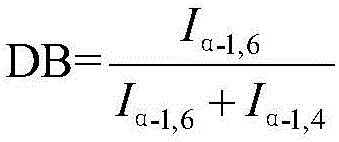Patents
Literature
142 results about "Peritoneal dialysis solutions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The two types of peritoneal dialysis differ mainly in the schedule of exchanges. In continuous ambulatory peritoneal dialysis (CAPD), the patient empties a fresh bag of dialysis solution into the abdomen. After 4 to 6 hours of dwell time, the patient returns the solution containing wastes to the bag.
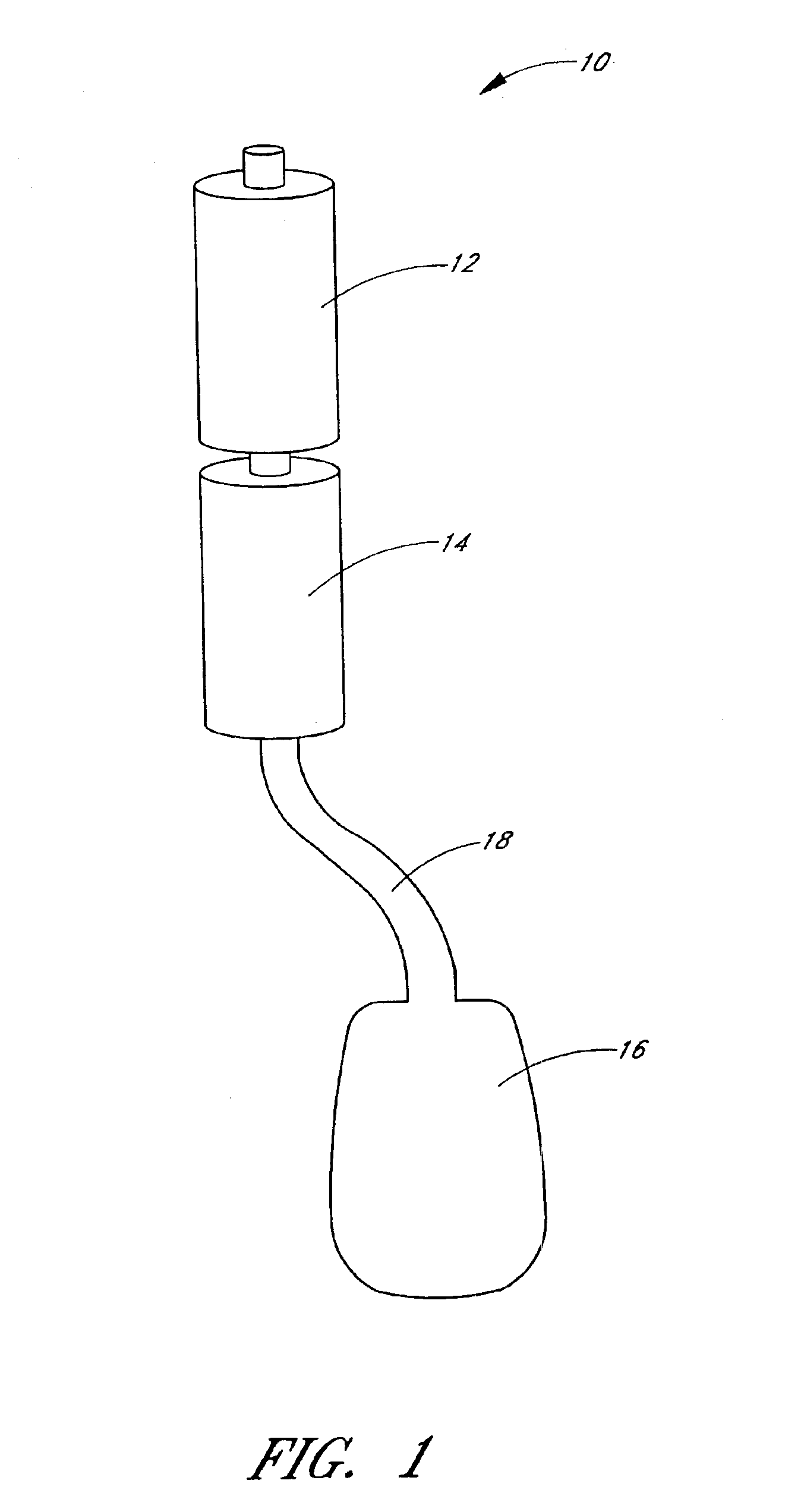
Artificial kidney dialysis system
ActiveUS20080051696A1Cumbersome to wearImprove the quality of lifeMedical devicesPeritoneal dialysisNephrosisMetabolite
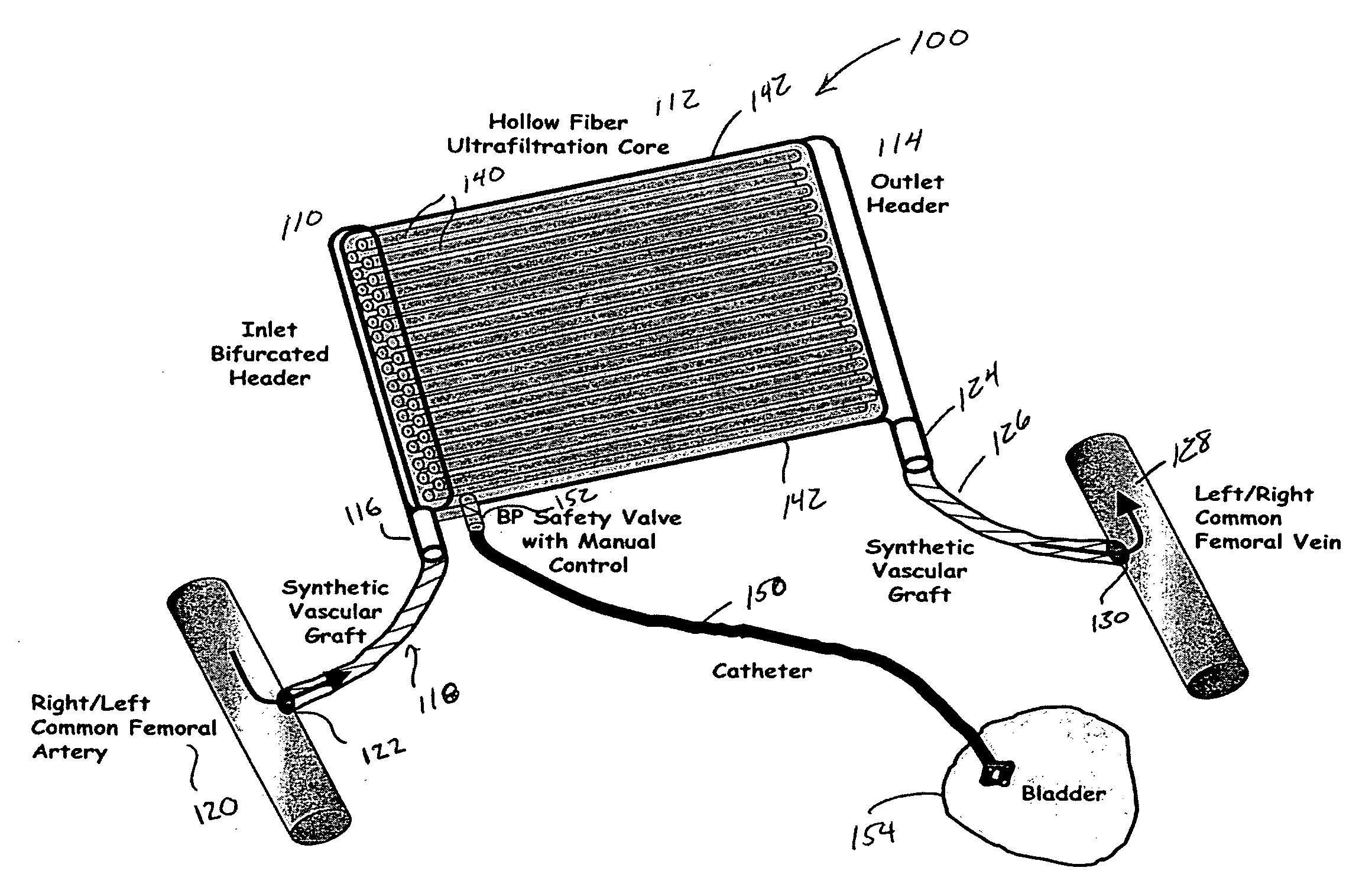
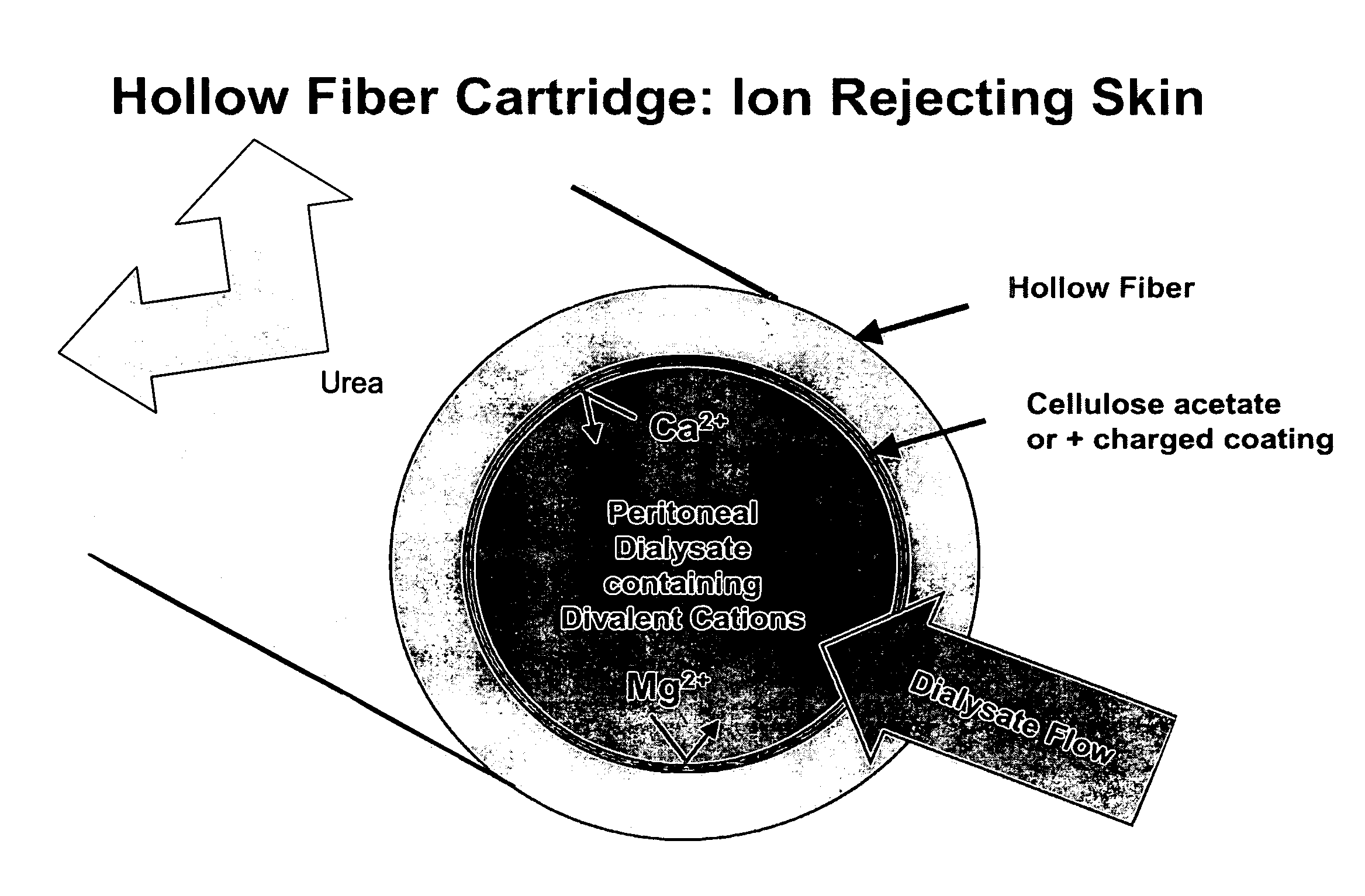
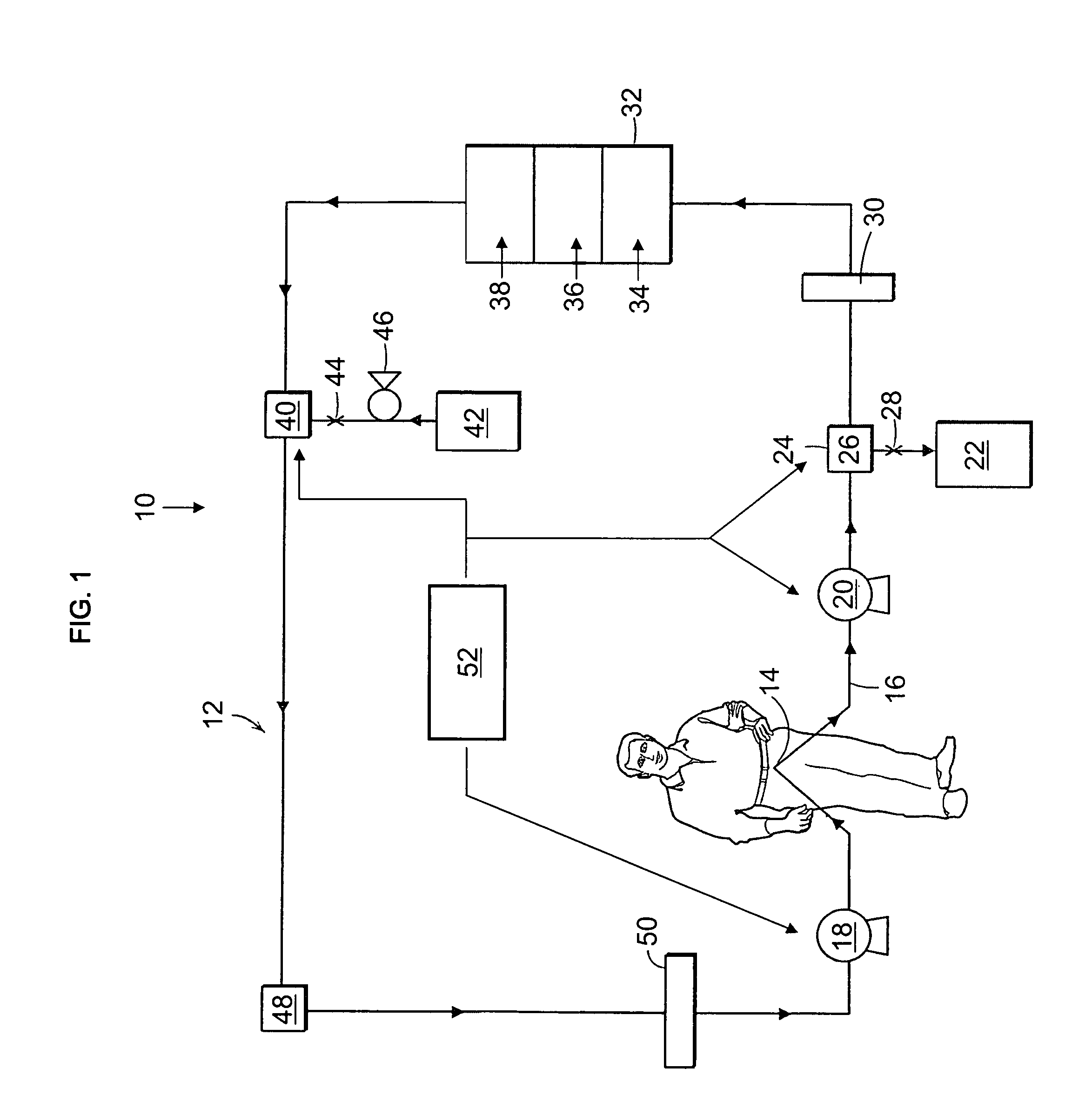
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Peritoneal dialysis method
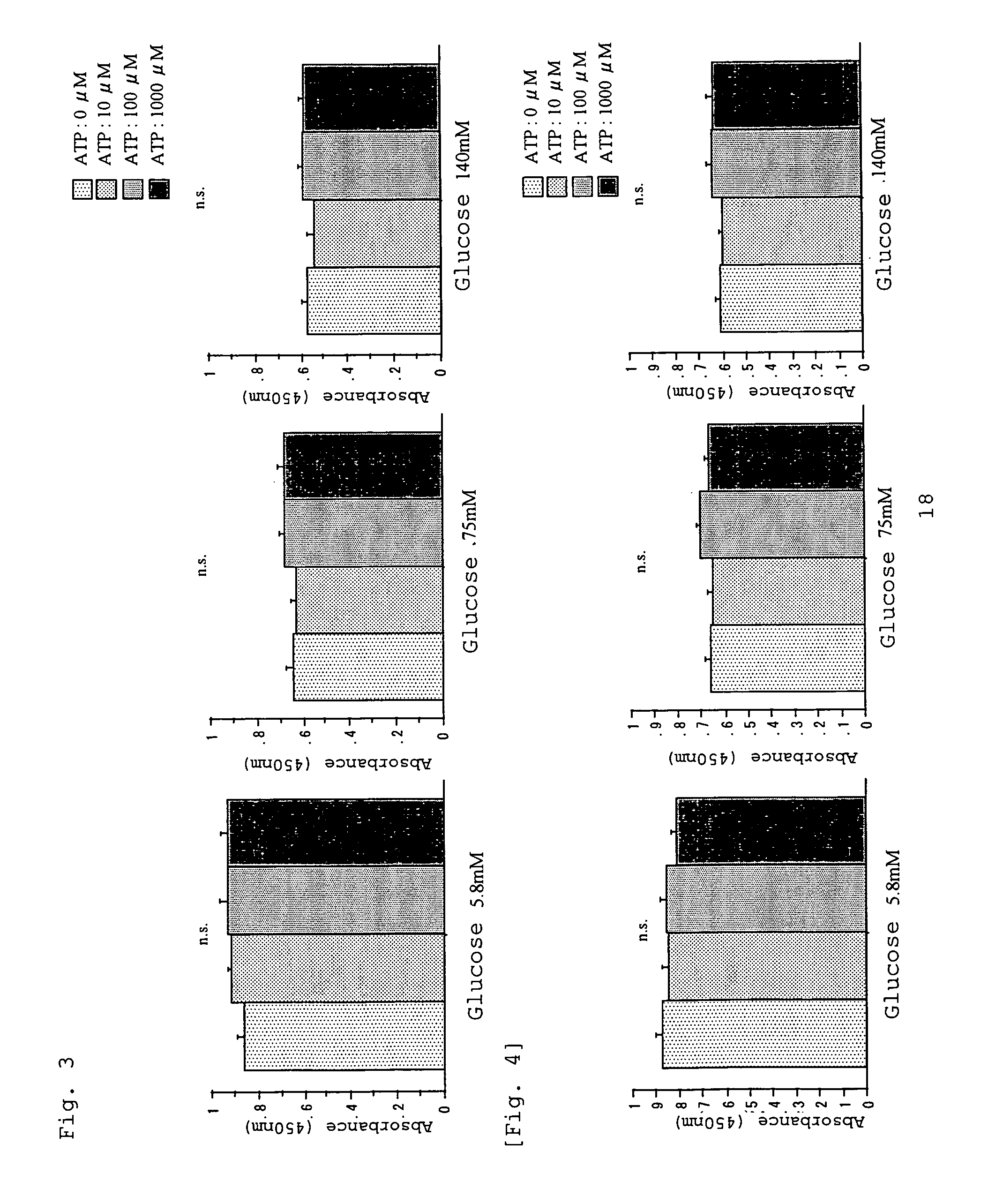
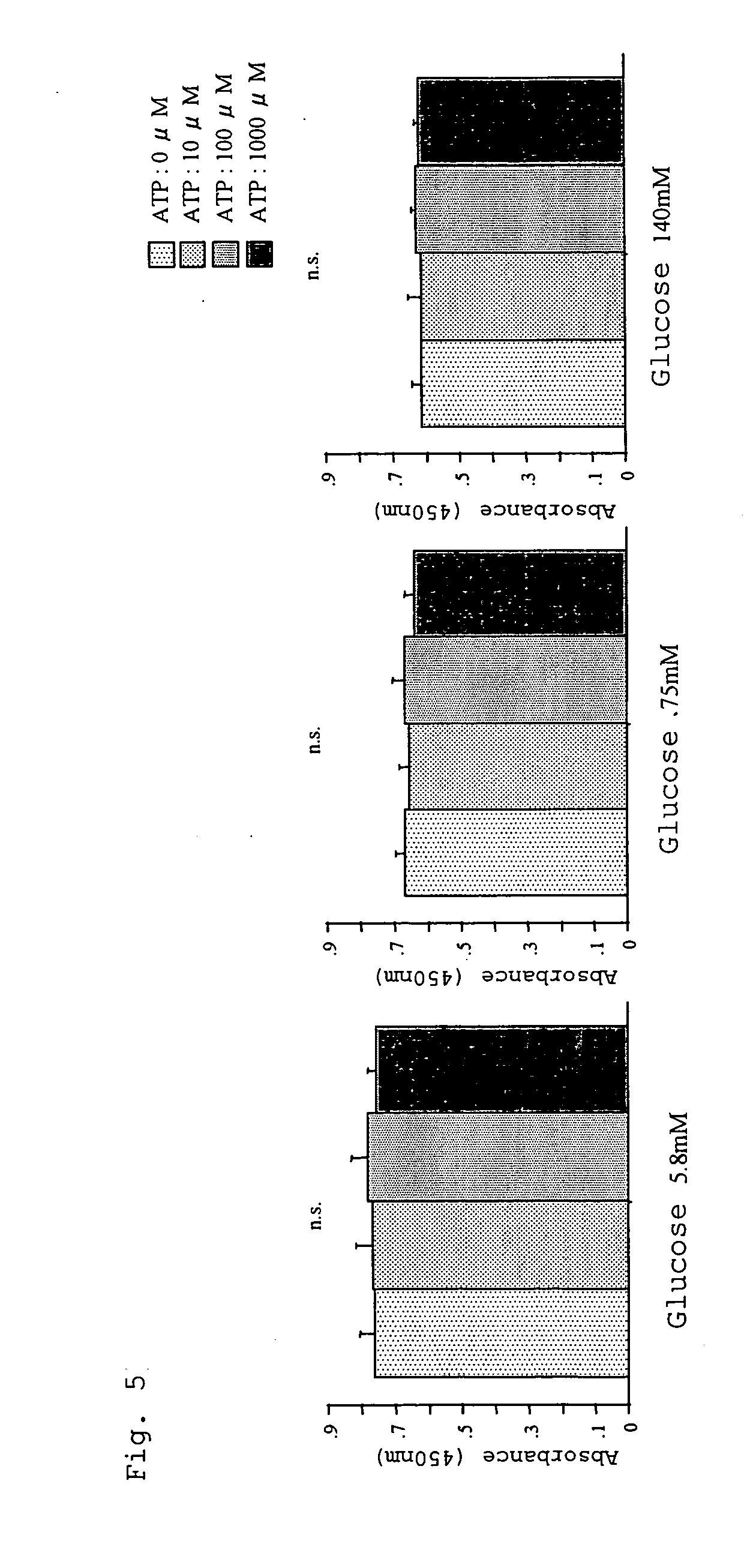
InactiveUS20060019925A1Less peritoneal injuryAvoid excessive injuryBiocideAntipyreticIntensive care medicineDialysis fluid
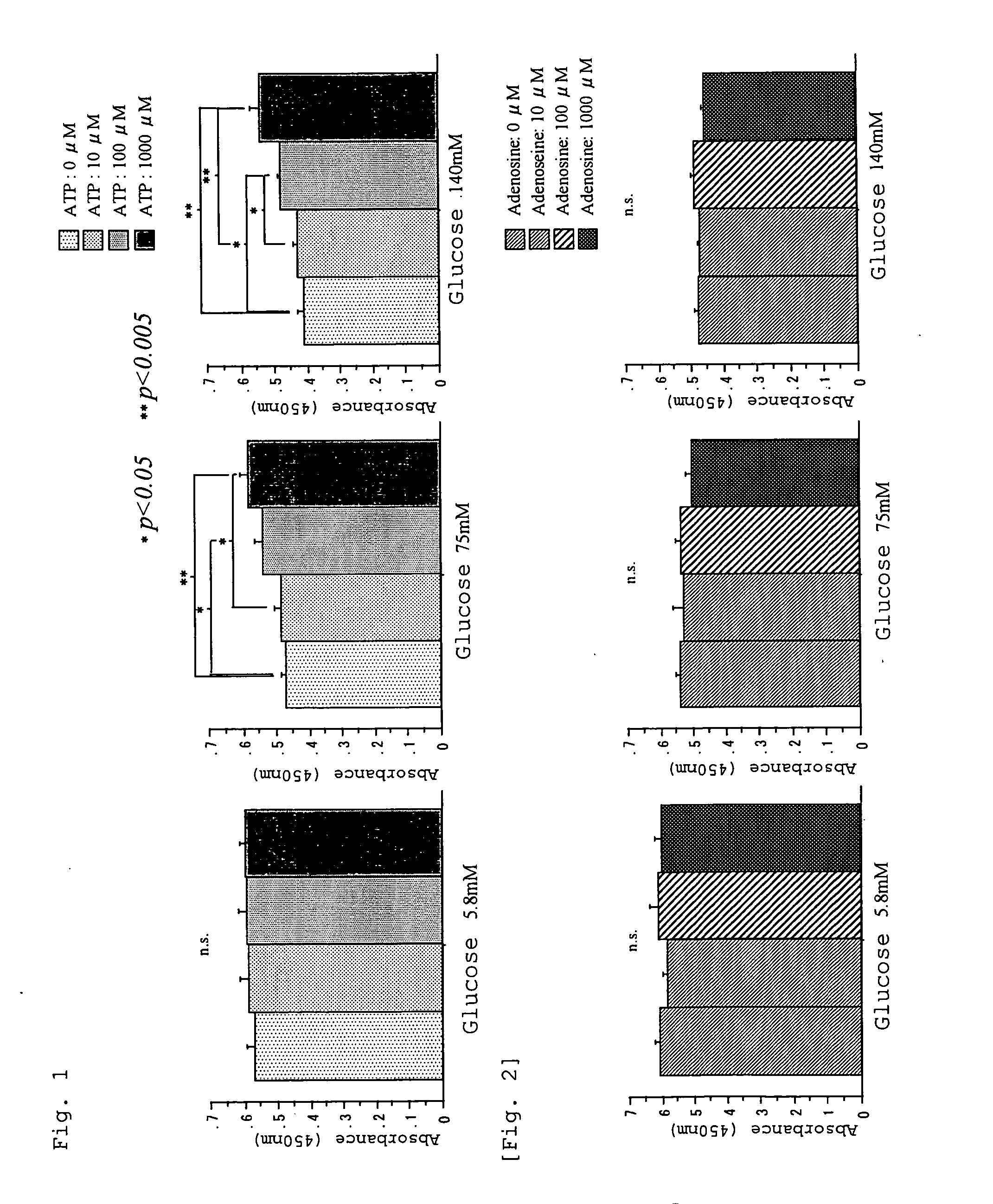
A peritoneal dialysate containing adenosine triphosphate or a salt thereof, and a peritoneal dialysis method using the dialysate. The peritoneal dialysate is safe and causes less peritoneal injuries even when employed in peritoneal dialysis over a long period of time.
