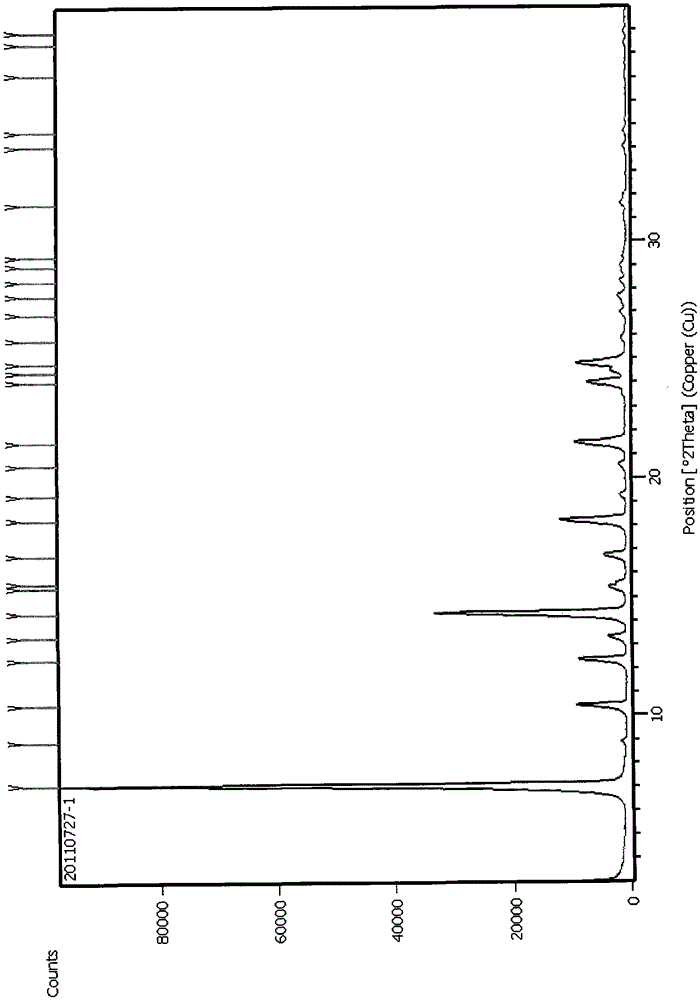
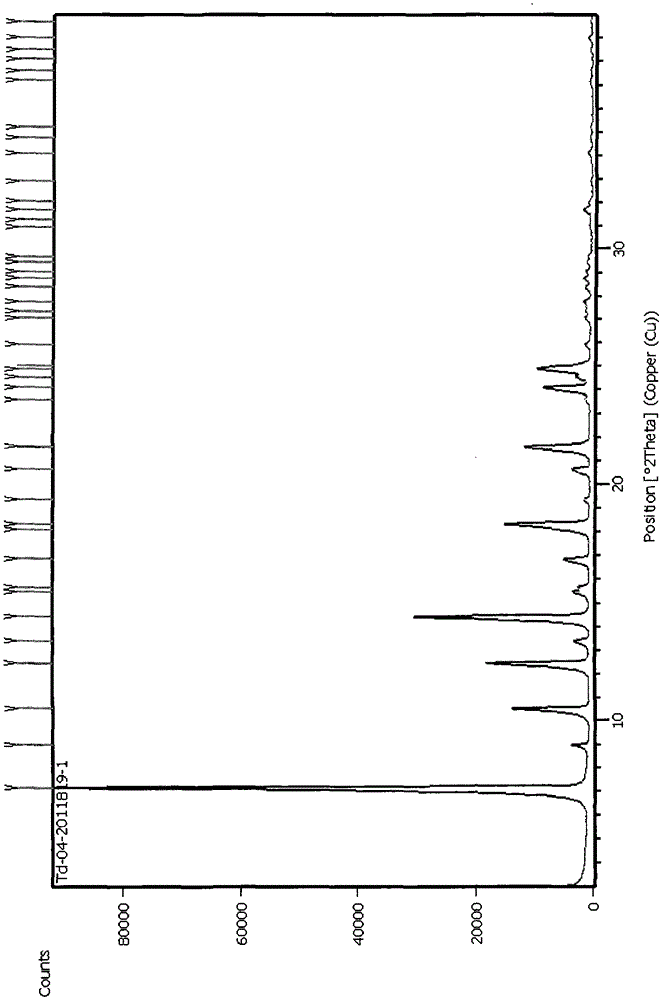
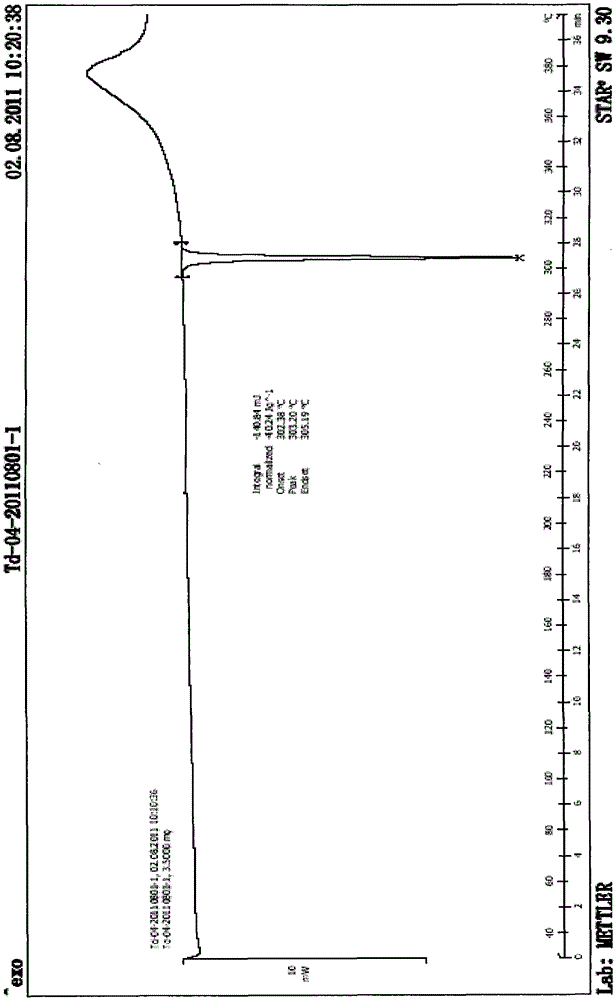
Patents
Literature
267 results about "Tadalafil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
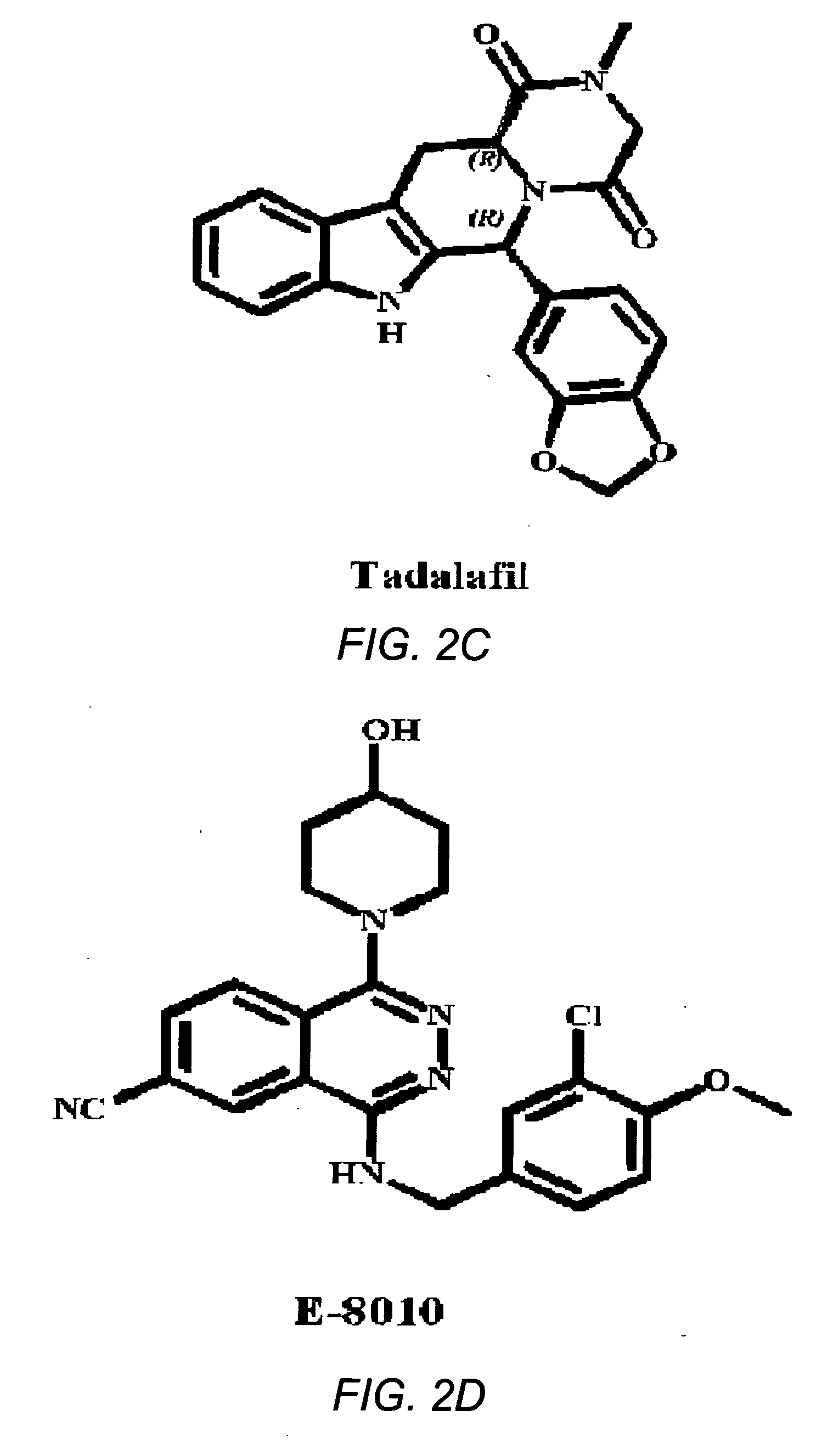
Tadalafil is used to treat high blood pressure in the lungs (pulmonary hypertension).
Oral fast dissolving films for erectile dysfunction bioactive agents
InactiveUS20090047330A1Improved ease of handlingIncrease usageBiocideAnimal repellantsVardenafilActive agent
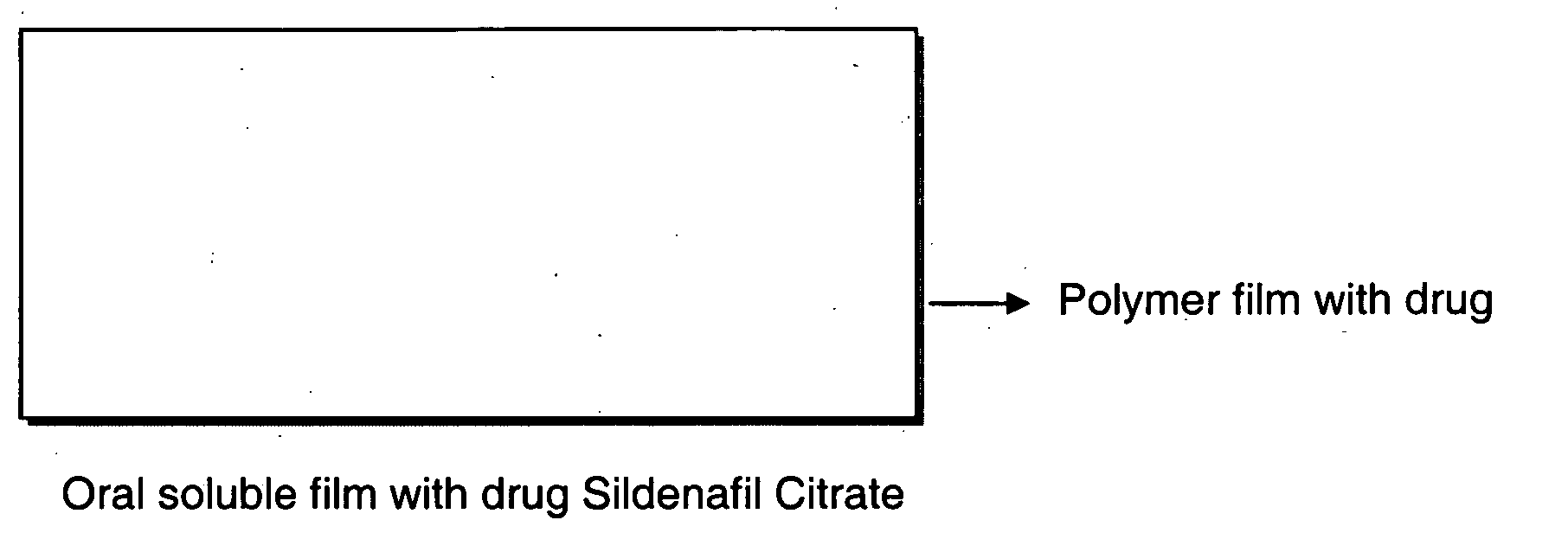
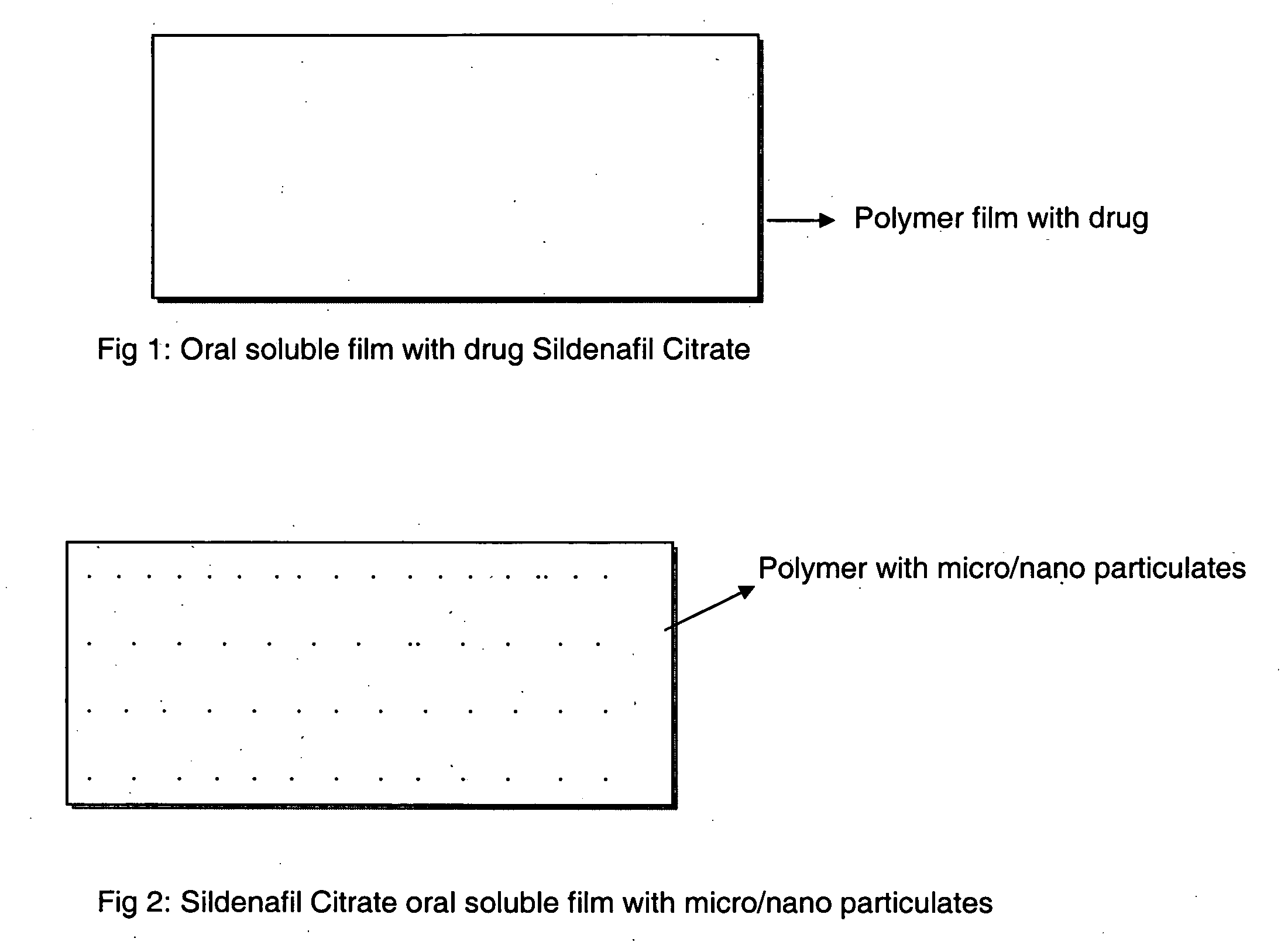
A novel edible polymer based film dosage form manufactured using natural, synthetic, semisynthetic, pharmaceutically acceptable polymers addressing the issues of swallowing difficulties (Dysphagia and Dynaphagia), of tablet or capsule dosage forms and handling and storage difficulties associated with liquid dosage forms, that also includes materials such as emulsifying agents, suspending agents, buffering agents, effervescence agents, colorants, flavorants, sweeteners and specified amounts of bioactive agents, for erectile dysfunction. A flexible film dosage form containing sildenafil citrate, tadalafil or Vardenafil is presented. The film system is enabled to be used in various applications such as oral, mucosal and external environments.
