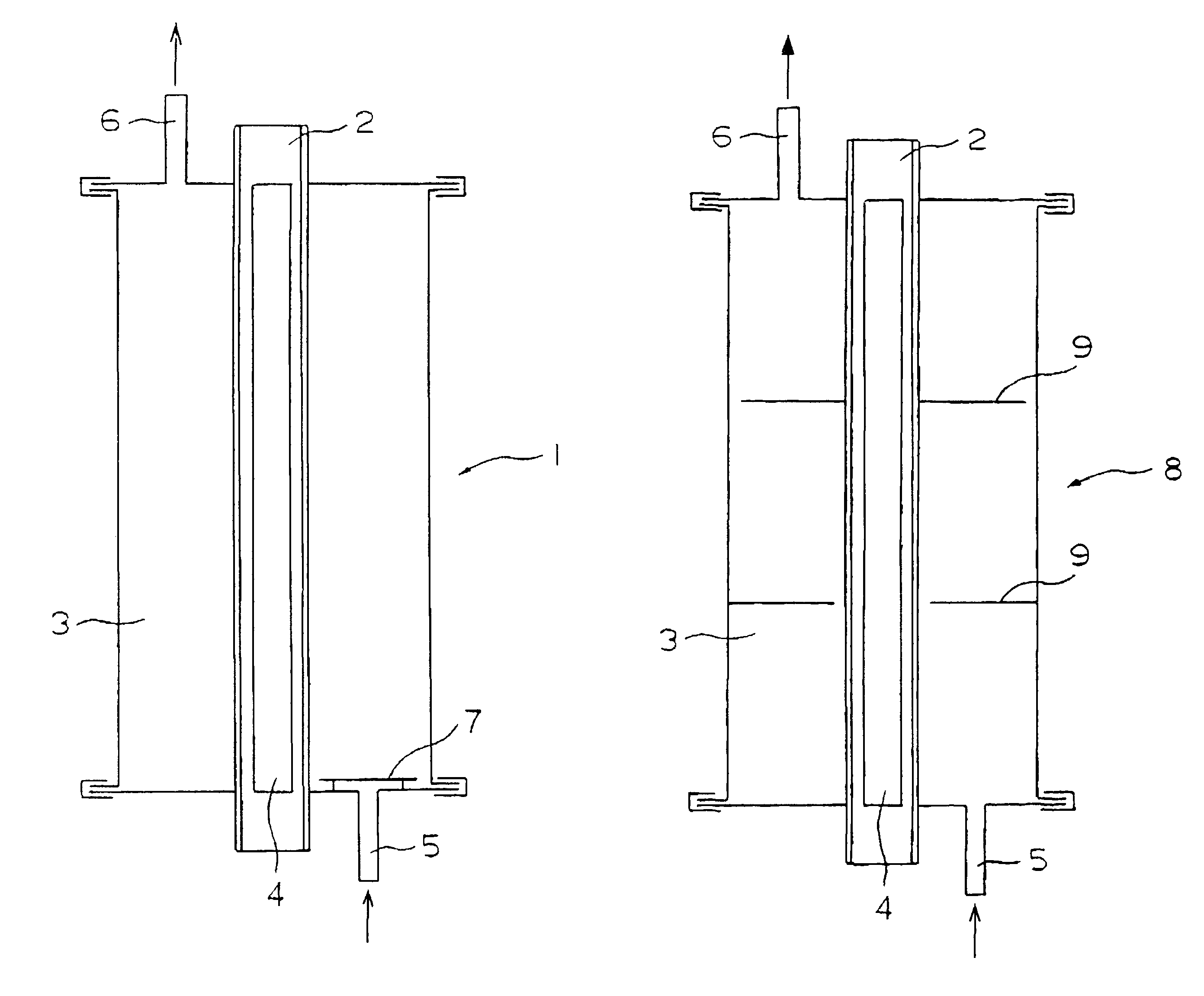
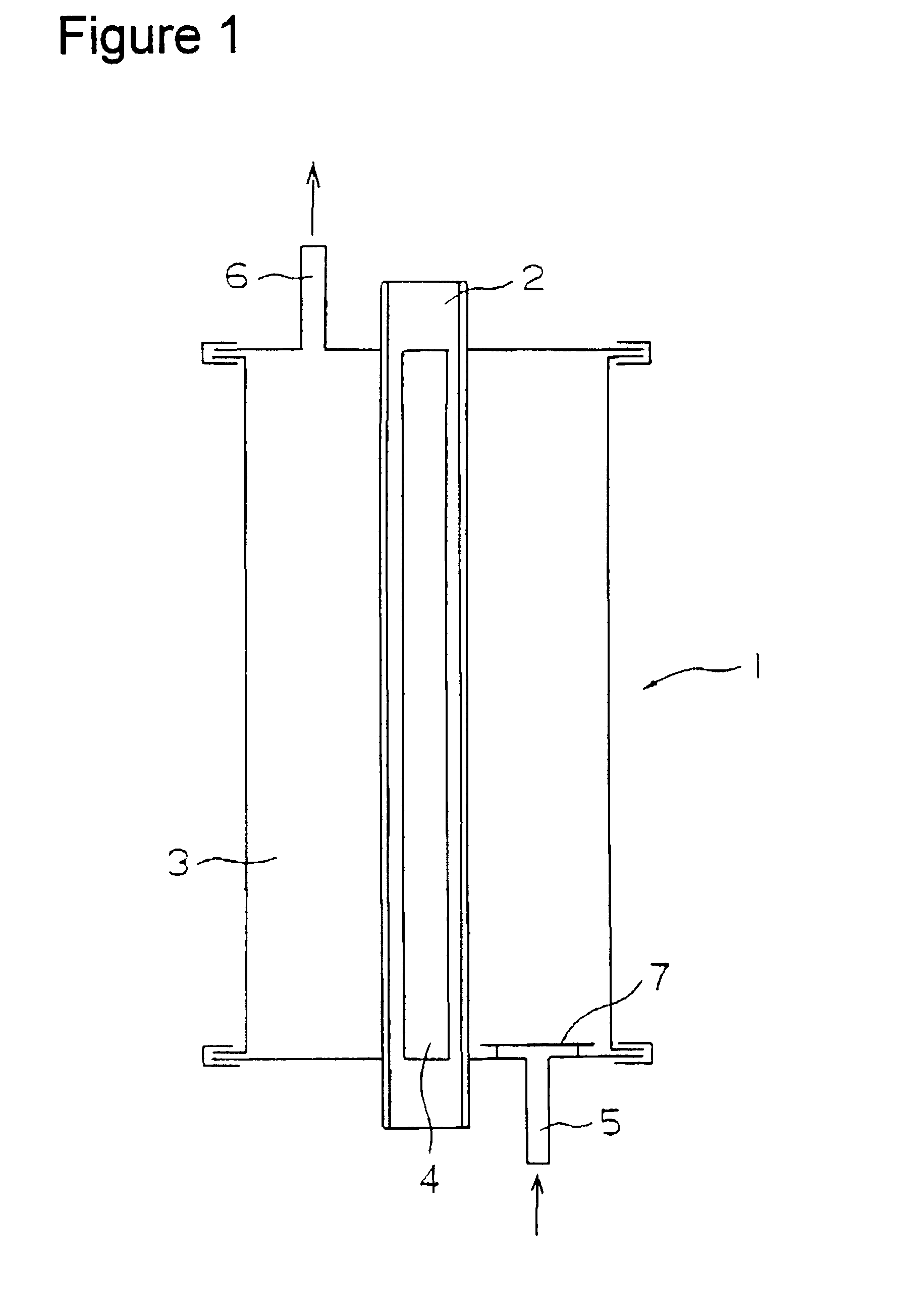
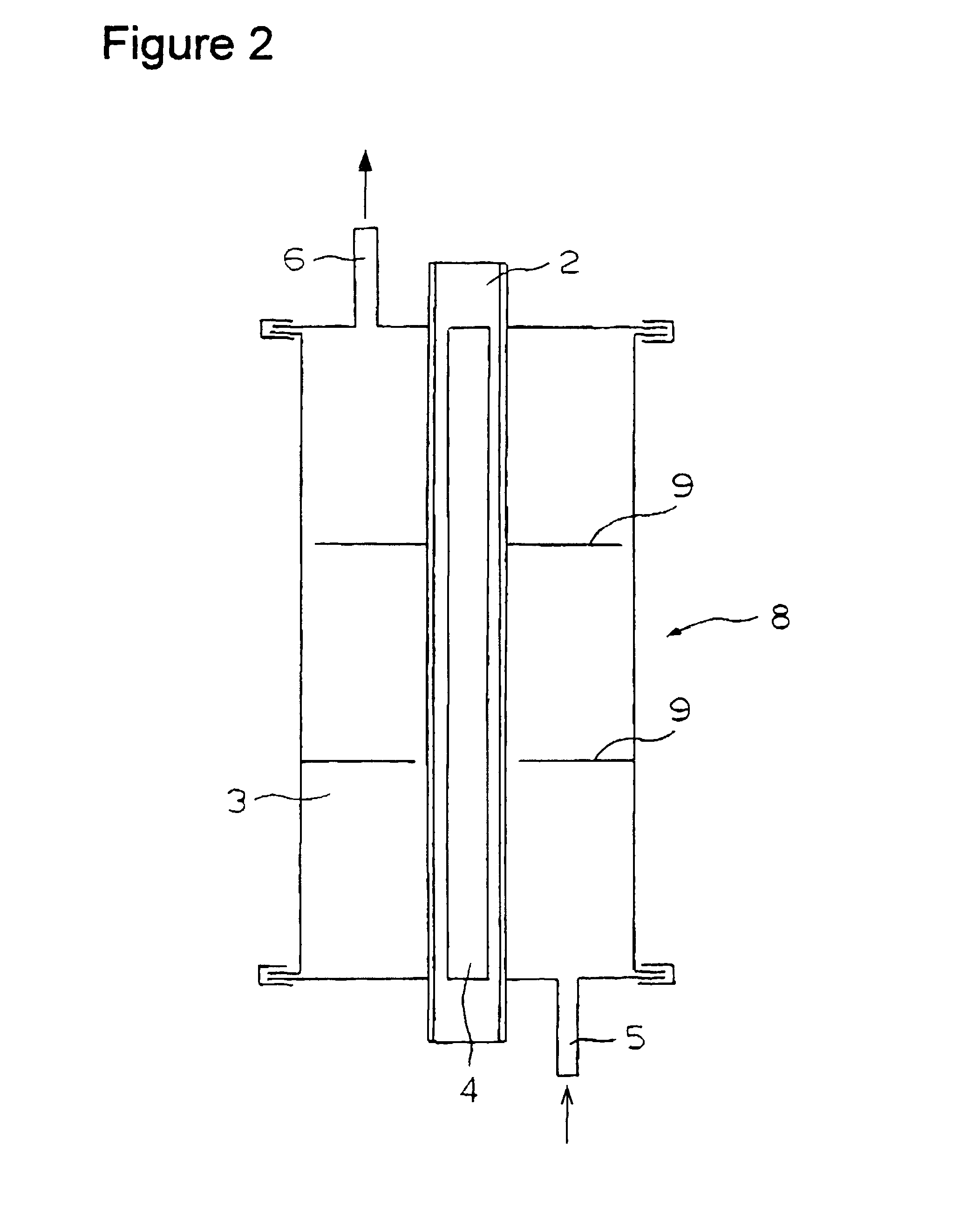
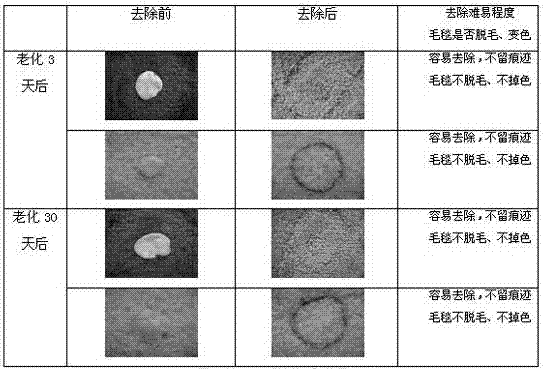
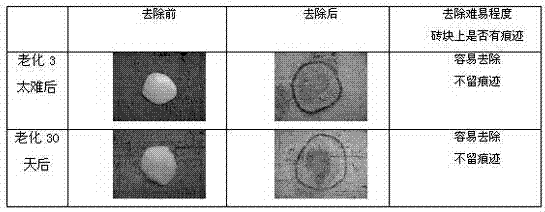
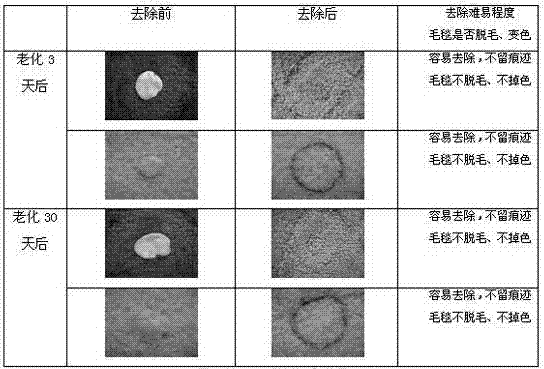
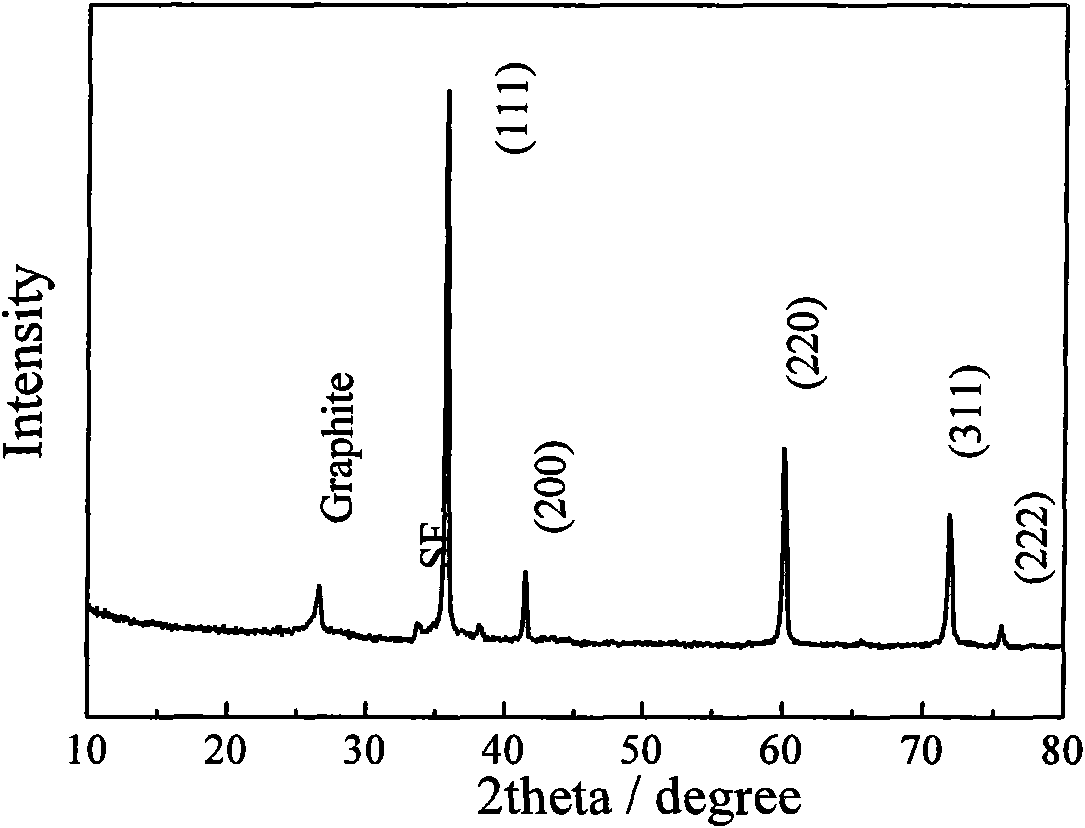
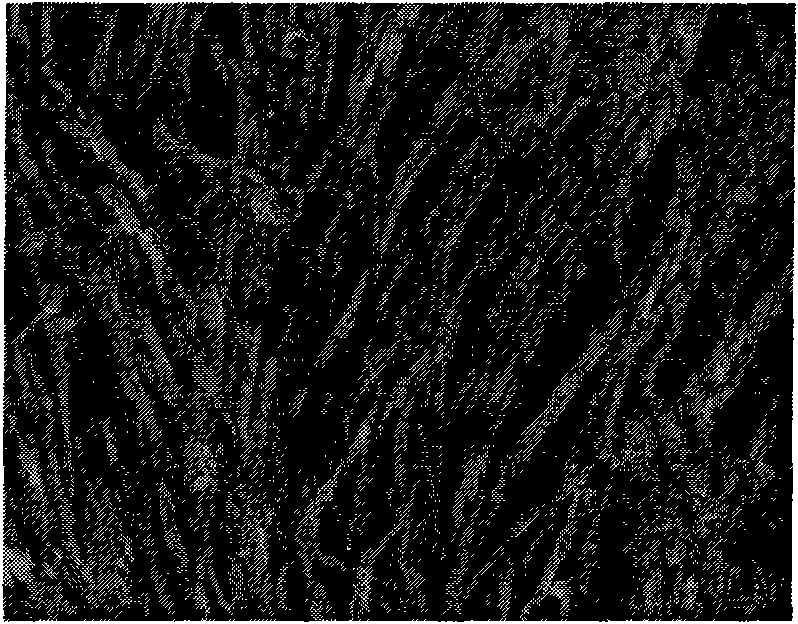
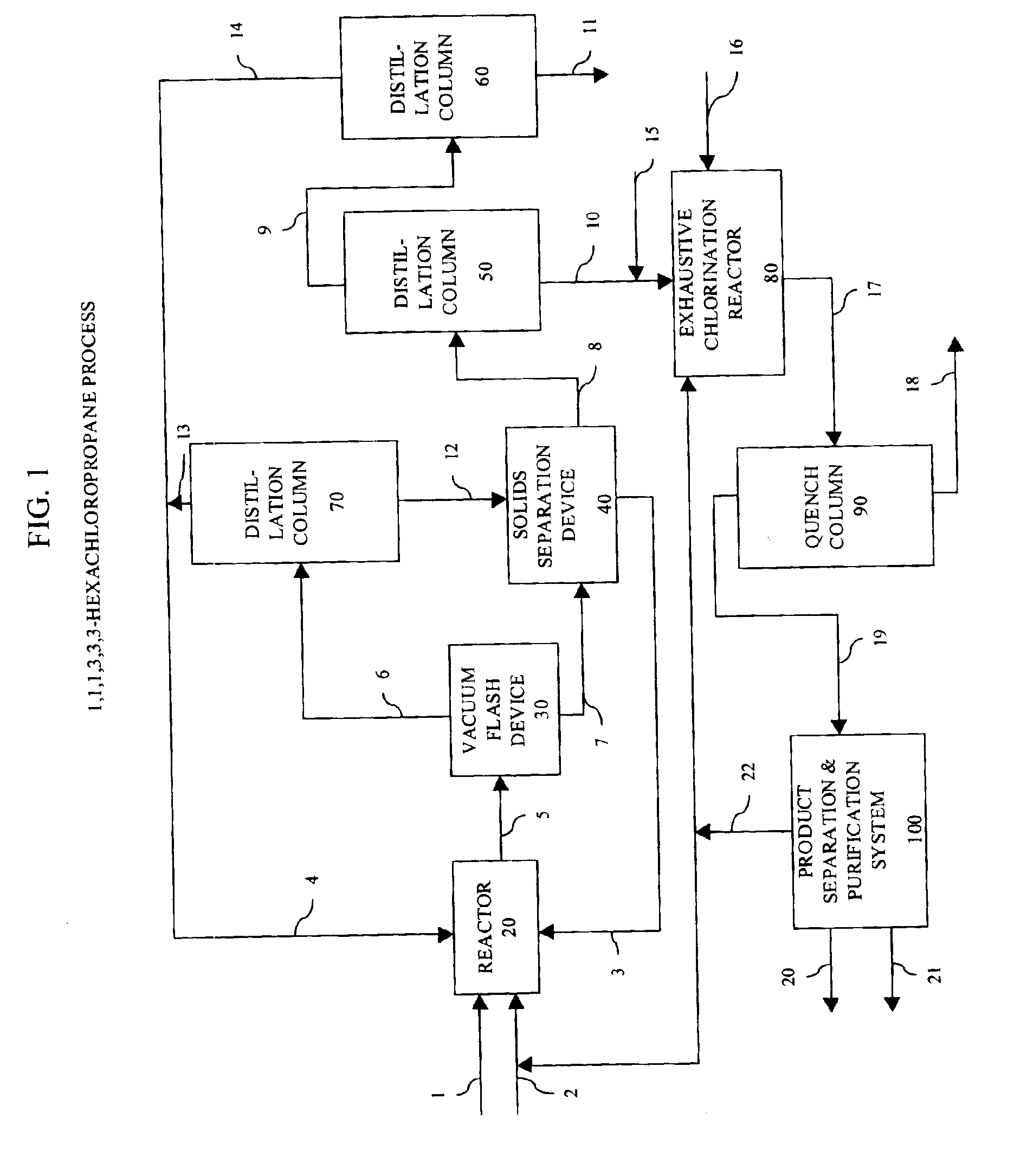
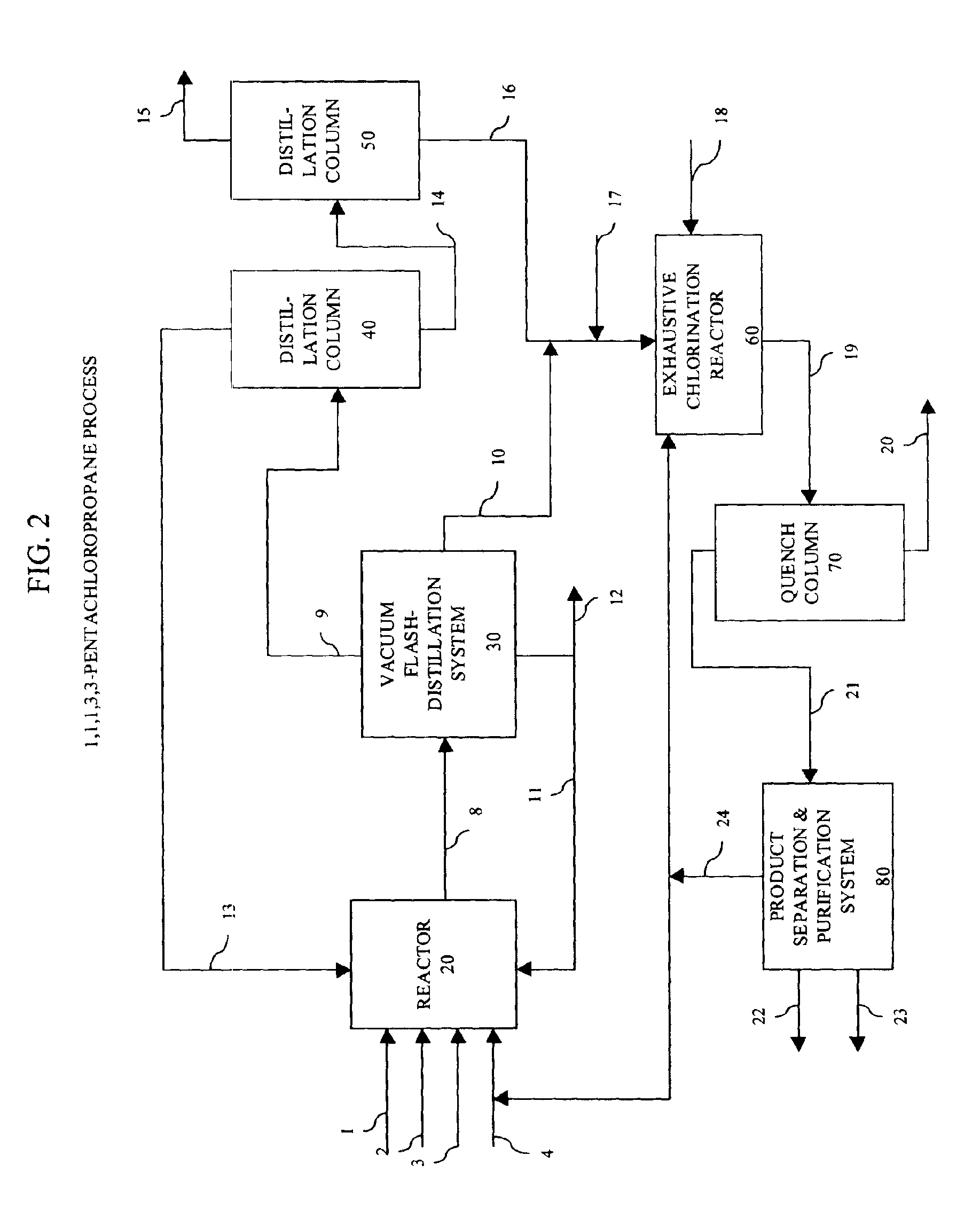
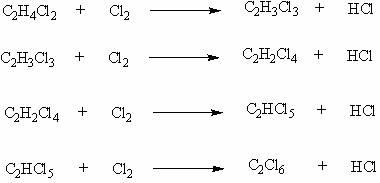
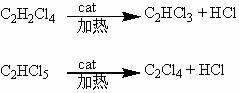
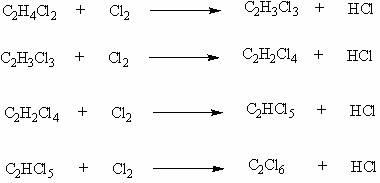
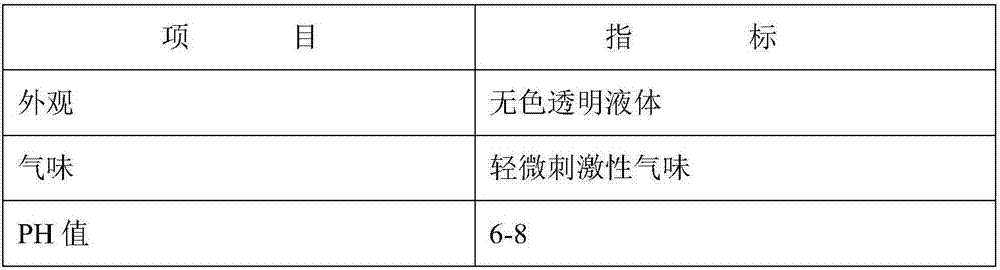
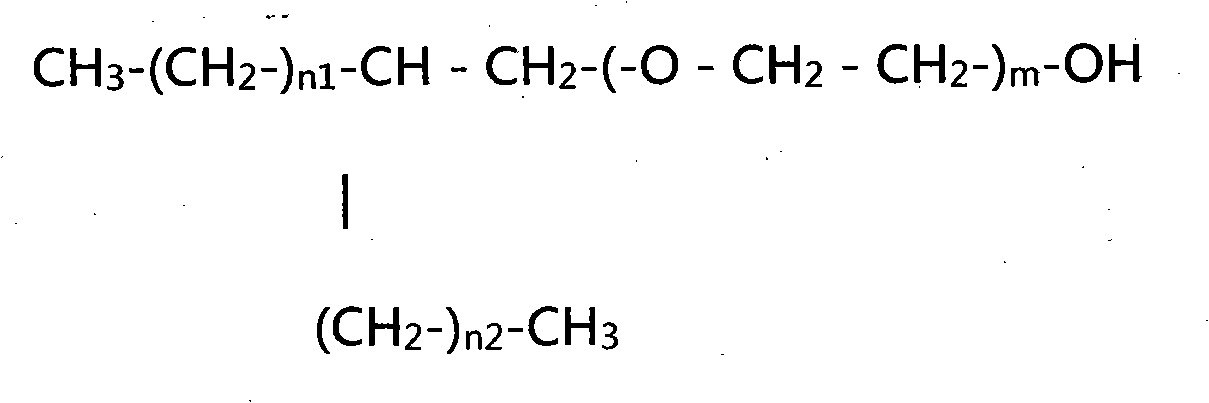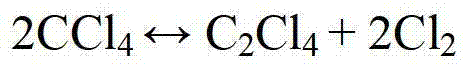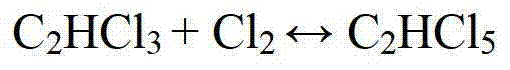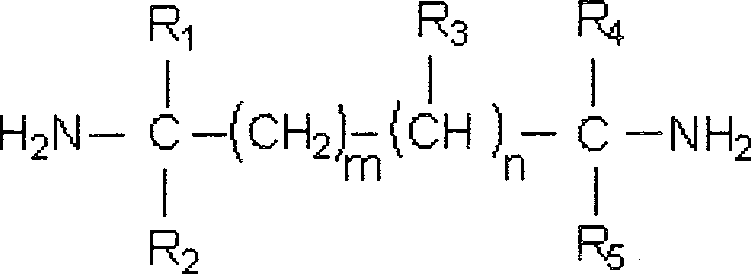Patents
Literature
539 results about "Tetrachloroethylene" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetrachloroethylene, also known under the systematic name tetrachloroethene, or perchloroethylene, and many other names (and abbreviations such as "perc" or "PERC", and "PCE"), is a chlorocarbon with the formula Cl₂C=CCl₂. It is a colorless liquid widely used for dry cleaning of fabrics, hence it is sometimes called "dry-cleaning fluid". It has a sweet odor detectable by most people at a concentration of 1 part per million (1 ppm). Worldwide production was about 1 million metric tons (980,000 long tons; 1,100,000 short tons) in 1985.
Preparation of graphene
The invention discloses a method for preparing graphene, which belongs to the technical field of chemical synthesis. The method is characterized in that sodium metal and halogenated hydrocarbon are taken as raw materials to react in a solvent in inert atmosphere so as to prepare the grapheme; reaction temperature is preferably between 120 and 400 DEG C, and is more preferably between 160 and 360 DEG C; the molar ratio of the sodium metal to the halogenated hydrocarbon is preferably between 1:1 and 100:1, wherein the halogenated hydrocarbon can be added before reaction or