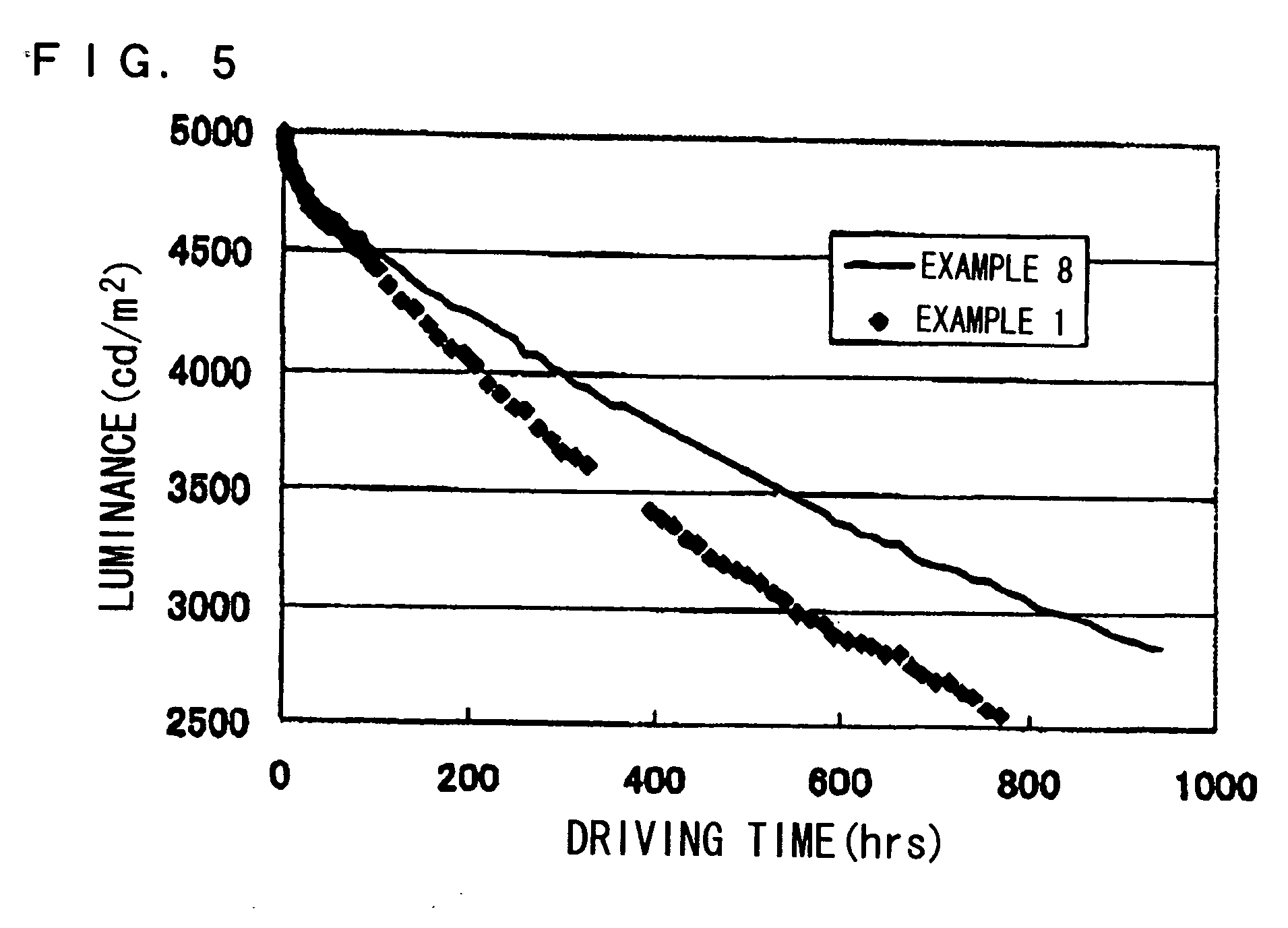
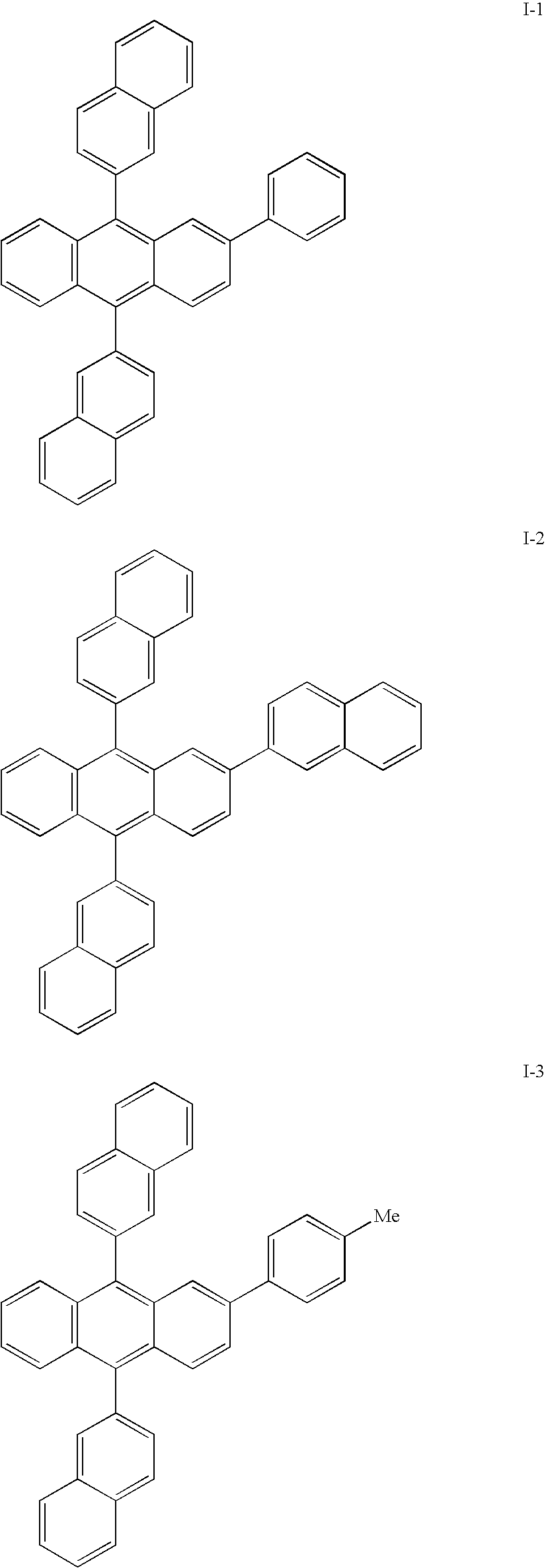
Patents
Literature
1420 results about "Phenanthroline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
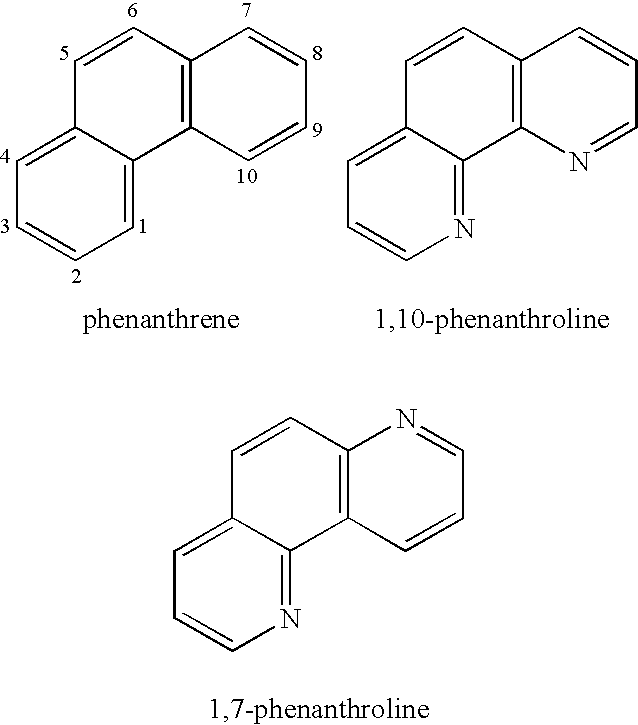
Phenanthroline (phen) is a heterocyclic organic compound. It is a white solid that is soluble in organic solvents. It is used as a ligand in coordination chemistry, forming strong complexes with most metal ions.
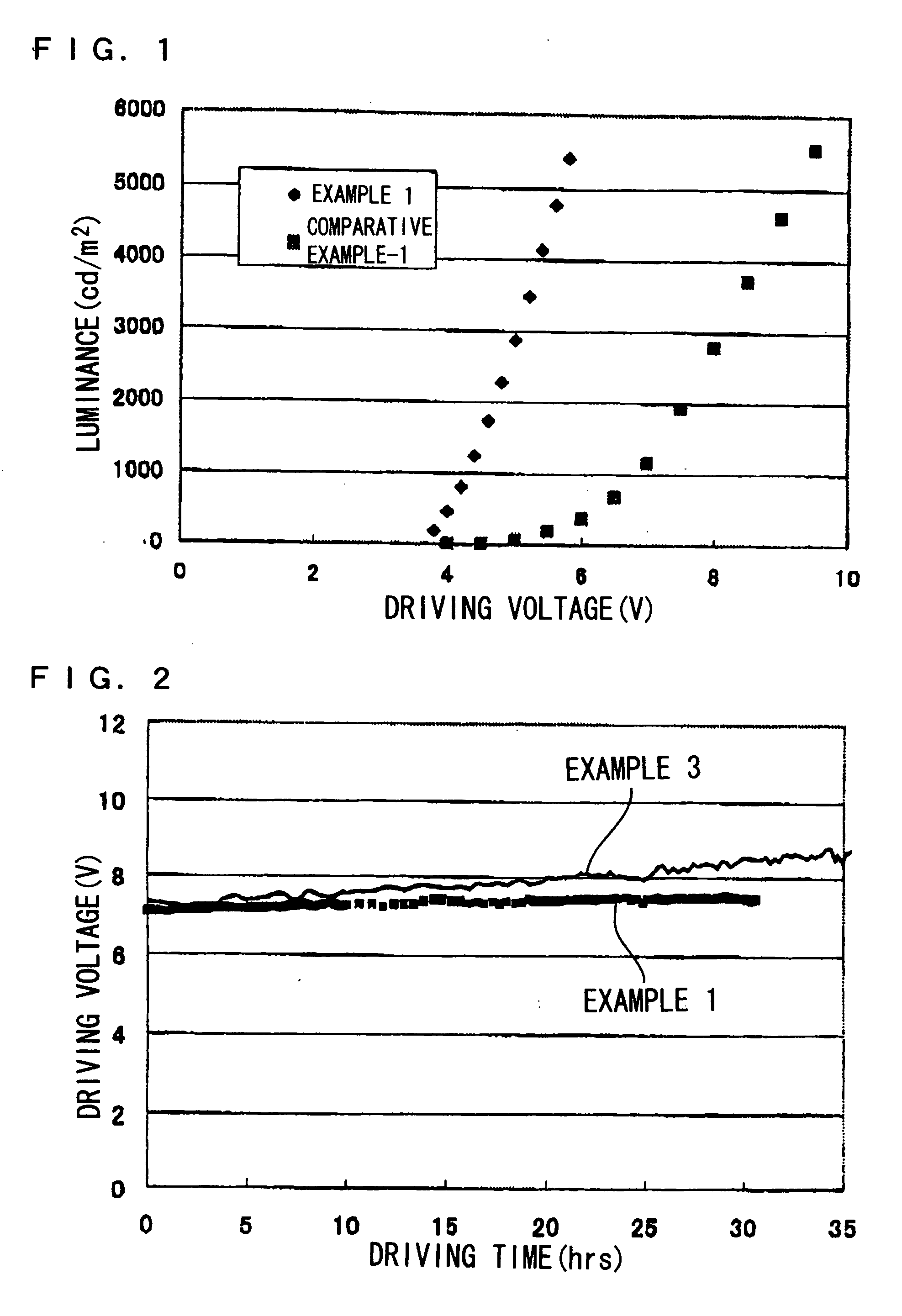
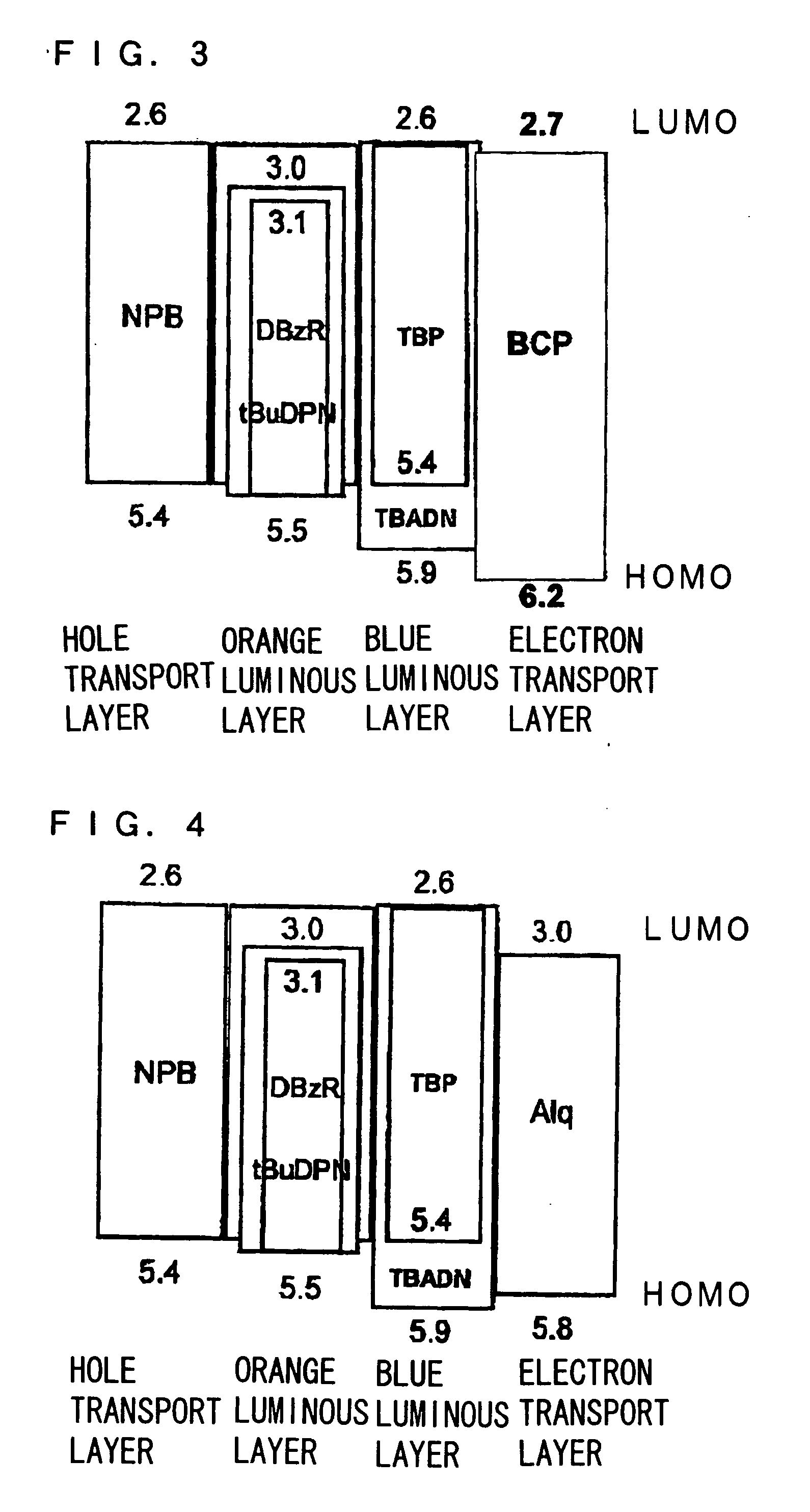
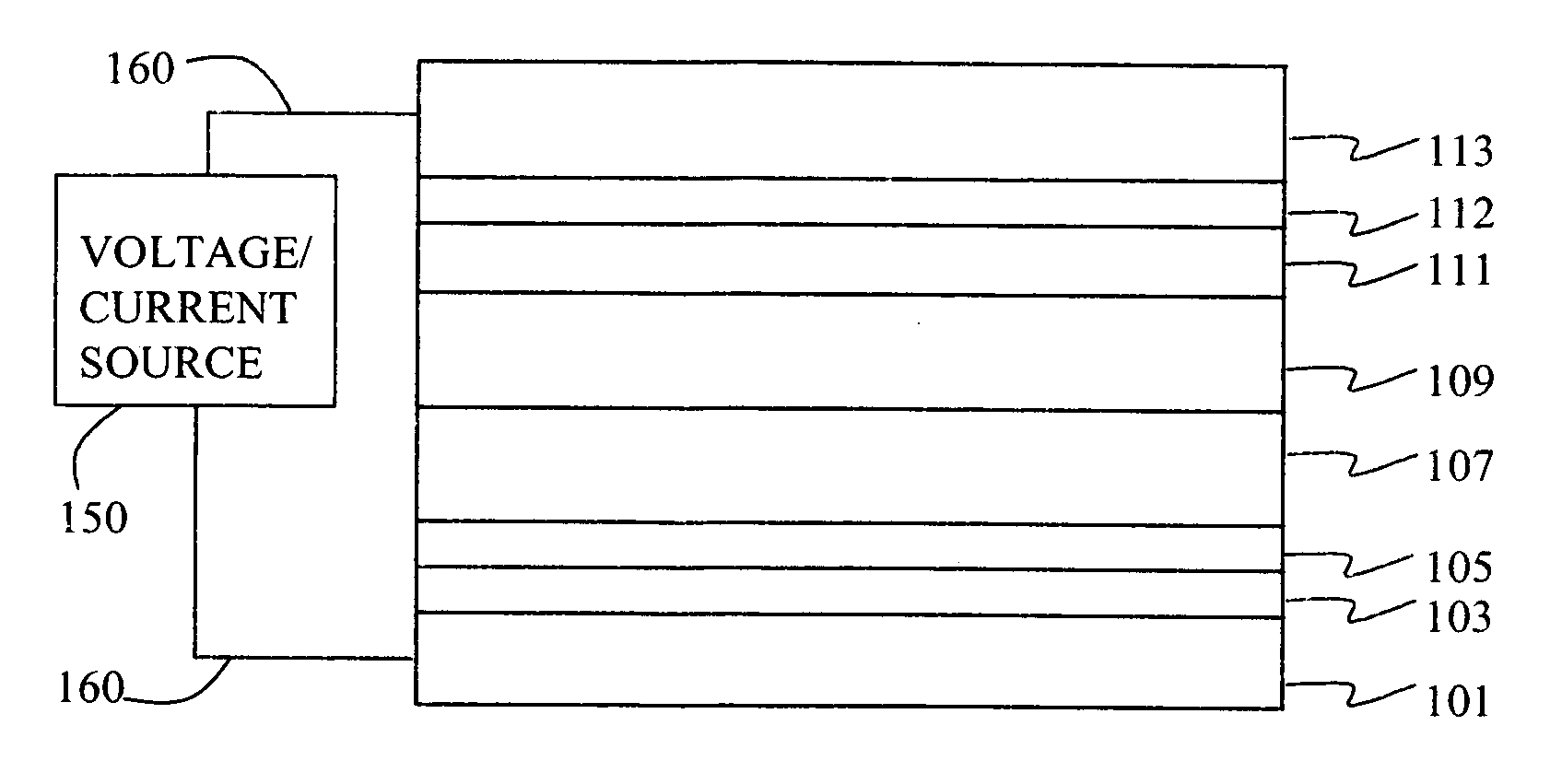
Electroluminescent device containing an anthracene derivative
ActiveUS20070122656A1Good equipment stabilityDischarge tube luminescnet screensElectroluminescent light sourcesAnthracenePhenanthroline
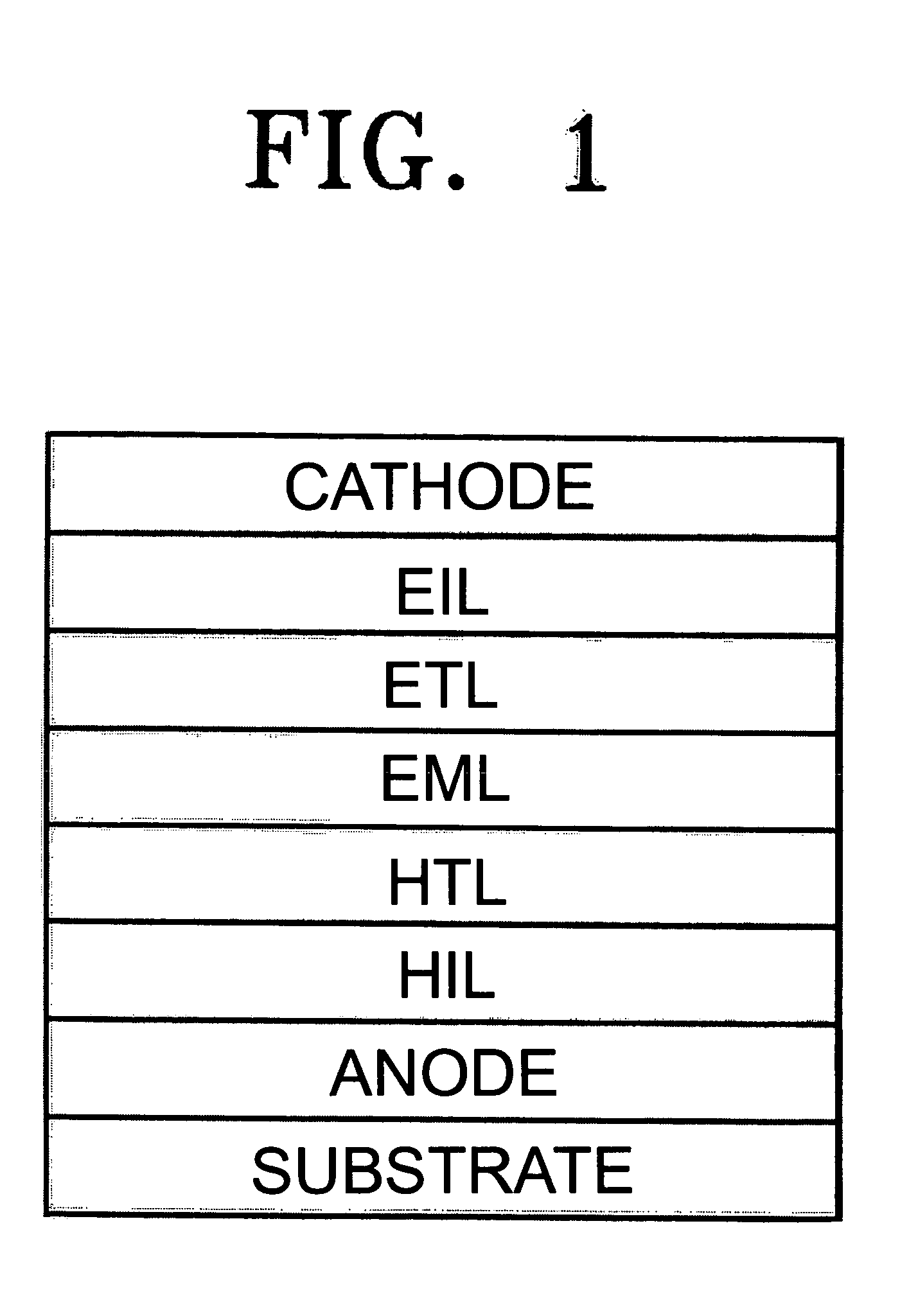
An OLED device comprises a cathode, an anode, and a light-emitting layer therebetween, and additionally comprises a layer between the cathode and the light-emitting layer including a compound comprising one and only one anthracene nucleus bearing no more than two phenanthroline-containing substituents wherein said anthracene nucleus is substituted in the 2-, 3-, 6-, or 7-position with a phenanthroline-containing substituent. When such materials are included in a layer, such as an electron-transporting layer, that provide both desirable electroluminescent properties as well as good device stability.
