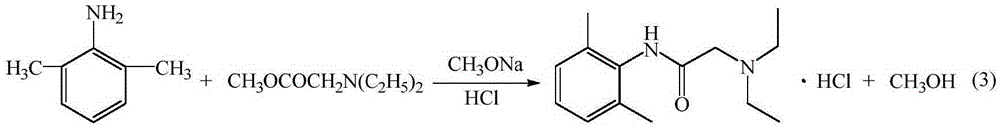
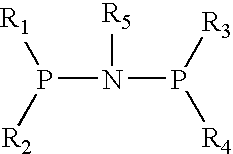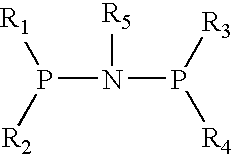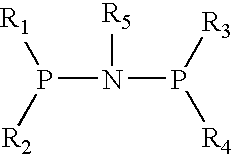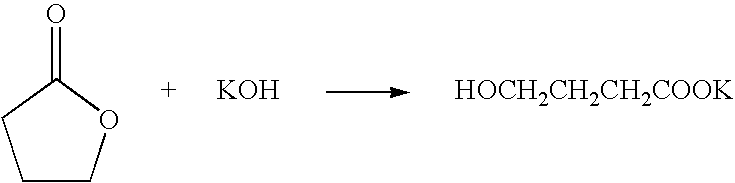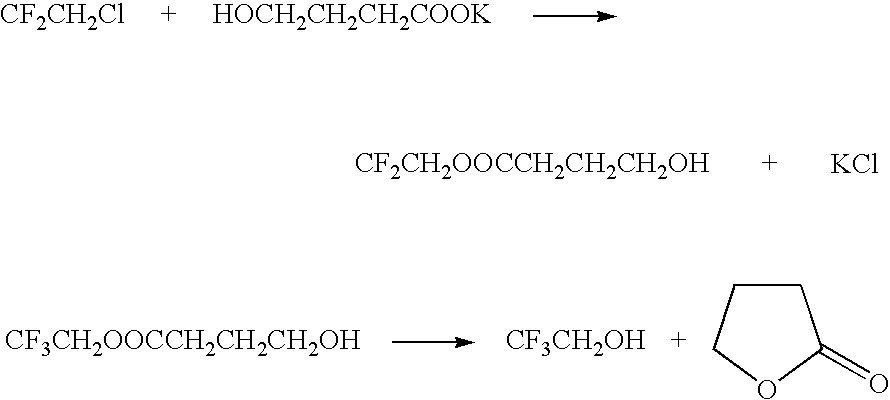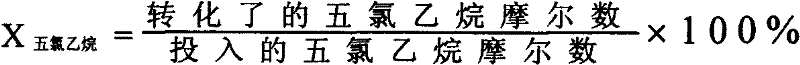Patents
Literature
756 results about "Chloroethane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Chloroethane, commonly known by its old name ethyl chloride, is a chemical compound with chemical formula CH₃CH₂Cl, once widely used in producing tetraethyllead, a gasoline additive. It is a colorless, flammable gas or refrigerated liquid with a faintly sweet odor.
Aluminum-silicon based aluminum section and preparation technology thereof
The invention discloses an aluminum-silicon based aluminum section. The aluminum section is composed of, by weight, 5.0-14.0% of silicon, 0.2-0.7% of magnesium, less than 0.03% of boron, less than 0.06% of strontium, 0.1-6.55% of strengthening elements, less than 0.25% impurity elements, and the balance aluminum. The aluminum-silicon based aluminium section has the advantages of high strength, high hardness, good wear resistance, etc. the invention also discloses a preparation technology for the aluminum-silicon based aluminum section, which comprises a first step of adding aluminum-silicon alloy to a graphite crucible and heating the alloy to form melt; a second step of adding the magnesium, the silicon and the strengthening elements; a third step of adding hexachloroethane for refinement; a forth step of adding the strontium for deterioration, casting to a cast iron die to form an ingot casting; and a fifth step of hot extruding, hot rolling for deformation, solid solution treating and aging treating in sequence after annealing the ingot casting, thereby obtaining the aluminum-silicon based aluminum section. The preparation technology is simple.
Owner:SOUTHEAST UNIV
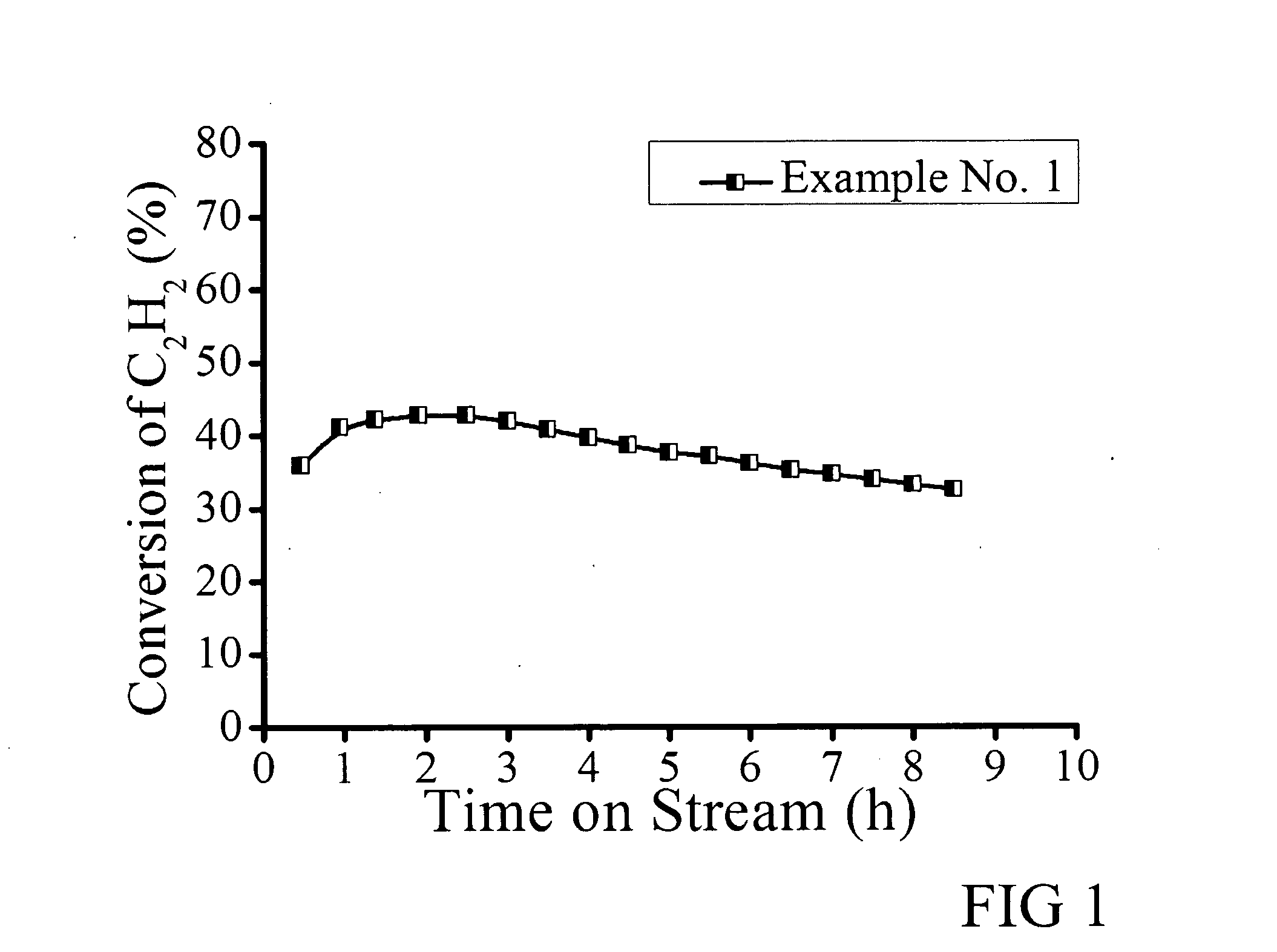
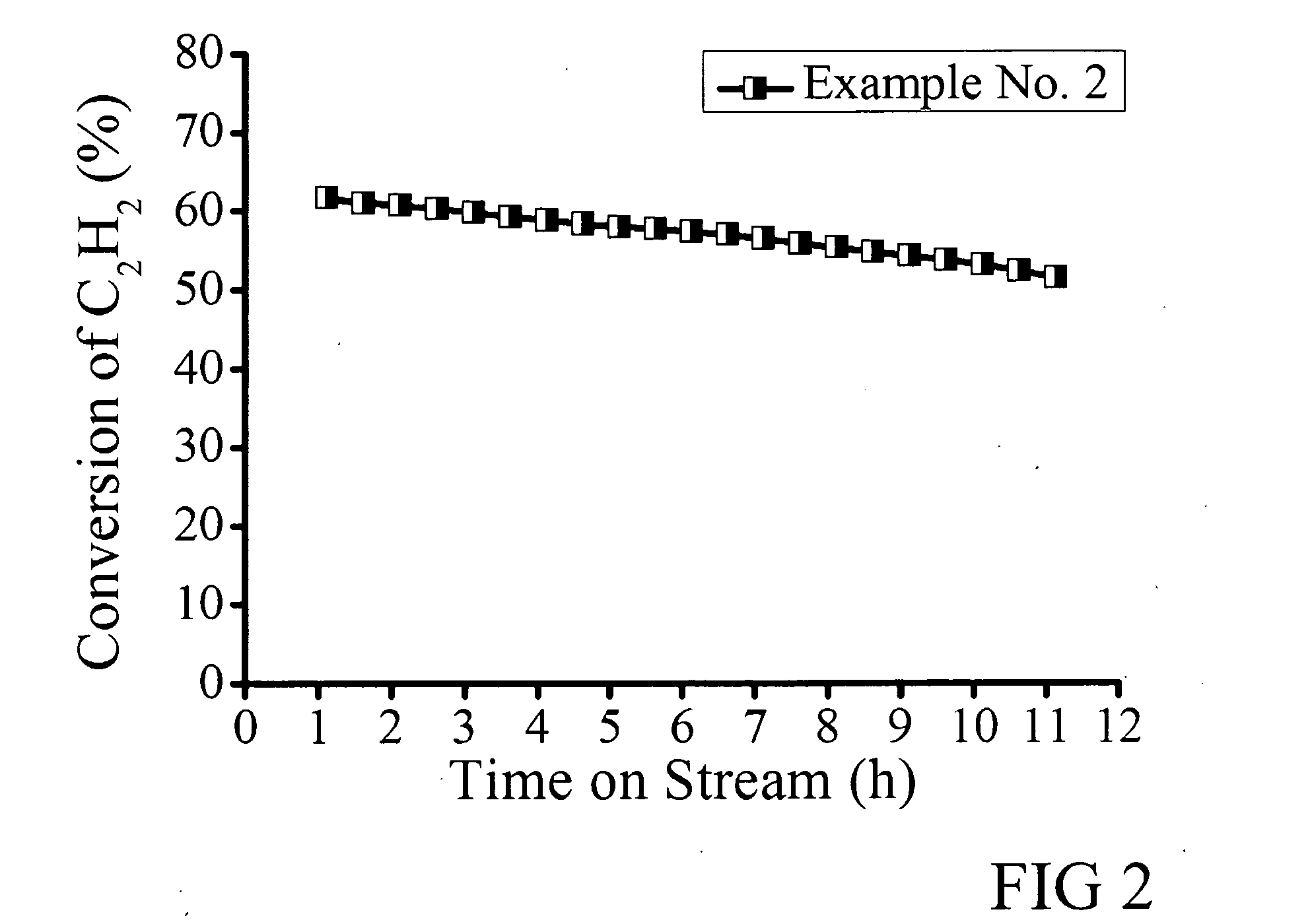
Gold-based catalysts for acetylene hydrochlorination
InactiveUS20140213437A1Molecular sieve catalystsMetal/metal-oxides/metal-hydroxide catalystsHydrogenChemical compound
Powder catalysts that comprise particles of chemical compounds of Au and Cu deposited on acid-washed carbon-based supports are effective catalysts in ethyne hydrochlorination to produce vinyl chloride monomers (VCMs). They give a high selectivity and productivity of VCM and decreased amounts of the byproducts of chloroethane, dichloroethane and others. Thiocyanates are used as complexing agents to extend the catalyst lifetime. The activity of the catalyst is enhanced by doping nitrogen atoms into the support.
Owner:TSINGHUA UNIV
Novel mercury-free catalyst and application of mercury-free catalyst in synthesis of vinyl chloride
ActiveCN104289246AGood catalytic performanceImprove catalytic performancePreparation by hydrogen halide split-offPhysical/chemical process catalystsIonPyrrole
The invention discloses a novel mercury-free catalyst, and application of the mercury-free catalyst in synthesis of vinyl chloride. The novel mercury-free catalyst is prepared by a method comprising the steps of pickling and drying active carbon, and then taking the active carbon as a carrier for later use; dissolving a nitrogen-containing compound in deionized water so as to prepare impregnation liquid, wherein the nitrogen-containing compound is selected from one or several of ammonia water, ammonia gas, pyridine, pyrrole, imidazole, acrylamide, polyacrylamide and polyvinylpyrrolidone; adding the pickled active carbon into the impregnation liquid containing the nitrogen-containing compound, and impregnating, wherein the content of the nitrogen-containing compound and the active carbon is 0.01-20% by weight part; and completely drying the impregnated active carbon at 60-140 DEG C, then calcining the active carbon at 400-800 DEG C for 4-6 hours in the nitrogen atmosphere to prepare the novel mercury-free catalyst. The prepared catalyst shows good catalytic performance in the aspect of catalyzing the reaction of synthesis of vinyl chloride by 1,2-dichloroethane and acetylene.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Non-flammable Electrolyte Containing Liquefied Gas and Lithium Secondary Batteries Containing Same
ActiveUS20180375156A1Flammability of any liquefied gas solvent can be effectively suppressedImprove solubilityOrganic chemistryCell electrodesElectrolytic agentDifluoroethyne
