Patents
Literature
73 results about "Bioburden" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
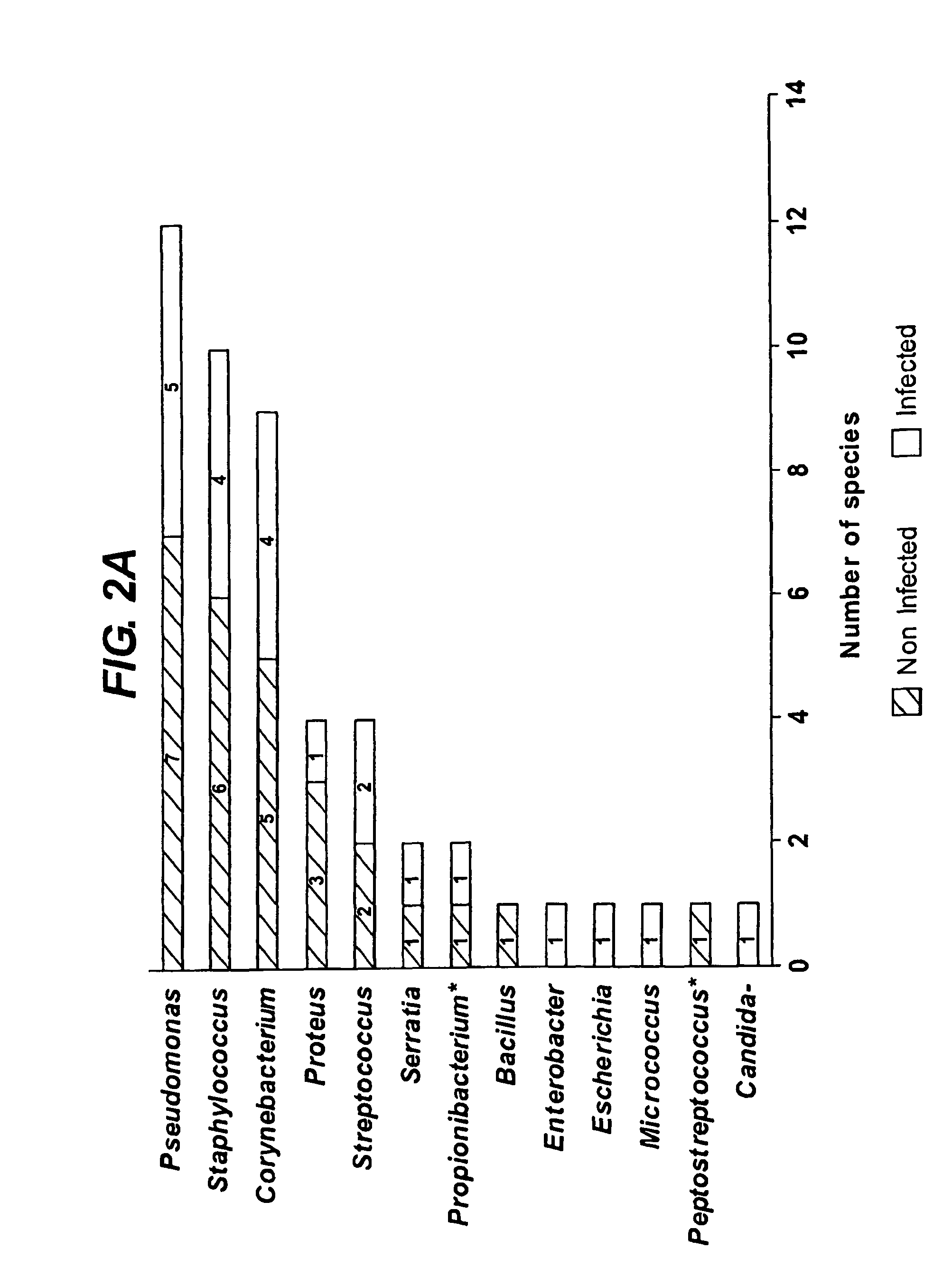
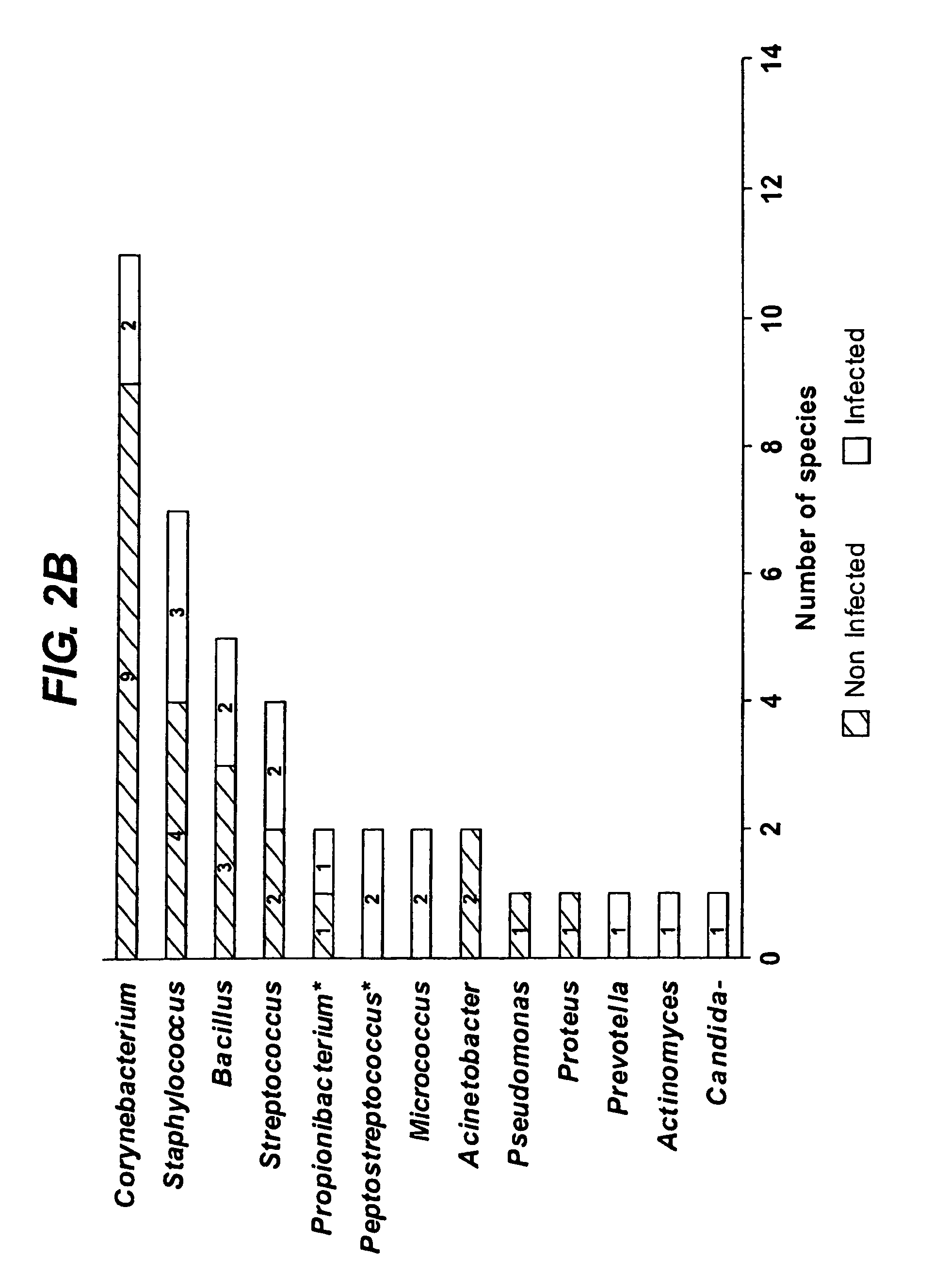
Bioburden is normally defined as the number of bacteria living on a surface that has not been sterilized. The term is most often used in the context of bioburden testing, also known as microbial limit testing, which is performed on pharmaceutical products and medical products for quality control purposes. Products or components used in the pharmaceutical or medical field require control of microbial levels during processing and handling. Bioburden or microbial limit testing on these products proves that these requirements have been met. Bioburden testing for medical devices made or used in the USA is governed by Title 21 of the Code of Federal Regulations and worldwide by ISO 11737.
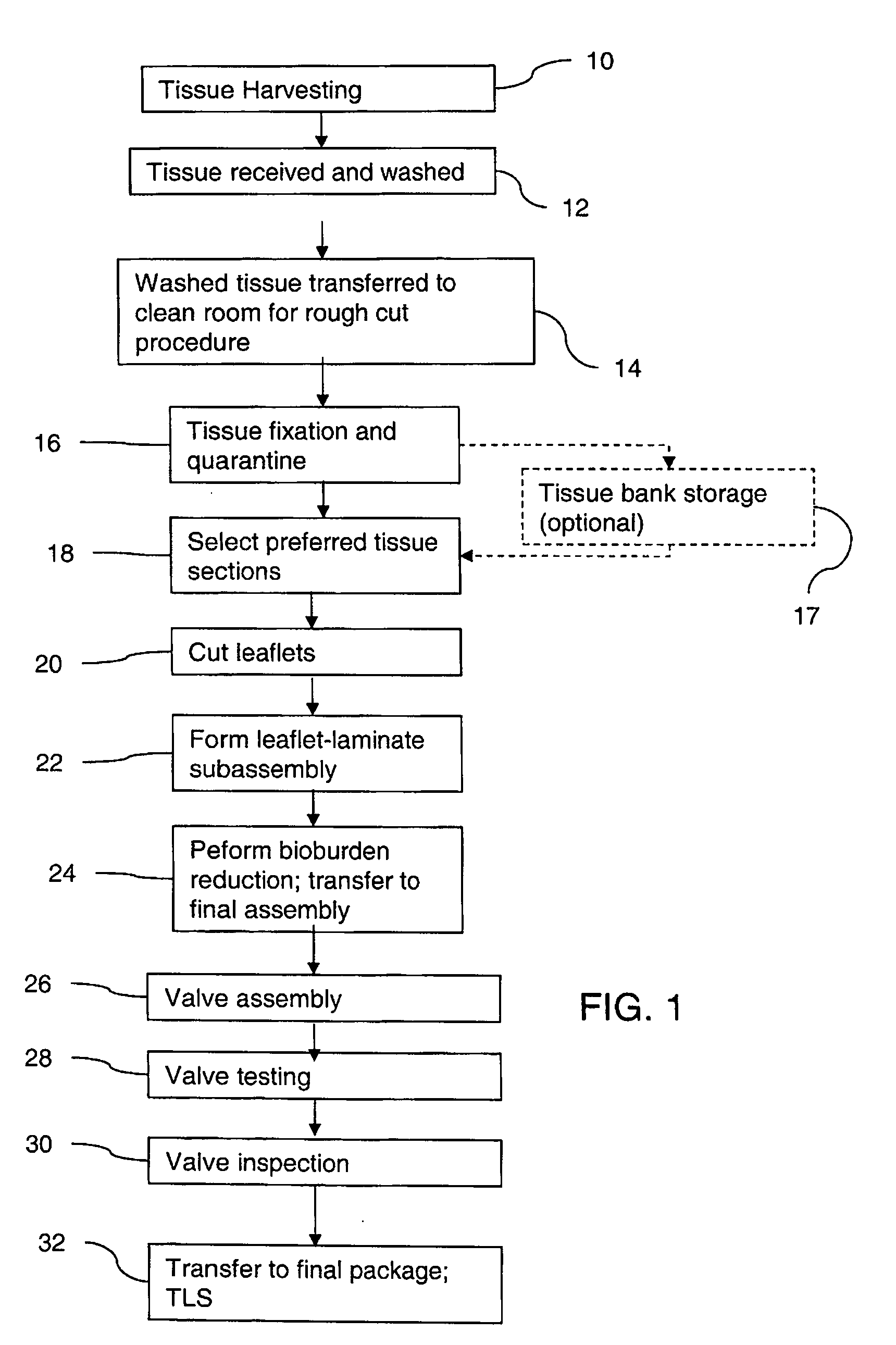
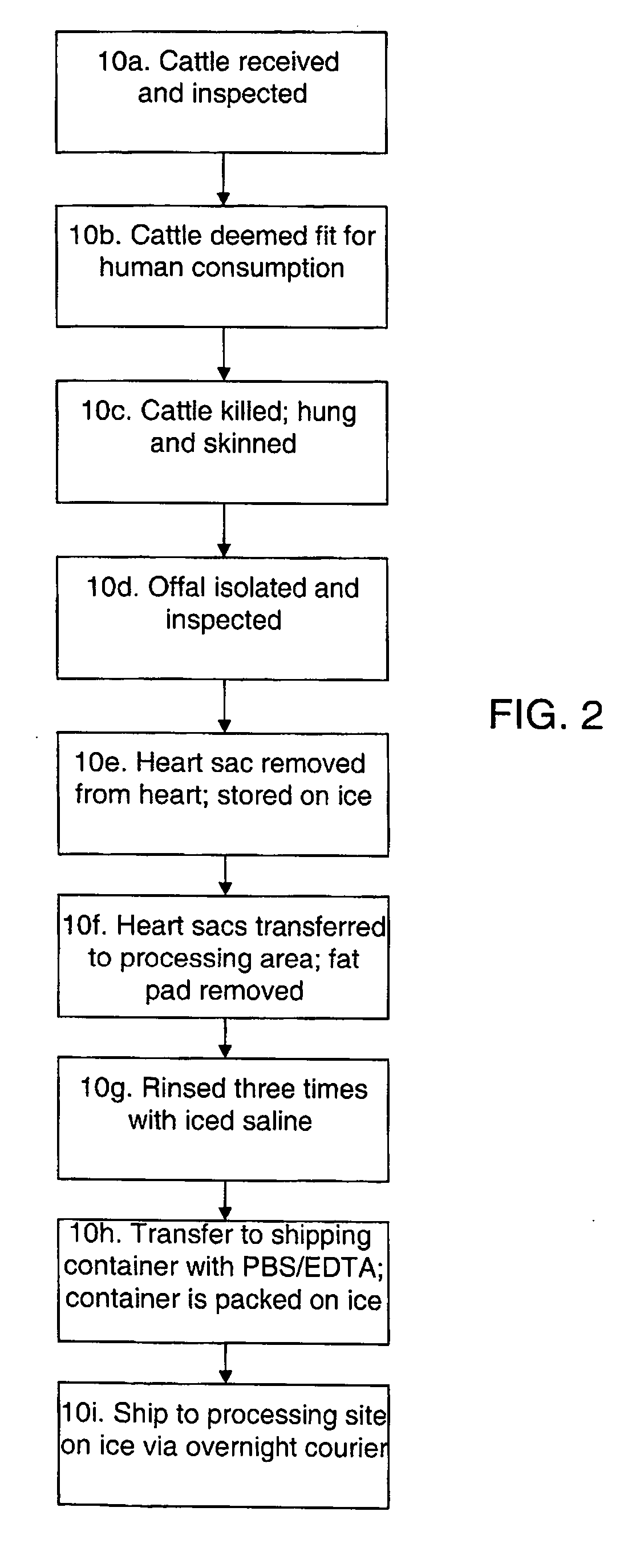
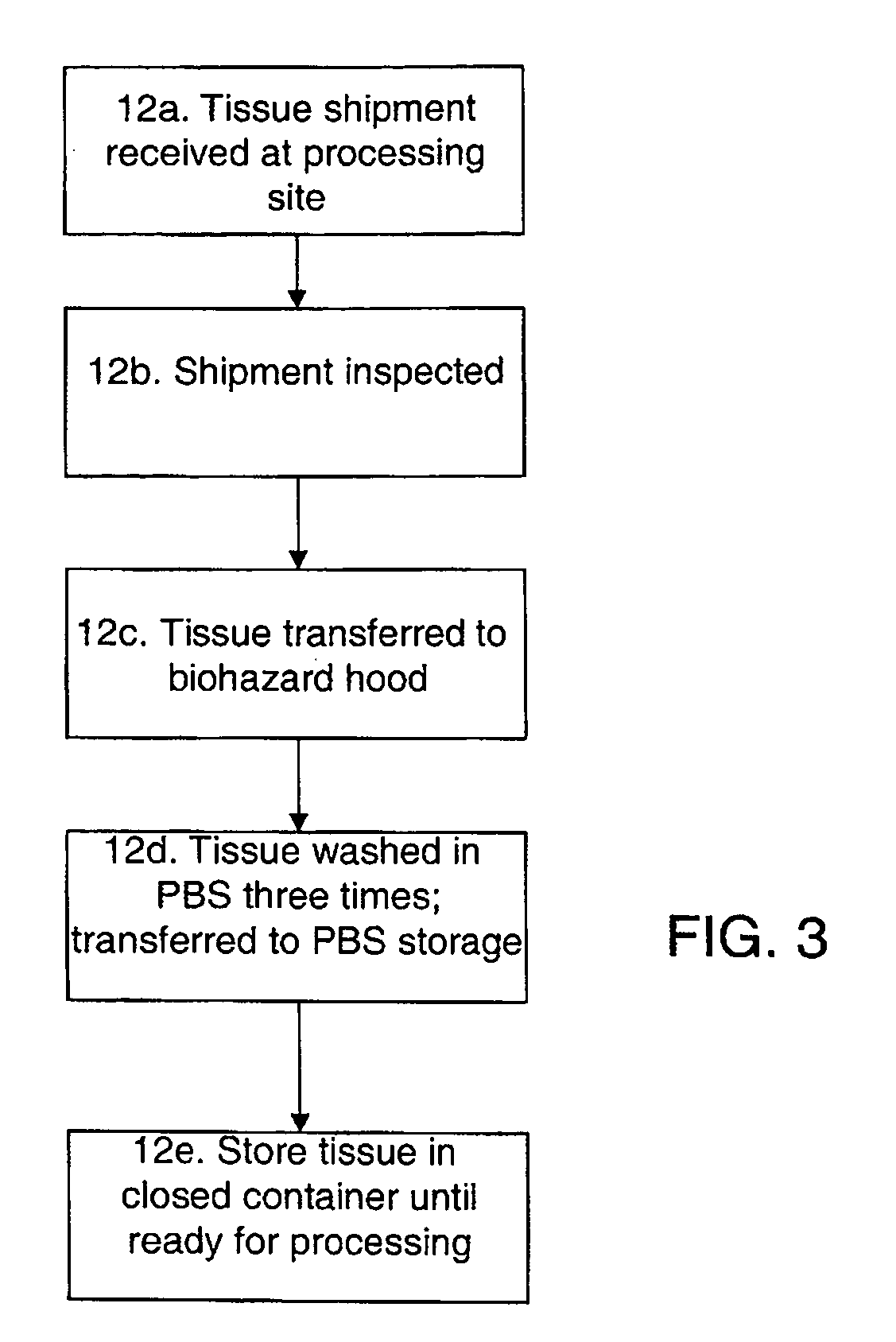
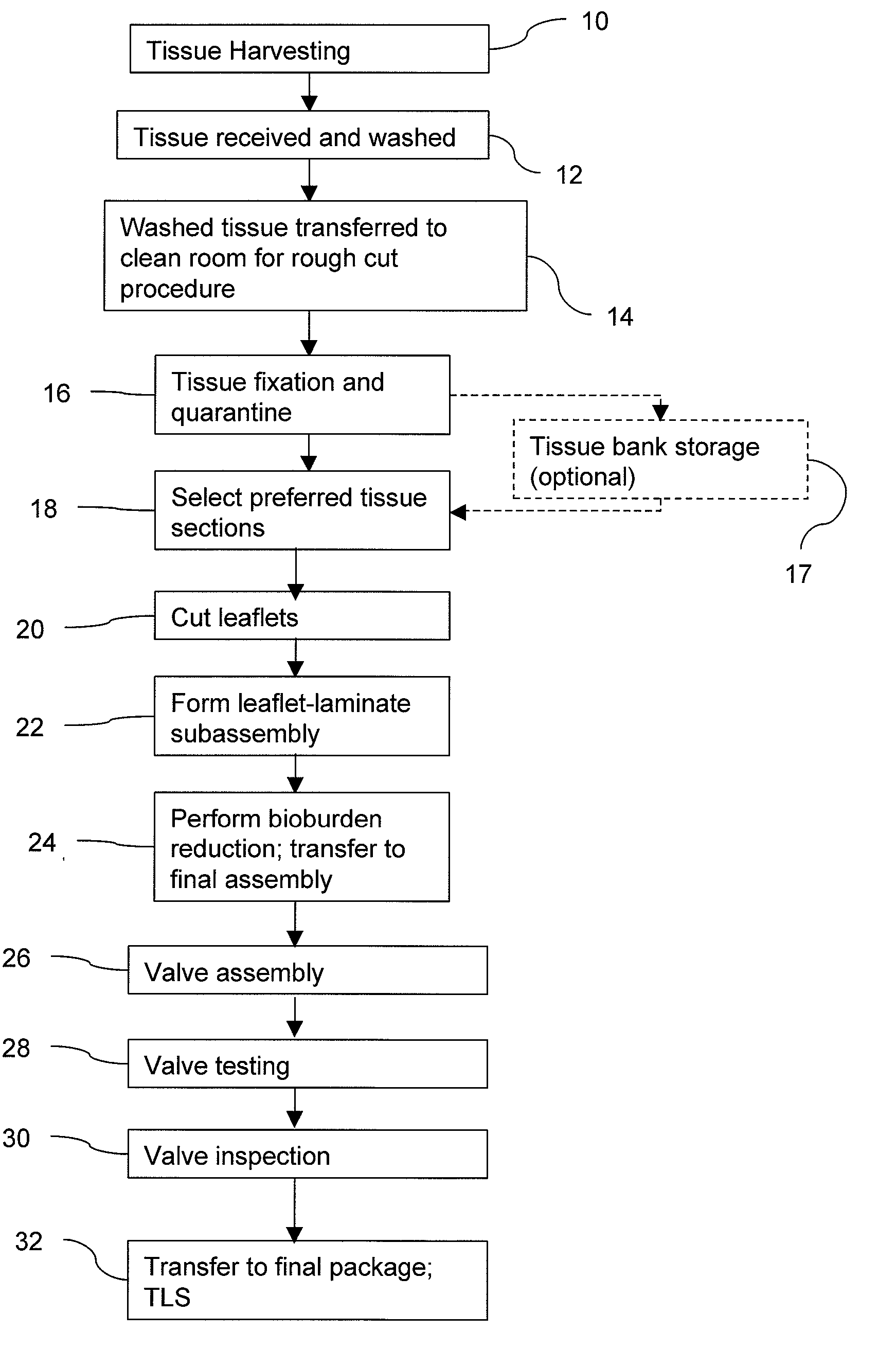
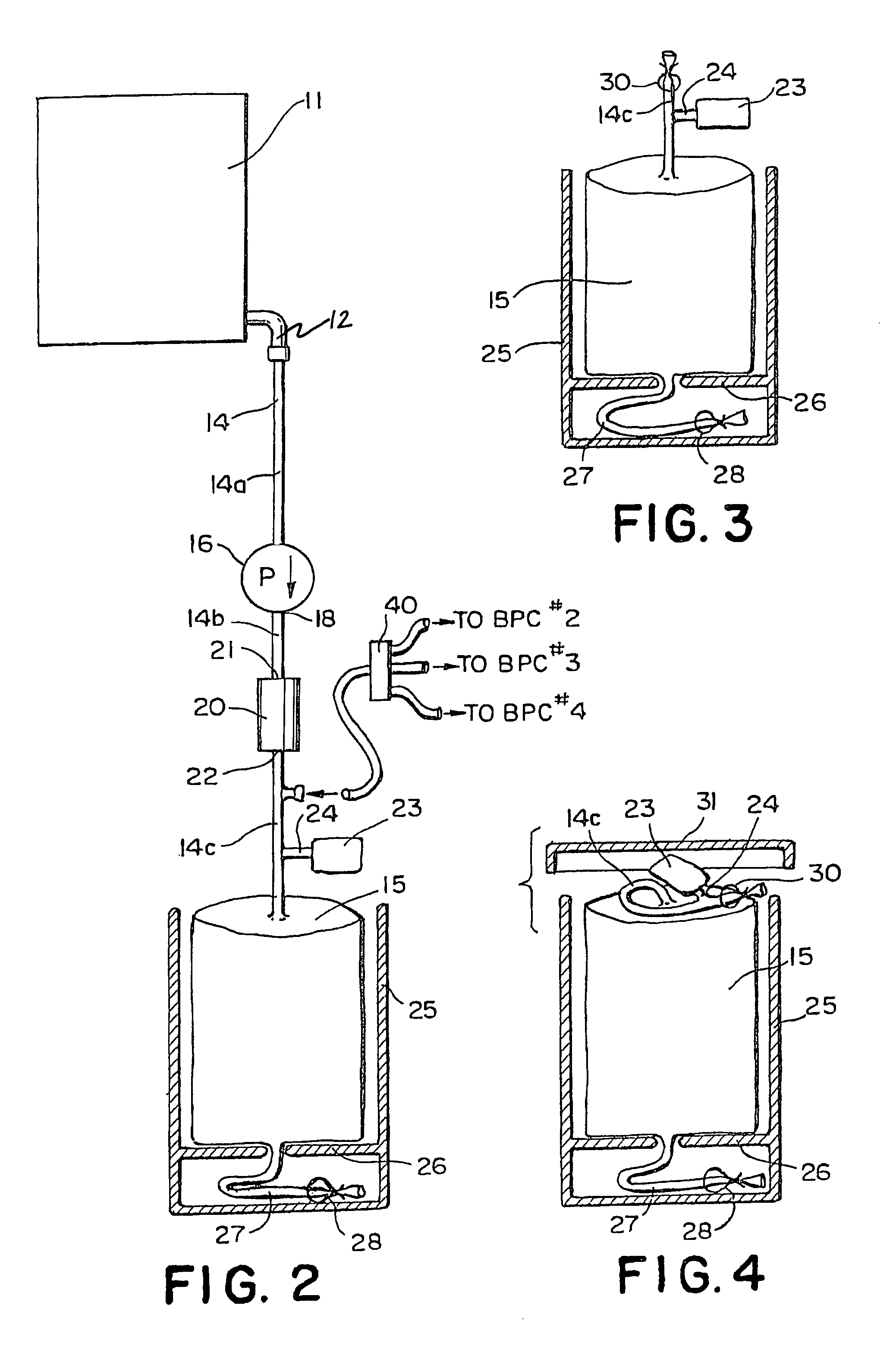
Methods for processing biological tissue
ActiveUS20060154230A1Remove background levelAvoid degradationDead animal preservationPhosphateCalcification
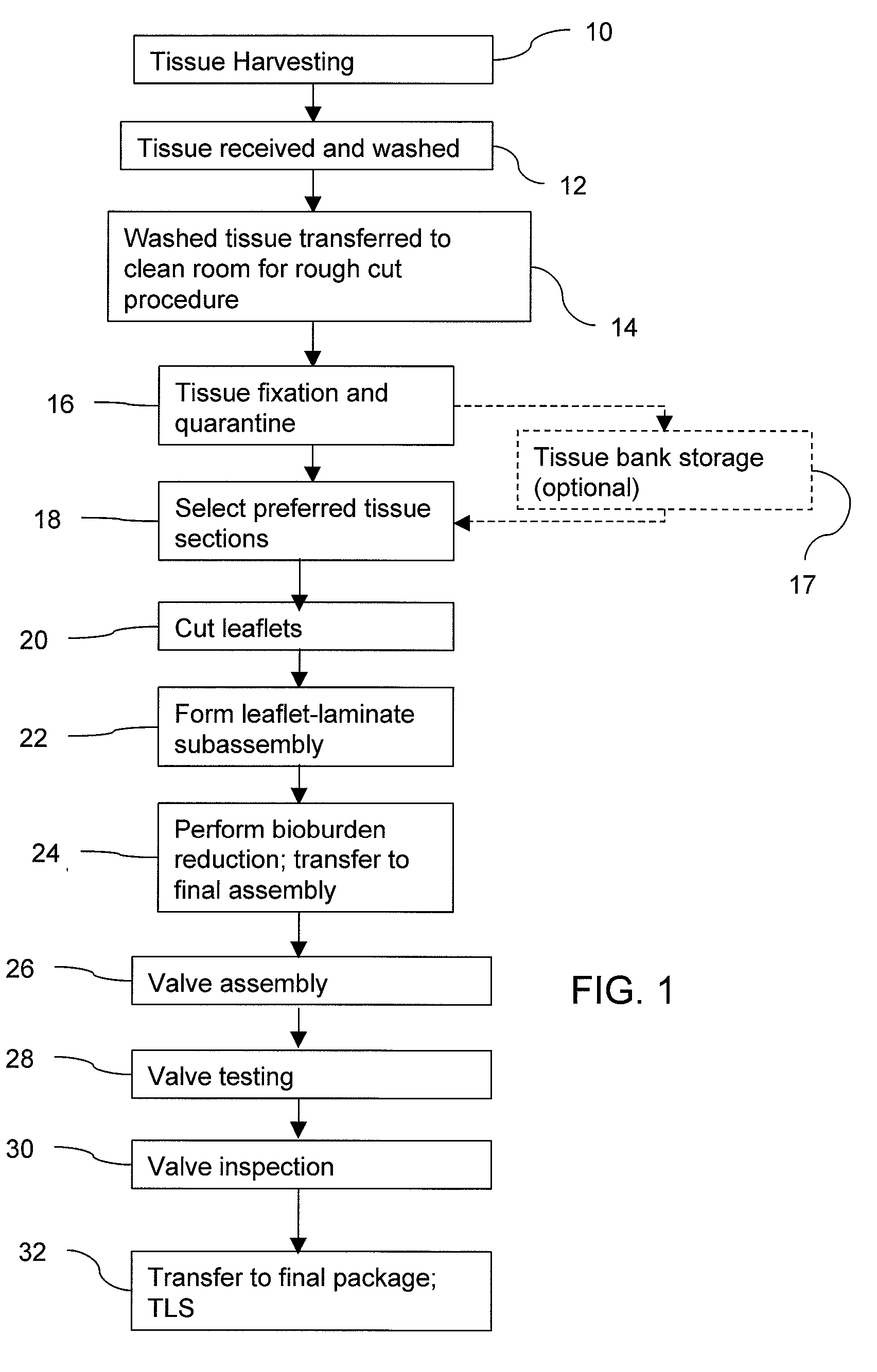
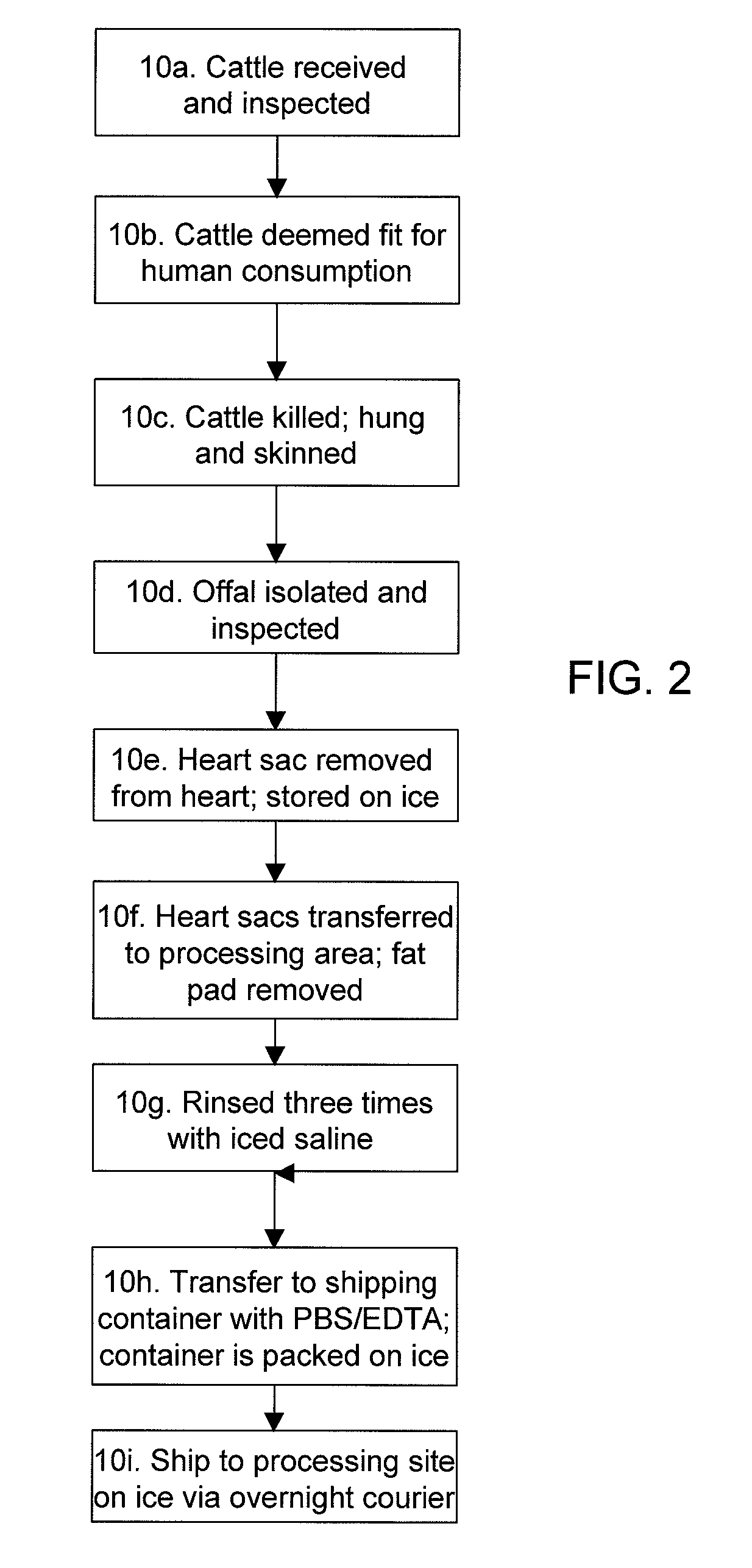
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Methods for processing biological tissue
ActiveUS20060207031A1Remove background levelAvoid degradationSuture equipmentsDead animal preservationCalcificationPhospholipid
A method for processing biological tissue used in biological prostheses includes providing a tissue procurement solution formed from a phosphate buffered saline (PBS) solution and a chelating agent. The tissue is transferred from the tissue procurement solution and undergoes chemical fixation. The fixed tissue is then immersed in a series of fresh bioburden reduction process (BRP) solutions to extract phospholipids. The tissue procurement solution reduces the bioburden on the stored tissue and preserves tissue architecture by minimizing tissue swelling. The tissue procurement solution further reduces calcium from the incoming water and / or tissue, and inhibits enzymes that digest the collagen matrix. The serial immersion of the tissue in the fresh bioburden solutions ensures optimal extraction of phospholipids thereby mitigating subsequent calcification of the tissue.
