Patents
Literature
200 results about "Bromosuccinimide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
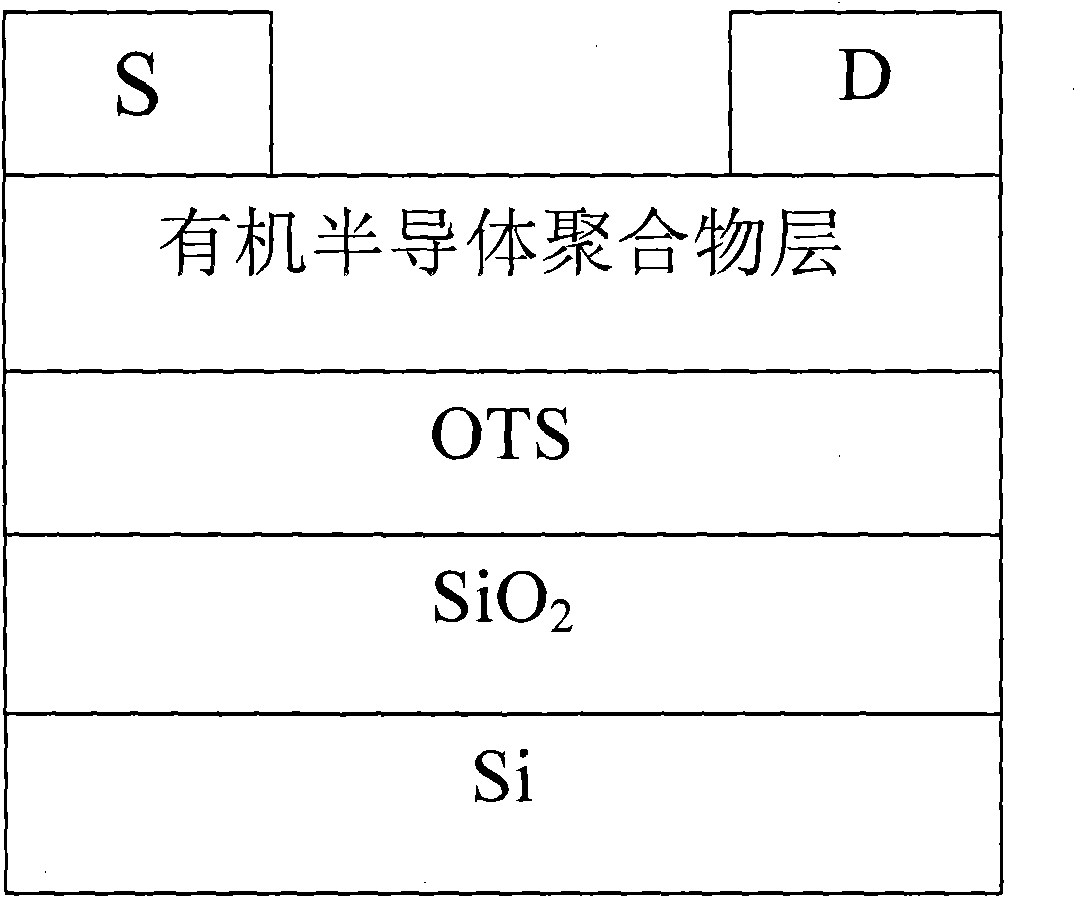
A brominating agent that replaces hydrogen atoms in benzylic or allylic positions. It is used in the oxidation of secondary alcohols to ketones and in controlled low-energy brominations. (From Miall's Dictionary of Chemistry, 5th ed; Hawley's Condensed Chemical Dictionary, 12th ed,).
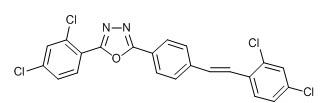
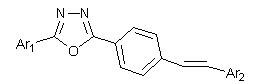
Stilbene derivative with 1,3,4-oxadiazole and preparation method and application thereof
InactiveCN102161646AMild preparation conditionsEasy to synthesizeBiocideOrganic chemistryPhosphorous acidCabbage looper
The invention provides a stilbene derivative with 1,3,4-oxadiazole and a preparation method and application thereof. The preparation method of the stilbene derivative with 1,3,4-oxadiazole comprises four specific reaction steps, namely oxidation cyclization reaction, NBS (N-bromosuccinimide) bromination reaction, triethyl phosphite esterification reaction and Wittig-Honner reaction. The stilbene derivative with 1,3,4-oxadiazole disclosed by the invention can be used to obviously inhibit the growing activities of Lepidopteron such as beet armyworm and cabbage looper and have important application value in the developments of environmentally friendly, efficient and new pesticides.
Owner:SOUTH CHINA UNIV OF TECH
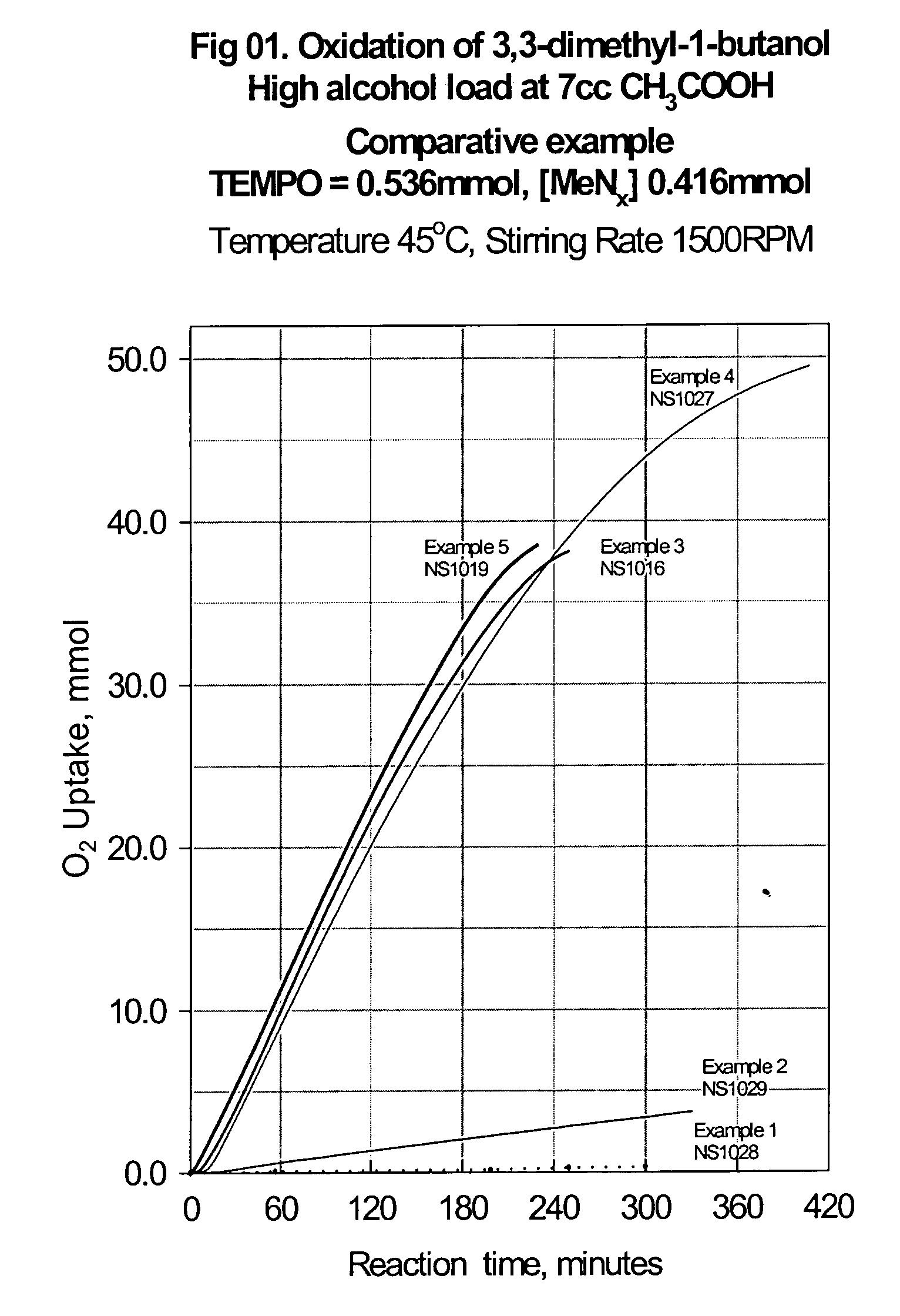
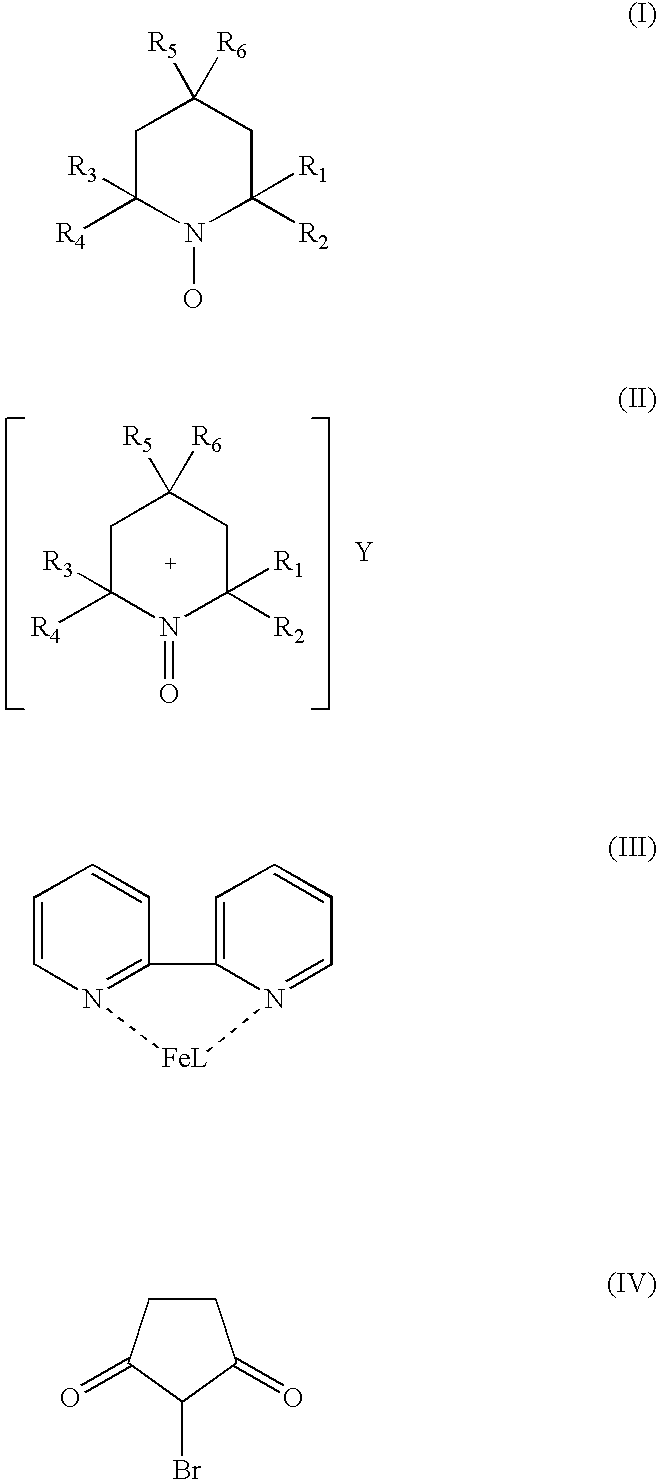
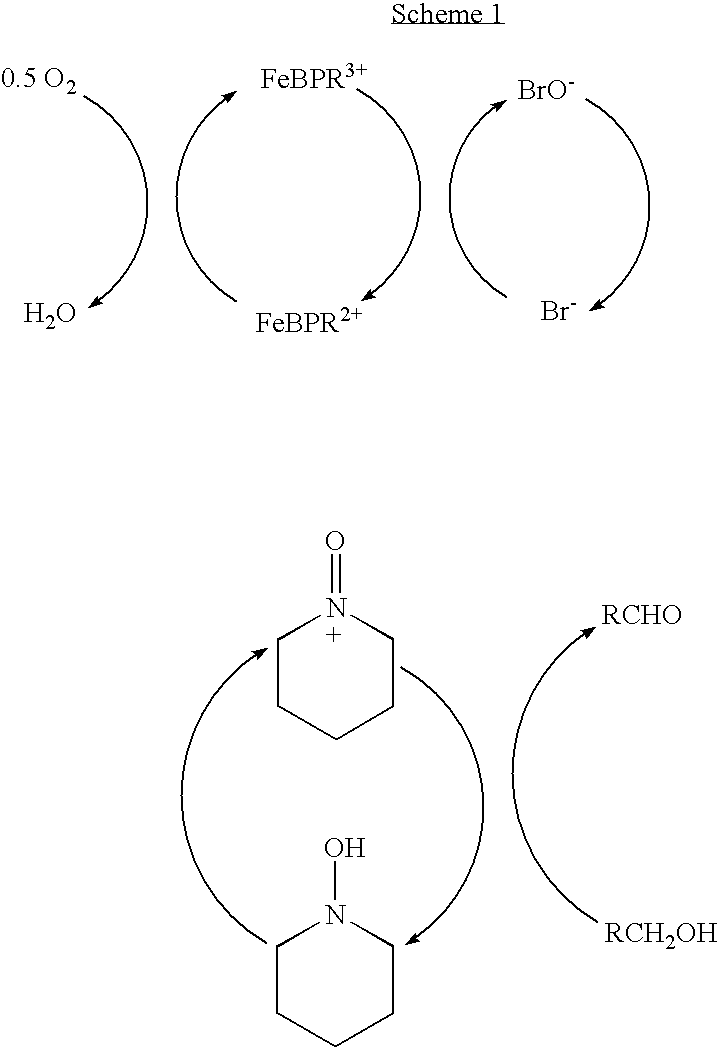
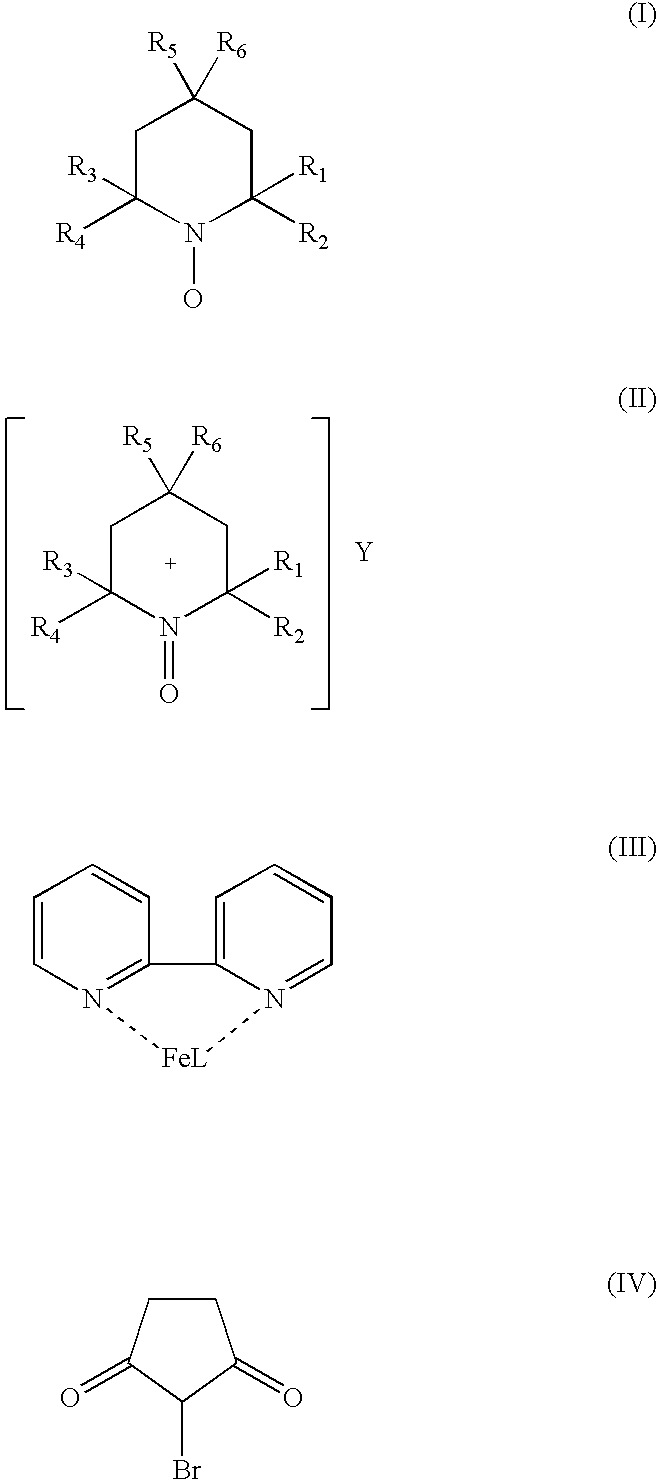
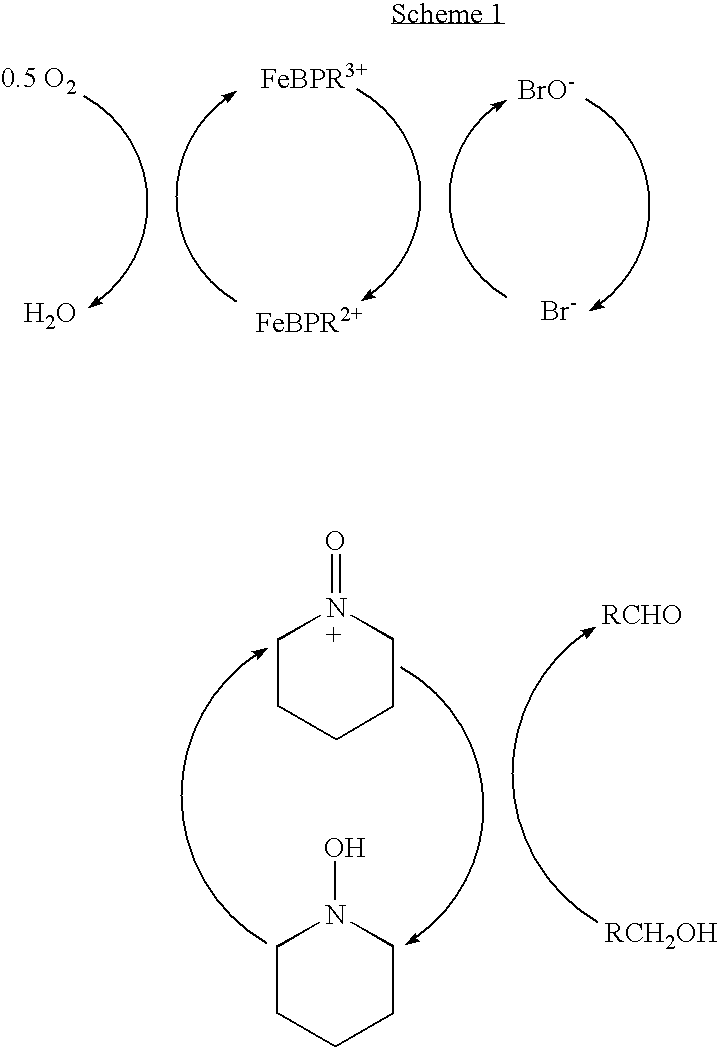
Catalyst system for aerobic oxidation of primary and secondary alcohols
InactiveUS20070078284A1Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic acidN-Bromosuccinimide
The present invention relates to a process of oxidation of alcohols selectively to aldehydes or ketones with molecular oxygen using a TEMPO based catalyst, Fe-bipyridyl or Fe-phenantroline co-catalyst and N-bromosuccinimide promoter in acetic acid solvent. The oxidation takes places at high rates and high aldehyde selectivity at temperatures in the range 45-50° C. and oxygen or air pressures of 0-15 psi. The alcohol conversion of 95-100% and aldehyde selectivity higher than 95% are achieved over 3-4 hours reaction time. Aldehydes such as 3,3-dimethyl-1-butanal can be produced efficiently using the present invention.
Owner:NUTRASWEET PROPERTY HLDG
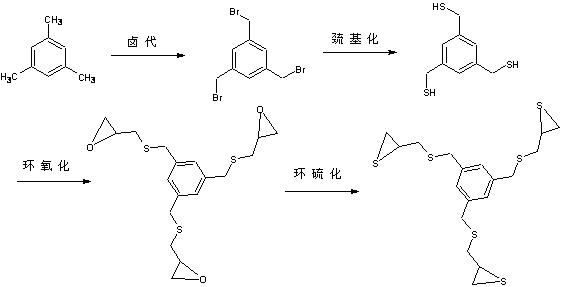