A rechargeable lithium cell comprising a cathode, an anode, an optional ion-permeable membrane disposed between the anode and the cathode, a non-flammable salt-retained liquefied gas electrolyte in contact with the cathode and the anode, wherein the electrolyte contains a lithium salt dissolved in or mixed with a liquefied gas solvent having a lithium salt concentration greater than 1.0 M so that the electrolyte exhibits a vapor pressure less than 1 kPa when measured at 20° C., a vapor pressure less than 60% of the vapor pressure of the liquefied gas solvent alone, a flash point at least 20 degrees Celsius higher than a flash point of the liquefied gas solvent alone, a flash point higher than 150° C., or no flash point, wherein the liquefied gas solvent is selected from methane, fluoromethane, difluoromethane, chloromethane, dichloromethane, ethane, fluoroethane, difluoroethane, tetrafluoroethane, chloroethane, dichloroethane, tetrachloroethane, propane, fluoropropane, chloropropane, ethylene, fluoroethylene, chloroethylene, or a combination thereof.
Owner:GLOBAL GRAPHENE GRP INC
Catalyst composition for ethylene oligomerization and the use thereof
ActiveUS7786336B2High activityGood choiceOrganic-compounds/hydrides/coordination-complexes catalystsHydrocarbons from unsaturated hydrocarbon addition1-OcteneAluminoxane
The present invention relates to a catalyst composition for ethylene oligomerization and the use thereof. Such catalyst composition includes chromium compound, ligand containing P and N, activator and accelerator; wherein the chromium compound is selected from the group consisting of acetyl acetone chromium, THF-chromium chloride and Cr(2-ethylhecanoate)3; general formula of the ligand containing P and N is shown as:in which R1, R2, R3 and R4 are phenyl, benzyl, or naphthyl. R5 is isopropyl, butyl, cyclopropyl, cyclopentyl, cyclohexyl or fluorenyl; the activatior is methyl aluminoxane, ethyl aluminoxane, propyl aluminoxane and / or butyl aluminoxane; the accelerator is selected from the group consisting of 1,1,2,2,-tetrachloroethane, 1,1,2,2-tetrabromoethane, 1,1,2,2-tetrafluoroethane, and compounds having a formula of X1R6X2, in which X1 and X2 are F, Cl, Br, I or alkoxyl, R6 is alkylene or arylene group; the molar ratio of chromium compound, ligand containing P and N, activator and accelerator is 1:0.5˜10:50˜3000:0.5˜10. After mixing the four components mentioned previously under nitrogen atmosphere for 10 minutes, they are incorporated to the reactor, or these four components are incorporated directly into the reactor. Then ethylene is introduced for oligomerization. Such catalyst can be used in producing 1-octene through ethylene oligomerization. It is advantageous in high catalysing activity, high 1-octene selectivity, etc. The catalytic activity is more than 1.0×106 g product·ma−1 Cr·h−1, the fraction of C8 linear α-olefin is more than 70% by mass.
Owner:PETROCHINA CO LTD
Efficient composite modifying-refining agent for hypoeutectic cast aluminium-silicon alloy and treatment process
InactiveCN1936044ASimplify the melt handling processImprove performanceStrontium carbonateSilicon alloy
The invention relates to a high efficiency compounded refiner for hypoeutectic aluminum-silicon alloy and the method of application. It contains 10-30wt% NaCl, 10-15wt% KCl, 10-20wt% sodium fluoride, 1-30wt% potassium fluoborate, and 1-20wt% potassium fluotitanate, 1-20wt% strontium carbonate, 1-15wt% cerium fluoride, 5-20wt% granular lanthanum abundant mixed rare earths and 1-5% heachloroethane. After taking the process of heating, dehydrating, mixing according to the ratio to equal, pressing to molding, it could be sealed to use. The technology includes the following steps: after the magnesium alloy melting in crucible, heating to 720-740 degree centigrade and removing the slag, standing for 3-5 minutes, pressing the refiner into alloy liquid to take refining process for 5-10 minutes, standing for 5-10 minutes after process to gain hypoeutectic aluminum-silicon alloy melt. The invention simplifies melt process technology, lowers cost and improves the capability of alloy.
Owner:重庆工学院
Cellulose ester blends
This invention relates to a blend comprising(a) about 2% to about 98% by weight of at least one ester of cellulose comprising an alkanoyl chain having from about 1 to about 10 carbon atoms, and having at a D.S. of about 2.3 to about 3.0, and an inherent viscosity of about 0.2 to about 3.0 deciliters / gram as measured at a temperature of 25° C. for a 0.5 g sample in 100 ml of a 60 / 40 parts by weight solution of phenol / tetrachloroethane, and(b) about 98% to about 2% by weight of at least one ester of cellulose comprising an alkanoyl chain having from about 1 to about 10 carbon atoms, and having a D.S. of about 1.5 to about 2.2, and an inherent viscosity of about 0.2 to about 3.0 deciliters / gram as measured at a temperature of 25° C. for a 0.5 g sample in 100 ml of a 60 / 40 parts by weight solution of phenol / tetrachloroethane, said percentages being based on the weight of component (a) plus component (b).