Owner:MEDTRONIC INC
Live bioload detection using microparticles
ActiveUS20120009588A1Quick checkDetect presenceBioreactor/fermenter combinationsBiological substance pretreatmentsMicroparticleBiology
The present invention provides methods to concentrate cells onto microparticles, to concentrate the microparticles, and to detect the cells. The present invention also includes unitary sample preparation and detection devices to be used in accordance with the methods.
Owner:3M INNOVATIVE PROPERTIES CO
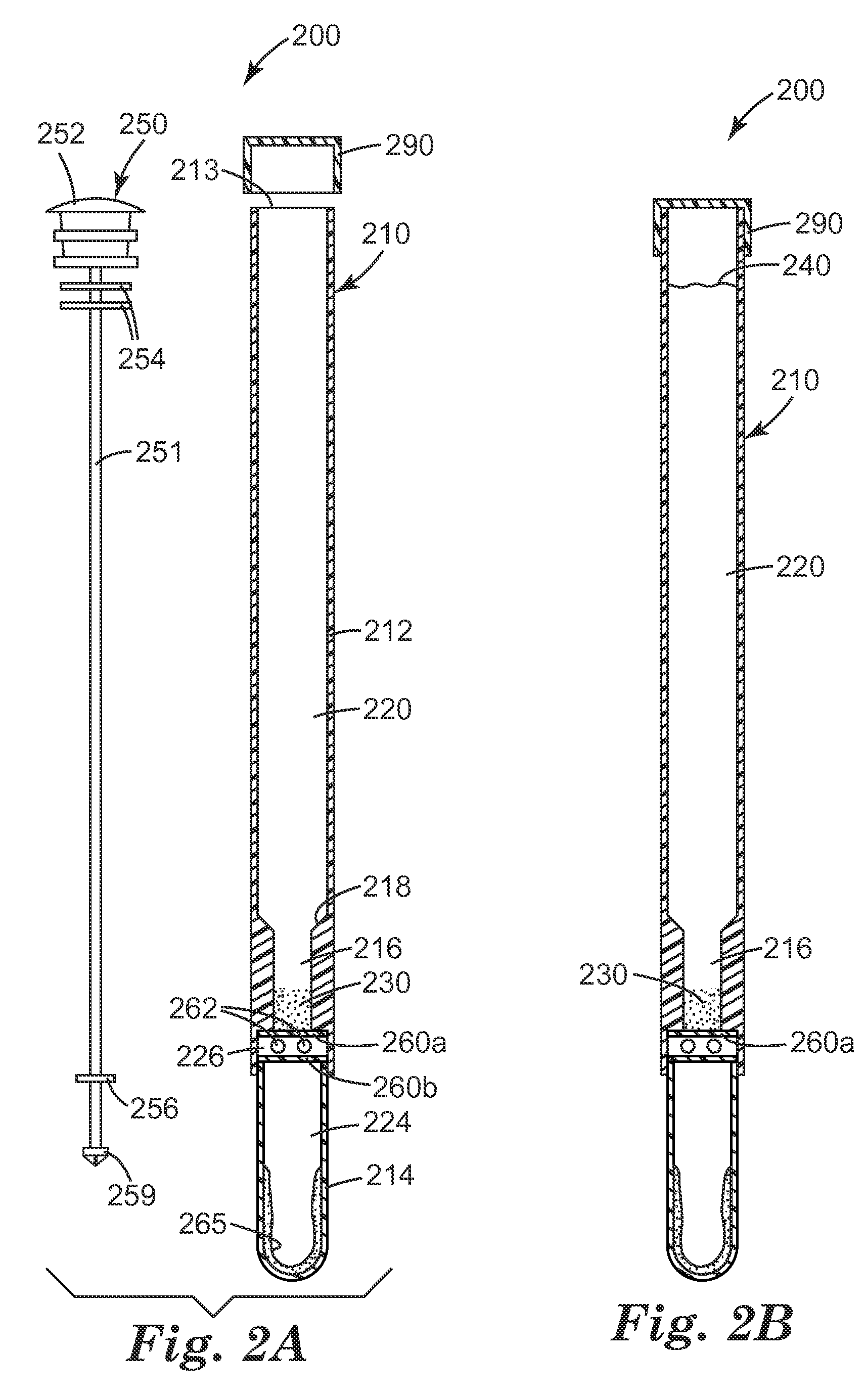
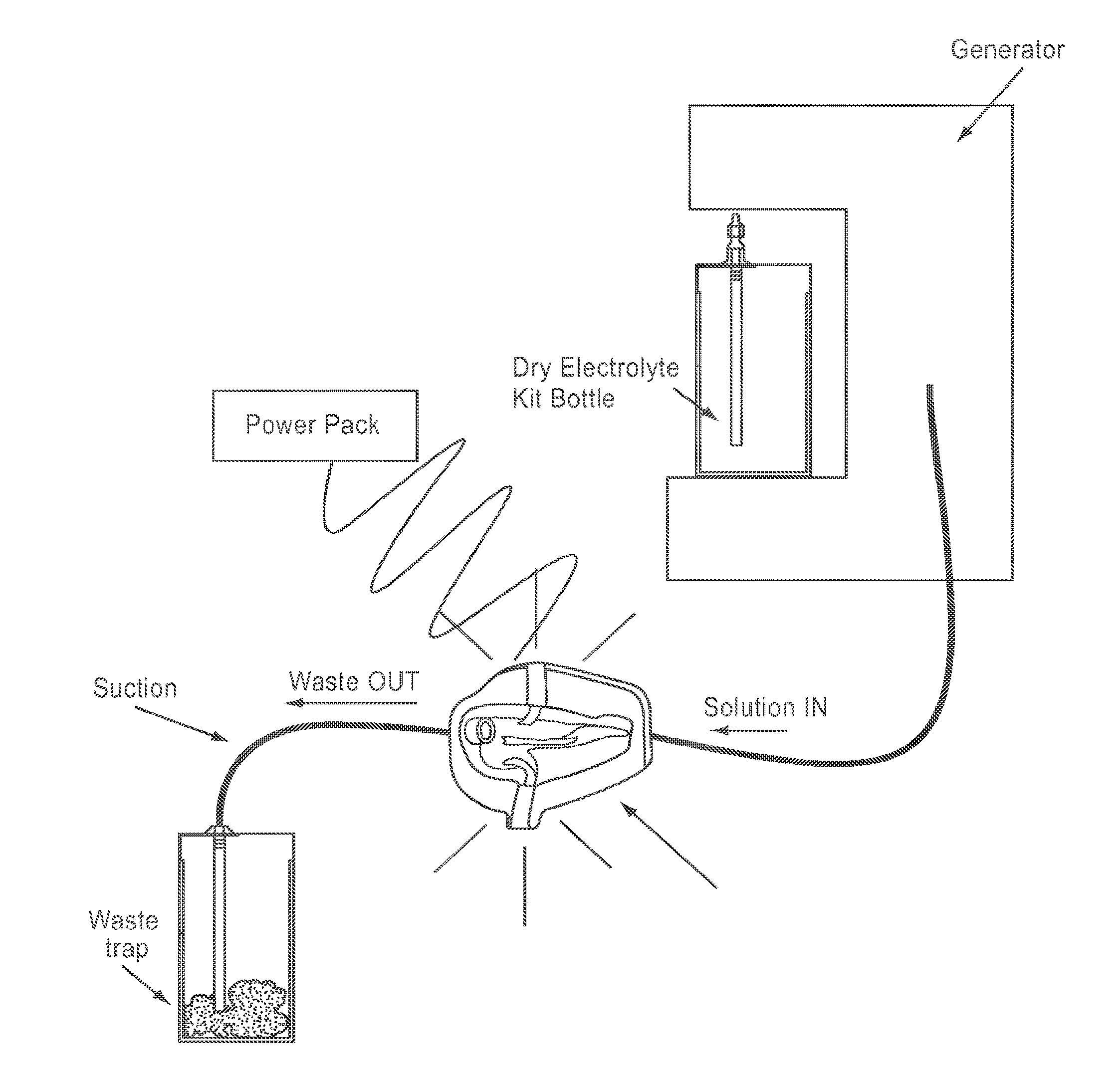
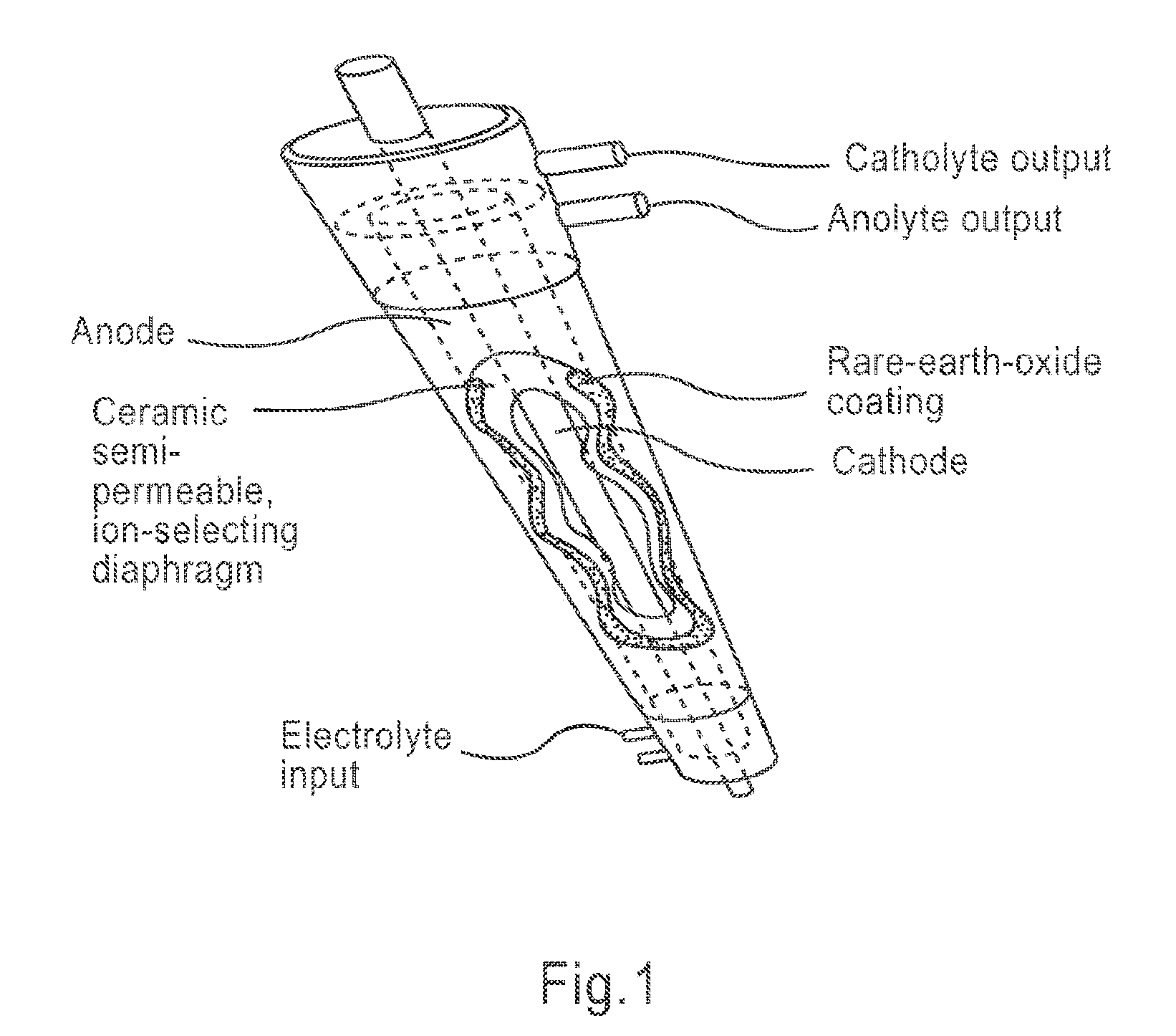
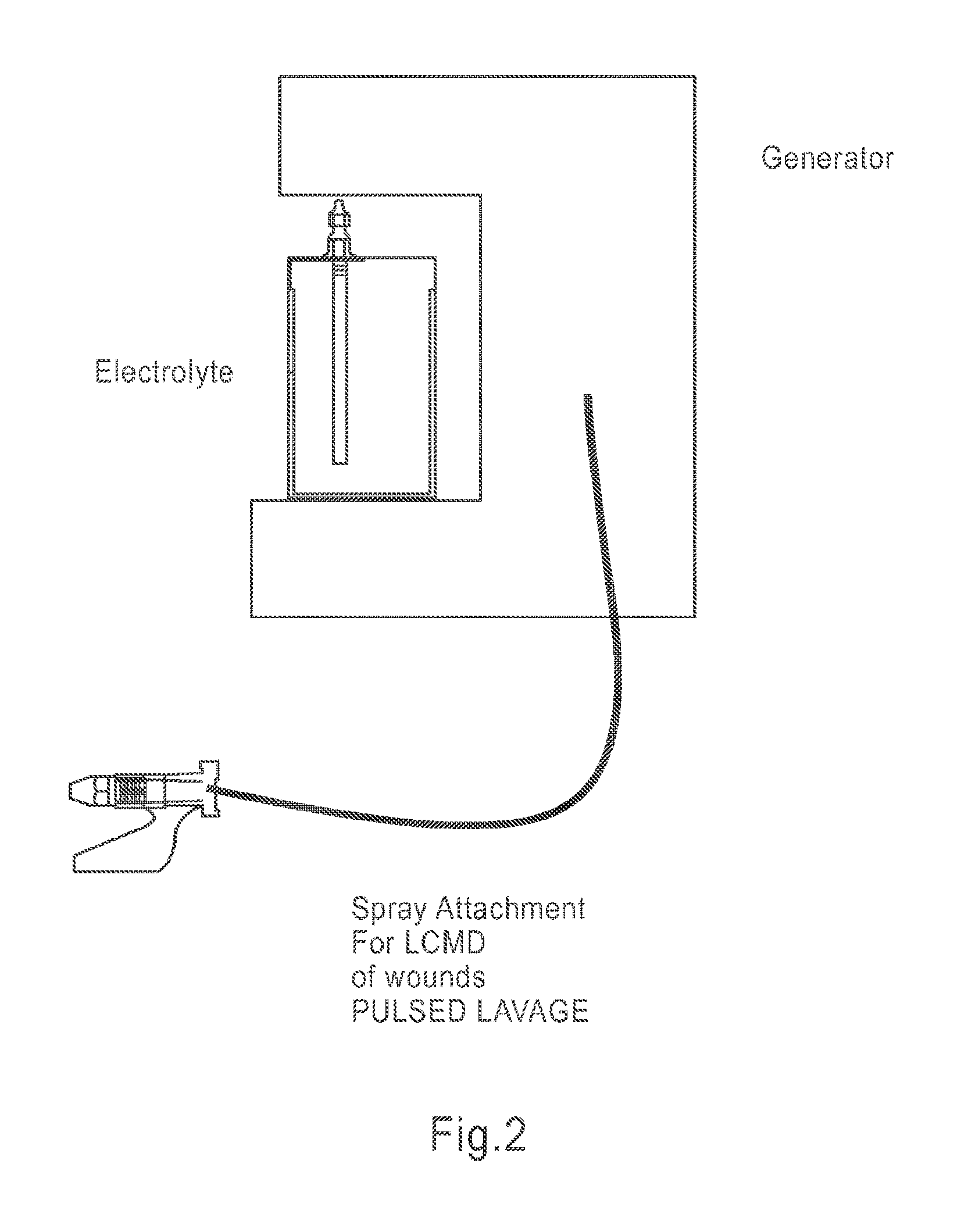
Apparatus and method for wound, cavity, and bone treatment
InactiveUS20130261534A1Environment safetyPromote formationElectrolysis componentsCannulasWound dressingWound care
Owner:PURICORE
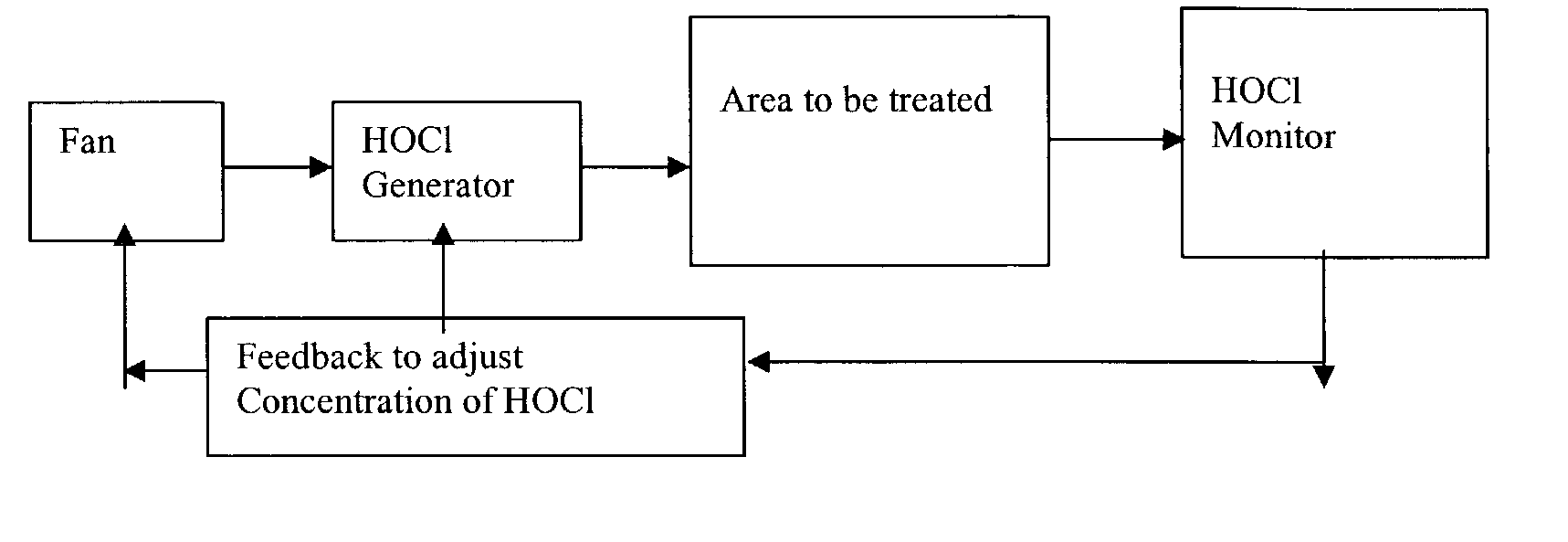
Mold remediation system and method
Provided herein are systems and methods associated therewith for killing molds and reducing other contaminating bioburden that may include bacteria and their spores, yeast, and viruses within various dwellings including homes, office buildings, institutions, and any other enclosed space in which humans reside, either temporarily or permanently. The technology of the present invention can be used to treat vehicles, airplanes, and ships. A process according to the invention includes the generation of a gaseous oxyhalogen species and distribution of the gaseous oxyhalogen species throughout a selected dwelling. It has been unexpectedly found that the concentration level of gaseous oxyhalogen species necessary to kill molds according to the inventive methods is far below that previously recognized in the art as being necessary for the killing of such molds. Thus, mold infestations may be killed in dwellings with minimal disruption to the usual business of the inhabitants. Further, fabrics such ad drapes and upholsteries contained within such dwellings may be carried out without causing any detrimental color changes to the fabrics.
Owner:DH TECH
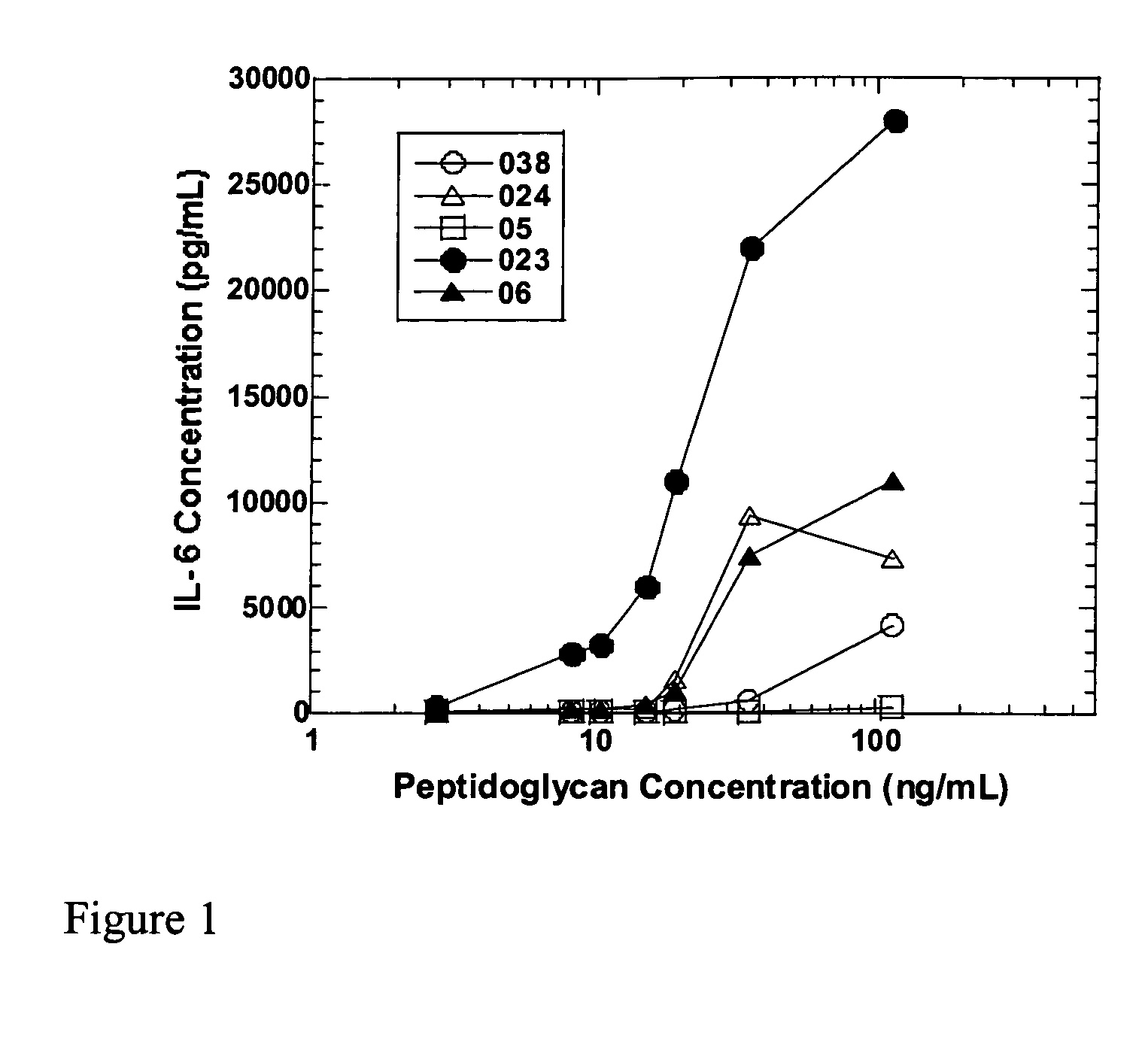
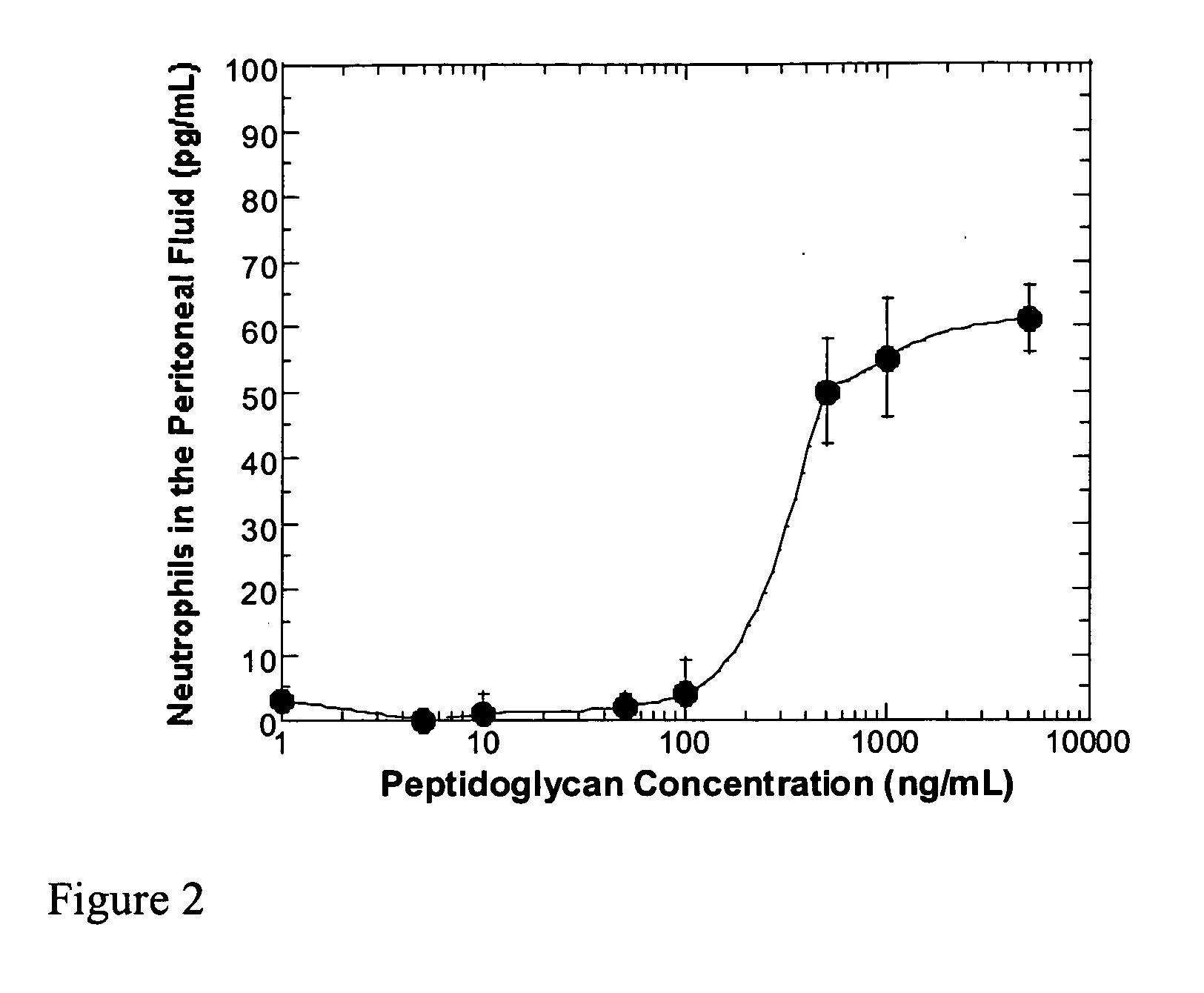
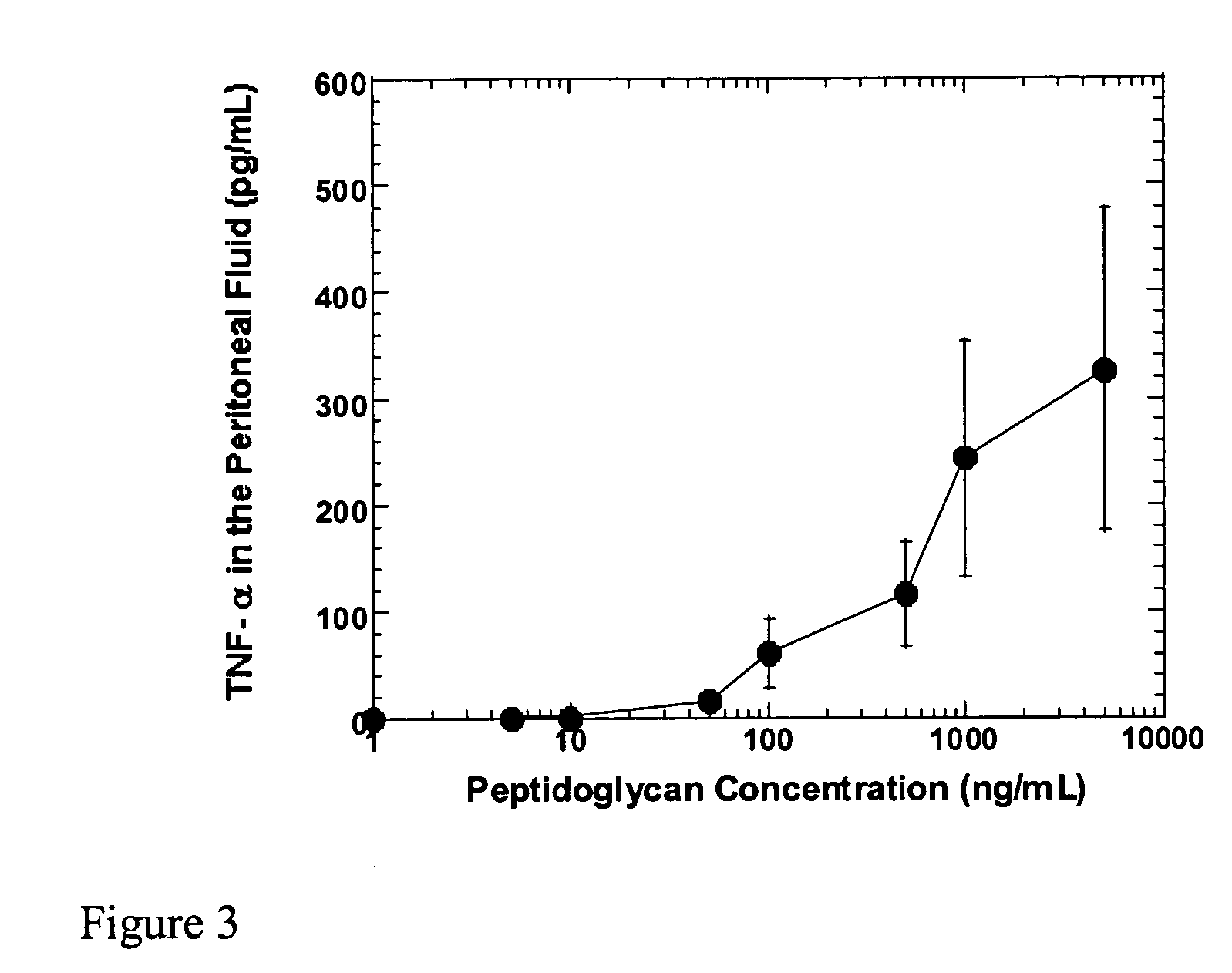
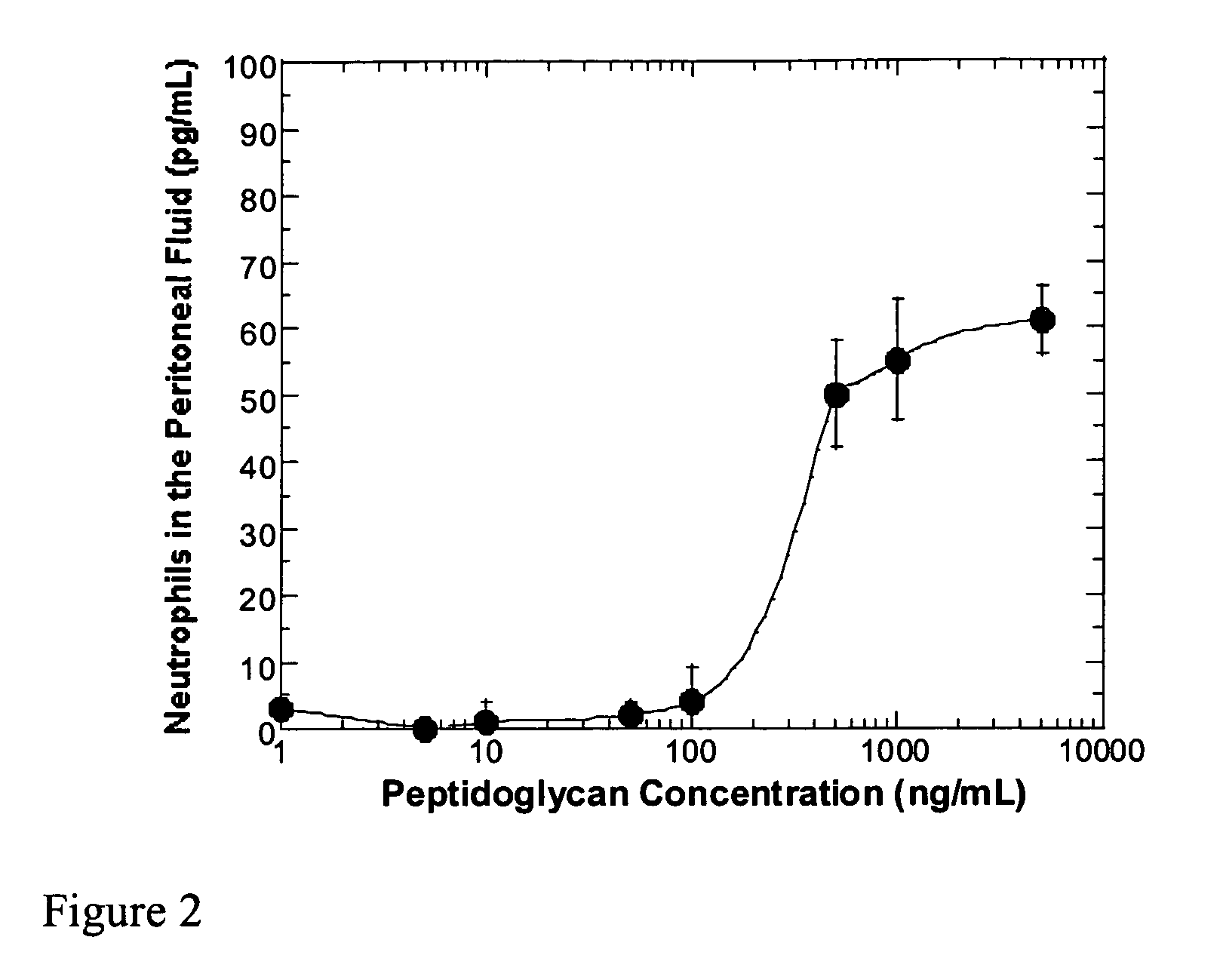
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS20050191717A1Improved peritoneal dialysis solutionImproved testing procedureAntibacterial agentsBiocideBiologyPeritoneal dialysis solutions
