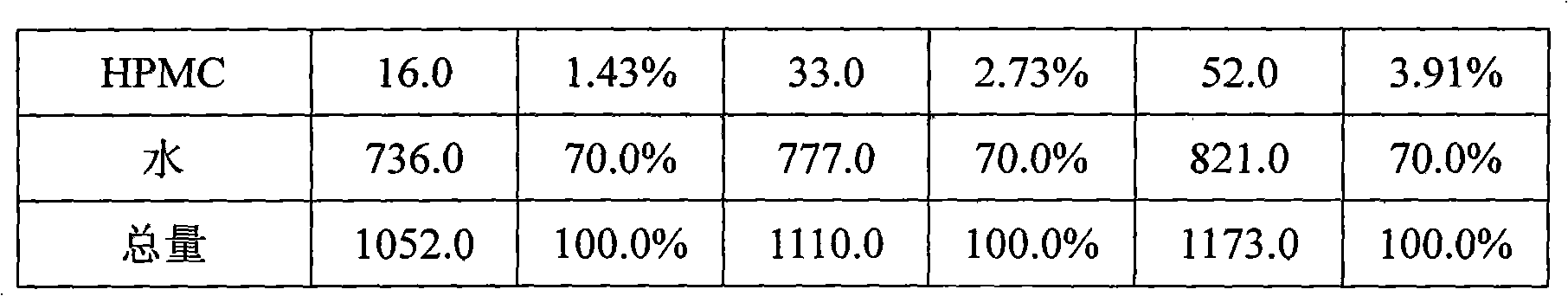
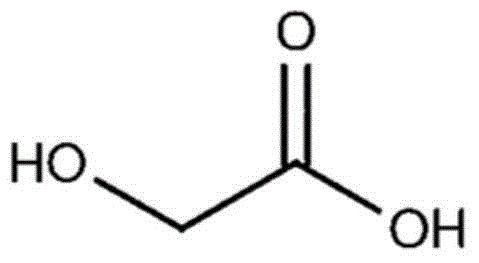
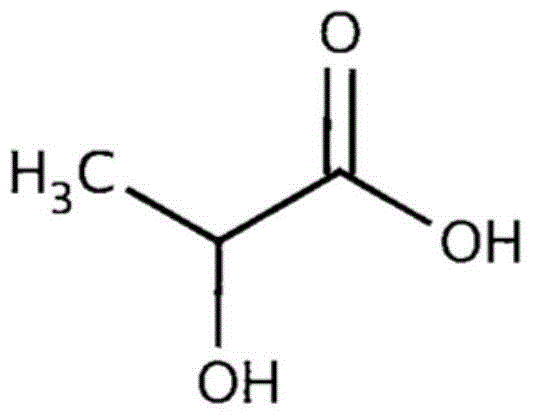
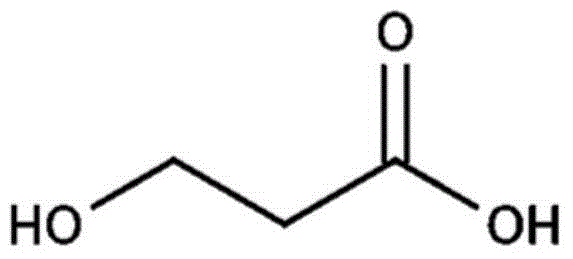
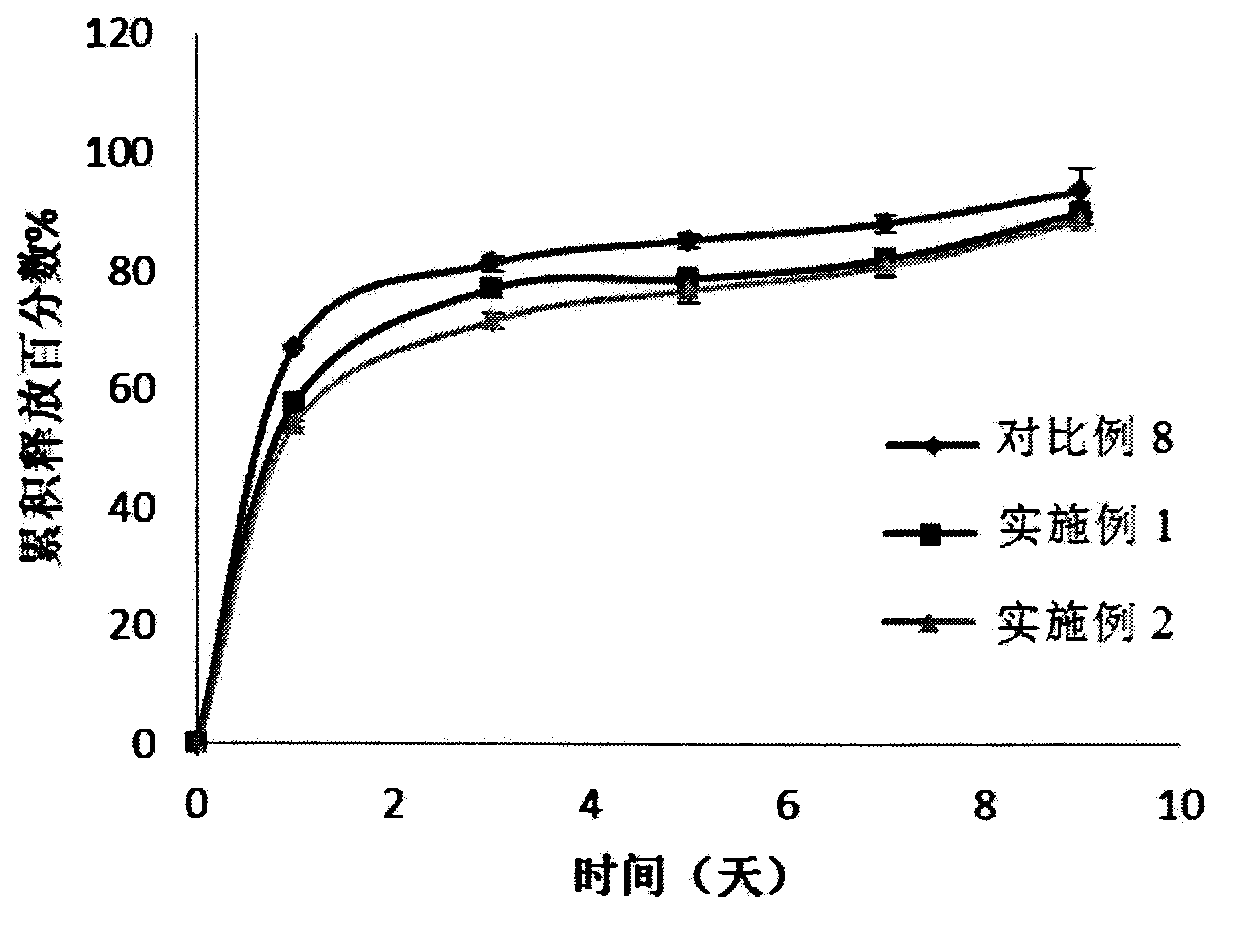
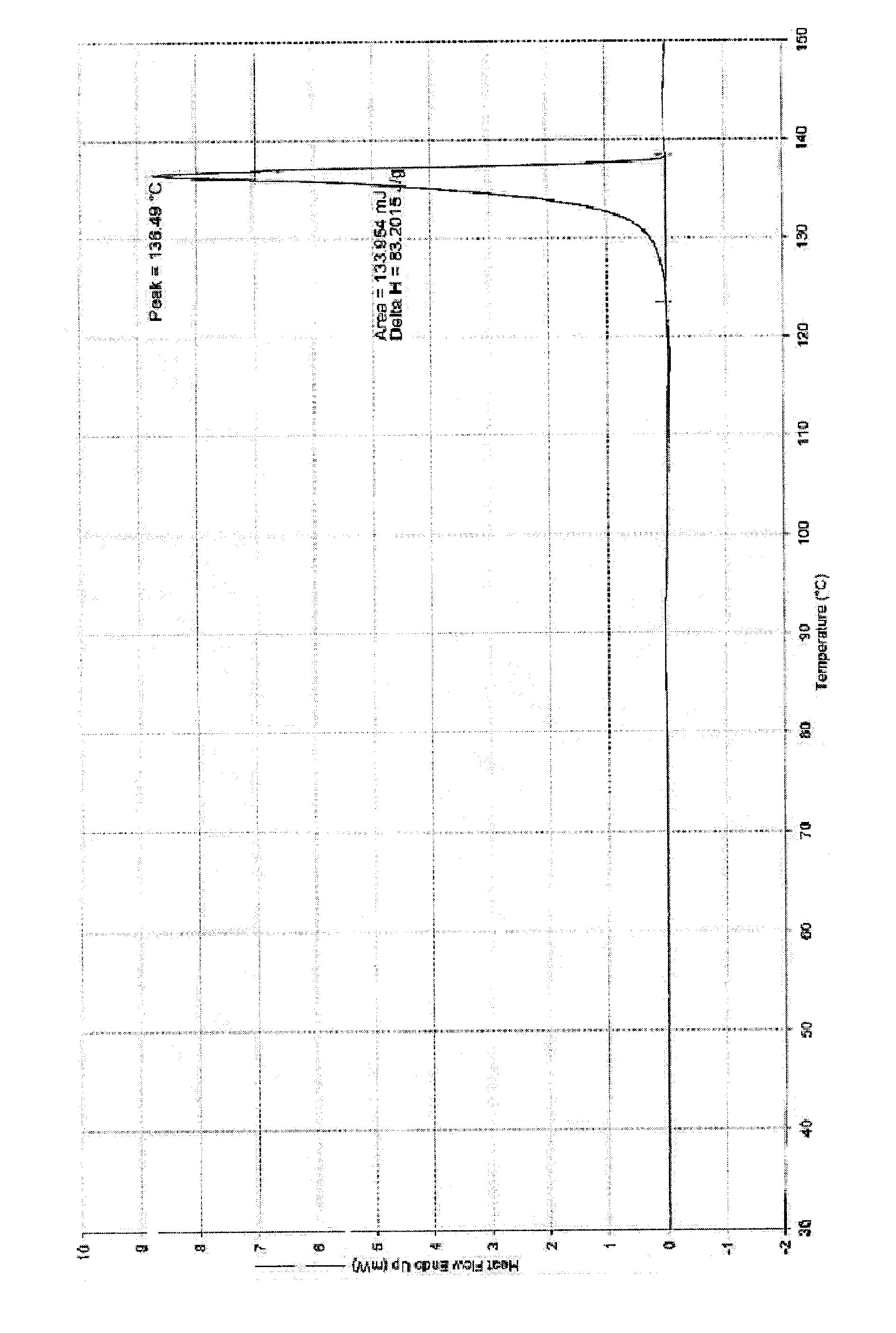
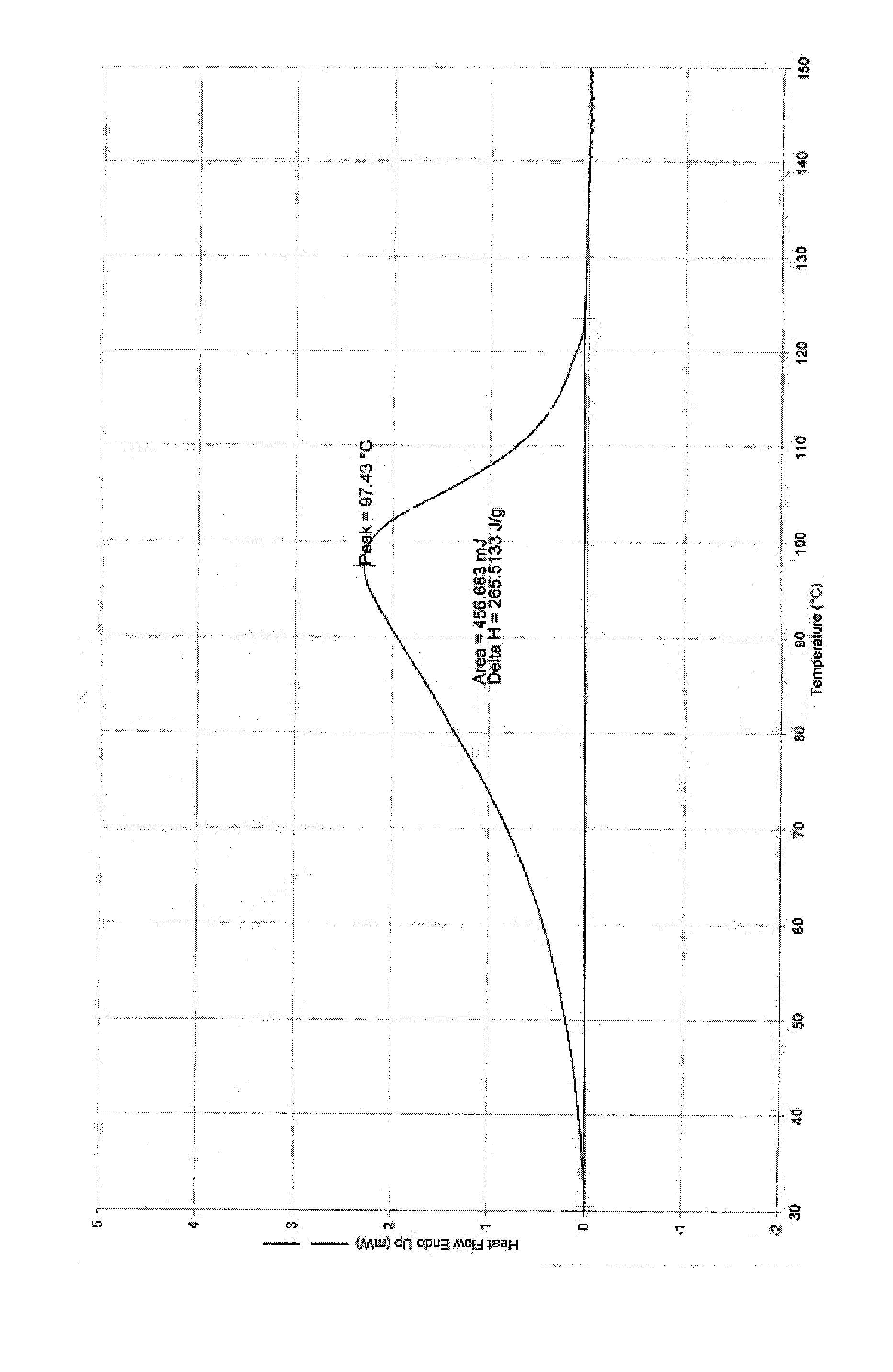
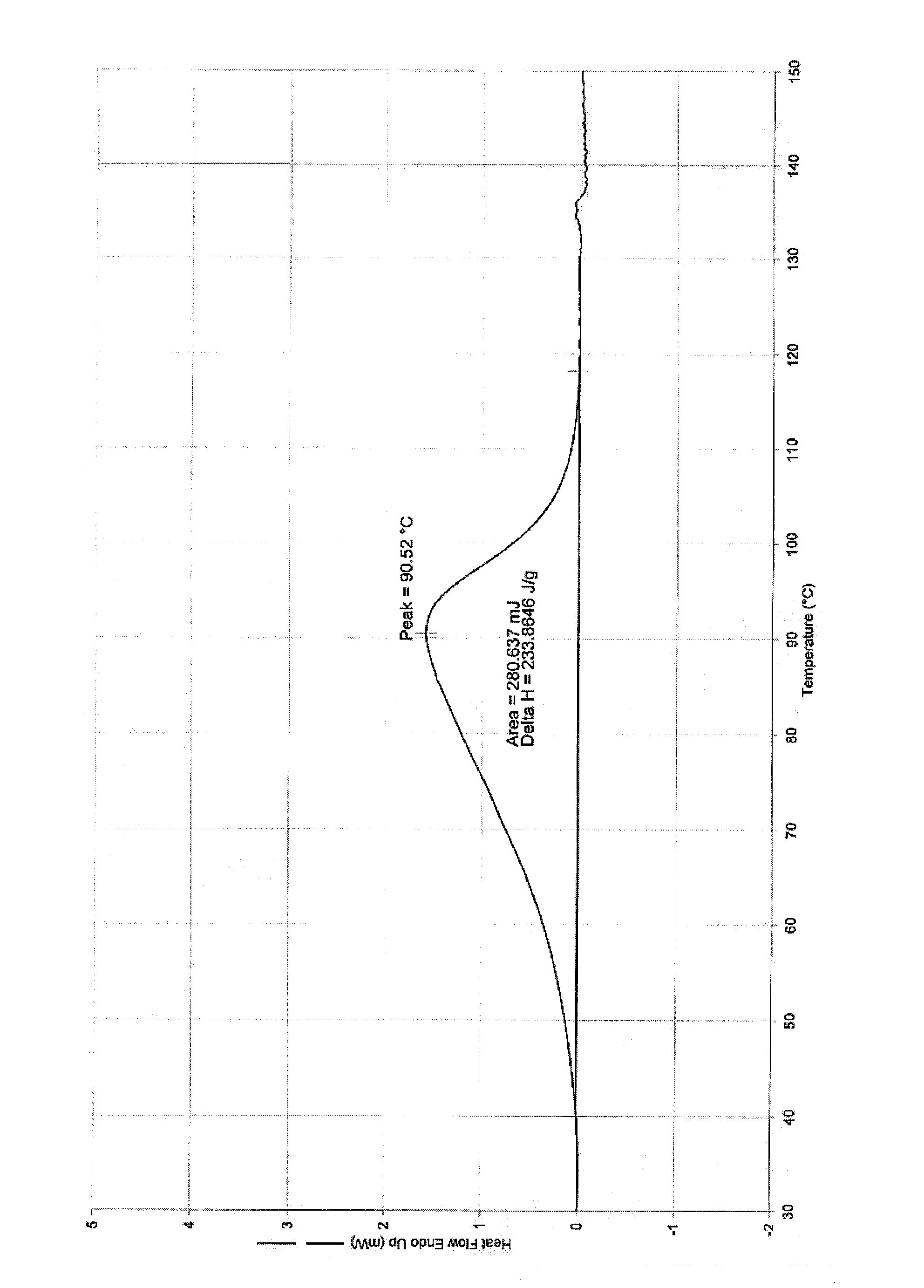
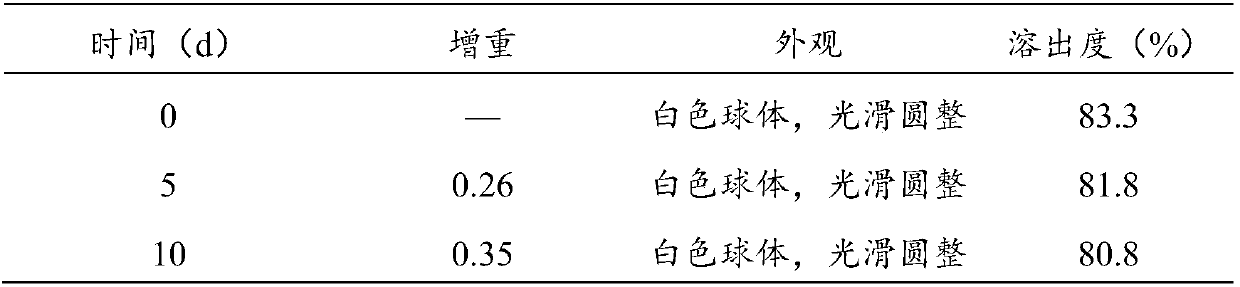
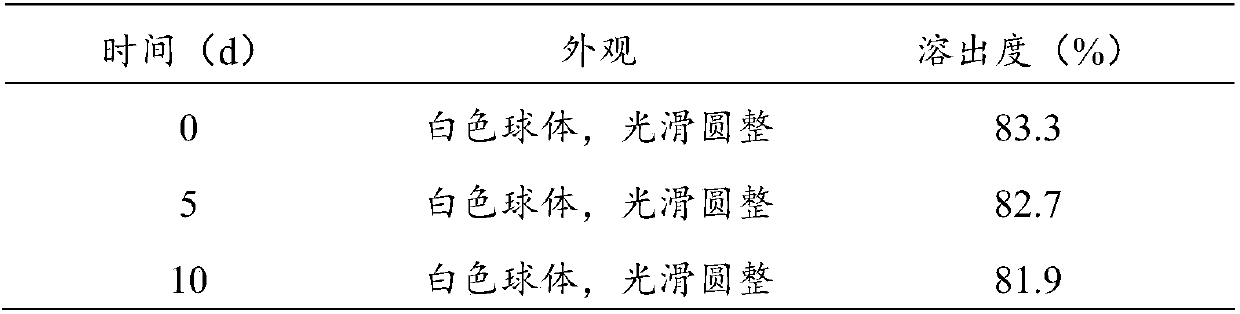
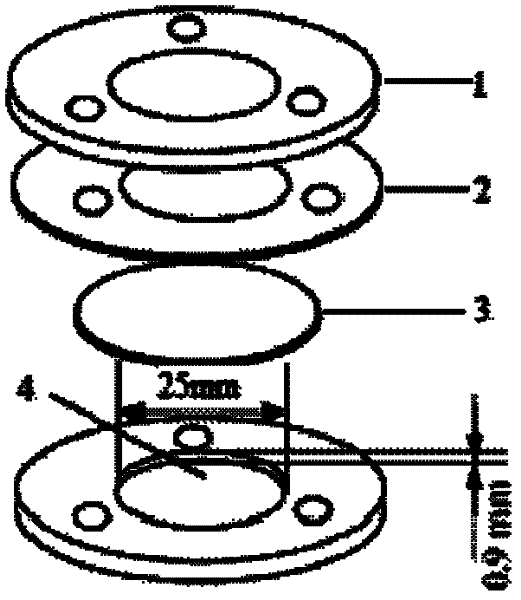
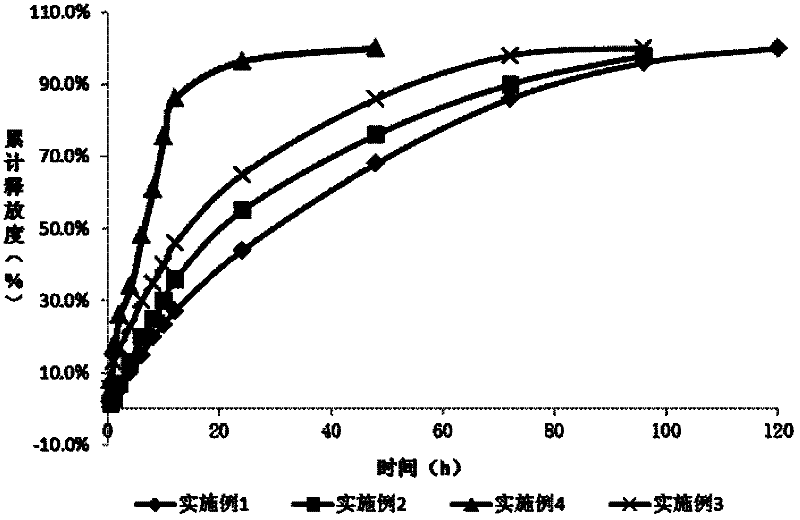
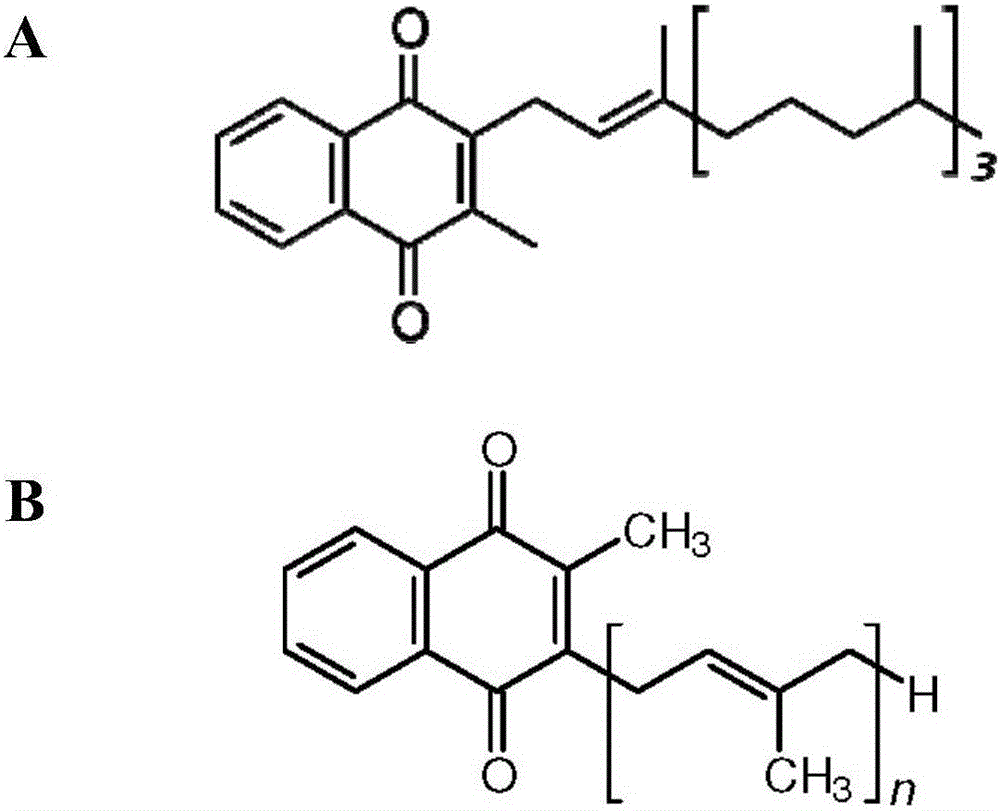
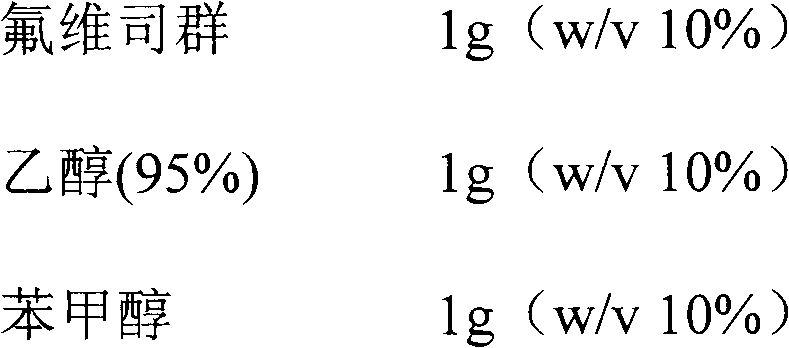Patents
Literature
374results about How to "Improve medication compliance" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Oral quick-dissolving film preparation and preparation method thereof
InactiveCN102824333AImprove disintegration time limitSolve the shortcomings of taking waterPharmaceutical non-active ingredientsSexual disorderVardenafilTadalafil
The invention discloses an oral quick-dissolving film preparation and a preparation method thereof. The oral quick-dissolving film comprises the following components in percentage by weight: 20 to 40 percent of medicinal active component, 40 to 75 percent of water-soluble film forming material, 10 to 25 percent of plasticizer, 0 to 25 percent of disintegrating agent, and 0.1 to 8 percent of water, wherein the medicinal active component is one of sildenafil, tadalafil, vardenafil or salts thereof. According to the oral quick-dissolving film preparation, the disintegration time limited of the film preparation can be remarkably accelerated, the problem that most oral solid preparations should be taken with water can be solved, medicine taking time cannot be delayed under a condition without water, and the taking compliance of a patient can be improved.