Owner:EASTMAN CHEM CO
Method for producing 2,2,2-trifluoroethanol
InactiveUS7208641B2MinimizationEffectively removing/separatingOrganic compound preparationHydroxy compound preparationHydroxybutyric acidSolvent
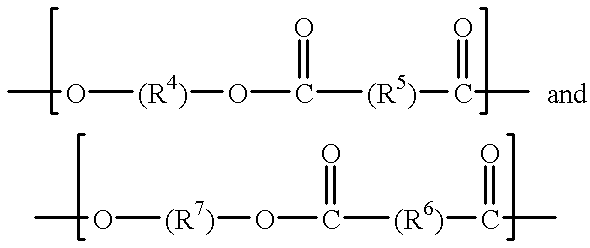
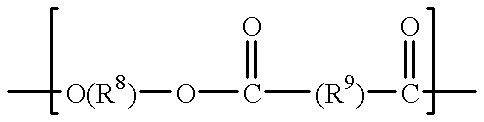
A method for producing 2,2,2-trifluoroethanol in which a γ-hydroxybutyric acid salt is reacted with 1,1,1-trifluoro-2-chloroethane to generate 2,2,2-trifluoroethanol is provided. This method leads to increased yields of 2,2,2 -trifluoroethanol, facilitates the separation of salt byproducts and allows the recycling of an aprotic polar solvent.The present invention concerns a method for producing 2,2,2-trifluoroethanol in which a γ-hydroxybutyric acid salt is reacted with 1,1,1-trifluoro-2-chloroethane in an aprotic polar solvent to generate 2,2,2-trifluoroethanol. This method is characterized in that the γ-hydroxybutyric acid salt used contains no more than 6 wt % of 4,4′-oxybis(butyric acid).
Owner:TOSOH F TECH INC
Process for preparing 1,1,1-trifluoro-2,2-dichloroethane
InactiveUS7053252B2Good choicePreparation by halogen halide additionOrganic chemistry methodsHydrogen fluoridePolymer science
The invention relates to a process for preparing 1,1,1-trifluoro-2,2-dichloroethane (F123).This process consists in placing 1,1,1-trifluoro-2-chloroethane (F133a) in contact with chlorine in the presence of hydrogen fluoride and a fluorination catalyst.F133a may be obtained by fluorination of trichloroethylene, and the F123 may be subsequently fluorinated to F125.
Owner:ATOFINA
Optical film
InactiveUS6210872B1Easily fixSuperior in orientation hold abilityLiquid crystal compositionsPolarising elementsChemistryPolyester
A novel optical film capable of readily vitrifying the tendency and state of liquid crystal alignment, excellent in the ability to retain the alignment state, and suitable for the application to optical elements. The film is produced from a liquid-crystal material containing as the essential ingredient a liquid-crystal polyester having structural units (A) and (B) as the essential unit, exhibiting a vitrified state at a temperature lower than the liquid-crystal transition point, and having a logarithmic viscosity number (eta) of 0.04-0.4 dl / g as measured at 30° C. in a phenol / tetrachloroethane solvent (60 / 40 by weight), wherein each X represents independently O or C=O; each Y represents independently a group selected from among F, Cl, Br and 1-4C alkyls; and n is 0 or 1.
Owner:NIPPON MITSUBISHI OIL CORP
Low-melting-point and high-strength aluminum-based brazing filler metal and preparation method thereof
InactiveCN102000924AReduce brittlenessReduce surface tensionWelding/cutting media/materialsSoldering mediaNitrogen gasToughness
The invention discloses a low-melting-point and high-strength aluminum-based brazing filler metal and a preparation method thereof. The brazing filler metal consists of the following components in percentage by mass: 6 to 13 percent of Si, 6 to 13 percent of Cu, 1 to 3 percent of Ni, 0.01 to 0.1 percent of Sr, 0.01 to 0.2 percent of Ti, 0.01 to 0.2 percent of Y and the balance of Al. The preparation method comprises the following steps of: weighing each component; adding the components into a graphite crucible melting furnace according to a certain sequence; melting and then refining twice, wherein argon gas and hexachloroethane are used as refining agents during the refining; introducing the hexachloroethane from bottom of solution by the argon gas, wherein the introduction pressure of the argon gas is 5 to 7KPa; and continuously casting solution which is subjected to the second refining process under the protection of nitrogen gas as required or performing gas atomization to form the aluminum-based brazing filler metal in different forms. The aluminum-based brazing filler metal prepared by the method has the superior characteristics of low melting point, high strength, toughness, wettability and spreadability and the like.
Owner:GUILIN QINGTONG NON FERROUS METAL ARTS & CRAFTS MATERIAL DEV CO LTD
Method for producing metafluoroethylene by pyrolysis of difluoro-chloroethane
InactiveCN1428320AImprove conversion rateCoking and clogging will not occurPreparation by hydrogen halide split-offPhysical chemistryFluoride
The method for producing vinylidene fluoride by using difluorine-chloroethane includes the following steps: adopting superheated steam, mixing it with difluorine-chloroethane and making them produce cracking reaction in tube reactor, then making the crackate undergo the processes of quenching, low-pressure condensation dewatering, drying treatment, removing high boiler, removing liquid component, rectifying vinylidene fluoride and recovering vinylidene fluoride and difluorine-chloroethane from residue so as to implement goal of said invention. The invented difluorine-chloroethane conversion rate can be up to 95%, selectivity of vinylidende fluoride is greater than 99% and its vinylidene fluoride monomer purity is greater than or equal to 99.99%.
Owner:ZHONGHAO CHENGUANG RES INST OF CHEMICALINDUSTRY CO LTD
Preparation method of trichlorosucrose-6-acetate
InactiveCN102070678AReduce dosageLess side effectsEsterified saccharide compoundsSugar derivativesFood sweetenersSide reaction
The invention relates to a preparation method of an intermediate of the food sweetening agent trichlorosucrose, belonging to the technical field of fine chemicals. The theory of the invention is as follows: chloroethane is used as reaction solvent, thus replacing dimethyl formamide (DMF) which is generally used as solvent; and DMF is used as catalyst, thereby greatly reducing the dosage of DMF and saving the cost; and the generated hydrogen chloride and sulfur dioxide can fast leave the reaction system, thereby reducing side reactions. The preparation method has the following beneficial effects; the method has low cost and less reaction steps, the yield is obviously improved by about 20% compared with the method using DMF as solvent, and the preparation method has obvious advantages.
Owner:CHANGZHOU NIUTANG CHEM PLANT CO LTD +1
Method for preparing bromination polystyrene
The invention discloses a method for preparing bromination polystyrene, which includes (1) adding 10-30 parts by weight of styrene into 100 parts by weight of dichloroethane and adding 0.1 to 0.5 parts by weight of BPO or AIBN to obtain polystyrene dichloroethane solution; (2) adding 0.1 to 3 parts by weight of lewis acid catalyst and 0.1 to 3 parts by weight of backbone protective agent in the above prepared solution and then dropping 30 to 90 parts of bromine chloride dichloroethane solution to obtain bromination polystyrene dichloroethane solution; (3) adding 00.5 to 0.1 parts by weight ofneutralizer into the above prepared solution, conducting washing and static layering, adding 0.1 to 3 parts by weight of fat removing bromine preventing agent in the layered organic layers, conducting flash evaporation, cooling, centrifugation and drying on the organic layer to obtain the product. The process is capable of manufacturing polystyrene with narrow molecular weight distribution, and the polystyrene can obtain bromination polystyrene with controllable molecular weight after bromination.
