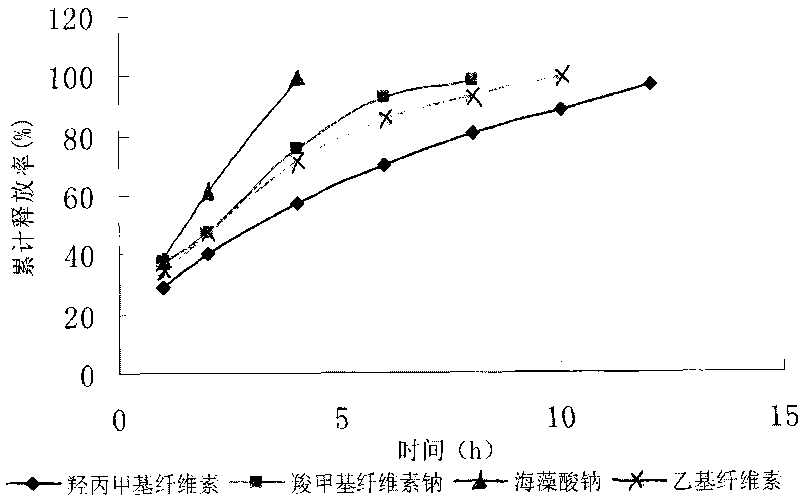
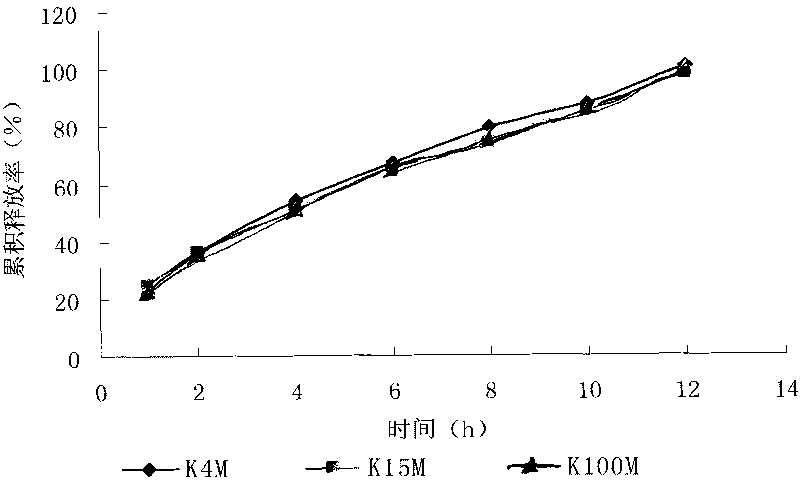
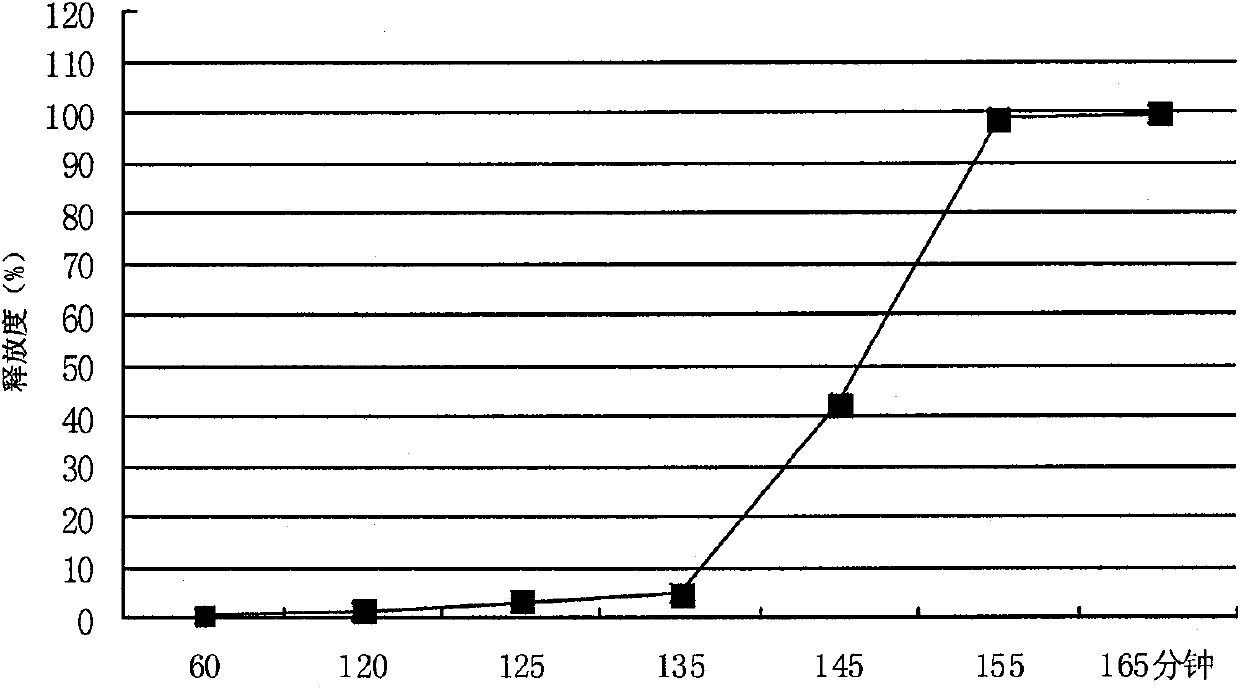
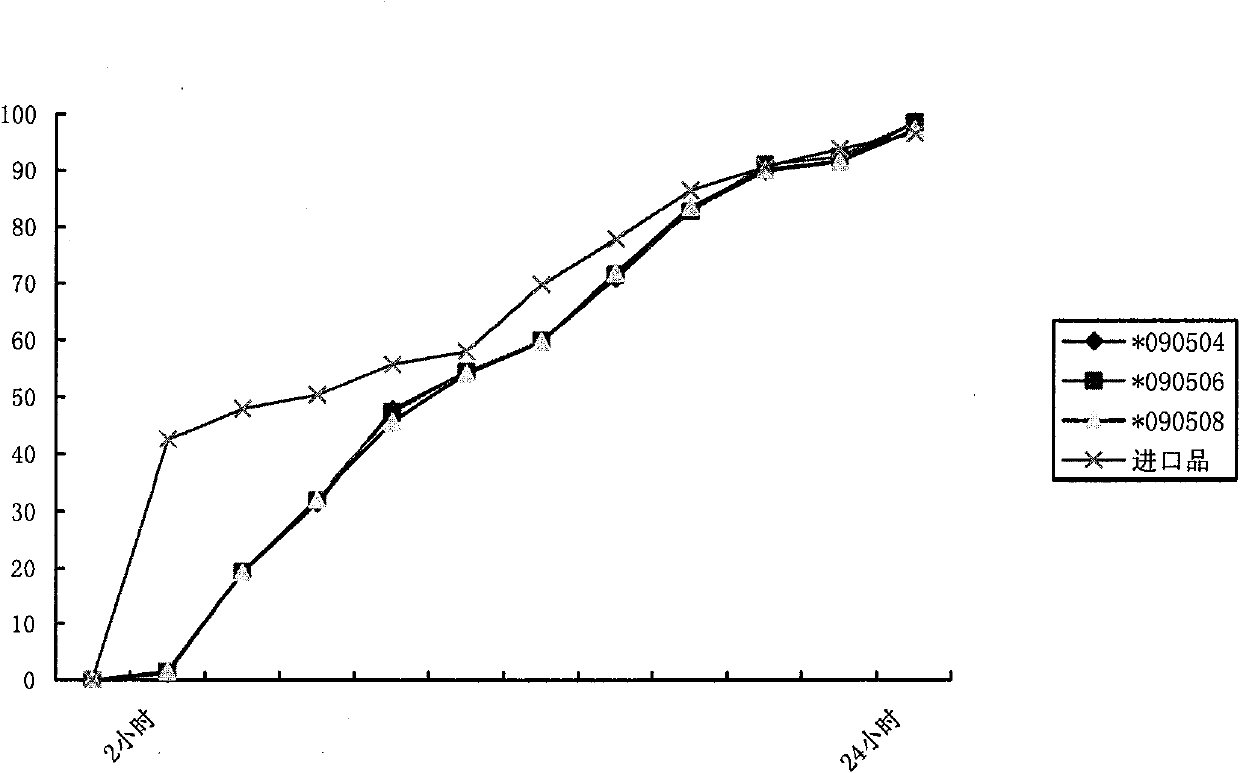
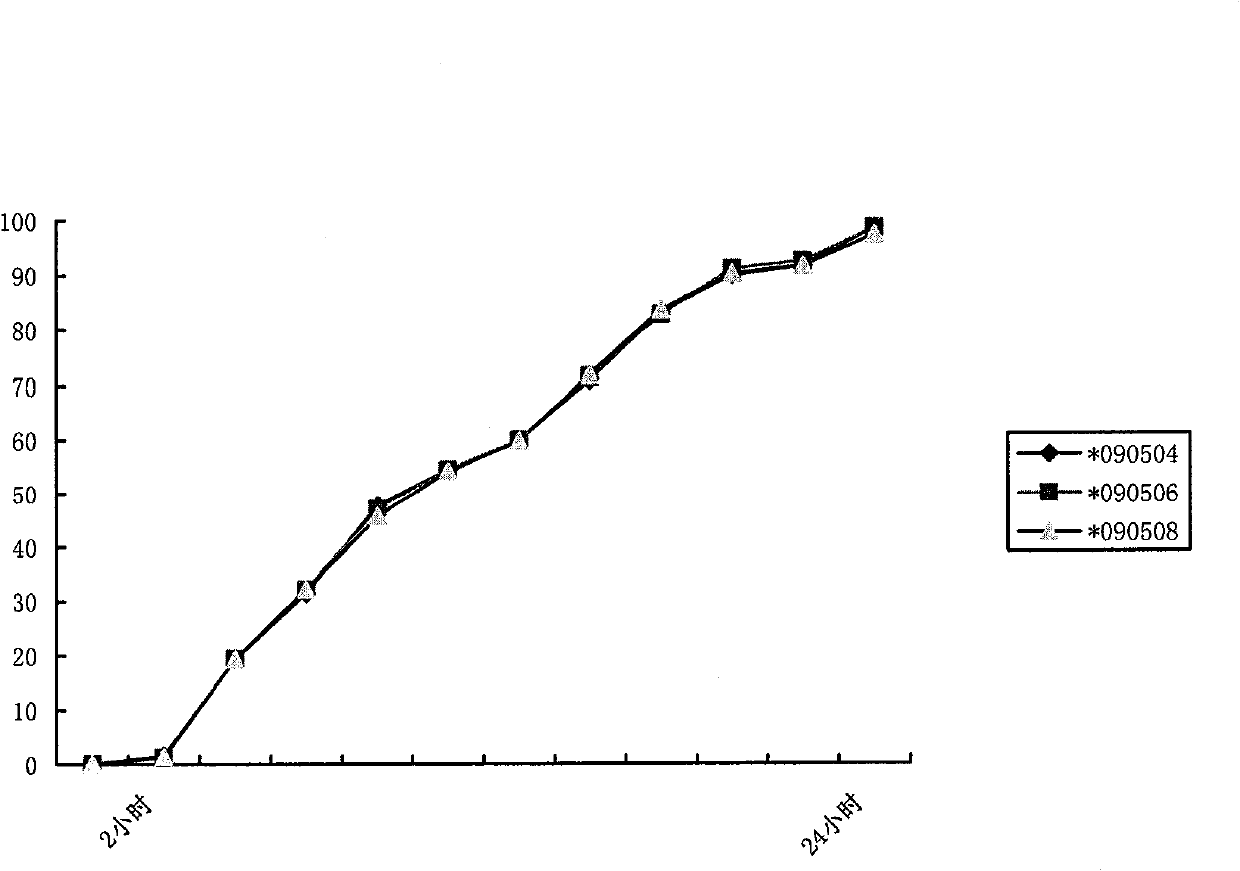
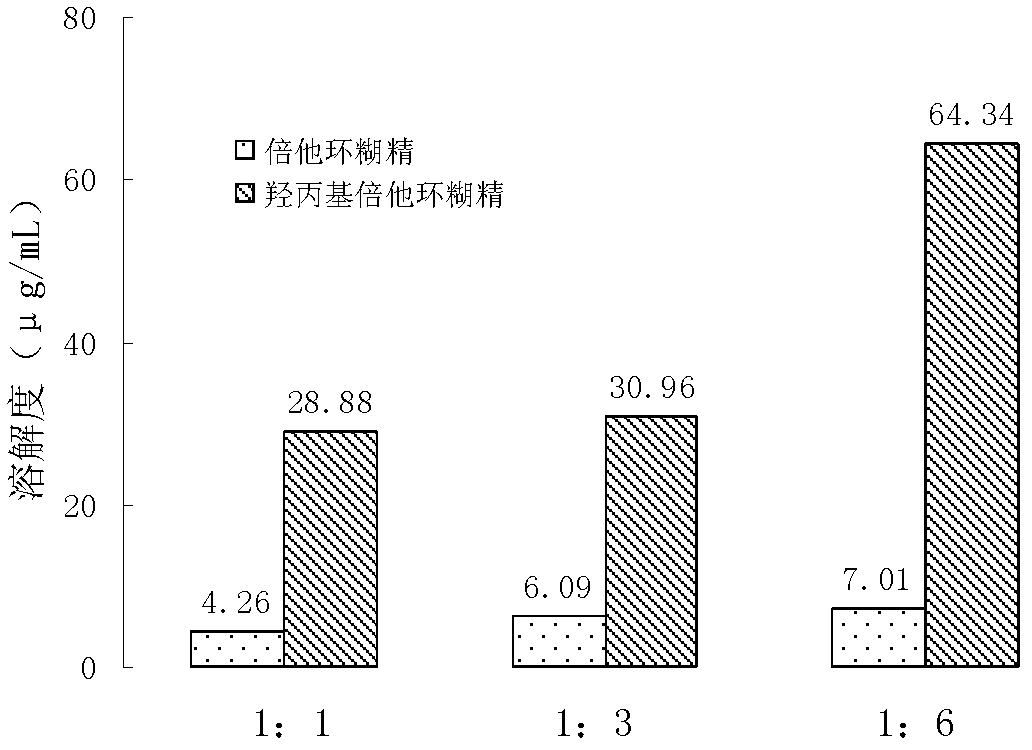
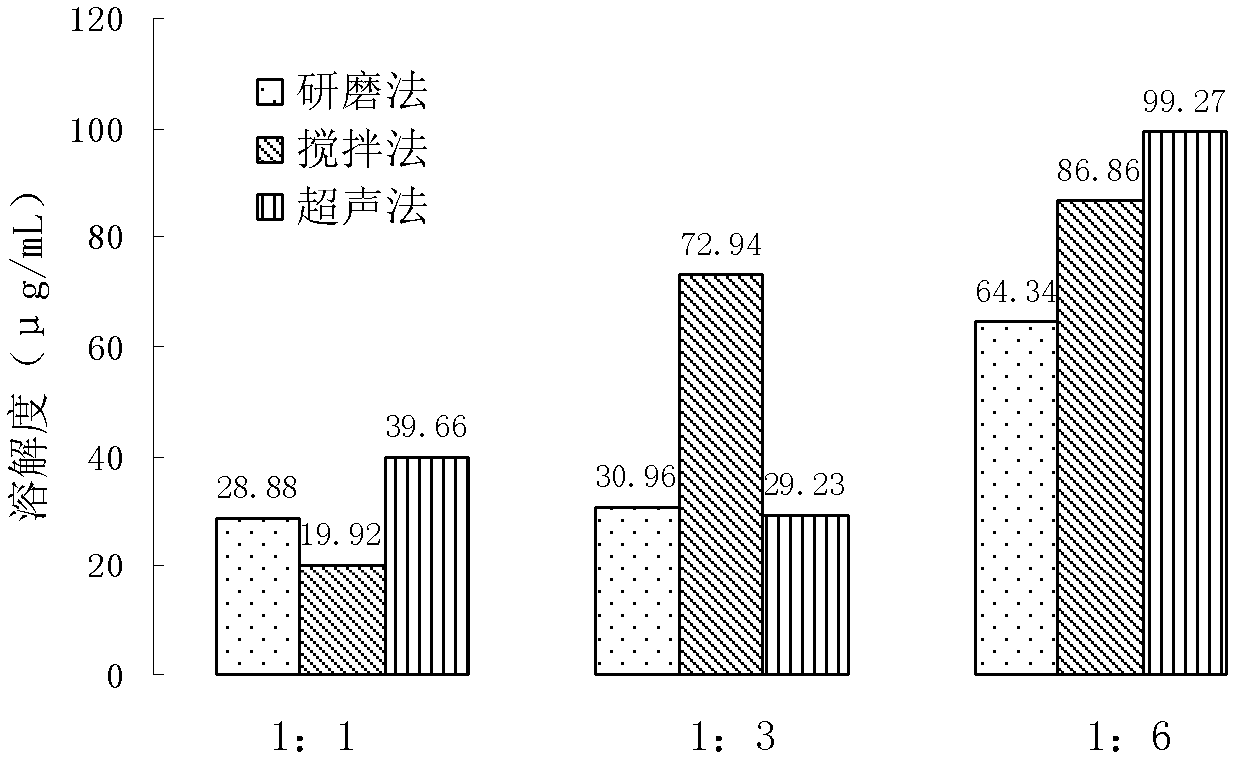
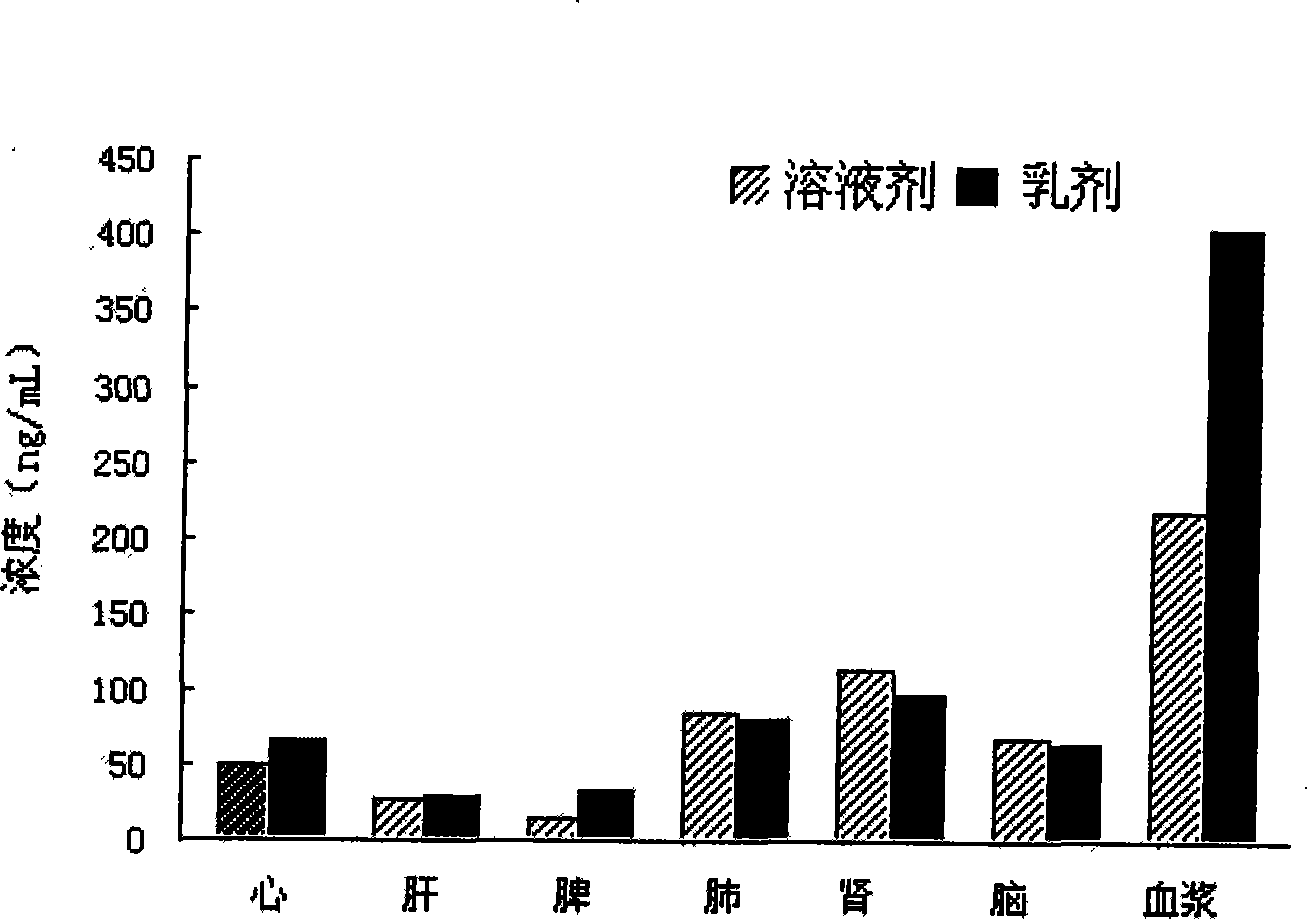
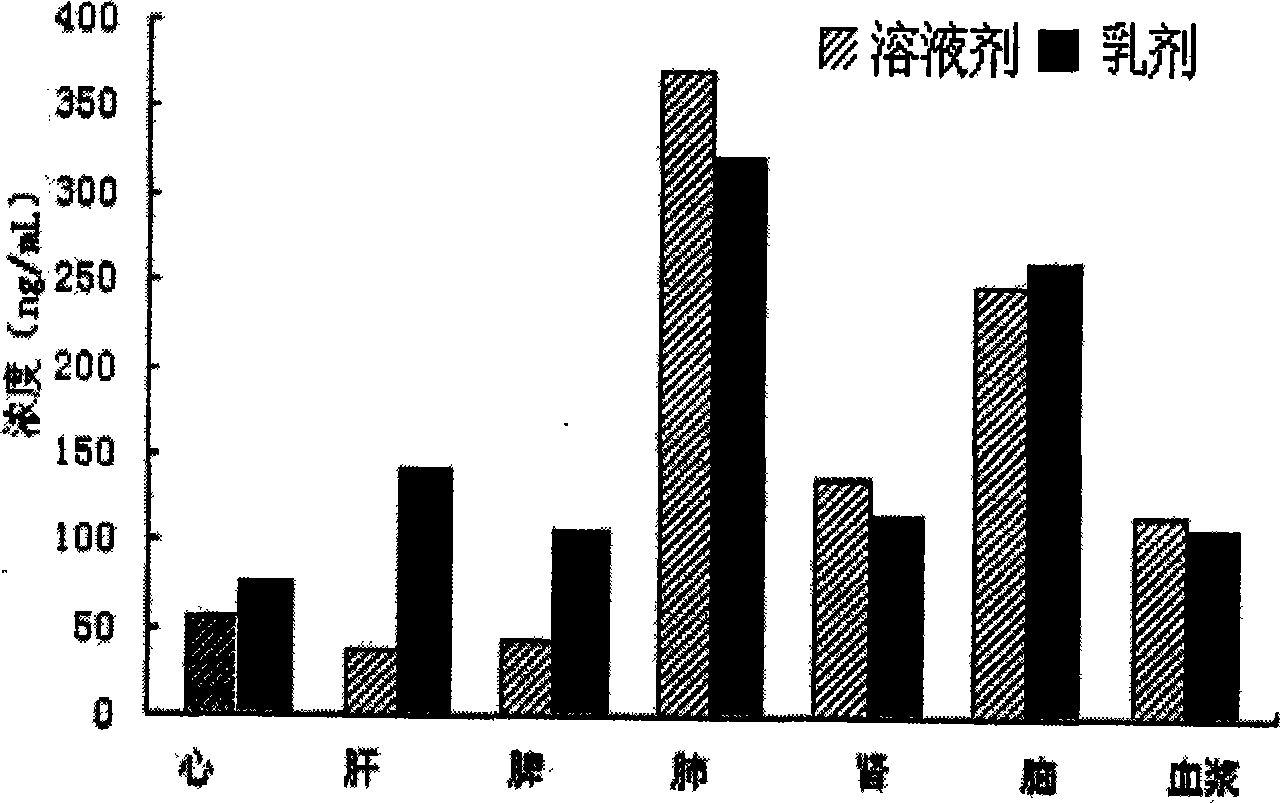
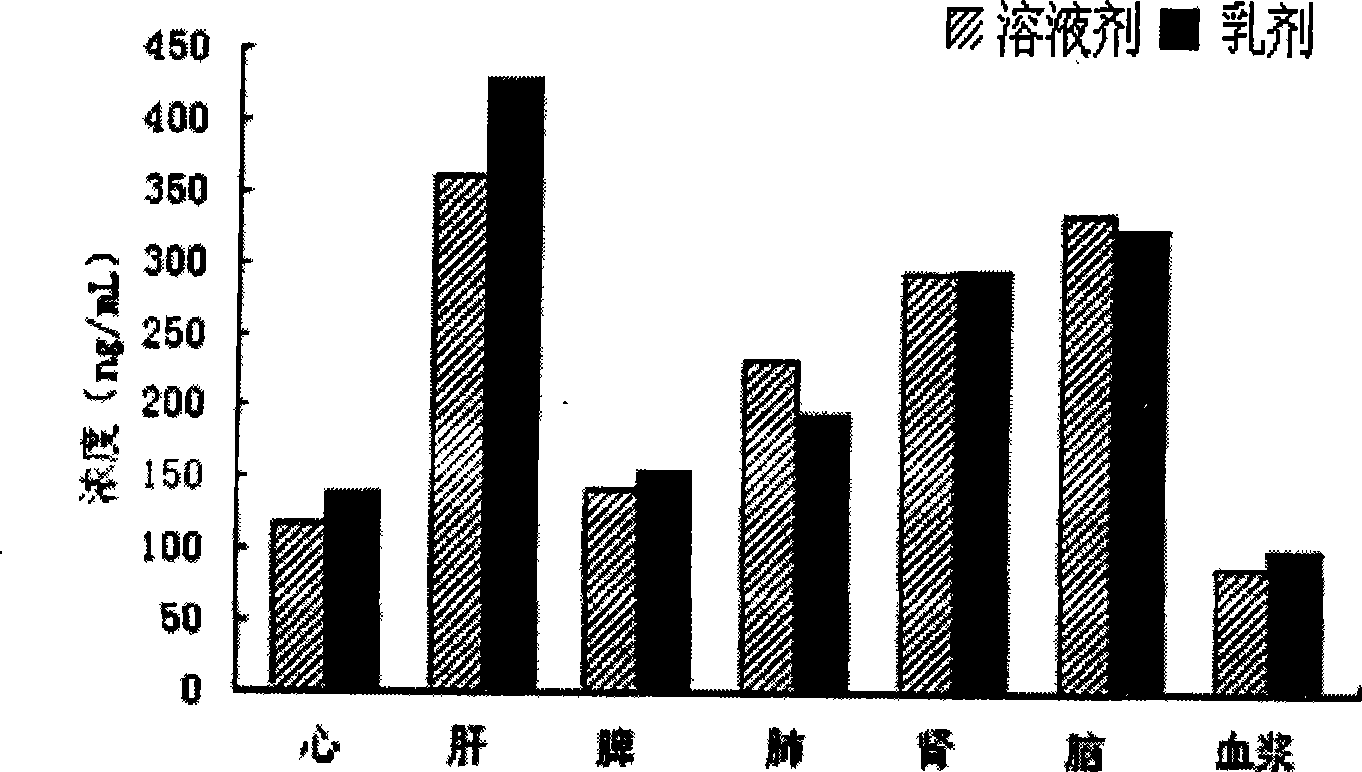
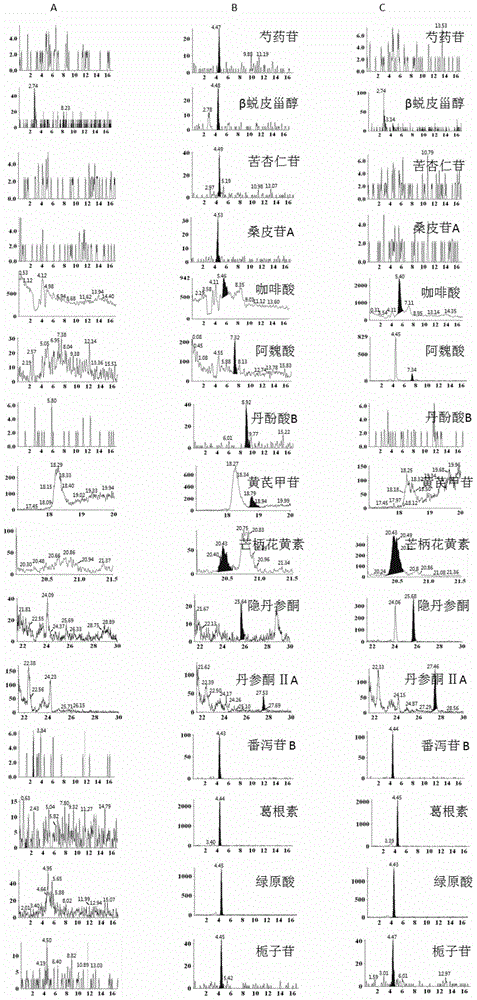
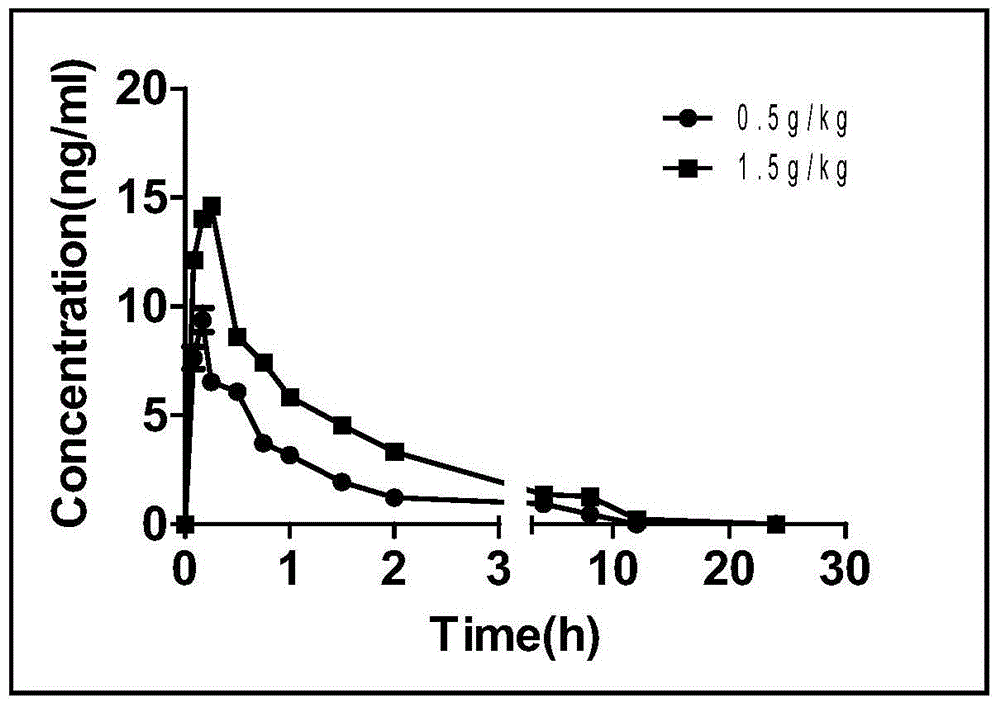
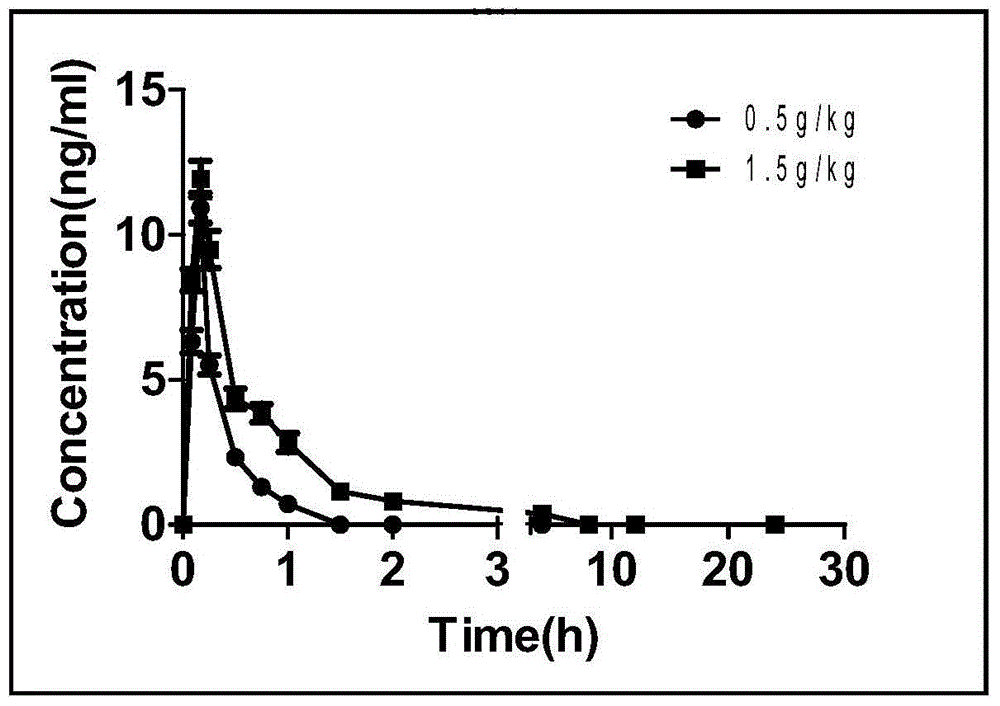
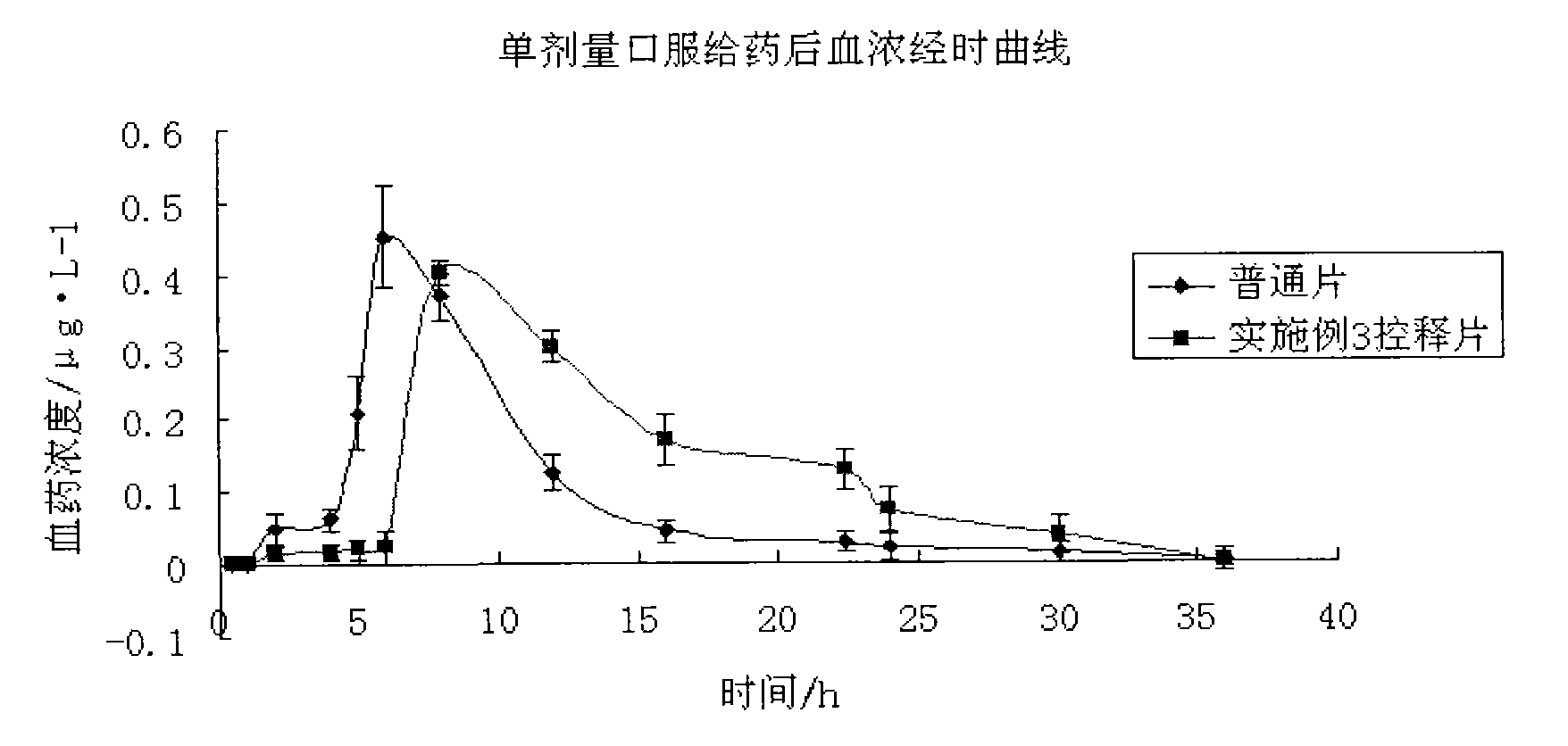
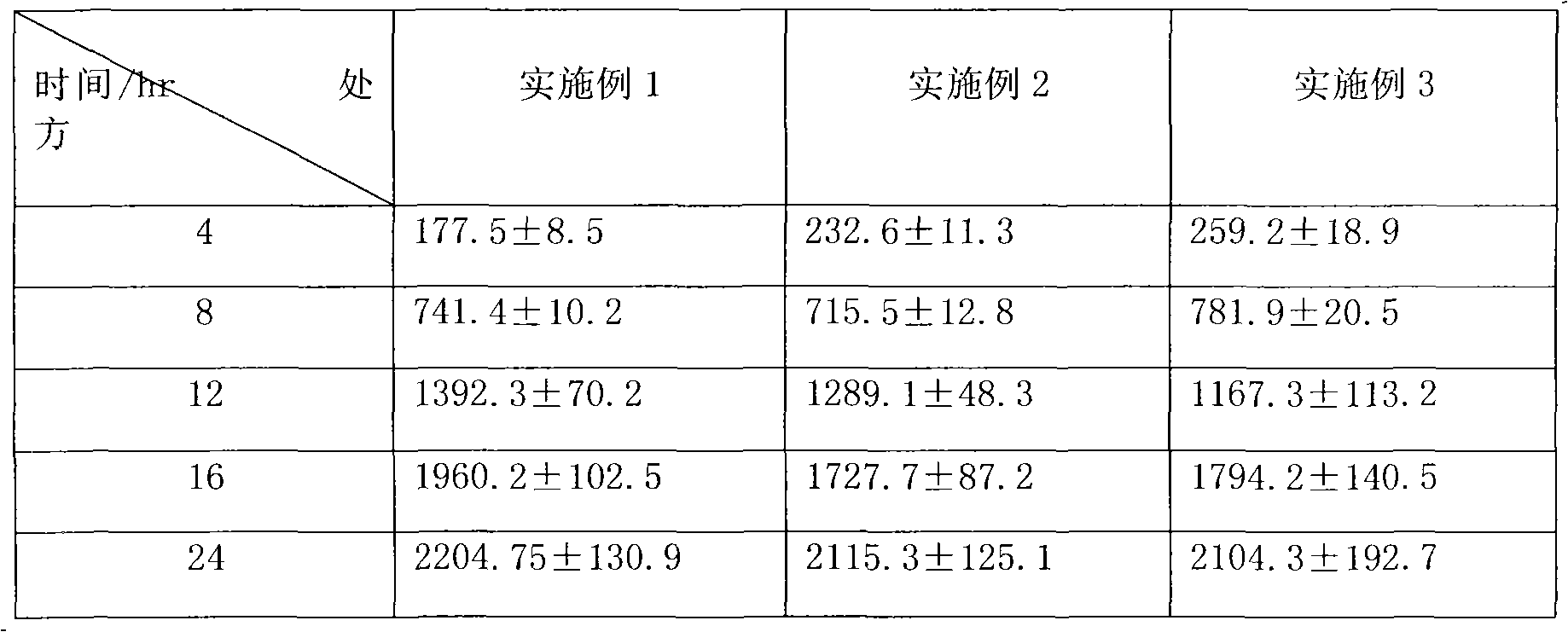
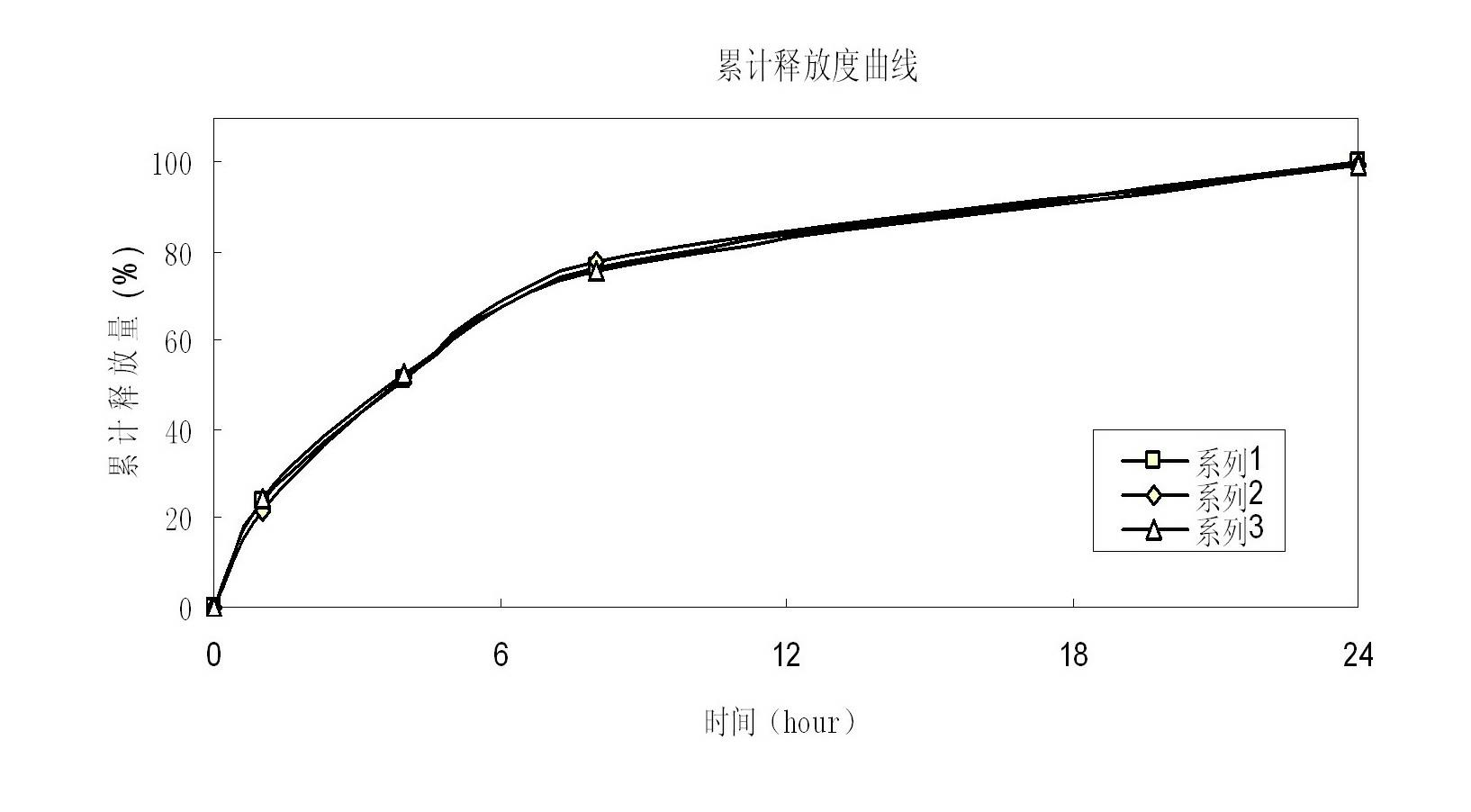
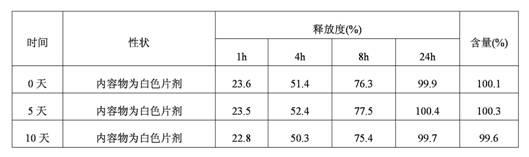
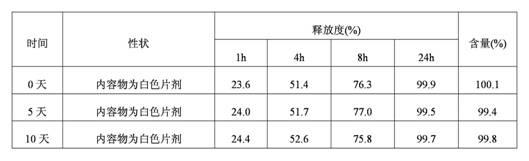
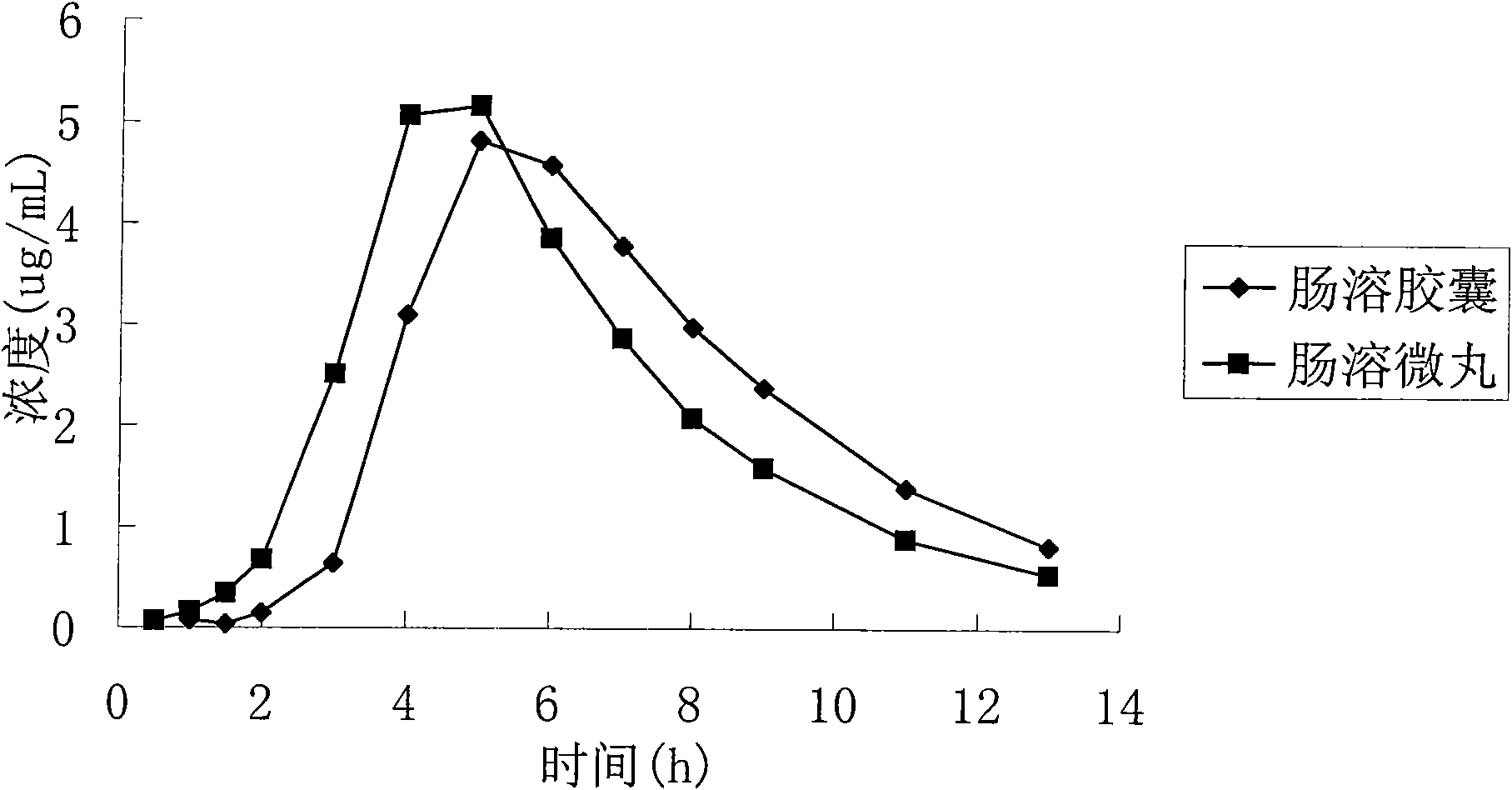
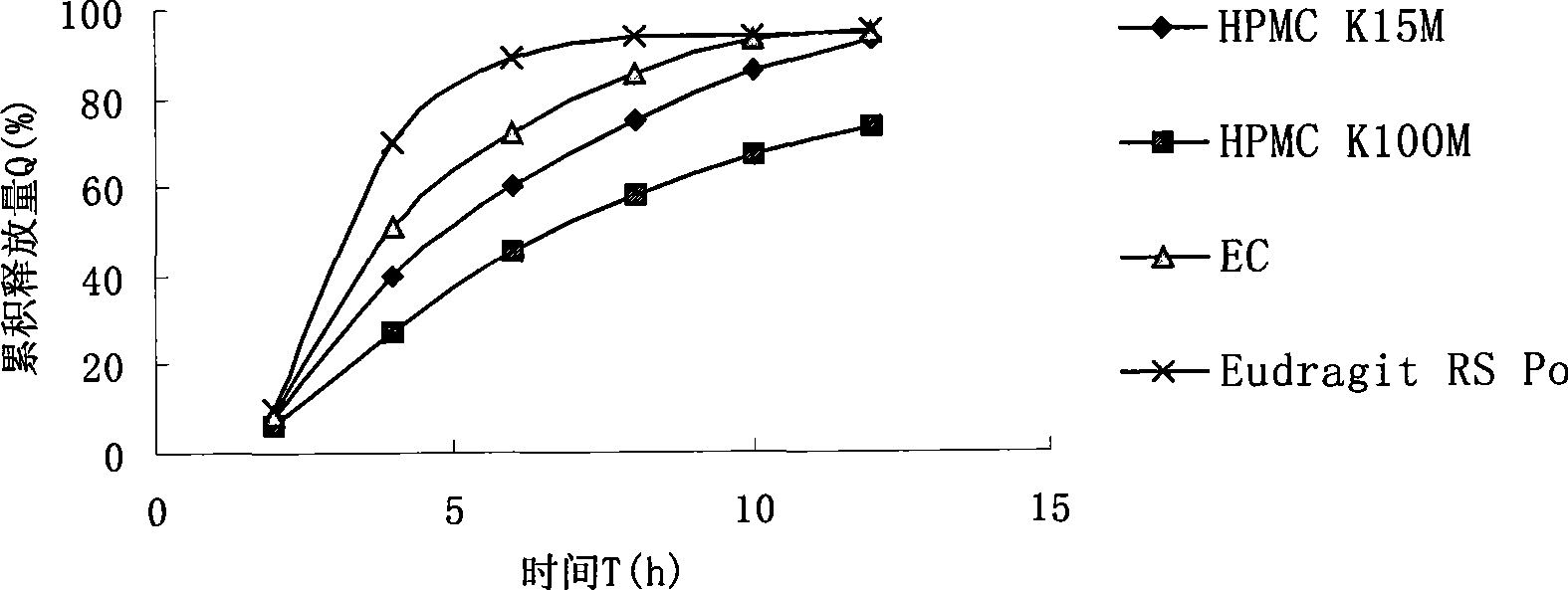
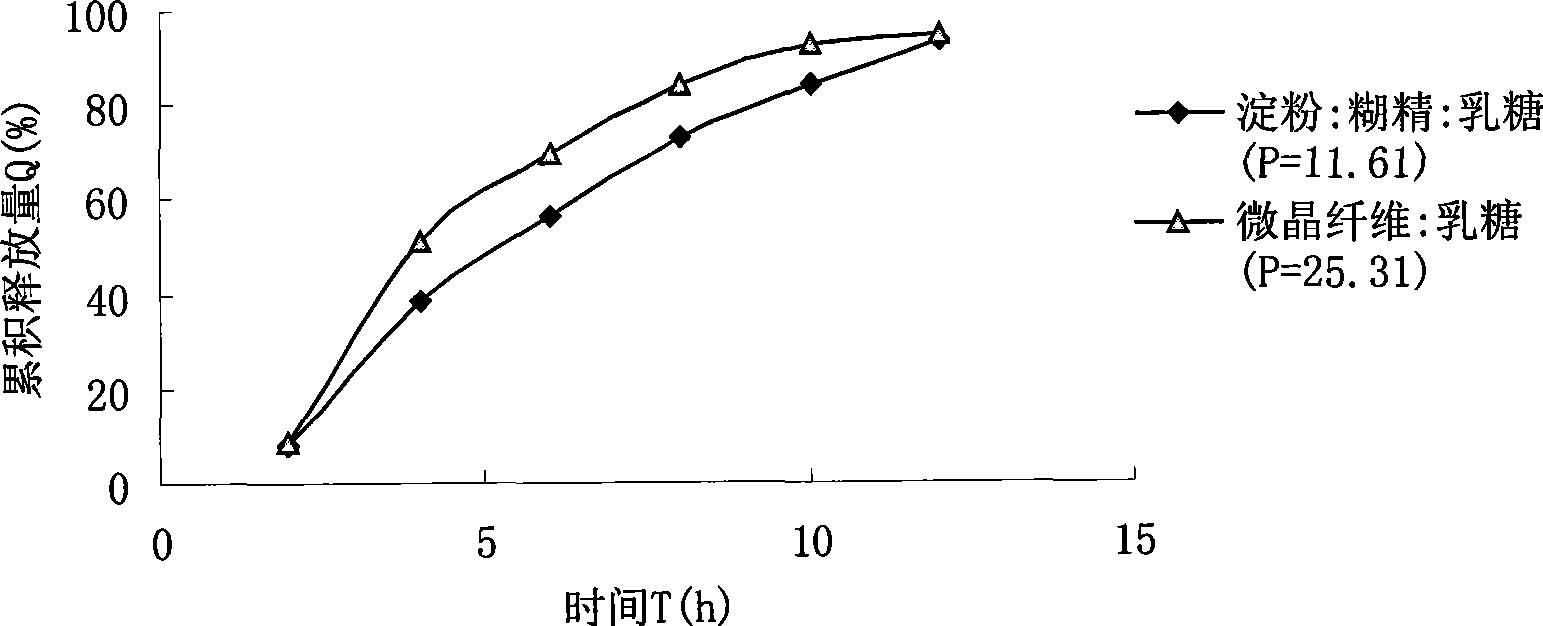
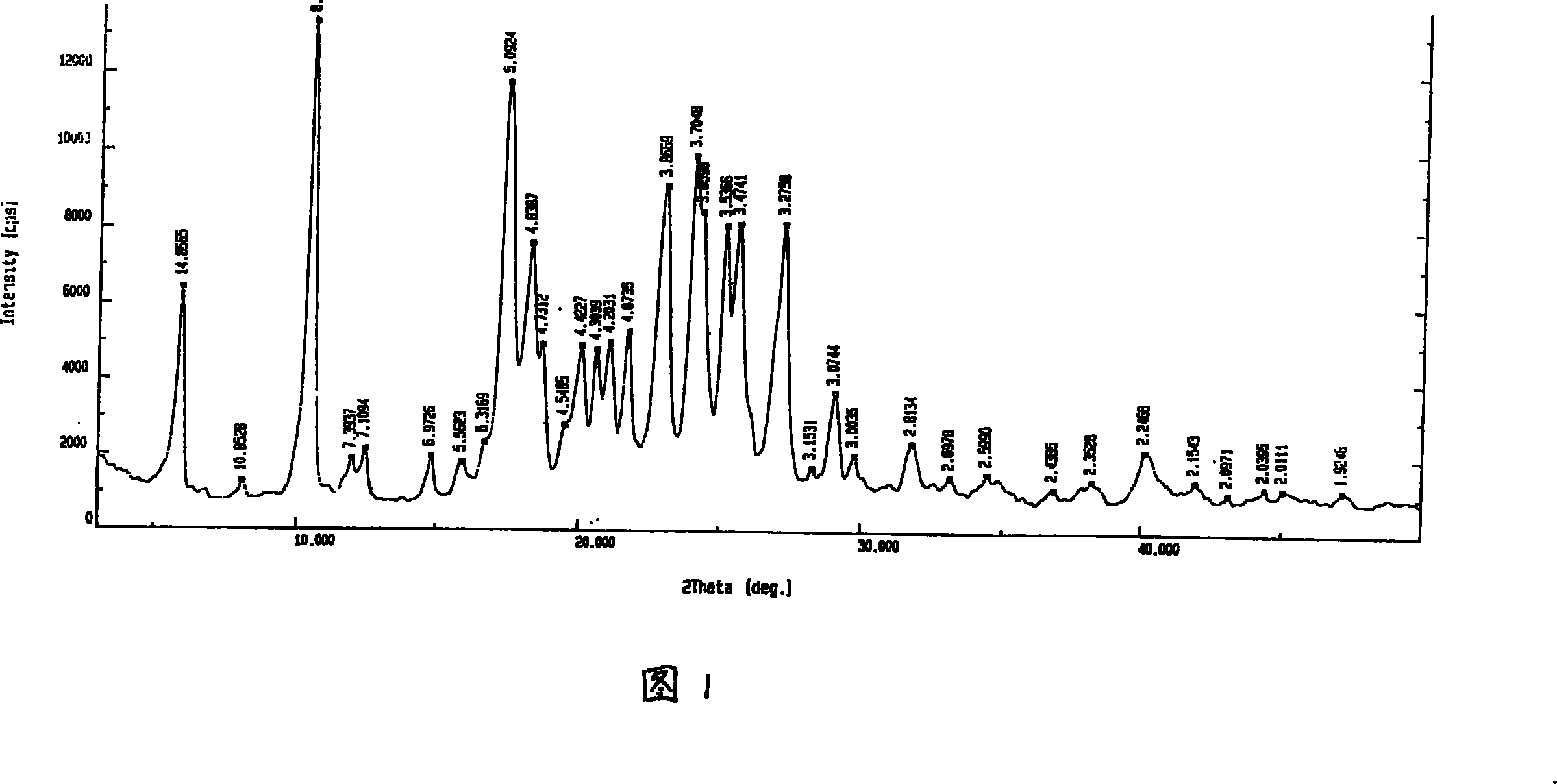
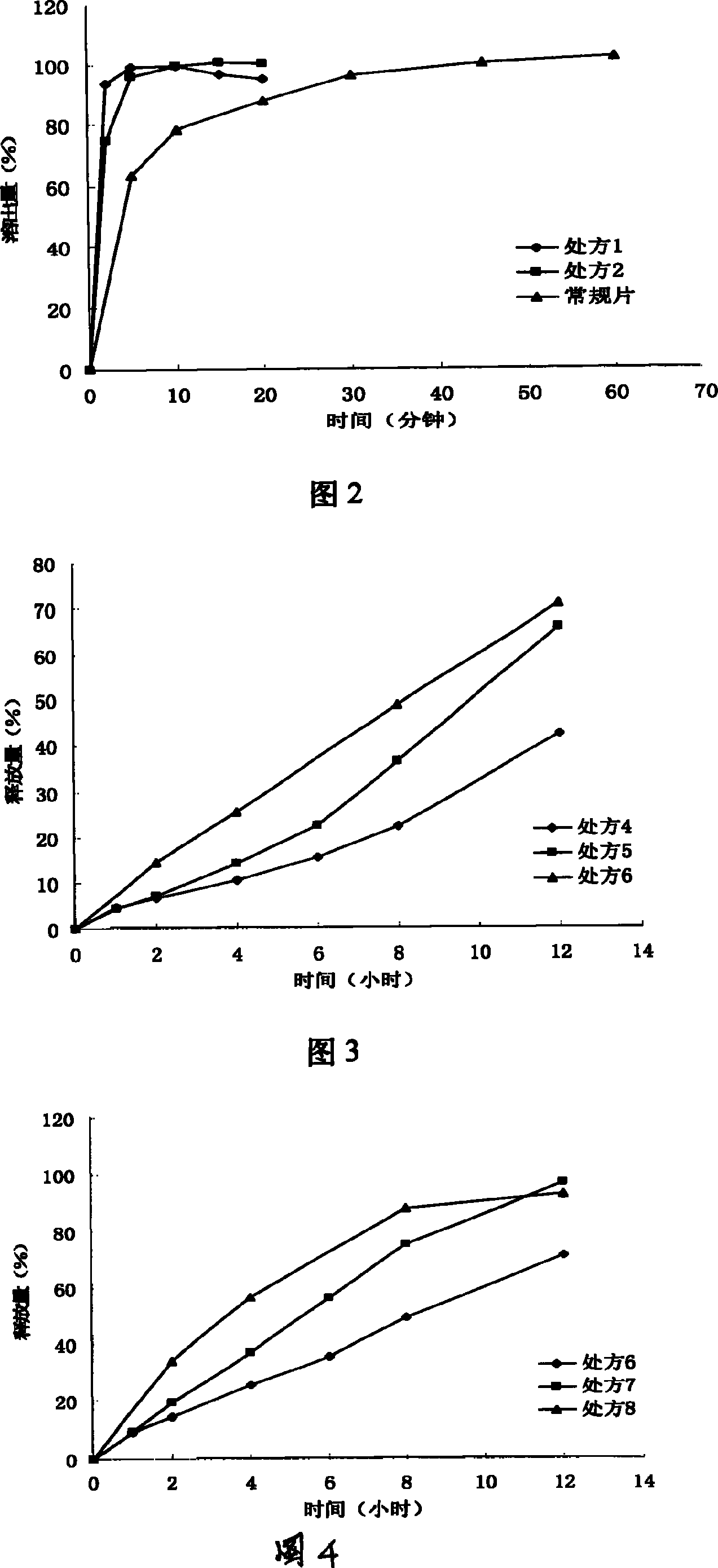
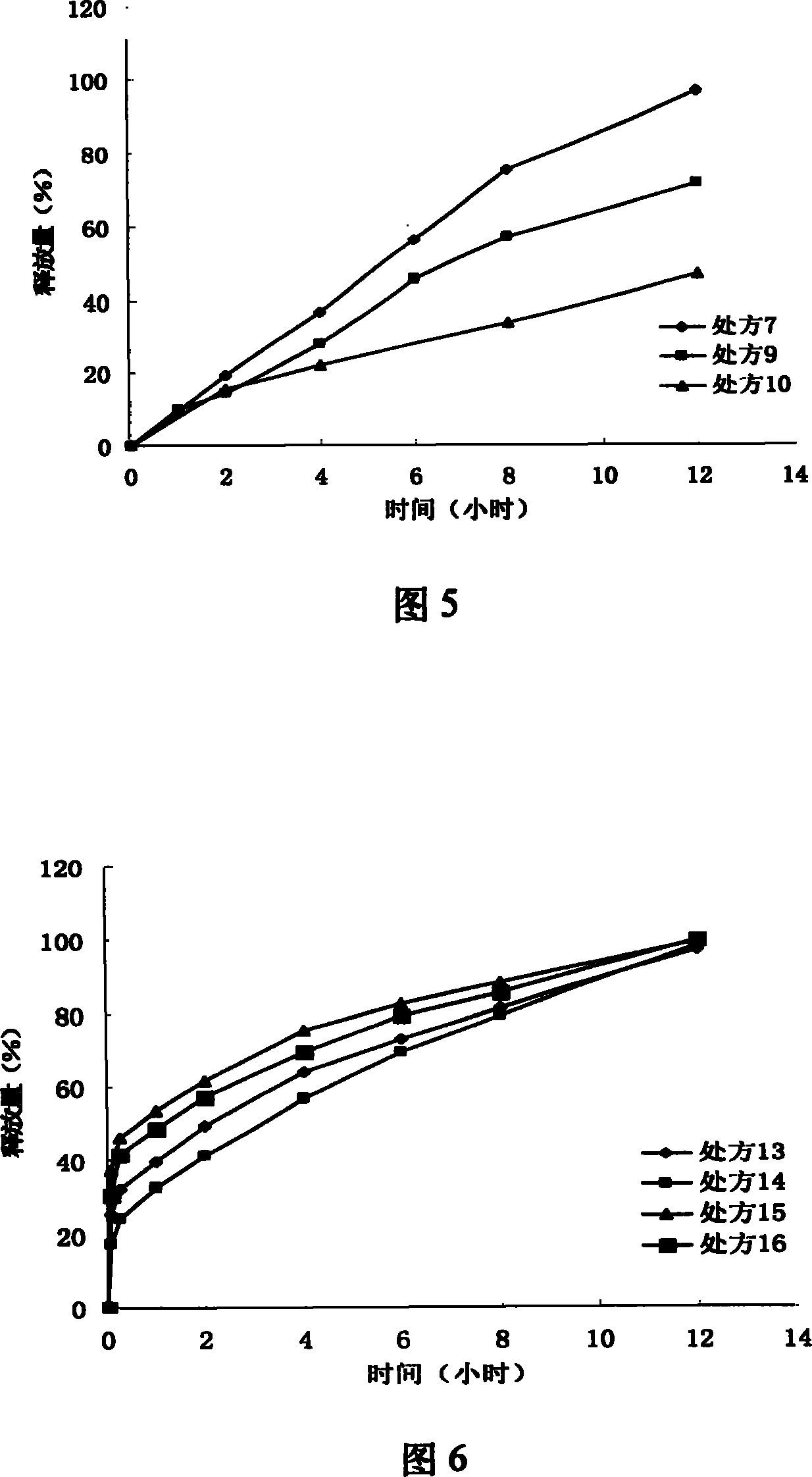
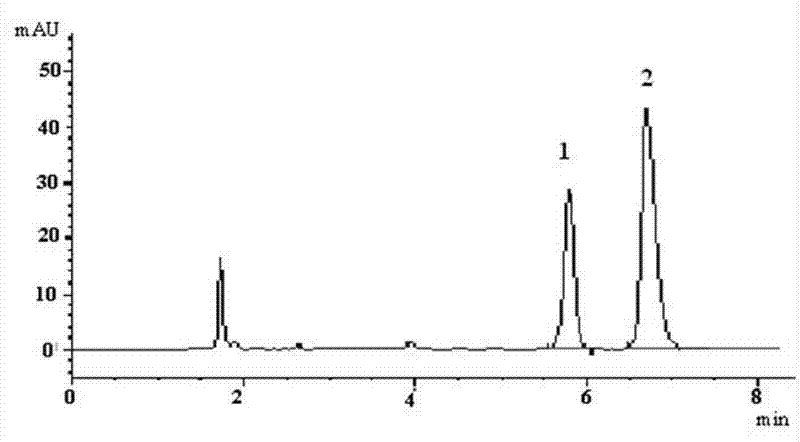
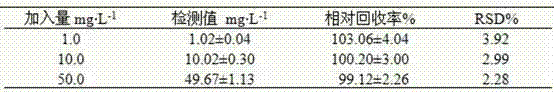
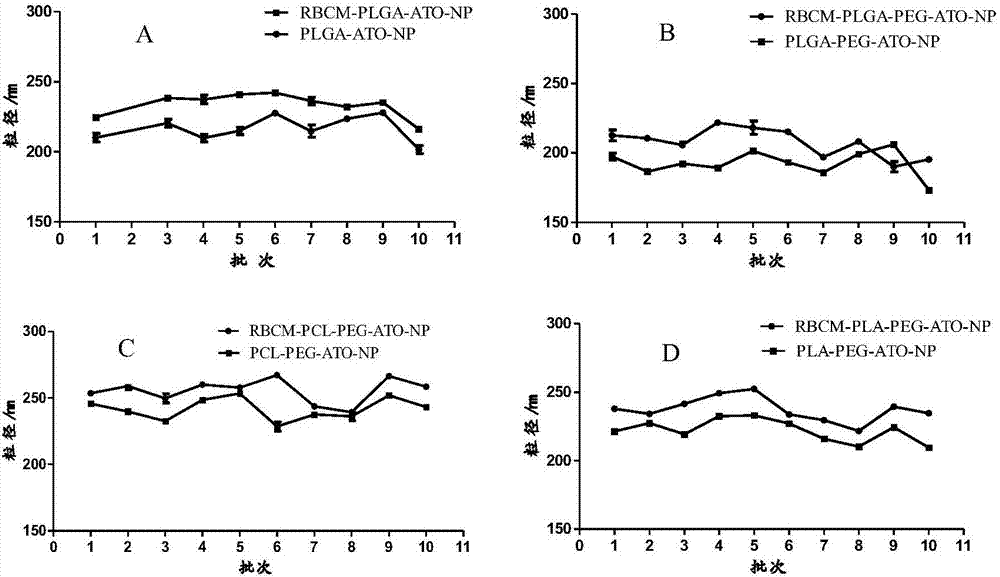
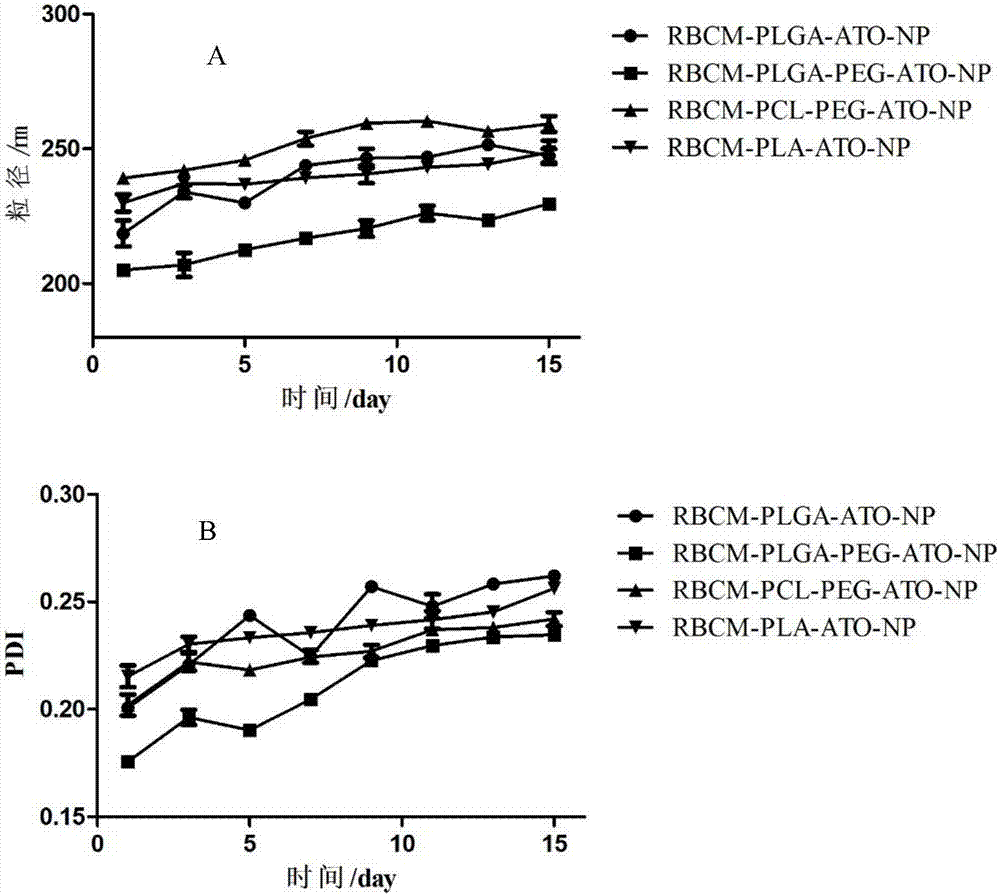
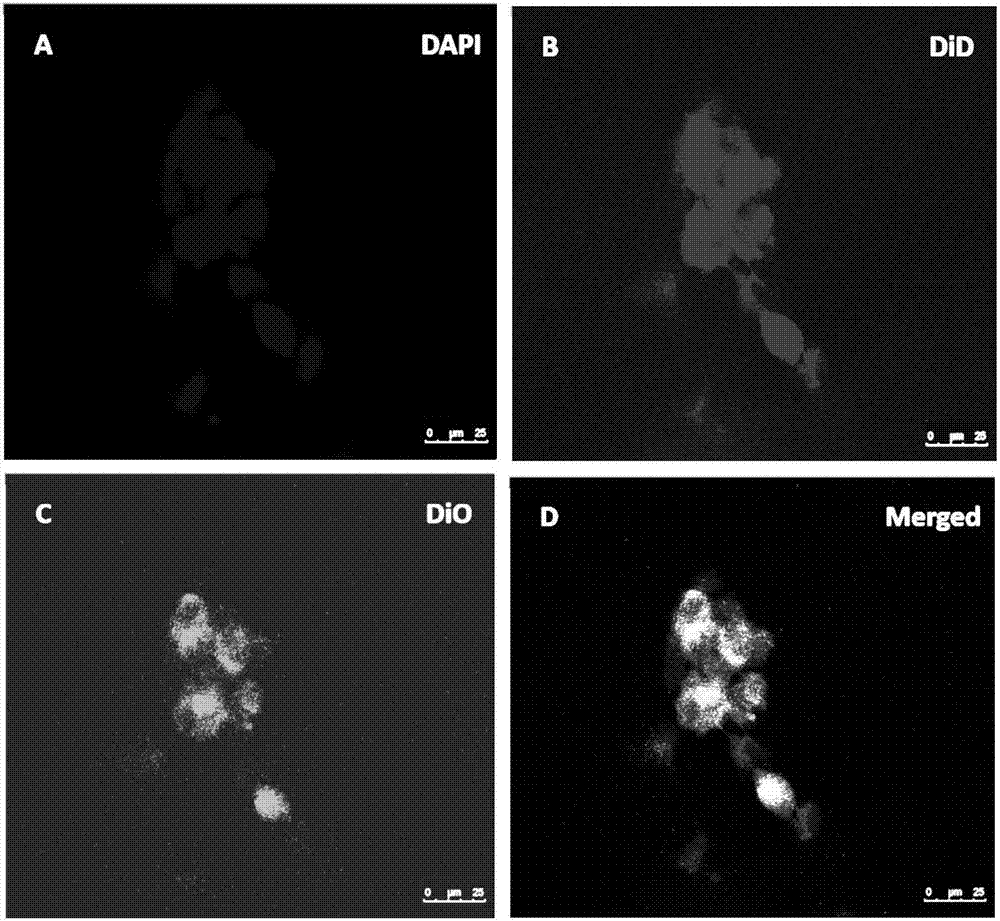
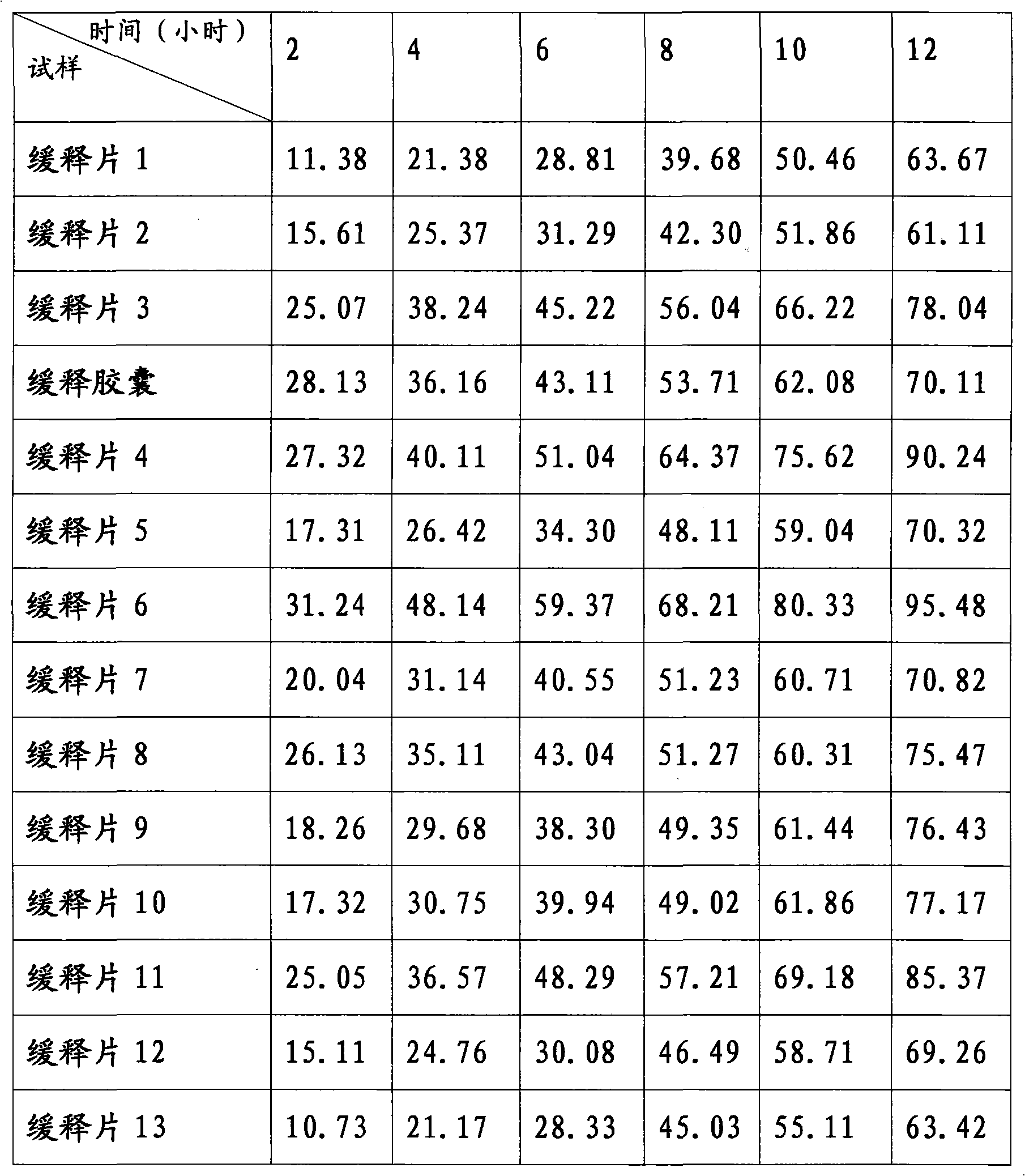
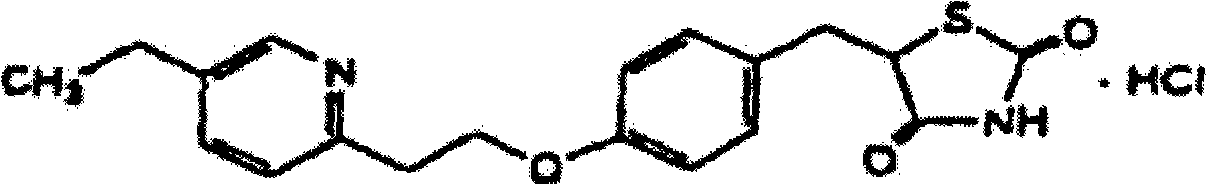
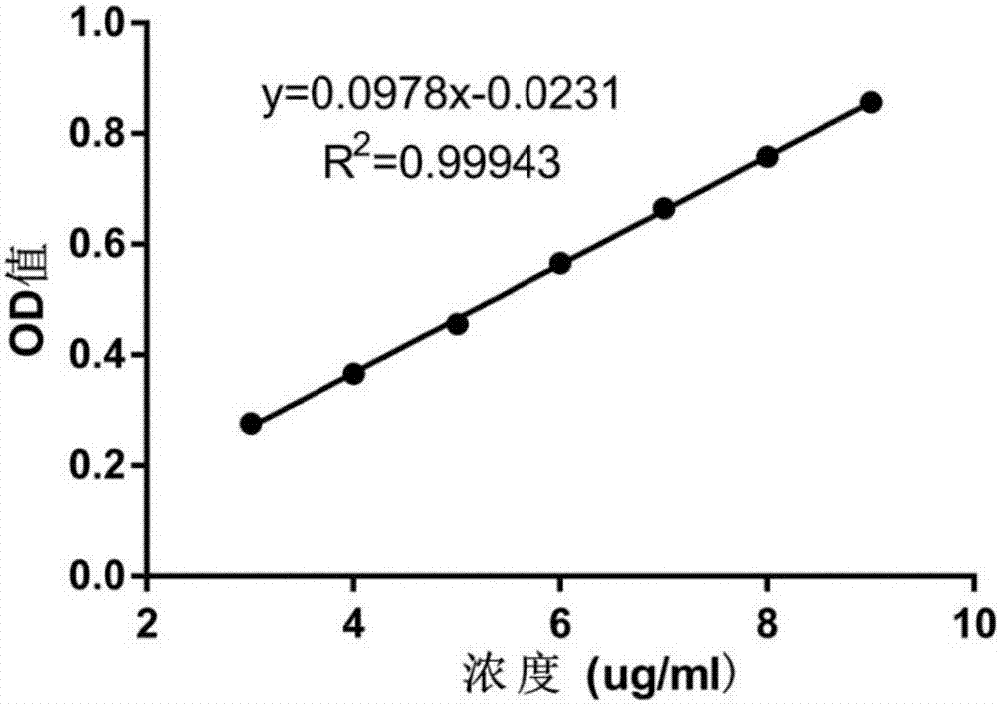
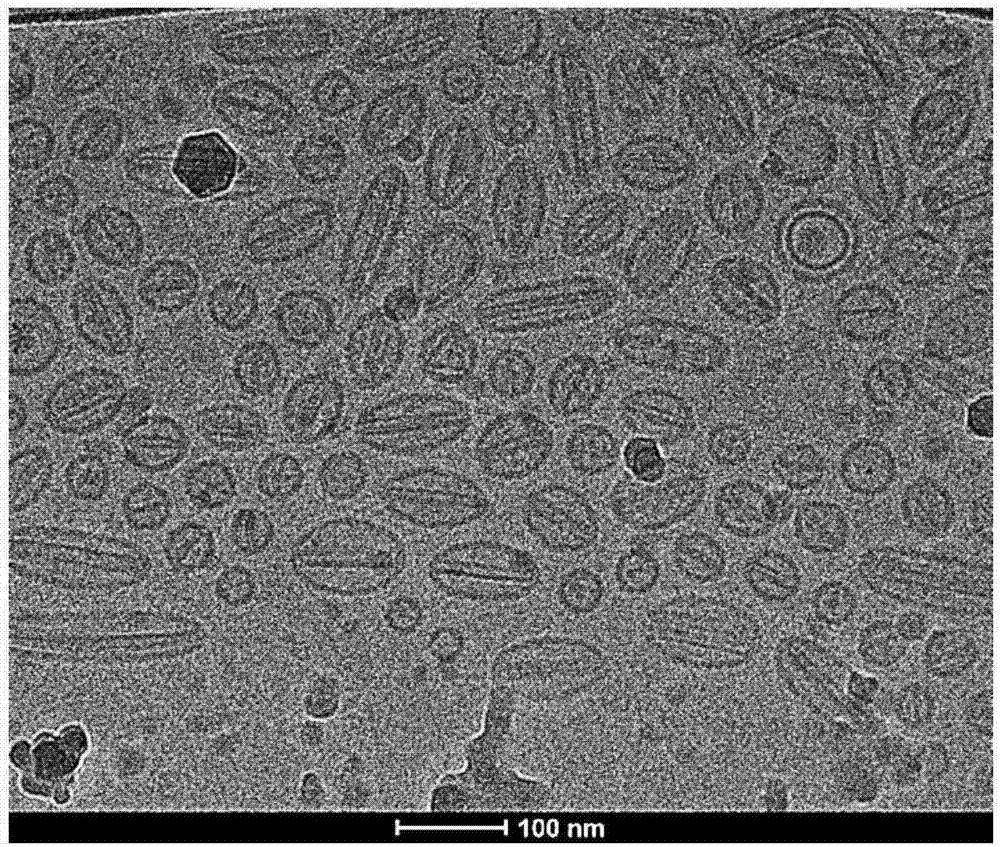
Patents
Literature
943 results about "Blood drug concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral medicine for treating cardio-cerebral vascular disease and preparation process thereof
InactiveCN1502337AReduce the number of daily dosesMedication convenienceOrganic active ingredientsPharmaceutical delivery mechanismOral medicineDisease
