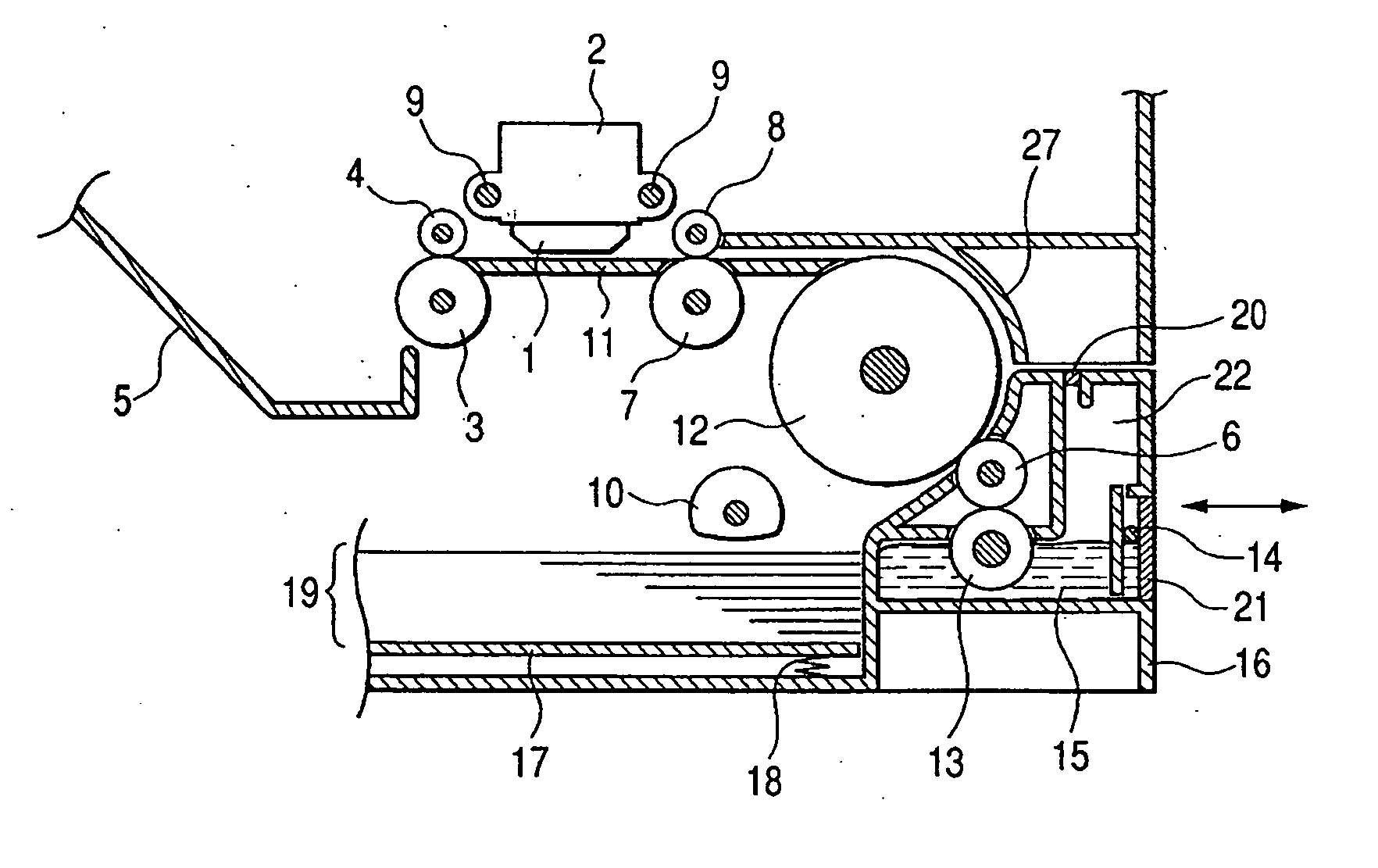
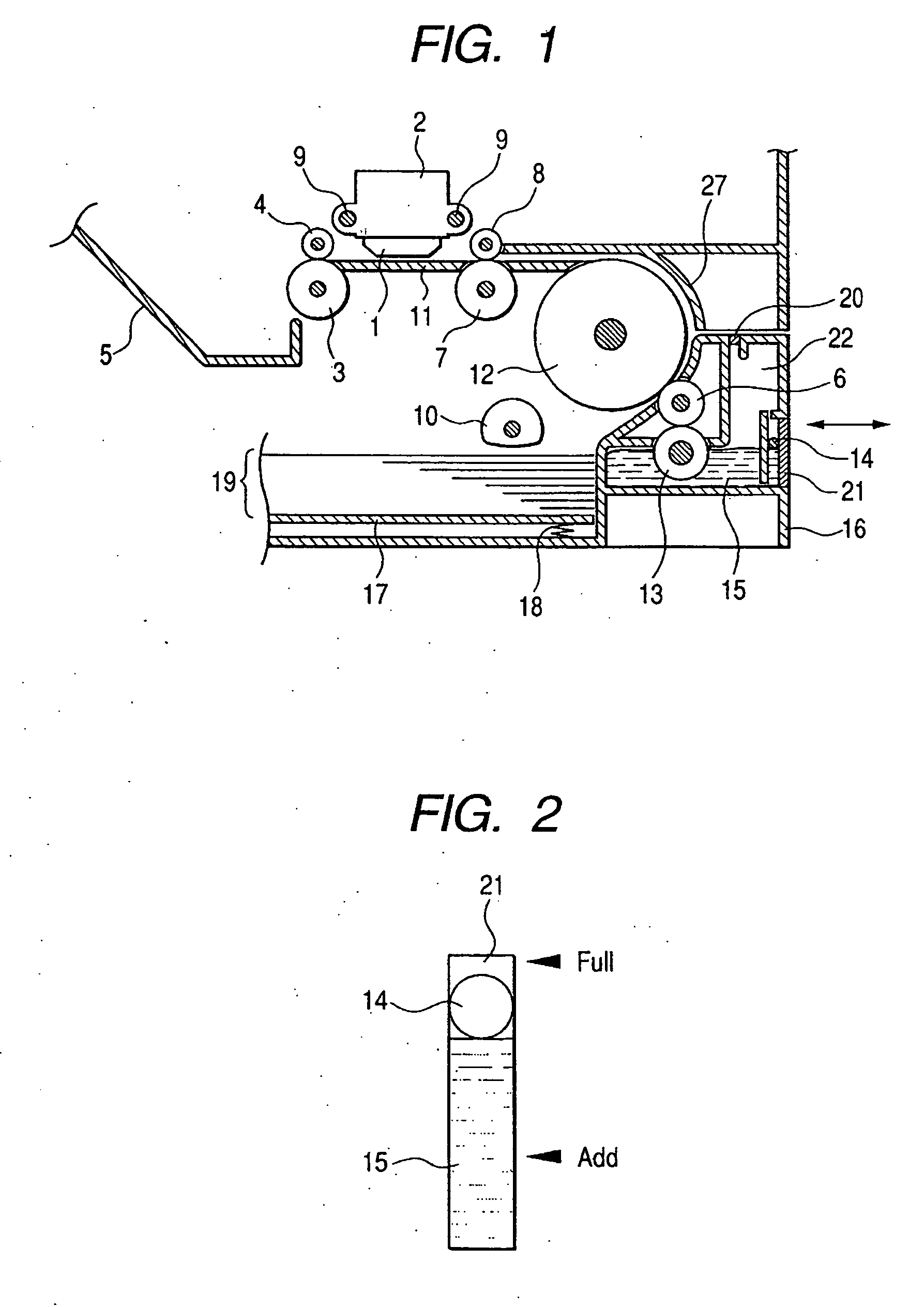
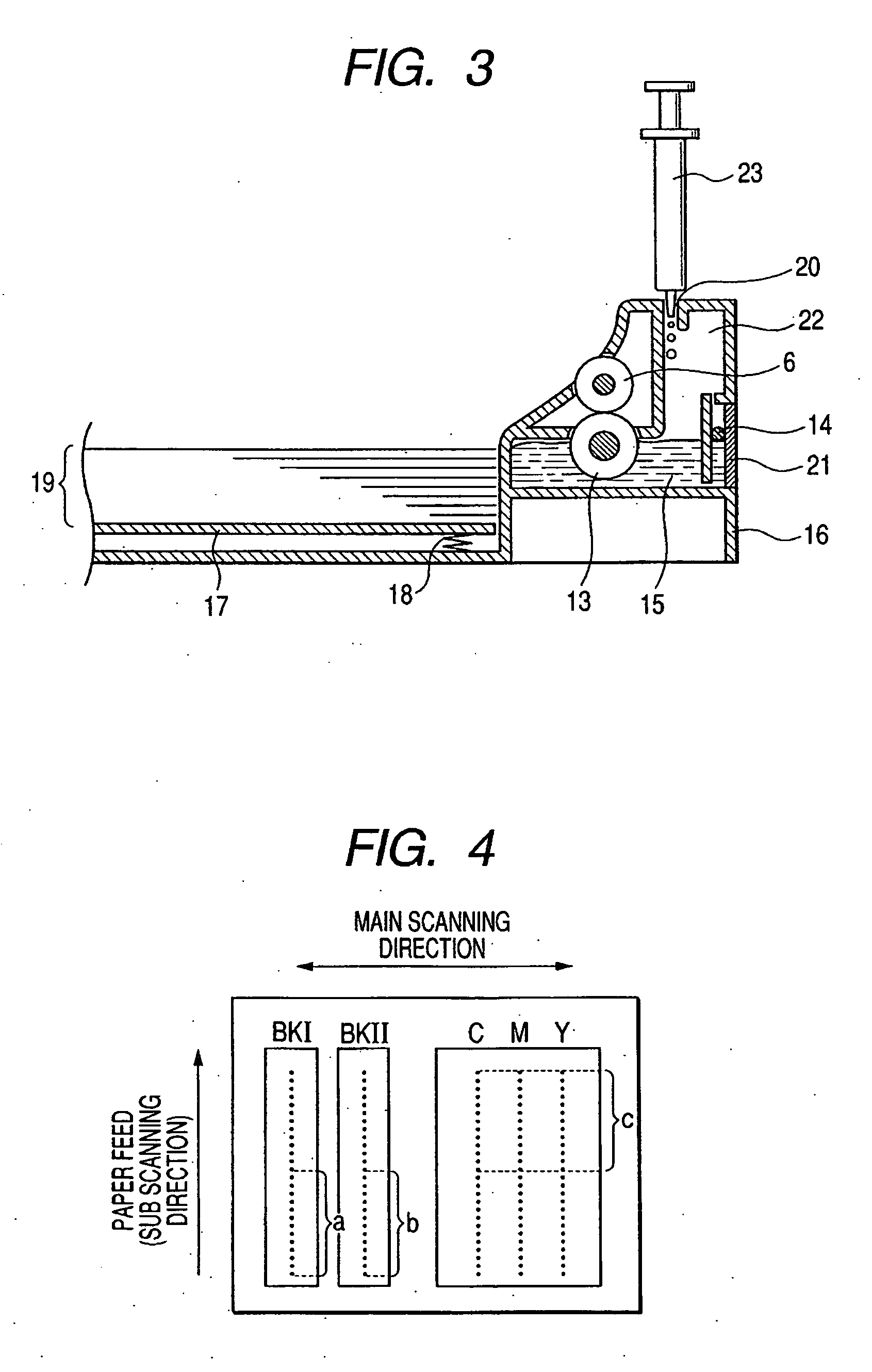
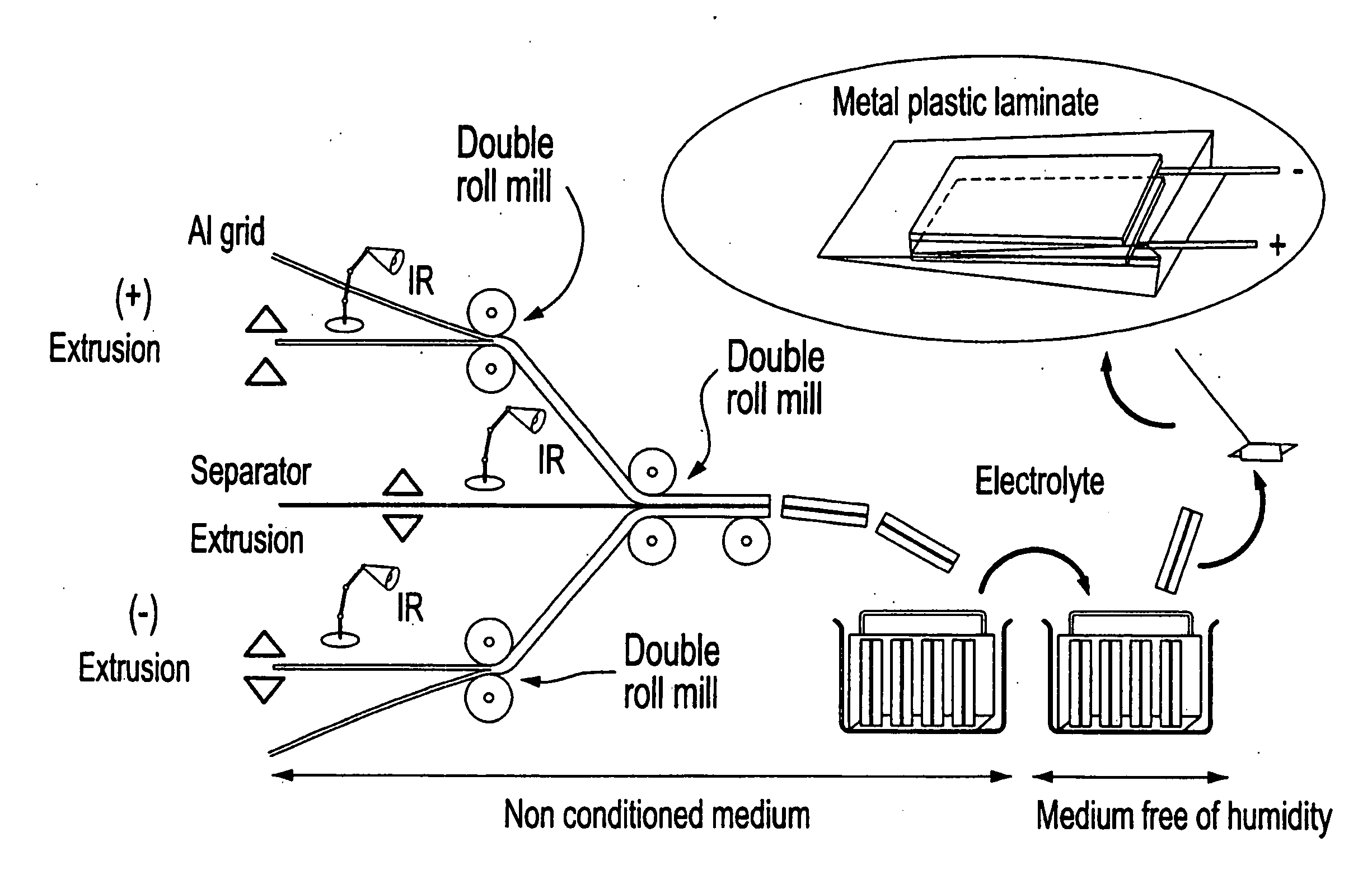
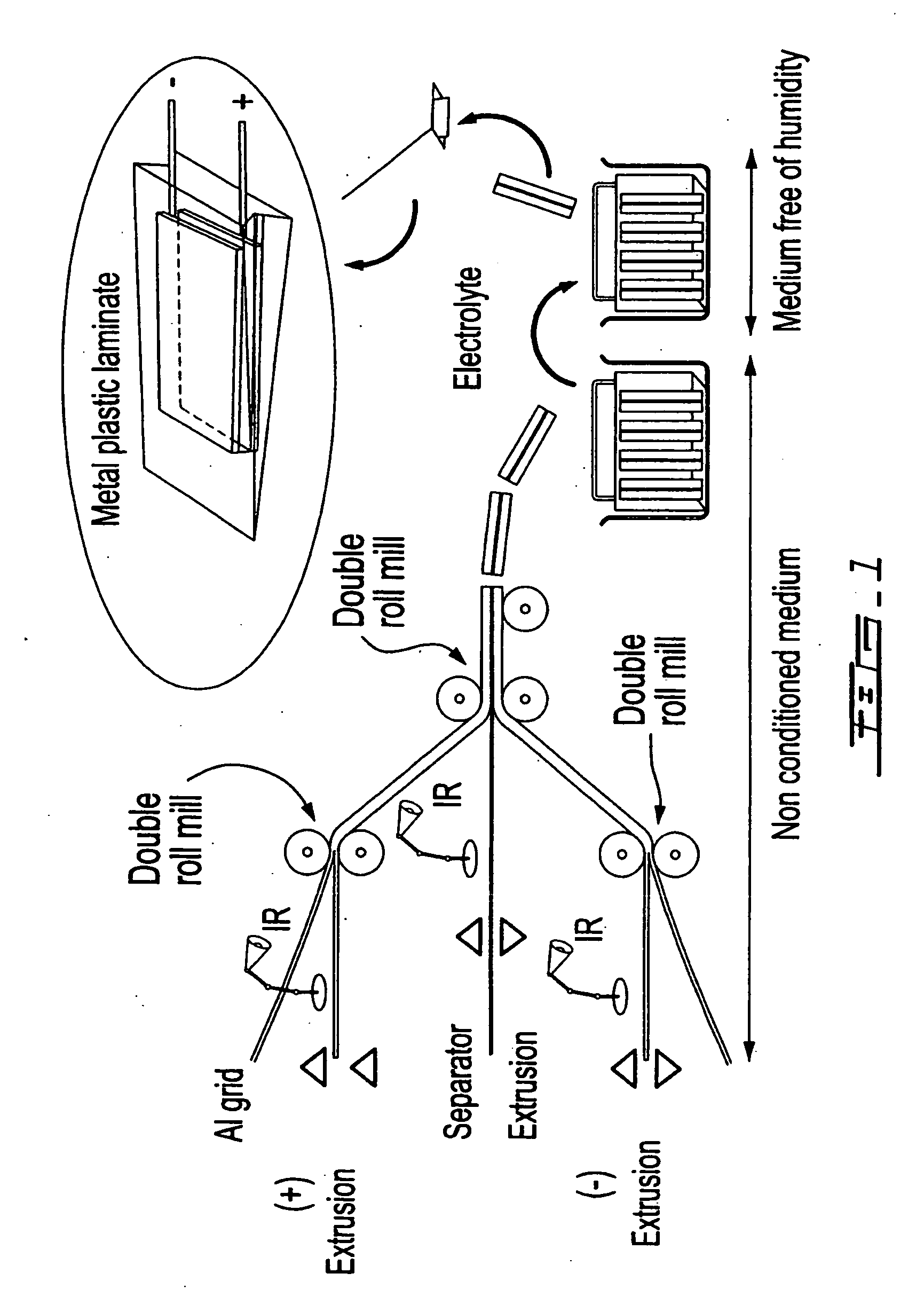
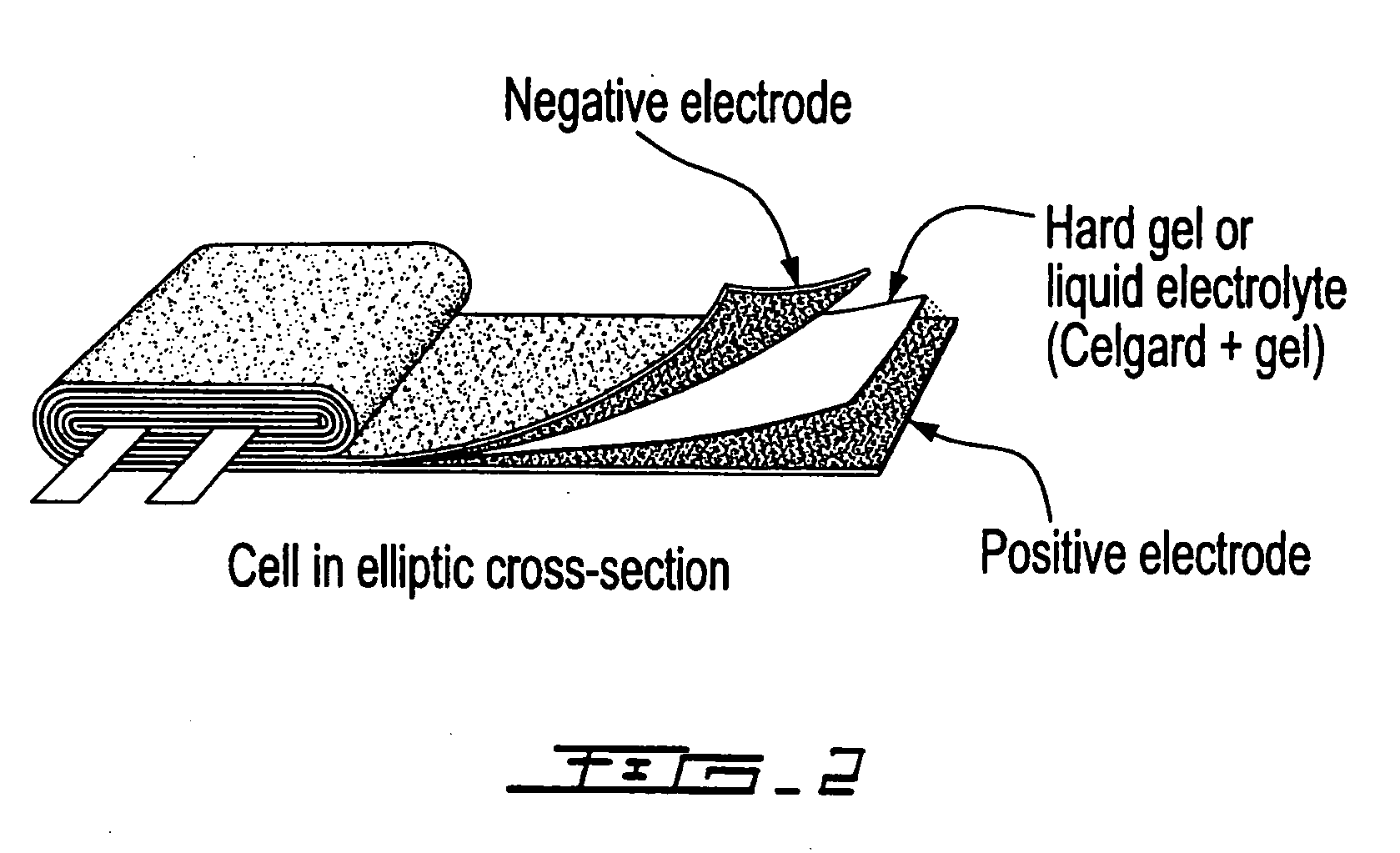
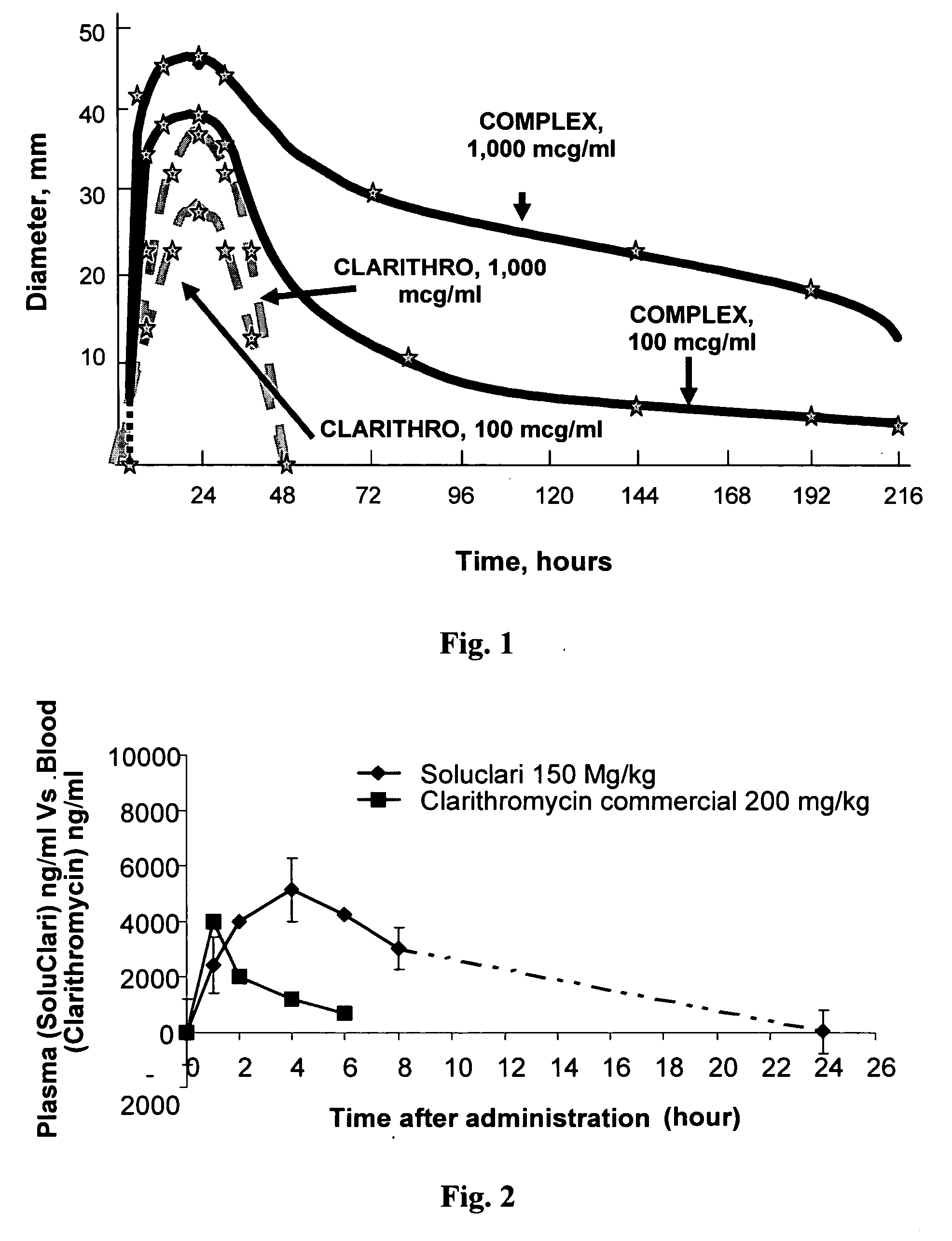
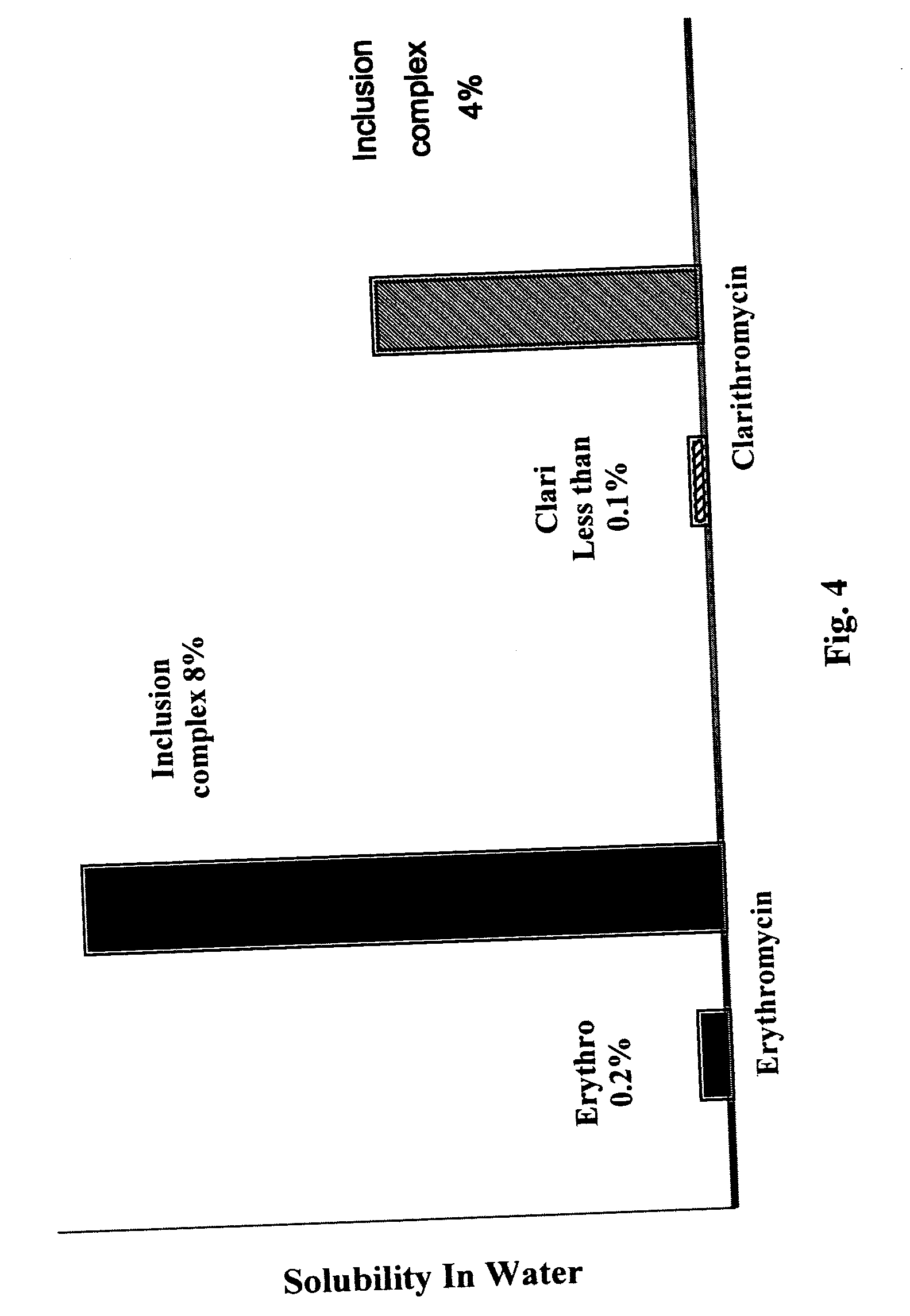
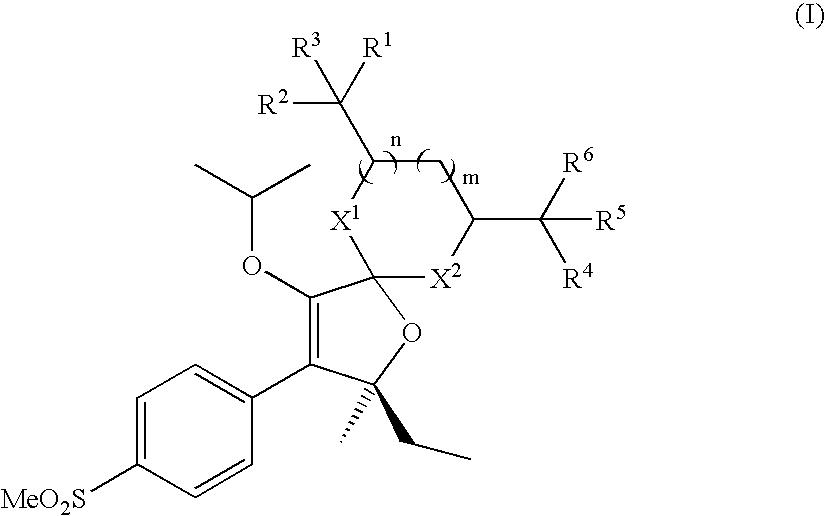
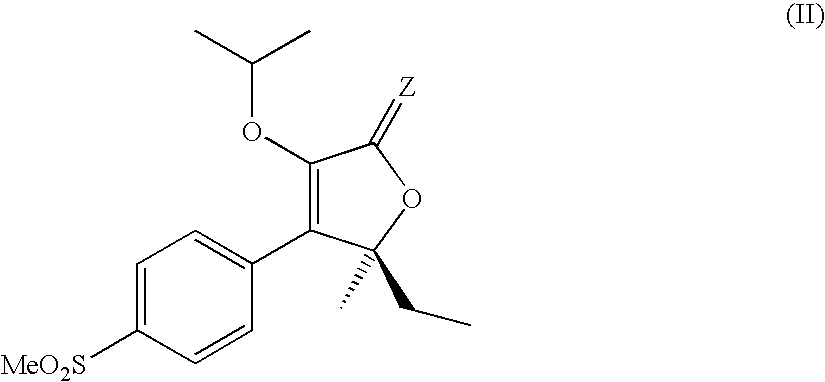
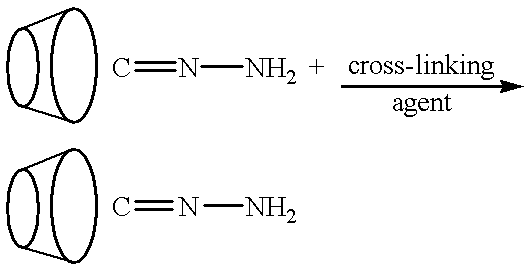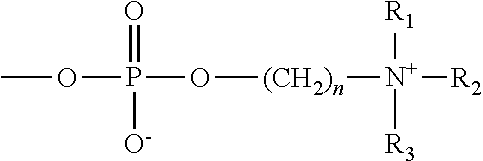Patents
Literature
10981 results about "Aqueous solubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cyclodextrin polymers for carrying and releasing drugs
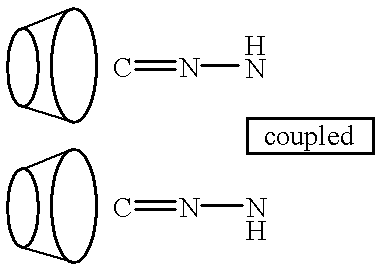
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Generating Acid Downhole in Acid Fracturing
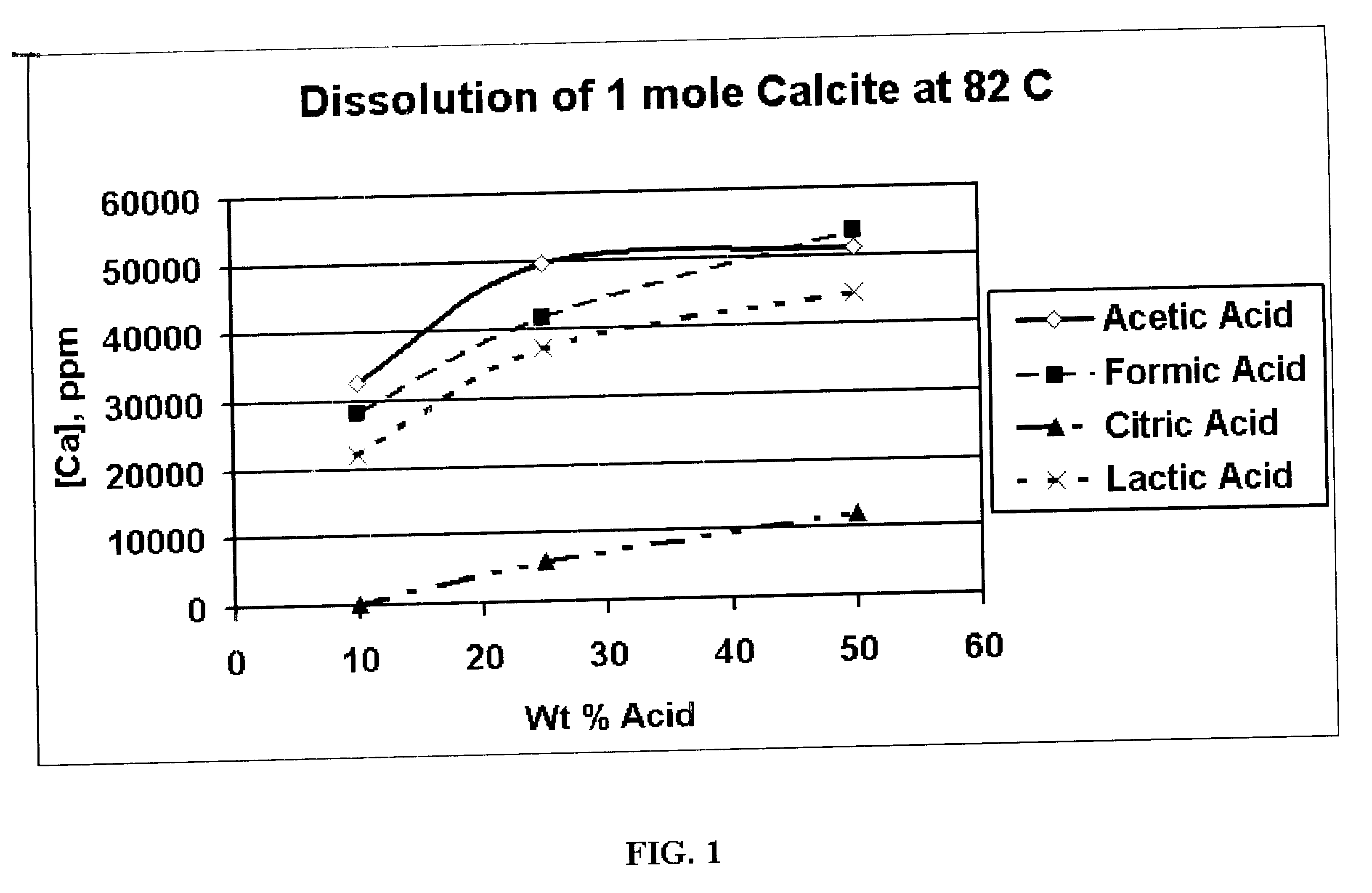
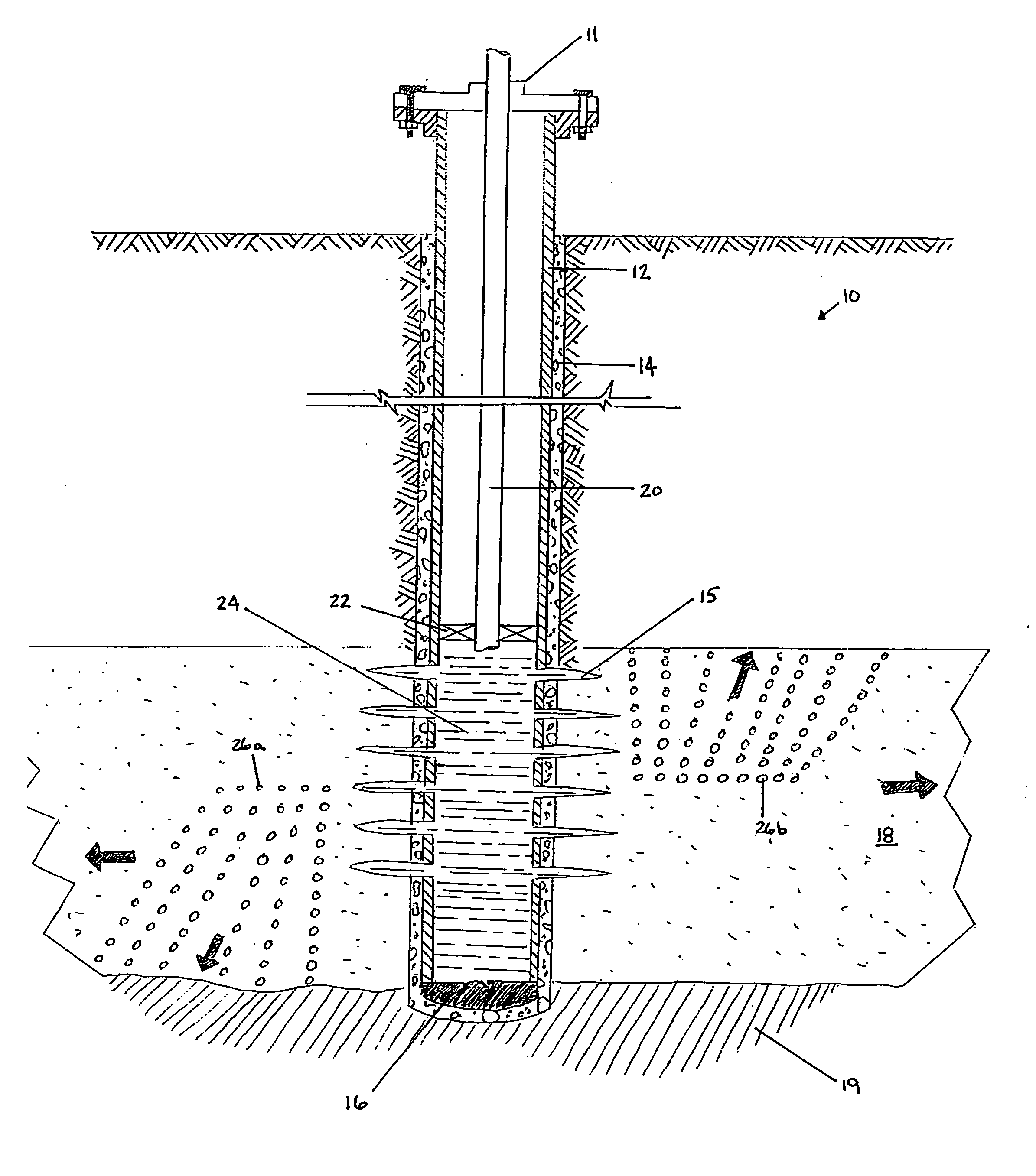
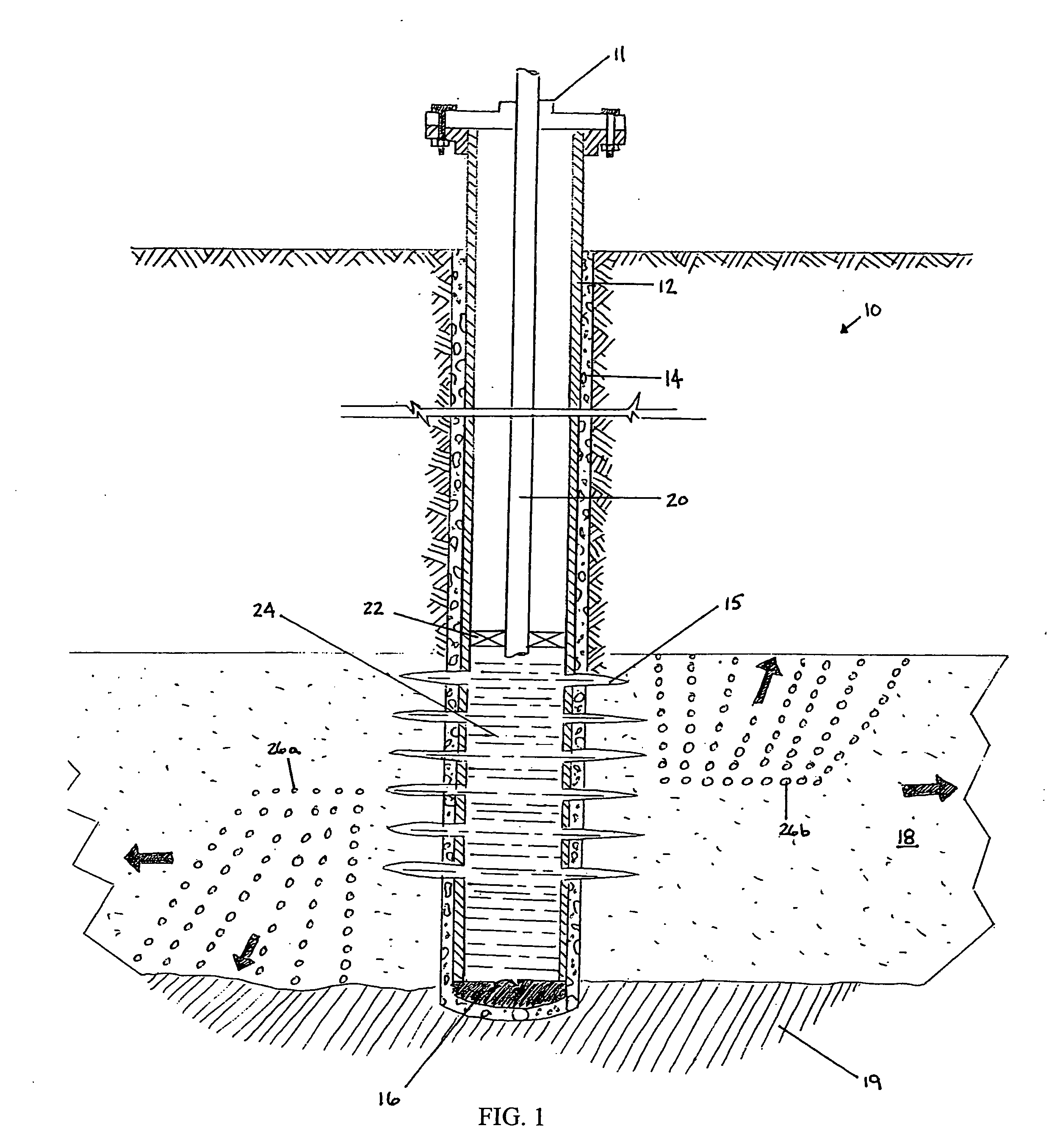
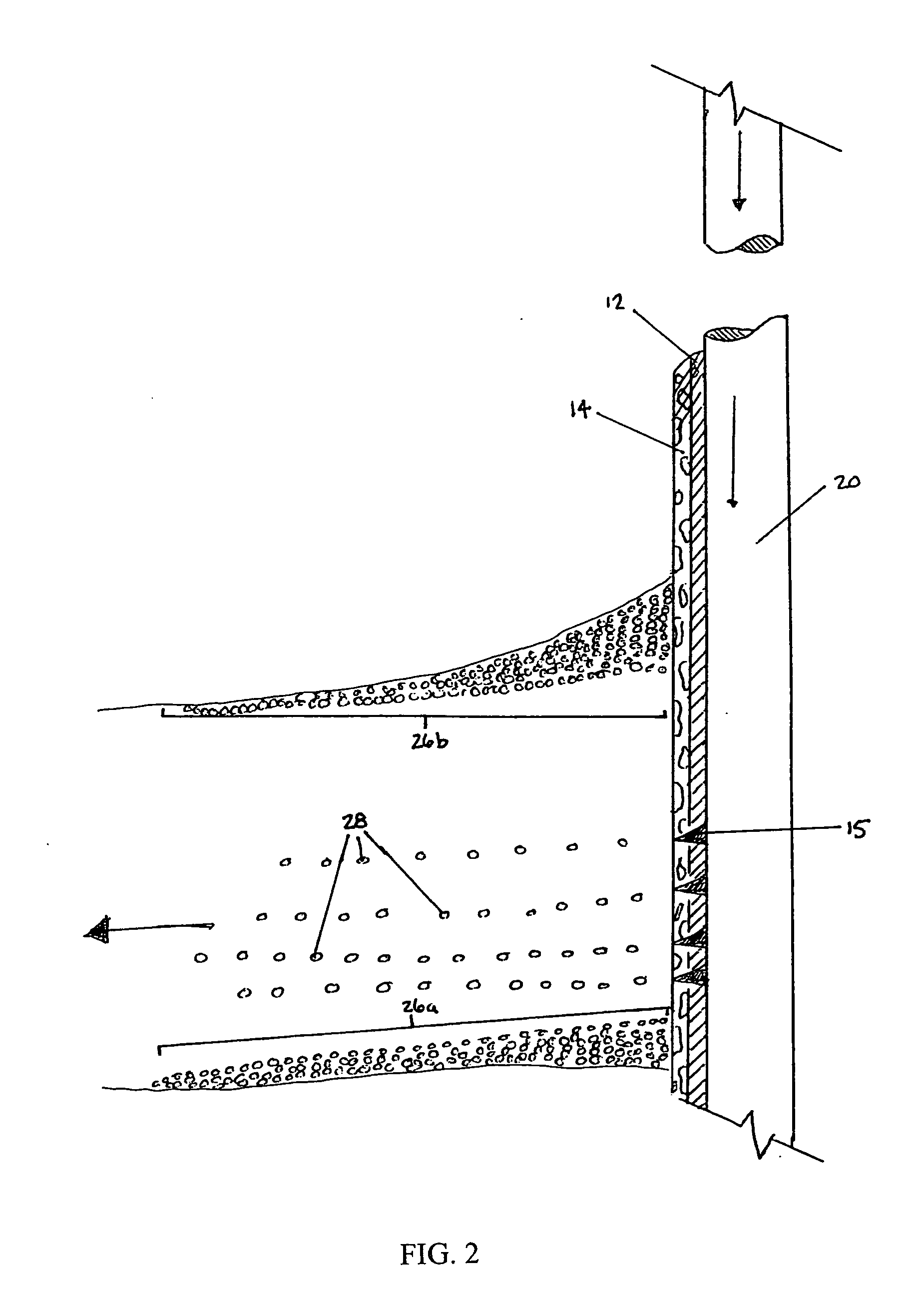
An acid fracturing method is provided in which the acid is generated in the fracture by hydrolysis of a solid acid-precursor selected from one or more than one of lactide, glycolide, polylactic acid, polyglycolic acid, a copolymer of polylactic acid and polyglycolic acid, a copolymer of glycolic acid with other hydroxy-, carboxylic acid-, or hydroxycarboxylic acid-containing moieties, and a copolymer of lactic acid with other hydroxy-, carboxylic acid or hydroxycarboxylic acid-containing moieties. The solid acid-precursor may be mixed with a solid acid-reactive material to accelerate the hydrolysis and / or coated to slow the hydrolysis. Water-soluble liquid compounds are also given that accelerate the hydrolysis. The method ensures that the acid contacts fracture faces far from the wellbore.
Owner:SCHLUMBERGER TECH CORP
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
Soluble deverting agents
