Organic photoelectric material and organic electroluminescent device containing material
An organic optoelectronic material and electroluminescence technology, applied in the field of material science, can solve the problems of limiting the luminous efficiency, service life and operating voltage of devices, preventing the full practical application of OLED technology, etc., and achieve good industrialization prospects, good application effects, power Efficiency and Quantum Efficiency Improvement Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
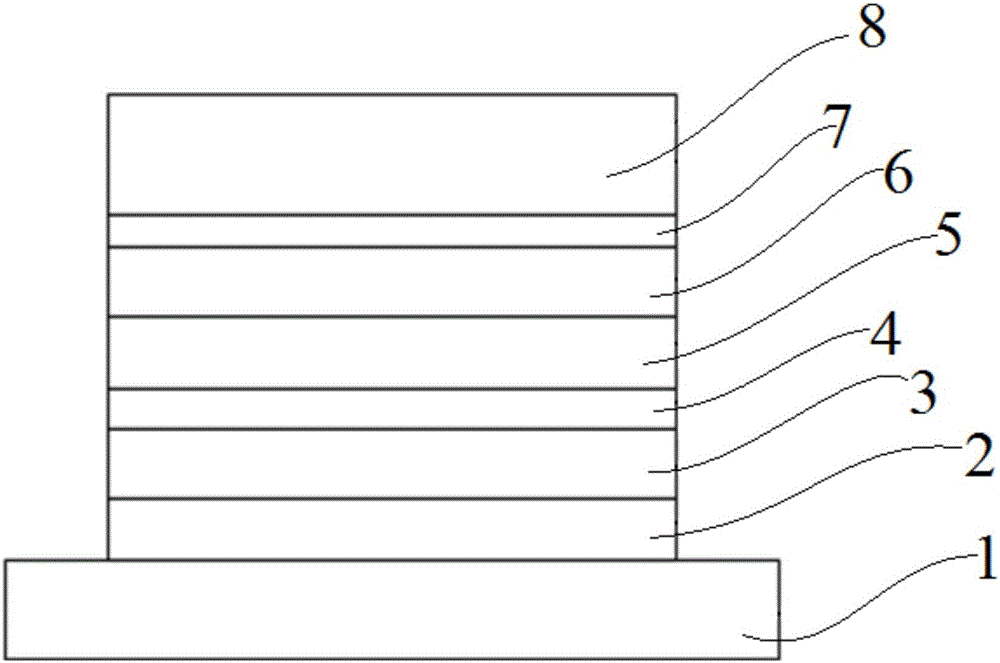
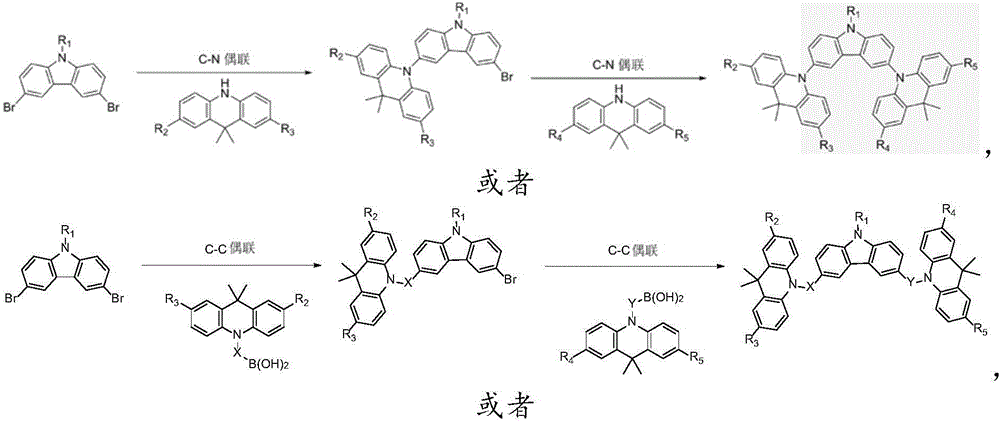
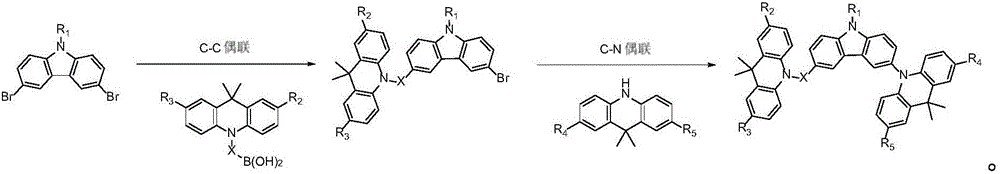
Image
Examples
Embodiment 110
[0053] Example 110, 10'-(9-(dibenzo[b,d]furan-4-yl)-9H-carbazole-3,6-diyl)bis(9,9-dimethyl-9, 10-dihydroacridine) (compound 1) synthesis:
[0054]
[0055] Preparation of compound 1-a:
[0056] Take 3,6-dibromocarbazole (32.5g, 0.1mol), potassium carbonate (20.7g, 0.15mol), cuprous iodide (1.9g, 0.01mol), o-phenanthroline (3.6g, 0.02mol) , 4-iododibenzofuran (38.2g, 0.13mol) and 400g DMF, under the protection of nitrogen, the mixture was reacted at 120°C for 15.0 hours. After the reaction was completed, the insoluble matter was filtered off, the filtrate was desolvated under reduced pressure until there was no fraction, and the residue was purified by column chromatography with petroleum ether ethyl acetate mixture (volume ratio 7:1), and then recrystallized by toluene ethanol to obtain the compound 1-a (29.9 g, 60.89%), GC-MS: 491.23, calculated 491.18.
[0057] Preparation of Compound 1:
[0058] Take compound 1-a (24.5g, 0.05mol), potassium carbonate (20.7g, 0.15mol)...
Embodiment 310
[0060] Example 310, 10'-((9-(dibenzo[b,d]furan-4-yl)-9H-carbazole-3,6-diyl)bis(4,1-phenylene)) Synthesis of bis(9,9-dimethyl-9,10-dihydroacridine) (compound 3):
