Patents
Literature
5437 results about "Crystal growth" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Crystal growth, is the process where a pre-existing crystal becomes larger as more molecules or ions add in their positions in the crystal lattice or a solution is developed into a crystal and further growth is processed. A crystal is defined as being atoms, molecules, or ions arranged in an orderly repeating pattern, a crystal lattice, extending in all three spatial dimensions. So crystal growth differs from growth of a liquid droplet in that during growth the molecules or ions must fall into the correct lattice positions in order for a well-ordered crystal to grow. The schematic shows a very simple example of a crystal with a simple cubic lattice growing by the addition of one additional molecule.
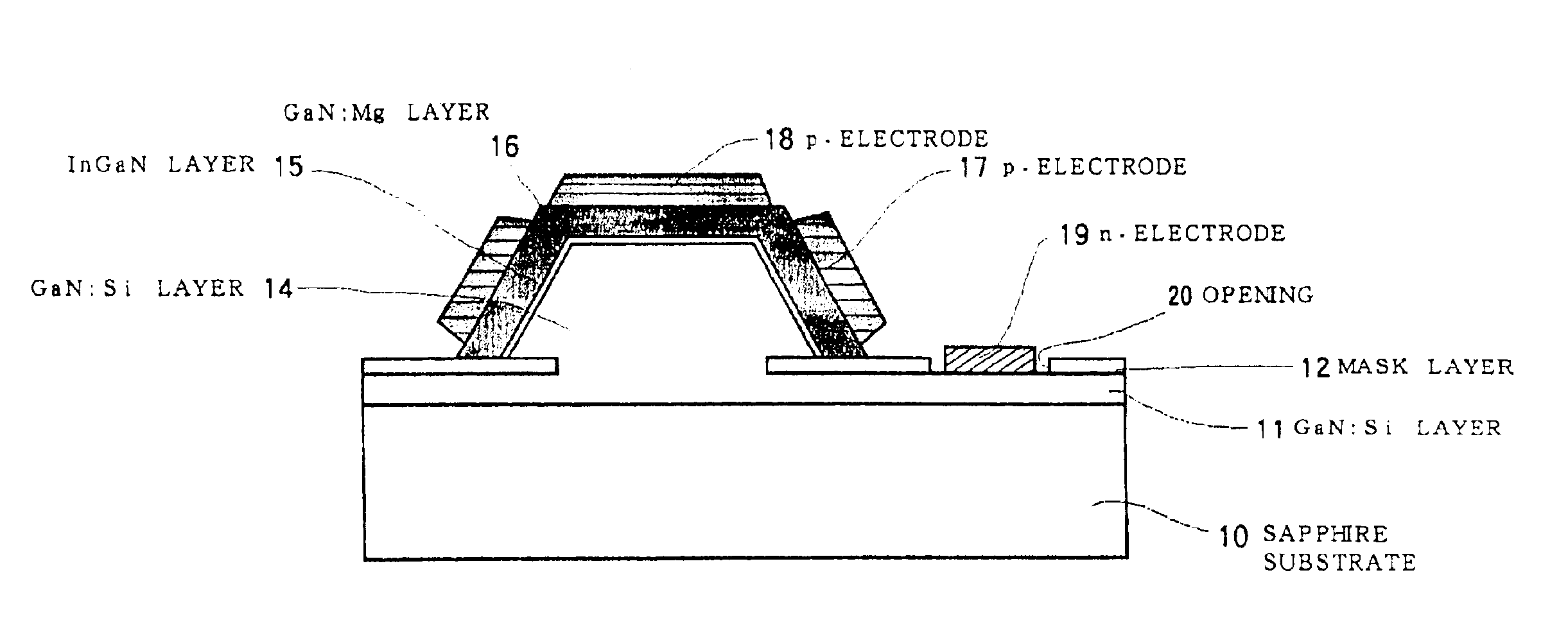
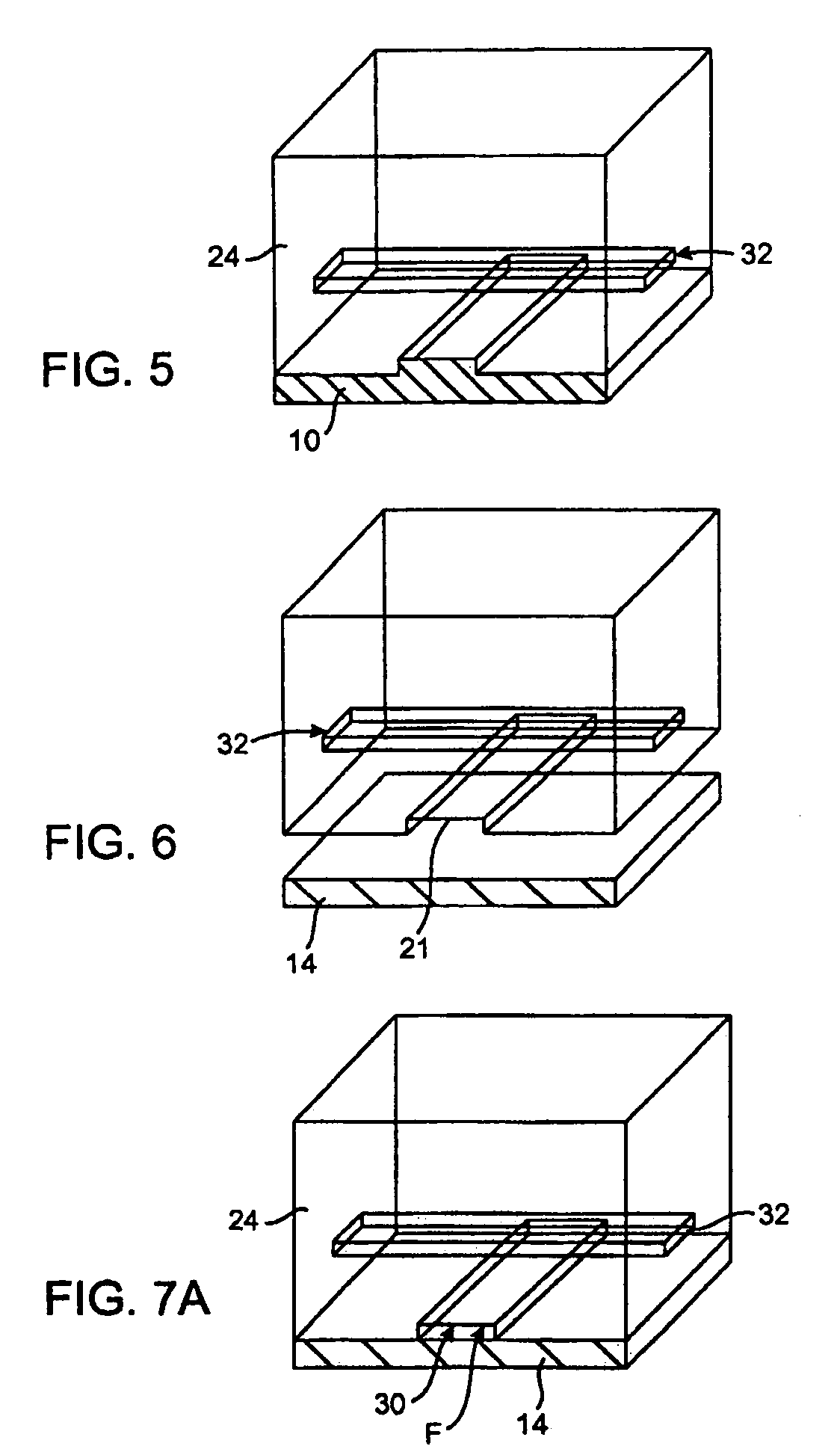
Semiconductor light-emitting device and semiconductor light-emitting device
InactiveUS7087932B2Well formedSolid-state devicesSemiconductor/solid-state device manufacturingCrystal planeCrystallinity
Owner:SAMSUNG ELECTRONICS CO LTD
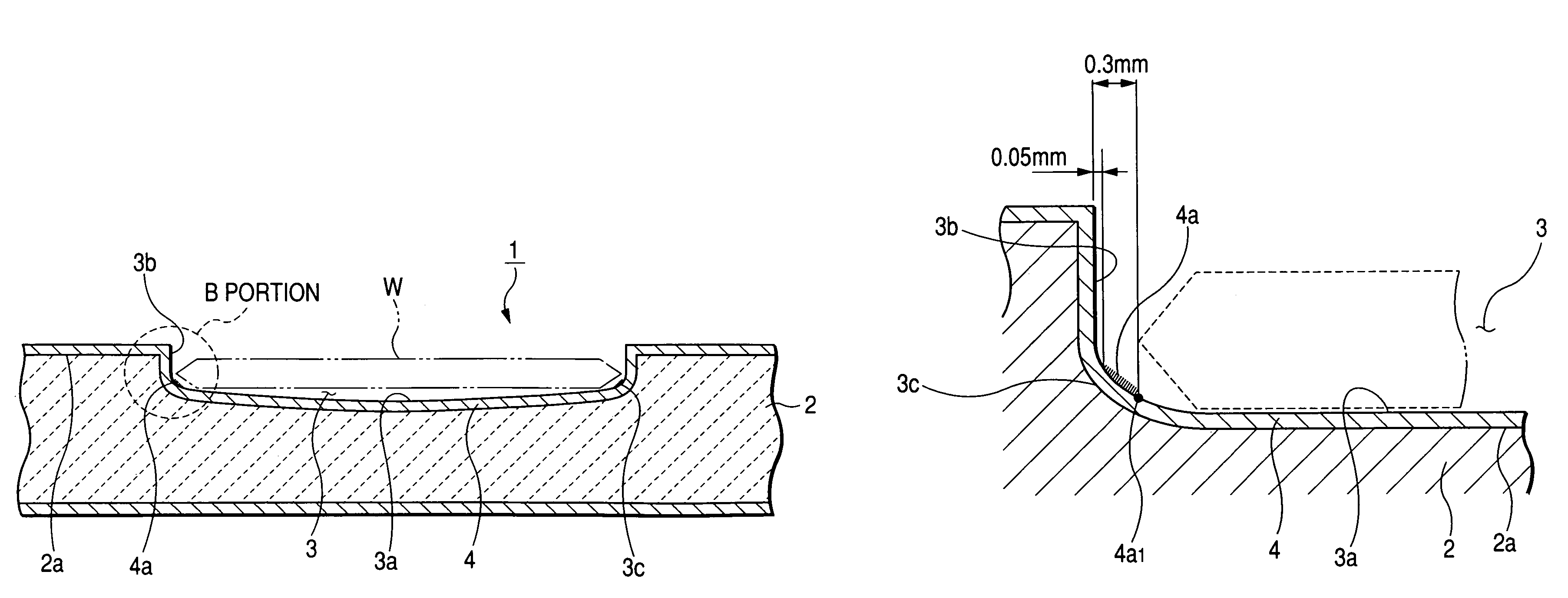
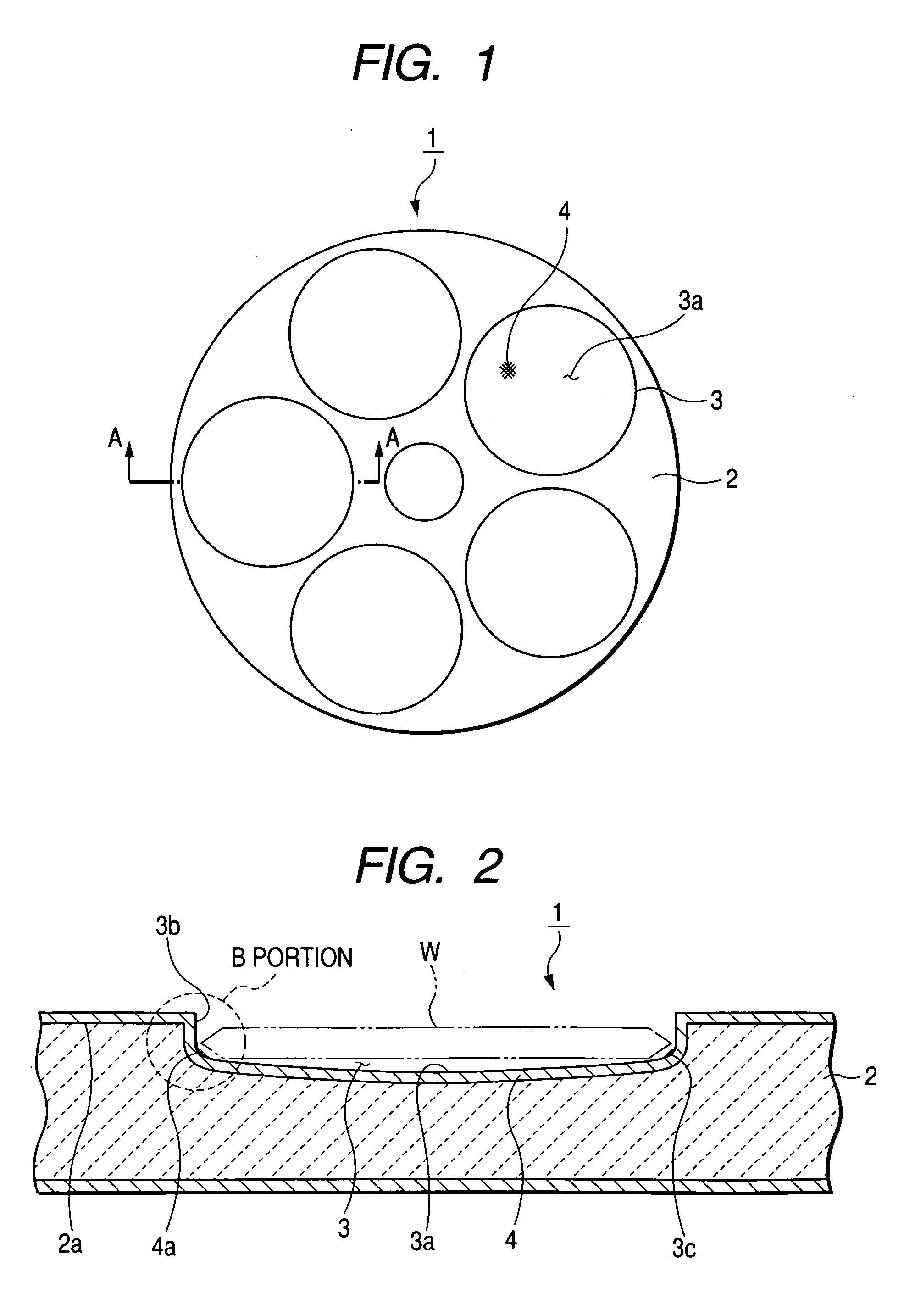
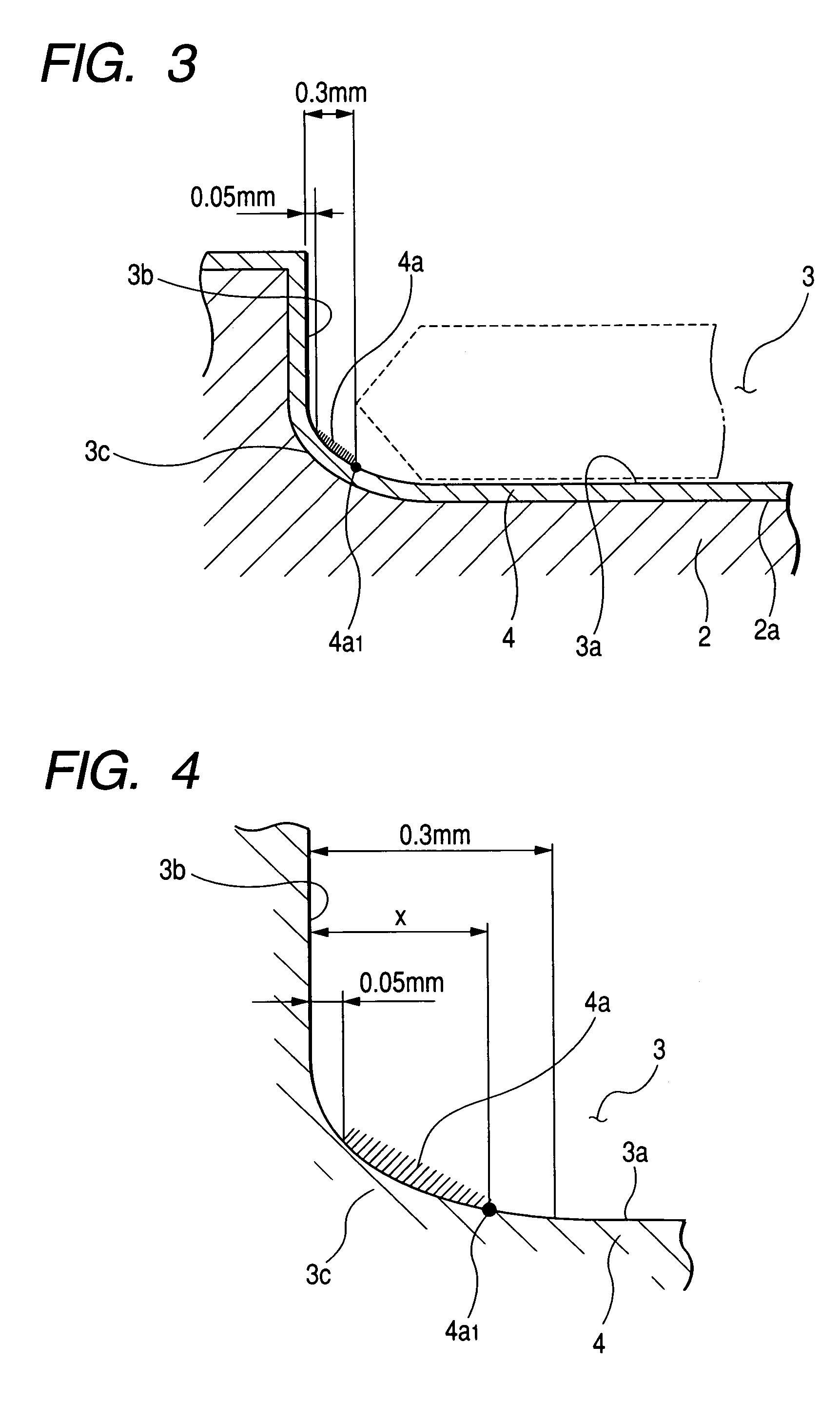
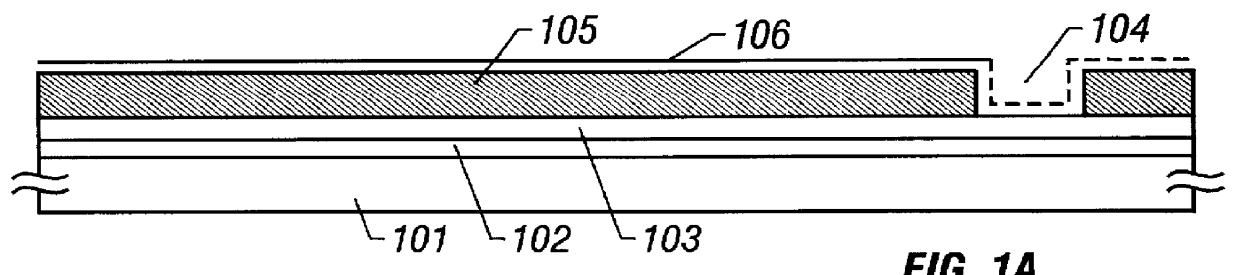
Susceptor
ActiveUS7393418B2Stably holdUnified performanceLiquid surface applicatorsDiffusion/dopingSusceptorSurface roughness
A susceptor at least a surface thereof being coated with SiC, includes a recess where an wafer is mounted, the recess having an round portion disposed on a lower portion of an outer circumferential portion of the recess, a ring-shaped SiC crystal growth surface portion provided within the round portion in a range of 0.05 mm or more and 0.3 mm or less defined from an outer circumference vertical portion of the recess and a contact portion, where the susceptor contacts with the wafer on the recess, having a surface roughness Ra in a range of 0.5 μm or more and 3 μm or less.