Methods and compositions for stimulating single and multiple intervals in subterranean wells by diverting well treatment fluids into a particular direction or into multiple intervals using water soluble coated diverting agents are described. The water soluble coating of the diverting material is preferably a collagen, poly(alkylene) oxide, poly(lactic acid), polyvinylacetate, polyvinylalcohol, polyvinylacetate / polyvinylalcohol polymer or a mixture thereof applied as a coating on any number of proppants. The method allows for the diverting of the flow of fluids in a downhole formation during a well treatment, such as during a fracturing process. Following completion of a treatment such as a hydraulic stimulation, the soluble diverting agent can be dissolved and removed by the water component of the well production.
Owner:FAIRMOUNT SANTROL
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20120026457A1Increased durabilityPicture framesSpecial ornamental structuresSilicone hydrogelWater soluble
Owner:ALCON INC
Fast dissolving orally consumable films
InactiveUS6923981B2Dissolve fastGood curative effectAntibacterial agentsCosmetic preparationsPolymer sciencePullulan
Physiologically acceptable films, including edible films, are disclosed. The films include a water soluble film-forming polymer such as pullulan. Edible films are disclosed that include pullulan and antimicrobially effective amounts of the essential oils thymol, methyl salicylate, eucalyptol and menthol. The edible films are effective at killing the plaque-producing germs that cause dental plaque, gingivitis and bad breath. The film can also contain pharmaceutically active agents. Methods for producing the films are also disclosed.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Sustained release pharmaceutical compositions for highly water soluble drugs
