Patents
Literature
114 results about "Microbial contaminants" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Microbiological contaminants are made up of bacteria, parasites and viruses. The use of disinfectants such as chlorine have significantly reduced the risk of waterborne disease, but certain contaminants like Cryptosporidium are resistant to chlorine.
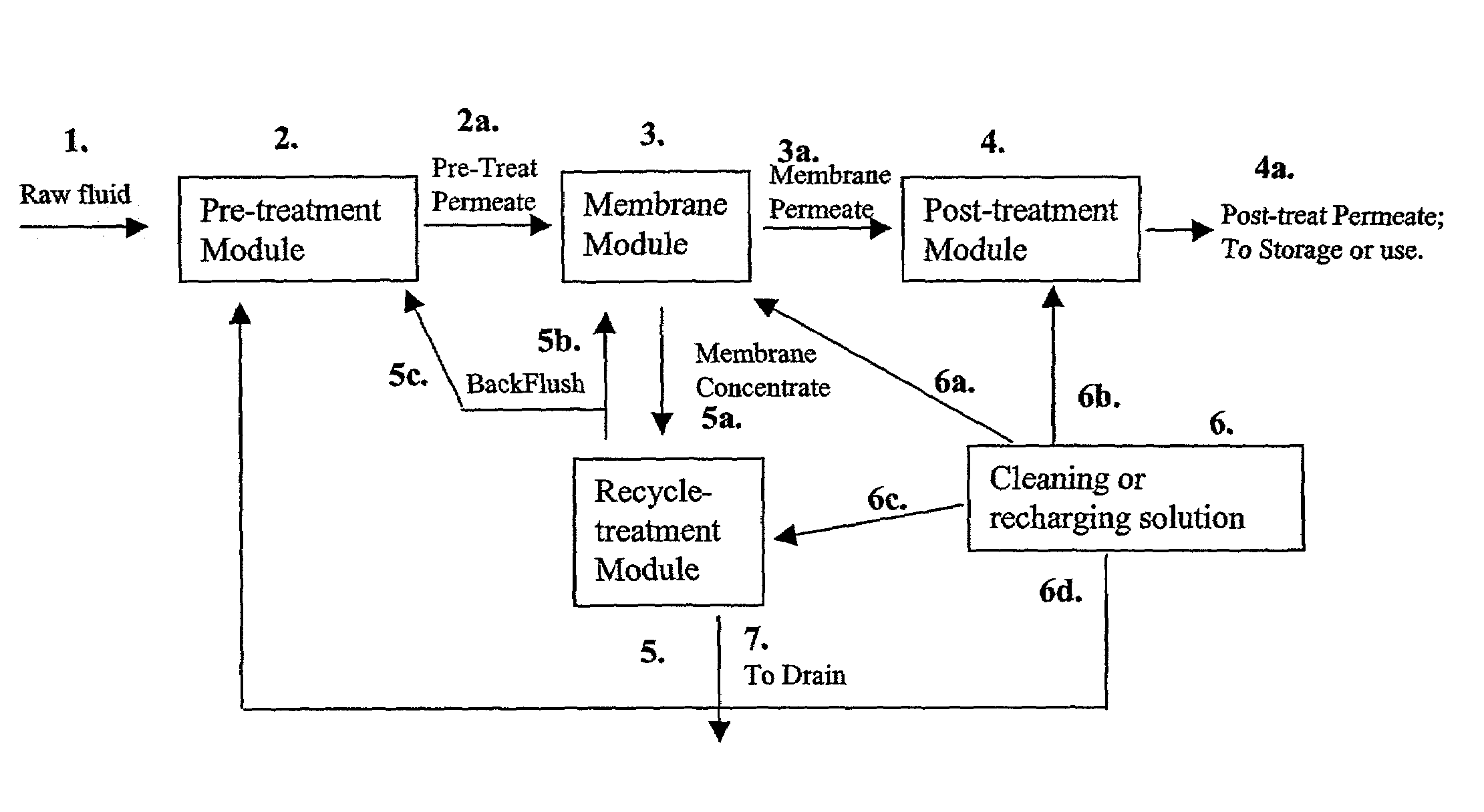
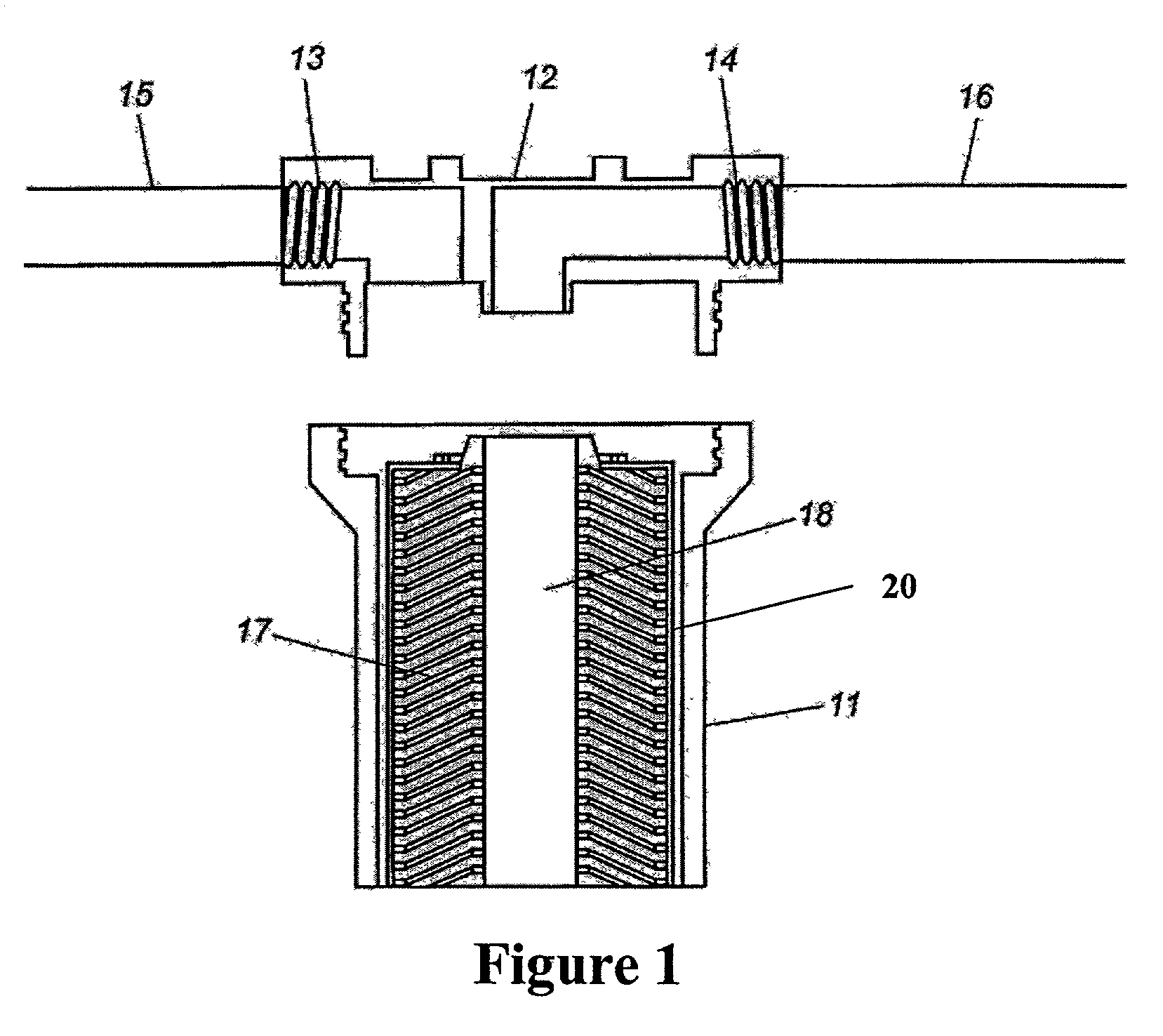
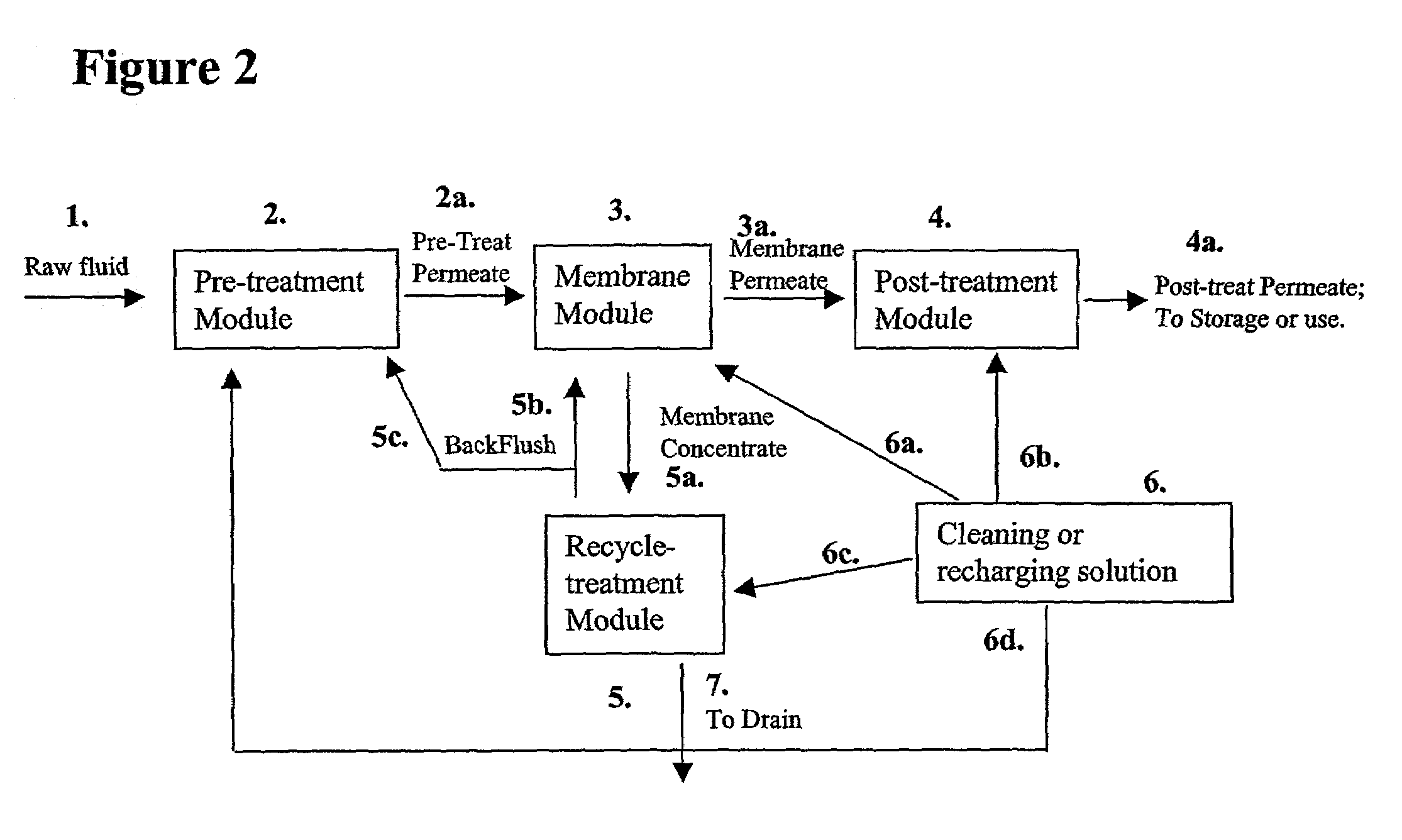
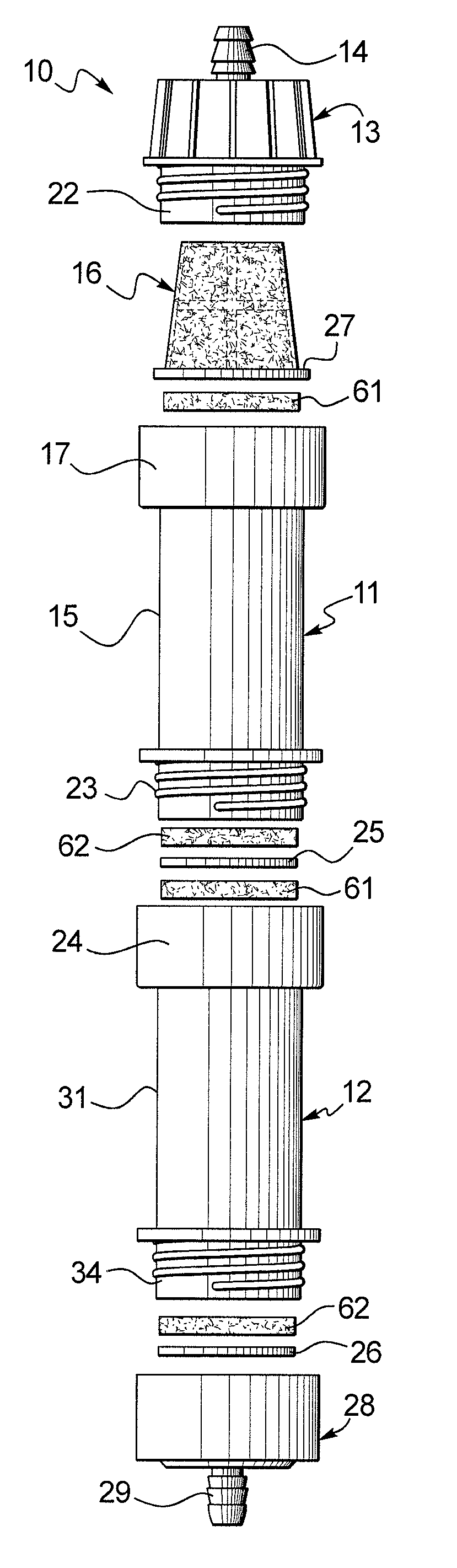
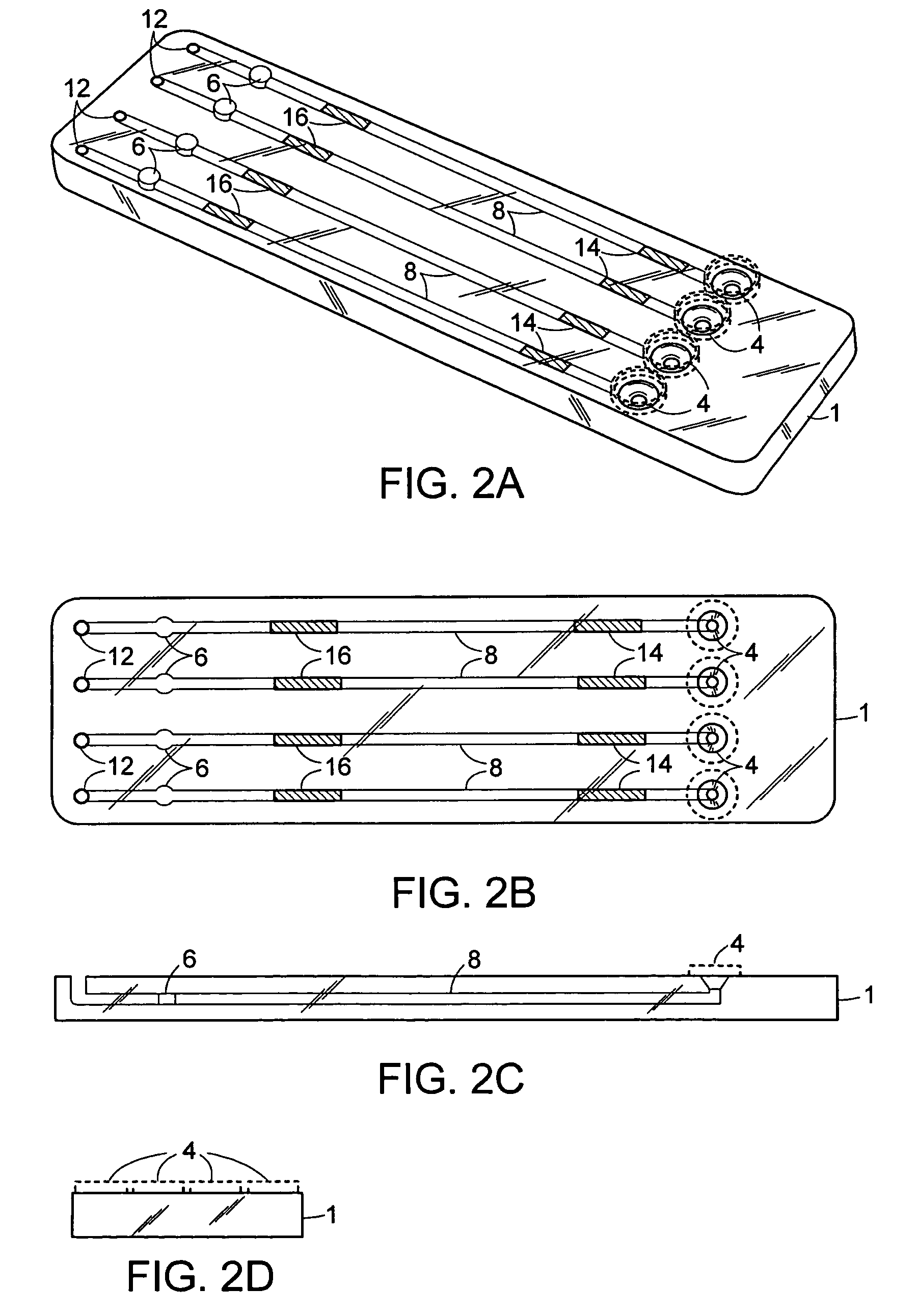
Membrane based fluid treatment systems
InactiveUS7186344B2Readily availableImprove the level ofLiquid separation auxillary apparatusIon-exchanger regenerationMembrane foulingTreatment system
A process for removing soluble and insoluble inorganic, organic, and microbiological contaminants from a fluid stream employing a pretreatment module, a post-treatment module, a recycle stream module or any combination thereof, and a membrane module, is provided. The process provided reduces the problems associated with membrane fouling and increases contaminant removal capacity.
Owner:WATERVISIONS INT
Modular Water Purification and Delivery System
InactiveUS20090008318A1Maximize capacityFlattening or eliminating the parabolic fluid flow profileUltrafiltrationWater/sewage treatment by ion-exchangeFiberHollow fibre
A modular filter system is provided with one or more modules that can be interchangeable, depending upon the specific application or specific health or environmental issue presented. Disclosed combinations can include one or more of any of the following modules in any relative position to one another: (a) a microbiological contaminant mitigation module, preferably in the form of an inverted u-shaped hollow fiber filter module wherein the fibers have ends potted on the downstream side and that consists essentially of hydrophilic fibers for water filtration with a small amount of hydrophobic fibers for venting of entrapped air; (b) a first chemical mitigation module, preferably in the form of an adsorption module comprising carbon or the combination of carbon and a deionization resin; and (c) a second chemical mitigation module, preferably in the form of a deionization resin module. Modules including a carbon bed or a resin bed may be equipped with a pair of hydrophobic foam bed restraints that apply opposing axial pressure to the bed in all operating conditions.
Owner:PRISMEDICAL CORP
Concentrated human milk fortifier liquid
InactiveUS20060204632A1Reduce riskReduce usageMilk preparationVitamin food ingredientsHuman milk fortifierMicroorganism
Disclosed are concentrated, liquid, human milk fortifier compositions comprising from about 15% to about 45% by weight of protein, on a dry weight basis, and having a caloric density of from about 1.25 kcauml to about 6.0 kcau / ml, wherein the liquid human milk fortifier composition is added to human milk in a volume-volume ratio of from about 1:3 to about 1:9. These composition include embodiments comprising carbohydrate and fat, that are formulated with improved stability by selecting any one of the following variations: 1) certain whey-casein protein blends, 2) water insoluble calcium-containing materials, 3) protein hydrolysates, and 4) aseptically packaged concentrates. The liquid concentrates are especially useful for providing nutrition to preterm infants in neonatal intensive care units or similar other institutional setting, and to minimize the risk of introducing microbial contaminants such as Enterobacter sakazakii to infant feedings during preparation in such institutional settings.