Owner:COORSTEK INC
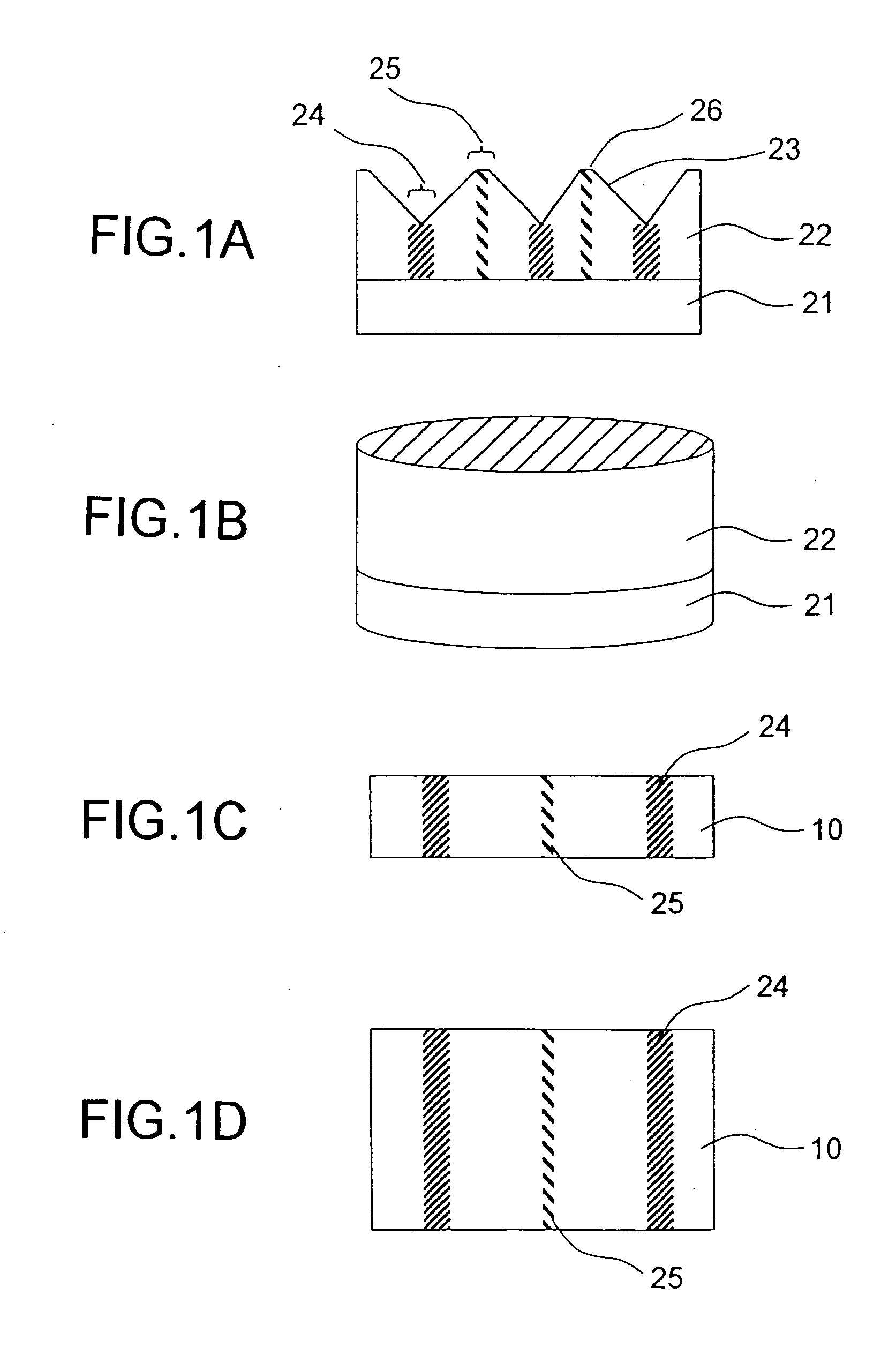
Method of manufacturing a semiconductor device
InactiveUS6165824ATransistorSemiconductor/solid-state device manufacturingCrystallinityCrystal growth
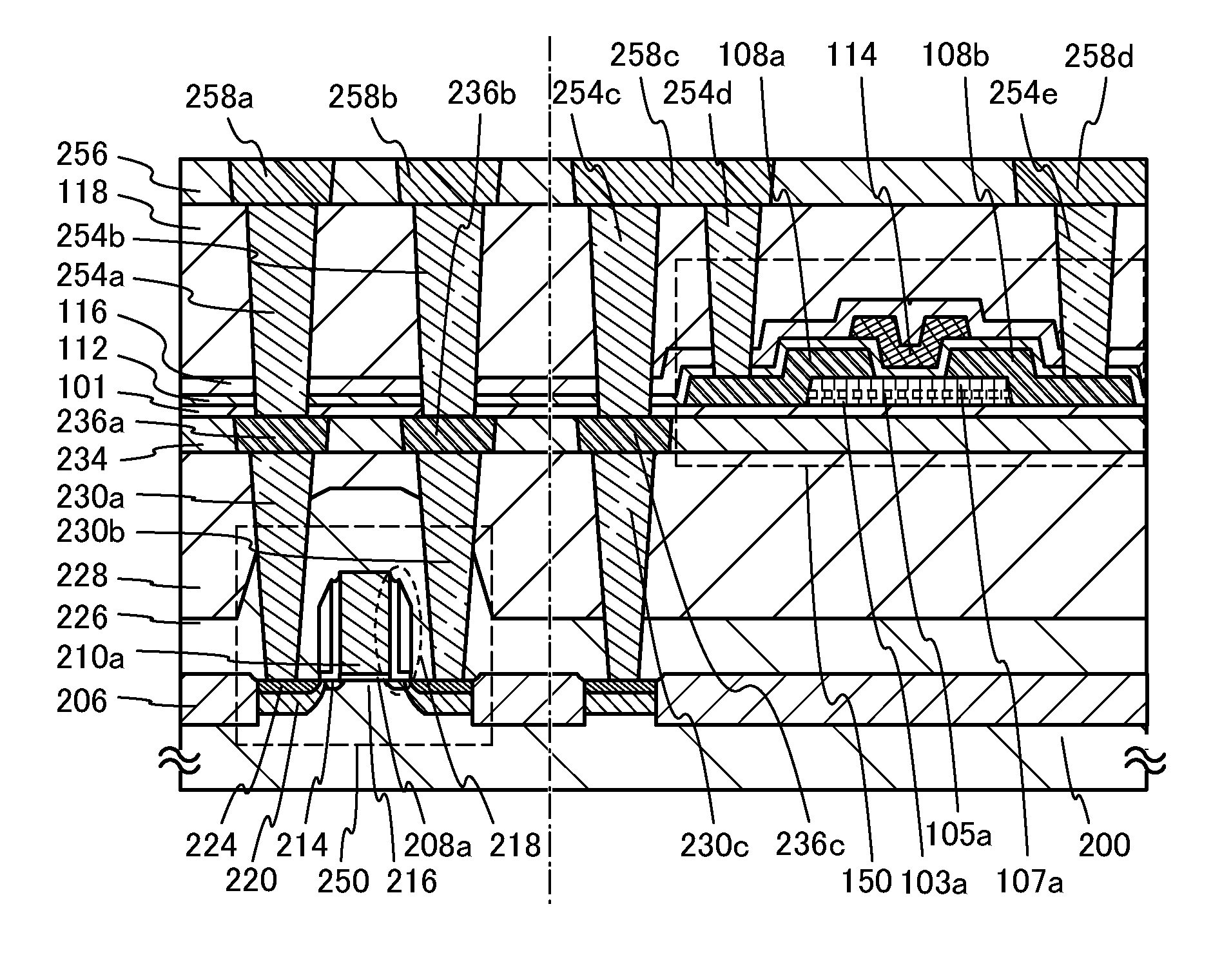
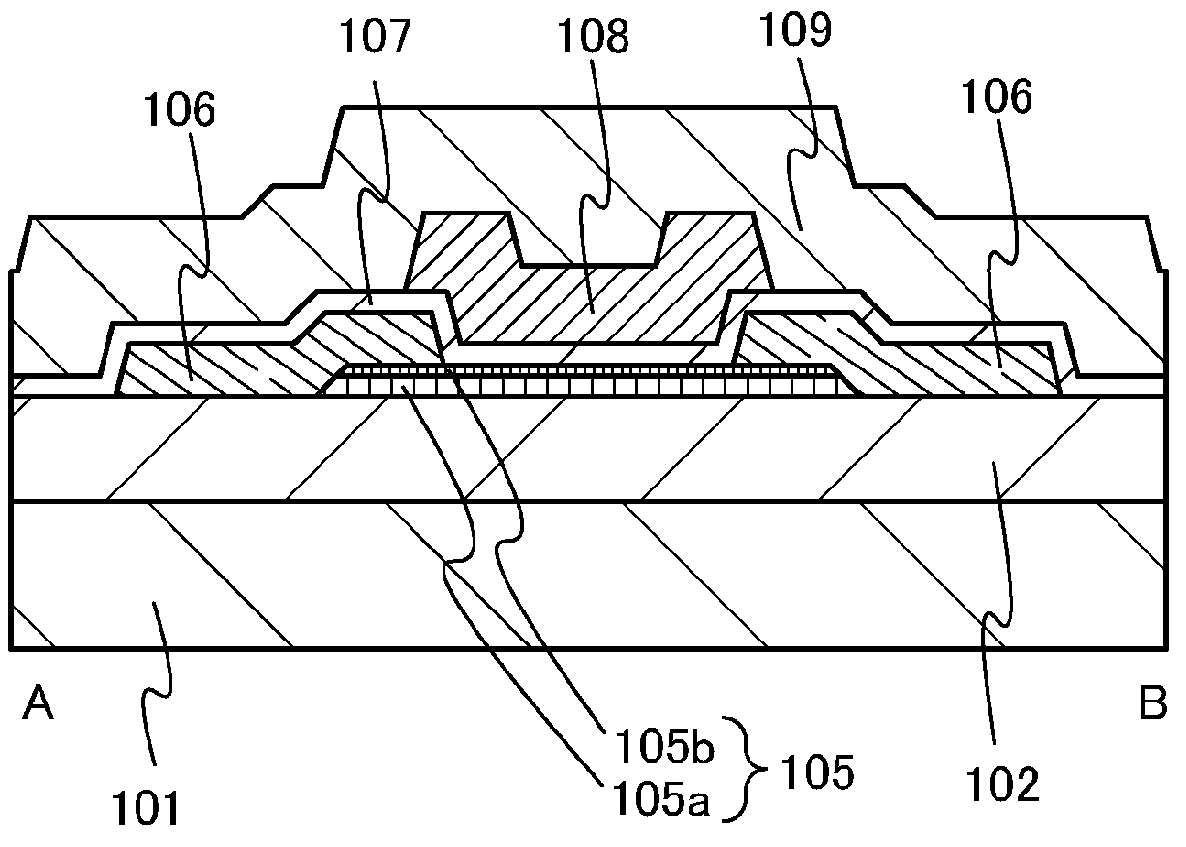
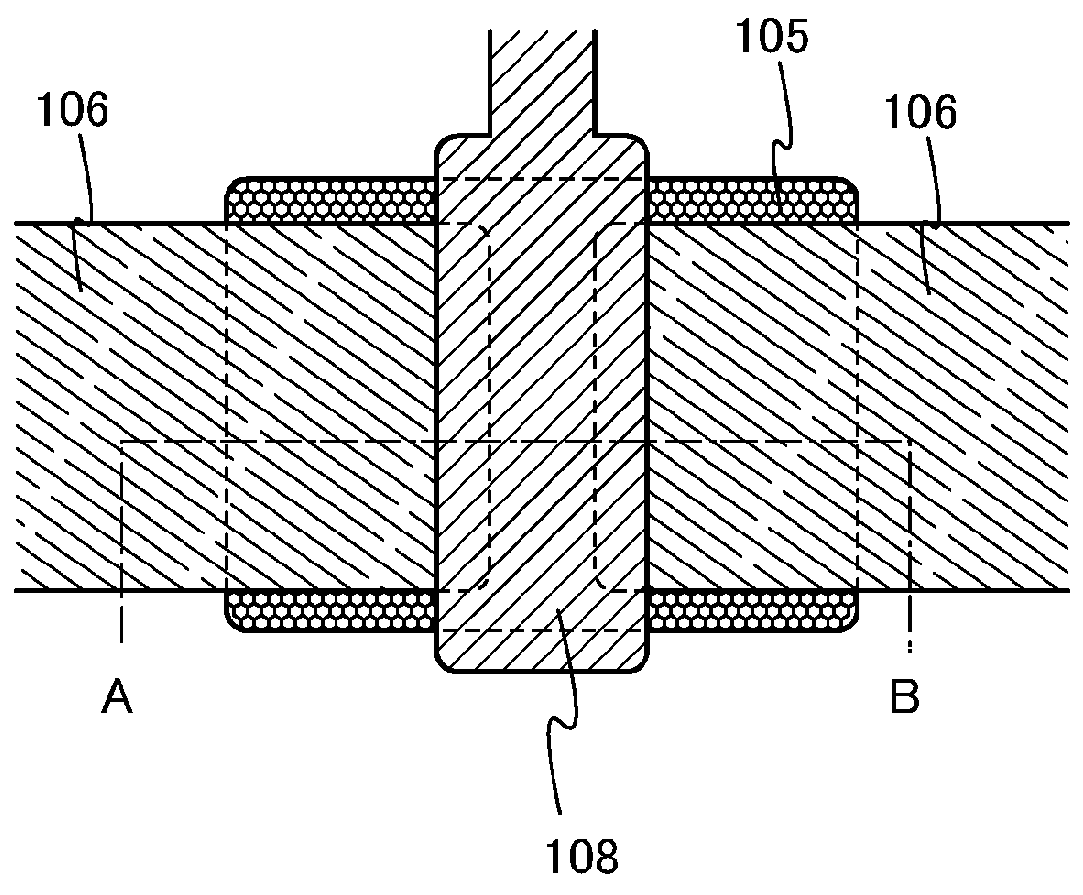
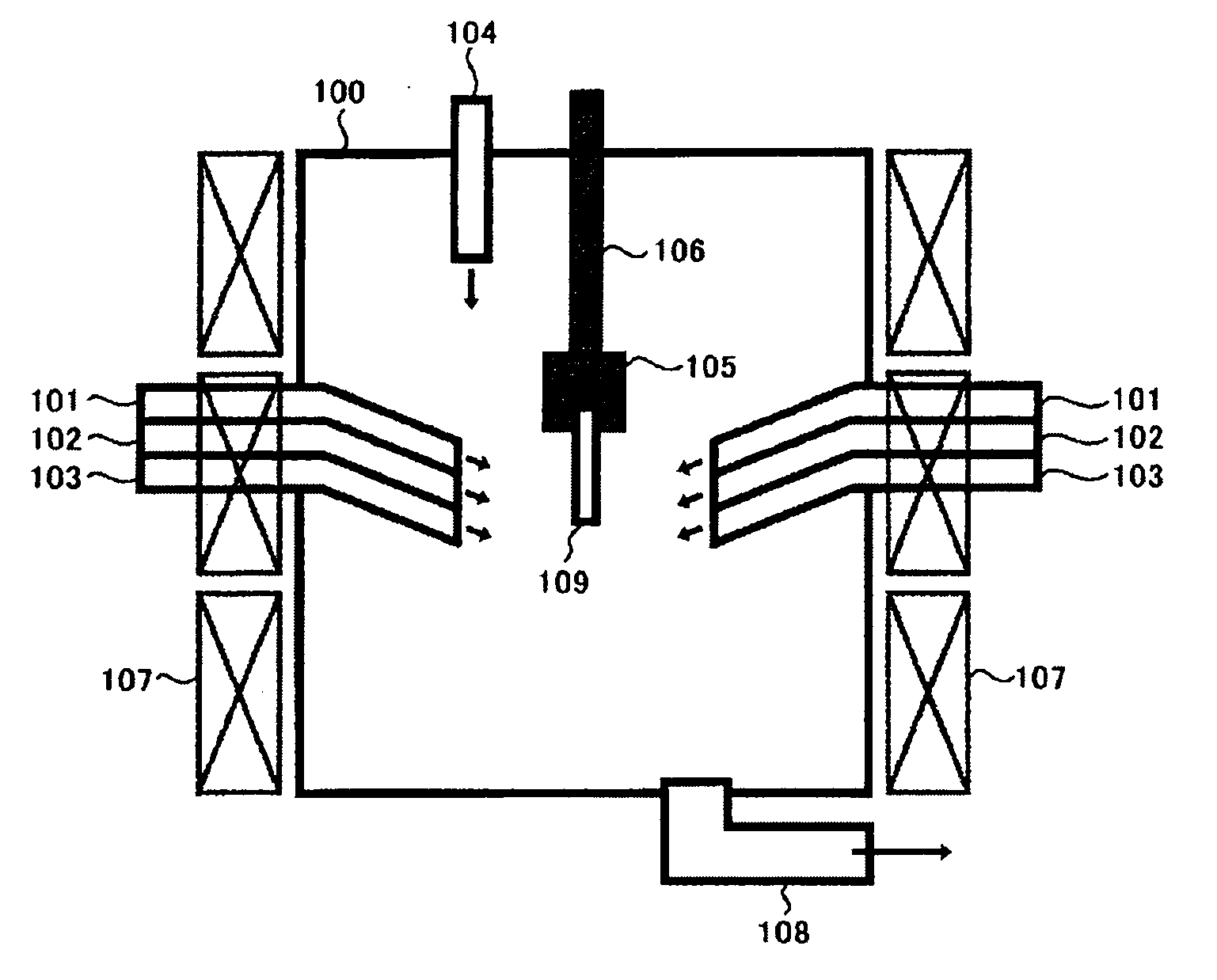
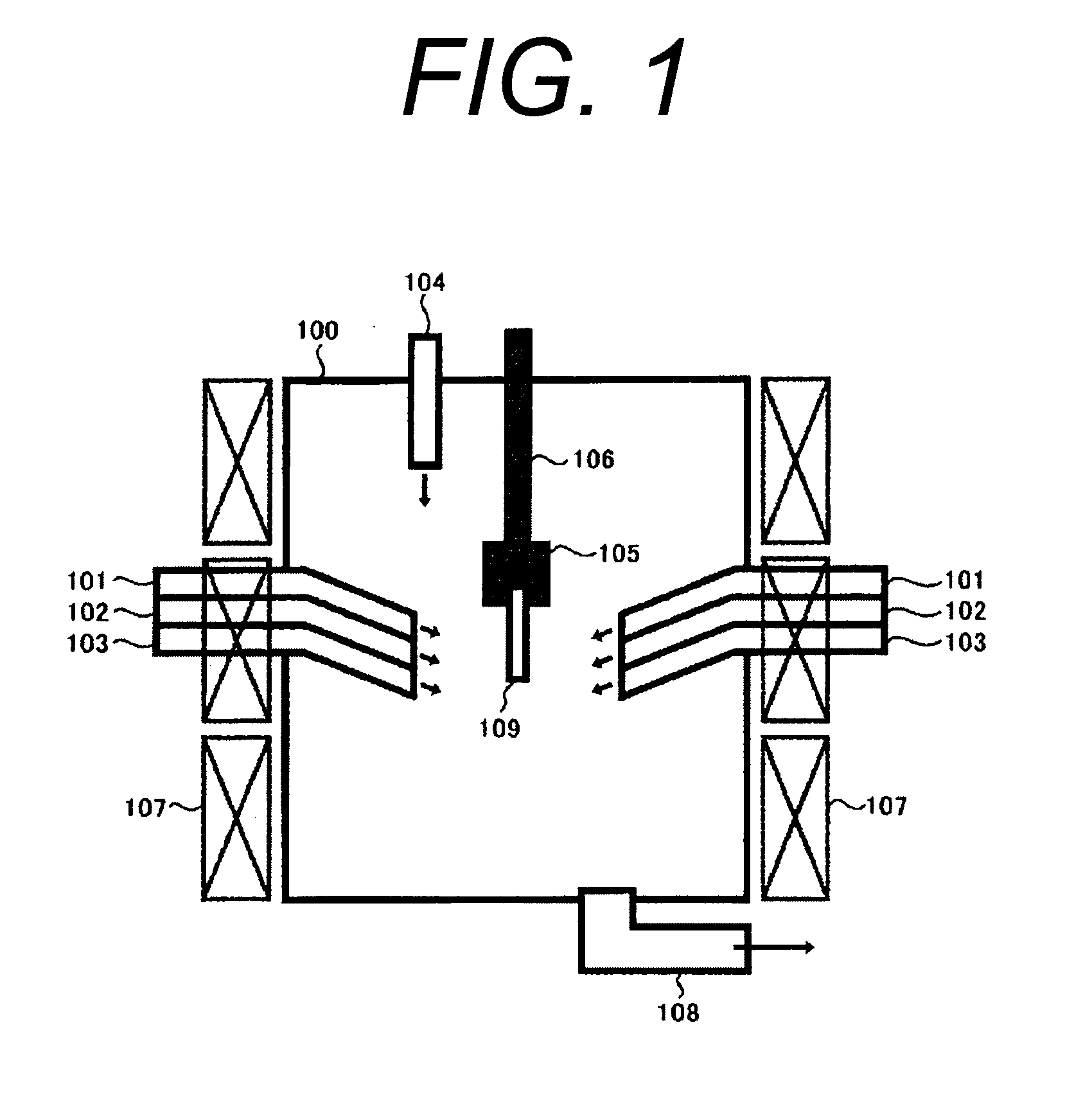
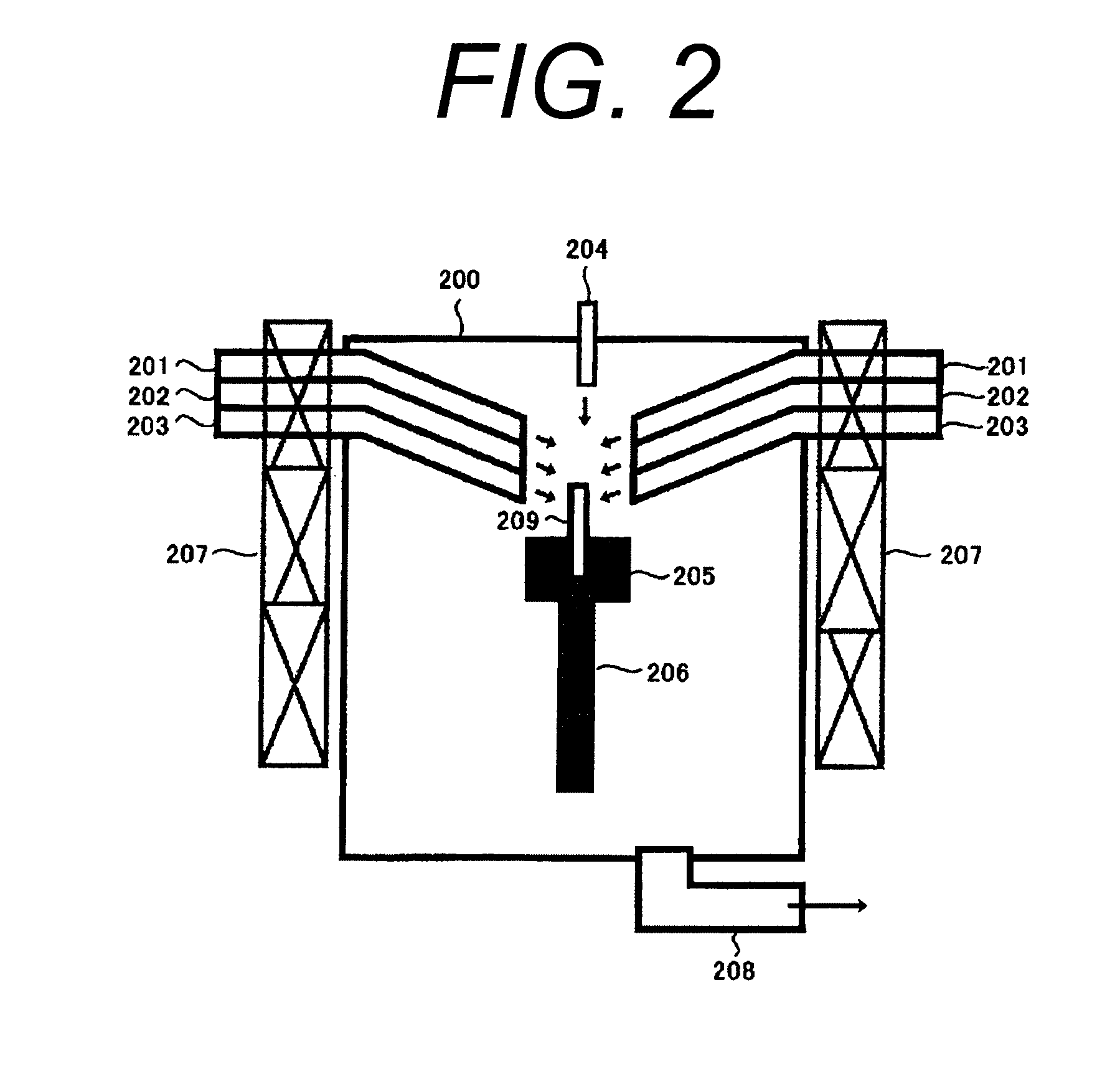
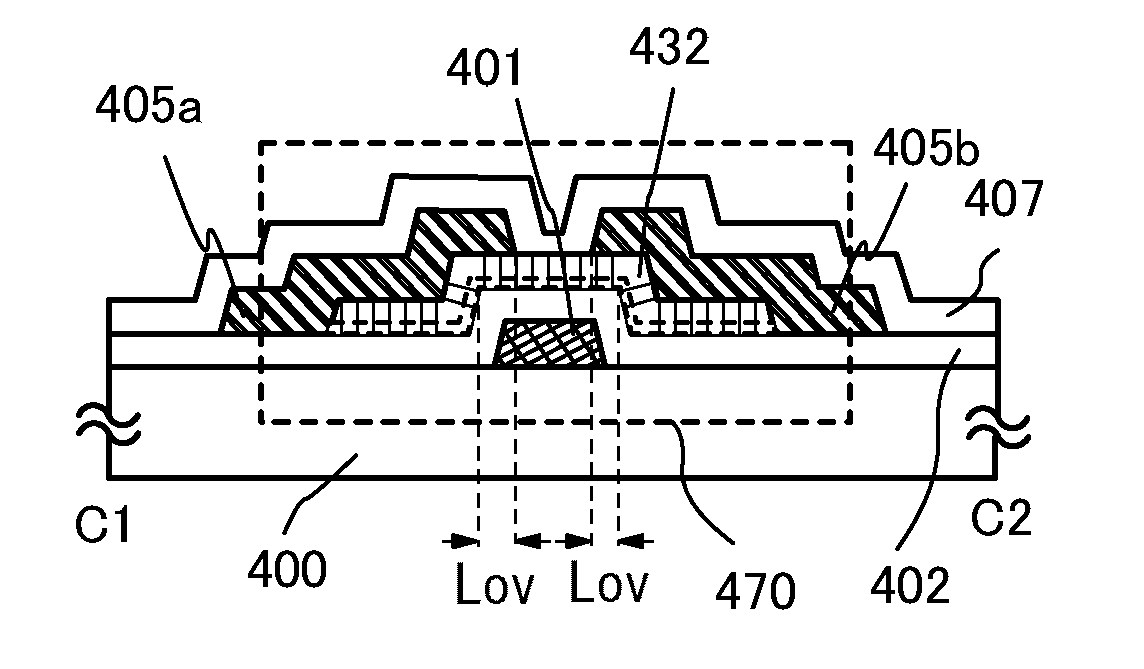
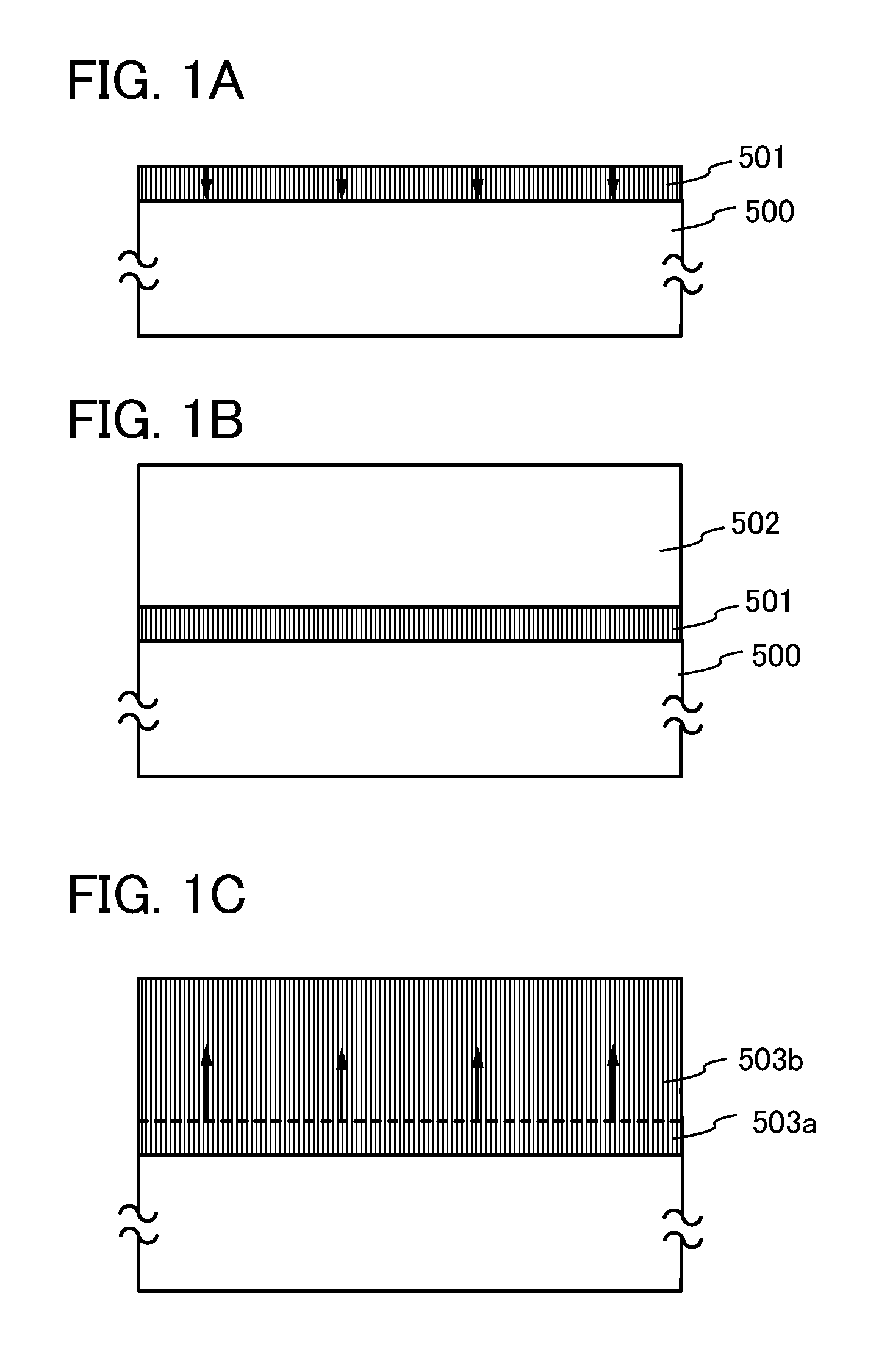
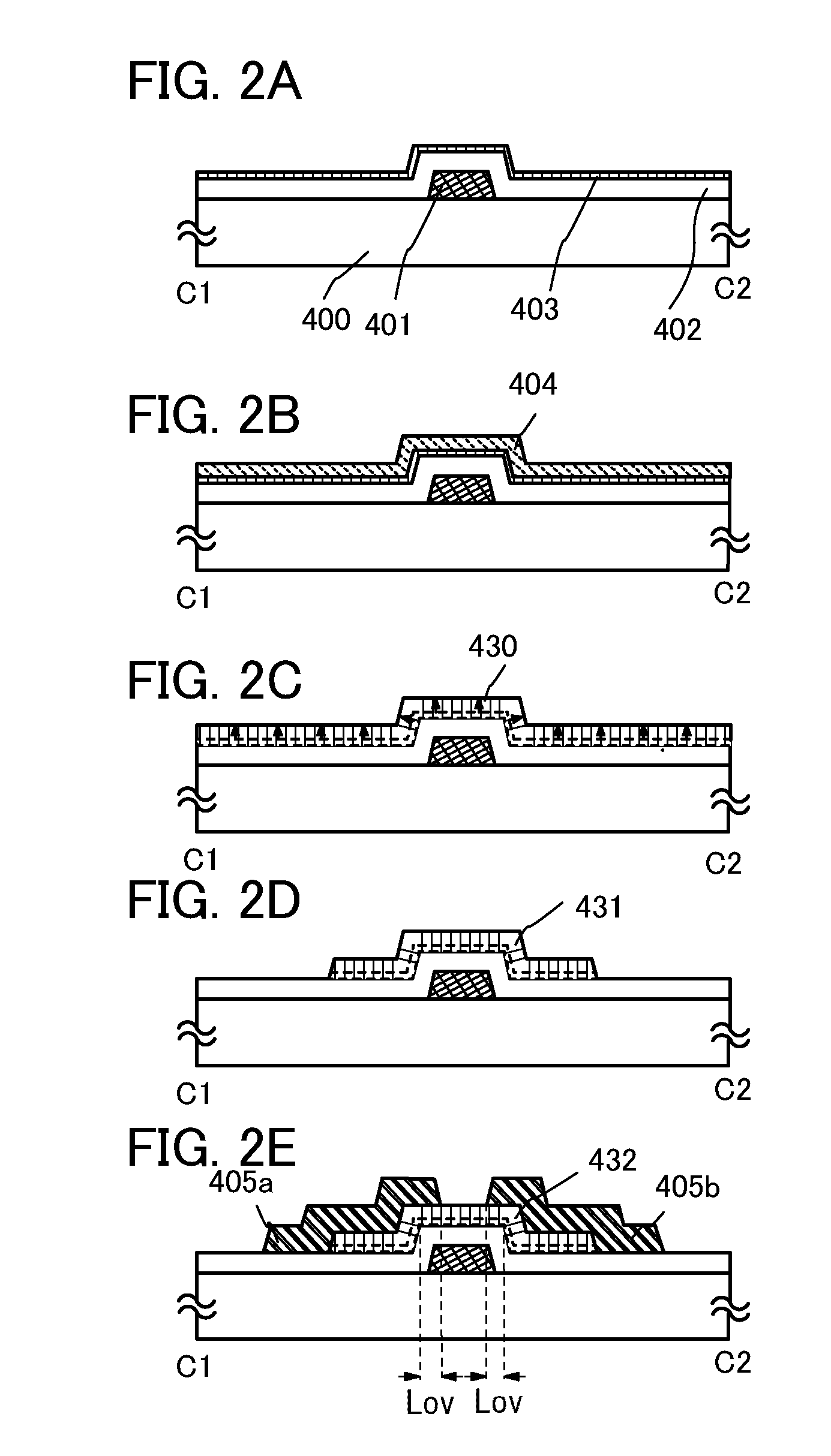
A crystal growth 301 is carried out by diffusing a metal element, and a nickel element is moved into regions 108 and 109 which has been doped with phosphorus. An axis coincident with the moving directions 302 and 303 of the nickel element at this time is made to coincide with an axis coincident with the direction of the crystal growth, and a TFT having the regions as channel forming regions is manufactured. In the path of the region where nickel moved, since high crystallinity is obtained in the moving direction, the TFT having high characteristics can be obtained by this way.