Owner:SHANDONG RUNKE CHEM
Method and device for refining chlorine hydride byproduct and recovering trifluoromethane in production of monochlorodifluoromethane
ActiveCN102101651AAchieve cycle productionAvoid pollutionChlorine/hydrogen-chloride purificationHalogenated hydrocarbon separation/purificationPolyvinyl chlorideWater resources
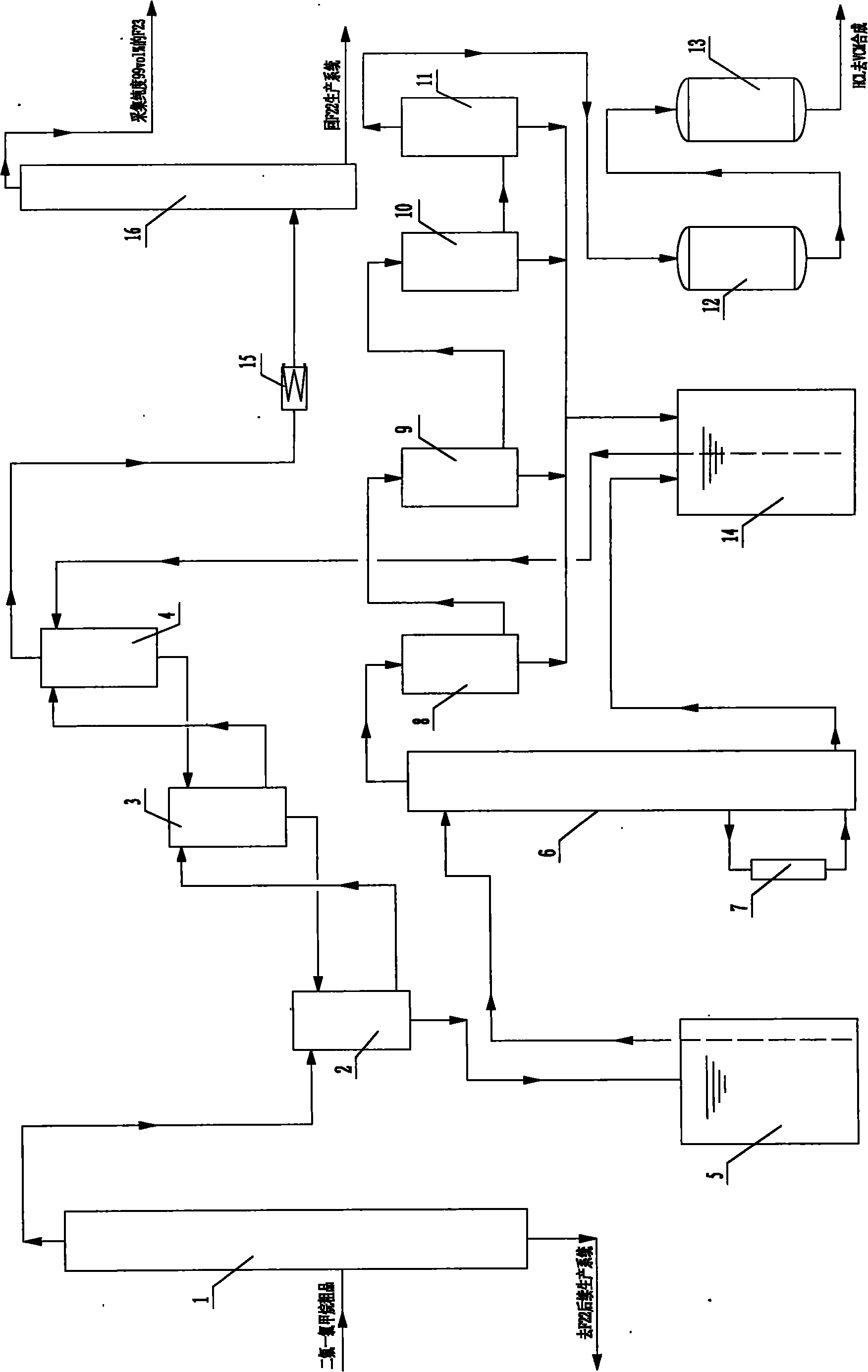
The invention relates to a method for refining a chlorine hydride byproduct and recovering trifluoromethane in production of monochlorodifluoromethane. Refined high-purity chlorine hydride can directly serve as a raw material for synthesizing vinyl chloride monomers; and the trifluoromethane byproduct separated in the refining process can be effectively recovered, wherein the refining of the HCl comprises the following steps of: crude separation, absorption, analysis, condensation, capture of acid mist, and adsorption; and after a F23 byproduct in the production of the monochlorodifluoromethane is separated in the absorption process in the refining of the HCl, the F23 byproduct is subjected to lossless compression and rectification to form the trifluoromethane with the purity of over 99 vol percent. By combining the recovery and comprehensive utilization of the HCl and the recovery of the trifluoromethane, the high consumption of water resources and liquid caustic soda is avoided, and the production cost of the monochlorodifluoromethane is reduced; and the effective recovery of the trifluoromethane prevents environmental pollution, and the chloroalkali, the monochlorodifluoromethane and polyvinyl chloride (PVC) can be circularly produced.
Owner:SHANDONG DONGYUE CHEM
Preparation of 3D rapid prototyping alumina-zirconia-carbon ceramic powder material
InactiveCN104725046ASmall median diameterNarrow particle size distributionAdditive manufacturing apparatusAdhesiveSilanes
The invention discloses a method for preparing a 3D rapid prototyping alumina-zirconia-carbon ceramic powder material. The method is characterized by comprising the following steps: firstly, pre-treating alumina-zirconia-carbon ceramic powder with N-(beta-aminoethyl)-gamma-amino propyl trimethoxy silane and stearic acid to obtain pre-treated alumina-zirconia-carbon ceramic powder; then, adding the following components in percentage by mass: 60-70 percent of trichloroethane and 2-5 percent of bisphenol A polycarbonate into a reactor, stirring and dissolving, and adding 26-36 percent of the pre-treated alumina-zirconia-carbon ceramic powder, uniformly stirring and mixing, intensively stirring at constant temperature of 50+ / -5 DEG C, refluxing to react for 5-7 hours, and drying by spraying to obtain the rapid prototyping alumina-zirconia-carbon ceramic powder material. The material does not need to spray adhesive, can be directly molded in a molding temperature range of 220-230 DEG C, has the advantages of simple preparation process, easily controlled condition and low production cost, and is easy for industrial production.
Owner:UNIV OF JINAN
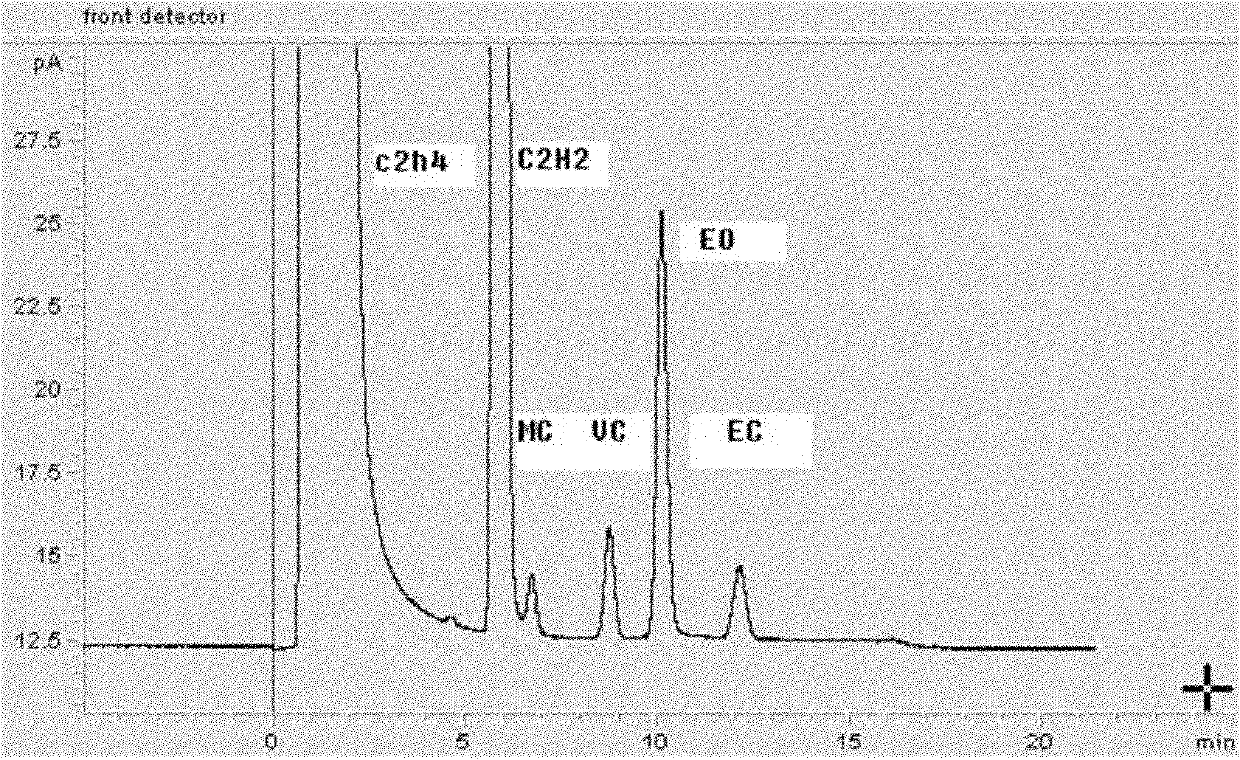
Chromatographic column, preparation method thereof and application thereof to analysis of mixture comprising 1,2-dichloroethane, ethyl chloride, methyl chloride and/or vinyl chloride
InactiveCN102565248AEfficient separation and accurate quantificationEasy to separateComponent separationChemistryChromatography column
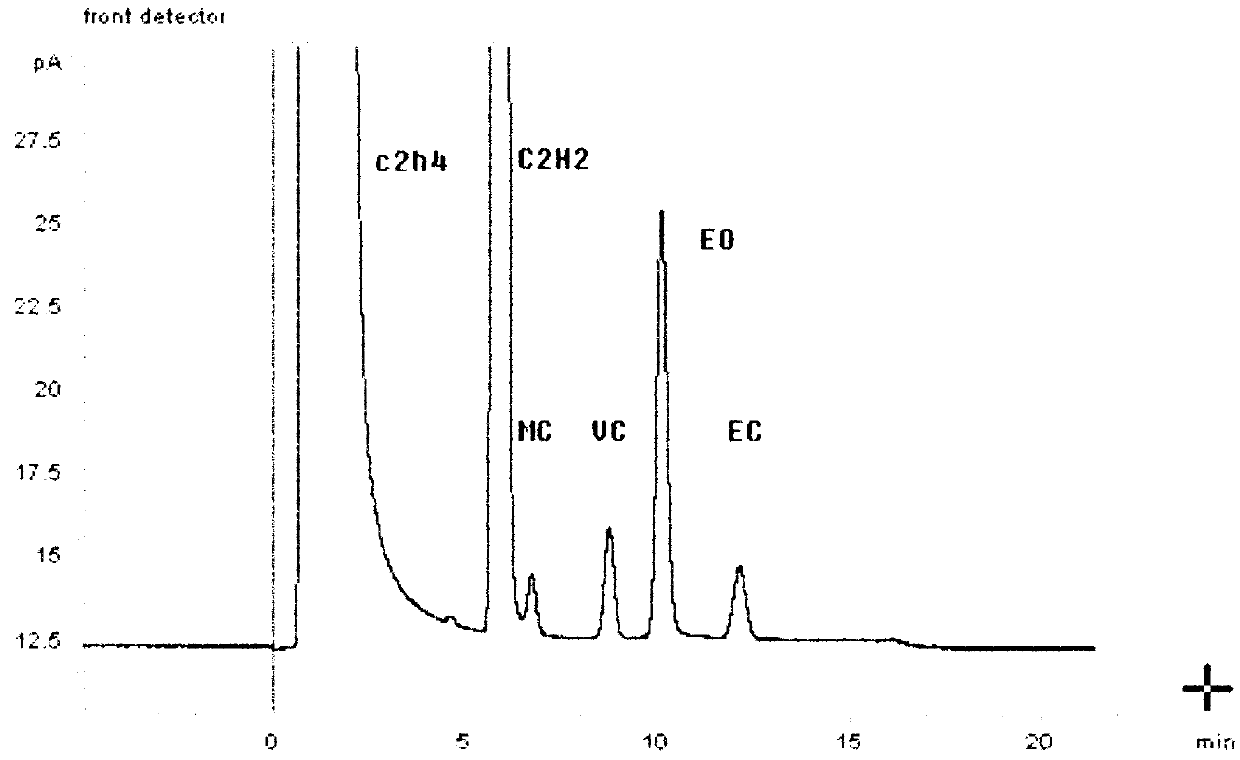
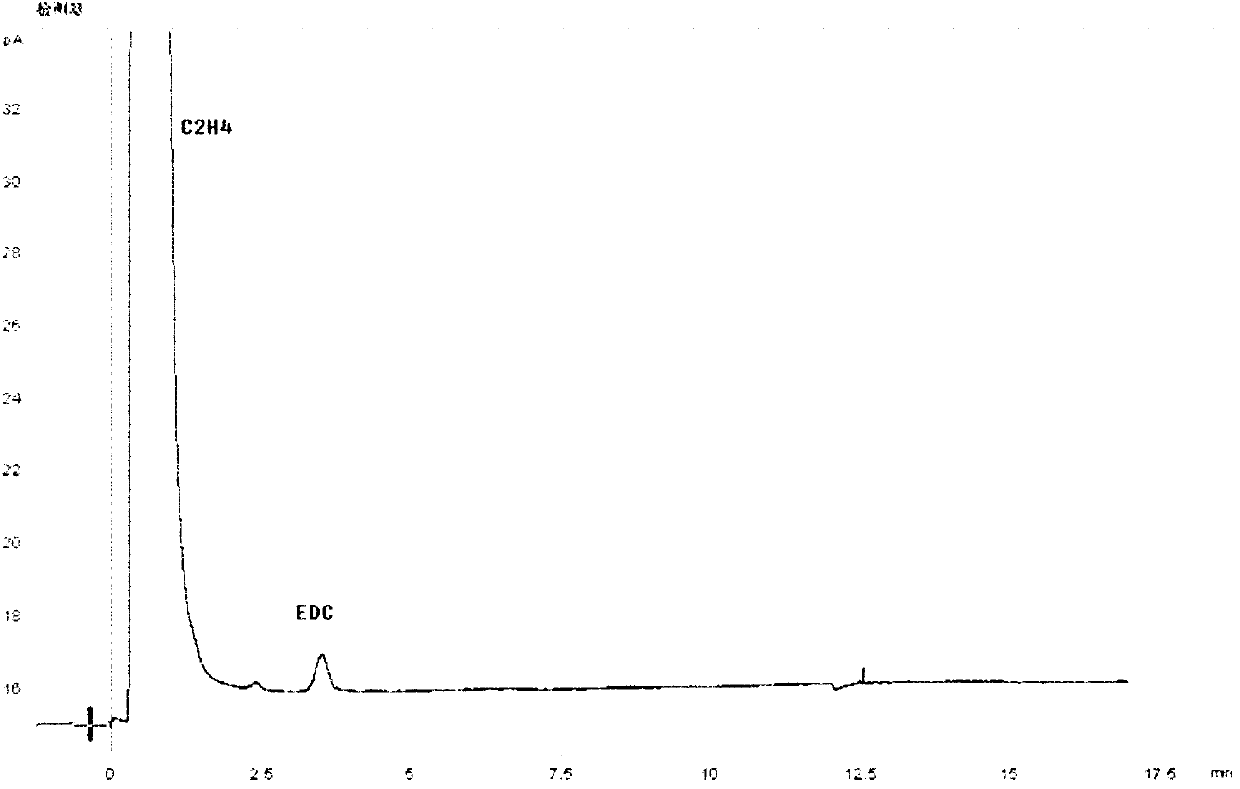
