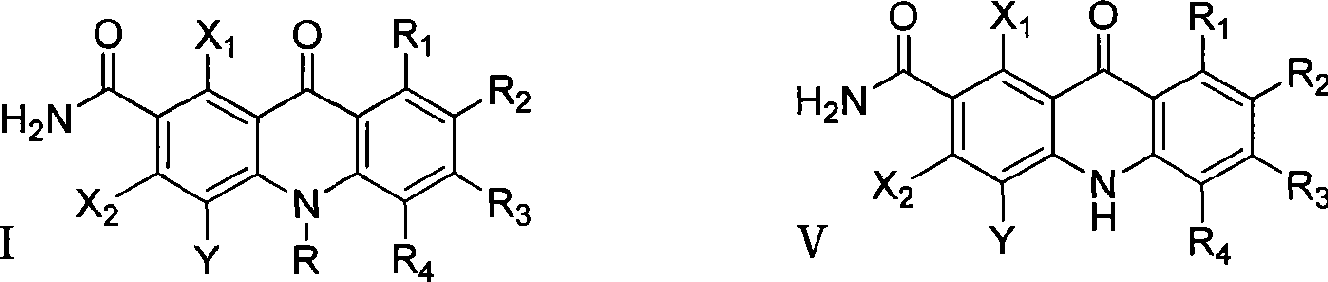Patents
Literature
894 results about "Acridine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Acridine is an organic compound and a nitrogen heterocycle with the formula C₁₃H₉N. Acridines are substituted derivatives of the parent ring. It is a planar molecule that is structurally related to anthracene with one of the central CH groups replaced by nitrogen. Like the related molecule pyridine and quinoline, acridine is mildly basic. It is an almost colorless solid. There are no commercial applications of acridines but at one time acridine dyes were popular. It crystallizes in needles.
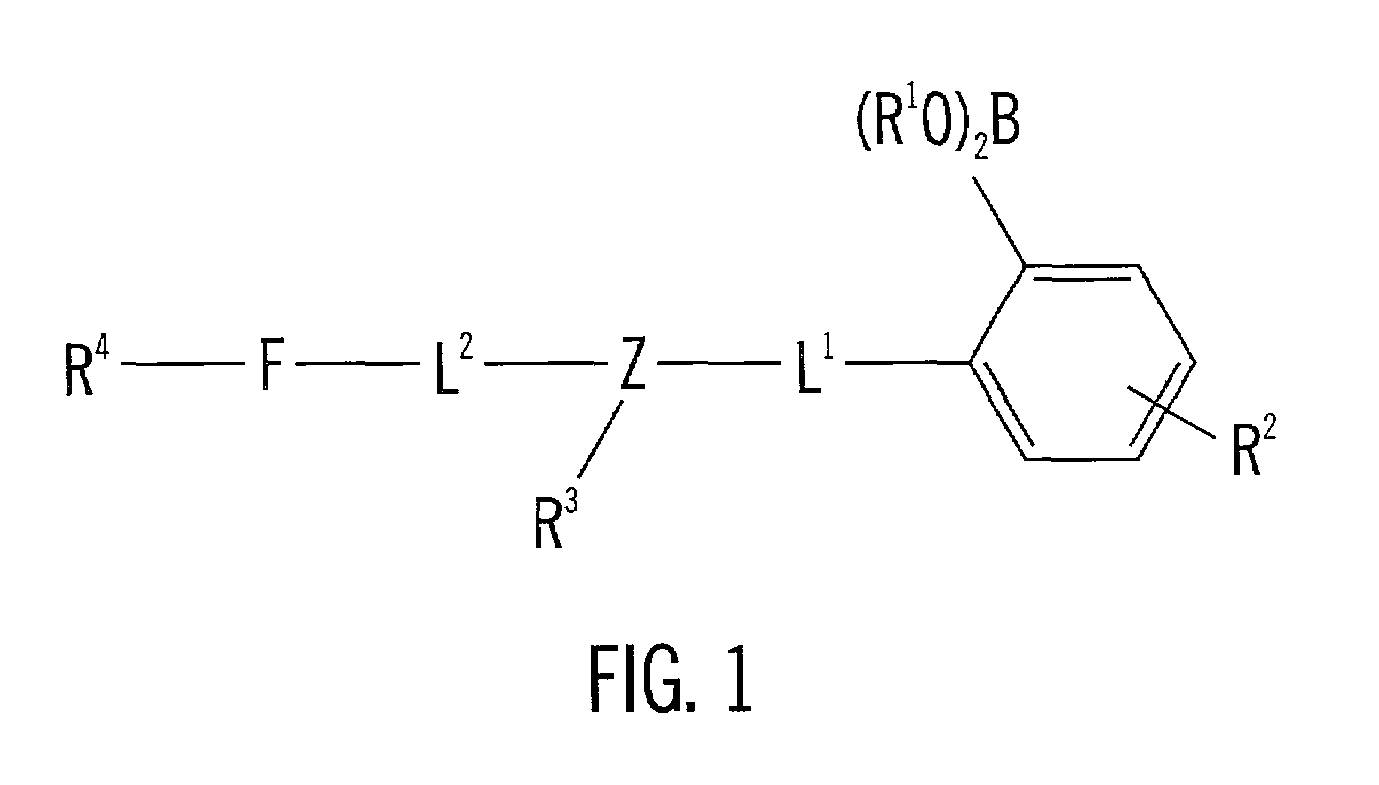
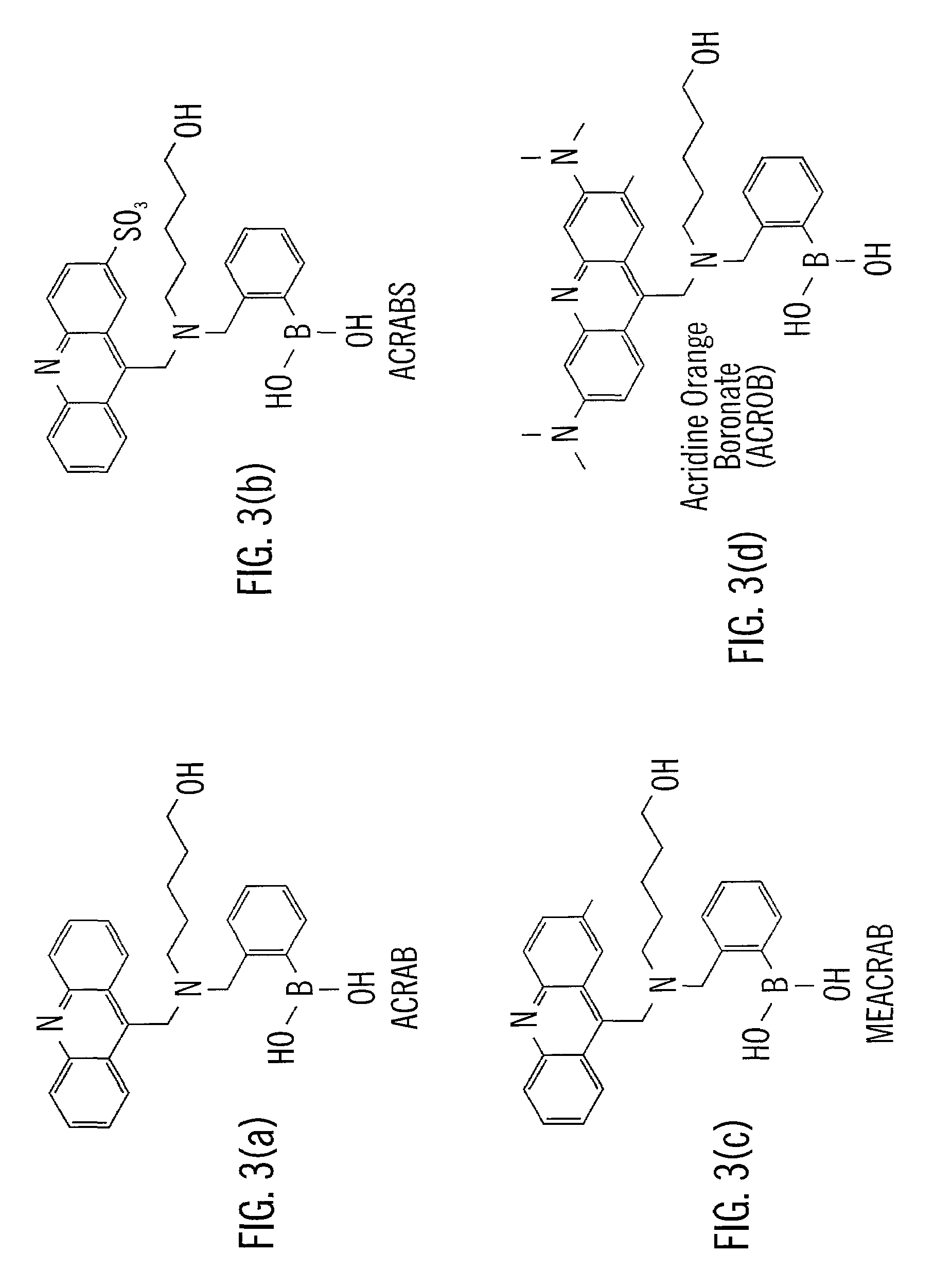
Analyte sensing via acridine-based boronate biosensors
InactiveUS7045361B2Improve sensor sensitivityIncrease overall biocompatibility and functioningMicrobiological testing/measurementChemiluminescene/bioluminescenceConcentrations glucoseFluorophore
Fluorescent biosensor molecules, fluorescent biosensors and systems, as well as methods of making and using these biosensor molecules and systems are described. These biosensor molecules address the problem of obtaining fluorescence emission at wavelengths greater than about 500 nm. Biosensor molecules generally include an (1) an acridine-based fluorophore, (2) a linker moiety and (3) a boronate substrate recognition / binding moiety, which binds polyhydroxylate analytes, such as glucose. These biosensor molecules further include a “switch” element that is drawn from the electronic interactions among these submolecular components. This fluorescent switch is generally “off” in the absence of bound polyhydroxylate analyte and is generally “on” in the presence of bound polyhydroxylate analyte. Thus, the reversible binding of a polyhydroxylate analyte essentially turns the fluorescent switch “on” and “off”. This property of the biosensor molecules, as well as their ability to emit fluorescent light at greater than about 500 nm, renders these biosensor molecules particularly well-suited for detecting and measuring in-vivo glucose concentrations.
Owner:MEDTRONIC MIMIMED INC
Method for immunological detection by combining acridinium ester labeling technology with general magnetic particles
The invention relates to the field of analyzing immunological determination, in particular to a method for immunological detection by combining an acridinium ester labeling technology with general magnetic particles and an immunological determination (quantitative / qualitative) kit which is prepared by utilizing the method. According to the method disclosed by the invention, substances, such as acridinium ester or acridine sulfonamide are used for labeling and detecting an antigen or an antibody, biotin is used for labeling and capturing the antigen or the antibody, and the immunological determination of biomolecules is carried out by combining the general (chain enzyme) avidin labeled magnetic particles. The method disclosed by the invention has the advantages of high sensitivity, wide detection range and capabilities of realizing the automation easily and having a wide application prospect in the field of the immunological detection.
Owner:苏州长光华医生物试剂有限公司
Derivatives of fluorene, anthracene, xanthene, dibenzosuberone and acridine and uses thereof
Owner:ALLERGAN SALES LLC
Thermal activation delayed fluorescent material and organic electroluminescent device
ActiveCN105503766AShort lifeImprove efficiencyOrganic chemistryBenzene azine dyesAcridineTriplet state
The invention relates to a thermal activation delayed fluorescent material with a general formula of the structure shown as the formula (I) or the formula (II). D is one of phenoxazinyl, phenothizainyl, 9,9-dimethyl acridine, 9-methyl phenazinyl, 9-phenyl phenazinyl, 4-phenoxazinyl-1-phenyl, 4-phenothizainyl-1-phenyl, 4-(9,9-dimethyl)acridinyl-1-phenyl, 4-(9-methyl)-phenazinyl-1-phenyl, 4-(9-phenyl)phenazinyl-1-phenyl and 3,5-bis-carbazolyl-1-phenyl. The invention further relates to an organic electroluminescent device which comprises a light-emitting layer, and luminescent dye of the light-emitting layer is the thermal activation delayed fluorescent material. The singlet state-triplet state energy gap (delta EST) of the thermal activation delayed fluorescent material is very small, triplet state excitors can be converted into singlet state excitors through inverse intersystem crossing (RIST) to emit light, and the efficiency and stability of an OLED device can be improved. The formula is shown in the description.
Owner:KUNSHAN GO VISIONOX OPTO ELECTRONICS CO LTD +1
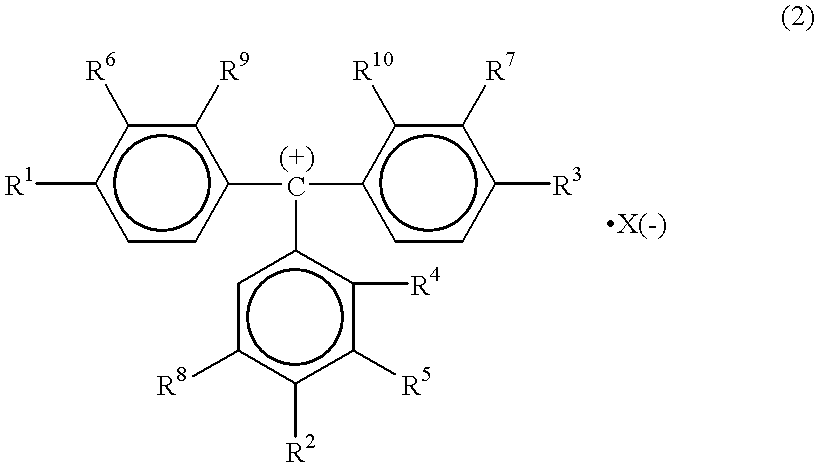
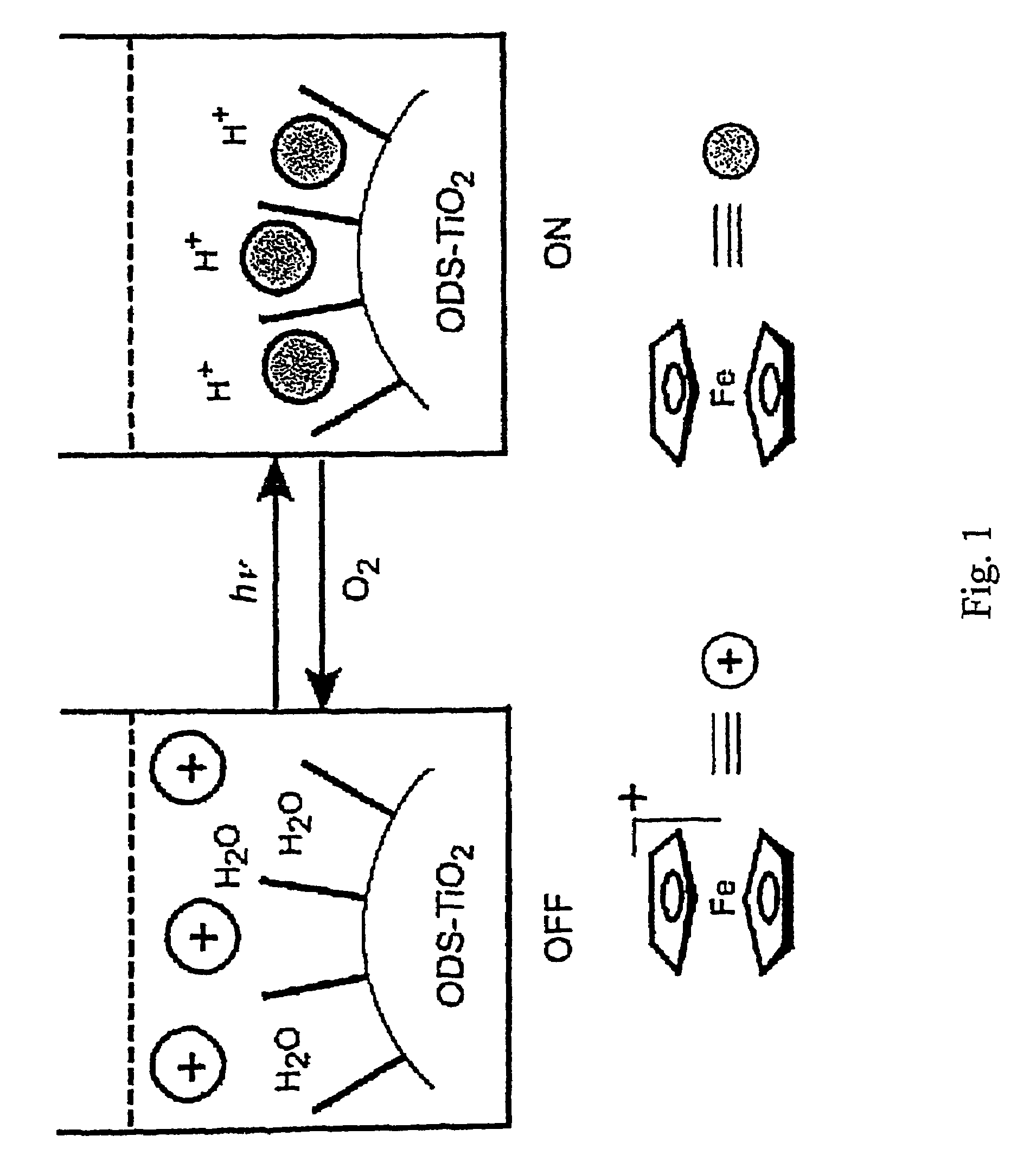
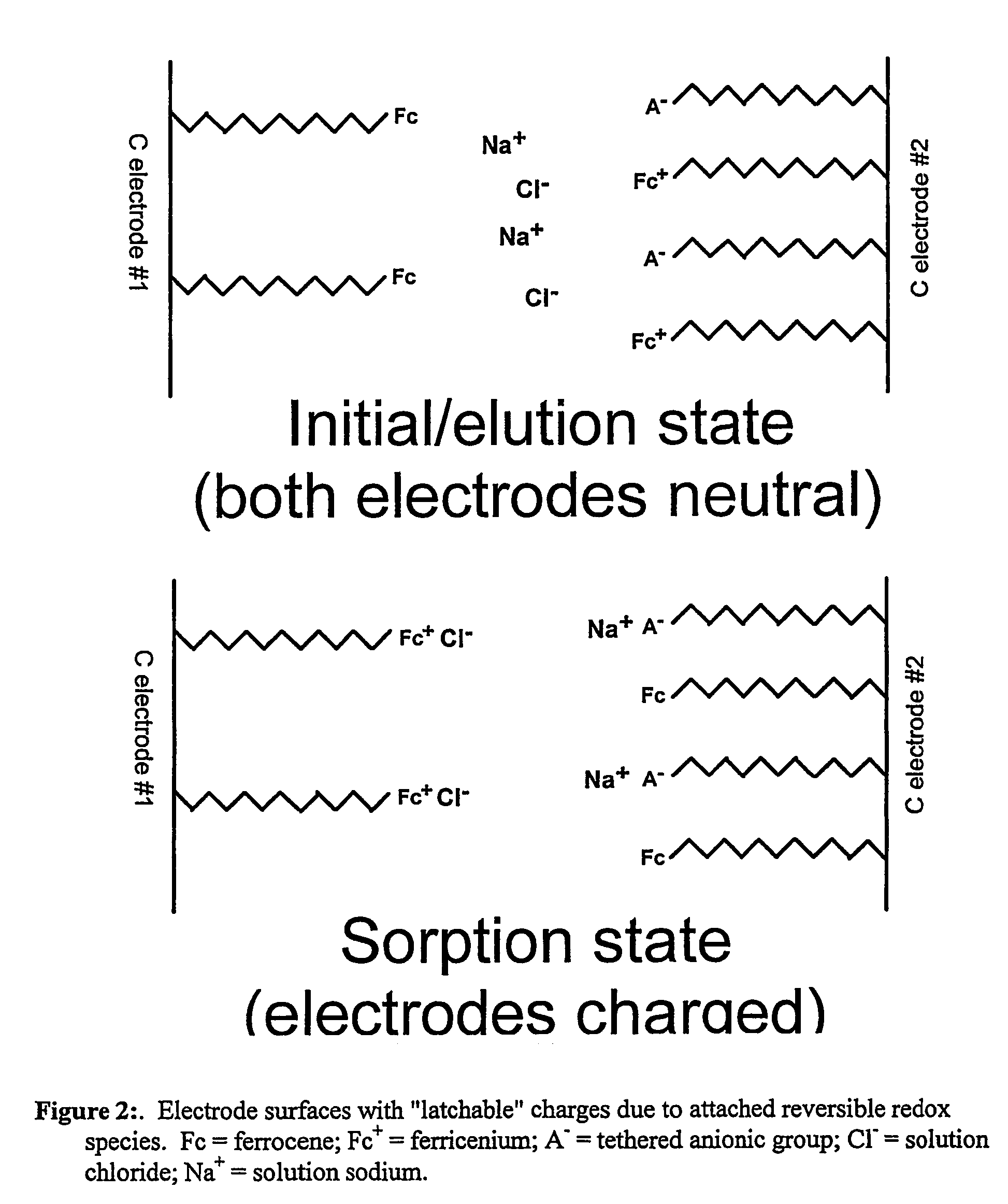