Owner:GLOBAL OLED TECH
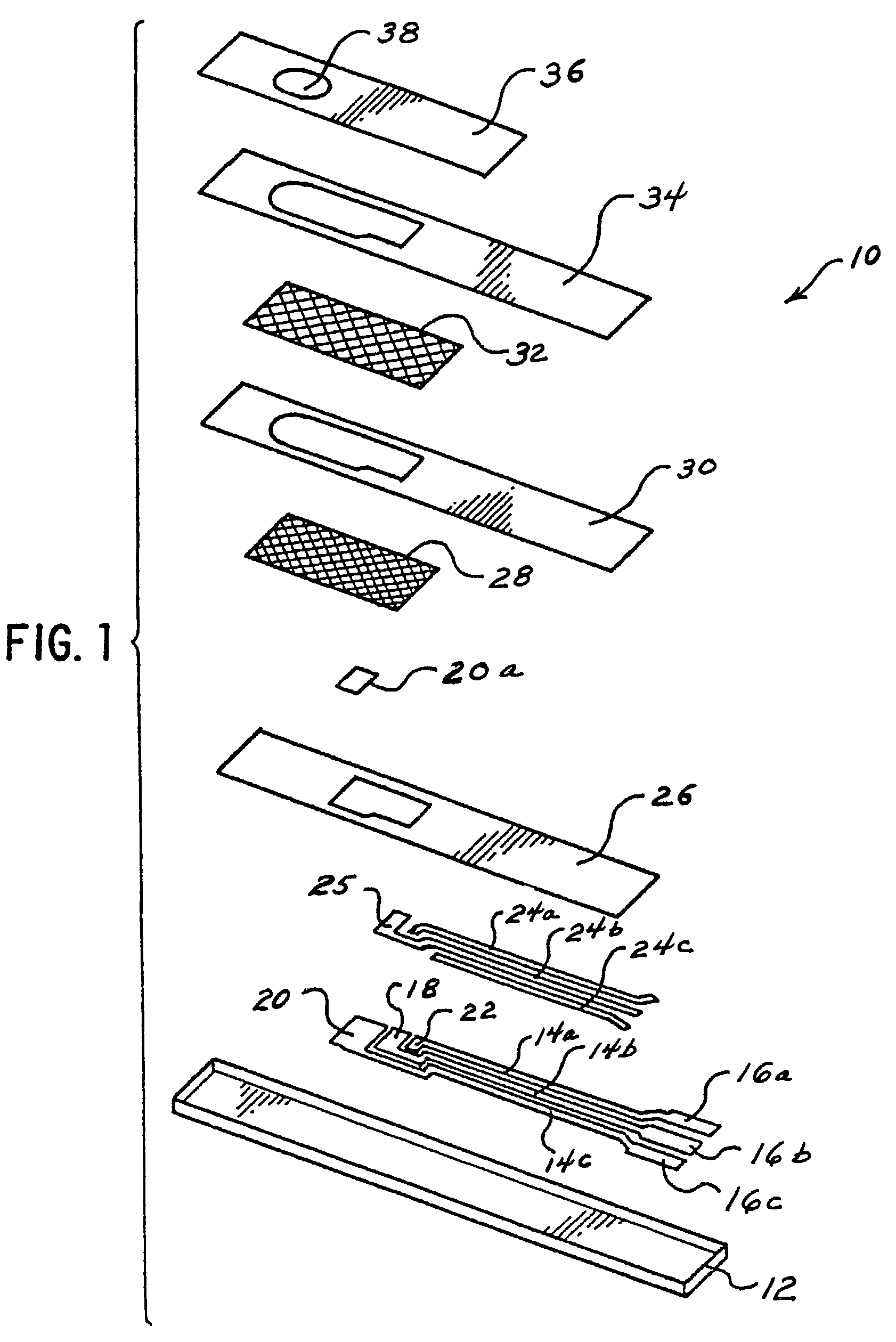
Biosensor having improved hematocrit and oxygen biases
ActiveUS7501053B2Accurate responseDesensitizationImmobilised enzymesBioreactor/fermenter combinationsQuinoneManganese
A biosensor that utilizes a mediator, i.e., an isomer of phenanthroline quinone, 1,10-phenanthroline-5,6-dione, and a metal ion, such as manganese, with an enzyme dependent upon NAD(P)+, such as, for example, glucose dehydrogenase, for improving the hematocrit bias and oxygen bias of biosensors. The electrodes of the biosensors employing this mediator and a metal ion provide an accurate clinical response over a hematocrit range that ranges from about 20% to about 70% and over an oxygen tension range that ranges from about 1 kPa to about 20 kPa.
Owner:ABBOTT DIABETES CARE INC
Organic electroluminescent display element, finder screen display device, finder and optical device
InactiveUS6468676B1Discharge tube luminescnet screensElectroluminescent light sourcesNitrosoAlkaline earth metal
An organic electroluminescent display element has at least a positive electrode, an organic luminescent film, an electron injection layer and a negative electrode. Each of the positive and negative electrodes is formed of a transparent conductive film, the electron injection layer is formed of a thin transparent film made of a halogenide of an alkali metal or an alkaline earth metal, or an organic metal complex containing an alkali metal or an alkaline earth metal as a metal, and the organic metal complex is at least one complex selected from the group consisting of acetylacetonate complexes, alpha-nitroso-beta-naphthol complexes, salicylaldoxime complexes, cupferron complexes, benzoinoxime complexes, bipyridine complexes, phenanthroline complexes, crown complexes, proline complexes and benzoylacetone complexes.
Owner:KONICA MINOLTA INC
Luminescent element material and luminescent element comprising the same
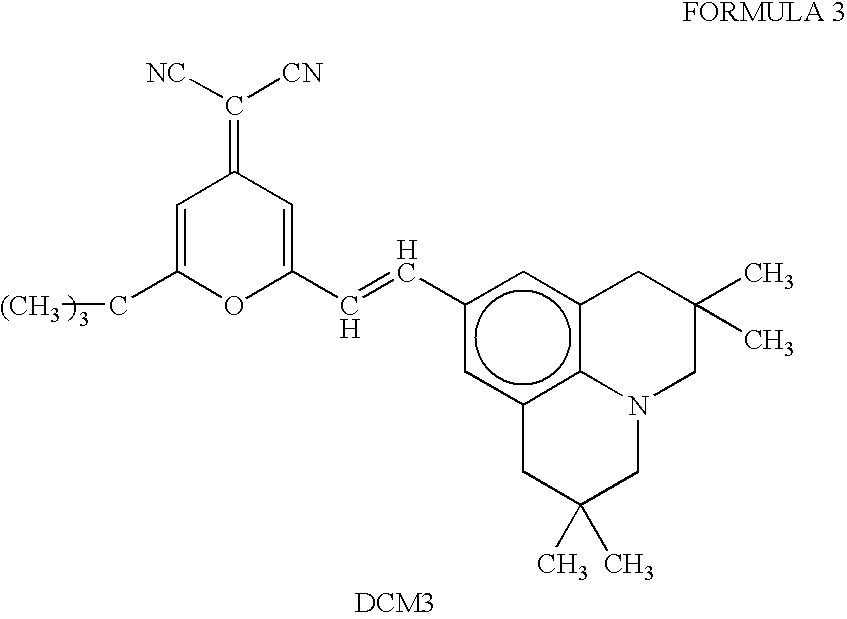
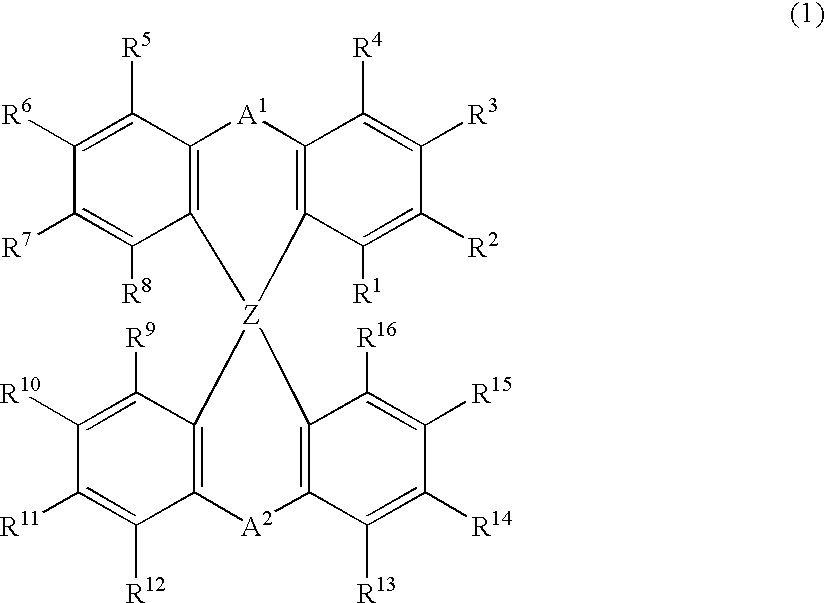
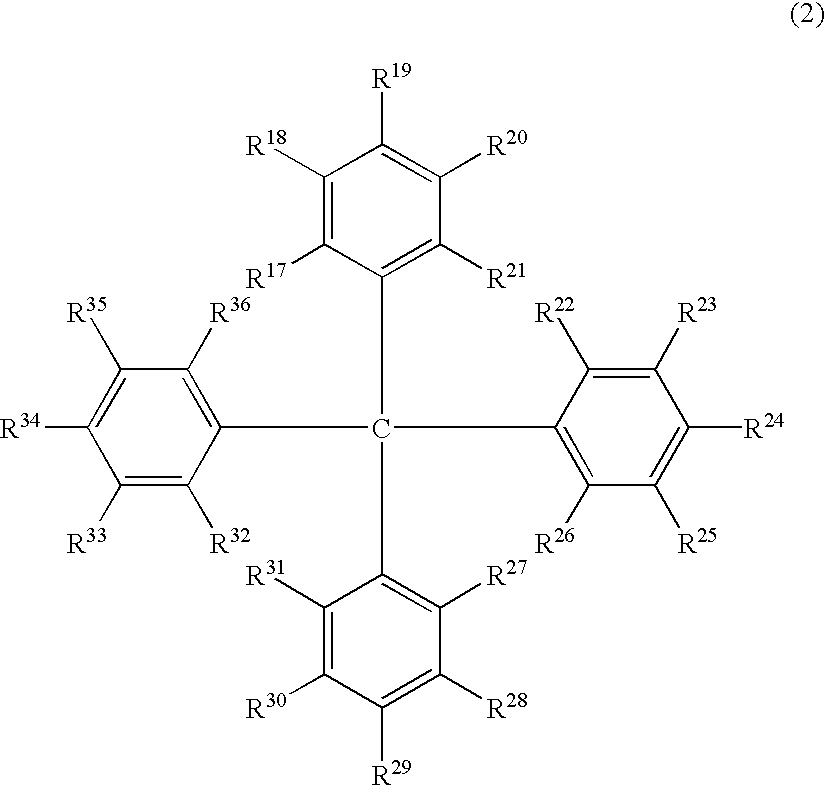
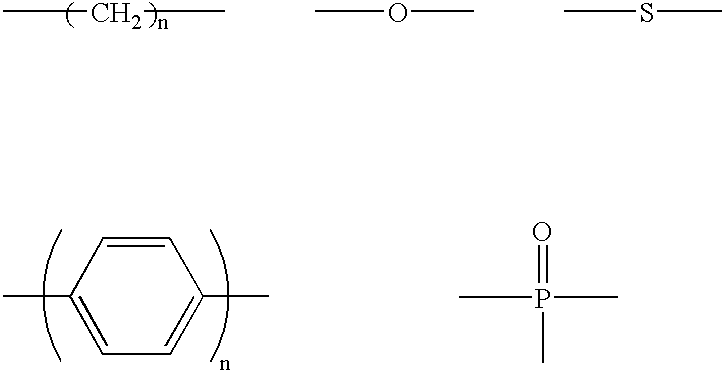
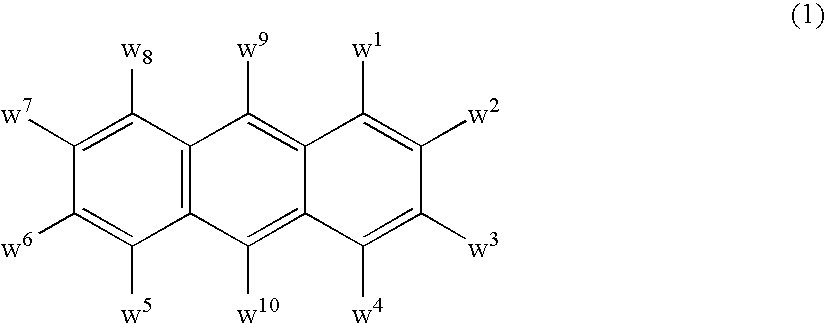
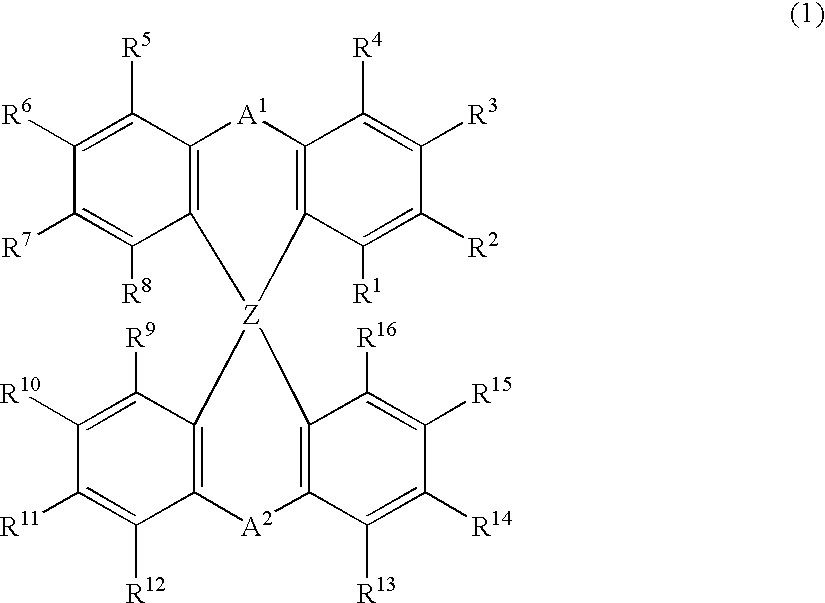
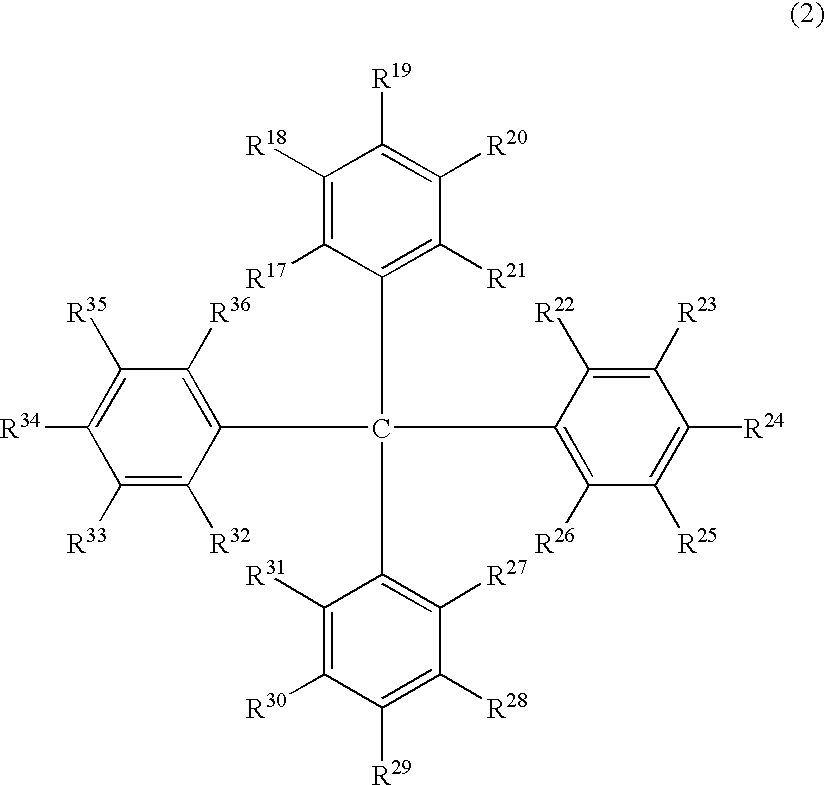
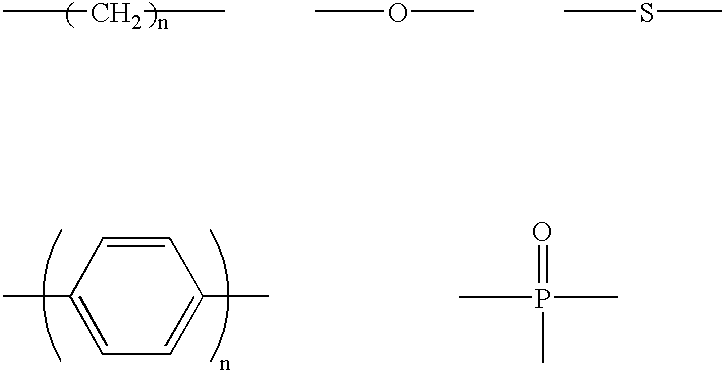
The light emitting device of the present invention relates to a light emitting device which is characterized in that it is a device with an emissive substance present between an anode and cathode, and which emits light by means of electrical energy, and said device has a least one type of compound denoted by (a) to (d) below. (a) A compound having a plurality of 1,7-phenanthroline skeletal structures (b) A benzoquinoline derivative (c) A spiro compound represented by general formula (1) A1 and A2 are each selected from single bonds, substituted or unsubstituted alkyl chains, ether chains, thioether chains, ketone chains and substituted or unsubstituted amino chains. However, A1<> A2. Z represents carbon or silicon. R1 to R16 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. (d) A tetraphenylmethane derivative represented by general formula (2) R17 to R36 are each selected from hydrogen, alkyl group, cycloalkyl group, aralkyl group, alkenyl group, cycloalkenyl group, alkynyl group, hydroxyl group, mercapto group, alkoxy group, alkylthio group, aryl ether group, aryl thioether group, aryl group, heterocyclic group, halogen, haloalkane, haloalkene, haloalkyne, cyano group, aldehyde group, carbonyl group, carboxyl group, ester group, carbamoyl group, amino group, nitro group, silyl group, siloxanyl group and a cyclic structure formed with an adjacent substituent. However, at least one of R17 to R36 is selected from substituents represented by general formula (3). -X-Ar (3) X is a single bond or is selected from the following, and Ar denotes a condensed aromatic ring or heteroaromatic ring. In the case where X is phosphorus oxide, then Ar represents an aromatic hydrocarbon or heteroaromatic ring. n is an natural number.
