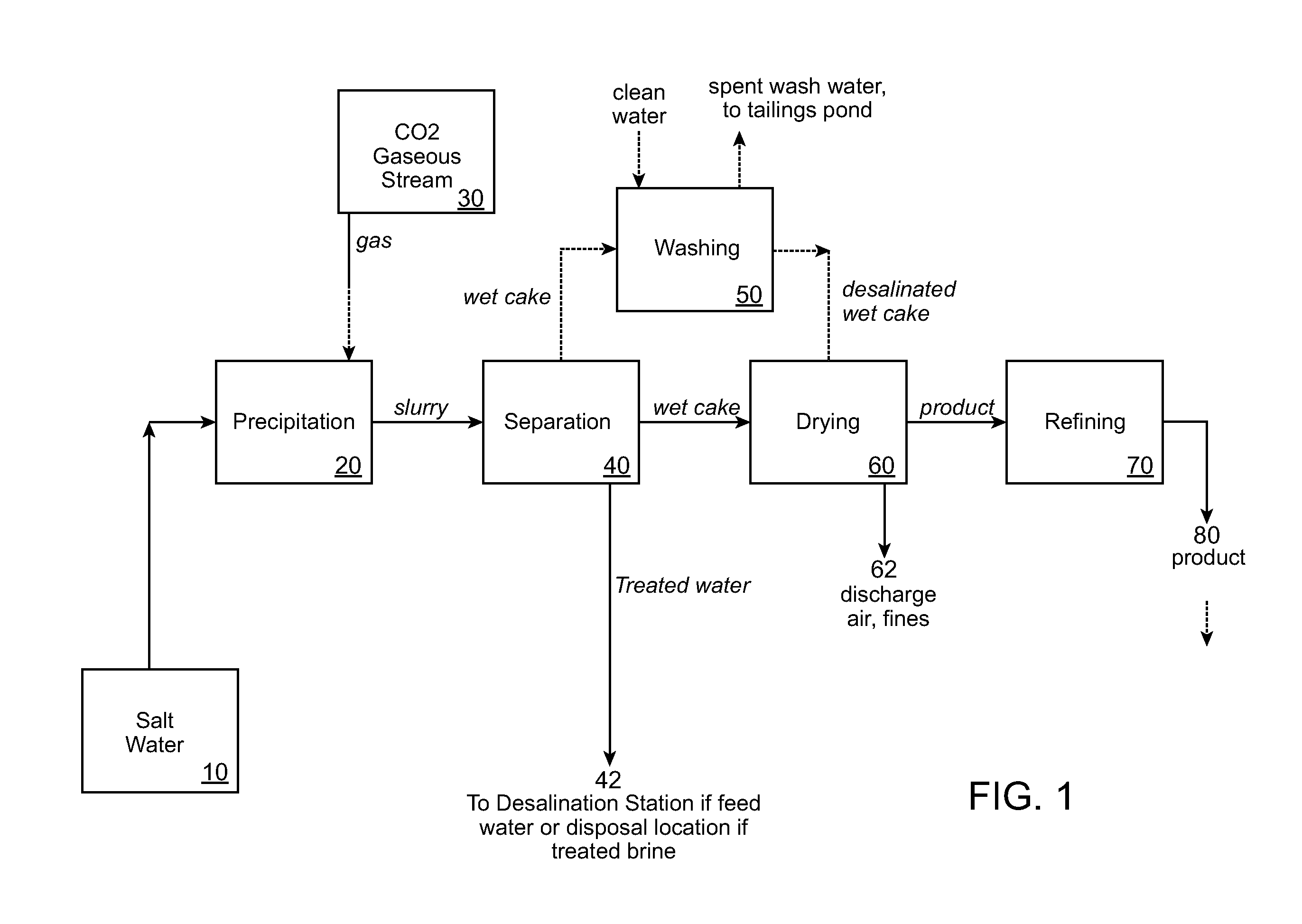
Desalination methods and systems that include carbonate compound precipitation
ActiveUS20090001020A1Easy complianceImprove desalination efficiencyGeneral water supply conservationSeawater treatmentSaline waterDesalination
Desalination methods that include carbonate compound precipitation are provided. In certain embodiments, feed water is subjected to carbonate compound precipitation conditions prior to desalination. In certain embodiments, desalination waste brine is subjected to carbonate compound precipitation conditions. In yet other embodiments, both feed water and waste brine are subjected to carbonate compound precipitation conditions. Aspects of embodiments of the invention include carbone dioxide sequestration. Embodiments of the invention further employ a precipitate product of the carbonate compound precipitation conditions as a building material, e.g., a cement. Also provided are systems configured for use in methods of the invention.
Owner:ARELAC INC
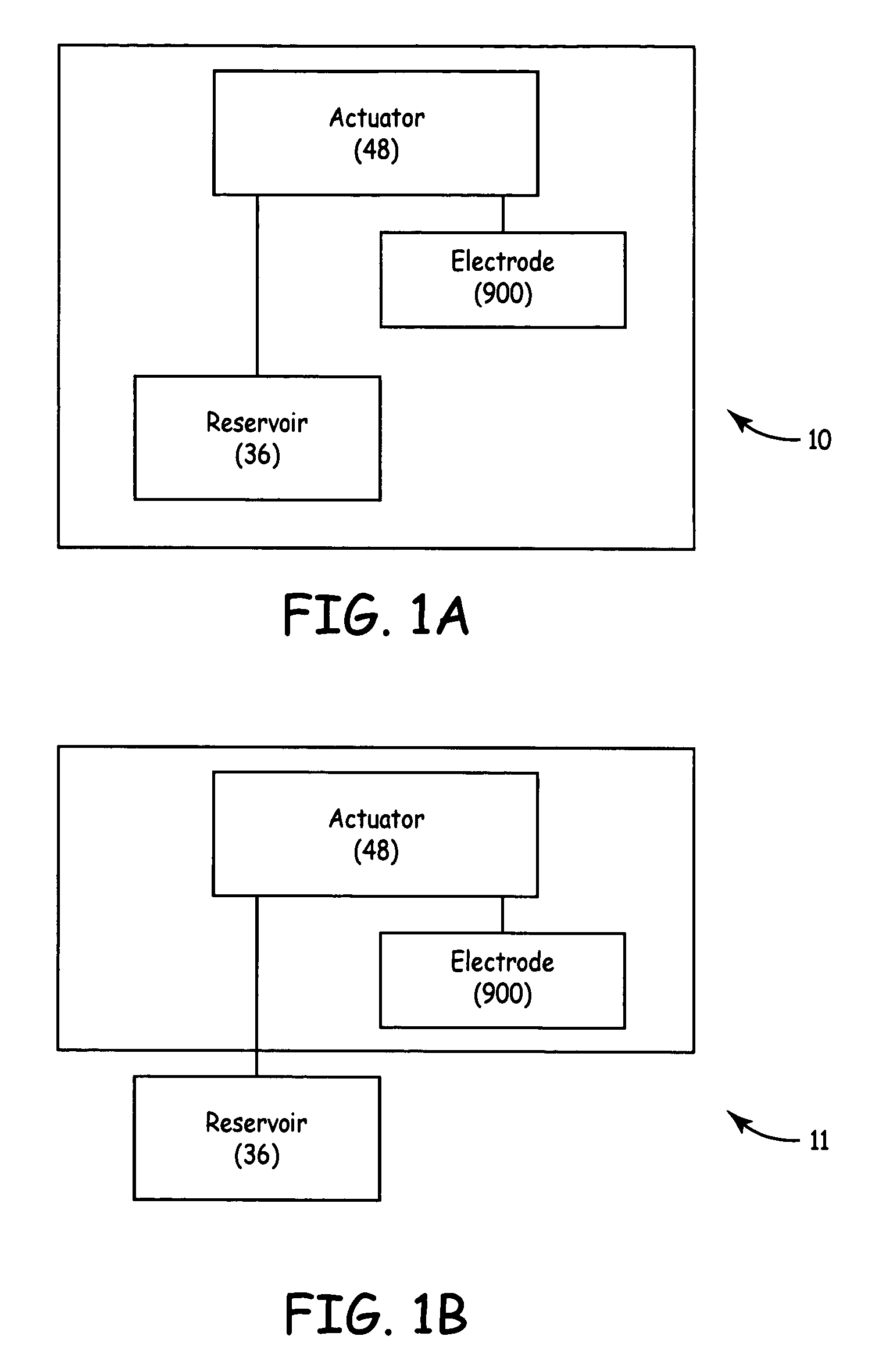
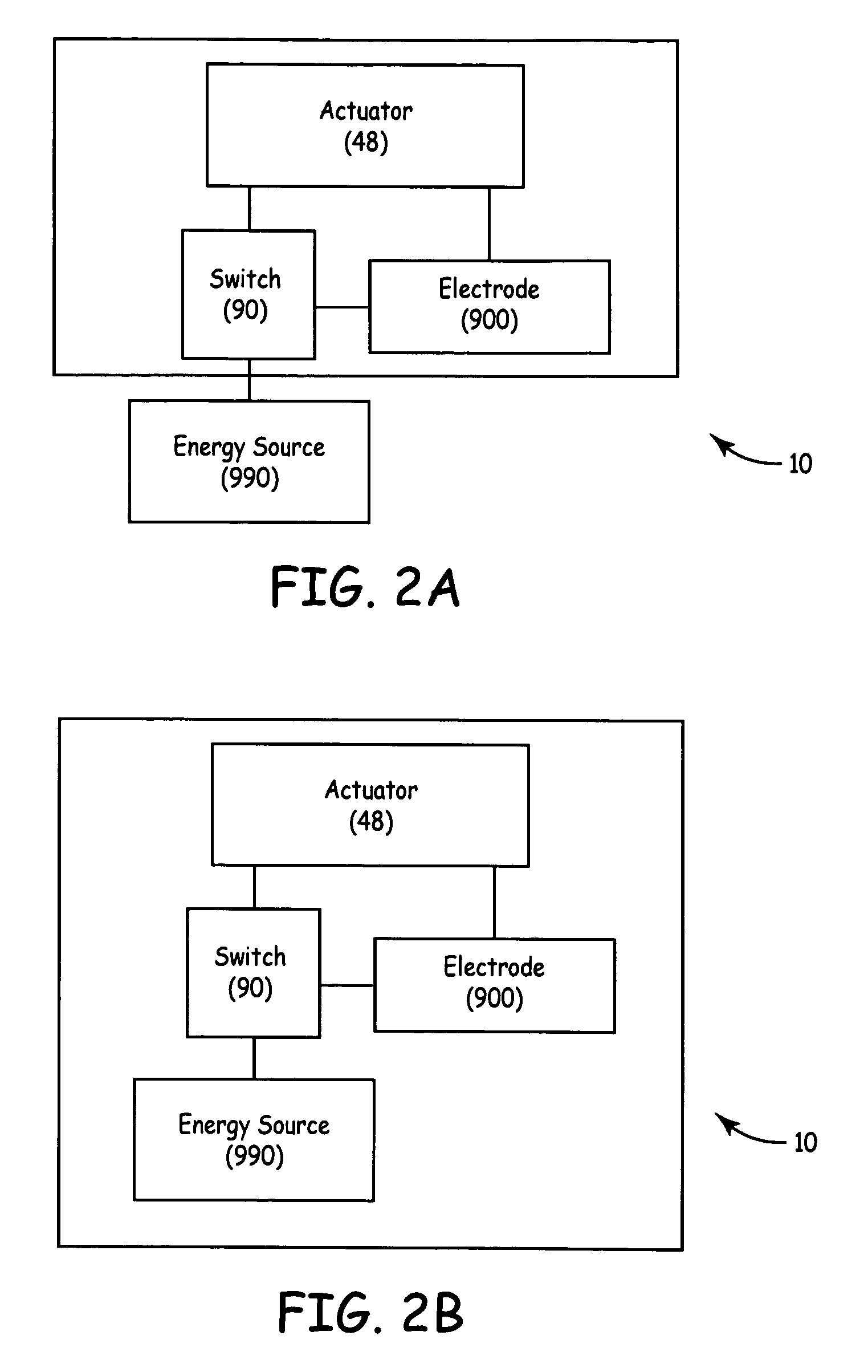
TUNA device with integrated saline reservoir
ActiveUS7322974B2Simplify wet electrode tissue ablation proceduresSimplify dry electrode techniquesSurgical needlesSurgical instrument detailsSaline waterActuator
Methods and apparatus for ablating a target tissue are discussed. Such methods and apparatus include those that simplify tissue ablation. For example, a tissue ablation device having an actuator, such as a trigger mechanism, coupled to a power source and an electrode is discussed. A single step of engaging the actuator causes the electrode to be introduced into the target tissue and causes energy to be delivered from the power supply to the tissue via the electrode. By way of additional example, a tissue ablation device having an actuator coupled to a fluid source and an electrode is discussed. A single step of engaging the actuator causes conductive fluid to flow from the fluid source to the target tissue location and causes the electrode to be introduced to the target tissue location. The fluid source may be a conductive fluid, such as saline, which may increase the efficiency of ablation. Various other configurations and methods that simplify tissue ablation are also discussed.
Owner:MEDTRONIC INC
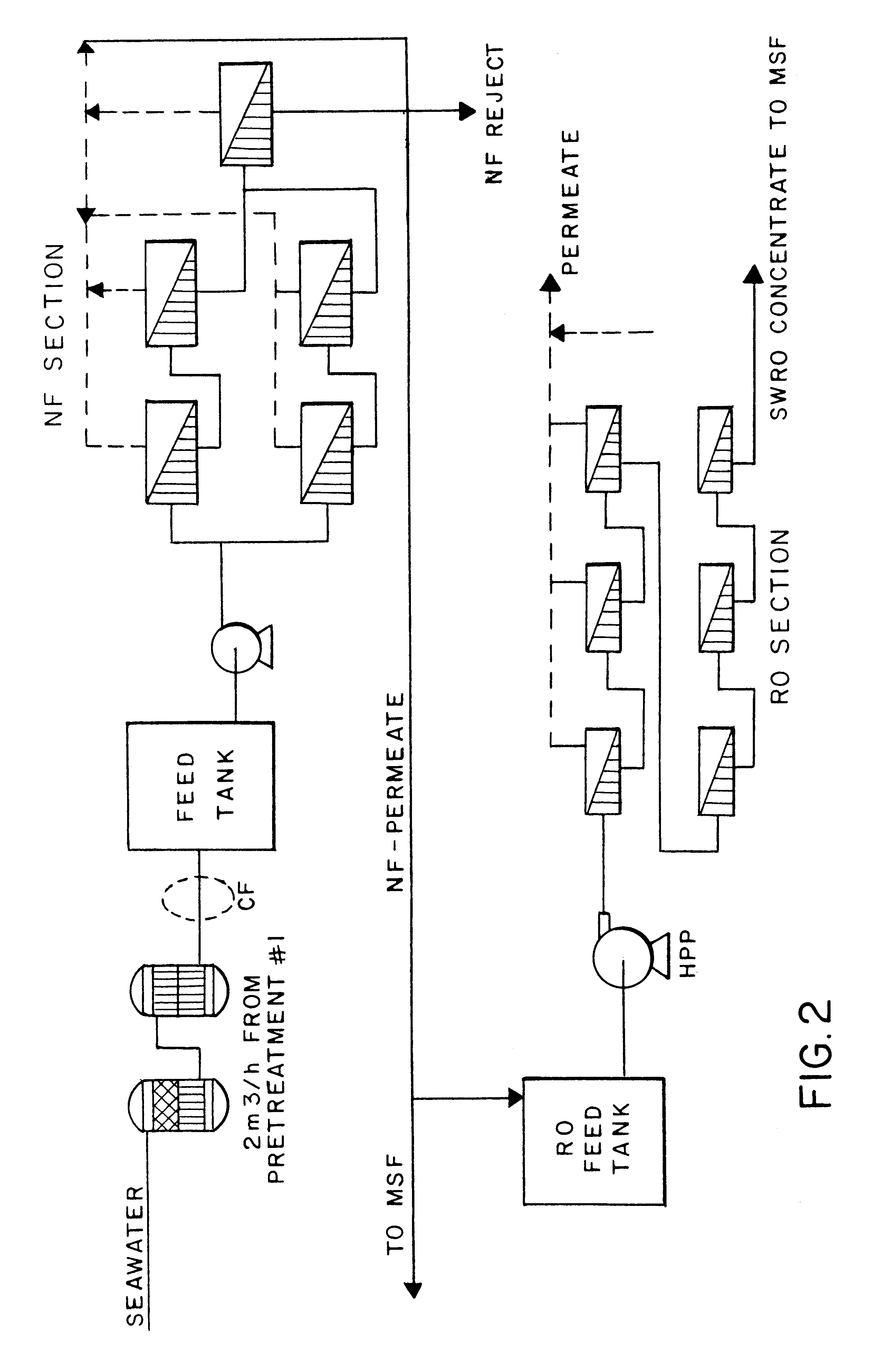
Process for desalination of saline water, especially water, having increased product yield and quality
InactiveUS6508936B1General water supply conservationSeawater treatmentTotal dissolved solidsDistillation
A desalination process is disclosed which combining two or more substantially different water treatment processes in a unique manner to desalinate saline water, especially sea water, to produce a high yield of high quality fresh water, including potable water, at an energy consumption equivalent to or less than much less efficient prior art desalination processes. In this process a nanofiltration step is synergistically combined with at least one of sea water reverse osmosis, multistage flash distillation. multieffect distillation of vapor compression distillation to provide an integrated desalination system by which sea water can be efficiently and economically converted to high quality potable water in yields which are at least 70%-80% greater than the yields available from the prior art processes. Typically a process of this invention using the nanofiltration initial step will produce, with respect to sea water feed properties, calcium, magnesium, sulfate and bicarbonate ion content reductions of 63%-94%, pH decreases of about 0.4-0.5 units and total dissolved solids content reductions of 35%-50%.