Owner:BANGALORE RAMESH
Use of phosphodiesterase 5 (PDE5) inhibitors in the treatment of schizophrenia
The use of phosphodiesterase 5 (PDE5) inhibitors for treatment of schizophrenia is described. Suitable PDE5 inhibitors for use for treatment of schizophrenia include sildenafil, vardenafil, tadalafil, E-8010, zaprinast, and E-4021. In one embodiment, for example, a method is described for treating schizophrenia in a patient which comprises treating the patient with an effective amount of a PDE5 inhibitor, or a pharmaceutically acceptable salt, solvate, or composition thereof. The PDE5 inhibitor may be administered orally. The PDE5 inhibitor may also be administered together with one or more conventional antipsychotic medications such as risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine, haloperidol, and fluphenazine.
Owner:SHARY CIRCLE
Solid oral dosage forms comprising tadalafil
InactiveUS20110263606A1Improved solubilization and stabilizationPromote absorptionBiocidePharmaceutical delivery mechanismTadalafilBioavailability
Owner:INTELGENX CORP
Oral quick-dissolving film preparation and preparation method thereof
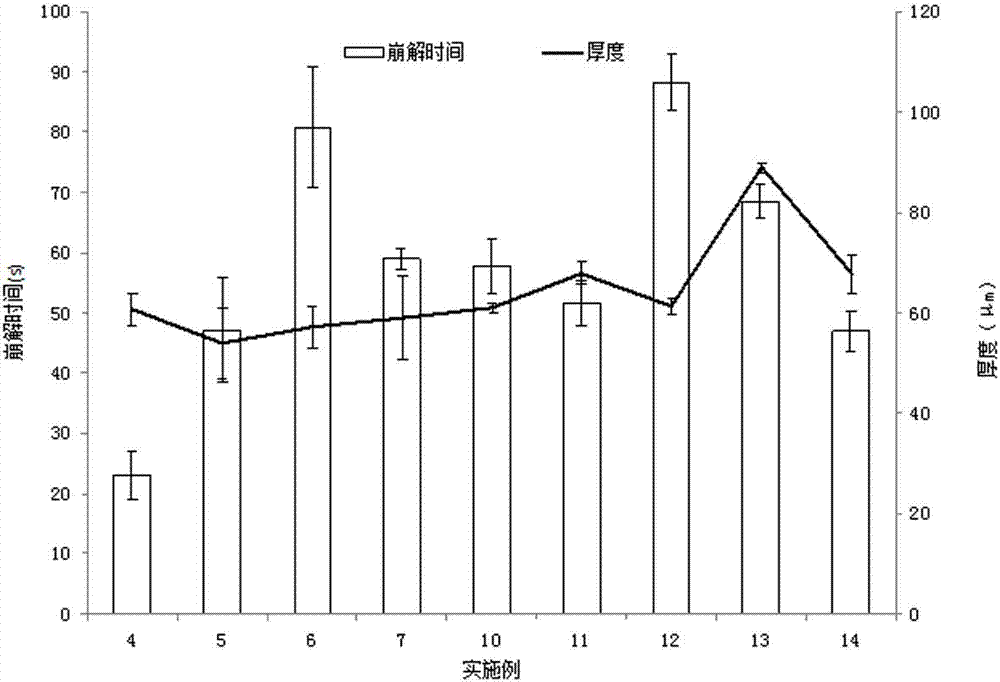
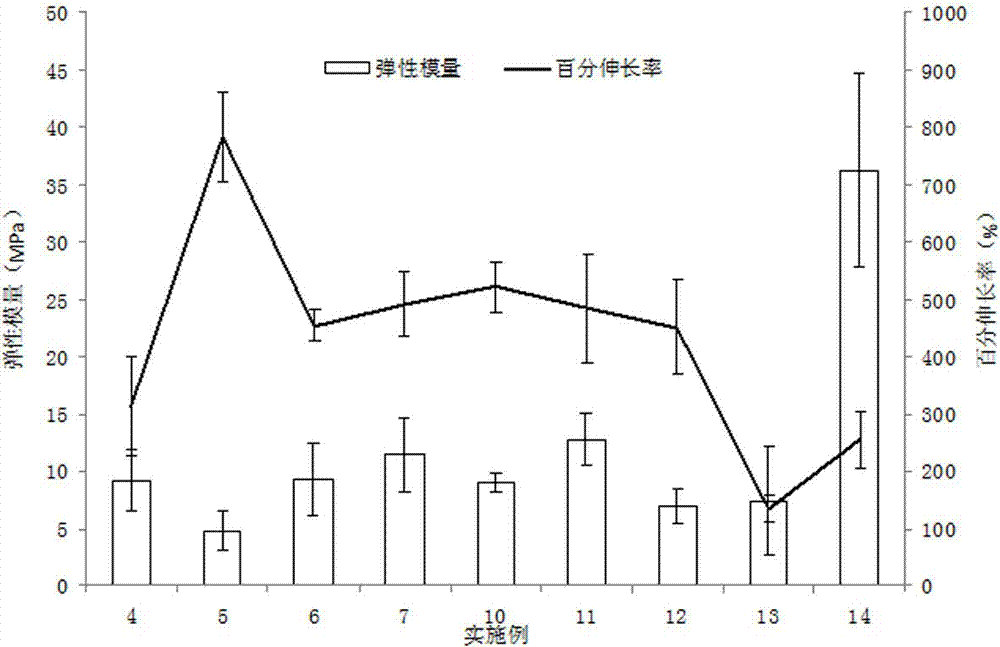
InactiveCN102824333AImprove disintegration time limitSolve the shortcomings of taking waterPharmaceutical non-active ingredientsSexual disorderVardenafilTadalafil
The invention discloses an oral quick-dissolving film preparation and a preparation method thereof. The oral quick-dissolving film comprises the following components in percentage by weight: 20 to 40 percent of medicinal active component, 40 to 75 percent of water-soluble film forming material, 10 to 25 percent of plasticizer, 0 to 25 percent of disintegrating agent, and 0.1 to 8 percent of water, wherein the medicinal active component is one of sildenafil, tadalafil, vardenafil or salts thereof. According to the oral quick-dissolving film preparation, the disintegration time limited of the film preparation can be remarkably accelerated, the problem that most oral solid preparations should be taken with water can be solved, medicine taking time cannot be delayed under a condition without water, and the taking compliance of a patient can be improved.
Owner:SUZHOU UNIV
Tadalafil orally disintegrating tablet and preparation method thereof
InactiveCN103271885AEasy to takeFast absorptionOrganic active ingredientsPill deliveryTadalafilFlavouring agent
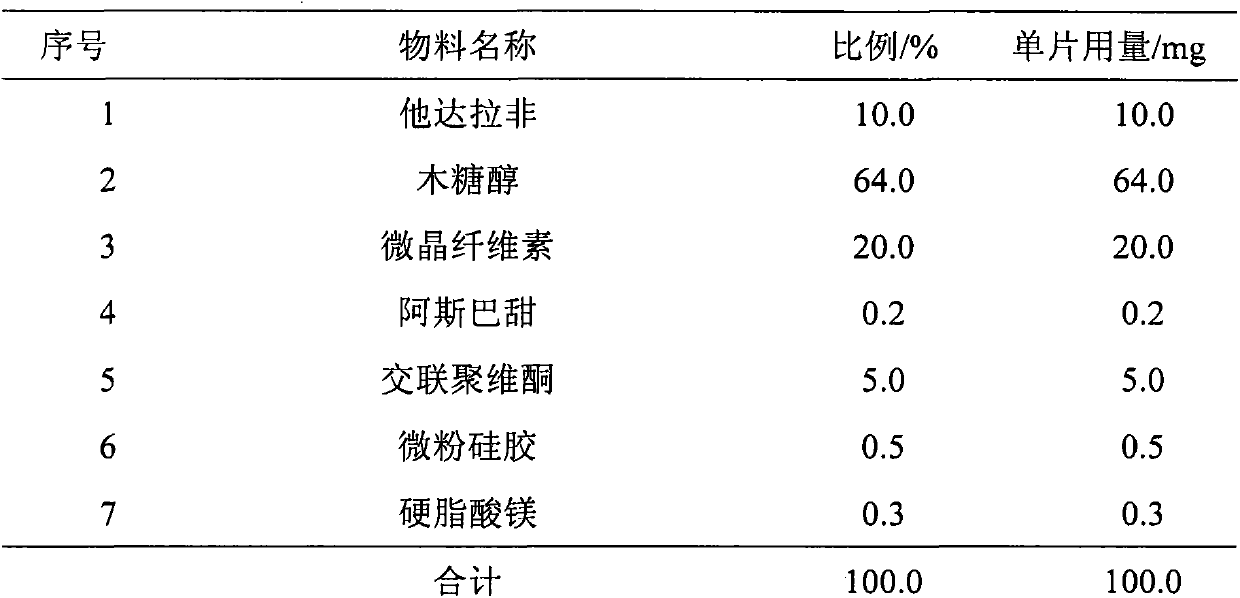
The invention relates to a tadalafil orally disintegrating tablet and a preparation method thereof. Aiming to improve the deficiency of existing tadalafil oral dosage forms, the invention provides the tadalafil orally disintegrating tablet which is convenient to take, is quick in absorption, takes effect quickly and has high bioavailability for doctors and patients to help the patients achieve requirements for natural and satisfactory sexual lives. The tadalafil orally disintegrating tablet takes tadalafil or medicinal salts thereof as an active ingredient, contains pharmaceutically acceptable auxiliary materials such as filling agents, disintegrating agents, flavouring agents and lubricating agents, and when being taken, dispenses with water, can be quickly disintegrated or dissolved in oral cavities only in a few seconds and is ingested along with saliva.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
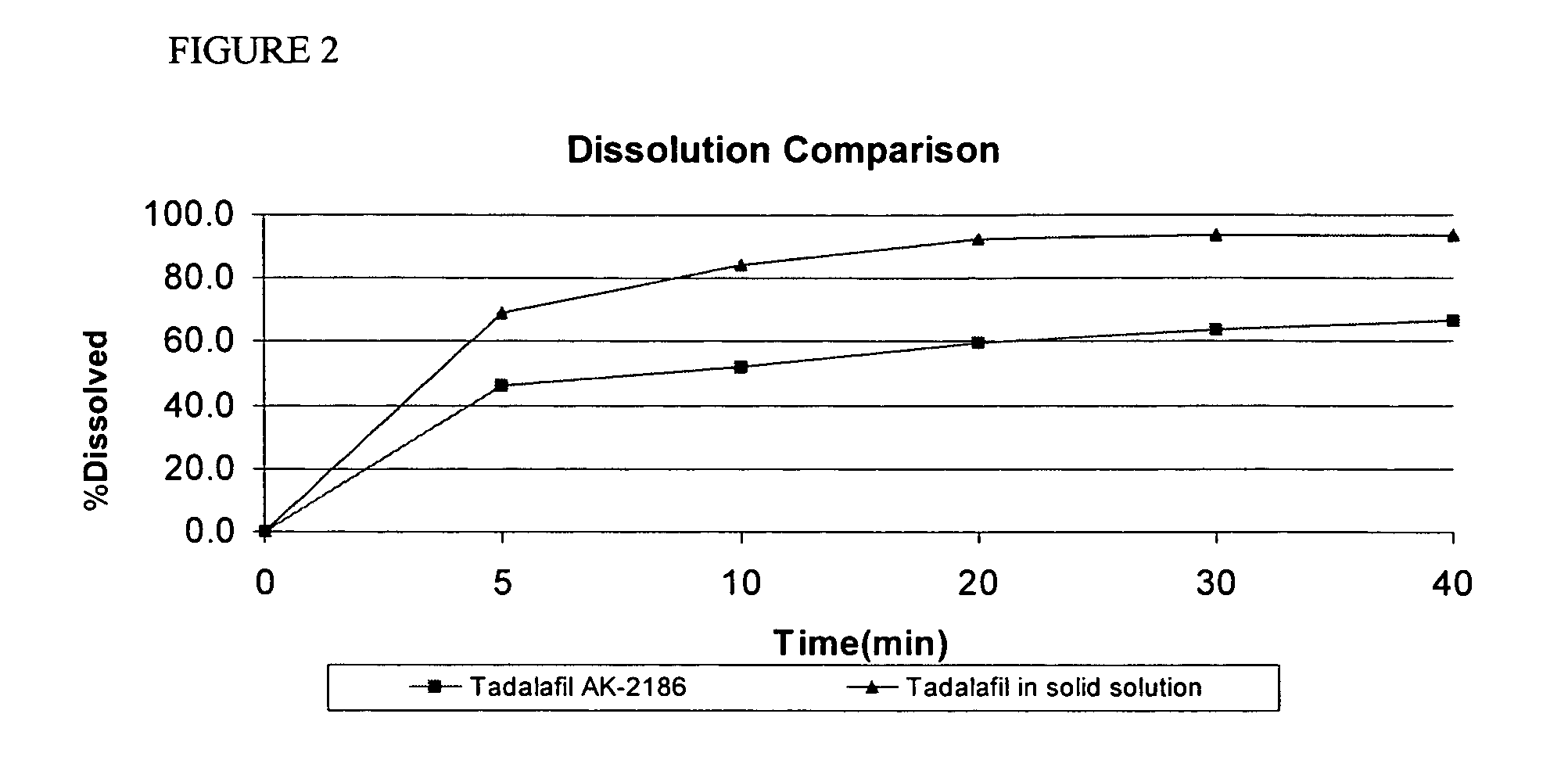
Tadalafil solid composites
This invention relates to oral pharmaceutical compositions suitable for making pharmaceutical formulations for oral administration that provide for the rapid dissolution of the phosphodiesterase 5 inhibitor tadalafil. In particular, the pharmaceutical compositions comprise solid composites of tadalafil exhibiting high solubility and rate of dissolution. The invention further relates to methods of preparing these pharmaceutical formulations and the use of such pharmaceutical formulations for treating diseases associated with PDE5 inhibitors.