[0061]
[0062] Preparation of compound 3
[0063] Get compound 1-a (24.5g, 0.05mol), (4-(9,9-dimethylacridin-10(9H)-yl) phenyl) phenylboronic acid (36.2g, 0.11mol), potassium carbonate ( 20.73g, 0.15mol) and 100g of water were dissolved with 200mL of toluene and 100mL of ethanol, and stirred under nitrogen gas for 1 hour to remove the oxygen in the reaction flask. Then add Pd(PPh 3 ) 4 0.58g (0.5mmol), reflux under vigorous stirring, and the reaction process was tracked and detected by TLC. After the reaction was completed, the aqueous phase was extracted with 200 mL of ethyl acetate, and the organic phase was desolvated under reduced pressure until there was no fraction. The residue was purified by column chromatography with pure toluene, and then recrystallized by toluene ethanol to obtain compound 3 (37.8 ...
Embodiment 81
[0065] Example 810-(4-(9-(dibenzo[b,d]furan-4-yl)-6-(4'-(9,9-dimethylacridin-10(9H)-yl) -[1,1'-biphenyl]-4-yl)-9H-carbazol-3-yl)phenyl)-9,9-dimethyl-9,10-dihydroacridine (compound 8) Synthesis:
[0066]
[0067] Preparation of Compound 8-b
[0068] Get compound 1-a (24.5g, 0.05mol), (4-(9,9-dimethylacridin-10(9H)-yl) phenyl) phenylboronic acid (6.5g, 0.02mol), potassium carbonate ( 10.4 g, 0.075 mol) and 100 g of water were dissolved with 200 mL of toluene and 100 mL of ethanol, and stirred under nitrogen gas for 1 hour to remove oxygen in the reaction flask. Then add Pd(PPh 3 ) 4 0.12g (0.1mmol), reflux under vigorous stirring, and the reaction process was tracked and detected by TLC. After the reaction was completed, the aqueous phase was extracted with 200 mL of ethyl acetate, the organic phase was desolvated under reduced pressure until there was no fraction, and the residue was purified by column chromatography with petroleum ether ethyl acetate mixture (volume ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com