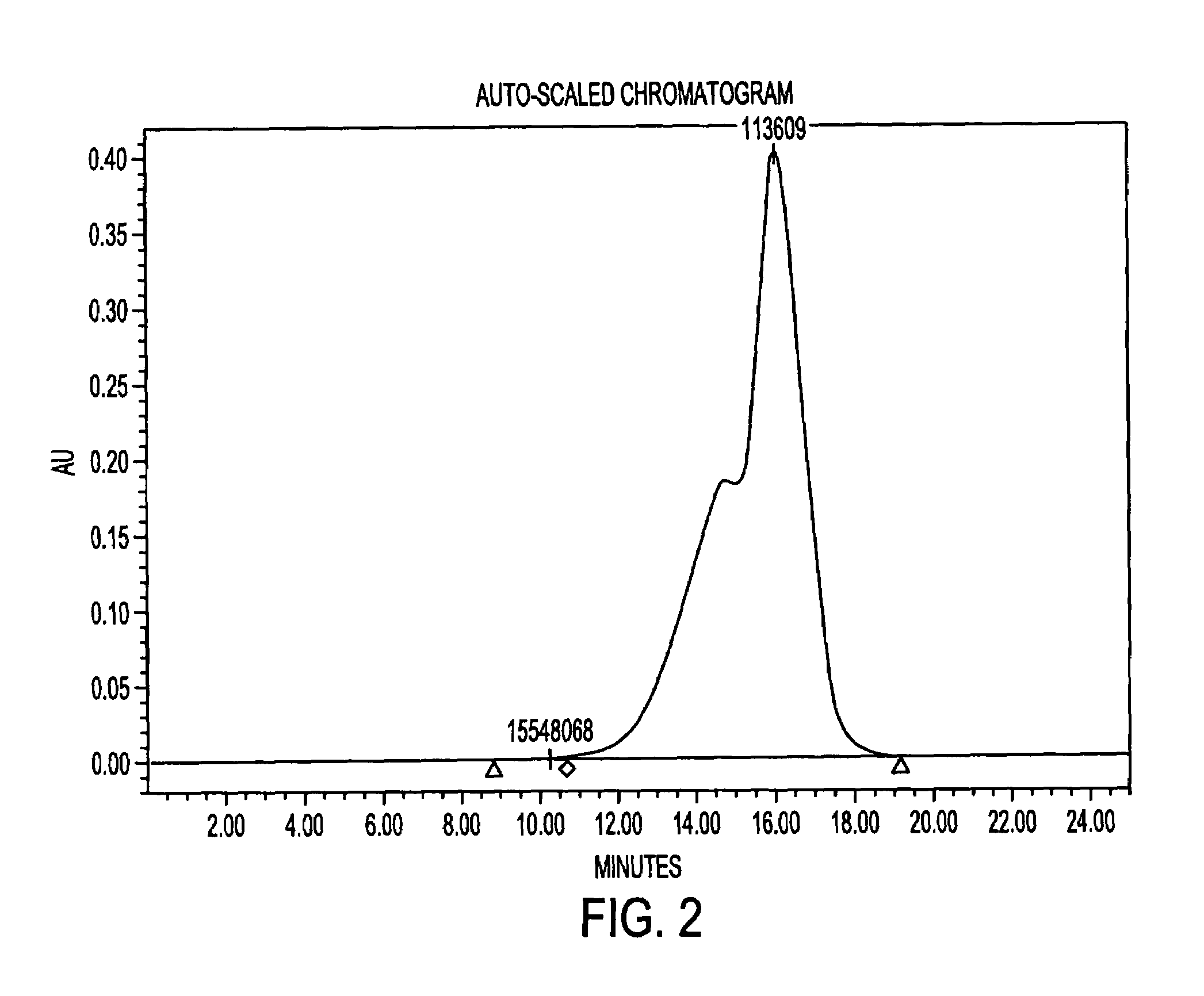
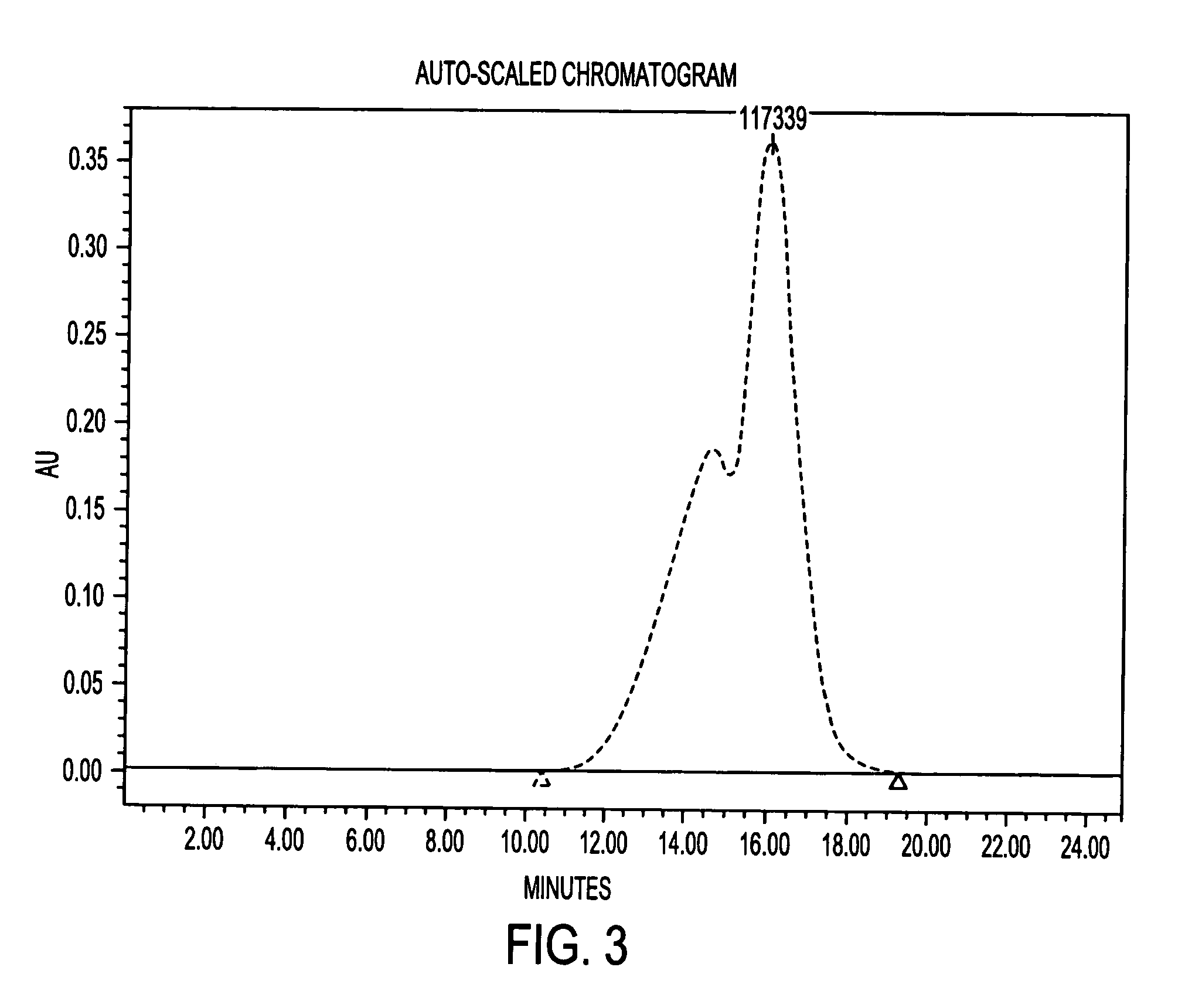
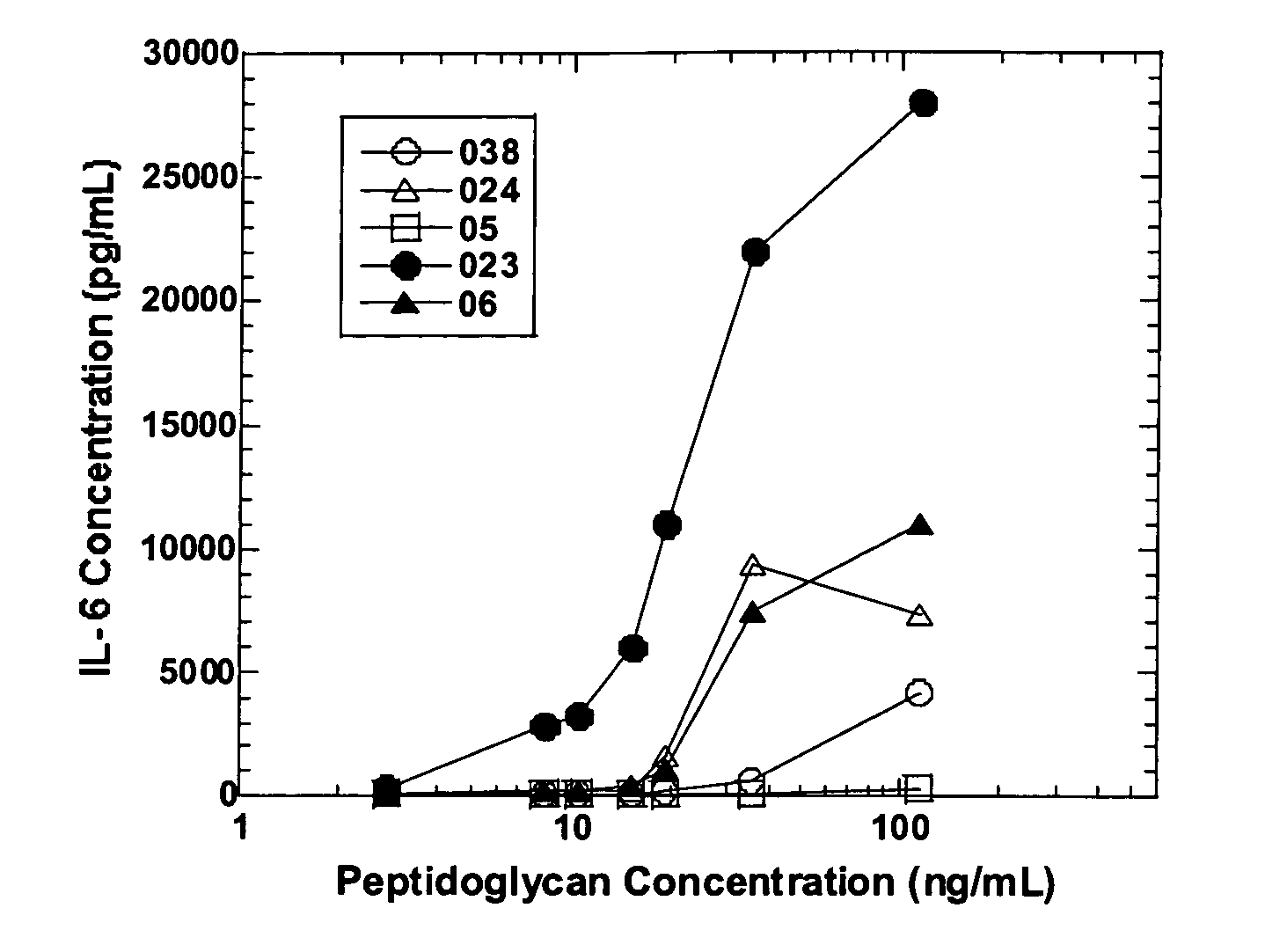
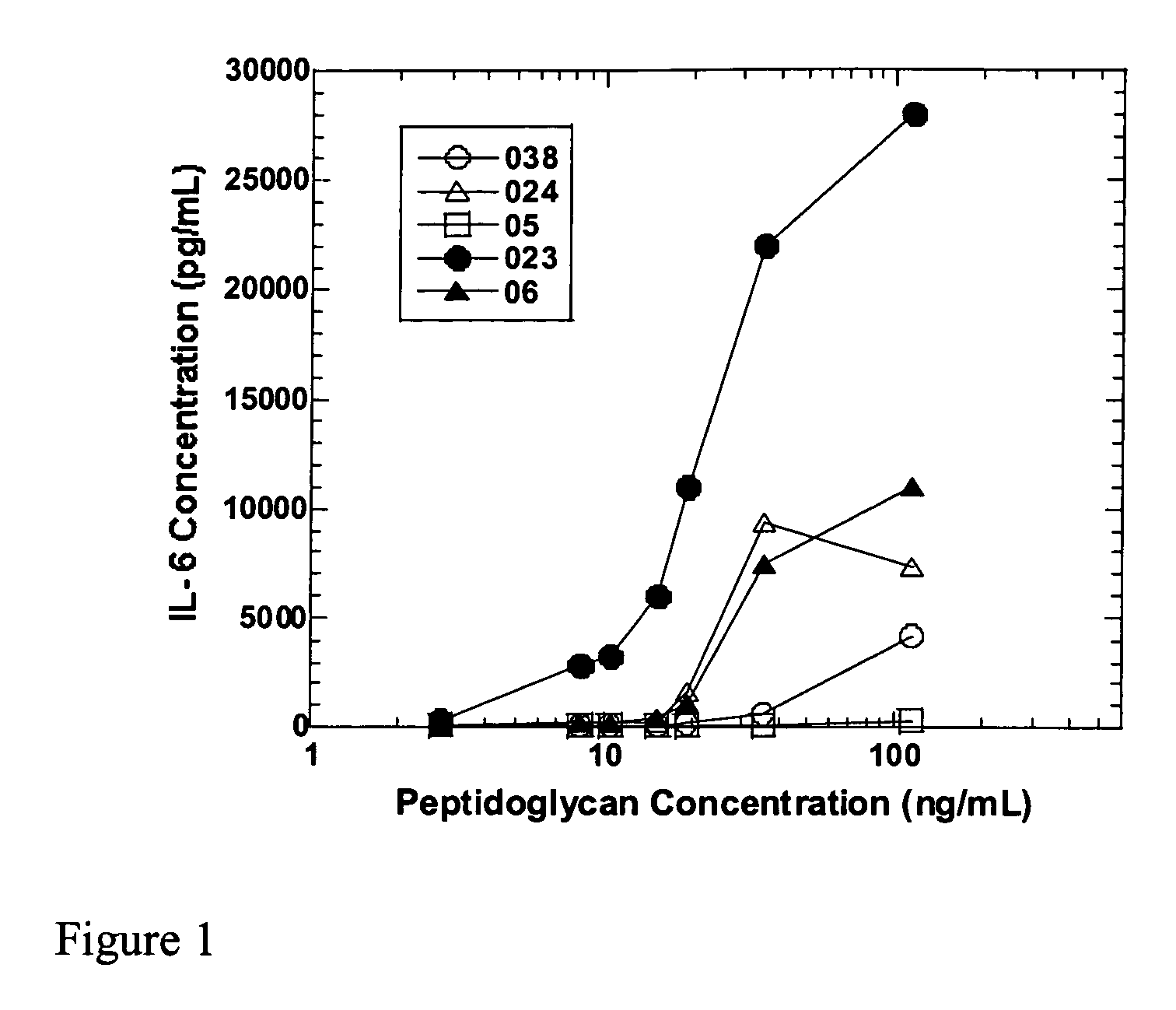
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
Net cage type biological carrier, immobilized microbiological treatment sewage device and application of immobilized microbiological treatment sewage device
ActiveCN104724817AAvoid short flowPrevent floatingTreatment using aerobic processesTreatment with anaerobic digestion processesHigh concentrationWater flow
The invention discloses a net cage type biological carrier, an immobilized microbiological treatment sewage device and application of the immobilized microbiological treatment sewage device. The net cage type biological carrier comprises a net cage and biological stuffing filled in the net cage, wherein the net cage is in the shape of a rectangle without a top cover, and a hollow-out mesh holes are formed in the side surface and bottom surface of the net cage. The immobilized microbiological treatment sewage device comprises a sewage treatment tank, a perforated mud discharge tube, a perforated aeration tube, a plurality of lower supporting beams and a sealing net, wherein a plurality of the net cage type biological carriers are stacked in the sewage treatment tank and arranged above the lower supporting beams. The net cage type biological carrier disclosed by the invention is large in specific surface area, low in accumulation, caking and blockage possibility, good in gas-water permeability, and convenient to mount and maintain. The immobilized microbiological treatment sewage device is good in gas-water flow state, conductive to bringing mass transfer effect into play, large in biological load amount, good in treatment effect and suitable for high-concentration degradation-resistant sewage treatment and advanced sewage treatment.
Owner:北京三泰正方生物环境科技发展有限公司
Compositions and methods of crop protection
InactiveUS7052708B2Avoid lostIncreased repellencyBiocidePharmaceutical delivery mechanismAbsorbable polymersGround hog