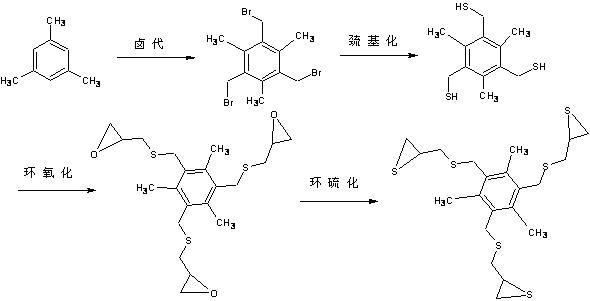
Optical resin monomer with high refractive index and preparation method thereof
ActiveCN102329298AImprove heat resistanceOrganic chemistryOptical elementsBenzoyl peroxidePotassium thiocyanate
The invention provides an optical resin monomer with high refractive index. The monomer has the structural formula shown in the specification, wherein in the structural formula, R is hydrogen, alkyl, alcoxyl, halogen and aryl; Ph is a benzene ring; m and n are integers; m is not less than 1 and not more than 6; and n ranges from 0 to (6-m). A preparation method comprises the following steps: taking the following raw materials: aromatic hydrocarbon, NBS (N-bromosuccinimide), BPO (benzoyl peroxide), thiourea, sodium hydroxide, hydrochloric acid, epichlorohydrin and potassium thiocyanate, wherein the mole ratio of aromatic hydrocarbon to NBS to thiourea to sodium hydroxide to hydrochloric acid to epichlorohydrin to potassium thiocyanate is 1:(3-5):0.1:(3-5):(9-12):(6-10):(3-5):(10-15); step 1. carrying out halogenation; step 2. carrying out halogen methylated thiolation: adopting a thiourea hydrocarbylation hydrolysis method or thiocyanate direct substitution method; 3. carrying out epoxidation: using a universal epichlorohydrin preparation method; and 4. preparing cyclic sulfide products: adopting a universal cyclic sulfide preparation method.
Owner:JIANGSU JUNSHI OPTICS CO LTD
Preparation method of ozagrel bulk drug
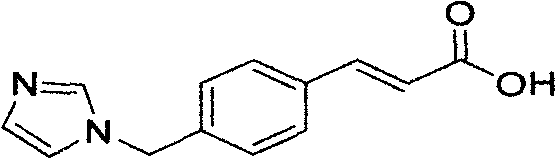
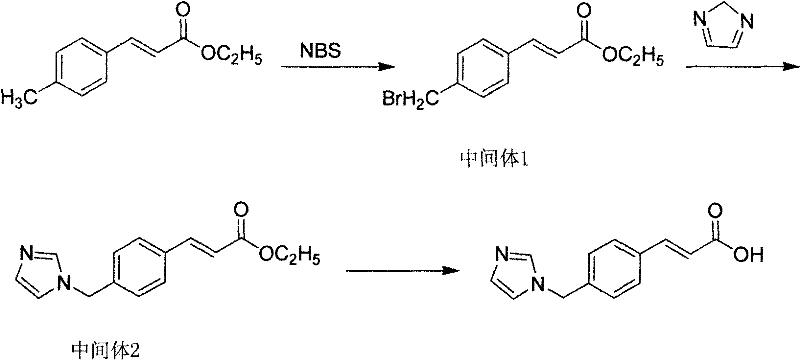
The invention discloses a preparation method of an ozagrel bulk drug. The method comprises the following steps of: bromating methyl ethyl cinnamate serving as a starting material with NBS (B-Bromosuccinimide) to obtain ethyl 4-bromomethylcinnamate; undergoing a condensation reaction on the ethyl 4-bromomethylcinnamate and imidazole to generate ozagrel ethyl ester; hydrolyzing under an alkaline condition; performing acid precipitation to obtain crude ozagrel; and refining to obtain an ozagrel bulk drug. Due to the adoption of the method, a method for refining catalysts, solvents and crude products used in each reaction step is improved, the product yield is high, the product quality is good, the use of toxic reagents and expensive reagents is avoided, little environmental pollution is caused, and the production cost is low. The method is suitable for industrial production, and is an improved and environmentally-friendly method for preparing the ozagrel bulk drug.
