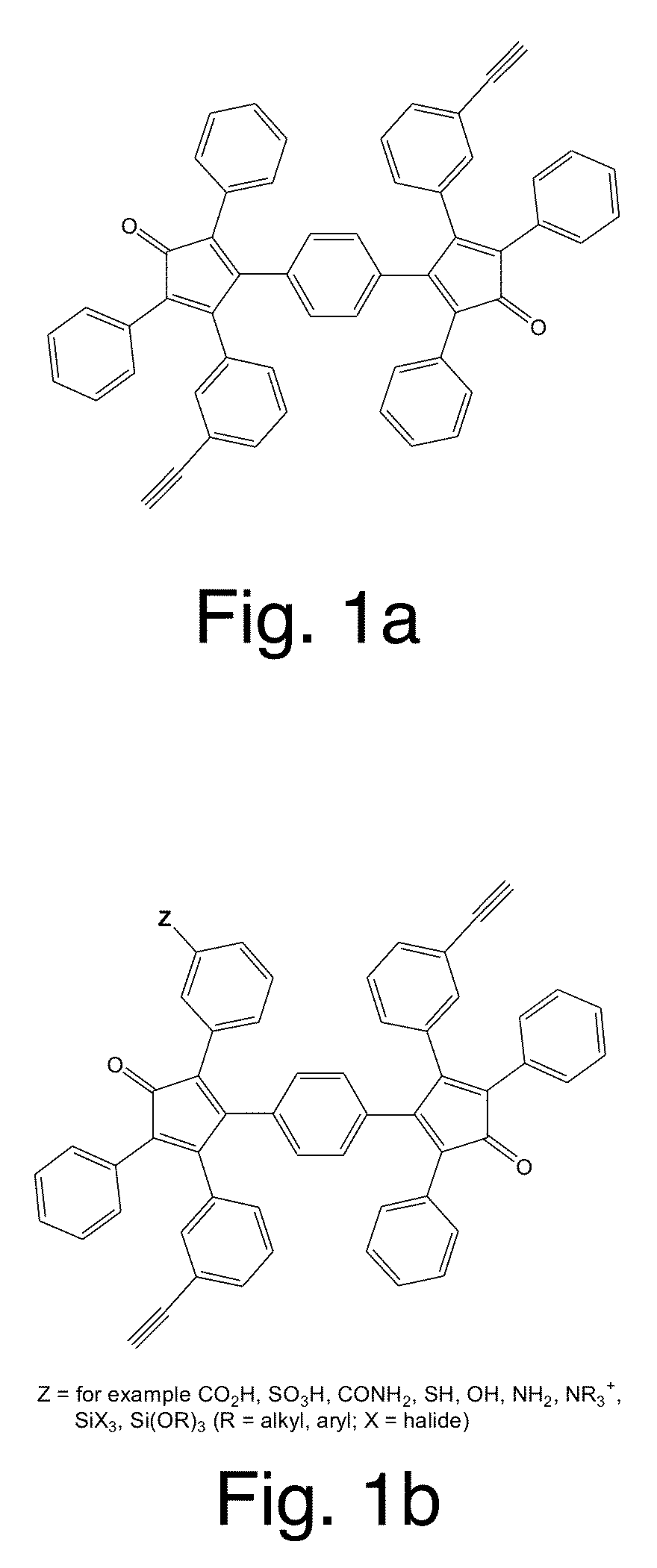
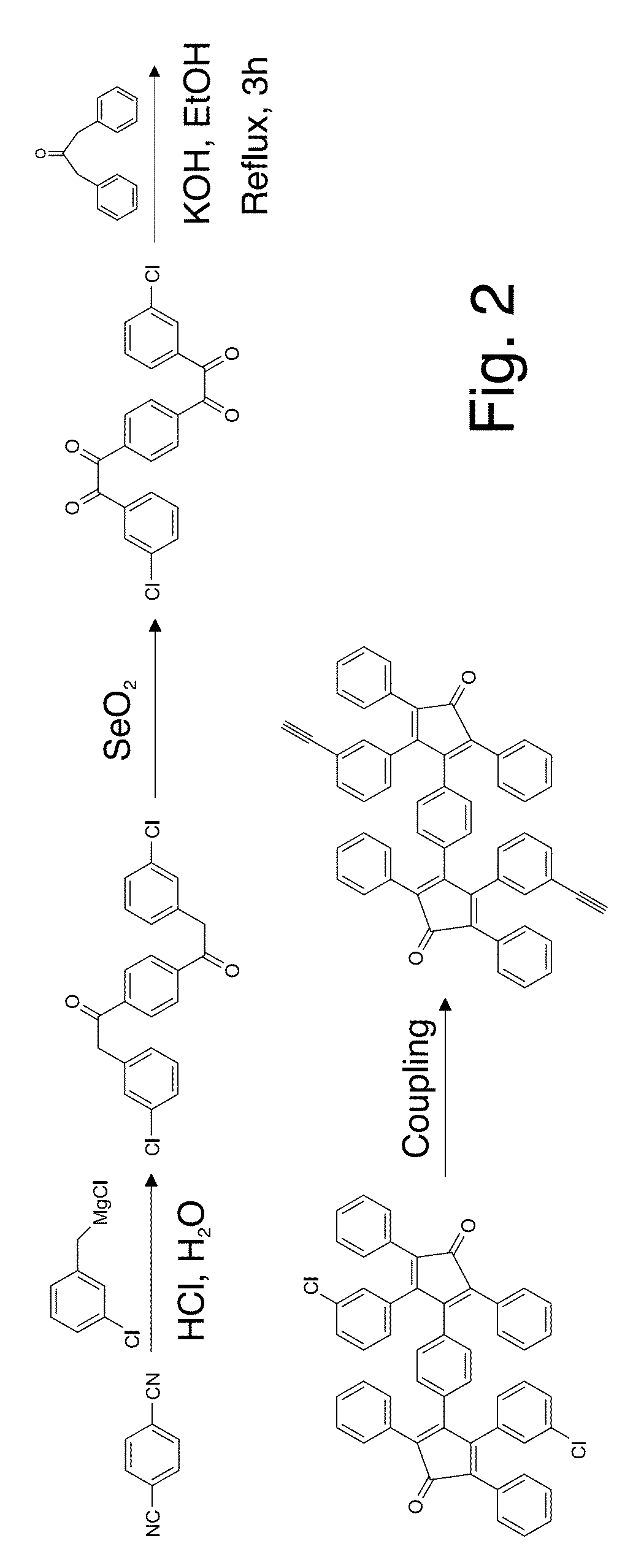
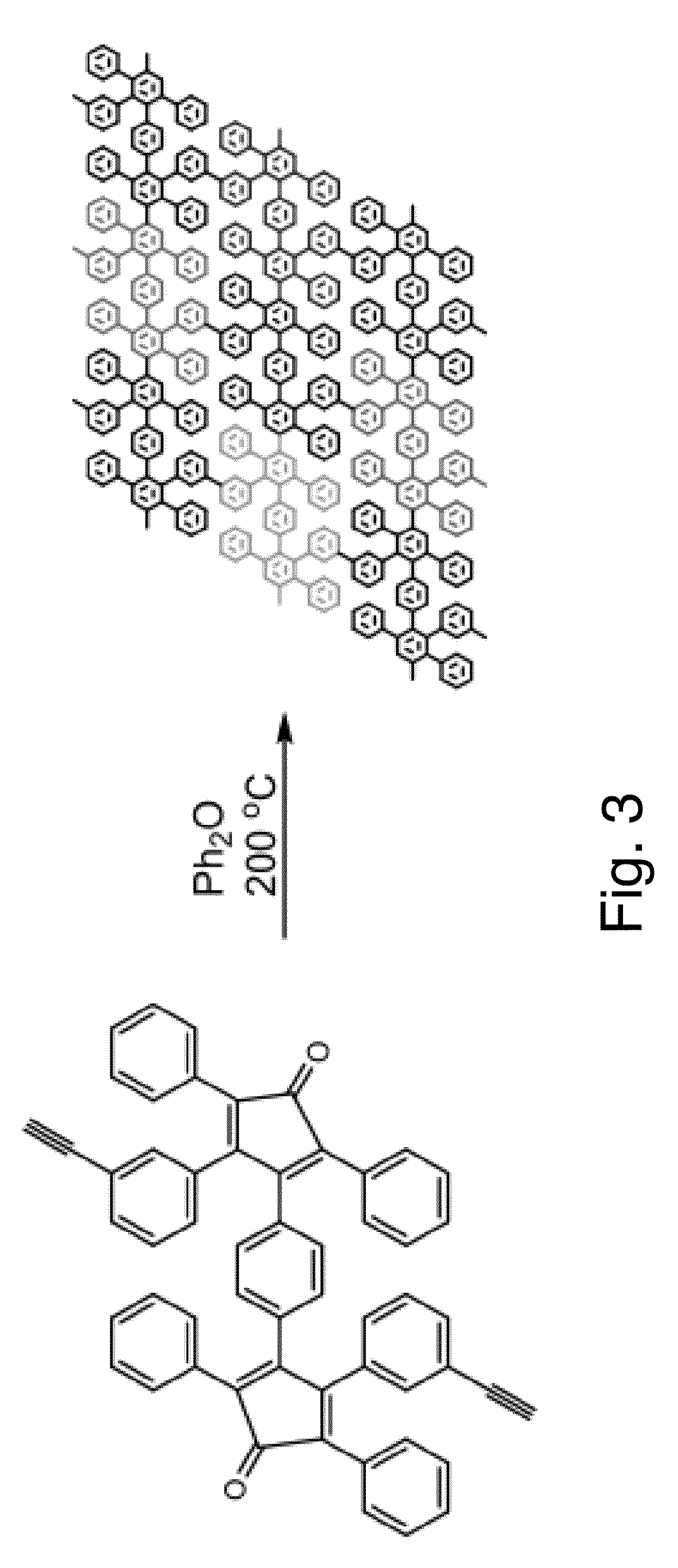
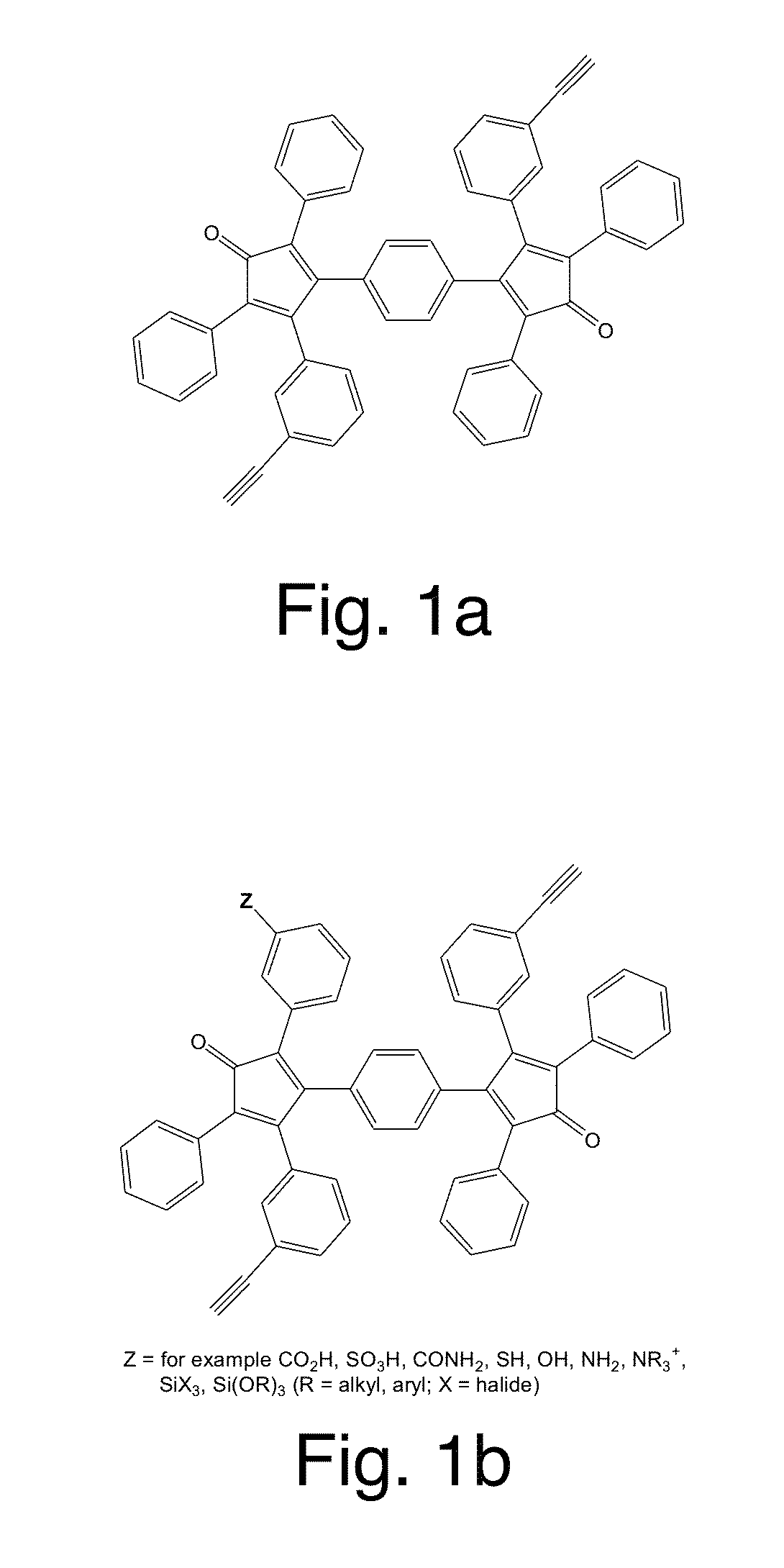
Patents
Literature
44 results about "Coronene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
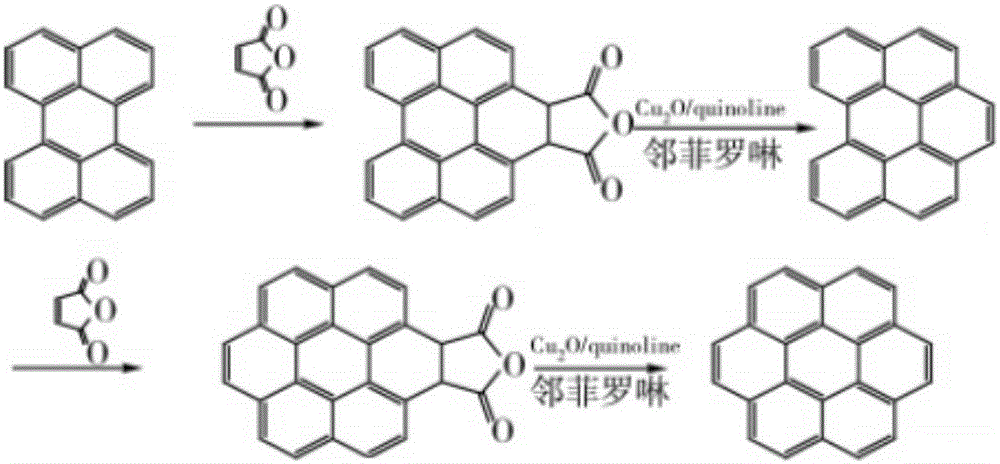
Coronene (also known as superbenzene) is a polycyclic aromatic hydrocarbon (PAH) comprising six peri-fused benzene rings. Its chemical formula is C₂₄H₁₂. It is a yellow material that dissolves in common solvents including benzene, toluene, and dichloromethane. Its solutions emit blue light fluorescence under UV light. It has been used as a solvent probe, similar to pyrene.
