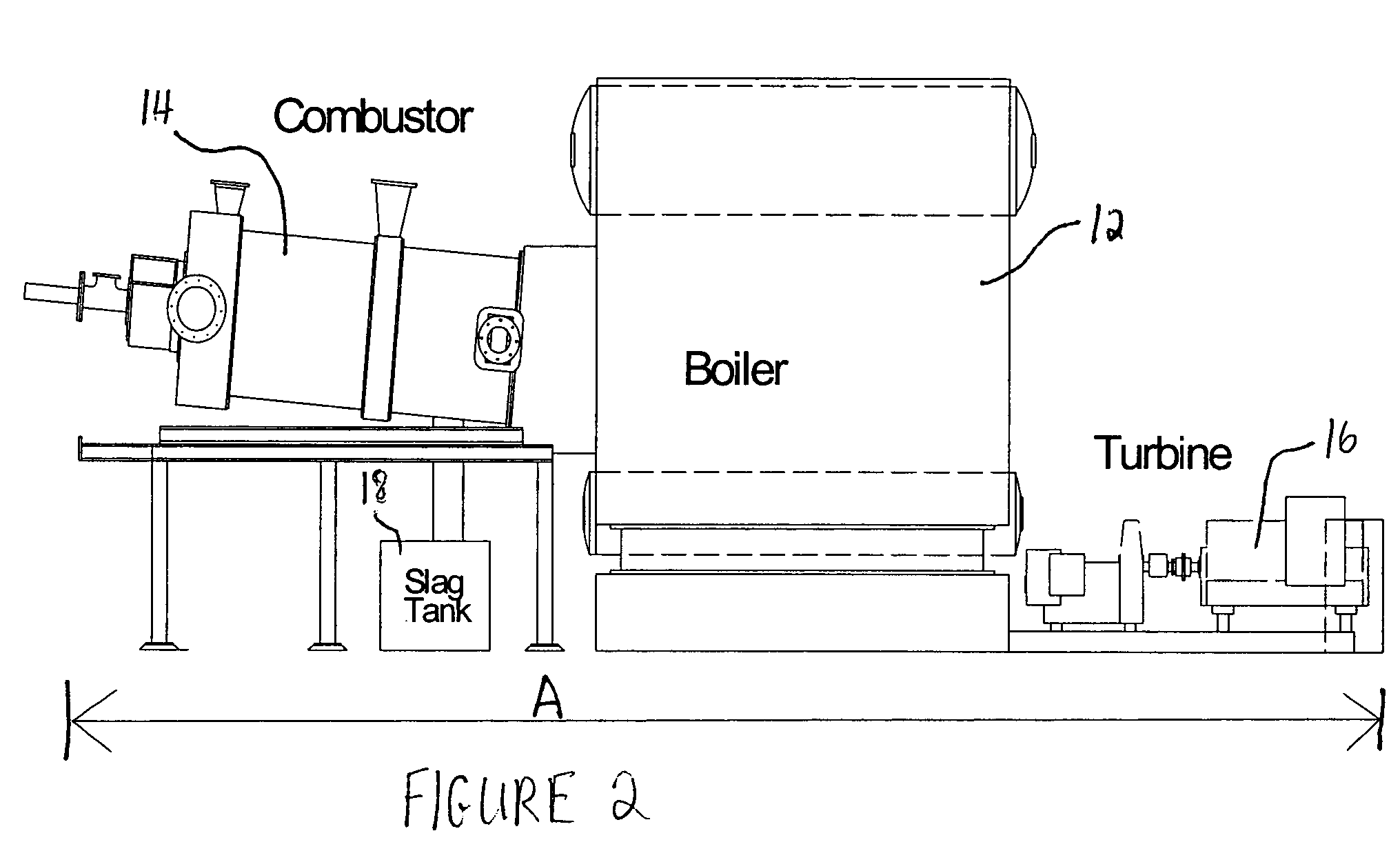
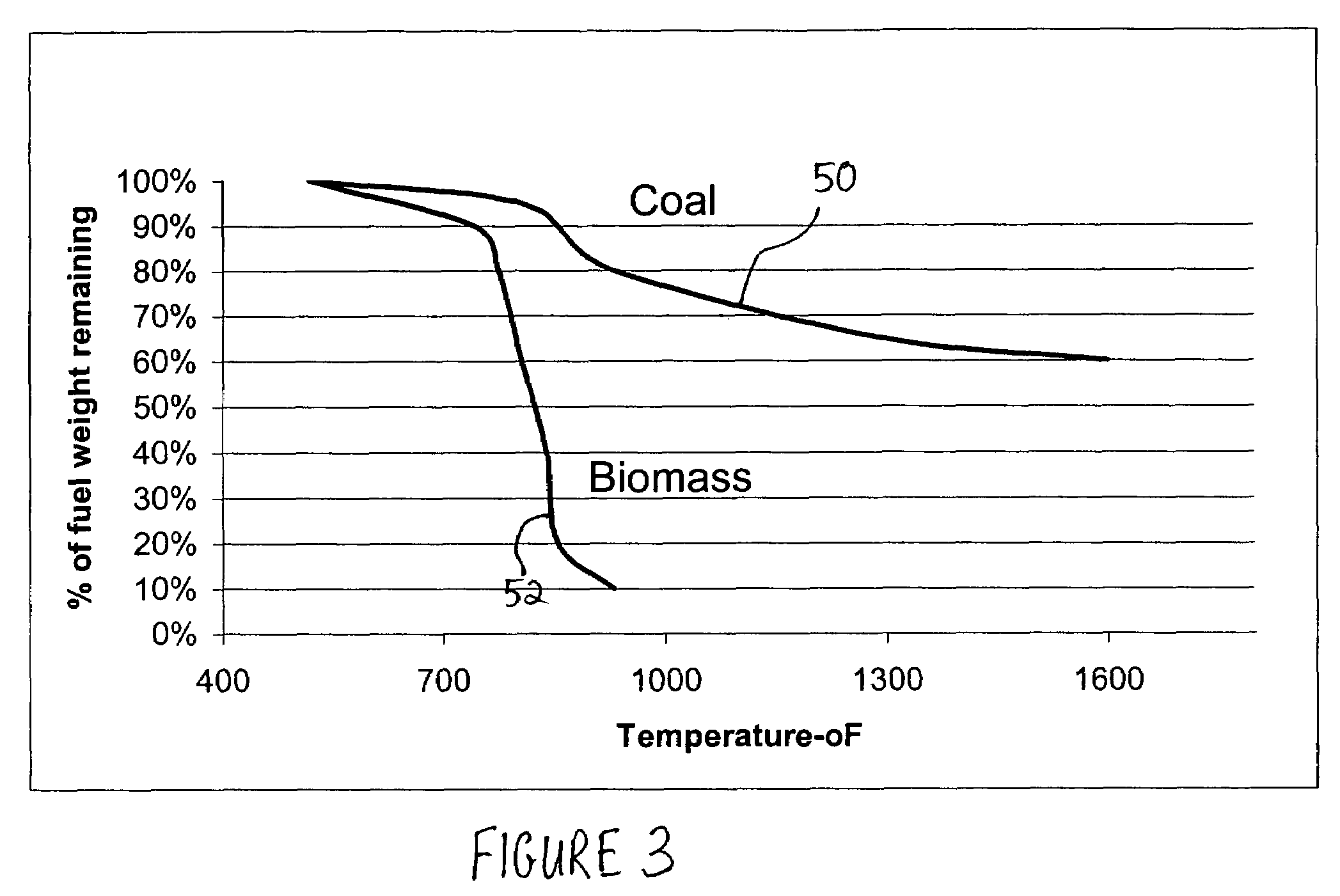
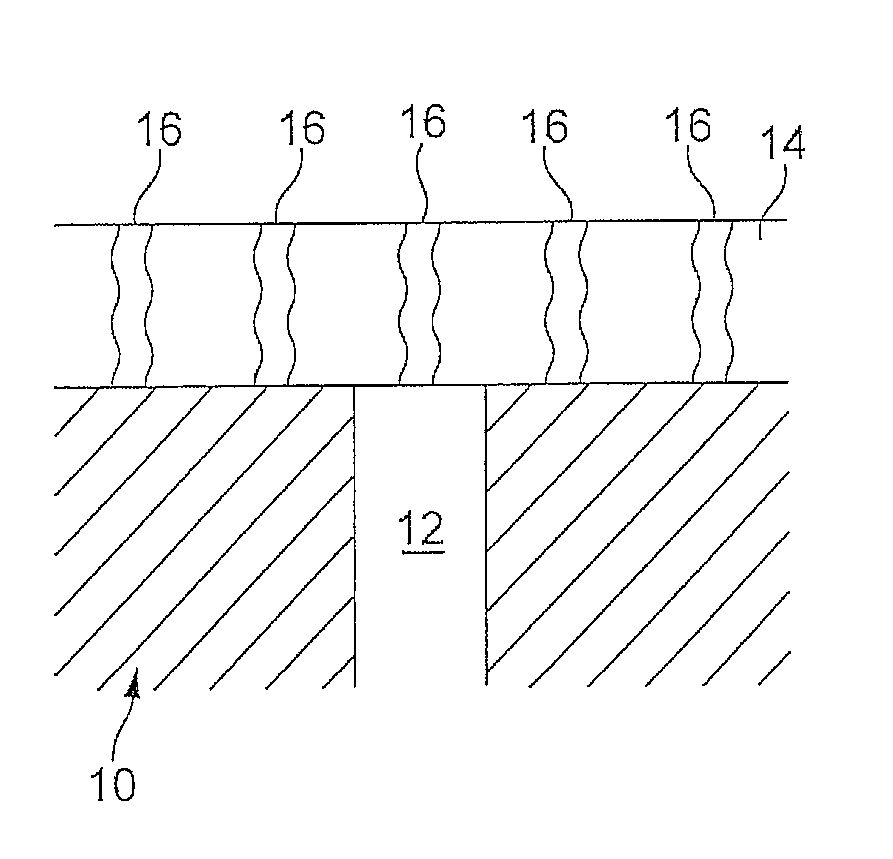
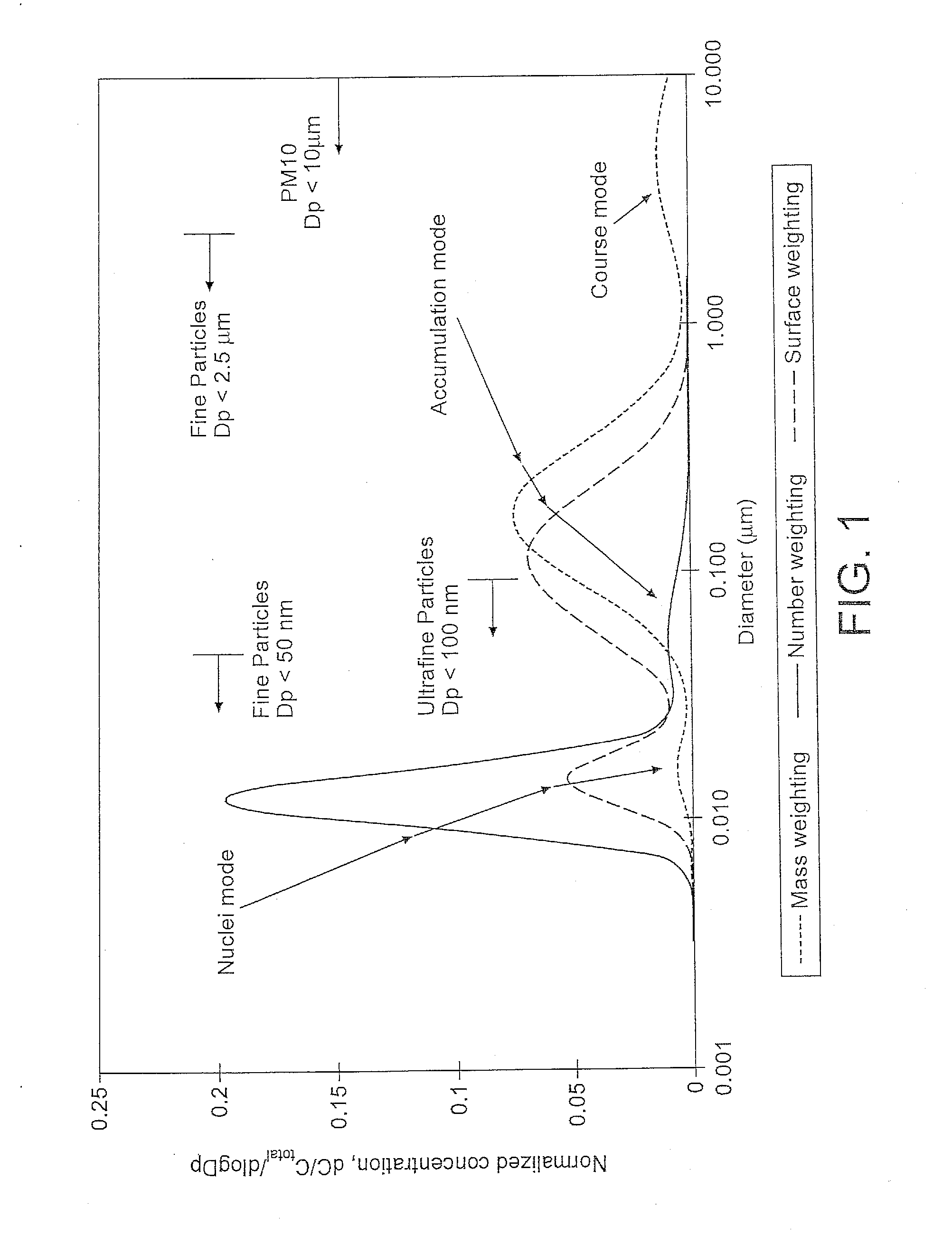
Patents
Literature
4297results about "Nitrogen compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
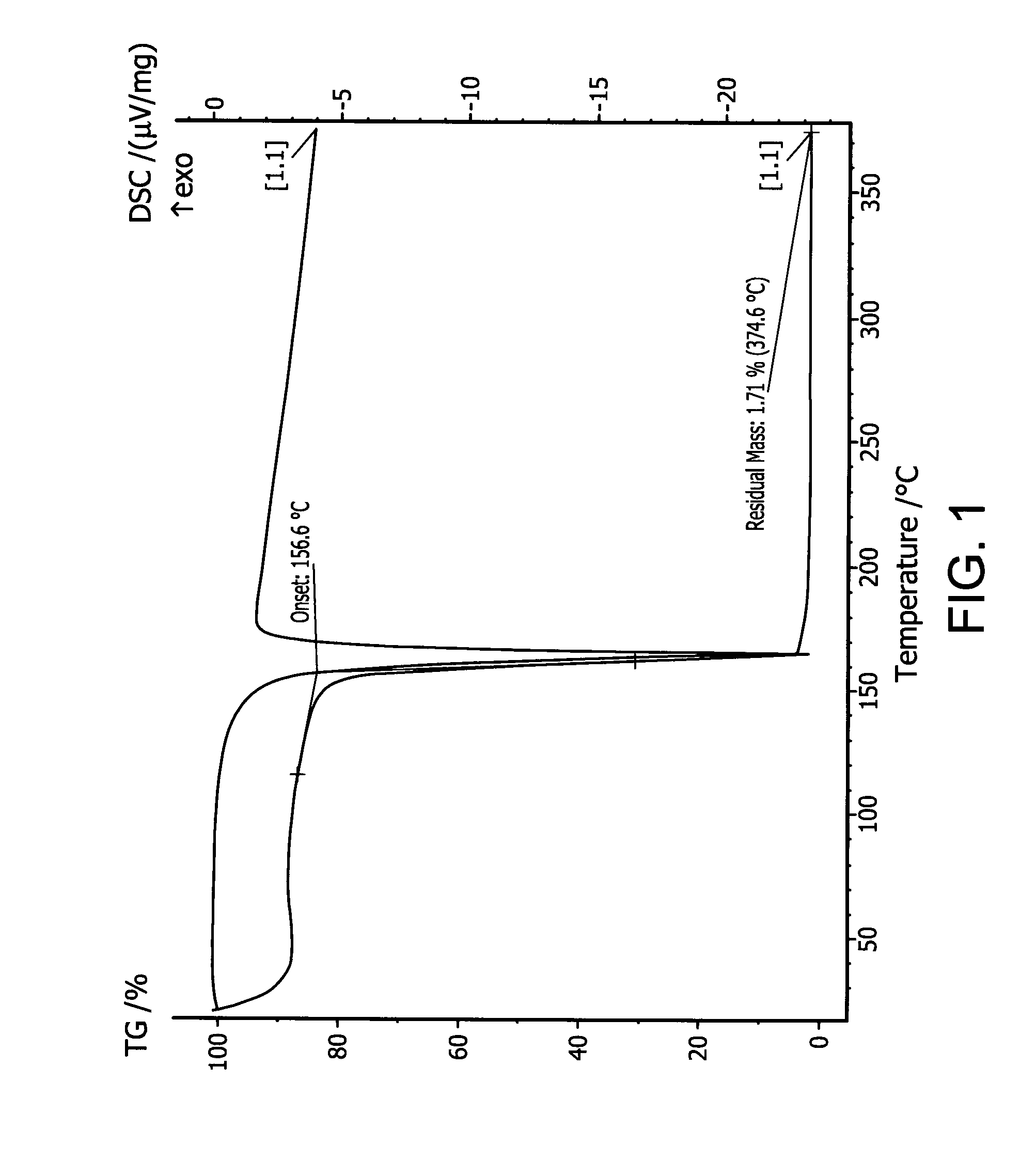
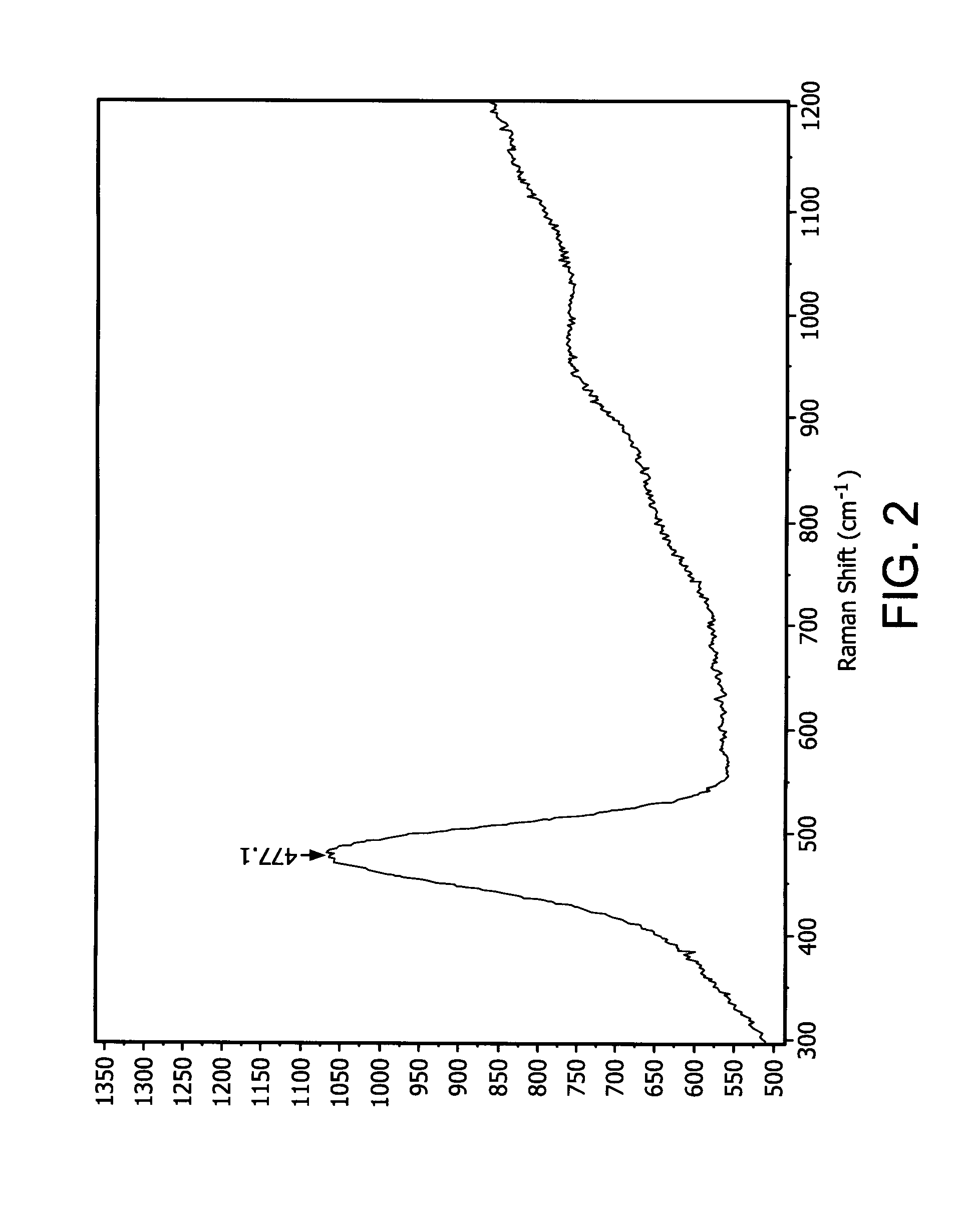
Organoaminodisilane precursors and methods for depositing films comprising same
Described herein are precursors and methods for forming silicon-containing films. In one aspect, there is provided a precursor of Formula I:wherein R1 is selected from linear or branched C3 to C10 alkyl group, linear or branched C3 to C10 alkenyl group, linear or branched C3 to C10 alkynyl group, C1 to C6 dialkylamino group, electron withdrawing group, and C6 to C10 aryl group; R2 is selected from hydrogen, linear or branched C1 to C10 alkyl group, linear or branched C3 to C6 alkenyl group, linear or branched C3 to C6 alkynyl group, C1 to C6 dialkylamino group, C6 to C10 aryl group, linear or branched C1 to C6 fluorinated alkyl group, electron withdrawing group, and C4 to C10 aryl group; optionally wherein R1 and R2 are linked together to form ring selected from substituted or unsubstituted aromatic ring or substituted or unsubstituted aliphatic ring; and n=1 or 2.
Owner:VERSUM MATERIALS US LLC
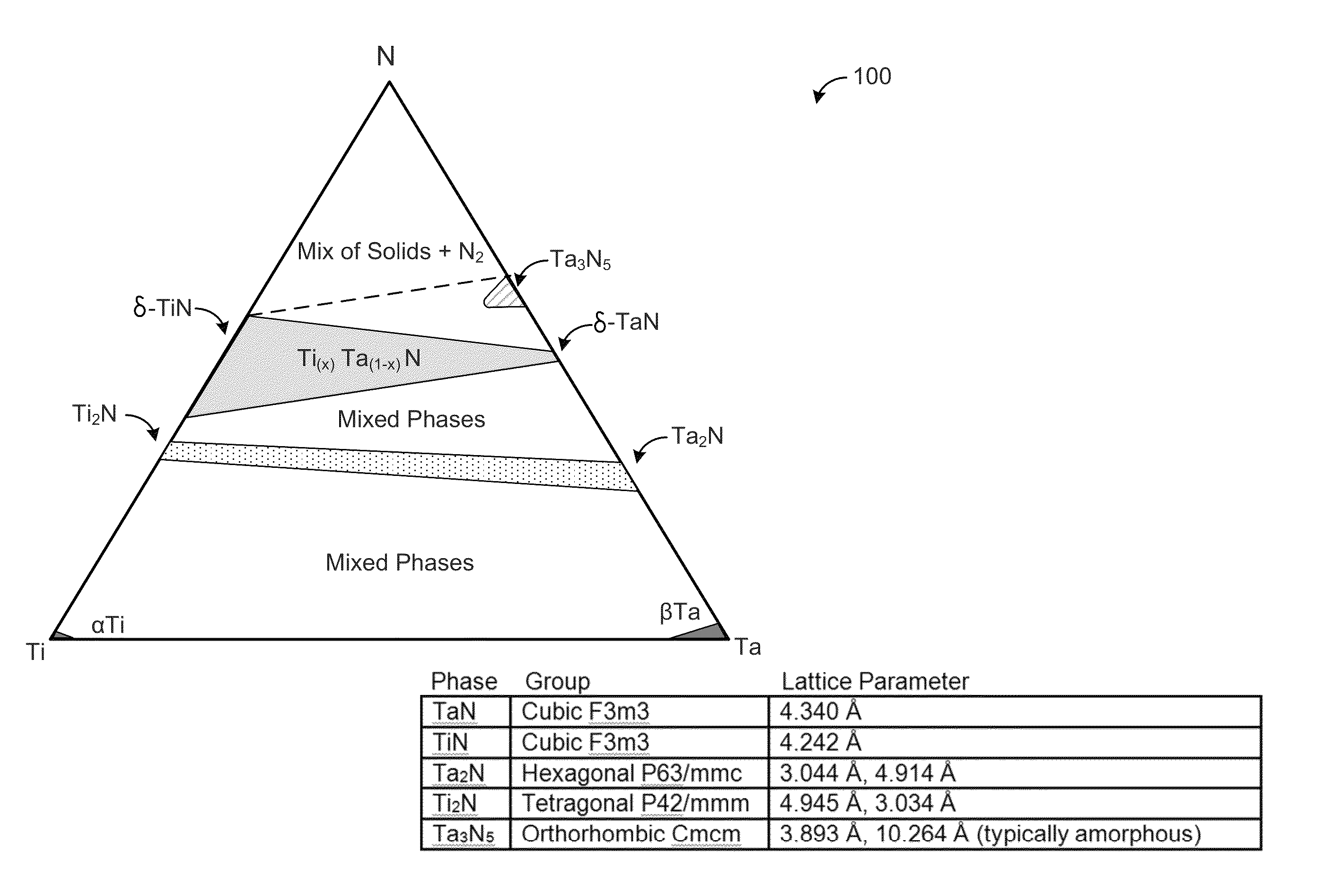
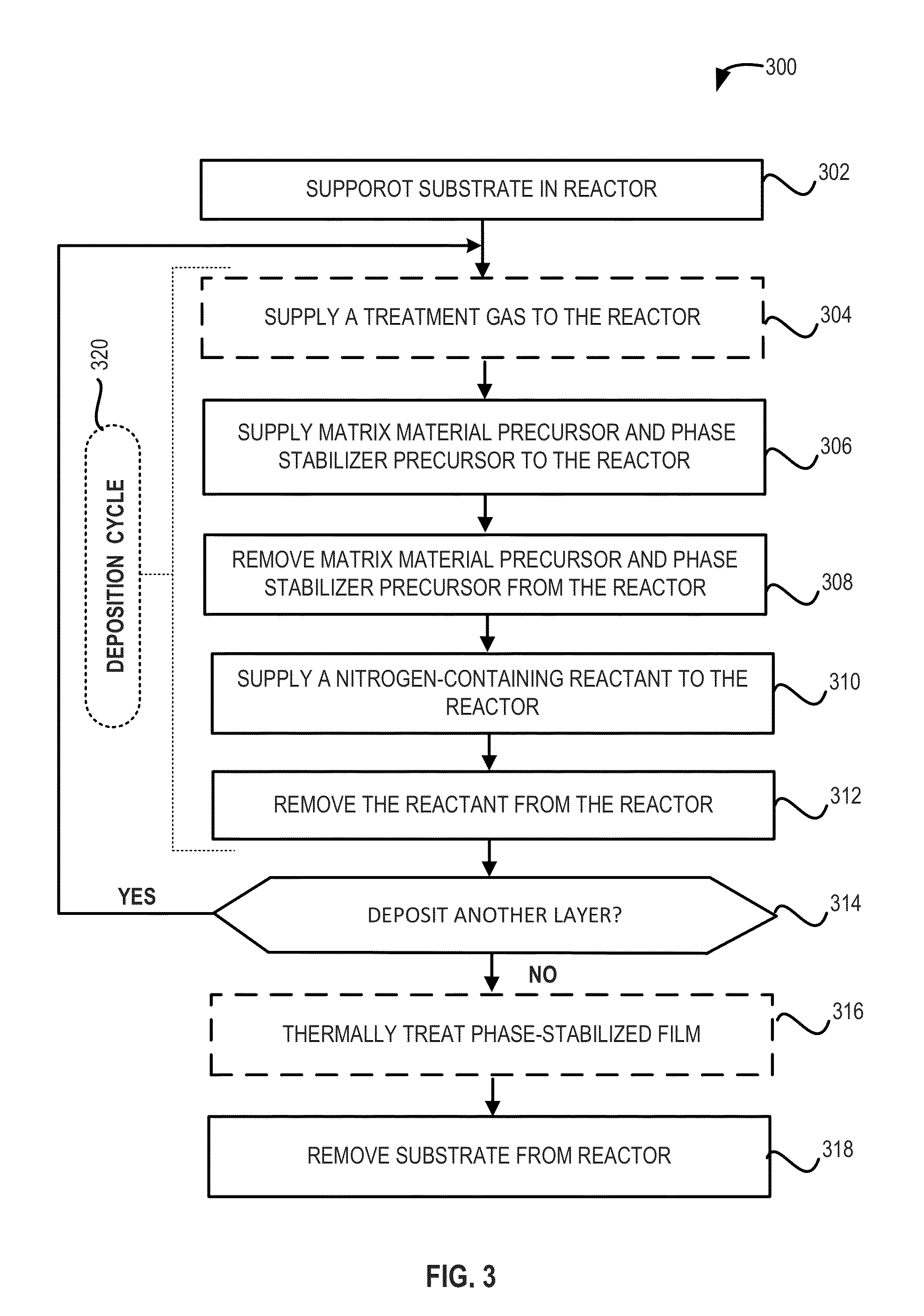
Phase-stabilized thin films, structures and devices including the thin films, and methods of forming same
Nitrogen-containing phase-stabilized films, methods of forming phase-stabilized films, and structures and devices including the phase-stabilized films are disclosed. The phase-stabilized films include a matrix material and a phase stabilizer, which provides a morphologically stabilizing effect to a matrix material within the films. The phase-stabilized films may be used as, for example, gate electrodes and similar films in microelectronic devices.