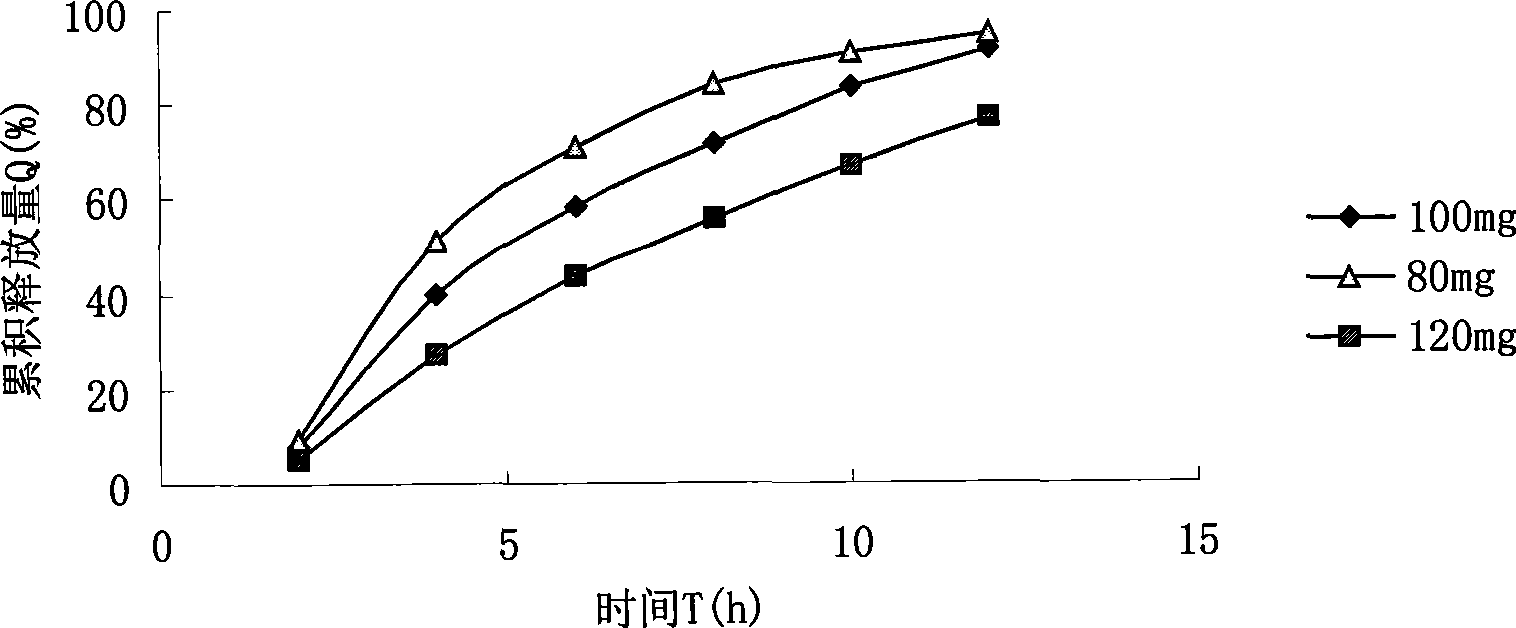
The present invention discloses a slowly-released oral medicine preparation which is made up by using breviscapine as main raw material and has the functions of promoting blood circulation and removing blood stasis, removing obstruction in the channels to relieve pain for curing angiocardiopathy and cerebrovascular disease with obvious therapeutic effect and its preparation method. Said slowly-released tablet (by one tablet) contains 60-120 mg of breviscapine, 20-75 mg of diluent, 50-150 mg of filling agent and 10-50 mg of slow release material. Said invention also provides its preparation method and concrete steps.
Owner:CHENGDU LIST PHARMA
Sustained-release preparations and method for producing the same
The present invention relates to sustained-release preparations prepared by double granulation and methods for producing the same. The sustained-release preparation according to the present invention enables maintenance of effective blood concentration of drug for many hours via sustained release of the drug over 12 hours or more, and further its production is easy owing to convenience of process.
Owner:AMOREPACIFIC CORP
Powdery composition for nasal administration
InactiveUS20020012688A1Promote absorptionHigh maximum blood concentrationPowder deliveryMicrocapsulesNasal cavitySolubility
The present invention relates to a powdery composition for nasal administration, which is characterized in that (1) the composition contains (i) a drug, (ii) a water-absorbing and gel-forming base material such as hydroxypropyl cellulose or