The invention relates to a chromatographic column for performing gas chromatographic analysis on a mixture comprising 1,2-dichloroethane, ethyl chloride, methyl chloride and / or vinyl chloride. A fixed phase is a mixture of GDX 502 of 60-80 meshes and Porapak P of 80-100 meshes, wherein the weight ratio of GDX 502 to Porapak P is (0.3-0.6):1. The invention further relates to a method for preparing the chromatographic column, which comprises the following step of: preparing a mixture of GDX 502 of 60-80 meshes and Porapak P of 80-100 meshes for serving as the fixed phase. The invention further relates to a method for performing gas chromatographic analysis on the mixture comprising 1,2-dichloroethane, ethyl chloride, methyl chloride and / or vinyl chloride, which comprises the following step of: making the mixture pass through the chromatographic column. The GDX 502 with a good separating effect on methyl chloride and Porapak P with good separating effects on ethyl chloride and vinyl chloride are combined together, and the mixture is taken as the fixed phase, so that 1,2-dichloroethane, ethyl chloride, methyl chloride and / or vinyl chloride in the mixture can be separated effectively and quantified accurately.
Owner:CHINA PETROLEUM & CHEM CORP +1
Compound 6013-type aluminum alloy microalloyed by zirconium and strontium and preparation method thereof
The invention discloses a 6013-type aluminum alloy microalloyed by zirconium and strontium, which mainly comprises Al, 1.22% to 1.52% of Mg, 0.90% to 1.15% of Si, 0.804% to 1.04% of Cu, 0.451% to 0.661% of Mn, 0.0311% to 0.135% of Zn, 0.0915% to 0.135% of Zr and 0.0157% to 0.0391% of Sr. The preparation method of the alloy comprises the steps of: melting pure Al; sequentially adding Al-Cu intermediate alloy, Al-Si intermediate alloy, Al-Mn intermediate alloy, Al-Zr intermediate alloy, Al-Sr intermediate alloy, pure Zn and pure Mn; melting and adding hexachloroethane for refining; standing and keeping the temperature for 5 to 10 minutes; removing residues; casting into ingots; and treating the cast ingots. The hardness of the alloy reaches 156.0 to 159.1 HV; the performance of resisting intergranular corrosion is in fourth level; and the performance of resisting exfoliation corrosion is not less than PB level. The alloy can be widely applied to the fields of modern aerospace, weaponry and the like, and has wide application prospect.
Owner:NANTONG JIANGZHONG PHOTOELECTRIC
Recovery method and recovery device for sulfur dioxide in sucralose chlorination reaction
InactiveCN108373139AReduce consumptionReal-time control of the reaction cycleChlorine/hydrogen-chloride purificationSulfur compoundsWater chlorinationEnvironmental engineering
The invention relates to a recovery method for sulfur dioxide in sucralose chlorination reaction. The method includes steps: 1, adding concentrated sulfuric acid into a drying tower, removing moisturefrom tail gas of a gas-liquid separator, and allowing dried sulfur dioxide and trichloroethane tail gas to enter a primary condenser; 2, allowing residual tail gas and condensate flowing through theprimary condenser to enter a trichloroethane separation tank, allowing sulfur dioxide and trichloroethane condensate to enter a collection tank, and enabling tail gas sulfur dioxide to enter a secondary condenser; 3, enabling tail gas and sulfur dioxide condensate flowing through the secondary condenser to enter a tertiary condenser at a temperature ranging from -25 DEG C to -30 DEG C, condensinggaseous sulfur dioxide into liquid sulfur dioxide, and sending into a recovery storage tank. The recovery method has advantages that hydrogen chloride in the tail gas is subjected to three-stage waterabsorption to obtain high-concentration hydrochloric acid while sulfur dioxide and trichloroethane in the tail gas are separated out, and accordingly the product conversion rate is increased, raw material and energy consumption is reduced, and cost is saved to the maximum extent.