Owner:ASM IP HLDG BV
Phase-stabilized thin films, structures and devices including the thin films, and methods of forming same
ActiveUS20130292676A1Desirable propertyPromote formationNitrogen compoundsSemiconductor/solid-state device manufacturingNitrogenMaterials science
Nitrogen-containing phase-stabilized films, methods of forming phase-stabilized films, and structures and devices including the phase-stabilized films are disclosed. The phase-stabilized films include a matrix material and a phase stabilizer, which provides a morphologically stabilizing effect to a matrix material within the films. The phase-stabilized films may be used as, for example, gate electrodes and similar films in microelectronic devices.
Owner:ASM IP HLDG BV
Red Nitride Phosphor and Production Method Thereof
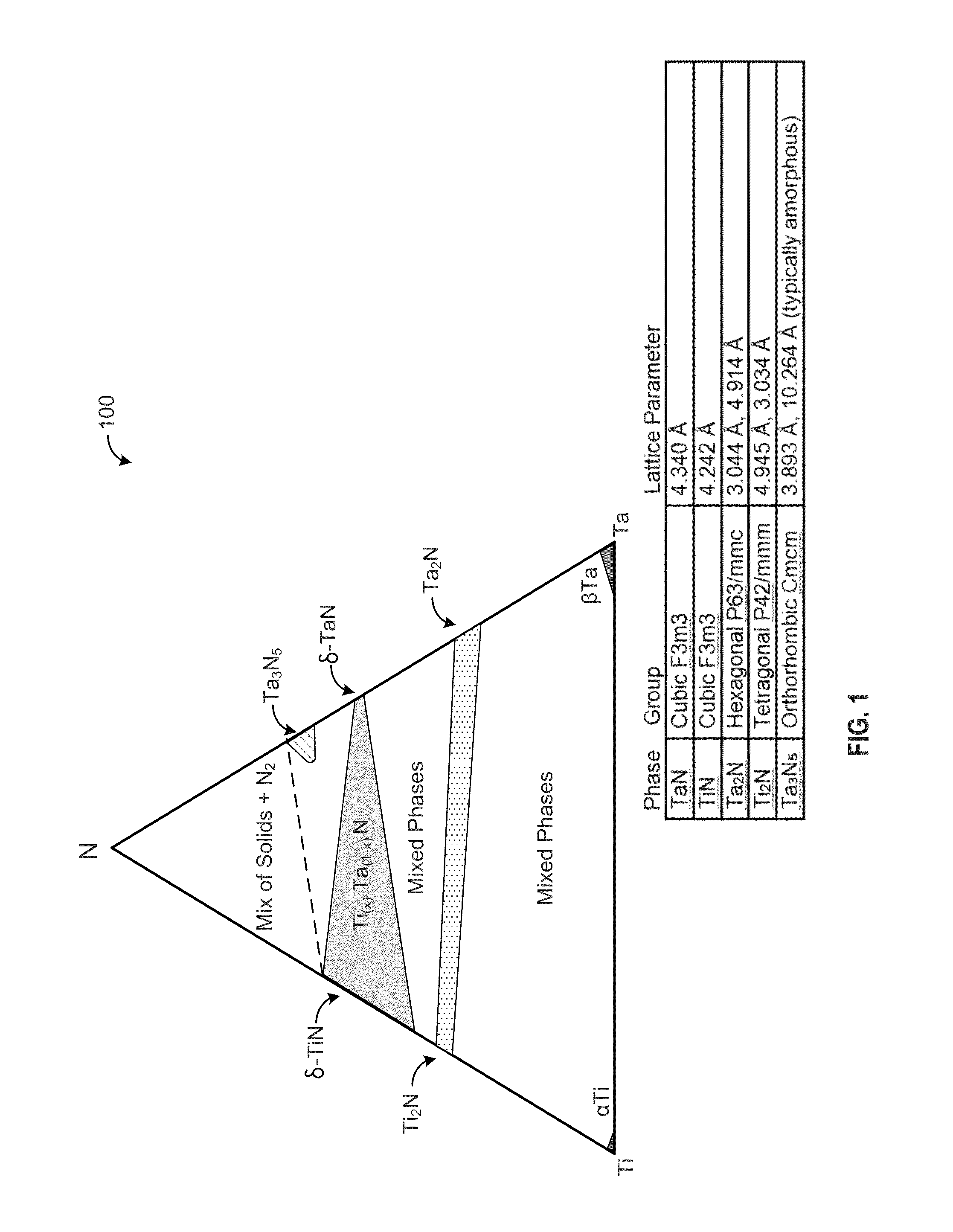
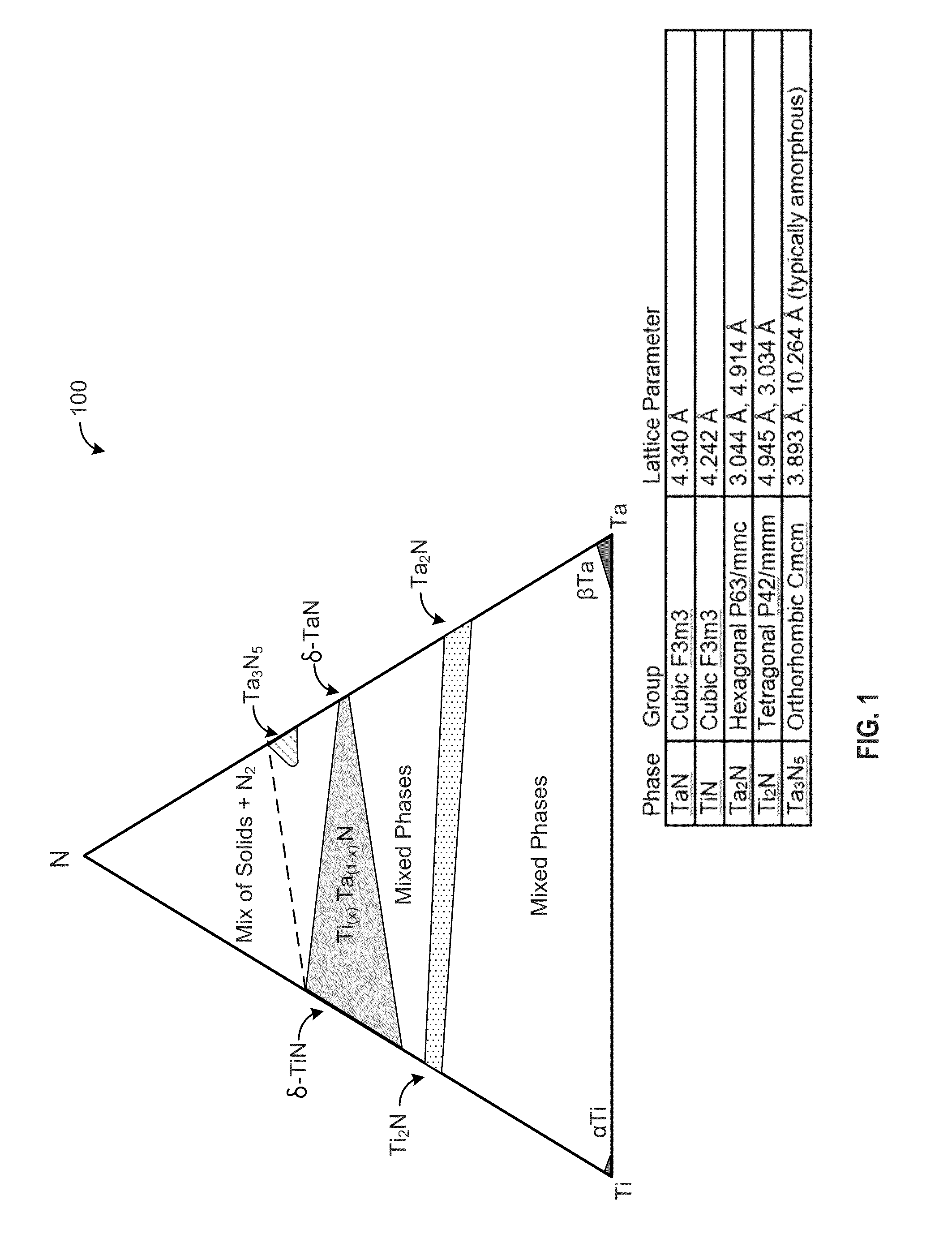
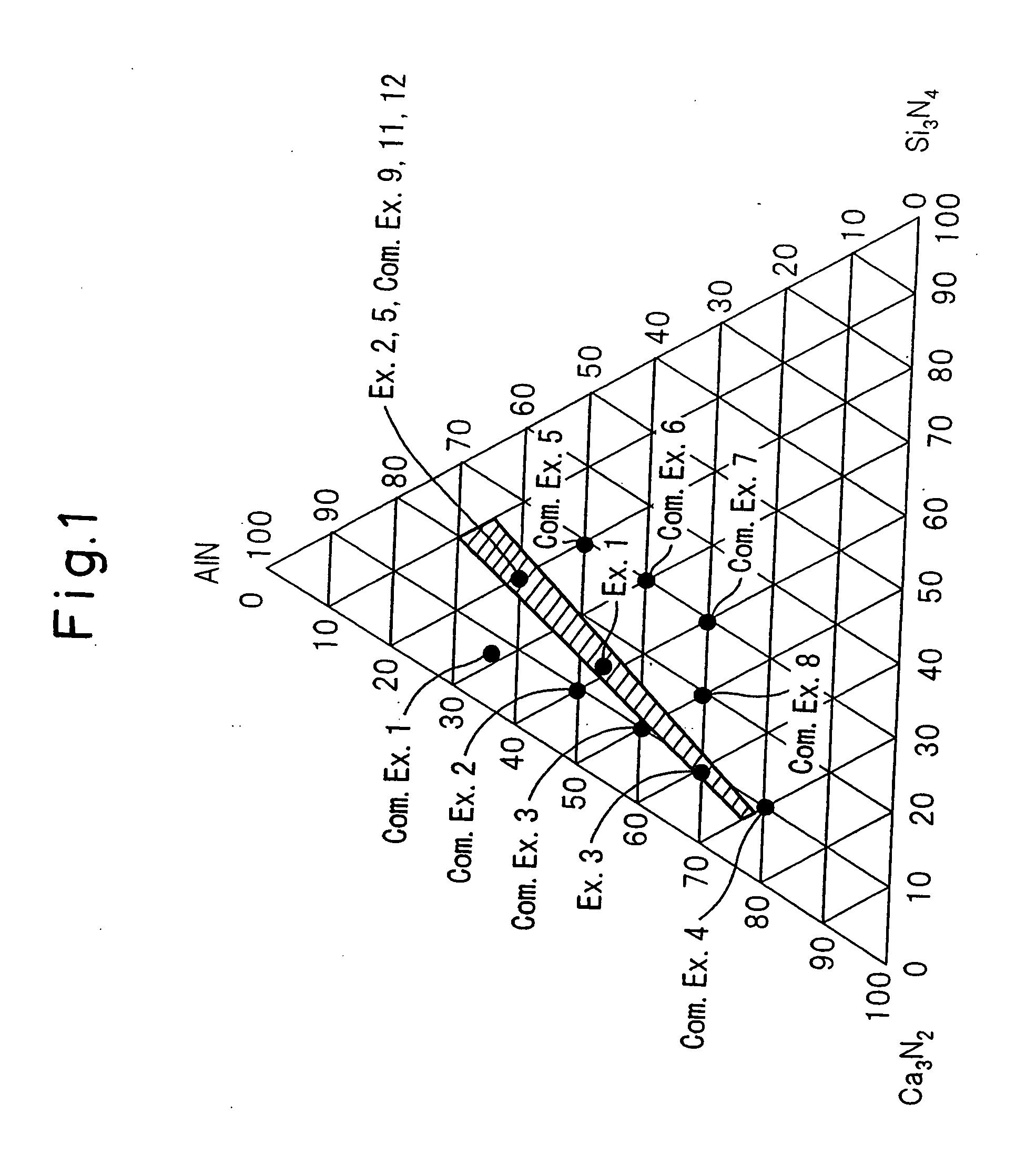
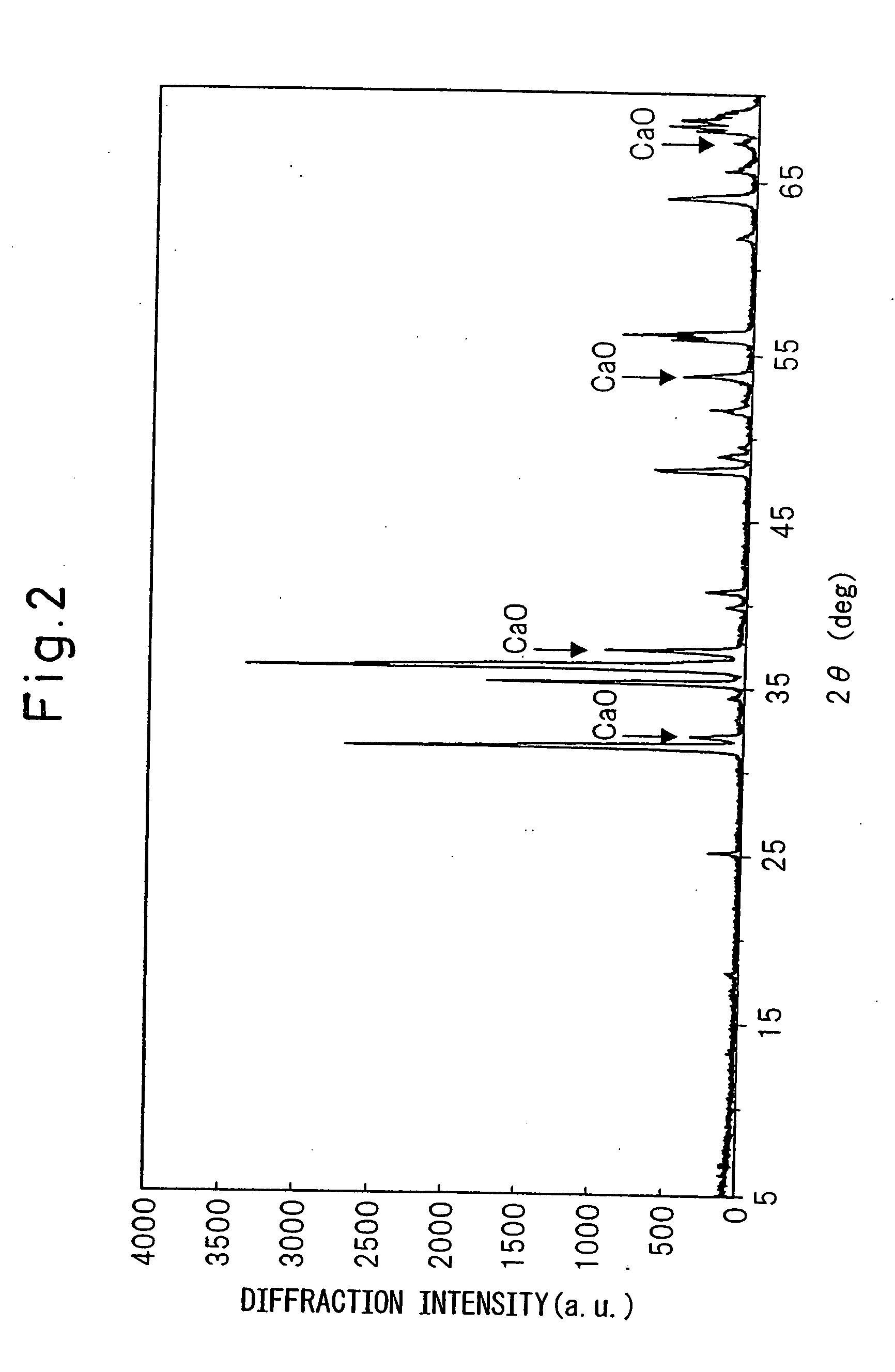
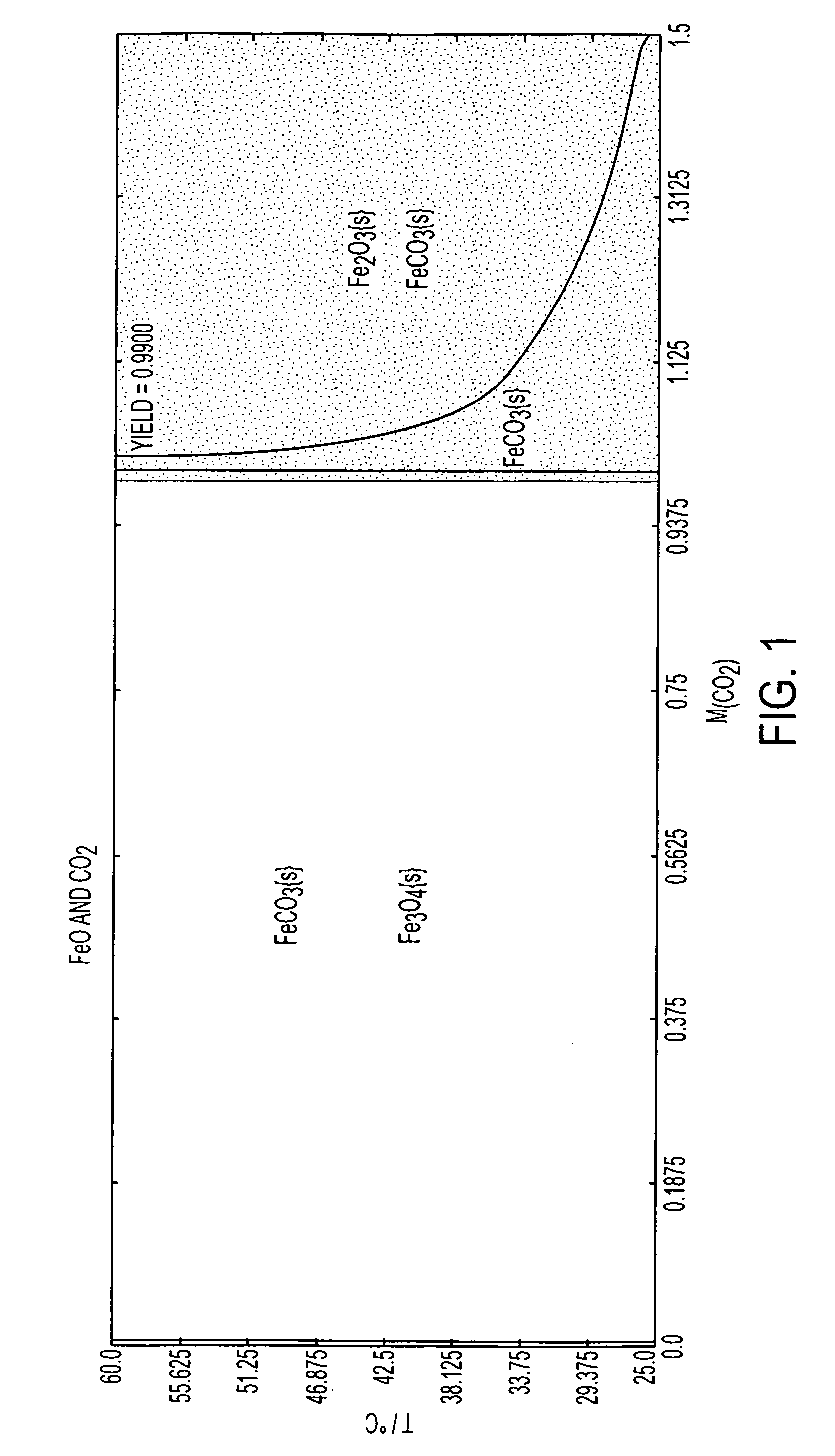
A red phosphor where the crystal phase constituting the phosphor is monoclinic Eu-activated CaAlSiN3. A red phosphor which is Eu-activated CaAlSiN3 powder having an average particle diameter of 10 μm or less as measured in the non-pulverized state by the laser scattering particle size distribution analysis. A light-emitting device comprising a blue light-emitting element, a yellow phosphor capable of converting the blue light emitted from the blue light-emitting element into yellow light, and the above-described red phosphor capable of converting the blue light emitted from the blue light-emitting element into red light. A method for producing Eu-activated CaAlSiN3, comprising firing a raw material powder comprising Ca3N2, AlN, Si3N4 and EuN at 1,400 to 2,000° C. in a nitrogen-containing atmosphere, the Ca3N2, AlN and Si3N4 giving a composition falling in the region surrounded by a straight line connecting the following four points A to D in the composition diagram of FIG. 1 and EuN being contained in an amount of 0.01 to 10 parts by weight as Eu per 100 parts by weight in total of Ca3N2, AlN and Si3N4.