The invention is an animal repellant composition containing one or more toxins, including for example one or more alkaloids isolated from for example, one or more members of the family Narcissus, and optionally containing one or more polymers, including for example one or more bioerodible polymers and / or more non-absorbable polymers. The animal repellant composition is useful for repelling animals from vegetation and for rendering vegetation unpalatable to animals. The invention also includes methods for repelling animals, as well as methods for treating vegetation to render the vegetation unpalatable to animals, such animals including for example, deer, voles, moles, ground hogs, mice, rats, rodents, raccoons, nematodes, larvae, worms, fungi, molds, bacteria, vegetative organisms, and insects. A suberin-polymer-repellant chemical complex and a method for treating damaged vegetation are also disclosed. Various other systems and methods for complexing biopolymers, toxins, and polymers for treating plants, microbially inactivating the bioburden of vegetation, and for repelling animals are disclosed.
Owner:THE CORATO FOUND
SARS-CoV-2 combination air purifier and decontamination and bioburden reduction system for surgical masks/respirators
ActiveUS10905790B1Material analysis using wave/particle radiationBreathing filtersAir purifiersRespirator
The present invention in an embodiment relates generally to an air purifier having an ultraviolet wave chamber which is accessible from the exterior designed for the placement of masks / respirators in need of decontamination. Such device preferably makes use of filters comprising copper foam.
Owner:MOORE CULLEN THOMAS +1
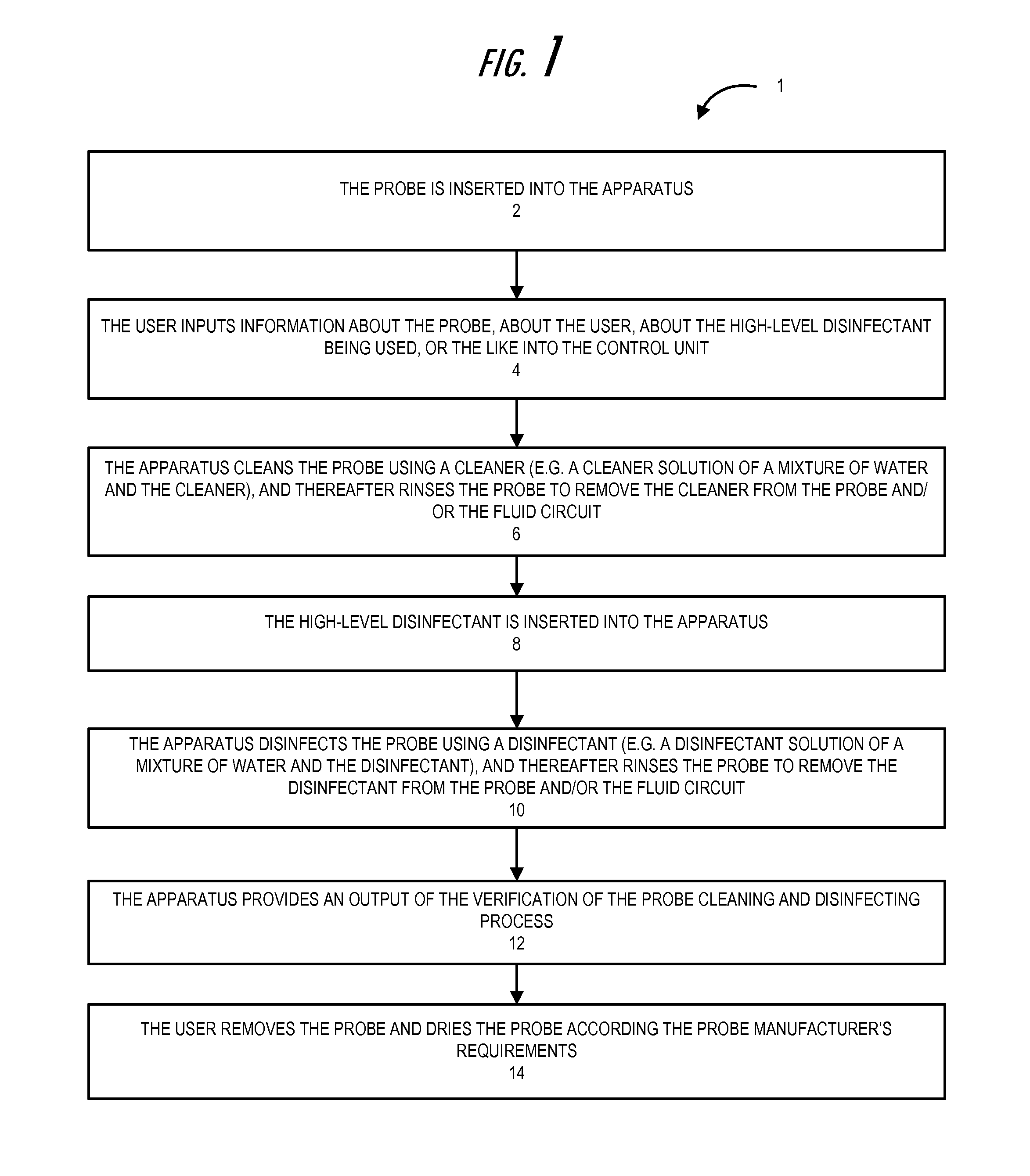
System and method for probe cleaning and disinfecting
Embodiments of the invention are directed to apparatuses and methods for cleaning and disinfecting probes. A cleaner is utilized to remove foreign materials (e.g., bioburden, soil, and other like material) from the probe after it is removed from the patient by soaking and / or flushing the foreign materials from the probe. The cleaner may be an enzymatic detergent that has bacteriostatic properties to inhibit bacterial growth within the apparatus. The multiple enzymes in the cleaner attack the foreign material, and include low-foam properties for effective recirculation across cycles within the cleaning step. The probe is rinsed after the cleaning step, and after rinsing a disinfectant process is applied to the probe. The disinfectant soaks and / or flushes the probe for a specified amount of time across cycles of recirculation to disinfect the surface of the probe, and afterwards the probe is rinsed thoroughly to remove the disinfectant from the probe.
