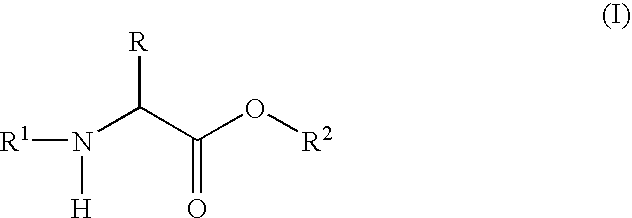
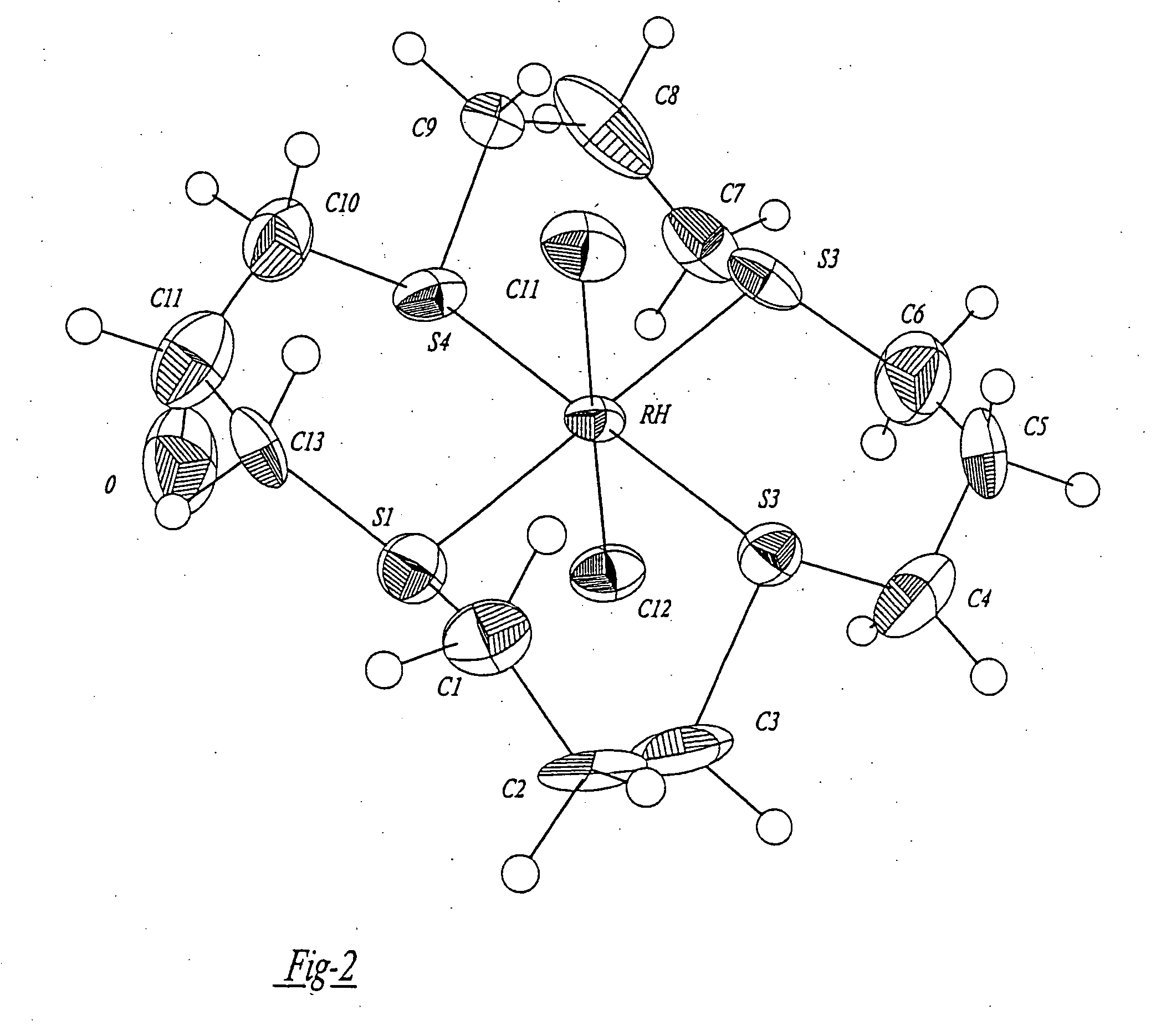
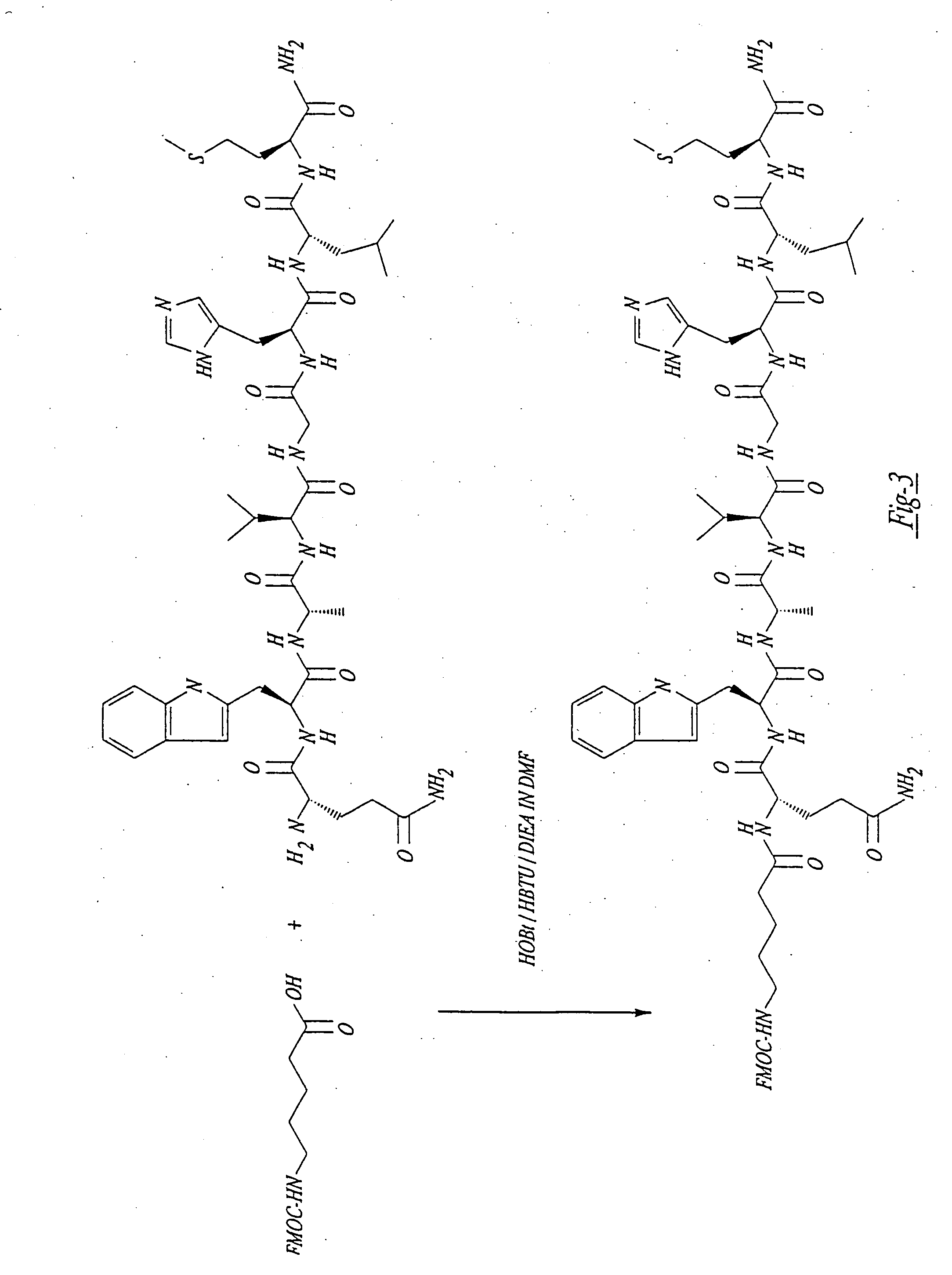
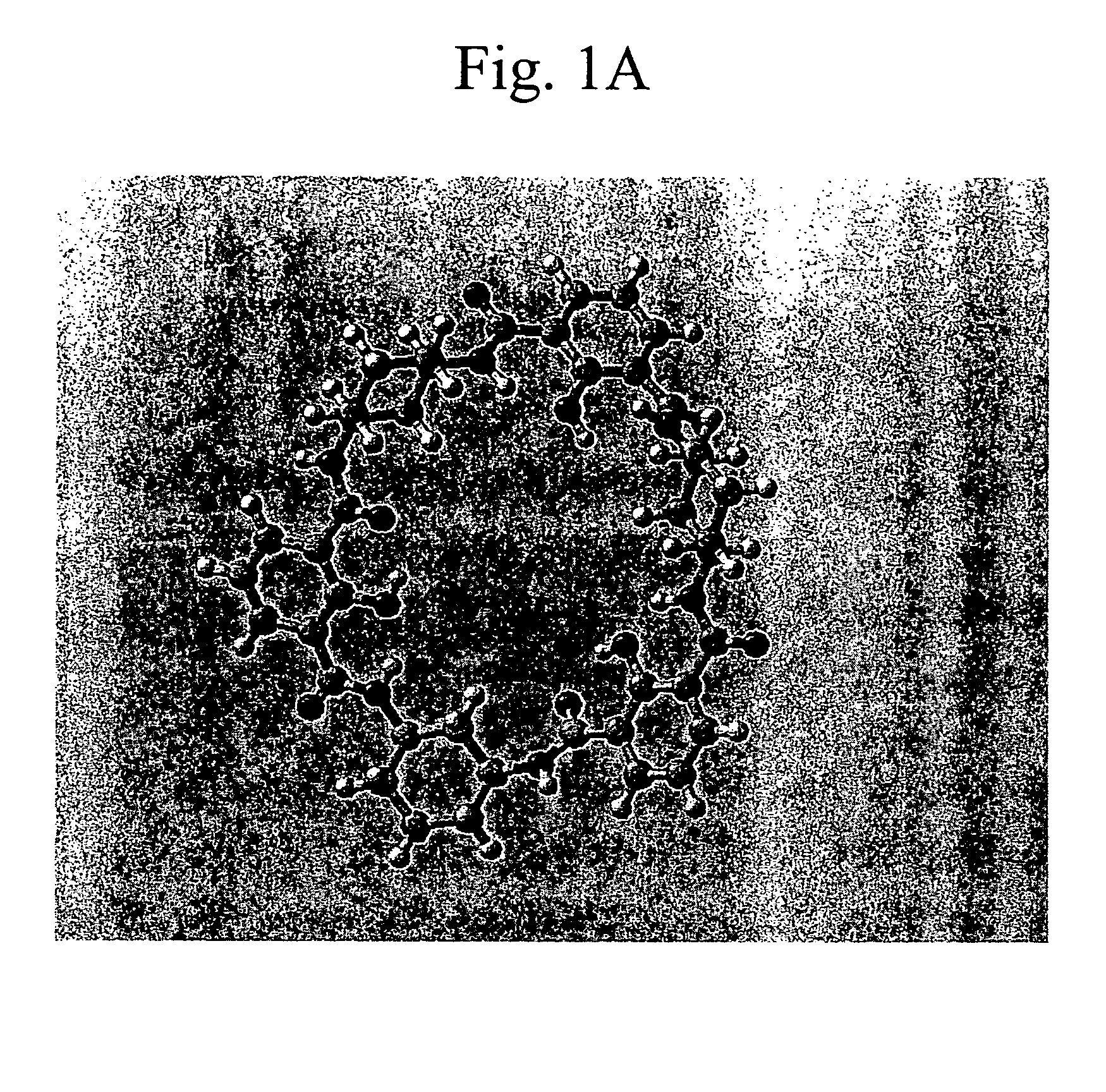
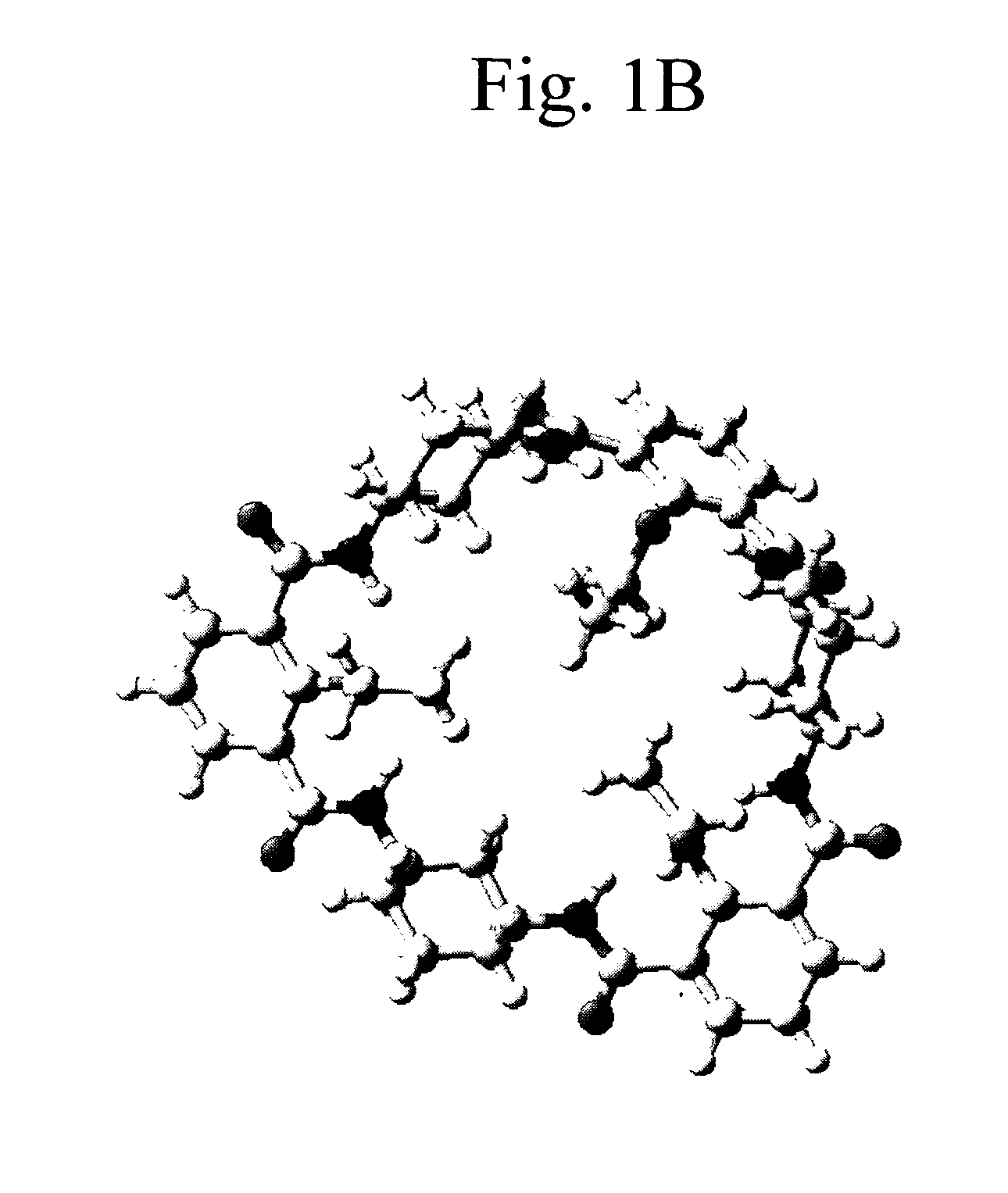
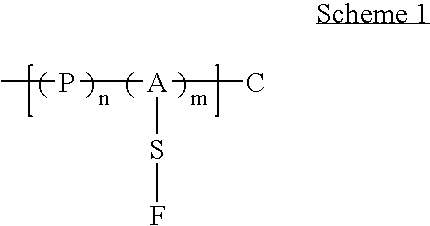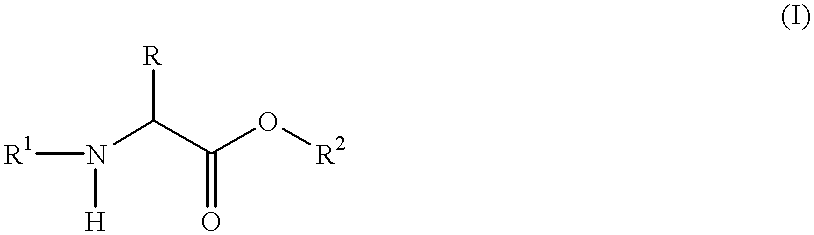Patents
Literature
127 results about "Synthon" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
In retrosynthetic analysis, a synthon is a destructural unit within a molecule which is related to a possible synthetic operation. The term was coined in 1967 by E. J. Corey. He noted in 1988 that the "word synthon has now come to be used to mean synthetic building block rather than retrosynthetic fragmentation structures". It was noted in 1998 that the phrase did not feature very prominently in Corey's 1981 book The Logic of Chemical Synthesis, as it was not included in the index. Because synthons are charged, when placed into a synthesis a neutral form is found commercially instead of forming and using the potentially volatile charged synthons.
