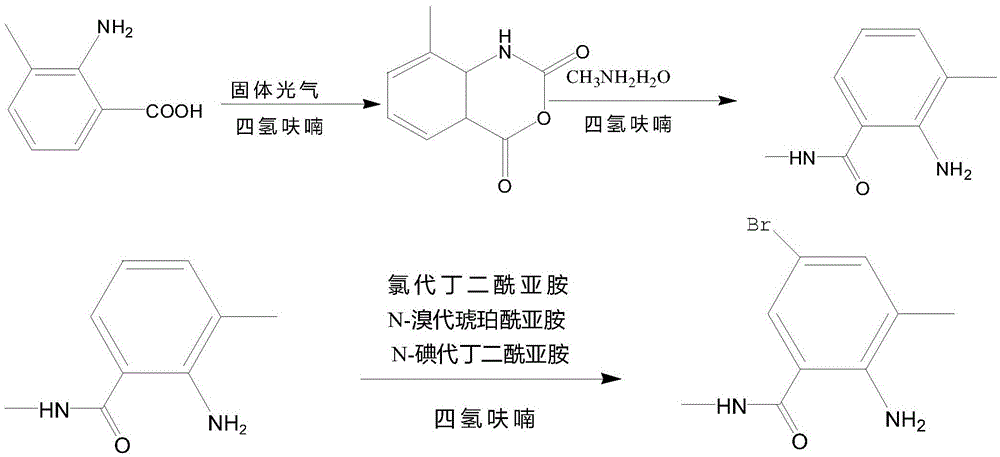
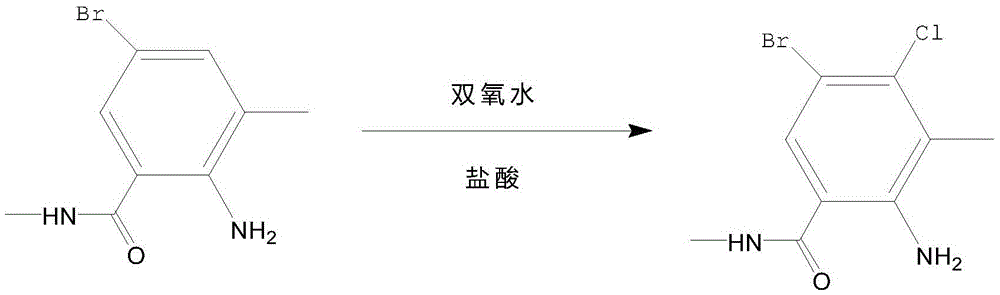
Synthetic method of 2-amino-N,3-dimethyl-4-chloro-5-bromobenzamide
A technology of bromobenzamide and synthesis method, which is applied in the field of chemical synthesis, and can solve the problems of no ideal industrial production method, difficulty in treatment, backward technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0018] Add 100mL of tetrahydrofuran to a 250mL three-necked flask equipped with a thermometer, and then add 8g of methyl anthranilate, and stir and mix at 800r / min for 10min at 20°C; after the stirring and mixing are completed, slowly add 2g of bis(trichloromethyl)carbonate was stirred and reacted at 20°C, and at a speed of 1mL / s, slowly dropwise added a sodium hydroxide solution with a mass concentration of 25% into a three-necked flask, and continued to stir for 45min; After the stirring is completed, it is monitored by thin-layer chromatography. If the reaction is not completed, continue to stir until the reaction is completed. After the reaction is completed, continue to dropwise add a mass concentration of 25% sodium bicarbonate solution until the pH of the reaction solution is 8; After the adjustment is completed, weigh 20 g of an aqueous solution of methylamine with a mass concentration of 20% into a three-necked flask, stir and react for 30 minutes, then pass through ni...
example 2
[0020] Add 110mL of tetrahydrofuran to a 250mL three-necked flask equipped with a thermometer, and then add 9g of methyl anthranilate, and stir magnetically at a speed of 1000r / min for 12min at 25°C; after the stirring and mixing are completed, slowly add 2.5 g of bis(trichloromethyl)carbonate was stirred and reacted at 25°C, and a sodium hydroxide solution with a mass concentration of 25% was slowly dropped into a three-necked flask at a rate of 1 mL / s, and the stirring was continued for 52 min; After the stirring is completed, it is monitored by thin-layer chromatography. If the reaction is not completed, continue to stir until the reaction is completed. After the reaction is completed, continue to add dropwise a sodium bicarbonate solution with a mass concentration of 25% until the pH of the reaction solution is 8.5; After the pH adjustment is completed, weigh 22g of an aqueous solution of methylamine with a mass concentration of 20% into a three-necked flask, stir and react...
example 3
[0022]Add 120mL of tetrahydrofuran to a 250mL three-necked flask equipped with a thermometer, and then add 10g of methyl anthranilate, and stir and mix with a magnetic force at a speed of 1200r / min for 15min at 30°C; after the stirring and mixing are completed, slowly add 3g of bis(trichloromethyl)carbonate was stirred and reacted at 30°C, and at a speed of 1mL / s, slowly dropwise added a sodium hydroxide solution with a mass concentration of 25% into a three-necked flask, and continued to stir for 60min; After the stirring is completed, it is monitored by thin-layer chromatography. If the reaction is not completed, continue to stir until the reaction is completed. After the reaction is completed, continue to add dropwise a sodium bicarbonate solution with a mass concentration of 25% until the pH of the reaction solution is 9; After the adjustment is completed, weigh 25g of a 20% methylamine aqueous solution into a three-necked flask, stir and react for 45min, and then pass thro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com