Owner:ABBOTT LAB INC
Method for the production of a fermentation product from a pretreated lignocellulosic feedstock
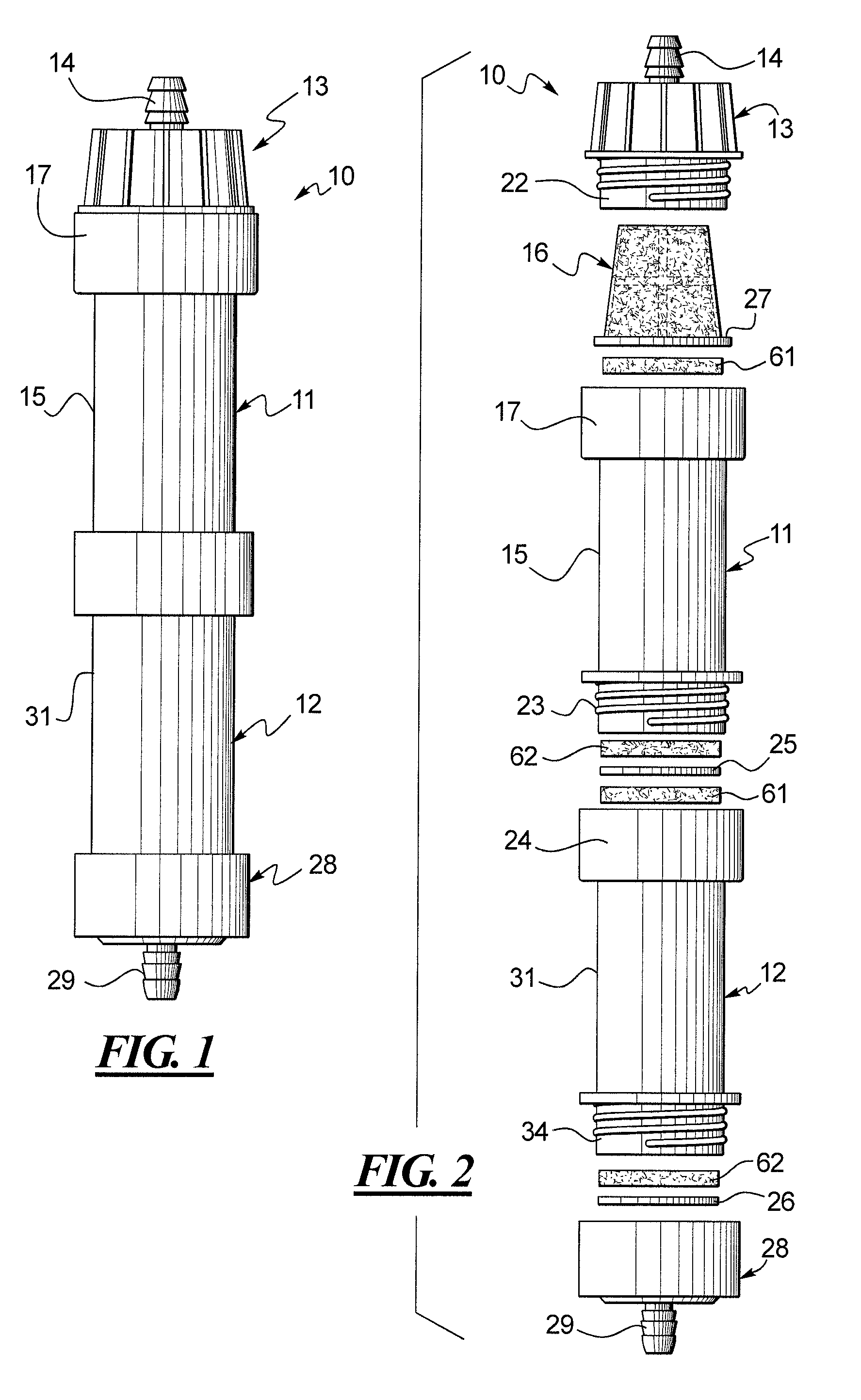
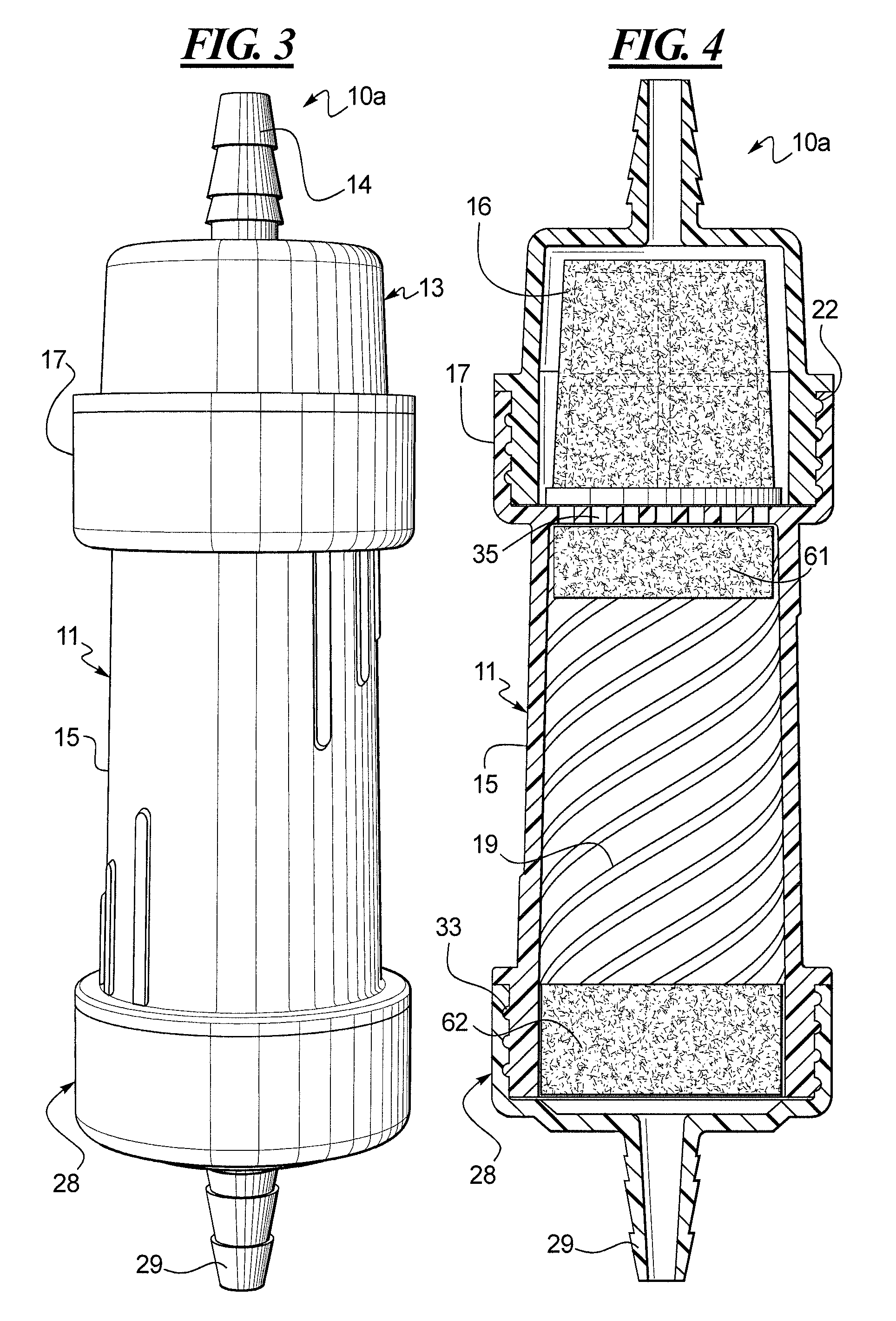
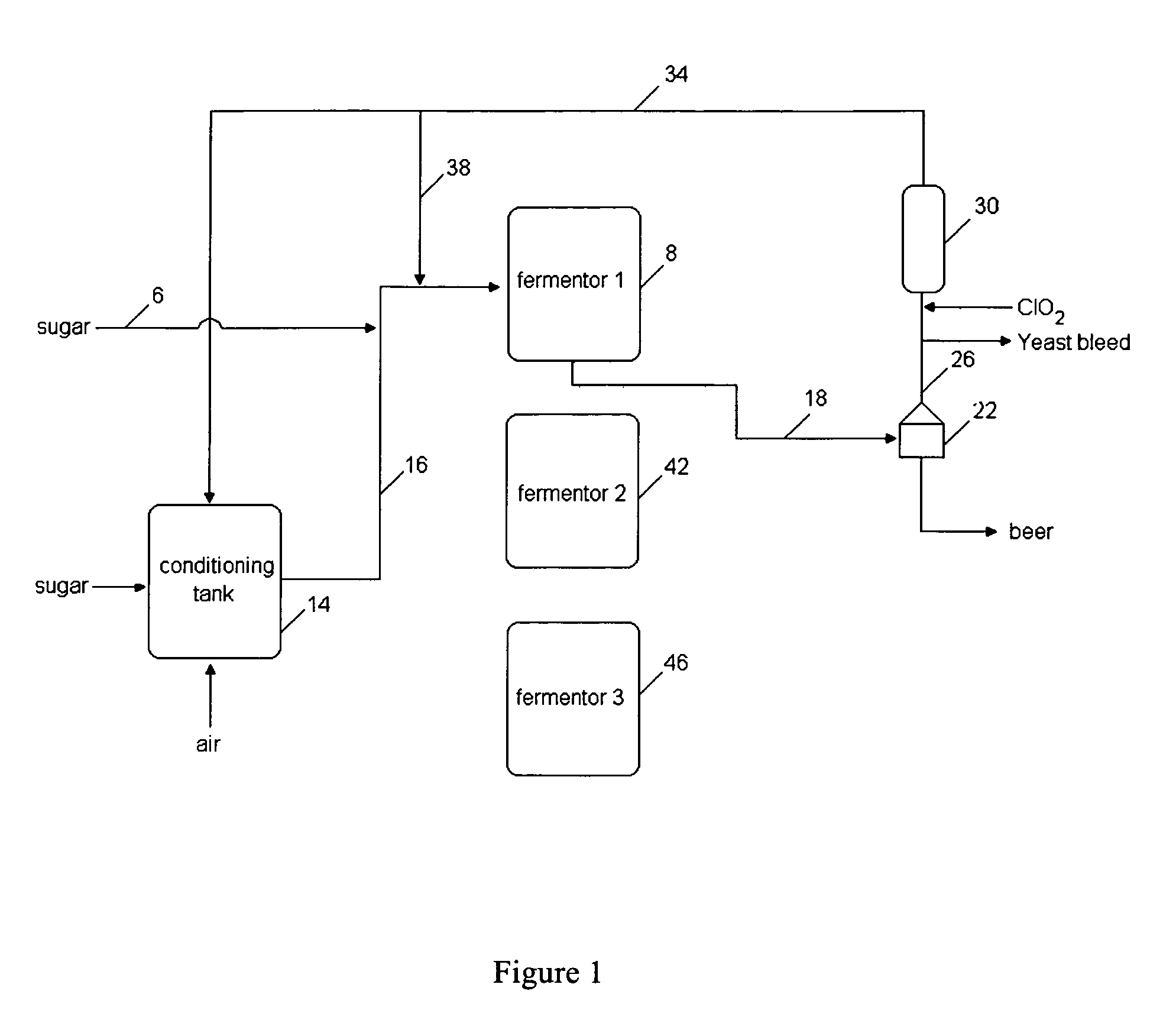
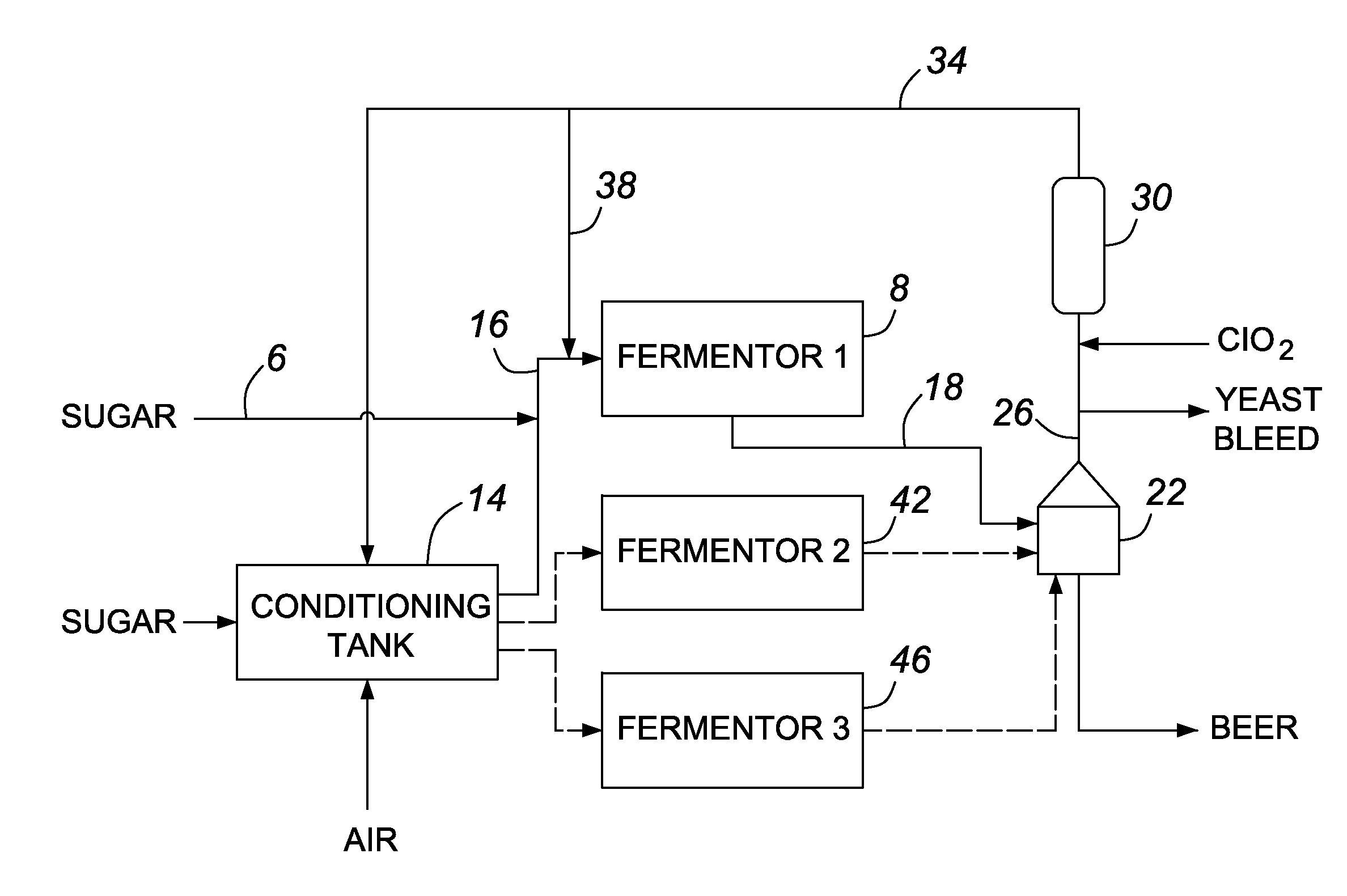
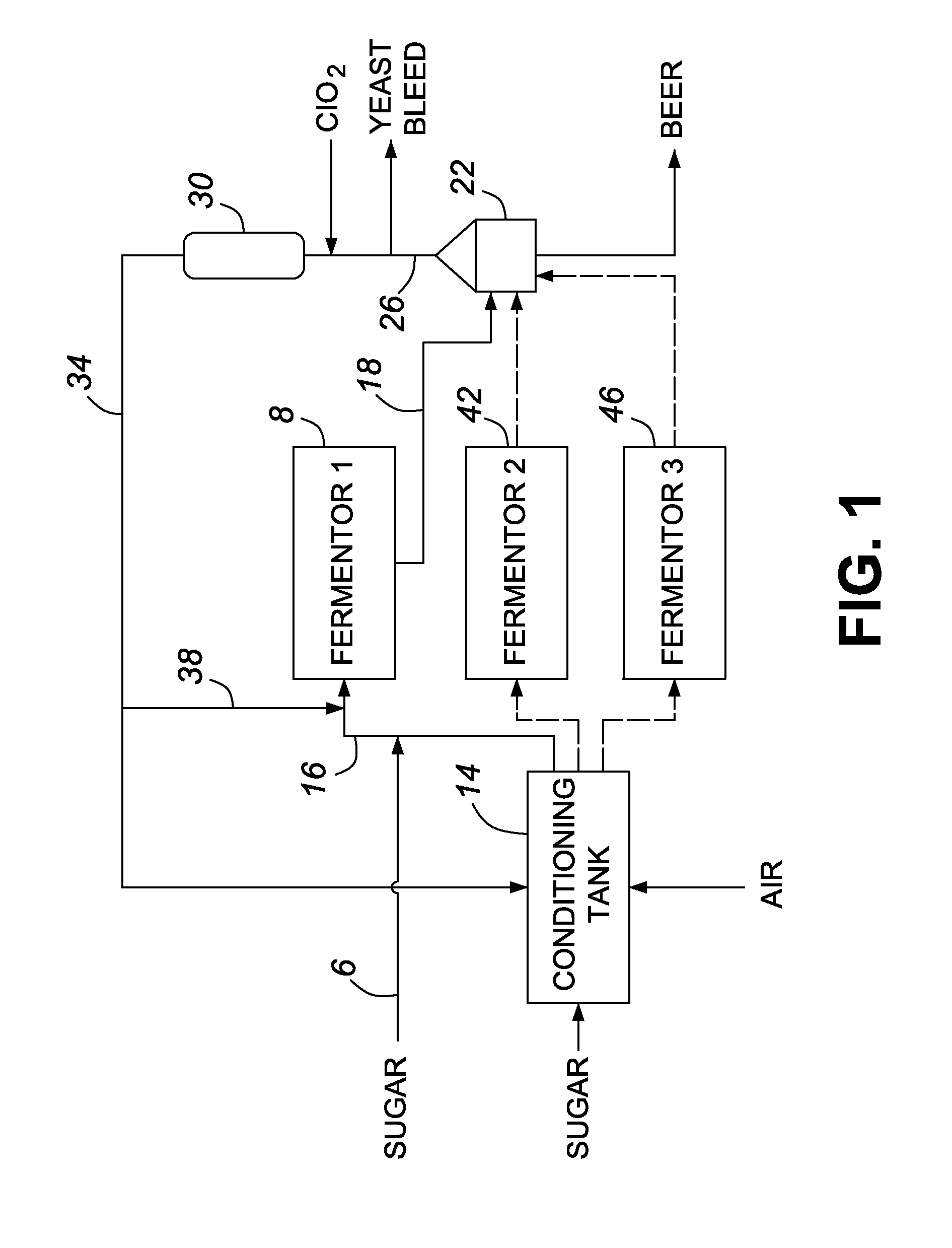
A method for obtaining a fermentation product from a sugar hydrolysate obtained from a feedstock containing hemicellulose, by (i) removing suspended fiber solids from said sugar hydrolysate to obtain a clarified sugar solution; (ii) fermenting xylose in the clarified sugar solution in a fermentation reaction with yeast to produce a fermentation broth comprising the fermentation product; (iii) separating the yeast from the fermentation broth to produce a yeast slurry; (vi) treating the yeast slurry thus obtained with an oxidant to kill microbial contaminants, thereby an oxidant-treated yeast slurry; (v) re-introducing at least a portion of the oxidant-treated yeast back to step (ii) to increase the concentration of yeast in said fermentation reaction; and (vi) recovering the fermentation product.
Owner:IOGEN ENERGY CORP
Method of and equipment for washing, disinfecting and/or sterilizing health care devices
InactiveUS20040037737A1Inexpensive, but effectiveLavatory sanitoryDeodrantsPathogenic microorganismBiological membrane
The invention provides a method for automatically washing, disinfecting and / or sterilizing health care equipment and / or cooking and catering utensils. The method includes the steps of placing the equipment to be washed in an enclosure; introducing a first electrochemically activated aqueous solution into the enclosure; and either sequentially or simultaneously introducing a second electrochemically activated aqueous solution into the enclosure. The first solution is characterised therein that it has dispersing or surfactant characteristics for at least partially dispersing a biofilm, pathogenic microorganisms, contamination or the like. The second solution is characterised therein that it has biocidal characteristics for killing microorganisms and disinfecting and / or sterilizing the equipment. The invention also extends to an apparatus for use in the above method.
Owner:RADICAL WATERS IP
Purification materials and method of filtering using the same
InactiveUS20050098495A1Reduce recordsRule out the possibilityMembrane filtersLoose filtering material filtersParticulatesCyst
The invention relates to a purification material (1) comprising filtration particulate matter aggregated with a first binder and further processed with a second binder to generate a porous fluid filtration material or a non-pourous coating, a filtering device comprising a housing (11) and the purification material (1), and a method of filtering and / or purifying a fluid including water or other solutions containing chemical and microbiological contaminants, such as fluids containing heavy metals, pesticides, by products of oxidation chemicals and including cysts, bacteria and / or viruses, where the fluid is passed through ot made to contact a surface of the purification material (1).
Owner:HUGHES KENNETH D
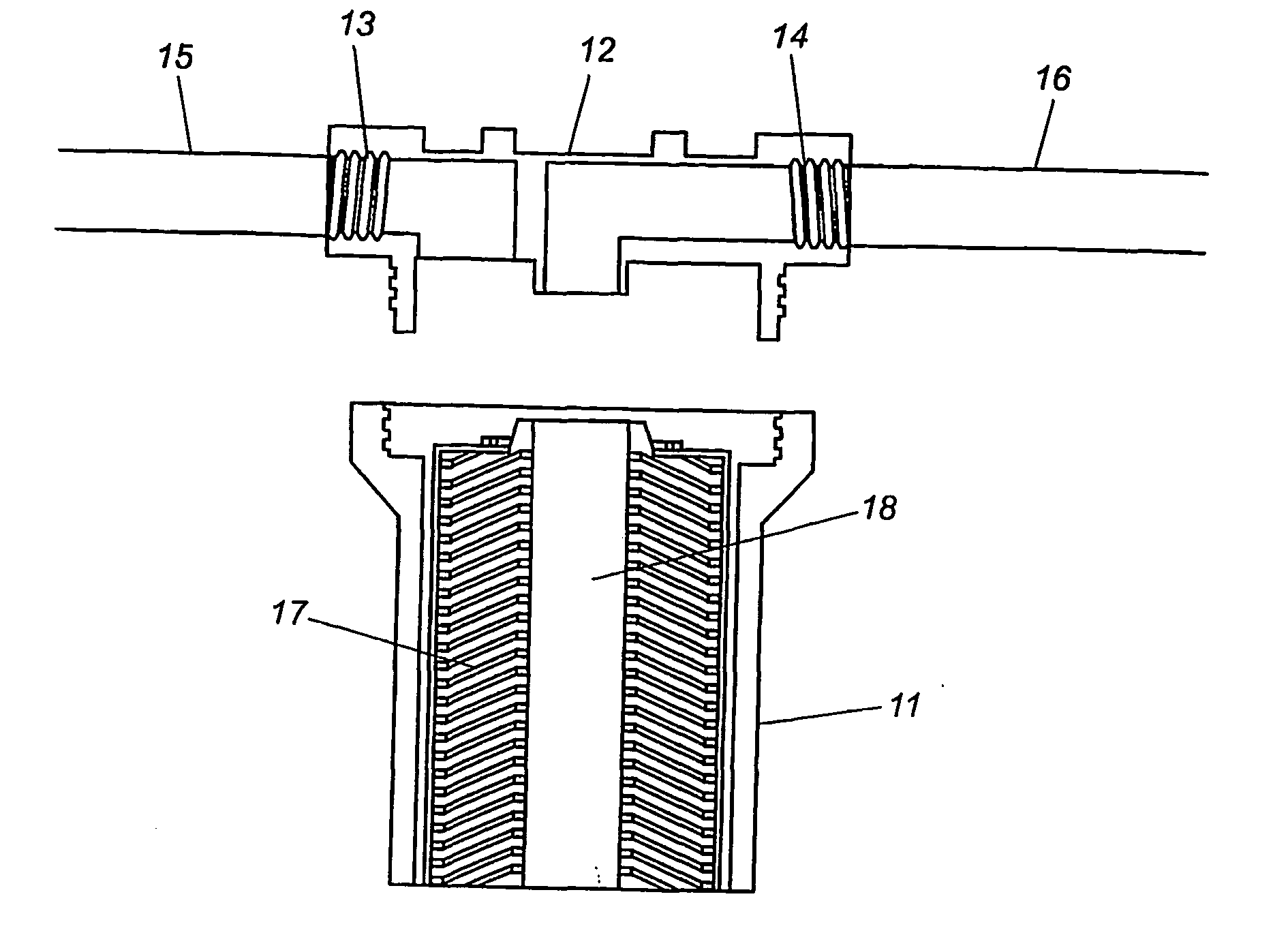
