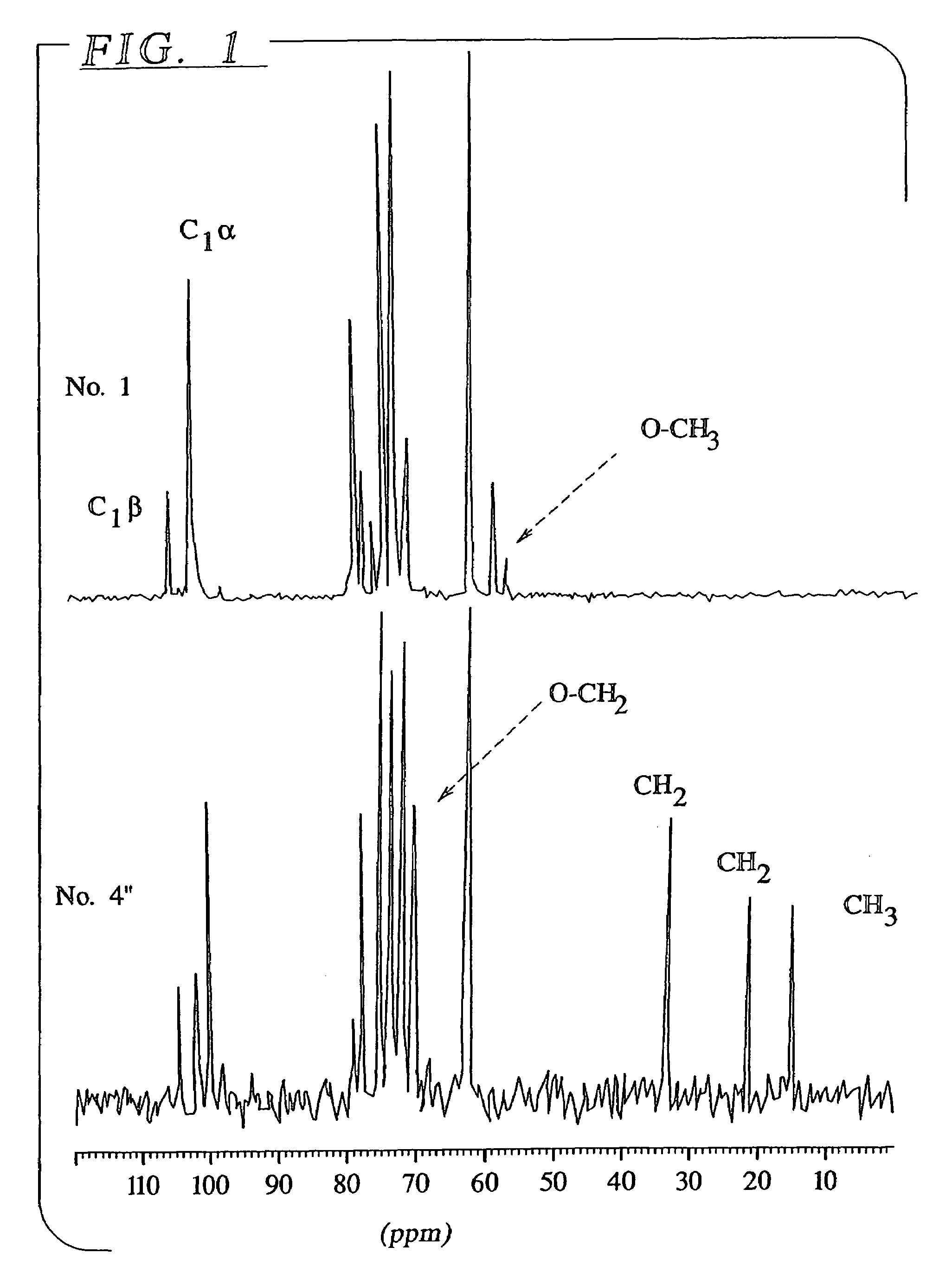
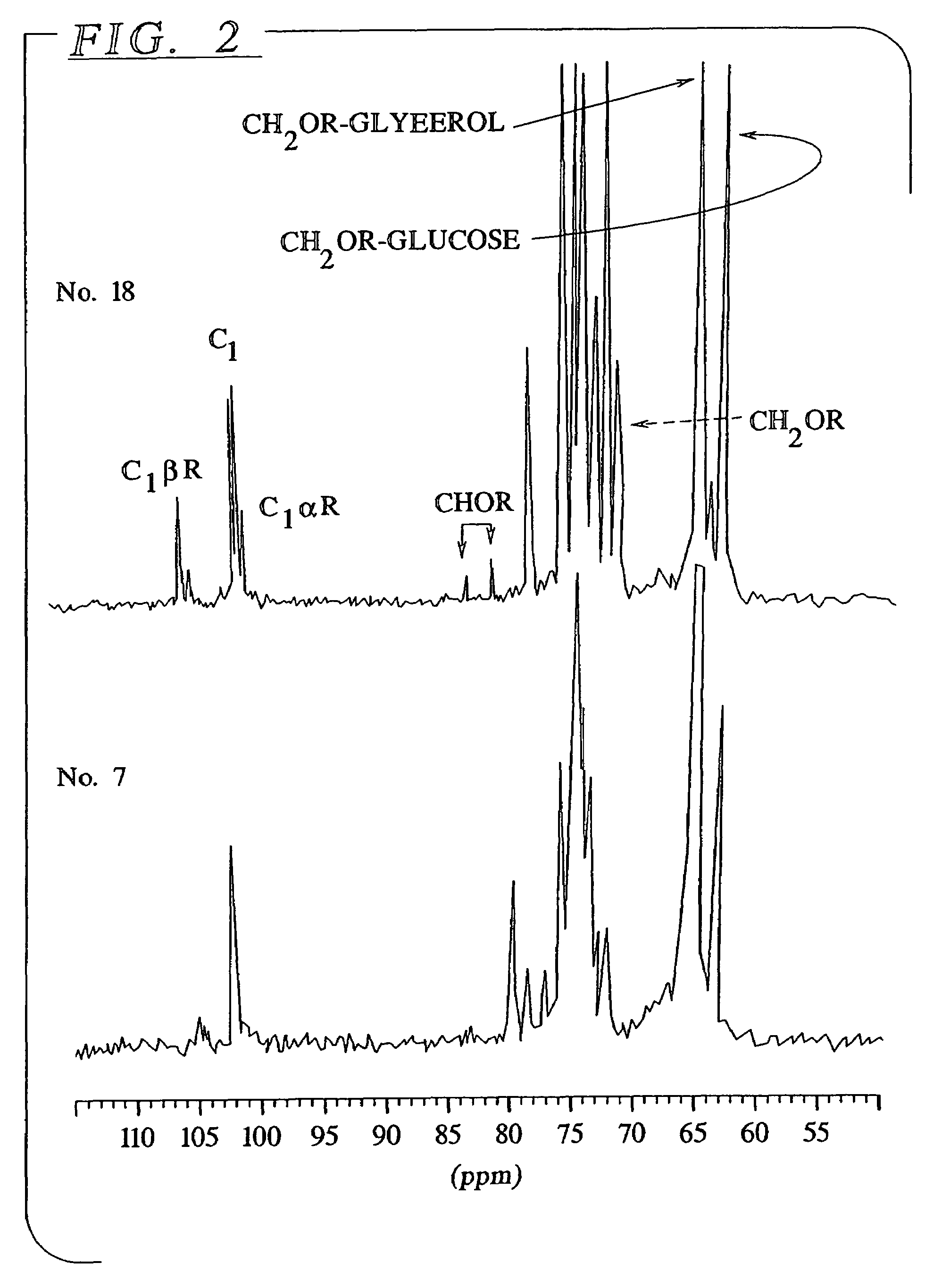
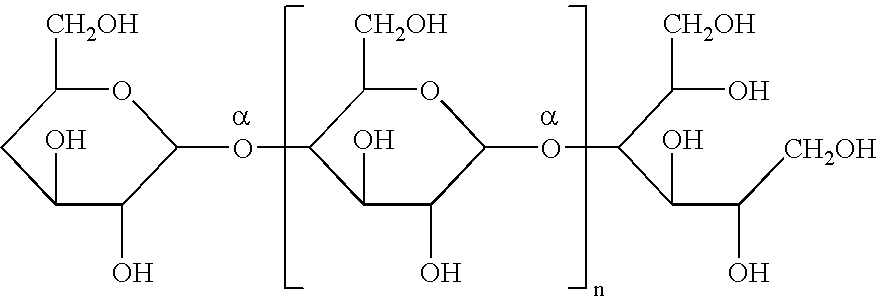
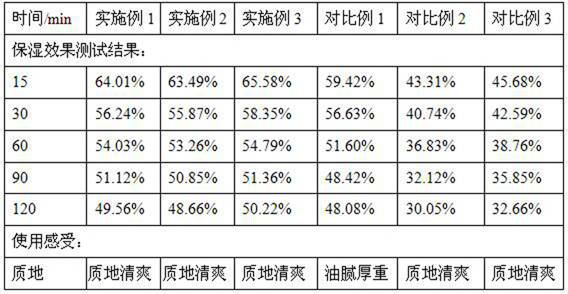
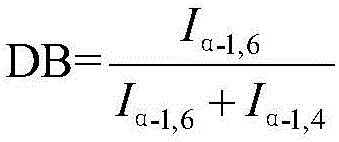Patents
Literature
49 results about "Icodextrin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Icodextrin (INN, USAN) is a colloid osmotic agent, derived from maltodextrin, used in form of an aqueous solution for peritoneal dialysis under the trade name Extraneal, and after gynecological laparoscopic surgery for the reduction of post-surgical adhesions (fibrous bands that form between tissues and organs) under the trade name Adept.
Icodextrin and preparing method thereof
ActiveCN103467608AMolecular weight concentrationUniform particle sizeSubstrate concentrationHydrolysis
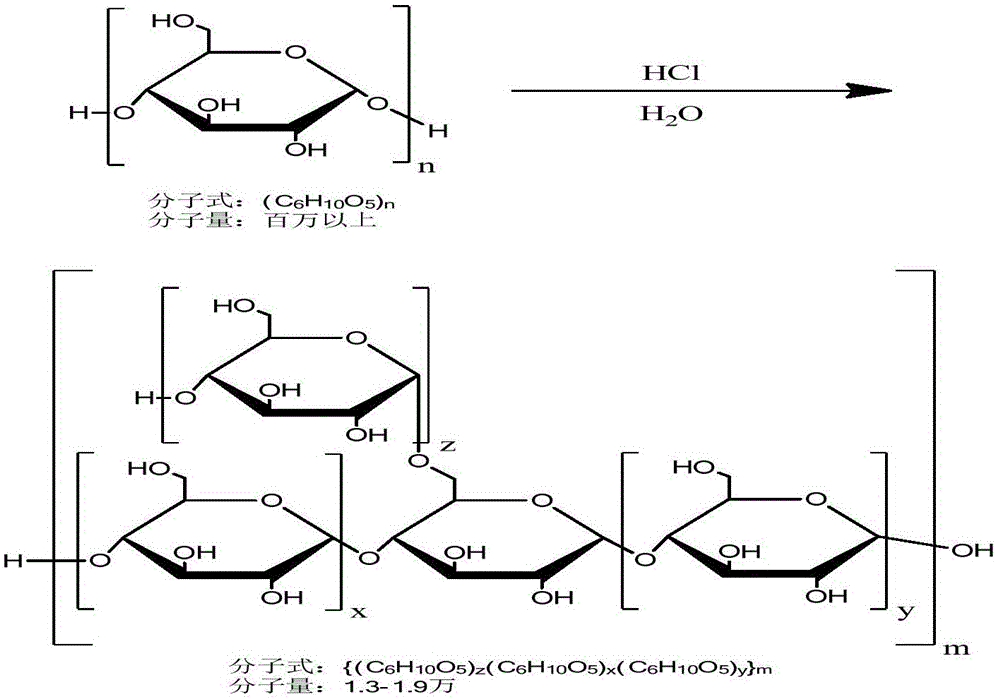
The invention discloses an icodextrin and a preparing method thereof. The preparing method comprises the first step of mixing cereal starch and water into a solution with the substrate concentration ranging from 20wt% to 50wt%, and adding acid to the mixture of the cereal starch and the water to form a reaction solution, wherein the final concentration of the acid ranges from 0.1% to 1.5% (V / V), the hydrolysis reaction is carried out at the temperature ranging from 70 DEG C to 93 DEG C, the reaction process is monitored at the same time, when the flow-out time duration of the reaction solution, measured with an Ubbelohde viscometer with the internal diameter of a capillary tube being 0.9mm to 1.0mm, is 2-4 minutes, the reaction solution is neutralized to pH7 through the aqueous alkali, the hydrolysis reaction is ended to obtain a product 1, and the reaction duration ranges from 0.5 hour to 4 hours; the second step of carrying out molecular weight screening on the product 1 to obtain a product 2 with the weight-average molecular weight ranging from 13 thousand Da to 19 thousand Da; the product 2 is dried and solidified to obtain the icodextrin. The distribution range of the molecular weight of the icodextrin is narrower, and the molecular weight distribution is more centralized.