Owner:ANHUI JINGHE IND
Preparation method of 1,1-difluoroethylene
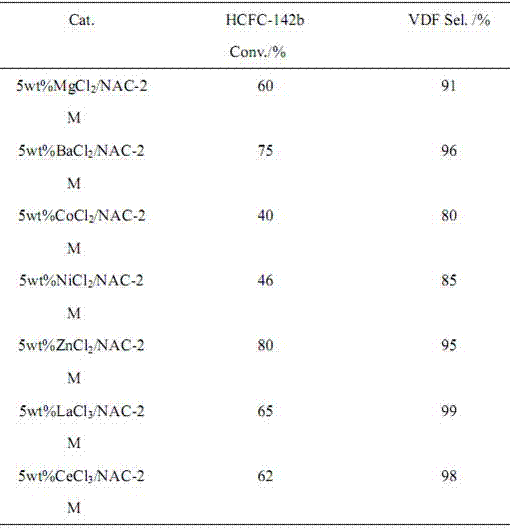
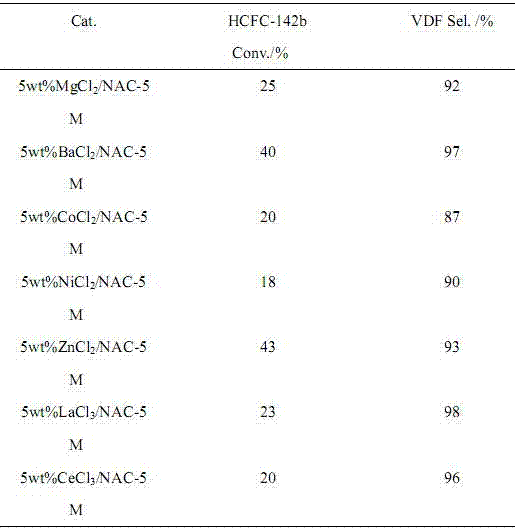
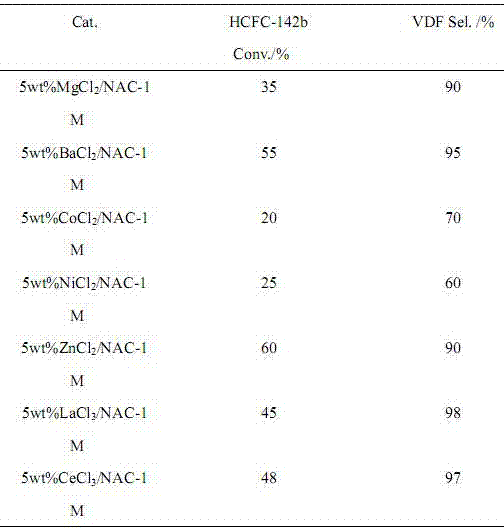
ActiveCN106866354AIncrease added valueEnvironmentally friendlyPreparation by hydrogen halide split-offPhysical/chemical process catalystsMetal chlorideAlkaline earth metal
The invention provides a preparation method of 1,1-difluoroethylene (vinylidene fluoride (VDF)). Under the action of a catalyst, a gas phase of 1,1-difluoro-1-chloroethane (HCFC-142b) is subjected to dehydrochlorination to generate the 1,1-difluoroethylene. The catalyst is composed of a main catalyst and a co-catalyst; the main catalyst is a nitrogen-containing carbon material; the co-catalyst is chloride of transition element, alkali-earth metal and lanthanide metal. The method for preparing the 1,1-difluoroethylene, provided by the invention, has the characteristics of low reaction temperature, high product selectivity, high yield, uneasiness of blocking a pipeline, moderate conditions of a preparation process, simplicity in operation and the like.
Owner:SHANDONG HUAXIA SHENZHOU NEW MATERIAL
Monoazo compounds, preparation method and uses thereof
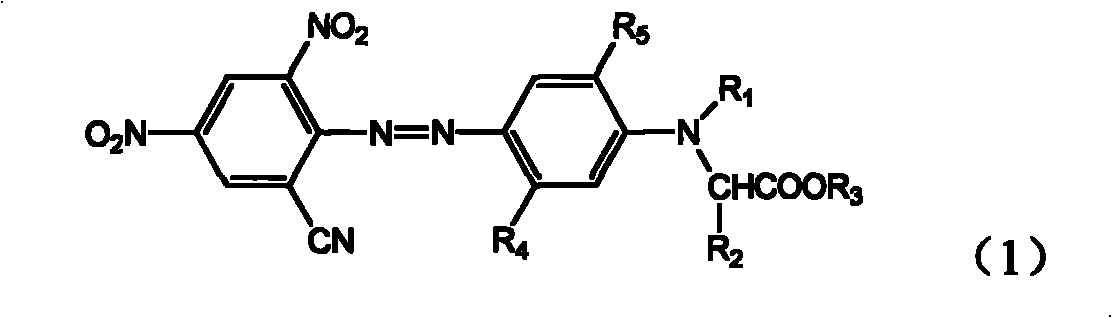
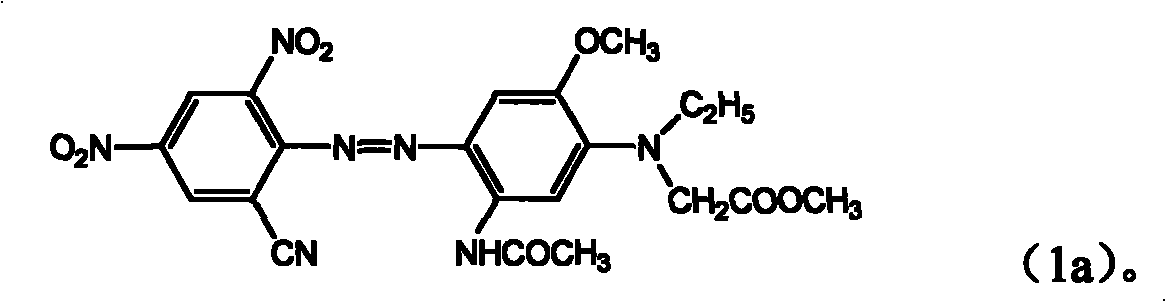
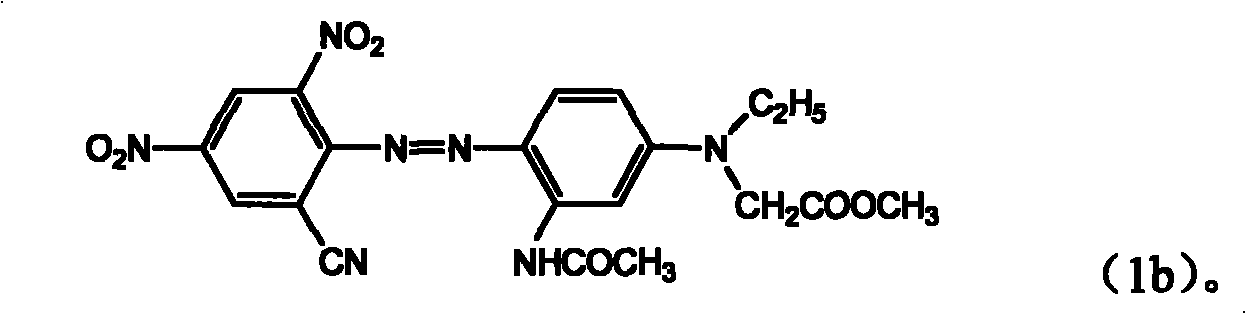
ActiveCN101289414AGood washing fastnessGood thermal migration resistanceOrganic chemistryReactive dyesPolyesterHalogen
The invention relates to a monoazo compound, a preparation method and an application thereof. The azo compound is shown in the formula (1), wherein, R1 is hydrogen, methyl, ethyl, allyl, butyl, methoxy carbonyl ethyl and an ethoxy carbonyl ethyl; R2 is the hydrogen, the methyl and the ethyl; R3 is C1-6 alkyl, wherein, atoms can be included or not included for the alkyl chain; R4 is the hydrogen, the methyl, the ethyl, -NHCO-C1-4 alkyl and halogen, and the C1-4 alkyl in the -NHCO-C1-4 alkyl is the methyl, the ethyl, 2-methicillin ethyl and chloroethane; R5 is the hydrogen, the methoxy, the ethoxy, the 2-methicillin ethyl and the halogen. The preparation method comprises: the monoazo dye which contains the halogen is first prepared; and then cyan is used for replacing the halogen substituent. The monoazo compound of the invention which can be applied in polyester dyeing has the advantage of excellent application fastness.
