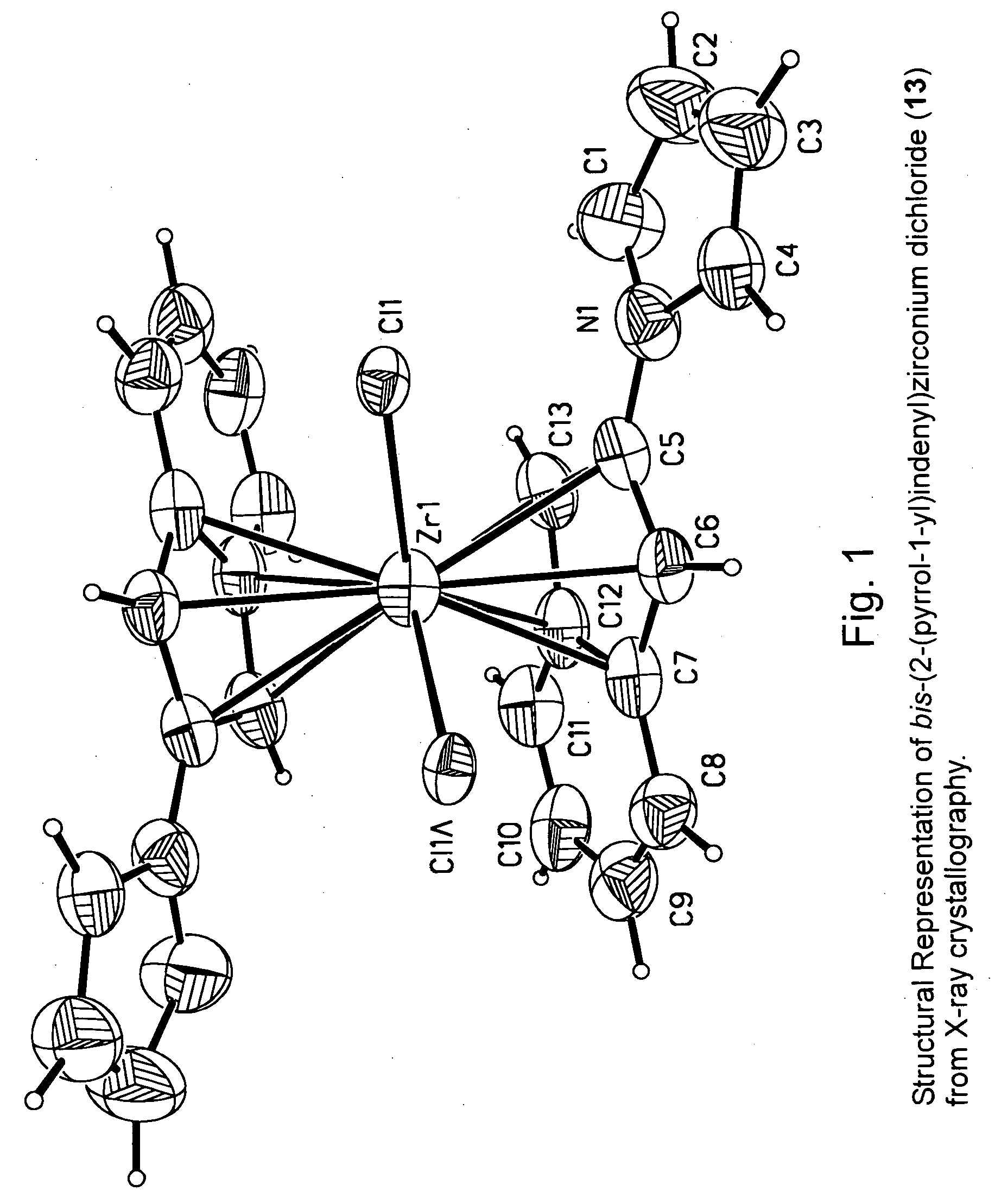
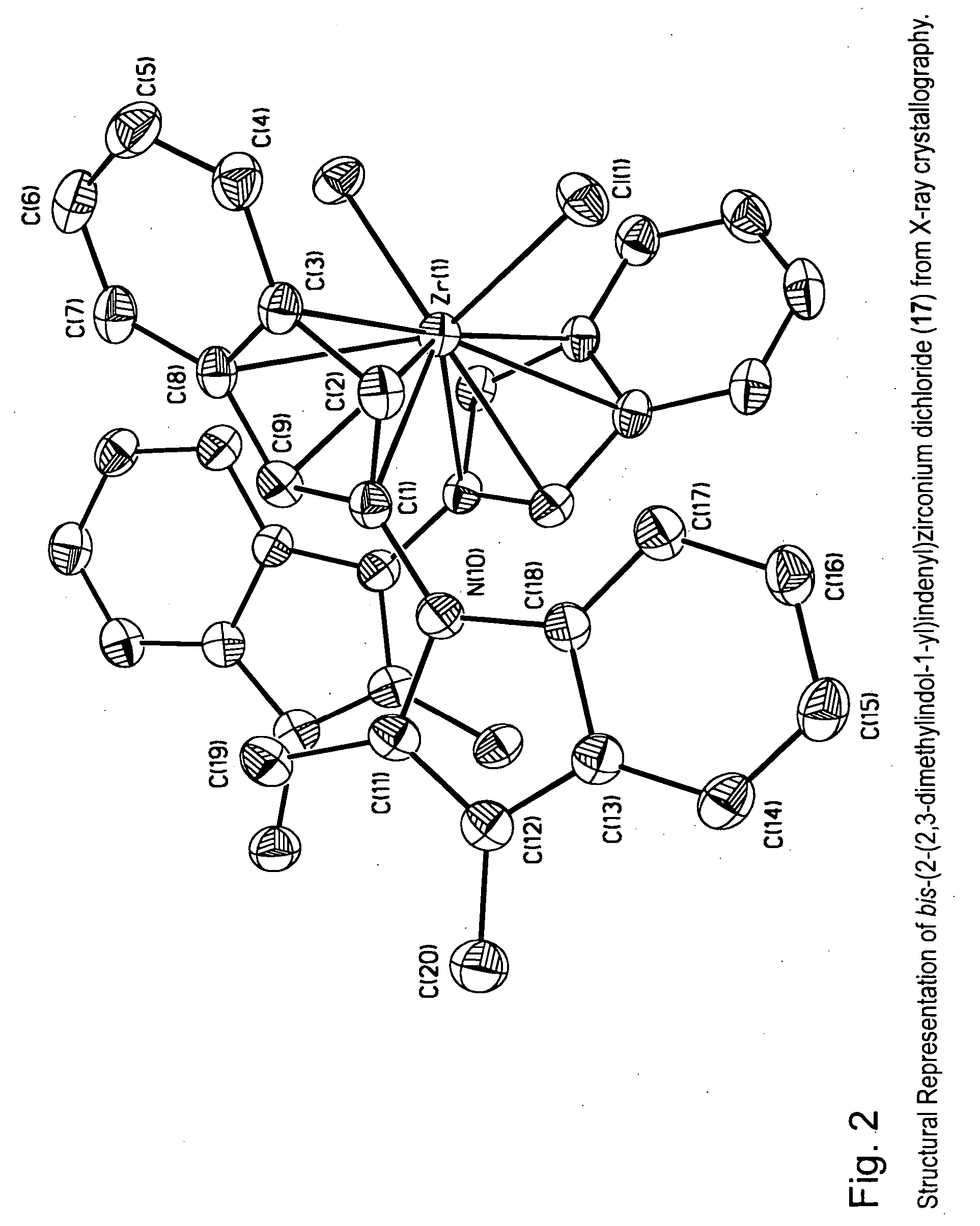
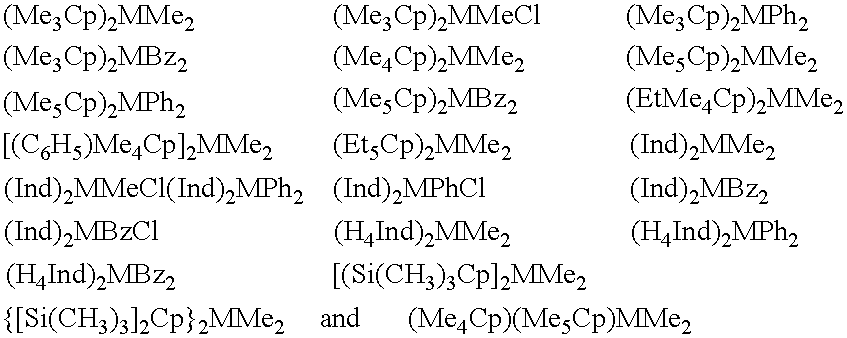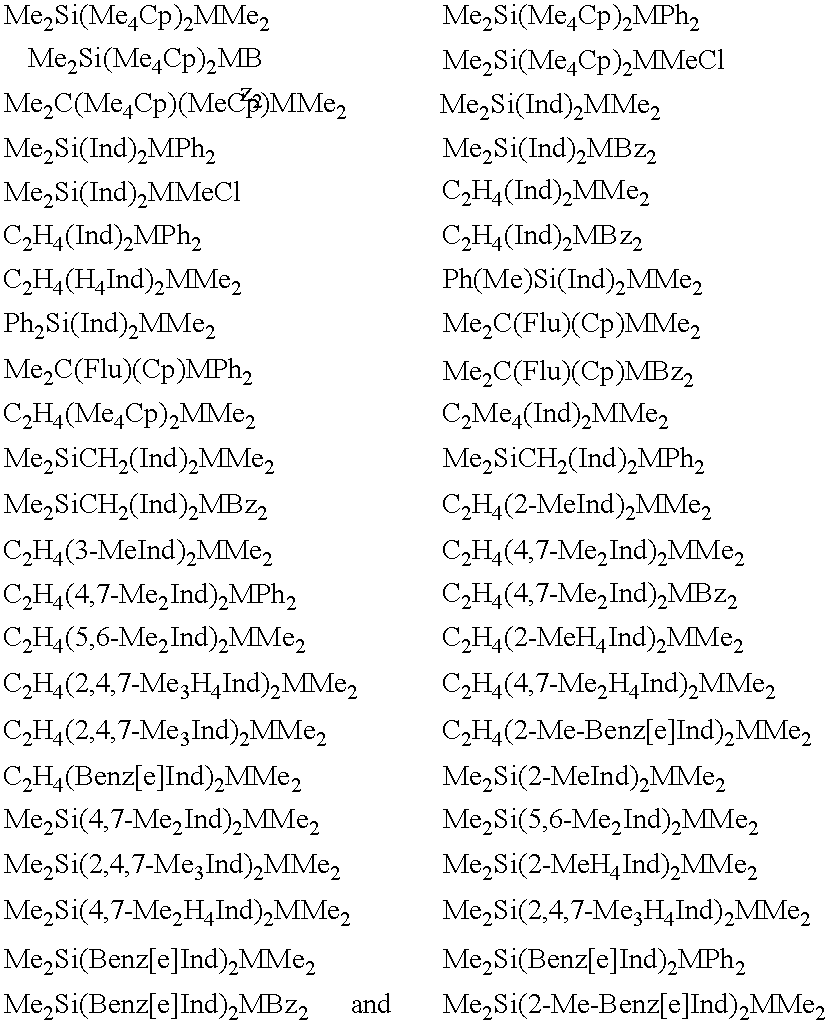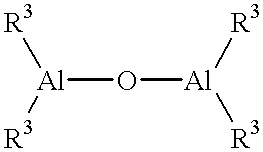Patents
Literature
355 results about "Actinide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The actinide /ˈæktɪnaɪd/ or actinoid /ˈæktɪnɔɪd/ (IUPAC nomenclature) series encompasses the 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through lawrencium. Strictly speaking, both actinium and lawrencium have been labeled as group 3 elements, but both elements are often included in any general discussion of the chemistry of the actinide elements. Actinium is the more often omitted of the two, because its placement as a group 3 element is somewhat more common in texts and for semantic reasons: since "actinide" means "like actinium", it has been argued that actinium cannot logically be an actinide, but IUPAC acknowledges its inclusion based on common usage.
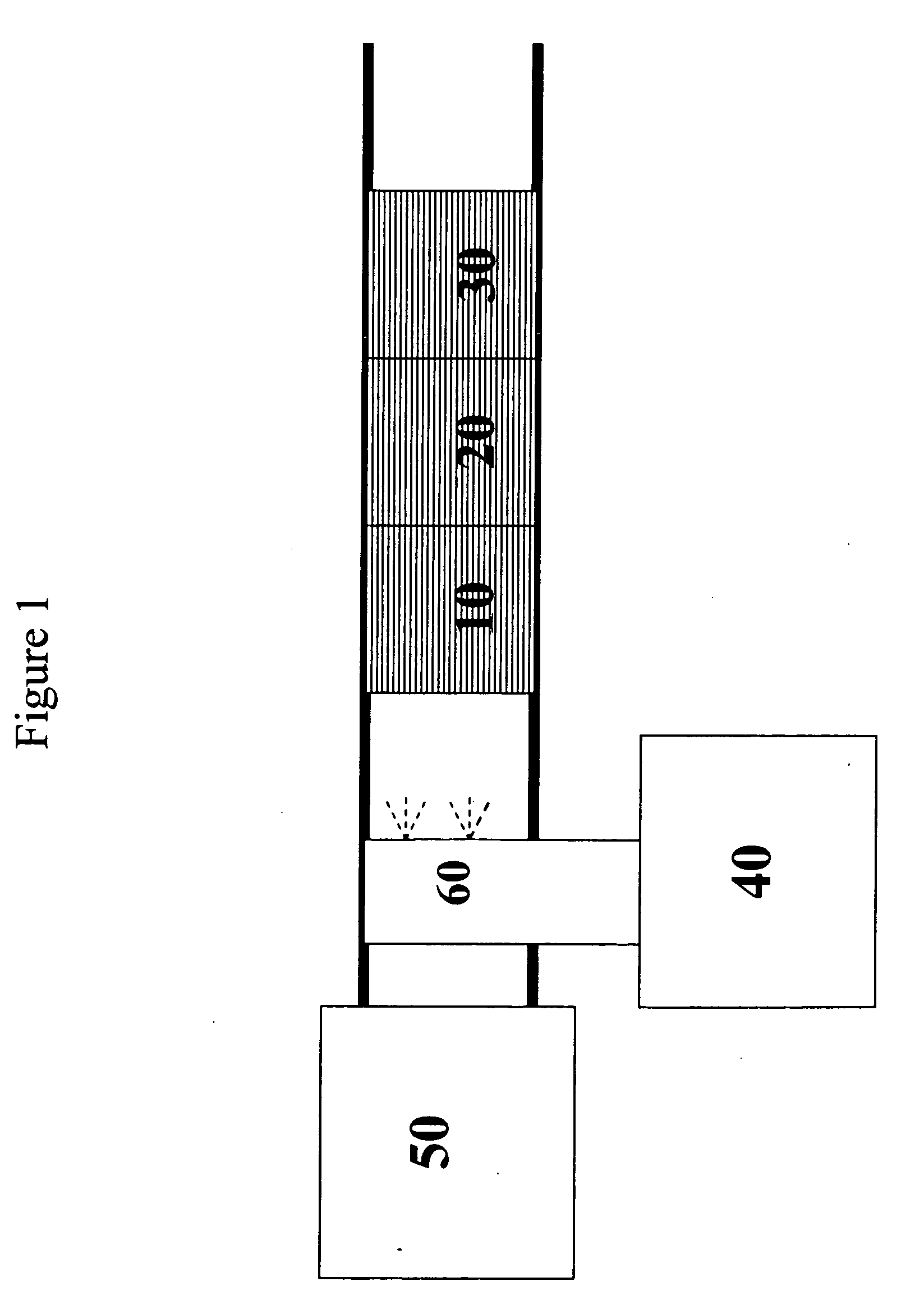
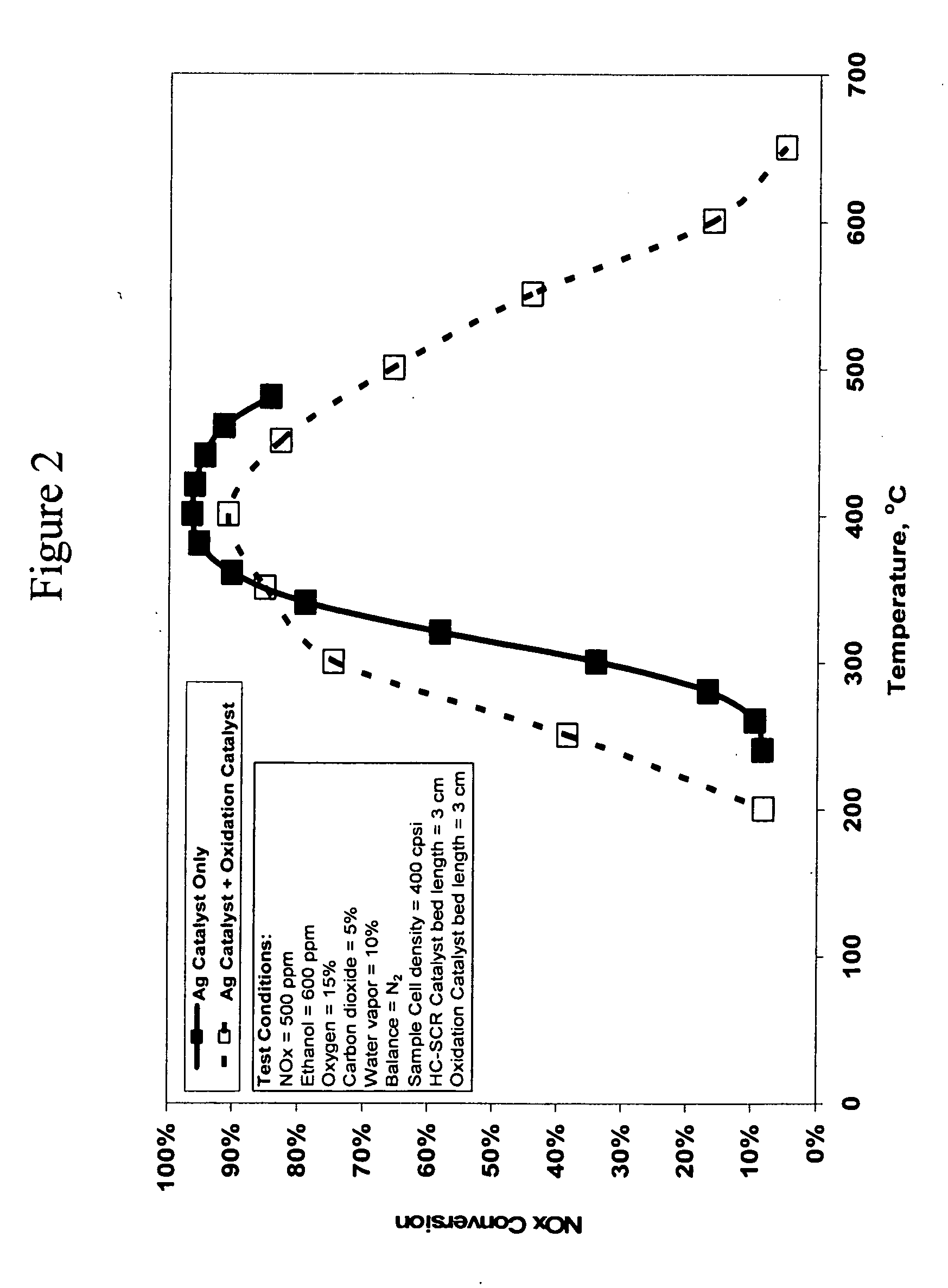
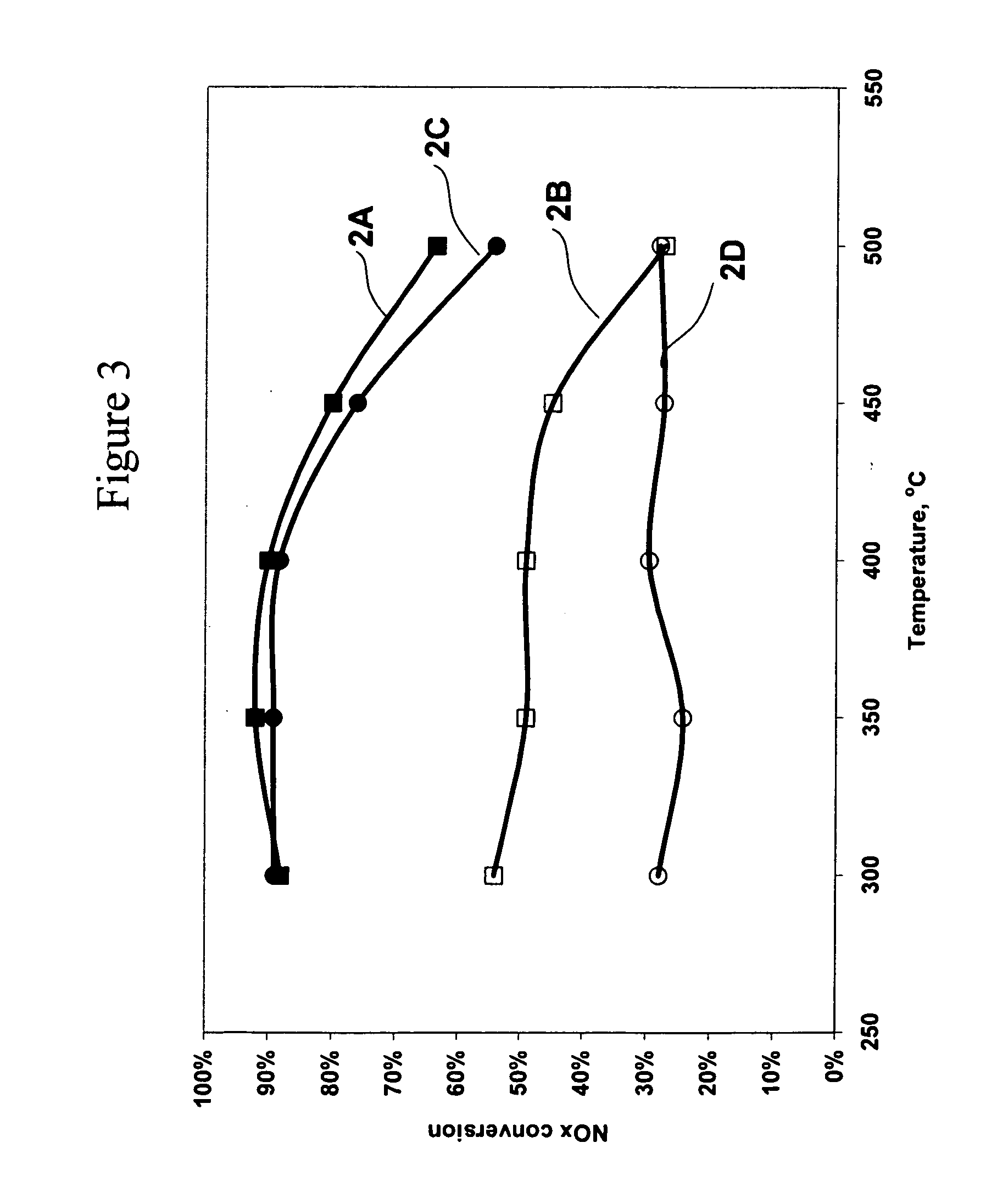
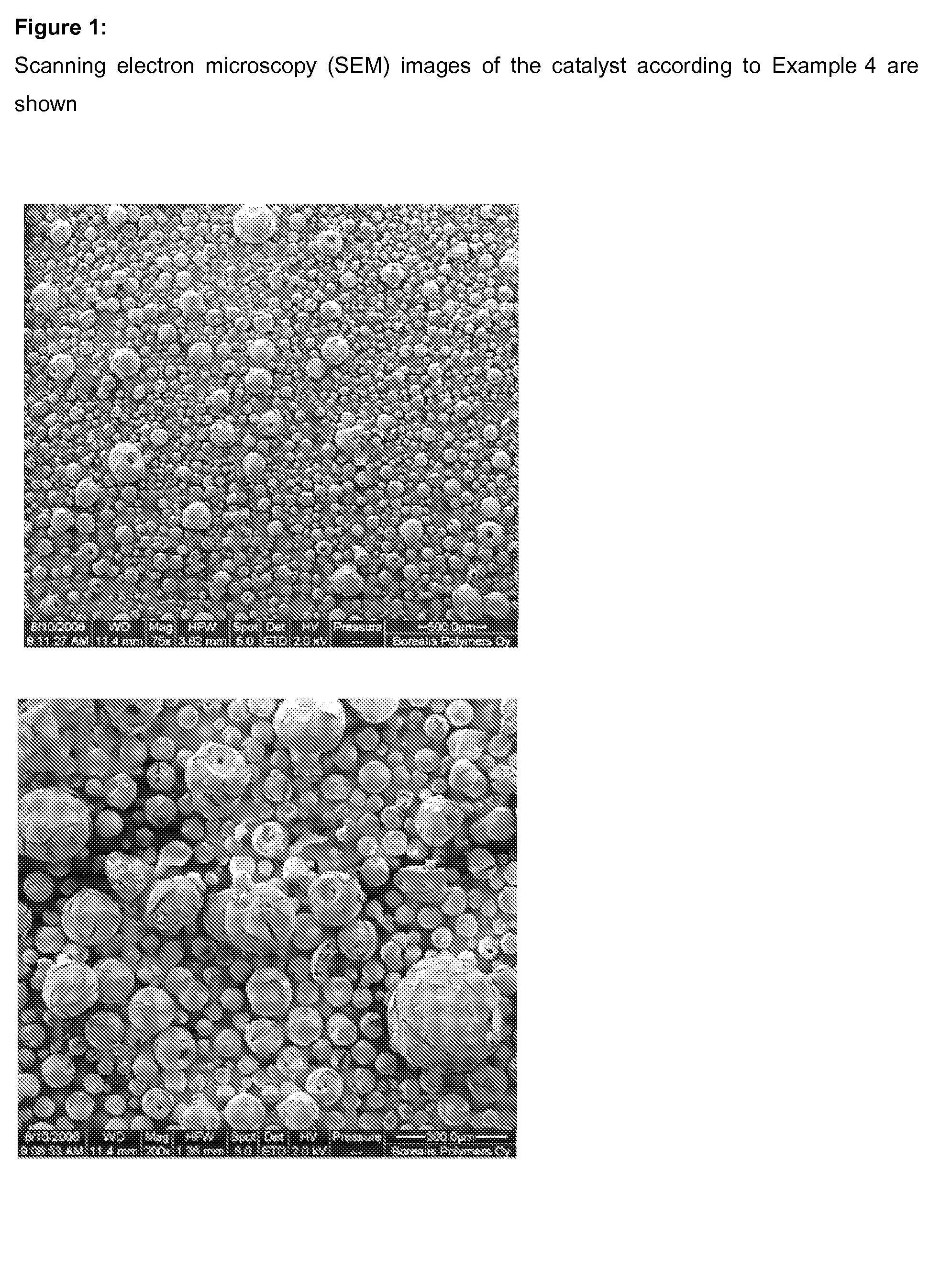
Catalyst and method for reducing nitrogen oxides in exhaust streams with hydrocarbons or alcohols
A catalyst system and a method for reducing nitrogen oxides in an exhaust gas by reduction with a hydrocarbon or oxygen-containing organic compound reducing agent are provided. The catalyst system contains a silver catalyst and a modifier catalyst, where the modifier catalyst contains a modifier oxide, where the modifier oxide is selected from the group consisting of iron oxide, cerium oxide, copper oxide, manganese oxide, chromium oxide, a lanthanide oxide, an actinide oxide, molybdenum oxide, tin oxide, indium oxide, rhenium oxide, tantalum oxide, osmium oxide, barium oxide, calcium oxide, strontium oxide, potassium oxide, vanadium oxide, nickel oxide, tungsten oxide, and mixtures thereof. The modifier oxide is supported on an inorganic oxide support or supports, where at least one of the inorganic oxide supports is an acidic support. The catalyst system of the silver catalyst and the modifier catalyst provides higher NOx conversion than either the silver catalyst or the modifier catalyst alone.