Owner:辽宁远大诺康医药有限公司
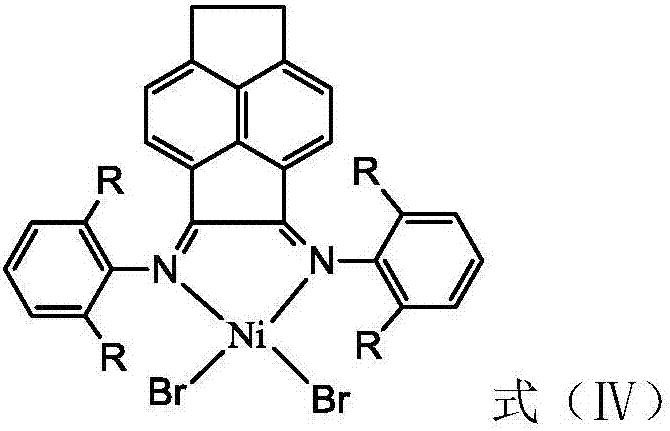
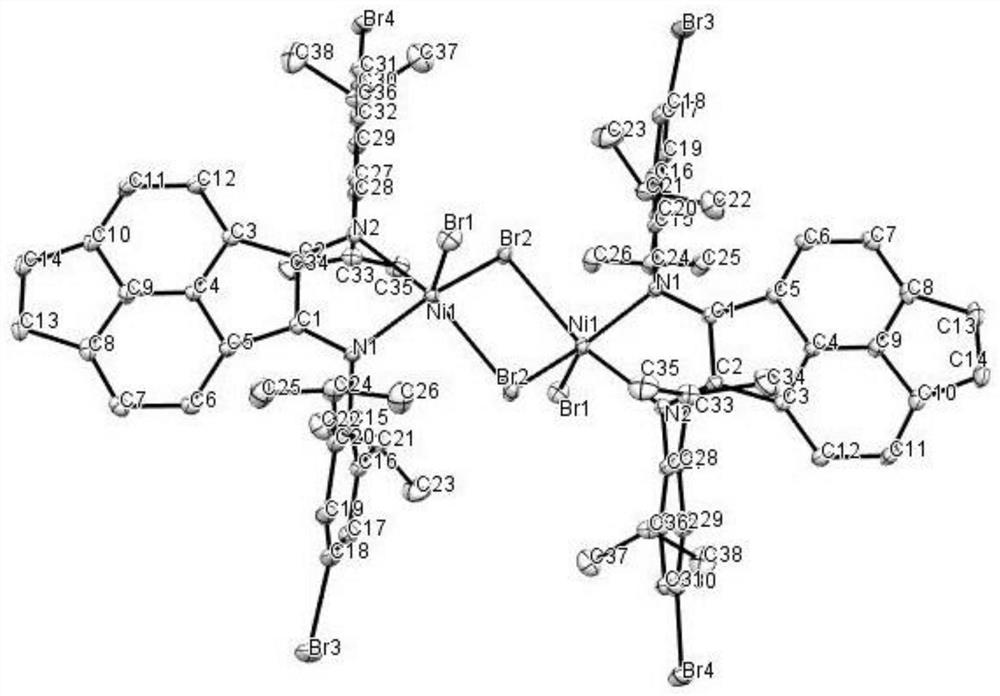
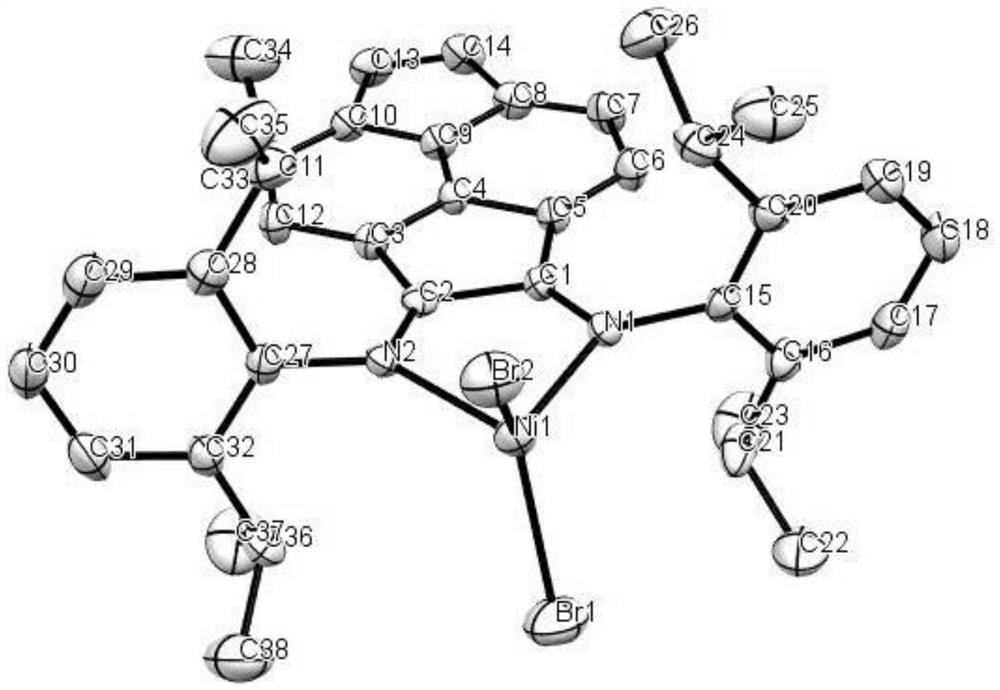
Vinylene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation method and application thereof
ActiveCN108003259AHigh activityImprove thermal stabilityNickel organic compoundsBulk chemical productionKetoneAniline
The invention relates to the field of olefin catalytic polymerization, and aims at providing a vinylene acenaphthene (alpha-diimine) nickel olefin catalyst, and preparation and an application thereof.The preparation comprises the typical synthetic steps: carrying out a diacylation reaction of acenaphthene to obtain a compound C1; carrying out a bromination reaction of the compound C1 and N-bromosuccinimide (NBS) to obtain a compound C2; carrying out an elimination reaction of the compound C2 to obtain a compound C3; carrying out ketone amine condensation reaction of the compound C3 with symmetric aniline, to obtain alpha-diimine ligands C4-C8; and under anhydrous and anaerobic conditions, complexing the alpha-diimine ligands C4-C8 with ethylene glycol dimethyl ether nickel dibromide to obtain a final product. The catalyst has higher activity and better thermal stability, and can catalyze ethylene with high activity at the temperature of greater than or equal to 60 DEG C to obtain high-molecular-weight hyperbranched polyethylene. Under the same polymerization conditions, ethylene can be catalyzed to polymerize to obtain branched polyethylene with higher molecular weight, so as to meet more application requirements. The cost of raw materials is low, the reaction yield is high and industrialized production can be achieved.
Owner:ZHEJIANG UNIV
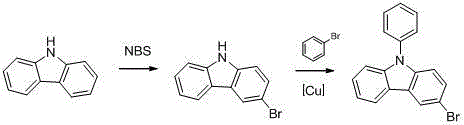
Preparation method of N-phenyl-3-bromocarbazole
Owner:DALIAN NETCHEM CHIRAL TECH
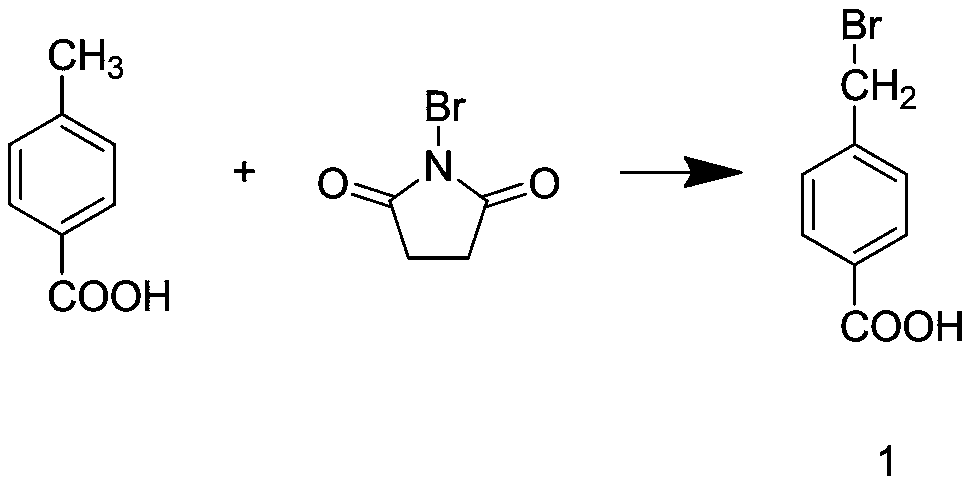
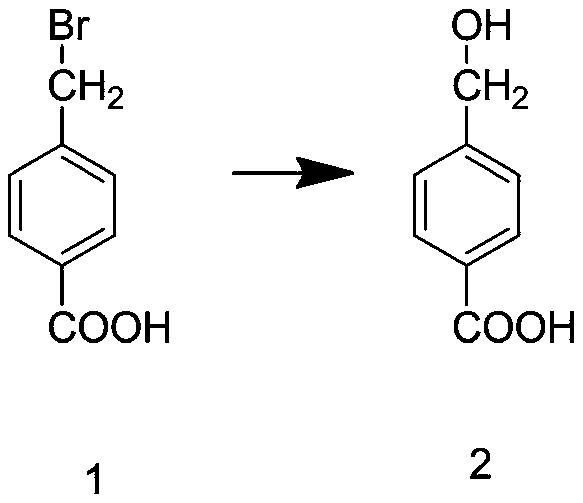
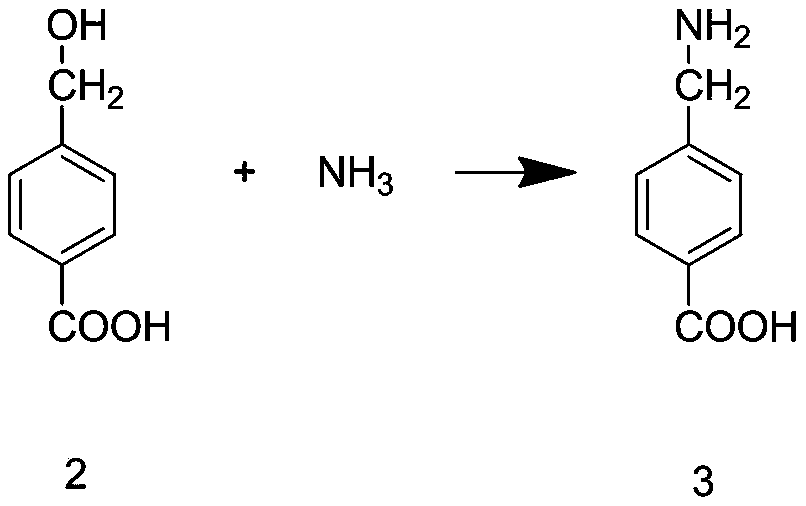
Tranexamic acid and preparation method thereof
InactiveCN111574388AHigh yieldReduce manufacturing costOrganic compound preparationAmino-carboxyl compound preparationBenzoic acidPtru catalyst
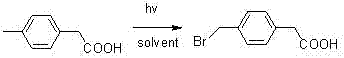
The invention discloses tranexamic acid and a preparation method thereof. P-bromotoluene used as a raw material is prepared into a Grignard reagent; the Grignard reagent is further subjected to addition reaction with carbon dioxide, and the reaction product is hydrolyzed under an acidic condition to obtain p-toluic acid; bromine substitution reaction is carried out on p-toluic acid and N-bromosuccinimide to obtain an intermediate 1; phase transfer catalytic reaction is carried out on the intermediate 1 to obtain an intermediate 2; the intermediate 2 reacts with a saturated toluene solution ofammonia gas to replace an alcoholic hydroxyl group on the intermediate 2 with an amino group to obtain an intermediate 3; and the intermediate 3 and hydrogen are hydrogenated under the action of a supported nickel catalyst to obtain tranexamic acid. The yield of tranexamic acid prepared by the preparation method of tranexamic acid is high, and compared with an existing preparation method, most ofthe used raw materials are low-price raw materials, so that the production cost of tranexamic acid is greatly reduced.
Owner:安徽鼎旺医药有限公司
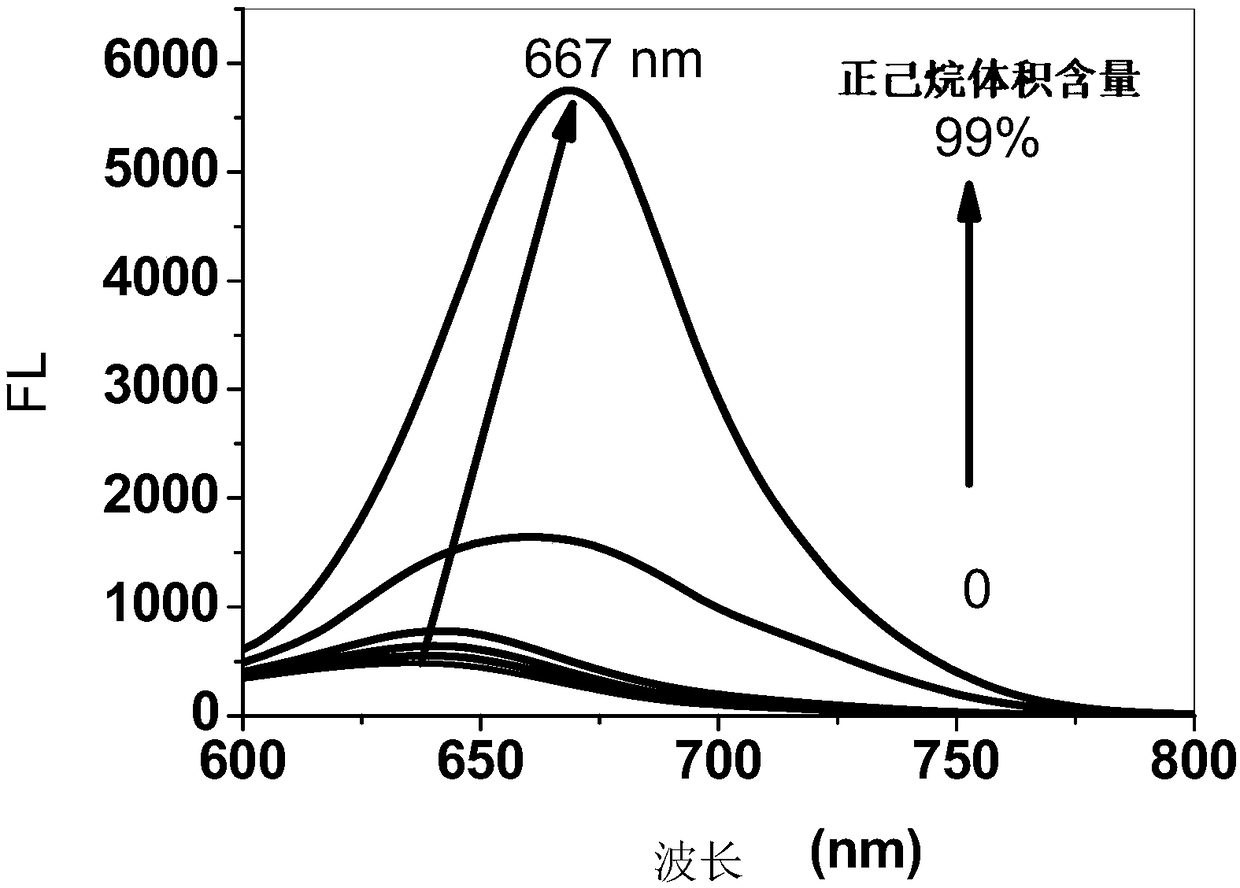
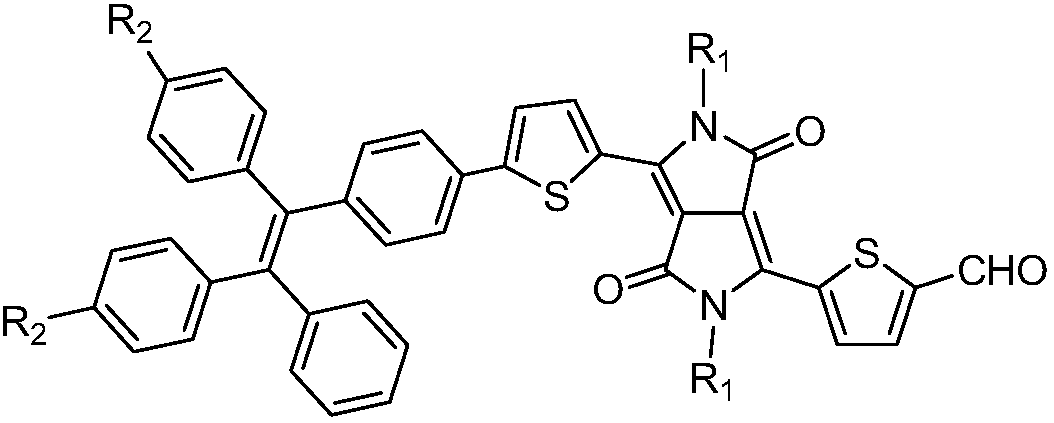
Aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and preparation method thereof
InactiveCN108912126AAdjustment rangeAdjust the fluorescence intensityOrganic chemistryLuminescent compositionsN dimethylformamideKetone
The invention discloses an aggregation-induced emission near-infrared emission diketopyrrolopyrrole compound and a preparation method thereof. The method comprises the steps that 3,6-dithiophene diketopyrrolopyrrole reacts with twice molar weight of alkyl bromide so as to obtain 2,5-dialkyl-3,6-dithienopyrrolopyrrole, then the 2,5-dialkyl-3,6-dithienopyrrolopyrrole sequentially undergoes Vilsmeierreaction with phosphorus oxychloride / N,N-dimethylformamide and undergoes substitution reaction with N-bromosuccinimide so as to obtain 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole, and then the 2,5-dialkyl-3-(5-formyl)thienyl-6-(5-bromine)thienyl diketopyrrolopyrrole undergoes Suzuki reaction with tetraphenyl ethylene boric acid ester so as to obtain 2,5-dialkyl-3(4-((E)-2-phenyl-1,2-disubstituted styryl)phenyl)thienyl-6-(5-formyl)thienyl diketopyrrolopyrrole. The compound has the emission range greater than 650 nm and has the aggregation-induced emission property.