Owner:TORAY IND INC
Compositions comprising rigid-rod polymers and carbon nanotubes and process for making the same
InactiveUS6900264B2High modulusHigh stiffnessMaterial nanotechnologySpecial tyresFiberLiquid crystalline
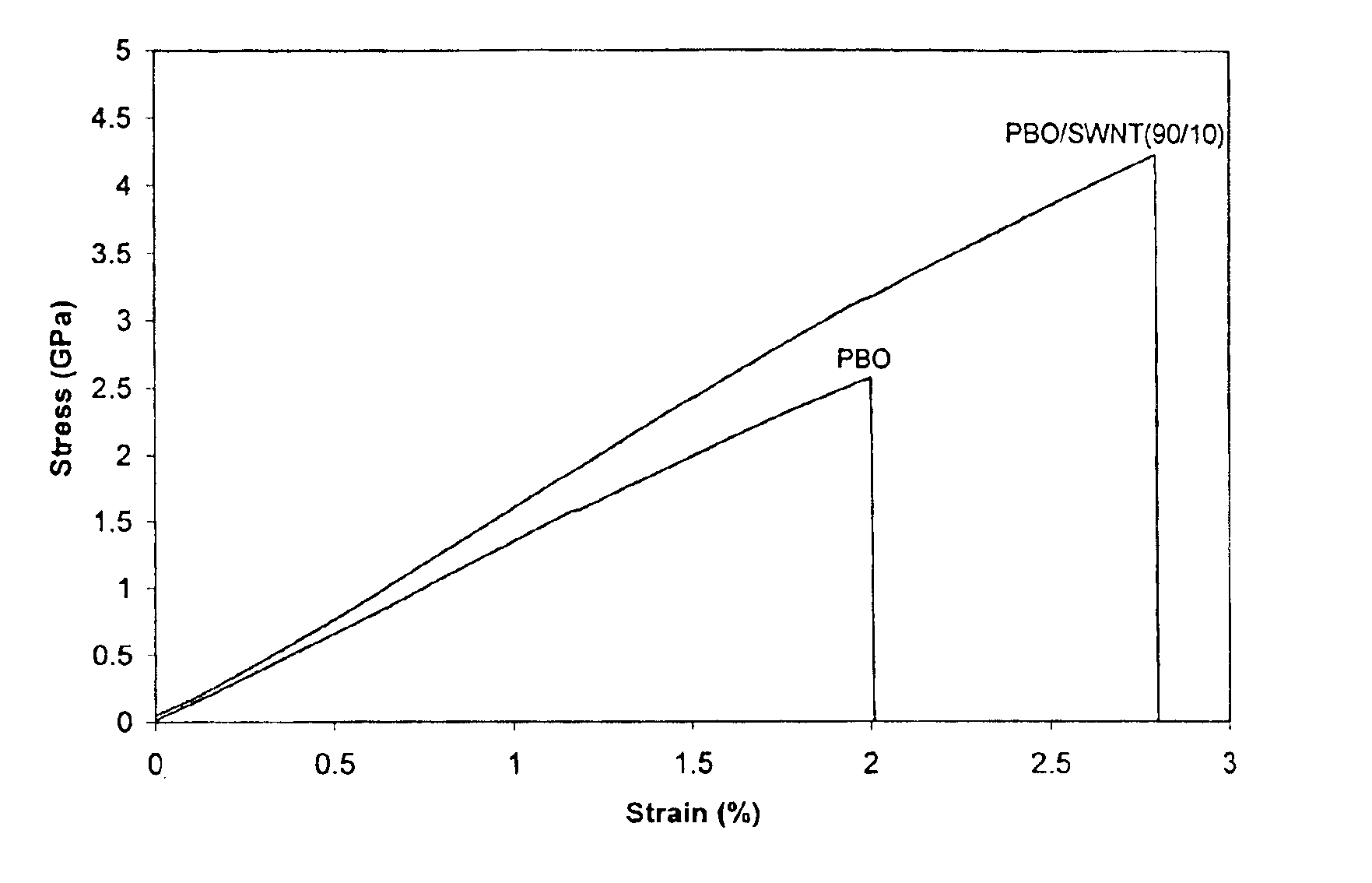
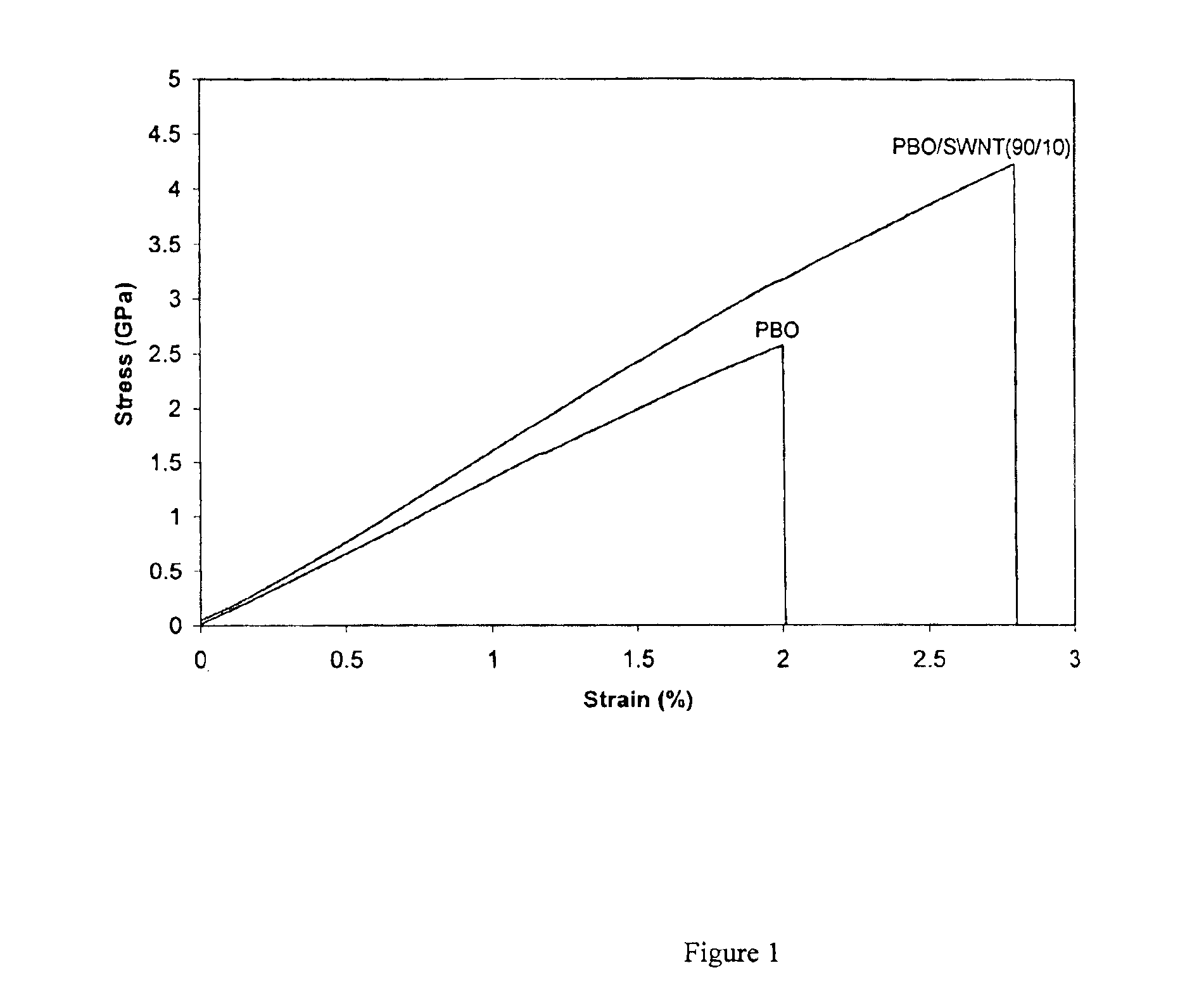
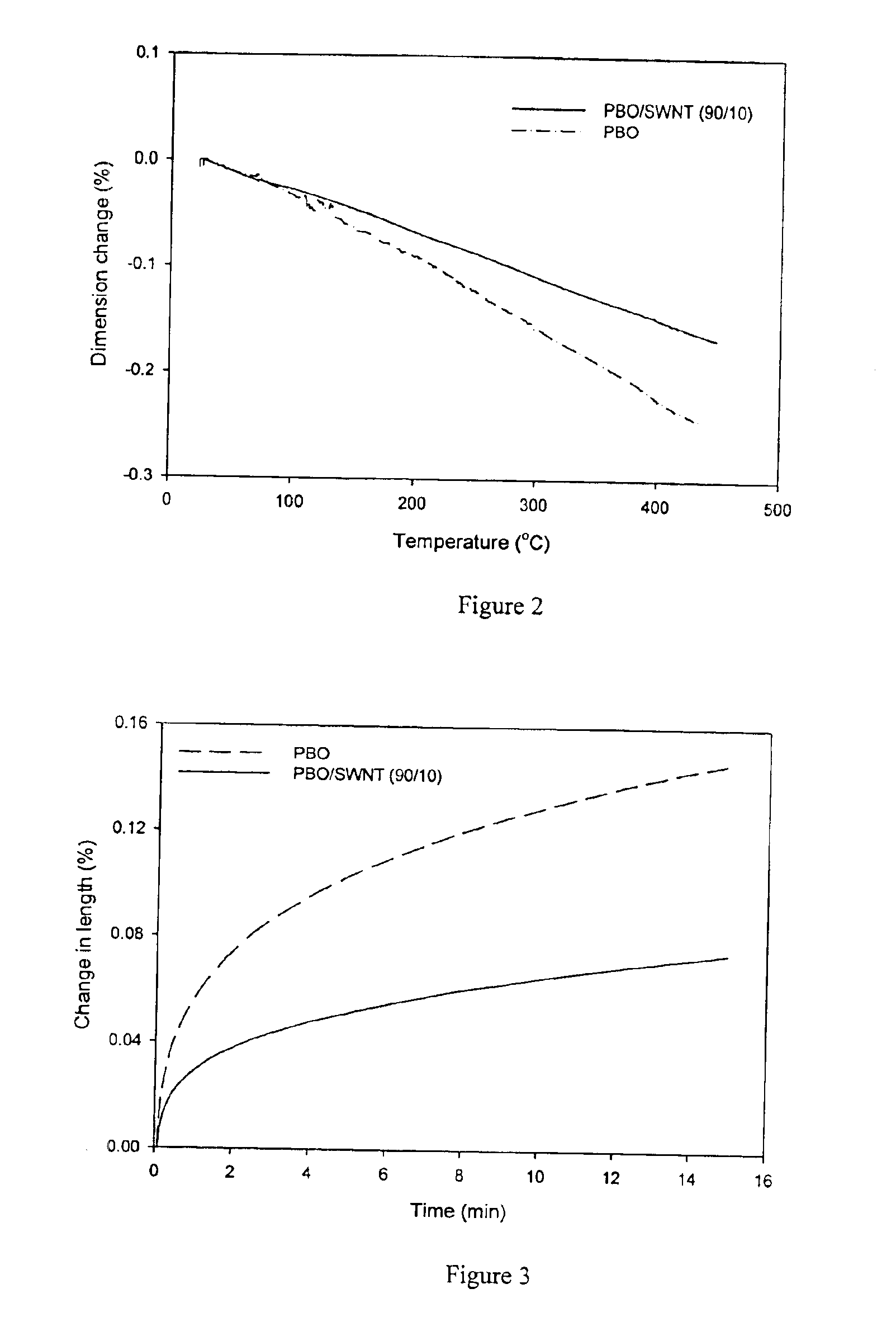
The present invention relates to compositions comprising rigid-rod polymers and carbon nanotubes. The compositions comprise dispersed carbon nanotubes aligned with rigid-rod polymers. The alignment of the nanotubes and polymers can be liquid crystalline. The rigid-rod polymers of this invention include, but are not limited to, polymers and copolymers comprising benzobisazole, pyridobisimidazole and benzamidazobenzo-phenanthroline repeat units. Dispersion of carbon nanotubes is achieved by in-situ polymerization in the presence of the carbon nanotubes, which may be either single-wall or multi-wall or a combination of both. The polymer compositions comprising carbon nanotubes may be spun into fibers or formed into films. The strength of the resulting fibers of the present invention is significantly greater than that of fibers without carbon nanotubes.