Owner:HUAREN PHARMACEUTICAL CO LTD
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS20050191717A1Improved peritoneal dialysis solutionImproved testing procedureAntibacterial agentsBiocideBiologyPeritoneal dialysis solutions
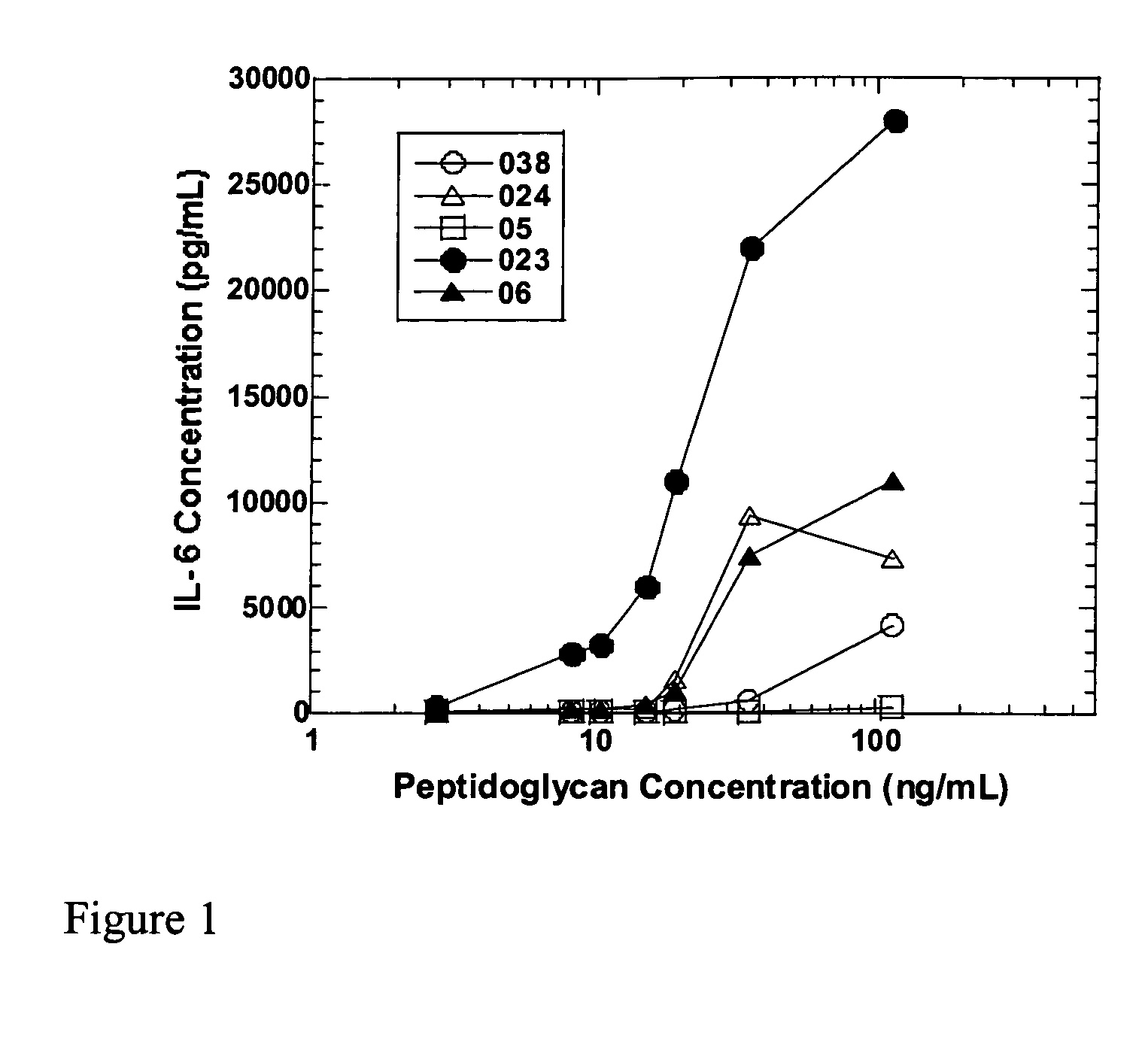
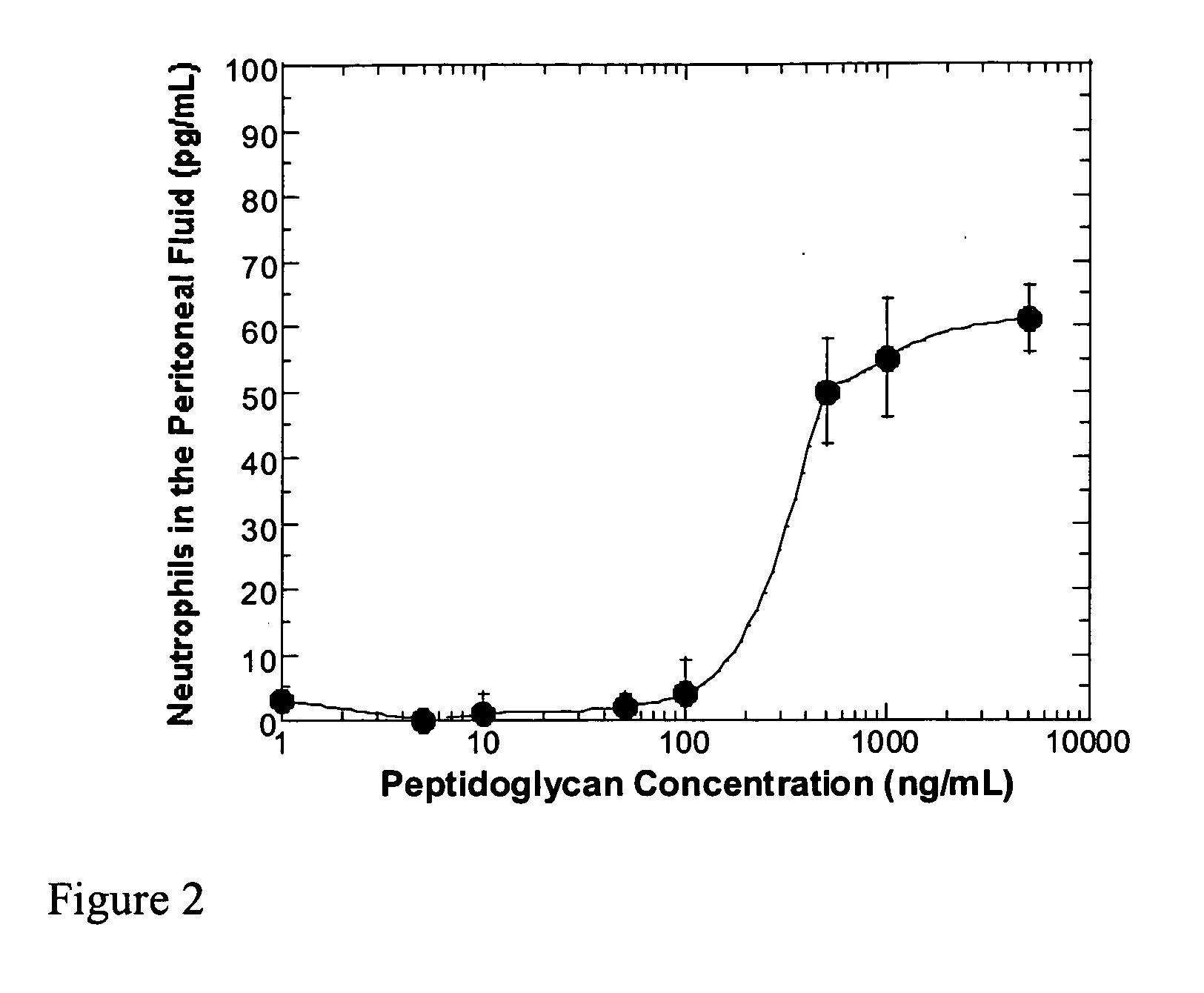
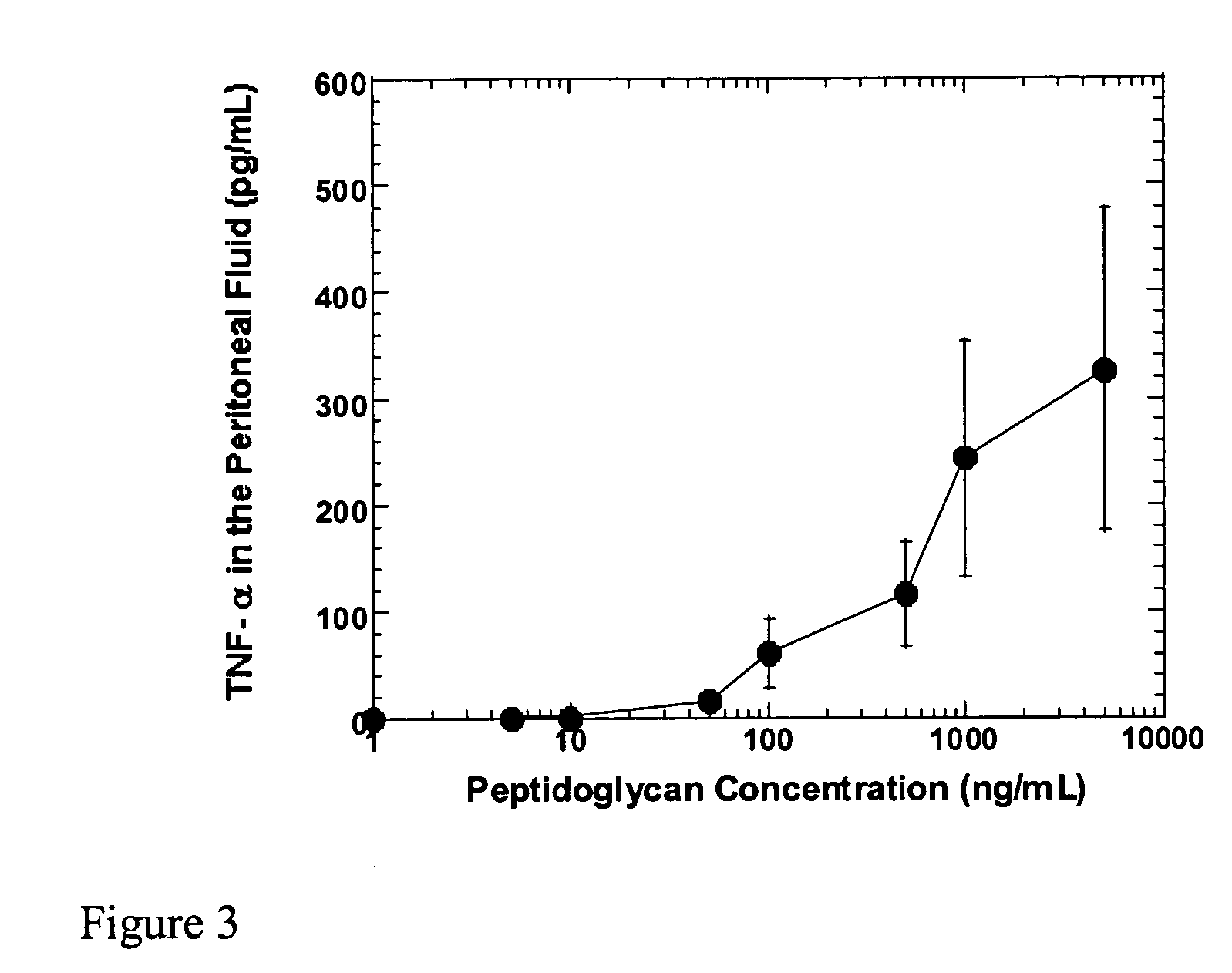
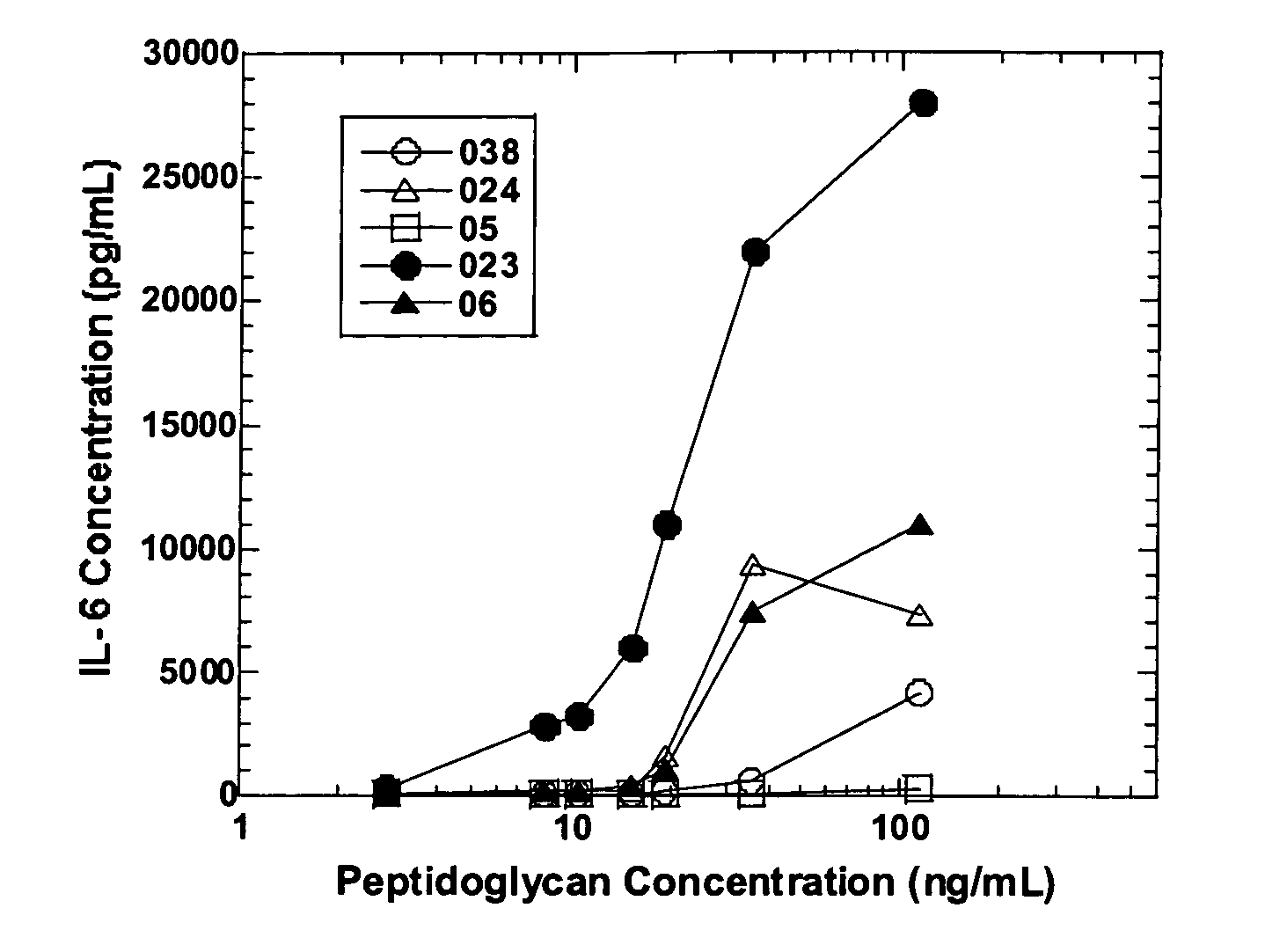
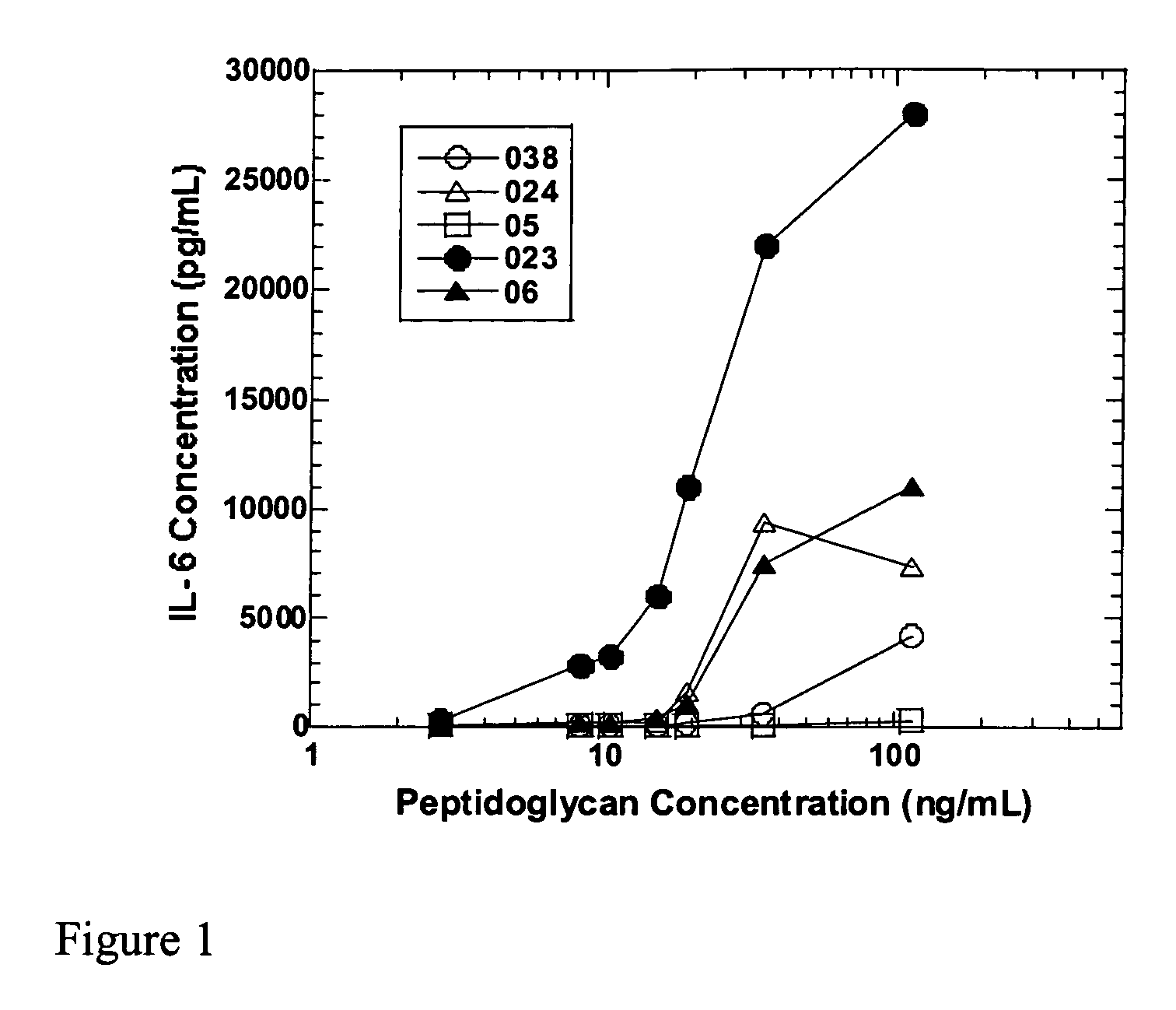
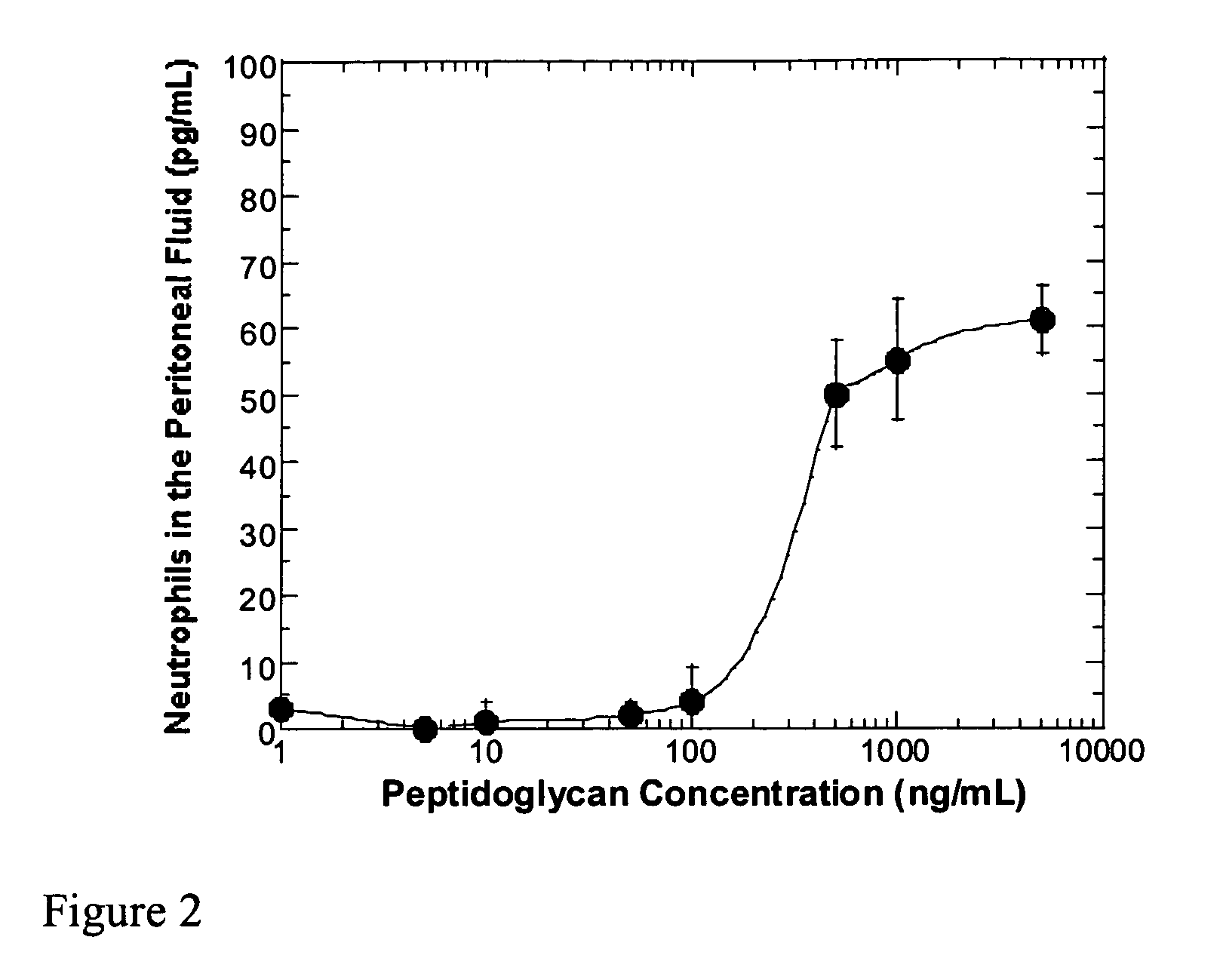
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
Industrial production method of icodextrin
The invention provides an industrial production method of icodextrin. The industrial production method comprises steps as follows: a, an acid solution with the concentration of 0.3%-1.5% is prepared; b, 1,000 parts by weight of the acid solution are taken, 400-450 parts by weight of corn starch are added to the acid solution during stirring, the mixture has a hydrolysis reaction for 1-2 h at the hydrolysis temperature of 90-95 DEG C, and then pH is regulated to 5-7, and a hydrolysis product is obtained; c, the hydrolysis product prepared in the step b is cooled to 55-60 DEG C, 12-13.5 parts by weight of activated carbon is added, decoloration is performed for 0.5-1 h, and filtration is performed after decoloration; d, a filtrate prepared in the step c is subjected to ultrafiltration and drying, and icodextrin is obtained. According to the industrial production method of icodextrin, the preparation process is simple, the production cost is low, a prepared product has good quality, and the industrial production method is applicable to industrial mass production of icodextrin.