Owner:CATALYTIC SOLUTIONS INC
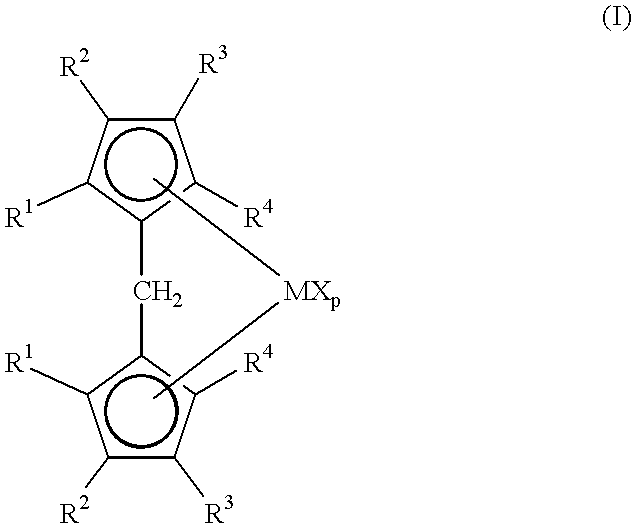
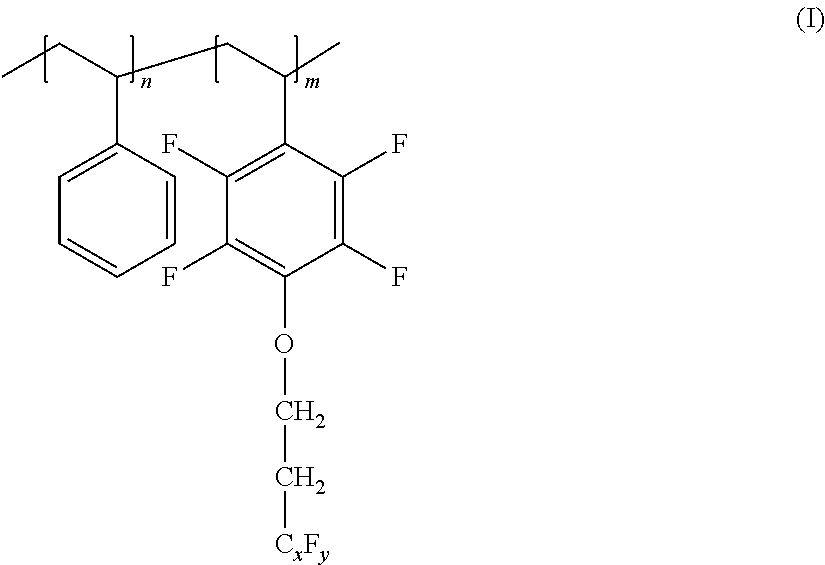
Metallocene compounds, process for their preparation and their use in catalytic systems for the polymerization of olefins
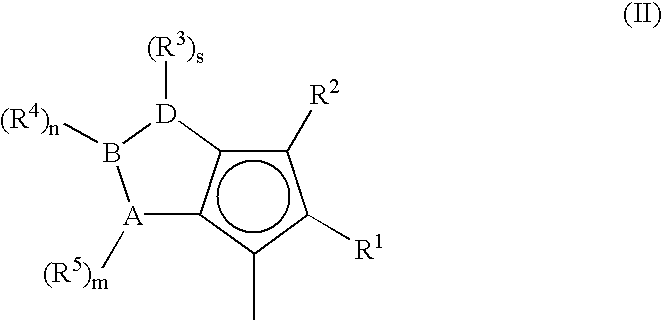
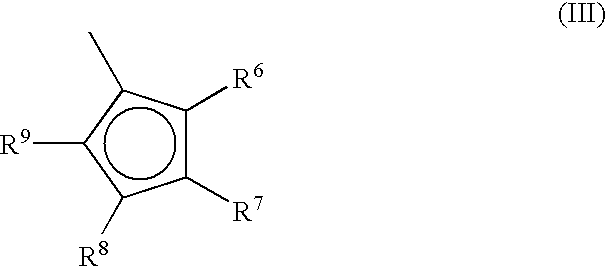
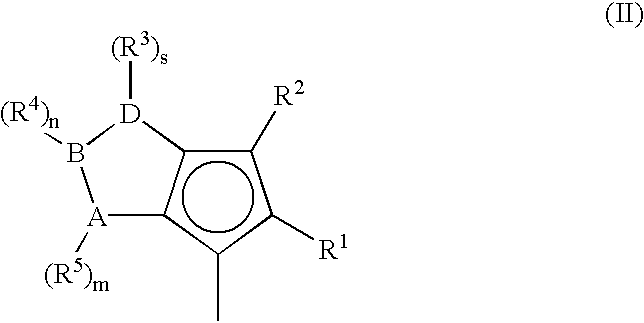
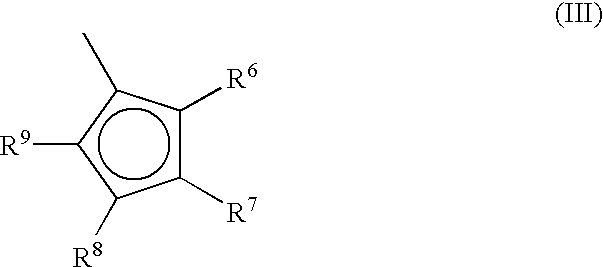
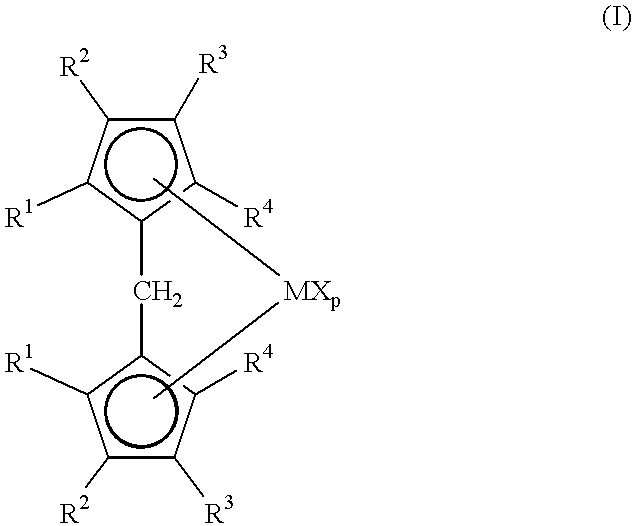
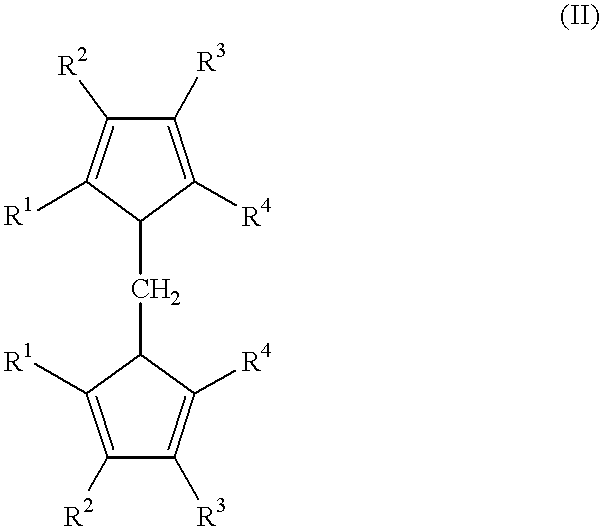
A class of metallocene compounds is disclosed having the general formula (I):wherein Y is a moiety of formula (II)wherein A, B and D, same or different from each other, are selected from an element of the groups 14 to 16 of the Periodic Table of the Elements (new IUPAC version), with the exclusion of nitrogen and oxygen; R1, R2, R3, R4 and R5 are hydrogen or hydrocarbon groups, Z is selected from a moiety of formula (II) as described above and from a moiety of formula (III):wherein R6, R7, R8 and R9, are hydrogen or hydrocarbon groups; L is a divalent bridging group; M is an atom of a transition metal selected from those belonging to group 3, 4, 5, 6 or to the lanthanide or actinide groups in the Periodic Table of the Elements (new IUPAC version), X, same or different, is hydrogen, a halogen, a R10, OR10, OSO2CF3, OCOR10, SR10, NR102 or PR102 group, wherein the substituents R10 are hydrogen or alkyl groups; p is an integer of from 0 to 3, being equal to the oxidation state of the metal M minus 2.The above metallocenes are particular useful in the polymerization of propylene.
Owner:BASELL POLYOLEFINE GMBH
Metallocene compounds, process for their preparation and their use in catalytic systems for the polymerization of olefins
A class of metallocene compounds is disclosed having general formula (I) wherein Y is a moiety of formula (II) wherein A, B, and D, same or different from each other, are selected from an element of the groups 14 to 16 of the Periodic Table of the Elements (new IUPAC version), with the exclusion of nitrogen and oxygen; R<1>, R<2>, R<3>, R<4 >and R<5 >are hydrogen or hydrocarbon groups, Z is selected from a moiety of formula (II) as described above and from a moiety of formula (III) wherein R<6>, R<7>, R<8 >and R<9>, are hydrogen or hydrocarbon groups; L is a divalent bridging group; M is an atom of a transition metal selected from those belonging to group 3, 4, 5, 6 or to the lanthanide or actinide groups in the Periodic Table of the Elements (new IUPAC version), X, same or different, is hydrogen, a halogen, a R<10>, OR<10>, OSO2CF3, OCOR<10>, SR<10>, NR<10>2 or PR<10>2 group, wherein the substituents R<10 >are hydrogen or alkyl groups; p is an integer of from 1 to 3, being equal to the oxidation state of the metal M minus 2. The above metallocenes are particularly useful in the polymerization of propylene.
Owner:BASELL POLYOLEFINE GMBH
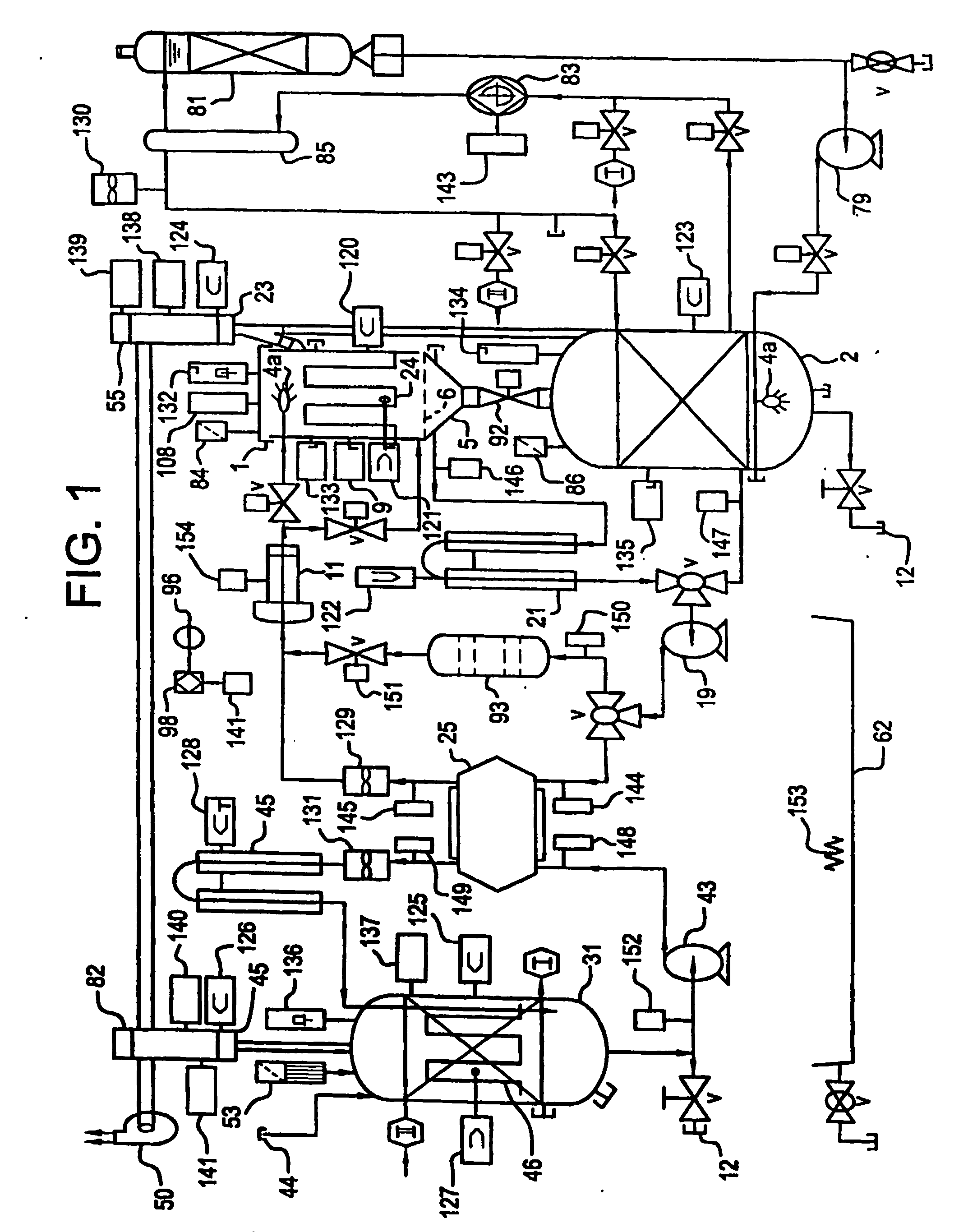
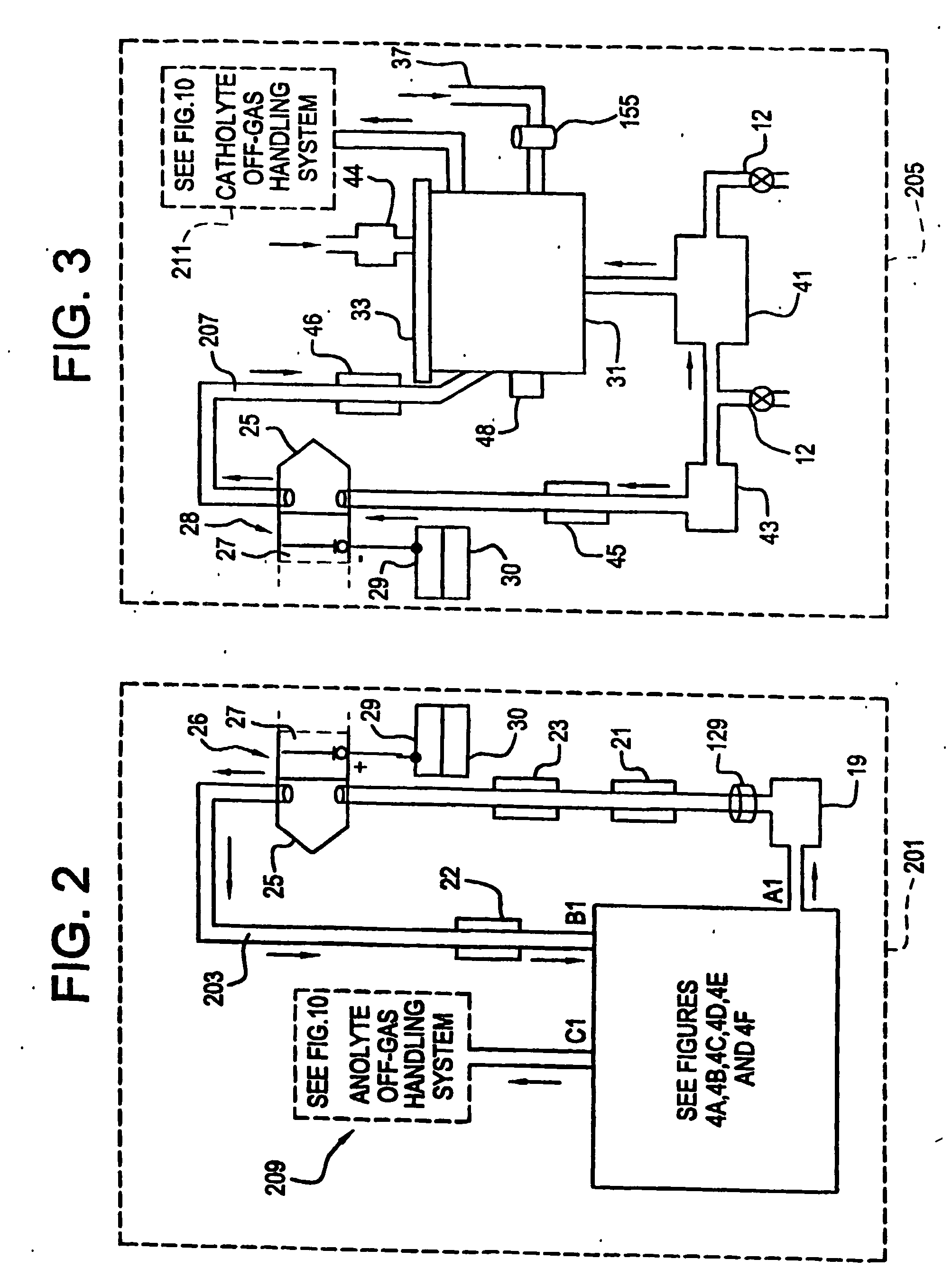
Apparatus and process for mediated electrochemical oxidation of materials
A unique apparatus unique apparatus and process that uses mediated electrochemical oxidation (MEO) for: (1) Destruction of: a) nearly all organic solid, liquid, and gases materials, except fluorinated hydrocarbons; b) all biological solid, liquid, and gases materials; c) and / or dissolution and decontamination (such as cleaning equipment and containers, etc.) of nearly all inorganic solid, liquid, or gas where higher oxidation states exist which includes, but is not limited to, halogenated inorganic compounds (except fluorinated), inorganic pesticides and herbicides, inorganic fertilizers, carbon residues, inorganic carbon compounds, mineral formations, mining tailings, inorganic salts, metals and metal compounds, etc.); and d) combined materials (e.g. a mixture of any of the foregoing with each other); henceforth collectively referred to as materials. (2) Sterilization / disinfection of equipment, glassware, etc., by destroying all existing infectious materials. (3) Dissolution of transuranic / actinide materials and / or destruction of the oxidizable components in the hazardous waste portion of mixed waste. (4) Generation of hydrogen and oxygen from MEO of materials. (5) Alteration of organic, biological, and inorganic materials by MEO to produce other compounds from these materials. The materials are introduced into an apparatus for contacting the materials with an electrolyte containing the oxidized form of one or more reversible redox couples, at least one of which is produced electrochemically by anodic oxidation at the anode of an electrochemical cell. The oxidized forms of any other redox couples present are produced either by similar anodic oxidation or reaction with the oxidized form of other redox couples present and capable of affecting the required redox reaction. The oxidized species of the redox couples oxidize the materials molecules and are themselves converted to their reduced form, whereupon they are reoxidized by either of the aforementioned mechanisms and the redox cycle continues until all oxidizable material species, including intermediate reaction products, have undergone the desired degree of oxidation. The entire process takes place at temperatures between ambient and approximately 100° C. The oxidation process may be enhanced by the addition of reaction enhancements, such as: ultrasonic energy and / or ultraviolet radiation.