Owner:DOBBYN GREGORY JOHN
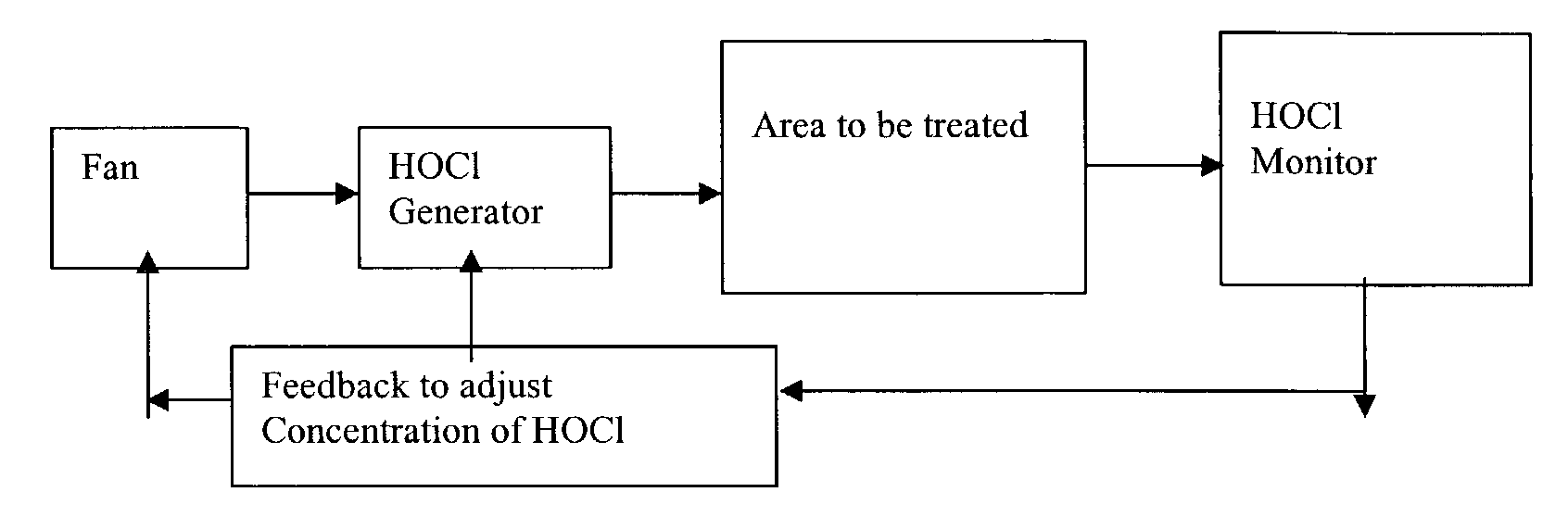
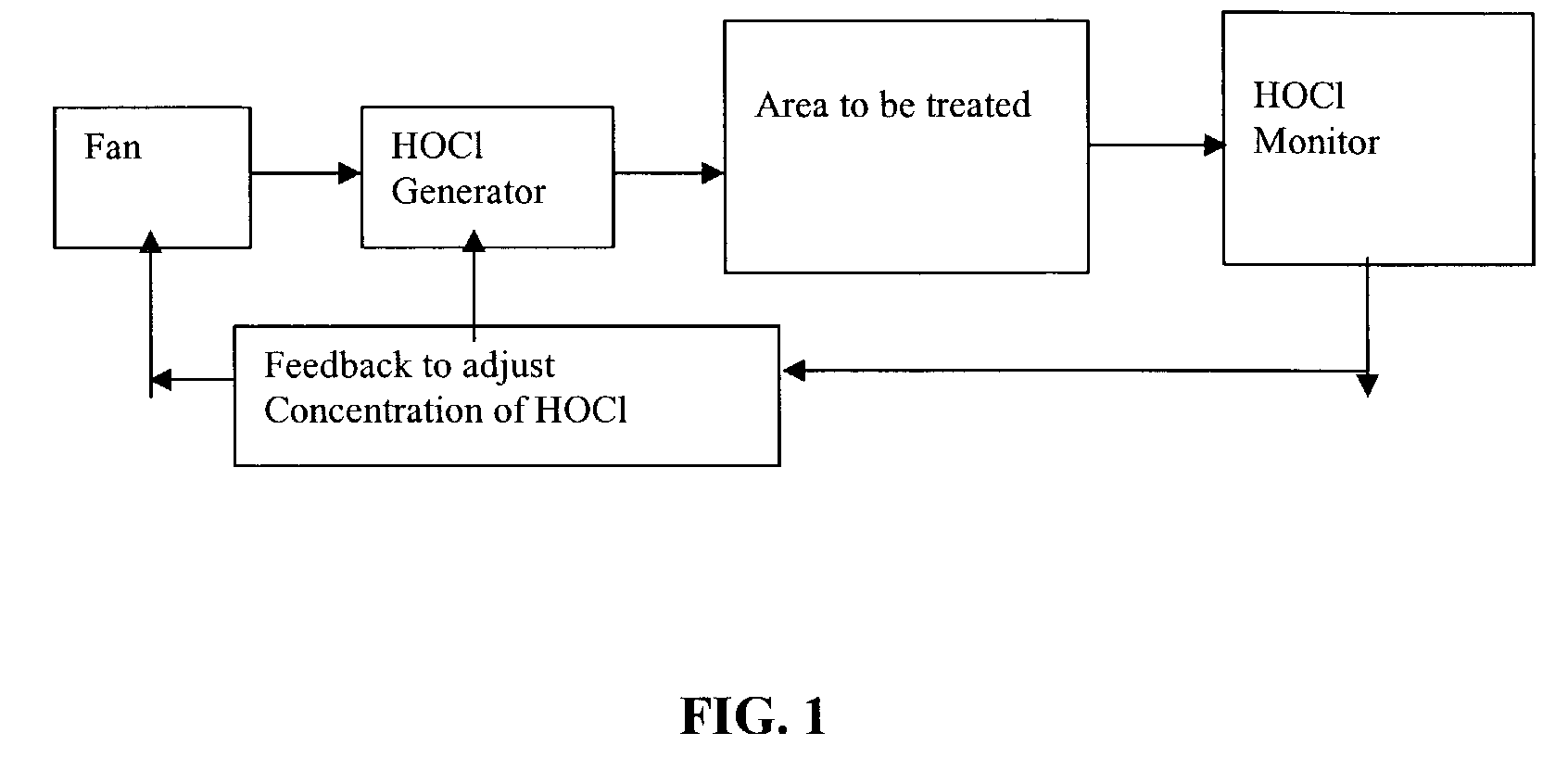
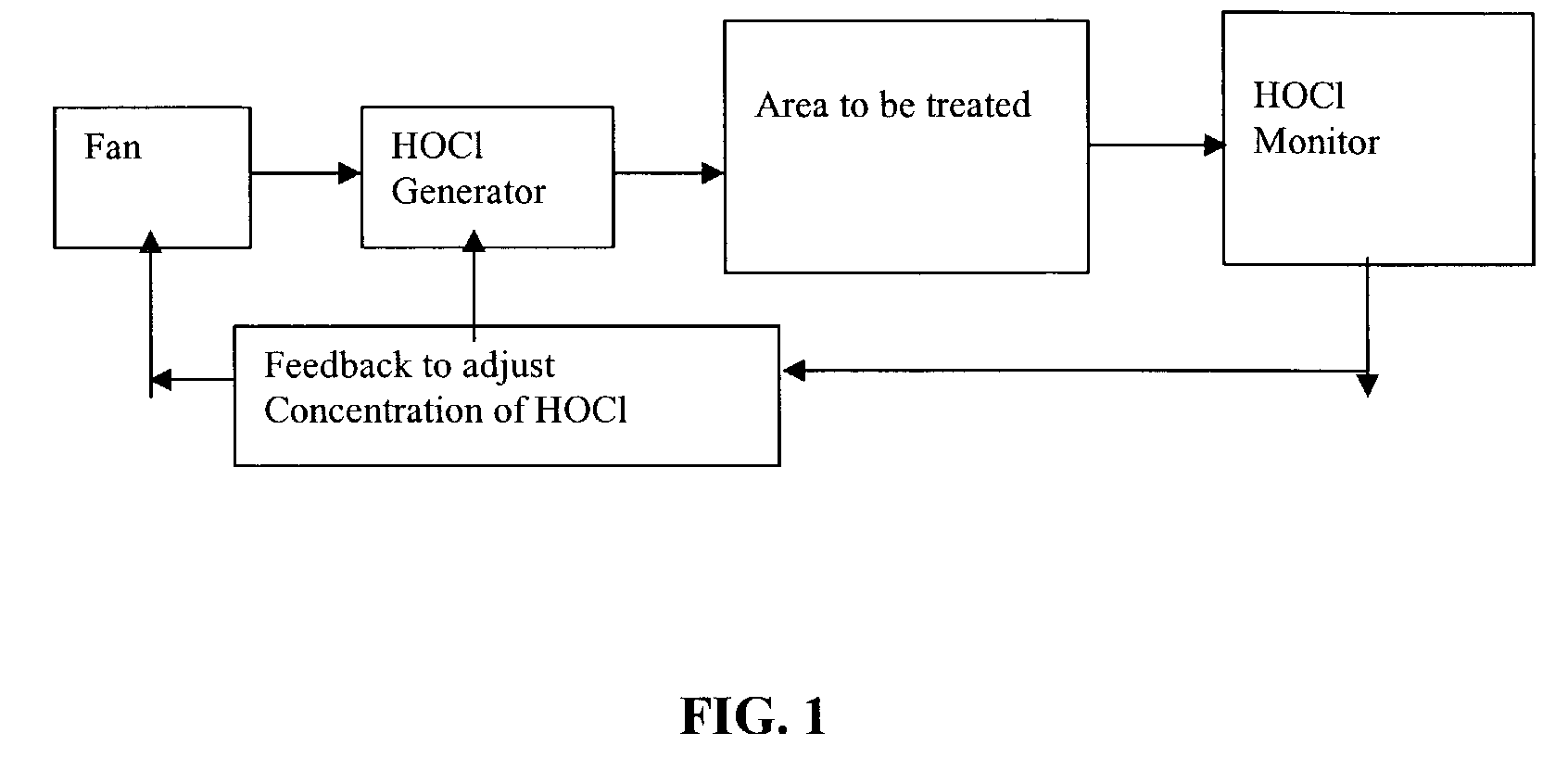
Method and apparatus for decontaminating a region without dehumidification
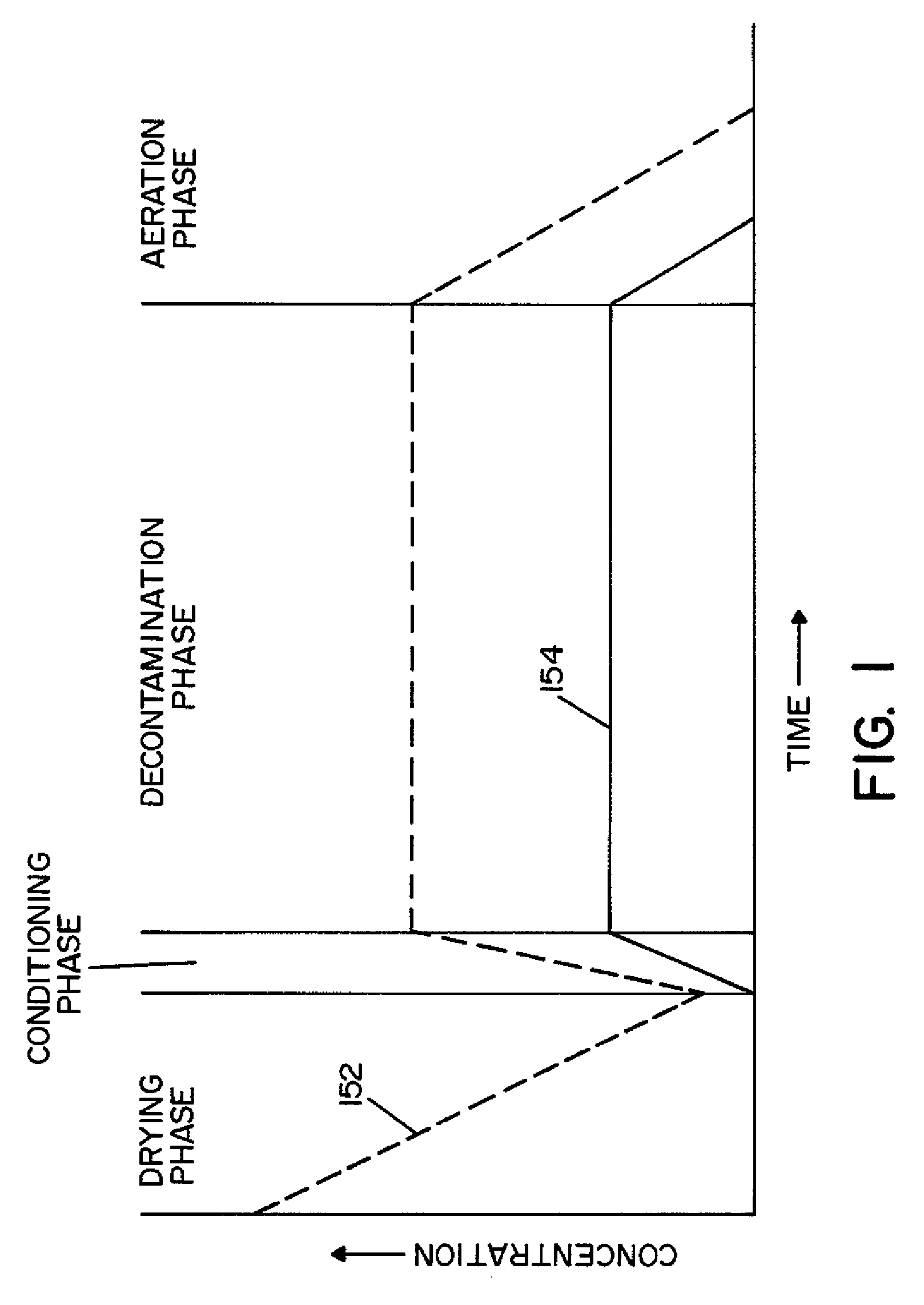
ActiveUS8007717B2Continuous monitoringExhaust apparatusElement comparisonSuccessful completionBioburden
A method and apparatus for decontaminating a region with a gaseous or vaporous decontaminant (e.g., vaporized hydrogen peroxide) without dehumidification (e.g., without the use of a dryer). The saturation concentration of the decontaminant inside the region is monitored, and the concentration of the decontaminant inside the region is regulated to prevent condensation of the decontaminant. The bioburden reduction is continuously monitored during a decontamination phase to ascertain successful completion of a decontamination process in accordance with a target bioburden reduction.
Owner:AMERICAN STERILIZER CO
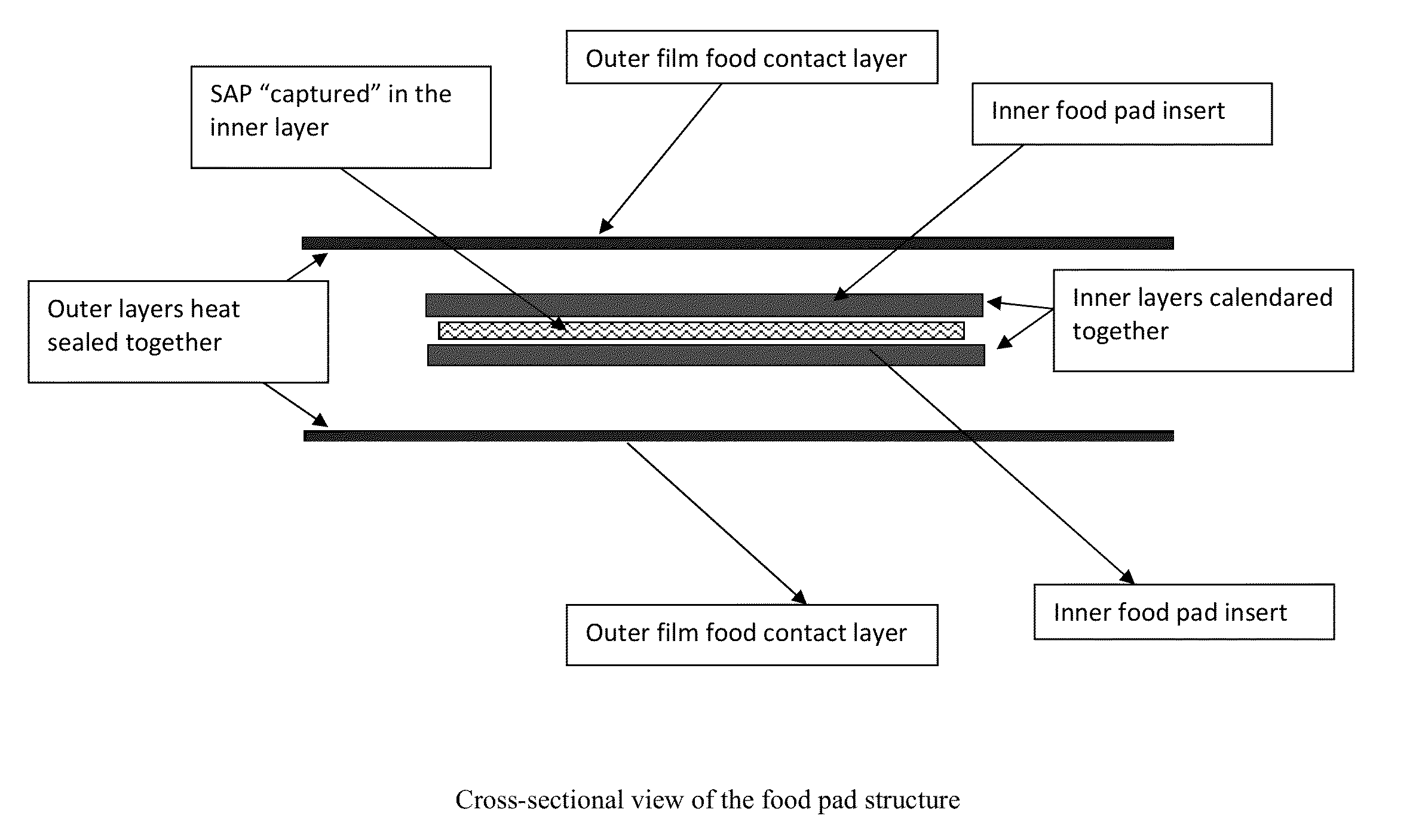
Biodegradable Polymer Non-woven Absorbent Pad with Absorbency and Antimicrobial Chemistry
InactiveUS20130295315A1Ensure robustnessIncreased manufacturing flexibilitySynthetic resin layered productsDomestic containersBiotechnologyAbsorbent Pads
Disclosed are food packaging materials and processes that are useful for commercial products to extend the freshness and preserve the integrity and shelf-life of packaged foods. Said food packaging materials utilize a low bioburden, biodegradable and / or compostable shock absorbing / cushioning nonwoven structure and some form or forms of an antimicrobial and / or antifungal agent consisting of silver or silver-based species that destroy microbes which would otherwise spoil the food. The shelf-life extension process involves silver-based antimicrobial agents that function to mitigate the spread of food spoilage pathogens when they come in contact with the said food packaging materials. Fluid absorbing or superabsorbent, capabilities may be incorporated in the structure to control excess fluids.
Owner:BIOVATION II
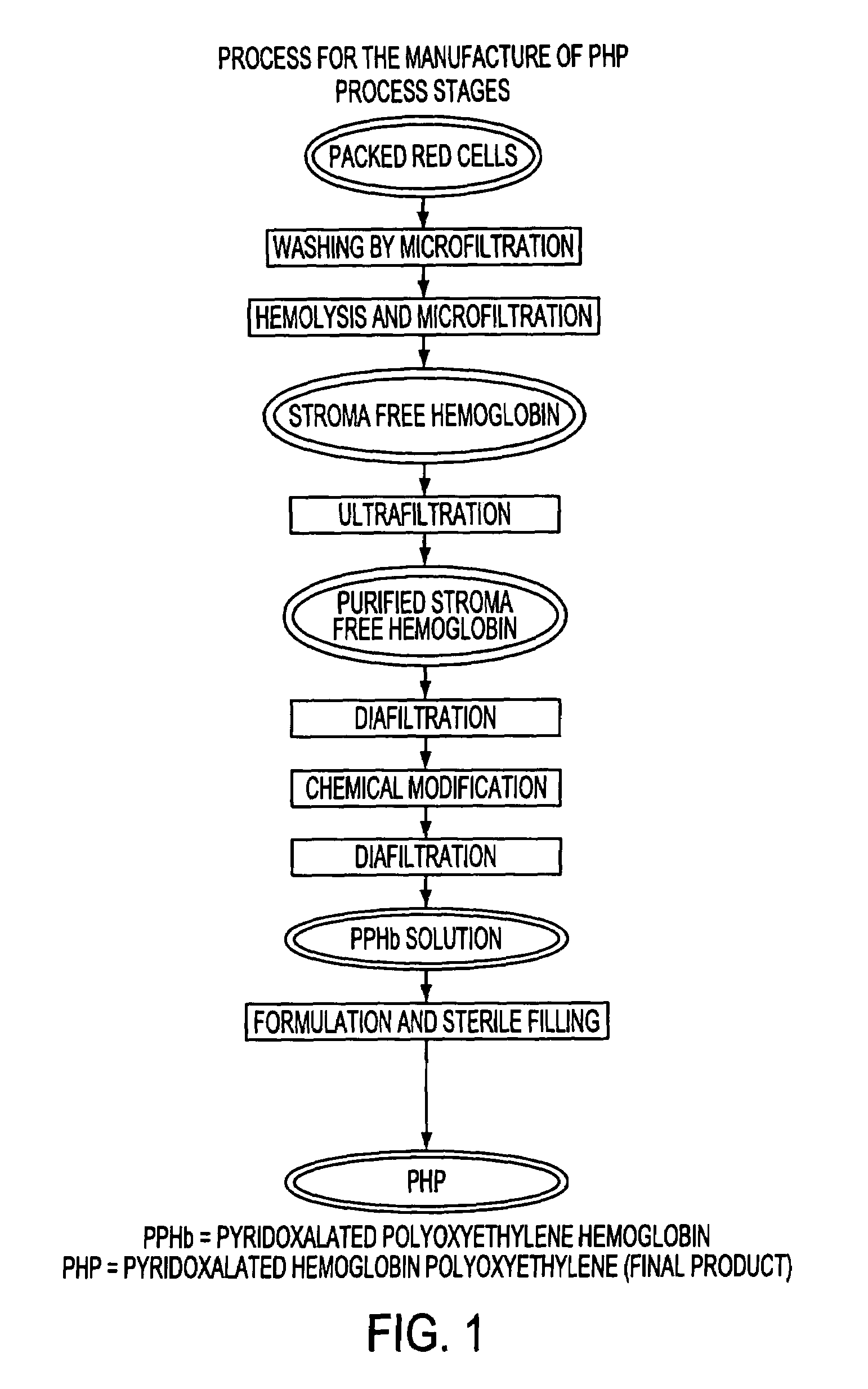
Methods for purification of an activated PEG solution and for the synthesis of a modified hemoglobin solution
InactiveUS7038016B2Lower Level RequirementsDecrease in levelCosmetic preparationsImpression capsFiltrationPolyethylene glycol
The present invention employs a dissolved activated polyethylene glycol (aPEG) or related molecule that has been passed through a filtration means for the substantial reduction of bioburden or endotoxin levels in the aPEG solution. The resulting filtered aPEG solution can be used for the preparation of a PEGylated hemoglobin solution containing substantially reduced levels of bioburden or endotoxin.
