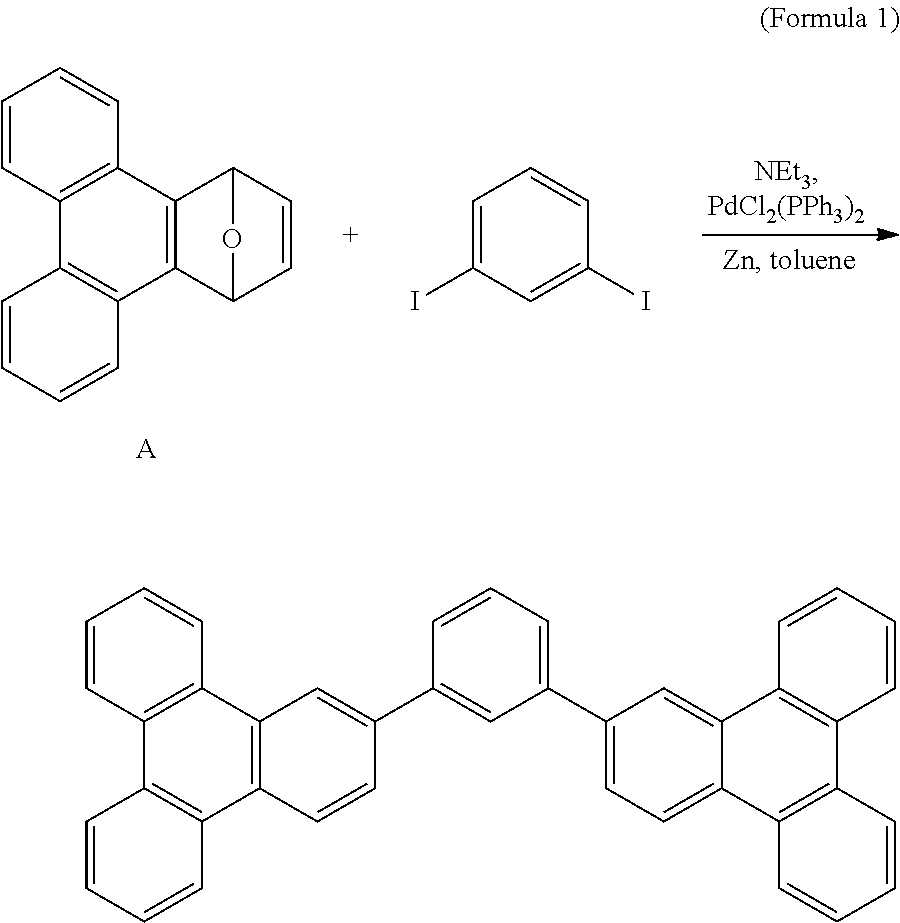
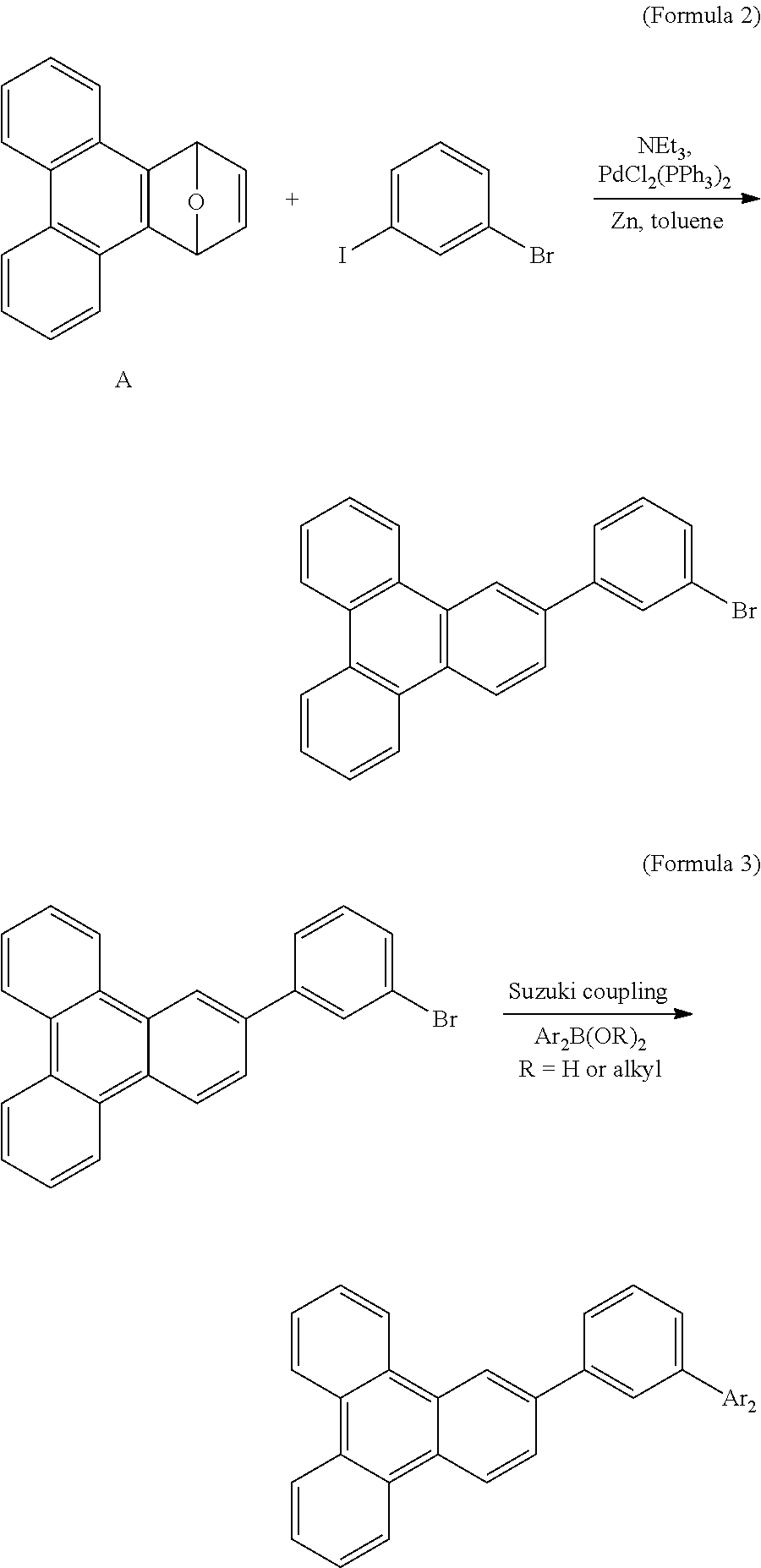
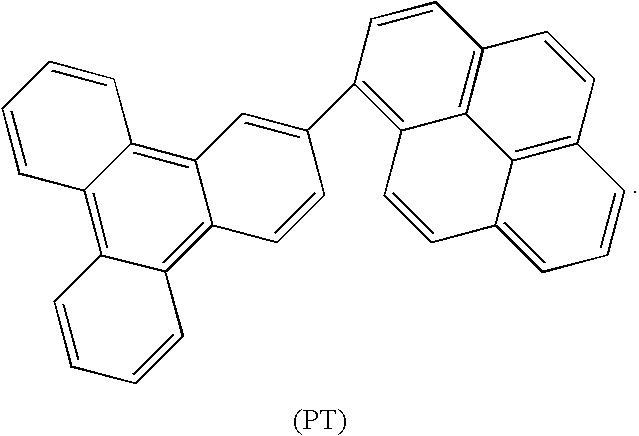
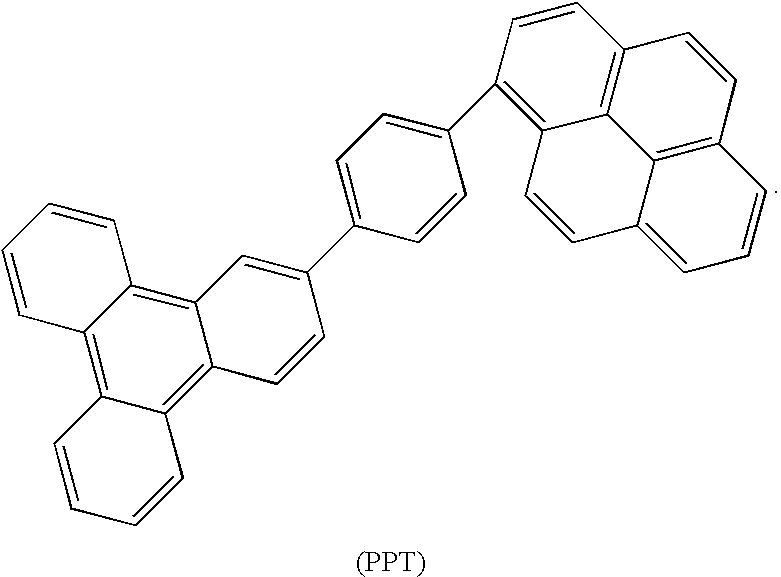
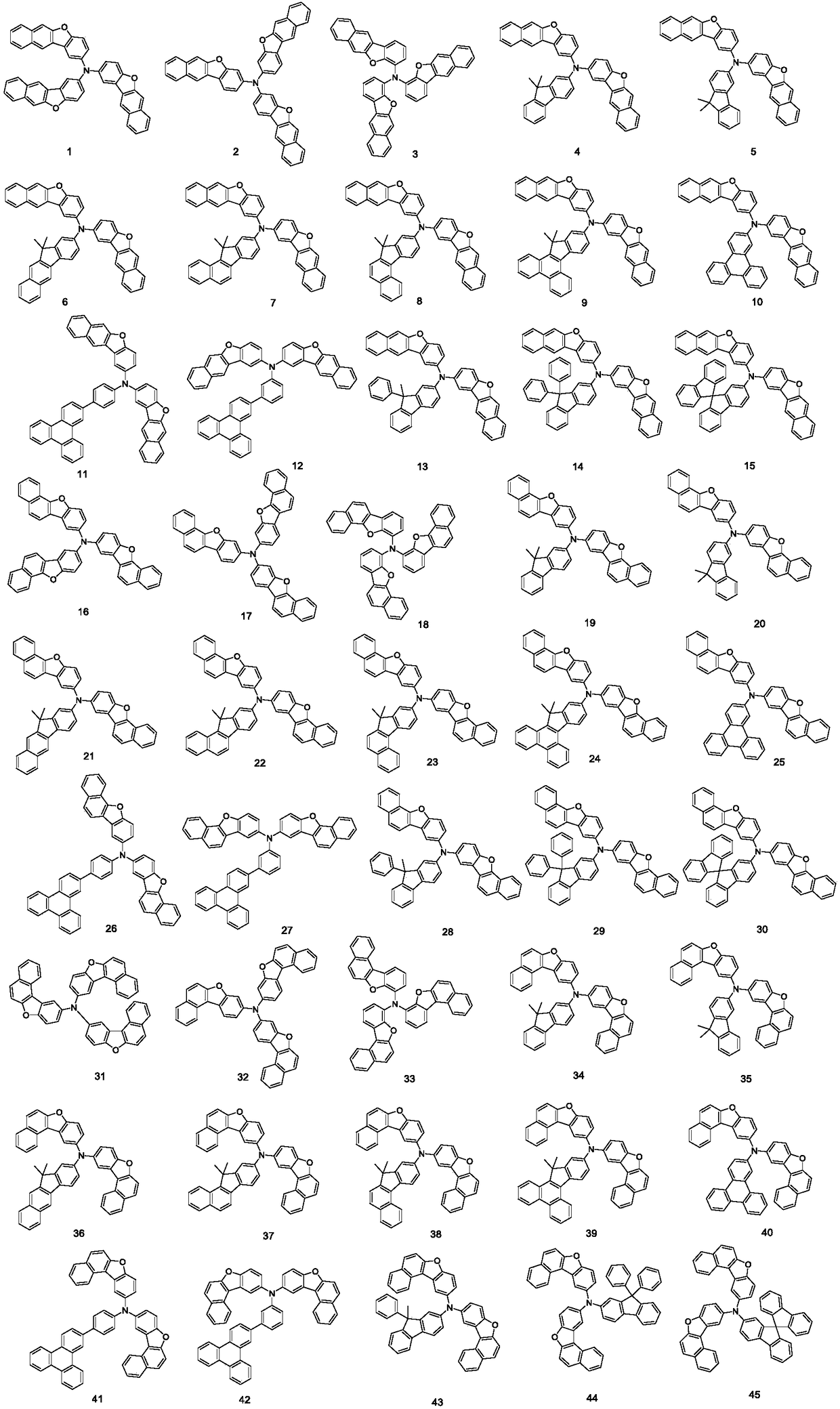
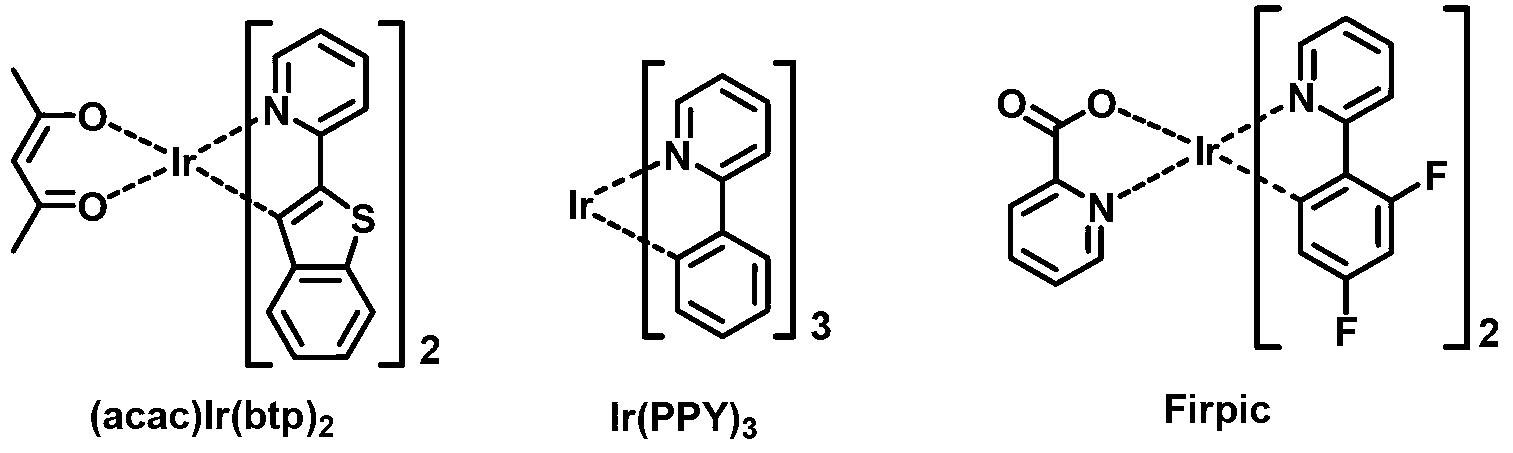
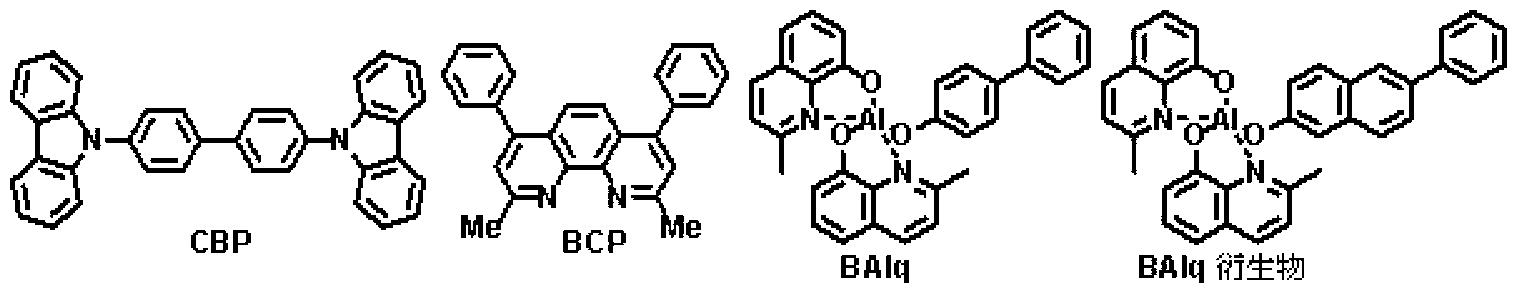
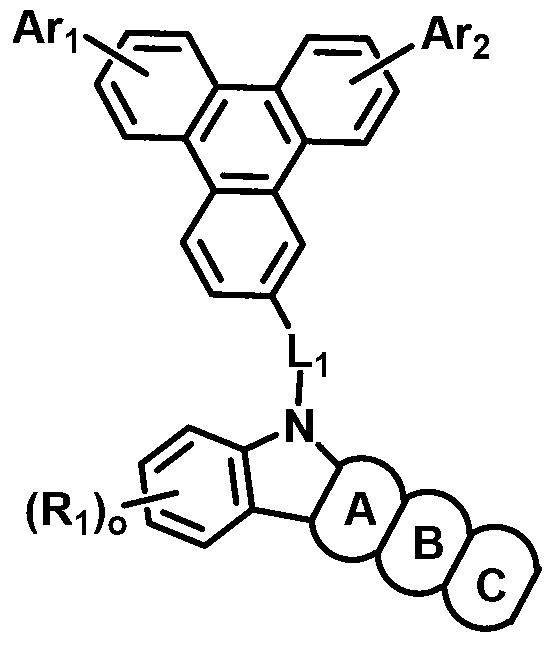
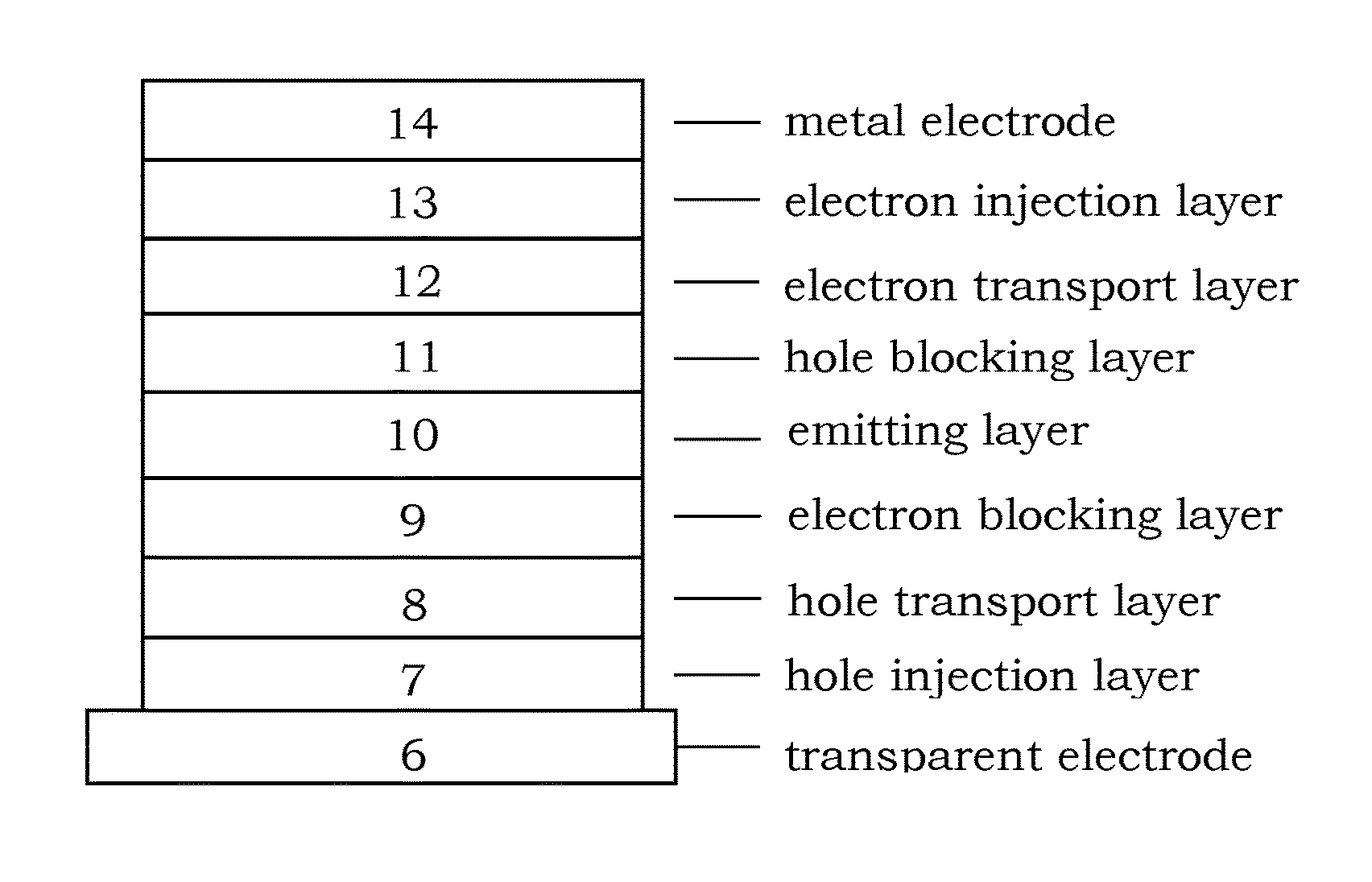
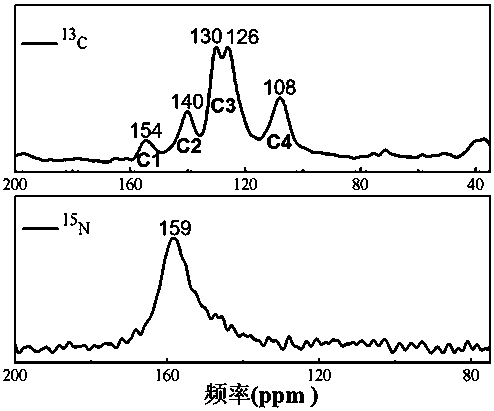
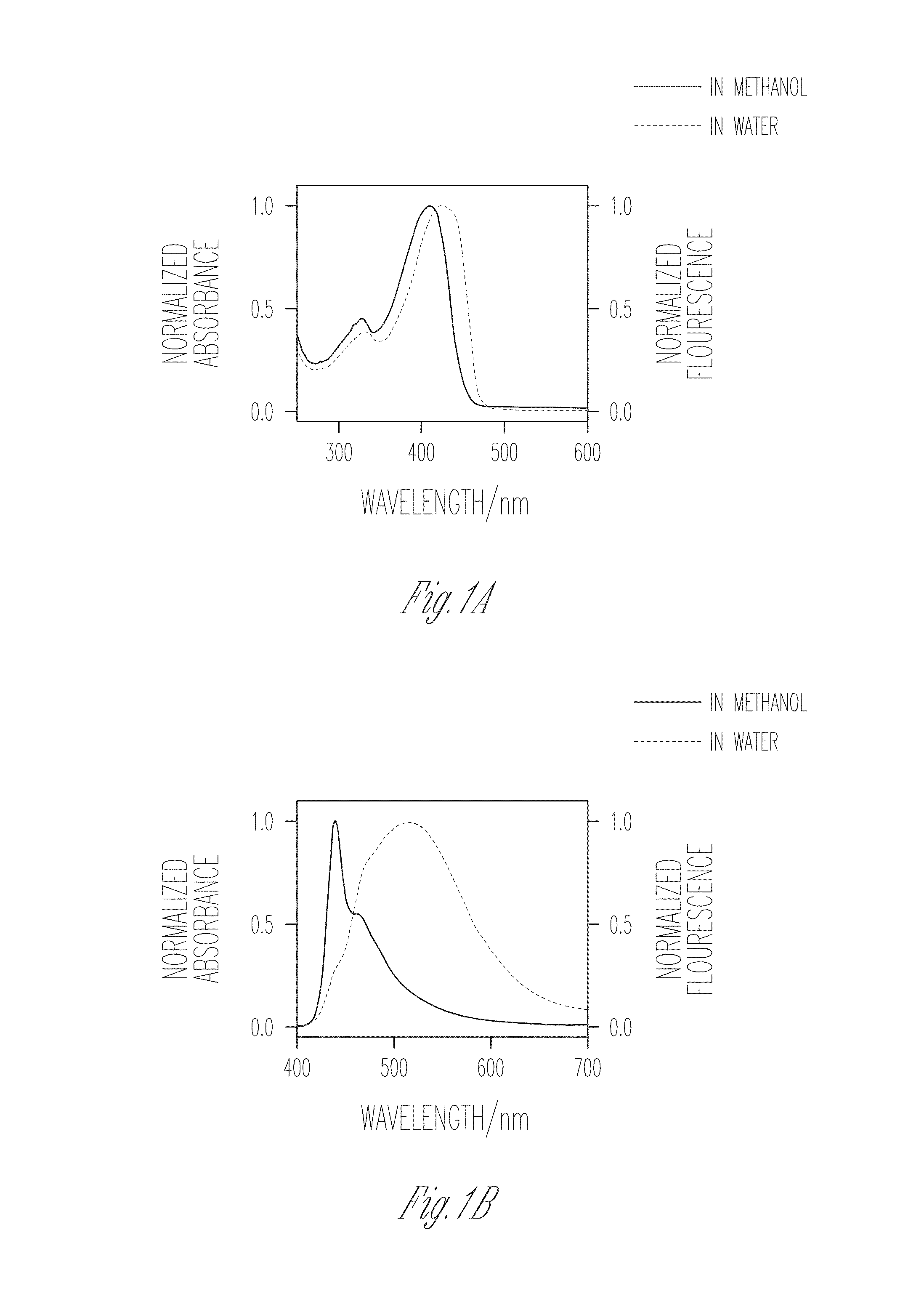
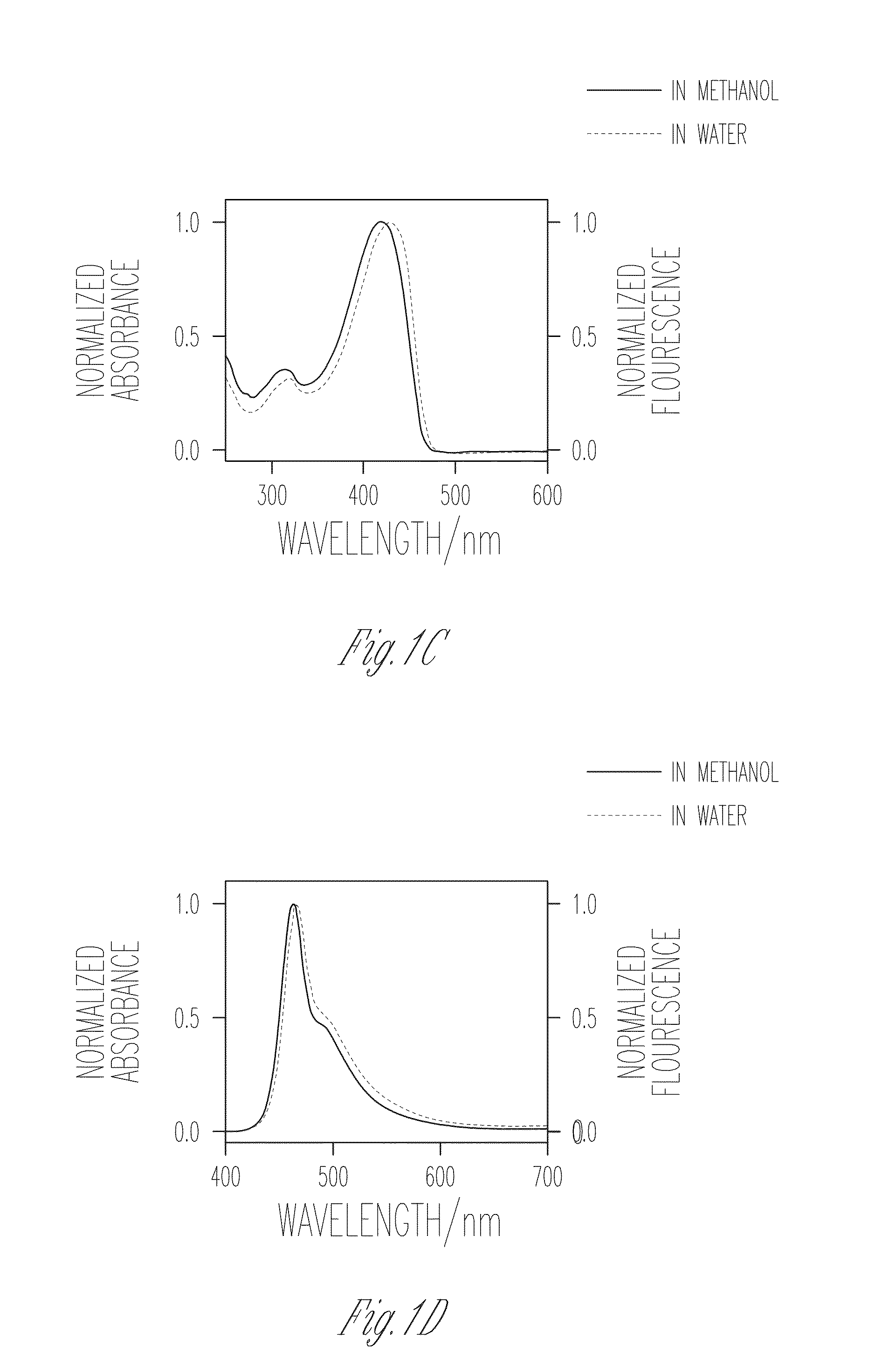
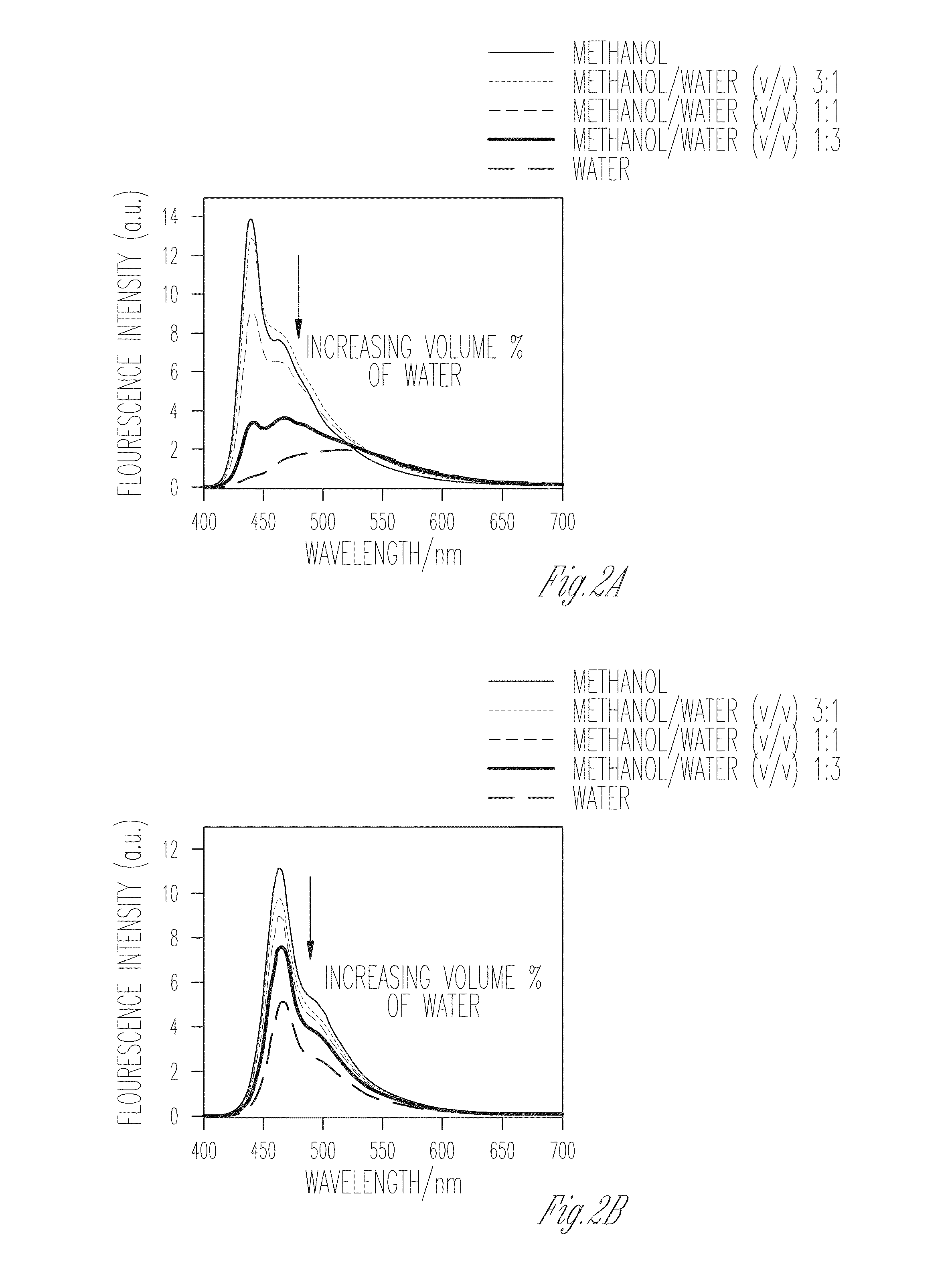
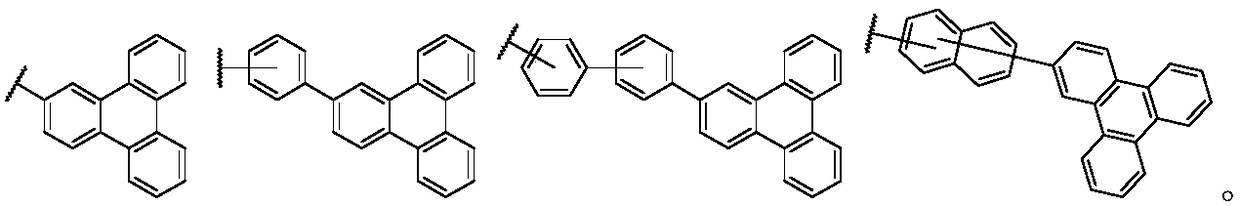
Patents