Owner:UBE IND LTD
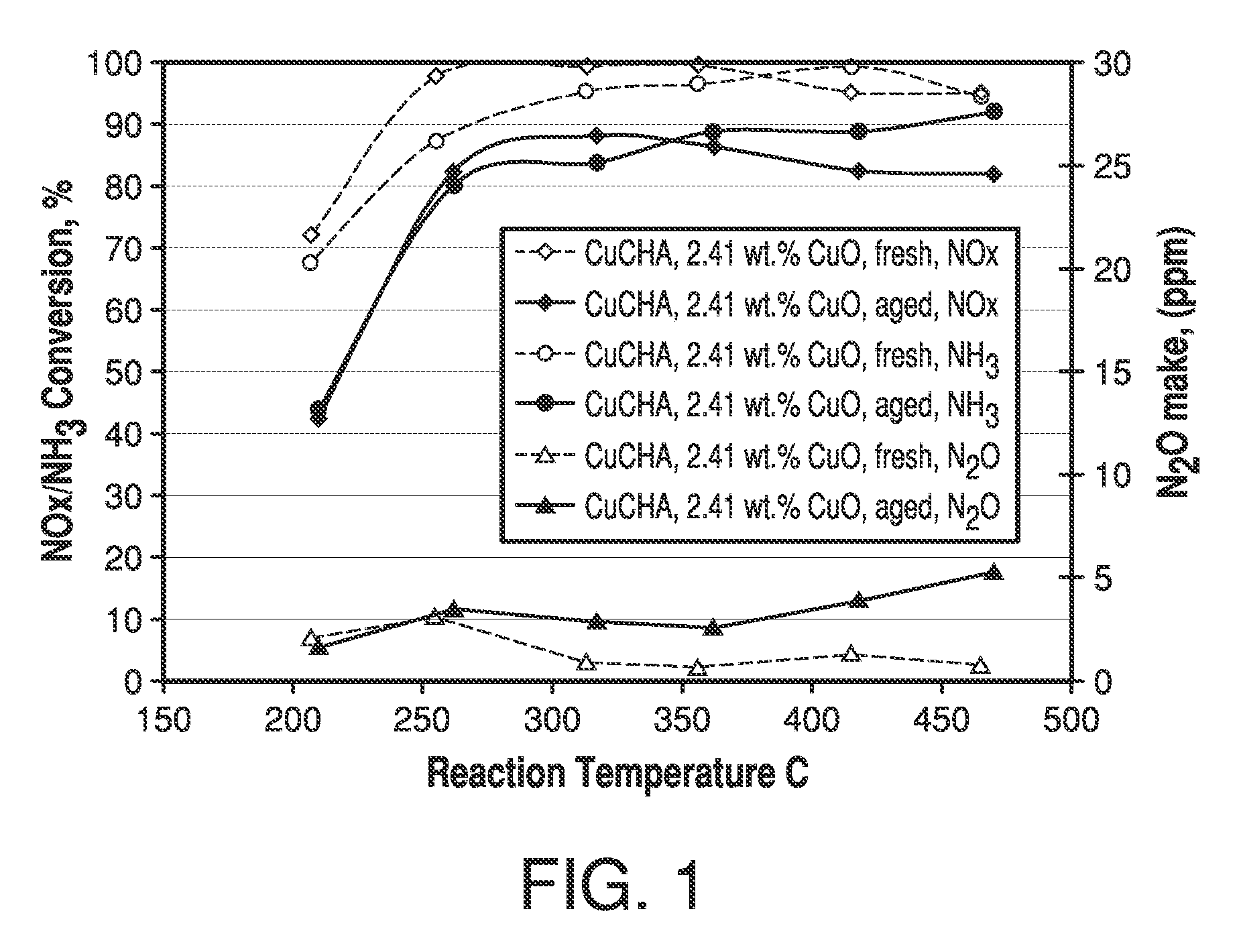
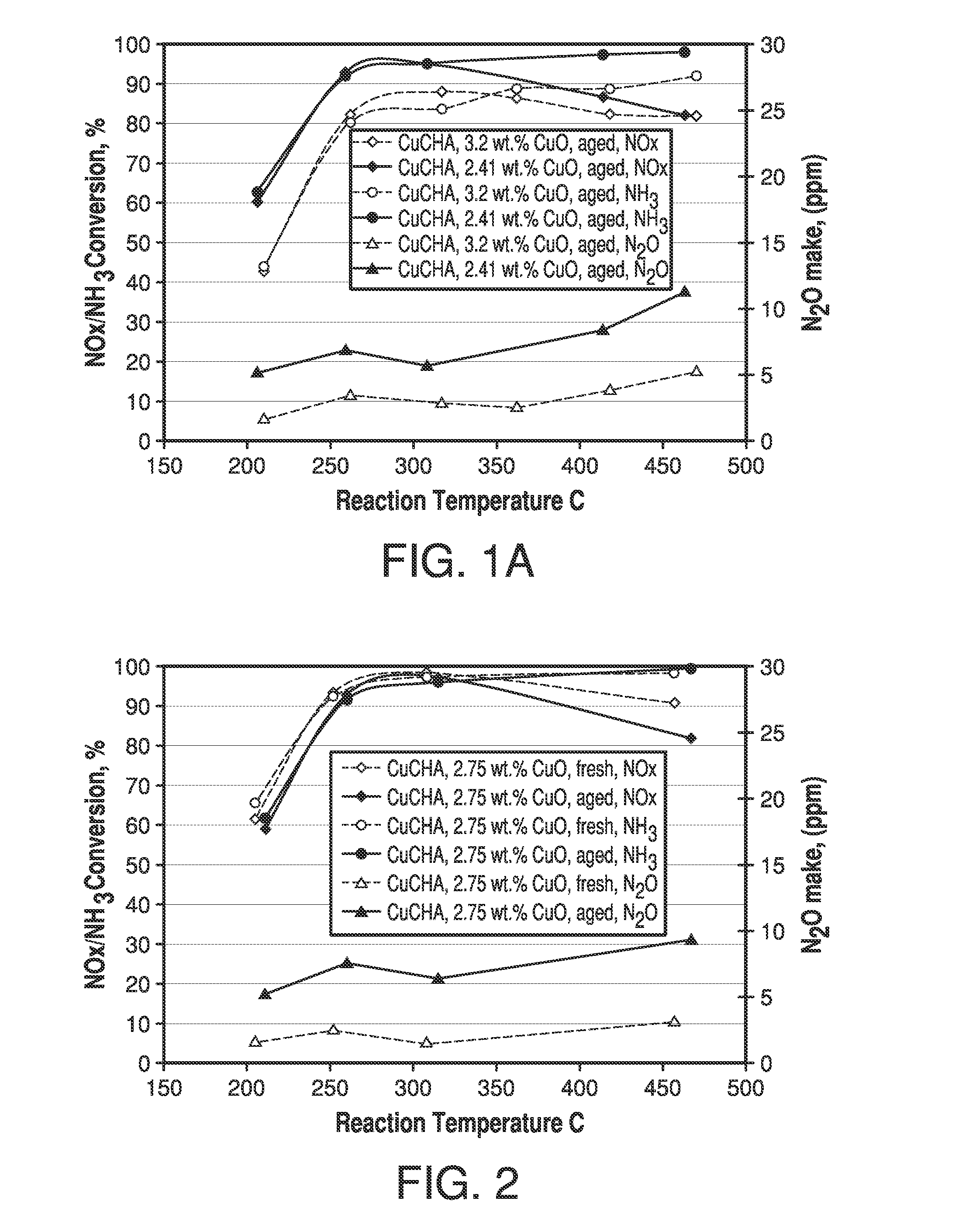
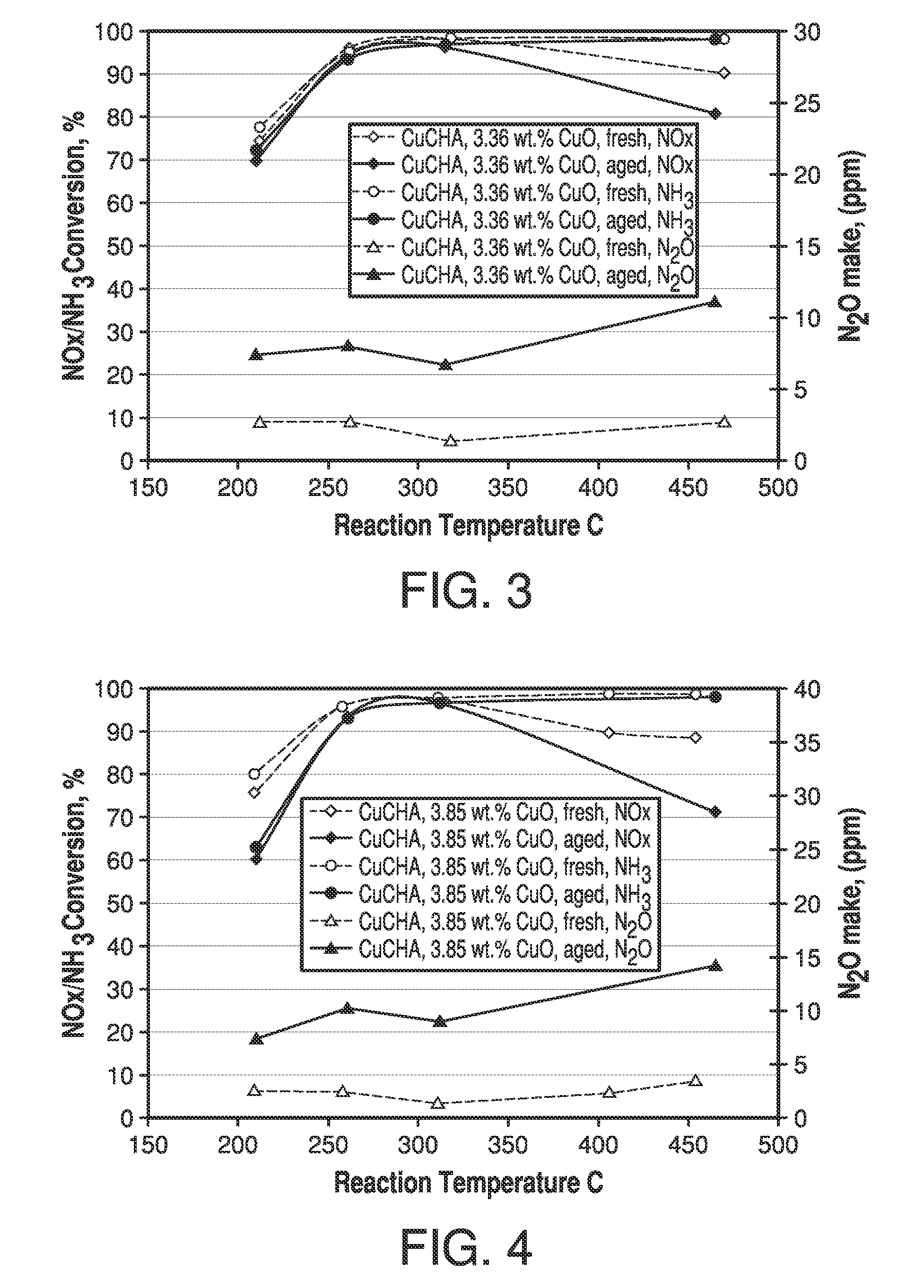
Copper CHA zeolite catalysts
ActiveUS7601662B2Good hydrothermal stabilityHigh catalytic activityCombination devicesAluminium compoundsReaction temperatureCrystal structure
Zeolite catalysts and systems and methods for preparing and using zeolite catalysts having the CHA crystal structure are disclosed. The catalysts can be used to remove nitrogen oxides from a gaseous medium across a broad temperature range and exhibit hydrothermal stable at high reaction temperatures. The zeolite catalysts include a zeolite carrier having a silica to alumina ratio from about 15:1 to about 256:1 and a copper to alumina ratio from about 0.25:1 to about 1:1.
Owner:BASF CORP
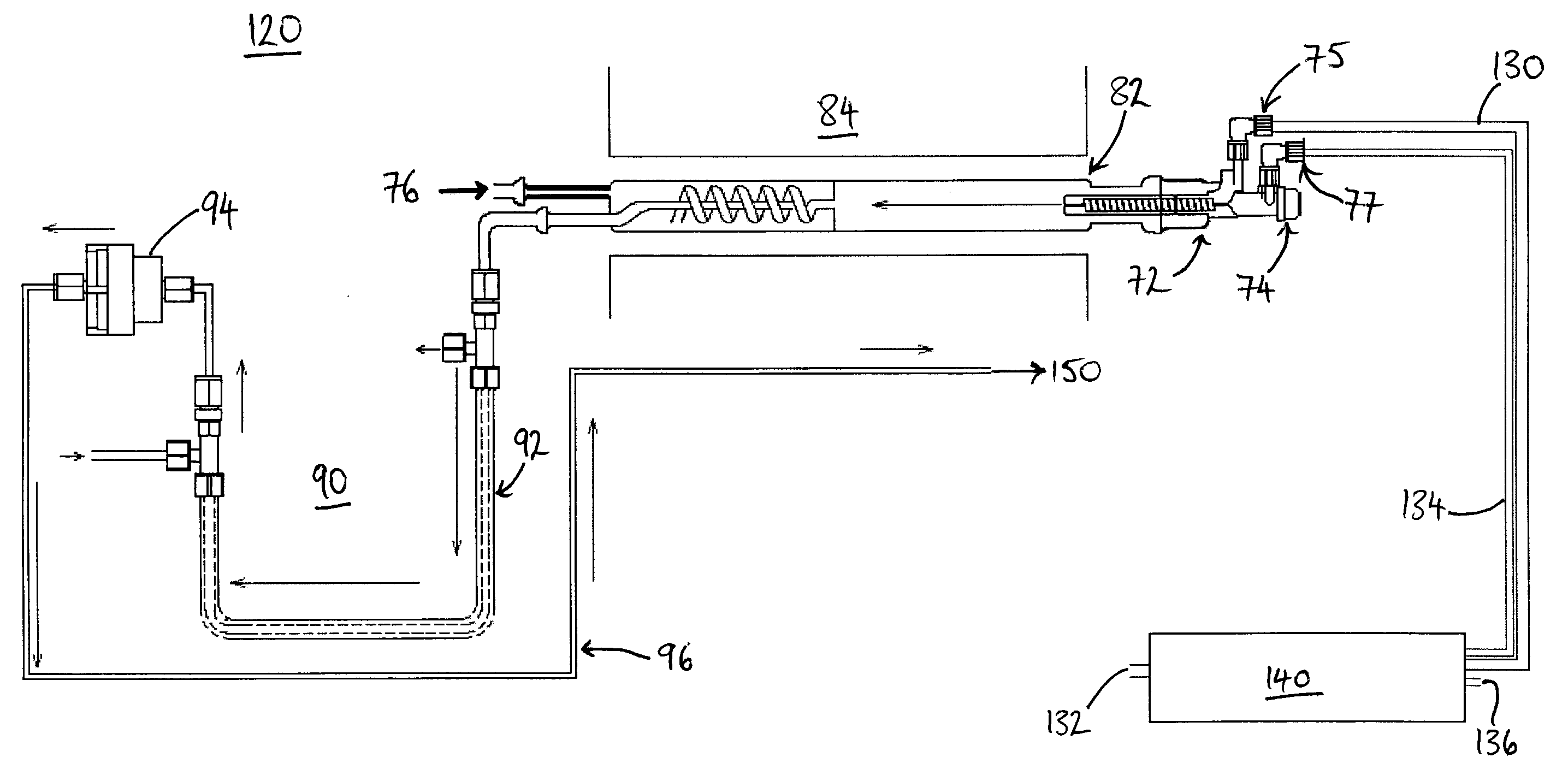
Apparatus and method for generating nitrogen oxides
InactiveUS20080176335A1Possible to useIncrease productionChemical analysis using combustionNitrogen compoundsCombustion chamberWorking temperature
A combustion analyzer apparatus and method for combustion analysing a sample, the analyzer comprising a combustion chamber (82) for receiving a sample for combustion therein to form combustion products, and a fluid supply apparatus for supplying fluid(s) into the chamber. The fluid supply apparatus (130-140) comprises a nitrogen oxides (NOx) generating apparatus (140,190,210,240) and is arranged to supply NOx into the combustion chamber. A yield of sulphur dioxide in the combustion products may thereby be improved. The NOx generating apparatus may be operated at a raised working temperature. The NOx generating apparatus may be provided by an ozonator with a supply of nitrogen and oxygen. A Venturi tube arrangement (246) may draw the generated NOx into a (carrier or oxygen) gas line to the combustion chamber. Ozone may be supplied to the combustion products to convert nitrogen monoxide therein to nitrogen dioxide. The NOx and ozone may be supplied by a single device (210,240).
Owner:THERMO ELECTRON MFG
Zone coated catalyst to simultaneously reduce NOx and unreacted ammonia
ActiveUS20060039843A1Efficient conversionReduce riskCombination devicesNitrogen compoundsEngineeringInternal combustion engine
Provided is an emissions treatment system and method for reducing NOx emissions in the exhaust stream produced from an internal combustion engine. The system has an injector for periodically metering ammonia or an ammonia precursor into an exhaust stream; and a first substrate with a first SCR catalyst composition, downstream of the injector. The first substrate has an inlet end, an outlet end, a length extending between the inlet end to the outlet end, wall elements and a plurality of passages defined by the wall elements. The first SCR catalyst composition is disposed on the wall elements from the inlet end toward the outlet end to a length that is less than the substrate's axial length to form an inlet zone. The first substrate also has an NH3 destruction catalyst composition with a platinum group metal component dispersed on a refractory metal oxide. The NH3 destruction catalyst is disposed on the wall elements from the outlet end toward the inlet end to a length that is less than the substrate's axial length to form an outlet zone. Generally, there is from 0.1 to 10 g / ft3 of platinum group metal component in the outlet zone.
Owner:BASF CATALYSTS LLC
Adhesion method and electronic component
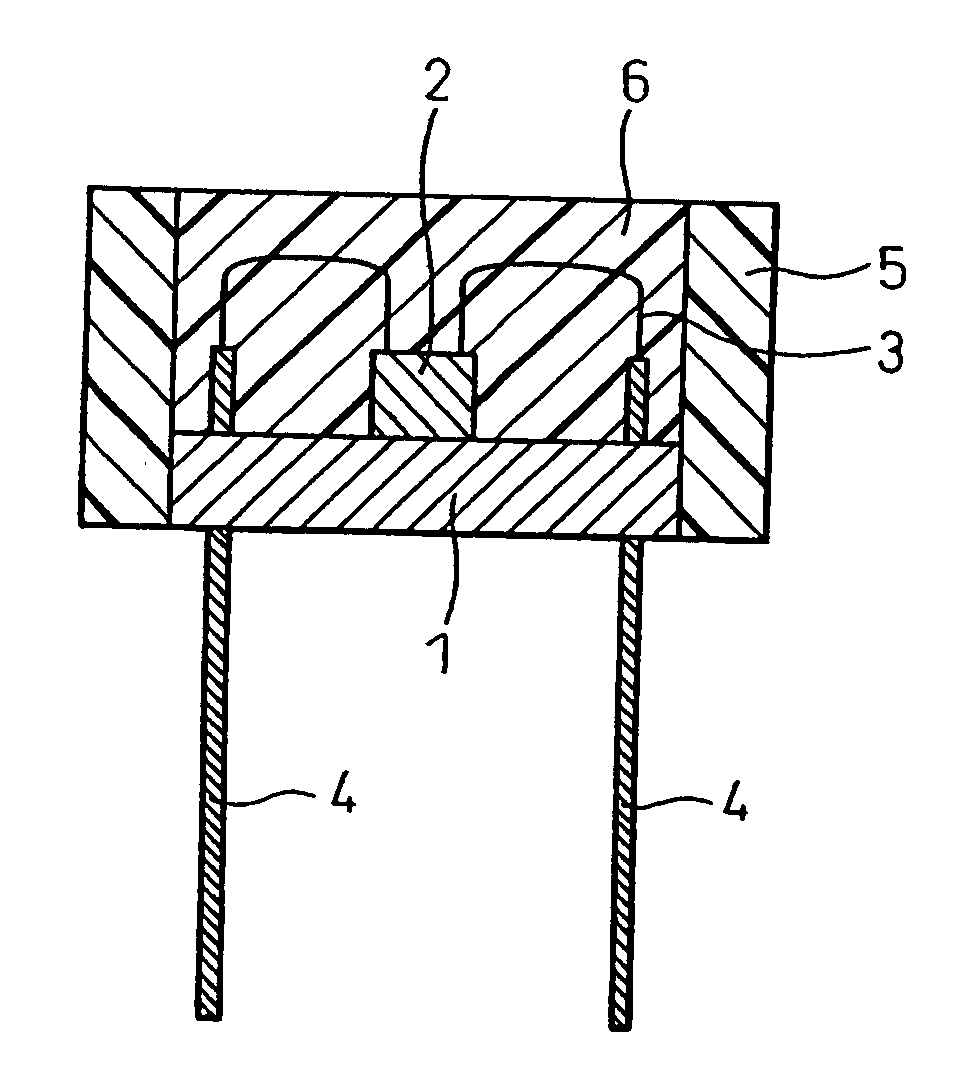
InactiveUS20010004131A1Efficiently dissipatedEnhanced radiationAdhesive processesLamination ancillary operationsAdhesiveBoron nitride
The present invention provides an adhesion method of improving the heat conduction in a fixed direction by using a heat conductive adhesive made by blending boron nitride powder and adhesive polymer and adhering by orienting boron nitride powder in the heat conductive adhesive to the fixed direction under the magnetic atmosphere and an electronic component for effectively dissipating heat generated from semiconductor device 2, power source 4, light source or other components used for the electric products, and an electronic component excellent in radiation.