during the reaction; and the halogenated hydrocarbon is preferably halogenated C1-4 aliphatic hydrocarbon and halogenated benzene, such as tetrachloroethylene, hexachlorobenzene, trichloroethylene, bromobenzene, ethylenetetrabromide and the like. The method also preferably performs post-treatment on the prepared graphene so as to improve purity. The method has the advantages of simple equipment, easy operation, low cost, high yield and good product properties, can play an important role in the industrial production of graphene and related products such as lithium ion batteries and the like, and is broad in application prospects.
Owner:PEKING UNIV
Copper (i) complexes for optoelectronic devices
ActiveUS20130150581A1Short possible emission decay timeDiminished roll-off behaviorFinal product manufactureGroup 5/15 element organic compoundsSolubilityChlorobenzene
The invention relates to neutral mononuclear copper (I) complexes for emitting light and with a structure according to formula (A) in which: M represents: Cu(I); L∩L represents: a single, negatively charged, bidentate ligand; N∩N represents: a diimine ligand (substituted with R and FG), in particular a substituted 2,2′-bipyridine derivative (bpy) or a substituted 1,10-phenanthroline derivative (phen); R represents: at least one sterically demanding substituent for preventing the planarisation of the complex in the excited state; FG=functional group, and represents: at least one second substituent for increasing solubility in organic solvents. The substituent can also be used for electron transport or alternatively for hole transport, said functional group being bound to the diimine ligands either directly or by means of suitable bridges; and the copper (I) complex: having a ΔE(S1−T1) value of less than 2500 cm−1 between the lowest excited singlet state (S1) and the triplet state (T1) which lies below; having an emission lifespan of at most 20 μs; having an emission quantum yield of greater than 40%, and a solubility of at least 1 g / L in organic solvents, in particular polar organic hydrocarbons such as acetone, methyl ethyl ketone, benzene, toluene, chlorobenzene, dichlorobenzene, dichloromethane, chloroform, dichloroethane, tetrachloroethylene, alcohols, acetonitrile or water.
