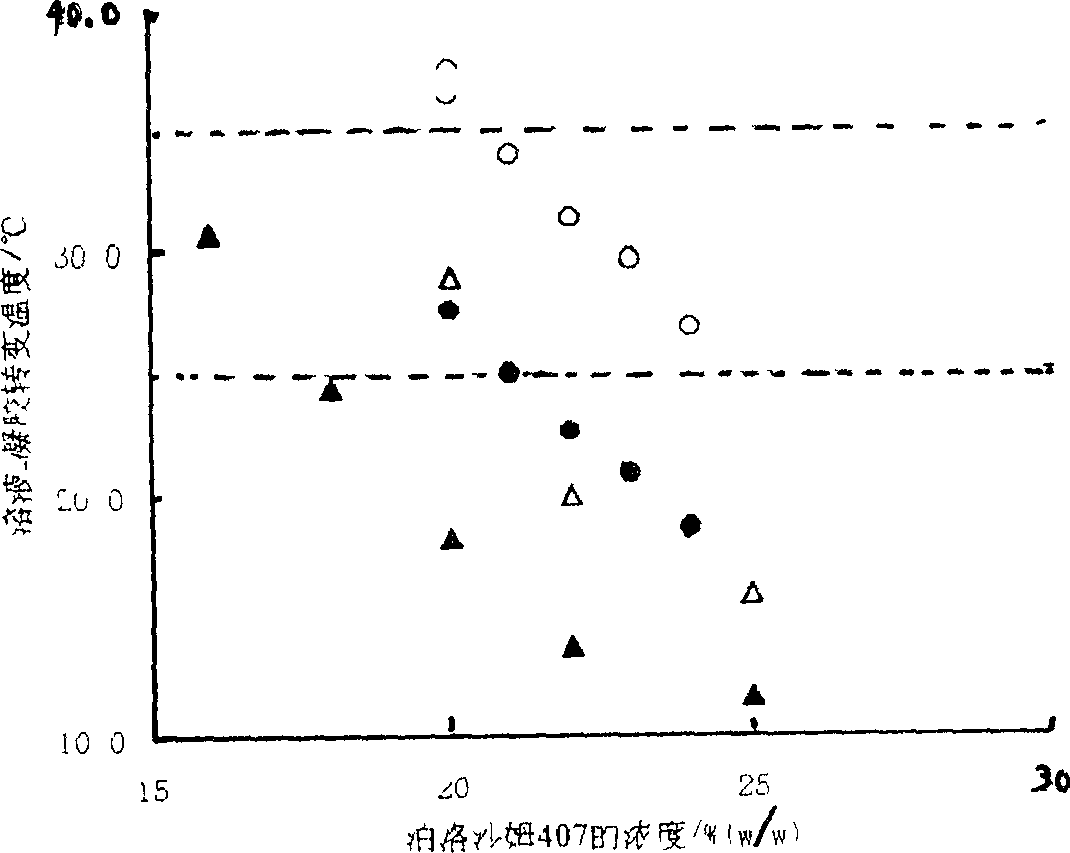
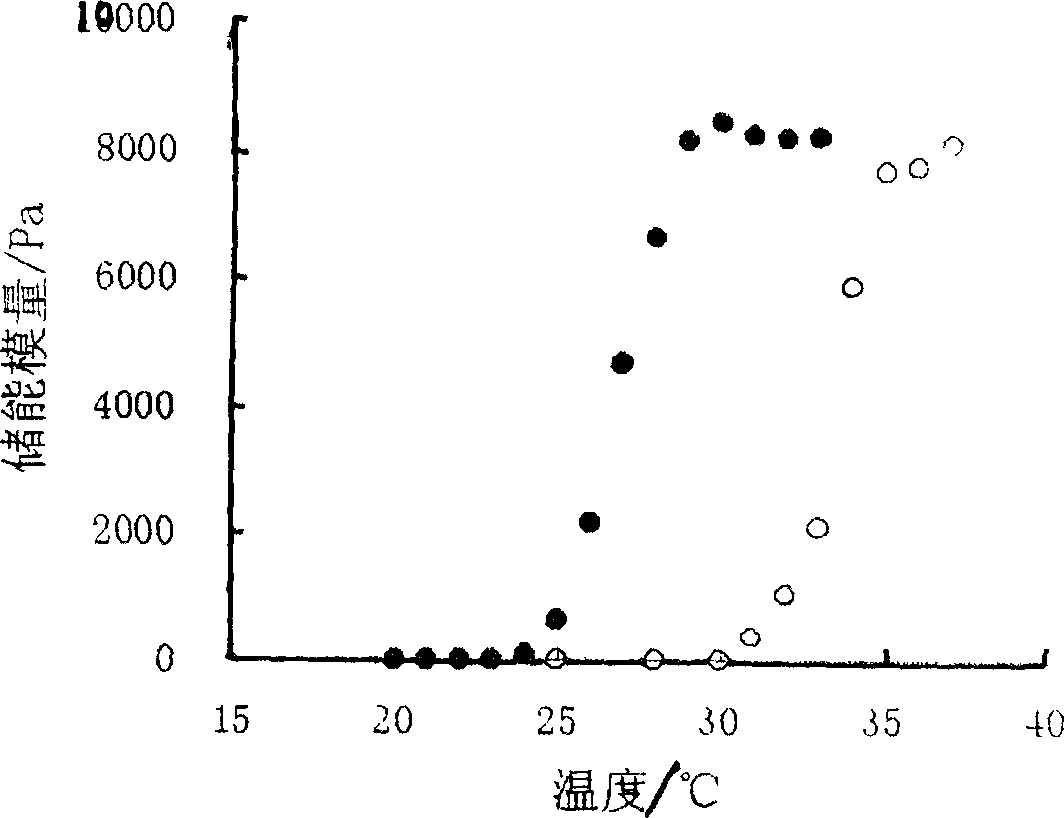
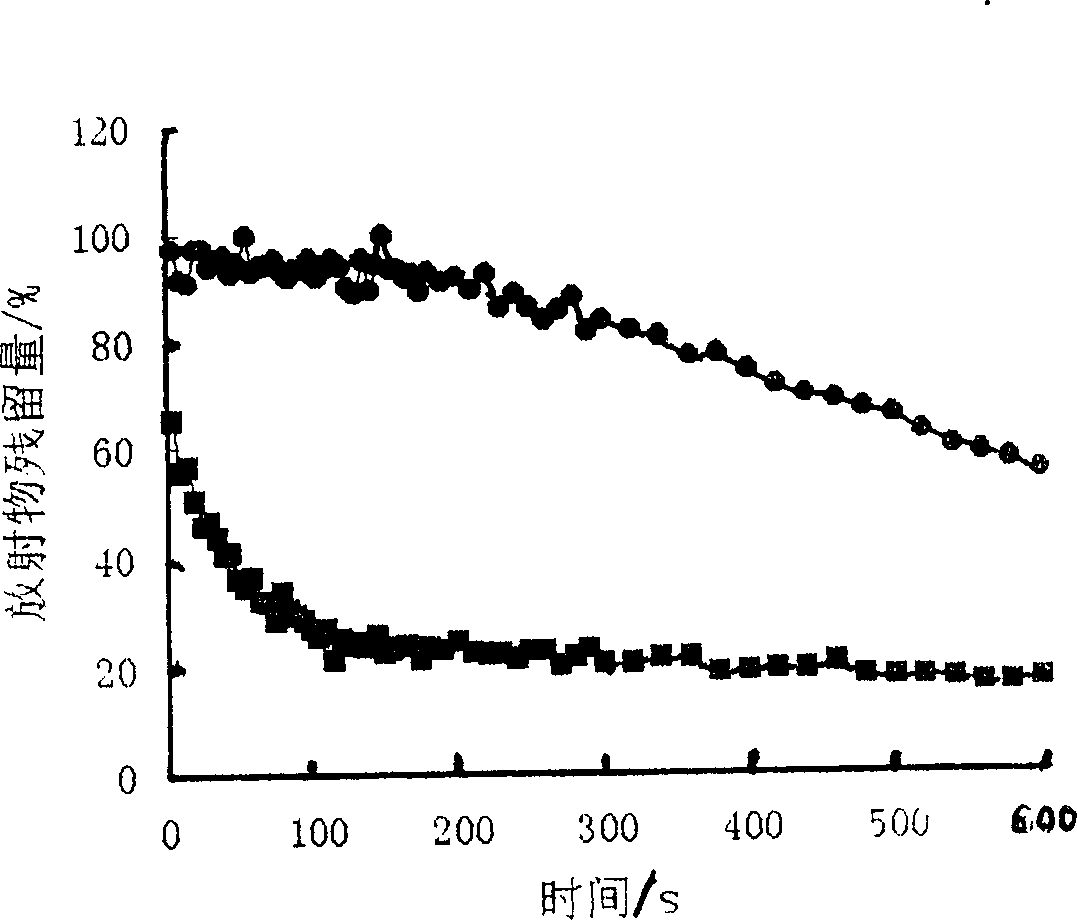
Ocular in-situ gel preparatino with proper phase conversion temperature
A phase transition temperature, in-situ gel technology, which is used in medical preparations with non-active ingredients, sensory diseases, drug combinations, etc., to achieve the effects of improving local bioavailability, delaying drug elimination, and facilitating medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Timolol maleate ophthalmic in situ gel; take timolol maleate 0.25%, brogeramine 0.02%, 21% poloxamer 407, 10% poloxamer 188, sterile isotonic Phosphate buffer solution (pH7.4) to full volume. The drug and benzalkonium chloride are dissolved in water, and the poloxamer is slowly added to the above solution under ice bath and stirring conditions, and stored under low temperature refrigeration until a clear solution is formed.
Embodiment 2
[0033]Ofloxacin ophthalmic in situ gel with hyaluronic acid; take ofloxacin 0.3%, sodium hyaluronate 0.2%, 21% poloxamer 407, 10% poloxamer 188, sterile Isotonic phosphate buffer solution (pH7.4) to full volume. Sodium hyaluronate was dissolved in the buffer solution, and then ofloxacin ophthalmic in situ gel was prepared according to the process of Example 1.
Embodiment 3
[0035] Acyclovir Ophthalmic In Situ Gel Containing Carbomer; Take Acyclovir 2%, Brogeramine 0.02%, Carbomer 934P 0.2%, Mannitol 5%, Poloxamer 407 22%, 10 % Poloxamer 188, sterile water to full volume. Disperse carbomer in water, adjust the pH to neutral with sodium hydroxide solution, add drugs, benzalkonium chloride and mannitol, slowly add poloxamer to the above solution under ice bath and stirring conditions, and store in low temperature refrigeration until a clear solution is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com