Owner:TEVA PHARM USA INC
Simple preparation process of tadalafil
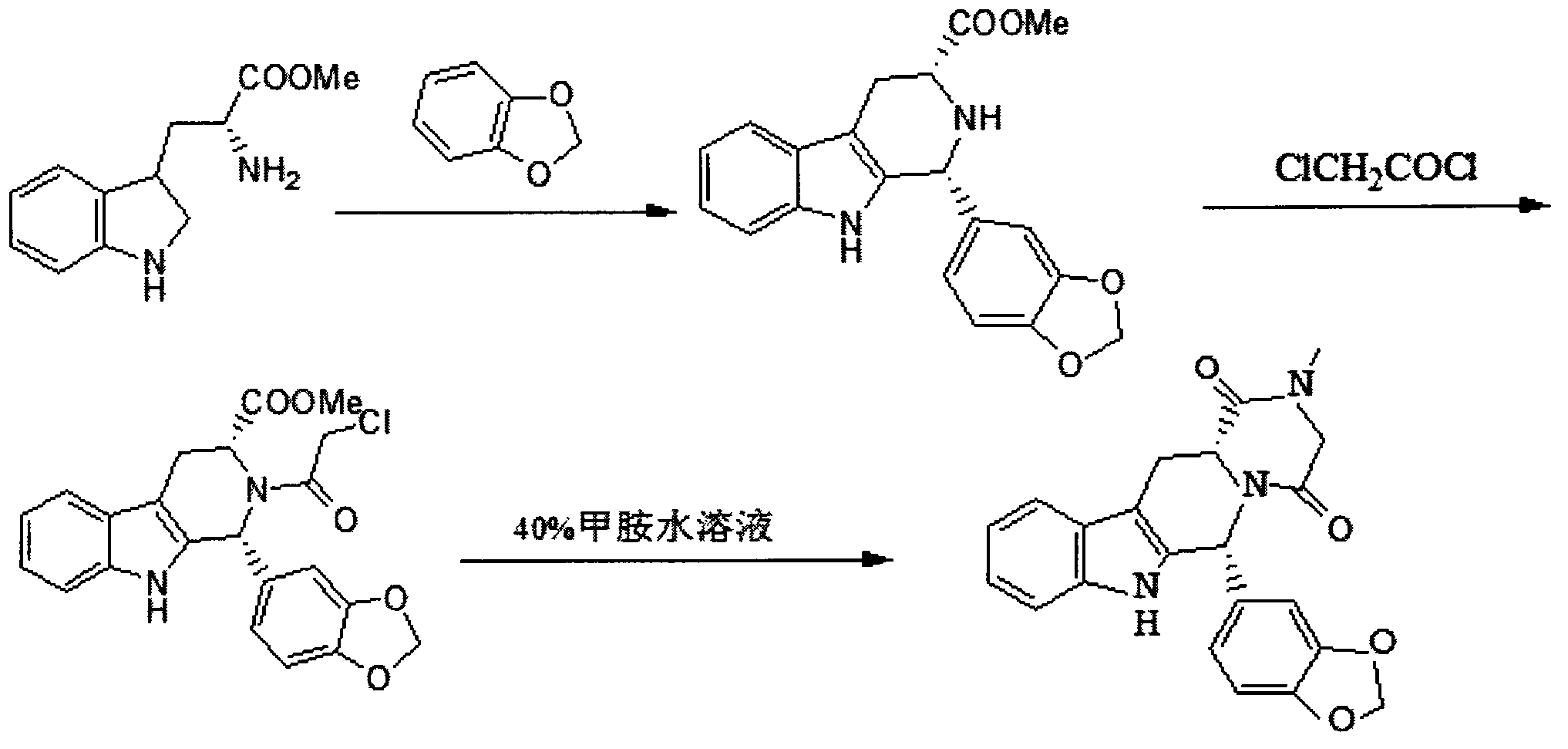
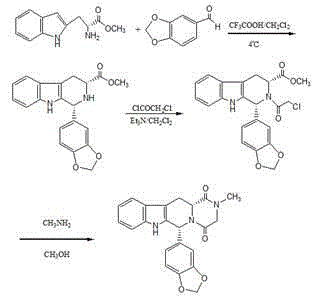
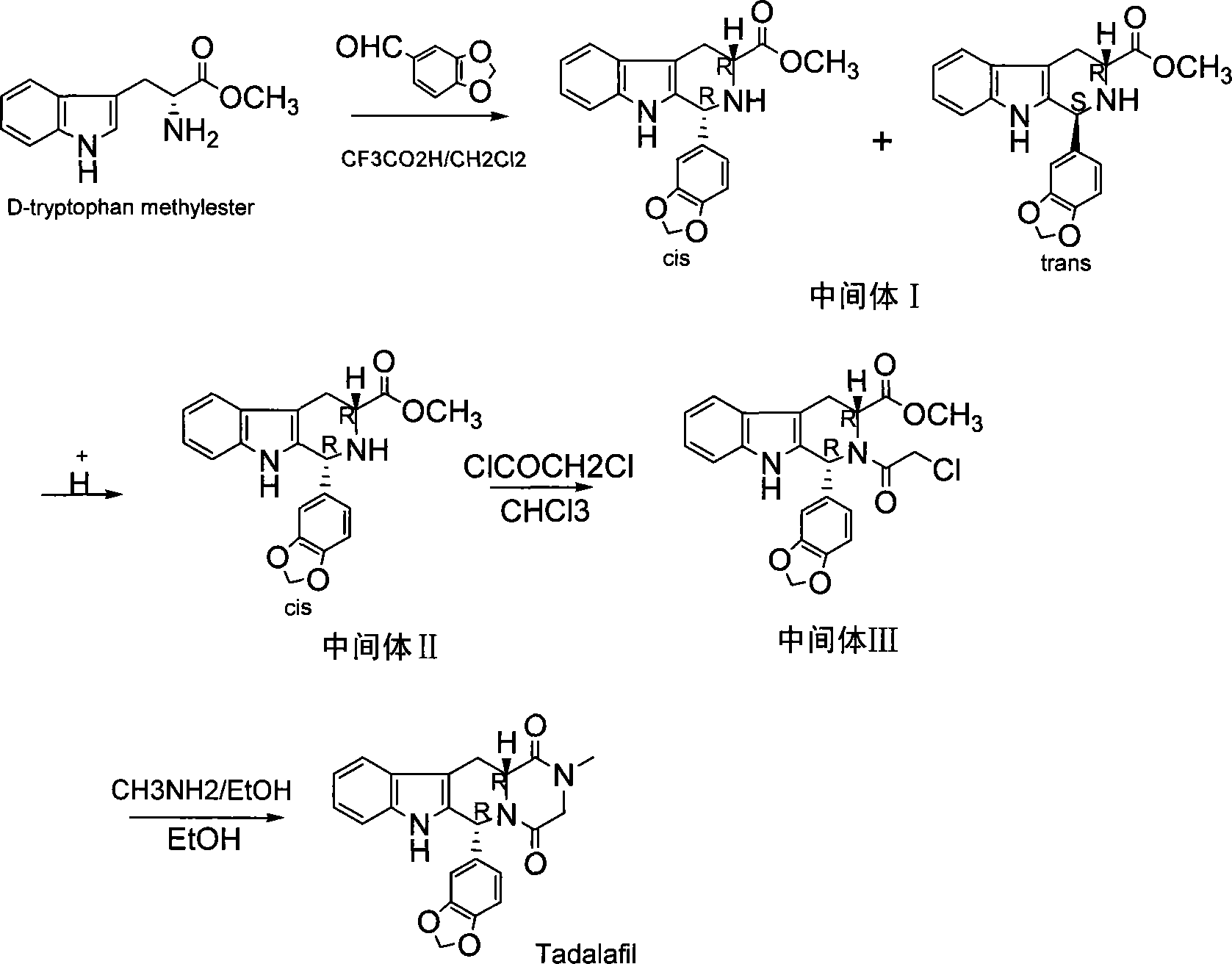
The invention relates to a preparation method of tadalafil. A product is obtained through condensation and cyclization, chloromethylation and aminolysis cyclization reaction based on D-tryptophan methyl ester hydrochloride and heliotropin as starting preparation materials. The simple preparation process is characterized in that condensation and cyclization are used for solving an isomer problem caused by Pictet-Spengler reaction by using isopropanol or nitromethane as a solvent; the yield of ethyl acetate in the aminolysis cyclization reaction is obviously improved; the aminolysis cyclization route includes three preparation steps, wherein the reaction yield of each step is high, the relevant impurities are easy to separate, the reaction conditions are simple, the production period is shorter, and toxic and highly corrosive reagents are not used, and therefore, the simple preparation process is safe and environment-friendly and easy to industrially produce, so that the high-purity qualified products are obtained.
Owner:ZHANG JIA GANG VINSCE BIO PHARM
Preparation method of tadalafil
The invention discloses a preparation method of tadalafil. The concrete preparation method is used for successfully synthesizing tadalafil by taking L-tryptophan methyl ester hydrochloride as an initial raw material and utilizing the characteristics that an ortho-position of an ester group has the chiral inversion property under an alkaline condition, the reaction between ester group and Pictet-Spengler is reversible reaction, and the ester group is easily converted into a cis-form product with relatively small solubility, therefore, the preparation method is a brand new technology. The price of L-tryptophan methyl ester hydrochloride is only less than 1 / 5 of that of D-tryptophan methyl ester hydrochloride, so that the cost of the overall route is greatly lower than that of the prior art, and then the preparation method is suitable for industrial production.
