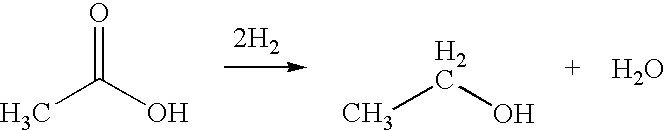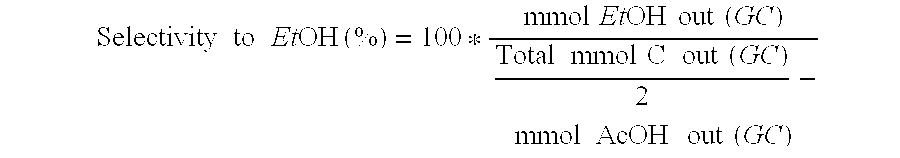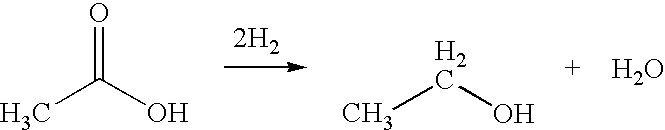Patents
Literature
3689 results about "Pt element" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Platinum is a chemical element with symbol Pt and atomic number 78. It is a dense, malleable, ductile, highly unreactive, precious, silverish-white transition metal.
Organic electroluminescent device
ActiveUS20060263635A1Group 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsPlatinumNitrogen
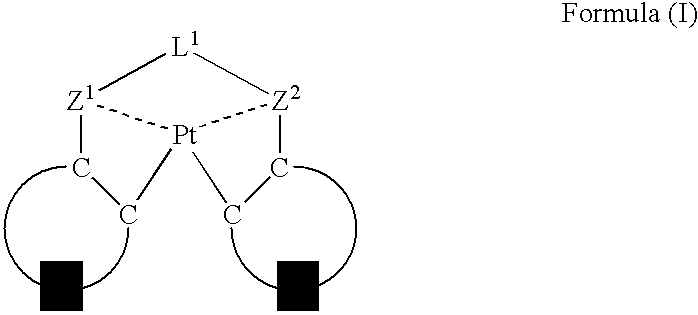
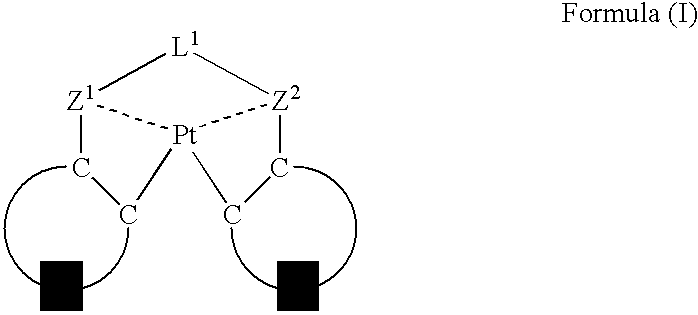
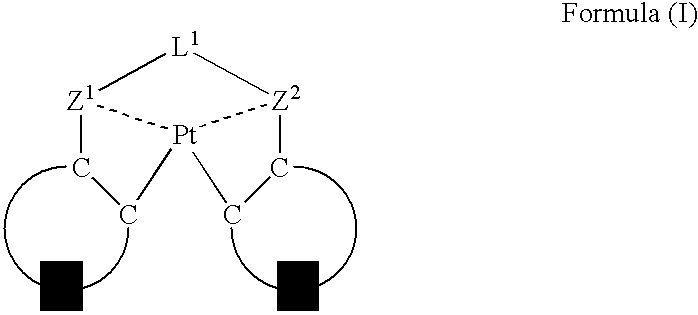
An organic electroluminescent device having a pair of electrodes and at least one organic layer interposed between the pair of electrodes, in which the at least one organic layer contains at least one compound represented by formula (I): wherein, Z1 and Z2 each independently represent a nitrogen-containing aromatic six-membered ring coordinated to the platinum through a nitrogen atom; Q1 represents a group of atoms necessary for forming, together with the —C—C—, a nitrogen-containing aromatic five-membered ring; L1 represents a single bond or a divalent linking group; and n is 0 or 1.
Owner:UDC IRELAND +1
Platinum complex and light emitting device
ActiveUS20070103060A1Enhanced glowSolve low luminous efficiencyDischarge tube luminescnet screensElectroluminescent light sourcesOxygenLight emission
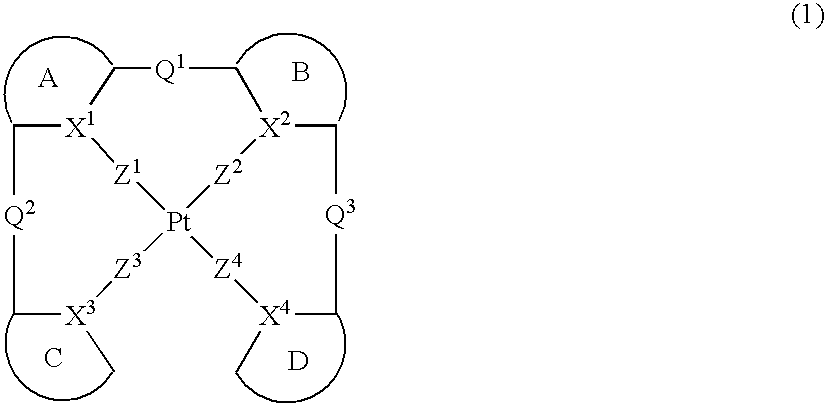
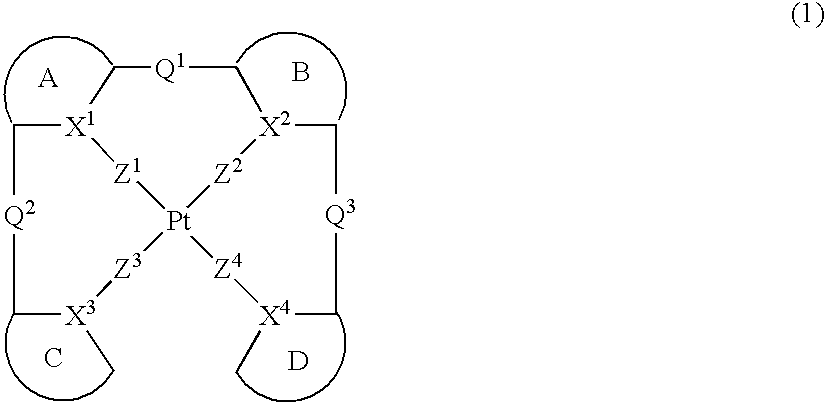
Provision of a novel platinum complex which is useful as a material for a light-emitting device of good light emission characteristic and light emission efficiency, and a novel light-emitting material that may be utilized in various fields. A platinum complex represented by the following general formula (1): (in which two rings of ring A, ring B, ring C, and ring D represent nitrogen-containing heterocyclic rings which may have a substituent and the remaining two rings of them represent aryl rings or hetero aryl rings which may have a substituent, the ring A and the ring B, the ring A and the ring C or / and the ring B and the rind D may form condensed rings. Two of X1, X2, X3, and X4 represent nitrogen atoms coordination bonded to a platinum atom and the remaining two of them represent carbon atoms or nitrogen atoms. Q1, Q2, and Q3 each represents a bond, oxygen atom, sulfur atom or bivalent group, two of Z1, Z2, Z3, and Z4 represent coordination bonds, and the remaining two of them represent covalent bonds, oxygen atoms or sulfur atoms), and a light-emitting device containing the platinum complex.
Owner:TAKASAGO INTERNATIONAL CORPORATION
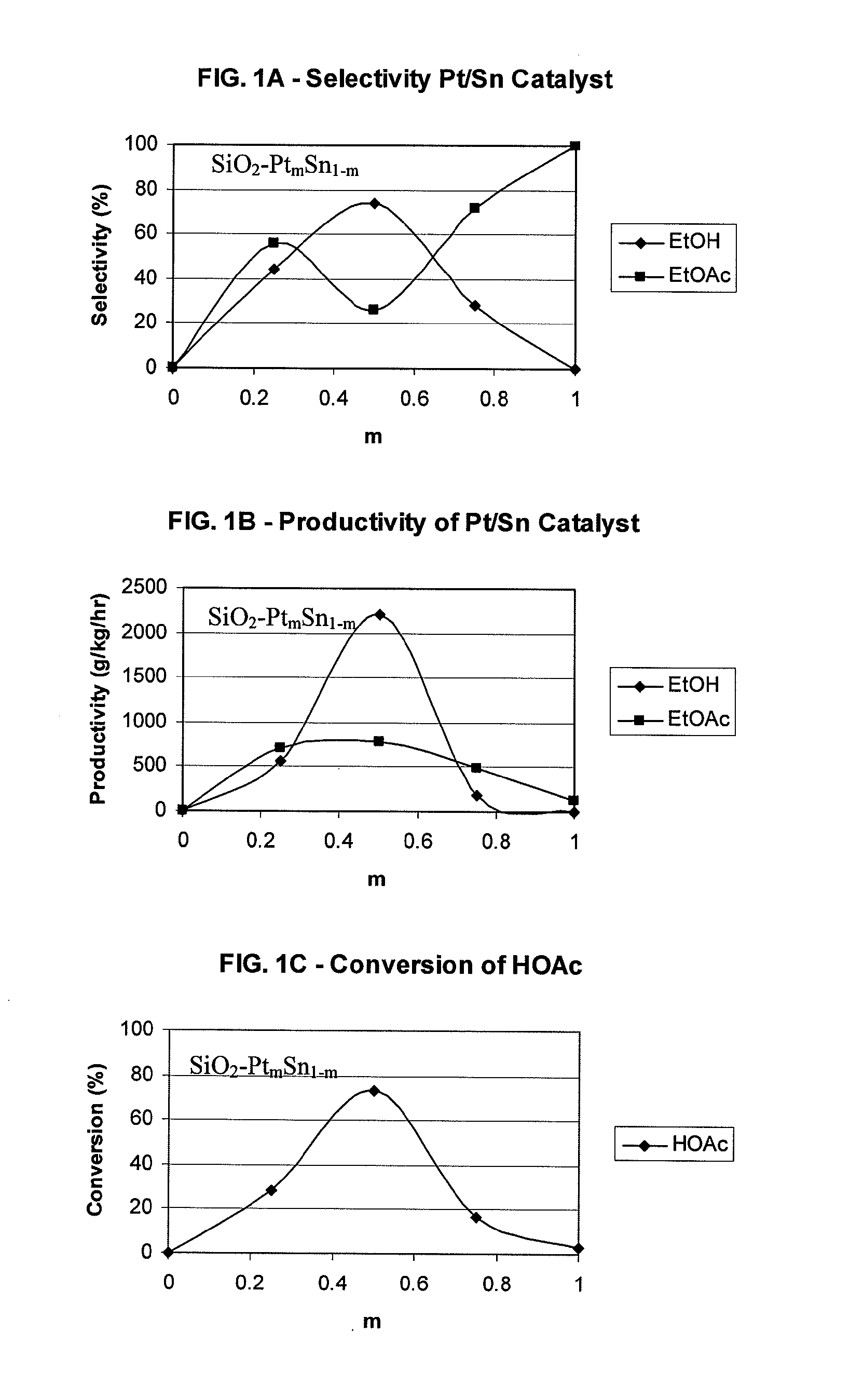
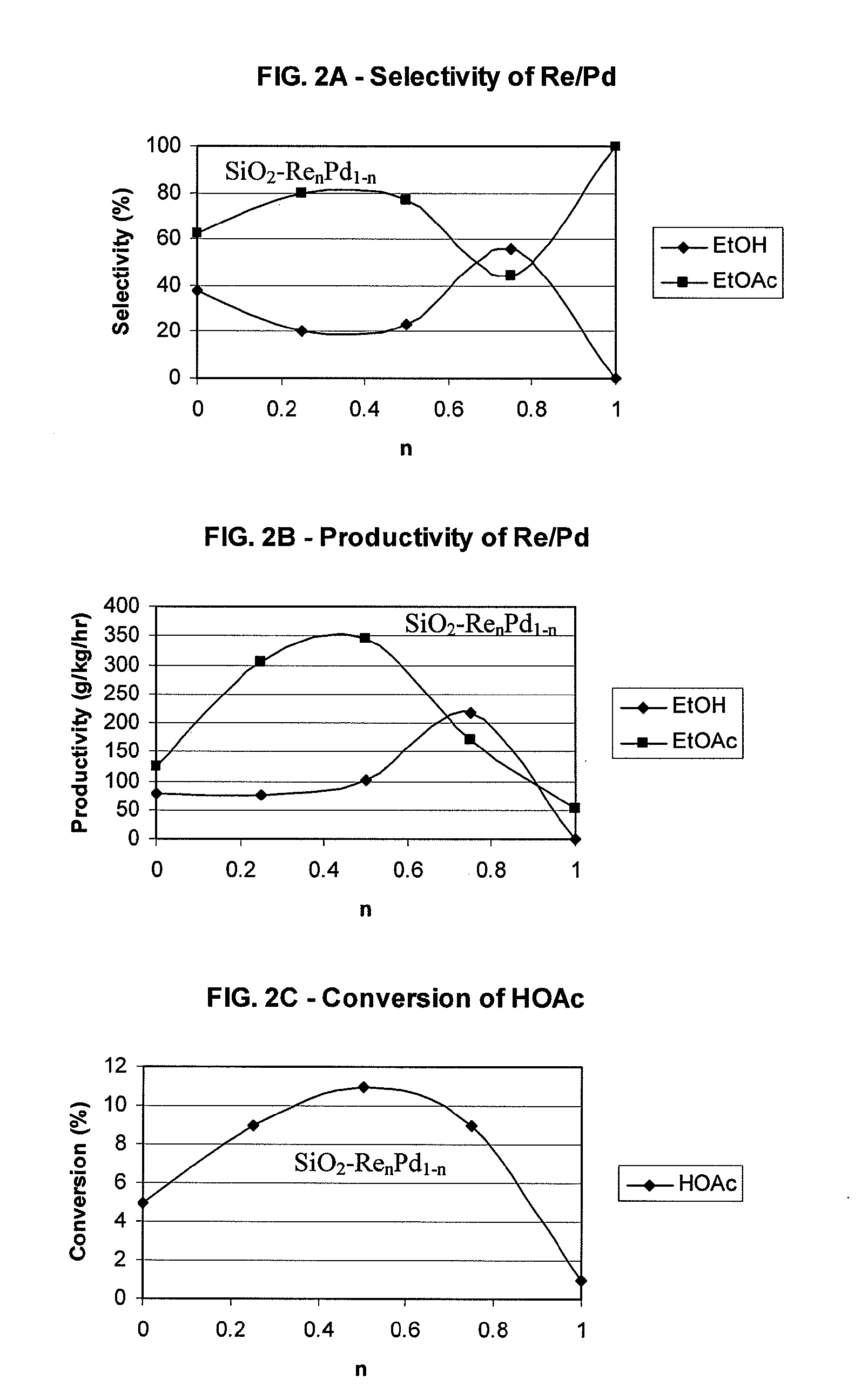
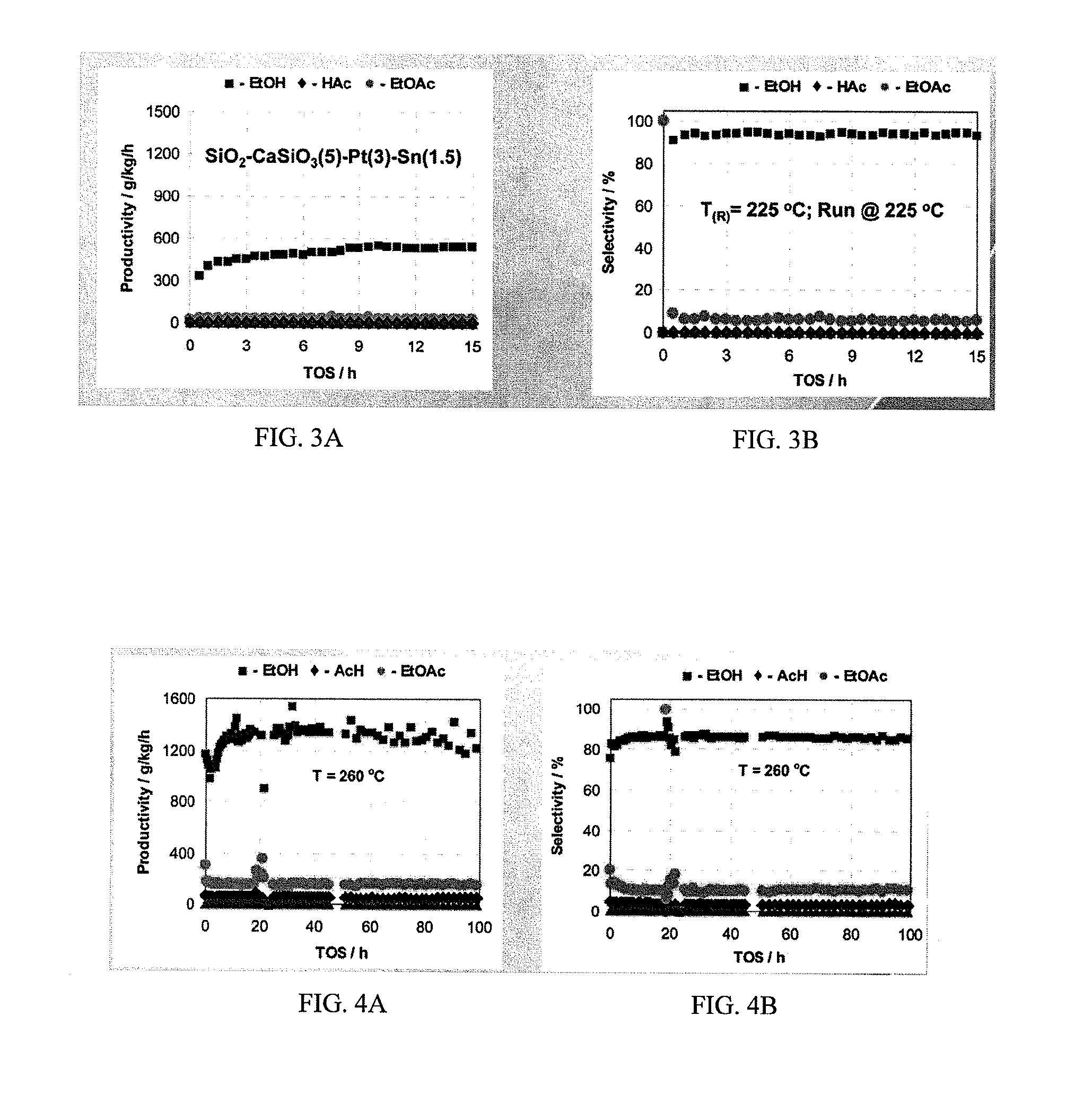
Direct and selective production of ethanol from acetic acid utilizing a platinum/tin catalyst
InactiveUS7863489B2High selectivityHigh yieldOrganic compound preparationOxygen compounds preparation by reductionCalcium silicateAcetic acid
A process for the selective production of ethanol by vapor phase reaction of acetic acid over a hydrogenating catalyst composition to form ethanol is disclosed and claimed. In an embodiment of this invention reaction of acetic acid and hydrogen over a platinum and tin supported on silica, graphite, calcium silicate or silica-alumina selectively produces ethanol in a vapor phase at a temperature of about 250° C.
Owner:CELANESE INT CORP
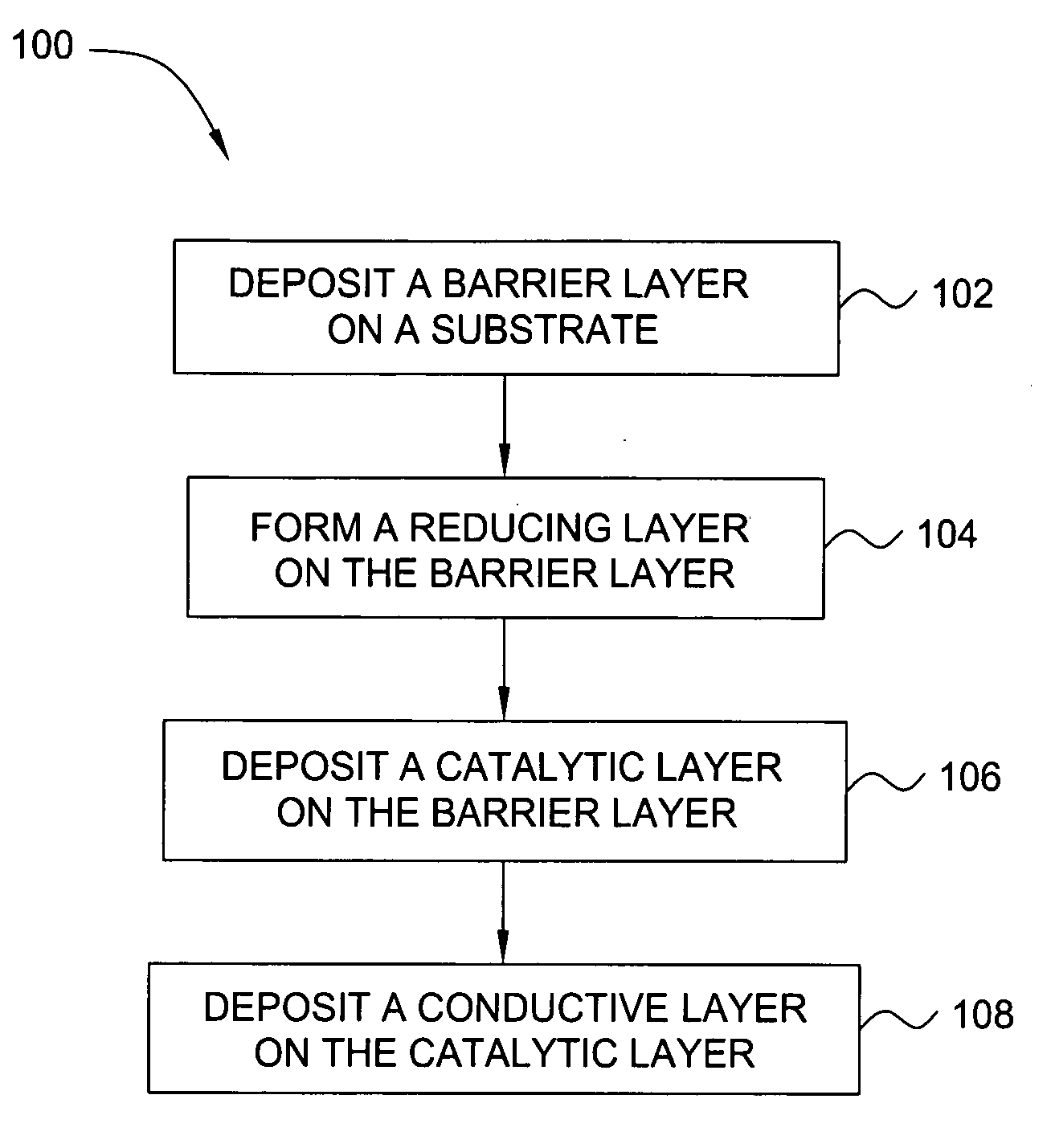
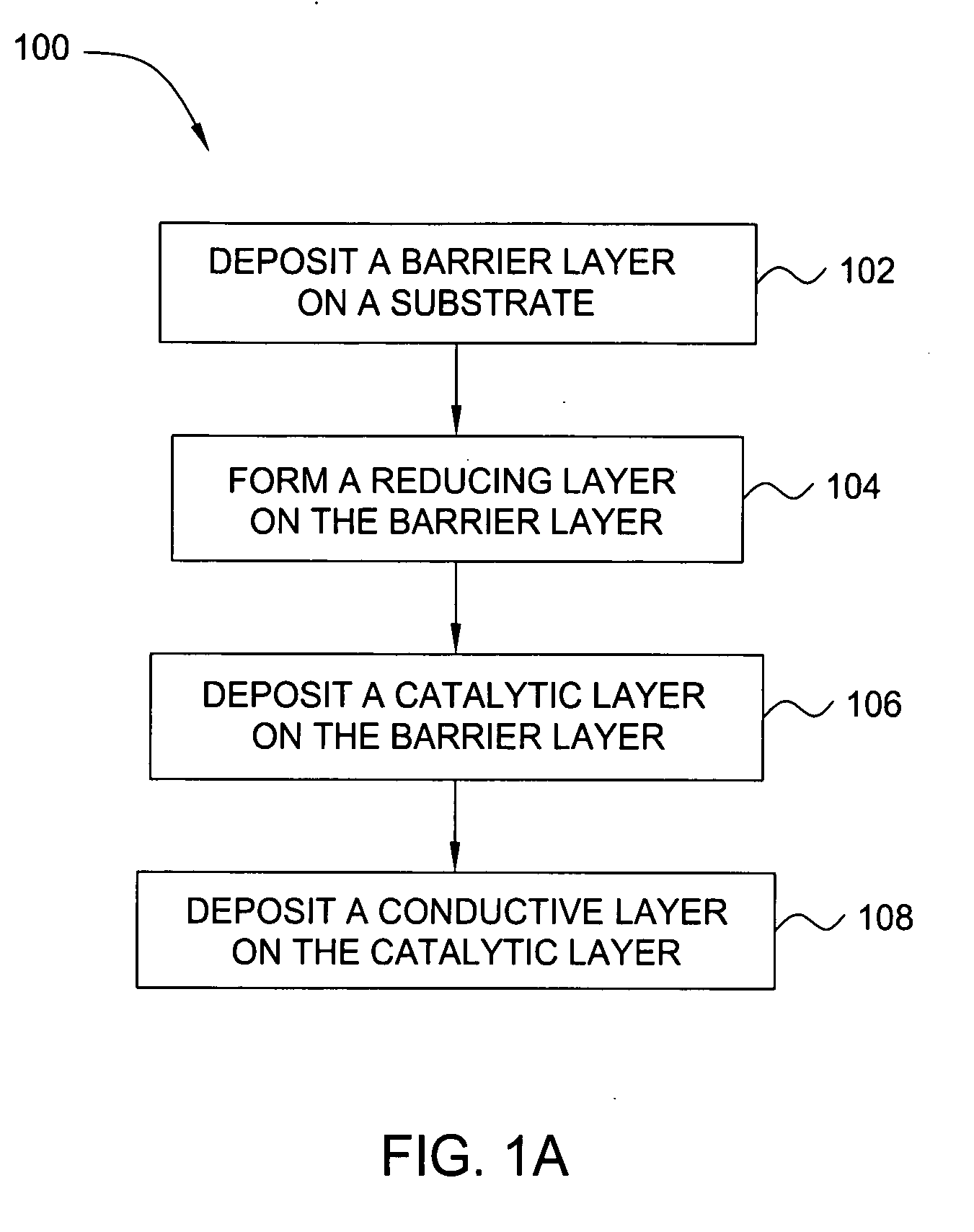
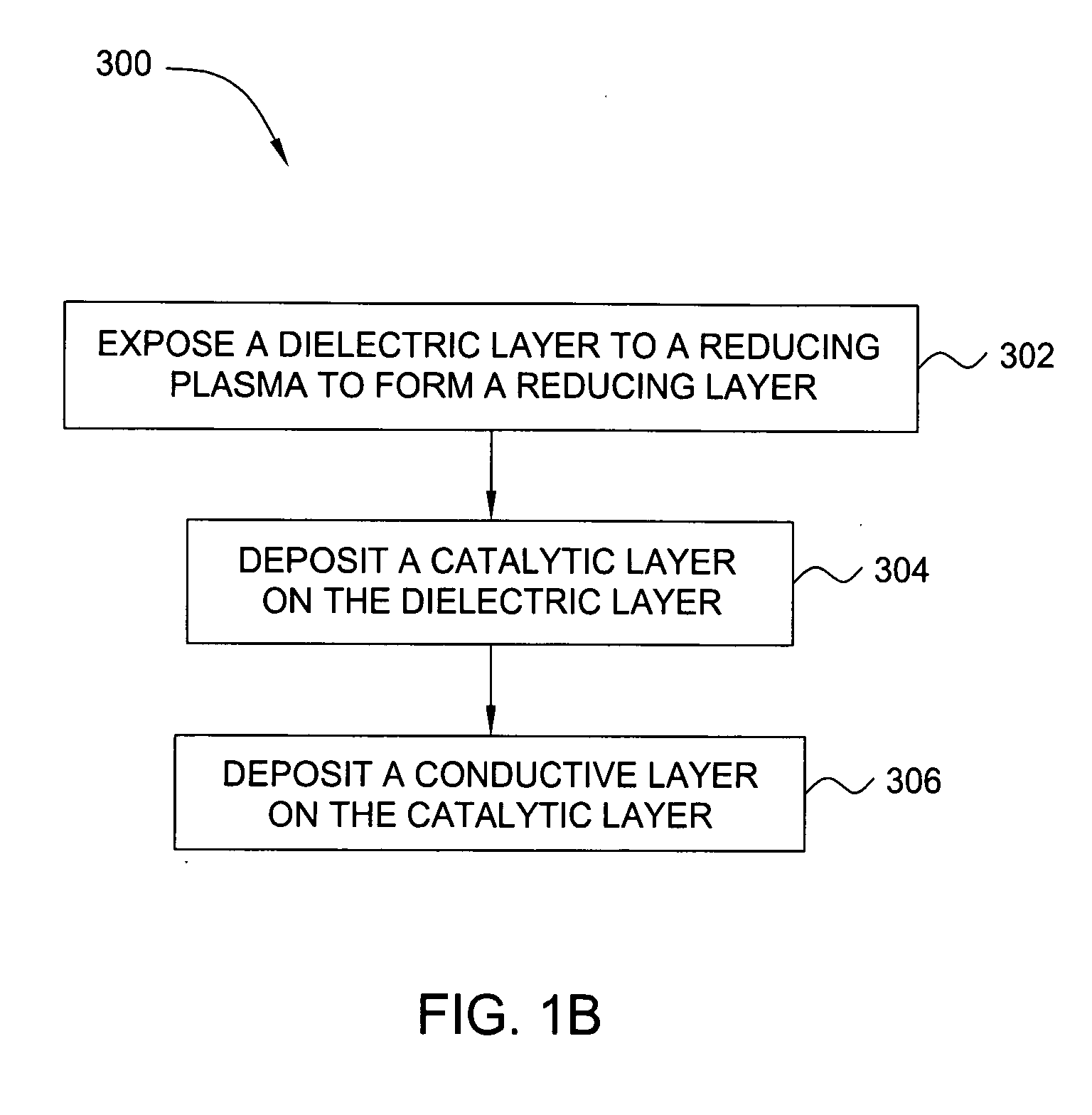
Deposition of an intermediate catalytic layer on a barrier layer for copper metallization
InactiveUS20060240187A1Solid-state devicesSemiconductor/solid-state device manufacturingIridiumSilanes
In one embodiment, a method for depositing a conductive material on a substrate is provided which includes exposing a substrate containing a barrier layer to a volatile reducing precursor to form a reducing layer during a soak process, exposing the reducing layer to a catalytic-metal precursor to deposit a catalytic metal-containing layer on the barrier layer, and depositing a conductive layer (e.g., copper) on the catalytic metal-containing layer. The volatile reducing precursor may include phosphine, diborane, silane, a plasma thereof, or a combination thereof and be exposed to the substrate for a time period within a range from about 1 second to about 30 seconds during the soak process. The catalytic metal-containing layer may contain ruthenium, cobalt, rhodium, iridium, nickel, palladium, platinum, silver, or copper. In one example, the catalytic metal-containing layer is deposited by a vapor deposition process utilizing ruthenium tetroxide formed by an in situ process.