Owner:KOWA CO LTD
Dialysis solution for peritoneal dialysis
InactiveUS6284140B1Easy to degradeDwell timeBiocideSolvent extractionHydroxyethyl starchUltrafiltration
The present invention relates to dialysis solutions for peritoneal dialysis, containing hydroxyethyl starch as the osmotically-active substance, electrolytes and, optionally, conventional additives, where the hydroxyethyl starch has a molecular weight Mw in the range from 10,000 to 150,000, a substitution MS in the range from 0.10 to 0.40, a substitution DS in the range from 0.09 to 0.35 and a substitution ratio C2 / C6>=8. With this peritoneal dialysis solution it is possible, with an outstanding ultrafiltration, to maintain a longer dwell time, for example the dialysis solution can be utilized for a period of 12 hours in the CAPD without replacement. In addition, the inventive dialysis solution is also particularly advantageous for patients with residual kidney function. The resorption of the osmotically active substance is clearly diminished and even after a dwell time of 12 hours it amounts to a maximum of 60-70%.
Owner:FRESENIUS AG
Artificial kidney dialysis system
The present disclosure relates to a wearable dialysis system and method for removing uremic waste metabolites and fluid from a patient suffering from renal disease. Uremic waste metabolites can be removed by a wearable peritoneal dialysis device that regenerates the peritoneal dialysis solution without removing positively charged, essential ions from the solution and, consequently, the patient. Fluids can be removed from the blood of the patient by an implantable fluid removing device. Fluids are delivered to the bladder and preferably removed from the body of the patient through urination. The wearable dialysis system may be operated continuously or semi-continuously and be comfortably adapted to the body of the patient while allowing the patient to perform normal activities.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Powered sterile solution device
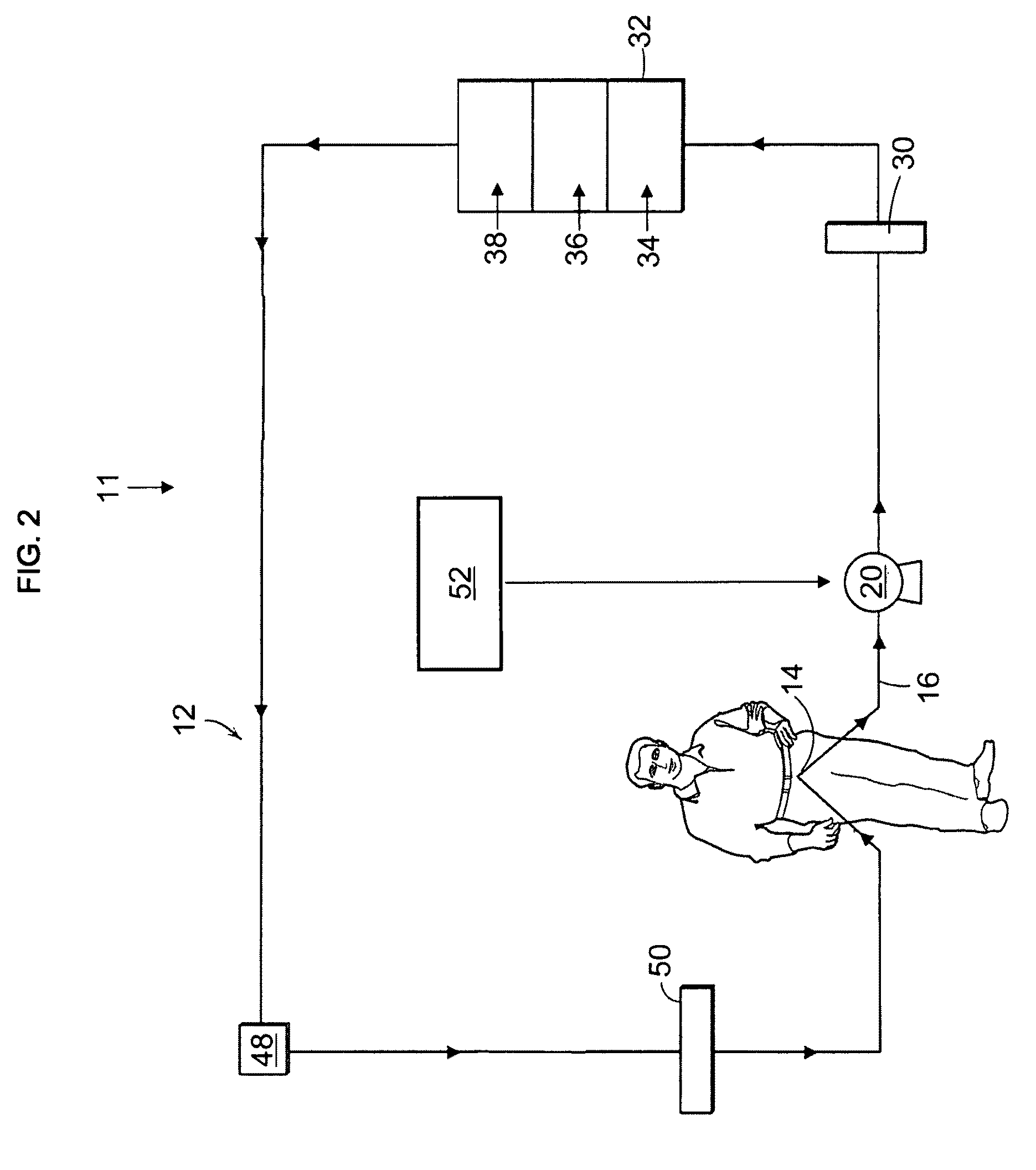
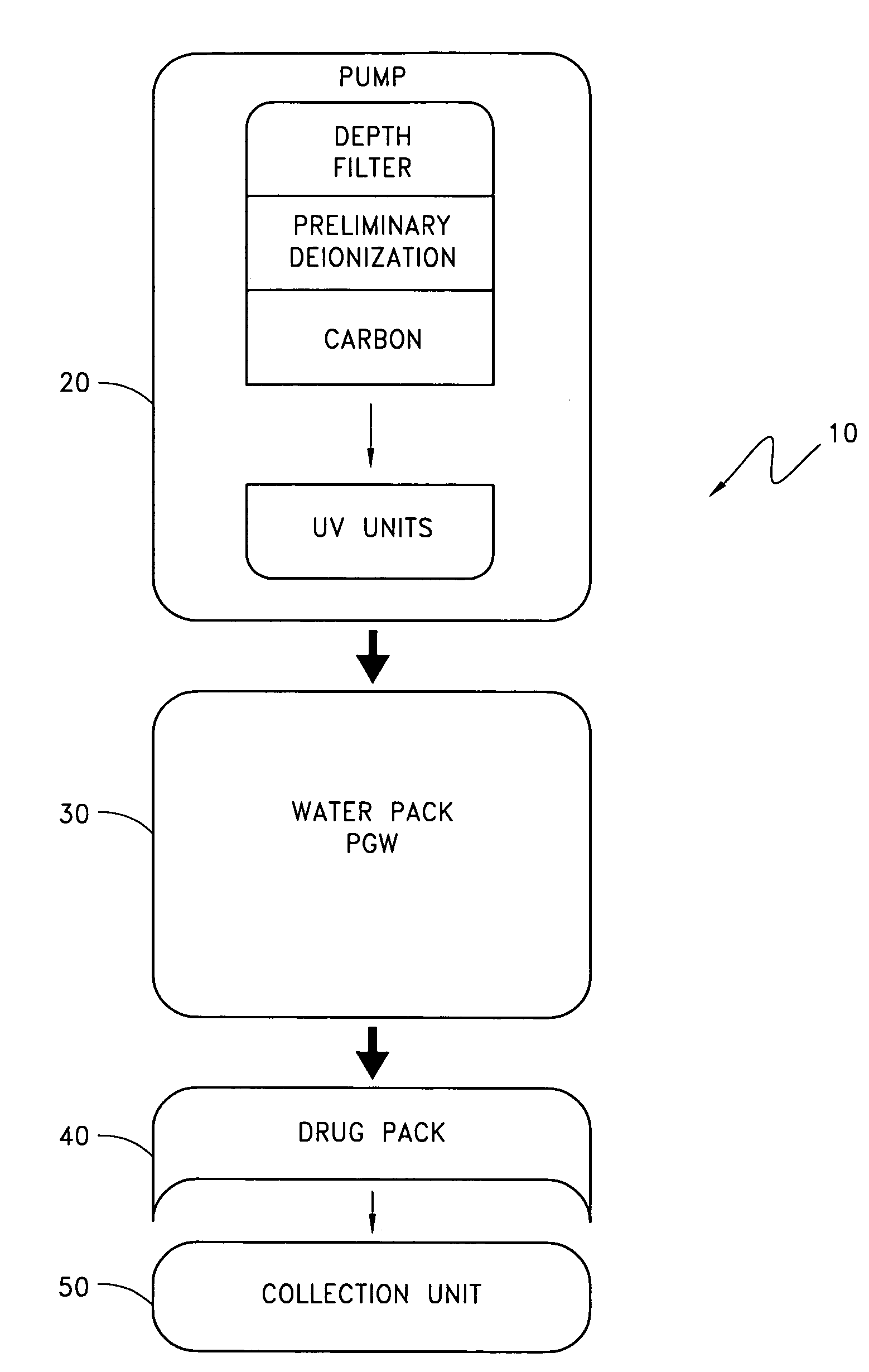
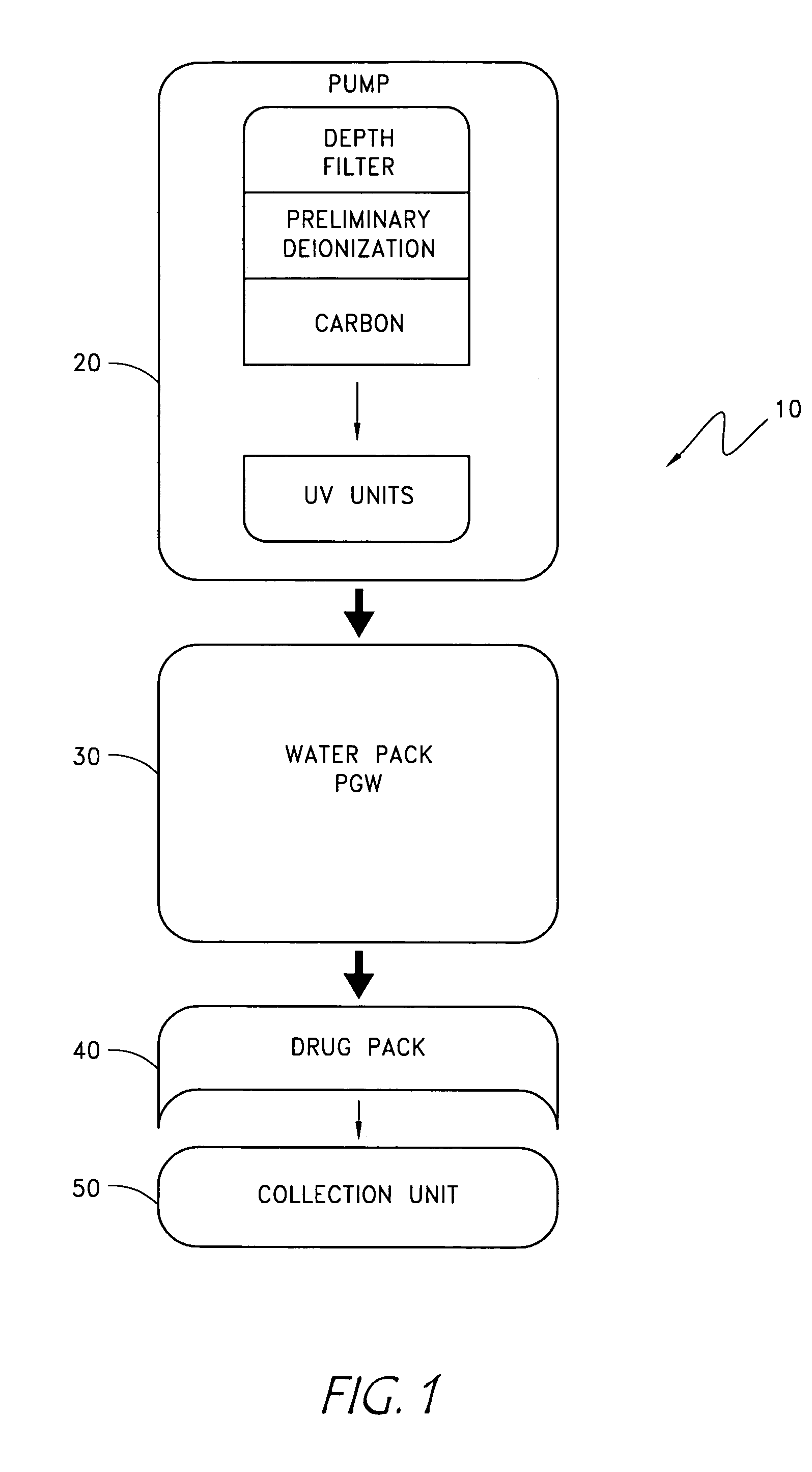
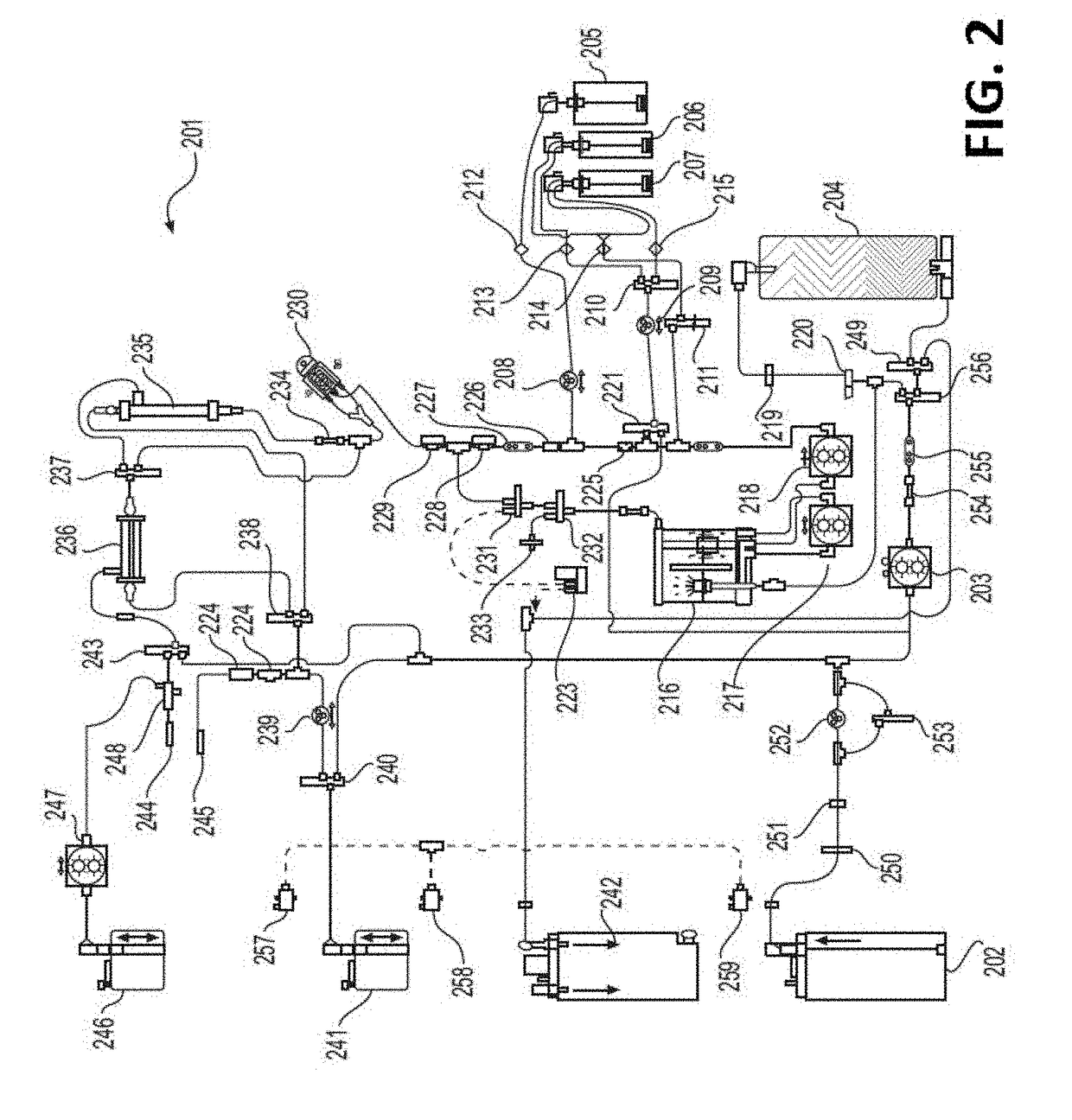
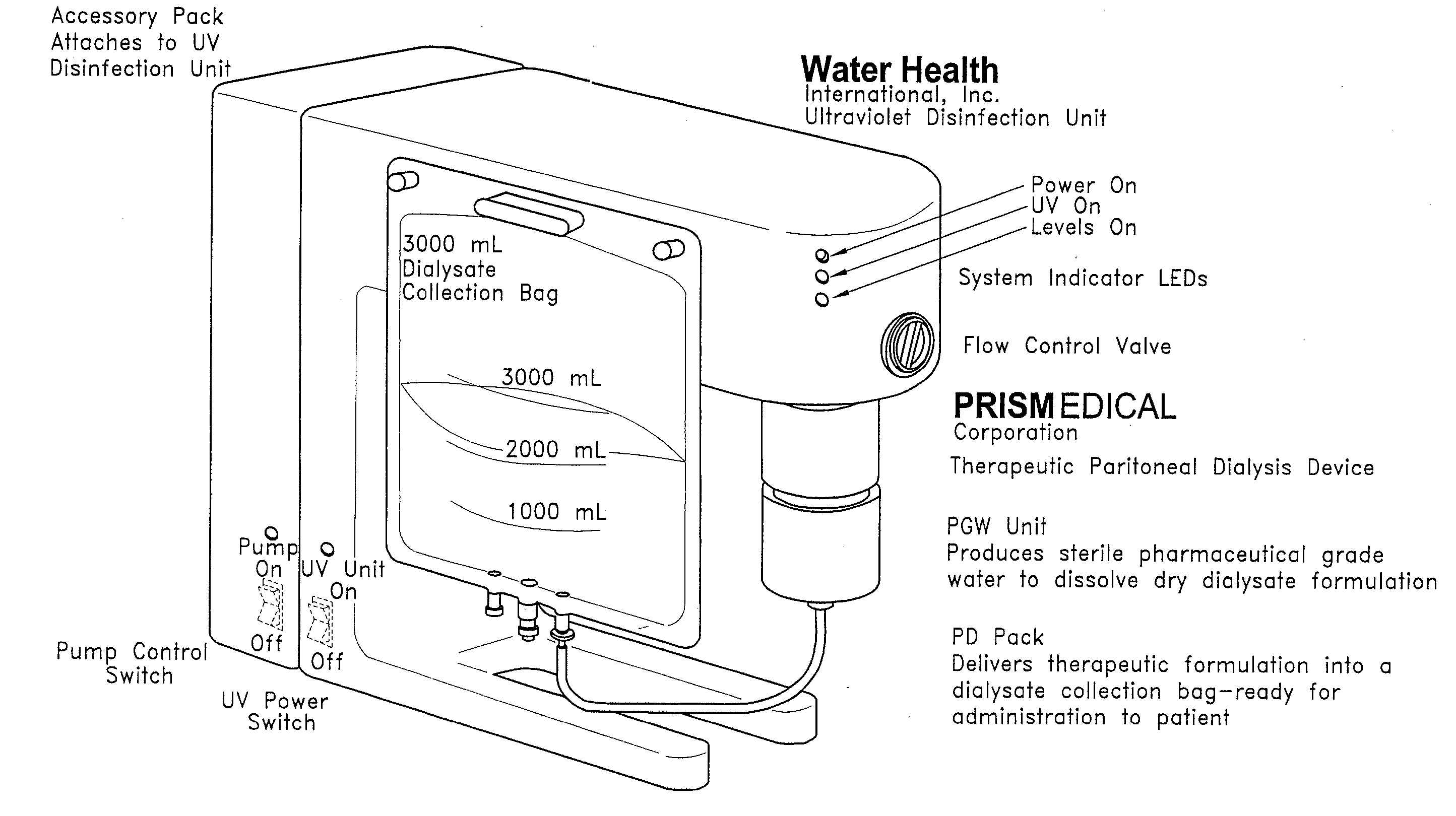
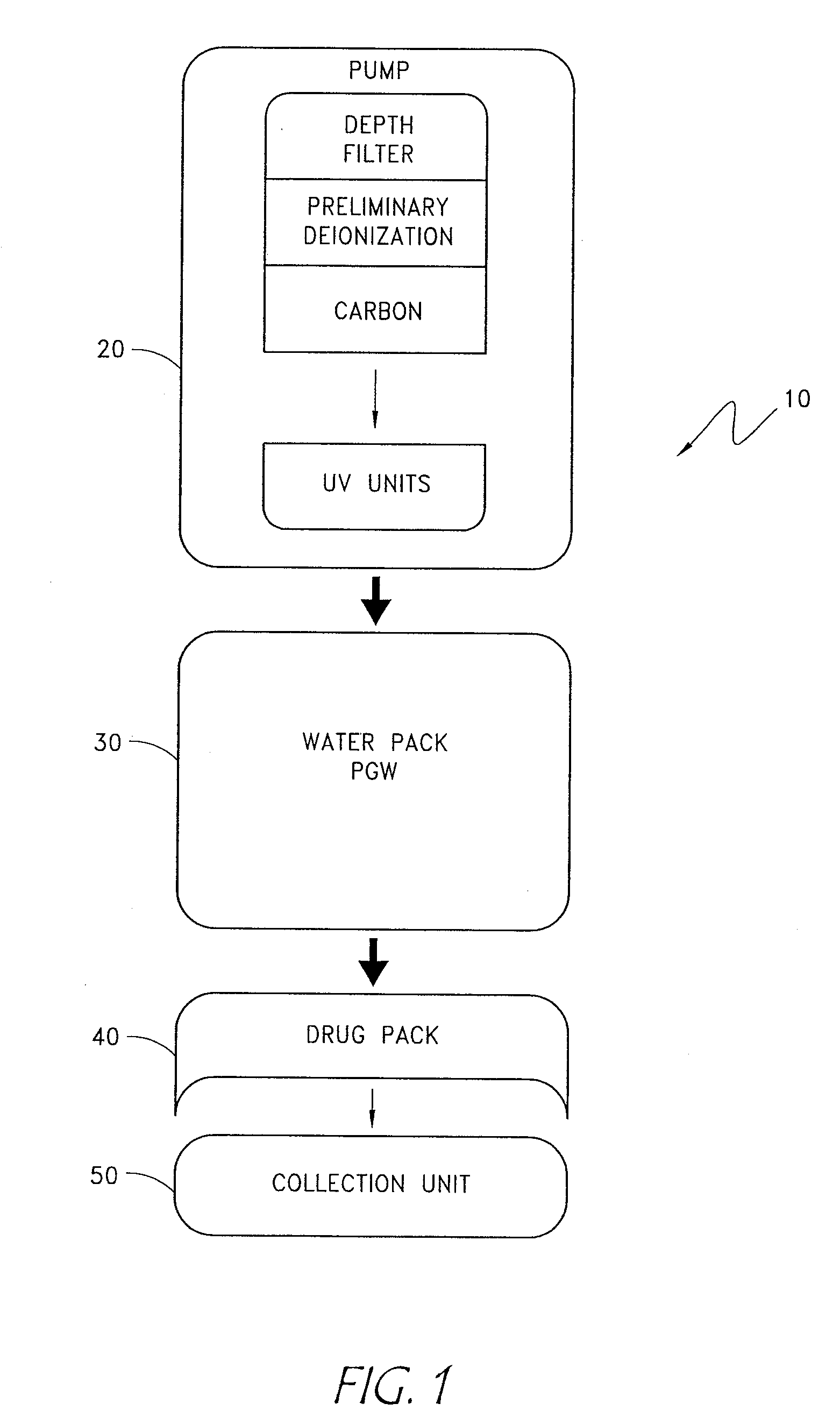
The disclosure below relates to apparatus and methods for producing medicament using sub-optimal water sources. One embodiment of the disclosure is directed to an apparatus comprising a preliminary purification component, a disinfection component, a pharmaceutical grade water preparation (PGW) component, and a drug pack. Another disclosed embodiment relates to a method for producing a peritoneal dialysis solution, comprising, passing diluent through a preliminary purification component, passing diluent through a disinfection component, passing diluent through a PGW preparation component, passing diluent through a drug pack, and collecting solute produced by the drug pack.
Owner:WATERHEALTH INTERNATIONAL +1
Biochemically balanced peritoneal dialysis solutions
InactiveUS7011855B2Improved peritoneal dialysis solutionAvoid lostBiocideSolvent extractionMedicinePeritoneal dialysis solutions
A peritoneal dialysis solution that is biochemically balanced to correct metabolic acidosis associated with chronic renal failure in a more physiological manner. The peritoneal dialysis solution has a physiological pH, e.g., pH of 7.0 to 7.4, and contains bicarbonate at a concentration that is found in normal blood. Additionally, the solution contains carbon dioxide at a partial pressure that is similar to partial pressure of carbon dioxide found in normal blood. The peritoneal dialysis solution also contains a weak acid with a pKa of less than 5.0.
Owner:BAXTER INT INC
Method for testing peritoneal function
InactiveUS7303541B2Efficiently and accurately evaluate the current conditionVaccination/ovulation diagnosticsMedical devicesPeritoneal equilibration testAbdominal cavity
Owner:JMS CO LTD
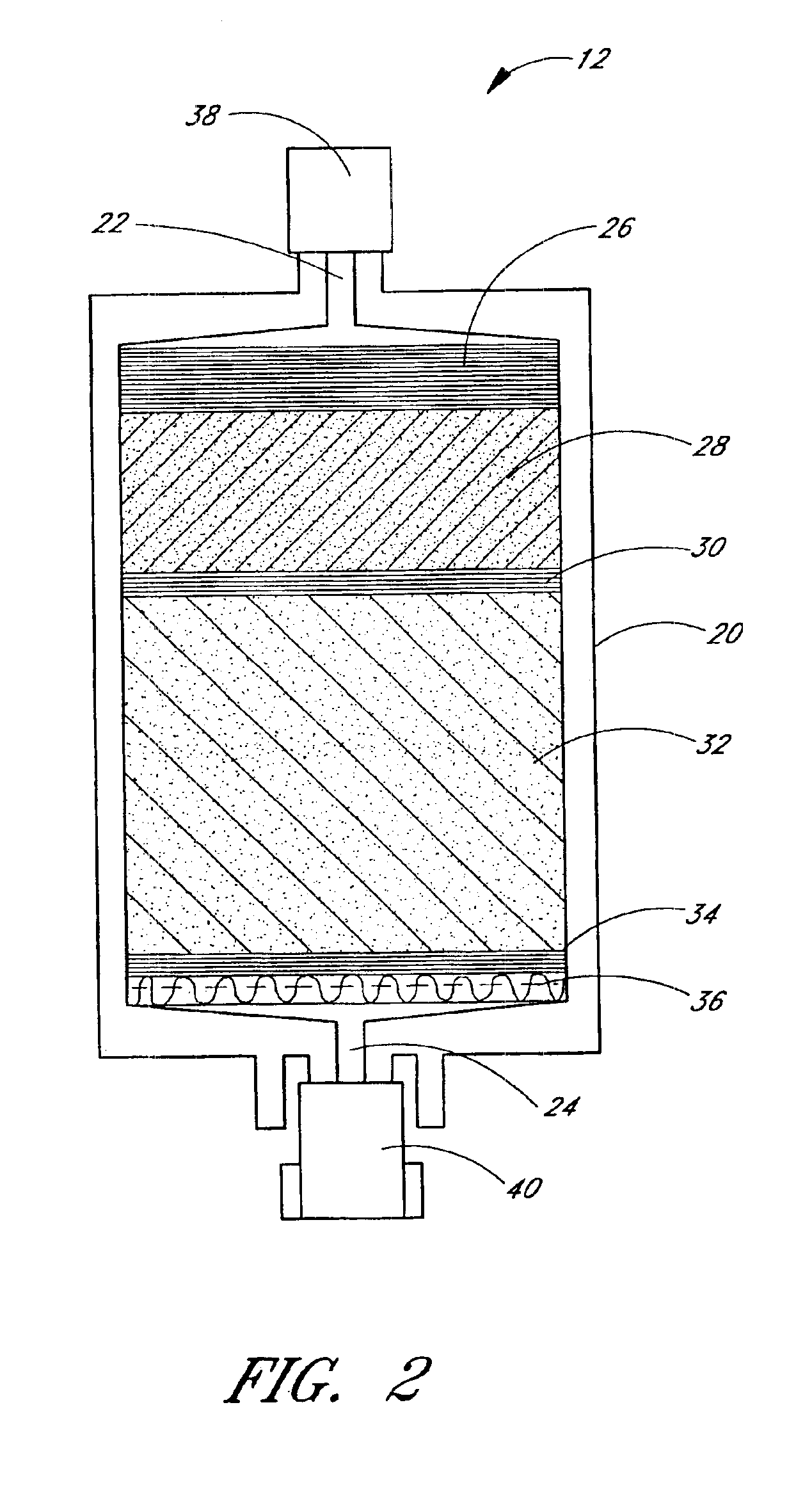
Apparatus and method for preparation of a peritoneal dialysis solution
InactiveUS6986872B2Minimize the possibilityEasy to separateGeneral water supply conservationDiagnosticsPeritoneal dialysateMagnesium salt
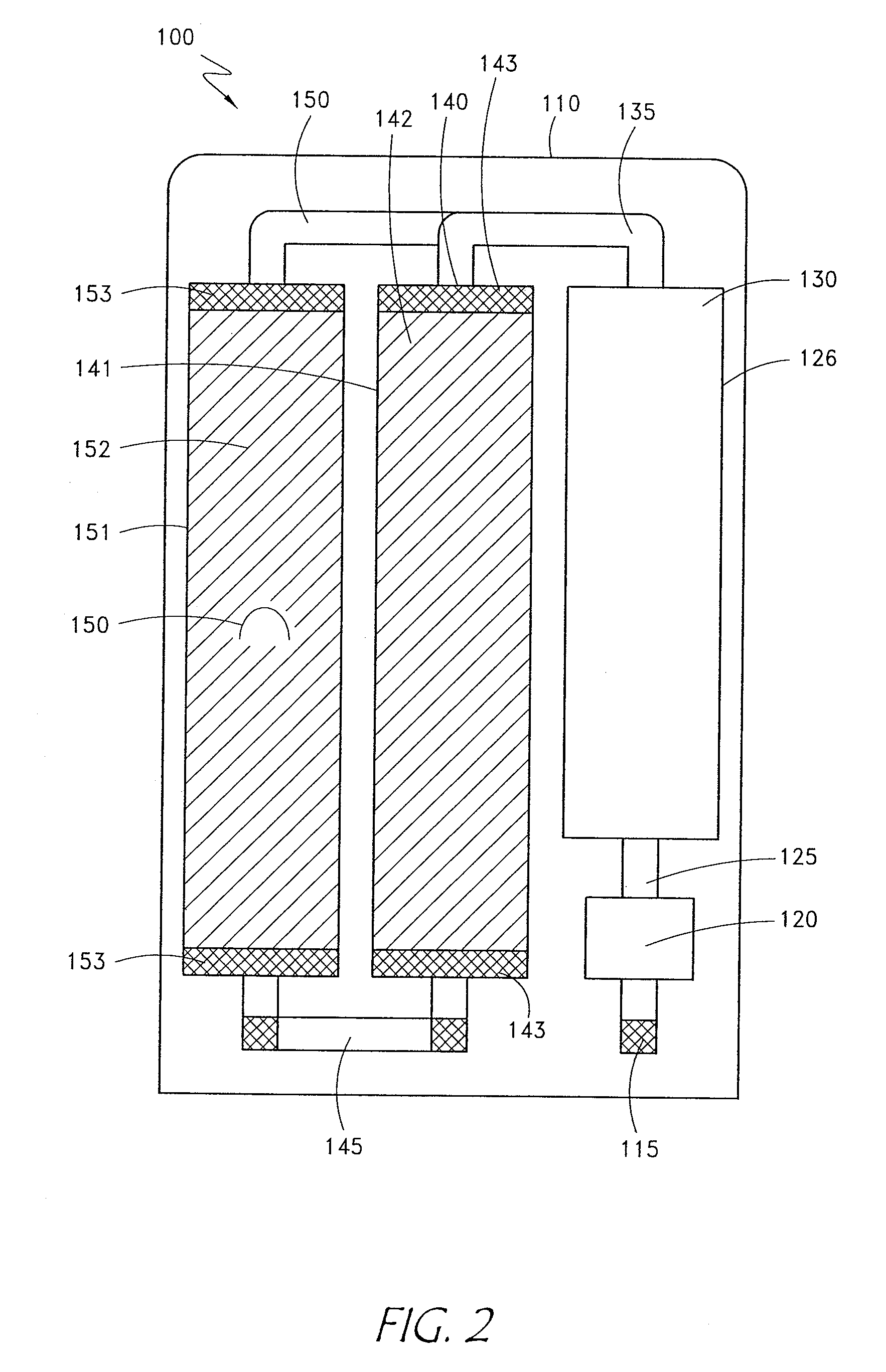