Owner:SEMICON ENERGY LAB CO LTD
Semiconductor laser diode
InactiveUS20080285609A1Improve luminous efficiencyAvoid polarizationLaser detailsLaser active region structureLight-emitting diodeCrystal growth
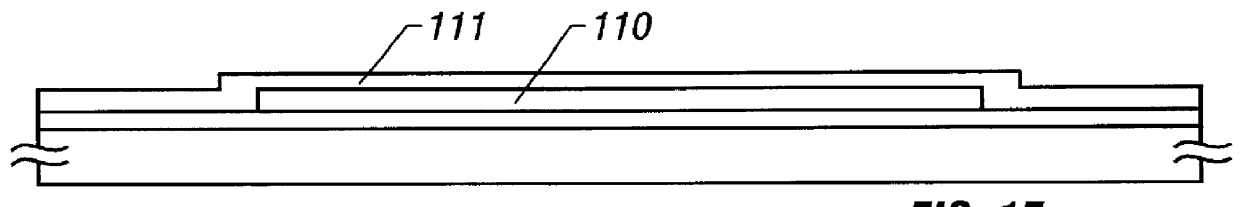
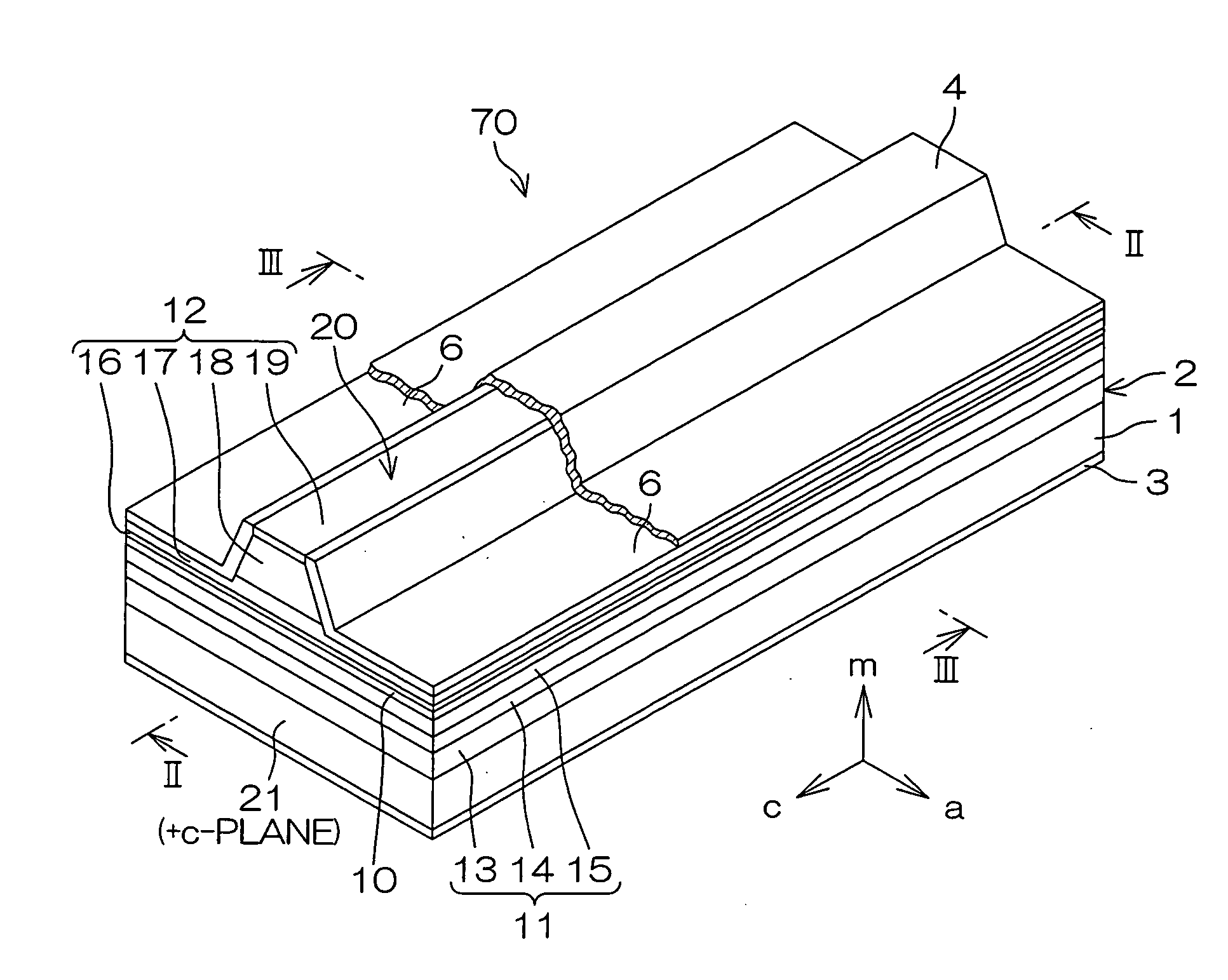
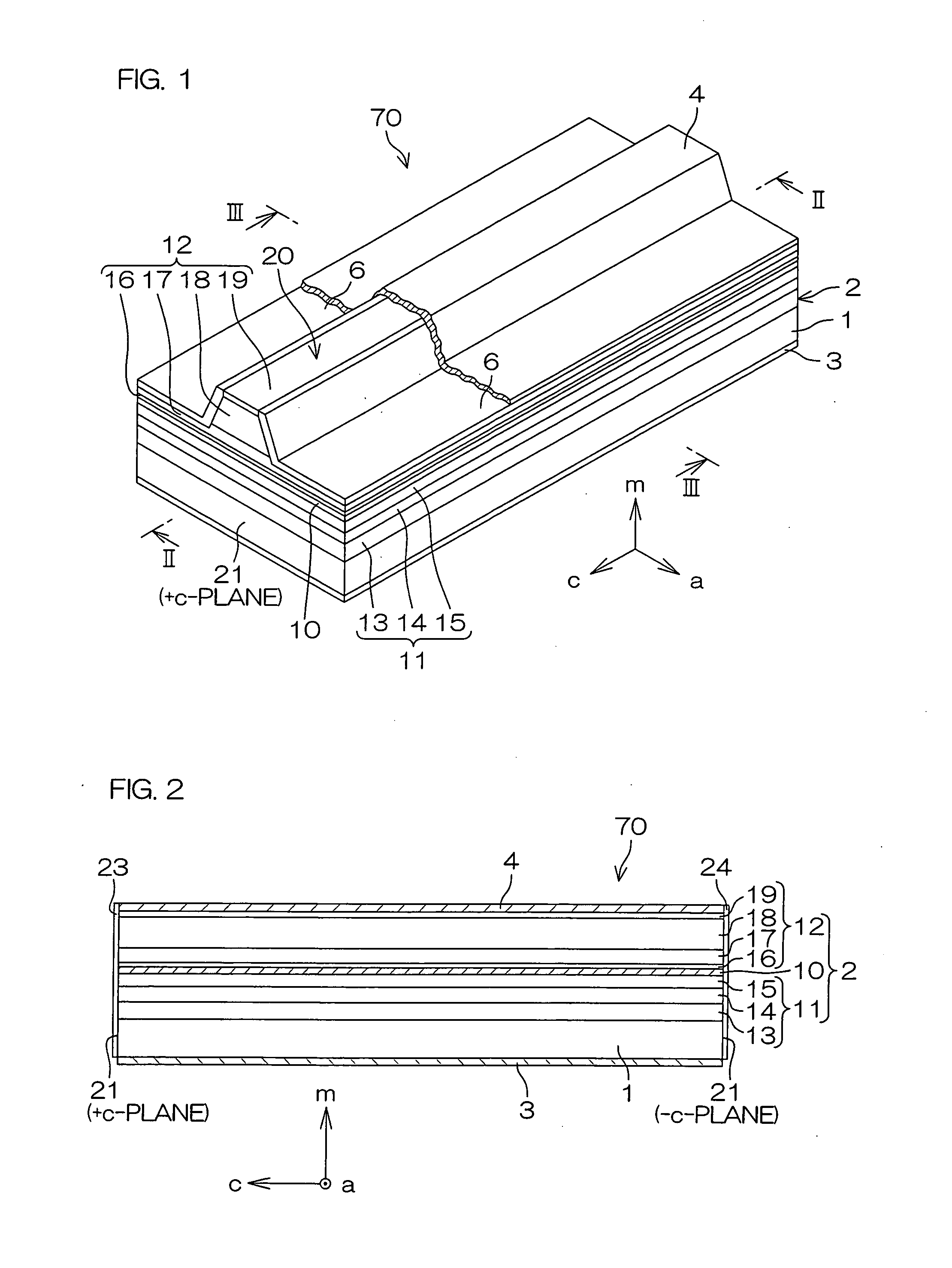
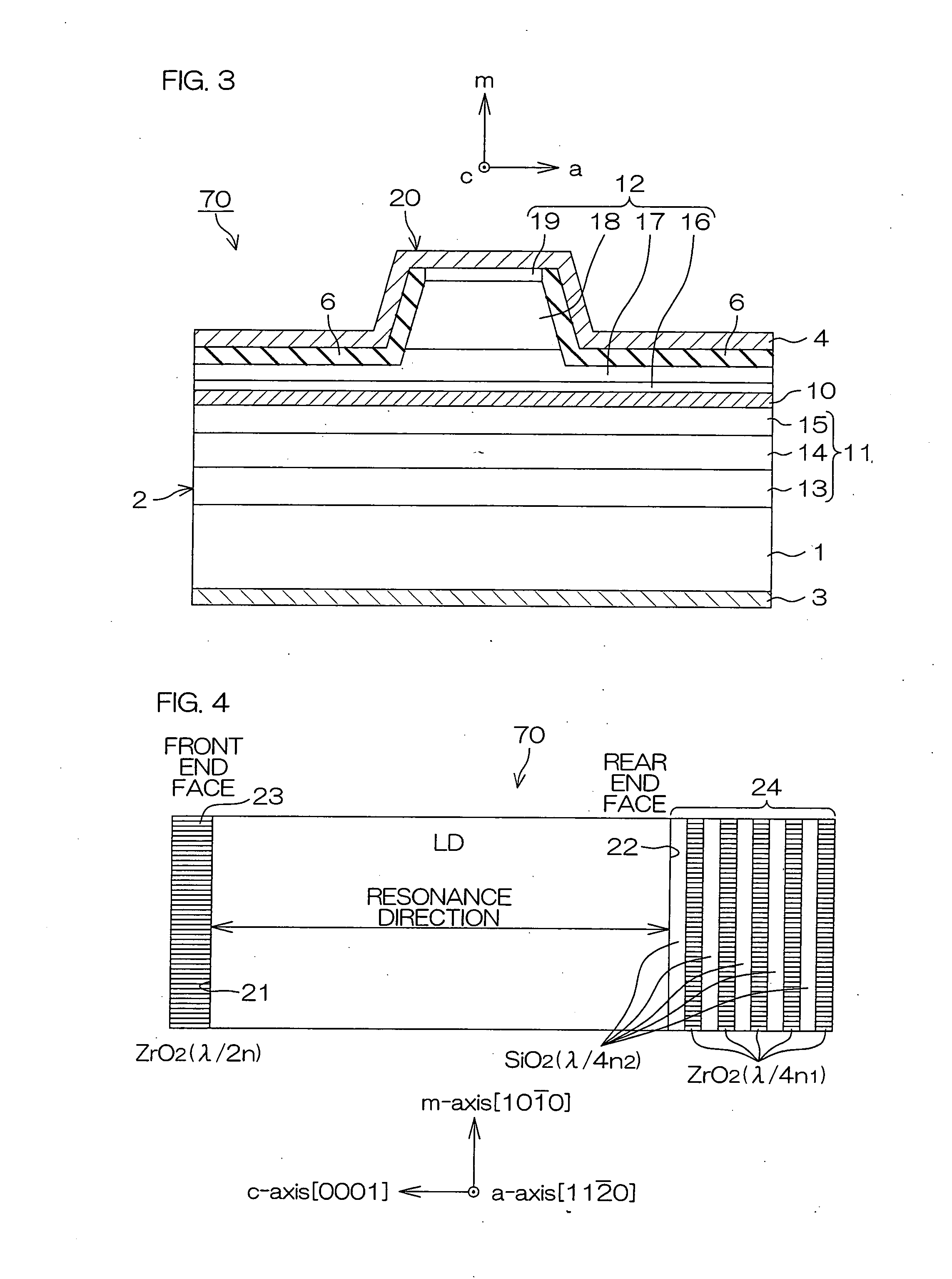
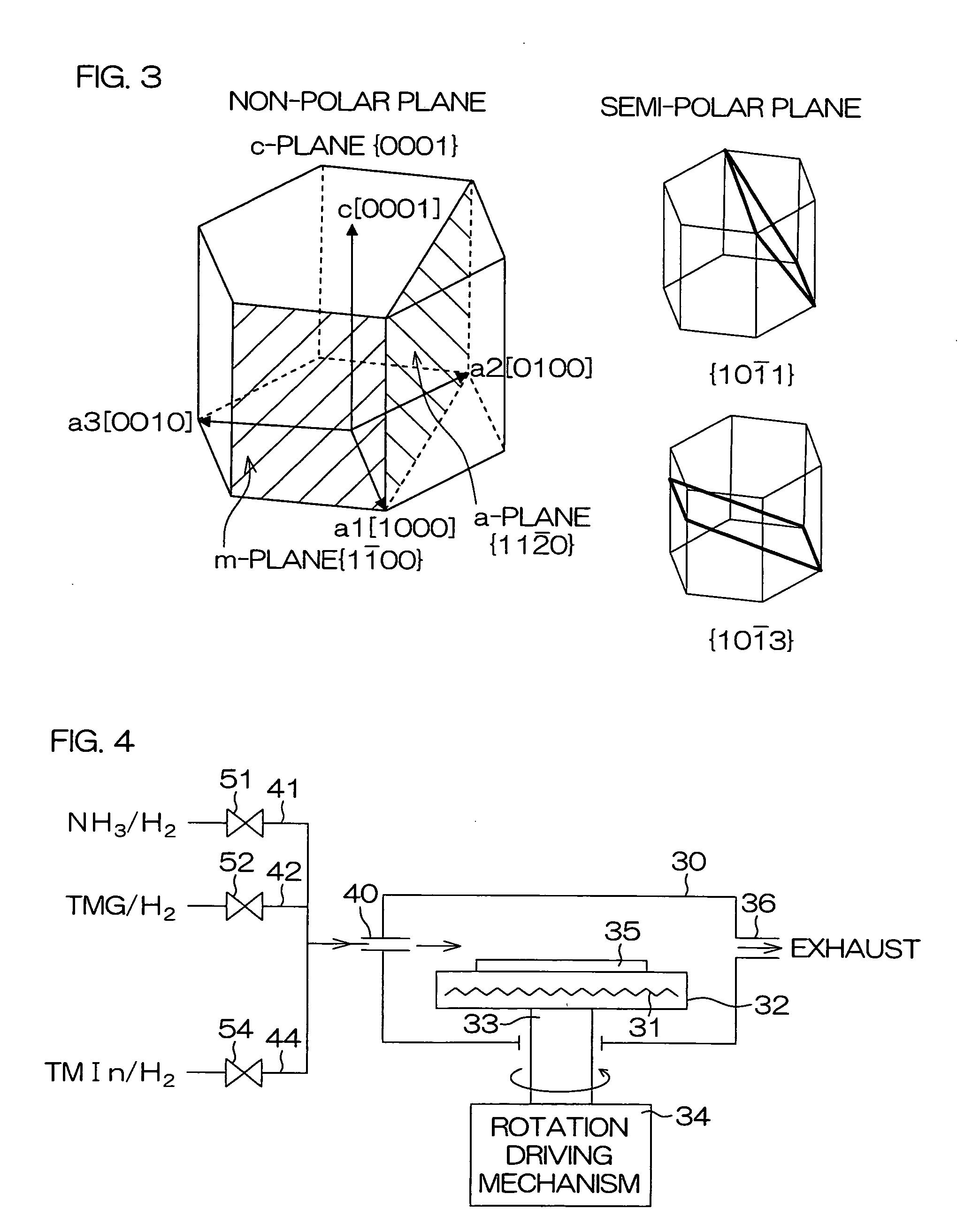
An inventive semiconductor laser diode includes a Group III nitride semiconductor layered structure having a major crystal growth plane defined by a non-polar or semi-polar-plane. The Group III nitride semiconductor layered structure includes: a p-type cladding layer and an n-type cladding layer; an In-containing p-type guide layer and an In-containing n-type guide layer held between the p-type cladding layer and the n-type cladding layer; and an In-containing light emitting layer held between the p-type guide layer and the n-type guide layer.
Owner:ROHM CO LTD
Light emitting device
InactiveUS20080232416A1Improve efficiencyImprove propertiesLaser detailsLaser active region structureLength waveWide band
Owner:ROHM CO LTD
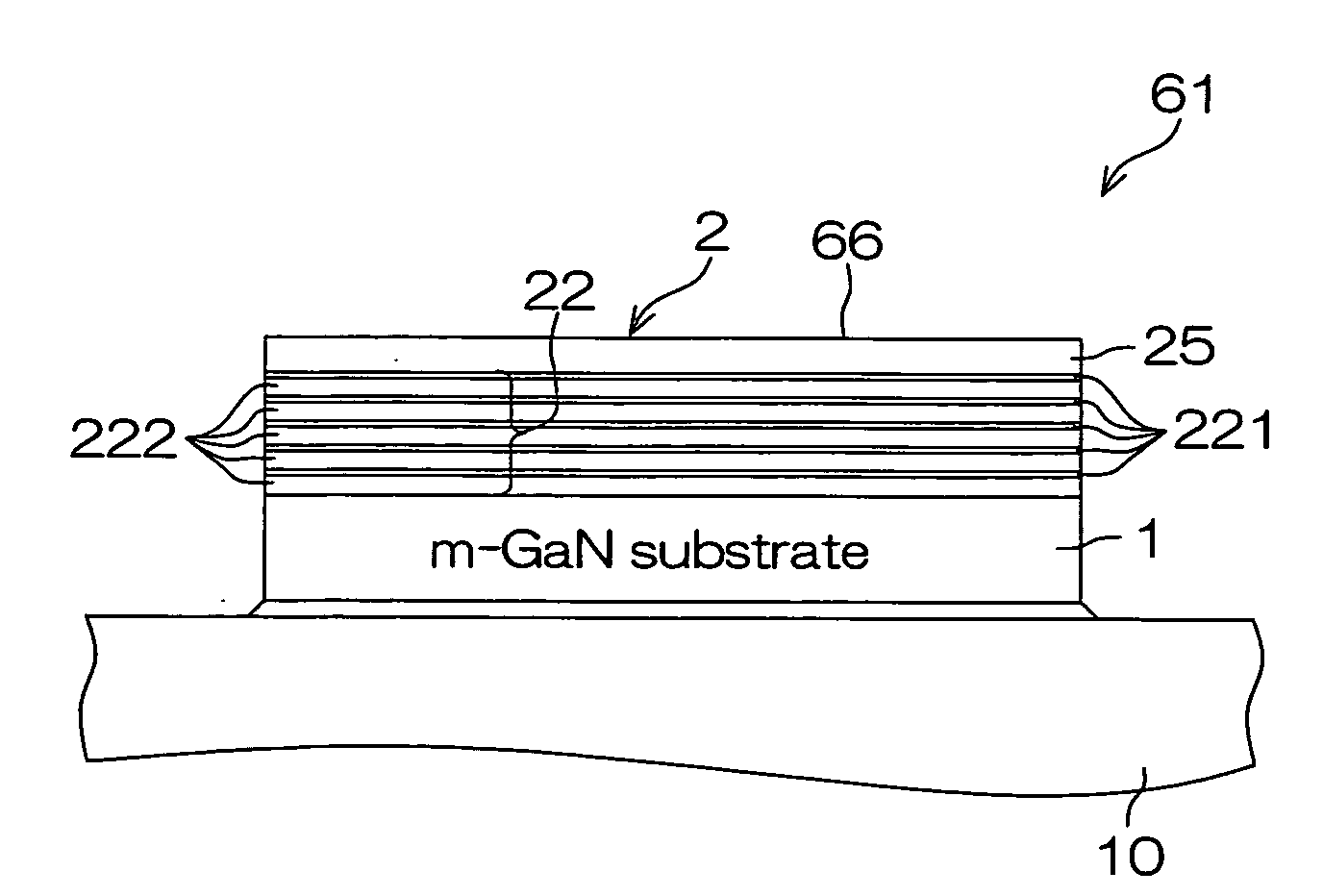
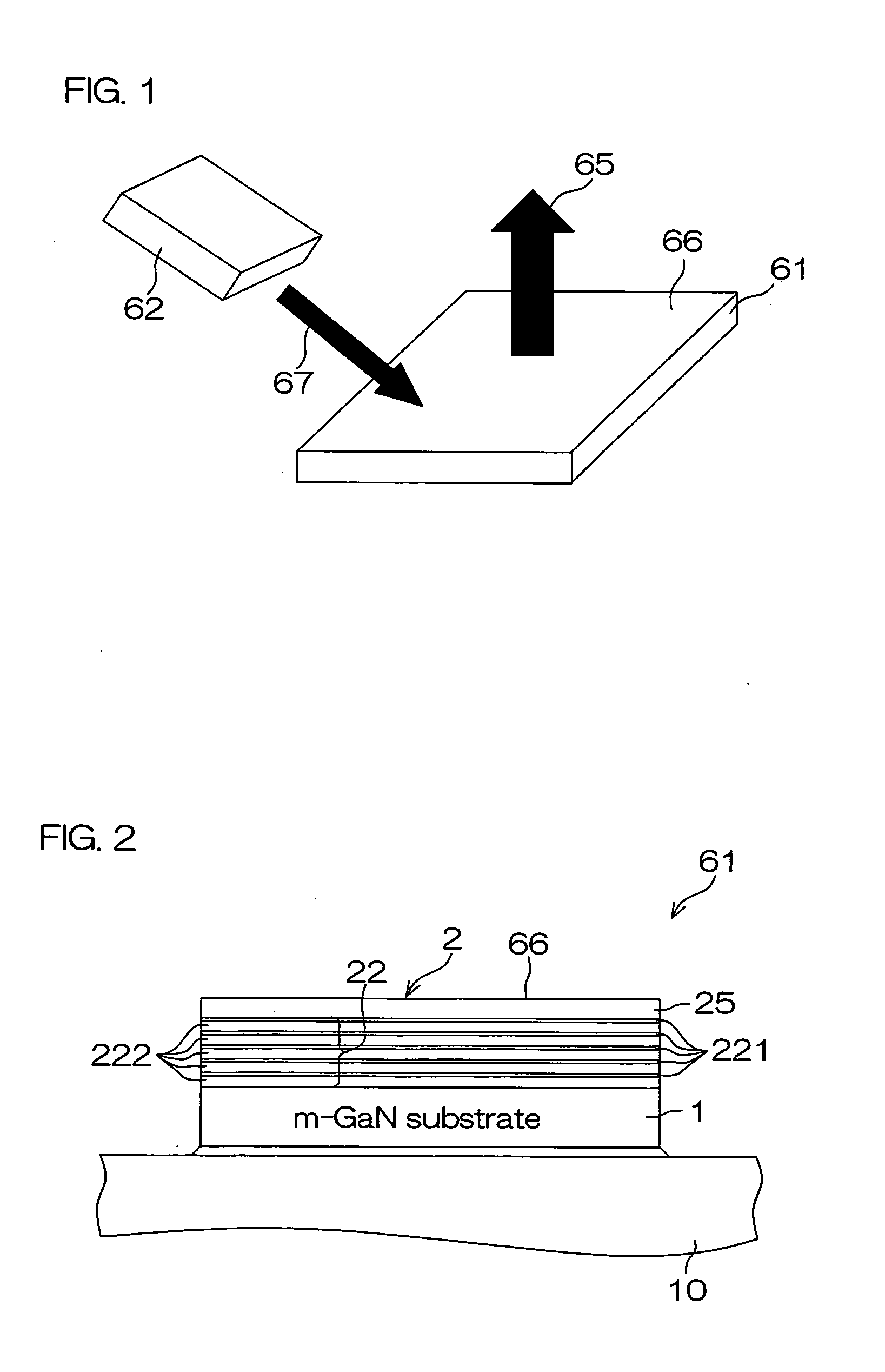
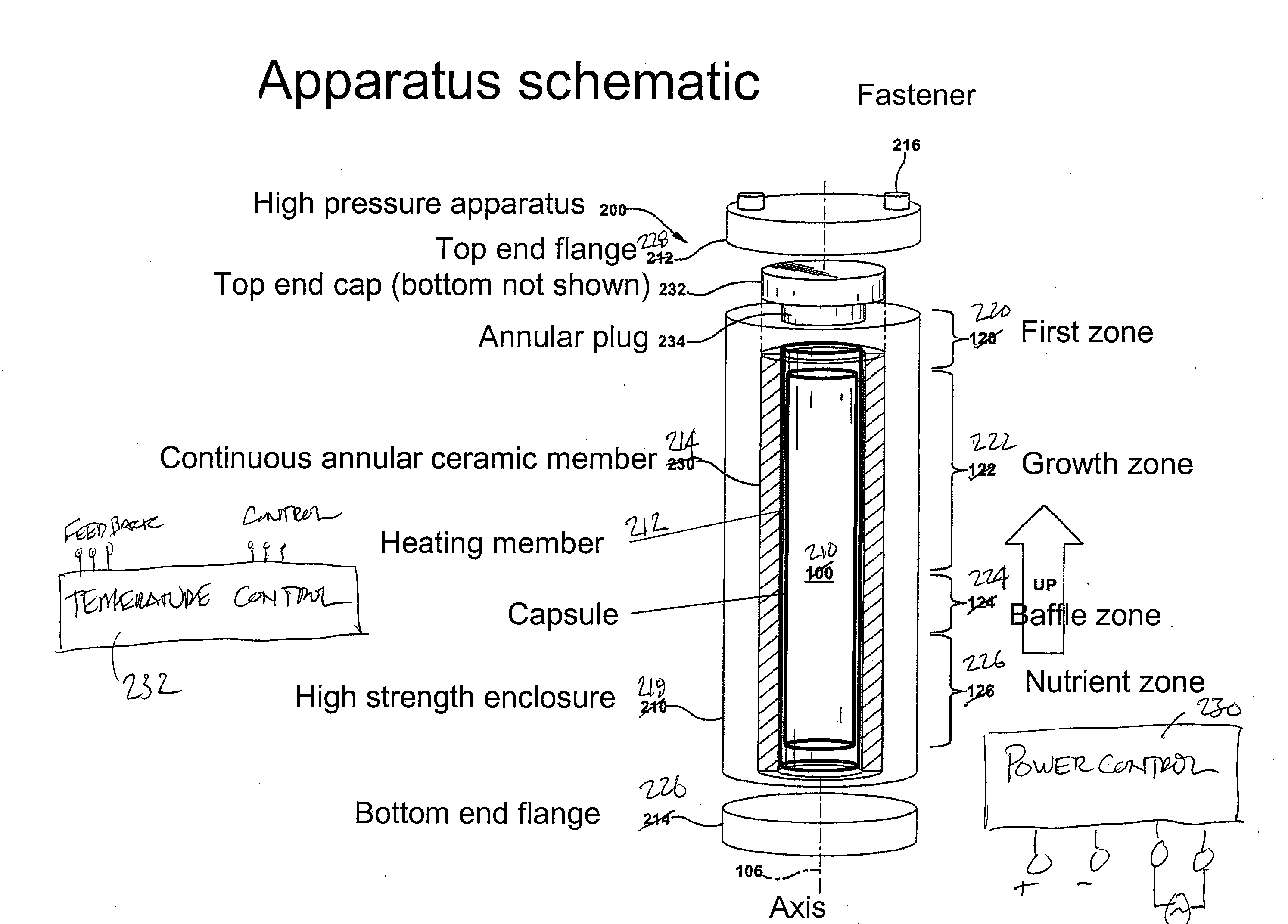
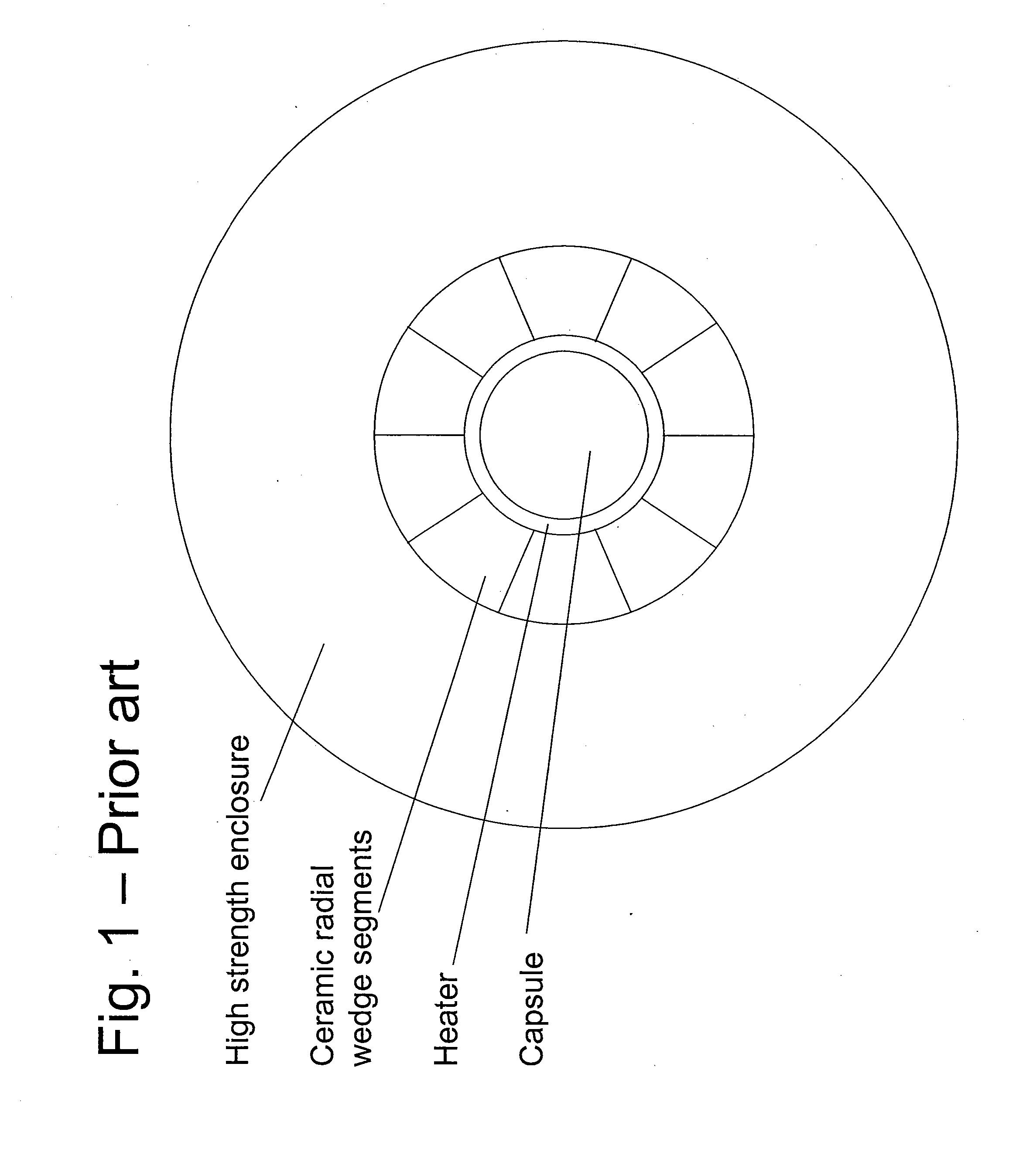
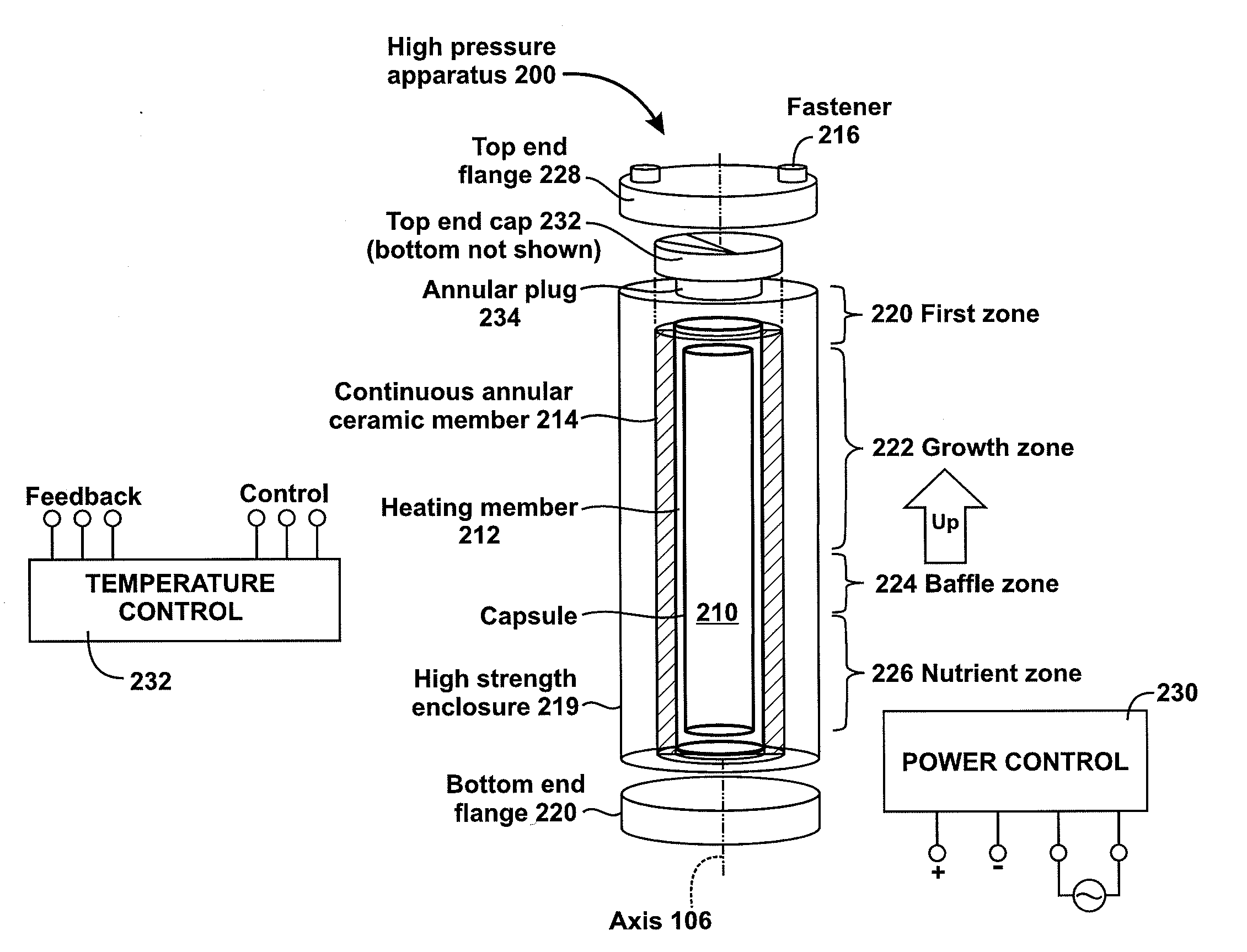
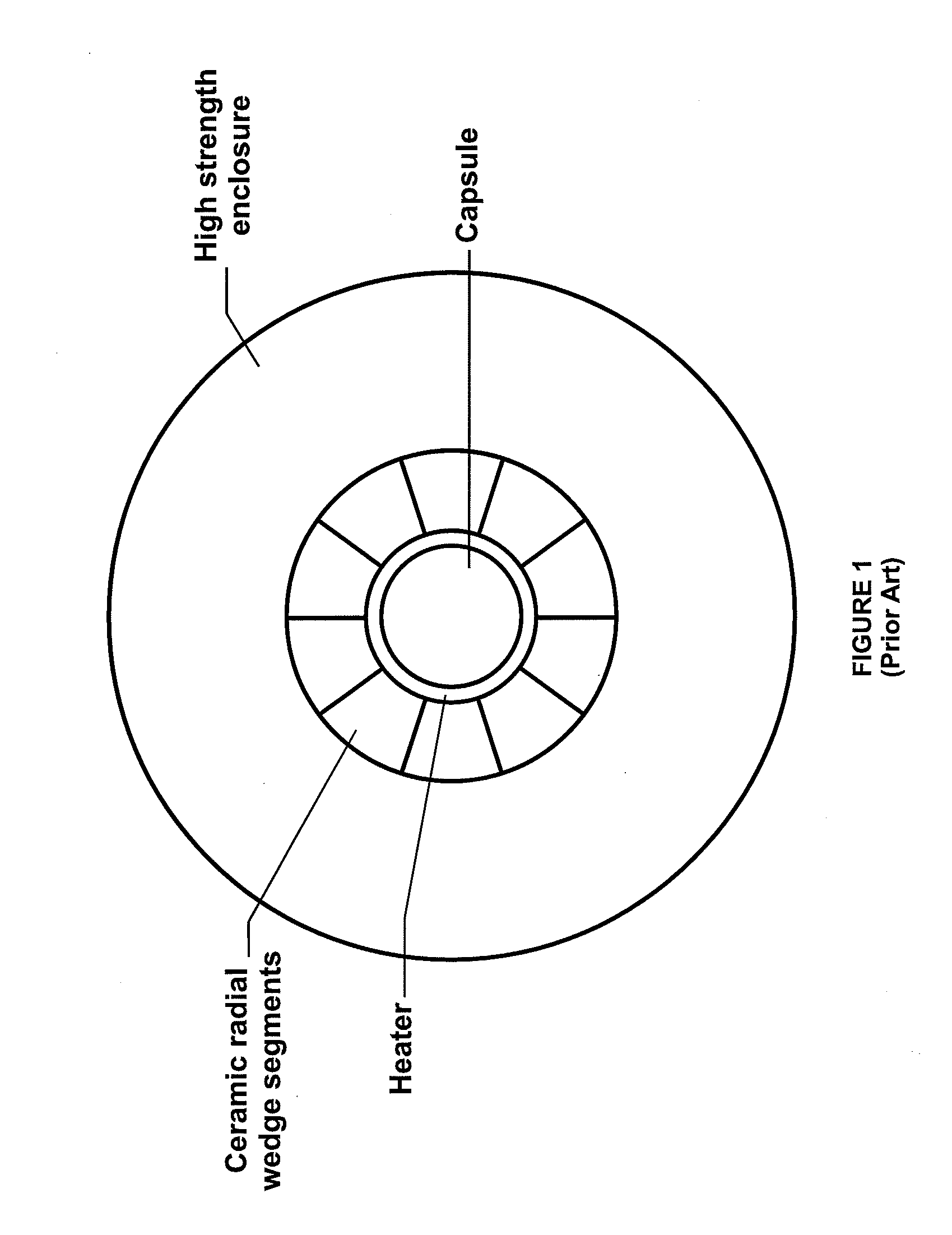
High pressure apparatus and method for nitride crystal growth
ActiveUS20090301387A1Simple and cost-effective to manufactureIncrease pressureAfter-treatment apparatusPolycrystalline material growthControl systemPower control system
A high pressure apparatus and related methods for processing supercritical fluids. In a specific embodiment, the present apparatus includes a capsule, a heater, at least one ceramic ring but can be multiple rings, optionally, with one or more scribe marks and / or cracks present. In a specific embodiment, the apparatus optionally has a metal sleeve containing each ceramic ring. The apparatus also has a high-strength enclosure, end flanges with associated insulation, and a power control system. IN a specific embodiment, the apparatus is capable of accessing pressures and temperatures of 0.2-2 GPa and 400-1200° C., respectively.