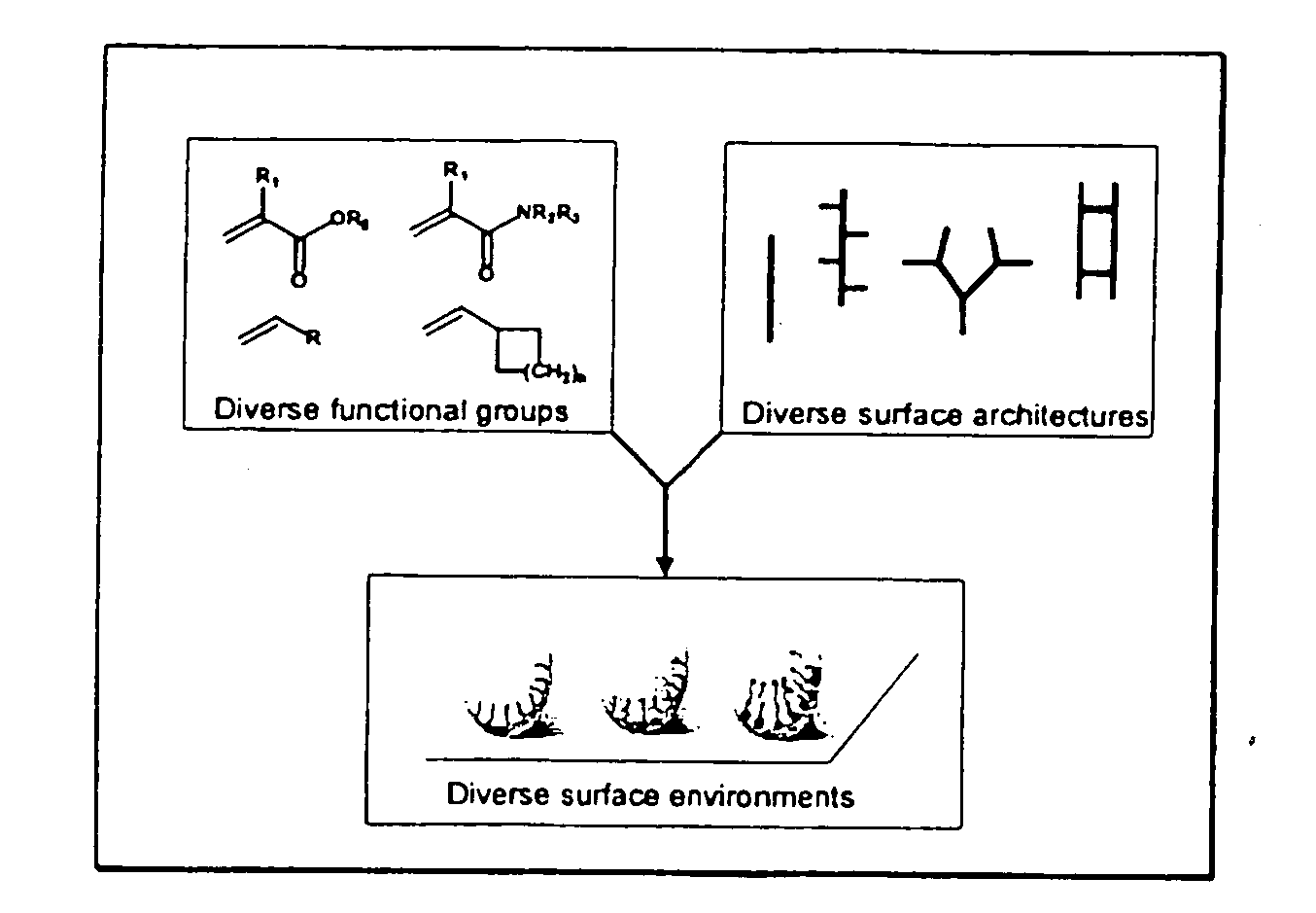
Generation of surface coating diversity
InactiveUS20060083858A1Generation of surfaceControl generationMembranesSequential/parallel process reactionsChemical compositionSynthon
The present invention relates to a surface discovery system comprising chemical compositions and high-throughput combinatorial synthesis methods for generating large numbers of diverse surface coatings on solid substrates. This surface discovery platform is built upon a fundamental chemical unit refereed to as a synthon. Each synthon comprises at least three elements: a chemical backbone coating on the solid substrate that comprises a passive (P) constituent and an active (A) constituent; a spacer unit (S) separating the backbone from a functional group; and a functional group (F). Variation of these synthon elements allows generation of large libraries surface coatings with a broad range of molecular and macroscopic properties. Further the spectrum of surfaces provided by the invention permits optimization of the wide range of solid-phase applications that involve surface immobilization of molecules.
Owner:ANTEO TECH
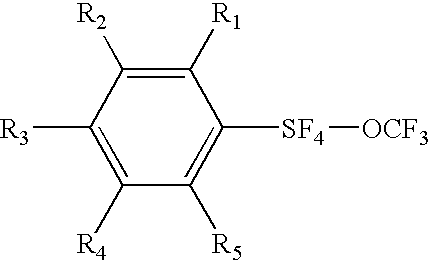
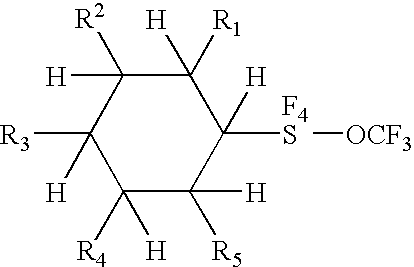
Alkyl and aryl trifluoromethoxytetrafluorosulfuranes
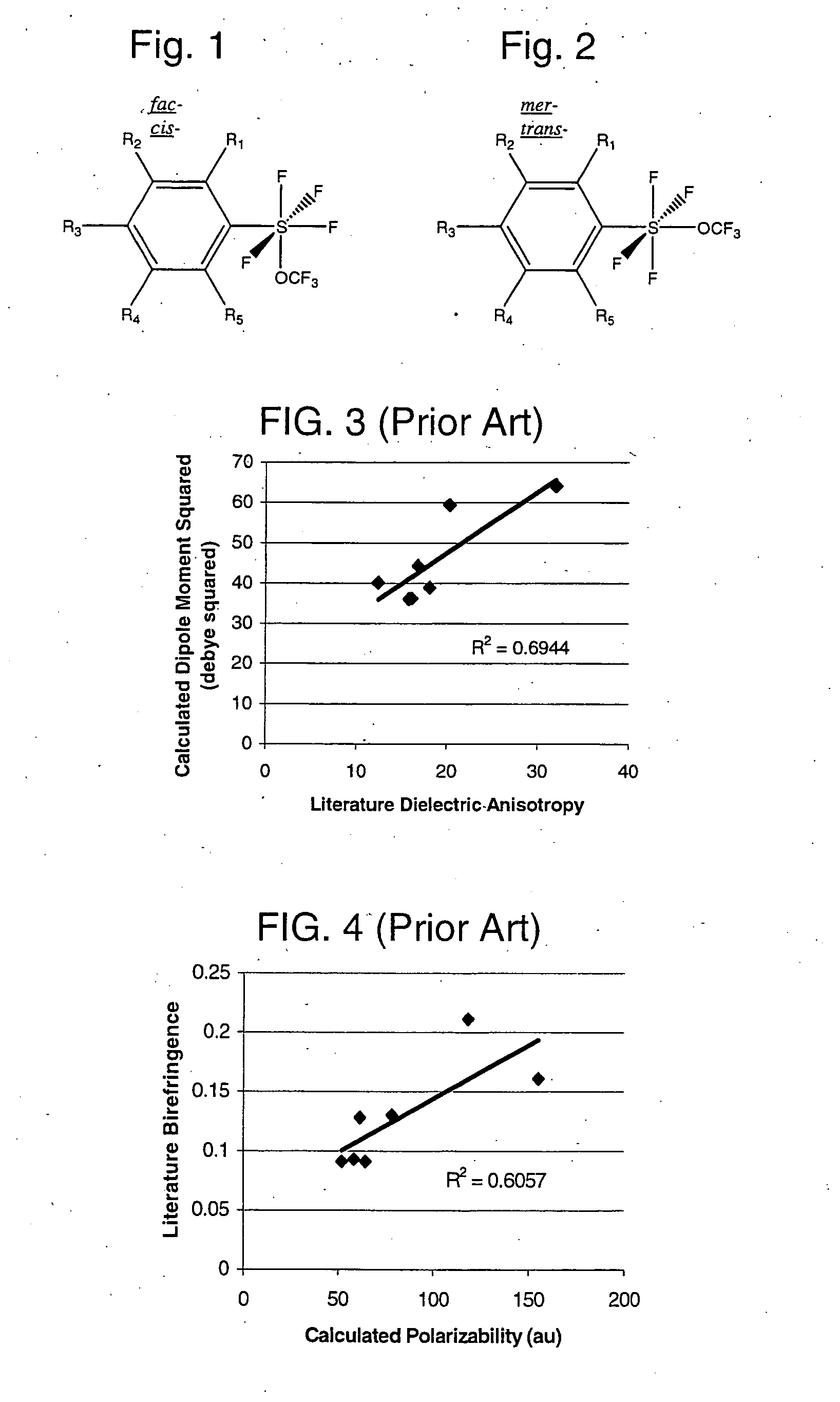
Novel compositions containing SF4—O—CF3 bonded to an organic group are disclosed. Aryl trifluoromethoxytetrafluorosulfuranes (or Ar—SF4—O—CF3), have been synthesized via the reaction of an aryl disulfide or thiol with fluoroxytrifluoromethane (F3COF). The compositions are useful synthons, which may be derivatized to yield highly electrically polar molecules, particularly novel liquid crystal compositions having high dielectric anisotropies. Cycloalkyl trifluoromethoxytetrafluorosulfuranes have similar utility.
Owner:AIR PROD & CHEM INC
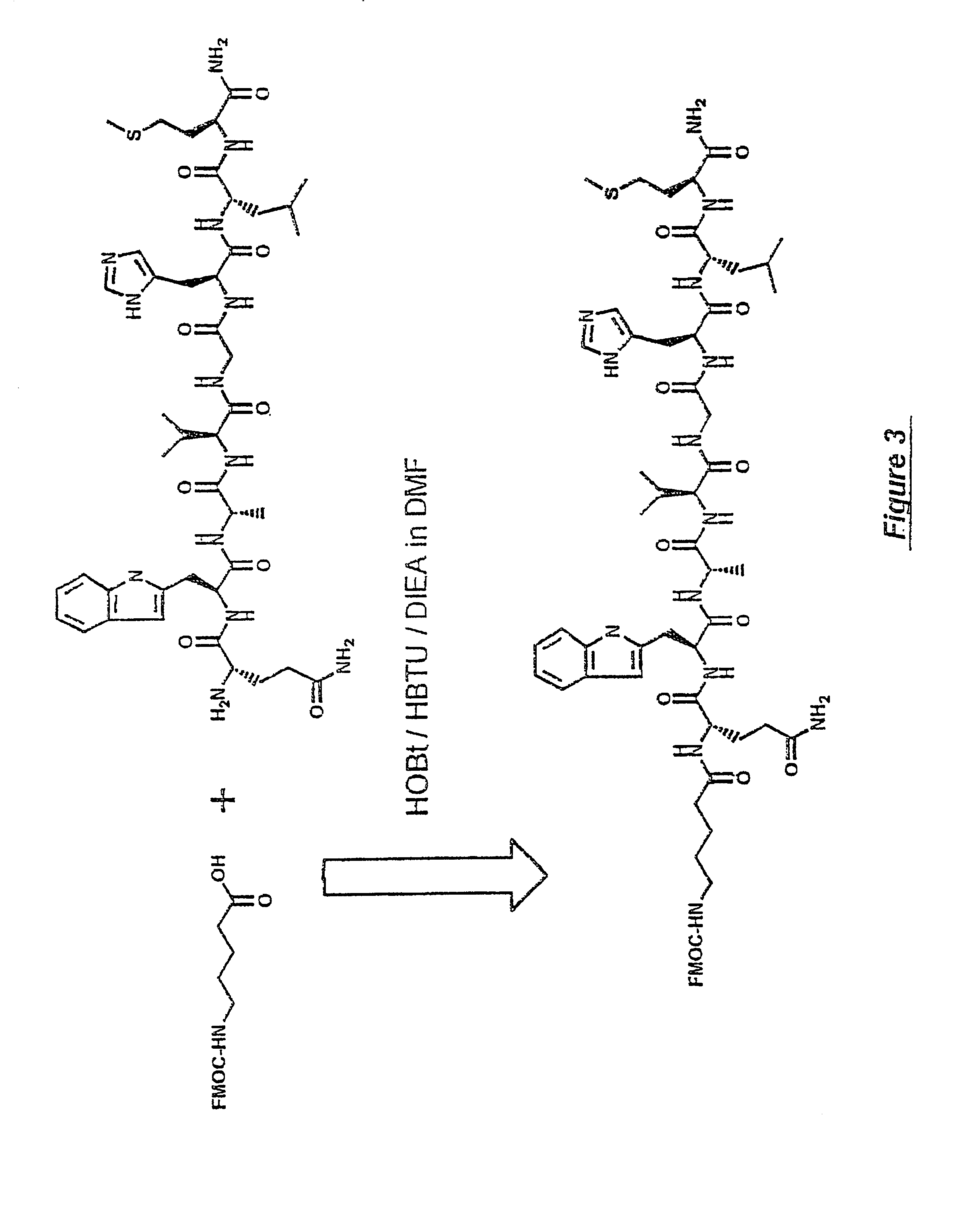