Owner:SOUTH CHINA UNIV OF TECH
Preparation method of phloroglucinol
ActiveCN103641687AShort reaction timeReduce manufacturing costOrganic chemistryOrganic compound preparationImidePtru catalyst
The invention discloses a preparation method of phloroglucinol, which comprises the following steps: dissolving resorcinol in reaction solvent, then adding an N-bromosuccinimide solution, heating to perform bromination reaction, and then treating to obtain 4-bromoresorcinol; adding the obtained 4-bromoresorcinol into a container, adding reaction solvent, strong alkali and a catalyst, heating to perform hydrolysis reaction, and then treating to obtain a phloroglucinol salt solution; and adding hydrochloric acid into the phloroglucinol salt solution to regulate the pH value and precipitate solid, filtering to obtain the solid phloroglucinol crude product, and finally purifying to obtain the phloroglucinol product. According to the preparation method, the low-price accessible resorcinol is used as the raw material and is subjected to bromination, strong alkali hydrolysis and acidification, thus preparing the phloroglucinol. According to the invention, the reaction time is shortened, the production cost is lowered, and remarkable economic benefits are achieved. By preparing the phloroglucinol through the preparation method, the yield is up to 70% or above, and the purity of the prepared product is up to 99.9% or above.
Owner:KAIFENG MINGREN PHARMA
Catalyst system for aerobic oxidation of primary and secondary alcohols
InactiveUS7351867B2Organic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystPhenanthroline
The present invention relates to a process of oxidation of alcohols selectively to aldehydes or ketones with molecular oxygen using a TEMPO based catalyst, Fe-bipyridyl or Fe-phenantroline co-catalyst and N-bromosuccinimide promoter in acetic acid solvent. The oxidation takes places at high rates and high aldehyde selectivity at temperatures in the range 45-50° C. and oxygen or air pressures of 0-15 psi. The alcohol conversion of 95-100% and aldehyde selectivity higher than 95% are achieved over 3-4 hours reaction time. Aldehydes such as 3,3-dimethyl-1-butanal can be produced efficiently using the present invention.
Owner:NUTRASWEET PROPERTY HLDG
O-carborane derivative, synthesis method and application thereof
ActiveCN111217846AIncrease polarityIncrease cell affinityGroup 3/13 element organic compoundsAntineoplastic agentsChemical synthesisHigh concentration
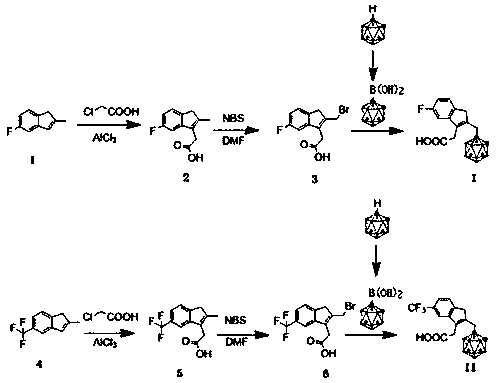
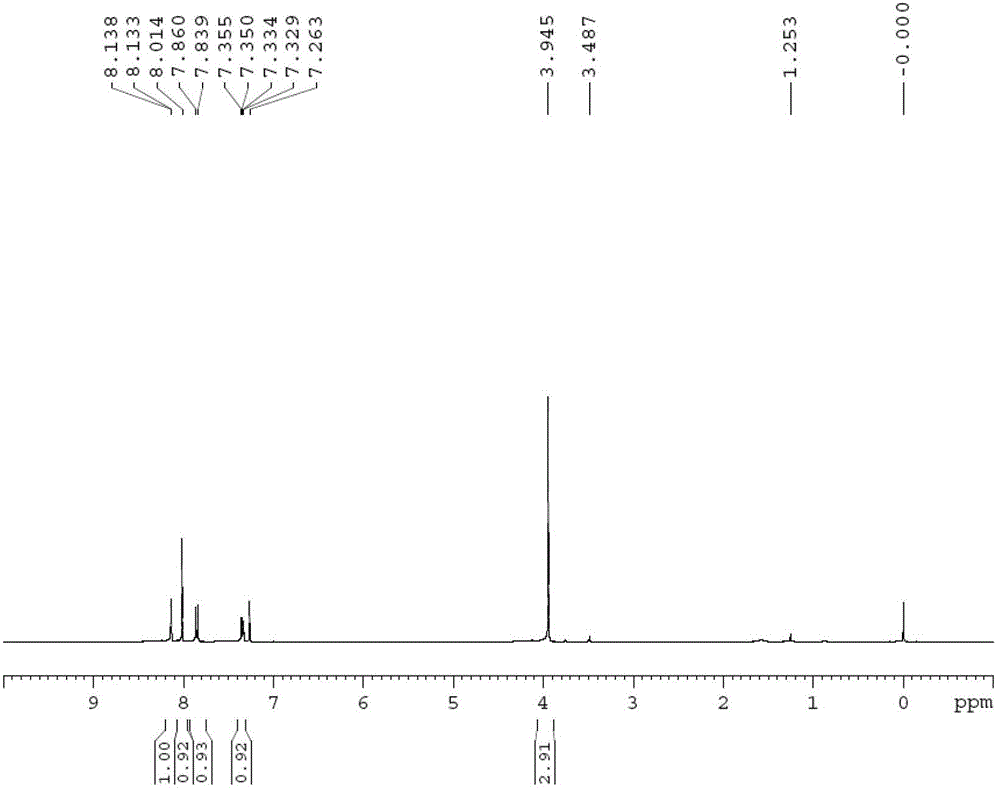
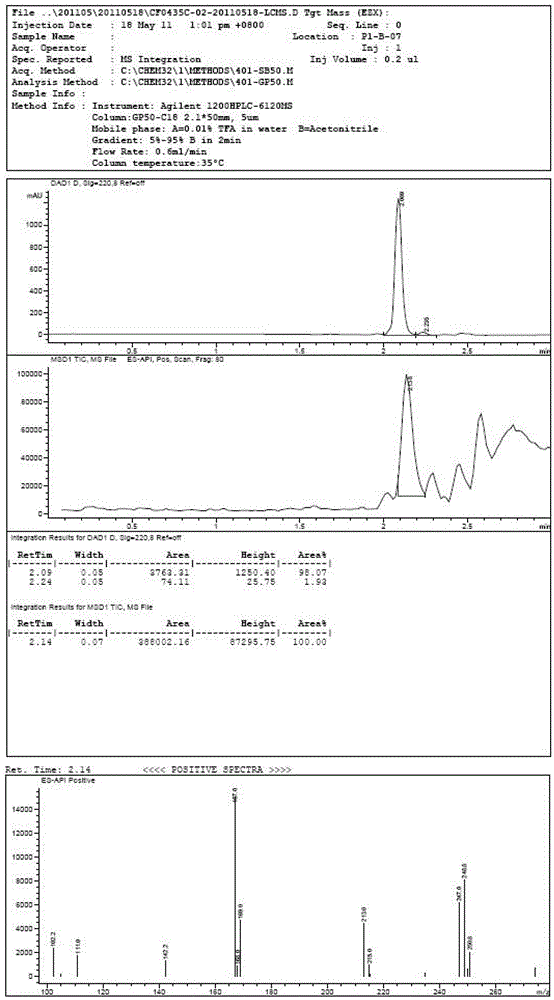
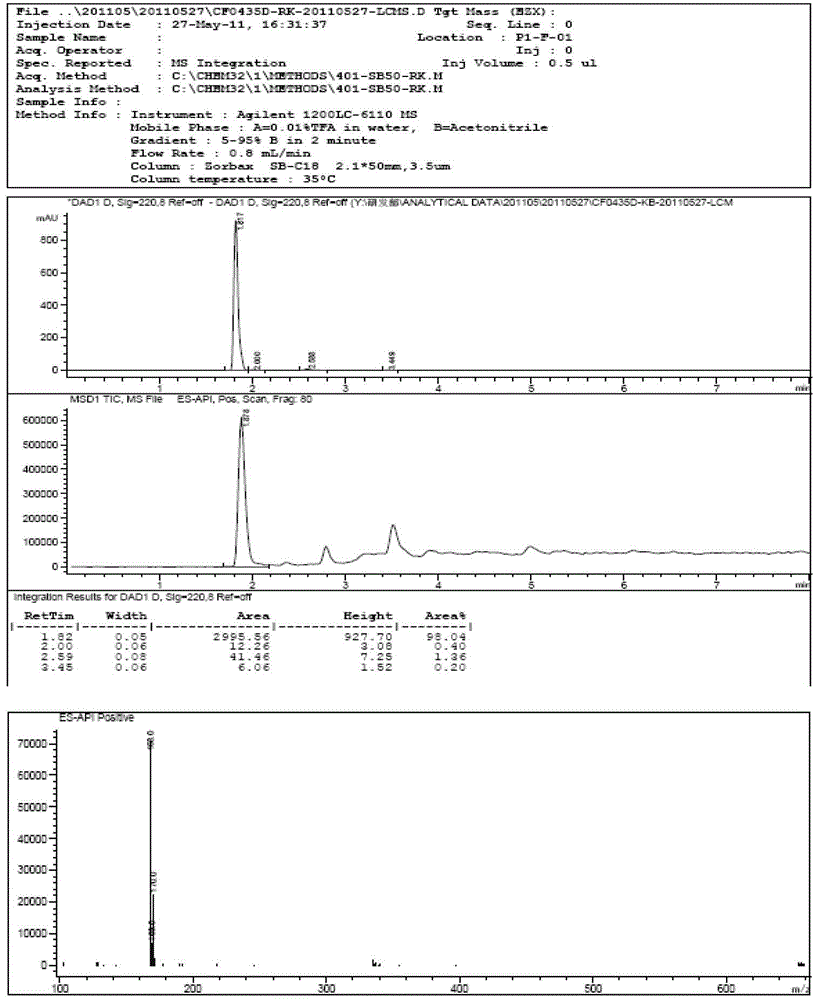
The invention discloses an o-carborane derivative and a synthesis method thereof. The method includes: step 1, taking 5-fluoro-2-methyl-1H-indene or 5-trifluoromethyl-2-methyl-1H-indene as a reactioninitiator, adopting aluminum trichloride as a catalyst, and carrying out reaction with 1-chloroacetic acid to obtain an intermediate 2; step 2, reacting the intermediate 2 with N-bromosuccinimide by taking DMF as a reaction solvent to obtain an intermediate 3; and step 3, reacting the intermediate 3 with o-carborane in an organic solvent or ionic liquid under an alkaline condition to obtain the corresponding o-carborane derivative. According to the invention, fluorine ions and relative hydrophilic groups are introduced for modification through chemical synthesis, the purposes of reducing toxicity and increasing compound polarity and cell affinity are achieved, so that the technical effect of targeted enrichment and high concentration of boron ions in tumor cells is achieved, and then the technical bottleneck that p-borane phenylalanine (BPA) is discharged by an "anti-transport" mechanism after the concentration of the BPA in the tumor cells reaches a certain number 1 is solved.