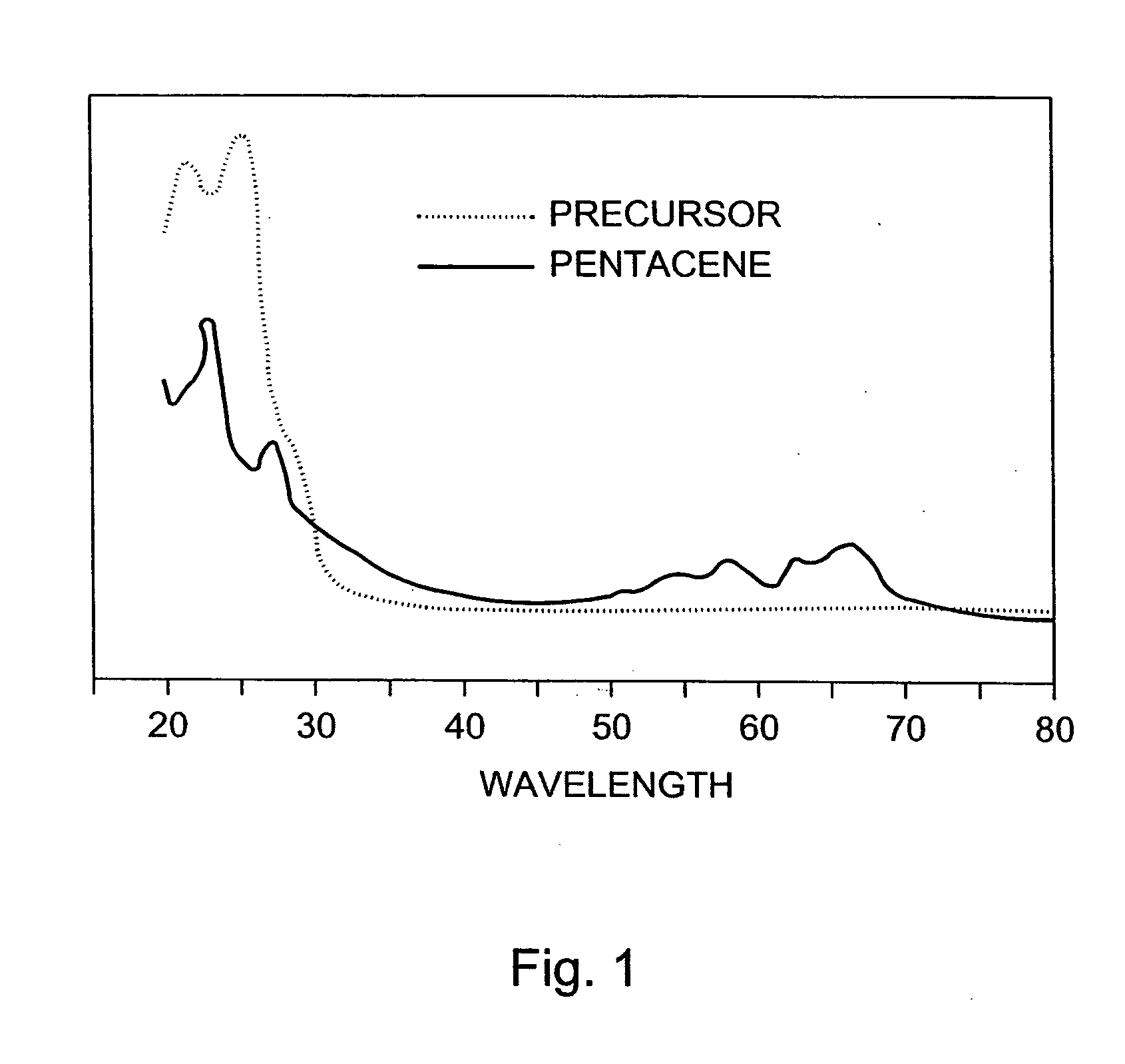
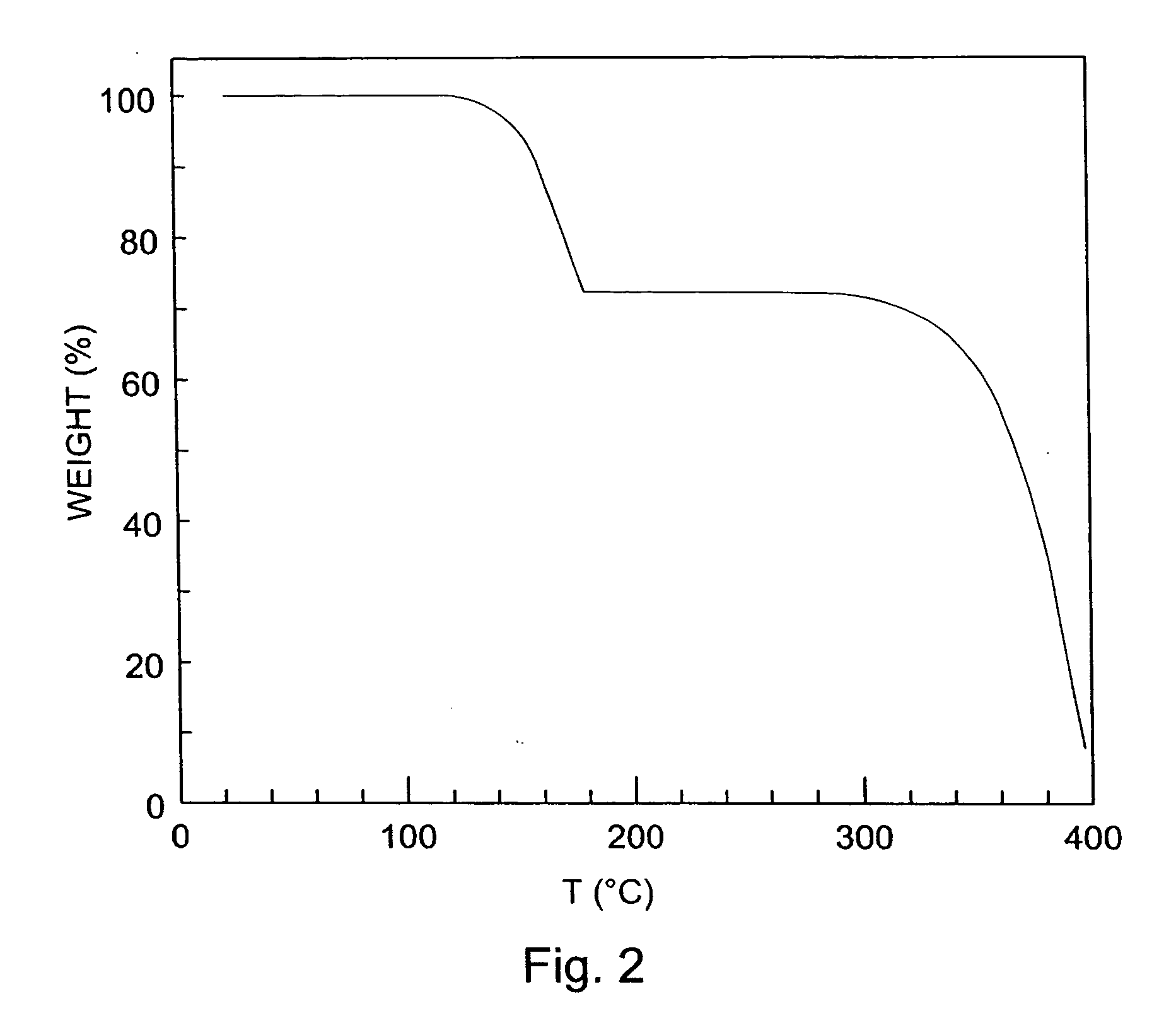
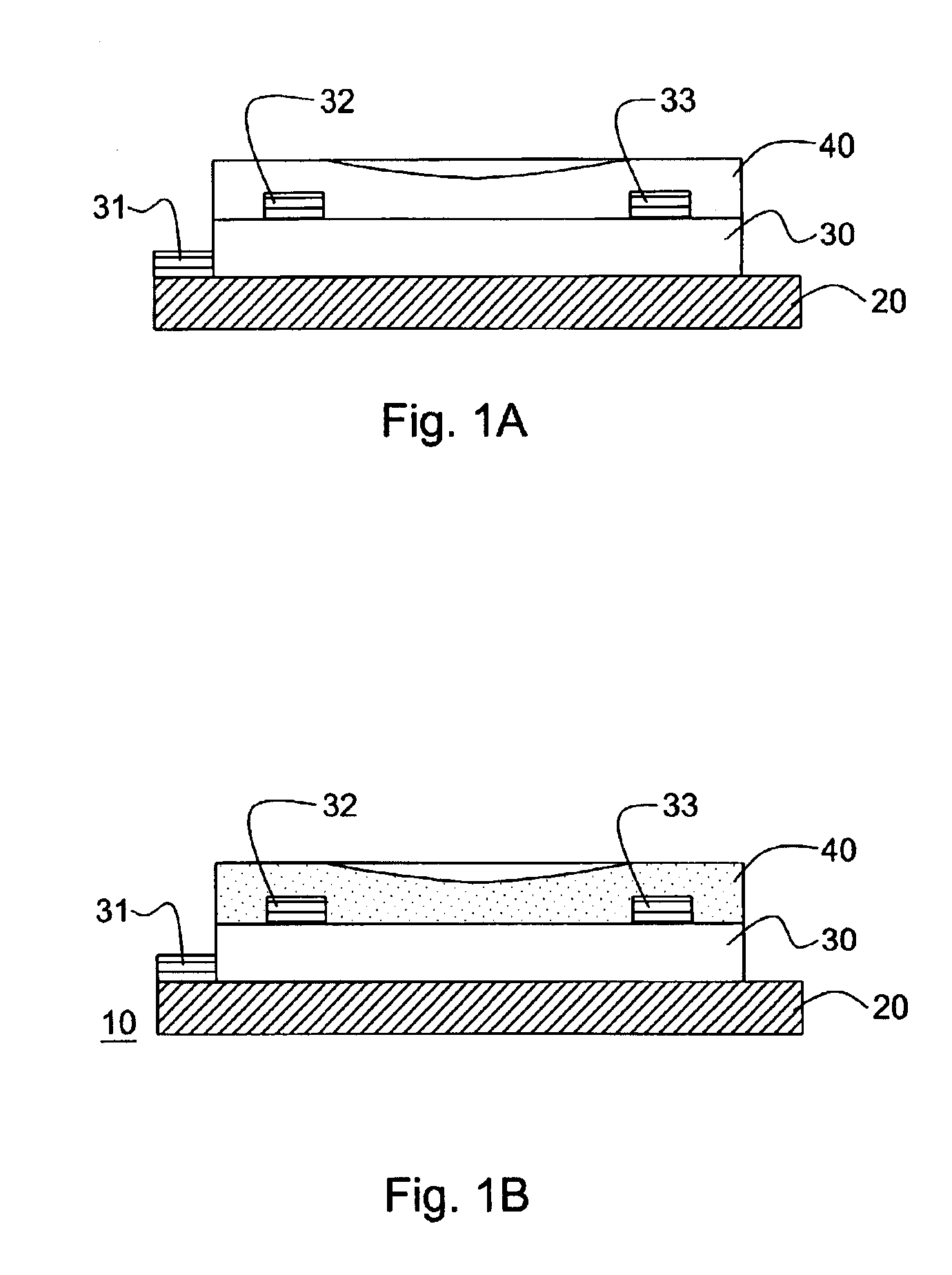
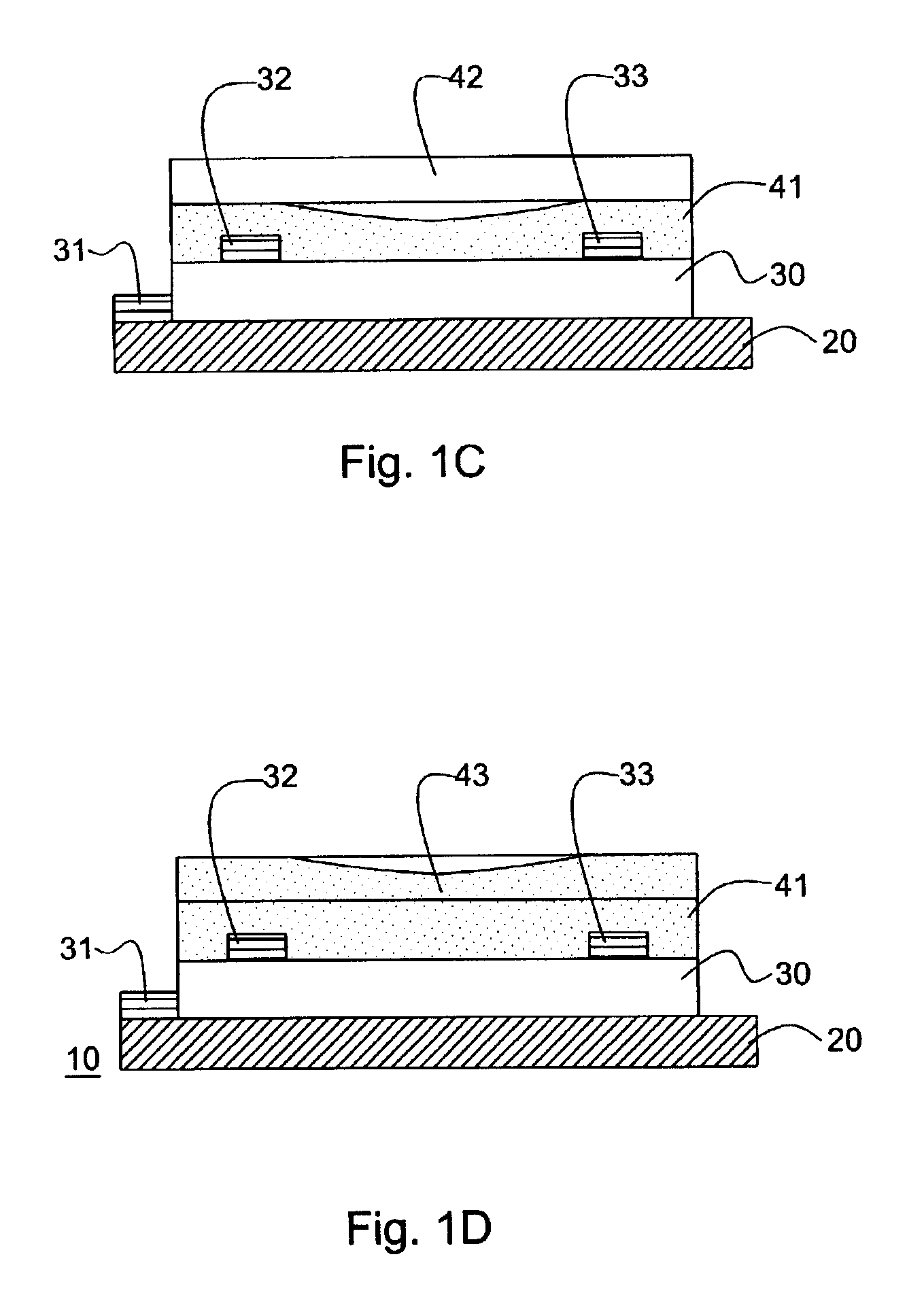
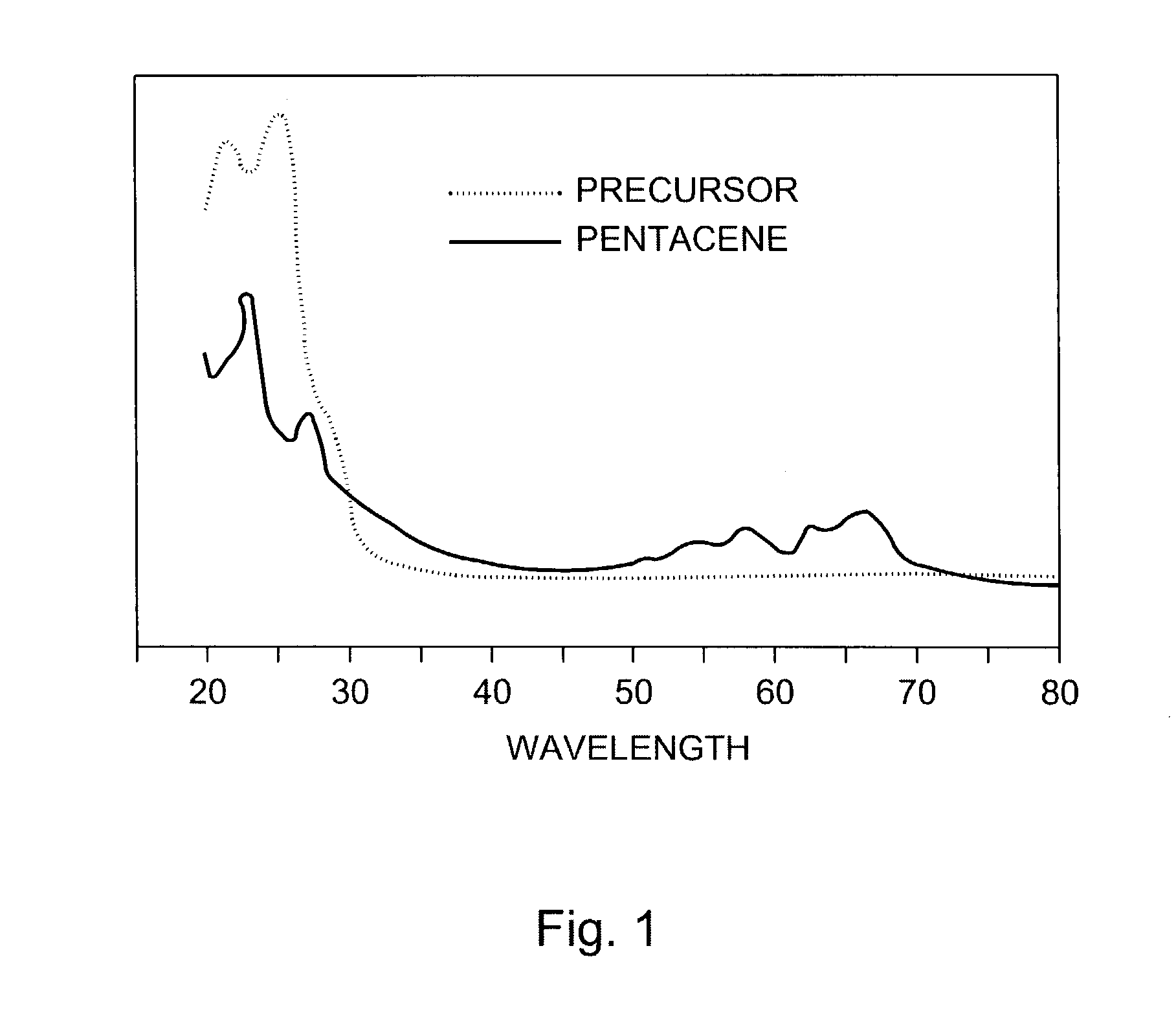
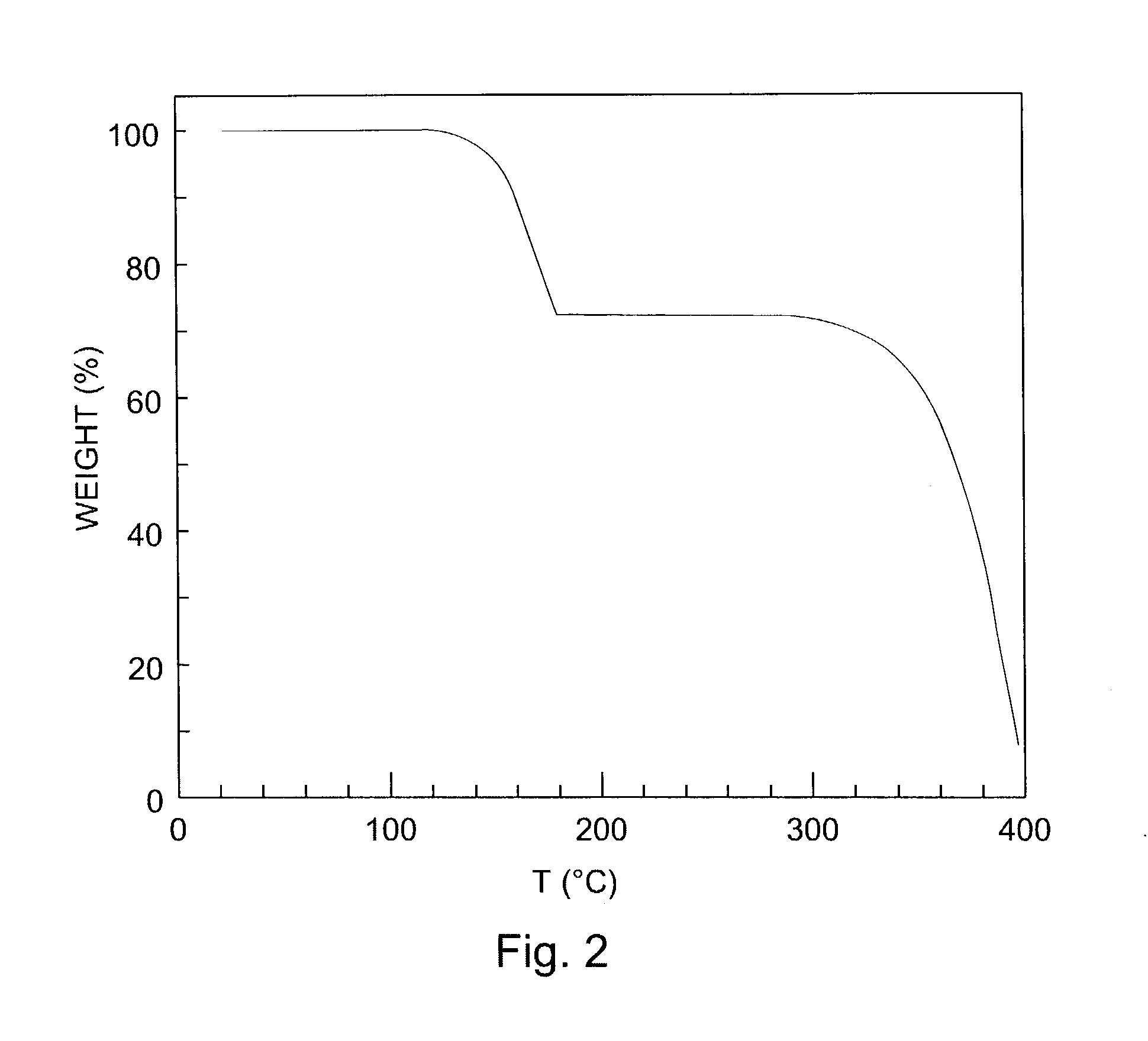
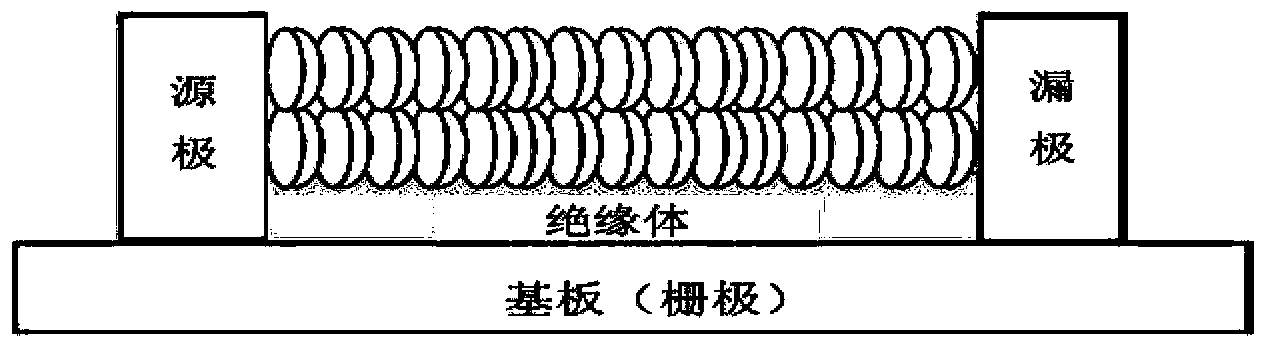
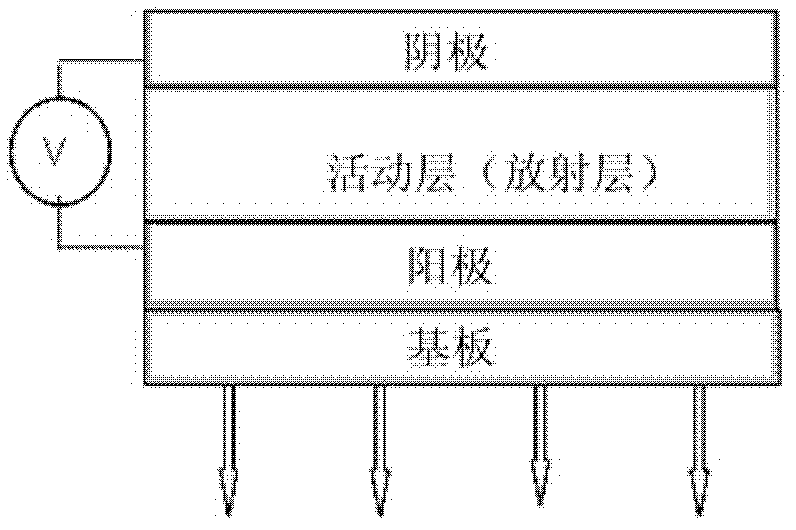
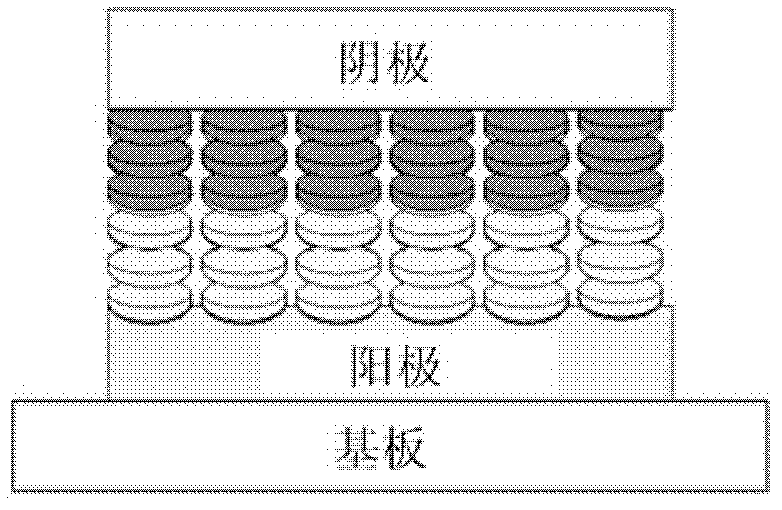
Thin film transistors using solution processed pentacene precursor as organic semiconductor
InactiveUS6963080B2Inexpensive approachLight weightMaterial nanotechnologyOrganic chemistryCoroneneArame
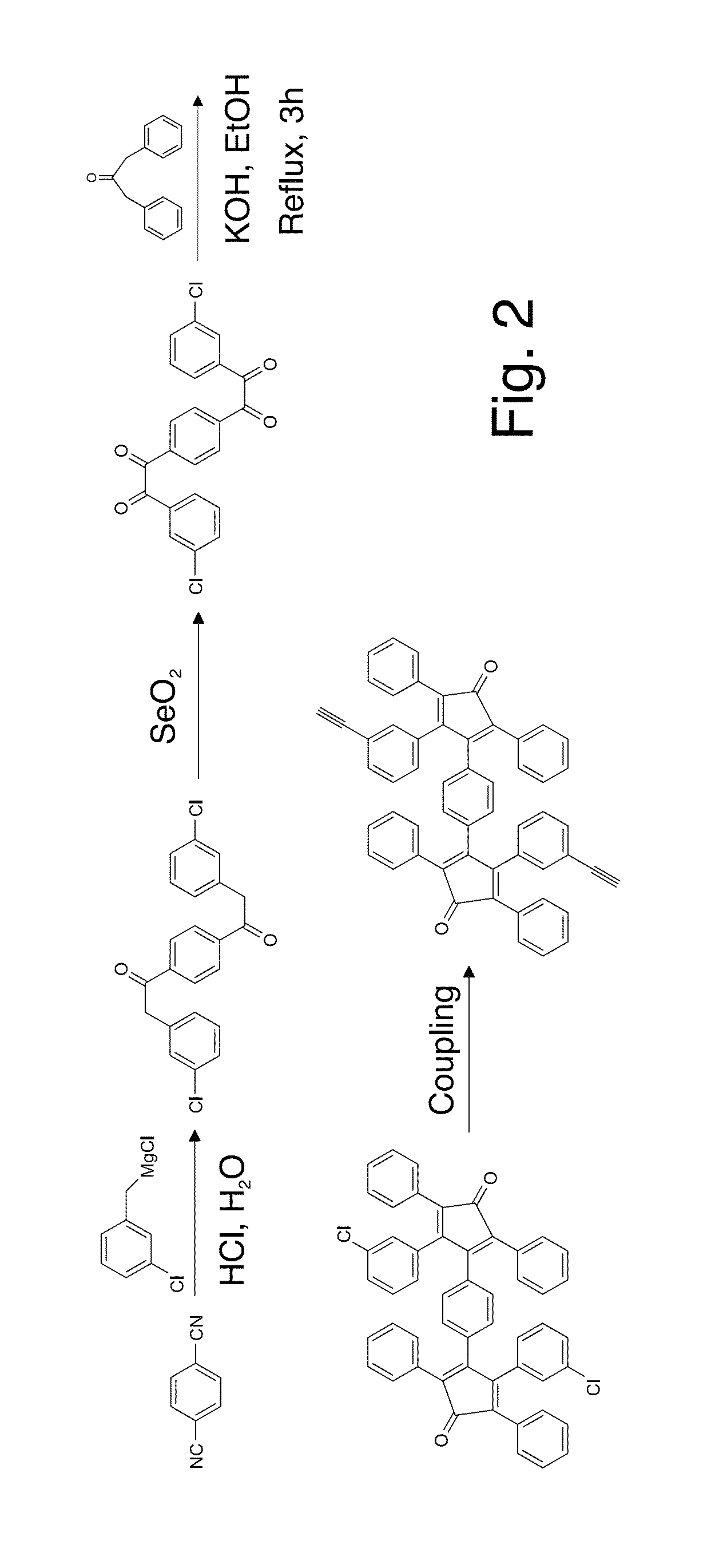
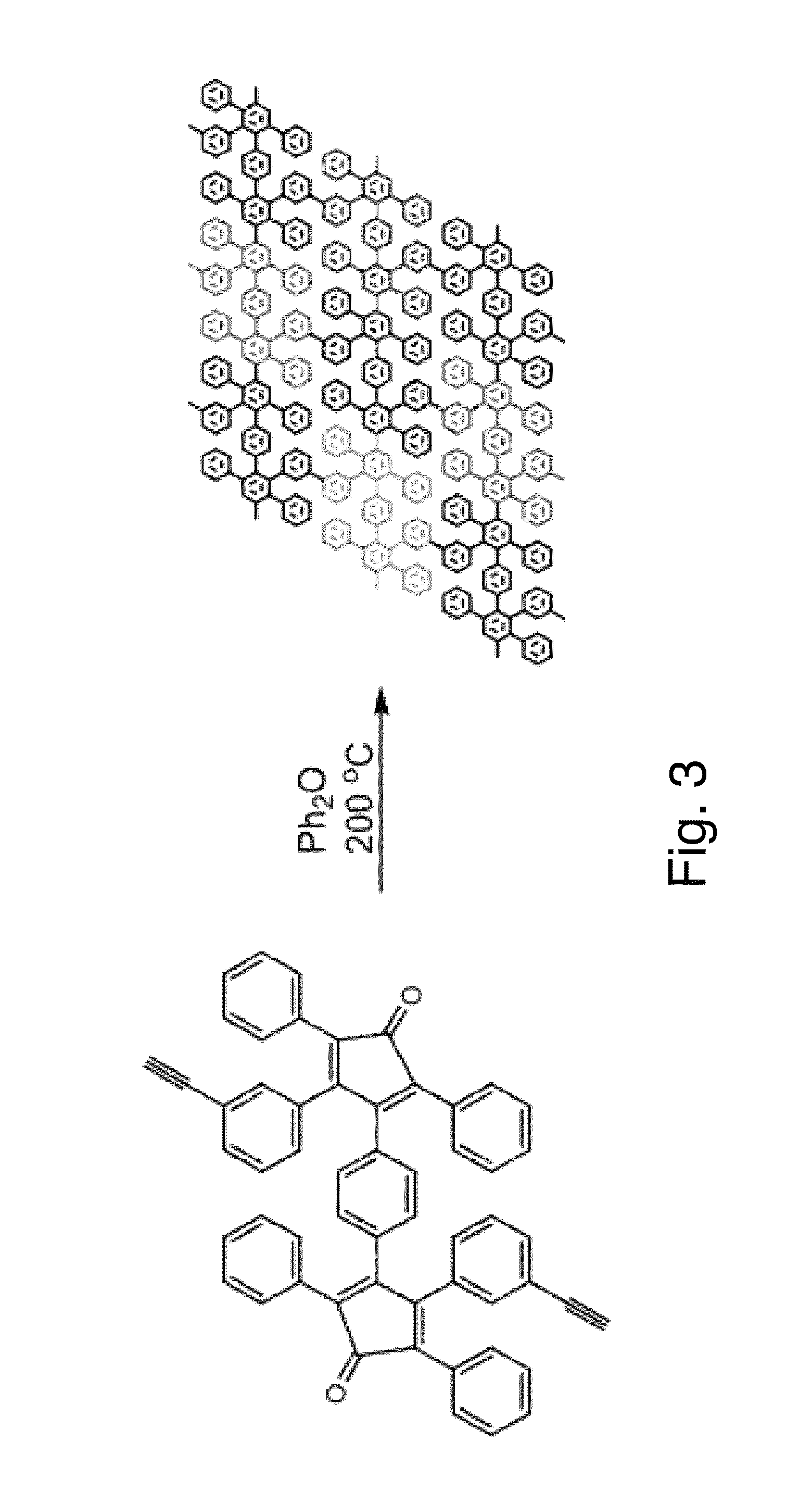
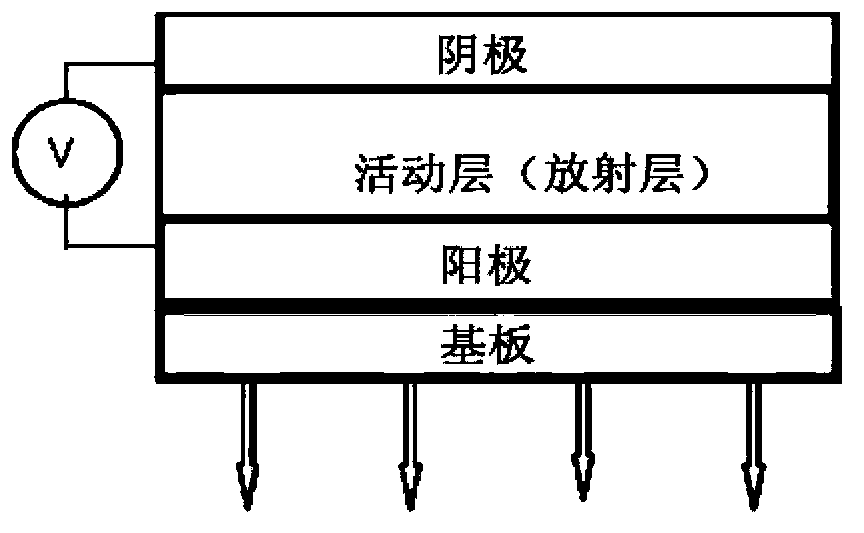
The present invention describes thin film transistors in which the active channel layer is a thin film of a polycyclic aromatic compound, such as, pentacene, prepared by solution processing a soluble precursor of the polycyclic aromatic compound on a substrate followed by heating to a moderate temperature to convert the precursor back to the polycyclic aromatic compound. The soluble precursors of the polycyclic aromatic compounds are organic solvent-soluble Diels-Alder adducts of polycyclic aromatic compounds, such as, oligothiophene, perylene, benzo[ghi]perylene, coronene and a polyacene with a variety of dienophiles that contain at least one heteroatom. The Diels-Alder adducts can be converted back to pentacene by retro-Diels-Alder reaction at moderate (60-250° C.) temperatures both in bulk, in solution or as thin-films.
Owner:GLOBALFOUNDRIES US INC
Hetero diels-alder adducts of pentacene as soluble precursors of pentacene
The present invention describes organic solvent-soluble Diels-Alder adducts of polycyclic aromatic compounds, such as, oligothiophene, perylene, benzo[ghi]perylene, coronene and polyacenes, with variety of dienophiles containing at least one heteroatom and in some cases two heteroatoms bonded to aromatic moiety, such as, thioxomalonates, azodicarboxylates, thialdehyde, acylnitroso and N-sulfinylamides. The Diels-Alder adducts are prepared by a simple, one step cycloaddition reaction of the polycyclic aromatic compounds, such as, pentacene, or other fused aromatic compounds, with heterodienophiles. The Diels-Alder adducts according to the present invention all form soluble adducts with pentacene and can be converted back to pentacene by retro-Diels-Alder reaction at moderate (60–250° C.) temperatures both in bulk, in solution or as thin-films.