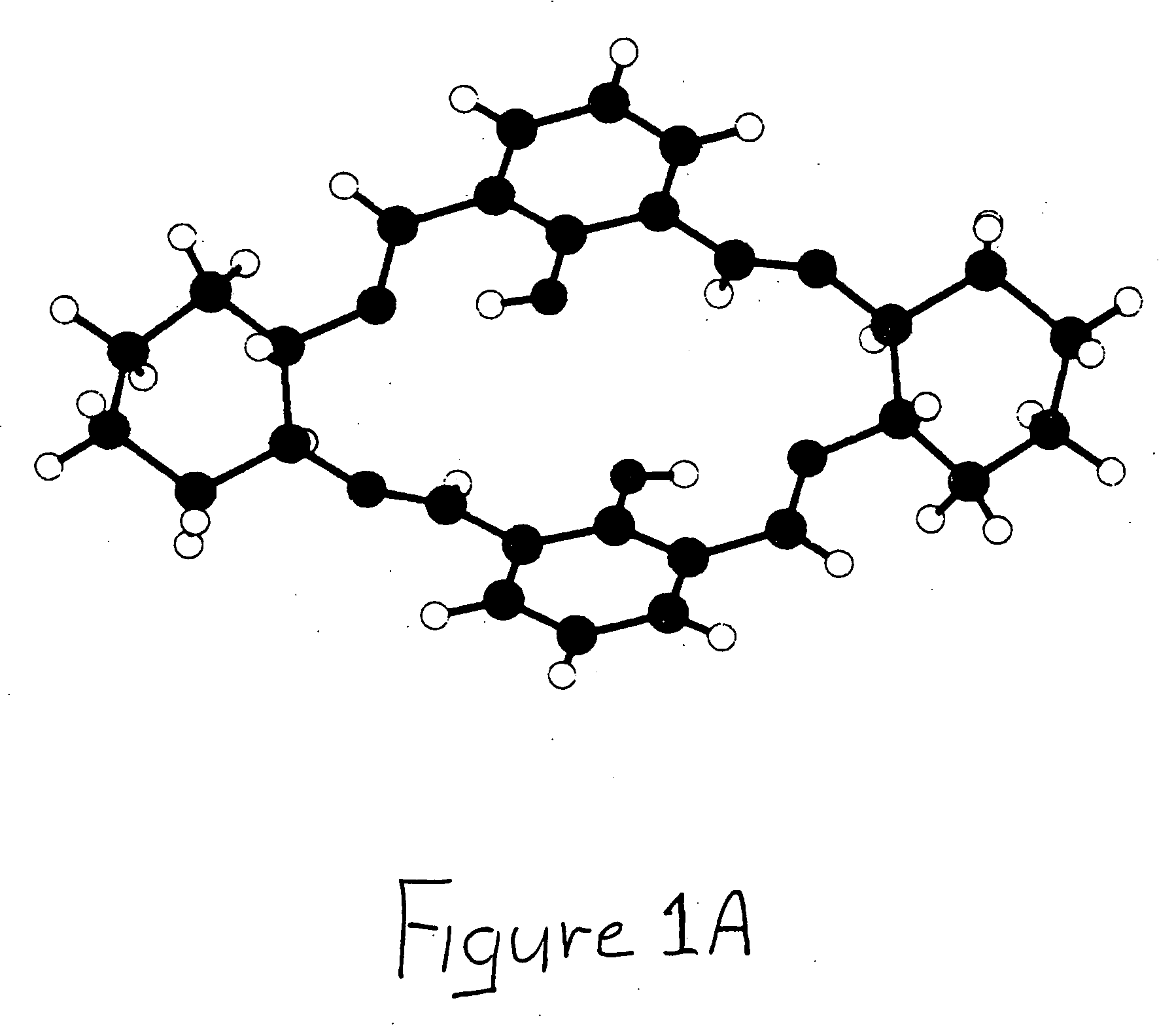
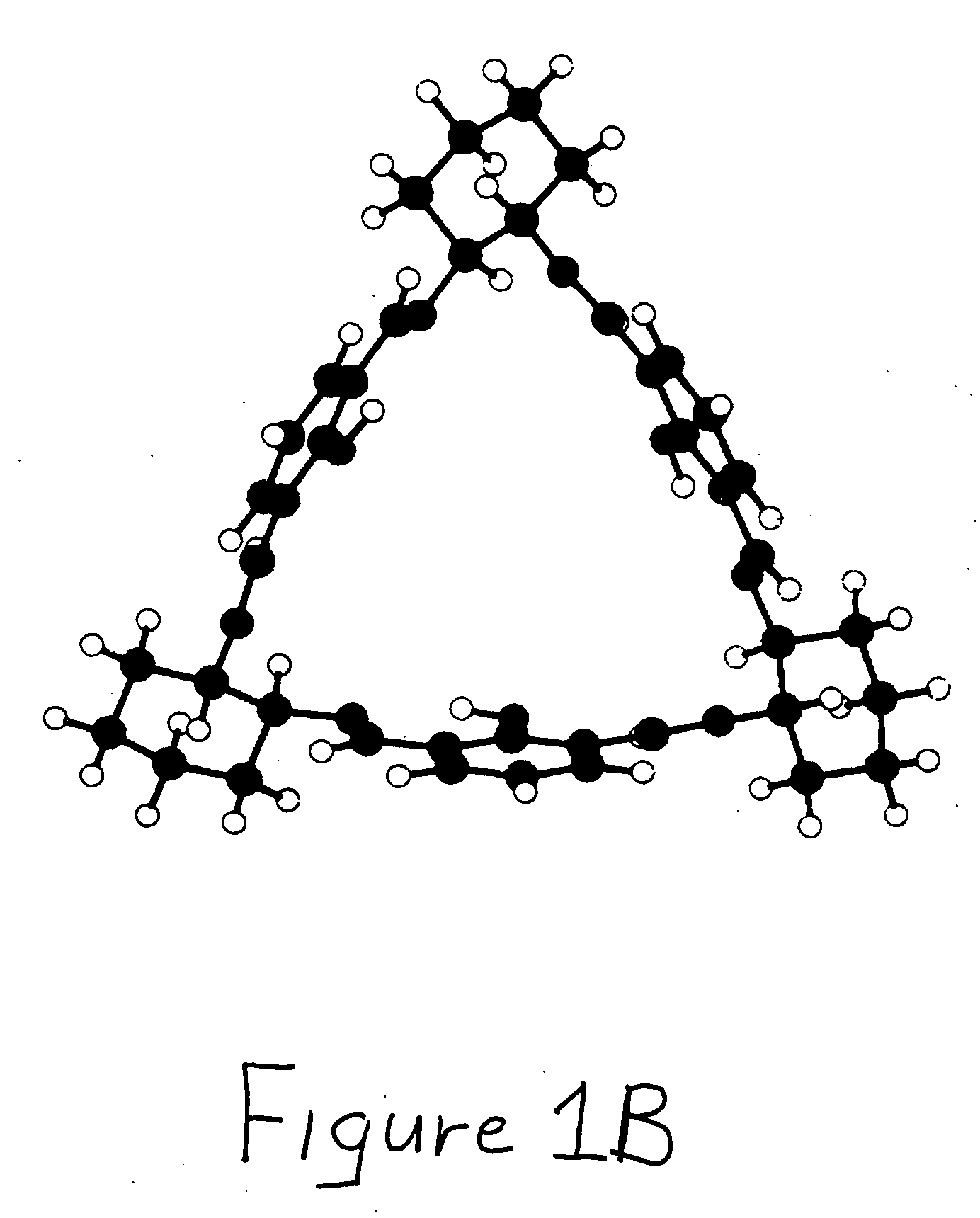
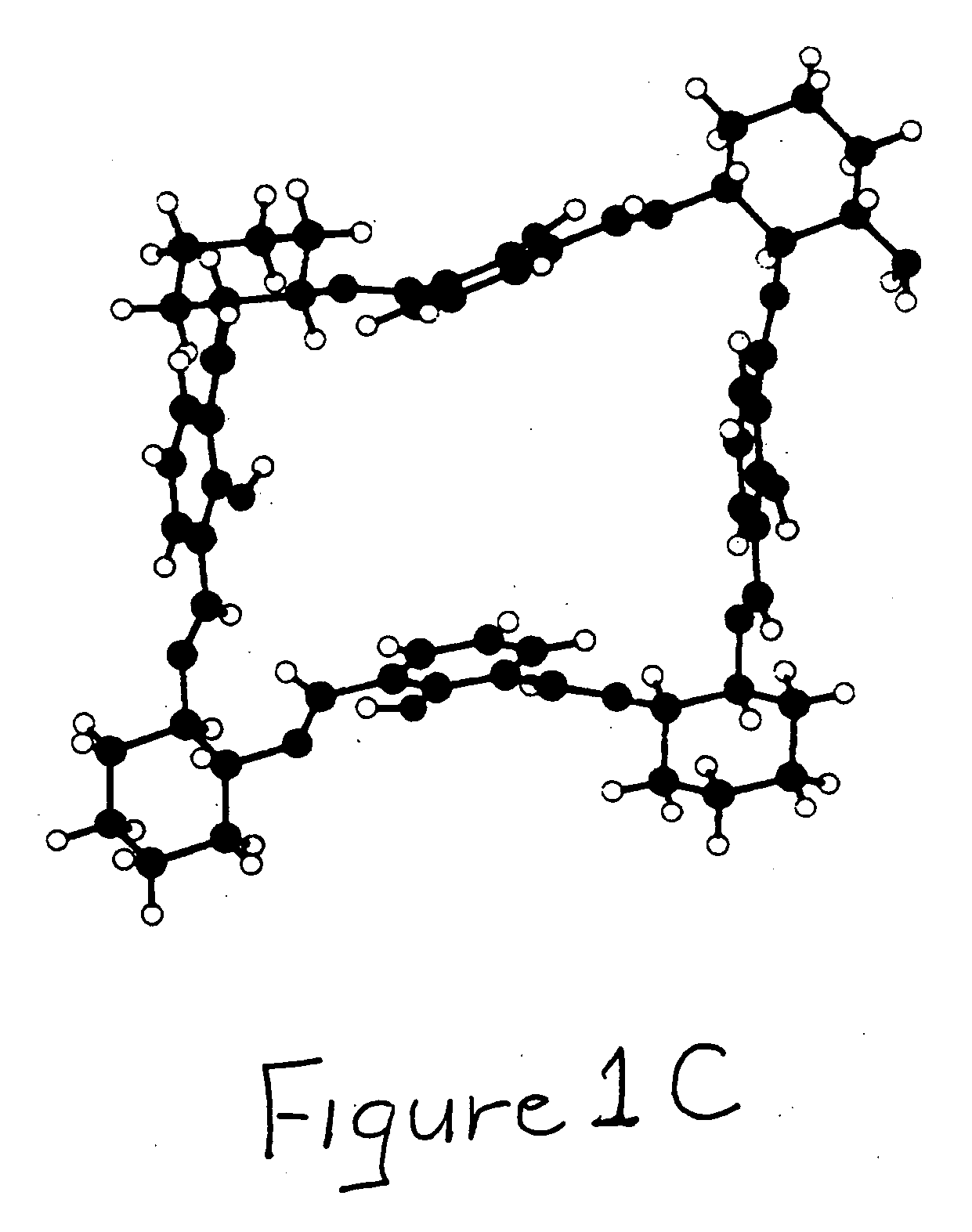
Bridged macrocyclic module compositions
This invention is related to the fields of organic chemistry and nanotechnology. In particular, it relates to materials and methods for the preparation of organic synthons and bridged macrocyclic module compounds. The bridged macrocyclic module compounds may be used to prepare macrocyclic compositions such as nanofilms, which may be useful for filtration.
Owner:COVALENT PARTNERS LLC
Forward synthetic synthon generation and its useto identify molecules similar in 3 dimensional shape to pharmaceutical lead compounds
ActiveUS20080172216A1Increase richnessIncrease diversityMolecular designChemical structure searchChemical reactionSynthesis methods
A forward synthetic method is described that utilizes recursive application of established organic chemical reactions to derive more complex synthons from available reagents than are available from the reagent synthons themselves. The product of each reaction serves as the starting point for further reactions thereby permitting the generation of multiple complex molecular structures. This synthon generation procedure typically yields 20 ? 30 new structures within the limits of easily accessible syntheses based upon each starting reagent. More complex syntheses yield even more structures. The generated synthons are characterized with a molecular structural descriptor possessing a neighborhood property and can be further characterized with features. The synthons are searched for three dimensional shape and feature similarity to molecular fragments derived from query molecules, typically pharmacological molecules of interest. Identified synthons can be assembled into molecules possessing the same three dimensional shape and likely activity as the molecule of interest.