Owner:SALINE WATER CONVERSION CORP
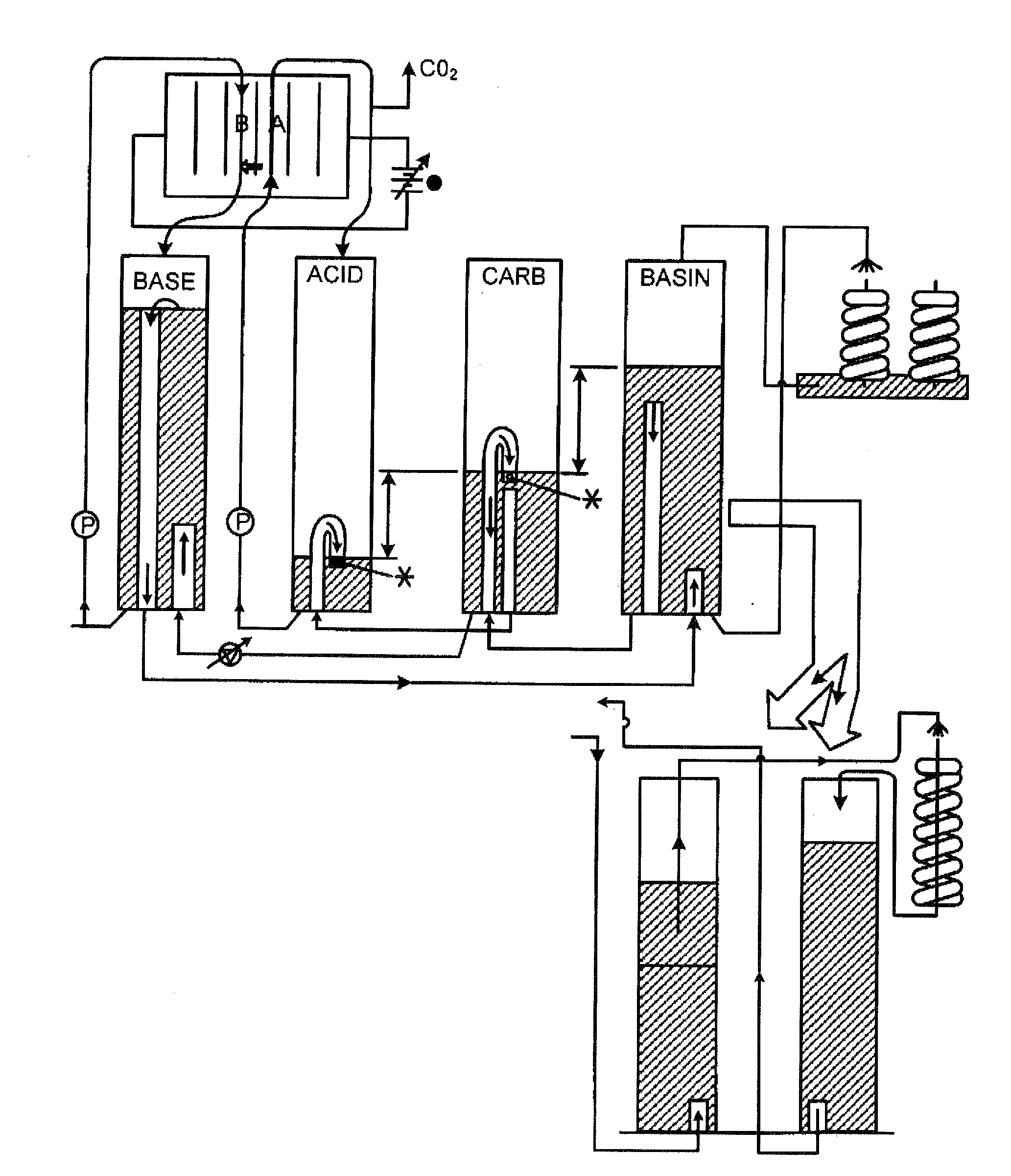
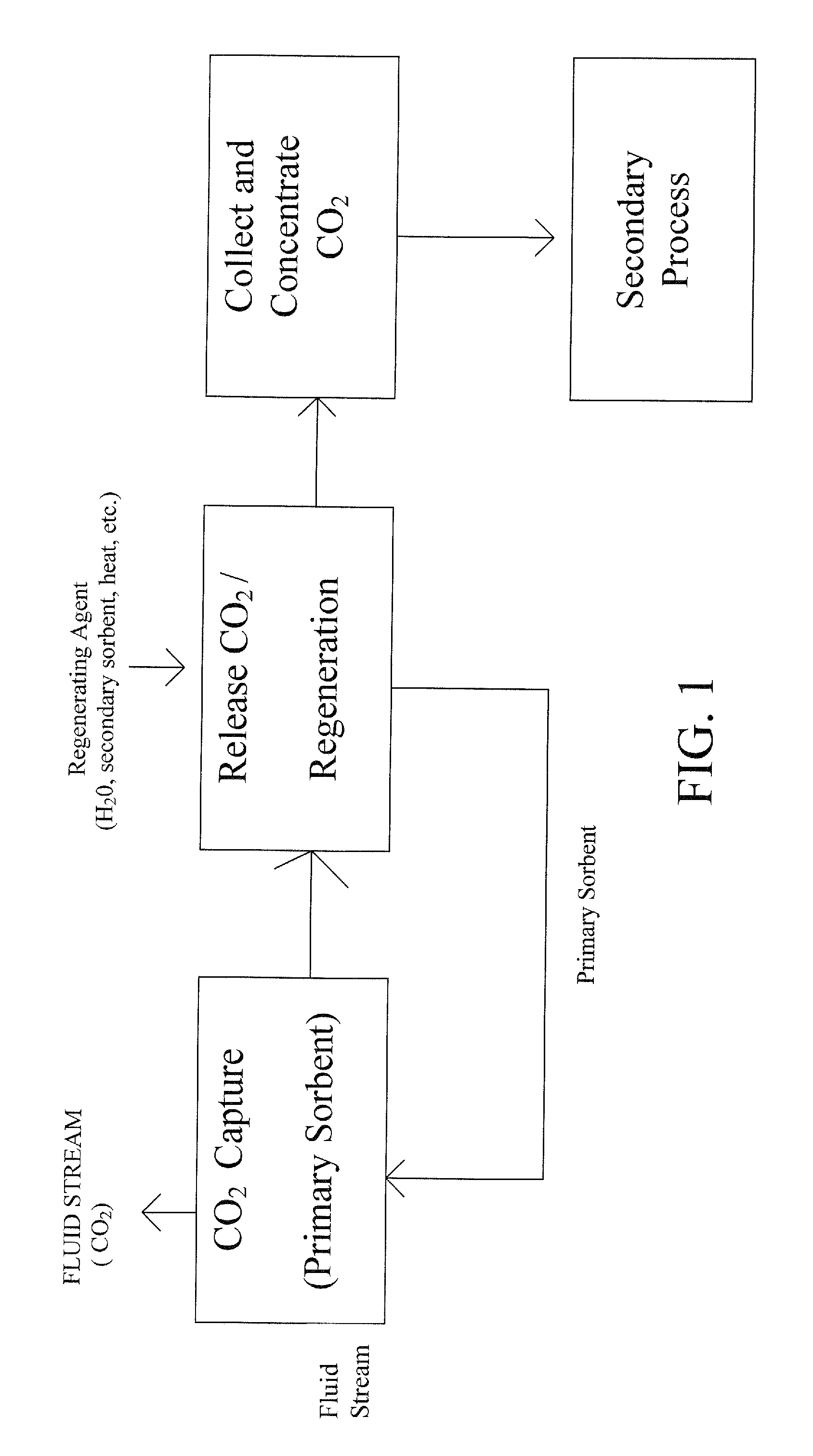
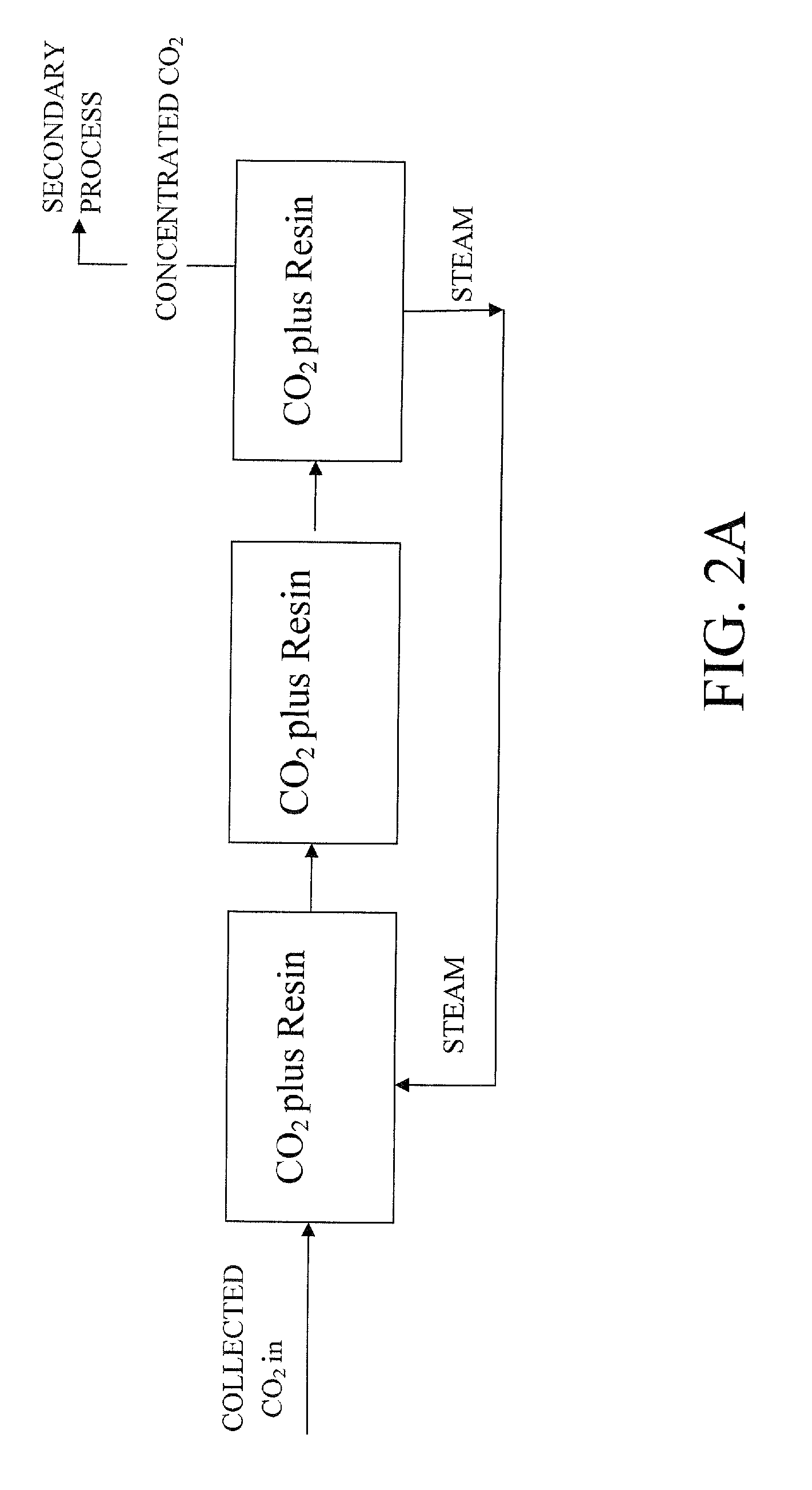
Extraction and sequestration of carbon dioxide
The present disclosure provides a method and apparatus for extracting carbon dioxide (CO2) from a fluid stream and for delivering that extracted CO2 to controlled environments for utilization by a secondary process. Various extraction and delivery methods are disclosed specific to certain secondary uses, included the attraction of CO2-sensitive insects, the ripening and preservation of produce, and the neutralization of brine.
Owner:KILIMANJARO ENERGY
Xanthan gels in brines and methods of using such xanthan gels in subterranean formations
Owner:HALLIBURTON ENERGY SERVICES INC
Methods to de-sulfate saline streams
ActiveUS7789159B1Avoid pollutionWaste water treatment from quariesSludge treatmentSaline waterInorganic materials
Methods are disclosed to de-sulfate saline streams such as seawater, brine from seawater desalination plants, and the like. The disclosed methods can also co-produce de-ionized water and inorganic materials from such de-sulfated saline streams.
Owner:BADER MANSOUR S
Dispensing and injection system for radiopharmaceuticals
InactiveUS20050203329A1Enhanced Radiation ProtectionEasy to effect automatic controlIn-vivo radioactive preparationsInfusion devicesSaline waterRadiation shield
Disclosed is a dispensing and injection system for radiopharmaceuticals, wherein a dosage calibrator is arranged inside a radiation-shielded hermetic chamber for detecting the radioactivity of radiopharmaceuticals contained in a vial. At least one saline water cartridge is arranged inside the casing and includes an internal passage, radiopharmaceuticals discharge end, a radiopharmaceuticals injection end, a and saline water reservoir outlet end. The saline water cartridge forms a saline water reservoir. A movable dispensing and injection mechanism controls radiopharmaceuticals dispensing operation of a syringe and controls movement of the syringe between a radiopharmaceuticals dispensing position and an injection position. When the movable dispensing and injection mechanism moves the syringe to the radiopharmaceuticals dispensing position, the syringe withdraws a predetermined amount of radiopharmaceuticals from the vial. When moved to the injection position, the syringe injects the withdrawn radiopharmaceuticals into the radiopharmaceuticals injection end of the saline water cartridge. After the radiopharmaceuticals is injected into a patient, the saline water stored in the saline water reservoir of the saline water cartridge is withdrawn for effecting flushing process.
Owner:SHIH CHI DAU
Method of acidizing a subterranean formation with diverting foam or fluid
ActiveUS7303018B2Potential damageHigh viscosityFluid removalDrilling compositionSaline waterFluid viscosity
A method of acidizing a subterranean formation with a diverting agent composed of a gelled or thickened viscoelastic foam or fluid generated from (i.) an amidoamine oxide gelling agent and (ii.) an acid or foam, water and / or brine. The gelled or thickened foam or fluid may be generated in-situ or introduced directly into the formation by mixing of the amidoamine oxide gelling agent and acid or foam, water and / or brine. As the acid spends, the acidizing fluid thickens. When the acid is further spent, the fluid viscosity declines eventually returning to a low viscosity state, allowing for easy cleanup. The process allows for selective acidizing of less permeable zones of the formation and more uniform stimulation of the hydrocarbon bearing formation.
Owner:BAKER HUGHES HLDG LLC
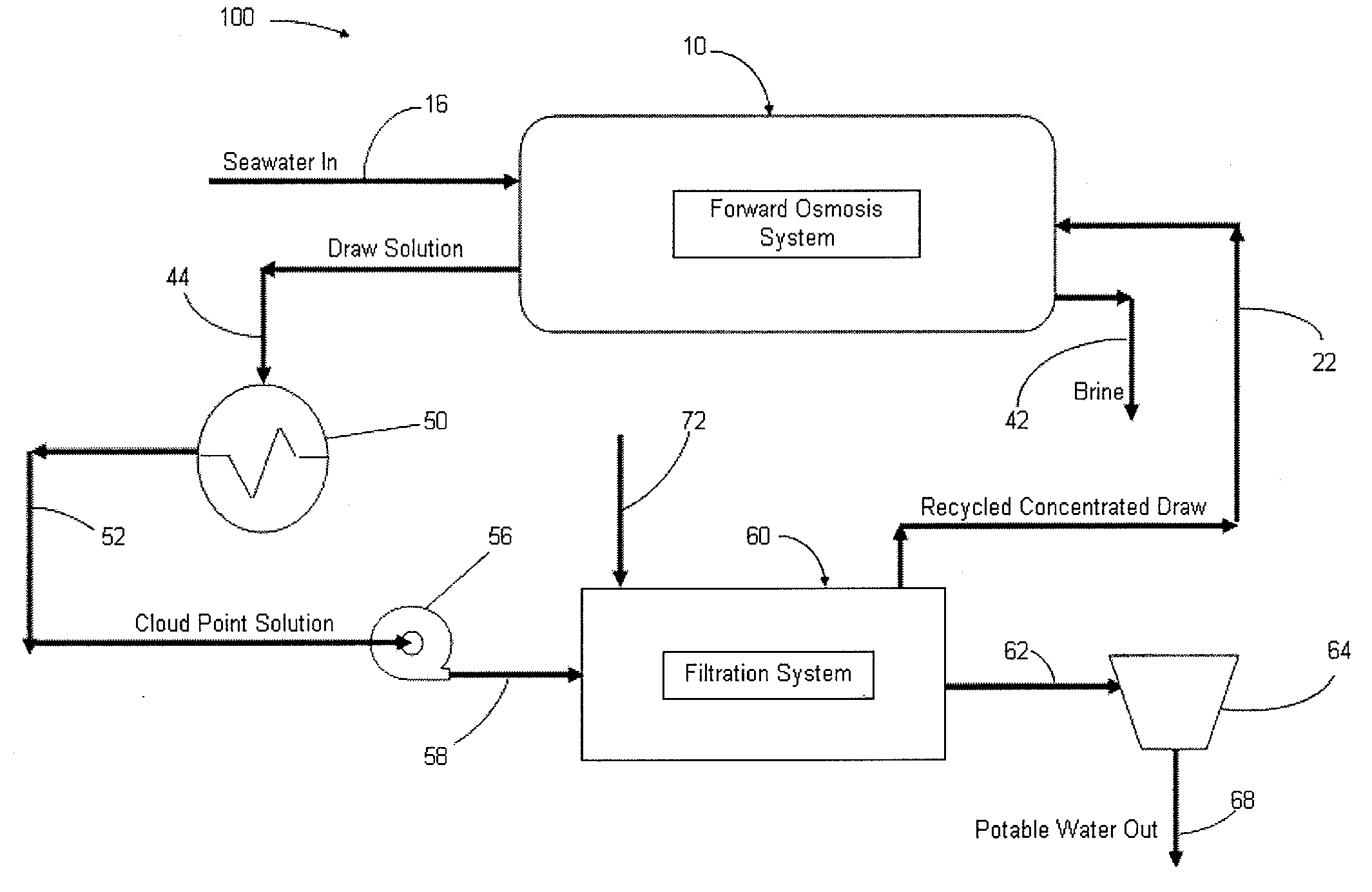
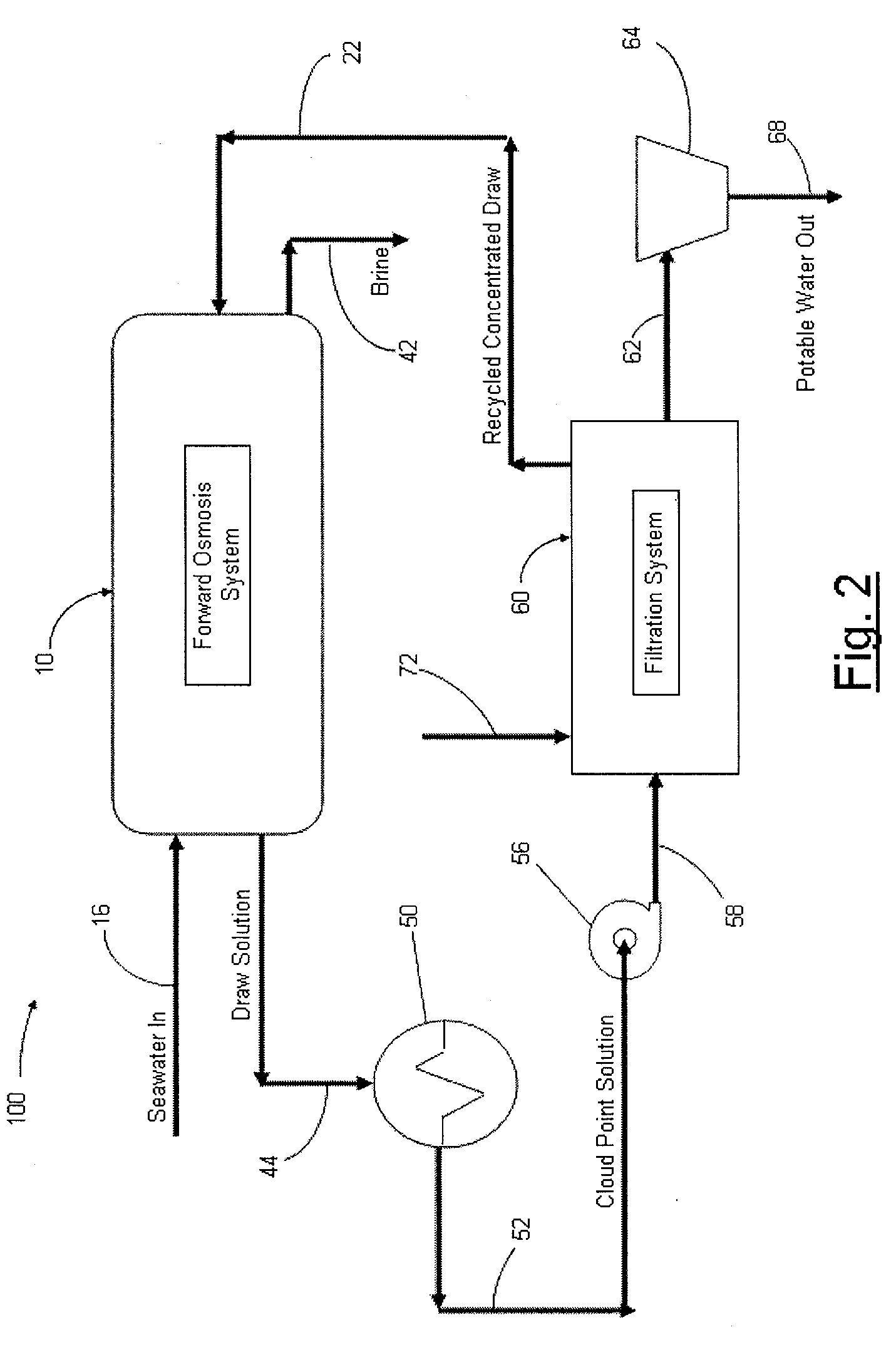
Systems and methods for forward osmosis fluid purification
ActiveUS20100155329A1Improve efficiencyFunction increaseGeneral water supply conservationSeawater treatmentSaline waterDesalination
A process for purification of fluids, for example, desalination of seawater or brackish water, using organic solutes in a concentrated water solution for use in a forward osmosis process, to extract fresh water out of salt water through the forward osmosis membrane, and subsequently separating the organic solutes out of the diluted forward osmosis permeate by cloud point extraction, thereby regenerating a concentrated organic solution for recycling to the forward osmosis process, and fresh water for potable water use.
Owner:JFEENG CORP
Use of nanoparticles for labelling oil field injection waters
InactiveUS20130084643A1Effective sizeEasy to implementOptical radiation measurementSurveyInjection wellOrganosilicon
The present invention relates to the development of tracer fluids, more generally, that of aqueous liquids, intended to be injected under pressure in an oil reservoir, for example from an injection well up to a production well.The object of the invention is to propose a new method of study of a solid medium, i.e. an oil reservoir, by diffusion of a liquid (i.e. injection waters) containing tracers, through said solid medium, which is simple to implement and economical and which remedies the drawbacks of the known tracers for injection waters of oil reservoirs.This method essentially consisting of injecting, in this solid medium, an injection liquid comprising a nanoparticle-based tracer having average dimensions comprised between 20 and 200 nm, detectable by means of one or several S signals at dilutions of less than or equal to 10−7, adapted to form a stable colloidal suspension in a saline medium, at least a portion of which is constituted of a core and a coating provided with an adjustable hydrophilic-lipophilic balance (HLB) and comprising at least one organic and / or organosilicon component; recovering the liquid having diffused; and analyzing this liquid having diffused to measure the quantity of tracer by detection of the signal or signals S.
Owner:TOTAL PUTEAUX FR
Brine-based viscosified treatment fluids and associated methods
The present invention relates to viscosified treatment fluids used in industrial and oil field operations, and more particularly, to brine-based viscosified treatment fluids comprising xanthan gelling agents, and their use in industrial and oil field operations. In one embodiment, the present invention provides a method of treating a portion of a subterranean formation comprising the steps of: providing a viscosified treatment fluid that comprises a brine and a gelling agent that comprises a clarified xanthan; and treating the portion of the subterranean formation. The present invention also provides methods of fracturing, gravel packing, and making viscosified treatments fluids. Also provided are viscosified treatment fluid compositions, and gelling agent compositions.
