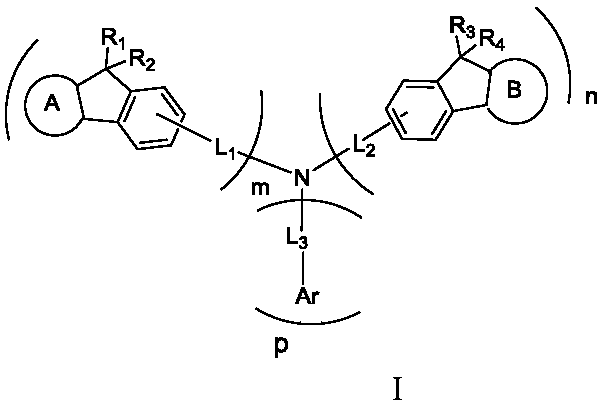
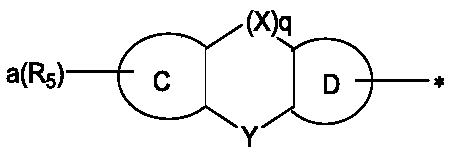
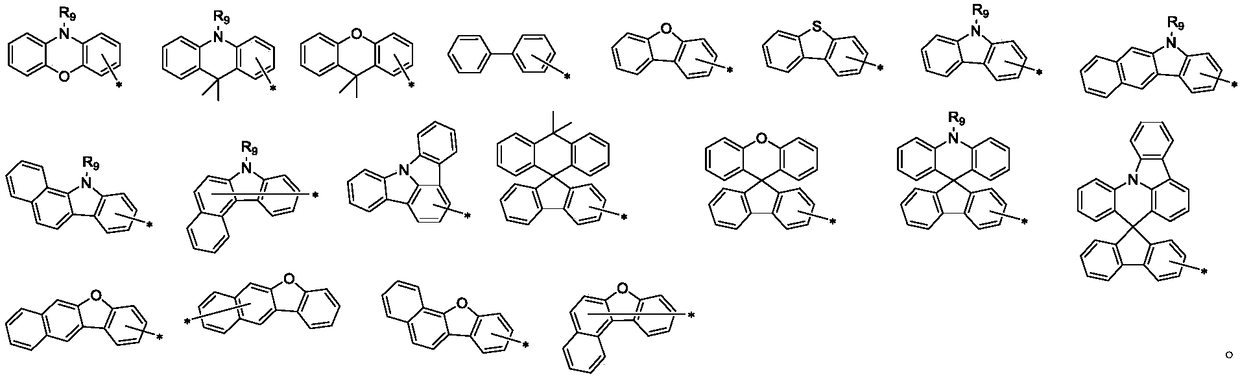
Arylamine derivative and organic electroluminescent device thereof
A technology of derivatives and aromatic amines, which is applied in the field of aromatic amine derivatives and their organic electroluminescent devices, can solve the problems of reduced device efficiency, poor thermal stability, and difficulty in film formation, and reduce coplanarity and thermal stability. Good, easy film-forming effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078][Example 1] Synthesis of Compound 1
[0079]
[0080] Step1: Add 3-amino-11,11-dimethyl-11H-benzofluorene (2.59g, 10mmol), 3-bromo-11,11-dimethyl-11H-benzofluorene (3.23g , 10mmol), Pd 2 (dba) 3 (0.21g, 0.25mmol), P(t-Bu) 3 (0.18g, 0.84mmol), NaOt-Bu (2.8g, 25mmol), 100mL of toluene solution, reacted at 100°C for 24h, extracted the organic phase with diethyl ether and water after the reaction, and washed the organic layer with MgSO 4 Drying, concentration of organic matter, column chromatography and recrystallization gave intermediate 1-1 (3.96 g, 79%).
[0081] Step2: Add intermediate 1-1 (5.02g, 10mmol), 3-bromo-10-phenyl-10H-phenoxazine (3.38g, 10mmol), Pd to the reactor 2 (dba) 3 (0.21g, 0.25mmol), P(t-Bu) 3 (0.18g, 0.84mmol), NaOt-Bu ((2.8g, 25mmol), 100mL of toluene solution, reacted at 100°C for 24h, extracted the organic phase with ether and water after the reaction, and washed the organic layer with MgSO 4 Dry, concentrate the organic matter, go throug...
Embodiment 2
[0082] [Example 2] Synthesis of Compound 6
[0083] Compound 6 (5.14 g, 77%) was obtained according to the synthesis method of compound 1 in Example 1.
[0084]
Embodiment 3
[0085] [Example 3] Synthesis of compound 24
[0086] Compound 24 (5.23 g, 80%) was obtained according to the synthesis method of compound 1 in Example 1.
[0087]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com