Owner:SUZHOU UNIV
Azithromycin for suspension and its preparing method
ActiveCN1813683AUniform sizeNarrow particle size distributionAntibacterial agentsOrganic active ingredientsAdhesiveDosage form
The present invention relates to a dried suspension preparation of medicinal azithromycin and its preparation method. Said dried suspension preparation includes (by wt%) 0.1-20% of azithromycin, 1-10% of lubricant flow aid, 0.1-10% of pH value regulating agent, 10-40% of high-molecular adhesive, 50-85% of sweetener and 0.5-15% of aromatic corrective.
Owner:HAINAN PULIN PHARMA +1
Transdermal patch containing pramipexole
ActiveCN103432104AGood solubilityImprove uniformityOrganic active ingredientsNervous disorderSolventMicrogram
The invention discloses a transdermal patch containing pramipexole. The transdermal patch comprises a medicine carrying pressure-sensitive adhesive layer, the pramipexole, solvent and penetration enhancer, wherein the medicine carrying pressure-sensitive adhesive layer comprises acrylate pressure-sensitive adhesive containing carboxyl base groups and acrylate pressure-sensitive adhesive containing hydroxyl base groups, which form mixed pressure-sensitive adhesive; the pramipexole is dissolved in the acrylate mixed pressure-sensitive adhesive, with the content being 10 to 30 weight percent; the content of the mixed pressure-sensitive adhesive is 50 to 80 weight percent; the content of the solvent is 5 to 20 weight