Owner:HALLIBURTON ENERGY SERVICES INC
Production of purified water and high value chemicals from salt water
ActiveUS7083730B2Effective recoveryElectrolysis componentsSolvent extractionReverse osmosisHigh pressure
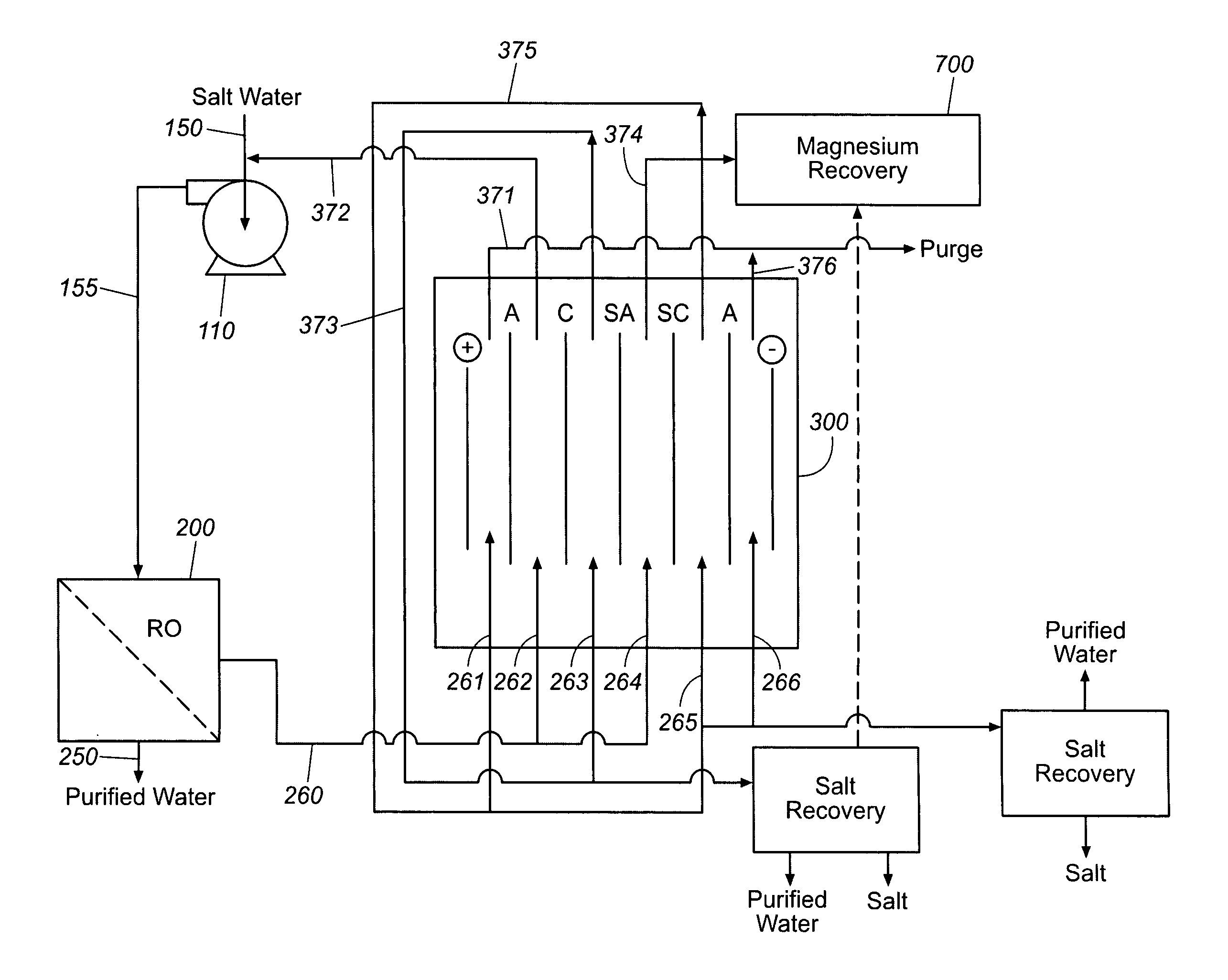
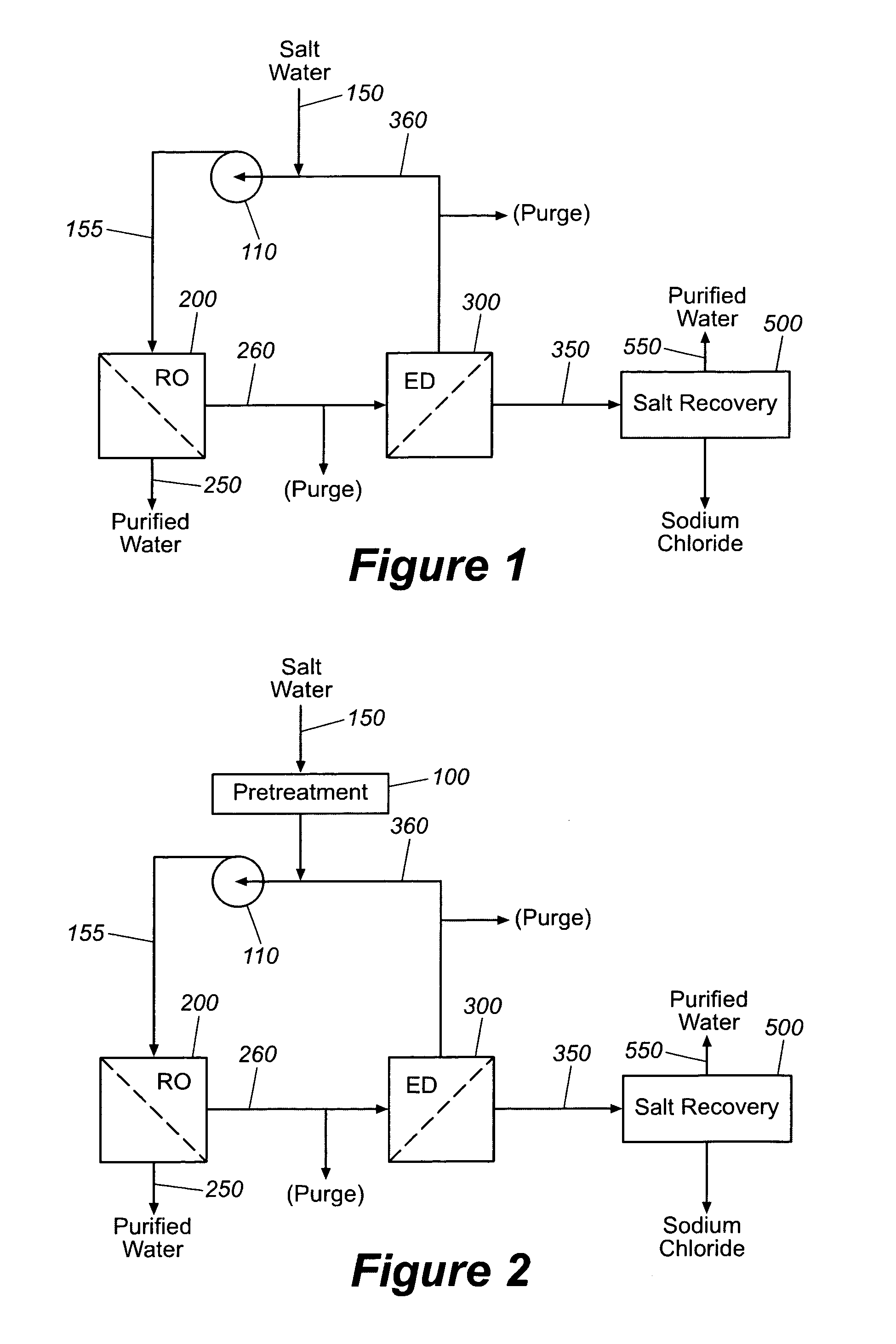
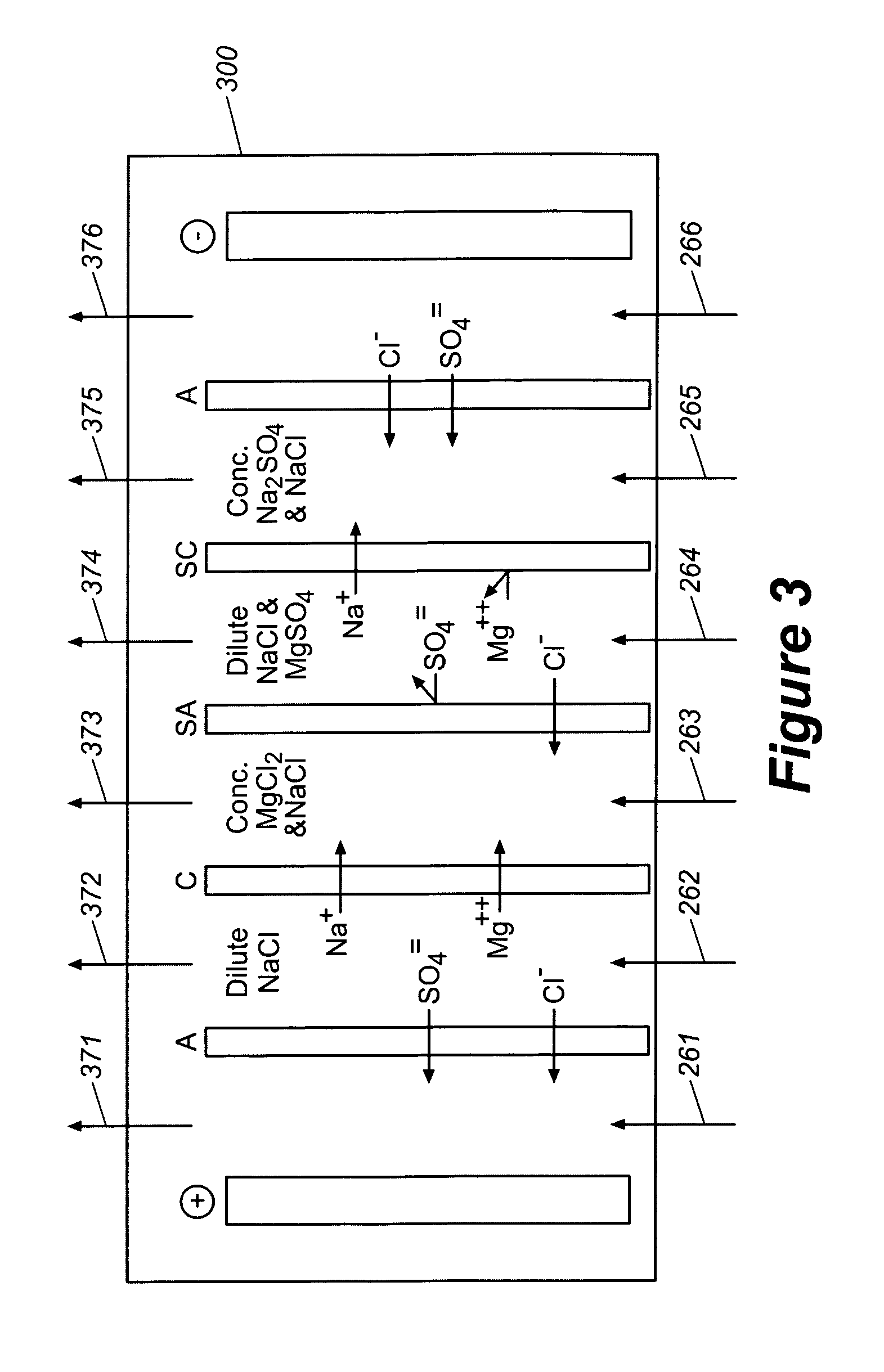
Sodium chloride and purified water are recovered by treating salt water that contains sodium chloride with an integrated reverse osmosis and electrodialysis system, which includes an efficiency-enhancing feature that is one or more of the following: the use of univalent anion and univalent cation selective membranes in the electrodialysis unit; the addition of a nanofiltration unit to process the diluate from the electrodialysis unit; or operation of the electrodialysis unit at an elevated pressure. Magnesium and bromine can optionally be produced when the salt water contains these materials.
Owner:SOUTH CAROLINA UNIV OF
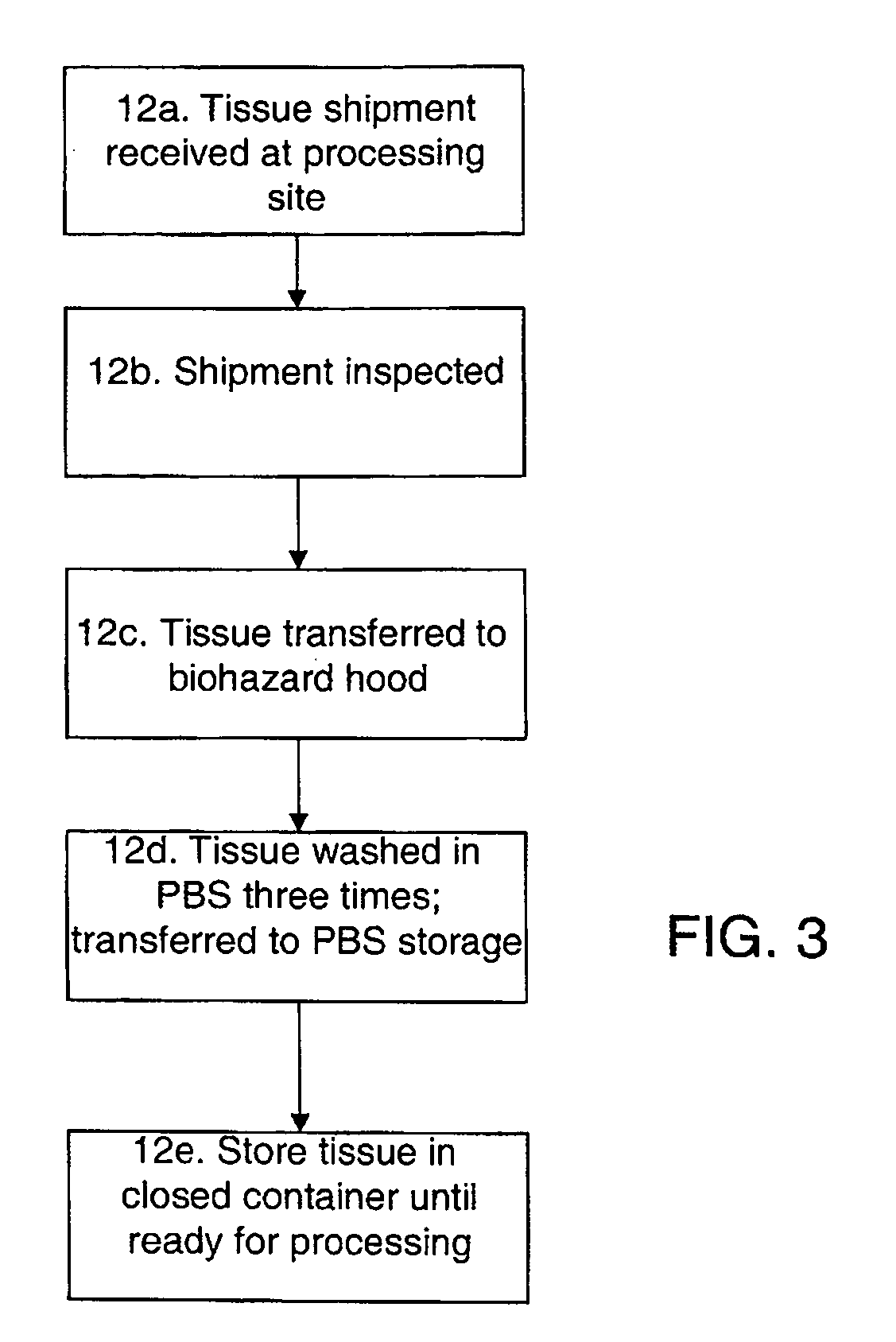
Methods for processing biological tissue
ActiveUS20060154230A1Remove background levelAvoid degradationDead animal preservationPhosphateCalcification
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
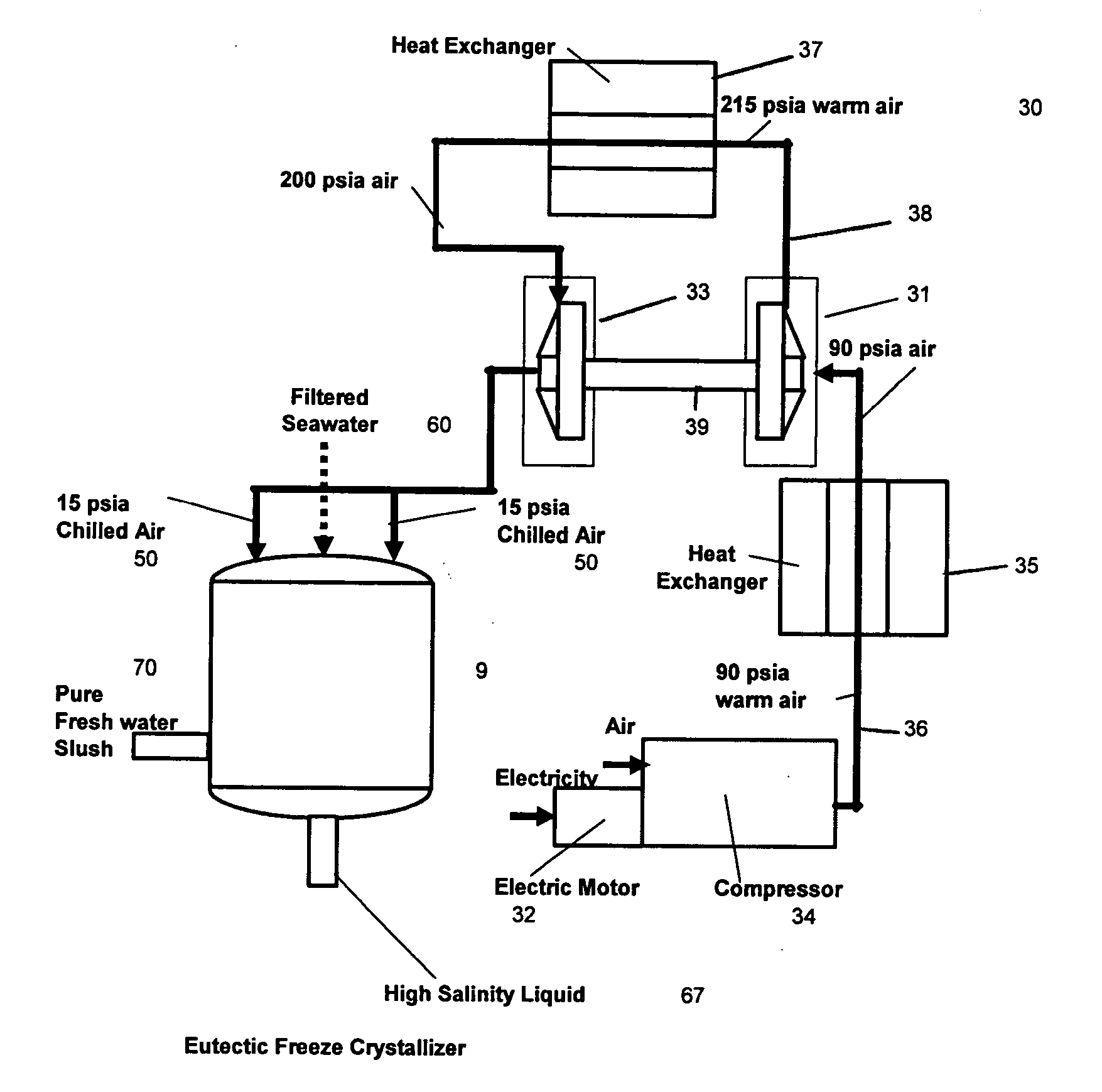
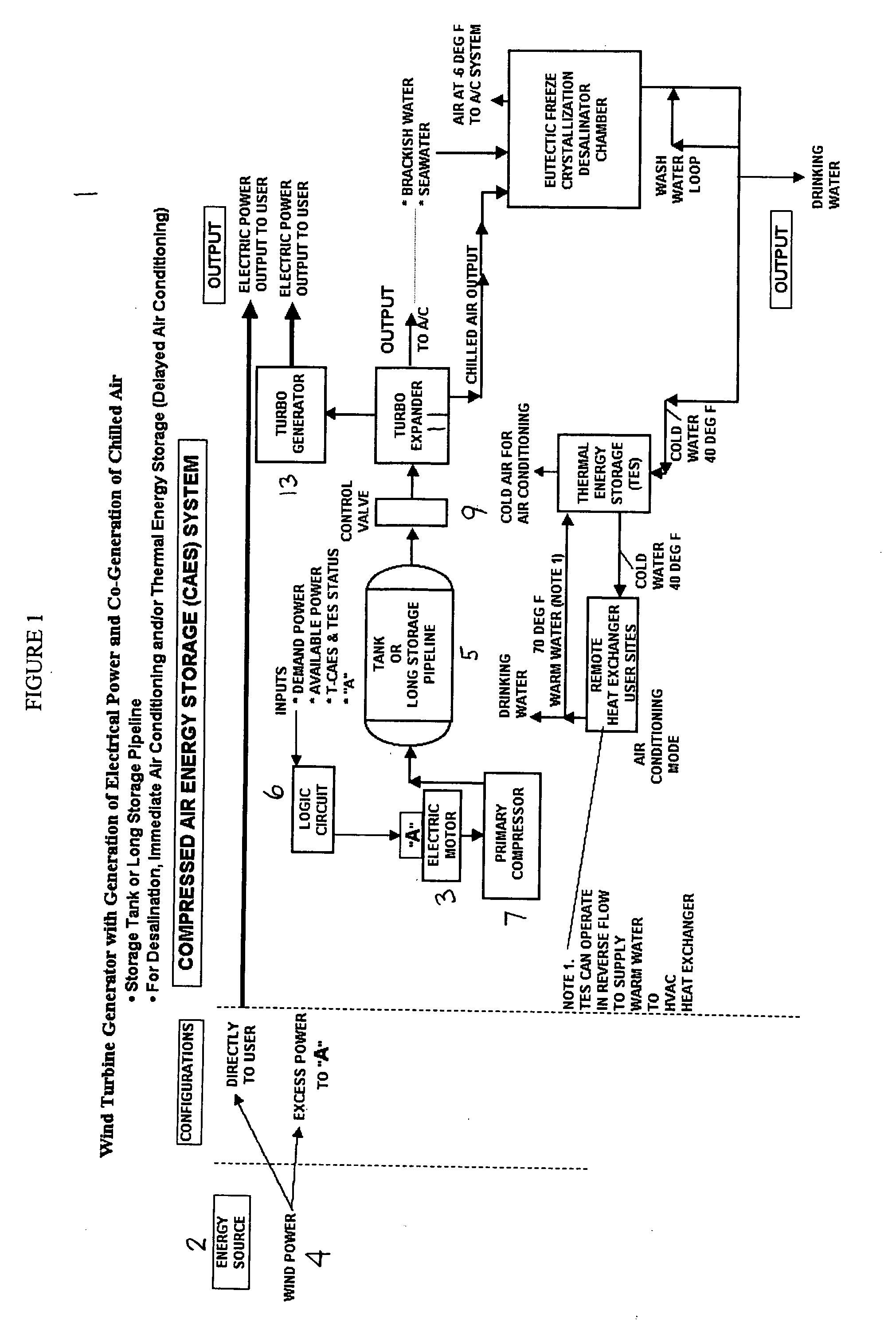
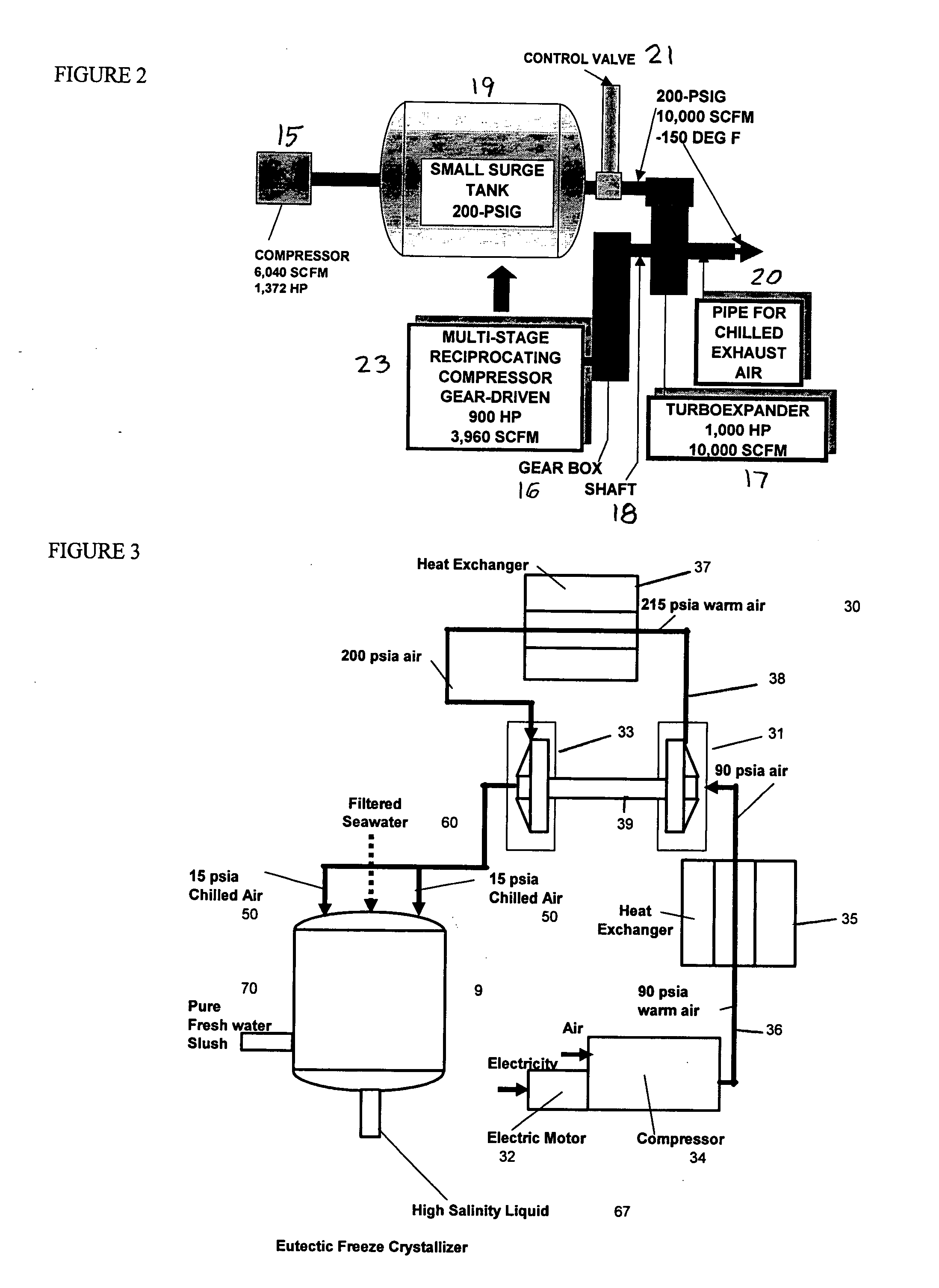
Desalination method and system using compressed air energy systems
InactiveUS20070295673A1Promote meltingFacilitate runoffWater treatment parameter controlWater cleaningSaline waterDesalination
The invention relates to a desalination method and system that uses freeze crystallization technology that incorporates the use of compressed air energy as the source for freezing temperatures. When compressed air is released by a turbo expander, chilled air is produced as a by-product, wherein the chilled air is introduced into a crystallization chamber. Also injected into the chamber is a spray cloud of seawater droplets, which has been pre-chilled by heat exchange with the cold chamber walls, and which is then circulated and exposed to the chilled air in the chamber. The sizes of the droplets can vary, but are preferably predetermined, along with the relative temperatures, flows and speeds of the spray and chilled air, such that when the droplets are circulated within the chilled air, and settle at the bottom of the chamber, they are deposited at slightly above the eutectic temperature. This way, the ice / snow mass that forms at the bottom of the chamber will consist of frozen ice crystals, and a residue of salt water brine, which can runoff from the mass, either from the sides, or through any voids or channels that may form within the mass.
Owner:ENIS BEN M +1
Method and apparatus for producting negative and positive oxidative reductive potential (orp) water
ActiveUS20050121334A1Effective and efficient and economicalCellsWater treatment parameter controlParticulatesElectrolysis
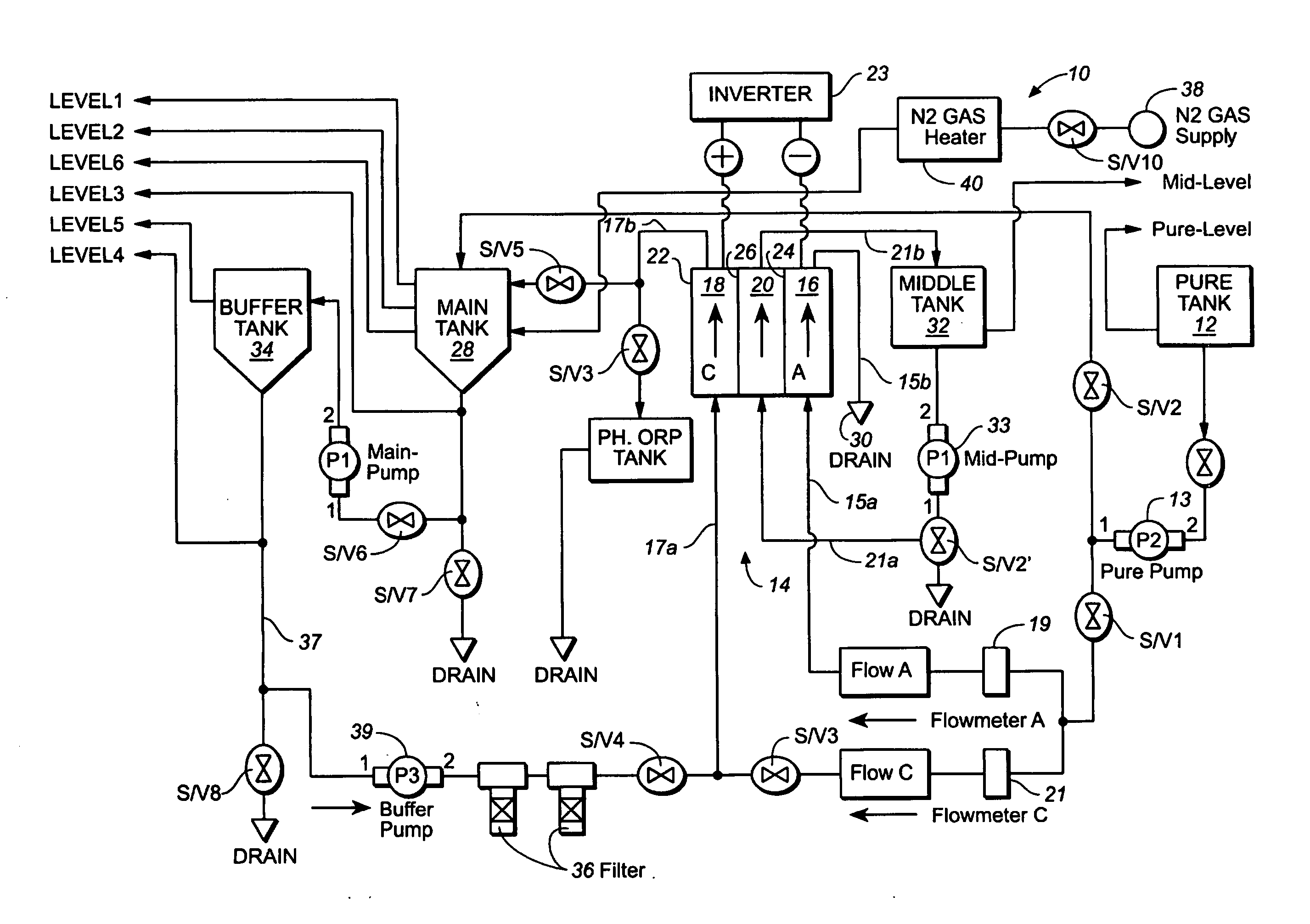
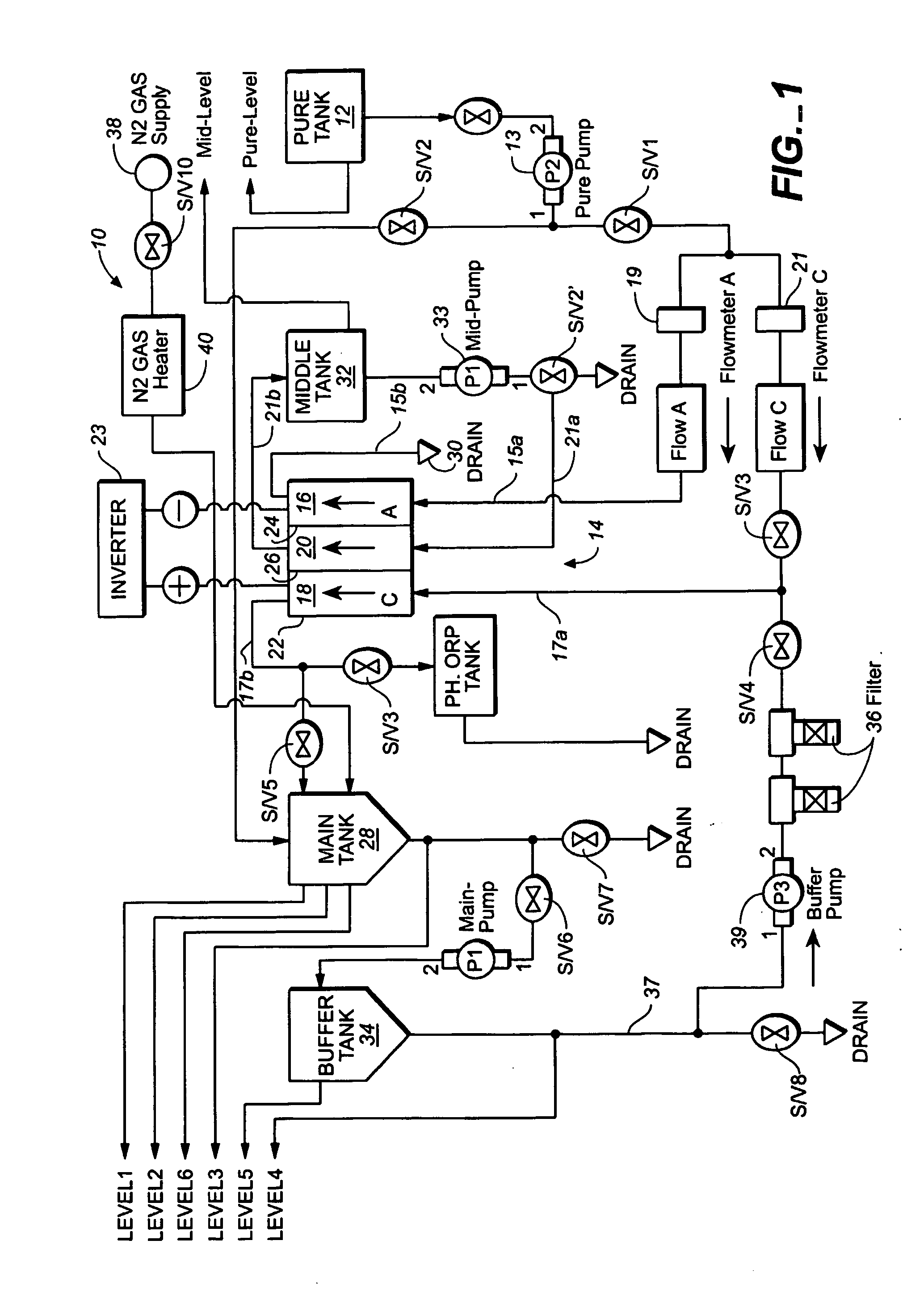