Owner:SLT TECH
Method and system for the localized deposit of metal on a surface
ActiveUS20170226636A1Desired patternPrecise patternPolycrystalline material growthSemiconductor/solid-state device manufacturingControl mannerCrystal growth
The present disclosure is directed to a method and system for the localized deposition of a metal layer on a surface. The method involves introducing at least two gaseous reactants to a substrate surface that is locally heated by a laser. The surface is heated to a temperature at which the gaseous reactants undergo a reaction that results in metal crystal growth on the substrate surface. The reaction is maintained for a desired period of time and under desired conditions to produce a localized deposit of a metal layer on the heated zone of the substrate. In some embodiments, the gas outlets and the laser may be moved in a controlled manner so that a metal layer may be deposited in a desired pattern on the substrate surface.
Owner:ILLINOIS TOOL WORKS INC
Apparatus for manufacturing semiconductor thin film
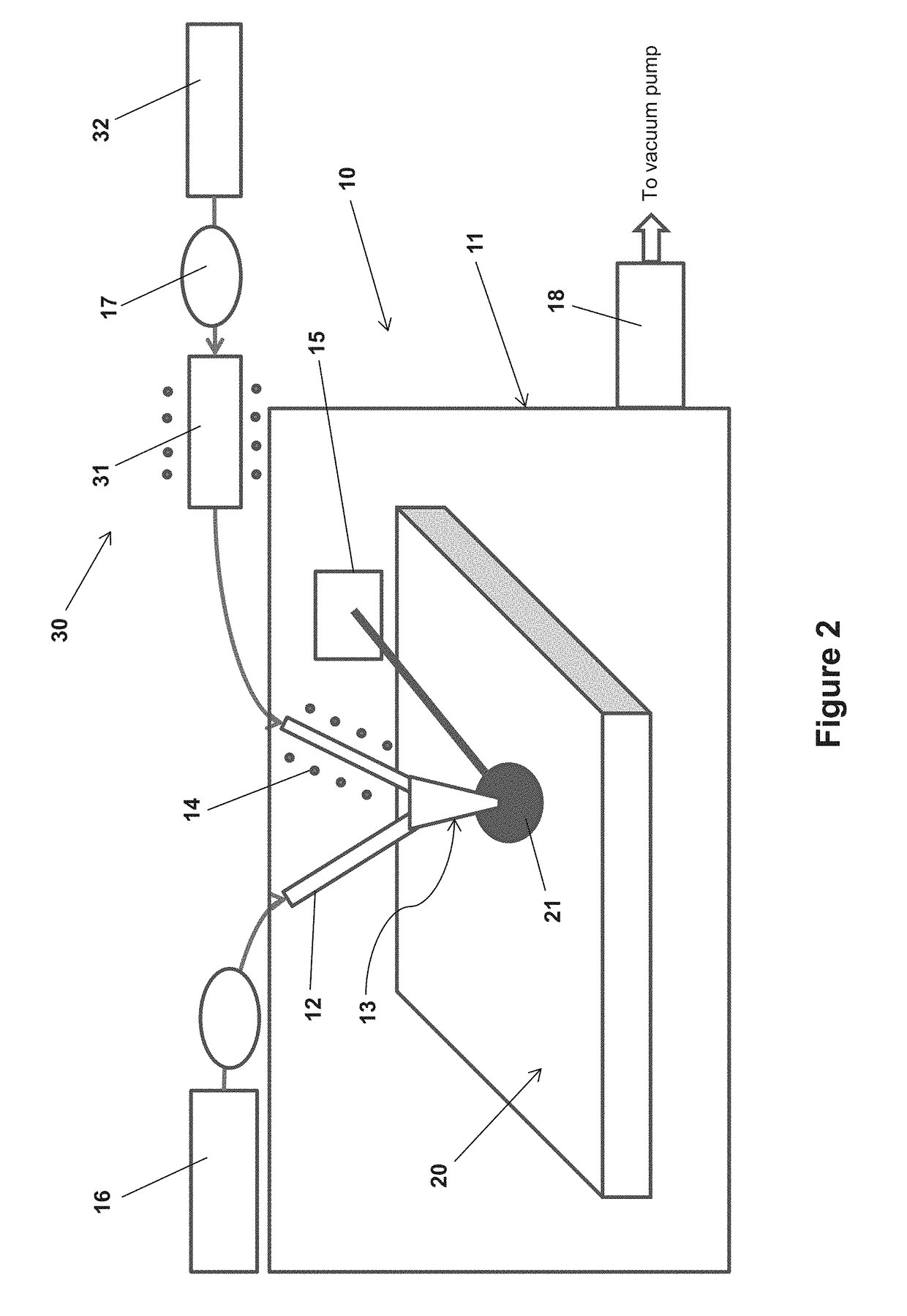
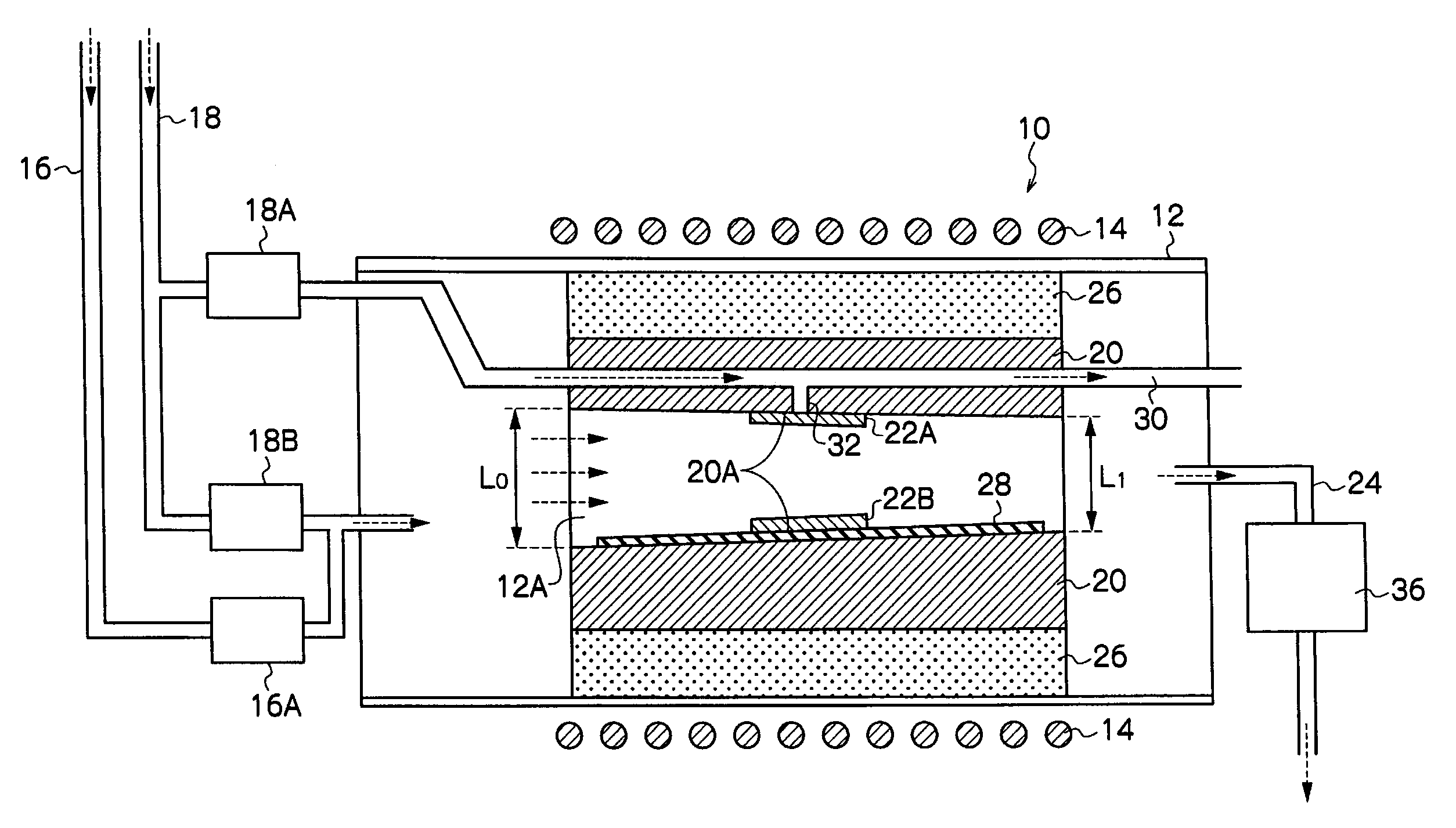
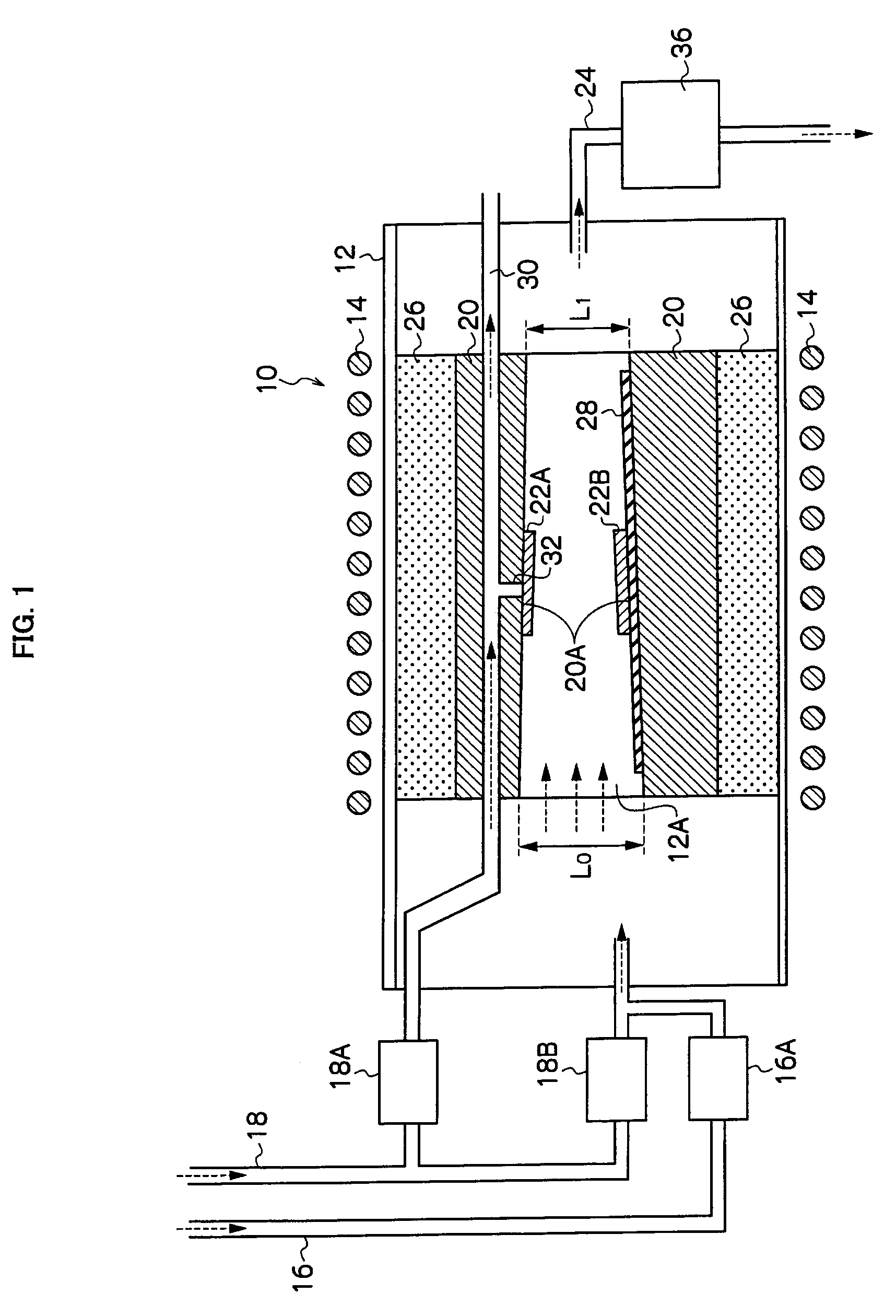
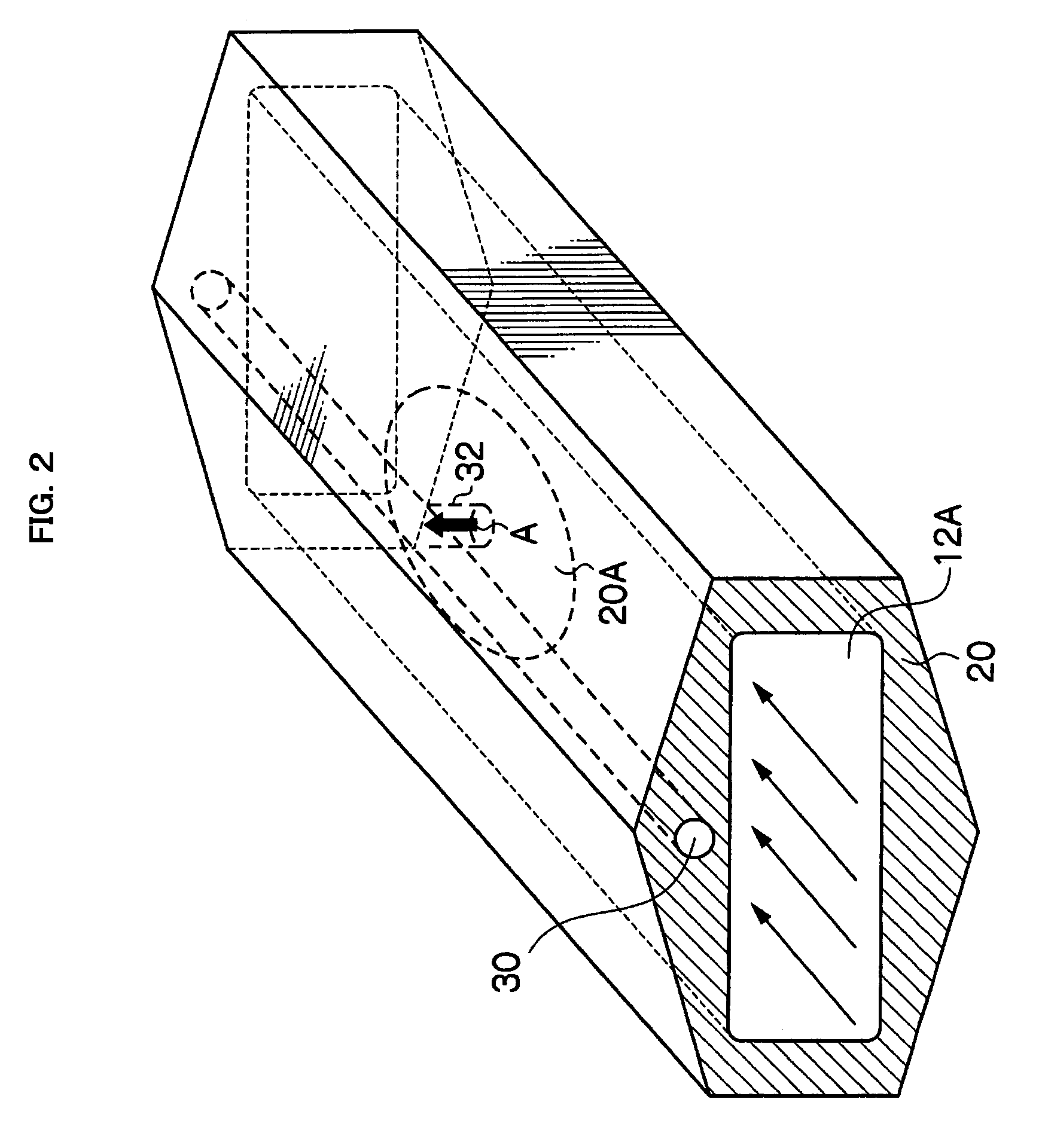
InactiveUS20090229519A1Improve in-plane evennessPolycrystalline material growthSemiconductor/solid-state device manufacturingIn planeSusceptor
The present invention provides an apparatus for manufacturing a semiconductor thin film that is capable of manufacturing an even thin film with substantially no adhesion of impurities, and is capable of improving in-plane evenness of a grown thin film. The invention is an apparatus for manufacturing a semiconductor thin film includes a reaction tube 12, a susceptor 20 disposed in the reaction tube 12, and a negative pressure generator, the negative pressure generator applying a negative pressure to a substrate 22A placed on the susceptor 20 to hold the substrate, and the substrate 22A is placed so that an angle of a normal line to a crystal growth face of the substrate 22A to a vertical downward direction is less than 180°.