Owner:四川博佳制药有限公司
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions
InactiveUS7118857B2Simple methodPrevent peritonitisAntibacterial agentsOrganic active ingredientsBiologyPeritoneal dialysis solutions
Methods and compositions for detection of microbial contaminants in peritoneal dialysis solutions are provided. The methods and compositions employ modified bioburden testing and the detection of peptidoglycan. A novel cause of aseptic peritonitis is provided—aseptic peritonitis associated with gram positive microbial contamination of a dialysis solution. Peptidoglycan is a major component of a gram positive bacterial cell wall and thus can serve as a marker for gram positive bacteria. In this regard, testing for peptidoglycans can be utilized to effectively prevent peritonitis in patients that use the peritoneal dialysis solutions, such as peritoneal dialysis solutions that contain a glucose polymer including an icodextrin and the like.
Owner:BAXTER INT INC +1
Icodextrin biomaterial and preparation method thereof
InactiveCN107308184AIncrease profitImprove the bactericidal effectInorganic boron active ingredientsHydroxy compound active ingredientsLymphatic SpreadIrritation
The invention discloses an icodextrin biomaterial with antibacterial and bactericidal effects in a perioperative period. The icodextrin biomaterial is a macromolecular biomaterial which has antibacterial, bactericidal and anti-adhesive effects when applied to surgery in the perioperative period. In the aspect of tumor surgery, the icodextrin biomaterial further has the effect of preventing the implantation metastasis caused by tumor cells which scatter on an inner membrane of a human body cavity during the tumor operation. According to the icodextrin biomaterial, water for injection is used as a carrier, and water-soluble icodextrin and iodine are used as main raw materials to prepare an icodextrin complex iodine biopolymer material. The icodextrin iodine biomaterial is prepared from icodextrin iodine and NaCl for regulating osmotic pressure substance or mannitol or glycine through using the water for injection. The icodextrin biomaterial does not have toxicity, irritation or sensitization for a human body, and has good biodegradability; and in clinic, the icodextrin biomaterial has good effects in antibiosis or sterilization, anti-adhesion and control of cancer cell implantation metastasis.
Owner:JIANGSU HAIERZI BIOTECH CO LTD
Preparation method of icodextrin for starch-based peritoneal dialysis solution
ActiveCN106397616ANarrow distributionHigh purityBlood disorderExtracellular fluid disorderHydrolysateUltrafiltration
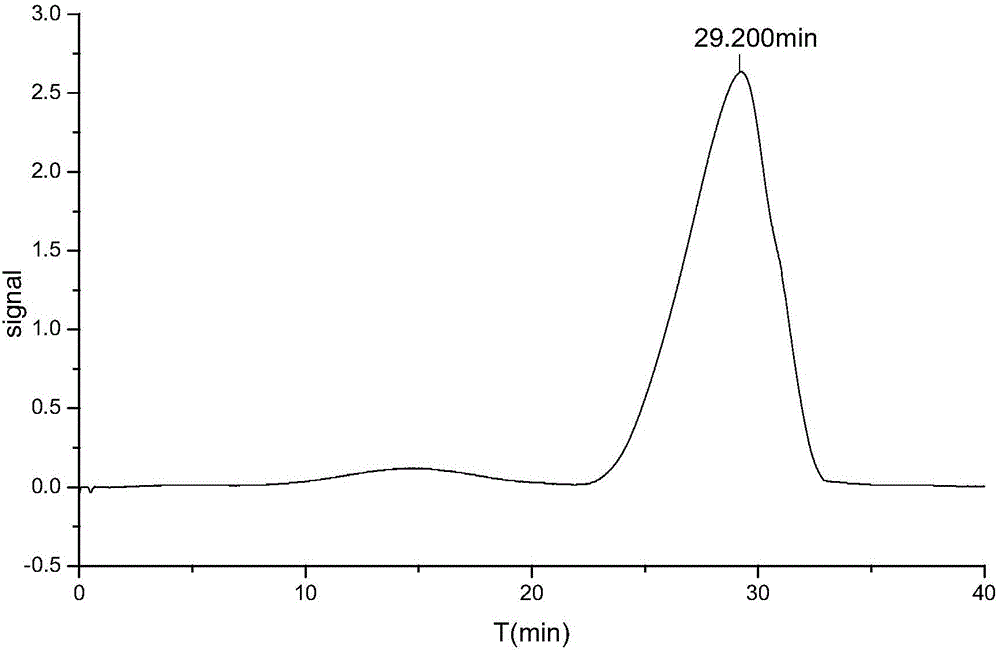
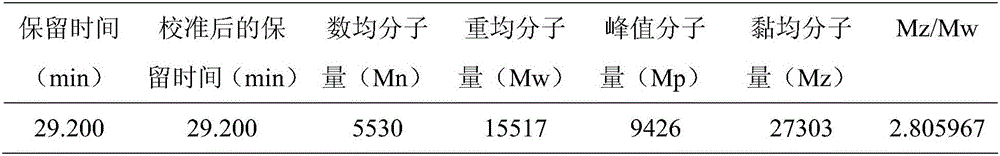
The present invention discloses a preparation method of icodextrin for a starch-based peritoneal dialysis solution. According to the technical scheme of the invention, native starch is adopted as a raw material, and then a starch solution with the concentration thereof to be 5% to 15% is prepared by using a phosphate buffered solution. At a certain temperature and a certain pH value, the starch solution is firstly subjected to enzymolysis by using alpha-amylase, and then the gelatinized starch is subjected to debranching by using a debranching enzyme. The enzymatic hydrolysate is subjected to alcohol precipitation, ultrafiltration, gel chromatographic column separation and purification, and then the weight-average and number-average molecular weights thereof and the alpha-1, 6 glycosidic bond thereof meet the requirements at the same time. The content of alpha-1, 6 glycosidic bond in icodextrin is smaller than 10%, and the weight-average molecular weight thereof is 13000 to 19000 Da. The number-average molecular weight thereof is 5000 to 6500 Da. The preparation method of icodextrin for the starch-based peritoneal dialysis solution is high in yield, good in quality and relatively low in cost. Meanwhile, the defects of the conventional icodextrin preparation process in the prior art are overcome.
Owner:SOUTH CHINA UNIV OF TECH
Peritoneal dialysis solution containing modified icodextrins
InactiveUS7208479B2Easy to optimizeSimple methodOrganic active ingredientsBiocideGluconic acidD-glucitol
The present invention provides a peritoneal dialysis solution that contains heat stable osmotic agents such as D-glucitols, gluconic acids and alkylglycosides produced the reduction, oxidation or glycosylation of icodextrins respectively. As a result, osmotic agents that are stable under autoclaving or heat sterilization conditions are provided which reduces the amount of bioincompatible materials in the sterilized peritoneal dialysis solutions. Methods of preparing the D-glucitols, gluconic acids and alkylglycosides are disclosed.
Owner:BAXTER INT INC
Peritoneal dialysis fluid
ActiveUS20150231321A1Improve stabilityHeat suppressionBiocideOrganic active ingredientsPh regulationPeritoneal dialysis fluid
The present invention is a sterile peritoneal dialysis fluid, including an acidic first liquid containing only icodextrin and 0 to 2.34 g / L of sodium chloride, and an alkaline second liquid containing an alkaline pH regulator, in which the first liquid after sterilization has a pH of 5.0 to 5.5, the second liquid after sterilization has a pH of 6.5 to 7.5, and a mixture of the first liquid and the second liquid after sterilization has a pH of 6.0 to 7.5. The present invention can provide a peritoneal dialysis fluid containing icodextrin, in which the stability of icodextrin during the heat sterilization and the subsequent storage can be improved to the maximum, and the pH of the peritoneal dialysis fluid is close to the physiological range.
Owner:TERUMO KK
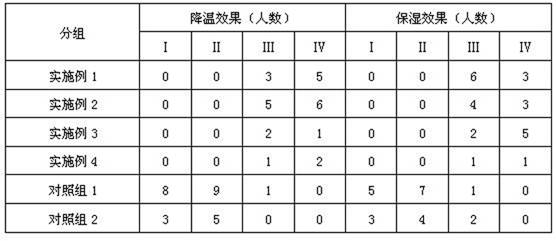
Moisturizing skincare lotion containing icodextrin and application of moisturizing skincare lotion
ActiveCN111643396AGood biocompatibilityRefreshing textureCosmetic preparationsToilet preparationsPhysiologyLotion
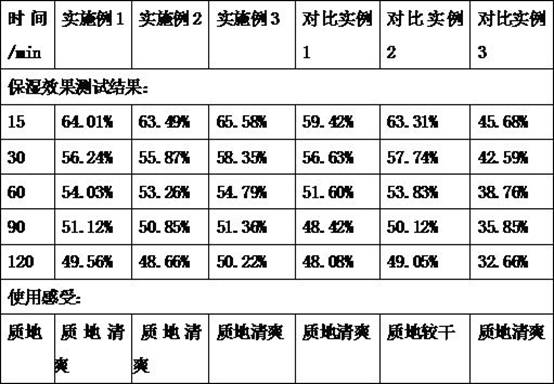
The invention belongs to the field of cosmetics, and relates to a moisturizing skincare lotion containing icodextrin and application of the moisturizing skincare lotion. The skin moisturizer disclosedby the invention contains icodextrin, and the content of the icodextrin is 3%-8%. The icodextrin used as a moisturizing component of the moisturizing skincare lotion is alpha-glucan, and the molecular weight of the icodextrin is smaller than that of common glucan, so that the water solubility is good, a larger amount of icodextrin can be added, a larger moisturizing effect can be achieved, and the texture of the skincare lotion is kept fresh and cool. The problems that the moisturizing ingredients of the moisturizing skincare lotion on the market at present mainly comprise beta-glucan, the water solubility is poor, and the moisturizing skincare lotion is viscous when the additive amount is about 1% are solved.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Anti-adhesion flushing fluid for gynecological operation and preparation method thereof
InactiveCN106176805ANormal permeabilityNormal pHSurgical drugsPharmaceutical delivery mechanismSodium lactateWound healing
The invention discloses anti-adhesion flushing fluid for a gynecological operation and a preparation method thereof and belongs to the technical field of flushing fluid for operation. Every 1L of the flushing fluid disclosed by the invention is prepared from the following raw materials in grams by weight: 30-50g of icodextrin, 4.5-6.5g of sodium chloride, 0.1-0.5g of calcium chloride, 0.01-0.1g of magnesium chloride, 4.0-5.0g of sodium lactate and a proper amount of water for injection. The invention also provides a method for preparing the flushing fluid. According to the invention, an anti-adhesion effect of icodextrin is fully utilized, and the calcium ion, magnesium ion and sodium lactate are added; due to the addition of the calcium ion and magnesium ion, the antibacterial and bacteriostatic abilities are enhanced, and wound healing is promoted; the sodium lactate serves as a pH buffer agent, so that normal osmotic pressure and pH value in the abdominal cavity can be guaranteed, and side reactions are reduced; and moreover, the flushing fluid disclosed by the invention is high in stability, difficult to degrade and denature, good in flushing effect and anti-adhesion effect, non-irritating to skin and convenient to use.
Owner:HUAREN PHARMACEUTICAL CO LTD
Peritoneal dialysis fluid
ActiveUS10010563B2Improve stabilityHeat suppressionOrganic active ingredientsPharmaceutical containersPh regulationPeritoneal dialysis fluid
Owner:TERUMO KK
Whitening mask and preparation method thereof
InactiveCN112294673AEasy to prepareReduce manufacturing costCosmetic preparationsToilet preparationsWhitening AgentsLotion
The invention provides a whitening mask and a preparation method thereof. The whitening mask comprises a whitening skincare lotion and a mask base material, and is characterized in that the whiteningskincare lotion comprises the following components in percentage by mass: 0.01%-5% of icodextrin, 0.1%-15% of a humectant, 0.1%-10% of a whitening agent, 0.01%-10% of a skin conditioner, 0.01%-0.1% oftriethanolamine, 0.01%-0.5% of a thickener, 0.1%-0.5% of a preservative, 0.001%-0.1% of daily essence and the balance of deionized water. The preparation method of the whitening mask comprises the following steps: preparing the mask base material and the whitening skincare lotion, and preparing the raw materials according to the raw material ratio of the whitening skincare lotion to prepare the whitening skincare lotion; and soaking the mask base material, in the whitening skincare lotion to obtain the whitening mask. The whitening mask has good whitening and moisturizing effects. The preparation method is simple, low in preparation cost and suitable for large-scale industrial production.