Owner:阮伟刚
Method for preparing lidocaine hydrochloride
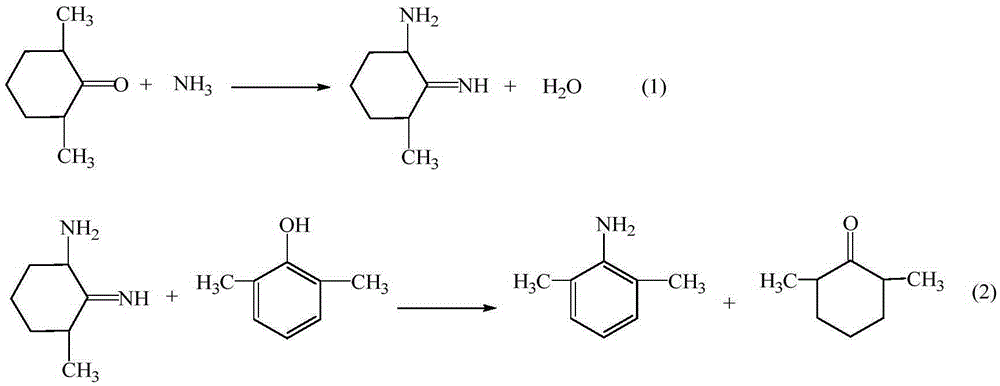
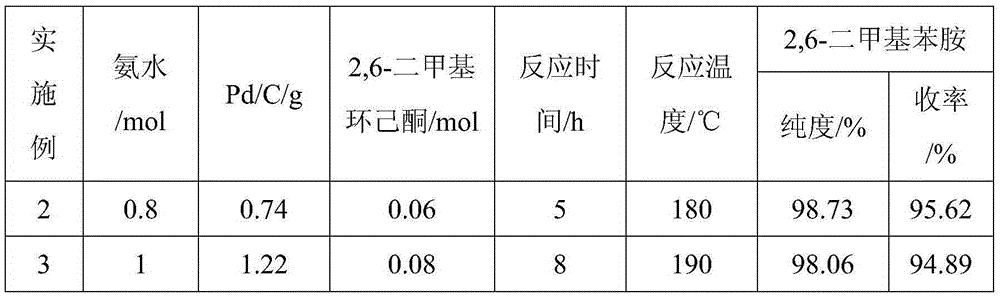
ActiveCN105294477AThe synthesis process is simpleHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideOrganic layer
The invention provides a method for preparing lidocaine hydrochloride, and belongs to the technical field of anesthetic synthesis. The method comprises the following steps: by taking 2,6-xylenol as a raw material, Pd / C as a main catalyst and 2,6-dimethylcyclohexanone as a promoter, performing liquid phase amination with ammonia water at high temperature, thereby obtaining a midbody 2,6-dimethylaniline; enabling sodium methylate, 2,6-dimethylaniline and N,N-lignocaine methyl acetate as raw materials to react at 90-95 DEGC, distilling while reaction is performed to remove methanol till no methanol can be evaporated out, continuously reacting for 30 minutes, cooling to the room temperature, adding dichloroethane, washing with water, and leaving to stand to layer, thereby obtaining an organic layer, namely, a lidocaine based dichloroethane solution; further adding hydrochloric acid into the lidocaine based dichloroethane solution, adjusting the pH value to be 3.5-4 by using hydrogen chloride, adding activated carbon to reflux for 20-40 minutes, filtering, concentrating the filtrate, cooling, crystallizing, and dying, thereby obtaining lidocaine hydrochloride. The lidocaine hydrochloride prepared by using the method is simple in synthesis process and high in product purity, that is, the purity can be greater than 99%, and the total yield is greater than 84%.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
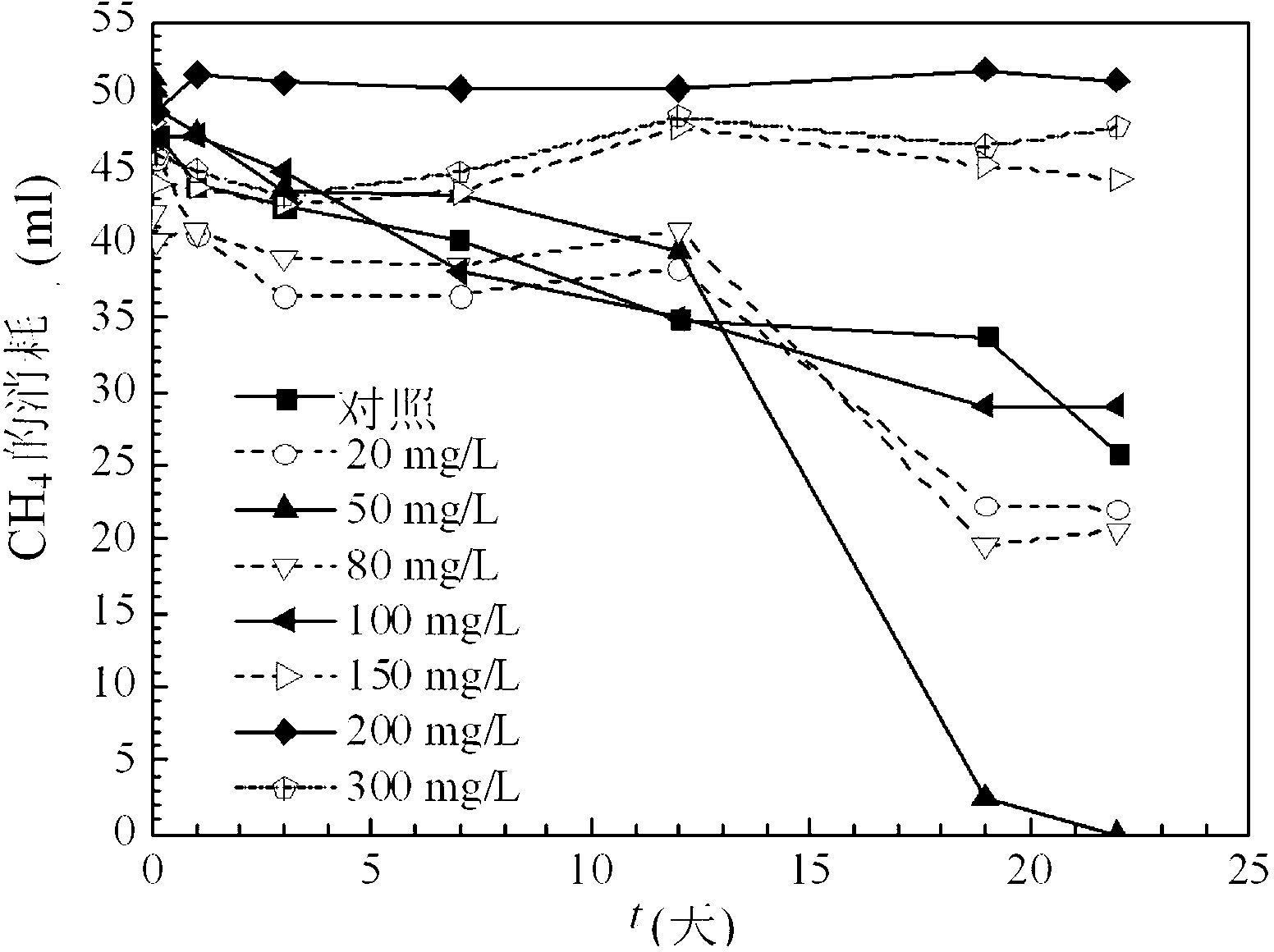
Facultative methanotroph capable of degrading chlorohydrocarbons, and its applications
InactiveCN103224896AImprove toleranceEfficient degradationBacteriaWater contaminantsSodium acetateAlkane
The invention relates to a facultative methanotroph capable of degrading chlorohydrocarbons, and its applications. The strain of the methanotroph has a preservation number of CCTCCNO:M2013062. The methanotroph can utilize carbon-rich carbon sources comprising ethanol, sodium acetate and the like, and overcomes application difficulties comprising low methane-oxidizing bacterium thallus density, difficult thallus enlargement culture and the like. The methanotroph can degrade chloroolefins comprising trichloroethylene, dichloroethylene, chloroethylene and the like, can also degrade chloralkanes comprising tetrachloromethane, trichloromethane, dichloroethane and the like, and is especially suitable for the fields of wastewater, drinking water and soil restoration.
Owner:CHONGQING UNIV OF TECH
Process for producing high-purity methane chloride
InactiveCN102153439ANo pollution in the processHigh purityHalogenated hydrocarbon preparationAcid washingChloride
The invention provides a process for producing high-purity methane chloride. The process comprises the following steps of: introducing vaporized and overheated methanol and hydrogen chloride a reactor containing an alumina catalyst and reacting in the reactor to generate a mixture of methane chloride, methane, chloroethane and dichloromethane; introducing the generated mixture to a chilling device, carrying out chilling separation and then introducing into an acid-washing tower, an alkaline washing tower and a sulfuric acid drying system, compressing to prepare coarse methane chloride; leading the coarse methane chloride into a treating column and separating out heavy components from the bottom of the treating column; evaporating light-component methane and methane chloride to the top of the treating column together; then performing water cooling and deep freezing so that the methane chloride is liquefied, completely discharging the methane which is not liquefied into an exhaust washing tower by a deep freezer so as to separate methane chloride from methane and finally obtain high-purity methane chloride. In the invention, a single-tower rectification manner is adopted, therefore, equipment investment is not increased greatly, but also the content of methane chloride is increased to be higher than 99.98%.