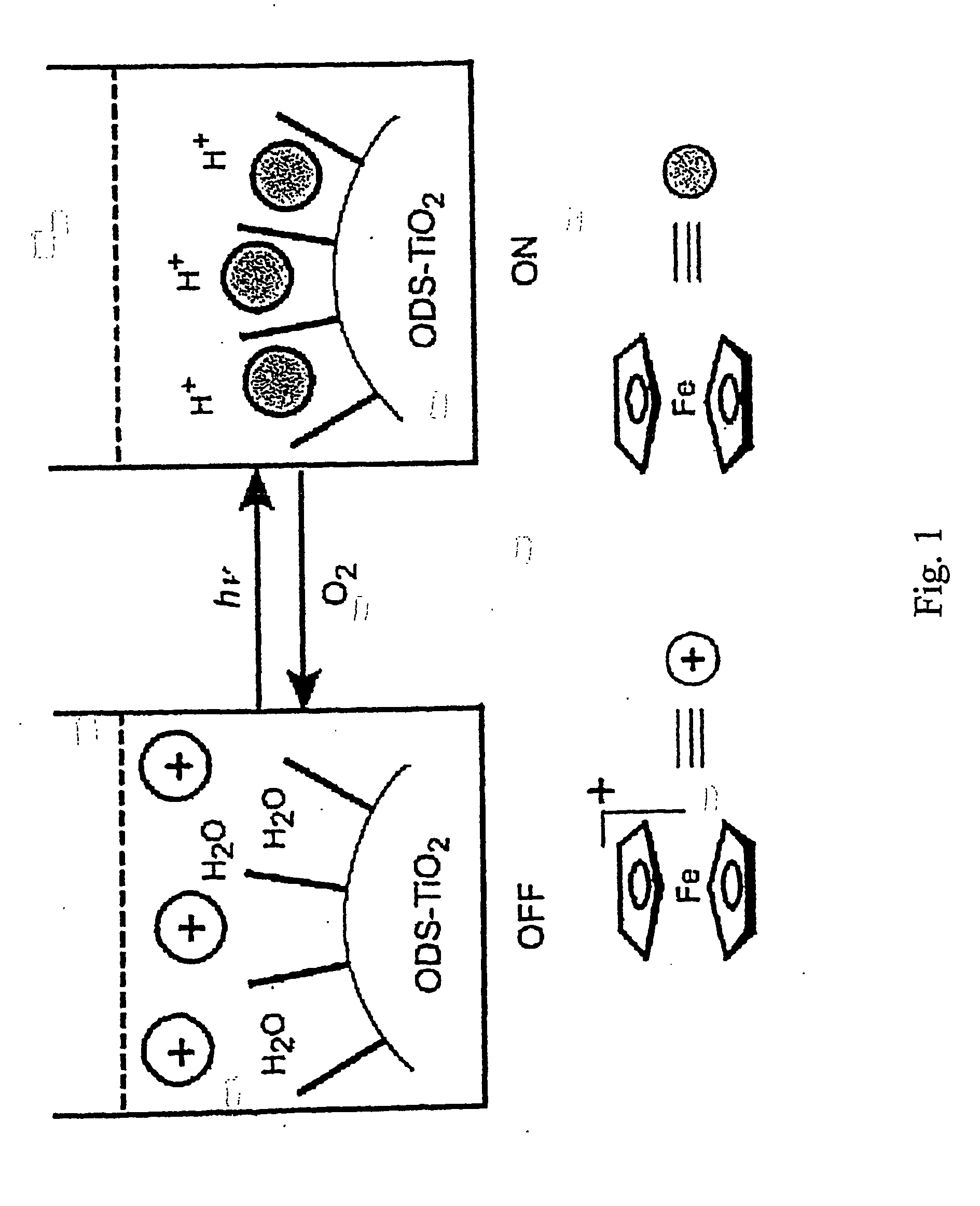
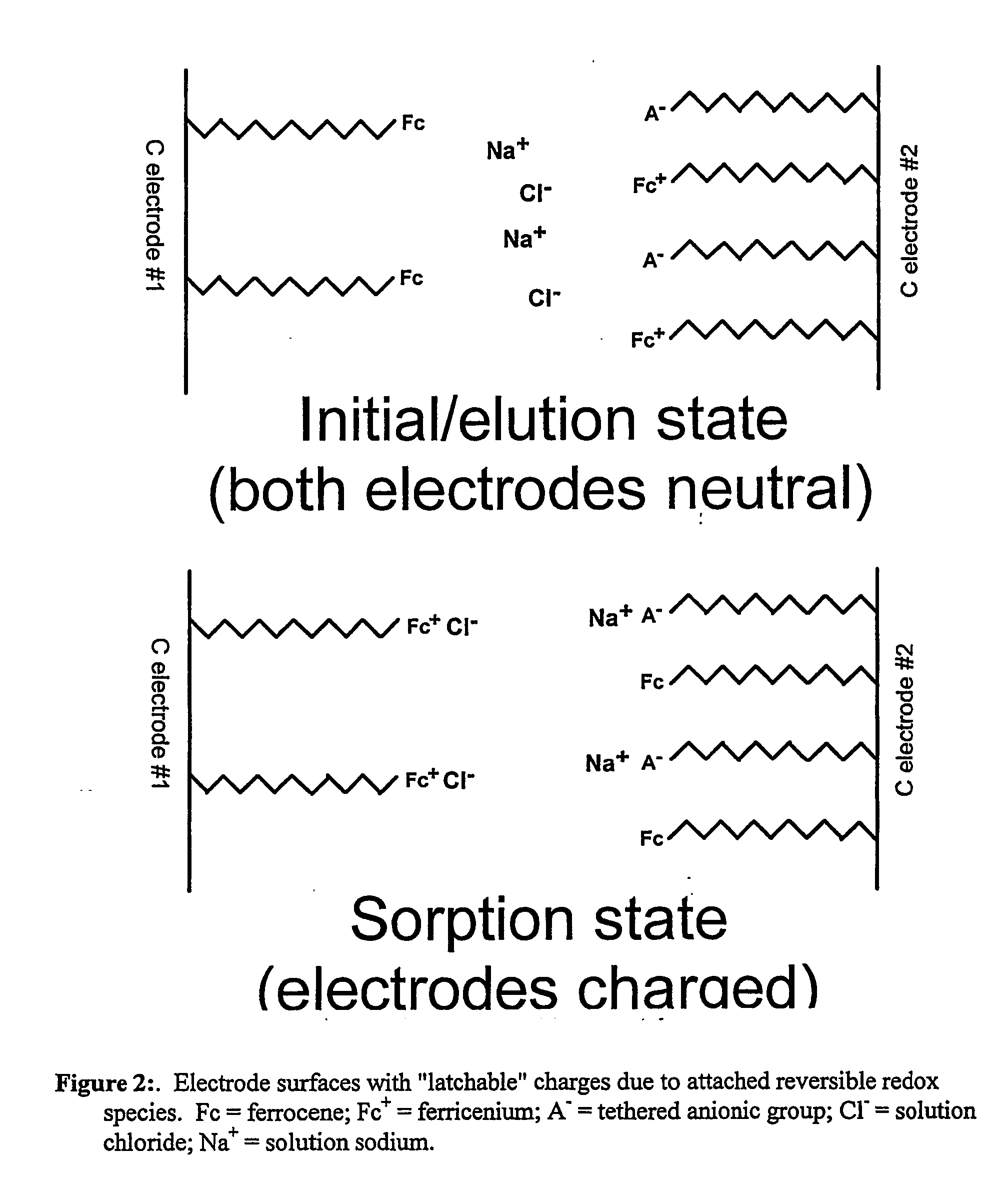
Redox-switchable materials
InactiveUS20050227071A1Improve efficiencyNanoinformaticsSynthetic resin layered productsQuinoneSemiconductor materials
The invention relates to redox-switchable material comprising a redox-active moiety adsorbed, bonded or both, to a semiconductor material. Among the preferred redox-active moieties disclosed herein are ferrocenes, acridines, and quinones though any such moiety may be employed. The redox-switchable material of this invention may be used to selectively remove one or more selected solutes from an aqueous solution wherein adsorption / complexation of the solute is influenced by the oxidation state of the redox-active moiety. In an alternative embodiment, inclusion moieties that are covalently bound to the redox-active moiety are employed to achieve selective complexation of the desired solute. Other possible applications of the disclosed materials are photoerasable writing media, electrochromic or photochromic materials, catalysis, and solar energy storage.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
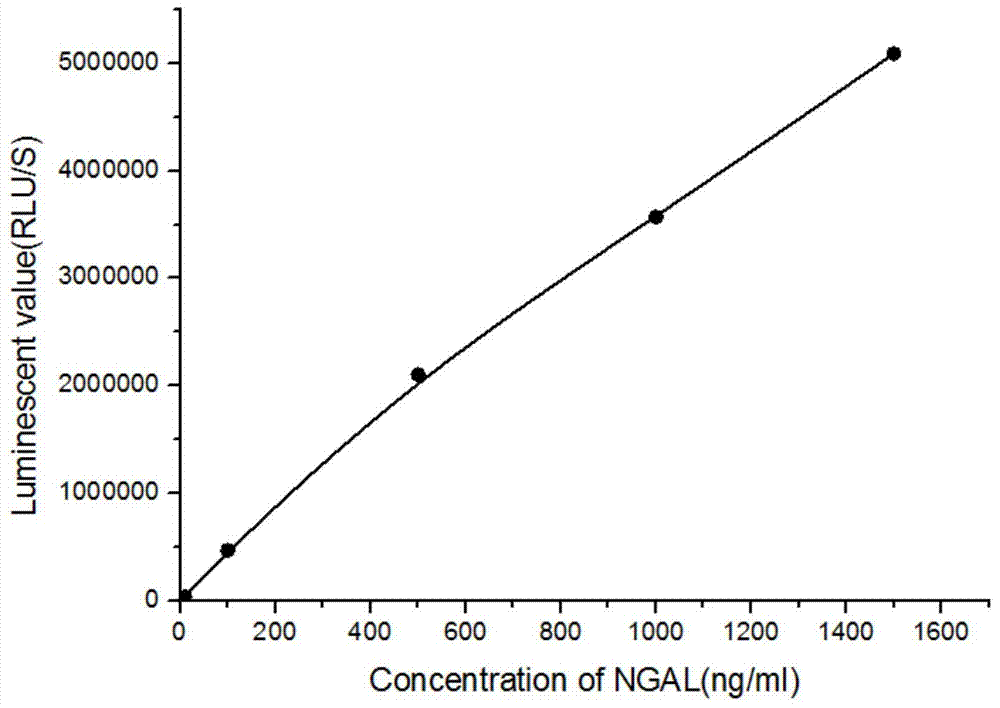
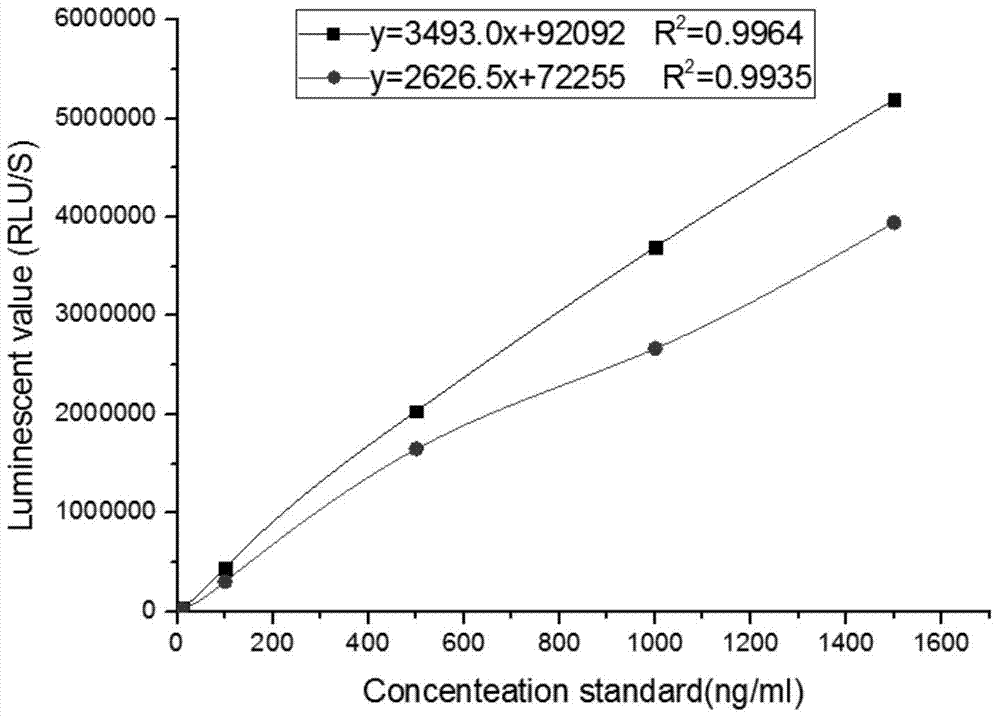
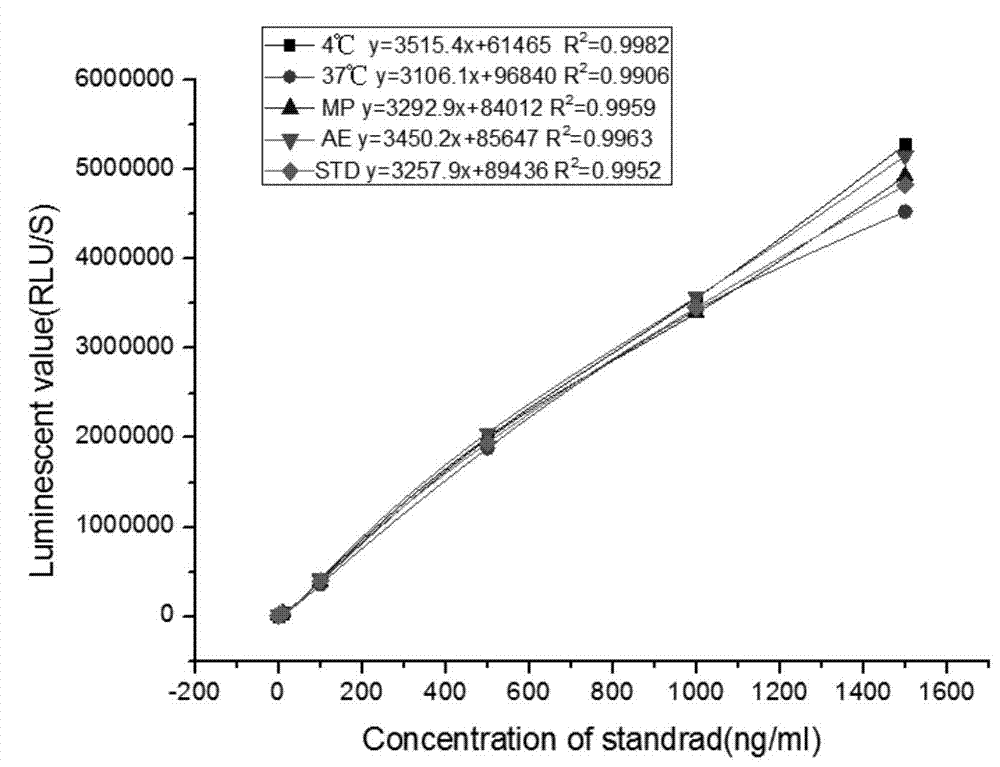
Kit for detecting NGAL content and preparation method thereof
The invention relates to a kit for detecting neutrophil gelatinase-associated lipocalin content based on chemiluminescence immunoassay. According to the invention, by employing a double-antibody sandwich immunization analysis method, a chemiluminescence magnetic microspheres immunization technology is used, anti-NGAL antibody-coated magnetic microspheres for specifically combining with NGAL antigen of a standard substance / sample in a reaction cup, then are reacted to another strain anti-NGAL antibody labelled with acridine salt to form an immunization compound, through an acid-base chemical reaction of a pre-Trigger and a Trigger, relative light unit (RLU / s) of the chemiluminescence reaction can be measured; the NGAL antigen content in the sample is in direct proportion to the relative light unit (RLU / s) measured by an optical system, determination of NGAL content in an urine specimen can be determined through standard curve fitting; and the method has the obvious advantages of high sensitivity, strong specificity, good stability, simple operation and low cost.
Owner:GUANGZHOU DARUI BIOTECH
Absorbing pathogen-inactivating compounds with porous particles immobilized in a matrix
InactiveUS20050142542A1Reduce risk of leakageEasy to manufactureImmobilised enzymesOther blood circulation devicesWhole blood productSorbent
Methods and devices are provided for reducing the concentration of low molecular weight compounds in a biological composition, while substantially maintaining a desired biological activity of the biological composition. The device comprises highly porous adsorbent particles, and the adsorbent particles are immobilized by an inert matrix. The matrix containing the particles is contained in a housing, and the particles range in diameter from about 1 μm to about 200 μm. The matrix can be fibrous, and the particles can have a surface area greater than 750 m2 / g and a pore diameter between about 25 and 800 Å. The device can be used to adsorb and remove a pathogen-inactivating compound that is a nucleic acid-binding compound such as psoralen, an acridine derivative or a dye from a biological composition such as a blood product.
Owner:CERUS CORP
Liquid crystal aligning agent, polyorganosiloxane, liquid crystal aligning film, forming method thereof and liquid crystal display element
ActiveCN101735825AImprove stabilityLiquid crystal compositionsPhotomechanical apparatusEpoxyAcetophenone
The present invention relates to a liquid crystal aligning agent, polyorganosiloxane, a liquid crystal aligning film, a forming method thereof and a liquid crystal display element. The present invention provides the liquid crystal aligning agent which can form the liquid crystal aligning film that can generate a pretilt angle with long time stability through an optical aligning method. The liquid crystal aligning agent comprises radiation-sensitive linear polyorganosiloxane which is prepared through the reaction of the following components: specific polyorgansiloxane with epoxy radical; (A) cinnamic acid derivative; and (B) a specific compound with a radiation-sensitive structure that is preferably selected from: acetophenone structure, benzophenone structure, anthraquinone structure, biphenyl structure, carbazole structure, nitro-aryl structure, fluorenes structure, naphthalene structure, anthracene structure, acridine structure and indole structure.