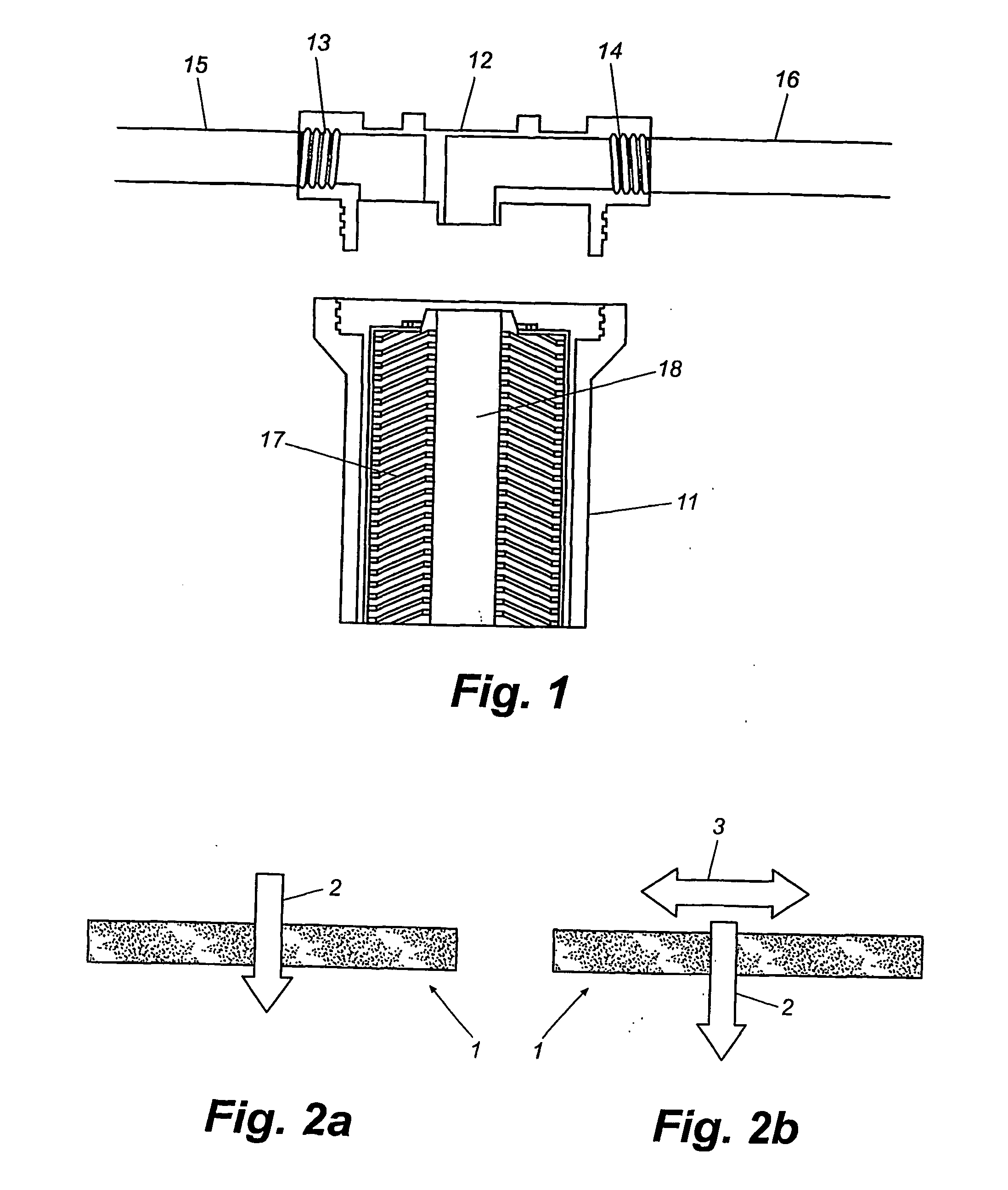
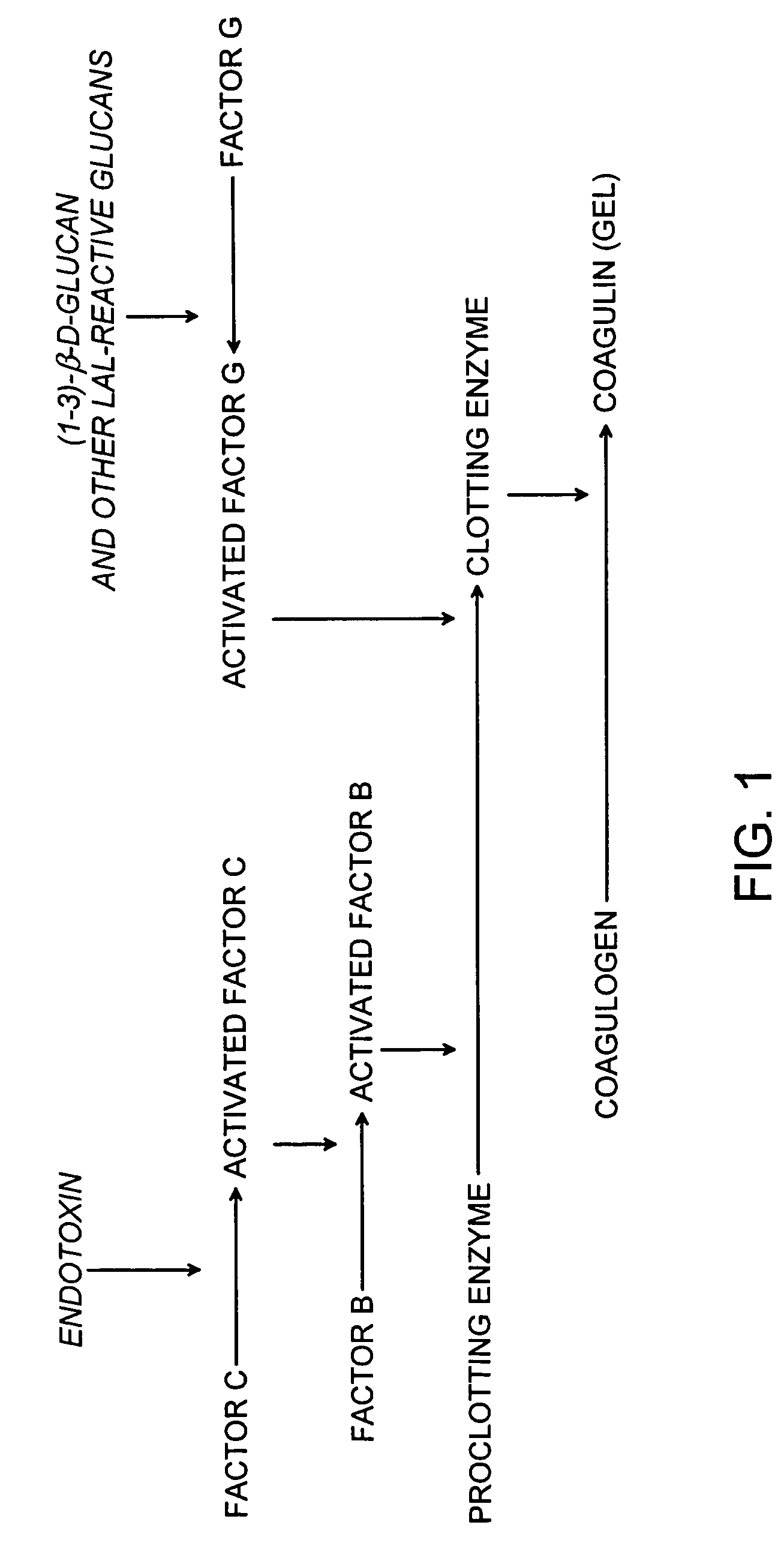
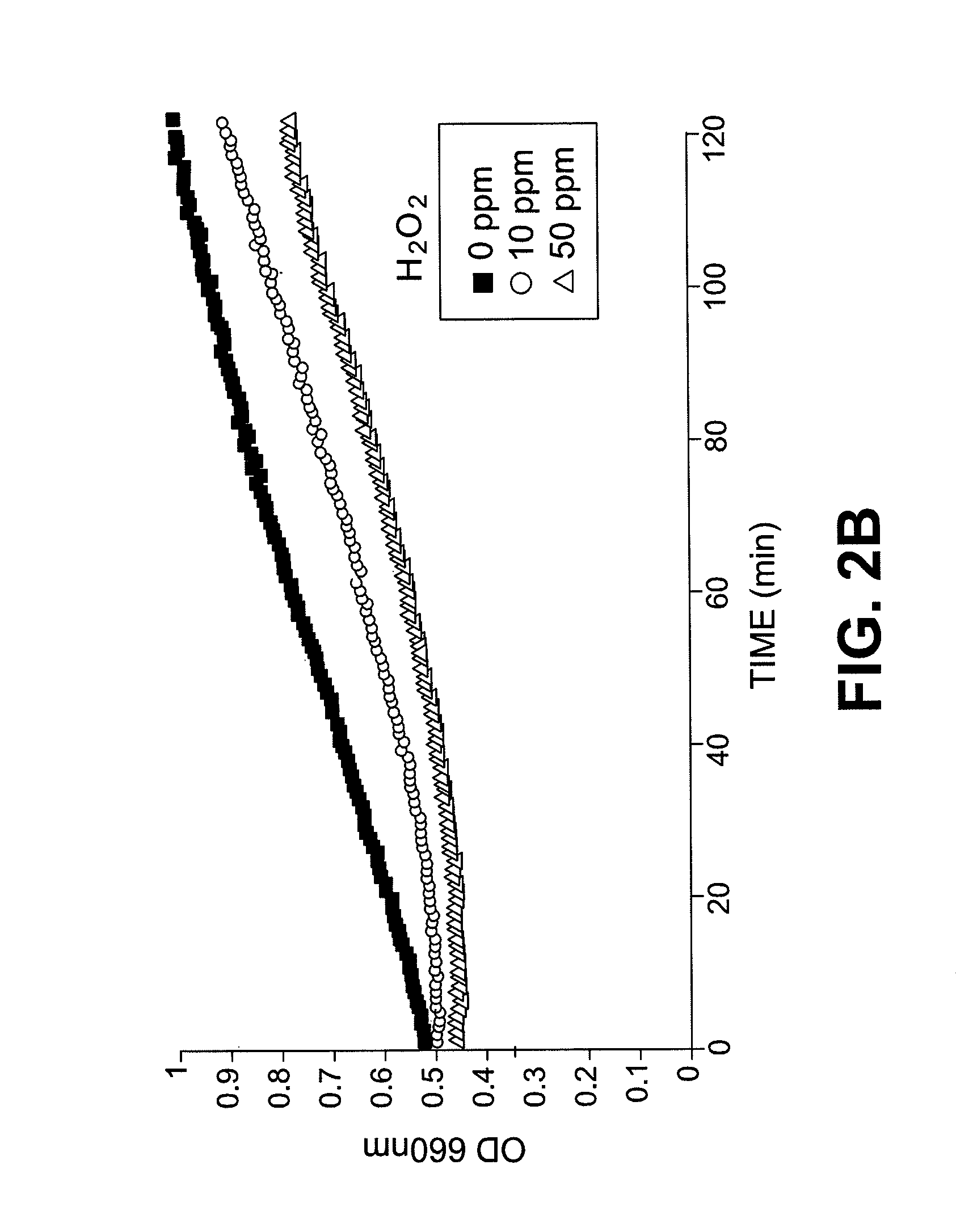
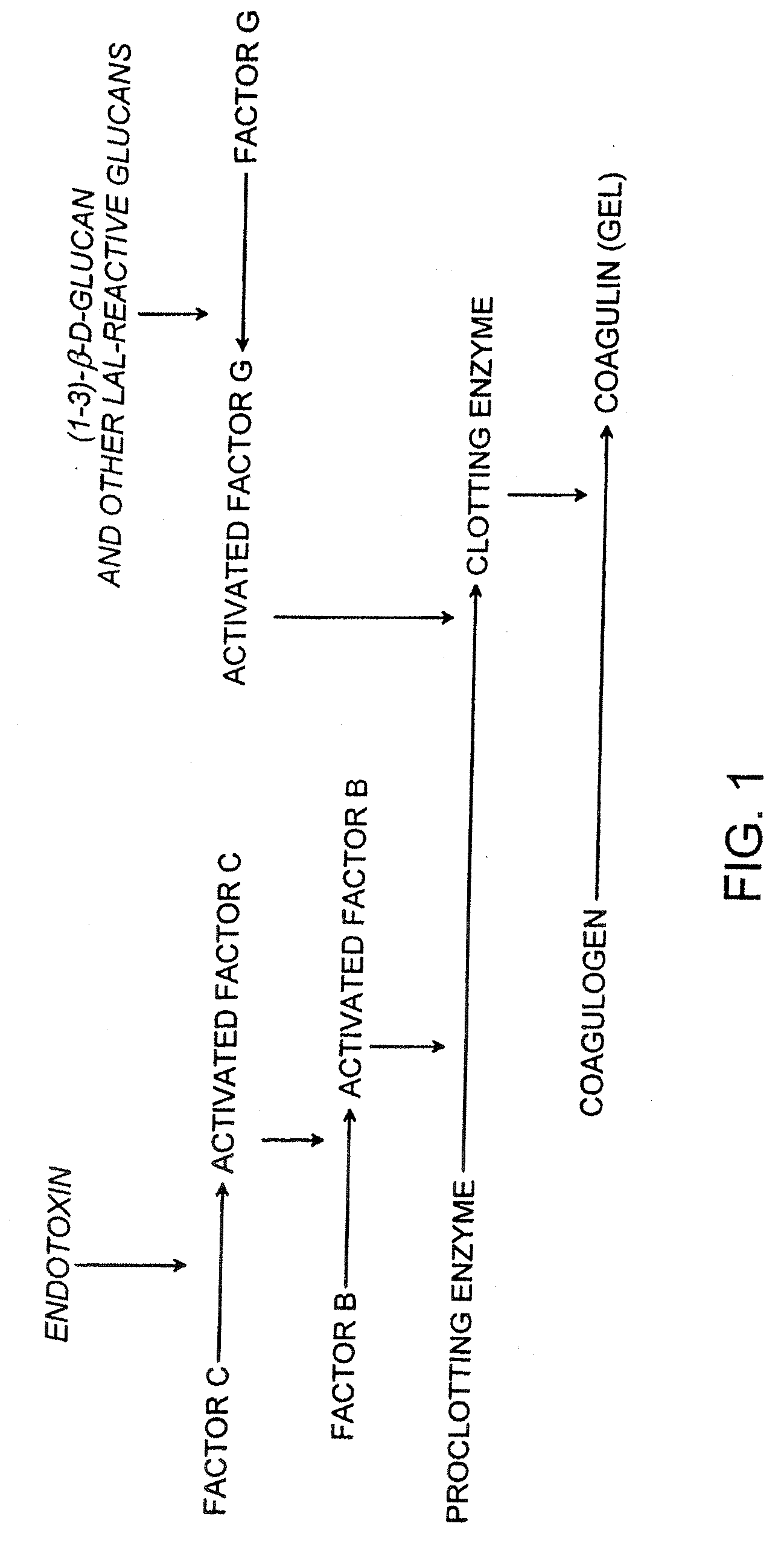
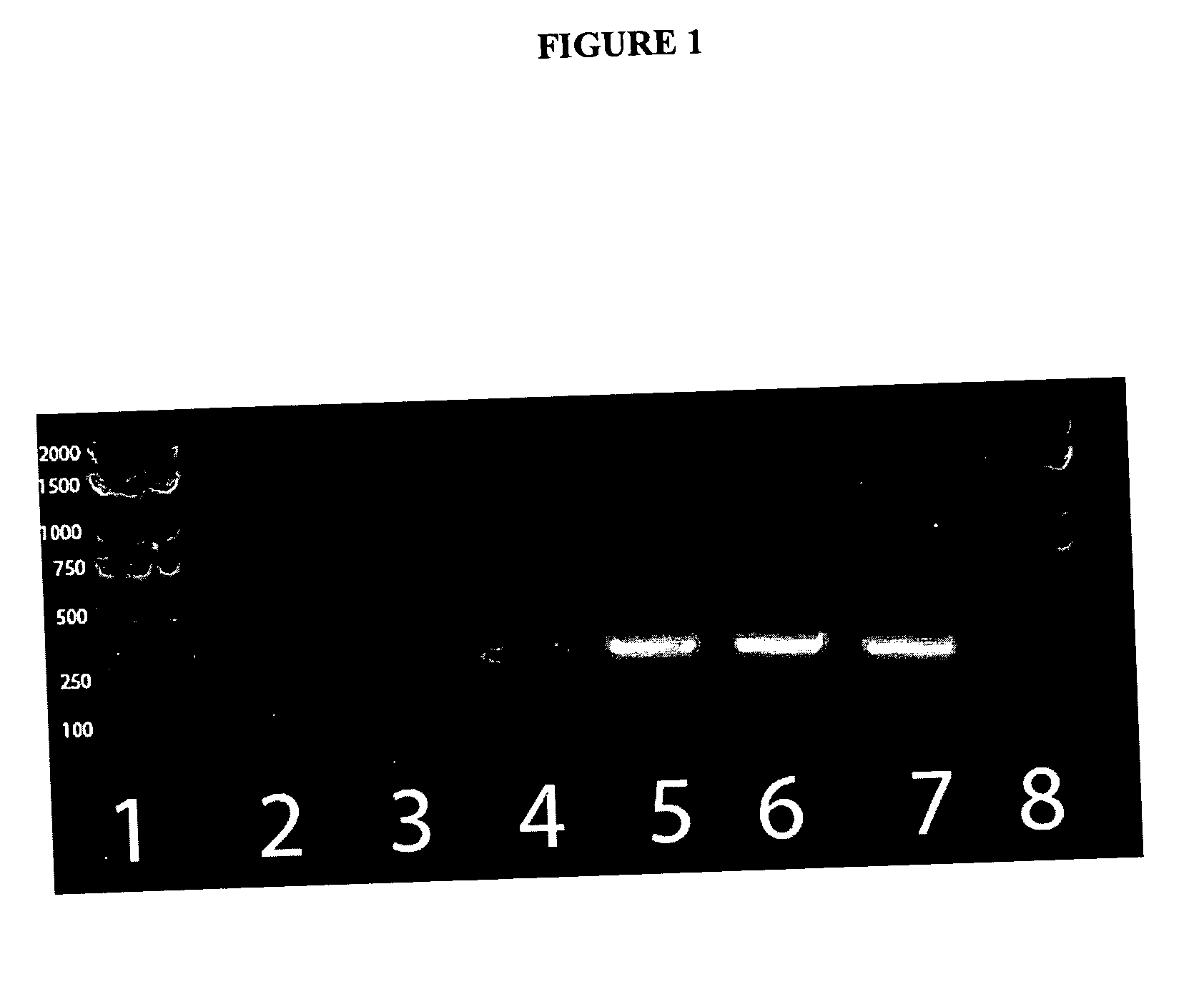
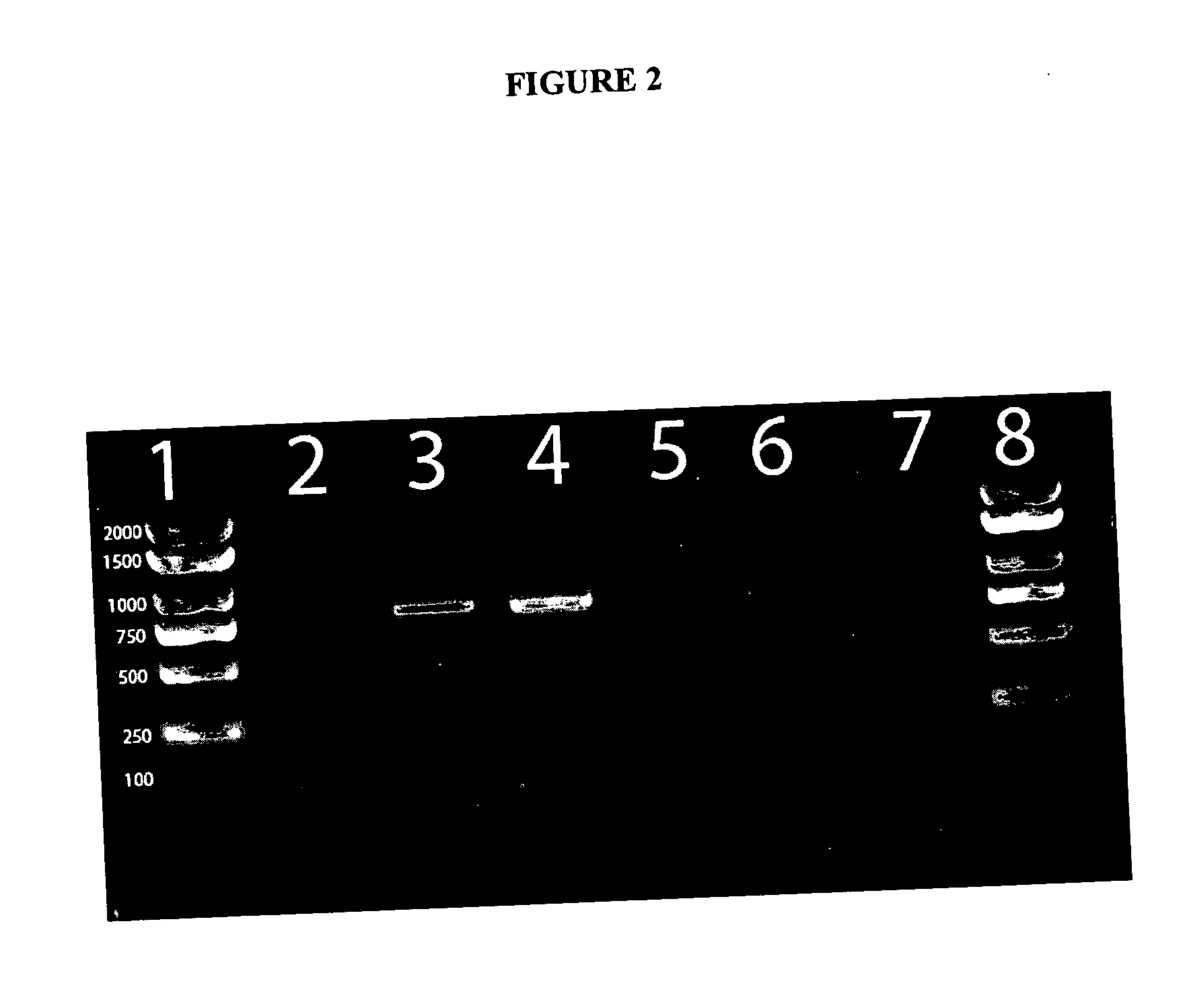
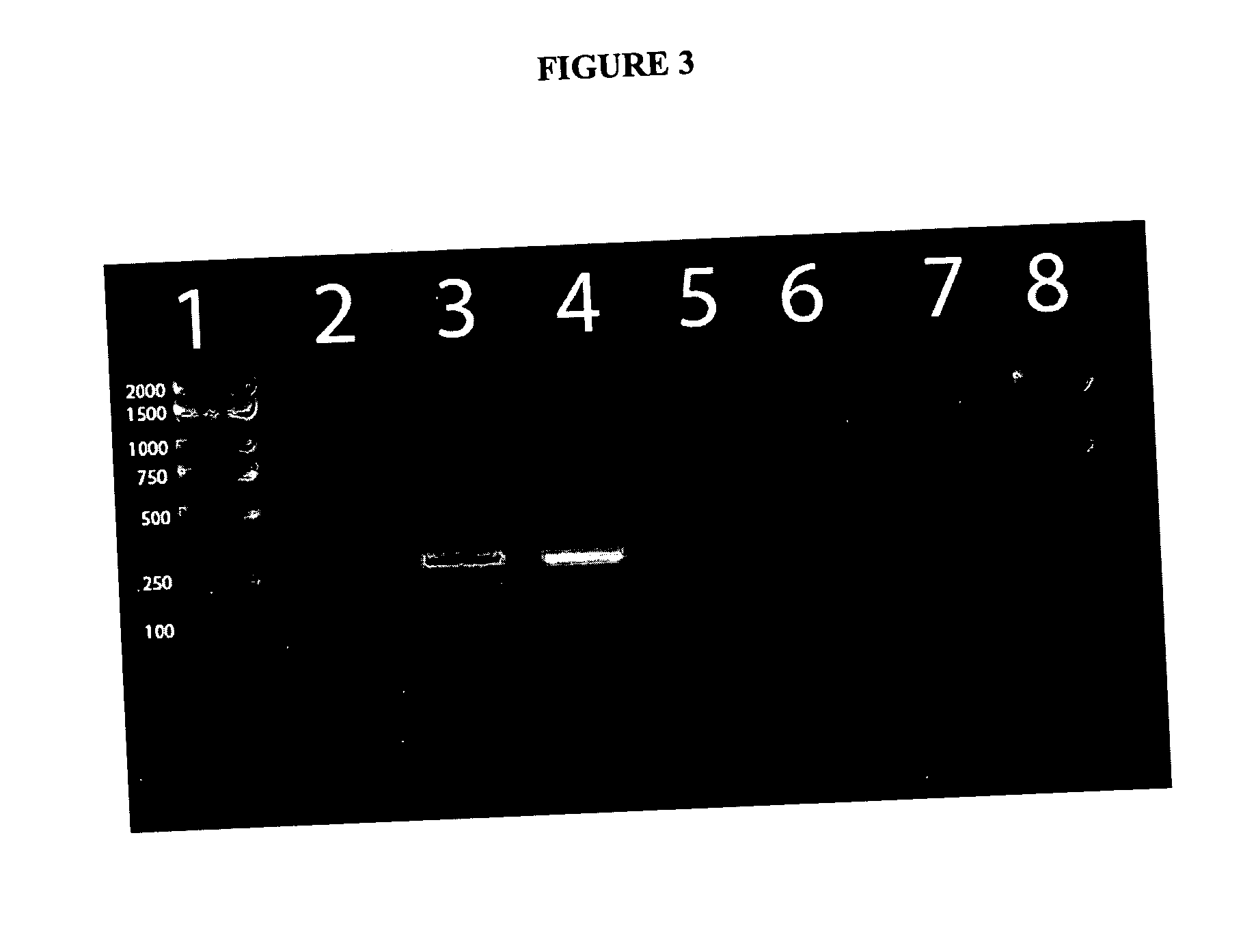
Methods and compositions for the detection of microbial contaminants
ActiveUS7329538B2Bioreactor/fermenter combinationsBiological substance pretreatmentsMicroorganismCell lysates
The invention provides methods and compositions for the detection and / or quantification of a microbial contaminant, for example, a bacterial endotoxin or a glucan, in a sample. In particular, the invention provides a test cartridge useful in the practice of hemocyte lysate-based assays for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides methods of making and using such cartridges. In addition, the invention provides a rapid, sensitive, multi-step kinetic hemocyte lysate-based assay for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides a glucan-specific lysate that can be used in a variety of assay formats, including, for example, a test cartridge, optionally configured to perform a kinetic assay.
Owner:CHARLES RIVER LAB INC
Method and apparatus for pollution control of confined spaces
A method of improve the removal of particulate matter, heavy metals, neutralizing acid, and kill microorganism pollutants, known to be in contaminated air volumes of occupied confined spaces, when exposed in close contact under pressure to a mixture of alkaline sorbent materials, having a known synergism between said pollutants using a self propelled fluidized bed reactor and packed bed filter apparatus system to optimize the contact collection efficiency of submicron particles and organic compounds.
Owner:AVINA DAVID CHRISTOPHER
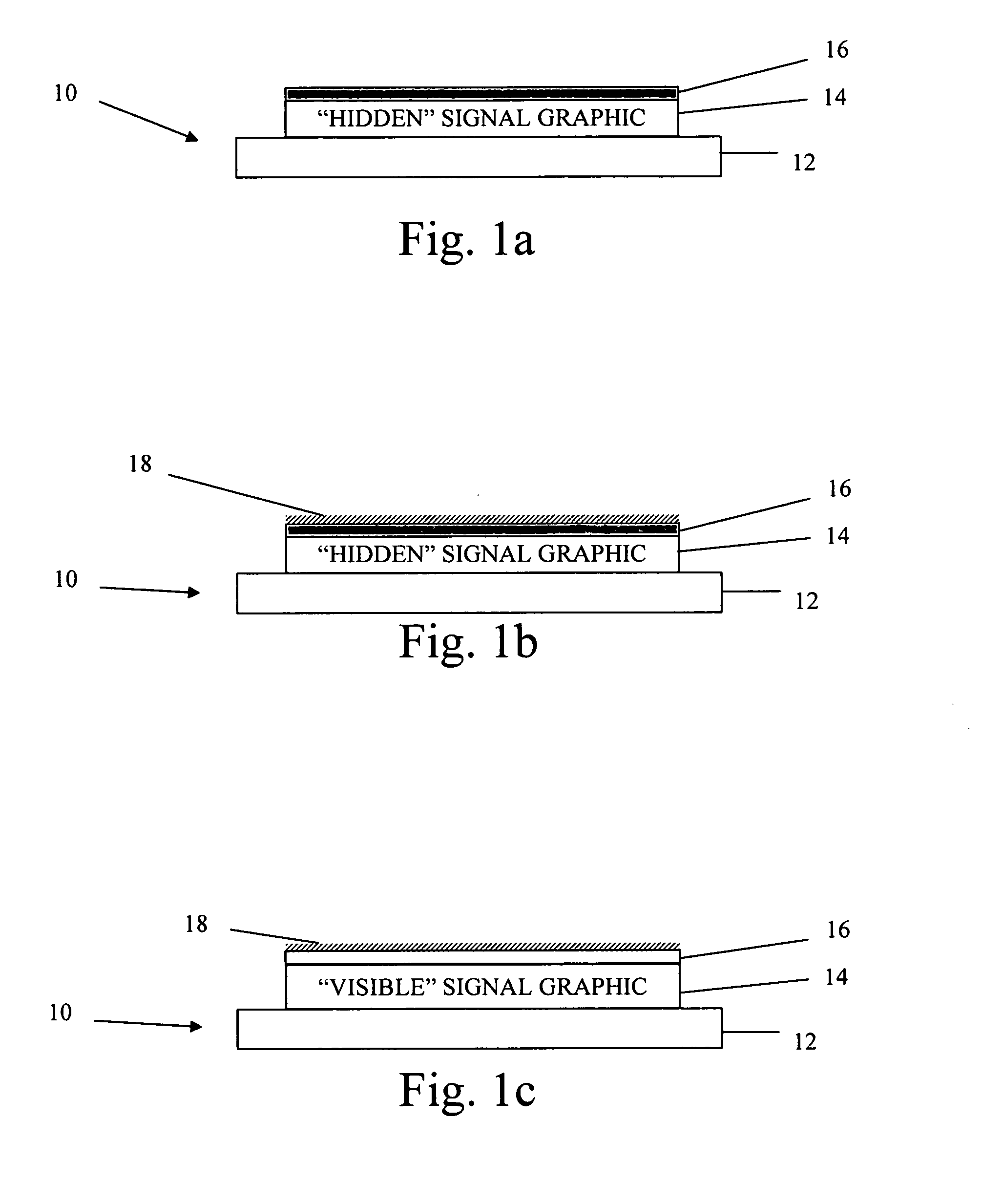
Microbe-sensitive indicators and use of the same
InactiveUS20080057534A1Microbiological testing/measurementSynthetic resin layered productsGraphicsChange color
A microbe contamination detection system is described. The microbe contamination detection system alerts a user or other individual in the event of microbial contamination of the system. The signal graphic is revealed when an obscuring graphic reacts with the microbial contamination to change color and / or become at least substantially transparent. The microbe contamination detection system may be used as a stand-alone device or may be incorporated as part of various articles or products, for instance, health care or food service preparation protective garments. Methods for using the microbe contamination detection system are also described.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Microbiological water filter
InactiveUS6957743B2Rule out the possibilityReadily availableLiquid degasificationMicroorganismsMicroorganismFiltration
A method and device for the filtration and / or purification of fluids water or other solutions containing microbiological contaminants, such as fluids containing including bacteria and / or viruses, where the fluid water is passed through a purification material composed of apatite and absorption media in a fixed binder matrix.