Owner:华仁健康产业(青岛)有限公司 +1
Liquid surgical dressing containing icodextrin
The invention provides a liquid surgical dressing containing icodextrin. Purified water or water used for injection is taken as a carrier, and the icodextrin, polyhydric alcohols, preservatives and thickening agents are added and can be made into a form of mist spray or essence. The liquid surgical dressing disclosed by the invention is high in safety and biocompatibility, can be used for moisturizing and repairing the skin and also can be directly used on a wound or a scar, a skin protection barrier is formed, the wound is accelerated to recover, and scar pigment deposition is alleviated.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Biocompatible peritoneal dialysate
ActiveUS11020420B2Improve stabilityGood biocompatibilityOrganic active ingredientsInorganic non-active ingredientsPeritoneal dialysateBiocompatibility
A biocompatible peritoneal dialysate according to the present invention is a sterilized biocompatible peritoneal dialysate composed of an acidic first solution containing icodextrin and a second solution containing a pH adjuster, wherein the pH after mixing of the sterilized first solution with the sterilized second solution is 6.0 to 7.5.
Owner:TERUMO KK
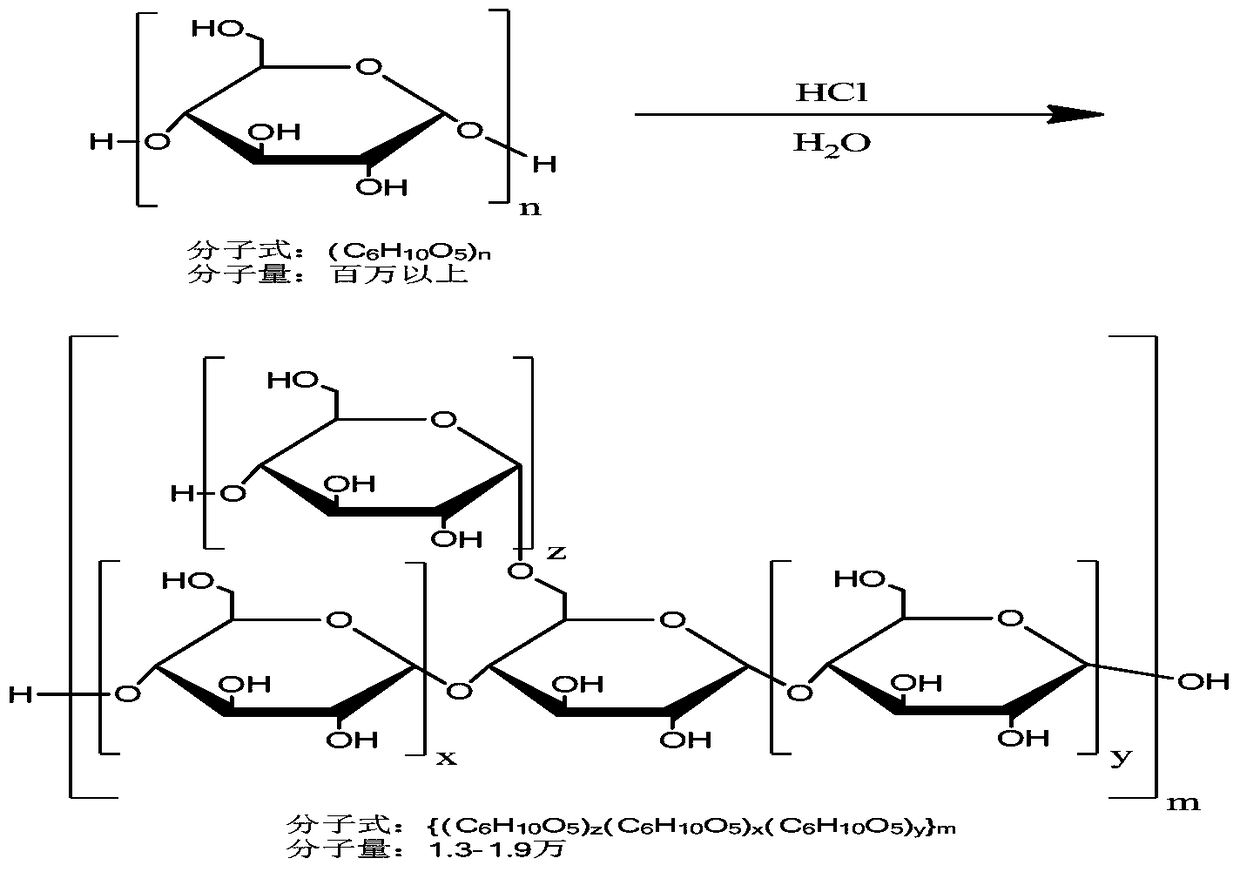
Icodextrin and its preparation method
The invention discloses an icodextrin and a preparing method thereof. The preparing method comprises the first step of mixing cereal starch and water into a solution with the substrate concentration ranging from 20wt% to 50wt%, and adding acid to the mixture of the cereal starch and the water to form a reaction solution, wherein the final concentration of the acid ranges from 0.1% to 1.5% (V / V), the hydrolysis reaction is carried out at the temperature ranging from 70 DEG C to 93 DEG C, the reaction process is monitored at the same time, when the flow-out time duration of the reaction solution, measured with an Ubbelohde viscometer with the internal diameter of a capillary tube being 0.9mm to 1.0mm, is 2-4 minutes, the reaction solution is neutralized to pH7 through the aqueous alkali, the hydrolysis reaction is ended to obtain a product 1, and the reaction duration ranges from 0.5 hour to 4 hours; the second step of carrying out molecular weight screening on the product 1 to obtain a product 2 with the weight-average molecular weight ranging from 13 thousand Da to 19 thousand Da; the product 2 is dried and solidified to obtain the icodextrin. The distribution range of the molecular weight of the icodextrin is narrower, and the molecular weight distribution is more centralized.
Owner:HUAREN PHARMACEUTICAL CO LTD
Mask powder with moisturizing function
InactiveCN112263508AMaintain cleanliness and healthAvoid churnCosmetic preparationsToilet preparationsEngineeringBiology
The invention provides mask powder with a moisturizing function. The mask powder is characterized by comprising the following components in percentage by mass: 1%-50% of icodextrin, 5%-20% of binder,10%-80% of filler, 1%-10% of auxiliary agent and 1%-10% of skin conditioner. Mask with moisturizing function prepared by the mask powder has good moisturizing effect, makes skin smooth, and can provide a layer of barrier against foundation, make-up, external environmental pollution and dust for the skin, thereby maintaining the skin in clean and healthy state.
Owner:华仁健康产业(青岛)有限公司 +1
Method for measuring content of magnesium salt in icodextrin bulk drug
InactiveCN106525819AHigh sensitivityGood repeatabilityPreparing sample for investigationAnalysis by thermal excitationMagnesium saltVolumetric flask
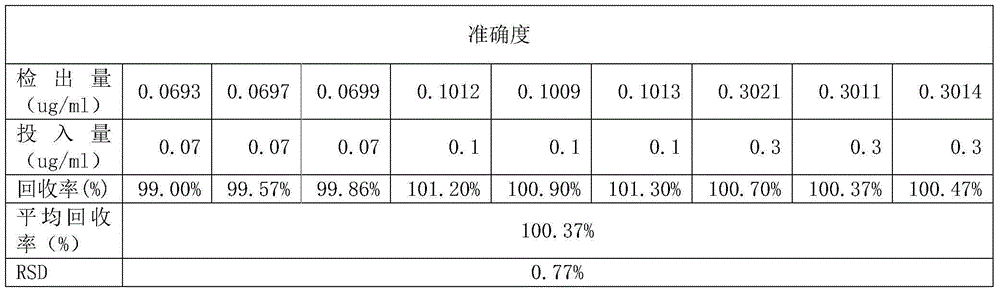
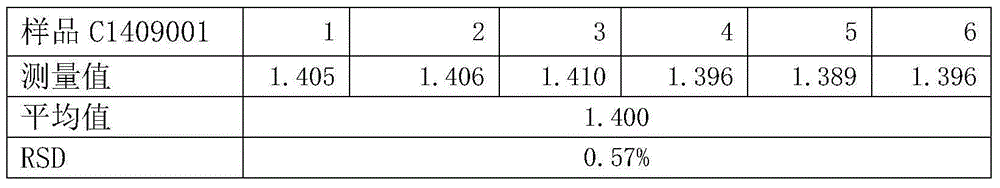
The invention belongs to the field of medical technology and relates to a method for measuring content of magnesium salt in icodextrin bulk drug. The method comprises the following steps: accurately weighing 0.1 g of icodextrin bulk drug, carrying out microwave digestion, transferring a sample solution into a 100ml PET volumetric flask, and keeping a constant volume by the use of water; preparing standard solutions with magnesium concentrations respectively being 0 ug / ml, 0.05 ug / ml, 0.1 ug / ml, 0.5 ug / ml, 1 ug / ml and 3 ug / ml; successively measuring strengths of the standard solutions and the sample solution under the condition of set inductively coupled plasma emission spectrometer parameters, drawing a calibration curve according to the strengths of the standard solutions, and comparing the calibration curve with a standard curve to obtain content of magnesium salt in the icodextrin bulk drug. The measurement method has high sensitivity and good repeatability, and realizes accurate measurement of magnesium salt in the icodextrin bulk drug. The method is simple to operate, is beneficial to reduce test cost and helps enhance product quality and safety.
Owner:HUAREN PHARMACEUTICAL CO LTD
Gel dressing containing icodextrin
The invention provides a gel dressing containing icodextrin. The dressing is prepared by using water for injection or purified water as a carrier and adding the icodextrin, carbomer or carboxymethylcellulose sodium, triethanolamine, butanediol, caprylhydroxamic acid, 3-[2-(Ethylhexyl)oxyl]-1,2-propandiol, 1,2-hexanediol, flavoring agent, etc. The gel dressing containing icodextrin provided by theinvention is in gelatinous form, and can be applied to new scars and minimally invasive wounds to prevent scar hyperplasia and external pollution, reduce the probability of scar formation and avoid pigmentation scars.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Moisturizing mask and preparation method thereof
InactiveCN112245346AEasy to prepareReduce manufacturing costCosmetic preparationsToilet preparationsBiologyDermatology
The invention provides a moisturizing mask and a preparation method thereof. The moisturizing mask comprises moisturizing skin lotion and a mask base material and is characterized in that the moisturizing skin lotion comprises the following components in percentage by mass of 0.01%-5% of icodextrin, 0.1%-15% of a humectant, 0.01%-10% of a skin conditioner, 0.01%-0.1% of triethanolamine, 0.01%-0.5%of a thickener, 0.1%-0.5% of a preservative, 0.001%-0.1% of daily essence, and the balance of deionized water. The preparation method of the moisturizing mask comprises the following steps: preparingthe mask base material and the moisturizing skin lotion, and preparing the raw materials according to the raw material ratio of the moisturizing skin lotion to obtain the moisturizing skin lotion. The produced moisturizing mask has a very good moisturizing effect, can also provide a layer of barrier for resisting foundation make-up, make-up, external environmental pollution, dust and the like forthe skin, and maintains the clean and healthy state of the skin. The preparation method is simple, and the preparation cost is low.
