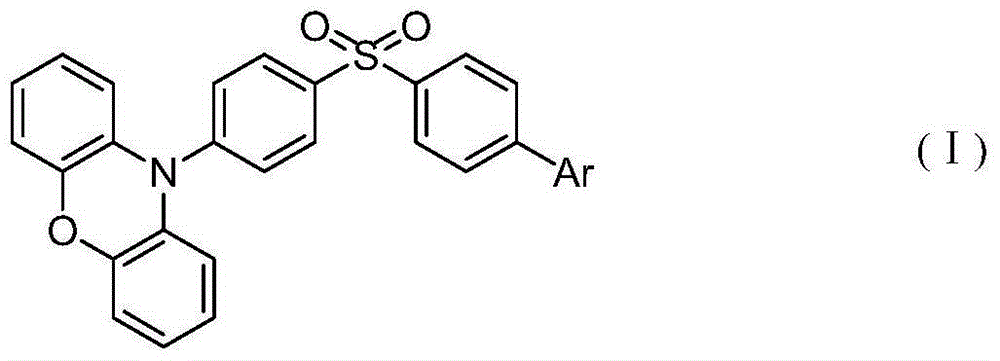
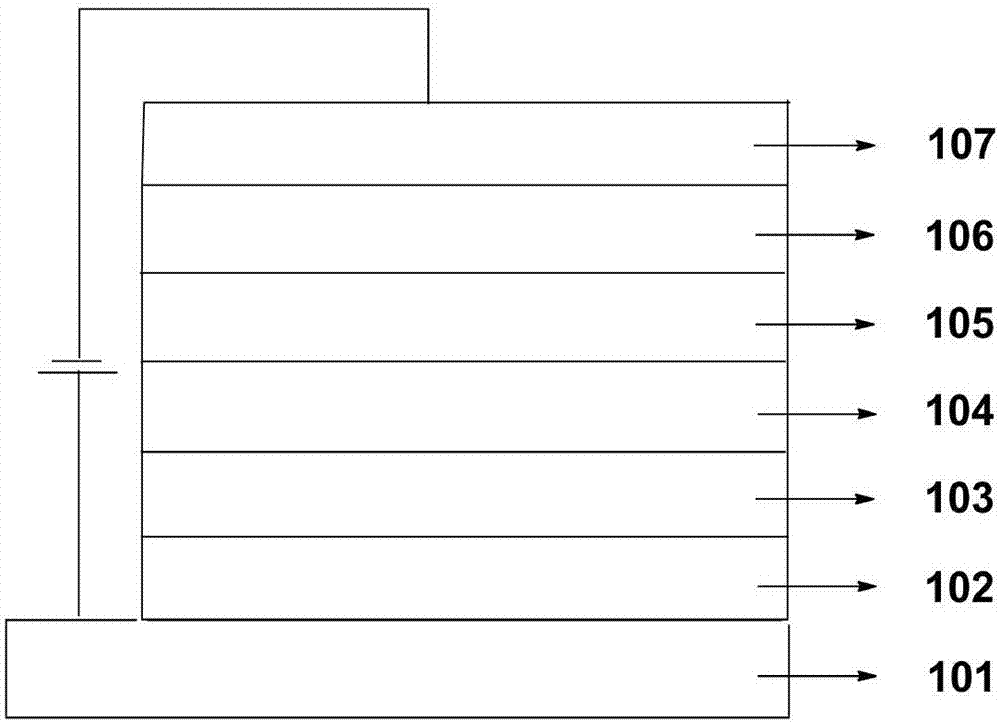
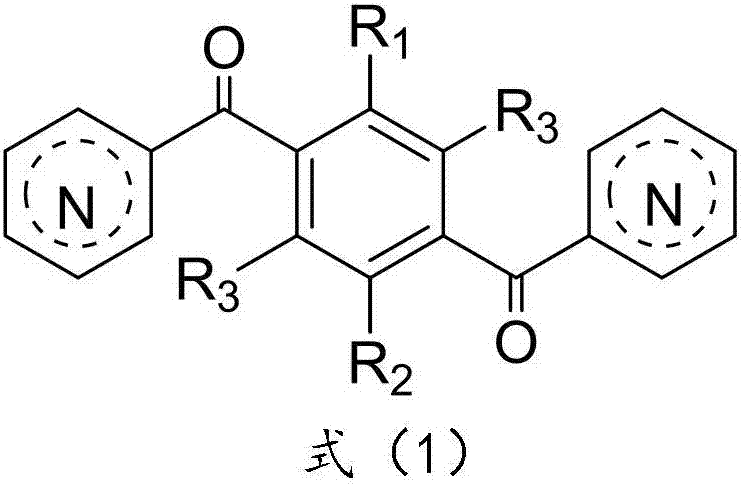
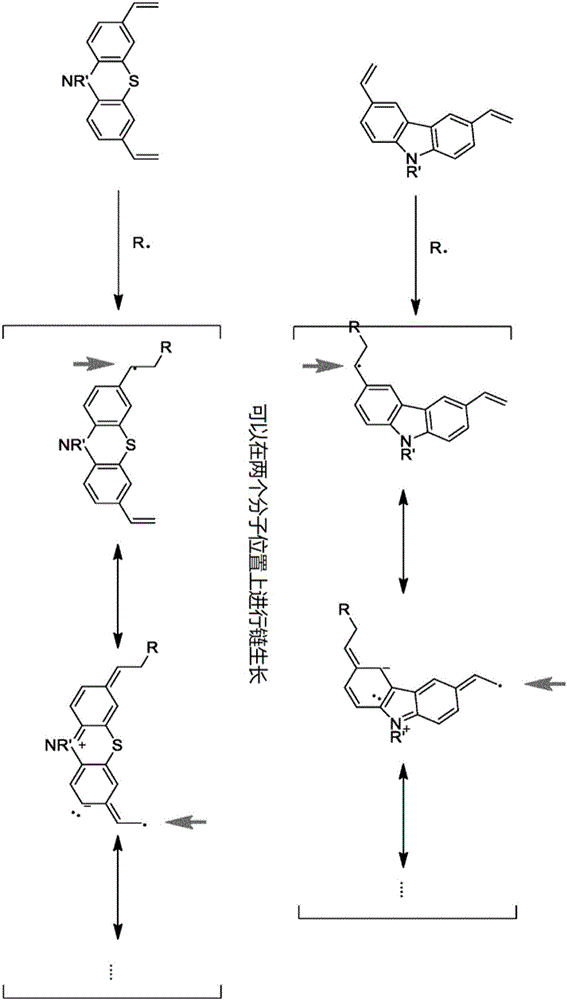
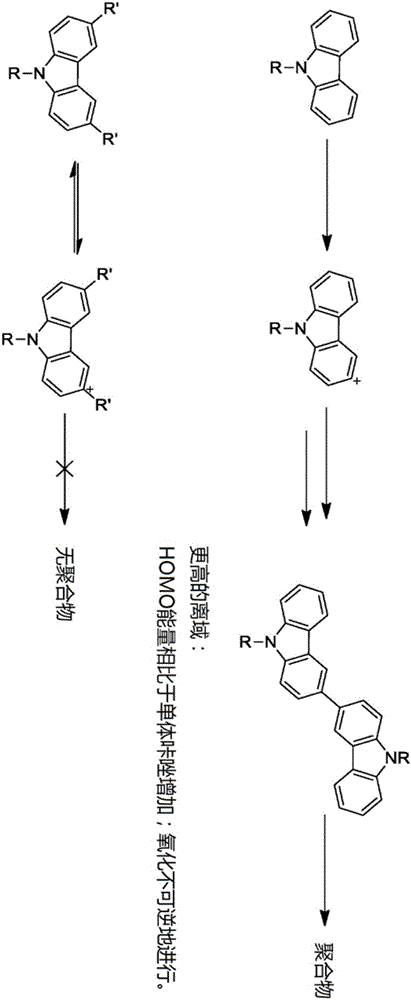
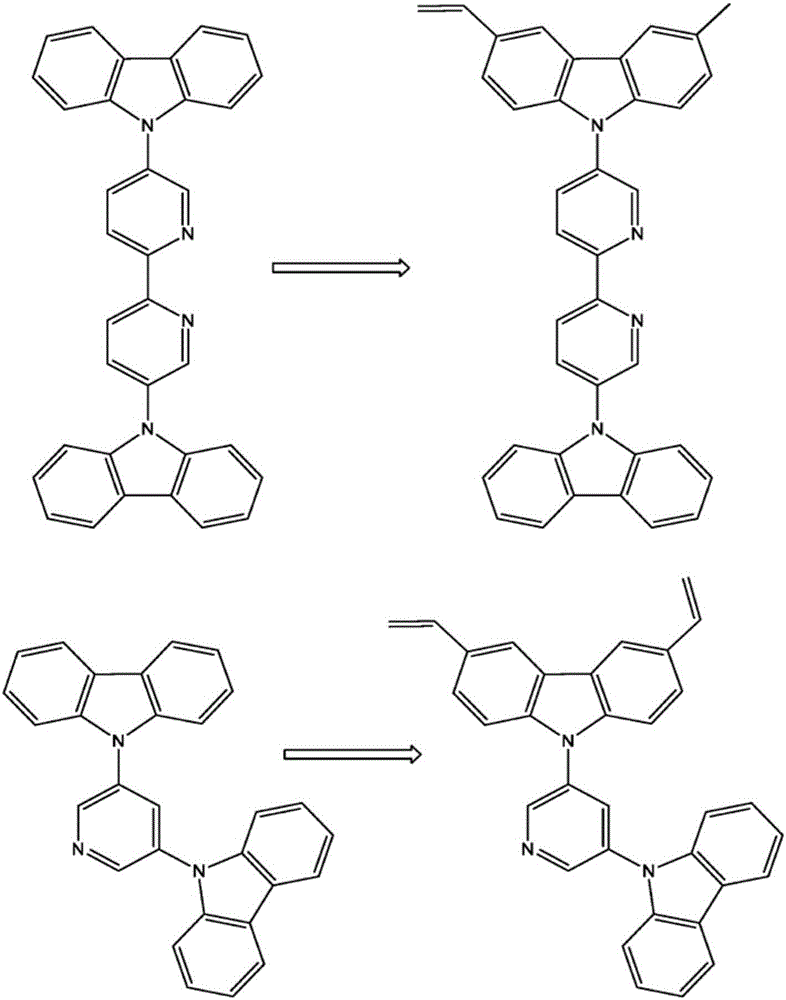
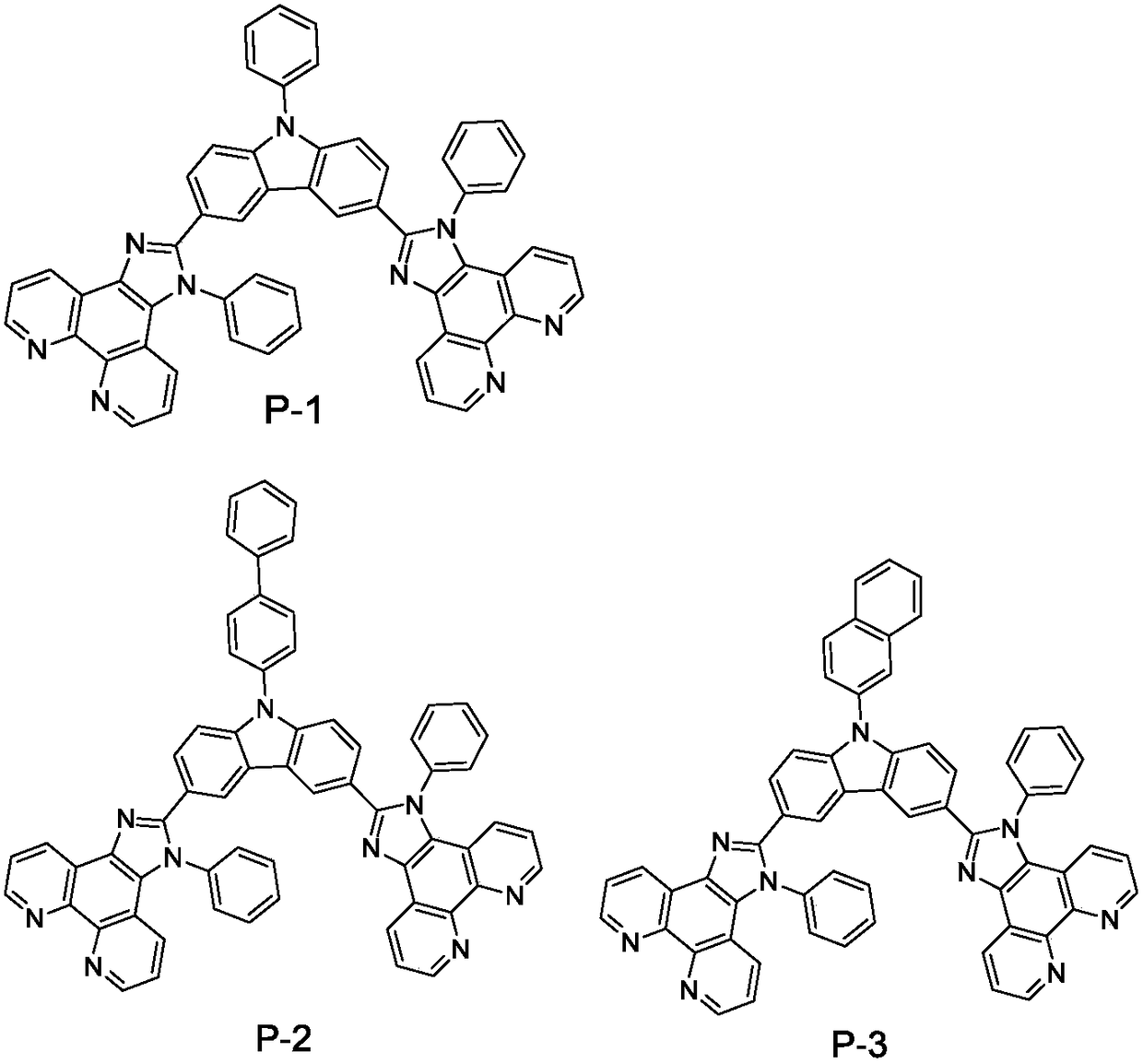
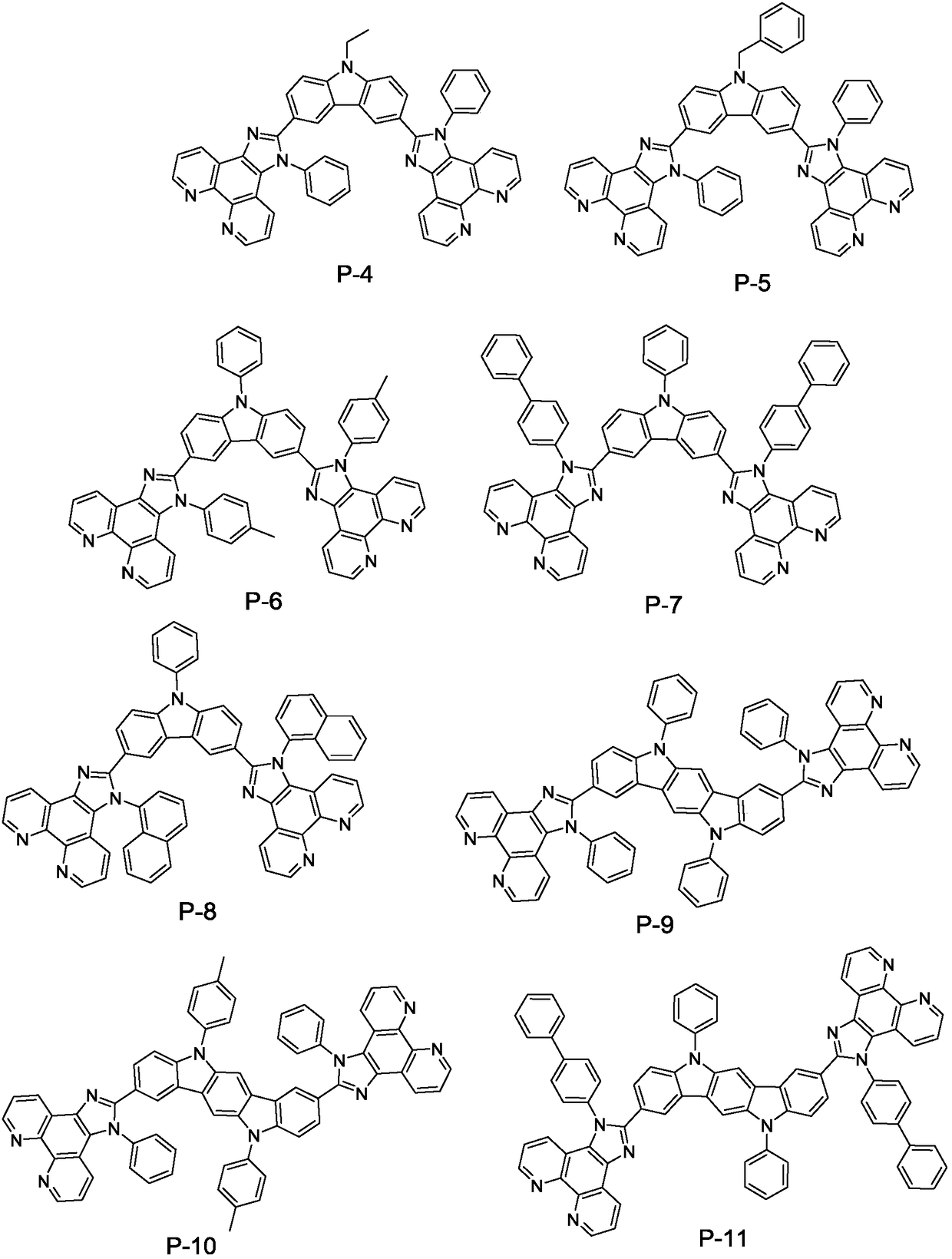
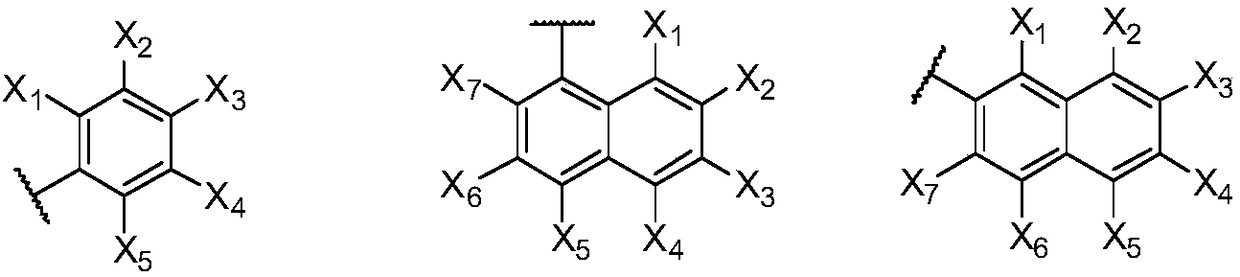
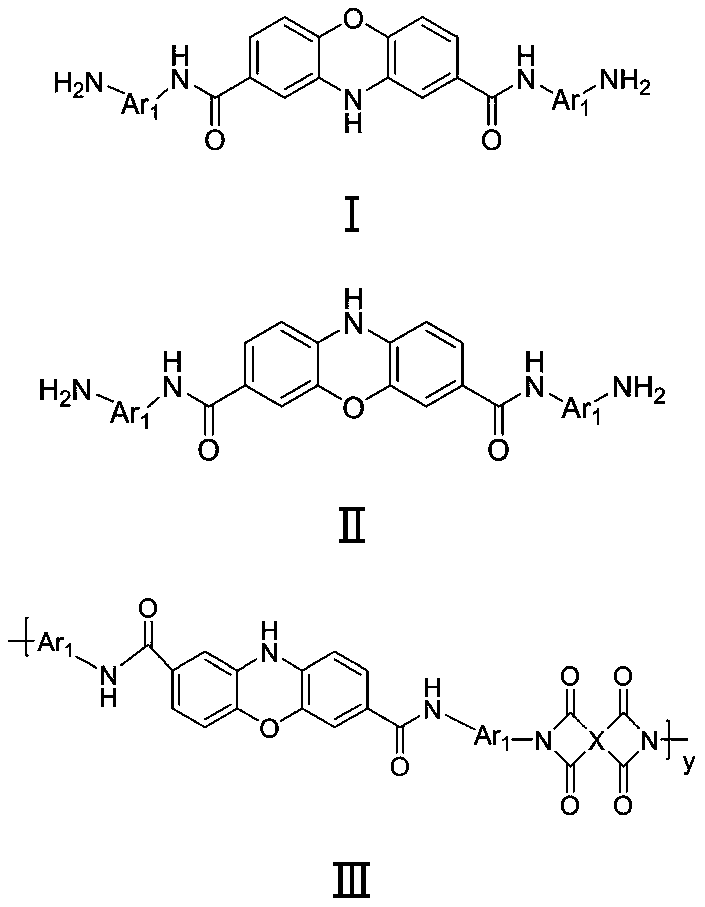
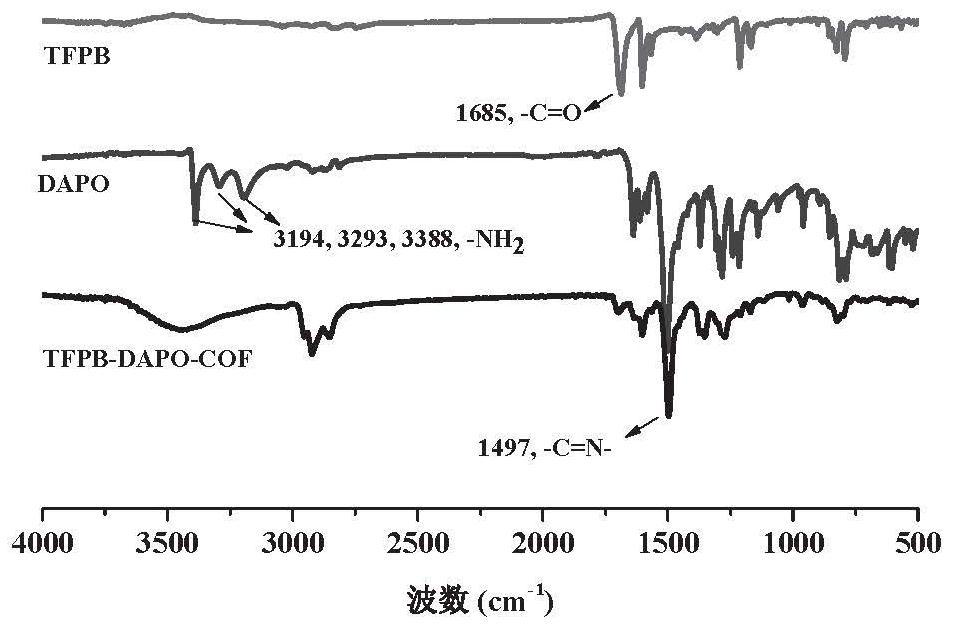
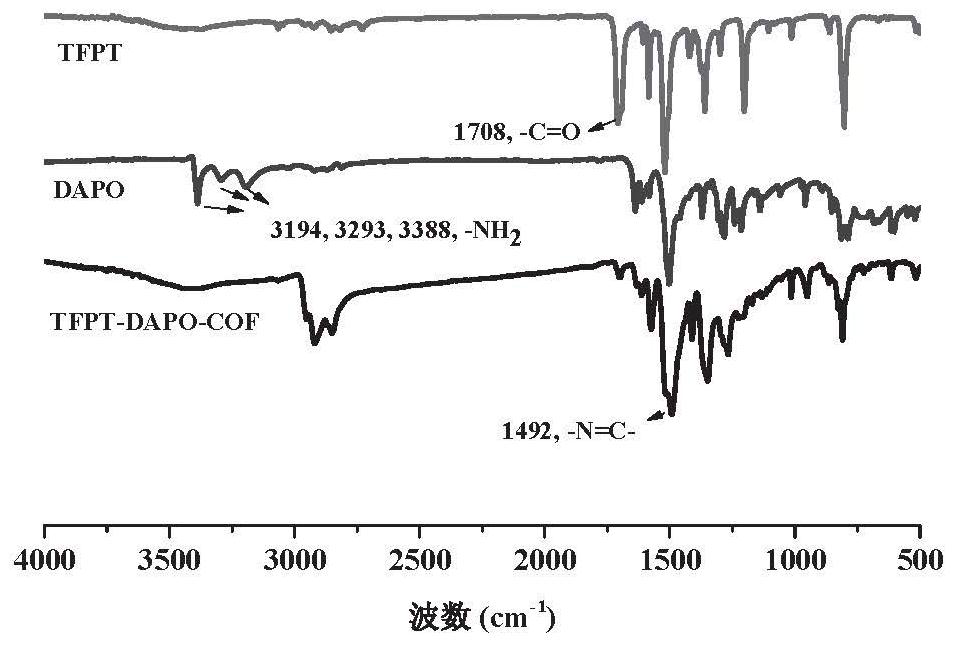