Owner:GLOBALFOUNDRIES INC
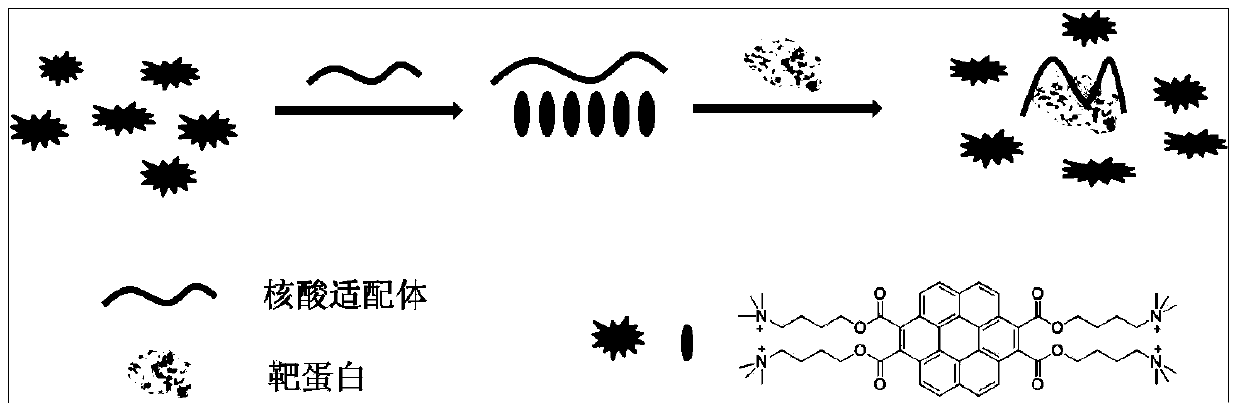
Coronene derivative probe and preparation method thereof, and protein detection method based on coronene derivative probe and aptamer
ActiveCN103992788AGood water solubilityEasy to prepareOrganic compound preparationFluorescence/phosphorescenceSolubilityProtein detection
The invention provides a coronene derivative probe and a preparation method thereof and a protein detection method based on the coronene derivative probe and aptamer. The objective of the invention is to overcome the problems of tedious operation, complex process and low sensitivity of a conventional protein detection method. The probe is 1,2,7,8-tetra-(4-trimethylamine butyloxycarbonyl)-coronene and has four charges and water solubility. The invention provides the preparation method for the coronene derivative probe. The invention further provides the protein detection method based on the coronene derivative probe and the aptamer. The protein detection method is marker-free, simple and fast and does not need covalent modification; moreover, a fluorescence enhanced signal is given out in detection, so compared with a fluorescence weakened signal, higher sensitivity is obtained.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
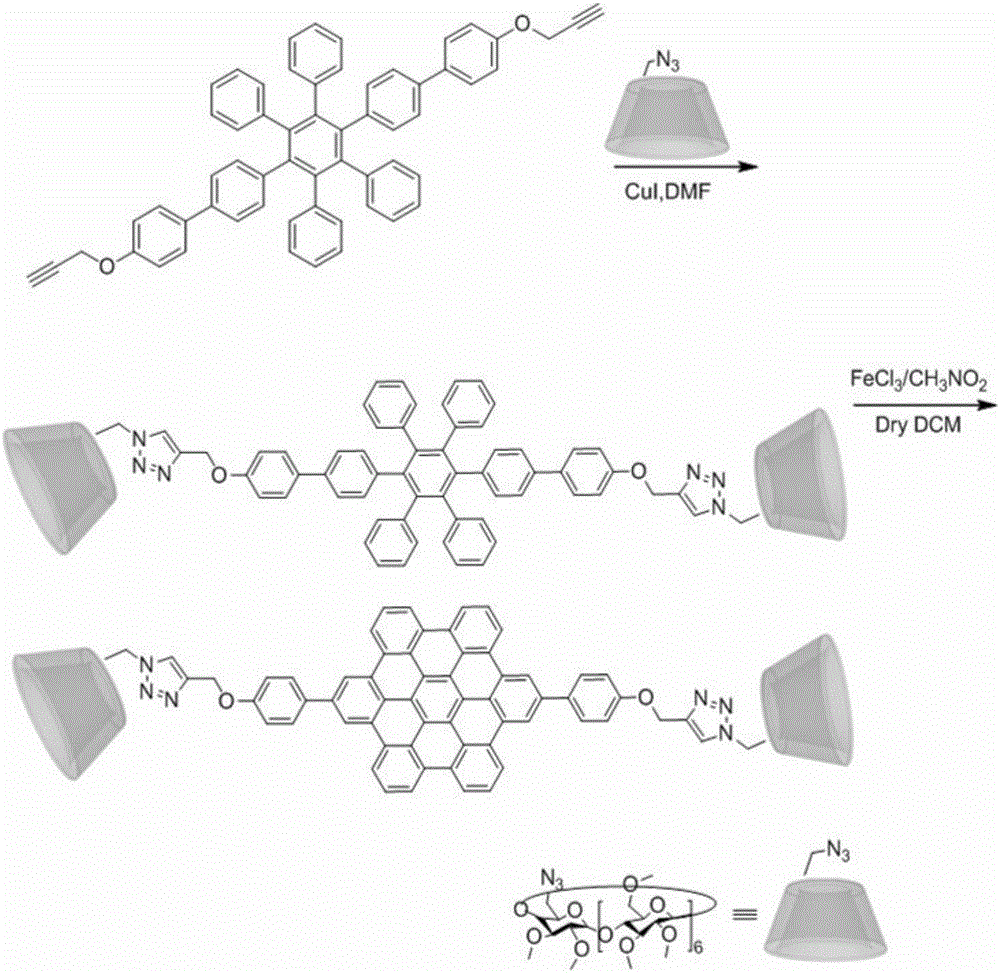
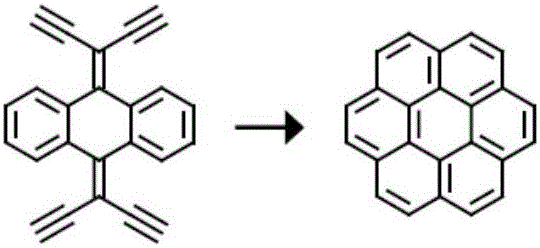
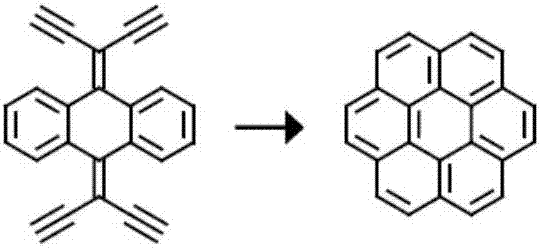
Three-dimensional nanometer graphene based on triptycene and preparation method thereof
ActiveCN103373892AStrong fluorescenceHigh yieldHydrocarbon by isomerisationFluorescence/phosphorescenceOrganic solventCoronene
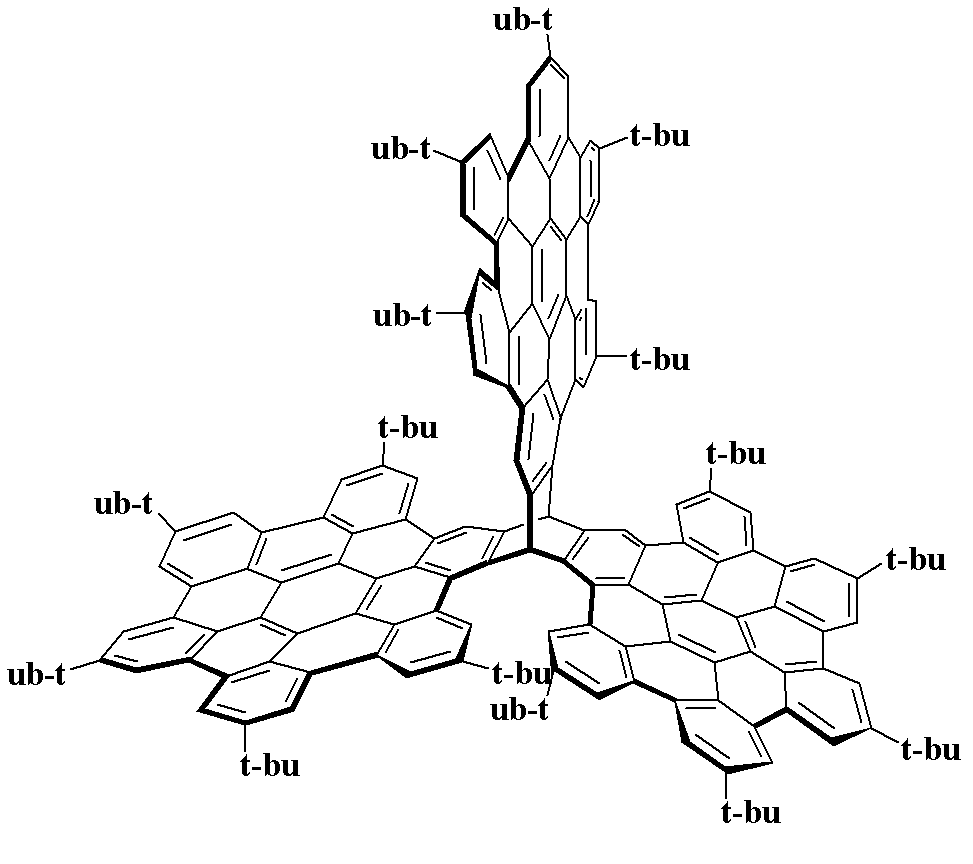
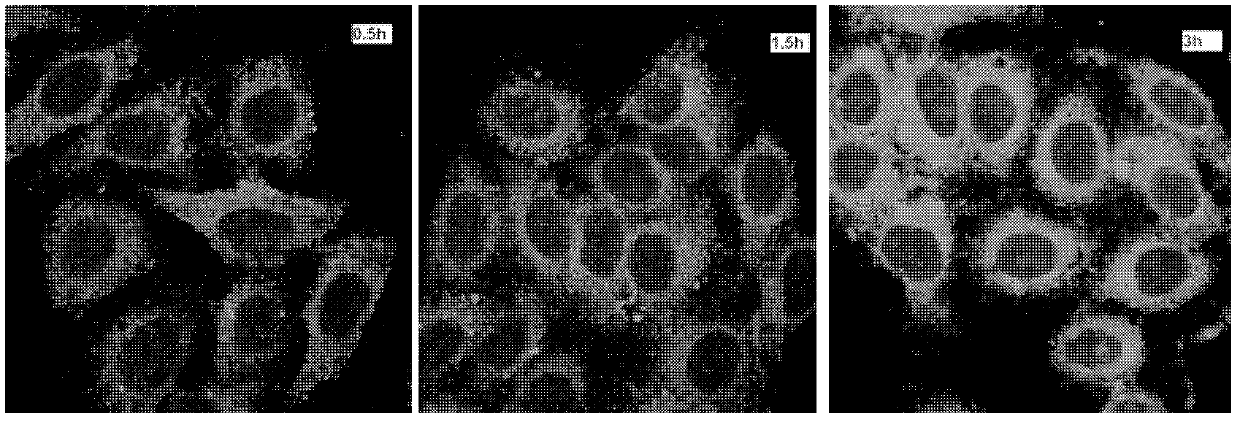
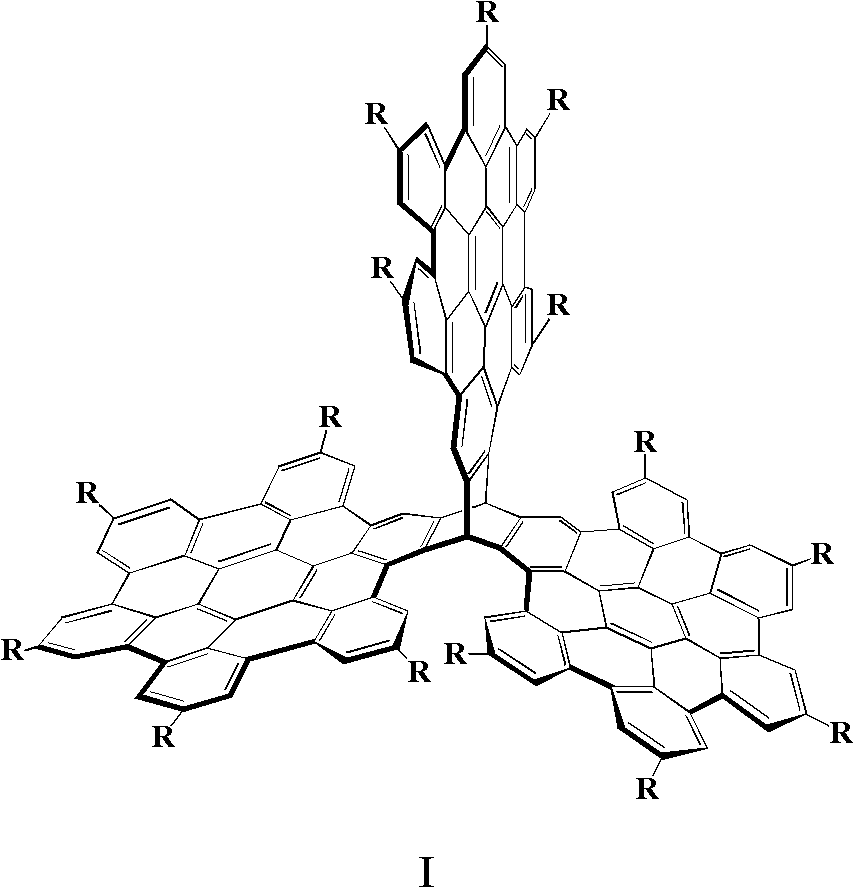
The invention discloses three-dimensional nanometer graphene based on triptycene and a synthetic method thereof. The three-dimensional nanometer graphene based on the triptycene is a novel coronene-modified triptycene derivative. The preparation method comprises the following steps of: firstly carrying out a Sonogashira coupling reaction on triiodo triptycene to obtain a tri-acetenyl triptycene derivative; then carrying out a Diels-Alder reaction on the tri-acetenyl triptycene derivative to obtain a coronene triptycene derivative; finally carrying out an FeCl3 oxidation and cyclization reaction in an organic solvent (dichloromethane) under the conditions of gas protection (Ar) and normal temperature for 15-240 minutes to obtain the three-dimensional nanometer graphene based on the triptycene. The preparation method disclosed by the invention is novel and higher in yield. The prepared three-dimensional nanometer graphene based on the triptycene disclosed by the invention has an outstanding effect on the aspect of cell imaging.
Owner:HUAZHONG UNIV OF SCI & TECH
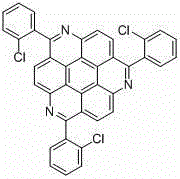
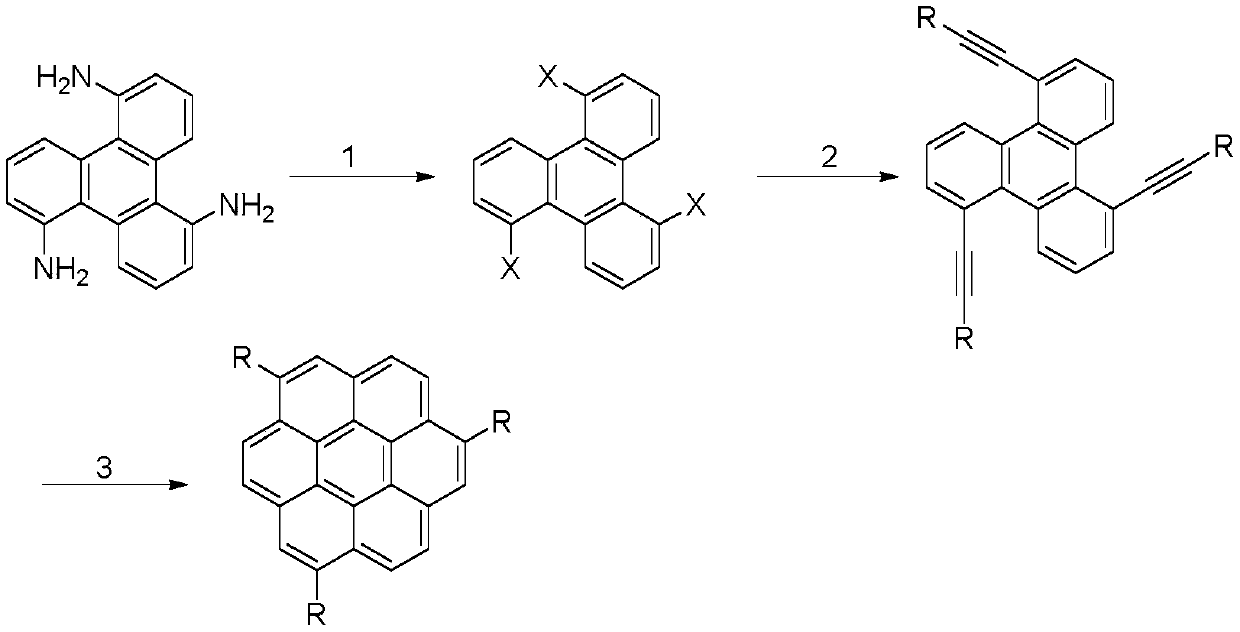
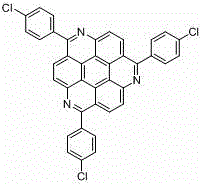
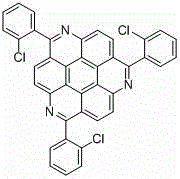
1, 5, 9-trisubstituted coronene compound and synthesis method thereof
ActiveCN106565408AReduce pollutionImprove thermal stabilityOrganic compound preparationHydrocarbonsFuranCoronene
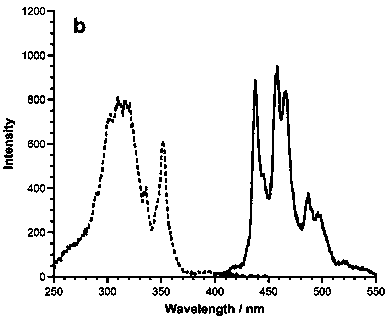
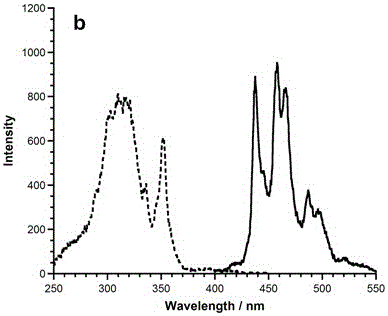
The present invention relates to a 1, 5, 9-trisubstituted coronene compound and a synthesis method thereof. The structural formula of the compound is shown in img file = 'dest _ path _ image 001. TIF 'wi = '109 'he = '108', wherein R represents H, C1-C18 alkyl, phenyl, 4-methylphenyl, 4-methoxy phenyl, benzyl, cyclohexyl, 4-trifluoromethylphenyl, thiophene, furan and the like. According to the technical scheme of the invention, the easily prepared 1, 5, 9-triamido triphenylene is subjected to diazotization and halogenation reaction to obtain the tri-halogenated triphenylene. After that, the tri-halogenated triphenylene is subjected to Sonogashira reaction with various alkynes to generate atriyne-triphenylene compounds. Finally, through the metal-catalyzed reaction and the cyclization reaction under the effect of an organic base, various 1, 5, 9-trisubstituted coronene compounds, novel in structure, can be obtained. According to the technical scheme of the invention, raw materials are easy for mass preparation. Meanwhile, the synthesis step is relatively short and the operation is convenient. The obtained trisubstituted coronene compound is good in thermal stability and chemical stability, and the trisubstituted coronene emits the relatively strong fluorescence within the range of 420-550 nm according to the fluorescence emission spectrum of the trisubstituted coronene compound. Therefore, the trisubstituted coronene is an excellent fluorescent material for preparing UV ultraviolet charge-coupled devices (UV-CCD) and organic light-emitting diodes (OLEDs), and has a wide application prospect in the field of electronic materials.
Owner:SHANGHAI UNIV
Synthon and method for preparing dibenzocoronene compounds by same
ActiveCN103570714AImprove solubilityEasy to purifyLiquid crystal compositionsOrganic chemistryCoroneneSynthon
The invention relates to a synthon and a method for preparing dibenzocoronene compounds by the same. The synthon is a tetrahydroxy-substituted perylene bisimide compound, and has a structure as shown in general formula (I), wherein R is a C1-C18 linear chain primary alkyl or a primary alkyl with a branched chain. According to the invention, dibromo-perylene anhydride or mixtures are used for preparing dibromo-imide; dibromo-imide and phenylboronic acid substituted by alkoxys at 3 and 4 positions are subjected to a Suzuki coupling reaction under the catalysis of Pd(PPh3)4 to prepare aryl-substituted perylene bisimide compounds; further dealkylation reaction is carried out under the action of boron tribromide so as to form tetrahydroxy-substituted perylene bisimide compound synthon; then the synthon is used as a raw material to synthesize charge transfer compounds of coronene compounds with imide groups at two sides of the molecule. The synthetic method of the invention is simple, high in yield, and widely applicable to organic light-emitting diodes, field effect transistors, and solar cells. The general formula (I) is shown in the description.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
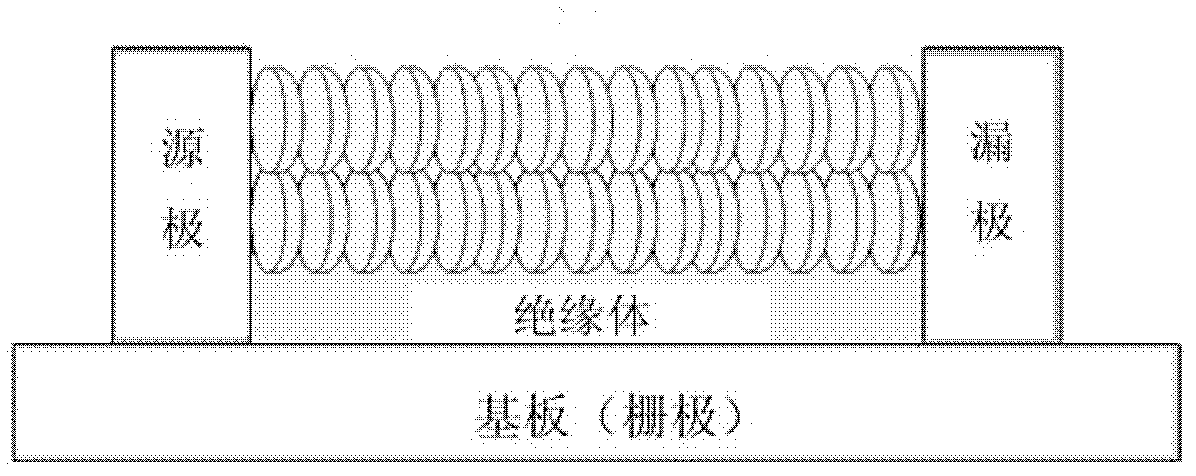
Method for bottom-up graphene sheet preparation and bandgap engineering
A combination of a substrate selected from silicon, silicon carbide or a metal and a graphene precursor having the following properties: (a) an aromatic structure that forms the basis of the graphene structure, said aromatic structure being selected from the group consisting of: benzene, naphthalene, pyrene, anthracene, chrysene, coronene, and phenanthrene, or a cyclic or acyclic structures which can be converted to aromatic structures and (b) functional groups that can react with each other to form additional aromatic structures.