Owner:POLYMATECH CO LTD
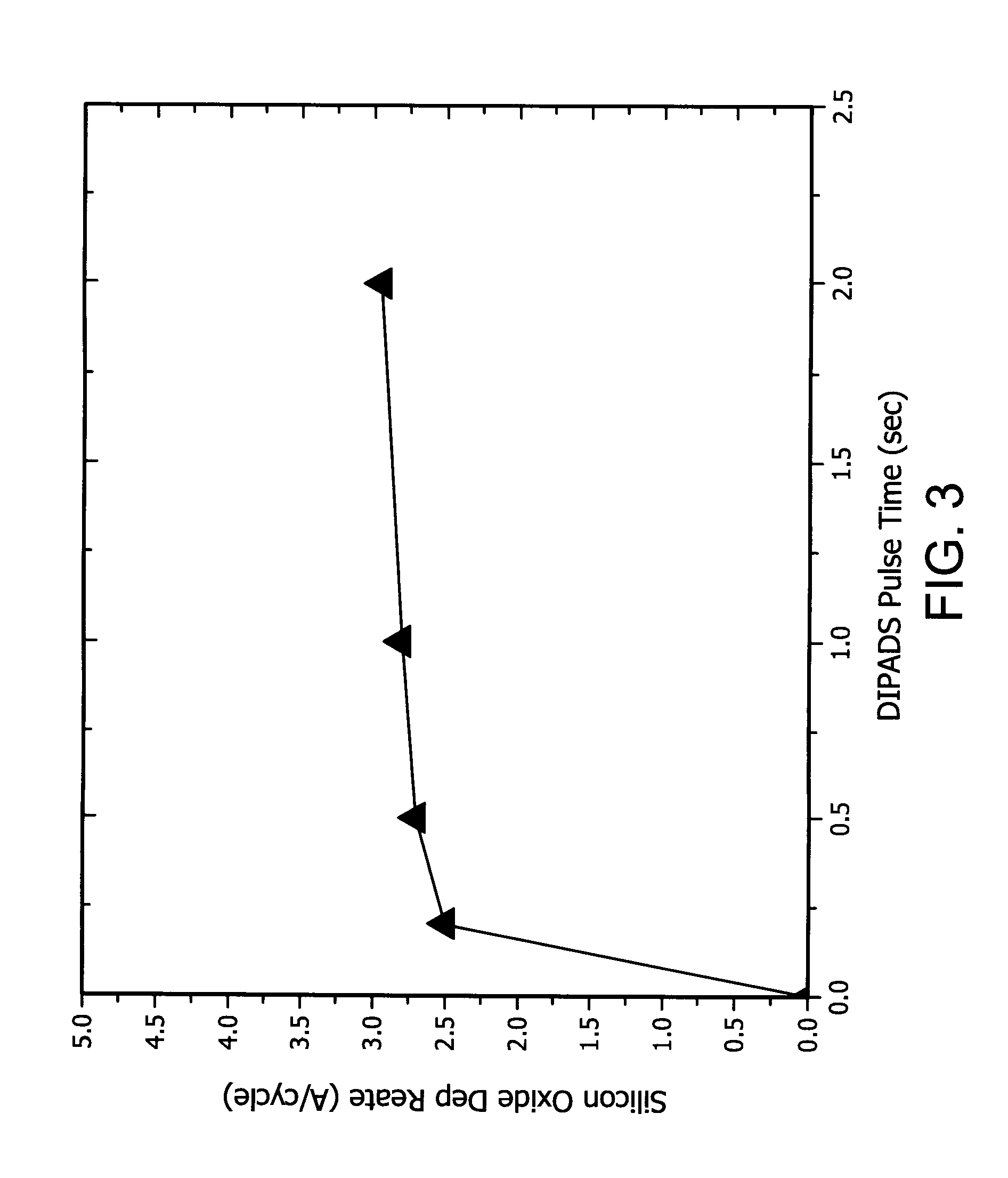
Vapor deposition reactor and method for forming thin film
Owner:VEECO ALD
Hybrid catalyst system for exhaust emissions reduction
InactiveUS20060010857A1Improve efficiencySpeed up the conversion processHydrogenGas treatmentExhaust fumesEngineering
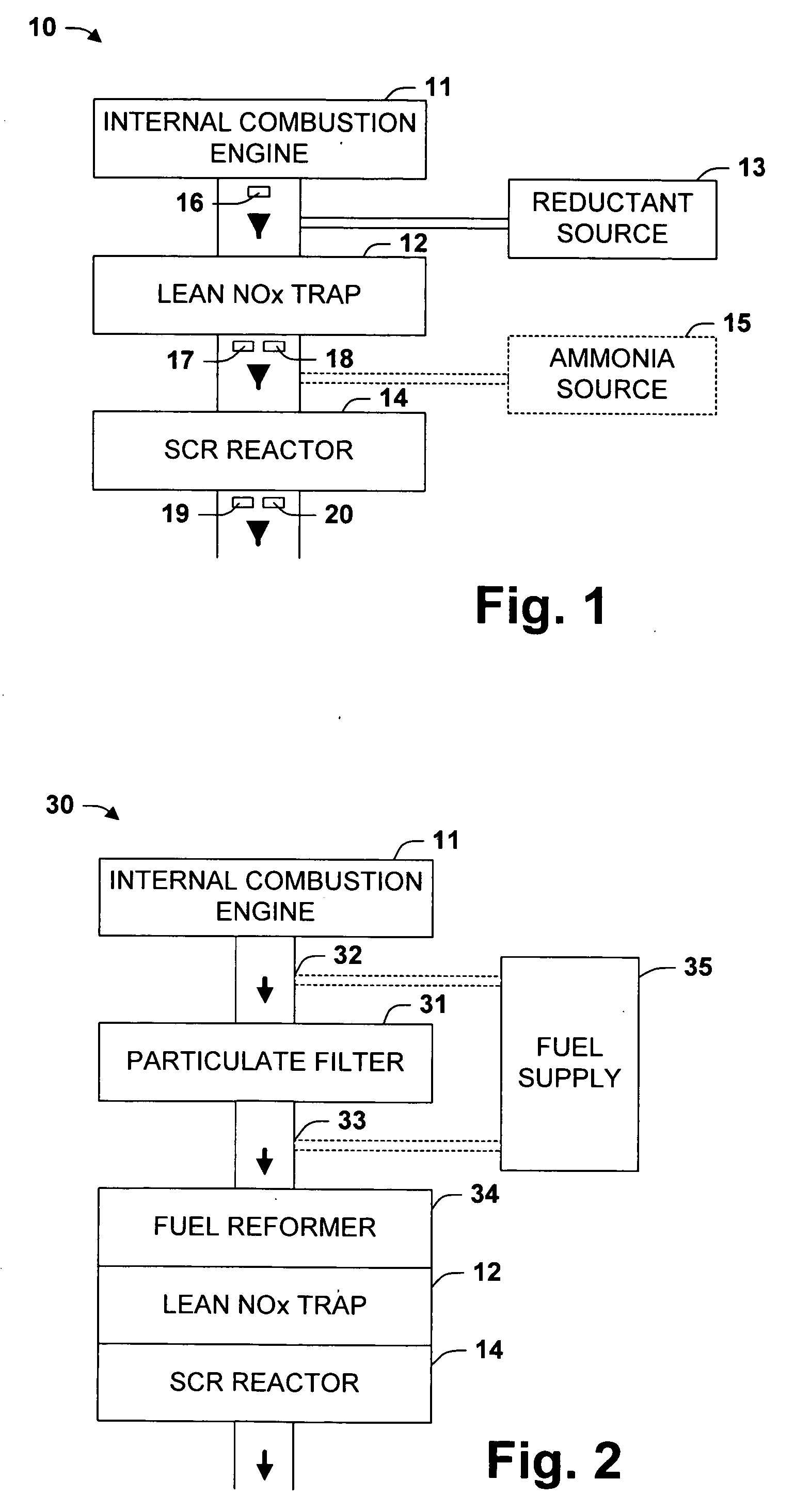
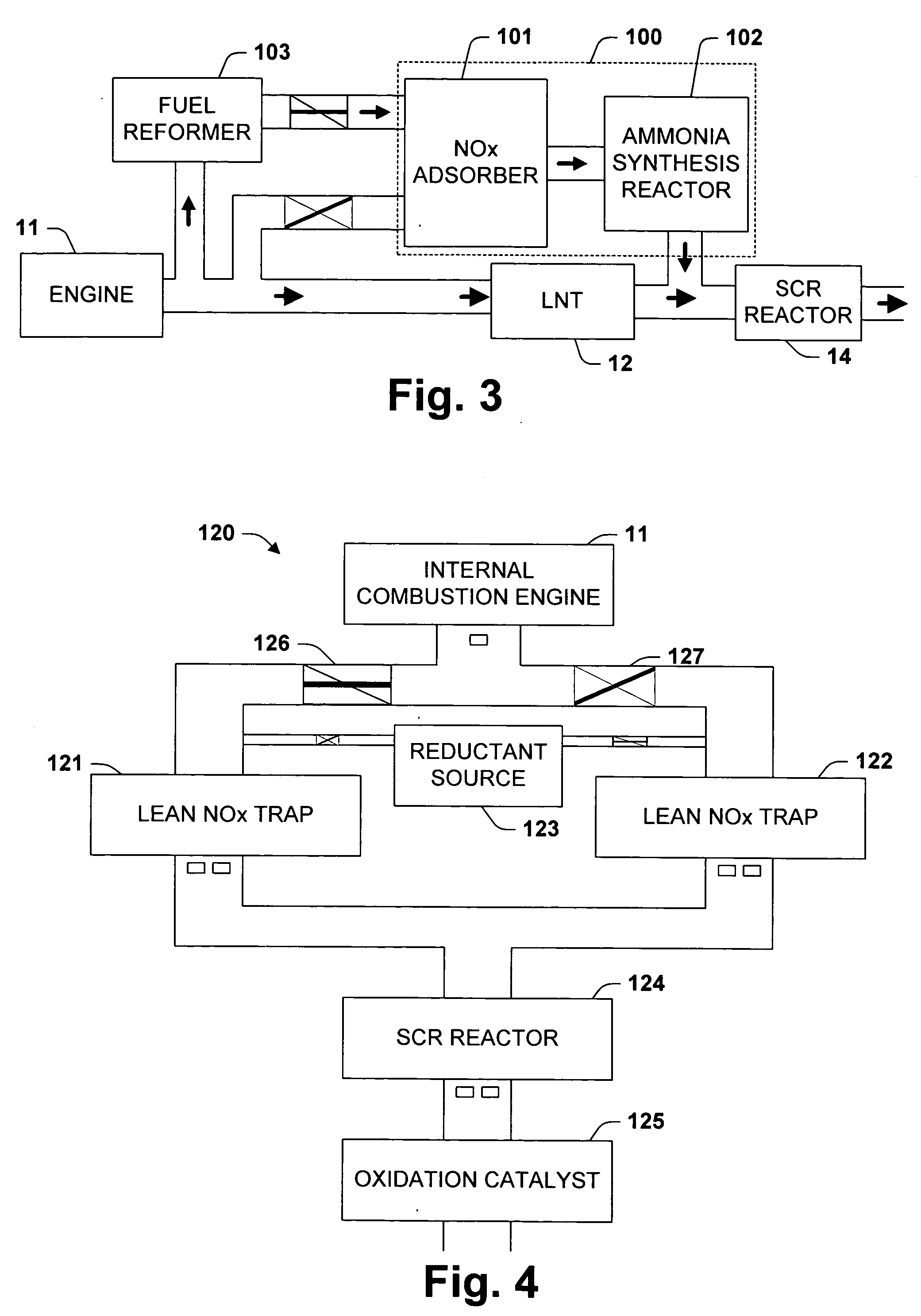
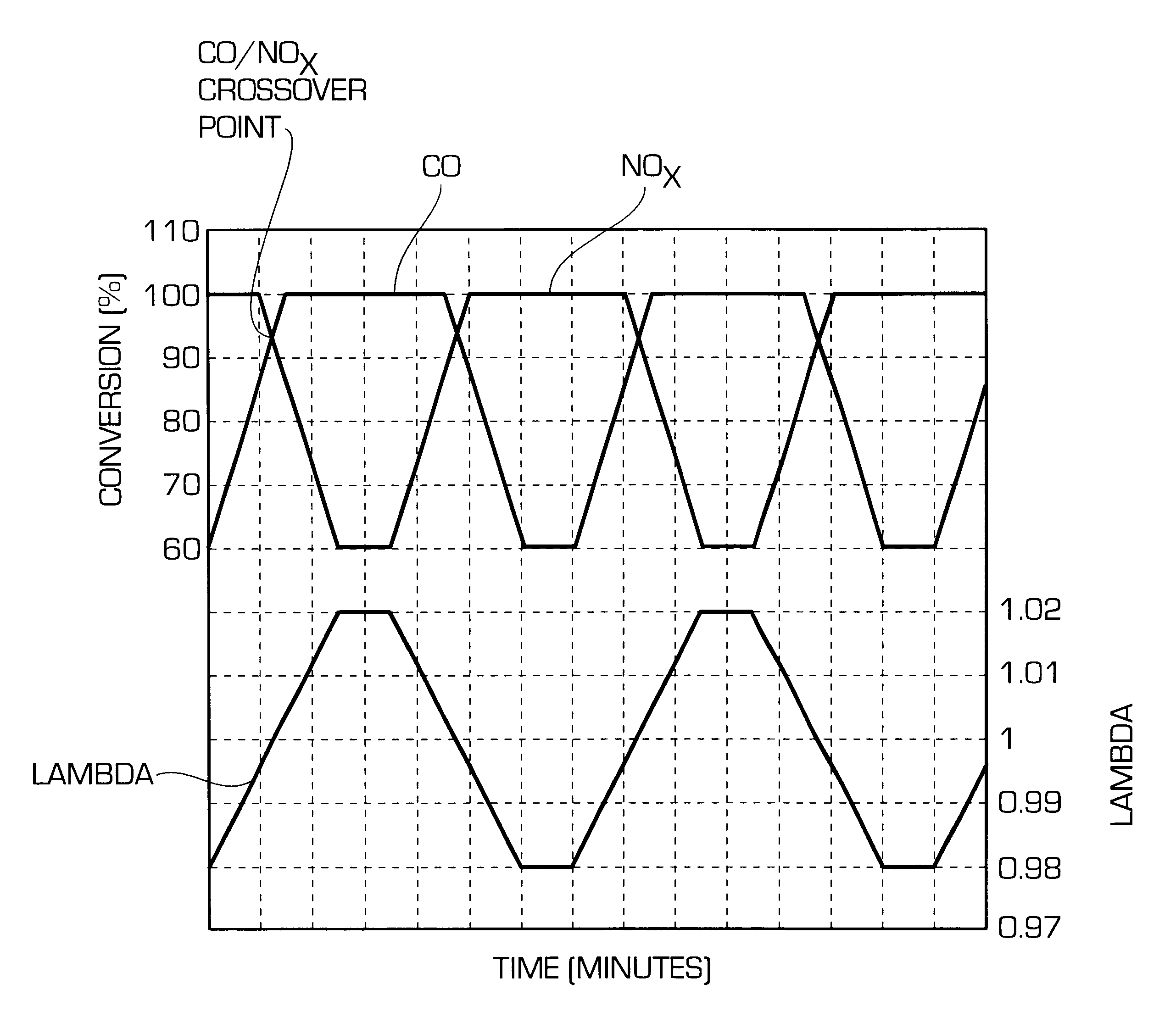
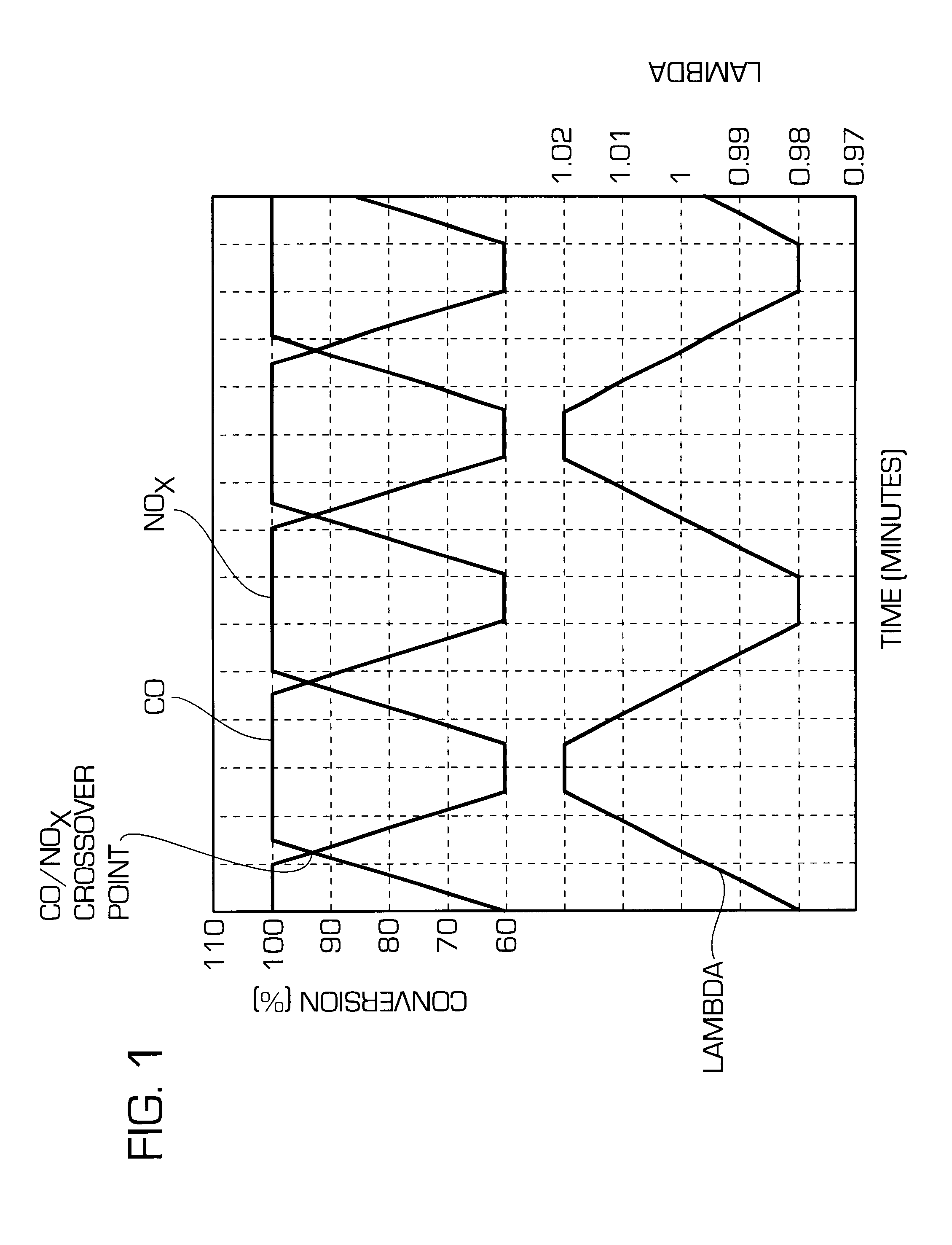
One aspect of the invention relates an exhaust treatment system having an SCR reactor following a NOx adsorber. Syn gas is used to regenerate the NOx adsorber. Another aspect relates to an LNT / SCR provided with an ammonia source separate from the LNT. A further aspect relates to a system comprising first and second LNTs and one or more SCRs downstream of the LNTs. A still further aspect relates to a device comprising first and second NOx adsorbers contained in a single housing. Another aspect relates to coating a surface of a moving part in an exhaust system with an oxidation catalyst to mitigate fouling. Additional aspects of the invention relate to strategies for controlling one or more of the time to initiate a regeneration cycle, the time to terminate a regeneration cycle, and the reductant injection rate during regeneration of LNT / SCR exhaust treatment systems.
Owner:INT ENGINE INTPROP CO LLC
Layered noble metal-containing exhaust gas catalyst and its preparation
InactiveUS6294140B1Shorten recovery timeImprove conversion efficiencyOrganic chemistryNitrogen compoundsCerium(IV) oxideEngineering
A catalyst for treating exhaust gas from an internal combustion engine includes a carrier body coated with an inner layer and an outer layer. The inner layer includes platinum deposited on a first support material and on a first oxygen storage component, and the outer layer includes platinum and rhodium deposited on a second support material and on a second oxygen storage component. The first and second support materials may be the same or different, and may be selected from the group of: silica, alumina, titania, zirconia, mixed oxides or mixtures thereof, and zirconia-rich zirconia / ceria mixed oxide. The first and second oxygen storage components may include ceria-rich ceria / zirconia mixed oxide compounds, optionally including praseodymia, yttria, neodymia, lanthana or mixtures thereof.
Owner:DMC2 DEGUSSA METALS +1
Thin film battery and electrolyte therefor
A solid amorphous electrolyte composition for a thin-film battery. The electrolyte composition includes a lithium phosphorus oxynitride material containing a sulfide ion dopant wherein the atomic ratio of sulfide ion to phosphorus ion (S / P) in the electrolyte ranges greater than 0 up to about 0.2. The composition is represented by the formula: where 2x+3y+2z=5+w, x ranges from about 3.2 to about 3.8, y ranges from about 0.13 to about 0.46, z ranges from greater than zero up to about 0.2, and w ranges from about 2.9 to about 3.3. Thin-film batteries containing the sulfide doped lithium oxynitride electrolyte are capable of delivering more power and energy than thin-film batteries containing electrolytes without sulfide doping.
Owner:OAK RIDGE MICRO ENERGY
Method for combined removal of mercury and nitrogen oxides from off-gas streams
InactiveUS7118720B1Promote oxidationProcess economyGas treatmentNitrogen compoundsNitrogen dioxideNitrogen oxide
A method for removing elemental Hg and nitric oxide simultaneously from a gas stream is provided whereby the gas stream is reacted with gaseous chlorinated compound to convert the elemental mercury to soluble mercury compounds and the nitric oxide to nitrogen dioxide. The method works to remove either mercury or nitrogen oxide in the absence or presence of each other.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Control of mercury emissions from solid fuel combustion
InactiveUS6848374B2Remove pollutantsEasy to captureCombination devicesGas treatmentSorbentSolid fuel
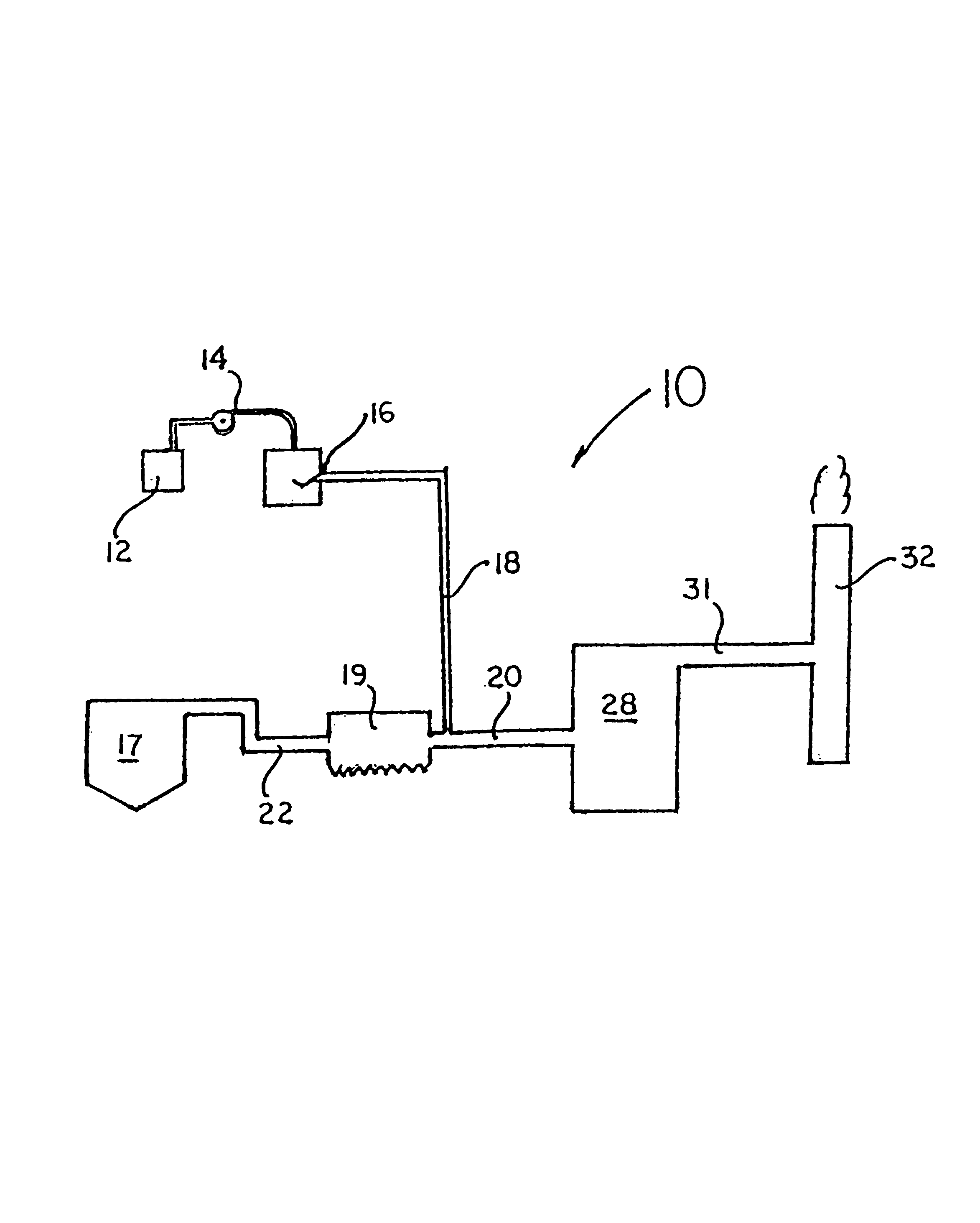
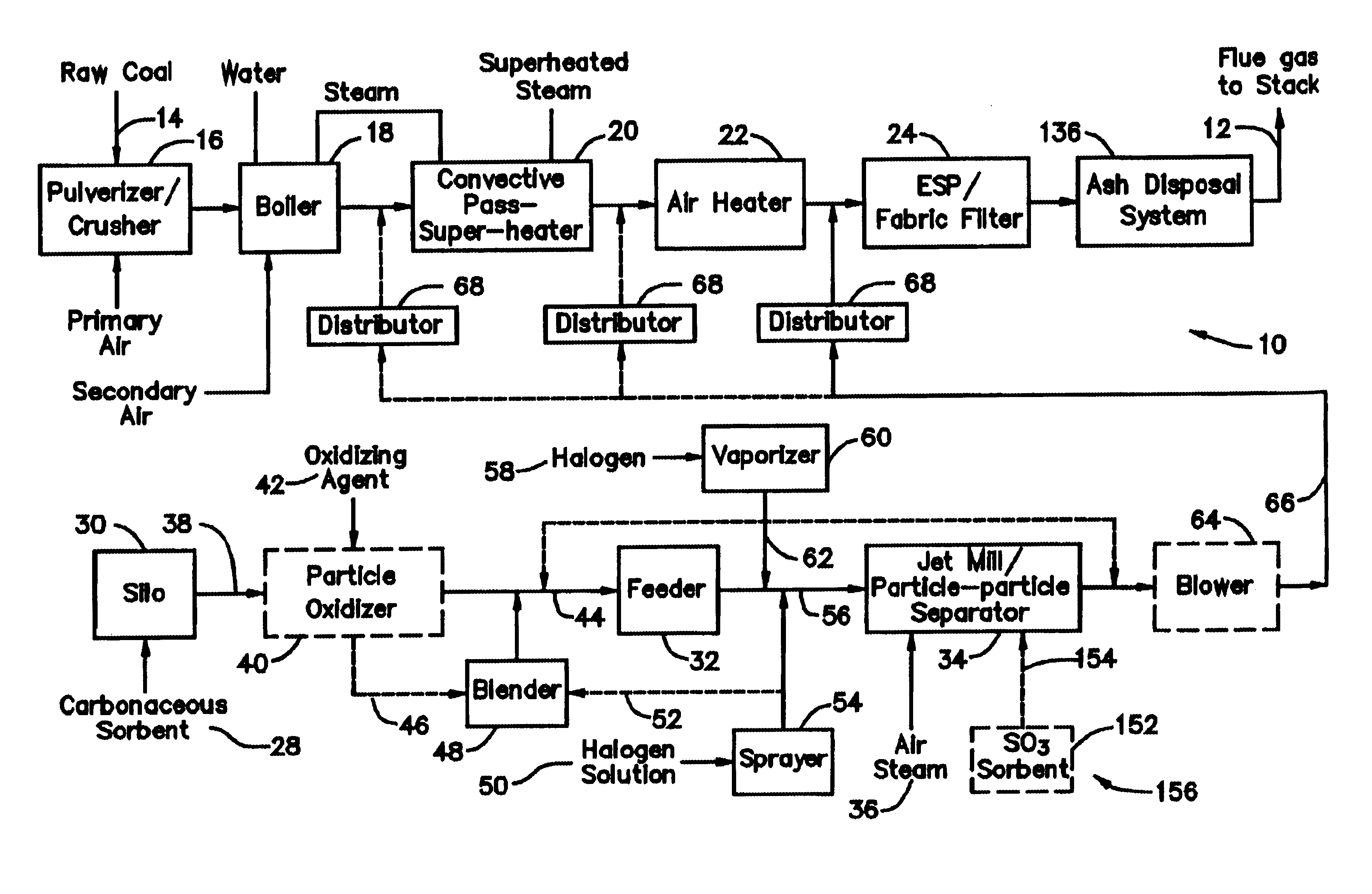
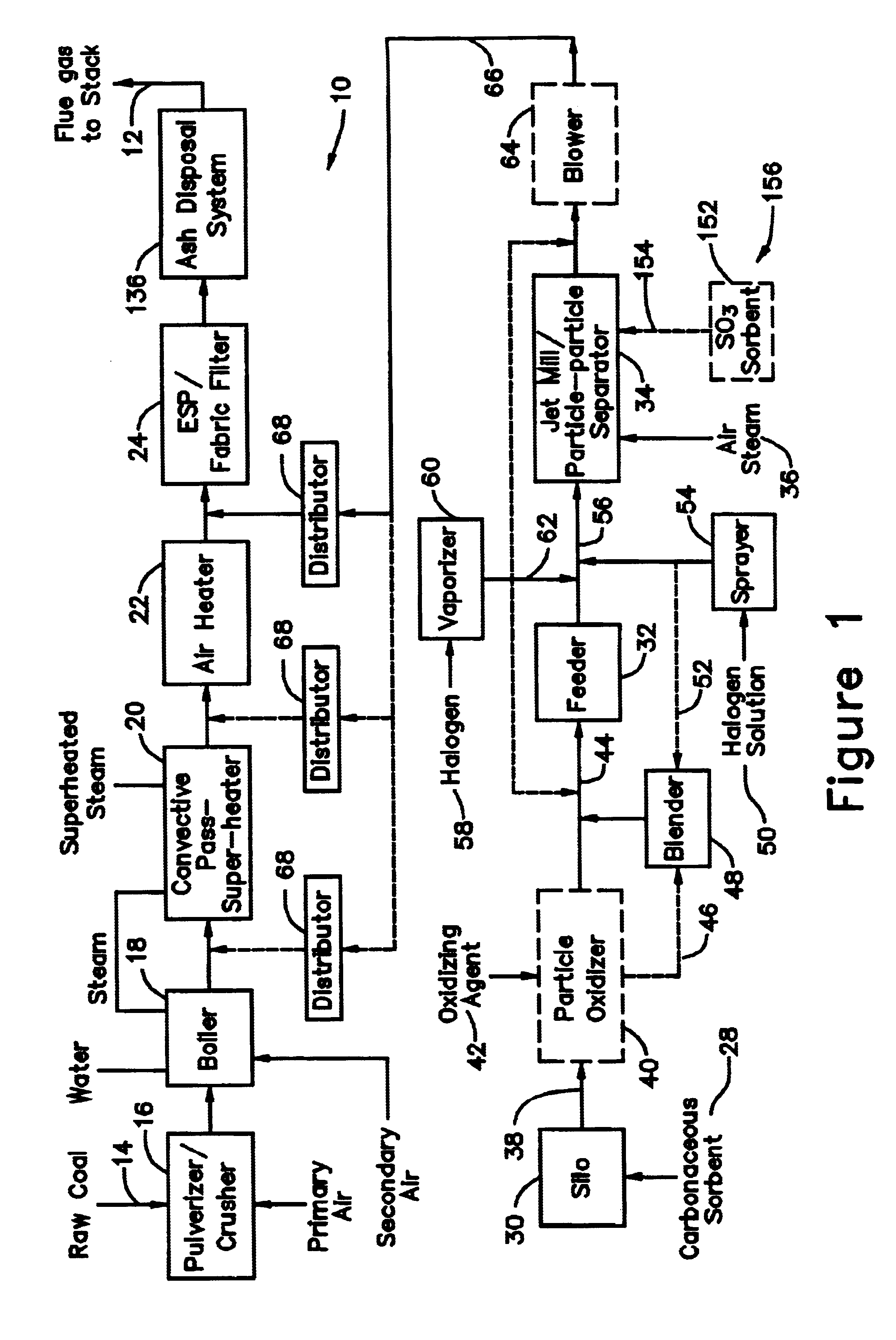
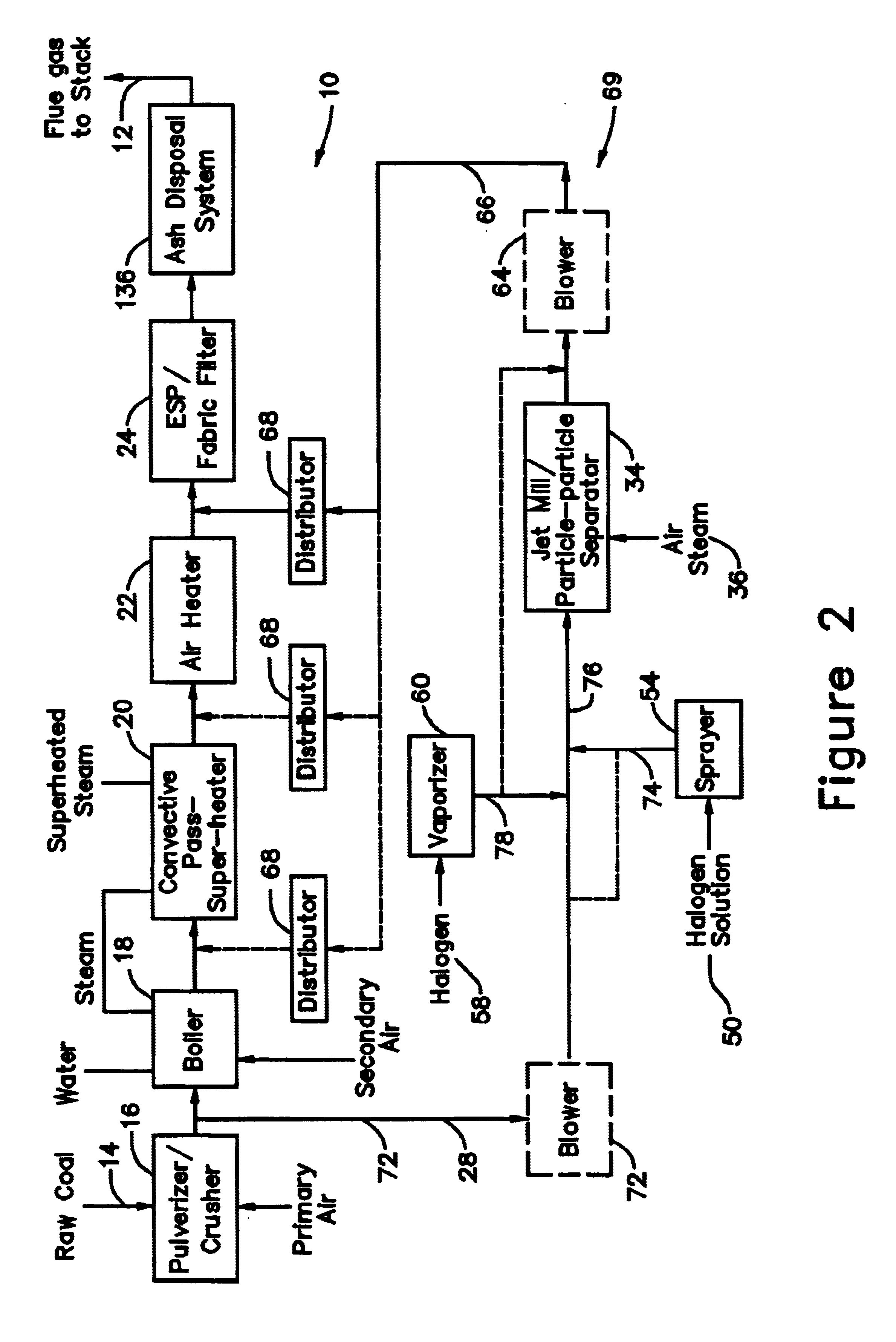
A system 26 for removing elemental mercury or mercury compounds handles carbonaceous sorbent 28 of a starter batch stored in a silo 30 in an agglomerated state. The sorbent 28 is fed by a feeder 32 to a separation device 34, which comminutes (if necessary) and de-agglomerates the sorbent particles 28 to their primary size distribution. This device 34 may be a particle-particle separator or a jet mill, where compressed air or high-pressure steam is the energy source. The de-agglomerated sorbent 28 of a contact batch created from the starter batch is conveyed by an airsteam for injection at a contact location 66 in a flue gas duct whereat carbonaceous sorbent of the contact batch adsorbs mercury from the flue gas.