Owner:SAMSUNG DISPLAY CO LTD
Photolytic device for breakdown of organic chlorine compounds
InactiveUS6238628B1Efficient decompositionReduce amountCombination devicesGas treatmentCompound aTetrachloroethylene
A photolytic device for breaking down organic chlorine compounds, such as trichloroethylene and tetrachloroethylene, in a fluid includes a first reaction chamber, a second reaction chamber, and an acid component absorption column. The first reaction chamber and the second reaction chamber are connected in series, and the second reaction chamber and the acid component absorption column are connected in series. The first reaction chamber is a perfect mixing type chamber, and the second reaction chamber is an imperfect mixing type chamber. The first reaction chamber and the second reaction chamber each have an ultraviolet light source capable of emitting light of wavelength 300 nm or less.
Owner:KURITA WATER INDUSTRIES LTD
Environmentally friendly adhesive remover
ActiveCN102260599AGood value for moneyQuick removalOrganic detergent compounding agentsSurface-active detergent compositionsShoe polishEngineering
The invention relates to an environment-friendly degumming agent. The product can clear away residual chewing gums on clothes and waste chewing gums on the ground, also has good effects on label illegal advertisements, oil pen illegal advertisements, lipsticks, shoe polish, adhesive tape remaining trace and complex mixed oil blackspots, has a neutral formula, is safe and poisonless to the environment and human bodies, and is easy to use; and through experiments of comparing effects with those of the traditional degumming products which are prepared from tetrachloroethylene, acetic