Literature
147 results about "Triphenylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
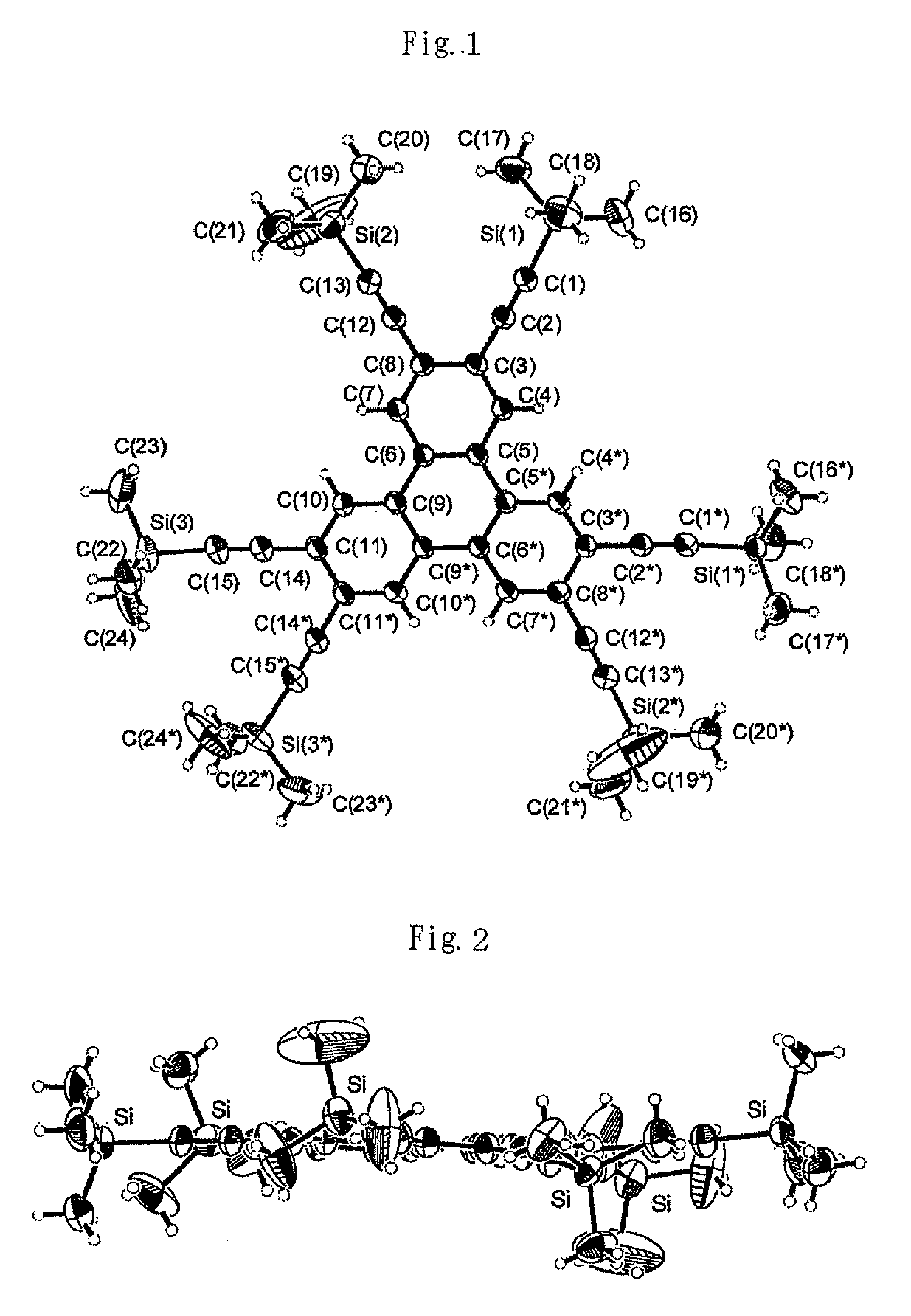
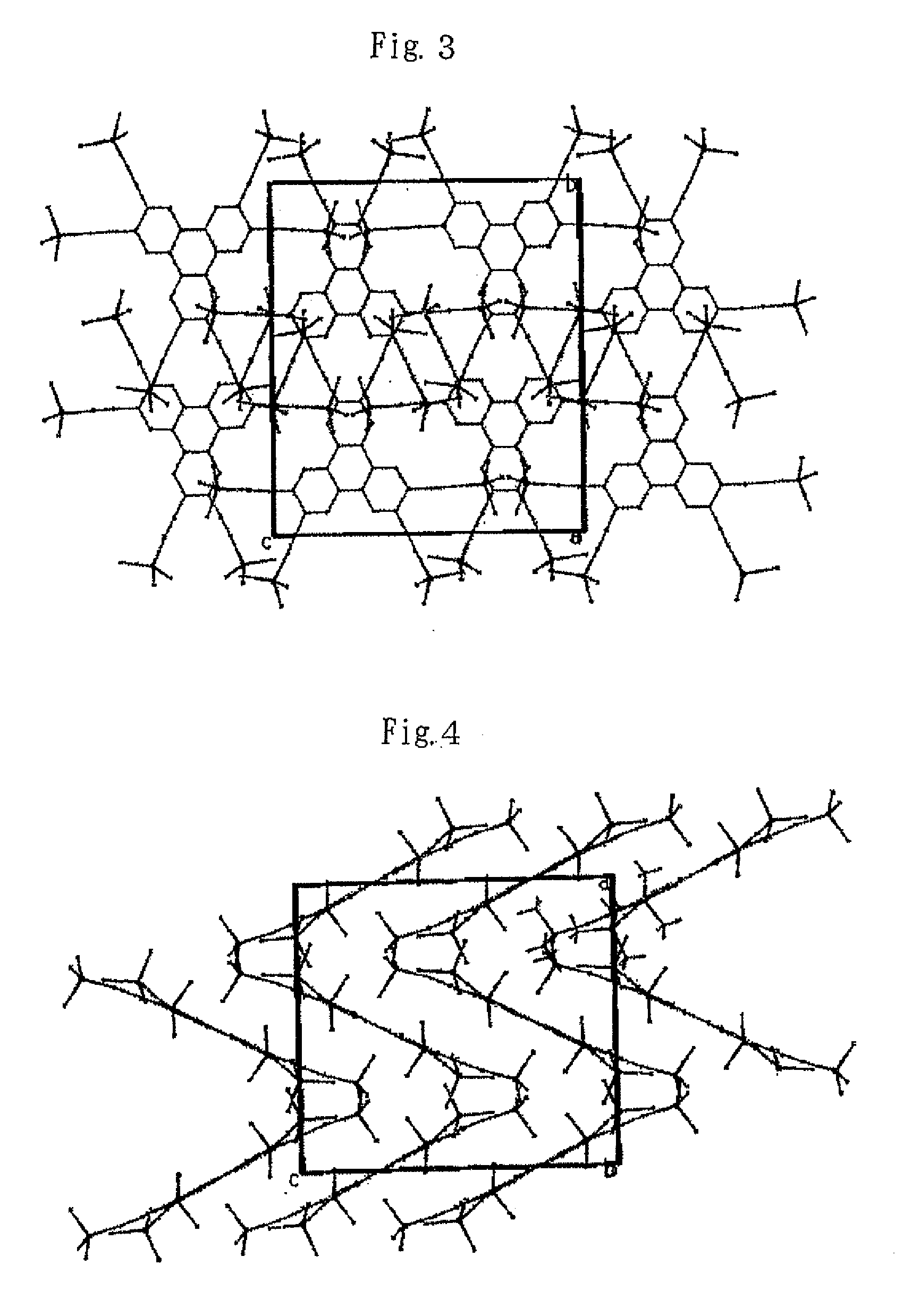
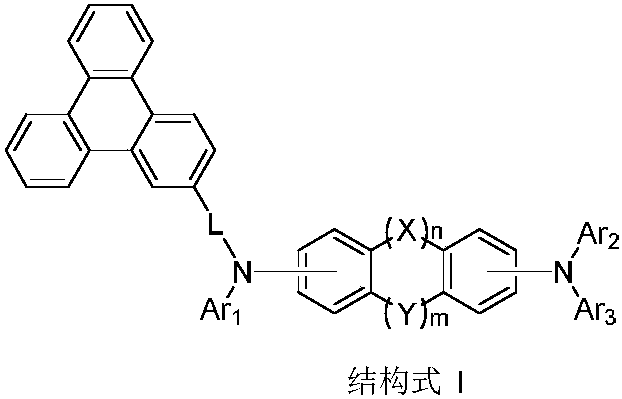
In chemistry, the organic compound triphenylene is a flat polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings. Triphenylene can be isolated from coal tar. It is also made synthetically by synthesis and trimerization of benzyne. One molecule of triphenylene has delocalized 18-π-electron systems based on a planar structure. It has the molecular formula C₁₈H₁₂.