Owner:GENERAL ELECTRIC TECH GMBH
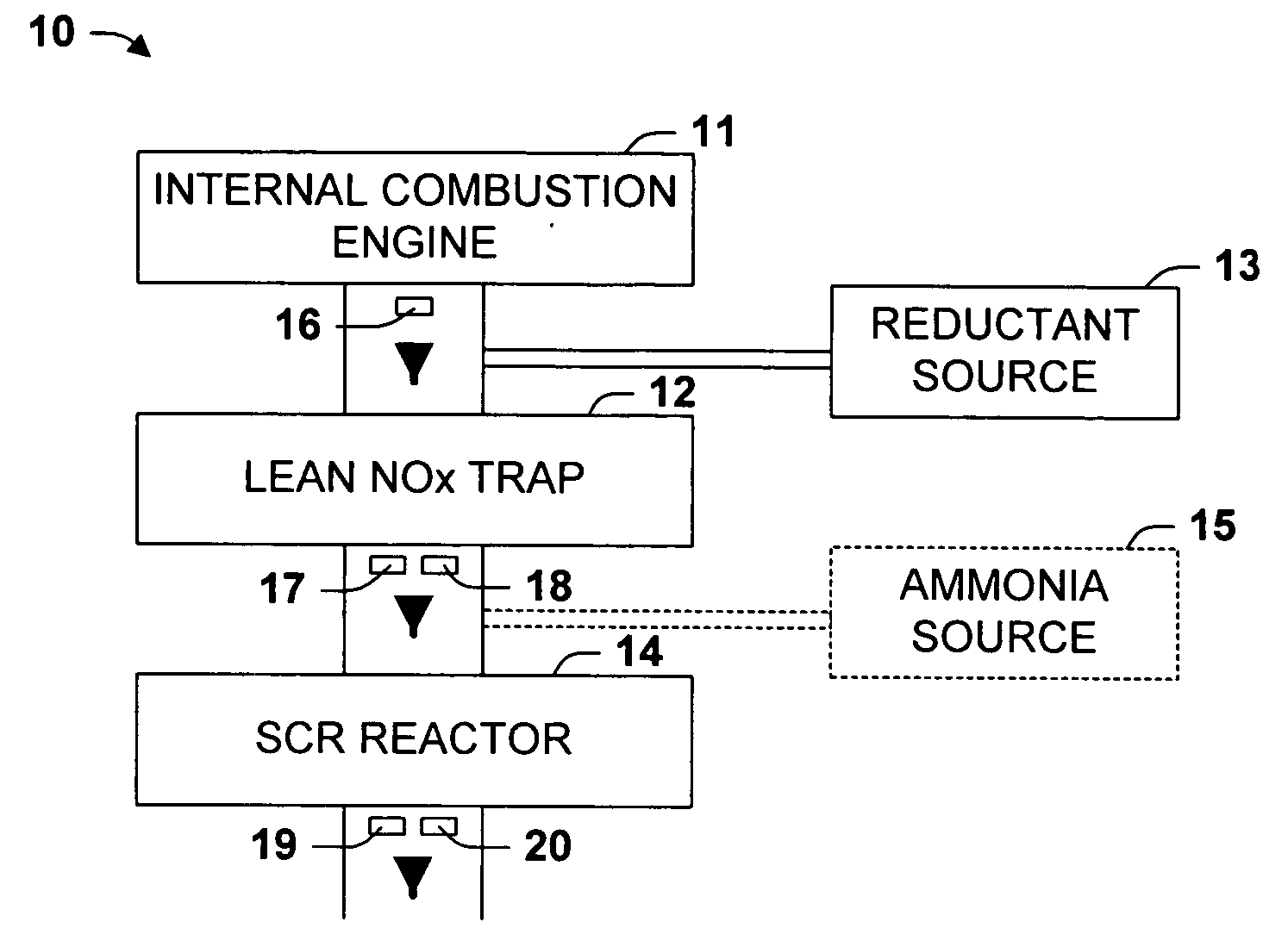
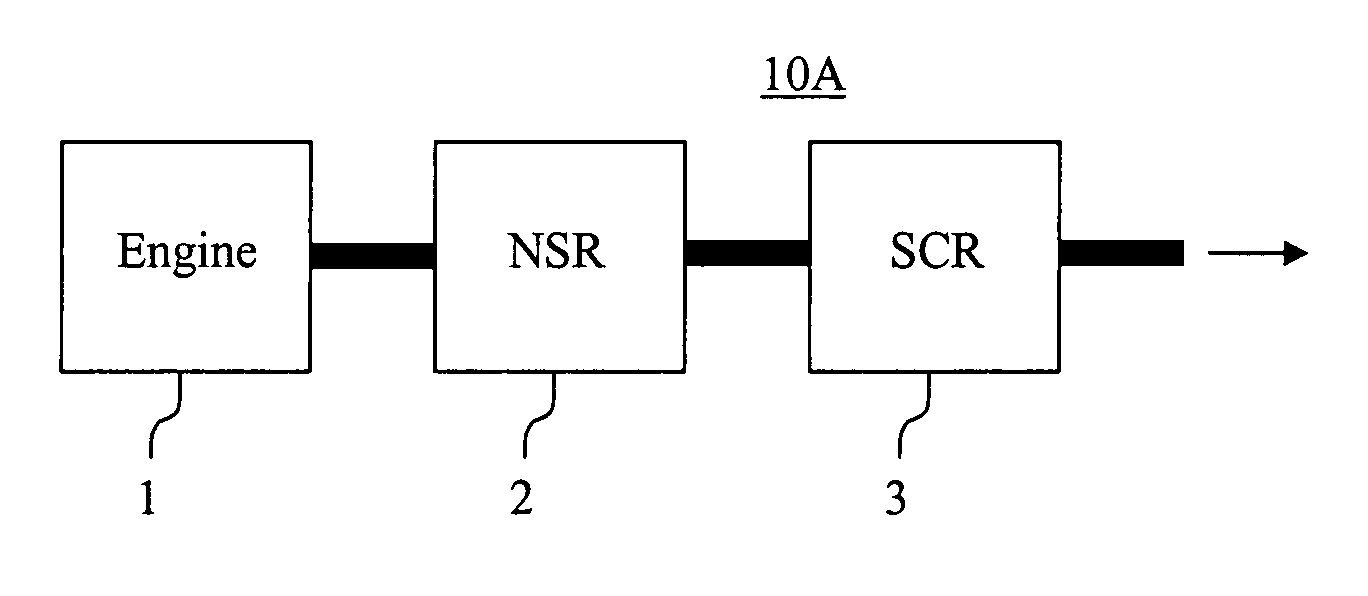
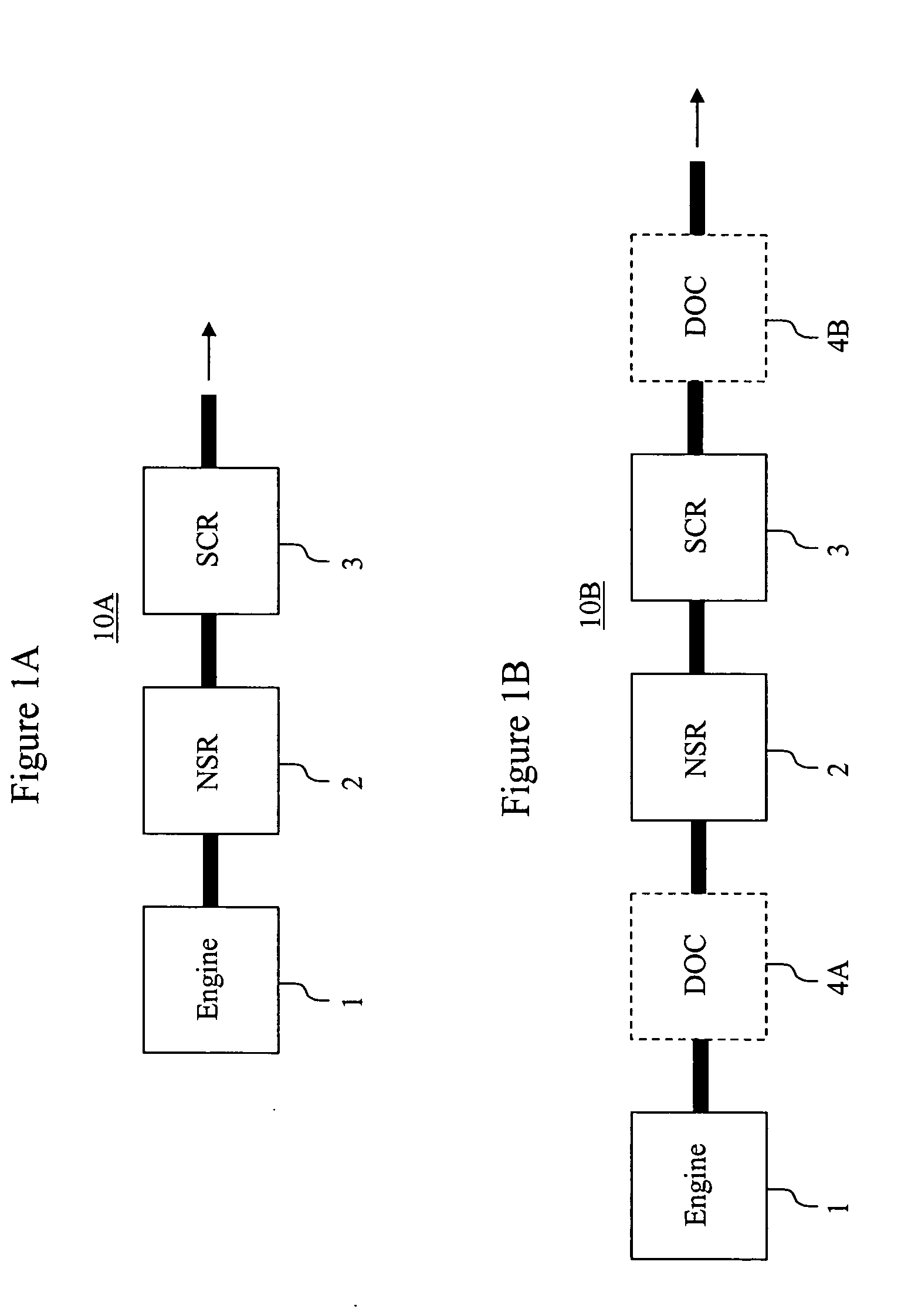
Emissions treatment system with NSR and SCR catalysts
ActiveUS20050129601A1Small sizeReduce amount particulate matterGas treatmentNitrogen compoundsSorbentGasoline
Provided is an emissions treatment system for an exhaust stream, having a NOx storage reduction (NSR) catalyst with a NOx sorbent at a concentration of at least 0.1 g / in3 and a platinum group metal component dispersed on a refractory metal oxide support; and, an SCR catalyst disposed downstream of the NSR catalyst. The emissions treatment system is advantageously used for the treatment of exhaust streams from diesel engines and lean burn gasoline engines.
Owner:BASF CATALYSTS LLC
Systems and methods for carbon capture and sequestration and compositions derived therefrom
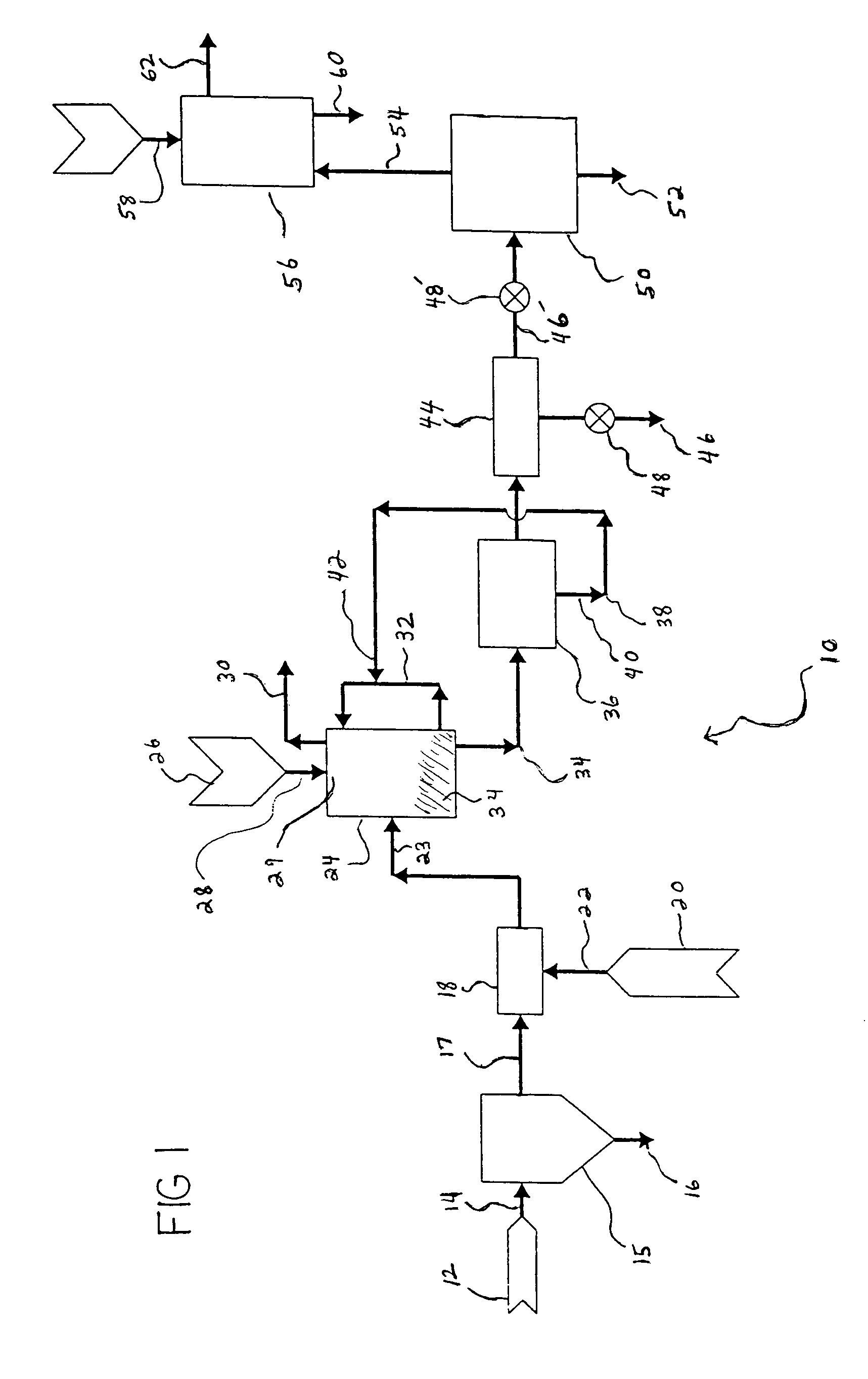
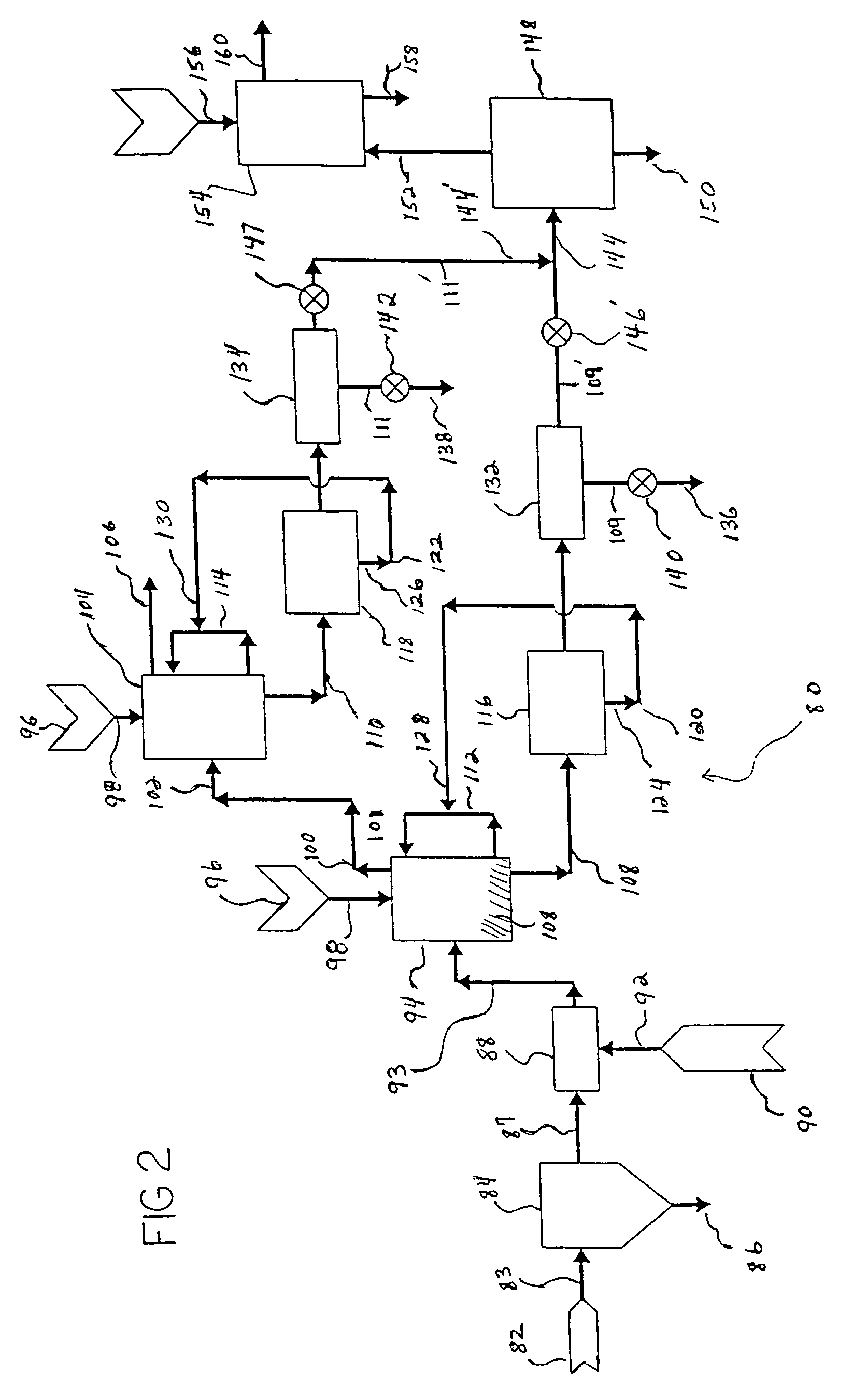
ActiveUS20090143211A1Promote formationCalcium/strontium/barium carbonatesNitrogen compoundsInterior spaceProduct formation
A method of sequestering a greenhouse gas is described, which comprises: (i) providing a solution carrying a first reagent that is capable of reacting with a greenhouse gas; (ii) contacting the solution with a greenhouse gas under conditions that promote a reaction between the at least first reagent and the greenhouse gas to produce at least a first reactant; (iii) providing a porous matrix having interstitial spaces and comprising at least a second reactant; (iv) allowing a solution carrying the at least first reactant to infiltrate at least a substantial portion of the interstitial spaces of the porous matrix under conditions that promote a reaction between the at least first reactant and the at least second reactant to provide at least a first product; and (v) allowing the at least first product to form and fill at least a portion of the interior spaces of the porous matrix, thereby sequestering a greenhouse gas.