percent; the content of the penetration enhancer is 2 to 15 weight percent. The pramipexole can be dissolved in a mixed pressure-sensitive adhesive patch in a high concentration way and is not crystallized, the medicine availability is high, the pramipexole has good stability in pressure-sensitive adhesive matrix, and can be continuously administrated for 5 to 7 days in a transdermal way at a relatively stable permeation rate of being larger than 5.0 micrograms / cm2 / h, and the application area of a pramipexole patch is smaller than 40cm2.
Owner:GUANGDONG HONGSHANHU PHARM CO LTD
Chewing tablet of proton pump inhibitor
InactiveCN101066279AEffective in timeImprove medication complianceOrganic active ingredientsDigestive systemSodium bicarbonateDuodenal ulcer
The present invention relates to one kind of chewing tablet of proton pump inhibitor for treating gastric ulcer, duodenal ulcer, stomal ulcer and other indications. The chewing tablet contains proton pump inhibitor in 1-5 wt%, carbonate in 35-60 wt% and hydroxide in 35-60 wt%. The proton pump inhibitor is one selected from omeprazole, S-omeprazole, pantoprazole, lansoprazole, rabeprazole, leminoprazole, tenatoprazole and their salts. The biologically acceptable buffering agent is selected from sodium bicarbonate, sodium carbonate, magnesium carbonate, calcium carbonate and their mixture, preferably sodium bicarbonate. The hydroxide is selected from magnesium hydroxide, calcium hydroxide, aluminum hydroxide, sodium hydroxide and their mixture, preferably magnesium hydroxide.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Medicinal composition containing insoluble medicament