Owner:华仁健康产业(青岛)有限公司 +1
Novel cold compress patch
PendingCN111617307AReduce harmKeep calmAbsorbent padsTherapeutic coolingCold compressesNonwoven fabric
The invention relates to a novel cold compress patch. The novel cold compress patch comprises a cloth layer and a stock solution soaked on the cloth layer, wherein the stock solution comprises icodextrin; the cloth layer is a non-woven fabric or medical gauze. The novel cold compress patch has the advantages that the icodextrin serving as a novel moisturizing raw material with a definite structurecan provide a water-retaining barrier for the skin, so that the skin is kept in a moist state, and the damage of a dry environment to the skin is reduced. In addition, due to the film-forming effectof the icodextrin, a layer of barrier for resisting foundation make-up, cosmetics, external environment pollution, dust and the like can be provided for the skin, and the clean and healthy state of the skin is maintained. A hydration film can be formed on the skin, so that water loss on the skin surface is prevented, skin calming is kept, and the skin is helped to resist external pollution.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
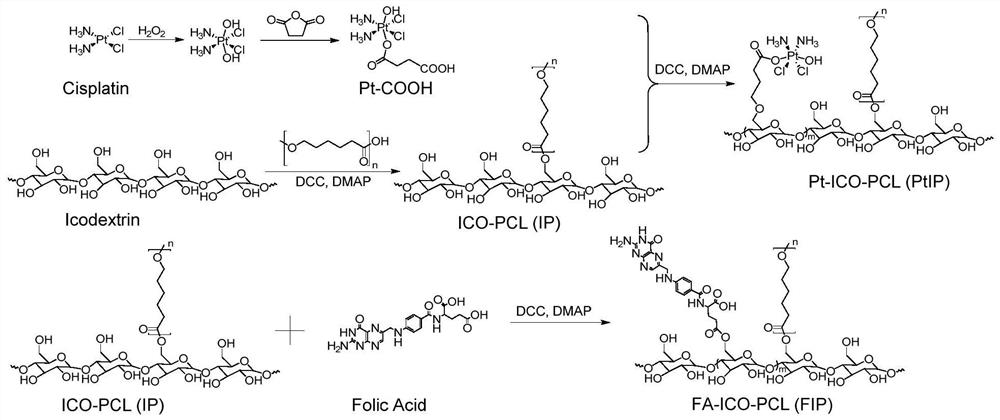
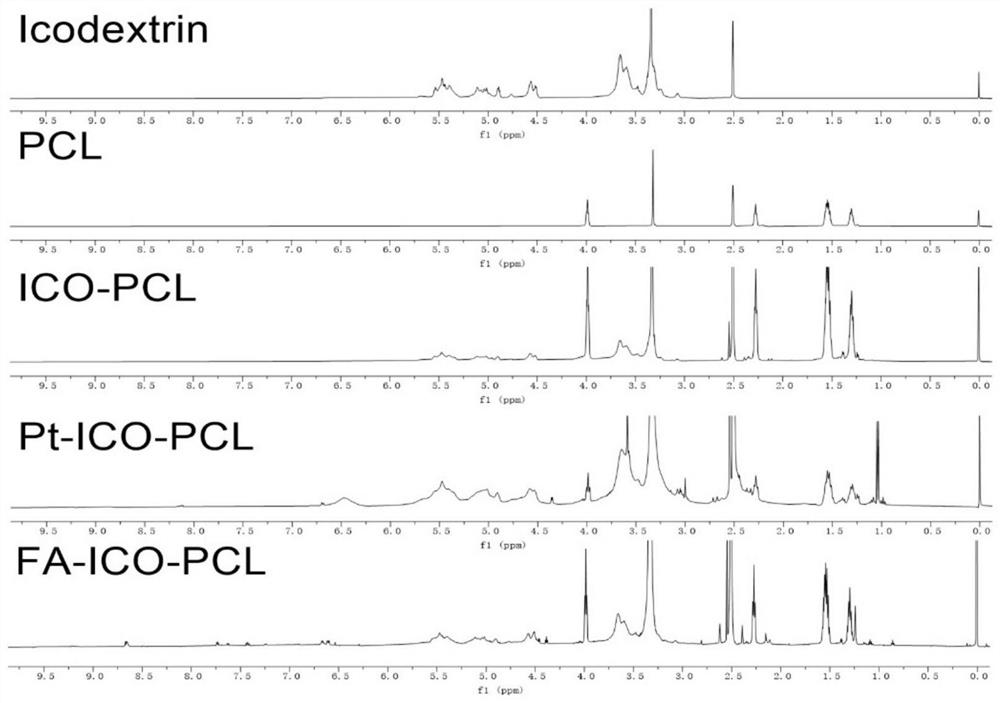
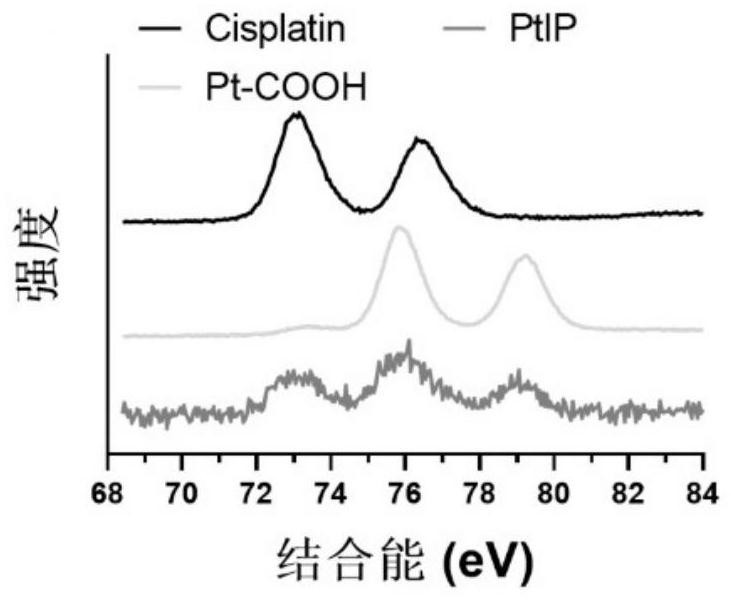
Platinum-icodextrin-polycaprolactone macromolecular compound, nano drug delivery system and application of nano drug delivery system
ActiveCN113616806AAchieve long cycle stabilityImprove bioavailabilityPowder deliveryInorganic active ingredientsPlatinumCisplatin
The invention belongs to the field of nano drug delivery systems, and discloses a platinum-icodextrin-polycaprolactone macromolecular compound, a nano drug delivery system and application of the nano drug delivery system. The compound is a polycaprolactone modified icodextrin-platinum conjugate, the icodextrin-platinum conjugate is formed by connecting a carboxylated tetravalent platinum compound to icodextrin through an ester bond, and the polycaprolactone is coupled with the icodextrin through the ester bond; the molecular weight of the polycaprolactone is 1 kDa to 10 kDa, and the molecular weight of the icodextrin is 10 kDa to 20 kDa; the grafting rate of the polycaprolactone on the icodextrin is 0.5-1, and the mass percentage of a platinum element in the macromolecular compound is 1%-5%. The compound contains a hydrophilic fragment icodextrin, a hydrophobic fragment polycaprolactone and tetravalent platinum, as a tumor treatment drug, the toxic and side effects of cis-platinum are reduced, the stability of circulation in blood is improved, so that the bioavailability of platinum is improved, and the anti-tumor effect is greatly enhanced.
Owner:HUAZHONG UNIV OF SCI & TECH
Collagen dressing containing icodextrin
PendingCN111743788AFast healthEasy to useCosmetic preparationsToilet preparationsNonwoven fabricBiology
The invention provides a collagen dressing containing icodextrin. The collagen dressing is prepared from icodextrin, hydrolyzed collagen, polyhydric alcohol, a preservative, a thickening agent and a flavoring agent. The collagen dressing is prepared by mixing the raw materials with purified water. The obtained dressing is prepared by combining icodextrin with collagen, is used for closed soft tissues, provides a healthy and clean microenvironment for the skin surface, can provide collagen required by skin recovery and assist the skin to recover more quickly, can be made into a non-woven fabric / gauze + stock solution form, is matched with different fragrances, and is good in use feeling, convenient to use and good in commercialization effect.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Industrial production method of icodextrin
Owner:四川博佳制药有限公司
New synthesis process of icodextrin API
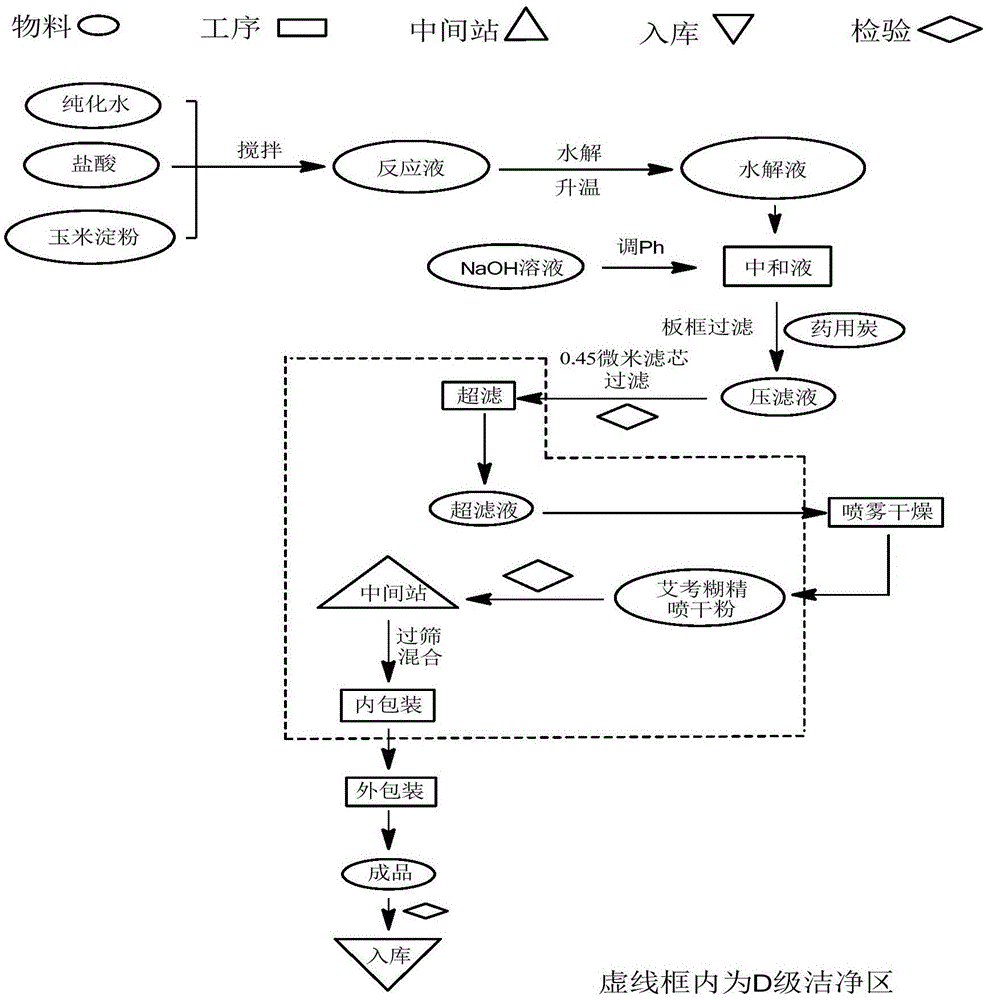
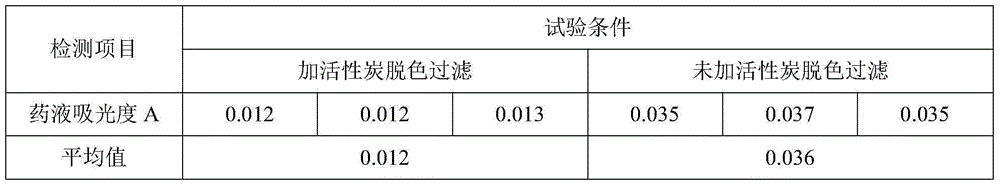
The invention relates to the field of medicine preparation, in particular to a new synthesis process of icodextrin raw material drug, which uses cornstarch as the starting material, hydrolyzes it to a certain extent by adding hydrochloric acid, and ultrafilters it with an ultrafiltration membrane with a molecular weight cut-off of 5000; adds activated carbon , by adjusting the pH value and controlling the temperature, spray-drying can obtain qualified products for each quality control index, especially for endotoxin control. The addition method and decarburization procedure, and the product with endotoxin controlled at less than 1.2 EU / g can be obtained by adjusting the temperature and pH value.