Owner:HRL LAB
Coronene compound containing thioether in lateral chain and preparation method thereof
ActiveCN102584655AHigh carrier mobilitySolid-state devicesSemiconductor/solid-state device manufacturingCoroneneHexaphenylbenzene
The invention relates to coronene compound containing thioether in lateral chain and the preparation method thereof. The structural formula of the compound is shown in the descriptiopn: R refers to alkyl. In the method, ring trimerization- ferric trichloride coronene method, namely 2- (4'- bromophenyl) ethanol is taken as raw material, through hydroxyl bromo, thioether is formed, palladium-catalyzed is selected for direct coupled reaction, so as to obtain diphenylacetypene ramification, under the catalysis of cobalt carbonyl, corresponding hexaphenylbenzene is produced by trimerization cyclization. Anhydrous ferric trichloride is oxidized in methylene dichloride and nitromethane, then reduction is performed by using iodine and sodium borohydride system, and after drying, THF (tetrahydrofuran) is used for recrystal. The synthetic method provided by the invention is simple, is high in yield, and can be extensively used in organic LBD, field-effect tubes and solar batteries.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
Method for preparing graphene aqueous solution by using water soluble coronene derivative as solubilizer
ActiveCN102303862AStructure will not breakImprove electrical performanceTransportation and packagingMixingCoroneneHydrazine compound
The invention discloses a method for preparing graphene aqueous solution by using a water soluble coronene derivative as a solubilizer, which comprises: dispersing graphene solid in ultrapure water, adding the water soluble coronene derivative, mixing, adding hydrazine hydrate, stirring, centrifuging, removing supernate, dissolving precipitate in ultrapure water, and thus, obtaining graphene aqueous solution. In the invention, the operation is simple, the property of the prepared graphene aqueous solution is stable, the dispersion state of the graphene in water can be regulated by changing the used amount of solubilizer, and the method has a bright application prospect in nano science, biomedicine and material science.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
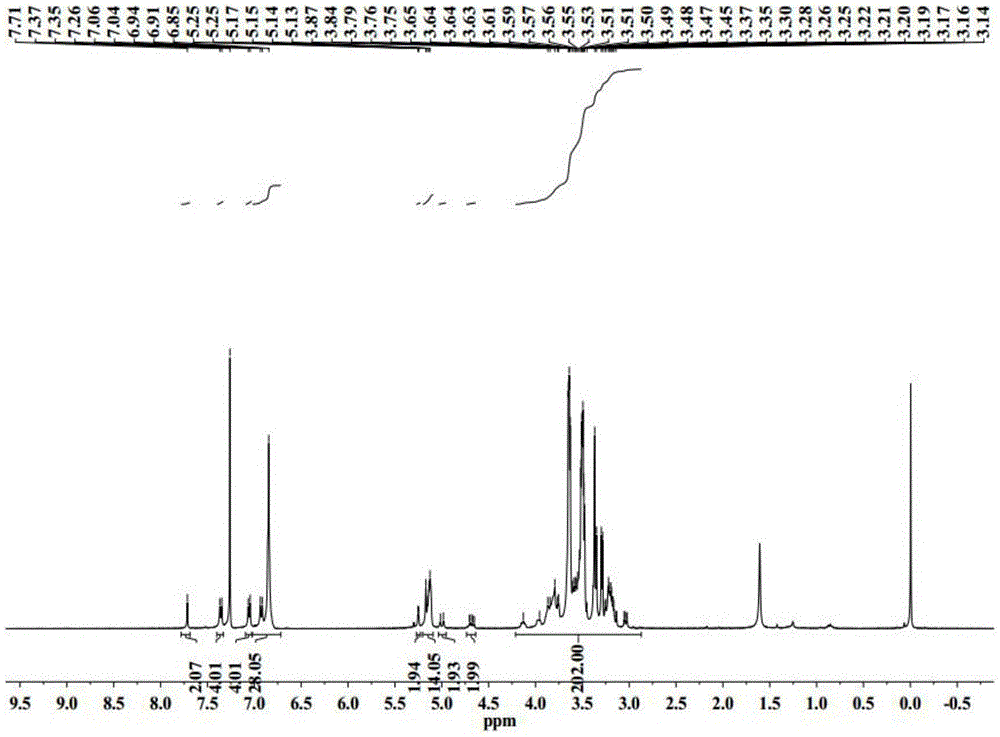
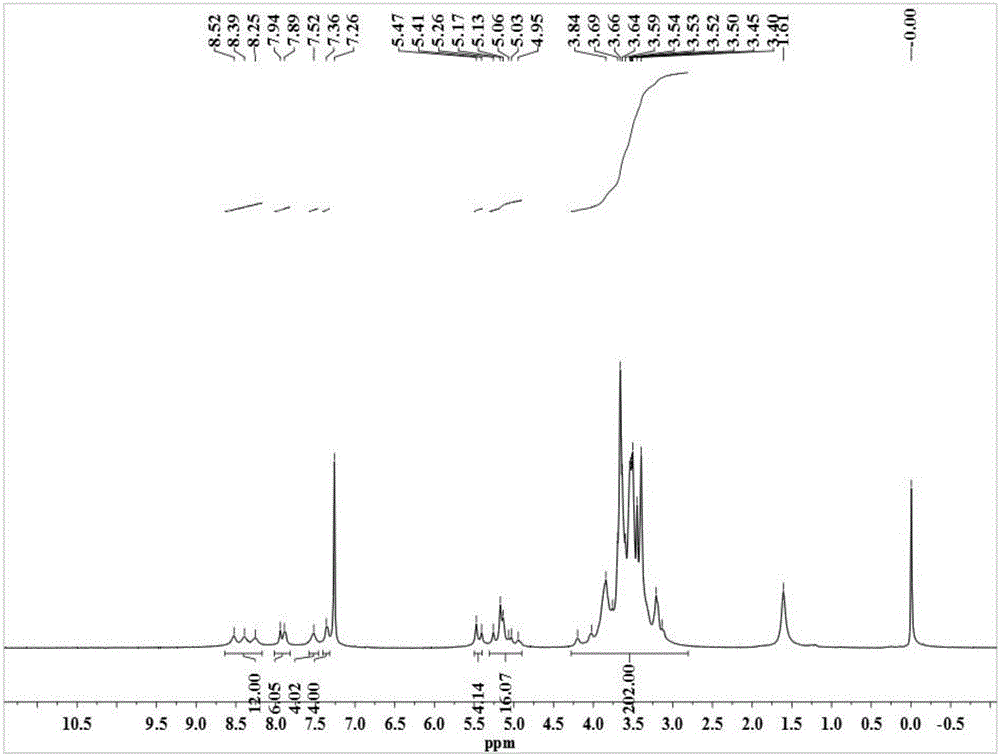
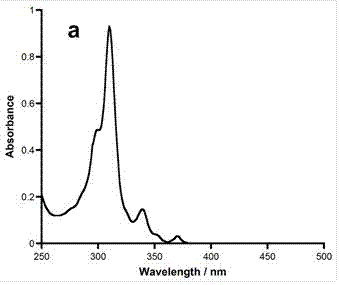
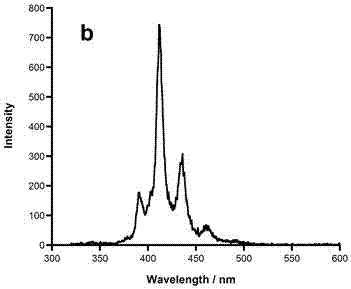
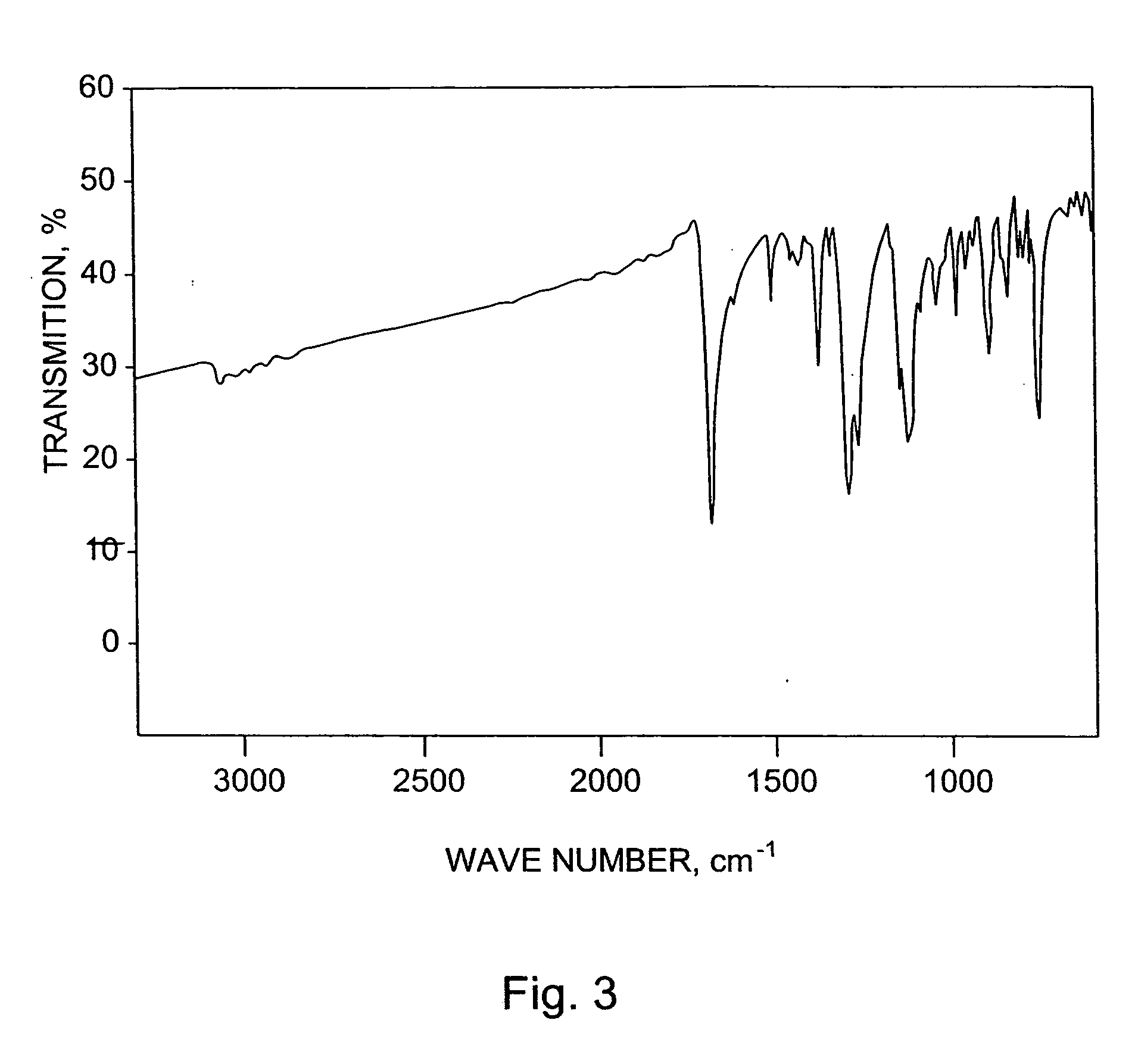
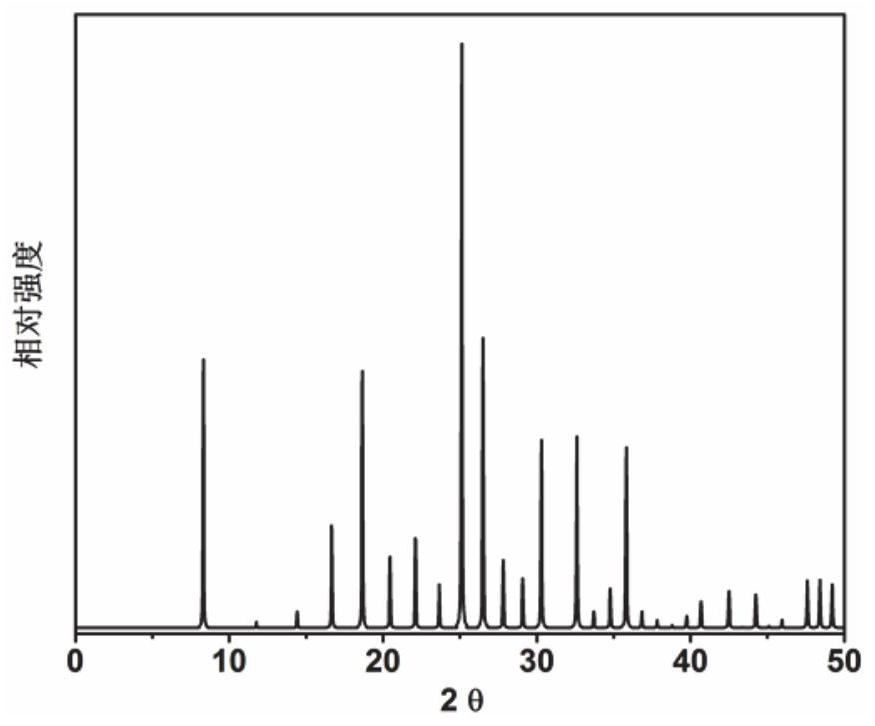
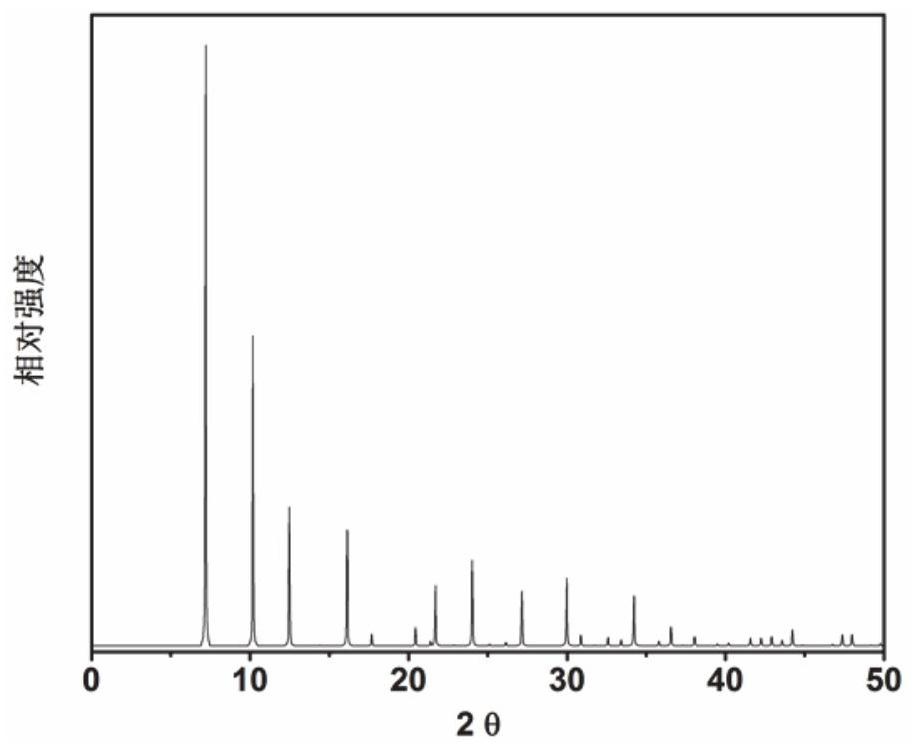
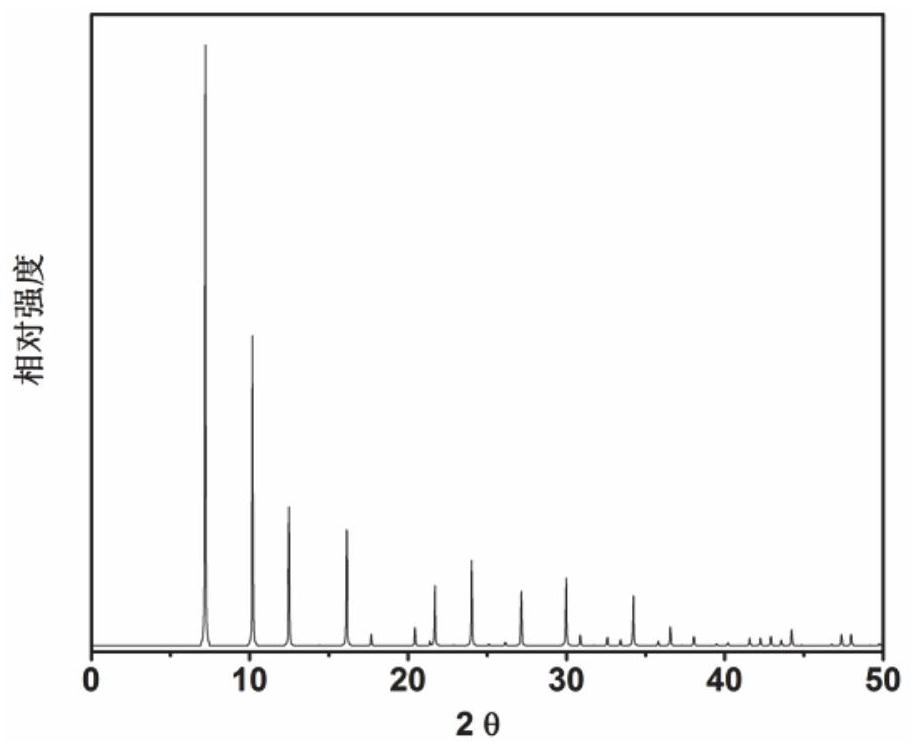
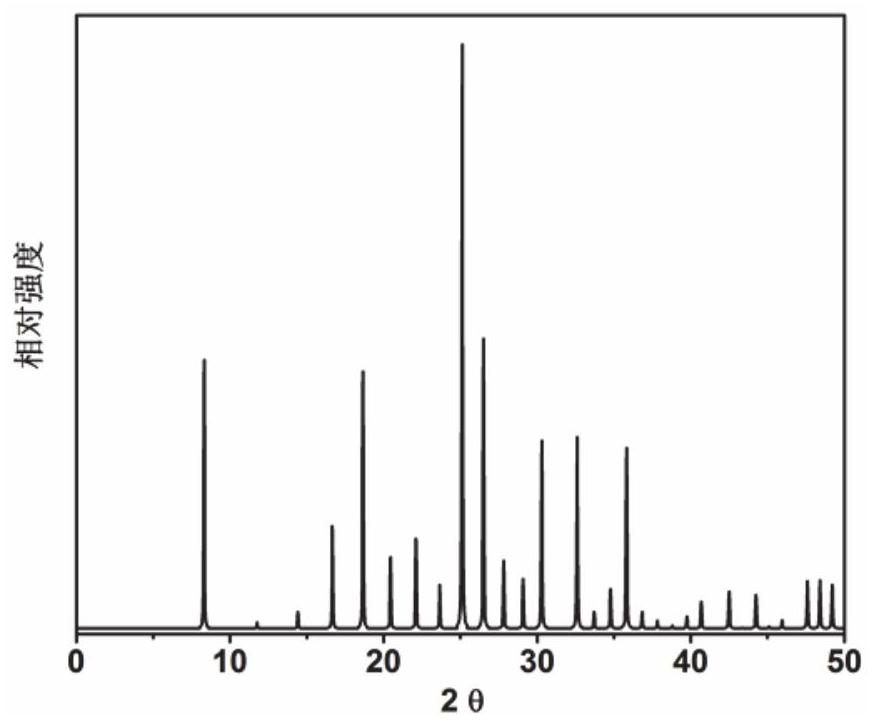
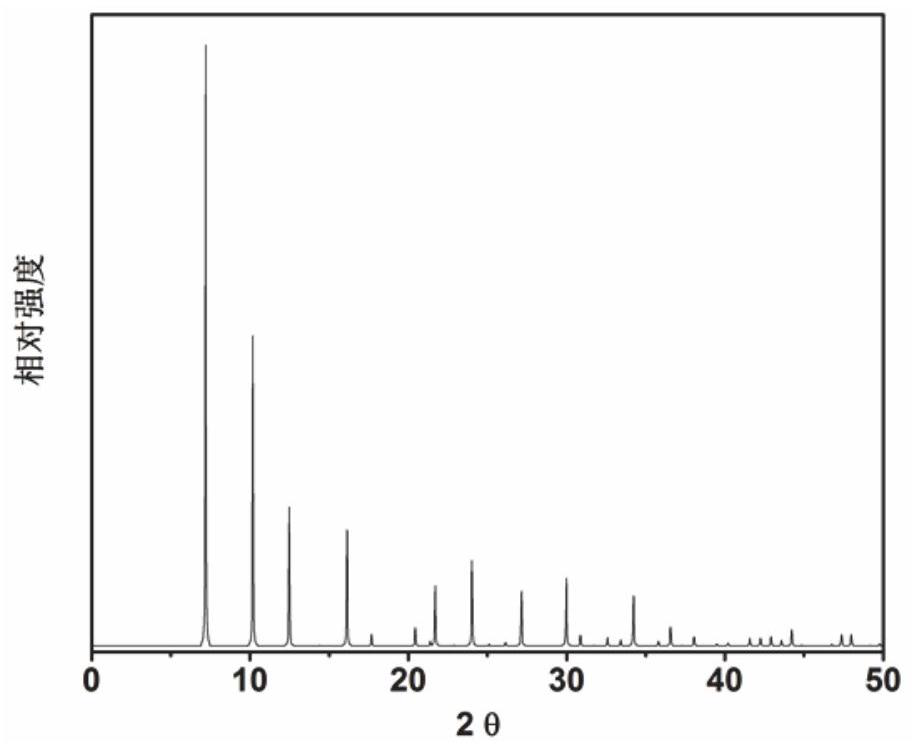
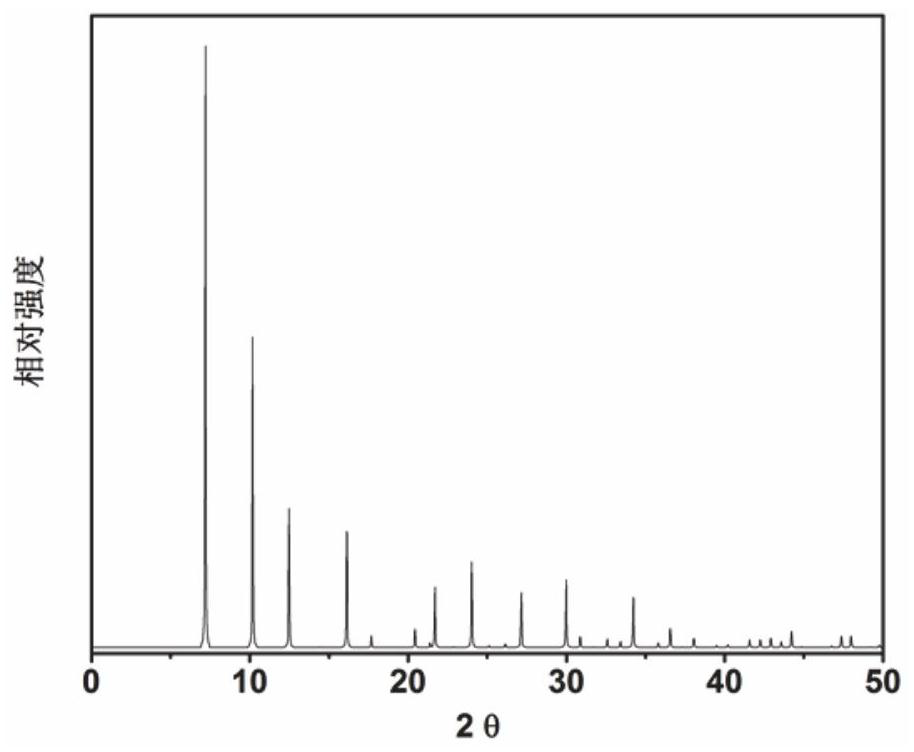
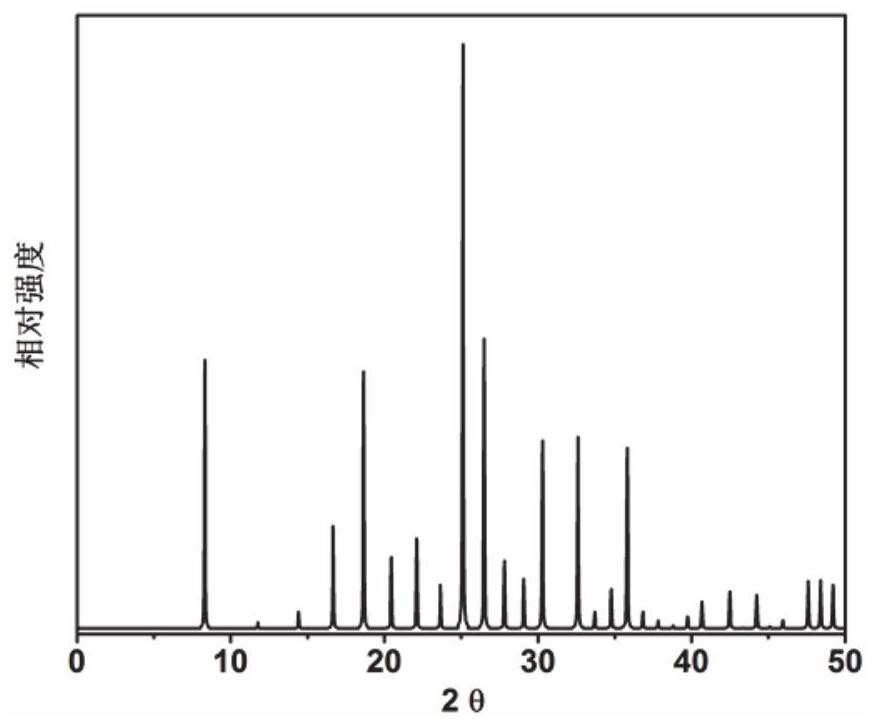
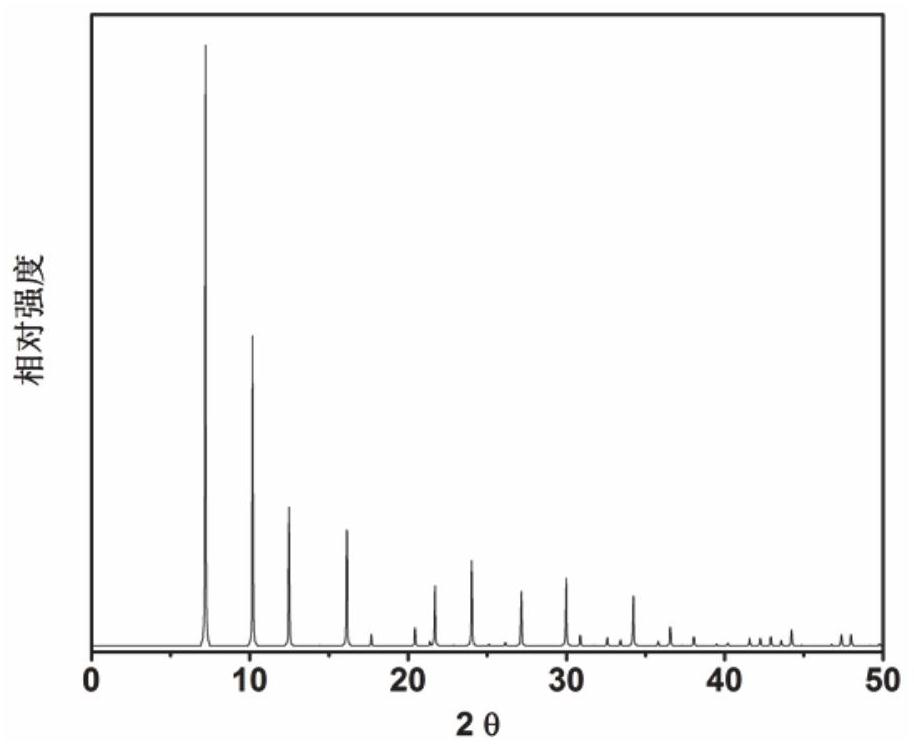
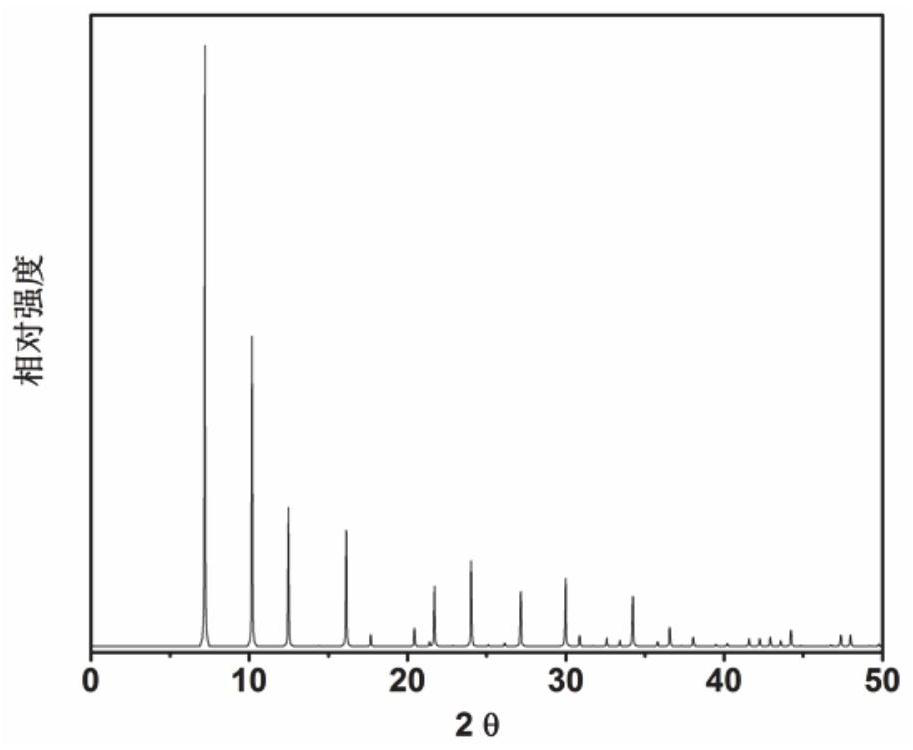
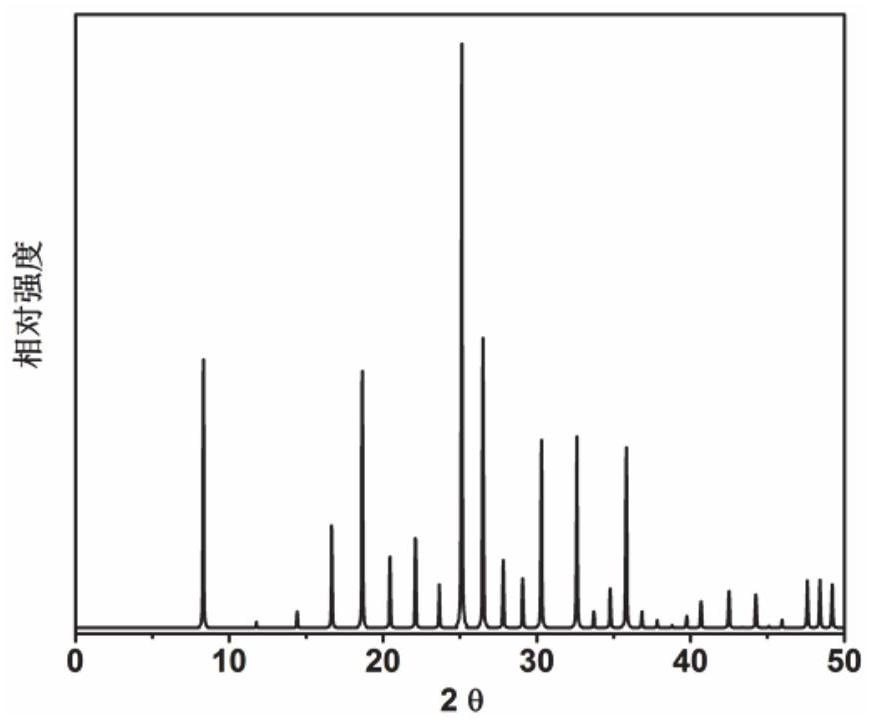
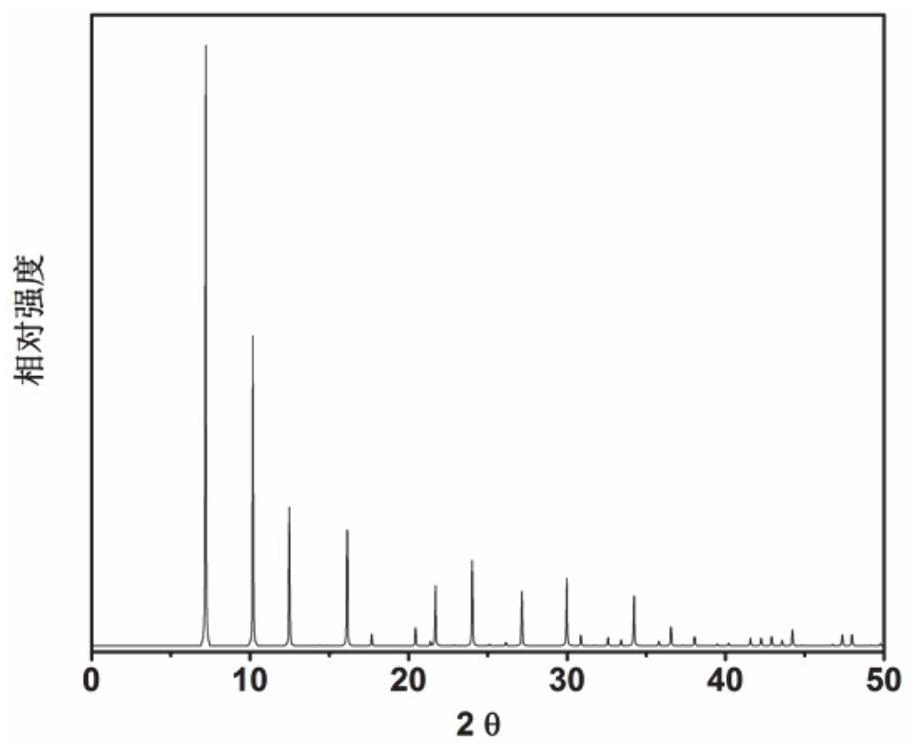
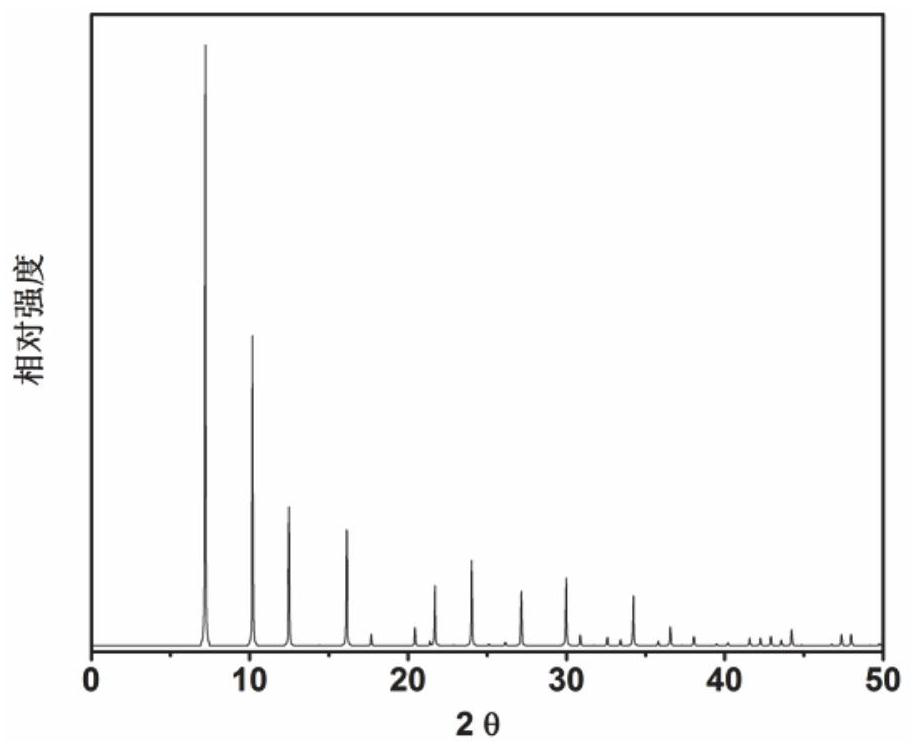
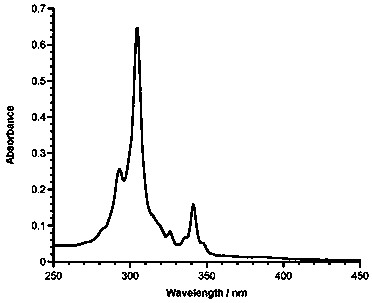
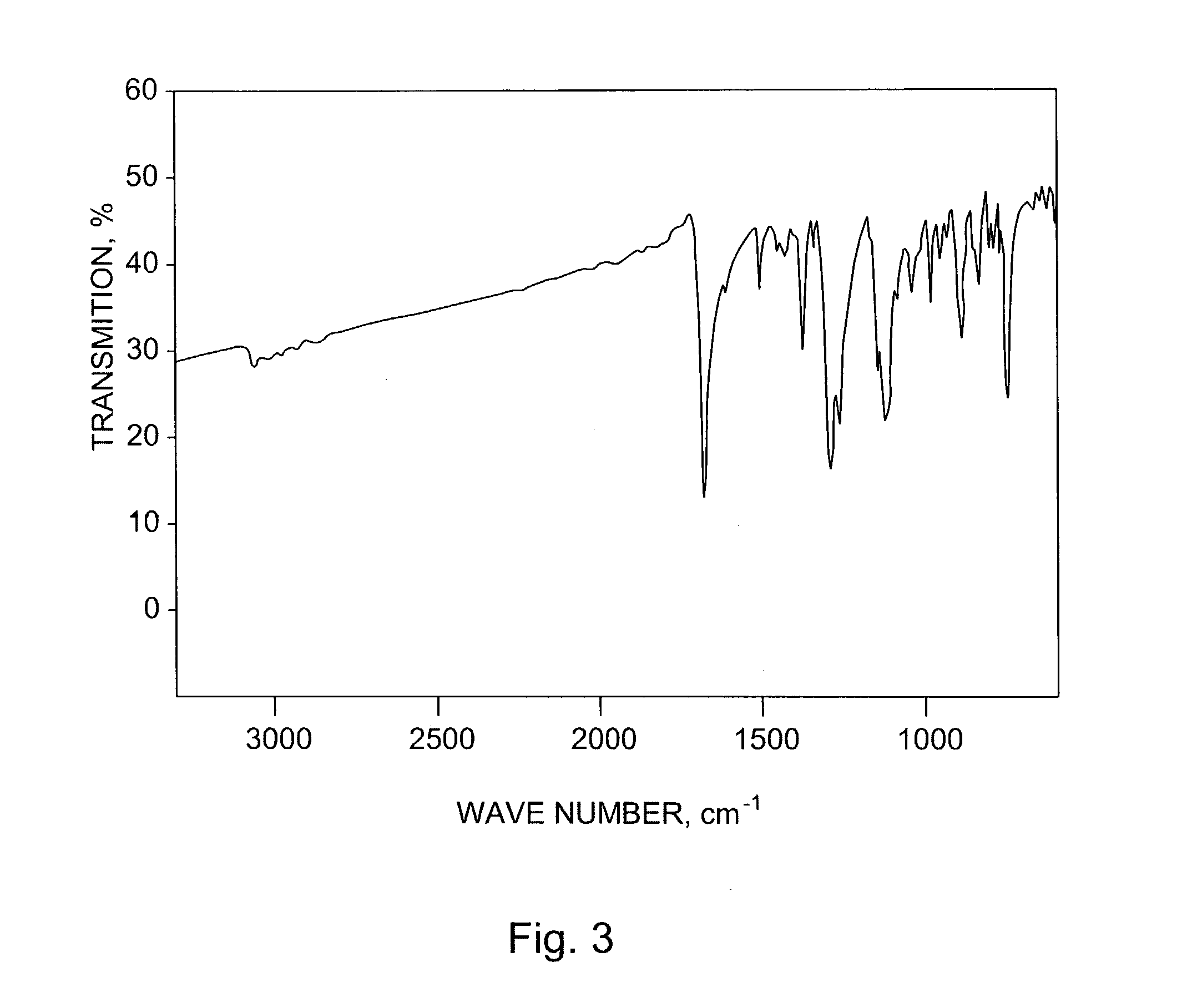
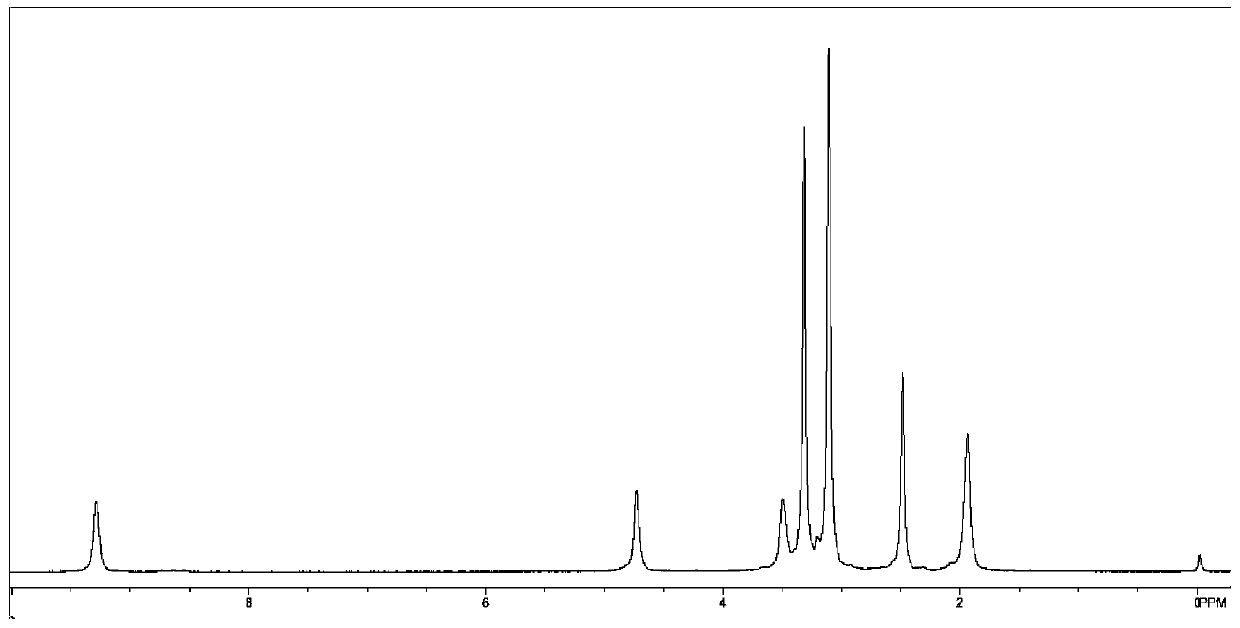
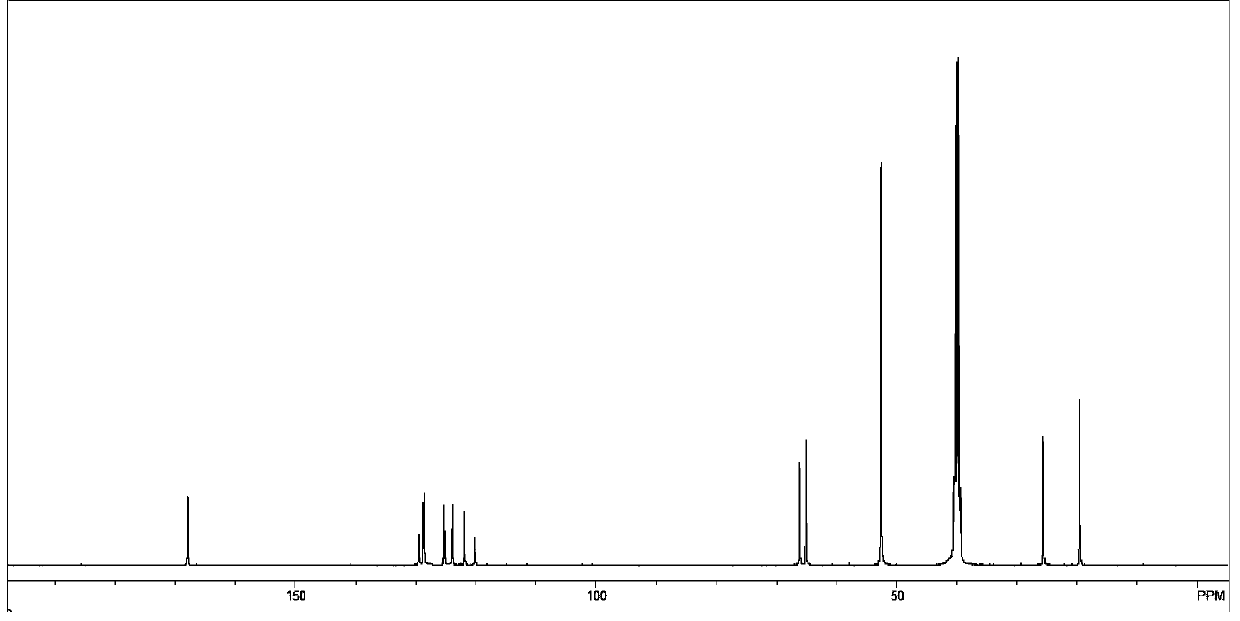
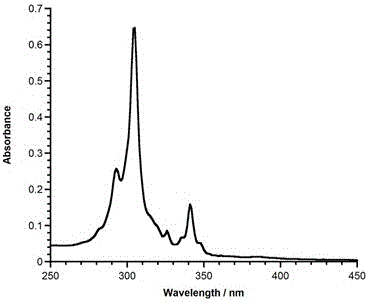
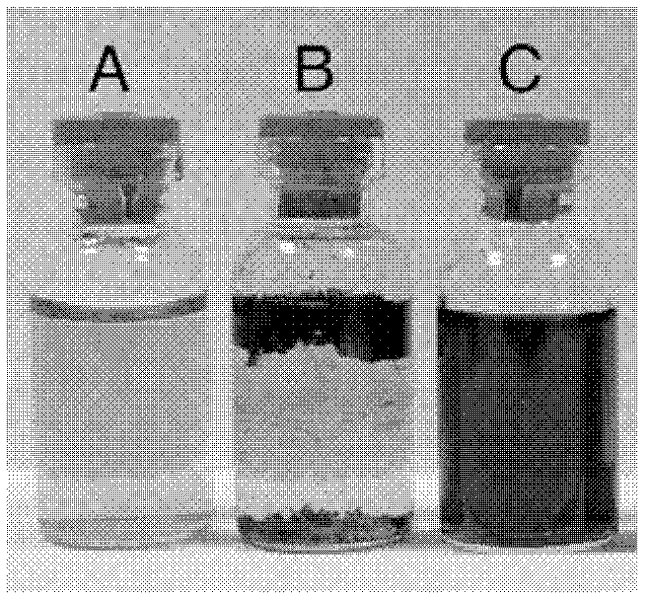