Owner:南京艾斯特医药科技有限公司
Method for synthesizing 5-isoindolone chloride
The invention discloses a method for synthesizing 5-isoindolone chloride, comprising the following steps: using 4-chlor-2-methyl benzoic acid as an initial reactant; obtaining products through methylation, NBS (N-bromosuccinimide) bromination reaction and amidation reaction in sequence; and obtaining a monobromo pure product through firstly double bromination and then debromination in the NBS bromination reaction. According to the method for synthesizing, the doping of double brominated products when NBS is excessive in the prior art is overcome, and processing is ensured after raw materials are completely reacted without detecting and tracking reaction under the condition that NBS is excessive, so that the cost is reduced, the reaction is easy to control, the complexity of operation is reduced, the purification is relatively simple, and the 5-isoindolone chloride is easy to produce in large scale.
Owner:CHEMFUTURE PHARMATECH JIANGSU
Metal organic framework based on imidazole sulfonic acid as well as preparation method and application
InactiveCN106883422ARealize functional integrationIncrease varietyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsBenzaldehydeSynthesis methods
The invention provides a metal organic framework based on imidazole sulfonic acid as well as a preparation method and application. The metal organic framework based on the imidazole sulfonic acid is obtained by synthesizing and modifying a metal organic framework based on an imidazole ligand through a sulfonic compound; a chemical structural formula of the metal organic framework based on the imidazole ligand is [Zr6O4(OH)4L6]n, wherein n is a natural number more than 0; L is an organic ligand L and a chemical structural formula of the organic ligand L is show in the description; a synthesis method of the organic ligand L comprises the following steps: firstly, enabling 3-methyl-terephthalic acid to react to obtain an intermediate A; secondly, taking the intermediate A and bromosuccinimide as raw materials and reacting to obtain an intermediate B; thirdly, taking the intermediate B and imidazole as raw materials and reacting to obtain an intermediate C; finally, carrying out hydrolysis reaction on the intermediate C to prepare the organic ligand L. The metal organic framework based on the imidazole sulfonic acid has a catalytic effect on benzaldehyde under normal pressure and has the characteristics of moderate reaction conditions, short reaction time, less dosage of a catalyst and capability of being recycled and repeatedly utilized.
Owner:SHANDONG NORMAL UNIV
Perylene diimide-fluorene-thiophene and (3, 4-b) pyrazine conjugated polymer and preparation method and application thereof
InactiveCN102134307AWide light absorption rangeIncrease profitOrganic chemistrySolid-state devicesSolubilitySuccinchlorimide
The invention discloses a perylene diimide-fluorene-thiophene and (3, 4-b) pyrazine conjugated polymer shown in a general formula (I) and a preparation method and application thereof. The invention comprising the following steps: causing 1, 7-dibromo-3, 4, 9, 10-perylene tetracid anhydride to react with 3, 4, 5-trialkyl (tri-oxygroup)-1-anline to obtain a monomer; then causing a 3', 4'-diamido-2,2': 5', 2''-terthienyl compound to react with a benzil compound to obtain a midbody; and then causing the midbody to react with N-bromosuccinimide to obtain another monomer; and under the oxygen-freecondition, causing the two monomers and the bi-boric acid ester to conduct polymerization to obtain a target product. The preparation method is simple, easy to operate and control, and suitable for industrialized production; the target product prepared by the method has good dissolubility, has strong absorbance and wide absorption range when being used in the field of organic solar cell, and can be extended to a near infrared region, thus improving the utilization rate of the sunlight.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
Polymethacrylate, preparation method and application thereof
InactiveCN101429260AAbility to promote endocytosisFacilitated releaseGenetic material ingredientsPharmaceutical non-active ingredientsAminolysisMethyl group
The invention relates to reductive cation polymethacrylate, a preparation method thereof, application of the polymethacrylate to establishment of gene nano-complexes and application of the polymethacrylate to gene therapy. The method comprises the following steps: 1, 3, 5-triisopropylphenyl is taken as a raw material and reacts with N- bromosuccinimide and potassium ethyl xanthate to obtain substituted 1, 3, 5- triisopropylphenyl potassium ethyl xanthate; and the substituted 1, 3, 5- triisopropylphenyl potassium ethyl xanthate is catalyzed by azo-bis-iso-butyrynitrile, and subjected to reversible addition-broken strand transfer polymerization, aminolysis and oxidation reaction with 2-(dimethylamino ethyl)metacrylic acid ester to obtain poly (1, 3, 5-three-terminal sulfhydryl (2-propyl phenyl -2-(dimethylamino)ethyl)) methacrylic acid ester) and disulfide bond crosslinking products of the poly (1, 3, 5-three-terminal sulfhydryl (2-propyl phenyl -2-(dimethylamino)ethyl)) methacrylic acid ester). The polymethacrylate can condense plasmid genes into nano complexes; the transfection efficiency of HEK293T cells and Hella cells is more than 30 percent; the toxicity is low; and the survival rate of the cells is more than 80 percent.
Owner:SHANGHAI INST OF APPLIED PHYSICS - CHINESE ACAD OF SCI
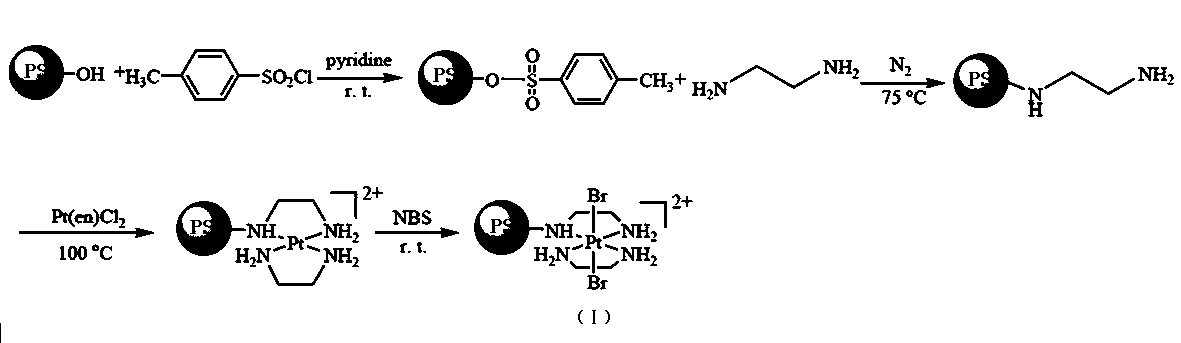
Supported platinum complex oxidizing agent easy to recycle and preparation method and application thereof
InactiveCN107892710AEasy to makeYield impactPeptide preparation methodsEthylenediaminePlatinum complex
The invention discloses a supported platinum complex oxidizing agent easy to recycle. The supported platinum complex oxidizing agent is provided with the structure shown in the formula I. The invention further provides a preparation method of the oxidizing agent. The method comprises the steps that 1, pyridine is taken as solvent, and polystyrene microsphere is reacted with toluene sulfonyl chloride to obtain a yellow solid substance; 2, ultrasonic dispersion is conducted on the obtained yellow solid substance in ethylenediamine, and the yellow solid substance is subjected to stirring reactionto obtain a deep yellow solid substance; 3, DMF is taken as solvent, and the obtained deep yellow solid substance and a two-valence platinum complex are subjected to stirring reaction to obtain a black solid substance; 4, after ultrasonic dispersion is conducted on the black solid substance in ethyl alcohol, the black solid substance is fully reacted with N,N'-bromosuccinimide to obtain a light black solid substance, namely the supported platinum complex oxidizing agent. The oxidizing agent preparation method is simple, recycle is easy, and the reaction yield is high.
Owner:HEBEI UNIVERSITY
2-bromine-4,6-dichloroaniline preparation method
ActiveCN103224452AHigh reaction yieldLow costOrganic compound preparationAmino compound preparationPotassium channelCombinatorial chemistry
The invention relates to a preparation method for a medicine KCNQ potassium channel conditioning agent critical material 2-bromine-4,6-dichloroaniline. According to the invention, 2,4-dichloroaniline is taken as a raw materials, and is subjected to NBS (N-bromosuccinimide) bromination to obtain a target object, and the preparation method of the invention has the advantages of low preparation method and simple operation, and has industrial production value.
Owner:INSIGHT HIGH TECH JIANGSU CO LTD
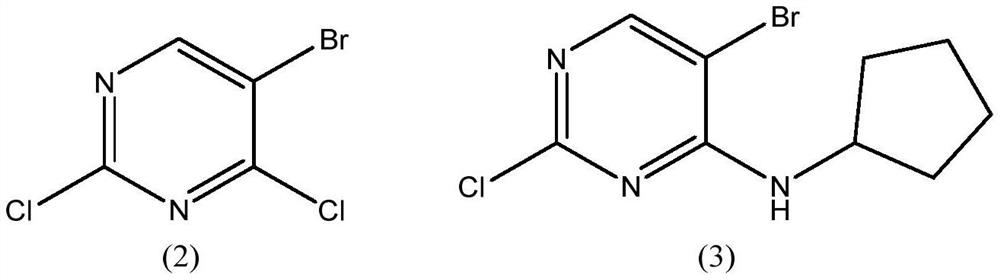
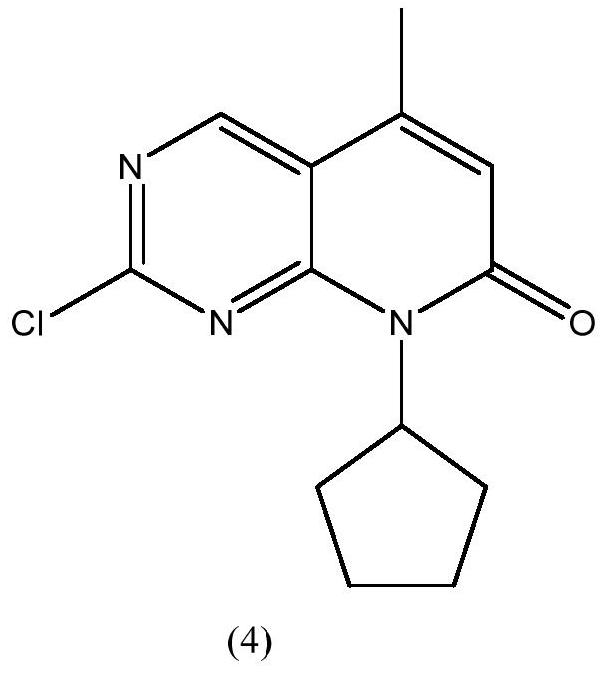
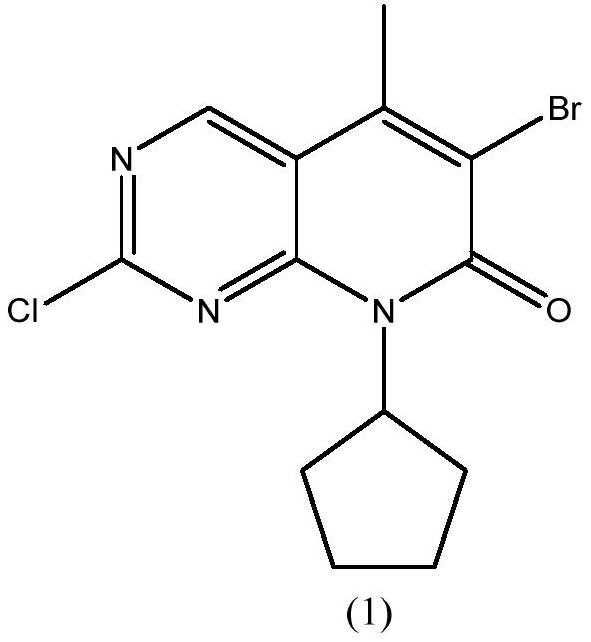
Preparation method of palbociclib intermediate
ActiveCN112898299AMild reaction conditionsSimple post-processingOrganic chemistryAcetic anhydrideKetone
The invention discloses a preparation method of a palbociclib intermediate. The method comprises the following steps: preparing 5-bromine-2-chloro-N-cyclopentylamine pyrimidine-4 amine from 5-bromine-2,4-dichloropyrimidine and cyclopentylamine by taking solvents such as dichloromethane and water as solvents and taking inorganic base as an acid-binding agent; with DIEA as an acid-binding agent, DMF as a solvent and TBAB as a phase transfer catalyst, in the presence of water, catalyzing with a trace amount of palladium, and carrying out normal hexane reflux dehydration; further subjecting the acetic anhydride to dehydration cyclization, such that 2-chloro-8-cyclopentyl-5-methylpyridino[2,3-D]pyrimidine-7-(8H)-ketone is obtained; and reacting the obtained compound with NBS (N-bromosuccinimide) in acetonitrile to obtain the 6-bromo-2-chloro-8-cyclopentyl-5-methylpyridino[2, 3-D]pyrimidine-7(8H)-ketone. The method is mild in reaction, simple and convenient to operate, recyclable in solvent, less in environmental pollution, high in yield, low in cost, high in product quality and suitable for industrial production.