hydroxypropylmethyl cellulose and (iii) a water-absorbing and water-insoluble base material such as crystalline cellulose or alpha-cellulose, (2) wherein the amount of the water-absorbing and gel-forming base material is about 5-40 wt % based on the total of the water-absorbing and gel-forming base material and the water-absorbing and water-insoluble base material, and (3) wherein the drug is unevenly dispersed more on / in the water-absorbing and water-insoluble base material than on / in the water-absorbing and gel-forming base material. The present invention provides the powdery composition for nasal administration excellent in absorption of the drug from the nasal cavity and having an extremely increased maximum blood concentration comparing a conventional composition for nasal administration even for a drug having a high solubility in water, a drug having a high lipophilicity or a peptide / proteinaceous drug having a large molecular weight.
Owner:TEIJIN LTD
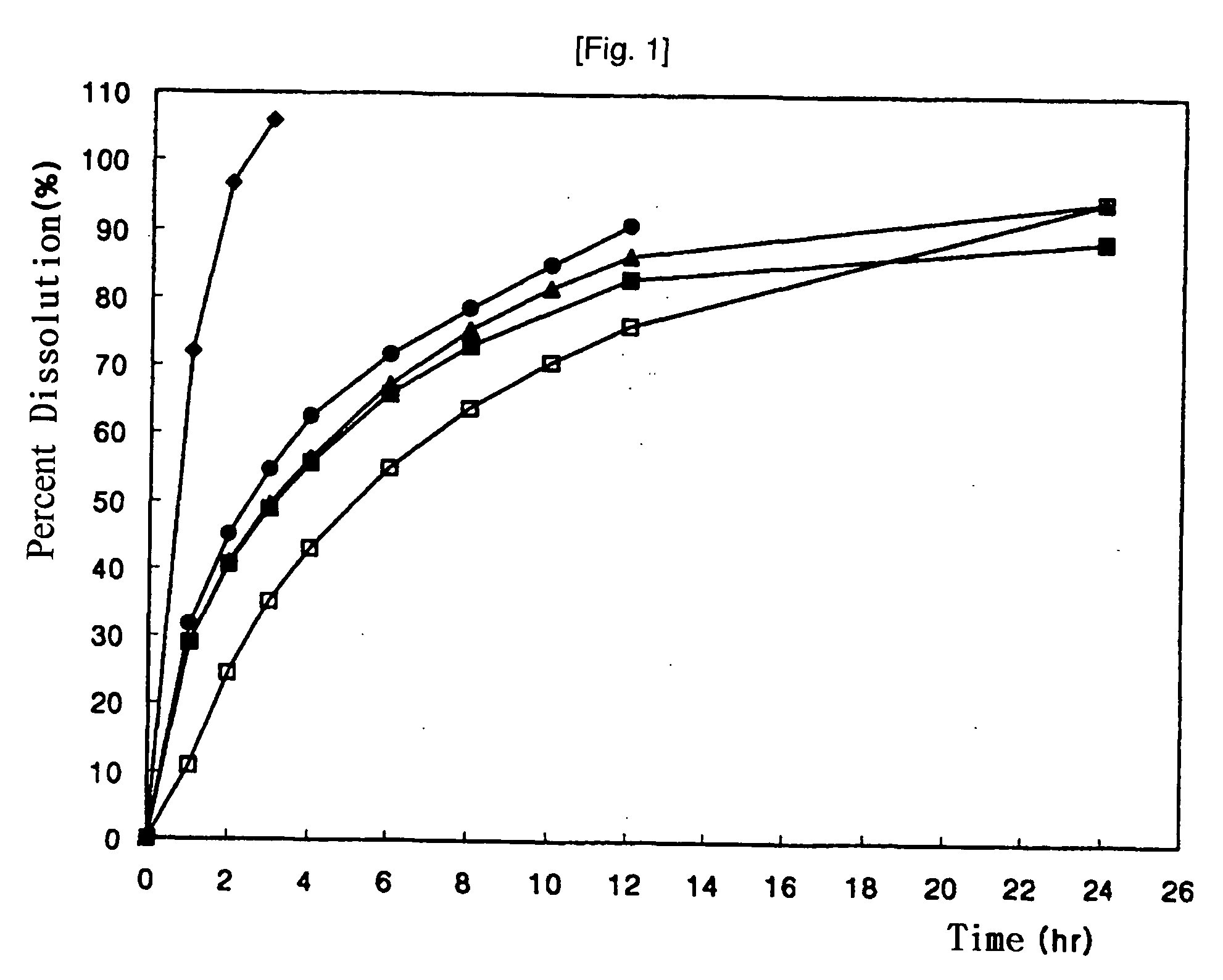
Colchicine bilayer sustained-release tablet and preparing method thereof
InactiveCN101732274AEffective plasma concentration stableStable blood concentrationOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletMedicine
The invention discloses a colchicine bilayer sustained-release tablet which comprises a quick release layer and a sustained-release layer, wherein the quick release layer mainly consists of colchicine, a disintegrant, a filler, a lubricant and an adhesive; the sustained-release tablet mainly consists of colchicine, a sustained-release matrix, a retarding agent, a filler and an adhesive; the weight ratio of the