Patents
Literature
6149 results about "Hydrogen chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The compound hydrogen chloride has the chemical formula HCl and as such is a hydrogen halide. At room temperature, it is a colourless gas, which forms white fumes of hydrochloric acid upon contact with atmospheric water vapor. Hydrogen chloride gas and hydrochloric acid are important in technology and industry. Hydrochloric acid, the aqueous solution of hydrogen chloride, is also commonly given the formula HCl.
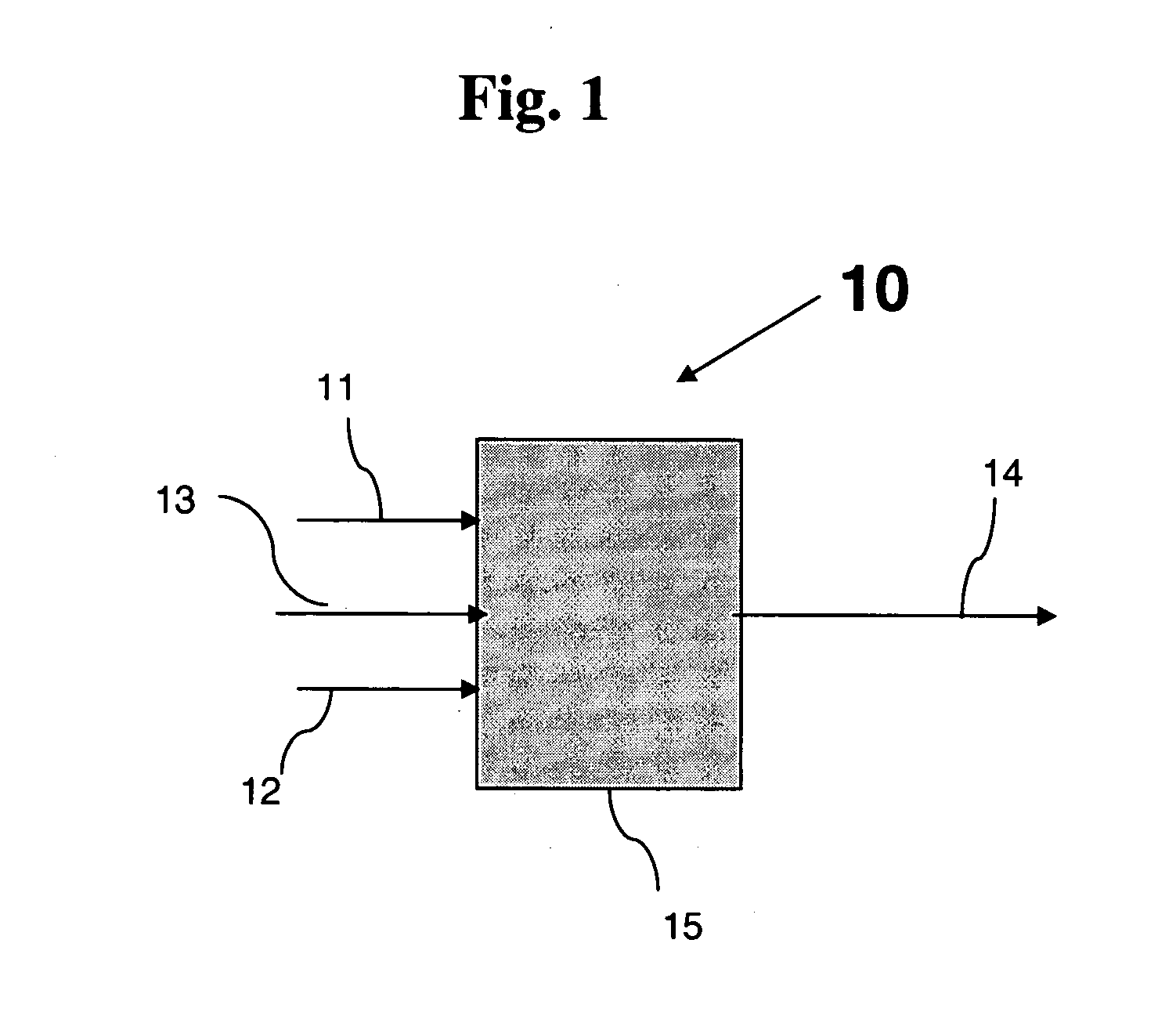
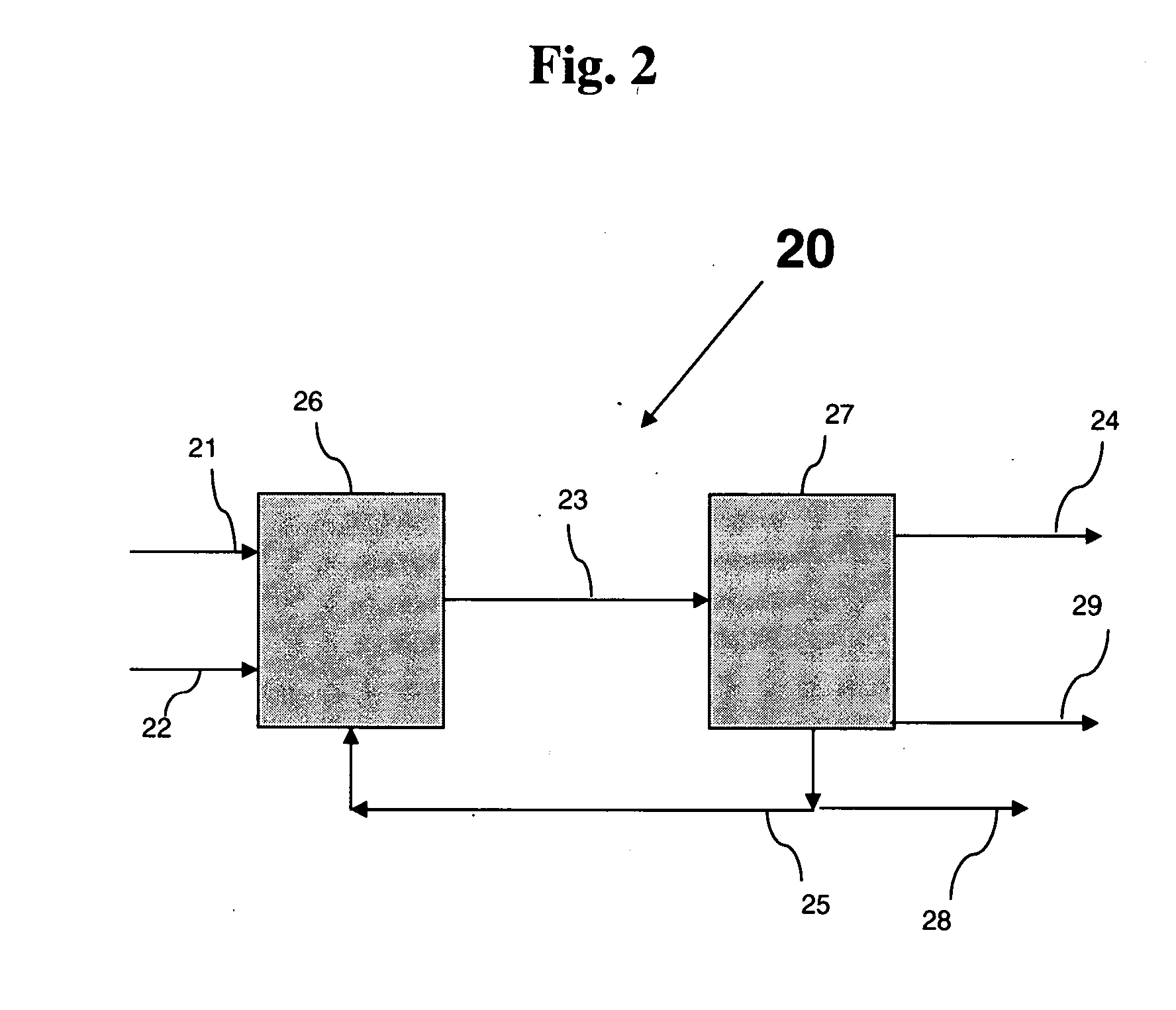
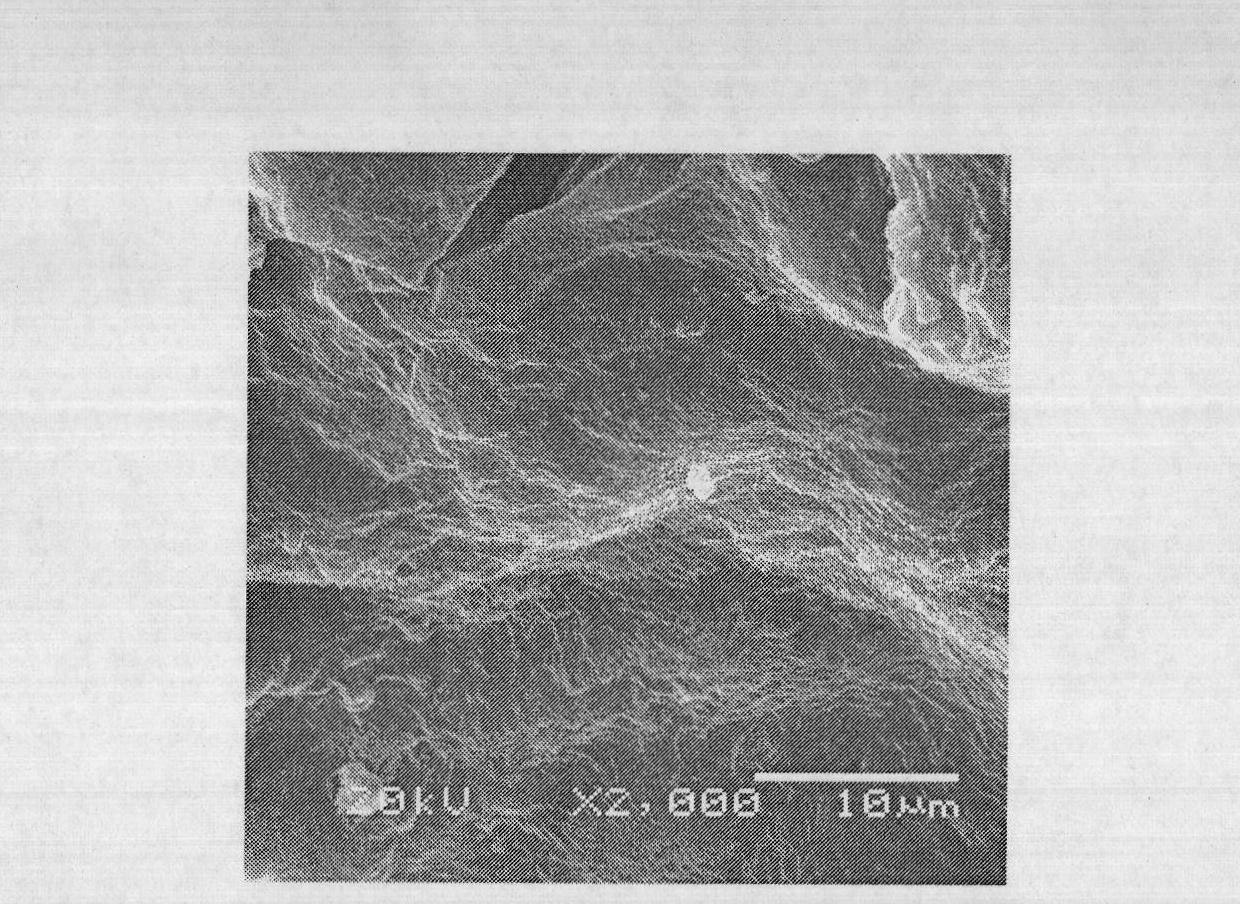
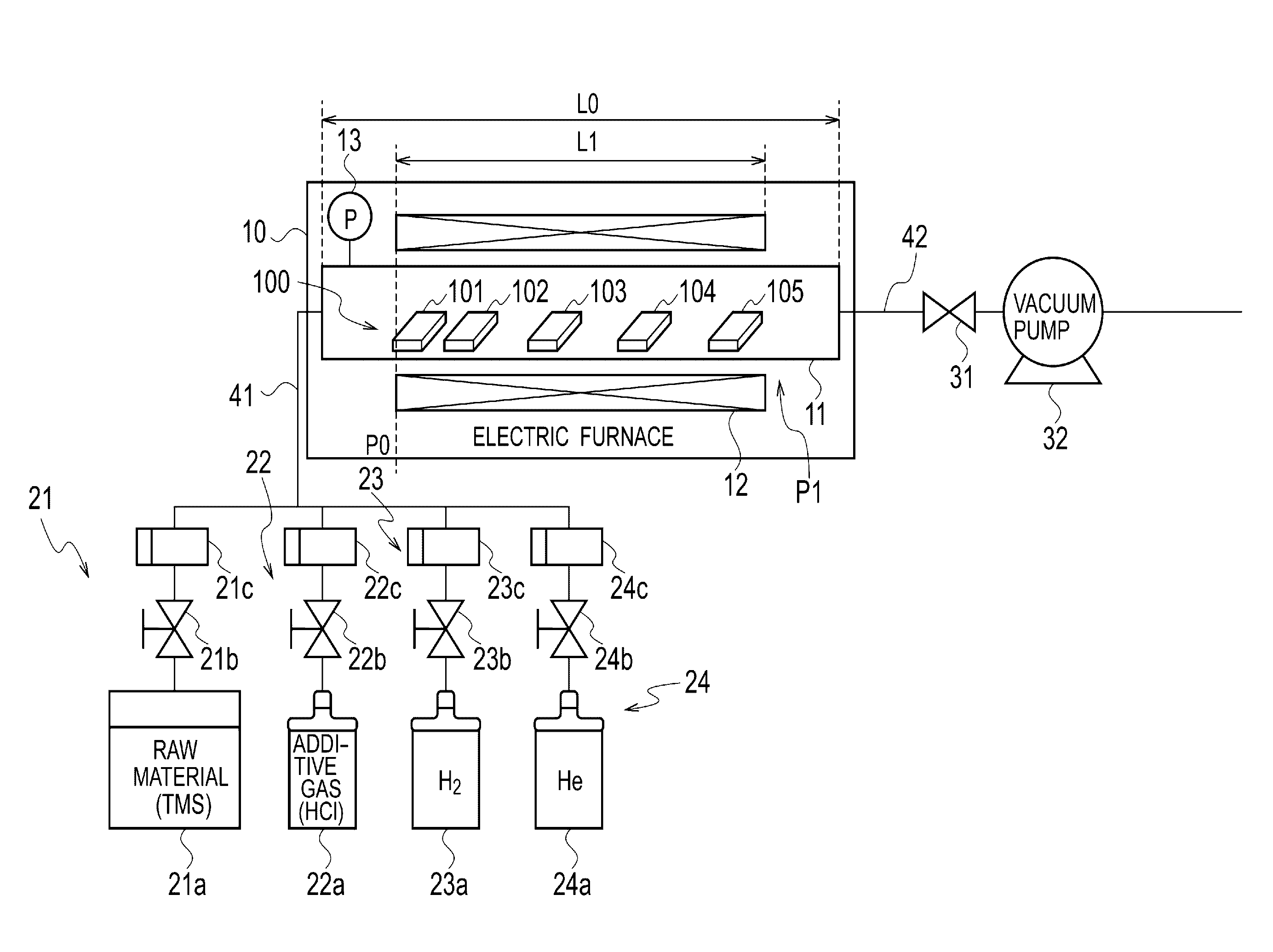
Heat-resistant composite material production method and production device
ActiveUS20160305015A1Reduce generationImprove mass productionSemiconductor/solid-state device manufacturingChemical vapor deposition coatingChlorideChemical vapor deposition
In the present embodiment, in the production of a heat-resistant composite material resulting from impregnating a ceramic fiber preform with silicon carbide, a mixed gas containing starting material gas, an additive gas, and a carrier gas is supplied to a substrate having a minute structure such as a preform stored in an electric furnace, silicon carbide is deposited to form a film by means of a chemical vapor deposition method or a chemical vapor infiltration method, and the film formation growth speed and embedding uniformity are controlled by means of the amount of additive gas added to the starting material gas, the starting material gas contains tetramethylsilane, and the additive gas contains a molecule containing chlorine such as methyl chloride or hydrogen chloride. The film formation growth speed and embedding uniformity of the silicon carbide are both achieved.
