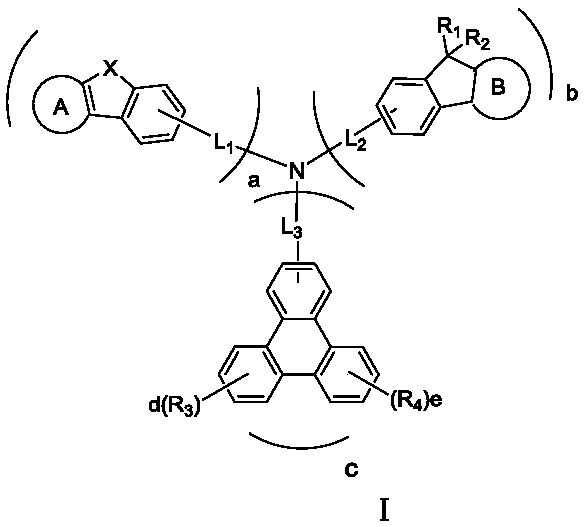
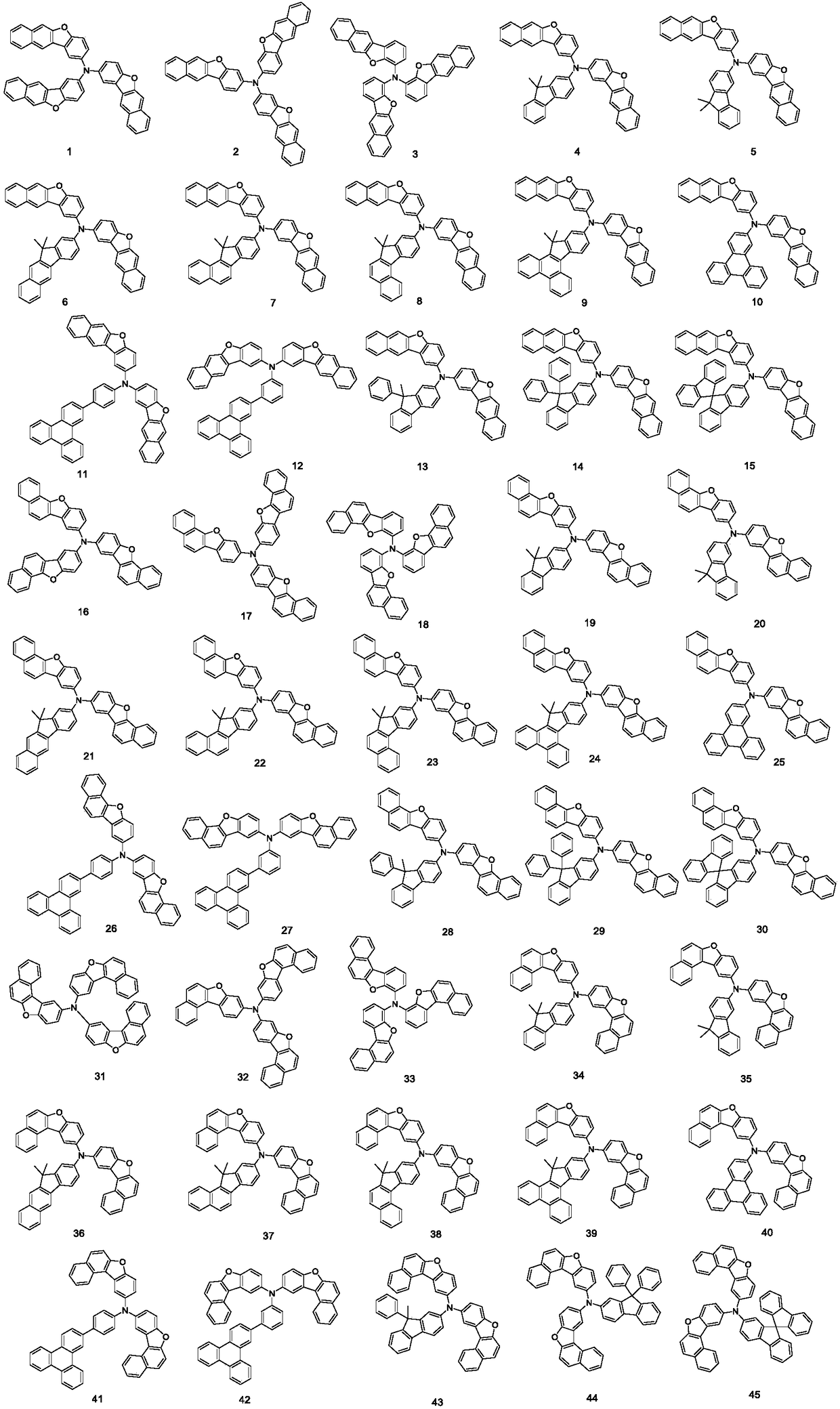
Triarylated amine derivative and organic light-emitting device
A technology for electroluminescent devices and derivatives, applied in the fields of electro-solid devices, electrical components, organic chemistry, etc., can solve the problems of reduced device efficiency, shortened life, poor thermal stability, etc., and achieve the effect of good hole transport ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] [Example 1] Synthesis of compound 1
[0076]
[0077]Step1: Add 2-amino-benzo[B]naphtho[2,3-D]furan (2.33g, 10mmol), 2-bromo-benzo[B]naphtho[2,3-D] into the reactor furan) (2.97g, 10mmol), Pd 2 (dba) 3 (0.21g, 0.25mmol), P(t-Bu) 3 (0.18g, 0.84mmol), NaOt-Bu (2.8g, 25mmol), 100mL of toluene solution, reacted at 100°C for 24h, extracted the organic phase with diethyl ether and water after the reaction, and washed the organic layer with MgSO 4 Dry, concentrate the organic matter, go through column chromatography, and recrystallize to obtain intermediate 10-1 (3.51 g, 78%).
[0078] Step2: Add intermediate 10-1 (4.50g, 10mmol), 2-bromotriphenylene (3.07g, 10mmol), Pd to the reactor 2 (dba) 3 (0.21g, 0.25mmol), P(t-Bu) 3 (0.18g, 0.84mmol), NaOt-Bu (2.8g, 25mmol), 100mL of toluene solution, reacted at 100°C for 24h, extracted the organic phase with diethyl ether and water after the reaction, and washed the organic layer with MgSO 4 Dry, concentrate the organic matte...
Embodiment 2
[0079] [Example 2] Synthesis of compound 30
[0080] Compound 30 (5.96 g, 78%) was obtained according to the synthesis method of compound 10 in Example 1.
[0081]
Embodiment 3
[0082] [Example 3] Synthesis of compound 94
[0083] Compound 94 (5.34 g, 80%) was obtained according to the synthesis method of compound 10 in Example 1.
[0084]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com