Owner:APPLIED MATERIALS INC
Direct and selective production of ethanol from acetic acid utilizing a platinum/tin catalyst
InactiveUS20100029995A1High selectivityHigh yieldOrganic compound preparationOxygen compounds preparation by reductionCalcium silicatePlatinum
A process for the selective production of ethanol by vapor phase reaction of acetic acid over a hydrogenating catalyst composition to form ethanol is disclosed and claimed. In an embodiment of this invention reaction of acetic acid and hydrogen over a platinum and tin supported on silica, graphite, calcium silicate or silica-alumina selectively produces ethanol in a vapor phase at a temperature of about 250° C.
Owner:CELANESE INT CORP
Electroless plating of metal caps for chalcogenide-based memory devices
InactiveUS20060094236A1Good electrical contactSemiconductor/solid-state device manufacturingPlatinumSulfur
A method of forming a metal cap over a conductive interconnect in a chalcogenide-based memory device is provided and includes, forming a layer of a first conductive material over a substrate, depositing an insulating layer over the first conductive material and the substrate, forming an opening in the insulating layer to expose at least a portion of the first conductive material, depositing a second conductive material over the insulating layer and within the opening, removing portions of the second conductive material to form a conductive area within the opening, recessing the conductive area within the opening to a level below an upper surface of the insulating layer, forming a cap of a third conductive material over the recessed conductive area within the opening, the third conductive material selected from the group consisting of cobalt, silver, gold, copper, nickel, palladium, platinum, and alloys thereof, depositing a stack of a chalcogenide based memory cell material over the cap, and depositing a conductive material over the chalcogenide stack.
Owner:MICRON TECH INC
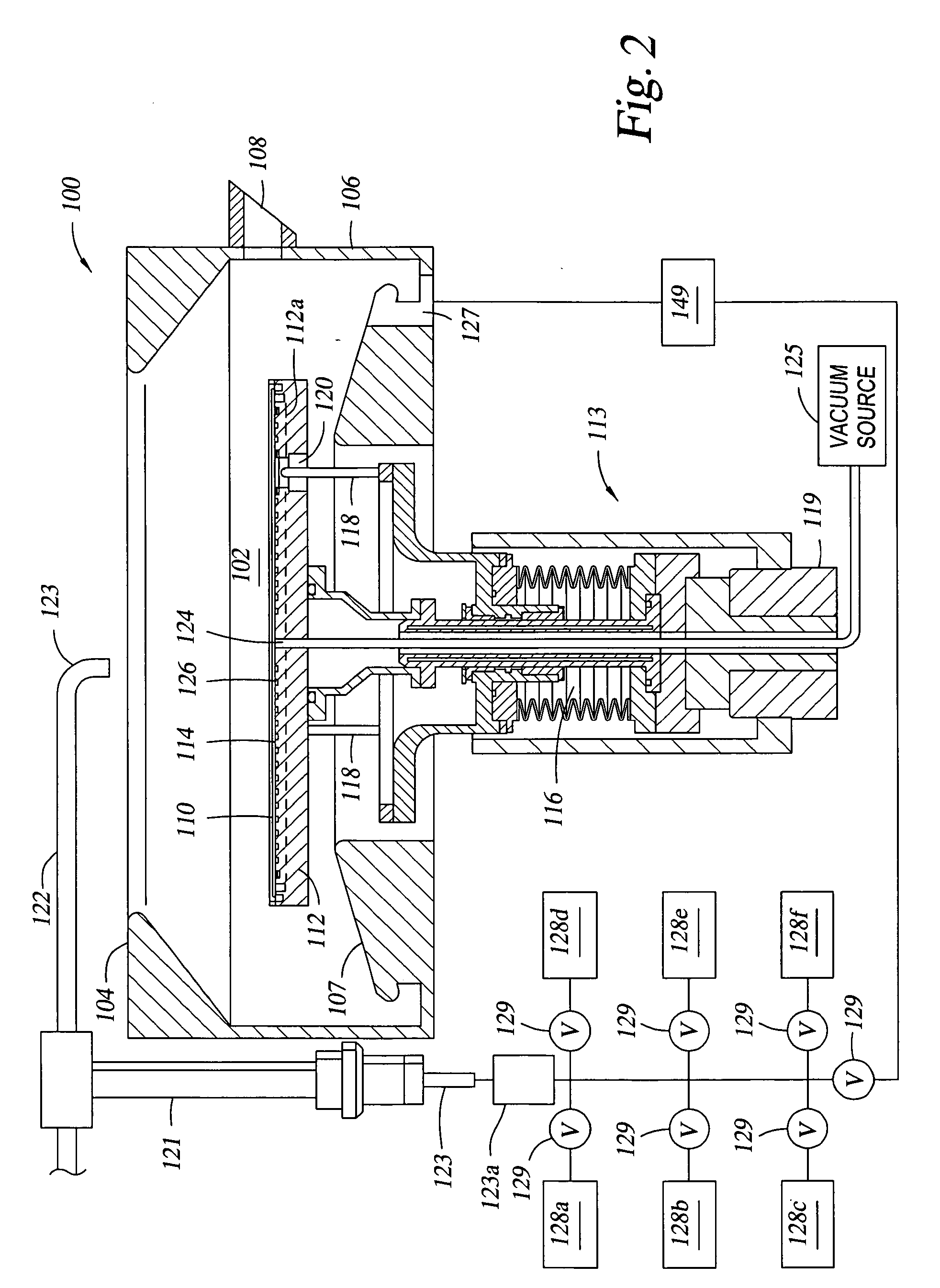
Electroless deposition apparatus
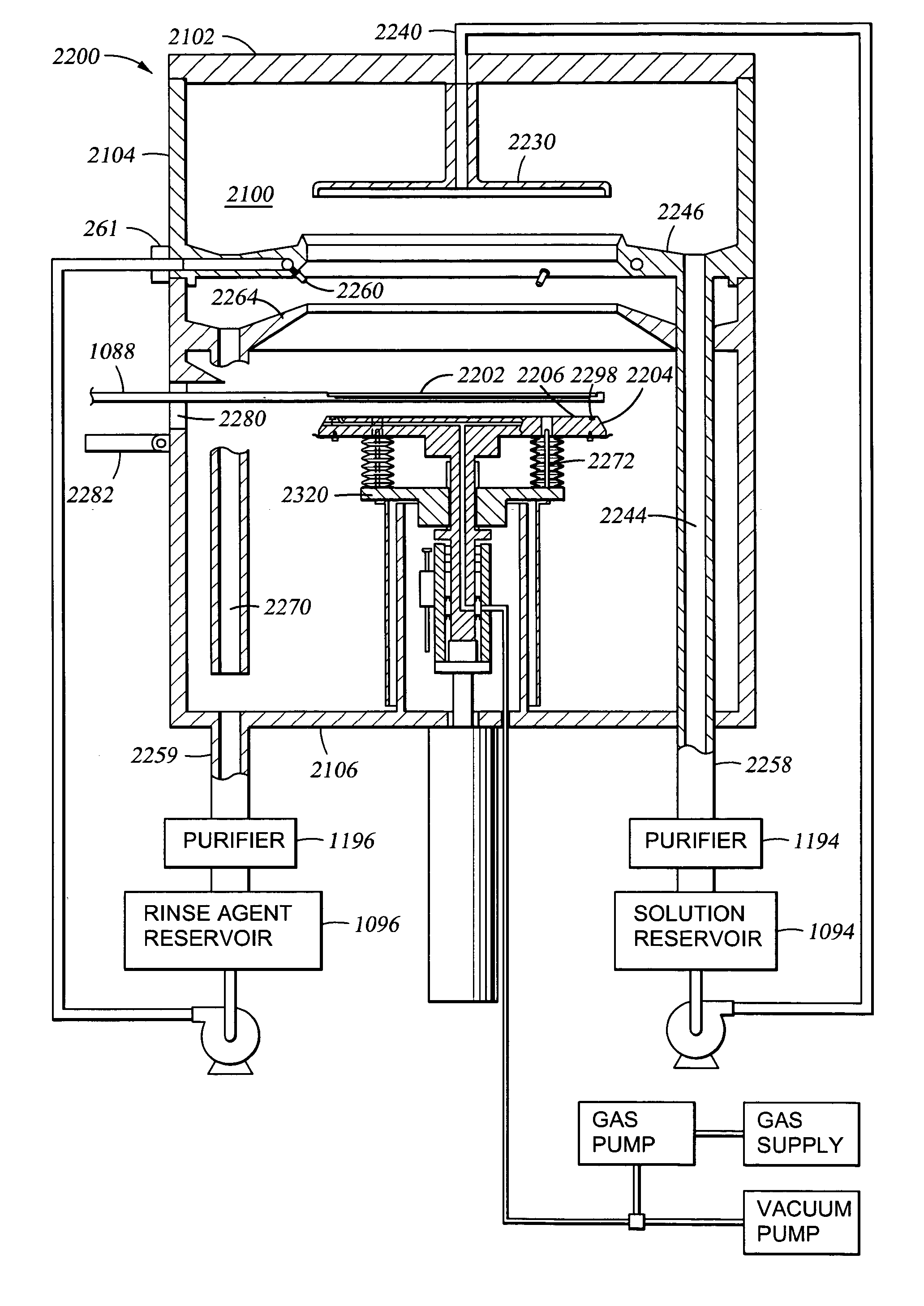
An apparatus and a method of depositing a catalytic layer comprising at least one metal selected from the group consisting of noble metals, semi-noble metals, alloys thereof, and combinations thereof in sub-micron features formed on a substrate. Examples of noble metals include palladium and platinum. Examples of semi-noble metals include cobalt, nickel, and tungsten. The catalytic layer may be deposited by electroless deposition, electroplating, or chemical vapor deposition. In one embodiment, the catalytic layer may be deposited in the feature to act as a barrier layer to a subsequently deposited conductive material. In another embodiment, the catalytic layer may be deposited over a barrier layer. In yet another embodiment, the catalytic layer may be deposited over a seed layer deposited over the barrier layer to act as a “patch” of any discontinuities in the seed layer. Once the catalytic layer has been deposited, a conductive material, such as copper, may be deposited over the catalytic layer. In one embodiment, the conductive material is deposited over the catalytic layer by electroless deposition. In another embodiment, the conductive material is deposited over the catalytic layer by electroless deposition followed by electroplating or followed by chemical vapor deposition. In still another embodiment, the conductive material is deposited over the catalytic layer by electroplating or by chemical vapor deposition.
Owner:APPLIED MATERIALS INC
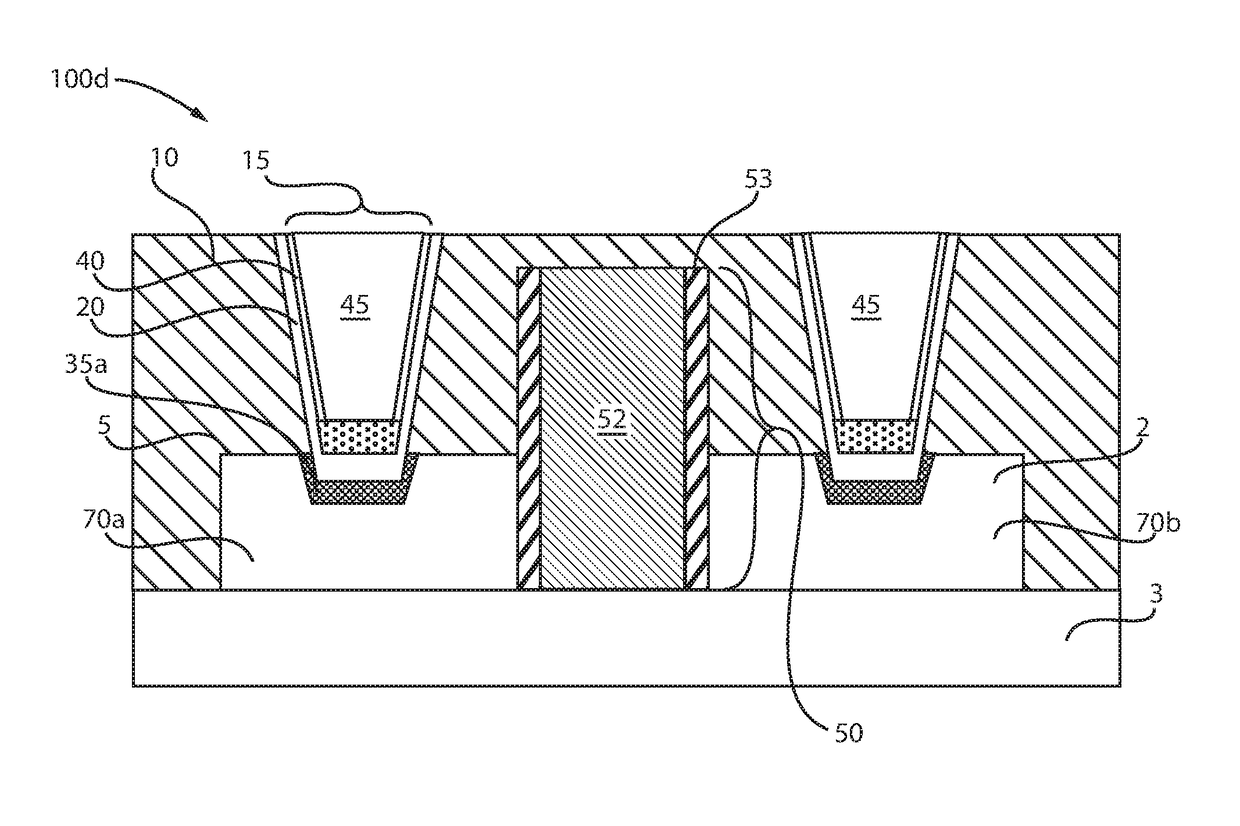
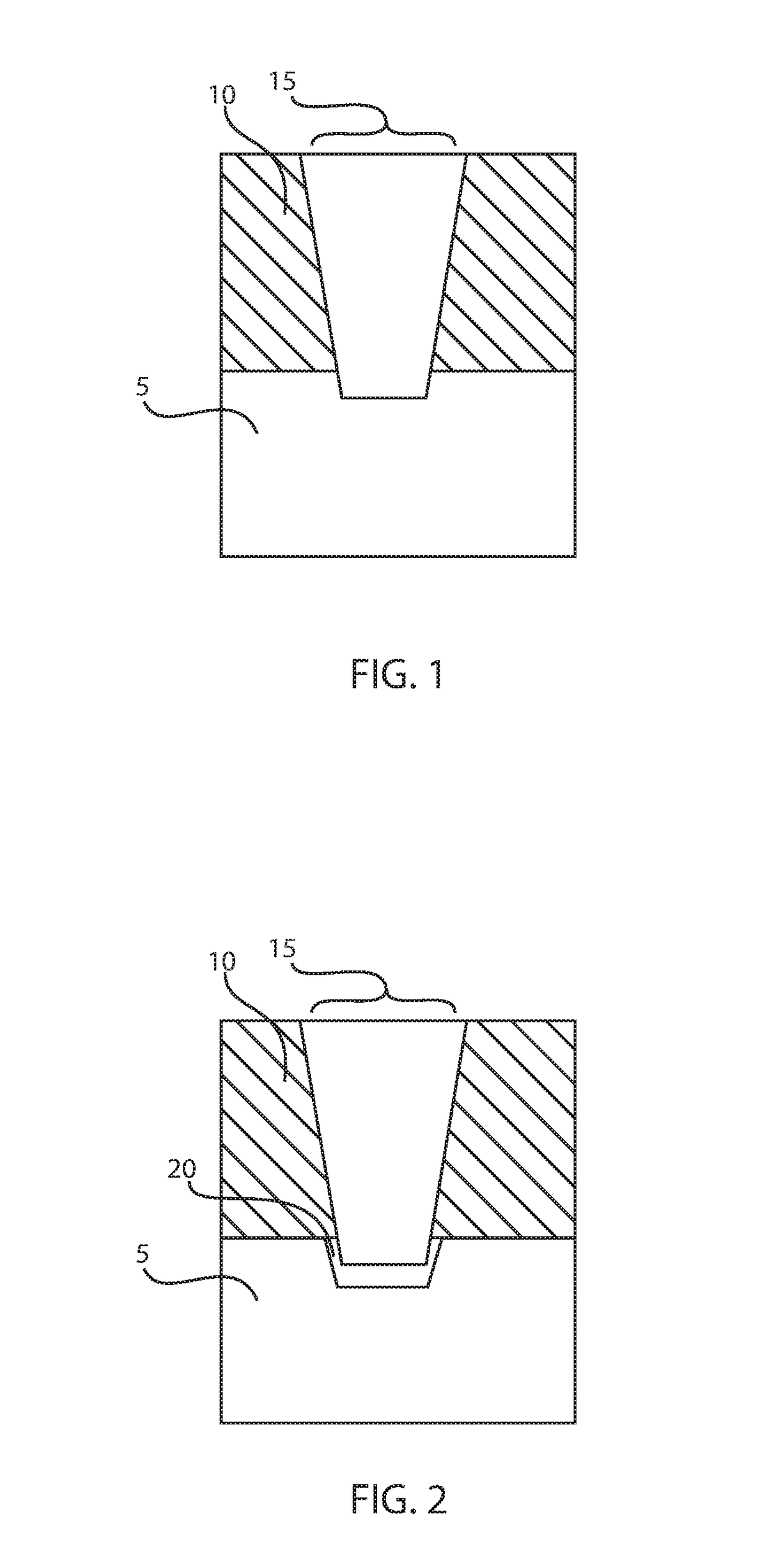
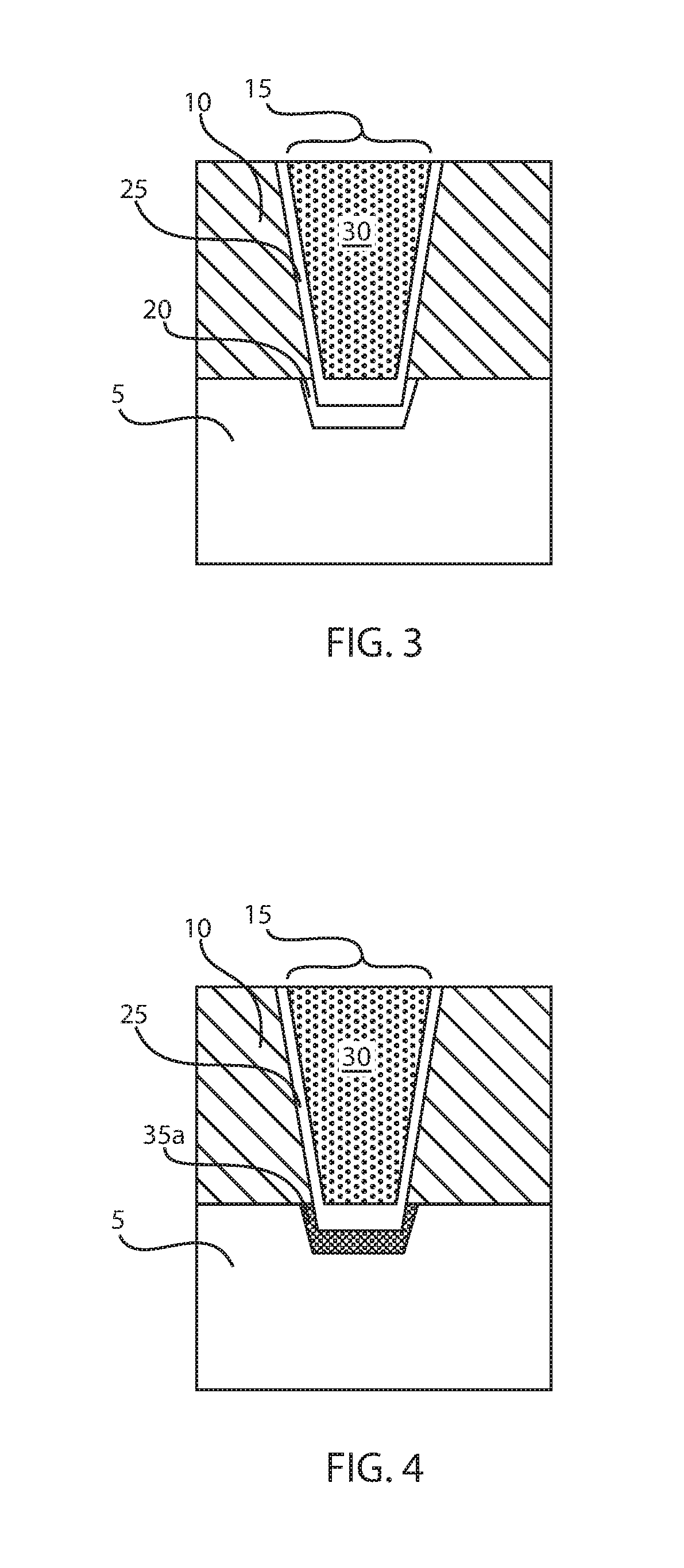
Low resistance contacts including intermetallic alloy of nickel, platinum, titanium, aluminum and type iv semiconductor elements
ActiveUS20180068950A1Semiconductor/solid-state device detailsSolid-state devicesPlatinumSemiconductor materials
A method of forming a contact to a semiconductor device is provided that forms an alloy composed of nickel (Ni), platinum (Pt), aluminum (Al), titanium (Ti) and a semiconductor material. The methods may include forming a nickel and platinum semiconductor alloy at a base of a via. A titanium layer having an angstrom scale thickness is deposited in the via in contact with the nickel platinum semiconductor alloy. An aluminum containing fill is deposited atop the titanium layer. A forming gas anneal including an oxygen containing atmosphere is applied to the structure to provide a contact alloy comprising nickel, platinum, aluminum, titanium and a semiconductor element from the contact surface of the semiconductor device.