ActiveUS20070020335A1Reduce spreadReduce erosionPowder deliveryOrganic active ingredientsControlled releaseActive agent
The present invention provides pharmaceutical compositions for controlled release of pharmaceutically active agents, especially those with a high water solubility, high dose, and / or short half-life. In addition, the present application provides methods for preparing and using such pharmaceutical compositions.
Owner:FARNAM +1
Oilwell sealant compositions comprising alkali swellable latex
Sealant compositions comprising an alkali swellable latex and a pH increasing material and methods of using the same to service a wellbore are provided. In one embodiment, the sealant composition can be used in a wellbore and includes an alkali swellable latex and a pH increasing material. The sealant composition can have a pH of from about 7 to about 14. In other embodiments, the pH increasing material includes a base-producing material. The base-producing material can include alkali and alkali earth metal carbonates, alkali and alkali earth metal bicarbonates, alkali and alkali earth metal hydroxides, alkali and alkali earth metal oxides, alkali and alkali earth metal phosphates, alkali and alkali earth metal hydrogen phosphates, alkali and alkaline earth metal sulphides, alkali and alkaline earth metal salts of silicates, alkali and alkaline earth metal salts of aluminates, water soluble or water dispersible organic amines, polymeric amine, amino alcohols, or combinations thereof.
Owner:HALLIBURTON ENERGY SERVICES INC
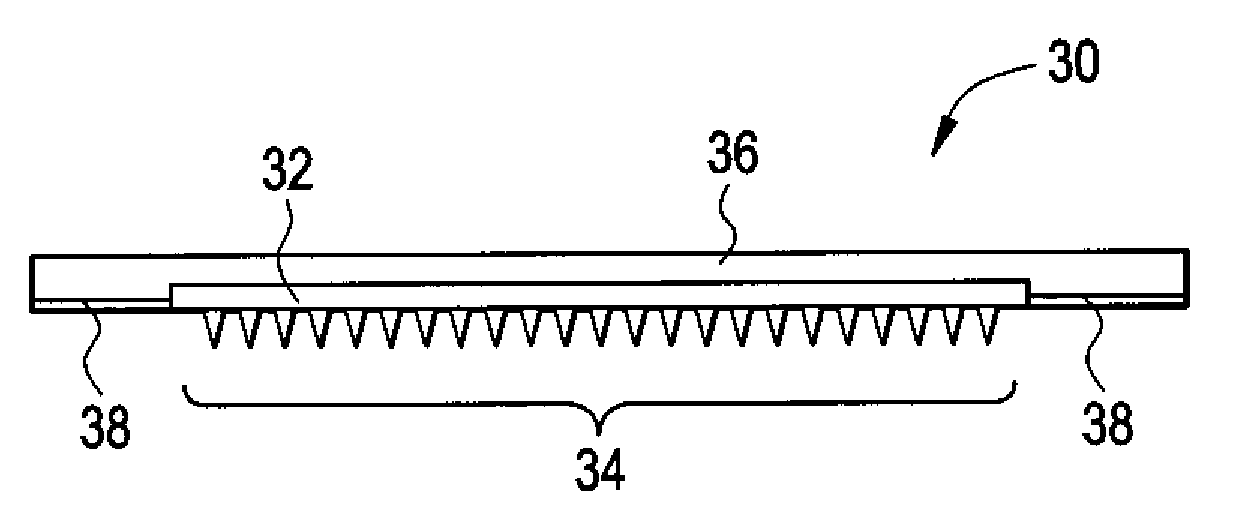
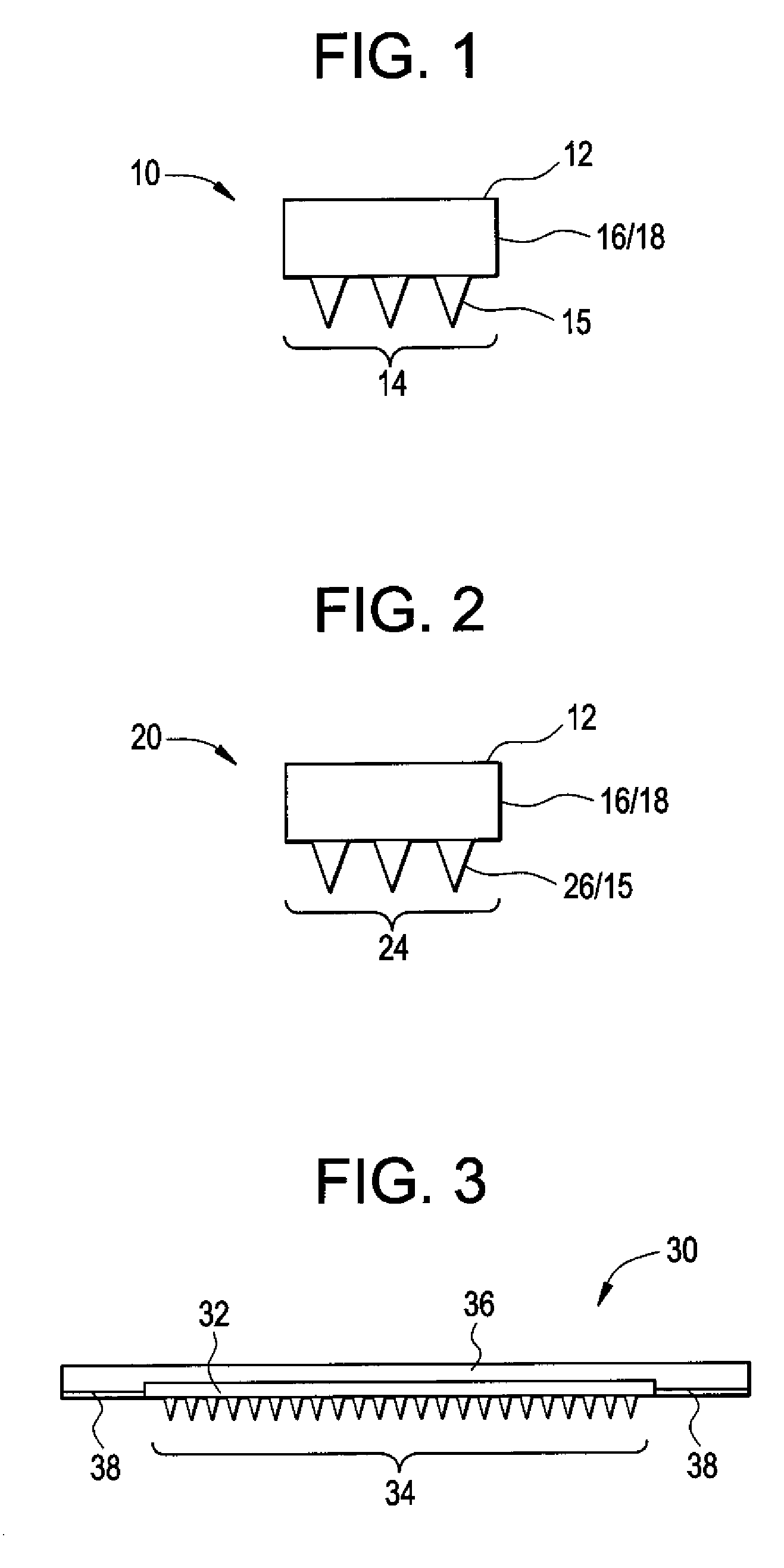
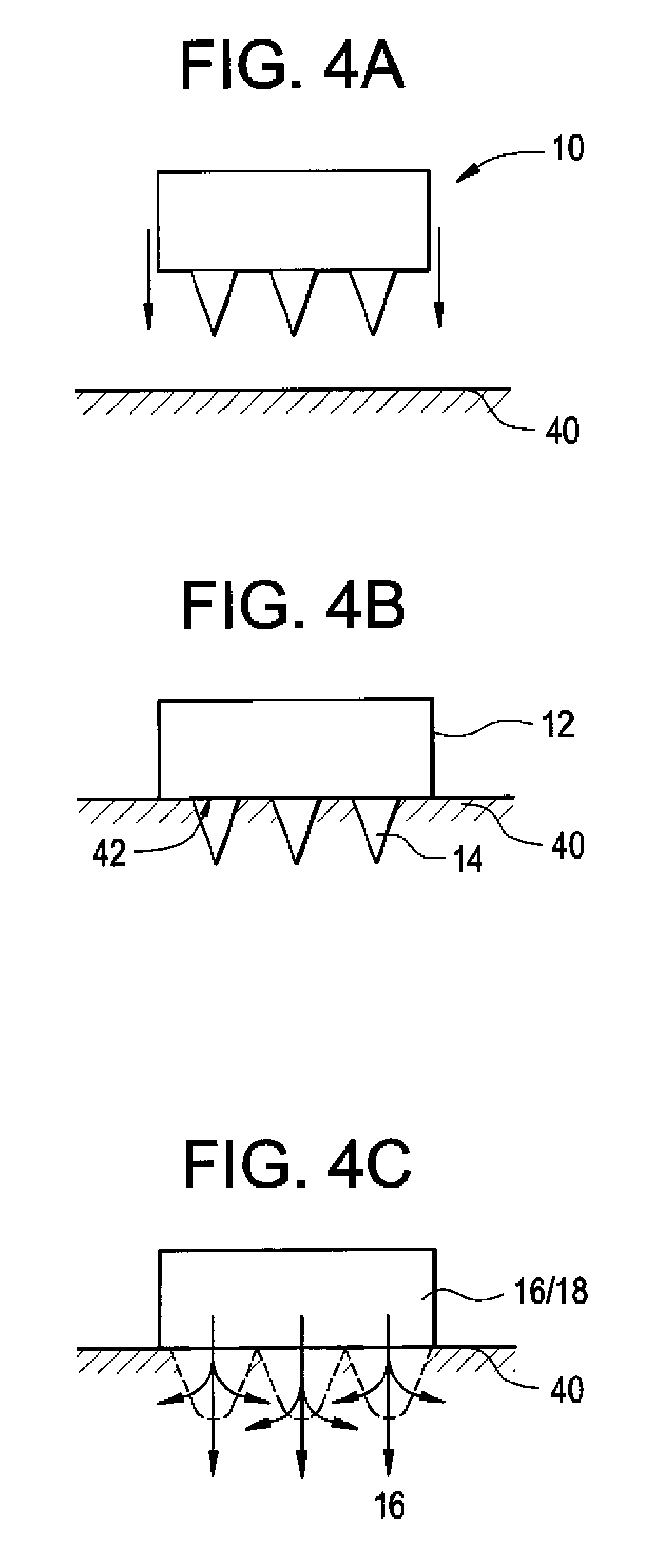
Microneedle Devices and Methods of Drug Delivery or Fluid Withdrawal