colchicine in the quick release layer to the colchicine in the sustained-release layer is (0.25-0.42) : 1; and the weight ratio of the quick release layer to the sustained-release layer is 1: 2 to 1: 4. The invention also discloses a method for preparing the colchicine bilayer sustained-release tablet. The colchicine bilayer sustained-release tablet releases the drugs through the quick release layer to quickly achieve the effective blood drug concentration, slowly releases the drugs through the sustained-release layer to exert and sustain the stable and uniform effective blood drug concentration, reduces the drug taking times and lightens the toxic or side function.
Owner:普尔药物科技开发(深圳)有限公司
Metformin hydrochloride enteric-coated sustained release tablet and preparation method thereof
ActiveCN101785763AOrganic active ingredientsMetabolism disorderPatient complianceSustained-Release Preparations
The invention discloses a metformin hydrochloride enteric-coated sustained release tablet which is prepared by enteric coating the metformin hydrochloride sustained release tablet. Compared with the prior art, the sustained release tablet integrates with the enteric coating technology to prepare a new form of the metformin hydrochloride enteric-coated sustained release tablet. By using the enteric coating technology, the metformin hydrochloride does not disintegrate in the stomach or stimulate the gastric mucosa, and the adverse reaction of nausea, stomachache and diarrhea caused by medicine taking can be avoided; meanwhile, the metformin hydrochloride is prevented from being damaged by gastric juice, and the bioavailability is improved. The