Owner:GEORGIA TECH RES CORP
Coenzyme-binding glucose dehydrogenase
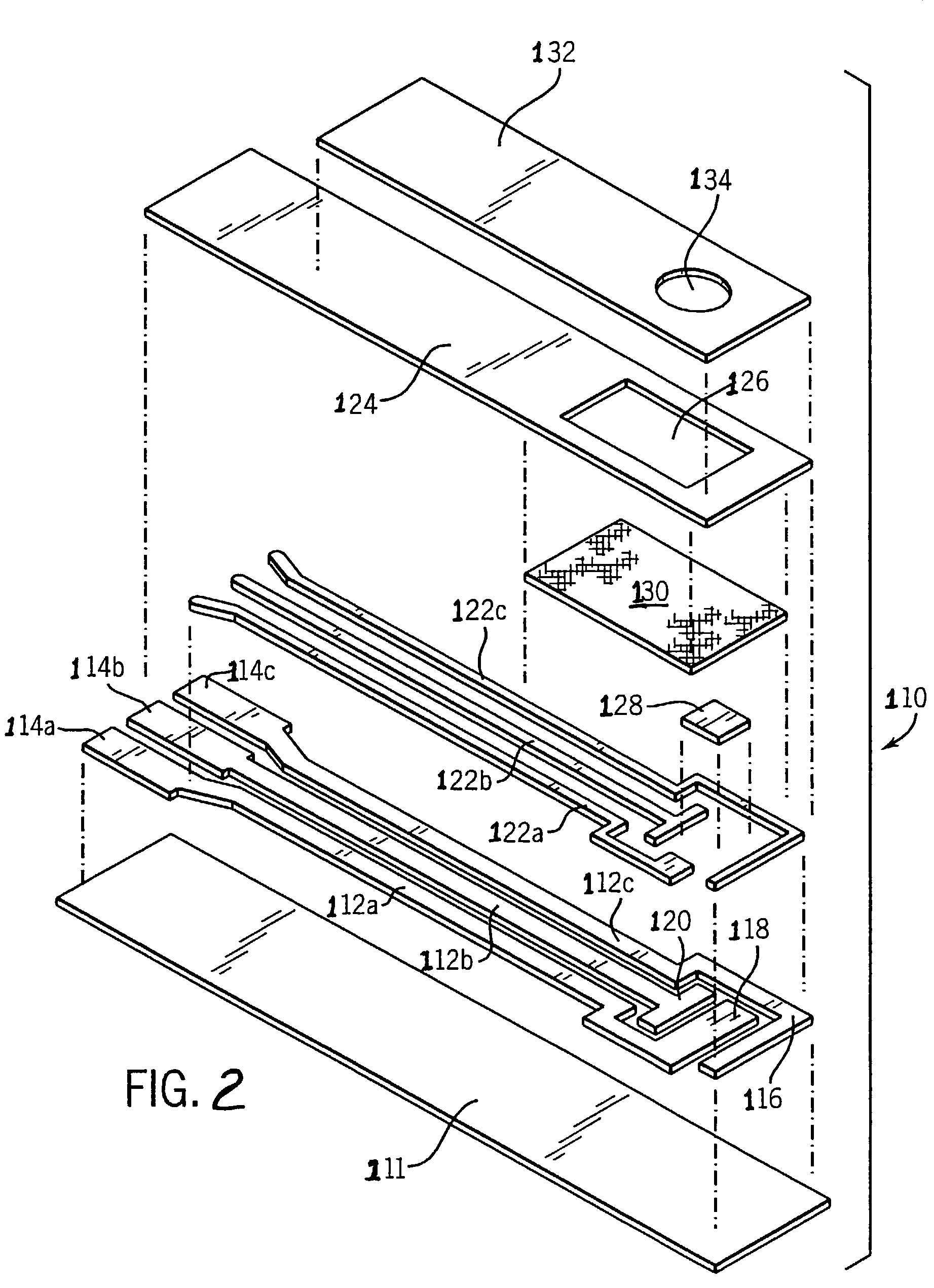
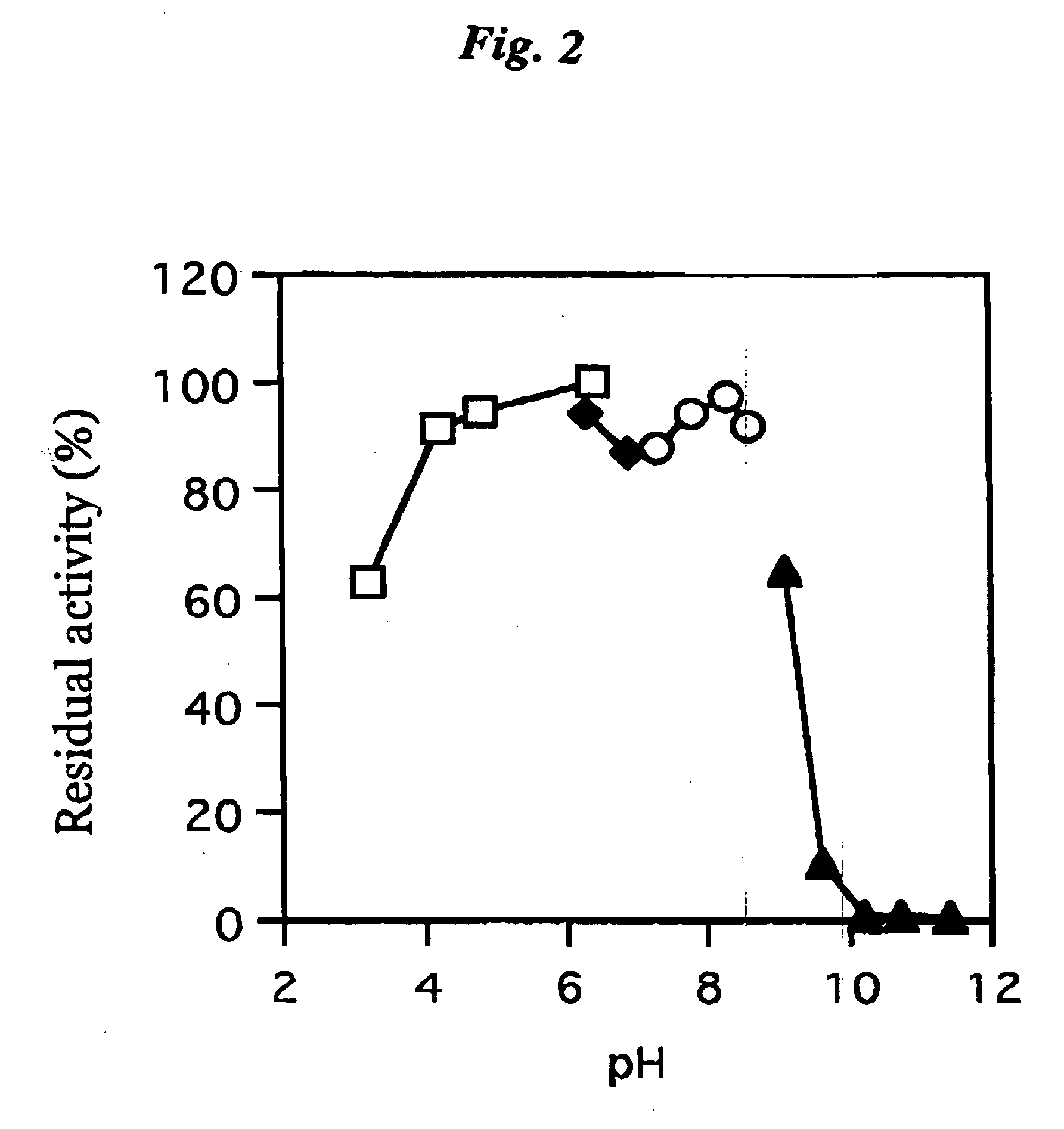
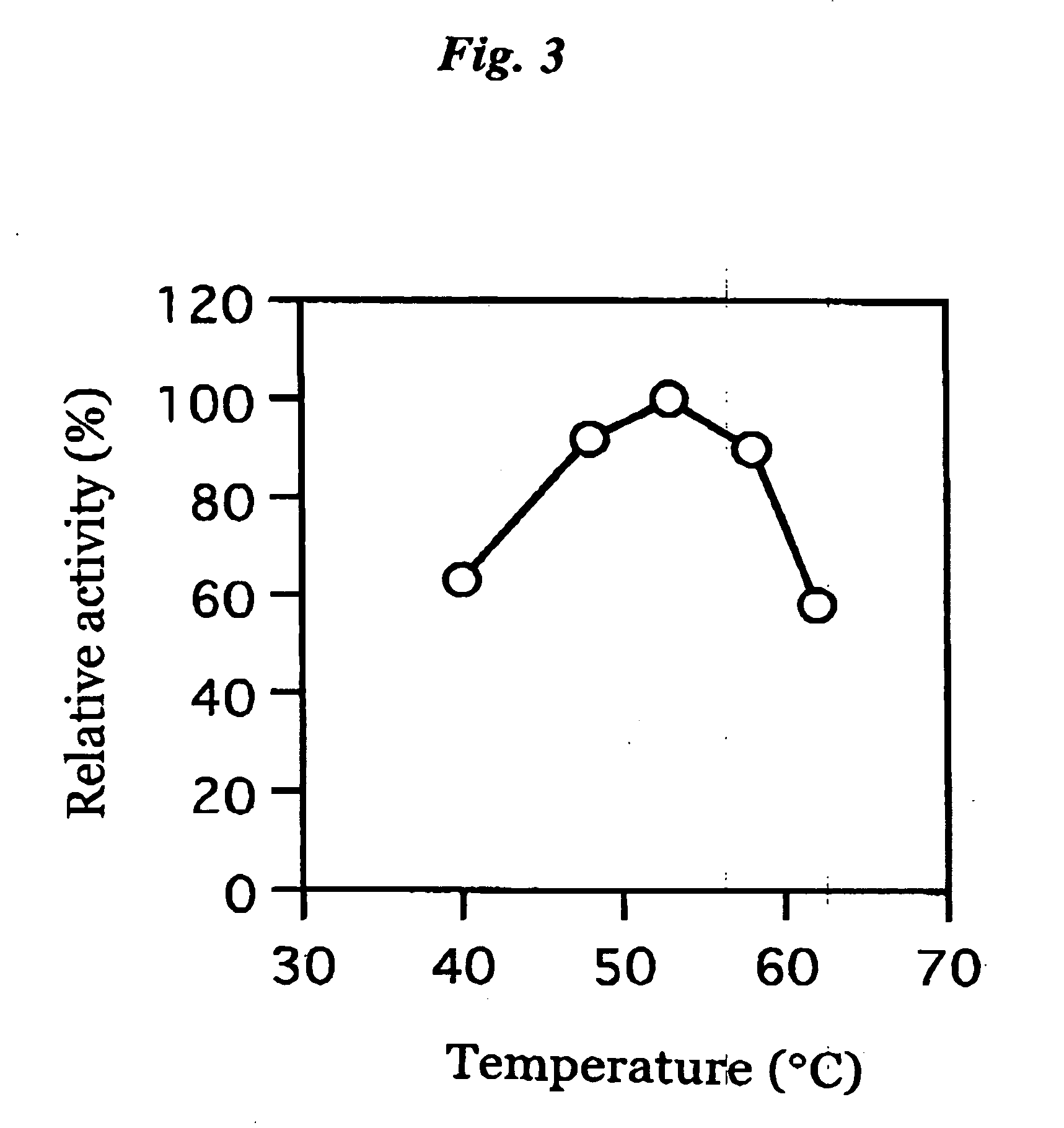
ActiveUS20060063217A1The process is convenient and fastImprove accuracyFungiMicrobiological testing/measurementGlucose sensorsPhenanthroline
The present invention provides a microorganism-derived soluble coenzyme-binding glucose dehydrogenase which catalyzes a reaction for oxidizing glucose in the presence of an electron acceptor, has an activity to maltose as low as 5% or less, and is inhibited by 1,10-phenanthroline. The invention also provides a method for producing the coenzyme-binding glucose dehydrogenase, and a method and a reagent for measuring employing the coenzyme-binding glucose dehydrogenase. According to the invention, the coenzyme-binding glucose dehydrogenase can be applied to an industrial field, and a use becomes possible also in a material production or analysis including a method for measuring or eliminating glucose in a sample using the coenzyme-binding glucose dehydrogenase as well as a method for producing an organic compound. It became also possible to provide a glucose sensor capable of accurately measuring a blood sugar level. Therefore, it became possible to provide an enzyme having a high utility, such as an ability of being used for modifying a material in the fields of pharmaceuticals, clinical studies and food products.
Owner:PHC CORP
Organic electroluminescent element
InactiveUS20060051615A1Improve balanceImprove luminous efficiencyDischarge tube luminescnet screensElectroluminescent light sourcesElectron holeHole injection layer
In an organic electroluminescent element in which a light emitting layer is disposed between a hole injection electrode and an electron injection electrode, and a hole injection layer is provided between the hole injection electrode and the light emitting layer, and an electron transport layer is provided between the electron injection electrode and the light emitting layer, the organic electroluminescent element is characterized in that a fluorocarbon layer is provided between the hole injection layer and the light emitting layer, and the electron transport layer is formed from a phenanthroline compound.
Owner:SANYO ELECTRIC CO LTD
Iridium complex phosphor material taking phthalazine derivative as ligand and preparation method thereof
InactiveCN102180909AImprove solubilityImprove hole transport abilityGroup 8/9/10/18 element organic compoundsLuminescent compositionsQuantum yieldIridium
The invention discloses an iridium complex phosphor material taking a phthalazine derivative as a ligand. The structure of the material is shown as formulas (I) and (II), wherein Ar represents aryl, substituted aryl, heterocycle aryl and substituted heterocycle aryl; R can be one of a halogen atom, alkyl, substituted alkyl, alkoxyl, aryloxy, an alkylthio group, arylthio, aromatic amino, aliphatic amino or a heterocyclic substituent; L^Y can be one of N-COOH, 8-hydroxyquinoline, beta-diketone, N^NH and the like; N^N can be bipyridyl, diquinoline, 1,10-phenanthroline, a derivative of the 1,10-phenanthroline and the like; and Z can be hexafluorophosphate and perchlorate. A dicyclic or ionic iridium complex phosphorescent electroluminescent device taking the phthalazine derivative as the ligand obtained with the preparation method has high internal and external quantum yields, high luminance and high stability. A dicyclic iridium complex is shown as a formula (I), i.e., (C^N)2Ir(L^Y); and an ionic iridium complex is shown as a formula (II), i.e., (C^N)2Ir(N^N)<+>Z<->.
Owner:NANJING UNIV OF POSTS & TELECOMM
Phenanthroline compound and light-emitting device
InactiveUS20060115676A1Improve efficiencyIncrease brightnessOrganic chemistryDischarge tube luminescnet screensArylHydrogen atom
A novel phenanthroline compound is provided which is suitable as a compound for an organic EL device and is represented by the general formula (I): wherein R1, R2, R3, R4, R5, R6, R7, and R8 are each independently a hydrogen atom, an alkyl group, a substituted or unsubstituted aralkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, or a halogen atom with the proviso that at least one of R1, R2, R3, R4, R5, R6, R7, and R8 is a group represented by the general formula (II): and R9, R10, R11, R12, R13, R14, R15, R16, R17, R18, R19, and R20 are each independently a hydrogen atom, an alkyl group, a substituted or unsubstituted aryl group, a substituted or unsubstituted heterocyclic group, or a halogen atom.
Owner:CANON KK
Method for bleaching lignocellulosic fibers
InactiveUS6136041AReduce processingSignificant to usePulp bleachingBleaching apparatusBleachPhenanthroline
PCT No. PCT / EP97 / 01865 Sec. 371 Date Dec. 30, 1998 Sec. 102(e) Date Dec. 30, 1998 PCT Filed Apr. 14, 1997 PCT Pub. No. WO97 / 39179 PCT Pub. Date Oct. 23, 1997A method of bleaching lignocellulosic fibers is disclosed which comprises the step of treating the lignocellulosic fibers with a bleaching composition comprising at least one oxidizing bleaching agent in aqueous solution in the presence of at least one additive which activates delignification or bleaching wherein the activating additive is a phenanthroline selected from the group consisting of 2,9-dimethyl-1,10-phenanthroline, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline, 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline-disulfonic acid-disodium salt-hydrate, and 3,4,7,8-tetramethyl-1,10-phenanthroline.
Owner:JASCHINSKI THOMAS +1
Copper (i) complexes for optoelectronic devices
ActiveUS20130150581A1Short possible emission decay timeDiminished roll-off behaviorFinal product manufactureGroup 5/15 element organic compoundsSolubilityChlorobenzene
The invention relates to neutral mononuclear copper (I) complexes for emitting light and with a structure according to formula (A) in which: M represents: Cu(I); L∩L represents: a single, negatively charged, bidentate ligand; N∩N represents: a diimine ligand (substituted with R and FG), in particular a substituted 2,2′-bipyridine derivative (bpy) or a substituted 1,10-phenanthroline derivative (phen); R represents: at least one sterically demanding substituent for preventing the planarisation of the complex in the excited state; FG=functional group, and represents: at least one second substituent for increasing solubility in organic solvents. The substituent can also be used for electron transport or alternatively for hole transport, said functional group being bound to the diimine ligands either directly or by means of suitable bridges; and the copper (I) complex: having a ΔE(S1−T1) value of less than 2500 cm−1 between the lowest excited singlet state (S1) and the triplet state (T1) which lies below; having an emission lifespan of at most 20 μs; having an emission quantum yield of greater than 40%, and a solubility of at least 1 g / L in organic solvents, in particular polar organic hydrocarbons such as acetone, methyl ethyl ketone, benzene, toluene, chlorobenzene, dichlorobenzene, dichloromethane, chloroform, dichloroethane, tetrachloroethylene, alcohols, acetonitrile or water.
Owner:SAMSUNG DISPLAY CO LTD
High efficiency transparent organic light emitting devices
InactiveUS6885149B2Improve efficiencyHighly efficient transparent cathodeSolid-state devicesSemiconductor/solid-state device manufacturingElectron injectionPhenanthroline
A highly transparent non-metallic cathode is disclosed that comprises a metal-doped organic electron injection layer that is in direct contact with a transparent non-metallic electron injecting cathode layer, such as indium tin oxide (ITO), wherein the metal-doped organic electron injection layer also functions as an exciton blocking or hole blocking layer. The metal-doped organic electron injection layer is created by diffusing an ultra-thin layer of about 5-10 Å of a highly electropositive metal such as Li throughout the layer. A representative embodiment of the highly transparent non-metallic cathode comprises a layer of ITO, a layer of 2,9-dimethyl-4,7-diphenyl-1,10-phenanthroline (BCP), which acts as an electron injection, exciton blocking, and hole blocking layer, and an ultra-thin layer of lithium, which degenerately dopes the layer of BCP, improving the electron injecting properties of the BCP layer. This cathode is demonstrated for use in an OLED having a transparency of about 90% or higher combined with a device external quantum efficiency of about 1% or higher.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Electroluminescent device including an anthracene derivative
ActiveUS20070252522A1Improve electroluminescence performanceDrive voltage and efficiencyDischarge tube luminescnet screensLamp detailsAnthracenePhenanthroline
An electroluminescent device comprises a cathode, an anode, and has therebetween a light emitting layer (LEL), the device further containing an electron transport layer (ETL) comprising an anthracene compound on the cathode side of the LEL and an organic electron injection layer (EIL) between the ETL and the cathode comprising a phenanthroline compound, wherein the thickness of the EIL and LEL are such that the ratio of the thickness of the EIL to LEL is greater than 0.125.