Owner:JSR CORPORATIOON
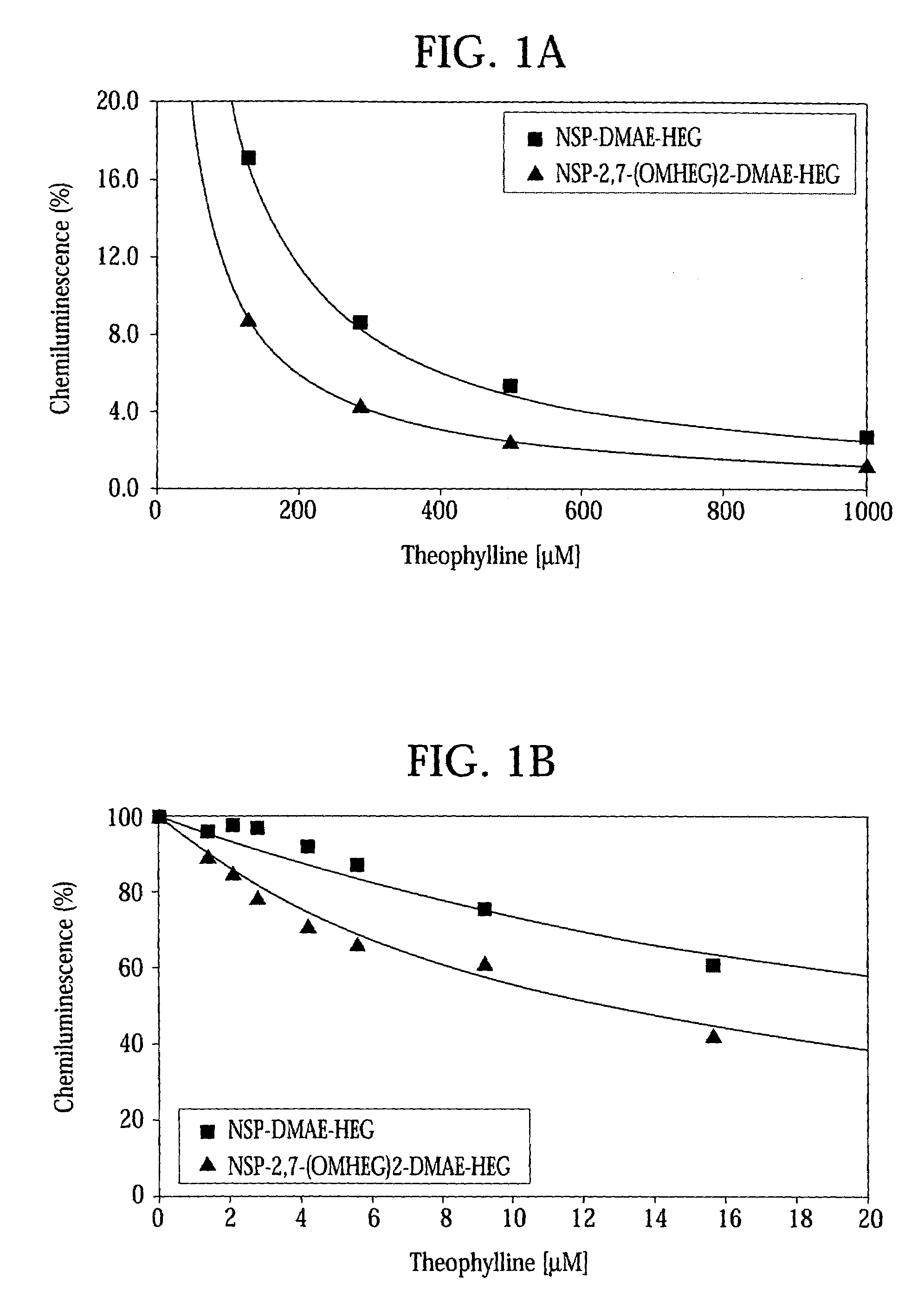
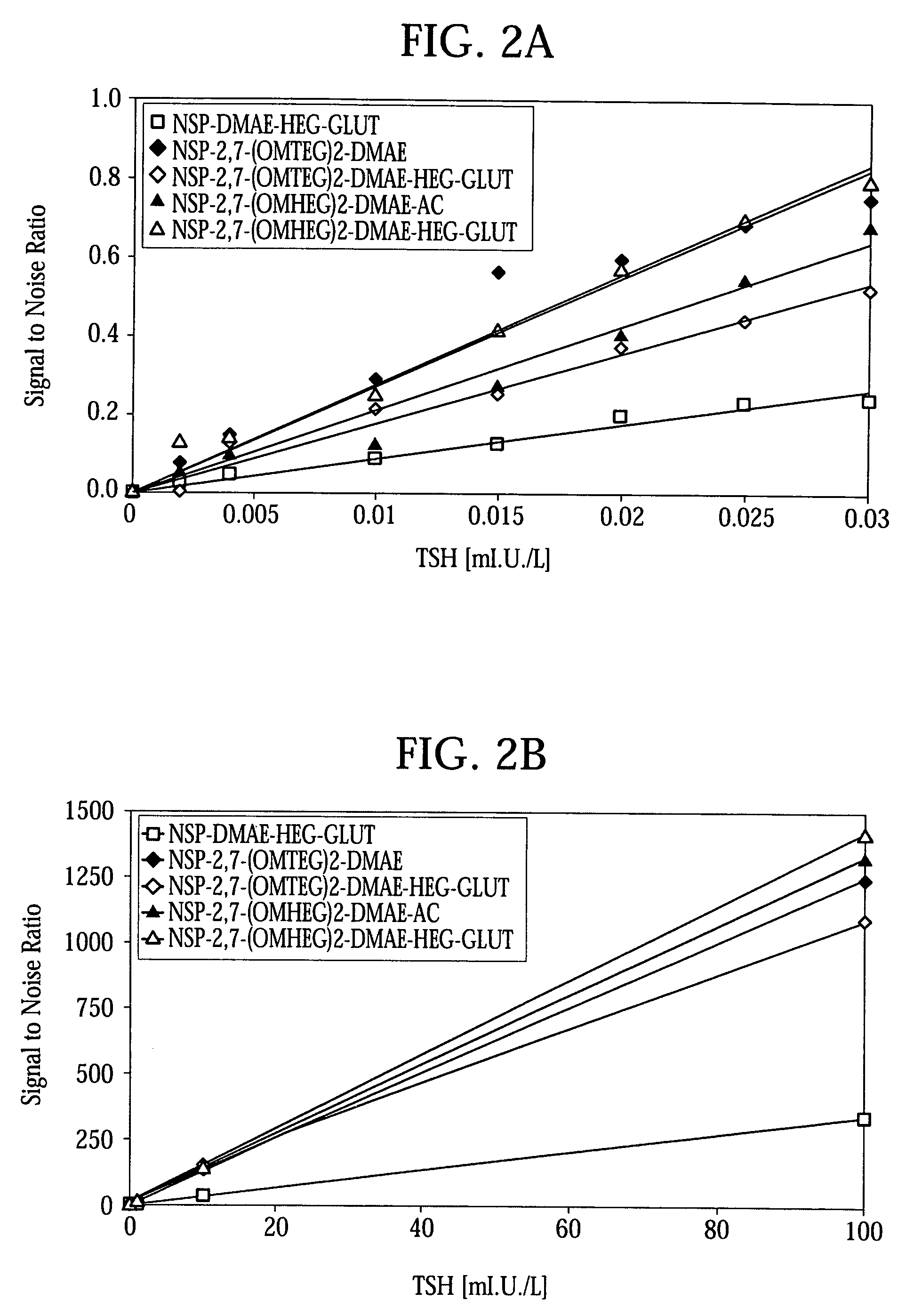
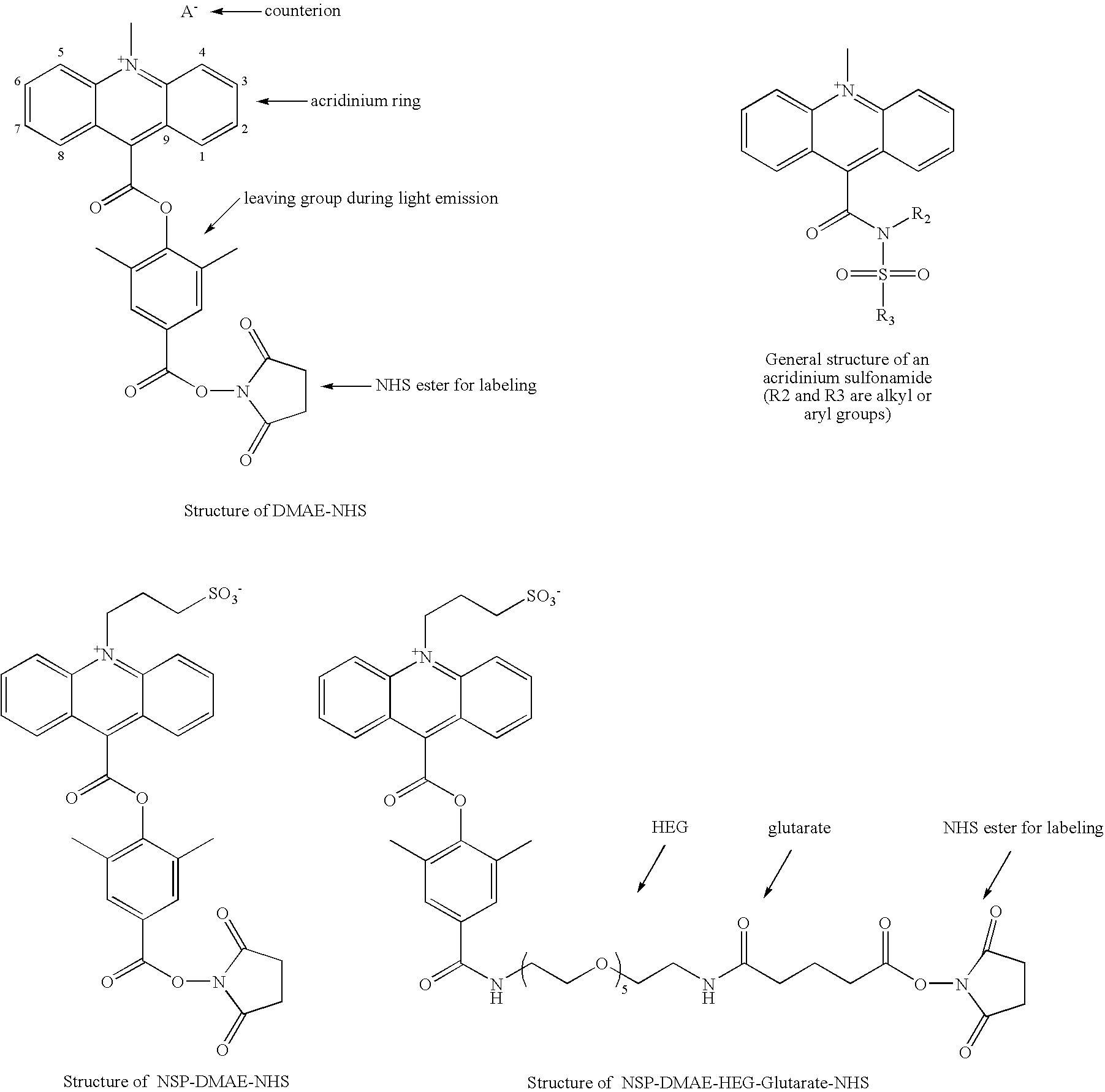
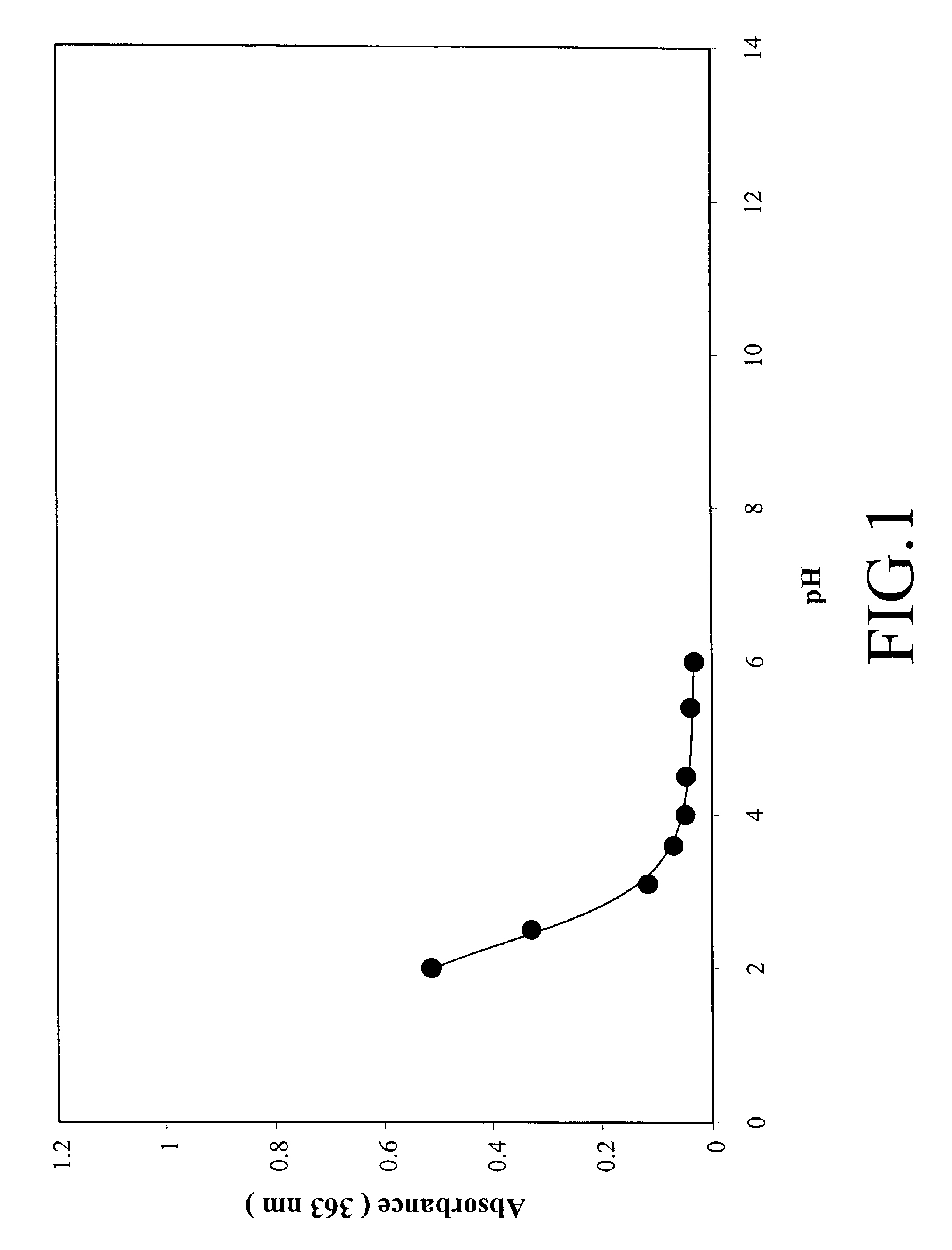
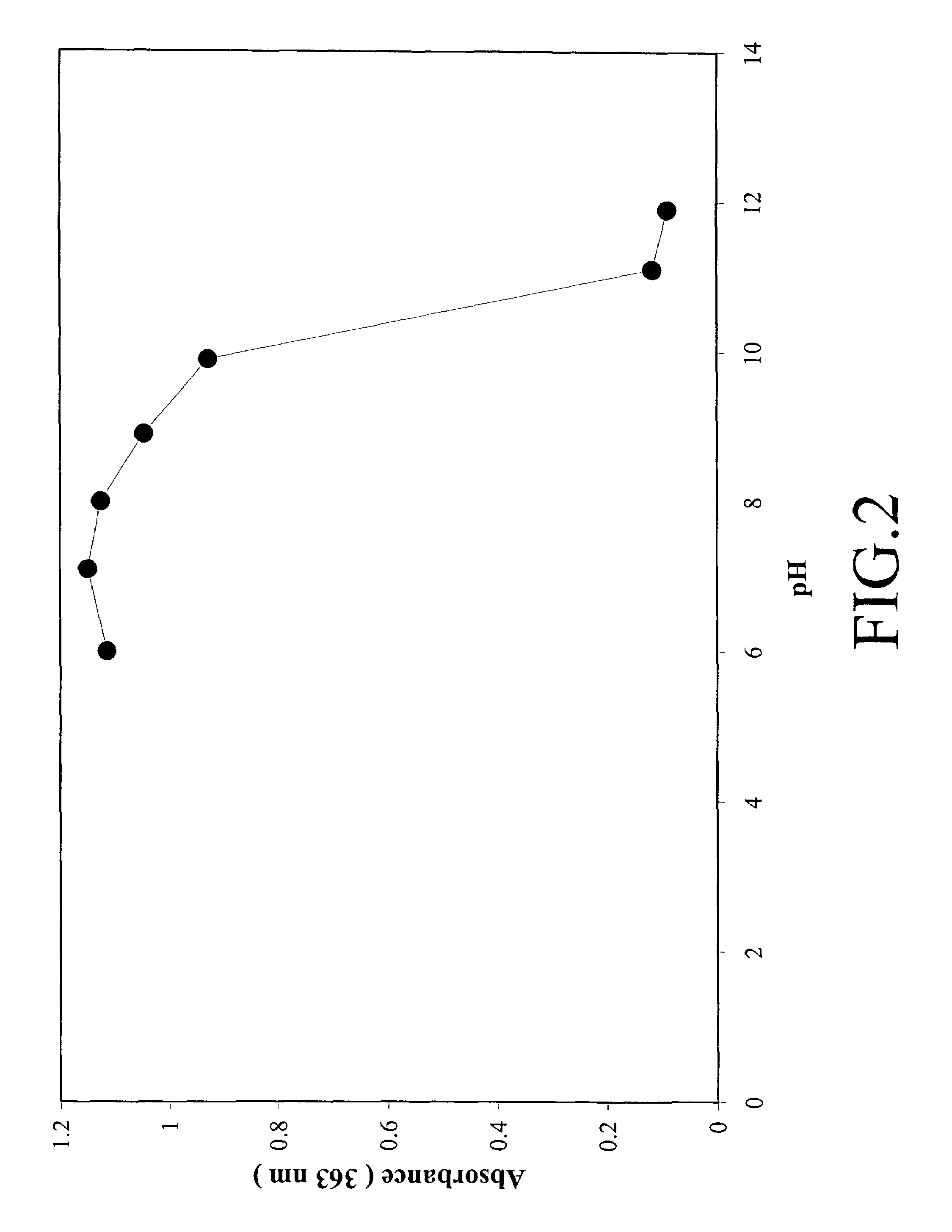
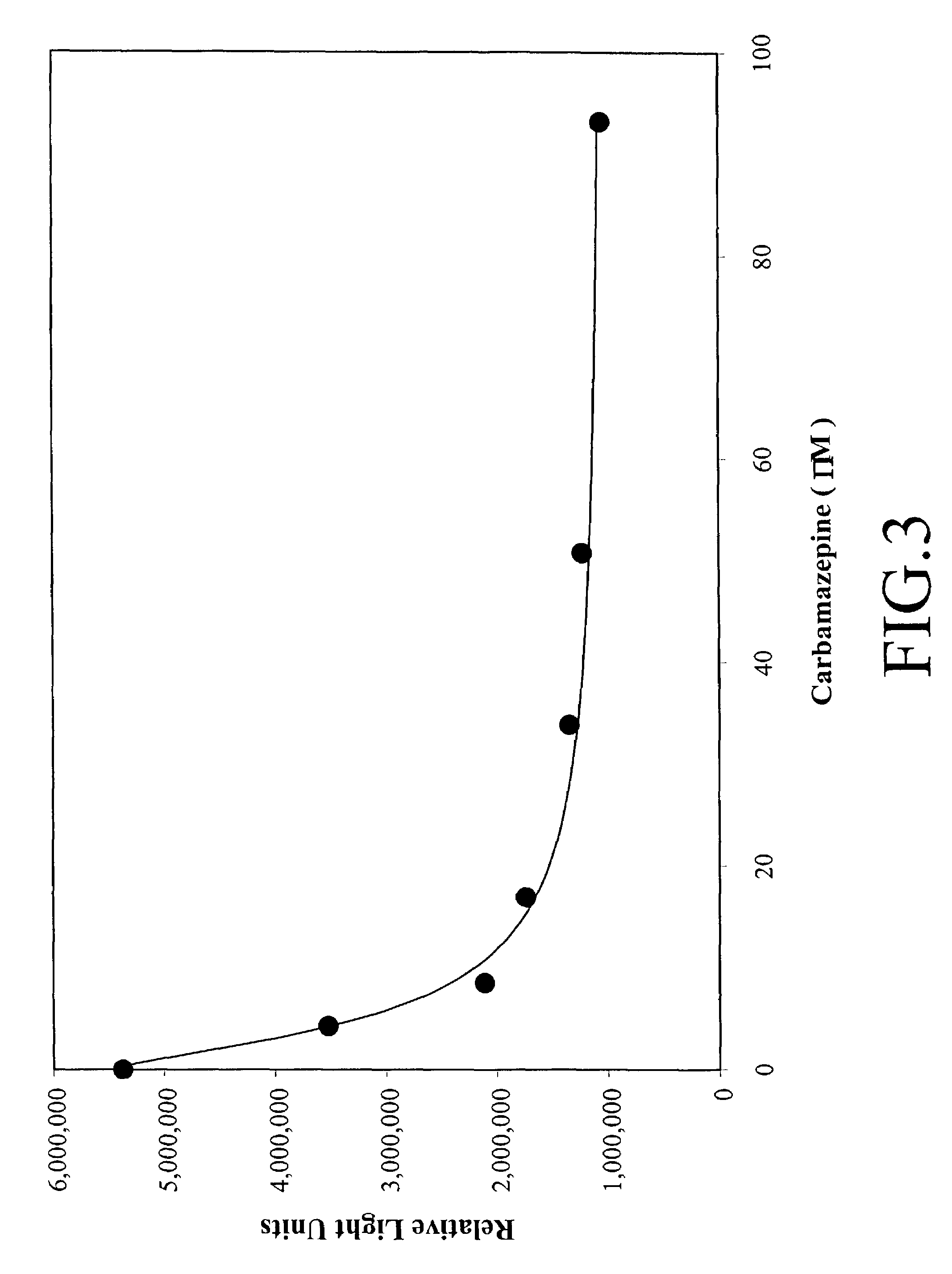
High quantum yield acridinium compounds and their uses in improving assay sensitivity
InactiveUS7309615B2Organic chemistryMaterial analysis by observing effect on chemical indicatorAcridineQuantum yield
The present invention relates to hydrophilic, high quantum yield acridinium compounds. It has been discovered that the placement of electron-donating groups in the acridinium ring system increases the amount of light that is emitted by the corresponding acridinium compound when its chemiluminescence is triggered by alkaline peroxide. More specifically, it has been found that the placement of one or two hydrophilic, alkoxy groups at the C-2 and / or C-7 position of the acridinium ring system of acridinium compounds increases their quantum yield and enhances the aqueous solubility of these compounds. The present hydrophilic, high quantum yield, acridinium compounds are useful chemiluminescent labels for improving the sensitivity of immunoassays.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
Applications of acridinium compounds and derivatives in homogeneous assays
ActiveUS7319041B2Organic chemistryMaterial analysis by observing effect on chemical indicatorAcridineAnalyte
Chemiluminescent acridinium compounds are used in homogeneous assays to determine the concentration of an analyte in a sample without strong acid or strong base treatment. The chemiluminescent acridinium compounds include acridinium esters with electron donating functional groups at the C2 and / or C7 position on the acridinium nucleus to inhibit pseudo-base formation, or acridinium sulfonamides with or without electron donating functional groups at the C2 and / or C7 position on the acridinium nucleus.
