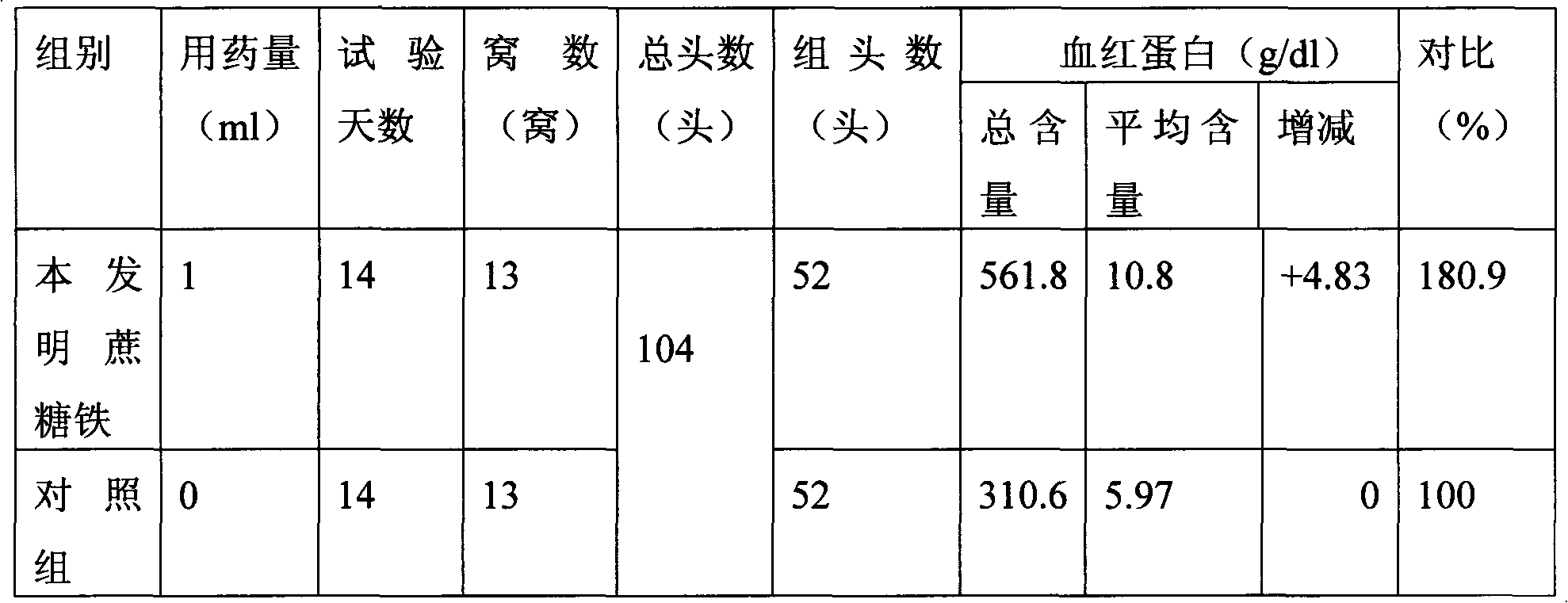
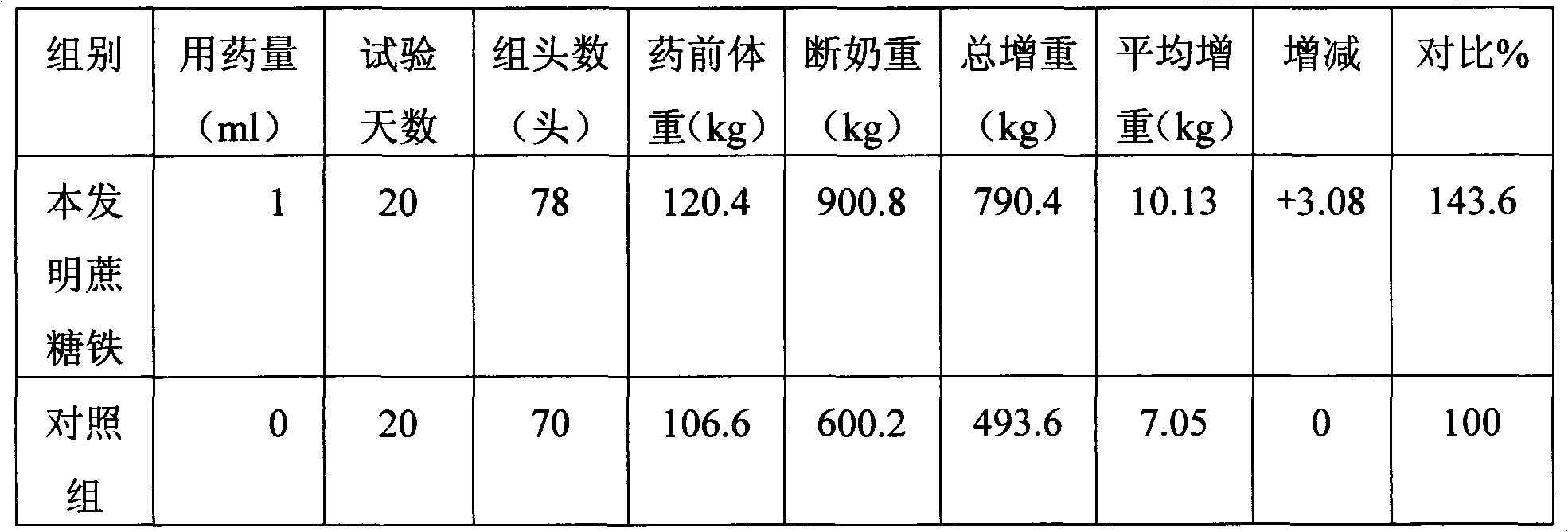
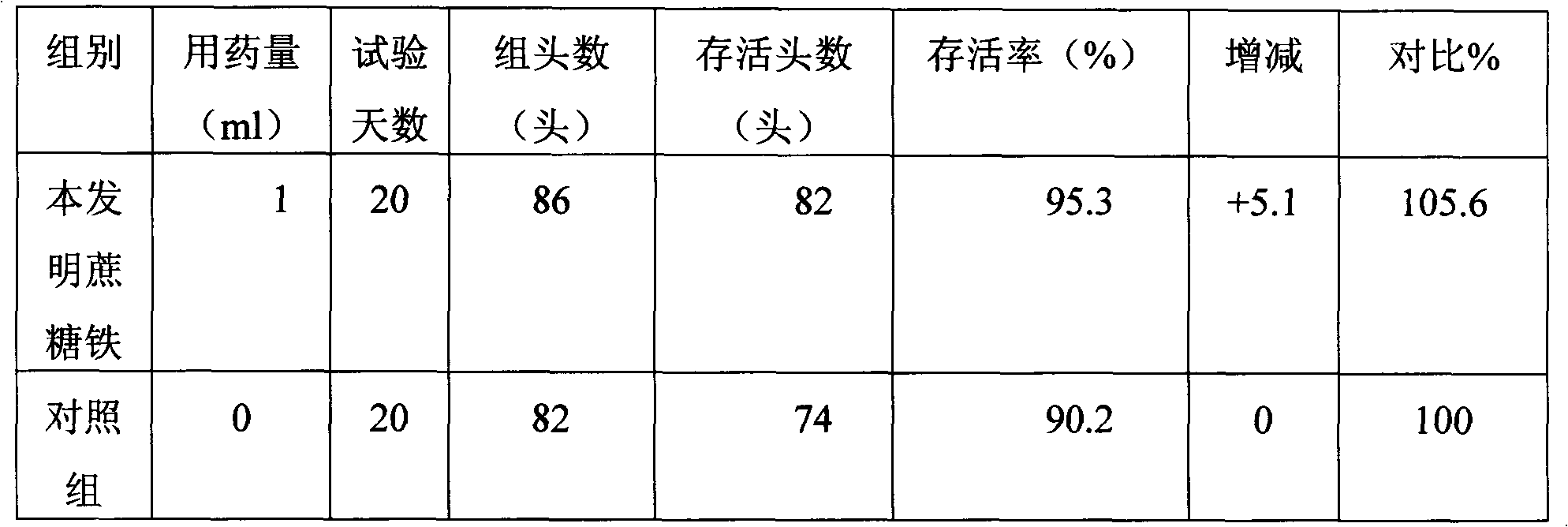
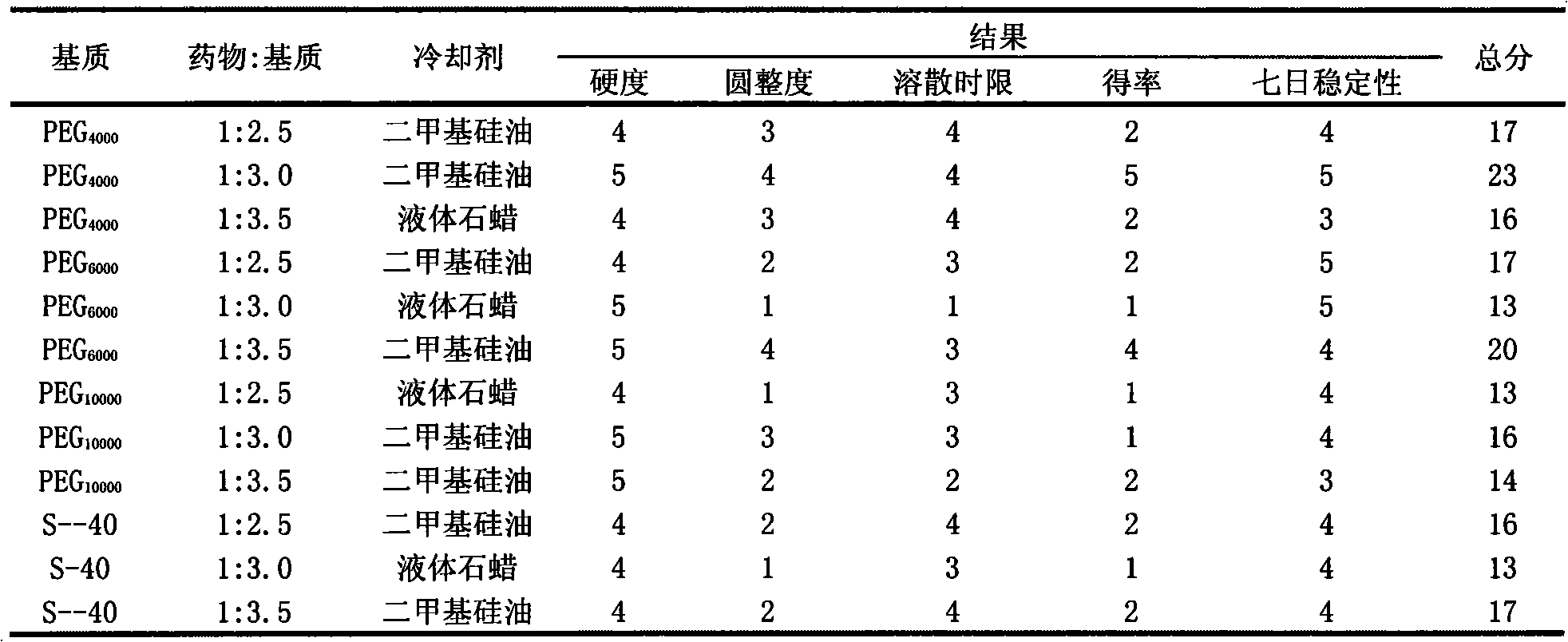
Patents
Literature
1475 results about "Bulk drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Definition of Bulk Drug Substance Bulk Drug Substance means any substance that is represented for use in a drug and that, when used in the manufacturing, processing, or packaging of a drug, becomes an active ingredient or a finished dosage form of the drug. The term does not include intermediates used in the synthesis of such substances.