Owner:IBM CORP
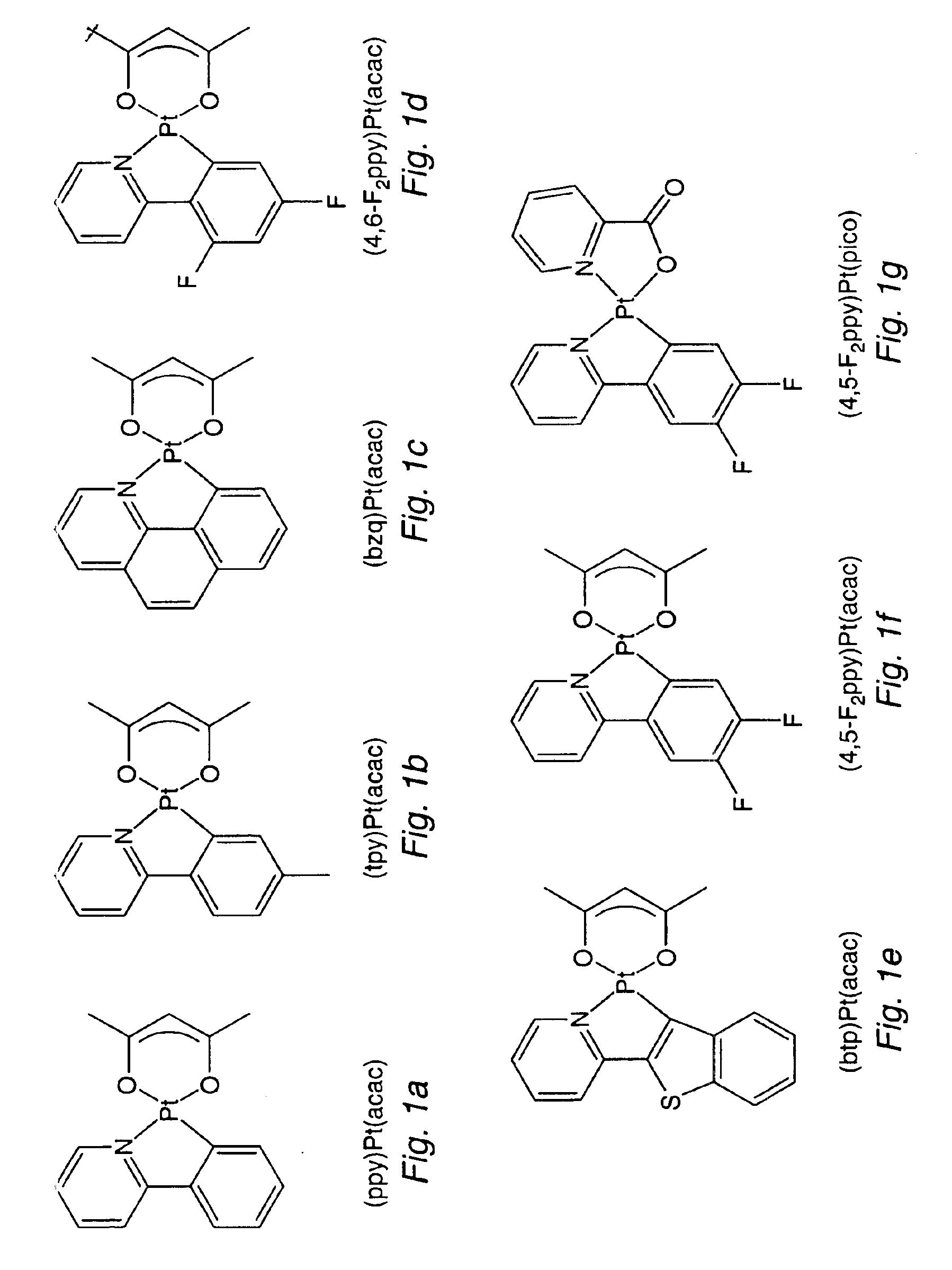
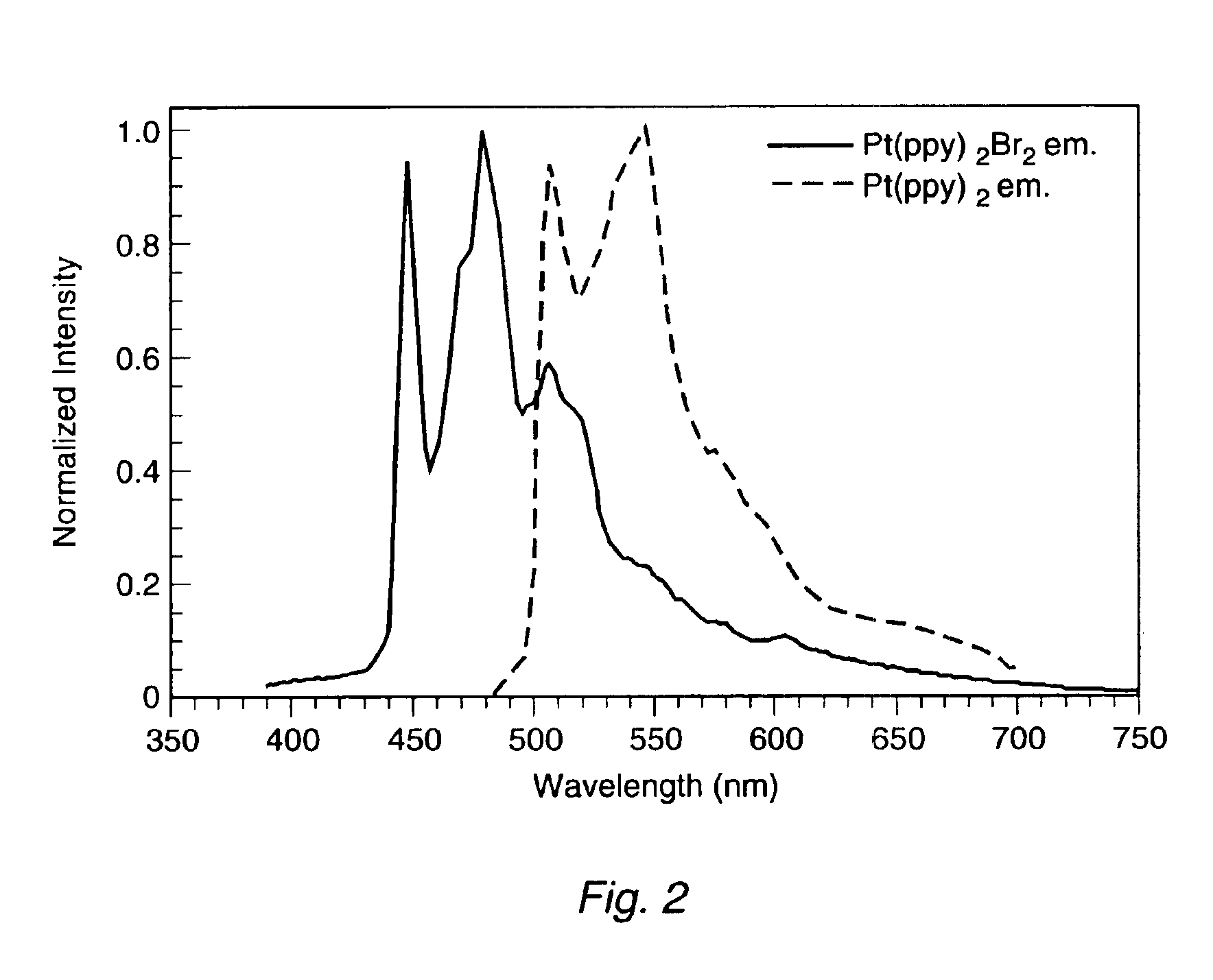
Organometallic complexes as phosphorescent emitters in organic LEDs
InactiveUS7001536B2Easy to combineDeterioration in emission qualityGroup 8/9/10/18 element organic compoundsElectroluminescent light sourcesIridiumPt element
Organic light emitting devices are described wherein the emissive layer comprises a host material containing an emissive molecule, which molecule is adapted to luminesce when a voltage is applied across the heterostructure, and the emissive molecule is selected from the group of phosphorescent organometallic complexes, including cyclometallated platinum, iridium and osmium complexes. The organic light emitting devices optionally contain an exciton blocking layer. Furthermore, improved electroluminescent efficiency in organic light emitting devices is obtained with an emitter layer comprising organometallic complexes of transition metals of formula L2MX, wherein L and X are distinct bidentate ligands. Compounds of this formula can be synthesized more facilely than in previous approaches and synthetic options allow insertion of fluorescent molecules into a phosphorescent complex, ligands to fine tune the color of emission, and ligands to trap carriers.
Owner:THE TRUSTEES FOR PRINCETON UNIV +1
Method for producing alcohols by hydrogenation of carbonyl compounds
InactiveUS6486366B1Less amountHigh strengthSugar derivativesOrganic compound preparationCobaltPt element
A method for preparation of alcohols by catalytic hydrogenation of carbonyl compounds with hydrogen or hydrogen-containing gases in the presence of a hydrogenation catalyst of Raney type, where the catalyst is used in the form of hollow bodies, Preferred as catalytically active components are nickel, cobalt, copper, iron, platinum, palladium or ruthenium.
Owner:DEGUSSA AG
Radiopaque nitinol alloys for medical devices
Owner:ABBOTT CARDIOVASCULAR
Organometallic platinum complexes for phosphorescence based organic light emitting devices
InactiveUS6911271B1Improved electroluminescenceStrong couplingIndium organic compoundsDischarge tube luminescnet screensPlatinum complexOrganic light emitting device
A device for producing electroluminescence comprising an organic light emitting device including an emissive layer comprising an organometallic compound comprised of a metal bound to a single carbon-coordination ligand, with the single carbon-coordination ligand being a mono-anionic carbon-coordination ligand.
Owner:THE TRUSTEES FOR PRINCETON UNIV
Layered noble metal-containing exhaust gas catalyst and its preparation
InactiveUS6294140B1Shorten recovery timeImprove conversion efficiencyOrganic chemistryNitrogen compoundsCerium(IV) oxideEngineering
A catalyst for treating exhaust gas from an internal combustion engine includes a carrier body coated with an inner layer and an outer layer. The inner layer includes platinum deposited on a first support material and on a first oxygen storage component, and the outer layer includes platinum and rhodium deposited on a second support material and on a second oxygen storage component. The first and second support materials may be the same or different, and may be selected from the group of: silica, alumina, titania, zirconia, mixed oxides or mixtures thereof, and zirconia-rich zirconia / ceria mixed oxide. The first and second oxygen storage components may include ceria-rich ceria / zirconia mixed oxide compounds, optionally including praseodymia, yttria, neodymia, lanthana or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
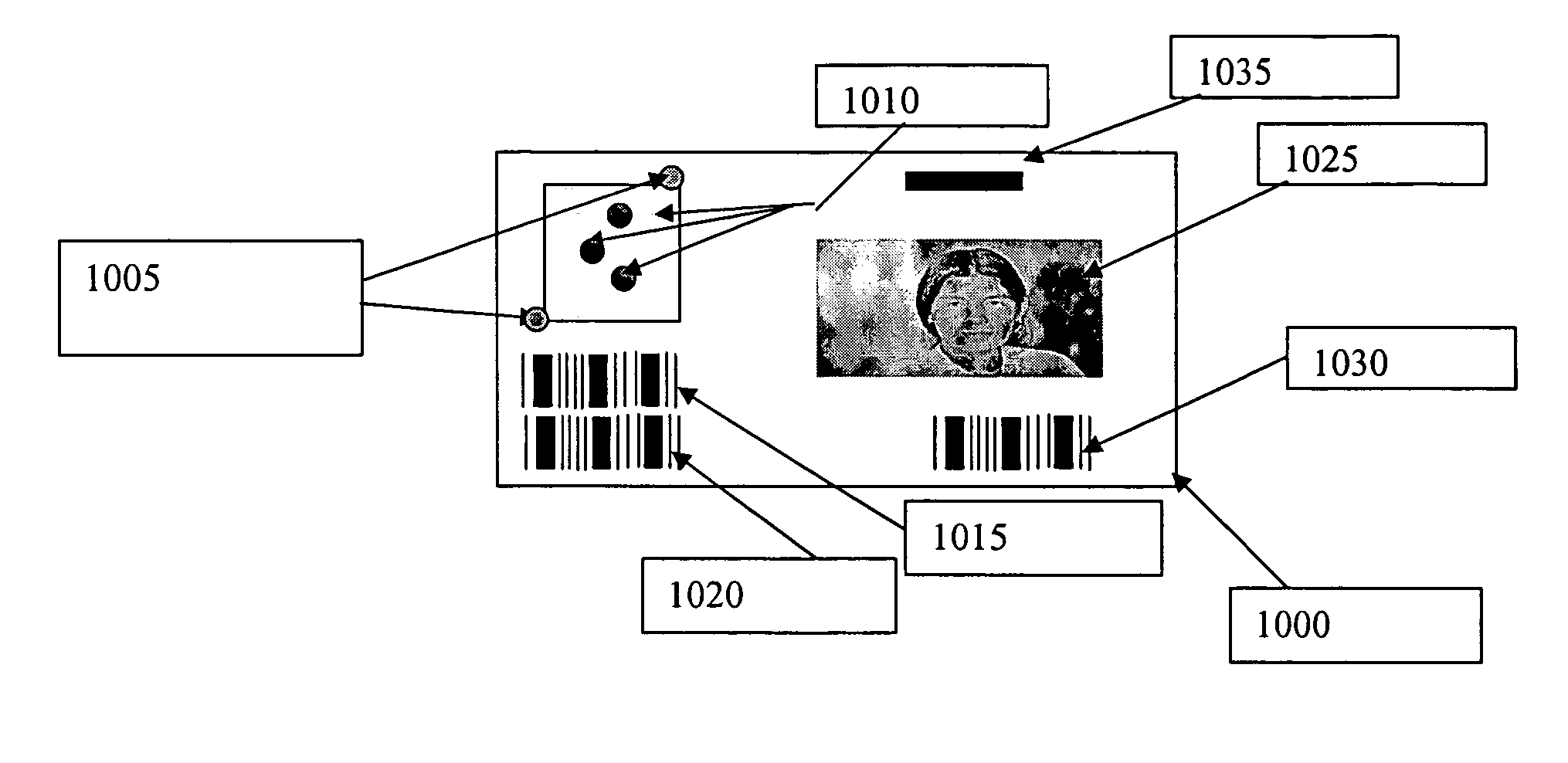
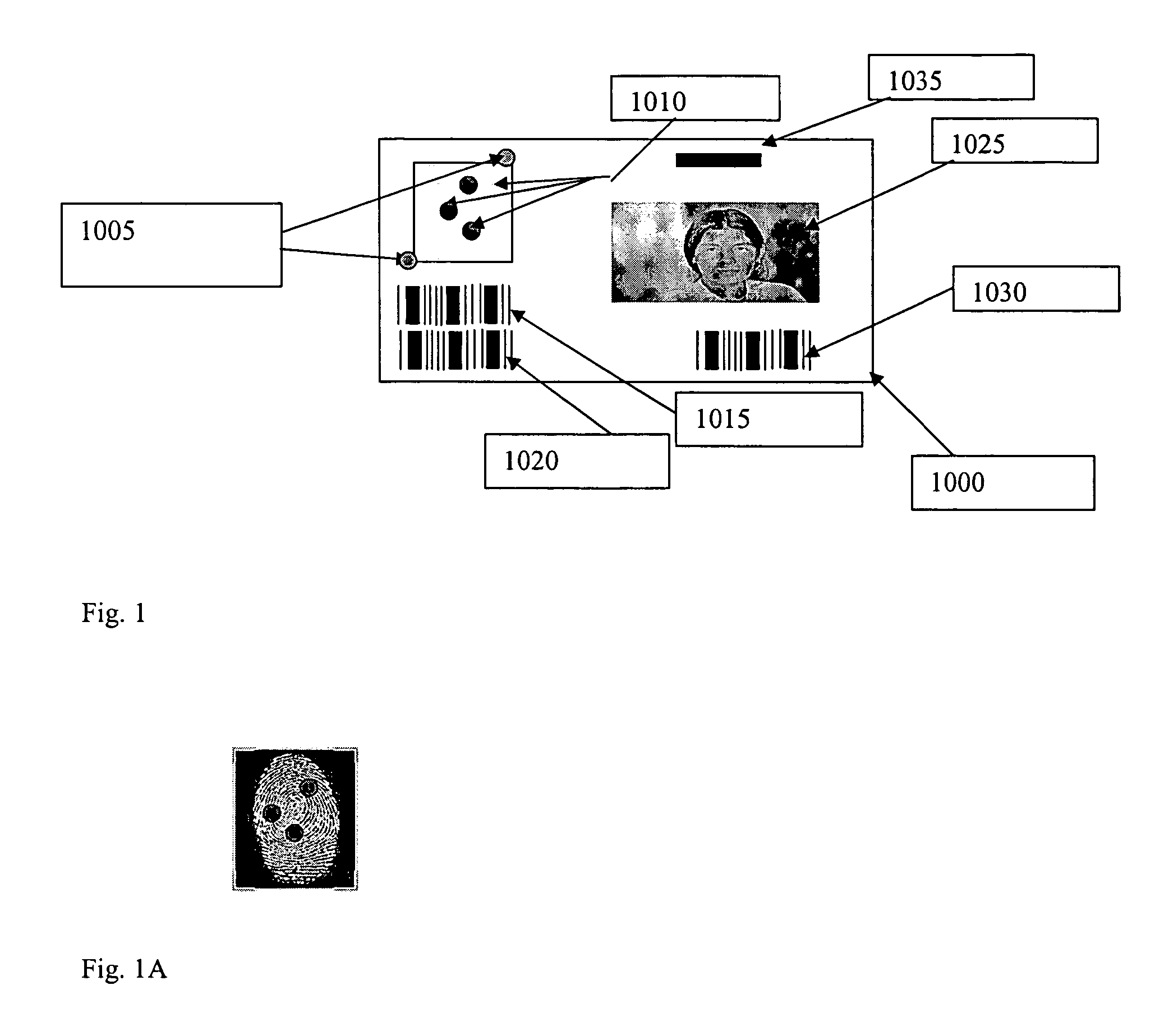
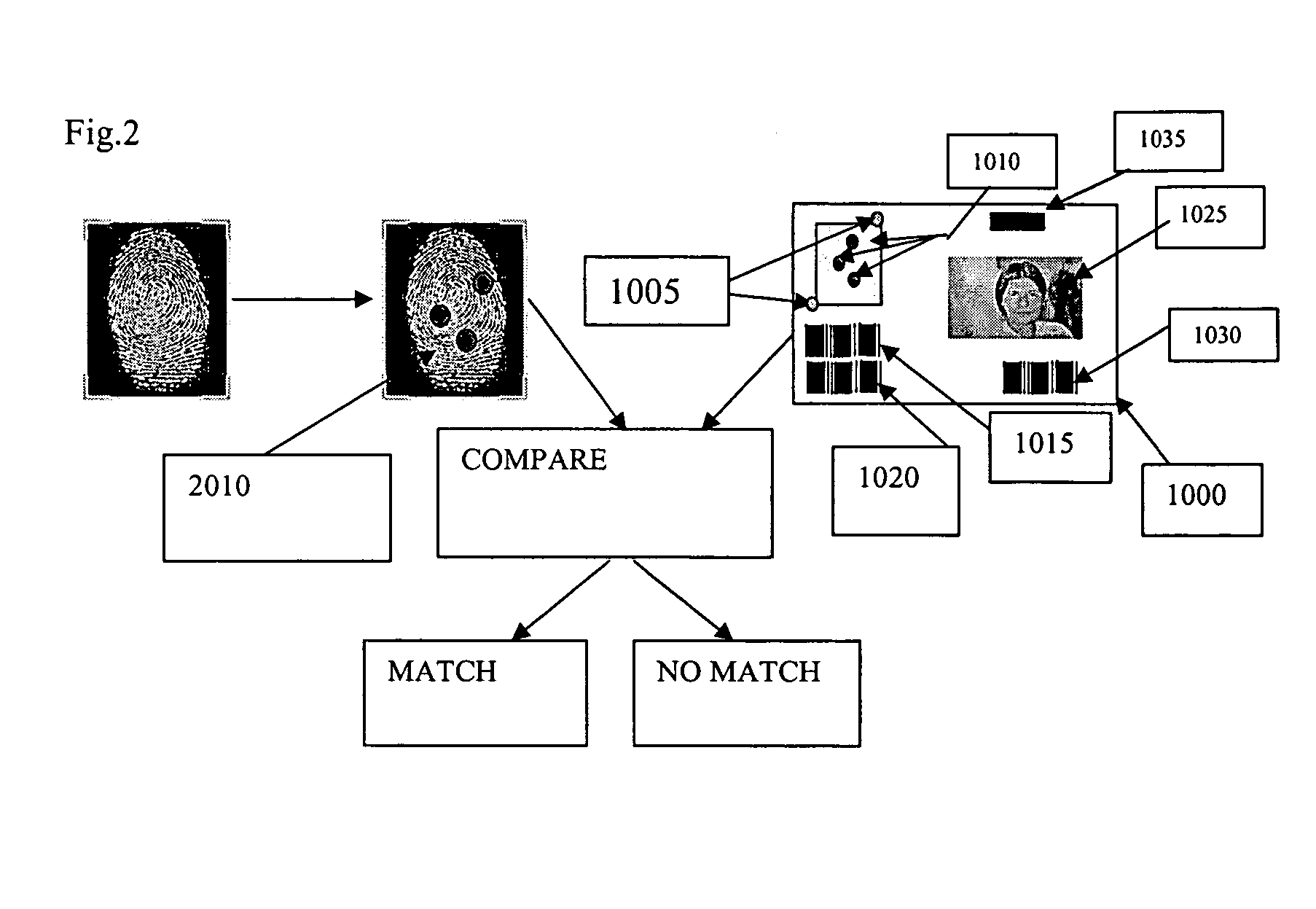
Security marking and security mark
InactiveUS20050156318A1Semiconductor/solid-state device detailsNanoinformaticsPlatinumProduct Identifier
The application discloses a security mark consisting of a plurality of layers, of which the cover layers are highly conductive films and the layers of the card core are films of varying transparency. One layer carries information, which can be read directly, if desired, above a security print, while the transparent conductive layer has an additional security markings, such as biometric or product identifiers marking which can be read conductively only with the aid of a special reader. All the layers consist of polymers, papers or mixtures which can be fused together to form a laminate which is fused together. The conductive layers form conductive traces which may be formed with single-walled or multi walled nano tubes or they can be formed from multiple layers or dispersions containing, carbon nano tubes, carbon nano tubes / antimony tin oxide, carbon nano tubes / platinum, or carbon nano tubes / silver, carbon, silver or carbon nano tubes / silver-cloride. An alternative layer can be formed from a separate conductive layer or suitable dispersion and the encoding accomplished by overlaying a nonconductive trace.