The invention provides an apparatus and method for storing and transporting peritoneal dialysate in dry or lyophilized form, and for forming a deliverable peritoneal dialysis solution therefrom. In one embodiment, a dry reagent bed, including reagents sufficient to produce a dialysis solution, is suspended in a diluent flow path through the apparatus housing. Continuous pressure on the reagent bed causes the bed to compact as it erodes when purified water is passed through the housing. The pressure ensures complete and even dissolution of the reagents. Through dry storage and simple dissolution, even in a home, the invention enables a wider variety of solution constituents, including reduced acid content and the use of bicarbonate as a stable buffer component. The latter is illustrated in a double-bed embodiment, where bicarbonate is stored separately from calcium or magnesium salts within a single housing.
Owner:PRISMEDICAL CORP
Fluid for peritoneal dialysis
InactiveUS20140148409A1Good biocompatibilityImprove removabilityBiocideOrganic active ingredientsBiocompatibility TestingBody fluid
The present invention has an object to provide a fluid for peritoneal dialysis with satisfactory body fluid removability, high biocompatibility, and improved storage stability, and the object is attained by a fluid for peritoneal dialysis containing one or more saccharides selected from cyclonigerosylnigerose, cyclomaltosylmaltose, and L-ascorbic acid 2-glucoside.
Owner:HAYASHIBARA BIOCHEMICAL LAB INC
Regenerative peritoneal dialysis system
PendingUS20170281847A1Organic active ingredientsInorganic active ingredientsEngineeringMechanical engineering
Systems and methods of generating and regenerating peritoneal dialysate are provided. The systems and methods use a dialysate regeneration module, a sterilization module and concentrates to prepare peritoneal dialysate from used peritoneal dialysate or source water. An optional integrated cycler for direct infusion of the generated peritoneal dialysate is included. Optional dialysate storage containers are provided for storage of the peritoneal dialysate prior to use.
Owner:MOZARC MEDICAL US LLC
Peritoneal dialysate fluid generation system with integrated cycler
PendingUS20170281846A1Specific water treatment objectivesMedical devicesPeritoneal dialysis solutionsDialysis fluid
Systems and methods of generating peritoneal dialysate and using the peritoneal dialysate with an integrated cycler are provided. The systems and methods use a water purification module, a sterilization module and concentrates to prepare peritoneal dialysate from source water and infuse the prepared peritoneal dialysate into a patient with an integrated cycler. Optional dialysate storage containers are provided for storage of the peritoneal dialysate prior to use.
Owner:MOZARC MEDICAL US LLC
Low-sodium peritoneal dialysis liquid
InactiveCN101485683AImprove scalabilityPromote popularizationHydroxy compound active ingredientsPeptide/protein ingredientsMedicineChloride
The invention discloses a low-sodium peritoneal dialysis solution. The solution does not contain bicarbonate ions, and components of the solution calculated as mmol / L comprise the following: 124 to 128 mmol / L of sodium ions, 1 to 2 mmol / L of calcium ions, 0.2 to 0.8 mmol / L of magnesium ions, 90 to 110 mmol / L of chloride ions, 37 to 47 mmol / L of lactate ions, and a corresponding isotonic agent. The low-sodium peritoneal dialysis solution, during the preparation, overcomes the defect that a special membrane material must be used in order to solve the stability problem of producing pH by decomposing bicarbonate radicals, reduces the cost, and is favorable for further expansion and popularization of clinical application.
Owner:CHENGDU QINGSHAN LIKANG PHARMA CO LTD
Parenteral administration of pyrophosphate for prevention or treatment of phosphate or pyrophosphate depletion
Phosphate depletion, a physiological condition commonly seen in certain patient populations, including alcoholics, malnourished, acutely ill patients, patients receiving parenteral nutrition, patients being re-fed after prolonged fasting, and dialysis patients, requires intravenous supplementation when oral repletion is not feasible. This invention provides a method and pharmaceutical composition for therapeutic administration of pyrophosphate, instead of phosphate, for phosphate or pyrophosphate repletion. During hemodialysis or peritoneal dialysis significant removal of phosphate and pyrophosphate occurs. Pyrophosphate depletion predisposes patients to vascular calcification. This invention further provides a method and pharmaceutical composition for therapeutic administration of pyrophosphate for phosphate or pyrophosphate repletion by addition of pyrophosphate to hemodialysis or peritoneal dialysis solutions.