Owner:BREONICS
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS7118857B2Simple methodPrevent peritonitisAntibacterial agentsOrganic active ingredientsBiologyPeritoneal dialysis solutions
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
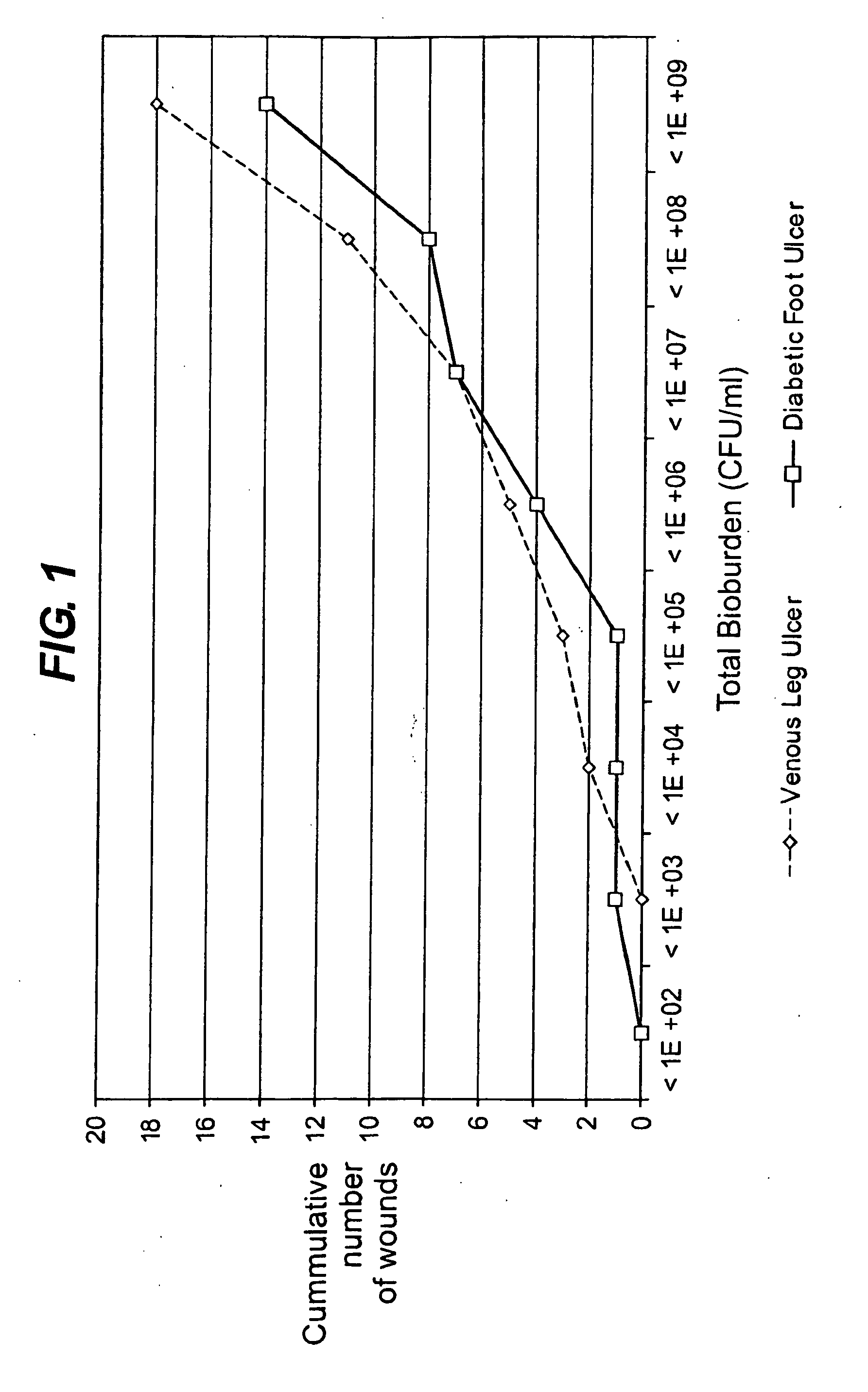
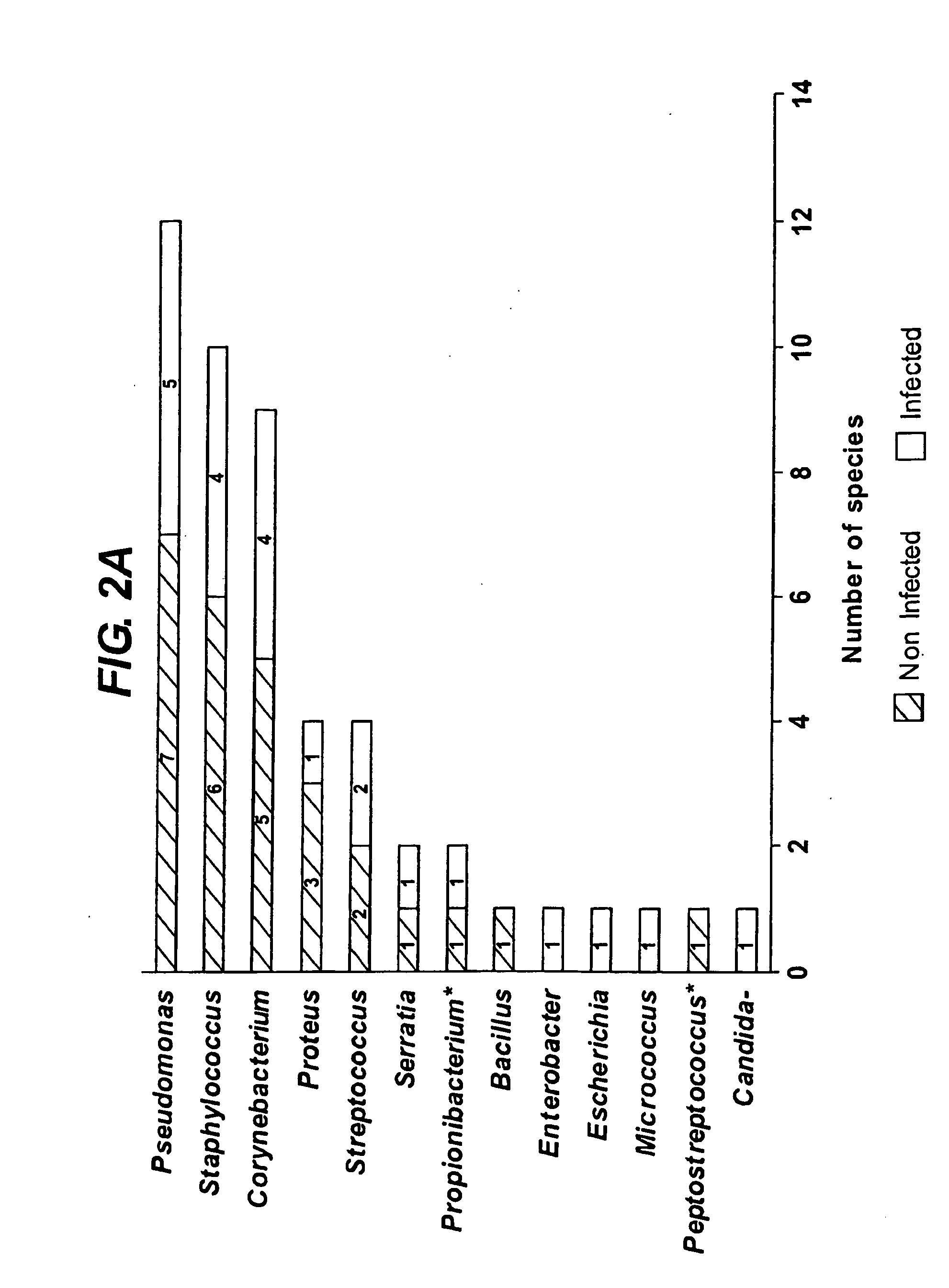
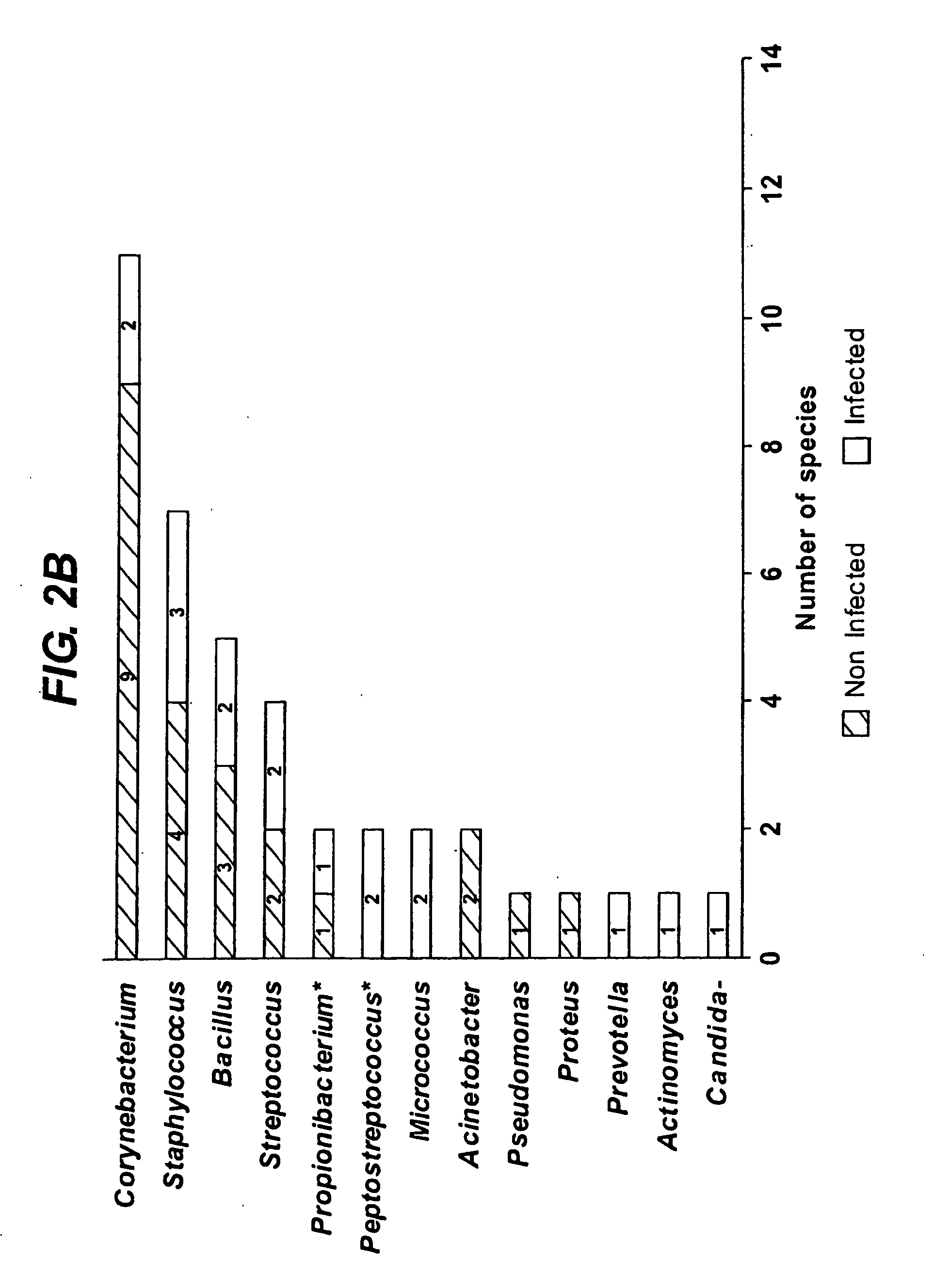
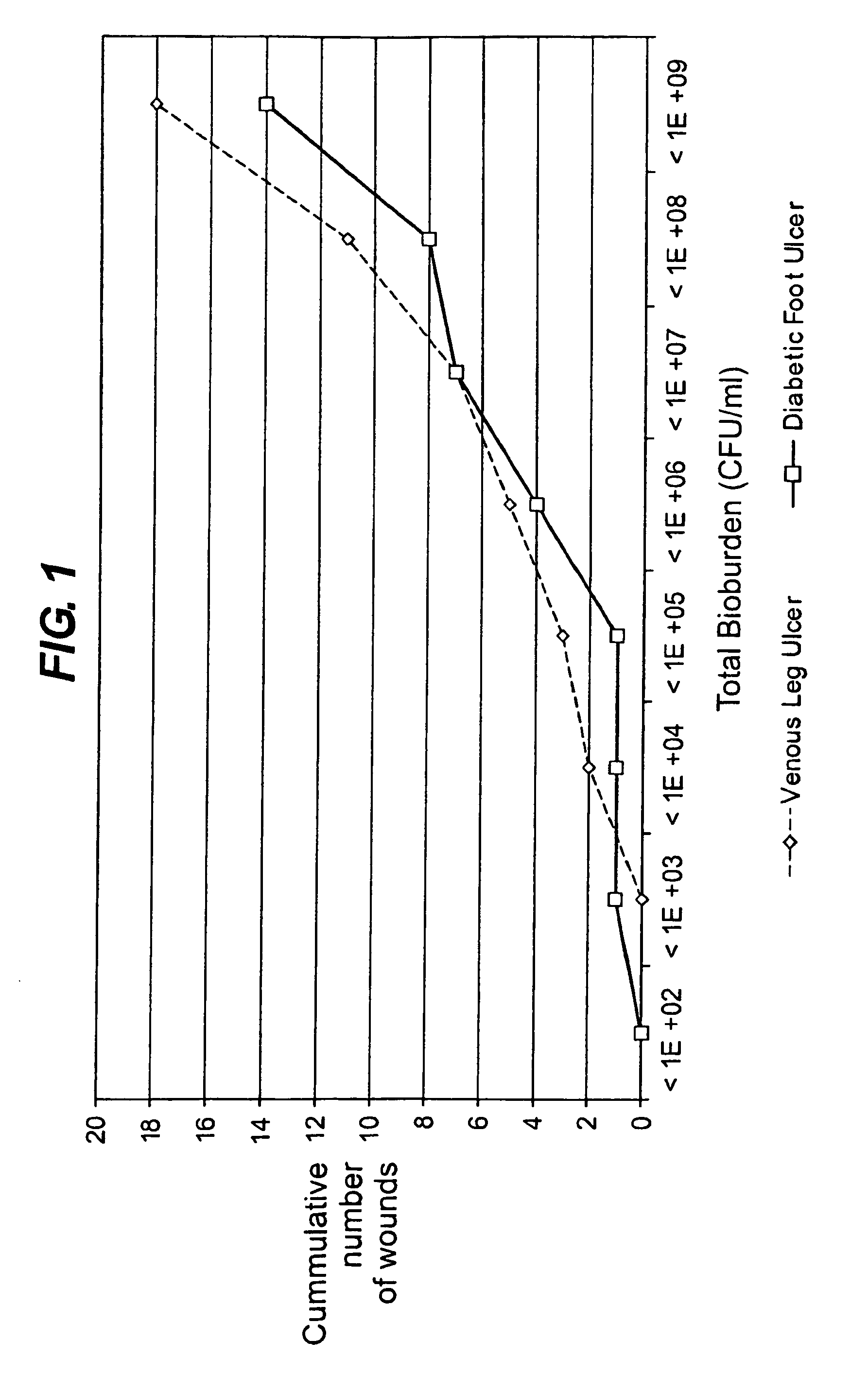
Diagnostic markers of wound infection
InactiveUS20100166694A1Low levelLow cytokine levelAntibacterial agentsBiocideMicroorganismDiabetic ulcer
The present invention relates to a method of determining the microbial bioburden in a wound (in particular a diabetic ulcer) in a test subject, the method comprising the step of measuring the level of a cytokine in a wound sample, wherein a cytokine level lower than a reference level indicates a significant microbial bioburden in the wound (or a cytokine level higher than a reference level indicates an insignificant microbial bioburden in the wound). The invention provides methods of diagnosis, prognosis and treatment of wound infection, and devices and kits for use in such methods.
Owner:WOUNDCHEK LAB US
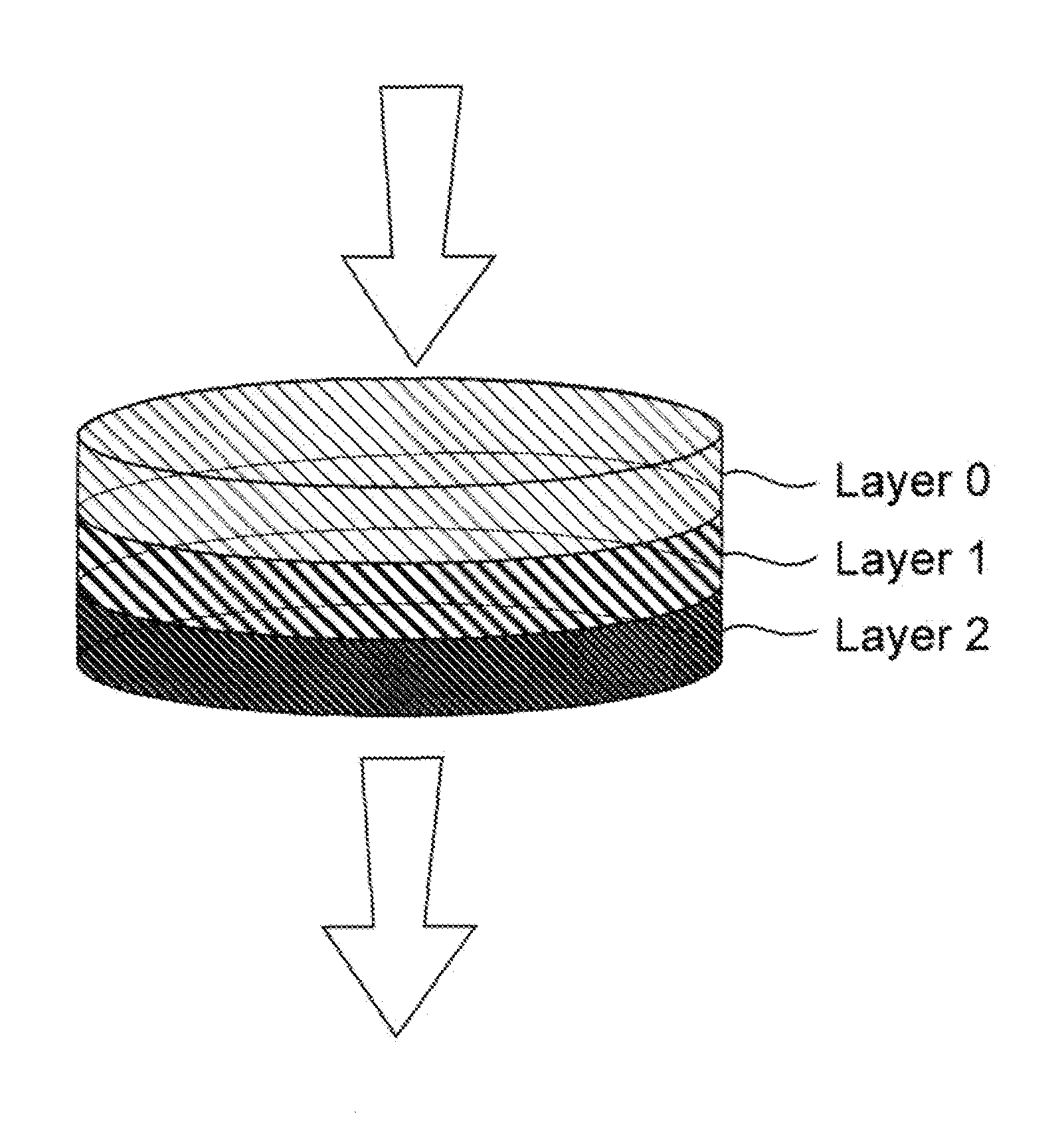
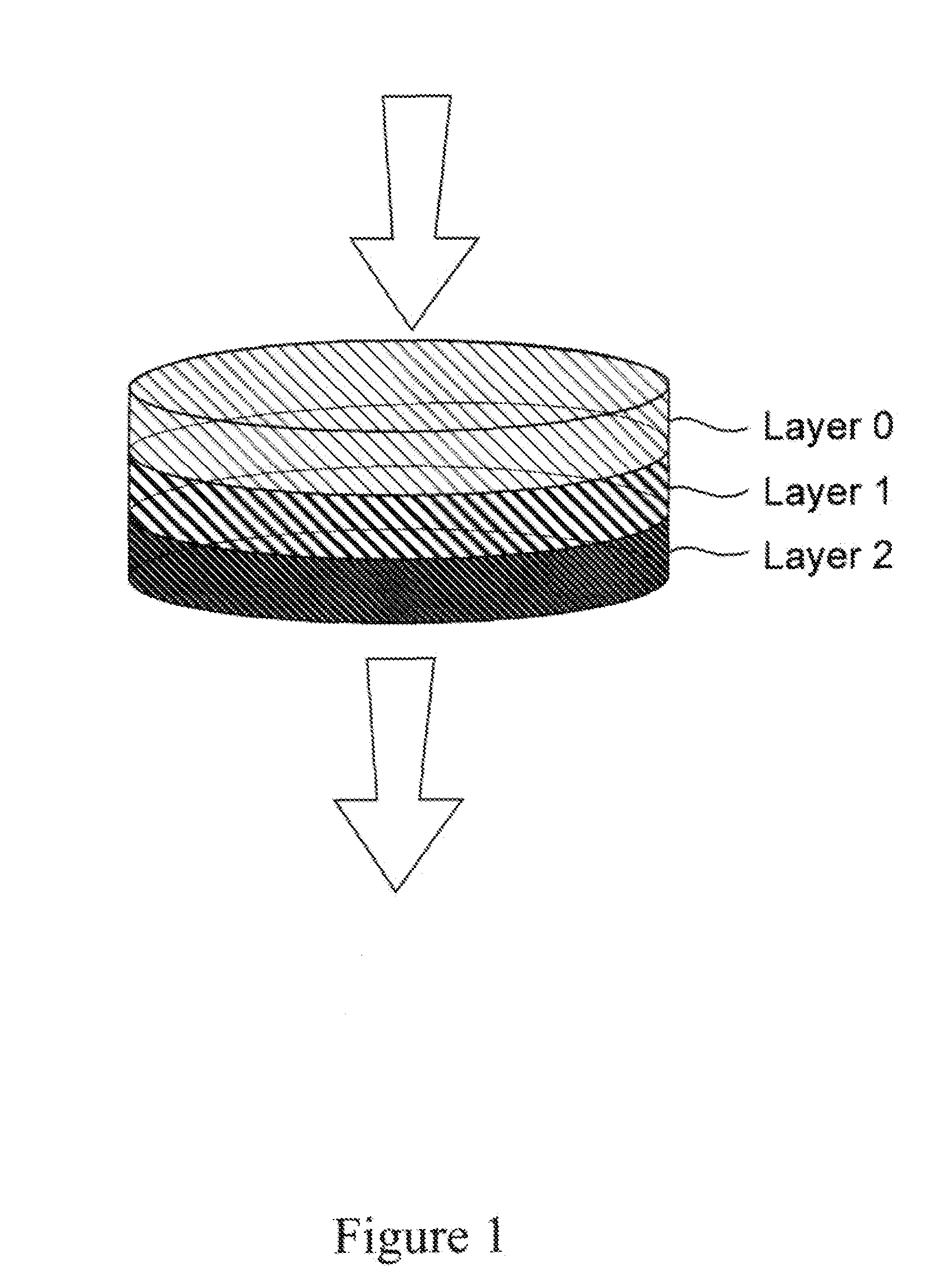
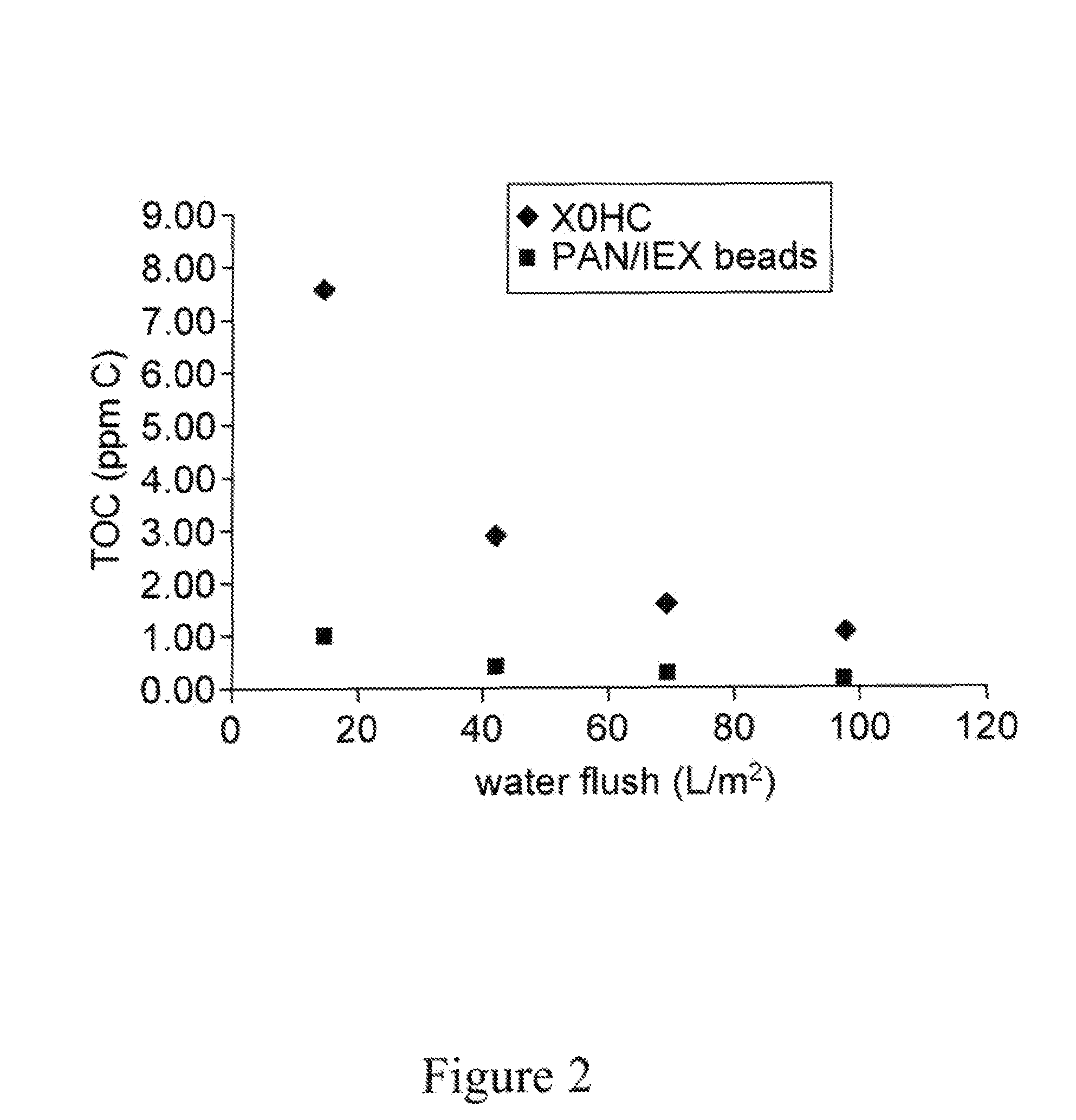
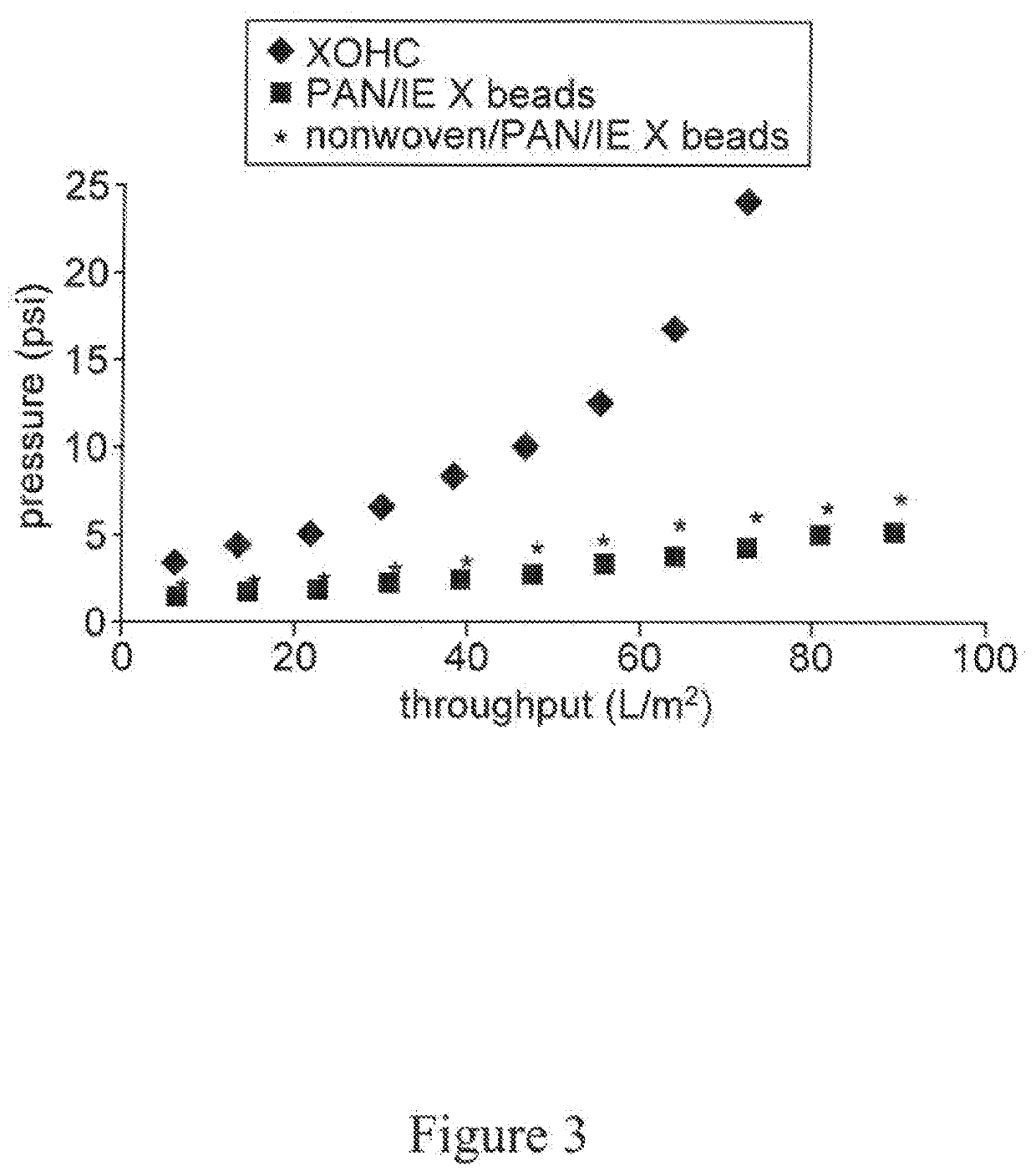
High capacity composite depth filter media with low extractables
ActiveUS20160114272A1Reduce the amount of waterHigh binding capacityIon-exchanger regenerationWater/sewage treatment by ion-exchangeFiberCulture fluid
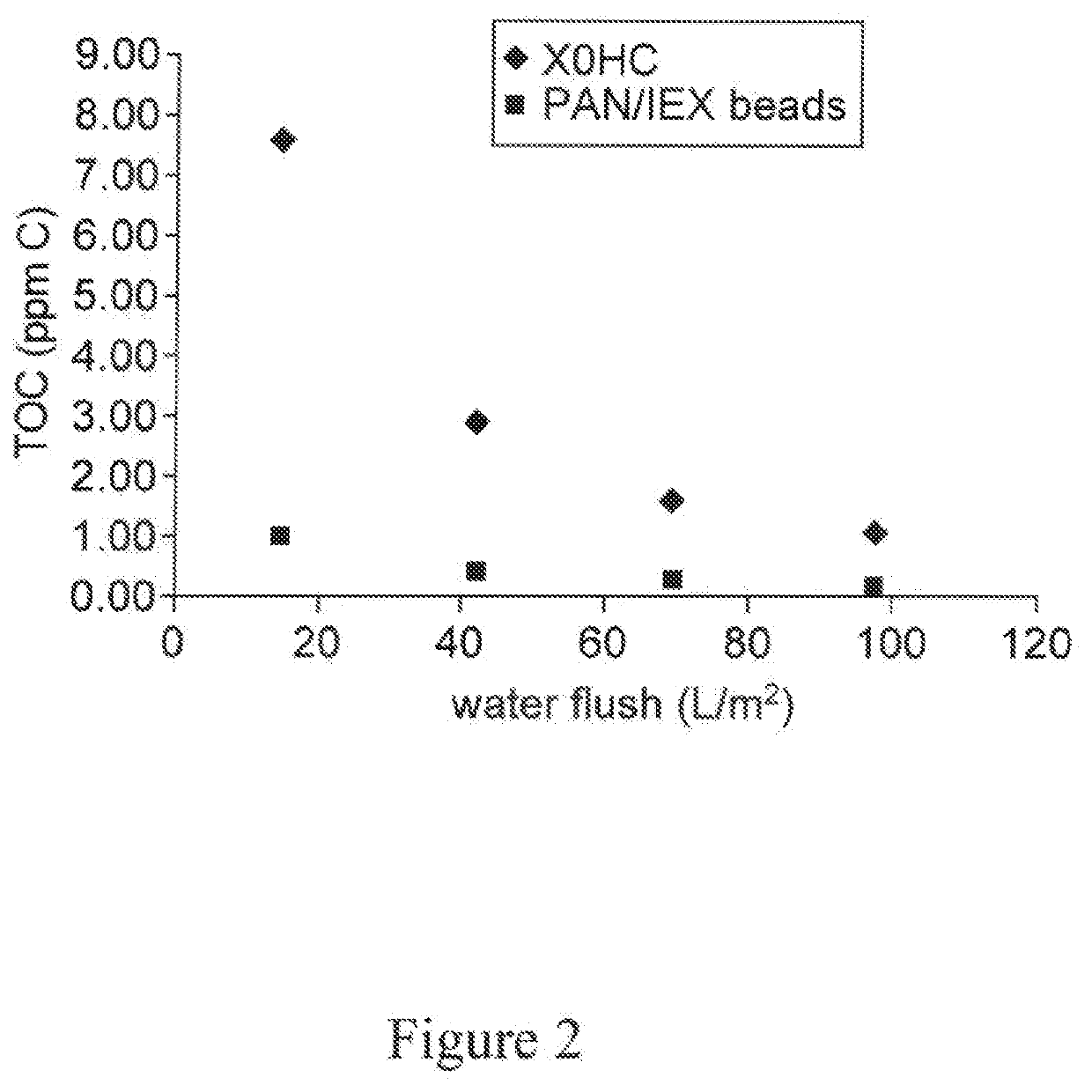
A depth filtration device for the clarification of biological fluids including a composite depth filter media having a nonwoven first layer integral with a second layer containing a polyaeryionitrile (PAN) fibers, a filter aid, and a wet-strength resin. The depth filter media exhibits increased creased binding capacity for soluble impurities such as DNA and host cells proteins from biological / cell culture feed-streams during secondary clarification and low-level impurity clearance of harvested cell culture fluids, such as those used for the manufacture of monoclonal antibodies. The depth filter media additionally exhibits significantly lower flushing requirements, resulting in lower levels of organic, inorganic and bioburden extractables released, high dirt holding capacities and good chemical and / or radiation resistance.
Owner:MILLIPORE CORP
Mold remediation system and method
Provided herein are systems and methods associated therewith for killing molds and reducing other contaminating bioburden that may include bacteria and their spores, yeast, and viruses within various dwellings including homes, office buildings, institutions, and any other enclosed space in which humans reside, either temporarily or permanently. The technology of the present invention can be used to treat vehicles, airplanes, and ships. A process according to the invention includes the generation of a gaseous oxyhalogen species and distribution of the gaseous oxyhalogen species throughout a selected dwelling. It has been unexpectedly found that the concentration level of gaseous oxyhalogen species necessary to kill molds according to the inventive methods is far below that previously recognized in the art as being necessary for the killing of such molds. Thus, mold infestations may be killed in dwellings with minimal disruption to the usual business of the inhabitants. Further, fabrics such as drapes and upholsteries contained within such dwellings may be carried out without causing any detrimental color changes to the fabrics.
Owner:DH TECH
Nano Fe(OH)3 composite biological filler as well as preparation method and application thereof
ActiveCN103951968AAvoid easy reunion problemsEvenly dispersedBiological water/sewage treatmentNanoparticleWastewater