product is a sustained release preparation, the medicine can stably release in vivo, the effective blood concentration can be maintained for a long time, the toxic and side effects caused by over-high blood concentration in a short time are avoided, the medicine taking frequency is decreased, and the patient compliance is improved as well.
Owner:贵州天安药业股份有限公司
Tecovirimat dry suspension and preparation method thereof
ActiveCN102406617AGood liquidityLess foamOrganic active ingredientsPowder deliveryChemistryHalf-life
The invention discloses a tecovirimat dry suspension and a preparation method thereof. The tecovirimat dry suspension provided by the invention comprises the following components in parts by mass: 20 to 30 parts of monoclinic crystal micro-powder of tecovirimat monohydrate, 40 to 80 parts of filler, 5 to 10 parts of suspending aid, and 1 to 5 parts of wetting agent, wherein, in the monoclinic crystal micro-powder of tecovirimat monohydrate, the space group of a monoclinic system is C2 / c, cell parameters are as follows: a is 28.724 (2), b is 10.533 (1), c is 12.902 (1) tenthmeter and beta is 112.18 (1) degrees, the cell volume V is 3614.7 (6) cubic tenthmeter, and the number Z of molecules in unit cell is 8. The dry suspension solid powder has good liquidity, can be quickly transformed into a uniform and stable suspension after being mixed with water, has less foam, low deposition speed and excellent redispersibility, and is convenient for administration of patients. Animal experimentsprove that: the preparation has long half-life period, high blood concentration and strong mousepox virus resisting activity.