Microneedle devices and methods of manufacture and use thereof are provided. The devices may be used in controlled delivery of drug across or into a biological barrier, such as skin, or fluid withdrawal from a biological barrier. In one case, the device includes a base substrate which comprises a drug dispersed in a swellable matrix material; and one or more microneedles extending from the base substrate, wherein the one or more microneedles comprise a water-soluble or water-swellable material, wherein the one or more microneedles will dissolve or swell following insertion into the biological barrier, providing a transport pathway for the drug to pass from the base substrate into the biological barrier.
Owner:GEORGIA TECH RES CORP
Disintegrant assisted controlled release technology
InactiveUS20060024361A1Controlled release rateEasy to controlOrganic active ingredientsBiocideControlled releaseWater insoluble
A disintegrant assisted controlled release device is disclosed. The device is a combination of a swelling disintegrant or super-disintegrant and water insoluble polymer or water soluble polymer, or both, and one or more water soluble or water insoluble active pharmaceutical ingredient(s). The said device is stabilized by a humectant or trehalose.
Owner:INTELLIPHARMACEUTICS
Isolatable, water soluble, and hydrolytically stable active sulfones of poly(ethylene glycol) and related polymers for modification of surfaces and molecules
A poly(ethylene glycol) derivative is disclosed that is activated with a sulfone moiety for selective attachment to thiol moieties on molecules and surfaces. The activated PEG is water soluble, hydrolytically stable for extended periods, and forms hydrolytically stable linkages with thiol moieties. The linkages generally are not reversible in reducing environments. The PEG derivative is useful for modifying the characteristics of substances including modifying biologically active molecules and surfaces for biocompatibility. Methods for synthesizing the active PEG and for preparing conjugates of the active PEG and other substances, including biologically active substances, are also disclosed.
