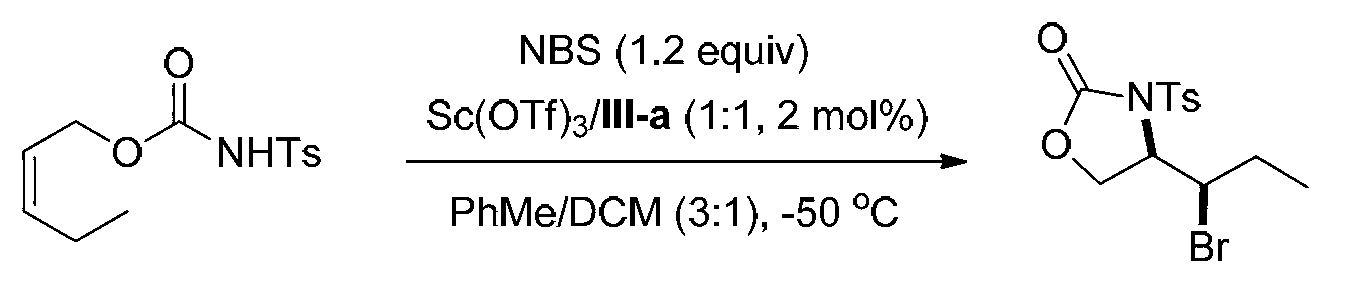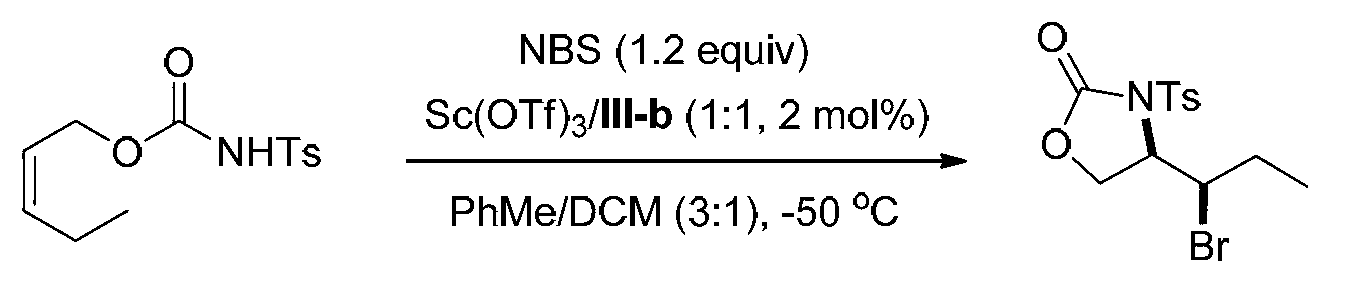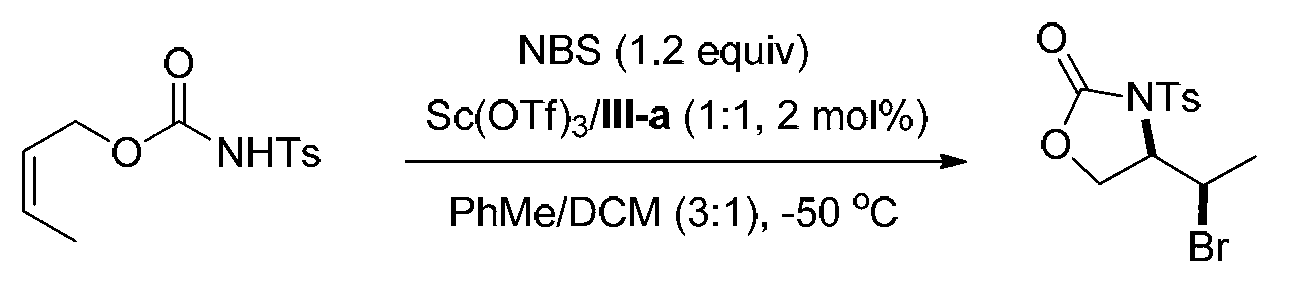Derivative containing halogen light active oxazolidinone and preparation method thereof
A kind of compound, the technology of basic conditions, applied in the field of halogen-containing photoactive oxazolinone derivatives and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Embodiment 1, synthetic compound formula I-a (see structural formula I-a)
[0079]
[0080] Ts in the above formula represents p-toluenesulfonyl.