A method and apparatus for electrolytically producing oxidation reduction potential water from aqueous salt solutions for use in disinfection, sterilization, decontamination, wound cleansing. The apparatus includes an electrolysis unit having a three-compartment cell (22) comprising a cathode chamber (18), an anode chamber (16), and a saline solution chamber (20) interposed between the anode and cathod chambers. Two communicating (24, 26) membranes separate the three chambers. The center chamber includes a fluid flow inlet (21a) and outlet (21b) and contains insulative material that ensures direct voltage potential does not travel through the chamber. A supply of water flows through the cathode and anode chambers at the respective sides of the saline chamber. Saline solution flows through the center chamber, either by circulating a pre-prepared aqueous solution containing ionic species, or, alternatively, by circulating pure water or an aqueous solution of, e.g., aqueous hydrogen chloride and ammonium hydroxide, over particulate insulative material coated with a solid electrolyte. Electrical current is provided to the communicating membranes separating the chambers, thus causing an electrolytic reaction that produces both oxidative (positive) and reductive (negative) ORP water.
Owner:SONOMA PHARMA INC
Carbonate products for carbon capture and storage
InactiveUS20110030586A1Gaseous chemical processesLiquid-gas reaction of thin-film typeCarbon capture and storageChemistry
Owner:CALERA CORP
Methods to improve heteroatom lattice substitution in large and extra-large pore borosilicate zeolites
InactiveUS6790433B2Controlled catalytic propertyAluminium compoundsMolecular sieve catalystsIron saltsAluminosilicate
The invention, in one embodiment, is a method for preparing crystalline zeolites by (a) contacting a calcined essentially aluminum free borosilicate zeolite with an aqueous acid solution, thereby producing an at least partially deboronated zeolite; (b) contacting said at least partially deboronated zeolite with a solution selected from the group consisting of an aqueous aluminum salt solution, thereby producing an aluminosilicate zeolite; an aqueous gallium salt solution, thereby producing a gallosilicate zeolite; an aqueous iron salt solution, thereby producing a ferrosilicate zeolite; and mixtures thereof; and (c) where the contacting in step (b) occurs at a pH of not greater than about 3.5. In another embodiment, the present invention provides a method for preparing crystalline zeolites by contacting a calcined essentially aluminum free large or extra-large pore borosilicate zeolite with a solution selected from the group consisting of an aqueous aluminum salt solution, thereby producing an aluminosilicate zeolite; an aqueous gallium salt solution, thereby producing a gallosilicate zeolite; an aqueous iron salt solution, thereby producing a ferrosilicate zeolite; and mixtures thereof; and where the contacting occurs at a pH of not greater than about 3.5.