Owner:IHI CORP +1
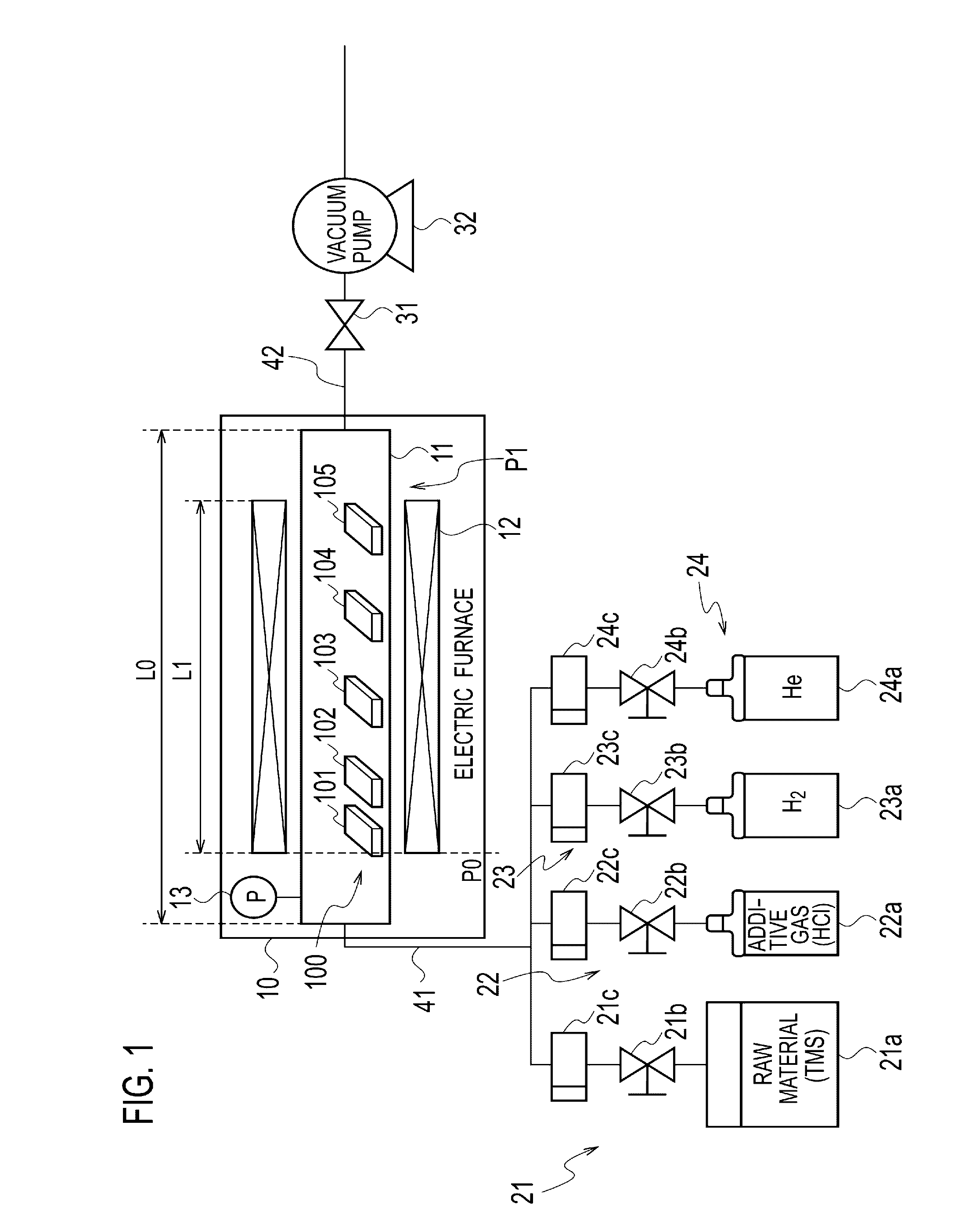
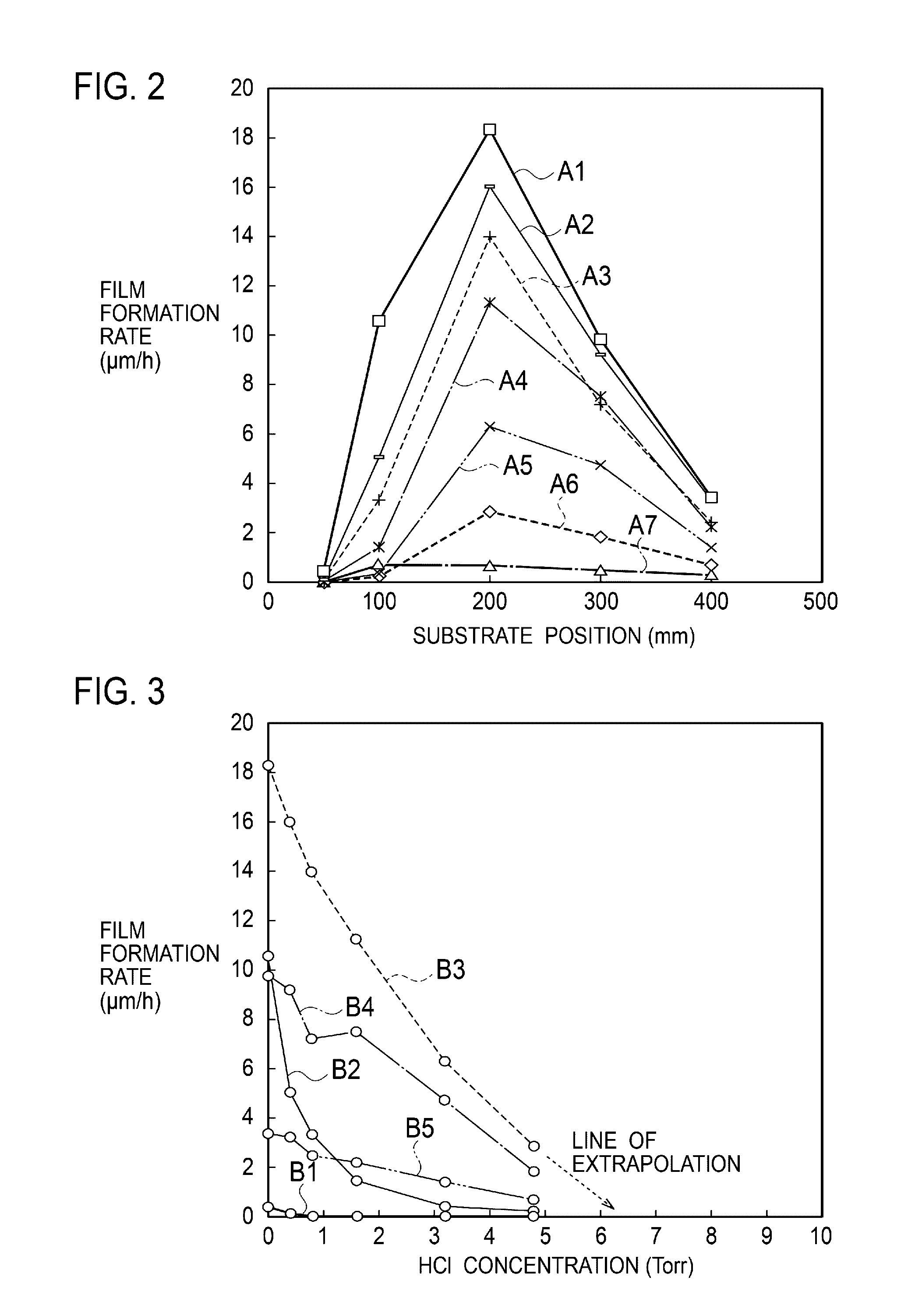
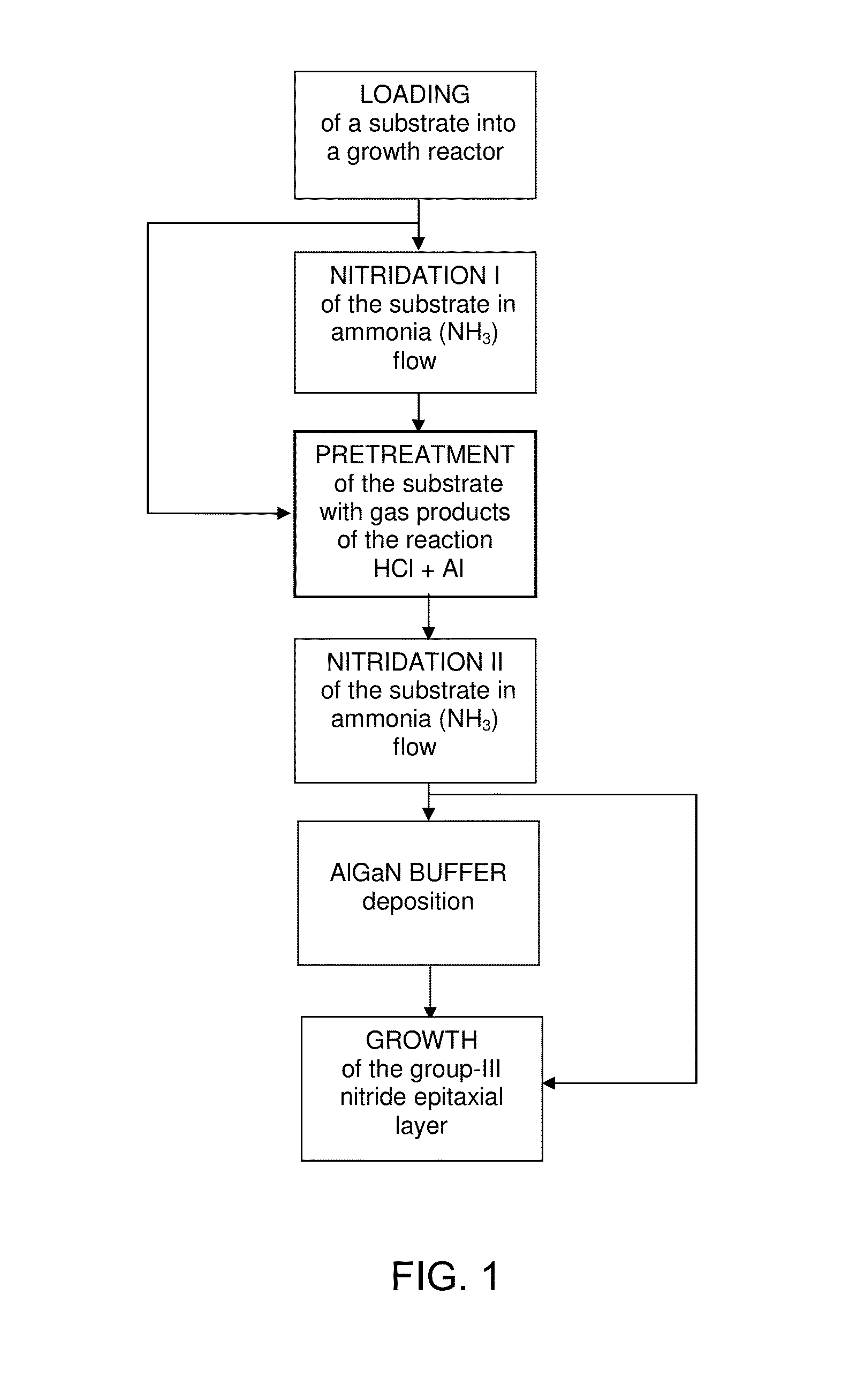
Method for Substrate Pretreatment To Achieve High-Quality III-Nitride Epitaxy
The present invention relates to a method for producing a modified surface of a substrate that stimulates the growth of epitaxial layers of group-III nitride semiconductors with substantially improved structural perfection and surface flatness. The modification is conducted outside or inside a growth reactor by exposing the substrate to a gas-product of the reaction between hydrogen chloride (HCl) and aluminum metal (Al). As a single-step or an essential part of the multi-step pretreatment procedure, the modification gains in coherent coordination between the substrate and group-III nitride epitaxial structure to be deposited. Along with epilayer, total epitaxial structure may include buffer inter-layer to accomplish precise substrate-epilayer coordination. While this modification is a powerful tool to make high-quality group-III nitride epitaxial layers attainable even on foreign substrates having polar, semipolar and nonpolar orientation, it remains gentle enough to keep the surface of the epilayer extremely smooth. Various embodiments are disclosed.
Owner:OSTENDO TECH INC
Integrated process to produce 2,3,3,3-tetrafluoropropene
InactiveCN101597209APreparation by hydrogen halide split-offChemical industryHydrogen chlorideChlorine
A method for preparing 2,3,3,3-tetrafluoroprop-1-ene comprising (a) providing a starting composition comprising at least one compound having a structure selected from Formulae I, II and III: CX 2 =CCl-CH 2 X (Formula I) CX 3 -CCl=CH 2 (Formula II) CX 3 -CHCl-CH 2 X (Formula III) wherein X is independently selected from F, Cl, Br, and I, provided that at least one X is not fluorine; (b) contacting said starting composition with a first fluorinating agent to produce a first intermediate composition comprising 2-chloro-3,3,3-trifluoropropene and a first chlorine-containing byproduct; (c) contacting said first intermediate composition with a second fluorinating agent to produce a second intermediate composition comprising 2-chloro-1,1,1,2-tetrafluoropropane and a second chlorine-containing byproduct; and (d) catalytically dehydrochlorinating at least a portion of said 2-chloro-1,1,1,2-tetrafluoropropane to produce a reaction product comprising 2,3,3,3-tetrafluoroprop-1-ene.
Owner:HONEYWELL INT INC
Process for the manufacture of 1,3,3,3-tetrafluoropropene
ActiveUS20050020862A1Physical/chemical process catalystsPreparation by hydrogen halide split-offPentafluoropropaneChemistry
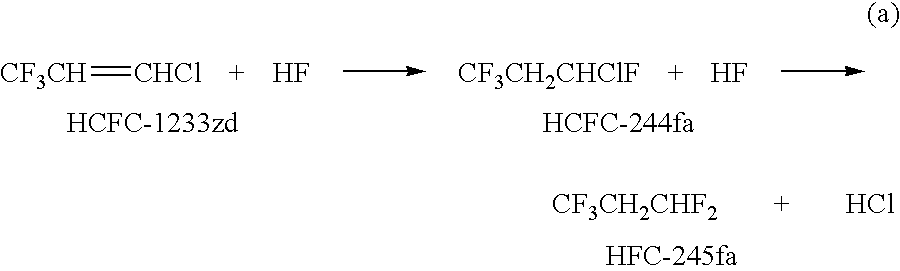
The invention provides an economic process for the manufacture of 1,3,3,3-tetrafluoropropene (HFC-1234ze) by a two stage process. A hydrofluorination of 1-chloro-3,3,3-trifluoropropene (HCFC-1233zd) into 1-chloro-1,3,3,3-tetrafluoropropane (HCFC-244fa) and 1,1,1,3,3-pentafluoropropane (HFC-245fa) is conducted, followed by the dehydrochlorination of HCFC-244fa and dehydrofluorination of HFC-245fa into HFC-1234ze.
Owner:HONEYWELL INT INC
PROCESS TO MANUFACTURE 2-CHLORO-1,1,1,2-TETRAFLUOROPROPANE (HCFC-244bb)
InactiveUS20100036179A1Preparation by dehalogenationPreparation by hydrogen halide split-offHydrogen fluorideHydrogen chloride
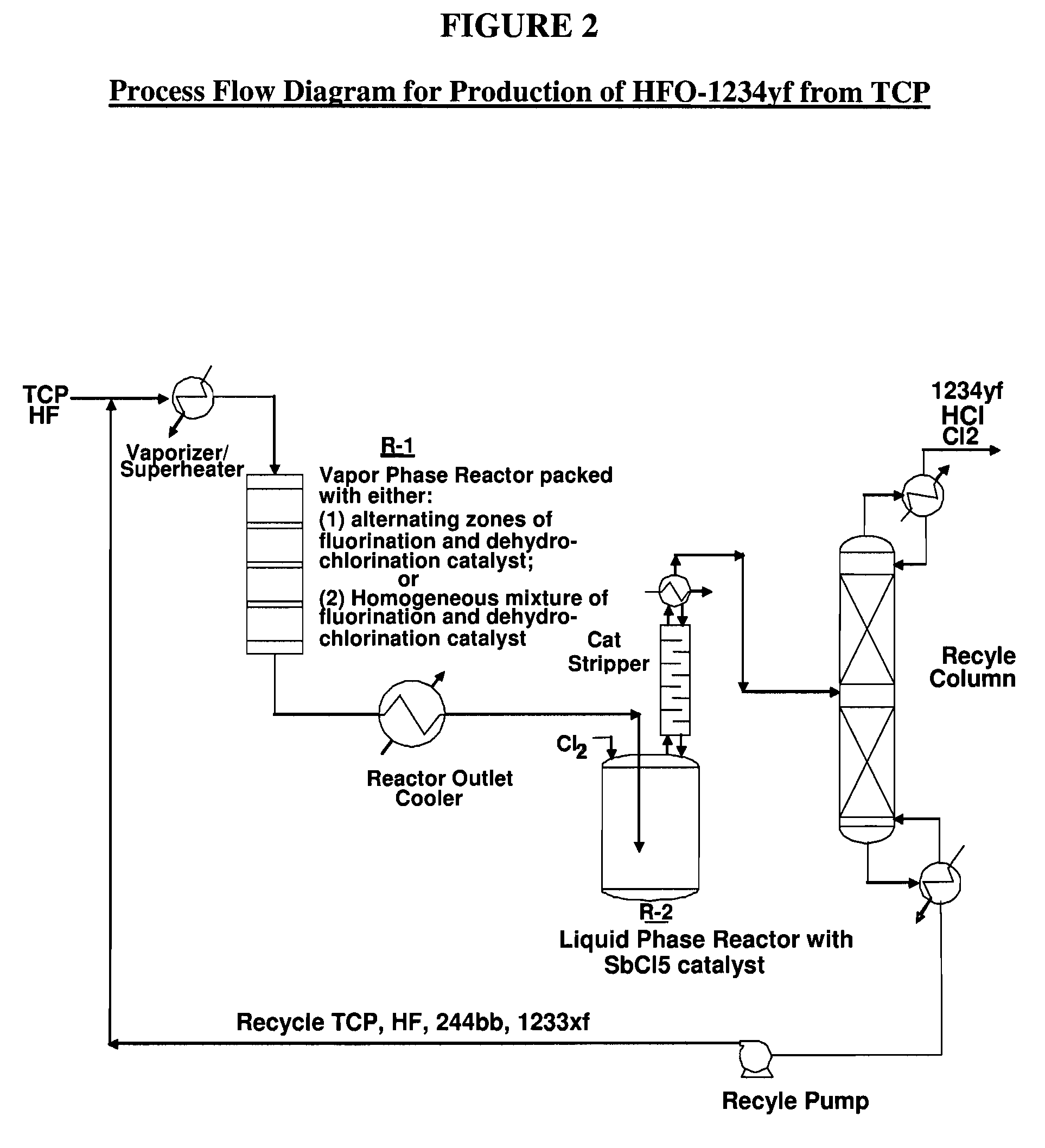
The invention provides an improved process to manufacture 2-chloro-1,1,1,2-tetrafluoropropane (HCFC-244bb) by reacting 2-chloro-3,3,3,-trifluoropropene (HCFO-1233xf) with hydrogen fluoride, in a liquid phase reaction in the presence of hydrogen chloride and a liquid phase fluorination catalyst. The hydrogen chloride is added into the reaction from an external source at a pressure of about 100 psig or more. The HCFC-244bb is an intermediate in the production of 2,3,3,3-tetrafluoropropene-1 (HFO-1234yf).