Owner:SCIMIST LNC
Process for the carbonylation of epoxides
InactiveUS20050014977A1Oxygen-containing compound preparationOrganic compound preparationLanthanideCobalt
The present invention pertains to a process for the carbonylation of an epoxide by reacting it with carbon monoxide in the presence of a catalyst system containing two components, wherein the first component is a source of one or more metals selected from the group consisting of cobalt, ruthenium and rhodium, and the second component is a coordination complex of a tetrapyrrole compound with one or more of the metals belonging to the group consisting of groups IIIA and IIIB of the periodic system, lanthanides and actinides. The present invention also pertains a process for the preparation of a catalyst system.
Owner:SHELL OIL CO
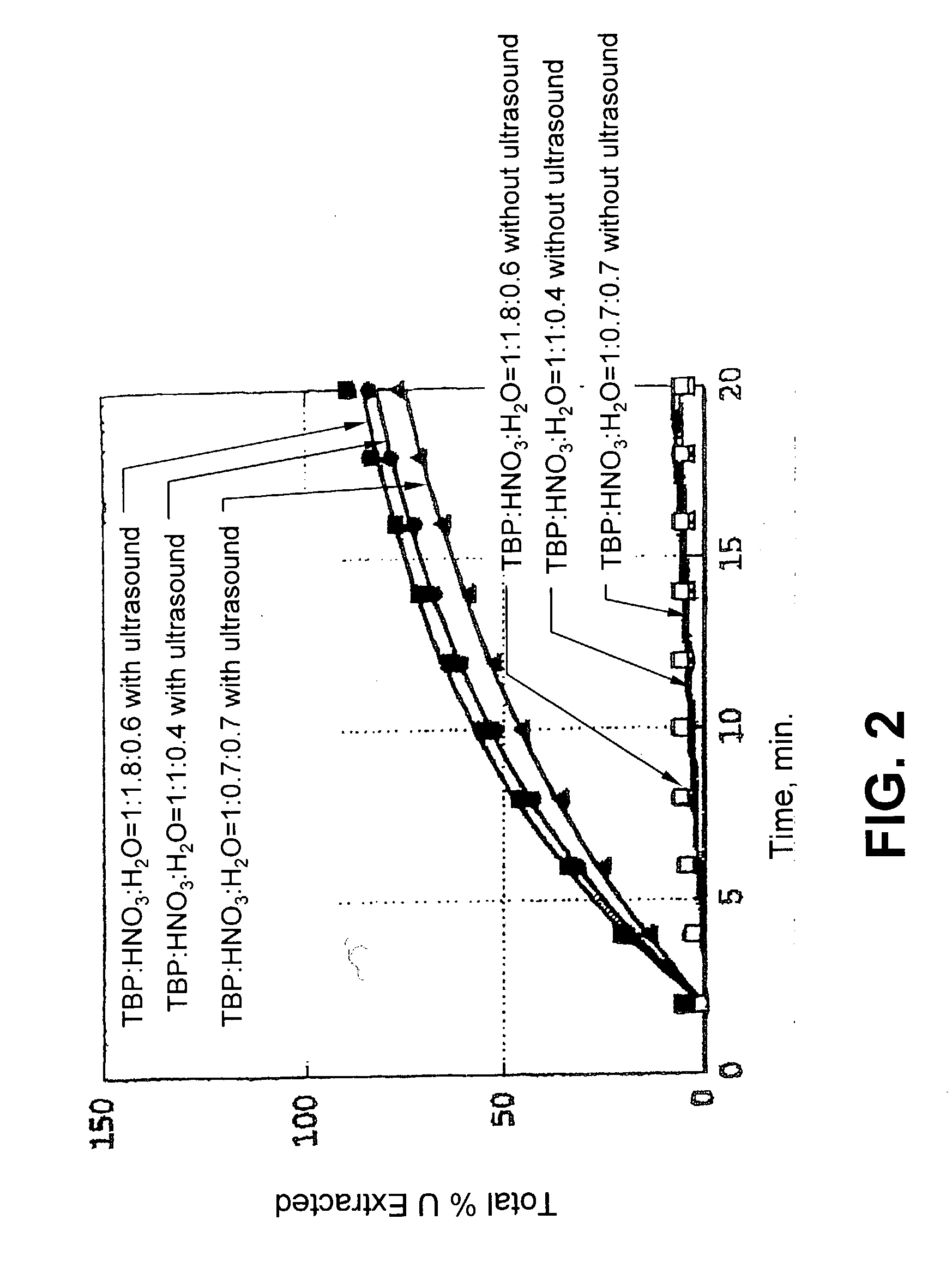
Ultrasound enhanced process for extracting metal species in supercritical fluids
InactiveUS20030183043A1Enhances rate and efficiencyReduce probabilitySolid sorbent liquid separationGold compoundsUranium oxidePresent method
Improved methods for the extraction or dissolution of metals, metalloids or their oxides, especially lanthanides, actinides, uranium or their oxides, into supercritical solvents containing an extractant are disclosed. The disclosed embodiments specifically include enhancing the extraction or dissolution efficiency with ultrasound. The present methods allow the direct, efficient dissolution of UO2 or other uranium oxides without generating any waste stream or by-products.
Owner:NAGOYA INDUSTRIAL SCIENCE RESEARCH INST +1
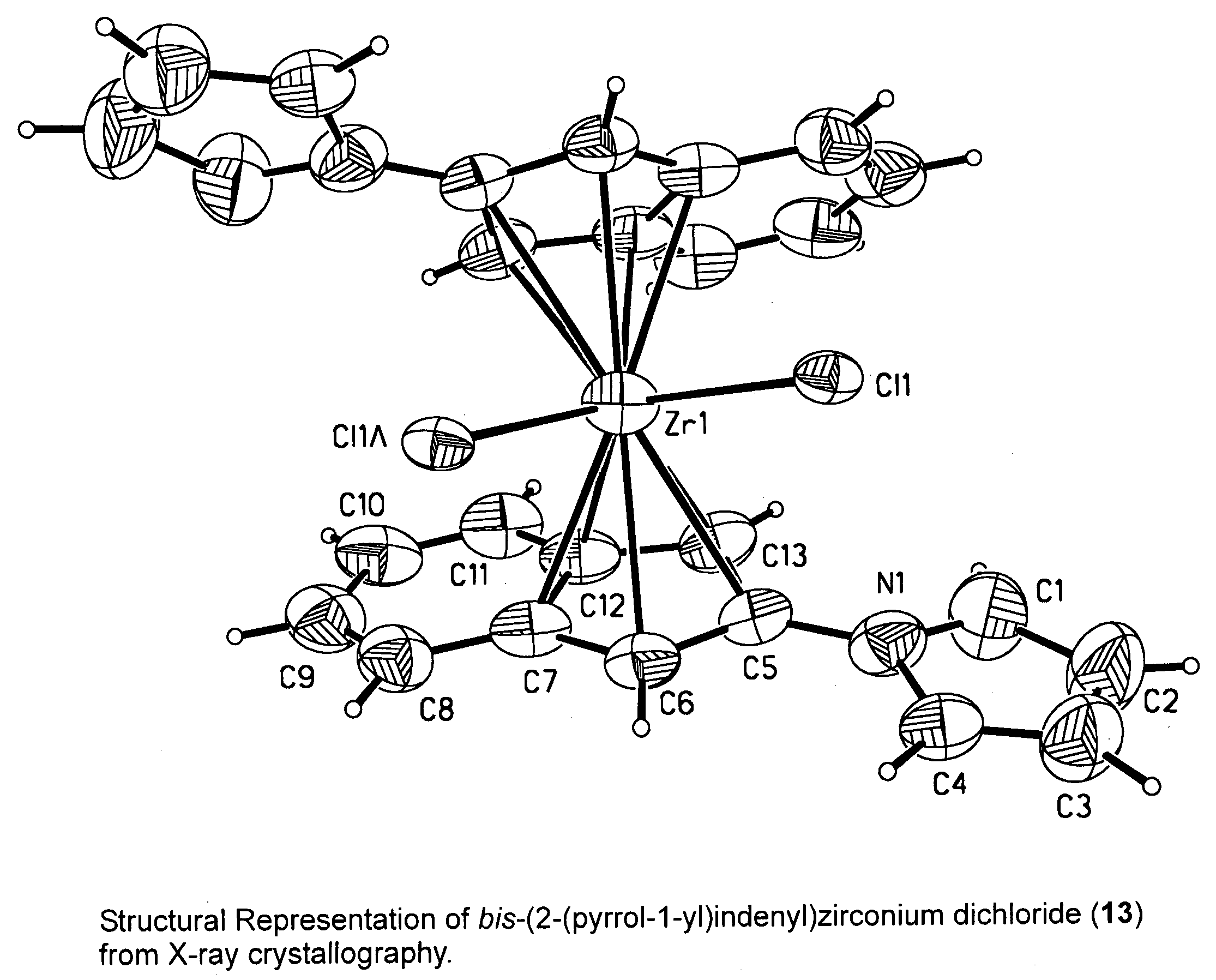
Heterocyclic substituted metallocene compounds for olefin polymerization
ActiveUS7276567B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLanthanideNitrogen
This invention relates to compounds represented by formula:whereinM is a group 3, 4, 5 or 6 transition, lanthanide, or actinide metal atom;E is an indenyl ligand substituted in any position with at least one aromatic or pseudoaromatic heterocyclic substituent that is bonded to the indenyl ring through a nitrogen or phosphorous ring heteroatom;A is a substituted or unsubstituted cyclopentadienyl, heterocyclopentadienyl, indenyl, heteroindenyl, fluorenyl, or heterofluorenyl ligand, or other mono-anionic ligand, or A may, independently, be E;Y is an optional bridging group;y is zero or one;X are, independently, univalent anionic ligands, andprovided that when A is E, and y is one, and Y is bonded to the one position of each indenyl ligand, and per indenyl ligand there is only one aromatic heterocyclic or pseudoaromatic heterocyclic that is bonded to the indenyl ligand.
Owner:EXXONMOBIL CHEM PAT INC
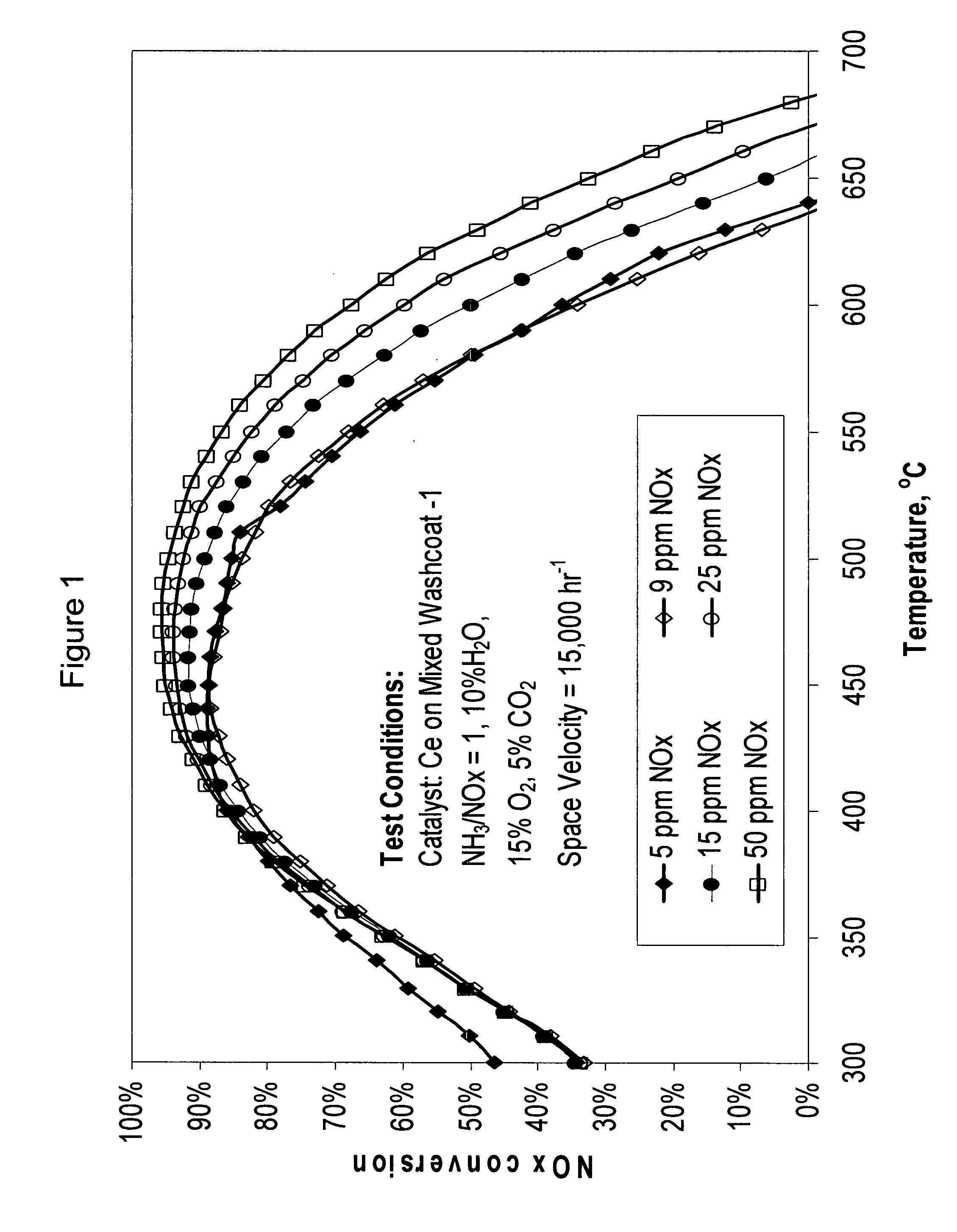
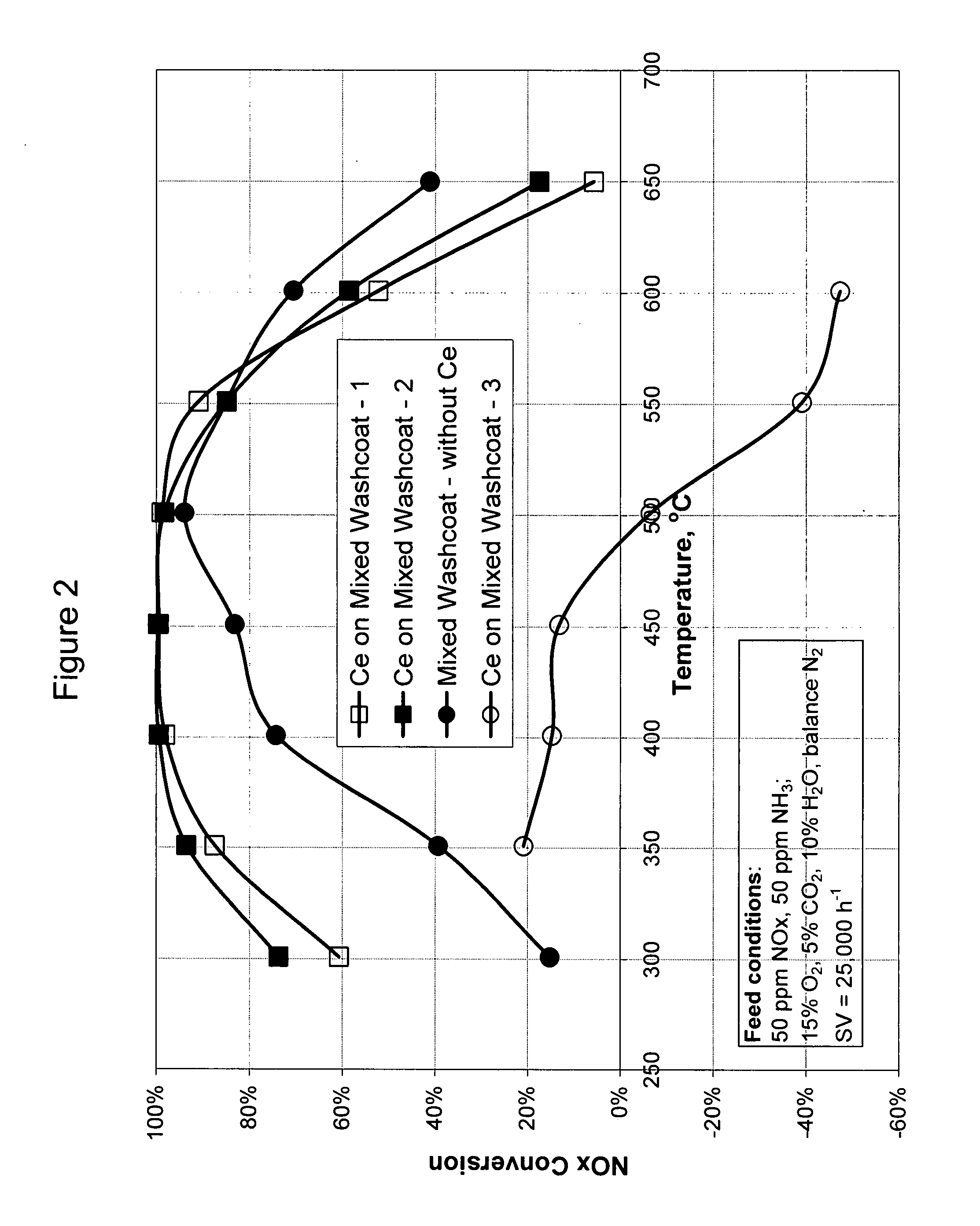
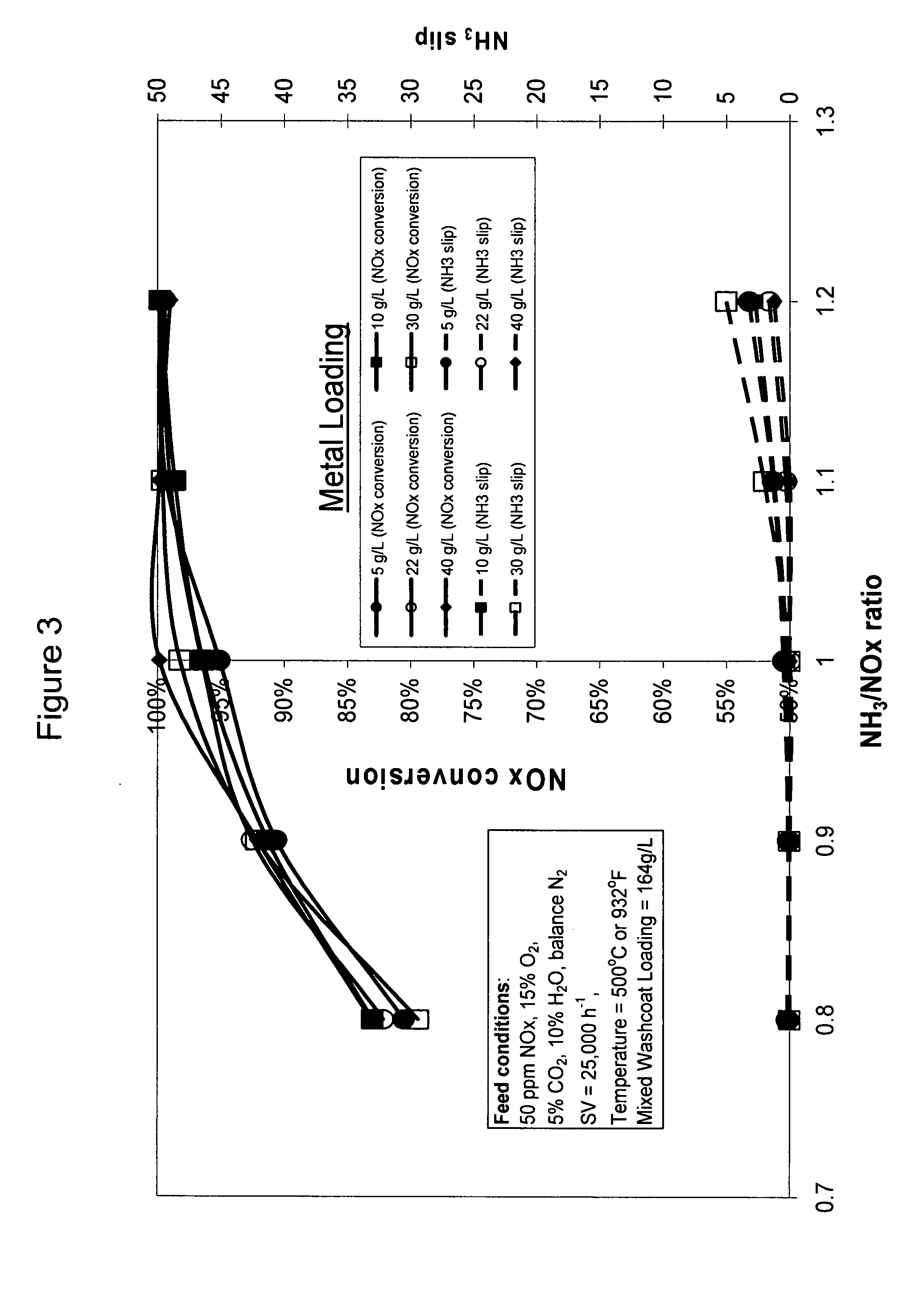
High temperature ammonia SCR catalyst and method of using the catalyst
ActiveUS20080167178A1Reduce selection requirementsMolecular sieve catalystsInternal combustion piston enginesCeriumMordenite
A catalyst and a method for selectively reducing nitrogen oxides (“NOx”) with ammonia are provided. The catalyst includes a first component comprising a zeolite or mixture of zeolites selected from the group consisting of ZSM-5, ZSM-11, ZSM-12, ZSM-18, ZSM-23, MCM-zeolites, mordenite, faujasite, ferrierite, zeolite beta, and mixtures thereof; a second component comprising at least one member selected from the group consisting of cerium, iron, copper, gallium, manganese, chromium, cobalt, molybdenum, tin, rhenium, tantalum, osmium, barium, boron, calcium, strontium, potassium, vanadium, nickel, tungsten, an actinide, mixtures of actinides, a lanthanide, mixtures of lanthanides, and mixtures thereof; optionally an oxygen storage material and optionally an inorganic oxide. The catalyst selectively reduces nitrogen oxides to nitrogen with ammonia at high temperatures. The catalyst has high hydrothermal stability. The catalyst has high activity for conversion of low levels of nitrogen oxides in exhaust streams. The catalyst and the method may have special application to selective reduction of nitrogen oxides in exhaust gas from gas turbines and gas engines, although the catalyst and the method have broad application to a wide range of gas streams that have excess oxygen and high temperatures. The temperature of exhaust gas from gas turbines and gas engines is high. Both the high temperature and the low levels of inlet NOx are challenging for selective catalytic reduction (SCR) catalysts.