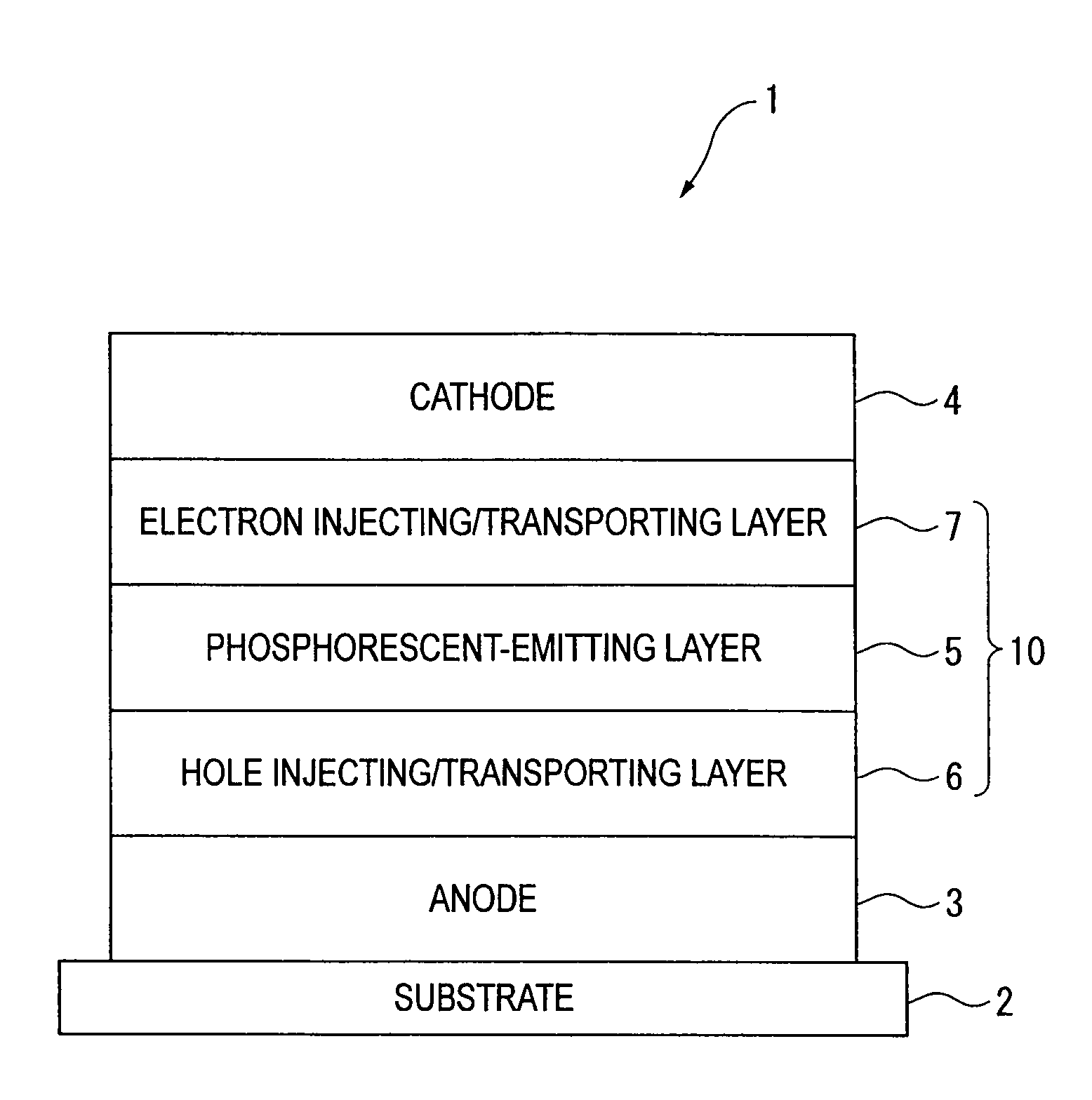
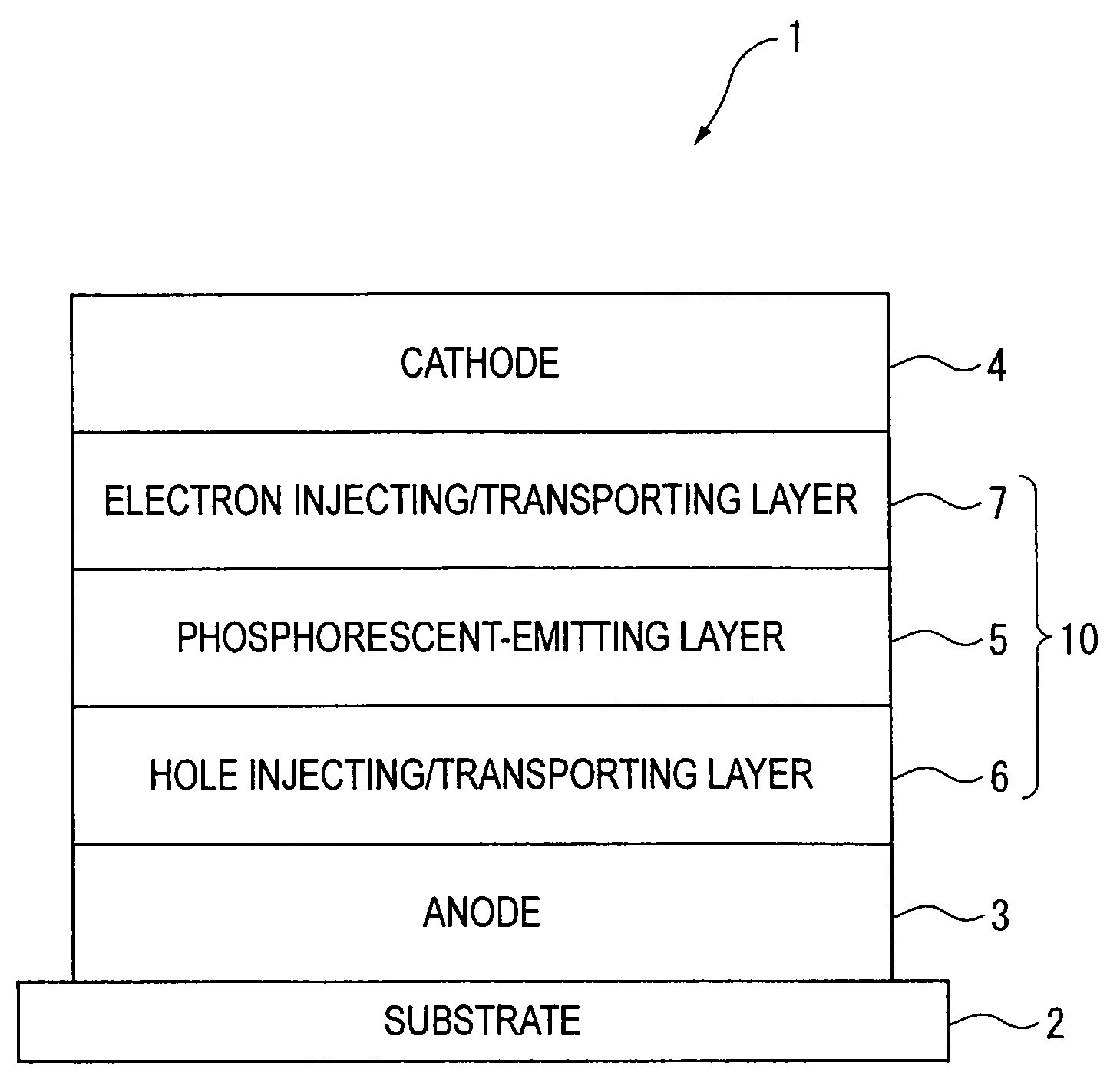
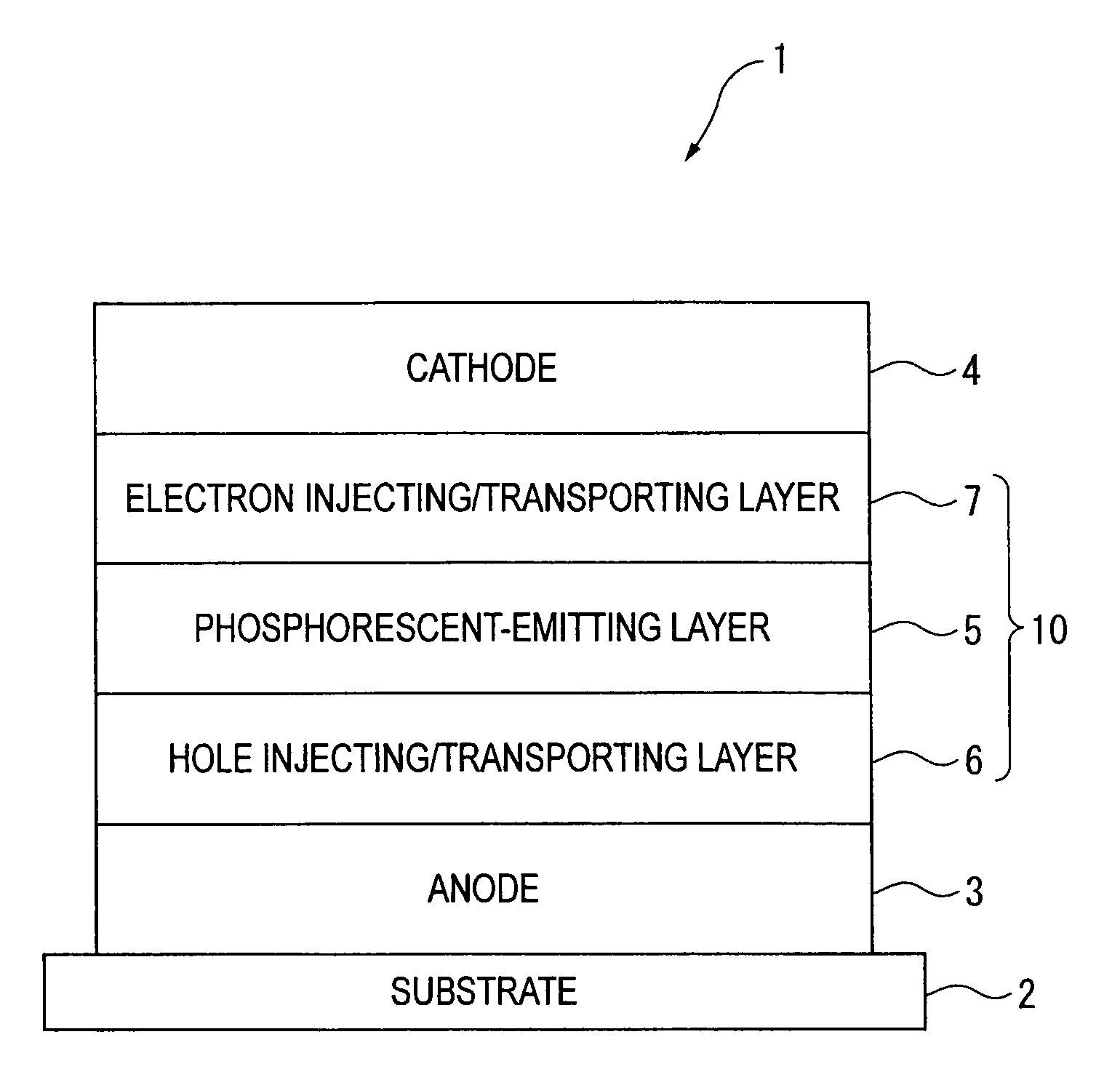
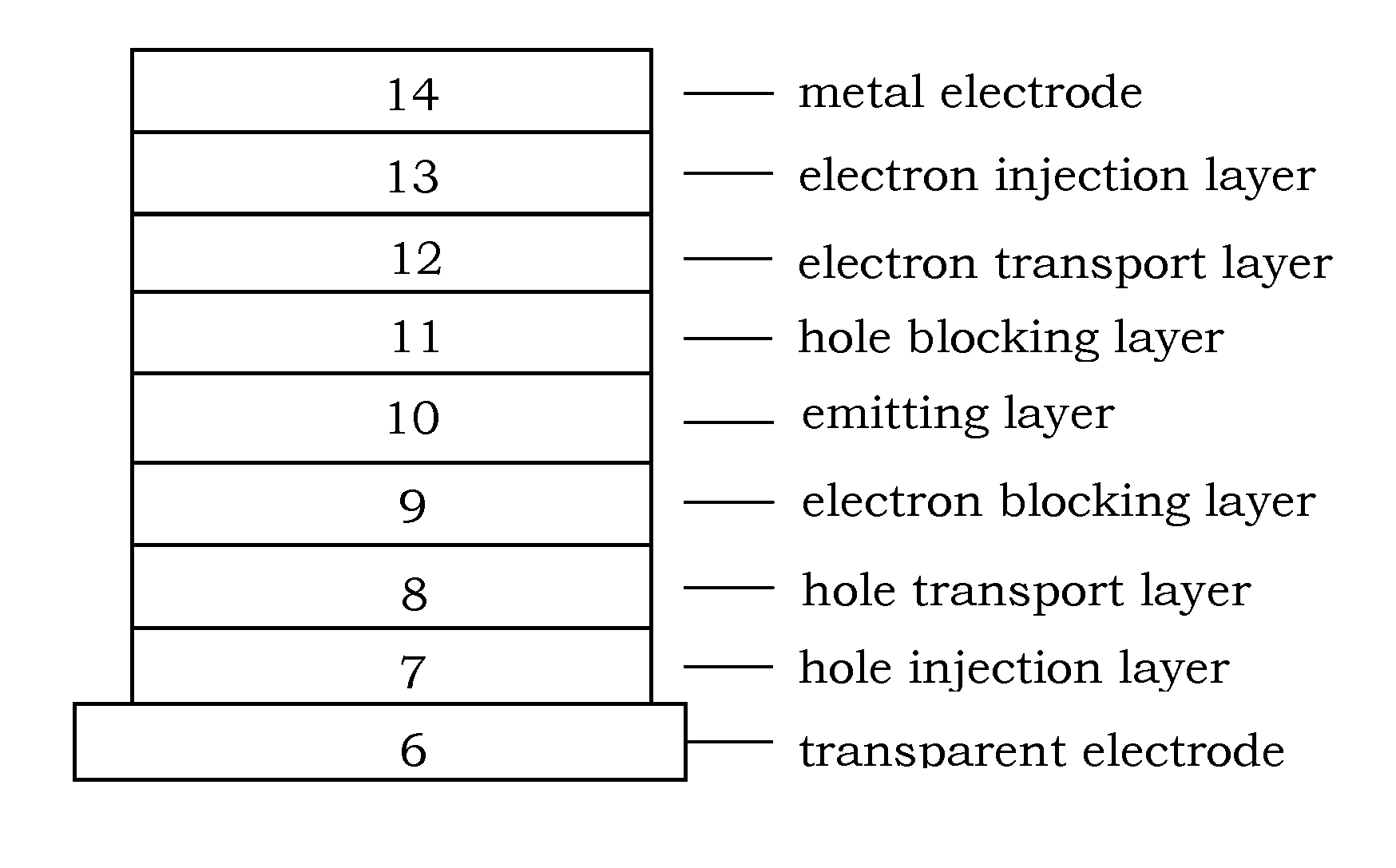
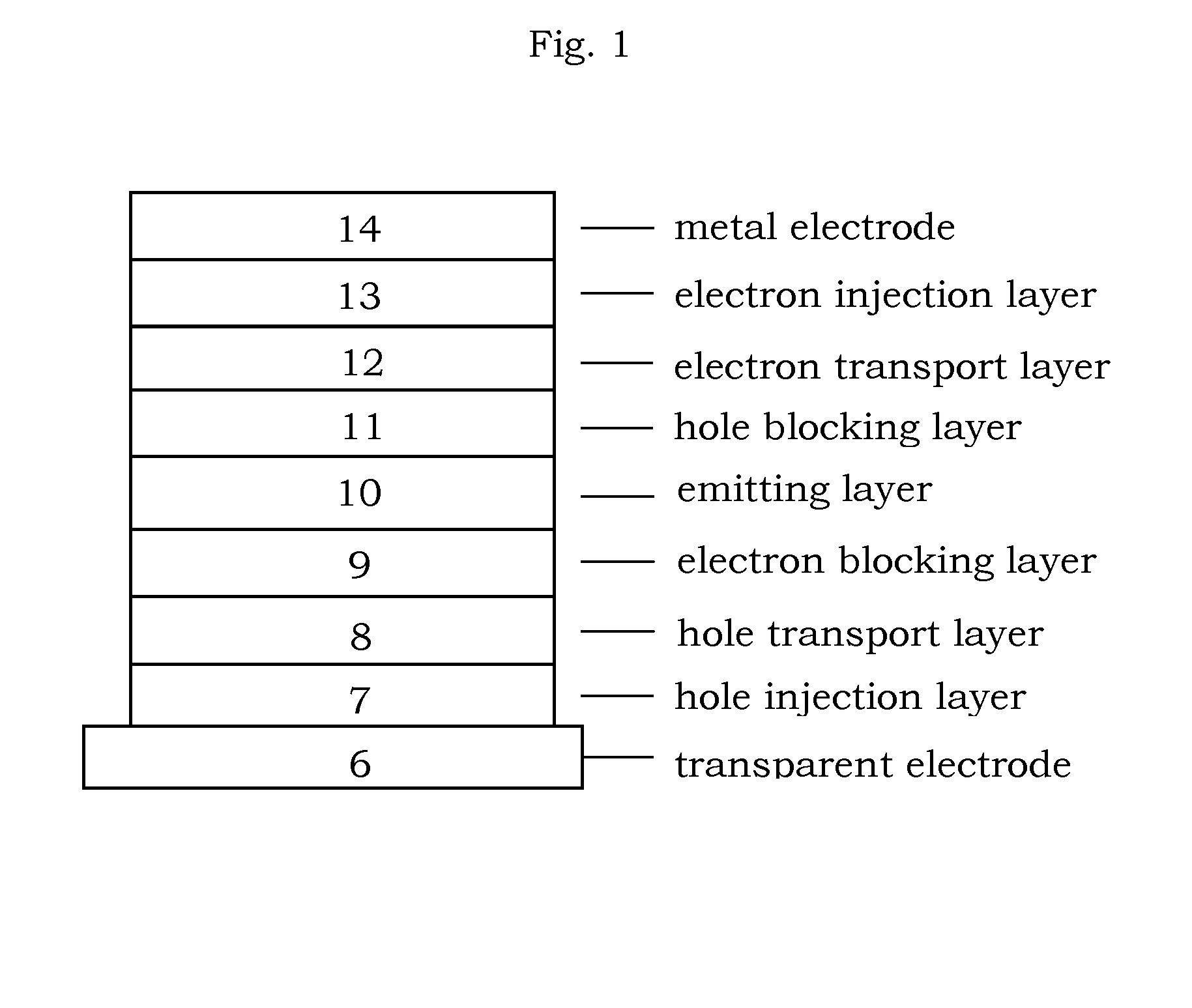
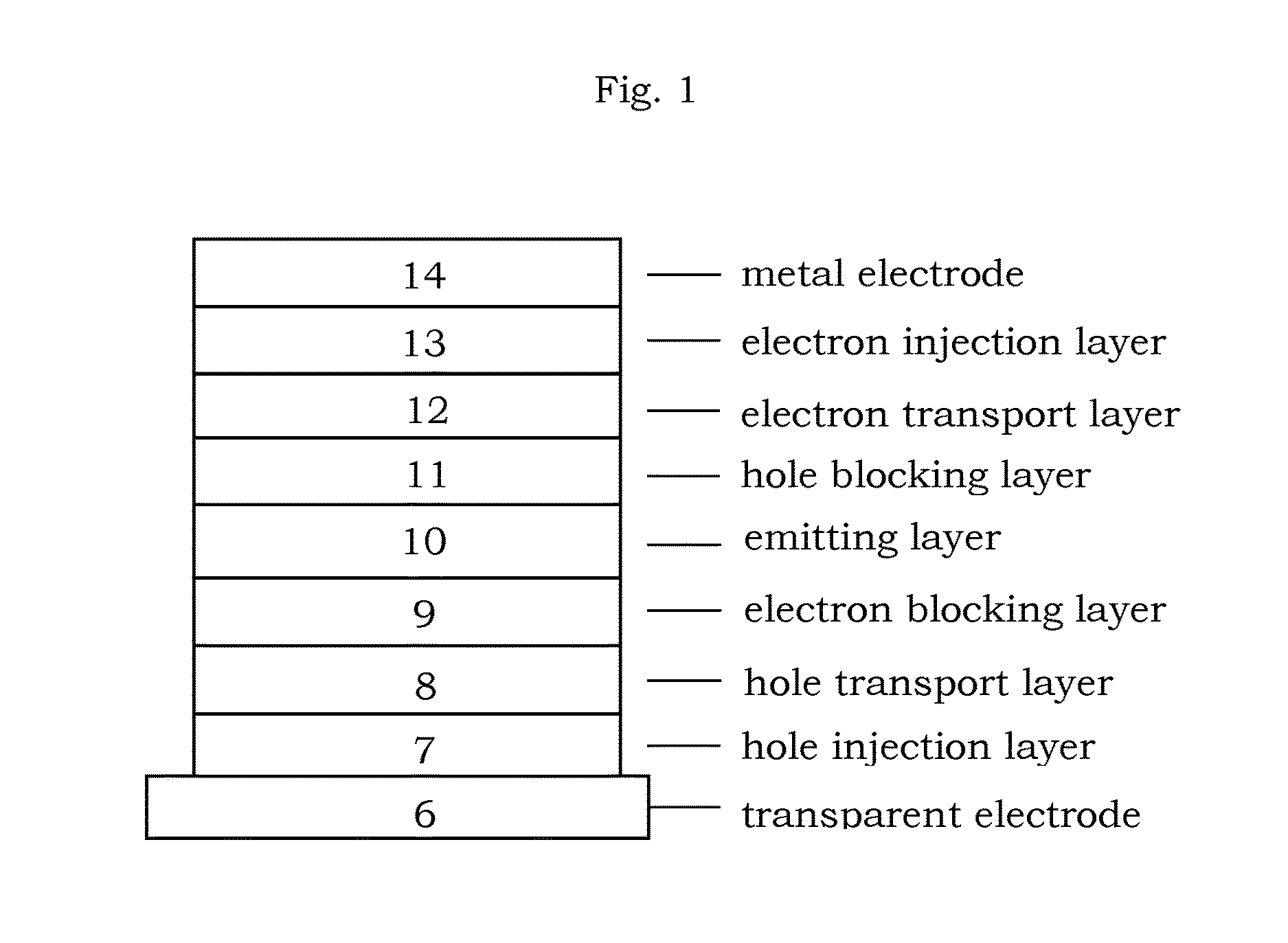
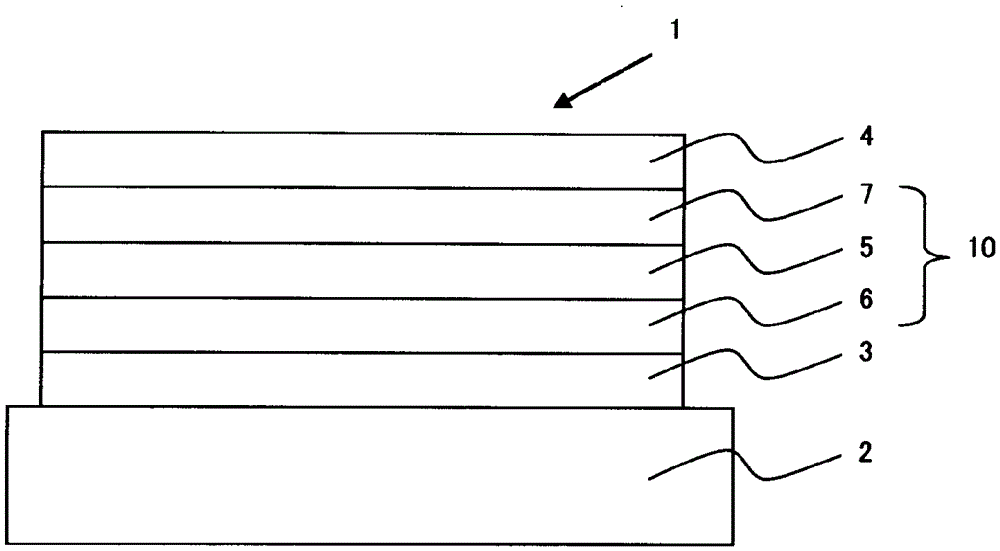
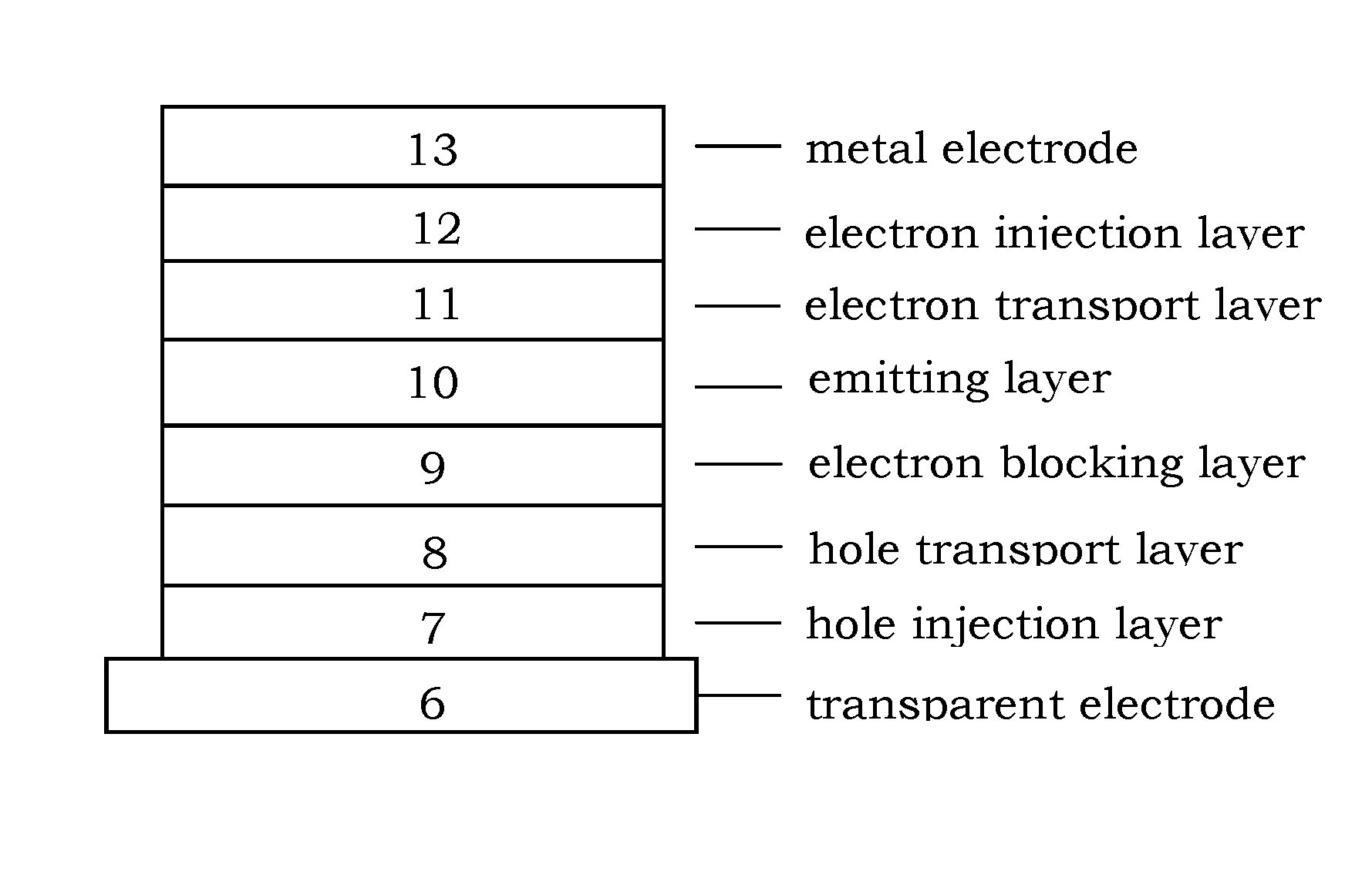
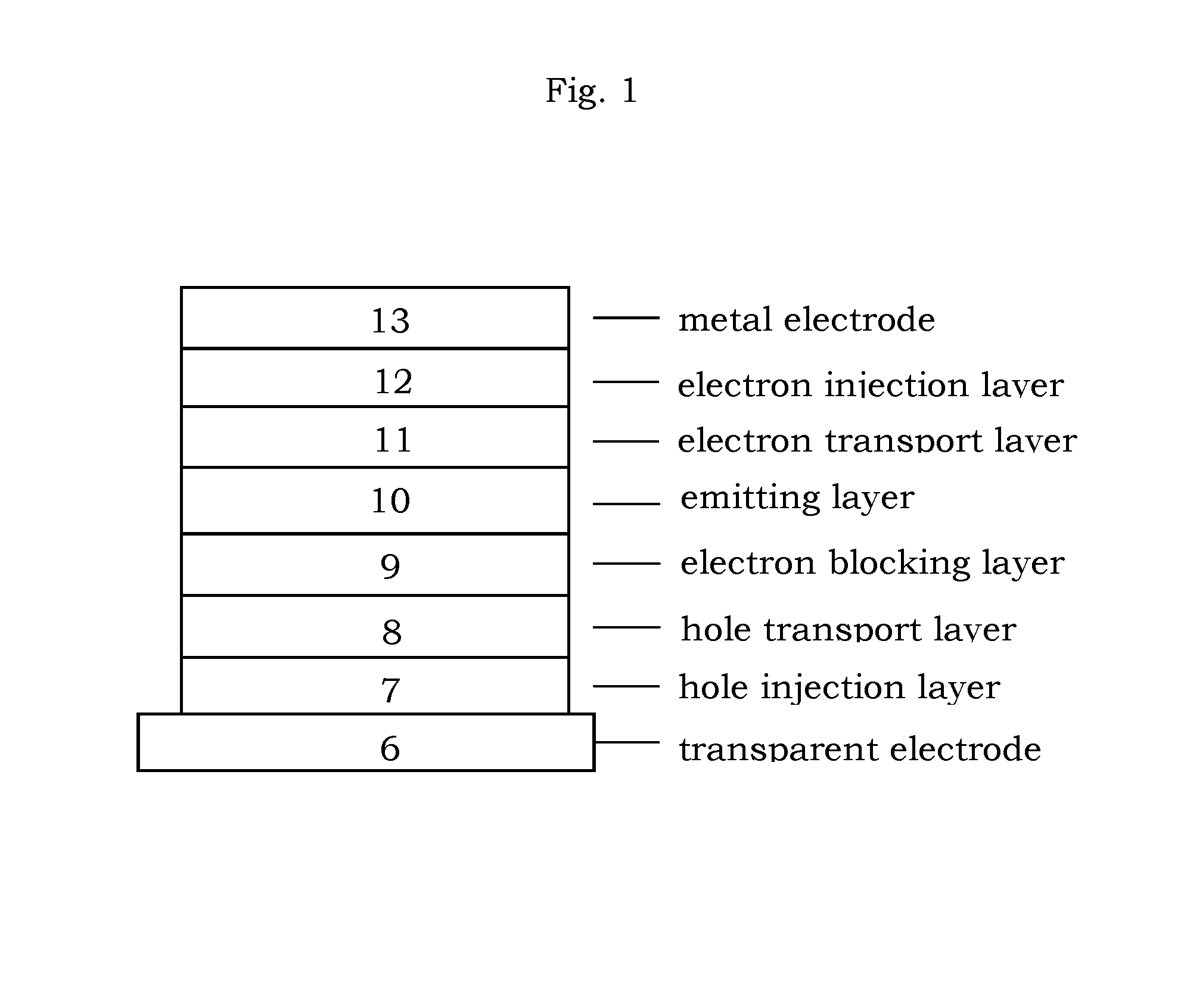
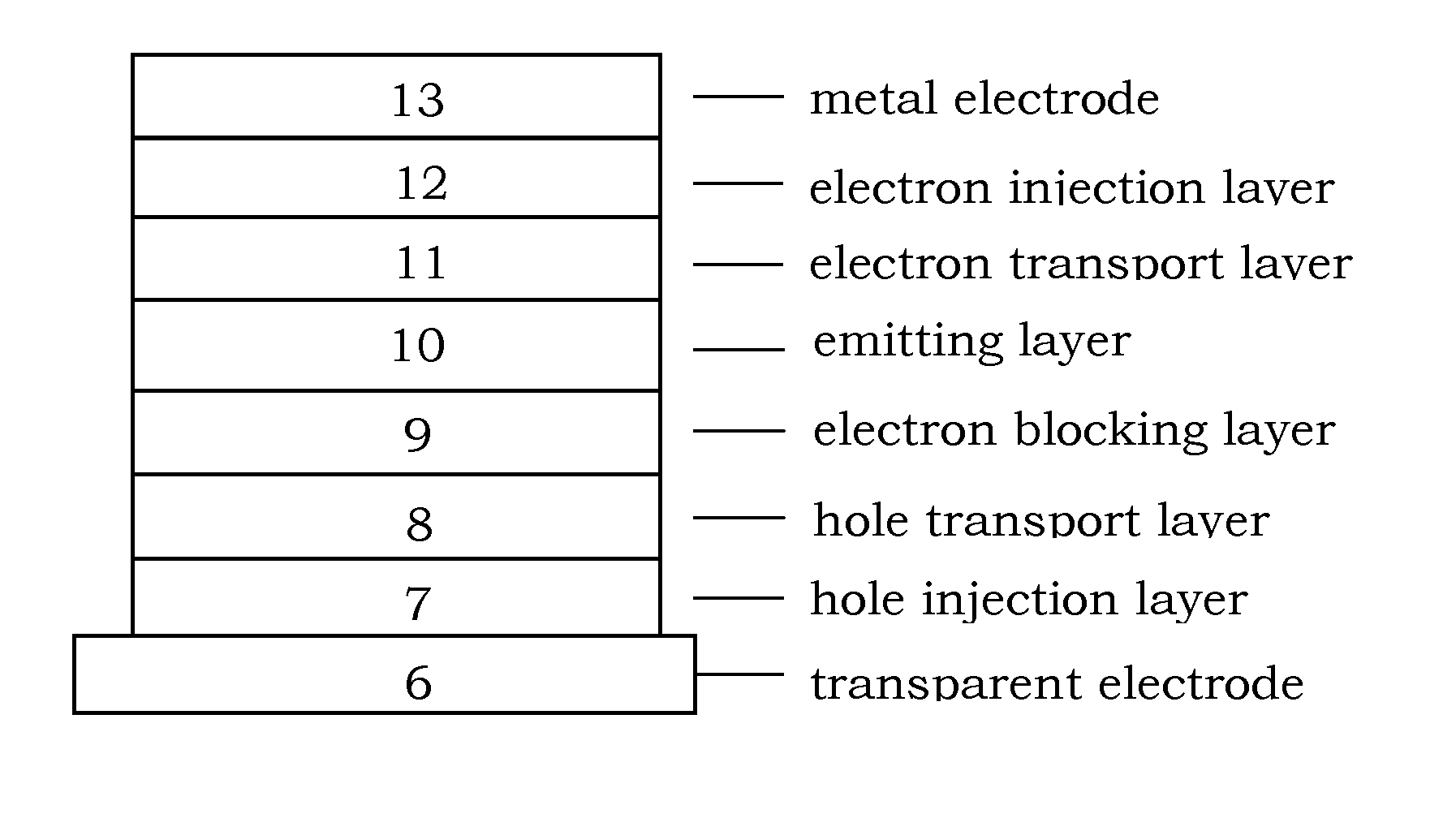
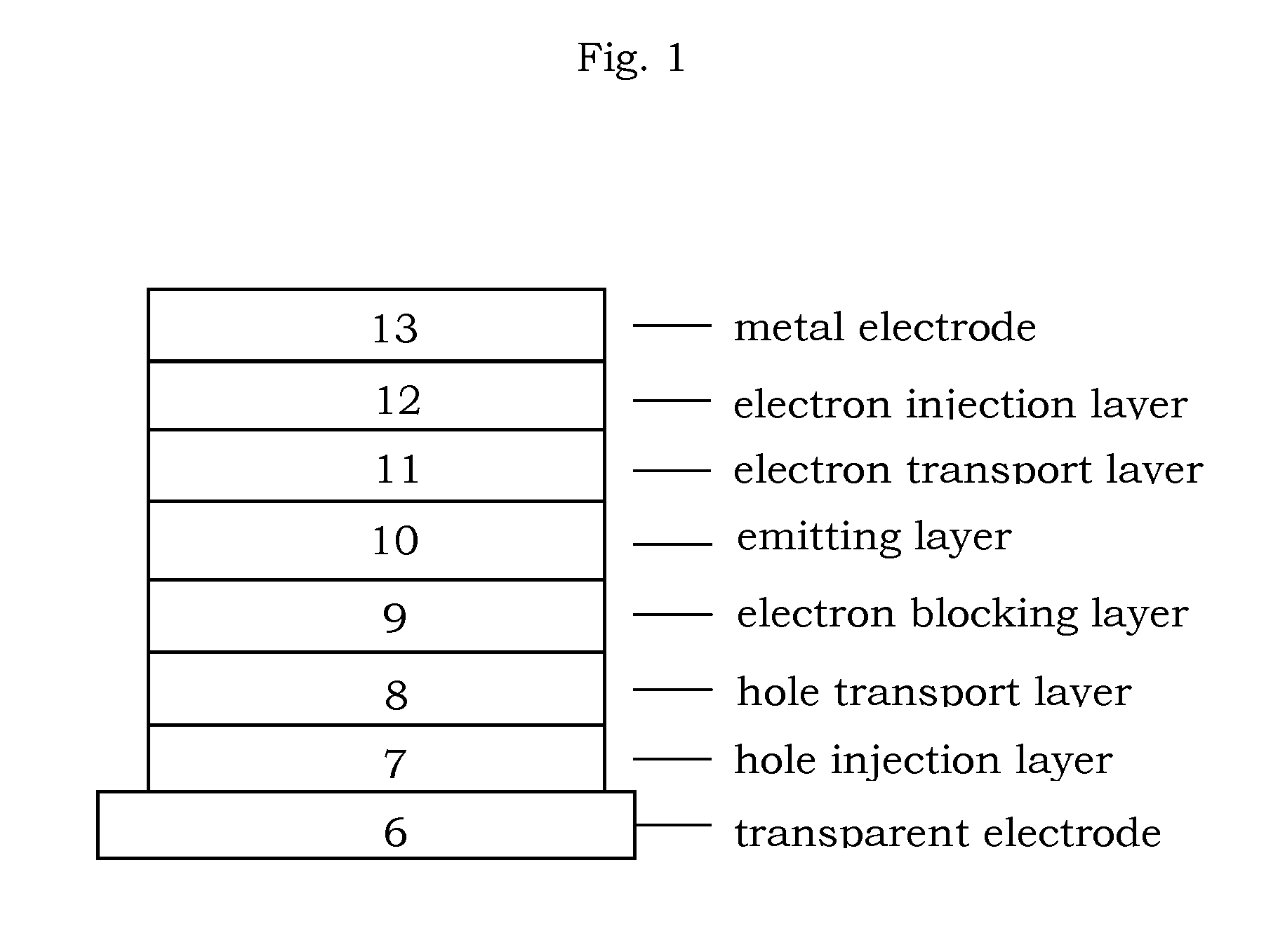
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009065A1Improve efficiencyLong lastingSilicon organic compoundsDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescence device and material for organic electroluminescence device
InactiveUS20090045731A1Improve efficiencyLong life-timeDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmFluoranthene
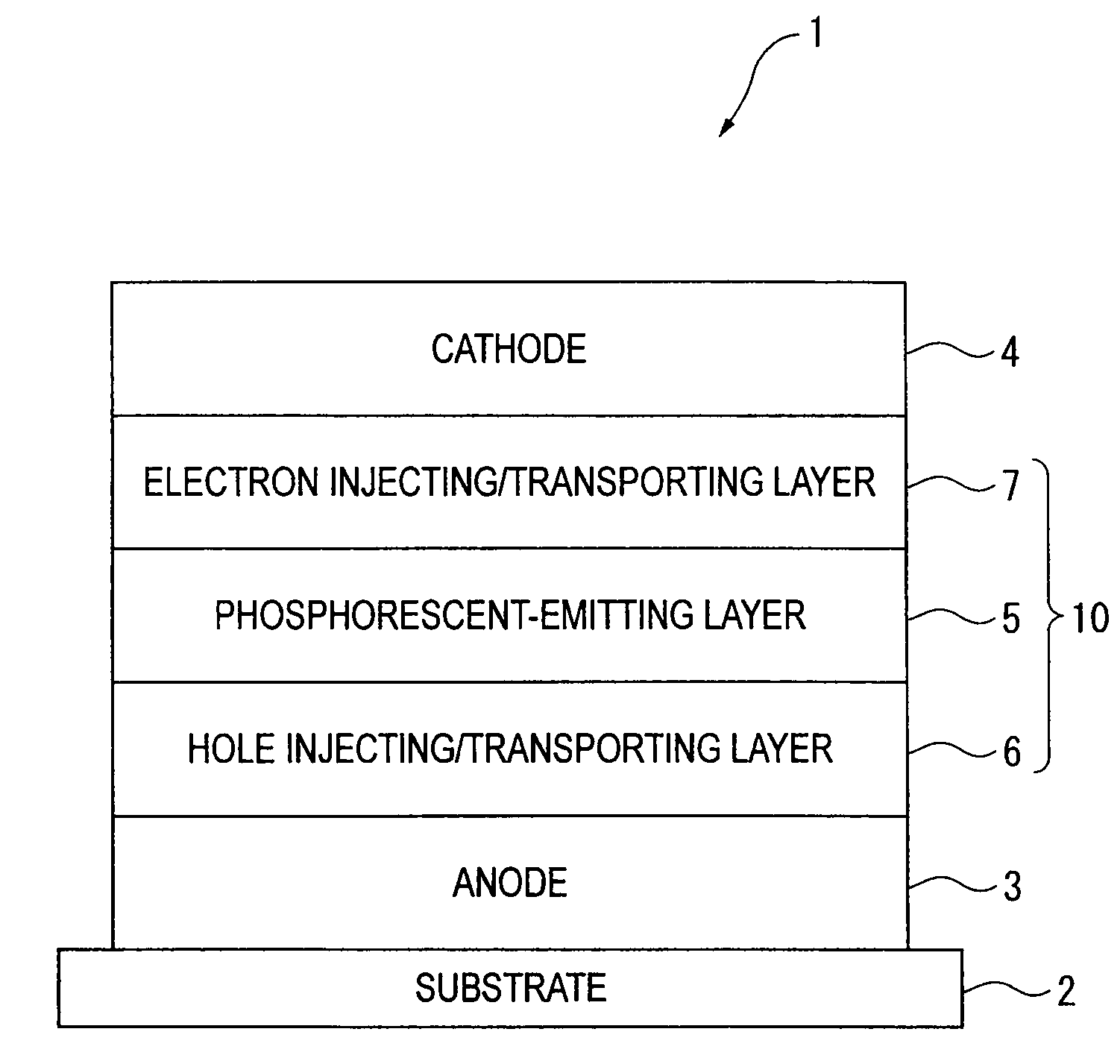
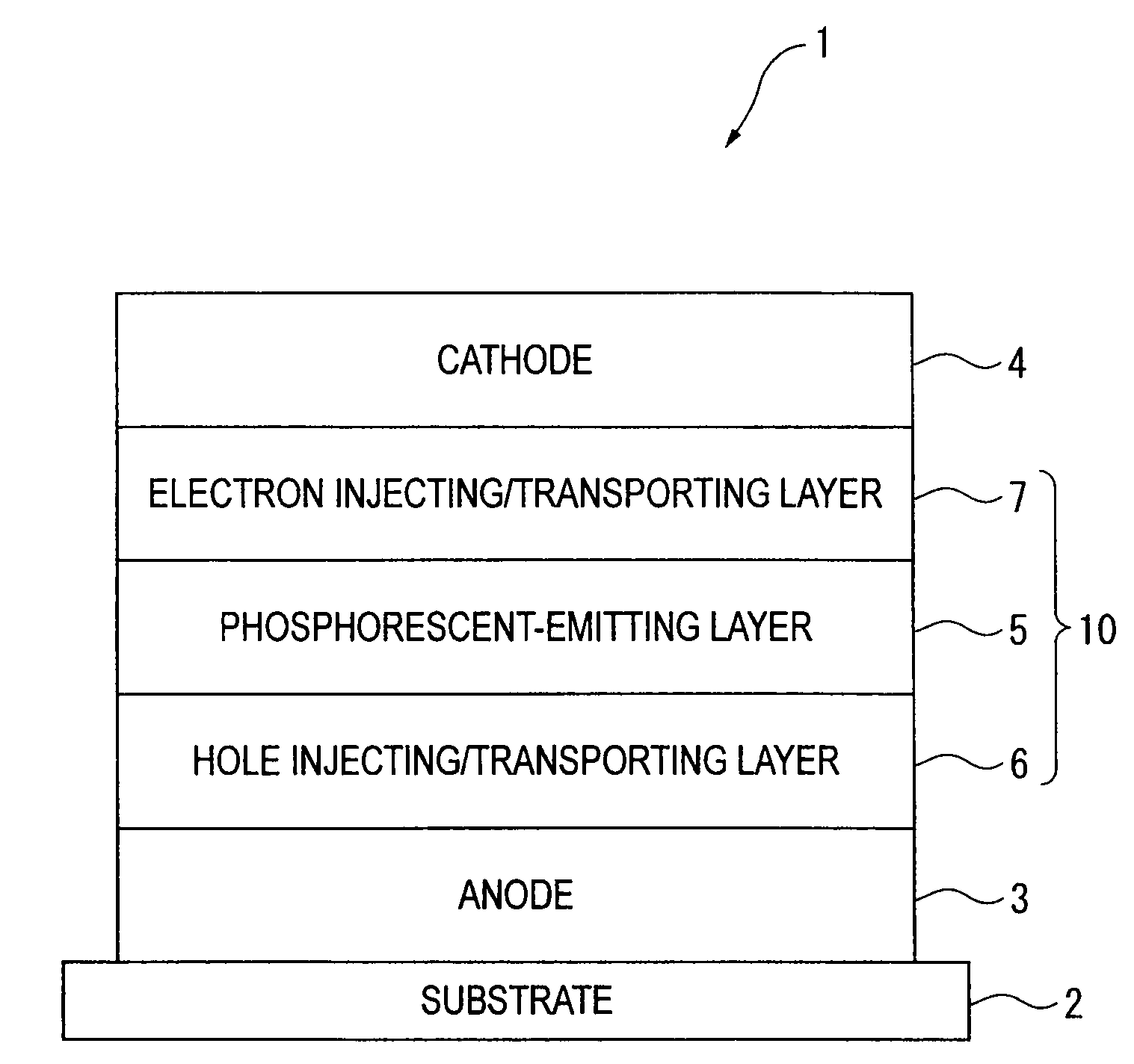
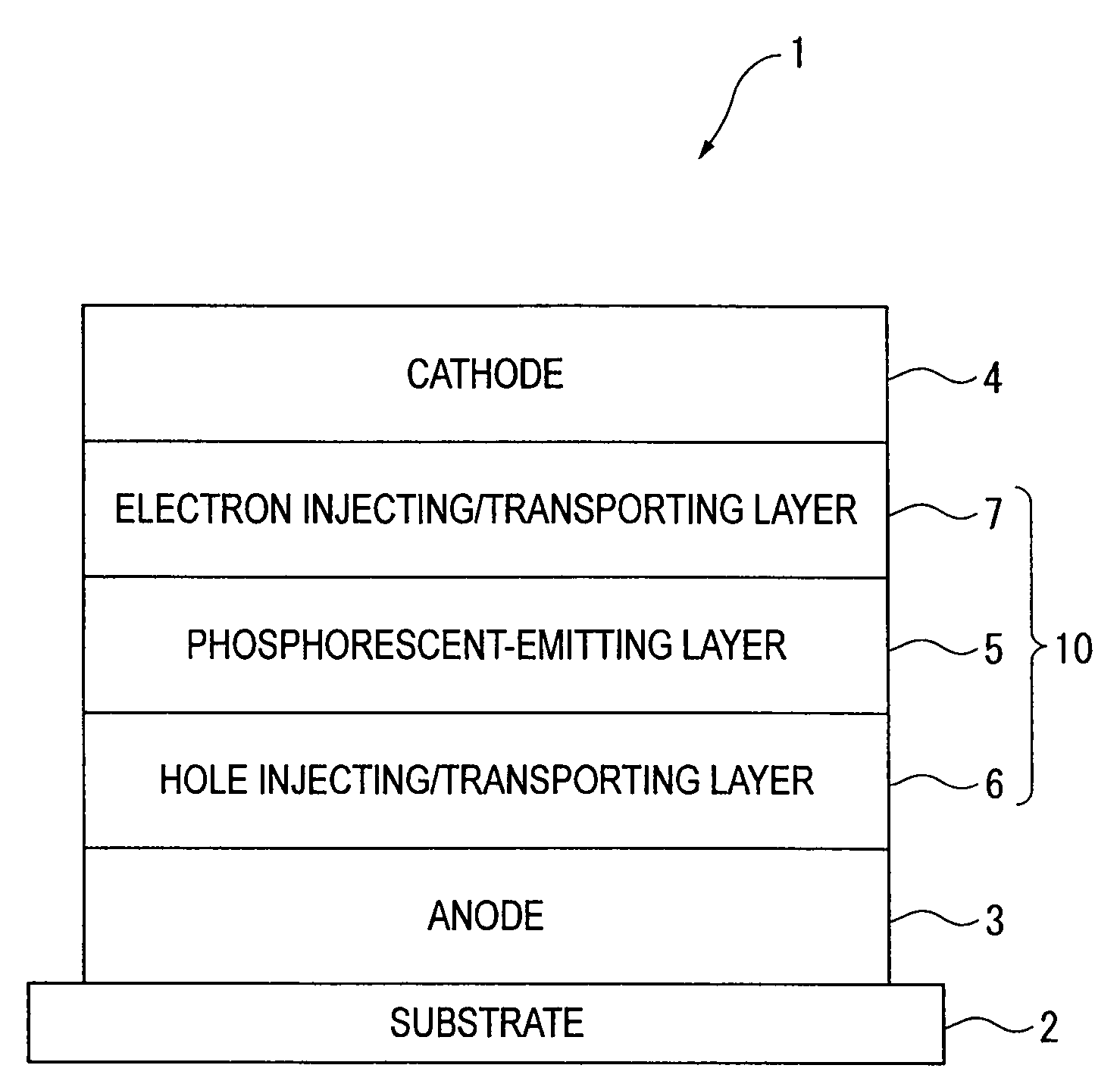
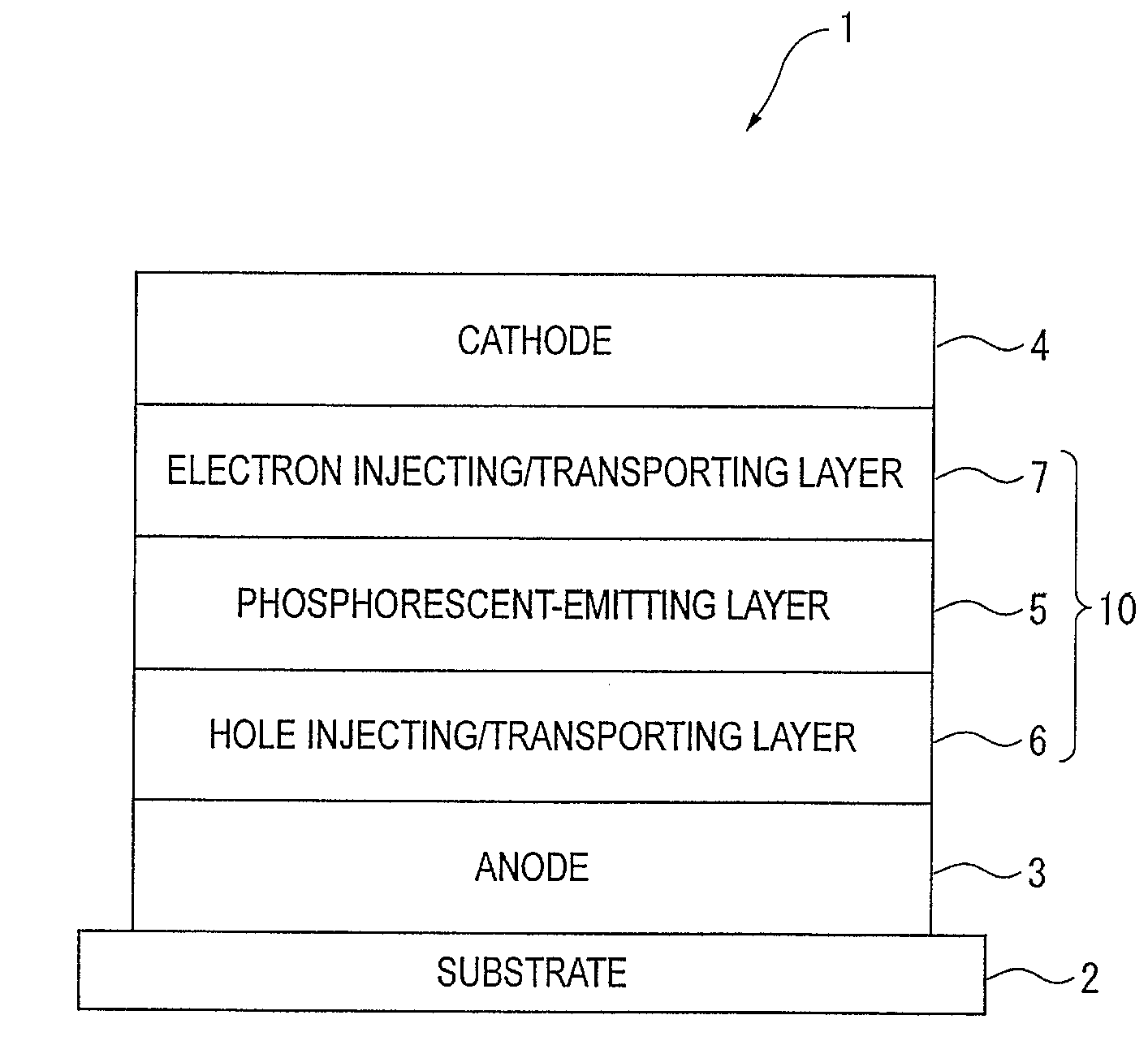
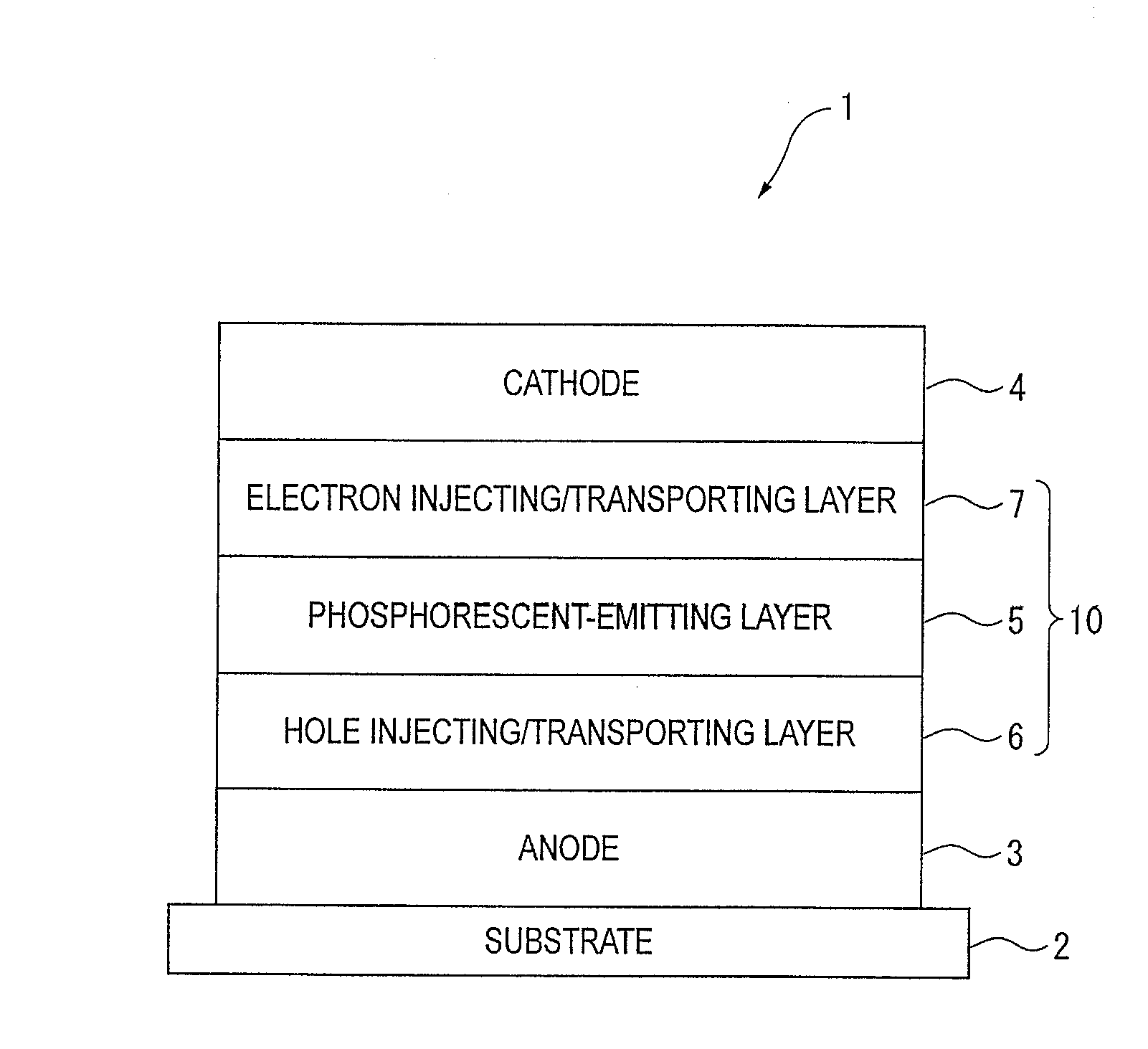
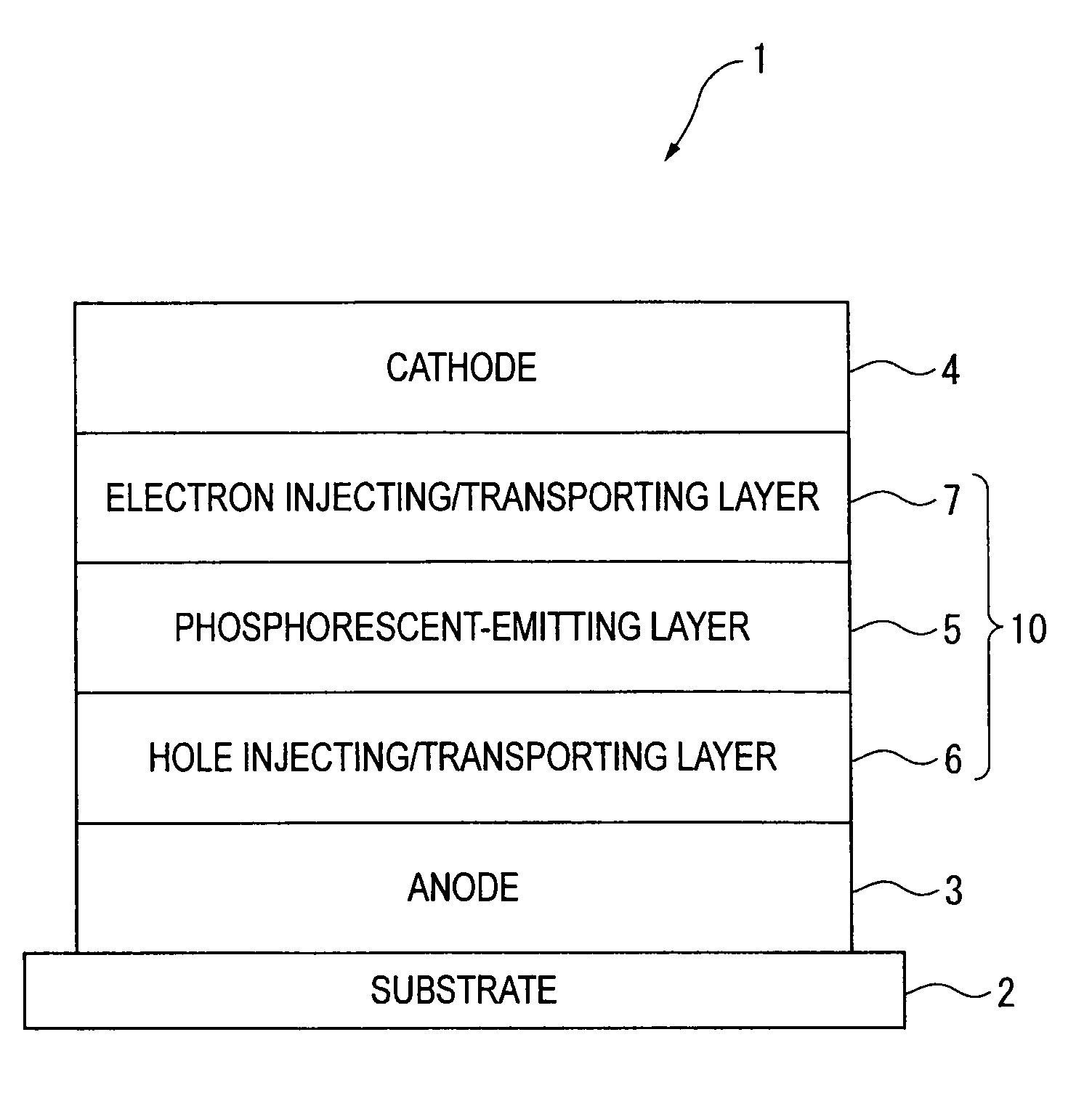
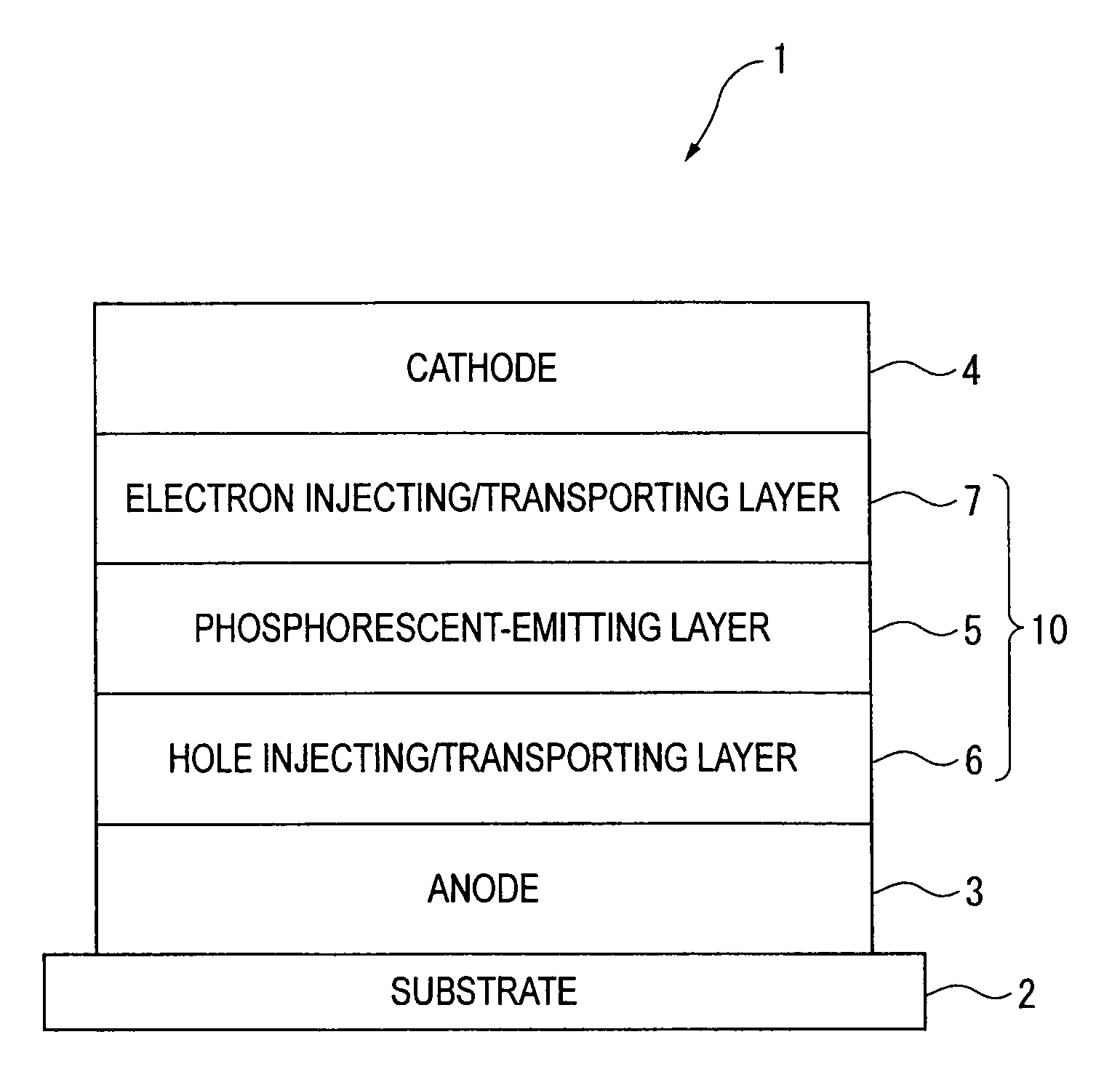
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).In the formula, Ar1, Ar2, Ar3, B1, B2, B3 and B4 each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon ring selected from a naphthalene ring, a chrysene ring, a fluoranthene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a triphenylene ring, a benzo[a]triphenylene ring, a benzochrysene ring, a benzo[b]fluoranthene ring and a picene ring. p is 0 or 1.
Owner:IDEMITSU KOSAN CO LTD