Owner:HONEYWELL INT INC
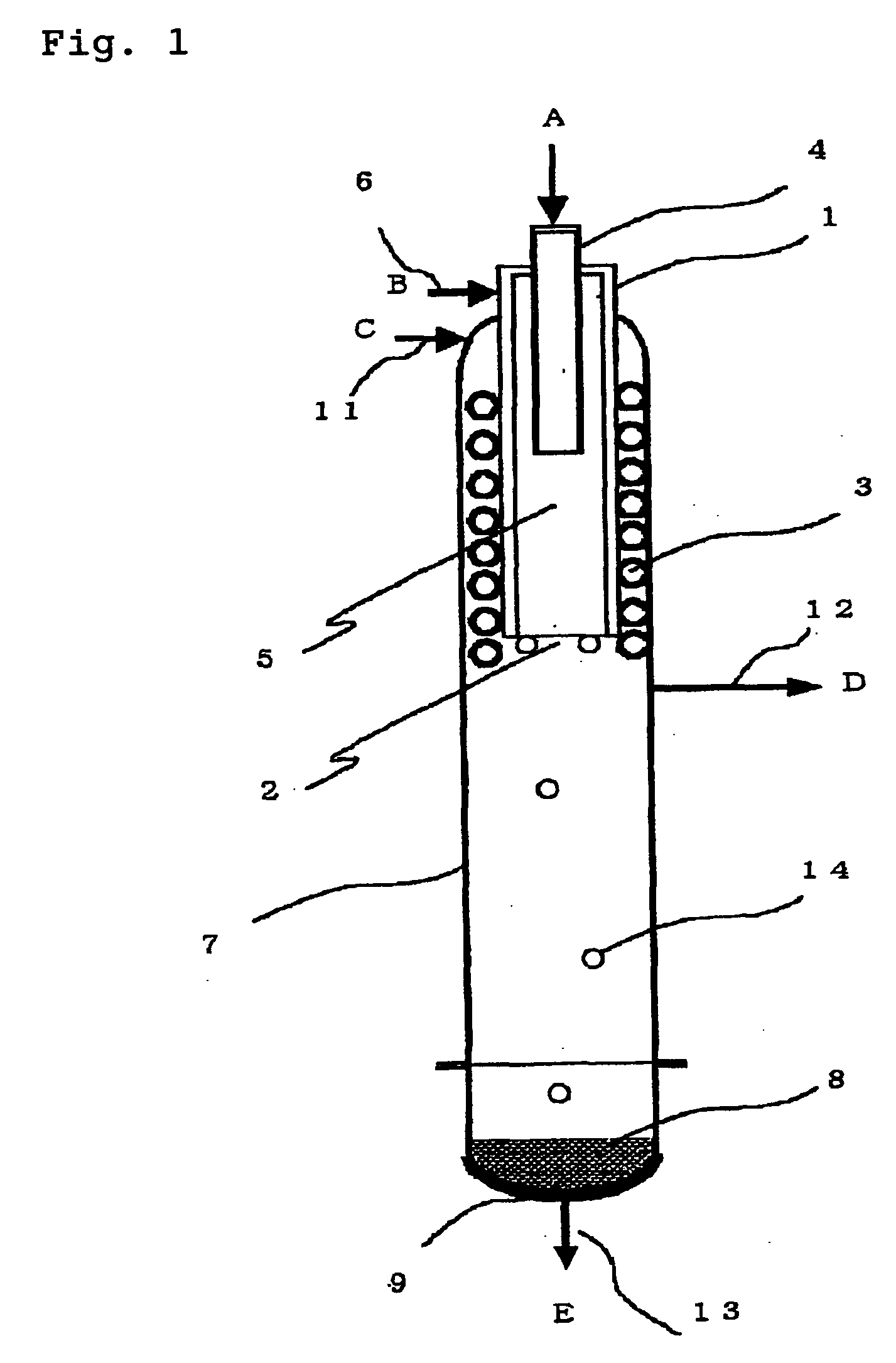
Flue gas control system of coal combustion boiler and operating method thereof
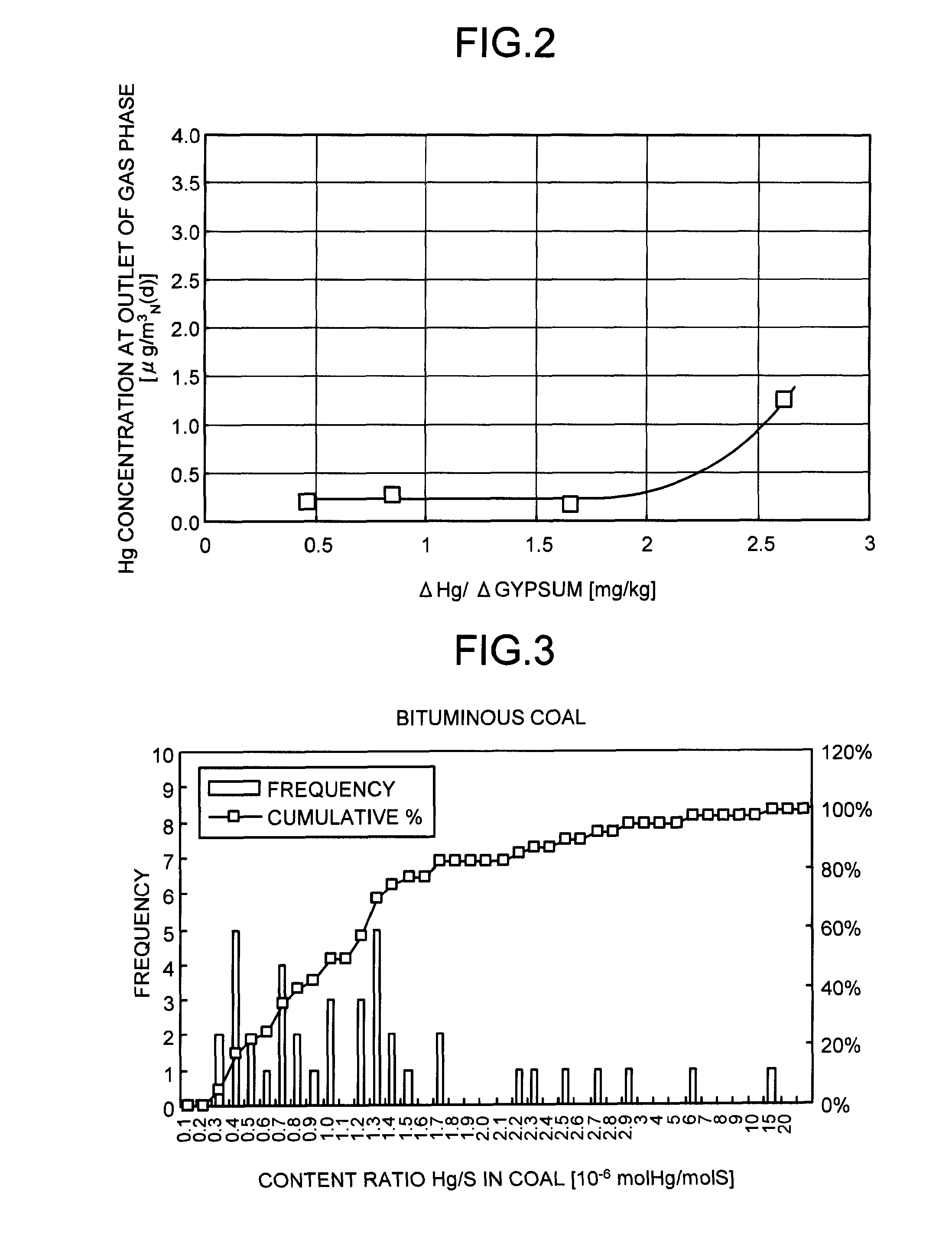
ActiveUS8071060B2Reduce operating costsCombination devicesNitrogen compoundsAir preheaterParticulates
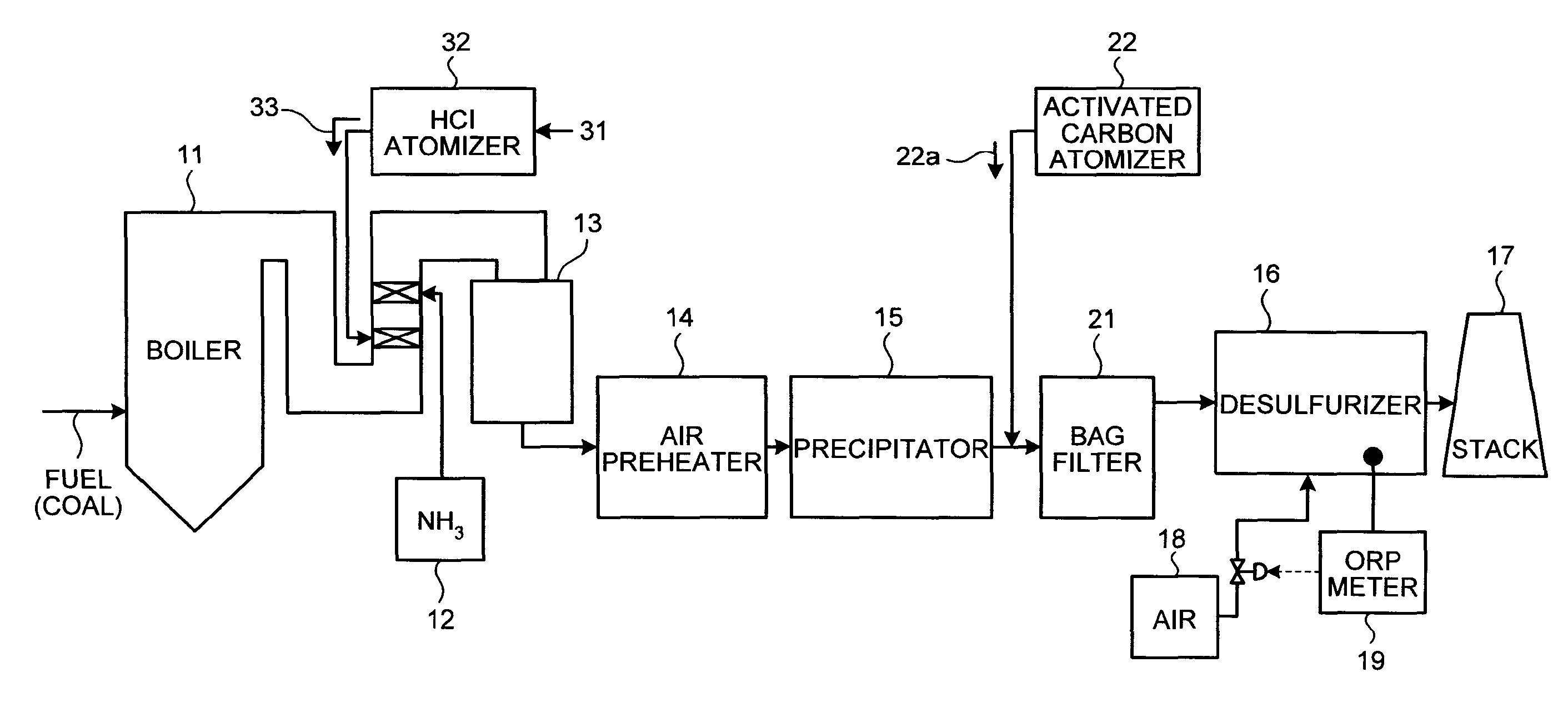
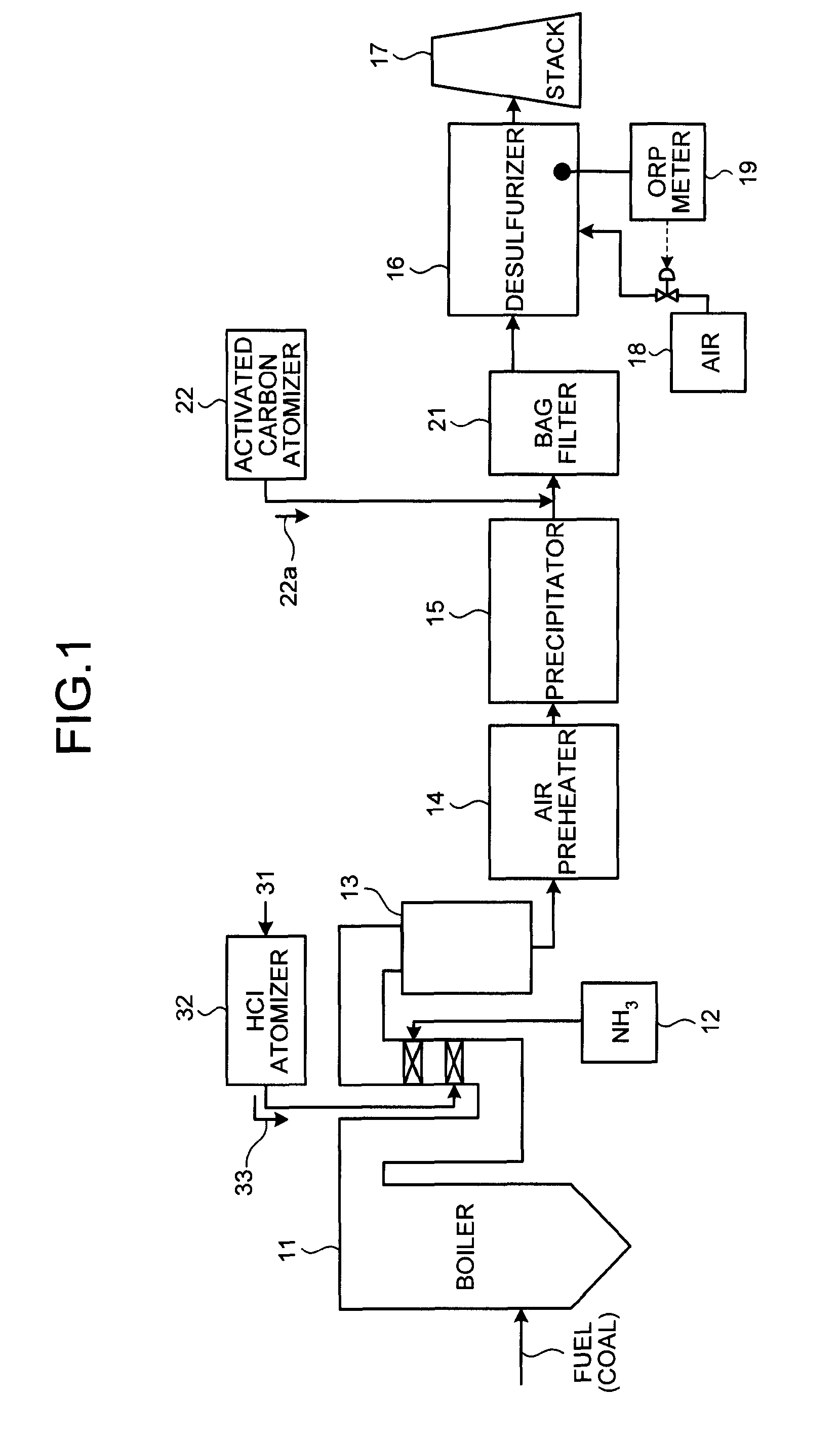
A flue gas control system of a coal combustion boiler comprises an HCl atomizer that sprays hydrogen chloride to flue gas from a coal combustion boiler that uses coal as a fuel; NOx removing apparatus that removes nitrogen oxides by ammonia denitration by adding ammonia to the flue gas after spraying hydrogen chloride and oxidizes mercury; an air preheater that recovers heat in the gas after removal of nitrogen oxides; a precipitator that removes particulates in the gas; an activated carbon atomizer that sprays activated carbon into the gas after particulate collection; a bag filter that collects activated carbon having adsorbed mercury; a desulfurizer that removes sulfur oxides in the flue gas after removal of activated carbon; a stack that discharges the gas which has undergone desulfurization to outside; and an ORP meter that measures an oxidation reduction potential for feeding air to a slurry absorbent in the desulfurizer.