ether, dichloromethane, methylbenzene, dimethylbenzene and the like, the using amount is small under the condition that the same removal effect is achieved, and the environment-friendly degumming agent also has no pungent peculiar smell. The environment-friendly degumming agent comprises the following raw materials in part by weight: 20 to 60 parts of solvent, 5 to 20 parts of mixture containing cocamide, 1 to 10 parts of lauryl alcohol alkoxylate, 0.1 to 1.0 part of alkyl amine oxide, 10 to 30 parts of cosolvent, and the balance of water.
Owner:于文 +1
Polyamide thermosol for clothing and preparation method thereof
The invention discloses a polyamide hot melt adhesive used for clothing a preparation method thereof. With the protection of nitrogen-gas, an unsaturated fatty acid dimers comprising carbon numbers between 12 and 20, an dicarboxylic acid comprising carbon numbers between 6 and 12, a molecular weight regulator, a diamine, a lactam, water and a stabilizer are subject to lower temperature and pressure and dehydration in reactions, next, an N-diethylamine- propyl-Octadecanamide, a nonyl phenol, condensation products of formaldehyde and tetraethylenepentamine are added in and stirred. After the series of reactions, the polyamide hot melt adhesive is obtained. The polyamide hot melt adhesive of the invention of used for clothing has the advantages of narrow molecular weight range, rapid solidification and excellent performance of water resistance and solvent resistance, etc.; the copolyamide is modified by adding appropriate proportions of condensation products of N-diethylamine propyl-Octadecanamide and mnnnich amide, so as to improve the water resistance and solvent resistance substantially. The loss rate of peeling strength of the products obtained is less than 20 percent after alkaline cleaning above 60 DEG C; the loss rate of peeling strength of the products obtained is less than 10 percent after dry cleaning by tetrachloroethylene.