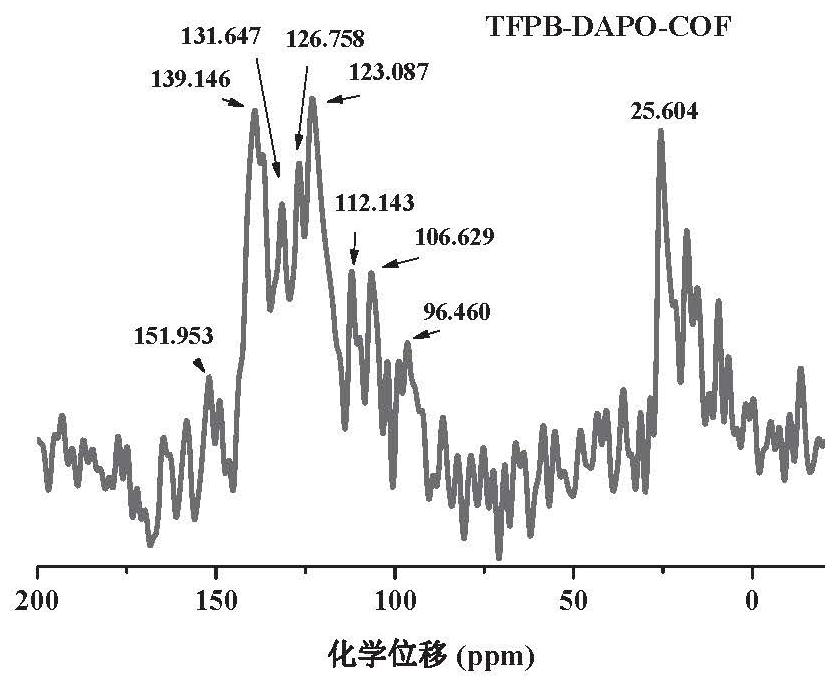
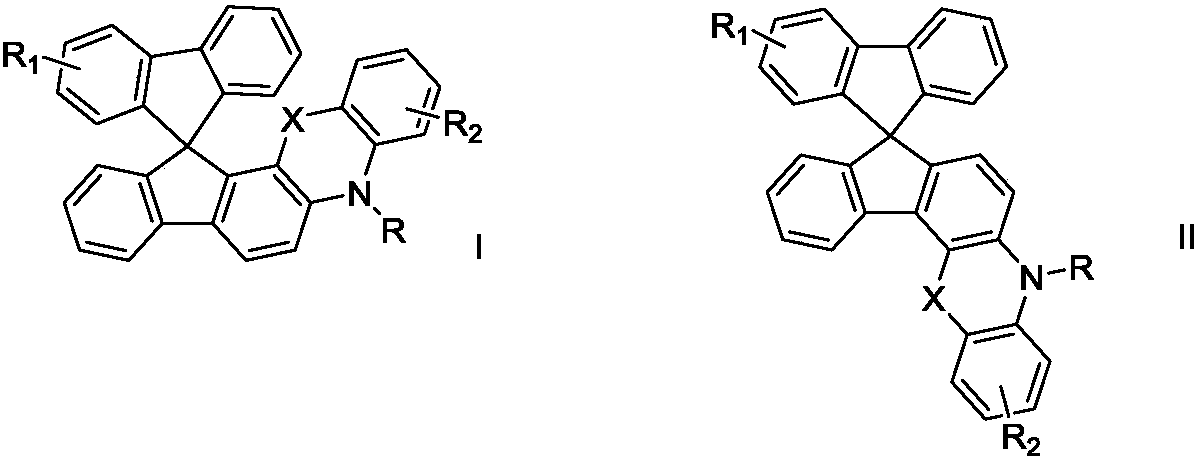
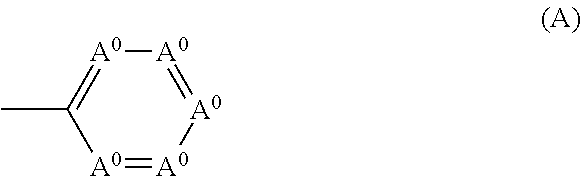Patents
Literature
119 results about "Phenoxazine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Phenoxazine is a heterocyclic compound. The structure of phenoxazine consists of an oxazine fused to two benzene rings. It occurs as the central core of a number of naturally occurring chemical compounds such as dactinomycin and litmus. The dyes Nile blue and Nile red are also based on a phenoxazine core.