Owner:MITSUBISHI HEAVY IND LTD
Method and apparatus for producting negative and positive oxidative reductive potential (orp) water
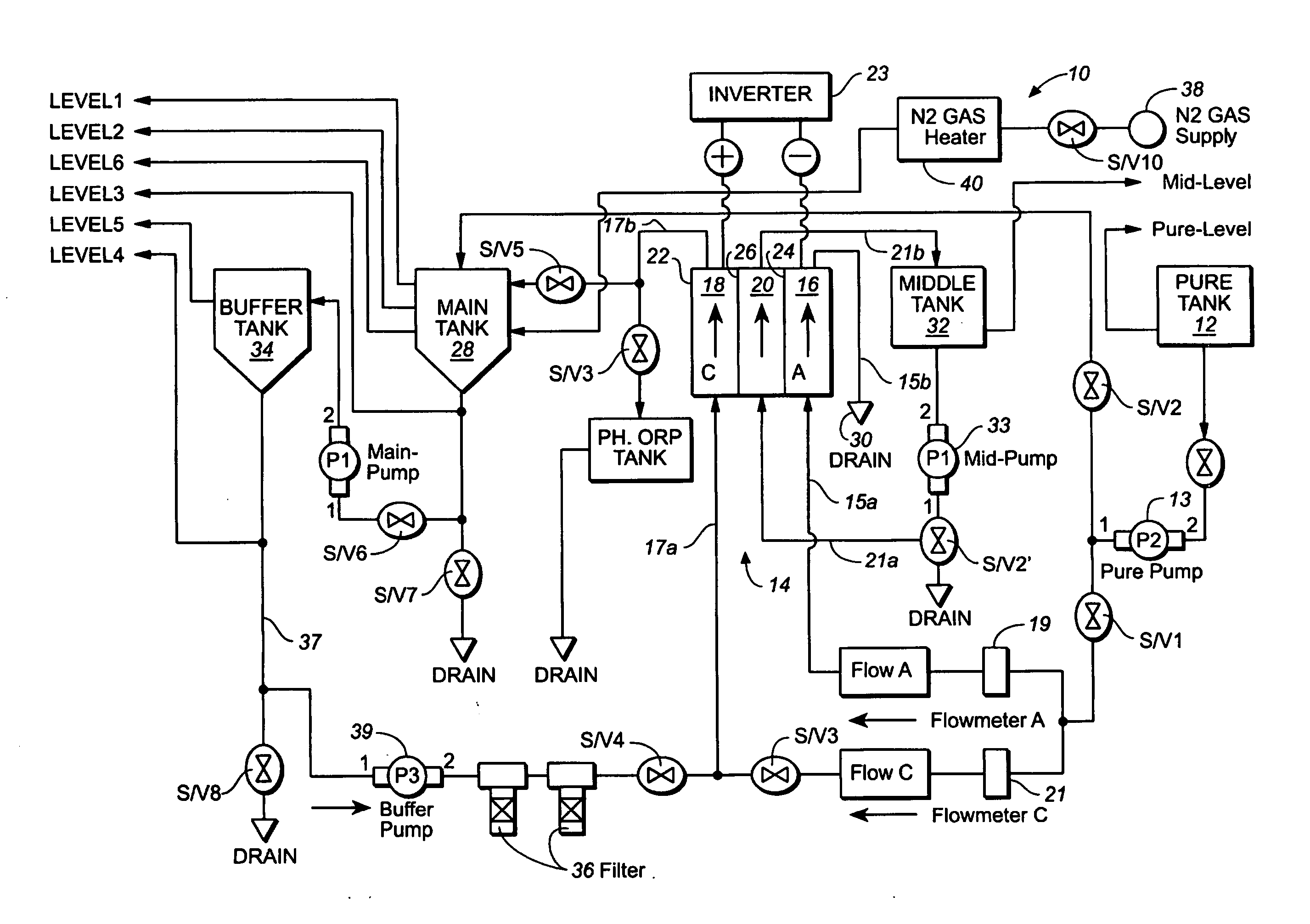
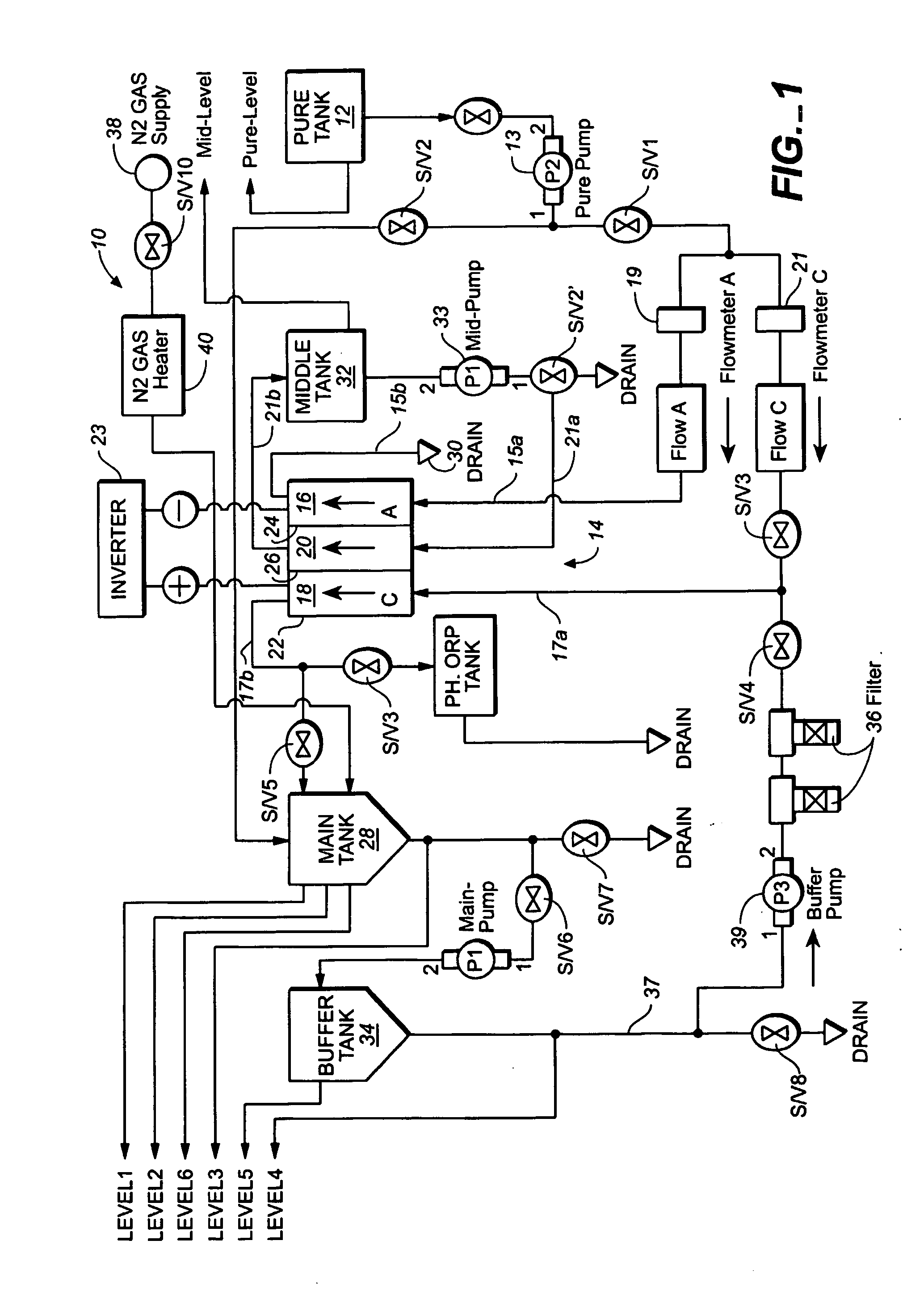
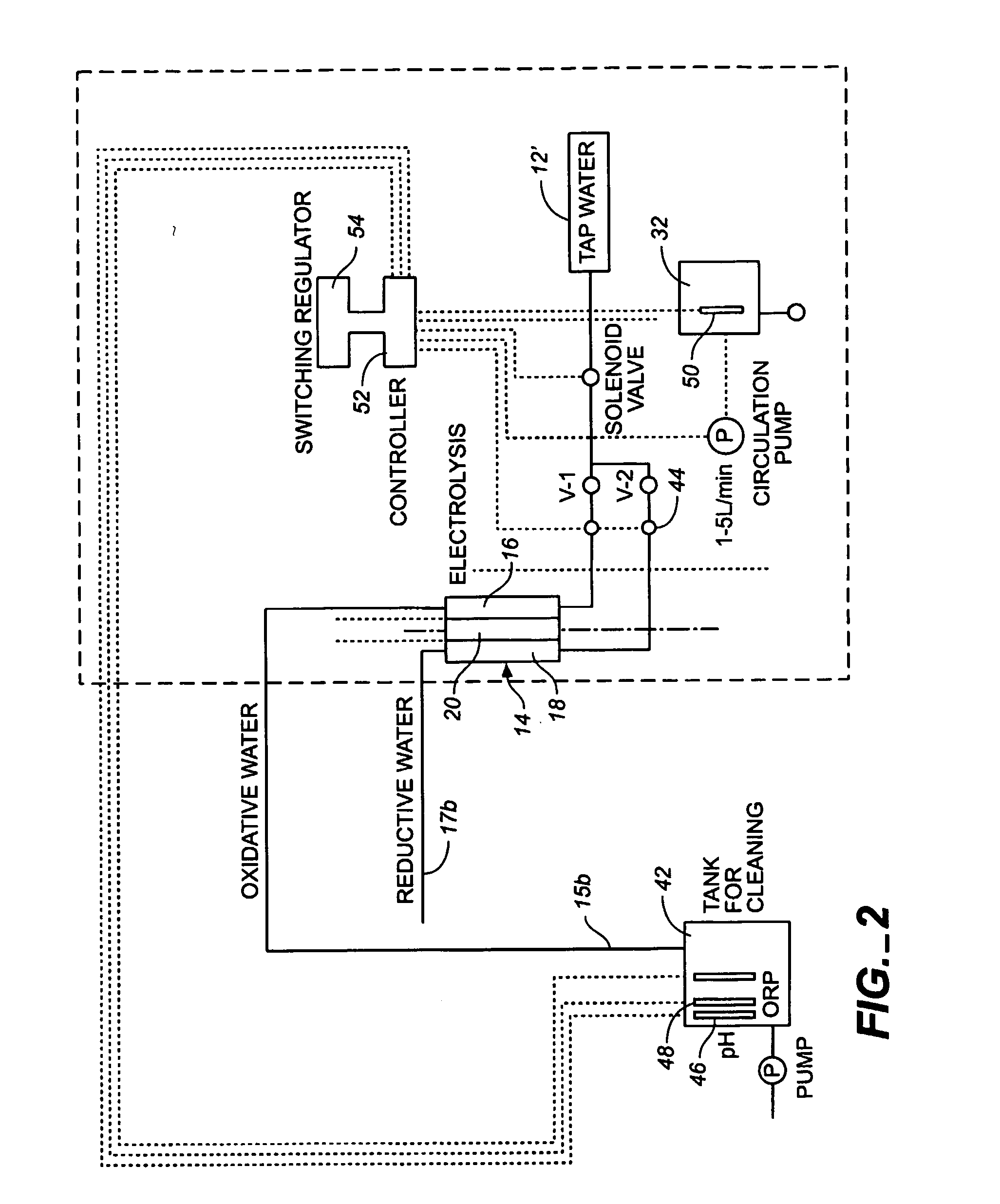
ActiveUS20050121334A1Effective and efficient and economicalCellsWater treatment parameter controlParticulatesElectrolysis
A method and apparatus for electrolytically producing oxidation reduction potential water from aqueous salt solutions for use in disinfection, sterilization, decontamination, wound cleansing. The apparatus includes an electrolysis unit having a three-compartment cell (22) comprising a cathode chamber (18), an anode chamber (16), and a saline solution chamber (20) interposed between the anode and cathod chambers. Two communicating (24, 26) membranes separate the three chambers. The center chamber includes a fluid flow inlet (21a) and outlet (21b) and contains insulative material that ensures direct voltage potential does not travel through the chamber. A supply of water flows through the cathode and anode chambers at the respective sides of the saline chamber. Saline solution flows through the center chamber, either by circulating a pre-prepared aqueous solution containing ionic species, or, alternatively, by circulating pure water or an aqueous solution of, e.g., aqueous hydrogen chloride and ammonium hydroxide, over particulate insulative material coated with a solid electrolyte. Electrical current is provided to the communicating membranes separating the chambers, thus causing an electrolytic reaction that produces both oxidative (positive) and reductive (negative) ORP water.
Owner:SONOMA PHARMA INC
Novel wood plastic composite decorative building material and manufacturing method thereof
InactiveCN101955614AImproved performance characteristicsGood environmental characteristicsAntioxidantPolyvinyl chloride
The invention relates to a novel wood plastic composite decorative building material and a manufacturing method thereof. The novel wood plastic composite decorative building material comprises the following components in part by weight: 100 parts of polyvinyl chloride (PVC) resin powder, 30 parts of mixture formed by mixing one or more of wood powder, bamboo powder, rice bran powder and crop straw powder in an arbitrary ratio, 30 parts of calcium powder, 5 to 10 parts of chlorinated polyethylene or MBS resin, 2 to 6 parts of rear earth compound stabilizer, 0.5 to 3 parts of polyethylene wax or oxidized polyethlene wax, 0.5 to 3 parts of stearic acid, 0.5 to 2.0 parts of foaming agent, 5 to 10 parts of blowing promoter, 0.5 to 1 part of antioxidant, 0.5 to 1 part of reinforcing agent, 1 to 3 parts of toner and 0.5 to 1 part of weather resistant agent. PVC plastic is modified by adding the auxiliaries, and the influence of unstable components (such as a vinyl chloride monomer, hydrogen chloride gas and the like) in the PVC on the performance of the building material is eliminated, so that the building material has better performance and environmental protection characteristics on the basis of keeping the original performance.
Owner:CHENGDU AOKINGTH TECH
Preparation of fluorinated olefins via catalytic dehydrohalogenation of halogenated hydrocarbons
ActiveUS20090043136A1Physical/chemical process catalystsPreparation by hydrogen halide split-offHalohydrocarbonHydrogen atom
A process for making a fluorinated olefin having the step of dehydrochlorinating a hydrochlorofluorocarbon having at least one hydrogen atom and at least one chlorine atom on adjacent carbon atoms, preferably carried out in the presence of a catalyst selected from the group consisting of (i) one or more metal halides, (ii) one or more halogenated metal oxides, (iii) one or more zero-valent metals / metal alloys, (iv) a combination of two or more of the foregoing.
Owner:HONEYWELL INT INC
Methods for removal of water from gases using superheated zeolites
InactiveUS6110258AHigh silica-to-alumina ratioReduce metal contentGas treatmentMolecular sieve catalystsBoiling pointSilicon dioxide
A method for removing trace moisture from a gas is disclosed. The method involves heating a zeolite having a high silica-to-alumina ratio to about 400 DEG C. to remove physically absorbed water from the zeolite, followed by heating the zeolite to a temperature in excess of 650 DEG C., to form a superheated zeolite. Heating to temperatures of 650 DEG C. or above is believed to cause dehydroxylation of the zeolite. A method for the preparation of a dehydroxylated zeolite is also disclosed. The superheated zeolite is contacted with the gas, thereby adsorbing water from the gas. A dehydroxylated zeolite for removing trace moisture from a gas wherein the zeolite has a high silica-to-alumina ratio and a low level of metallic impurities is also disclosed. The zeolite and methods of the invention are particularly useful for removing trace water from acid gases such as hydrogen chloride and hydrogen bromide.
Owner:MATHESON TRI GAS INC
Catalyst system of chloroethylene prepared by hydrochlorinating acetylene and preparation method and application thereof
ActiveCN101716528AEasy to prepareImprove conversion ratePreparation by halogen halide additionOrganic-compounds/hydrides/coordination-complexes catalystsPt elementIonic liquid
The invention discloses a catalyst system of chloroethylene prepared by hydrochlorinating acetylene and a preparation method and application thereof. The catalyst system comprises a catalyst carrier and a catalyst, wherein the catalyst carrier is imidazole ion liquid and the catalyst is any combination of one or more of gold, platinum, palladium, tin, mercury, copper or rhodium chlorides. The preparation method of the catalyst system is to dissolve the catalyst into the catalyst carrier. Acetylene and chlorine hydride are mixed and react in the presence of the catalyst system. The preparation process realizes a liquid phase reaction for preparing chloroethylene by hydrochlorinating acetylene, avoids the loss of the catalyst system, is more environment-friendly and safer, and improves the conversion rate of the acetylene and the selectivity of the chloroethylene.
Owner:于志勇
Manufacturing method for 1,1,1,3,3-pentafluoropropane
InactiveUS6060628AImprove economyImprove efficiencyPreparation by dehalogenationOrganic chemistry methodsHydrogenHydrogen chloride
PCT No. PCT / JP96 / 00273 Sec. 371 Date Aug. 26, 1997 Sec. 102(e) Date Aug. 26, 1997 PCT Filed Feb. 8, 1996 PCT Pub. No. WO96 / 26914 PCT Pub. Date Jun. 9, 1996A manufacturing method for 1,1,1,3,3-pentafluoropropane in which the method is composed of: step A wherein 2,3-dichloro-1,1,1,3,3-pentafluoropropane is reduced with hydrogen under the presence of hydrogenation catalyst in gaseous phase; step B wherein all of the products of the said step A are introduced into a cooler condenser, so that either a component of hydrogen and hydrogen chloride as non-condensation component and another compoment of 1,1,1,3,3-pentafluoropropane as condensation components or a component of hydrogen as non-condensation component and another component of hydrogen chloride and 1,1,1,3,3-pentafluoropropane as condensation component are obtained; step C wherein hydrogen is separated from the non-condensation component of the said step B, and it is recycled to the said step A; and step D wherein 1,1,1,3,3-pentafluoropropane is separated from the condensation component of the said step B. The producing method based on a manufacturing process of 1,1,1,3,3-pentafluoropropane can be provided with good efficiency and economy in industrial scales.