Owner:SIEMENS HEALTHCARE DIAGNOSTICS INC
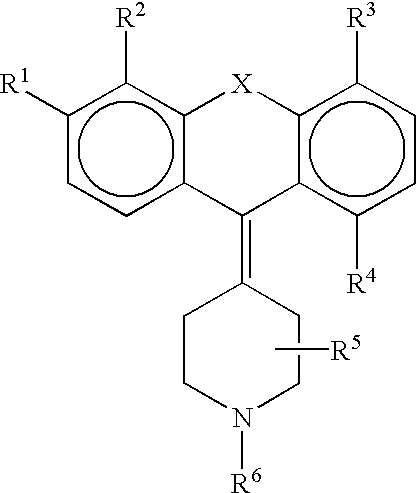
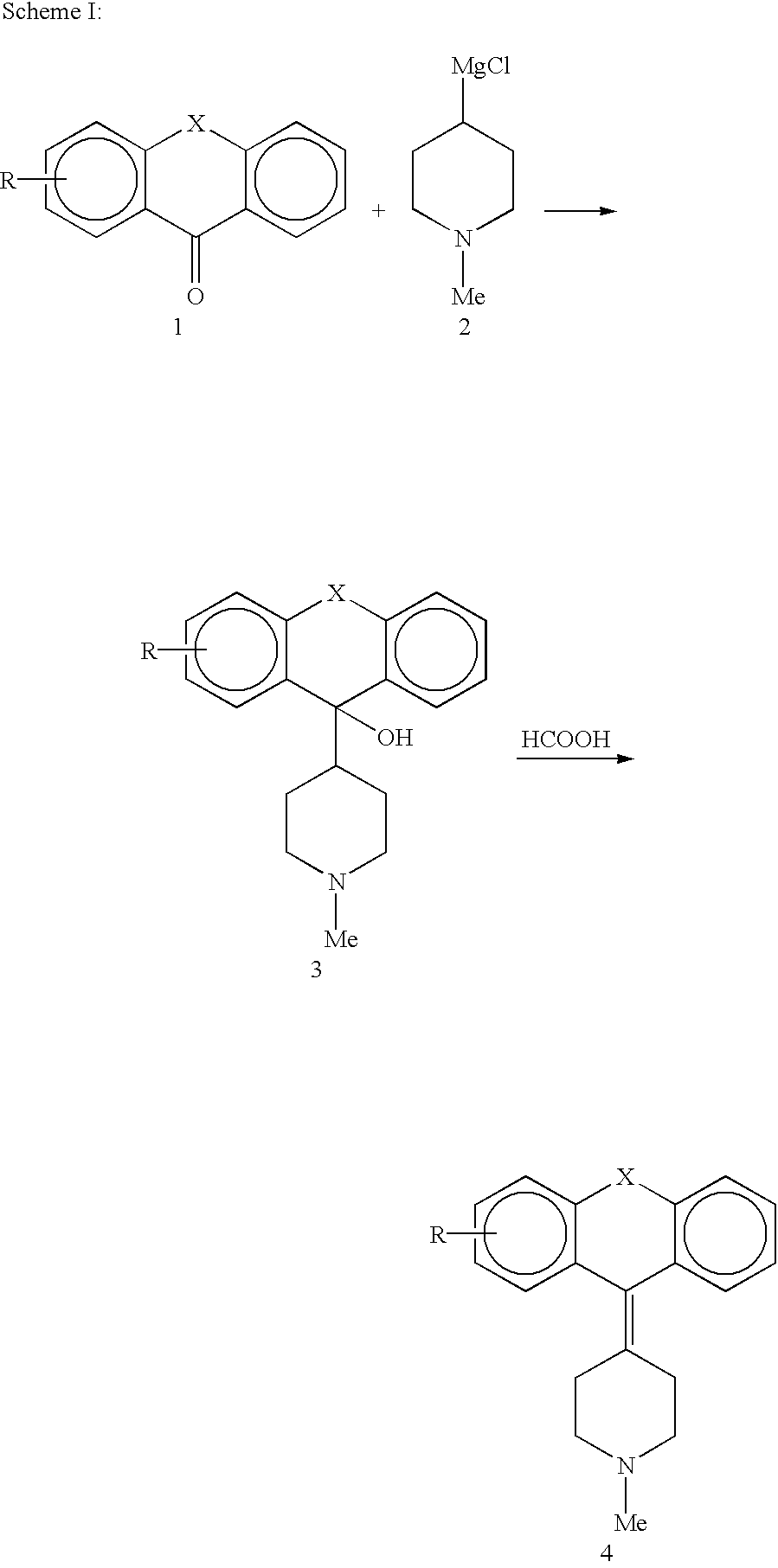
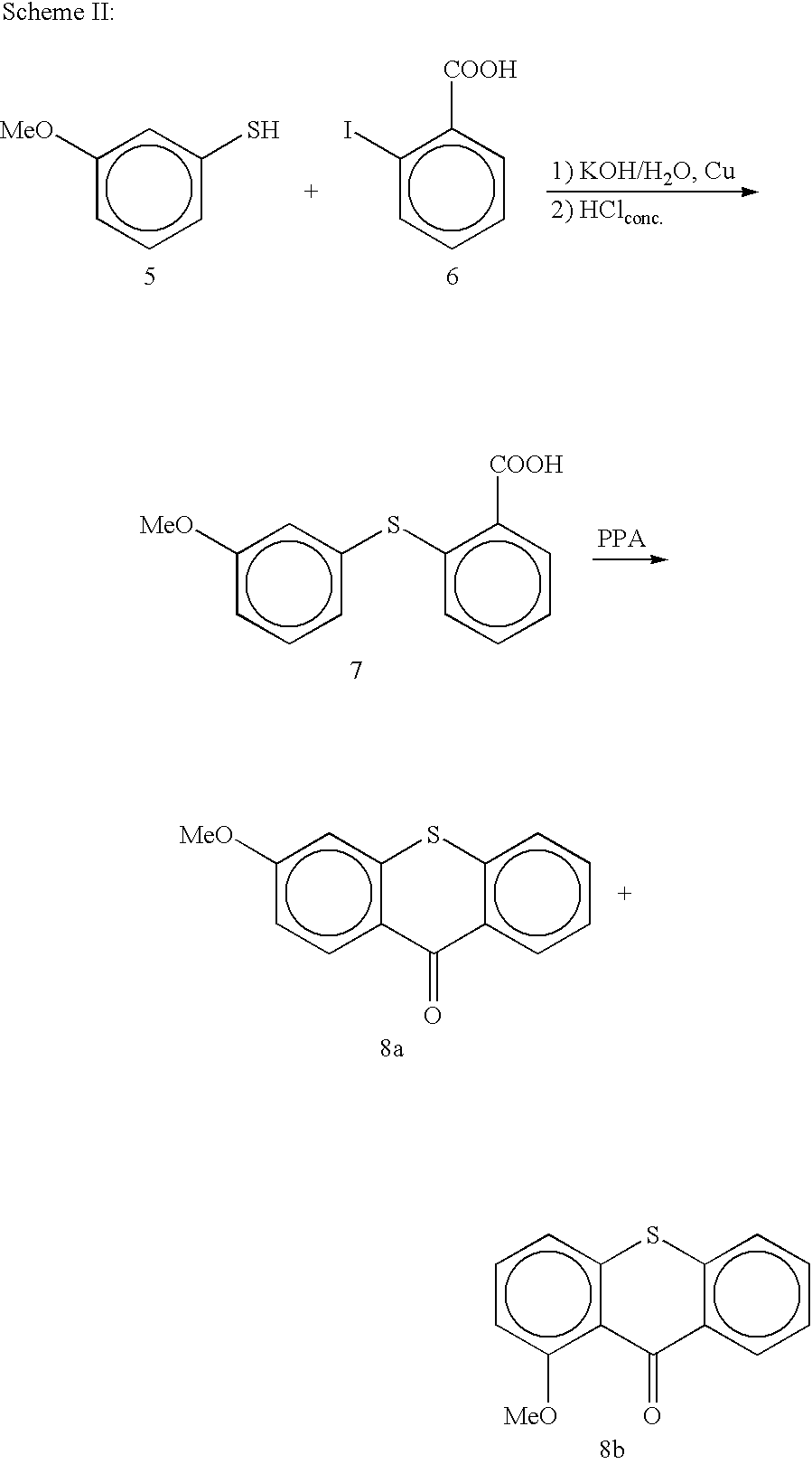
Derivatives of 4-(Thio- or Selenoxanthene-9-ylidene)-piperidine or acridine and its use as a selective 5-HT2B receptor antagonist
The present invention relates to derivatives of 4-(Thio- or Selenoxanthene-9-ylidene)-piperidine or acridine and pharmaceutically acceptable salts thereof, use of these compounds as a medicament and for the manufacture of a medicament for treatment of a disease state which can be alleviated by treatment with a 5-HT2B antagonist.
Owner:BIOFRONTERA BIOSCIENCE GMBH
Quinacridone pigment compositions comprising unsymmetrically substituted components
The present invention relates to a novel quinacridone pigment compositions, a process using a mixed amine synthesis for the ultimate production of the compositions and to their use as colorants for pigmenting high molecular weight organic materials.
Owner:CIBA SPECIALTY CHEM CORP
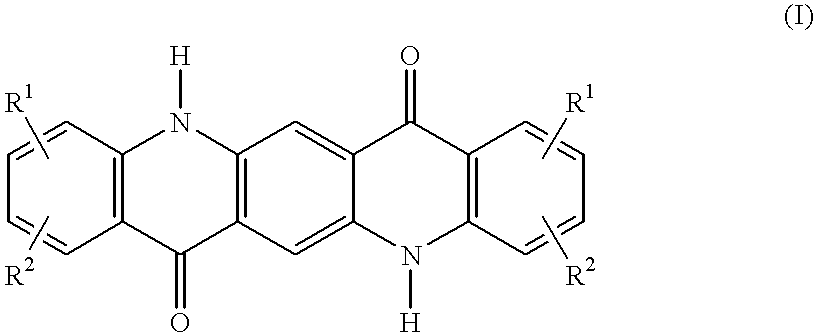
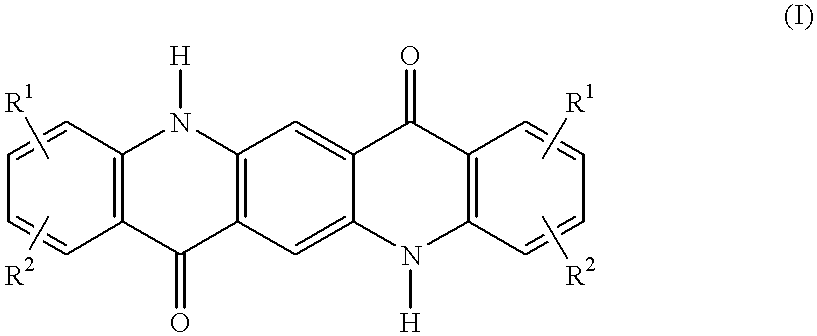
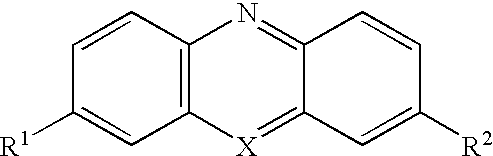
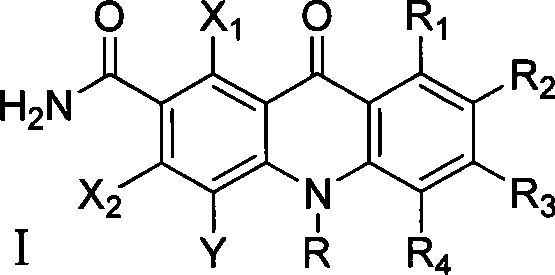
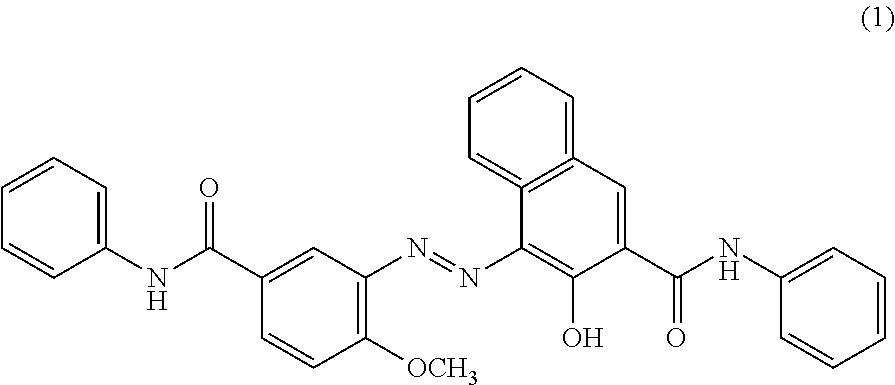
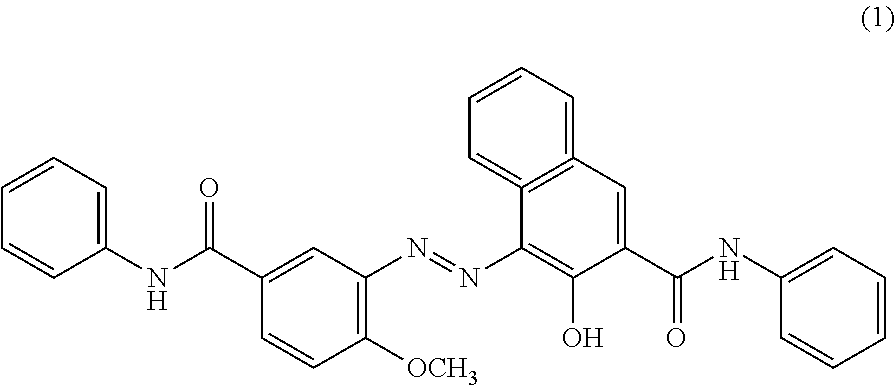
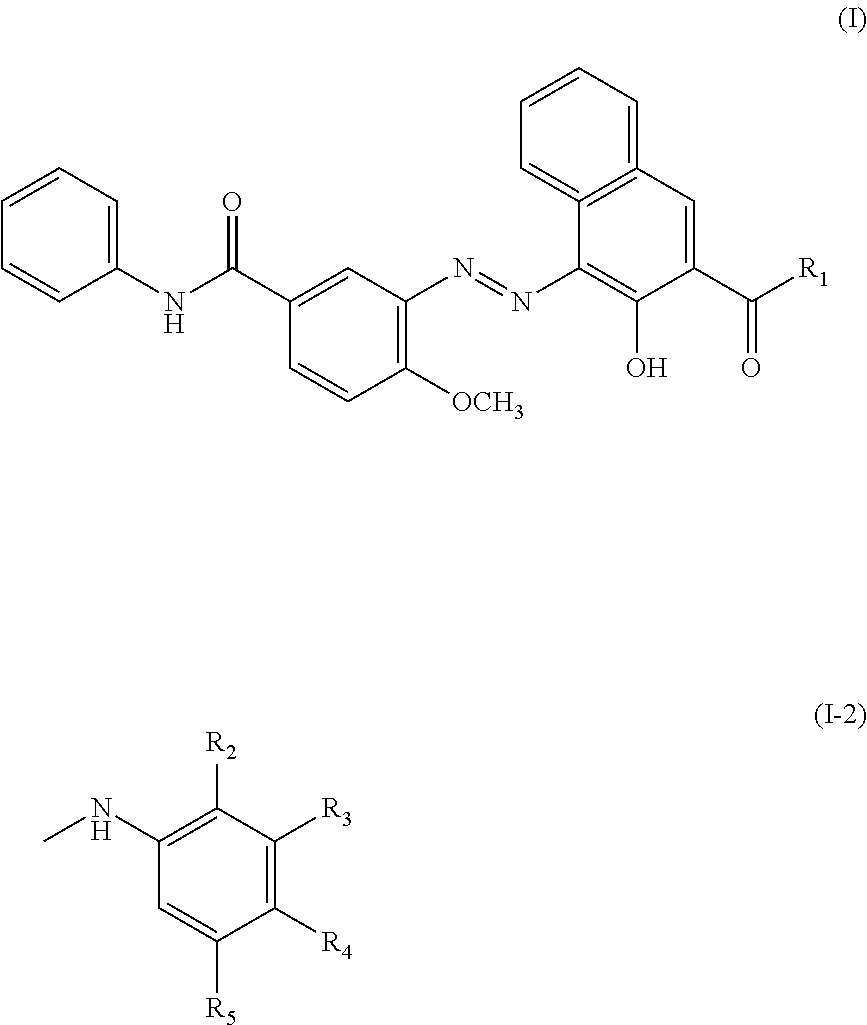
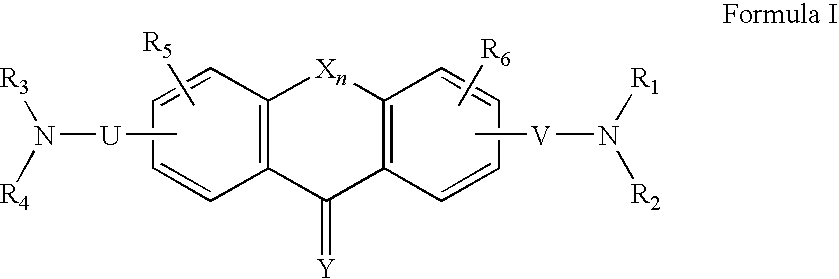
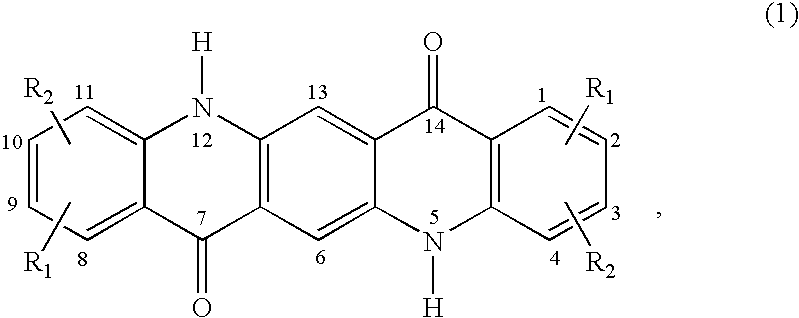
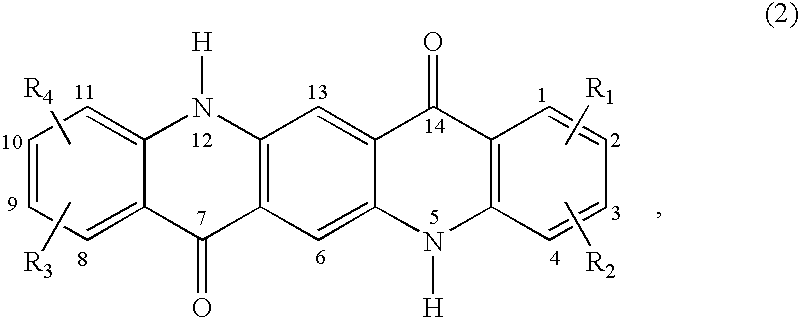
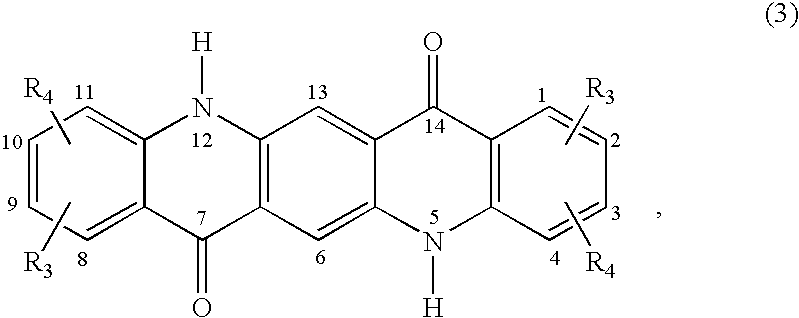
C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids
The present invention provides novel pyrrolo-[2,1-c][1,4]benzodiazepine hybrids useful as anti-tumour agents and a process for the preparation thereof.