Owner:HUAREN PHARMACEUTICAL CO LTD +1
A composition containing icodextrin and its application
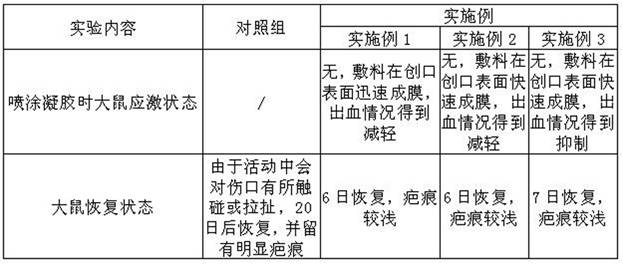
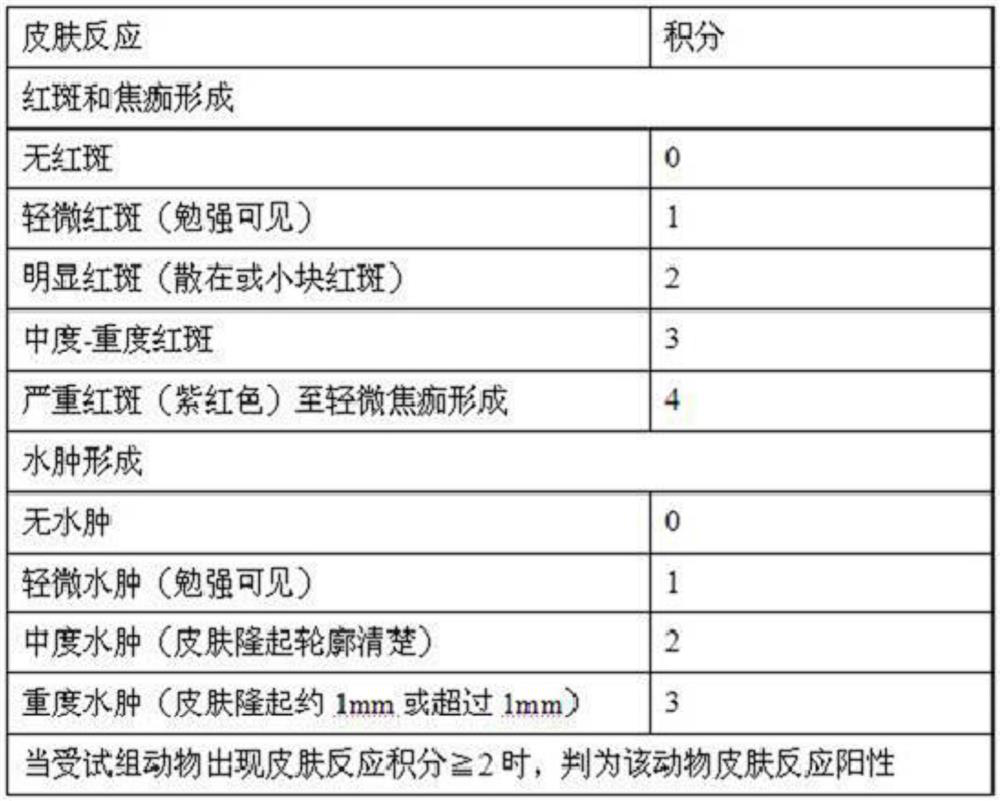
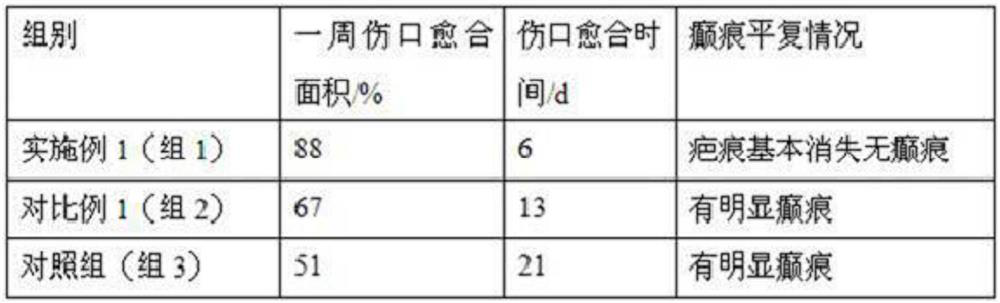
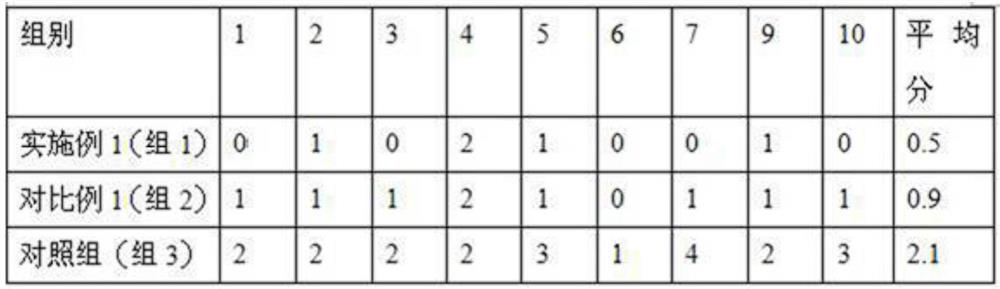
The invention belongs to the field of medical dressings, and relates to a composition containing icodextrin for promoting wound healing and preventing scars, in particular to a formula containing the composition and an application method. An icodextrin-containing composition of the present invention is characterized in that: the composition contains icodextrin; and the mass content of the icodextrin is 0.001-8%. The composition containing icodextrin of the present invention has the effect of promoting wound healing and preventing scar. As a medical dressing, icodextrin has good biocompatibility, good permeability and hydration floating effect, so it has good anti-adhesion properties. At the same time, the raw material of icodextrin is starch, which is convenient and easy to obtain. It is more convenient to widely promote and apply. At the same time, hyaluronic acid has good moisturizing properties, and the combination of the two will play a synergistic role in promoting wound healing, anti-adhesion and moisturizing.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Use of a mixture of modified glucose polymers for reducing tumor metastasis
PendingCN107206016AHydrocarbon active ingredientsPharmaceutical delivery mechanismLymphatic SpreadMedicine
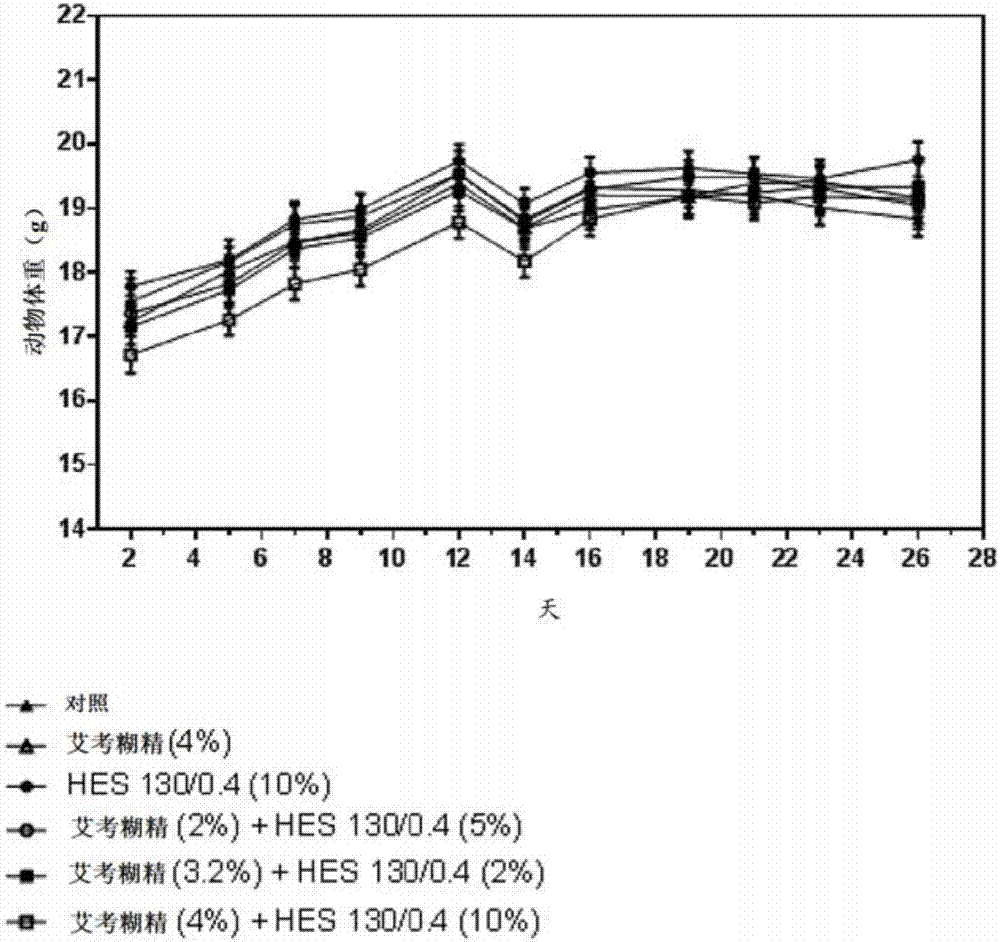
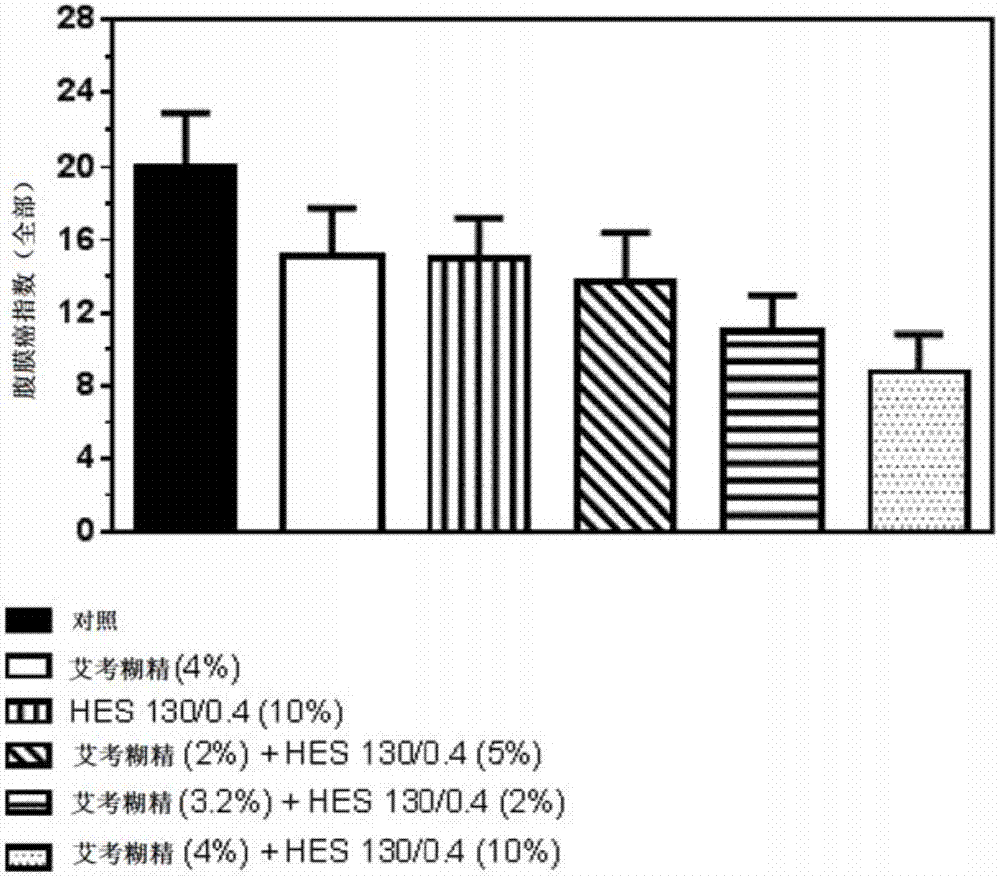
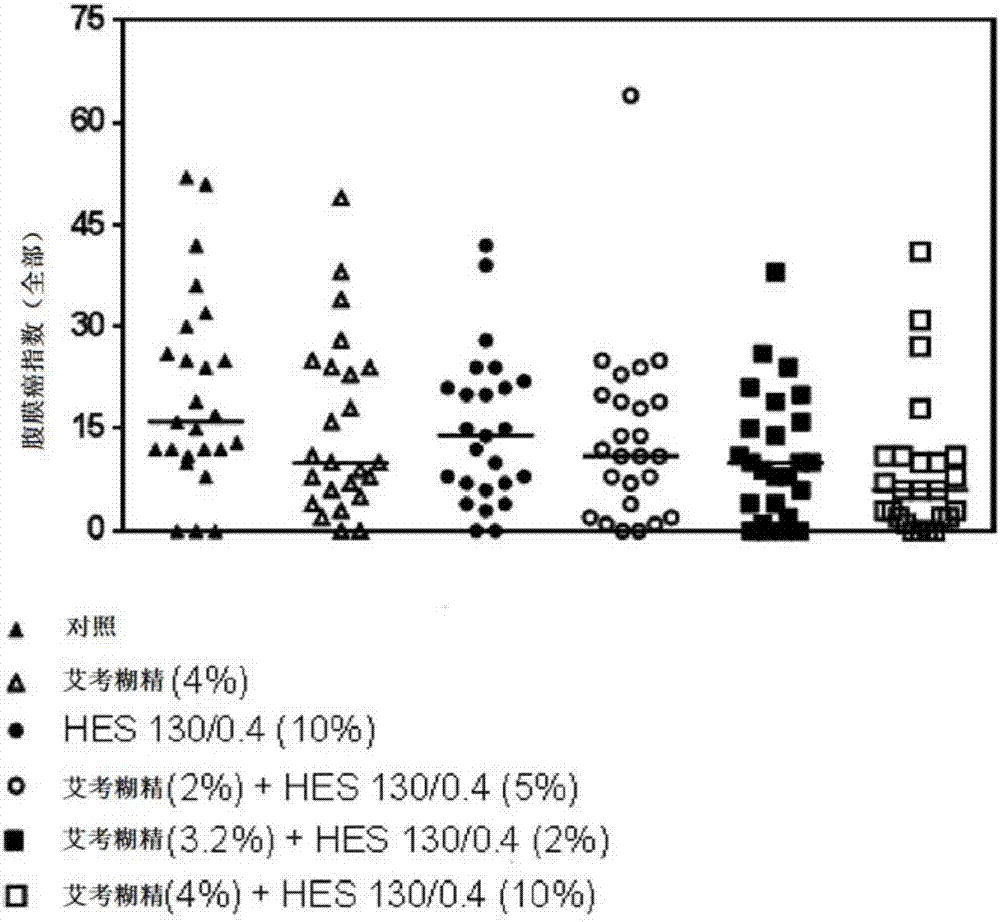
The present invention relates to a solution, in particular a pharmaceutically acceptable solution, comprising icodextrin and hydroxyalkyl starch (HAS), wherein the icodextrin is present at a concentration of from 1 to 7.5 % (w / v) and wherein said HAS is present at a concentration of from 1 to 15 % (w / v). The present invention further relates to the aforesaid pharmaceutically acceptable solution for use as a medicament and for use in (e.g., in methods of) preventing metastasis formation and / or relapse by administration to a body cavity of a subject afflicted with cancer. The present invention further relates to a kit comprising icodextrin and HAS in pre- weighed amounts and a pharmaceutically acceptable means of dissolving the same, to a device comprising a pharmaceutically acceptable solution of the present invention and means for administering the same, as well as to a pharmaceutically acceptable solution comprising icodextrin and hydroxyalkyl starch at a total concentration in the range of from 1 to 20 % (w / v), wherein the weight ratio of the icodextrin relative to the hydroxyalkyl starch is in the range of from 0.05:1 to 5:1, for use in preventing metastasis formation and / or relapse by administration to a body cavity of a subject afflicted with cancer.