Traditional Chinese medicine preparation for treating cancer and preparation method thereof
InactiveCN101670071AReduce manufacturing costLow cure rateAmphibian material medical ingredientsAnthropod material medical ingredientsCauses of cancerSide effect
The invention discloses a traditional Chinese medicine preparation for treating cancer, which is prepared by 103 types of bulk drugs such as solidago decurrens, Chinese sage herb, hypericum japonicumand the like. A preparation method of the traditional Chinese medicine preparation comprises the following steps: preparing the bulk drugs into water extract by a conventional method; using the prepared water extract for preparing oral solution by a traditional method; drying the obtained water extraction, then conducting grinding and sieving; taking the throughs for extracting powder, and using the extracted powder for preparing tablets, granules, electuaries or capsules by a traditional method. The preparation method also can be as follows: sieving out the dust of the bulk drugs, conductinggrinding and sieving, taking the throughs, conducting disinfection and sterilization to obtain raw powder, and using the raw powder for preparing tablets, granules, electuaries or capsules by a traditional method. With drugs mixed rationally, the traditional Chinese medicine preparation addresses both the symptoms and root causes of cancer. The bulk drugs are frequently-used common Chinese drugs,have low preparation cost and small side effect, and can effectively enhance own immunity, attack pathogen while do not damage body. The traditional Chinese medicine preparation can cure various typesof cancer, particularly lung cancer, stomach cancer, liver cancer, esophageal cancer, pancreatic cancer, cervical cancer and breast cancer.
Owner:卢速江
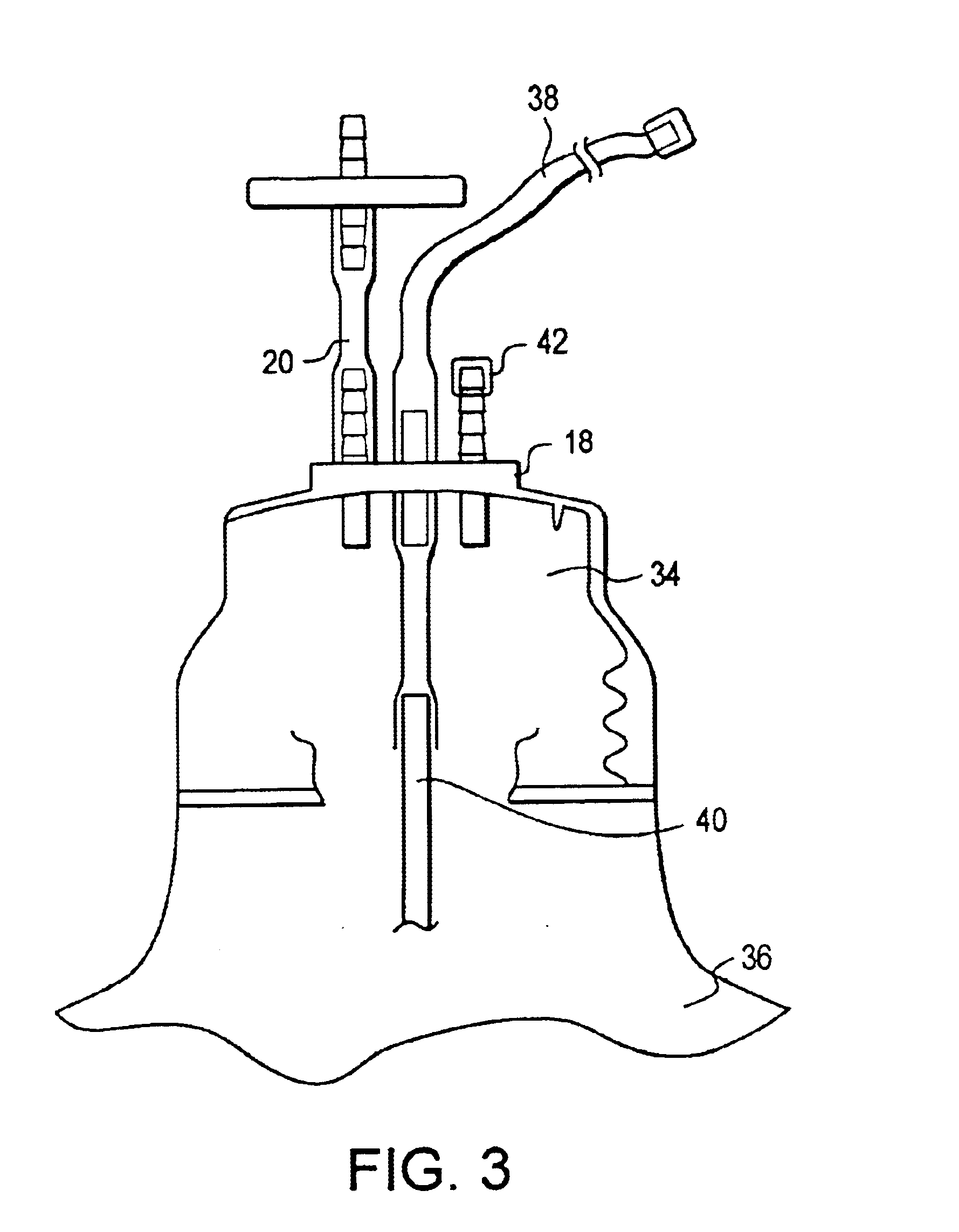
Automated apparatus for dispensing medicaments
Apparatus for delivering a medicament to a user has a drug vault including pre-packaged product storage containers holding inventory pre-packaged medicament products, and containers holding inventory bulk medicament. A control system is operable to dispense bulk medicament from the bulk product storage containers, to package the dispensed inventory bulk medicament as dispensed packaged medicament products, and to pick and deliver medicament products to a delivery zone.
Owner:PCAS PATIENT CARE AUTOMATION SERVICES
Traditional Chinese medicine composition for treating respiratory tract haze disoperation and preparation method
InactiveCN103127369AEffectively treat or prevent respiratory diseasesNo side effectsPill deliveryGranular deliveryMedicinal herbsFritillaria thunbergii
The invention discloses a traditional Chinese medicine composition for treating respiratory tract haze disoperation and a preparation method, the composition comprises the following bulk drugs by weight: 10-30 g of balloonflower root, 1-15g of Zhejiang fritillaria bulb, 1-15g of grosvenor momordica fruit and 1-15g of licorice. The traditional Chinese medicine composition has the efficacy of dispersing lung Qi, lifting airway self-cleaning capability and eliminating harmful substances, in vitro and clinical verifies that the traditional Chinese medicine composition has the accurate curative effect for preventing and treating respiratory tract haze disoperation without toxic and side-effect. The above materials are ground to crude powder and packed in a tea bag, and brewed by boiled water, or water is used for immersing the bulk drugs for 20 minutes, the water decoction is carried out for 15 minutes, the above materials are filtered for drinking as tea, and auxiliary materials and a corrigent are cooperated, an oral liquid, a syrup, a capsule, a particulate agent, a tablet and effervescence particles which can be accepted on pharmacy are prepared directly or indirectly through conventional procedures, and the traditional Chinese medicine composition can be clinically used for treating or preventing the respiratory tract diseases caused by the haze disoperation.
Owner:高雪 +1
Automated pharmacy drug handling and prescription verification system and method
InactiveUS20110146835A1Guaranteed accuracyMaximum safetyLiquid fillingDrug and medicationsDispensaryDispensing medications
An intake to exit security system for high-volume pharmacies provides maximum security from tampering and assures accuracy. The system immediately assigns bar codes to shipments upon arrival and then tracks them through warehousing, bulk distribution, prescription dispensing and shipping to patients, hospitals and drugstores. Bar-coded lock neck devices secure bulk drug canisters to bar-coded dispensing machines at specified dispensing stations where the machines dispense drugs into pre-labeled prescription bottles according to prescription indicia on the labels. Bottles then undergo content analysis and certification before packaged and shipped to customers. A Ramon laser spectral analysis contrasts the bottle contents to a library of known spectral signatures of drugs, and the pharmacist is alerted to any detected difference. A simultaneously captured visual image of the pills enables the pharmacist visually to compare the contents to a library of known visual appearances of the drugs. Both analyses are recorded for prescriptions certified and forwarded to customers. Deviations are excised without disrupting flow of other prescriptions, and the system automatically reassigns an incorrectly filled prescription to another bottle which starts anew through the system. Full bottles of commonly used drugs and specialized containers for irregularly shaped objects, creams and ointments may be pre-filled and inventoried for later collation with prescription bottles at the packaging and shipping stage.
Owner:TENSION INT
Methods of producing carbon-13 labeled biomass
InactiveUS6872516B2Preventing oxygen buildupHigh saturationCompounds screening/testingBiocideVolumetric Mass DensityWater soluble
A method and apparatus for preparing uniform carbon-13 labeled biomass using a water soluble carbon-13 labeled carbon source, such as a [13C]-bicarbonate or [13C]-carbonate salt, is disclosed. The biomass is prepared in one or more sterile carboys filled with growth medium, in which acidity, oxygen, and biomass density are carefully monitored and maintained. By using a solid, water-soluble [13C]-bicarbonate or [13C]-carbonate salt as the sole carbon source, a biomass is provided which is uniformly and efficiently labeled with carbon-13. This method and apparatus is particularly useful for the growth of an edible carbon-13 labeled algal mass, with Spirulina platensis being a specific alga species. The biomass may be prepared in conformance with FDA current good manufacturing practice regulations, and may be harvested and formed into lyophilized bulk drug powder which may be further processed into various drug product forms which are useful for diagnostic tests or in pharmaceutical compositions.