Owner:CRAMER RICHARD D +1
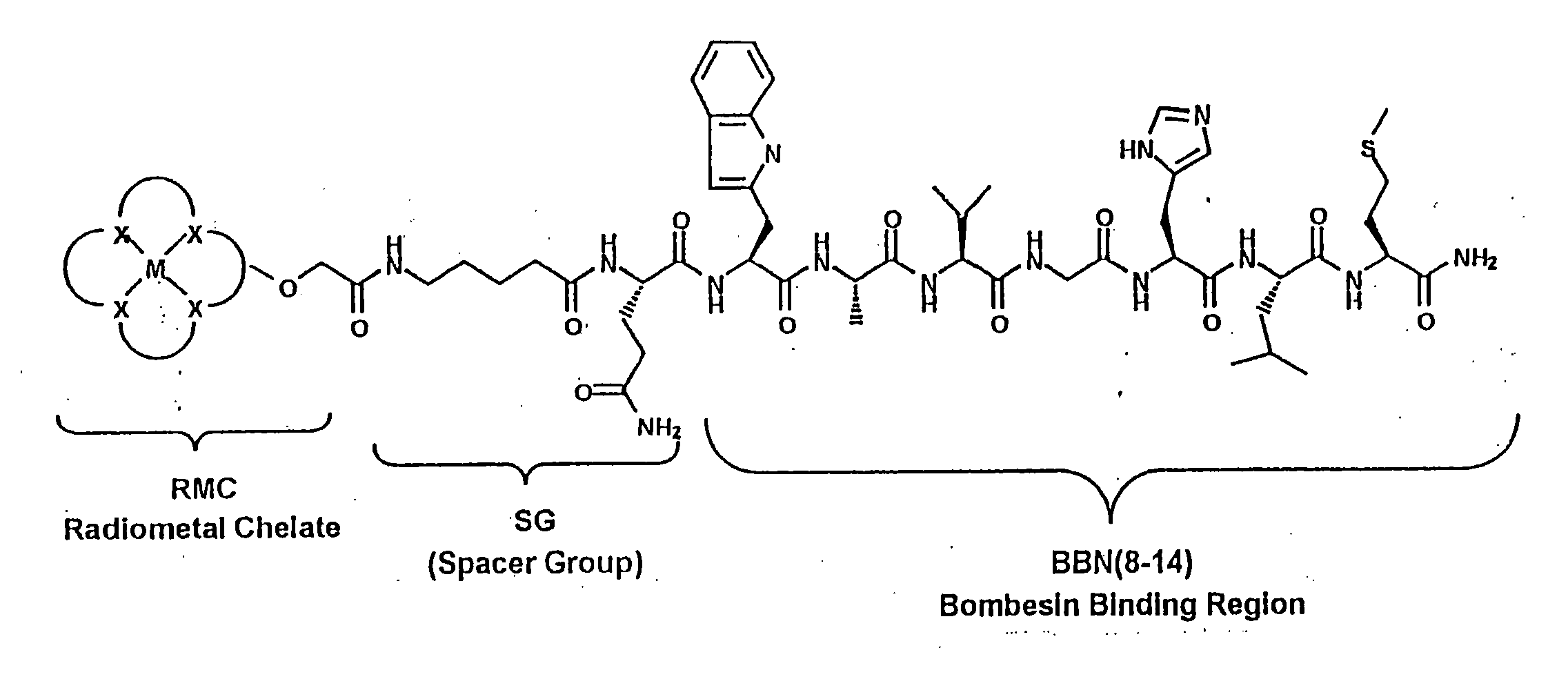
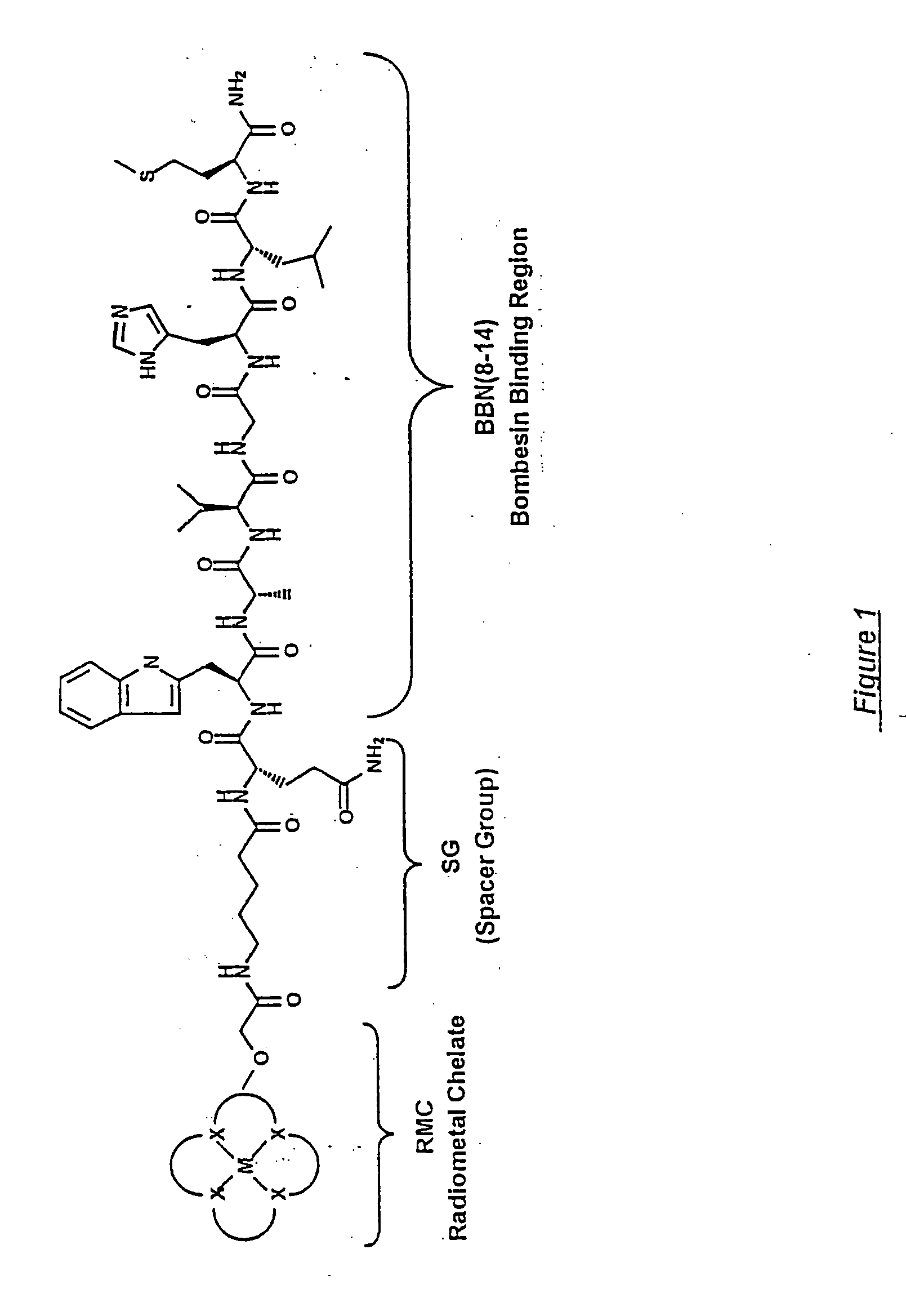
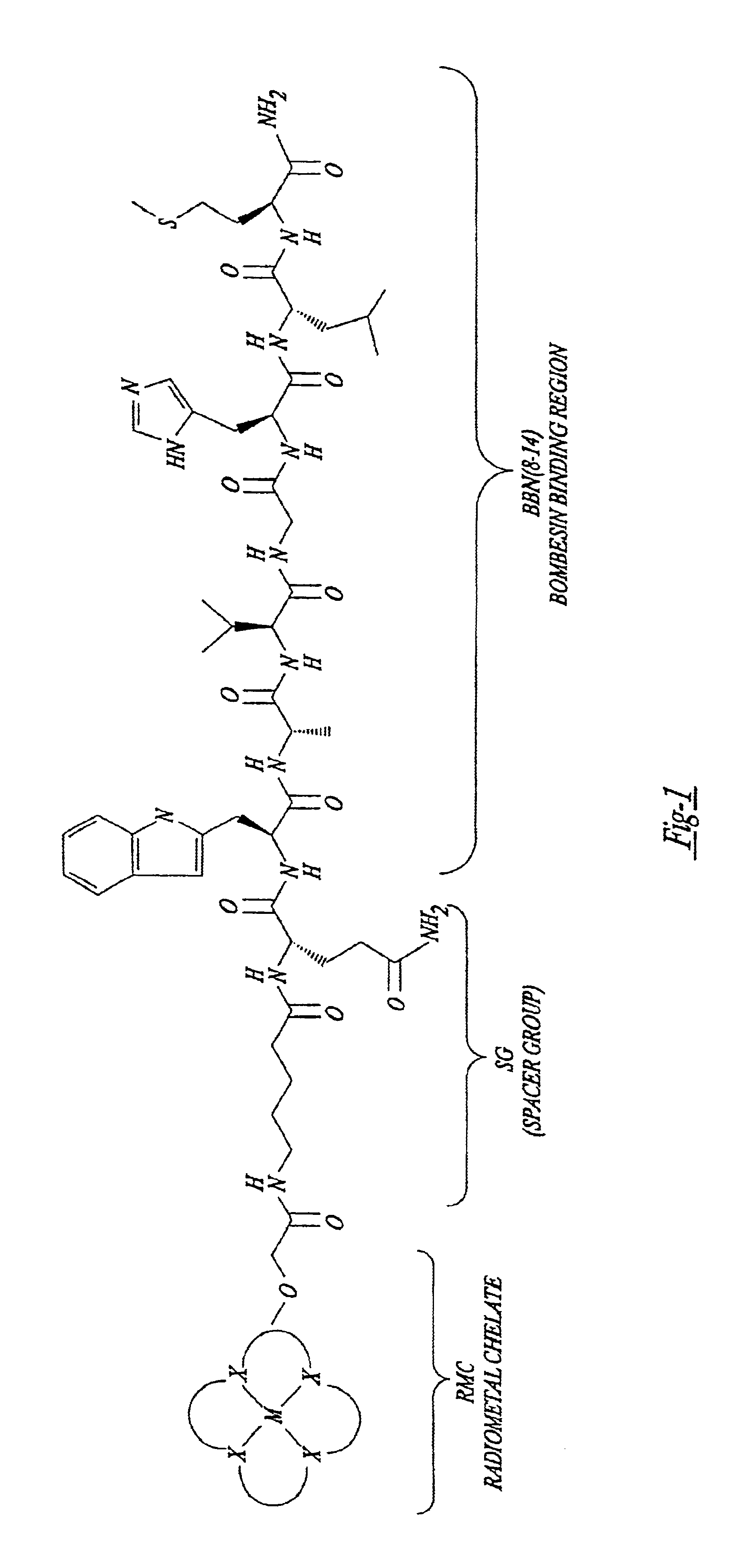
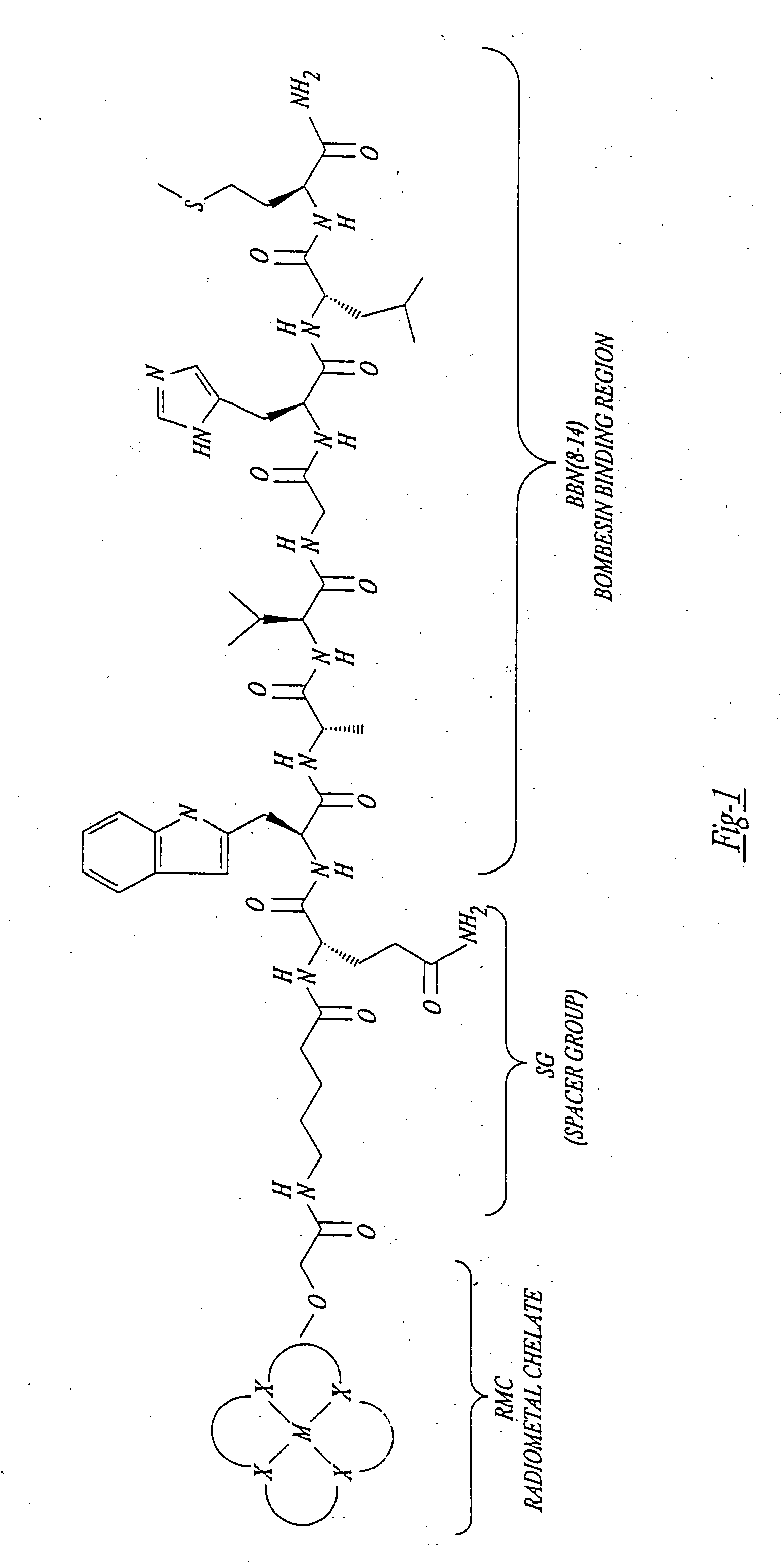
Gastrin receptor-avid peptide conjugates
InactiveUS20060067886A1Peptide/protein ingredientsRadioactive preparation carriersDiseaseGastrin-releasing peptide receptor
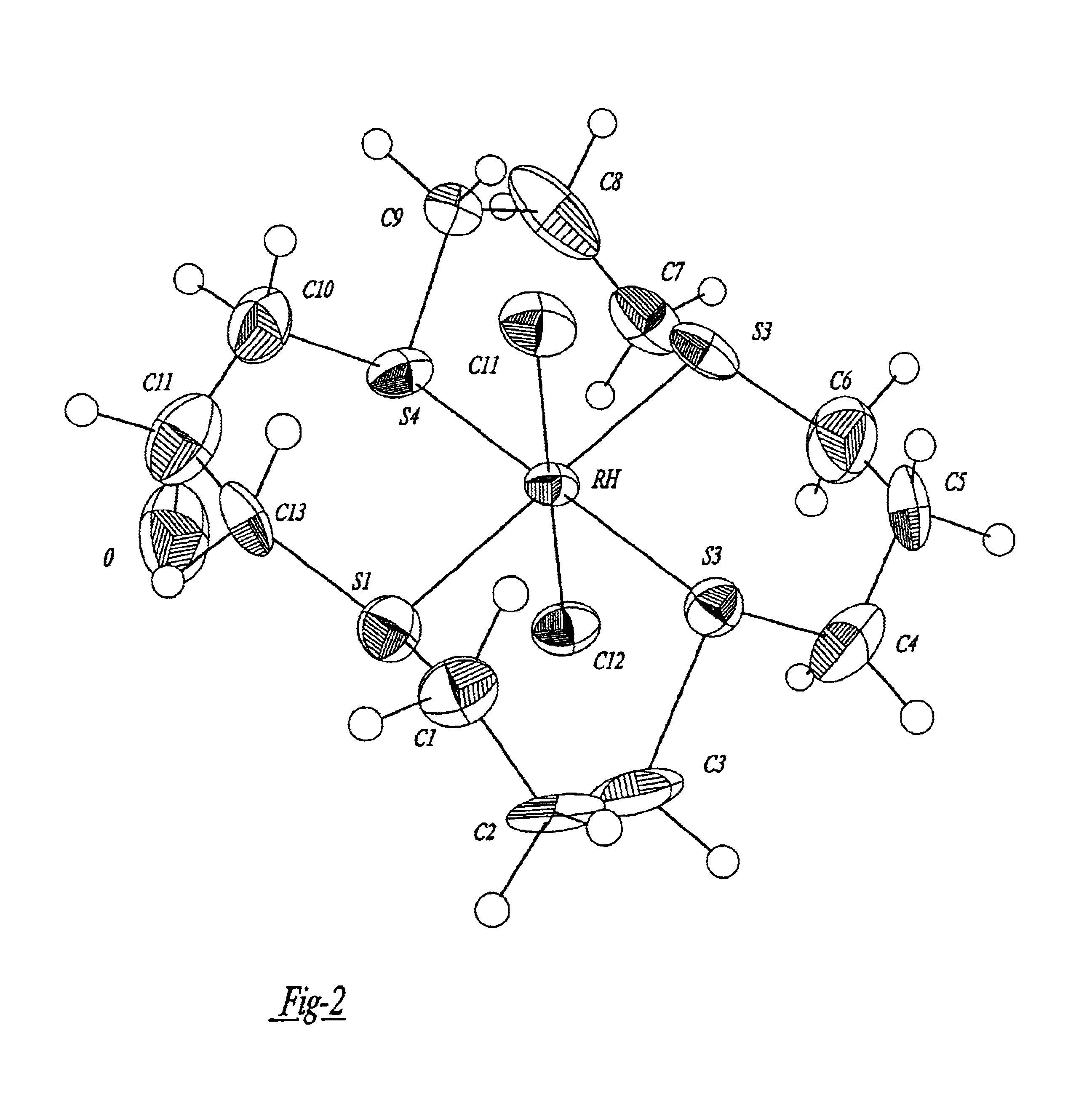
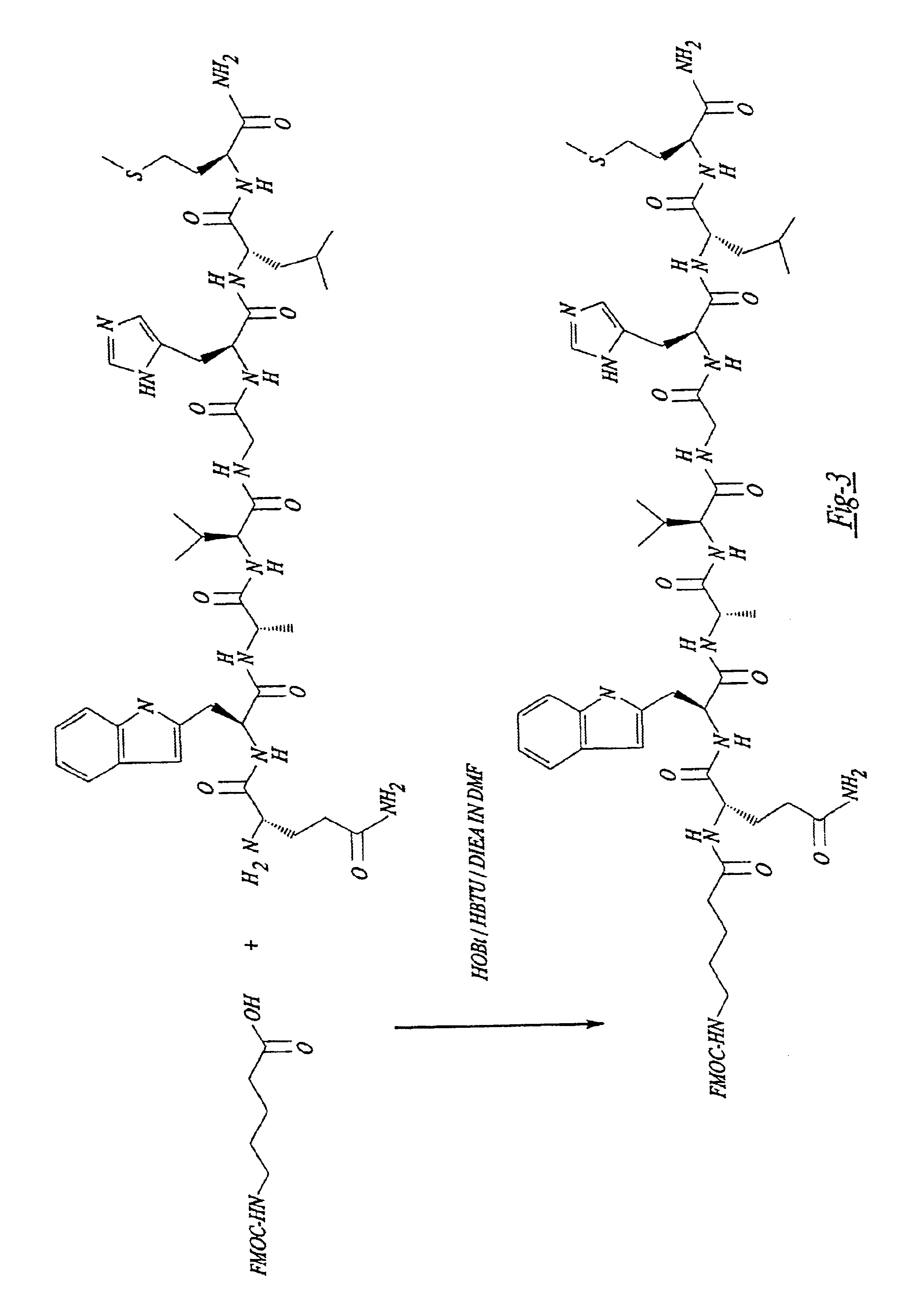
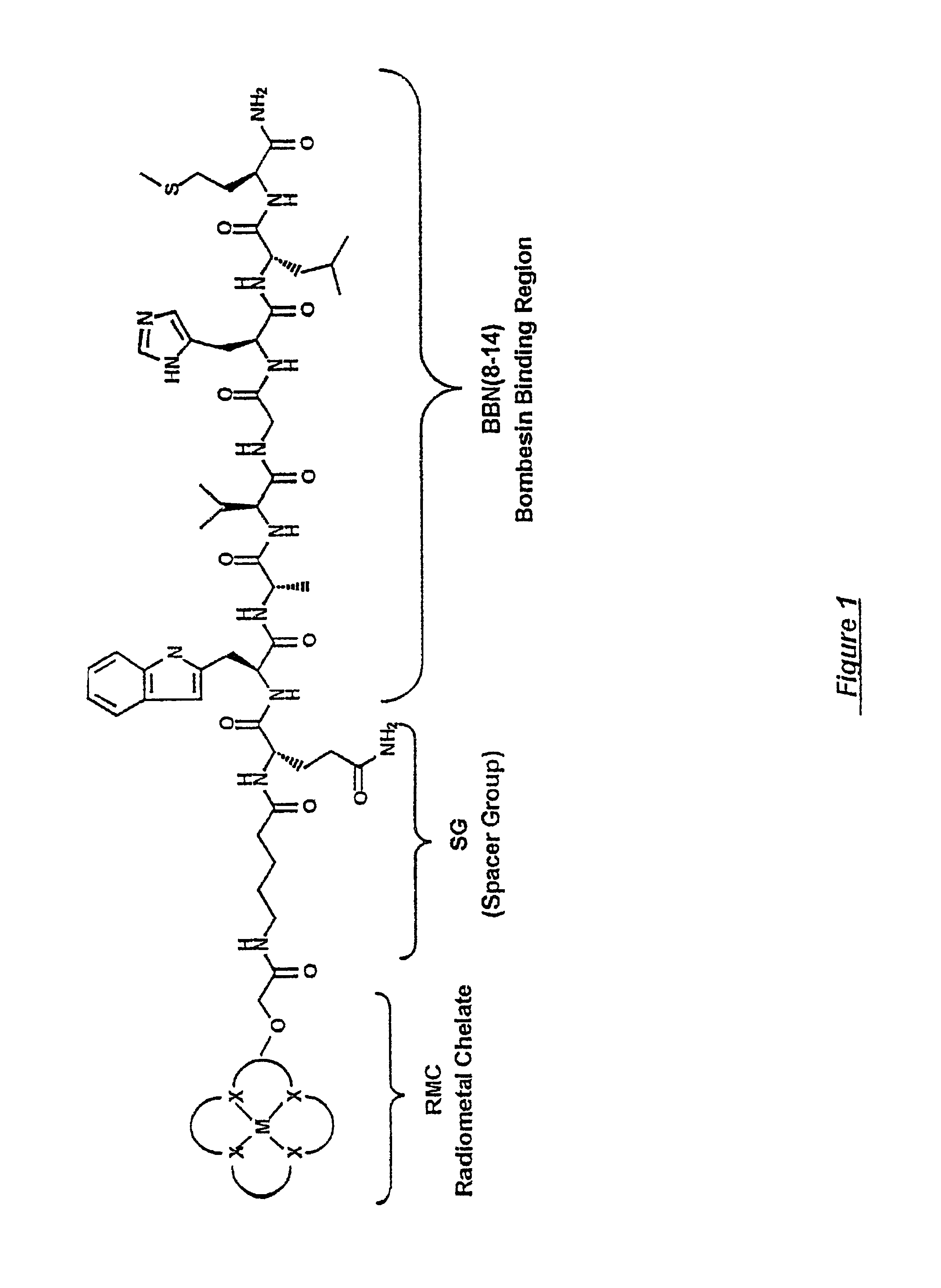
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:HOFFMAN TIMOTHY J +4
Gastrin receptor-avid peptide conjugates
InactiveUS6921526B2Peptide/protein ingredientsGastrin releasing peptideSynthonGastrin-releasing peptide receptor
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:UNIVERSITY OF MISSOURI
Gastrin receptor-avid peptide conjugates
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a-moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:UNIVERSITY OF MISSOURI
Backbone modified oligonucleotide analogs and preparation thereof through reductive coupling
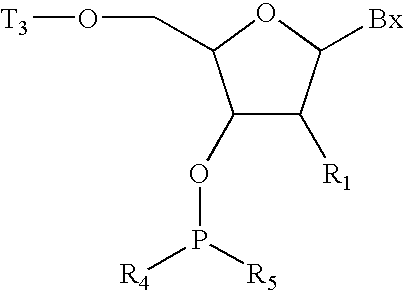
InactiveUS6025482ASequencing is facilitatedEnhanced cellular uptakeGroup 4/14 element organic compoundsSugar derivativesSynthonCoupling
Methods for preparing oligonucleotide analogs which have improved nuclease resistance and improved cellular uptake are provided. In preferred embodiments, the methods involve reductive coupling of 3'- and 4'- substituted or 4'- and 3'-substituted nucleosidic synthons.
Owner:IONIS PHARMA INC
Amphiphilic molecular modules and constructs based thereon
Owner:COVALENT PARTNERS LLC
Bridged macrocyclic module compositions