Owner:GUPTA AJAY
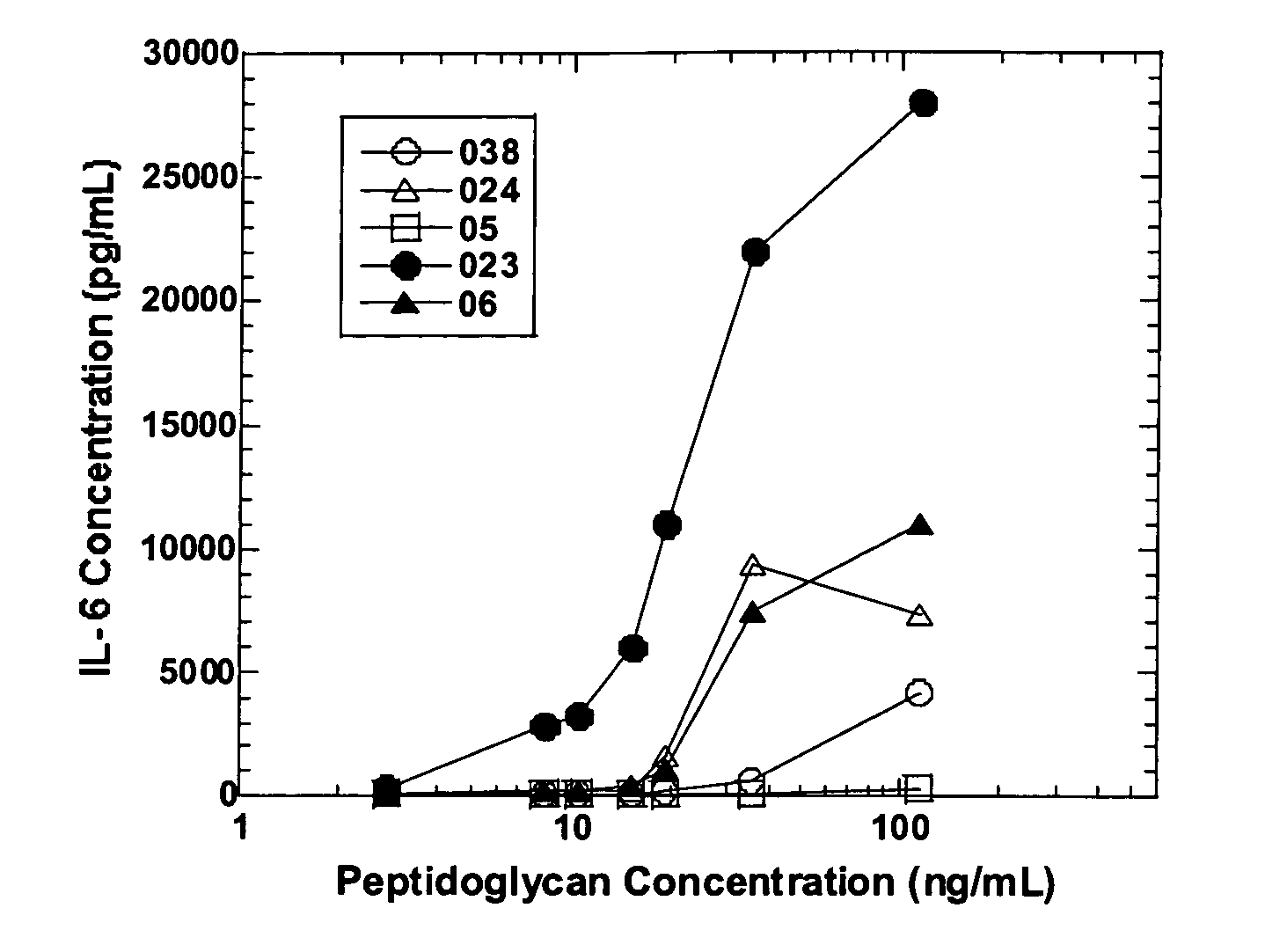
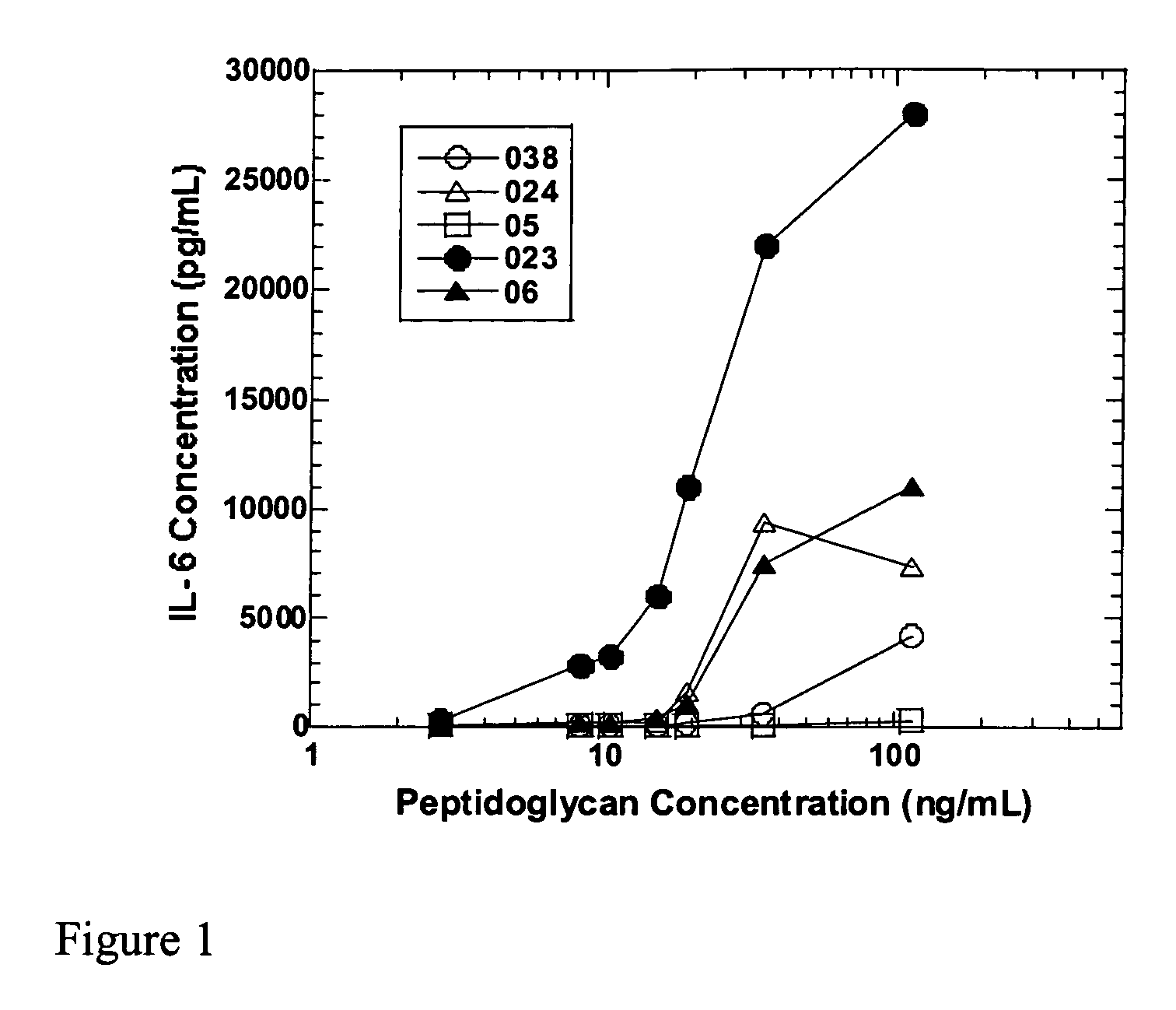
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
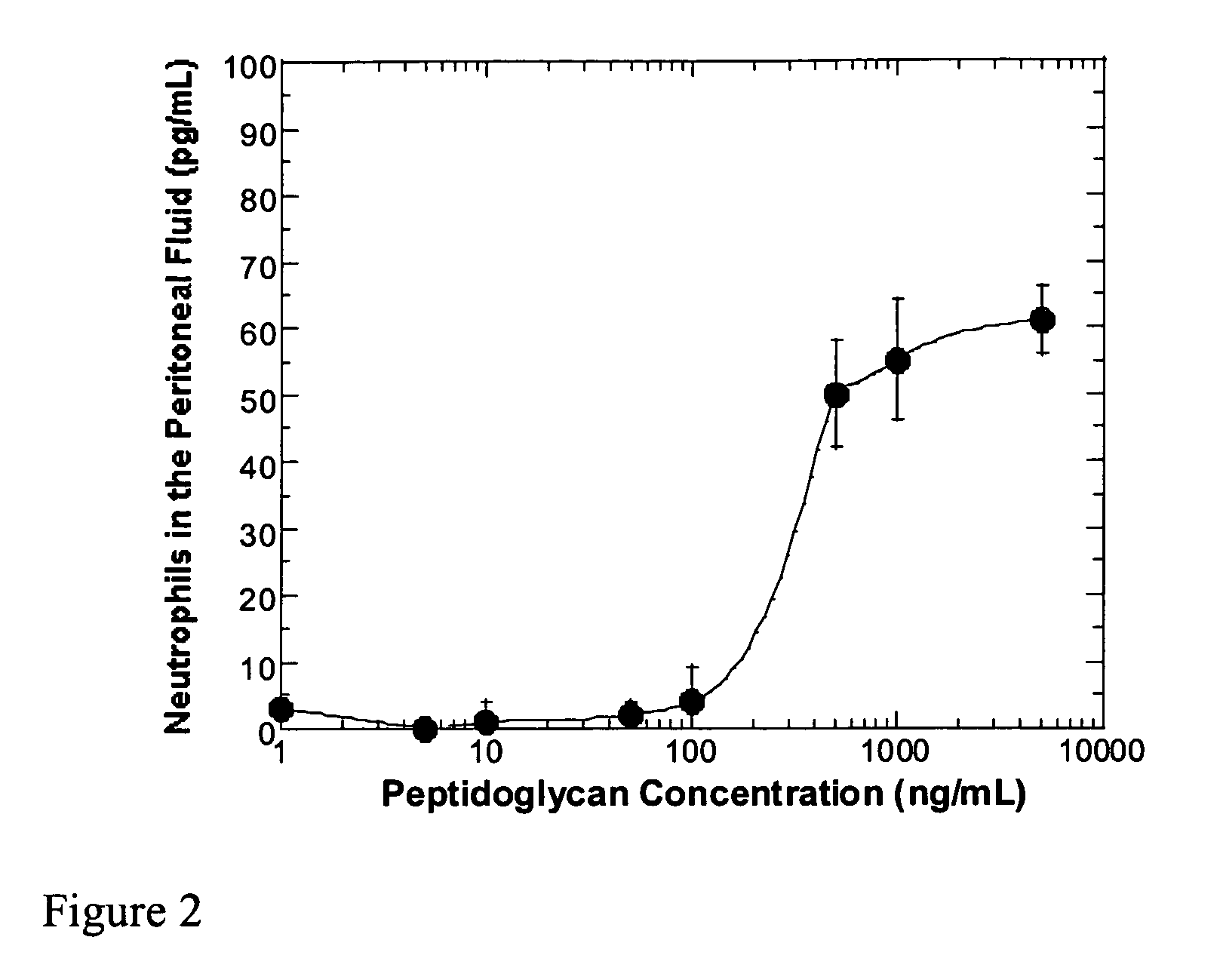
InactiveUS20050191717A1Improved peritoneal dialysis solutionImproved testing procedureAntibacterial agentsBiocideBiologyPeritoneal dialysis solutions
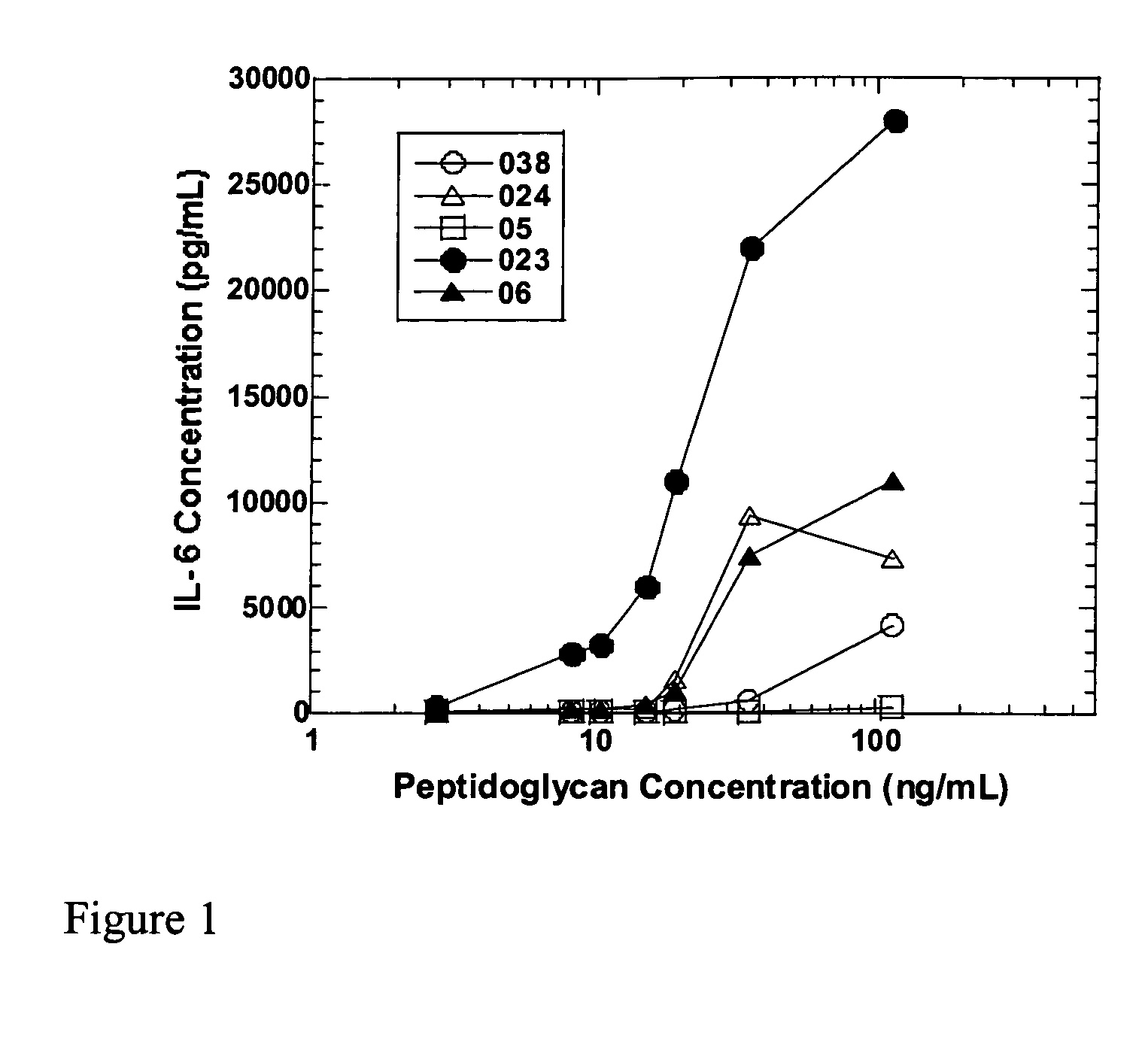
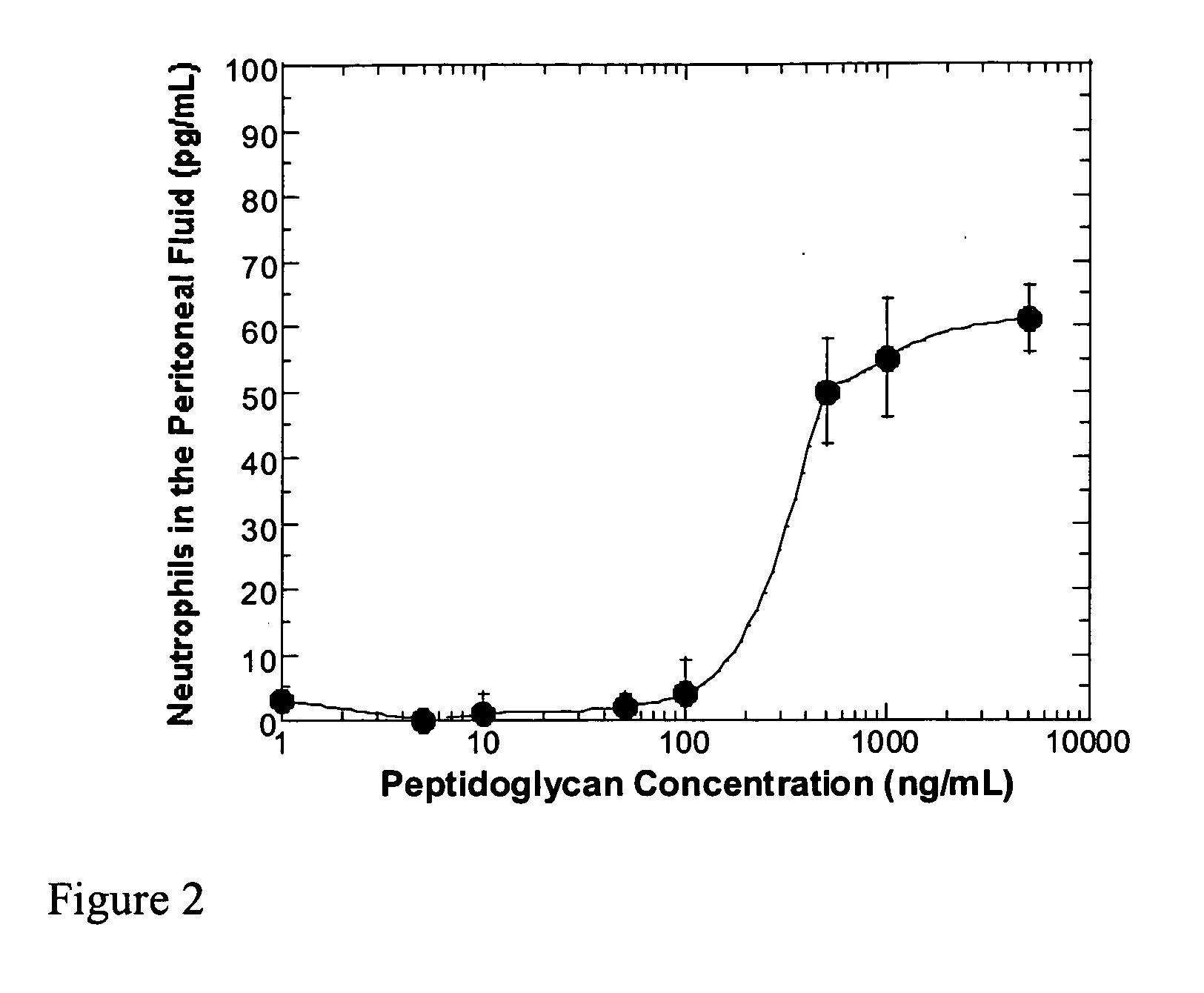
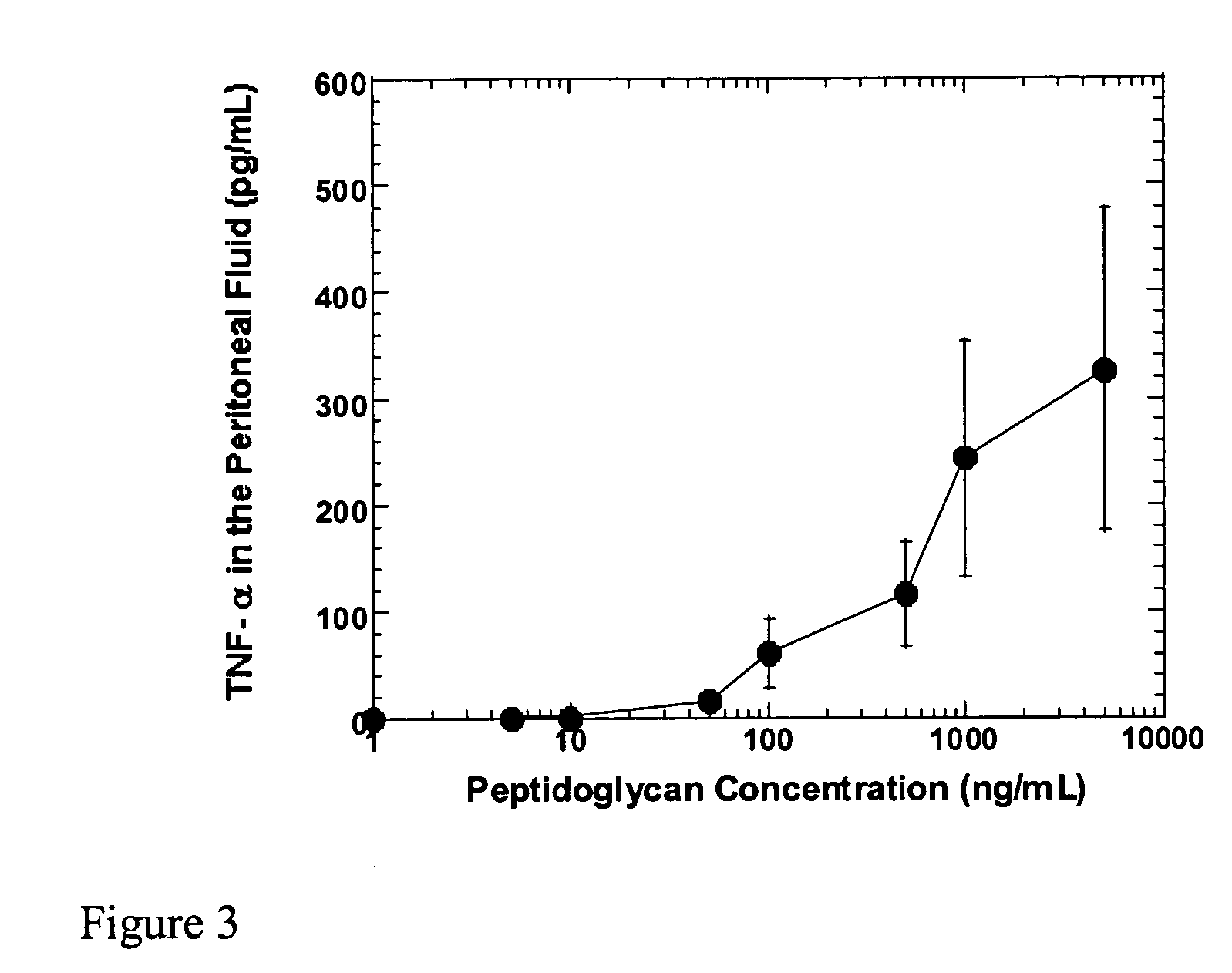
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
Household peritoneal dialysis liquid manufacture equipment
InactiveCN102989047AReduced Pollution ChancesReduce financial burdenPeritoneal dialysisMedical equipmentLiquid storage tank
The invention relates to medical equipment, and in particular relates to household peritoneal dialysis liquid manufacture equipment. The equipment comprises a water purifier, a medical distilled water boiler and a peritoneal dialysis liquid preparation cabinet, wherein the peritoneal dialysis liquid preparation cabinet is divided into an upper chamber and a lower chamber, the upper chamber is a preparation bin which is provided with a powdered preparation container and an ultraviolet sterilization lamp, a peritoneal dialysis liquid storage tank is arranged in the lower chamber, and the upper chamber and the lower chamber are communicated through a tubular connecting part; the water purifier is used for purifying raw water into purified water; the medical distilled water boiler is used for further producing the purified water into distilled water to prepare the peritoneal dialysis liquid; the peritoneal dialysis liquid preparation cabinet is used for mixing peritoneal dialysis liquid concentrate and the prepared injection water in the storage tank strictly in a proportion under an aseptic condition to prepare the peritoneal dialysis liquid. The peritoneal dialysis liquid manufacturing process is carried out in a closed pipeline from the beginning to the end before the peritoneal dialysis liquid which is prepared from the raw water in the water purifier enters a peritoneal dialysis machine, so that the chance of pollution can be reduced. According to the equipment, medical cost for household application can be greatly reduced, economic burden of patients can be reduced, so that the equipment is in accordance with the national condition of China with vast area and scattered patients, and is advantageous to technical popularization of APD (automatic peritoneal dialysis).