Owner:ADVANCED BREATH DIAGNOSTICS
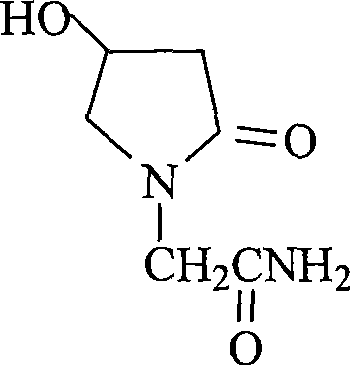
Oxiracetam injection
ActiveCN101396358AGood storage stabilityQuality improvementOrganic active ingredientsNervous disorderInjection solutionIntermediate product
The invention relates to an oxiracetam injection solution which comprises 1 weight portion of oxiracetam, 1.5 to 5.5 weight portions of glucose and 50 to 100 weight portions of injection water, wherein, the pH value of the injection solution is 3.8 to 4.5. The invention also provides a preparation method of the oxiracetam injection solution which comprises the steps as follows: a) the bulk drug oxiracetam and the glucose or sodium chloride are solved in the injection water under the temperature of 40 DEG C to 70 DEG C to obtain dissolved solution; b) the dissolved solution is cooled to the room temperature, active carbon is added for decolorizing, the active carbon is removed by filtering, proper quantity of water is complemented, and citric acid and sodium citrate are added to adjust the pH value to 3.8 to 4.5 to obtain the dissolved solution with adjusted pH value; c) an intermediate product after encapsulation is sterilized; and d) the product after the sterilization is packed. The oxiracetam injection solution manufactured by the prescription and the preparation method of the invention has good storage stability.
Owner:广东世信药业有限公司 +1
Oil-soluble pesticide nano capsules and preparation method thereof
InactiveCN101461358AMild conditionsEase of industrial productionBiocideAnimal repellantsCypermethrinAbamectin
Disclosed is an oil soluble pesticide nm capsule and preparation method, having weight ration of: pesticide: 0.5-4%; high molecular compound: 8-25%; mixed-surfactant: 8-20%; cosurfactant 3-10%; crosslinking agent 0.5-1.0%; organic solvent 3-10%; water: added to 100%, wherein the high molecular compound is selected from sodium lignosulfonates, chitosan, gum arabic or glutin, one or two is / are selected for agglutination reaction, forming capsule skin of the nm capsule; the capsule core is pesticide bulk drug, selected from diflubenzuron, emamectin benzoate, abamectin, imidacloprid, ivermectin or highly active cypermethrin. The mixed-surfactant adopts nonionic surfactant and anionic surfactant for compound. The pesticide nm controlled release preparation prepared by the invention is easy in storage, high in stability, large in drug-loading rate and high in dispersion.
Owner:NANJING NORMAL UNIVERSITY
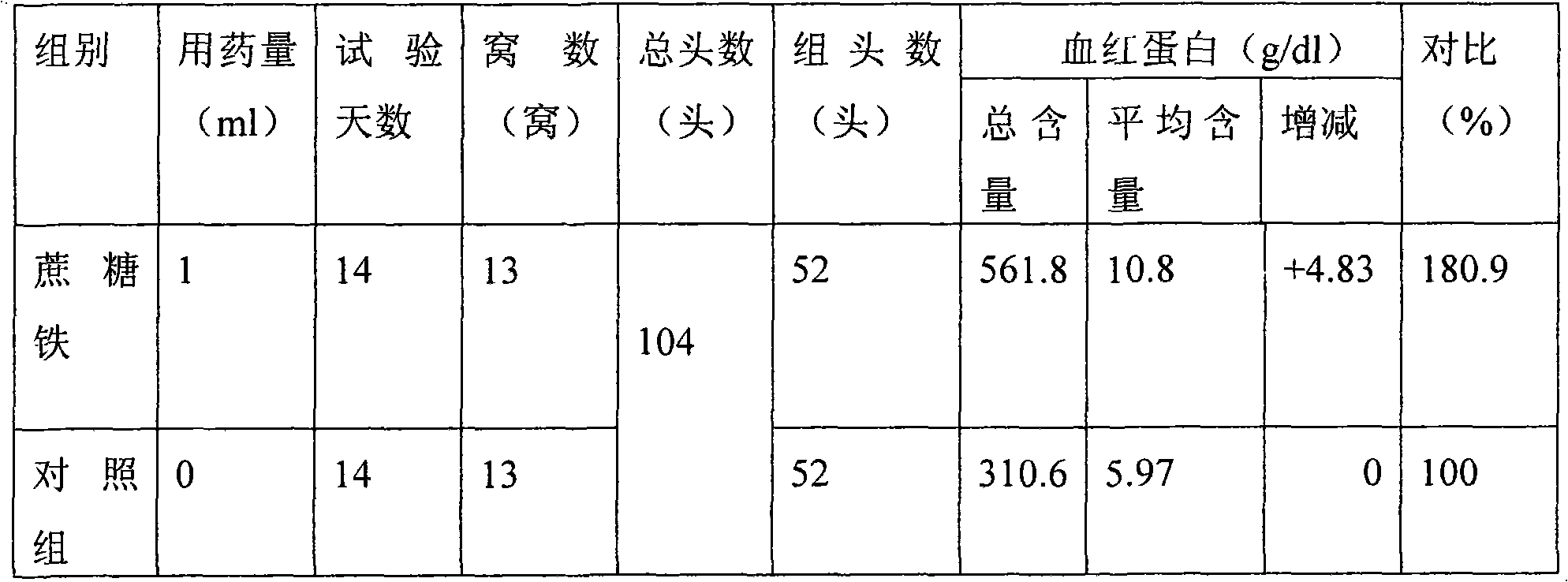
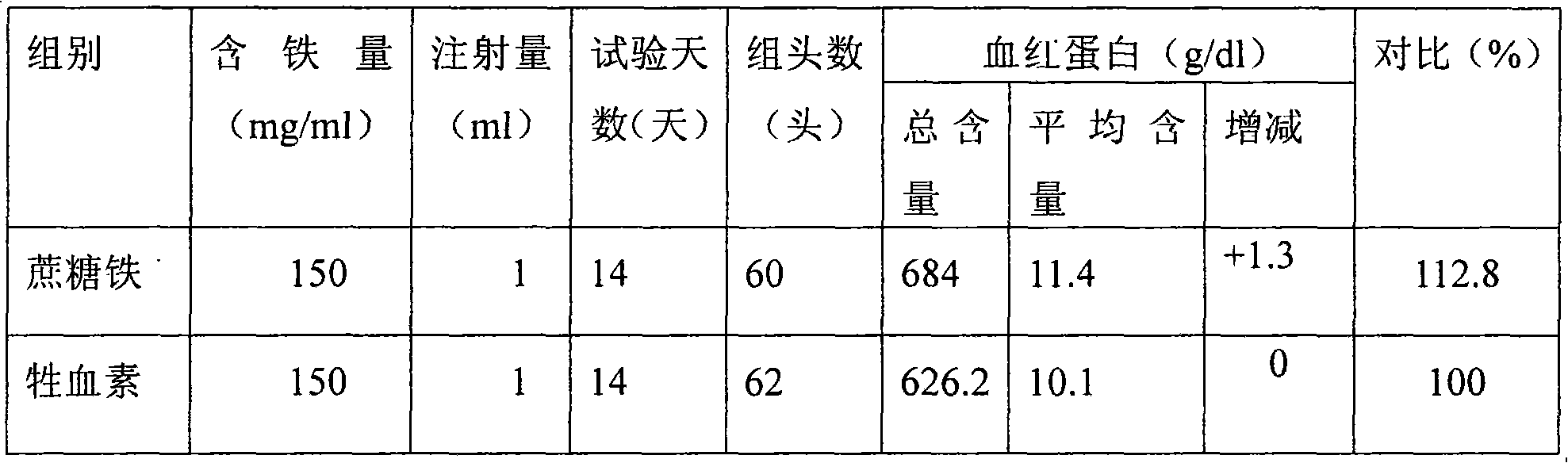
Preparation method for iron sucrose bulk drug and injection thereof
InactiveCN101671373AImprove stabilityGood water solubilityOrganic active ingredientsSugar derivativesAcetic acidIron salts
The invention relates to a preparation method for an iron sucrose bulk drug. The method comprises the following steps: (1) crystallization: the pH value of sucrose solution is adjusted to 1-4 with acetic acid, iron salt solution is added to the sucrose solution to form mixed liquor, the pH value of the mixed liquor is adjusted to 1-3 with Na2CO3 solution, then the mixed liquor is stirred and is continued to be added with the Na2CO3 solution until turbidity appears in the mixed liquor, the pH value of the mixed liquor is adjusted to 4-7, and then the mixed liquor is filtered and added with water to obtain iron cake solution; (2) complexing: the sucrose solution is heated and added with alkaline liquor for alkalization, and then the alkalized sucrose solution is added with the iron cake solution and stands for the night to obtain complexing solution; and (3) refining: the complexing solution after standing for the night is filtered, and the filtrate precipitates with 2-3 times of 95 percent of ethanol and is filtered to obtain the iron sucrose bulk drug. The invention has the advantages of simple process steps, easy control of the reaction processes, good product quality, high yieldand lower production cost and is a high-novelty preparation method for the iron sucrose bulk drug and the injection thereof.
Owner:TIANJIN ZHONGAO BIOTECH
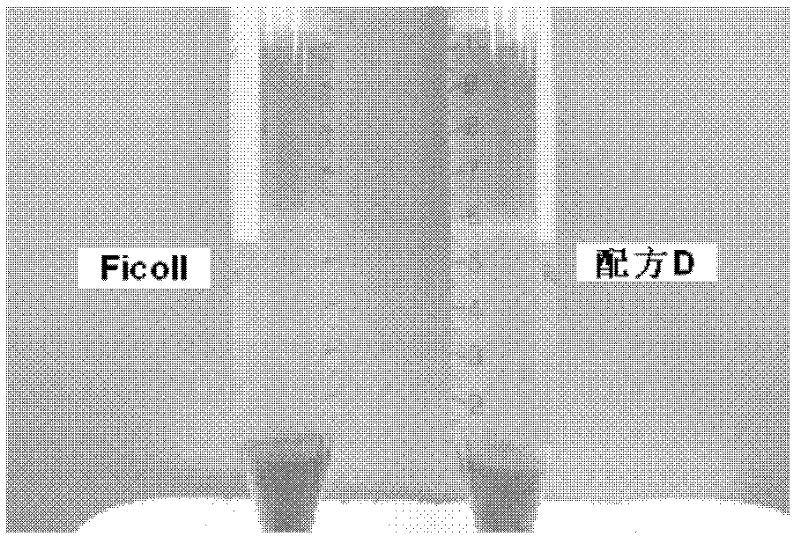
Cell separation medium and cell separation method
The invention discloses a composition for separating a mononuclear cell and a compound lymphocyte separation medium. All raw materials in a formula of the compound lymphocyte separation medium respectively meet the standard of intravenous injection-grade bulk drugs and are high in safety; the purity of the recovered lymphocyte and the recovery rate of the lymphocyte have no remarkable difference from those of a conventional separation medium control group; and the lymphocyte obtained by separating respectively has equivalent effects to those of the control group in four indexes, namely multiplication, morphology, a surface marker and cytotoxicity activity, and is favorable in clinical application prospect.