Owner:DOUGLAS JOEL S
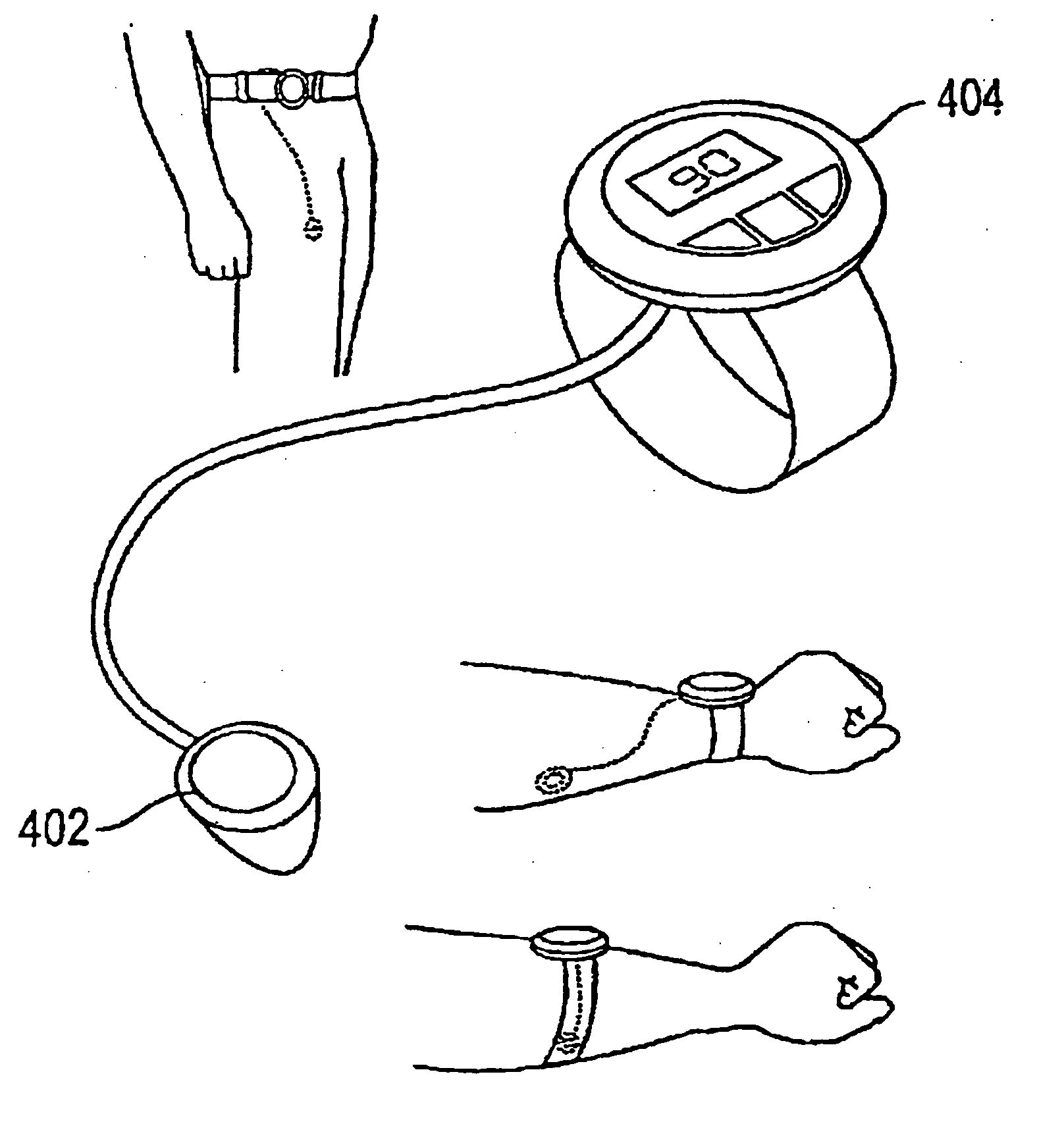
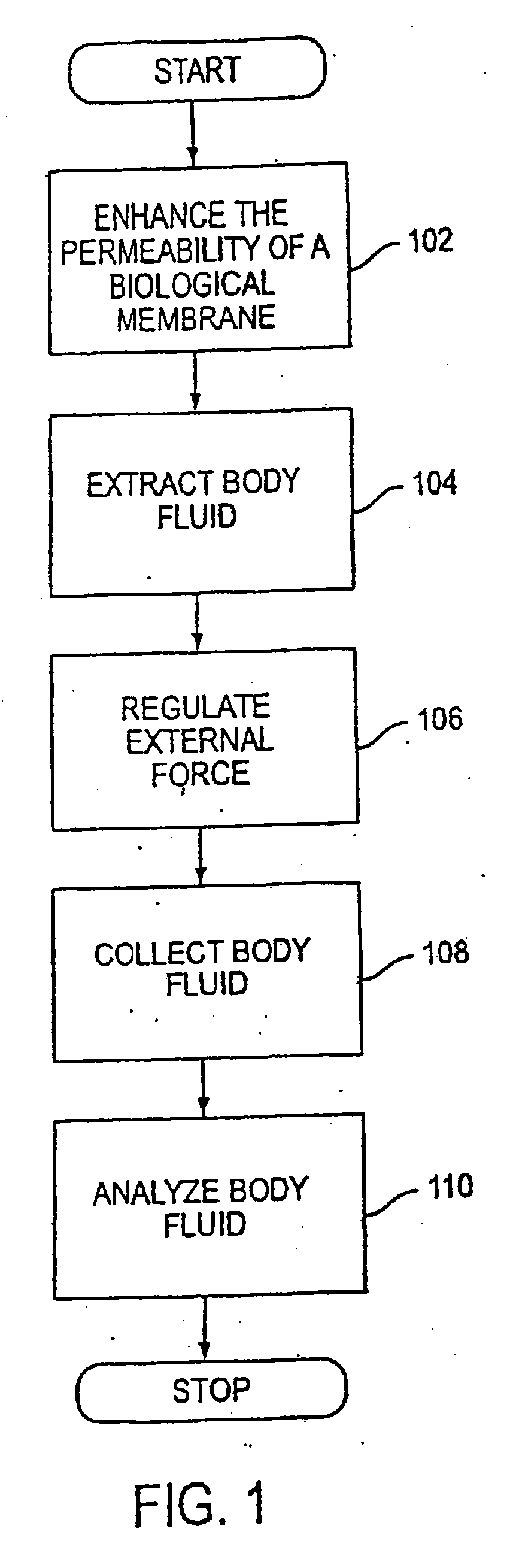
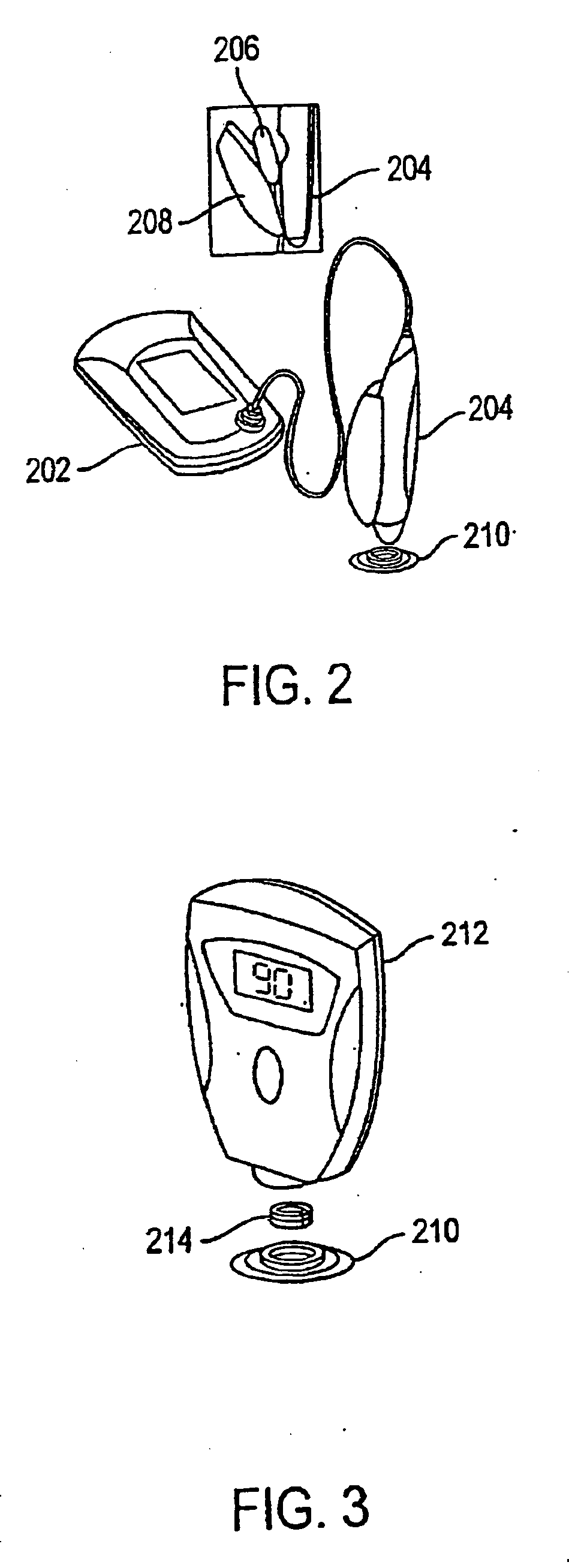
System and method for analyte sampling and analysis with hydrogel
InactiveUS20060094946A1Microbiological testing/measurementVolume/mass flow measurementPolyethylene glycolCorrection method
The invention relates to a transdermal analyte monitoring system comprising a medium adapted to interface with a biological membrane and to receive an analyte from the biological membrane and an electrode assembly comprising a plurality of electrodes, wherein the medium is adapted to react continuously with the analyte, an electrical signal is detected by the electrode assembly, and the electrical signal correlates to an analyte value. The analyte value may be the flux of the analyte through the biological membrane or the concentration of the analyte in a body fluid of a subject. The medium may comprise a vinyl acetate based hydrogel, an agarose based hydrogel, or a polyethylene glycol diacrylate (PEG-DA) based hydrogel, for example. The surface region of the electrode may comprise pure platinum. The system may include an interference filter located between the biological membrane and the electrode assembly for reducing interference in the system. The system may comprise a processor programmed to implement an error correction method that corrects for sensor drift.
Owner:ECHO THERAPEUTICS INC
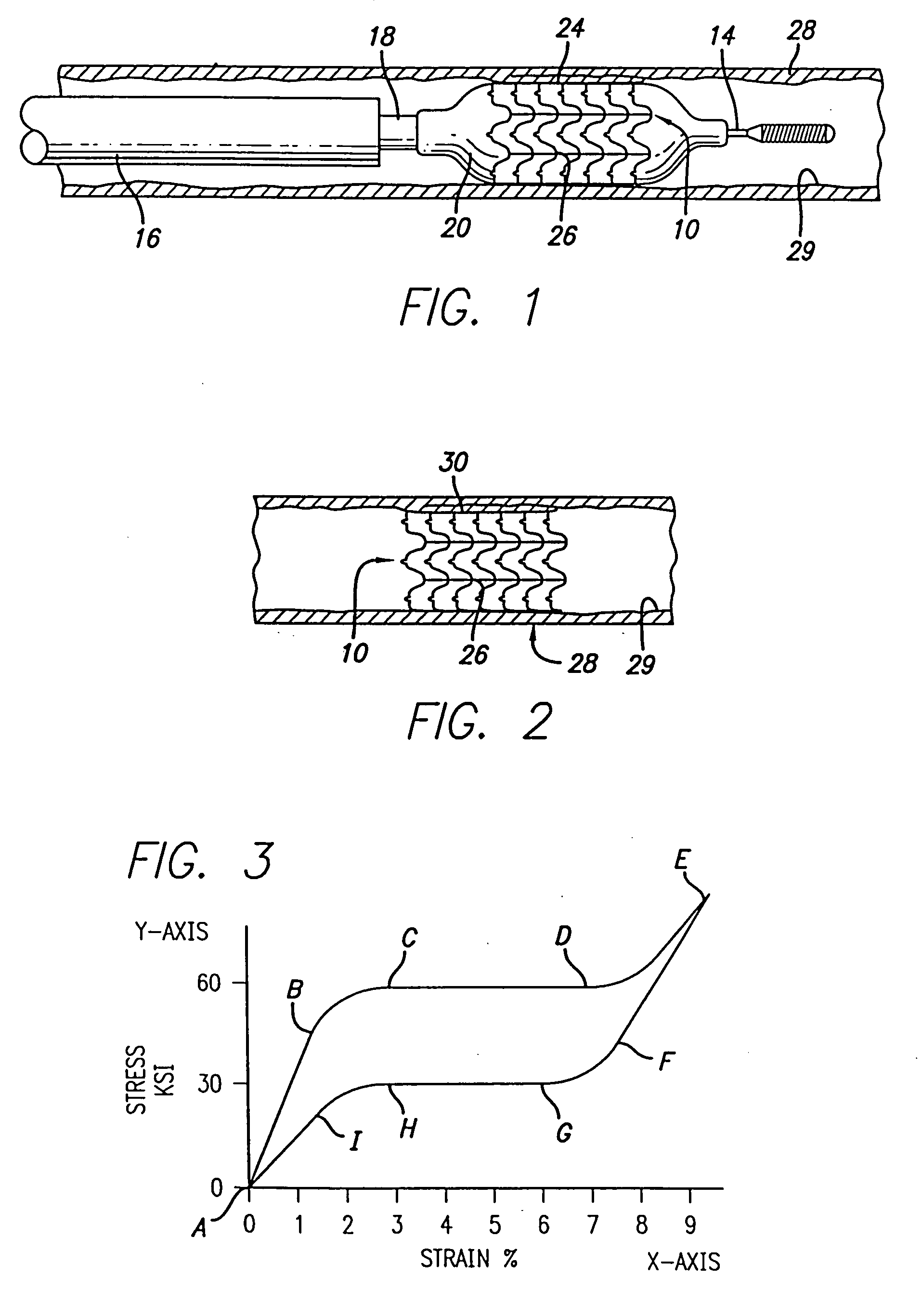
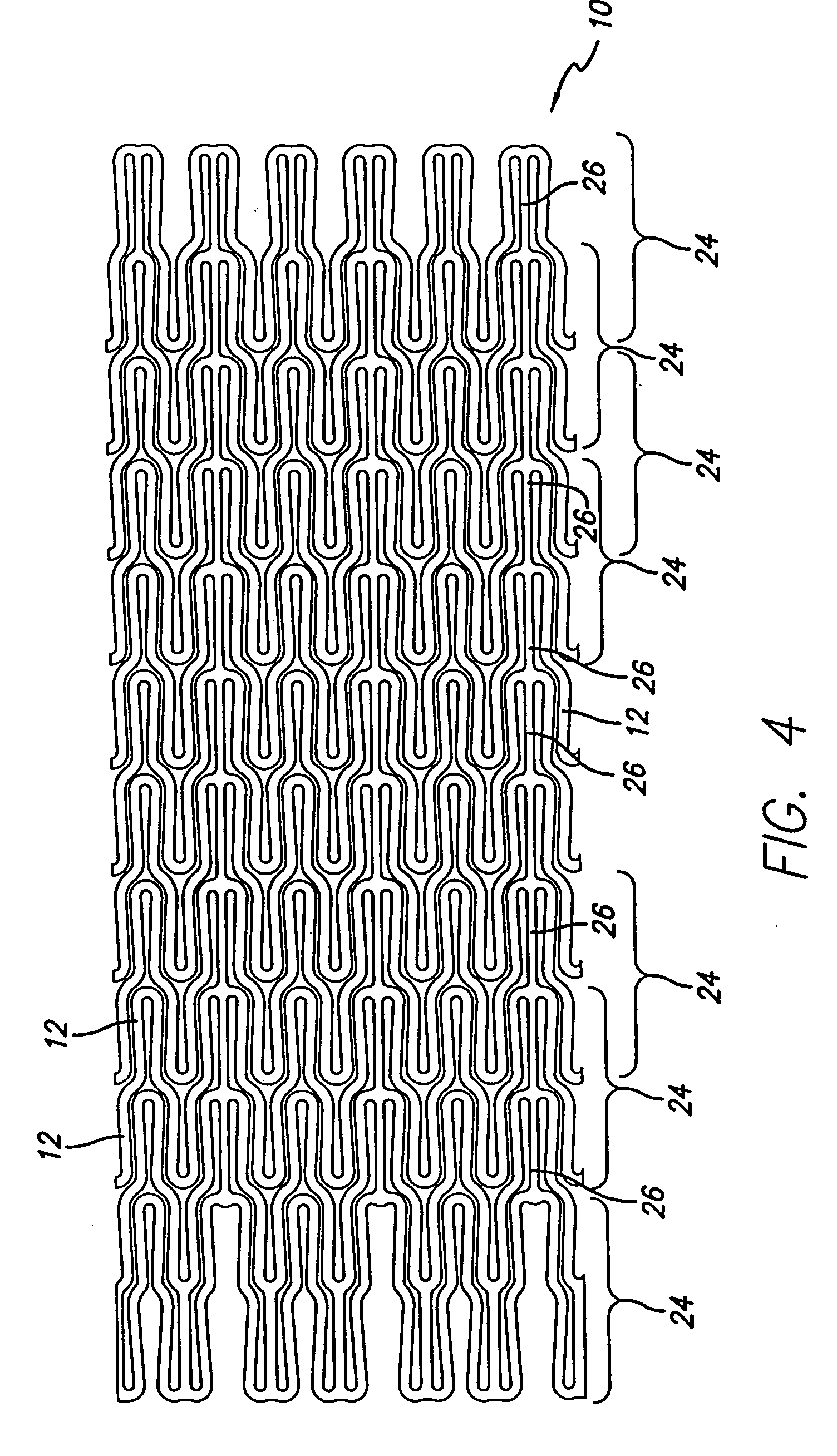
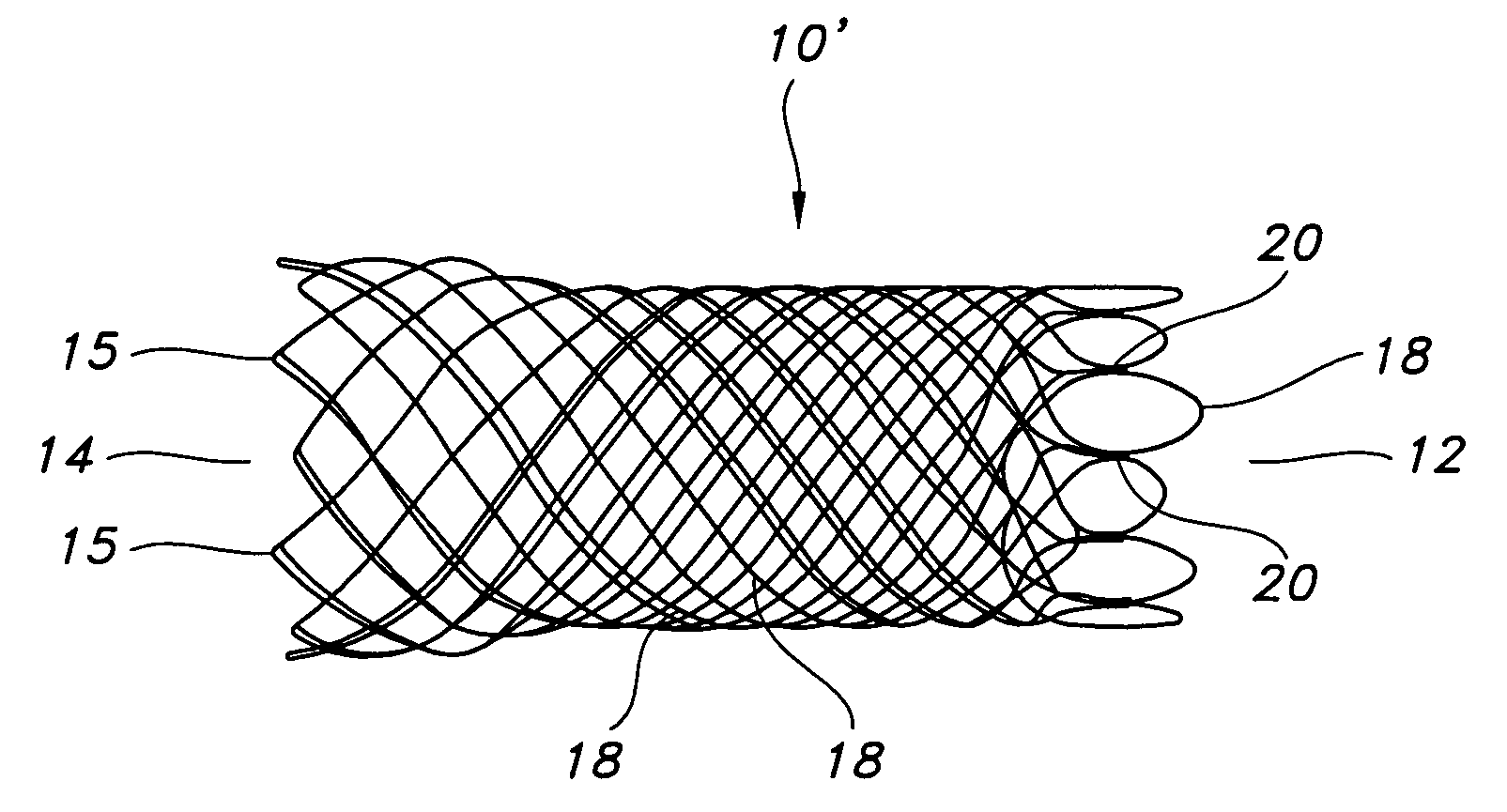
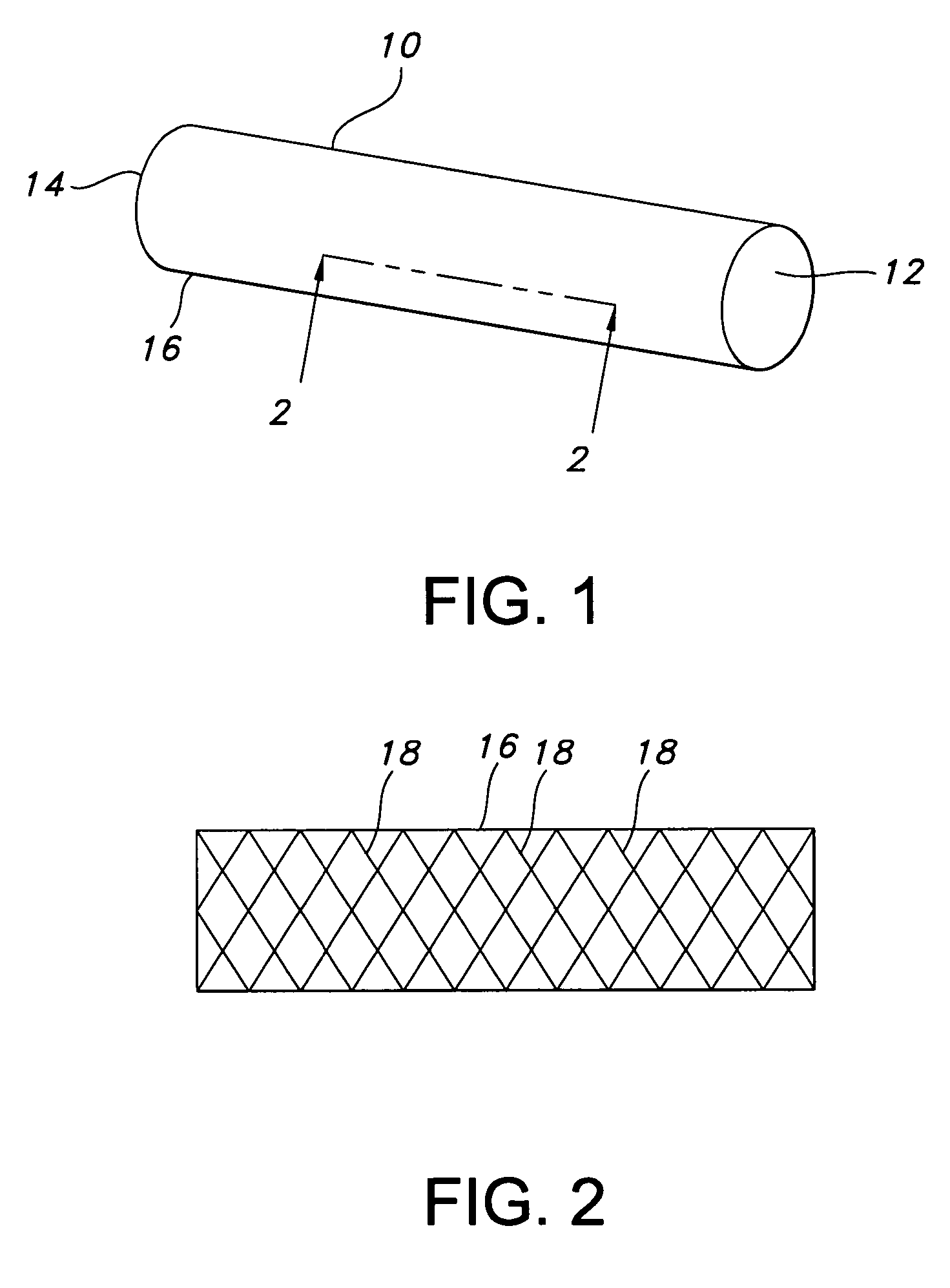
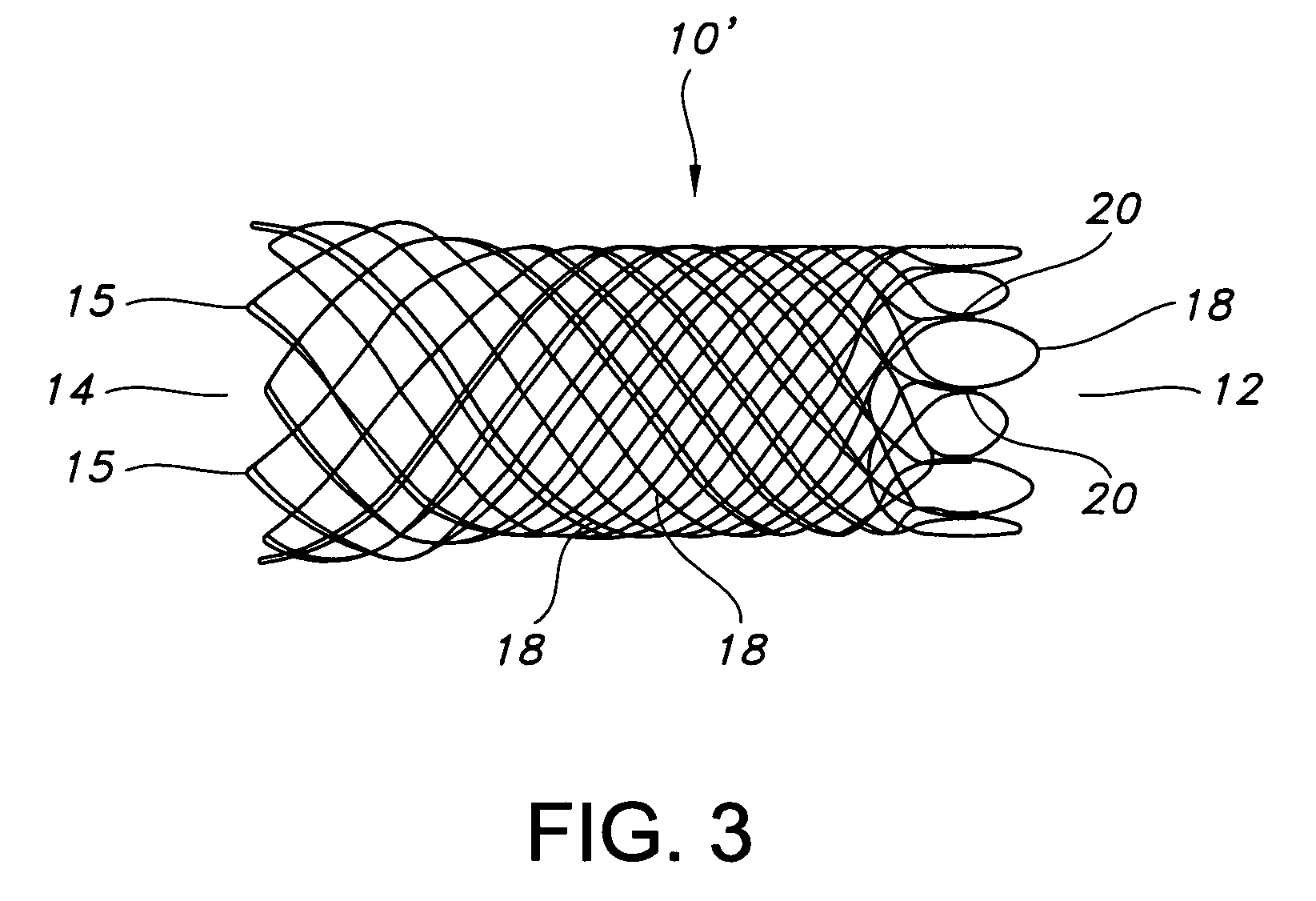
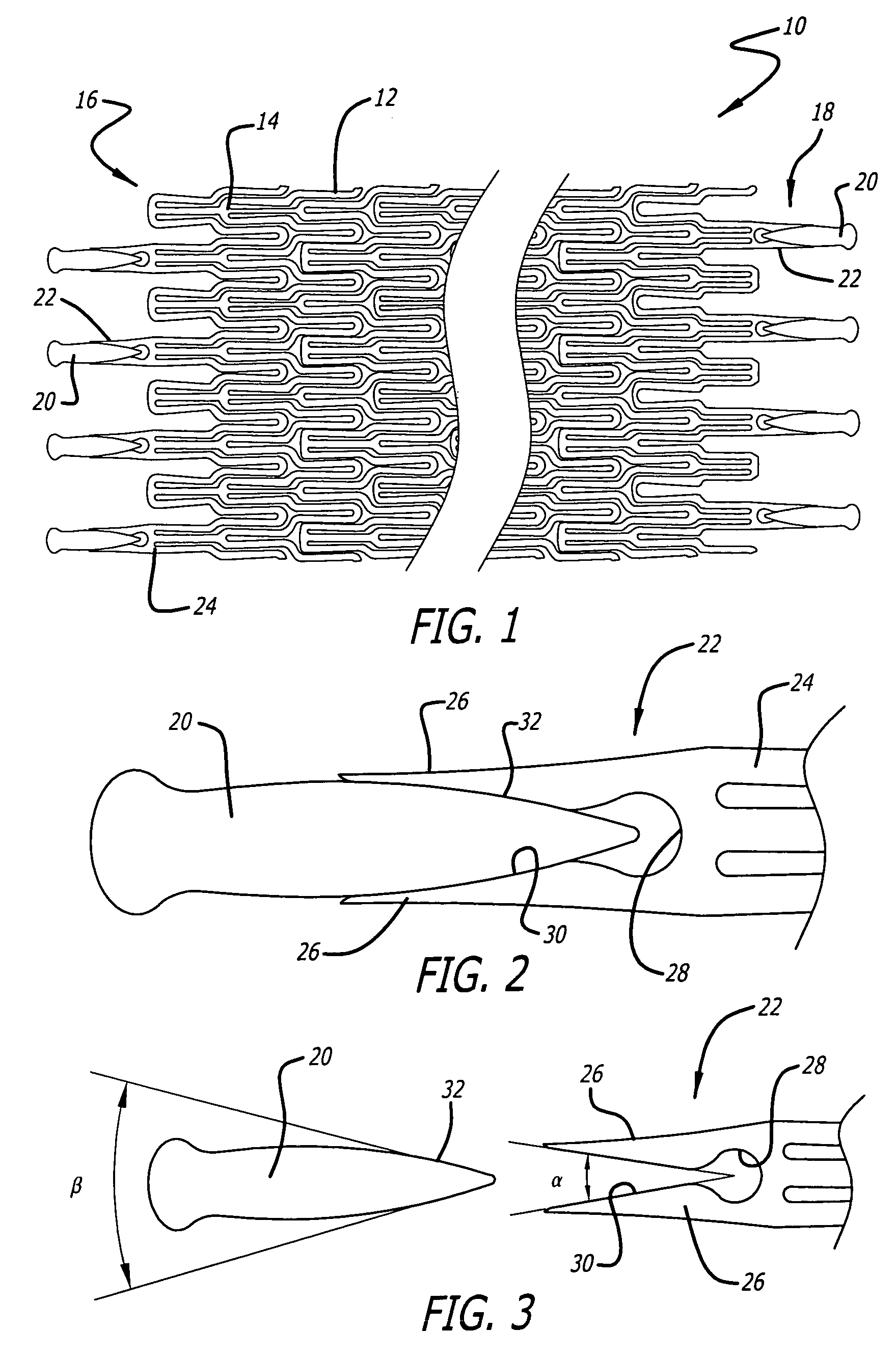
Atraumatic stent with reduced deployment force, method for making the same and method and apparatus for deploying and positioning the stent
An implantable stent includes a plurality of elongate wires braided to form a hollow tubular structure having a tubular wall to define an interior surface and an exterior surface and having opposed open first and second ends, wherein the opposed open first and second ends are atraumatic ends The atraumatic ends of the stent are desirably free of any loose wire ends. The wires include composite wires to enhance visibility of the wires to provide improved external imaging of the wires in the body. The elongate composite wires of the stent may be metallic wires having an outer metallic portion including a first metal, such as nitinol, and an inner metallic core portion including a second metal, which is a radiopaque material, such as gold, barium sulfate, ferritic particles, platinum, platinum-tungsten, palladium, platinum-iridium, rhodium, tantalum or combinations thereof.