This invention is related to the fields of organic chemistry and nanotechnology. In particular, it relates to materials and methods for the preparation of organic synthons and bridged macrocyclic module compounds. The bridged macrocyclic module compounds may be used to prepare macrocyclic compositions such as nanofilms, which may be useful for filtration.
Owner:COVALENT PARTNERS LLC
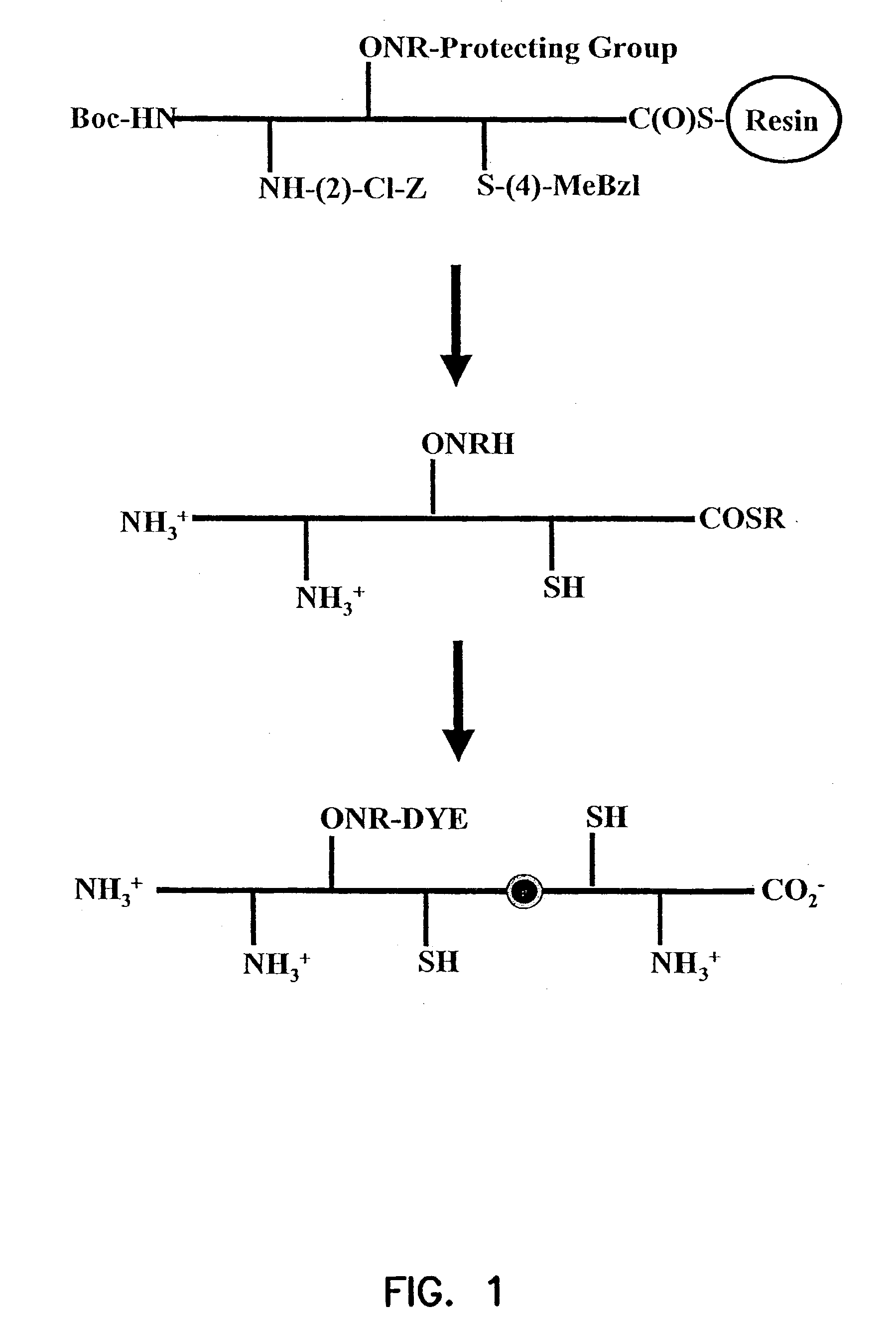
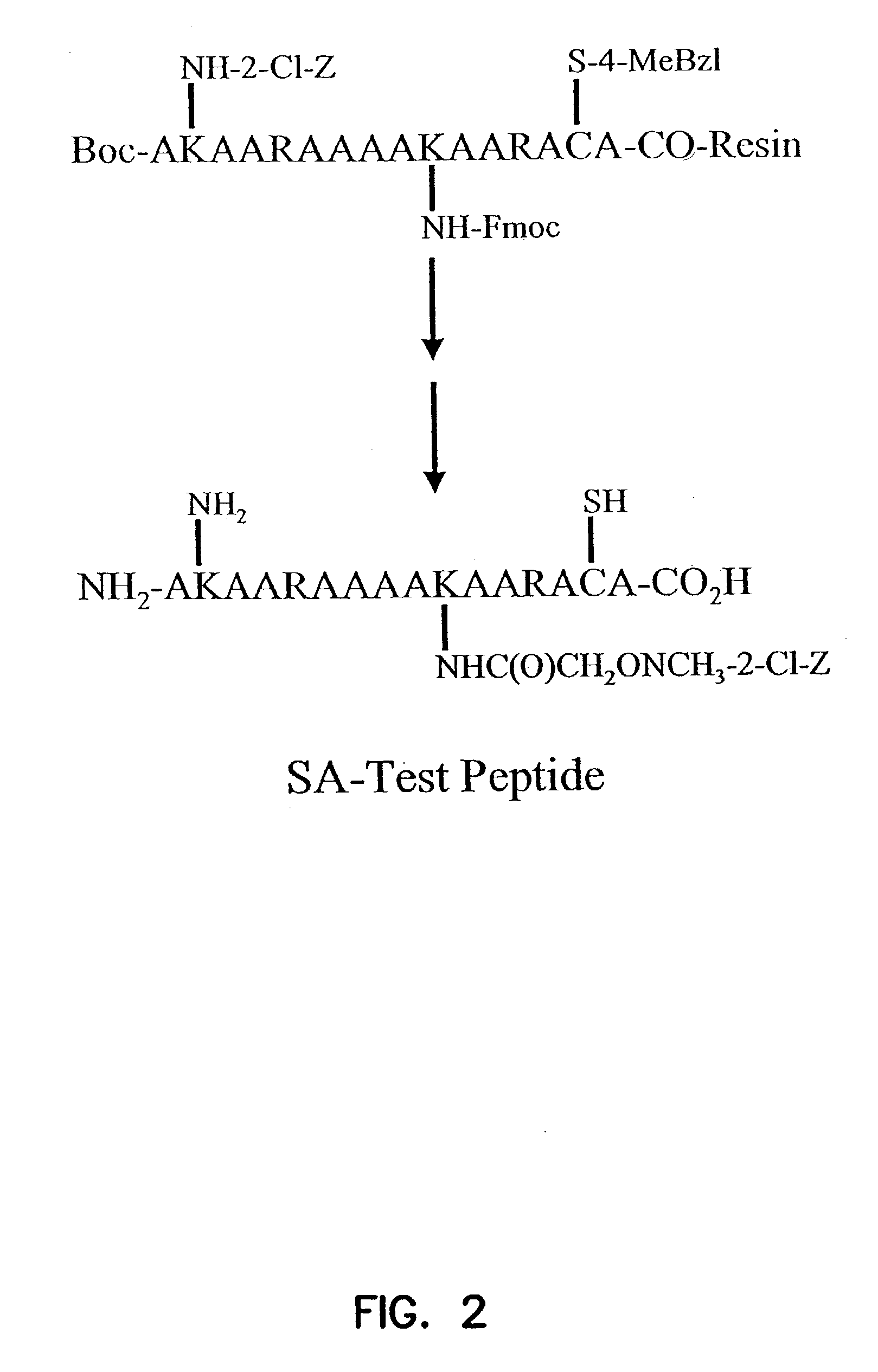
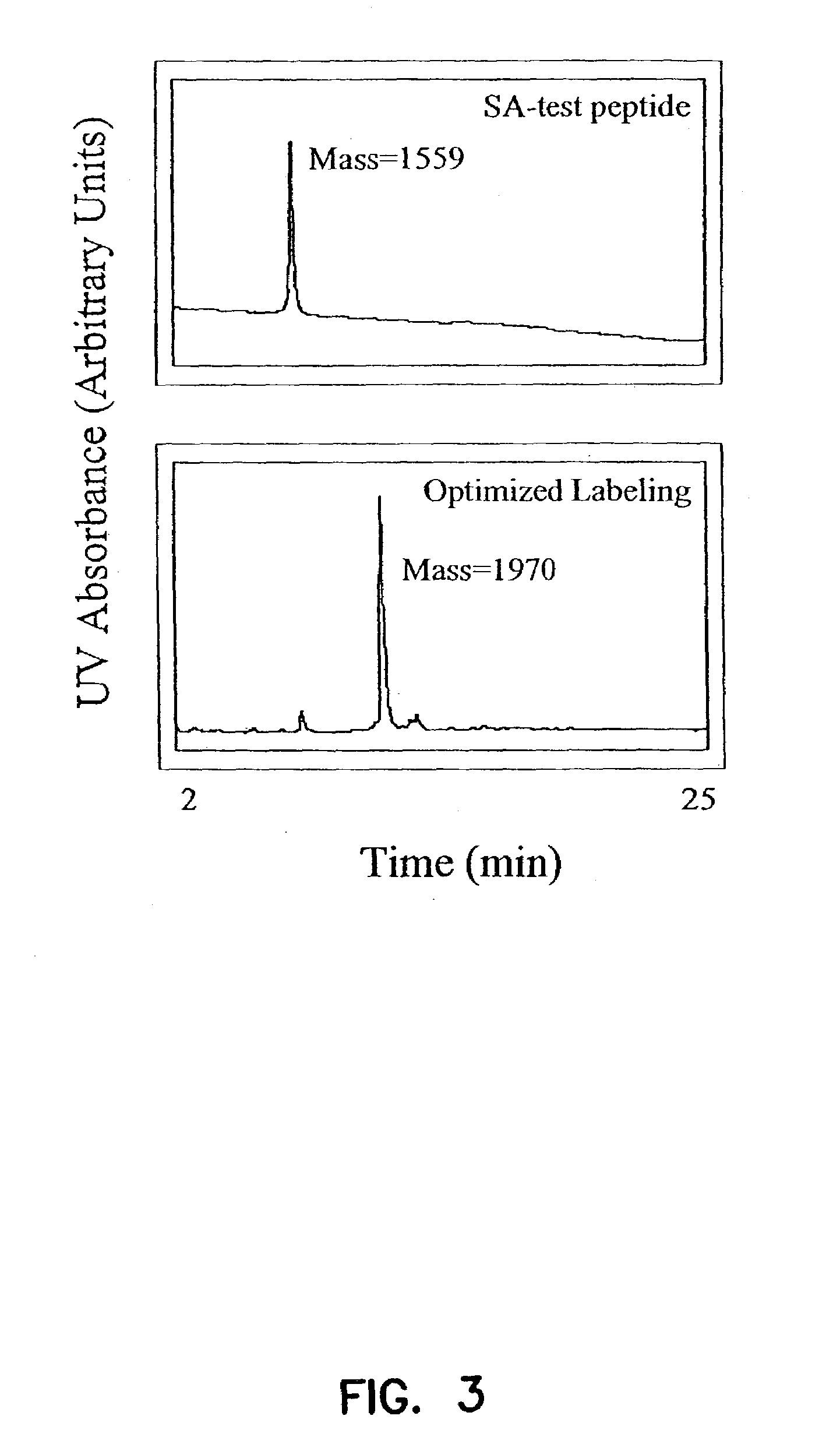
Labeled peptides, proteins and antibodies and processes and intermediates useful for their preparation
InactiveUS6951947B2Improve efficiencyMinimal perturbationDepsipeptidesPeptide preparation methodsSynthonFluorophore
The invention provides peptide synthons having protected functional groups for attachment of desired moieties (e.g. functional molecules or probes). Also provided are peptide conjugates prepared from such synthons, and synthon and conjugate preparation methods including procedures for identifying the optimum probe attachment site. Biosensors are provided having environmentally sensitive dyes that can locate specific biomolecules within living cells and detect chemical and physiological changes in those biomolecules as the living cell is moving, metabolizing and reacting to its environment. Methods are included for detecting GTP activation of a Rho GTPase protein using polypeptide biosensors. When the biosensor binds GTP-activated Rho GTPase protein, the environmentally sensitive dye emits a signal of a different lifetime, intensity or wavelength than when not bound. New fluorophores whose fluorescence responds to environmental changes are also provided that have improved detection and attachment properties, and that can be used in living cells, or in vitro.