Owner:BEIJING JING MENG STEM CELL TECH +1
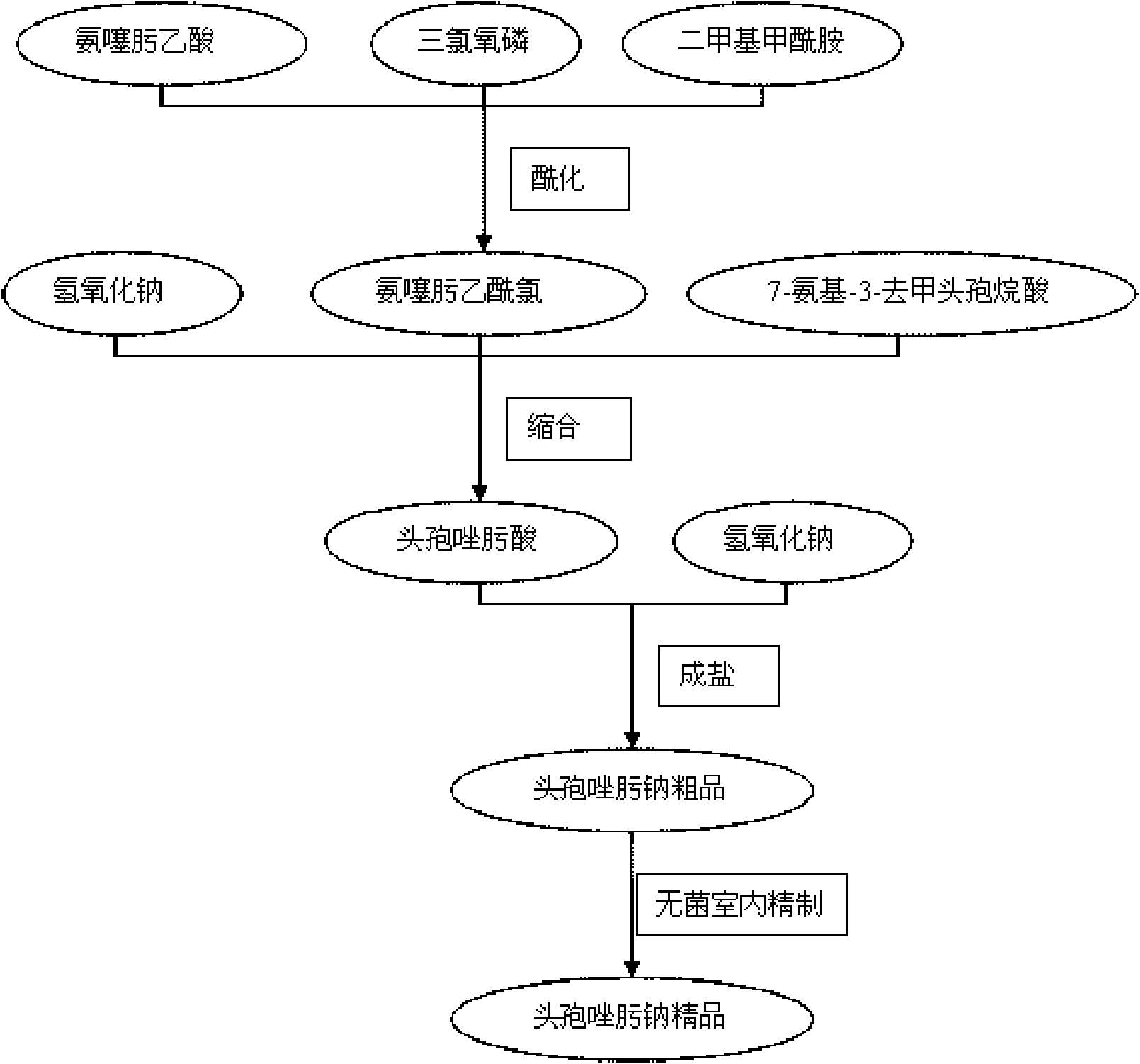
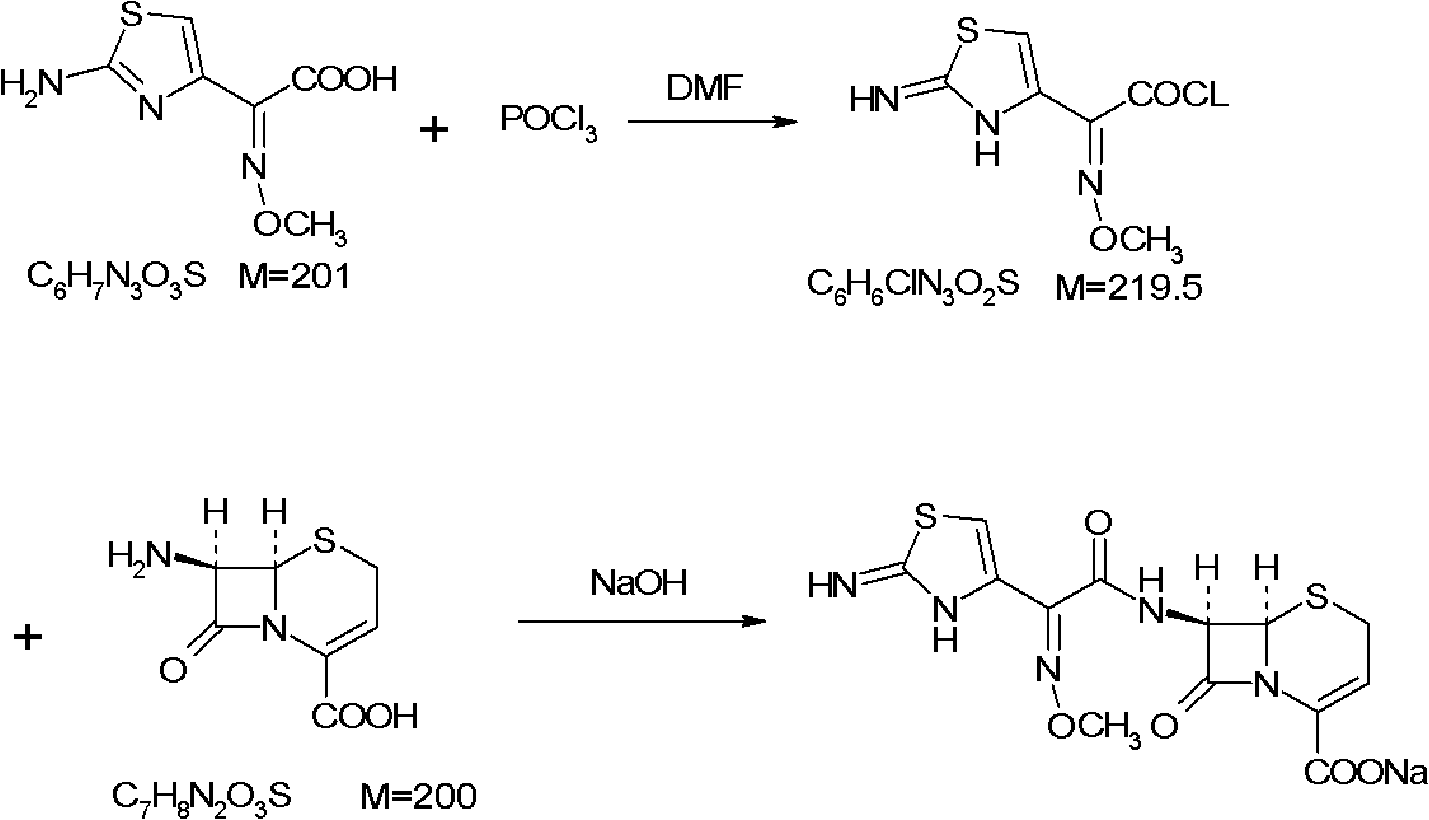
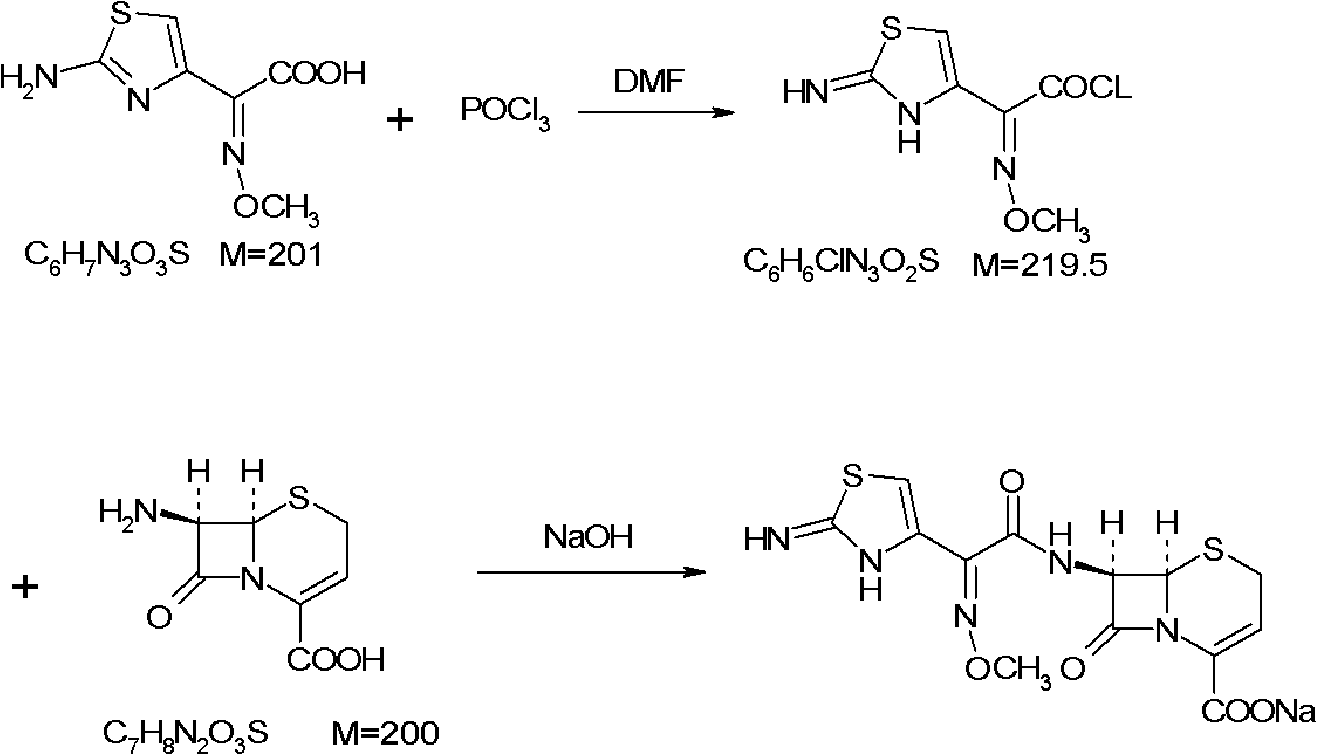
Ceftizoxime sodium drug injection powder and preparation method thereof, as well as synthetic method of bulk drug ceftizoxime sodium
ActiveCN101606910AUniform colorHigh purityAntibacterial agentsOrganic active ingredientsDrug injectionCLARITY
The invention relates to a ceftizoxime sodium drug injection powder and a preparation method thereof, as well as a synthetic method of bulk drug ceftizoxime sodium. The ceftizoxime sodium drug injection powder consists of 100% ceftizoxime sodium, wherein the ceftizoxime sodium is pretreated, and the pretreatment is aseptic refining and / or grinding. The ceftizoxime sodium drug has the advantages of high purity, almost no impurity, better and more stable quality, better clarity and the like; and the synthetic method of bulk drug ceftizoxime sodium has lower bulk cost, less synthesis technology difficulty, mild reaction condition, stable and reliable yield and quality, and high purity and yield of products.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Daily ration concentrated feed of egg-laying sheldrake and preparation method thereof
ActiveCN103039745AEasy to shrinkPromote digestion and utilizationFood processingAnimal feeding stuffYeastFodder
The invention discloses a daily ration concentrated feed of an egg-laying sheldrake. The daily ration concentrated feed comprises wheat bran, beer yeast, rice bran, fish meal, shell powder, bean dregs, rape seed cake, mineral additive premix, vitamin additive premix, salt and Chinese herb feed additive, wherein the Chinese herb feed additive comprises following raw bulk drugs: rhizoma polygonati, pericarpium citri reticulatae, Chinese angelica, fructus psoraleae, folium isatidis, bletilla striata, calcined oyster shell, bighead atractylodes rhizome, radix salviae miltiorrhizae, semen cuscutae, dandelion, leonurus, red paeonia, medical stone, astragalus mongholicus, patrinia, scutellaria baicalensis, fructus cnidii and hawthorn. Aiming to the physiological characteristics that during egg laying, the vital energy and blood of a sheldrake body is weak, and the function of ovary is gradually declined and the physical characteristic that a sheldrake lays an egg in the small hours certainly, on the basis of theories of the Chinese Veterinary Medicine, the invention adopts the component with the purposes of eliminating dampness, strengthening the spleen and stomach, tonifying middle-Jiao and Qi and enhancing resistance to diseases, so that reproductive endocrine system is improved, follicular development is promoted, uterine contraction is adjusted, egg laying is facilitated, egg laying rate is effectively improved, egg quality is improved, and good economic benefits and social benefits are created.