Owner:SHANDONG BOYUAN PHARM CO LTD
Preparation method of 2, 3-thiophenedicarboxaldehyde
ActiveCN102627626AMild reaction conditionsRaw materials are easy to getOrganic chemistryBenzoyl peroxidePtru catalyst
The invention discloses a preparation method of 2, 3-thiophenedicarboxaldehyde and belongs to the field of organic synthesis. The preparation method is characterized in that 2, 3-thiophenedicarboxaldehyde as a raw material and N-bromosuccinimide undergo a bromination reaction in the presence of benzoyl peroxide as a catalyst and carbon tetrachloride or trichloromethane as a solvent at a certain temperature to produce 2, 3-di(bromomethyl)thiophene; and 2, 3-di(bromomethyl)thiophene and hexamethylenetetramine undergo a Sommelet reaction in the presence of trichloromethane as a solvent at a certain temperature to produce 2, 3-thiophenedicarboxaldehyde. Compared with the prior art, the preparation method has the advantages that raw materials are cheap and easily available; reaction conditions are mild and can be controlled easily; and a product yield is high.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD +1
Method for preparing 2-bromo-fluorobenzyl bromide
InactiveCN102070397AReduce manufacturing costImprove securityHalogenated hydrocarbon preparationOrganic synthesisSulfite salt
The invention relates to the field of organic synthesis, in particular to a method for preparing 2-bromo-fluorobenzyl bromide, which comprises the following steps: (1) in the presence of an organic or inorganic solvent, adding a 2-bromo-6-fluorotoluene compound and 40-mass-percent hydrobromic acid and dripping 30-mass-percent hydrogen peroxide under a lighting condition, and reacting for 6 to 24 hours, wherein the molar ratio of 2-bromo-6-fluorotoluene to HBr is 1:(1-3.5) and the molar ratio of the 2-bromo-6-fluorotoluene to H2O2 is 1:(1-3.5); and (2) washing the reaction solution with saturated sodium sulfite solution and water, drying the reaction solution with anhydrous sodium sulfate, evaporating solvent under a reduced pressure, and obtaining the 2-bromo-6-fluorotoluene compound by silica gel column chromatography. In the method, the hydrobromic acid and hydrogen peroxide are used in place of the traditional N-bromosuccinimide brominating agent, so the production cost is reduced; and lighting is used in place of a benzoyl peroxide initiator, so method has the advantages of mild reaction conditions, high product purity, high yield and the like.
Owner:CHANGZHOU UNIV
Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one
InactiveCN104781244AGood atom economyCarbamic acid derivatives preparationOrganic compound preparationBlood Coagulation Factor XCarboxylic acid
The present invention relates to an improved and industrially advantageous process for the preparation of (1S, 4S, 5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one represented by the following formula (I) which is a key intermediate in the synthesis of edoxaban, a compound that exhibits an inhibitory effect on activated blood coagulation factor X (also referred to as activated factor X or FXa), and is useful as a preventive and / or therapeutic drug for thrombotic diseases. The process includes reacting (1S)-cyclohex-3-ene-1-carboxylic acid of formula (II) with a brominating agent selected from the group consisting of N-bromosuccinimide or 1,3-dibromo-5,5-dimethylhydantoin in the presence of a base selected from calcium oxide or calcium hydroxide in a solvent selected from the group comprising of dichloromethane, toluene, tetrahydrofuran, ethy1 acetate, hexanes, cyclopentyl methyl ether (CPME) or a mixture thereof to get (1S, 4S, 5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one of formula (I).
Owner:DAIICHI SANKYO CO LTD
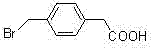
P-bromomethyl phenylacetic acid preparation method
InactiveCN106905135AHigh yieldHigh purityOrganic compound preparationCarboxylic compound preparationBenzoyl peroxideLight irradiation
The present invention provides a p-bromomethyl phenylacetic acid preparation method, which comprises: dissolving p-methyl phenylacetic acid in a high boiling point solvent, wherein the solvent is benzene, chlorobenzene or dichlorobenzene; adding a brominating agent and a catalyst, wherein the brominating agent is bromine or bromosuccinimide, the catalyst is azobisisobutyronitrile or benzoyl peroxide, and a molar ratio of the p-methyl phenylacetic acid to the brominating agent is 1:0.8-2.0; heating to a temperature of 80-130 DEG C, carrying out a reaction for 6-12 h through the initiation with incandescent light irradiation, and cooling; and filtering, and carrying out water washing to obtain the p-bromomethyl phenylacetic acid. According to the present invention, the high boiling point reaction solvent is used, a lot of the generated heat can be taken away by the high boiling point solvent through the circulation after the reaction is initiated by the illumination, the product p-bromomethyl phenylacetic acid is precipitated from the solvent after the cooling, and the water washing and the drying are performed to obtain the product, wherein the HPLC purity of the product is greater than 98.5%, and the yield is up to 90%.
Owner:SHANGHAI INST OF TECH
Preparation method of 6-dehydronandrolone acetate
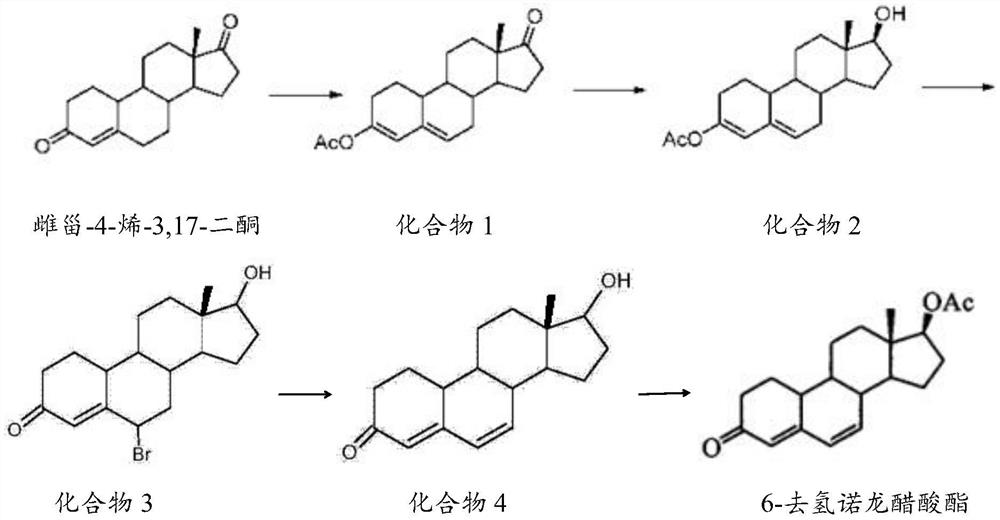
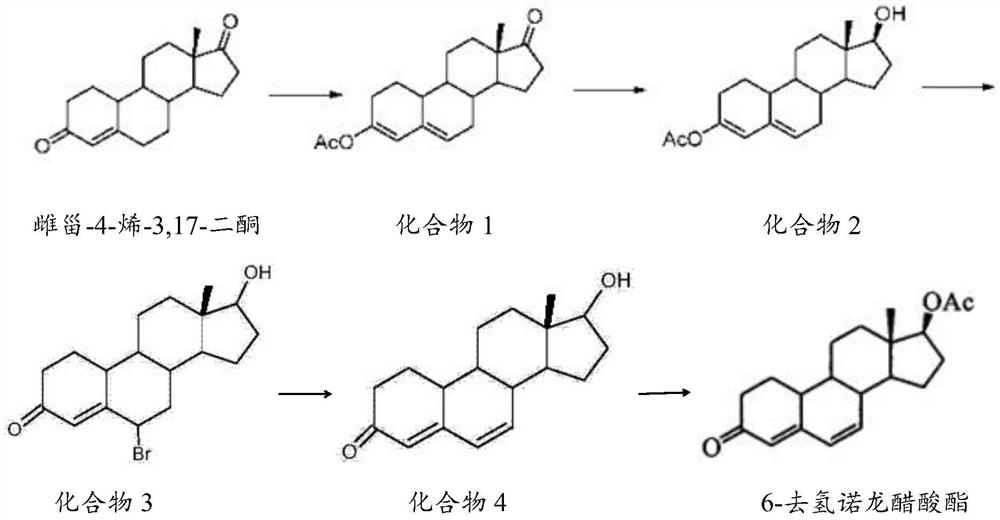
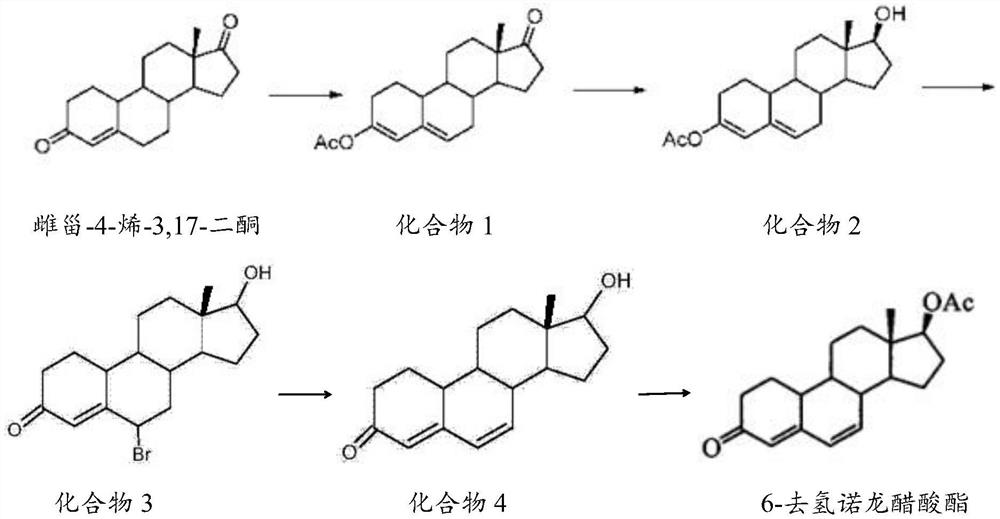
The invention belongs to the technical field of steroid drug intermediates, and provides a preparation method of 6-dehydronandrolone acetate. The preparation method comprises the following steps: carrying out a catalytic reaction on estra-4-ene-3,17-dione, acetic anhydride and p-toluenesulfonic acid to obtain a compound 1; carrying out a reduction reaction on the compound 1, hydroboron and aluminum trichloride to obtain a compound 2; subjecting the compound 2 and N-bromosuccinimide to reacting with DMF to obtain a solution of a compound 3; carrying out an addition reaction on the solution of the compound 3 and alkali to obtain a compound 4; and subjecting the compound 4, acetic anhydride, triethylamine and dichloromethane to a catalytic reaction to obtain 6-dehydronandrolone acetate. By adding borohydride and the aluminum trichloride in a reasonable ratio, hydrolysis of a 3-site ester group is effectively avoided, and side reactions are few; and meanwhile, the yield and the purity of a target product are remarkably improved by reasonably setting a synthesis route and controlling a reaction temperature.
Owner:YICHENG GOTO PHARMA
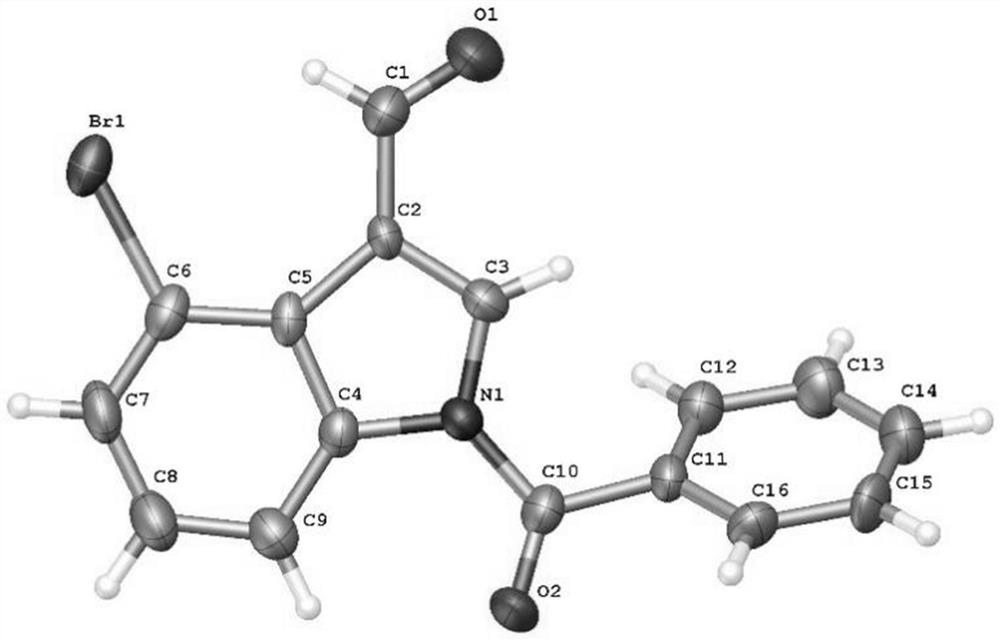
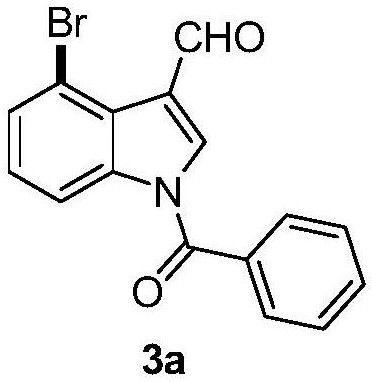
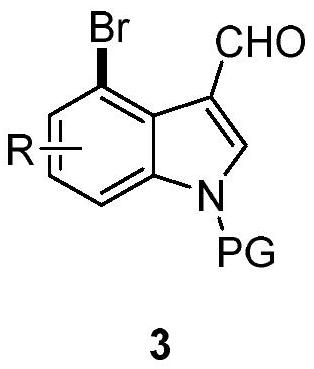
4-bromoindole compound and preparation method thereof
ActiveCN112028812AHigh reaction yieldHigh chemoselectivityAntibacterial agentsNervous disorderImideBiochemical engineering
The invention discloses a 4-bromoindole compound and a preparation method thereof. Various N-protected indolo-3-formaldehyde and N-bromosuccinimide (NBS) are used as reaction substrates to prepare the4-bromoindole compound. The reaction yield can reach medium to excellent, the chemical selectivity and regioselectivity of the reaction are excellent, the reaction conditions are mild, and the application range of a substrate is wide. The method has the advantages of simple operation, low cost, few side reactions, high product purity, convenient separation and purification, and suitableness for large-scale preparation, so the obtained product has a very good application prospect in the field of biological medicines.