Owner:优标易站(苏州)电子商务有限公司
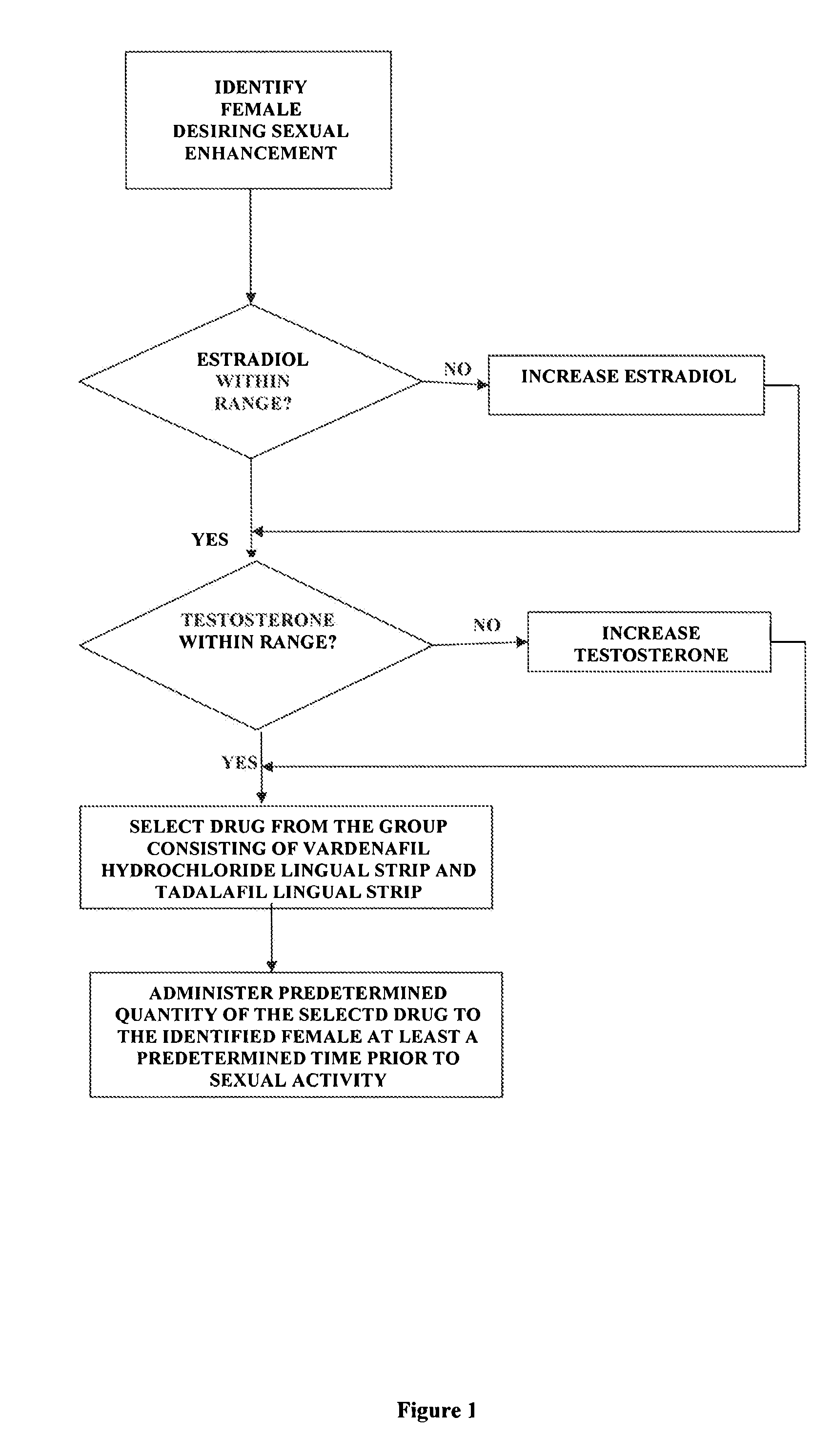
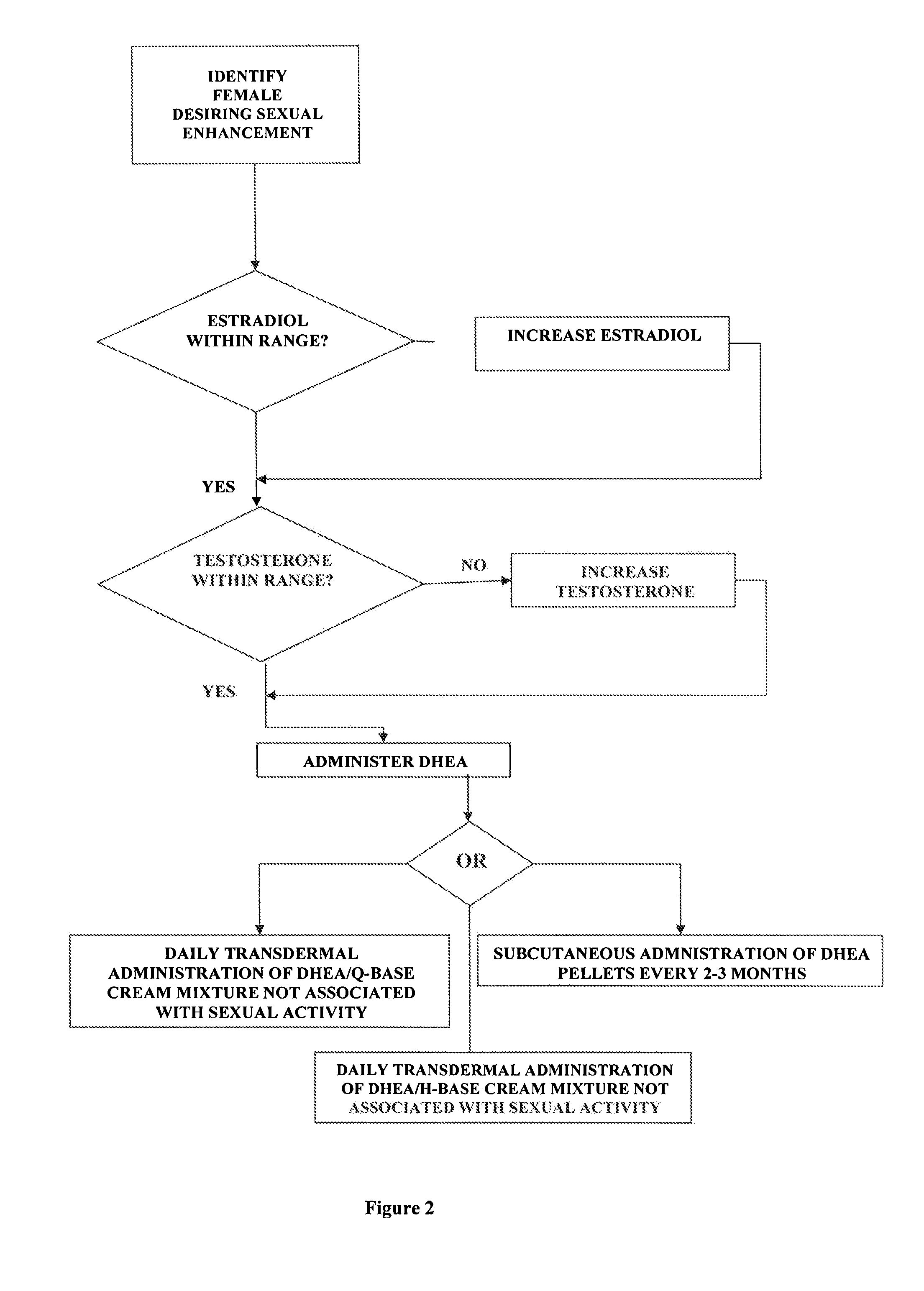
Methods of female sexual enhancement
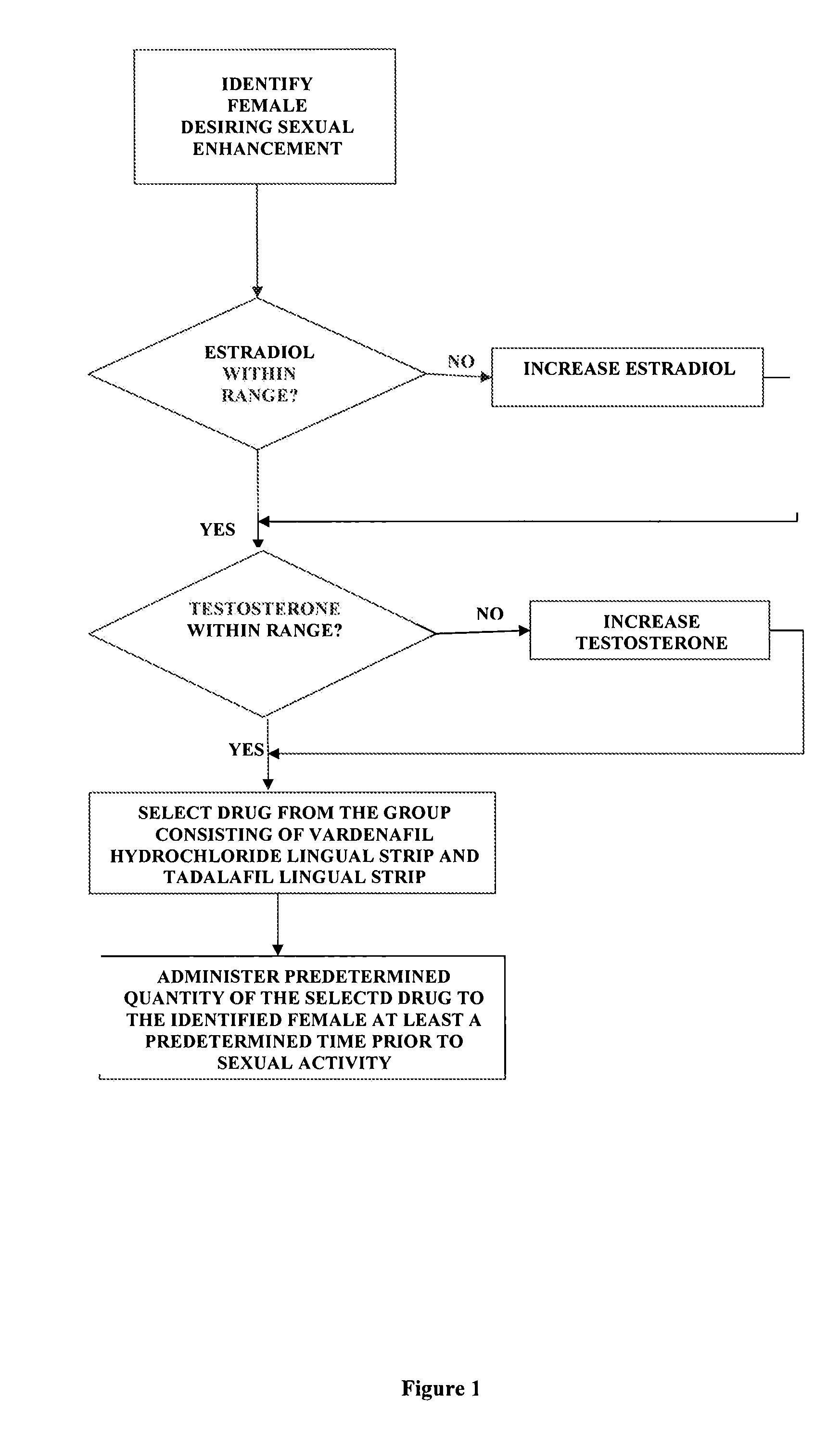
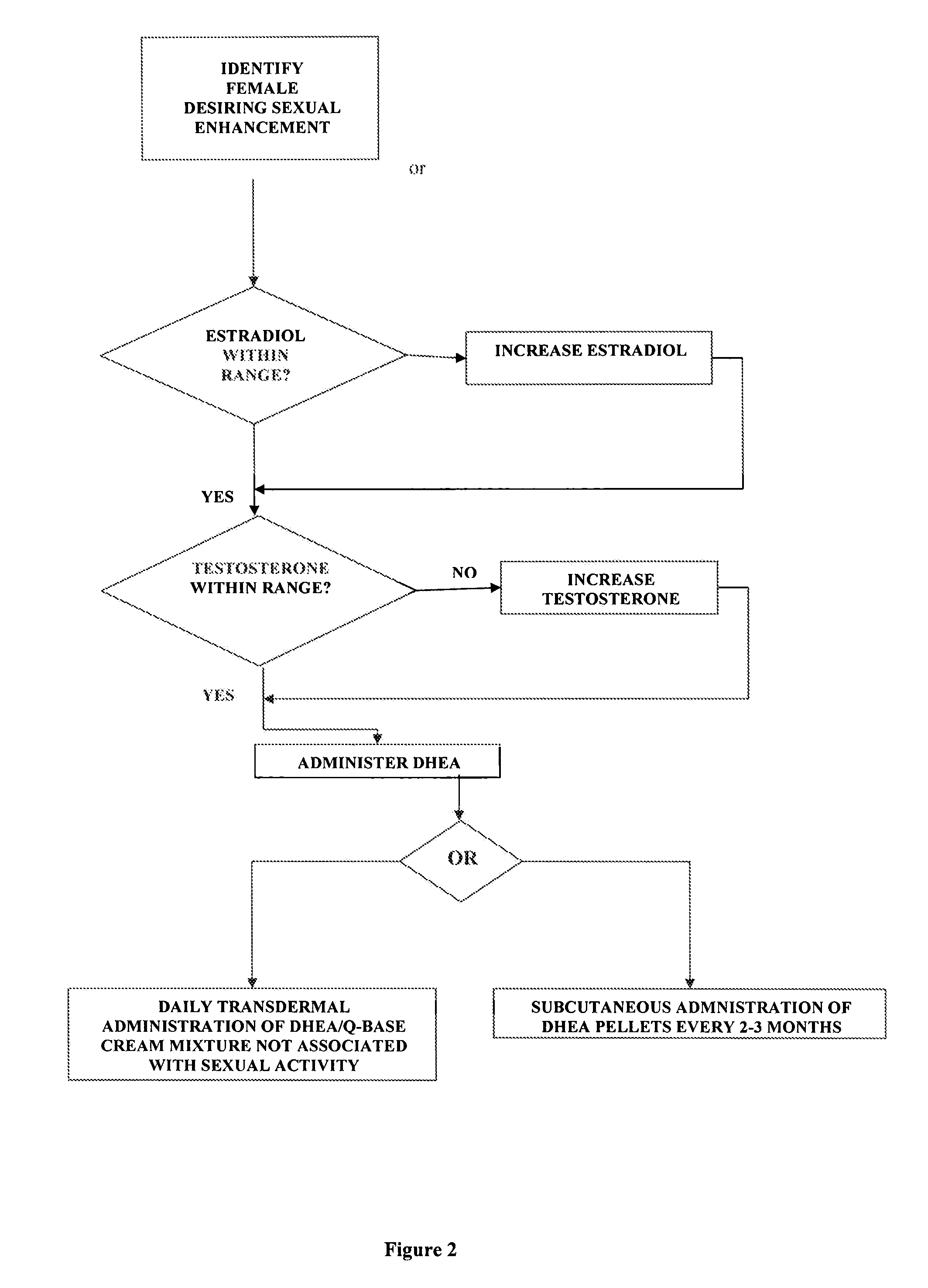
A method of sexual enhancement in women includes the steps of identifying a woman requesting sexual enhancement, assuring that the woman's blood includes estradiol within a first predetermined range and testosterone within a second predetermined range, and thereafter administrating a drug selected from the group consisting of vardenafil hydrochloride and tadalafil prior to sexual activity. The selected drug may be loaded into a starch strip which is then applied to the woman's tongue. Sexual enhancement in women can also be achieved by transdermal or subcutaneous application of the steroid hormone DHEA.
Owner:LES MEDECINS
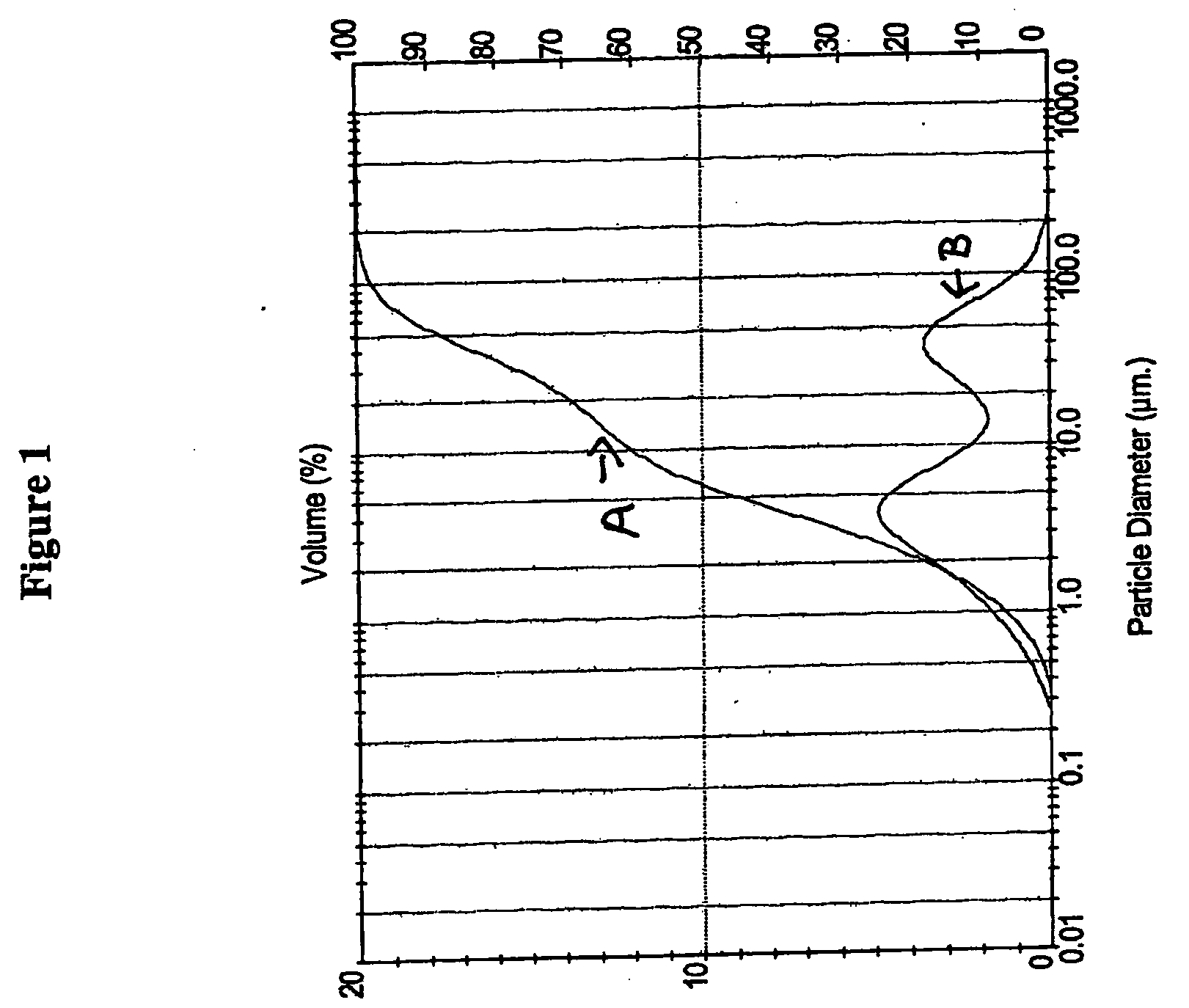
Solid particulate tadalafil having a bimodal particle size distribution
Owner:TEVA PHARM USA INC
Preparing method of phosphodiesterase 5 inhibitor tadalafil
ActiveCN103980275AGood removal effectHigh chiral purityOrganic chemistryPhosphodiesterase 5 inhibitorTadalafil
The invention relates to a preparing method of a phosphodiesterase 5 inhibitor tadalafil. D-methyl tryptophanate hydrochloride is adopted as an initial raw material, and is subjected to cyclization with heliotropin, N-acylation, aminolysis-cyclization, and other reactions to obtain a tadalafil crude product. The tadalafil crude product is recrystallized to obtain a tadalafil finished product. The method has characteristics of mild reaction conditions, short reaction time, high yield, good product stability and convenience for industrial production.