Owner:南通天洋光伏材料科技有限公司
Process for carrying out vacuum cleaning, oil removal and wax removal by modified hydrocarbon cleaner
The invention relates to the technical field of electroplating pretreatment, in particular to a process for carrying out vacuum cleaning, oil removal and wax removal by a modified hydrocarbon cleaner. The process comprises degassing ultrasonic cleaning, vacuum cleaning, vacuum steam flushing, vacuum drying and the like. A plurality of flows are carried out in the vacuum environment with a certain vacuum degree and are matched with a degassing ultrasonic cleaning and vacuum cleaning technology and the modified hydrocarbon cleaner. For the cleaning process, in the whole process, harmful substances of halogen and the like, such as trichloroethylene, methylene chloride, tetrachloroethylene and the like, cannot be used and additional drying equipment is not required. Particularly, the process has excellent cleaning effect on irregular articles with blind holes, joints, gaps, cracks and the like. Meanwhile, the defects of oil removal and wax removal of the conventional process are overcome.
Owner:广东新球清洗科技股份有限公司
Method for synthesizing carbide nano powder by solid-phase reaction
InactiveCN101891192AControl morphologyRaw materials are easy to getTungsten/molybdenum carbideReaction temperatureMixed materials
The invention relates to a method for synthesizing carbide nano powder by a solid-phase reaction, which comprises the following steps of: mixing a silicon source or a metal source, a carbon source, magnesium powder and elementary iodine according to a mol ratio of 1: (0.05-2): (1-25): (1.25-12), sealing the mixed material in an autoclave, and placing the autoclave in a drying box to perform a reaction for 6 to 48 hours at the temperature of between 100 and 500 DEG C; and washing, separating and drying the product to obtain the carbide nano powder. In the invention, glucose or maltose which universally exists in the natural world is used as the carbon source to avoid using a chlorine-containing carbon source such as carbon tetrachloride and tetrachloroethylene which have bad influences on an operation environment, and the reaction temperature and the reaction pressure are relatively mild, so that the method for synthesizing carbide nano powder by the solid-phase reaction has a potential for mass production.
Owner:SHANDONG UNIV
Metal cleaner
The invention discloses a metal cleaner. The metal cleaner is prepared from, by weight, 50%-90% of halohydrocarbon cleaners, 0%-10% of ester additives, 2%-30% of hydrocarbon additives, 0%-20% of alcohol additives, 0%-25% of alcohol ether additives and 0%-5% of amine additives, wherein the halohydrocarbon cleaners are chlorohydrocarbon and / or chloro-fluoro-hydrocarbon, chlorohydrocarbon is tetrachloroethylene and / or dichloromethane, and chloro-fluoro-hydrocarbon is 1-fluoro-1,1-dichloroethane. The metal cleaner is large in cleaning force, high in permeability and dispersity, capable of cleaning wax, ink, punching oil, cutting oil and lubricating oil, small in smell and free of toxicity.
Owner:SHENZHEN XINJUNXIANG TECHCAL
Catalyst used for preparing tetrachloroethylene and its preparation method and use
InactiveCN101143328AHalogenated hydrocarbon preparationMetal/metal-oxides/metal-hydroxide catalystsGas phaseCobalt
The invention relates to novel catalyst to prepare tetrachloroethylene in carbon tetrachloride gas-phase catalysis. The invention is characterized in that the catalyst carrier is prepared from soluble salt solution or mixed solution with ammonia in coprecipitation method. And then one or two of the mixtures such as the compounds of platinum, palladium and nickel, etc, together with one or a plurality of the mixtures such as the compounds of silver, cobalt, tin, zinc, copper or iron is / are loaded on the catalyst carrier in conventional equivalent impregnation method or excessive impregnation method. One or two of the composite oxides of alumina, zirconia, silica and titania is / are used as the carrier or carriers of the catalyst. One or two of platinum, palladium or nickel and one or two of silver, cobalt, tin, zinc, copper and iron are used as the active components to form multi-metal catalyst. The catalyst prepared in the method of the invention maintains high transformation rate of carbon tetrachloride, at the same time reduces the production of by-products such as methane, maintains high selectivity of tetrachloroethylene, and maintains a long service life of the catalyst.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
High efficiency low-foaming blue leather degreaser
InactiveCN101831362ASuitable for a wide temperature rangeFacilitates cleaning removalNon-ionic surface-active compoundsDetergent compounding agentsWater basedKerosene
The invention relates to a degreaser used for degreasing blue leather. The degreaser is a washing composite mainly using surfactant as base material and is clear and transparent microemulsion. The formula of the degreaser comprises the following components by weight percent: 20-60% of surfactant (one or two of non-ionic surfactants such as fatty alcohol polyoxyethylene ether, fatty alcohol polyoxypropylene ether and alkanolamide, 10-40% of solvent (one or two of toluene, xylene, turpentine, C8-C12 of solvent oil, trichloroethylene, tetrachloroethylene and solvent kerosene), 0-5% of cosolvent ( one or two of ethanol, n-propanol, isopropanol and ethylene glycol monobutyl ether, 0-10% of acidic lipase, 0-2% of defoaming agent and 40-60% of water. The application temperature of the microemulsion degreaser is wide, and can be stored for long time without layering and have stable state; and the degreaser has the advantages of solvent degreasing and emulsion degreasing, the fat dissolving and cleaning functions of organic solvents and the cleaning characteristics of water-based surfactants. Therefore, the natural fat in blue leather can be reduced to less than 1%.
Owner:CHINA LEATHER & FOOTWEAR IND RES INST
Cleaning agent for electromechanical equipment
InactiveCN101724520AReduce surface tensionGood degreasing effectOrganic non-surface-active detergent compositionsElectrical devicesMechanical equipment
The invention discloses a cleaning agent for electromechanical equipment. The cleaning agent comprises the following components in percentage by weight: 20-40 percent of halogenated hydrocarbon, 55-75 percent of petroleum solvent, 3-8 percent of butyl cellosolve and 0.05-1 percent of benzotriazole, wherein the halogenated hydrocarbon is one or the mixture of components comprising tetrachloroethylene and dichloromethane. The cleaning agent is not easy to burn at normal temperature, has good insulating property and voltage resistance, can be widely applied to the cleaning of various electrical equipment and mechanical equipment and has convenient raw material sources, low cost and simple operation. Large equipment can be cleaned by an injection method, and small equipment or parts can be cleaned by a dipping method.
Owner:SHAANXI UNIV OF SCI & TECH
Industrial preparation method for tetrachloroethylene
InactiveCN102816046AImprove conversion rateUtilize maximumPreparation by hydrogen halide split-offResource utilizationEthyl Chloride
The present invention provides an industrial production method for tetrachloroethylene. The method is characterized by adopting monochloromethane or natural gas, excess chlorine gas, and a methane chloride as raw materials, and carrying out a reaction to produce tetrachloroethylene, wherein the methane chloride is any one or a mixture selected from dichloromethane, trichloromethane, and carbon tetrachloride according to any ratios. The preparation method of the present invention has the following advantages that: raw material conversion rate is high, the produced heavy component is decomposed, and then can return to a reactor to generate tetrachloroethylene, and the by-produced hydrogen chloride can be converted into monochloromethane for providing a raw material for a methane chloride apparatus or a tetrachloroethylene synthesis apparatus so as to achieve maximum chlorine resource utilization.
Owner:自贡鸿鹤化工股份有限公司
Decarburization cleaner
ActiveCN101538511AReduce consumptionEasy to useOrganic detergent compounding agentsSurface-active detergent compositionsDissolutionSolvent
The invention discloses a decarburization cleaner. Perchloroethylene and methylen chloride or dichloropropane without flammable risk are used as decarburization solvents of the invention; on the basis of the permeability and dissolution of the solvents, the surfactant sodium alkyl sulfonate is compounded, thus promoting and improving the dirt-removing power, and trolamine is taken as antirusting agent without affecting the activity of the surfactant. The invention also discloses a formula and a preparation method of the decarburization cleaner. No heating or stirring is needed in using the invention, excellent using effect can be achieved by soaking at room temperature, energy consumption can be reduced remarkably. The pH value is neutral, thus having no corrosion on the surface of the material. The product has the characteristics of low toxicity, non-combustion and the like, thus the safety is improved greatly. The invention can be recycled only by simple filtering, thus saving using cost.
Owner:SHANGHAI JIUSHENG IND
Machinery equipment cleaning compound
InactiveCN103834483AReduce corrosionWith sterilizationNon-ionic surface-active compoundsDetergent compounding agentsSodium metasilicateMass ratio
The invention discloses an industrial level machinery equipment cleaning compound comprising 20-35% of waterless sodium metasilicate in mass, 10-25% of cosolvent in mass, 10-25% of surfactant in mass, 3-5% of antiseptic in mass, 2-4% of corrosion inhibitor in mass, 1.2% of thickening agent in mass and remaining deionized water; the surfactant is a mixture composited by RF-25-1 surfactant, aliphatic acid methyl esters ethoxylate (FMEE) and alkyl glucosides FC-41 according to mass ratio of 1:2:1; the cosolvent comprises at least two from a group formed by isopropanol, glycerol, dichloromethane and tetrachloroethylene; the antiseptic is chlorothalonil powder; the corrosion inhibitor is methyl benzotriazole TTA; the thickening agent is carboxymethylcellulose sodium. Through the said mode, the machinery equipment cleaning compound is anti-bacterium, environmental protection, and can clean the machine equipment with high efficiency.