Owner:TOYOTA JIDOSHA KK
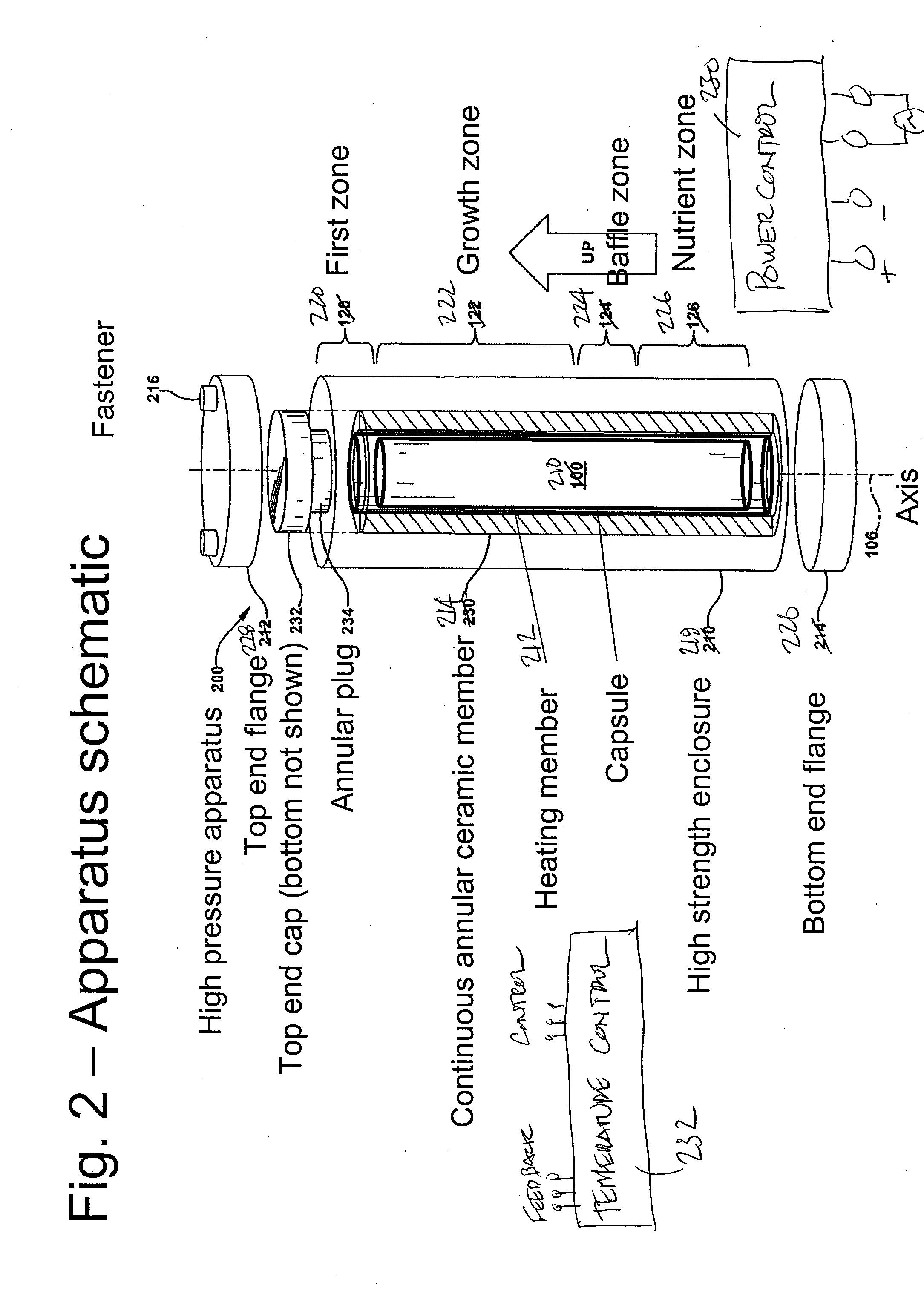
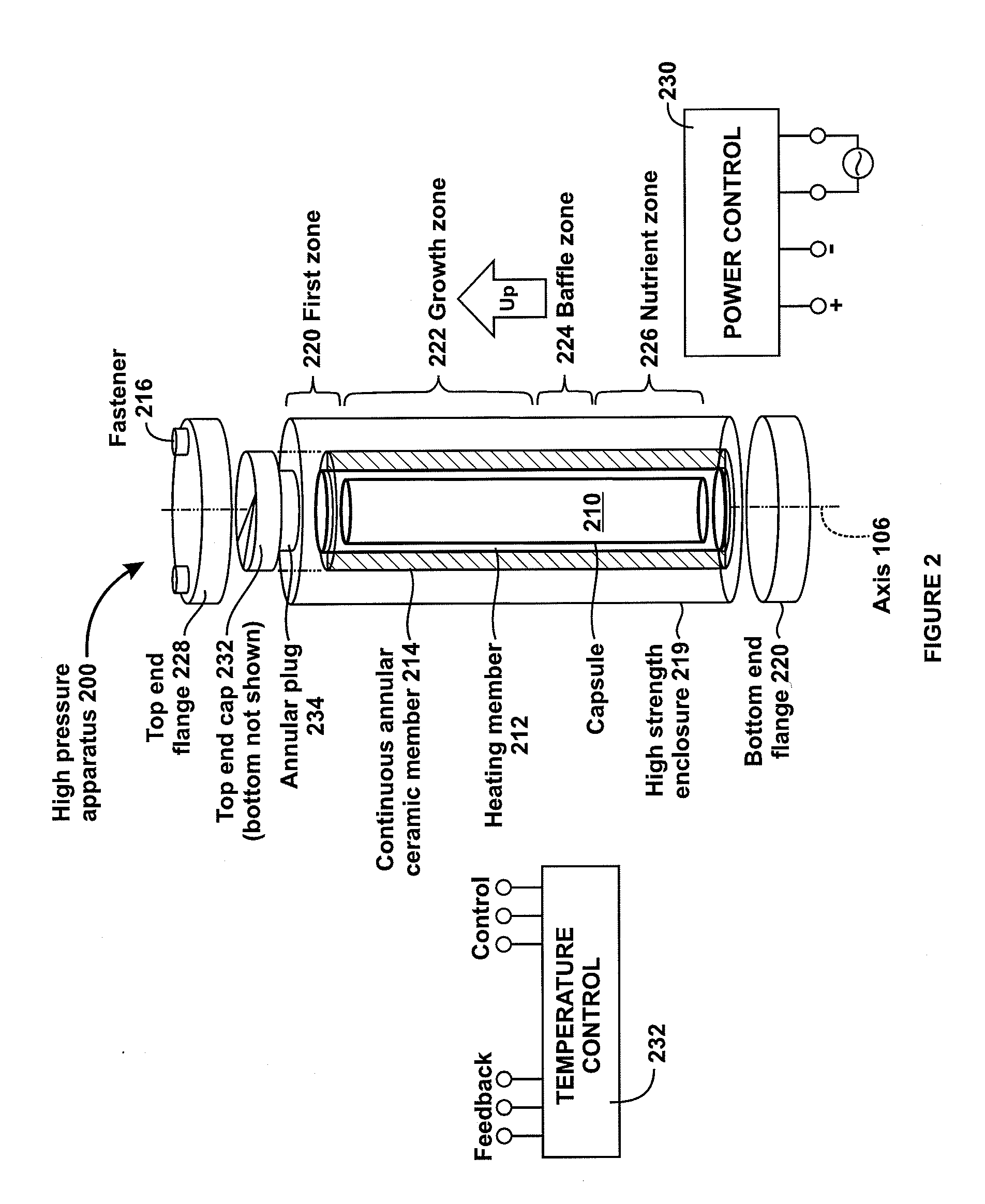
High pressure apparatus and method for nitride crystal growth
ActiveUS20090320744A1Cost-effectiveSimple and cost-effective to manufactureAfter-treatment apparatusPolycrystalline material growthControl systemPower control system
A high pressure apparatus and related methods for processing supercritical fluids. In a specific embodiment, the present apparatus includes a capsule, a heater, at least one ceramic ring but can be multiple rings, optionally, with one or more scribe marks and / or cracks present. In a specific embodiment, the apparatus optionally has a metal sleeve containing each ceramic ring. The apparatus also has a high-strength enclosure, end flanges with associated insulation, and a power control system. In a specific embodiment, the apparatus is capable of accessing pressures and temperatures of 0.2-2 GPa and 400-1200° C., respectively.
Owner:SLT TECH
Concentric-ring sprayer structure for material vapor phase epitaxy
InactiveCN103014846ANo effect on growthGrowth impactFrom chemically reactive gasesVapour phase epitaxySprayer
The invention discloses a concentric-ring sprayer structure for material vapor phase epitaxy, which solves the problem that the large-area deposition region provides a uniform flow field of a precursor gas mixture in a large-substrate or multi-substrate crystal growth process. The sprayer structure comprises more than one independent air inlet pipeline, wherein each air inlet pipeline is provided with a controller for monitoring and regulating inlet gas flow speed and flow rate; the bottom of the sprayer is provided with an air outlet baffle; more than one concentric ring is arranged in the sprayer; independent cavities are formed among the concentric rings and are mutually separated; the top end of each concentric ring is connected with one independent air inlet pipeline; and the air outlet baffle at the bottom end of each concentric ring is provided with one or more air outlets. The air sources are mutually separated and independently controlled; and the multi-sprayer integrated use mode obviously improves the quality of the large-area deposited grown crystal, and greatly enhances the production efficiency.
Owner:SINO NITRIDE SEMICON +1
Crystal growth devices and systems, and methods for using same
Owner:FLUIDIGM CORP
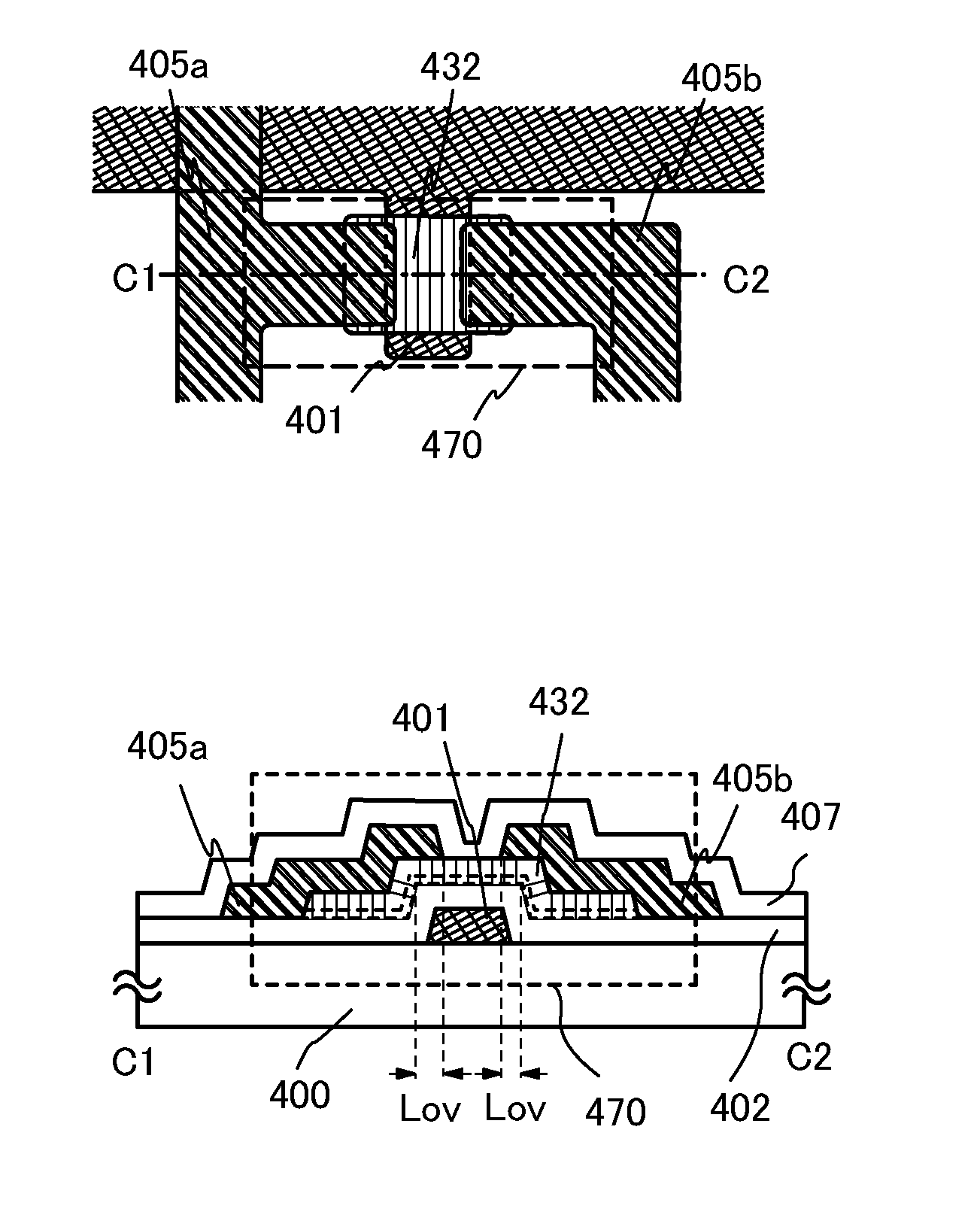
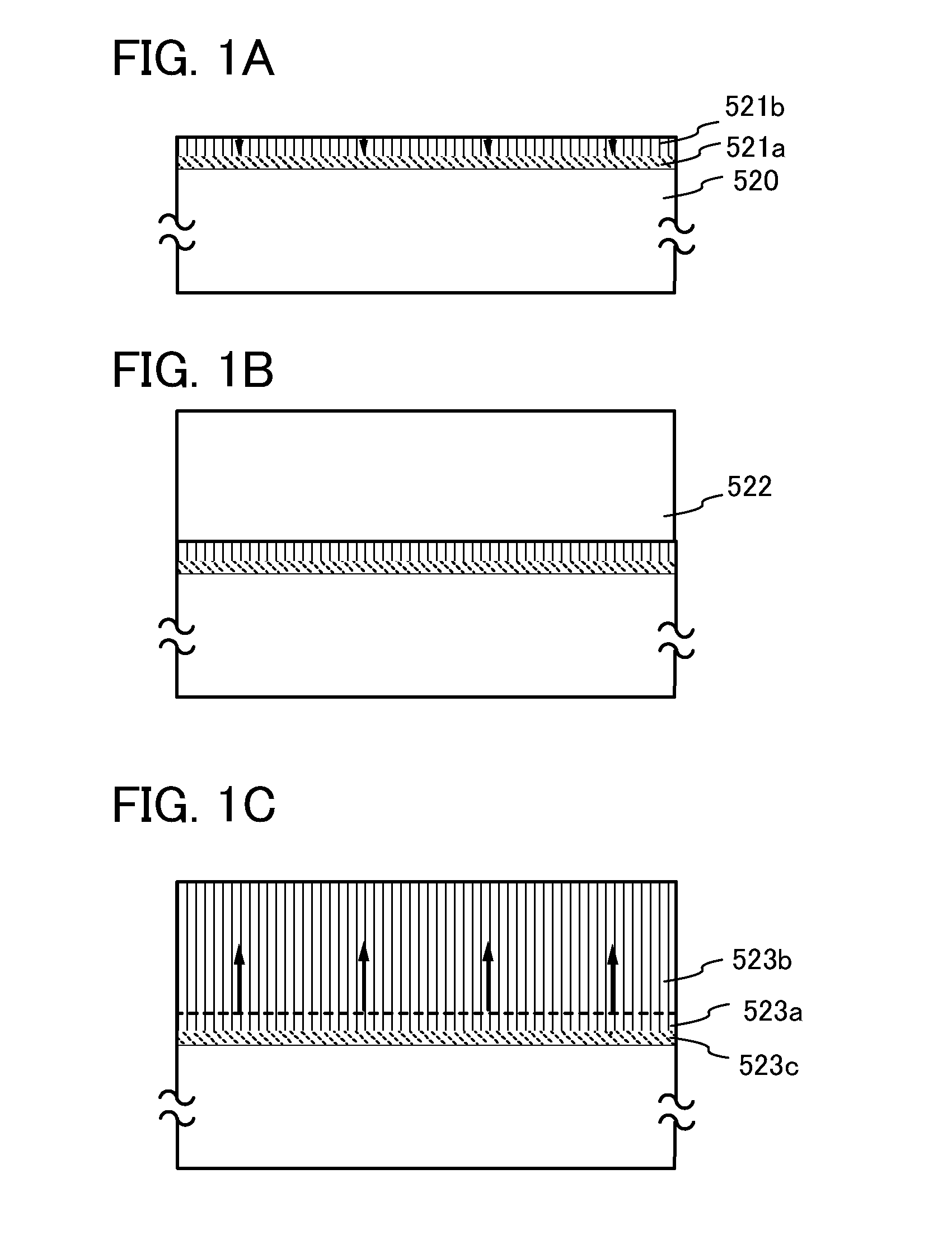
Stacked oxide material, semiconductor device, and method for manufacturing the semiconductor device
ActiveUS20110127521A1Improve mobilityReduced shutdown currentSolid-state devicesVacuum evaporation coatingDevice materialCrystal growth
One embodiment is a method for manufacturing a stacked oxide material, including the steps of forming an oxide component over a base component; forming a first oxide crystal component which grows from a surface toward an inside of the oxide component by heat treatment, and leaving an amorphous component just above a surface of the base component; and stacking a second oxide crystal component over the first oxide crystal component. In particular, the first oxide crystal component and the second oxide crystal component have common c-axes. Same-axis (axial) growth in the case of homo-crystal growth or hetero-crystal growth is caused.
Owner:SEMICON ENERGY LAB CO LTD
Method for manufacturing semiconductor device
ActiveUS20110156026A1High field-effect mobilitySufficient performanceSolid-state devicesSemiconductor/solid-state device manufacturingDisplay deviceSingle crystal
A larger substrate can be used, and a transistor having a desirably high field-effect mobility can be manufactured through formation of an oxide semiconductor layer having a high degree of crystallinity, whereby a large-sized display device, a high-performance semiconductor device, or the like can be put into practical use. A first multi-component oxide semiconductor layer is formed over a substrate and a single-component oxide semiconductor layer is formed thereover; then, crystal growth is carried out from a surface to an inside by performing heat treatment at 500° C. to 1000° C. inclusive, preferably 550° C. to 750° C. inclusive so that a first multi-component oxide semiconductor layer including single crystal regions and a single-component oxide semiconductor layer including single crystal regions are formed; and a second multi-component oxide semiconductor layer including single crystal regions is stacked over the single-component oxide semiconductor layer including single crystal regions.