Owner:湖北省医药工业研究院有限公司
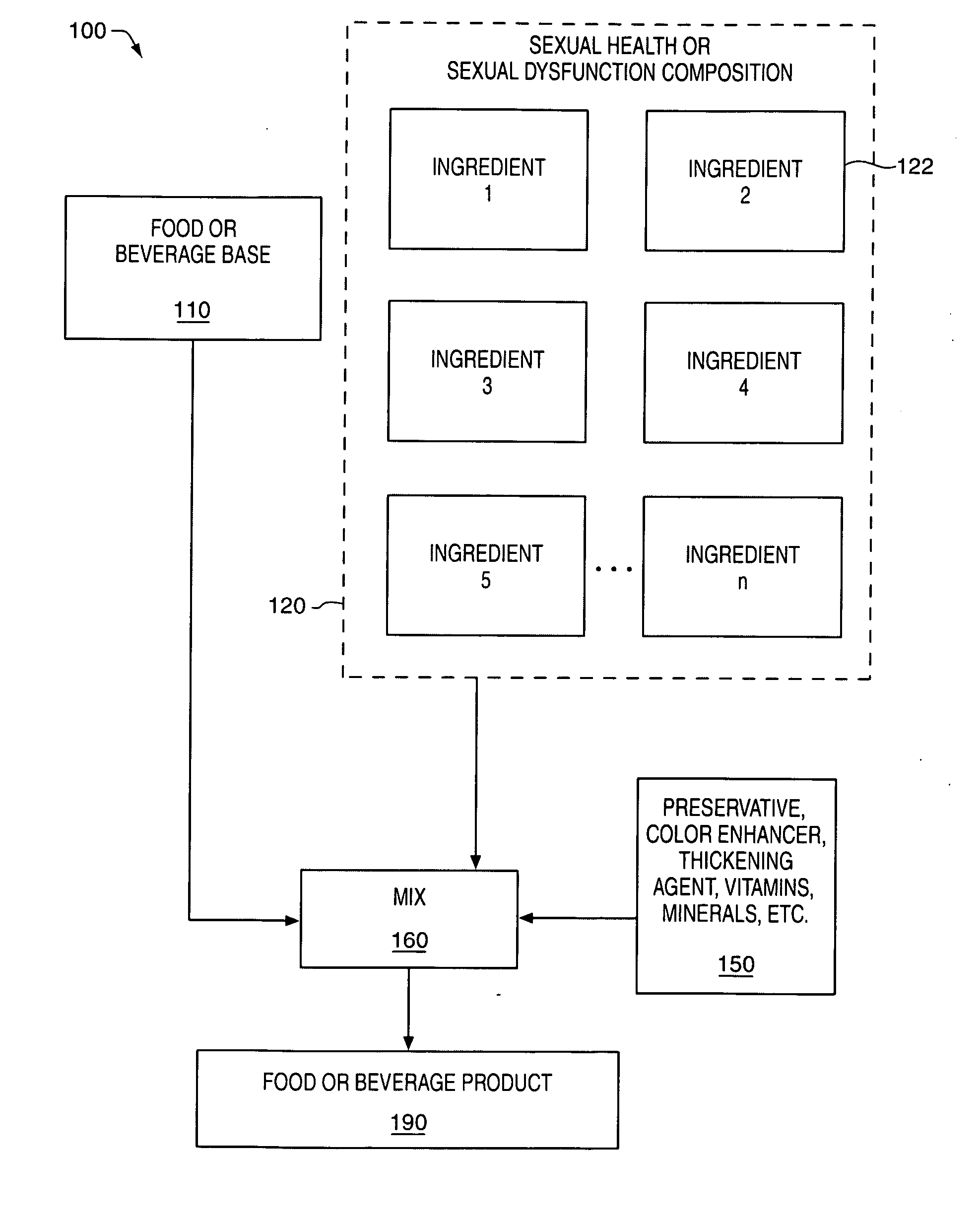
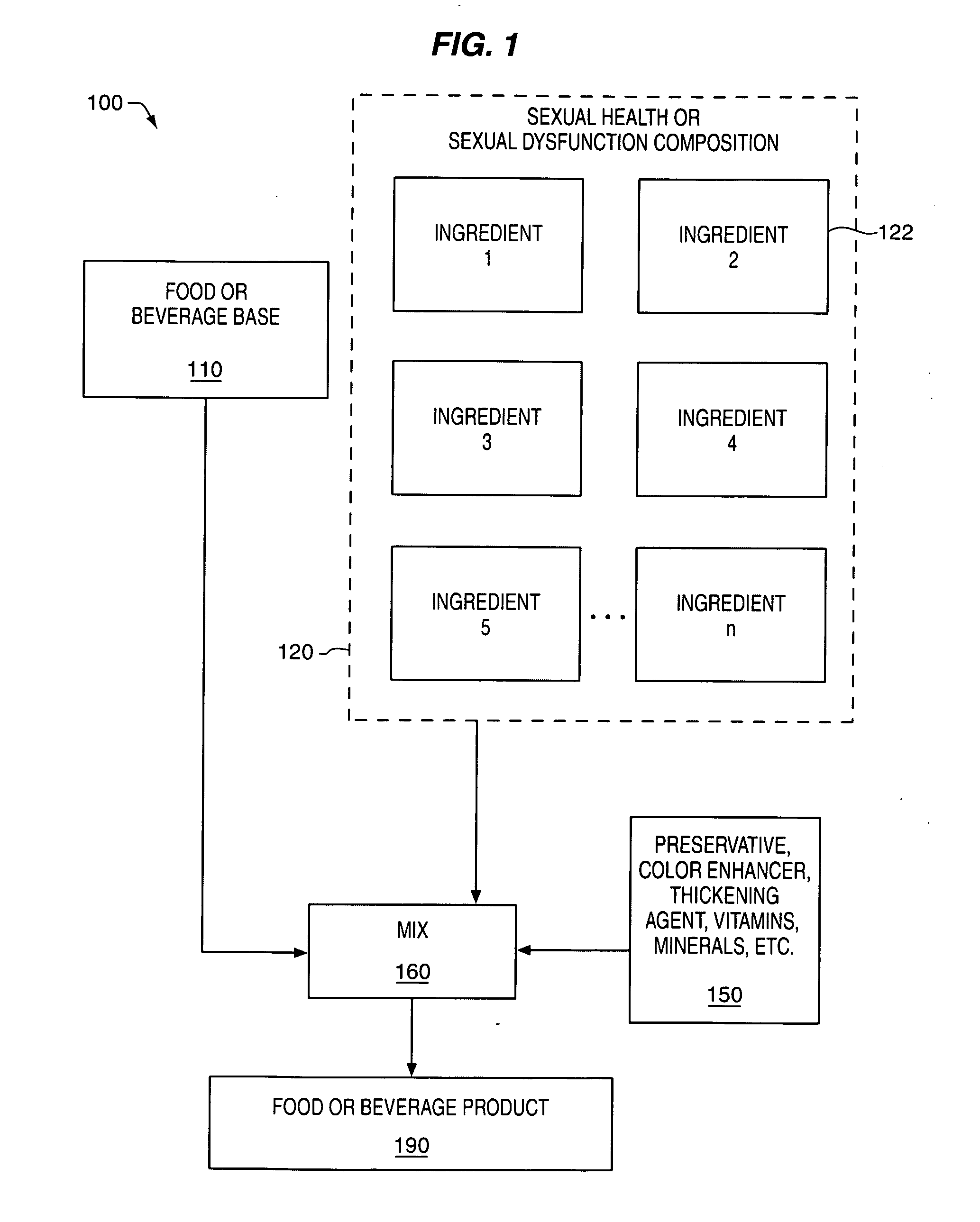
Delivery system and method for supporting and promoting healthy sexual function and prevention and treatment of sexual dysfunction
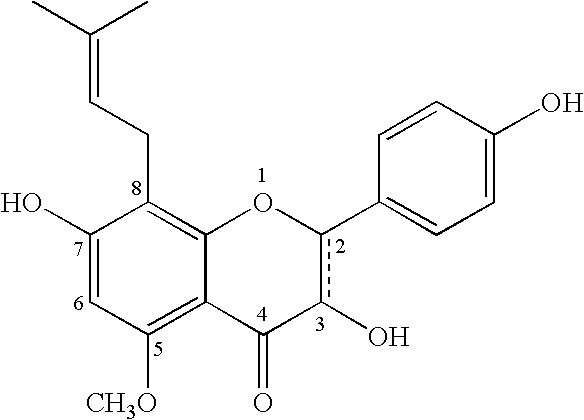
InactiveUS20060110478A1Increase in cGMPGood curative effectFood ingredient as antioxidantBiocideSexual functionVardenafil
Improved delivery systems and delivery methods for supporting and promoting healthy sexual function, for preventing sexual dysfunction, or for treatment of sexual dysfunction. A compositions including one or more cGMP-specific PDE5 inhibitors and / or dopaminergic agonists is administered in the form of a breath-care strip, mint or lozenge, or a food or beverage product. The cGMP-specific PDE5 inhibitor comprises an ingredient selected from the group consisting of sophoflavescenol, vardenafil, tadalafil, and sildenafil. The dopaminergic agonist comprises apomorphine. Vitex agnus-castus extract, and one or more of lipoic acid, L-Arginine, folic acid, trimethylglycine, policosanol, carnitine, biotin, and acetyl L-Carnitine may also be included in the delivery vehicle.
Owner:MCCLEARY EDWARD LARRY +2
Oral medicinal preparation of tadalafil
ActiveCN103191075AImprove complianceImprove bioavailabilityOrganic active ingredientsPill deliveryTadalafilOrally disintegrating tablet
The invention relates to an oral medicinal preparation of tadalafil and application of the preparation in treatment of male erectile dysfunction. The preparation is characterized in that the main medicne is tadalafil or salt thereof, and the particular formulation can be orally disintegrating tablets, chewable tablets, oral membranes and other preparations capable of being quickly released in the oral cavity; tadalafil is an insoluble medicament, and is low in bioavailability, so that the administrated infective dose is large, multiple adverse responses can be generated, and visual impairment or loss can be caused due to unreasonable administration. Regarding the researches of tadalafil medicinal preparation for improving medicament bioavailability and reducing occurrence of adverse responses, the wide clinical application of tadalafil in treatment of male erectile dysfunction is particularly important. Aiming at the fact that the tadalafil medicinal preparation brings great favor to broad ED patients in improvement of the compliance when the tadalafil medicinal preparation is taken by a patient, particularly in administration in an environment without water and in a non-swallow mode. The oral medicinal preparation is prepared through massive studies based on patients.
Owner:NANJING CHIA TAI TIANQING PHARMA
Methods of female sexual enhancement
InactiveUS20060252734A1Enhanced female sexual pleasureImprove satisfactionOrganic active ingredientsBiocideVardenafilGynecology
Owner:LES MEDECINS
Solid dosage forms
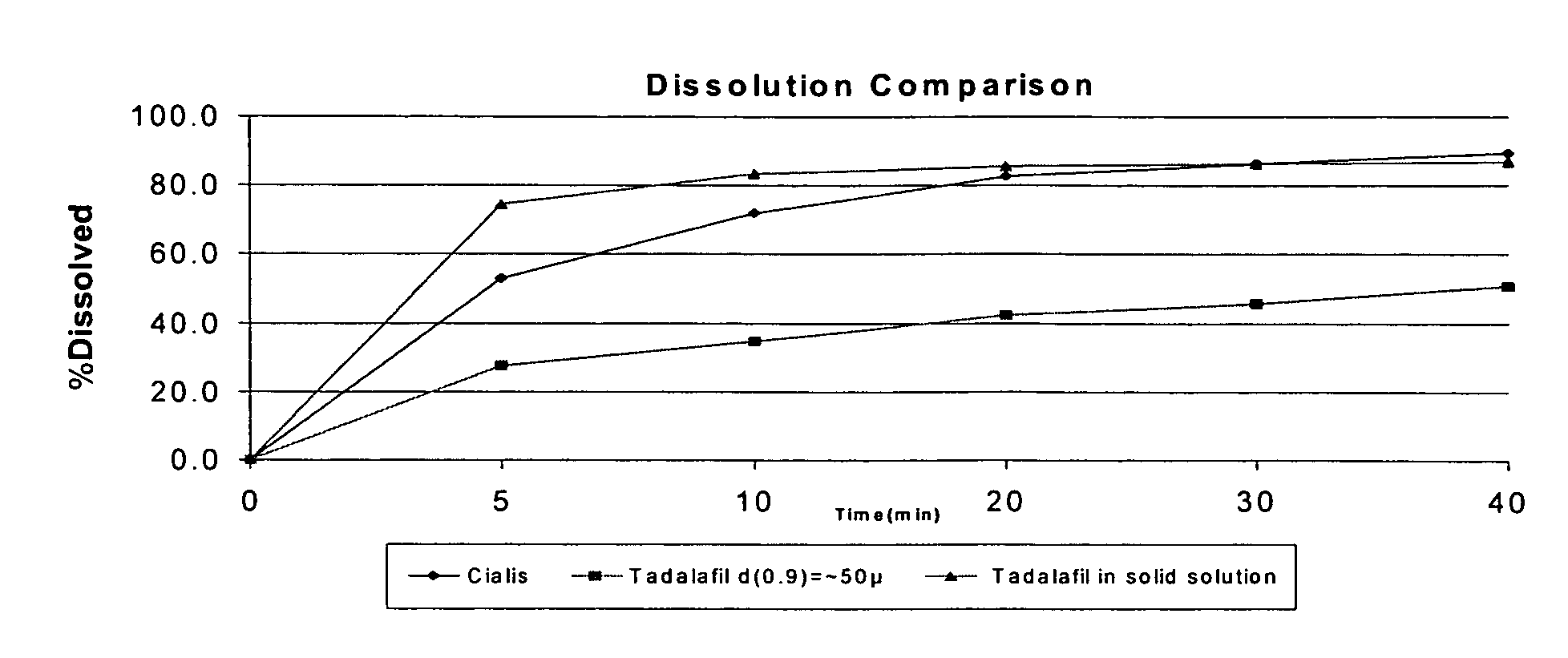
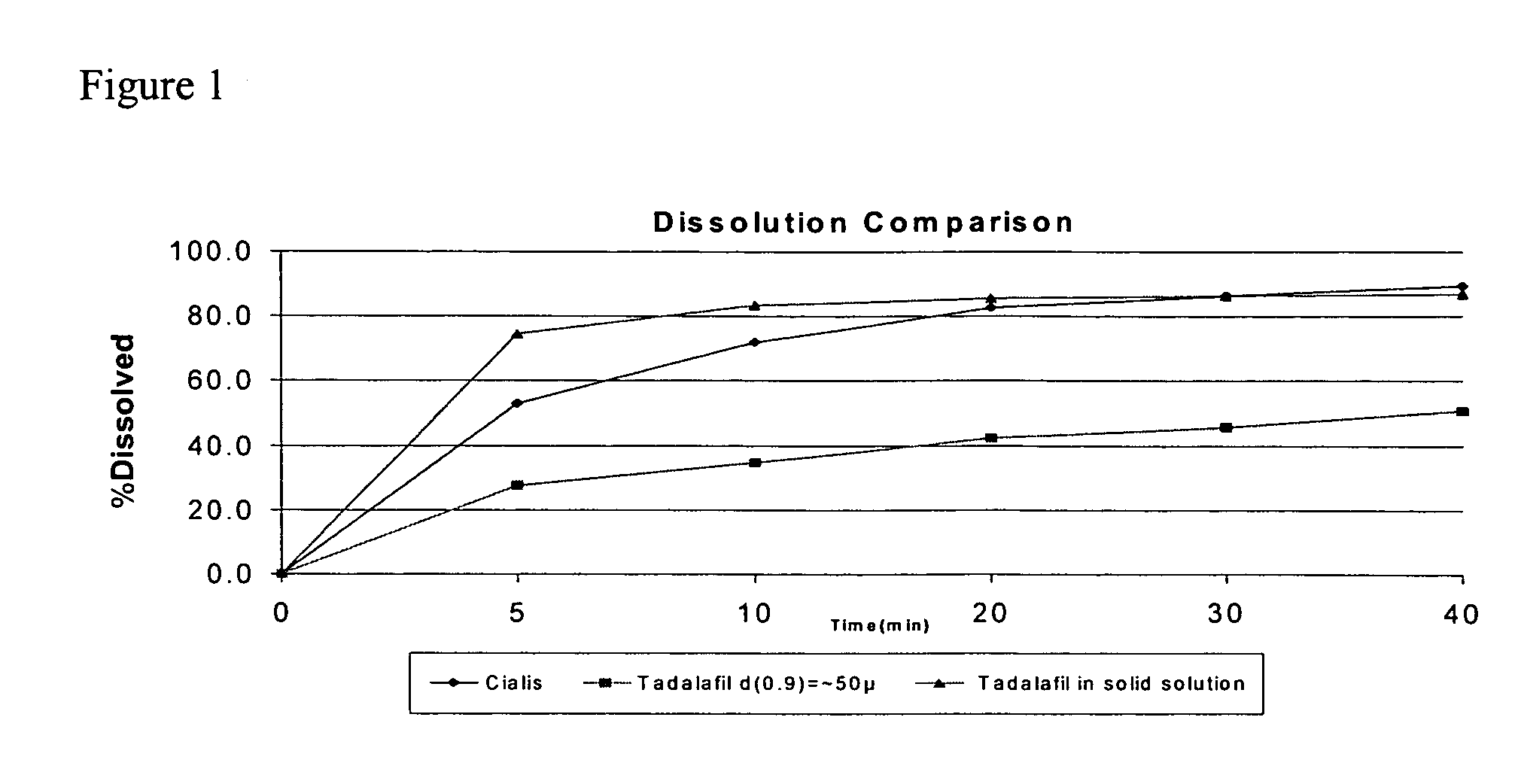
Pharmaceutical dosage forms comprising tadalafil are described. Preferred dosage forms are bioequivalent to Cialis® notwithstanding a large particle size.
Owner:TEVA PHARM USA INC
Method for detecting Tadalafei and derivative thereof
ActiveCN101021483ARapid responseEnough sensitivityMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationTest sampleTadalafil
The invention discloses a method to detect Tadalafil and its derivative. It contains steps: (1) Color reaction. Make color reaction by adding concentrated sulfuric acid. (2) Result estimation. If color reaction presents blue violet, there are Tadalafil and its derivative in test samples. The method has merits of quick, simple, high sensitivity and strong property etc.