InactiveCN102145003AAvoid degradationAvoid Absorption IrregularitiesUrinary disorderAmide active ingredientsPoor complianceSide effect
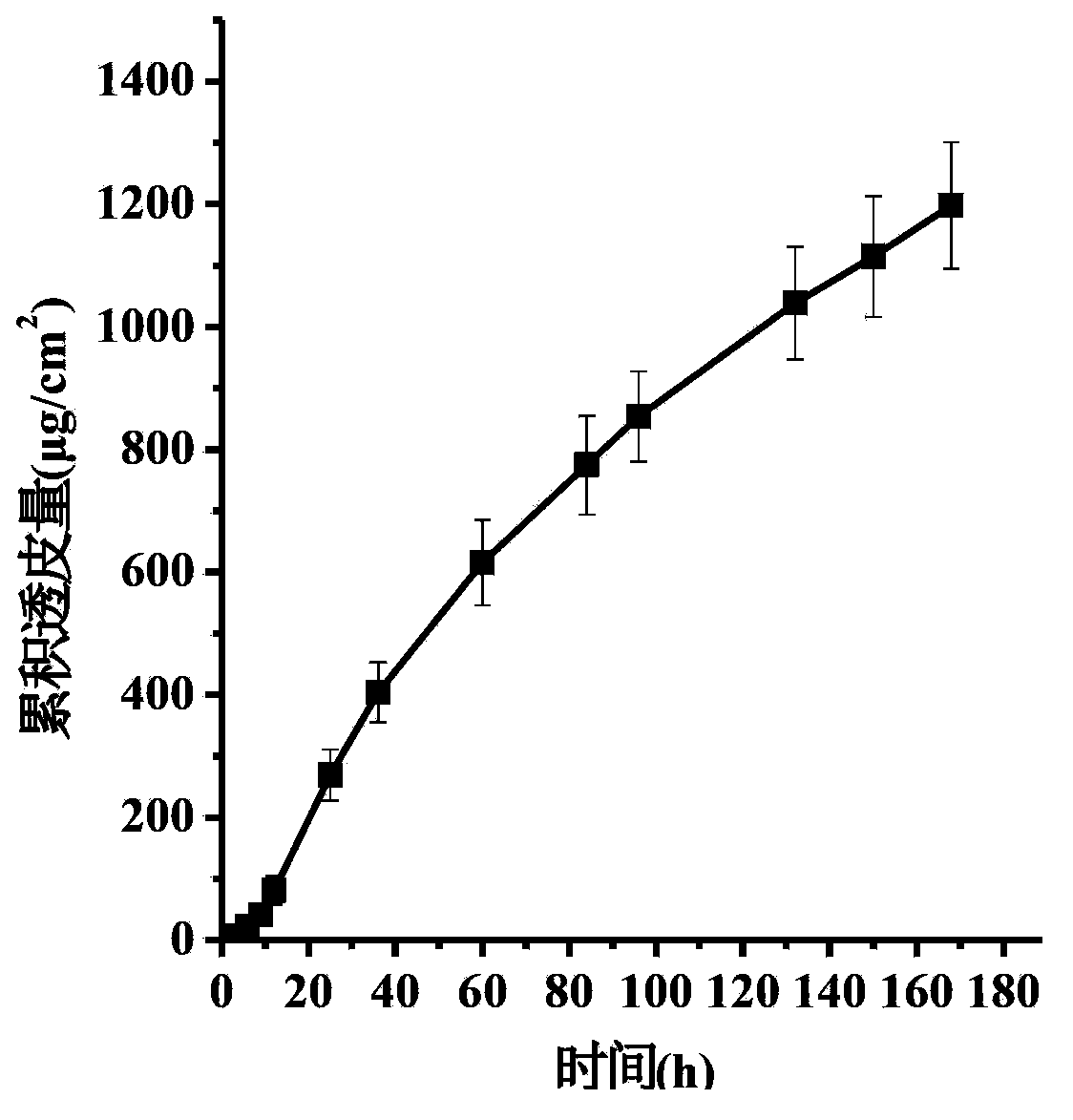
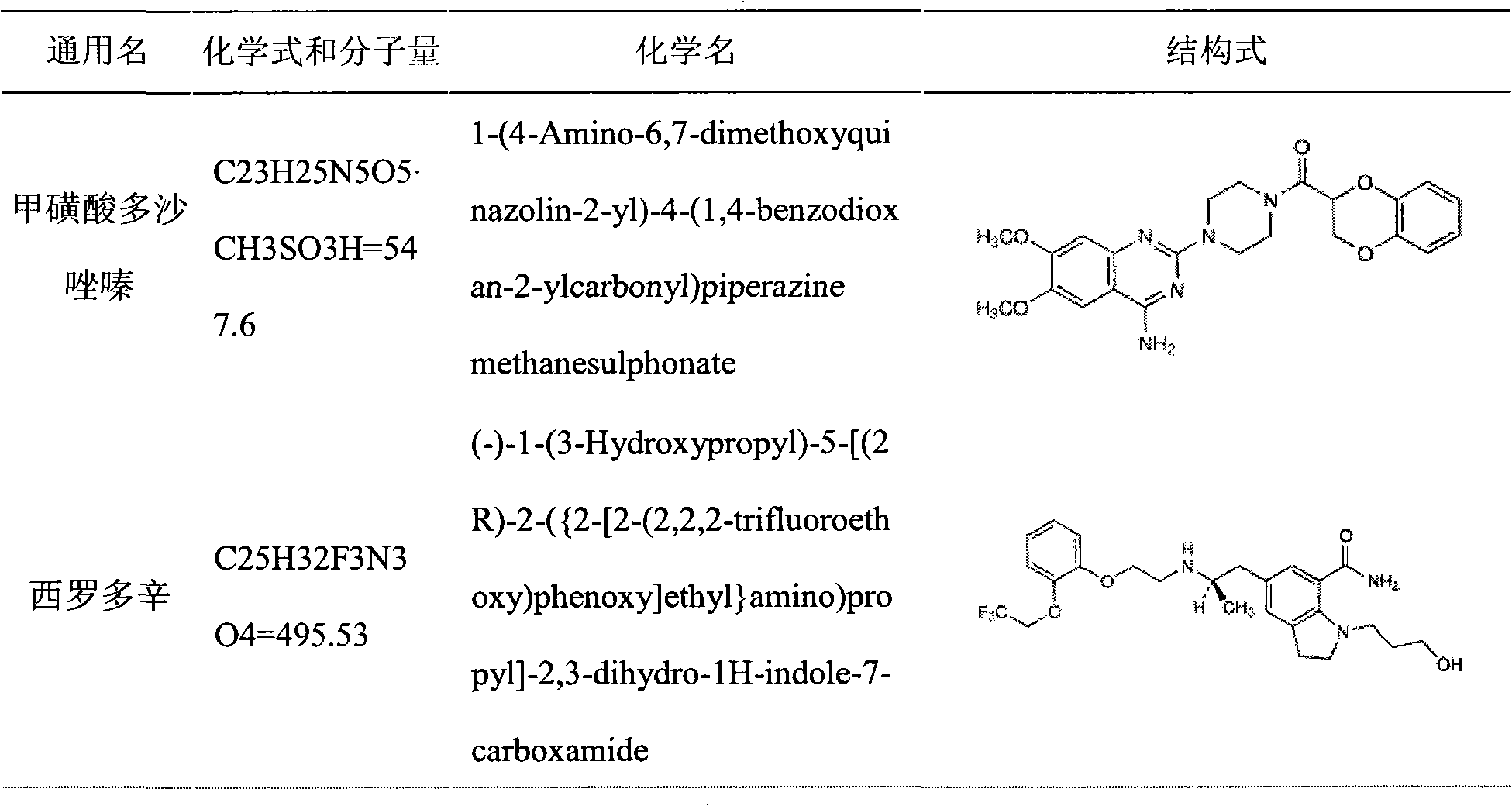
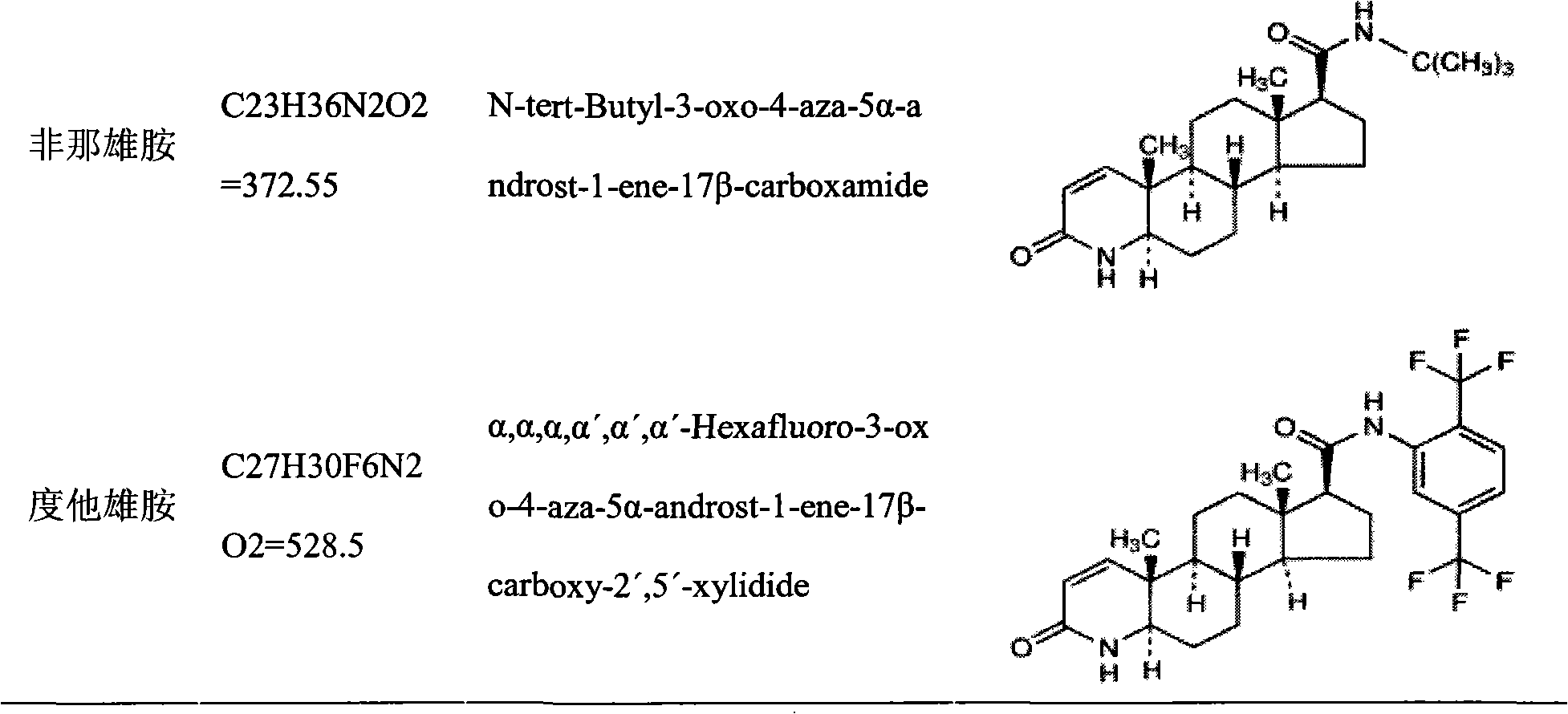
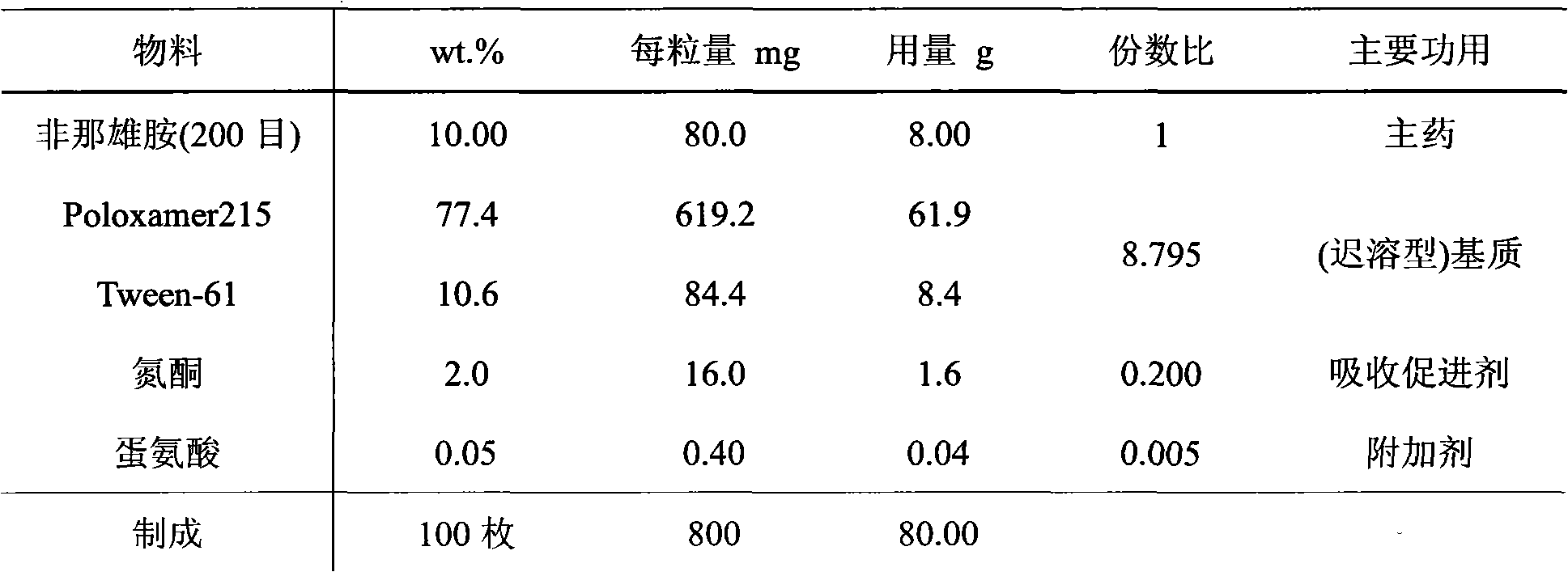
The invention discloses a medicinal composition containing an insoluble alpha-receptor retardant and / or 5alpha-reductase inhibitor with an effective dose, which comprises the following components of: principal ingredients, a substrate, a solubilizer, a sorbefacient and an additive in a weight ratio of 1:(8-7,000):(0-460):(0-150):(0-150), wherein the medicinal composition at least contains one of the solubilizer and the sorbefacient. The effective dose of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor is in 0.05 to 80 milligrams of parent compounds of the insoluble alpha-receptor retardant and / or the 5alpha-reductase inhibitor, the weight of a preparation per unit is between 0.8 and 4.2 grams. The medicinal composition can be subjected to oral administration or rectum administration, so the defects of poor curative effect and large toxic and side effect in the conventional oral administration and systemic administration of injection and large side effect and poor compliance of patients in local injection administration can be overcome, the lasting time of the medicinal effect can be increased, and better treatment means can be provided for medical care personnel and patients; and a product process is simple and is suitable for industrial batch production.