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090045730A1Improve efficiencyLong life-timeOrganic chemistryDischarge tube luminescnet screensOrganic filmBenzo(c)phenanthrene
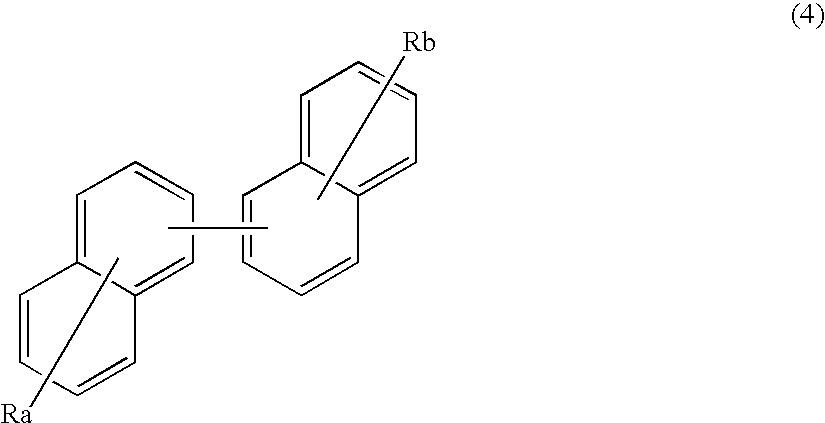
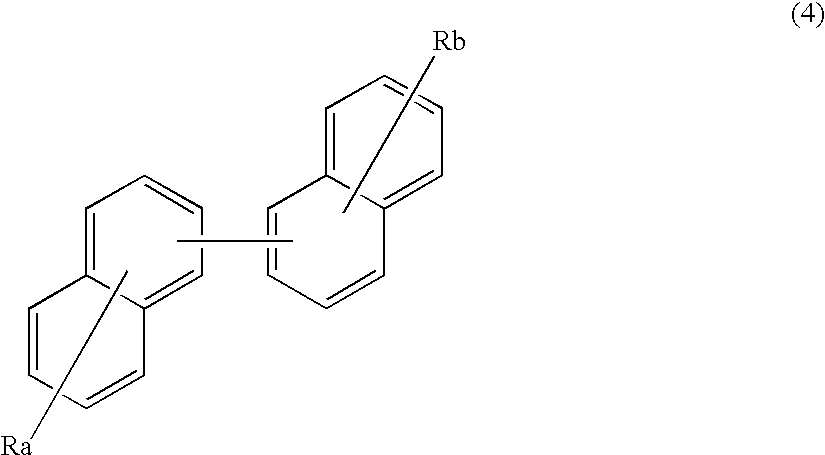
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. The organic thin-film layer includes at least one emitting layer. The at least one emitting layer contains at least one phosphorescent material and a host material represented by the following formula (1).Ra-Ar1-Rb (1)In the formula, Ar1, Ra and Rb each represent a substituted or unsubstituted benzene ring or a condensed aromatic hydrocarbon ring selected from a substituted or unsubstituted naphthalene ring, a substituted or unsubstituted chrysene ring, a substituted or unsubstituted fluoranthene ring, a substituted or unsubstituted phenanthrene ring, a substituted or unsubstituted benzophenanthrene ring, a substituted or unsubstituted dibenzophenanthrene ring, a substituted or unsubstituted triphenylene ring, a substituted or unsubstituted benzo[a]triphenylene ring, a substituted or unsubstituted benzochrysene ring, a substituted or unsubstituted benzo[b]fluoranthene ring and a substituted or unsubstituted picene ring. Substituents for Ra and Rb are not aryl groups.
Owner:IDEMITSU KOSAN CO LTD
Electrolytic solution for non-aqueous type battery and non-aqueous type secondary battery
InactiveUS20030118912A1Excellent in high-temperature storage characteristicReduced responseCell electrodesOrganic electrolyte cellsNon aqueous electrolytesTerphenyl