Owner:WATERVISIONS INT
Reverse osmosis water purifier
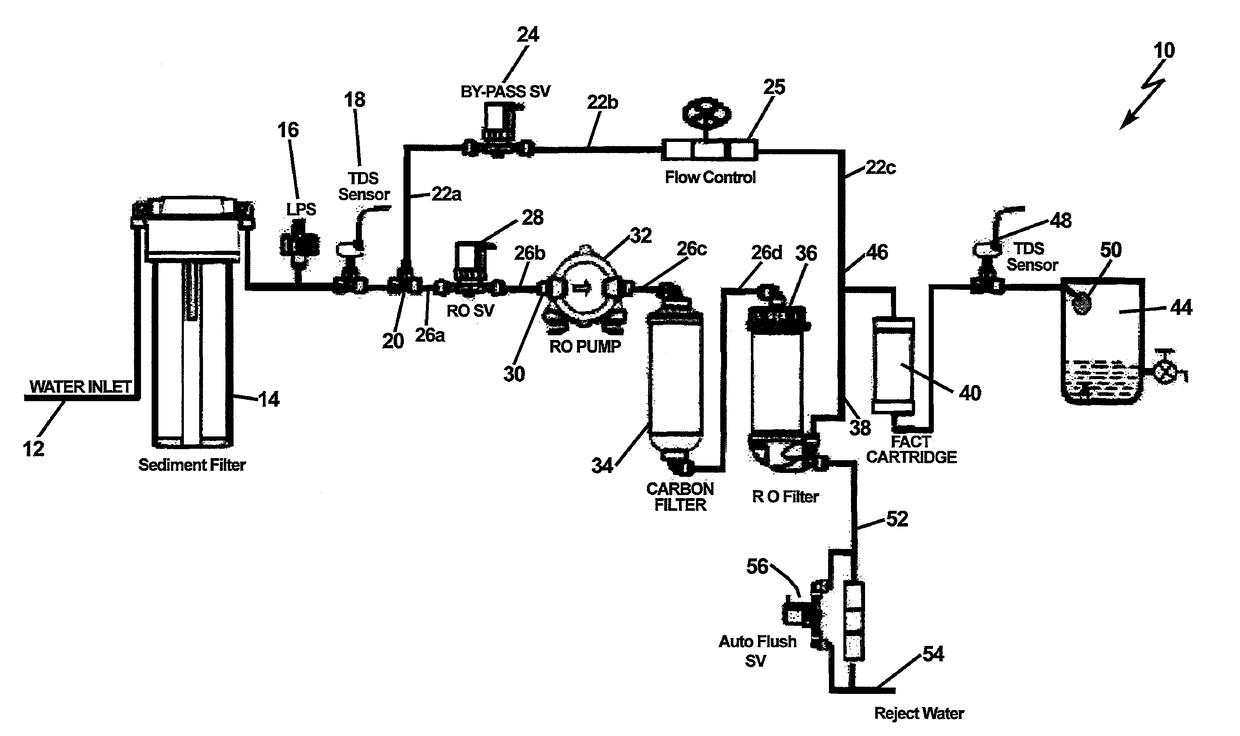
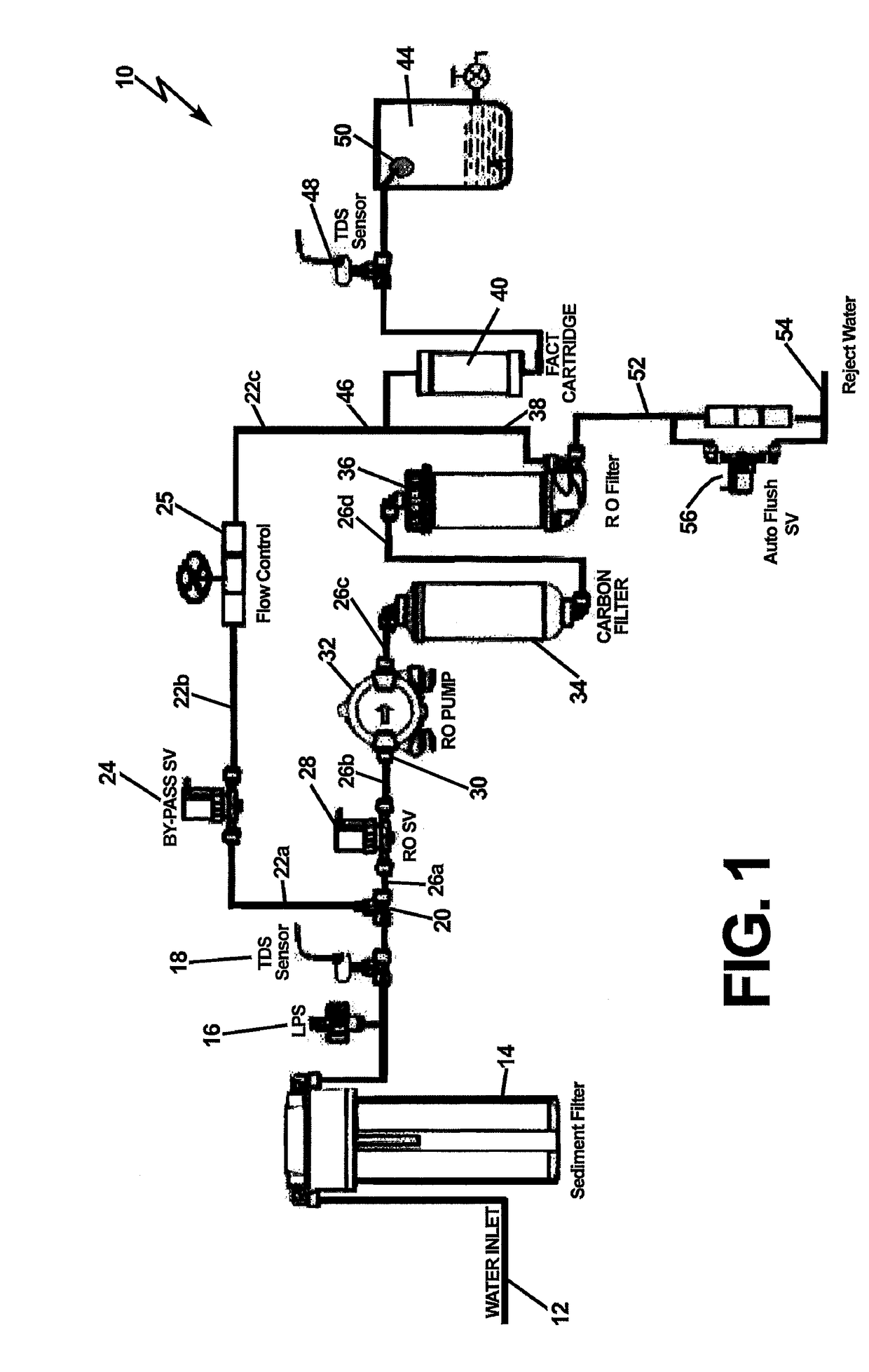
InactiveUS20170129795A1Treatment involving filtrationReverse osmosisFiltrationTotal dissolved solids
A reverse osmosis water purifier that monitors Total Dissolved Solids (TDS) at the onset of entering the water filtration system and downstream upon exiting the system. A comparison of the TDS levels is made to each other or predetermined levels, and action is taken regarding whether to bypass the RO filter, or continue filtering through the RO membrane, or combine the two fluid streams. A microbiological barrier filter is introduced in-line with the egress port of a reverse osmosis filter, and downstream of the bypass water circuit. The microbiological filter is utilized to remove microbiological contaminants from the output water, either directly from the RO filter output, or the bypass filter circuit, or both.
Owner:MARMON WATER SINGAPORE
Portable chemical sterilizer
InactiveUS20060099121A1Avoid accumulationSafe removalBiocideDead animal preservationContaminated equipmentEngineering
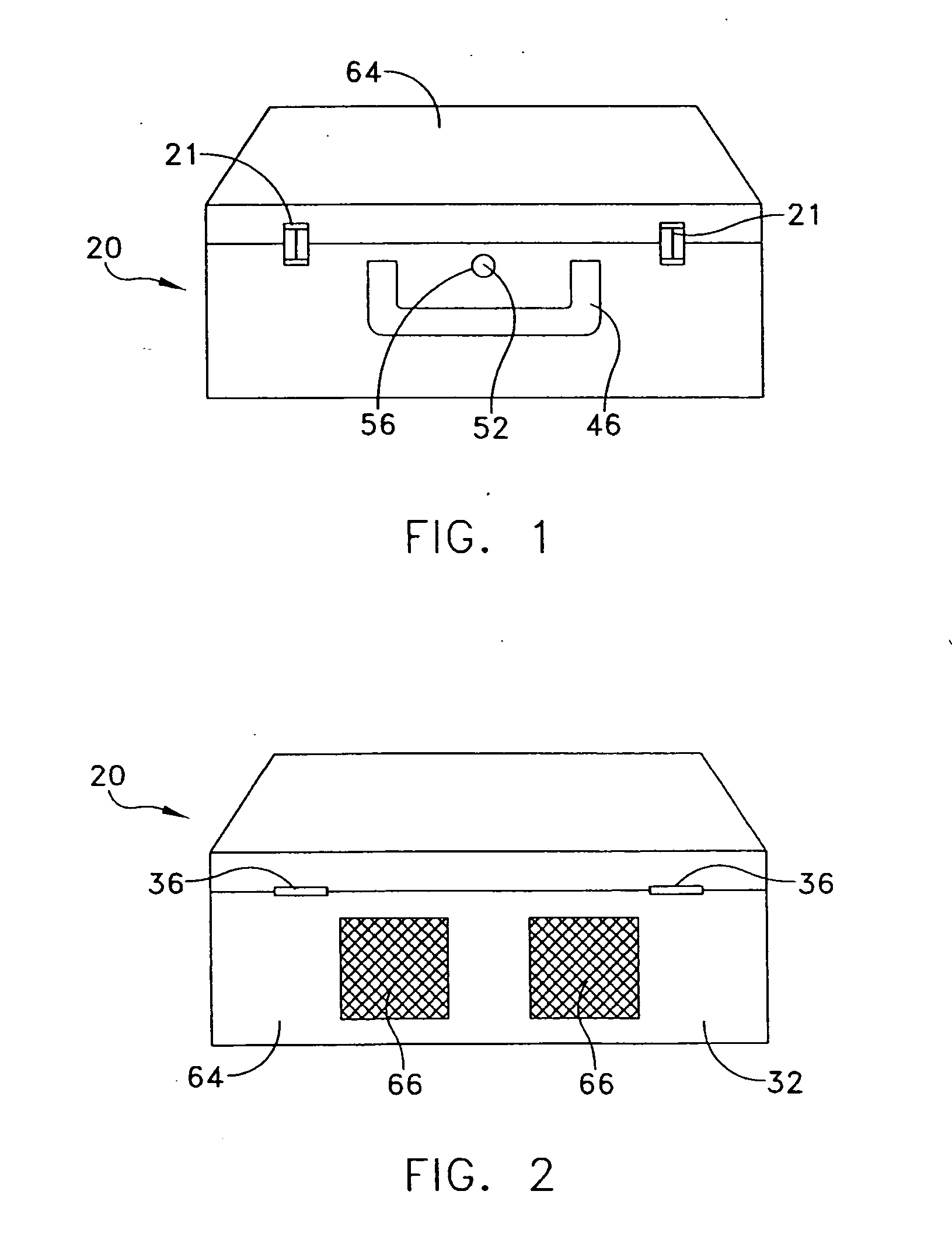
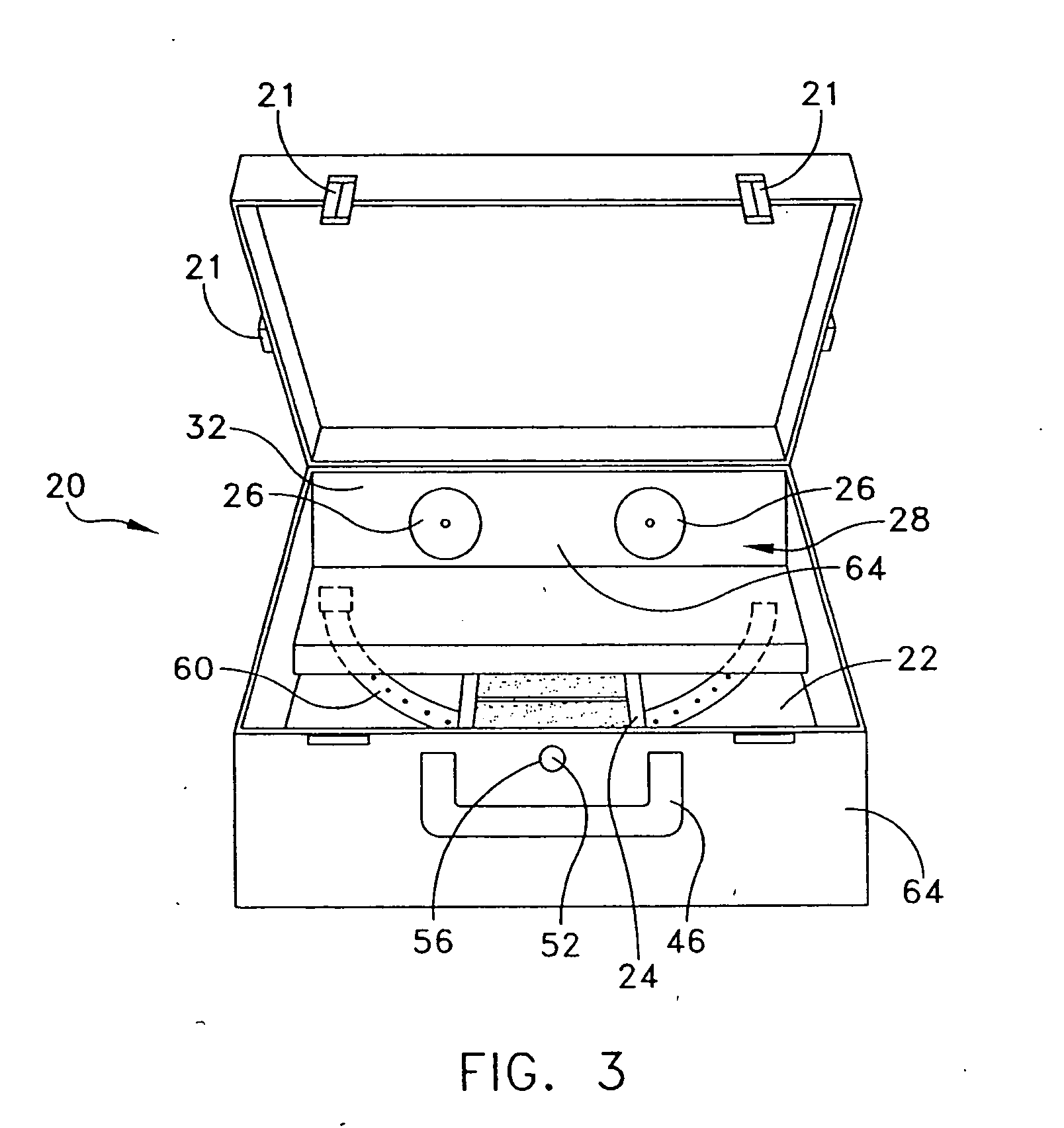
A portable, lightweight, easy-to-carry, reusable, durable, and environmentally-friendly assembly for sterilizing contaminated equipment using conditions of a chemical sterilant, heat, and humidity generated in situ without requiring external electricity, fuels, or other exogenous energy sources for operation. The carry assembly includes a plastic carry-case or insulated aluminum pressure vessel having an inner chamber for accepting microbiologically contaminated objects, a vessel disposed in the chamber for serving as a reaction chamber and / or boiler, a chemical combination which upon mixing generates at least minimally sufficient conditions of the sterilant, heat, and humidity to effect sterilization of the objects, and outlet valves mounted on the carry-case for controllably venting pressures above ambient air pressure.
Owner:ARMY USA AS REPRESENTED BY THE SEC OF THE
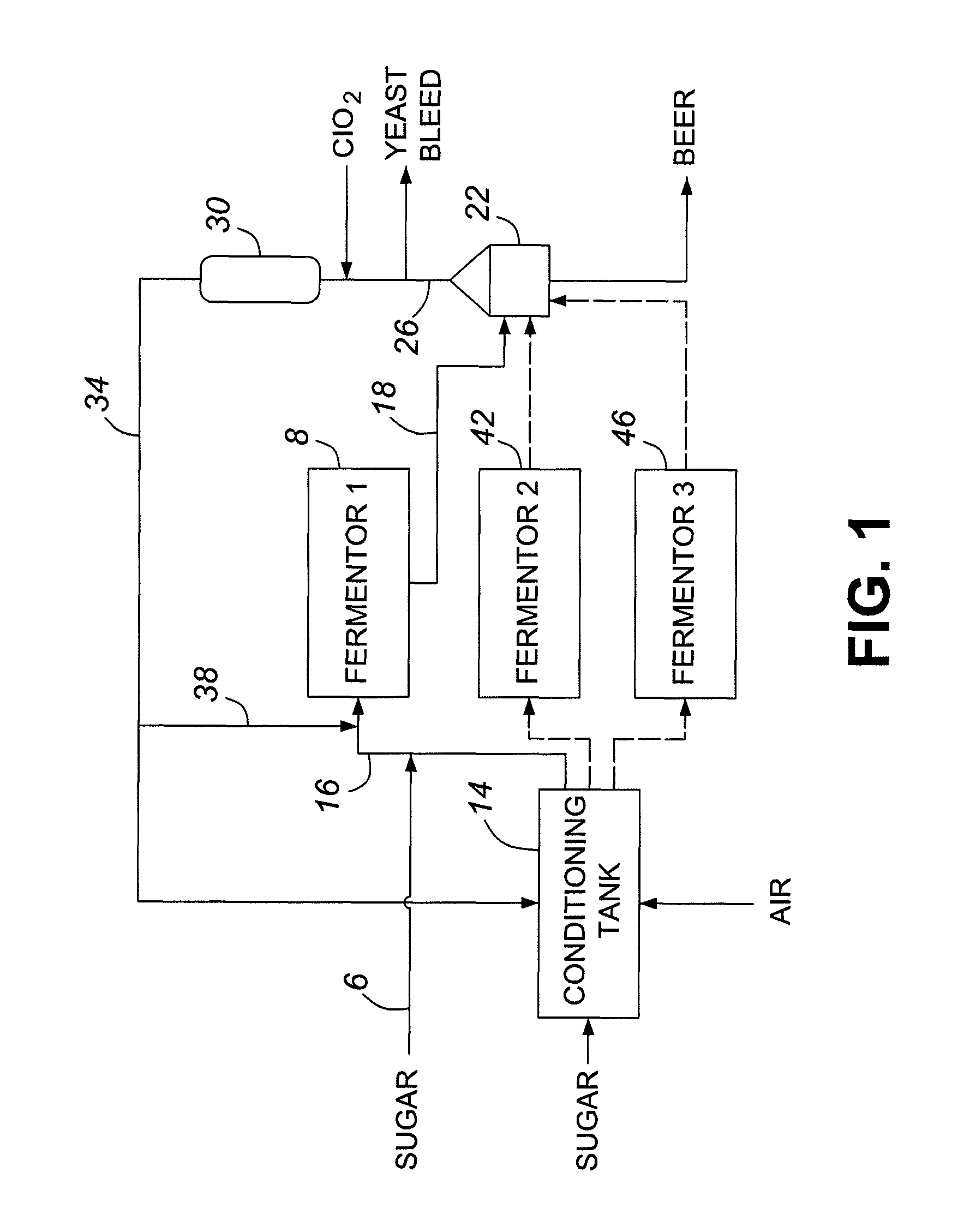
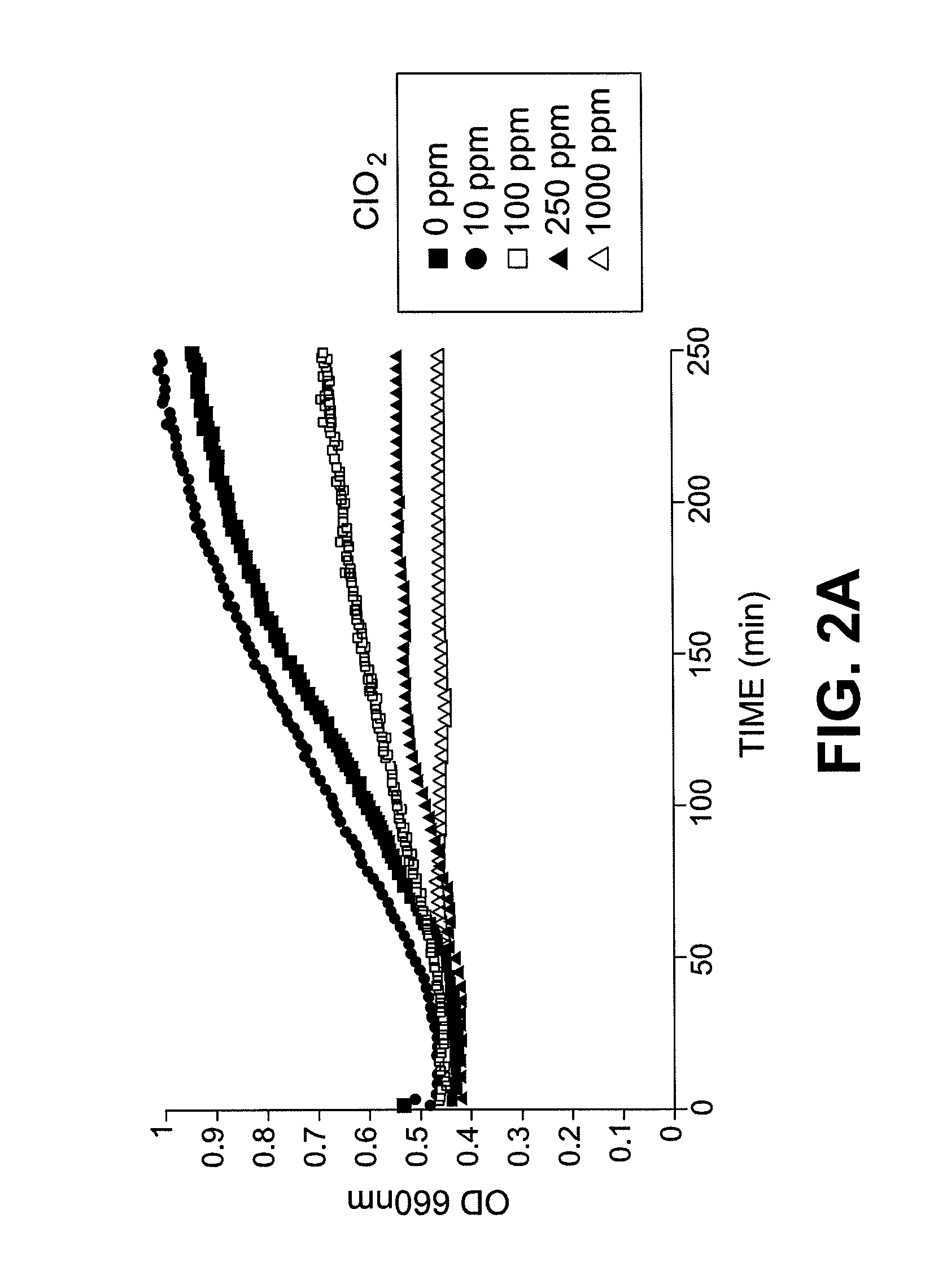
Method for the production of a fermentation product from a sugar hydrolysate
InactiveUS20110207192A1Improve viabilityReduction in fermentative capacityBiofuelsFermentationChlorine dioxideHydrolysate
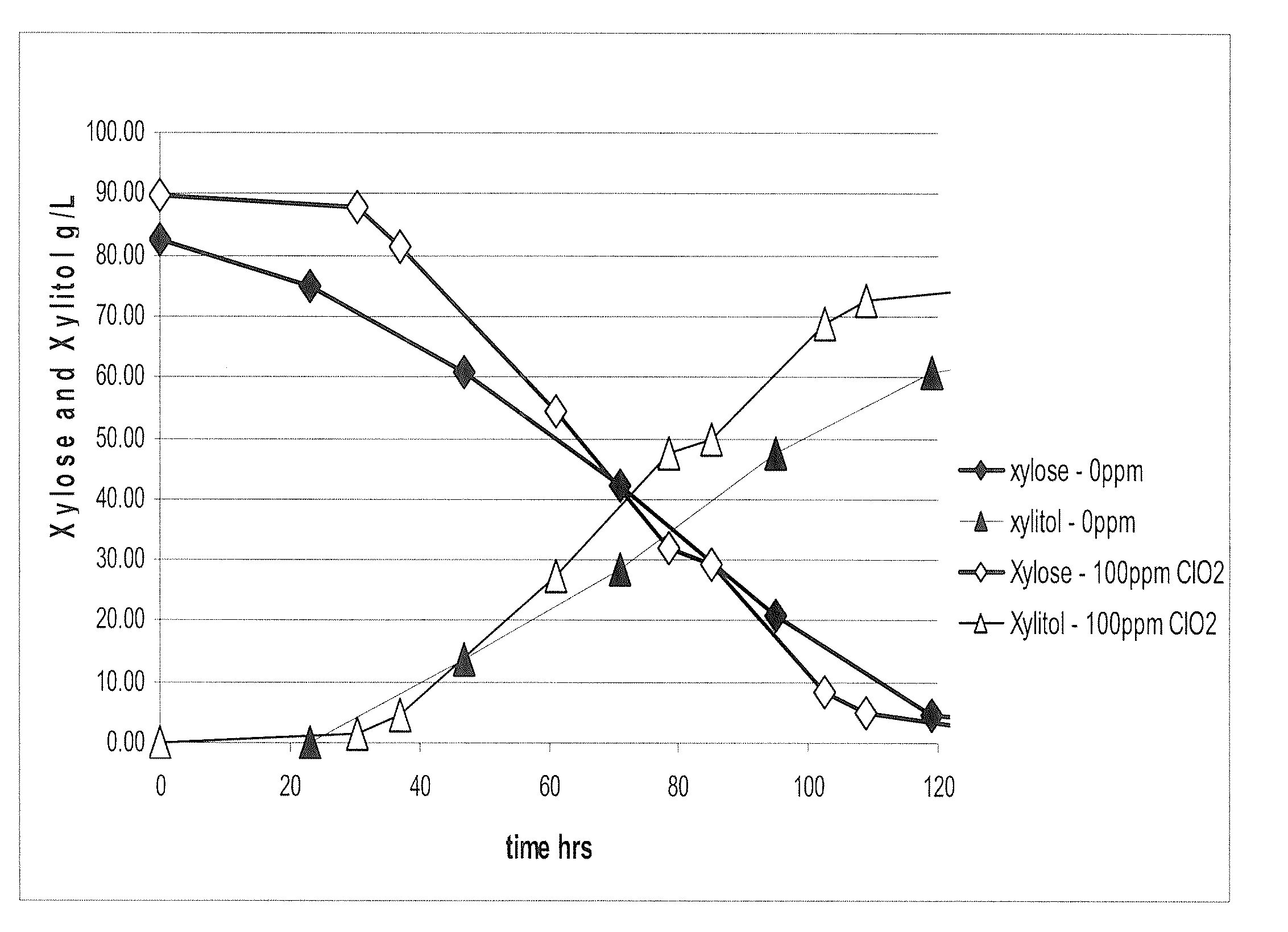
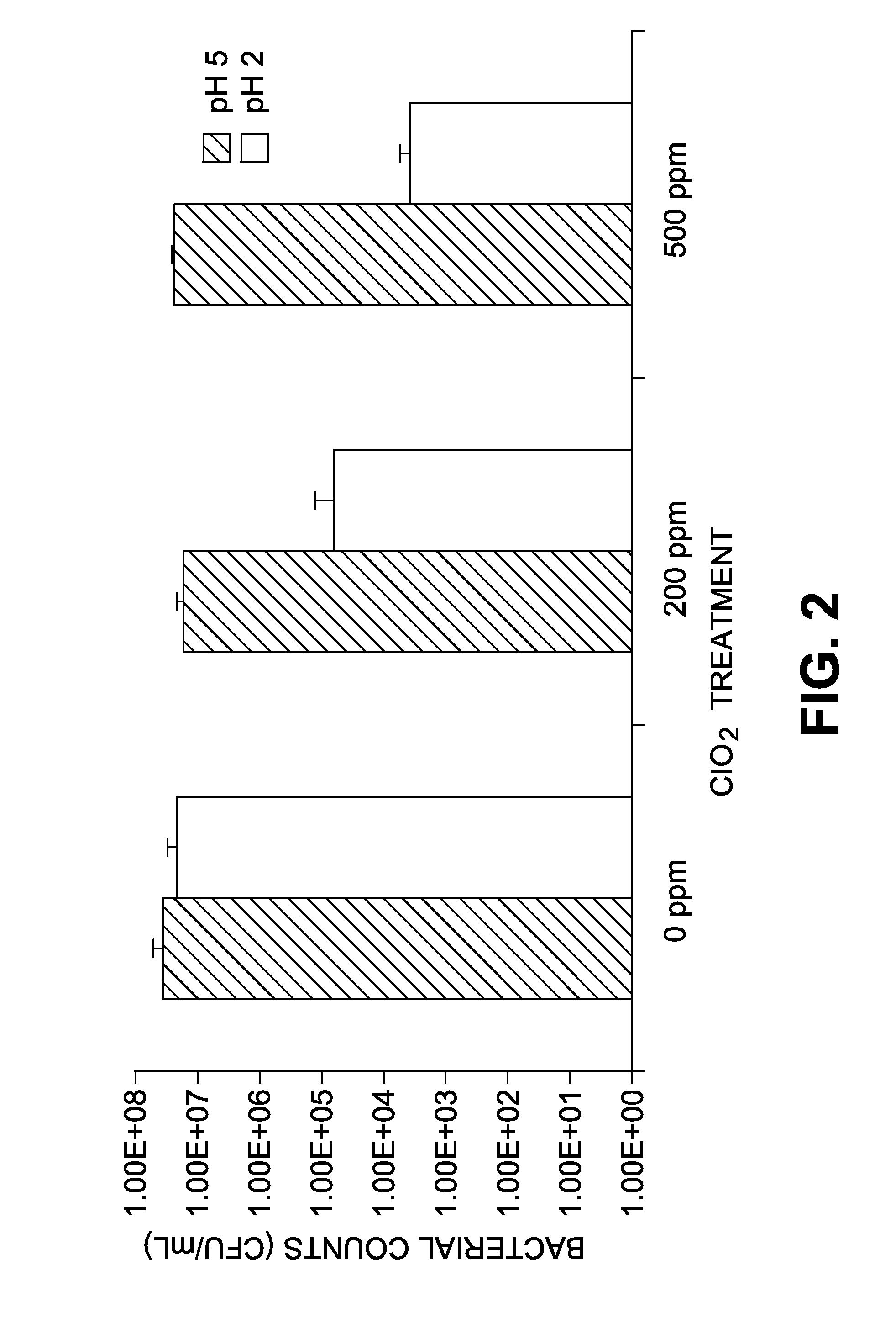
The present invention relates to a method for producing a fermentation product from a sugar hydrolysate. The method comprises fermenting the sugar hydrolysate in a fermentation system with yeast to produce a fermentation broth comprising a fermentation product; introducing acid and an oxidant, such as chlorine dioxide, to the fermentation system so as to expose microbial contaminants in the fermentation system at one or more stages to chlorine dioxide and a pH of less than 3.0; and recovering the fermentation product. In one example of the invention, a yeast slurry obtained from a yeast recycle step is treated with acid and the oxidant.