Owner:董大泉
Bicarbonate-based peritoneal dialysis solutions
InactiveUS20050276868A1Improved dialysis solutionGood biocompatibilityBiocideUrinary disorderMedicineReady to use
Dialysis solutions and methods of manufacturing and using same are provided. The dialysis solution at least includes three solution parts that are separately stored and sterilized at an effective pH to promote the stabilization of the solution parts including a pH-sensitive solution part that has, for example, a pH-sensitive osmotic agent, such as a glucose polymer. The solution parts are admixed to form a ready-to-use solution with a physiologically acceptable pH.
Owner:BAXTER INT INC +1
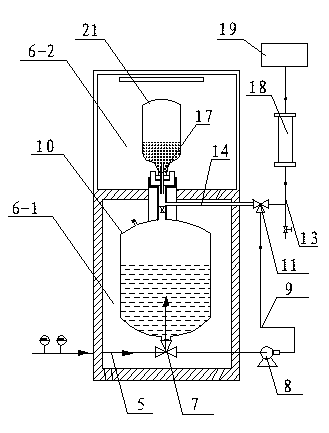
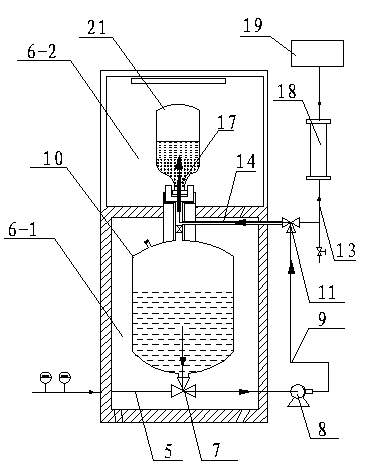
Fully automatic carbonate peritoneal dialysis device and dialysis method
ActiveCN105013031AReduced Chances of ContaminationReduced Pollution ChancesMedical devicesPeritoneal dialysisPolyesterAbdominal cavity
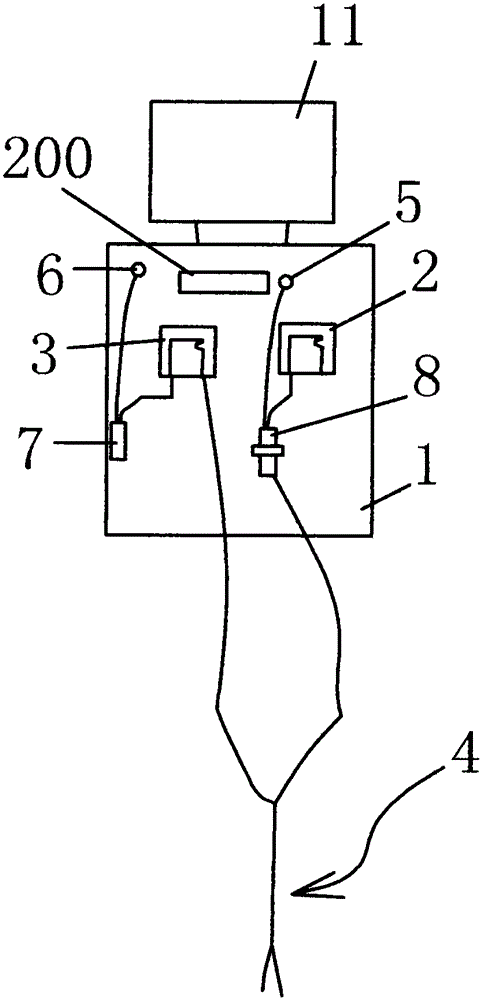
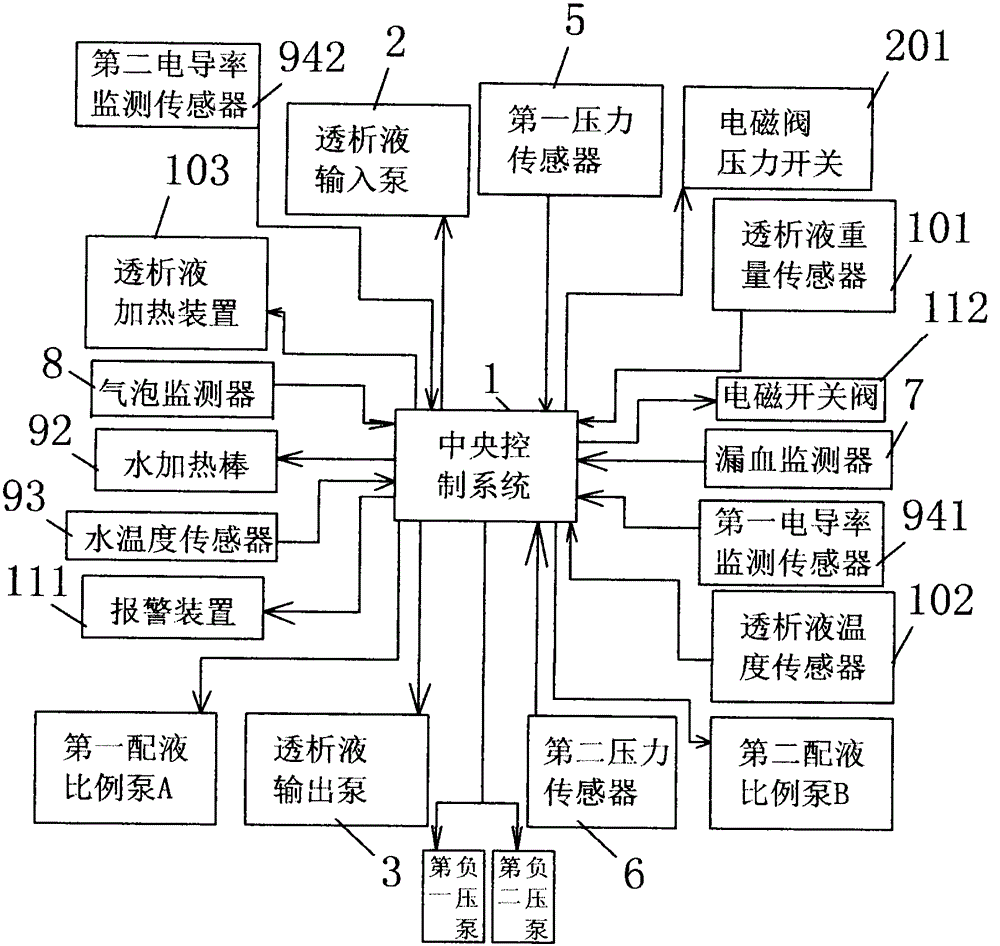
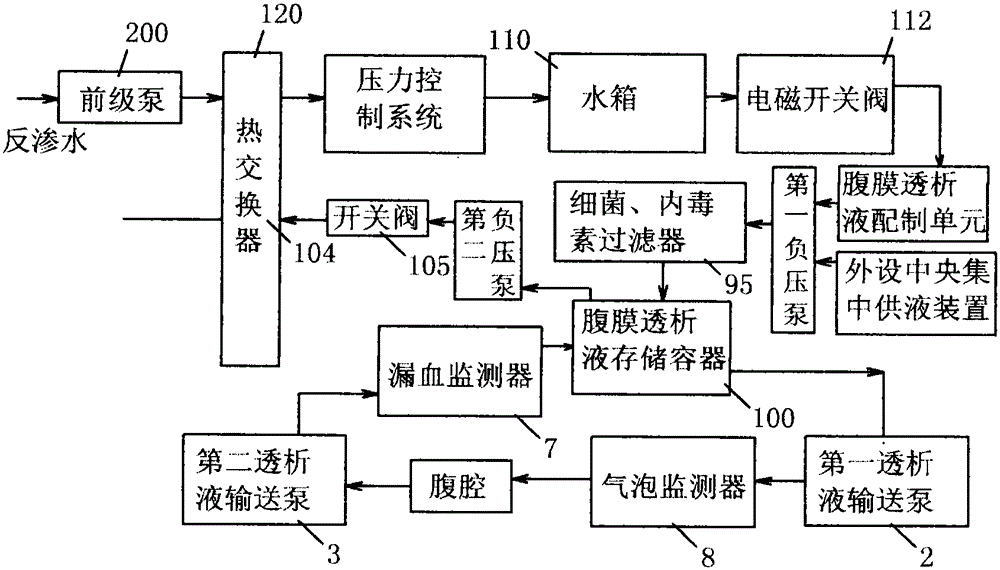
The present invention relates to a fully automatic carbonate peritoneal dialysis device. The design element of the device is that the device comprises a peritoneal dialysate storage container, a dialysate input pump, a dialysate output pump, a dual-channel X-type polyester sleeve pipe, a blood leakage monitor and a bubble monitor. A dialysate weight sensor, a dialysate temperature sensor and a dialysate heating device are arranged in the peritoneal dialysate storage container. The dialysate input pump sends peritoneal dialysate into a human body abdominal cavity according to a set rate, and the dialysate output pump pumps the peritoneal dialysate out of the human body abdominal cavity according to the consistent rate of the dialysate input pump. One channel of the dual-channel X-type polyester sleeve pipe communicates with the dialysate input pump, and the other channel of the dual-channel X-type polyester sleeve pipe communicates with the dialysate output pump. The invention provides the fully automatic carbonate peritoneal dialysis device and a fully automatic carbonate peritoneal dialysis method.
Owner:广州市红十字会医院
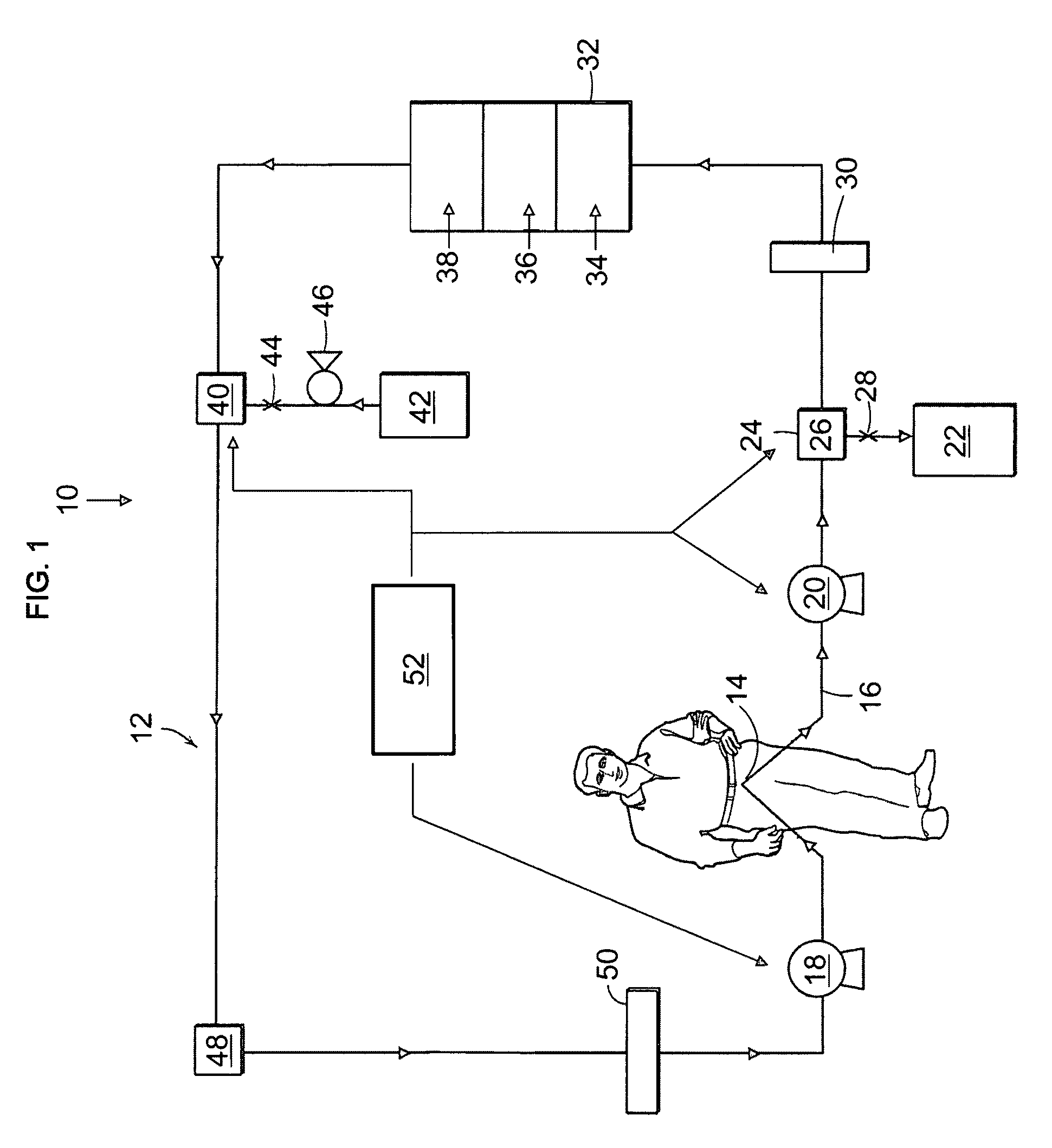
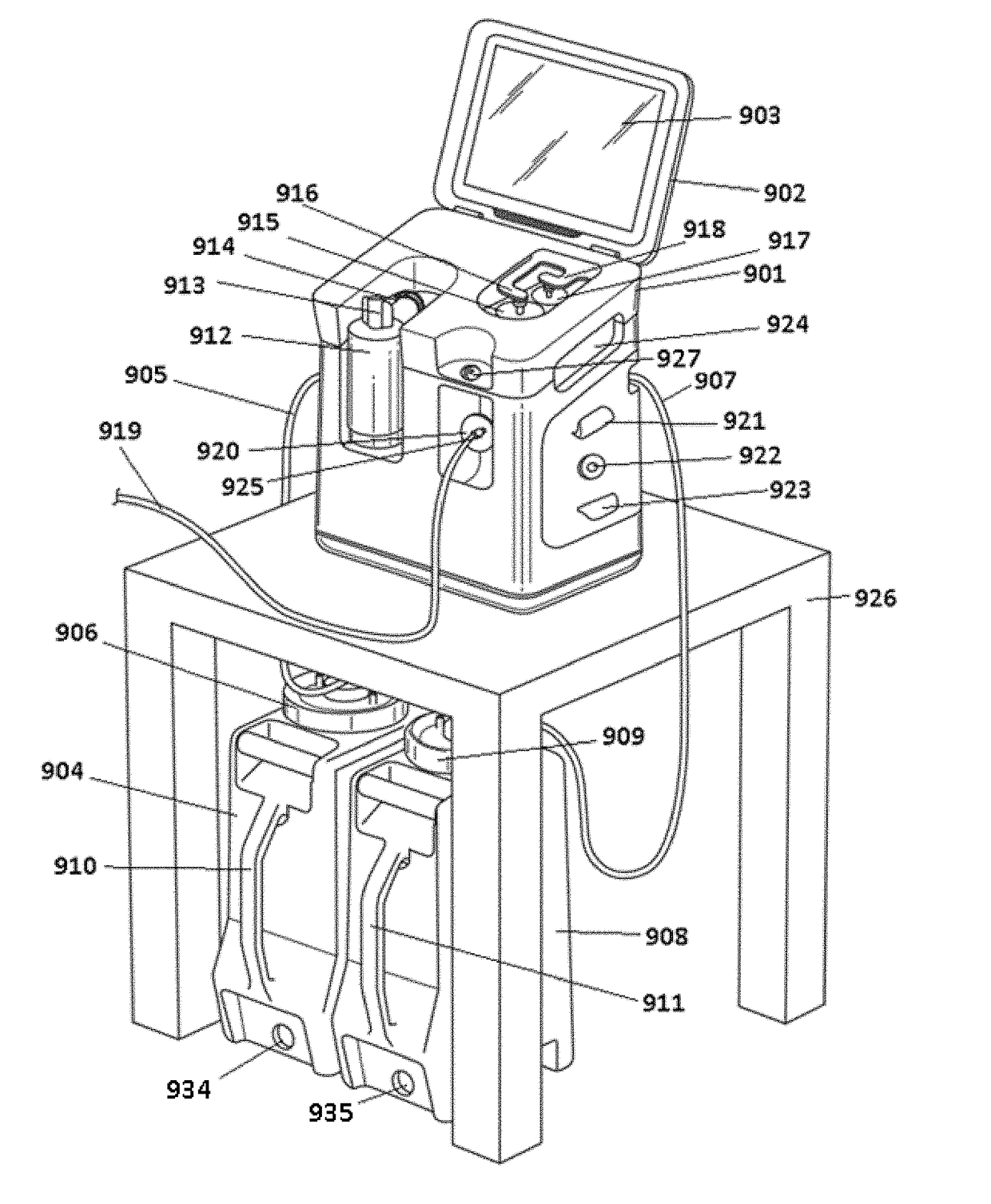
Peritoneal Dialysis System and Method
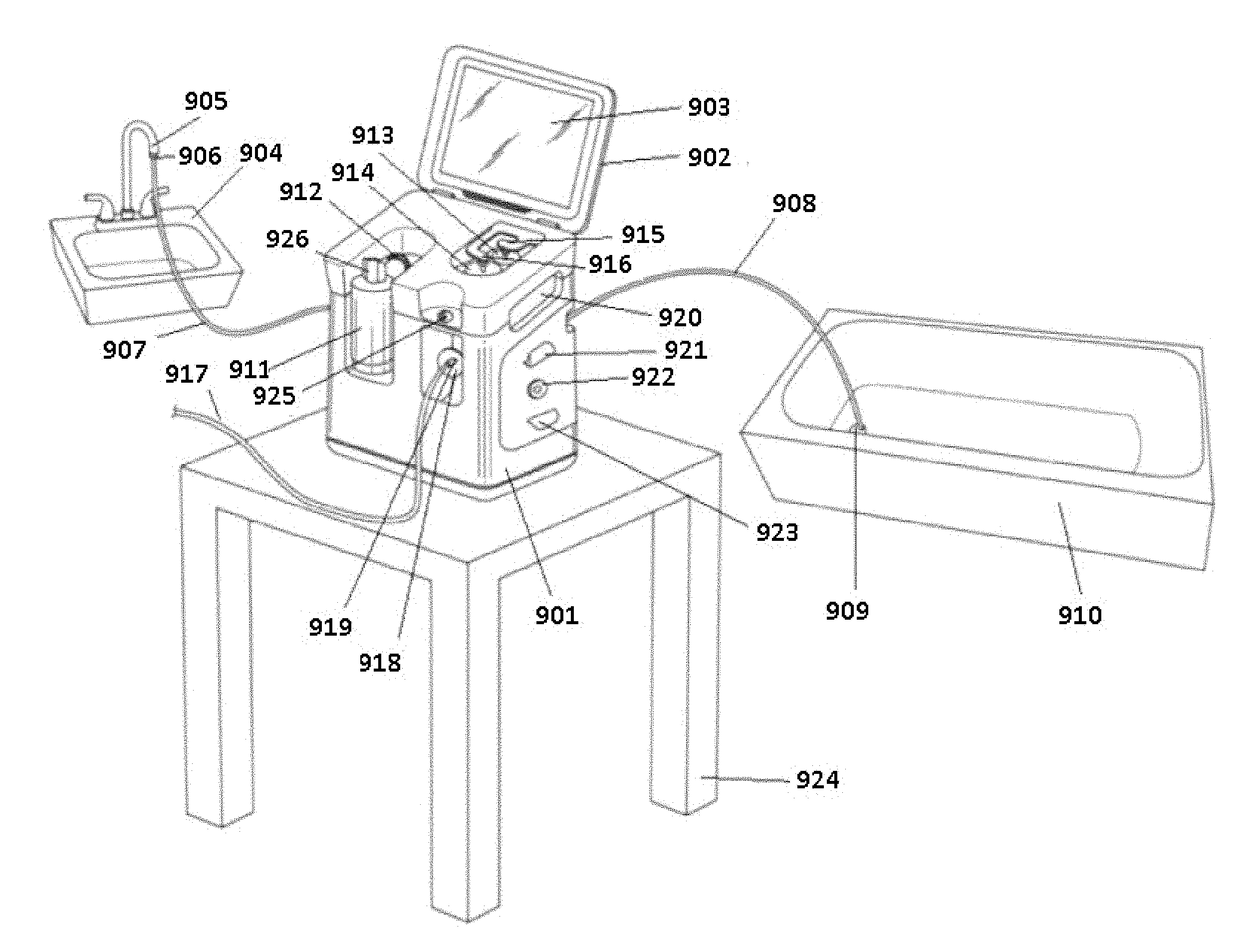
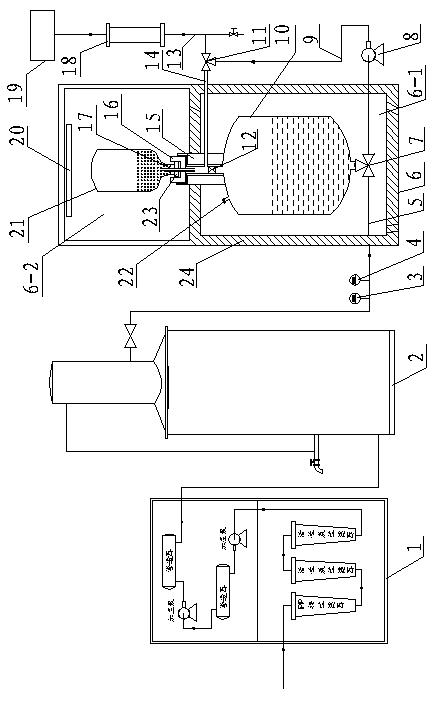
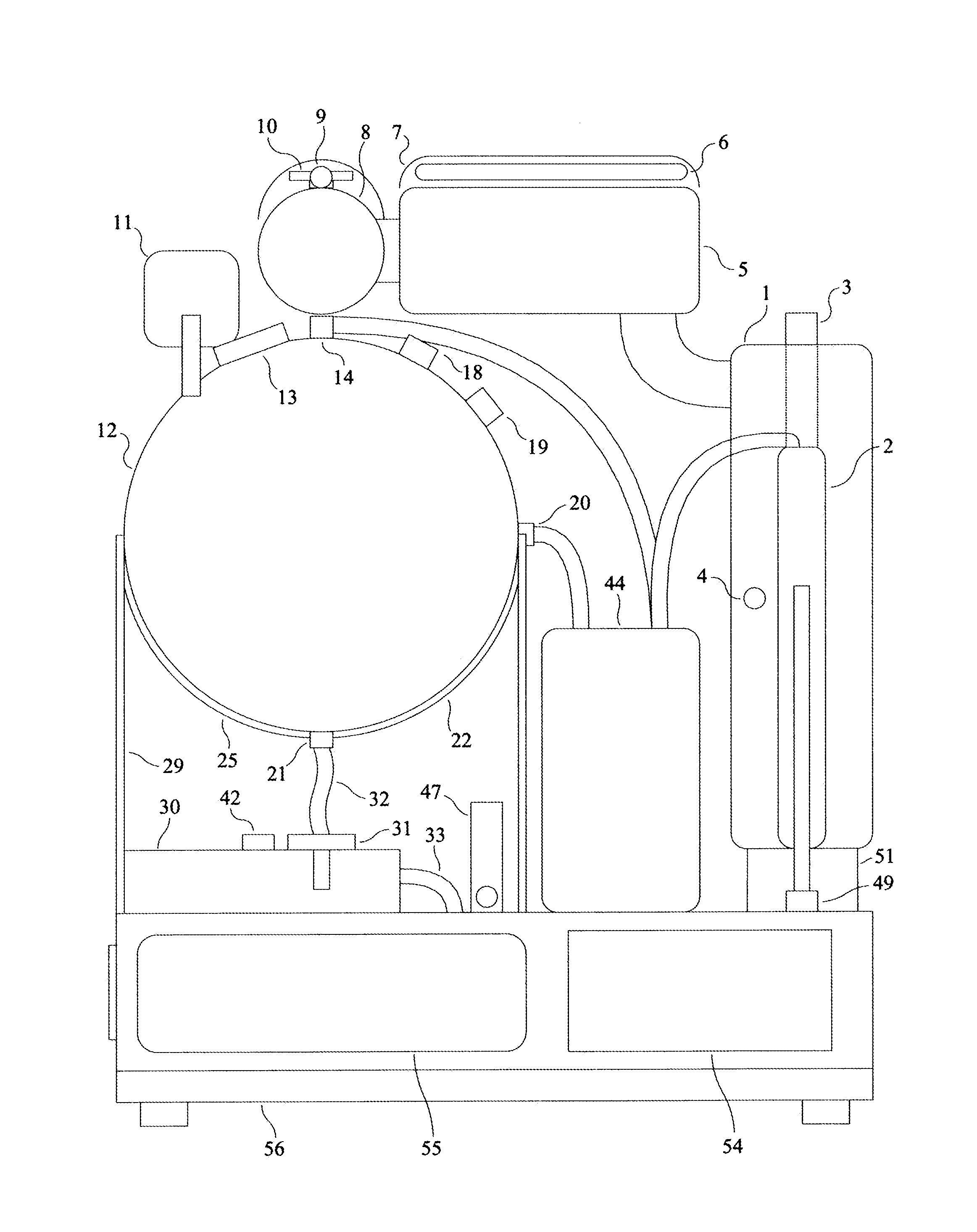
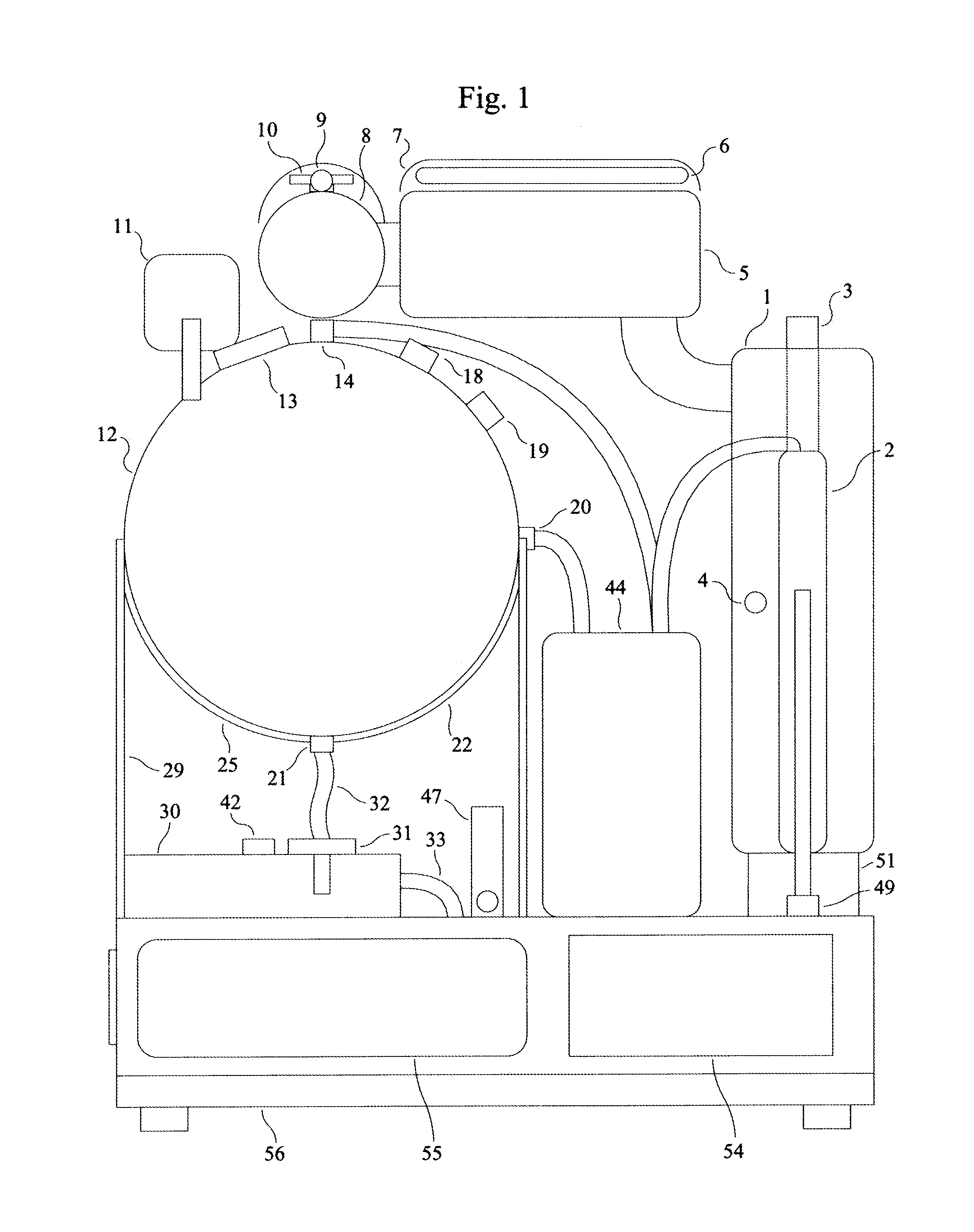
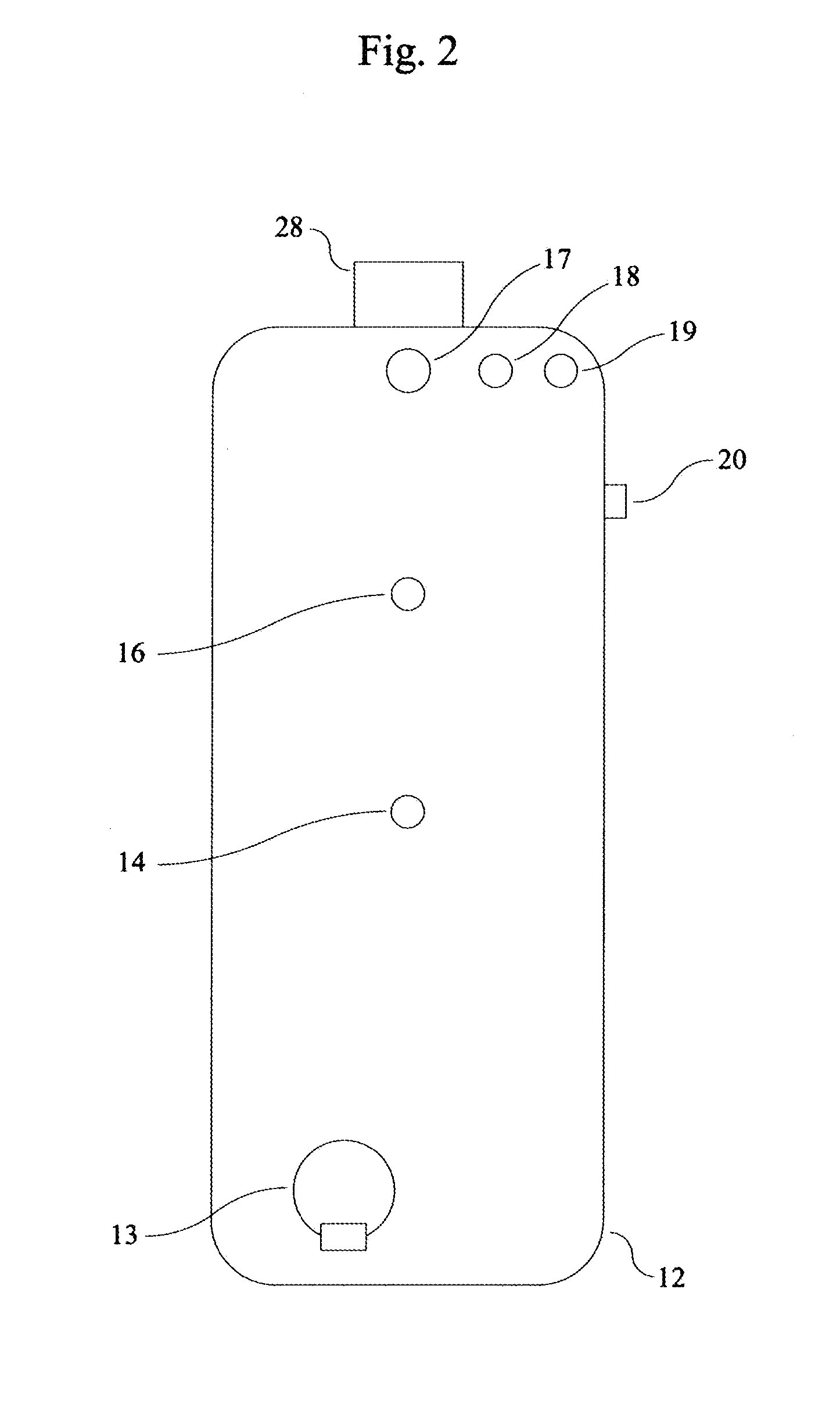
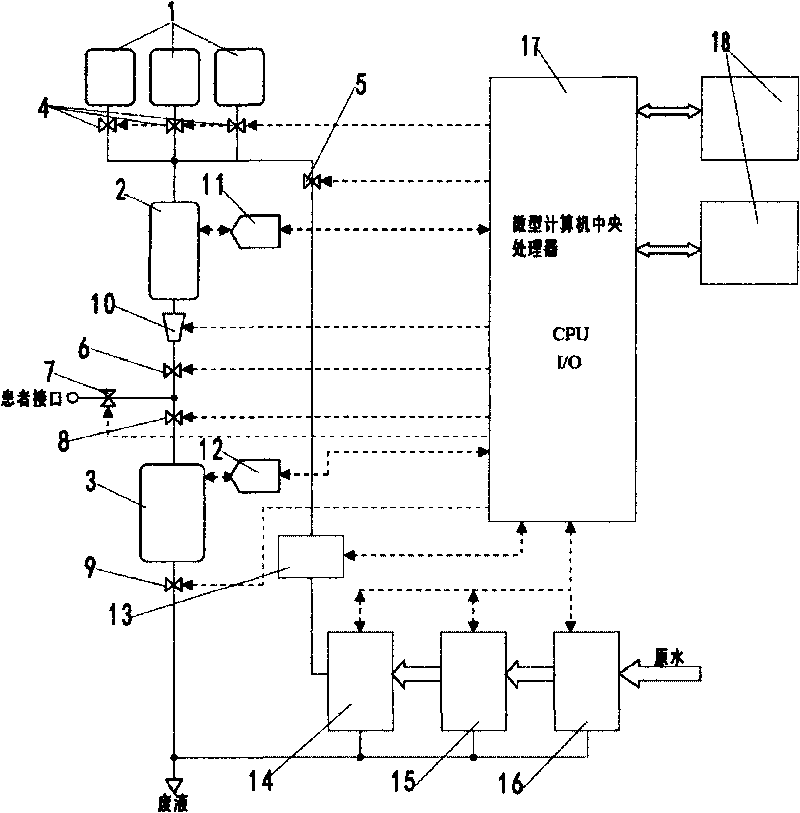