Owner:INST OF BIOENG ACAD OF MILITARY MEDICAL SCI OF THE CHINESE
Nimodipine lipid microsphere injection and preparation method thereof
ActiveCN101485632AImprove solubilityImprove stabilityOrganic active ingredientsNervous disorderSolubilityLipid formation
The invention provides a nimodipine lipid microsphere injection, which is prepared from the following components in percentage by weight: 0.08 percent of nimodipine, 0.5 to 2.3 percent of lecithin for injection, 2 to 8 percent of soybean oil for injection, 2 to 8 percent of medium chain fatty acid for injection, 1 to 3 percent of glycerin, 0.1 to 0.2 percent of tween-80, 0.03 to 0.05 percent of sodium oleic acid, and the balance being water for injection. The preparation method comprises steps of preparation of an oil phase, preparation of water phase, preparation of colostrum, homogenization and canning. In the nimodipine lipid microsphere injection, the soybean oil for injection and the medium chain fatty acid for injection are used to prepare the oil phase, the nimodipine is a fat soluble drug and can be better dissolved in the oil phase, the lipid microsphere in which the soybean oil for injection is the main component has solvent characteristics, is non-toxic, and can guide the fat soluble drugs to be dissolved in emulsion particles and perform the metabolism along with lipid oil drops and slowly release, thereby maintaining the effective blood concentration, lowering toxic and side effects of the drugs, increasing the solubility and stability of the nimodipine drug, improving the drug-loading rate and reducing the hydrolysis of the drugs.
Owner:沈阳信康药物研究有限公司
Method for simultaneously detecting main components of Naoxintong capsule in plasma
ActiveCN104614456AInhibit aggregationImprove neurological deficitsComponent separationAstragalosideSalvianolic acid B
The invention provides a method for simultaneously detecting main components of paeoniflorin, beta ecdysterone, laetrile, mulberroside A, caffeic acid, ferulic acid, salvianolic acid B, astragaloside, formononetin, cryptotanshinone and tanshinone IIA of a Naoxintong capsule in a plasma sample by a liquid chromatography-tandem mass spectrometry (HPLC-MS / MS). In a liquid chromatogram, a mobile phase consists of acetonitrile and a formic acid aqueous solution of which the volume fraction is 0.1%, and gradient elution is used. A mass spectrum uses a quick positive and negative ions switching and analyzing mode and an MRM (Multiple Reaction Monitoring) scanning manner. After the Naoxintong capsule is taken, the situations of the changes of the blood-medicine concentration of several kinds of main components in the plasma of a rat are detected at the same time. The methodological survey results indicate that the established method conforms to determination requirements on biological samples in a body; the method is good in sensitivity, high in specificity, stable, reliable, and suitable for detecting substances with lower contents.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE +1
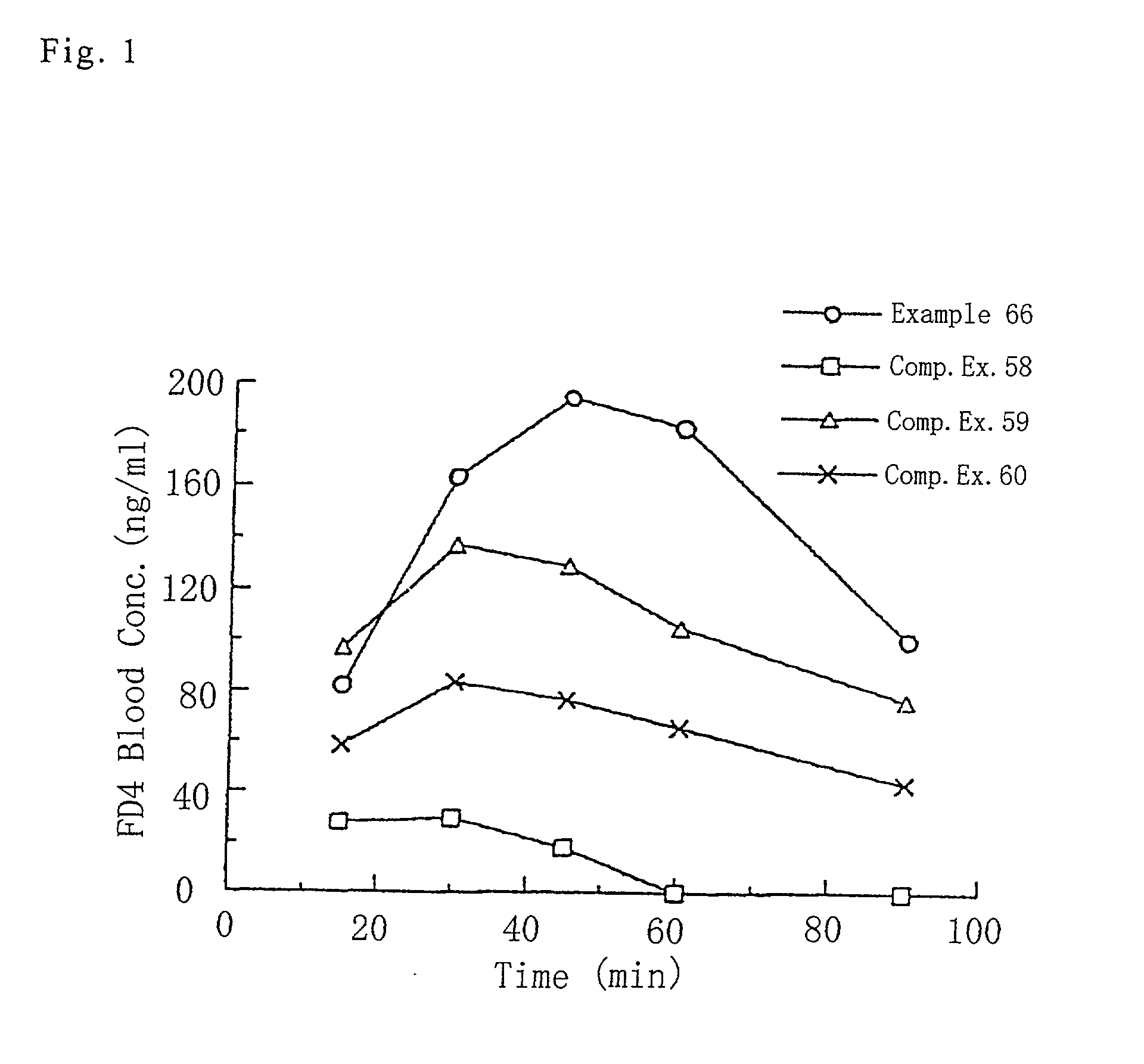
Coated preparation
ActiveUS20100166853A1Increase maximum blood concentration and AUCReduce deviationBiocideMetabolism disorderSolubilityBULK ACTIVE INGREDIENT
The present invention provides a preparation containing pioglitazone or a salt thereof as an active ingredient, which shows high bioavailability of pioglitazone and less interindividual variation in blood drug concentration, as well as a preparation with suppressed color change during preservation. The preparation contains a core containing a pharmaceutically acceptable organic acid with water solubility at 20° C. of not less than 10 mg / mL and pKa1 (a negative common logarithm of the first acid dissociation constant Ka1) at 25° C. of not more than 5, and a coating layer containing pioglitazone or a salt thereof. The coating layer may further contain mannitol or trehalose.
Owner:TAKEDA PHARMA CO LTD
Transdermal plaster of aryl propionic non-steroid antiphlogistic
InactiveCN1387842AImprove adhesionImprove stabilityOrganic active ingredientsAntipyreticTransdermal patchWhole body
The present invention relates to medicine technology and is especially one kind of new preparation form. The transdermal plaster is noe kind of antiphlogistic containing Flubiprofen, Ketoprofen, Ibuprofen, Rosorolfen, Naproxan and other aryl propionic non-steroid. It has three parts including non-sticking layer, medicine layer and lining layer. The medicine layer incldues medicine dispersed in matrix, and the matrix consists of non-polar polymer and plasticizer and may contains tackifier, transdermal promoter and oxidant. It has accurate admistration amount, no stimulation to gastrointestinaltract, relatively higher local medicine density in the affected part and controllable medicine release.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
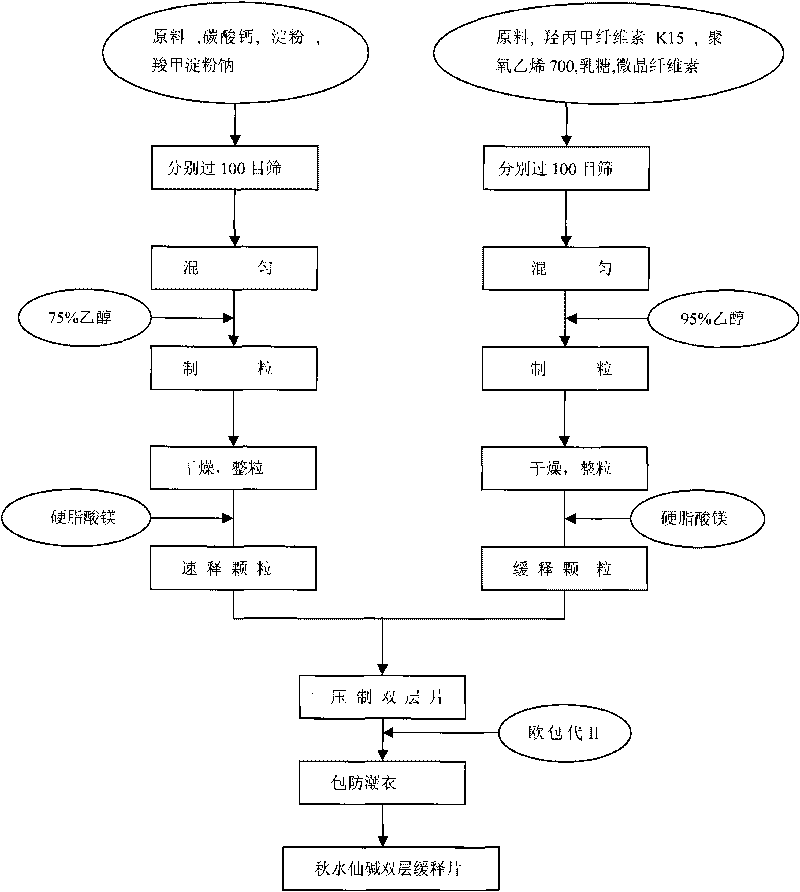
Colchicines gastric floating sustained-release tablet and method for preparing same
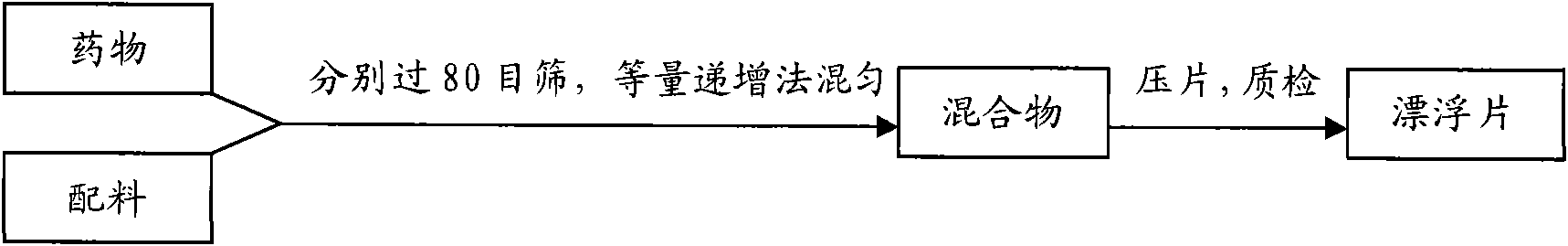
InactiveCN101536990ASchematic diagram of the preparation processOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a colchicines gastric floating sustained-release tablet, which comprises active components of colchicines and pharmaceutic adjuvant according to the weight ratio of 1:24-1,999, wherein the pharmaceutic adjuvant comprises a hydrophilic gel framework material, a effervescing agent, a floating assistant material, a filler, a pH value regulator and a lubricant. The colchicines gastric floating sustained-release tablet can swell quickly in gastric juice or a similar gastric juice medium and can float on the gastric juice for at least 4 hours. The invention also relates to a method for preparing the colchicines gastric floating sustained-release tablet. The colchicines gastric floating sustained-release tablet can reach the effective blood-drug concentration quickly after being taken and then release drugs slowly, and can maintain the balanced blood-drug concentration so as to reduce the dose times, relieve the toxic side effect and improve the bioavailability.
Owner:普尔药物科技开发(深圳)有限公司
Nifedipine osmotic pump controlled release tablet and preparation method thereof
InactiveCN102138912AMaintain blood levelsGood curative effectOrganic active ingredientsPharmaceutical delivery mechanismNifedipineControlled Release Tablet
The invention provides a nifedipine osmotic pump controlled release tablet comprising a drug-containing layer tablet core, a booster layer tablet core, a coating membrane and a single drug-release pore on the surface of the controlled release tablet at one side of the drug-containing layer tablet core. The nifedipine osmotic pump controlled release tablet provided by the invention has stable medicament release velocity, basically realizes zero drug release within 0-20h and basically fully releases drug; therefore, the dosing number of a patient is reduced, and more stable blood drug concentration can be realized after a patient takes the drug. The nifedipine osmotic pump controlled release tablet provided by the invention is a safe, effective, stable, controllable and conveniently-applied medicament new preparation for clinically treating hypertension.
Owner:CHINA PHARM UNIV
Guanidine hydrochloride sustained release preparation and preparation method thereof
ActiveCN102579381AImprove stabilitySimple manufacturing processOrganic active ingredientsGranular deliveryBlood drug concentrationTableting
The invention discloses guanidine hydrochloride sustained release preparation and a preparation method thereof. The guanidine hydrochloride sustained release preparation is mainly prepared by guanidine hydrochloride, a filling agent, sustained release materials and potential of hydrogen (pH) sensitive materials. The preparation method comprises the steps of firstly preparing all raw materials according to proportion, evenly mixing the guanidine hydrochloride, the filling agent, the sustained release materials and the pH sensitive materials at high speed, granulating and drying the evenly mixed powder, finally adding a flow agent, a lubrication agent and an adhesion agent, tableting according to a general method, and achieving guanidine hydrochloride sustained release tablets. The guanidine hydrochloride sustained release preparation is small in side effect, obviously reduces difference of preparation of different batches, improves stability of samples, is convenient long term curing of patients and improves compliance of medicine. A patient can take the guanidine hydrochloride sustained release tablets once a day, so that effective drug concentration in bodies can be guaranteed for 24 hours, and nervous centralis side reactions caused by the fact that a blood concentration peak value is high due to general preparation is reduced.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Aspirin enteric-coated pellet
ActiveCN101596166AEasy accessAvoid stimulationOrganic active ingredientsAntipyreticBlood concentrationBioavailability
The invention discloses an aspirin enteric-coated pellet which comprises the following components in percentage by weight from inside to outside: 5-22 blank core pellet, 55-75 medicinal layer consisting of aspirin and medicinal excipients and 20-35 enteric-coated layer. The invention also discloses a method for preparing the aspirin enteric-coated pellet and an aspirin enteric-coated capsule containing the aspirin enteric-coated pellets. The aspirin enteric-coated capsule has the advantages of good stability, small stimulation, steady blood concentration, high bioavailability, and the like.
Owner:Yung Shin Pharm Ind (Kunshan) Co Ltd
Novel dosage form of sinomenine medicament or hydrochlorate thereof and preparation technique thereof
The invention discloses a sinomenine or an enteric-coated controlled-release tablet of hydrochloride thereof. The prepared enteric-coated controlled-release tablet hardly releases the drug in artificial simulated gastric juice, but can slowly and smoothly release the drug in artificial simulated intestinal juice; the sustained release time of the drug can achieve more than 12 hours or even 24 hours; the enteric-coated controlled-release tablet is taken once or twice daily, the plasma drug concentration in vivo is smooth, and the peak-valley phenomenon of the plasma drug concentration is reduced; as the prepared enteric-coated controlled-release tablet hardly releases the drug in stomach, the contacted concentration of the drug with the gastric mucosa is small, the stimulation of the stomach caused by the drug is alleviated. As the prepared enteric-coated controlled-release tablet sustainedly slowly releases the drug in intestinal tract, the times of the drug administration are reduced, and the patient compliance is improved, thereby being applicable to the needs of the clinical development.
Owner:HUNAN ZHENGQING PHARM GRP CO LTD
Controlled release dosage form of tacrolimus
InactiveUS20080181947A1Improved controlled releaseConstant blood concentration profileBiocidePowder deliveryBlood concentrationWater soluble
A controlled release dosage form of tacrolimus, comprising a solid dispersion of tacrolimus, wherein a controlled release base, which is selected from the group consisting of a water-soluble macromolecule, a gum base, and a membrane forming agent and does not form the solid dispersion of tacrolimus, is further contained, is disclosed. The controlled release dosage form of tacrolimus has an excellent controlled release and shows a stable blood concentration.
Owner:ASTELLAS PHARMA INC
Immunomodulator slow-release preparation and preparation method thereof
ActiveCN103610658AProlong the action timeUniform and constant action timeOrganic active ingredientsPill deliveryBlood concentrationProlonged-release tablet
The invention discloses an immunomodulator slow-release preparation and a preparation method thereof. A lenalidomide slow-release tablet is composed of a slow-release layer and an optional quick-release layer, wherein the slow-release layer contains active ingredients of lenalidomide and a slow-release framework material simultaneously; the quick-release layer does not contain the slow-release framework material. The lenalidomide slow-release tablet disclosed by the invention is capable of slowly and uniformly releasing medicines by virtue of the slow-release framework material, so as to reduce the release speed, delay the time to peak, prolong the action time of lenalidomide, and provide a uniform and constant blood concentration. Moreover, The lenalidomide slow-release tablet disclosed by the invention is simple in prescription and excellent in quality stability; the preparation process is simple to operate, free from special treatment and production equipment, low in production cost, and beneficial to batch-enlarged industrial production for the product; the preparation method is high in yield, the granulation and crushing procedures are simple and practicable to operate, the intermediate material is good in stability, flowability, compressibility and content uniformity, and completely meets the requirements of tabletting, and the surface of the prepared tablet is smooth and beautiful.