Owner:IOGEN ENERGY CORP
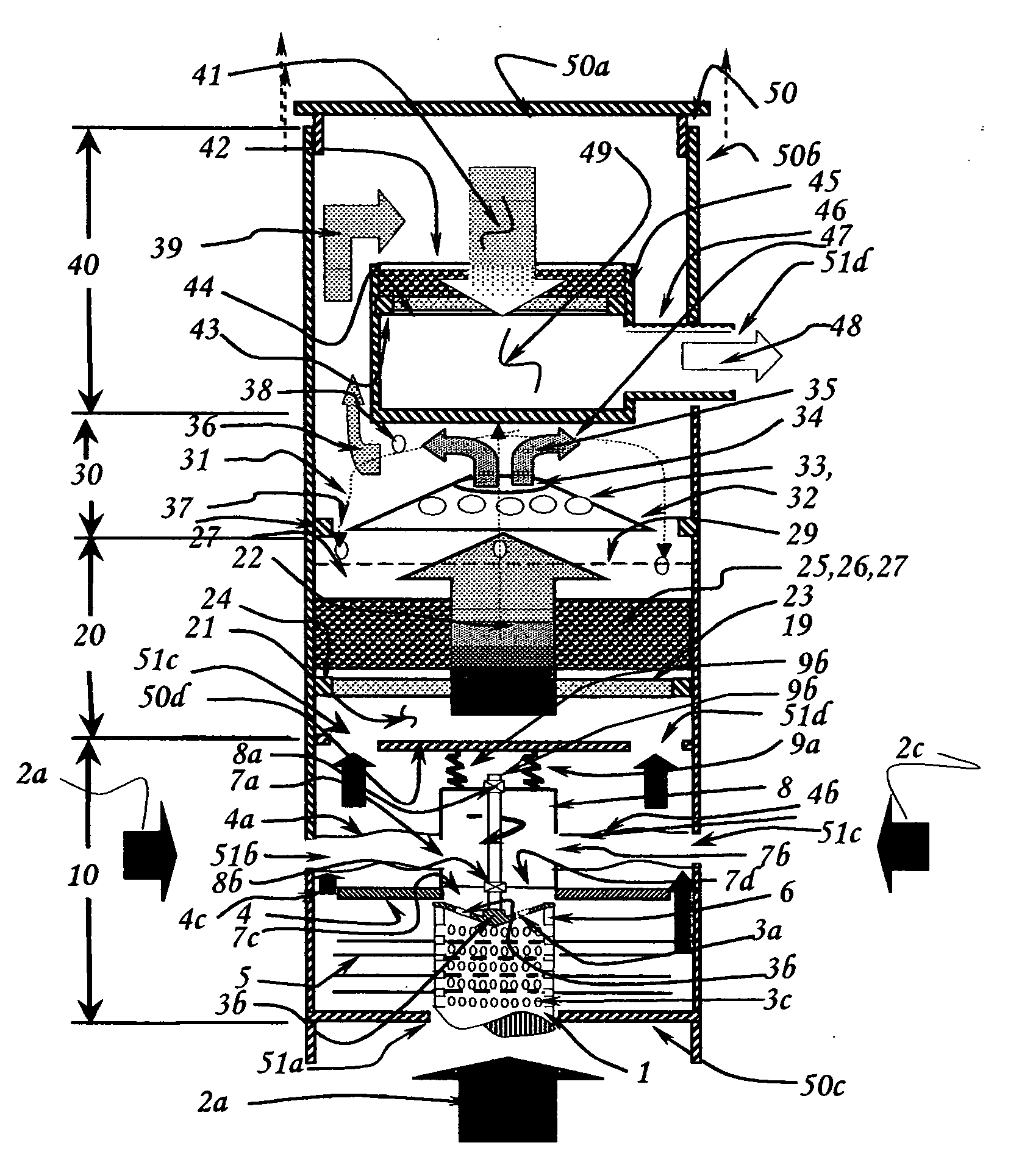
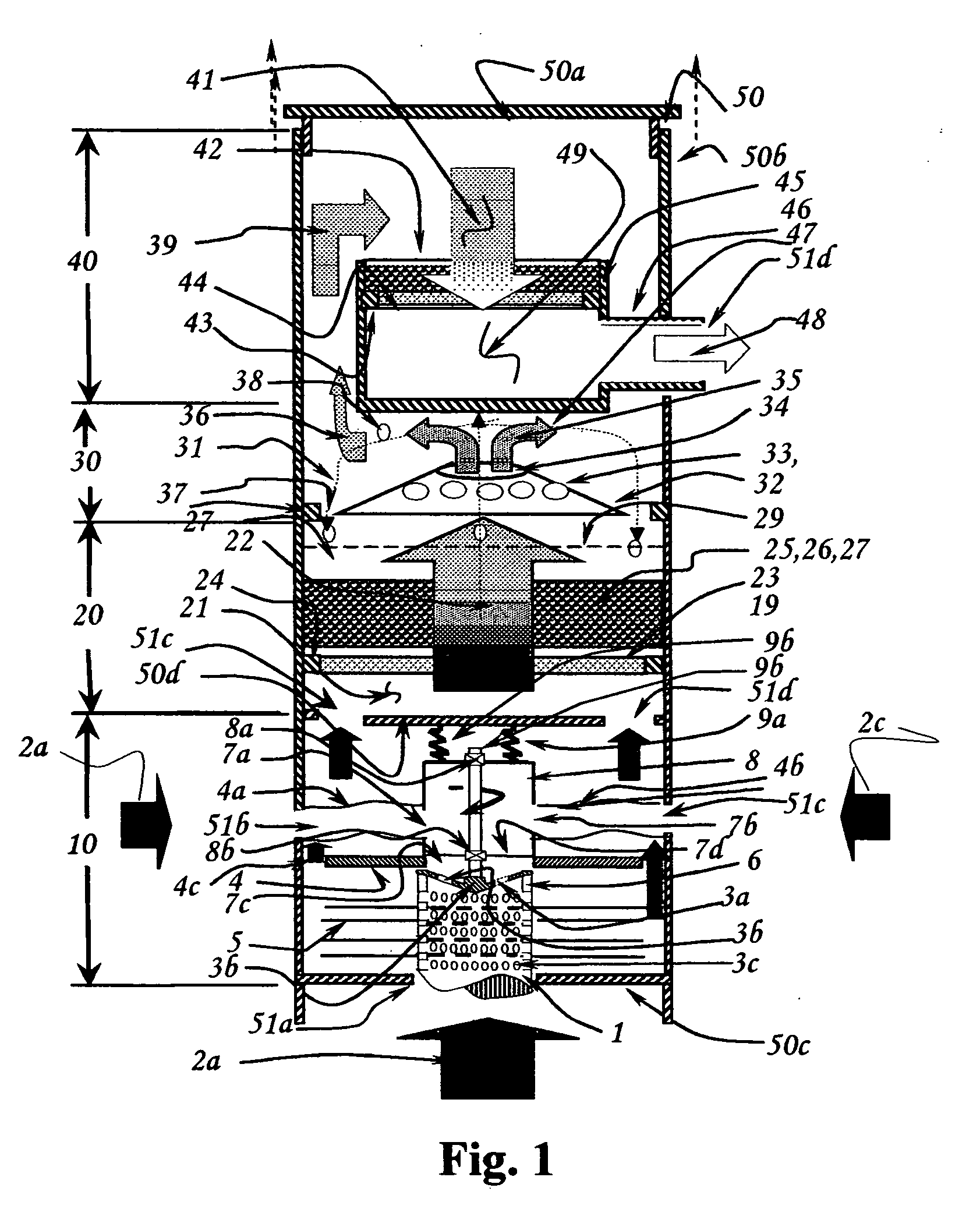
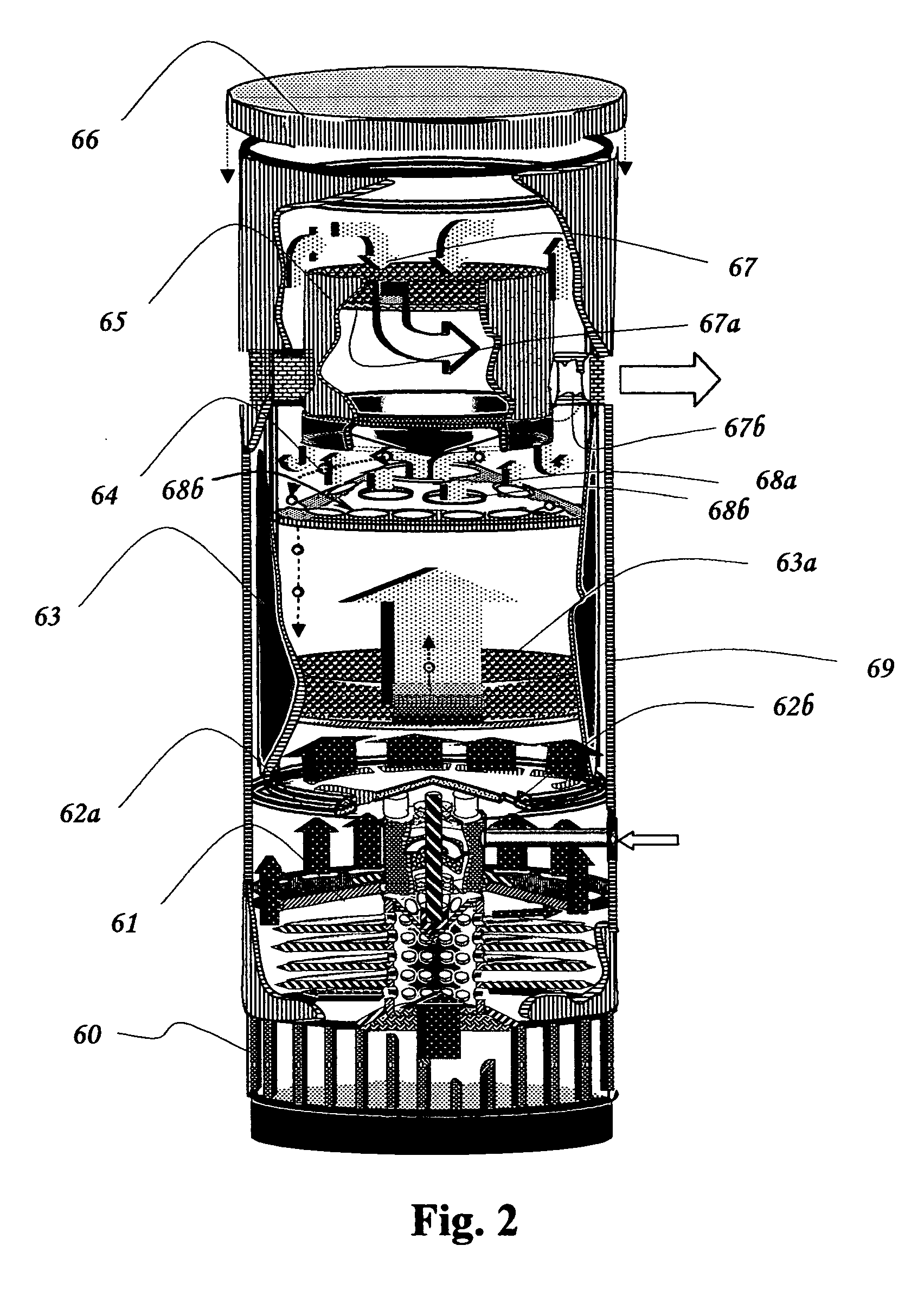
Fluid sterilization apparatus
InactiveUS6454952B1Increase resistanceLower resistanceWater/sewage treatment by irradiationOther chemical processesMomentumUltraviolet lights
Apparatus for treating water or other fluid with radiant energy from an ultraviolet lamp includes a helical shaped input fluid flow guide or helical ramp disposed at or near one end an elongated annular chamber around the lamp. The guide or ramp serves to impart input spiral flow momentum to the fluid upon entry to the chamber. A corresponding output fluid flow guide or helical ramp may be disposed at or near the opposed end of the chamber to impart output spiral flow momentum to the fluid as it approaches discharge from the chamber. Spiral flow serves to extend the time that the fluid is exposed to ultraviolet light and thereby increases the probability that any microbiological contaminants present in the fluid will be killed. The use of helical shaped guides or ramps better serves to establish such a flow in circumstances where the available fluid pressure is relatively low.
Owner:INT WATER GUARD INDS
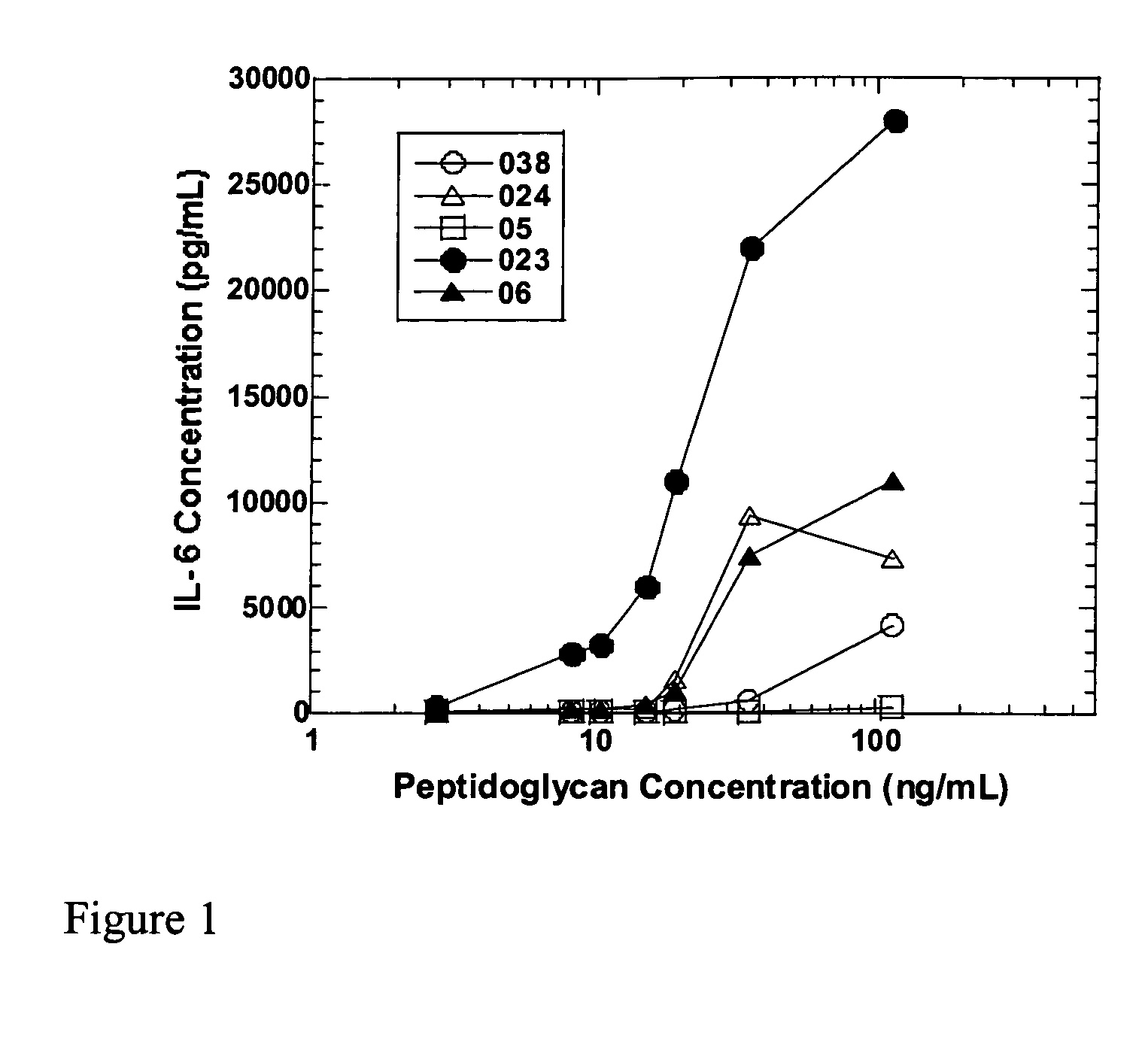
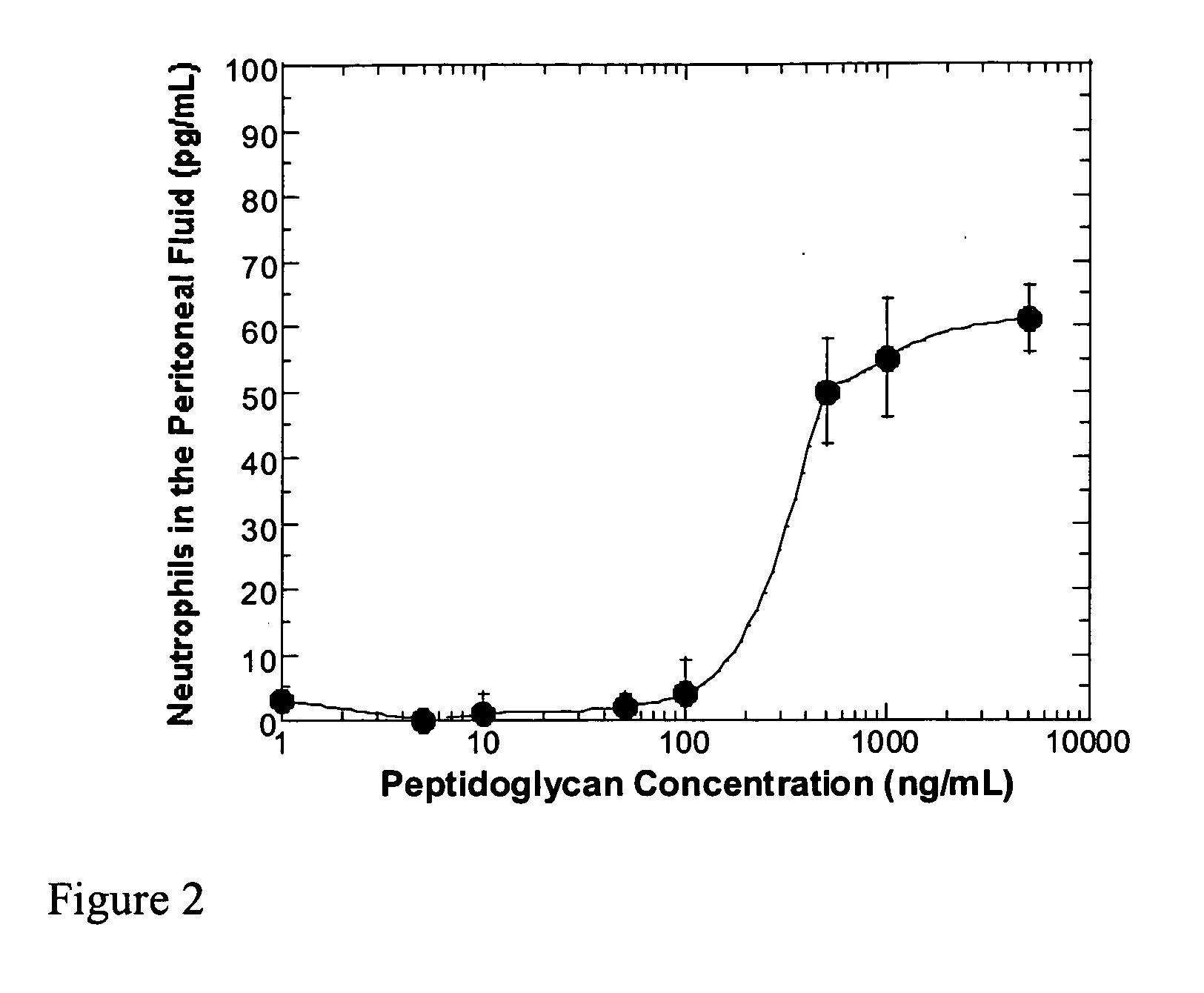
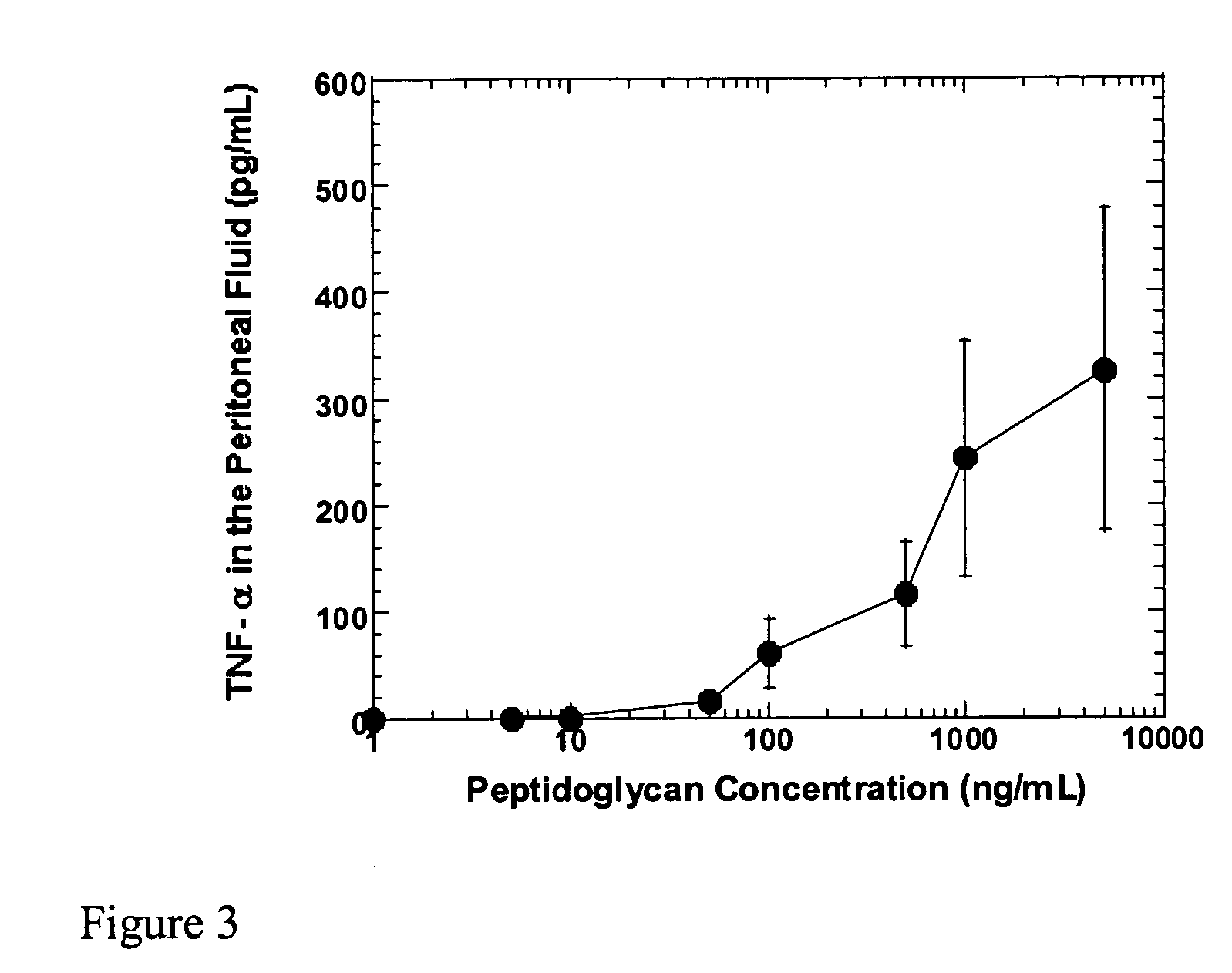
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS20050191717A1Improved peritoneal dialysis solutionImproved testing procedureAntibacterial agentsBiocideBiologyPeritoneal dialysis solutions
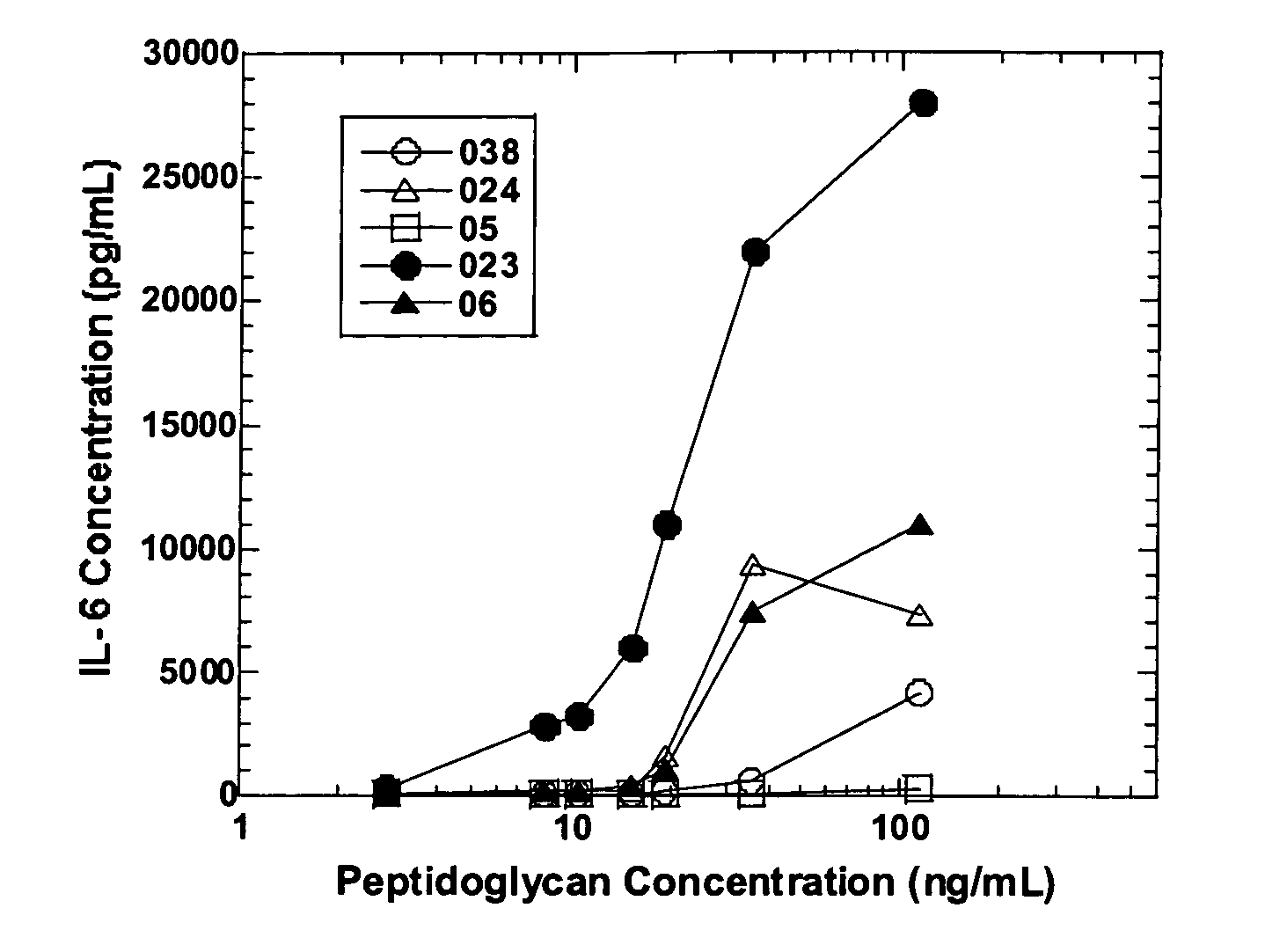
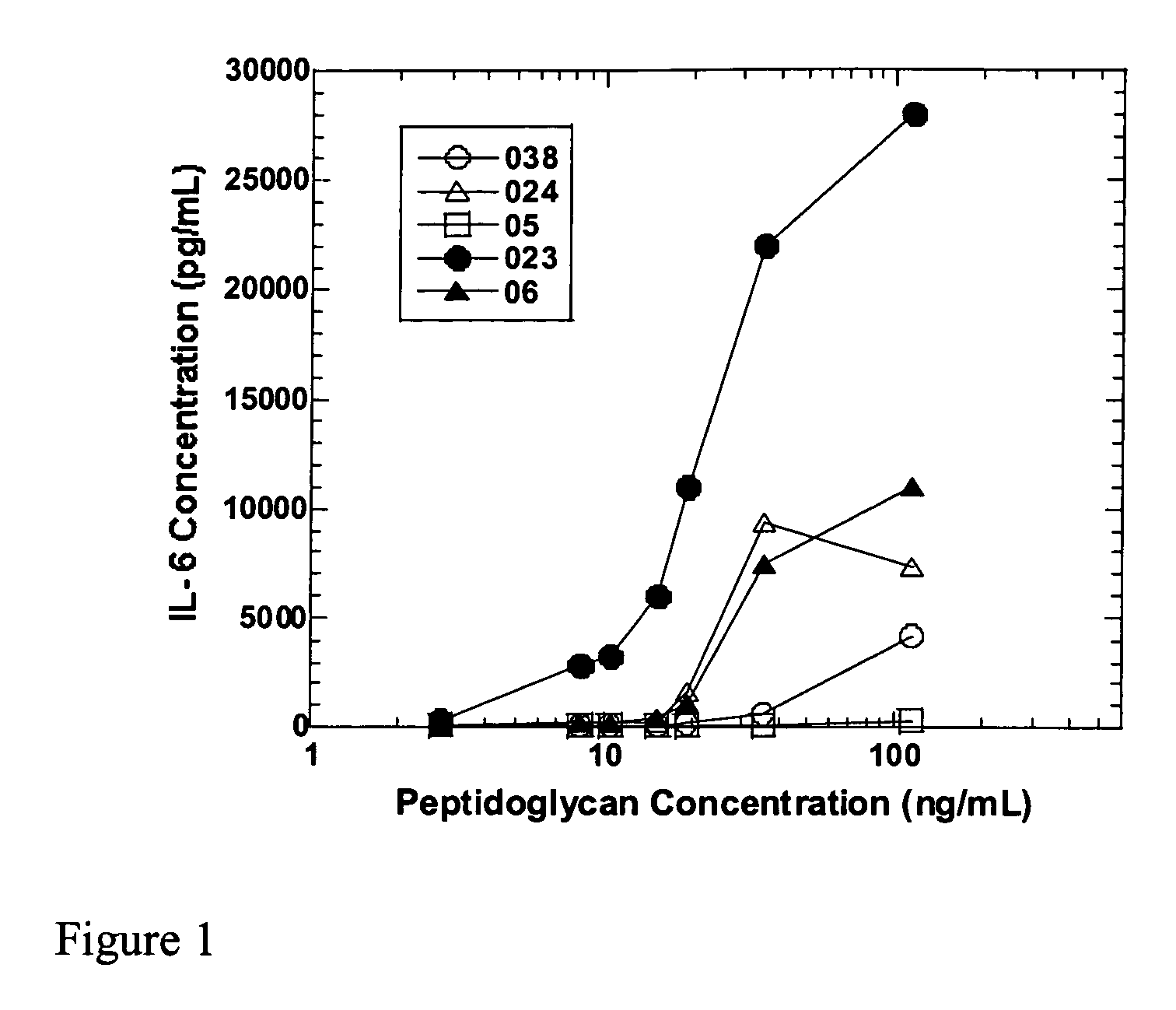
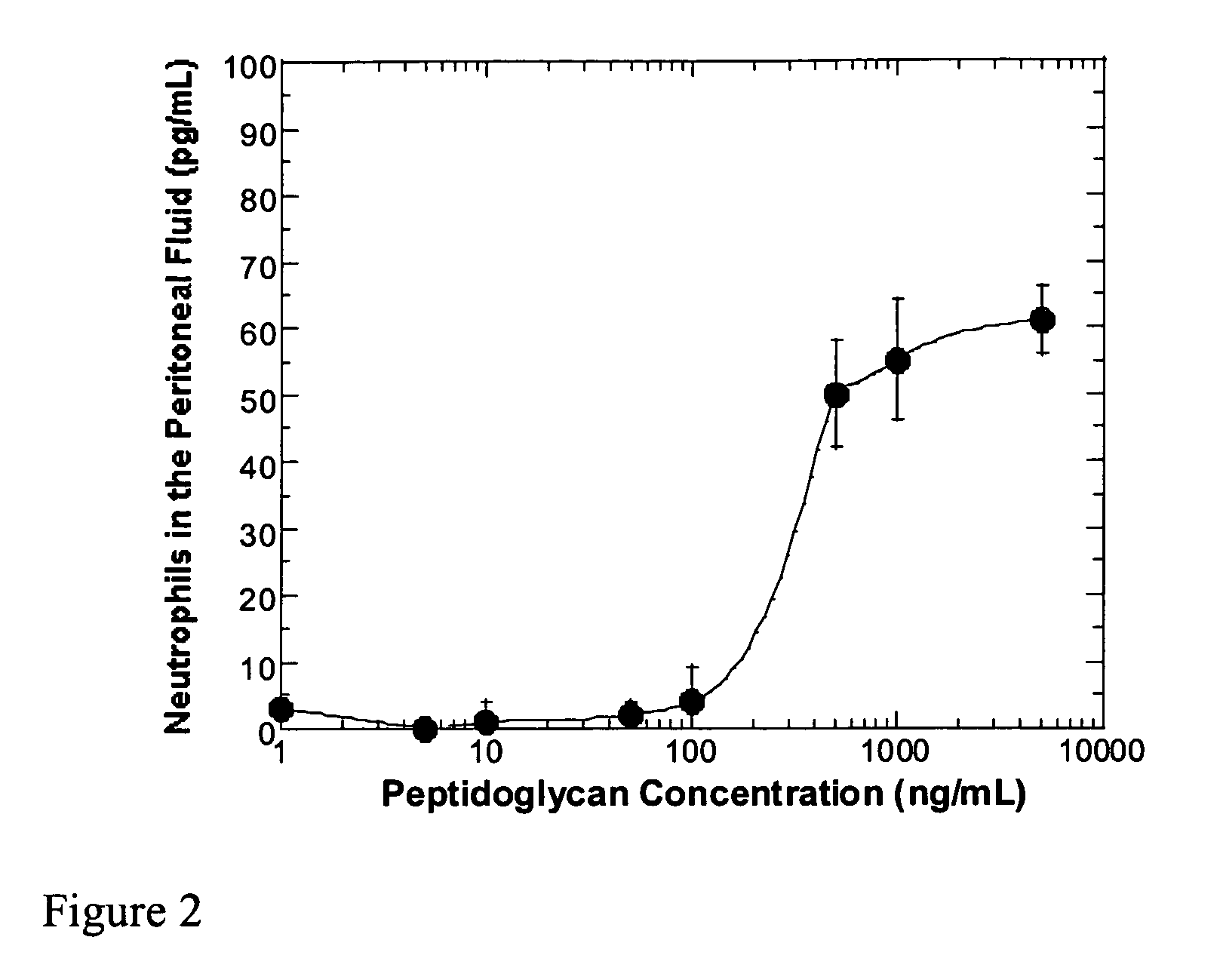
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
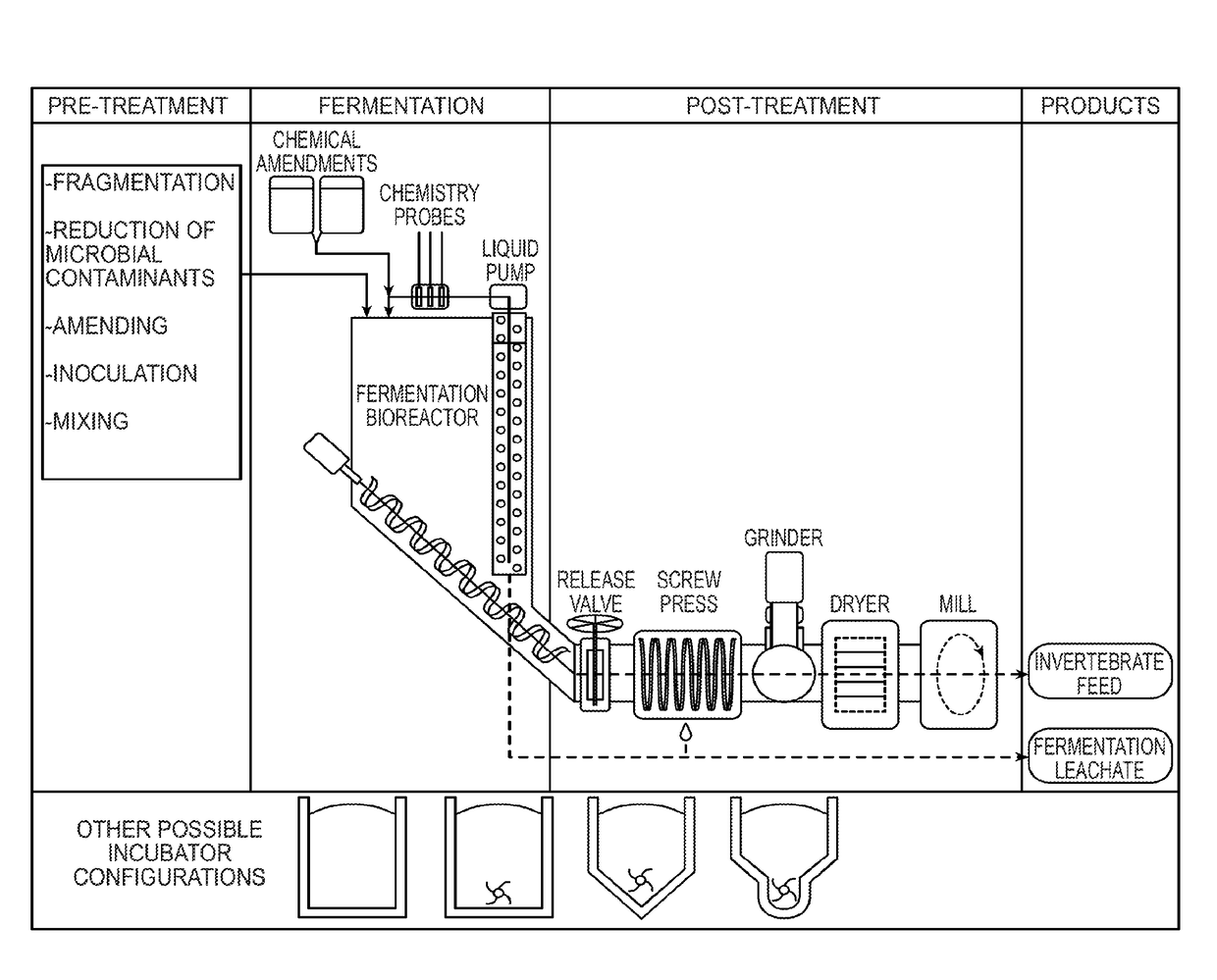
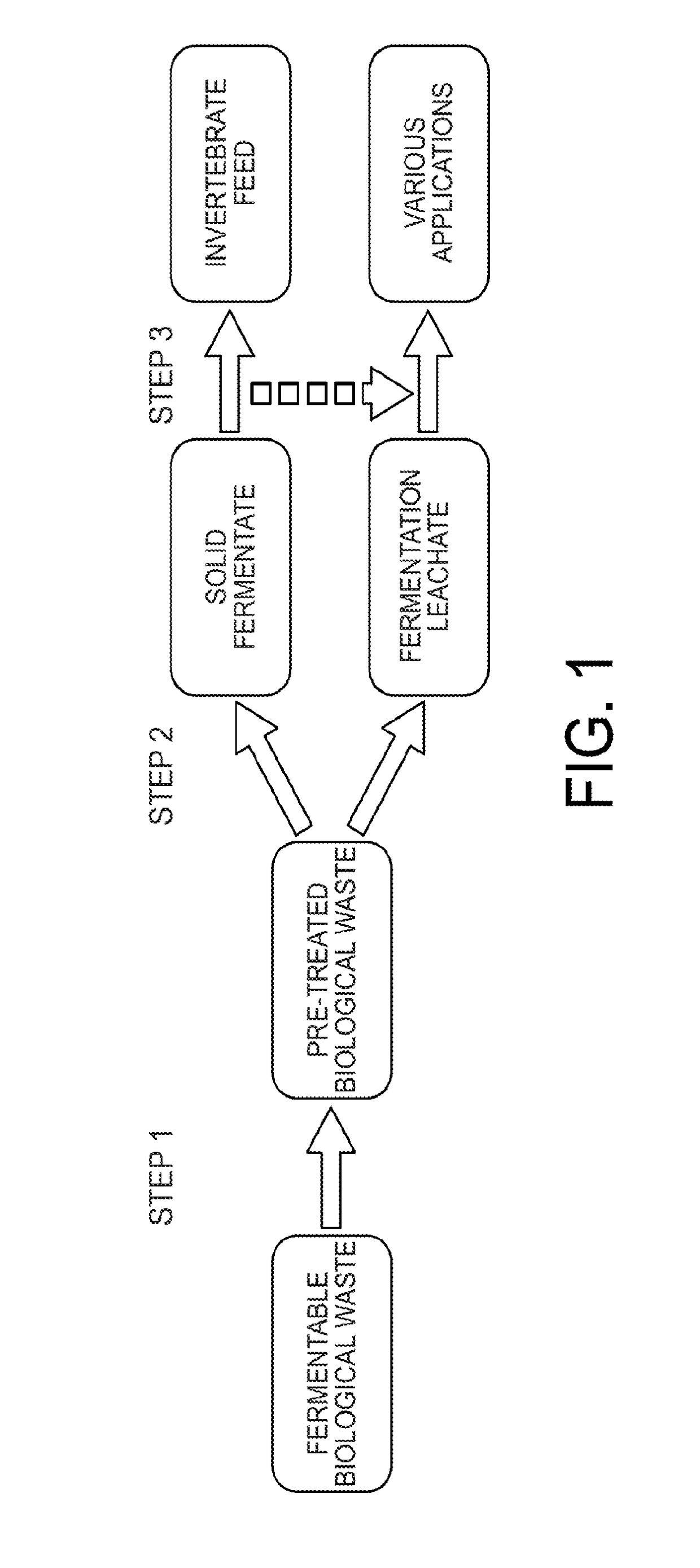
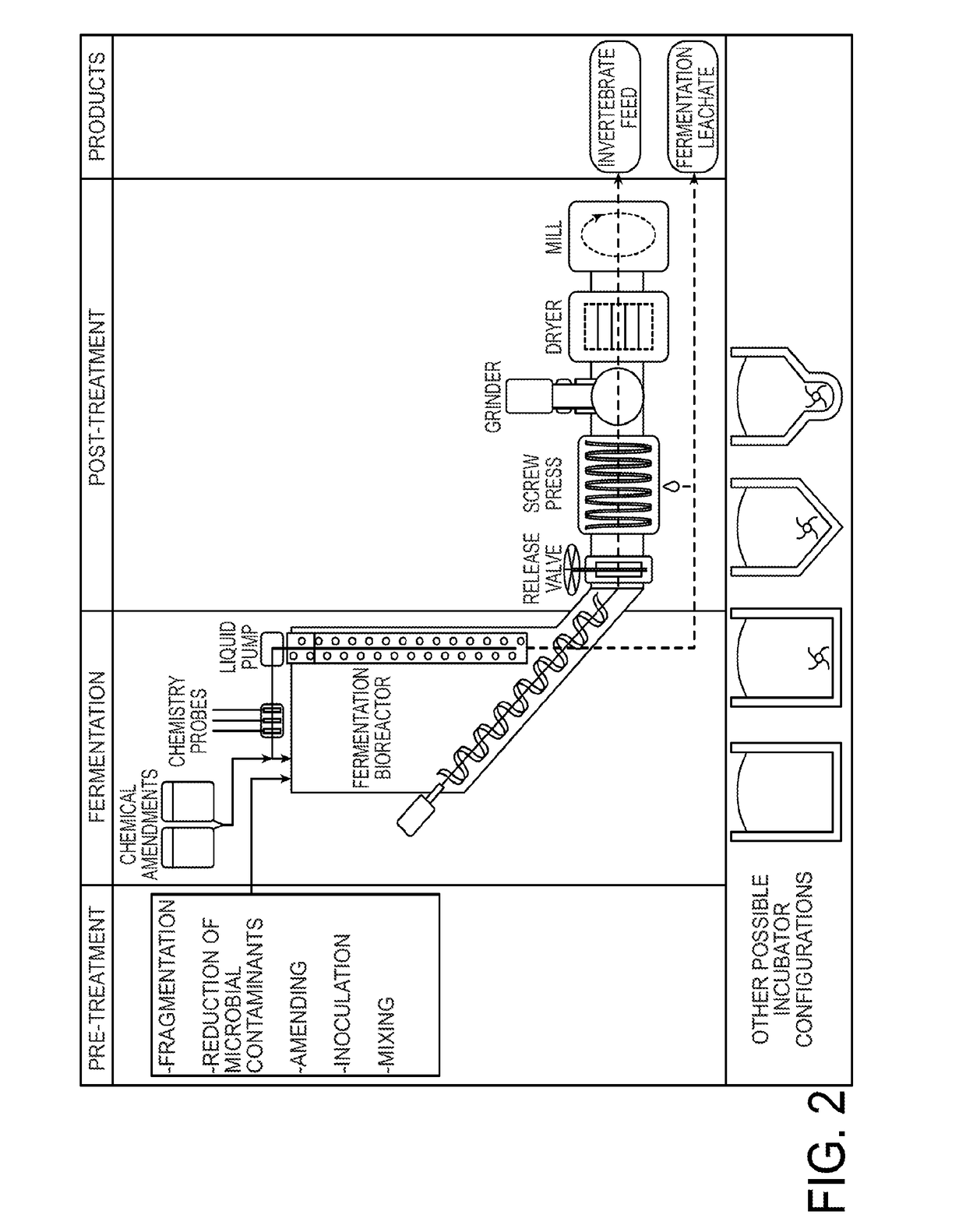
Method for converting food waste and other biological waste into invertebrate feed