The invention discloses a nano Fe(OH)3 composite biological filler as well as a preparation method and application thereof. The method is characterized by combining Fe(OH)3 nanoparticle preparation with the polyurethane (PU) foaming technique, firstly preparing a Fe(OH)3 nano PU prepolymer in situ and then completing foaming, thus avoiding the problem that Fe(OH)3 particles are easy to aggregate. The nano Fe(OH)3 composite biological filler and the preparation method have the beneficial effects that the prepared composite biological filler has large specific surface area and good biological load performance; Fe(OH)3 in the filler is uniformly dispersed, has a stable structure and can accelerate the metabolic activity of microorganisms, accelerate the biofilm formation of the microorganisms on the surface of the filler, simultaneously avoid the toxic effects of nanoparticles on the microorganisms due to small particle sizes, improve the skeleton strength of the PU foam filler and improve the stability and impact resistance of the filler; by adjusting the content of Fe(OH)3, the density of the prepared filler is convenient to control, thus ensuring the filler to be in a state of suspension in a wastewater treatment process and increasing the specific surface area of the filler.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Antipathogenic Biomedical Implants, Methods and Kits Employing Photocatalytically Active Material
InactiveUS20100331978A1Reducing and avoiding infectionOvercome disadvantagesDental implantsSurgical adhesivesLight irradiationBiomedical engineering
An antipathogenic biomedical implant is formed throughout its structure of a matrix material comprising at least about 1 weight percent of a photocatalytically active filler which exhibits an antipathogenic effect upon irradiation with light. The photocatalytically active filler is arranged in the matrix material in the implant to receive light irradiated from an external light source. In another embodiment, an antipathogenic biomedical implant comprises at least about 1 weight percent of a photocatalytically active material which exhibits an antipathogenic effect upon irradiation with light, wherein the photocatalytically active material is arranged in the implant to receive light irradiated from an external light source. Methods for providing an antipathogenic biomedical implant, methods for reducing pathogens on a biomedical implant, methods for reducing the bioburden in a biomedical implant installation, and kits for providing an antipathogenic biomedical implant employ the antipathogenic biomedical implants.
Owner:STROMME MARIA +2
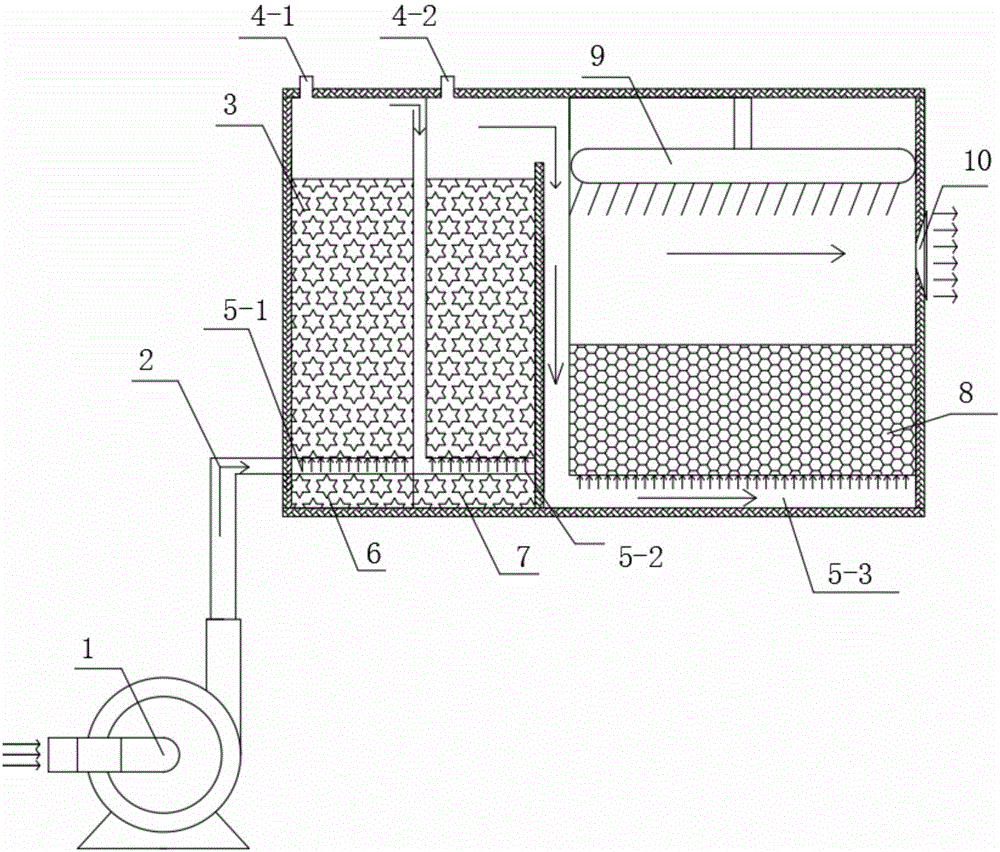
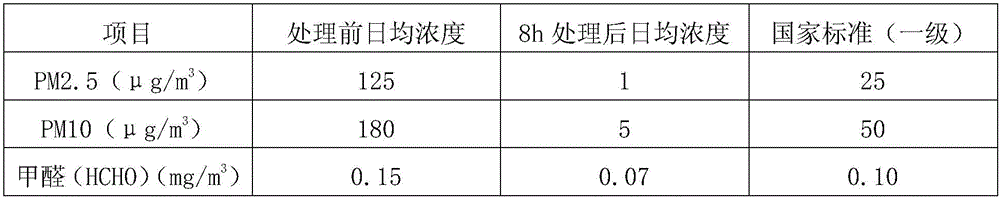
Indoor air treatment device and method
InactiveCN107174946ADiversity guaranteedHigh activityCombination devicesGas treatmentActivated carbonParticulates
The invention relates to an indoor air treatment device and method. The method comprises the following steps of carrying out microorganism treatment on the indoor air, adsorbing by activated carbon, and disinfecting by ultraviolet rays, wherein the microorganism treatment adopts an immobilized microorganism treatment technique. The device comprises an immobilized microorganism filter tank, an activated carbon adsorbing assembly and a space ultraviolet radiating assembly, wherein the immobilized microorganism filter tank adopts the microorganism immobilizing technique. The method and the device have the advantages that an adopted microorganism immobilizing carrier has strong microorganism immobilizing ability, the biological loading amount is large, and the immobilized microorganism can simultaneously present the aerobiotic, facultative anaerobic and anaerobic bacteria coexistence state; the particulate pollutants, organic matters, ammonia and nitrogen, nitrate nitrogen and sulfide pollutants in the waste gas can be simultaneously removed, the treatment speed is high, the purifying efficiency is high, and the cost is low.
Owner:BEIJING FENGZELVYUAN ENVIRONMENT TECH
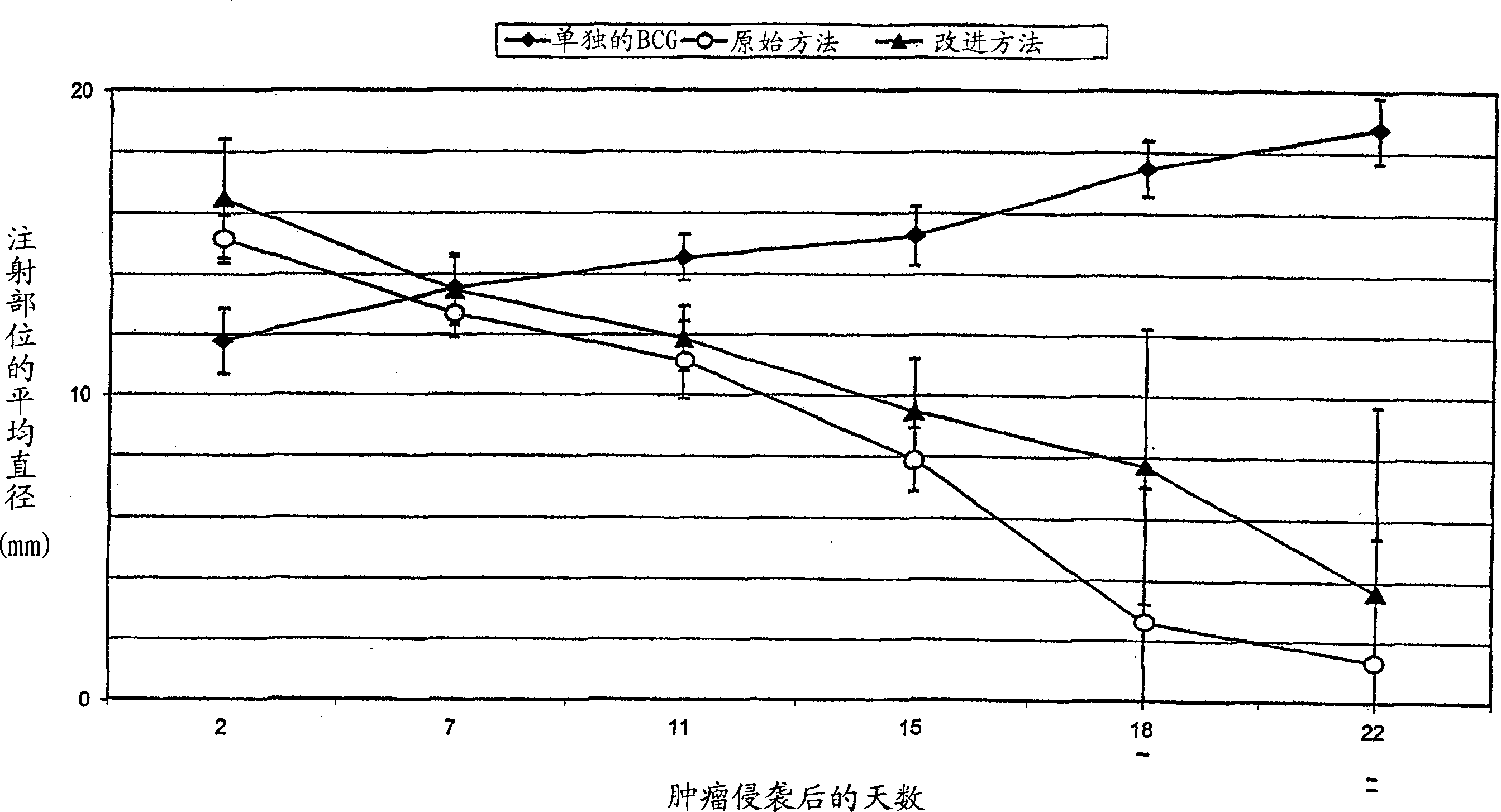
Sterile immunogenic non-tumorigenic tumor cell compositions and methods
InactiveCN1774255AStay aliveMaintain metabolic activityMammal material medical ingredientsCancer antigen ingredientsCell AggregationsMetastasis tumor
This invention relates to methods of removing bioburden from an aggregate of cells to obtain sterile cells that remain viable and immunogenic for the production of vaccines. This invention further relates to a method of eliciting an immune response to prevent a recurrence of metastases that involves preparing and administering a sterile vaccine derived from solid tumors. The vaccine is prepared by excising a solid tumor from a cancer patient, digesting the tumor cells with an enzyme to obtain dissociated cells, irradiating the dissociated cells to render the cells non-tumorigenic, and sterilizing the cells.