Owner:于淑芳
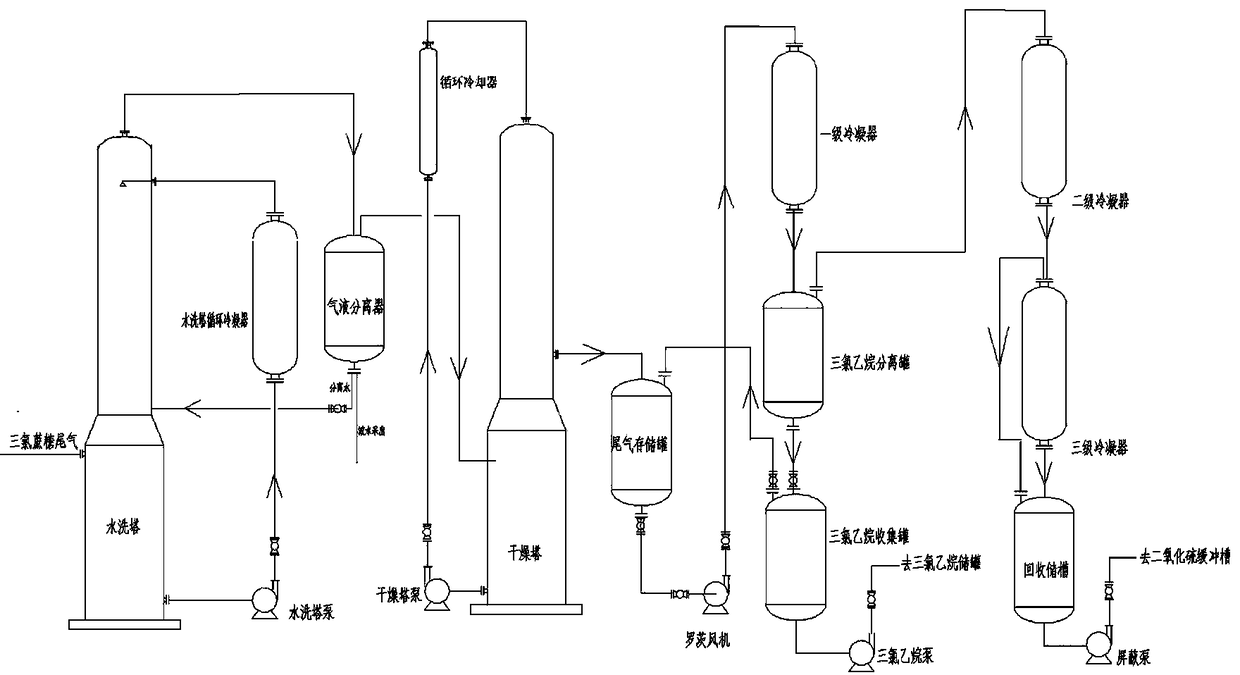
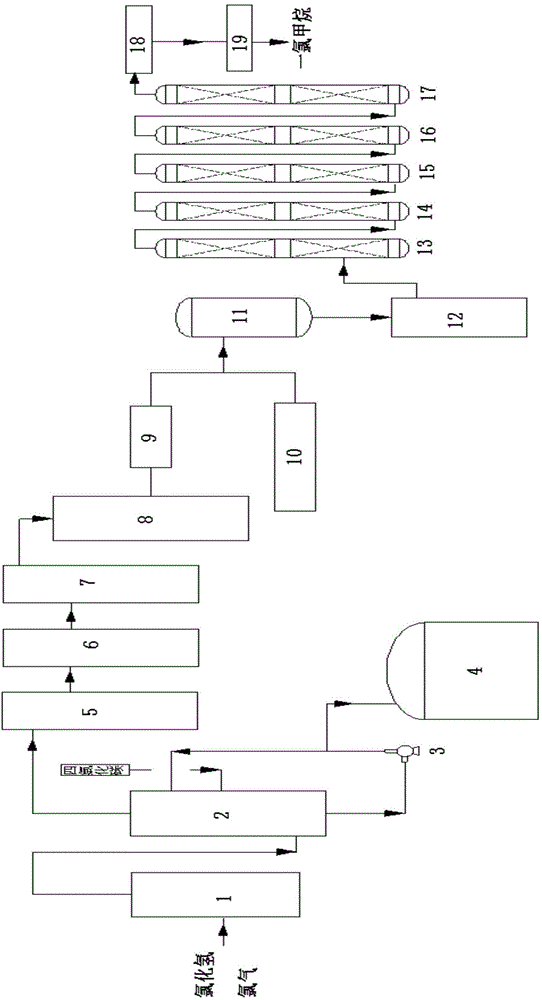
Preparation method of difluoro chloroethane and its production equipment
ActiveCN1556083AImprove conversion rateReduce unit consumptionHalogenated hydrocarbon preparationChemical reactionIt equipment
A process for preparing difluorochloro ethane from difluoroethane and chlorine gas includes proportionally mixing, three-class photo-chemical reaction while regulating light flux for presenting the high-boiling-point substance peaks with different heights, removing acid, alkali washing, compressing, degassing, rectifying and drying. Its equipment is disclosed also. Its advantages are high conversion rate of difluoroethane and high selectivity and purity of product.
Owner:ZHEJIANG AIKESHENG CHEM
Nitrogen-doped activated carbon catalyst and application thereof
ActiveCN104475143AImprove catalytic performanceImprove conversion ratePreparation by hydrogen halide split-offPhysical/chemical process catalystsGas phaseTetrachloroethane
The invention discloses a nitrogen-doped activated carbon catalyst and application thereof. The preparation method of the nitrogen-doped activated carbon catalyst comprises the following steps: adding a nitrogen-containing compound into water to dissolve to obtain a nitride solution, wherein the nitrogen-containing compound is one or more of ammonia water, ammonia gas, pyridine, pyrrole, imidazole, acrylamide, polyacrylamide and polyvinylpyrrolidone; adding activated carbon into the nitride solution to soak; completely drying the soaked activated carbon, and calcining in nitrogen atmosphere, thereby preparing the nitrogen-doped activated carbon catalyst. The nitrogen-doped activated carbon catalyst disclosed by the invention has very good catalysis property in reaction of catalyzing tetrachloroethane and acetylene to generate trichloro ethylene and chloroethylene and catalyzing tetrachloroethane gas phase cracking to generate trichloro ethylene.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Process for the preparation of sucralose by the chlorination of sugar with triphosgene (BTC)
In one embodiment of the invention a method to prepare sucralose-6-acylate through chlorinating sucrose-6-acylater by BTC in the process of sucralose preparation is disclosed. In this embodiment a Vilsmeier reagent is firstly prepared below 0° C. by dissolving BTC in DMF or in component solvent, containing DMF, toluene, dichloroethane, chloroform and carbon tetrachloride. Consequently, sucrose-6-ester was chlorinated by Vilsmeier reagent. BTC can also be dissolved in one or several organic solvent such as toluene, dichloroethane, chloroform and carbon tetrachloride, and added to a DMF solution of sucrose-6-acylate for chlorination. Sucralose was prepared through de-esterifying the obtained sucralosed 6-ester using sodium methoxide / methanol or sodium ethoxide / ethanol.
Owner:MAMTEK INT
Method for producing methane chloride through byproduct hydrogen chloride in production process of tetrachloroethylene
ActiveCN104311383AResolve disposal issuesEasy to corrodeHalogenated hydrocarbon preparationGas phaseMixed gas
The invention relates to a method for producing methane chloride through a byproduct hydrogen chloride in production process of tetrachloroethylene. The method comprises the steps of (1) condensing the mixed gas of excess chlorine and the byproduct hydrogen chloride in production process of tetrachloroethylene, then absorbing the chlorine by carbon tetrachloride between -5DEG C and -21DEG C, condensing again, eliminating the foam, buffering, and compressing; (2) heating up the hydrogen chloride and methyl alcohol, then reacting for 2-5 seconds under the action of a catalyst aluminum oxide so as to obtain the mixed product of methane chloride, methane, chloroethane and dichloromethane, and (3) chilling and separating the mixed product, sequentially water washing, alkali washing, drying by sulfuric acid and compressing the gas phase at 0.6-0.95MPa, condensing at 25-40DEG C, so as to prepare the methane chloride. According to the method, the chlorine is adsorbed by carbon tetrachloride, so that the chlorine is prevented from entering into a reaction system of the hydrogen chloride and methyl alcohol to affect the purity and selectivity of the reaction product methane chloride.
Owner:SHANDONG DONGYUE FLUO SILICON MATERIALS CO LTD
Method for preparing tetrachloroethylene by liquid-phase catalysis of pentachloroethane
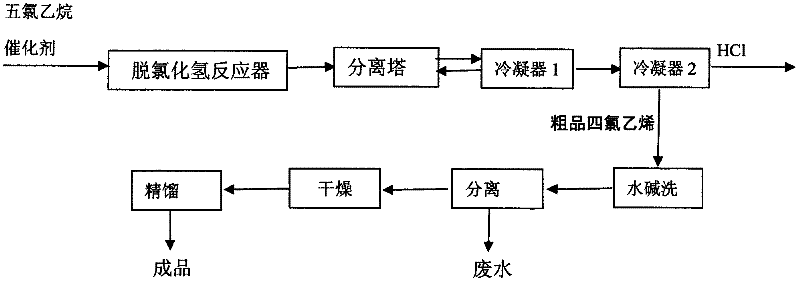
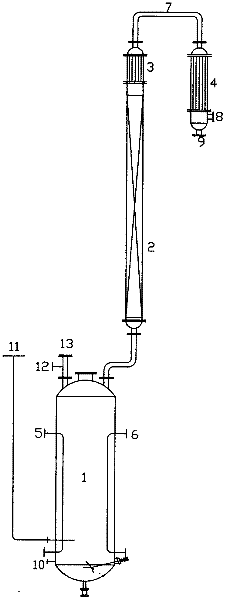
ActiveCN102295521ASolve the environmental problems of serious pollutionIncrease profitPreparation by hydrogen halide split-offCalcium hydroxideTetrachloroethylene
The invention relates to a method for preparing tetrachloroethylene through pentachloroethane liquid-phase catalysis, which comprises the following steps of: an intermittent method: by using high-purity pentachloroethane as a raw material, adding a metal halide as a catalyst according to the percentage by weight, stirring and heating in a reactor, and intermittently reacting and distilling under a normal pressure to obtain a crude tetrachloroethylene product; or a continuous method: by using the prepared crude tetrachloroethylene product as a mother solution, adding the metal halide as the catalyst, stirring, heating, then adding the high-purity pentachloroethane, maintaining the reaction, then heating to prepare the crude tetrachloroethylene product, and continuously adding the high-purity pentachloroethane to continuously produce the crude tetrachloroethylene products with the same volume; and carrying out water-alkali washing for the crude products prepared by the two methods to be neutral, separating, drying and rectifying to obtain a tetrachloroethylene finished product. The environmental-protection difficult problem of serious environmental pollution brought by traditional calcium-hydroxide saponification is radically solved, particularly the usage amount of the catalyst is small in the continuous method, materials are continuously fed and produced, the method is simpler and more labor-saving to operate, the cost is low, the method is suitable for large-batch production, the utilization ratio of the materials is high, and the purity of the prepared product reaches up to more than 99.8 percent.
Owner:江西国宏化工有限公司
Open type gear oil
InactiveCN1594517AImprove the disadvantages of poor viscosity-temperature propertiesProvide adhesionAdditivesPhysical chemistryEnvironmental engineering
Disclosed is an open type gear oil comprising asphalt based basic oiling material, adhesive, extreme pressure antiwearing agent, heavy alkyl benzene solvent and 2# solvent including chlorine-containing methane, chlorine-containing ethane and chlorine-containing ethane. The open type gear oil is prepared through mixing the components.
Owner:刘端 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com