Owner:COUNCIL OF SCI & IND RES
Method for analyzing acridine ester labeled antigen or acridine ester labeled antibody
InactiveCN101609090AReduce consumptionProduct quality and safetyChemiluminescene/bioluminescenceBiological testingChemical structureAntigen
The invention relates to a chemiluminescent immunoassay method, in particular to a method for analyzing an acridine ester labeled antigen or an acridine ester labeled antibody and an immunoassay kit prepared by same. An acridine ester label is provided with a special luminescent group in a chemical structure, and the special group directly participates in luminescent reaction in the analyzing process of luminescent immunization; the substance does not have background luminescence usually, can be used for detecting a sample with low concentration or trace concentration in the reaction and is a luminescent agent with high luminescent efficiency; and molecules of acridine ester I and acridine ester II and acridine amide III can be combined with an antibody (antigen) to generate the labeled antibody with high chemiluminescent activity and immunological reaction specificity.
Owner:BEIJING ELCOTEQ BIO TECH
Method for production of polymer-encapsulated pigments
The invention relates to a method for production of an aqueous dispersion of polymer-encapsulated pigments, characterised in that (a) an aqueous pigment dispersion, containing at least one organic pigment (P), selected from the group of azo, isoindolinone, isoindoline, anthanthrone, thioindigo, thiazinindigo, triarylcarbonium, quinophthalone, anthraquinone, dioxazine, phthalocyanine, quinacridone, quinacridonquinone, indanthrone, perylene, perinone, pyranthrone, diketopyrrolopyrrole, isoviolanthrone and azomethine pigments, at least one detergent (T), and water is prepared, (b) a monomer miniemulsion, stabilised by a hydrophobic organic compound with a water solubility at 20 DEG C of at most 5x10<-5> g / l, is prepared from a polymerisable monomer (M) and at least one detergent (T) in water, (c) a monomer pigment emulsion is prepared, whereby the aqueous dispersion from (a) and the monomer miniemulsion from (b) are mixed and homogenised and (d) the pigment-containing monomer from (c) is polymerised in the presence of a polymerisation initiator and / or by heat, whereupon an encapsulation of the pigment with the polymer thus formed occurs.
Owner:CLARIANT PROD DEUT GMBH
N-benzyl-acridone, derivatives of N-benzyl-acridone and preparation methods and application of N-benzyl-acridone and derivatives
ActiveCN102146056AGrowth inhibitionHigh activityOrganic active ingredientsOrganic chemistryCancer cellAcridone
The invention discloses N-benzyl-acridone, derivatives of the N-benzyl-acridone and preparation methods and application of the N-benzyl-acridone and the derivatives. A structural formula of a compound is shown as a formula (I), wherein R1, R2, R3, R4 and R5 are all randomly selected from the following substituent groups: H, OH, NH2, Cl, F, Br, I, CN, NO2, CH3, OCH2Ph and O(CH2)nCH3; n in O(CH2)nCH3 is an integer in the range of 0 to 3; Z is randomly selected from the following substituent groups: CH2 and SO2; A is randomly selected form the following substituent groups: S, O and NH; R6, R7, R8, R9, R10, R11, R12 and R13 are all randomly selected from the following substituent groups: H, OH, NH2, Cl, F, Br, I, CN, NO2, CH3 and the following a and b; necessarily, only one substituent group of the R6, the R7, the R8, the R9, the R10, the R11, the R12 and the R13 is a or b; a represents NHCO(CH2)nR or CONH(CH2)nR, wherein n is an integer in the range of 0 to 3 and R represents tertiary amine; and b represents NHCOBNH2 or CONHBNH2, wherein B is an alkyl connection chain or an alkyl connection chain substituted heteroatom. A cell proliferation inhibition test result shows that the compound in the formula I can better inhibit cancer cell CCRF-CEM from growing, wherein part of compounds show higher activities than a parent compound (10-(3,5-dimethoxyphenyl) acridone). (the formula I).
Owner:SHENZHEN GRADUATE SCHOOL TSINGHUA UNIV
Use of mixed-crystal pigments of the quinacridone series in electrophotographic toners and developers, powder coatings and inkjet inks
The use of mixed-crystal pigments of the quinacridone series consisting ofa) from 85 to 99% by weight of unsubstituted beta-phase quinacridone of the formula (I)in which R1 and R2 are hydrogen atoms, andb) from 1 to 15% by weight of one or more substituted quinacridones of the formula (I) in which the substituents R1 and R2 are identical or different and are chlorine, bromine or fluorine atoms orC1-C4-alkyl, C1-C4-alkoxy or carboxamido groups, which can be substituted by C1-C6-alkyl groups, and R1 can additionally be hydrogen,as colorants in electrophotographic toners and developers, powder coating materials, inkjet inks, electret fibers, and color filters.
Owner:CLARIANT PROD DEUT GMBH
Redox-switchable materials
InactiveUS7435362B2Improve efficiencyDiffusing elementsNanoinformaticsQuinoneSemiconductor materials
The invention relates to redox-switchable material comprising a redox-active moiety adsorbed, bonded or both, to a semiconductor material. Among the preferred redox-active moieties disclosed herein are ferrocenes, acridines, and quinones though any such moiety may be employed. The redox-switchable material of this invention may be used to selectively remove one or more selected solutes from an aqueous solution wherein adsorption / complexation of the solute is influenced by the oxidation state of the redox-active moiety. In an alternative embodiment, inclusion moieties that are covalently bound to the redox-active moiety are employed to achieve selective complexation of the desired solute. Other possible applications of the disclosed materials are photoerasable writing media, electrochromic or photochromic materials, catalysis, and solar energy storage.
Owner:BOARD OF RGT NEVADA SYST OF HIGHER EDUCATION ON BEHALF OF THE UNIV OF NEVADA RENO
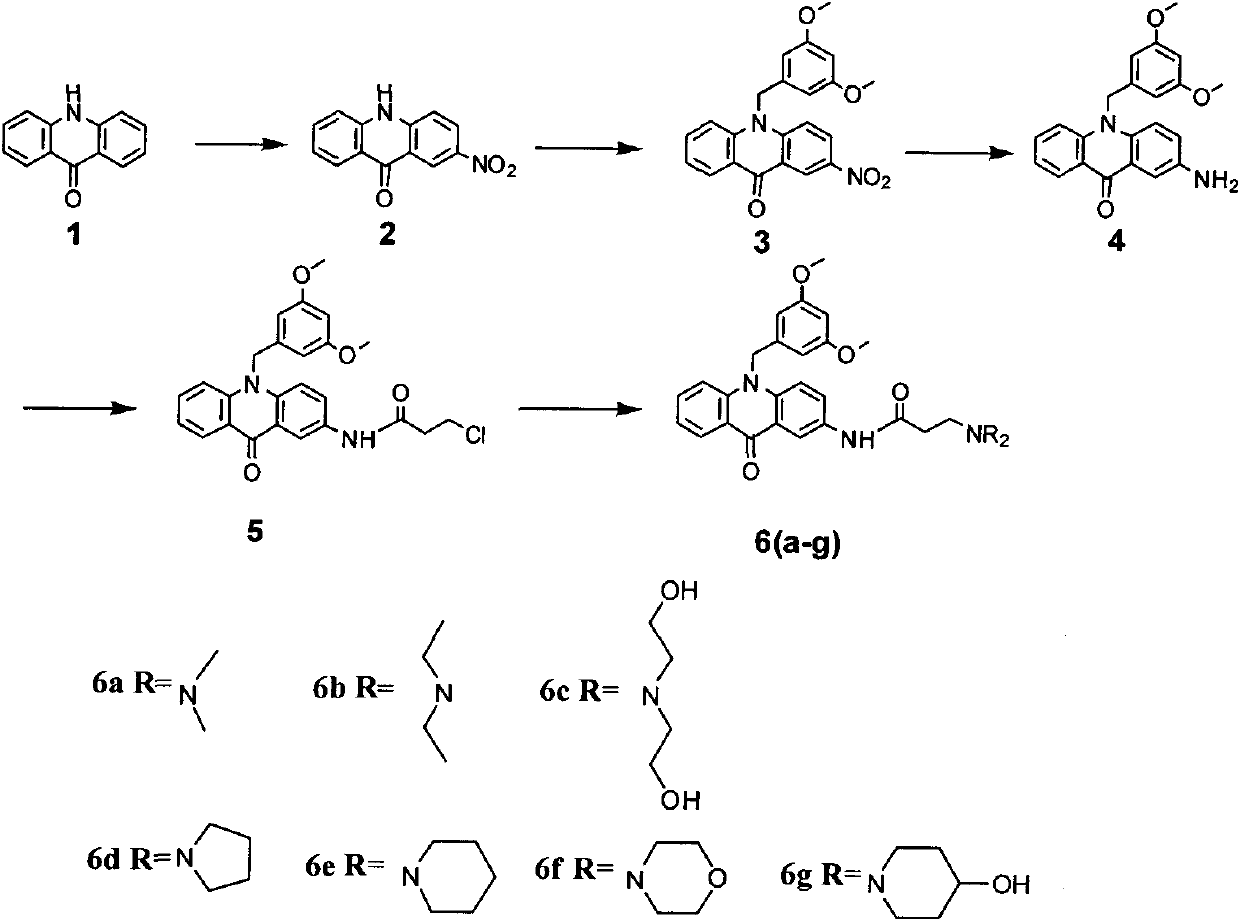
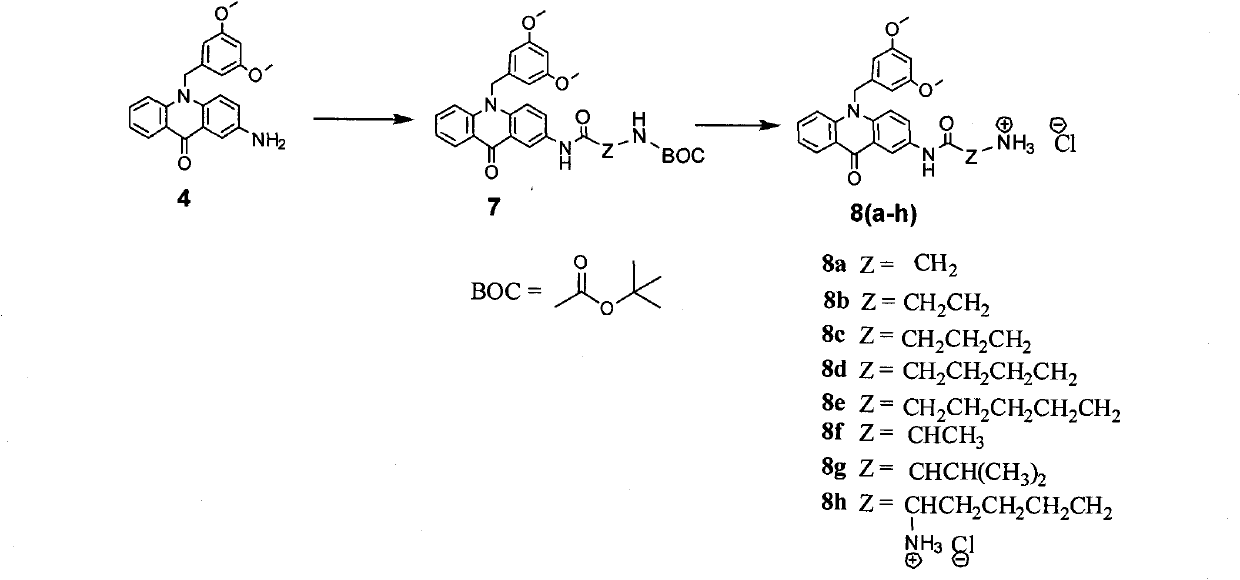
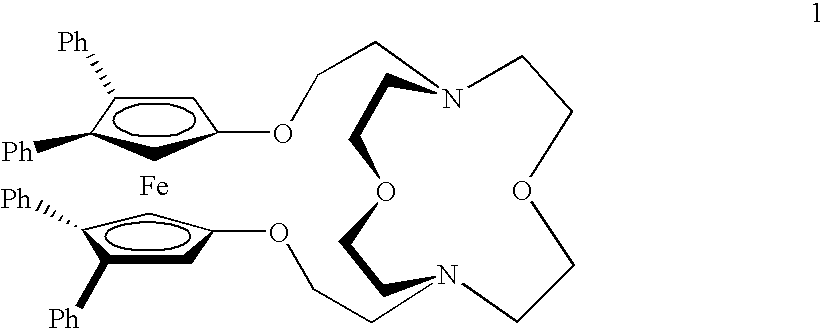
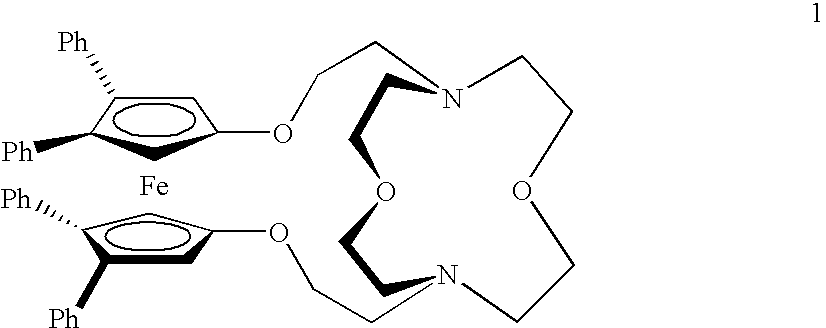
Acridine derivatives and their use as medicaments
The invention relates to novel acridine derivatives of the formula 1, to their preparation and to their use as medicaments, in particular for the treatment of tumors.
Owner:ZENTARIS GMBH
Chemiluminescence immunity analysis detecting myocardium calcium protein T hypersensitization method for acridine ester and alkaline phosphatase
InactiveCN101226200AChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexCalcium protein
The invention relates to a chemical illumination immunity analysis method for checking human cTnT, which uses acridiniumester and / or alkaline phosphatase as label. The immunity reaction uses two-site immunoassay and / or competition law. The immunity reaction can use streptavidin-biotin two-site immunoassay to improve sensitivity. The invention uses NaOH and H2O2 as acridiniumester illumination initiating agent, uses 1, 2-dioxo cyclohexane derivative (adamantine derivative) as the illumination substrate of alkaline phosphatase, and uses fluorescein derivative and surface activator as illumination renforcing agent. The solid carrier is orifice plate, macromolecule polymer tube (ball) and ferriferrous oxide magnetic particles or the like.
Owner:天津天美生物技术有限公司
Oxidative dye composition and indicator
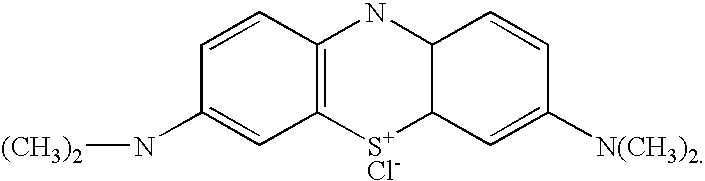
ActiveUS20070054412A1Material analysis by observing effect on chemical indicatorMicrobiological testing/measurementAcridineAlkaline earth metal
An oxidative dye solution comprises an indicating dye that has been reacted with a reducing agent. The pre-reacted dye when subjected to an oxidizing disinfection or sterilization agent such as peracetic acid or hydrogen peroxide under goes a visible color change and thus can serve as a chemical, process, or chemical integrator, indicator. The dyes include various azines, thiazines, and oxazines compounds and the reducing agents include alkali metal alkyloxides and alkaline earth metal alkyloxide compounds.