Owner:CHEVROU USA INC
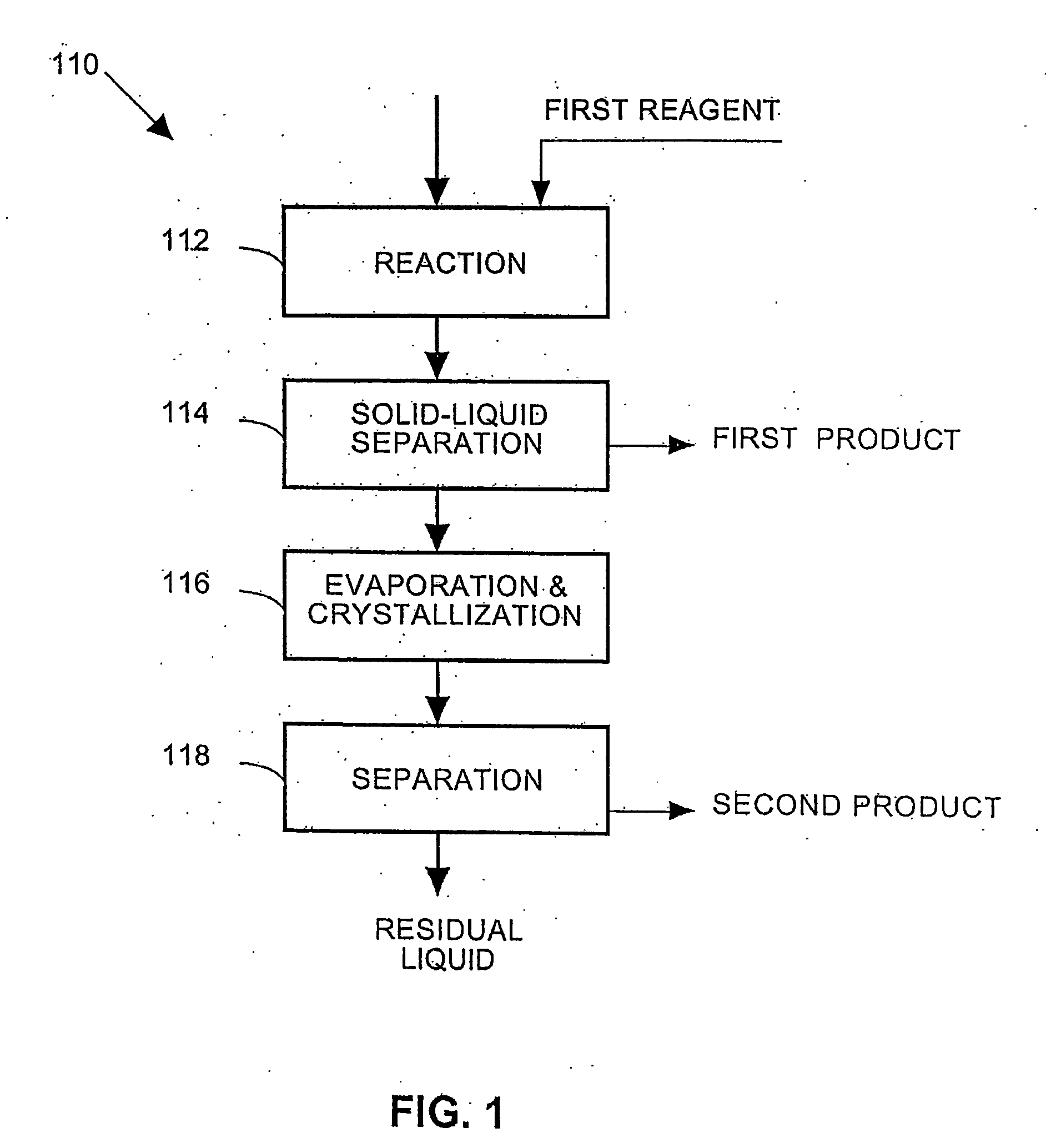
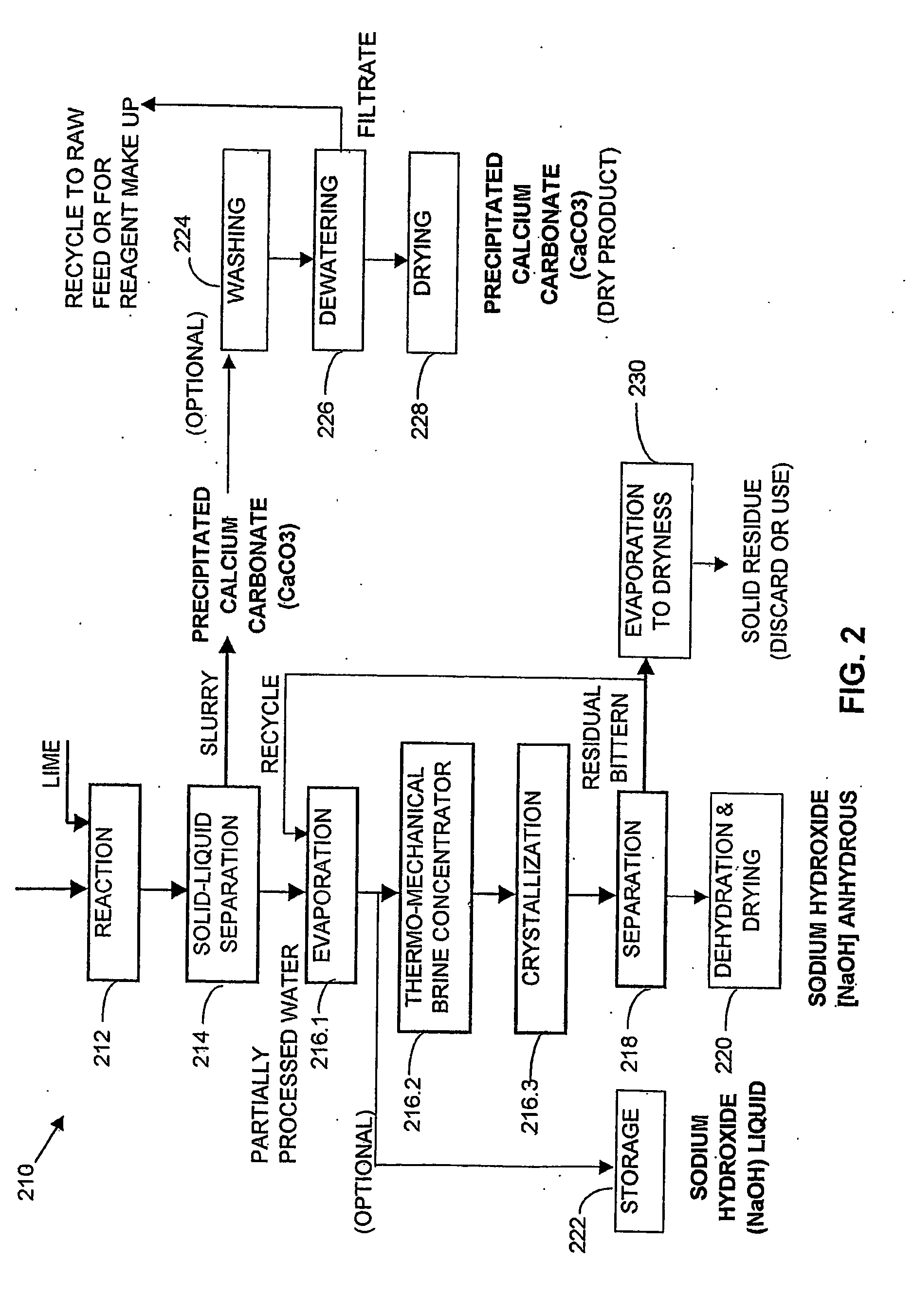
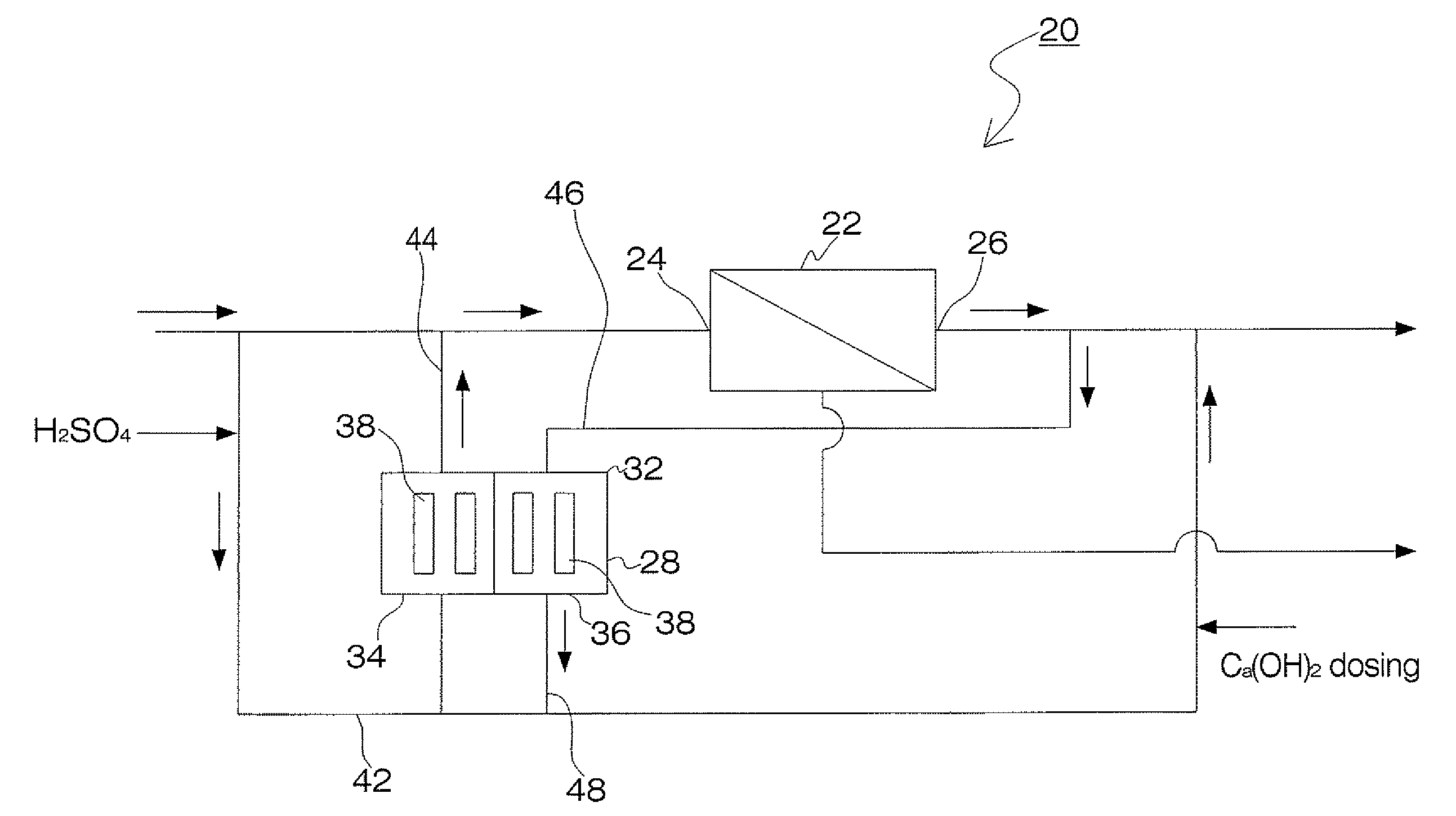
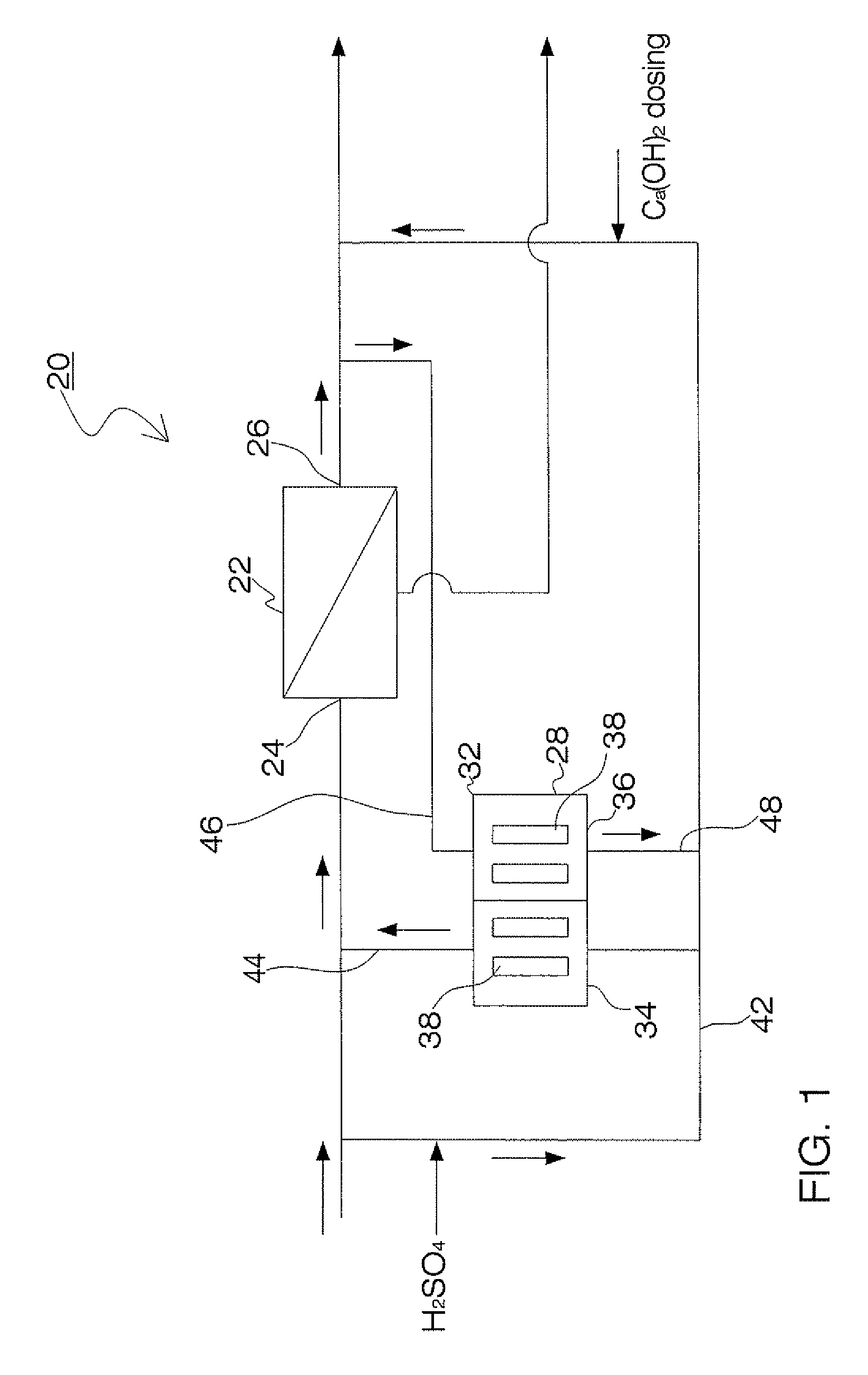
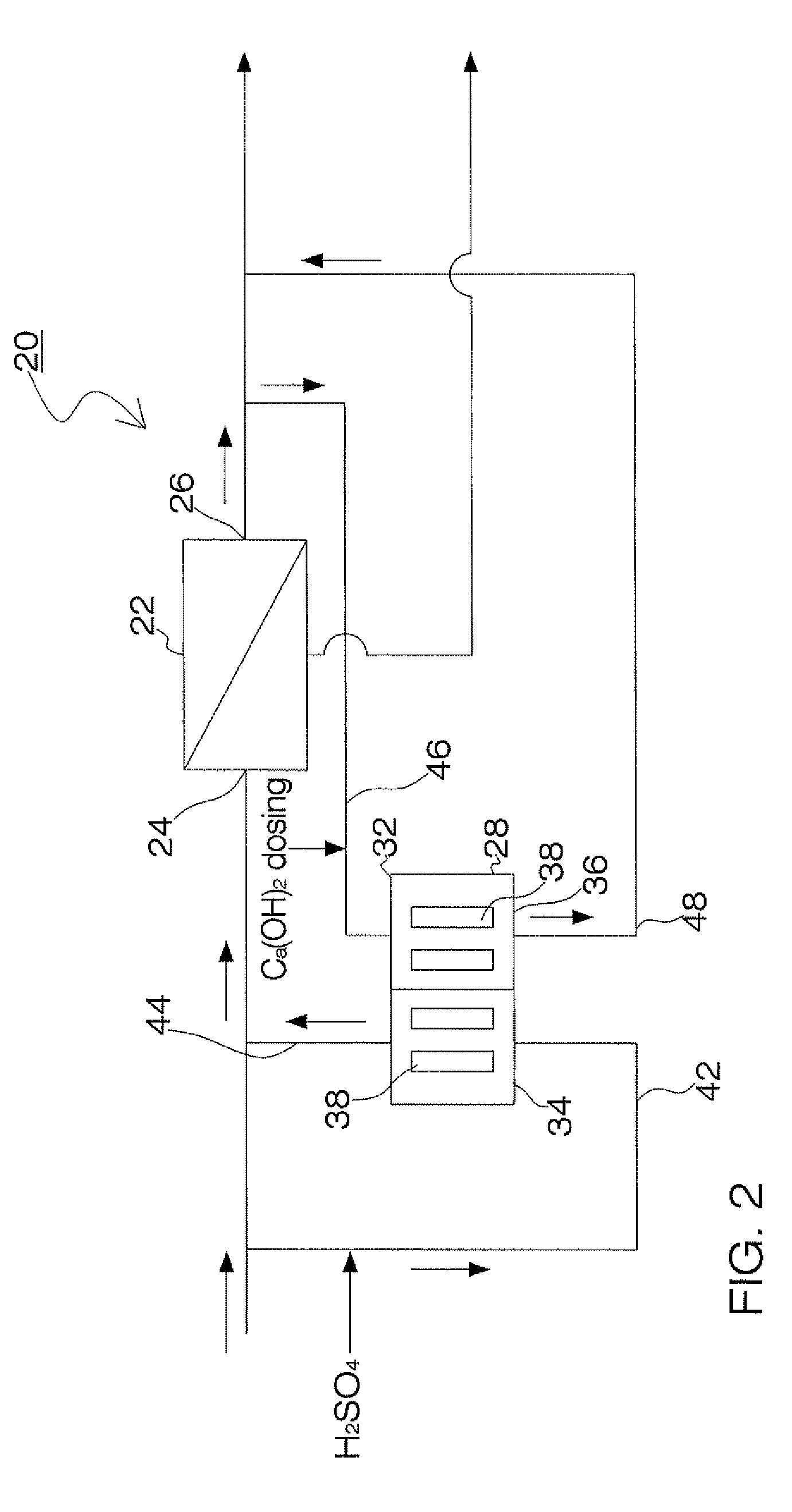
Process and apparatus for the treatment of saline water
InactiveUS20060196836A1Reduce ZLD costReduce loadWaste water treatment from quariesGeneral water supply conservationTotal dissolved solidsEvaporation
A process and an apparatus are described for treating seven types of saline waters each having a concentration of total dissolved solids exceeding 1 g / L, wherein the concentration of total dissolved solids, the ratio of the chloride ion concentration to the bicarbonate ion concentration and the ratio of the chloride ion concentration to the sulphate ion concentration of each of the water types are as indicated in Table 1. The process includes the steps of contacting the water with a first reagent comprising a source of calcium ions selected from calcium oxide and calcium hydroxide to form a first solid product which is recovered. The process includes a further step of subjecting at least a portion of the partially processed water to at least partial evaporation so as to promote the formation of a precipitate and a mother liquor. The precipitate is recovered as a second product.
Owner:GEO PROCESSORS
Dressing material containing medicine chitoholosida and its preparation method
InactiveCN1579559AHas therapeutic effectSustained releaseAbsorbent padsBandagesSolid componentPhosphate
The invention produces polyethylene alcohol hydrogel dressing containing medicine and chitosan with 60Co gamma-radial or high energy electron beam radial cross linking. Additional, adds in some humectant, plasticizer, medicine, the solvent is the secondary distilled water, physiological saline or phosphate neutral buffer liquid. The product can release medicine slowly and has natural amylose chitosan with biology sterilization activity, it has active sterilization function, at the same time, it has high water quantity, and good water reserving performance, the mechanical intensity is moderate, and the light penetration and air penetration are excellent. It can accord the demands for curing each kind of wound. It can used as the permanent dressing for light skin injuries, and it also can be applied to the temporally close of severe skin organization wound or burn wound.
Owner:CHANGZHOU INST OF ENERGY STORAGE MATERIALS &DEVICES
Particulate water absorbent agent and production method thereof, and water absorbent article
ActiveUS20050209352A1Improve liquidityImprove water absorptionOther chemical processesAbsorbent padsParticulatesSaline water
A particulate water absorbing agent of the present invention includes a water absorbent resin, having a cross-linking structure, whose surface has been cross-linked by adding a surface treatment agent, wherein: (i) a mass average particle diameter (D50) ranges from 200 to 600 μm and 95 to 100 wt % of a particulate water absorbing agent whose particle diameter ranges from less than 850 μm to not less than 150 μm is contained with respect to 100 wt % of whole the particulate water absorbing agent, and (ii) a logarithmic standard deviation (σζ) of particle size distribution ranges from 0.25 to 0.45, and (iii) a compressibility rate defined by a following equation ranges from 0 to 18%, and (iv) a surface tension of a supernatant liquid obtained in 4 minutes after dispersing 0.5 g of the particulate water absorbing agent in 50 ml of physiological saline whose temperature is 20° C. is 55 mN / m or more, the compressibility rate (%)=(P−A) / P×100 where P represents a tapped bulk density of the particulate water absorbing agent and A represents a loose bulk density of the particulate water absorbing agent.