Owner:张立英
Aryl propionic acid derivative composition and pharmaceutical purpose
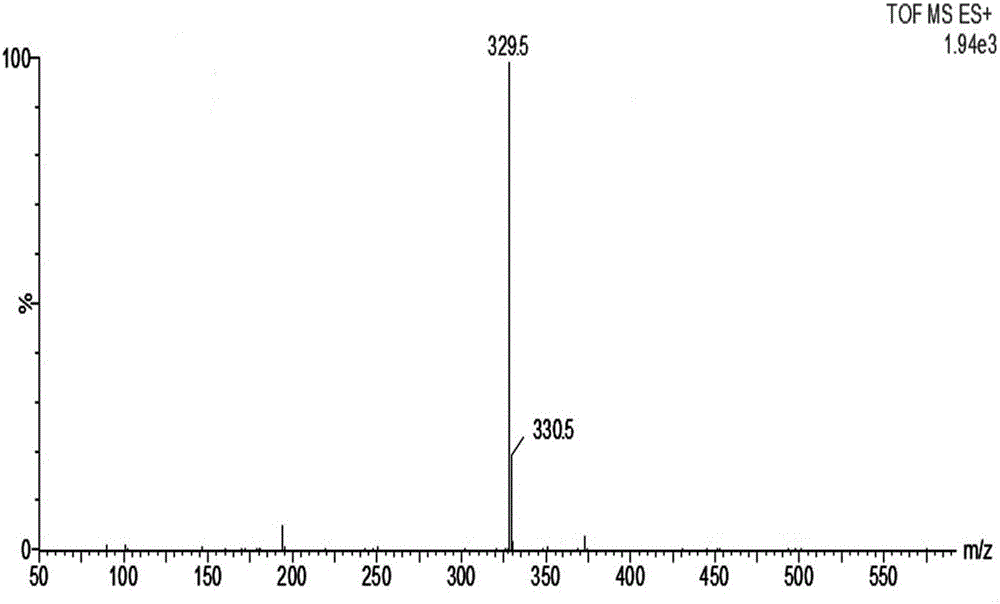
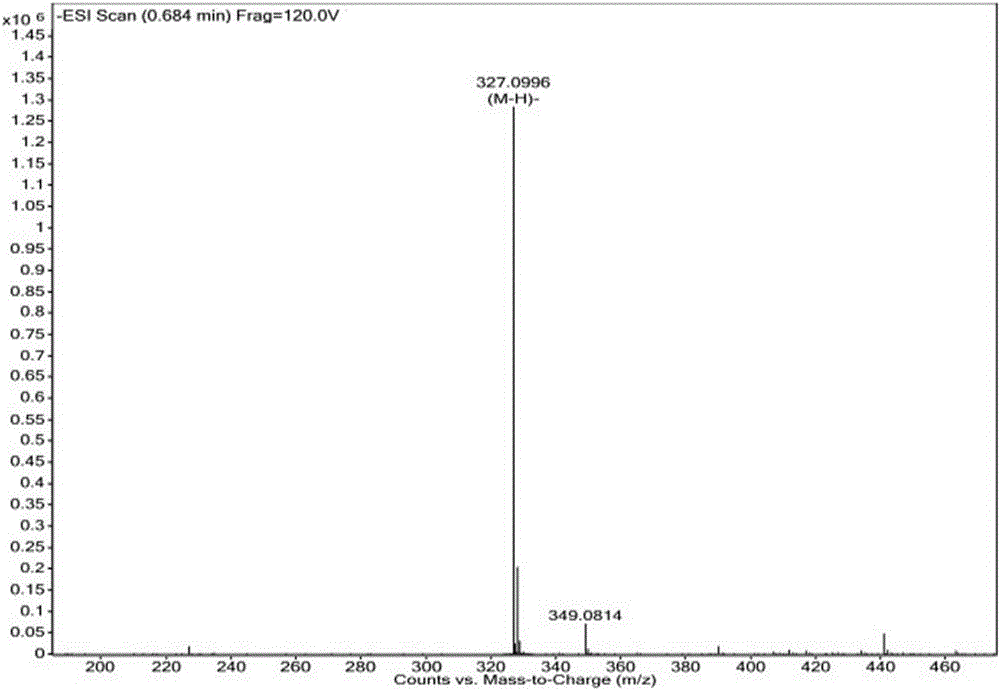
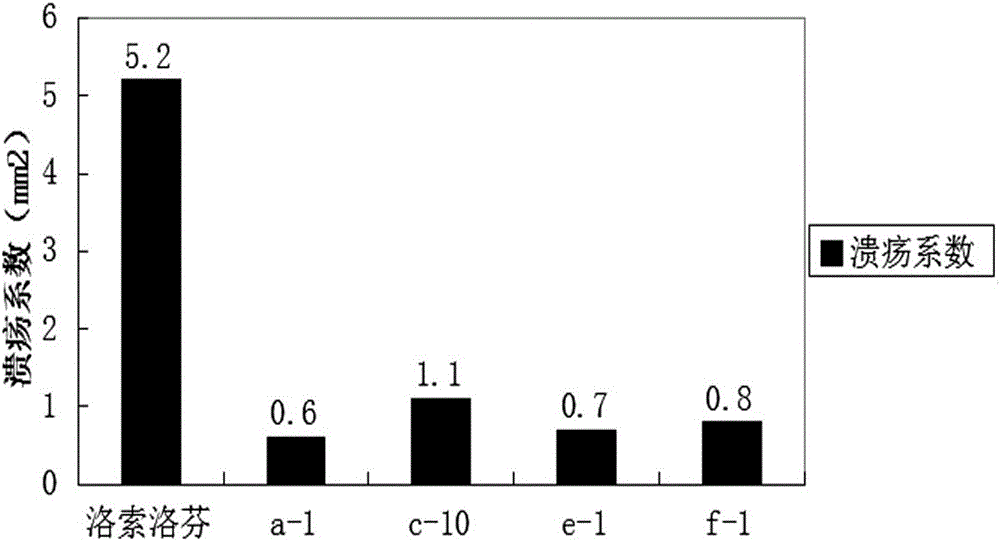
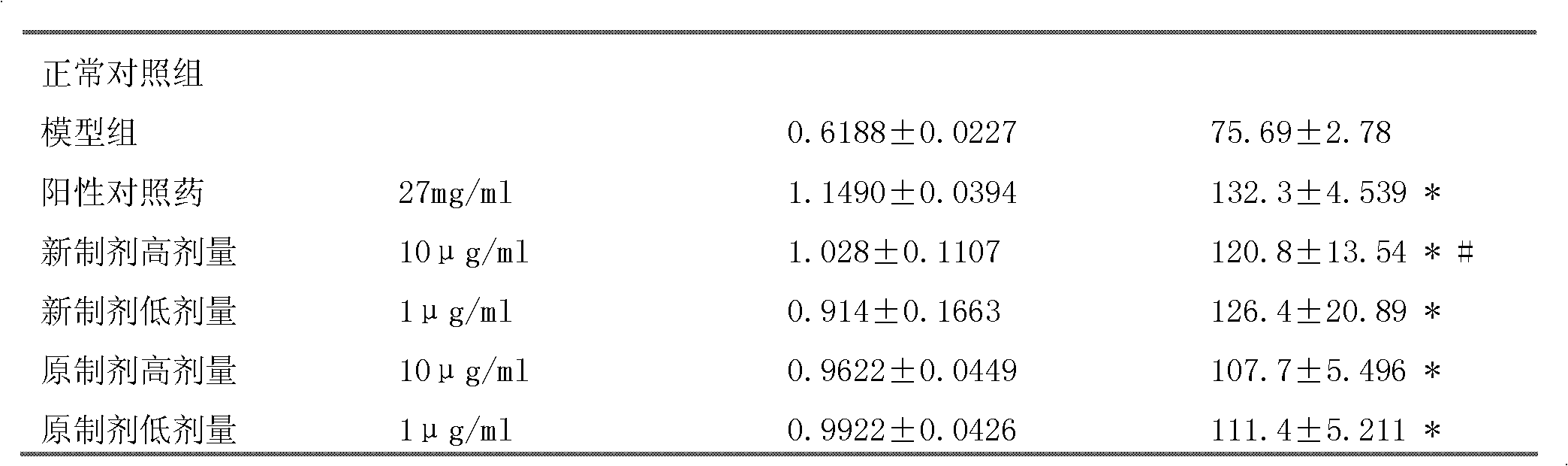
ActiveCN106661061ALess irritatingImprove medication complianceOrganic active ingredientsAntipyreticMetabolitePhosphate
The invention discloses an aryl propionic acid derivative composition and a pharmaceutical purpose. Alcohol metabolites of Loxoprofen having highest activity in metabolism is used as a nuclear parent to produce a series of derivatives of phosphate, sulphonate, carbonic ester, and amino-acid ester. The derivatives are used to enhance pesticide effect of anti-inflammatory and analgesic effects, and reduce toxic reaction, and have good pharmacokinetic property. And at the same time, the stimulation of the medicine on the gastrointestinal tract, and medication compliance of patients is improved, and therefore the aryl propionic acid derivative composition is the non-steroidal anti-inflammatory drugs having potentials.
Owner:NANJING HERON PHARMA SCI & TECH CO LTD +1
Preparation method of pharmaceutical composition preparation treating apoplexy sequelae
ActiveCN103182049AGood curative effectImprove medication complianceHeavy metal active ingredientsMammal material medical ingredientsBioavailabilityShale oil extraction
The invention relates to a preparation method of a pharmaceutical composition preparation treating apoplexy sequelae. The method includes volatile oil extraction and inclusion, ethanol extraction, water extraction, medicinal fine powder crushing, preparation molding and other steps. Compared with traditional preparations, the medication compliance of patients can be improved obviously. Under the premise of retaining original preparation efficacy, the pharmaceutical preparation prepared by the method provided in the invention can realize rapid absorption, effective components can intensively reach nidi to take fast effect, and the bioavailability is high. The pharmaceutical preparation prepared by the method disclosed in the invention has improved efficacy.