Owner:AMERICAN STERILIZER CO
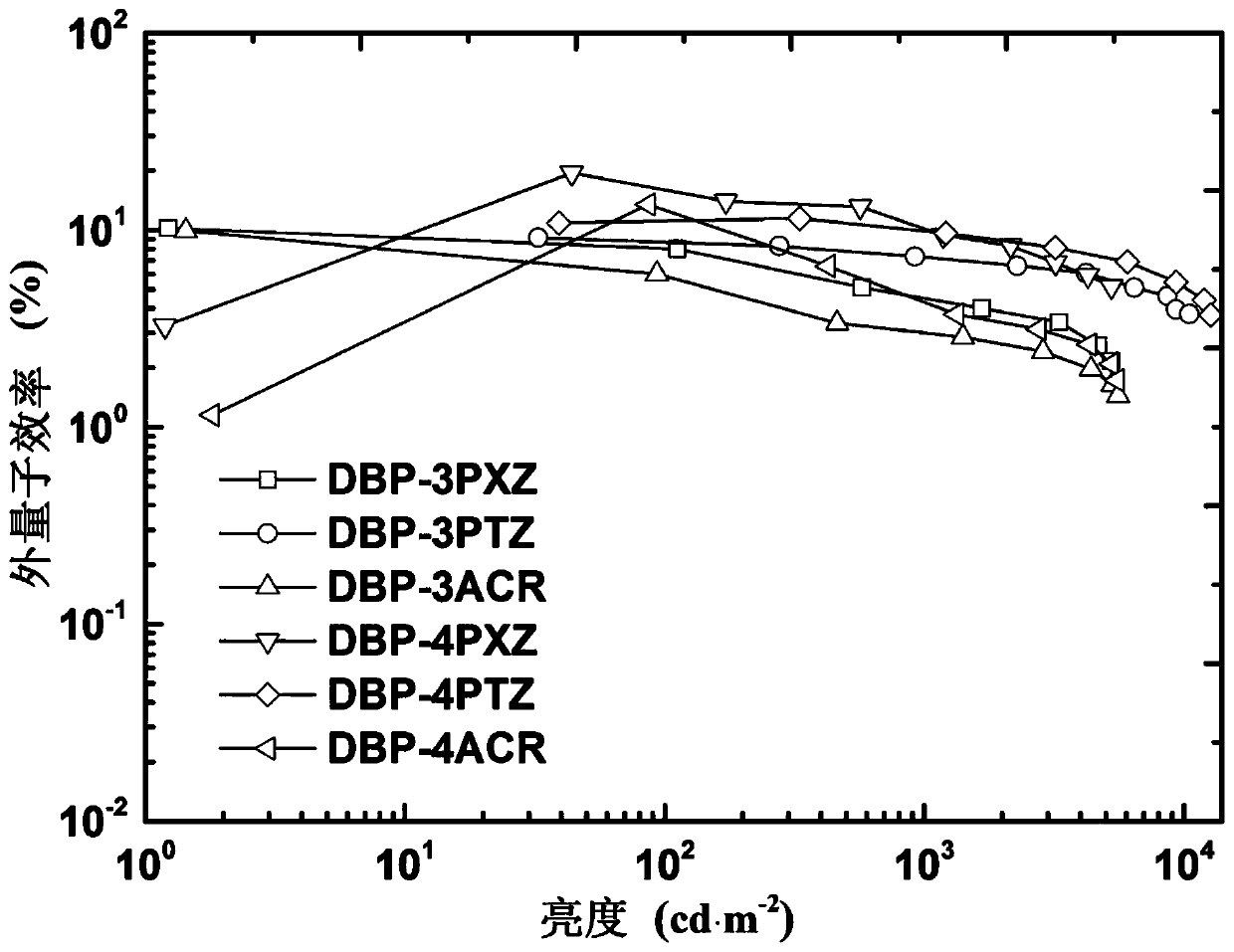
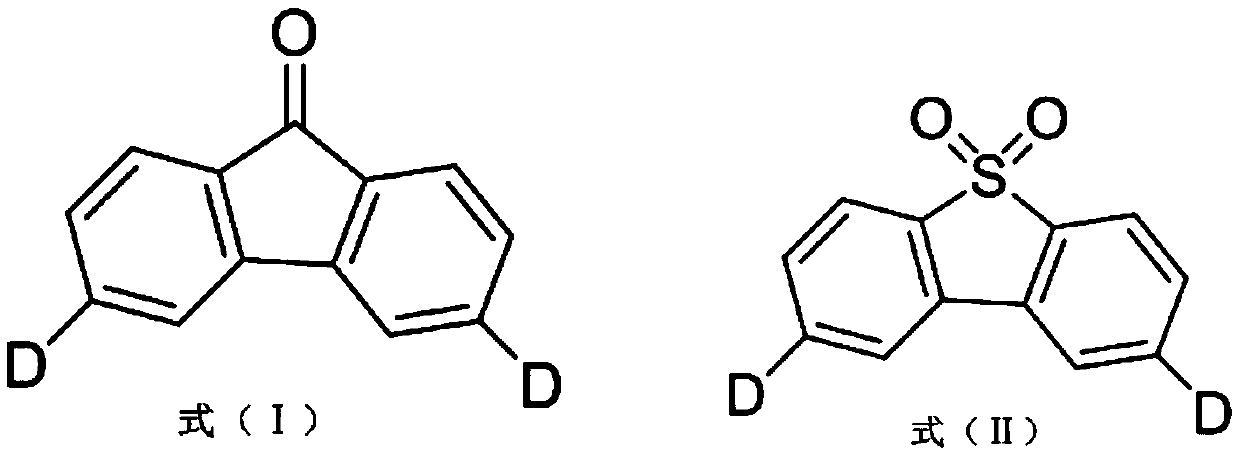
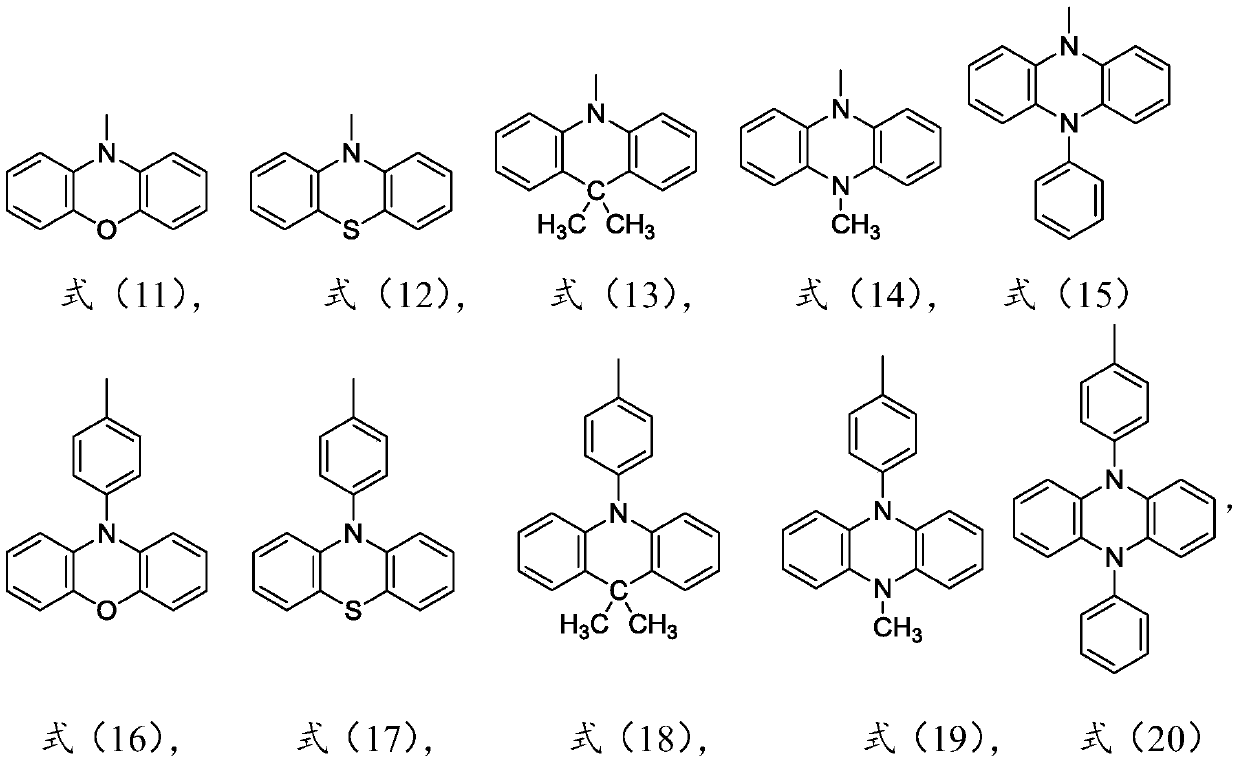
Thermal activation delayed fluorescence material based on dibenzophenazine derivative as well as preparation method and application of thermal activation delayed fluorescence material
ActiveCN111574514AEfficient use ofEfficient transferOrganic chemistrySolid-state devicesQuantum yieldElectron donor
The invention discloses a thermal activation delayed fluorescence material based on a dibenzophenazine derivative as well as a preparation method and application of the thermal activation delayed fluorescence material, and belongs to the technical field of organic photoelectric materials and devices. The structural formula of the material is shown as a formula (I), in the formula (I), R1 is phenoxazinyl, phenothiazinyl or dimethyl acridinyl, R1' is phenoxazinyl, phenothiazinyl or dimethyl acridinyl, R1 '' is phenoxazinyl, phenothiazinyl or dimethyl acridinyl, and R2 is H, phenoxazinyl, phenothiazinyl or dimethyl acridinyl. The material provided by the invention has the advantages that the electron acceptor and the electron donor unit show a highly distorted rigid structure, 100% theoretical internal quantum efficiency can be realized, the fluorescence quantum yield is high, a guarantee is provided for a high-efficiency long-waveband OLED device, and the like. And a polysubstitution mode is adopted, so that red shift of molecular emission wavelength is facilitated, long-waveband luminescence is realized, and a guarantee is provided for an efficient long-waveband OLED device.
Owner:山西穿越光电科技有限责任公司
Organic photoelectric material and organic electroluminescent device containing material
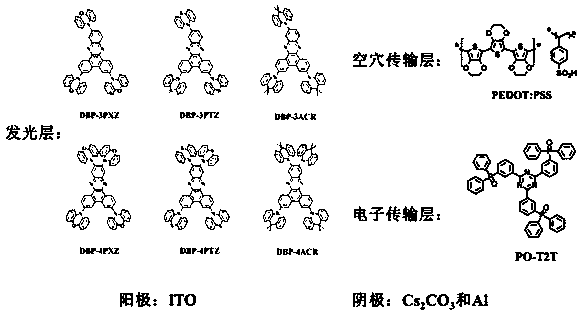
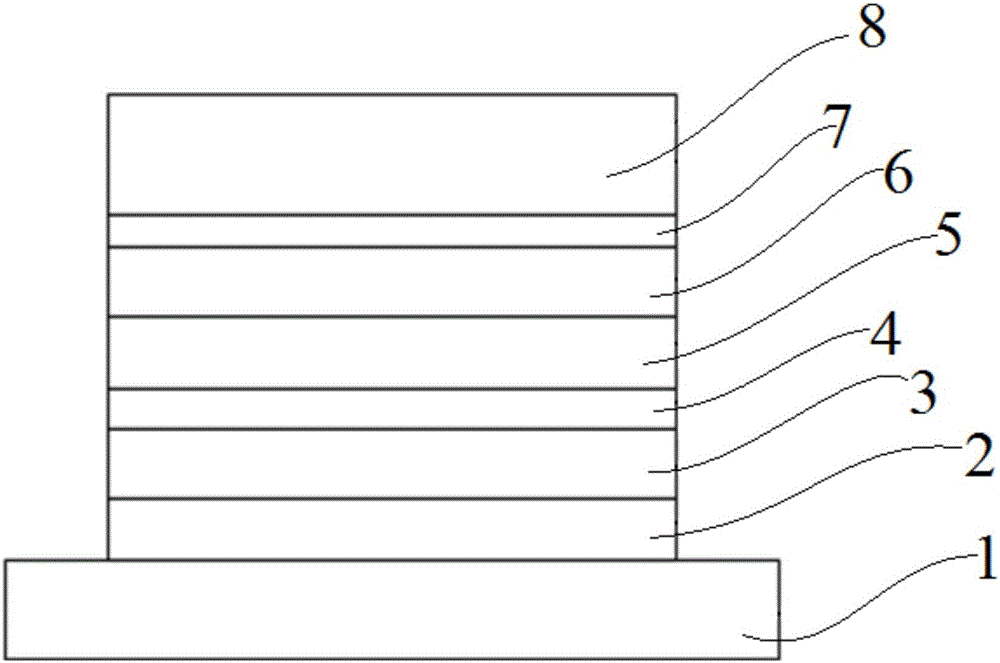
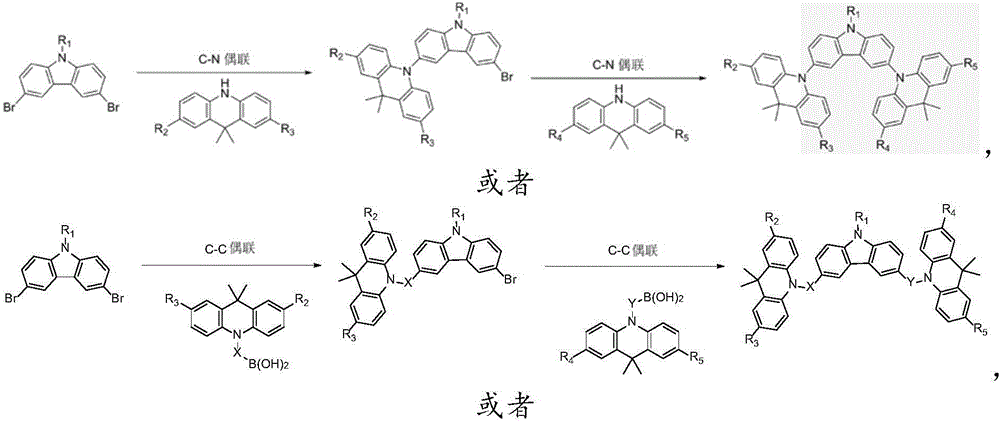
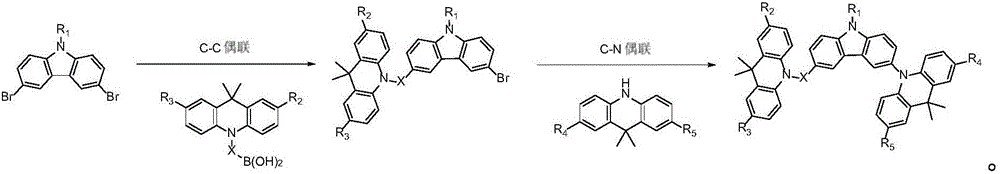
ActiveCN105801569AImprove thermal stabilityHigh glass transition temperatureOrganic chemistrySolid-state devicesAcridineCarbazole
The invention discloses an organic photoelectric material.The general structural formula of the organic photoelectric material is as shown in the formula (I), wherein R1 is selected from a phenyl group, C7-14 alkyl phenyl groups, C10-60 polycyclic conjugate aromatic groups and aromatic heterocyclic radicals at least containing one of N, S and O; carbazole and acridine structural units of a matrix structure are connected through X and Y, wherein X and Y are both independently selected from a C-N single bond, phenylene, biphenylene, C6-24 polycyclic conjugate arylidene and aromatic heterocyclic radicals at least containing one of N and O, and R2, R3, R4 and R5 are independently selected from a hydrogen group, a halogen group, a cyano group, a nitro group, C1-10 alkyl groups, substituted or unsubstituted C1-12 alkoxyl groups, a phenyl group, C7-14 alkyl phenyl or C7-14 alkyl methoxyphenyl.
Owner:VALIANT CO LTD
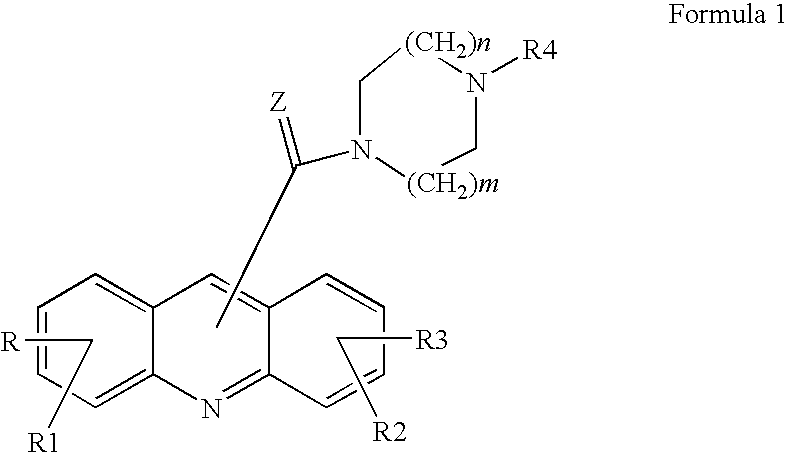
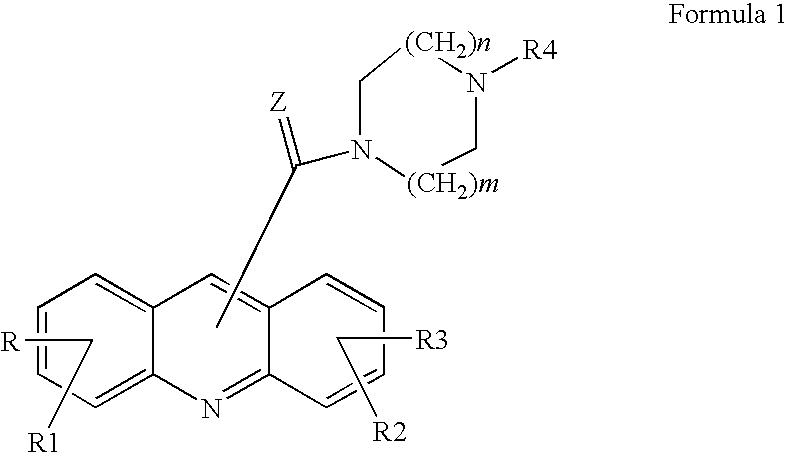
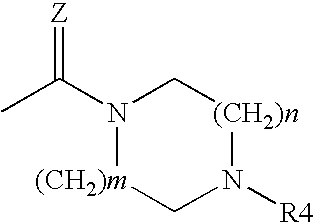
Acridine derivatives, and preparation method and application thereof
ActiveCN104650032AExcellent electron transport materialGood electronic acceptanceOrganic chemistrySolid-state devicesAcridineElectronic transmission
The invention relates to acridine derivatives of which the structure is disclosed as Formula (1). When the electronic transmission layer of the electroluminescent device is prepared from the material provided by the invention, the turn-on voltage of the device can be lowered, the luminescence efficiency of the device can be enhanced, and the service life of the device can be prolonged.