Owner:BEIJING INST FOR DRUG CONTROL
Use of phosphodiesterase inhibitors for treating multidrug resistance
InactiveUS20120252816A1Suppression problemInhibiting ABCG transporter activityBiocideAnimal repellantsVardenafilPde5 inhibition
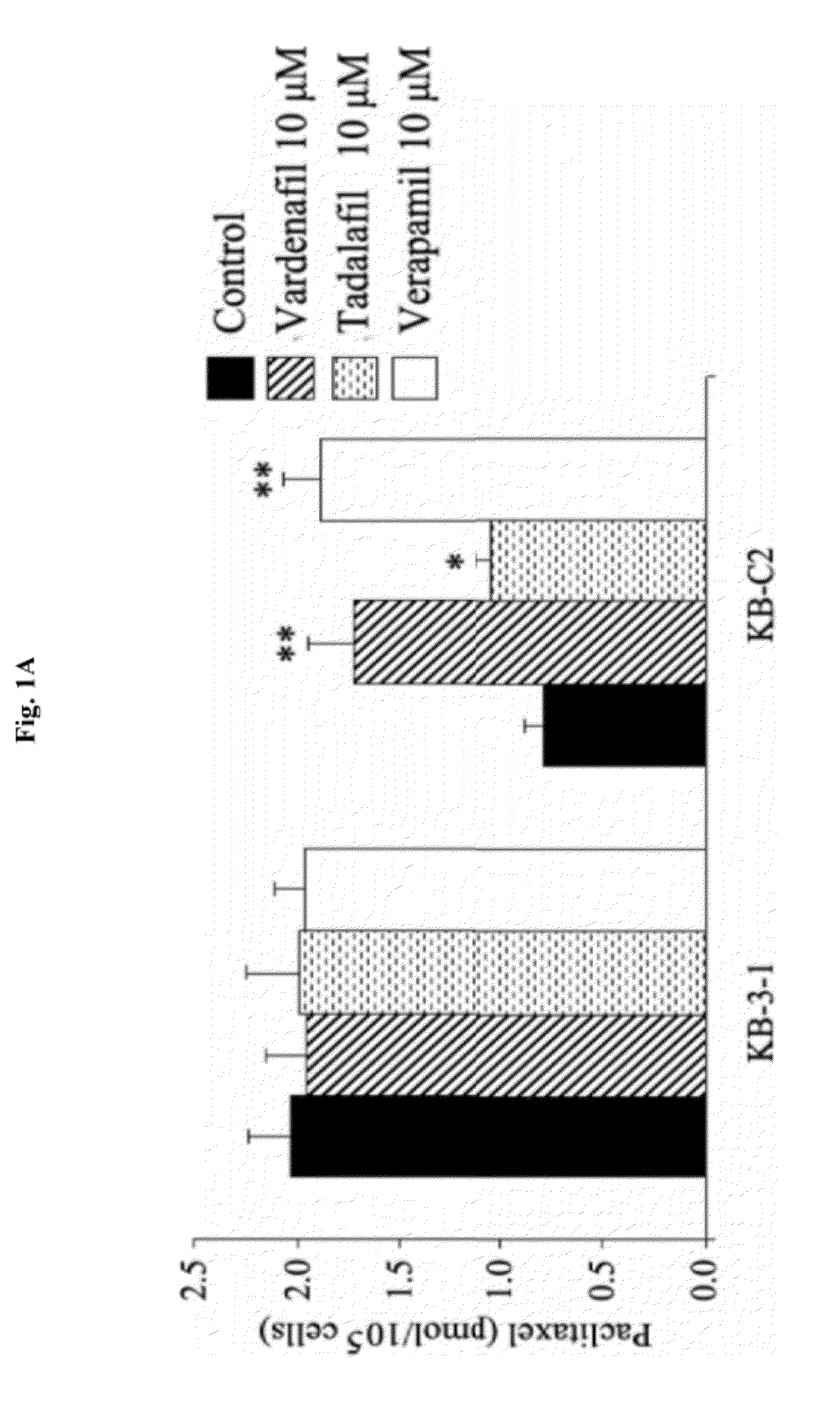
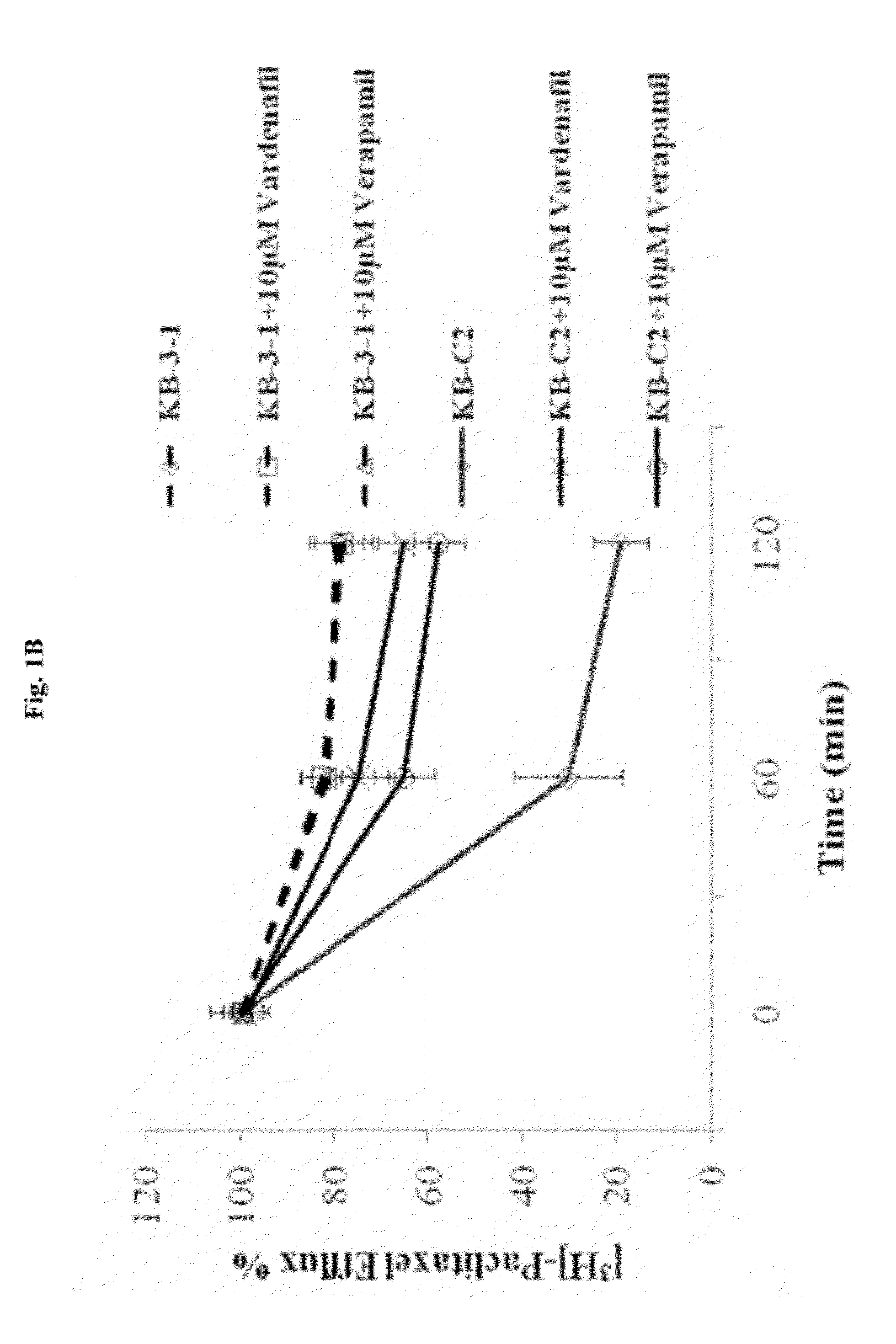
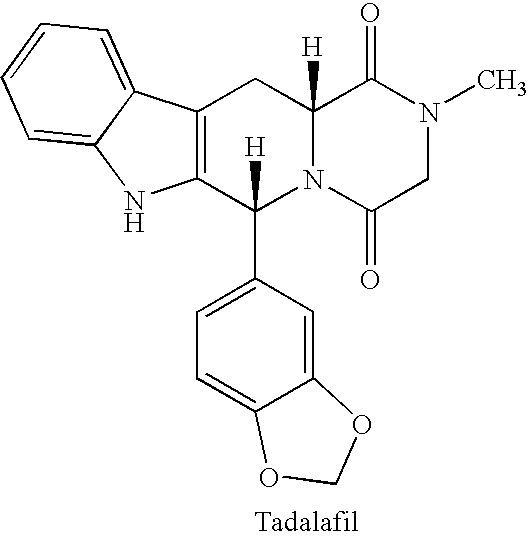
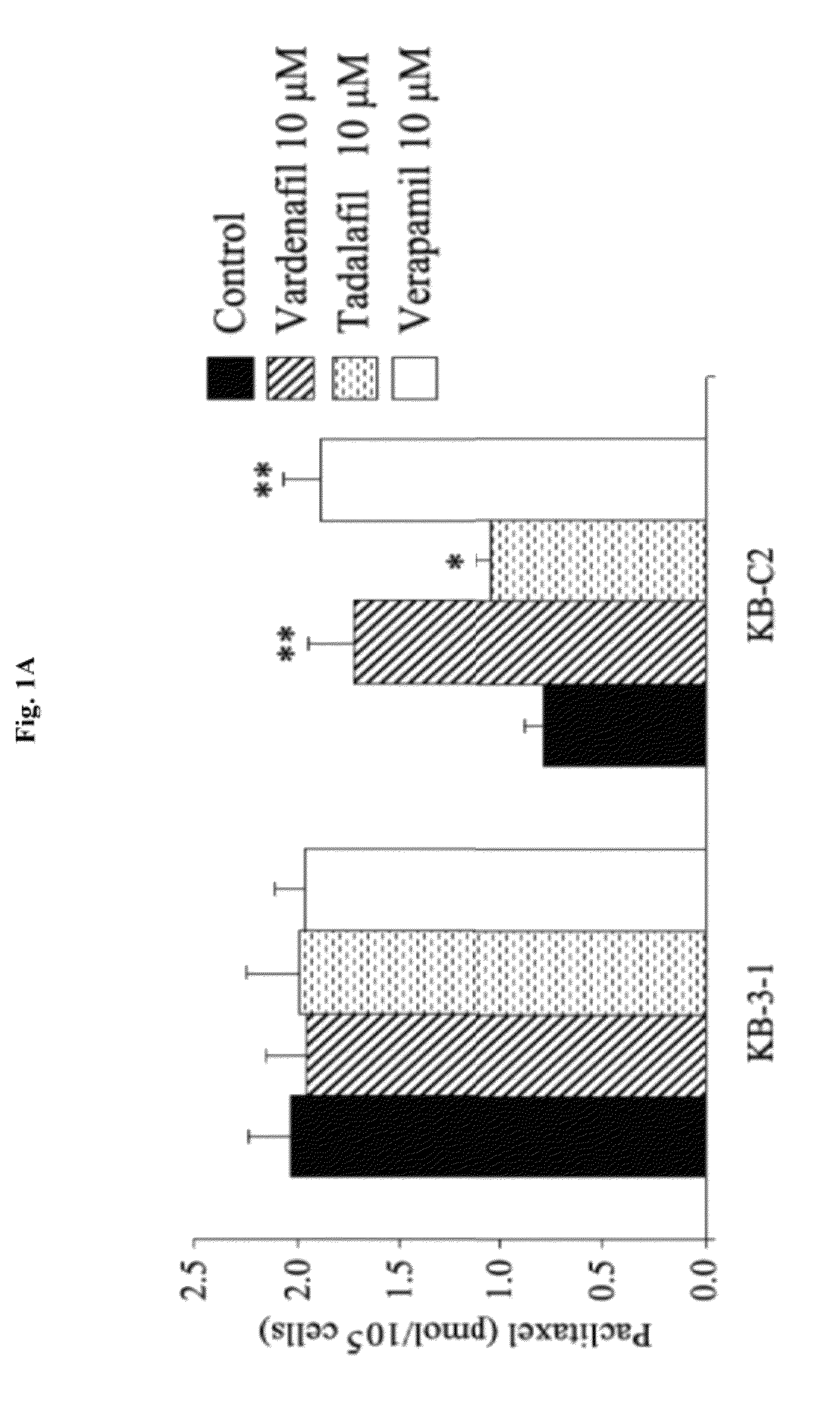
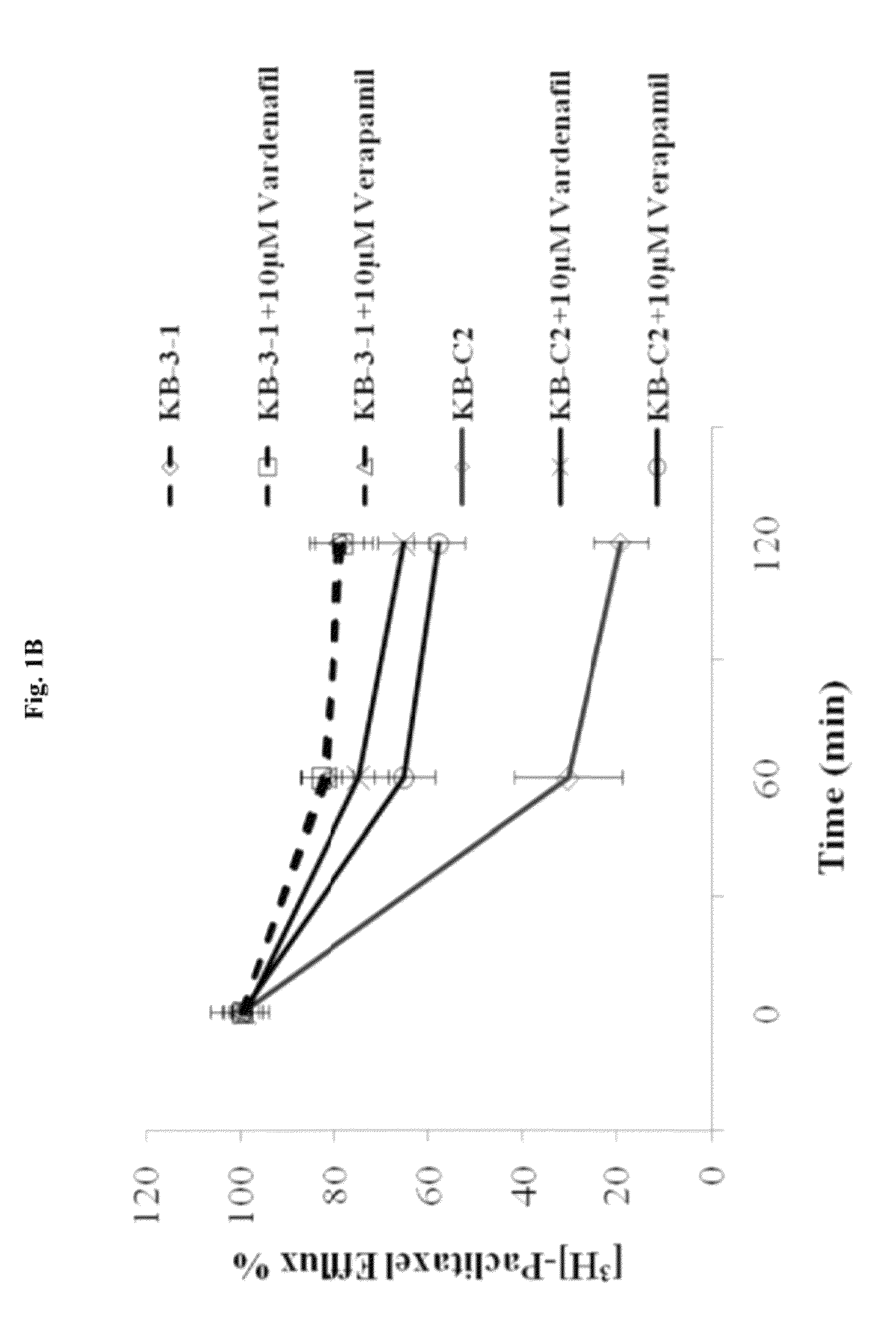
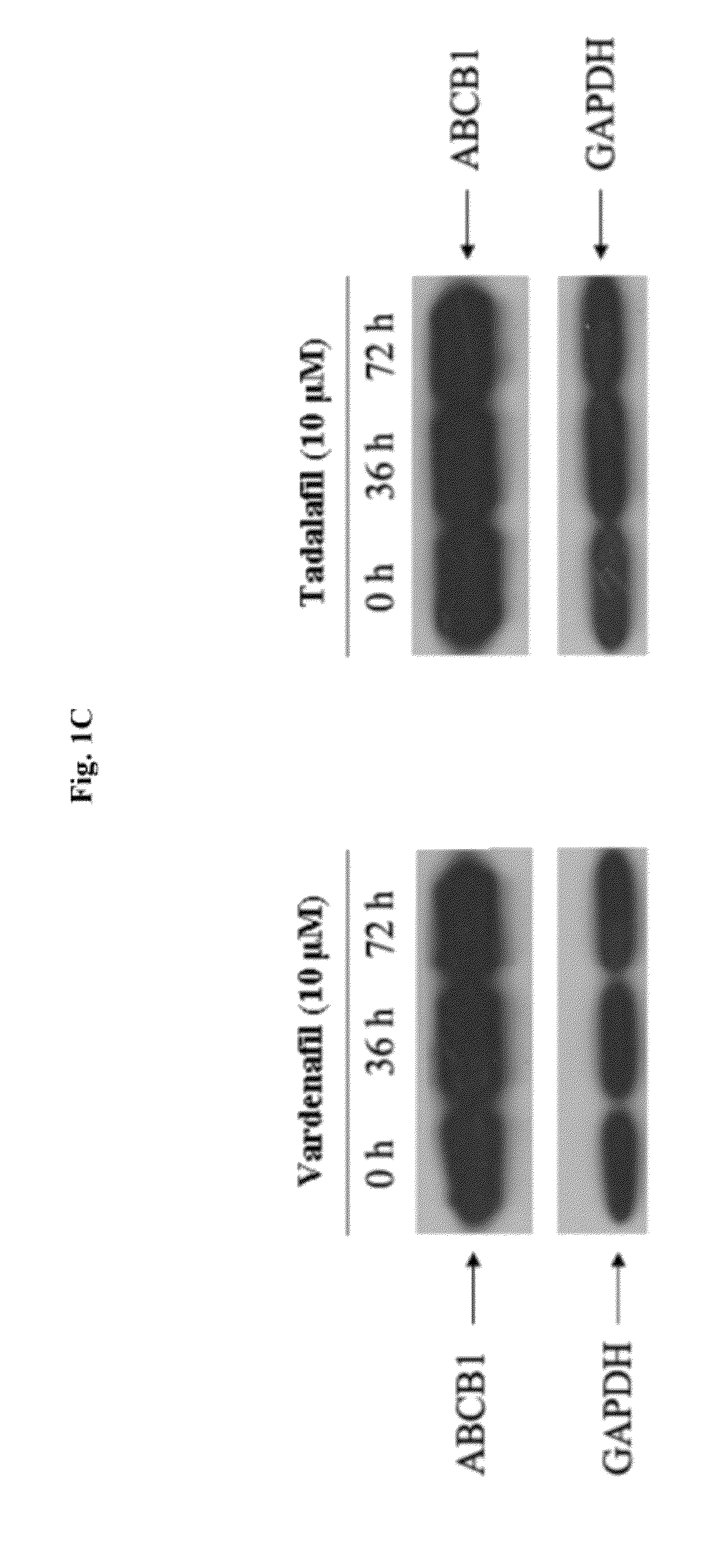
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
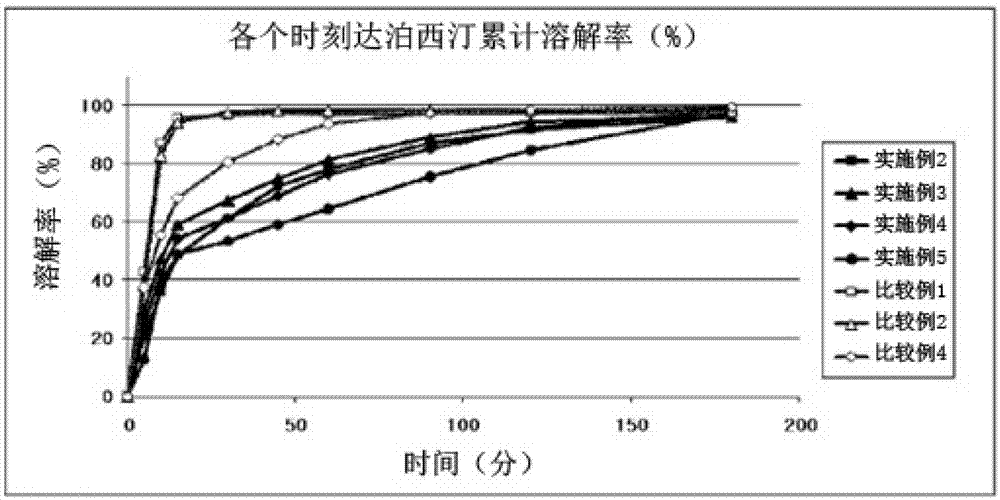
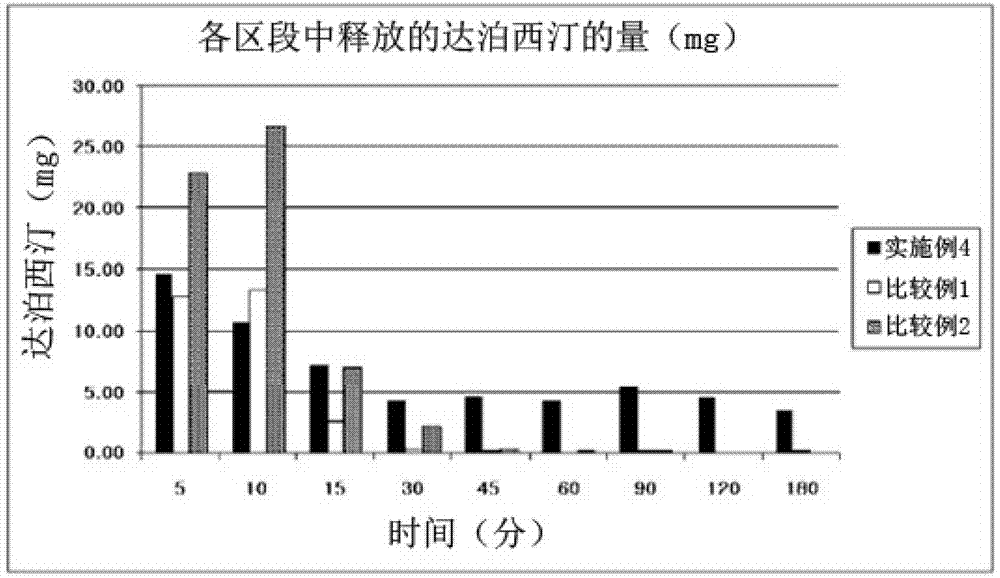
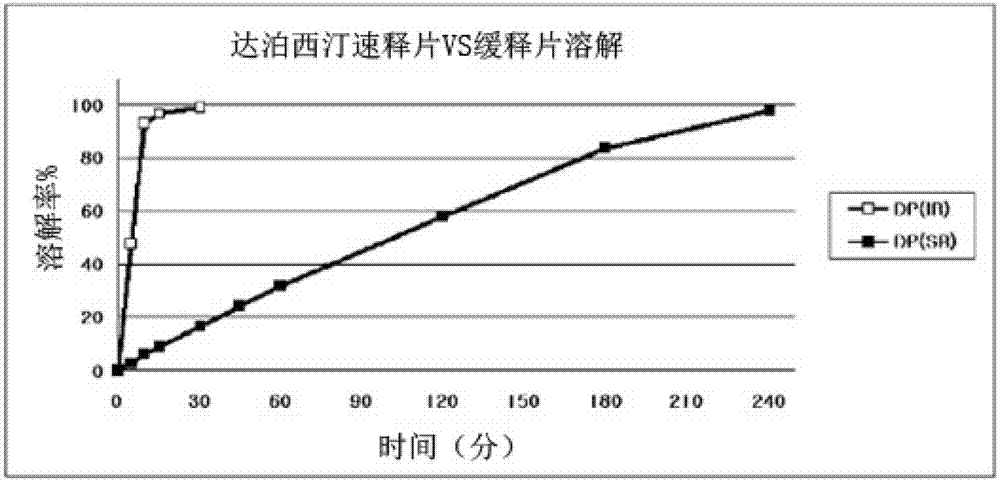
Time-delayed sustained release pharmaceutical composition comprising dapoxetine for oral administration
InactiveCN102958513ASatisfy sexual desireLittle side effectsOrganic active ingredientsPill deliverySexual impotenceOral medication
The present invention relates to a time-delayed sustained release pharmaceutical composition for oral administration, which comprises an immediate release phase and a prolonged sustained release phase, wherein said immediate release phase and prolonged sustained release phase respectively comprise dapoxetine therein as an active ingredient.; The pharmaceutical composition of the present invention comprises dapoxetine, which is an agent for treating premature ejaculation, in both the immediate release phase and the prolonged sustained release phase thereof, to thereby immediately exhibit the effectiveness of the pharmaceutical composition of the present invention in order to enable a patient to achieve sexual satisfaction during the early stage of administration, as well as to reduce side effects by means of the time-delayed sustained release of the prolonged sustained release phase during the early stage of administration and enable a continuous in vivo absorption of dapoxetines, to thereby lengthen the duration of the effectiveness of the pharmaceutical composition of the present invention.; Further, agents for treating erectile dysfunction, such as sildenafil, tadalifil or the like can be added to the immediate release phase so as to allow for a coincidence of the durations of the effectiveness of a premature ejaculation treatment agent and erectile dysfunction treatment agents, even though a half-life difference exists between the two types of treatment agents, thus maximizing patient satisfaction.
Owner:NAVIPHARM CO LTD
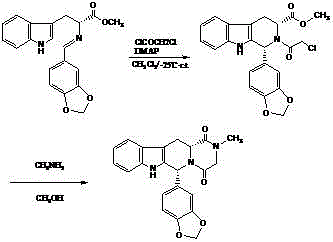
Improved tadalafil preparation method
The invention belongs to the field of preparation of chemical raw medicaments, and more in particular relates to an improved preparation method for a phosphodiesterase 5 inhibitor tadalafil. A specific synthesis route is shown in the specification. The method comprises the following steps of performing Pictet-Spengler cyclization reaction and chloroacetyl chloride acylation on starting reactants (D-tryptophan methyl ester hydrochloride and piperonal) to obtain an intermediate product, directly performing subsequent reaction on the intermediate product without purification, preparing an intermediate 1-(1,3-benzodioxol-5-yl)-2-(chloracetyl)-2,3,4,9-tetrahydro-1H-pyridino-[3,4,-B]indol-3-thiophenate methyl by using a one-pot reaction method, performing column chromatography purification to obtain a single cis-isomer, and finally reacting the single cis-isomer with methylamine hydrochloride in the presence of an inorganic base to obtain the tadalafil.
Owner:ANHUI WANBANG MEDICAL TECH
Method for synthesizing phosphodiesterase 5 inhibitor tadanafil
InactiveCN101205228AIncreased Stereospecific YieldThe separation method is simpleOrganic chemistryDiketoneBenzene