Owner:CATALYTIC SOLUTIONS INC
Metallocene compounds and their use in catalysts for the polymerization of olefins
InactiveUS6268518B1Group 5/15 element organic compoundsHydrocarbon by hydrocarbon condensationArylHydrogen
It is disclosed a new class of bridged metallocene compounds of formula (I):wherein R1 and R2 can be hydrogen, alkyl, cycloalkyl, aryl, alkylaryl or arylalkyl radicals; R3 and R4 form a condensed, 5- to 8-membered, aliphatic, aromatic or heterocyclic ring; M is a transition metal of groups 3, 4, 5, lanthanide or actinide; X is hydrogen, halogen, -R, -OR, -OSO2CF3, -OCOR, -SR, -NR2 or PR2, wherein R is an hydrocarbon substituent; and p is 0-3. Furthermore, a catalyst system for olefin polymerization based on the above bridged metallocene compounds and the ligands for their preparation are disclosed.
Owner:MONTELL TECH CO BV
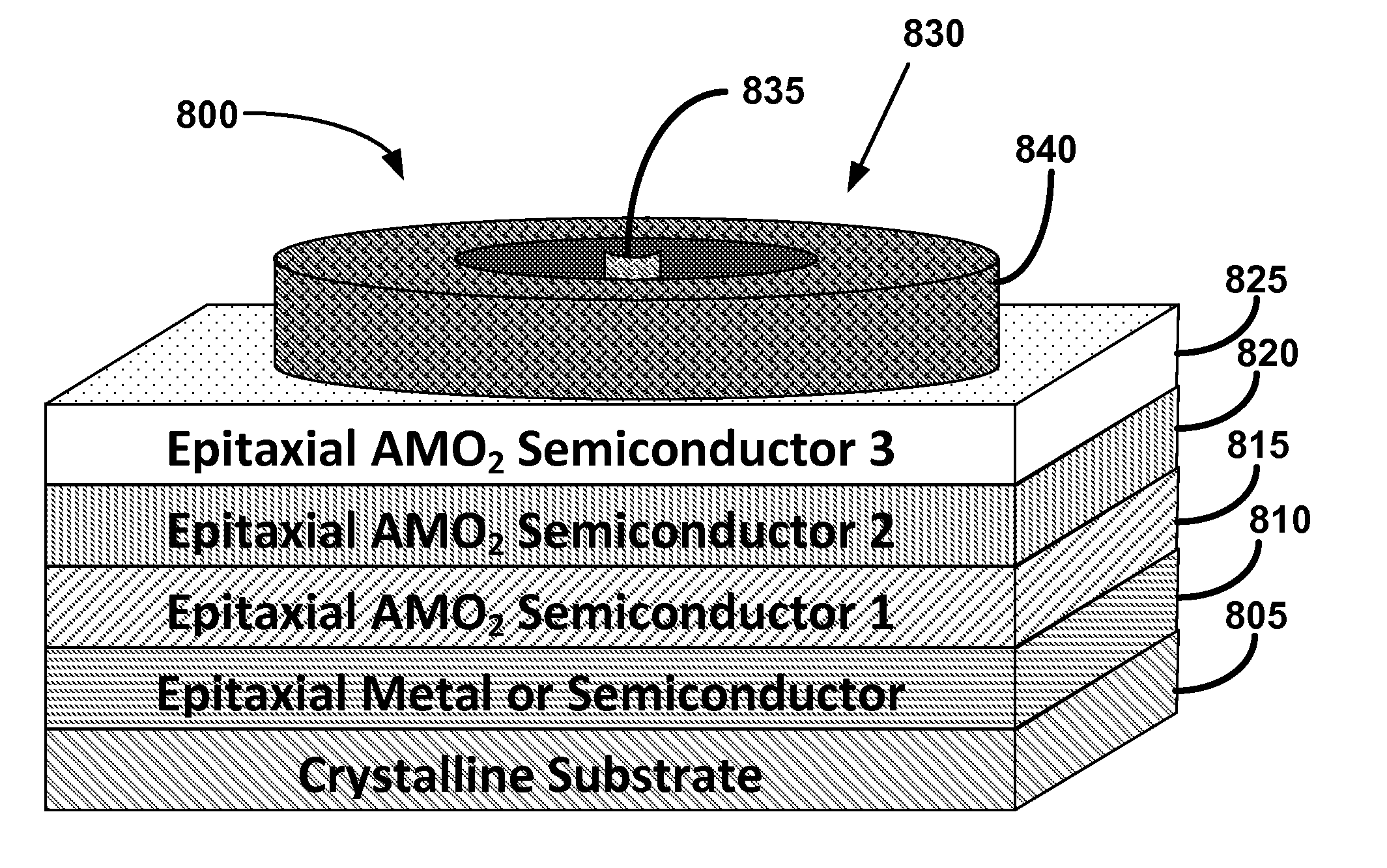
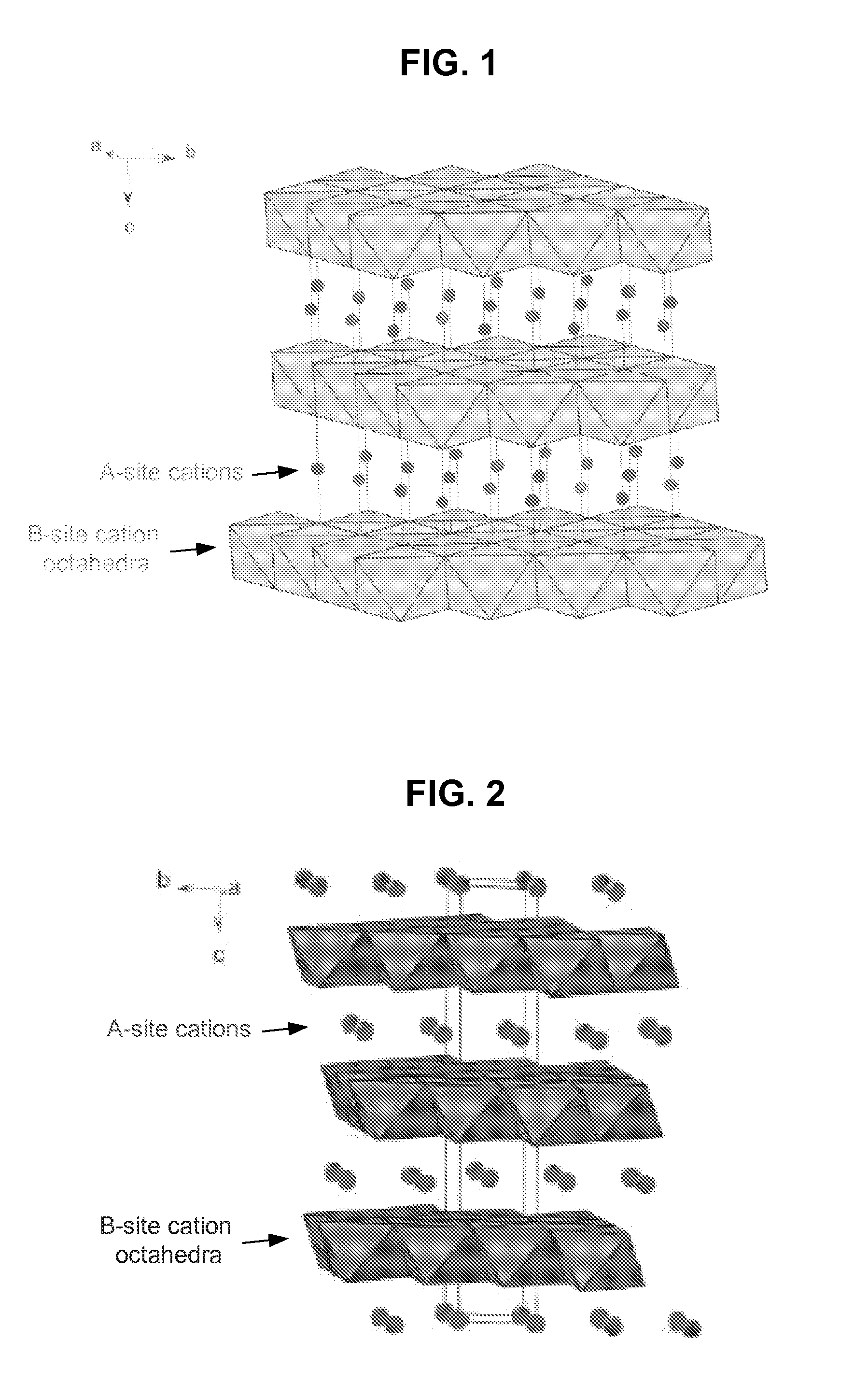
Metal oxide structures, devices, and fabrication methods
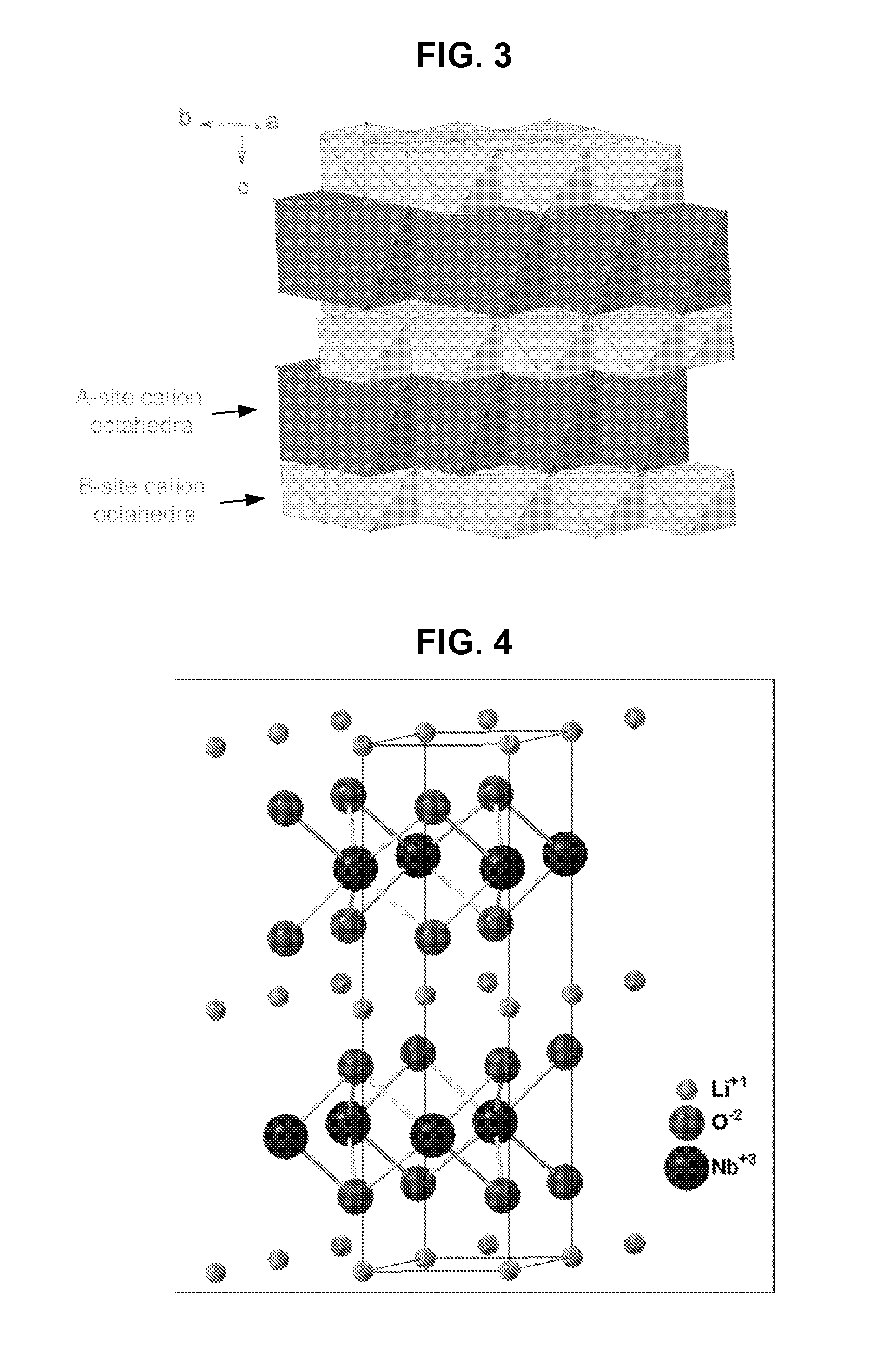
Metal oxide structures, devices, and fabrication methods are provided. In addition, applications of such structures, devices, and methods are provided. In some embodiments, an oxide material can include a substrate and a single-crystal epitaxial layer of an oxide composition disposed on a surface of the substrate, where the oxide composition is represented by ABO2 such that A is a lithium cation, B is a cation selected from the group consisting of trivalent transition metal cations, trivalent lanthanide cations, trivalent actinide cations, trivalent p-block cations, and combinations thereof, and O is an oxygen anion. The unit cell of crystal structure of the oxide composition can be characterized by first layer of a plane of lithium cations and a second layer of a plurality of edge-sharing octahedra having a B cation positioned in a center of each octahedron and an oxygen anion at each corner of each octahedron. The first layer and the second layer of the unit cell are alternatingly stacked along one axis of the unit cell. Other aspects, features, and embodiments are also claimed and described.
Owner:GEORGIA TECH RES CORP
Use of photocatalytic preparations of colloidal titanium dioxide for preserving the original appearance of cementitious, stone, or marble products
Use of photocatalytic preparations of colloidal titanium dioxide optionally doped with a metal chosen from groups I-VA, and the lanthanide and actinide series of the periodic table, for preserving the original appearance of cementitious, stone, and marble products.
Owner:ITALCEMENTI
Organic light emitting diode
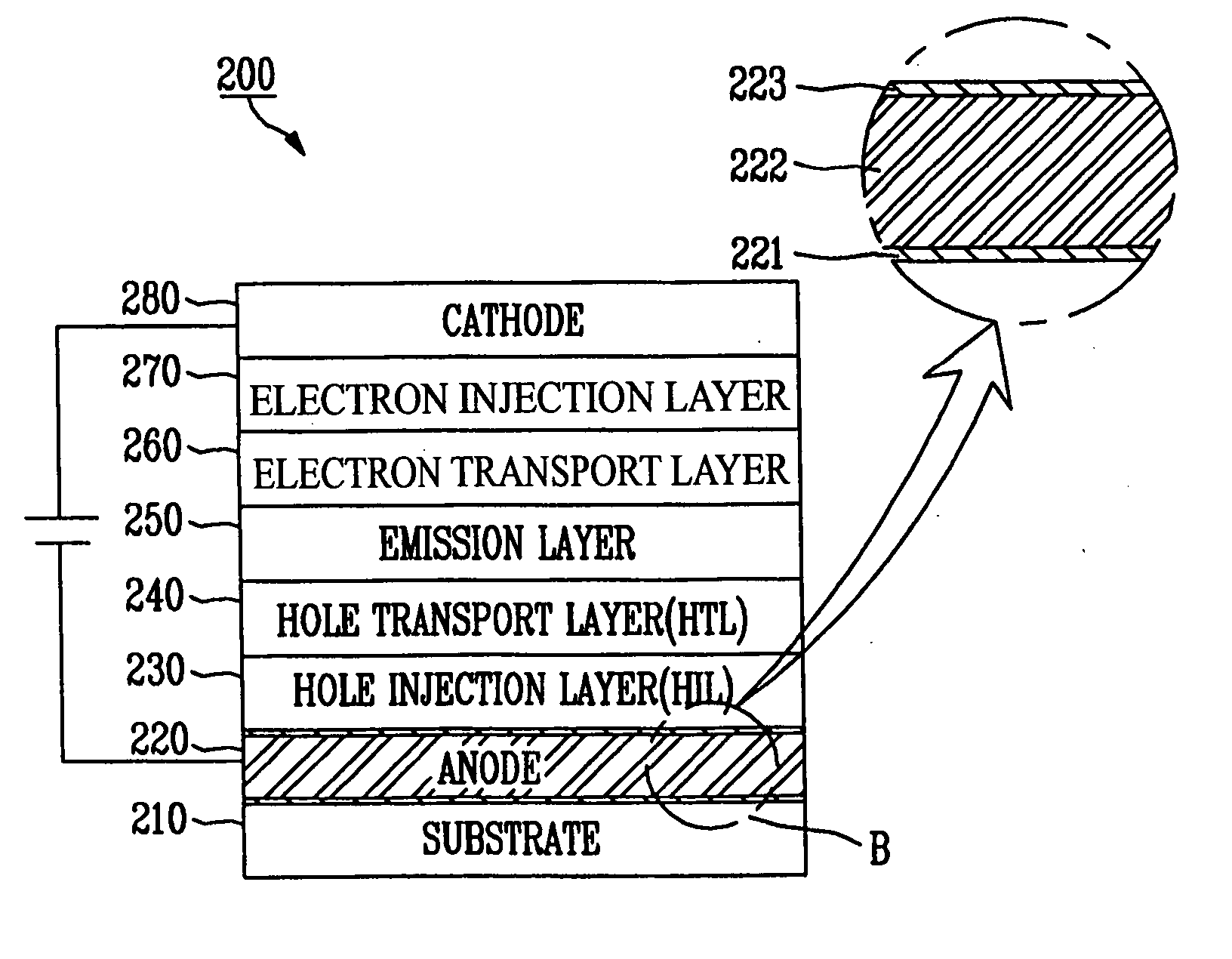
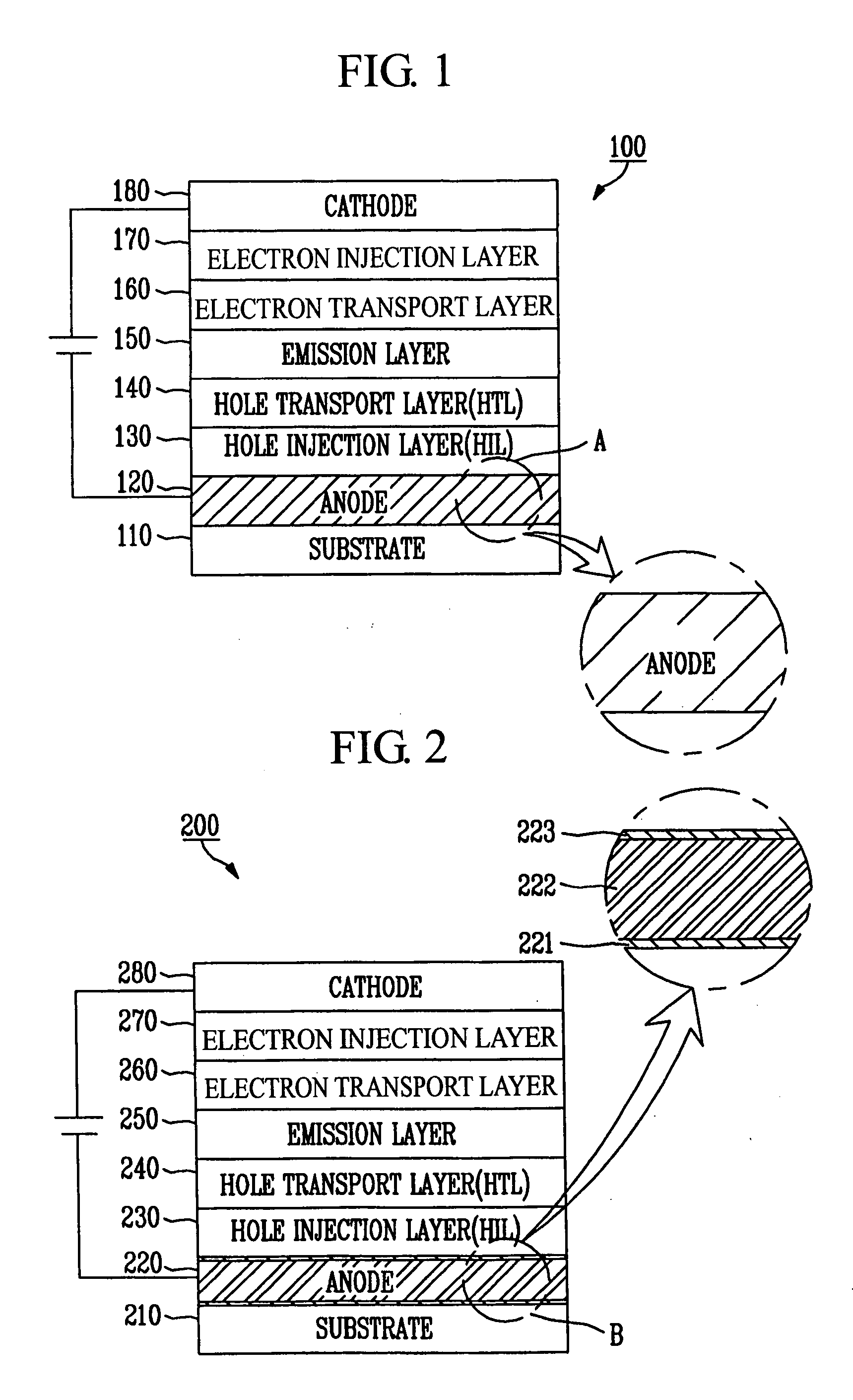
ActiveUS20060091791A1Improve productivityReflectance can be improvedDischarge tube luminescnet screensElectroluminescent light sourcesLanthanideAlloy
An organic light emitting diode, which has a pixel electrode, the pixel electrode constructed with a first layer comprising metal oxide on the substrate; a second layer comprising silver alloyed with at least one metal selected from a group consisting of lanthanide series elements and actinide series elements on the first layer; and a third layer comprising metal oxide on the second layer. As such, there are provided the second layer comprising the silver alloy, and the first and third layer comprising the metal oxide and formed above and below the second layer so that adhesion of a silver alloy (e.g., ATD alloy) may be enhanced, and an anode having enhanced reflectance may also be provided by using silver with increased reflectance.