Owner:BEIJING ETERNAL MATERIAL TECH +1
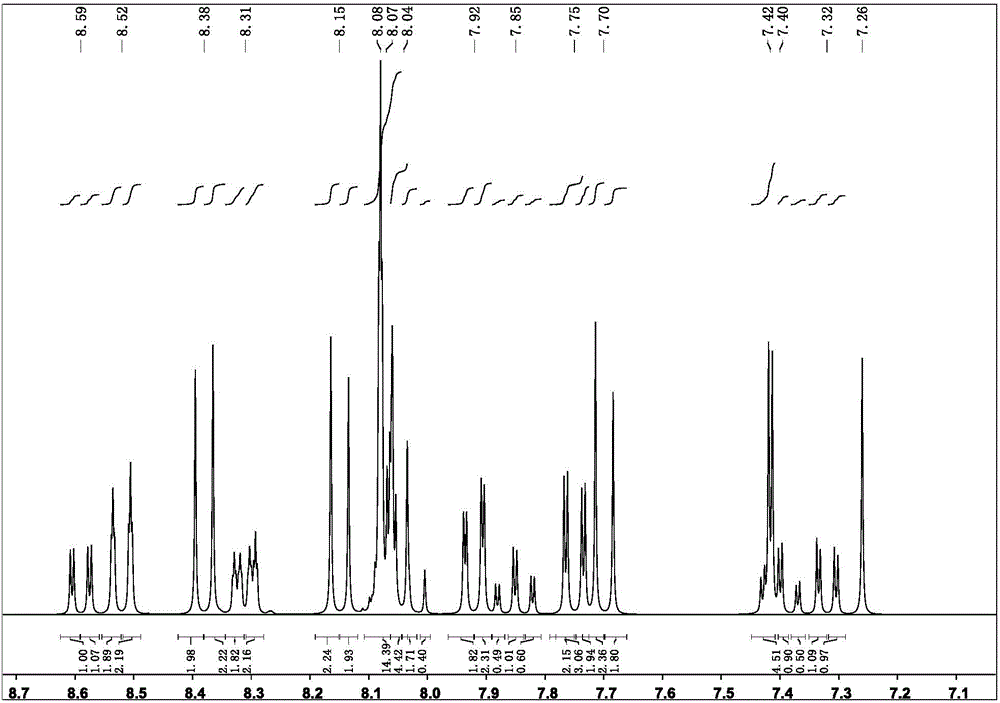
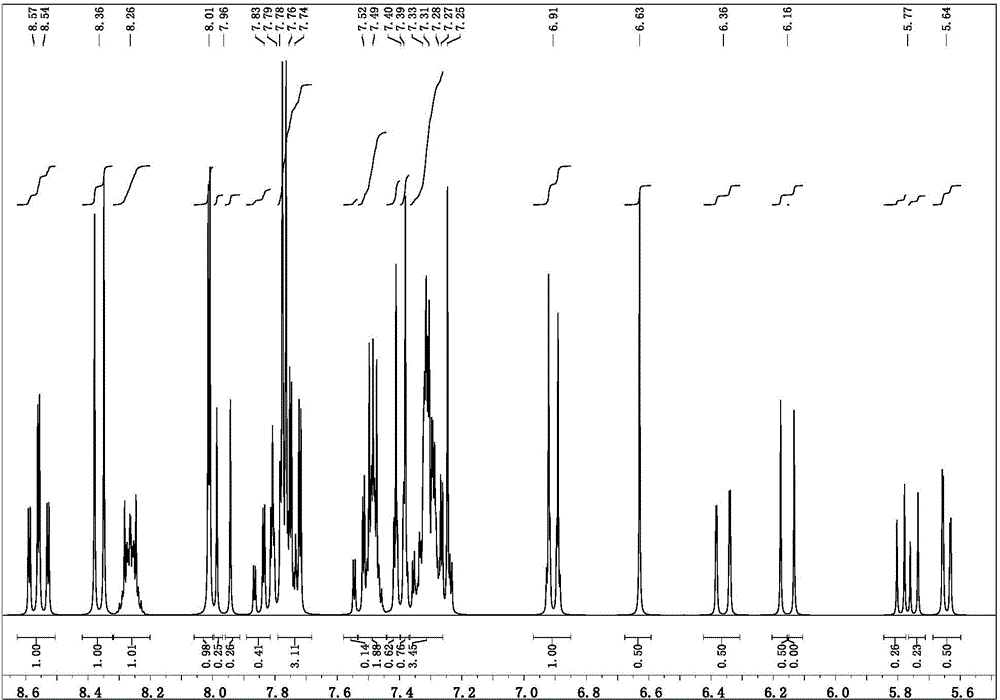
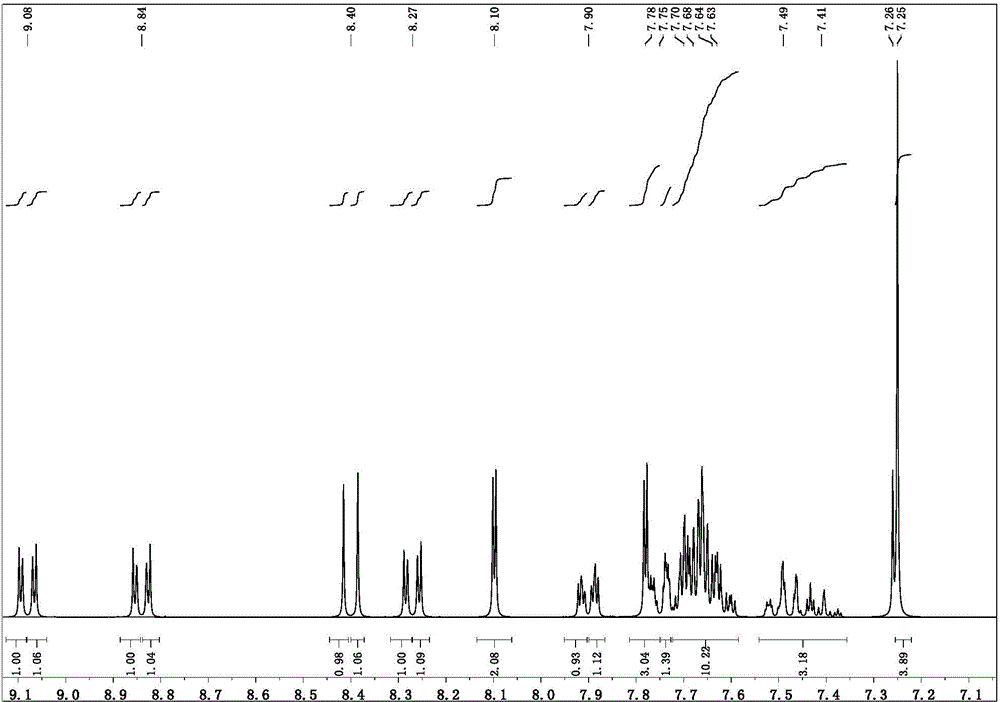
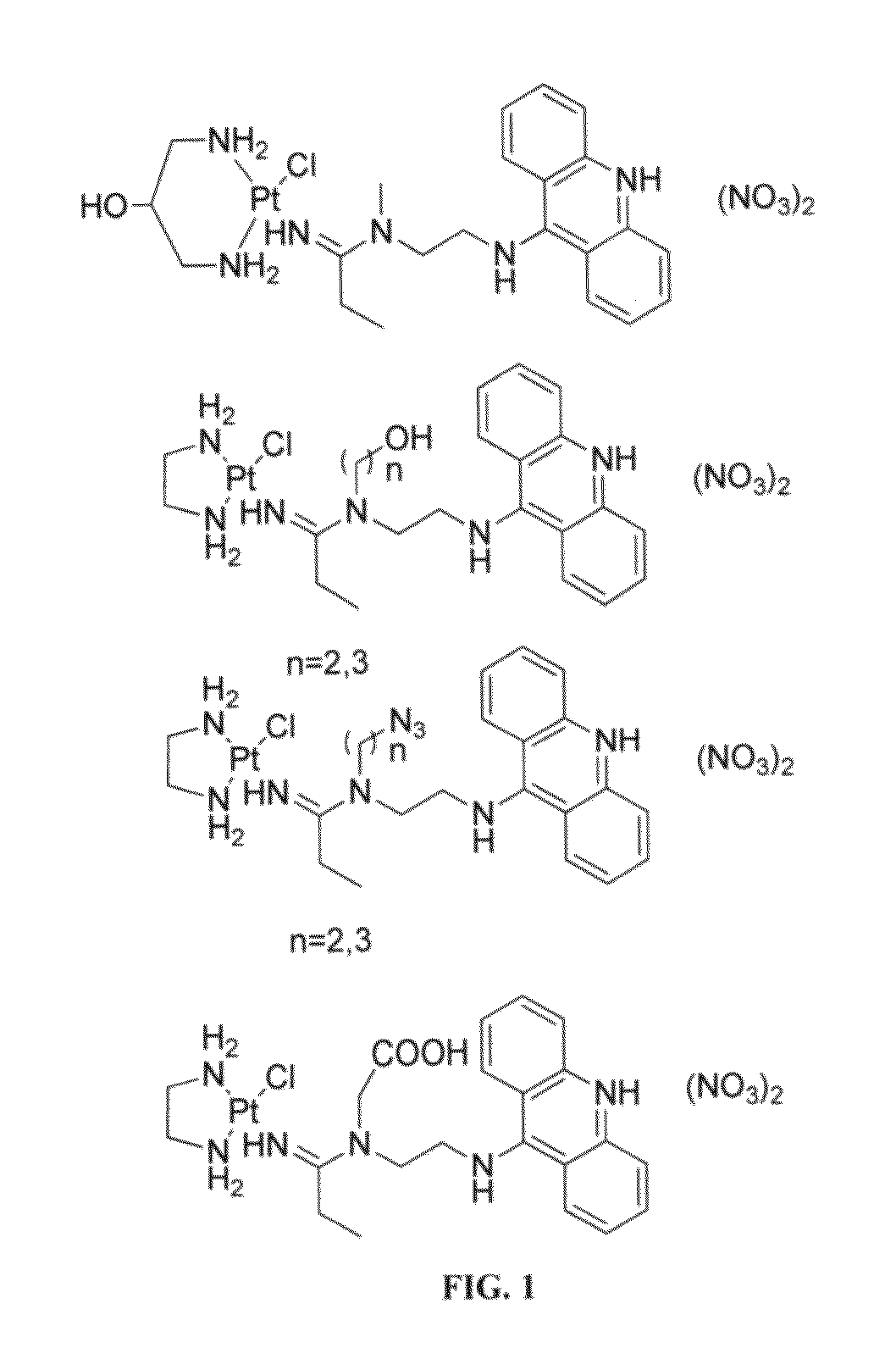
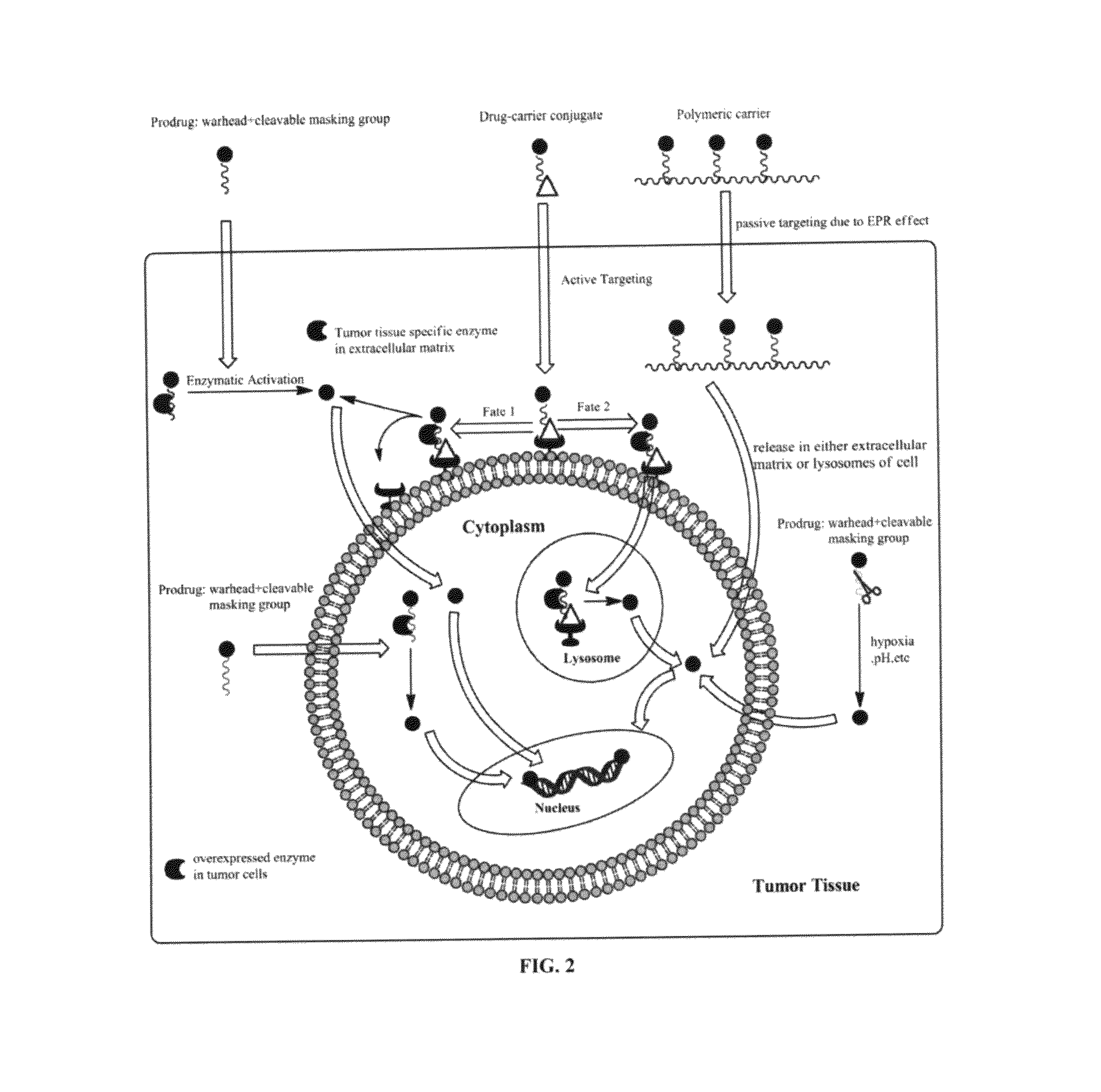
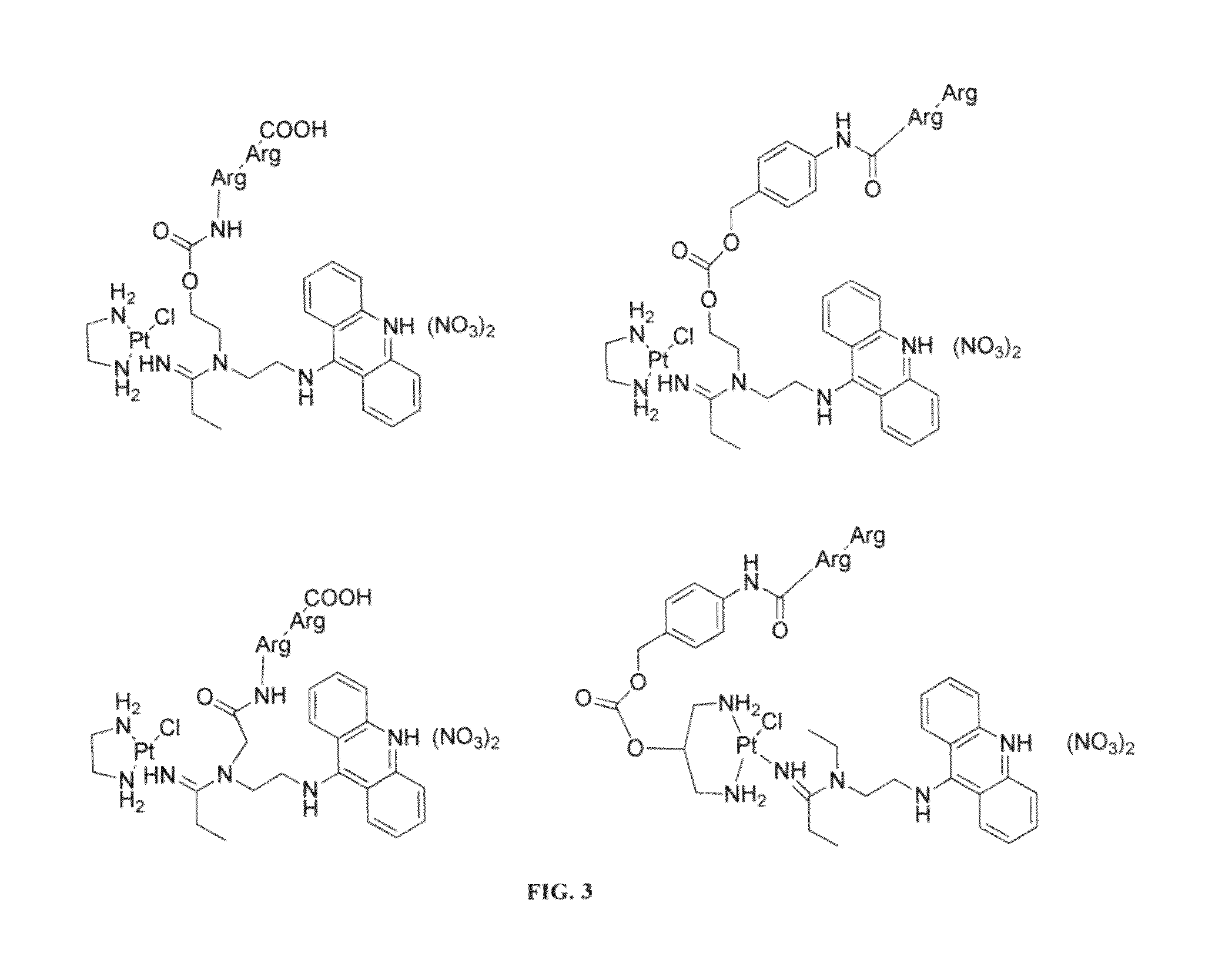
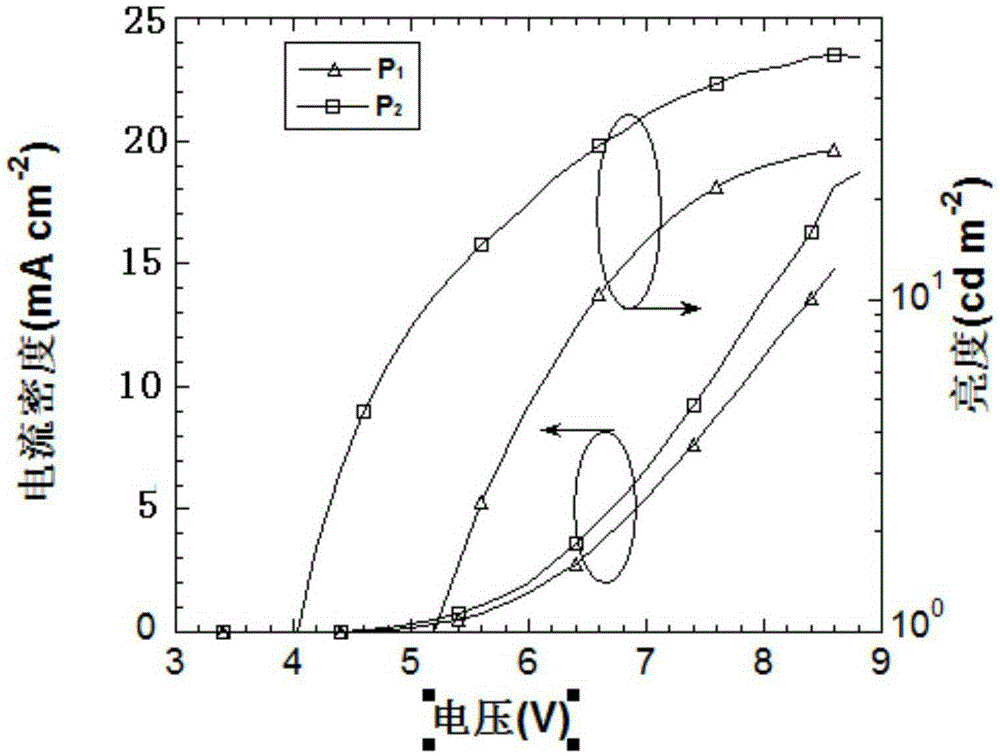
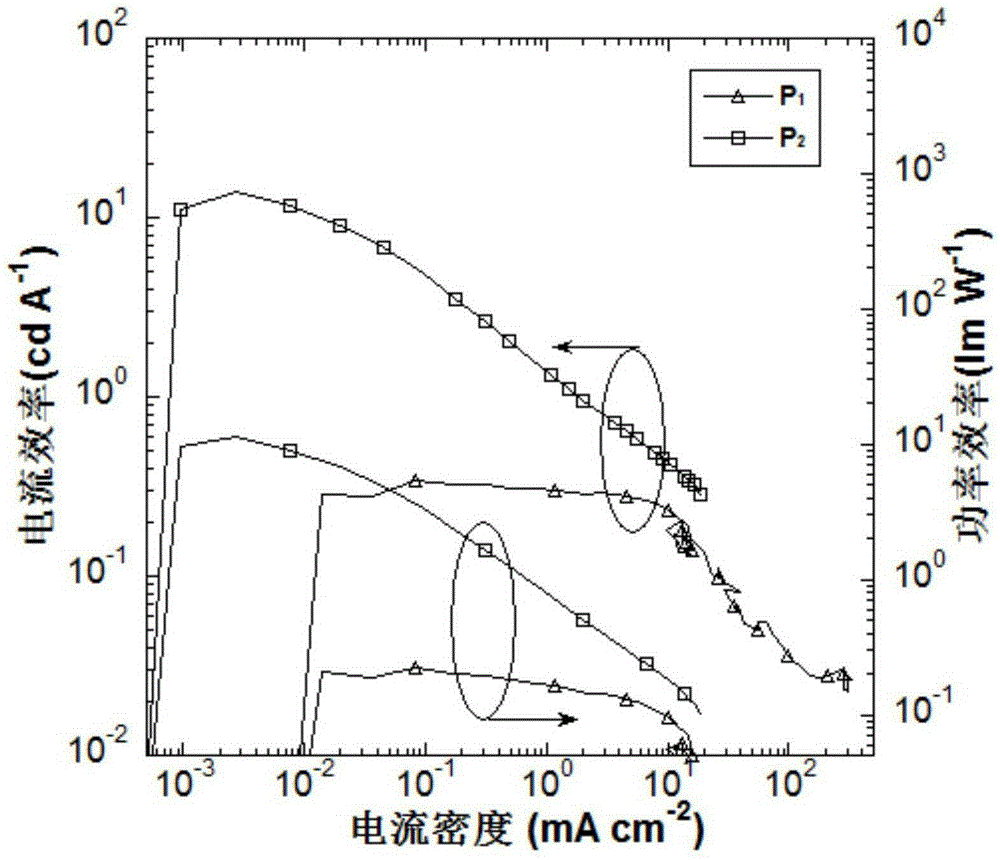
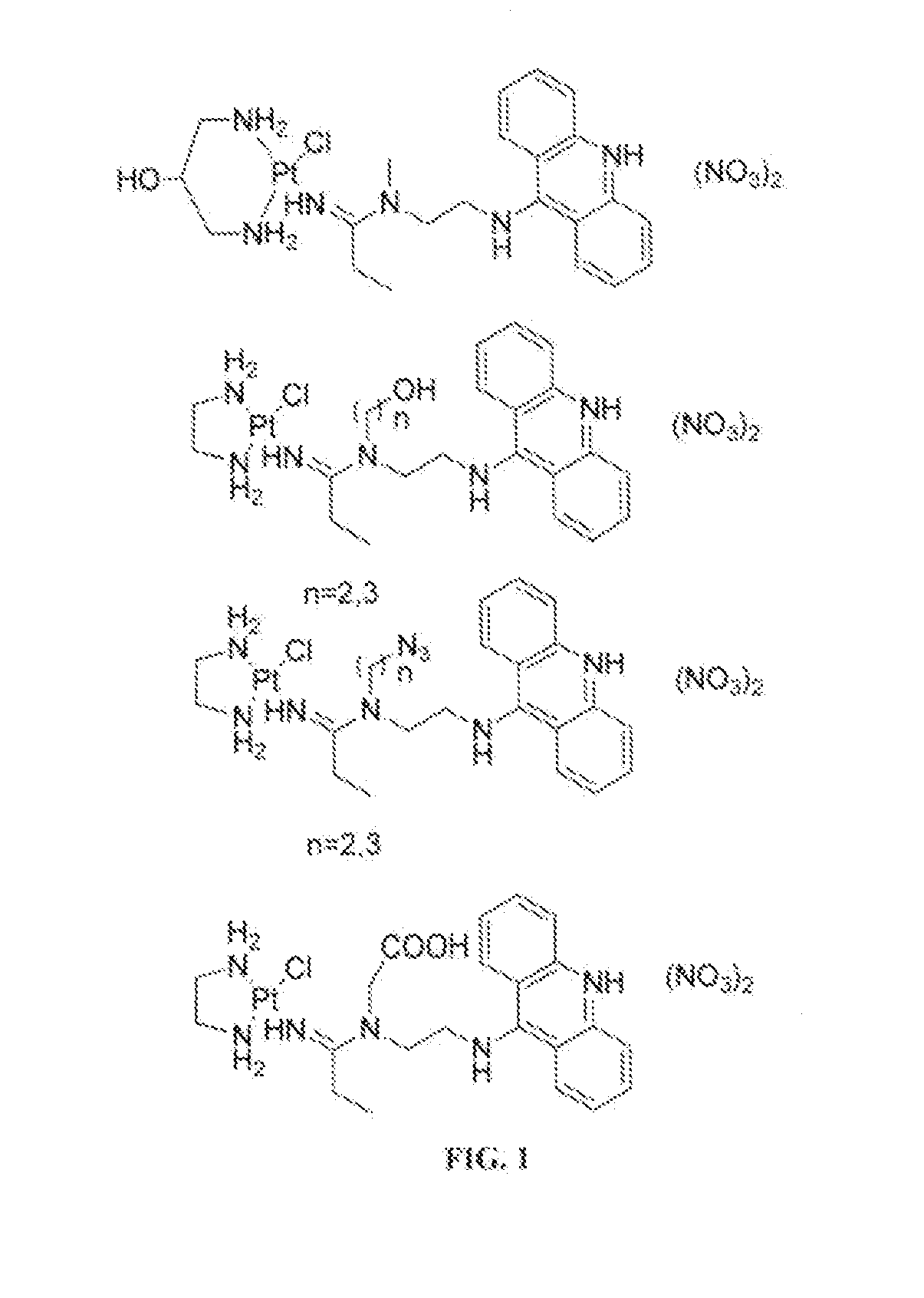
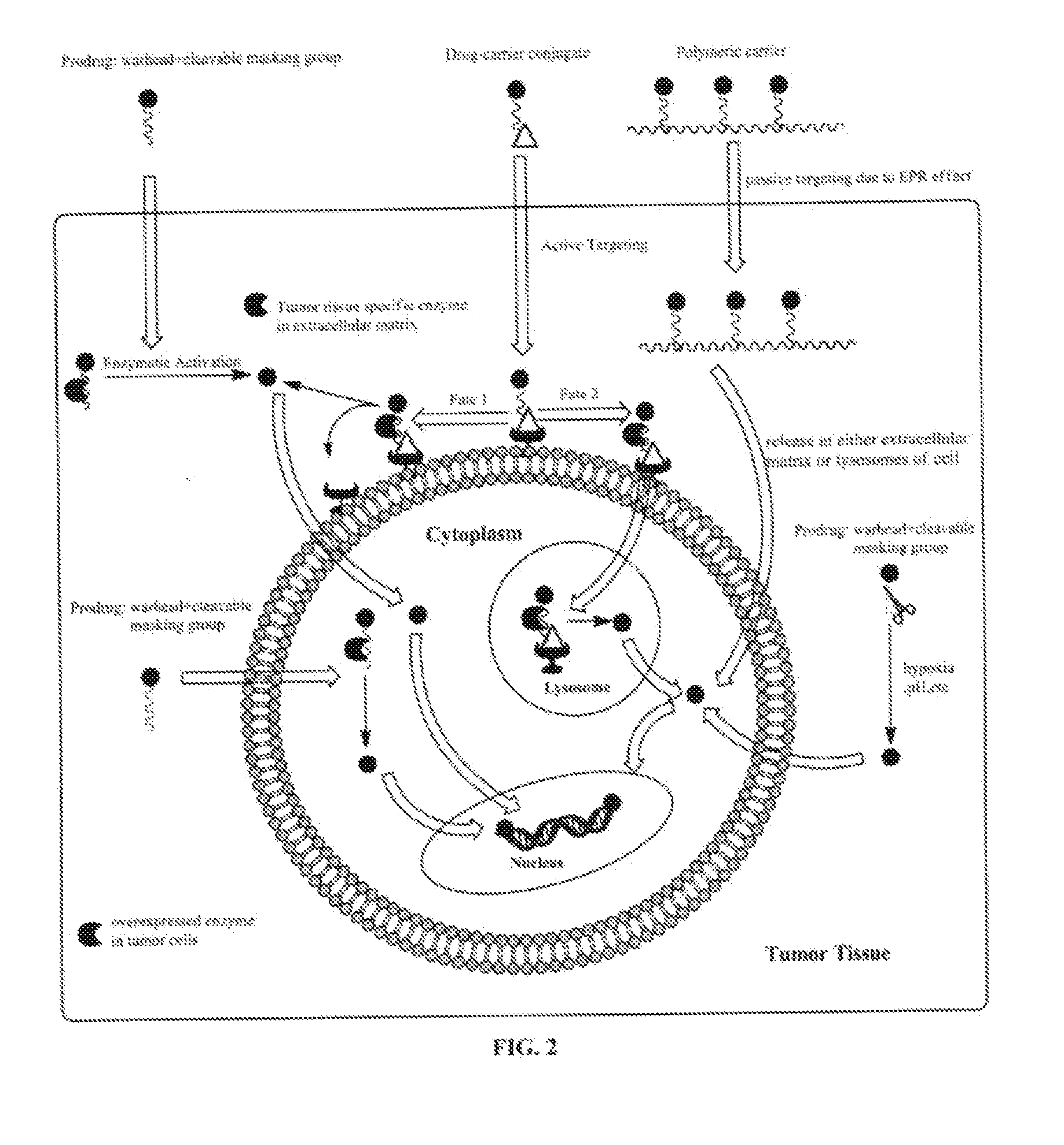
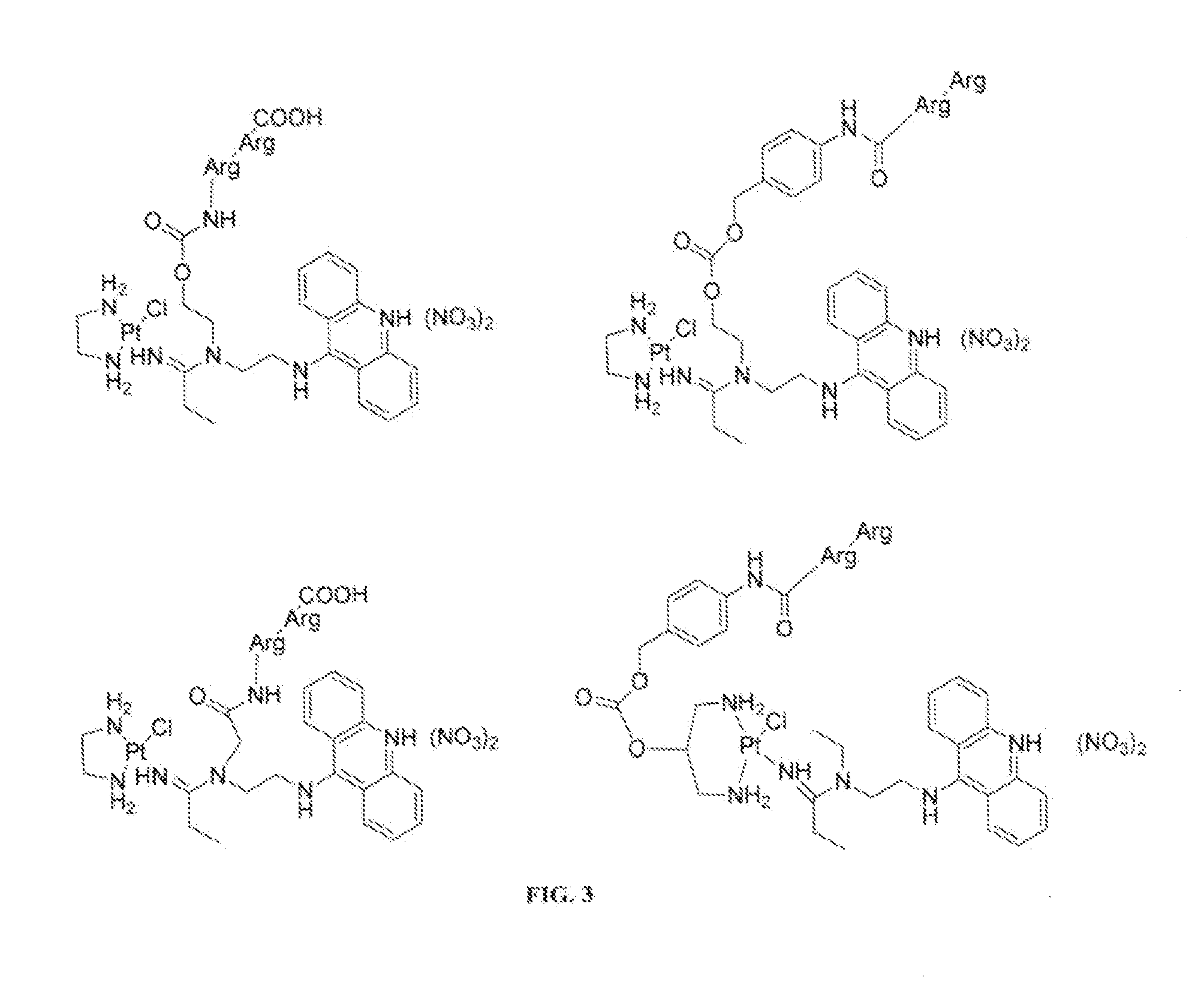
Targeted delivery and prodrug designs for platinum-acridine anti-cancer compounds and methods thereof
ActiveUS9090640B2Not induce DNA cross-linksStrong cytotoxicityOrganic active ingredientsPlatinum organic compoundsErlotinibCancer cell
Acridine containing cisplatin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective cisplatin compounds to the nucleus in cancer cells is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C1-6alkylene-phenyl-NH—C(O)—R15, folic acid, αvβ3 integrin RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazoles, oxazoles, erlotinib, and / or mixtures thereof; wherein R15 is a peptide.
Owner:WAKE FOREST UNIV
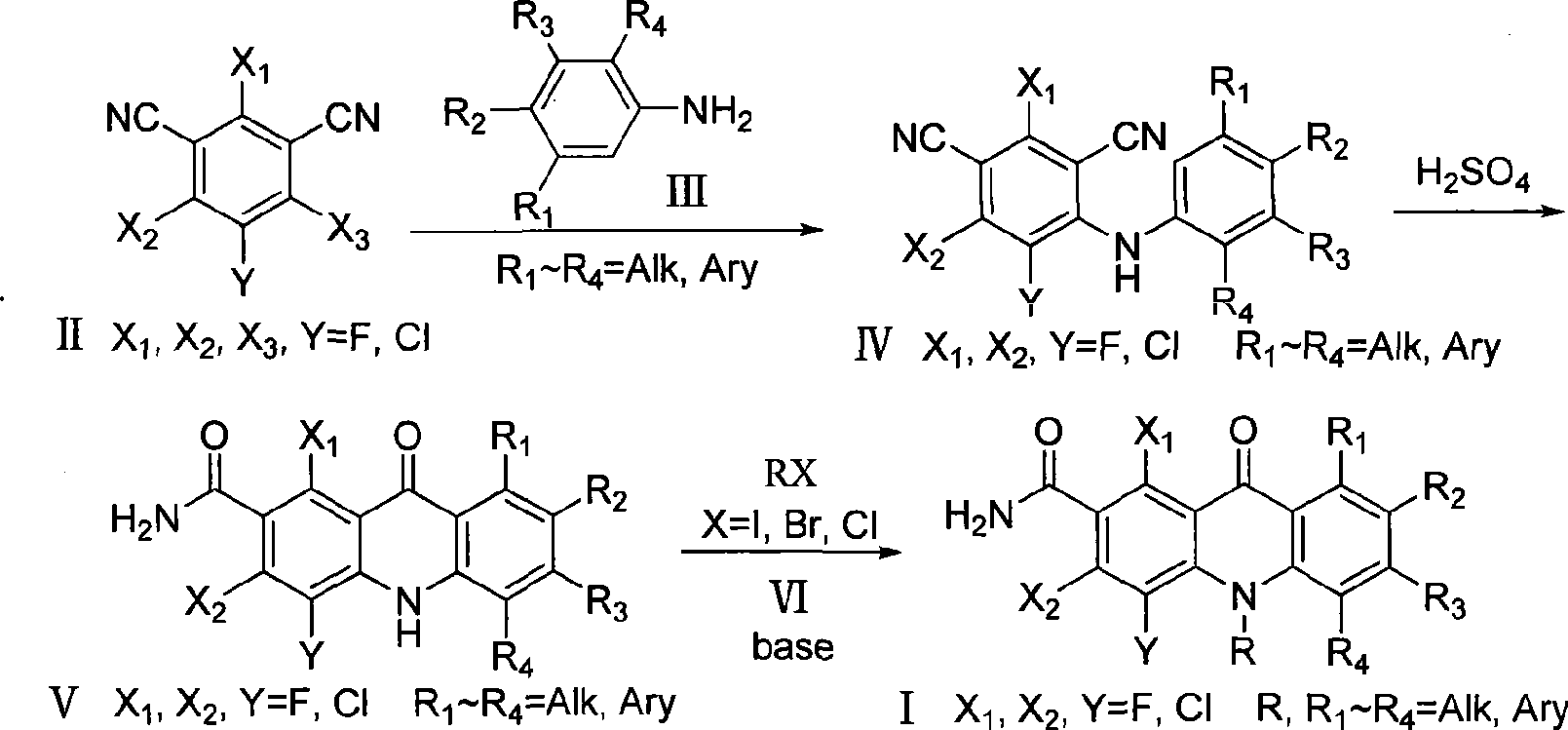
Polyhaloacridones compound, intermediate and synthetic method thereof
The invention discloses multi-halogenated acridone compounds, intermediates and a synthetic method thereof, wherein, the synthetic method comprises the steps: under the condition of alkali or no alkali, multi-halogenated m-phthalonitrile with the structural formula of (II) and phenylamine compound with the structural formula of (III) are reacted in a non-protonic solvent at certain temperature ( 0 to 90 DEG C ), thus generating the intermediate with the structural formula of (IV); then the intermediate (IV) is reacted with sulphuric acid to synthetize the multi-halogenated acridone compound with the structural formula of (V), and then the formula (V) is treated with alkylation or arylation reaction of nitrogen atoms to synthetize the multi-halogenated acridone compound (I).
Owner:YUNNAN UNIV
Magenta toner
Provided is a magenta toner having a toner particle containing a binder resin, a wax and a colorant, wherein the colorant contains a specific amount of a compound (1), the colorant also contains one or more compounds selected from the group consisting of a naphthol compound, a quinacridone compound and a lake compound thereof in addition to the compound (1), and the binder resin contains a specific amount of a polyester resin.
Owner:CANON KK
9,9'-spiral acridine-containing compound and preparation method and application thereof
InactiveCN106831773ALow singlet-triplet energy level differenceImprove exciton utilizationOrganic chemistrySolid-state devicesAcridineHalogen