Owner:SUZHOU LOTTE CHEM TECH
Electrical appliance cleaner and preparation thereof
The invention discloses an electrical appliance cleaner, which selects comparatively safe methylen chloride, perchloroethylene, F141B to be constituents. Perchloroethylene has high KB value and strong decontaminating and degreasing ability, can form a layer of waterproof protecting film on the surface of material before full volatilization to prevent water vapour from accumulating, and can be used as the cleaner of the invention. F141B has very strong volatility, which can be used for adjusting the volatility of the product. Methylen chloride also has volatility, decontaminating ability and excellent permeability, which is used for improving the resultant force of the product. The electrical appliance cleaner of the invention is safe in use without flash point or fire point, does not burn while encountering with open fire; the electrical appliance cleaner can replace gasoline, xylene and other flammable organic solvents; the electrical appliance cleaner has no obvious effect on vast majority of plastics, rubber and other high molecular compounds.
Owner:SHANGHAI JIUSHENG IND
Method for reusing heavy end by-products in the manufacture of polychlorinated alkanes
ActiveUS7112709B2Efficient productionReduce the number of materialsHalogenated hydrocarbon separation/purificationPreparation by halogen additionAlkaneHexachlorobutadiene
A method for recovering much of the carbon and chlorine value in the heavy ends and other undesired by-products formed during the production of a C3 or higher polychlorinated alkane through the reaction of carbon tetrachloride with an olefine or chlorinated olefine, the improvement comprising the step of first separating the heavy ends and any other higher or lower boiling chlorohydrocarbon impurities from most of the desired product, and subjecting the separated heavy ends and impurities therewith to a high temperature exhaustive chlorination to produce carbon tetrachloride, tetrachloroethene, and minor amounts of hexachlorobutadiene and hexachlorobenzene by-products.
Owner:OCCIDENTAL CHEM CORP
Glue removing agent for LED (light emitting diode) encapsulation residue glue
InactiveCN105695150AEfficient removalSimple configurationInorganic/elemental detergent compounding agentsCationic surface-active compoundsEpoxyMethylene Dichloride
The invention discloses a glue removing agent for LED (light emitting diode) encapsulation residue glue. The glue removing agent is basically prepared from the following ingredients in percentage by weight: 30 to 80 percent of solvents, 10 to 60 percent of diluting agents, 5 to 10 percent of penetrating agents, 0.1 to 5 percent of metal protecting agents, 0.1 to 5 percent of catalysts and 0.1 to 0.5 percent of surface active agents. The product can be used for removing silica gel and epoxide resin encapsulation glue remained by an LED encapsulation material on equipment; the effect is good; no corrosion is left on the equipment; toxicity on the human body is low; compared with conventional methylene dichloride, carbon tetrachloride, tetrachloroethylene, acetone, toluene, xylene and vinyl acetate, the effect is equivalent; the consumption is low; the environment-friendly effect is achieved; the use is convenient.
Owner:SHENZHEN SHENGYUAN SEMICON
A kind of method that takes dichloroethane as raw material to produce trichloroethylene and tetrachloroethylene
InactiveCN102267863ATo satisfy the market's needsSimple processPreparation by hydrogen halide split-offTetrachloroethaneEthylene Dichloride
The invention relates to a method for preparing trichloroethylene and perchloroethylene from dichloroethane as a raw material. The existing method has the defects of difficult realization and large amount of investment. The method of the invention comprises the following steps that dichloroethane undergoes chlorination to produce a mixture of dichloroethane, trichloroethane, tetrachloroethane, pentachloroethane and hexachloroethane, and hydrogen chloride gas under a dichloroethane gaseous or liquid phase; the mixture is transferred into a rectifying tower to be rectified; a tetrachloroethane / pentachloroethane mixture fraction with a top temperature of 147 to 162 DEG C is collected; the collected tetrachloroethane / pentachloroethane mixture fraction is vaporized and then is fed into a fixedbed reactor to form a trichloroethylene / perchloroethylene gas mixture; the trichloroethylene / perchloroethylene gas mixture is condensed into a liquid mixture; the liquid mixture and hydroquinone are fed into a rectifying tower to be rectified; a fraction with a top temperature of 86 to 87.5 DEG C is collected to obtain trichloroethylene finished products; and a fraction with a top temperature of 120 to 121.5 DEG C is collected to obtain perchloroethylene finished products. The method has the advantages of simple process, good controllability, easy acquirement of raw materials, low cost and little or no process wastewater or waste residue.
Owner:内蒙古磐迅科技有限责任公司
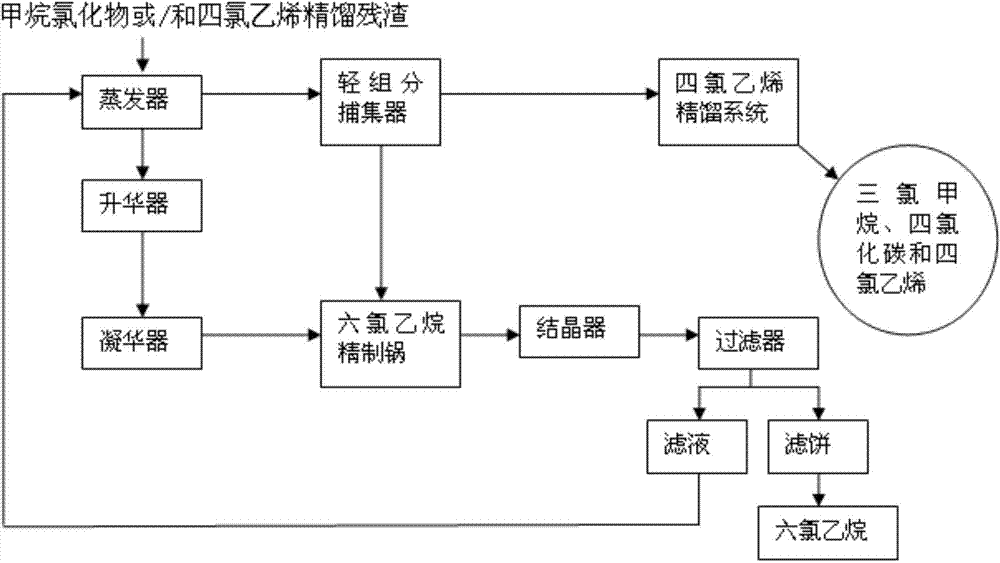
Method for recovering organic chloride from rectification residues of methane chloride or/and tetrachloroethylene
ActiveCN103787823AEfficient recyclingDisposal savingsHalogenated hydrocarbon separation/purificationOrganic chloride compoundEvaporation
Owner:山东中盛药化设备有限公司
Deadhesive cleaning agent and preparation method thereof
InactiveCN106906082AEasy to removePromote polymerizationOrganic detergent compounding agentsAnionic surface-active compoundsChemical industrySurface-active agents
The invention belongs to the field of cleaning agents, and particularly relates to a BS-791 deadhesive cleaning agent and a preparation method thereof. The deadhesive cleaning agent comprises, by weight, 50-60 parts of dichloromethane, 2-10 parts of ethyl alcohol, 20-30 parts of tetrachloroethylene, 3-8 parts of ethyl acetate, 2-8 parts of xylene, 1-3 parts of acetone, 1-3 parts of surface active agents and 1-3 parts of additives. The BS-791 deadhesive cleaning agent can rapidly clean glue scales and other scales of various positions of various chemical devices and pipelines and the like, the glue scales on the devices can soften, expand and dissolve, so that the glue scales are cleaned, and the cleaning agent solves the problem of cleaning of material scales of chemical industries. The cleaning agent is obvious in cleaning effect, free from residue, wide in popularization and application prospect and remarkable in economic benefit.
Owner:齐齐哈尔百思特科技有限责任公司
Iron composite particles for purifying soil or ground water, purifying agent containing the iron composite particles, and method for purifying soil or ground water
InactiveUS20060081811A1Efficient and economicalMaterial nanotechnologyWater contaminantsPolychlorinated biphenylMaterials science
Iron composite particles for purifying soil or ground water, comprise an iron component, at least one noble metal selected from the group consisting of ruthenium, rhodium and palladium, and carbon or aluminum, and having a noble metal content of 0.01 to 5.00% by weight and a particle diameter of 0.01 to 1.0 μm. The iron composite particles and the purifying agent according to the present invention are capable of decomposing aliphatic organohalogen compounds such as dichloromethane, carbon tetrachloride, 1,2-dichloroethane, 1,1-dichloroethylene, cis-1,2-dichloroethylene, 1,1,1-trichloroethane, 1,1,2-trichloroethane, trichloroethylene, tetrachloroethylene, 1,3-dichloropropene or the like, and aromatic organohalogen compounds such as dioxins, PCB (polychlorinated biphenyl) or the like, which are contained in soil, ground water or waste water, at ordinary temperature in an efficient and economical manner.