Owner:INTRACEL RESOURCES
High capacity composite depth filter media with low extractables
ActiveUS20200129901A1Avoid the needReduce the amount of waterMembrane filtersStationary filtering element filtersAntiendomysial antibodiesAntibodies monoclonal
A depth filtration device for the clarification of biological fluids including a composite depth filter media having a nonwoven first layer integral with a second layer containing a polyacrylonitrile (PAN) fibers, a filter aid, and a wet-strength resin. The depth filter media exhibits increased binding capacity for soluble impurities such as DNA and host cell proteins from biological / cell culture feedstreams during secondary clarification and low-level impurity clearance of harvested cell culture fluids, such as those used for the manufacture of monoclonal antibodies. The depth filter media additionally exhibits significantly lower flushing requirements, resulting in lower levels of organic, inorganic and bioburden extractables released, high dirt holding capacities and good chemical and / or radiation resistance.
Owner:MILLIPORE CORP
Dual parallel optical axis modules sharing sample stage for bioburden testing
InactiveUS20200183140A1Easy to moveMicrobiological testing/measurementMicroscopesFluorescenceOptical axis
Filter membranes and the like carrying fluorescent targets suspected of being bioburden are tested by mounting the membranes on a sample holder within a cabinet for bioburden verification and discrimination from false positives. Fluorescence is excited by a fluorescence excitation module having a first optical axis perpendicular to the sample, with three dimension locations of fluorescent targets that are probable bioburden stored for use by a microscope imaging module having a second optical axis parallel to the first optical axis, with dual wavelength illumination for autofocus and then for stimulating fluorescence without photo bleaching of the targets. The probable bioburden targets are moved for inspection and identification in the microscope imaging module as bioburden or not by sharing a common sample holder in the cabinet.
Owner:REAMETRIX
Sterile chromatography resin and use thereof in manufacturing processes
ActiveCN106068150AChromatographic cation exchangersChromatographic anion exchangersChromatography columnPharmacology
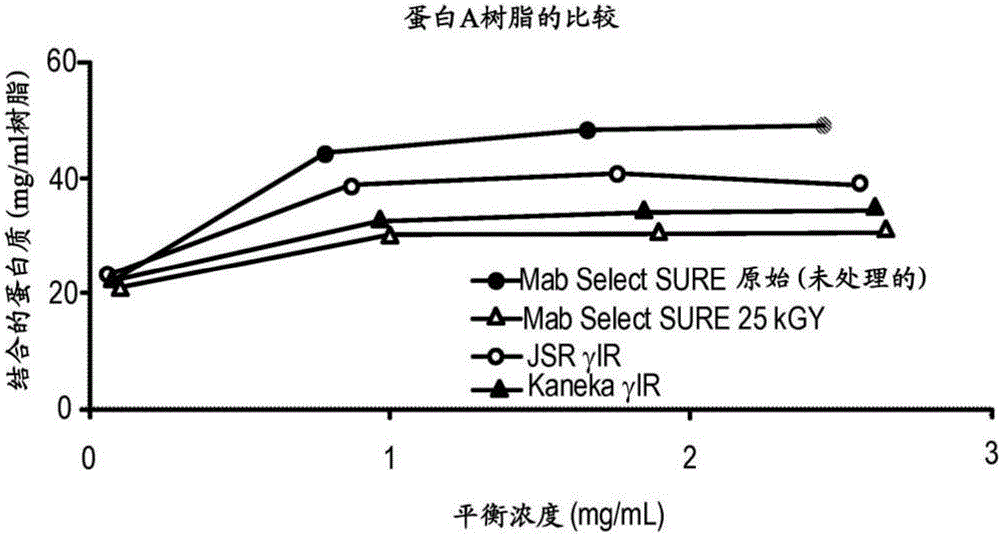
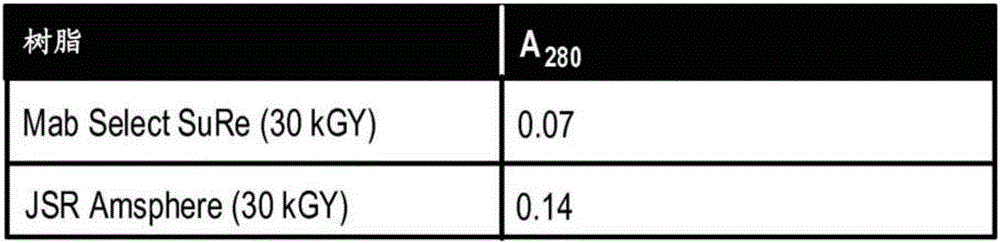
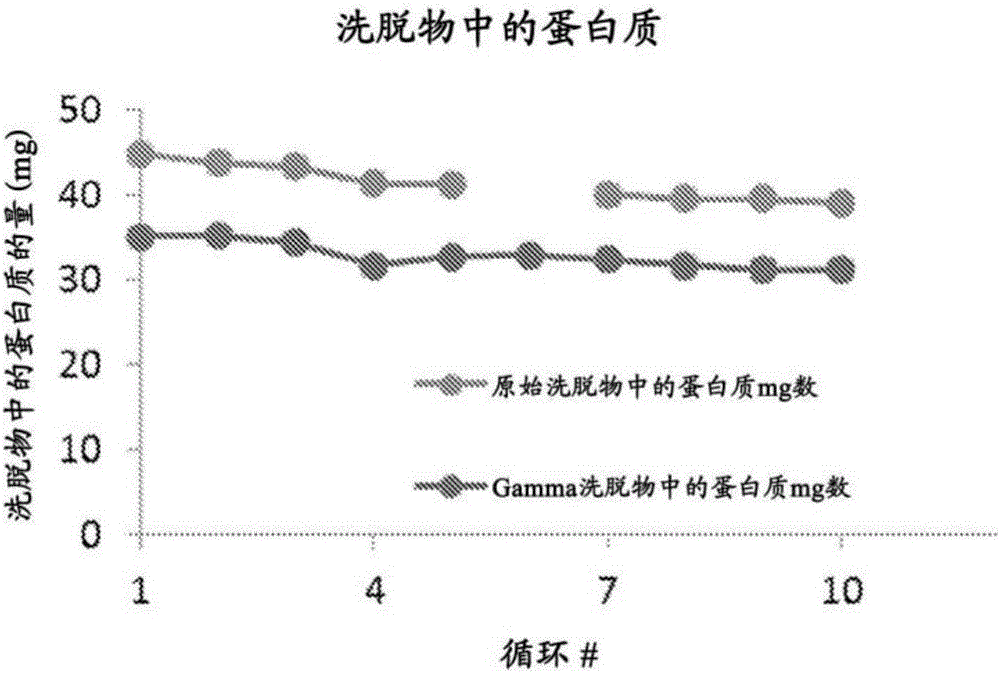
Provided herein are methods of reducing bioburden of (e.g., sterilizing) a chromatography resin that include exposing a container including a composition including a chromatography resin and at least one antioxidant agent and / or chelator to a dose of gamma-irradiation sufficient to reduce the bioburden of the container and the chromatography resin, where the at least one antioxidant agent and / or chelator are present in an amount sufficient to ameliorate the loss of binding capacity of the chromatography resin after / upon exposure to the dose of gamma-irradiation. Also provided are reduced bioburden chromatography columns including the reduced bioburden chromatography resin, compositions including a chromatography resin and at least one chelator and / or antioxidant agent, methods of performing reduced bioburden column chromatography using one of these reduced bioburden chromatography columns, and integrated, closed, and continuous processes for reduced bioburden manufacturing of a purified recombinant protein.
Owner:GENZYME CORP
Method for establishing a sterilizing dose for radiation sensitive products
ActiveUS20100166599A1Adequate dosageEnsure sterilizationLavatory sanitoryMedical waste disposalMicroorganismDose level
A method provides for sterilizing with radiation objects which have a low bioburden and which are sensitive to radiation. A dosage of radiation sufficient to ensure sterilization without damaging the object is determined by determining the bioburden upon one or more samples of the objects, determining an estimate of the dose that results in a preselected probability (e.g., 0.1, 0.01, 0.001, 0.0003162 . . . etc. (i.e., SAL=10−1, 10−2, 10−3, 10−3.5 . . . etc.)) of a surviving microorganism on the objects preferably by testing a quantity of samples of the objects at varying dosage levels of radiation, confirming the estimate by testing a quantity of samples of the objects at the dose that was estimated; and calculating a dosage for the sterility assurance level of 10−6 by adding a factor to the dose that was confirmed to result in said preselected probability of a surviving microorganism on the objects and wherein the factor is proportional to the dose that yields said preselected probability of a surviving microorganism on the objects and inversely proportional to a log of the bioburden.
Owner:ETHICON INC
Diagnostic markers of wound infection
InactiveUS8012698B2Improve material stabilityImprove accuracyAntibacterial agentsBiocideMedicineDiabetic ulcer
The present invention relates to a method of determining the microbial bioburden in a wound (in particular a diabetic ulcer) in a test subject, the method comprising the step of measuring the level of a cytokine in a wound sample, wherein a cytokine level lower than a reference level indicates a significant microbial bioburden in the wound (or a cytokine level higher than a reference level indicates an insignificant microbial bioburden in the wound). The invention provides methods of diagnosis, prognosis and treatment of wound infection, and devices and kits for use in such methods.
Owner:WOUNDCHEK LAB US
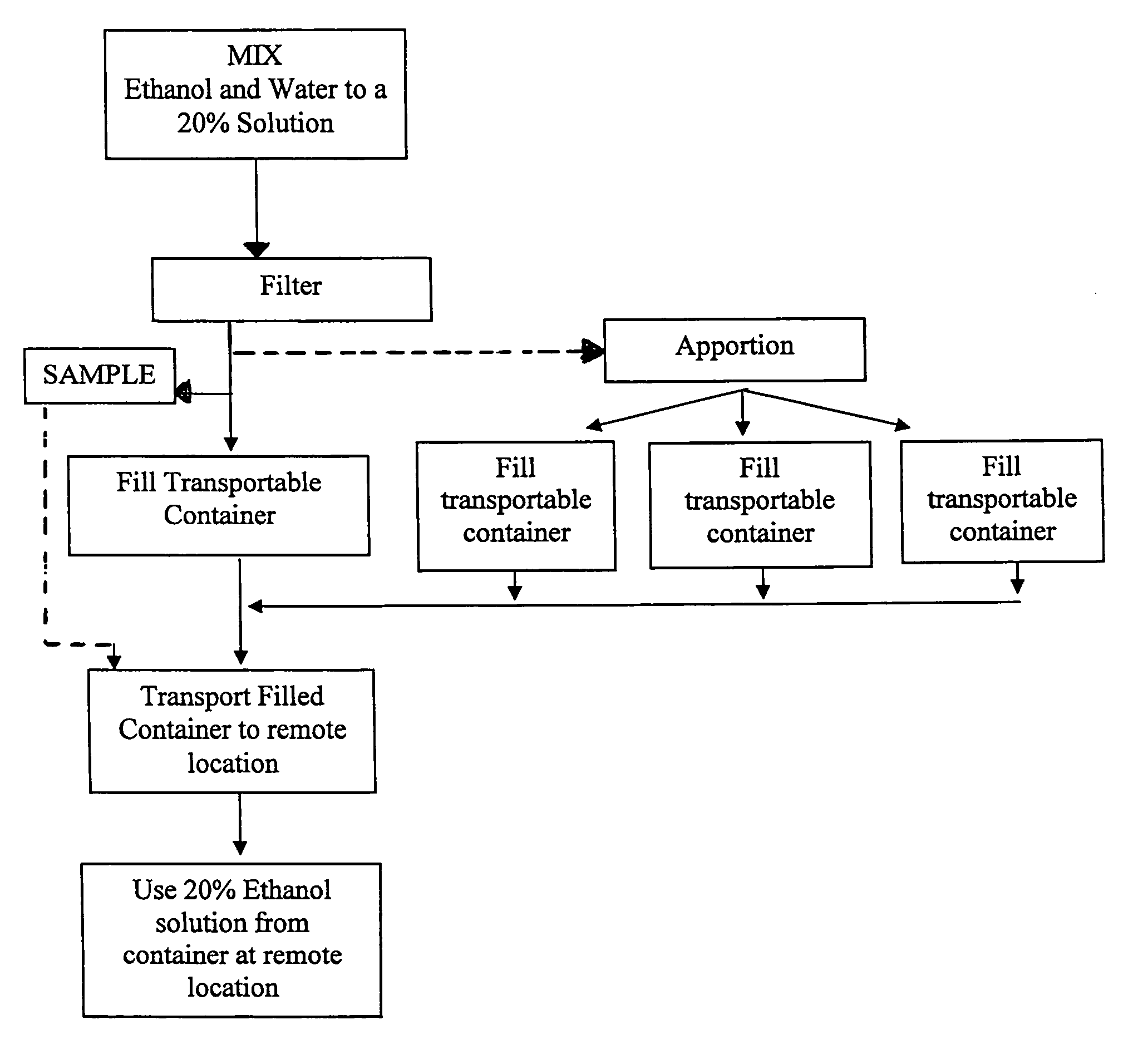
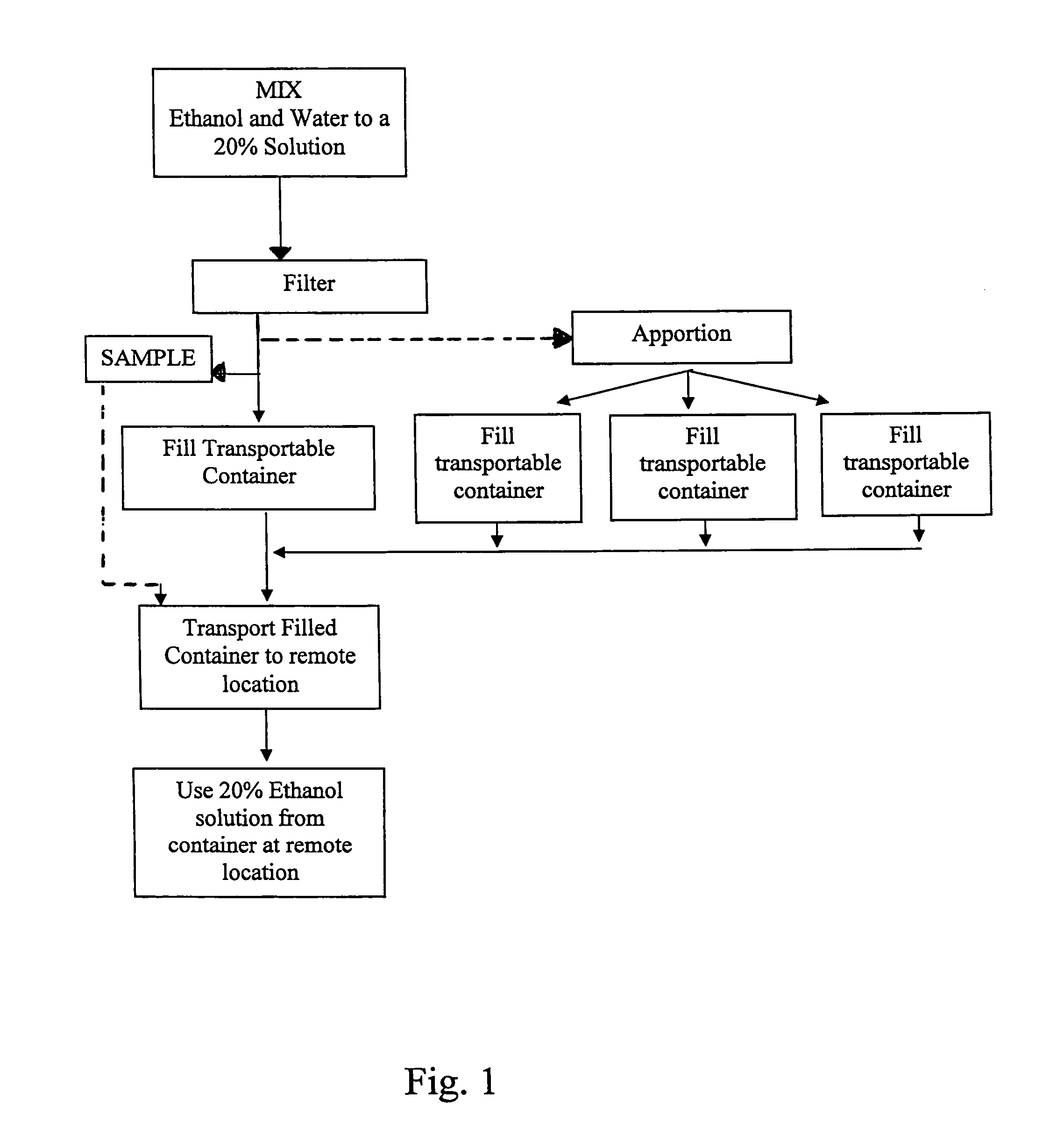
System for providing 20% ethanol solutions that meet bioburden and endotoxin requirements
InactiveUS8003768B1Meet the requirementsSafe and efficientIon-exchanger regenerationLarge containersReady to useBioburden
A system and method for providing 20% ethanol solutions meeting bioburden and endotoxin specifications, which provides the 20% ethanol solution in a ready-to-use form which may be dispensed directly from the container in which the solution is shipped, and which may be used in connection with storage, reuse and / or rejuvination of Protein A.
Owner:DECON LABS
Treatment method and system for wastewater containing polyacrylamide
ActiveCN109502933AImprove biodegradabilityConducive to habitatWater contaminantsWater/sewage treatment with mechanical oscillationsActivated carbonChemical reaction
The invention provides a treatment method and system for wastewater containing polyacrylamide. The treatment method mainly comprises following steps: 1), biological supported particles are put in wastewater for impregnation, and adsorb polyacrylamide and indigenous microorganisms in the wastewater; 2), a microelement solution is added to the biological supported particles obtained in step 1), andthe indigenous microorganisms are promoted to perform anaerobic degradation on polyacrylamide; 3), activated carbon and biological sewage obtained in step 2) are subjected to ozone oxidation, and thetreatment is finished. The system comprises an anaerobic degradation device, an ozone oxidation device and a discharge device which are connected in sequence, and further comprises a biological supported particle reduction device. According to the method and the system, organic combination of a biological method, physical absorption and chemical reaction is realized, the probability of blockage caused by directly introducing polyacrylamide wastewater into a reactor is reduced, ecological destruction caused by polyacrylamide can be reduced fundamentally, activated carbon is repeatedly utilized,and the green, economical, energy-saving and environment-friendly treatment effects are realized.
Owner:OCEAN UNIV OF CHINA
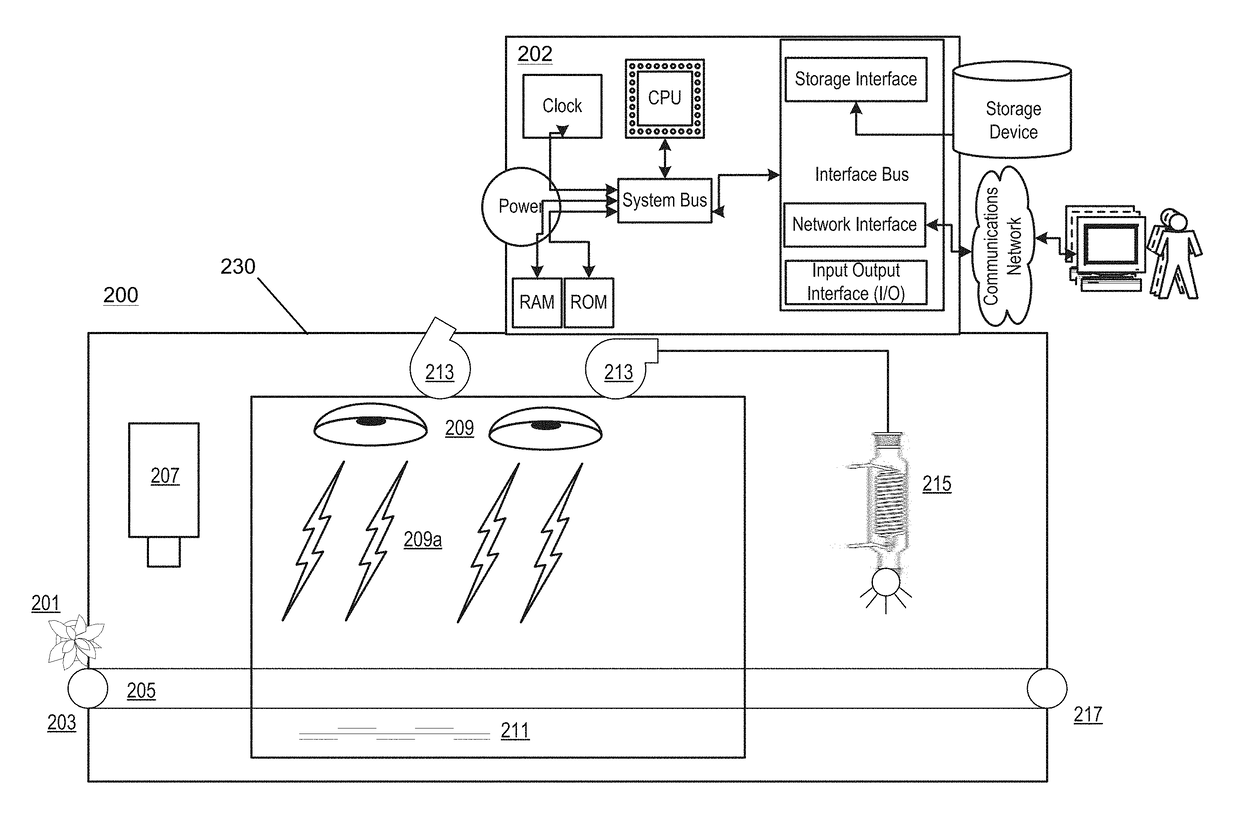
Methods and Apparatus For Low-Pressure Radiant Energy Processing Of Cannabis
ActiveUS20180221522A1Sufficient microbial killingReduce stressDrying solid materials with heatDrying solid materials without heatCannabisMicrowave
Methods and apparatuses for reducing the bioburden of cannabis using low-pressure radiant energy processing. The present invention achieves sufficient microbial killing and / or adequate drying without impacting product quality (terpene loss, smell, color, texture, etc.) by appropriate determination and application of pressure(s) and radiant energy (e.g., microwave intensity).
Owner:TILRAY INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com