The invention relates to a synthesis method for (6R, 12aR)-6-(1, 3-benzodioxane heterocyclas-5-base)-2-methyl-2, 3, 6, 7, 12, -12a-hexahydro pyrazinyl [1', 2':1, 6] pyridyl [3, 4-b] indole-1, 4-diketone in the technical field of the pharmaceutical engineering. With the D-methyl tryptophan and piperonal as raw materials, the cis-1, 3-disubstituted carboline intermediates are prepared and generated through the Pictet-Spengler cyclization and then the products called tadalafil are obtained through the acylation ammoniation of the 3-secondary amino group and the final MTX substitution and condensation close-loop. By adopting the acidic condition protic solvents in the invention, the trans-1, 3-disubstituted carboline intermediates generated through the Pictet-Spengler cyclization, which is a key step in the existing process, are converted into those with a cis-configuration, thus greatly enhancing the stereospecific configuration yield of the carboline intermediates so as to enhace the overal yield of the tadalafil with reaching the goal of optimizing and improving the synthesis process.
Owner:SHANGHAI JIAO TONG UNIV
Method for refining tadalafil
InactiveCN106674223AAchieve recyclingSignificant economyOrganic chemistry methodsAcetic acidTadalafil
The invention provides a method for refining tadalafil. The method comprises the following steps: 1) dispersing a tadalafil crude product into a mixed solvent of methanol and acetic acid, heating the material to perform dissolved clarification on the crude product; 2) steaming the material to remove a part of the solvent, gradually precipitating crystals; 3) slowly cooling the material, continuously crystallizing the product; and 3) filtering the material, washing the material, and drying the material to obtain the tadalafil crystal form A. Compared with a direct cooling crystallization method, the product yield is obviously increased, production power is increased, recovery and utilization of the mixed solvent are realized, and the method has obvious economic value and environmental protection value.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
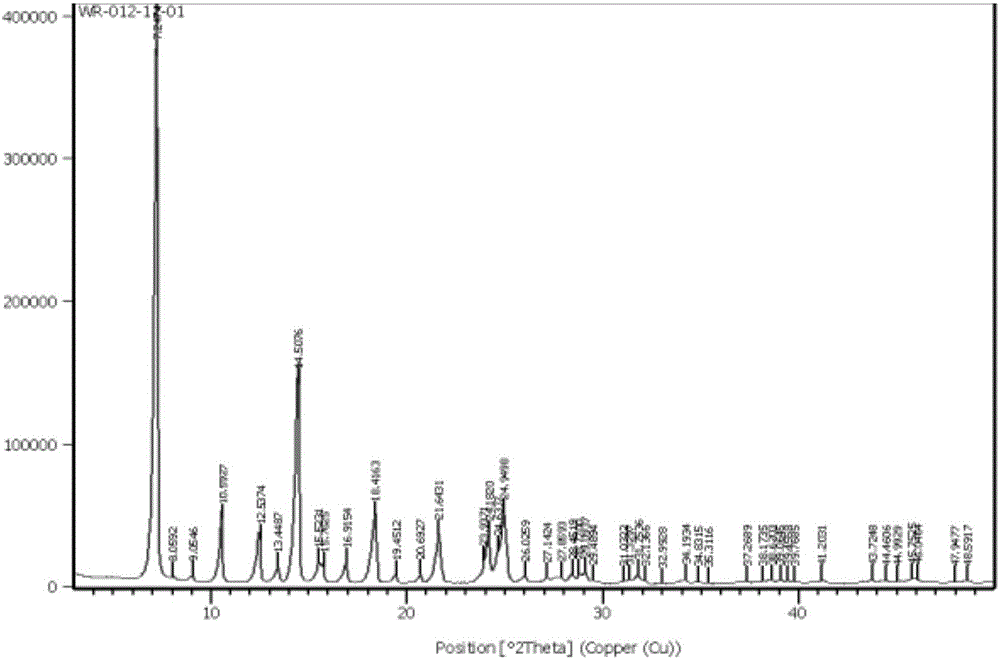
Method for preparing Tadalafil crystal form A
The invention discloses a method for preparing Tadalafil crystal form A. The method is characterized in that the crystallization solvent is a binary mixed solvent of a C1-C4 alcohol and acetic acid or a ternary mixed solvent of a C1-C4 alcohol, acetic acid and water. The method has the characteristics that: a mixed solvent system is used, thus the dosage of the crystallization solvent can be greatly reduced, the drying process can be simplified and the corrosion of acidic materials to the equipment can be reduced.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Use of Sildenafil, Vardenafil and Other 5-Phosphodiesterase Inhibitors to Enhance Permeability of the Abnormal Blood-Brain Barrier
InactiveUS20080188480A1Improve blood-brain barrier permeabilityImprove breathabilityBiocideNervous disorderVardenafilMedicine
This invention relates to compositions, methods and kits for enhancing the permeability of the blood-brain barrier. Particularly, compositions comprising 5-phosphodiesterase inhibitors, such as sildenafil, vardenafil, or tadalafil, when administered to a mammal, will selectively enhance the permeability of the blood-brain barrier in abnormal brain tissue. This selective enhancement allows for selective delivery of therapeutic agents to treat the abnormal brain tissue; for example, a brain tumor.
Owner:CEDARS SINAI MEDICAL CENT
Tadalafil having a large particle size and a process for preparation thereof
Owner:TEVA PHARM USA INC
Use of phosphodiesterase inhibitors for treating multidrug resistance
The present invention relates to methods of treating multidrug resistance in cancerous cells with phosphodiesterase (PDE) inhibitors, e.g., PDE5 inhibitors. More specifically, the invention relates to methods of treating multidrug resistance that arises, e.g., during administration of chemotherapeutic / antineoplastic (anticancer) agents for treatment of cancer, with a PDE5 inhibitor (e.g., sildenafil, vardenafil, and tadalafil). The invention also relates to methods of treating cancer, e.g., multidrug resistant cancer, using a PDE5 inhibitor in combination with an antineoplastic therapeutic agent. Further, the invention relates to pharmaceutical compositions for treating multidrug resistant cancers comprising a PDE5 inhibitor, or a combination of a PDE5 inhibitor and an antineoplastic agent.