In a rechargeable non-aqueous electrolyte secondary battery using positive electrodes, negative electrodes and a non-aqueous electrolytic solution, additives to the electrolytic solution are used in combination, preferably in combination of at least two compounds selected from o-terphenyl, triphenylene, cyclohexylbenzene and biphenyl, and thus there are provided batteries excellent in safety and storage characteristics.
Owner:PANASONIC CORP +1
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS20090009067A1Improve efficiencyLong life-timeDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmBenzo(c)phenanthrene
An organic electroluminescence device includes: a cathode; an anode; and a single-layered or multilayered organic thin-film layer provided between the cathode and the anode. In the organic electroluminescence device, the organic thin-film layer includes at least one emitting layer, and the at least one emitting layer contains: at least one phosphorescent material; and a host material represented by the following formula (1).Ra—Ar1—Ar2—Rb (1)In the formula, Ar1, Ar2, Ra and Rb each represent a substituted or unsubstituted benzene ring or a substituted or unsubstituted condensed aromatic hydrocarbon group selected from a group consisting of a naphthalene ring, a chrysene ring, a fluoranthene ring, a triphenylene ring, a phenanthrene ring, a benzophenanthrene ring, a dibenzophenanthrene ring, a benzotriphenylene ring, a benzochrysene ring, a picene ring and a benzo[b]fluoranthene ring.