Owner:NIPPON SHOKUBAI CO LTD
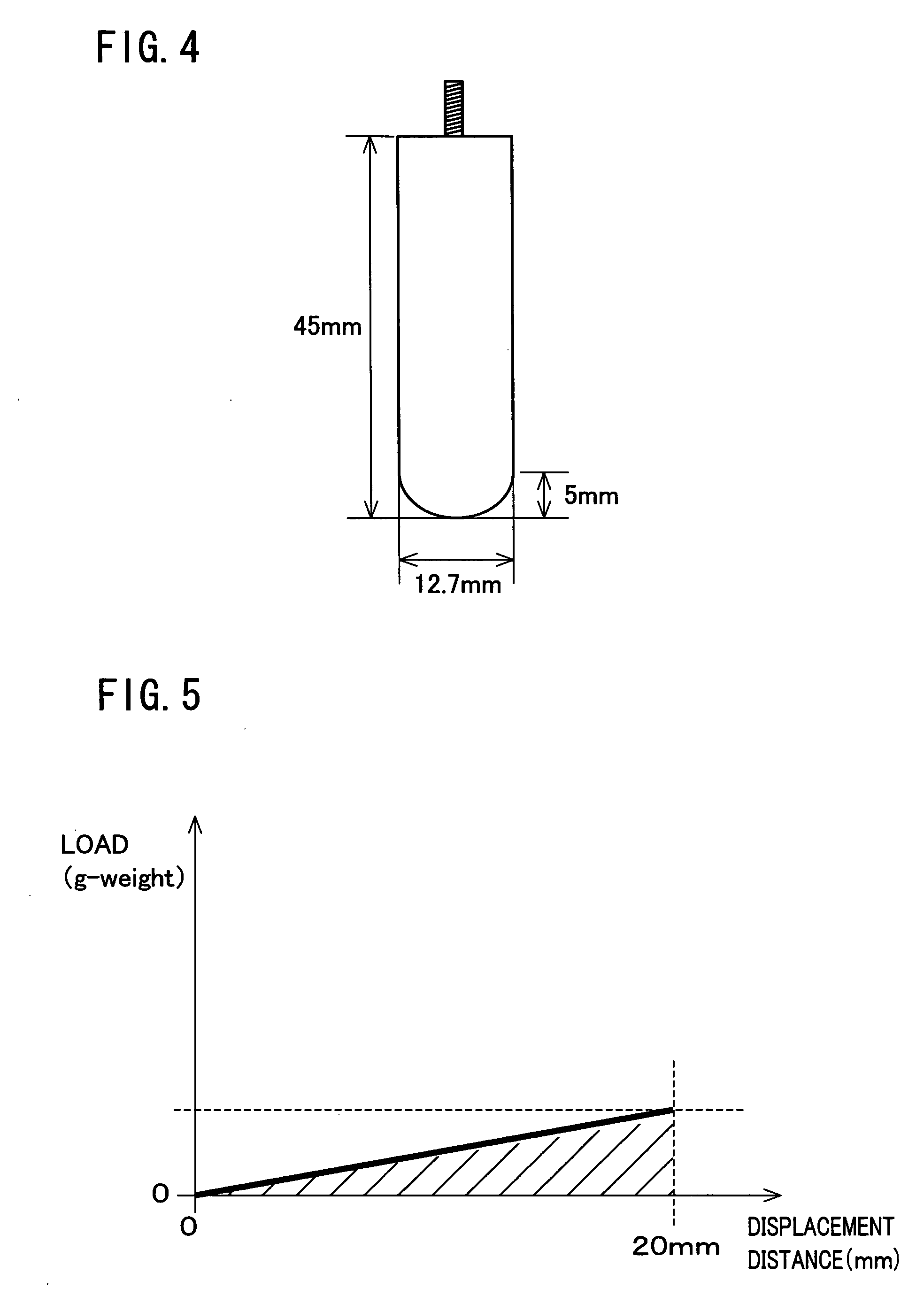
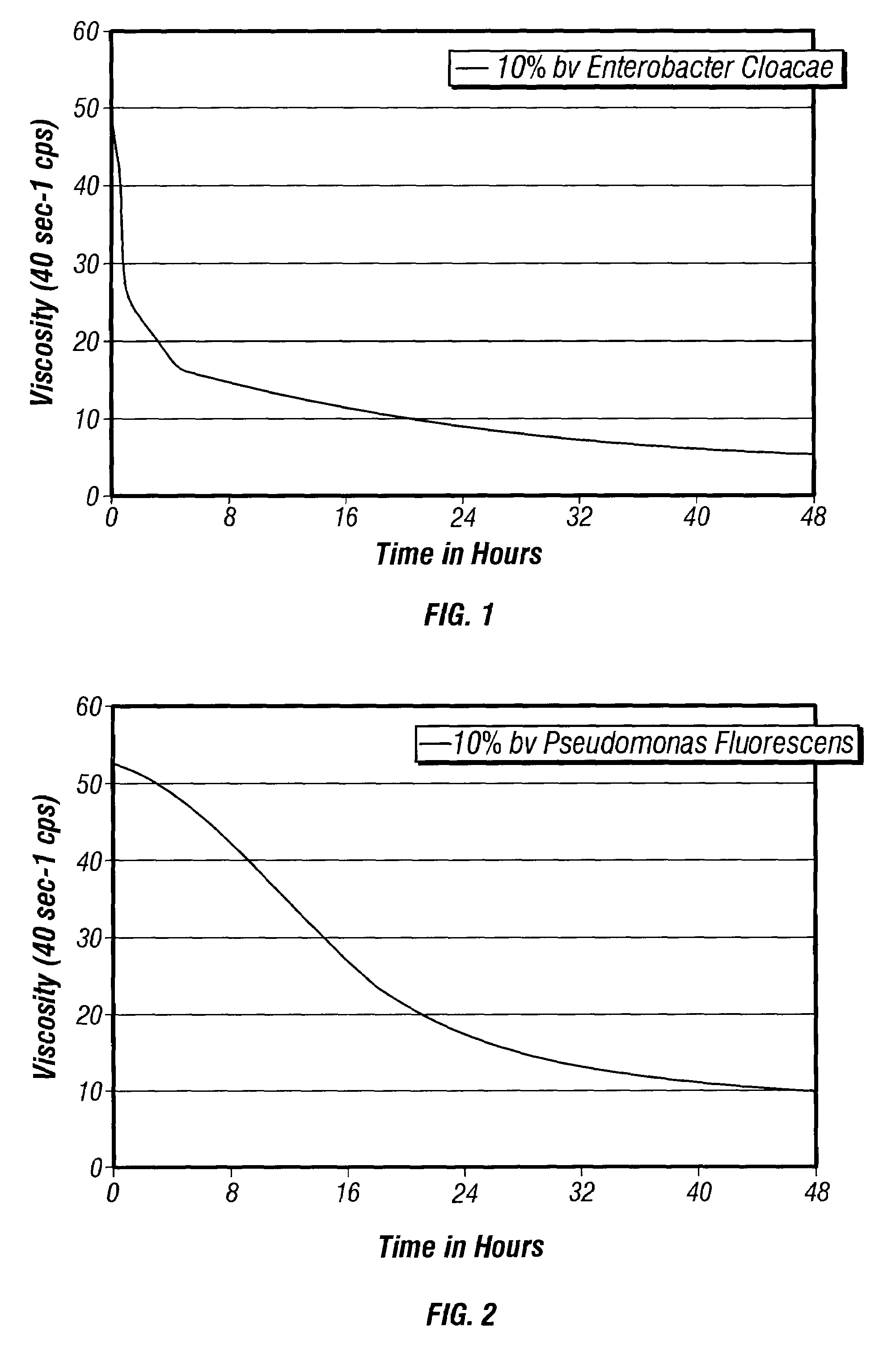
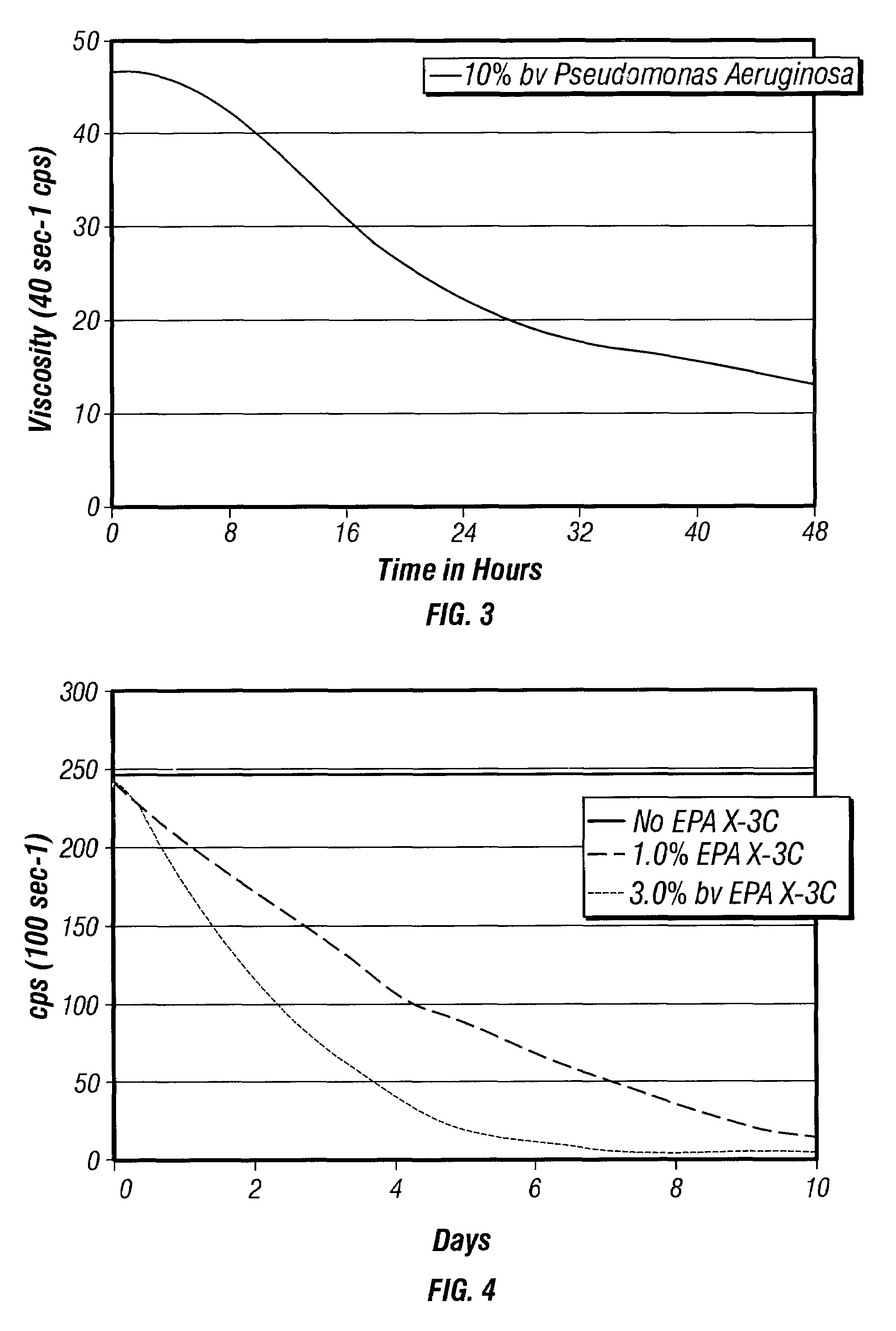
Bacteria-based and enzyme-based mechanisms and products for viscosity reduction breaking of viscoelastic fluids
ActiveUS7052901B2Low viscosityBreak viscosityDeodrantsFlushingIndirect actionPseudomonas fluorescens
It has been discovered that fluids viscosified with viscoelastic surfactants (VESs) may have their viscosities reduced (gels broken) by the direct or indirect action of a biochemical agent, such as bacteria, fungi, and / or enzymes. The biochemical agent may directly attack the VES itself, or some other component in the fluid that produces a by-product that then causes viscosity reduction. The biochemical agent may disaggregate or otherwise attack the micellar structure of the VES-gelled fluid. The biochemical agent may produce an enzyme that reduces viscosity by one of these mechanisms. A single biochemical agent may operate simultaneously by two different mechanisms, such as by degrading the VES directly, as well as another component, such as a glycol, the latter mechanism in turn producing a by-product (e.g. an alcohol) that causes viscosity reduction. Alternatively, two or more different biochemical agents may be used simultaneously. In a specific, non-limiting instance, a brine fluid gelled with an amine oxide surfactant can have its viscosity broken with bacteria such as Enterobacter cloacae, Pseudomonas fluorescens, Pseudomonas aeruginosa, and the like.
Owner:BAKER HUGHES INC
System and method for using carbon dioxide sequestered from seawater in the remineralization of process water
ActiveUS7771599B1Reduce scaleReduce inorganicLiquid degasificationGeneral water supply conservationSaline waterAlkalinity
Disclosed is an improved method for the remineralization of process water in a desalination system. The method sequesters carbon dioxide gas (CO2) from seawater or concentrate (brine) of desalination process via a gas transfer membrane. The sequestered carbon dioxide gas (CO2) is thereafter used in the production of soluble calcium bicarbonate (Ca(HCO3)2). The calcium bicarbonate (Ca(HCO3)2) adds hardness and alkalinity to the resulting process water.
Owner:DOOSAN HEAVY IND & CONSTR CO LTD
Recovery of common salt and marine chemicals from brine
InactiveUS6776972B2High purityLow costGeneral water supply conservationSeawater treatmentSaline waterEvaporation
A new process for recovery of common salt, potassium chloride, concentrated magnesium chloride with enriched bromide, and high purity magnesia from brine in an integrated manner, said process comprises preparation of calcium chloride by reaction of hydrochloric acid generated in the process with limestone, desulfatation of brine with calcium chloride, production of sodium chloride of superior quality in solar pans, solar evaporation of bittern thereby producing carnallite and end bittern, processing carnallite through established processes to produce potassium chloride, recovering end bittern containing highly concentrated magnesium chloride and enriched bromide and calcination of a part of the end bittern after solidification to produce high purity magnesia and hydrochloric acid utilizable in the process.
Owner:COUNCIL OF SCI & IND RES
Antimicrobial salt solutions for food safety applications
ActiveUS7090882B2Reduce in quantityInhibit microorganismsMilk preservationDough treatmentBiologyFood processing
Owner:CARGILL INC
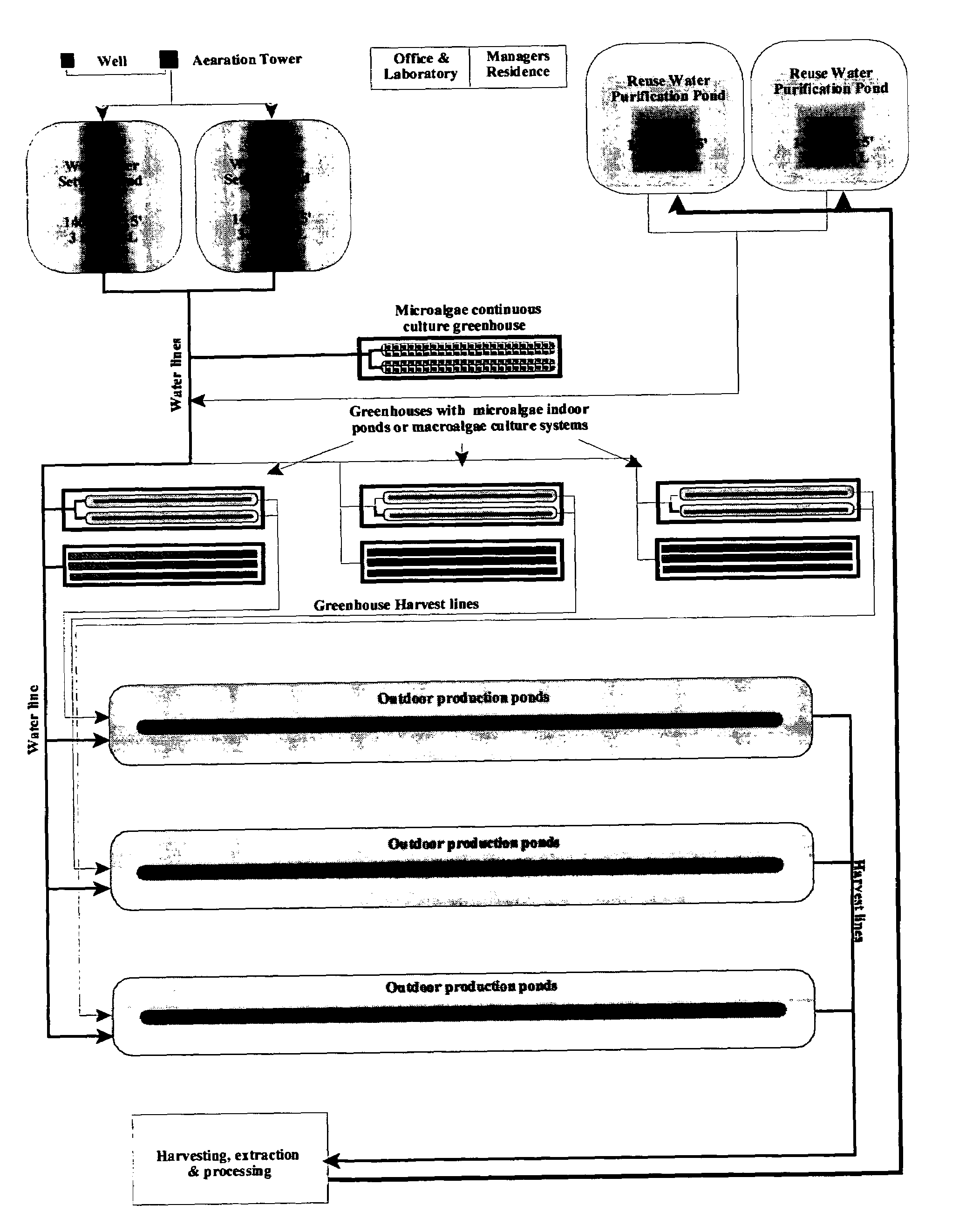
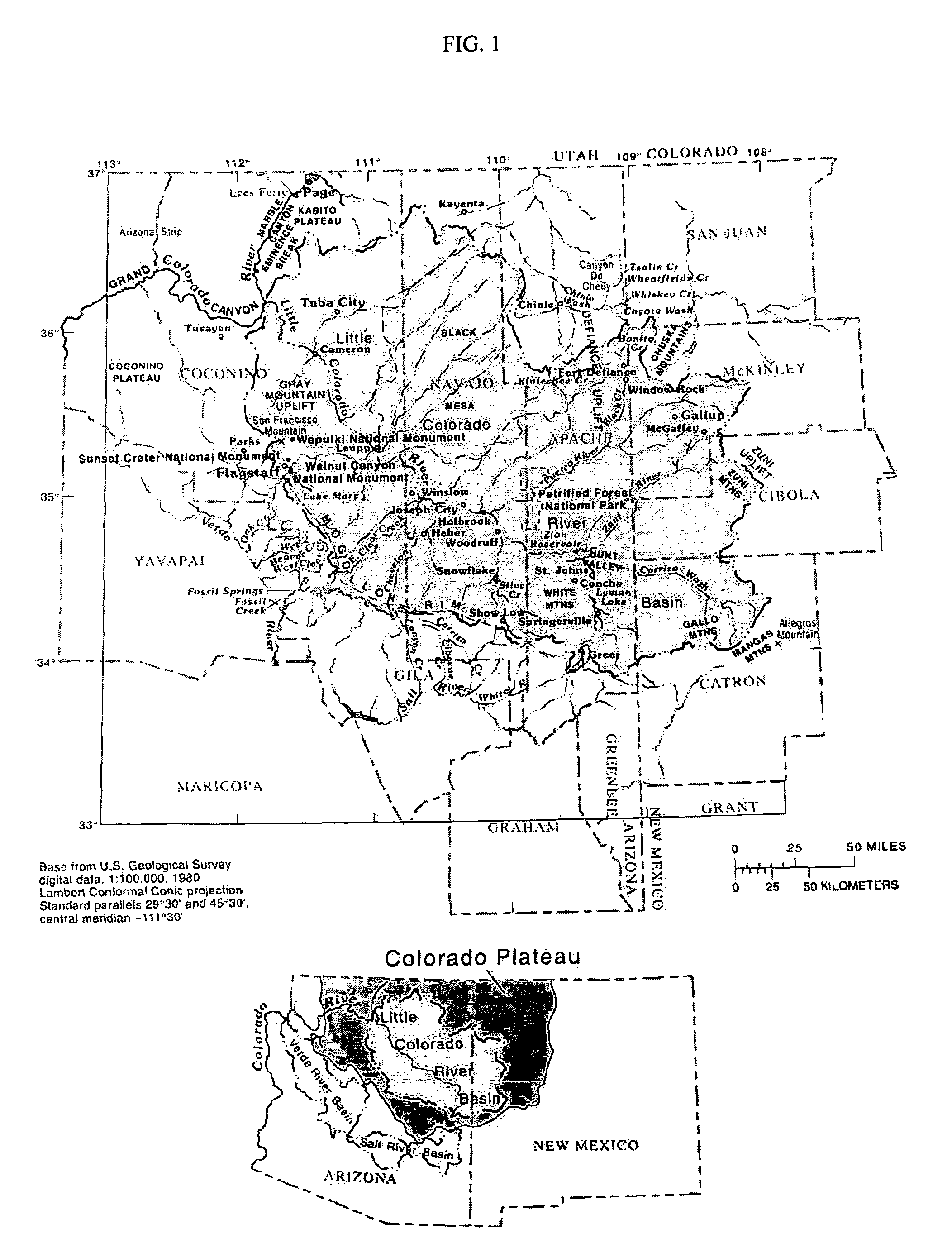
Inland aquaculture of marine life using water from a saline aquifer
InactiveUS6986323B2Overcome disadvantagesClimate change adaptationPisciculture and aquariaInland saline aquacultureWater layer
A method and system for the inland aquaculture of marine species using water from a saline aquifer having a heavy metals content within the acceptable limits of the EPA guidelines for drinking water. The aquifer is preferably the Coconino aquifer located in Arizona and New Mexico. The system can be used to culture microalgae, macroalgae, fish, shrimp and many other marine species. Nutrients and fertilizers can be added to the water to optimize culture conditions for particular species. Useful products can be isolated from the marine species or the cultured marine species can be harvested as useful products themselves.
Owner:ALGAE BIOSCI
Flexible ceramic wear-resistant heat-proof dual-anticorrosive coating
InactiveCN1528844AIncreasing the thicknessReduce the probability of water seepageAnti-corrosive paintsEpoxy resin coatingsCoated surfaceSaline water
The invention is a kind of flexible ceram wearing and heat resisting heavy corrosion preventing paint, which is made up of epoxy resin and several kinds of ceram powder, rust-protection paint and firming agent, the weights of each ingredient are: (1) epoxy resin: 100; (2) additive: 50-120; (3) rust protection paint: 8-25; (4) ceram powder: 100-200; (5) compound solvent: 40-70; paint: firming agent=(35-50):1. The paint needn't base coat, it can be painted directly or brushed on the surface of metal under normal temperature, it has excellent wearing and corrosion prevention performance, impact resisting performance and flexibility, it can insulate acid, alkali, salt, saline water. The surface is smooth; it can be applied to oil pipe, oil pot, and chemical device, ship, wheel vane, pump, dust catcher, etc.