Owner:THE SCRIPPS RES INST
Amphiphilic molecular modules and constructs based thereon
Owner:COVALENT PARTNERS LLC
Methods for preparing oligonucleotides having chiral phosphorothioate linkages
Methods are provided for preparing internucleotide phosphorothioate linkages that are enriched in the Sp or Rp enantiomer comprising coupling a synthon with a 2′-substituted nucleoside in the presence of coupling agent that is selected to enhance either the Rp or Spenantiomer according to its pKa.
Owner:IONIS PHARMA INC
Labeled peptides, proteins and antibodies and processes and intermediates useful for their preparation
InactiveUS20050287518A1Minimal perturbationHigh yieldMicrobiological testing/measurementDepsipeptidesSynthonFluorophore
The invention provides peptide synthons having protected functional groups for attachment of desired moieties (e.g. functional molecules or probes). Also provided are peptide conjugates prepared from such synthons, and synthon and conjugate preparation methods including procedures for identifying the optimum probe attachment site. Biosensors are provided having environmentally sensitive dyes that can locate specific biomolecules within living cells and detect chemical and physiological changes in those biomolecules as the living cell is moving, metabolizing and reacting to its environment. Methods are included for detecting GTP activation of a Rho GTPase protein using polypeptide biosensors. When the biosensor binds GTP-activated Rho GTPase protein, the environmentally sensitive dye emits a signal of a different lifetime, intensity or wavelength than when not bound. New fluorophores whose fluorescence responds to environmental changes are also provided that have improved detection and attachment properties, and that can be used in living cells, or in vitro.
Owner:THE SCRIPPS RES INST
Gastrin receptor-avid peptide conjugates
A compound for use as a therapeutic or diagnostic radiopharmaceutical includes a group capable of complexing a medically useful metal attached to a moiety which is capable of binding to a gastrin releasing peptide receptor. A method for treating a subject having a neoplastic disease includes administering to the subject an effective amount of a radiopharmaceutical having a metal chelated with a chelating group attached to a moiety capable of binding to a gastrin releasing peptide receptor expressed on tumor cells with subsequent internalization inside of the cell. A method of forming a therapeutic or diagnostic compound includes reacting a metal synthon with a chelating group covalently linked with a moiety capable of binding a gastrin releasing peptide receptor.
Owner:UNIVERSITY OF MISSOURI
Macrocyclic module compositions
Owner:COVALENT PARTNERS LLC
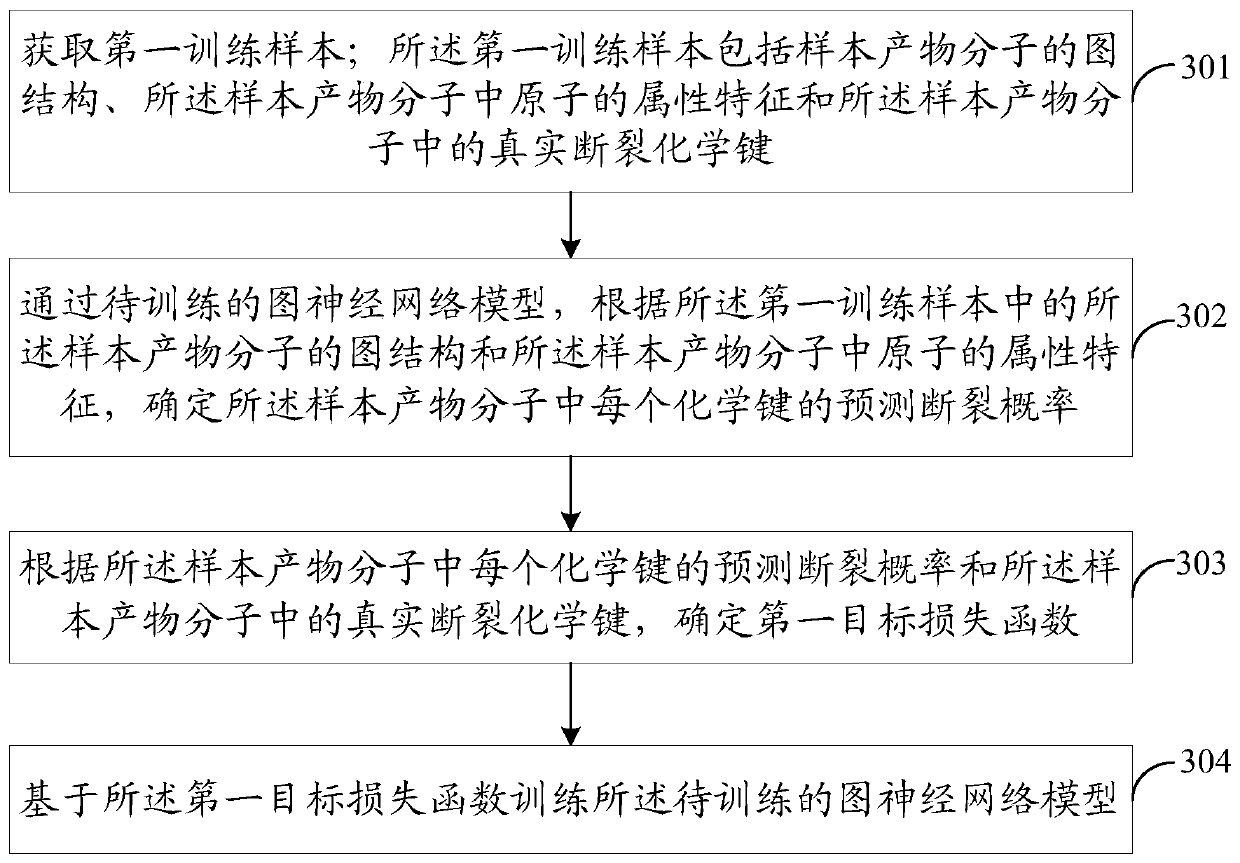
Artificial intelligence-based retrosynthesis prediction method and device, equipment and storage medium
PendingCN111524557AImprove forecast accuracyImprove visualizationMolecular entity identificationChemical processes analysis/designAlgorithmSynthon
Embodiments of the invention disclose an artificial intelligence-based retrosynthesis prediction method and device, equipment and a storage medium. The method comprises the following steps of: acquiring a graph structure of a product molecule and attribute features of atoms in the product molecule; predicting a fracture chemical bond in the product molecule at least according to the graph structure of the product molecule and the attribute features of atoms in the product molecule through a graph neural network model; carrying out bond breaking treatment on the product molecule based on the fracture chemical bond to obtain at least one synthon; and predicting a reactant molecule at least according to a character string corresponding to a retrosynthesis reaction type, a character string corresponding to the product molecule and a character string corresponding to the at least one synthon through a sequence learning model. According to the method, the prediction precision of the retrosynthesis reaction of an organic compound can be effectively improved, so the prediction process of the retrosynthesis reaction of the organic compound is easier to visualize and is more interpretable.
Owner:TENCENT TECH (SHENZHEN) CO LTD
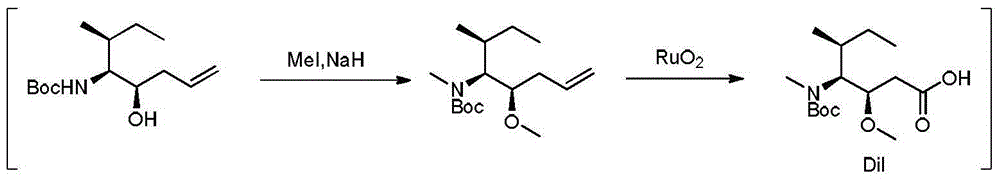
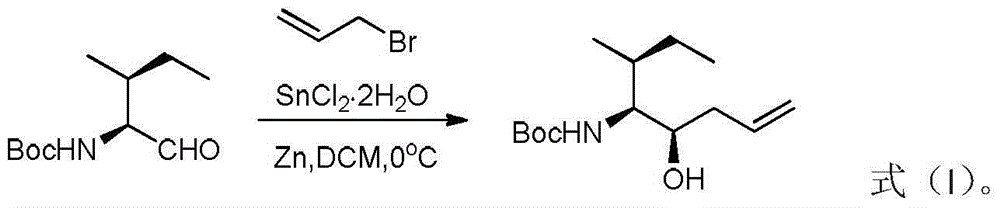
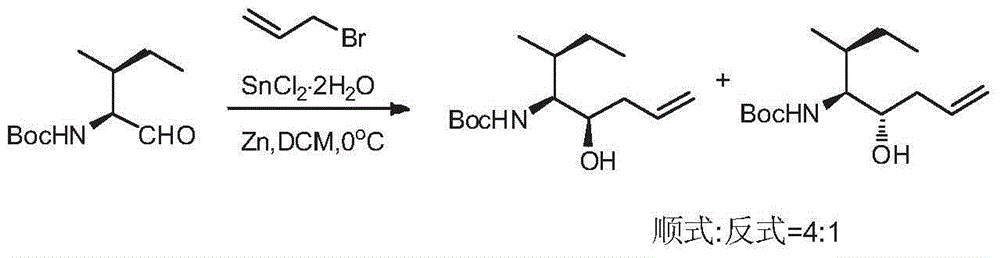
Preparation method of tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-ocentyl-4-carbamate
InactiveCN105566166ARaw materials are cheap and easy to getLow costCarbamic acid derivatives preparationOrganic compound preparationCarbamateTert-Butyloxycarbonyl protecting group
The invention discloses a preparation method of tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-ocentyl-4-carbamate. The preparation method comprises the following steps: using N-tert-butoxycarbonyl-L-isoleucinal and allyl bromide as raw materials to prepare a crude product with a higher diastereoselectivity through a Barbier reaction; then performing column chromatography separation and purification to obtain the tert-butyl (3R, 4S, 5S)-5-hydroxy-3-methyl-7-ocentyl-4-carbamate. The synthetic product of the invention can be used as an intermediate for synthesizing a key synthon Dil of aplysiatoxin 10; the method of the invention is low in raw material cost, mild in reaction condition, short in reaction time, and easy to operate.
Owner:EAST CHINA NORMAL UNIV
Synthetic fiber modified pavement asphalt and its preparing process
InactiveCN1966581AOvercoming simple shearOvercoming rateIn situ pavingsBuilding insulationsSynthonSynthetic fiber
The invention disclosed a kind of asphalt material that is used in paving the highway as well as its preparing method, especially a kind of synthon reshaped asphalt as well as its preparing method which belongs to the high polymer material domain. The ingredient of the asphalt includes 30-98.95wt% of synthon, 0.5-60wt% of way asphalt, 0.05-3wt% anti-oxidant and 0.5-30wt% of filling. After previously mix the materials, charge them into the high speed shearing device, shearing the material at 80-220 DEG C for 3-120min to prepare the product. Because of the high speed shearing device and the add of fillings such as talcum powder, during the melting, shearing, leveling, mixing and dispersing process of the materials, the invention has overcome the problem of tangling of the synthon; because of the antioxidant, the comprehensive qualities of the asphalt concrete has been substantially improved. The preparing procedure is simple, practical, and needs low investment and operating cost which is easier to be extended and universalized.