Owner:SAMSUNG DISPLAY CO LTD
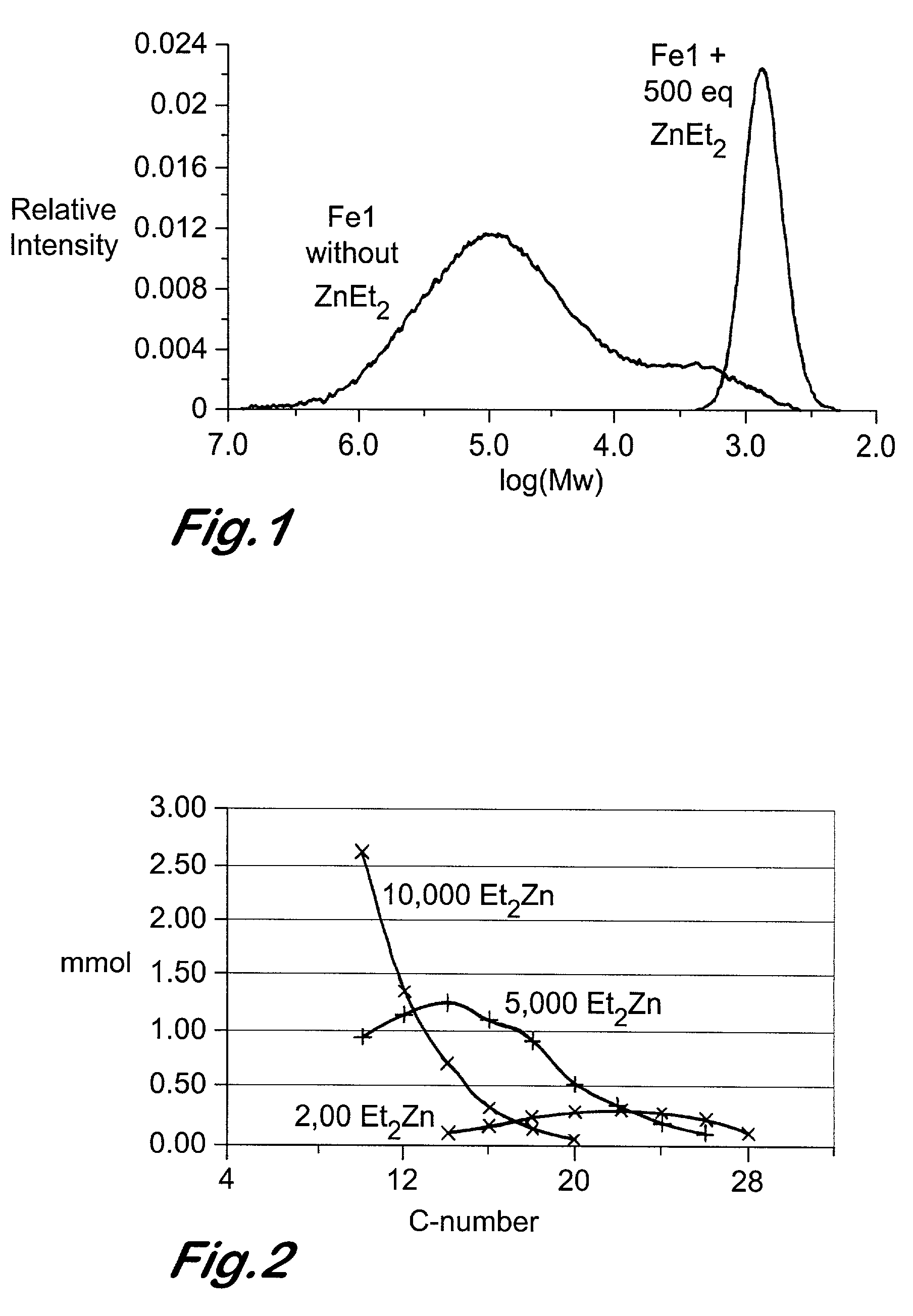
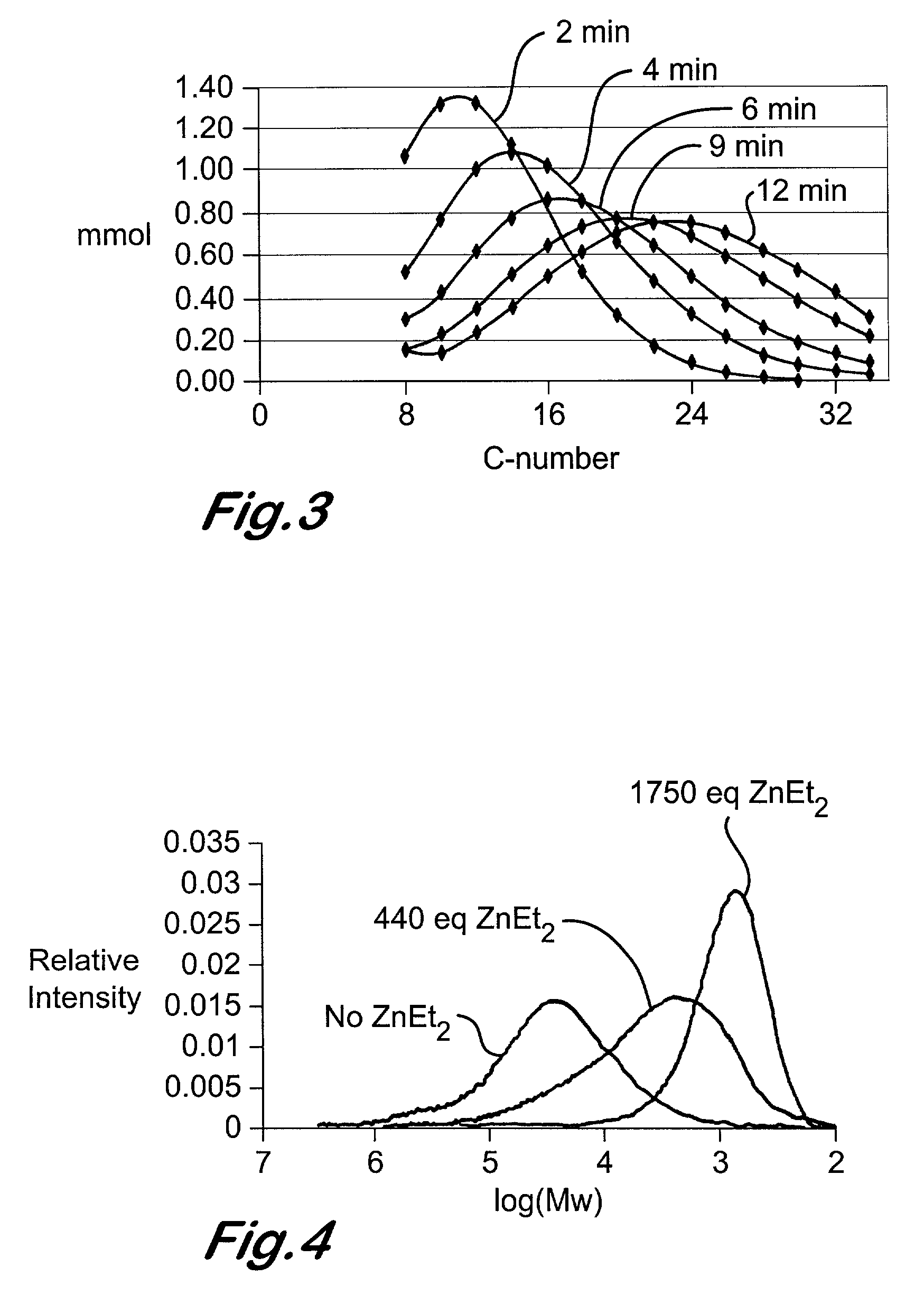
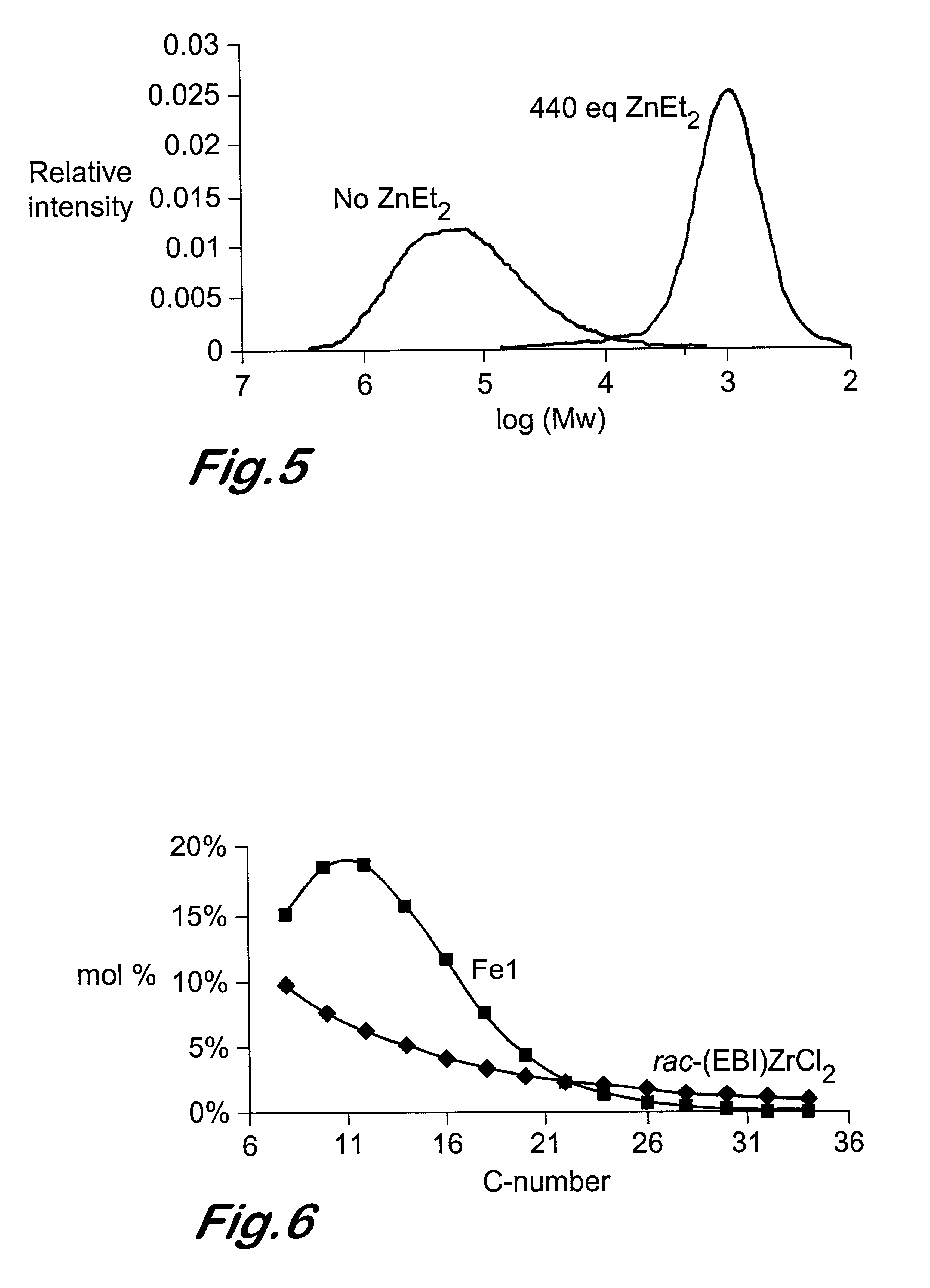
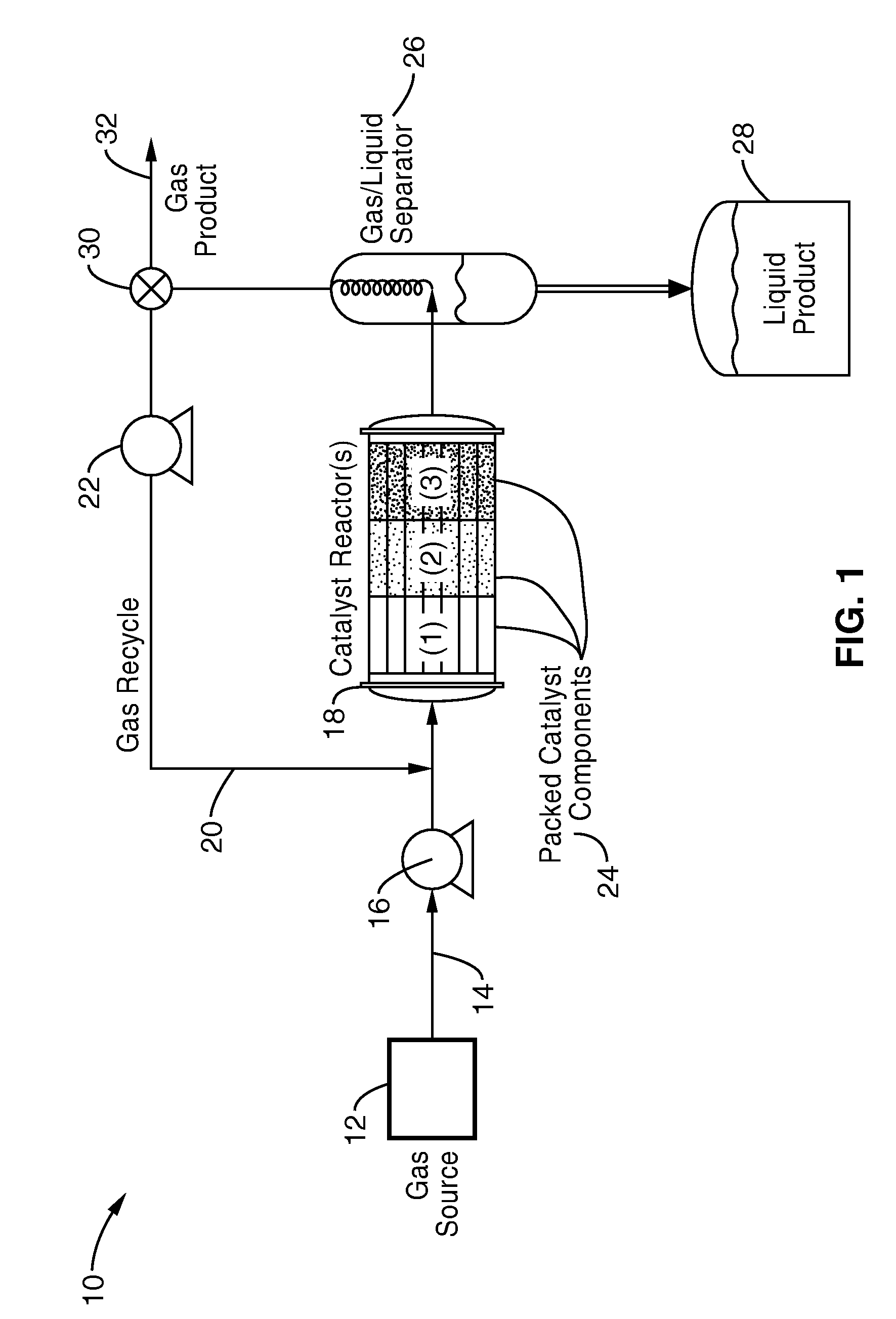
Chain growth reaction process
InactiveUS7087686B2Preparation by oxidation reactionsOxygen-containing compound preparationAlcoholLanthanide
A process is disclosed for the preparation of zinc alkyl chain growth products via a catalysed chain growth reaction of an alpha-olefin on a zinc alkyl, wherein the chain growth catalyst system employs a group 3-10 transition metal, or a group 3 main group metal, or a lanthanide or actinide complex, and optionally a suitable activator. The products can be further converted into alpha-olefins by olefin displacement of the grown alkyls as alpha-olefins from the zinc alkyl chain growth product, or into primary alcohols, by oxidation of the resulting zinc alkyl chain growth product to form alkoxide compounds, followed by hydrolysis of the alkoxides.
Owner:INEOS SALES (UK) LTD
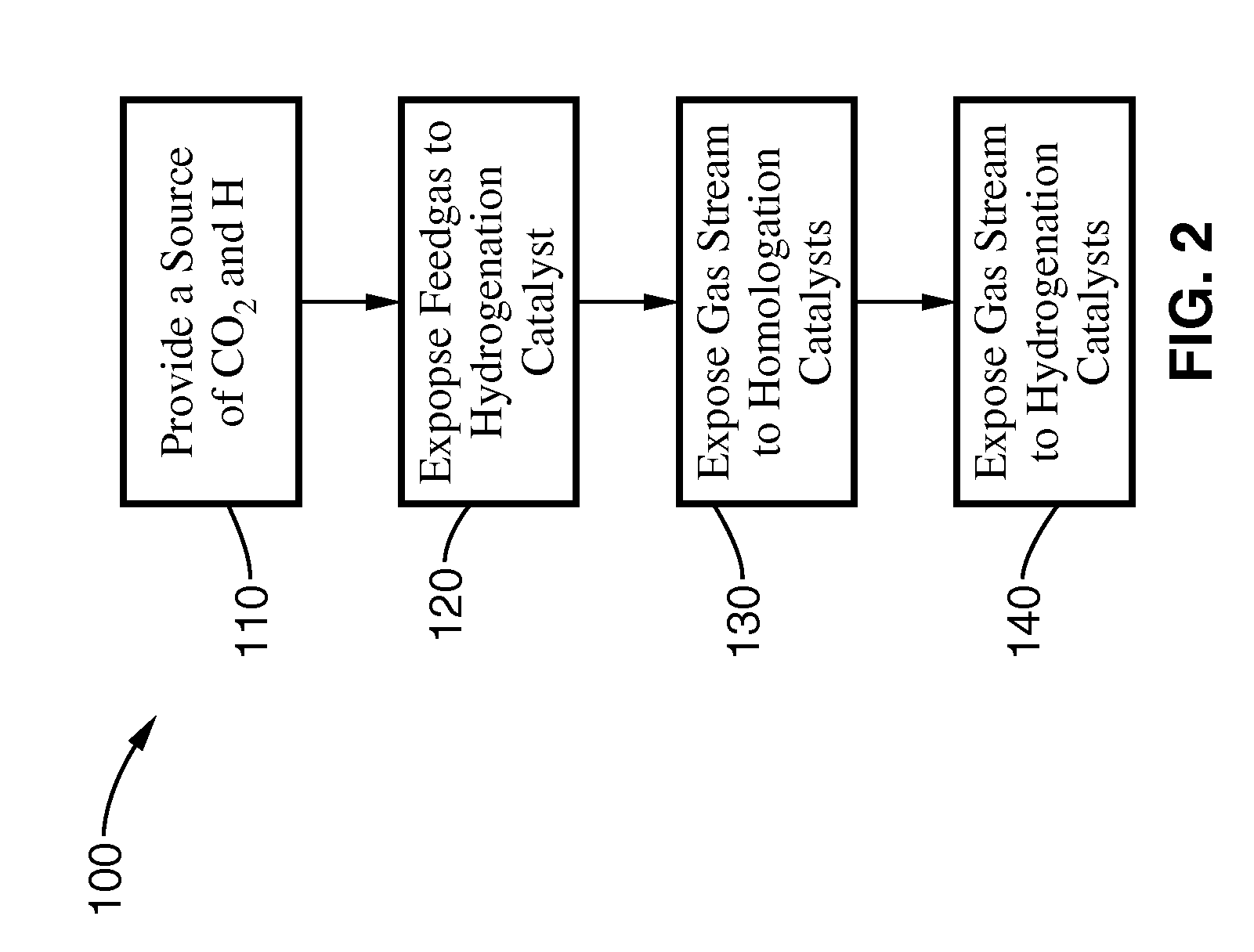
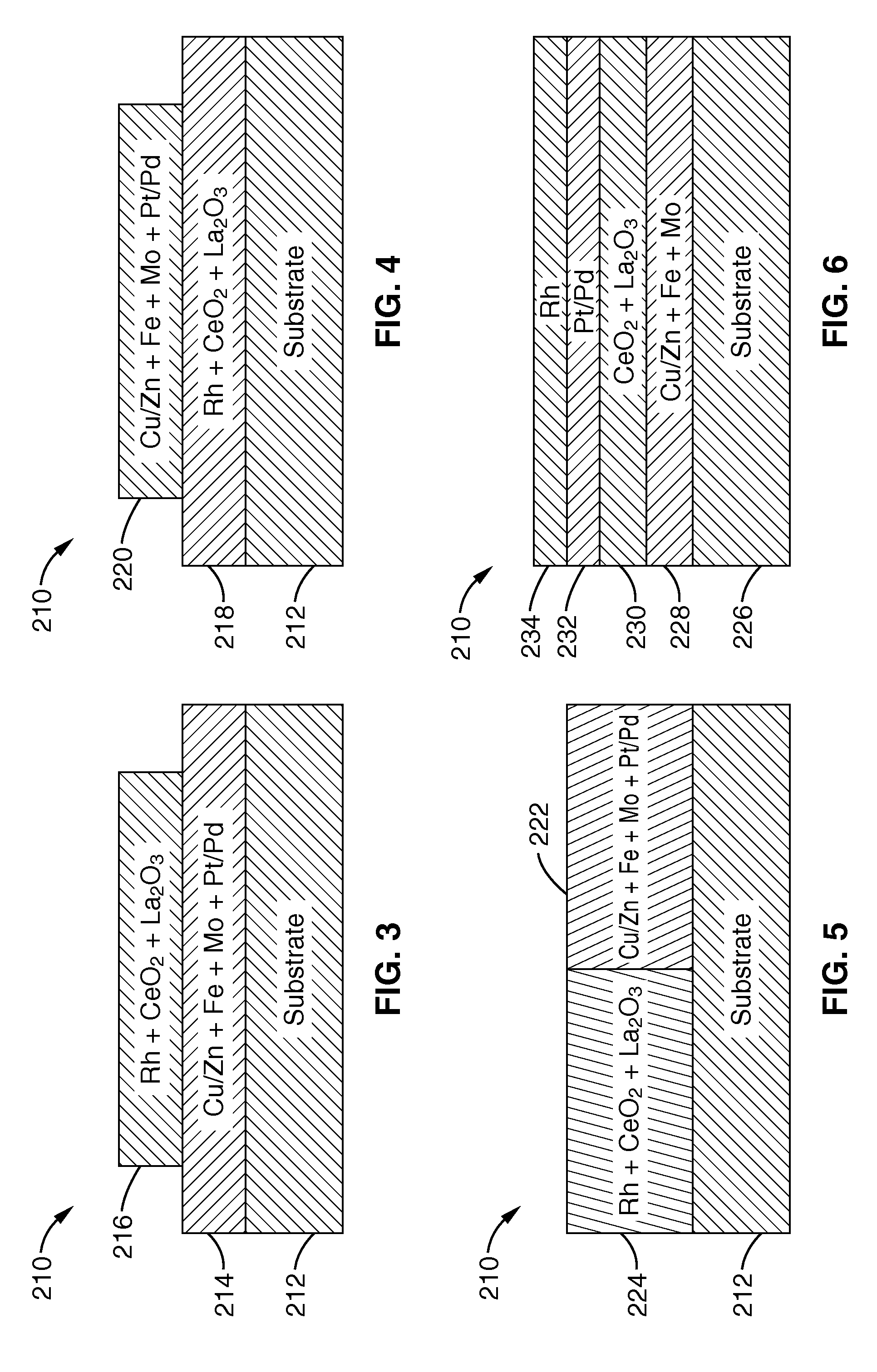
Combination catalytic process for producing ethanol from synthesis gas
ActiveUS7718832B1High selectivityHigh yieldOrganic compound preparationOxygen compounds purification/separationSyngasLanthanide
A catalytic process selectively produces ethanol by contacting synthesis gas (syngas), composed primarily of hydrogen and carbon monoxide, with three catalysts within a reactor. The first catalyst is a hydrogenation promoter comprising Cu—Zn, Mo or Fe with an optional alkali metal additive and an optional support of aluminum oxide, silica, zeolite or clay. The second catalyst is a homologation promoter comprising one or more of the Group VIII metals in free or combined form with a co-catalyst metals consisting of Y or lanthanide or actinide series metals with optional additives and support. The third catalyst is a hydrogenation promoter. This series of catalysts improves the selectivity and yield for ethanol from syngas.