Owner:BOSTON SCI SCIMED INC
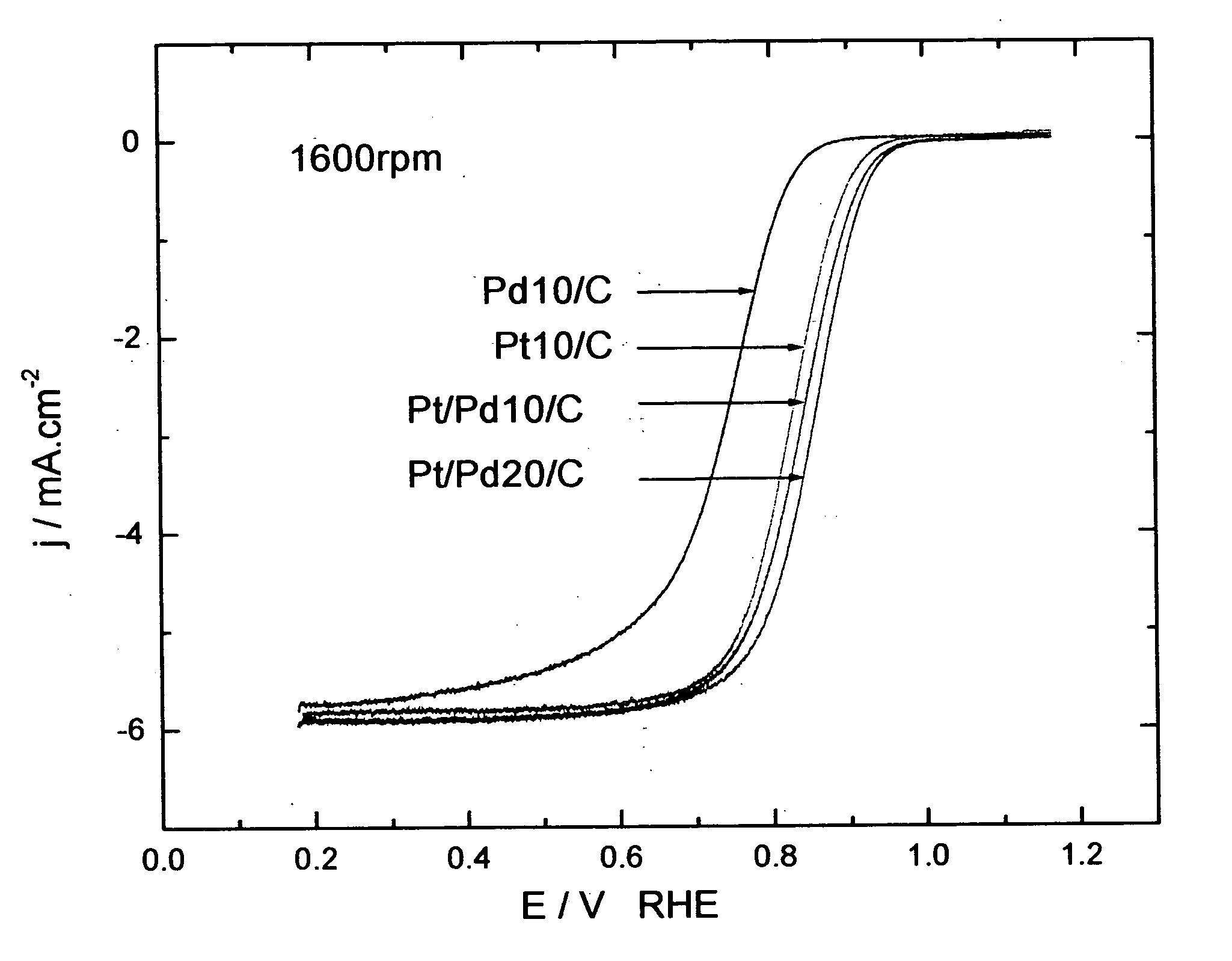
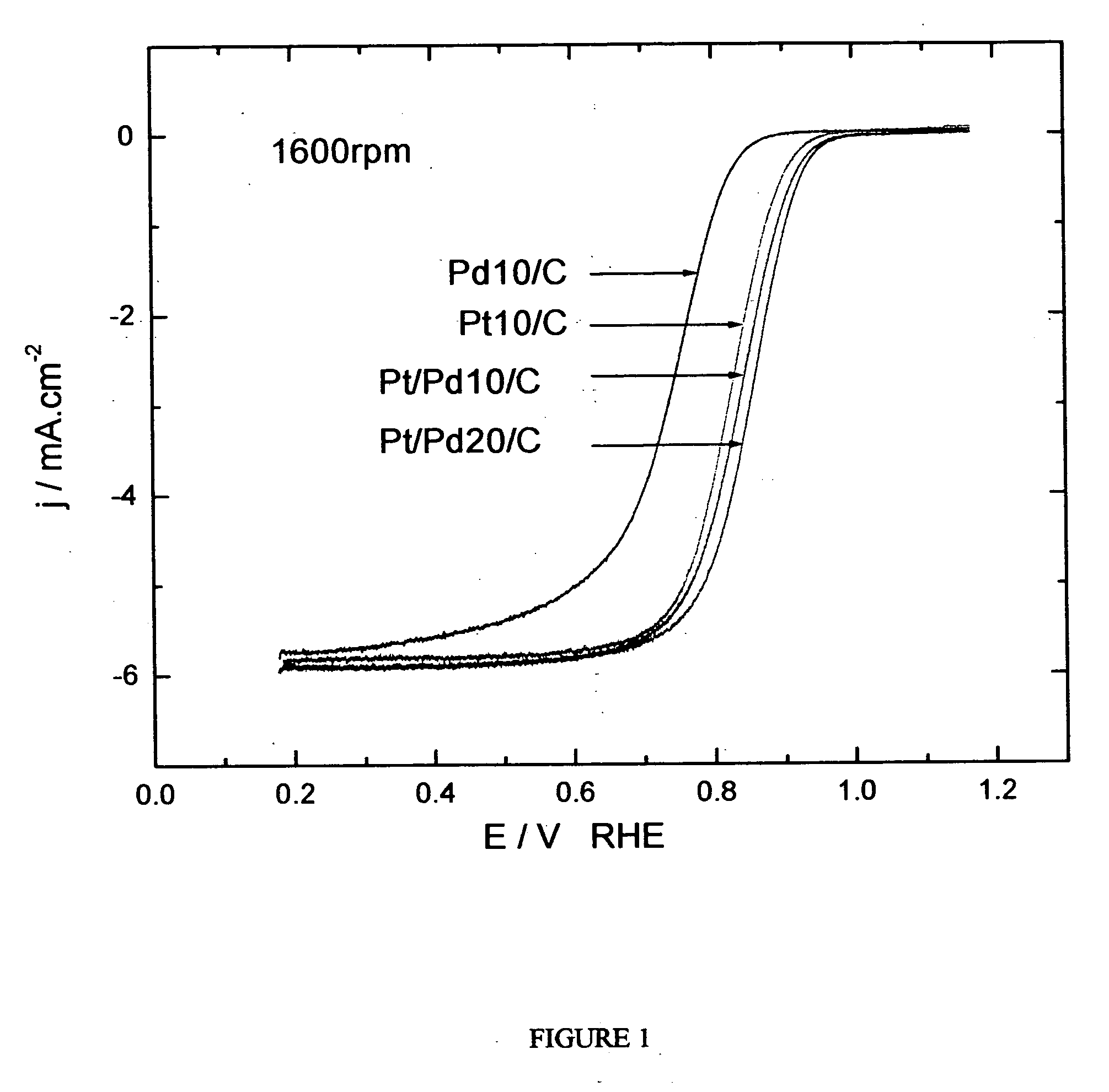
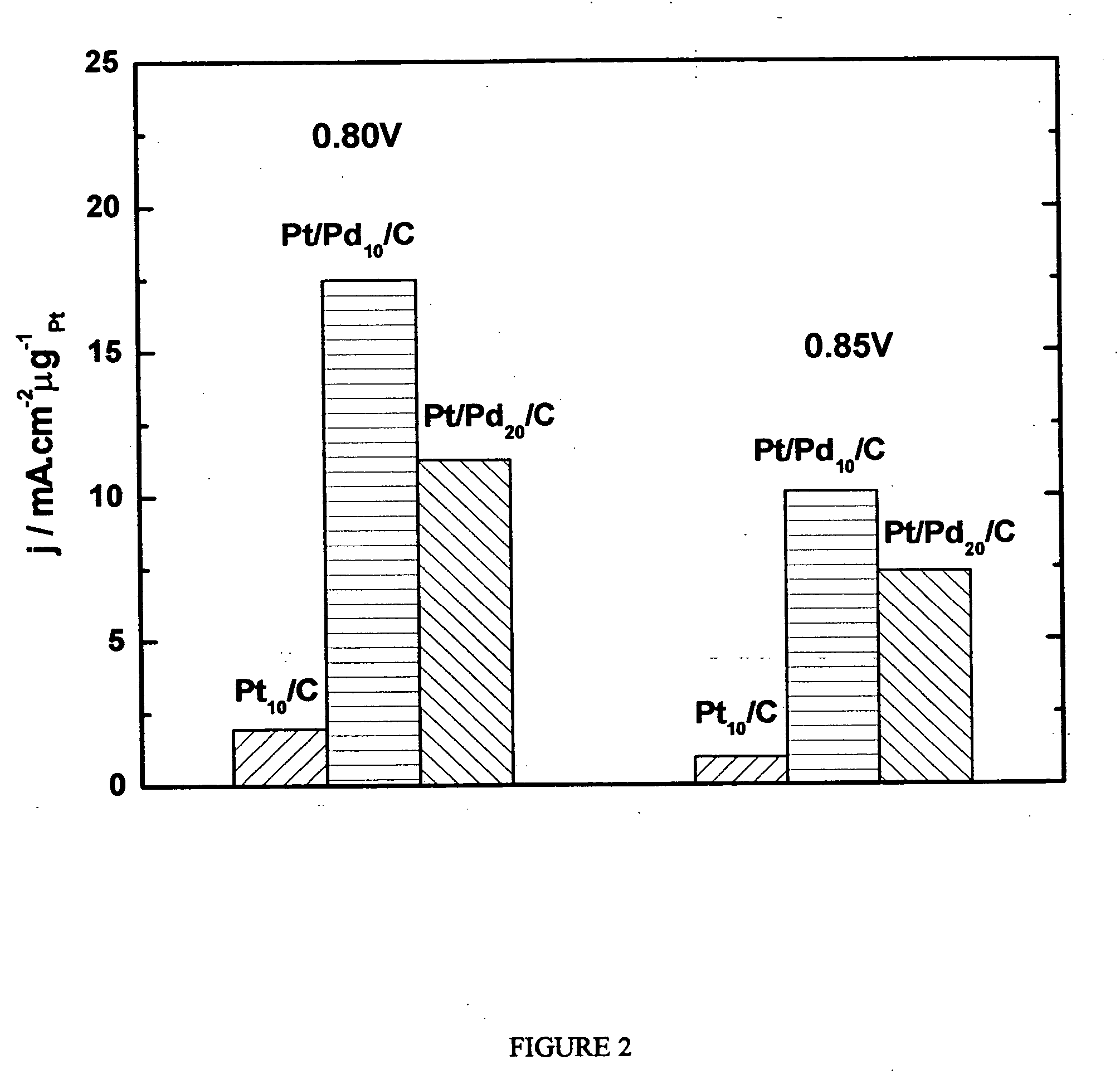
Electrocatalysts having platinum monolayers on palladium, palladium alloy, and gold alloy nanoparticle cores, and uses thereof
ActiveUS20070031722A1Improved oxygen-reducing catalytic activityLow platinum loadingMetal-working apparatusActive material electrodesRheniumGold alloys
The invention relates to platinum-coated particles useful as fuel cell electrocatalysts. The particles are composed of a noble metal or metal alloy core at least partially encapsulated by an atomically thin surface layer of platinum atoms. The invention particularly relates to such particles having a palladium, palladium alloy, gold alloy, or rhenium alloy core encapsulated by an atomic monolayer of platinum. In other embodiments, the invention relates to fuel cells containing these electrocatalysts and methods for generating electrical energy therefrom.
Owner:BROOKHAVEN SCI ASSOCS
Radiopaque markers for medical devices
InactiveUS20050060025A1High level of radiopacitySufficient radiopacityStentsBlood vesselsIridiumRhenium
An implantable medical device includes a structural body made from a superelastic material and includes one or more marker holders integrally formed on the structural body. Each marker holder is designed to hold a radiopaque marker which has a level of radiopacity greater than the superelastic material. The radiopaque marker can be made from a nickel-titanium alloy which includes a ternary element. The ternary element can be selected from the group of elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, and hafnium. In one form, the marker holder includes a pair of projecting fingers connected together at a notched region to cooperatively create a particular-shaped opening. This opening, in turn, is adapted to receive a similarly shaped portion formed on the radiopaque marker. In one form, the radiopaque marker includes an inner core which is partially, or completely, encased by an outer layer. This inner core can be made from a highly radiopaque material while the outer layer is formed from a material that is easier to weld to the marker.
Owner:ABBOTT VASCULAR SOLUTIONS
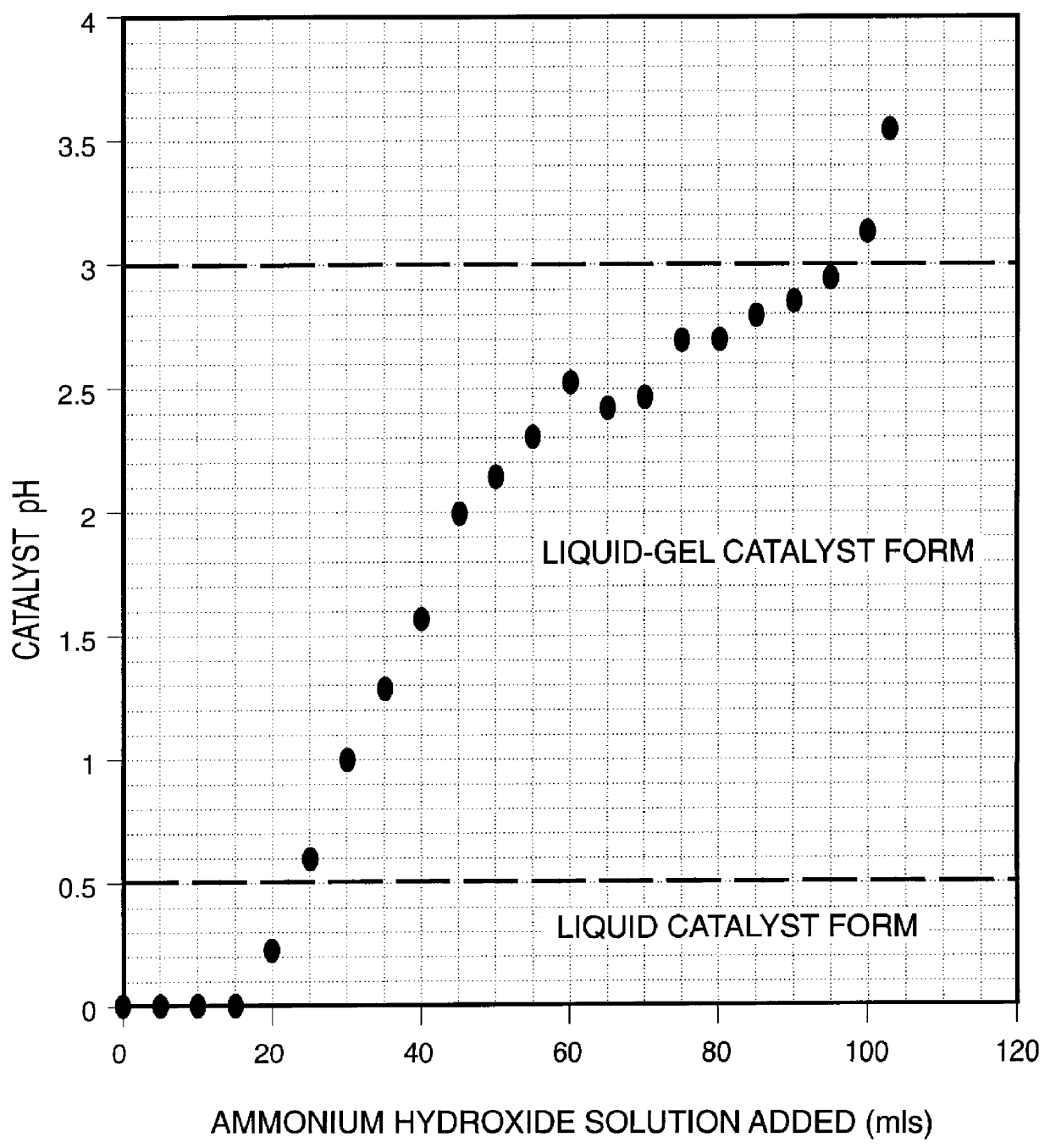
Iron-based ionic liquid catalysts for hydroprocessing carbonaceous feeds
InactiveUS6139723AIncrease hydrocracking ability of catalystIndirect and direct heating destructive distillationCatalyst activation/preparationLiquid productIron salts
A highly dispersed iron-based ionic liquid or liquid-gel catalyst which may be anion-modified and metals-promoted has high catalytic activity, and is useful for hydrocracking / hydrogenation reactions for carbonaceous feed materials. The catalyst is produced by aqueous precipitation from saturated iron salt solutions such as ferric sulfate and ferric alum, and may be modified during preparation with anionic sulfate (SO42-) and promoted with small percentages of at least one active metal such as cobalt, molybdenum, palladium, platinum, nickel, or tungsten or mixtures thereof. The resulting catalyst may be used in a preferred ionic liquid form or in a liquid-gel form, and either fluidic form can be easily mixed and reacted with carbonaceous feed materials such as coal, heavy petroleum fractions, mixed plastic waste, or mixtures thereof. The invention includes methods for making the ionic liquid or liquid-gel catalyst, and processes for using the fluidic catalysts for hydroprocessing the carbonaceous feed materials to produce desirable low-boiling hydrocarbon liquid products.