Owner:REAR SERVICE TECH EQUIP INST NAVY PLA
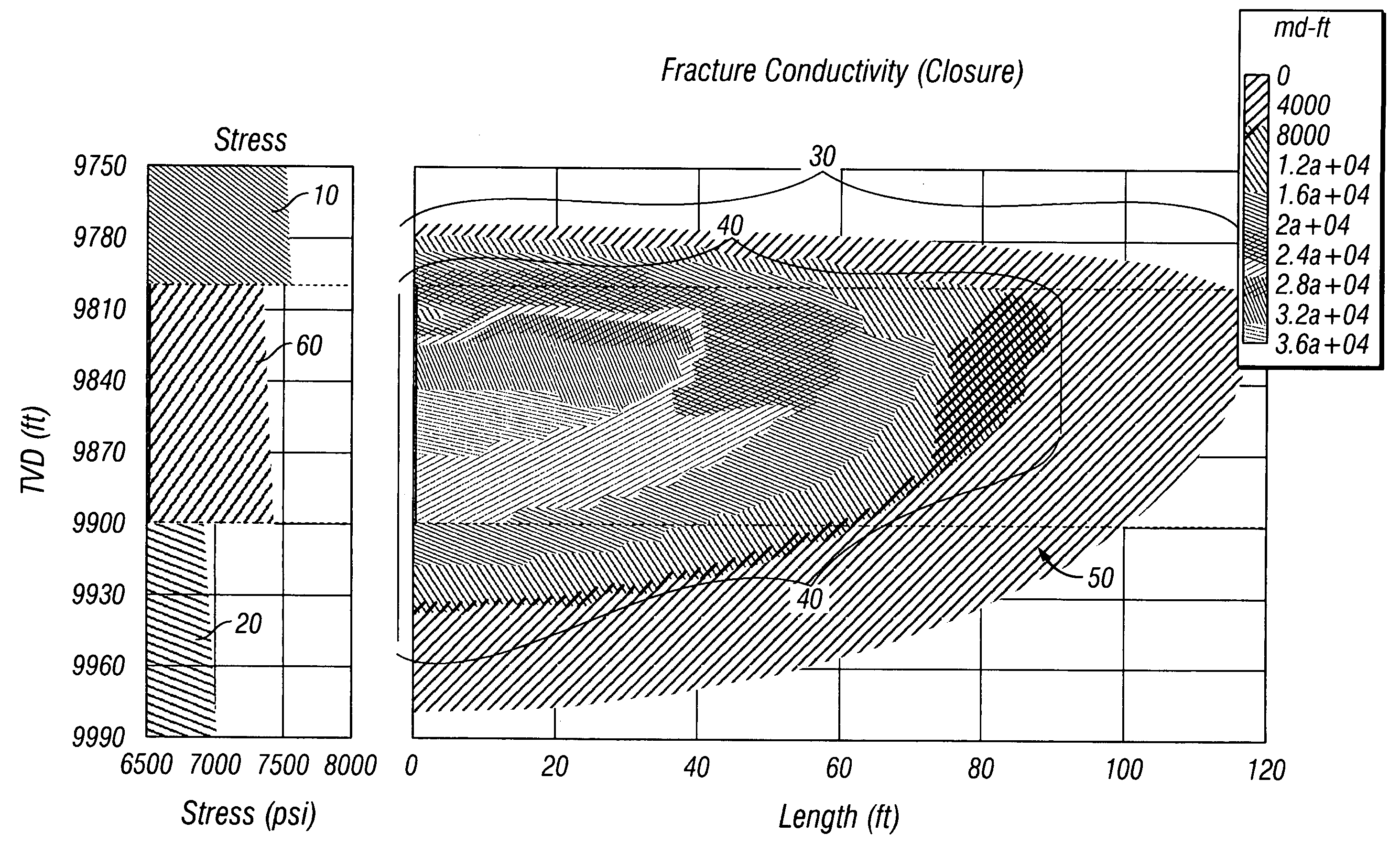
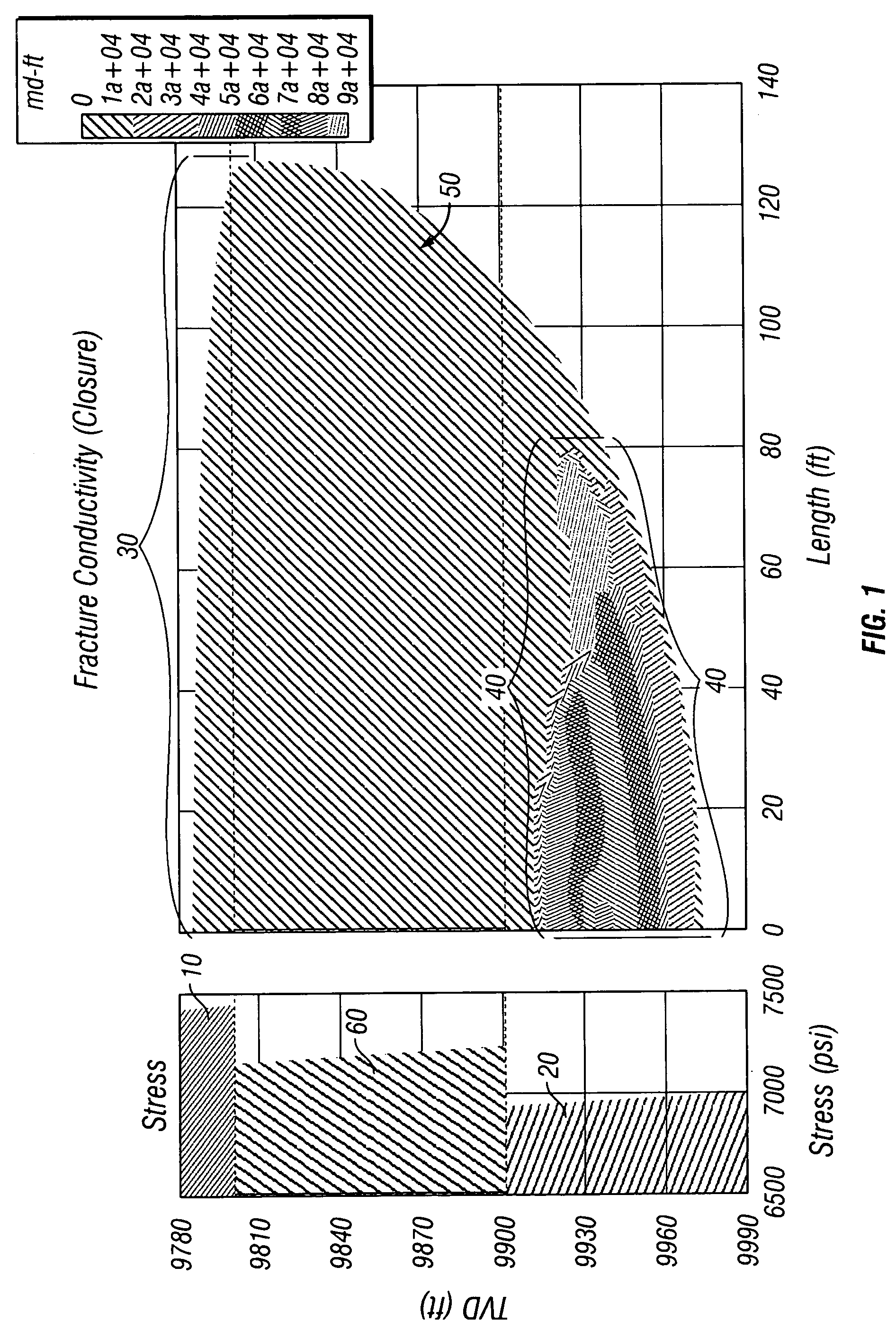
Method of hydraulic fracturing to reduce unwanted water production
ActiveUS7207386B2Limit undesirable height growthFluid removalDrilling compositionSaline waterGeomorphology
A method of hydraulically fracturing a hydrocarbon-bearing subterranean formation ensures that the conductivity of water inflow below the productive zone of the subterranean formation is reduced. The method consists of two principal steps. In the first step, a fracture in and below the productive zone of the formation is initiated by introducing into the subterranean formation a fluid, free of a proppant, such as salt water, fresh water, brine, liquid hydrocarbon, and / or nitrogen or other gases. The proppant-free fluid may further be weighted. In the second step, a proppant laden slurry is introduced into the subterranean formation which contains a relatively lightweight density proppant. Either the fluid density of the proppant-free fluid is greater than the fluid density of the proppant laden slurry or the viscosity of the proppant-free fluid is greater than the viscosity of the proppant laden slurry. The method limits undesirable fracture height growth in the hydrocarbon-bearing subterranean formation during the fracturing.
Owner:BAKER HUGHES HLDG LLC
Methods to produce sulfate-free saline water and gypsum
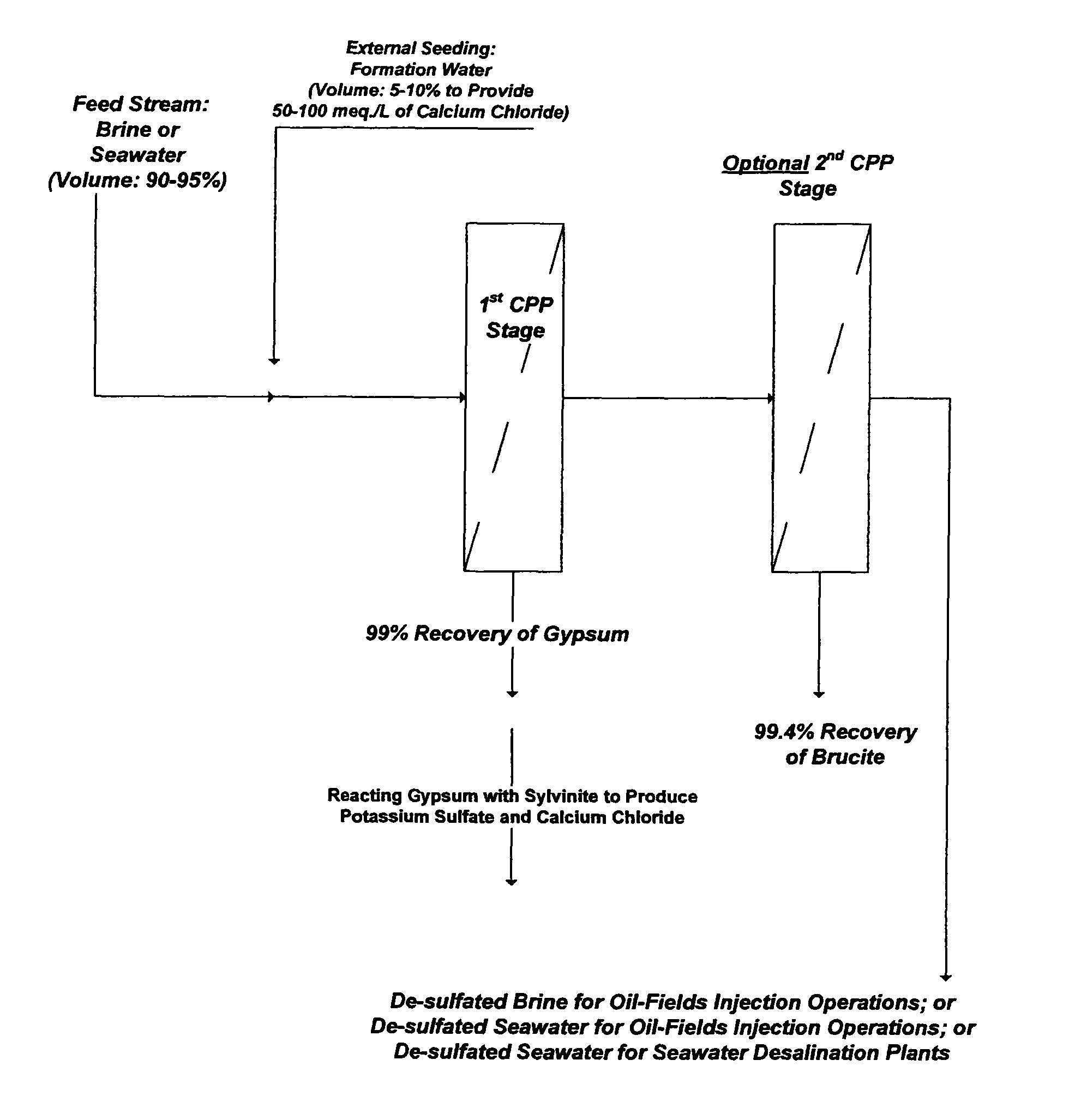
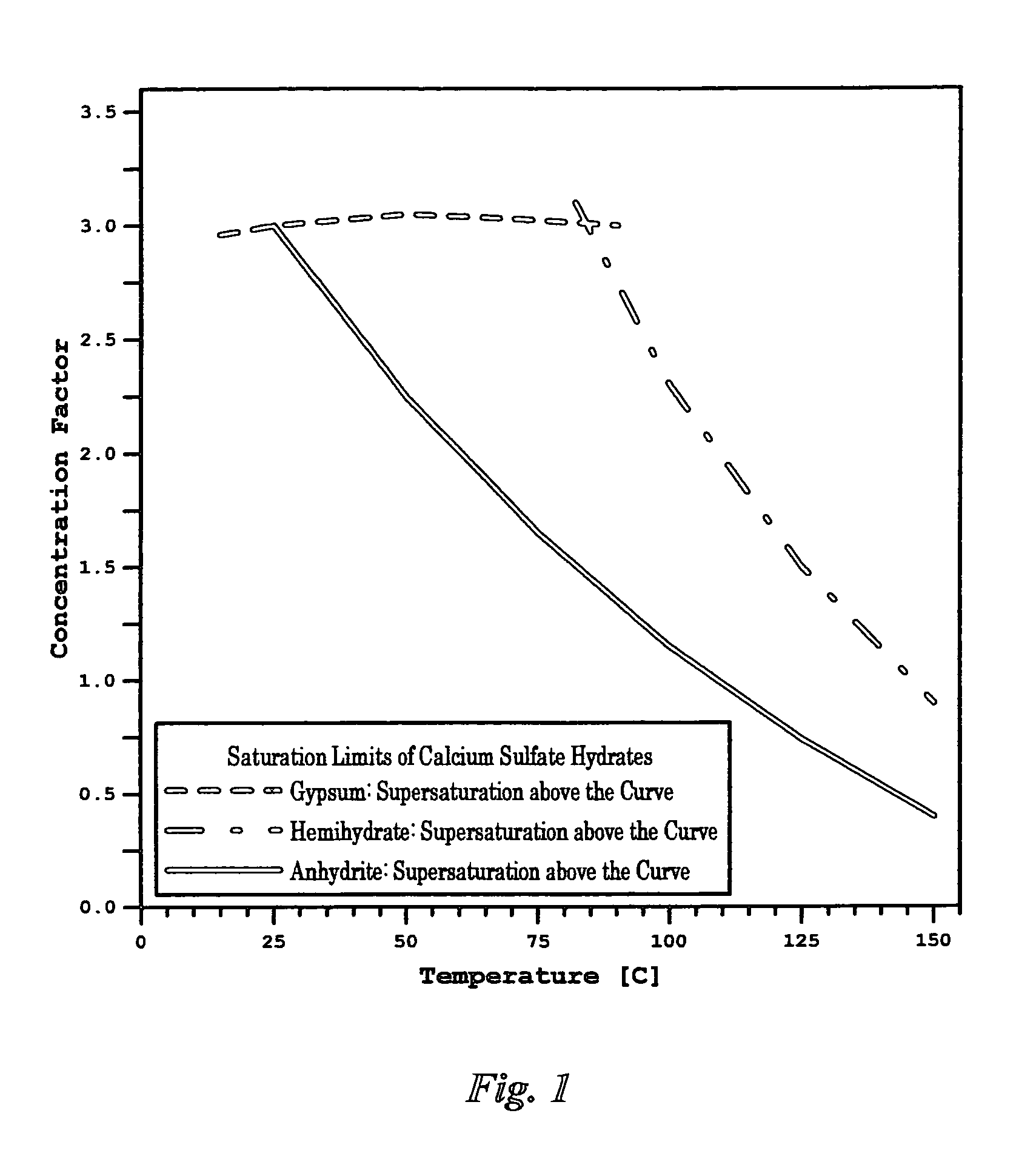
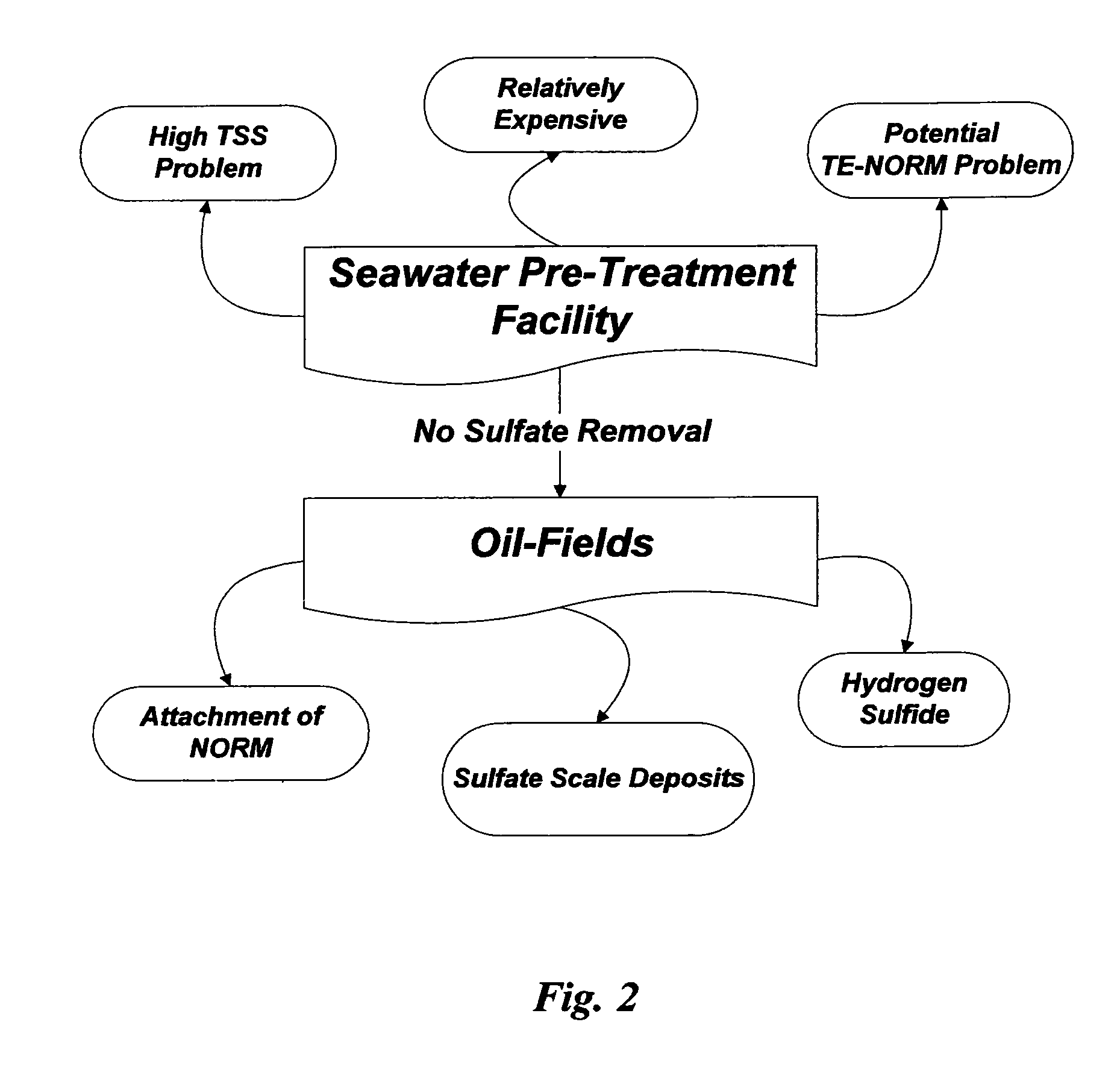
ActiveUS7392848B1Avoid pollutionWaste water treatment from quariesSludge treatmentSaline waterSulfate
Methods are disclosed for the selective separation of sulfate from a saline stream such as seawater to produce nearly sulfate-free saline stream for oil-fields water injection operations. The separated sulfate in the form of gypsum from the treated saline stream can be used in different applications.