Owner:RUTGERS THE STATE UNIV
Multi-component removal in flue gas by aqua ammonia
InactiveUS7255842B1Regeneration process is less-costlyIncrease load capacityGas treatmentNitrogen compoundsNitric oxideSlurry
A new method for the removal of environmental compounds from gaseous streams, in particular, flue gas streams. The new method involves first oxidizing some or all of the acid anhydrides contained in the gas stream such as sulfur dioxide (SO2) and nitric oxide (NO) and nitrous oxide (N2O) to sulfur trioxide (SO3) and nitrogen dioxide (NO2). The gas stream is subsequently treated with aqua ammonia or ammonium hydroxide which captures the compounds via chemical absorption through acid-base or neutralization reactions. The products of the reactions can be collected as slurries, dewatered, and dried for use as fertilizers, or once the slurries have been dewatered, used directly as fertilizers. The ammonium hydroxide can be regenerated and recycled for use via thermal decomposition of ammonium bicarbonate, one of the products formed. There are alternative embodiments which entail stoichiometric scrubbing of nitrogen oxides and sulfur oxides with subsequent separate scrubbing of carbon dioxide.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
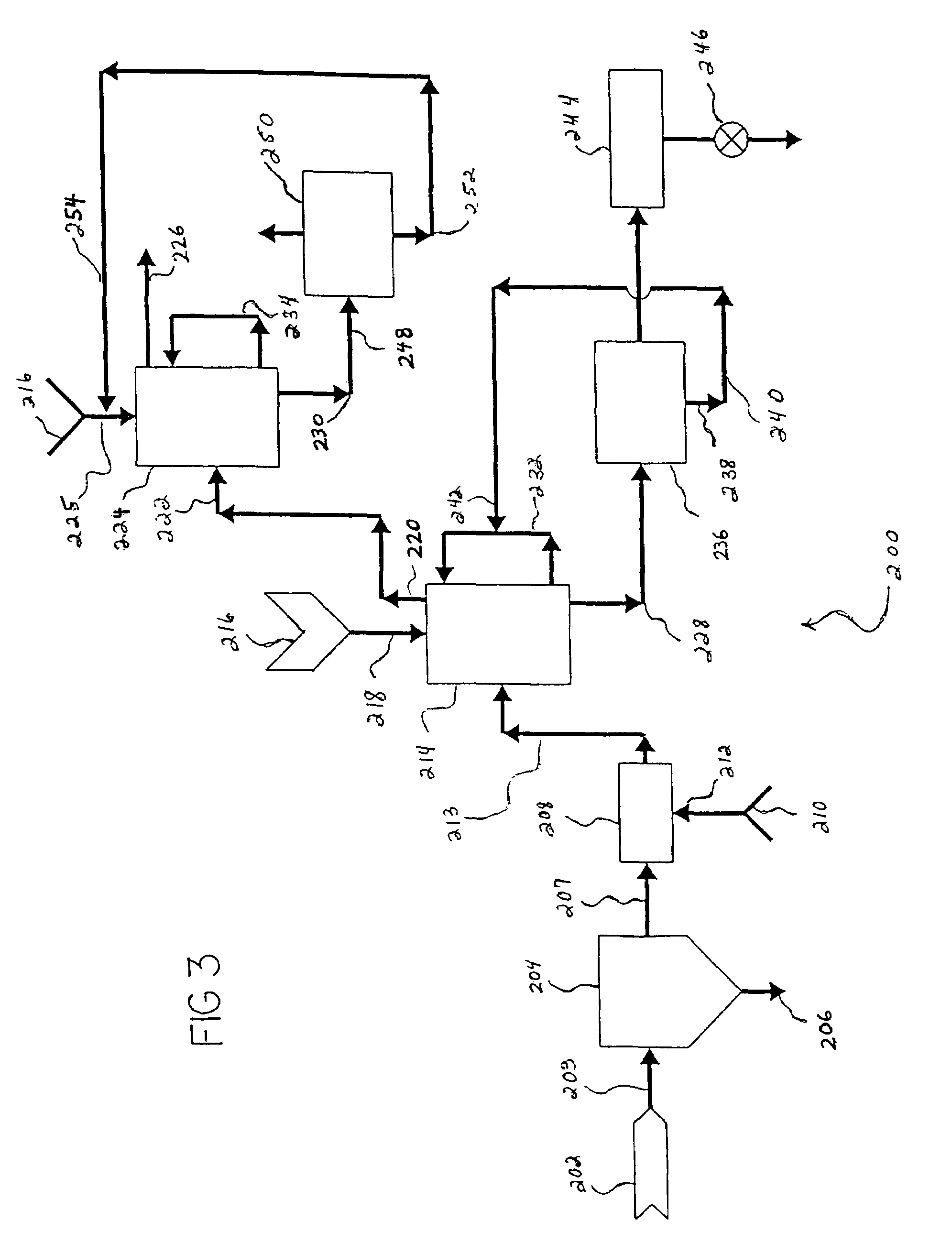
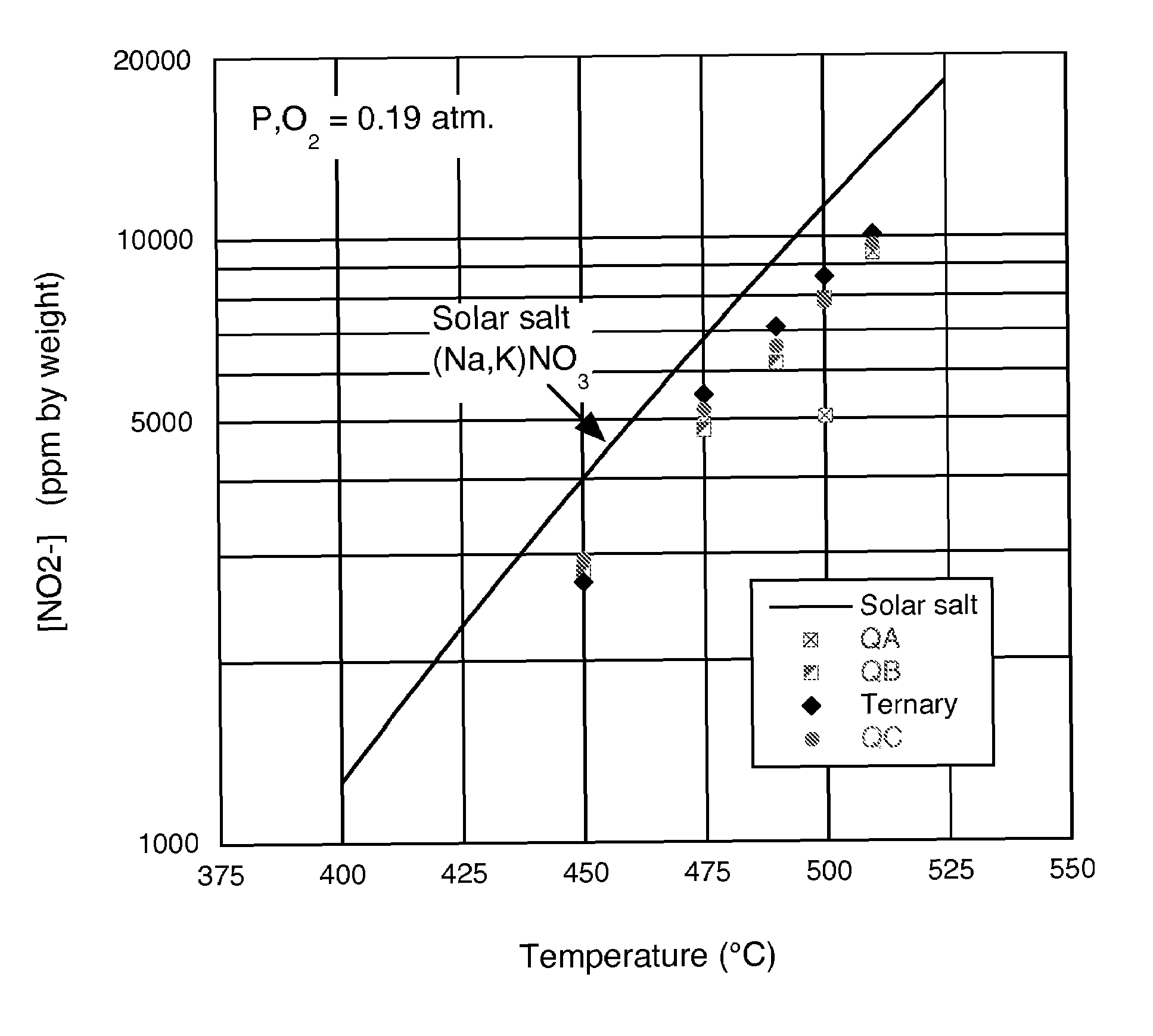
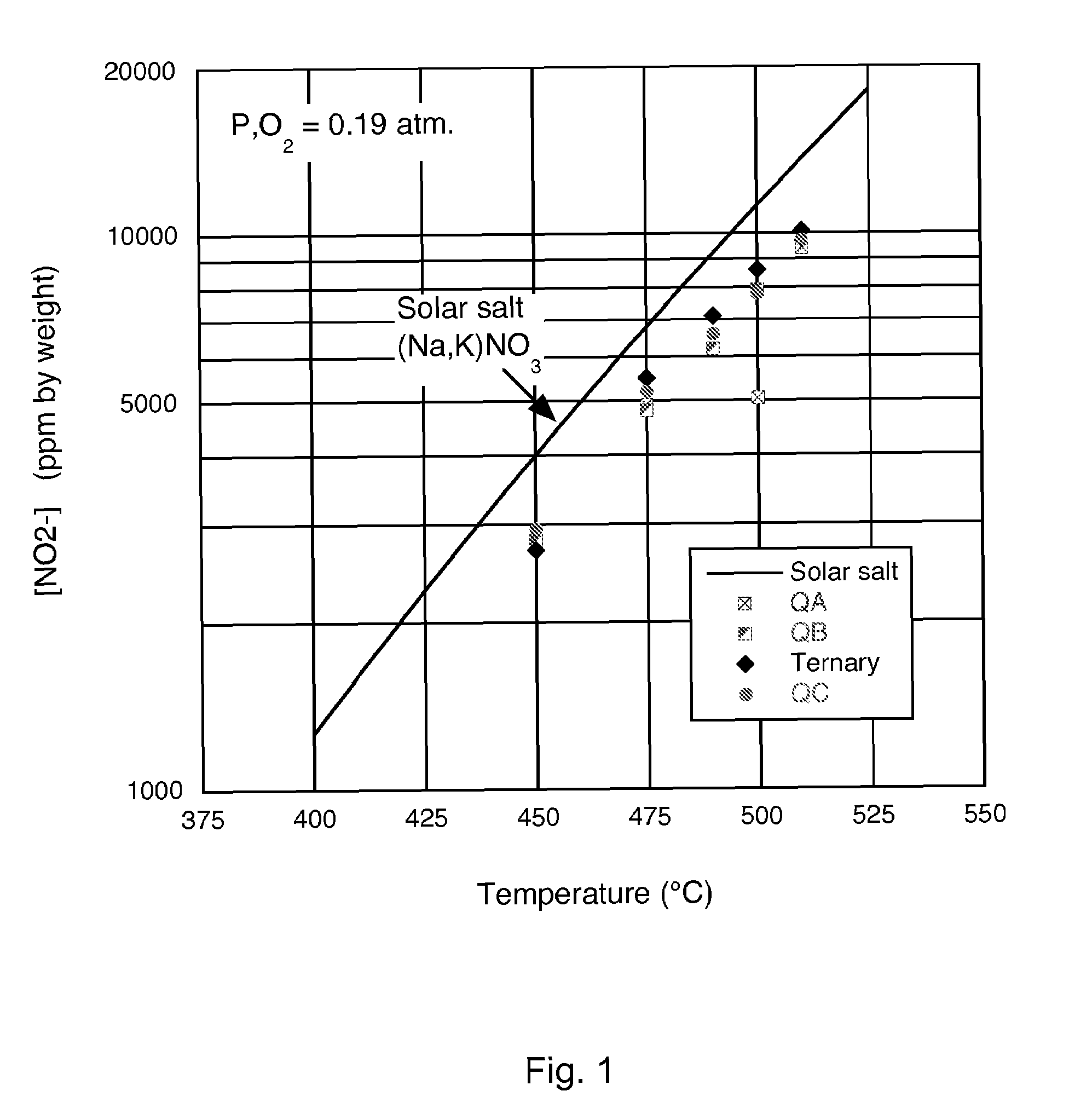
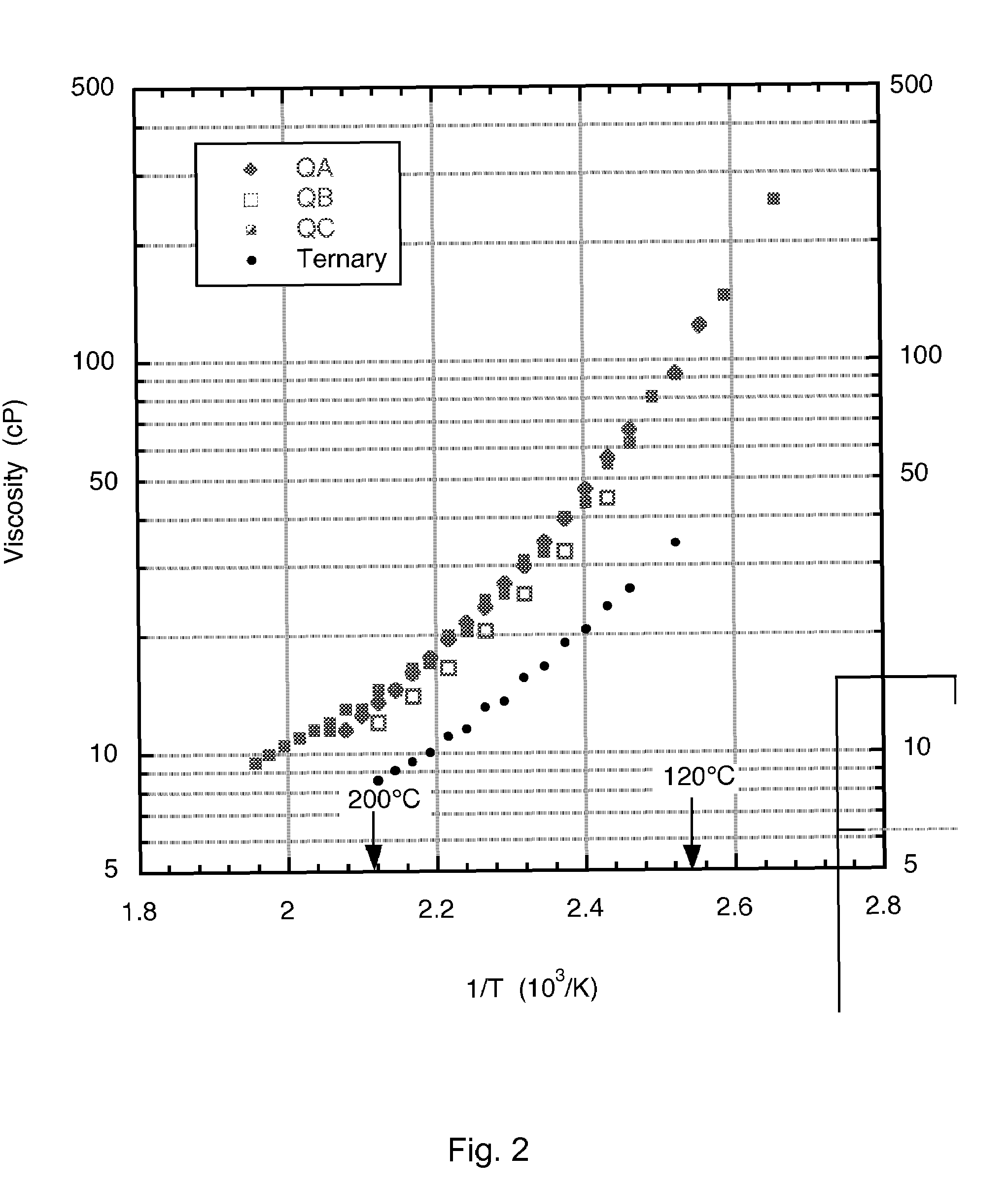
Low-melting point inorganic nitrate salt heat transfer fluid
A low-melting point, heat transfer fluid made of a mixture of four inorganic nitrate salts: 9-18 wt % NaNO3, 40-52 wt % KNO3, 13-21 wt % LiNO3, and 20-27 wt % Ca(NO3)2. These compositions can have liquidus temperatures less than 100 C; thermal stability limits greater than 500 C; and viscosity in the range of 5-6 cP at 300 C; and 2-3 cP at 400 C.
Owner:NAT TECH & ENG SOLUTIONS OF SANDIA LLC
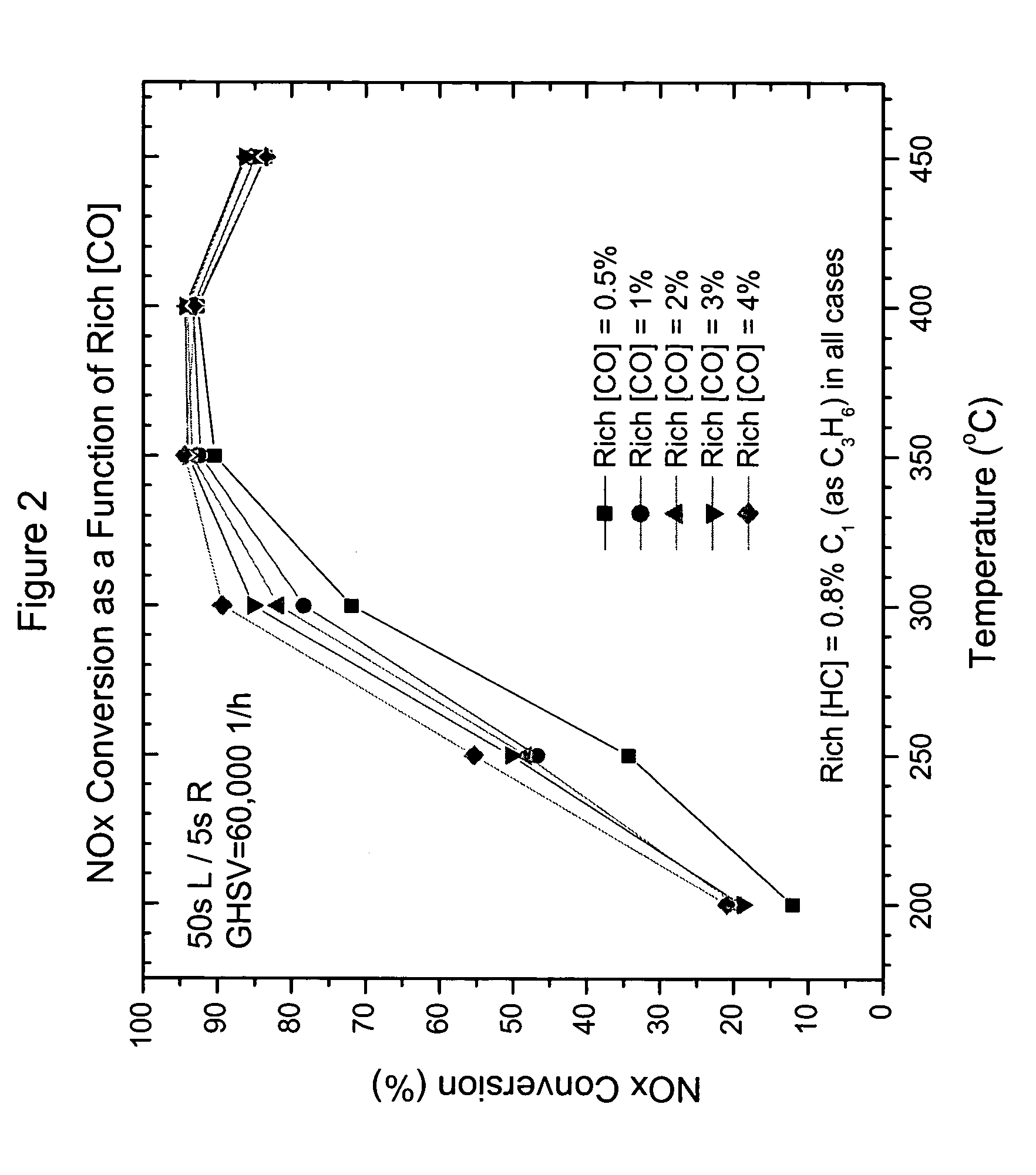
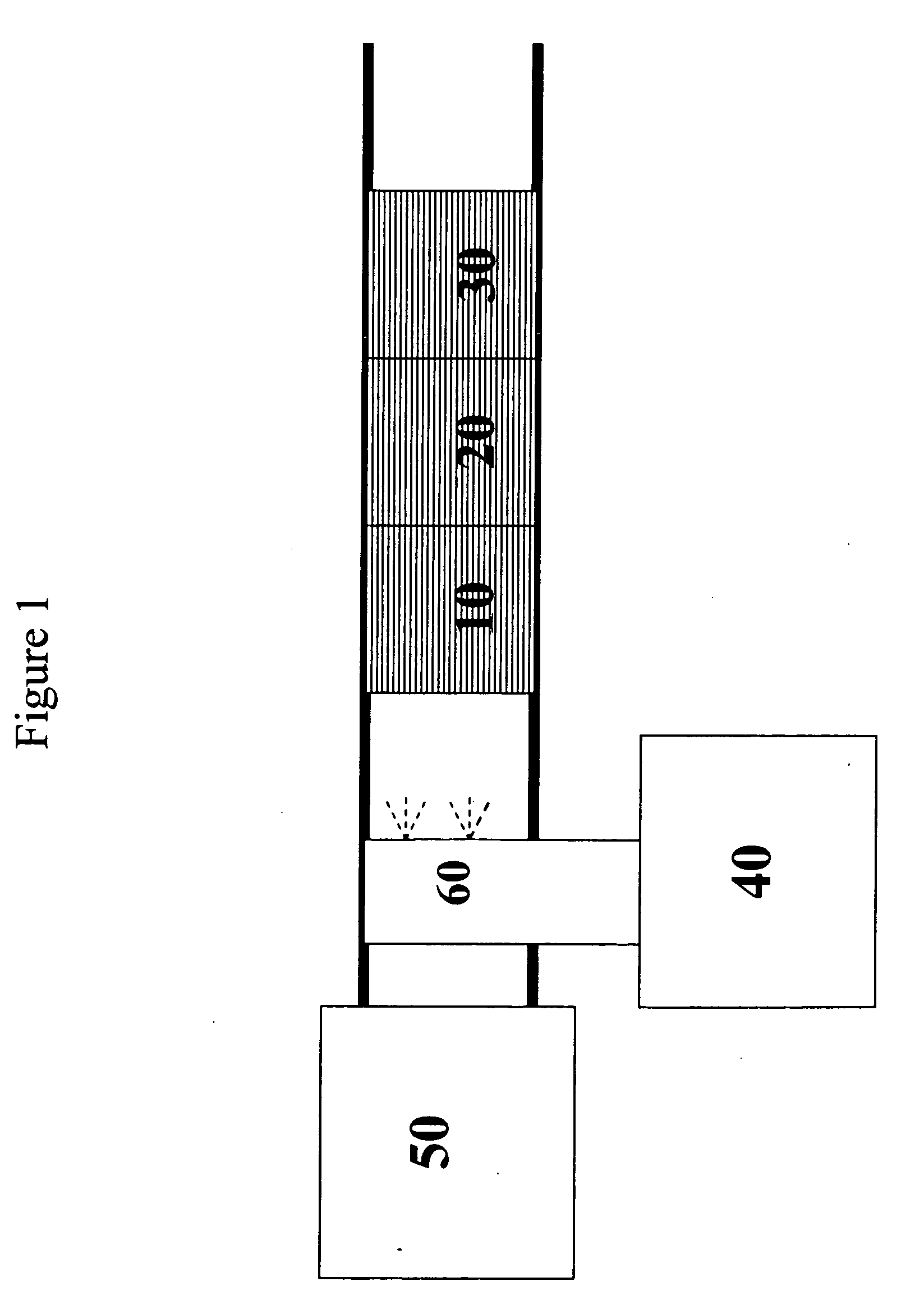
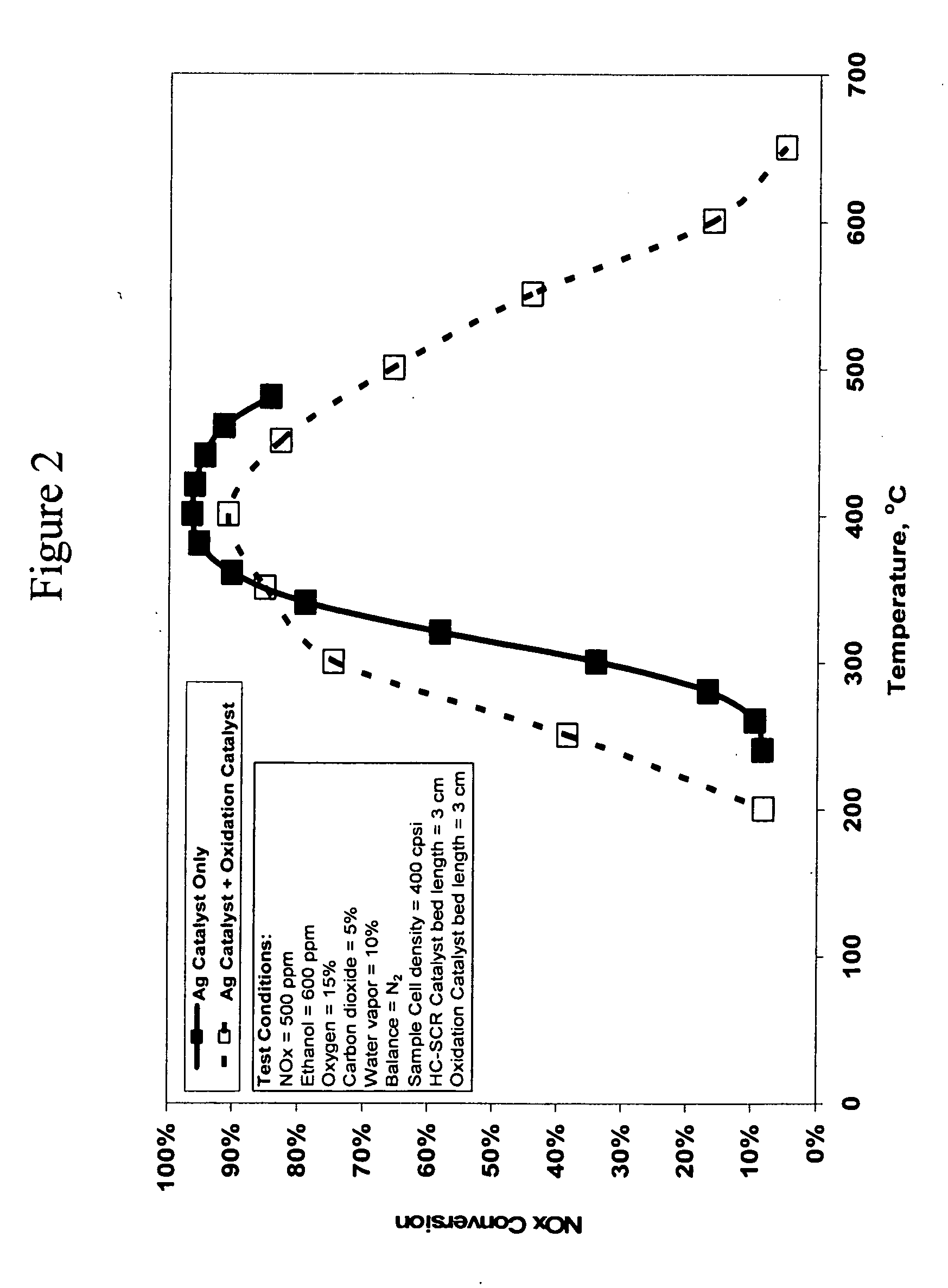
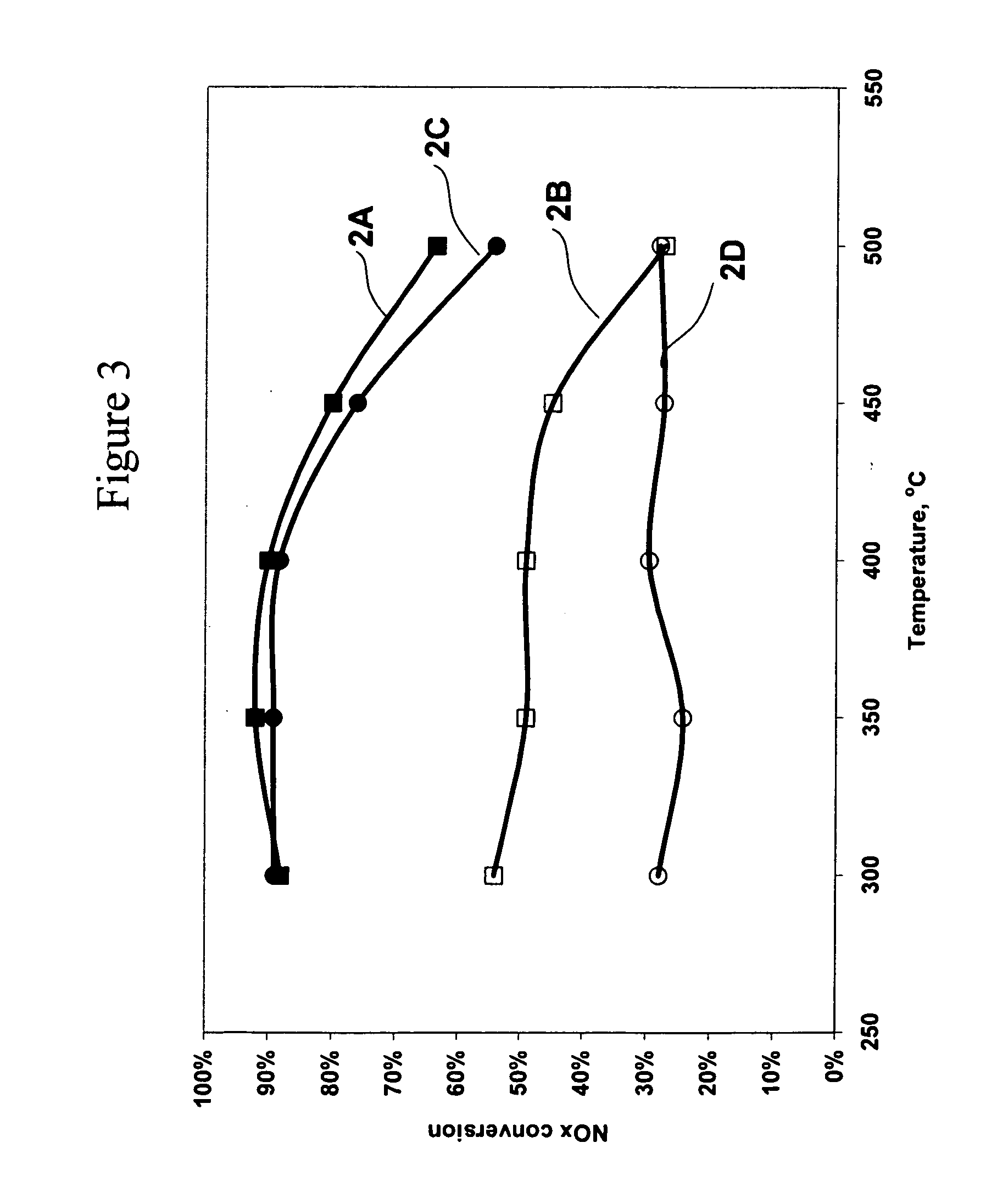
Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols
A catalyst system and a method for reducing nitrogen oxides in an exhaust gas by reduction with a hydrocarbon or oxygen-containing organic compound reducing agent are provided. The catalyst system contains a silver catalyst and a modifier catalyst, where the modifier catalyst contains a modifier oxide, where the modifier oxide is selected from the group consisting of iron oxide, cerium oxide, copper oxide, manganese oxide, chromium oxide, a lanthanide oxide, an actinide oxide, molybdenum oxide, tin oxide, indium oxide, rhenium oxide, tantalum oxide, osmium oxide, barium oxide, calcium oxide, strontium oxide, potassium oxide, vanadium oxide, nickel oxide, tungsten oxide, and mixtures thereof. The modifier oxide is supported on an inorganic oxide support or supports, where at least one of the inorganic oxide supports is an acidic support. The catalyst system of the silver catalyst and the modifier catalyst provides higher NOx conversion than either the silver catalyst or the modifier catalyst alone.