Owner:PINGDINGSHAN UNIVERSITY +1
Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof
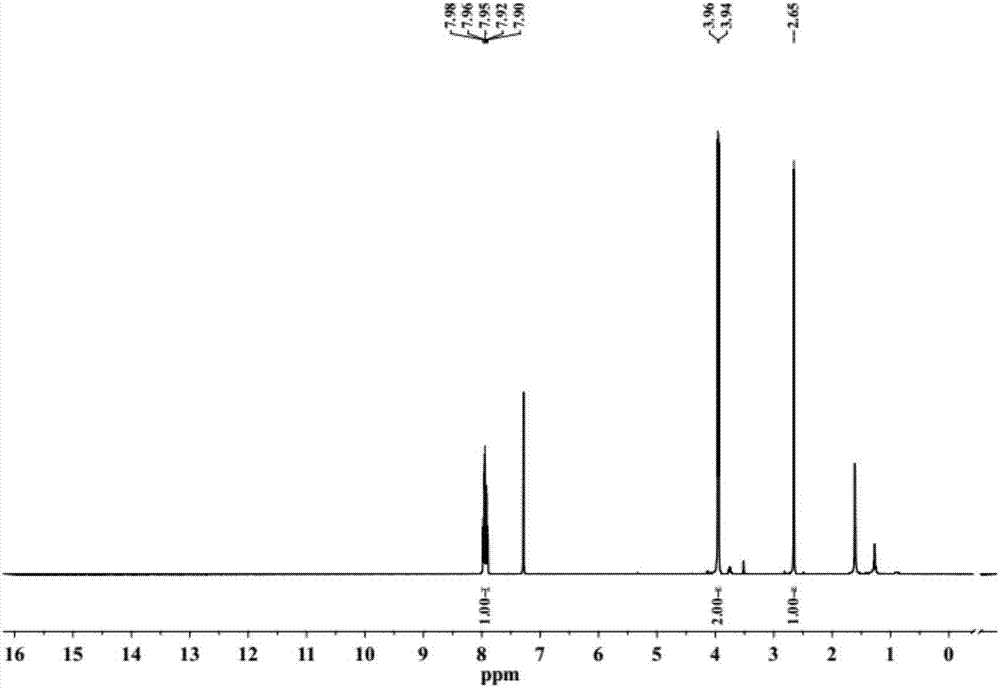
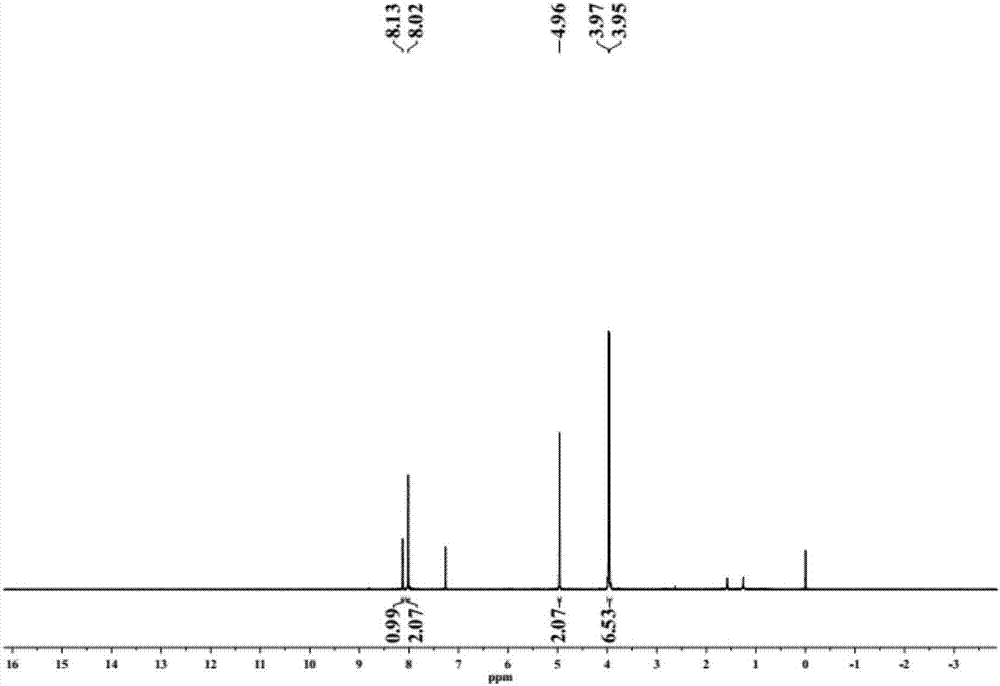
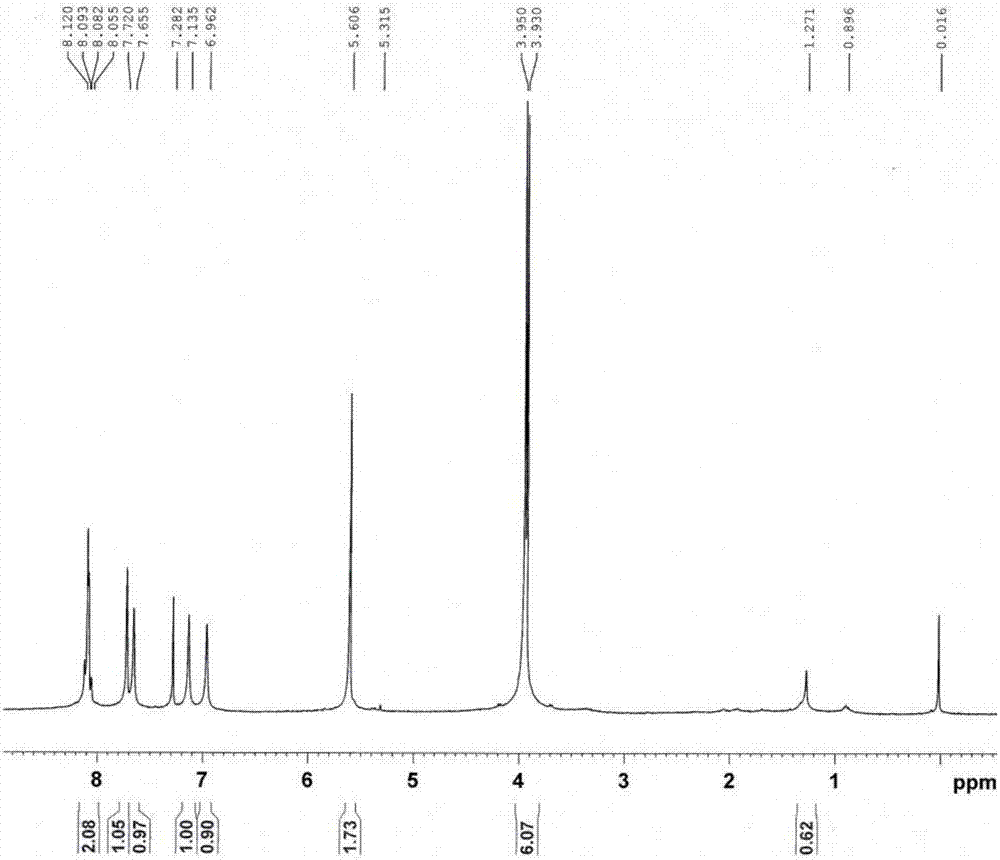
InactiveCN102127208AImprove solubilityEasy to manufactureFinal product manufactureSolid-state devicesSolubilityPolymer science
The invention discloses a cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer of a general formula (I) and a preparation method and use thereof. The preparation method comprises: reacting a diketone compound with 3,4-diaminothiophene.hydrogen chloride; subjecting the product of the reaction to bromination with N-bromosuccinimide (NBS) to obtain a target monomer; and underan oxygen-free condition, subjecting the 4,4-dialkyl-2,6-bis(trimethylstannyl)-cyclopenta(2,1-b:3,4-b')dithiophene, 4,4-dialkyl-2,6-dibromo-cyclopenta(2,1-b:3,4-b')dithiophene and the target monomer to polymerization to obtain the product disclosed by the invention. The preparation method disclosed by the invention is simple, makes operation and control easy, and is suitable for industrial production. Because a long-chain alkyl is introduced into the molecule of the polymer, the solubility and processability of the polymer are improved. The polymer can be widely used in fields of polymer solar cells and the like.
Owner:OCEANS KING LIGHTING SCI&TECH CO LTD +1
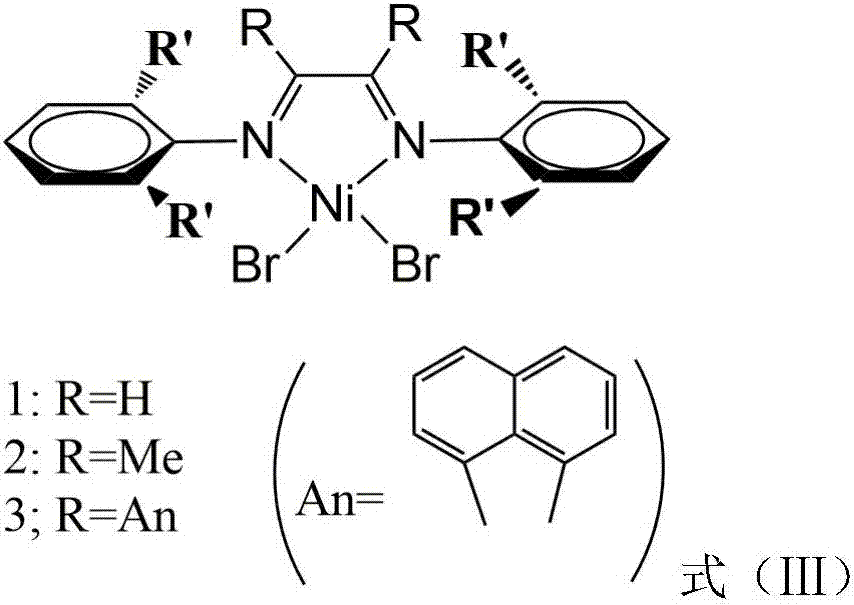
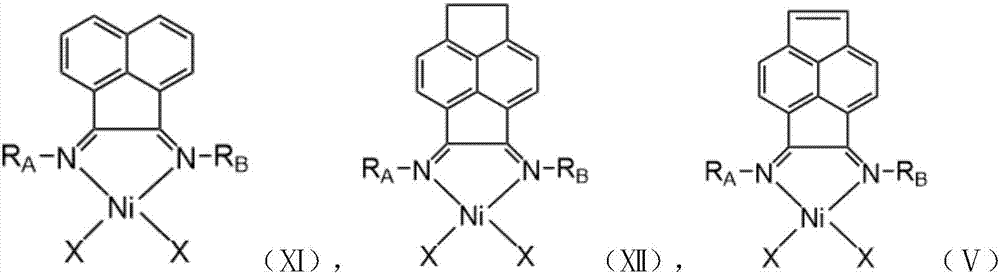
Improved (alpha-diimine) nickel catalyst as well as preparation method and application thereof
ActiveCN113429502AIncrease the degree of branchingHigh molecular weightNickel organic compoundsBulk chemical productionPolymer sciencePtru catalyst
Owner:ZHEJIANG UNIV
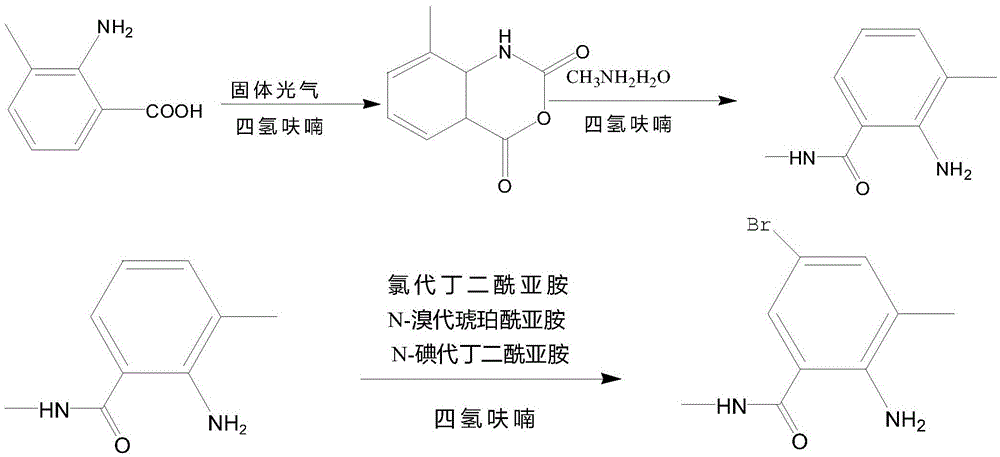
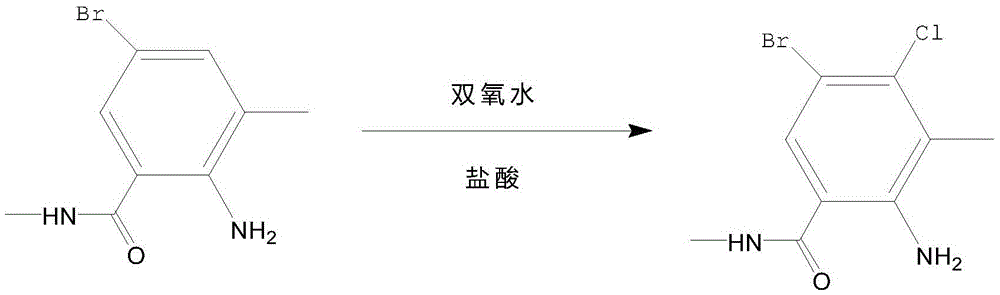
Synthetic method of 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide
InactiveCN105523951AOrganic compound preparationCarboxylic acid amides preparationN-BromosuccinimideChemical synthesis
The invention relates to a synthetic method of 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide and belongs to the field of chemical synthesis. The method comprises the following steps: using tetrahydrofuran and tetrahydrofuran as raw materials to react under phosgene catalytic action, adjusting pH by dropwise adding a sodium bicarbonate solution, simultaneously carrying out a reaction between chlorosuccinimide, N-iodosuccinimide and N-bromosuccinimide, carrying out suction filtration, and oxidizing so as to prepare 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide. The method has advantages of simple operation and high synthesis rate.