Owner:FRESENIUS KABI DEUT GMBH
A kind of preparation method of icodextrin
A method for preparing icodextrin, belonging to the technical field of cyclodextrin production, comprising: gelatinization, acidolysis, decolorization and ultrafiltration processes in sequence, characterized in that: the gelatinization process includes mixing insoluble organic particles, starch and The water is mixed and stirred at 90-100°C, and the slurry is adjusted at normal temperature, wherein the added amount of the insoluble organic particles is 1-2% of the weight of the starch. The insoluble organic particles are polystyrene microspheres, insoluble cellulose particles or poly(N-isopropylacrylamide) microgel particles. Compared with the prior art, the starch liquid is gelatinized before the acid hydrolysis, and insoluble organic particles are added in the gelatinization to make the starch disperse evenly, prevent the aging of the starch, reduce the generation of insoluble starch particles, and improve the conversion rate and yield of the product .
Owner:ZIBO QIANHUI BIOTECH
Peritoneal dialysis fluid
ActiveUS11020520B2Improve stabilityHeat suppressionOrganic active ingredientsInorganic non-active ingredientsPeritoneal dialysis fluidSurgery
The present invention is a sterile peritoneal dialysis fluid, including an acidic first liquid containing only icodextrin and 0 to 2.34 g / L of sodium chloride, and an alkaline second liquid containing an alkaline pH regulator, in which the first liquid after sterilization has a pH of 5.0 to 5.5, the second liquid after sterilization has a pH of 6.5 to 7.5, and a mixture of the first liquid and the second liquid after sterilization has a pH of 6.0 to 7.5. The present invention can provide a peritoneal dialysis fluid containing icodextrin, in which the stability of icodextrin during the heat sterilization and the subsequent storage can be improved to the maximum, and the pH of the peritoneal dialysis fluid is close to the physiological range.
Owner:TERUMO KK
Dialysis concentrate and preparation and quality detection method thereof
PendingCN114869906AOsmosis helpsEnsure Assay AccuracyComponent separationMacromolecular non-active ingredientsSodium lactateCyclodextrin
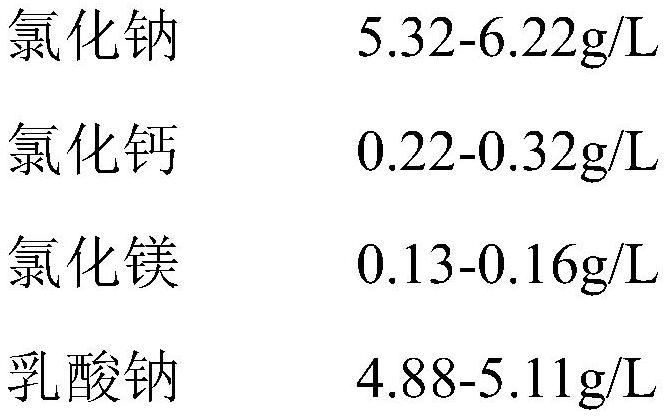
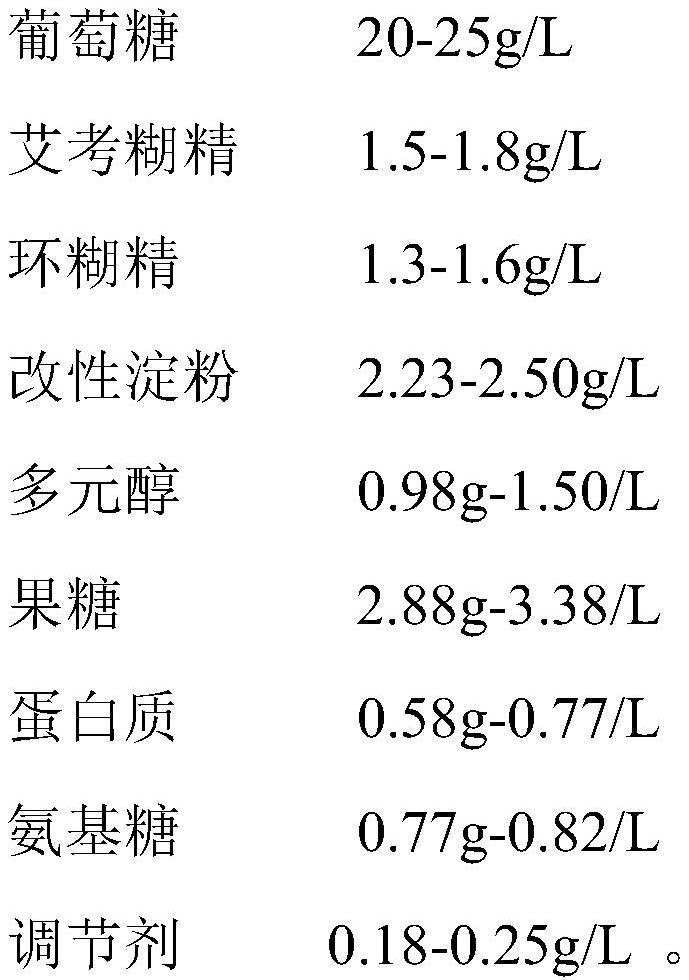
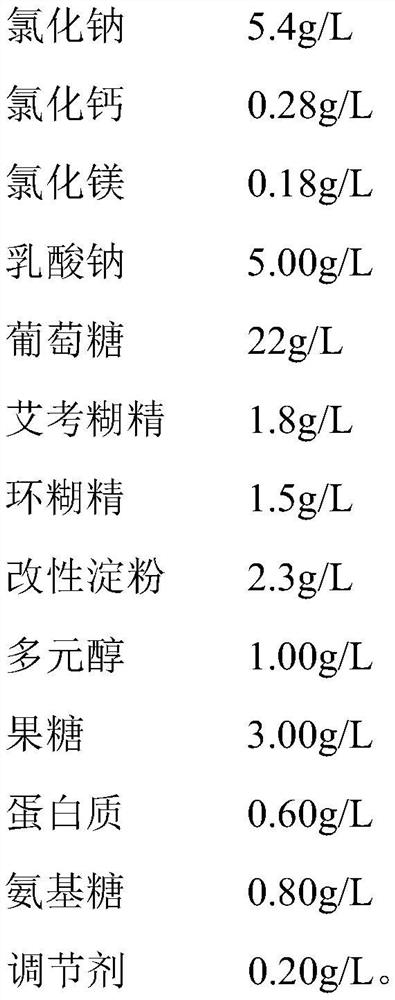
The invention provides a dialysis concentrate and a preparation and quality detection method thereof, and belongs to the technical field of dialyzates, the dialysis concentrate comprises lactate, a penetrant and a buffer agent; the lactate comprises sodium chloride, calcium chloride and magnesium chloride; the penetrant comprises glucose, icodextrin, cyclodextrin, modified starch, polyol, fructose, protein and amino sugar; the penetrant further comprises a regulator; the buffering agent is at least one of sodium lactate, bicarbonate, pyruvate and citrate. The dialysis concentrate is formed by mixing the lactate, the penetrant and the buffering agent, the sodium chloride, the calcium chloride and the magnesium chloride are the ion content of the dialysate, the added glucose, icodextrin and cyclodextrin penetrant can contribute to the permeation of the dialysate, and the penetrant hardly interferes with the sodium lactate, so that the dialysis concentrate has the advantages that the dialysis concentrate is safe and reliable. And the content accuracy of the mixed sodium lactate is ensured.
Owner:HUAYU WU XI PHARMA
Icodextrin-containing traditional Chinese medicine functional beverage capable of neutralizing effect of alcoholic drinks
InactiveCN111700196ALow sweetnessReduce heatNatural extract food ingredientsFood ingredient functionsBiotechnologyTraditional medicine
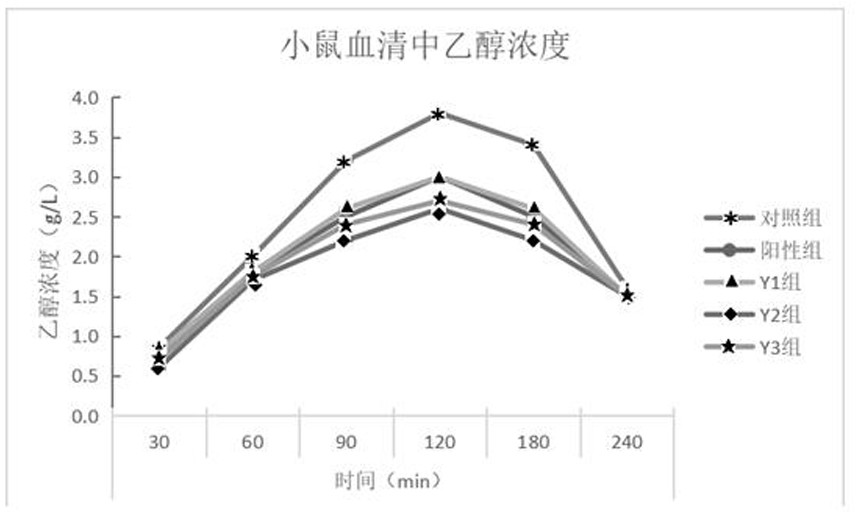
The invention relates to the field of foods, in particular to an icodextrin-containing traditional Chinese medicine functional beverage capable of neutralizing the effect of alcoholic drinks. The icodextrin-containing traditional Chinese medicine functional beverage capable of neutralizing the effect of alcoholic drinks contains the icodextrin, a radix puerariae extract, a kudzu vine flower extract, a Chinese wolfberry fruit extract, white granulated sugar, edible salt, auxiliary materials and water. The icodextrin-containing electrolyte beverage disclosed by the invention is good in the effect of neutralizing the effect of alcoholic drinks, safe and good in quality stability, has the advantages of being low in sugariness, good in mouth feel, good in moisturizing properties and not liableto crystallize, can be continuously hydrolyzed in intestinal tracts, and has the efficacy of prolonging energy supply of human bodies and reinforcing endurance and capacity for work of organisms.
Owner:HUAREN PHARMACEUTICAL CO LTD +3
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com