Preparation method of 1,1-difluoroethylene
A technology of vinylidene fluoride and ethylene chloride, which is applied in the field of preparation of 1,1-ethylene difluoride, can solve the problems that catalytic cracking cannot be industrialized, catalyst stability and service life are poor, etc., and achieves significant social and economic benefits and high cost Low, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
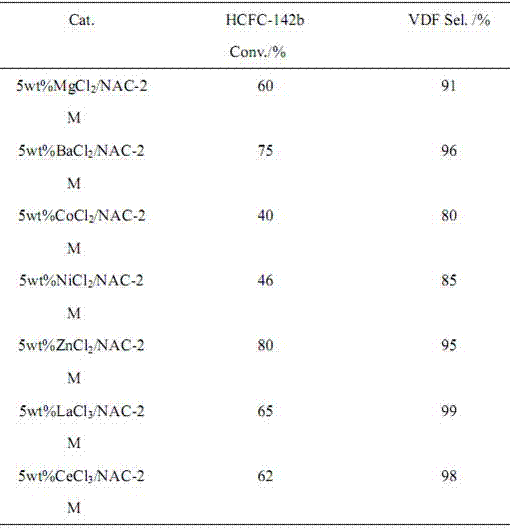
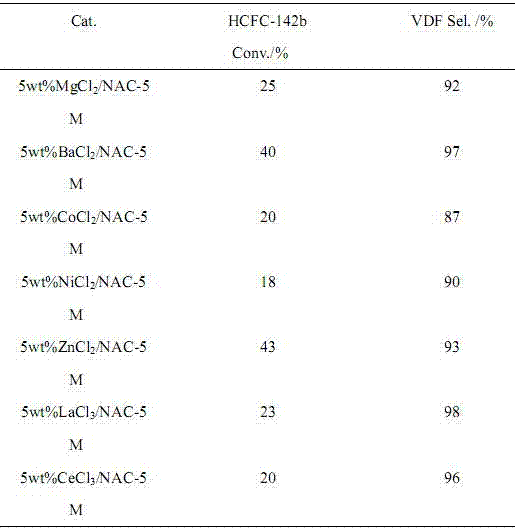
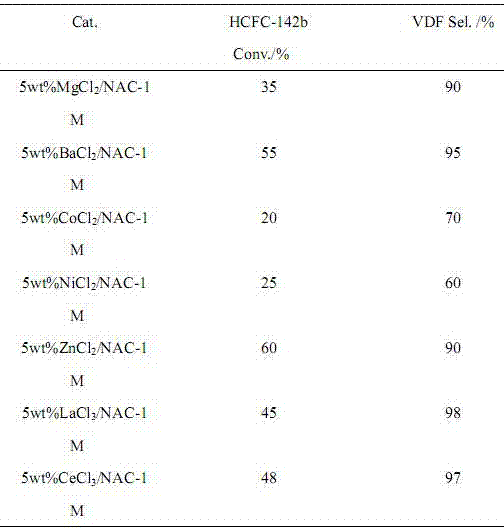
Image
Examples
Embodiment 1
[0029] First, the coconut shell activated carbon was treated with 20 wt% nitric acid solution at 85 °C for 2 h, and washed to neutral. The nitric acid-treated coconut shell charcoal and melamine were stirred and mixed in absolute ethanol at a mass ratio of 1:1, and then the ethanol was evaporated to dryness to obtain a mixture of activated carbon and melamine. The mixture was calcined at 800°C for 4h to obtain nitrogen-doped carbon catalyst.
[0030] Then, the obtained nitrogen-doped carbon catalyst is used to catalyze the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the tube reactor is a Ni tube with an inner diameter of 20mm and a length of 800mm, and is placed in a heating furnace to heat up to 400°C for reaction. The reaction pressure is normal pressure. Before the reaction, pass N 2 (airspeed is 1000h -1 ), the temperature of the catalyst bed was raised fro...
Embodiment 2
[0032] The coconut shell charcoal treated with nitric acid and melamine were mixed in absolute ethanol at a mass ratio of 1:1, the ethanol was evaporated to dryness and calcined at 800°C for 4 hours to obtain a nitrogen-doped carbon catalyst.
[0033]Then, the obtained nitrogen-doped carbon catalyst is used to catalyze the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the tube reactor is a Ni tube with an inner diameter of 20mm and a length of 800mm, and is placed in a heating furnace to heat up to 400°C for reaction. The reaction pressure is normal pressure. Before the reaction, pass N 2 (with an airspeed of 1000 h -1 ), the temperature of the catalyst bed was raised from room temperature to 200 °C at a rate of 5 °C / min and dried for 2 h. Then, the temperature was raised to 400°C at a rate of 5°C / min and the catalyst was treated for 2 hours, then the temperature ...
Embodiment 3
[0035] The coconut shell charcoal treated with nitric acid and melamine were mixed in absolute ethanol at a mass ratio of 1:1, the ethanol was evaporated to dryness and calcined at 800°C for 4 hours to obtain a nitrogen-doped carbon catalyst.
[0036] Then, the obtained nitrogen-doped carbon catalyst is used to catalyze the removal of HCl from 1,1-difluoro-1-chloroethane to prepare vinylidene fluoride. The reaction is carried out in a plug-flow tube reactor. The reaction tube of the tube reactor is a Ni tube with an inner diameter of 20mm and a length of 800mm, and is placed in a heating furnace to heat up to 400°C for reaction. The reaction pressure is normal pressure. Before the reaction, pass N 2 (with an airspeed of 1000 h -1 ), the temperature of the catalyst bed was raised from room temperature to 200 °C at a rate of 5 °C / min and dried for 2 h. Then, the temperature was raised to 400°C at a rate of 5°C / min and the catalyst was treated for 2 hours, then the temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com