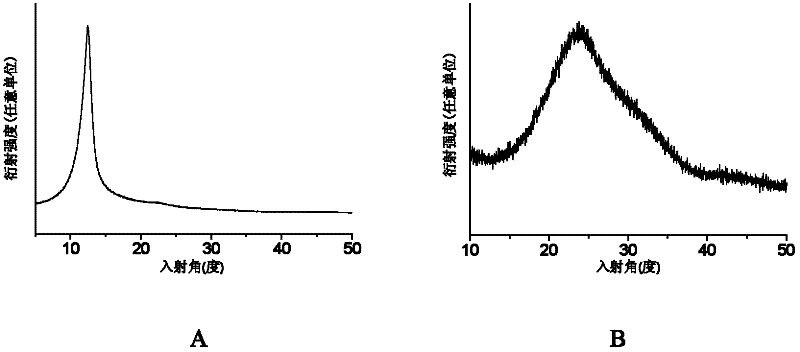
Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection
InactiveCN105203623AOvercome the disadvantage of only analyzing macromolecular substancesMaterial analysis by electric/magnetic meansBenzeneCoronene
The invention discloses an application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as an MALDI-TOF MS matrix in micromolecule detection. Small molecules comprise amino acid, medicine micromolecules, saccharide micromolecules, aromatic hydrocarbon compounds and the like. 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene based on nano graphene has the characteristic of graphene, can be dissolved into an organic solvent, is not prone to fly away from a target plate as the MALDI-TOF MS matrix, and rarely has pollution to an ion source, the defect that a traditional matrix is poor in micromolecule measuring effect is overcome, and 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene has the good effect when used for detecting MALDI-TOFMS of multiple kinds of micromolecules.
Owner:SHAANXI NORMAL UNIV
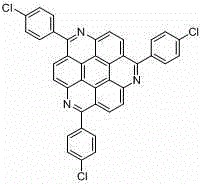
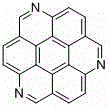
1, 5, 9-triazanaphthalene coronene compound and synthetic method thereof
InactiveCN104974156AReduce pollutionImprove thermal stabilityOrganic chemistryLuminescent compositionsCoroneneFluorescence
The invention relates to a 1, 5, 9-triazanaphthalene coronene compound and a synthetic method thereof. The compound has a structural formula as shown in the description, wherein R represents any one of hydrogen, alkyl, aryl, heterocyclic aryl, trifluoromethyl and perfluoroalkyl. The synthetic method disclosed by the invention comprises the following steps of: utilizing cheap 2, 3- dichloronitrobenzene which is easy to obtain as a raw material to prepare trinitro type substances; reducing the trinitro type substances so as to obtain triamine type substances; enabling the triamine type substances to react with acyl chloride or anhydride so as to obtain triamide type compounds; and cyclizing the triamide compounds so as to obtain substituted 1, 5, 9-triazanaphthalene coronene compounds in novel structures. The synthetic method is few in synthetic steps, mild in reaction conditions, convenient to operate and high in yield. The fluorescence emission spectrum of the triazanaphthalene coronene compound in an organic solvent has stronger fluorescence within the range of 420-550nm; the triazanaphthalene coronene compound has favorable heat stability and chemical stability, and can be used for preparing luminescent materials of organic light-emitting diodes and organic semiconductor materials.
Owner:SHANGHAI UNIV
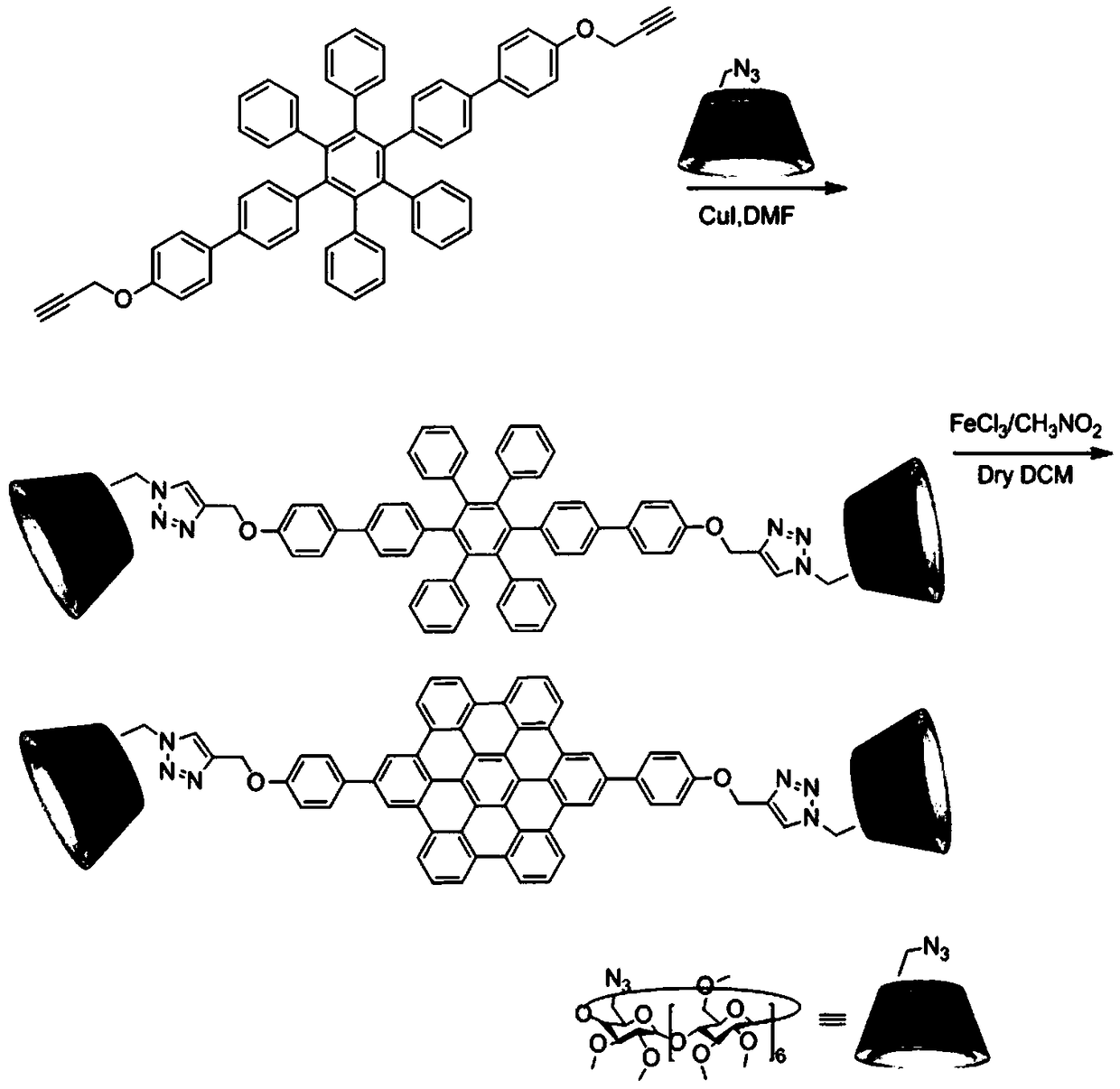
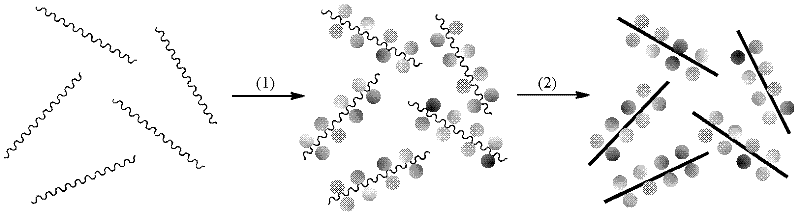
Nano supramolecular assembly body of fully methylated beta-cyclodextrin modified coronene derivative
InactiveCN106632737ALow detection limitEnables selective sensingFluorescence/phosphorescenceLuminescent compositionsChemical structureCoronene