Owner:SUZHOU UNIV +1
Synthesis of poly-(p-aryleneethynylene)s in neat water under aerobic condit
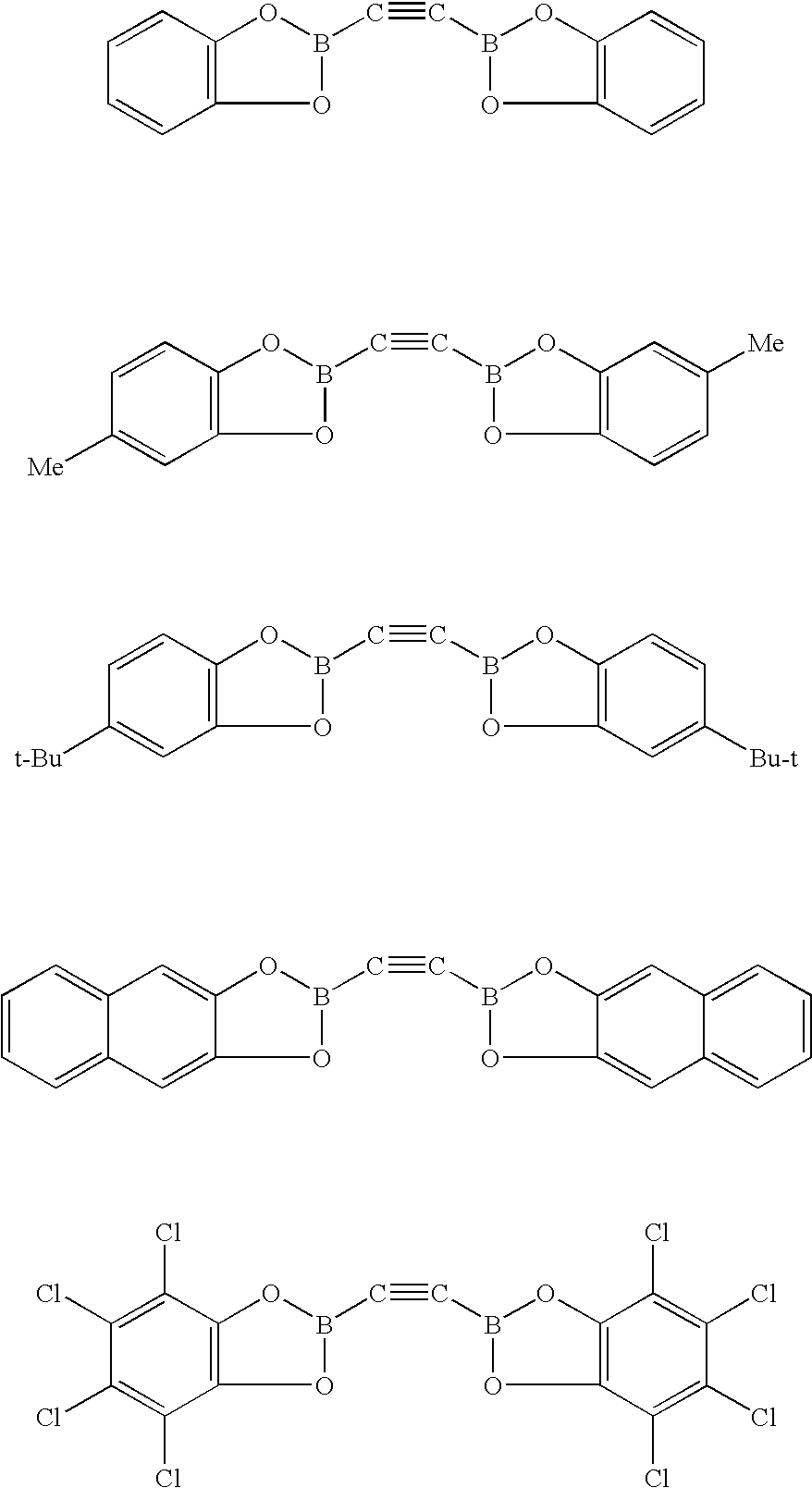
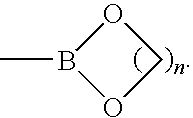
Provided are ethyne synthons comprising boron and related methods. Also provided are related water-soluble arylethynylene polymers capable of being synthesized in neat water under aerobic conditions.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
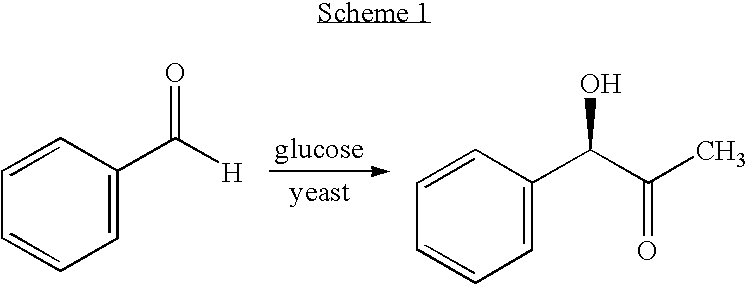
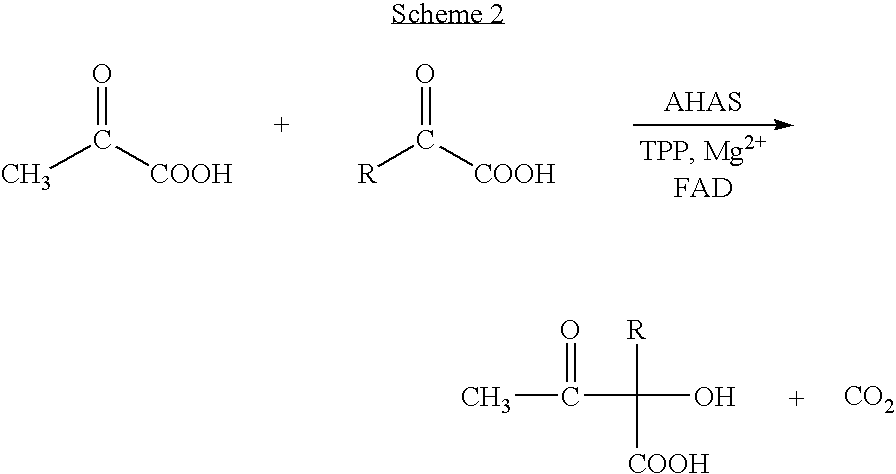
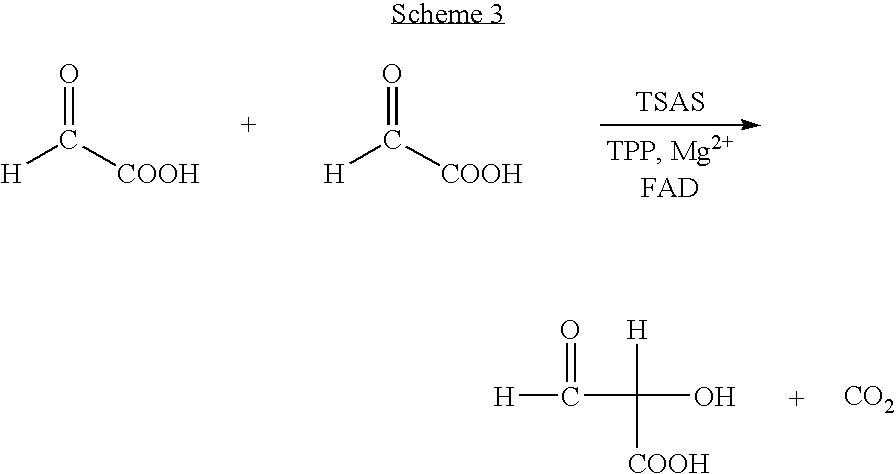
Process for preparing chiral aromatic alpha-hydroxy ketones using 2-hydroxy-3-oxoacid synthase
InactiveUS20060148042A1Improve efficiencyOrganic compound preparationRecombinant DNA-technologySynthonEnantiomer
A biotransformation process for preparing chiral aromatic-hydroxy ketones in high yields is described, using 2-hydroxy-3-oxoacid synthase, such as AHAS or TSAS. Optionally substituted arylaldehydes and -oxoacids react in this process to provide pure enantiomers, useful as synthons in the production of various drugs, an example being (R)-phenylacetyl carbinol.
Owner:BEN GURION UNIVERSITY OF THE NEGEV
Silver nanoparticle binding agent conjugates based on moieties with triple cyclic disulfide anchoring groups
InactiveUS20100234579A1Sharper profileImprove stabilitySugar derivativesMicrobiological testing/measurementMaterial synthesisSynthon
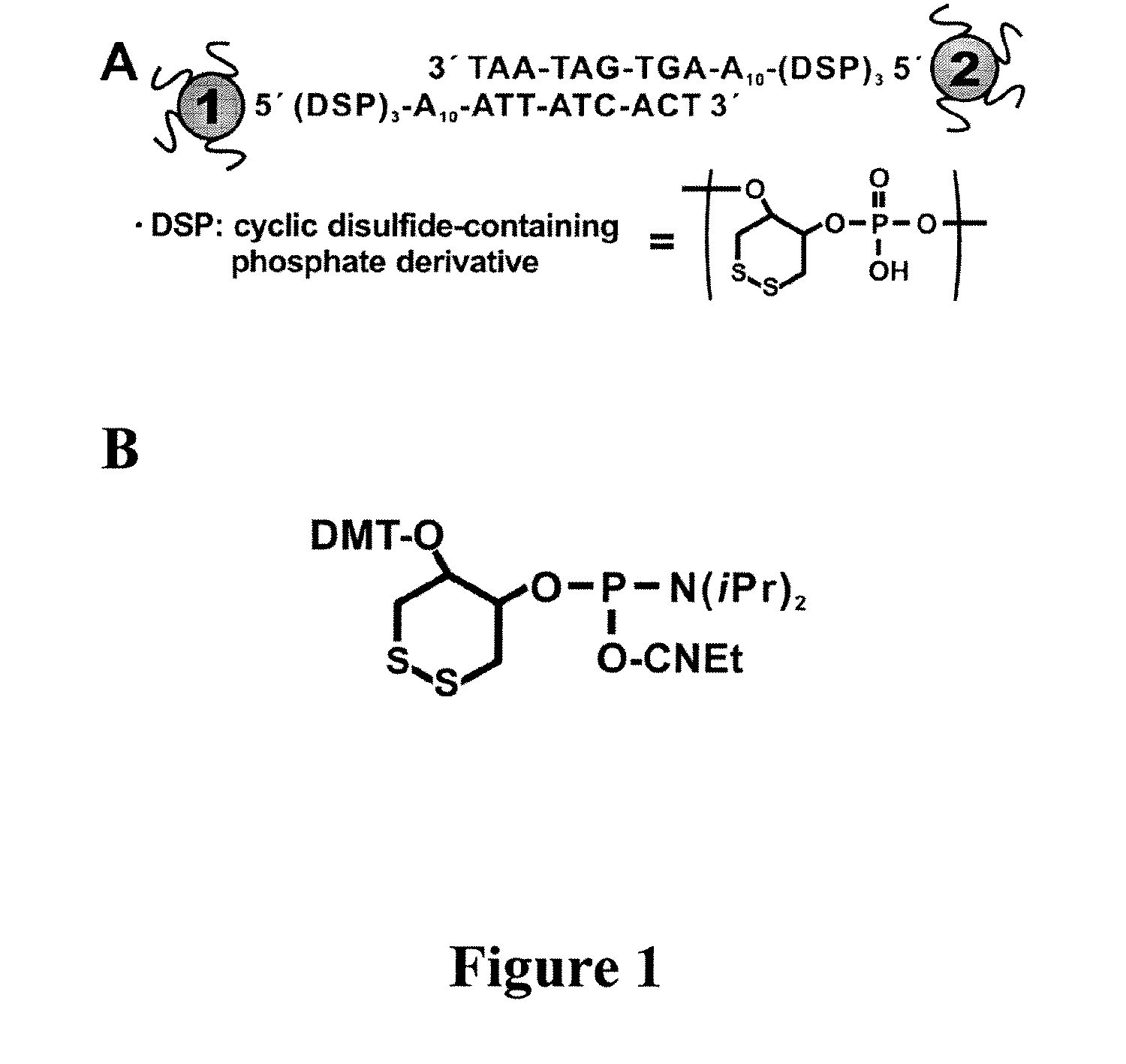
The present invention concerns the use of binding agent-functionalized silver nanoparticles for a variety of uses, including molecular diagnostic labels, synthons in programmable materials synthesis approaches, and functional components for nanoelectronic devices. More specifically, the invention provides a new strategy for preparing silver nanoparticle-binding agent conjugates that are based upon moieties with triple cyclic disulfide-anchoring groups.
Owner:NORTHWESTERN UNIV
Synthon and method for preparing dibenzocoronene compounds by same
ActiveCN103570714AImprove solubilityEasy to purifyLiquid crystal compositionsOrganic chemistryCoroneneSynthon
The invention relates to a synthon and a method for preparing dibenzocoronene compounds by the same. The synthon is a tetrahydroxy-substituted perylene bisimide compound, and has a structure as shown in general formula (I), wherein R is a C1-C18 linear chain primary alkyl or a primary alkyl with a branched chain. According to the invention, dibromo-perylene anhydride or mixtures are used for preparing dibromo-imide; dibromo-imide and phenylboronic acid substituted by alkoxys at 3 and 4 positions are subjected to a Suzuki coupling reaction under the catalysis of Pd(PPh3)4 to prepare aryl-substituted perylene bisimide compounds; further dealkylation reaction is carried out under the action of boron tribromide so as to form tetrahydroxy-substituted perylene bisimide compound synthon; then the synthon is used as a raw material to synthesize charge transfer compounds of coronene compounds with imide groups at two sides of the molecule. The synthetic method of the invention is simple, high in yield, and widely applicable to organic light-emitting diodes, field effect transistors, and solar cells. The general formula (I) is shown in the description.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
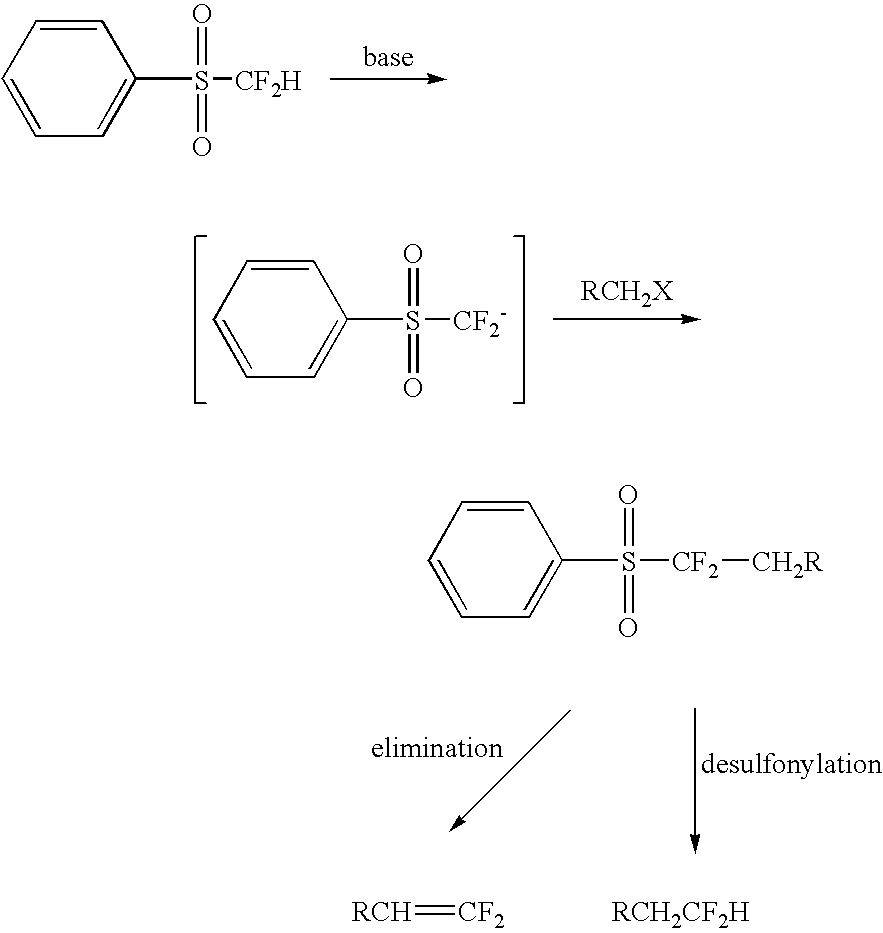
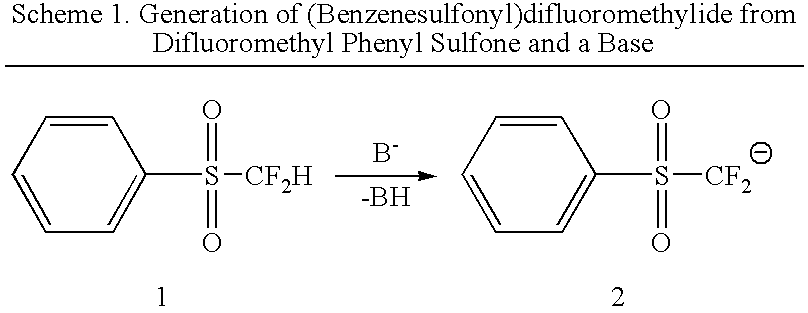
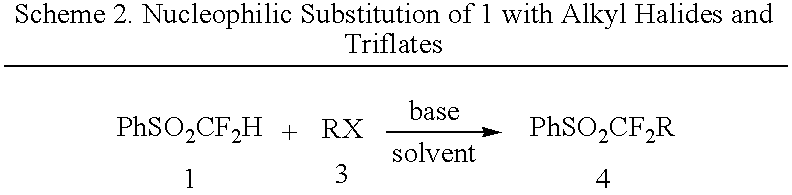
Nucleophilic substitution reactions of difluorormethyl phenyl sulfone with alkyl halides leading to the facile synthesis of terminal 1,1-difluoro-1-alkenes and difluoromethylalkanes
(Benzenesulfonyl)difluoromethyl anion, in situ generated from difluoromethyl phenyl sulfone and a base, was found to easily undergo nucleophilic substitution reactions (SN2) with primary alkyl halides, elemental halogens, and perfluoroalkyl halides with good selectivity. The formed (benzenesufonyl)difluoromethylalkanes are useful intermediates for the facile preparation of 1,1-difluoro-1-alkenes and difluorometbylalkanes. Thus, difluoromethyl phenyl sulfone acts as both “CF2═” and “CF2H−” synthons.