Owner:JIANGSU DAXIANG FEED
Forage for controlling hemorragic disease of grass carp
InactiveCN103599392ASmall side effectsImprove securityAnthropod material medical ingredientsAnimal feeding stuffDiseaseAnimal Foraging
A provided forage for controlling hemorragic disease of grass carp comprises the following bulk drugs: radix astragali, epimedium, lycium barbarum, sophora flavescens, andrographis paniculata, cacumen platycladi, coptis, acanthopanax senticosus, Chinese gall, herba plantaginis, lygodium japonicum, ligusticum wallichii, haw, mint, artemisia capillaris, pasque flower, purslane, caulis dendrobii, ferula, bighead atractylodes rhizome, conyza japonica, alternanthera sessilis and licorice. The traditional Chinese medicine composition provided by the invention has the advantages of being efficient, small in toxic and side effects, high in safety, small in residue and the like, and is wide in source and low in cost.
Owner:青岛智慧农大技术服务有限公司
Headspace gas chromatography method for determining residual quantity of organic solvent in glycine bulk drug
InactiveCN108614058AEffective quality controlEasy to operateComponent separationNitrogen gasCapillary column
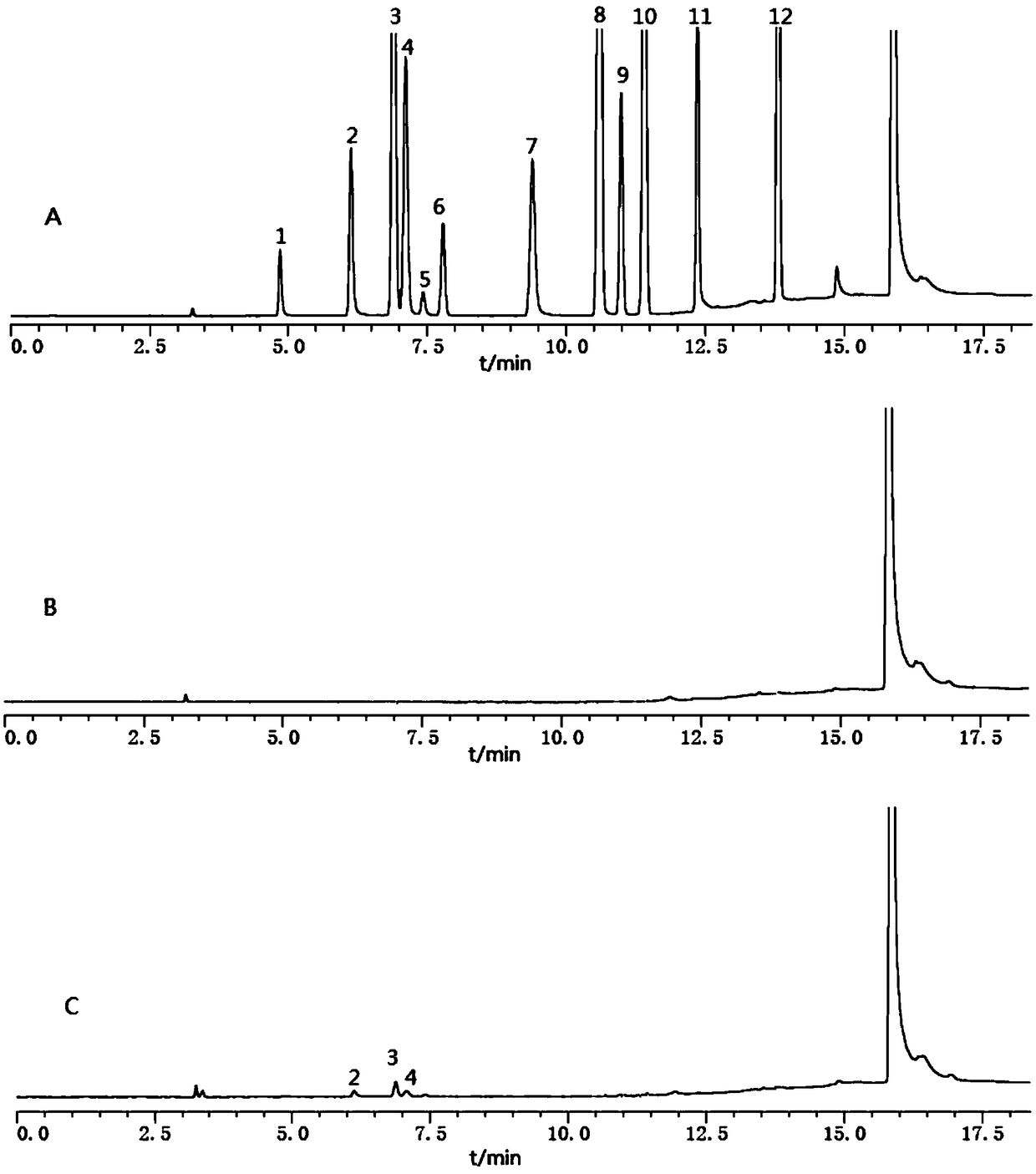
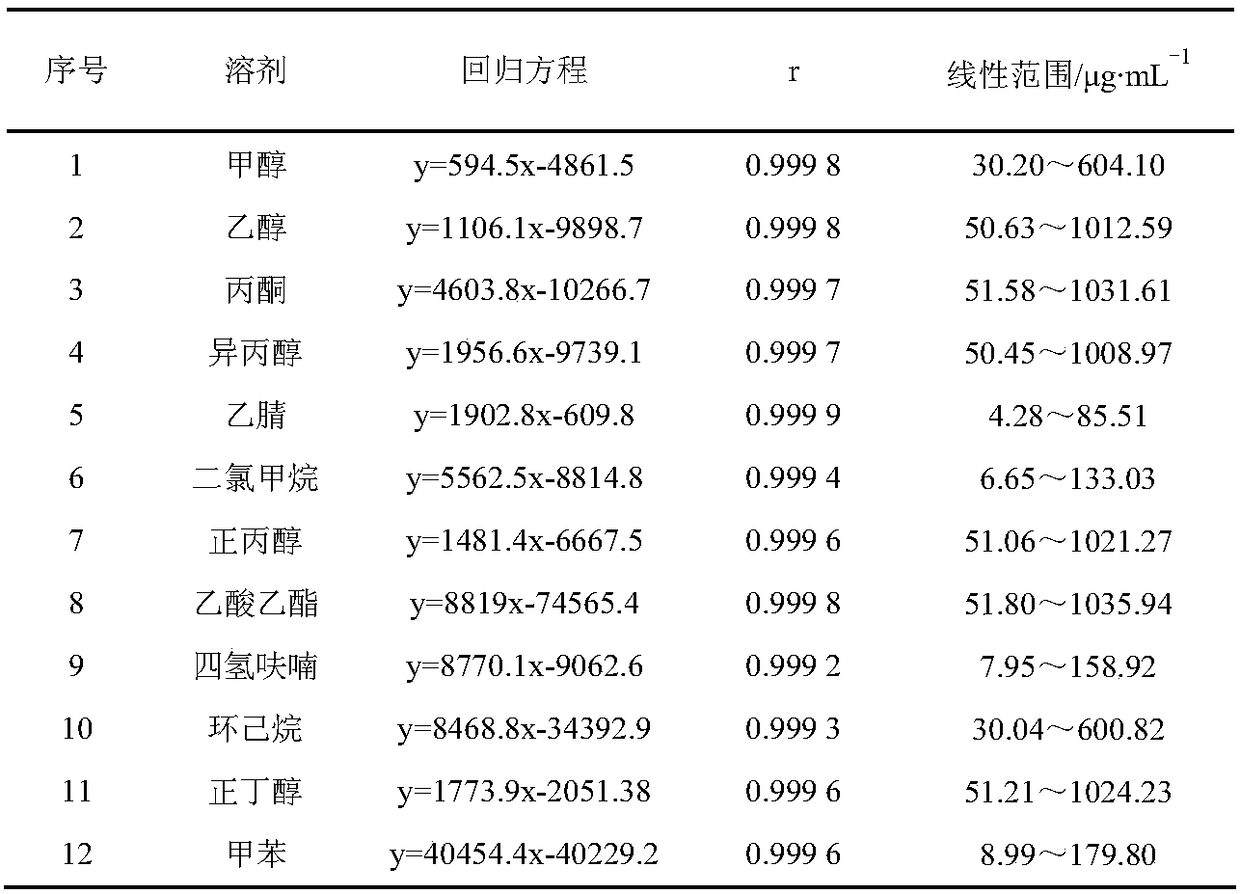
The invention discloses a headspace gas chromatography method for determining the residual quantity of an organic solvent in a glycine bulk drug. The method is implemented under conditions that a DB-624 capillary column and a hydrogen flame ionization detector are used, sample inlet temperature is 150 DEG C, the detector temperature is 250 DEG C, high purity nitrogen is taken as carrier gas with flow rate being 2.2 mL / min, the split ratio is 10:1, headspace heating temperature is 80 DEG C, and equilibrium time is 35 min; besides, glycine is easily soluble in water and the 12 solvents are soluble in water to a certain degree, so that an aqueous solvent is adopted. The method can be used for simultaneously determining the residual quantity of the 12 organic solvents including methanol, ethanol, acetone, isopropanol, acetonitrile, dichloromethane, n-propanol, ethyl acetate, tetrahydrofuran, cyclohexane, n-butanol and toluene in the glycine bulk drug, has the characteristics of being easyto operate, quick, sensitive and accurate in result, and provides reference for effectively controlling the quality of the glycine raw material and ensuring medication safety.