Owner:GREYROCK TECH LLC
Quasi-crystalline boehmites containing additives
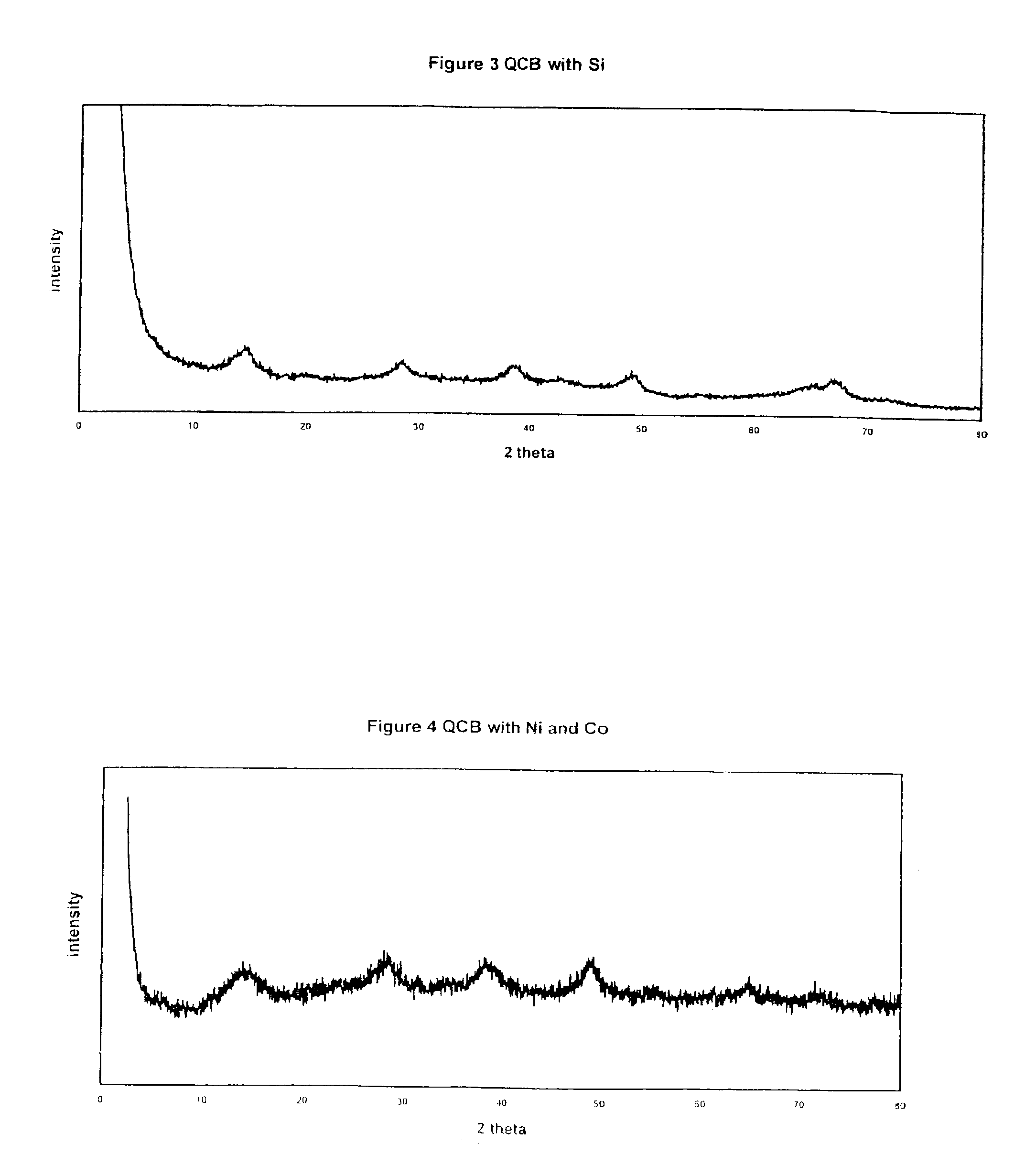
The present invention pertains to a quasi-crystalline boehmite containing additive in a homogeneously dispersed state. Suitable additives are compounds containing elements selected from the group of alkaline earth metals, alkaline metals, rare earth metals, transition metals, actinides, silicon, gallium, boron, titanium, and phosphorus. Said QCBs according to the invention may be prepared in several ways. In general, a quasi-crystalline boehmite precursor and an additive are converted to a quasi-crystalline boehmite containing the additive in a homogeneously dispersed state.
Owner:ALBEMARLE NETHERLANDS BV
Method for separating in an aqueous medium lanthanides and/or actinides by combined complexing-nanofiltration, and novel complexing agents therefor
InactiveUS6843917B1Easy to implementEasily degradableMembranesOrganic chemistryWaste processingLanthanide
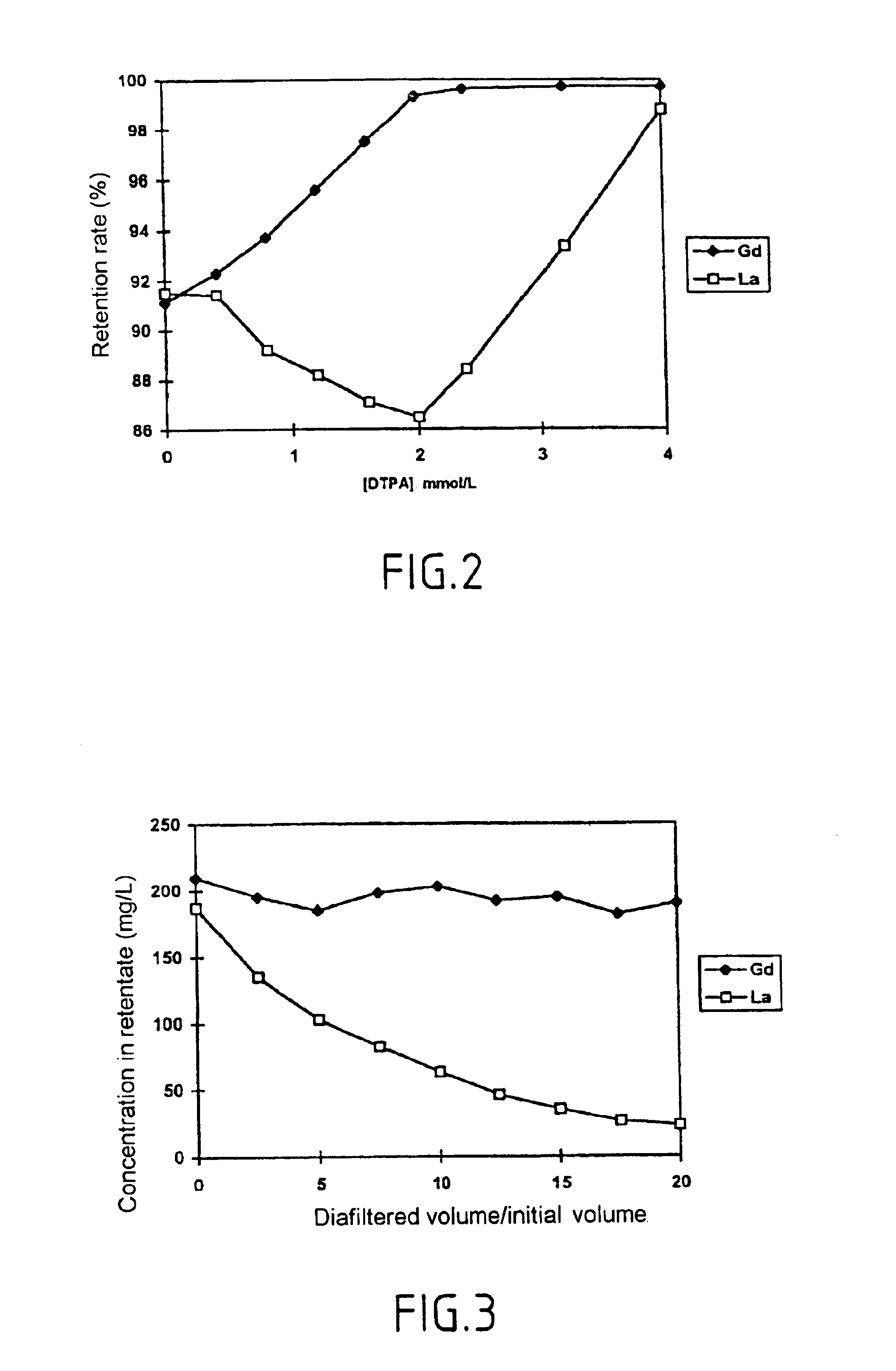
The invention relates to the separation of lanthanides and actinides by nanofiltration complexation. The object of the invention is to satisfy the existing need for a simple, efficient and economical technique for separating lanthanides and actinides. This object is achieved by a process consisting of using ligands of the polyamino acid type, such as EDTA or DTPA, for complexing lanthanides and / or actinides before separating them by nanofiltration. The invention further relates to novel polyamino acid ligands incorporating ligand structures additional to EDTA and DTPA. Application to the production of rare earths or nuclear waste processing, especially to recycling operations carried out on spent nuclear fuels is also discussed.
Owner:UNIV CLAUDE BERNARD LYON 1 +1
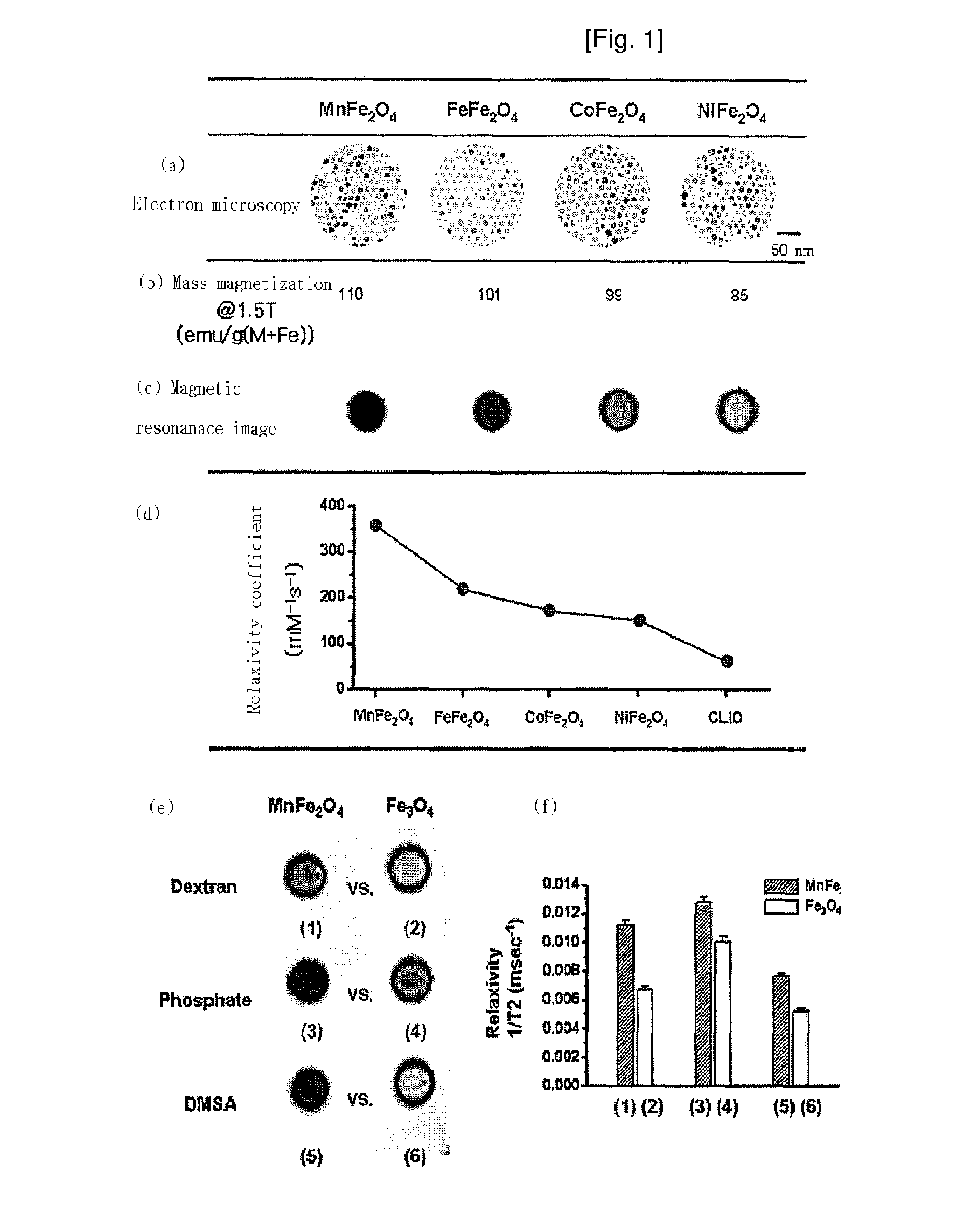
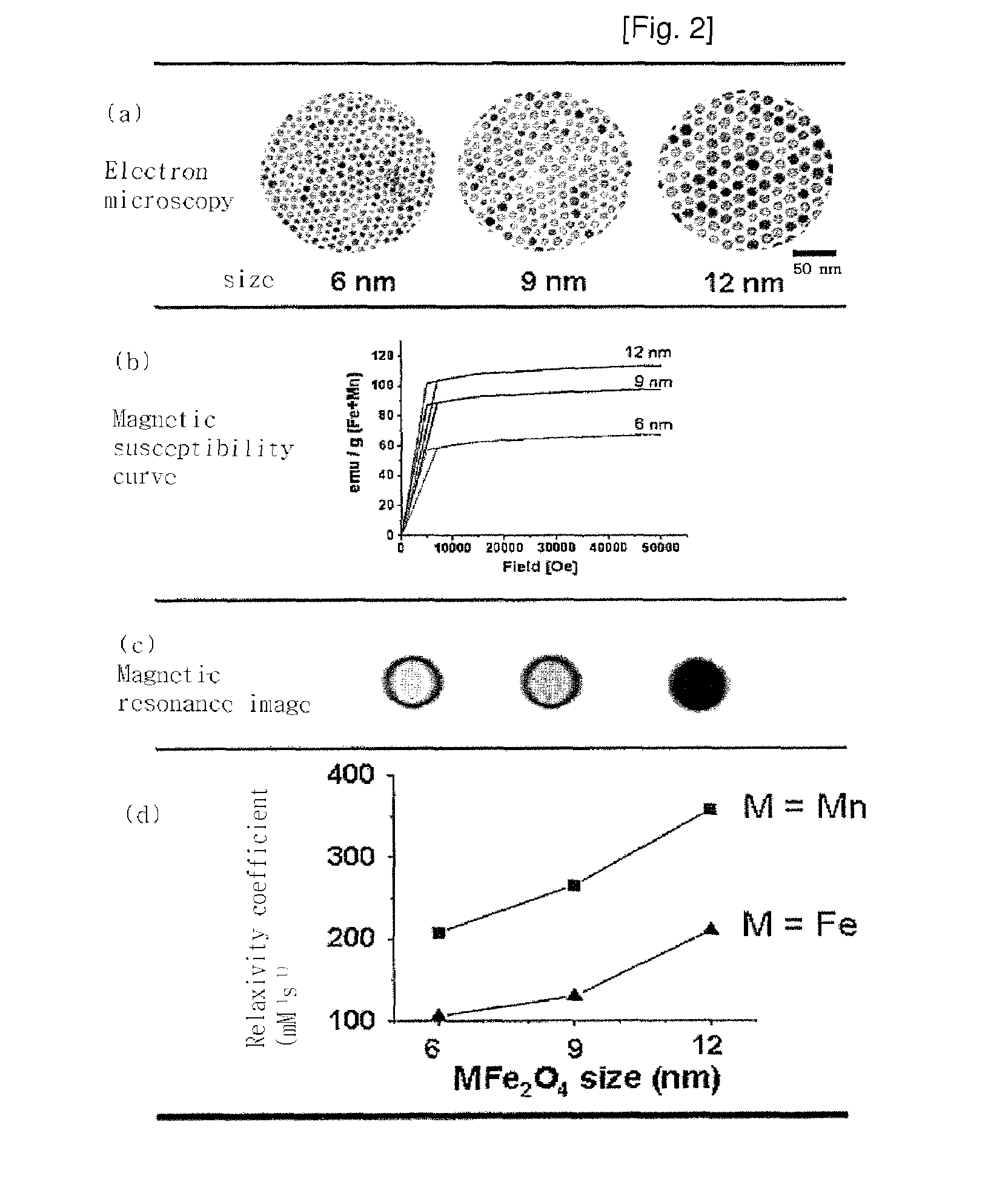
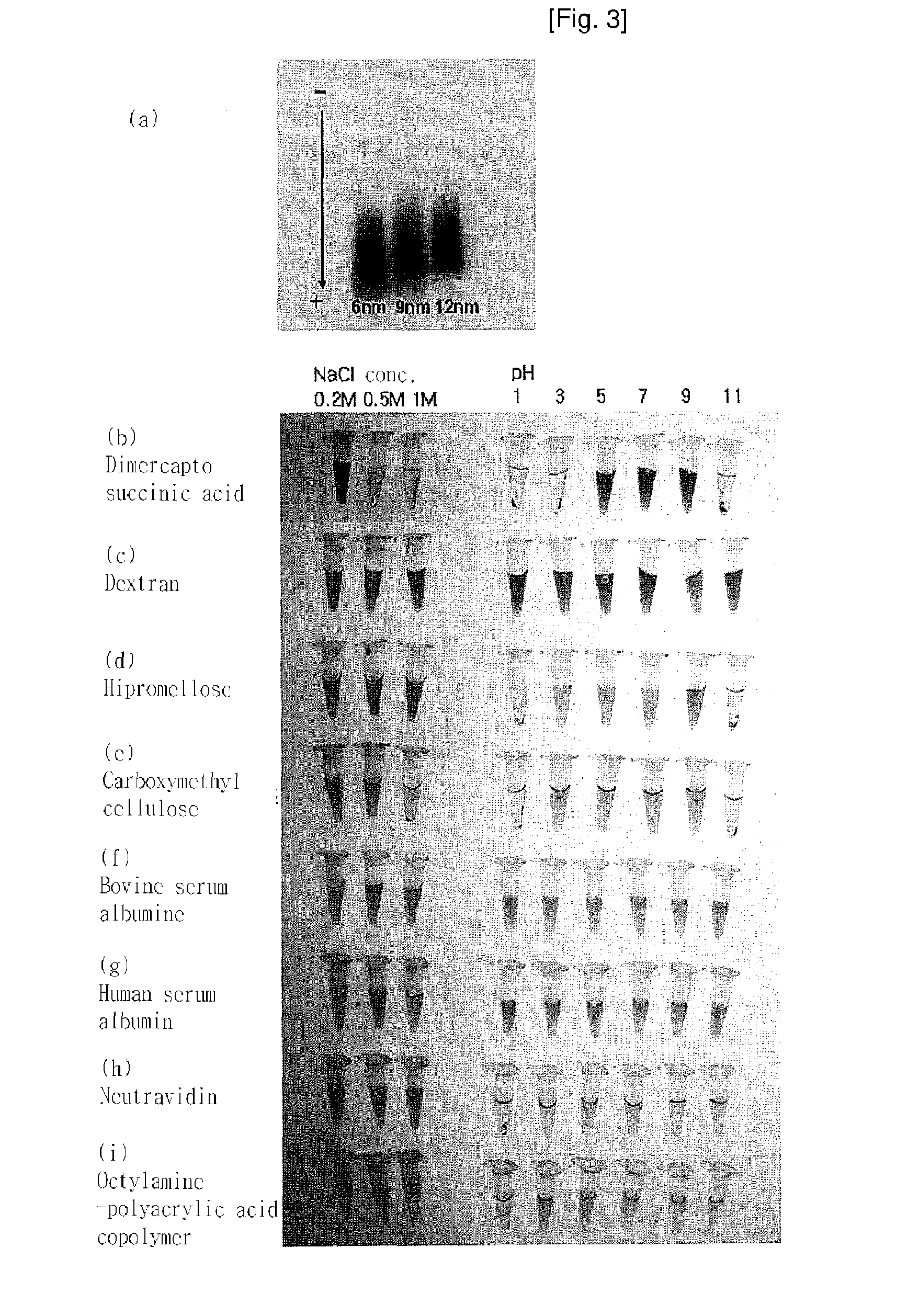
Magnetic resonance imaging contrast agents containing water-soluble nanoparticles of manganese oxide or manganese metal oxide
InactiveUS20090220431A1Uniform sizeImprove magnetic propertiesPowder deliveryMaterial nanotechnologyElemental compositionMRI contrast agent
The present invention relates to a manganese-containing metal oxide nanoparticle-based magnetic resonance imaging (MRI) contrast agent, which is characterized in that: The core of it comprises 1 to 1000 nm-sized manganese-containing metal oxide nanoparticles which include MnO a (0<a<5) or MnMbOe (wherein M is at least one metal atom selected from the group consisting of a Group 1 or 2 element such as Li, Na, Be, Ca, Ge, Mg, Ba, Sr and Ra, a Group 13 element such as Ga and In, a transition metal element such as Y, Ta, V, Cr, Co, Fe, Ni, Cu, Zn, Ag, Cd and Hg, and lanthanide or actinide group elements such as La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm and Yb, 0<b<5 and 0<c<10); preferably MnM′dFeeOf (wherein M′ is at least one metal atom selected from the group consisting of a Group 1 or 2 element such as Li, Na, Be, Ca, Ge, Mg, Ba, Sr and Ra, a Group 13 element such as Ga and In, a transition metal element such as Y, Ta, V, Cr, Co, Fe, Ni, Cu, Zn, Ag, Cd and Hg, and lanthanide or actinide group elements such as La, Ce, Pr, Nd, Pm, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm and Yb, 0<d<5, 0<e<5, and 0<f<15). In addition, the nanoparticles include water-soluble manganese-containing metal oxide nanoparticles which is characterized in that they are soluble in water themselves or stable in an aqueous media as being coated with a water-soluble ligand and they possess enhanced magnetic properties and MRI contrast effect. Also the water soluble manganese-containing metal oxide nanoparticles are coupled with an bioactive material such as chemical molecules or bio-functional molecules, and thus the nanoparticles can be used as an MRI contrast agent for target specificity and cell tracking.