Owner:CHANGZHOU UNIV
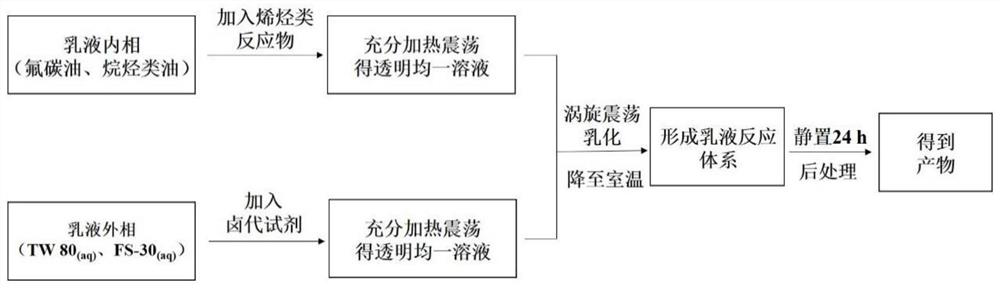
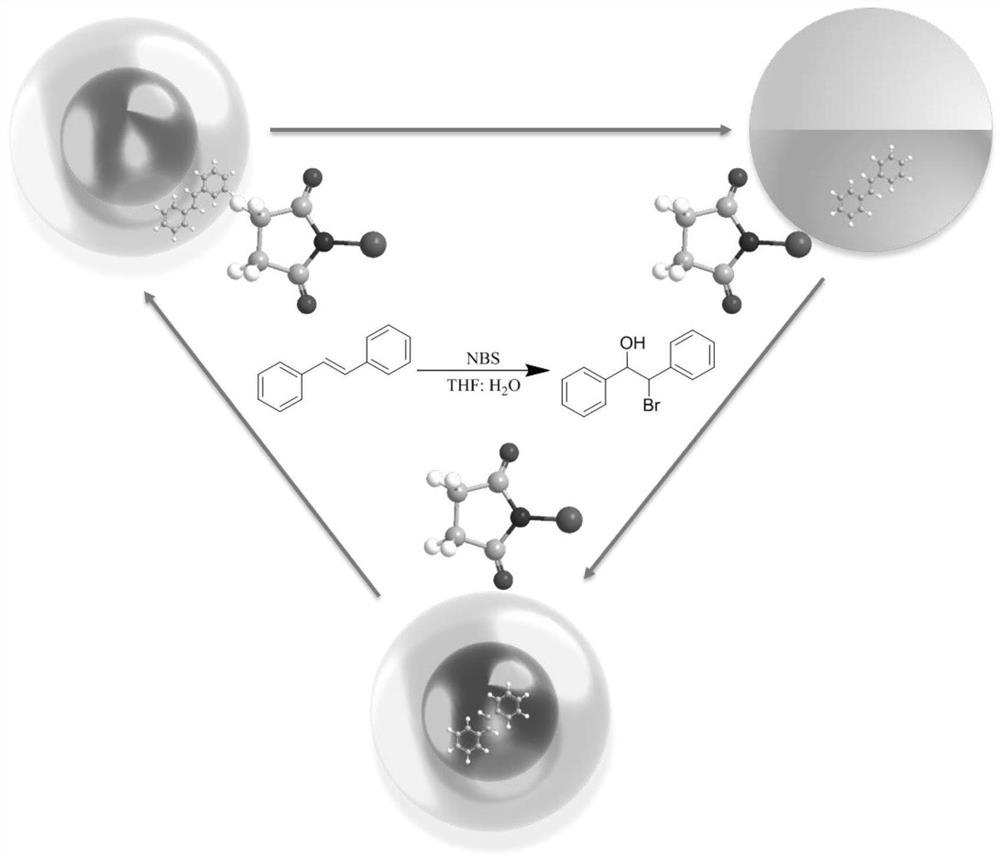
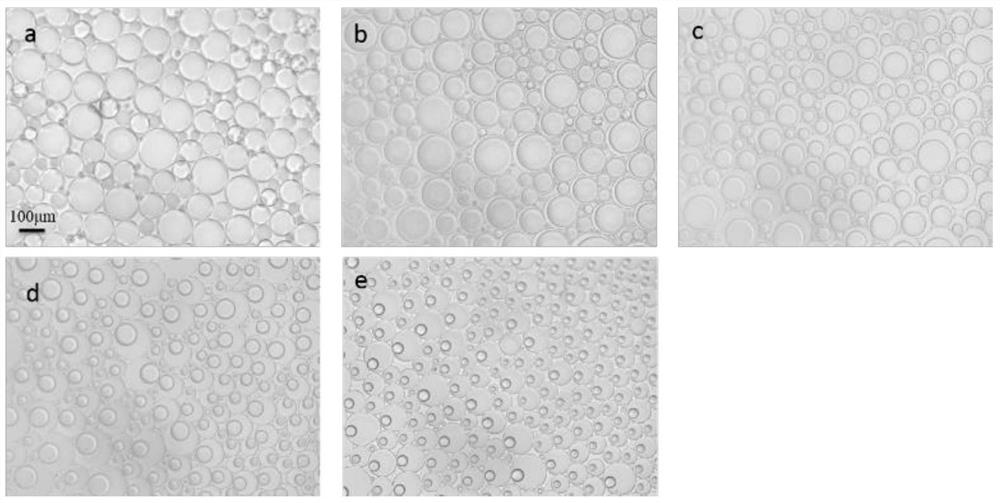
Method for synthesizing beta-brominated alcohol by anisotropic emulsion microreactor
ActiveCN113149817AChemical/physical/physico-chemical microreactorsPreparation by OH and halogen introductionActive agentDissolution reaction
The invention discloses a method for synthesizing beta-brominated alcohol by an anisotropic emulsion microreactor. Anisotropic emulsion liquid drops are used as a micro-reactor, reaction raw materials, namely olefin and N-bromosuccinimide (NBS), are selectively dissolved in an n-heptane phase and a surfactant aqueous solution phase which are mutually insoluble respectively, fluorocarbon oil is selected as a third phase, an anisotropic emulsion with fluorocarbon oil / n-heptane as an internal phase and a surfactant aqueous solution as an external phase is prepared by a one-step vortex oscillation method; two reaction raw materials are selectively solubilized and isolated by a multi-phase microcell of anisotropic emulsion droplets, meanwhile, the characteristics of multi-phase interfaces and multi-microstructures of the anisotropic droplets are fully exerted, so that static controllable synthesis of beta-brominated alcohol is realized without continuous stirring. The method has the advantages of simplicity and convenience in operation, low energy consumption, controllable reaction rate and easiness in product recovery.
Owner:YANGZHOU UNIV
Preparation method of high-performance carboxyl-terminated low-molecular-weight fluorine-containing polymer
ActiveCN112279944AAchieve saturationHigh saturation rateAdditive manufacturing apparatusPolymer scienceTetrafluoroborate
The invention discloses a preparation method of a high-performance carboxyl-terminated low-molecular-weight fluorine-containing polymer, belonging to a polymer preparation method. According to the invention, a carboxyl-terminated low-molecular-weight fluorine-containing polymer containing double bonds in a chain and prepared by an oxidative degradation method is used as a raw material, a fluorinated addition reaction system and method for the fluorine-containing polymer are created, N-fluoro-N'-(chloromethyl)triethylenediamine bis(tetrafluoroborate) or a hydrogen fluoride complex and the likeare used as fluorinating reagents, lithium aluminum hydride or borohydride is used as a hydrogenation reagent, silver fluoride and the like are used as nucleophilic reagents, and N-bromosuccinimide and the like are used as electrophilic reagents, so a fluorine content is increased while saturation of double bonds in a chain is realized, and the thermal stability of the polymer is greatly improved.The method is simple in process, mild in reaction conditions and high in efficiency; a saturation rate reaches 80% or above, the fluorine content of the produced polymer is higher than 66%, and the polymer can serve as a fluorine-containing precursor of a functional polymer and an additive manufacturing (3D printing) raw material and can also be applied as a high-performance adhesive, a joint mixture, a coating, a processing compounding agent and the like.
Owner:SHENYANG INSTITUTE OF CHEMICAL TECHNOLOGY
Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof
The invention relates to a tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof. The derivative is prepared by the steps that ethyl cyanoacetate serves as an initial raw material, a hydroxylamine compound (A) is generated under the action of sodium nitrite and phosphoric acid, ethyl 2-aminocyanoacetate (B) is obtained through reduction of sodium hydrosulfite and reacts with acetic anhydride and a Lawesson's reagent separately to obtain5-amino-4-formate thiazole compounds (D), NBS (N-bromosuccinimide) bromination is conducted, a 2-bromo-dihydropyrrolo[1,2-a]thiazolo[5,4-d]pyrimidinone compound (F) and a 2-bromo-7,8-dihydro-5H-pyridino[1,2-a]thiazolo[5,4-d]pyrimidin-10(6H)-one derivative (G) are obtained under the action of phosphorus oxychloride, and finally Suzuki coupling reaction is conducted to obtain 64 differently-substituted tricyclic thiazolo[5,4-d]pyrimidone derivatives H1-H32 and I1-I32. The inhibitory activity of the 64 compounds on acetylcholinesterase / butyrylcholinesterase in Alzheimer's disease and the antibacterial activity of Candida albicans, and the result shows that 46 compounds have strong inhibitory activity on acetylcholinesterase / butyrylcholinesterase, and 27 compounds have inhibitory activity on candida albicans.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
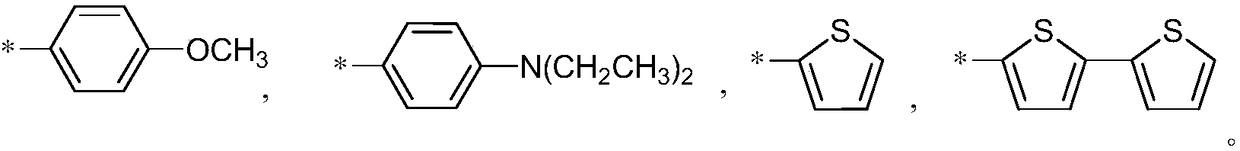

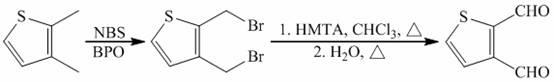
![Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one](https://images-eureka.patsnap.com/patent_img/ed4ea338-e424-46e2-9058-737b9b871a63/2013800611386100002DEST_PATH_IMAGE002.PNG)
![Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one](https://images-eureka.patsnap.com/patent_img/ed4ea338-e424-46e2-9058-737b9b871a63/2013800611386100002DEST_PATH_IMAGE004.PNG)
![Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one Process for the preparation of (1S,4S,5S)-4-bromo-6-oxabicyclo[3.2.1]octan-7-one](https://images-eureka.patsnap.com/patent_img/ed4ea338-e424-46e2-9058-737b9b871a63/2013800611386100002DEST_PATH_IMAGE006.PNG)
![Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof](https://images-eureka.patsnap.com/patent_img/1e24a6bd-cff5-452f-ba99-1ae2bf6dca9c/H2010100444362E00011.png)
![Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof](https://images-eureka.patsnap.com/patent_img/1e24a6bd-cff5-452f-ba99-1ae2bf6dca9c/H2010100444362E00012.png)
![Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof Cyclopenta(2,1-b:3,4-b')dithiophene-thieno[3,4-b]pyrazine conjugated polymer and preparation method and use thereof](https://images-eureka.patsnap.com/patent_img/1e24a6bd-cff5-452f-ba99-1ae2bf6dca9c/E2010100444362B00011.png)
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/FDA0003323019360000011.png)
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/FDA0003323019360000021.png)
![Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof Tricyclic thiazolo[5,4-d]pyrimidone derivative and application thereof](https://images-eureka.patsnap.com/patent_img/2b2a1369-2132-497a-9110-e6d46991144f/BDA0003323019370000041.png)