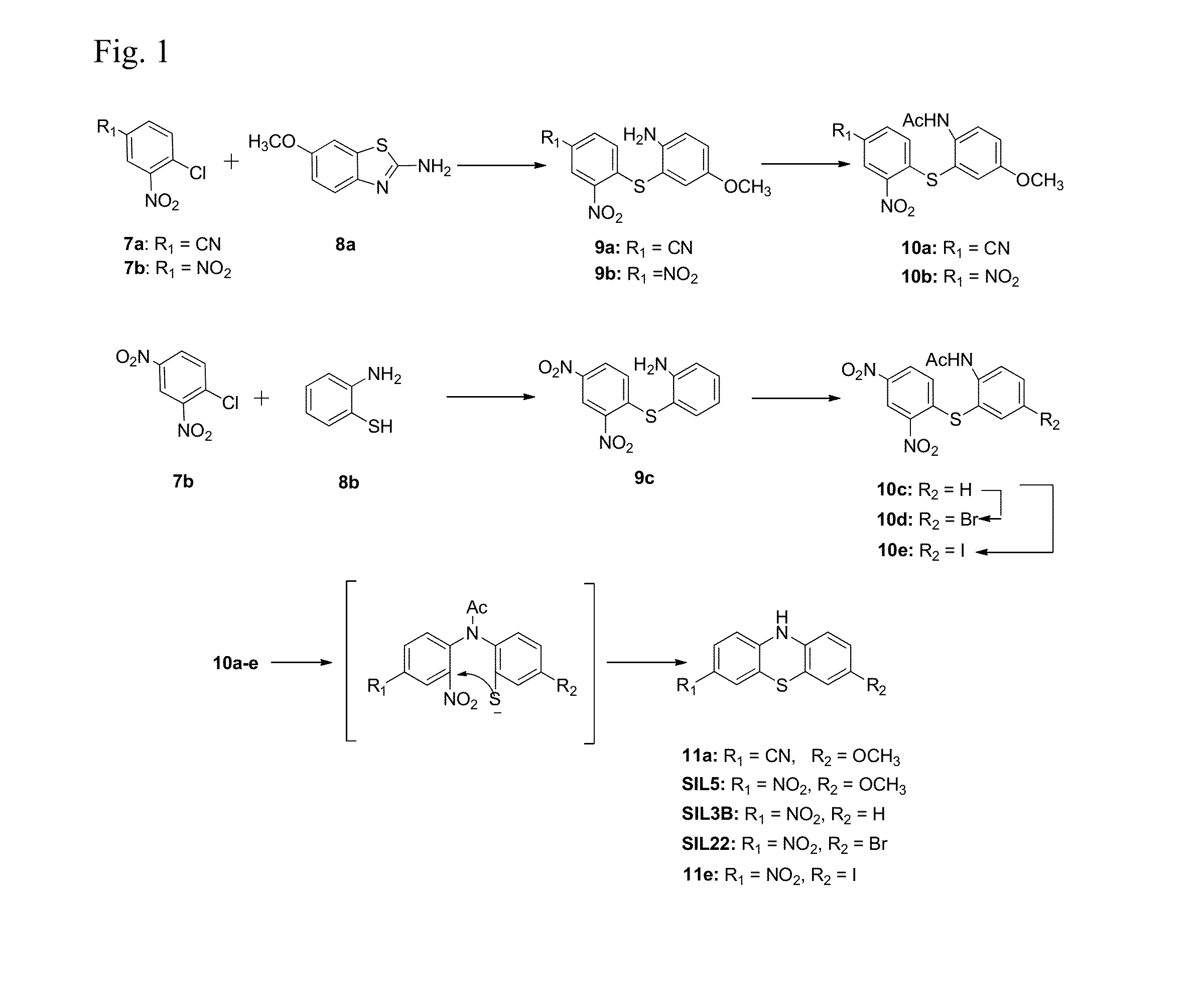
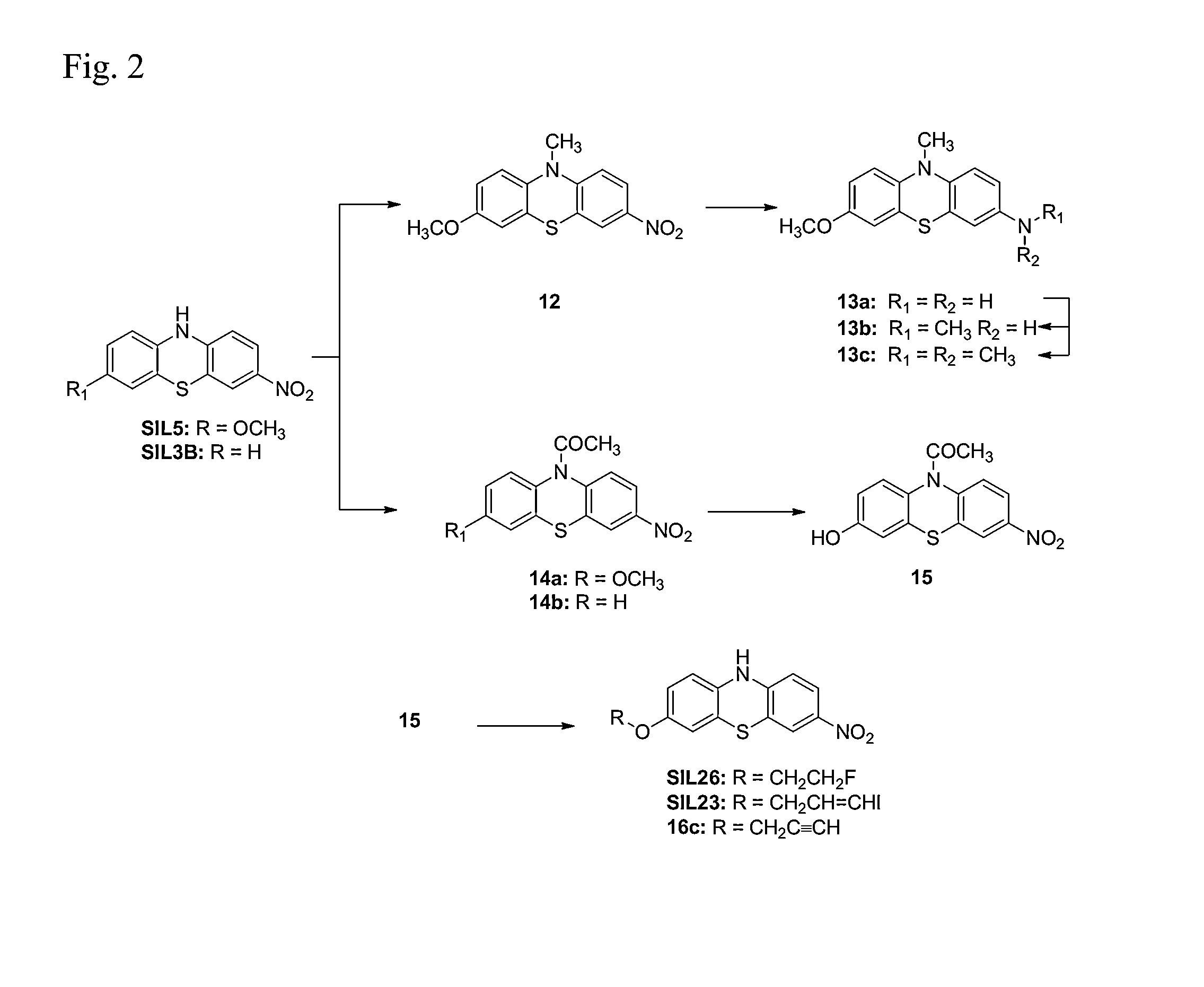
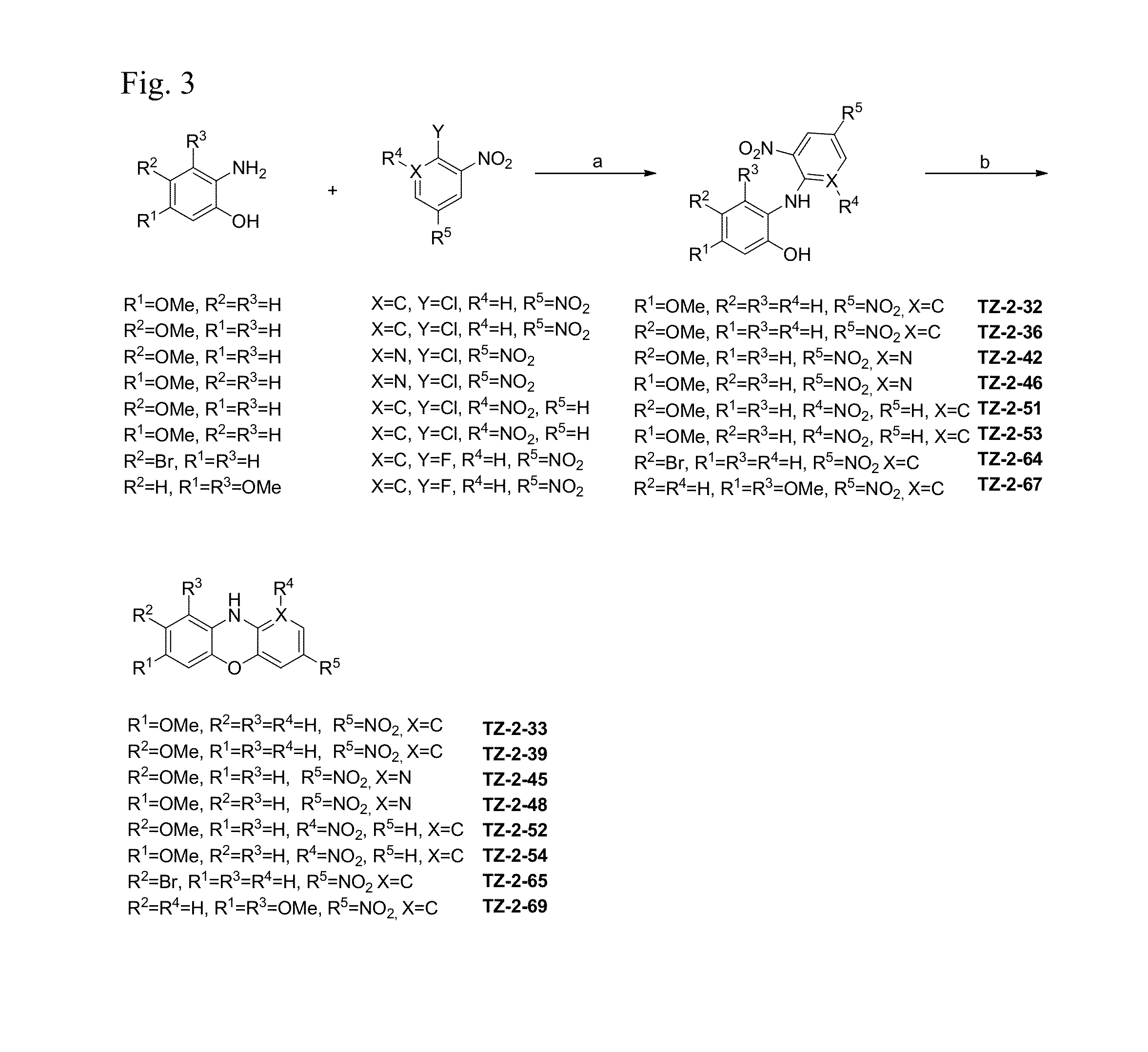
Synthesis of quinobenzoxazine analogues with topoisomerase II and quadruplex interactions for use as antineoplastic agents
InactiveUS6528517B1Potentiated antibiotic activityPotentiated anticancer activityBiocideOrganic chemistryDNA underwindingType II topoisomerase
The present invention discloses a novel quinobenzoxazine self-assembly complex on DNA and on the topoisomerase II-DNA complex. The related model is used to design a new series of quinobenzoxazines, pyridobenzophenoxazines, pyrridonaphthophenoxazines, and other related compounds that may exhibit anticancer or antibiotic activity. The anticancer activity of these compounds is thought to operate via stabilization of the topoisomerase II-DNA complex and / or interaction with G-quadruplexes, while the antibiotic activity of these compounds derives from their ability to inhibit gyrase, the bacterial type II topoisomerase.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Asymmetrical thermal-activation-delayed aggregation-induced emission material based on diphenyl sulfone phenoxazine, as well as synthesis method and application of material
ActiveCN105038764AHas aggregation-induced luminescent propertiesHigh thermal decomposition temperatureOrganic chemistrySolid-state devicesSynthesis methodsNon doped
The invention discloses an asymmetrical thermal-activation-delayed aggregation-induced emission material based on a diphenyl sulfone phenoxazine, as well as a synthesis method and application of the material. A core structure of the material comprises a diphenyl sulfone unit and a phenoxazine group, and further comprises an electronic structure unit made from aryl heterocyclic groups except a phenoxazine substituent. The synthesis method and the purification process of the material are simple, the thermal performance, luminous performance and the like of an end product can be adjusted according to the connection of different groups, the obtained emission material has thermal-activation-delayed fluorescent and aggregation-induced emission performance at the same time, is good in heat stability, higher in glass transition temperature and excellent in luminous performance. A non-doped OLED device manufactured by using the emission material as the luminous layer is high in luminous brightness and good in stability, so that the luminous efficiency and the service life of the OLED device can meet the practical requirements.
Owner:SUN YAT SEN UNIV
Organic small molecule luminescent material and organic electroluminescent device prepared from same
ActiveCN105254562AChanges in intramolecular charge transfer propertiesSingle structureOrganic chemistrySolid-state devicesAcridineOrganic electroluminescence
The invention belongs to the field of organic photoelectric material technology, and discloses an organic small molecule luminescent material and an organic electroluminescent device prepared from same. The organic small molecule luminescent material uses 9,9-dimethyl-10- phenyl acridine or 10-phenyl phenoxazine as an electron providing unit, and is connected with a different electron withdrawing unit at 2-site of the acridine group or the 3-site of the phenoxazine group to obtain a series of novel efficient organic small molecule luminescent materials. The organic small molecule luminescent material can change the characteristic of intramolecular charge transfer in the material, can make the luminescence peak of the luminescent material has a blue-shift, can be used as a luminescent layer for the organic electroluminescent device, and has a high device efficiency.