Owner:广西壮族自治区食品药品检验所
Method for refining lansoprazole bulk drug
InactiveCN101289443AHigh refining yieldHigh clarityOrganic chemistryDigestive systemActivated carbonLansoprazole
The invention relates to a refining method for a lansoprazole crude drug, which is characterized in that the lansoprazole sample is dissolved in anhydrous alcohol and stirred to complete dissolution, and activated carbon is added to be stirred, pumped and filtered; then the solution which is placed in a freezing chamber to freeze and crystallize is pumped and filtered to be dry, the filter cake is washed to be dry by the anhydrous alcohol, and white solid can be obtained; finally, the obtained white solid is dried in an oven, and the solid is turned over discontinuously during the drying until reaching constant weight. The preparation method for the lansoprazole crude drug is simple and convenient, high in refining yield, low in the total impurities of the refined lansoprazole and good in clarity after being prepared into injections.
Owner:上海慈瑞医药科技股份有限公司
Iron sucrose injection and preparation method thereof
InactiveCN103040730AImprove stabilityGood water solubilityOrganic active ingredientsMetabolism disorderActivated carbonFiltration membrane
The invention relates to a preparation method of iron sucrose injection. The method comprises the steps of (1) alkalization; (2) complexation; (3) alcohol precipitation; (4) powder collection, comprising the procedures of performing solid-liquid separation on alcohol precipitation solution by a centrifugal machine, spray drying the solid phase and collecting powder to obtain a bulk drug of the iron sucrose; (5) injection preparation, comprises the steps of adding the bulk drug of the iron sucrose in injection water to prepare a solution containing 100-300 mg of iron element in every milliliter, adding a 20-40% NaOH solution for alkalization, controlling a pH value at 11, heating and controlling a temperature at 40-80 DEG C, then adding 1-0.3% of activated carbon, stirring for 15-60 minutes, filtering with a 0.22 [mu]m micro-filtration membrane, heating with steam and controlling the temperature at 110-120 DEG C, and keeping the temperature for 30 minutes to obtain a finished product of the iron sucrose injection. The preparation method of the iron sucrose injection is simple in process steps, has easily controllable reaction process, good product quality, high yield and low production cost, and is a preparation method of the iron sucrose injection, with relatively high innovativeness.
Owner:TIANJIN ZHONGAO BIOTECH
Acyclovir sustained-release preparation composition and method for preparing the same
InactiveCN101502521ASlow release rateReduce the frequency of takingAntiviralsCapsule deliveryMedicinePatient compliance
The invention discloses a group of acyclovir sustained-release preparation combinations and a preparation method thereof. The group of acyclovir sustained-release preparation combinations is mainly prepared from acyclovir bulk drugs, medicinal sustained-release materials and other appropriate auxiliary materials. The acyclovir enteric sustained-release preparation provided by the invention can deaccelerate the release rate of main drugs, reduce the frequency of administration and improve the patient compliance. The acyclovir sustained-release preparation combination provided by the invention has the advantages of high quality controllability and stability of the preparation process.
Owner:山东淄博新达制药有限公司
Medicinal dripping pill for treating hypertension
ActiveCN101579480ASignificant effect of drop pill treatmentGood treatment effectPill deliveryPharmaceutical non-active ingredientsSide effectTreatment effect
The invention discloses a medicinal dripping pill for treating hypertension, which consists of composition intermediates of red infested rice, uncaria, dendrobium and polyethylene glycol. The intermediates of the dripping pill are prepared from dry paste of extract of bulk drug prepared by the prior method. The medicinal dripping pill has remarkable treatment effect and no side effects.
Owner:BEIJING WBL PEKING UNIV BIOTECH
Pharmaceutical compositions of atorvastatin
The present invention provides stable pharmaceutical compositions comprised of atorvastatin and sodium bicarbonate or L-arginine. The compositions are prepared as bulk drug compositions and also as oral dosage units, such as tablets or capsules. The compositions are useful for preparation of monolithic and bi-layer tablets containing atorvastatin as the only active agent or combined with one or more additional active agents. The compositions are useful for treating hypercholesterolemia and related conditions.
Owner:MERCK SHARP & DOHME CORP
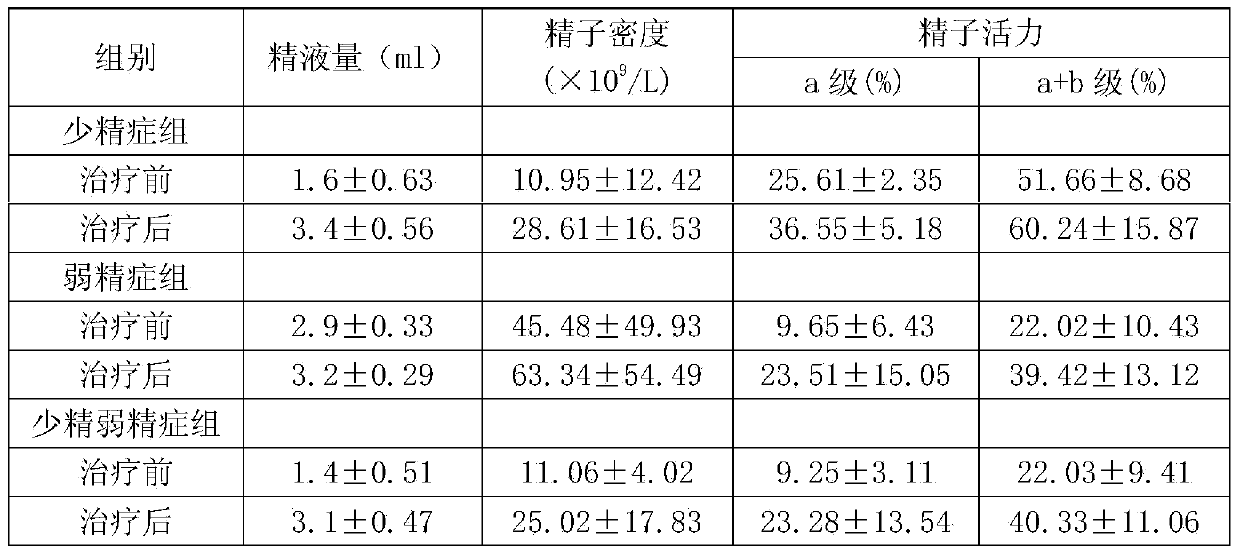
Medicine for treating male oligospermia and asthenozoospermia and improving sperm quality
ActiveCN104083468AImprove survival rateAdapt to treatment requirementsMammal material medical ingredientsSexual disorderVitamin CIrritation
The invention discloses a medicine for treating male oligospermia and asthenozoospermia and improving sperm quality. The medicine for treating male oligospermia and asthenozoospermia and improving sperm quality takes L-carnitine, maca, corduceps militaris, zinc-rich selenium-rich whole egg powder, vitamin C, citric acid, zizyphus jujube, deer blood, folic acid, taurine, fructose and coenzyme Q10 as active compositions, and is prepared according to a certain weight ratio. The medicine aims at the disadvantages of conventional spermatogenic medicines, and is reasonable in formula. By properly combining multiple bulk drugs and utilizing the mutual synergic effect of the bulk drugs, the treatment effect is improved, the medicine adapts to treatment requirements of complex disease conditions, adverse reaction is reduced, and the medicine is durable in treatment effect, fast in absorption, rapid in effectiveness, strong in effect, free of stimulation, safe, reliable and simple for administration, is capable of effectively promoting generation of sperms, improving the survival rate of sperms, and is an effective medicine for treating oligospermia, asthenozoospermia and sperm quality badness caused by various reasons. The total effective rate of the medicine reaches 95.6% based on 5730 examples treated by using the medicine.
Owner:SHANDONG XINXIAN PHARMA
Medicine for curing burn dressing and method of preparing the same
InactiveCN101249215AGood treatment effectStrong anti-infection effectAnthropod material medical ingredientsHydroxy compound active ingredientsVegetable oilCoptis
Owner:喻永强
Medicament for curing cancer
InactiveCN101554462ARestoring a clean environmentThorough detoxificationSolution deliveryPill deliveryLymphatic SpreadAdemetionine
The invention relates to a medicament for curing cancer and a preparation method thereof. The medicament comprises a No.1 polypharmaceutical, a No.2 polypharmaceutical and a No.3 polypharmaceutical, wherein the No.1 polypharmaceutical comprises 31 flavors of traditional Chinese medicines including Glauber salt, kudzuvine root, red sange root, mongolian snakegourd, rhubarb, eucommia bark, and the like; the No.2 polypharmaceutical comprises 26 flavors of traditional Chinese medicines including Chinese clematis, dried rehamnnia root, sesame kernel, schisandra fruit, tangerine peel, coix seed, and the like; and the No.3 polypharmaceutical comprises 6 flavors of traditional Chinese medicines including liquorice, cogongrass rhizome, dandehon herb, mint, dendrobium stem and cyperus rotundus linn. The bulk drugs consisting of the No.1 polypharmaceutical, the No.2 polypharmaceutical and the No.3 polypharmaceutical are crushed firstly, the No.1 polypharmaceutical and the No.2 polypharmaceutical can be respectively prepared into capsules, tablets, drop pills, granulas, oral liquid and injection by corresponding technologies; and the No.3 polypharmaceutical can be prepared into fine powder medicinal tea or granulas. The medicament has the unique effects of inhibiting tumor and removing pain, suppressing metastasis, removing necrosis and promoting granulation, balancing nutrition, expelling toxin, increasing immunity, and the like. The surgery can be replaced for curing the cancer generally, and the radiotherapy and the chemotherapy are not used; the effective rate of preventing the caner is 100 percent, the radical rate to the early cancer is 100 percent, and the curative ratios of the caner of medium and late stages are respectively over 98 percent and over 70 percent; and the invention also has the advantages of omni-directional treatment, high curative ratio and no toxic side effect.