A peritoneal dialysis machine that includes a base, a boiling vessel, a condenser, a fluid storage vessel, a sterilizing UV lamp, a fluid mixer, a dialysate cassette, a boiling vessel demineralizing system, and an optional fluid storage vessel rinsing system. The peritoneal dialysis machine automatically generates a predetermined volume of distilled water each day. Pre-weighed quantities of low-endotoxin dialysate chemicals are then mixed into the warm distilled water, and the resulting peritoneal dialysate is sterilized via exposure to ultraviolet radiation. These actions are complete by the patient's indicated bed time. The peritoneal dialysis machine then exchanges spent peritoneal dialysate in the user's peritoneal cavity with fresh peritoneal dialysate throughout the night, causing each infusion of fresh dialysate to dwell in the user's peritoneal cavity for a predetermined time. All spent fluids are routed from the peritoneal dialysis machine to a toilet or a sewer drain.
Owner:HOFFMAN JOSEF C A
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS7118857B2Simple methodPrevent peritonitisAntibacterial agentsOrganic active ingredientsBiologyPeritoneal dialysis solutions
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
Peritoneal dialysis solution and preparation method thereof
ActiveCN103432164AAvoid damageGuaranteed stabilityPharmaceutical containersBagsSodium bicarbonateHigh resistance
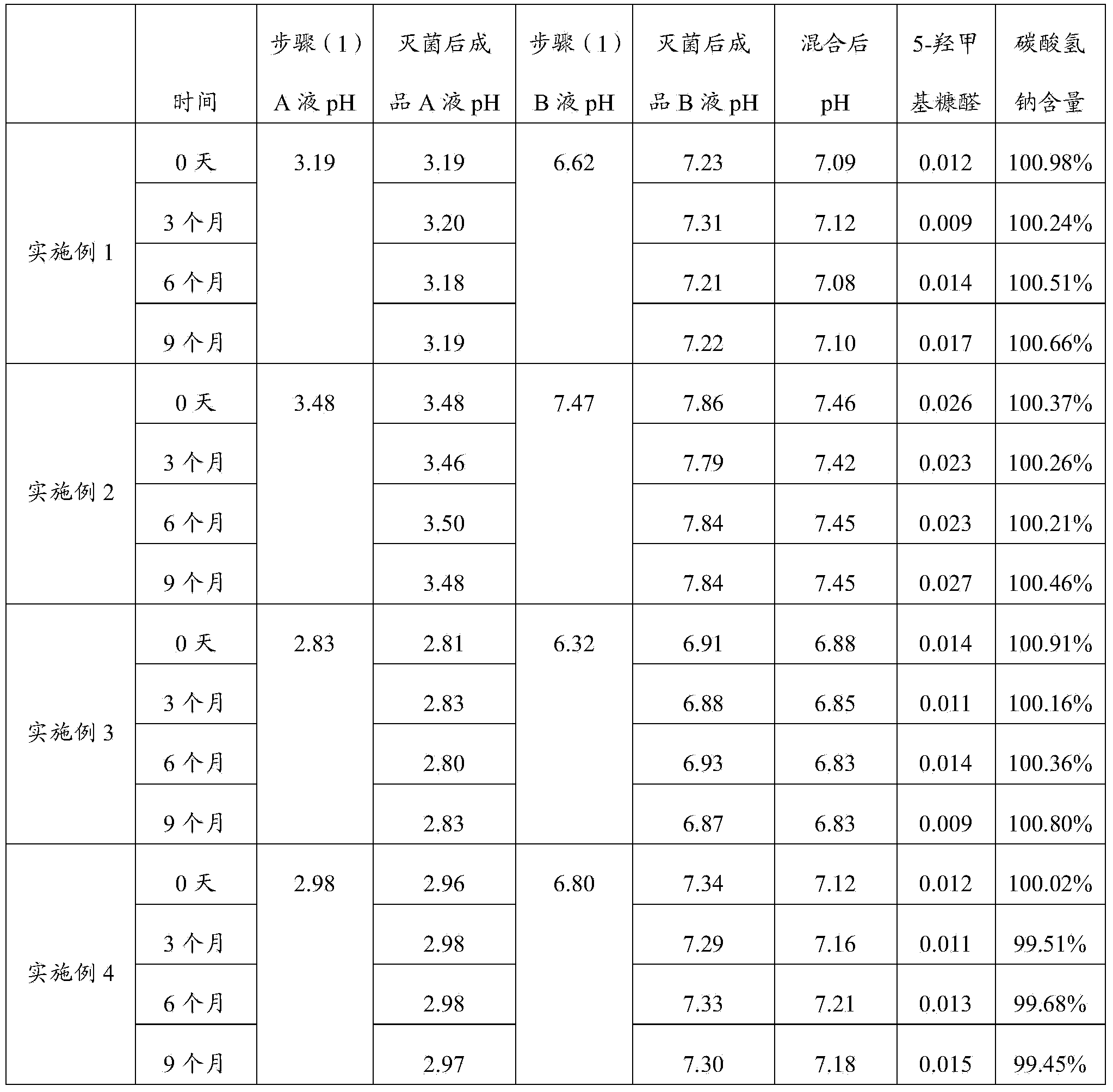
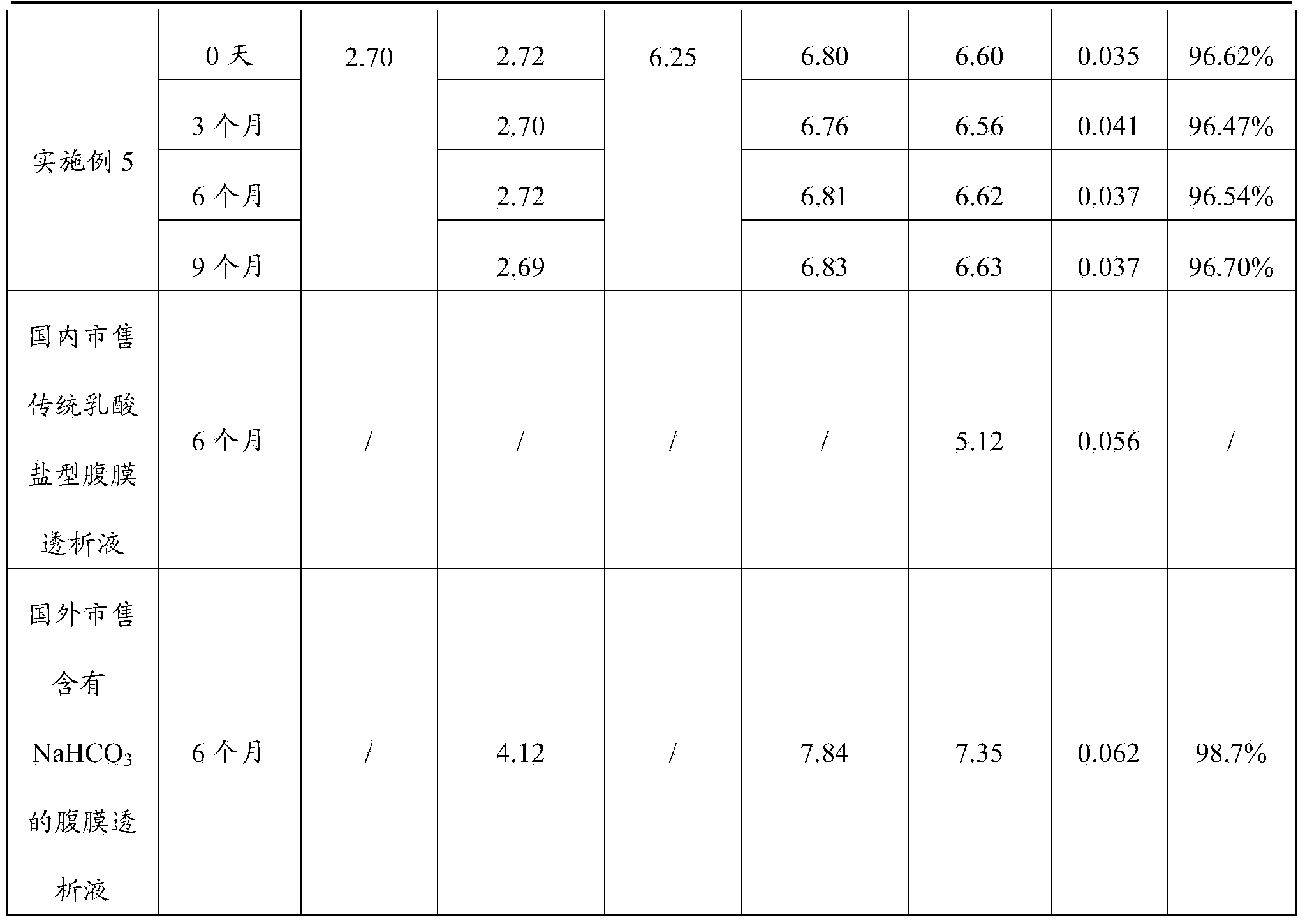
The invention discloses a peritoneal dialysis solution and a preparation method thereof. The preparation method comprises the following steps: dissolving pharmaceutically acceptable amounts of anhydrous glucose, calcium chloride and magnesium chloride into water for injection, and adjusting the pH value to 2.8-3.5 to obtain a solution A; dissolving pharmaceutically acceptable amounts of sodium chloride, sodium bicarbonate and sodium lactate into water for injection, introducing CO2, and adjusting the tank pressure to 0.05-0.2MPa and the pH value to 6.3-7.5 to obtain a solution B; and packaging by using a double-chamber bag to obtain the peritoneal dialysis solution. The peritoneal dialysis solution prepared by using the preparation method disclosed by the invention is few in degradation products of glucose and stable in quality of sodium bicarbonate; and a non-PVC (Polyvinyl Chloride) double-chamber bag and an external packaging bag with high resistance are adopted, so that the stability of sodium bicarbonate in high-temperature sterilization and storage processes can be further ensured.