Owner:HEADWATERS CTL
Platinum- and platinum alloy-coated palladium and palladium alloy particles and uses thereof
ActiveUS20060135359A1Improved oxygen-reducing catalytic activityLow platinum loadingSynthetic resin layered productsCellulosic plastic layered productsAlloyOxygen
The present invention relates to particle and nanoparticle composites useful as oxygen-reduction electrocatalysts. The particle composites are composed of a palladium or palladium-alloy particle or nanoparticle substrate coated with an atomic submonolayer, monolayer, bilayer, or trilayer of zerovalent platinum atoms. The invention also relates to a catalyst and a fuel cell containing the particle or nanoparticle composites of the invention. The invention additionally includes methods for oxygen reduction and production of electrical energy by using the particle and nanoparticle composites of the invention.
Owner:BROOKHAVEN SCI ASSOCS
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
Platinum electrode and method for manufacturing the same
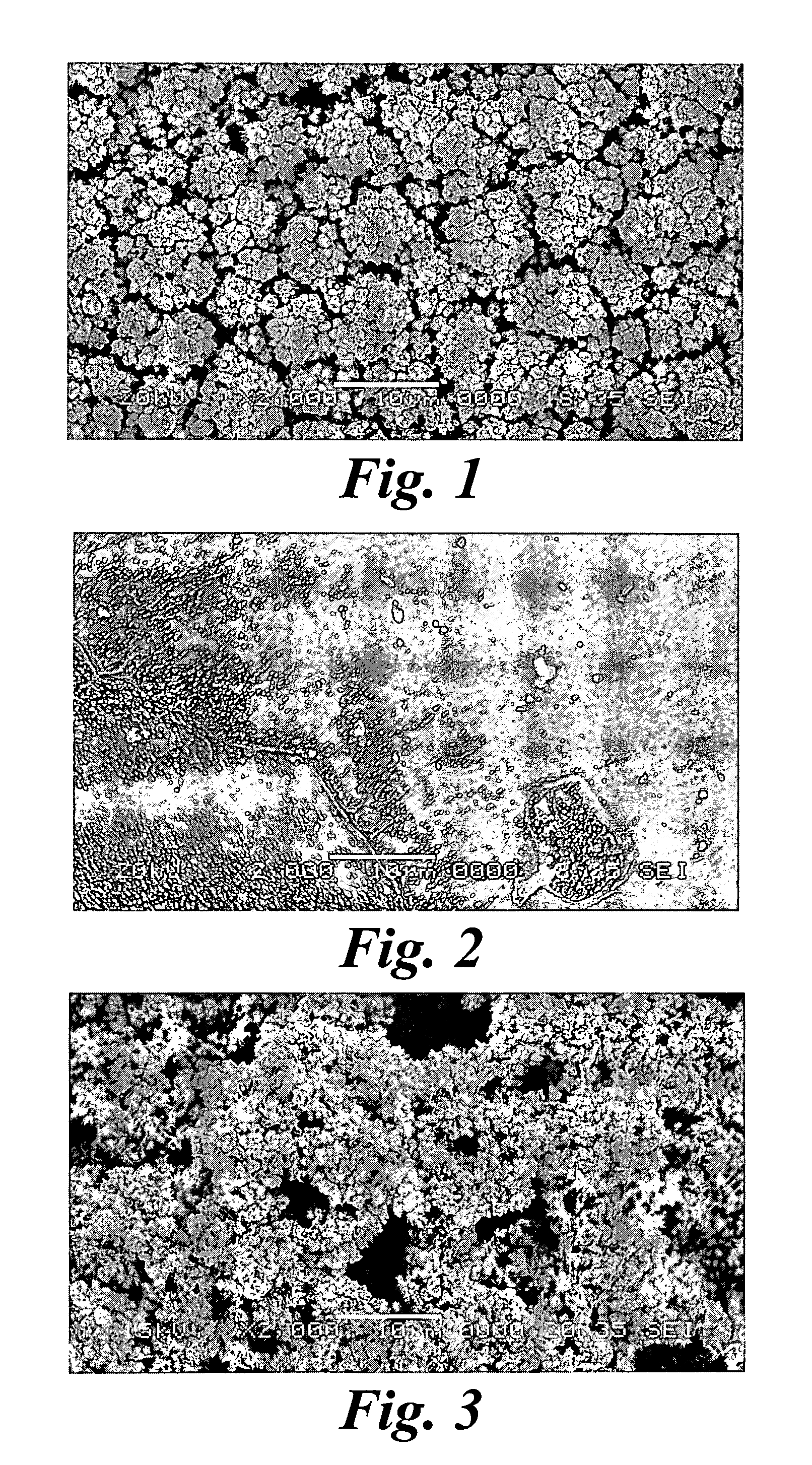
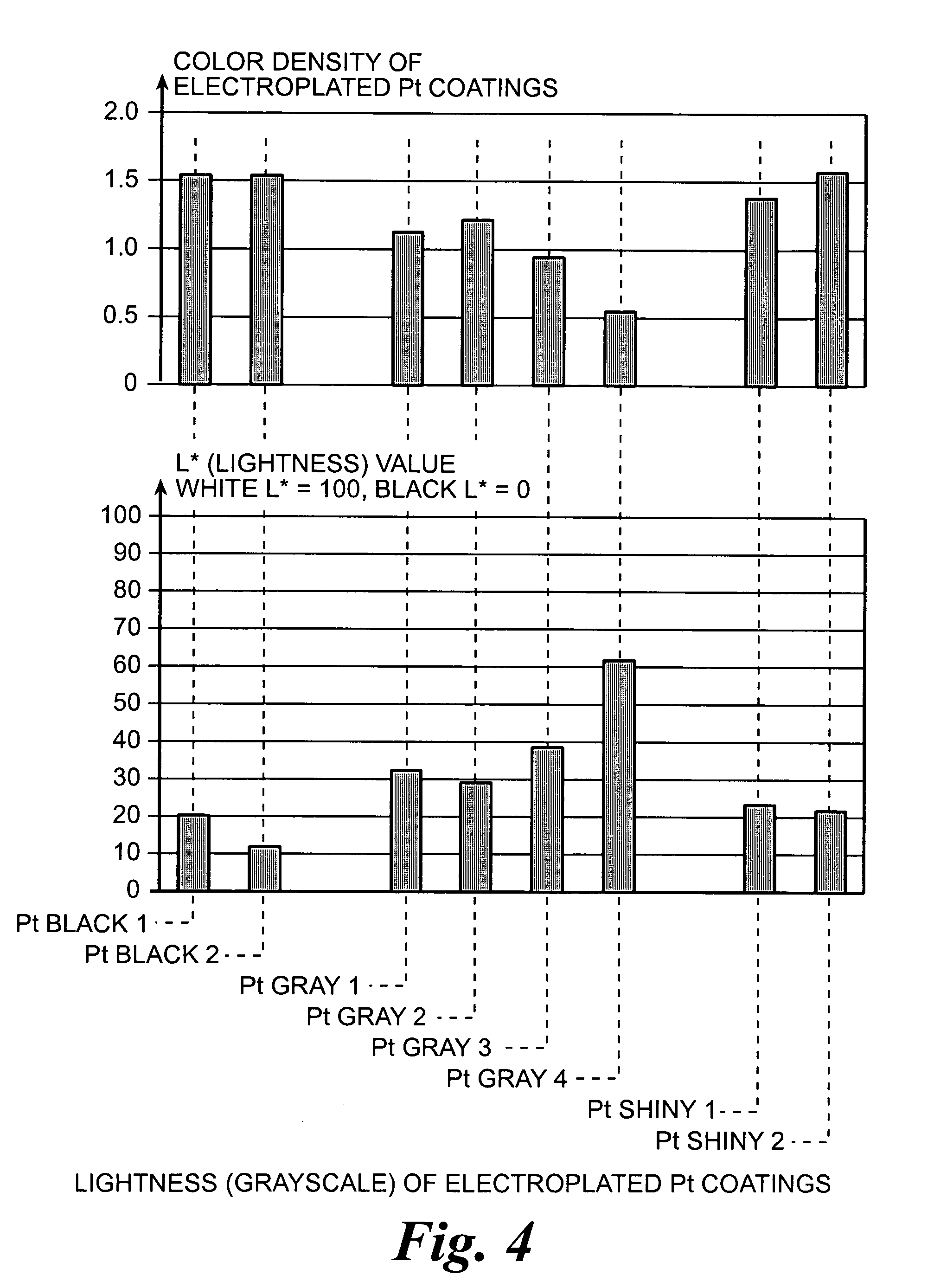
InactiveUS6974533B2Increase surface areaSufficient physical and structural strengthCellsHead electrodesMetallurgyPt element
An improved electrode and method for manufacturing the improved electrode wherein the electrode having a fractal surface coating of platinum [which the present inventor refers to as “platinum gray”] with a increase in surface area of at least 5 times when compared to shiny platinum of the same geometry and also having improved resistance to physical stress when compared to platinum black having the same surface area. The process of electroplating the surface coating of platinum gray comprising plating at a moderate rate, i.e., at a rate that is faster than the rate necessary to produce shiny platinum and that is less than the rate necessary to produce platinum black.
Owner:CORTIGENT INC
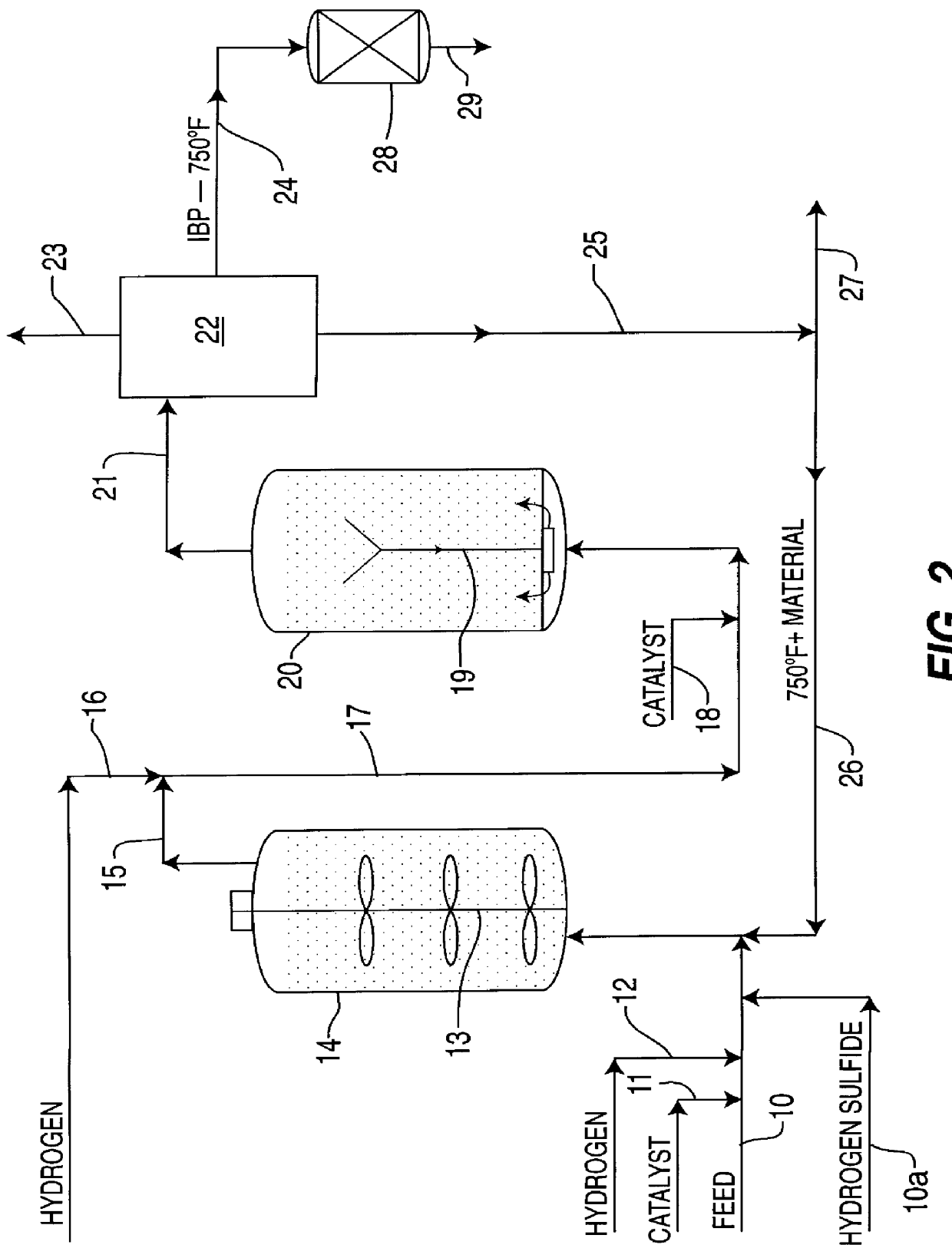
Isoparaffinic base stocks by dewaxing fischer-tropsch wax hydroisomerate over Pt/H-mordenite
A high VI and low pour point lubricant base stock is made by hydroisomerizing a high purity, waxy, paraffinic Fischer-Tropsch synthesized hydrocarbon fraction having an initial boiling point in the range of 650-750° F., followed by catalytically dewaxing the hydroisomerate using a dewaxing catalyst comprising a catalytic platinum component and an H-mordenite component. The hydrocarbon fraction is preferably synthesized by a slurry Fischer-Tropsch using a catalyst containing a catalytic cobalt component. This combination of the process, high purity, waxy paraffinic feed and the Pt / H-mordenite dewaxing catalyst, produce a relatively high yield of premium lubricant base stock.
Owner:EXXON RES & ENG CO
Low platinum fuel cell catalysts and method for preparing the same
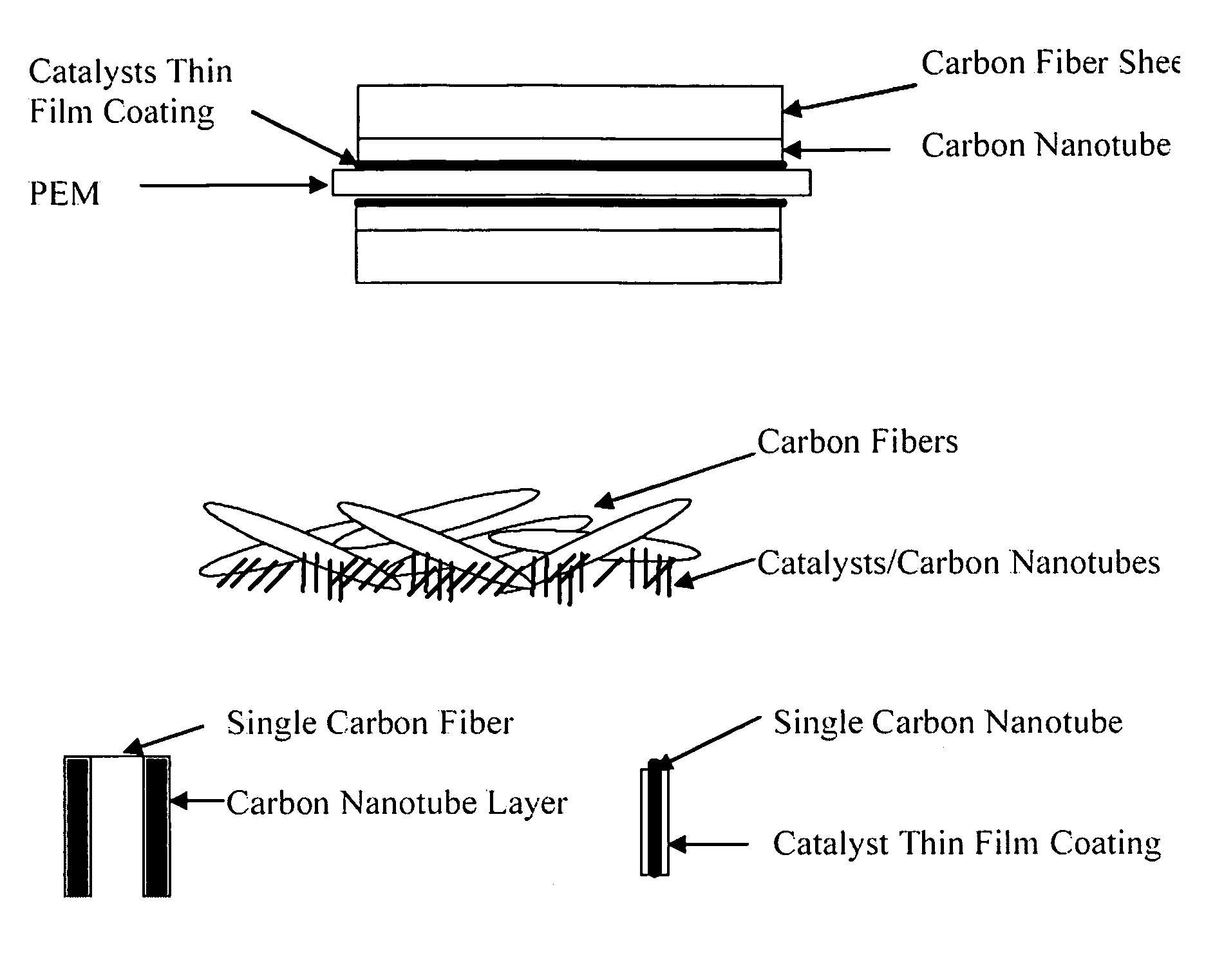
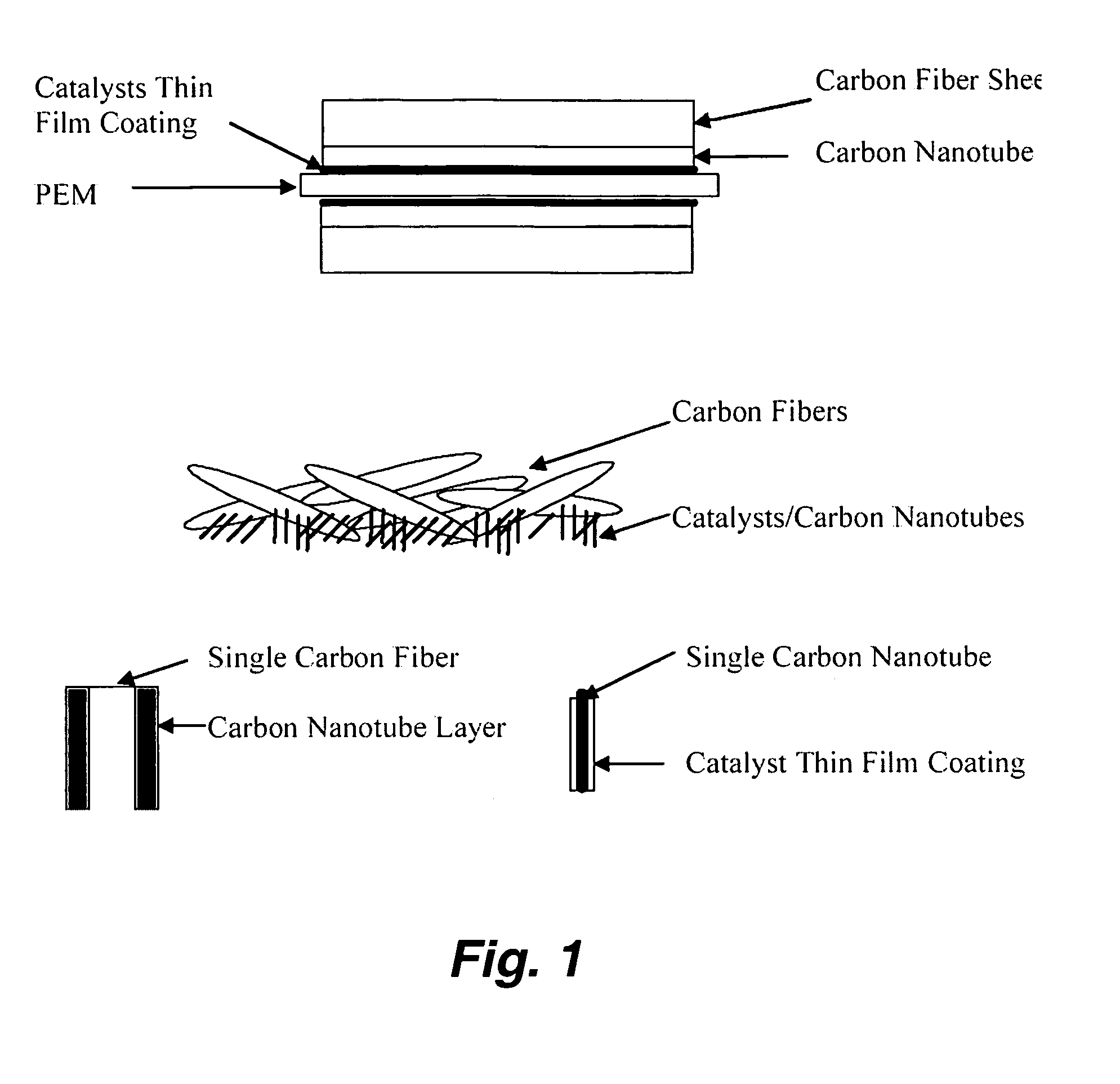
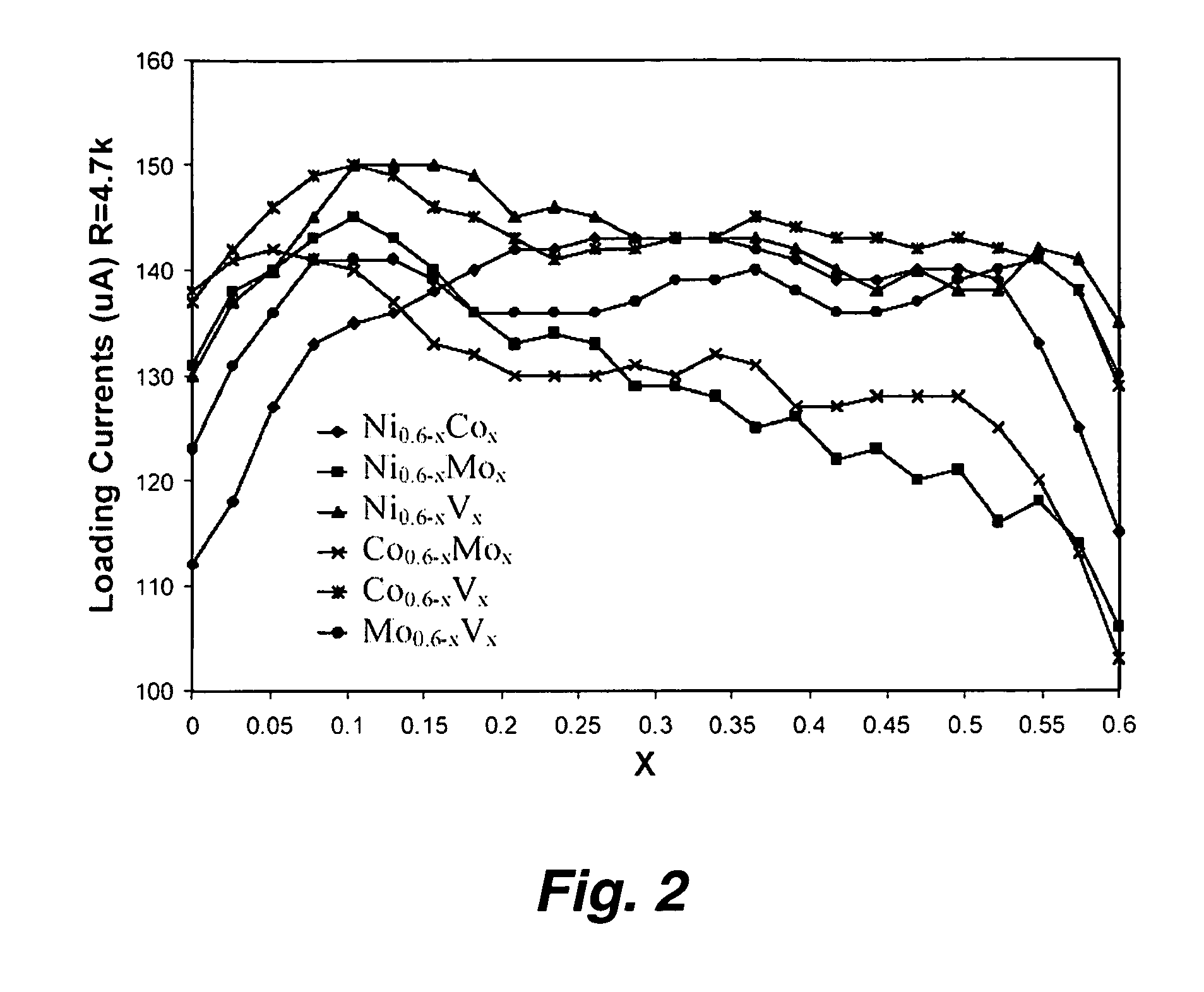
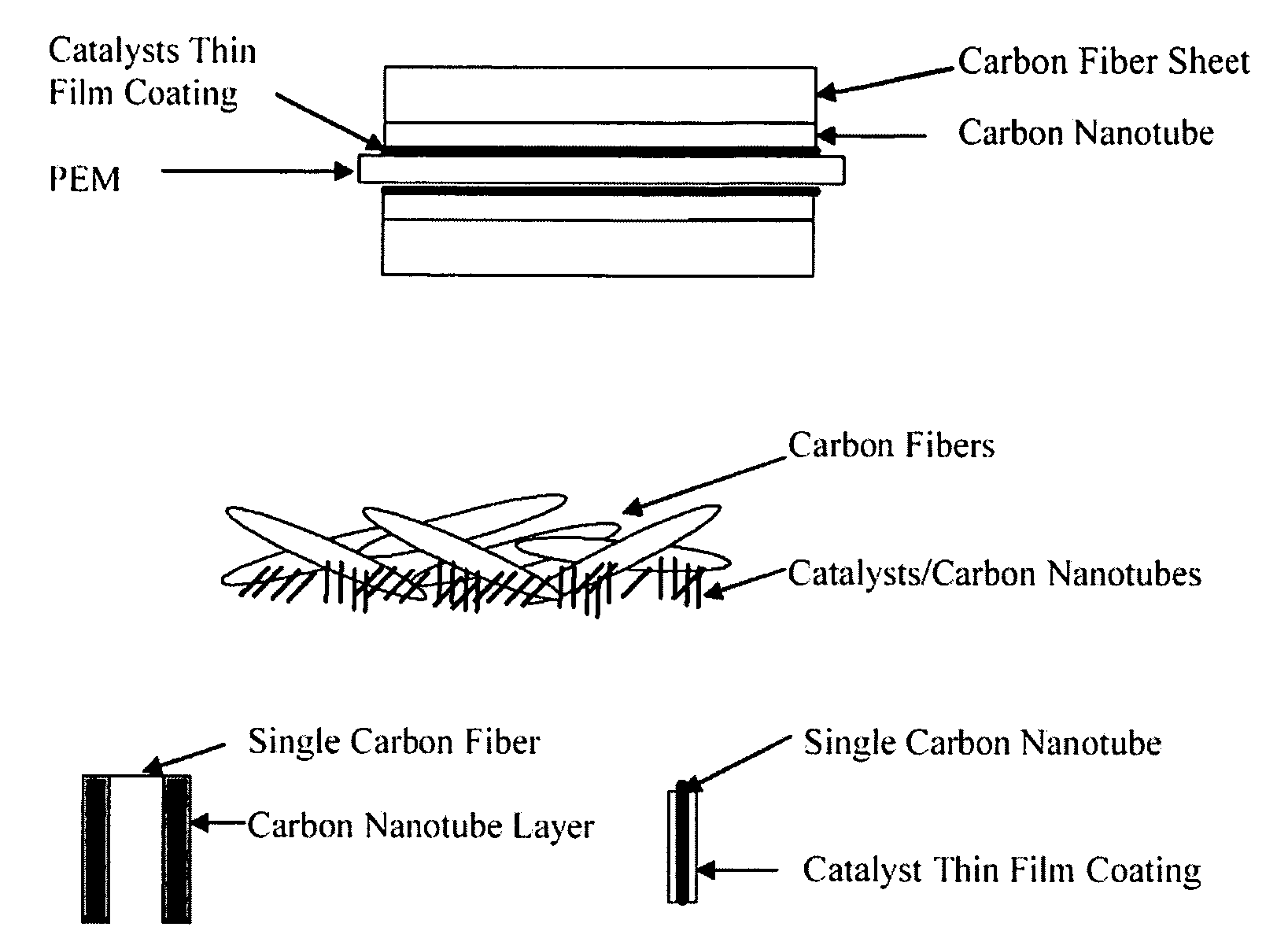
InactiveUS20050053826A1Good dispersionEfficient CatalysisMaterial nanotechnologyConductive materialFiberCarbon fibers
This invention provides novel fuel cell catalysts comprising new series of catalytically active thin-film metal alloys with low platinum concentration supported on nanostructured materials (nanoparticles). In certain embodiments, an integrated gas-diffusion / electrode / catalyst layer can be prepared by processing catalyst thin films and nanoparticales into gas-diffusion media such as Toray or SGL carbon fiber papers. The catalysts can be placed in contact with an electrolyte membrane for PEM fuel cell applications.