Owner:JINHE TIBETAN MEDICINE
Pharmaceutical composition of fulvestrant
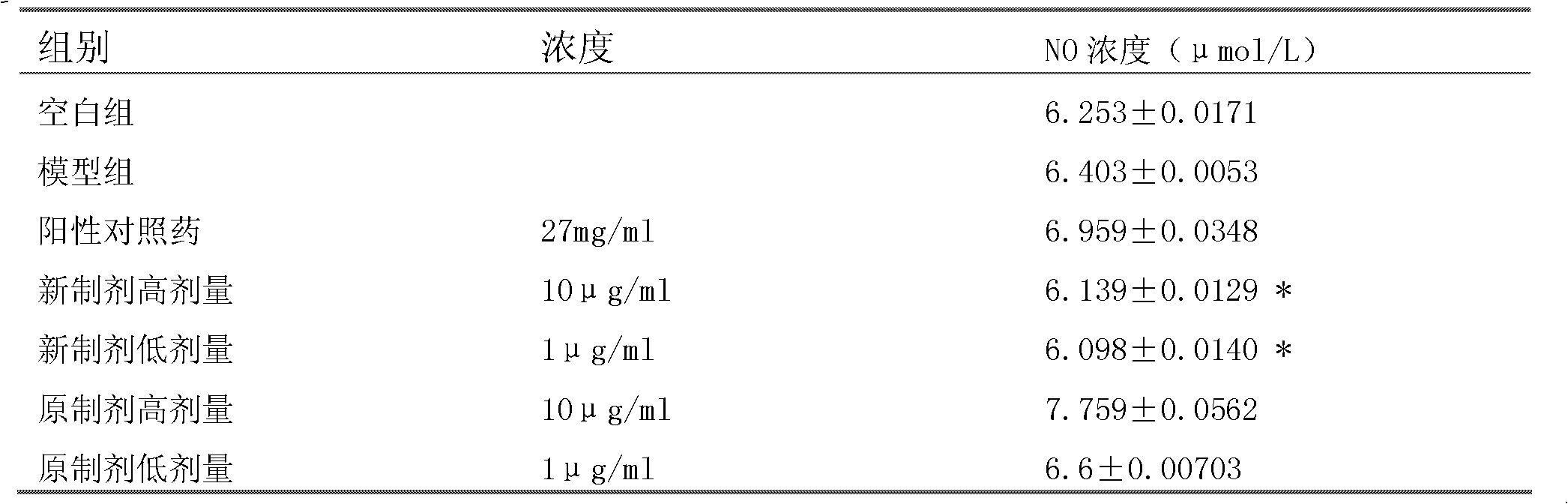
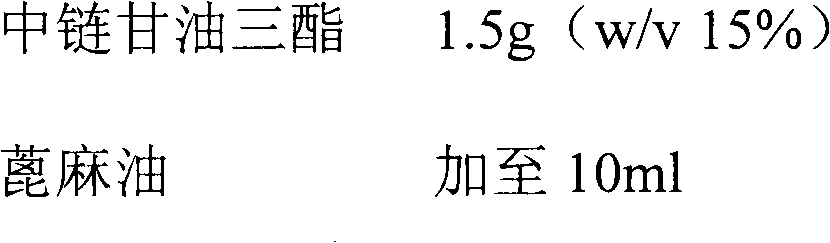
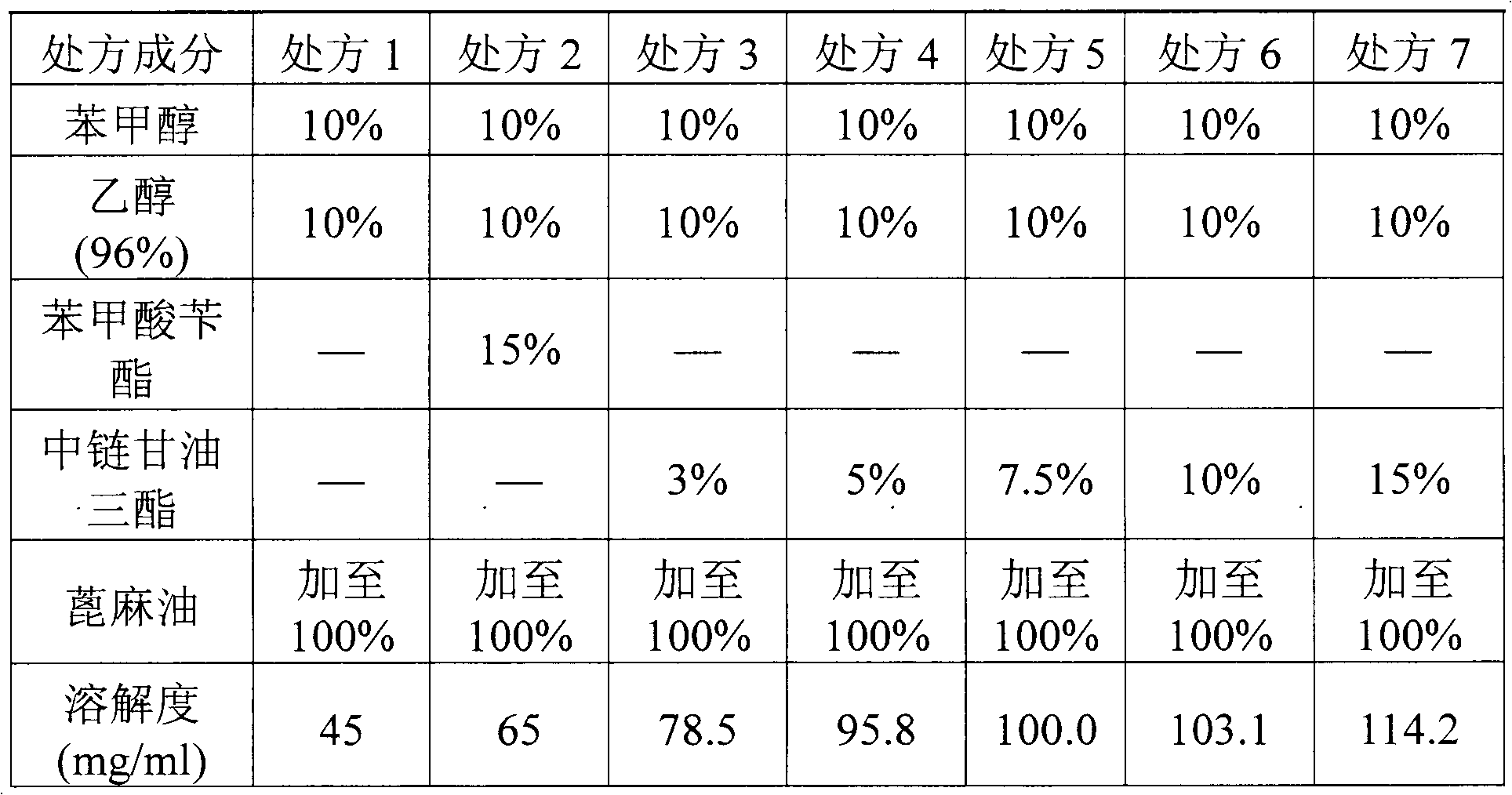
ActiveCN103070871AImprove solubilityMedication convenienceOrganic active ingredientsPharmaceutical non-active ingredientsAlcoholMedium-chain triglyceride
The invention relates to a pharmaceutical composition of fulvestrant. The pharmaceutical composition comprises: (1) fulvestrant; (2) at least one pharmaceutically acceptable alcohol; (3) medium-chain triglyceride; and (4) a castor oil matrix.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Composition of malaytea scurfpea extract as well as preparation method and use thereof
InactiveCN102228506AKeep dryEasy to crushAntibacterial agentsAntinoxious agentsFructus psoraleae extractCyclodextrin
The invention discloses a composition of malaytea scurfpea extract as well as the preparation method and use thereof. The composition contains a malaytea scurfpea extract, cyclodextrin and an optional excipient acceptable in the physiology. The extract of the invention has an excellent processing performance.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Novel Olanzapine orally disintegrating tablet
ActiveCN102178657APrevent oxidationAvoid degradationOrganic active ingredientsNervous disorderParticulatesOrally disintegrating tablet
The invention relates to a novel Olanzapine orally disintegrating tablet. The tablet comprises (I) a stabilizing Olanzapine coating particulate and (II) an additive, wherein the stabilizing Olanzapine coating particulate contains: (i) a neutral core; (ii) an active layer surrounding the neutral core; and (iii) a protective layer surrounding the active layer. The invention also relates to a preparation method of the stabilizing Olanzapine coating particulate and the novel Olanzapine orally disintegrating tablet containing the stabilizing Olanzapine coating particulate.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Film-forming gel composition, application and wound protecting material
InactiveCN105012960AGood wound protectionPromote healingAntibacterial agentsAntipyreticCross-linkCellulose
The invention discloses a film-forming gel composition, its application and a wound protecting material. The film-forming gel composition comprises the following ingredients: hydroxy propyl cellulose, an esterifying agent, a cross-linking agent and a solvent, wherein the esterifying agent contains saturated fatty hydroxy carboxylic acid and tannic acid. The chemical general formula of the saturated fatty hydroxy carboxylic acid is CnH2n+2-m-L(OH)m(COOH)L, wherein n, m and L are integers; n is greater than or equal to 1; m is greater than or equal to 1; L is greater than or equal to 1; m+L is 2-5; and n+L is 2-6. The film-forming gel composition can be used to prepare a drug for treating skin or oral mucosal diseases, or can be used as a drug carrier to prepare a drug film-forming gel composition and a wound protecting material. The film-forming gel composition contains no aromatic organic carboxylic acid with pharmacological activity, will not produce pharmaceutical action on human body and also will not cause adverse reactions on human body.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Quick release percutaneous absorption Chinese traditional medicine paster for reducing ever and curing cold
InactiveCN1733148ALittle side effectsMaintain a constant plasma concentrationAntipyreticAnalgesicsMedicinal herbsFast release
The invention provides a fast release percutaneous absorption Chinese traditional medicine paster for reducing ever and treating cold, which comprises clear grease of concentrated Chinese medicinal herbs, novel macromolecular material, chemical penetration accelerating agent.
Owner:刘安西
Oral preparation containing amisulpride
ActiveCN102600132AMask bitternessImprove medication complianceNervous disorderMacromolecular non-active ingredientsDrugWater soluble
Owner:QILU PHARMA
Compound medicine contg. famotidine cyclodextrin clathrate, and its prepn. method
InactiveCN1868473AMask bitternessImprove medication complianceOrganic active ingredientsDigestive systemMedicineFamotidine
A compound medicine taken orally is prepared from famotidine, cyclodextrin and antiacid through including the famotidine particles by cyclodextrin, and mixing the inclusion compound with antiacid.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
A temperature-sensitive gel medicine preparation and a preparation method thereof
ActiveCN103622902AImprove Medication ComplianceGood reference value and application significancePeptide/protein ingredientsMetabolism disorderDivalent metalWater soluble
The invention discloses a temperature-sensitive gel medicine preparation and a preparation method thereof. A raw material formula of the temperature-sensitive gel medicine preparation comprises a medicine, a temperature-sensitive gel composition, and an auxiliary material C. The auxiliary material C is one or more selected from a metal salt, a carbohydrate and a hydrophilic polymer used for injection medicines. The metal salt is a pharmaceutically acceptable metal salt which can dissociate divalent metal cation in a water solution. The medicine is a compound A which exists in an anion state in a water solution. The compound A is one or more selected from a protein type medicine, a polypeptide type medicine and a glycopeptides type antibiotic. The preparation method comprises a step of dissolving the medicine into the temperature-sensitive gel composition existing in a solution form according to the raw material formula. The temperature-sensitive gel medicine preparation is especially suitable for water-soluble medicines, and is capable of diminishing burst release effects and releasing the medicine slowly, thus achieving an objective of slow release.