ActiveUS20170265496A1Solid waste disposalTransportation and packagingMicrobial inoculationBiological waste
Biological waste such as food, organic or other biologically-derived waste is converted into shelf-stable and health-safe invertebrate feed. The method for converting includes pre-treating waste by fragmenting, reducing microbial contaminants, optionally amending with components that optimize fermentation, inoculating with microorganisms and mixing. Fermentation takes place in a bioreactor and produces fermentation leachate and solid fermentate. In the post-treatment steps, the solid fermentate is separated from the fermentation leachate. The solid fermentate is ground, dewatered and milled. The solid fermentate can be used as an invertebrate feed with or without further processing.
Owner:RIVER ROAD RES
Method for the production of a fermentation product from a pretreated lignocellulosic feedstock
Owner:IOGEN ENERGY CORP
Methods and Compositions for the Detection of Microbial Contaminants
ActiveUS20080020422A1Microbiological testing/measurementBiological material analysisMicroorganismDextran
The invention provides methods and compositions for the detection and / or quantification of a microbial contaminant, for example, a bacterial endotoxin or a glucan, in a sample. In particular, the invention provides a test cartridge useful in the practice of hemocyte lysate-based assays for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides methods of making and using such cartridges. In addition, the invention provides a rapid, sensitive, multi-step kinetic hemocyte lysate-based assay for the detection and / or quantification of a microbial contaminant in a sample. In addition, the invention provides a glucan-specific lysate that can be used in a variety of assay formats, including, for example, a test cartridge, optionally configured to perform a kinetic assay.