Owner:NEKTAR THERAPEUTICS INC
Cyclodextrin polymer compositions for use as drug carriers
Owner:KOSAK KENNETH M
Cosmetic compositions having improved wear and beauty benefits
InactiveUS6503495B1Maintain integrityInhibit growthCosmetic preparationsImpression capsWater insolubleWater soluble
Owner:NOXELL CORP
Silicone hydrogel lens with a crosslinked hydrophilic coating
ActiveUS20130118127A1Increased durabilitySpectales/gogglesPackage sterilisationHydrophilic coatingPolymer science
The invention is related to a cost-effective method for making a silicone hydrogel contact lens having a crosslinked hydrophilic coating thereon. A method of the invention involves autoclaving, in a sealed lens package, a silicone hydrogel contact lens having a base coating of polyacrylic acid thereon in an aqueous solution in the presence of a water-soluble, crosslinkable hydrophilic polymeric material having epoxide groups, for a period of time sufficient to covalently attach the crosslinkable hydrophilic polymeric material onto the surface of the silicone hydrogel contact lens through covalent linkages each formed between one epoxide group and one of the carboxyl groups on and / or near the surface of the silicone hydrogel contact lens.
Owner:ALCON INC
Formaldehyde free insulation binder
An aqueous binder composition containing a water-soluble and substantially infinitely water-dilutable free radical polymerized adduct of a monomeric carboxylic acid component and a monomeric hydroxyl component, polymerized in the presence of a chain transfer agent, and the related method of its use for making glass fiber products, especially fiberglass insulation.
Owner:GEORGIA PACIFIC CHEM LLC
Aqueous ink, aqueous ink set, ink cartridge, ink-jet recording apparatus, ink-jet recording method, and image-forming method