Owner:DAIKIN IND LTD
Method forming epitaxial silicon structure
InactiveUS20080286957A1Semiconductor/solid-state device detailsSemiconductor/solid-state device manufacturingHydrogen chlorideSilicon
A method of forming an epitaxial silicon structure is disclosed. The method includes performing a first epitaxial growth process using a first source gas including silicon (Si) and hydrogen chloride (HCl) to form a first epitaxial silicon layer on a substrate, and performing a second epitaxial growth process using a second source gas including silicon (Si) and chlorine (Cl) to form a second epitaxial silicon layer on the first epitaxial silicon layer.
Owner:SAMSUNG ELECTRONICS CO LTD
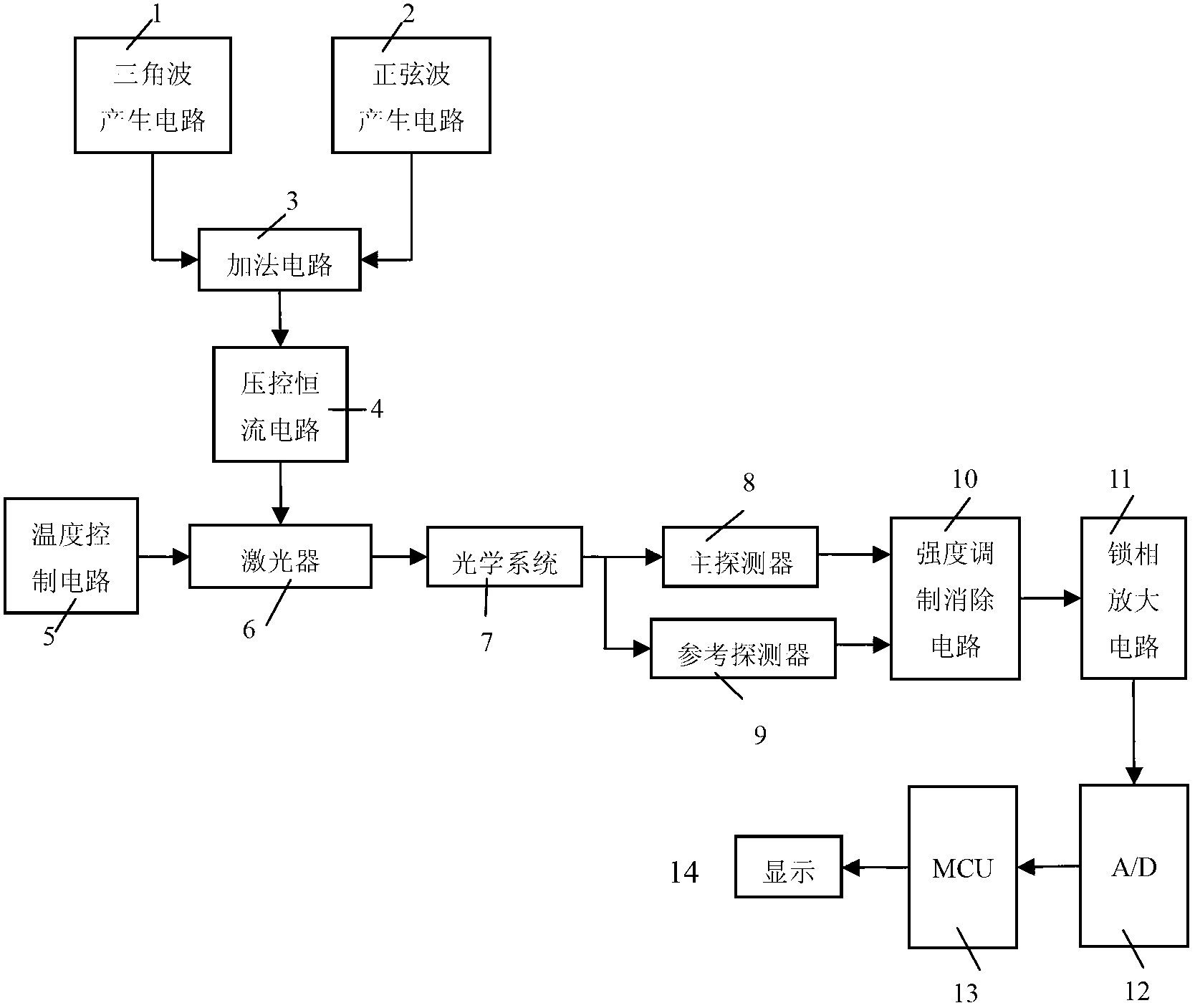
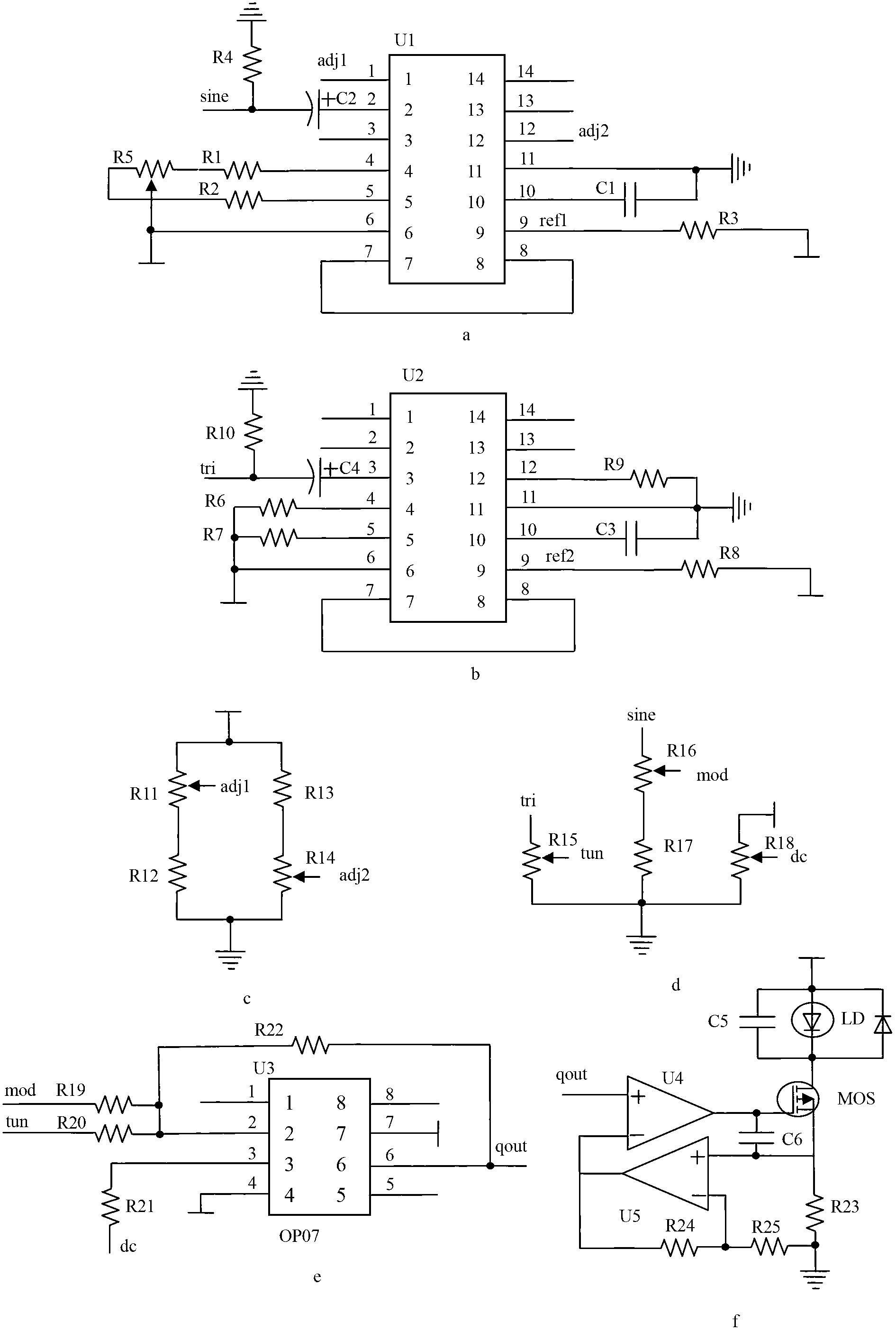
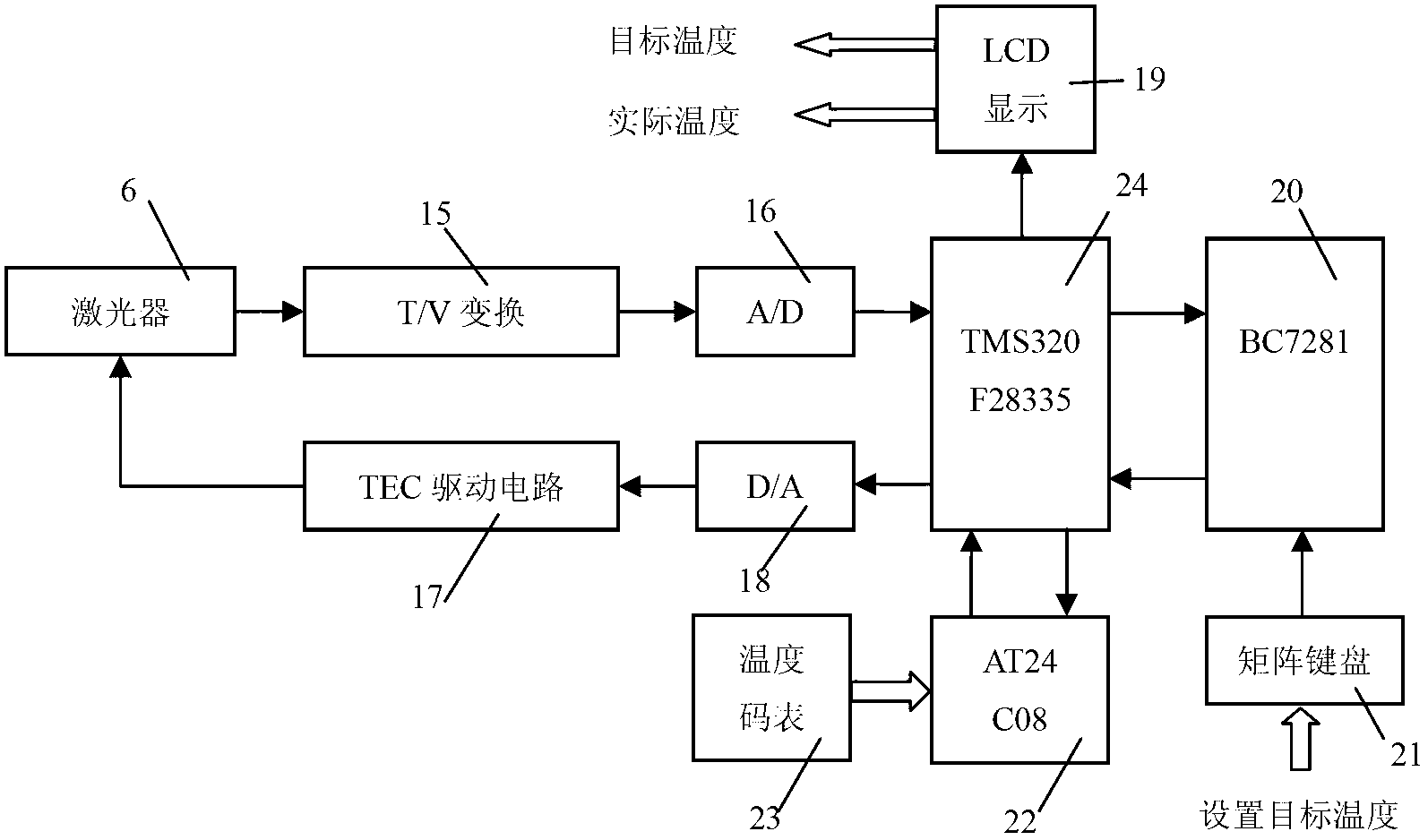
Laser infrared gas analyzer based on TDLAS-WMS (tunable diode laser absorption spectroscopy-wavelength modulation spectroscopy)
ActiveCN102706832AAdjustable temperatureAvoid damageColor/spectral properties measurementsWater vaporData acquisition
The invention belongs to the technical field of gas detection and relates to a laser infrared gas analyzer based on TDLAS-WMS (tunable diode laser absorption spectroscopy-wavelength modulation spectroscopy) for detecting hydrogen chloride, methane, carbon monoxide, water vapor and other gases. The laser infrared gas analyzer comprises a laser, a laser driving circuit, a temperature control circuit, an optical system with an optical cavity, a main detector, a reference detector, an intensity modulation and canceling circuit, a phase-locking and amplification circuit and a data acquisition and display circuit, wherein the laser driving circuit and the temperature control circuit are used for controlling the laser to emit light, the two ends of the optical system are respectively connected with the laser and the detector, the intensity modulation and canceling circuit is used for canceling the influence of intensity modulation in the system, the phase-locking and amplification circuit is used for extracting harmonic signals, and the data acquisition and display circuit is used for displaying the concentration of the gas to be detected. Compared with other detection instruments, the laser infrared gas analyzer has the advantages that division operation is introduced into the intensity modulation and canceling circuit, and the laser infrared gas analyzer is combined with a space double-optical path differential detection method, so that the influence of the intensity modulation can be fundamentally canceled.
Owner:JILIN UNIV
Methods for removal of impurity metals from gases using low metal zeolites
InactiveUS6395070B1High silica-to-alumina ratioReduce metal contentGas treatmentMolecular sieve catalystsBoiling pointTrace metal
A method for removing trace moisture from a gas is disclosed. The method involves heating a zeolite having a high silica-to-alumina ratio to about 400° C. to remove physically adsorbed water from the zeolite, followed by heating the zeolite to a temperature in excess of 650° C., to form a superheated zeolite. The superheated zeolite is contacted with the gas, thereby adsorbing water from the gas. A dehydroxylated zeolite for removing trace moisture from a gas wherein the zeolite has a high silica-to-alumina ratio and a low level of metallic impurities is also disclosed. A method for removing metallic impurities from a gas using the low metals zeolite is also disclosed. The zeolites and methods of the invention are particularly useful for removing trace water and trace metal impurities from acid gases such as hydrogen chloride and hydrogen bromide.
Owner:MATHESON TRI GAS INC
Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process
The present invention relates to a new industrial process for the synthesis of solvate- free 17a-acetoxy-11ss-[4-(N,N-dimethyl-amino)-phenyl]-19-norpregna-4,9-diene-3,20-dione [CDB -2914] of formula (I) which is a strong antiprogestogene and antiglucocorticoid agent. The invention also relates to compounds of formula (VII) and (VIII) used as intermediates in the process. The process according to the invention is the following: i) 3-(ethylene-dioxy)-estra-5(10),9(11)-diene-17-one of formula (X) is reacted with potassium acetilyde formed in situ in dry tetrahydrofuran by known method, ii) the obtained 3-(ethylene-dioxy)-17a-ethynyl-17ss-hydroxy-estra-5(10),9(11)-diene of formula (IX) is reacted with phenylsulfenyl chloride in dichloromethane in the presence of triethylamine and acetic acid, iii) the obtained isomeric mixture of 3-(ethylene-dioxy)-21-(phenyl-sulfinyl)-19-norpregna-5(10),9(11),17(20),20-tetraene of formula (VIII) is reacted first with sodium methoxide in methanol, then with trimethyl phosphite, iv) the obtained 3-(ethylene-dioxy)-17a-hydroxy-20-methoxy-19-norpregna-5(10),9(11),20-triene of formula (VII) is reacted with hydrogen chloride in methanol, then v) the obtained 3-(ethylene-dioxy)-17a-hydroxy-19-norpregna-5(10),9(11l); -diene-20- one of formula (VI) is reacted with ethylene glycol hi dichloromethane in the presence of trimethyl orthoformate and p-toluenesulfonic acid by known method, vi) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-19-norpregna- 5(10),9(11)-diene of formula (V) is reacted with hydrogen peroxide in a mixture of pyridine and dichloromethane in the presence of hexachloroacetone by known method, vii) the obtained 3,3,20,20-bis(ethylene-dioxy)-17a-hydroxy-5,10-epoxy-19-norpregn-9(11)-ene of formula (IV), containing approximately a 1:1 mixture of 5a,10a- and 5ss,10ss-epoxides, is isolated from the solution and reacted with a Grignard reagent obtained from 4-bromo-N,N-dimethyl-aniline in tetrahydrofuran.