Owner:HUAREN PHARMACEUTICAL CO LTD
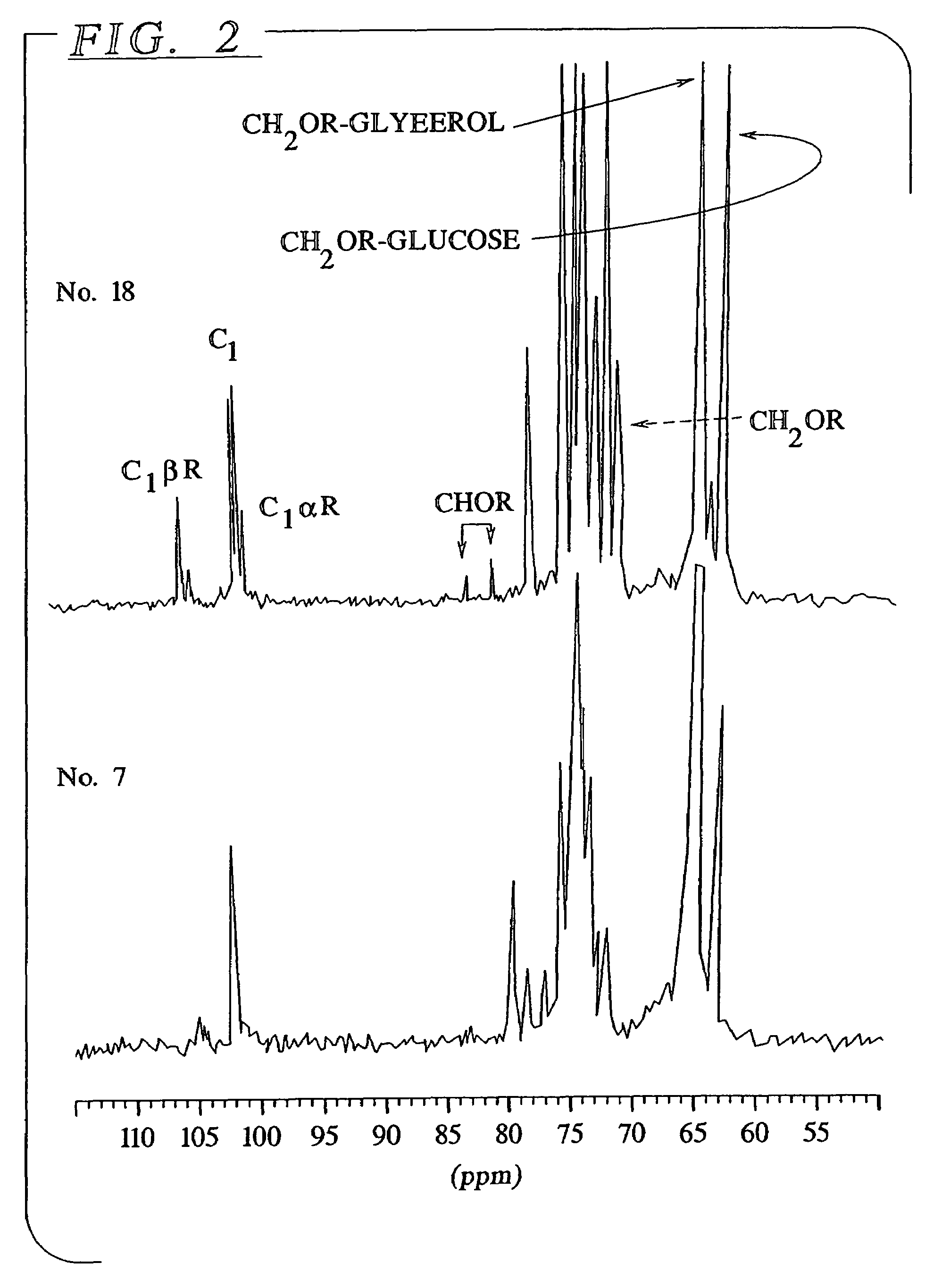
Preparation method of icodextrin for starch-based peritoneal dialysis solution
ActiveCN106397616ANarrow distributionHigh purityBlood disorderExtracellular fluid disorderHydrolysateUltrafiltration
The present invention discloses a preparation method of icodextrin for a starch-based peritoneal dialysis solution. According to the technical scheme of the invention, native starch is adopted as a raw material, and then a starch solution with the concentration thereof to be 5% to 15% is prepared by using a phosphate buffered solution. At a certain temperature and a certain pH value, the starch solution is firstly subjected to enzymolysis by using alpha-amylase, and then the gelatinized starch is subjected to debranching by using a debranching enzyme. The enzymatic hydrolysate is subjected to alcohol precipitation, ultrafiltration, gel chromatographic column separation and purification, and then the weight-average and number-average molecular weights thereof and the alpha-1, 6 glycosidic bond thereof meet the requirements at the same time. The content of alpha-1, 6 glycosidic bond in icodextrin is smaller than 10%, and the weight-average molecular weight thereof is 13000 to 19000 Da. The number-average molecular weight thereof is 5000 to 6500 Da. The preparation method of icodextrin for the starch-based peritoneal dialysis solution is high in yield, good in quality and relatively low in cost. Meanwhile, the defects of the conventional icodextrin preparation process in the prior art are overcome.
Owner:SOUTH CHINA UNIV OF TECH
Detecting method for 5-hydroxymethylfurfural in peritoneal dialysis solution
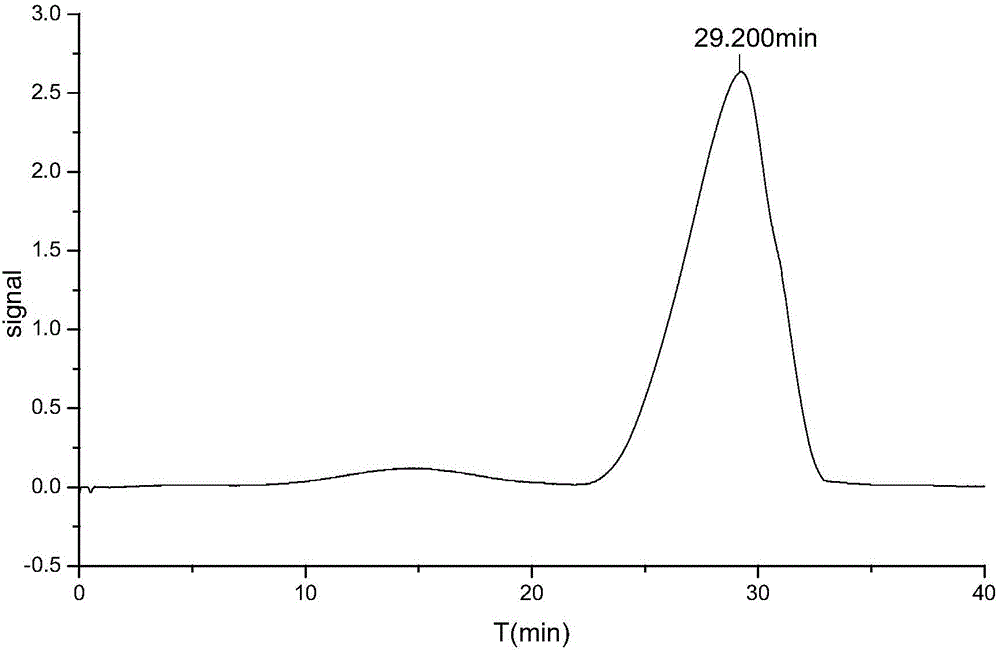
InactiveCN106546667ASimple processQuality improvementComponent separationBottleHydroxymethylfurfural
The invention belongs to the field of medical technologies and relates to a detecting method for 5-hydroxymethylfurfural in a peritoneal dialysis solution. A 5-hydroxymethylfurfural comparison product is precisely weighed and dissolved with water, a comparison product solution is prepared, an appropriate quantity of peritoneal dialysis solution is taken to be put into a measuring bottle and diluted with water to a scale, a solution for a testing product is prepared, 20 microliters of comparison fluid and 20 microliters of fluid for testing are precisely taken and injected into a chromatographic instrument, a chromatogram map is recorded, and the labeled amount of 5-hydroxymethylfurfural in the peritoneal dialysis solution is obtained according to an external standard method through peak area calculation; through efficient liquid chromatography, the content of 5-hydroxymethylfurfural in the peritoneal dialysis solution is accurately detected, accurate control over the quality of the peritoneal dialysis solution is achieved, the technology is easily optimized, and product quality and safety are improved.
Owner:HUAREN PHARMACEUTICAL CO LTD
Preparation method of extraneal
The invention relates to a preparation method of a peritoneal dialysis solution raw material-extraneal. The preparation method comprises the following steps: (1) adding lysozyme for treatment after washing starch; (2) after adopting debranching enzyme for hydrolysis, adding calcium chloride and alpha-amylase, and adding acid for rapidly deactivating the alpha-amylase after carrying out hydrolysis treatment; (3) adding modified activated carbon, and carrying out ultrasonic treatment; (4) carrying out molecular weight screening on a hydrolytic product in a filter solution C by using an ultra filtration membrane; (5) then adding the modified activated carbon, and filtering and drying after treatment, thus obtaining the extraneal. According to the preparation method of the extraneal, disclosed by the invention, the preparation efficiency and the yield are high, the pertinence is strong, reaction depth can be conveniently controlled, the hydrolytic product has good consistency with an originally-researched product, the generation of AGE (Advanced Glycation End Products) is completely eradicated, and peritoneal dialysis can be sustainably carried out.
Owner:青岛力腾医药科技有限公司
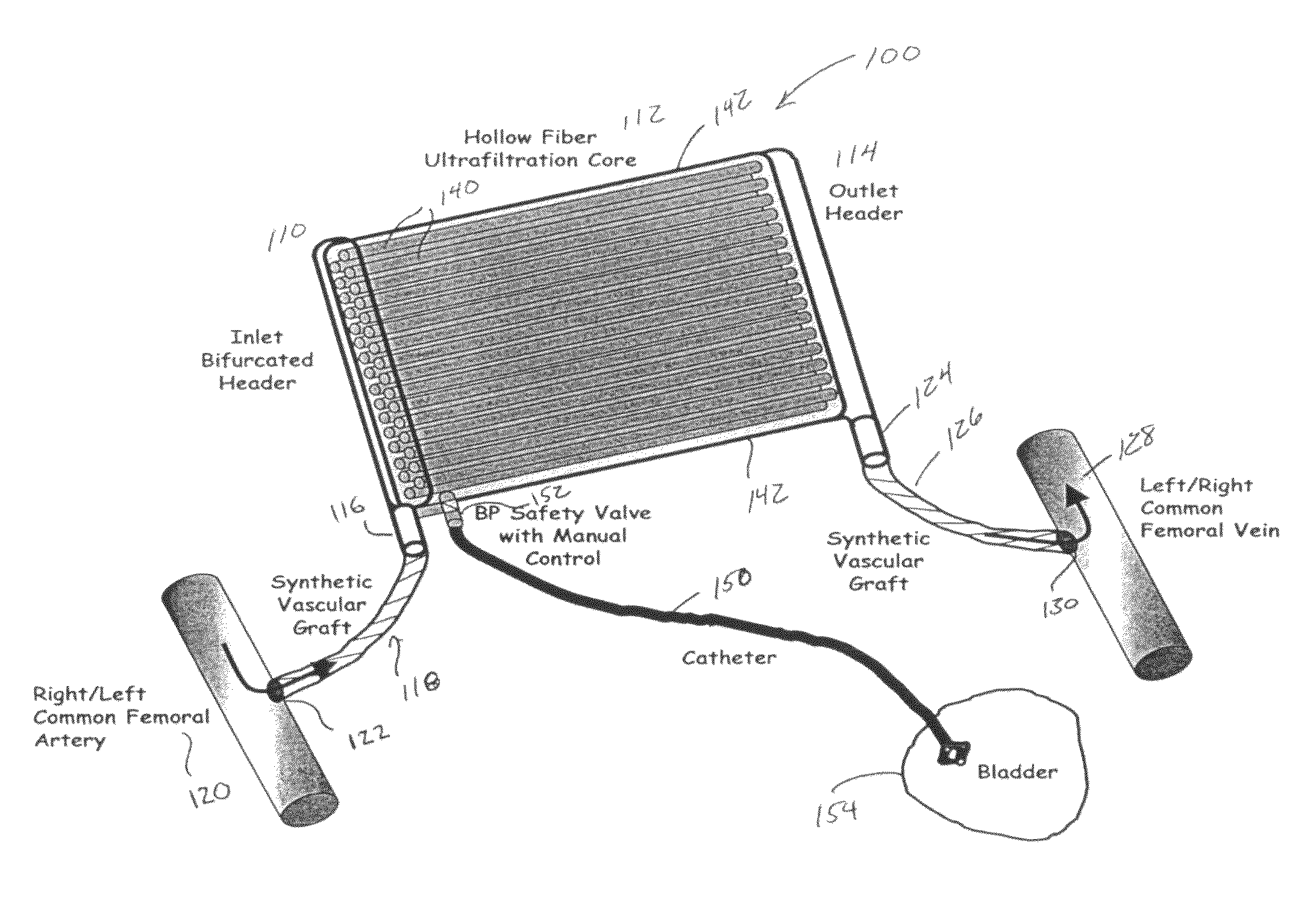
Wearable kidney
ActiveUS8715221B2Small sizeReduce loadMedical devicesPeritoneal dialysisMetaboliteIntensive care medicine
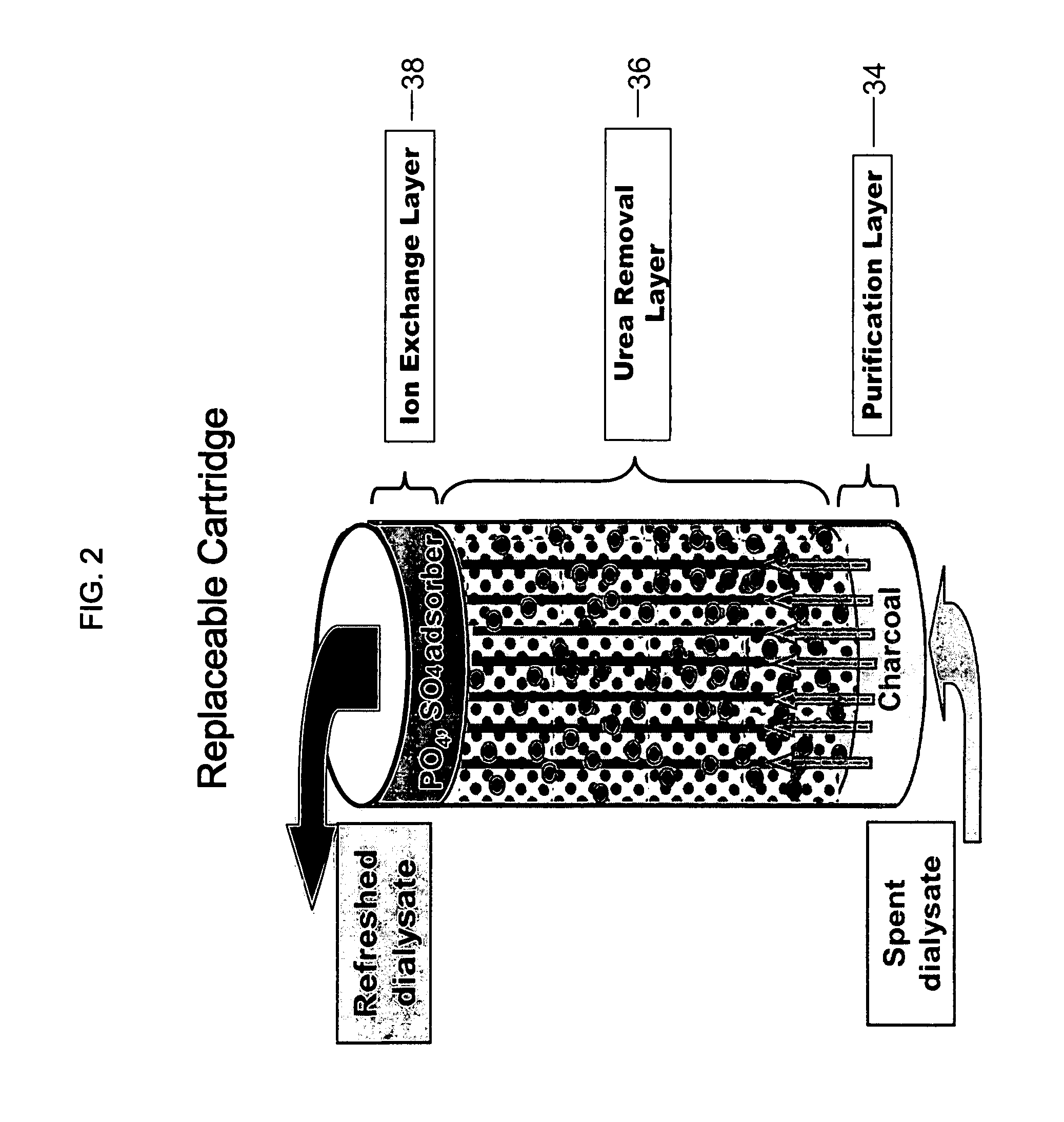
The present invention relates to a wearable peritoneal dialysis system and a replaceable cartridge in the wearable peritoneal dialysis system that regenerates the peritoneal dialysis solution without removing essential ions from the solution and, consequently, the patient. The invention also relates to methods of removing uremic waste metabolites from a patient using the wearable peritoneal dialysis system. A source of one or more enzymes that degrades uremic waste metabolites can be administered orally in conjunction with use of the wearable peritoneal dialysis system such that the load of toxins needing to be eliminated by the wearable peritoneal dialysis system is reduced. The wearable peritoneal dialysis system is meant to operate continuously or semi-continuously, its components small and light enough that it can be comfortably worn by a patient constantly, without burden.