Provides is a nano supramolecular assembly body of a fully methylated beta-cyclodextrin modified coronene derivative. A chemical formula of a construction unit of the assembly body is C184H248N6O70, a chemical structural formula of the assembly body is shown in the description, the supramolecular assembly body is established through intermolecular pi-pi interaction, the assembly body is a nanoscale and clubbed aggregate in morphological size. The nano supramolecular assembly body has the advantages that the preparation method of the nano supramolecular assembly body of the fully methylated beta-cyclodextrin modified coronene derivative is simple and convenient, the yield is high, the assembly body has good fluorescent properties; the supramolecular assembly body has good selectivity to nitro aroma compounds, has low detection limit to a polynitro explosive compound 2,4,6-trinitrophenol and has the wide application prospect in the field of fluorescence sensing detection.
Owner:NANKAI UNIV
Agricultural film with improved transmitted light quality
InactiveCN107434893AStrong fluorescenceStrong absorption capacityClimate change adaptationGreenhouse cultivationCoroneneFluorescence
The invention discloses an agricultural film with improved transmitted light quality. The agricultural film comprises a transparent plastic matrix, and the transparent plastic matrix contains 0.01-0.03 wt% of coronene and 0.01-0.03 wt% of an europium metal complex. A making method of the agricultural film comprises the following steps: uniformly mixing the europium metal complex, coronene and the transparent plastic, and melting and molding the obtained mixture to obtain the agricultural film. The maximum absorption wavelength of the coronene is 255 nm, the maximum emission wavelength of the coronene is 520 nm, the europium metal complex has strong absorption in a wavelength range of 260-420 nm, and the maximum emission wavelength of the europium metal complex is 613 nm. The fluorescence characteristics of the coronene and the europium metal complex are used to convert ultraviolet lights in sunshine into blue lights and red lights which facilitate the photosynthesis of crops, so the transmitted light quality of the agricultural film is improved, the production period of the crops is shortened, and the agricultural film has good economic benefits.
Owner:GUANGXI LIUCHENG CHENGLIN AGRI SCI & TECH
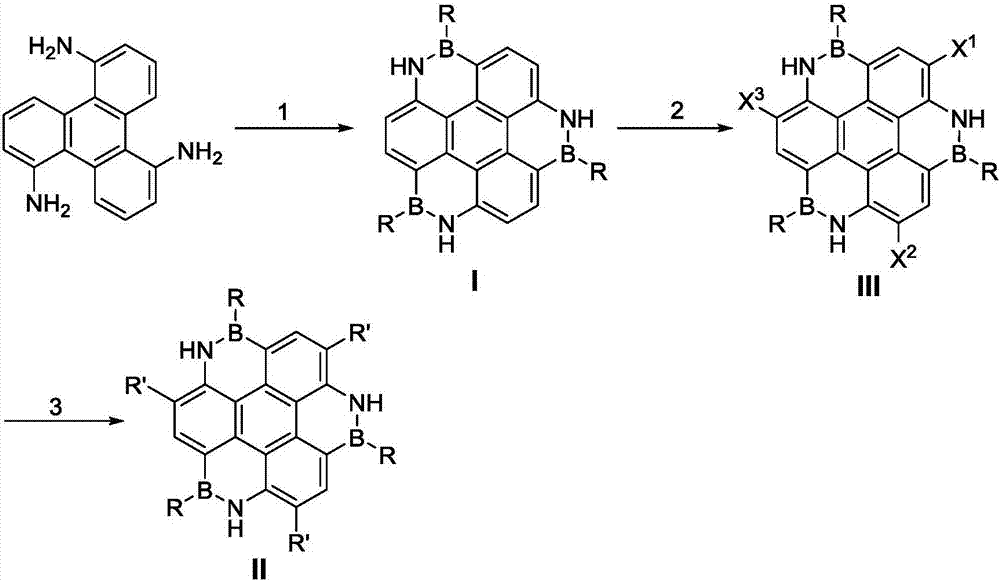
Boron-nitride doped coronene compound and preparation method thereof
InactiveCN107163071AReduce pollutionImprove thermal stabilityGroup 3/13 element organic compoundsCoroneneFluorescence
The invention relates to a boron-nitrogen doped pine compound and a preparation method thereof. The structural formula of the compound is: in the formula, R is phenyl, methylphenyl, tert-butylphenyl, trifluoromethylphenyl, bis(trifluoromethyl)phenyl, mesityl, naphthyl, etc.; R' is H, C4-C18 alkyl, phenyl, methylphenyl, methoxyphenyl, fluorophenyl, trifluoromethylphenyl, tert-butylphenyl, naphthyl, benzyl, ring Hexyl, pyridyl, thienyl or 2,4,6-trimethylphenyl, etc. The boron-nitrogen-doped pine compound of the present invention has good thermal stability, and the fluorescence emission spectrum in an organic solvent has strong fluorescence in the range of 380-500 nm, and its band gap can be opened after boron-nitrogen doping , and has great application prospects in the fields of hydrogen storage, biomedicine, coordination chemistry, catalysis, and electronic devices.