Owner:RICHTER GEDEON NYRT
Method for producing fluorinated organic compounds
ActiveUS20090287026A1Economical processReduce equipmentPreparation by hydrogen halide split-offPreparation by halogen halide additionChemistryHydrogen fluoride
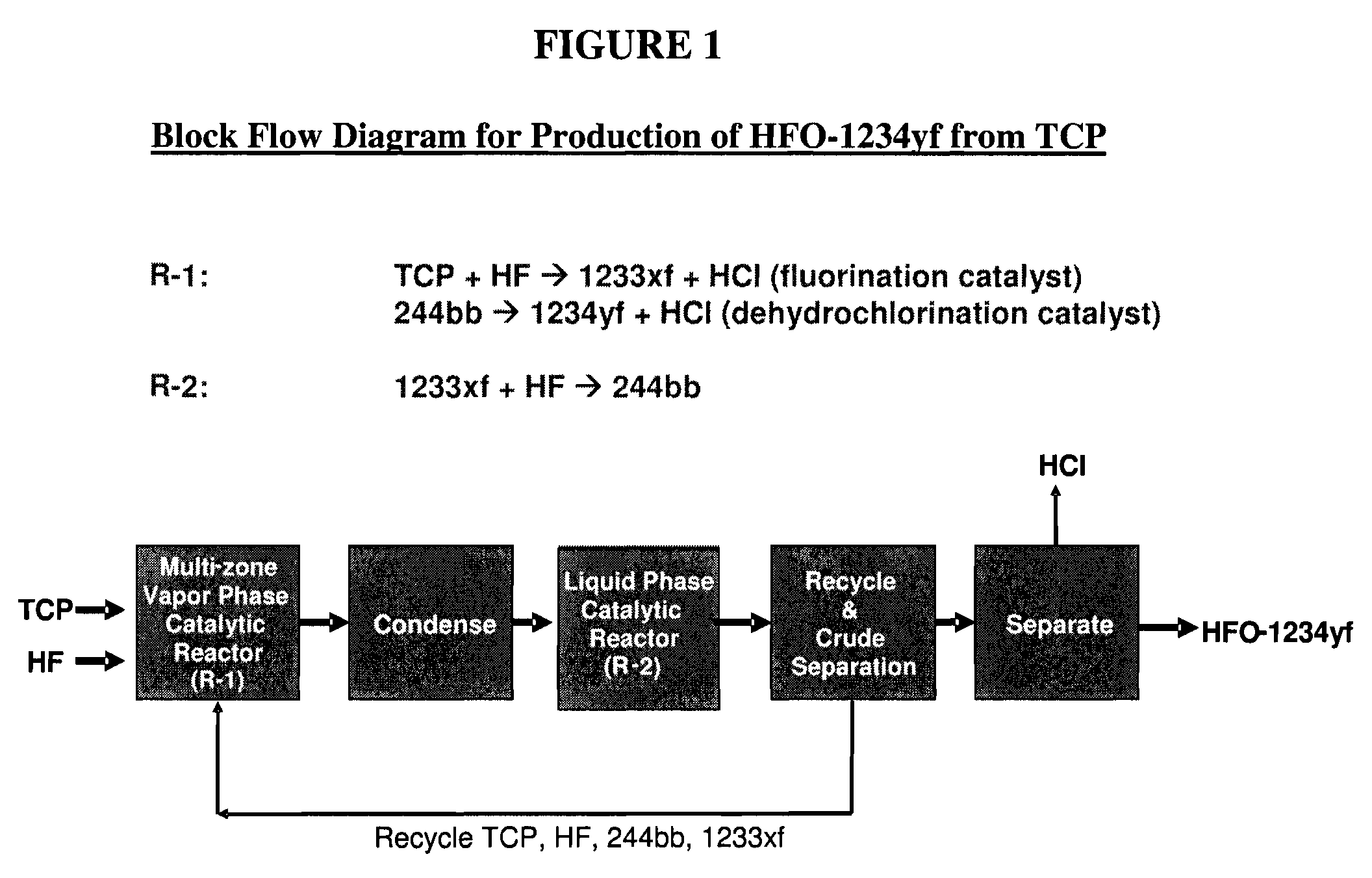
Disclosed is a process for producing tetrafluoropropene comprising: (a) catalytically fluorinating at least one tetrafluoropropene in a first reactor to produce HCFO-1233xf; (b) reacting said HCFO-1233xf with hydrogen fluoride in a second reactor to produce HCFC-244bb; (c) recycling at least a portion of said HCFC-244bb back to said first reactor as recycled HCFC-244bb; and (d) catalytically dehydrochlorinating said recycled HCFC-244bb in said first reactor to produce HFO-1234yf.
Owner:HONEYWELL INT INC
Porous silica granule, method for producing the same, and method for producing synthetic quartz glass powder using the porous silica granule
InactiveUS6849242B1Minimizes pore diameterHigh bulk densitySilicaGlass blowing apparatusMean diameterSlurry
The granule consists of individual granules approximately spherical in shape, having a pore volume of 0.5 cm3, a mean diameter of pores of 50 nm or less, a specific surface area of 100 m2 / g or less, and a bulk density of 0.7 g / cm3 or higher. It is produced by dispersing a fumed silica obtained by hydrolysis of a silicon compound into pure water to obtain a slurry, and drying the slurry. The granule is used for producing high purity synthetic quartz glass powder. The method further comprises: a first heat treatment under an oxygen-containing atmosphere, a second heat treatment in a temperature range of from 600 to 1100° C., and a third heat treatment in a temperature range of from 1100 to 1300° C. under an atmosphere containing hydrogen chloride; and a step of densification comprising calcining the product at a temperature not higher than 1500° C. under vacuum or in an atmosphere of gaseous hydrogen or gaseous helium. To calcine the powder without causing fusion adhesion of the particles, bubbling fluidization of said porous silica granule is conducted by supplying gaseous helium and calcining thereof in a temperature range of from 1000 to 1600° C.
Owner:HERAEUS QUARZGLAS +1
Aryl silicate ester flame retardant plasticizer and preparation method thereof
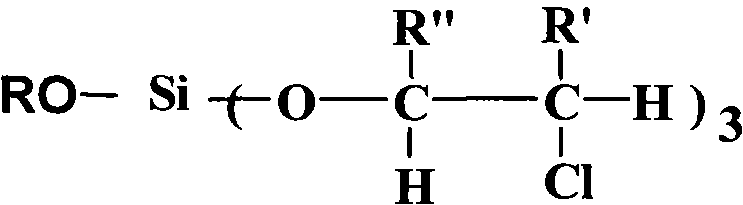
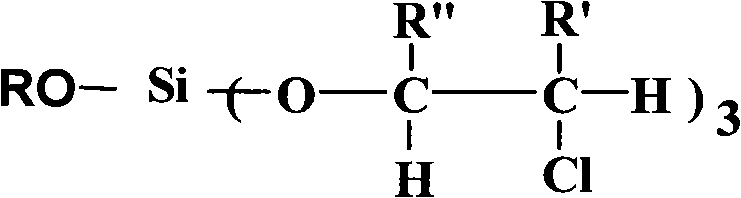
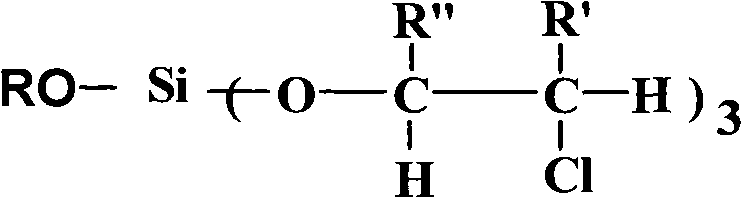
InactiveCN102050961AHydrolytic activity decreasedImprove stabilitySilicon organic compoundsArylHydrogen
The invention relates to an aryl silicate ester flame retardant plasticizer and a preparation method thereof. The structure of the compound is expressed with a general formula shown in the specification, wherein R is phenyl, tolyl and benzyl, R' is hydrogen and methyl, and R'' is hydrogen and methyl or chloromethyl. The preparation method comprises the following steps: using nitrogen to protect, and reacting silicon tetrachloride with a hydroxyl compound with an aryl having the same molar ratio as that of the silicon tetrachloride to release hydrogen chloride, and then with alkylene oxide with a certain molar ratio to obtain a target product. The target product also can be obtained through changing the feeding sequence. The compound has the advantages of stable physical and chemical properties, high flame retardant performance, good plasticity, simple process and easiness of large-scale production.
Owner:李兰敏
Method for producing silicon
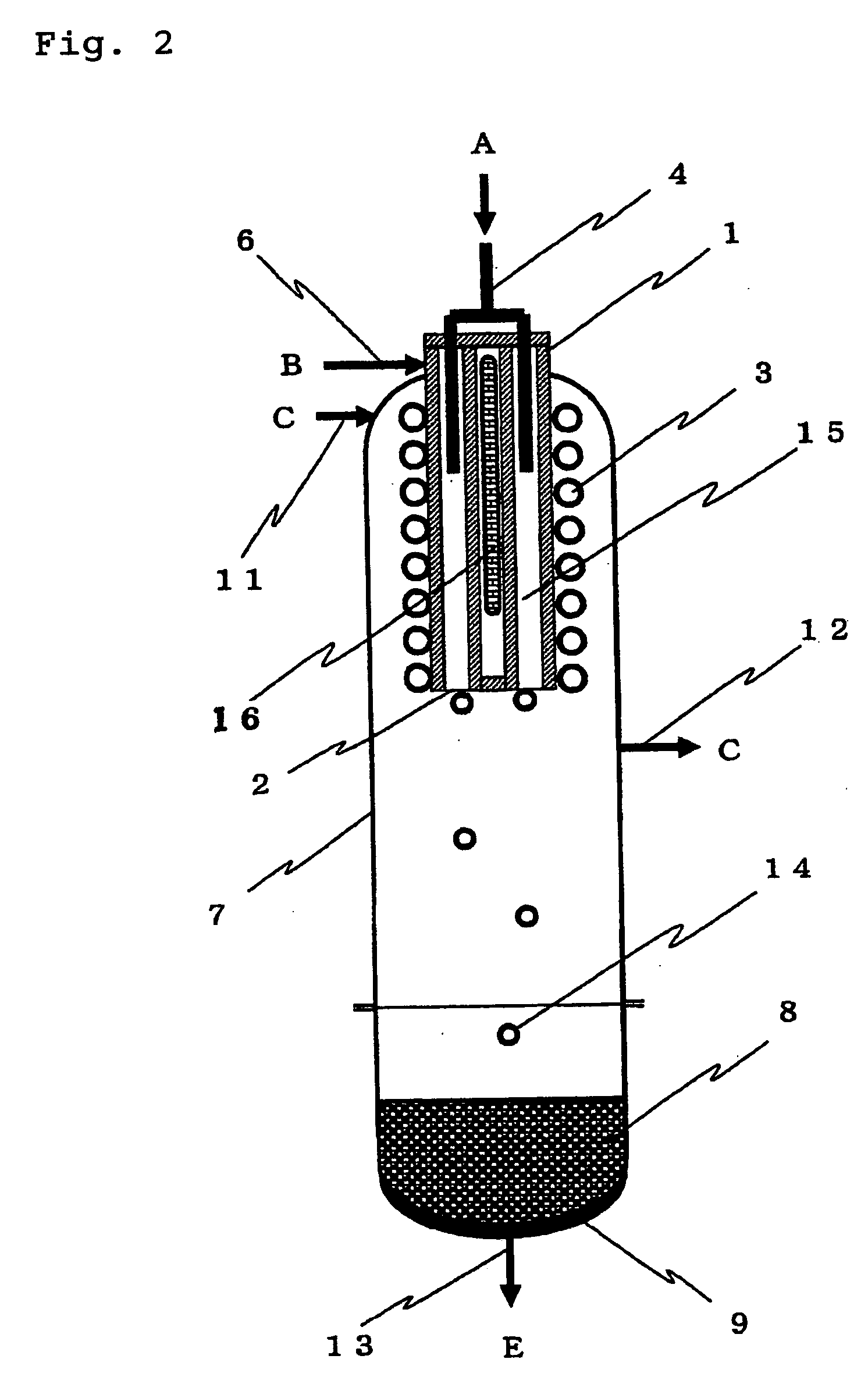
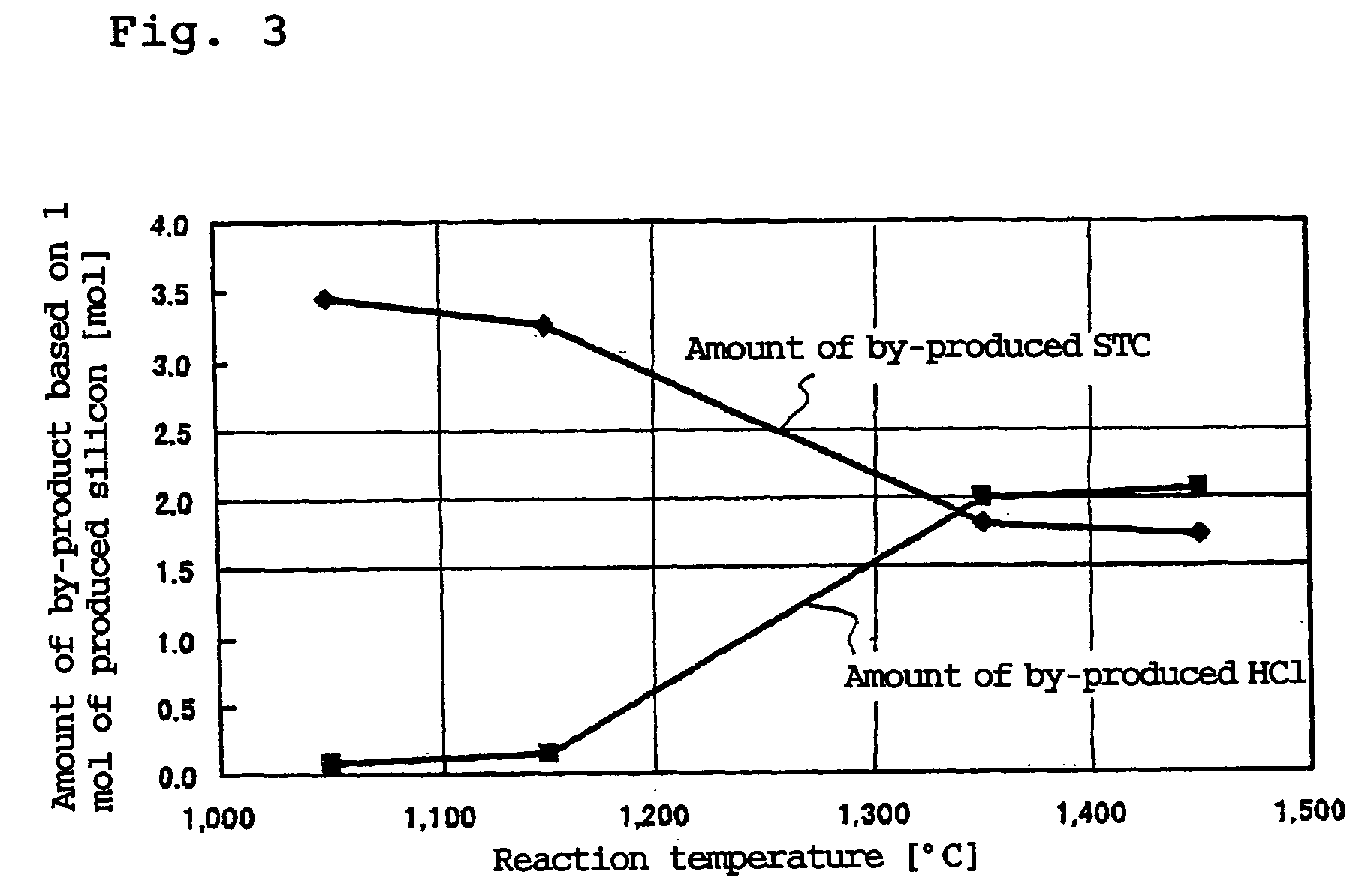
A silicon production process which improves the production efficiency of trichlorosilane while an industrially advantageous output is ensured and the amount of the by-produced tetrachlorosilane is suppressed. This process does not require a bulky reduction apparatus for the by-produced tetrachlorosilane, can construct a closed system, which is a self-supporting silicon production process, can easily control the amount of the by-produced tetrachlorosilane and therefore can adjust the amount of tetrachlorosilane to be supplied to a tetrachlorosilane treating system when the tetrachlorosilane treating system is used. This process comprises a silicon deposition step for forming silicon by reacting trichlorosilane with hydrogen at a temperature of 1,300° C. or higher, a trichlorosilane forming step for forming trichlorosilane by contacting the exhausted gas in the above silicon deposition step to raw material silicon to react hydrogen chloride contained in the exhausted gas with silicon, and a trichlorosilane first recycling step for separating trichlorosilane from the exhausted gas in the trichlorosilane forming step and recycling it to the silicon deposition step.