Owner:IND ACADEMIC CORP FOUND YONSEI UNIV
Method for separating actinides and lanthanides by liquid-liquid extraction using calixarenes
The invention involves a process for separating actinides and lanthanides by liquid-liquid extraction by means of calixarenes.These calixarenes have the formula:with R1 and R2 being alkyl groups or o-nitrophenoxy alkyl groups and R3 and R4 being aryl groups,and they are used in an organic liquid phase containing an organic diluent. The diluent and the calixarene concentration of the organic phase are chosen so as to ensure an enrichment of the organic phase with the actinide(s) and / or lanthanide(s) to be separated from an aqueous acid or saline solution.
Owner:COMMISSARIAT A LENERGIE ATOMIQUE ET AUX ENERGIES ALTERNATIVES
Magnetic particle extractive agent and method for isolating radionuclide
InactiveCN101231899AEasy to desorbEasy to operateOrganic/organic-metallic materials magnetismLiquid solutions solvent extractionLiquid wasteAdditive ingredient
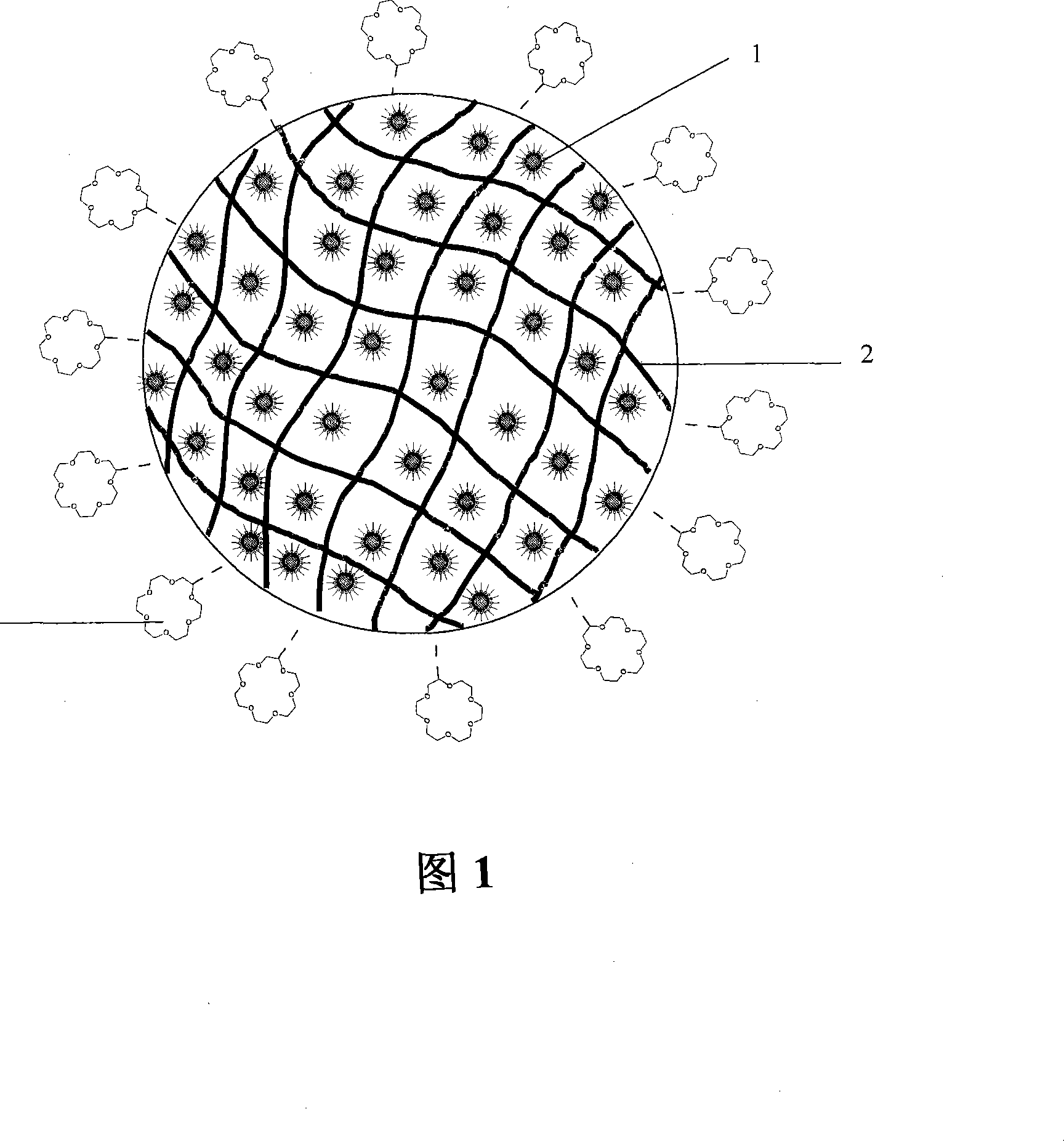
The invention discloses finished magnetic-particle extractant combined with magnetic nanometer microspheres, a preparation method thereof and application thereof in separation of radioactive species. The extractant consists of magnetic fine particles carrying separated functional groups of radioactive species. Nanometer particles of magnetism Fe3O4 are dispersed inside a polymer. A layer of ingredients selected from crown ether and ramification thereof or neutral phosphorus (TBP, TOPO) ramification and tertiary amine (TOA) ramification connected with chemical bonds is arranged on the surface of the polymer. The magnetic particle extractant can be directly added to solution containing radioactive species, and is stirred at a room temperature. The magnetic extractant is separated out with the applied magnetic field to separate radioactive species. The invention is integrated with the advantages of simple operation of magnetic separation and high selectivity of extraction separation, and can separate target radioactive species out from complicated radioactive liquid waste containing one or a plurality of kinds of <90>Sr and actinide elements. The magnetic-particles can be desorbed easily, and can be used repeatedly without producing secondary waste. In addition, by adopting the invention, continuous large-scale separation can be completed.
Owner:NAT INST FOR RADIOLOGICAL PROTECTION & NUCLEAR SAFETY CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Heterocyclic substituted metallocene compounds for olefin polymerization
ActiveUS20050261449A1Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationLanthanideNitrogen
This invention relates to compounds represented by formula: wherein M is a group 3, 4, 5 or 6 transition, lanthanide, or actinide metal atom; E is an indenyl ligand substituted in any position with at least one aromatic or pseudoaromatic heterocyclic substituent that is bonded to the indenyl ring through a nitrogen or phosphorous ring heteroatom; A is a substituted or unsubstituted cyclopentadienyl, heterocyclopentadienyl, indenyl, heteroindenyl, fluorenyl, or heterofluorenyl ligand, or other mono-anionic ligand, or A may, independently, be E; Y is an optional bridging group; y is zero or one; X are, independently, univalent anionic ligands, and provided that when A is E, and y is one, and Y is bonded to the one position of each indenyl ligand, and per indenyl ligand there is only one aromatic heterocyclic or pseudoaromatic heterocyclic that is bonded to the indenyl ligand.
Owner:EXXONMOBIL CHEM PAT INC
Sorption and separation of various materials by graphene oxides
Various aspects of the present invention pertain to methods of sorption of various materials from an environment, including radioactive elements, chlorates, perchlorates, organohalogens, and combinations thereof. Such methods generally include associating graphene oxides with the environment. This in turn leads to the sorption of the materials to the graphene oxides. In some embodiments, the methods of the present invention also include a step of separating the graphene oxides from the environment after the sorption of the materials to the graphene oxides. More specific aspects of the present invention pertain to methods of sorption of radionuclides (such as actinides) from a solution by associating graphene oxides with the solution and optionally separating the graphene oxides from the solution after the sorption.
Owner:ZONKO LLC
Method for synthesizing extremely high-temperature melting materials
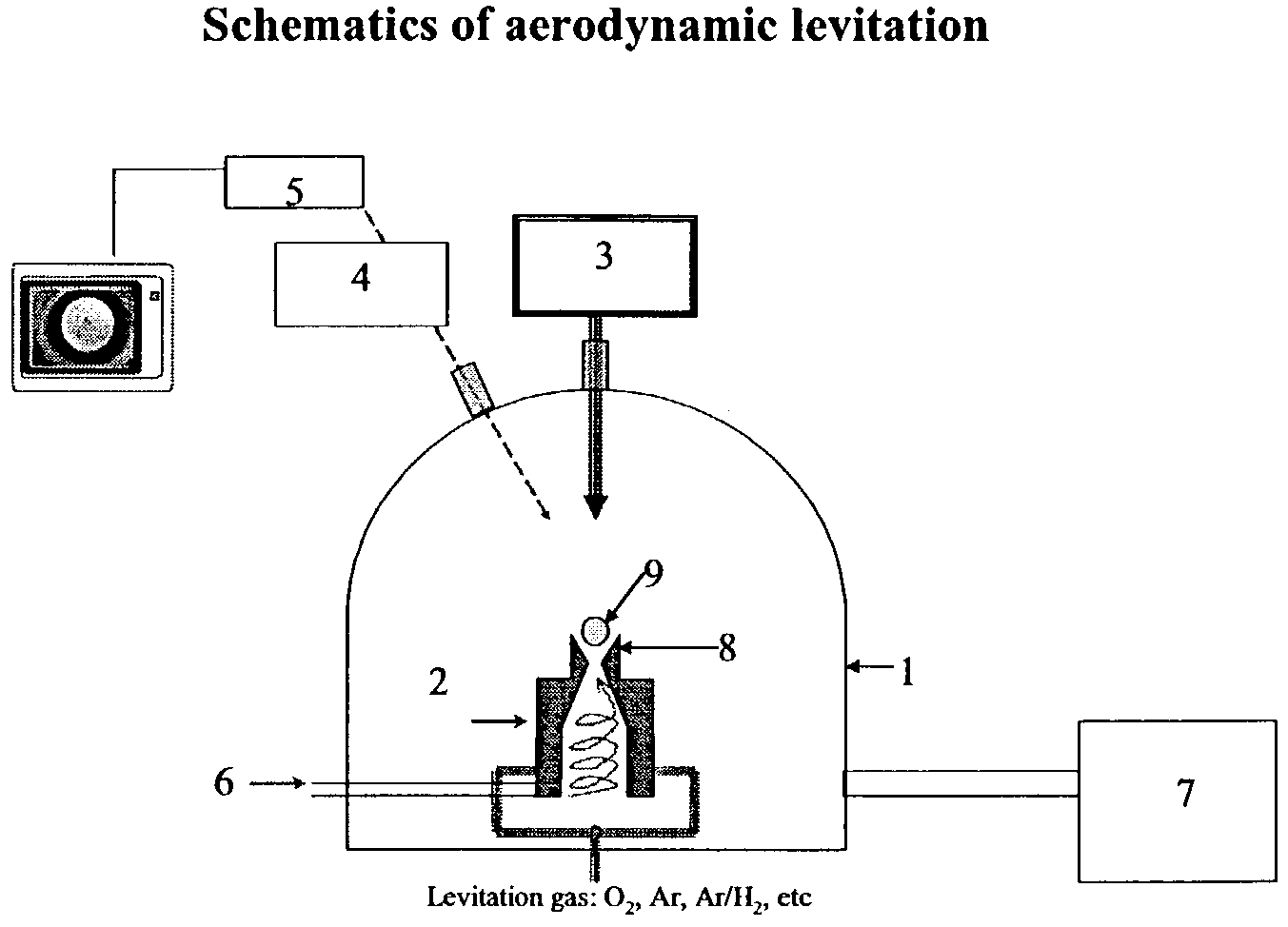
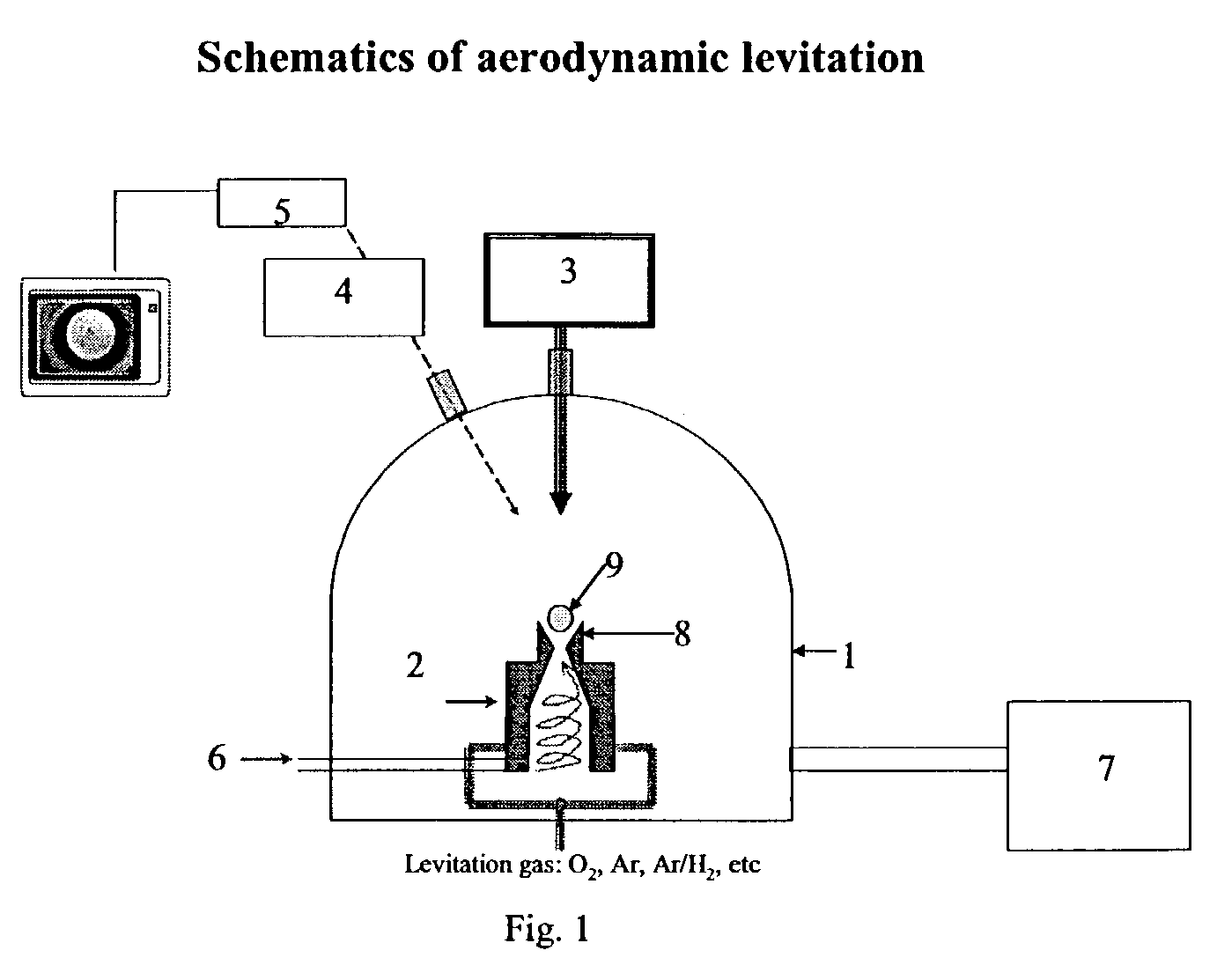
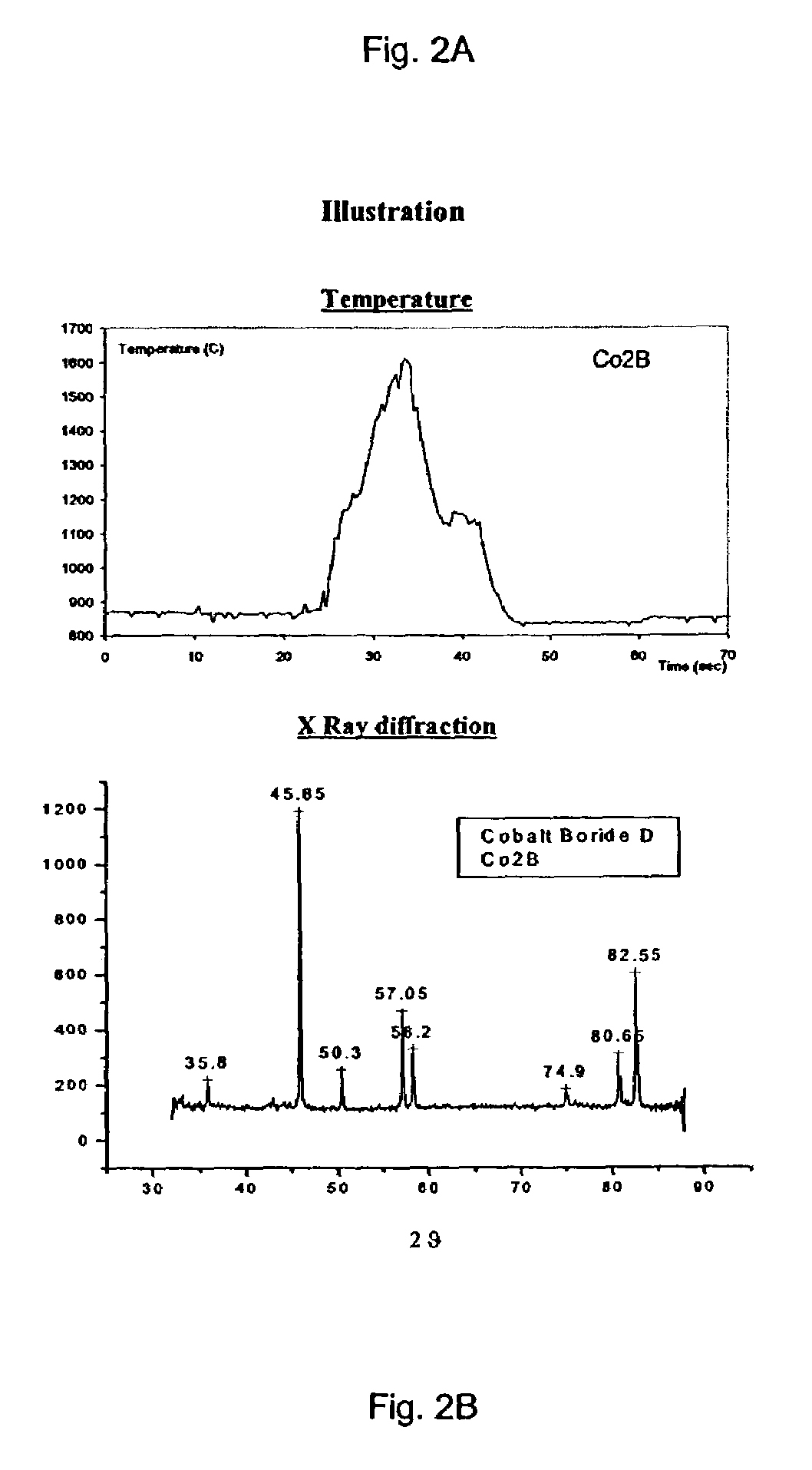
The invention relates to a method of synthesizing high-temperature melting materials. More specifically the invention relates to a containerless method of synthesizing very high temperature melting materials such as borides, carbides and transition-metal, lanthanide and actinide oxides, using an Aerodynamic Levitator and a laser. The object of the invention is to provide a method for synthesizing extremely high-temperature melting materials that are otherwise difficult to produce, without the use of containers, allowing the manipulation of the phase (amorphous / crystalline / metastable) and permitting changes of the environment such as different gaseous compositions.
Owner:THE UNITED STATES AS REPRESENTED BY THE DEPARTMENT OF ENERGY
Process for the preparation of metallocene compounds
InactiveUS6191294B1Silicon organic compoundsGroup 8/9/10/18 element organic compoundsLeaving groupAlkaline earth metal
A new process is disclosed, particularly simple, convenient and practical, for the direct synthesis of metallocenes of formula (I): (CP)(ZR1m)n(A)rMLpL'q wherein (ZR1m)n is a divalent group bridging Cp and A; Cp is a substituted or unsubstituted cyclopentadieryl group; A is -O-, -S-, -N(R2)-, wherein R2 is hydrogen, alkyl, cycloalkyl aryl, alkylaryl or arylalkyl, or A has the same meaning of Cp; M is a transition metal belonging to group 3, 4, 5, 6 or to the lanthanide or actinide groups; L is a monoanionic sigma ligand, such as alkyl, cycloalkyl, aryl, alkylaryl or arylalkyl, optionally containing Si or Ge; L' is halogen or -OR5, R5 being a hydrocarbon radical; m is 1 or 2; n is 0-4; r is 0 or 1; p is 1-3; q is 0-2. Said process comprises reacting the ligand (Y-Cp)(ZR1m)n(A-Y)r, wherein Y is a suitable leaving group, with at least 1 molar equivalent of ML's in the presence of at least (1+r+p) molar equivalents of LjB or LMgL', wherein B is an alkaline or alkaline-earth metal, s ranges from 3 to 6, and j is 1 or 2.
Owner:MONTELL TECH CO BV
Multi-element anode lithium battery material suitable for high voltage and preparation method for material
The invention provides a multi-element anode lithium battery material suitable for high voltage, and a preparation method for the material. The multi-element anode lithium battery material has the feature of a single-particle structure, and the particle size distribution is within the range of 0.5-15 mum; the chemical formula of the nickel-cobalt-manganese multi-element lithium battery anode material with composite doping elements is LiNiXCoyMnzMaNbO2, wherein the value range of x, y and z is that: x is not smaller than 0.2 and not larger than 0.9, y is not smaller than 0 and not larger than 0.4, z is not smaller than 0.1 and not larger than 0.5, 1-x-y-z equals to a+b, is larger than 0, and is not larger than 0.05, M is any one selected from titanium, aluminum, ferrum, vanadium, silicium, fluorine, lanthanide and actinide elements, and N is any one selected from calcium, magnesium, aluminum, zirconium, ferrum and titanium. Based on the preparation of the single-particle multi-element lithium battery anode material, the method of compositing specific doping elements is adopted, the structural stability under high voltage charge and discharge is improved and the cycle performance of the material is effectively improved without changing the electrochemical performance of the material.
Owner:CHENGDU JINGYUAN NEW MATERIALS TECH
Carbon nanotubes using for recovery of radionuclides and separation of actinides and lanthanides
ActiveUS20090093664A1High mechanical strengthExcellent chemical/physical stabilityFibre chemical featuresPhosphorus organic compoundsNuclear powerPhosphate
Methods and compositions to extract radionuclides such as various actinides and lanthanides from organic and / or aqueous solutions by utilizing extractant functionalized carbon nanotubes are disclosed. More particularly, phosphorous-containing (such as phosphine oxides, phosphoric acids or phosphates) organic extractants and other predesigned extractants (such as crown ethers, calncrown derivatives, malonamide and diglycolamide derivatives, polyethylene glycol derivatives, cobalt dicarbollite derivatives, and N-donating heterocyclic ligands) can be covalently and / or non-covalently employed on the surfaces and / or ends (tips) of carbon nanotubes for the purpose of removal radionuclides such as various actinides and lanthanides from organic and / or aqueous solutions. Extractant functionalized carbon nanotubes can be used for extracting radioactive nuclides from nuclear waste or spent nuclear fuel, which are produced and / or reprocessed from the nuclear power generation or other nuclear application. The invention also relates to the solid-liquid separation process based on the contact of liquid radioactive waste by using the invention extracting agents as the solid phase.