Owner:AC PHARMA CO LTD
Iguratimod oral double-layer sustained-release preparation
InactiveCN101095671AAccelerate time to peak blood concentrationImprove in vitro dissolutionOrganic active ingredientsAntipyreticSide effectEffective action
The invention relates to oral double iguratimod controlled release formulation, which comprises fast release layer and slow release layer that are composed of 8-30% micronizing iguratimod crystal powder and medical findings, and the granule size of iguratimod crystal powder is 1-10 um. The effective component in fast release layer is released in short time and reaches to effective blood chemical concentration for effective action; the iguratimod in sloe release layer is released gradually and maintains effective blood medical concentration for continuous effective action. The invention overcomes shortcomings of short effective action time and a little high toxic effect.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
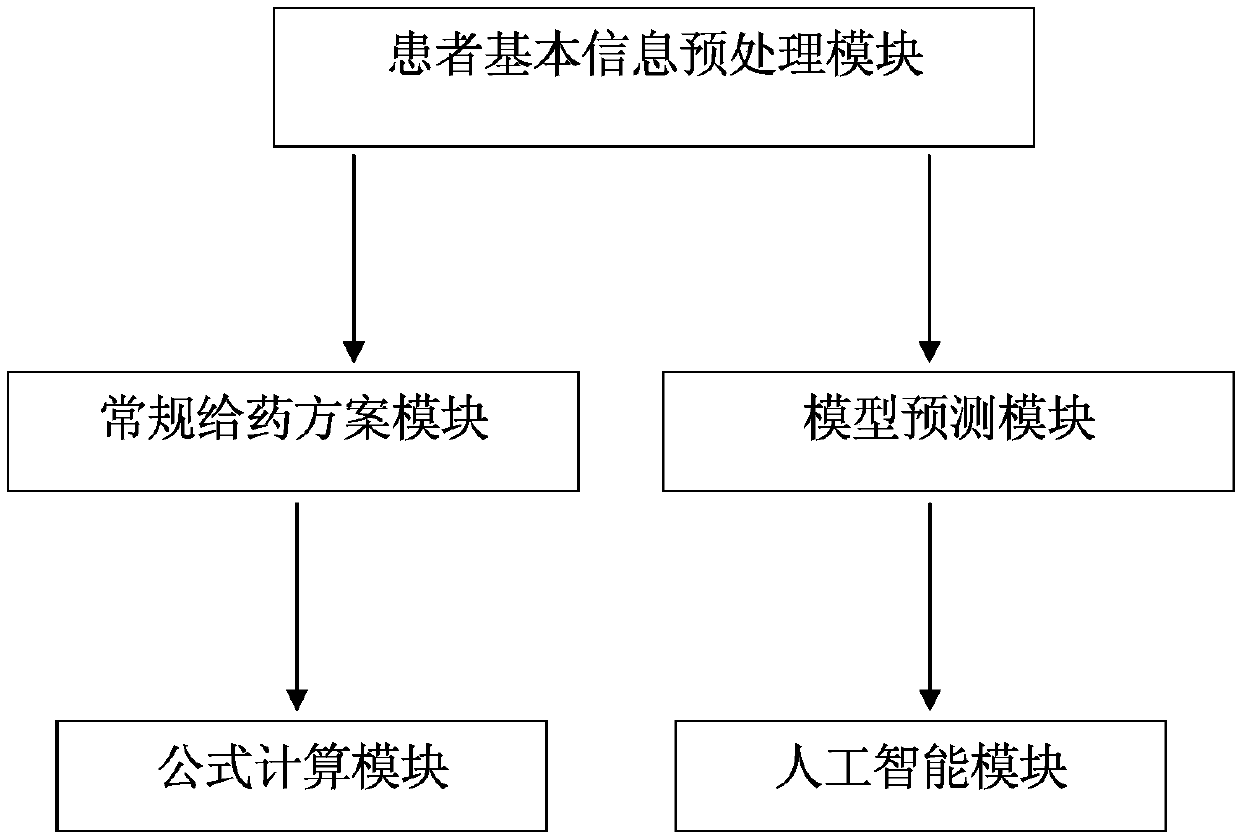
Clinical medication decision support system
InactiveCN107845411ATargetedImproving the decision-making level of clinical medicationDrug and medicationsMedical automated diagnosisMedication regimenRoutine practice
The invention provides a clinical medication decision support system, and the system comprises a patient basic information preprocessing module which is used for collecting and preprocessing the biological basic information of a patient who needs to adjust a medication scheme through a decision system; a routine dosage regimen module which is used for formulating and adjusting a common patient dosage regimen; a formula calculation module which is used for formulating and adjusting a medicine and group dosage regimen meeting a formula calculation condition; a model prediction module which is used for setting an initial scheme of a special patient and predicting the treatment effect or blood concentration; and an artificial intelligence module which is an advanced function and is used for the prediction of the overall treatment scheme of an individual concerned patient. The levels of the above function modules are set from the bottom to the top, the complexity of the above function modules is also gradually improved and the requirements for the parameters of the above function modules are also increased gradually. For most of patients, the modules at the low level can achieve the expected treatment effect. For the patients who have the complex diseases and need to be attended specially, the advanced modules are needed for support.
Owner:THE AFFILIATED HOSPITAL OF QINGDAO UNIV
Quantitative analysis method for ion mobility spectrum
ActiveCN106198704AGood repeatabilityImprove accuracyMaterial analysis by electric/magnetic meansCorrelation coefficientQualitative analysis
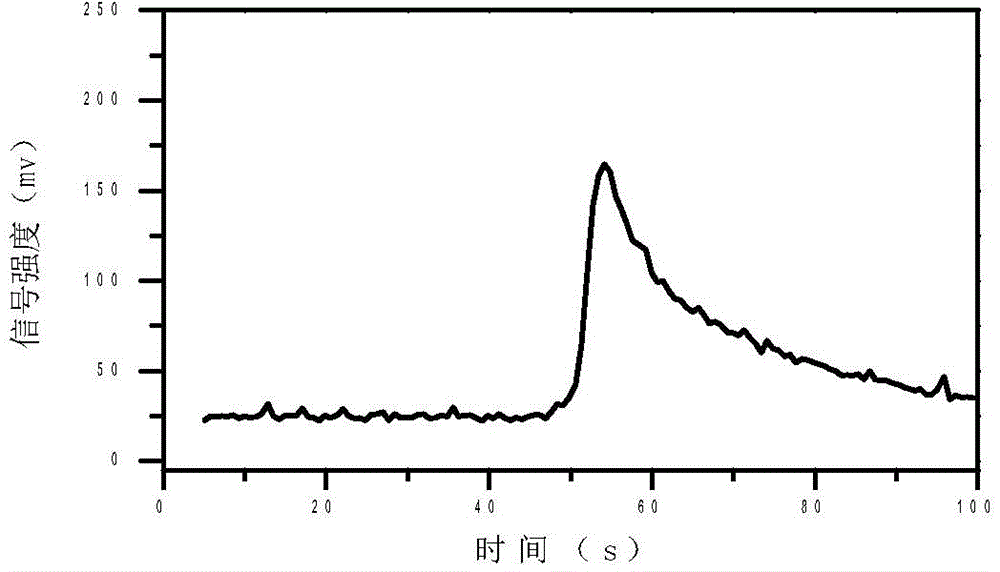
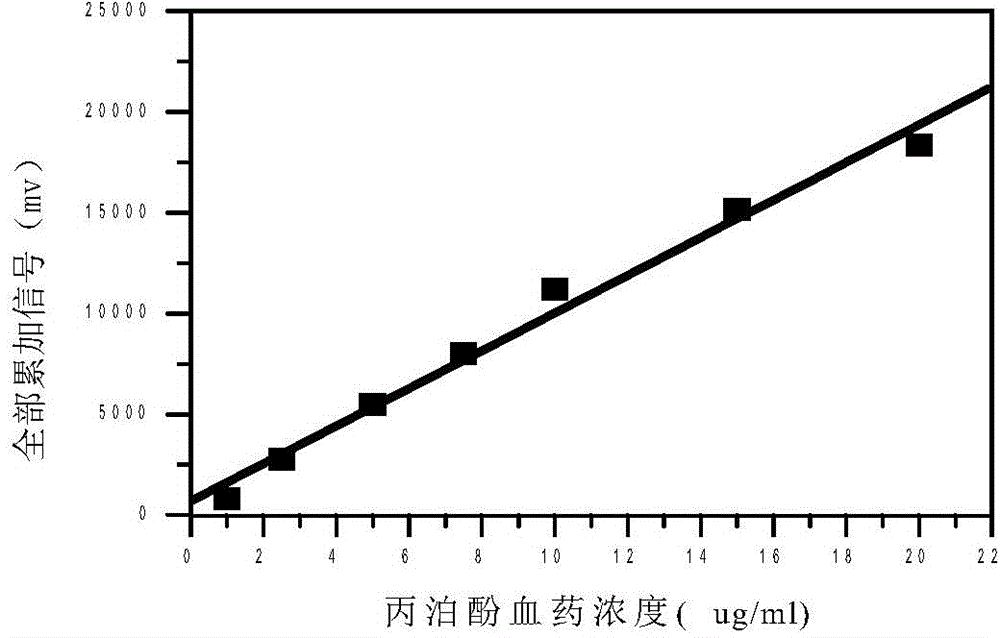
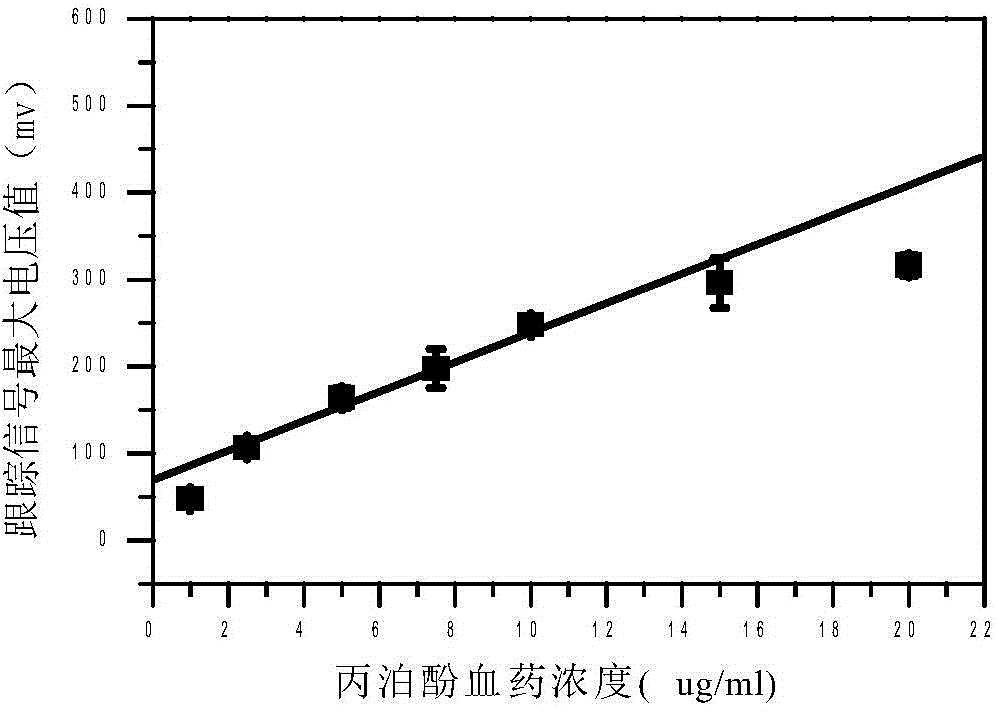
The present invention discloses an accurate quantitative analysis method for an ion mobility spectrum rapid detection sample. According to the method, based on the migration time obtained through qualitative analysis of an ion mobility spectrum, the thermal analysis process of the whole sample to be detected is completely recorded, and the recorded curve is defined as the tracking trend line of the thermal analysis of the sample; the samples with different concentrations are prepared by adopting the ion mobility spectrum technology as the base detection technology, and the tracking trend of the sample is subjected to data analysis; and by adopting the propofol in blood as an example, a standard curve equation y=671.48+934.42.x of the ion mobility spectrum detection is established between the plasma concentration of 1-20 ppm, wherein the correlation coefficient r is 0.9928. According to the present invention, the method is simple, rapid and efficient, and can be widely used for the quantitative analysis of the ion mobility spectrum rapid detection sample.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
A kind of method for measuring paraquat blood concentration
InactiveCN102288696AEasy pretreatmentThe pre-processing process is simpleComponent separationParaquatBlood plasma
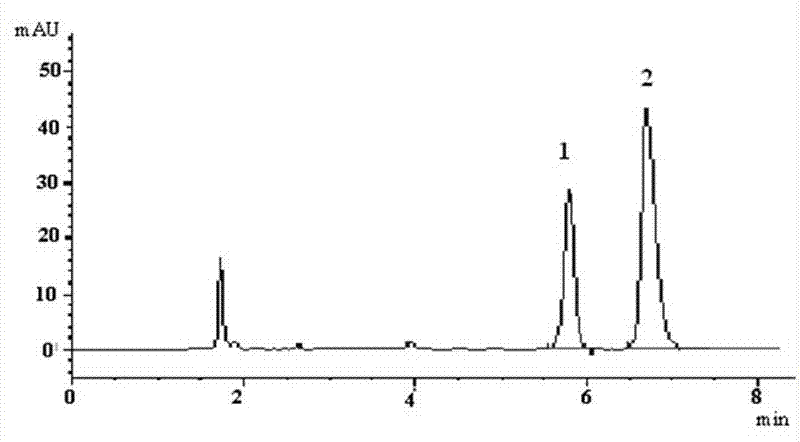
The invention discloses a method for measuring blood concentration of paraquat. The method comprises the following steps of: (1) pretreating a sample, namely adding aqueous solution of an internal standard substance and a protein precipitation agent acetonitrile into a plasma sample, performing whirl mixing, centrifuging and sampling supernatant; (2) separating the sample, namely adopting a universal C18 liquid chromatographic column, and using an ion pairing agent in an acid mobile phase, wherein the acid mobile phase is mixed solution of 3mmol.L<-1> aqueous solution of sodium dodecyl sulfate, 0.2 percent of aqueous solution of trifluoroacetic acid, acetonitrile and water; and (3) detecting by using a diode array detector, namely detecting by using the diode array detector at a detectionwavelength of 250 to 260nm, measuring peak areas of the internal standard substance and the paraquat, and calculating the blood concentration of the paraquat through least square method linear regression. The plasma sample is easy and convenient to pretreat, the detection process is sensitive and rapid, toxic substances and the blood concentration thereof can be rapidly determined in actual application, and the method is high in clinical application value.
Owner:HENAN UNIV OF SCI & TECH
Red cell membrane encapsulated polyester-type As2O3-supported nano particles and preparation method thereof
ActiveCN106943378AEvenly distributedGood sustained releaseInorganic active ingredientsPharmaceutical non-active ingredientsRed cell membraneDrug delivery
The invention discloses a red cell membrane encapsulated polyester-type As2O3-supported nano particles and a preparation method thereof. The nano particle employs the red cell membrane as shell and a polyester-type material as a core. The polyester-type material is supported with the As2O3. The particle size of the nano particle is about 200 nm. The nano particle has a round shape and is uniformly distributed, has a clear core-shell structure and good stability, is free of causing hemolysis and agglutination of red cells and can be used in intravenous injection. The drug delivery system, compared with a free medicine, has significantly sustained releasing effect, and can solve the problem of quick increase of blood concentration after intravenous injection of the As2O3. Because that substances, being 3-400 nm in size, are generally not discharged from kidney or a rejection system, so that the drug delivery system has advantages in circulation in blood system and achieves passive targeted effects; the blood concentration of a toxic medicine is reduced and further toxicity is reduced, so that a new strategy for development of toxic antitumor medicine preparations is provided.