Owner:TOKUYAMA CORP
Process for manufacturing methyl chloride
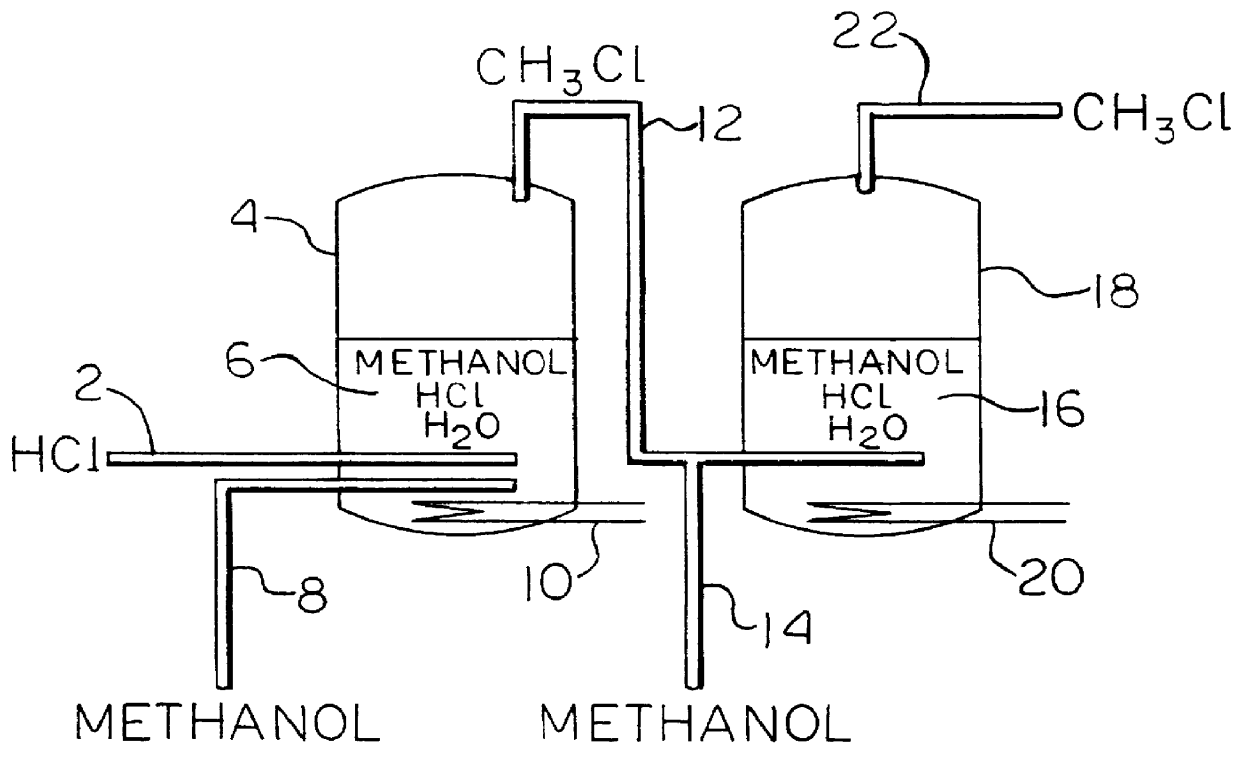
A process for manufacturing methyl chloride. The process consists essentially of contacting hydrogen chloride with at least a stoichiometric amount of methanol split into at least two portions with a first portion comprising about 60 to 95 percent of the methanol added to the process in the presence of a first liquid medium at a temperature in the range of about 115 DEG C. to 170 DEG C. to form a gaseous mixture containing methyl chloride, and contacting the gaseous mixture with a second methanol portion and adding to a second liquid medium at a temperature in the range of about 100 DEG C. to 160 DEG C., and recovering the methyl chloride.
Owner:DOW SILICONES CORP +1
Process for the conversion of a crude glycerol, crude mixtures of naturally derived multicomponent aliphatic hydrocarbons or esters thereof to a chlorohydrin
ActiveUS20080015370A1Minimize formationHigh selectivityOrganic compound preparationCarboxylic acid esters preparationSufficient timeAliphatic hydrocarbon
A process for converting a crude glycerol, crude mixtures of naturally derived multihydroxylated-aliphatic hydrocarbons or esters thereof to a chlorohydrin, by contacting the crude glycerol, crude mixtures of naturally derived multihydroxylated-aliphatic hydrocarbons or esters thereof starting material with a source of a superatmospheric partial pressure of hydrogen chloride for a sufficient time and at a sufficient temperature, and wherein such contracting step is carried out without substantial removal of water, to produce the desired chlorohydrin product; wherein the desired product or products can be made in high yield without substantial formation of undesired overchlorinated byproducts; wherein said crude glycerol, said ester of crude glycerol, or mixture thereof is derived from a renewable raw material. Chlorohydrins made by the process of the present invention are useful in preparing epoxides such as epichlorohydrins.
Owner:BLUE CUBE IP
Method of producing chlorobenzyl by photochlorination
ActiveCN1948245AIncrease profitPhotochlorination reduces power consumptionHalogenated hydrocarbon preparationSide chainHydrogen chloride
The present invention relates to a method for producing chlorobenzyl by utilizing photochlorination process. Said method includes the following steps: using LED as light source, making chlorine gas be contacted with the material from the bottom portion of the chlorination equipment by means of distributor, under the reaction temperature of 90 deg.C-150 deg.C making chlorine gas and aromatic compound produce side-chain chlorination reaction, firstly forming monochlorobenzyl, continuously introducing chlorine gas to obtain dichlorobenzyl, finally forming trichlorobenzyl, namely the invented product.
Owner:ZHEJIANG WEIHUA NEW MATERIAL CO LTD
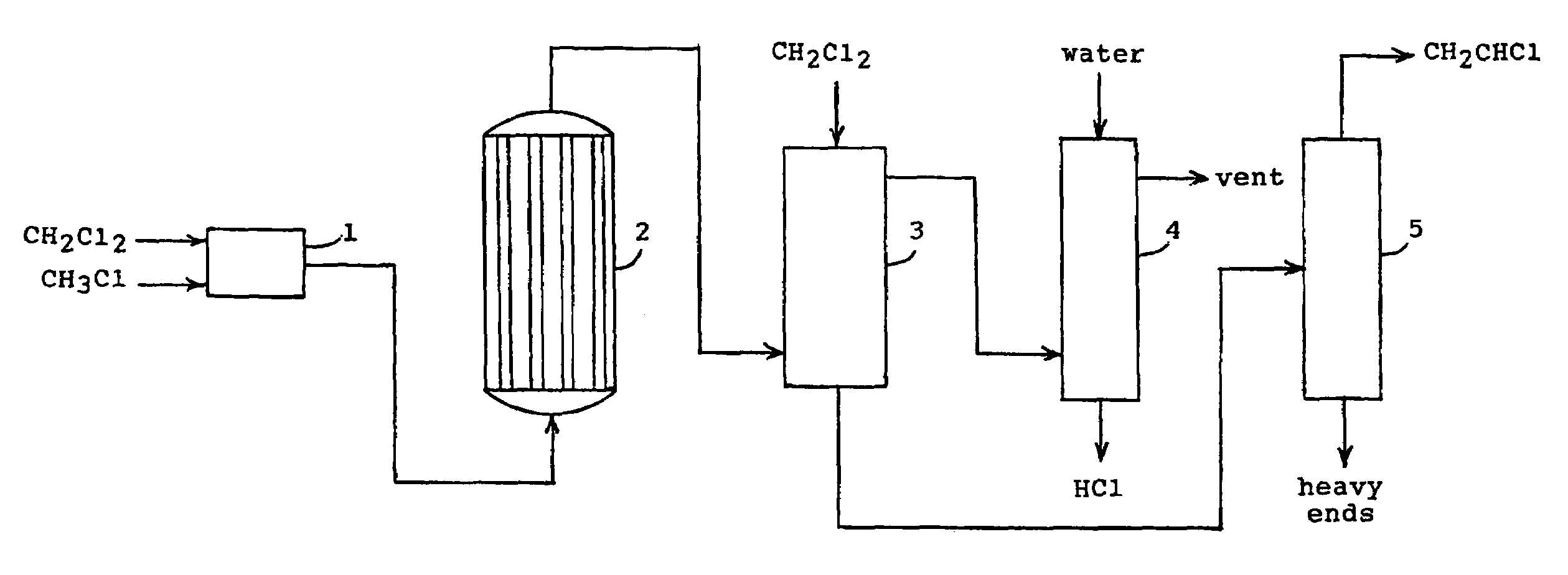
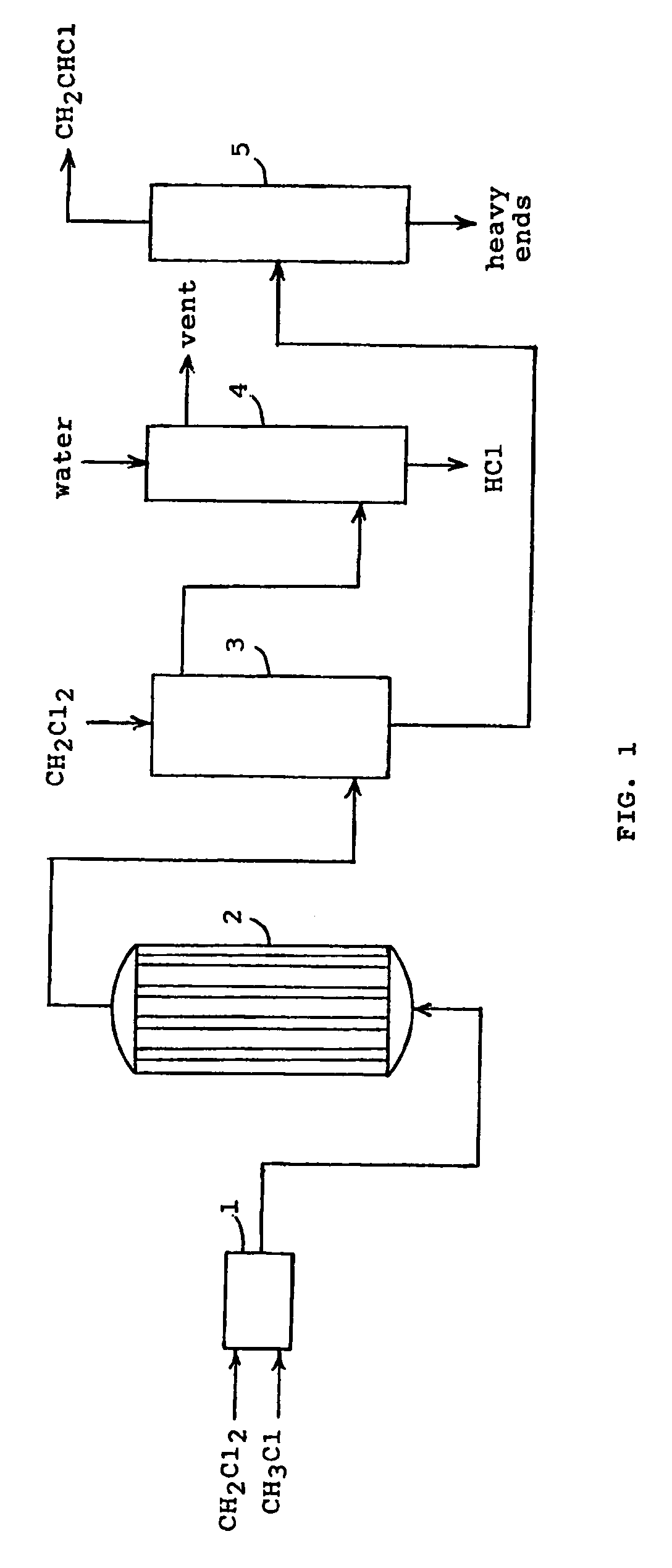
Method for producing vinyl chloride monomer
Owner:STAUFFER VALERIE +1
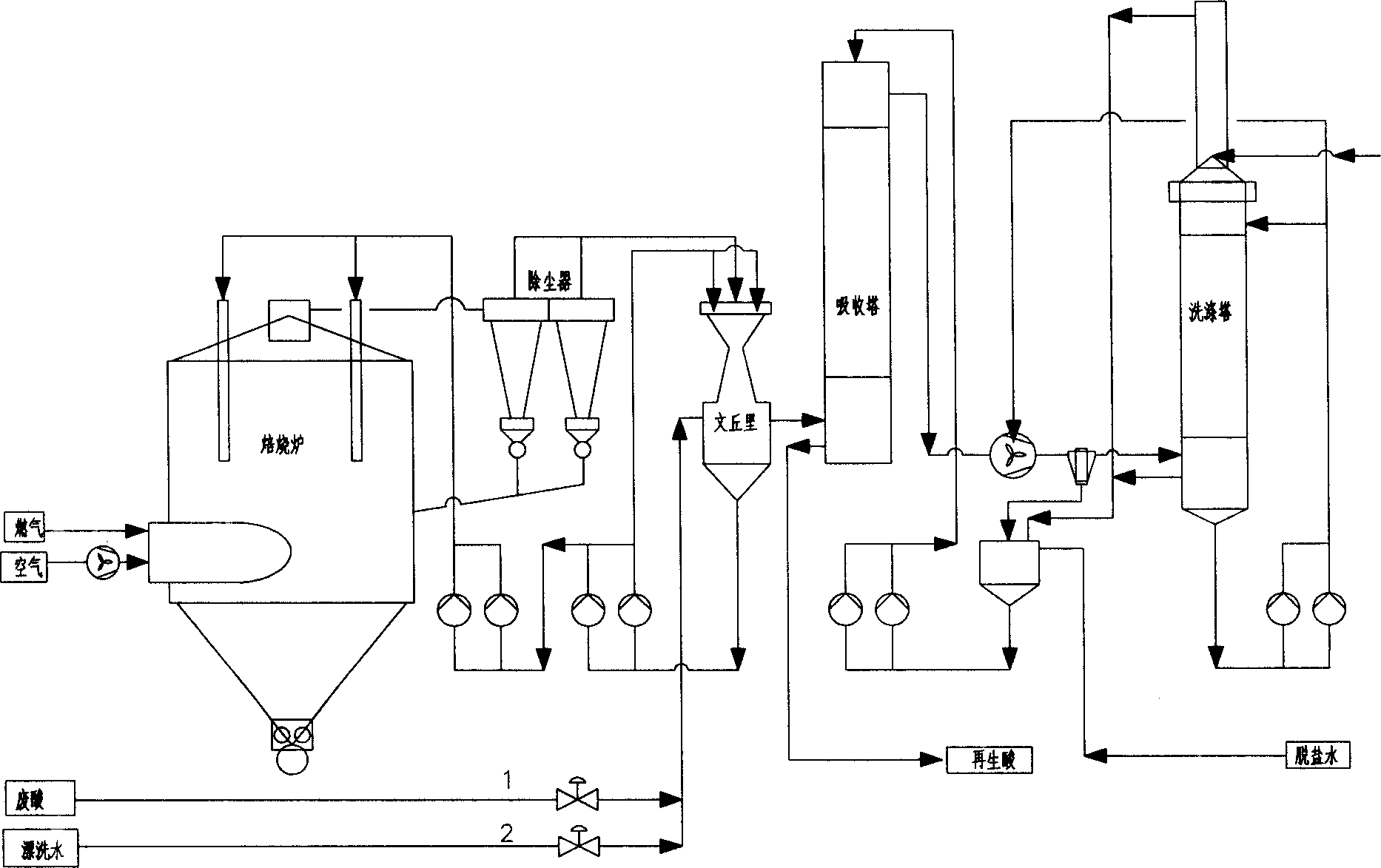
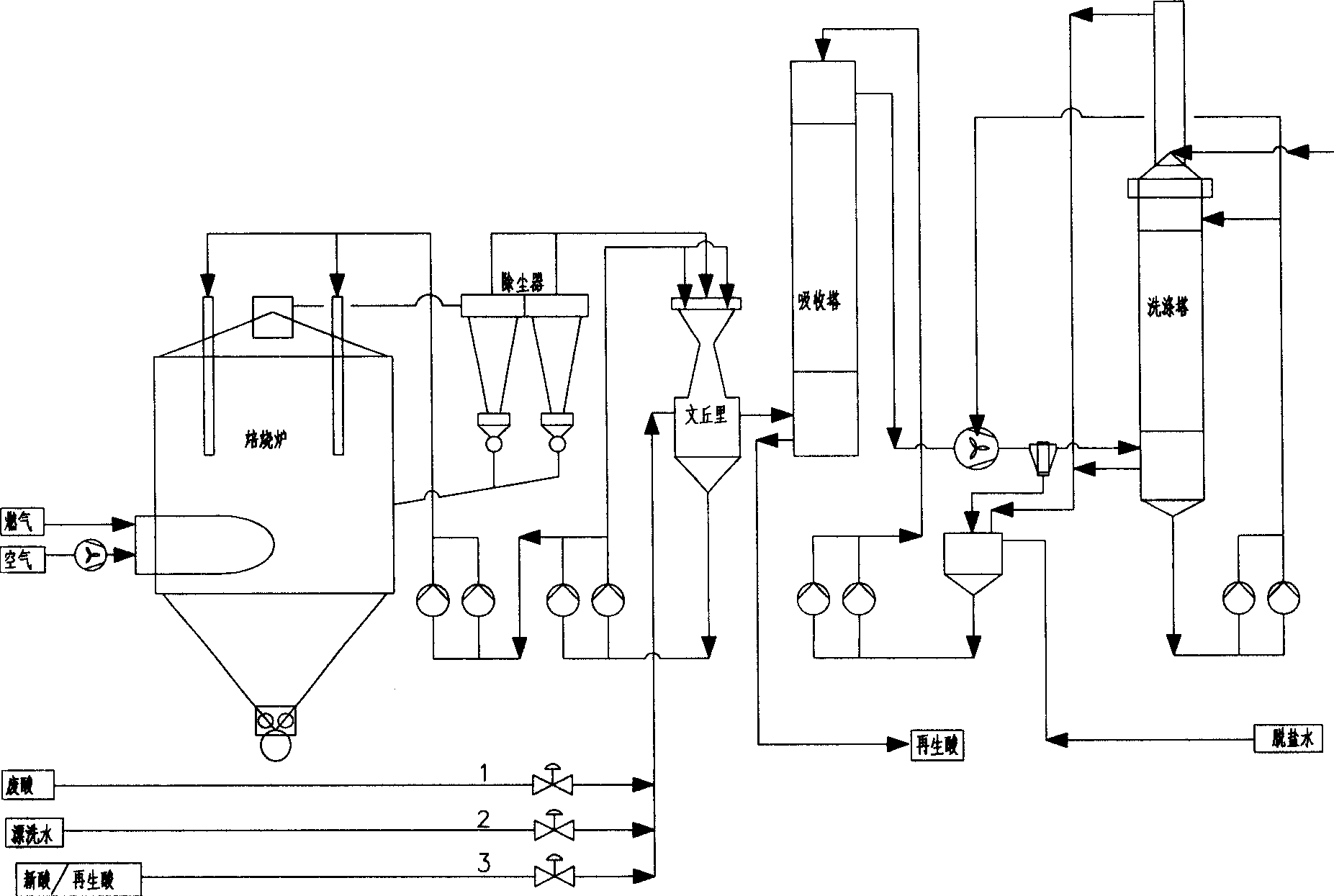
Hydrochloride waste regenerating process for spray roasting
ActiveCN1851320AReduce the concentration of iron oxide powderLower iron levelsIncinerator apparatusVolumetric Mass DensityHydrogen chloride
The invention relates to hydrochloric acid exhausted liquid reclaiming process. It includes the following steps: water operation after 390 centigrade degree; adding 18-20 percent hydrochloric acid before acid operation 5-10 minutes; acid operation; and blowing out. It can reduce brown iron oxide density of discharging gas, and the iron content in reclaimed acid.