Owner:TODA IND
Anaerobic biodegradation of unsaturated, saturated, aromatic and halogenated hydrocarbons
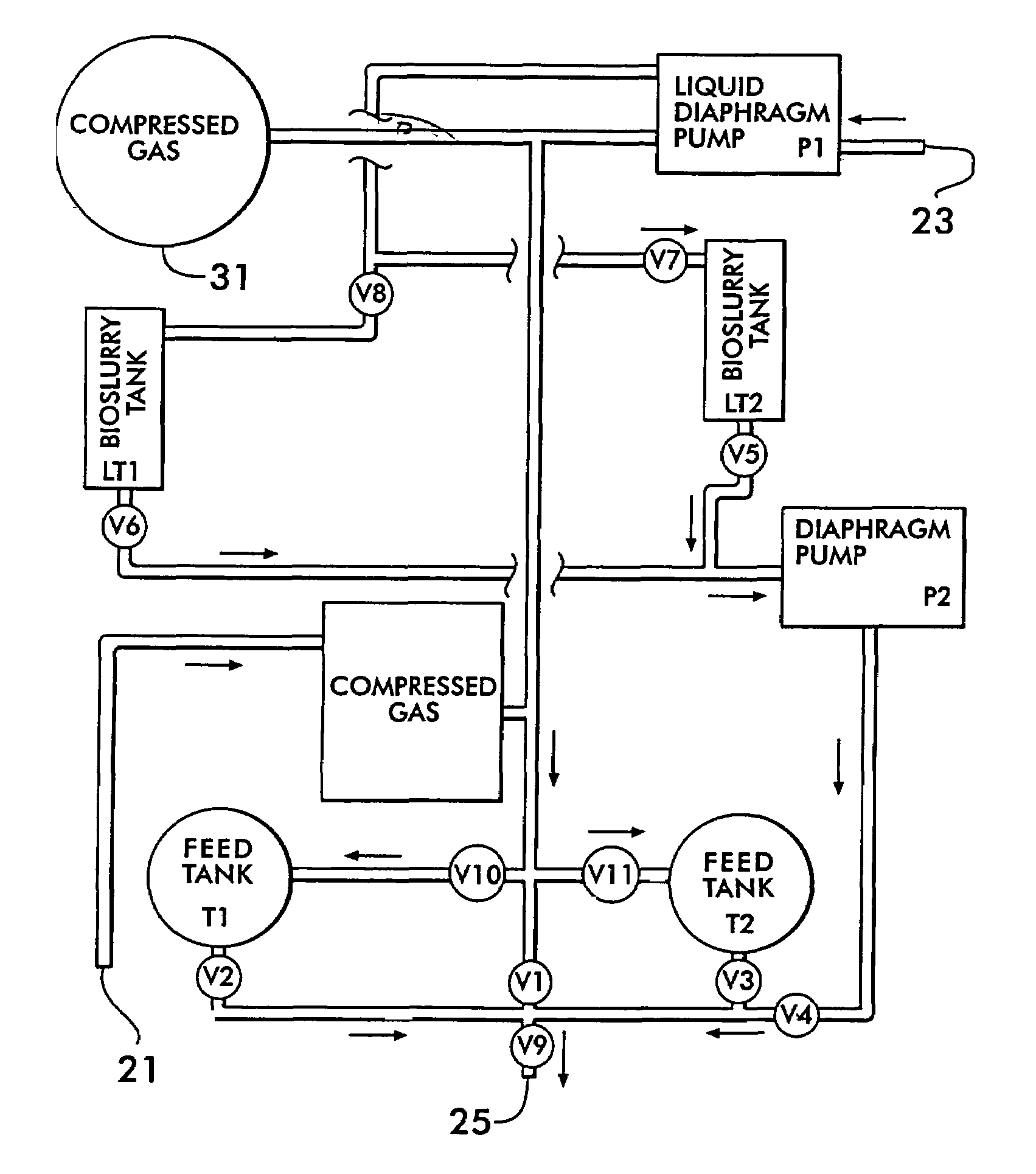
An apparatus and method for anaerobic biodegradation, bioremediation or bioprocessing of hydrocarbons dissolved in an aqueous matrix, such as wastewater, groundwater, or slurry. Dissolved alkanes (saturated hydrocarbons), alkenes (unsaturated hydrocarbons), aromatic hydrocarbons and / or halogenated hydrocarbons are metabolized or cometabolized. In one form, the invention involves introducing an aqueous stream comprising at least one dissolved aromatic hydrocarbon (such as benzene, toluene, ethylbenzene, o-xylene, m-xylene, p-xylene, phenol, o-cresol, m-cresol, or p-cresol) and a dissolved oxide of nitrogen [such as nitrate (NO3-), nitrite (NO2-), nitric oxide (NO) and nitrous oxide (N2O)] to a reactor, and operating said reactor under conditions that support denitrification of the aromatic hydrocarbon. Alternatively, the aqueous stream may comprise at least one alkane (such as ethane) and / or at least one alkene (such as ethene or ethylene) and biodegradation of these compounds is accomplished. In a preferred form, the aqueous stream also comprises at least one dissolved halogenated hydrocarbon (such as tetrachloroethylene, trichloroethylene, or 1,1,1-trichloroethane) and dehalogenation of the halogenated hydrocarbon is accomplished. The reactor may be a continuous stirred tank reactor, a batch (or sequencing batch) reactor, a plug-flow reactor, a fixed-film reactor, or a pore space in an underground aquifer in situ. The reactor is operated in such a way that molecular oxygen is excluded from the space or zone in which the biodegradation is occurring and the other requirements of denitrifying bacteria are met. In some implementations, kinetic control (control of mean cell residence time) is used to enrich a denitrifying culture in the reactor.
Owner:YESTECH
Method for accelerated dechlorination of matter
InactiveUS7129388B2Accelerated dechlorinationIncreasing rate of biological mineralizationContaminated soil reclamationSolventAnaerobic microorganisms
Accelerated dechlorination of soil and water contaminated with chlorinated solvents is achieved by stimulating anaerobic microorganisms and thus increasing the rate of biological mineralization of the solvents. This is accomplished by a treatment process consisting of colloidal suspension of iron powder, polylactate ester such as glycerol tripolylactate, xylitol pentapolylactate, and sorbitol hexapolylactate, chemical oxygen scavengers in solution with essential nutrients, and vitamin stimulants such as B2 and B12 delivered via compressed gases N or C02 so as not to oxygenate an environment targeted for anaerobic processes. The treatment stimulates naturally occurring microorganisms while oxidizing dissolved phase target compounds via the surface action of the iron particles resulting in the breakdown of chlorinated solvents such as tetrachloroethene, trichloroethene, carbon tetrachloride and their daughter products.
Owner:INNOVATIVE ENVIRONMENTAL TECH
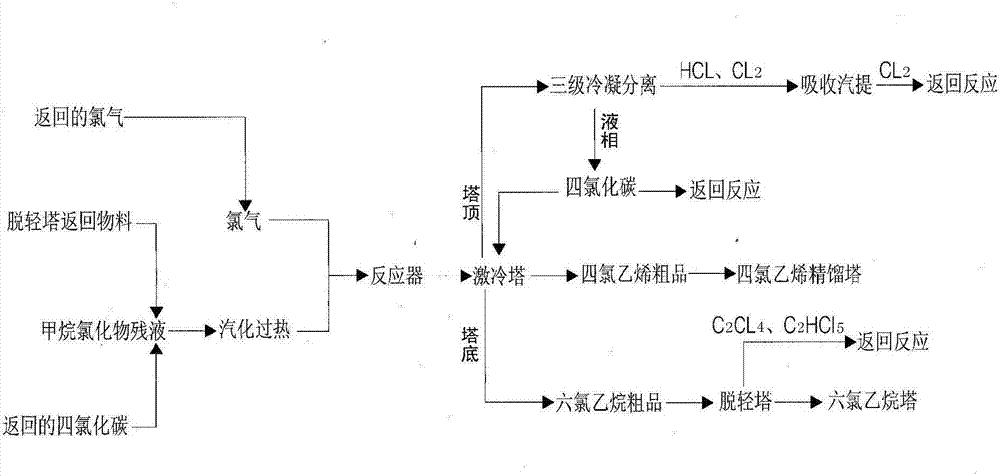
Production process for production of tetrachloroethylene and coproduction of hexachloroethane by using methane chloride residual liquid
ActiveCN103086839AAvoid pollutionIncrease profitabilityHalogenated hydrocarbon preparationGas phaseMixed gas
The invention discloses a production process for production of tetrachloroethylene and coproduction of hexachloroethane by using methane chloride residual liquid. According to the production process, the methane chloride residual liquid subjected to vaporization and overheating and excessive chlorine enter a reactor, the mixed gas after reaction enters a chilling tower, a tetrachloroethylene crude product in gas phase is extracted out from the top side line of the chilling tower, the gas containing carbon tetrachloride, excessive chlorine and hydrogen chloride passes through three stages of condensers and a separation tank in sequence, one part of condensed liquid-phase material carbon tetrachloride is used as chilling liquid, and the other part of condensed liquid-phase material carbon tetrachloride is returned to the system for reaction, so that the aim of consuming the methane chloride residual liquid through continuous circulation is achieved; hydrogen chloride and excessive chlorine are absorbed by using water and subjected to steam stripping, and chlorine is returned to the reaction system; the materials at the bottom of the chilling tower are treated in a lightness-removing column, so that the light components including tetrachloroethylene and pentachloroethane are separated and returned to the reactor for reaction; and hexachloroethane is subjected to rectification to obtain a hexachloroethane finished product. According to the production process, the methane chloride residual liquid is subjected to innocent treatment to prevent environmental pollution caused by improper treatment.