Owner:SHANGHAI MODERN PHARMA ENG INVESTIGATION CENT
Gellan gum and calcium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule
ActiveCN103394092AReduced risk of hygroscopicityReduce adhesionCosmetic preparationsToilet preparationsPutrefactionVegetable fibers
The invention relates to a gellan gum and calcium chloride gelled hydroxypropyl methylcellulose enteric-coated hollow capsule, and belongs to the technical field of the production of hollow capsules. The hollow capsule is characterized in that the hollow capsule is prepared through mixing a main raw material hydroxypropyl methylcellulose with the gellan gum and calcium chloride under a special condition, the hollow capsule has the characteristics of timely disintegration, low water content, suitableness for medicines having strong hygroscopic property and being sensitive to water, small influences of a dissolve-out medium to the shell of the capsule, high drug compliance, long storage duration data, no deterioration within 3-4 years, low condition requirements on the storage and the transportation, difficult crushing in the low humidity environment, difficult deformation, putrefaction and deterioration in the high temperature environment, according with the requirements of populations having all culture backgrounds and religious faiths because of pure vegetable fibers, almost no pollution in the production process, secondary re-dissolving utilization of tailings, omission of the commonly-used surfactants and plasticizers, acceleration of the gelling speed by reducing the gelling temperature only through utilizing calcium chloride to assist the gellan gum, additive reduction, production cost reduction, and suitableness for the mass popularization.
Owner:SICHUAN TIANSHENG PHARMA
Tebipenem pivoxil oral preparation and preparation method thereof
ActiveCN102860985AGreat tasteImprove medication complianceAntibacterial agentsPowder deliveryActive componentPatient compliance
The invention relates to a tebipenem pivoxil-containing oral preparation and a preparation method of the tebipenem pivoxil-containing oral preparation. The preparation provided by the invention consists of tebipenem pivoxil and a pharmaceutic adjuvant as the active components. The pharmaceutic adjuvant contains beta-cyclodextrin. The tebipenem pivoxil oral preparation provided by the invention overcomes the defects that the grit feeling is produced when the tebipenem pivoxil oral preparation is taken, the patient compliance is poor and the preparation technology is complicate in the prior art, so that the tebipenem pivoxil oral preparation is an excellent preparation with good taste, high dissolvability, good stability and simple preparation technology.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Medicine for treating chronic renal failure and preparation method thereof
ActiveCN102526324AReduce dosageImprove medication complianceAnthropod material medical ingredientsUrinary disorderDiseasePharmaceutical drug
The invention discloses a medicine for treating chronic renal failure and a preparation method thereof. The medicine for treating chronic renal failure comprises bulk pharmaceutical chemicals, namely rehmanniae praeparatum, fructus corni, Indian buead, astragali radix and rhizoma atractylodis macrocephalae which are proportionally processed and prepared into granules. The medicine for treating chronic renal failure provided by the invention has functions of replenishing qi, nourishing kidney, invigorating spleen, resolving stasis, detoxing and removing turbidity, and can be used for treating the diseases such as early-stage and middle-stage chronic renal failure, spleen and kidney deficiency of syndrome differentiation of chronic kidney disease, blood stasis and obstruction, toxin and turbidity accumulation and the like.
Owner:AIR FORCE MEDICAL CENT PLA
Olanzapine oral disintegration tablet and preparation method thereof
InactiveCN102631331ARapid disintegration in vitroEvenly dispersedOrganic active ingredientsNervous disorderHydrogen phosphateMANNITOL/SORBITOL
The present invention relates to an olanzapine oral disintegration tablet and a preparation method thereof. The olanzapine oral disintegration tablet comprises: olanzapine, mannitol, and calcium hydrogen phosphate. The olanzapine oral disintegration tablet uses the olanzapine as the raw material, comprises one or more pharmaceutically acceptable excipients and is prepared by a direct compression. The olanzapine oral disintegration tablet can be quickly disintegrated in vitro, and quickly and uniformly disintegrated and dispersed, and is slightly sweet and tastes good. The olanzapine oral disintegration tablet is conveniently taken, and is especially suitable for the patients with emergency in dysphagia or special environment such as the elderly, children, and coma patient. The olanzapine oral disintegration tablet has the advantages of simple preparation process and low cost, and is suitable for industrialized production.
Owner:BEIJING MEDISAN TECH +1
Sevelamer carbonate effervescent tablets and preparation method thereof
InactiveCN104434866AHigh hardnessImprove medication complianceOrganic active ingredientsMetabolism disorderEffervescent tabletUse medication
Owner:JINAN KANGHE MEDICAL TECH
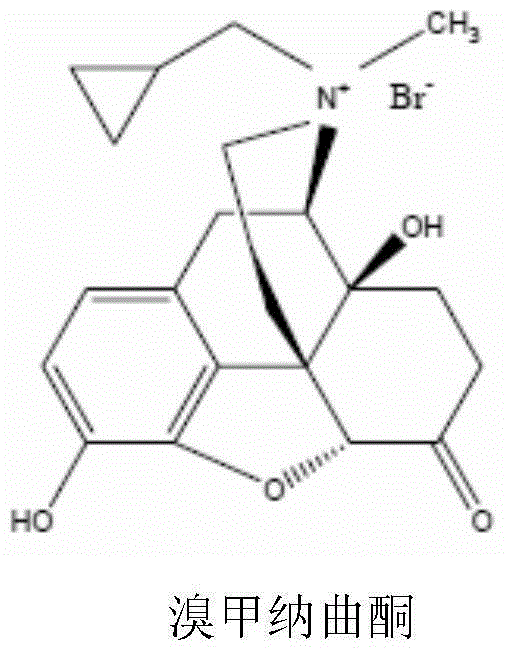
Methylnaltrexone bromide solid composition and preparation method thereof
InactiveCN105582011AFix stability issuesAddressing Content UniformityOrganic active ingredientsDigestive systemSocial benefitsOral medication
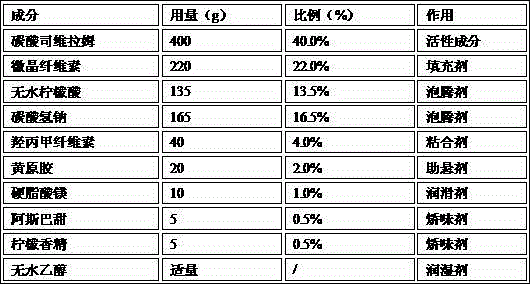
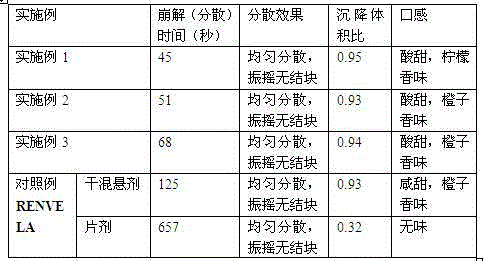
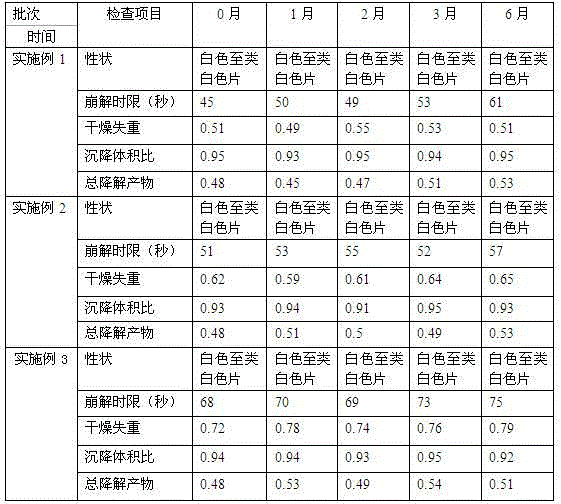
The invention relates to a methylnaltrexone bromide solid composition and a preparation method thereof. The solid composition comprises ingredients of methylnaltrexone bromide and an acidic stabilizing agent at a weight ratio being (10:1) to (1:10). The methylnaltrexone bromide solid composition has the advantages that the formula process is advanced; the product quality is stable; the problems of stability and the content uniformity of the methylnaltrexone bromide in the preparation process can be perfectly solved; and the problem of stability of a methylnaltrexone bromide preparation in the storage process can also be perfectly solved. The methylnaltrexone bromide solid composition invented by the research group has the beneficial effects that the solid preparation and the methylnaltrexone bromide injection on the market have the identical curative effect; the administration mode is changed into oral administration; the medicine administration compliance of a patient is improved; the production cost is reduced; the selling price of a product is reduced; and the economical burden of the patient is reduced. Therefore economic, safe, effective and convenient therapeutic medicine is provided for the patient with constipation due to opioid medicine, and good social benefits and economic benefits can be obtained.
Owner:江苏国丹生物制药股份有限公司
Bilobalide K dropping pill and preparation method thereof
InactiveCN107753445AHigh dissolution rateImprove medication complianceOrganic active ingredientsNervous disorderMedicineBilobalides
The invention relates to the field of medicine preparations and in particular to a bilobalide K dropping pill and a preparation method thereof. The bilobalide K dropping pill consists of the followingcomponents in parts by mass: 7.5-15.0 parts of bilobalide K, 70-87.5 parts of a water-soluble substrate and 5-15 parts of a solubilizer. The invention further discloses a preparation method of the bilobalide K dropping pill. As the bilobalide K is prepared into the bilobalide K dropping pill, the dissolution rate of the bilobalide K is increased, the bioavailability of oral taking is improved, and the bilobalide K dropping pill has the characteristics of being small in dosage, high in product stability, good in medicinal compliance of patients, and the like.
Owner:JIANGSU KANION PHARMA CO LTD
Pharmaceutical composition for treating periodontitis, and preparation method and application thereof
ActiveCN102240277ASpecial physical propertiesSensitive to temperatureOrganic active ingredientsAerosol deliveryControlled releaseLiquid state
The invention relates to a pharmaceutical composition for treating periodontitis and a preparation method thereof, wherein the pharmaceutical composition comprises chlorhexidine or derivatives thereof, poloxamer 407, a release speed adjusting agent, a stabilizer and other additives. The pharmaceutical composition has particular physical characteristics; when the temperature reaches the gel point, the composition can undergo phase transition from liquid to semisolid gel; the composition is liquid at the room temperature; after being used, the composition changes into semisolid gel quickly under the human body temperature condition and then is adhered on the surface of periodontal mucosa to release the medicine, thus a therapeutic effect is obtained; the composition has specific controlled-release property, and can achieve a controlled-release effect of 4 to 5 days of sustained medicine release by adjusting types and dosages of the release speed adjusting agents, thereby reducing the medicine using times, and improving the medication compliance of patients; and the composition is good in stability, and convenient to produce, transport and store.
Owner:SHENZHEN SOUTH CHINA PHARMA
Cefixime granule and preparation method thereof
InactiveCN102697736AGood quality and stabilityGood suspension effectAntibacterial agentsOrganic active ingredientsFluidized bedAdhesive
The invention relates to the field of pharmaceutical preparations, especially to a cefixime granule and a one-step granulating method of the cefixime granule. The cefixime granule comprises the following components by weight: 1-20% of cefixime, 56-95% of diluent, 0.5-1.0% of a buffer agent, 0.5-10% of adhesive and 0.1-5% of flavoring. The cefixime granule has the advantages of good quality stability, good suspension property and good content uniformity, and good taste simultaneously, so that the cefixime granule is especially suitable for children and crowd of dysphagia, and medication compliance is good. A preparation method of the cefixime granule further is provided by the invention; due to use of the one-step granulating method, steps of mixing, granulating and drying are finished in the same one fluidized bed by one step; operation method is simple, and production efficiency is high, so that the preparation method is especially suitable for industrial production.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
Stable vitamin K2 submicron emulsion and preparing method
InactiveCN106074377AImprove stabilityFreeze-thaw toleranceOrganic active ingredientsSkeletal disorderFreeze thawingEmulsion
Vitamin K2 submicron emulsion is prepared from, by weight, 0.0005-1% of vitamin K2, 1.0-10% of oil, 0.1-1.2% of phospholipid, 0.5-2.3% of emulgator, 0.5-3.0% of antifreezing agent and water according to the total weight of the vitamin K2 submicron, wherein emulgators except phospholipid can serve as the emulgator in the emulsion. The vitamin K2 submicron emulsion is good in sterilization stability and freeze-thaw stability, and the expected shelf life can reach two years or more.