Owner:CHEMNANO MATERIALS
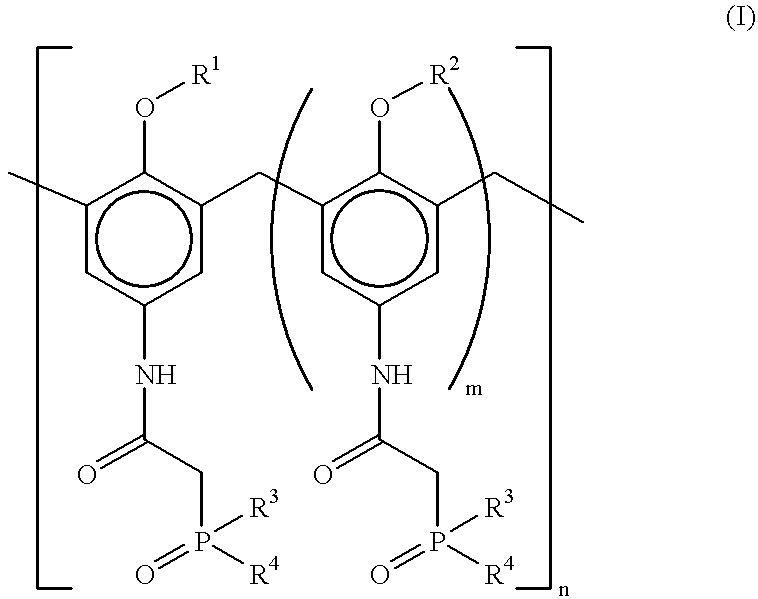
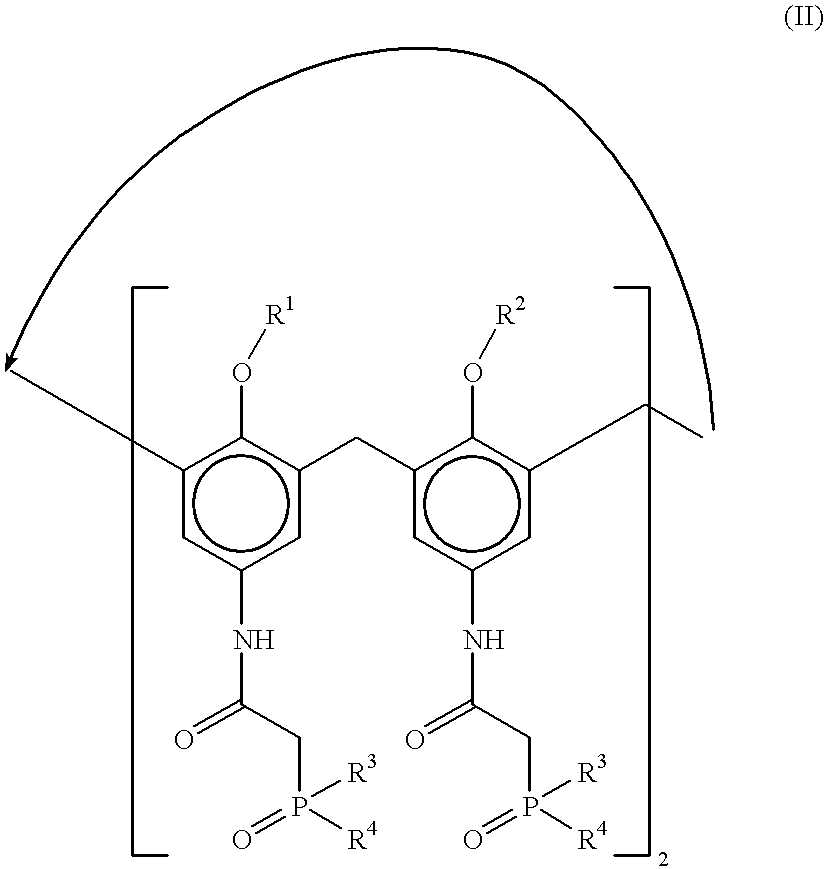
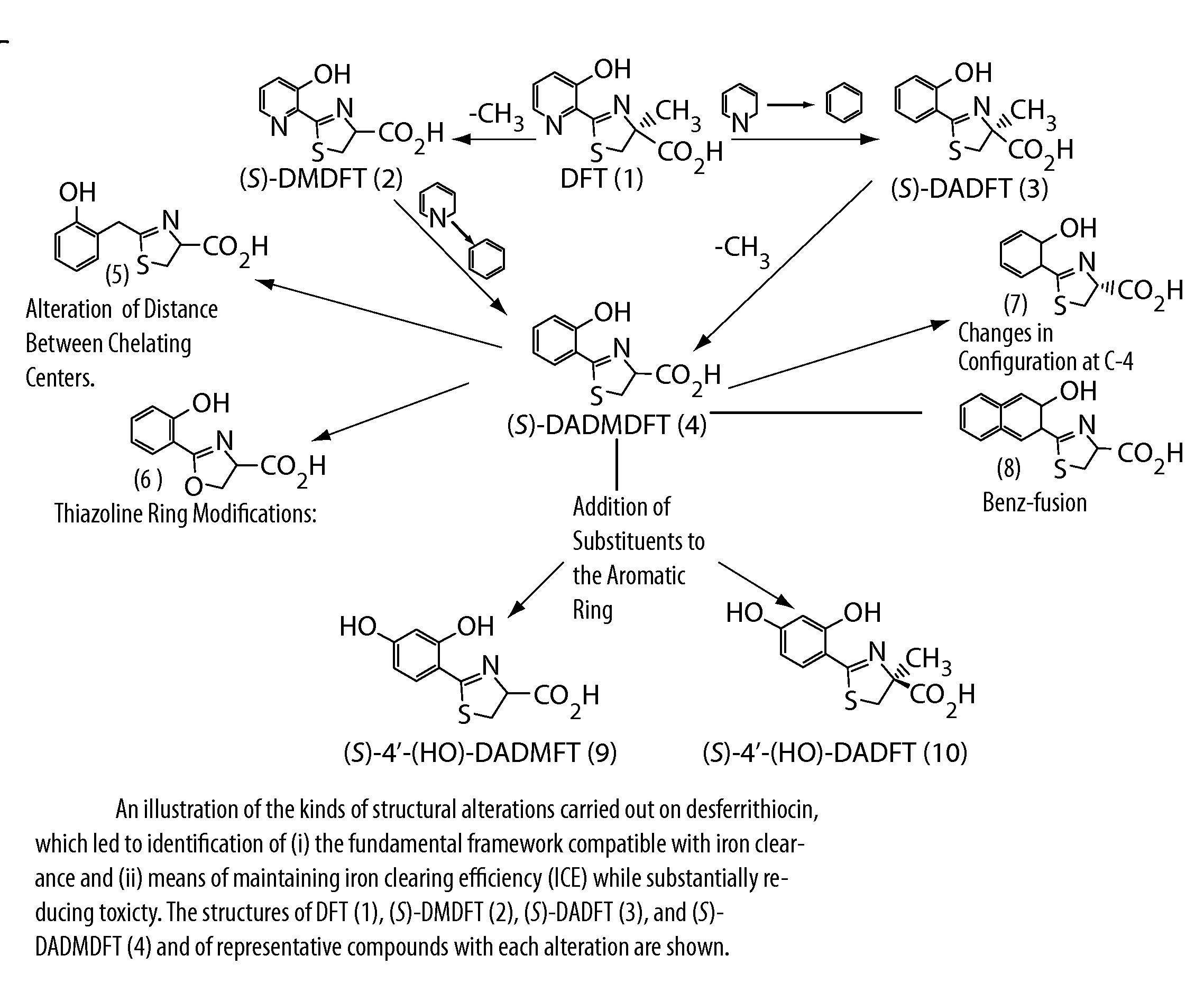
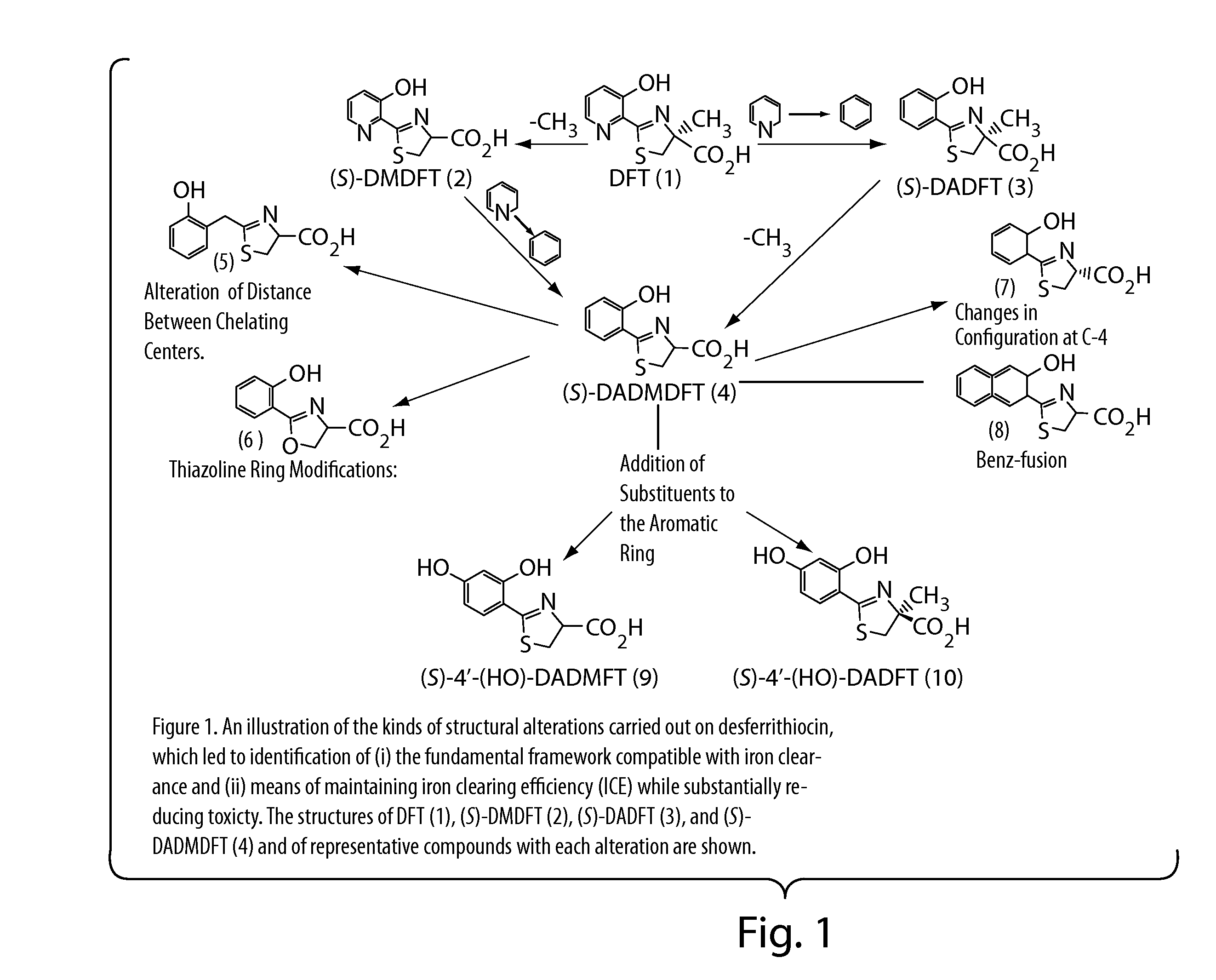
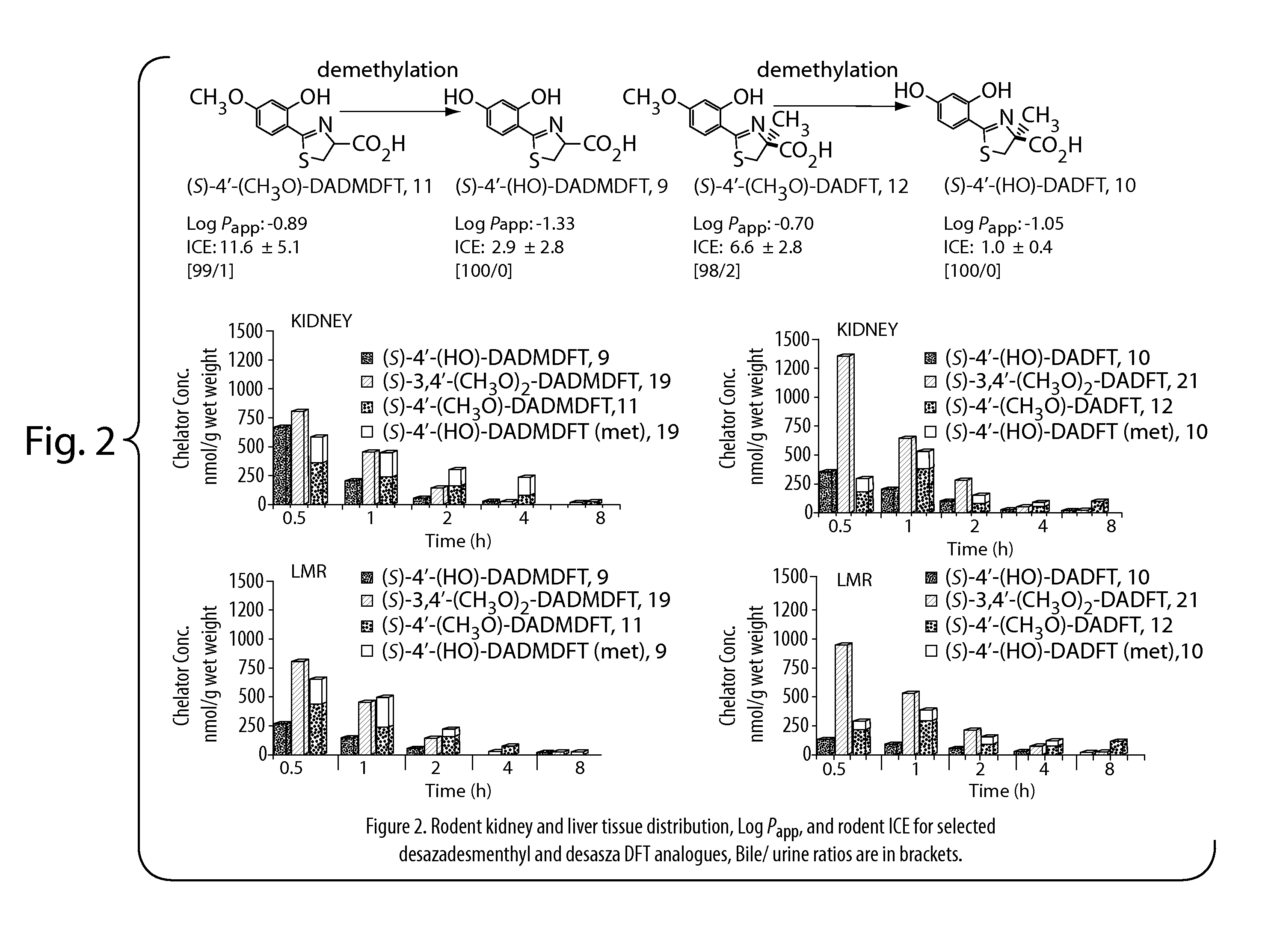
Desferrithiocin analogue actinide decorporation agents
A pharmaceutical composition comprising a non-toxic effective amount of an actinide decorporation agent and a pharmaceutically acceptable carrier therefore, the actinide decorporation agent comprising a hexacoordinate desferrithiocin analogue capable of chelating an actinide in vivo and a method for removing an actinide from the tissue of a human or nonhuman mammal.
Owner:UNIV OF FLORIDA RES FOUNDATION INC
Process for the preparation of an unsupported, solid metallocene catalyst system and its use in polymerization of olefins
InactiveUS8791042B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationPolystyreneLanthanide
Owner:BOREALIS AG
Heteroatom bridged metallocene compounds for olefin polymerization
InactiveUS20070135597A1Organic-compounds/hydrides/coordination-complexes catalystsGroup 5/15 element organic compoundsPolymer sciencePolyolefin
This invention relates to a transition metal compound represented by the formula: wherein M is a group 3, 4, 5 or 6 transition metal atom, or a lanthanide metal atom, or actinide metal atom; E is: 1) a substituted or unsubstituted indenyl ligand that is bonded to Y through the four, five, six or seven position of the indenyl ring, or 2) a substituted or unsubstituted heteroindenyl ligand that is bonded to Y through the four, five or six position of the heteroindenyl ring, provided that the bonding position is not the same as the position of the ring heteroatom, or 3) a substituted or unsubstituted fluorenyl ligand that is bonded to Y through the one, two, three, four, five, six, seven or eight position of the fluorenyl ring, or 4) a substituted or unsubstituted heterofluorenyl ligand that is bonded to Y through the one, two, three, four, five or six position of the heteroindenyl ring, provided that the bonding position is not the same as the position of the ring heteroatom; A is a substituted or unsubstituted cyclopentadienyl ligand, a substituted or unsubstituted heterocyclopentadienyl ligand, a substituted or unsubstituted indenyl ligand, a substituted or unsubstituted heteroindenyl ligand, a substituted or unsubstituted fluorenyl ligand, a substituted or unsubstituted heterofluorenyl ligand, or other mono-anionic ligand; Y is a Group 15 or 16 bridging heteroatom substituent that is bonded via the heteroatom to E and A; and X are, independently, univalent anionic ligands, or both X are joined and bound to the metal atom to form a metallocycle ring, or both X join to form a chelating ligand, a diene ligand, or an alkylidene ligand. This invention further relates to catalyst systems comprising the above transiotioon metal compounds, activators and optional supports and their use to polymerize or oligomerize olefins.
Owner:EXXONMOBIL CHEM PAT INC
Production of olefin polymerization catalysts
InactiveUS7341971B2Organic-compounds/hydrides/coordination-complexes catalystsCatalyst activation/preparationEmulsionLanthanide
The invention provides a process for producing an olefin polymerization catalyst, comprising an organometallic compound of a transition metal or of an actinide or lanthanide, in the form of solid catalyst particles, comprising forming a liquid / liquid emulsion system which comprises a solution of one or more catalyst components dispersed in a solvent immiscible therewith; and solidifying said dispersed phase to convert said droplets to solid particles comprising the catalyst and optionally recovering said particles.
Owner:BOREALIS TECH OY
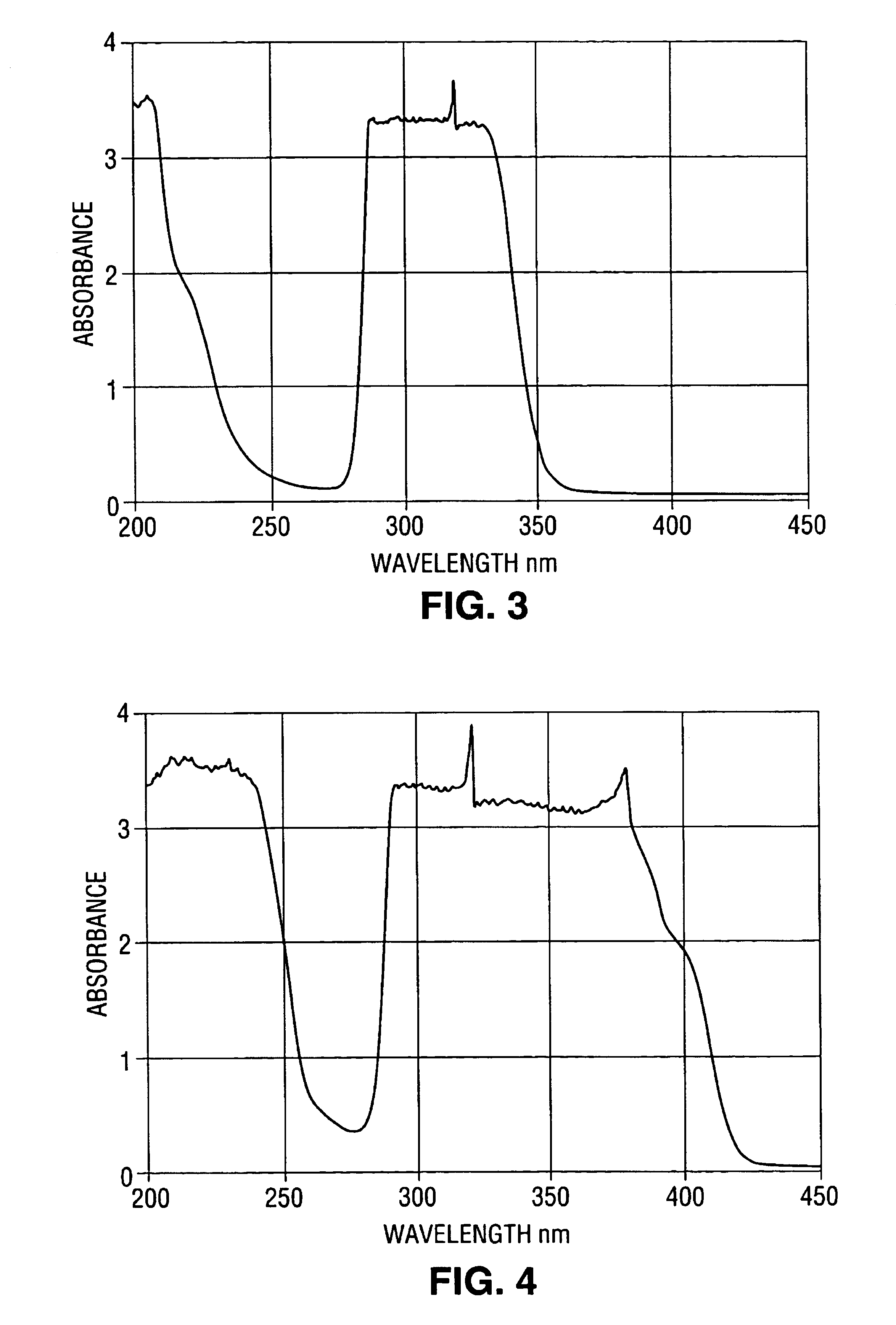
Crystalline filters for ultraviolet light sensors
A crystal filter and a method of making a crystal filter capable of transmitting radiation within a particular pass band is disclosed. The crystal filter is particularly appropriate for a UV detection system, where the pass band is between about 200 to about 350 nm. A UV detection system incorporating the crystal filter is also described. One embodiment of crystal filter is formed from a single-crystal transparent host, such as a fluoride host, codoped with lanthanide or actinide fluorides and lanthanide or actinide nitrides, oxides, borides, carbides or hydroxides. Filter crystals according to the present invention can be grown by various crystal growth methods, including Czochralski and Bridgeman crystal growth methods.
Owner:II VI DELAWARE INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com