Owner:SHANGHAI UNIV
Preparation method of mixed fluorescent film for broadband silicon-based detector
InactiveCN103289680ADoes not destroy crystal structure propertiesEnsure consistencyLuminescent compositionsSemiconductor devicesCoroneneAdhesive
The invention relates to a preparation method of a mixed fluorescent film for a broadband silicon-based detector, which comprises the following steps of: performing grinding pretreatment of Lumogen powder and Coronene powder respectively by use of an agate mortar, and then putting into a designated beaker; preparing a suspension colloidal solution by taking a toluene solution as a solvent and a nitrocotton colloidal solution as an adhesive; fetching a proper amount of the suspension colloidal solution, and dropwise adding on a photosensitive surface or quartz glass substrate; and meanwhile, performing vacuum adsorption of a sample on a spin coater, and performing spin coating to obtain a film. The preparation method does not damage the crystal structure characteristics of two fluorescent materials so as to guarantee the consistency of the luminescence characteristics of the two fluorescent materials before and after film formation; the film compensates for the defect of low quantum efficiency of a single fluorescent material at partial band; compared with the traditional thermal evaporation technology, the preparation technology is simple to operate, and the material utilization rate is remarkably improved; and the preparation method provided by the invention reduces the experimental cost, and is time-saving and efficient and favorable for the industrial development of a silicon-based imaging device.
Owner:UNIV OF SHANGHAI FOR SCI & TECH
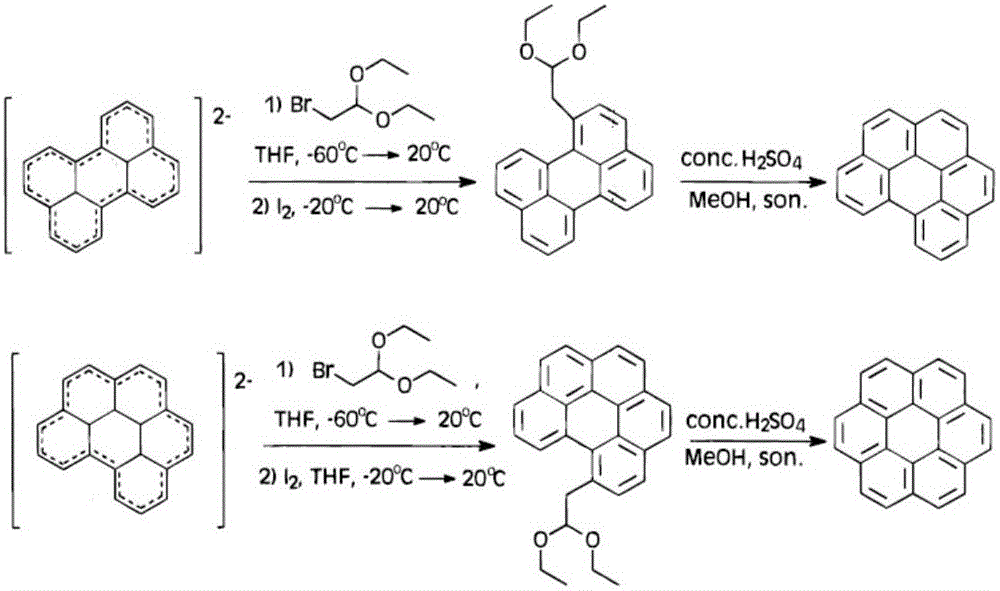
Preparation method of coronene
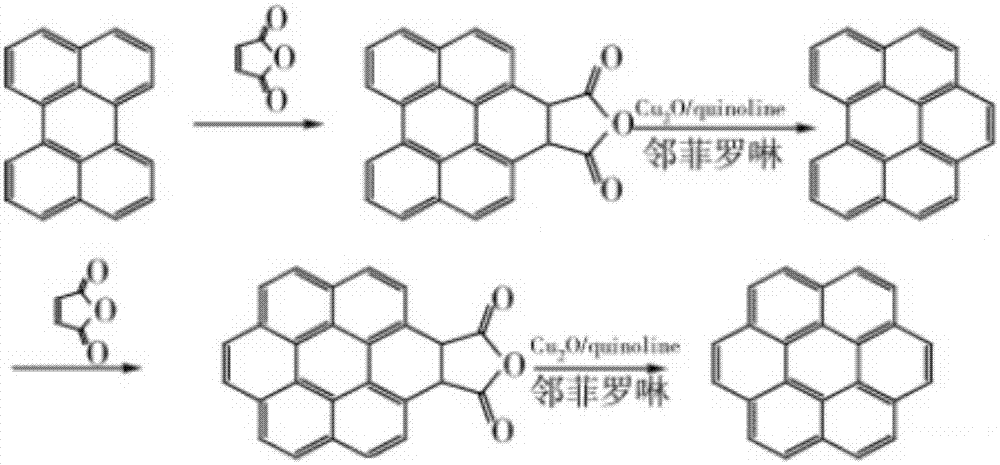
ActiveCN105801333ASimple processMild reaction conditionsOrganic compound preparationHydrocarbon from oxygen organic compoundsBenzeneCoronene
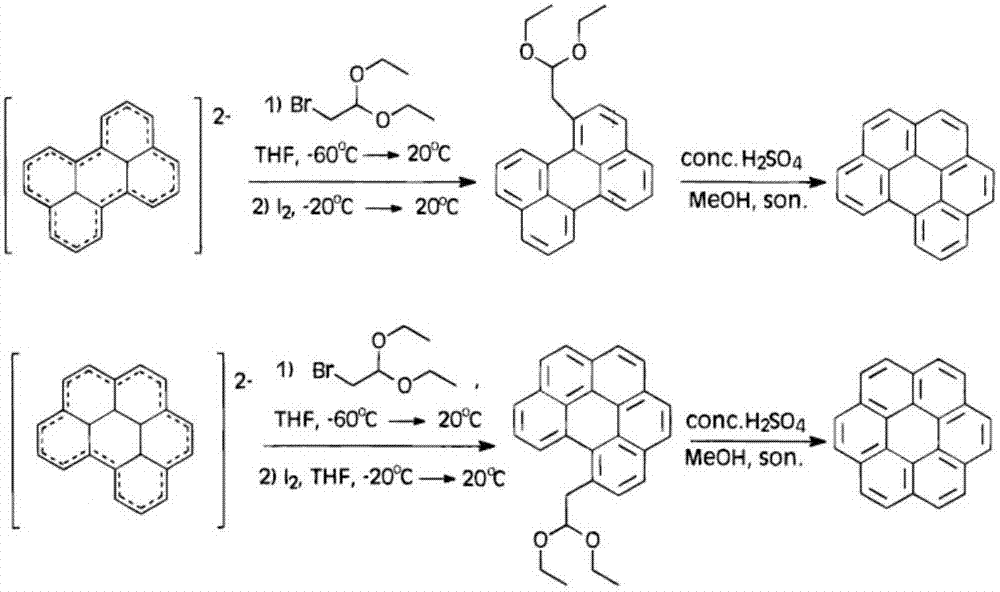
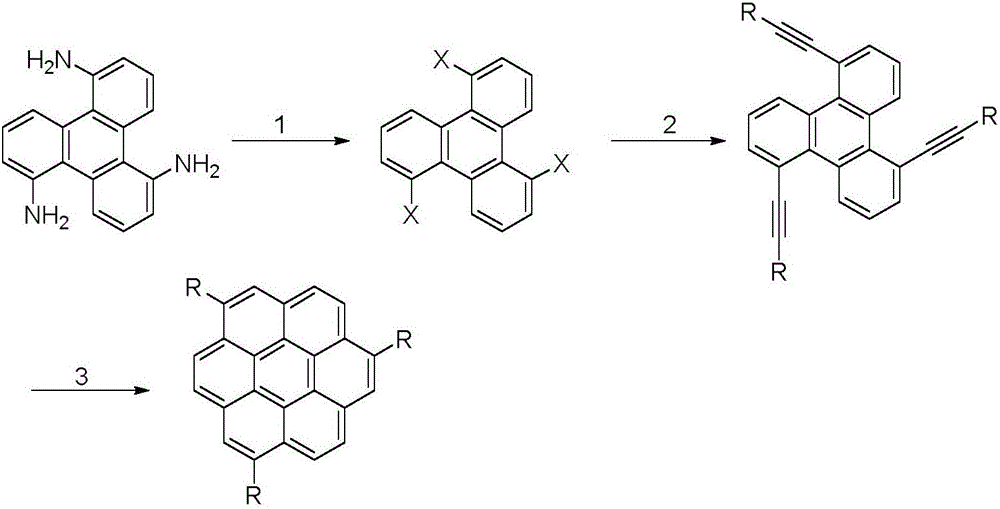
The invention discloses a preparation method of coronene. The preparation method comprises the following steps: coupling reaction: subjecting 1 equivalent of compound shown in a formula I and 4.2-5.6 equivalent of compound shown in a formula II to coupling reaction and converting a reaction product to a compound shown in a formula III; ring closing reaction: subjecting the compound shown in the formula III to ring closing reaction in the presence of acid and converting a reaction product to coronene shown in a formula IV, wherein the formulas I, II, III and IV are shown in the specification. The preparation method has the beneficial effects that the preparation method is simple in process and is easy to operate; the required raw materials are available and are low in costs; therefore the preparation method is suitable for large-scale production.
Owner:江苏佳麦化工有限公司
Method for bottom-up graphene sheet preparation and bandgap engineering
A combination of a substrate selected from silicon, silicon carbide or a metal and a grapheme precursor having the following properties: (a) an aromatic structure that forms the basis of the graphene structure, said aromatic structure being selected from the group consisting of: benzene, naphthalene, pyrene, anthracene, chrysene, coronene, and phenanthrene, or a cyclic or acyclic structures which can be converted to aromatic structures and (b) functional groups that can react with each other to form additional aromatic structures.
Owner:HRL LAB
Method for bottom-up graphene sheet preparation and bandgap engineering
A combination of a substrate selected from silicon, silicon carbide or a metal and a grapheme precursor having the following properties: (a) an aromatic structure that forms the basis of the graphene structure, said aromatic structure being selected from the group consisting of: benzene, naphthalene, pyrene, anthracene, chrysene, coronene, and phenanthrene, or a cyclic or acyclic structures which can be converted to aromatic structures and (b) functional groups that can react with each other to form additional aromatic structures.
Owner:HRL LAB
Hetero diels-alder adducts of pentacene as soluble precursors of pentacene
Owner:GLOBALFOUNDRIES INC
A kind of preparation method of coronene compound
ActiveCN111777482BSimple preparation processMild reaction conditionsMolecular sieve catalystsCatalyst activation/preparationMolecular sievePtru catalyst
The application discloses a method for preparing coronene compounds, the method comprising the following steps: (1) pre-treating a catalyst containing SAPO molecular sieves; (2) loading a raw material containing acyclic olefins into a reactor , contacting and reacting with the pretreated catalyst in step (1), to obtain the coronene compound; wherein, the pretreatment is to activate the catalyst. The method does not require expensive raw materials, complicated operations, and is suitable for large-scale production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A method for preparing coronene compounds from acyclic olefins
ActiveCN111777483BSimple processMild preparation conditionsMolecular sieve catalystCatalystsMolecular sievePtru catalyst
A method for preparing coronene compounds from acyclic olefins, characterized in that the method comprises: loading the reaction raw materials into a reactor, contacting and reacting with a catalyst comprising activated silica-alumina molecular sieves to obtain the coronets Benzene compounds; wherein, the reaction raw materials include acyclic olefins. The method has mild preparation conditions, easy operation and simple process; the raw materials used are low in cost and easy to obtain, and are suitable for large-scale production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Flame-retardant ethylene propylene diene monomer
Owner:佛山市高明区生产力促进中心
A method for preparing coronene compounds from alcohol raw materials
ActiveCN111777487BSimple preparation processMild reaction conditionsMolecular sieve catalystsMolecular sieve catalystMolecular sieveAlcohol
The invention relates to a method for preparing coronene compounds from alcohol raw materials. The method of the present invention comprises the following steps: contacting and reacting raw materials containing alcohol compounds and catalysts in a reactor to obtain coronene compounds; wherein, the catalysts include silicon-aluminum molecular sieves that have undergone activation treatment.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Coronene compound containing thioether in lateral chain and preparation method thereof
ActiveCN102584655BSolid-state devicesSemiconductor/solid-state device manufacturingCoroneneSide chain
The invention relates to coronene compound containing thioether in lateral chain and the preparation method thereof. The structural formula of the compound is shown in the descriptiopn: R refers to alkyl. In the method, ring trimerization- ferric trichloride coronene method, namely 2- (4'- bromophenyl) ethanol is taken as raw material, through hydroxyl bromo, thioether is formed, palladium-catalyzed is selected for direct coupled reaction, so as to obtain diphenylacetypene ramification, under the catalysis of cobalt carbonyl, corresponding hexaphenylbenzene is produced by trimerization cyclization. Anhydrous ferric trichloride is oxidized in methylene dichloride and nitromethane, then reduction is performed by using iodine and sodium borohydride system, and after drying, THF (tetrahydrofuran) is used for recrystal. The synthetic method provided by the invention is simple, is high in yield, and can be extensively used in organic LBD, field-effect tubes and solar batteries.
Owner:BEIJING INSTITUTE OF GRAPHIC COMMUNICATION
A kind of preparation method of coronene
ActiveCN105801333BSimple processMild reaction conditionsOrganic compound preparationHydrocarbon from oxygen organic compoundsBenzeneCoronene
The invention discloses a preparation method of coronene. The preparation method comprises the following steps: coupling reaction: subjecting 1 equivalent of compound shown in a formula I and 4.2-5.6 equivalent of compound shown in a formula II to coupling reaction and converting a reaction product to a compound shown in a formula III; ring closing reaction: subjecting the compound shown in the formula III to ring closing reaction in the presence of acid and converting a reaction product to coronene shown in a formula IV, wherein the formulas I, II, III and IV are shown in the specification. The preparation method has the beneficial effects that the preparation method is simple in process and is easy to operate; the required raw materials are available and are low in costs; therefore the preparation method is suitable for large-scale production.
Owner:江苏佳麦化工有限公司
Preparation method of coronene compound
ActiveCN111777482ASimple preparation processMild reaction conditionsMolecular sieve catalystsCatalyst activation/preparationMolecular sievePtru catalyst
The invention discloses a preparation method of a coronene compound. The method comprises the following steps of: (1) pretreating a catalyst containing an SAPO molecular sieve; and (2) filling a reactor with a raw material containing non-cyclic olefin, and carrying out contact reaction with the catalyst pretreated in the step (1) to obtain the coronene compound, wherein the pretreatment refers toactivation of the catalyst. The method does not need expensive raw materials or complex operation, and is suitable for large-scale production.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
1,5,9-trisubstituted pine compound and its synthesis method
ActiveCN106565408BReduce pollutionImprove thermal stabilityOrganic compound preparationHydrocarbonsFuranCoronene
The present invention relates to a 1, 5, 9-trisubstituted coronene compound and a synthesis method thereof. The structural formula of the compound is shown in img file = 'dest _ path _ image 001. TIF 'wi = '109 'he = '108', wherein R represents H, C1-C18 alkyl, phenyl, 4-methylphenyl, 4-methoxy phenyl, benzyl, cyclohexyl, 4-trifluoromethylphenyl, thiophene, furan and the like. According to the technical scheme of the invention, the easily prepared 1, 5, 9-triamido triphenylene is subjected to diazotization and halogenation reaction to obtain the tri-halogenated triphenylene. After that, the tri-halogenated triphenylene is subjected to Sonogashira reaction with various alkynes to generate atriyne-triphenylene compounds. Finally, through the metal-catalyzed reaction and the cyclization reaction under the effect of an organic base, various 1, 5, 9-trisubstituted coronene compounds, novel in structure, can be obtained. According to the technical scheme of the invention, raw materials are easy for mass preparation. Meanwhile, the synthesis step is relatively short and the operation is convenient. The obtained trisubstituted coronene compound is good in thermal stability and chemical stability, and the trisubstituted coronene emits the relatively strong fluorescence within the range of 420-550 nm according to the fluorescence emission spectrum of the trisubstituted coronene compound. Therefore, the trisubstituted coronene is an excellent fluorescent material for preparing UV ultraviolet charge-coupled devices (UV-CCD) and organic light-emitting diodes (OLEDs), and has a wide application prospect in the field of electronic materials.
Owner:SHANGHAI UNIV
Preparation method of coronene
ActiveCN105801329ASimple processMild reaction conditionsOrganic compound preparationHydrocarbon from oxygen organic compoundsBenzeneCoronene
The invention discloses a preparation method of coronene. The preparation method comprises the following steps: addition: subjecting 1 equivalent of compound shown in a formula I and 2.2-3.0 equivalent of compound shown in a formula II to addition reaction in the presence of alkali and converting a reaction product to a compound shown in a formula III through hydrolysis; ring closing: subjecting the compound shown in the formula III to ring closing reaction in the presence of acid and converting a reaction product to coronene shown in a formula IV, wherein the formulas I, II, III and IV are shown in the specification. The preparation method has the beneficial effects that the preparation method is simple in process and is easy to operate; the required raw materials are available and are low in costs; the yield is as high as 80-91%; therefore the preparation method is suitable for large-scale production.
Owner:FUYANG XINYIHUA MATERIAL TECH
1,5,9-Triazapine compound and its synthesis method
InactiveCN104974156BReduce pollutionImprove thermal stabilityOrganic chemistryLuminescent compositionsCoroneneFluorescence
The invention relates to a 1, 5, 9-triazanaphthalene coronene compound and a synthetic method thereof. The compound has a structural formula as shown in the description, wherein R represents any one of hydrogen, alkyl, aryl, heterocyclic aryl, trifluoromethyl and perfluoroalkyl. The synthetic method disclosed by the invention comprises the following steps of: utilizing cheap 2, 3- dichloronitrobenzene which is easy to obtain as a raw material to prepare trinitro type substances; reducing the trinitro type substances so as to obtain triamine type substances; enabling the triamine type substances to react with acyl chloride or anhydride so as to obtain triamide type compounds; and cyclizing the triamide compounds so as to obtain substituted 1, 5, 9-triazanaphthalene coronene compounds in novel structures. The synthetic method is few in synthetic steps, mild in reaction conditions, convenient to operate and high in yield. The fluorescence emission spectrum of the triazanaphthalene coronene compound in an organic solvent has stronger fluorescence within the range of 420-550nm; the triazanaphthalene coronene compound has favorable heat stability and chemical stability, and can be used for preparing luminescent materials of organic light-emitting diodes and organic semiconductor materials.
Owner:SHANGHAI UNIV
A Nanosupramolecular Assembly of Permethylated β-Cyclodextrin Modified Hexabenzocoronene Derivatives
InactiveCN106632737BEnables selective sensingGood choiceFluorescence/phosphorescenceLuminescent compositionsChemical structureCoronene
Provides is a nano supramolecular assembly body of a fully methylated beta-cyclodextrin modified coronene derivative. A chemical formula of a construction unit of the assembly body is C184H248N6O70, a chemical structural formula of the assembly body is shown in the description, the supramolecular assembly body is established through intermolecular pi-pi interaction, the assembly body is a nanoscale and clubbed aggregate in morphological size. The nano supramolecular assembly body has the advantages that the preparation method of the nano supramolecular assembly body of the fully methylated beta-cyclodextrin modified coronene derivative is simple and convenient, the yield is high, the assembly body has good fluorescent properties; the supramolecular assembly body has good selectivity to nitro aroma compounds, has low detection limit to a polynitro explosive compound 2,4,6-trinitrophenol and has the wide application prospect in the field of fluorescence sensing detection.
Owner:NANKAI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection](https://images-eureka.patsnap.com/patent_img/f57628bc-515c-4acc-89fa-78292022af0b/HDA0000793278600000011.PNG)
![Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection](https://images-eureka.patsnap.com/patent_img/f57628bc-515c-4acc-89fa-78292022af0b/HDA0000793278600000012.PNG)
![Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection Application of 6-tertiary butyl-6-hexyloxy-6-benzo[a,b,de,lm,op,rs,uv]coronene as MALDI-TOF MS matrix in micromolecule detection](https://images-eureka.patsnap.com/patent_img/f57628bc-515c-4acc-89fa-78292022af0b/HDA0000793278600000021.PNG)