Owner:SEMICON ENERGY LAB CO LTD
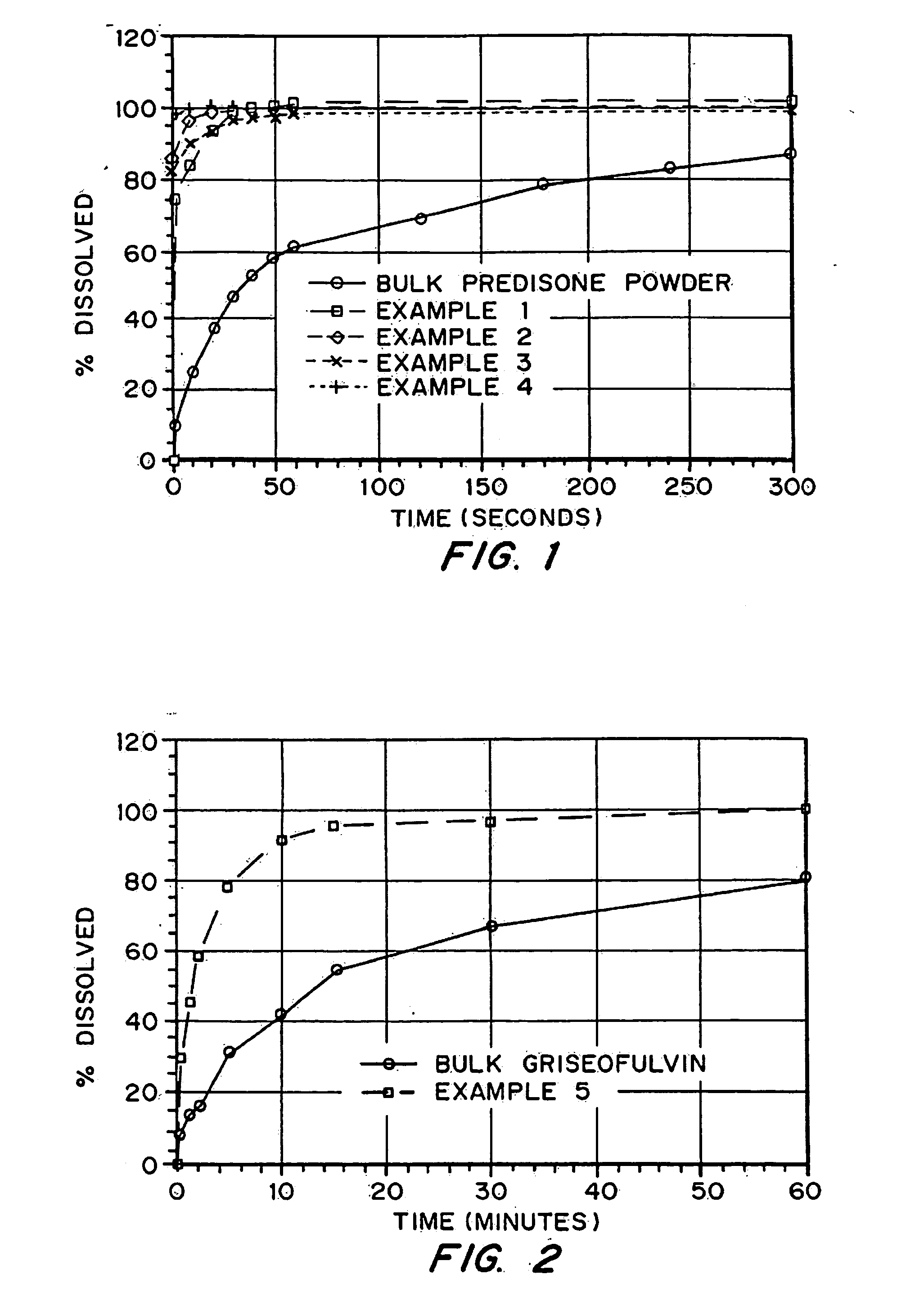
Porous drug matrices and methods of manufacture thereof
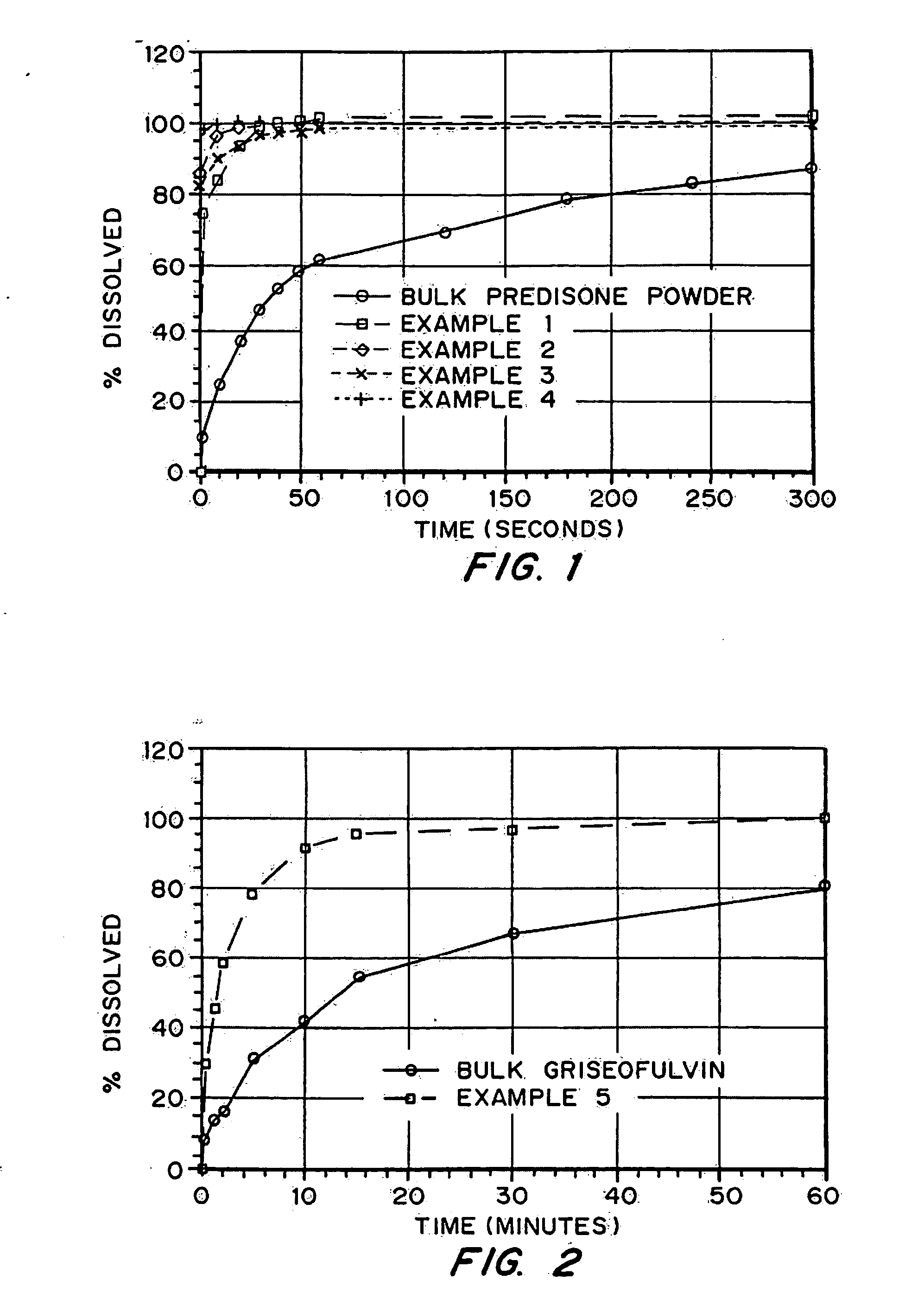
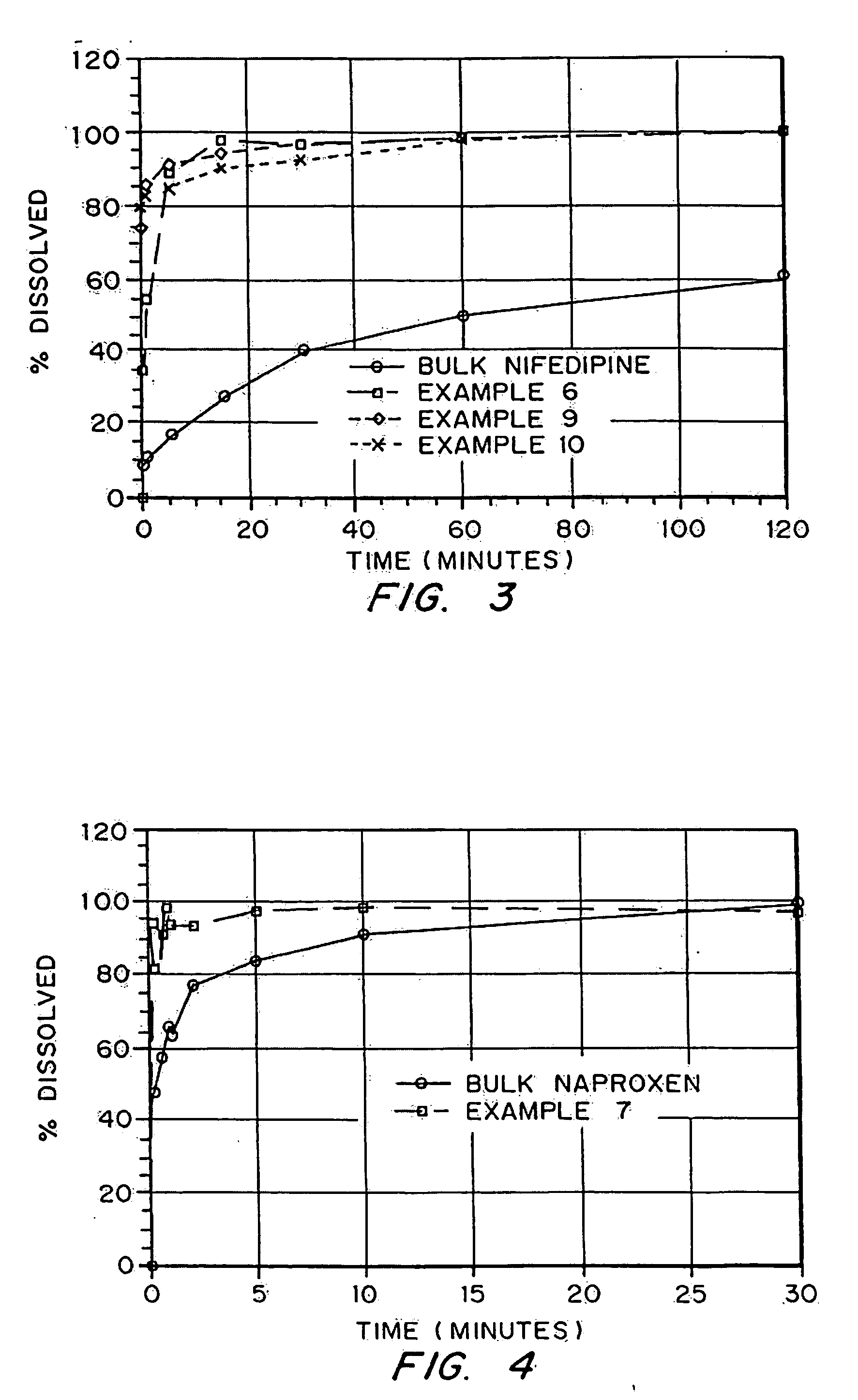
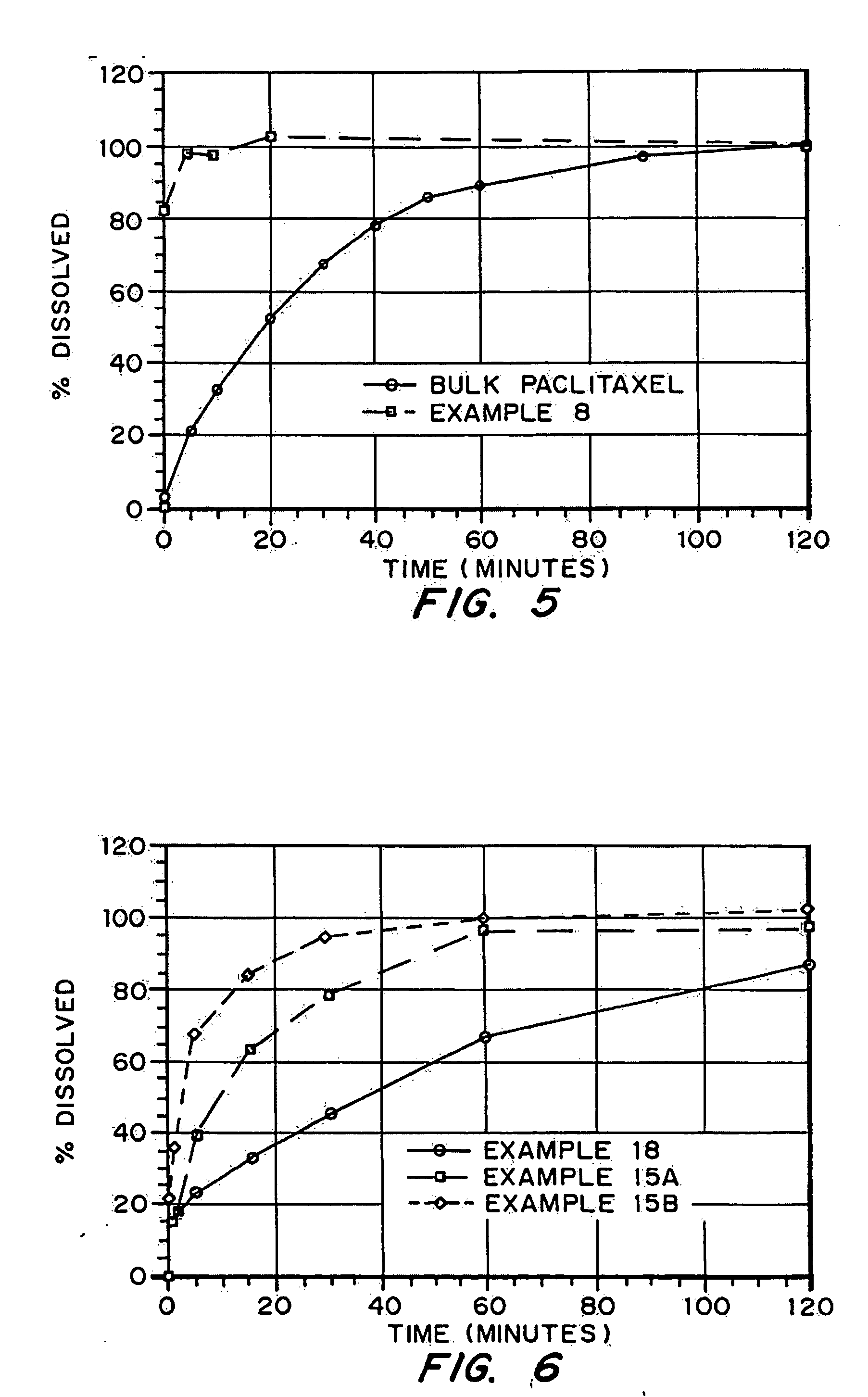
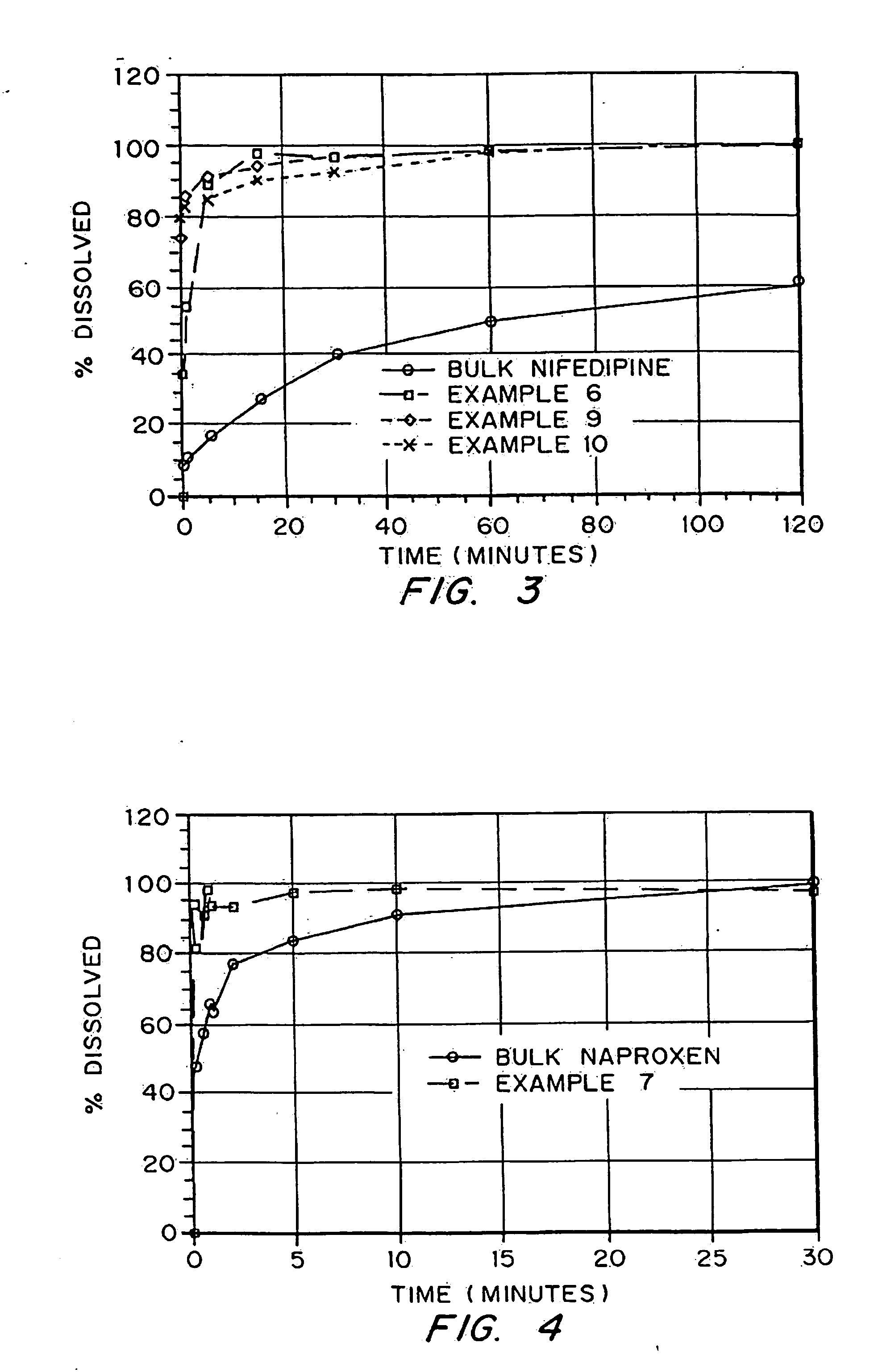
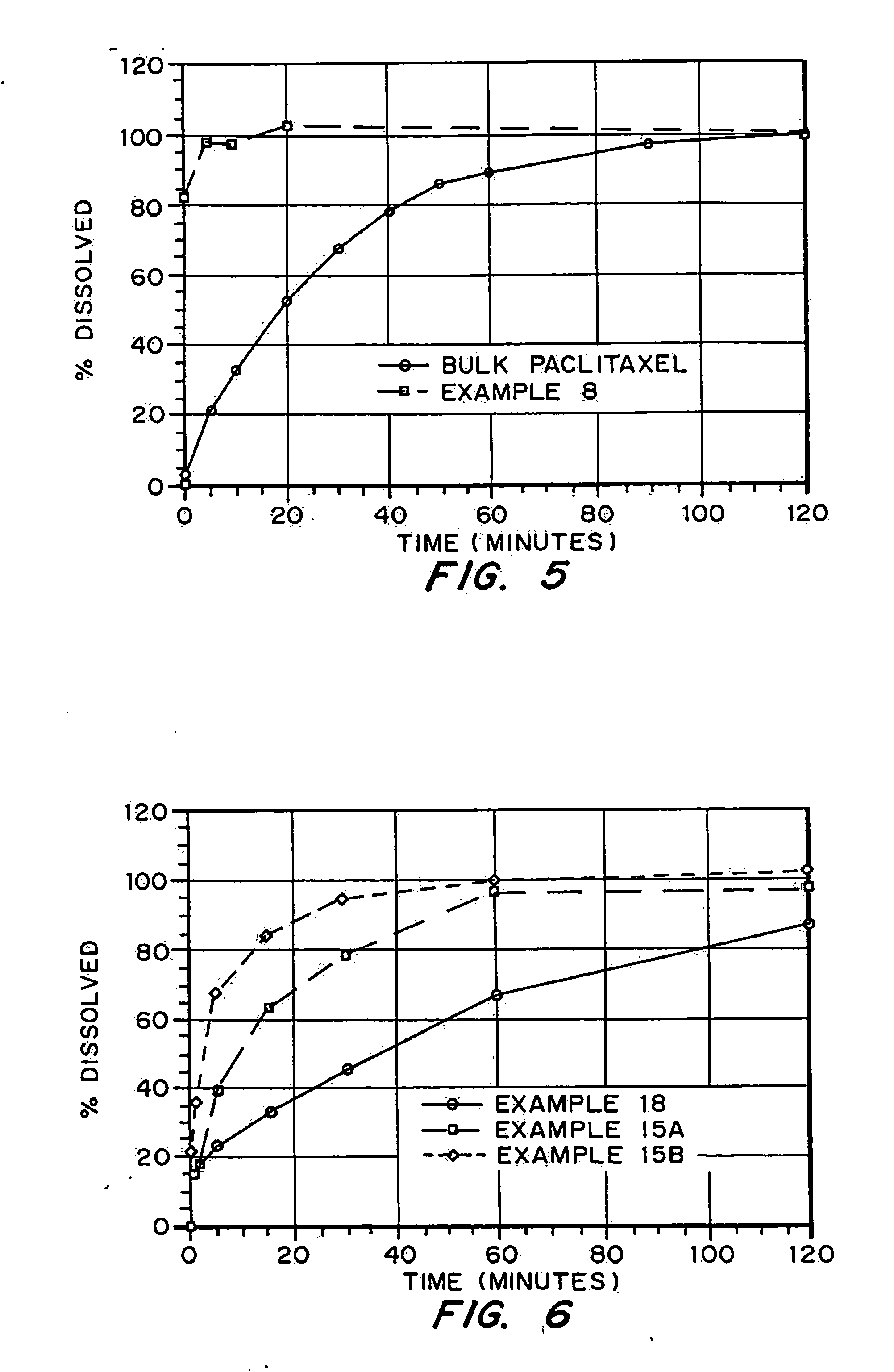
InactiveUS20050048116A1Fast dissolutionHigh dissolution ratePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid-compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Semiconductor device and method for manufacturing semiconductor device
ActiveUS20120132903A1Well representedUnstable characteristicTransistorSolid-state devicesCrystal structureEngineering
A highly reliable semiconductor device is manufactured by giving stable electric characteristics to a transistor in which an oxide semiconductor film is used for a channel. An oxide semiconductor film which can have a first crystal structure by heat treatment and an oxide semiconductor film which can have a second crystal structure by heat treatment are formed so as to be stacked, and then heat treatment is performed; accordingly, crystal growth occurs with the use of an oxide semiconductor film having the second crystal structure as a seed, so that an oxide semiconductor film having the first crystal structure is formed. An oxide semiconductor film formed in this manner is used for an active layer of the transistor.
Owner:SEMICON ENERGY LAB CO LTD
GaN field-effect transistor and method of manufacturing the same
InactiveUS20010040246A1Reduce dislocation densityTransistorPolycrystalline material growthLateral overgrowthEngineering
There are provided a GaN field effect transistor (FET) exhibiting an excellent breakdown voltage owing to the high quality of GaN crystal in a region where the electric lines of force concentrate during operation of the same, and a method of manufacturing the same. The FET has a layer structure formed of a plurality of GaN epitaxial layers. A gate electrode and a source electrode are disposed on the surface of the layer structure, and a drain electrode is disposed on the reverse surface of the same. A region of the layer structure in which the electric lines of force concentrate during operation of the FET has a reduced dislocation density compared with the other regions in the layer structure. The GaN FET is manufactured by forming, on a crystal-growing substrate having a surface formed with a plane pattern of a material other than a GaN-based material in an identical design to a plane pattern of an electrode determining the region in which the electric lines of force concentrate, a plurality of GaN epitaxial layers, one upon another, by using the epitaxial lateral overgrowth technique, thereby forming a layer structure, and then forming operational electrodes on the surface of the layer structure.
Owner:FURUKAWA ELECTRIC CO LTD
Nitride semiconductor crystal and its production method
InactiveUS20110129669A1Easy to produceEfficient productionSemiconductor/solid-state device manufacturingGlass/slag layered productsCrystal growthSeed crystal
A method for efficiently producing a plate-like nitride semiconductor crystal having the desired principal plane in a simple method is provided. A raw material gas is fed to a seed crystal in which a ratio (L / W) of length L in a longitudinal direction and maximum width W, of a plane of projection obtained by projecting a crystal growth face on the seed crystal in a growth direction is from 2 to 400, and the maximum width W is 5 mm or less, thereby growing a plate-like semiconductor crystal on the seed crystal.
Owner:MITSUBISHI CHEM CORP
Method of preparing a semiconductor having controlled crystal orientation
InactiveUS6756657B1TransistorSemiconductor/solid-state device manufacturingAmorphous siliconCrystal orientation
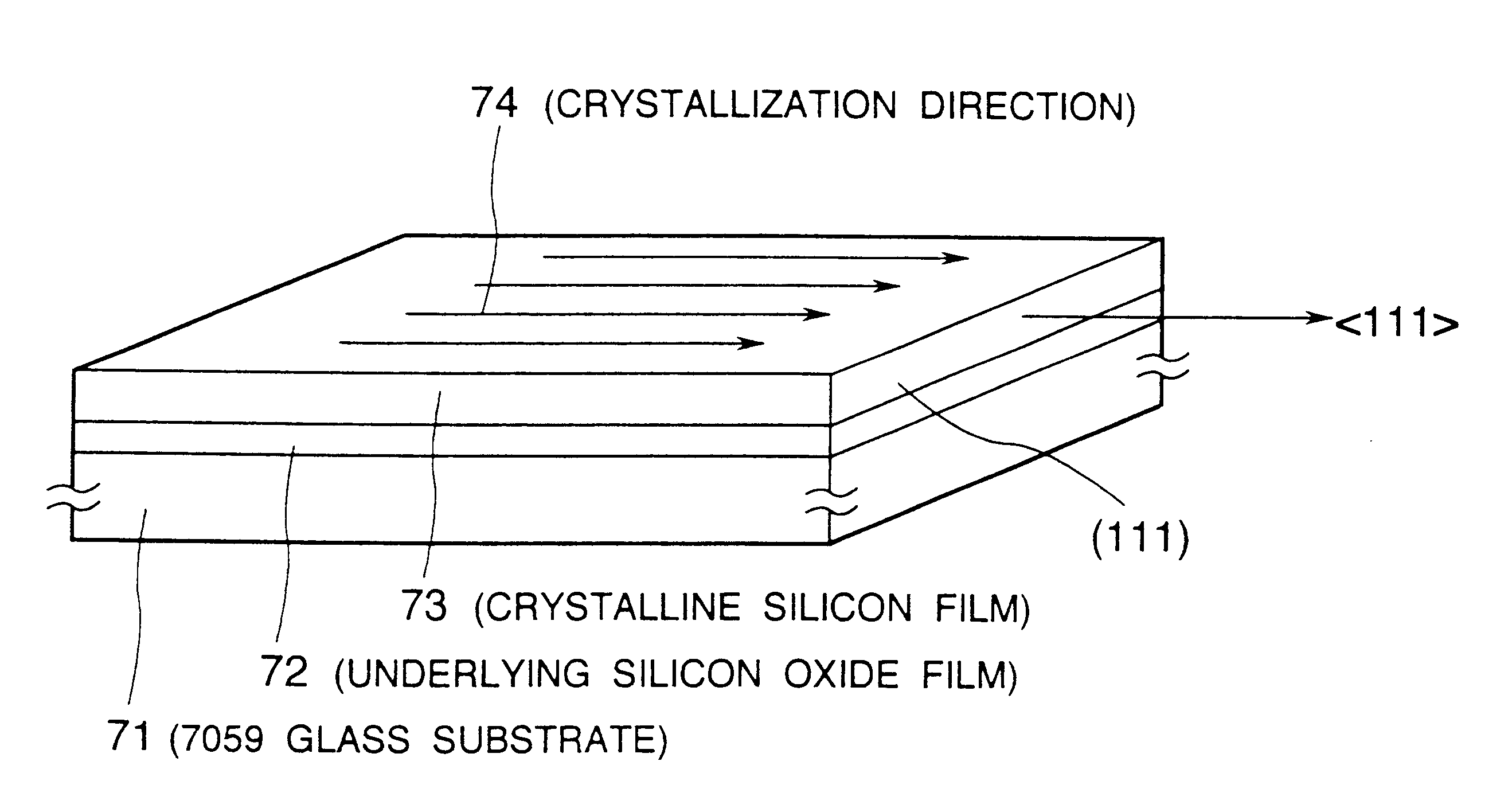
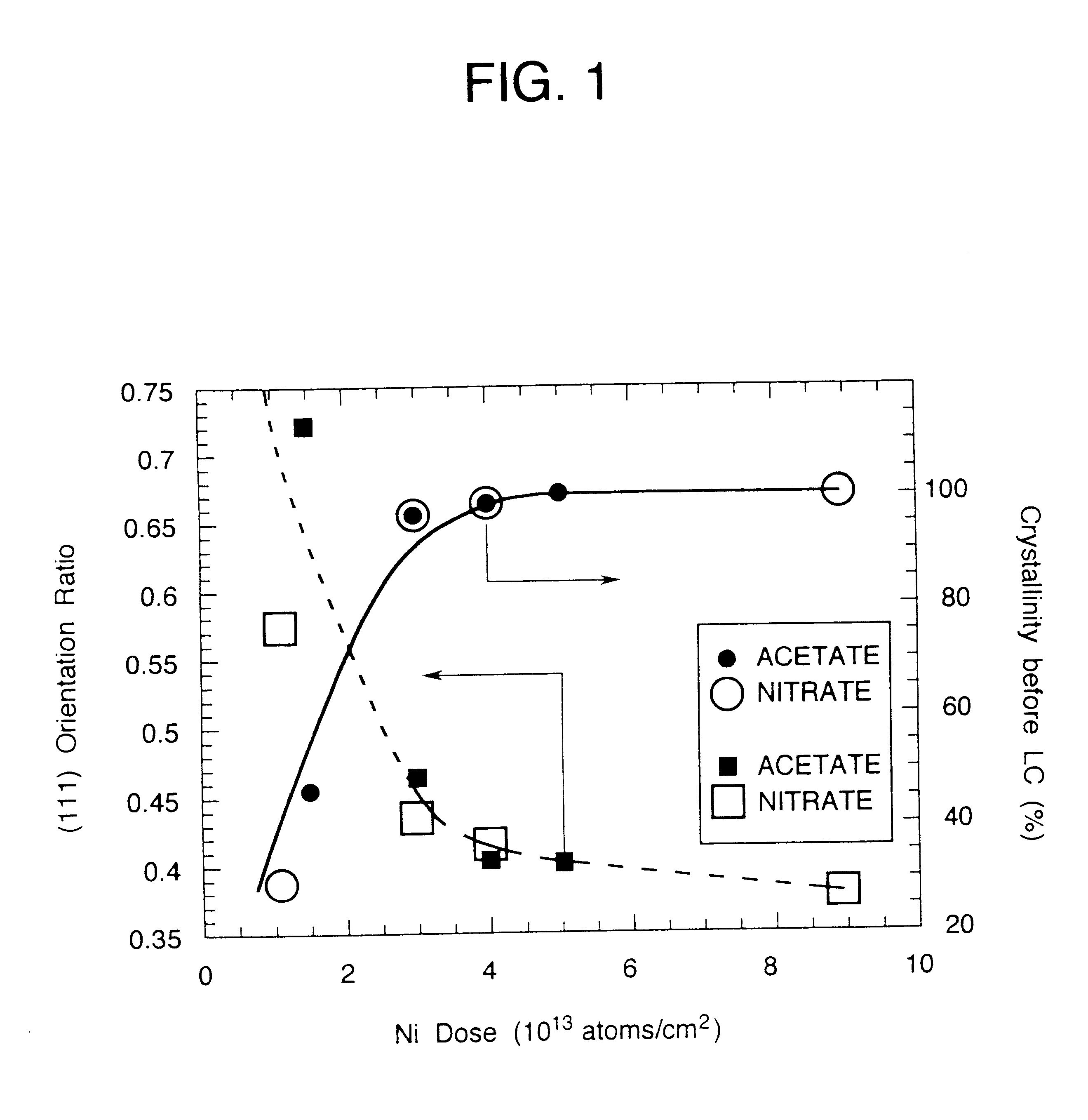
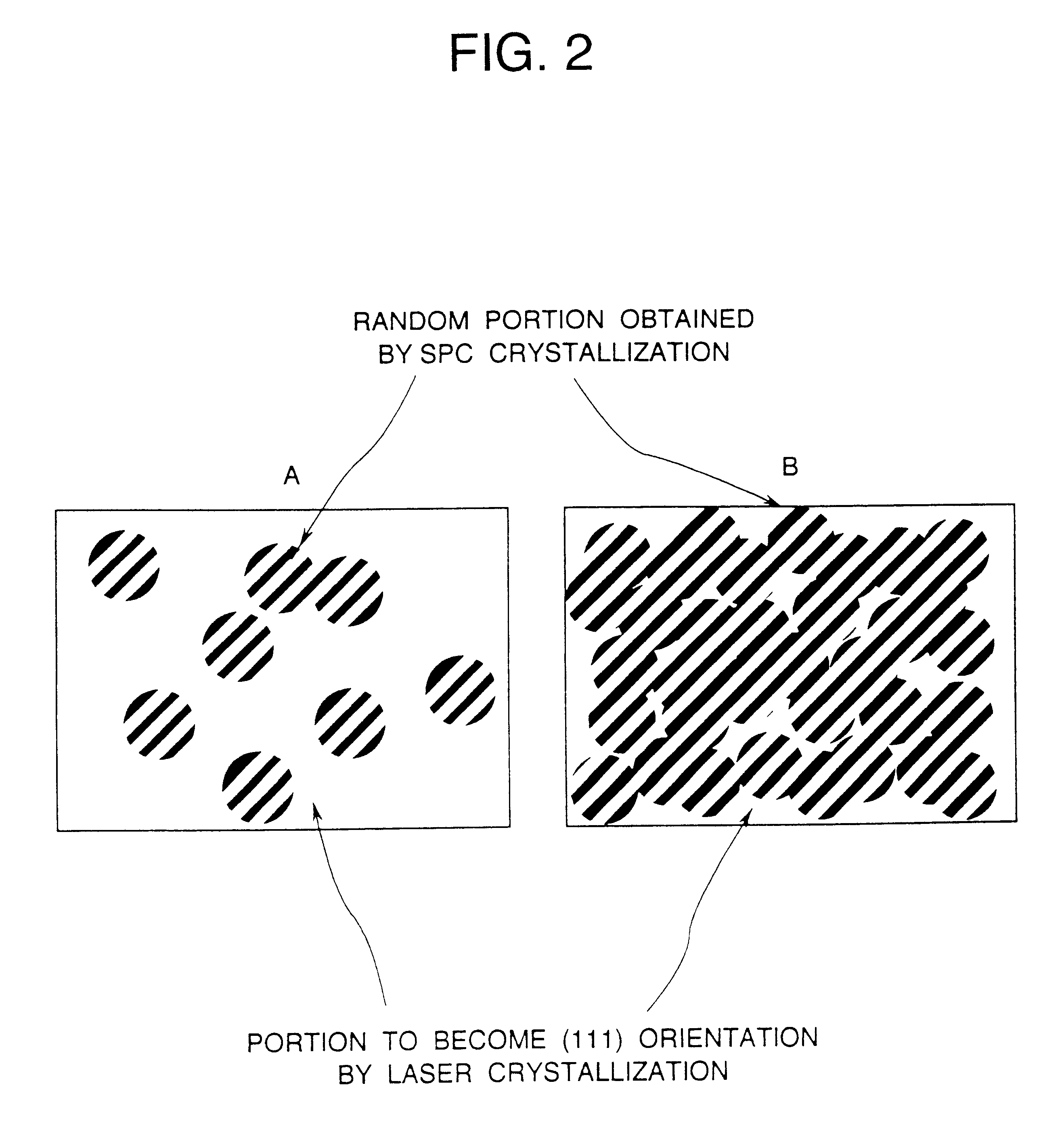
A semiconductor device is disclosed. The semiconductor device has a crystalline silicon film as an active layer region. The crystalline silicon film has needle-like or columnar crystals oriented parallel to the substrate and having a crystal growth direction of (111) axis. A method for preparing the semiconductor device comprises steps of adding a catalytic element to an amorphous silicon film; and heating the amorphous silicon film containing the catalytic element at a low temperature to crystallize the silicon film.