Owner:SHANGHAI JIAO TONG UNIV
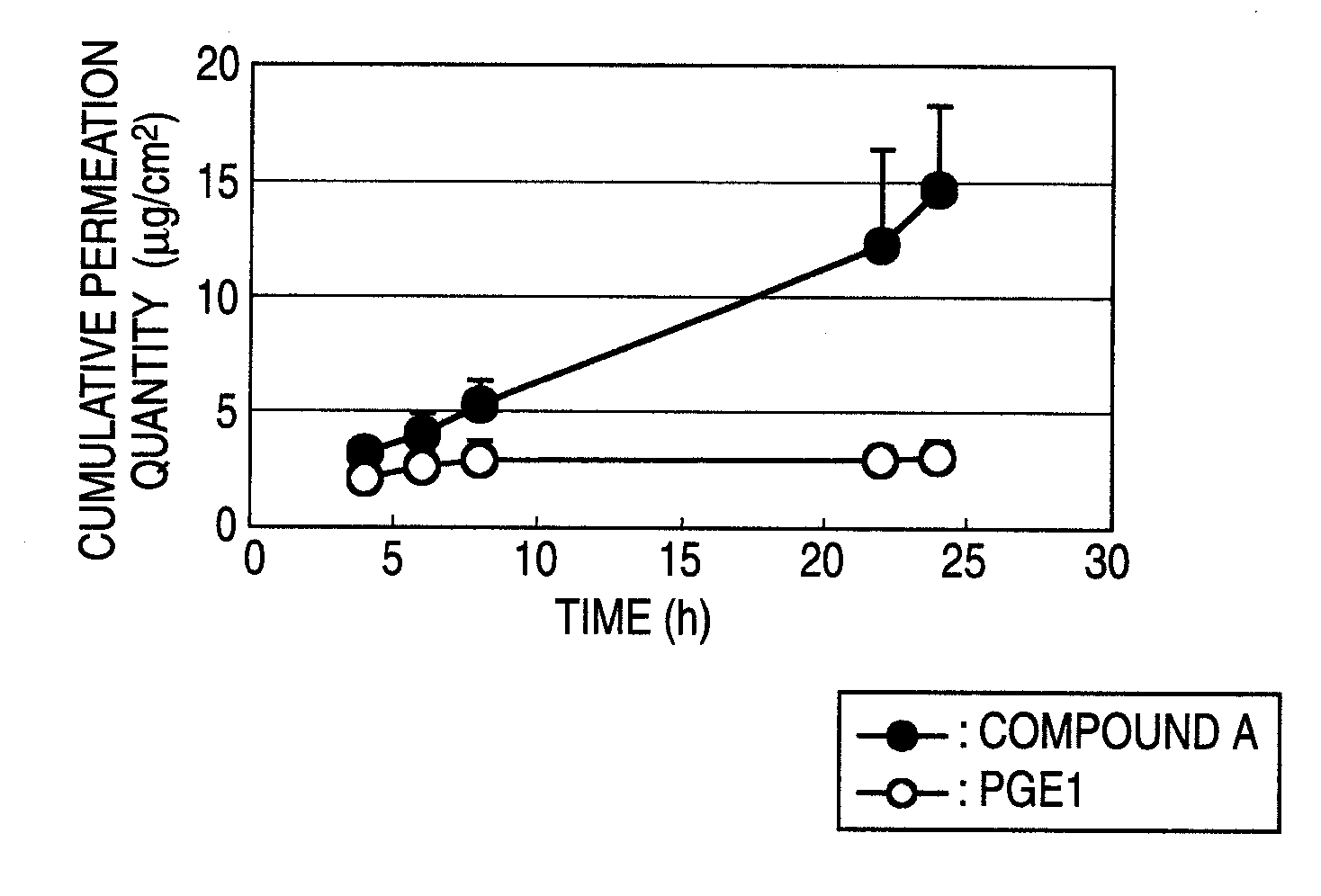
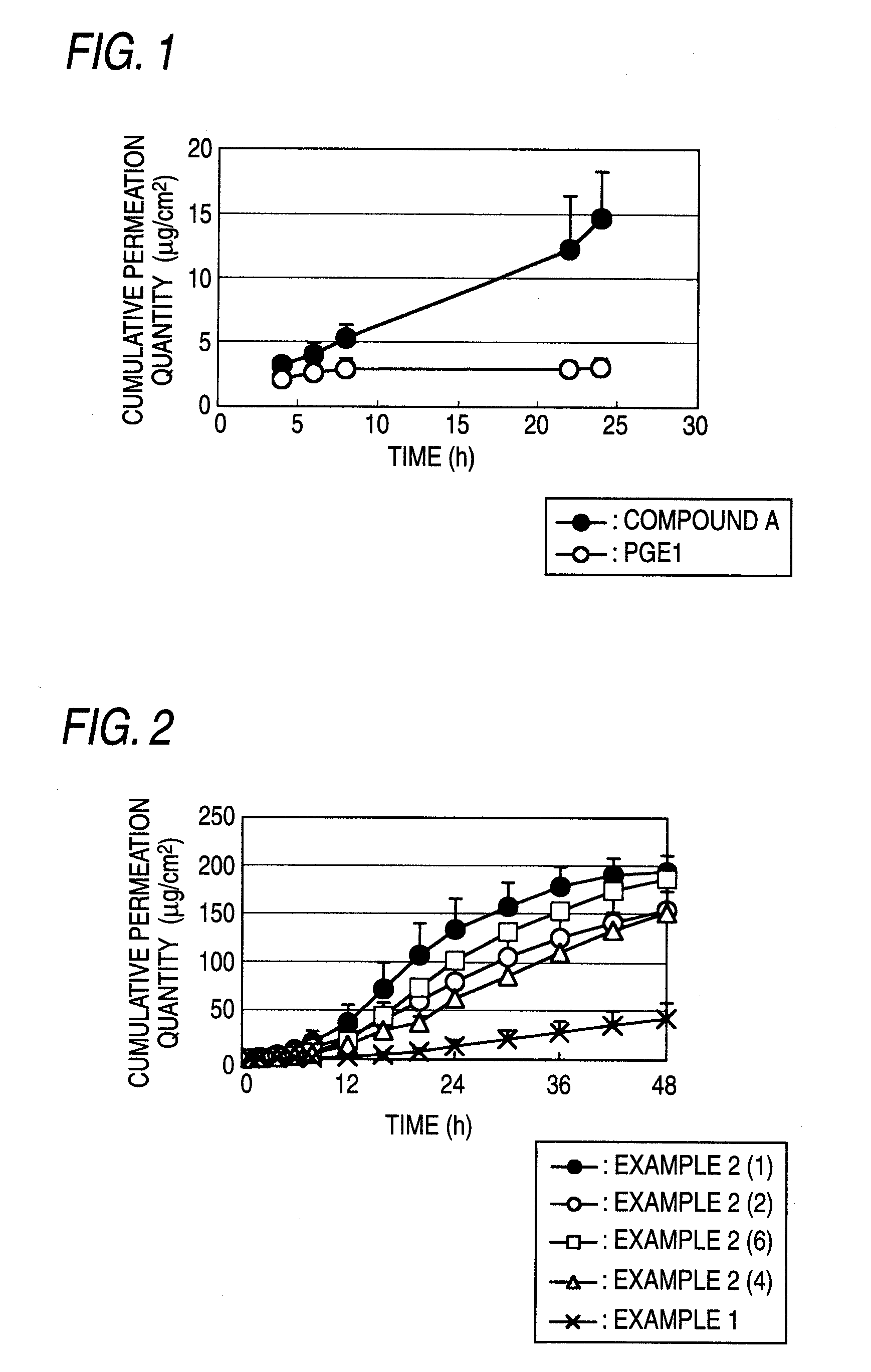
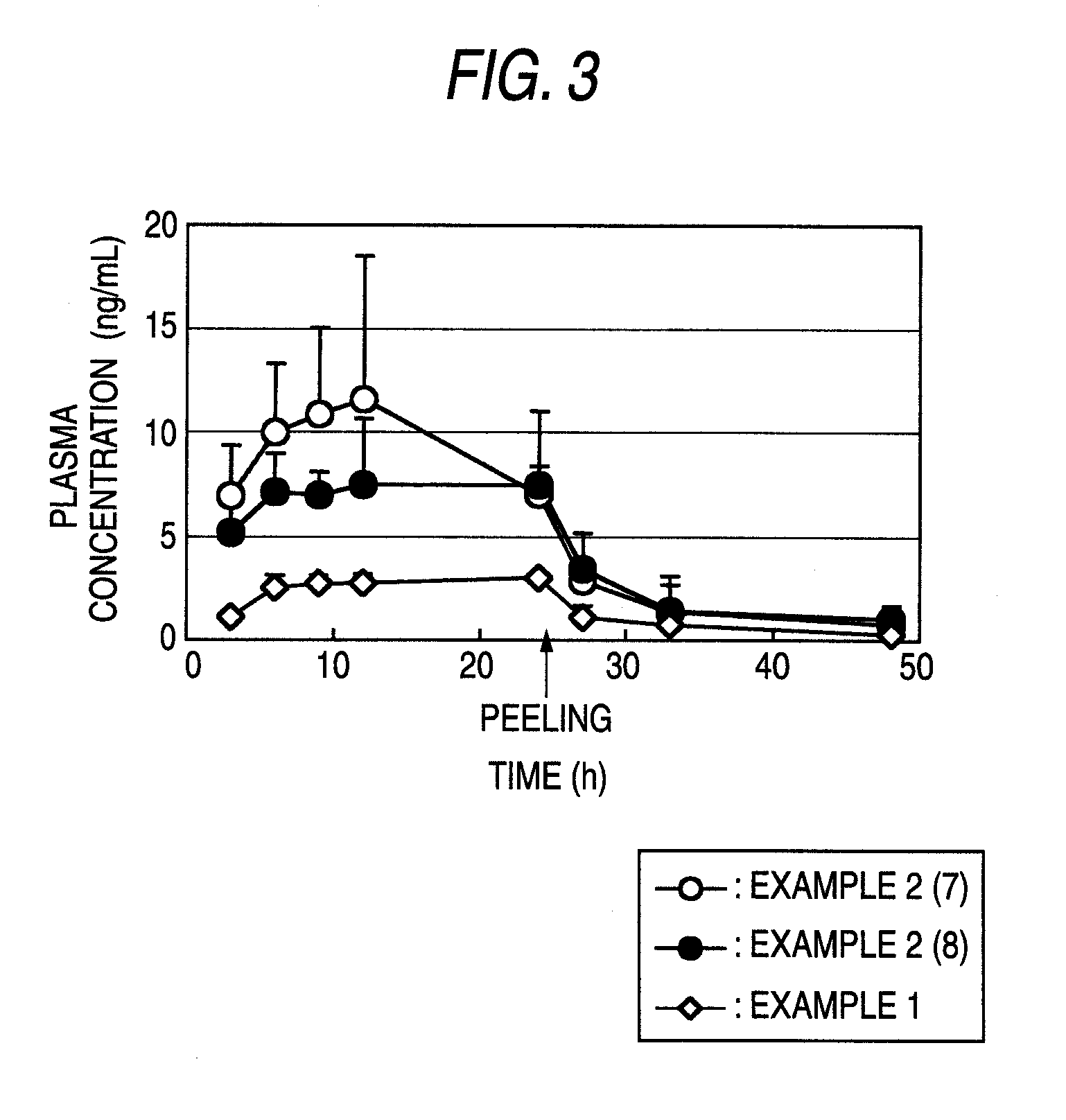
Trenadermal absorption preparation
The present invention relates to a blood concentration regulation type percutaneous absorption preparation, which comprises an ester form of 4-{[2-((1R,2R,3R)-3-hydroxy-2-{(1E,3S)-3-hydroxy-4-[3-(methoxymethyl)phenyl]but-1-enyl}-5-oxocyclopentyl)ethyl]sulfanyl}butanoic acid and a base for external preparations. The blood concentration regulation type percutaneous absorption preparation of the present invention can stably maintain blood concentration of the active form of the present invention and is safe since there are no side effects. Therefore, it can be used as a percutaneous absorption preparation which can be persistently administered.
Owner:ONO PHARMA CO LTD
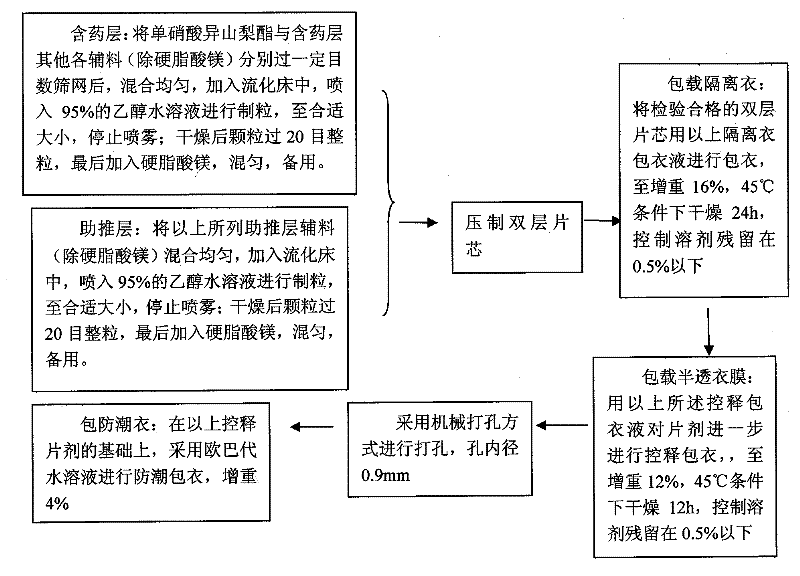
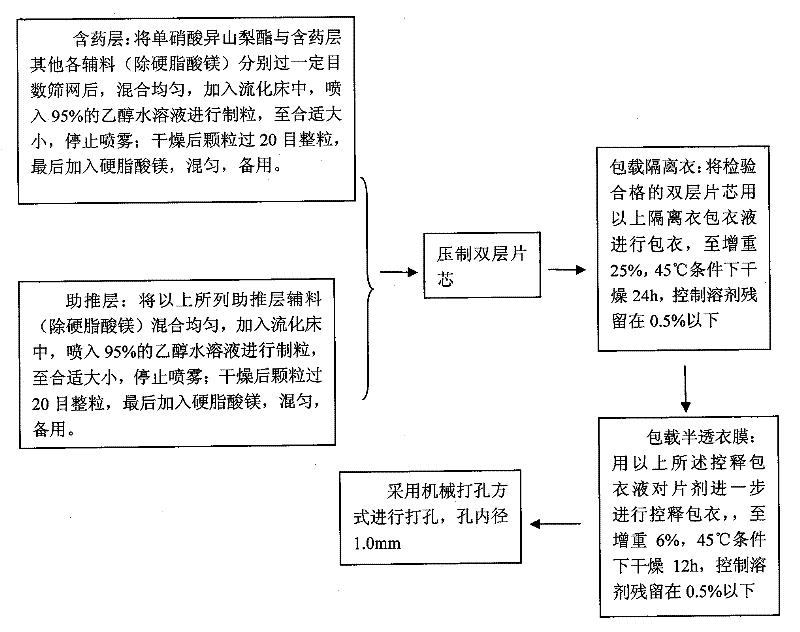
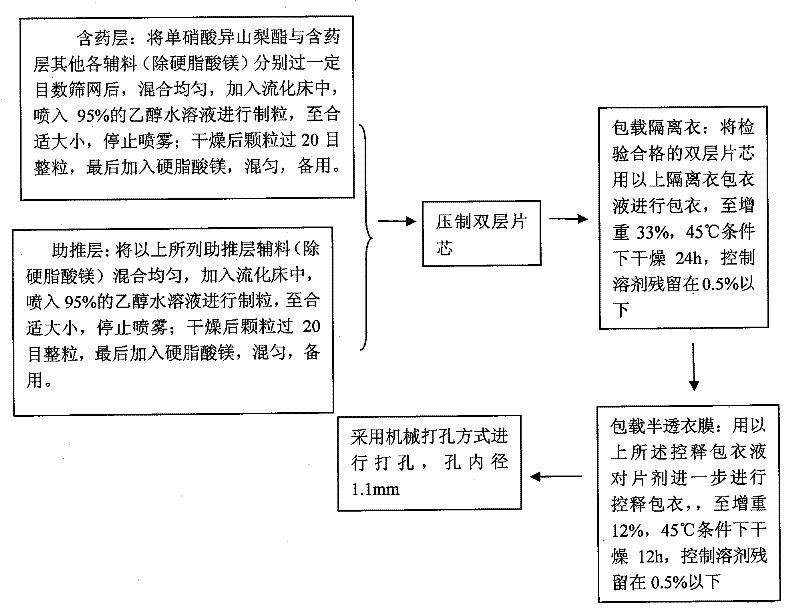
A kind of isosorbide mononitrate controlled release preparation and preparation method thereof
ActiveCN102293760AGood treatment effectAvoid drug resistancePill deliveryPharmaceutical non-active ingredientsEngineeringDrug release
The invention discloses an isosorbide mononitrate timing controlled release preparation and a preparation method thereof. The isosorbide mononitrate timing controlled release preparation of the present invention has certain time-lag and long-acting stationary release, and comprises a semi-permeable membrane with drug release apertures, an isolating coating and a double layer label including a drug contained layer and a boosting layer. The isosorbide mononitrate timing controlled release preparation of the present invention is an osmotic pump tablet with certain time-lag and long-acting stationary release, and can provide a more lasting and stationary plasma concentration and is bioequivalent compared with a sale long-acting preparation Elantan.
Owner:SHANGAI PHARMA GRP CO LTD
Zaltoprofen sustained-release preparation and preparation thereof
InactiveCN101297790ASimple production equipmentGood process reproducibilityOrganic active ingredientsAntipyreticNon steroid anti inflammatory drugAntiinflammatory drug
The invention discloses a new formulation of a new non-steroidal anti-inflammatory drug zaltoprofen-a sustained-release preparation of the zaltoprofen and a preparation method thereof; each tablet / each granule of the zaltoprofen sustained-release preparation contains 80 to 240mg of the zaltoprofen and 10 to 120mg of excipients which play the sustained-release function; the sustained-release preparation is conductive to the long-term maintenance of the effective plasma-drug concentration and can improve the efficacy, reduce the side effects, reduce the times of being taken and facilitate the taking and the carry.
Owner:SICHUAN DIHON MEDICAL DEV
Pioglitazone hydrochloride sustained-release dropping pill and preparation method thereof
InactiveCN101269040AIncrease surface areaHas a wetting effectOrganic active ingredientsMetabolism disorderPharmaceutical formulationBlood drug concentration
The invention discloses to a drug compound for treating diabetes and particularly relates to a drug compound oral pharmaceutical formulation adopting pioglitazone as the ingredient. The drug compound aims to supplement the deficiency of the prior oral pharmaceutical formulation used for treating Type-2 Diabetes and provide a drug compound oral pharmaceutical formulation, sustained-release pioglitazone dropping pill which has high bioavailability, controllable release time, long-acting effect, low frequency of drug taking, steady plasma concentration, low cost and absence of contamination during the production. The sustained-release pioglitazone dropping pill adopts pioglitazone as the chemical ingredient and is prepared jointly with the medicinal carriers of hydrophilic frame ingredients and hydrophobic frame ingredients used as the stroma.
Owner:北京博智绿洲医药科技有限公司
Tacrolimus sustained release tablets and preparation method thereof
InactiveCN101361722AImprove medication complianceReduce the number of dosesOrganic active ingredientsPharmaceutical delivery mechanismLiver and kidneySide effect
The invention relates to a sustained release tablet dosage, in particular to a Tacrolimus sustained release tablet used for liver and kidney transplantation patients and a preparation method thereof, belonging to the field of medicine technology. The invention is characterized in that Tacrolimus and adhesive are taken and dissolved in ethanol to prepare Tacrolimus solution; sustained release materials, thinner and flow agent are taken, mixed and dispersed uniformly and then added into the Tacrolimus solution to prepare wet particles, which are dried, ground and added and mixed with lubricant; finally, the Tacrolimus sustained release tablet is prepared after tabletting and drying. The sustained release tablet has the unique advantages of: reducing administration times, improving the administration adaptability of patients; after administration, having stable blood concentration, small toxic and side effects and improving the drug effect and safety; reducing the total dosage of drugs so as to achieve the greatest curative effect by the lowest dosage; being capable of being realized on most of tablet production lines and easily accepted by manufacturing units and manufacturers due to relatively simple preparation process; and having relatively low manufacturing cost and broad clinical application prospect.
Owner:贾祥波 +1
Dasatinib liposome preparation, and preparation method thereof
ActiveCN107260680AAchieve slow and controlled releaseImprove lipophilicityOrganic active ingredientsInorganic non-active ingredientsDasatinibBiocompatibility Testing
The invention relates to a dasatinib liposome preparation, and a preparation method thereof. The dasatinib liposome preparation is high in biocompatibility; target modification can be carried out; sustained and controlled release of dasatinib can be realized; and it is beneficial for maintenance of relatively high plasma drug concentration in a long term, improvement of drug distribution, and increasing of drug bioavailability. The dasatinib liposome preparation possesses excellent lipophilic performance, is capable of passing through phospholipid bilayer in a molecular form and entering into inner water phase; pH value of liposome inner water phase is relatively low, the dasatinib moleculars are capable of bonding with hydrogen ions so as to form dasatinib ions, and combination with anions in an ammonium salt solution and forming of insoluble salts are realized, diffusion of dasatinib in the inner water phase into an outer water phase is inhibited, dasatinib is coated by the liposome inner water phase steadily, encapsulation efficiency and storage stability are improved, and excellent in-vitro slow release effect is achieved.
Owner:SHANGHAI JIAO TONG UNIV
Ticagrelor pharmaceutical composition and preparing method thereof
ActiveCN105998026AImprove complianceQuick effectOrganic active ingredientsInorganic non-active ingredientsPatient complianceTicagrelor
The invention relates to a sustained-release preparation composition of an anticoagulant drug ticagrelor (also called brilinta) and a preparing method thereof. The composition comprises ticagrelor and other pharmaceutical accessories. The composition is characterized in that the composition can be quick in acting, also can ensure persistent effectivity within 24 hours, and further can reduce Cmax based on guarantee of the effective plasma concentration, thereby improving patient compliance and reducing drug safety problem in the premise without reduction of curative effect.
Owner:SICHUAN HAISCO PHARMA CO LTD
Composition of beta-nicotinamide mononucleotide or beta-nicotinamide mononucleotide precursor, and preparation method and application of composition
InactiveCN111166760AImprove internal functionTo promote metabolismOrganic active ingredientsMetabolism disorderLiver and kidneyBlood concentration
The invention relates to the field of biological medicines, and in particular to a composition of beta-nicotinamide mononucleotide or a beta-nicotinamide mononucleotide precursor, and a preparation method and application of the composition. The composition comprises the following raw materials in percentage by weight: 0.001 to 25 percent of beta-nicotinamide mononucleotide or a precursor thereof,0 to 20 percent of a transdermal absorption enhancer, 2 to 50 percent of an auxiliary component and the balance of water. After being absorbed by a human body in a mode of transdermal administration or external administration, the composition can exert effects of improving internal functions of a human body, improving metabolism of a human body or improving epidermal functions of a human body, andresisting skin inflammation, allergy and skin aging. Through the mode of transdermal administration and external administration, metabolic burden of liver and kidney can be avoided, blood concentration can be kept constant, and administration is convenient.
Owner:浙江安赛新材料科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com