Owner:IDEMITSU KOSAN CO LTD
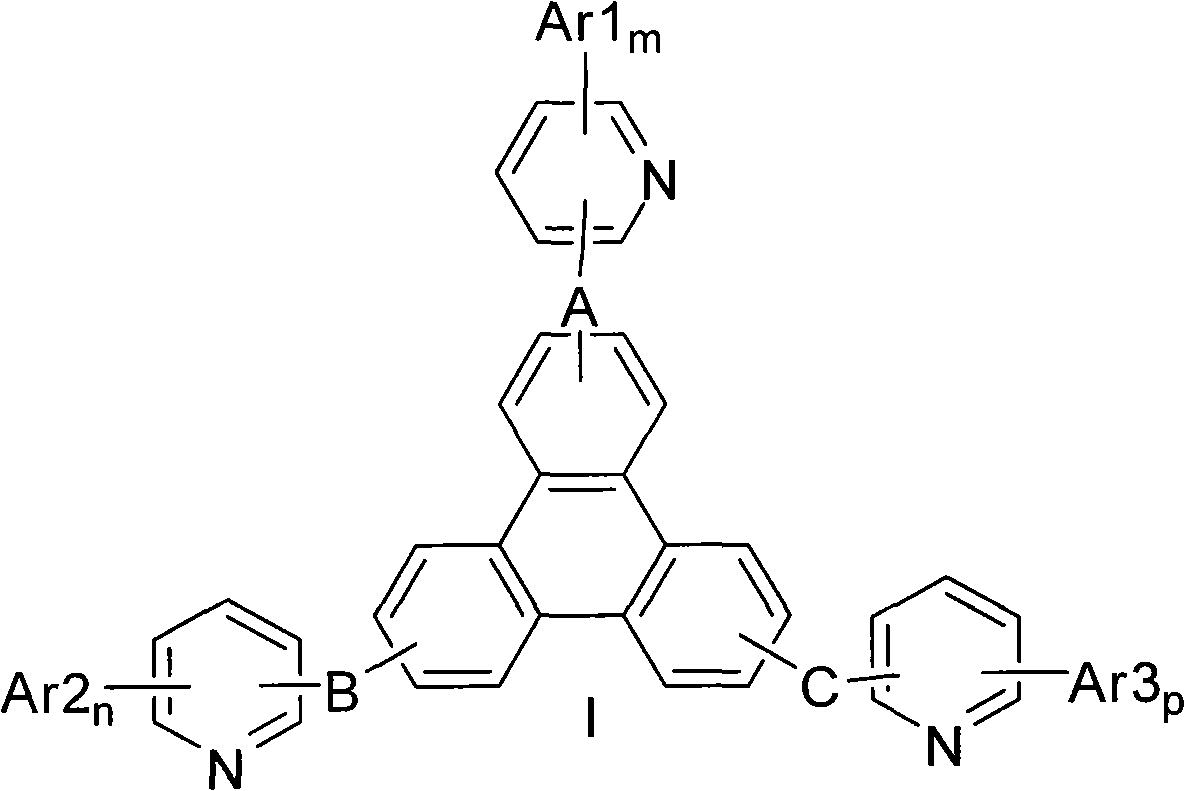
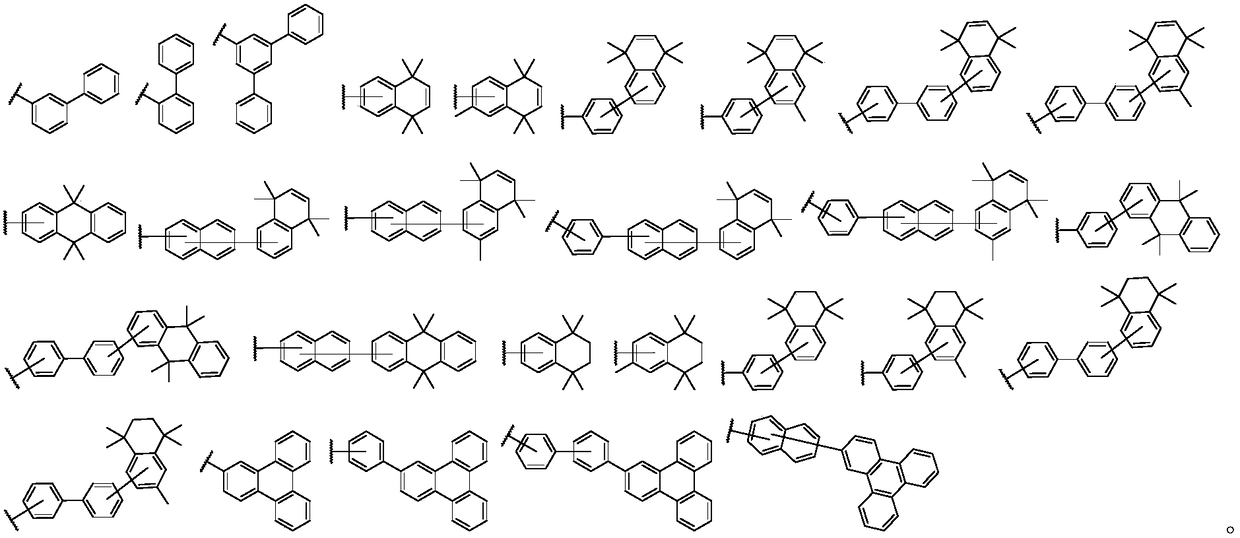
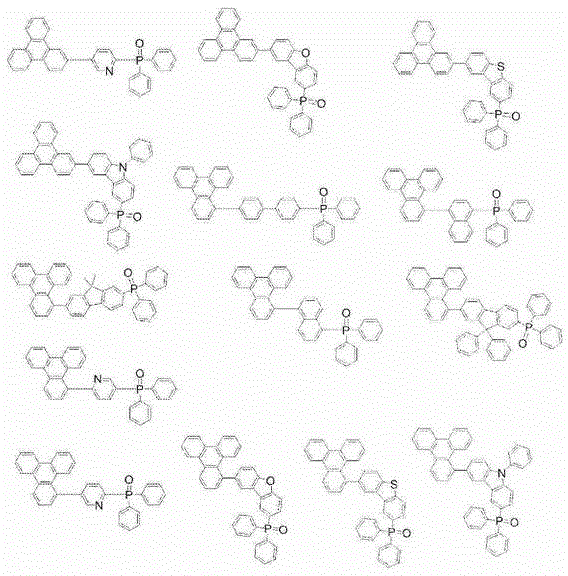
Triphenylene compound containing pyridine group and application thereof
InactiveCN102532105AHigh triplet energy levelImprove stabilityOrganic chemistrySolid-state devicesCharge carrier mobilityCompound (substance)
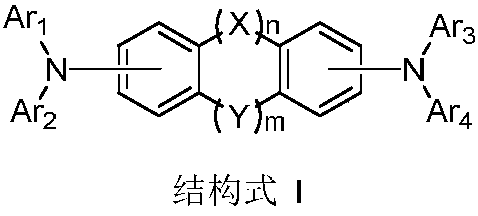
The invention provides a novel compound, which has stable compound property, a simple preparation process, high luminous efficiency and high carrier mobility and can be applied to a phosphorescent main body material and an electron transfer layer of an electroluminescent element. According to an applied device, the driving voltage can be reduced remarkably, and the current efficiency is increased. The structural general formula of the compound is shown as a formula I, wherein a mother nucleus is selected from triphenylene; terminal groups Ar1, Ar2 and Ar3 are selected from a pyridine group, a phenyl group, a biphenylyl group or a naphthyl group; A, B and C are chemical bonds or aromatic rings with 6-30 carbon atoms; and m, n and p are integers of 0-2.
Owner:TSINGHUA UNIV +2
Organic electroluminescence device and material for organic electroluminescence device
ActiveUS8779655B2Improve efficiencyLong lastingDischarge tube luminescnet screensElectroluminescent light sourcesOrganic filmBenzo(c)phenanthrene
Owner:IDEMITSU KOSAN CO LTD
Triphenylene based aromatic compounds and oleds utilizing the same
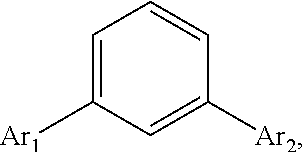
Disclosed is a triphenylene based aromatic compound, wherein a benzene center is substituted with a triphenylene group and another aromatic group such as triphenylenyl, pyrenyl, phenylvinyl, carbazolylphenyl, or arylanthryl in the meta position of the benzene center. The meta-substituted aromatic compound of the invention has better thermal stability (Tg) than the conventional para-substituted aromatic compound. The meta-substituted aromatic compound, served as a hole transporting layer or a host material applied in a light emitting layer in an OLED, is more preferable than the conventional para-substituted aromatic compound.
Owner:E INK HLDG INC
Synthesis of triphenylene and pyrene based aromatics and their application in oleds
InactiveUS20090169921A1Increase brightnessImprove power efficiencyOrganic chemistryElectrical apparatusArylBenzene
The present invention provides a compound of the general formulaAr1—R1—Ar2 (I)wherein Ar1 and Ar2 independently represent triphenylenyl or pyrenyl, and R1 represent a bond, aryl, or heteroaryl. The present invention also provides a process for the preparation of the compound formula (□), and an organic electroluminescence device utilizing luminescent material comprising the compound of formula (□) as an emitting layer.
Owner:CHENG CHIEN HONG
Triphenylene compounds, method of manufacturing the same and organic electroluminescent devices employing the same
InactiveUS8026663B2Improve the level ofReduce voltageGroup 4/14 element organic compoundsDischarge tube luminescnet screensHydrogen atomAliphatic hydrocarbon
The present invention provides a novel compound represented by general formula (I) below, a method of manufacturing the same and an organic electroluminescent device employing the same:where R1 to R6 are independent of one another and are each a hydrogen atom or a substituent represented by general formula (II) below, and at least one of R1 to R6 is a substituent represented by general formula (II):—C≡C—SiRaRbRc (II)where Ra, Rb and Rc are independent of one another and are each an aliphatic hydrocarbon group having 1 to 10 carbon atoms or an aromatic hydrocarbon group.
Owner:GUNMA UNIVERSITY
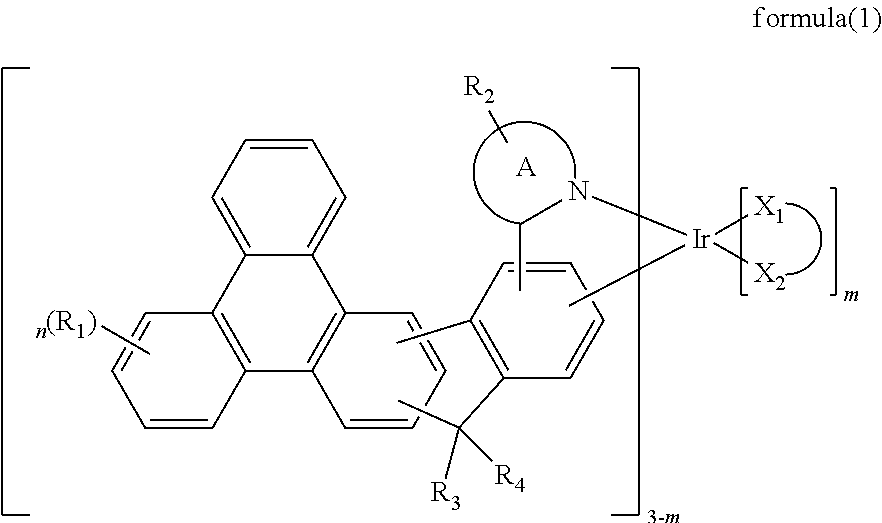
Indenotriphenylene-based iridium complexes for organic electroluminescence device
ActiveUS20160351835A1Improve thermal stabilityHigh carrier mobilityIndium organic compoundsSolid-state devicesDopantIsoquinoline
The present invention discloses an indenotriphenylene-based iridium complexes is represented by the following formula (1), the organic EL device employing the derivative as light emitting dopant of emitting layer can display good performance like as lower driving voltage and power consumption, increasing efficiency and half-life time.wherein A ring represents an imidazole, a pyridine, a quinoline and an isoquinoline, X1-X2 represents a bidentate ligand, and m, n and R1 to R4 are the same definition as described in the present invention.
Owner:LUMINESCENCE TECH
Triarylated amine derivative and organic light-emitting device
InactiveCN109467543ABack passGood hole transport propertiesOrganic chemistrySolid-state devicesFuranOrganic light emitting device
The invention provides a triarylated amine derivative and an organic light-emitting device, and relates to the technical field of organic light-emitting materials. A furan or thiophene derivative group, a fluorene derivative group, and a triphenylene group are connected on a triarylated amine main structure to obtain the triarylated amine derivative, the film forming property of the triarylated amine derivative is good, the thermal stability is good, the triarylated amine derivative has an excellent hole transfer capability, is simple to synthesize and easy to operate, can be applied into theorganic light-emitting device to serve as a hole transfer layer, effectively solves the problems of poor thermal stability of a hole transfer material in the organic light-emitting device, difficultyin film forming, low light emitting efficiency of the device and serious roll-off, and the organic light emitting device has the advantages of low drive voltage, high light emitting efficiency and long service life.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Triphenylene-perylene monoimide diformate binary compound, preparation method and applications thereof
ActiveCN105461628AWith opticsEasy to adjust temperatureLiquid crystal compositionsOrganic chemistryOrganic solar cellPerylene
The present invention provides a triphenylene-perylene monoimide diformate binary compound, which has a structure represented by a general formula I. The present invention further provides a preparation method of the triphenylene-perylene monoimide diformate binary compound, and applications of the triphenylene-perylene monoimide diformate binary compound as an organic solar cell active layer. Compared to the compound in the prior art, the compound of the present invention has advantages of easy adjustment of liquid crystal phase change temperature and temperature range, such that the compound has optical, electronic and electro-optical uses, and especially has uses as the organic solar cell active layer. The general formula (I) is defined in the specification.
Owner:JIANGSU HECHENG ADVANCED MATERIALS
Novel organic electroluminescent compounds and organic electroluminescent device using the same
InactiveCN103228661AImprove luminous efficiencyExcellent material lifeSilicon organic compoundsIndium organic compoundsOrganic electroluminescenceOperating life
Organic electroluminescent compounds comprising a triphenylene conjugated to a five-ring fused heterocyclic system, as depicted in formula 1 are provided. Also provided is an organic electroluminescent device comprising these compounds. The organic electroluminescent compounds disclosed herein exhibit good luminous efficiency and excellent material life. They can be used to manufacture OLED devices very superior in terms of operating life and which consume less power due to improved power efficiency.
Owner:ROHM & HAAS ELECTRONICS MATERIALS LLC
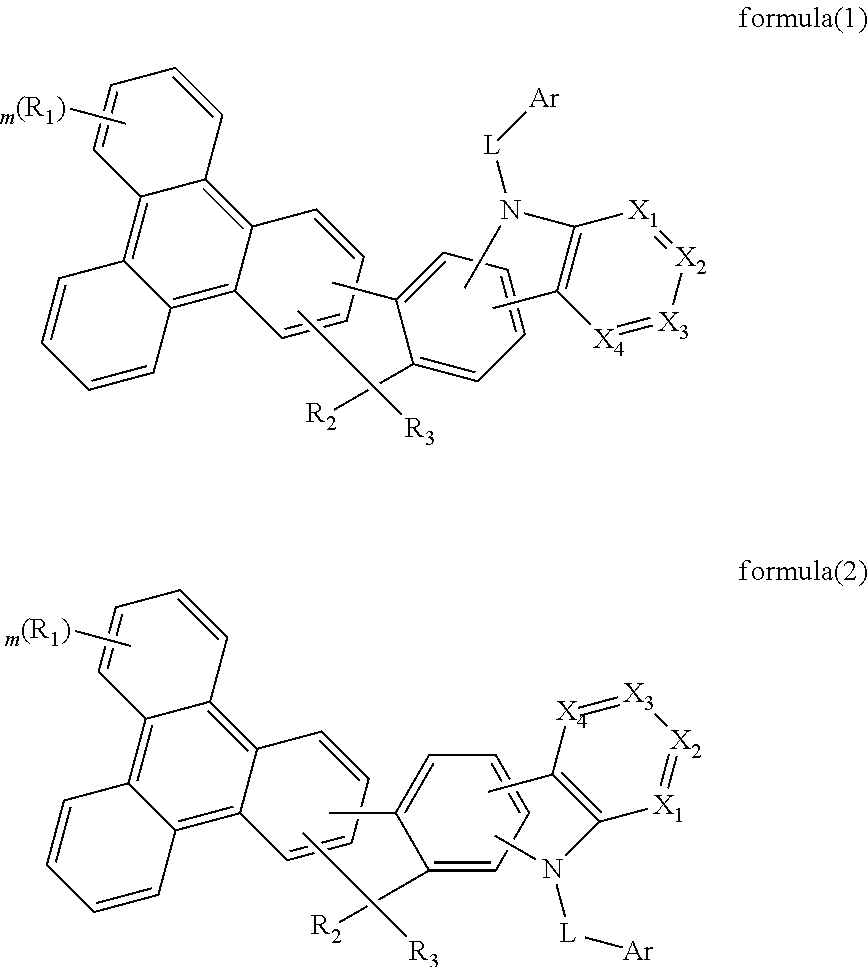
Compounds for organic electroluminescence device
ActiveUS20160372679A1Improve thermal stabilityHigh carrier mobilityOrganic chemistryElectroluminescent light sourcesCarbazoleOrganic electroluminescence
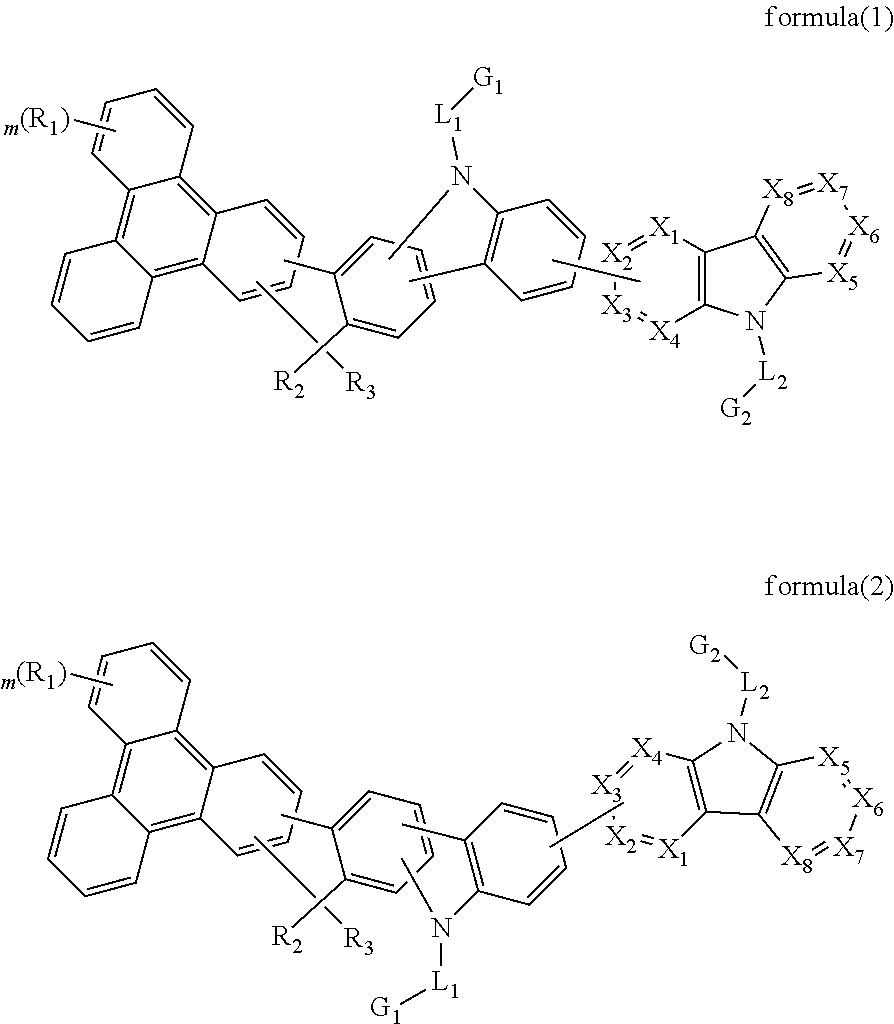
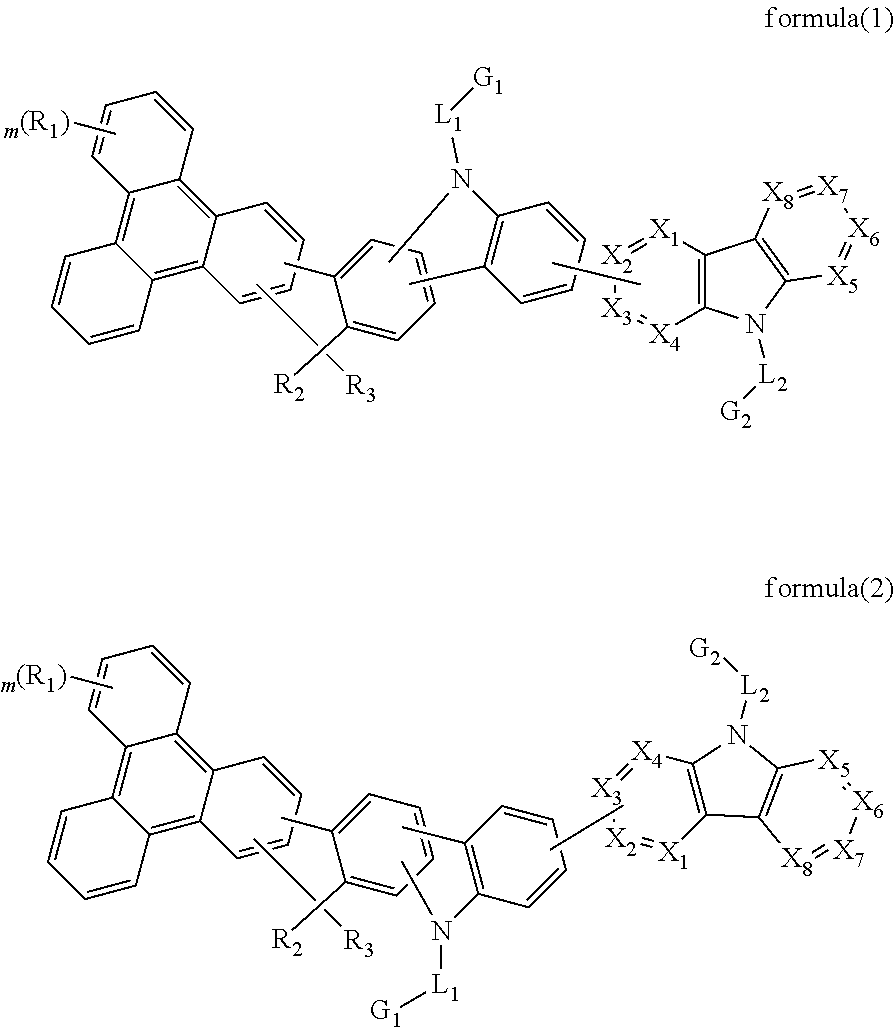
The present invention discloses an triphenylene-based fused carbazole compound is represented by the following formula (1) or formula (2), the organic EL device employing the derivative as light emitting host of emitting layer can display good performance like as lower driving voltage and power consumption, increasing efficiency and half-life time.wherein Ar, X1 to X4, m, and R1 to R3 are the same definition as described in the present invention.