Owner:UNIV OF SOUTHERN CALIFORNIA
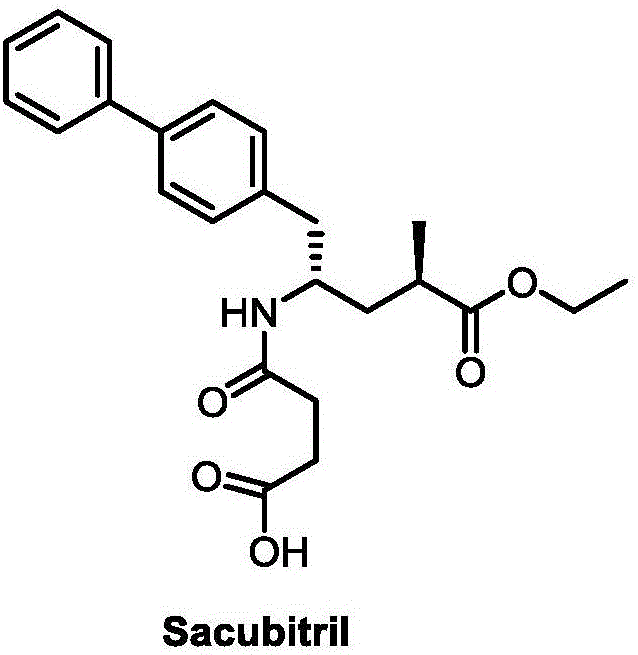
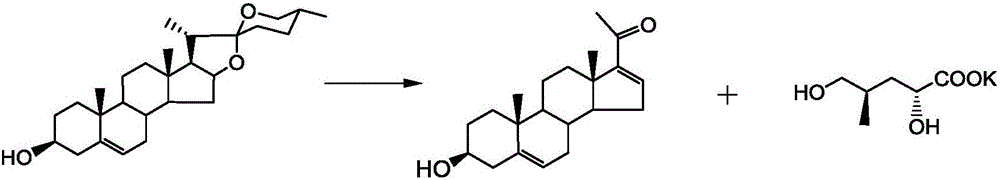
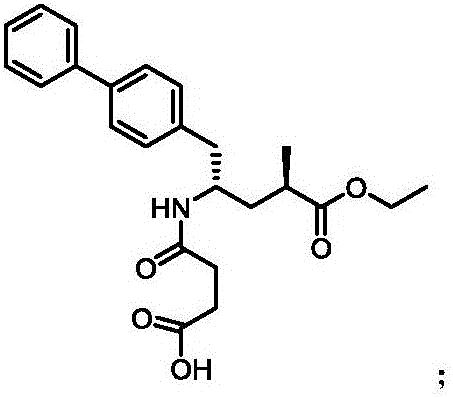
Synthetic method for Sacubitril
InactiveCN106380421AHigh reaction yieldLow priceOrganic compound preparationOrganic chemistry methodsSynthonSacubitril
The invention provides a preparation method for Sacubitril as one component of a novel hypotensive drug Entresto. According to the method, chiral synthons, obtained in the oxydative degradation wastes of steroid sapogenin, are adopted as raw materials. The raw materials are subjected to five steps of reactions, and then the high-yield synthesis of Sacubitril is realized. The raw materials of the method are simple, easily available and low in cost. Meanwhile, the synthetic method is characterized by being mild in condition, simple in operation, high in yield and few in by-product. The method can be applied to the industrial production.
Owner:SHANGHAI INST OF ORGANIC CHEM CHINESE ACAD OF SCI
Novel method for efficiently preparing beta-aminoketone by utilizing dimethyl sulfoxide
InactiveCN106699662AOvercome limitationsOvercome environmental problemsOrganic chemistryChemical recyclingN dimethylformamideSynthon
The invention belongs to the technical field of organic synthesis chemistry and discloses a simple and efficient novel method for preparing beta-aminoketone by utilizing a metal salt catalyst, dimethyl sulfoxide used as a one-carbon synthon, and imidazole and ketone derivatives. By utilizing the novel method, a series of beta-aminoketone compounds are prepared. The limitation of preparing the beta-aminoketone compounds at present is overcome; disadvantages of a traditional method that toxic effects on the environment and human bodies are relatively great when a toxic reagent, namely a formaldehyde analogue, participates in reaction are avoided; the low-toxicity dimethyl sulfoxide, N,N-dimethylformamide (DMF), N,N-dimethylacetamide (DMA) and N-methyl pyrrolidone (NMP) are used as the one-carbon synthons for synthesizing a series of beta-aminoketone for the first time, and a new way for synthesizing the beta-aminoketone compounds is expanded. The method provided by the invention has the advantages of simplicity in operation, high efficiency and easiness of obtaining raw materials, reagents and catalysts; the method is suitable for synthesizing various beta-aminoketone compounds and is suitable for large-scale industrial production.
Owner:ANYANG NORMAL UNIV
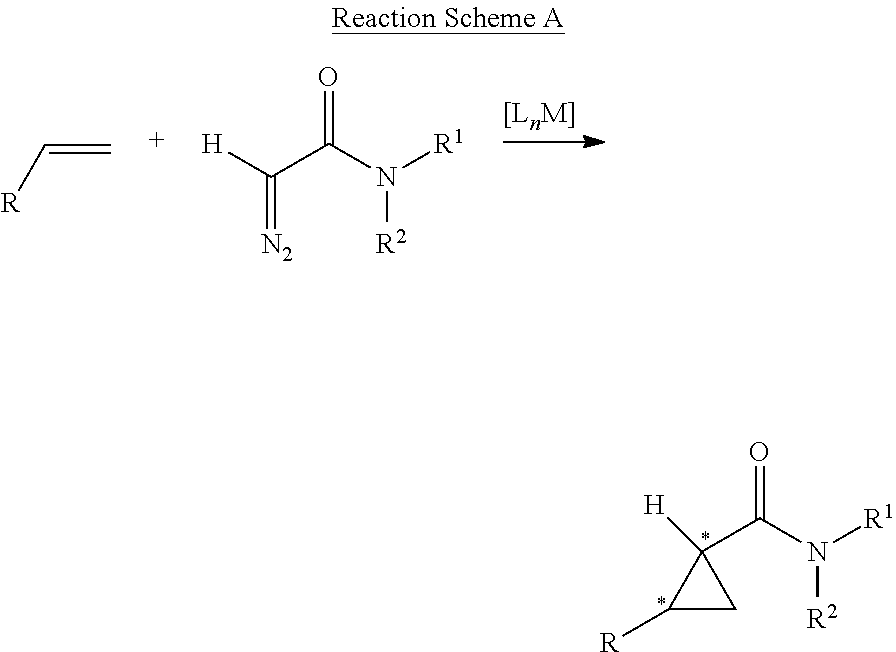
Asymmetric Cobalt-Catalyzed Cyclopropanation With Succinimidyl Diazoacetate
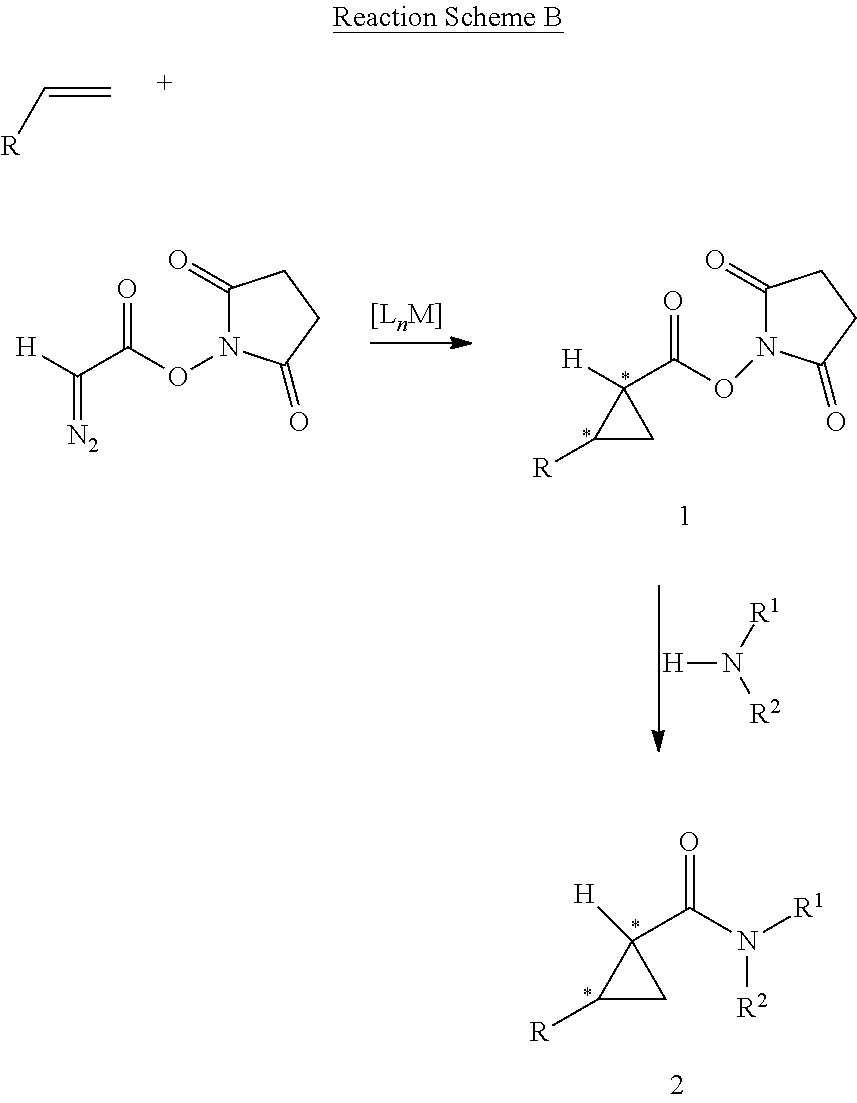
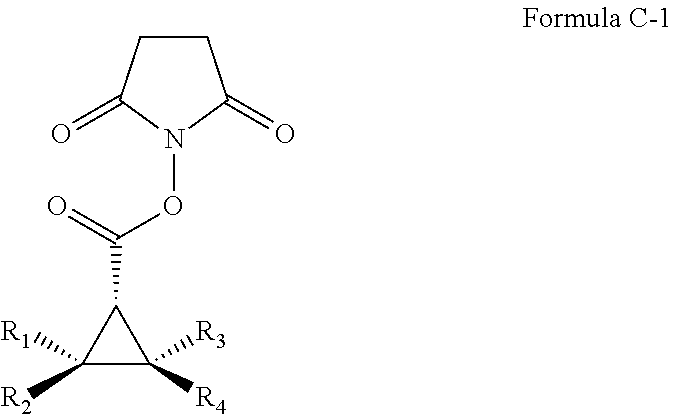
InactiveUS20120077959A1Excellent diastereoExcellent enantioselectivitiesSugar derivativesOrganic compound preparationPtru catalystSynthon
Cobalt(II) complexes of the D2-symmetric chiral porphyrins are effective catalysts for asymmetric cyclopropanation reactions with succinimidyl diazoacetate. The Co-catalyzed reaction is suitable for various olefins, providing the corresponding cyclopropane succinimidyl esters in high yields and excellent diastereo- and enantio-selectivity. The resulting enantioenriched cyclopropane succinimidyl esters can serve as convenient synthons for the general synthesis of optically active cyclopropyl carboxamides through mild reactions with a wide range of amine derivatives, including unprotected peptides and amino sugars
Owner:UNIV OF SOUTH FLORIDA
Universal method for constructing chiral organic molecular cage
The invention discloses a universal method for constructing a chiral organic molecular cage, which comprises the following steps: with a 2,6-dicarbonyl-4-substituted phenol structure as a parent nucleus, carrying out aldehyde ligand synthesis by coupling reaction, methoxy protection and deprotection reaction, Duff reaction and the like; and then introducing polyaldehyde synthons with different structures and functions are constructed by introducing rigid functional groups such as various benzene rings, naphthalene and anthracene, and then efficiently constructing the chiral porous molecular cage by the polyaldehyde synthons and chiral cyclohexanediamine under organic / water / acid conditions. The method has the advantages of simple and efficient ligand design, rich chiral molecular cage configuration, easiness in purification, wide ligand application range, easiness in reaction expansion and the like. By utilizing the modular synthesis method, a series of chiral molecular cages with richand adjustable structures can be prepared on a large scale for the fields of adsorption, separation, catalysis, energy and the like.
Owner:YANCHENG INST OF TECH
N-heterocyclic carbene catalytic functionalized imine as novel 1, 4-dipole synthon and synthesis application thereof
ActiveCN112778328ALower synthesis costAchieving Stereodiversity SynthesisOrganic chemistryChemical synthesisAchirality
The invention relates to an N-heterocyclic carbene catalytic functionalized imine as a novel 1, 4-dipole synthon and application thereof, and belongs to the field of chemical synthesis. Under the mild reaction condition, a chiral N-heterocyclic carbene catalyst is used for catalyzing and activating aldimine, a novel aza 1, 4-dipole synthon is obtained under the oxidation condition, and the novel organic synthon can be further subjected to 4 + 2 cyclization reaction with trifluoroacetophenone, isatin and an isatin-derived imine substrate to generate a heterocyclic compound with a novel structure and a chiral quaternary carbon center. According to the method disclosed by the invention, the N-heterocyclic carbene catalyst with the same chiral configuration and an achiral thiourea catalyst are used for co-catalysis, so that three-dimensional diverse synthesis of the trifluoroacetophenone compound can be realized. The method is mild in condition and efficient in reaction and has good substrate universality, and the reported aldimine-derived 1, 4-aza dipole synthon provides an important method for synthesizing various functional nitrogen heterocyclic compounds and has the potential of being applied to industrial production.
Owner:NANJING UNIV OF TECH
Labeled peptides, proteins and antibodies and processes and intermediates useful for their preparation
InactiveUS7176037B2Minimal perturbationHigh yieldChemiluminescene/bioluminescenceDepsipeptidesSynthonFluorophore
The invention provides peptide synthons having protected functional groups for attachment of desired moieties (e.g. functional molecules or probes). Also provided are peptide conjugates prepared from such synthons, and synthon and conjugate preparation methods including procedures for identifying optimum probe attachment sites. Biosensors are provided having functional molecules that can locate and bind to specific biomolecules within living cells. Biosensors can detect chemical and physiological changes in those biomolecules as living cells are moving, metabolizing and reacting to its environment. Methods are included for detecting GTP activation of a Rho GTPase protein using polypeptide biosensors. When the biosensor binds GTP-activated Rho GTPase protein, an environmentally sensitive dye emits a signal of a different lifetime, intensity or wavelength than when not bound. New fluorophores whose fluorescence responds to environmental changes are also provided that have improved detection and attachment properties, and that can be used in living cells, or in vitro.
Owner:THE SCRIPPS RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com