InactiveUS20060098068A1High image densityGood bleeding resistanceMeasurement apparatus componentsInksOrganic solventWater insoluble
An aqueous ink comprises water, a water-insoluble coloring material, and plurality of water-soluble organic solvents and the plurality of water-soluble organic solvents including a good medium or good mediums for the water-insoluble coloring material and a poor medium or poor mediums for the water-insoluble coloring material. The ratio of the content of the poor medium and the content of the good medium is in a specific range. A water-soluble organic solvent showing the maximum Ka value out of respective Ka values of the plurality of water-soluble organic solvents is the poor medium. When the aqueous ink is in contact with a specific reaction liquid, the dissolution state or dispersed state of the water-insoluble coloring material in the ink is made unstable. As a result, an image which has a sufficiently large area factor even with a small amount of ink droplets and is excellent in image density, bleeding resistance, and fixability can be obtained. In addition, the aqueous ink is excellent in storage stability.
Owner:CANON KK
Electrode coated with a film obtained from an aqueous solution comprising a water-soluble binder, production method thereof and uses of same
ActiveUS20060166093A1High performanceProblem be overcomeElectrode thermal treatmentConductive materialWater solubleAqueous solution
The invention relates to a method of preparing an electrochemical electrode which is partially or totally covered with a film that is obtained by spreading an aqueous solution comprising a water-soluble binder over the electrode and subsequently drying same. The production cost of the electrodes thus obtained is reduced and the surface porosity thereof is associated with desirable resistance values.