Owner:CHARLES RIVER LAB INC
Detection and source identification of microbial contaminants in water samples
InactiveUS20070122831A1Quick checkSufficient capturingMicrobiological testing/measurementMicroorganismBiology
The present invention relates generally to methods of microbial source tracking in environmental water samples. More specifically the present invention relates to methods of microbial source tracking using detection of Bifidobacterium species as markers of source contamination in environmental water samples. The present invention utilizes differences in DNA sequence between genes common to all Bifidobacterium as a means for detecting which species are present in an environmental water sample. This species specific information can then be used to determine the source of fecal contamination.
Owner:THE BOARD OF RGT UNIV SYST OF GEORGIA
Modular compositing-multiple lot screening protocols for detection of pathogens, microbial contaminants and/or constituents
ActiveUS7534584B2Microbiological testing/measurementPreparing sample for investigationValidation testMicroorganism
Particular aspects provide a method of sampling, testing and validating test lots (e.g., single-unit production lots), comprising: assembling a plurality of product portions from each of a plurality of test lots and combining the collected product portions to provide a corresponding set of test lot samples (wherein each test lot sample is attributed to a particular corresponding test lot); enriching the set of test lot samples; removing equal portions of each enriched sample, and combining the removed portions to provide a modular composite sample; and testing of the modular composite sample for the target agent / organism, wherein where such testing is positive, individual test lots may nonetheless yet be validated by further testing of a respective enriched test lot sample and obtaining a negative test result. The methods have broad utility for monitoring all sort of test lots (e.g., environmental lots, production lots, pharmaceutical lots, etc.) and for efficiently affecting informed, targeted remedial measures.
Owner:INST FOR ENVIRONMENTAL HEALTH
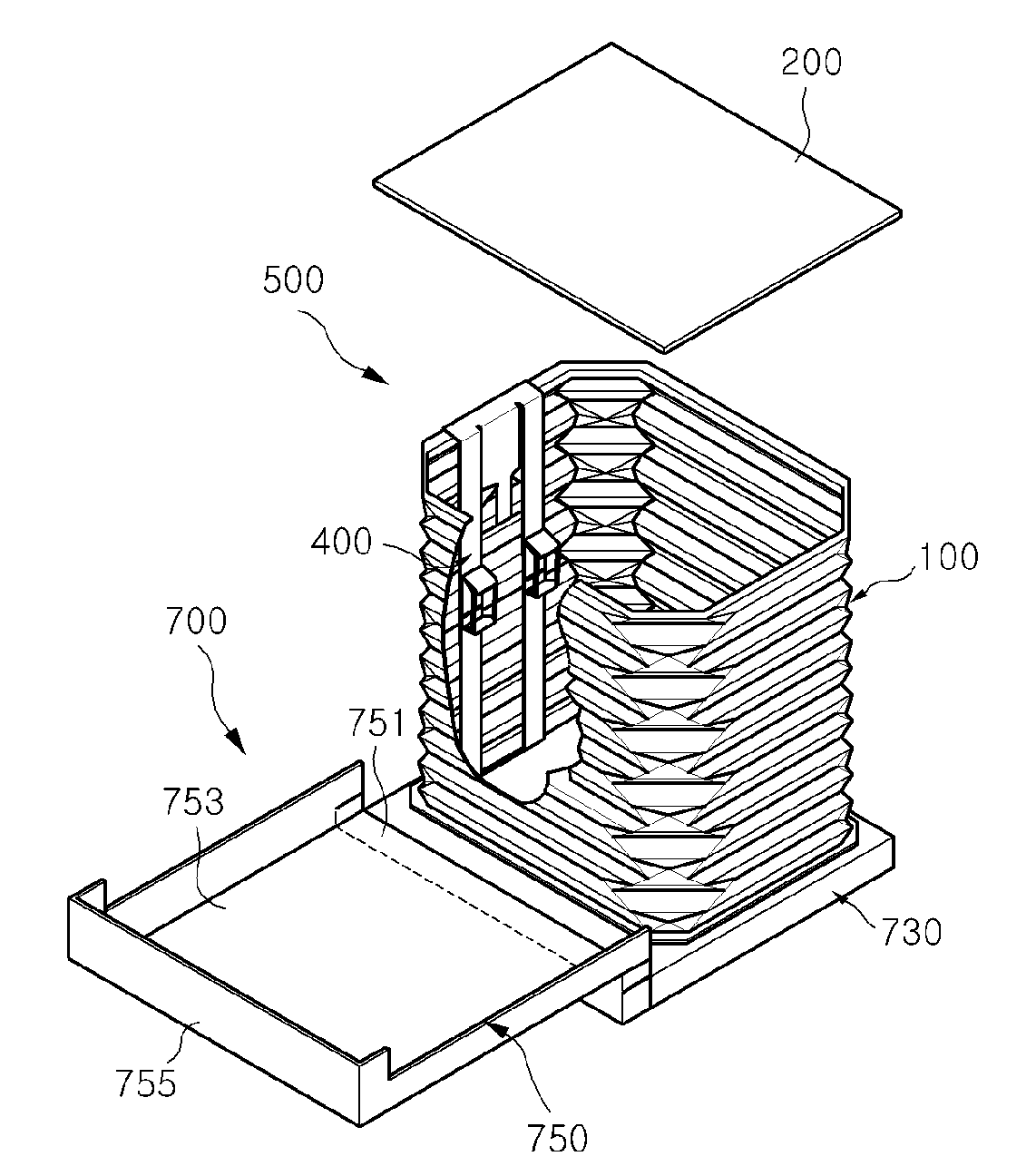
Disposable folding type sanitary toilet for pets
A disposable folding type sanitary toilet for pets includes a folding type receiving unit configured in the form of a box having a receiving space. A wall of the box is configured to fold such that the volume of the receiving unit is adjusted, thereby allowing the sanitary toilet to be easily stored and carried. The sanitary toilet further includes a case unit for containing the bottom of the receiving unit and protecting the bottom, top, and sides of the receiving unit. The receiving unit includes a top plate, a wall unit connected to the top plate, the wall unit having horizontal folds formed at its exterior for adjusting the vertical height of the wall unit, and a lower receiving part coupled to the bottom of the wall unit and having a receiving space. An attached filter may be deployed during closure to inhibit discharge of dust and / or microbial contaminants.
Owner:BJORNSON WILLIAM
Large-scale water purification and desalination
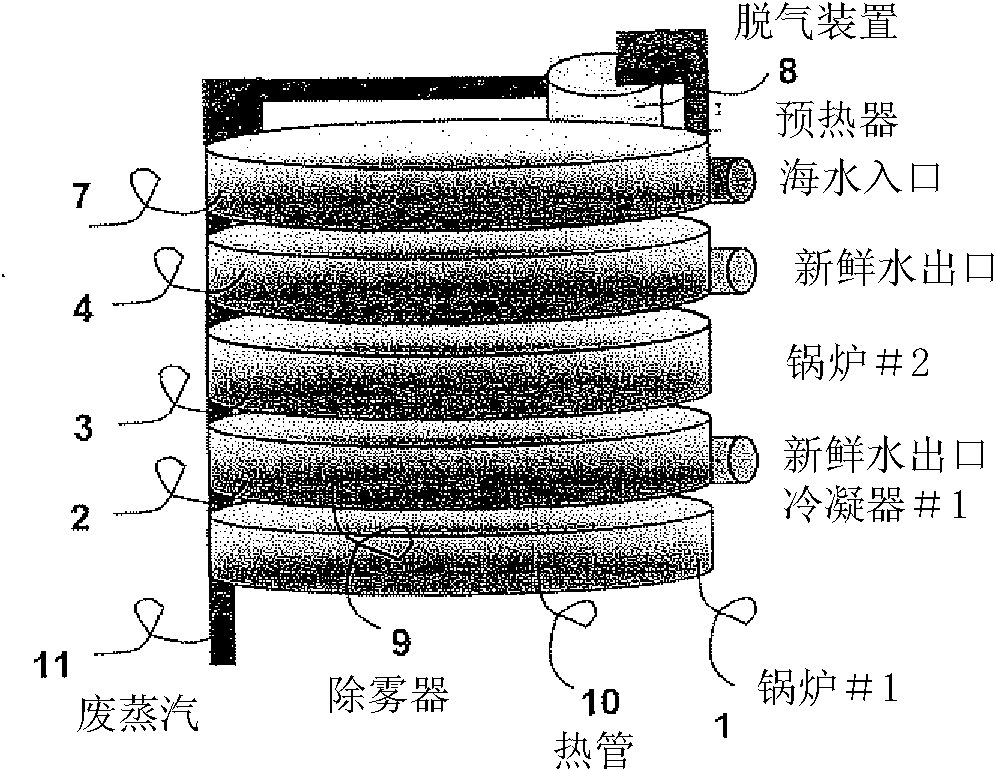
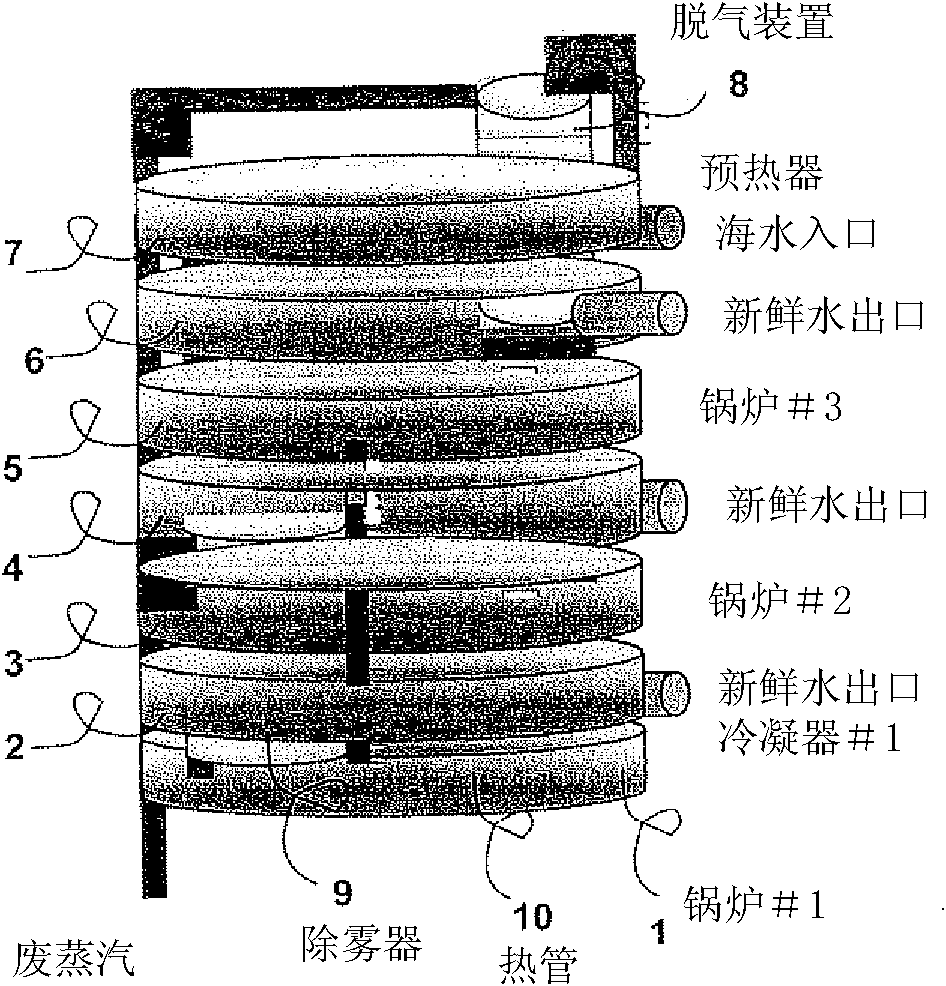
ActiveCN102215927AGeneral water supply conservationDistillation regulation/controlDistillationEvaporation chamber
Embodiments of the invention provide systems and methods for water purification and desalination. The systems have a preheater, a degasser, multiple evaporation chambers with demisters, heat pipes, and a control system, wherein the control system permits continuous operation of the purification and desalination system without requiring user intervention or cleaning. The system is capable of recovering heat from each distillation stage, while removing, from a contaminated water sample, a plurality of contaminant types including: microbiological contaminants, radiological contaminants, metals, salts, volatile organics, and non-volatile organics.
Owner:SYLVAN SOURCE
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS7118857B2Simple methodPrevent peritonitisAntibacterial agentsOrganic active ingredientsBiologyPeritoneal dialysis solutions
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
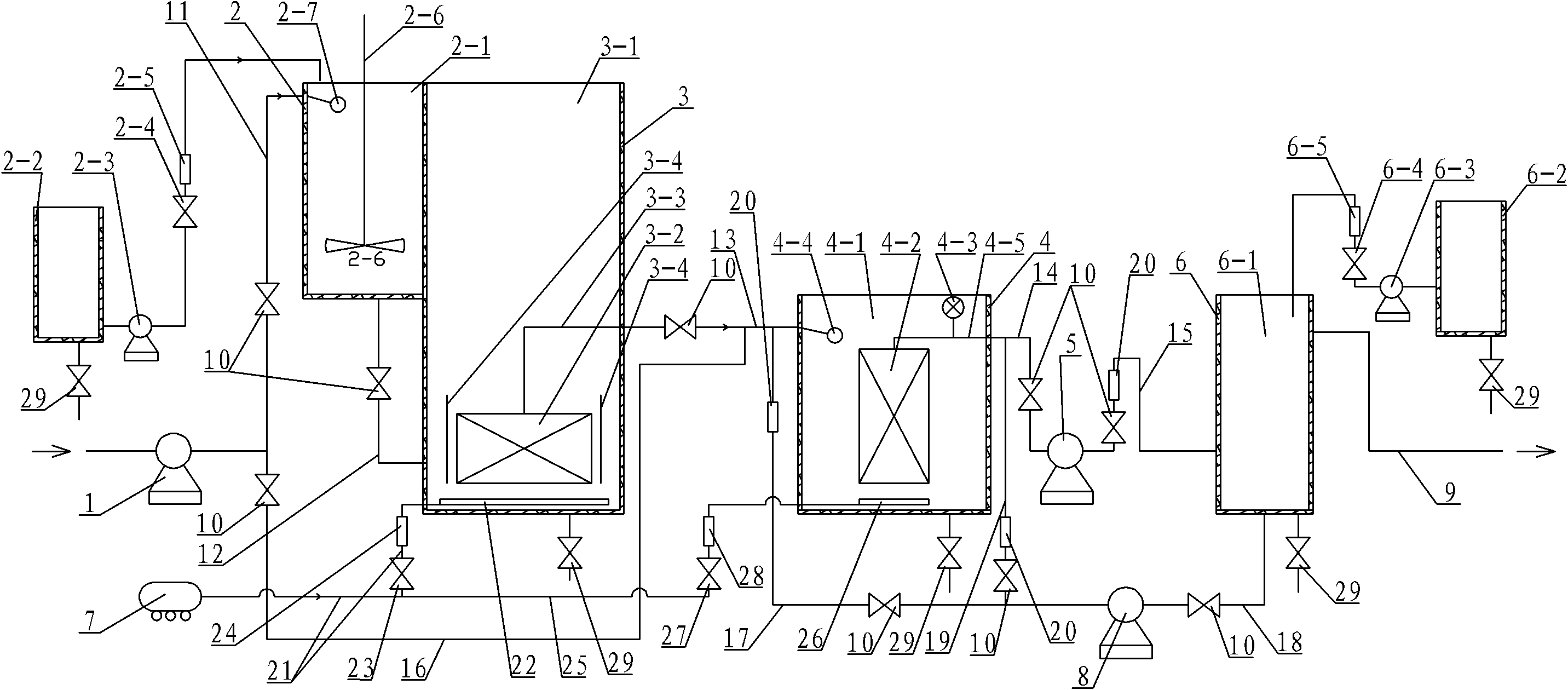
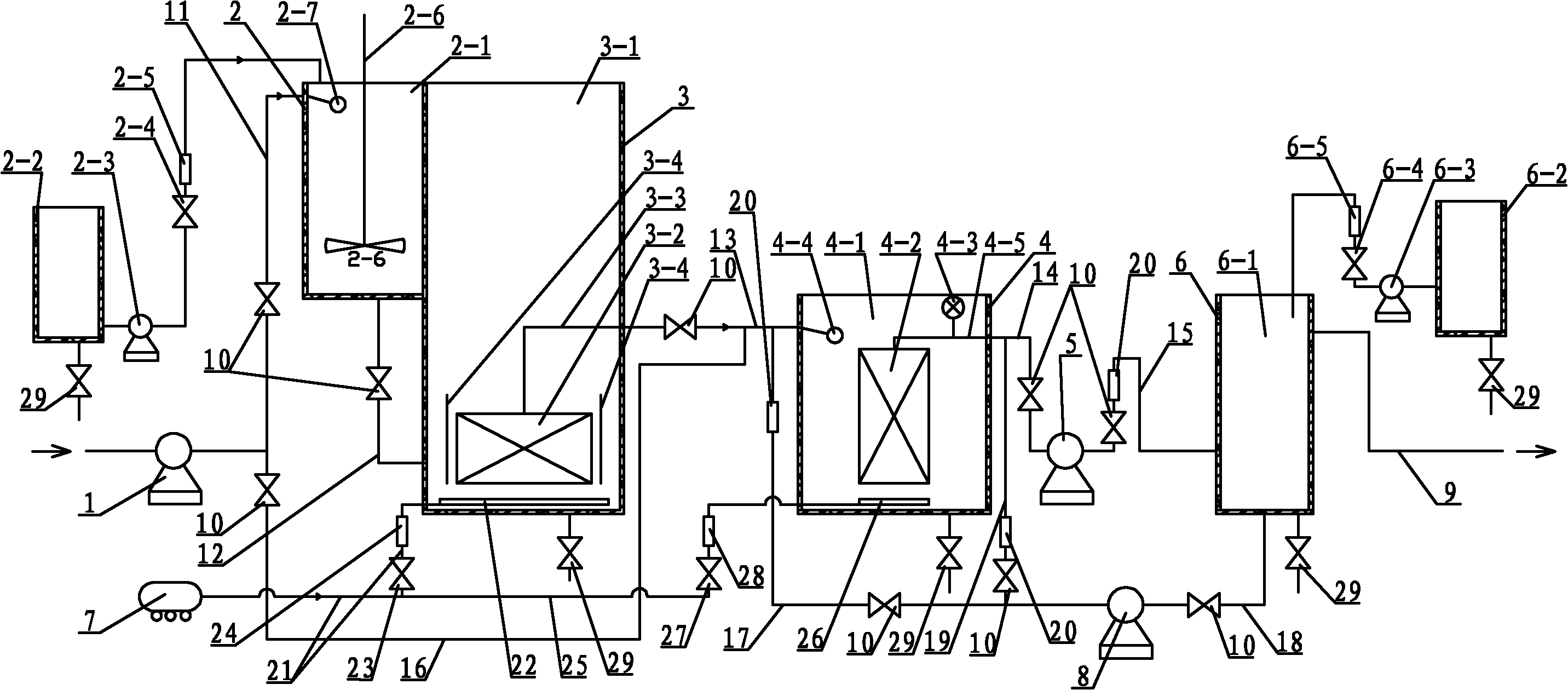
Dual-membrane processing system and method for micro-polluted raw water
InactiveCN102079591AReduce dosageHigh biosecurityWater/sewage treatment bu osmosis/dialysisMultistage water/sewage treatmentFiltration membraneWater source
The invention provides a dual-membrane processing system and method for micro-polluted raw water, relating to a processing system and method for drinkable water and aiming to solve problems that the prior drinkable water processing technology cannot ensure biological safety of water quality, can hardly control contaminants of biological origin such as bacteria, has complicated process and causes unstable effluent quality. Most of colloids and granular materials in raw water are removed by using a micro-filtration membrane module and micro-organic contaminants such as bacteria are completely entrapped by using an ultra-filtration membrane module so as to realize water processing by using two membranes. The method comprises the following steps: raw water and water processing medicines are mixed in a mixing pool and then the mixture is added in a micro-filtration membrane separating pond to completely react, the products of reaction are filtered by the micro-filtration membrane module to obtain filtered water, and then the filtered water is added in the ultra-filtration membrane separating pond to be filtered by the ultra-filtration membrane module to obtain clean water, and the clean water is added in a clean water tank to be sterilized by a sterilant to obtain drinkable water. The invention is used for production of drinkable water with micro-polluted surface water as a water source and water quality safeguard.
Owner:HARBIN INST OF TECH
Compositions and methods for detecting a biological contaminant
Provided are compositions and methods useful to the determination of whether a microbial contaminant is present in a biological therapeutic production process. Specifically, an artificial positive amplification control plasmid and unique quantitative PCR detection probe are provided, which enables the rapid and real-time detection of a false positive result.
Owner:REGENERON PHARM INC
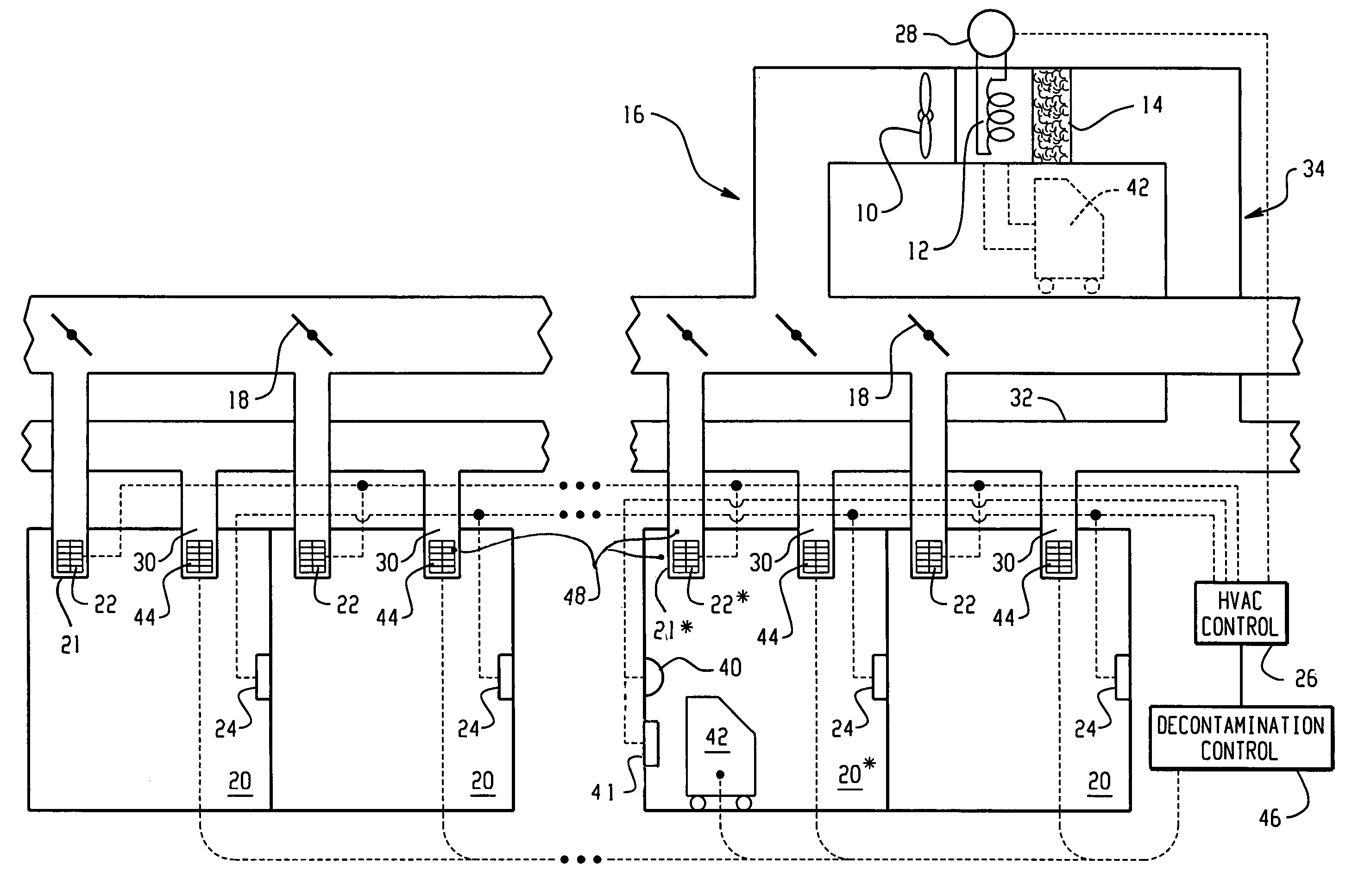
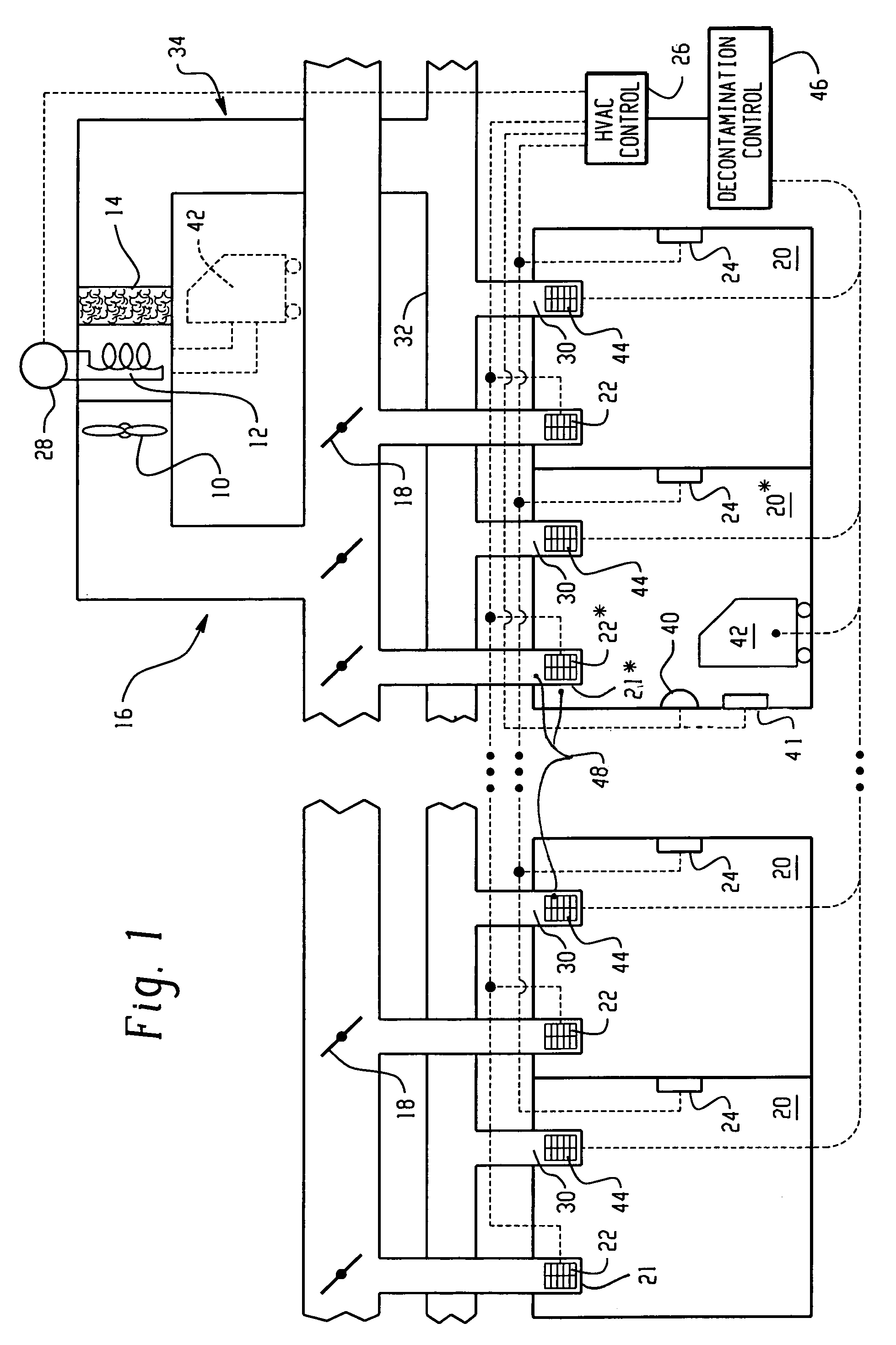
Building decontamination with vaporous hydrogen peroxide
ActiveUS7361304B2EfficacyApparent advantageMechanical apparatusLighting and heating apparatusEngineeringVapor generator
When microbial contamination is introduced into a room (20*) of an enclosure, such as a building, an HVAC system including supply ductwork (16) and a return ductwork (34) is decontaminated with hydrogen peroxide vapor. A decontamination controller (46) operates controllable baffles (22) at outlet registers (20), temporary controllable baffles (44) at inlet registers (30), and a blower system (10) to circulate hydrogen peroxide vapor from hydrogen peroxide vapor generators (42) through the ductwork in both forward and reverse directions. Further, at least portions of the baffles are closed to create dwell times in which the hydrogen peroxide vapor resides in the ductwork with minimal or turbulent flow.