Owner:GLOBAL OLED TECH
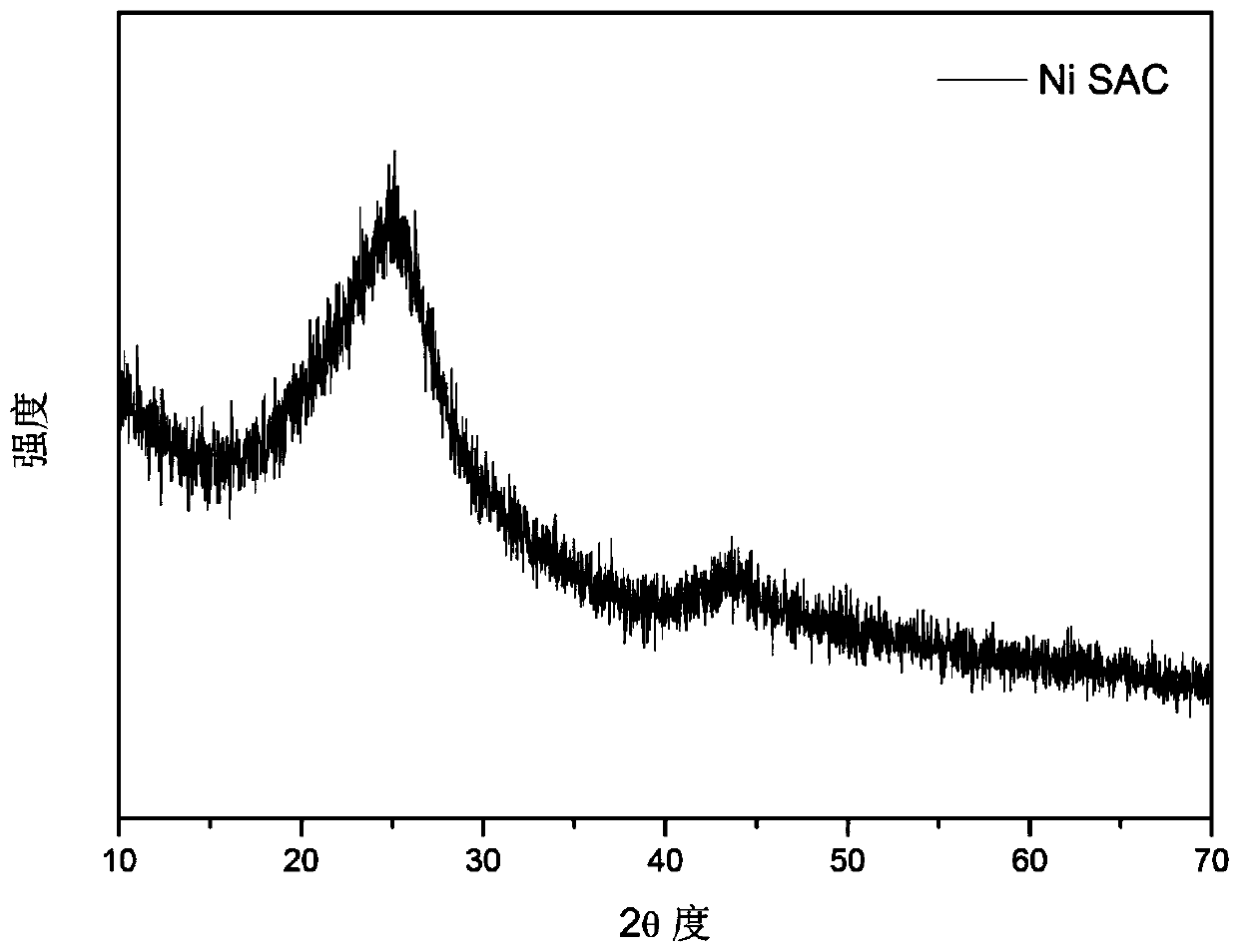
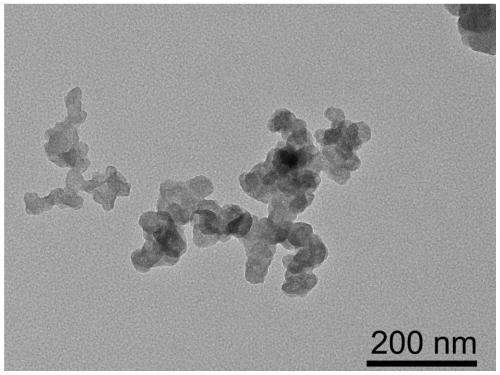
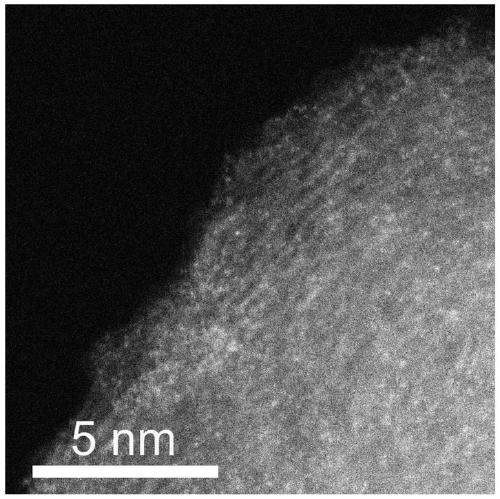
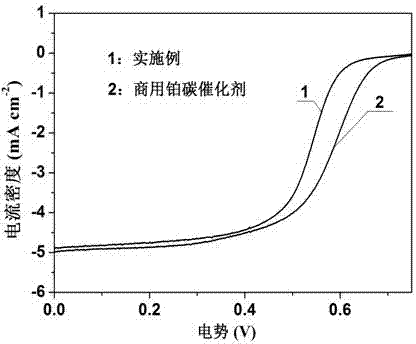
Transition metal single-atom catalyst as well as preparation method and application thereof
InactiveCN109908904AIncrease loadLow priceMetal/metal-oxides/metal-hydroxide catalystsElectrodesFaraday efficiencyPhenanthroline
The invention provides a transition metal single-atom catalyst. In the catalyst, transition metal single atoms are taken as active ingredients, carbon black is taken as a carrier, the loading capacityof the transition metal single atoms is 0.5-6wt%, and the highest loading capacity of the transition metal single atoms is higher than that of the prior art by 2-3 times. Various low-price transitionmetal single-atom catalysts with high dispersibility and high loading capacity are formed by inducing a limit range and by taking phenanthroline with strong complexation effect as a ligand and takingthe carbon black with high conductivity and high surface activity as the carrier. A preparation method of the transition metal single-atom catalyst has universality and can be used for preparing various low-price transition metal single-atom catalysts; according to the method, a Cr single-atom catalyst, an Nb single-atom catalyst, an Rh single-atom catalyst, a Cd single-atom catalyst, an Os single-atom catalyst and an Ir single-atom catalyst are prepared for the first time. Additionally, the preparation method has the advantages of being low in cost and simple in process and achieving large-scale production. Next, the catalyst has higher selectivity and Faraday efficiency in application of preparation of carbon monoxide by reducing carbon dioxide through electro-catalysis.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Electroluminescent devices including organic eil layer
ActiveUS20100084647A1Improved electroluminescent featureLow working voltageSolid-state devicesSemiconductor/solid-state device manufacturingElectronic transmissionFluoranthene
An OLED device comprises a cathode, an anode, and has therebetween a light emitting layer (LEL) comprising a phosphorescent emitting compound disposed in a host comprising a mixture of at least one electron transporting co-host which is a benzophenone derivative with a spiro substituent and at least one hole transporting co-host which is a triphenylamine which contains one trivalent nitrogen atom that is bonded only to carbon atoms, at least one of which is a member of an aromatic ring, wherein there is present an electron transporting layer contiguous to the LEL (HBL?) on the cathode side comprising an anthracene or a fluoranthene and wherein there is present an election injecting layer comprising a phenanthroline or a lithium quinolate contiguous to the cathode.
Owner:GLOBAL OLED TECH
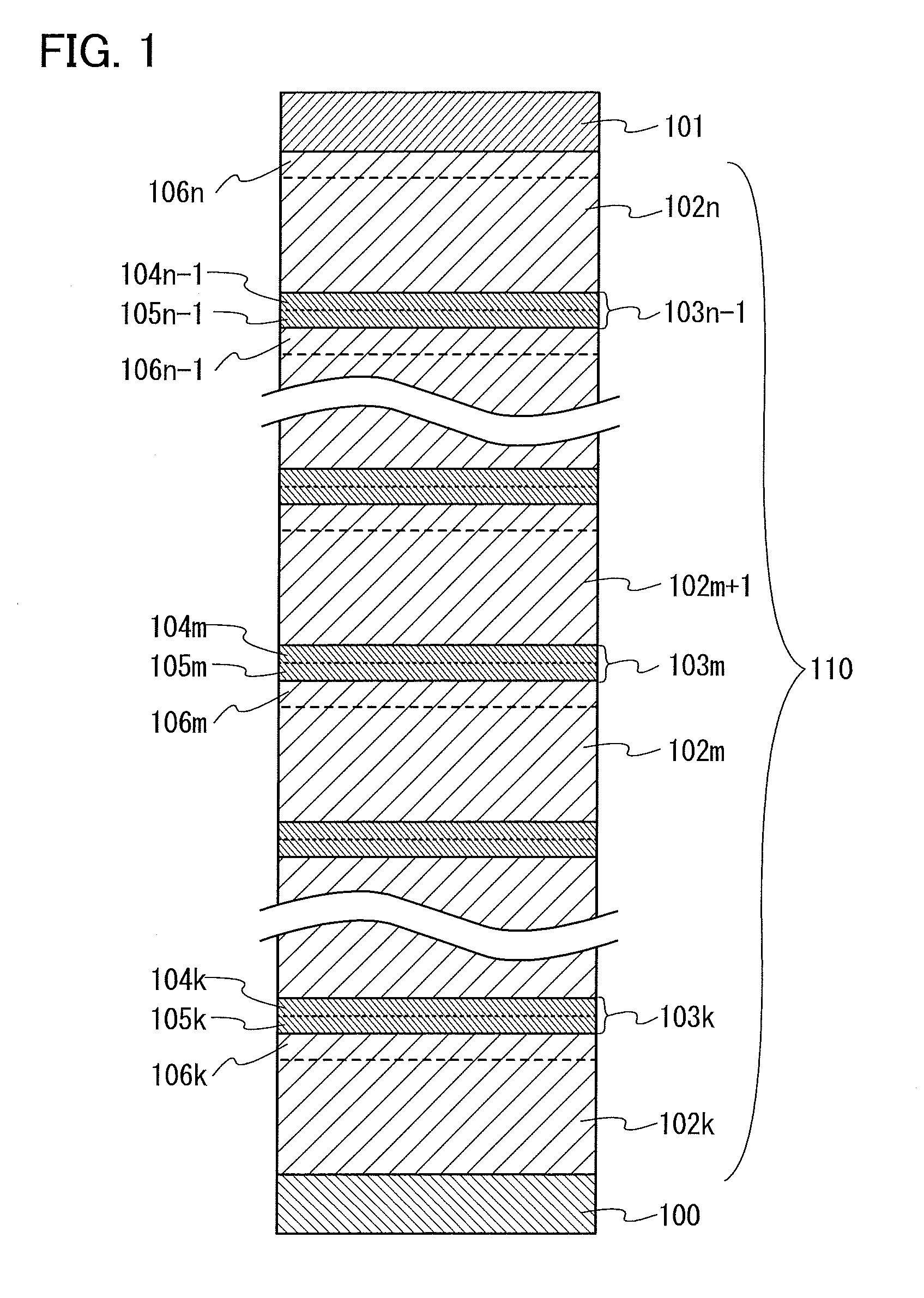
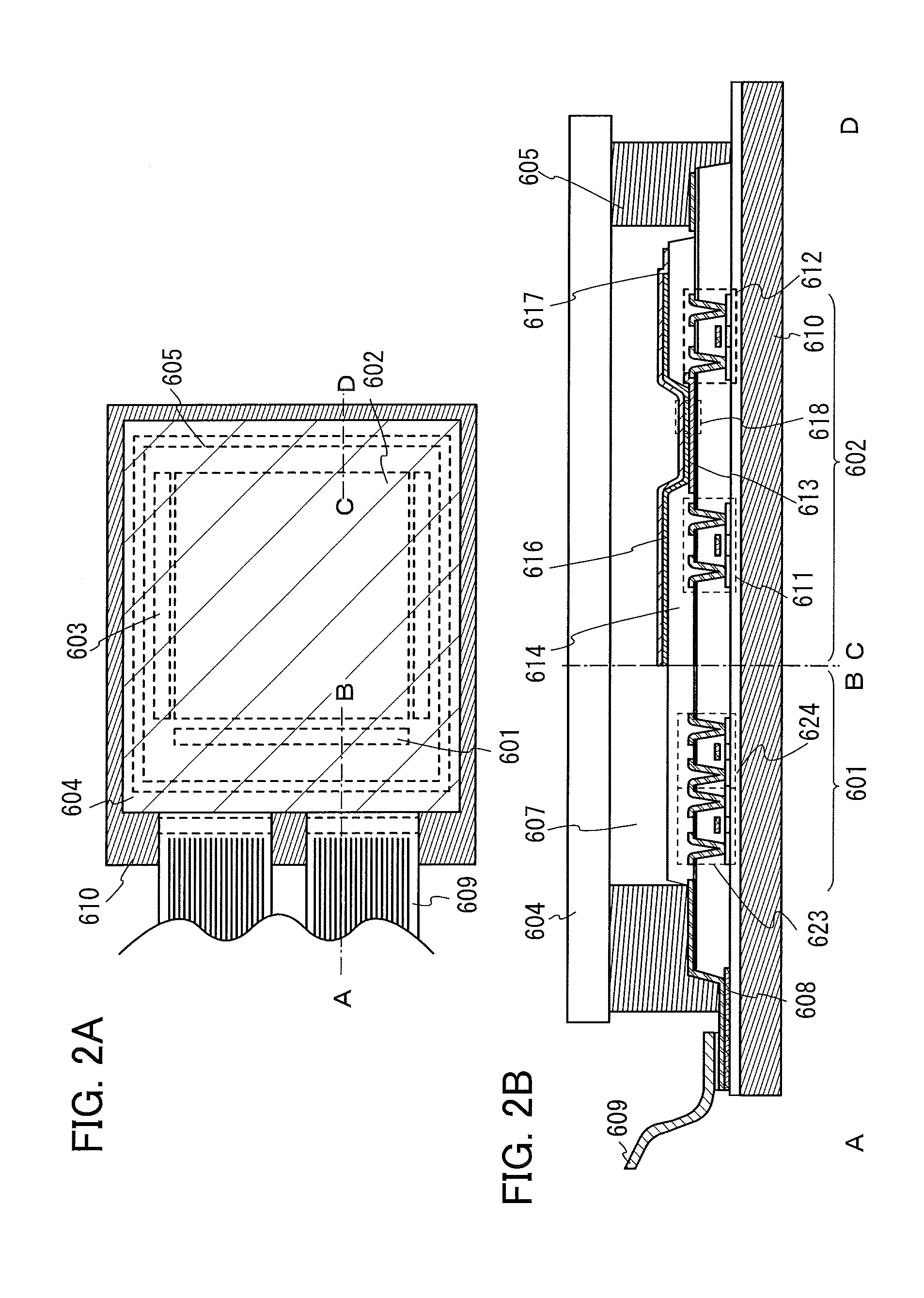
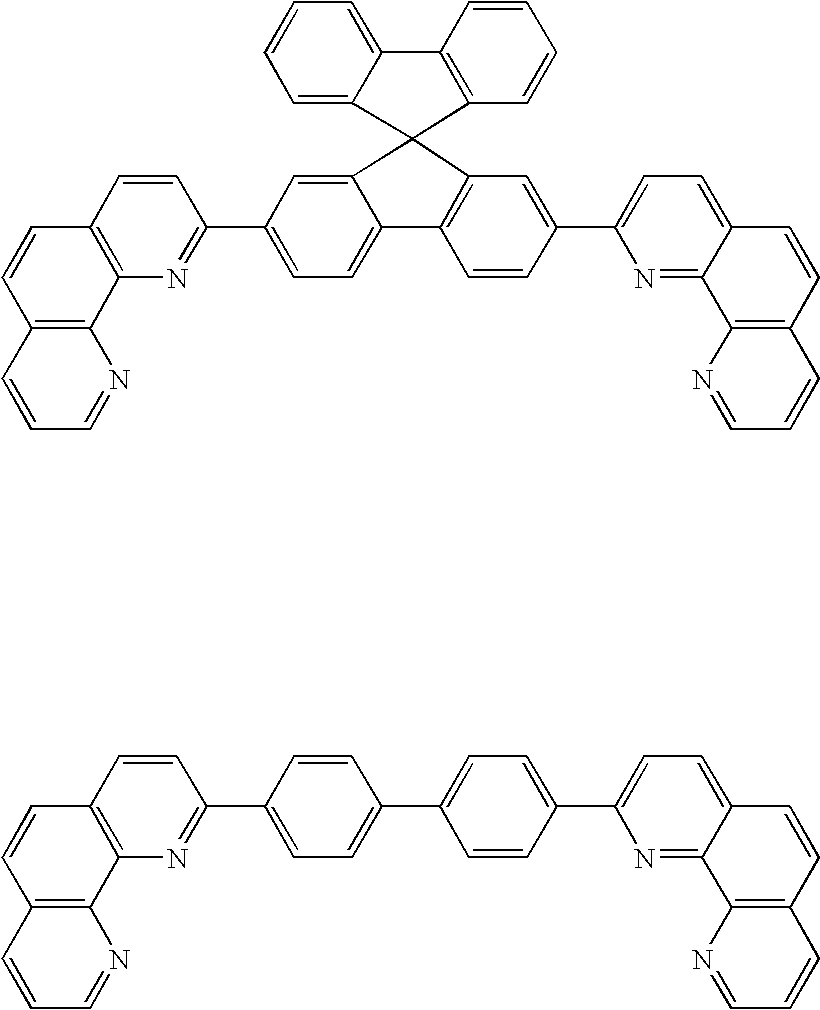
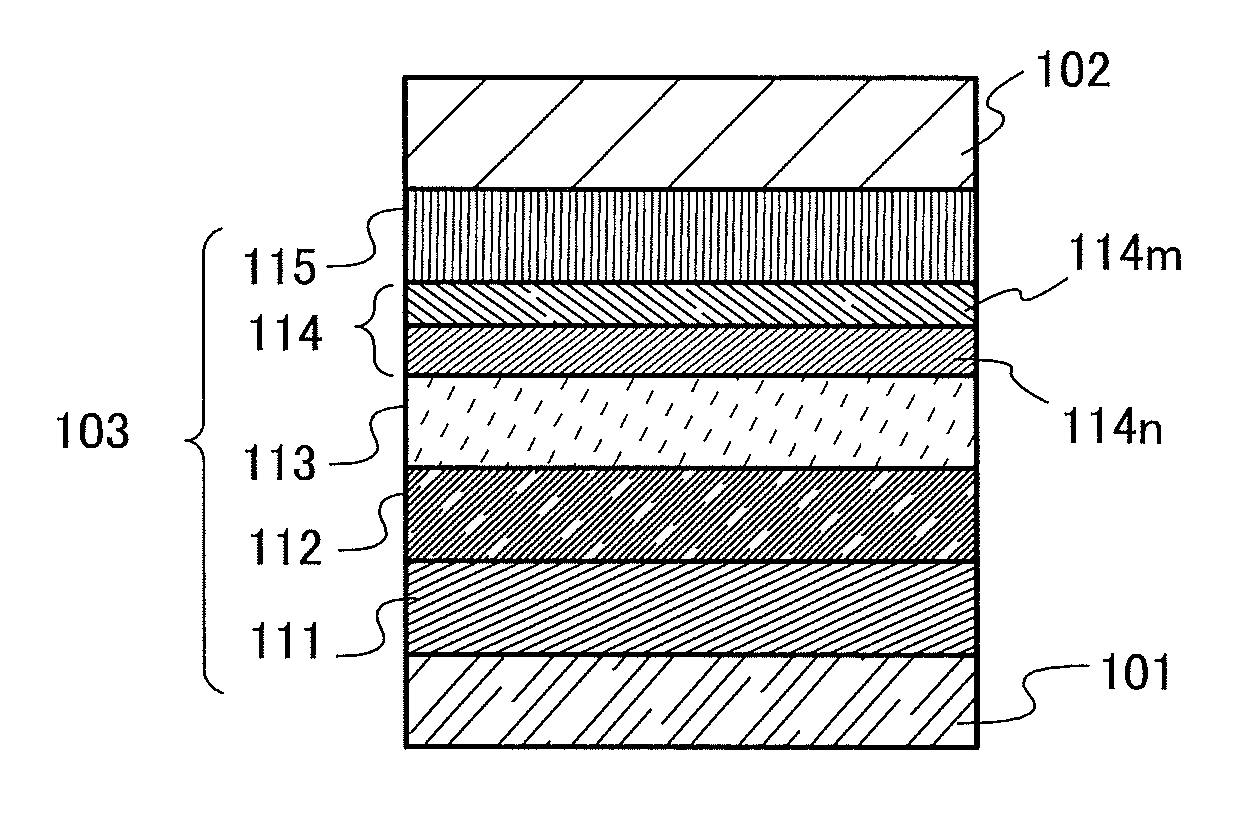
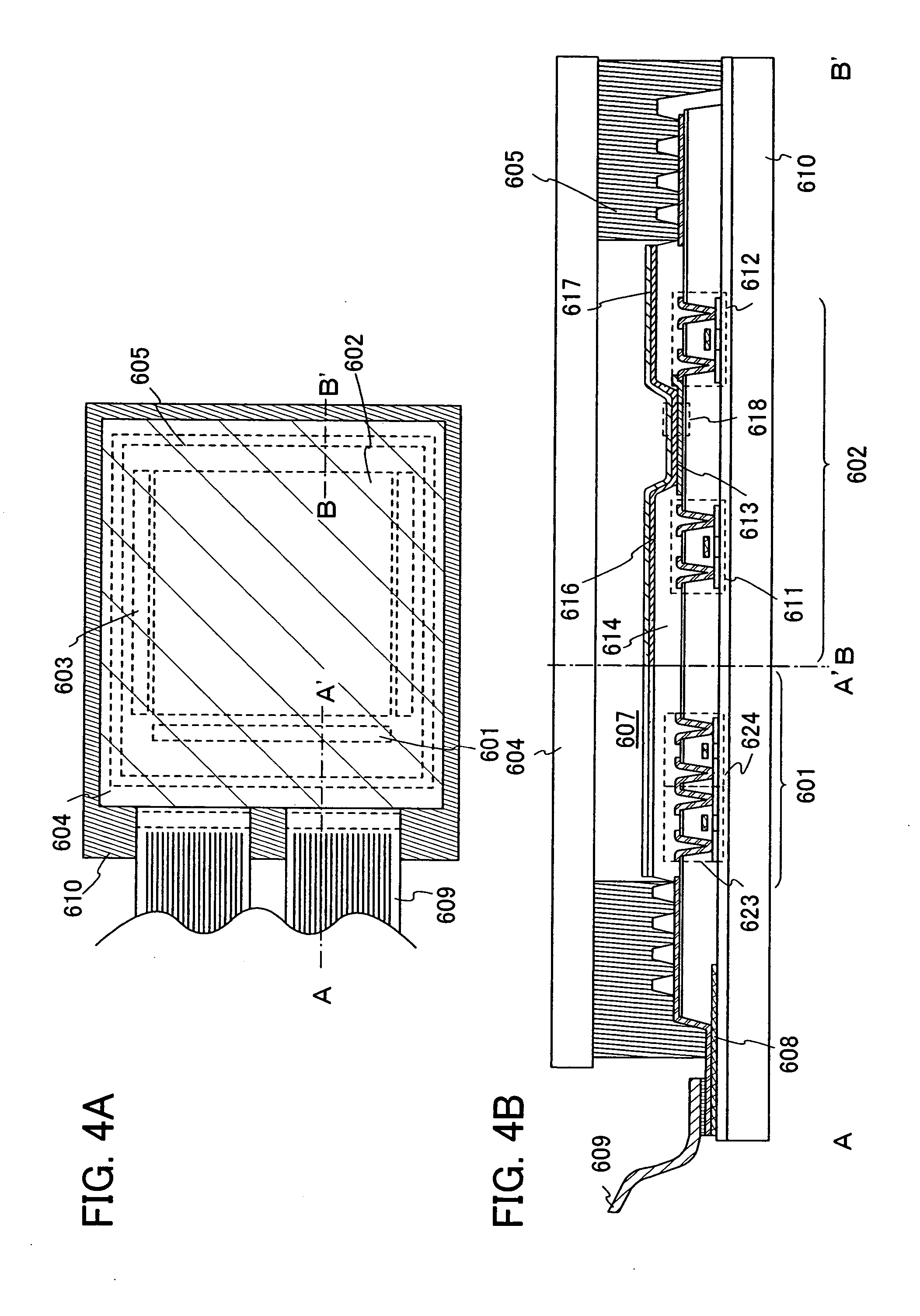
Light-emitting element, display module, lighting module, light-emitting device, display device, electronic device, and lighting device
ActiveUS9515278B2Suppress generationHigh quality imagingSolid-state devicesSemiconductor/solid-state device manufacturingPhenanthrolineComputer module
A tandem light-emitting element in which generation of crosstalk can be suppressed even when the element is applied to a high-definition display is provided. In the tandem light-emitting element, a layer in contact the anode side of an intermediate layer contains 2,9-bis(naphthalen-2-yl)-4,7-diphenyl-1,10-phenanthroline (abbreviation: NBPhen).
Owner:SEMICON ENERGY LAB CO LTD
Preparation method of general-purpose multi-metal sulfide nano-material
InactiveCN102633297AHigh yieldAdjustable phaseTin compoundsGallium/indium/thallium compoundsPhenanthrolineDiethyldithiocarbamic Acid
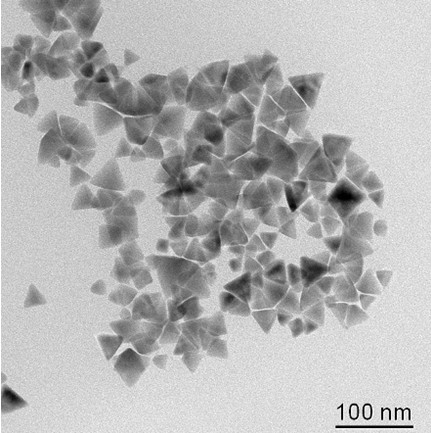
The invention discloses a preparation method of a general-purpose multi-metal sulfide nano-material. The preparation method comprises the following steps of: taking two or more different metal diethyldithiocarbamates, or two or more different metal phenanthroline diethyldithiocarbamates, or the combination of the metal diethyldithiocarbamates and the metal phenanthroline diethyldithiocarbamates as reaction predecessors, performing co-thermal decomposition in a mixed solution of surfactants with different coordination properties, and performing nucleation and growth so as to prepare a high-quality multi-metal sulfide nano-material by one step. The multi-metal sulfide prepared by the preparation method disclosed by the invention has the advantages of pure phase state, adjustable composition, uniform and controllable appearance and size, high yield, low toxicity or no toxicity, better dispersivity in a non-polar organic solvent, easiness in control of reaction conditions, mild preparation conditions, simple operation, good repeatability and capability of realizing large-scale production.
Owner:SUZHOU INST OF NANO TECH & NANO BIONICS CHINESE ACEDEMY OF SCI