Owner:LUMINESCENCE TECH
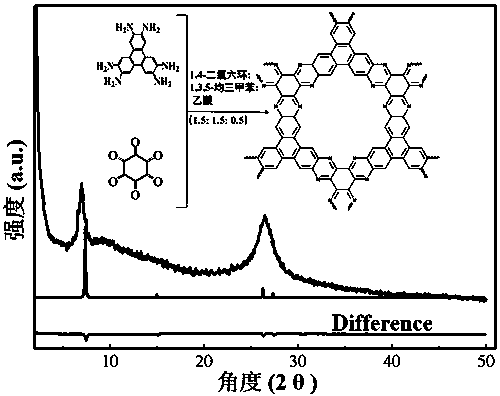
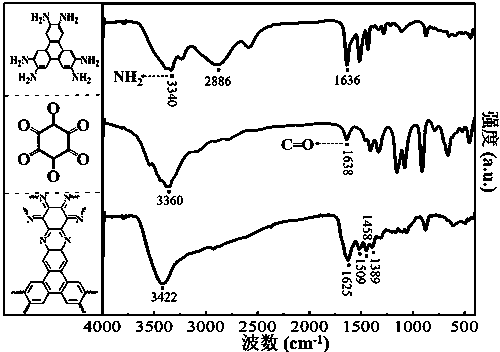
Preparation of phenazine-connecting two-dimensional covalent organic framework material of novel structure
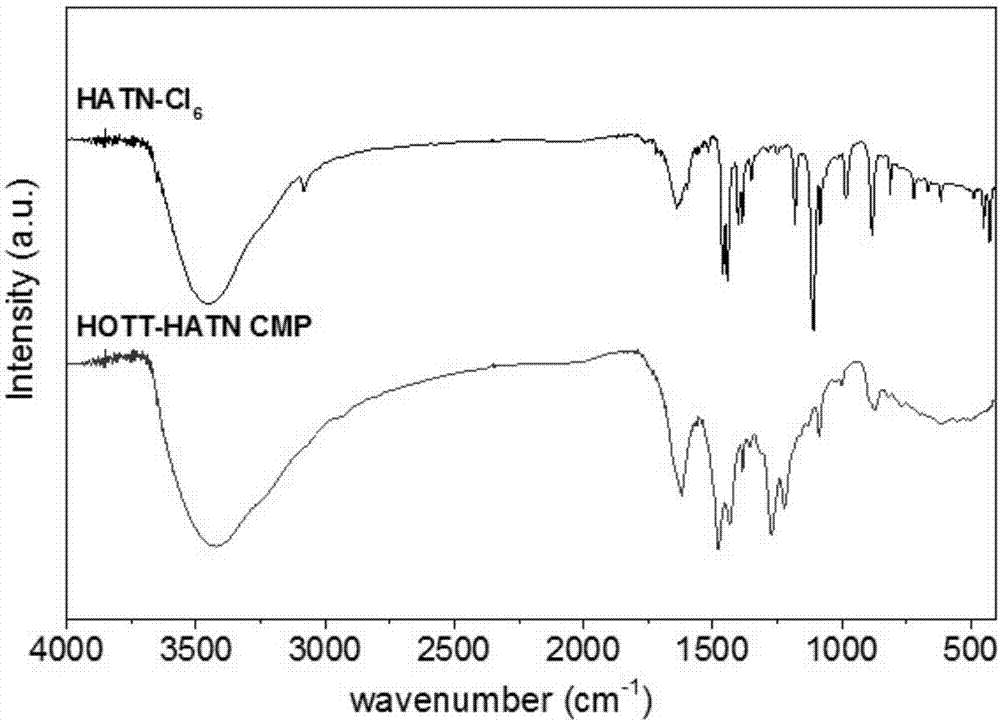
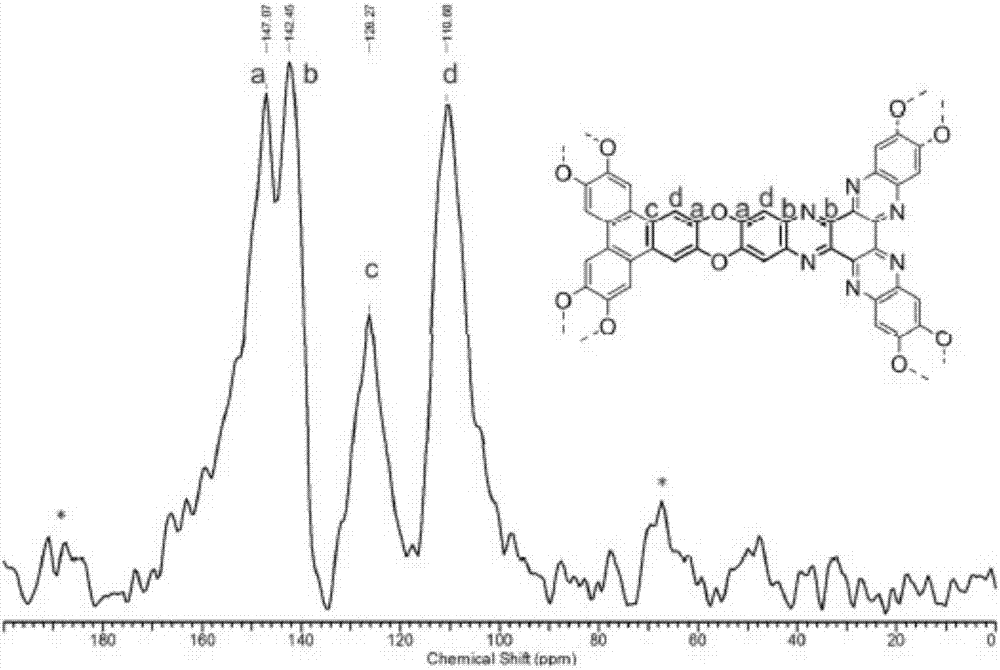
The invention discloses a preparation method of a phenazine-connecting two-dimensional covalent organic framework material of a novel structure. The synthesized phenazine-connecting two-dimensional covalent organic framework material of the novel structure has a highly planar rigid structure in a graphene-like single layer, and is also highly conjugated between layers. A synthesis method is a solvothermal method and is easy and safe to operate, the reaction is a molecular level reaction, and the synthesized product is more ordered and more stable. According to the method, triphenylene-2,3,6,7,10,11-hexaaminotriphenylene and hexaketocyclohexane octahydrate serve as raw materials, 1,4-dioxane and 1,3,5-mesitylene serve as organic solvents, and the phenazine-connecting two-dimensional covalent organic framework material of the novel structure is successfully prepared through the solvothermal method under the condition that acetic acid is used for adjusting the acid environment.
Owner:HARBIN UNIV OF SCI & TECH
Conjugated polyelectrolytes and methods of using the same
Various embodiments disclosed relate to conjugated polyelectrolytes and methods of using the same. Various embodiments provide a conjugated polyelectrolyte including a subunit having the structure —R1—Y—R2—Z—. At each occurrence, R1 is independently chosen from 1,4-bonded phenylene substituted by —X—R3—R4 j times and 2,5-bonded thiophene substituted by —X—R3—R4 j times. At each occurrence, Y is independently chosen from a bond and —C≡C—. At each occurrence, R2 is independently chosen from a bond, a substituted or unsubstituted phenylene, thiophenylene, azulenylene, heptalenylene, biphenylene, indacenylene, fluorenylene, phenanthrenylene, triphenylenylene, pyrenylene, naphthacenylene, chrysenylene, biphenylenylene, anthracenylene, and naphthylene. At each occurrence, Z is independently chosen from a bond and —C≡C—. The variables j, R3, and R4 are as defined herein.
Owner:STC UNM +1
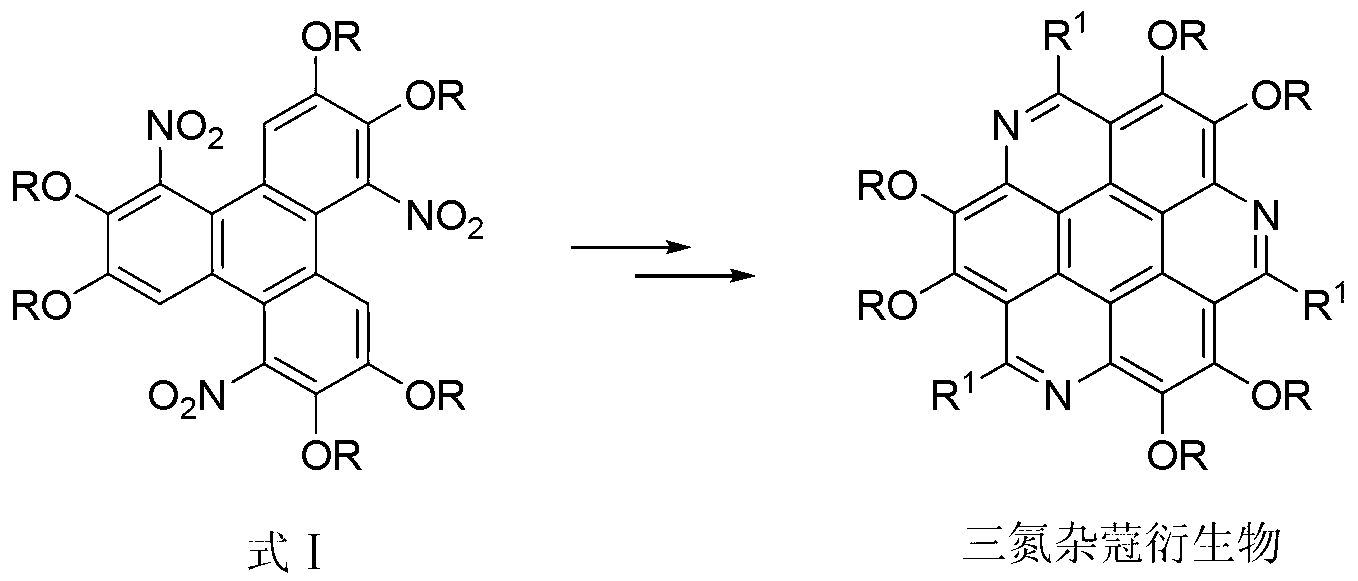
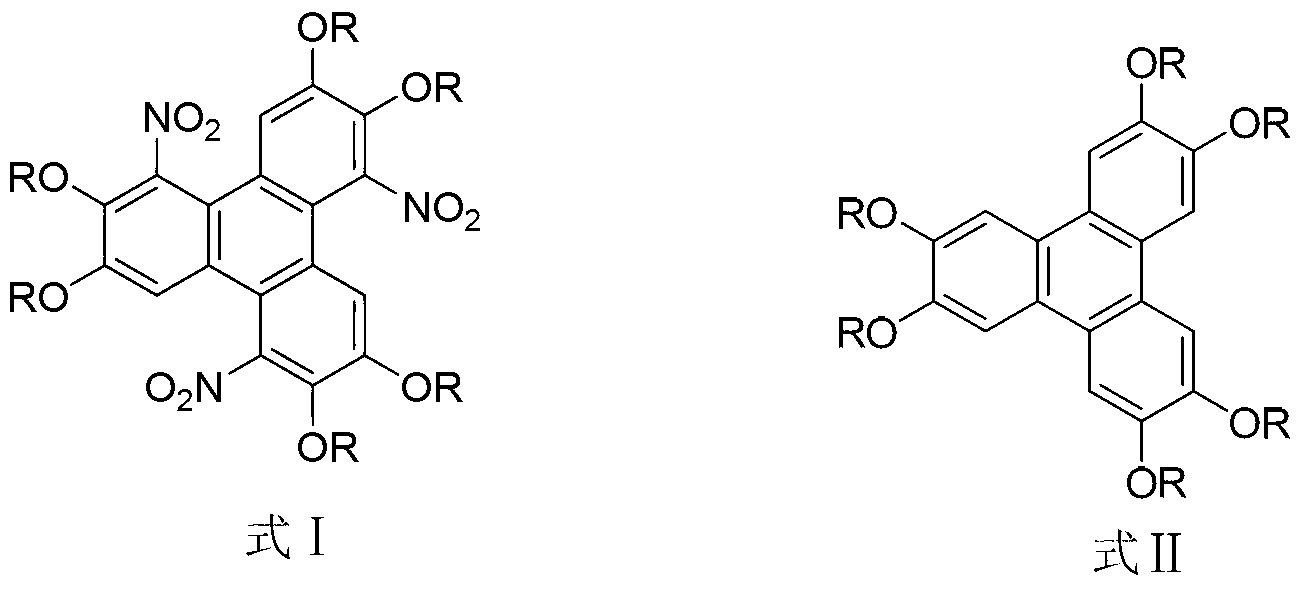
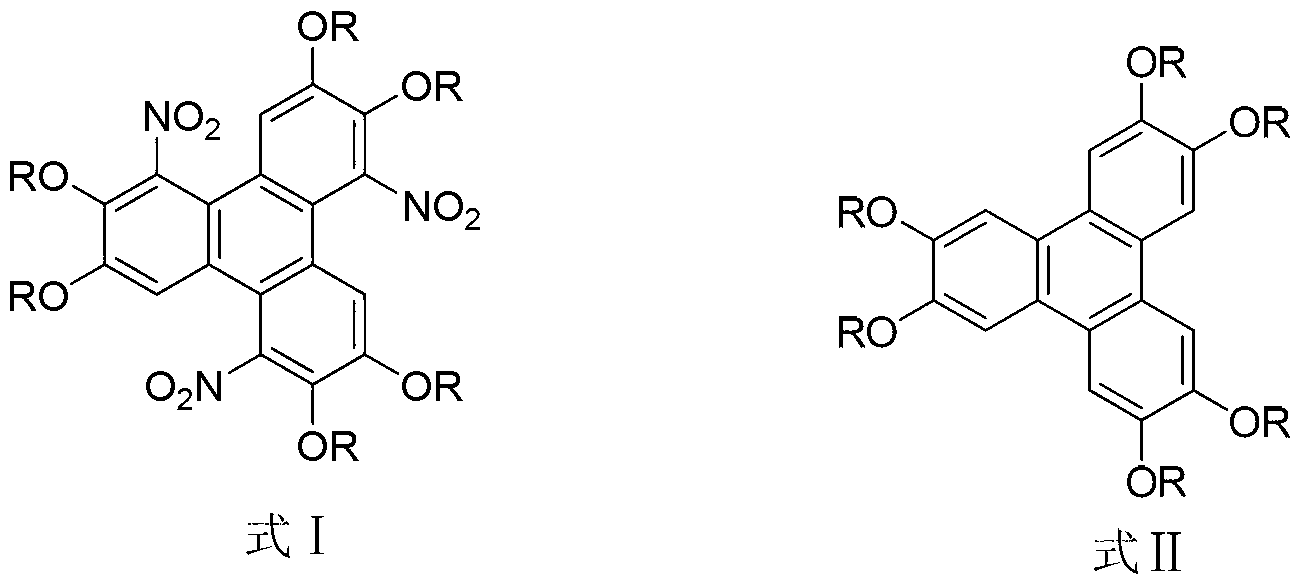
Method for synthesising 1,5,9-trinitro-2,3,6,7,10,11-hexa-alkoxy triphenylene
InactiveCN103265436AHigh yieldMild reaction conditionsNitro compound preparationSulfonyl chlorideNitrate
The invention discloses a method for synthesising 1,5,9-trinitro-2,3,6,7,10,11-hexa-alkoxy triphenylene. The method comprises the following step of synthesising 1,5,9-trinitro-2,3,6,7,10,11-hexa-alkoxy triphenylene by taking 2,3,6,7,10,11-hexa-alkoxy triphenylene as a raw material, and taking nitrate and alkyl sulfonyl chloride as nitration reagents. Compared with literature report methods, the method disclosed by the invention has the advantages of being moderate in reaction conditions, simple to operate, high in product yield, without the need of other strong acid reagents during a reaction process, and the like.
Owner:SHAANXI NORMAL UNIV
Nanometer organic microporous polymer and method for adsorbing heavy metal in drinking water
ActiveCN107417913AStable structureGood chemical stabilityOther chemical processesWater contaminantsEnvironmental resistanceBenzene
The invention belongs to the field of an organic microporous polymer, and particularly relates to a nanometer organic microporous polymer and a method for adsorbing heavy metal in drinking water. The nanometer organic microporous polymer provided by the invention has a structure shown as a formula (I) shown in description, and comprises a condensed polycyclic aromatic structure unit; the nanometer organic microporous polymer is prepared from triquinoyl octahydrate, 4,5-dichloro-1,2 phenylenediamine and 2,3,6,7,10,11 hexahydroxy triphenylene through reaction; the nanometer organic microporous polymer is fully organic aromatic framework solid capable of being highly recovered; no metal ions are contained; the synthesis does not need a transition metal catalyst; no halogen-containing by-products are generated; green and environment-friendly effects are achieved; a very important function is provided for the green and sustainable development technology. Experiments prove that the organic microporous polymer provided by the invention can fast adsorb heavy metal materials in the drinking water, so that the heavy metal content is lower than the drinkable limit; the nanometer organic microporous polymer scan be widely applied to drinking water purifications.
Owner:GUANGDONG UNIV OF TECH
Amine derivatives and OLED (organic light-emitting diode) containing same
InactiveCN109438258ALarge conjugated structureHigh hole mobilityOrganic chemistrySolid-state devicesHeat stabilityRefractive index
Owner:CHANGCHUN HYPERIONS TECH CO LTD
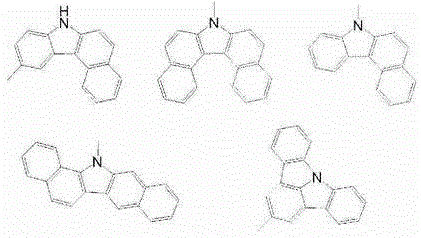
Triphenylene-based fused biscarbazole derivative and use thereof
The present invention discloses a triphenylene-based fused biscarbazole derivative expressed by a formula (1) or formula (2) and the use thereof. The triphenylene-based fused biscarbazole derivative can be used in an organic EL component mainly as a light emitting host of a light emitting layer, so that the organic EL component having low drive voltage, low power consumption, high efficiency and long half-life time. The invention also relates to an organic EL component containing the derivative expressed by the formula (1) or formula (2).
Owner:LUMINESCENCE TECH
1, 5, 9-trisubstituted coronene compound and synthesis method thereof
ActiveCN106565408AReduce pollutionImprove thermal stabilityOrganic compound preparationHydrocarbonsFuranCoronene
The present invention relates to a 1, 5, 9-trisubstituted coronene compound and a synthesis method thereof. The structural formula of the compound is shown in img file = 'dest _ path _ image 001. TIF 'wi = '109 'he = '108', wherein R represents H, C1-C18 alkyl, phenyl, 4-methylphenyl, 4-methoxy phenyl, benzyl, cyclohexyl, 4-trifluoromethylphenyl, thiophene, furan and the like. According to the technical scheme of the invention, the easily prepared 1, 5, 9-triamido triphenylene is subjected to diazotization and halogenation reaction to obtain the tri-halogenated triphenylene. After that, the tri-halogenated triphenylene is subjected to Sonogashira reaction with various alkynes to generate atriyne-triphenylene compounds. Finally, through the metal-catalyzed reaction and the cyclization reaction under the effect of an organic base, various 1, 5, 9-trisubstituted coronene compounds, novel in structure, can be obtained. According to the technical scheme of the invention, raw materials are easy for mass preparation. Meanwhile, the synthesis step is relatively short and the operation is convenient. The obtained trisubstituted coronene compound is good in thermal stability and chemical stability, and the trisubstituted coronene emits the relatively strong fluorescence within the range of 420-550 nm according to the fluorescence emission spectrum of the trisubstituted coronene compound. Therefore, the trisubstituted coronene is an excellent fluorescent material for preparing UV ultraviolet charge-coupled devices (UV-CCD) and organic light-emitting diodes (OLEDs), and has a wide application prospect in the field of electronic materials.
Owner:SHANGHAI UNIV
Triphenylene Compounds, Method of Manufacturing the Same and Organic Electroluminescent Devices Employing the Same
InactiveUS20080265750A1Increase heatAvoid low lightSilicon organic compoundsDischarge tube luminescnet screensHydrogen atomAliphatic hydrocarbon
The present invention provides a novel compound represented by general formula (I) below, a method of manufacturing the same and an organic electroluminescent device employing the same;where R1 to R6 are independent of one another and are each a hydrogen atom or a substituent represented by general formula (II) below, and at least one of R1 to R6 is a substituent represented by general formula (II):—C≡C—SiRaRbRc (II)where Ra, Rb and Rc are independent of one another and are each an aliphatic hydrocarbon group having 1 to 10 carbon atoms or an aromatic hydrocarbon group.