Owner:SOUTH CHINA UNIV OF TECH
Polymer and polymeric luminescent element employing the same
InactiveUS20100301310A1Maintain good propertiesImprove performanceSolid-state devicesSemiconductor/solid-state device manufacturingPolymer sciencePhenoxazine
Owner:SUMITOMO CHEM CO LTD
Phenazine oxazines dye and application of the same in dye sensitization of solar battery
InactiveCN101294004ASave raw materialsEasy to synthesizeSolid-state devicesSemiconductor/solid-state device manufacturingElectron donorElectrical battery
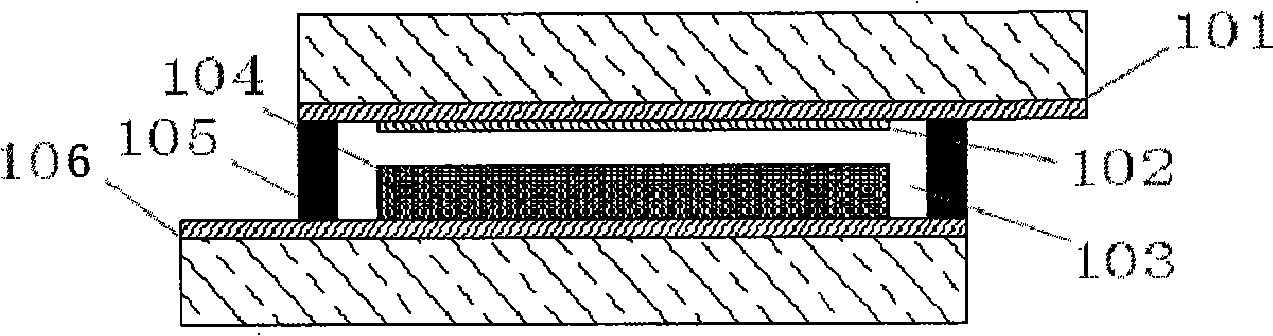
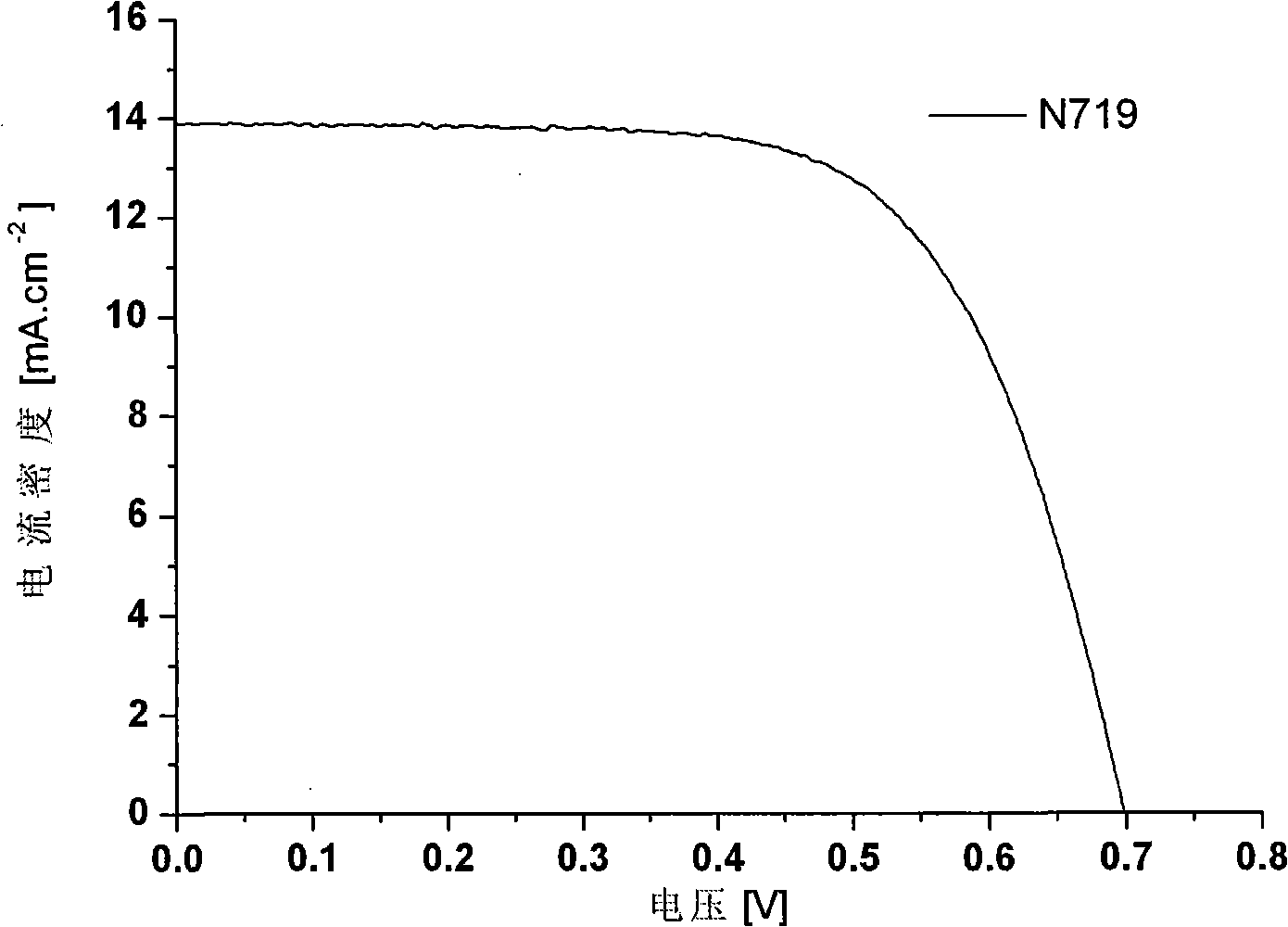
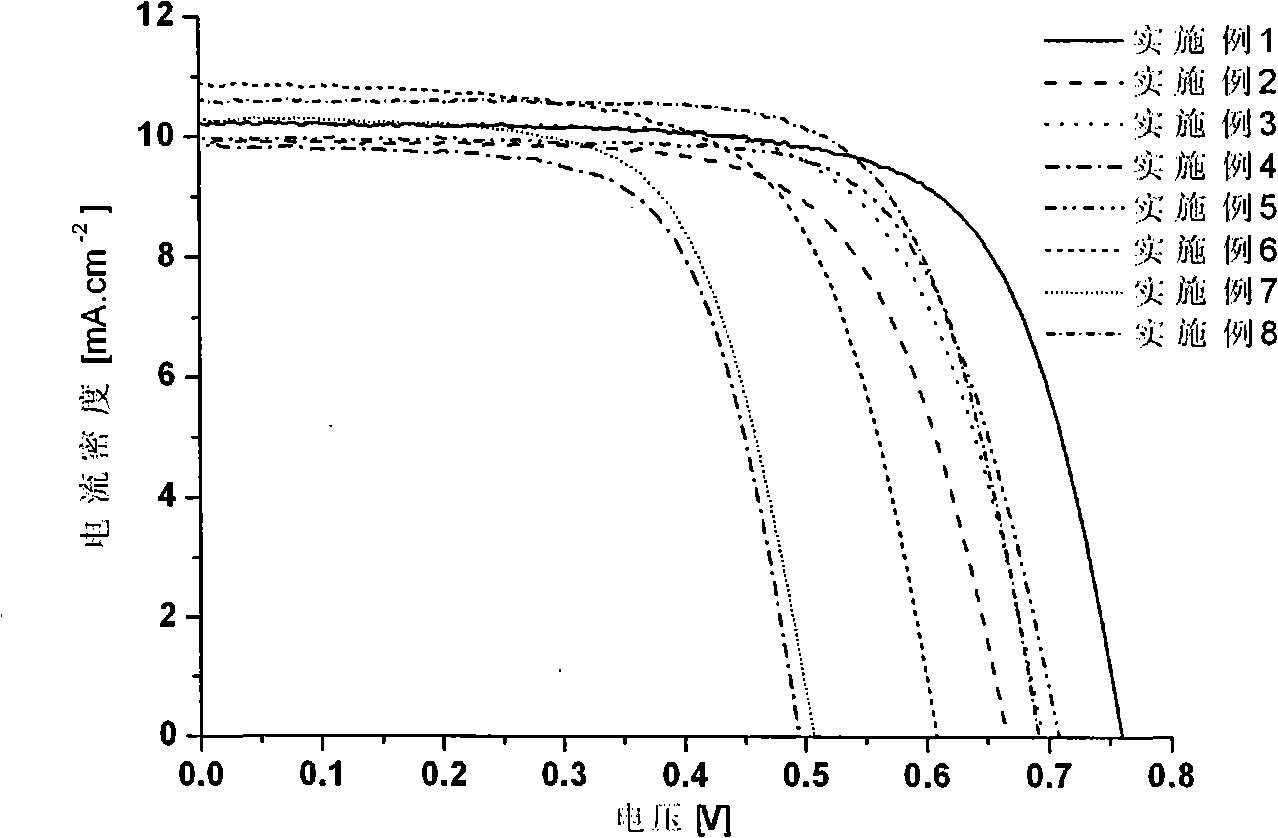
The invention relates to a phenoxazine dye and a photoelectric converter using the dye, and belongs to the technology field of phenoxazine dyes. The technical proposal is that: the chemical structure of the dye contains substituted phenoxazine and the derivative thereof as electron donors and different conjugated groups as bridging chains, wherein one end of the bridging chains are connected with different electron-withdrawing groups. An electron-donating group on a nitrogen atom and lone-pair electrons on an oxygen atom interact with a chromophoric conjugated system, thereby resulting in more easier charge transfer of the excited state of molecules and generating unique photoelectric property. The phenoxazine dye has good application performance in dye-sensitized solar cells, and can replace expensive noble metal photosensitive dye in dye-sensitized solar cells.
Owner:ZHONGKE ENTERPRISE DEV NINGDE
Preparation method and applications of hexatomic dinitrogen heterocyclic derivatives containing four identical substituent groups
InactiveCN106243091AHave electroluminescent propertiesOrganic chemistrySolid-state devicesPyridazinePyrazine
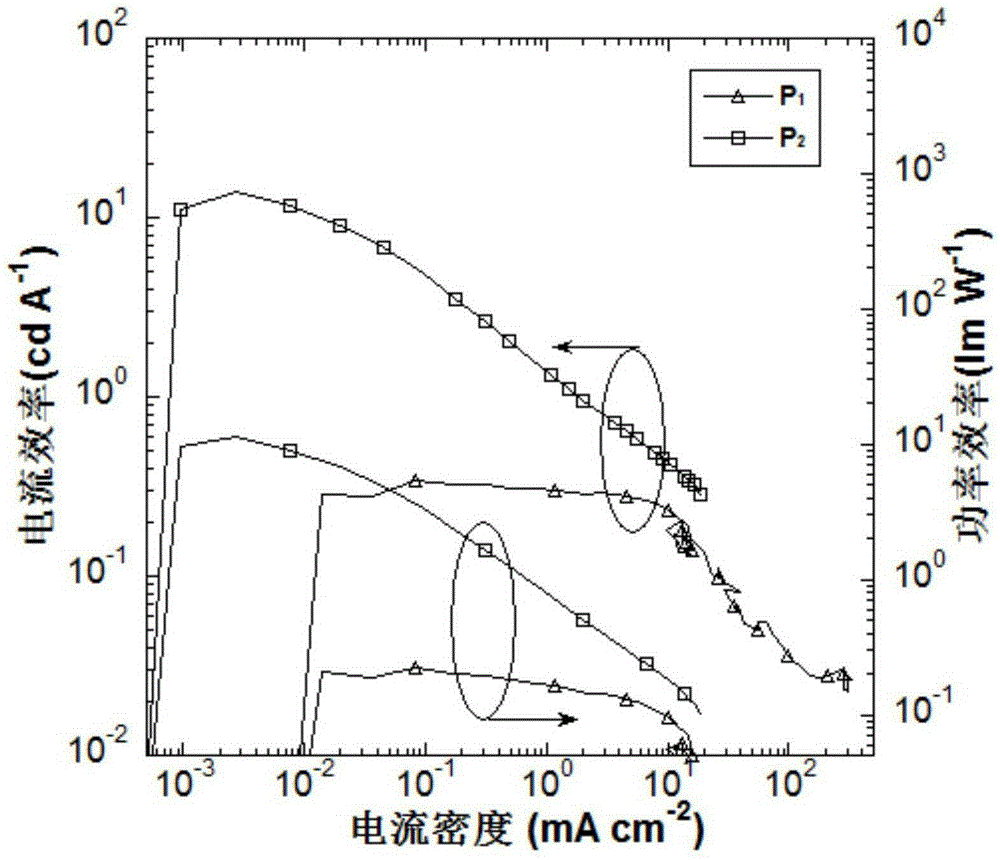
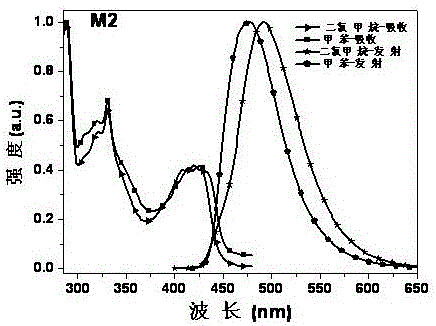
The invention discloses a preparation method and applications of hexatomic dinitrogen heterocyclic derivatives containing four identical substituent groups, and belongs to the field of application technology of luminescent materials in preparation of organic light-emitting devices. According to the preparation method, pyrazine and pyridazine are taken as electron acceptors of luminescent materials; the periphery of the electron acceptors is connected with electron donors such as carbazole, phenoxazine, and 9,9-dimethyl-9,10-dihydracridine; frontal orbital overlapping degree of HOMO and LUMO is adjusted, so that luminescent materials with thermally activated delayed fluorescence (TADF) characteristics are obtained. At room temperature, the hexatomic dinitrogen heterocyclic derivatives possess strong absorption at ultraviolet-visible region, a dilute solution of the hexatomic dinitrogen heterocyclic derivatives is capable of emitting strong fluorescence, the wavelength of emission peaks ranges from 450 to 615nm in the blue light-orange light range, the hexatomic dinitrogen heterocyclic derivatives can be applied to organic light-emitting devices as luminescent materials. According to devices based on compound M2, turn-on voltage is 3.9V, the maximum brightness 3846cdm<2> is achieved at a voltage of 12V; and the maximum luminous efficiency 17.4cdA<1> and the maximum power efficiency 12.21mW<1> are achieved when electric current density is 0.06mAcm<2>(4.5V).
