Preparation method and applications of hexatomic dinitrogen heterocyclic derivatives containing four identical substituent groups
A technology for diazoheterocycles and derivatives, applied in the field of preparation of six-membered dizazaheterocycle derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
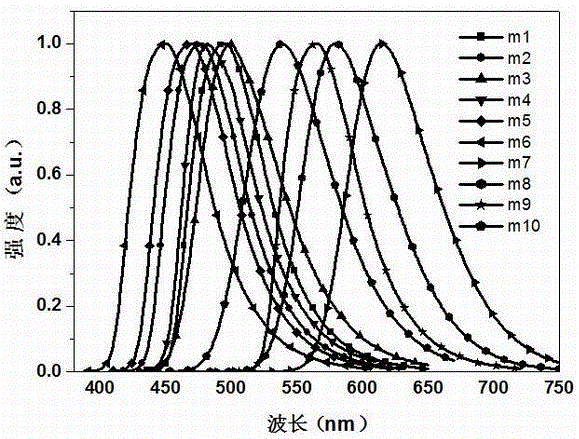
Examples
Embodiment 1
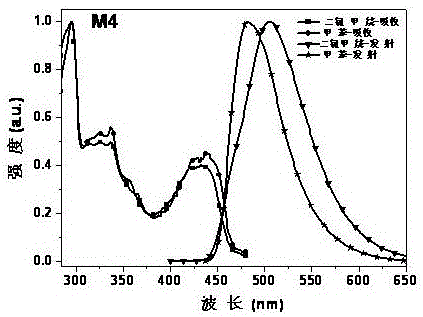
[0043] Embodiment 1: the synthesis of derivative M1
[0044]
[0045] Add 1.69g (10.10mmol) carbazole into a 250ml three-necked flask, then add 100mL N,N-dimethylformamide as a reaction solvent, and stir on a magnetic stirrer for 10min. Under the condition of ice bath, 0.49g (20.19mmol) NaH was added to the reaction flask in batches, and the stirring was continued for 1h. 0.50g (2.30mmol) of 3,4,5,6-tetrachloropyridazine was dissolved in 20ml of N,N-dimethylformamide, and added dropwise to the reaction system. After the addition was complete, under nitrogen protection, Reaction at 60°C for 15h. After the reaction is over, pour the reaction solution into 150ml of dilute hydrochloric acid with a concentration of 10% to quench, filter under reduced pressure, wash with water, and dry the crude product with petroleum ether and ethyl acetate (PE:EA=10:1) Perform column chromatography as the mobile phase to remove impurities, and then use pure dichloromethane (DCM) to flush the ...
Embodiment 2
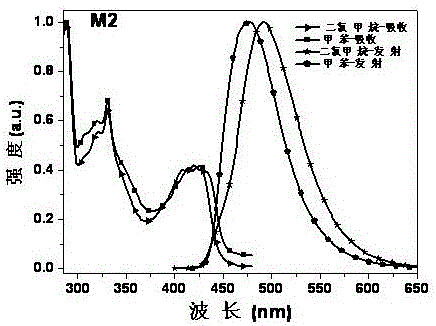
[0046] Embodiment 2: the synthesis of derivative M2
[0047] method 1)
[0048]
[0049]Add 1.35g (8.08mmol) carbazole into a 250ml three-neck flask, then add 100mL N,N-dimethylformamide as a reaction solvent, and stir on a magnetic stirrer for 10min. Under the condition of ice bath, 0.39g (16.16mmol) NaH was added to the reaction flask in batches, and the stirring was continued for 1h. 0.40g (1.84mmol) of 2,3,5,6-tetrachloropyrazine was dissolved in 20ml of N,N-dimethylformamide, and added dropwise to the reaction system. After the addition, under nitrogen protection, 60 Reaction at ℃ for 15h. After the reaction is over, pour the reaction solution into 150ml of dilute hydrochloric acid with a concentration of 10% to quench, filter under reduced pressure, wash with water, and dry the crude product with petroleum ether and ethyl acetate (PE:EA=10:1) Perform column chromatography as the mobile phase to remove impurities, and then use pure dichloromethane (DCM) to flush the...
Embodiment 3
[0053] Both method (1) and method (2) in Example 3 can obtain the target product M2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com