[0081] Weigh into the reaction test tube ligand (shown in formula III-a) (0.00691g, 0.01mmol) and Sc (OTf) 3 (0.00492g, 0.01mmol), a mixed solvent of PhMe / DCM=3 / 1 (v / v, 4.0mL) was added, and stirred at 0°C for 0.5h. Add NBS (0.1068g, 0.60mmol), stir at 0°C for 5min and cool to -50°C, then add the olefin substrate (shown in formula II-a) (0.1415g, 0.5mmol) in PhMe / DCM=3 / 1 (v / v, 1.0 mL) solution, reacted at -50°C for 48h. After the reaction, add triethylamine (0.5mL) to quench the reaction, directly spin dry and perform column chromatography (eluent is petroleum ether: ethyl acetate=5 / 1, v / v) to obtain 0.1589g white solid oxazole The phenone product (shown by formula I-a) has a yield of 88% and an enantiomeric excess of 96% (as determined by HPLC).
[0082] HPLC conditions: chiral AD-H column, mobile phase: mixed solven...
Embodiment 2
[0085] Embodiment 2, synthetic compound formula I-a (see structural formula I-a) by ligand formula III-b
[0086]
[0087] Ts in the above formula represents p-toluenesulfonyl.
[0088] Weigh the ligand (shown in formula III-b) (0.00788g, 0.01mmol) and Sc(OTf) in the reaction test tube 3 (0.00492g, 0.01mmol), a mixed solvent of PhMe / DCM=3 / 1 (v / v, 4.0mL) was added, and stirred at 25°C for 0.5h. Add NBS (0.1068g, 0.60mmol), stir at 25°C for 5min and cool to -50°C, then add the olefin substrate (shown in formula II-a) (0.1415g, 0.5mmol) in PhMe / DCM=3 / 1 (v / v, 1.0 mL) solution, reacted at -50°C for 48h. After the reaction, add triethylamine (0.5mL) to quench the reaction, directly spin dry and perform column chromatography (eluent is petroleum ether: ethyl acetate=5 / 1, v / v) to obtain 0.1532g white solid oxazole The phenone product (shown by formula I-a) has a yield of 85% and an enantiomeric excess of 97% (as determined by HPLC).
[0089] HPLC conditions: chiral AD-H column,...
Embodiment 3
[0091] Embodiment 3, synthetic compound formula I-b (see structural formula I-b)
[0092]
[0093] Ts in the above formula represents p-toluenesulfonyl.
[0094] Weigh into the reaction test tube ligand (shown in formula III-a) (0.00691g, 0.01mmol) and Sc (OTf) 3(0.00492g, 0.01mmol), a mixed solvent of PhMe / DCM=3 / 1 (v / v, 4.0mL) was added, and stirred at 0°C for 0.5h. Add NBS (0.1068g, 0.60mmol), stir at 0°C for 5min and cool to -50°C, then add the olefin substrate (shown by formula II-b) (0.1345g, 0.5mmol) in PhMe / DCM=3 / 1 (v / v, 1.0 mL) solution, reacted at -50°C for 72h. After the reaction, add triethylamine (0.5mL) to quench the reaction, directly spin dry and perform column chromatography (eluent is petroleum ether: ethyl acetate=5 / 1, v / v) to obtain 0.1381g white solid oxazole The phenone product (shown by formula I-b) has a yield of 79% and an enantiomeric excess of 96% (as determined by HPLC).
[0095] HPLC conditions: chiral AD-H column, mobile phase: mixed solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com