Owner:SEMICON ENERGY LAB CO LTD
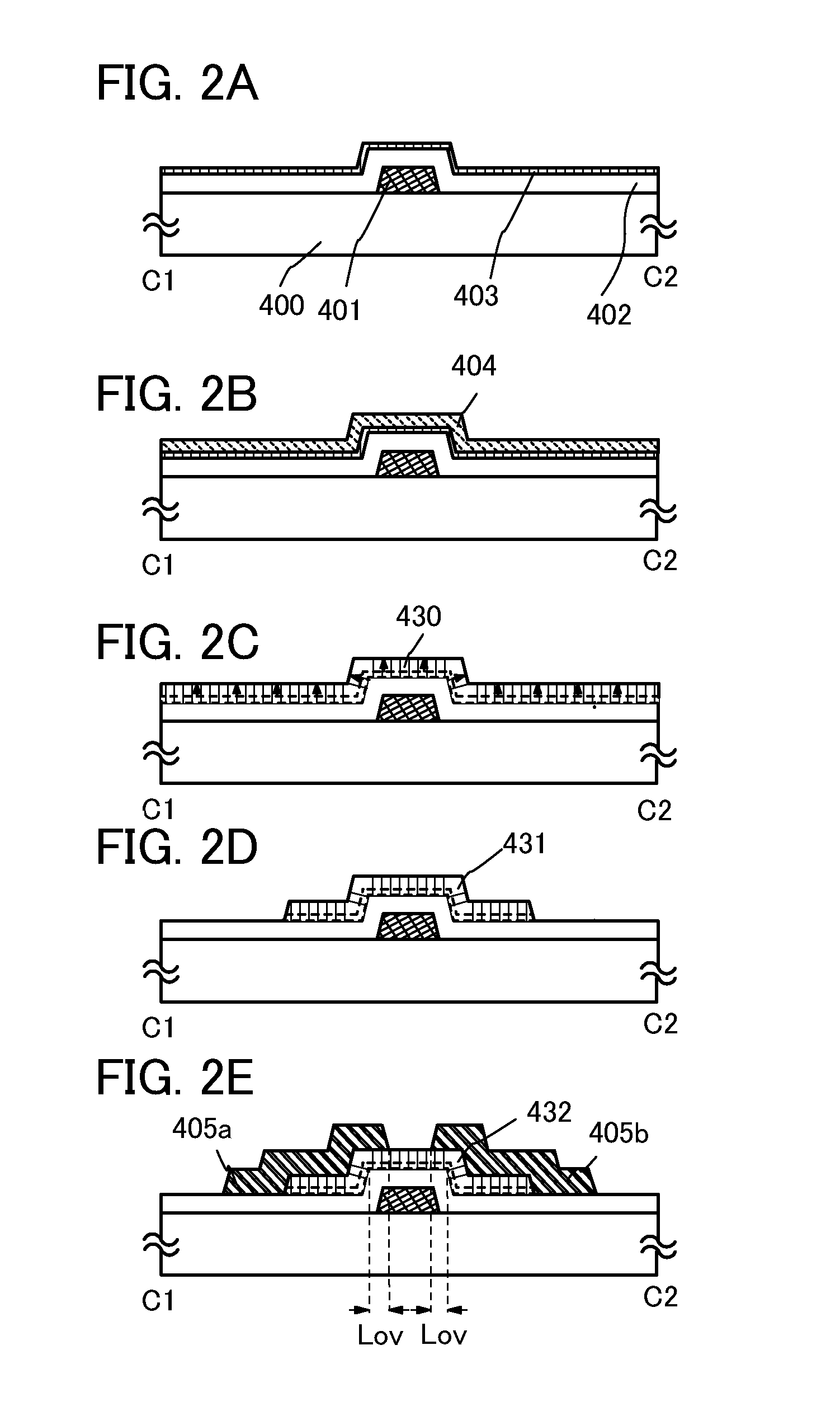
Stacked oxide material, semiconductor device, and method for manufacturing the semiconductor device
ActiveUS20110127579A1Improve mobilityReduced shutdown currentVacuum evaporation coatingSolid-state devicesDevice materialEngineering
One embodiment is a method for manufacturing a stacked oxide material, including the steps of forming a first oxide component over a base component, causing crystal growth which proceeds from a surface toward an inside of the first oxide component by first heat treatment to form a first oxide crystal component at least partly in contact with the base component, forming a second oxide component over the first oxide crystal component; and causing crystal growth by second heat treatment using the first oxide crystal component as a seed to form a second oxide crystal component.
Owner:SEMICON ENERGY LAB CO LTD
Method of manufacturing a semiconductor device
InactiveUS6479333B1TransistorSemiconductor/solid-state device manufacturingCrystallinityCrystal growth
A crystal growth 301 is carried out by diffusing a metal element, and a nickel element is moved into regions 108 and 109 which has been doped with phosphorus. An axis coincident with the moving directions 302 and 303 of the nickel element at this time is made to coincide with an axis coincident with the direction of the crystal growth, and a TFT having the regions as channel forming regions is manufactured. In the path of the region where nickel moved, since high crystallinity is obtained in the moving direction, the TFT having high characteristics can be obtained by this way.
Owner:SEMICON ENERGY LAB CO LTD
Semiconductor device and method for manufacturing the same
ActiveUS20110127522A1High on-off ratioHigh puritySolid-state devicesSemiconductor/solid-state device manufacturingSemiconductor materialsElectron donor
Objects are to provide a semiconductor device for high power application in which a novel semiconductor material having high productivity is used and to provide a semiconductor device having a novel structure in which a novel semiconductor material is used. The present invention is a vertical transistor and a vertical diode each of which has a stacked body of an oxide semiconductor in which a first oxide semiconductor film having crystallinity and a second oxide semiconductor film having crystallinity are stacked. An impurity serving as an electron donor (donor) which is contained in the stacked body of an oxide semiconductor is removed in a step of crystal growth; therefore, the stacked body of an oxide semiconductor is highly purified and is an intrinsic semiconductor or a substantially intrinsic semiconductor whose carrier density is low. The stacked body of an oxide semiconductor has a wider band gap than a silicon semiconductor.
Owner:SEMICON ENERGY LAB CO LTD
Porous drug matrices and methods of manufacture thereof
InactiveUS20050058710A1Fast dissolutionExtended half-lifePowder deliveryGranular deliveryDrugs solutionMicroparticle
Drugs, especially low aqueous solubility drugs, are provided in a porous matrix form, preferably microparticles, which enhances dissolution of the drug in aqueous media. The drug matrices preferably are made using a process that includes (i) dissolving a drug, preferably a drug having low aqueous solubility, in a volatile solvent to form a drug solution, (ii) combining at least one pore forming agent with the drug solution to form an emulsion, suspension, or second solution and hydrophilic or hydrophobic excipients that stabilize the drug and inhibit crystallization, and (iii) removing the volatile solvent and pore forming agent from the emulsion, suspension, or second solution to yield the porous matrix of drug. Hydrophobic or hydrophilic excipients may be selected to stabilize the drug in crystalline form by inhibiting crystal growth or to stabilize the drug in amorphous form by preventing crystallization. The pore forming agent can be either a volatile liquid that is immiscible with the drug solvent or a volatile solid compound, preferably a volatile salt. In a preferred embodiment, spray drying is used to remove the solvents and the pore forming agent. The resulting porous matrix has a faster rate of dissolution following administration to a patient, as compared to non-porous matrix forms of the drug. In a preferred embodiment, microparticles of the porous drug matrix are reconstituted with an aqueous medium and administered parenterally, or processed using standard techniques into tablets or capsules for oral administration.
Owner:ACUSPHERE INC
Method for manufacturing semiconductor device
ActiveUS20120064664A1Stable electrical characteristicsReduce the amount of variationSemiconductor/solid-state device detailsElectroluminescent light sourcesCrystalline oxidePower semiconductor device
An object is to manufacture a semiconductor device including an oxide semiconductor film, which has stable electric characteristics and high reliability. A crystalline oxide semiconductor film is formed, without performing a plurality of steps, as follows: by utilizing a difference in atomic weight of plural kinds of atoms included in an oxide semiconductor target, zinc with low atomic weight is preferentially deposited on an oxide insulating film to form a seed crystal including zinc; and tin, indium, or the like with high atomic weight is deposited on the seed crystal while causing crystal growth. Further, a crystalline oxide semiconductor film is formed by causing crystal growth using a seed crystal with a hexagonal crystal structure including zinc as a nucleus, whereby a single crystal oxide semiconductor film or a substantially single crystal oxide semiconductor film is formed.
Owner:SEMICON ENERGY LAB CO LTD
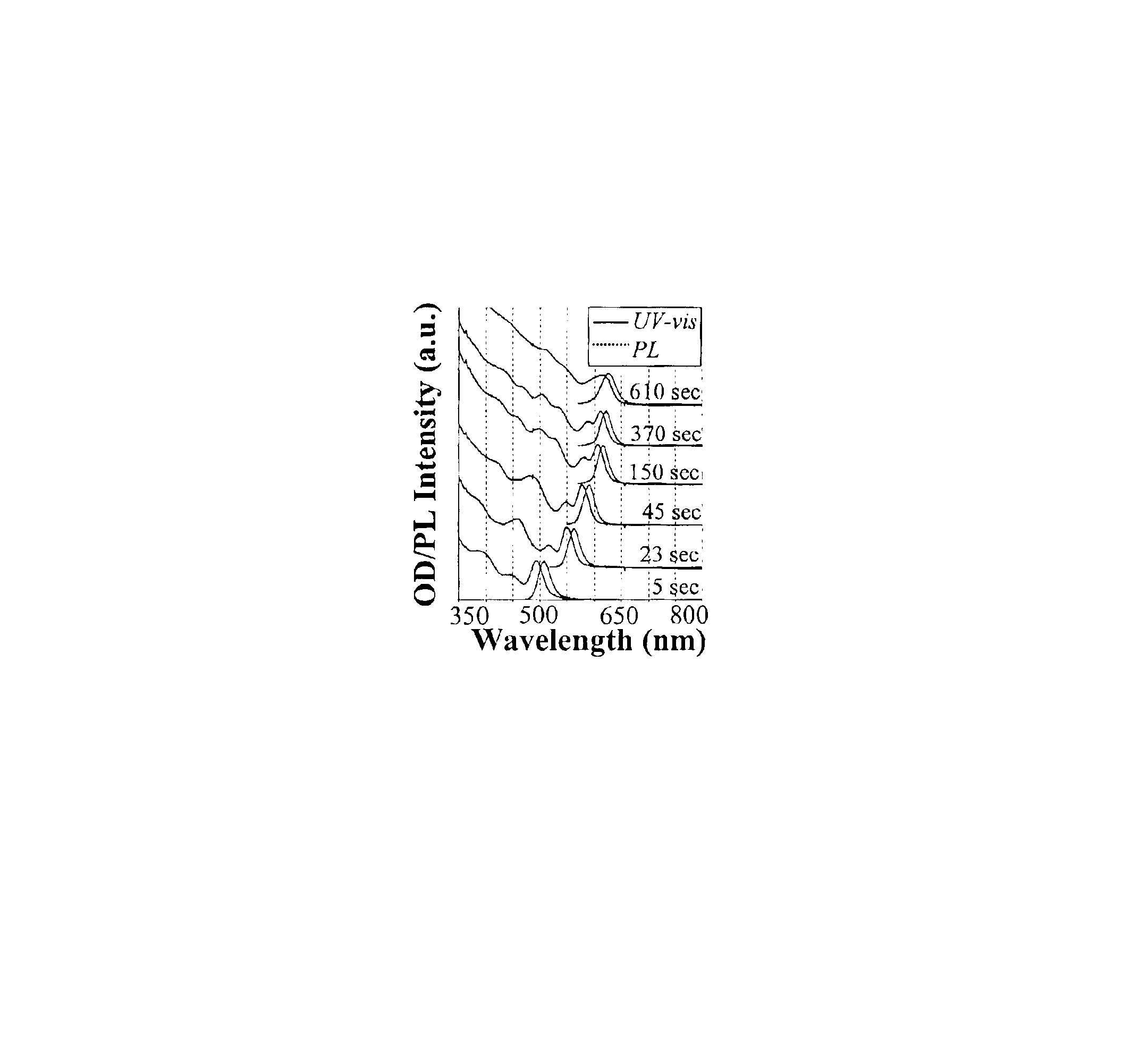
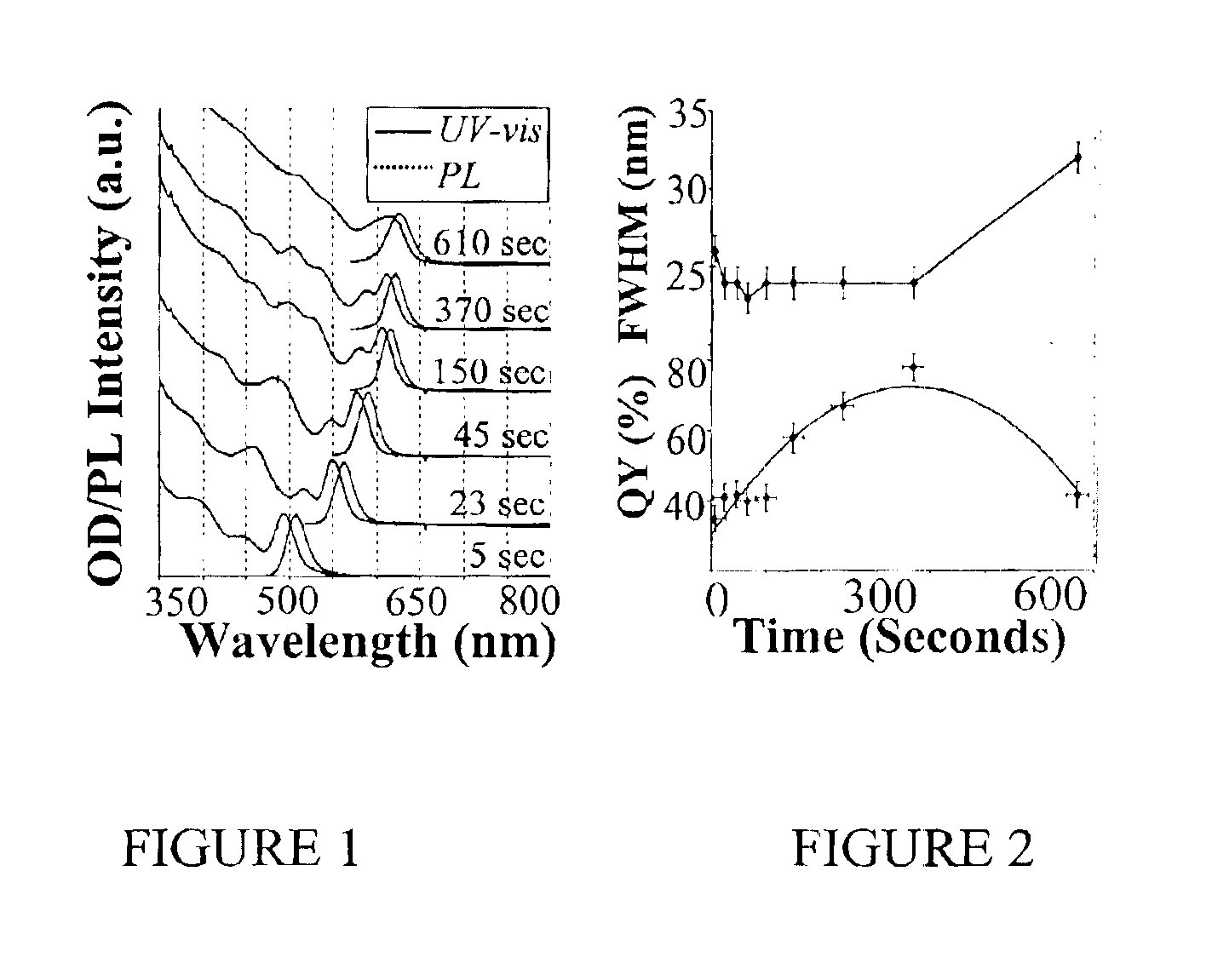
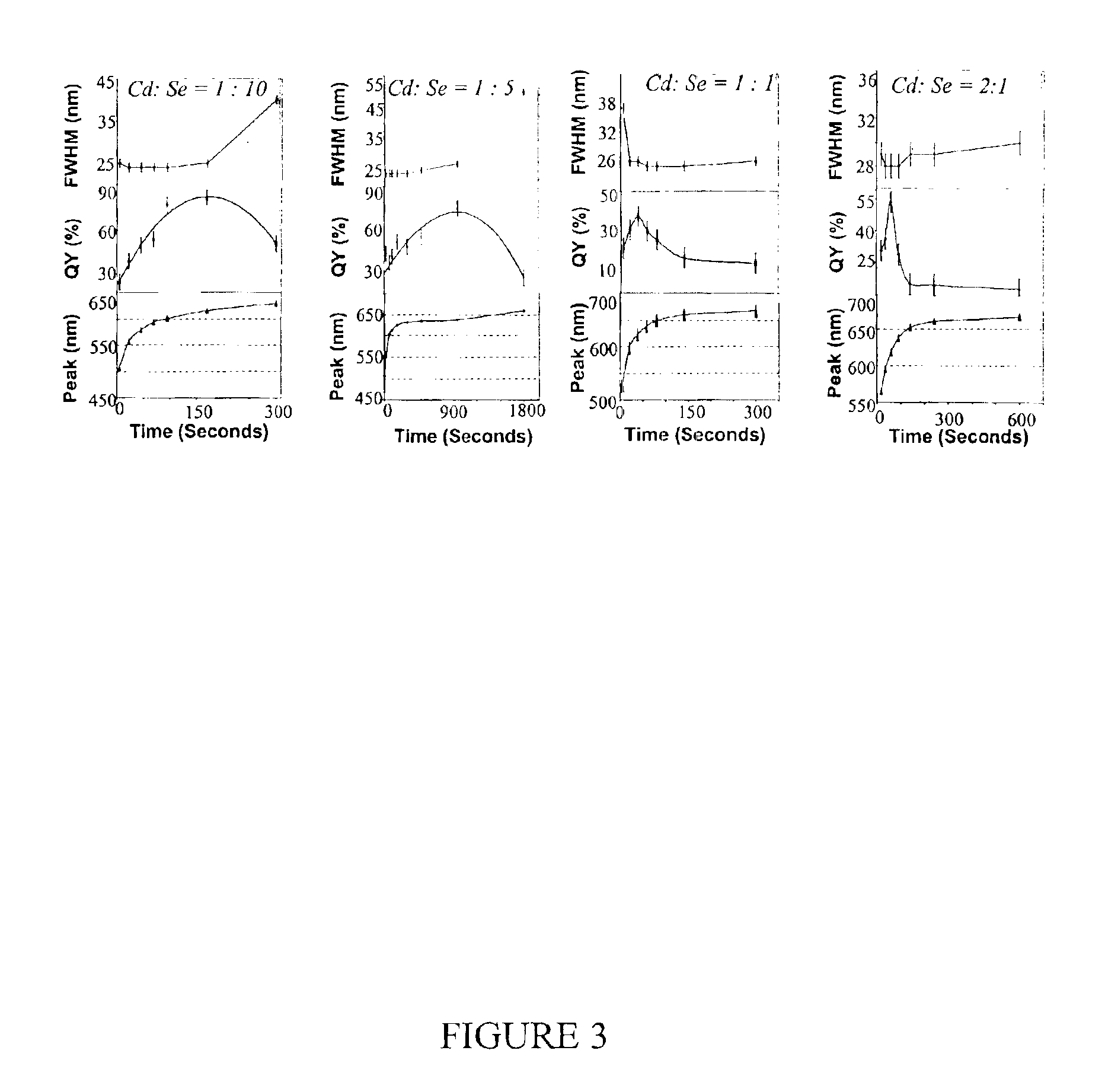
Colloidal nanocrystals with high photoluminescence quantum yields and methods of preparing the same
InactiveUS6869545B2Good monodispersityImprove launch performanceMaterial nanotechnologyNanoopticsQuantum yieldPhotoluminescence
The present invention provides new compositions containing colloidal nanocrystals with high photoluminescence quantum yields, new synthetic methods for the preparation of highly luminescent colloidal nanocrystals, as well as methods to control the photoluminescent properties of colloidal nanocrystals. The new synthetic methods disclosed herein allow photoemission brightness (quantum yield) to be correlated with certain adjustable nanocrystal growth parameters associated with a given synthetic scheme.
Owner:THE BOARD OF TRUSTEES OF THE UNIV OF ARKANSAS
Light Emitting Element and Method of Manufacturing the Same
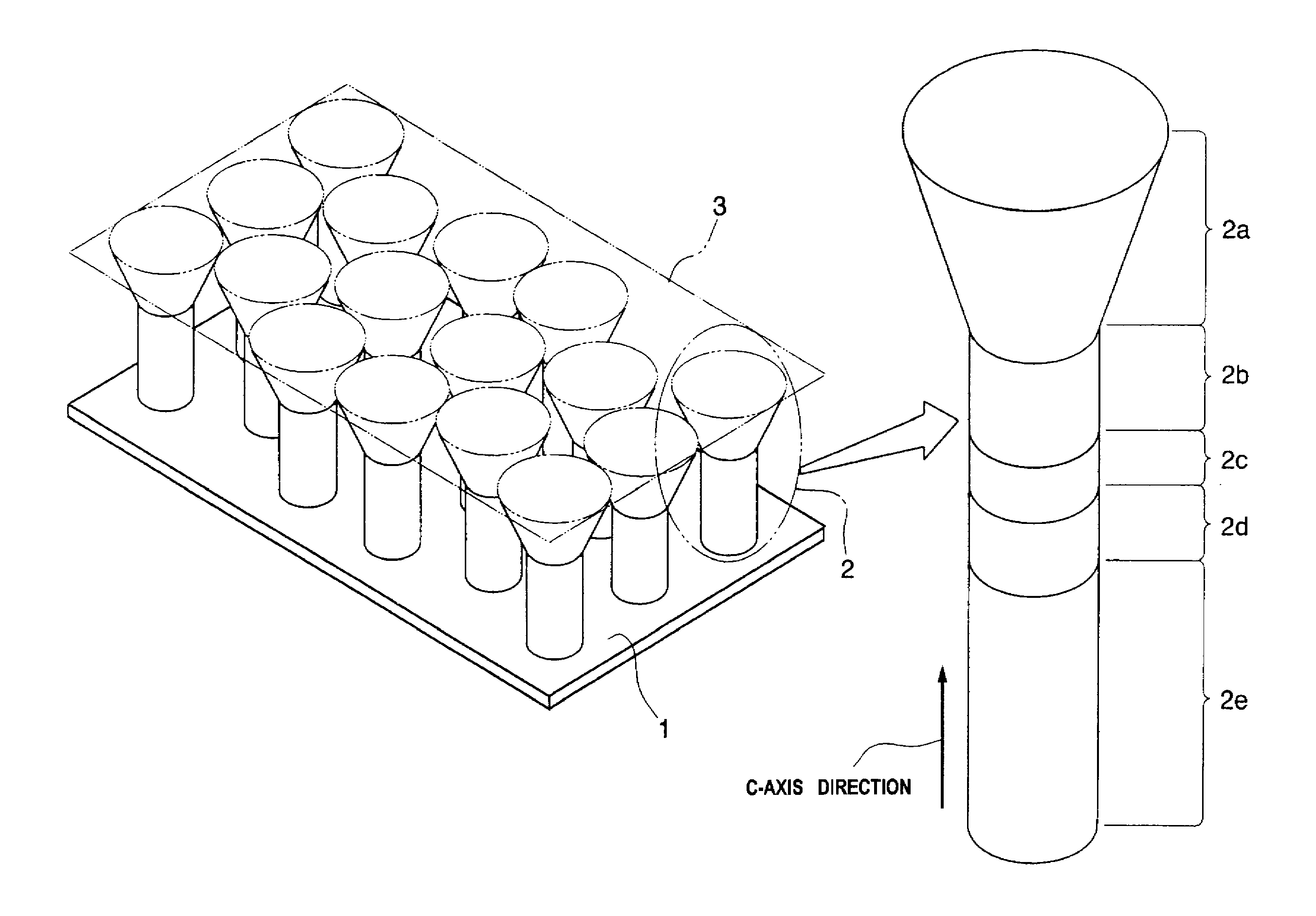
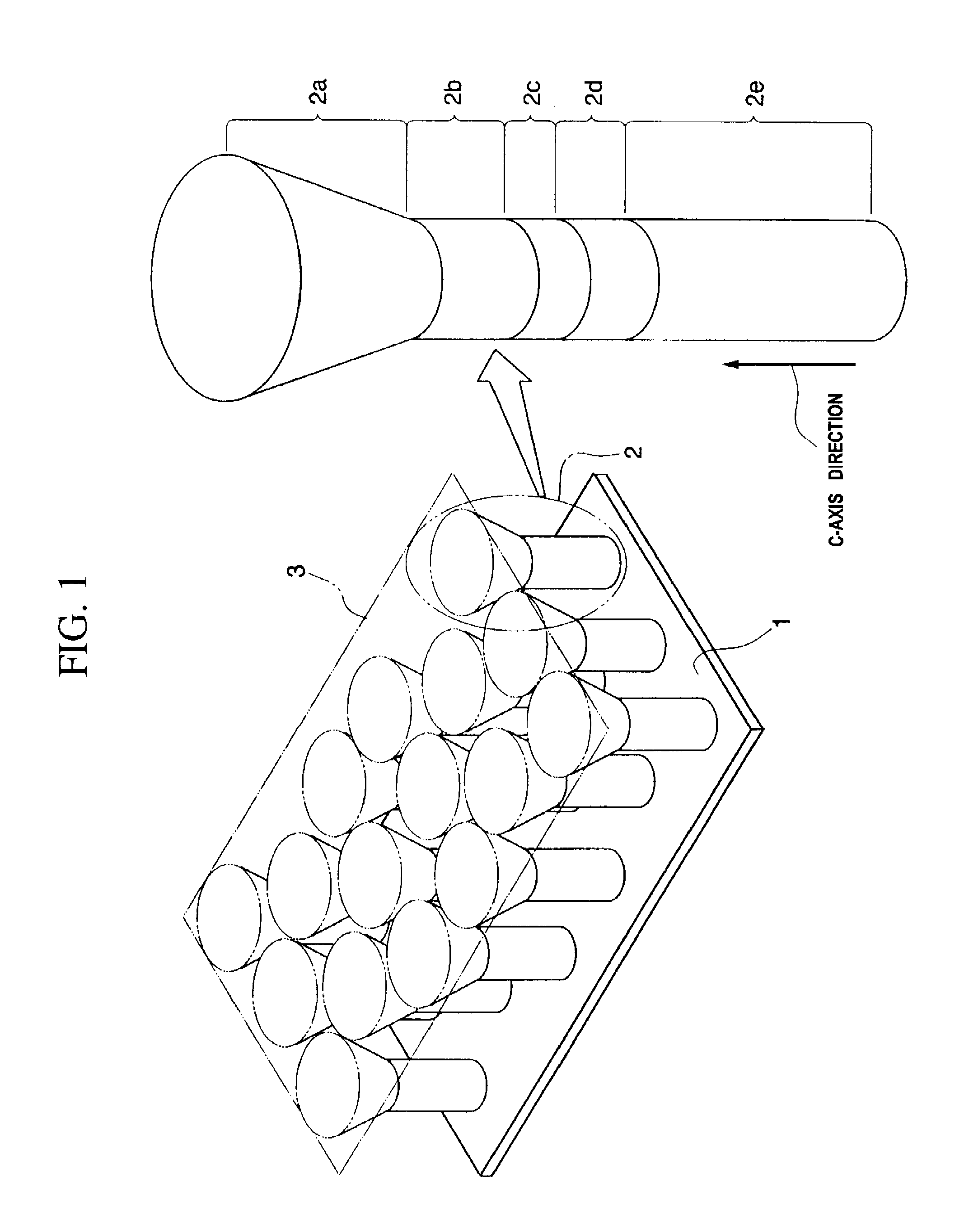
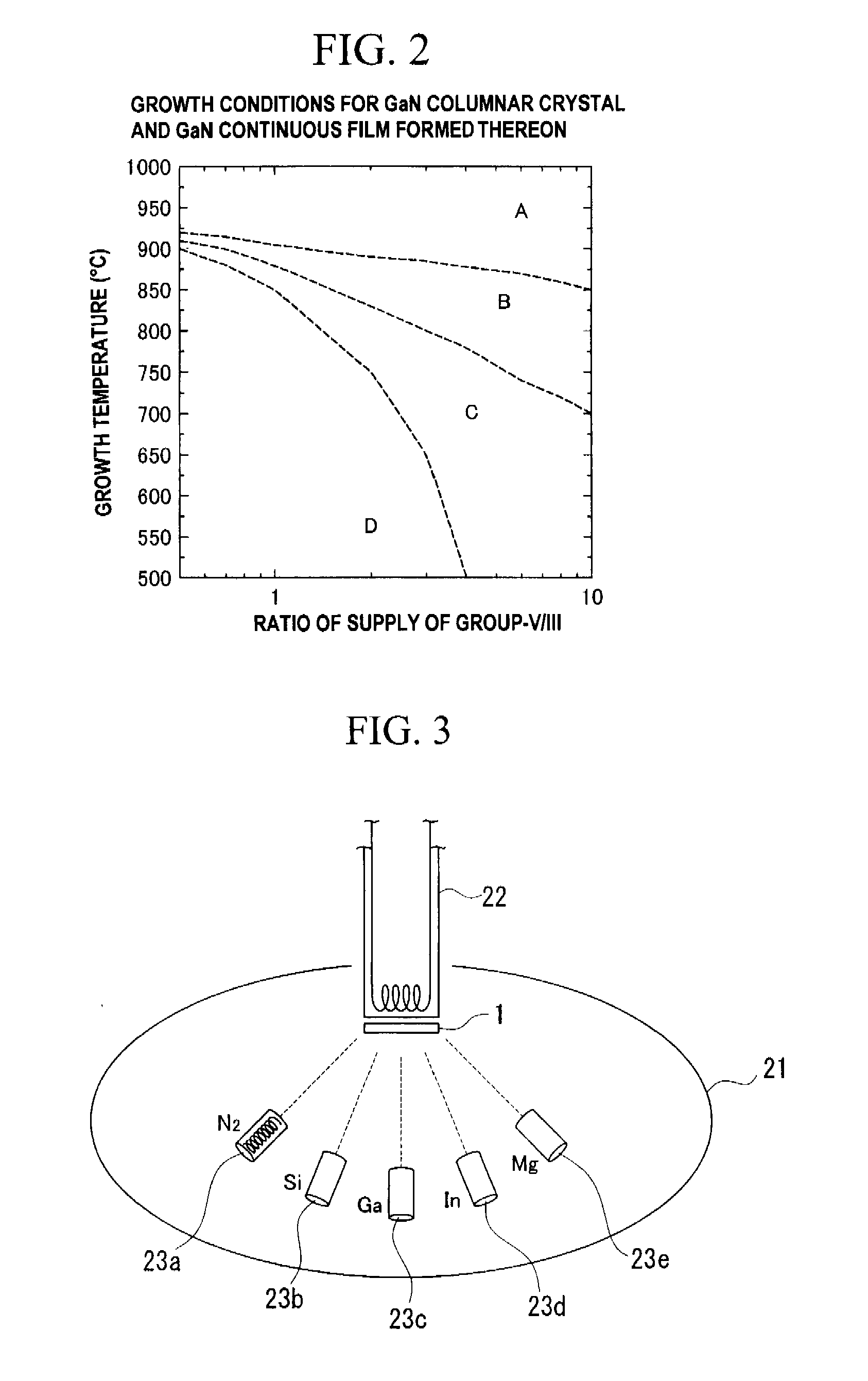
A method of manufacturing a semiconductor element by forming, on a substrate, columnar crystals of a nitride-base or an oxide-base compound semiconductor, and by using the columnar crystals, wherein on the surface of the substrate, the columnar crystals are grown while ensuring anisotropy in the direction of c-axis, by controlling ratio of supply of Group-III atoms and nitrogen, or Group-II atoms and oxygen atoms, and temperature of crystal growth, so as to suppress crystal growth in the lateral direction on the surface of the substrate.