Owner:DALIAN UNIV OF TECH
Compound, display panel and display device
ActiveCN110054644AAvoid gatheringImprove luminous efficiencySolid-state devicesSemiconductor/solid-state device manufacturingAcenaphthyleneThianthrene
The invention provides a compound, a display panel and a display device. The compound is in a structure shown as a formula (I), wherein R1 and R2 refer to electron-donating groups and independently selected from any one of C1-C20 alkyl groups, C3-C20 cycloalkyl groups, C1-C20 alkoxy groups, a phenyl, a biphenyl, a naphthyl, an anthryl, a phenanthryl, an acenaphthylene group, a pyrenyl, a perylenegrouop, a fluorenyl, a spirobifluorene group, a chrysene group, a benzo-phenanthryl, a benzo-anthryl, a fluoranthene group, a pyrene groupo, a furyl, a benzofuryl, a substituted or unsubstituted dibenzofuran group, a substituted or unsubstituted thienyl, a benzothiophene group, a dibenzothiophene group, a phenoxazine group, a phenazinyl, a phenothiazinyl, a thianthrene group, a carbazolyl, an acridinyl and a diarylamine group, and m is selected from an integer range of 1-4. The compound is a material with AIE characteristics and TADF characteristics, triplet-state excitons are converted into singlet-state excitons through a reverse intersystem conversion process to realize radioluminescence, and the exciton utilization rate of luminescent materials is increased.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
Novel thermal-activated-delayed-fluorescence luminescent material and application thereof
ActiveCN106946850AImprove luminosityHigh thermodynamic stabilityOrganic chemistrySolid-state devicesAlkaneAcridine
The invention belongs to the field of organic electroluminescent materials and particularly relates to a novel thermal-activated-delayed-fluorescence luminescent material and an application thereof. The material is characterized by comprising a structure represented by a formula (1) shown in the description, wherein R1 and R2 each is any one independently selected from a group consisting of a hydrogen atom, a C3-C30 substituted or unsubstituted carbazolyl group, a C3-C30 substituted or unsubstituted arylamine group, a C3-C30 substituted or unsubstituted phenothiazine group, a C3-C30 substituted or unsubstituted phenoxazine group, a C3-C30 substituted or unsubstituted phenazine group and a C3-C30 substituted or unsubstituted acridine group, and R1 and R2 are not hydrogen simultaneously; and R3 is any one selected from a group consisting of hydrogen and C1-C10aliphatic straight-chain or branched-chain alkane. The material provided by the invention is stable in property, has good luminescent performance and is applied to organic electroluminescent devices as a luminescent material.
Owner:VALIANT CO LTD
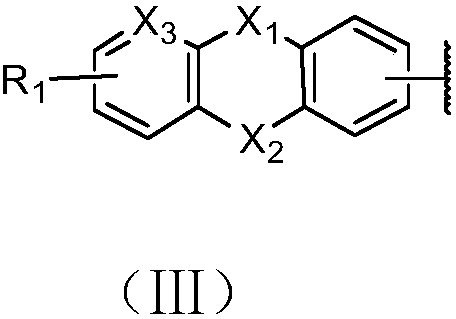
Organic electrosynthesis method of phenothiazine/phenoxazine compound
ActiveCN108863982AReduce processEasy to operateElectrolysis componentsOrganic chemistryPhenoxazineElectrosynthesis
The invention discloses an organic electrosynthesis method of a phenothiazine / phenoxazine compound. The organic electrosynthesis method adopts an organic electrosynthesis means for a compound shown ina formula I and a compound shown in a formula II to prepare the phenothiazine / phenoxazine compound. The organic electrosynthesis method disclosed by the invention has the beneficial effects that a target product shown in a formula III can be obtained by directly activating C-H without need of expensive halogenated raw materials and additional reduction and substitution processes, so that the process flow is obviously shortened, the operation is simplified, the preparation efficiency is improved, and the production cost is reduced; the reaction condition is simple, mild and environmentally friendly, the atom economy is high, the adaptation range of a reaction substrate is wide, and the yield of a target product is high.
Owner:NANCHANG HANGKONG UNIVERSITY
Compound, display panel and display device
ActiveCN110078755AImprove stabilityExtend your lifeSilicon organic compoundsSolid-state devicesAcenaphthyleneBenzanthracenes
The invention provides a compound, a display panel and a display device. The compound has the structure shown in formula (I), L represents substituted or unsubstituted phenyl, naphthyl, pyridyl, pyrimidinyl and pyrazinyl; D is an electron-donating group and independently selected from any one of substituted or unsubstituted phenyl, biphenyl, naphthyl, anthryl, phenanthryl, acenaphthylene, pyrenyl,peryl, flurenyl, spiro bifluorenyl, benzophenanthryl, benzanthracene, fluoranthryl, picene, furyl, benzofuryl, dibenzofuryl, thienyl, benzothienyl, dibenzothienyl, phenoxazine, thianthryl, carbazolyl, acridinyl and diarylamino. The designed compound has a TADF characteristic and can emit light by triplet exciton of traditional fluorescence molecular transition inhibition so as to improve the device efficiency.
Owner:WUHAN TIANMA MICRO ELECTRONICS CO LTD
Organic electroluminescence material comprising 4,5-diazaspiro thioxanthone structure, application of organic electroluminescence material, and device
ActiveCN106349251AEasy injectionImprove transmission performanceOrganic chemistrySolid-state devicesAcridineCarbazole
The invention belongs to the field of organic electroluminescence materials, and particularly relates to an organic electroluminescence material comprising a 4,5-diazaspiro thioxanthone structure, application of the organic electroluminescence material, and a device; the organic electroluminescence material can be used for OLEDs (Organic Light Emitting Diodes), has very good bipolar transmission property and can be used as a main material. A structural formula is as shown in the description, wherein Ar1 and Ar2 are independently selected from hydrogen atoms, substitutive or non-substitutive carbazole groups of C3 to C30, substitutive or non-substitutive arylamine groups of C3 to C30, substitutive or non-substitutive phenothiazine groups of C3 to C30, substitutive or non-substitutive phenoxazine groups of C3 to C30, substitutive or non-substitutive phenazine groups of C3 to C30 and substitutive or non-substitutive acridine groups of C3 to C30, and Ar1 and Ar2 are not hydrogen at the same time. The invention also relates to the device comprising at least one layer of bipolar compound as a main body, and a method for manufacturing the device.
Owner:VALIANT CO LTD
Crosslinkable host materials
InactiveCN106715420AEasy to moveImprove efficiencyGroup 5/15 element organic compoundsFinal product manufacturePyridazinePhenanthroline
The invention relates to a crosslinkable organic molecule having a structure of the formula (1) and to the use thereof, wherein Ar is independently of one another, an unsaturated or aromatic carbo- or heterocyclic unit with 5 to 30 ring atoms, selected from the group consisting of naphthalene, anthracene, phenanthrene, pyrene, dihydropyrene, chrysene, perylene, fluoranthene, benzanthracene, tetracene, pentacene, benzpyrene, furan, benzofuran, isobenzofuran, thiophene, benzothiophene, isobenzothiophene, dibenzothiophene, pyrrole, indole, isoindole, carbazole, pyridine, quinoline, isoquinoline, acridine, phenanthridine, benzo-5,6-quinoline, benzo-6,7-quinoline, benzo-7,8-quinoline, phenothiazine, phenoxazine, pyrazole, indazole, imidazole, benzimidazol, naphthimidazole, phenanthrimidazole, pyridimidazole, pyrazine-imidazole, quinoxalinimidazole, oxazole, benzoxazole, naphthoxazole, anthroxazole, phenanthroxazole, isoxazole, isothiazole, 1,3-thiazole, benzothiazole, pyridazine, benzopyridazine, pyrimidine, benzpyrimidine, quinoxaline, pyrazine, phenazine, naphthyridine, azacarbazole, benzocarboline, phenanthroline, 1,2,3-triazole, 1,2,4-triazole, benzotriazole, 1,2,3-oxadiazole, 1,2,4-oxadiazole, 1,2,5-oxadiazole, 1,3,4-oxadiazole, 1,2,3-thiadiazole, 1,2,4-thiadiazole, 1,2,5-thiadiazole, 1,3,4-thiadiazole, 1,3,5-triazine, 1,2,4-triazine, 1,2,3-triazine, tetrazole, 1,2,3,4- oxatriazole, 1,2,3,4-oxatriazole, 1,2,4,5-tetrazine, 1,2,3,4-tetrazine, 1,2,3,5-tetrazin, purine, pteridine, indolizine, benzothiadiazole, indenocarbazole, indenofluorene, spirobifluorene, and indolocarbazole; D1 is a donor group having a structure of the formula (1a); and D2 is a donor group having a structure of the formula (1b).
Owner:SAMSUNG DISPLAY CO LTD
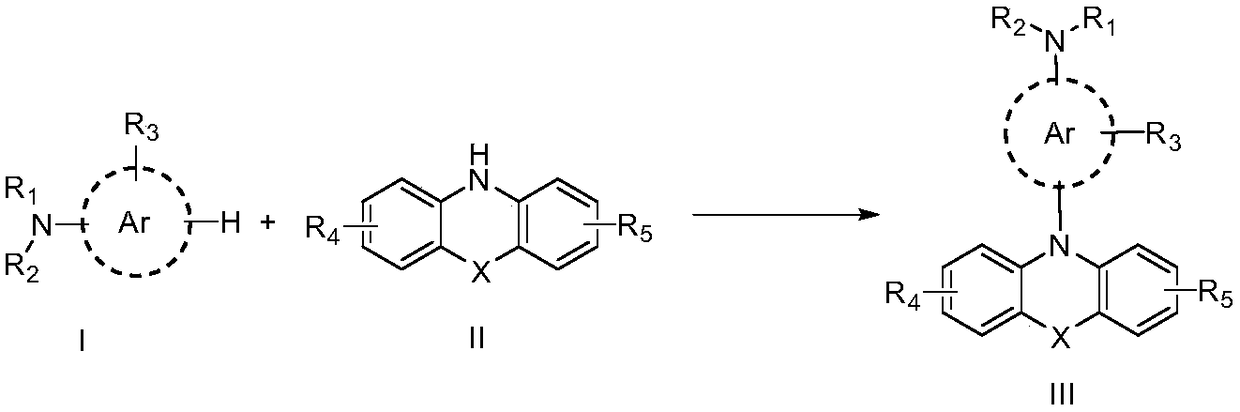
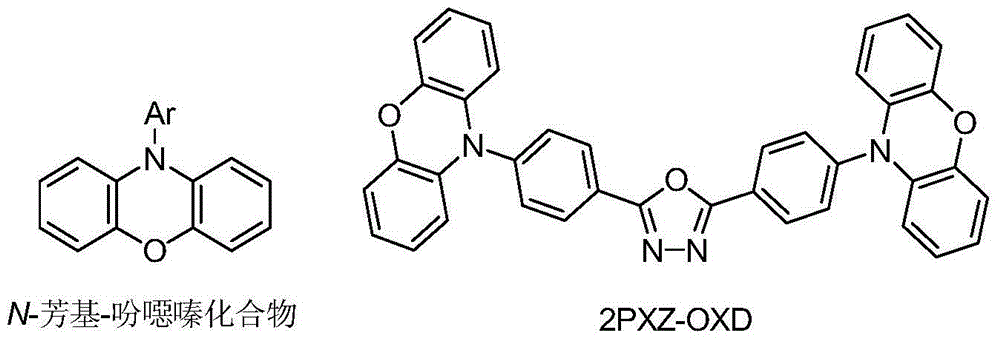
Method for synthesizing N-aryl-phenoxazine compounds
ActiveCN103819423AEfficiently control the sequence of cascade reactionsControlling the order of cascade reactionsOrganic chemistryChemical synthesisSolvent
The invention discloses a method for synthesizing N-aryl-phenoxazine compounds, and belongs to the chemical synthesis filed. The method comprises the following steps: taking a compound represented by the formula I, which is shown in the description, as the raw materials; mixing the compound with a solvent, a catalyst, and an alkali reagent, adding an arylation agent, and carrying out reactions at a temperature of 50 to 250 DEG C so as to obtain the target product; wherein the solvent is amides, benzenes, or nitriles; the catalyst is copper halide, cuprous halide, alkyl copper carboxylate, alkyl copper carboxylate hydrate, aryl copper carboxylate, cuprous oxide, or cuprous cyanide; and the arylation agent is iodobenzenes represented by formula II, which is shown in the description, mono-substituted iodopyridine, or 3-iodothiophene. The method has the advantages of simple reaction steps and high yield of target product.