Owner:CATALYTIC SOLUTIONS INC
Polymer nanocomposite implants with enhanced transparency and mechanical properties for administration within humans or animals
Polymer nanocomposite implants with nanofillers and additives are described. The nanofillers described can be any composition with the preferred composition being those composing barium, bismuth, cerium, dysprosium, europium, gadolinium, hafnium, indium, lanthanum, neodymium, niobium, praseodymium, strontium, tantalum, tin, tungsten, ytterbium, yttrium, zinc, and zirconium. The additives can be of any composition with the preferred form being inorganic nanopowders comprising aluminum, calcium, gallium, iron, lithium, magnesium, silicon, sodium, strontium, titanium. Such nanocomposites are particularly useful as materials for biological use in applications such as drug delivery, biomed devices, bone or dental implants.
Owner:PPG IND OHIO INC
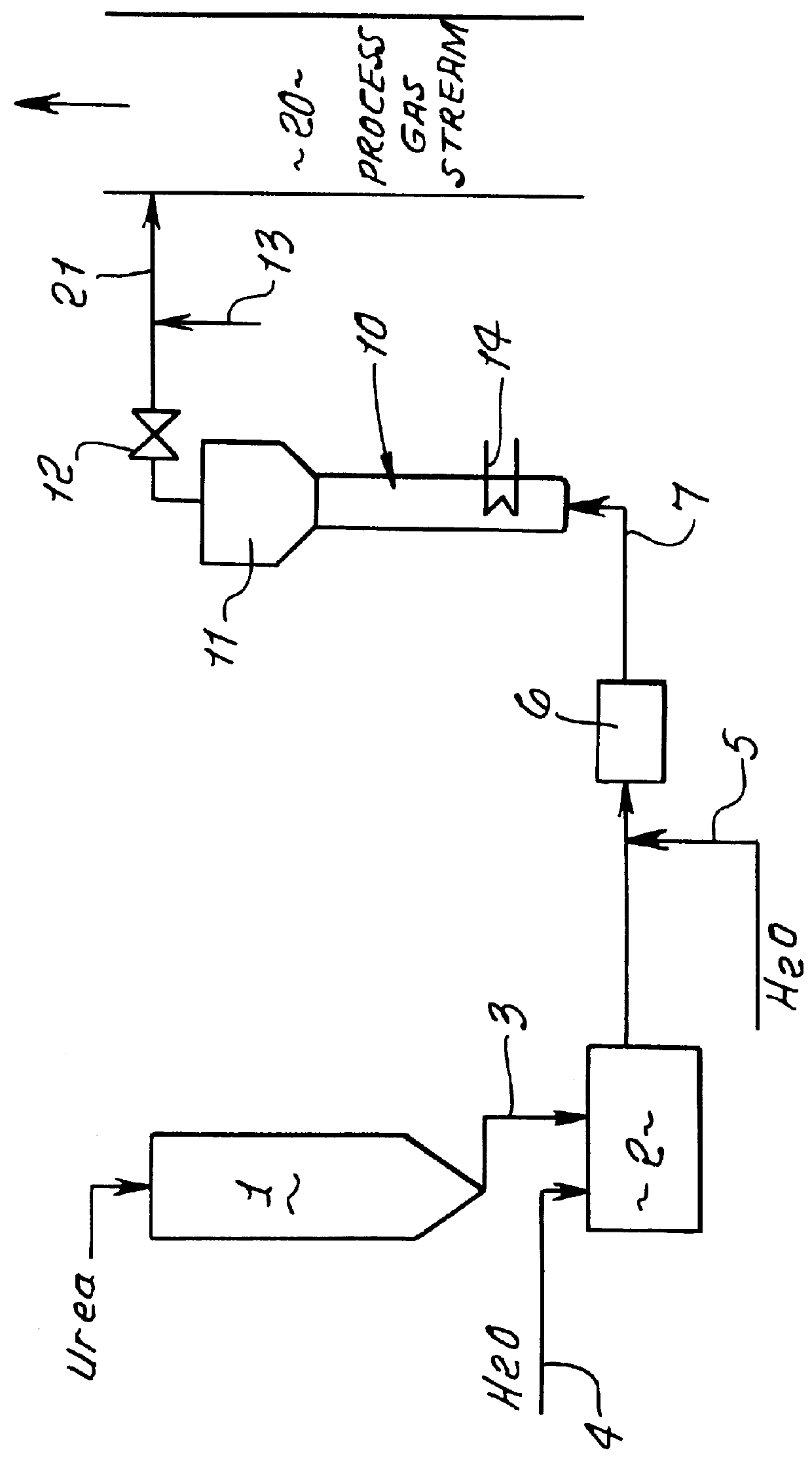
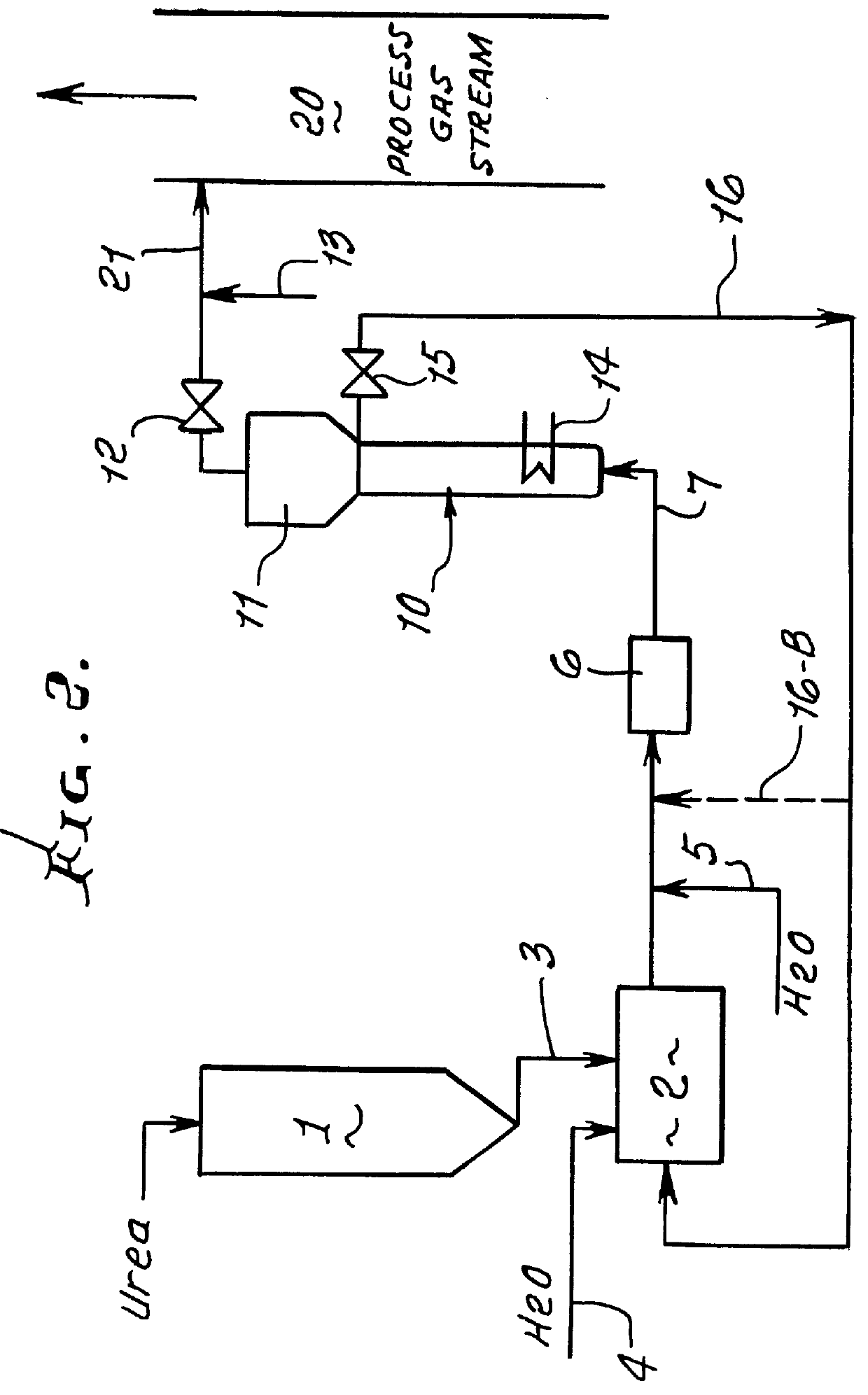
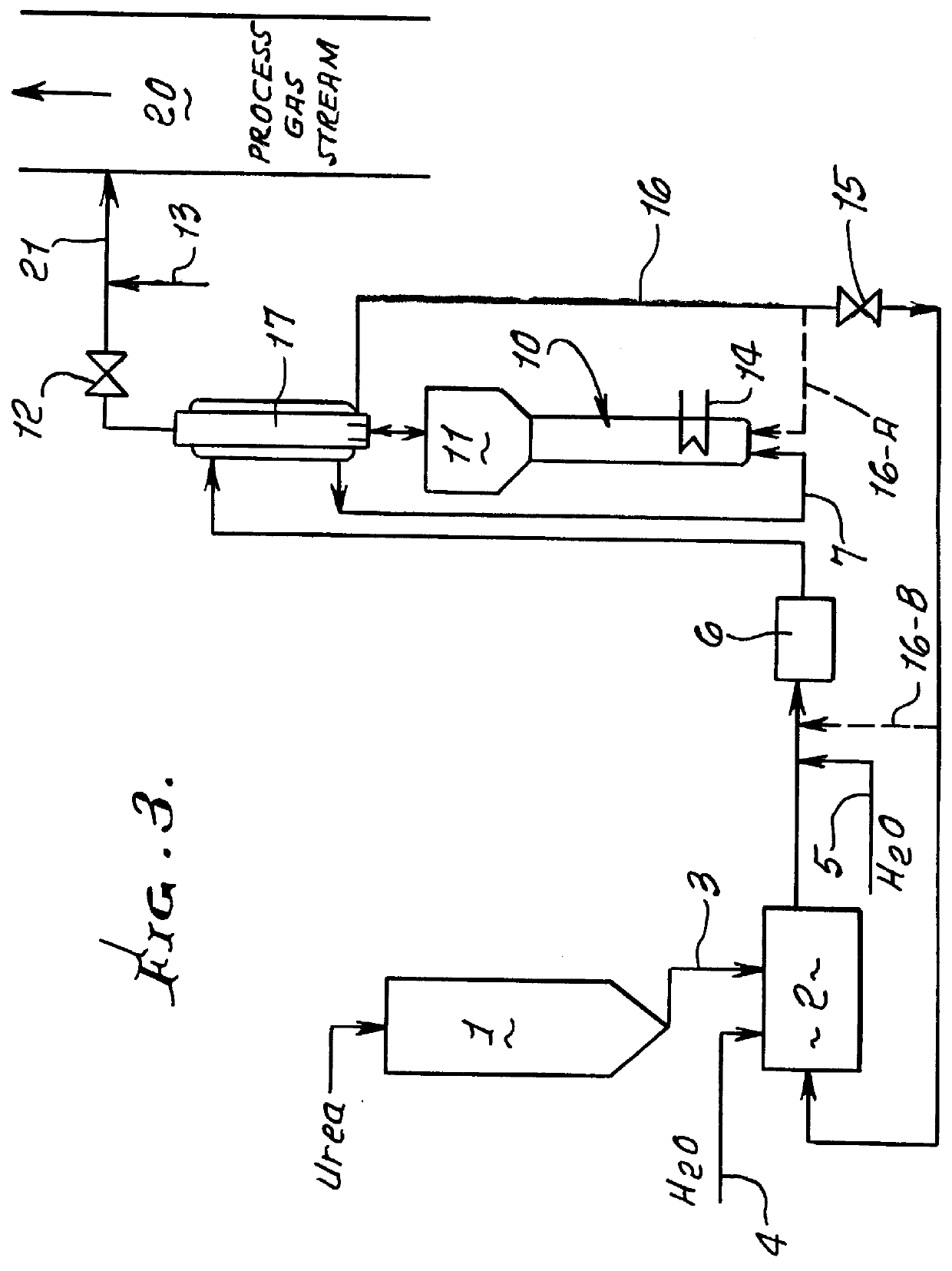
Methods for the production of ammonia from urea and/or biuret, and uses for NOx and/or particulate matter removal
InactiveUS6077491ARisk minimizationReduce amountNitrogen compoundsDispersed particle separationHydrolysisAmmonia
This patent describes technology for generating ammonia from urea. The method is based on the hydrolysis of an aqueous solution of urea and / or biuret by heating under pressure to form a mixture of ammonia, carbon dioxide and water. The gas mixtures produced are useful for supplying ammonia at controlled pressure and rate of flow for many industrial applications without the risks and hazards associated with the transportation and on-site storage of ammonia, thereby providing a significant safety advantage over present industrial practice.
Owner:EC&C TECH INC A
Method of purifying nanotubes and nanofibers using electromagnetic radiation
Disclosed are methods of purifying mixtures comprising nanofibers and / or nanotubes and residual catalyst particles that are covered by outer layers of the nanotube or nanofiber material. The mixtures are exposed to electromagnetic radiation, which induces localized heating in the residual catalyst particles. The localized heating creates breaches in the outer layers. Thereafter, the residual catalyst particles may be removed under relatively mild conditions that do not significantly affect the structural integrity of the nanotubes or nanofibers. The methods of the invention have been used to particular advantage in the purification of single wall carbon nanotubes (SWNTs) synthesized using metal catalysts. For these SWNTs, microwave radiation is preferably used to induce the localized heating, the outer layers are preferably removed at least in part by carrying out the localized heating under air, and the residual catalyst may be removed by exposure to relatively dilute aqueous acid.
Owner:PENN STATE RES FOUND
Silicon composite particles, preparation thereof, and negative electrode material for non-aqueous electrolyte secondary cell
ActiveUS20050214644A1Improve cycle performanceMinimize changesSilicaNitrogen compoundsSilicon alloyInorganic compound
Silicon composite particles are prepared by sintering primary fine particles of silicon, silicon alloy or silicon oxide together with an organosilicon compound. Sintering of the organosilicon compound results in a silicon-base inorganic compound which serves as a binder. Each particle has the structure that silicon or silicon alloy fine particles are dispersed in the silicon-base inorganic compound binder, and voids are present within the particle.
Owner:SHIN ETSU CHEM IND CO LTD
Nitride phosphor and method for preparation thereof, and light emitting device
InactiveUS7258816B2High luminous efficiencyDifficult to generateDischarge tube luminescnet screensNitrogen compoundsRare-earth elementNitride phosphor
Owner:NICHIA CORP
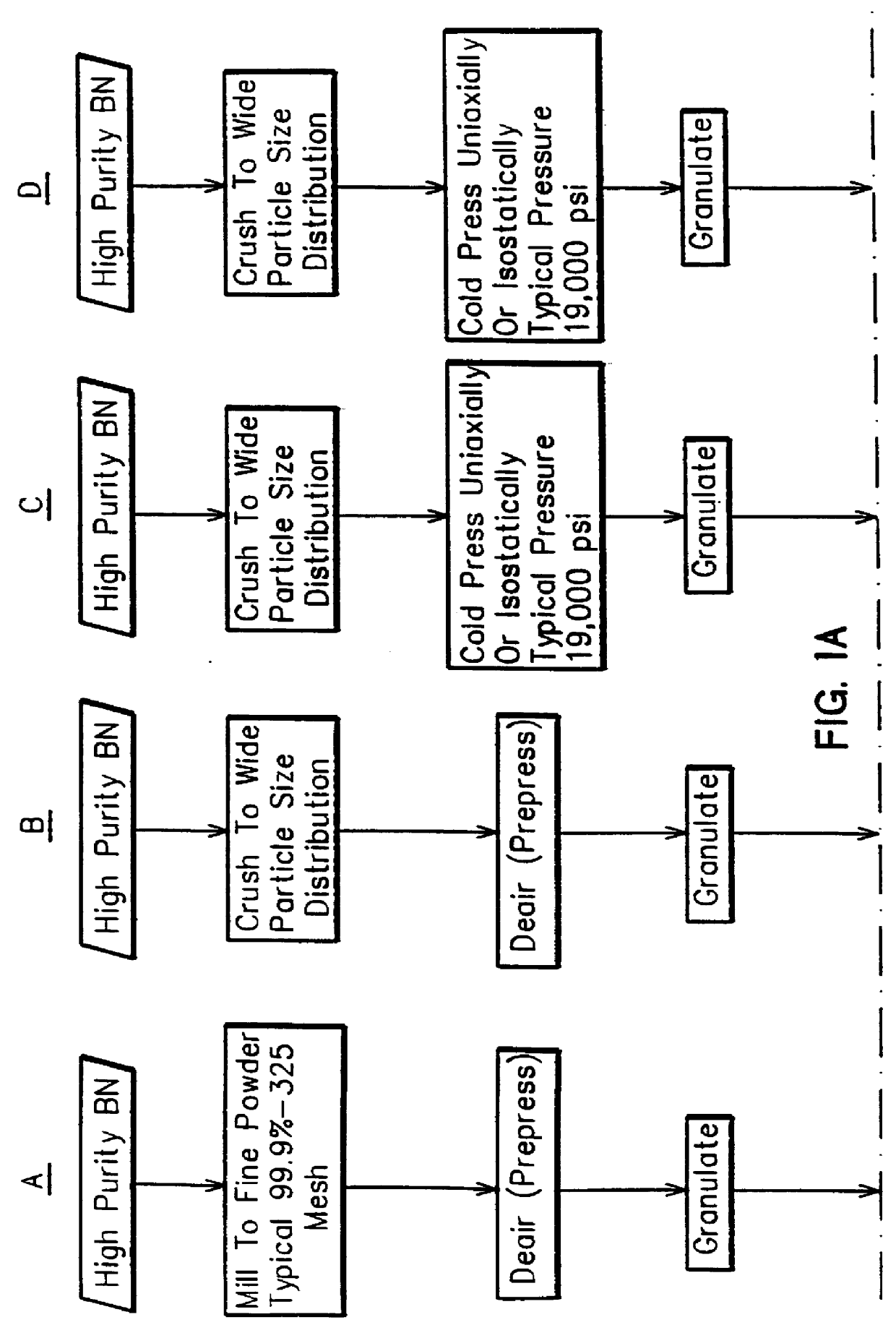
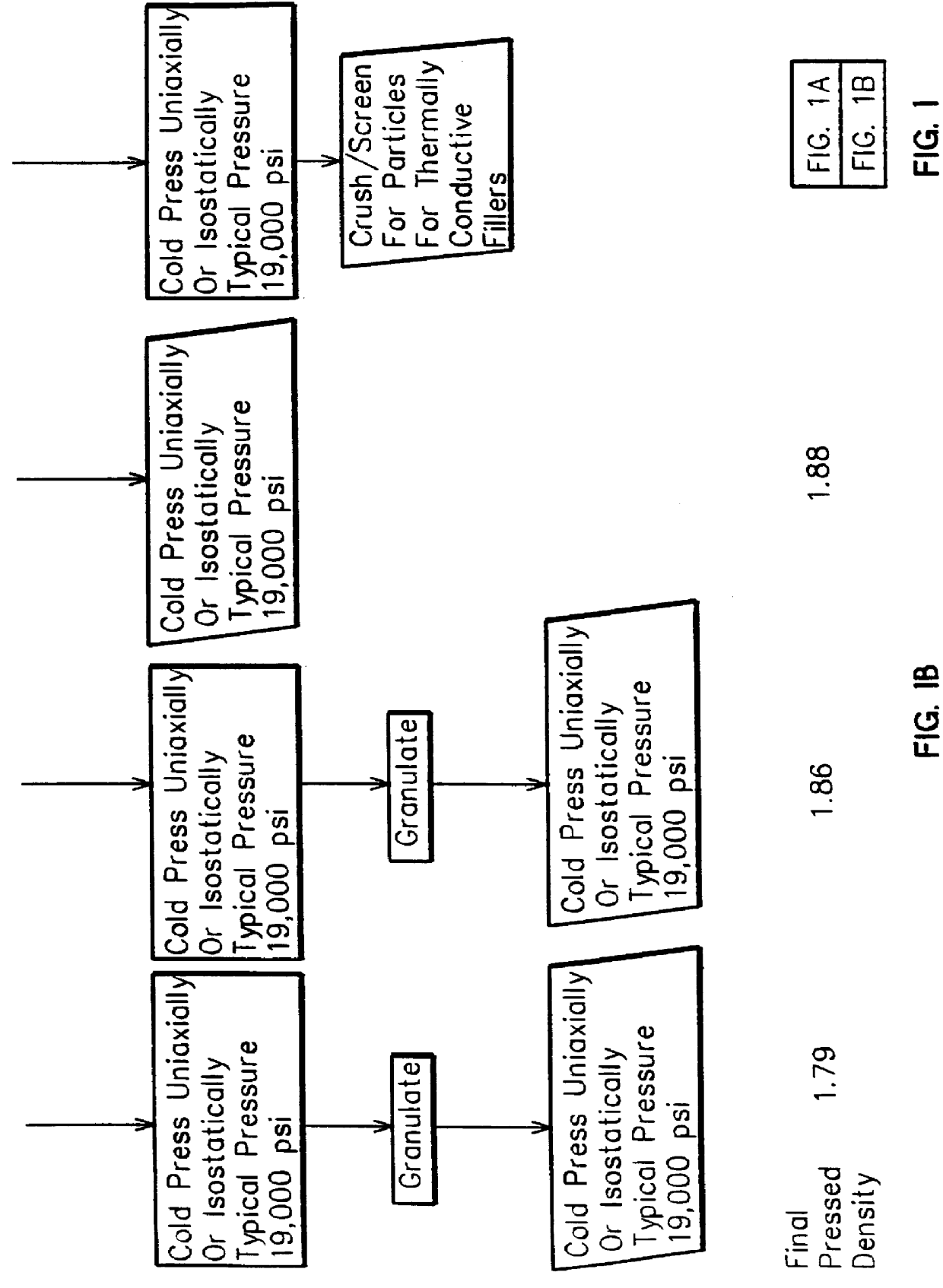
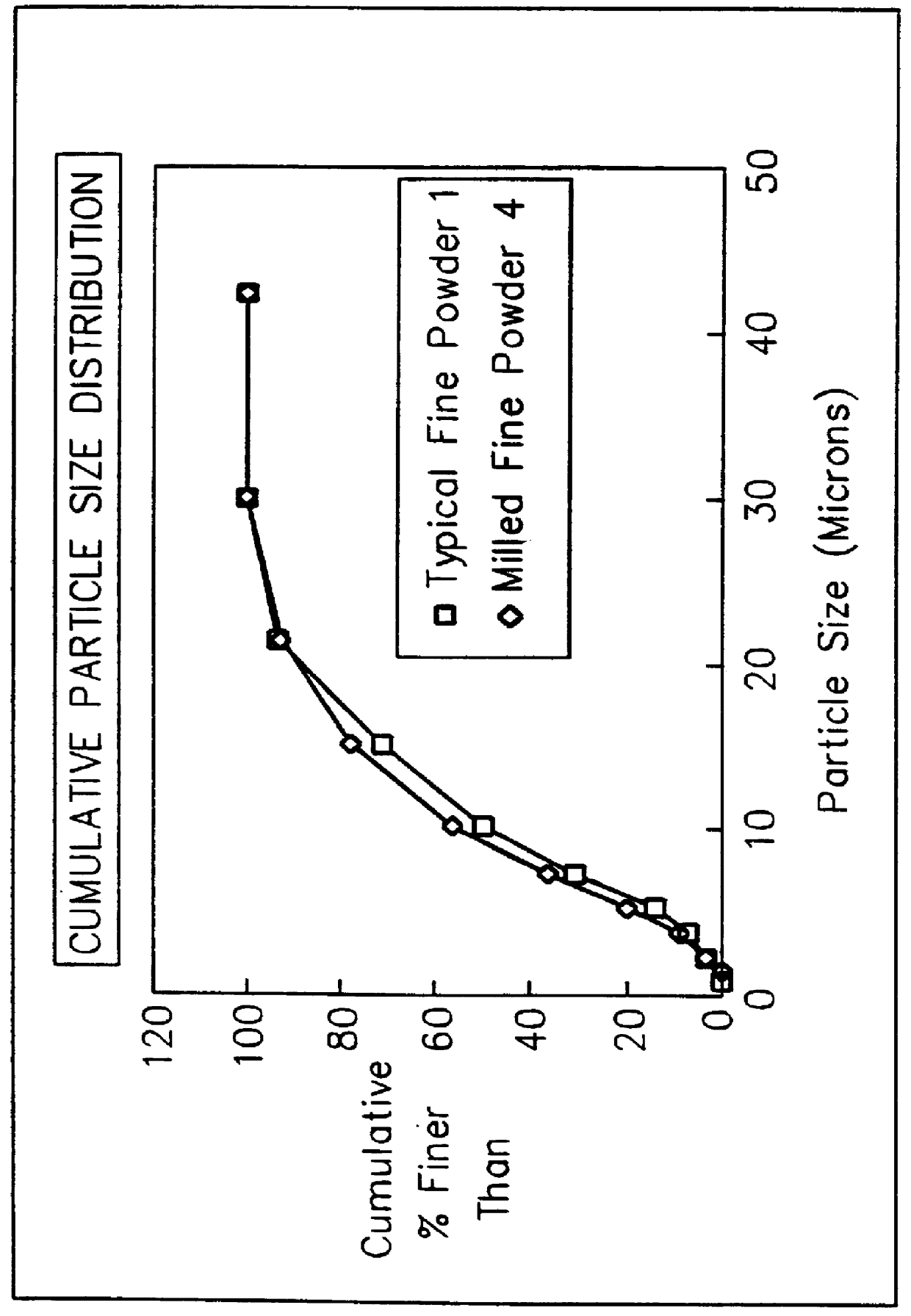
Method for forming high density boron nitride and high density agglomerated boron nitride particles
A method of forming pellets or agglomerates of high density boron nitride from high purity hexagonal boron nitride by crushing the high purity hexagonal boron nitride into boron nitride particles extending over a size range of at least 100 microns with the majority of the particles having a particle size above 50 microns and cold pressing the crushed particles into a compacted form. The compacted form is then granulated into a granulated powder and again cold pressed to form pellets or agglomerates of boron nitride particles with the operations of cold pressing and granulation occurring in one or more stages.