The invention discloses a 9,9'-spiral acridine-containing compound. 9,9'-spiral acridine is taken as a core and the 9,9'-spiral acridine is combined with peripheral electrophilic Ar and Ar' units. The invention further discloses a preparation method of the 9,9'-spiral acridine-containing compound. The 9,9'-spiral acridine-containing compound is obtained through selecting different halogen-substituted Ar unit and Ar' unit, carrying out Buchwald-Hartwig coupled reaction on 10H, 10'H-9,9'-spiral acridine, carrying out copper-catalyzed aryl halide amination reaction or carrying out nucleophilic substitution reaction under a strong alkaline condition. The invention further discloses an application of the 9,9'-spiral acridine-containing compound. The 9,9'-spiral acridine-containing compound has high fluorescence and certain conductivity, and can be used for fabricating a light-emitting layer of an organic light-emitting diode as a luminous body.
Owner:SOUTH CHINA UNIV OF TECH +1
Organic small molecule luminescent material and organic electroluminescent device prepared from same
ActiveCN105254562AChanges in intramolecular charge transfer propertiesSingle structureOrganic chemistrySolid-state devicesAcridineOrganic electroluminescence
The invention belongs to the field of organic photoelectric material technology, and discloses an organic small molecule luminescent material and an organic electroluminescent device prepared from same. The organic small molecule luminescent material uses 9,9-dimethyl-10- phenyl acridine or 10-phenyl phenoxazine as an electron providing unit, and is connected with a different electron withdrawing unit at 2-site of the acridine group or the 3-site of the phenoxazine group to obtain a series of novel efficient organic small molecule luminescent materials. The organic small molecule luminescent material can change the characteristic of intramolecular charge transfer in the material, can make the luminescence peak of the luminescent material has a blue-shift, can be used as a luminescent layer for the organic electroluminescent device, and has a high device efficiency.
Owner:SOUTH CHINA UNIV OF TECH
Targeted Delivery and Prodrug Designs for Platinum-Acridine Anti-Cancer Compounds and Methods Thereof
ActiveUS20140193334A1Strong cytotoxicityInhibition is effectiveOrganic active ingredientsBiocideCancer cellErlotinib
Acridine containing cisplatin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective cisplatin compounds to the nucleus in cancer cells is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C1-6alkylene-phenyl-NH—C(O)—R15, folic acid, αvβ3 integrin RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazoles, oxazoles, erlotinib, and / or mixtures thereof; wherein R15 is a peptide.
Owner:WAKE FOREST UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
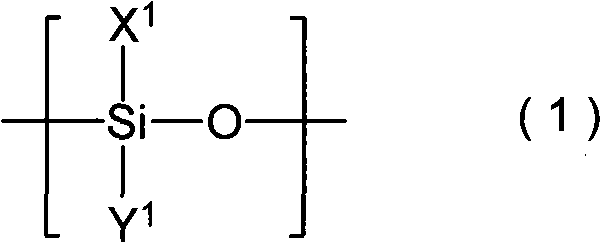
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-D00001.png)
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-C00001.png)
![C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids C8—linked pyrrolo[2,1-c][1,4]benzodiazepine-acridone/acridine hybrids](https://images-eureka.patsnap.com/patent_img/9137b741-81bb-4108-b21a-567f2cb25f70/US07056913-20060606-C00002.png)