Owner:陈星儒 +1
Method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products
The invention relates to a method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products, which comprises the following steps: 1)taking Radix Astragali and Ligusticum wallichii extract product fine powder, preciously weighing, adding methanol, performing ultrasonic extraction, filtering and drying a filtrate, dissolving residue by ethanol and metering volume, filtering by a filter membrane, taking a subsequent filtrate to obtain a tested object solution; 2)taking a ferulic acid reference substance, a senkyunolide I reference substance, a calycosin glucoside reference substance, an ononin reference substance, a calycosin reference substance and a fermlononetin reference substance, preciously weighing, respectively adding methanol to prepare a reference substance solution; and 3)respectively and preciously absorbing the tested object solution and the reference substance solution, and injecting a high efficiency liquid chromatography for determining to obtain the fingerprint chromatography of radix astragali and ligusticum wallichii extract products. The method has active effect for guiding clinical medication and effective feeding guidance to the bulk drugs during the production process, and ensuring the reliable quality; the method has the advantages of convenient and fast operation, so that similarity result can be obtained, the quality of the traditional Chinese medicinal materials radix astragali and ligusticum wallichii extract products can be evaluated, and the result is objective and accurate.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV +1
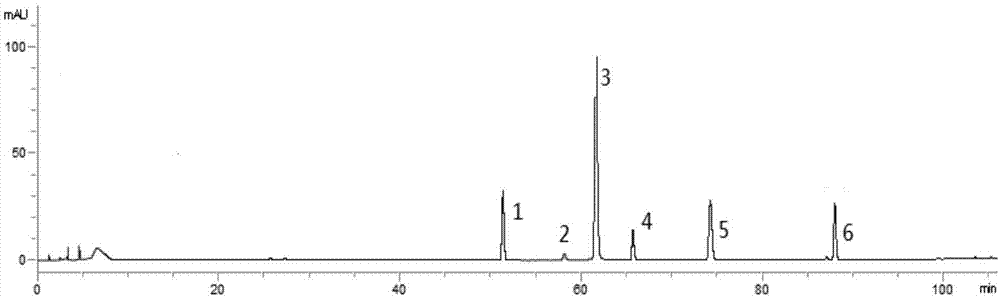
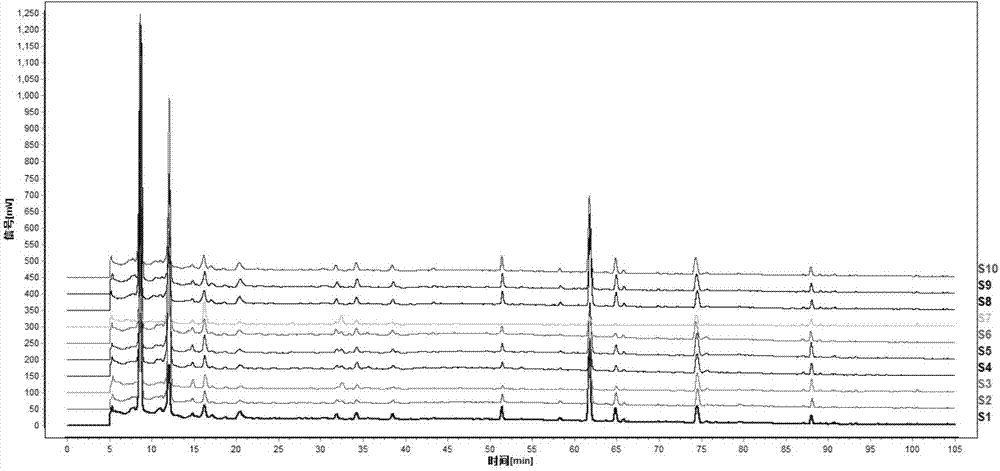
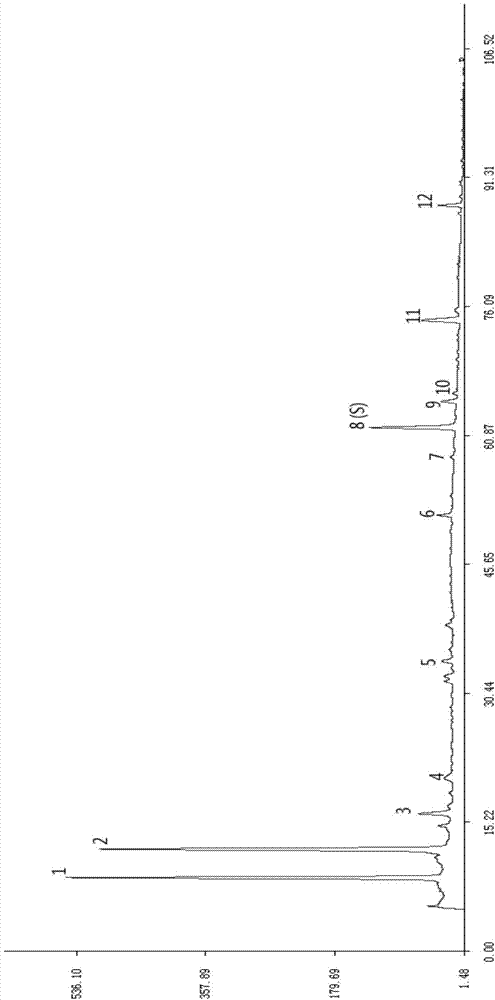
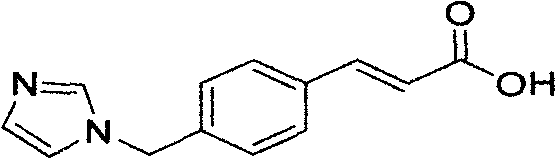
Preparation method of ozagrel bulk drug
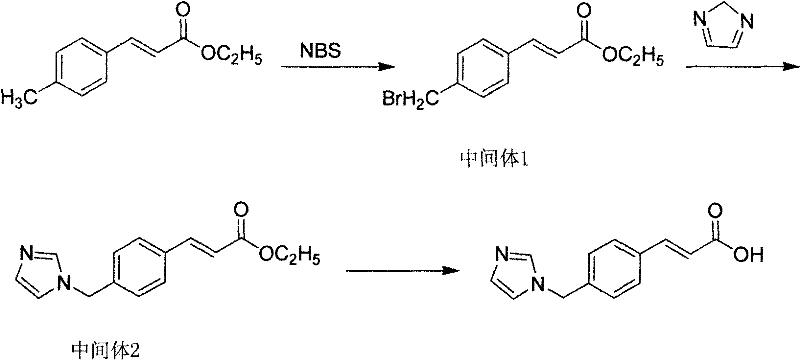
The invention discloses a preparation method of an ozagrel bulk drug. The method comprises the following steps of: bromating methyl ethyl cinnamate serving as a starting material with NBS (B-Bromosuccinimide) to obtain ethyl 4-bromomethylcinnamate; undergoing a condensation reaction on the ethyl 4-bromomethylcinnamate and imidazole to generate ozagrel ethyl ester; hydrolyzing under an alkaline condition; performing acid precipitation to obtain crude ozagrel; and refining to obtain an ozagrel bulk drug. Due to the adoption of the method, a method for refining catalysts, solvents and crude products used in each reaction step is improved, the product yield is high, the product quality is good, the use of toxic reagents and expensive reagents is avoided, little environmental pollution is caused, and the production cost is low. The method is suitable for industrial production, and is an improved and environmentally-friendly method for preparing the ozagrel bulk drug.
Owner:辽宁远大诺康医药有限公司
Traditional Chinese medicinal composition for traditional Chinese moxibustion treatment, preparation method and applications thereof
ActiveCN102430011AImprove clinical efficacyLarge temperature rangeNervous disorderHydroxy compound active ingredientsDiseaseMyrrh
The invention relates to a traditional Chinese medicinal composition for traditional Chinese moxibustion treatment, comprising the bulk drugs in part by weight: 5-10 parts of moxa, 0.5-1.5 parts of frankincense, 0.5-1.5 parts of myrrh, 0.5-1.5 parts of agilawood, 0.5-1.5 parts of borneol and 0.5-1.5 parts of cassia twig. The invention also provides a preparation method and applications of the traditional Chinese medicinal composition. The invention has the advantages that the double treatment action of moxa and traditional Chinese medicine is played, the clinic curative effect is greatly improved, the power is continuous and permeated, and the warm range is large, so that the invention is applicable to various cold syndromes, asthenia syndrome, painful syndrome and stasis syndrome, can beused for treating various common diseases and difficult miscellaneous diseases on clinic, and also can be used for daily health care; and in the invention, the carrying is convenient, the operation is easy, the price is far lower than that of the common clinic diagnosis, and considerable economic benefit can be brought.