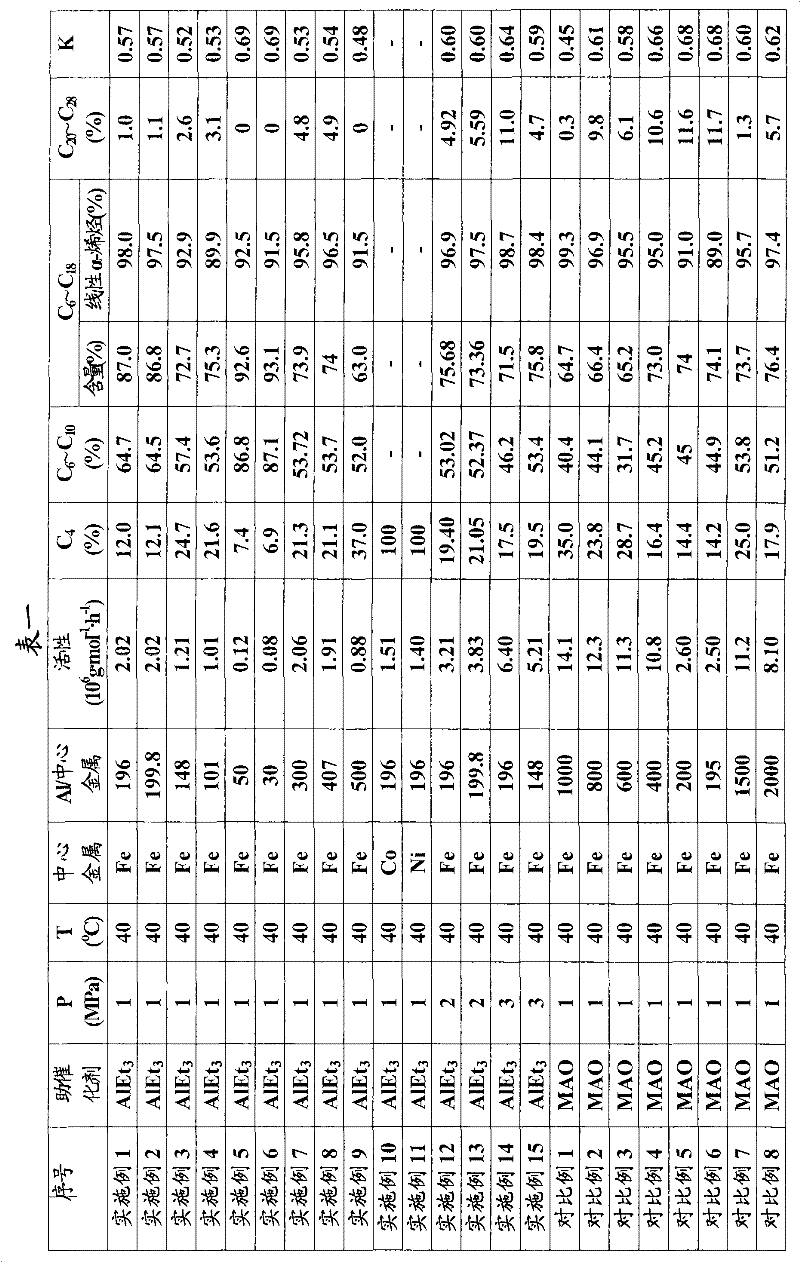
Ethylene oligomerization method
InactiveCN102206127ALow molar ratioMolar ratio (Al/M) decreasedHydrocarbonsHydrocarbon preparationPhenanthrolineChloride
The invention provides an ethylene oligomerization method. Chloride of Fe<2+>, Co<2+> and Ni<2+> with 2-imino-1, 10-phenanthroline coordinated is taken as a main catalyst, Triethylaluminium is taken as a cocatalyst for replacing a high-price methylaluminoxane and the like, and the mol ratio of aluminum in the cocatalyst of triethylaluminium to central metal in the main catalyst is more than 30 and less than 200 or more than 200 and less than 500, The amount of the cocatalyst is greatly reduced, therefore the cost of the ethene oligomerization reaction is greatly reduced, the invention has a broad industrialization prospect.
Owner:CHINA PETROLEUM & CHEM CORP
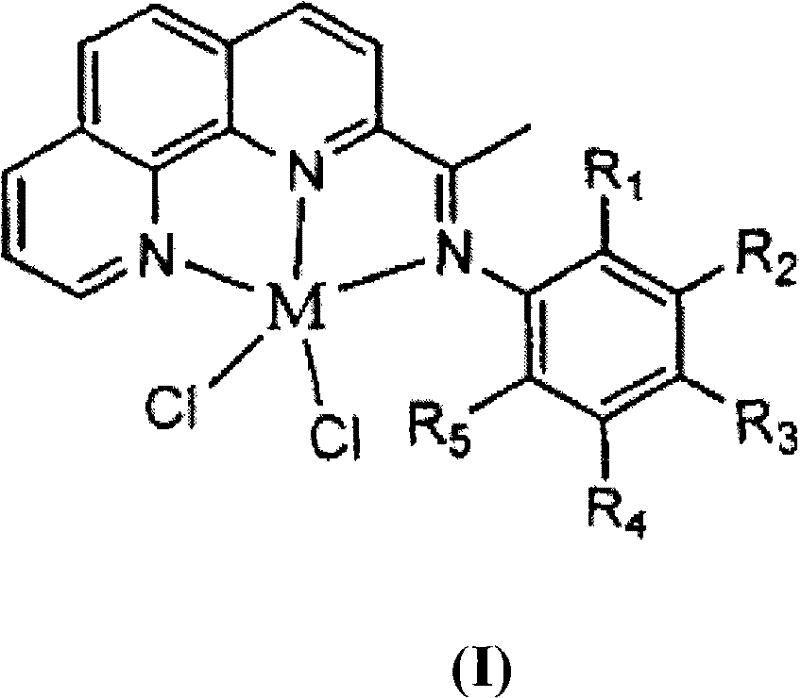
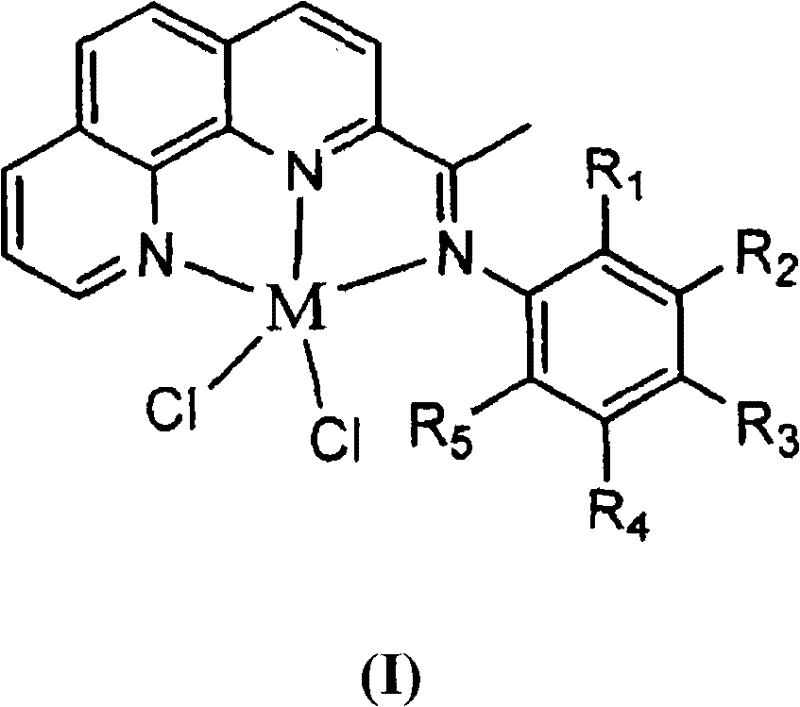
2-imino-9-phenyl-1,10-phenanthroline transient metal complex, and preparation and use thereof
InactiveCN101348501AHigh selectivityHigh catalytic activityGroup 8/9/10/18 element organic compoundsHydrogenPhenanthroline
The invention discloses a 2-imdo group-9-phenyl group-1, 10-phenanthrolines transition metal complex and a preparation method thereof and an application thereof. The structural formula of the 2-imdo group-9-phenyl group-1, 10-phenanthrolines transition metal complex of the invention is shown as the formula(I), wherein M is transition metal, and R and R<1> are independently selected from hydrogen, alkyl and phenyl respectively. In the invention, the ligand and the transition metal complex of 2-imdo group-9-phenyl group-1, 10-phenanthrolines containing N^N^N ligand are designed and synthesized; when the transition metal complex is used to catalyze the ethylene oligomerization reaction, the transition metal complex performs good catalytic activity and high selectivity of 1-butylene, so that the transition metal complex has broad industrial application prospect.
Owner:INST OF CHEM CHINESE ACAD OF SCI
Organic electroluminescent device
InactiveUS20050287393A1Improve emission efficiencyEnhanced lifetime characteristicDischarge tube luminescnet screensElectroluminescent light sourcesDopantCarbazole
An organic EL device which has a light emission layer between a pair of electrodes. The light emission layer is formed of a phosphorescent dopant and a host including (i) a carbazole compound and (ii) one or more selected from an oxadiazole compound, a phenanthroline compound, a triazine compound, and a triazole compound. Since the host has both hole transport property and electron transport property, the organic EL device has enhanced emission efficiency and lifetime characteristics even in the absence of a hole-blocking layer.
Owner:SAMSUNG SDI CO LTD
Preparation method and application of nitrogen-doped porous carbon nano sheet composite material
The invention discloses a preparation method of a nitrogen-doped porous carbon nano sheet composite material. The preparation method comprises the steps of performing high-temperature carbonization treatment on a mixture which consists of melamine and adjacent phenanthroline iron and serves as a precursor in a tubular furnace under an inert gas environment, and then removing dissolved iron compounds from an acidic solution to obtain a porous carbon nano sheet layer with carbon-coated iron carbide nano particles. The preparation method has the advantages that the technology is simple, the raw materials are cheap, and operation is easy to implement; in the prepared composite material, iron carbide is uniformly dispersed in the carbon nano sheet layer, so that the composite material is high in specific surface area and pore volume; iron carbide nano particles are completely coated by graphitized carbon, so that oxidization and corrosion are hardly caused; the composite material is stable in acidic electrolyte, and the battery activity can be effectively improved; when used as an electrocatalyst, the composite material is relatively high in electrocatalysis efficiency; the preparation method has an important value and significance in the field of preparation of doped carbon nano composite materials and electrocatalysis of proton membrane fuel batteries.
Owner:NANKAI UNIV
Electroluminescent device containing a phenanthroline derivative
InactiveUS20070122657A1Good equipment stabilityIncrease brightnessDischarge tube luminescnet screensLamp detailsPhenanthrolinePerylene derivatives
An OLED device comprises a cathode, an anode, and a light-emitting layer therebetween, and additionally comprises a layer between the cathode and the light-emitting layer including a phenanthroline compound comprising no more than two optionally substituted phenanthroline moieties wherein one and only one group substituted on a phenanthroline nucleus includes an aromatic system comprising four or more fused aromatic rings. Such a device provides desirable electroluminescent properties, such as increased luminance and reduced drive voltage, as well as good device stability.