Owner:JIANGSU AOLUNDA HIGH TECH IND
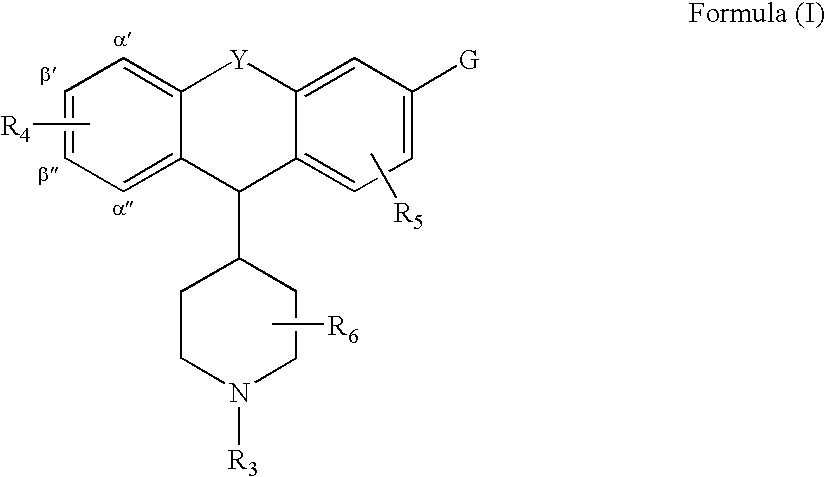
Piperdinyl-phenoxazine and phenothiazine derivatives as delta-opioid modulators
Owner:JANSSEN PHARMA NV
Polyfluorene derivative and organic light-emitting device thereof
InactiveCN108530418AHigh glass transition temperatureImprove film formationOrganic chemistrySolid-state devicesPolyfluoreneGlass transition
The invention provides a polyfluorene derivative and an organic light-emitting device thereof, and belongs to the technical field of organic photoelectric materials. The derivative has a structure shown as a formula (I). The polyfluorene derivative disclosed by the invention has a larger conjugate plane structure, so that high electron fluidity can be provided; electron-deficient group dibenzofuran, dibenzothiophene, acridine, phenoxazine and phenothiazine structures are introduced to better facilitate the acception of electrons and enable the polyfluorene derivative to have good transmissionperformance; by introducing a bridged structure, on the one hand, the molecular weight of the compound can be enlarged, and the obtained material is enabled to have high glass transition temperature and prevent the crystallization effect; on the other hand, the derivatives are enabled to have certain distortion in three-dimensional stereoscopic structure, and the film-forming property of the derivative is improved. The organic light-emitting device, which is prepared by using the compound as a main body material in a luminescent layer, shows the advantages of low driving voltage and high luminous efficiency; the polyfluorene derivative is an organic luminescent material with excellent performance.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Chiral thermal activation delayed fluorescence material and preparation method thereof
ActiveCN112079843ASignificant internal charge transferHigh fluorescence quantum yieldOrganic chemistry methodsSolid-state devicesQuinoxalineQuantum yield
The invention relates to a chiral thermal activation delayed fluorescence material and a preparation method thereof. The delayed fluorescence material is R / S-18,21-di(10H-phenoxazine-10-yl)-3,4,5,6,7,8,9,10-octahydrodibenzo[a,c]dinaphtho[2',1':5,6;1',2'':7,8][1,4]dioxin[2,3i]phenazine, or R / S-16,17-bis(4-(10H-phenoxazine-10-yl)phenyl)3,4,5,6,7,8,9,10-octahydronaphthalo[2',1':5,6; 1',2'':7,8][1,4]dioxane[2,3g] quinoxaline. The compound provided by the invention has the characteristics of a rigid large-plane twisted structure and a remarkable internal charge transfer (ICT) effect, and has the advantages of typical thermal activation delayed fluorescence (TADF) property, circular polarization (CPL) property, high fluorescence quantum yield (PLQY), good thermal stability and the like. The method has the advantages of few synthesis and preparation steps, readily available raw materials, simple synthesis and purification processes and high yield, and can be used for large-scale synthesis andpreparation.
Owner:SUZHOU UNIV
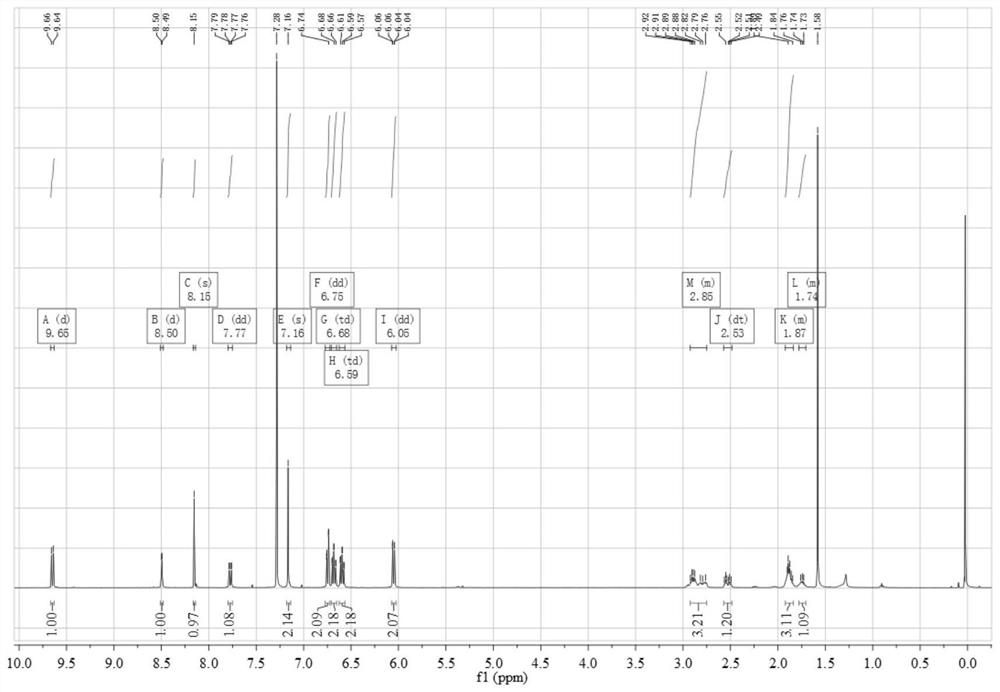
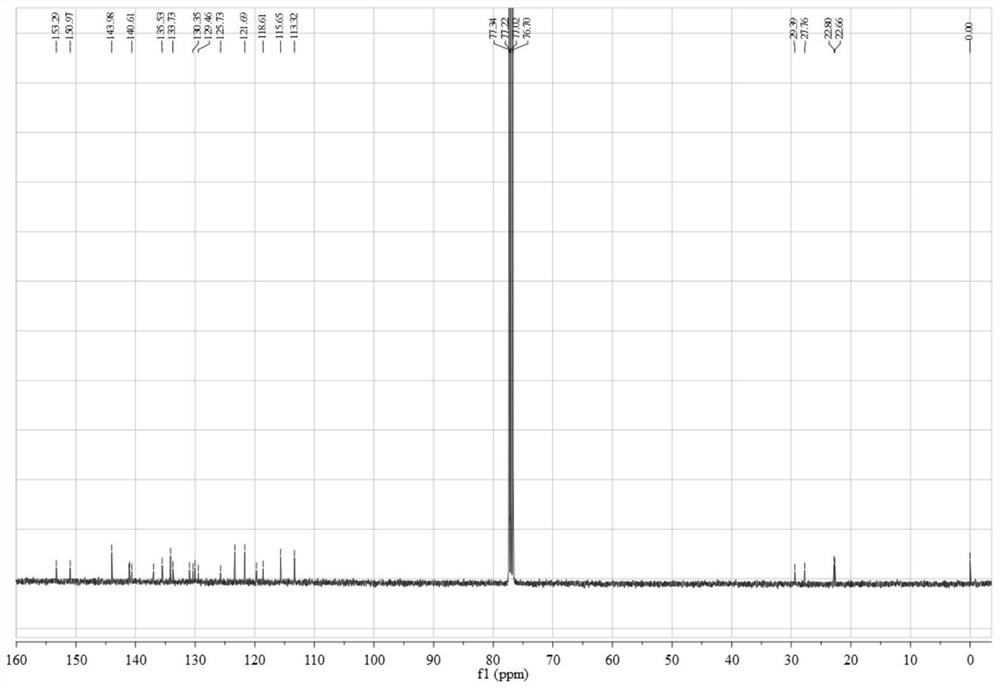
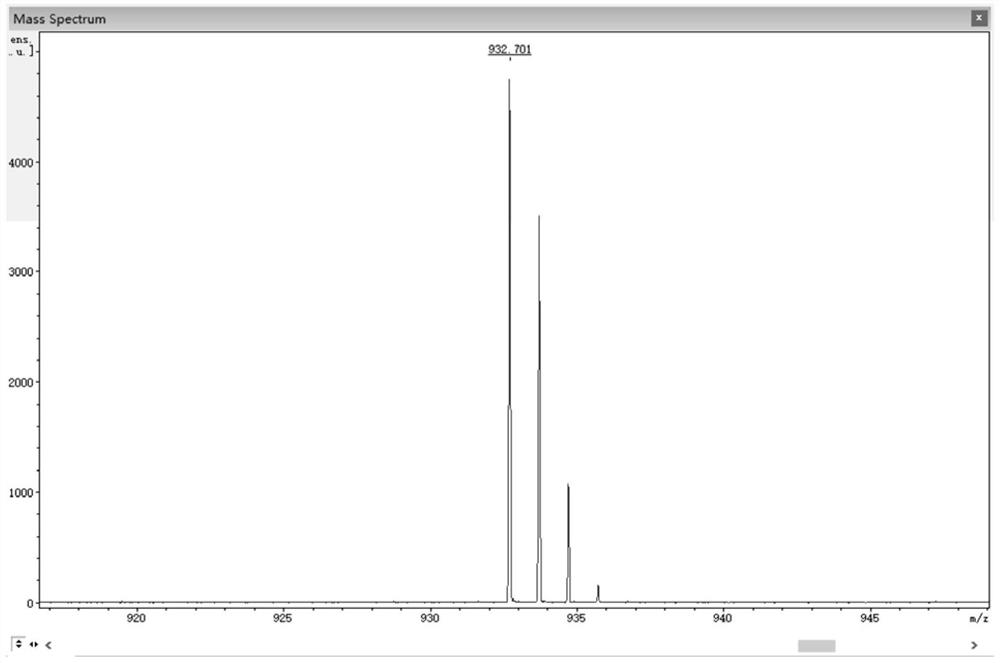
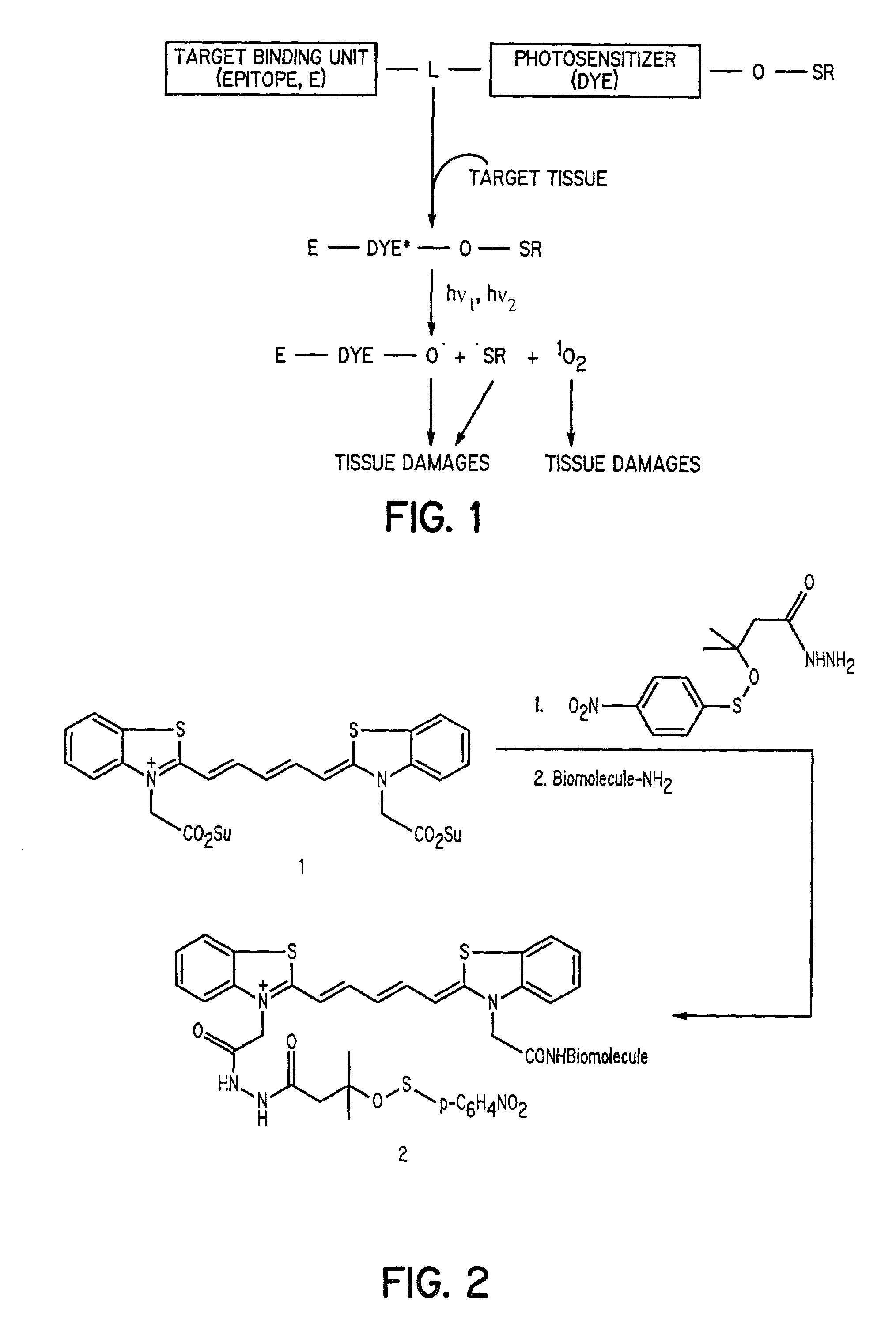
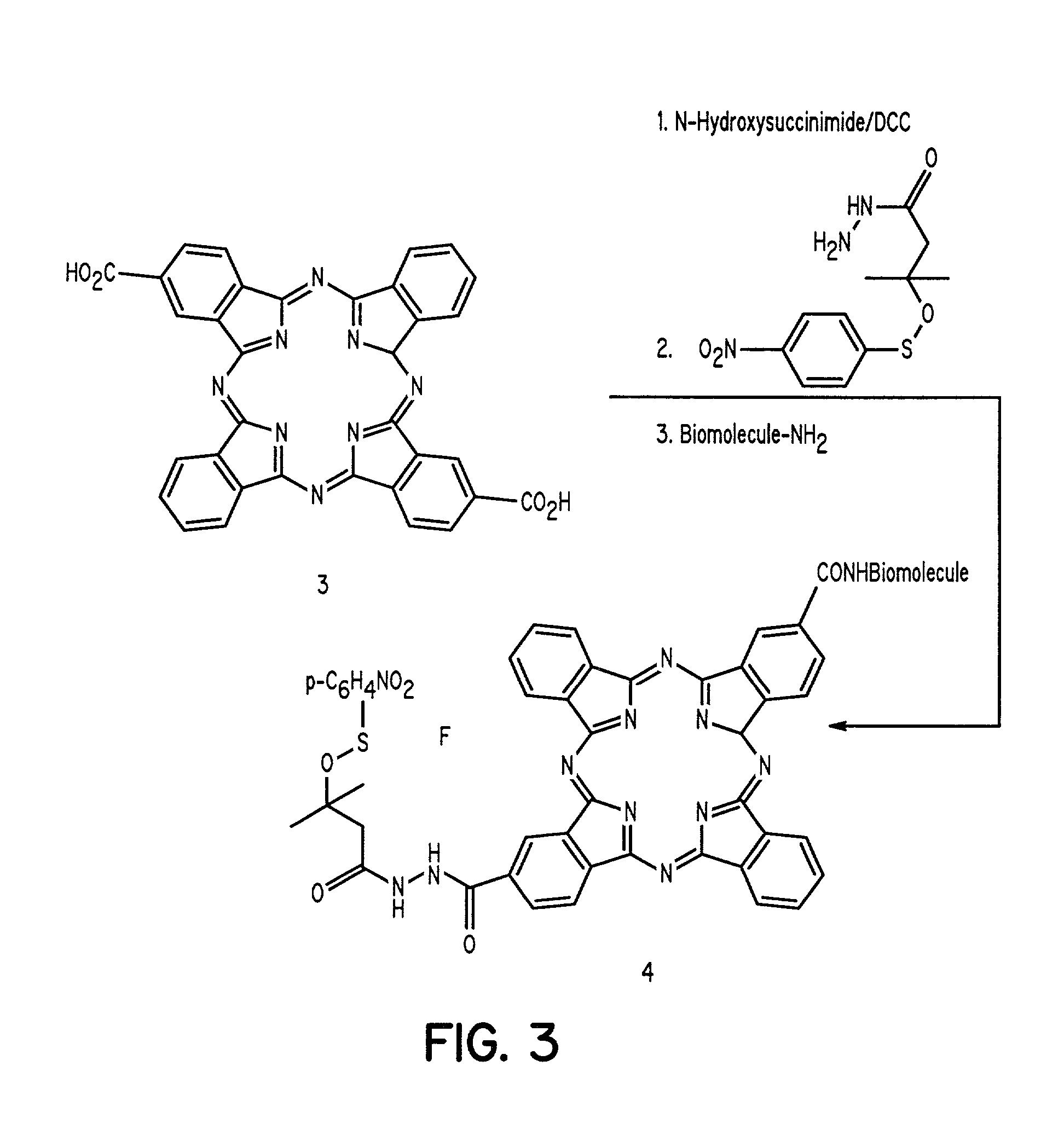
Cyanine-sulfenates for dual phototherapy
Dye-sulfenate derivatives and their bioconjugates for dual phototherapy of tumors and other lesions. The compounds comprise sulfenates having the formula, t,0080where E is selected from the group consisting of somatostatin receptor binding molecules, heat sensitive bacterioendotoxin receptor binding molecules, neurotensin receptor binding molecules, bombesin receptor binding molecules, cholecystekinin receptor binding molecules, steroid receptor binding molecules, and carbohydrate receptor binding molecules, and dihydoxyindolecarboxylic acid; L and X are independently selected from the group consisting of —(R5)NOC—, —(R5)NOCCH2O—, —(R5)NOCCH2CH2O—, —OCN(R5)—, —HNC(═S)NH—, and HNC(═O)NH—; DYE is an aromatic or a heteroaromatic radical derived from the group consisting of cyanines, indocyanines, phthalocyanines, rhodamines, phenoxazines, phenothiazines, phenoselenazines, fluoresceins, porphyrins, benzoporphyrins, squaraines, corrins, croconiums, azo dyes, methine dyes, indolenium dyes, crellins, and hypocrellins; R1 to R5 are independently selected from the group comprising hydrogen, C1-C10 alkyl, C5-C10 aryl, C1-C10 polyhydroxyalkyl, and C1-C10 polyalkoxyalkyl; and Ar is an aromatic or heteroaromatic radical derived from the group consisting of benzenes, naphthalenes, naphthoquinones, diphenylmethanes, fluorenes, anthracenes, anthraquinones, phenanthrenes, tetracenes, naphthacenediones, pyridines, quinolines, isoquinolines, indoles, isoindoles, pyrroles, imidiazoles, oxazoles, thiazoles, pyrazoles, pyrazines, purines, benzimidazoles, furans, benzofurans, dibenzofurans, carbazoles, acridines, acridones, phenanthridines, thiophenes, benzothiophenes, dibenzothiophenes, xanthenes, xanthones, flavones, coumarins, and anthacylines. The compounds are designed to produce both Type 1 and Type 2 phototherapeutic effects at once using a dual wavelength light source that will produce singlet oxygen and free radicals at the lesion of interest.