Owner:INTEMATIX
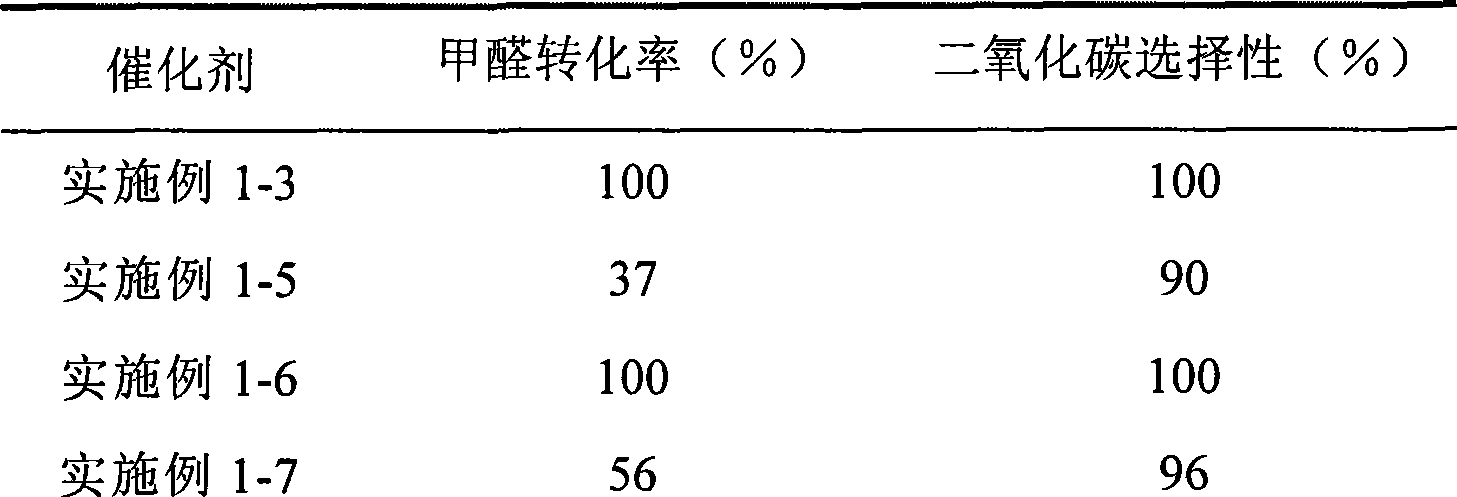
Catalyst for complete oxidation of formaldehyde at room temperature
ActiveCN101380574AEasy to makeEasy to operateDeodrantsMetal/metal-oxides/metal-hydroxide catalystsPorous carbonPt element
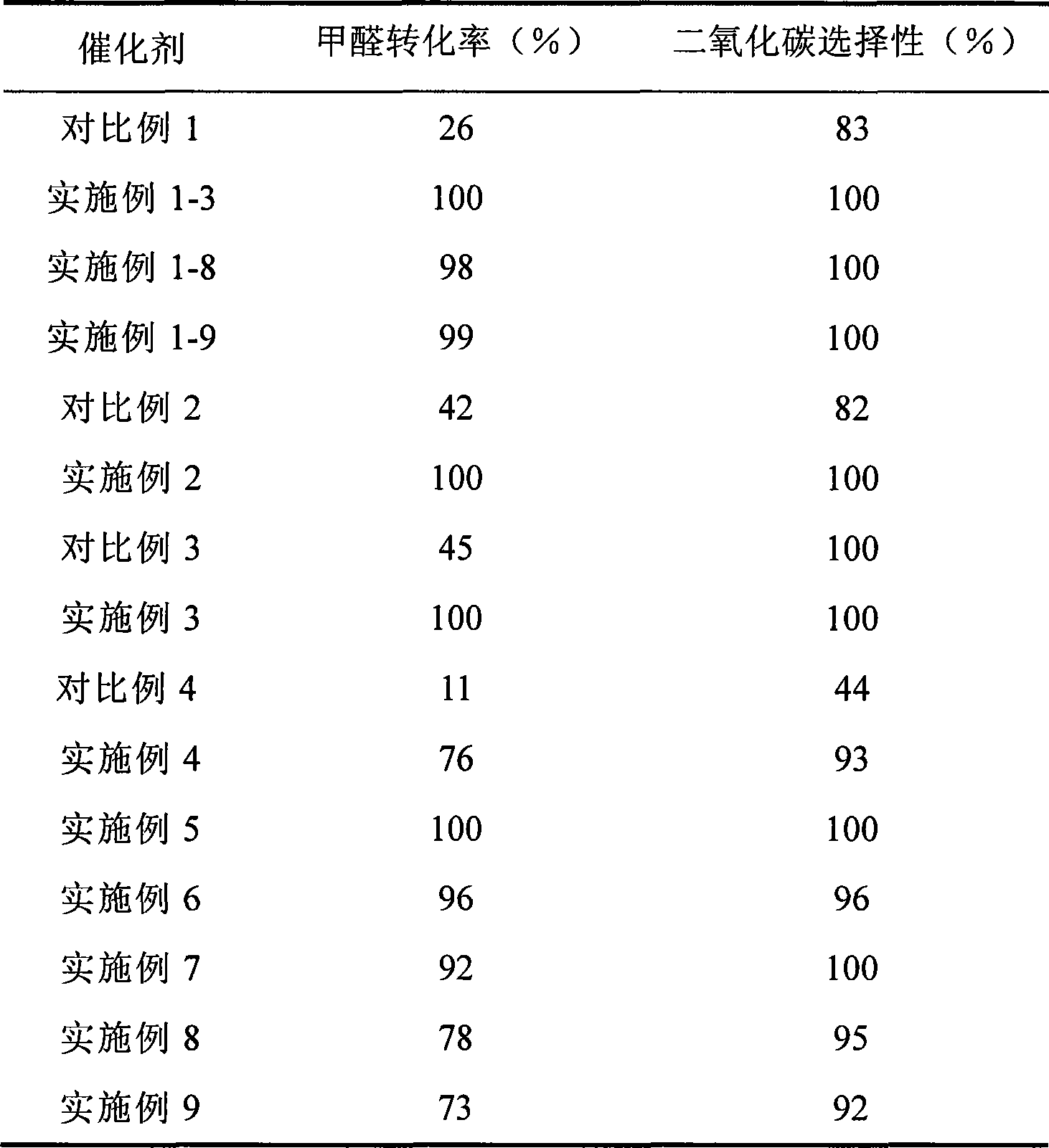
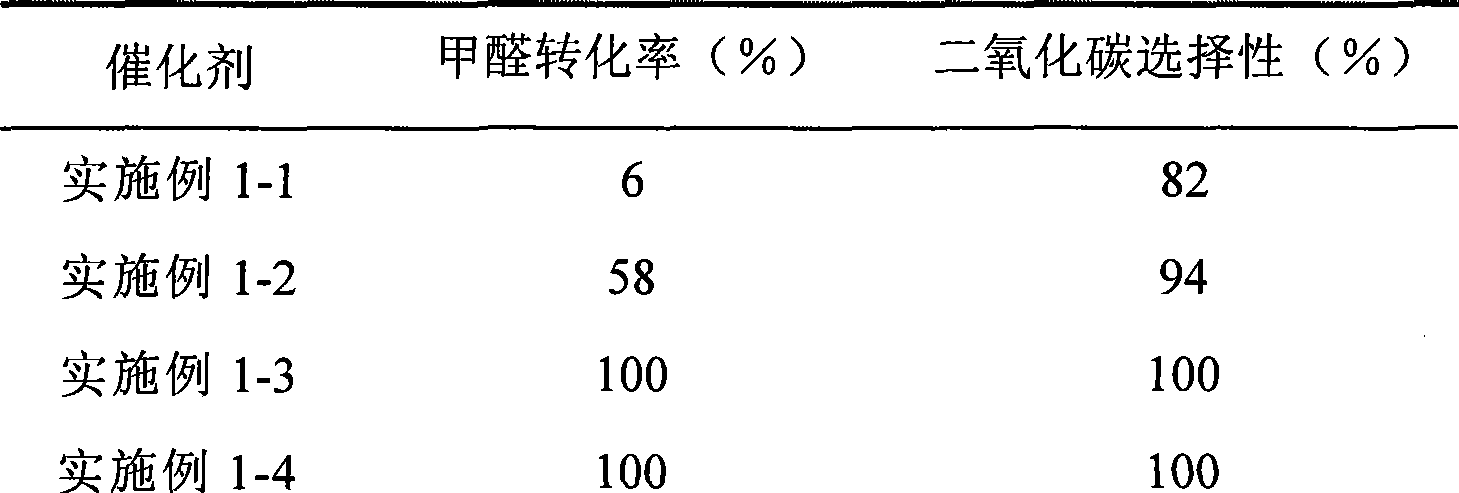
The invention provides a high selectivity catalyst used for catalyzing and completely oxidizing formaldehyde with low concentration at room temperature. The catalyst can catalyze formaldehyde completely so as to lead the formaldehyde to be converted into carbon dioxide and water at room temperature. In addition, the conversion rate of formaldehyde remains 100% within a long period of time, without complex auxiliary facilities such as light source, a heating oven and the like, and external conditions. The catalyst comprises three parts which are inorganic oxide carrier, noble metal component and auxiliary ingredient. Porous inorganic oxide carrier is one of cerium dioxide, zirconium dioxide, titanium dioxide, aluminium sesquioxide, tin dioxide, silicon dioxide, lanthanum sesquioxide, magnesium oxide and zinc oxide or the mixture thereof or composite oxide thereof, zeolite, sepiolite and porous carbon materials. The noble metal component of the catalyst is at least one of platinum, rhodium, palladium, gold and silver. The auxiliary ingredient is at least one of the alkali metals of lithium, sodium, kalium, rubidium and cesium. The loading of the noble metal component used in the catalyst of the invention is 0.1 to 10% according to weight converter of metal elements and the selective preference is 0.3 to 2%. The loading of the auxiliary ingredient is 0.2 to 30% according to weight converter of metal elements and the selective preference is 1 to 10%. When the loading of the auxiliary ingredient is lower than 0.2% or higher than 30%, the activity of the catalyst for catalyzing and oxidizing formaldehyde at room temperature is decreased remarkably.
Owner:广东顺德中科鸿图环境材料有限公司
Transparent electrode for the radiofrequency ablation of tissue
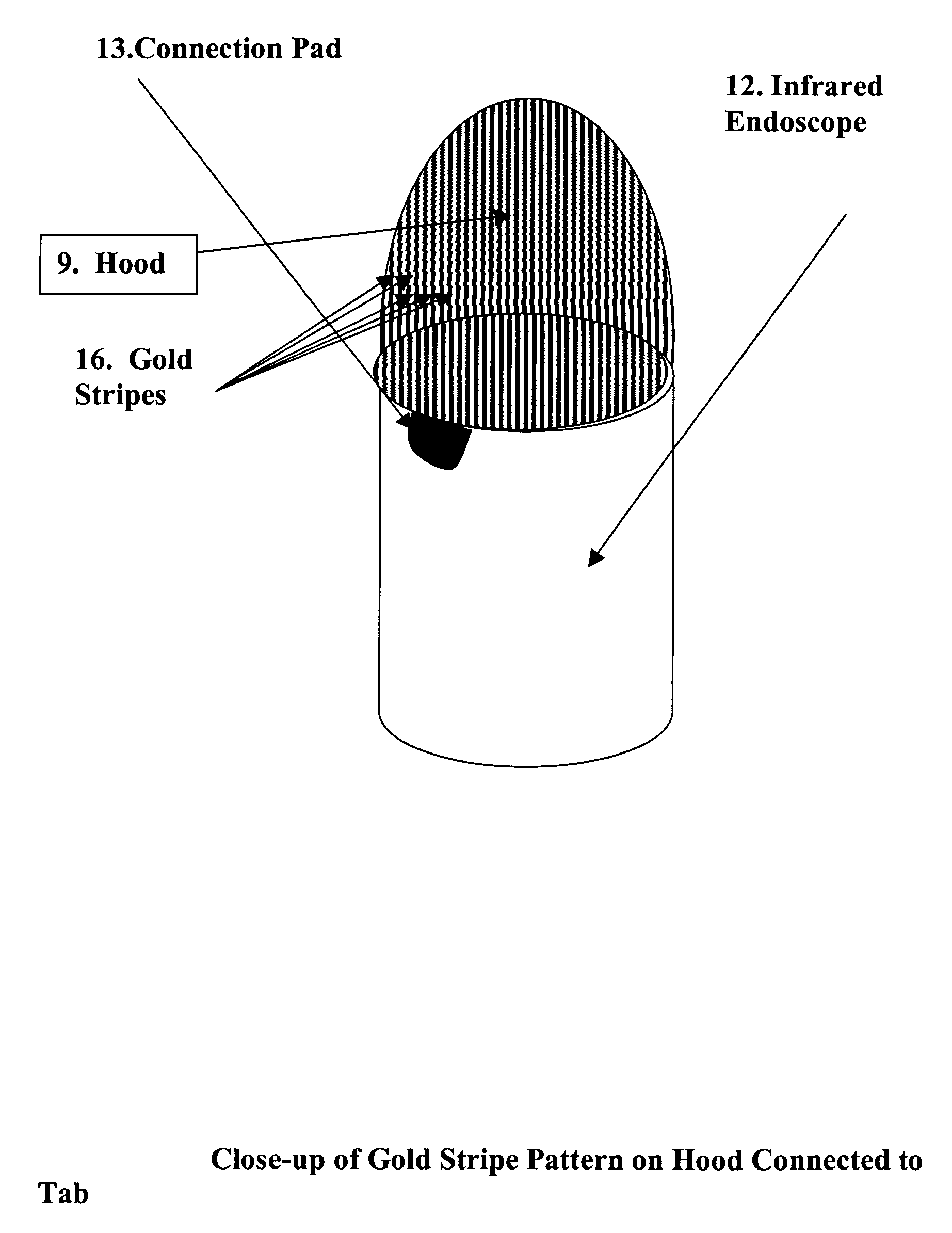
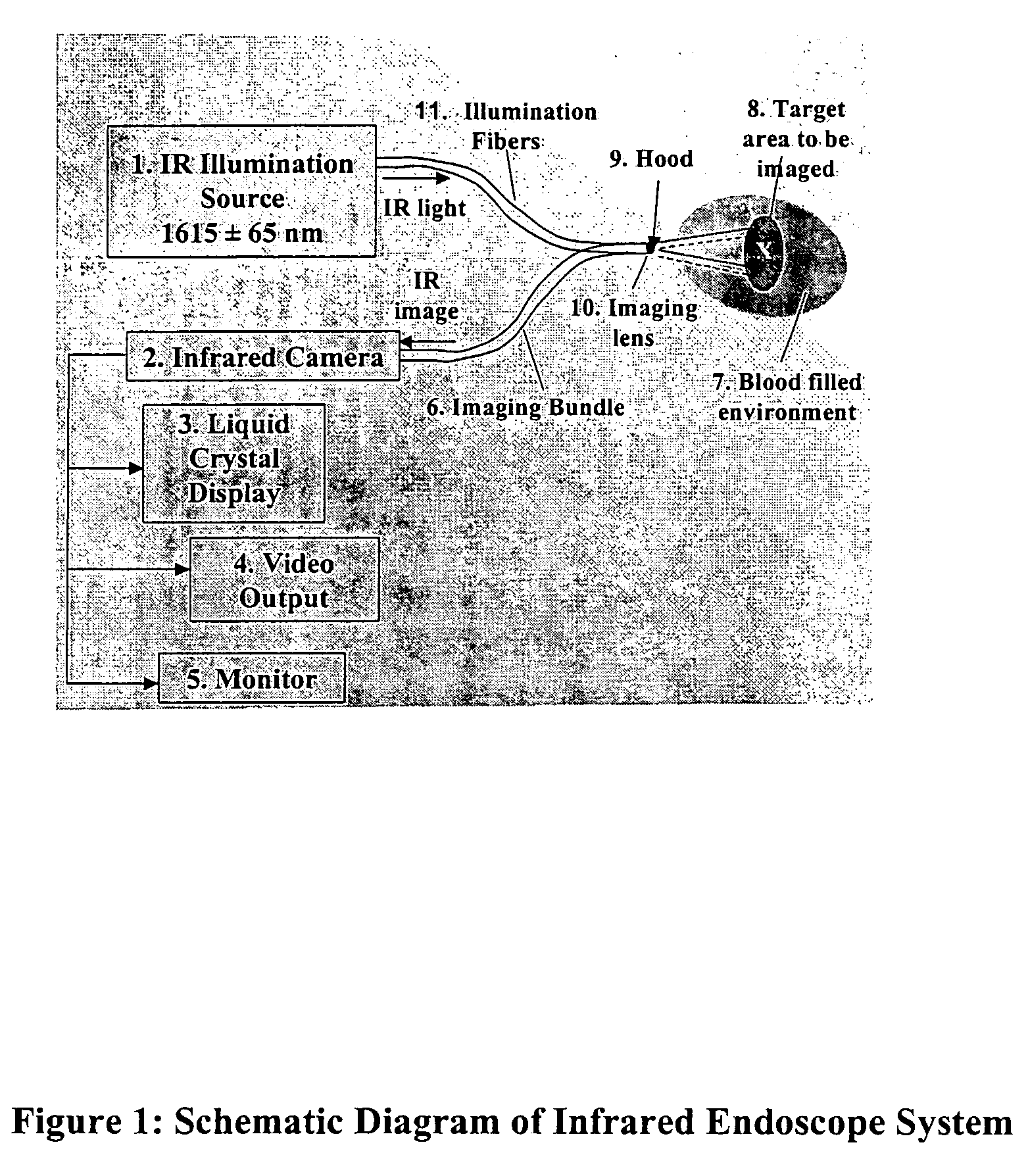
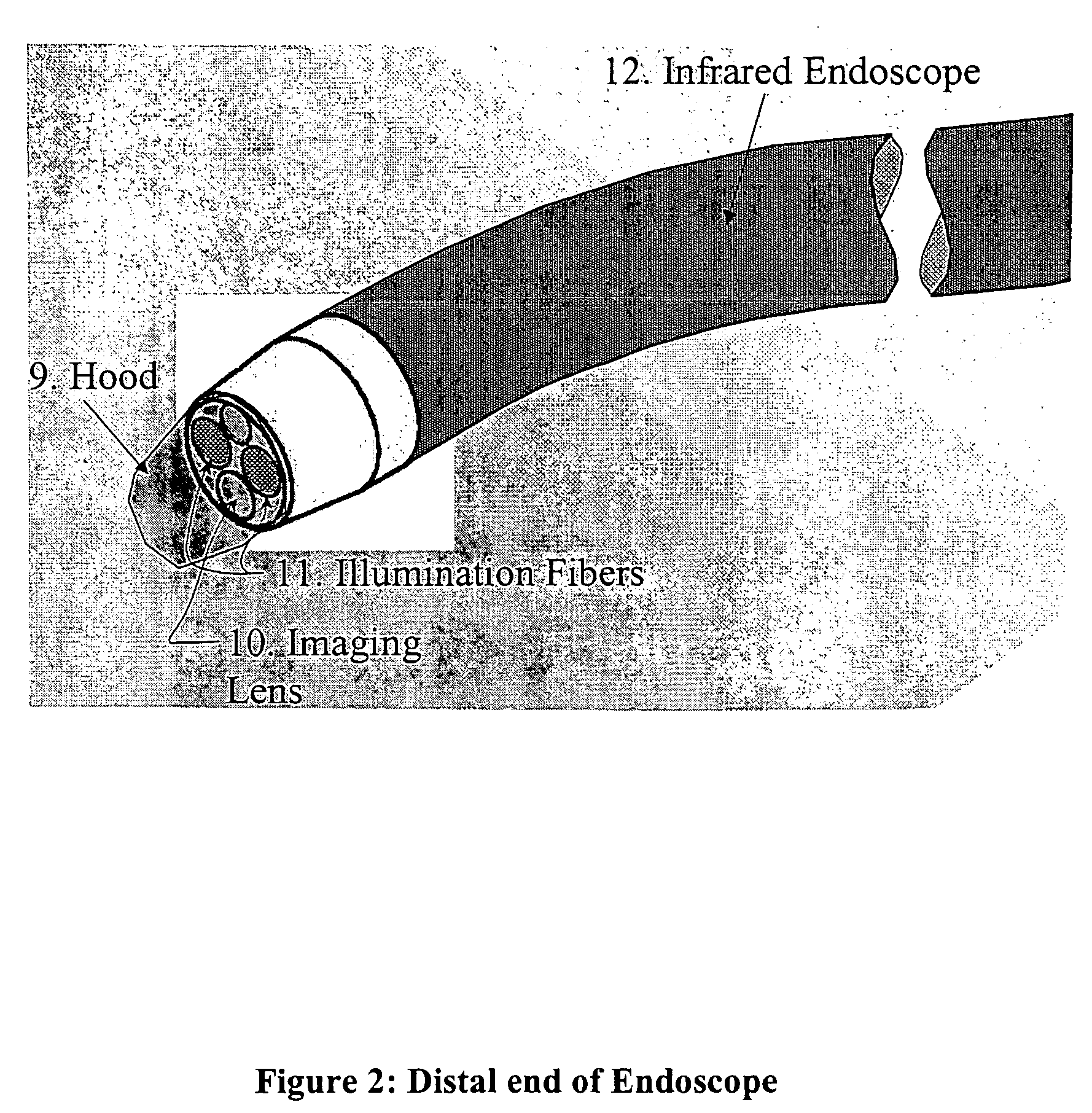
ActiveUS7527625B2Efficient use ofNo ionizing radiationSurgical instrument detailsCoatingsPlatinumIntracardiac echocardiography
A novel transparent electrode that uses a conductive coating to allow delivery of current to the heart as well as outward imaging through the electrode is described. The embodiments disclose a catheter incorporating an endoscope, whose imaging tip is coated with a conductive coating that is transparent in the endoscopic image. However, a transparent electrode may be fashioned for any imaging modality, such as intracardiac echocardiography (ICE), that finds the electrode to be transparent to the energy used. This electrode coating may be a thin, optically transparent or translucent coating of platinum or gold or may be a pattern with enough open spaces to see the underlying tissue, such as looking through a screen. A wire is connected to the conductive coating and routed to a radiofrequency generator.