Owner:GLOBAL OLED TECH
Light-Emitting Element, Display Module, Lighting Module, Light-Emitting Device, Display Device, Electronic Device, and Lighting Device
ActiveUS20150041795A1Improve heat resistanceReduce power consumptionSolid-state devicesSemiconductor/solid-state device manufacturingHeat resistanceDisplay device
A light-emitting element with improved heat resistance is provided without losing its advantages such as thinness, lightness, and low power consumption. A light-emitting element is provided which includes a first electrode, a second electrode, and an EL layer between the first electrode and the second electrode, in which the EL layer includes a layer containing a condensed aromatic compound or a condensed heteroaromatic compound, and a layer containing 2,9-bis(naphthalen-2-yl)-4,7-diphenyl-1,10-phenanthroline (abbreviation: NBPhen) in contact with the layer containing the condensed aromatic compound or the condensed heteroaromatic compound.
Owner:SEMICON ENERGY LAB CO LTD
Luminescent element material and luminescent element comprising the same
InactiveUS7318966B2Efficient transportEfficiently inhibit transportMethine/polymethine dyesSolid-state devicesPhenanthrolineLight emitting device
Owner:TORAY IND INC
Phenanthroline derivative compound
InactiveUS20070037983A1Efficient transferGood electron transport propertiesOrganic chemistryDischarge tube luminescnet screensArylPhenanthroline
The present invention provides a novel phenanthroline derivative compound, and a manufacturing method thereof, and an electron transporting material, a light emitting element, a light emitting device, and an electronic device using the phenanthroline derivative compound. The phenanthroline derivative compound is characterized by being represented by General Formula 1. Note in General Formula 1 that Ar1 represents an aryl group, and preferably a substituted or unsubstituted phenyl group, a substituted or unsubstituted naphthyl group, a substituted or unsubstituted anthryl group, or a substituted or unsubstituted phenanthryl group. As an example of a preferable compound, there is TMPBP, NaBP, PBP, or the like. A light emitting element, a light emitting device, and an electronic device preferably contain the compound in a light emitting layer, and in that case, the compound is preferably utilized as a host.
Owner:SEMICON ENERGY LAB CO LTD
Surface functionalized nano-particle and method for preparing its polymer nanometre composite material
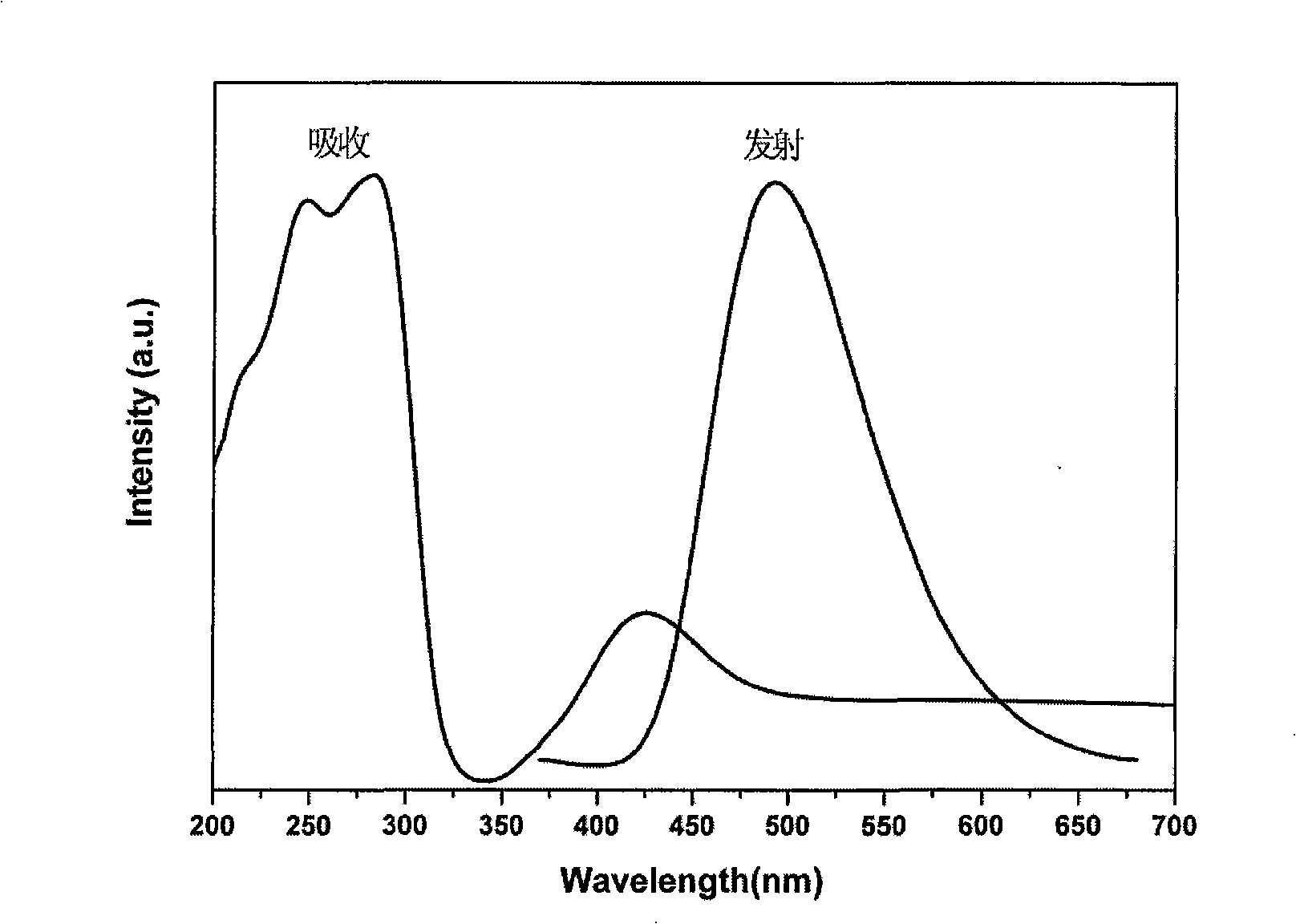
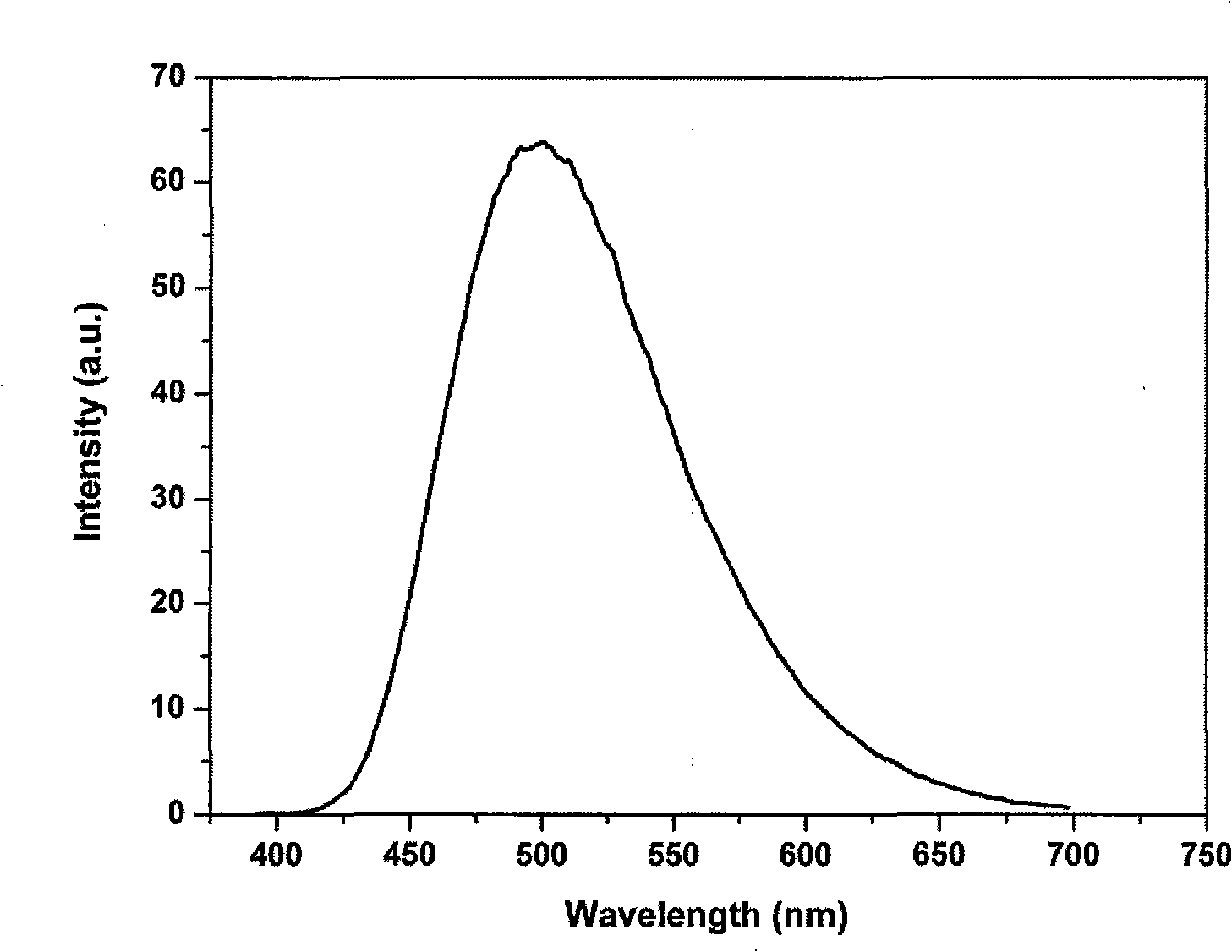
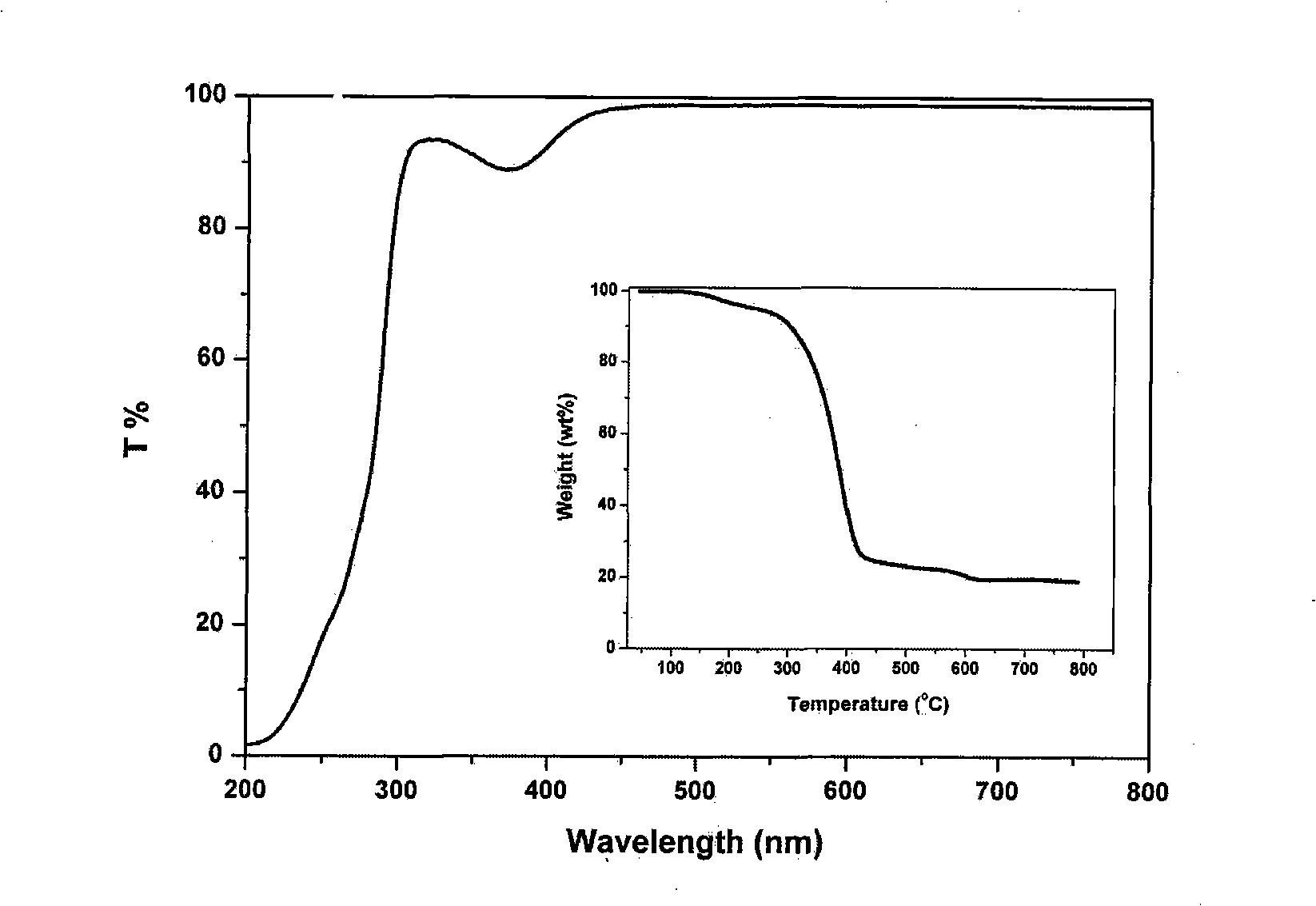
InactiveCN101343536AUnique optical propertiesImprove mechanical propertiesSolid-state devicesSemiconductor/solid-state device manufacturingFluorescencePhenanthroline
The invention relates to nanometer particle surface functionalization and the preparation method of the transparent polymer nanometer composite material, which belongs to the chemical material field. The functionalization for the surface of the nanometer particles can be performed by using an organic small molecule with a nitrogen-containing function (eg. 8-hydroxy quinoline and phenanthroline derivative) though a method of ligand exchanging or direct situ decorating. The functional nanometer particles can be coated directly during the synthetic process or synthetized through ligand exchanging later, and the nanometer particles have a good fluorescence property. The functionalized nanometer particles and the polymer are compounded for preparing the transparent polymer nanometer composite material through a solution co-blending method and a situ bulk polymerization method. The method for functionalizing nanometer particle integrate the functions of organic functional molecules and the functions of nanometer particles into one, and a novel approach for constructing novel functional nanometer particles is provided. The prepared functional nanometer particles / polymer composites material have important application value in the fields, such as photoelectric apparatus, display devices and solar batteries, and the like.