Owner:MEDIBEACON
Room temperature phosphorescent compound and composition and application thereof
The invention relates to a room temperature phosphorescent compound and composition and application thereof based on phenothiazine or phenoxazine and derivatives substituting naphthalene or naphthalene derivatives, and belongs to the technical field of phosphorescent compounds. The room temperature phosphorescent compound is represented in a formula (1) or (2), and has room temperature phosphorescence characteristics. The invention further provides a room temperature phosphorescent composition with high luminous efficiency, and the room temperature phosphorescent composition is composed of thecompound represented by the formula (1) or (2) and a compound containing a 1,3,5-triazine group. The phosphorescent compound has the characteristics of room temperature phosphorescence emission in both the crystalline state and the amorphous state, and the compound and the composition are used as a luminescent material for preparing a luminescent layer of an organic electroluminescent device, andthe prepared organic electroluminescence device achieves an important breakthrough in pure organic electrophosphorescent devices.
Owner:JILIN UNIV
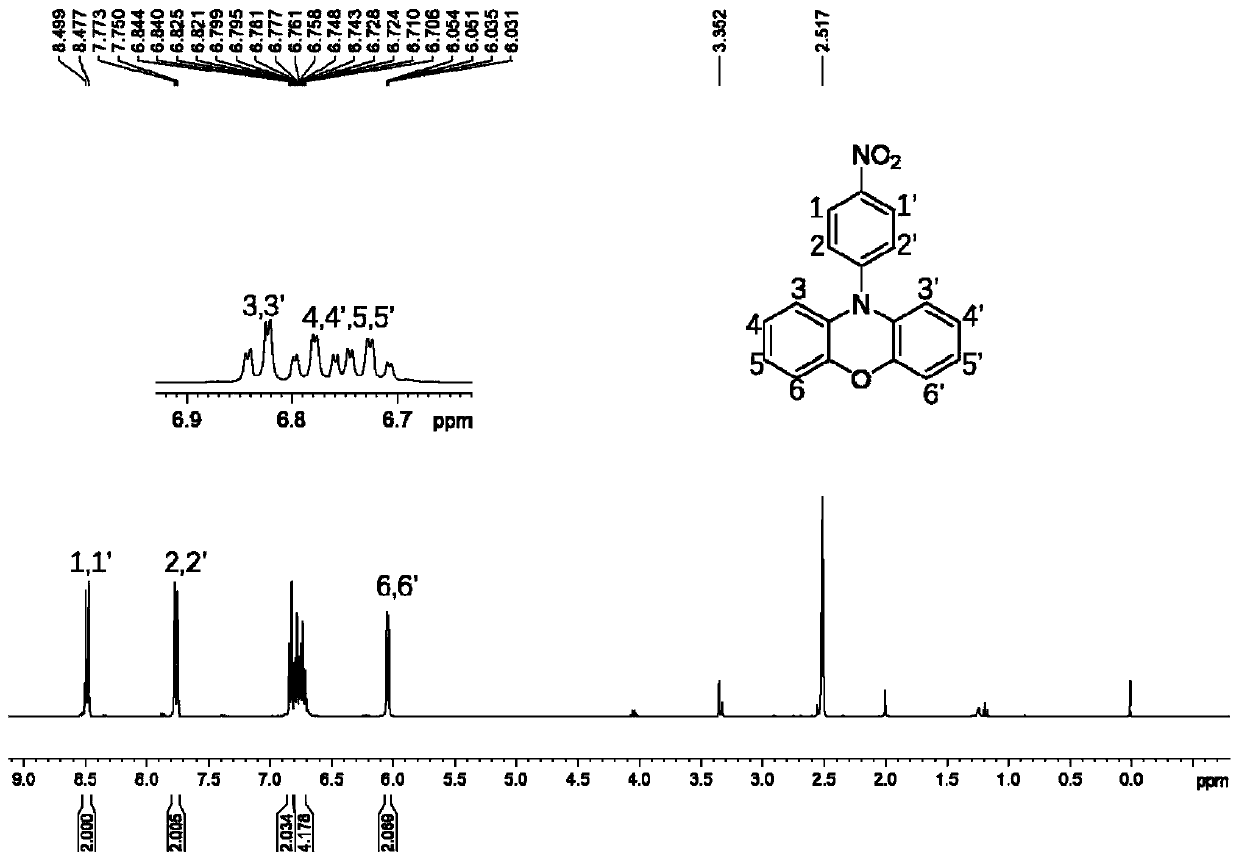
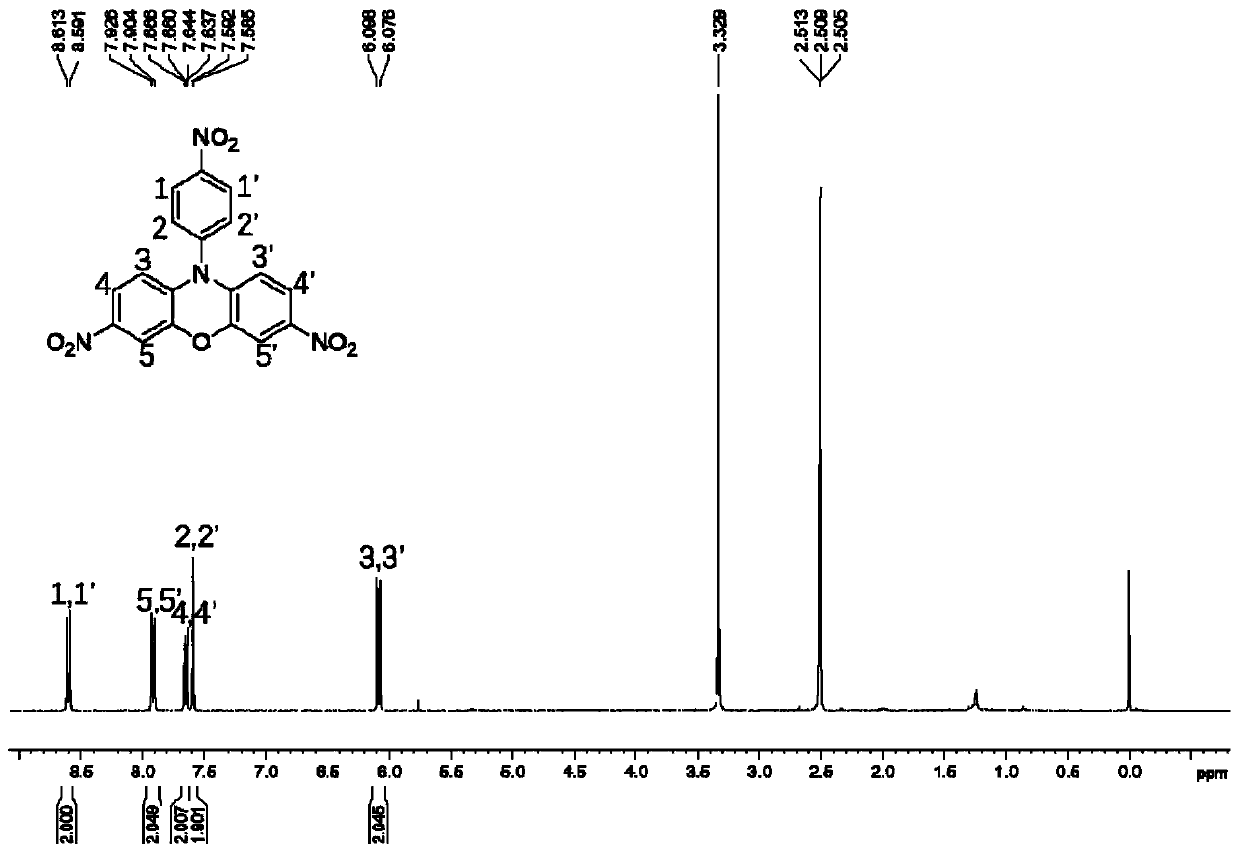
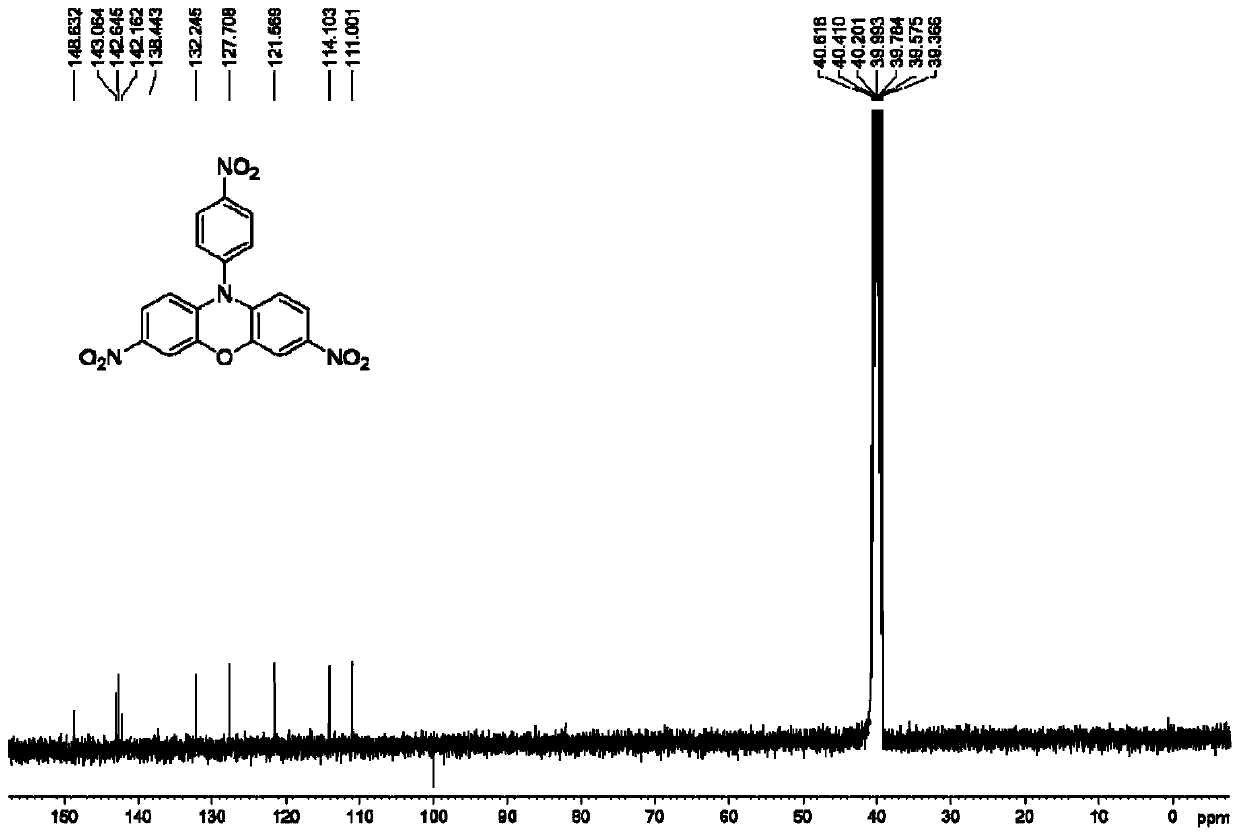
3,7-disubstituted phenoxazine derivatives and preparation method thereof
InactiveCN110437172AThe synthesis method is simpleOperational securityOrganic chemistryOxazine dyesNitrationSolar cell
The invention relates to the technical field of phenoxazine derivatives, and particularly relates to 3,7-disubstituted phenoxazine derivatives and a preparation method thereof. Phenoxazine adopted asa raw material is subjected to a coupling reaction to synthesize N-(4-aminophenyl) phenoxazine; the N-(4-aminophenyl) phenoxazine is subjected to a nitration reaction to synthesize 3,7-dinitro-N-(4-nitrophenyl) phenoxazine; the 3,7-dinitro-N-(4-nitrophenyl) phenoxazine is subjected to reduction to synthesize 3,7-diamino-N-(4-aminophenyl) phenoxazine. The obtained 3,7-dinitro-N-(4-nitrophenyl) phenoxazine and the 3,7-diamino-N-(4-aminophenyl) phenoxazine can be structurally modified easily, and novel compounds having different functions can be easily synthesized from the same. The synthesized phenoxazine derivatives can be widely applied in materials, dye, photosensitized solar cells, and other fields.