Owner:HYDRO QUEBEC CORP
Ocular in-situ gel preparatino with proper phase conversion temperature
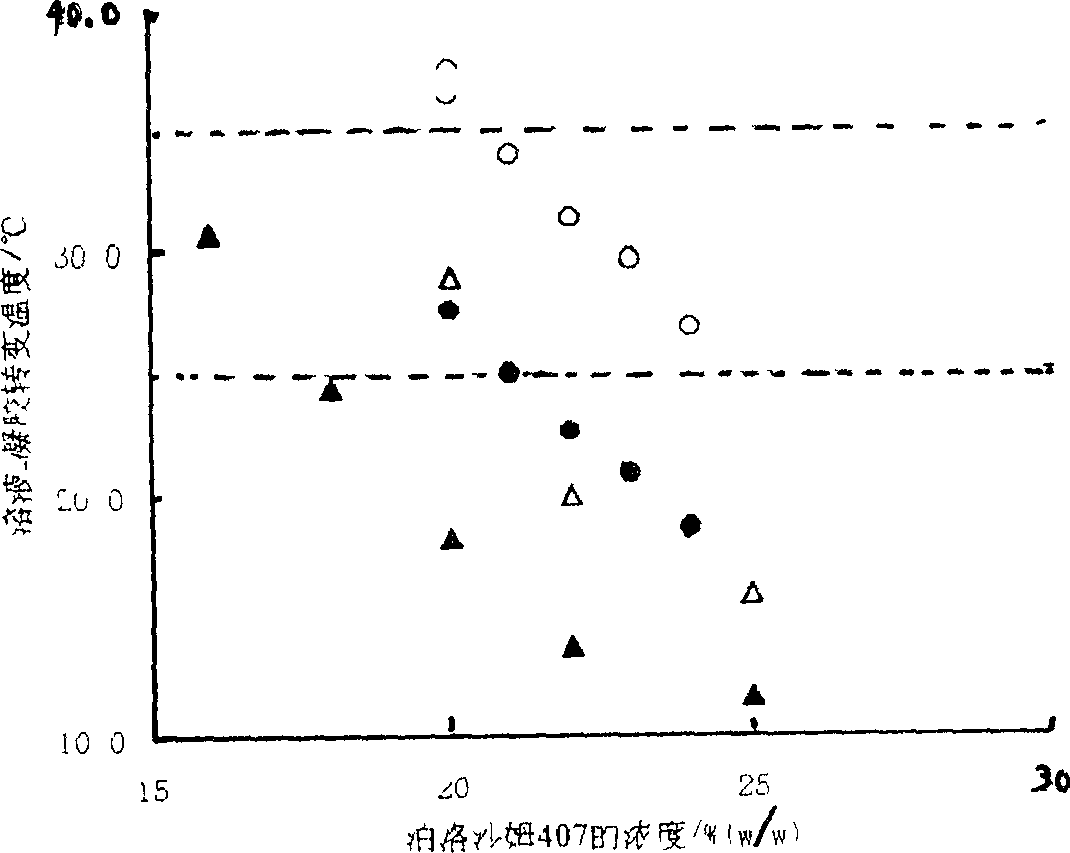
InactiveCN1377706AMedication convenienceReduce eliminateSenses disorderPharmaceutical delivery mechanismGel preparationTopical bioavailability
The gel preparation has the merits of both solution and gel. By means of the combination of different type poloxamers, ocular in-situ gel preparation containing medicine and water soluble polymer supplementary material is prepared. The preparation contains poloxamer 407 and poloxament 188 as well as water soluble polymer supplementary material less than 3%. The preparation has proper phase conversion temperatur and may form gel on the surface of cornea of living body after being applied in liquid state at room temperature. The present invention can delay the disappearance of medicine and raise the biological utilization in some local area and is suitable for various ocular medicines.
Owner:SHENYANG PHARMA UNIVERSITY
Continuous multi-microencapsulation process for improving the stability and storage life of biologically active ingredients
The invention relates to microcapsules, and a continuous micro-encapsulation water-in-oil-in-water microencapsulation process through in situ and interfacial polymerization of the emulsion. The formulation comprises a continuous water phase having a dispersion of microcapsules which contain oil drops and wherein the inside of each oil phase drop -containing optionally oil-soluble materials- there is a dispersion of water, or aqueous extract or water dispersible material or water soluble material. The oil drops are encapsulated with a polymerisable material of natural origin. Such microcapsules are appropriated for spray-dry processes, to be used as dry powder, lyophilised, self-emulsifiable powder, gel, cream and any liquid form. The active compounds included in te microcapsules are beneficial to the health and other biological purposes. Such formulations are appropriate to be incorporated in any class of food, specially for the production of nutraceuticals, as well as cosmetic products (such as rejuvenescence creams, anti-wrinkle creams, gels, bath and shower consumable products and sprays). The preparations are adequate to stabilise compounds added to the food, media for cultivating microbes and nutraceuticals, specially those which are easily degradable or oxidizable.
Owner:CHEM FAB BUDENHEIM AG
Process for forming clear, wettable silicone hydrogel articles
The present invention is a process for forming ophthalmic devices such as contact lenses, comprising the steps of curing a reactive mixture comprising at least one silicone-containing component, at least one hydrophilic component and at least one protonated diluent or protonatable diluent having a Hansen solubility parameter, δp between about 2 and about 7 to form an ophthalmic device having an advancing contact angle of less than about 80°; contacting the ophthalmic device with an aqueous solution which is capable of changing the Hansen solubility parameter, δp of the protonated or protonatable co-diluent to enhance water solubility and removing said diluent(s) with said aqueous solution.
Owner:JOHNSON & JOHNSON VISION CARE INC
Water soluble nanoparticles and method for their production
InactiveUS20050191359A1Readily bioavailableLow costPowder deliveryBiocidePolymer scienceNanoparticle
Hydrophilic dispersions of stable nano-sized particles are provided comprising: (a) a water-insoluble or water-soluble active compound, wherein said active compound is selected from the group consisting of a macrolide antibiotic, donepezil hydrochloride, an azole compound and a taxane; and (b) an amphiphilic polymer which wraps said active compound in a non-crystalline manner to form a nano-sized molecular entity in which no valent bonds are formed.
Owner:SOLUBEST
Water soluble prodrugs of COX-2 inhibitors
Disclosed are water soluble compounds which are useful as prodrugs of COX-2 inhibitors, and pharmaceutical compositions comprising them.
Owner:MERCK & CO INC
Powdered pharmaceutical formulations having improved dispersibility
InactiveUS6136346AGood dispersionReliable pulmonary delivery of drugPowder deliveryPeptide/protein ingredientsInhalationWater soluble
PCT No. PCT / US96 / 05265 Sec. 371 Date Mar. 17, 1998 Sec. 102(e) Date Mar. 17, 1998 PCT Filed Apr. 15, 1996 PCT Pub. No. WO96 / 32096 PCT Pub. Date Oct. 17, 1996Dispersibility of a respirable powder, administrable by inhalation, is increased by including a pharmaceutically acceptable water-soluble polypeptide.
Owner:NOVARTIS FARMA
Zinc salt compositions for the prevention of dermal and mucosal irritation
InactiveUS20050238602A1Minimize and prevent irritationReduce transmissionCosmetic preparationsBiocideMedicineIrritation
The present invention provides for compositions and methods that may offer protection from irritants, as well as antimicrobial protection. Preferred embodiments of the invention include topical antimicrobial compositions comprising two or more water-soluble zinc salts in low concentrations.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Nanocrystals, compositions, and methods that aid particle transport in mucus
ActiveUS20130323179A1Lower aqueous solubilityUltrasonic/sonic/infrasonic diagnosticsPowder deliveryNanocrystalAqueous solubility
Nanocrystals, compositions, and methods that aid particle transport in mucus are provided. In some embodiments, the compositions and methods involve making mucus-penetrating particles (MPP) without any polymeric carriers, or with minimal use of polymeric carriers. The compositions and methods may include, in some embodiments, modifying the surface coatings of particles formed of pharmaceutical agents that have a low water solubility. Such methods and compositions can be used to achieve efficient transport of particles of pharmaceutical agents though mucus barriers in the body for a wide spectrum of applications, including drug delivery, imaging, and diagnostic applications. In certain embodiments, a pharmaceutical composition including such particles is well-suited for administration routes involving the particles passing through a mucosal barrier.
Owner:ALCON INC +1
Pouched cleaning compositions
The present invention relates to a pouched cleaning composition wherein the pouch is constructed from a water-soluble film and contains a liquid composition comprising less than 5% by weight of the liquid composition, of water; an anionic surfactant; and at least 0.5% by weight of the liquid composition of a builder / chelant free of C8-22 alkyl or alkenyl chains characterized by a solubility in said liquid composition of less than 0.3% by weight. Such pouched cleaning compositions provide better cleaning performance and better pouch solubility while avoiding the formation of residues.
Owner:PROCTER & GAMBLE CO
Polymerization process and materials for biomedical applications
InactiveUS20050090612A1Avoid problemsPromote sportsOptical articlesOptical partsPolymer scienceAqueous solubility
A molded component or article for biomedical use is prepared from a crosslinkable non-water-soluble polymer which when crosslinked and saturated with water forms a hydrogel. The polymer is formulated as a composition containing a non-aqueous diluent in addition to the polymer, the diluent being present in a volumetric proportion that is substantially equal to the volumetric proportion of water in the hydrogel that would be formed when the polymer is crosslinked and saturated with water. The composition is cast in a mold where the composition is exposed to conditions that cause crosslinking to occur by a reaction to which the non-aqueous diluent is inert. The crosslinking reaction produces a molded non-aqueous gel which is then converted to a hydrogel by substituting an aqueous liquid such as water or physiological saline for the non-aqueous diluent. The use of a molding composition whose curing consists essentially entirely of crosslinking results in a molding process that entails little or no shrinkage, and dimensional integrity is maintained up through the formation of the hydrogel by using the non-aqueous diluent in essentially the same volumetric proportion as water in the hydrogel.