Owner:NORTHEAST NORMAL UNIVERSITY
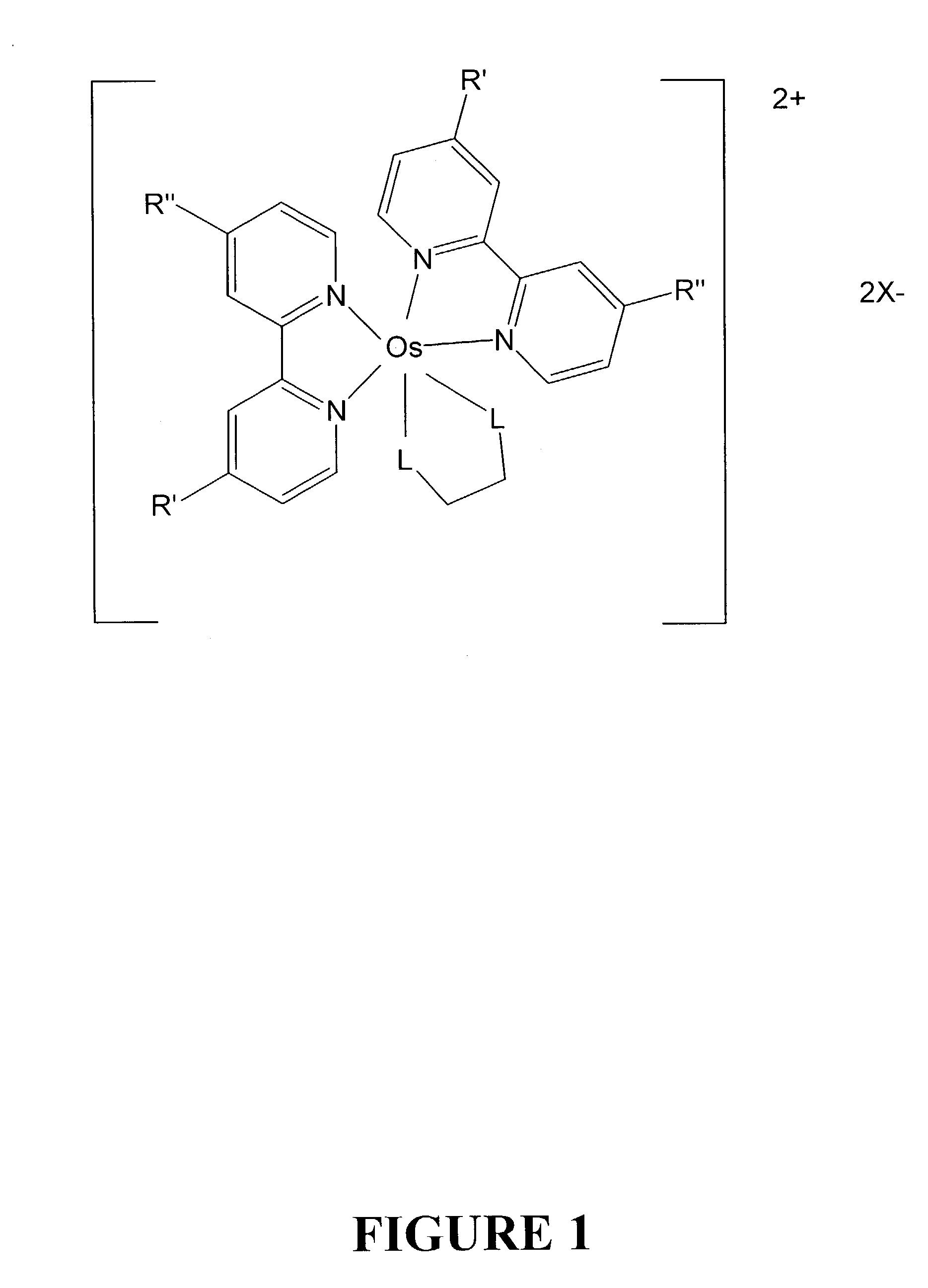
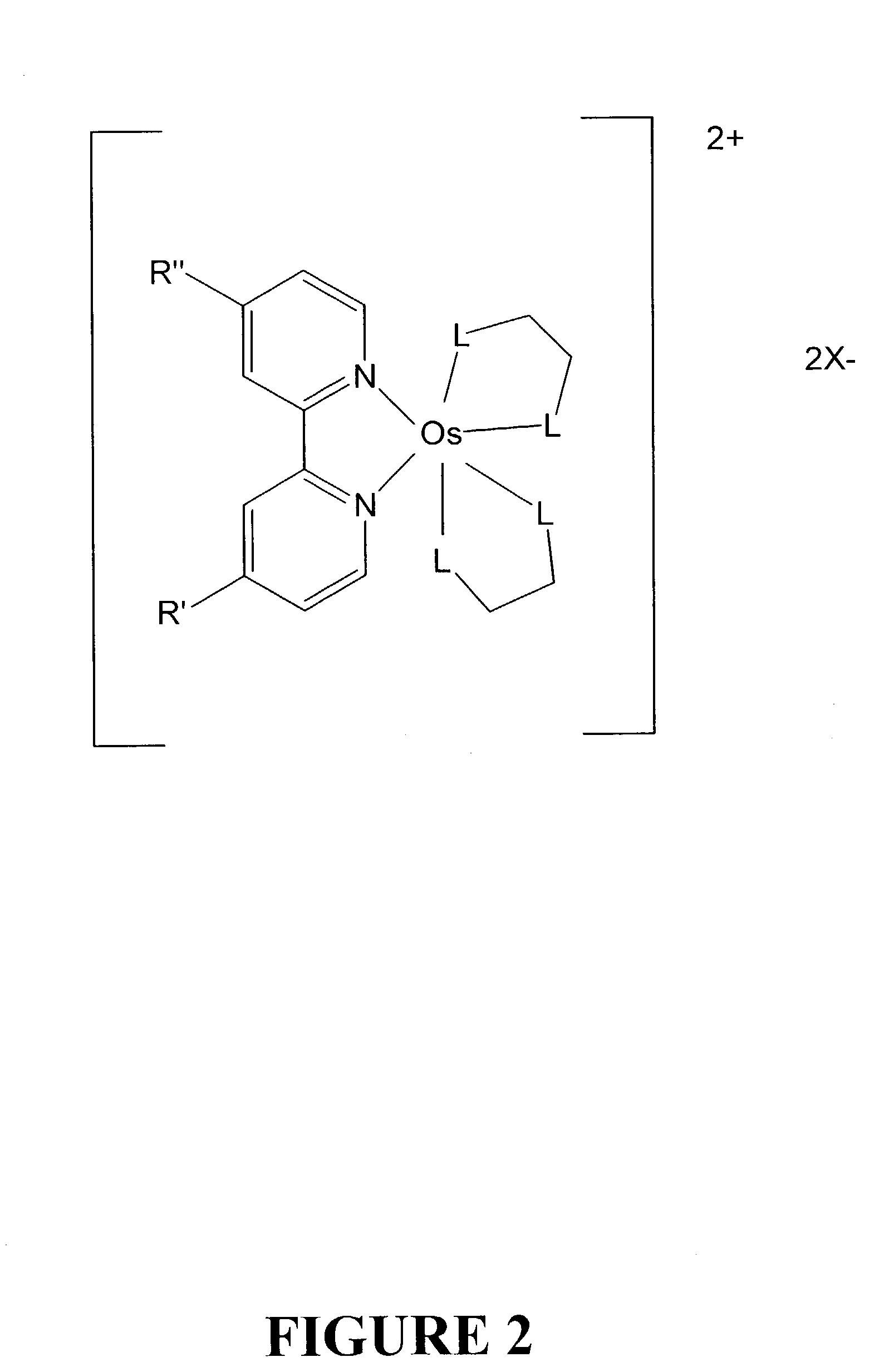
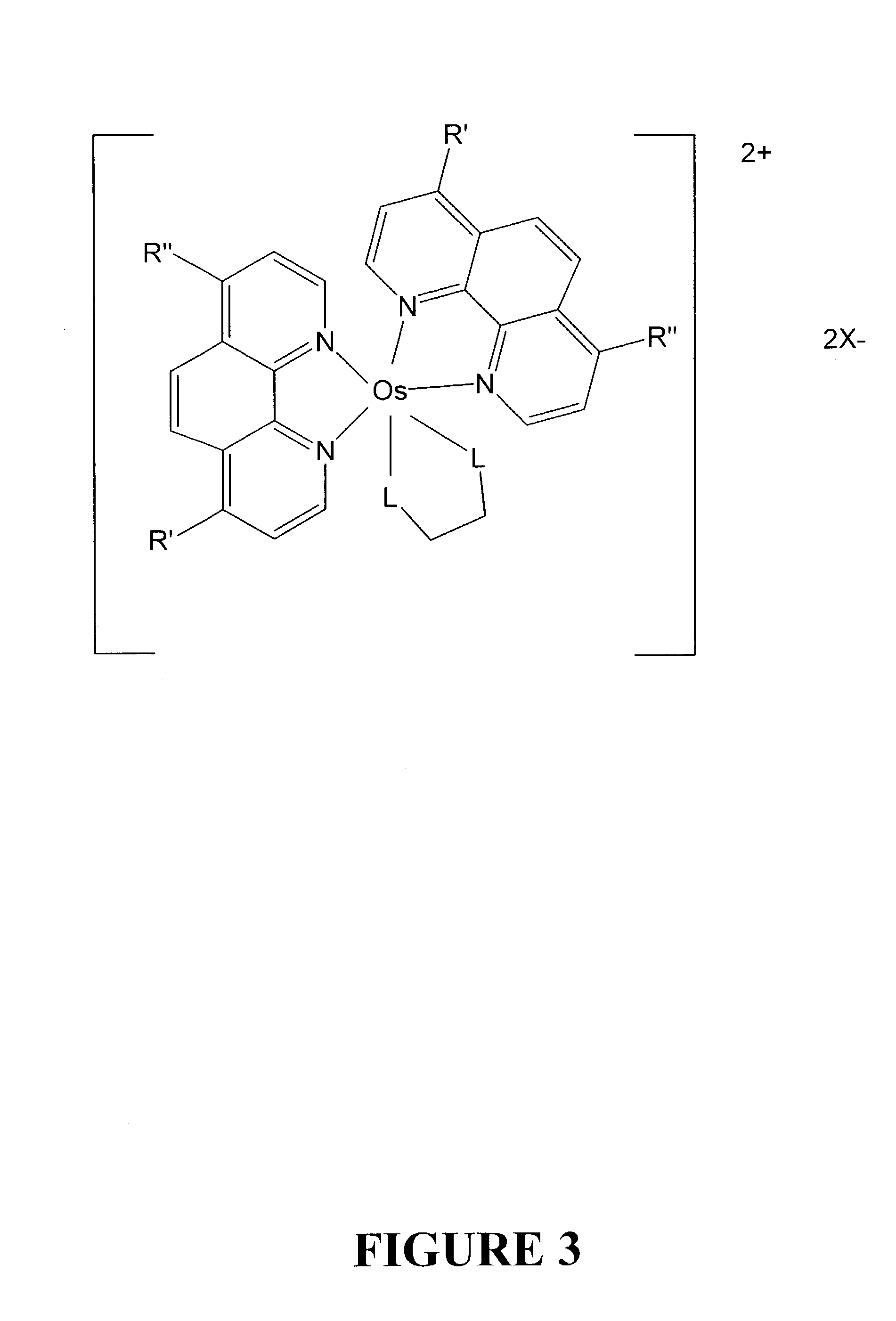
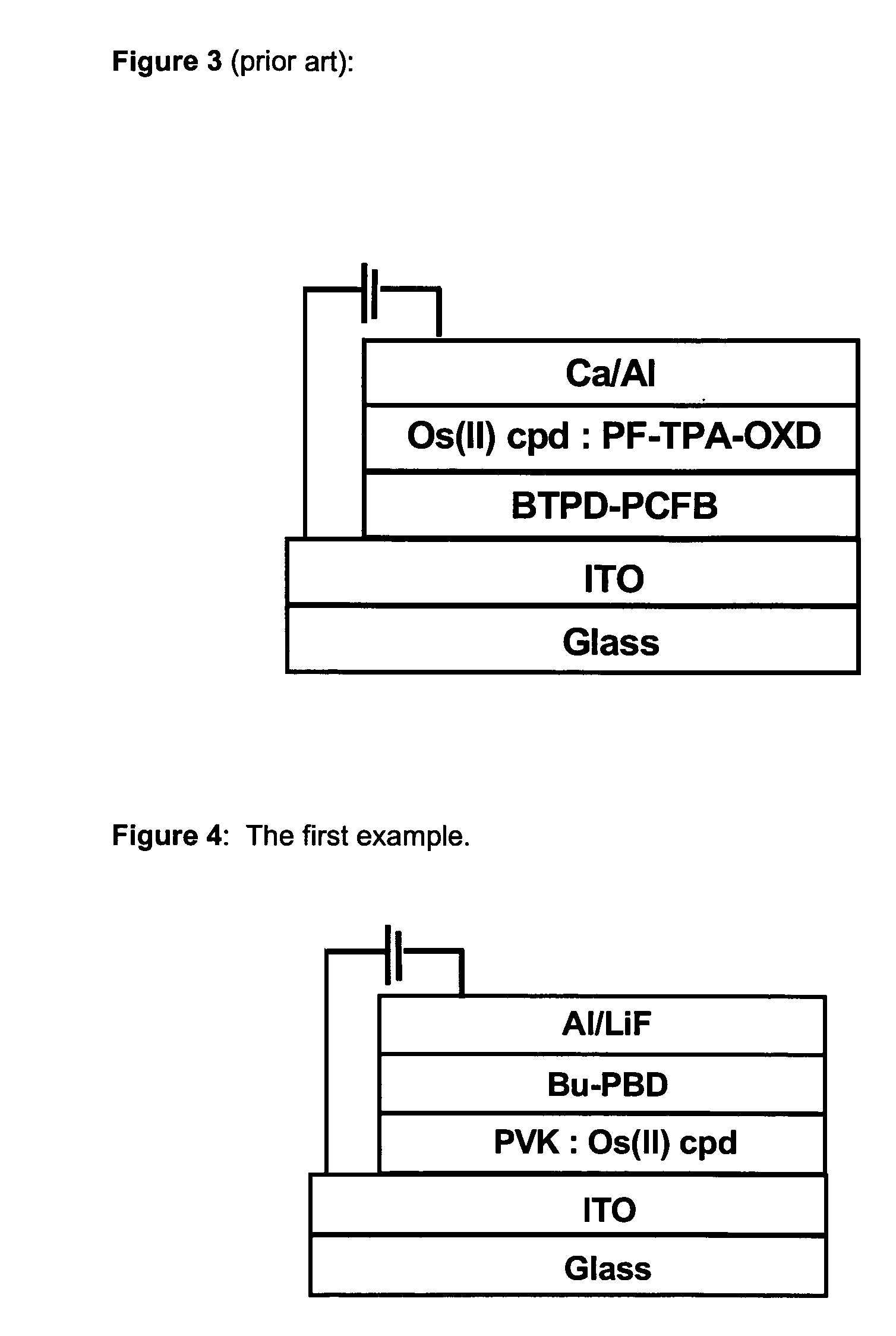
Osmium complexes and related organic light-emitting devices
InactiveUS7416791B1Discharge tube luminescnet screensGroup 8/9/10/18 element organic compoundsOrganic light emitting devicePhenanthroline
Osmium complexes having the formula [Os(II) (N—N)2L—L]2+ 2A− (or A2−), or [Os(II) N—N(L—L)2]2+ 2A− (or A2−), where N—N is a bipyridine or phenanthroline ligand, L—L is a π-acid bidentate ligand, and A is counter ion.
Owner:UNIV OF WASHINGTON
Diode-addressed color display with lanthanoid phosphors
InactiveUS6165631AImprove quantum efficiencyImprovement factorDischarge tube luminescnet screensCathode ray tubes/electron beam tubesEthylenediamineDisplay device
A diode-addressed color display comprising an UV-diode and a phosphor of the general formula LnL3X2, wherein Ln=Eu3+, Tb3+, Tm3+, Dys3+, Sm3+, L=4-R-4'-benzophenone carboxylic acid, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-R-4'-benzophenone acetylacetonate, wherein R=phenyl, benzyl, CH3, CF3, C2H5, F, Cl, OCH3, CH3CO; 4-acetophenone carboxylic acid, 4-trifluoroacetophenone carboxylic acid, 4-acetophenone acetylacetonate or 4-trifluoroacetophenone acetylacetonate and X=+E,fra 1 / 2+EE phenanthroline, +E,fra 1 / 2+EE diphenyl phenanthroline, +E,fra 1 / 2+EE 4-Cl-phenanthroline, +E,fra 1 / 2+EE bipyridine, +E,fra 1 / 2+EE ethylenediamine, triphenyl phosphineoxide, trimethyl phosphineoxide, triethyl phosphineoxide, +E,fra 1 / 2+EE diethylene glycol-dimethylether (diglyme) or ethanol.
Owner:US PHILIPS CORP
Phosphorescent Osmium (II) complexes and uses thereof
InactiveUS20070001166A1Discharge tube luminescnet screensElectroluminescent light sourcesPhenanthrolineCarbene
There is disclosed herein phosphorescent compounds, uses thereof, and devices including organic light emitting diode (OLEDs) including such compounds. Compounds of interest include: wherein A is Os or Ru The anionic chelating chromophores NˆN, which are formed by connecting one pentagonal ring structure containing at least two nitrogen atoms to a hexagonal pyridine type of fragment via a direct carbon-carbon linkage. L is a neutral donor ligand; the typical example includes carbonyl, pyridine, phosphine, arsine and isocyanide; two neutral L's can also combine to produce the so-called chelating ligand such as 2,2′-bipyridine, 1,10-phenanthroline and N-heterocyclic carbene (NHC) ligand, or bidentate phosphorous ligands such as 1,2-bis(diphenylphosphino)ethane, 1,2-bis(diphenylphosphino)benzene. L can occupy either cis or trans orientation. When L occupies the trans position, the preferred structure contains both the hexagonal fragment of NˆN as well as its pentagonal fragment located at the trans position respect to their counterparts of the second NˆN chromophore. When L occupies the cis position, the preferred structure consists of the pentagonal unit of NˆN chromophores residing opposite to the L. X,1 X2 and X3 independently are C or N; when X2 is N, R1 is omitted, when X3 is N, R2 is omitted, R1 is H, C1-C8 alkyl, C1-C8 substituted phenyl or C1-C4 perfluoroalkyl, R2 is H, F or cyano substituent, X4 is either C or N; X4 may locate at any position of the hexagonal ring, when X4 is N and R3 and R4 are not linked to X4, R3 is H, methyl or C1-C3 small alkyl, R4 is H, methyl or C1-C3 small alkyl, or R3 and R4 together form an additional conjugated unit with structure
Owner:TAO YE +3
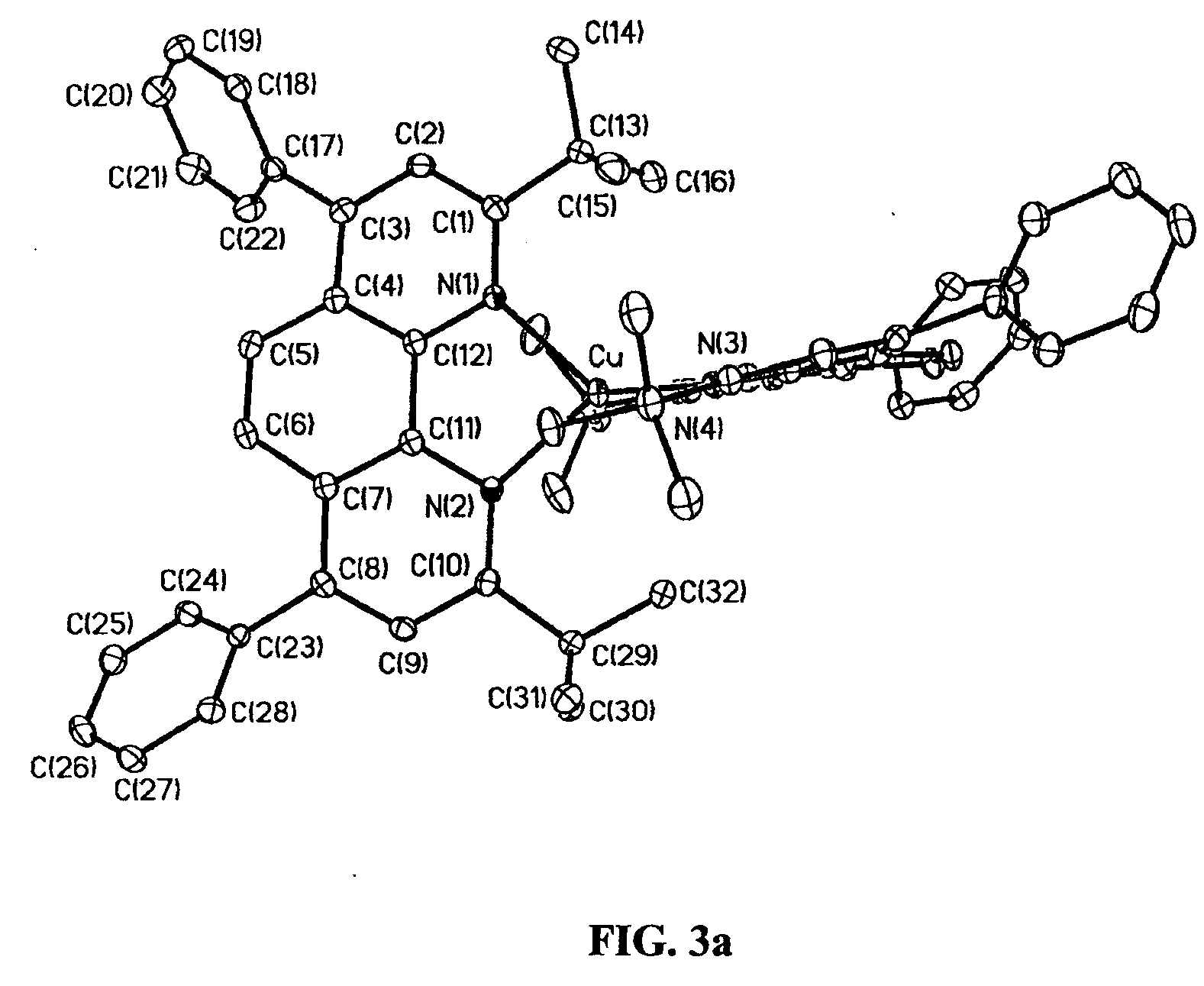
Bis(2,9-di-tert-butyl-1,10-phenanthroline)copper(i) complexes, methods of synthesis, and uses therof
InactiveUS20080206890A1Group 1/11 organic compounds without C-metal linkagesElectrolytic capacitorsAnalyteSynthesis methods
The present invention provides homoleptic and heteroleptic copper(I) complexes having sterically demanding ligands, such as the bis(2,9-di-tert-butyl-1,10-phenanthroline)copper(I) complex and related complexes, methods of synthesis of these complexes and uses thereof. These copper(I) complexes are useful for various applications including photovoltaic cells, light-emitting electrochemical cells, and analyte sensor systems.
Owner:WISCONSIN ALUMNI RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com