Owner:SUNGEN BIOSCIENCE CO LTD
Oral cavity instant film containing risperidone and preparation method for oral cavity instant film
InactiveCN104546806AMask bitternessImprove medication complianceOrganic active ingredientsNervous disorderActive componentPlasticizer
The invention provides a risperidone oral cavity instant film capable of shielding bad taste of medicine and a preparation method for the instant film. The oral cavity instant film mainly comprises medicine active components shielding taste and auxiliary materials applicable in pharmacology; the medicine active ingredients comprise risperidone; the auxiliary materials applicable in pharmacology comprise a film forming material, a plasticizer, a sweetener, a filling agent and the like. The oral cavity instant film has the advantages that the oral cavity instant film is favorable in taste, can be dissolved quickly in oral cavity without drinking water, and is high in bioavailability and convenient to use; the preparation technology is simple, convenient and feasible; the related auxiliary materials are easy to purchase; the oral cavity instant film has favorable social and economic benefits.
Owner:AVENTIS PHARMA HAINAN
Linagliptin composition and preparation method thereof
InactiveCN104644563ADisintegrates quicklyMask bitternessMetabolism disorderPharmaceutical non-active ingredientsMedicineDrug compliance
The invention discloses linagliptin particles and a preparation method thereof. Linagliptin is used for treating type 2 diebetes. At present, only linagliptin tablets are sold on the market. Aiming at dysphagia patients, compared with the above two dosage forms, the linagliptin particles have obvious advantages and can improve drug compliance of patients.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Hypromellose enteric empty capsule gelated by gellan gum and potassium chloride
ActiveCN103417979AReduced risk of hygroscopicityReduce the impactCosmetic preparationsToilet preparationsVegetable fibersSurface-active agents
A hypromellose enteric empty capsule gelated by gellan gum and potassium chloride belongs to technical field of empty capsule production, is characterized in that hypromellose is used as a main raw material and mixed with the gellan gum and potassium chloride to prepare the empty capsule, and has the benefits that the disintegration is timely, moisture content is low, the empty capsule is suitable for medicines which have strong hygroscopicity and are sensitive to water, the influence of dissolved mediums on a capsule shell is small, the medication compliance is high, the storage period is long, the empty capsule can not go bad within 3-4 years, requirements on storage and transportation conditions are low, the empty capsule is less susceptible to fragmentation at a low-moisture environment, is insusceptible to deformation at a high-temperature environment, and has low probabilities of rottenness and deterioration; the empty capsule is made from pure vegetable fibers, and can meet requirements of all cultural backgrounds and religious groups; the manufacturing process is almost free from pollution, tailings can be again dissolved for reuse; frequently-used surface-active agents and plasticizers are eliminated, the gelating speed is accelerated only by using the potassium chloride to help the gellan gum to condense so as to reduce the gelating temperature, accordingly, the additives are reduced, and the production cost is reduced; the empty capsule is suitable for mass promotion.
Owner:SICHUAN TIANSHENG PHARMA
Preparation method of Longmu Zhuanggu granule
ActiveCN104147344AReduce the amount requiredTake a small doseSkeletal disorderMammal material medical ingredientsAlcoholMacroporous resin
The invention discloses a preparation method of a Longmu Zhuanggu granule. The preparation method comprises the steps of adding a process of carrying out macroporous resin adsorption on clear paste on the basis of the original Longmu Zhuanggu granule preparation process, eluting by using 30-60% ethyl alcohol, collecting the eluant and concentrating to thick paste. According to the preparation method, the gross of the traditional Chinese medicine extract can be decreased; compared with the process without macroporous resin adsorption, the preparation method disclosed by the invention has the advantages that the gross of the extract can be decreased by 42.5%; most strikingly, the decrease of the gross of the extract does not cause effect reduction; on the premise of taking traditional Chinese medicinal material with same dosage, the effect is not much different from the original method, so that the taking dosage of the Longmu Zhuanggu granule can be decreased. The decrease of the taking dosage is beneficial to decreasing the accessories, reducing the cost, reducing the preparation specification, improving the taking compliance, reducing the bitterness of the drug and improving the taste of the granule during taking after mixing with water.
Owner:JIANMIN PHARMA GRP CO LTD
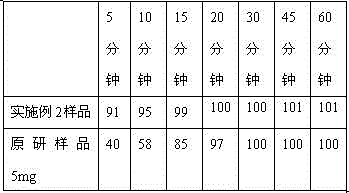
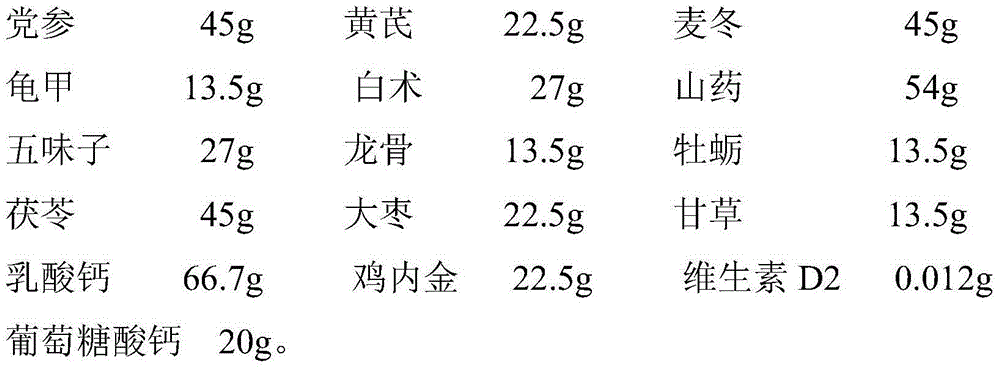
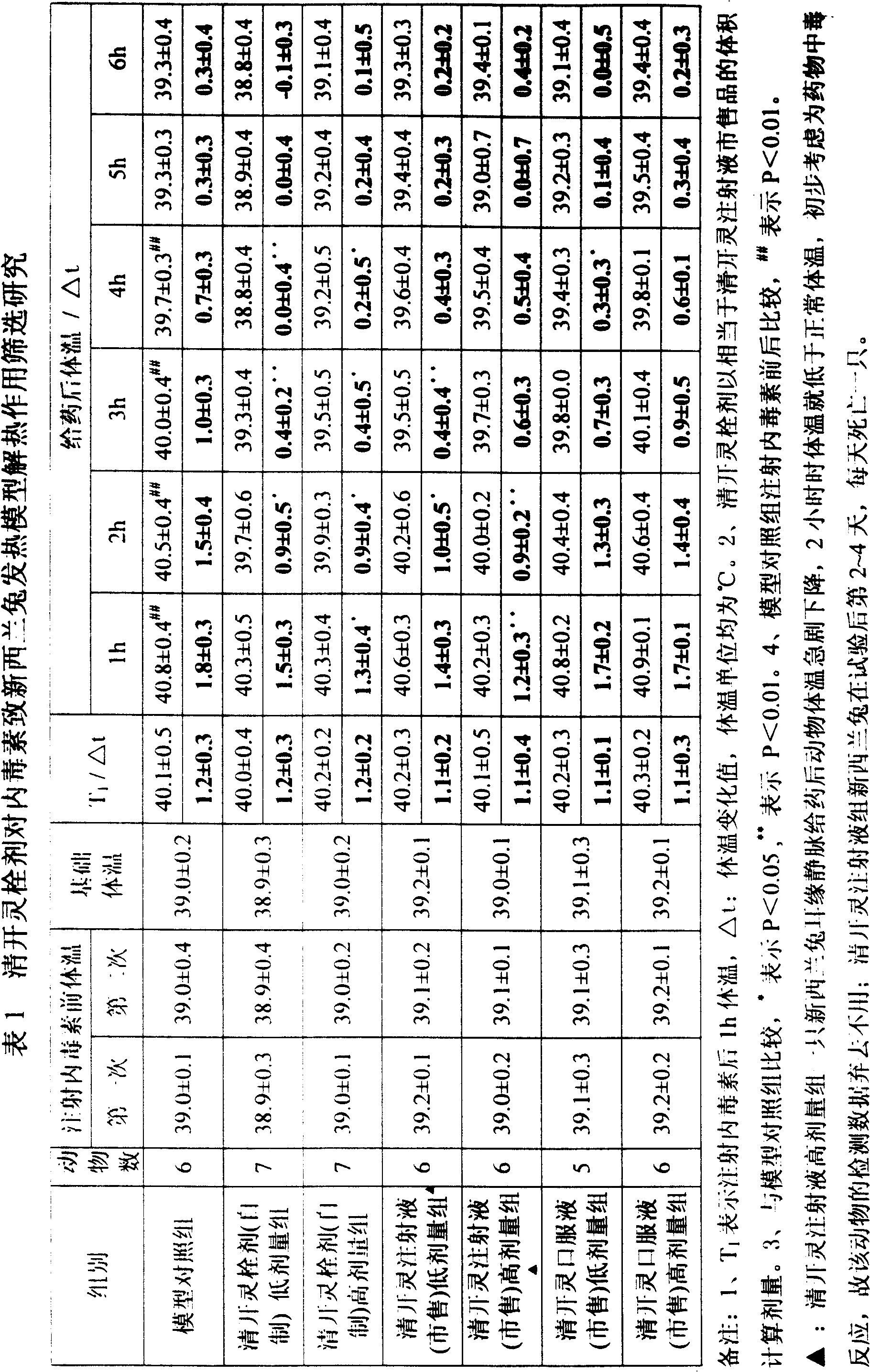
Qingkailing suppositorium
ActiveCN101327262AQuick effectLong duration of actionOrganic active ingredientsNervous disorderHydrolysateThird generation
The invention discloses a Qingkailing suppository, relates to a Chinese medicine compound preparation, the raw materials of which mainly come from plants and animals, in particular to a novel formulation of Qingkailing preparation. The Qingkailing suppository provided by the invention for supplying rectum with medicine adopts the following components of cholic acid, hyodeoxycholic acid, baicalin, buffalo horn, the hydrolysate of mother-of-pearl, gardenia, banlangen, the water purified extract of honeysuckle as Qingkailing combination. The suppository which is 1-3g weight is made from the raw materials and supplementary materials with the following weight proportions of 8-13 proportions of Qingkailing combination, 5-50 proportions of suppository base, 0.1-5 proportions of surfactant, 0.05-3 proportions of sorbefacient, and 0-3 proportions of additional agent. The Qingkailing suppository of the invention not only can compensate the defects that the oral taking preparation is slow to take effect and injection preparation has great toxicity, but also can prolong the duration of the medicine, provides a wider selection range for clinics and sufferers, has simple production technology, is suitable for industrialized batch production, and is suitable to be taken by infants.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com