Owner:ZHENGZHOU UNIV
Blue electroluminescent polymer and organic electroluminescent device using the same
ActiveUS20060166037A1Easily transport chargeGood colorOrganic chemistryDischarge tube luminescnet screensLuminescent polymersOrganic electroluminescence
A blue electroluminescent polymer having a phenoxazine-based unit in a polyarylene backbone and an organic electroluminescent device using the polymer. The organic electroluminescent device has improved luminous efficiency and color purity.
Owner:SAMSUNG DISPLAY CO LTD
Hole transport material with phenoxazine as core structure, and synthetic method and application thereof
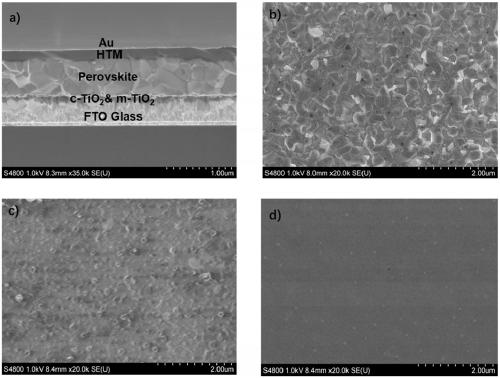
ActiveCN109265410AEasy to synthesizeLow costOrganic chemistrySolid-state devicesPerovskite solar cellPhenoxazine
The invention belongs to the technical field of organic functional materials, and discloses a hole transport material with phenoxazine as a core structure, a preparation method thereof, and application of the hole transport material in perovskite solar cells. The hole transport material with the phenoxazine derivative as the core structure, two end of which are connected with N,N-dimethoxyaniline,has the advantages of stable natural condition, low cost, high hole mobility, high electric conductivity and the like; the hole transport material with phenoxazine as a core structure has lower production cost and comparable photoelectric conversion efficiency as compared with a conventional hole transport material Spiro-OMeTAD; the novel hole transparent material with phenoxazine as a core structure has a broad application prospect, providing a new choice for manufacture of efficient and stable perovskite solar cells in terms of hole transport materials.
Owner:JIANGSU UNIV
Organic electroluminescence material with phenoxazine/thenoxazin type derivative and organic luminescent device
InactiveCN107312017AImprove luminous efficiencyExcellent OLED materialGroup 5/15 element organic compoundsSolid-state devicesState of artOrganic light emitting device
The invention provides an organic electroluminescence material with a phenoxazine / thenoxazin type derivative and an organic luminescent device, belongs to the technical field of organic photoelectric materials, and aims to solve the technical problems that in the prior art an organic photoelectric material is poor in light emission property such as low light emission efficiency, relatively high driving voltage and short service life. Compared with the prior art, the organic electroluminescence material based on the phenoxazine / thenoxazin type derivative, which is provided by the invention, has driving voltage of 3.8V at least and light emission efficiency as high as 18.8cd / A, and is an excellent OLED material.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Tricyclic heteroaromatic compounds as alpha-synuclein ligands
Derivatives of phenothiazine, phenoxazine, and phenazine compounds and their use as α-synuclein ligands are described. Also described are methods of using these compounds and their radiolabeled analogs for the detection, monitoring, and treatment of synucleinopathies, including Parkinson's disease.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Compound, organic light-emitting device and display device
InactiveCN108218867AImprove luminous efficiencyReduce the driving voltageOrganic chemistrySolid-state devicesOrganic light emitting deviceDisplay device
The invention relates to the field of display and especially relates to compound, an organic light-emitting device and a display device. The compound is shown as a formula I in the following image description, wherein X group is chosen from carbazolyl, dihydroacridine, dihydrophenazine, phenoxazine, phenothiazine, benzopyrrolocarbazolyl, benzofurocarbazolyl, benzothienocarbazolyl, indenocarbazolylor triphenylamine; the X perssadgroup is substituted or unsubstituted; A group is chosen from acryl with a carbon atom number as 6 to 30, alkyl with a carbon atom number as 1 to 20 or cycloalkyl witha carbon atom number as 3 to 20; the A group is substituted or unsubstituted.
Owner:FUYANG XINYIHUA MATERIAL TECH
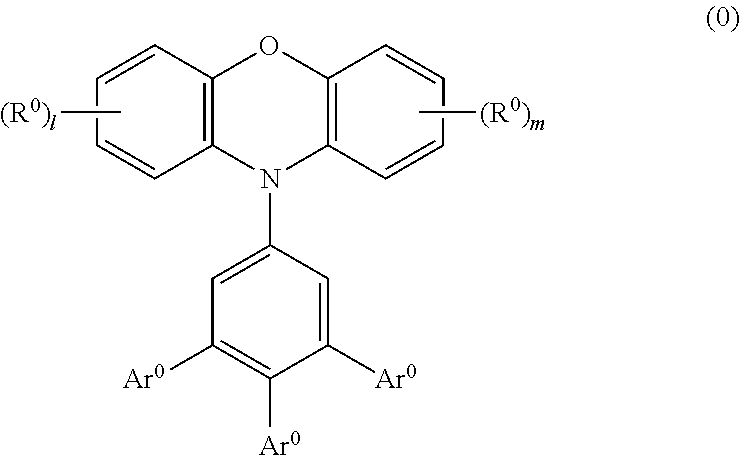
Phenoxazine polymer compound and light emitting device using the same
ActiveUS20110108820A1High color purityEasy to getOrganic chemistrySolid-state devicesArylPolymer science
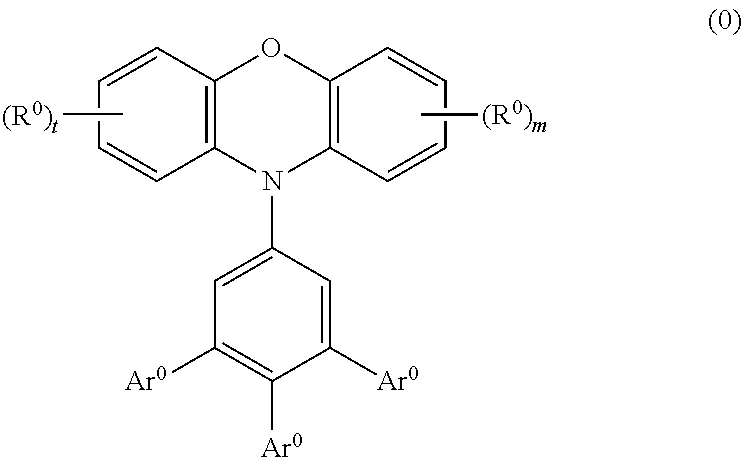
A polymer compound comprising a residue of a compound represented by the following formula (0):wherein Ar0 represents a substituent such as a hydrogen atom, an alkyl group, an alkoxy group, an aryl group and the like, or a group represented by the following formula (A), at least two Ar0s are groups represented by the following formula (A), R0 represents a substituent such as an alkyl group, an alkoxy group, an aryl group and the like, l and m represent an integer of 0 to 3,wherein A0 represents —N═ or —C(R2)═. R2 represents a substituent such as a hydrogen atom, an alkyl group, an alkoxy group, an aryl group and the like.
Owner:SUMITOMO CHEM CO LTD
Phenoxazine compounds, its pharmaceutical compositions and medical use
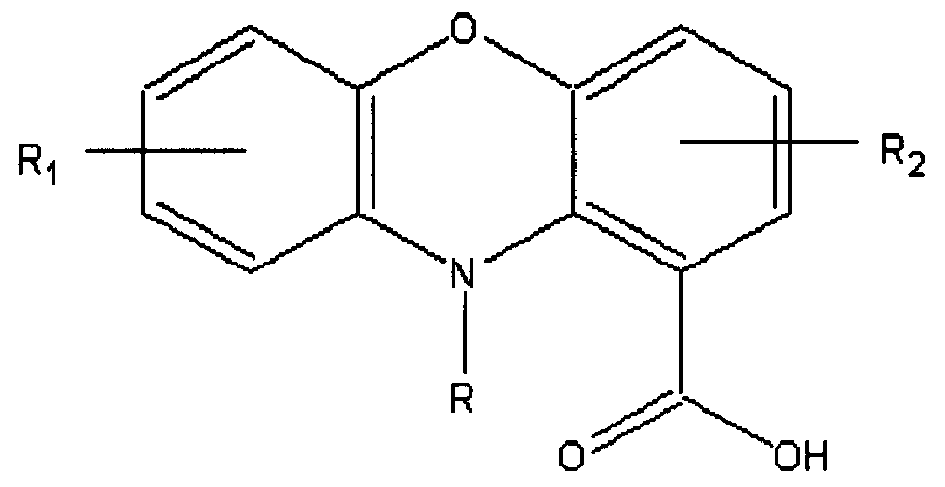
The invention relates to the phenoxazine compounds of formulae I, and the pharmaceutical acceptable salts, esters, amides and prodrugs thereof, to the pharmaceutical compositions containing them, to the use of the preparation of the medicines for inhibiting the starchy protein coagulate. The definitions of R1, R2 and R in formulae I are described in the specification.
Owner:陆鑑青
Phenoxazine derivative and organic light-emitting device thereof
InactiveCN109180602AImprove transmission performanceHigh molecular weightOrganic chemistrySolid-state devicesCharge carrierOrganic light emitting device
The invention provides a phenoxazine derivative and an organic light-emitting device thereof, and belongs to the technical field of organic photoelectric materials. The above compound has a structurerepresented by formula (I), and introduction of a phenoxazine structure to one end increases the transportability of carriers; introduction of a bridging structure to a specific position can increasethe molecular weight of the compound to make the obtained material have a high glass transition temperature and not crystallize, and also makes the compound have a certain distortion in a spatial three-dimensional structure in order to improve the film forming property; and introduction of a cyano structure capable of easily accepting electrons to the other end of the bridging structure further increases the transportability of carriers. The organic light-emitting device produced by using the compound as a light-emitting layer host material has the advantages of low driving voltage and high light-emitting efficiency, and is an organic light-emitting material with excellent performances.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Diamine containing phenoxazine and amide structures and polyimide thereof
InactiveCN111072585AStrong rigid structureIncrease functional diversityOrganic chemistryThermal expansionCarboxylic acid
The invention discloses diamine containing phenoxazine and amide structures and polyimide thereof. The preparation method comprises the following steps: converting halogen atoms of dihalogenated phenoxazine into cyano groups, carrying out hydrolysis to obtain a dicarboxylic acid monomer, then carrying out acylating chlorination, grafting a nitro-containing group through an amide reaction, finallycarrying out reduction to obtain a diamine monomer containing the phenoxazine and the amide structures, and polymerizing the prepared diamine monomer with dianhydride to obtain polyimide containing the phenoxazine and the amide structures. The planar rigid structure of the phenoxazine and the amide polar group are creatively introduced into a polyimide main chain, the planar rigid structure is beneficial to regular stacking of molecular chains and induction of polymer crystallization, and polar groups can enhance the hydrogen-bond interaction of the molecular chains and promote tight stackingof the molecular chains so that polyimide has excellent barrier property, higher glass-transition temperature and thermal stability, lower thermal expansion coefficient and good antibacterial property.
Owner:HUNAN UNIV OF TECH
Phenoxazine two-dimensional covalent organic framework material and preparation method and application thereof
ActiveCN113292690AHigh crystallinityIncrease the areaFluorescence/phosphorescenceLuminescent compositionsOrganic solventFluorescence
The invention discloses a phenoxazine-based two-dimensional covalent organic framework material and a preparation method and application thereof. The phenoxazine-based two-dimensional covalent organic framework material has a structure as shown in any one of the following formulas: the phenoxazine-based two-dimensional covalent organic framework material prepared by the invention has high crystallinity and specific surface area, uniform pore size distribution and better thermal stability; meanwhile, the phenoxazine-based two-dimensional covalent organic framework material has fluorescence emission with specific wavelength in an organic solvent, and the fluorescence emission intensity is related to the moisture content in the organic solvent. Therefore, the phenoxazine-based two-dimensional covalent organic framework material prepared by the method can be applied to detection of the moisture content in an organic solvent, and has a good application prospect in the field of functional organic materials.
Owner:NANJING UNIV OF TECH
Spirofluorene and nitrogen-containing hetercyclic organic electroluminescent material and organic luminescent device thereof
InactiveCN108409680AImprove performanceImprove hole transport abilityOrganic chemistrySolid-state devicesOrganic light emitting deviceNitrogen
The invention provides a spirofluorene and nitrogen-containing hetercyclic organic electroluminescent material and an organic luminescent device thereof and belongs to the technical field of organic photoelectric materials. The spirofluorene and nitrogen-containing hetercyclic organic electroluminescent material structurally contains a spirofluorene structure which is a blue-light structure with excellent property, not only has higher triplet energy level and wider energy gap, but also is higher in vitrifying conversion temperature and difficult in crystallization; by connection of groups suchas phenoxazine and phenothiazine and the like in molecules, the cavity transmission capability of the blue-light main body structure is added, so that the transportation of current carriers is balanced. Compared with the prior art, the spirofluorene and nitrogen-containing hetercyclic organic electroluminescent material is applied to the organic luminescent device, is especially used as a blue luminescent material in a luminescent layer and has relatively-higher luminescent efficiency.
Owner:CHANGCHUN HYPERIONS TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com