Owner:OLYMPUS CORP
Corrosion-Resistant Downhole Transmission System
An apparatus in accordance with the invention may include a downhole tool and a data transmission path incorporated into the downhole tool. The data transmission path may include one or more contact surfaces providing electrical continuity to the data transmission path. To protect the contact surfaces from corrosion while maintaining electrical conductivity, a coating may be attached to one or more of the contact surfaces. The coating may include any of various materials that increase the corrosion-resistance of the underlying base metal, including but not limited to cobalt, nickel, tin, tin-lead, platinum, palladium, gold, silver, zinc, or combinations thereof.
Owner:INTELLISERV
Low platinum fuel cells, catalysts, and method for preparing the same
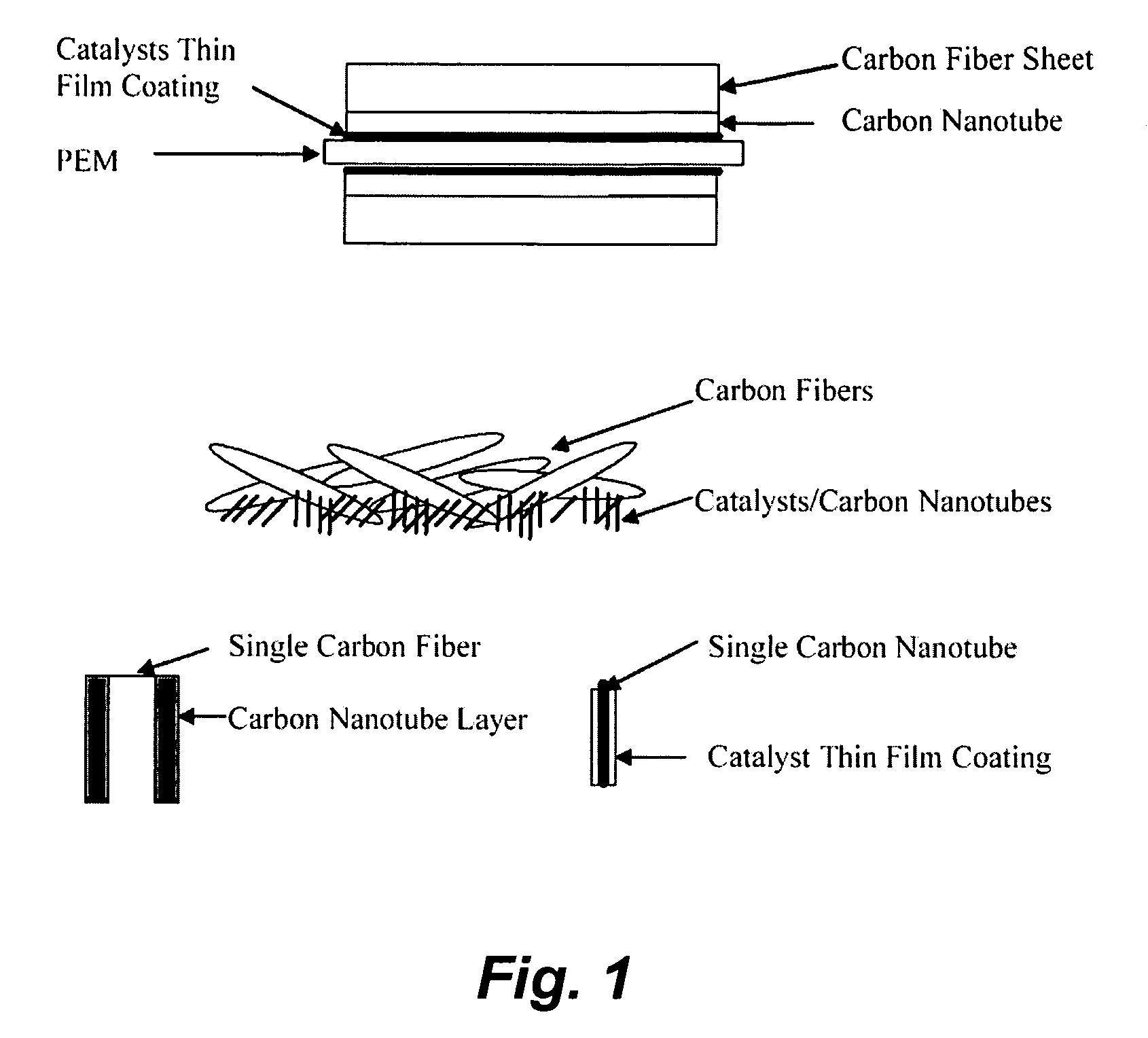
InactiveUS20060172179A1Efficient processingImprove throughputMaterial nanotechnologyConductive materialFiberCarbon fibers
This invention provides novel fuel cell electrodes and catalysts comprising a series of catalytically active thin-film metal alloys with low platinum concentration supported on nanostructured materials (nanoparticles). Processing of the electrodes and catalysts can include electrodeposition methods, and high-pressure coating techniques. In certain embodiments, an integrated gas-diffusion / electrode / catalyst layer can be prepared by processing catalyst thin films and nanoparticles into gas-diffusion media such as Toray or SGL carbon fiber papers. The catalysts can be placed in contact with an electrolyte membrane for PEM fuel cell applications.
Owner:INTEMATIX
Catalyst for purifying exhaust gas
InactiveUS6066587AImprove responseImprove purification effectMolecular sieve catalystsInternal combustion piston enginesPlatinumPartial oxidation
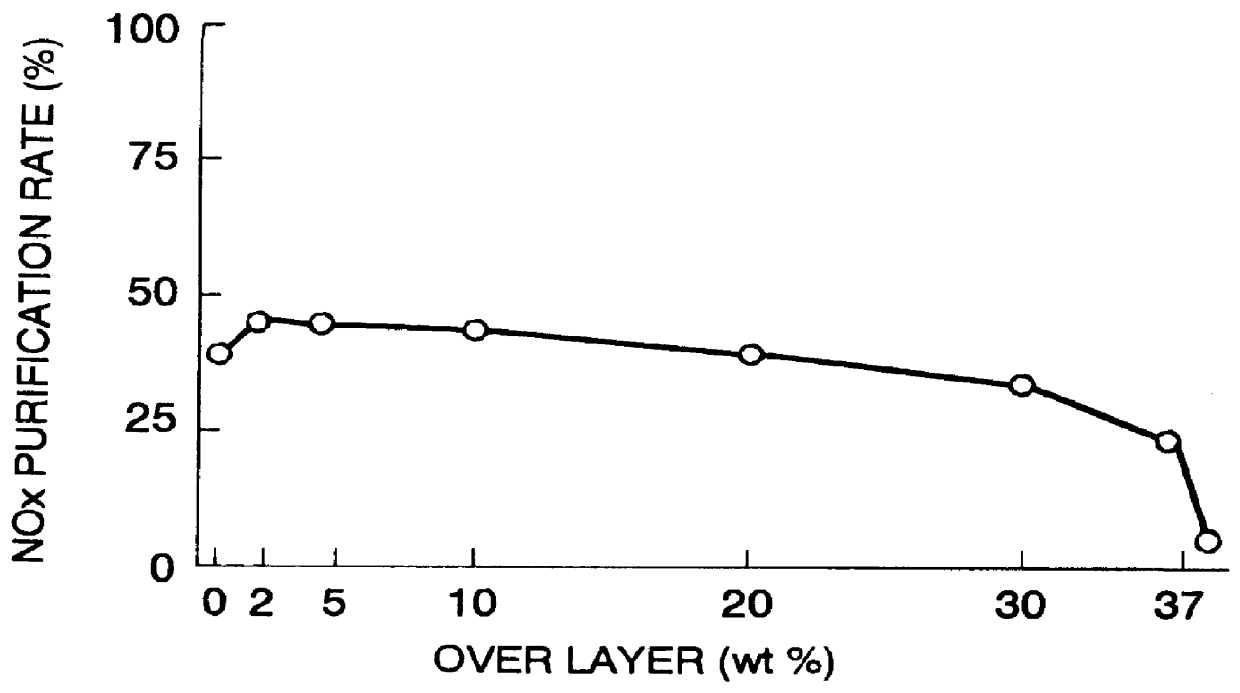
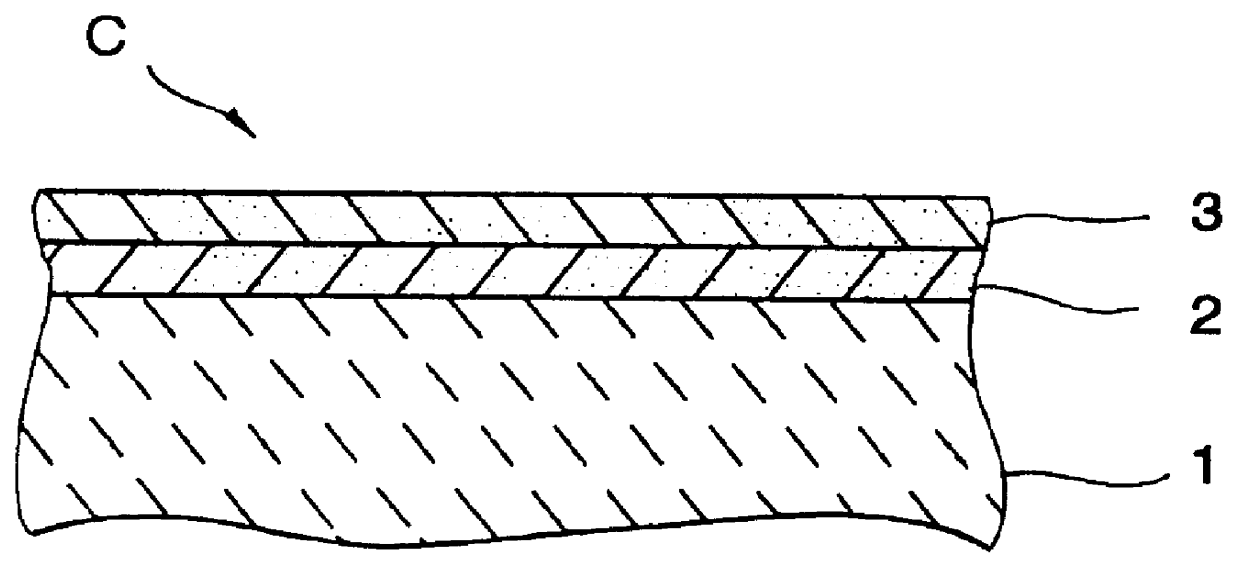
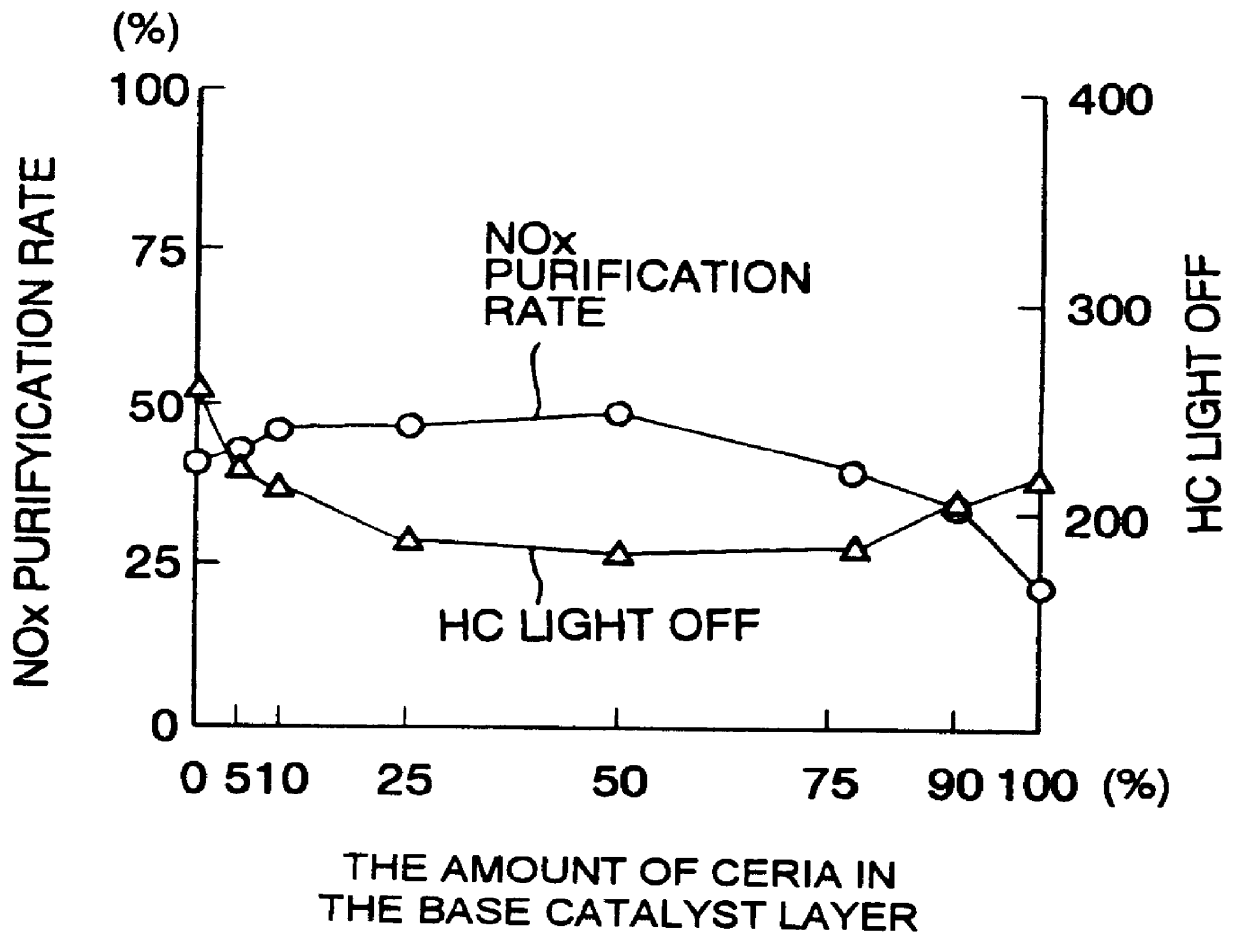
A catalyst has a base catalyst layer containing platinum and barium as precious metal supported by alumina and an over catalyst layer containing platinum and rhodium as precious metal supported by zeolitr. The platinum and rhodium in the over catalyst layer activate NOx and HC so as to make them more reactive in terms of energy, and the barium in the base catalyst layer makes the platinum be more dispersive in the base catalyst layer. Under the existence of dispersive platinum, NOx in exhaust gas is decomposed and purified by reaction with reactive NO2 and partially oxidized HC generated in the over catalyst layer.
Owner:MAZDA MOTOR CORP
Catalyst for manufacturing hydrogen or synthesis gas and manufacturing method of hydrogen or synthesis gas
InactiveUS6361757B1Produce hydrogenEfficient productionIron compoundsCobalt compoundsIridiumForming gas
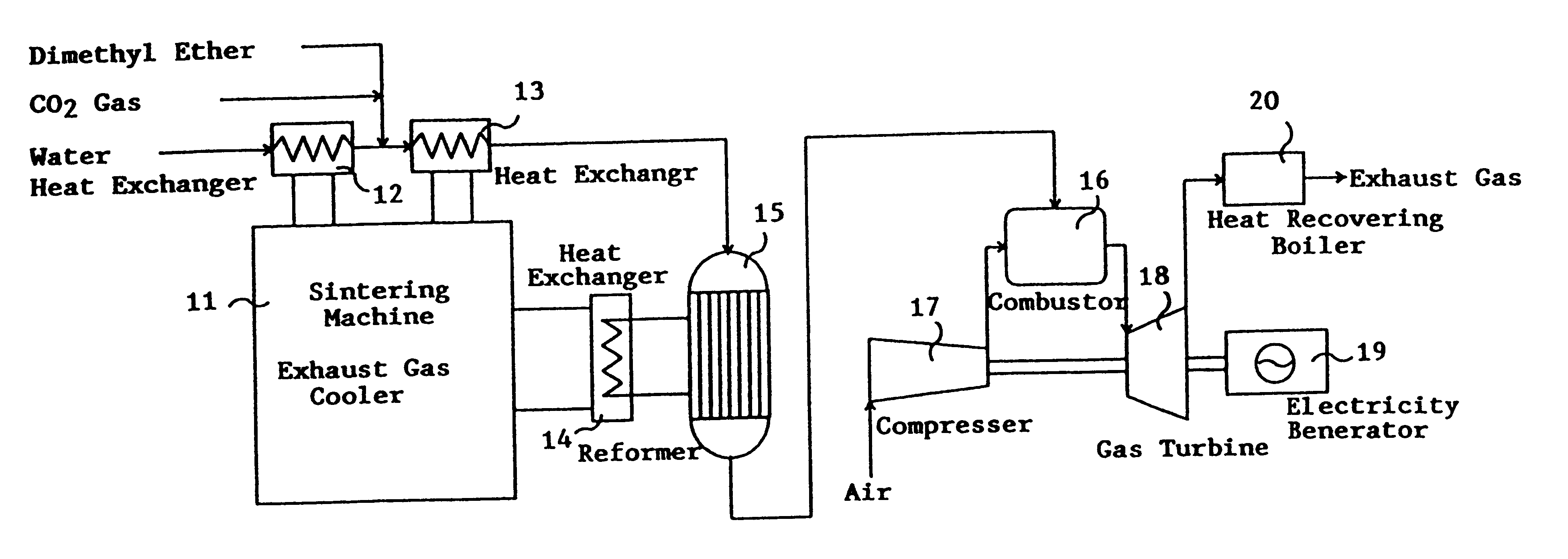
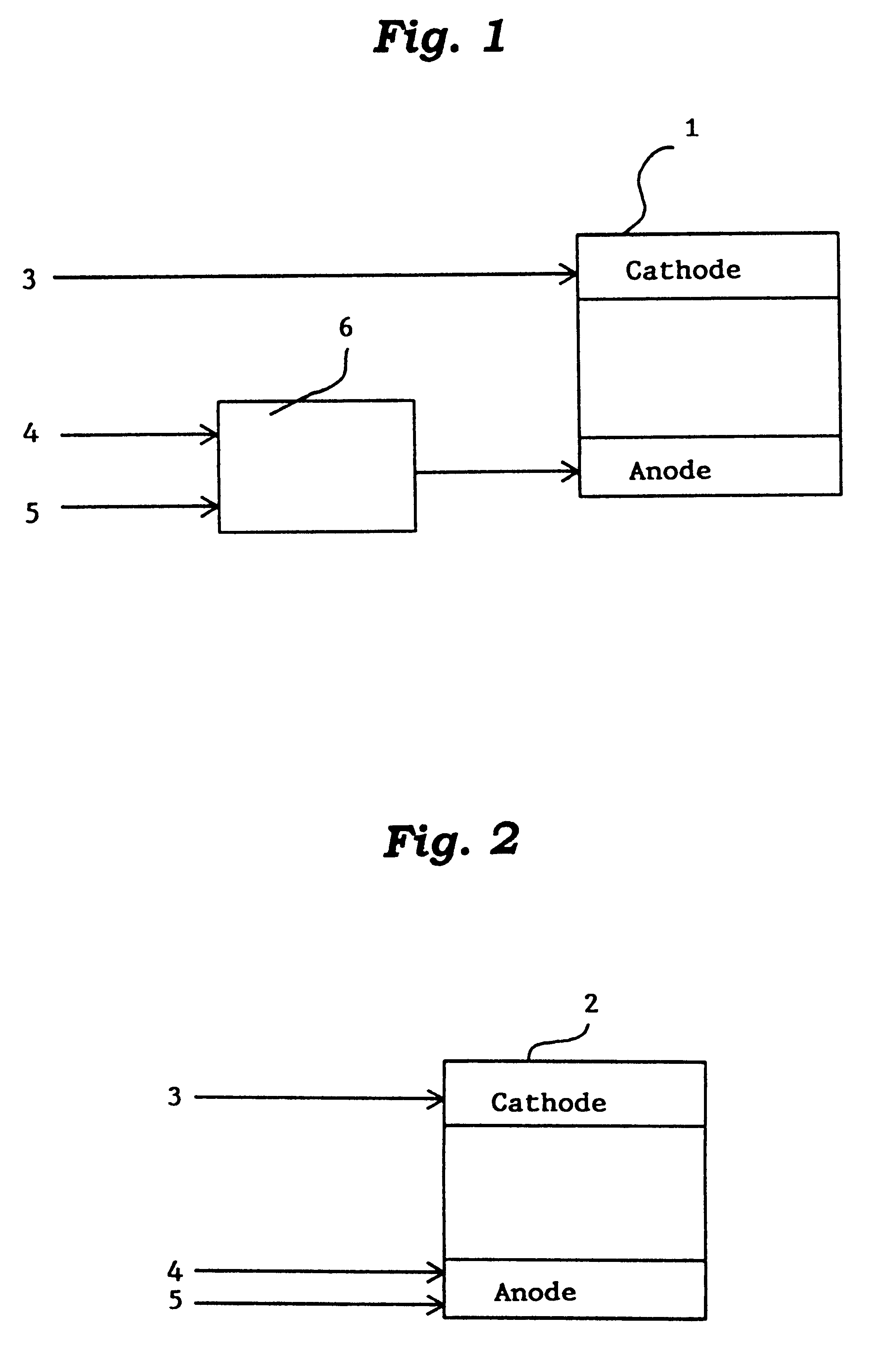
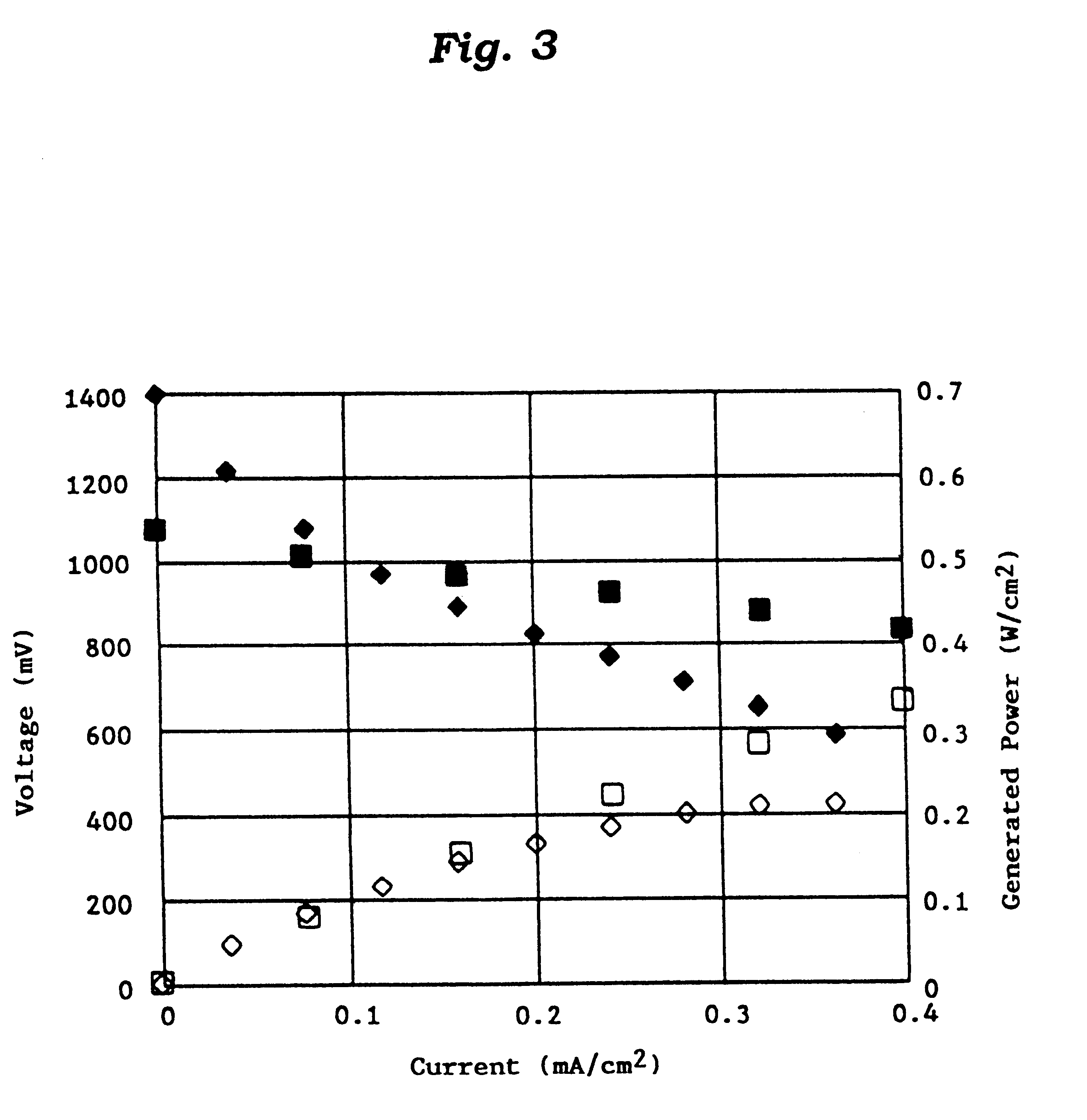
This invention provides a catalyst for producing hydrogen gas from a mixed gas comprising dimethyl ether and water vapor or carbon dioxide gas, which comprises copper, iron, cobalt, palladium, iridium, platinum, rhodium, or nickel as an active component, and a method of producing synthesis gas or hydrogen gas in a high yield at a low temperature. By using the catalyst, a fuel cell, electricity generation, reduction of iron ore and the like can be carried out.
Owner:NIPPON KOKAN KK
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com