Owner:GENERAL ELECTRIC CO
Production of hydrogen and removal and sequestration of carbon dioxide from coal-fired furnaces and boilers
InactiveUS7282189B2Increase ratingsValue maximizationOrganic chemistryNitrogen compoundsHydrogenProcess engineering
Methods for reducing and eliminating carbon dioxide from the emissions of solid fuel fired power plants, particularly coal fired power plants, and to sequester the carbon dioxide, typically by using existing equipment. In some embodiments, the methods involve pyrolyzing the solid fuel to remove volatile matter and using the volatile matter to produce hydrogen. Additionally, the methods may involve burning the solid fuel or pyrolized solid fuel at very fuel rich stoichiometric conditions. Sequestration may include the production of a carbon dioxide-containing solution and the pumping of the solution into the ground, particularly in areas high in limestone.
Owner:ZAUDERER BERT
Exhaust system for a vehicular positive ignition internal combustion engine
ActiveUS20110158871A1Reduce frequencyImprove filtering effectCombination devicesCation exchanger materialsParticulatesPorous substrate
Owner:JOHNSON MATTHEY PLC
Layered catalyst composite
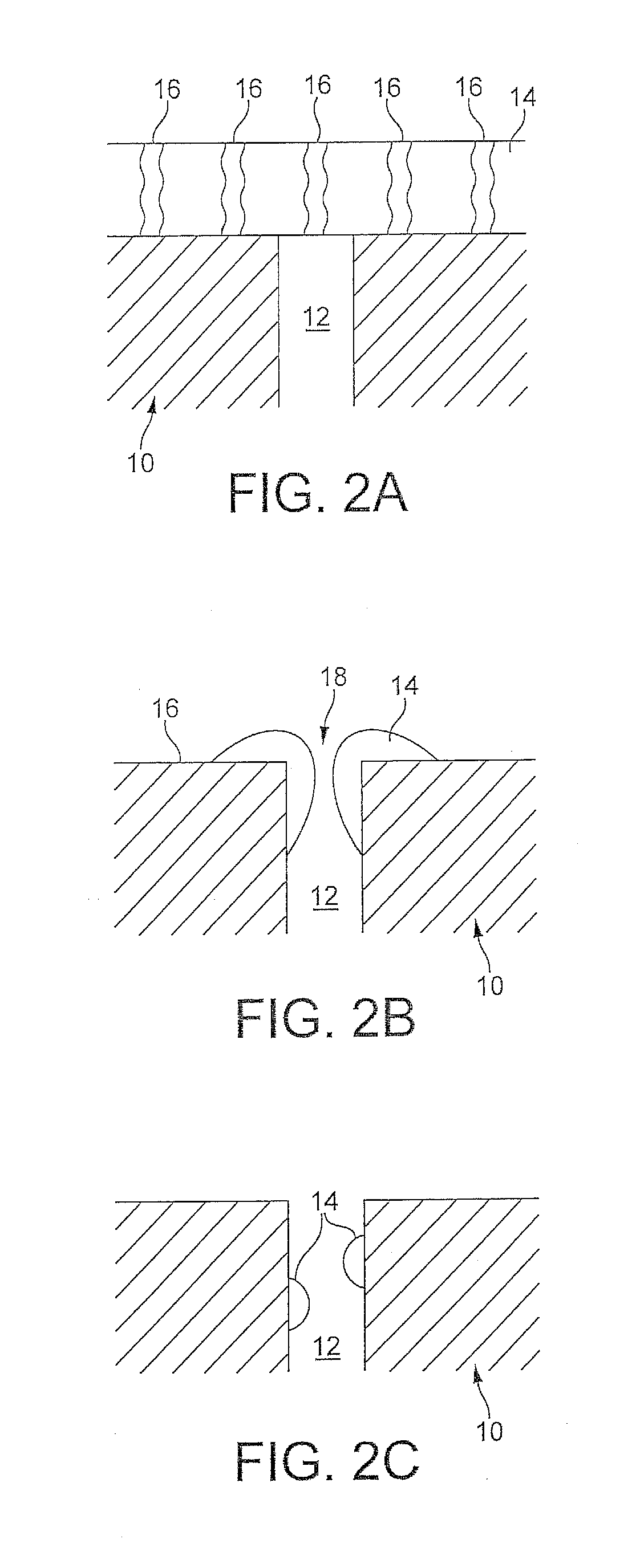
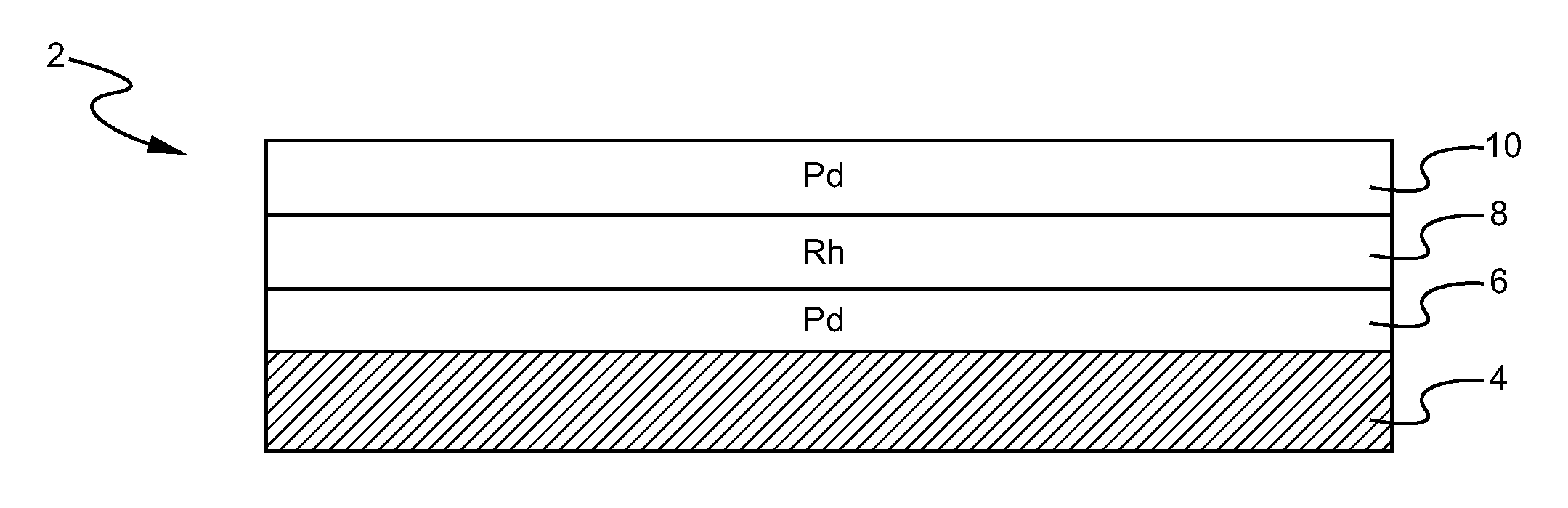
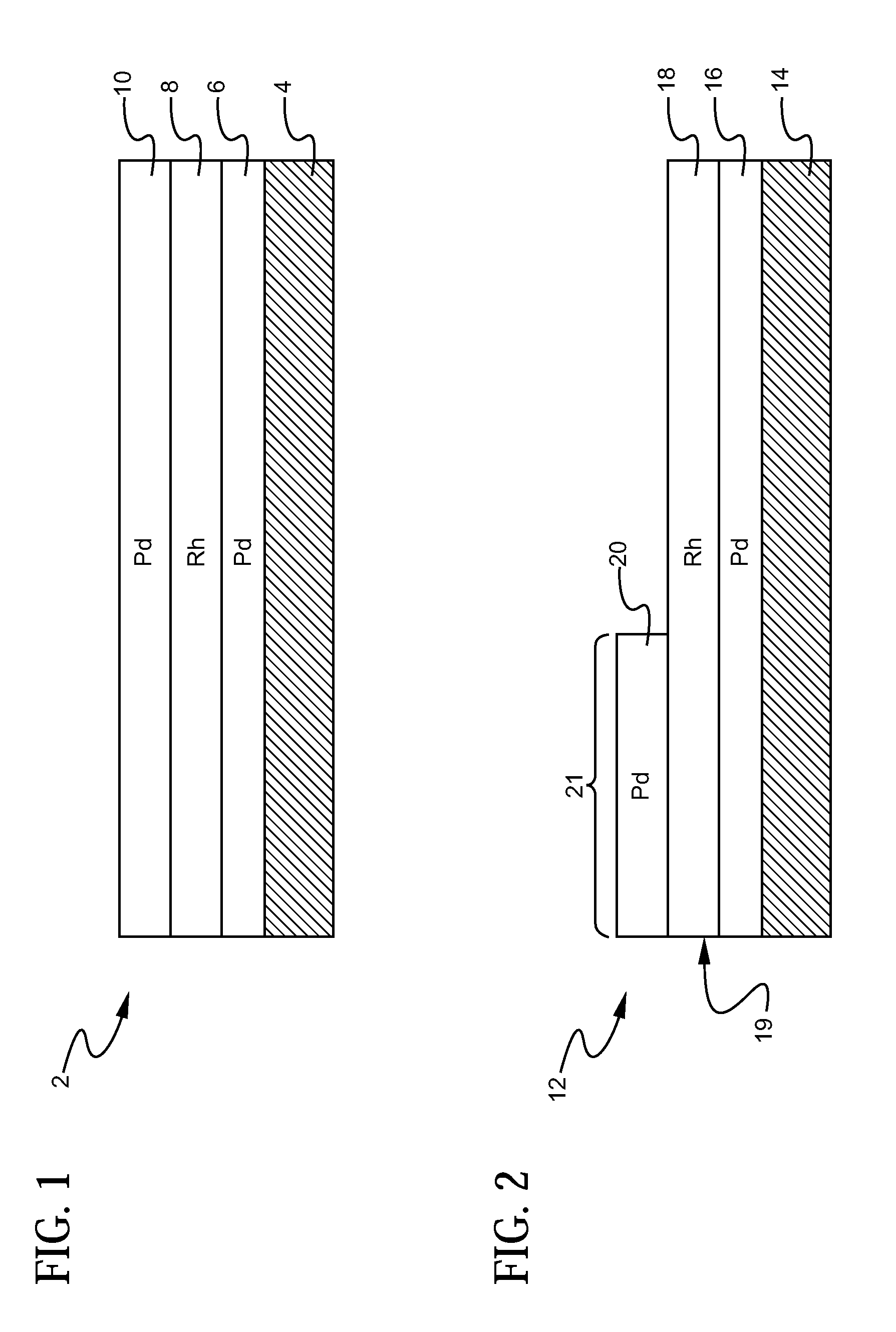
A layered, three-way conversion catalyst having the capability of simultaneously catalyzing the oxidation of hydrocarbons and carbon monoxide and the reduction of nitrogen oxides is disclosed. In one or more embodiments, the catalyst comprises three layers in conjunction with a carrier: a first layer deposited on the carrier and comprising palladium deposited on a refractory metal oxide and an oxygen storage component; a second layer deposited on the first layer and comprising rhodium deposited on a refractory metal oxide and an oxygen storage component; and a third layer deposited on the second layer and comprising palladium deposited on a refractory metal oxide.
Owner:BASF CATALYSTS LLC
Flue gas purification process using a sorbent polymer composite material
ActiveUS20050019240A1Easy to fixImprove removal efficiencyGas treatmentNitrogen compoundsSorbentFluoropolymer
This invention provides a process of removing sulfur oxides, mercury vapor, and fine particulate matters from industrial flue gases that contain such pollutants. The pollutants are removed by modules, which contain microporous adsorbent (i.e., sorbent) material that is held within a polymer matrix. The preferred polymers are fluoropolymers. The composite material that contains the microporous absorbent material held within a polymer matrix removes sulfur oxides by converting them into high concentration sulfuric acids. It also removes mercury vapor by chemically adsorbing the mercury into the matrix. It also removes fine particulate matters by surface filtration. The sulfuric acid that is produced inside the composite material is automatically expelled onto the external surfaces of the composite material and is drained into an acid reservoir together with the fine particulate matters which are washed from the external surfaces of the composite material by the constant dripping of the sulfuric acid along the external surfaces of the composite material.
Owner:WL GORE & ASSOC INC
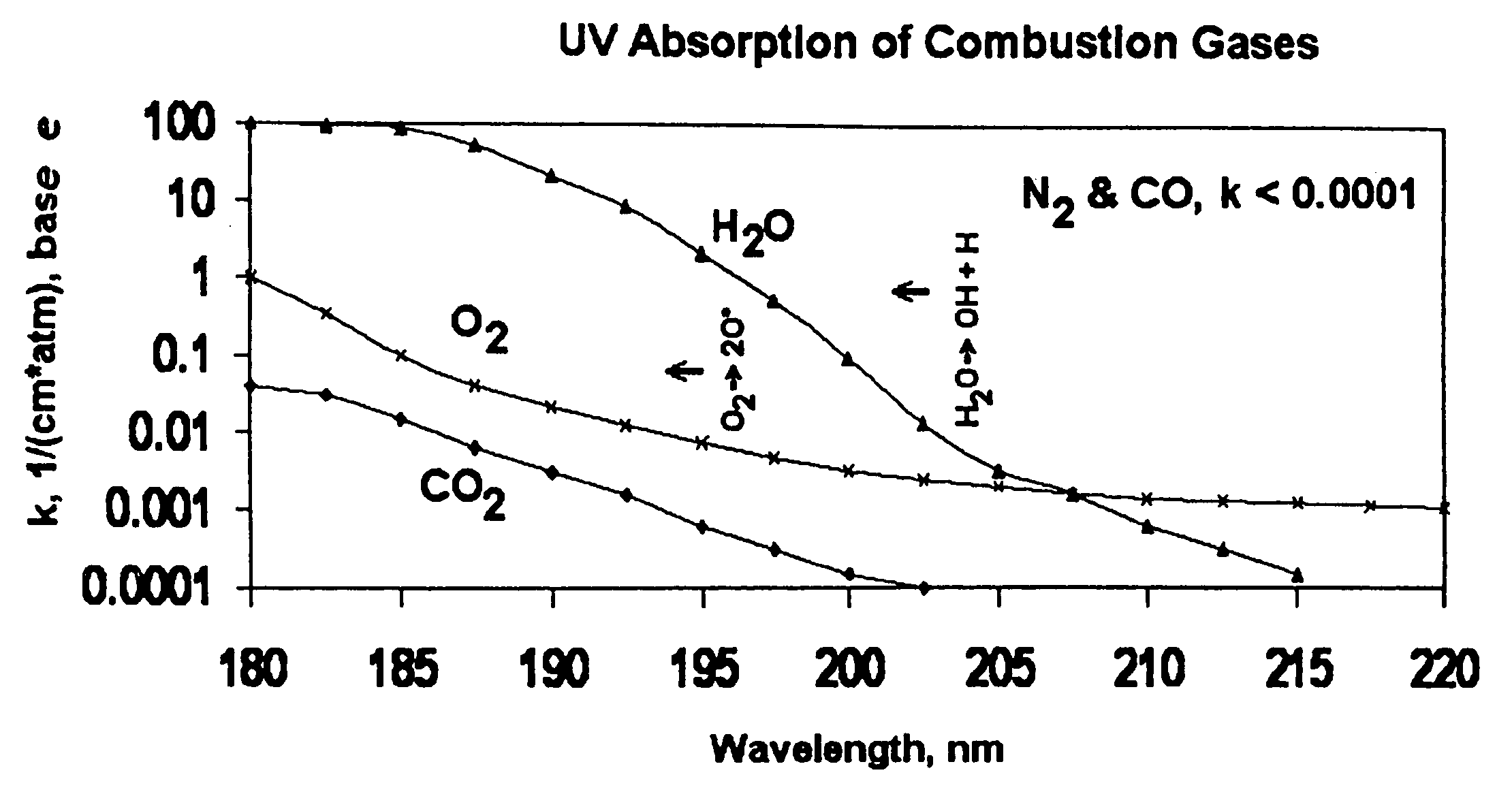
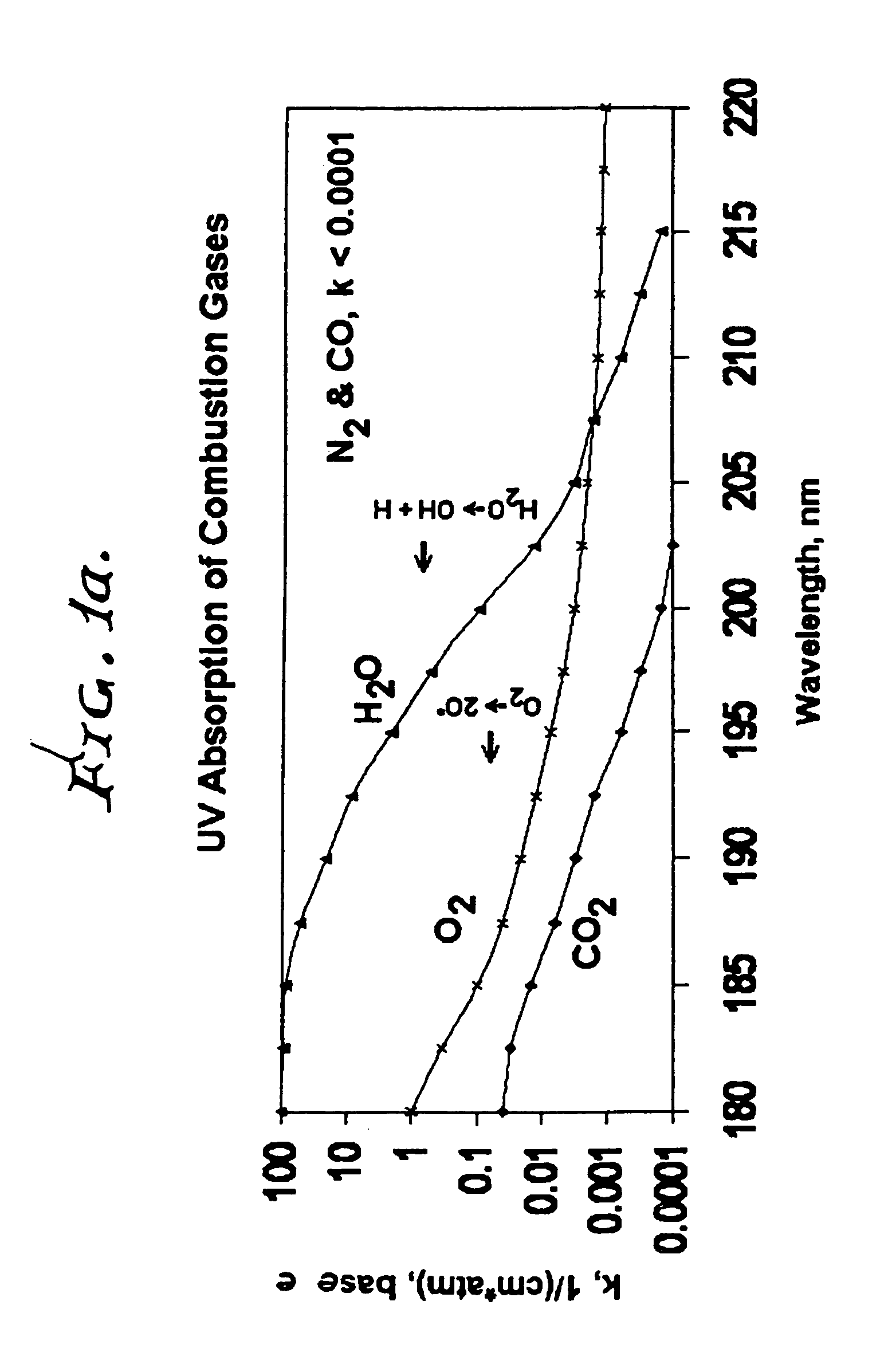
Controlled spectrum ultraviolet radiation pollution control process
InactiveUS7498009B2Cost effectiveEasy to adaptCombination devicesOrganic chemistryEnvironmental engineeringNitrogen oxide
A method for reducing or substantially eliminating oxides of nitrogen from an effluent gas stream, that includes providing a source of ultraviolet radiation with a precise wavelength, adding ammonia or an ammonia based reagent to the effluent stream, upstream of the ultraviolet radiation source, controllably operating the ultraviolet radiation source to irradiate the effluent stream flowing in the duct and substantially reducing or eliminating oxides of nitrogen by promotion a reaction of ammonia with the oxides of nitrogen to produce N2 and H2O, and also thereby destroying any surplus ammonia. This process can also be modified to oxidize carbon monoxide and VOC's to CO2 and H2O.
Owner:DANA UV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com