Owner:BADER MANSOUR S
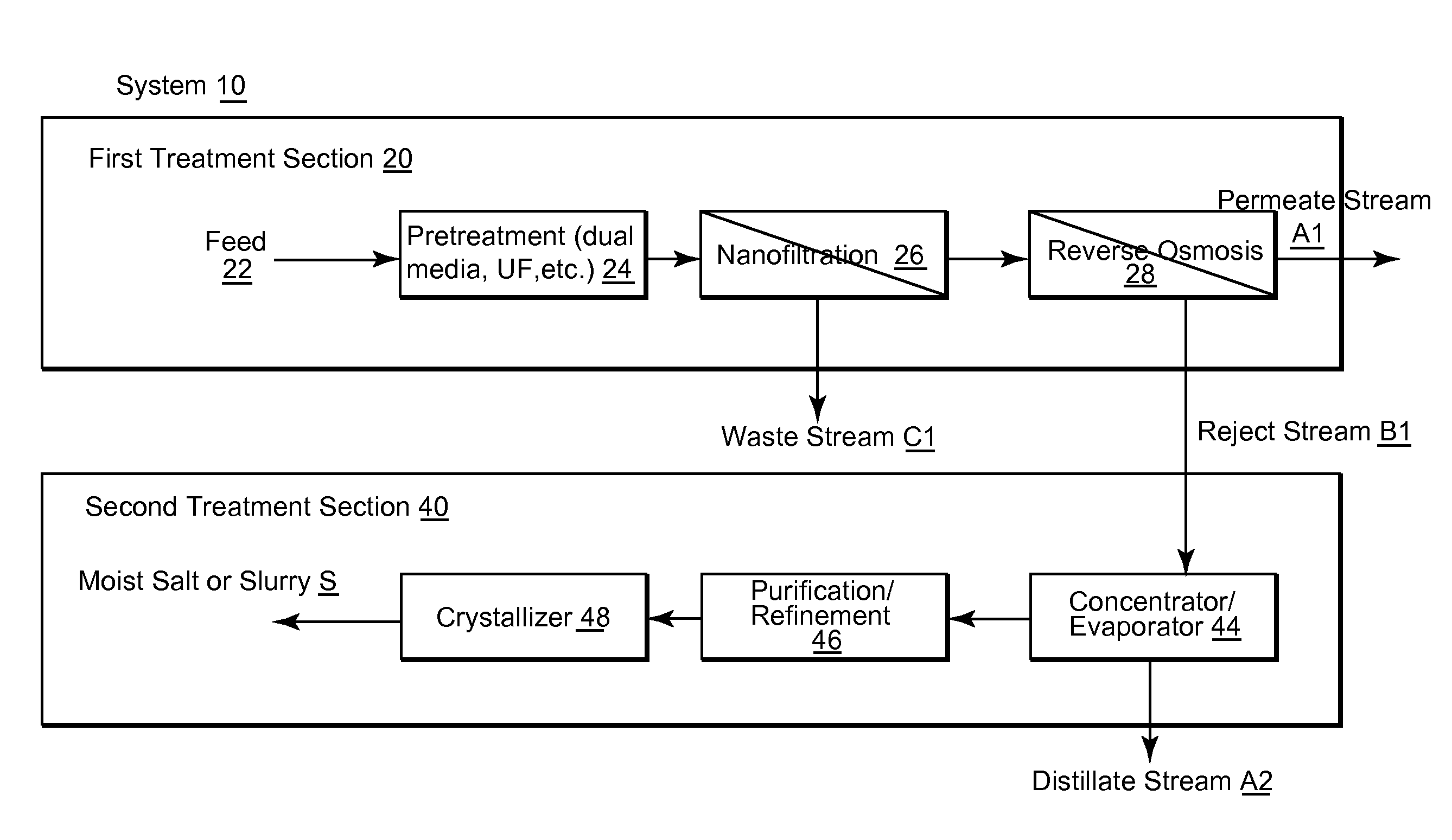
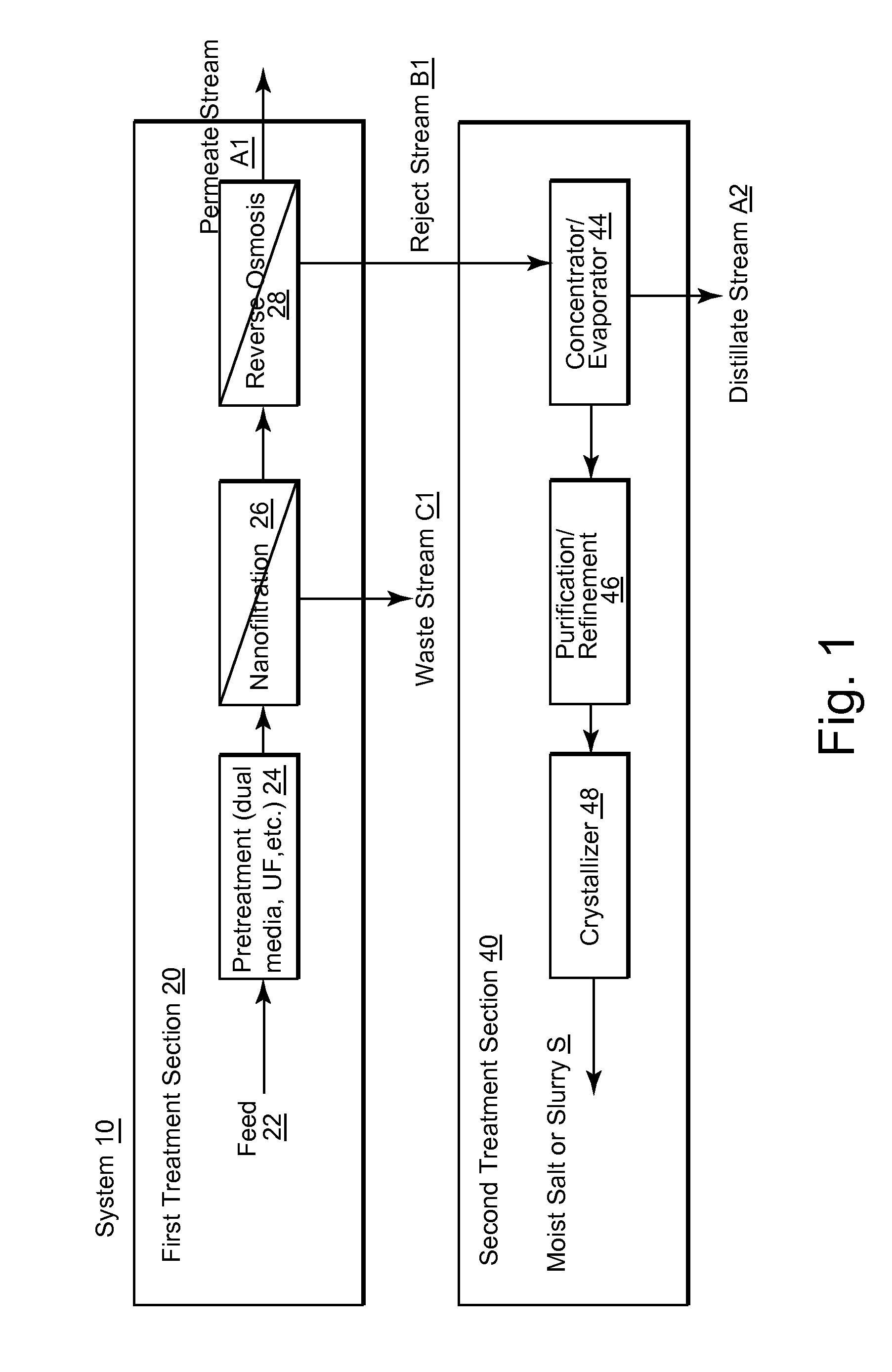
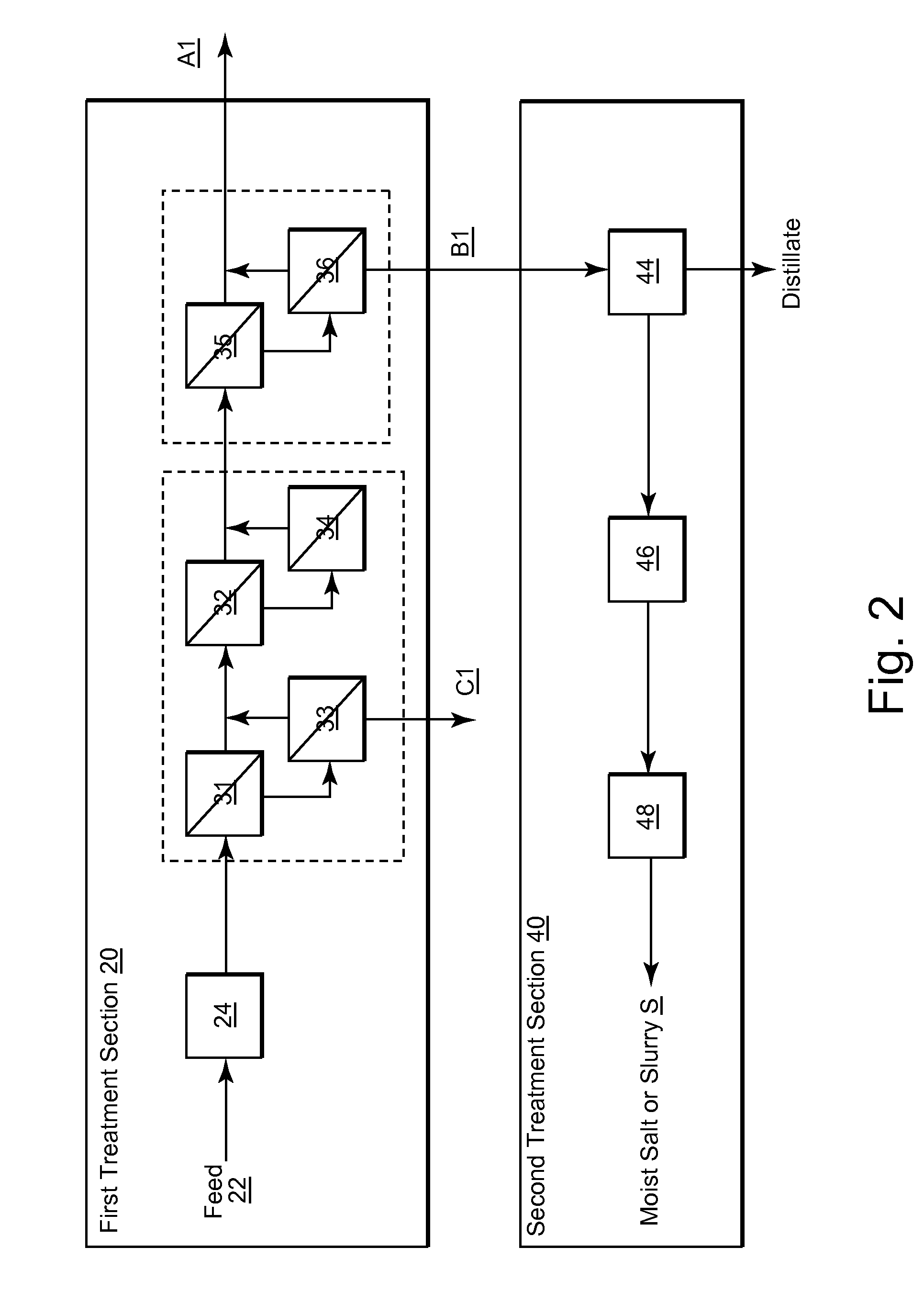
Water desalination plant and system for the production of pure water and salt
InactiveUS20120160753A1Enhanced commercial NaCl productionGeneral water supply conservationSeawater treatmentSaline waterWater desalination
A desalination plant for treating a sea water or brackish water feed is provided. The desalination plant includes a first treatment section to effectively remove scaling species, the first treatment section including nanofiltration section, the nanofiltration section including at least two stages and at least two passes; and a reverse osmosis section that operates at high recovery to produce a purified permeate stream and a selectively NaCl salt-enriched reject stream as a saline output.
Owner:VORA NISHITH +4
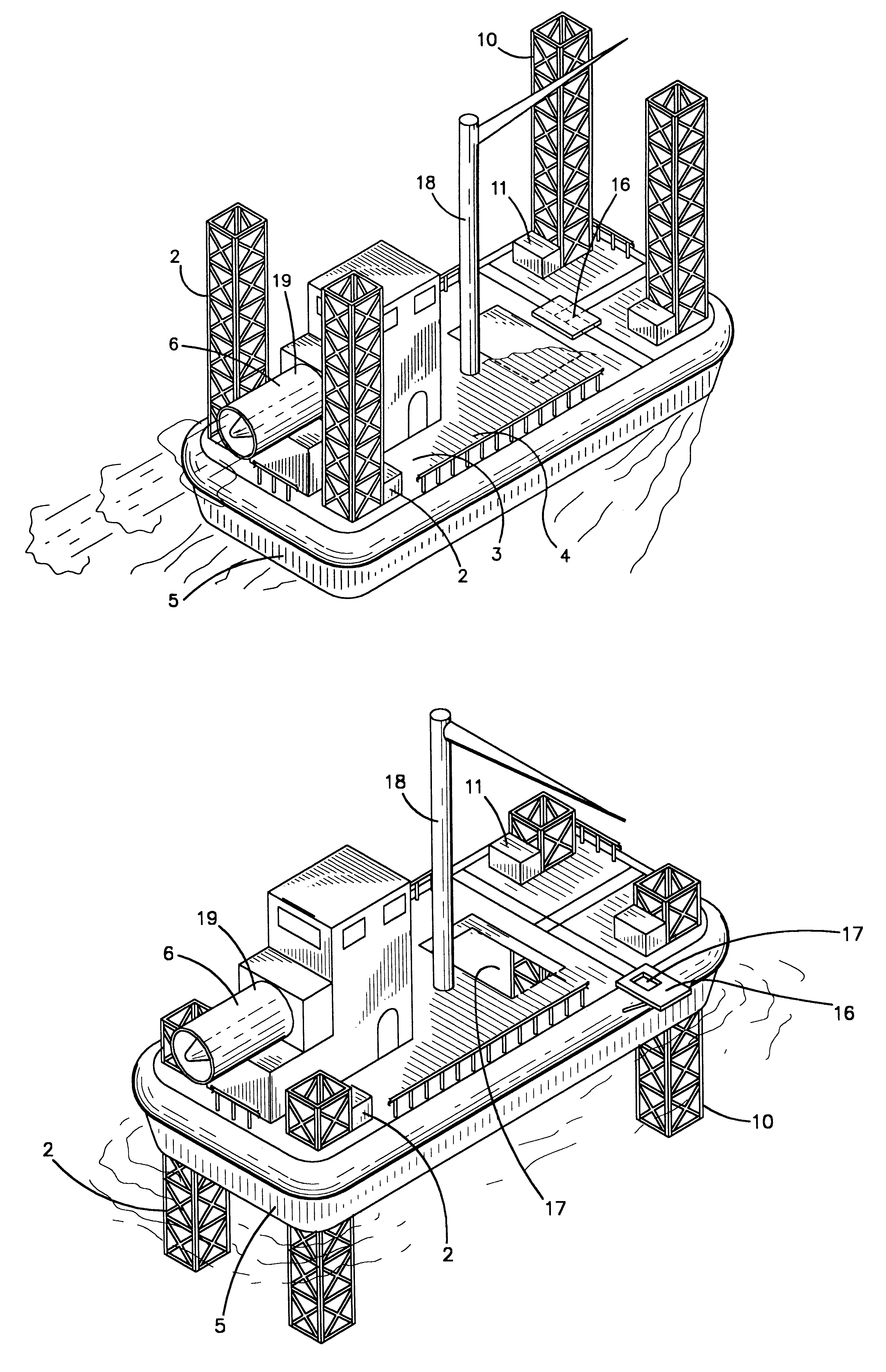
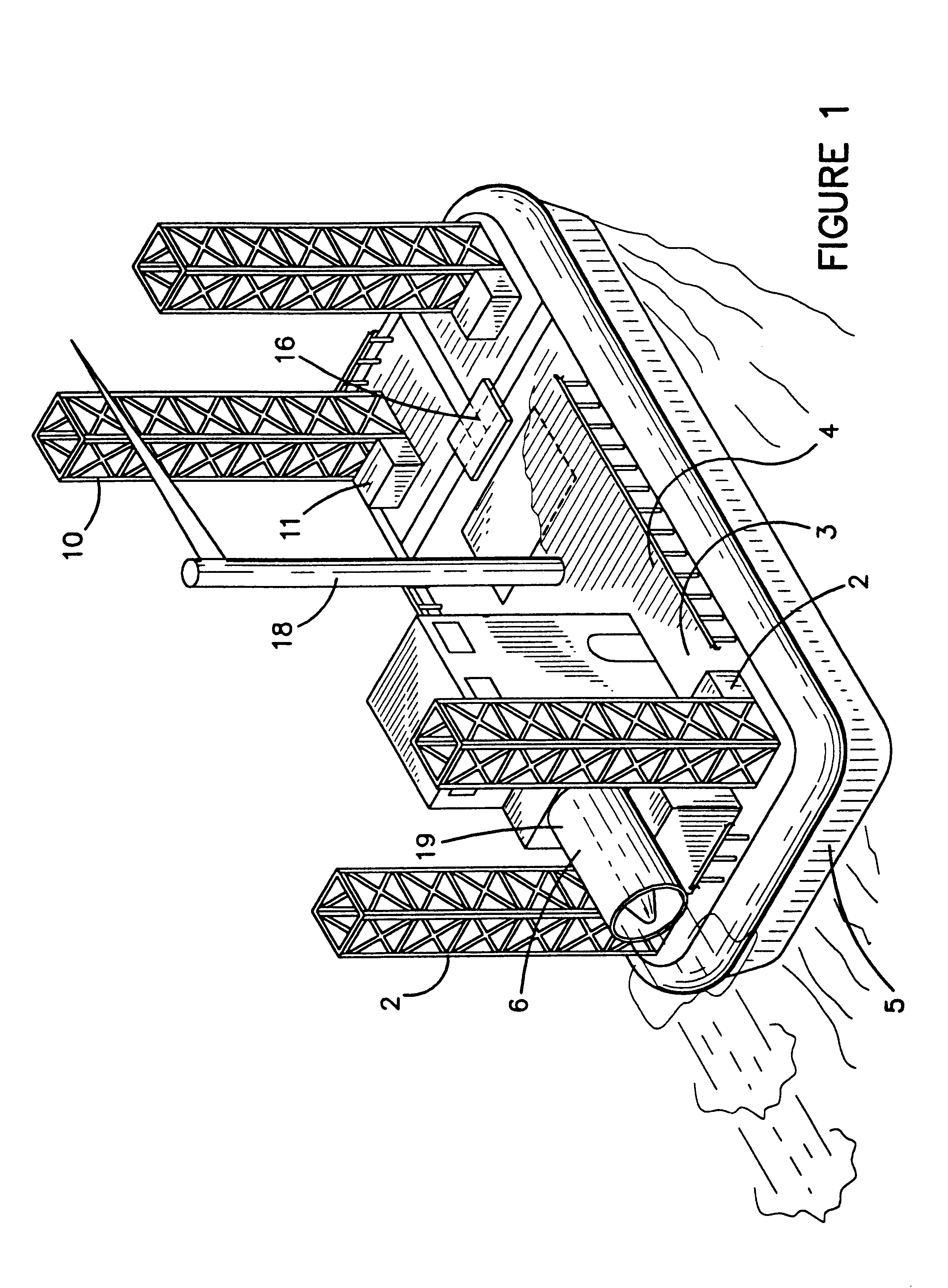
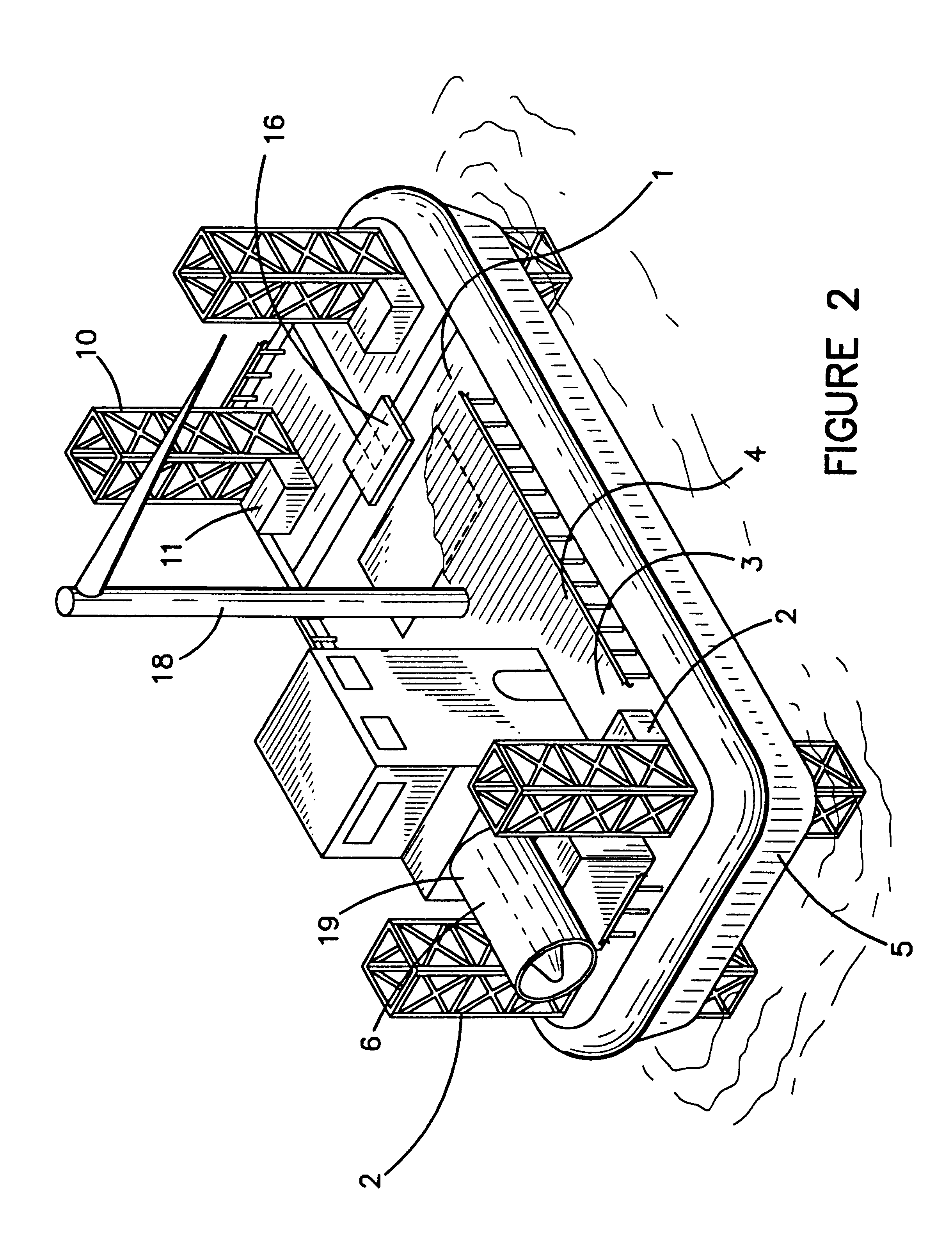
Hovercraft work platform
A jack up work platform is recited. The work platform comprises a hovercraft vessel outfitted with several jack up legs. The hovercraft can traverse environmentally sensitive terrain such a brackish and freshwater marshes without the need to dig canals that may cause or exacerbate salt water intrusion. Once the drilling or exploration site is reached, the jack up legs may be lowered, lifting the work platform above the surface. Once the vessel is secured on the legs, it may be used like a conventional oil field work platform for drilling, exploration, or construction jobs for which other work platforms or vessels are typically used. The deck of the hovercraft may serve as the work platform or the deck may be provided with a cantilevered work platform extension. The hovercraft will preferably be provided with typical oil field heavy machinery such as a crane for assembly of the legs, and drill stem as well as for work on other structures.
Owner:MILLER GEORGE AUSTIN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com