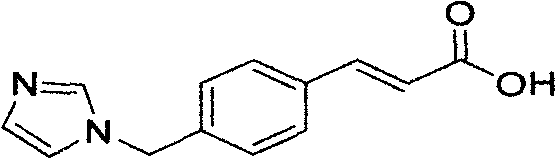
Preparation method of ozagrel bulk drug
The technology of raw material drug and ethyl radoxylate, which is applied in the field of improved preparation of ozagrel bulk drug, can solve the problems of human health threat, large environmental pollution, low refining yield and the like, so as to avoid toxic reagents and expensive reagents, environmental The effect of low pollution and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
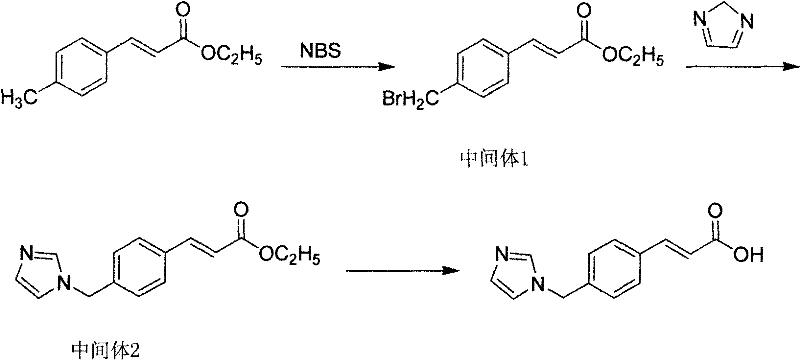
[0016] The preparation process of ethyl p-bromomethyl cinnamate (intermediate 1) described in the present embodiment is as follows:
[0017] (1), 950g (5mol) ethyl p-methyl cinnamate, 979g (5.5mol) N-bromosuccinimide, 47.5g benzoyl peroxide, and 5000ml ethyl acetate were added to a 10000ml four-necked bottle in turn , stirred and heated to reflux for 6 hours to obtain a light yellow turbid liquid, which was cooled and left to stand overnight. Filter out the white crystals, soak and wash the filter cake with a small amount of ethyl acetate, drain, the filtrate is yellow and transparent, add 200g of anhydrous magnesium sulfate to dry for 1 hour, filter to obtain a yellow transparent liquid, concentrate to dryness under reduced pressure to obtain a yellow viscous liquid, add Absolute ethanol, freeze and stand overnight, filter, soak and wash with a small amount of ice-free ethanol, and dry to obtain 1139 g (4.25 mol) of off-white intermediate 1 p-bromomethyl cinnamate ethyl ester...
Embodiment 2
[0021] The preparation process of ozagrel ethyl ester (intermediate 2) described in this embodiment is as follows:
[0022] (1), 1139g (4.25mol) of intermediate 1, 578g (8.5mol) of imidazole, 476g (8.5mol) of potassium hydroxide and 4500ml of anhydrous ether were sequentially added to a 10000ml four-necked flask, stirred and heated, and refluxed for 6 hours to obtain yellow Cloudy liquid, stand overnight. Filter out the slightly brown solid, concentrate the filtrate to dryness under reduced pressure, add n-hexane to crystallize, suction filter, wash with n-hexane, and dry to obtain 512 g (2 mol) of intermediate 2 ozagrel ethyl ester, melting point 88-90°C, yield 47.1% .
[0023] (2), 1139g (4.25mol) of intermediate 1, 578g (8.5mol) of imidazole, 901g (8.5mol) of anhydrous sodium carbonate and 4500ml of anhydrous ether were sequentially added to a 10000ml four-necked flask, stirred and heated, and refluxed for 6h to obtain Yellow cloudy liquid, stand overnight. The slightly ...
Embodiment 3
[0026] The preparation process of the crude ozagrel described in this embodiment is as follows:
[0027] (1) Dissolve 400g (10mol) of sodium hydroxide in 2500ml of water, stir and add 512g (2mol) of intermediate 2 below 40°C, heat up and reflux for 4h, drop to normal temperature, slowly add hydrochloric acid dropwise below 30°C, and control the pH value 4-5. Stand at room temperature for 2-3 hours, filter, wash with a small amount of water, and dry to obtain 341 g (1.5 mol) of yellow crude ozagrel with a yield of 75%.
[0028] (2) Dissolve 560g (10mol) of potassium hydroxide in 2500ml of water, stir and add 512g (2mol) of intermediate 2 below 40°C, heat up and reflux for 4h, drop to normal temperature, slowly add hydrochloric acid dropwise below 30°C, and control the pH value 4-5. Stand at room temperature for 2-3 hours, filter, wash with a small amount of water, and dry to obtain 316 g (1.39 mol) of yellow crude ozagrel, with a yield of 69.5%.
[0029] (3) Dissolve 480g (12m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com