Owner:FRESENIUS MEDICAL CARE HLDG INC
Online peritoneal dialysis unit and preparation method
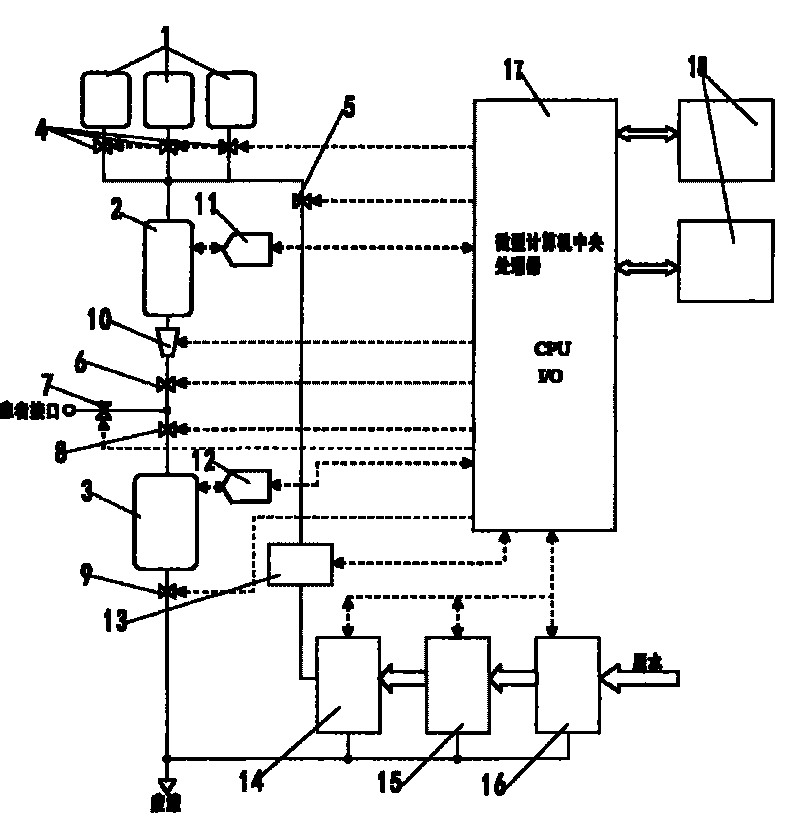
InactiveCN101745157AReduce storage capacityReduce capacityPeritoneal dialysisSquare meterWater processing
The invention discloses an online peritoneal dialysis unit and preparation method which integrates computer automation control technique, antiosmosis water processing technology and peritoneal dialysis solution concentrating technology. The unit can online prepare peritoneal dialysis solution equal to or more than 30L in 10-20 hour curing process with a peritoneal dialysis solution preparing speed which is equal to or more than 200ml / min, which meets the requirement that the clinic care exchange capacity reaches 50ml / min; automatically completes exchange operating process more than 15-18 times, solves the defects that using the current CAPD technology to realize large-volume exchange is difficult to operate frequently and the APD equipment does not have the function of 30L-36L large-volume exchange function, and provides technical supports for realizing the KT / V value of most patients to be equal to or more than 2.1, realizing the Ccr of more than 70 percent of patients to be equal to or more than 60ml / 1.73 square meters, realizing the elimination rate of carbamide to be more than 12ml / min, and improves the survive rate of the patients.
Owner:贺心雅
Peritoneal dialysis solution
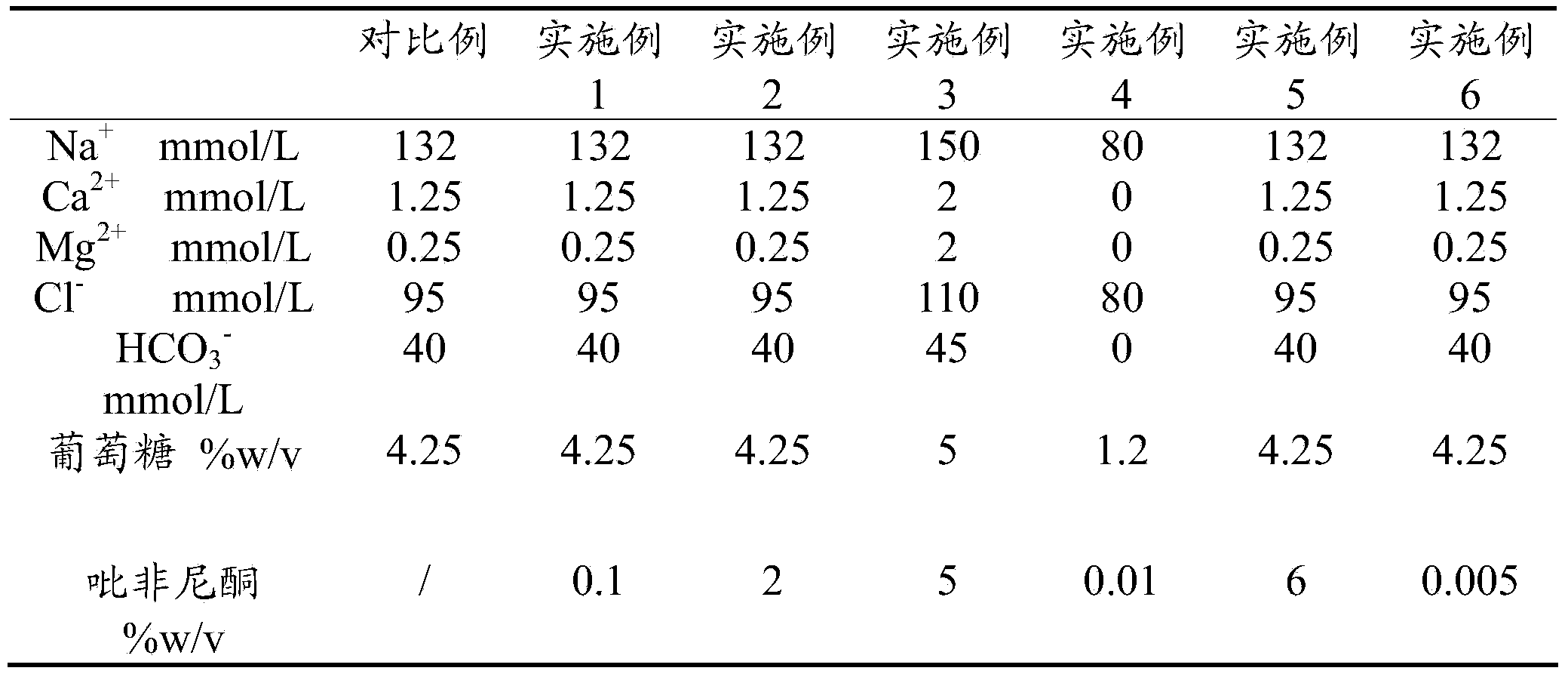
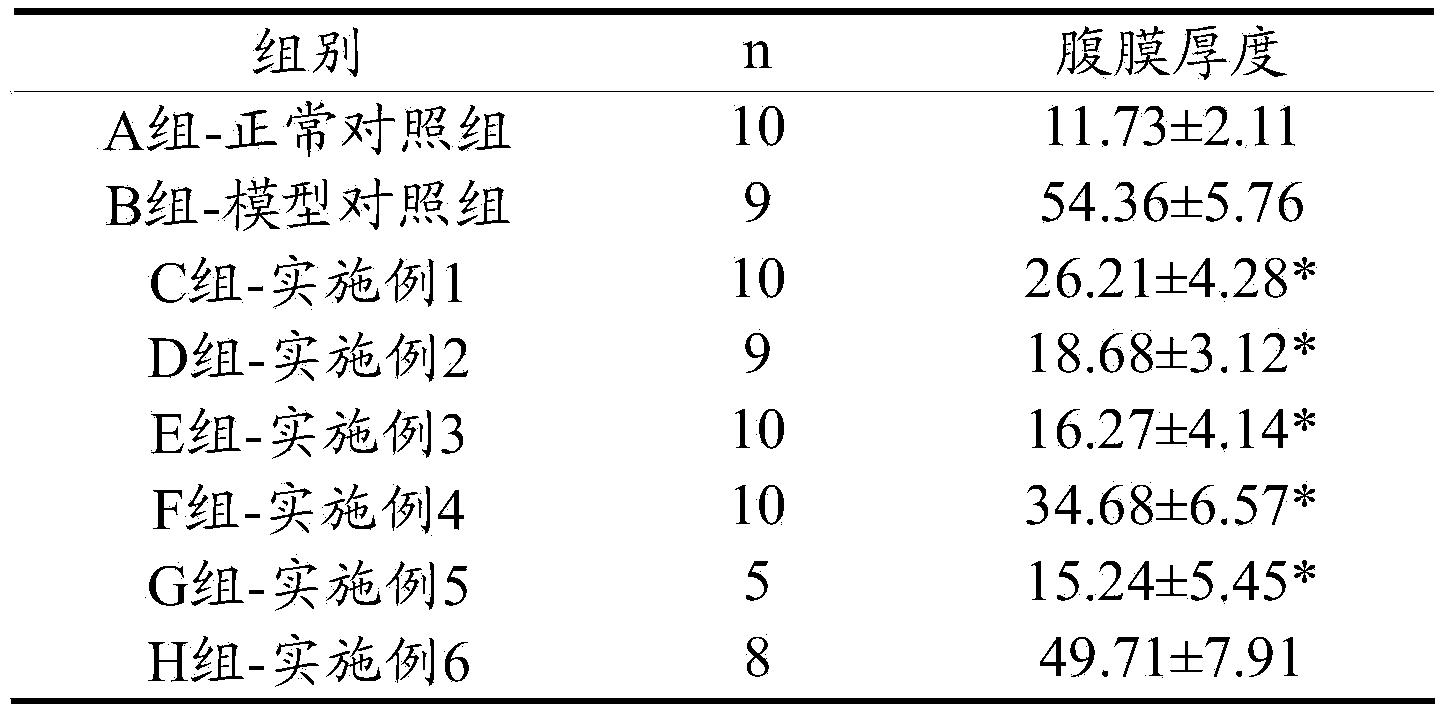
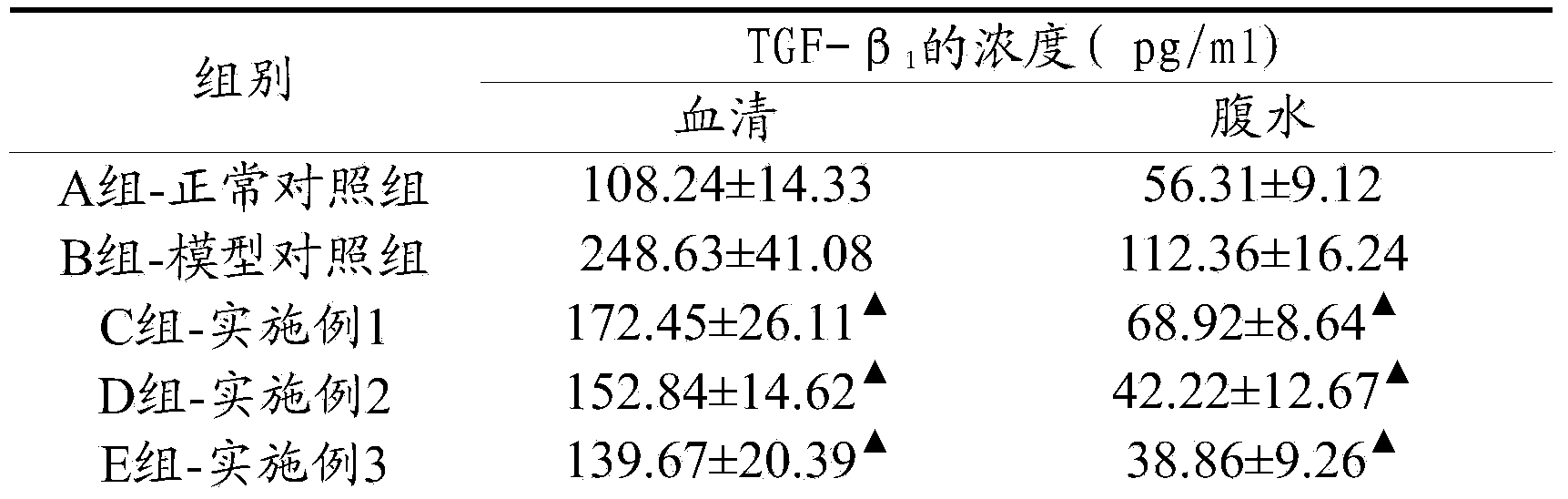
ActiveCN103463081ABlock and delay the process of peritoneal fibrosisOrganic active ingredientsAluminium/calcium/magnesium active ingredientsHigh concentrationExperimental research
The invention discloses a peritoneal dialysis solution which comprises 0.01-5%w / v pirfenidone. Through a good deal of experimental research and analysis, the process that the 0.01-5%w / v pirfenidone is added to a conventional peritoneal dialysis solution so that peritoneum fibrosis caused by high-concentration glucose in an existing peritoneal dialysis solution can be remarkably blocked and delayed is found out.
Owner:HUAREN PHARMACEUTICAL CO LTD
Powered sterile solution device
The disclosure below relates to apparatus and methods for producing medicament using sub-optimal water sources. One embodiment of the disclosure is directed to an apparatus comprising a preliminary purification component, a disinfection component, a pharmaceutical grade water preparation (PGW) component, and a drug pack. Another disclosed embodiment relates to a method for producing a peritoneal dialysis solution, comprising, passing diluent through a preliminary purification component, passing diluent through a disinfection component, passing diluent through a PGW preparation component, passing diluent through a drug pack, and collecting solute produced by the drug pack.
Owner:PRISMEDICAL CORP +1
Method for detecting content of glucose in peritoneal dialysis solution
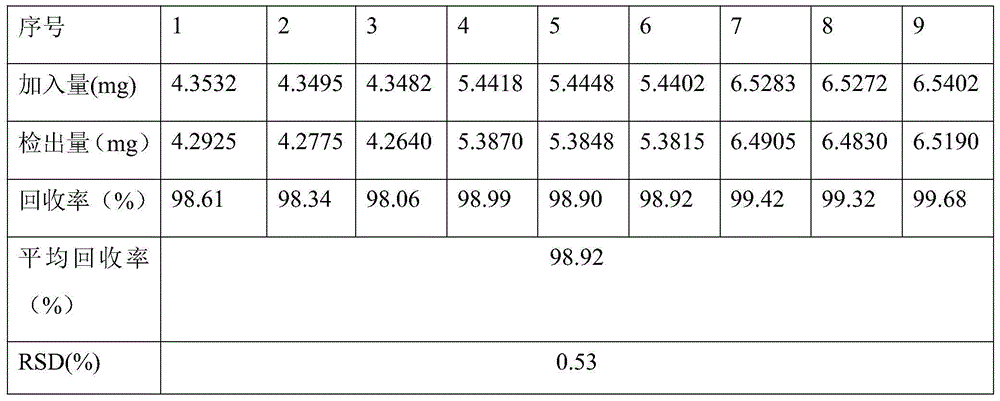
InactiveCN105203650ASimple processQuality improvementComponent separationRefractive indexColumn temperature
The invention relates to a method for detecting the content of glucose in a peritoneal dialysis solution. The method comprises the following steps: precisely weighing an anhydrous glucose reference substance, and dissolving and diluting through deionized water so as to obtain a solution, wherein 1 ml of the solution contains 5.0-6.5 mg of glucose; precisely weighing a neutral peritoneal dialysis solution and diluting through deionized water so as to obtain another solution, wherein 1 ml of the solution contains 5.0-6.5 mg of glucose; respectively and precisely taking 20 [mu]l of a reference solution and a test solution, injecting the reference solution and the test solution into a chromatographic instrument, recording a chromatogram, and calculating as a peak area according to an external standard method so as to obtain labelled amount of glucose, wherein chromatographic conditions are as follows: the chromatographic column is a WatersSugarParkI column, 300 mm*6.5 mm, 6.5 [mu] m; a mobile phase is deionized water; the flow velocity is 0.5+ / -0.1 ml / min; the column temperature is 80+ / -5 DEG C; the set temperature of a refractive index detector is 40+ / -5 DEG C and the sample amount is 20 [mu]l. According to the invention, the content of glucose in the peritoneal dialysis solution is detected through the high performance liquid chromatography, the quality of the peritoneal dialysis solution is controlled precisely, the process optimization is facilitated and the product quality and safety are improved.
Owner:HUAREN PHARMACEUTICAL CO LTD
Peritoneal dialysis solution (lactate) (low calcium) composition
ActiveCN102973600AOrganic active ingredientsAluminium/calcium/magnesium active ingredientsSodium lactateSulfite salt
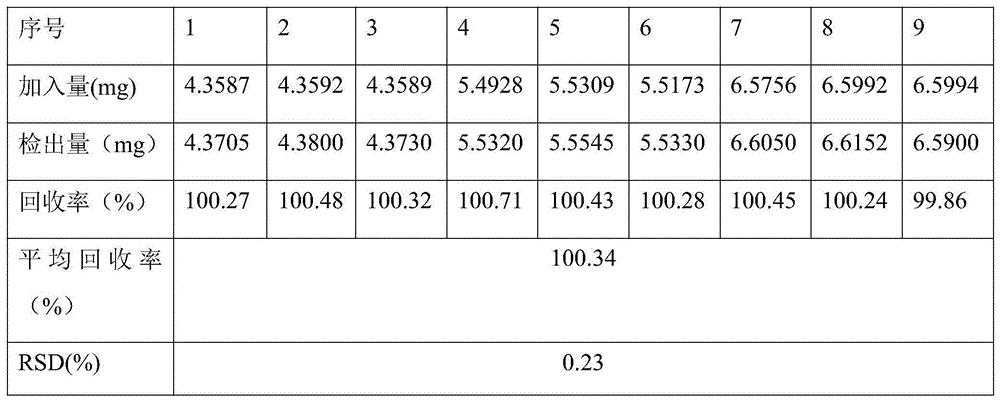
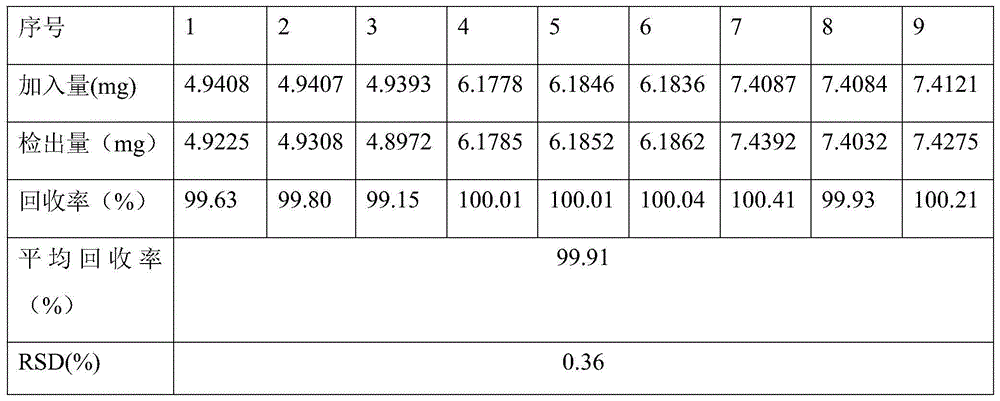
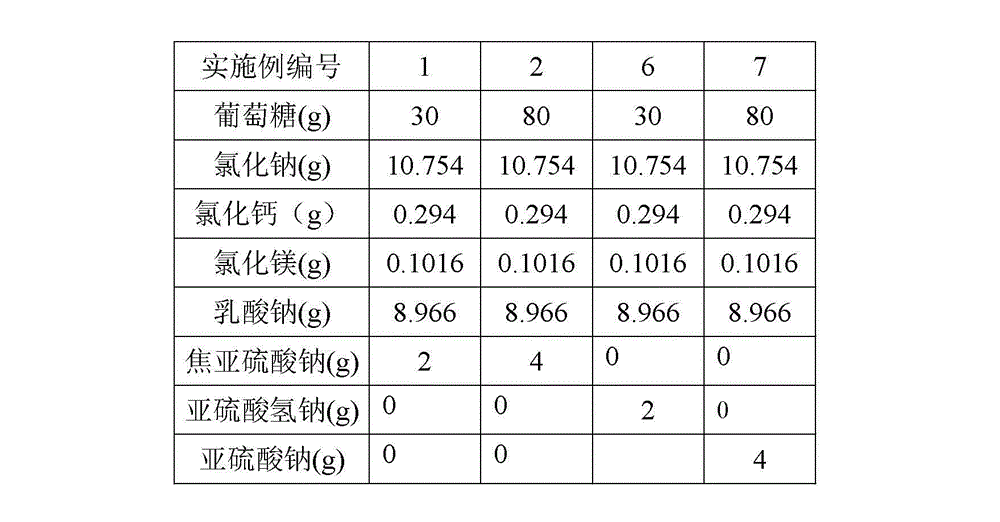
The invention relates to a peritoneal dialysis solution (lactate) (low calcium) composition which comprises 1.5-4.0% of glucose, 0.5-0.6% of sodium chloride, 0.0001-0.0002% of calcium chloride, 0.00004-0.00006% of magnesium chloride and 0.4-0.5% of sodium lactate, and is characterized by further comprising 0.01-0.02% of sodium metabisulfite, sodium sulfite or sodium hydrogen sulfite.
Owner:TIANJIN JINYAO GRP
Peritoneal dialysis solution containing modified icodextrins
InactiveUS7208479B2Easy to optimizeSimple methodOrganic active ingredientsBiocideGluconic acidD-glucitol
The present invention provides a peritoneal dialysis solution that contains heat stable osmotic agents such as D-glucitols, gluconic acids and alkylglycosides produced the reduction, oxidation or glycosylation of icodextrins respectively. As a result, osmotic agents that are stable under autoclaving or heat sterilization conditions are provided which reduces the amount of bioincompatible materials in the sterilized peritoneal dialysis solutions. Methods of preparing the D-glucitols, gluconic acids and alkylglycosides are disclosed.
Owner:BAXTER INT INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com