Owner:ZMS LLC
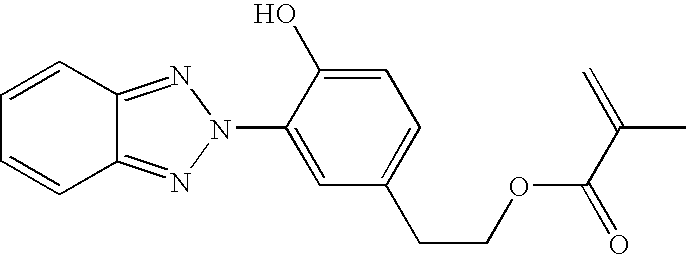
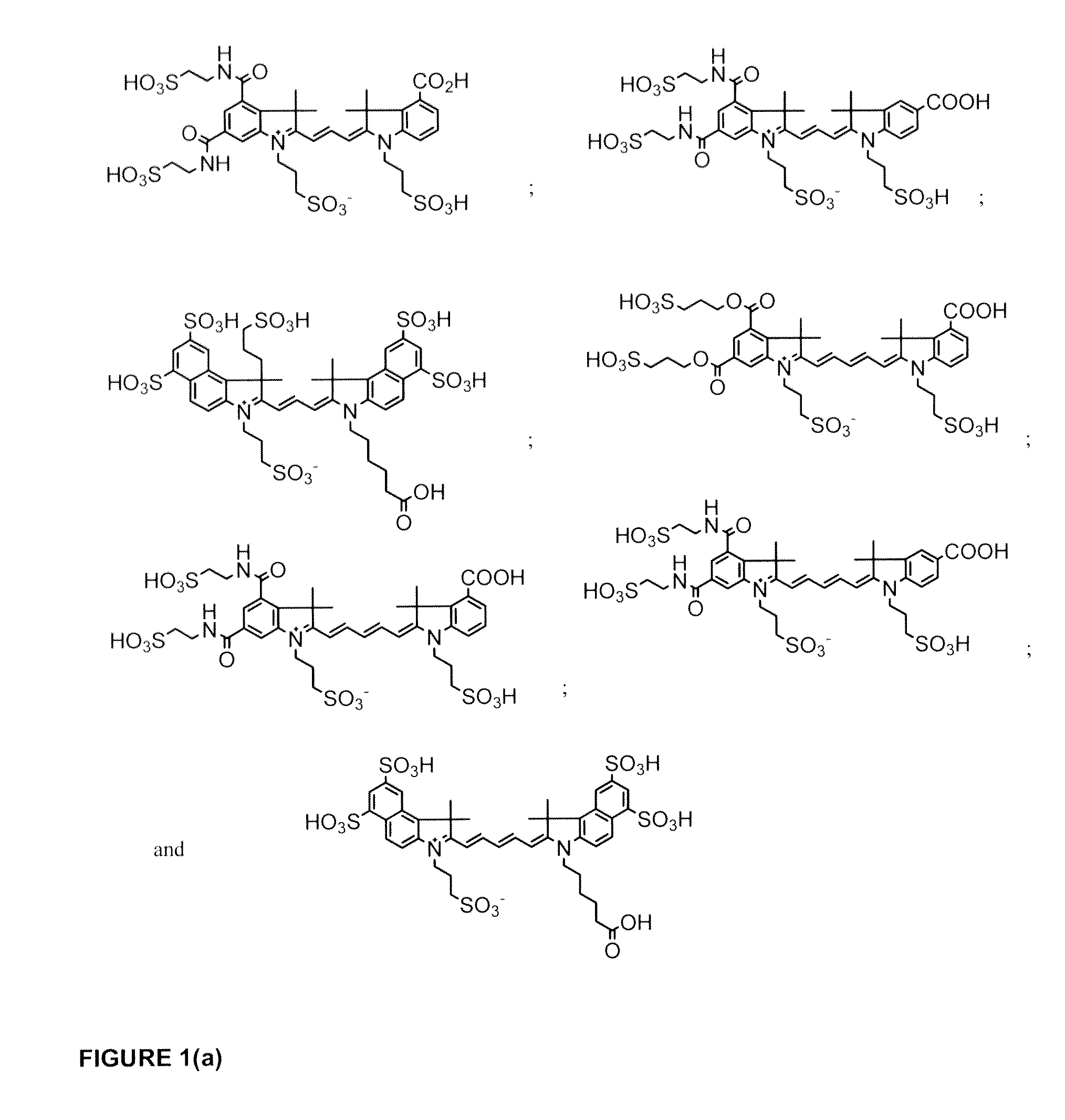
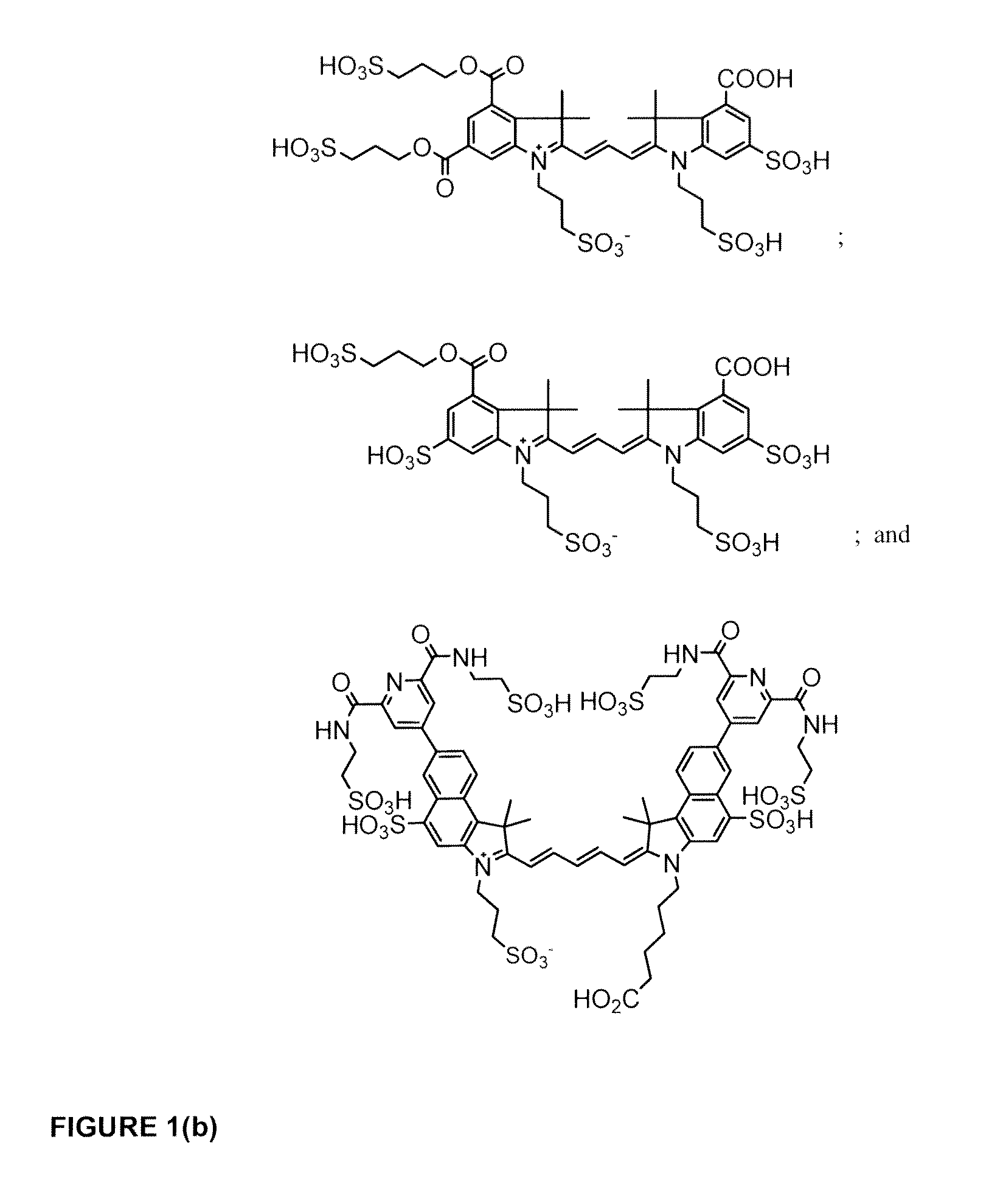
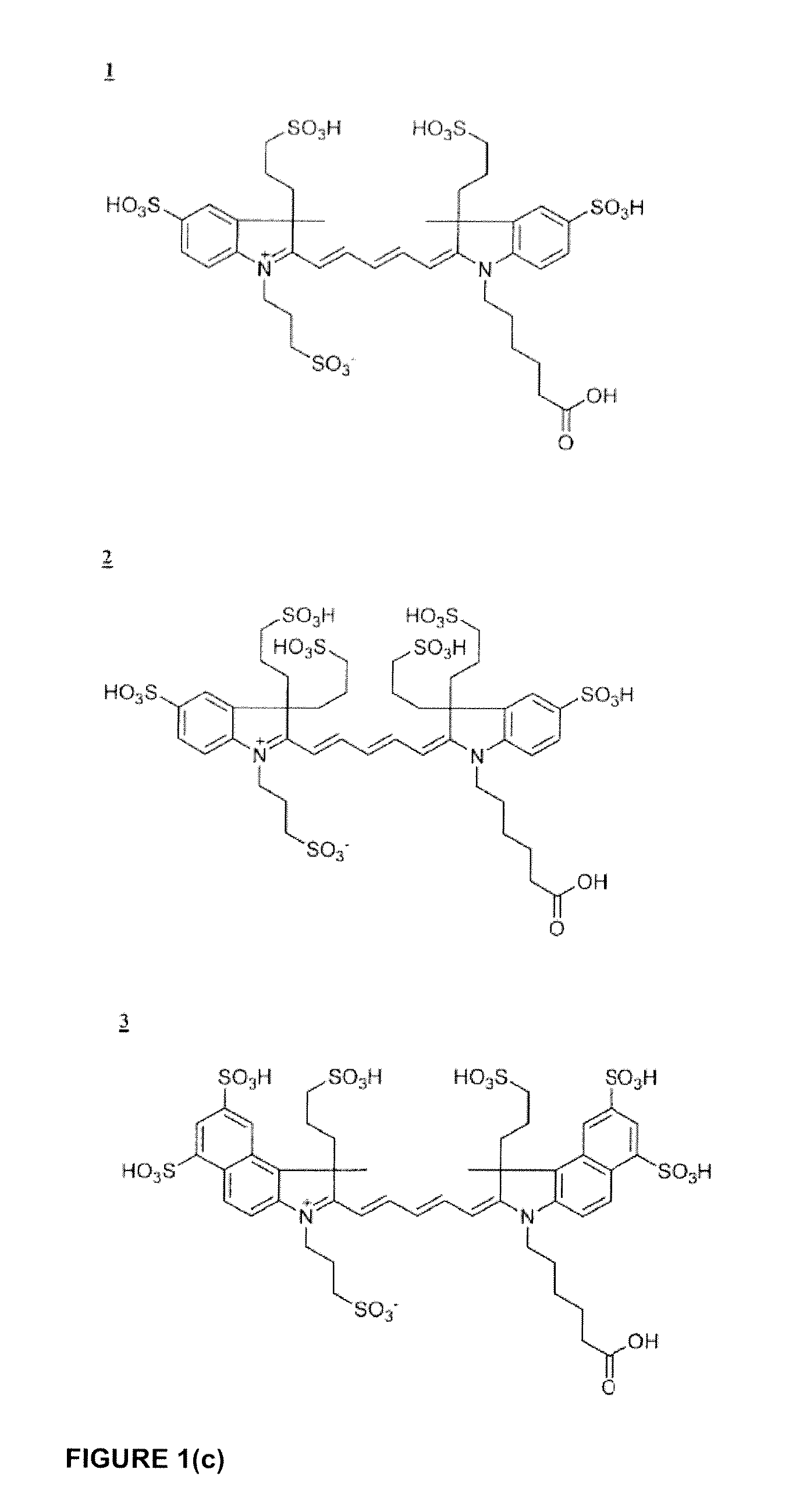
Cyanine dyes
ActiveUS20120052506A1Versatile assay designImprove analytical performanceMethine/polymethine dyesSugar derivativesSolubilityCyanine
The invention provides a novel class of cyanine dyes that are functionalized with sulfonic acid groups and a linker moiety that facilitates their conjugation to other species and substituent groups which increase the water-solubility, and optimize the optical properties of the dyes. Also provided are conjugates of the dyes, methods of using the dyes and their conjugates and kits including the dyes and their conjugates.
Owner:PACIFIC BIOSCIENCES
Aqueous suspension of agrochemical
InactiveUS6306414B1Low viscosityEffective dispersionOrganic active ingredientsBiocideSuspended particlesPhenyl Ethers
The present invention relates to an aqueous suspension comprising (i) a compound of the formula:or a salt thereof, (ii) a condensate of formaldehyde with an aromatic sulfonic acid or a salt thereof or a polyoxyalkylene allyl phenyl ether sulfate, and (iii) an absorptive water-soluble polymer. The aqueous suspension of the invention can be used with advantage as a stable aqueous suspension of low viscosity providing for excellent delivery from a container, with excellent dispersibility in diluent water and excellent long-term fluidity free from caking due to precipitation of the suspended particles.
Owner:SUMITOMO CHEM CO LTD
Injectable Depot Formulations And Methods For Providing Sustained Release Of Poorly Soluble Drugs Comprising Nanoparticles
Pharmaceutical formulations comprising: a compound of low water solubility, having a maximum average particle size; a carrier; and at least two surface stabilizers are disclosed. The present invention also comprises methods of treating various conditions with such a formulation and processes for making such a formulation.
Owner:PFIZER INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com