Owner:SHANGHAI RES INST OF ACUPUNCTURE & MERIDIANS
Ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel and preparation method thereof
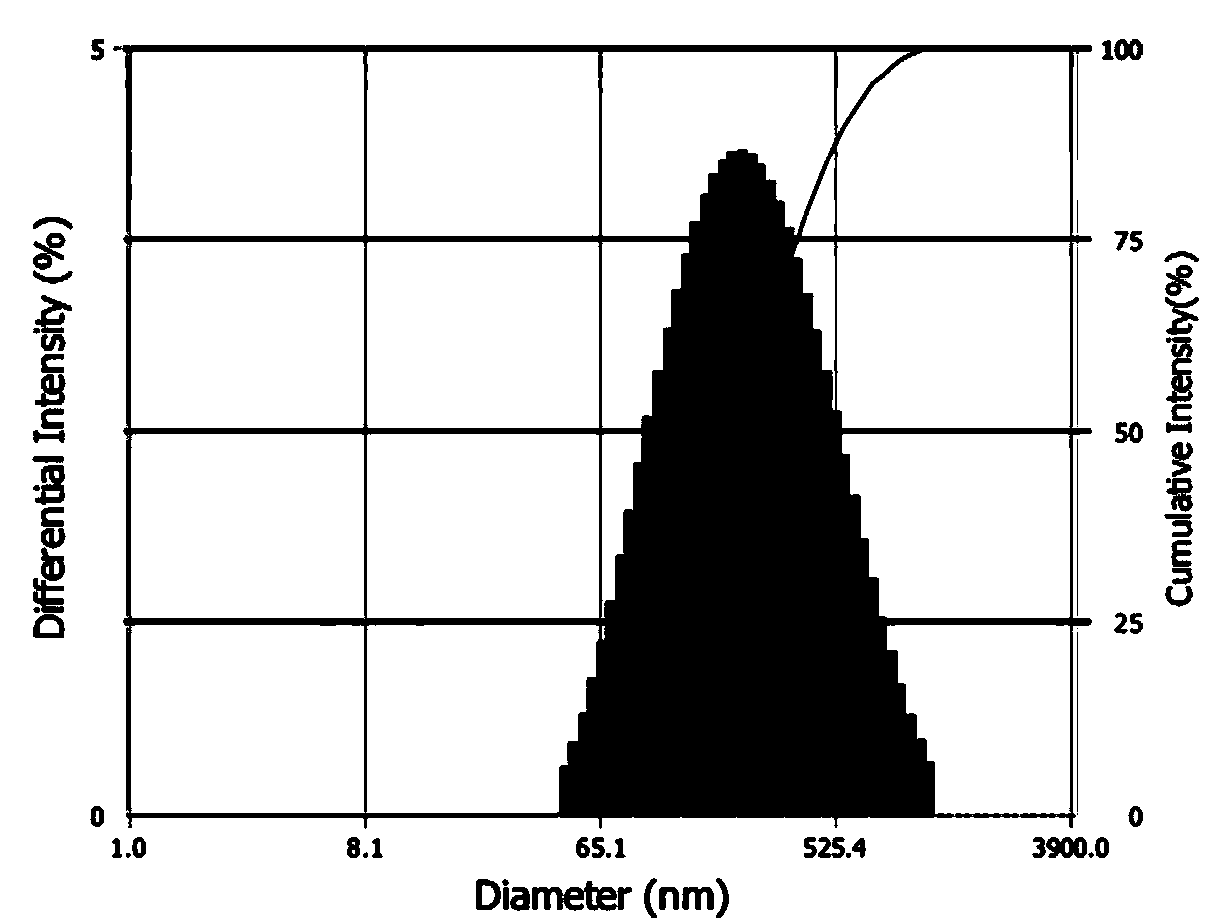
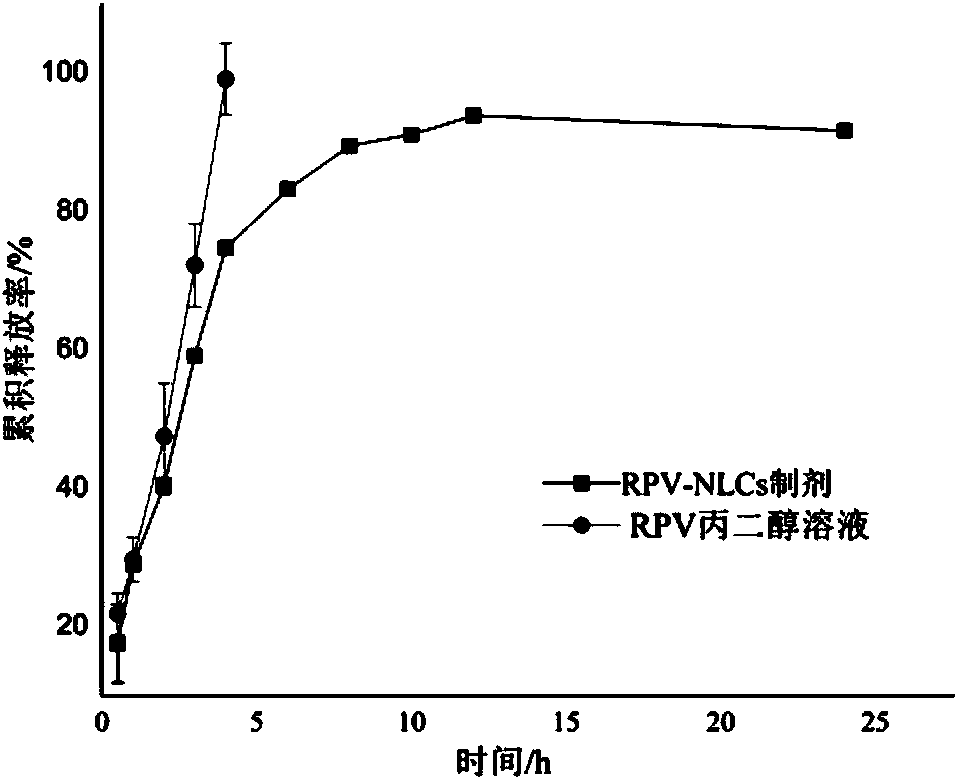
InactiveCN103816111AImprove solubilityReduce releaseAerosol deliveryOintment deliveryLipid formationBiocompatibility Testing
The invention belongs to the technical field of medicinal preparations and specifically to a ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel and a preparation method thereof. The ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel disclosed by the invention mainly comprises bulk drug ropivacaine, a solid lipid material, a liquid lipid material, a surfactant, a cosurfactant, a gel matrix and injection water. The preparation method is a high-temperature emulsification low-temperature setting and cold melt method. The ropivacaine nanometer lipid carrier temperature-sensitive in-situ gel prepared in the invention is applied through transdermal drug delivery and has the advantages of a slow release function, a high transdermal permeation rate, a high entrapment rate, high drug loading capacity, good biocompatibility and good stability.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Traditional Chinese medicine for treating hyperpiesis and preparation method
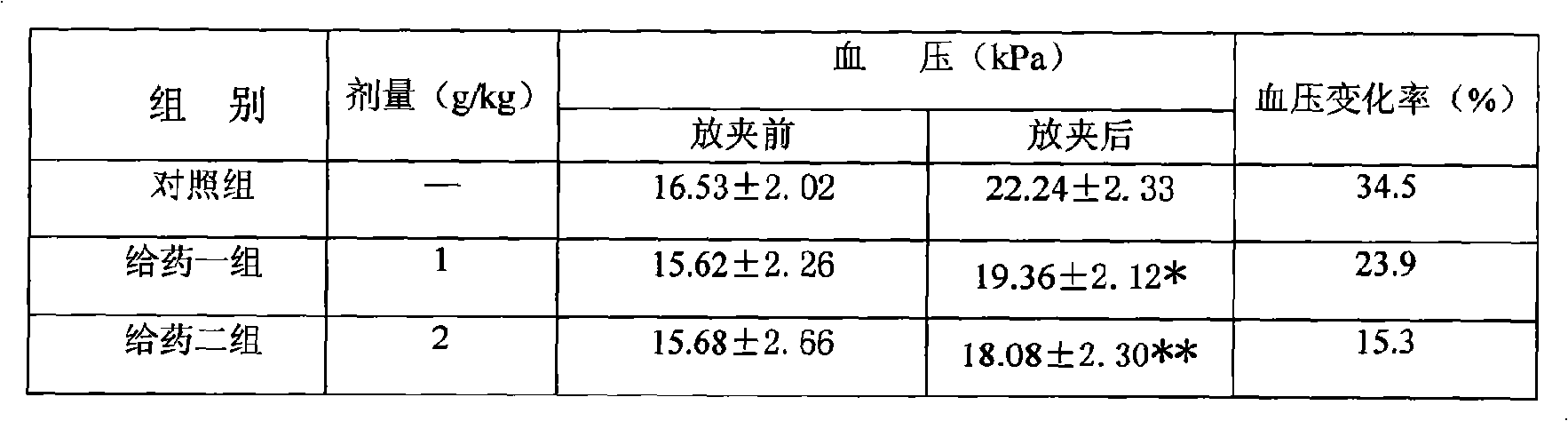
InactiveCN101559182AAccurate doseGood effectAnthropod material medical ingredientsCardiovascular disorderPropolisAdemetionine
The invention relates to a traditional Chinese medicine for treating hyperpiesis and a preparation method, the medicine can effectively treat hyperpiesis and has double efficacy of herbal cuisine and diet therapy in treating hyperpiesis. The invention comprises the following bulk drugs: 10-45 of propolis powder, 9-40 of natto powder, 9-40 of wild jujube seed, 9-40 of gastrodia tuber, 8-30 of corn stigma, 8-30 of cassia seed, 8-25 of pagodatree flower powder, 8-25 of kudzuvine root, 5-15 of gambir plant, 5-15 of scutellaria laterifolia, 5-15 of haw, 2-10 of apocynum, 2-10 of earthworm, 2-10 of evodia fruit, 8-25 of selfheal, 5-15 of Chinese gentian root, 5-15 of antelope horn, 5-15 of bupleurum, 5-15 of red-rooted salvia, 2-10 of radish seed and 2-10 of Chinese magnoliavine, wherein the unit of weight is parts by weight.
Owner:武兴战
Traditional Chinese medicine composition for treating liver cirrhosis and fibrosis and preparation method thereof
InactiveCN102772737AInhibition of replicationInhibit fibroplasiaDigestive systemAntiviralsLicorice rootsSpleen
The invention discloses a traditional Chinese medicine composition for treating liver cirrhosis and fibrosis and a preparation method thereof. The traditional Chinese medicine composition is mainly prepared by the bulk drugs of radix salviae miltiorrhizae, black soya bean, red paeony root, ophiopogon root, astragalus root, radix codonopsis pilosulae, angelica, carapax amydae, rhizoma zedoariae, twotooth achyranthes root, Chinese yam, giant knotweed, hedyotis diffusa, fructus amomi and honey-fried licorice root. The prescription disclosed by the invention is complete in the matching of a monarch, a minister, an adjuvant and a conductant drug, is excellent in the efficacy of drugs, is flexible in application and has the comparatively obvious characteristic of traditional Chinese medicine. The traditional Chinese medicine composition disclosed by the invention has the actions of resisting the fibrosis, resisting hepatitis B virus (HBV) replication, improving the liver function, regulating the blood circulation of the liver, reinforcing the liver, softening hard masses, strengthening the spleen and the like; and the traditional Chinese medicine composition also has the actions of lowering the portal pressure, reducing the thickness of the spleen and improving the liver function. The traditional Chinese medicine composition disclosed by the invention has an obvious curative effect in the therapeutic action to the liver cirrhosis and fibrosis. In addition, the traditional Chinese medicine composition disclosed by the invention has an obvious action of resisting the liver cirrhosis and fibrosis of children.
Owner:HUANGHUAI UNIV
Standard fingerprint of fleabane phenol bulk drug and preparation, establishment method and application
ActiveCN102650626AQuality assuranceGuaranteed pharmacological activityComponent separationPHENOL LIQUIDAsarone
The invention provides a standard fingerprint of fleabane phenol bulk drugs and preparations, an establishment method and application. The technical schemes comprise the determination of the standard fingerprint and the characteristic fingerprint peak of fleabane drug materials, the fleabane phenol bulk drugs and the preparations, and the application of the standard fingerprint in the aspects of extraction and processing, and quality control in the process of producing the fleabane phenol bulk drugs and the preparations. Therefore, the overall monitoring of the process and the quality from the fleabane drug materials to fleabane phenol bulk drugs and then the preparations can be realized, basic substances of the fleabane drug materials, the fleabane phenol bulk drugs and the preparations can be conveniently confirmed, and the production process and quality can be conveniently evaluated, and standard indications can be provided for subsequent process improvement and perfection.
Owner:YUNNAN SHIPURUI BIOLOGICAL ENG
Officinal and edible medicine and preparation method thereof
ActiveCN103495056AMild in natureWide range of useSenses disorderAntipyreticMedicinal herbsAcute Pharyngitis
The invention discloses an officinal and edible medicine, which is prepared from bulk drugs including folium mori, chrysanthemum, almond, selfheal, mint, platycodon grandiflorum, liquorice, rhizoma phragmitis, houttuynia cordata and honeysuckle. Ten bulk drugs are matched scientifically to achieve cooperative functions and are used for treating wind-heat type common cold, clearing away heat and toxic materials, clearing away the lung-heat, and treating hot eyes, headache, cough, dry mouth, swollen sore throat, phlegm-heat, breath with cough, chronic / acute pharyngitis, acute pneumonia and the like. In addition, nine bulk drugs are officinal and edible homologous bulk drugs and mild in property, can be eaten for a long time, has no side effect and can be eaten as food and medicine. The officinal and edible medicine can be prepared into herbal tea for eliminating summer-heat by cooling, healthy beverage or various dosage forms, and has wide taking range.
Owner:TIANSHENG PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com