Owner:WISDRI ENG & RES INC LTD
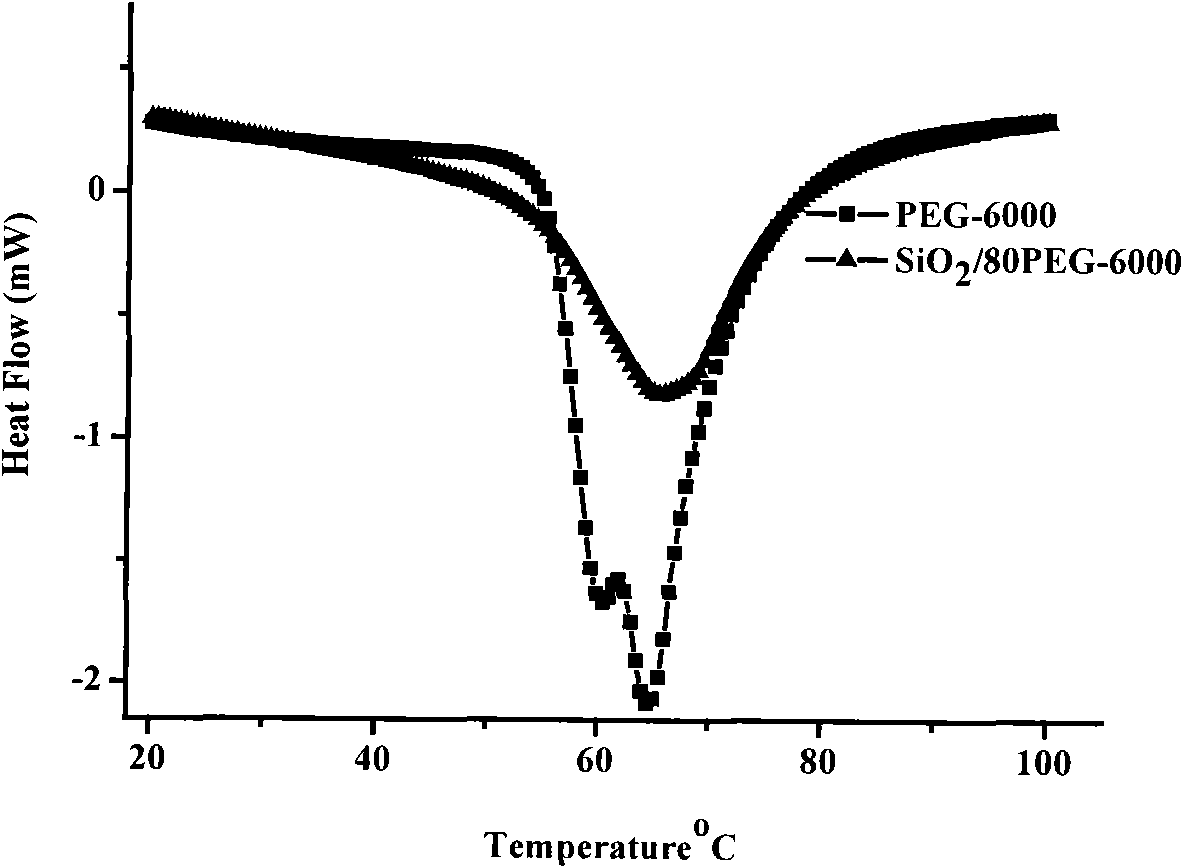
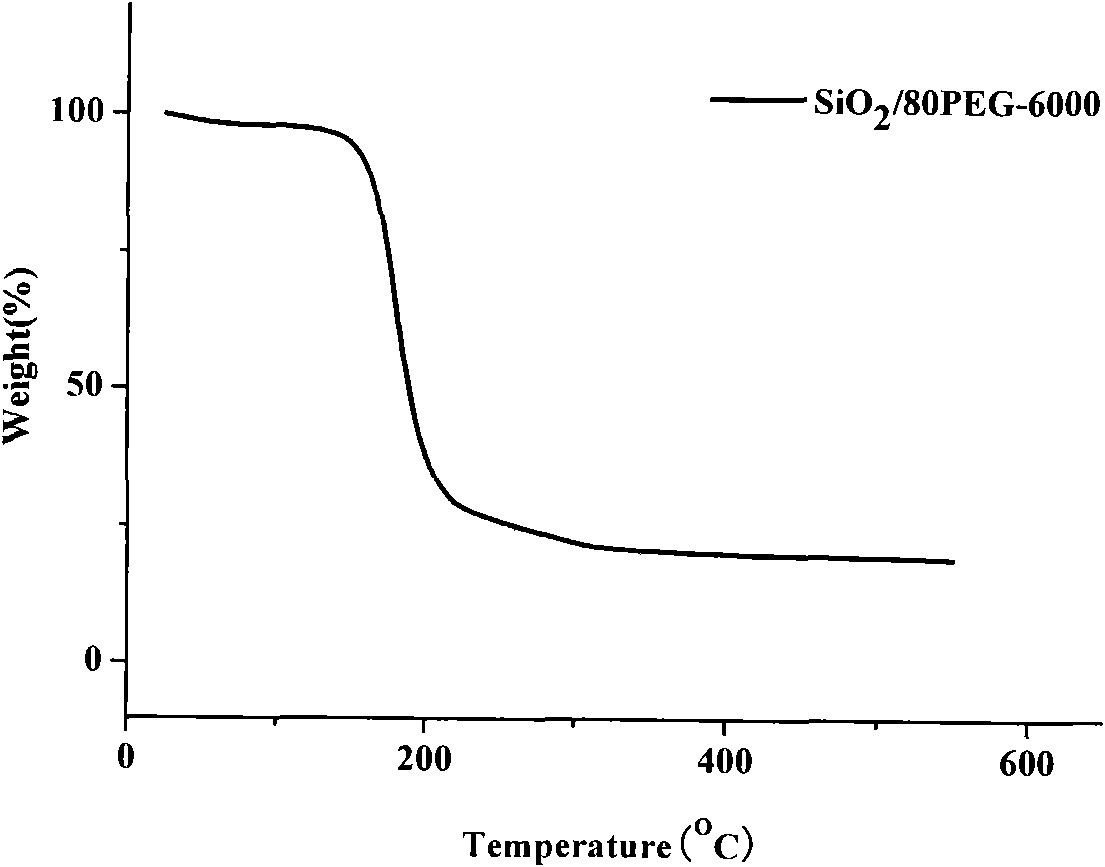
Method for preparing polyethylene glycol/silicon dioxide composite shape-stabilized phase change energy storage material and product thereof
InactiveCN101942290AEase of industrial productionReduce manufacturing costHeat-exchange elementsPolyethylene glycolHydrolysis
The invention discloses a method for preparing a polyethylene glycol / silicon dioxide composite shape-stabilized phase change energy storage material and a product thereof. The method comprises the following steps of: dissolving polyethylene glycol serving as a work substance into water in an amount of between 0.2 and 1.0g / ml to obtain water solution of the polyethylene glycol; dripping a side product SiCl4 of polycrystalline silicon into the obtained water solution of the polyethylene glycol for a hydrolysis and sol-gel reaction to form gel with a three-dimensional network structure; drying the gel to prepare powder, removing water and hydrogen chloride from the powder; and dripping a modifier for modifying the surface of the powder hydrophobically in an amount which accounts for 0.1 to 1.5 weight percent of the powder at the temperature of 80 DEG C under the protection of nitrogen for surface modification to obtain the polyethylene glycol / silicon dioxide composite shape-stabilized phase change energy storage material. The method can change wastes into valuables and reduce the manufacturing cost of the product by using the side product SiCl4 of the polycrystalline as a silicon source, and is favorable for the industrial production and wide popularization and application of the SiO2 shape-stabilized phase change energy storage material.
Owner:SOUTHWEST JIAOTONG UNIV +1
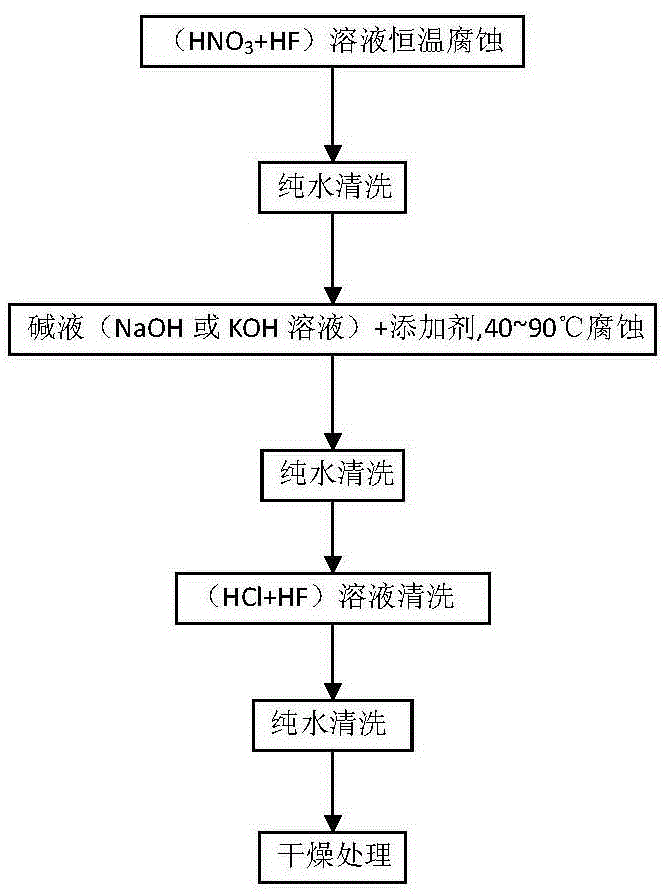
Texturing and cleaning process method of polysilicon wafer
ActiveCN103151423AImproving the effect of wool cleaningEasy to cleanAfter-treatment detailsFinal product manufactureHydrogen fluorideHydrogen Nitrate
The invention discloses a texturing and cleaning process method of a polysilicon wafer. The method comprises the following steps of 1, firstly, putting the polysilicon wafer in a mixing solution of HF (hydrogen fluoride) and HNO3 (hydrogen nitrate), and soaking; 2, putting the polysilicon wafer corroded by acid into pure water, and cleaning; 3, putting the polysilicon wafer in an alkaline solution, carrying out alkaline corrosion treatment, adding a texturing additive into the alkaline solution, and carrying out secondary texturing on the polysilicon wafer; 4, putting the polysilicon wafer corroded by alkali into the pure water, and cleaning; 5, putting the polysilicon wafer in a mixing solution of HCl (hydrogen chloride) and HF, and soaking; 6, putting the polysilicon wafer corroded by the acid into the pure water, and cleaning; and 7, drying the treated polysilicon wafer. The process method has the advantages that on the premise of not changing other processes, the final converting efficiency of the polysilicon wafer is improved by 0.2% to 0.3%, and the purpose of final efficiency stacking of a battery sheet is realized.
Owner:CHANGZHOU S C EXACT EQUIP
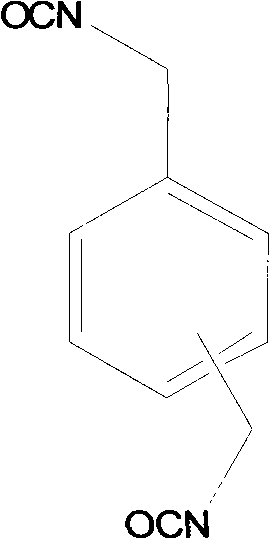
Method for preparing xylylene diisocyanate based on salification-phosgenation reaction
ActiveCN102070491ALarge particle sizeIncrease the rate of the phosgenation reactionIsocyanic acid derivatives preparationOrganic compound preparationXylyleneHigh concentration
The invention discloses a method for preparing xylylene diisocyanate (XDI) based on salification-phosgenation reaction. The method comprises the following steps of: (a) a salification reaction process of amine solution which is formed by xylylene diamine in an inert organic solvent and hydrogen chloride; (b) a hydrochloride centrifugal concentration process; (c) a high-pressure phosgenation process; and (d) a low-pressure phosgenation process. In the method, the salification reaction of the amine is kept performing in low-concentration hydrochloride solution all the time; and high-concentration hydrochloride solution participates in the phosgenation reaction to prepare the XDI. The process has a high time-space conversion rate and simultaneously ensures a good salification effect.
Owner:WANHUA CHEM GRP CO LTD +1
Catalyst system for preparing vinyl chloride by hydrochlorinating acetylene and preparation and application thereof
ActiveCN101879464AEasy to prepareImprove conversion rateCatalyst carriersPreparation by halogen halide additionManganeseIonic liquid
The invention discloses a catalyst system for preparing vinyl chloride by hydrochlorinating acetylene, and preparation and application thereof. The catalyst system comprises a catalyst carrier and a catalyst, wherein the catalyst carrier is pyridine ionic liquid, and the catalyst is one or any combination of more than two of tin, palladium, gold, copper, manganese, bismuth, mercury and rhodium. The preparation method for the catalyst system comprises a step of dissolving the catalyst in the catalyst carrier. Acetylene gas and hydrogen chloride gas are mixed and then reacted in the presence of the catalyst system. The preparation process realizes a liquid phase reaction for preparing the vinyl chloride by hydrochlorinating the acetylene, avoids the loss of the catalyst system, is more environment-friendly and safer, and improves the conversion rate of the acetylene and the selectivity of the vinyl chloride.
Owner:于志勇
Preparation method of lithium hexafluorophosphate
ActiveCN102009972ARich sourcesReduce manufacturing costPhosphorus compoundsPhosphoric acidNitrogen gas
The invention relates to a preparation method of lithium hexafluorophosphate. The preparation method comprises the following steps of: (1) distilling to obtain hydrogen fluoride liquid of which the purity is over 99.99 weight percent; (2) reacting the high-purity hydrogen fluoride liquid with phosphorus pentachloride to obtain mixed gas of the phosphorus pentafluoride and hydrogen chloride; (3) introducing the mixed gas of the phosphorus pentachloride and the hydrogen chloride into hydrogen fluoride and lithium fluoride, reacting at a certain temperature and under certain pressure to obtain solution of lithium hexafluorophosphate, exhausting hydrogen chloride gas at regular time, and absorbing by using water to prepare byproduct hydrochloric acid; and (4) crystallizing and separating, namely filtering the solution of lithium hexafluorophosphate, delivering filtrate into a crystallizing slot, separating the lithium hexafluorophosphate out at the temperature of between -70 and 80 DEG C, filtering, and performing primary drying and secondary drying to obtain a lithium hexafluorophosphate product, wherein the residual hydrogen fluoride gas is displaced by nitrogen. The preparation method has readily available raw materials and is easy to operate, the purity of the obtained lithium hexafluorophosphate product is over 99.9 percent, the moisture is lower than 10ppm, and the production requirements of lithium ion electrolytic cells are met.
Owner:MORITA NEW ENERGY MATERIALS ZHANGJIAGANG CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
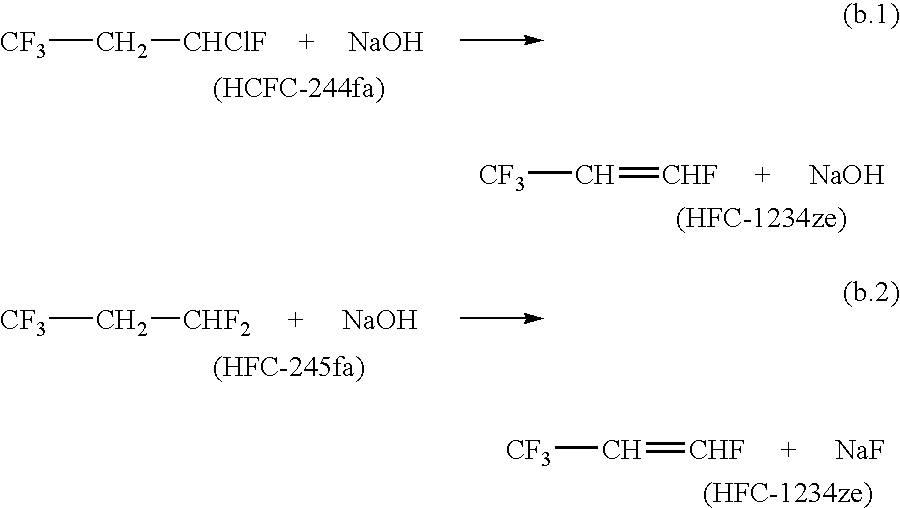
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00221.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00231.PNG)
![Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process Industrial process for the synthesis of 17a-acetoxy-11ss-[4-(n,n-dimethyl-amino)- phenyl]-19-norpregna-4,9-diene-3,20-dione and new intermediates of the process](https://images-eureka.patsnap.com/patent_img/9a66d1cf-4b54-4dee-bae5-7e40fd7cdedf/A200780021915E00232.PNG)