Owner:SOPHIA UNIVERSITY
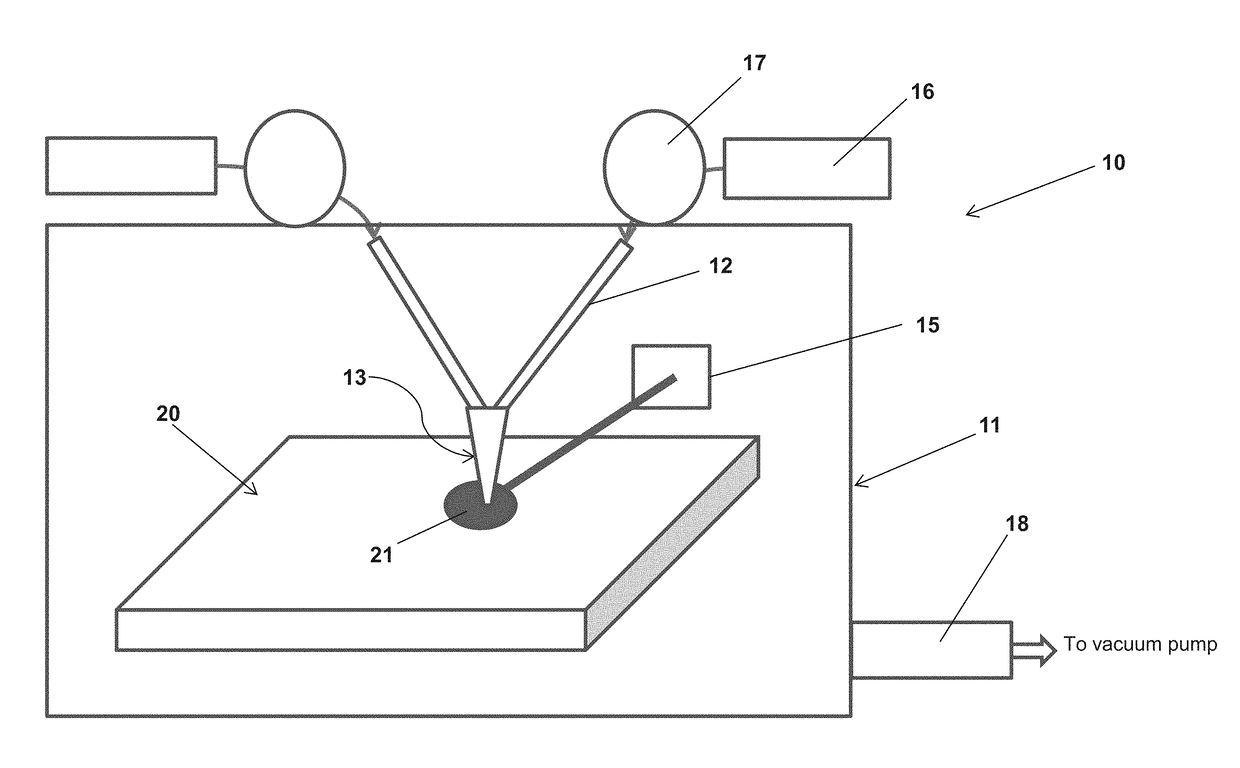
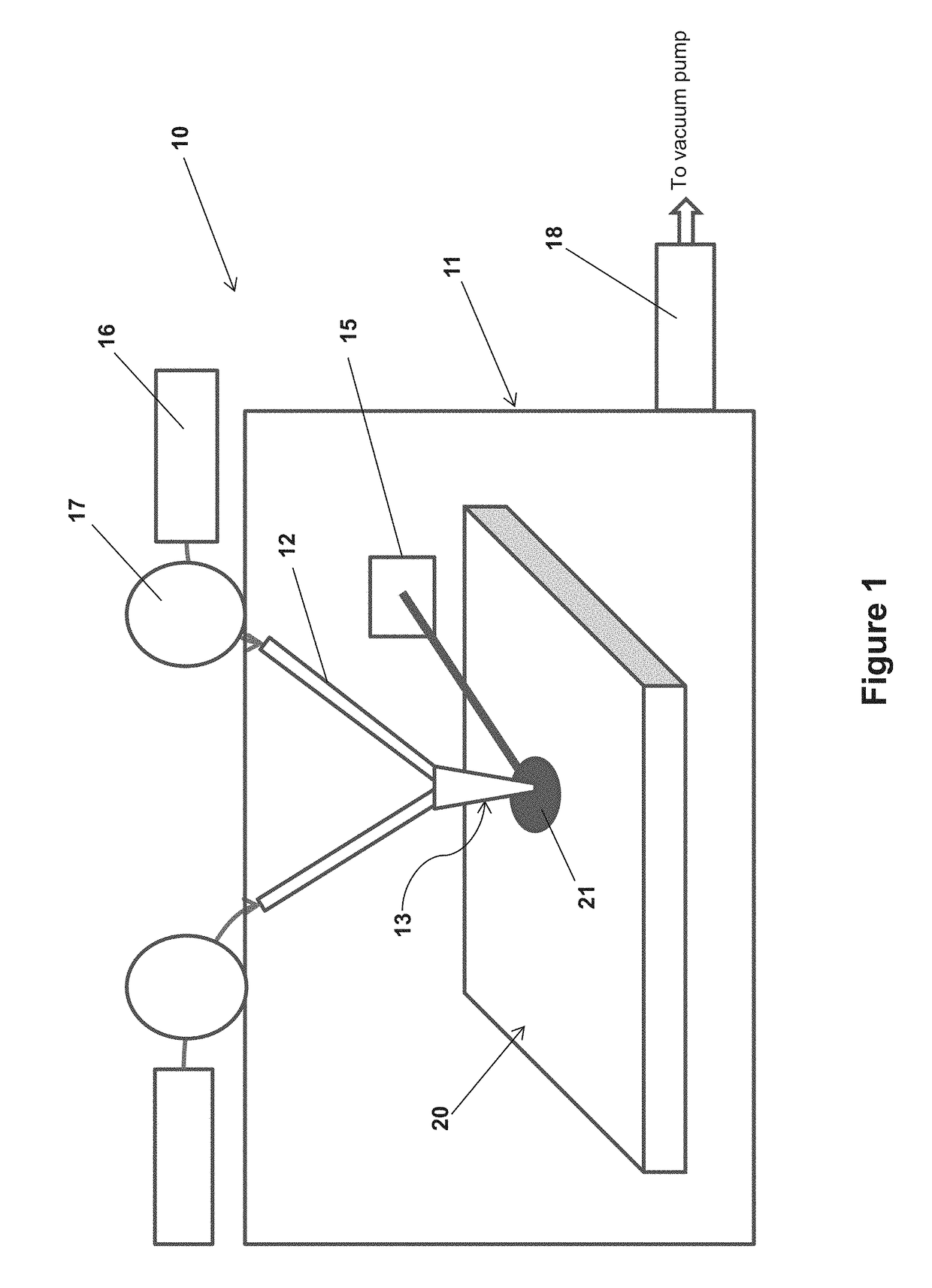
Method and apparatus for crystal growth using a membrane-assisted semi-closed reactor
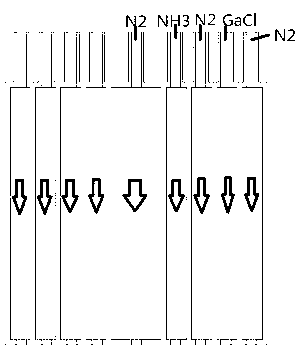
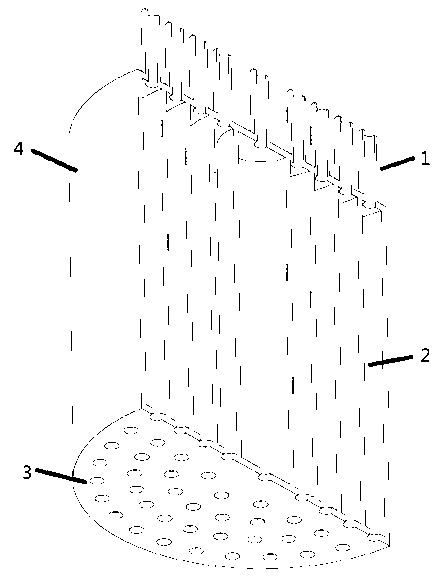
InactiveUS20120291696A1Polycrystalline material growthFrom chemically reactive gasesTemperature controlVapor phase
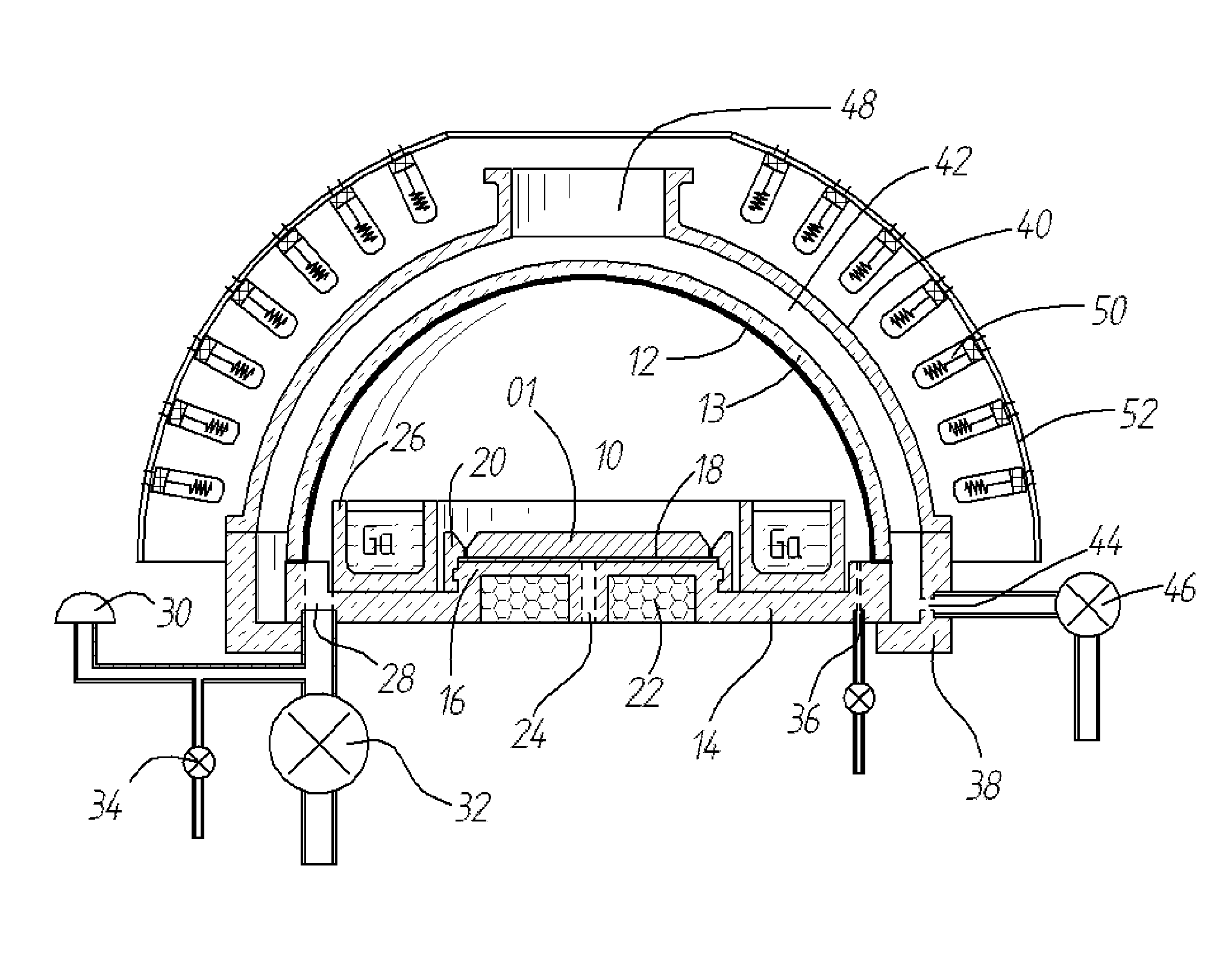
A method and apparatus for depositing III-V material is provided. The apparatus includes a reactor partially enclosed by a selectively permeable membrane 12. A means is provided for generating source vapors, such as a vapor-phase halide of a group III element (IUPAC group 13) within the reactor volume 10, and an additional means is also provided for introducing a vapor-phase hydride of a group V element (IUPAC group 15) into the volume 10. The reaction of the group III halide and the group V hydride on a temperature-controlled substrate 18 within the reactor volume 10 produces crystalline III-V material and hydrogen gas. The hydrogen is preferentially removed from the reactor through the selectively permeable membrane 12, thus avoiding pressure buildup and reaction imbalance. Other gases within the reactor are unable to pass through the selectively permeable membrane.
Owner:CLARKE ANDREW PETER
Apparatus for producing single crystal and quasi-single crystal, and associated method
An apparatus including a crucible, an energy source, and a controller is provided. The crucible may be sealed to a nitrogen-containing gas, and may be chemically inert to at least ammonia at a temperature in a range of about 400 degrees Celsius to about 2500 degrees Celsius. The energy source may supply thermal energy to the crucible. The controller may control the energy source to selectively direct sufficient thermal energy to a predefined first volume within the crucible to attain and maintain a temperature in the first volume to be in a range of from about 400 degrees Celsius to about 2500 degrees Celsius. The thermal energy may be sufficient to initiate, sustain, or both initiate and sustain growth of a crystal in the first volume. The first temperature in the first volume may be controllable separately from a second temperature in another volume within the crucible. The first temperature and the second temperature differ from each other. Associated methods are provided.
Owner:SLT TECH
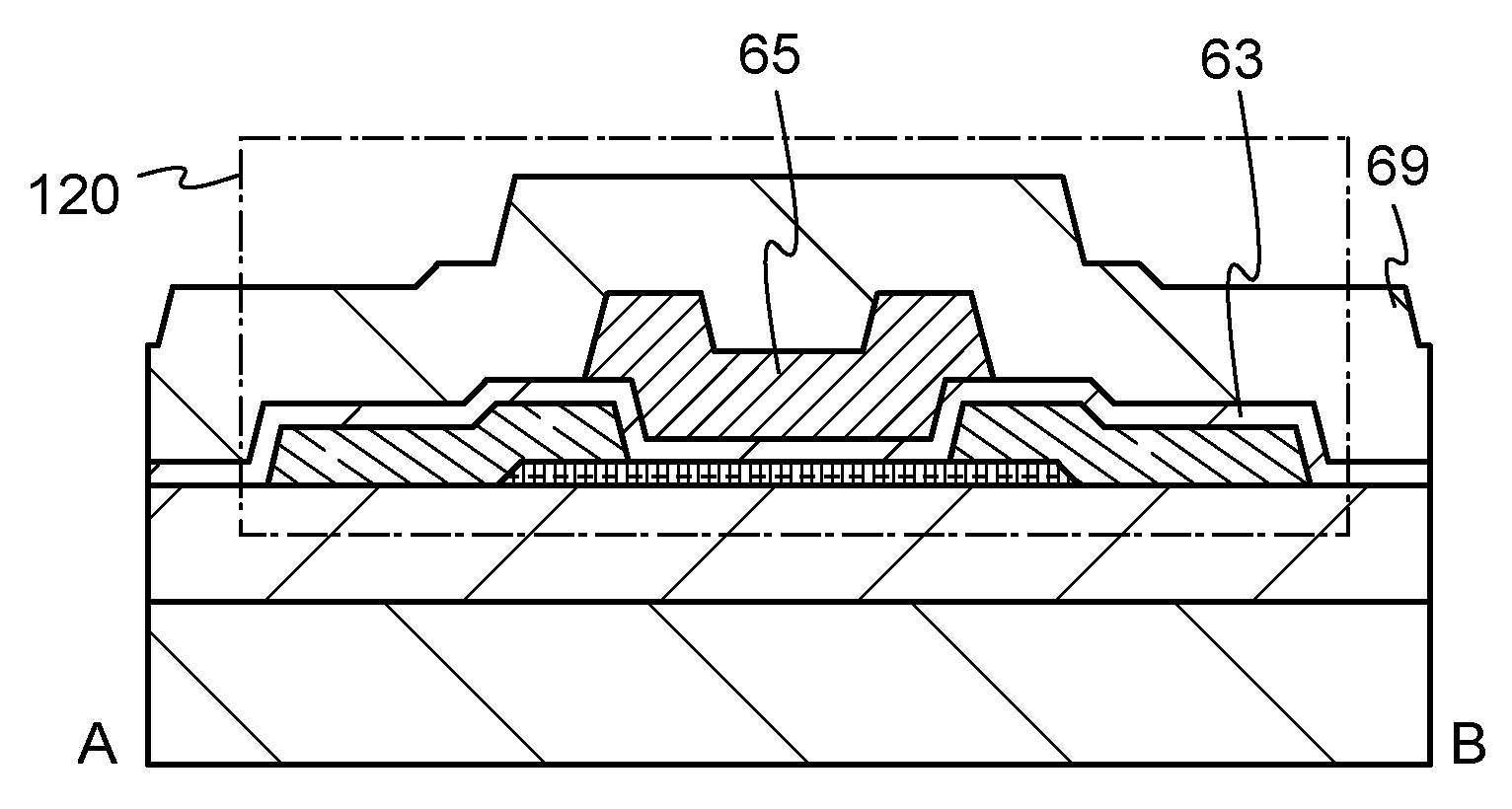
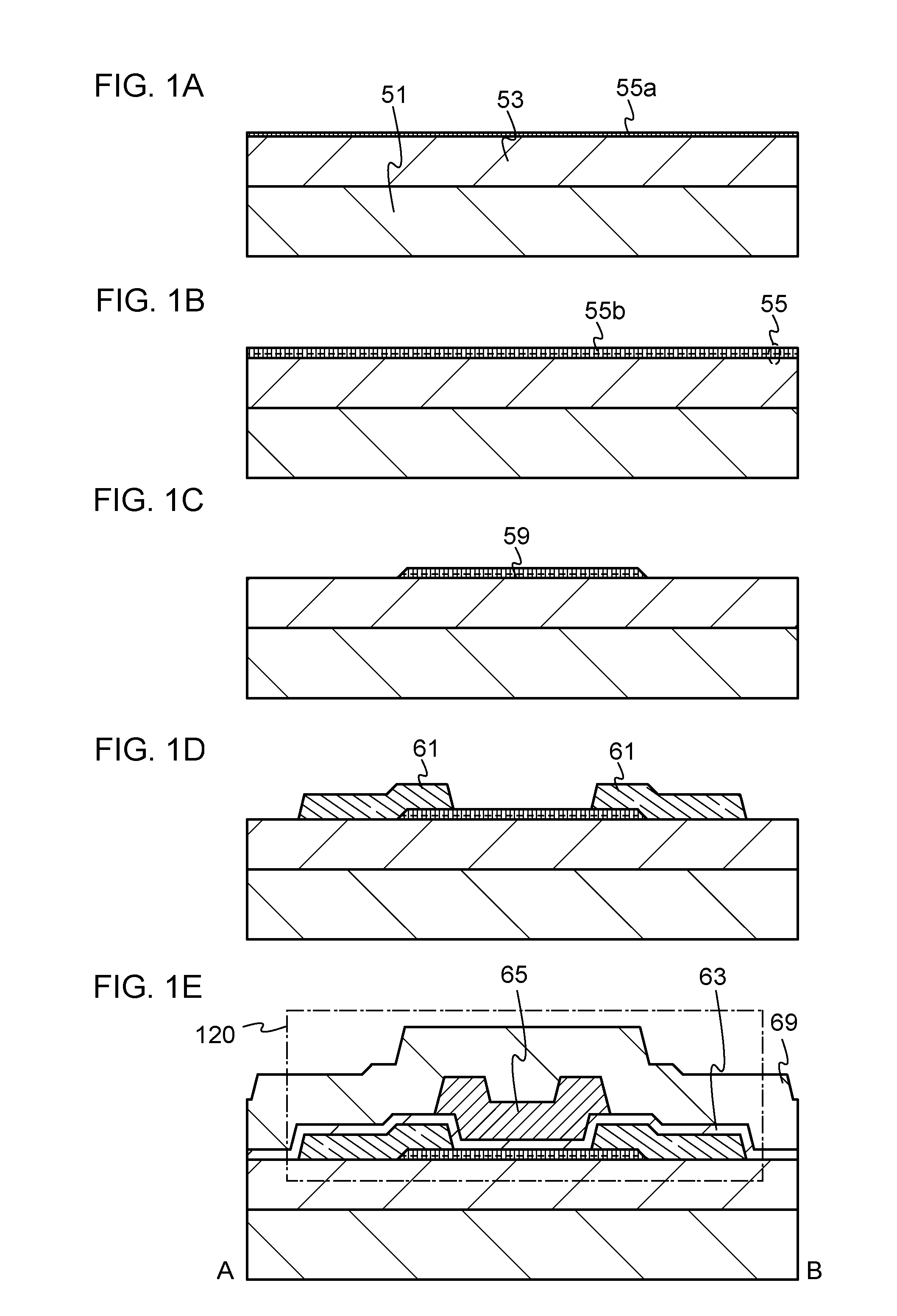
Nitride semiconductor, method for manufacturing the same and nitride semiconductor device
A facet-forming layer made of nitride semiconductor containing at least aluminum is formed on a substrate made of gallium nitride (GaN). A facet surface inclined with respect to a C-surface is formed on the surface of the facet-forming layer, and a selective growth layer laterally grows from the inclined facet surface. As a result, the selective growth layer can substantially lattice-match an n-type cladding layer made of n-type AlGaN and grown on the selective growth layer. For example, a laser structure without cracks being generated can be obtained by crystal growth.
Owner:PANASONIC CORP
Crystal growth devices and systems, and methods for using same
High throughput screening of crystallization of a target material is accomplished by simultaneously introducing a solution of the target material into a plurality of chambers of a microfabricated fluidic device. The microfabricated fluidic device is then manipulated to vary the solution condition in the chambers, thereby simultaneously providing a large number of crystallization environments. Control over changed solution conditions may result from a variety of techniques, including but not limited to metering volumes of crystallizing agent into the chamber by volume exclusion, by entrapment of volumes of crystallizing agent determined by the dimensions of the microfabricated structure, or by cross-channel injection of sample and crystallizing agent into an array of junctions defined by intersecting orthogonal flow channels.
Owner:FLUIDIGM CORP
Nitride semiconductor device, its manufacturing method, and semiconductor optical apparatus
InactiveUS20050042787A1Stress minimizationRelaxation stressLaser detailsSemiconductor laser structural detailsHigh densityCrystallographic defect
A nitride semiconductor laser device with a reduction in internal crystal defects and an alleviation in stress, and a semiconductor optical apparatus comprising this nitride semiconductor laser device. First, a growth suppressing film against GaN crystal growth is formed on the surface of an n-type GaN substrate equipped with alternate stripes of dislocation concentrated regions showing a high density of crystal defects and low-dislocation regions so as to coat the dislocation concentrate regions. Next, the n-type GaN substrate coated with the growth suppressing film is overlaid with a nitride semiconductor layer by the epitaxial growth of GaN crystals. Further, the growth suppressing film is removed to adjust the lateral distance between a laser waveguide region and the closest dislocation concentrated region to 40 μm or more.
Owner:SHARP KK +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com