Owner:STERIS CORP
Adherent Antimicrobial Barrier and Sanitizing Agent
ActiveUS20070297942A1Improve adhesionEliminate indirect transferBiocideDead animal preservationFood safetyCompound (substance)
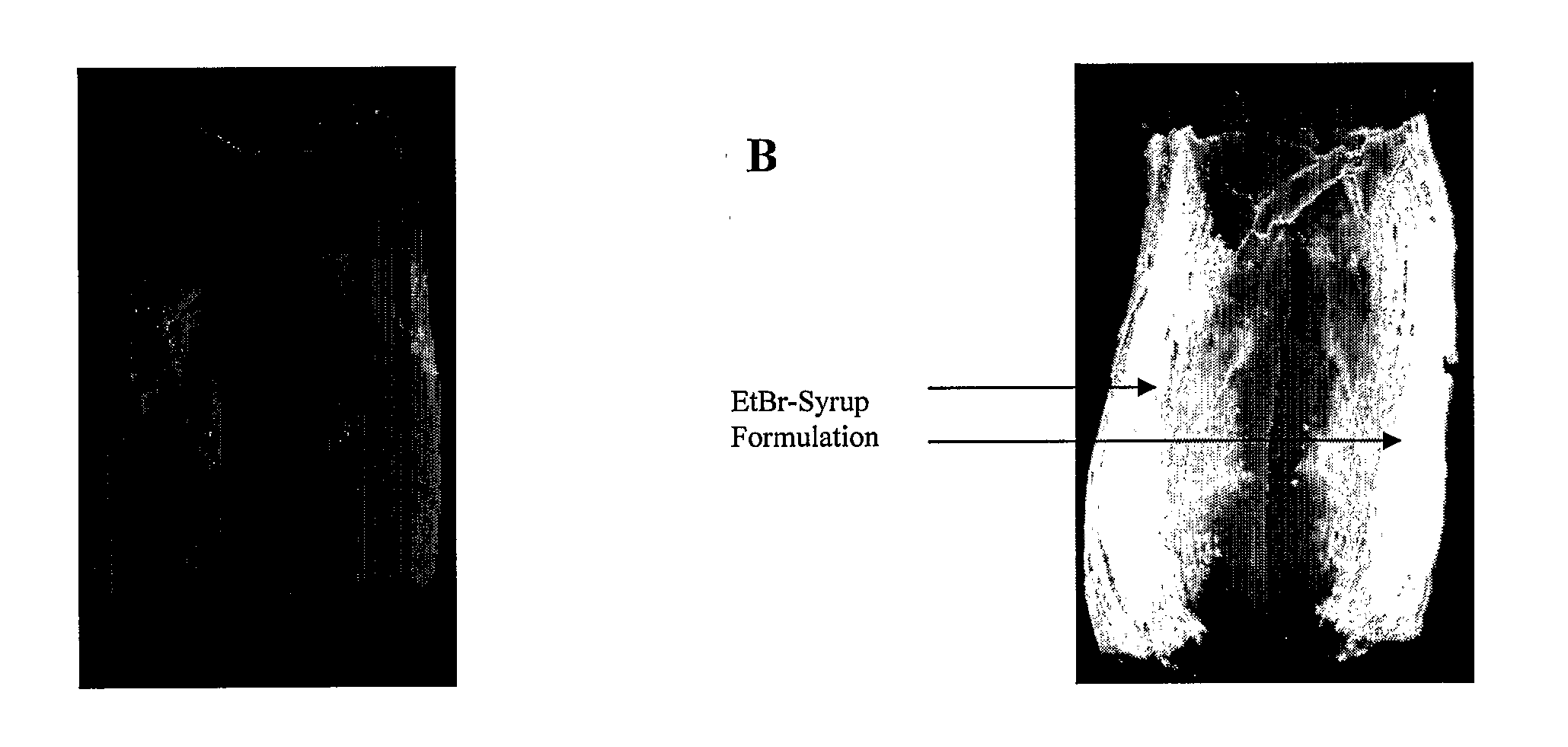
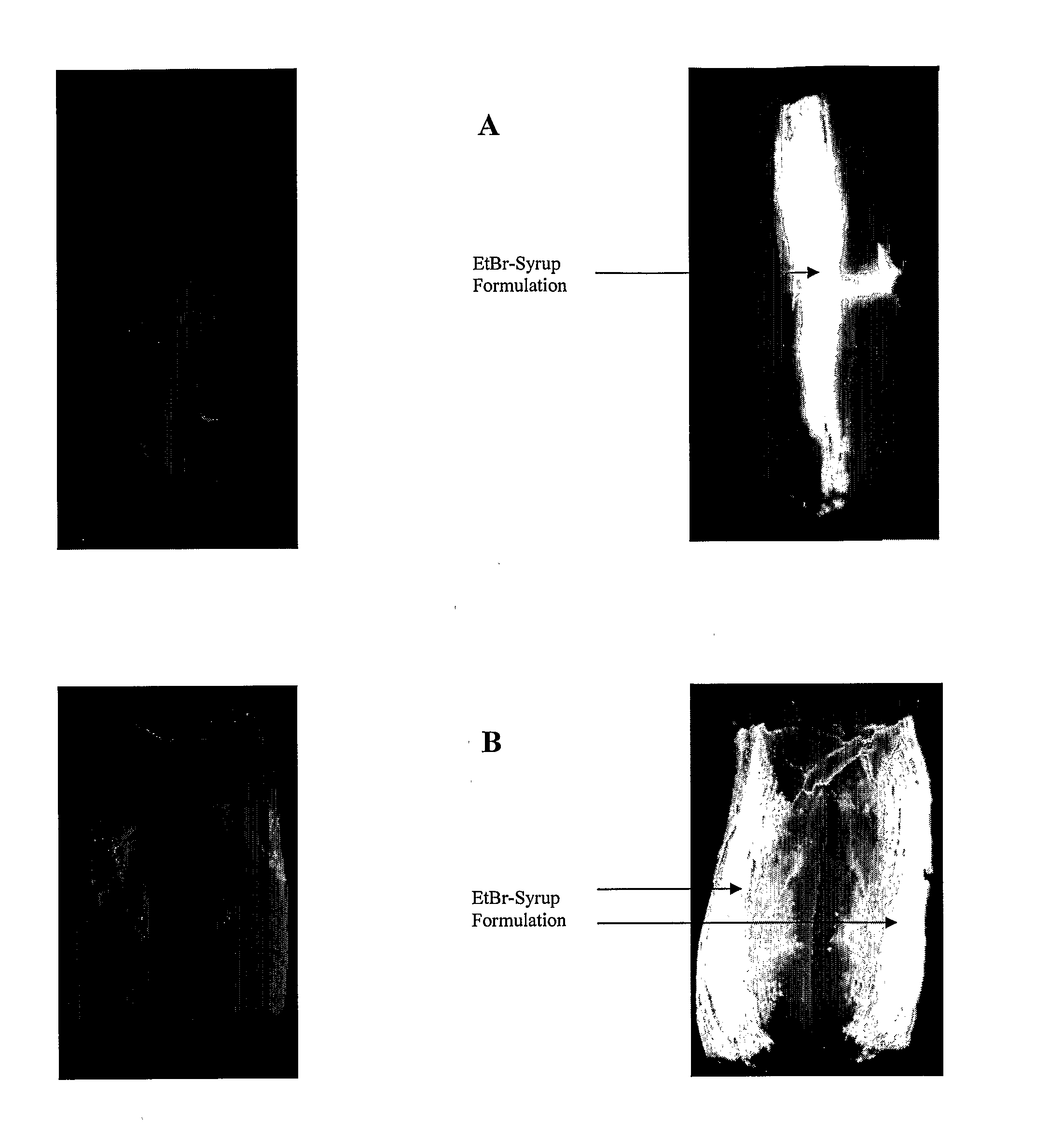
The present invention provides novel viscous and adherent food-safe antimicrobial compositions, and methods for using same in the immediate and residual decontamination of microbial-contaminated substrate surfaces, in reducing or precluding cutting implement-mediated transfer of surface contamination during cutting operations in the food industry, and for reducing or preventing transfer of contamination from contaminated surfaces in the food and pharmaceutical. Adherent antimicrobial protective layers are formed on substrate surfaces (e.g., processing equipment and utensils), providing a barrier (e.g., chemical and / or physical) to the passage or transport of microbial contamination between and among surfaces. The adherent formulations confer residual de-contaminating activity, providing for prolonged killing of associated microbial contamination. The inventive solutions, are preferably formulated and applied as a gel, syrup or foam, and are preferably prepared using materials which are ‘generally-regarded-as-safe’ (GRAS) in food products, obviating post-treatment removal prior to consumption. Preferably, the inventive formulations are heated to a temperature equal to or greater than about 80° C. prior to application.
Owner:INST FOR ENVIRONMENTAL HEALTH
Device for obtaining uniform samples of meat and methods of use thereof
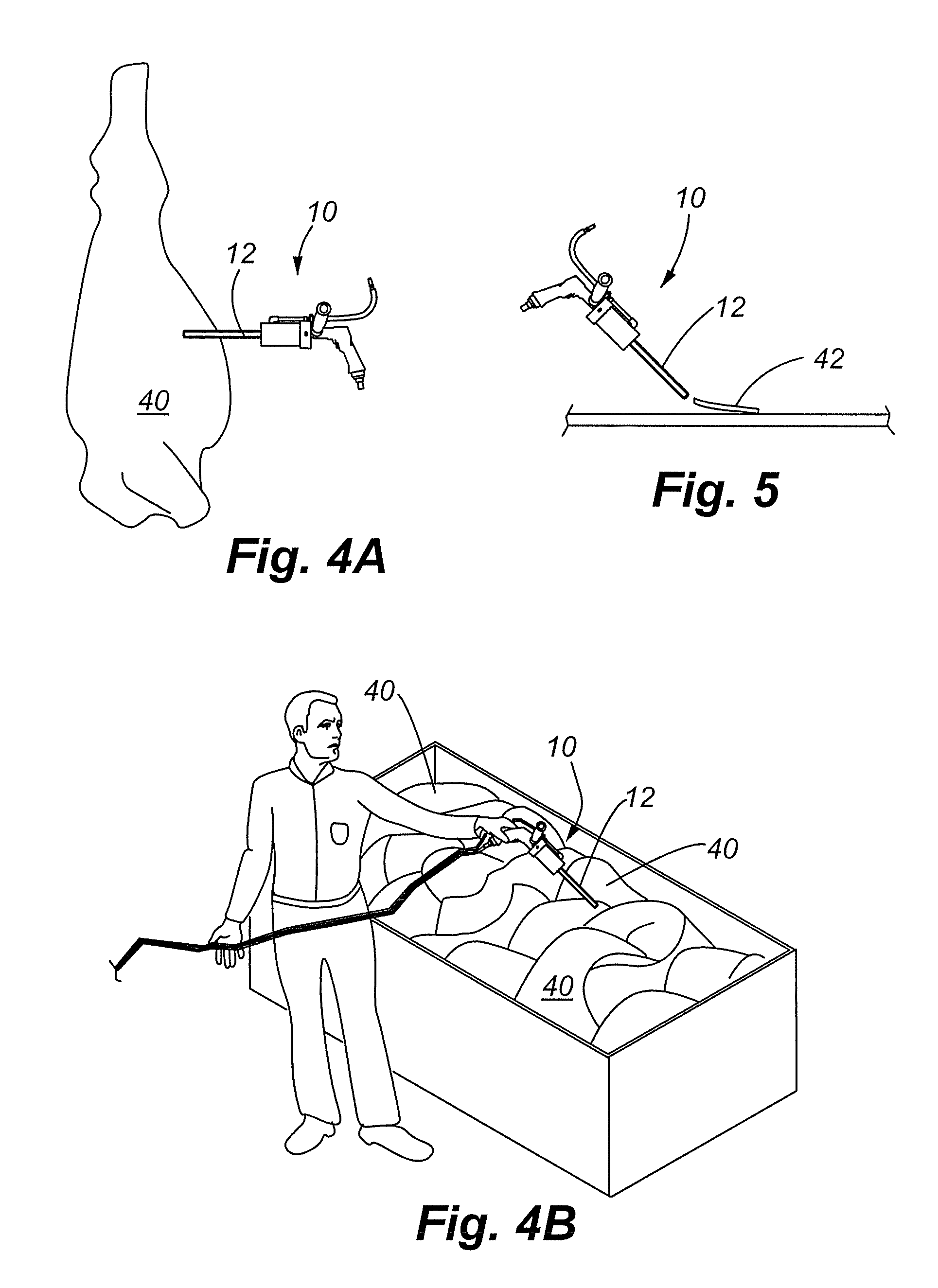
InactiveUS20080295619A1Improve scalabilityQuickly and easily complyTransportation and packagingMicrobiological testing/measurementHand heldEngineering
The present invention generally relates to a device for obtaining samples of meat during commercial meat production. More specifically, embodiments of the present invention provide for an automated, hand held device used to rapidly obtain samples of meat that are of essentially uniform size in order to test for the presence of microbiological contaminants in such meat, as well as methods of using the device. The device can be used to comply with laws and regulations concerning the testing of meat for microbial contamination during commercial meat production. The device is also lightweight and ergonomically constructed so that it may be operated by a single individual.
Owner:JBS USA FOOD CO +1
Method and apparatus for pollution control of confined spaces
A method of improve the removal of particulate matter, heavy metals, neutralizing acid, and kill microorganism pollutants, known to be in contaminated air volumes of occupied confined spaces, when exposed in close contact under pressure to a mixture of alkaline sorbent materials, having a known synergism between said pollutants using a self propelled fluidized bed reactor and packed bed filter apparatus system to optimize the contact collection efficiency of submicron particles and organic compounds.
Owner:AVINA DAVID CHRISTOPHER
Sterile packing and sterilization method using this packing
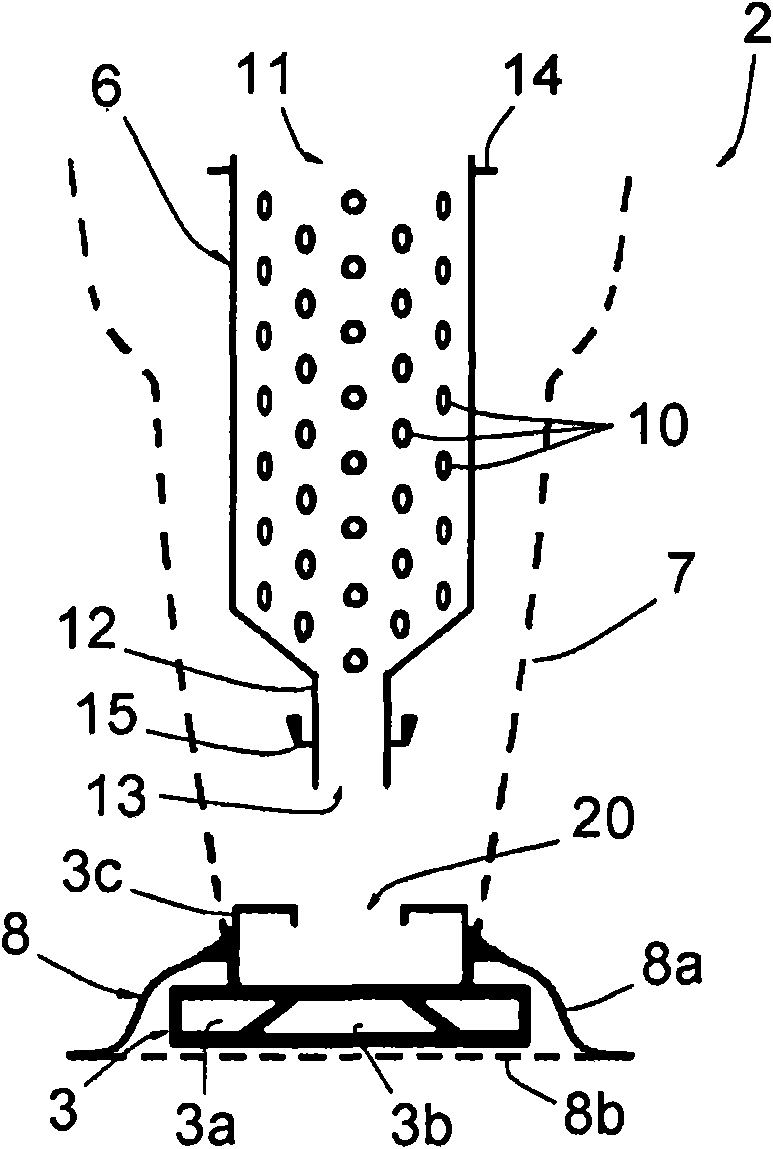
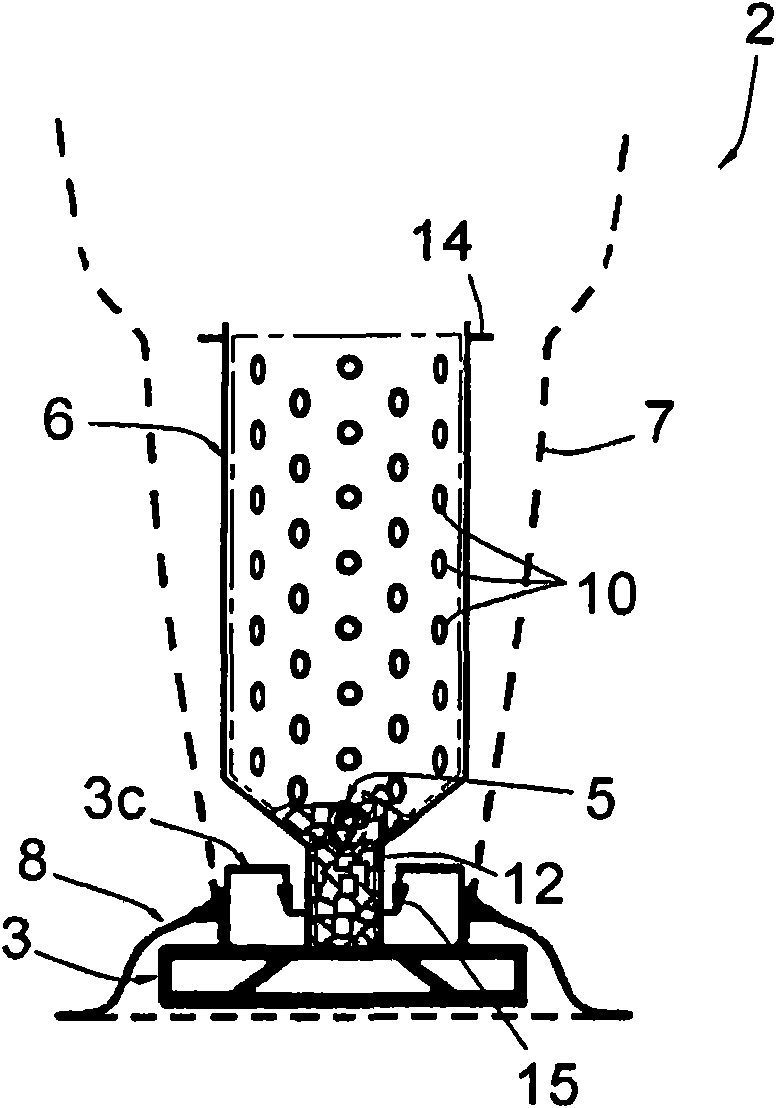
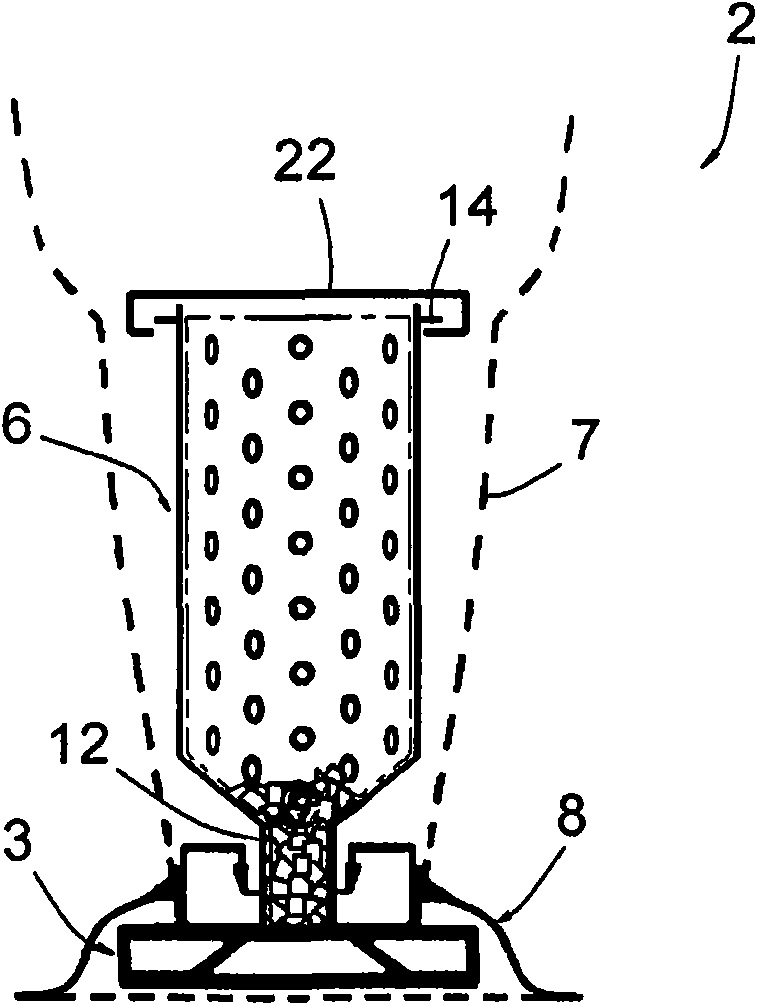
ActiveCN101678939AIntegrity guaranteedEfficient sterilizationIntravenous devicesPackaging under vacuum/special atmosphereEngineeringSmall hole
This sterile packing includes: a container (2) for holding the at least one object (5) to be sterilized and having an inlet opening (11 ) and a discharge opening (13) via which the at least one object (5) may pass into and out of the container (2), the container (2) comprising a rigid part (6) having a bored peripheral wall with a multitude of small holes (10) having dimensions lower than those of the the at least one object (5), and a non-rigid part (7) in a material porous to the sterilizing fluid and non-porous to microbial contamination, the non-rigid part (7) being able to contain the rigid part (6) and to be sealed thereon; and - at least one envelope (4) made in a flexible and airtight material, which is vacuum sealing fitted on the container (2).
Owner:BECTON DICKINSON FRANCE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com