Owner:GUNMA UNIVERSITY
Arylamine derivative and organic light-emitting device
InactiveCN109400488ALarge conjugated structureHigh hole mobilityOrganic chemistrySolid-state devicesRefractive indexGlass transition
The invention discloses an arylamine derivative and an organic light-emitting device, and relates to the technical field of organic photoelectric materials. The arylamine derivative is an electron-rich system, and has a large conjugation structure, and hole transport performance is good. In addition, the light refraction index of the arylamine derivative is high, and when the arylamine derivativeis used as a light taking-out layer of the organic light-emitting device, the light exiting efficiency of the device can be improved effectively. In addition, the arylamine derivative has a large rigid structure due to introduction of large-size triphenylene, the glass transition temperature and thermal stability of the material are improved effectively, and film forming of the material is facilitated. The organic light-emitting device comprises an anode, a cathode and an organic matter layer, and the organic matter layer is positioned between the anode and the cathode, and comprises the arylamine derivative. The organic light-emitting device has low driving voltage, high light-emitting efficiency and long service life.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
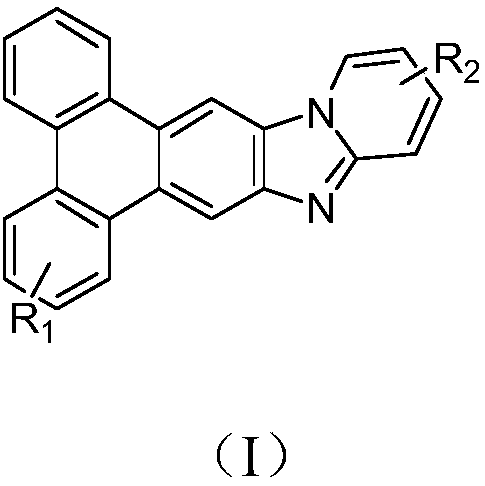
Triphenylene derivate and organic electroluminescence device
InactiveCN108440524AHigh glass transition temperatureImprove luminous efficiencyOrganic chemistrySolid-state devicesOrganic electroluminescenceGlass transition
The invention provides a triphenylene derivate and an organic electroluminescence device, and belongs to the technical field of organic electroluminescence materials. The compound has the structure asshown in formula (I). According to the triphenylene derivate, triphenylene and benzimidazole are condensed, so that the obtained material is high in glass transition temperature and is capable of avoiding crystalizing; the synthesizing method is simple, and easy to operate; the organic electroluminescence device prepared through the triphenylene derivate is high in luminescence efficiency and lowin driving voltage.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Compound, material for organic electroluminescent elements, organic electroluminescent element and electronic device
ActiveCN105473600AImprove performanceGood driving voltage and external quantum efficiencyGroup 5/15 element organic compoundsElectroluminescent light sourcesOrganic electroluminescenceVoltage
Provided are: an organic electroluminescent element which exhibits higher performance and has particularly good driving voltage and external quantum efficiency, while having a longer service life; and an electronic device which is provided with this organic electroluminescent element. Also provided is a compound which enables the achievement of the organic electroluminescent element and the electronic device. Specifically provided are: a compound of a specific structure having a triphenylene skeleton; an organic electroluminescent element which uses this compound; and an electronic device which is provided with this organic electroluminescent element.
Owner:IDEMITSU KOSAN CO LTD
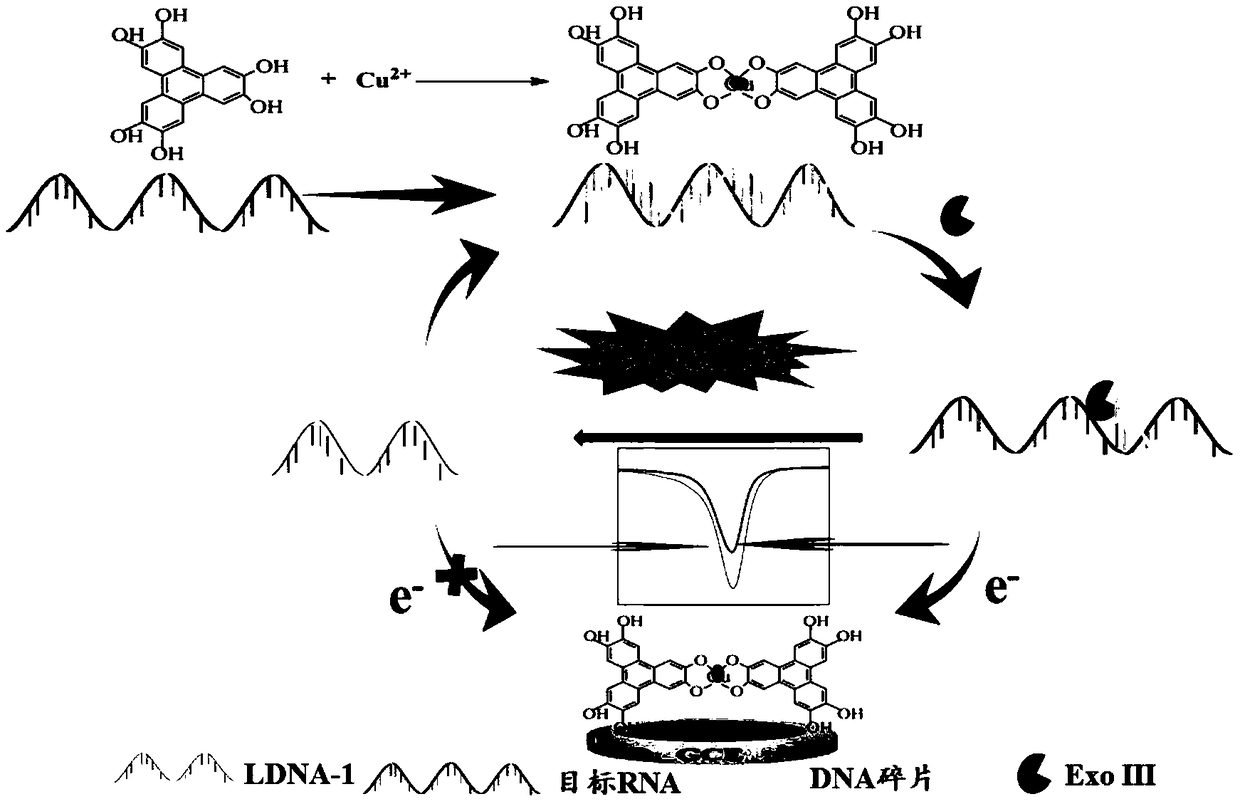
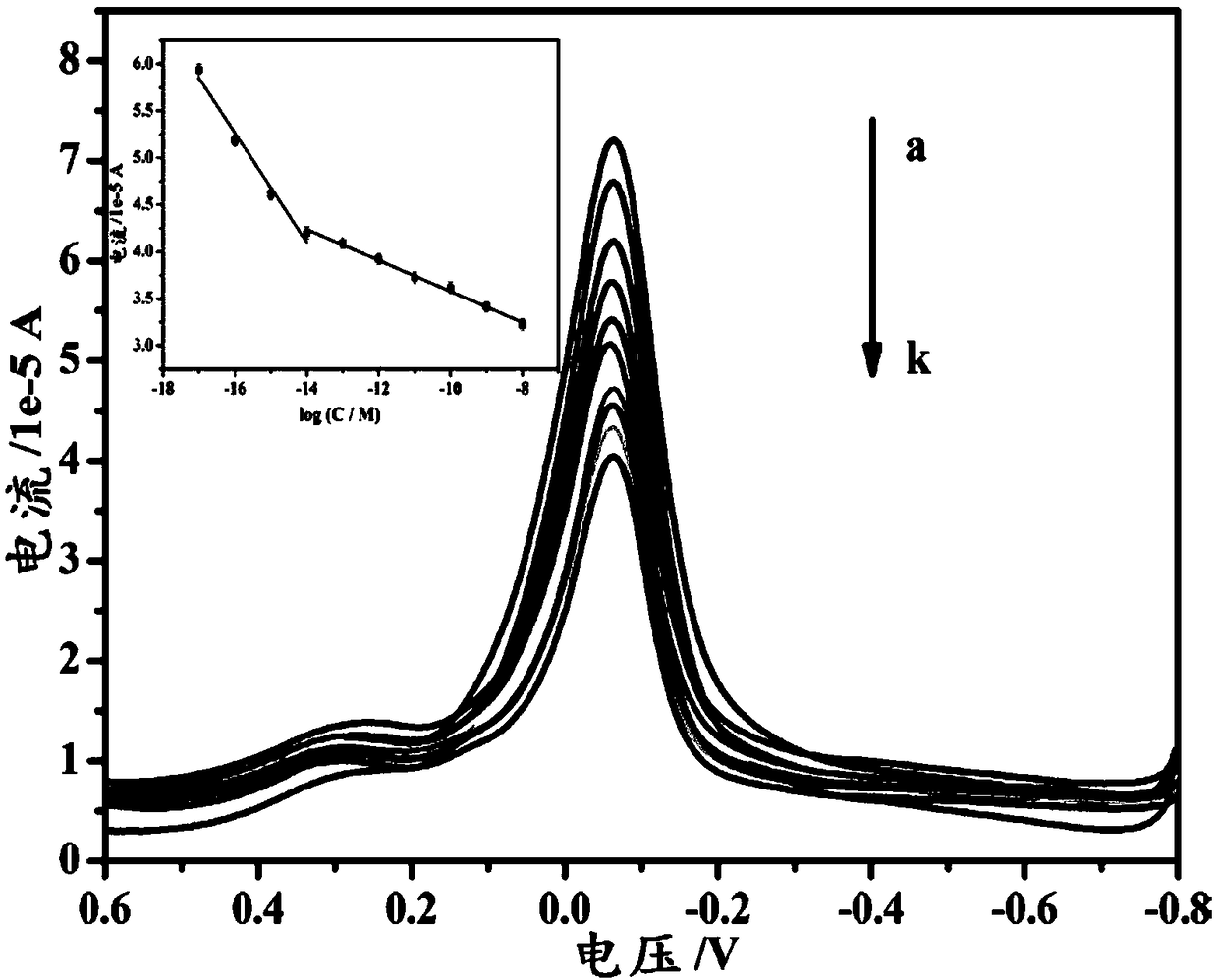
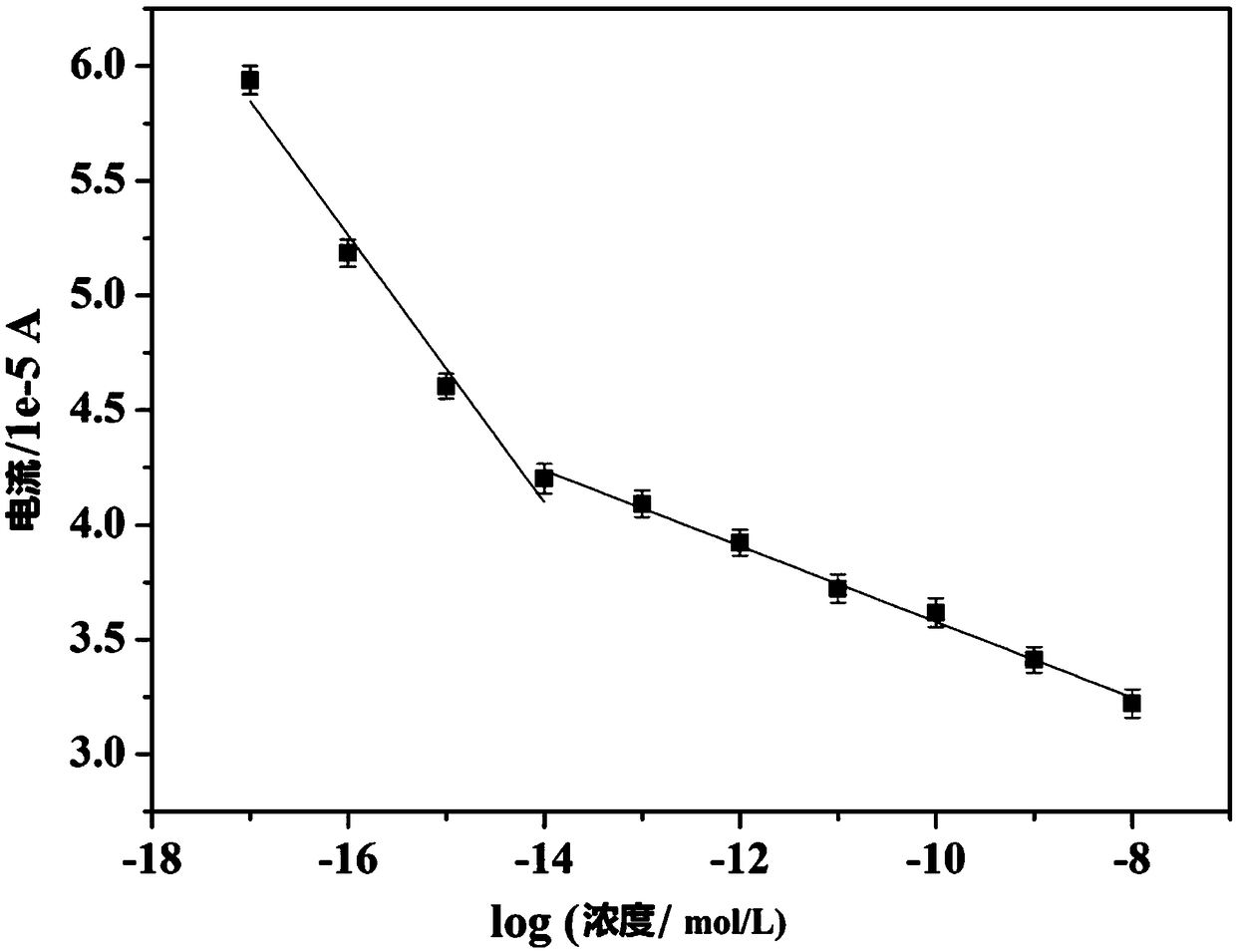
Copper-hexahydroxy triphenylene-based miRNA-21 (micro ribonucleic acid-21) electrochemical sensor and detecting method thereof
Disclosed are a copper-hexahydroxy triphenylene-based miRNA-21 electrochemical sensor and a detecting method thereof. The copper-hexahydroxy triphenylene-based miRNA-21 electrochemical sensor is in athree-electrode system, wherein an auxiliary electrode is a platinum electrode, a reference electrode is an Ag / AgCl electrode, and a working electrode is a copper-hexahydroxy triphenylene-based miRNA-21 eletrode. The copper-hexahydroxy triphenylene-based miRNA-21 electrochemical sensor is characterized in that the copper-hexahydroxy triphenylene-based miRNA-21 electrode is modified with a Cu-CAT (catalase) membrane, and the Cu-CAT membrane absorbs nucleotide products produced by shearing DNA / miRNA-21 (deoxyribonucleic acid / miRNA-21) with ExoIII (exonuclease III). Through the specific hydrophobic pi-pi stack effects between Cu-CAT and oligonucleotide products sheared by ExoIII, the copper-hexahydroxy triphenylene-based miRNA-21 electrochemical sensor can achieve high-sensitivity, rapid andprobe fixing-free sensing analysis on HCC (hepatocellular carcinoma) biomarkers.
Owner:MINNAN NORMAL UNIV
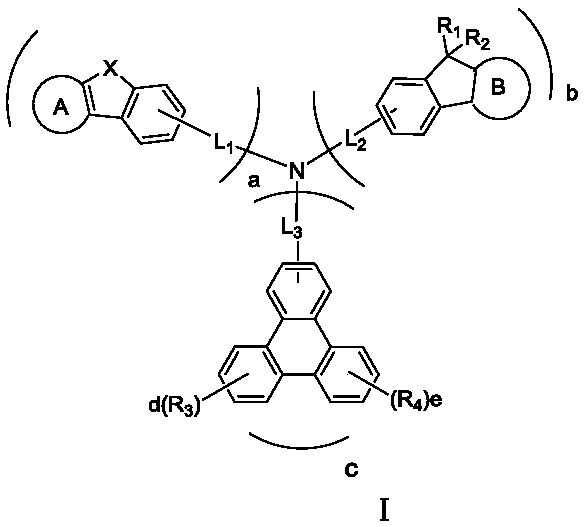
Indenotriphenylene-based amine derivative for organic electroluminescent device
ActiveUS20160240784A1Low efficiencyIncrease power consumptionOrganic chemistrySolid-state devicesOrganic electroluminescencePerylene derivatives
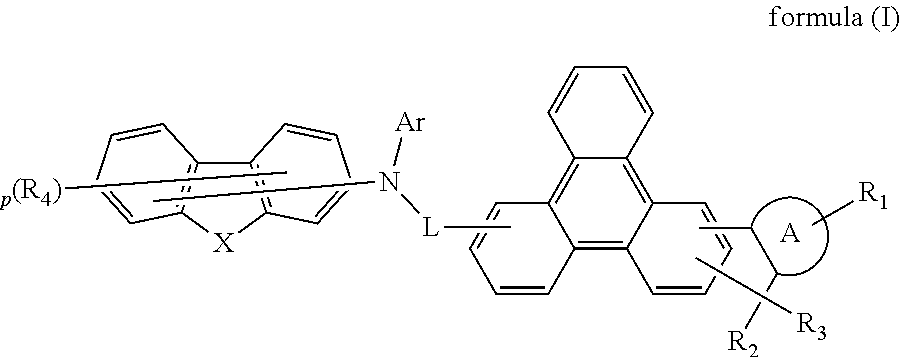
The present invention discloses a indenotriphenylene-based amine derivative is represented by the following formula(I), the organic EL device employing the derivative as hole transport material, electron blocking material, can increasing efficiency and half-life time.wherein R1 to R4, p, X, A ring, L and Ar are the same definition as described in the present invention.
Owner:NINGBO LUMILAN NEW MATERIAL CO LTD
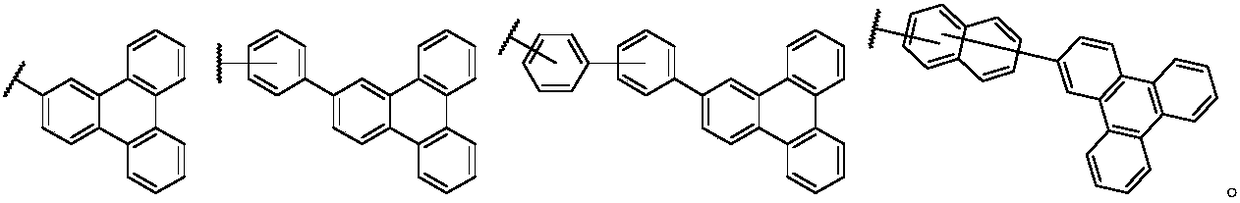
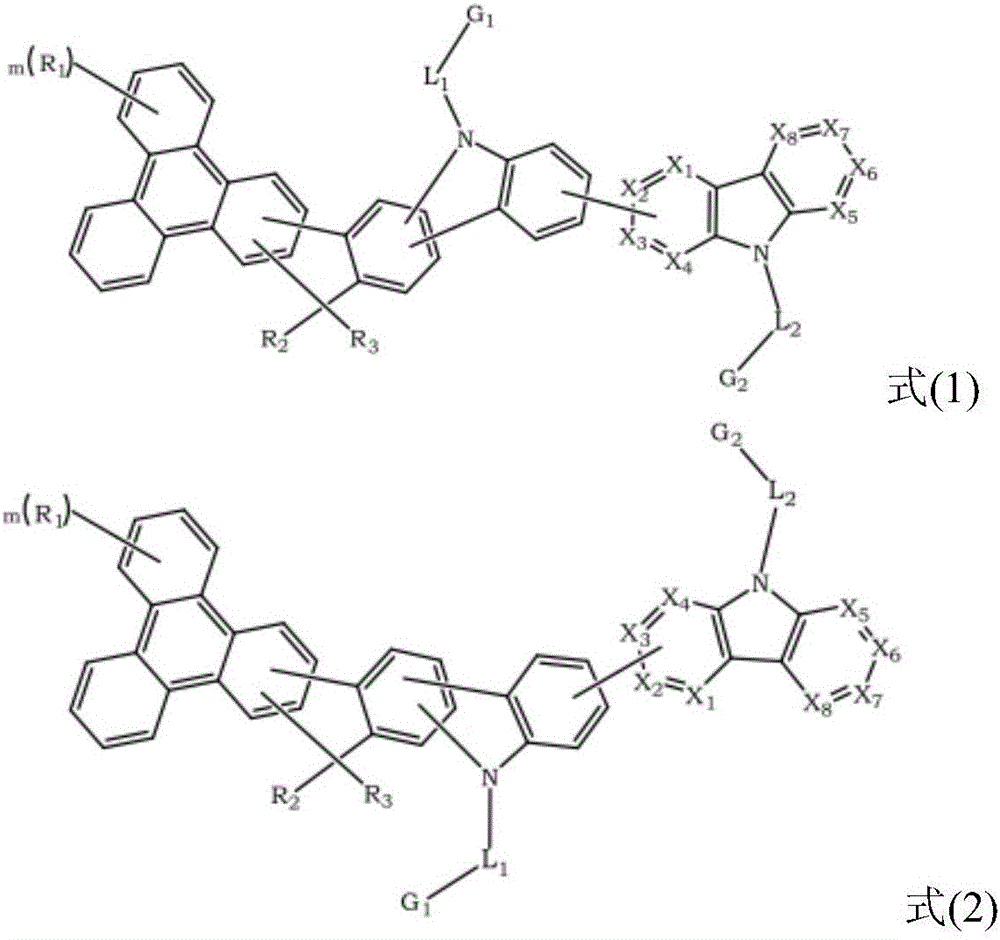
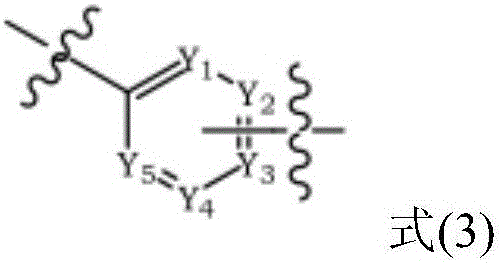
Triphenylene-based fused biscarbazole derivative and use thereof
InactiveUS20160380207A1Improve thermal stabilityHigh carrier mobilityOrganic chemistrySolid-state devicesEngineeringCombinatorial chemistry
The present invention discloses an triphenylene-based fused biscarbazole derivative is represented by the following formula(1) or formula(2), the organic EL device employing the derivative as light emitting host of emitting layer can display good performance like as lower driving voltage and power consumption, increasing efficiency and half-life time.wherein G1, G2, L1, L2, X1 to X8, m, and R1 to R3 are the same definition as described in the present invention.
Owner:LUMINESCENCE TECH
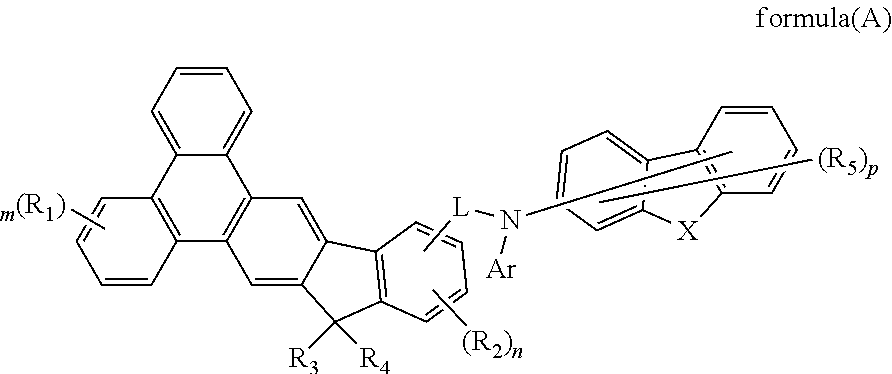
Indenotriphenylene-based amine derivative for organic electroluminescent device
ActiveUS20160240783A1Low efficiencyIncrease power consumptionOrganic chemistrySolid-state devicesOrganic electroluminescencePerylene derivatives
The present invention discloses a indenotriphenylene-based amine derivative is represented by the following formula (A), the organic EL device employing the compound as hole transport material, electron blocking material, can lower driving voltage and power consumption, increasing efficiency and half-life time.Wherein R1 to R5, m, n, p, L and Ar are the same definition as described in the present invention.
Owner:NINGBO LUMILAN NEW MATERIAL CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com