Owner:SHANDONG DONGYUE FLUO SILICON MATERIALS CO LTD
Modified polyamide thermosol based on polyester and preparation thereof
The invention provides a method for preparing a polyester modification-based polyamide hot melt adhesive, characterized by comprising the following steps: facilitating aliphatic dicarboxylic acid having 7-12 carbon atoms, aliphatic monocarboxylic acid having 7-12 carbon atoms, aliphatic diamine having 6-14 carbon atoms, lactam having 6-11 carbon atoms and water to react with a stabilizing agent; adding a proper amount of copolyester after the dehydration, continuing reaction for a particular time; discharging to acquire the polyester modification-based polyamide hot melt adhesive. The polyester modification-based polyamide hot melt adhesive of the invention has the characteristics of short opening hours, rapid curing, outstanding high temperature resistance and washability and the like; the polyester having a molecular weight of 8,100-18,300 is selected for the modification of the synthesized polyamide, which not only obviously shortens the opening hours of the adhesive, but also causes the obtained products to have less than 18% of the peeling strength loss rate after being washed with alkaline water above 80 DEG C, or to have less than 8% of the peeling strength loss rate after being dry-cleaned with tetrachloroethylene.
Owner:SHANGHAI TIANYANG HOT MELT ADHESIVE CO LTD
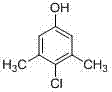
Preparation method of 3,5-dimethyl-4-chlorophenol
InactiveCN104326881AImprove conversion rateReduce generationOrganic chemistryOrganic compound preparationAluminium chlorideWater chlorination
The invention discloses a preparation method of 3,5-dimethyl-4-chlorophenol, which takes tetrachloroethylene as a solvent, benzyl thiophenol and aluminium chloride as cocatalysts, sulfuric chloride as a chloridizing agent, orientation chlorination is carried out through two phases of low-temperature chlorination and high-temperature chlorination, the mass ratio of tetrachloroethylene to MX is 0.5-4: 1; the mass ratio of the cocatalyst to MX is 2.5-6.5:1000; at low temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.9-1.2: 1; at high temperature chlorination phase, the mass ratio of sulfuric chloride dropping amount to MX is 0.1-0.2: 1; the temperature at the low temperature chlorination phase is controlled at 30-45 DEG C, chlorination is carried out for 4-6 hours; the temperature at high temperature chlorination phase is controlled at 50-65 DEG C, and chlorination is carried out for 1-2 hours, insulation reaction is carried out after the dropping process of sulfuric chloride is completed, tail gas is removed for 1-2 hours, and steps of water-washing layering, cooling and crystallizing, centrifuging and washing, and drying to obtain the product. According to the method, the conversion rate can reach more than 95%, finished product PCMX yield is increased, and the by-product can be effectively reduced.
Owner:RONGCHENG QINGMU CHEM MATERIALS
Method for producing methane chloride through byproduct hydrogen chloride in production process of tetrachloroethylene
ActiveCN104311383AResolve disposal issuesEasy to corrodeHalogenated hydrocarbon preparationGas phaseMixed gas
The invention relates to a method for producing methane chloride through a byproduct hydrogen chloride in production process of tetrachloroethylene. The method comprises the steps of (1) condensing the mixed gas of excess chlorine and the byproduct hydrogen chloride in production process of tetrachloroethylene, then absorbing the chlorine by carbon tetrachloride between -5DEG C and -21DEG C, condensing again, eliminating the foam, buffering, and compressing; (2) heating up the hydrogen chloride and methyl alcohol, then reacting for 2-5 seconds under the action of a catalyst aluminum oxide so as to obtain the mixed product of methane chloride, methane, chloroethane and dichloromethane, and (3) chilling and separating the mixed product, sequentially water washing, alkali washing, drying by sulfuric acid and compressing the gas phase at 0.6-0.95MPa, condensing at 25-40DEG C, so as to prepare the methane chloride. According to the method, the chlorine is adsorbed by carbon tetrachloride, so that the chlorine is prevented from entering into a reaction system of the hydrogen chloride and methyl alcohol to affect the purity and selectivity of the reaction product methane chloride.
Owner:SHANDONG DONGYUE FLUO SILICON MATERIALS CO LTD
Preparation method of 2-cyano-4'-bromomethylphenylbenzene
ActiveCN102746193AHigh active bromine contentImprove the safety of useCarboxylic acid nitrile preparationOrganic compound preparationTetrachloroethyleneReaction temperature
The invention provides a preparation method of 2-cyano-4'-bromomethylphenylbenzene, which comprises the following steps: (1) adding 2-cyano-4'-methylphenylbenzene, dibromohydantoin and tetrachloroethylene into a reactor, adding a catalyst, and evenly mixing, wherein the mol ratio of 2-cyano-4'-methylphenylbenzene to dibromohydantoin to tetrachloroethylene to catalyst is 1:(0.5-0.6):(8-12):(0.008-0.012), and the catalyst is tetrabutyl ammonium bromide, cetyl trimethyl ammonium bromide or benzyl triethyl ammonium bromide; (2) heating to react, wherein the reaction temperature is controlled at 60-120 DEG C, and the reaction time is controlled at 4-8 hours; and (3) after the reaction finishes, cooling, filtering, concentrating to recover the tetrachloroethylene, and recrystallizing with methanol or ethanol or isopropanol to obtain the 2-cyano-4'-bromomethylphenylbenzene. The production process provided by the invention has the advantages of high operation safety, simple technique, low cost and high yield.
Owner:TAINING SHENGDA INDAL
Method of preparing porous ceramics provided with amorphous pore surfaces
InactiveUS6042763AWaste water treatment from animal processingSeparation devicesLiquid wasteCadmium Cation
This invention relates to a method for treating various kinds of drain water and waste liquid which treatment now becomes a problem, for example, drain water and waste liquid containing hardly removable phosphorus and nitrogen, waste liquid containing organochlorine compounds such as tetrachloroethylene, etc., excretive drain water from a piggery containing organonitrogen compounds at a high level, waste liquid containing heavy metals such as lead, hexavalent chromium, cadmium and the like, drain water from dairy product plants, fishery processing plates, slaughterhouses, etc. which contains water soluble protein at a high level, drain water from pulp plants, photo developing waste liquid, car wash drain water containing a mixture of car polishing wax and detergent and the like by the use of porous ceramics provided with amorphous pore surfaces. Porous ceramics provided with amorphous pore surfaces are prepared by mixing clay, a pore forming material and water, molding into an arbitrary shape followed by drying, heating up an molded article thus dried, heating up an article temperature from normal temperature to 600 to 800 DEG C. over a time period of 5 to 15 hours, maintaining this temperature for 3 to 7 hours and then calcinating at 1,200 to 1,500 DEG C.
Owner:KUMAOKA SHUNICHI
Hydrogenation heat gas chemical yield increasing solution component for low-permeability carbonate reservoir oil well
ActiveCN102942914AImprove permeabilityImprove single well productivityFluid removalDrilling compositionHydrazine compoundAlkylphenol
The invention belongs to the technical field of oil exploitation, and particularly relates to a hydrogenation heat gas chemical yield increasing solution component capable of being used for increasing productivity of a low-permeability carbonate reservoir oil well. The solution component comprises first solution, second solution and third solution, the mass ratio of the first solution, the second solution and the third solution is 1:1:3, the first solution comprises urea CO(NH2)2, sodium nitrite NaNO2, hydrazine N2H4 and water H2O, the second solution comprises aluminum chloride AlCl3, sodium hydride NaH, ammonium chloride NH4Cl and tetrachloroethylene C2Cl4, and the third solution comprises hydrochloric acid HCl, methenamine (CH2)6N4 and alkylphenol ethoxylate C9H19C6H4O(C2H4O)nH. Compared with an acidizing and fracturing process, a hydrogenation heat gas chemical method is simple in process, has the advantage of combination of heat recovery, fracturing and acidizing, is long in yield increasing duration and high in recovery ratio, and can be applied to carbonate oil reservoir exploitation.
Owner:吉林贯通能源科技有限责任公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com