Owner:ST JOHNS UNIV
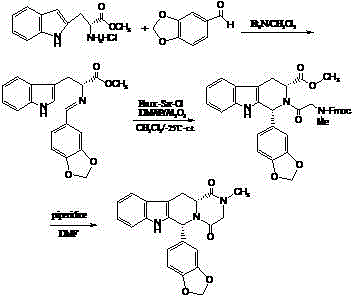
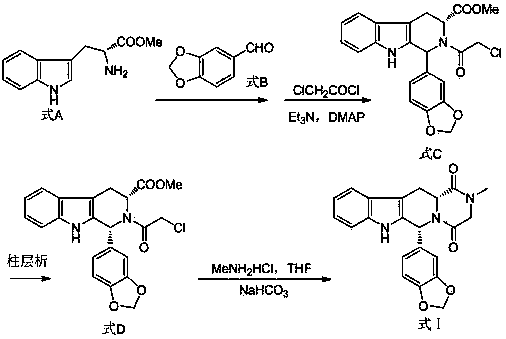
Process for preparing Tadalafil and its intermediate
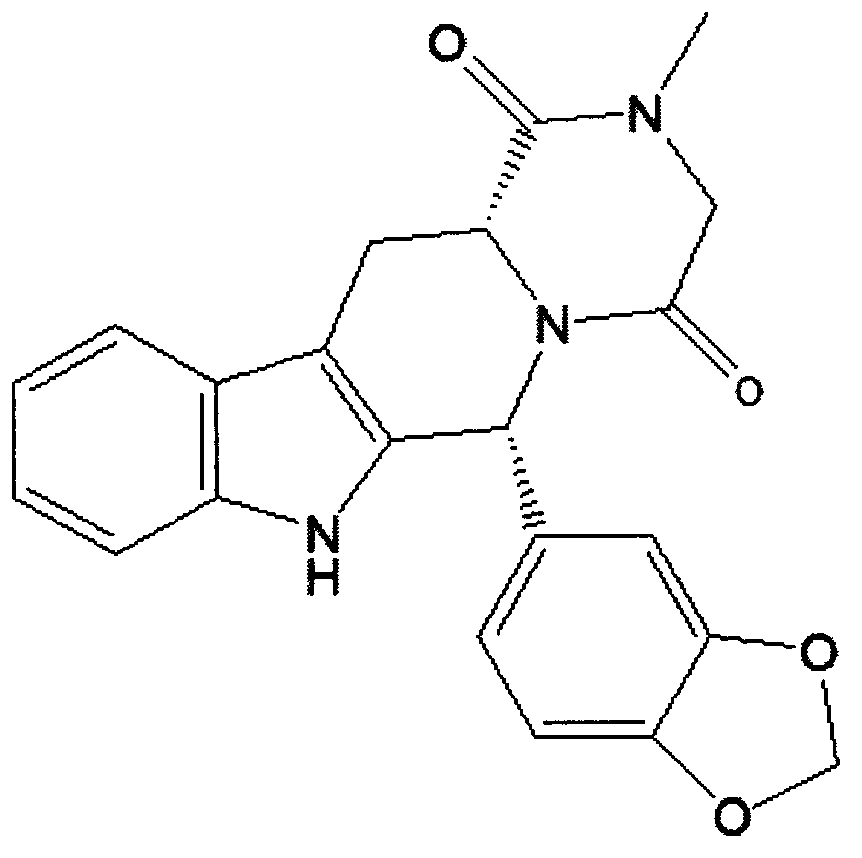
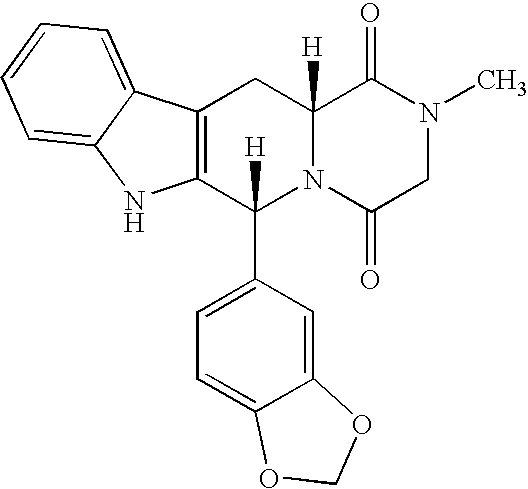
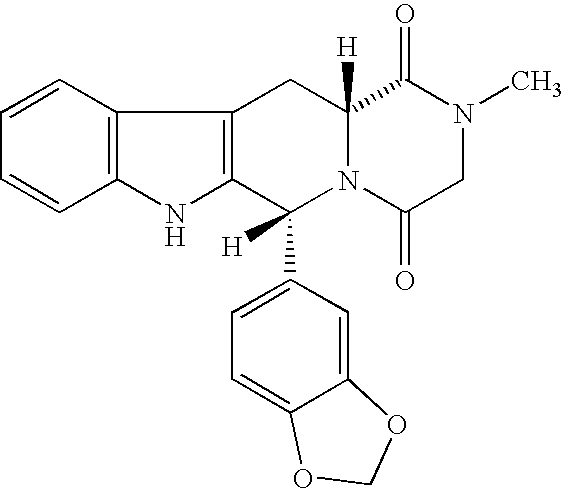
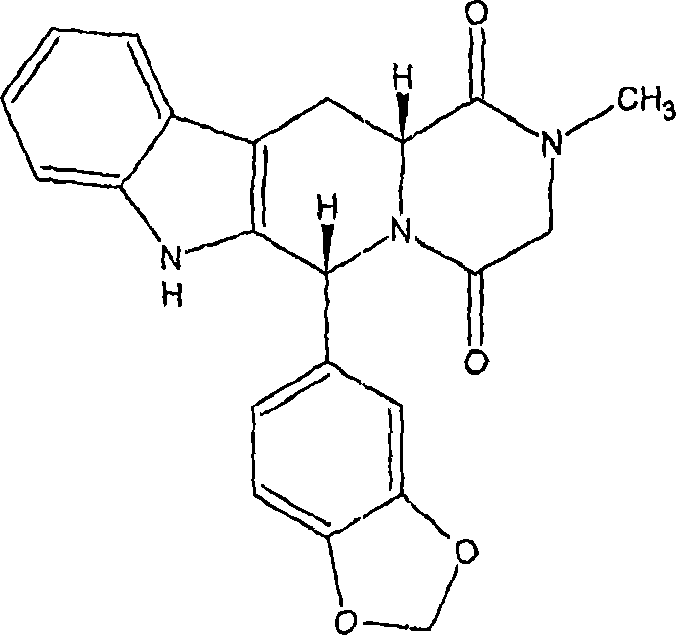
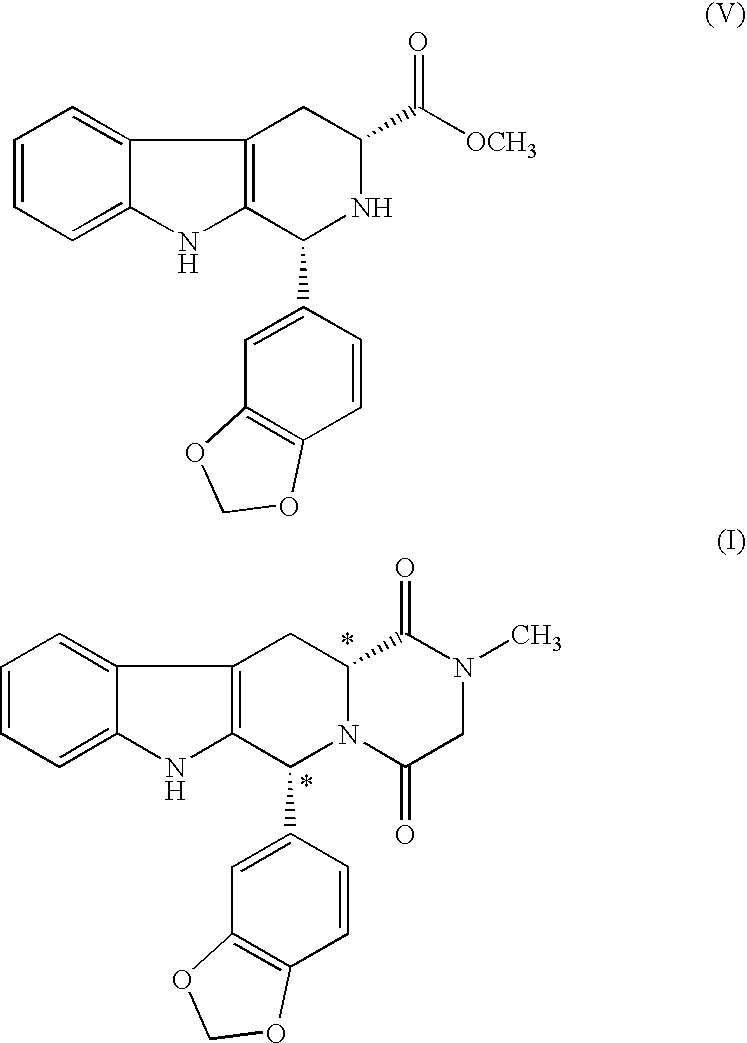
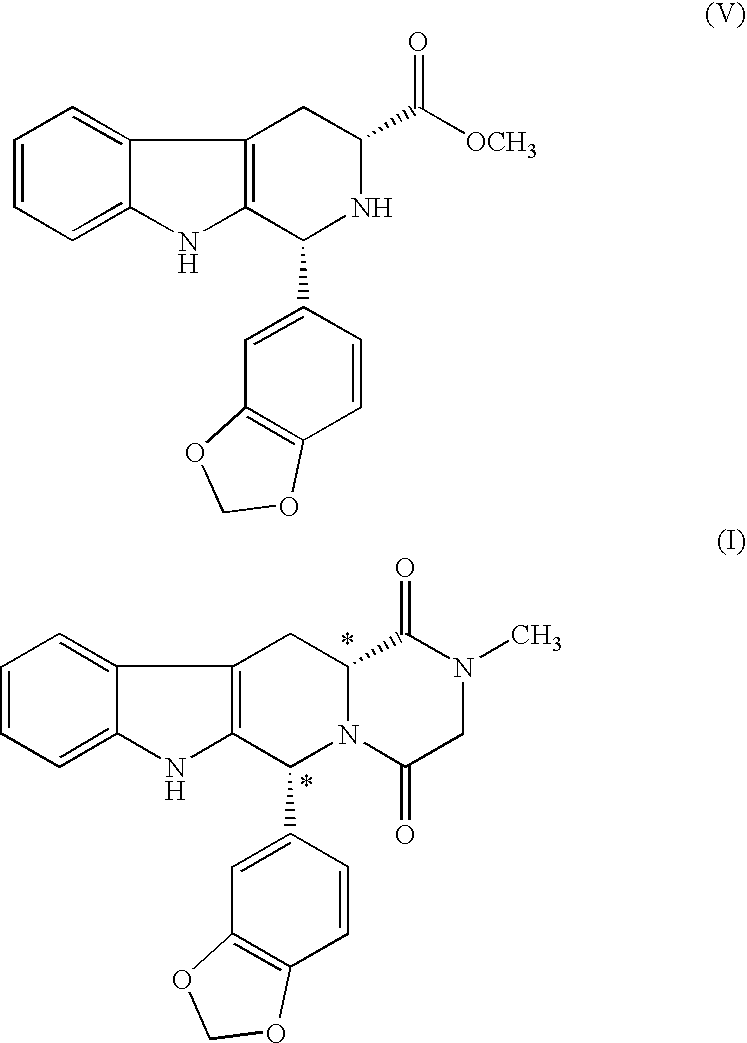
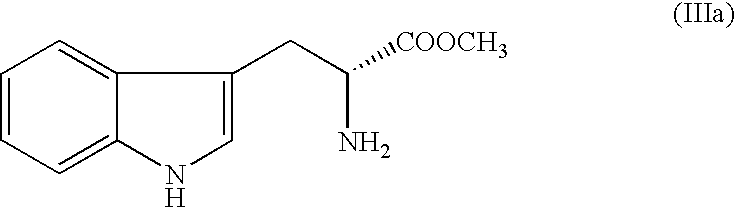
The present invention relates an improved process for the preparation of tetrahydro-beta-carboline derivative of formula (V) which is useful as an intermediate for the preparation of Tadalafil of formula (I). Moreover, the present invention relates to the process for the preparation of Tadalafil of formula (I)
Owner:ALEMBIC LTD
Immunoassay method for nano-colloidal gold marker of tadalafil and analogs thereof
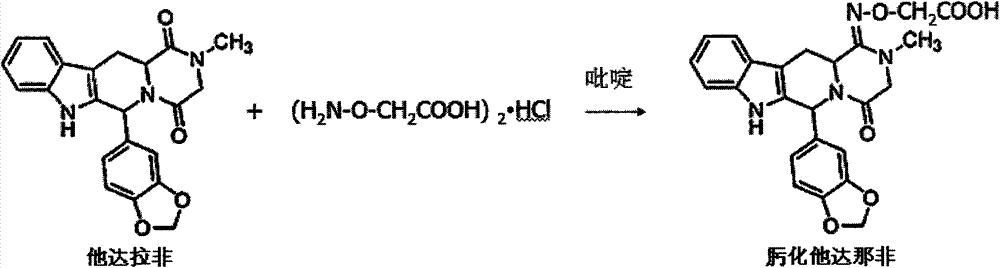
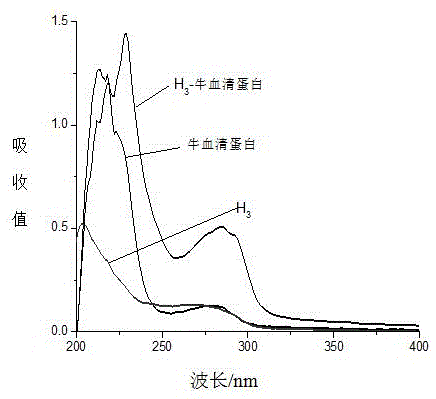
The invention discloses an immunoassay method for a nano-colloidal gold marker of tadalafil and analogs thereof. The determination method comprises the following steps: coupling oximate tadalafil with bovine serum albumin by adopting an activated ester method, synthesizing artificial immunity antigen which is used for immunizing animals, and preparing specific tadalafil and analogs thereof; in addition, coupling the oximate tadalafil with egg albumin to be used for construct an immunity detection method; separating, purifying and labeling the tadalafil and analogs thereof to the nano-colloidal gold, and then respectively curing the tadalafil and analogs detection antibodies thereof and goat-anti-rabbit IgG on a carrier, and adding a detection antigen into a detection solution. According to the method, the lowest detection limit of the tadalafil and analogs thereof legally added into health products and Chinese patent medicines is 10mu g / kg, and the fast detection can be completed within 5 minutes. The method is stable, fast and accurate, and suitable for one-step fast detection on legally added medicines.
Owner:SHAOGUAN COLLEGE
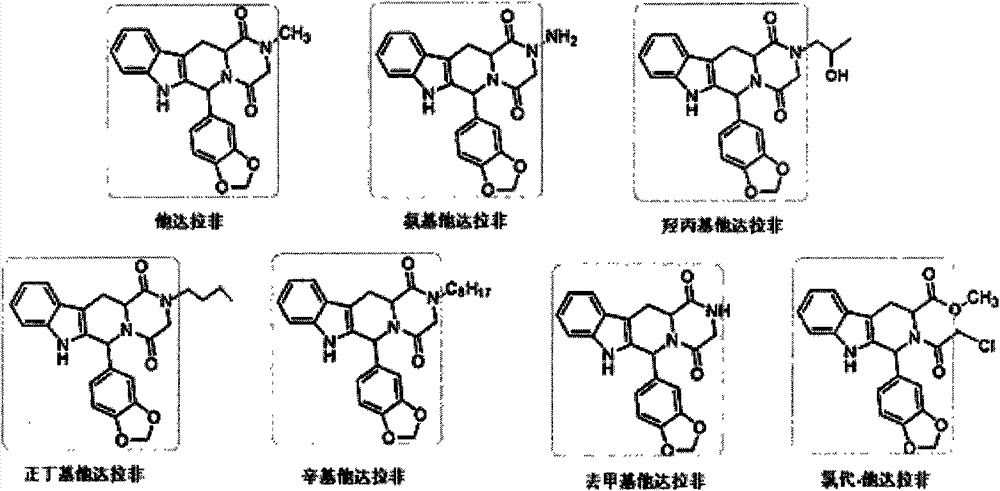
Artificial antigen of tadalafil and analogues thereof, antibody and ELISA kit thereof
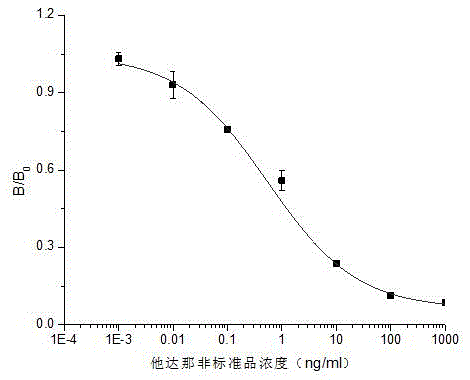
The invention discloses an artificial antigen of tadalafil and analogues thereof, an antibody and an ELISA kit thereof. The kit comprises an enzyme label plate coated with a tadalafil antigen, a tadalafil antibody operating fluid, a horseradish peroxidase labelled secondary antibody, a tadalafil standard-substance operating fluid, a substrate liquid, a substrate buffer, a reaction stopping liquid and a condensation washing liquid. The invention also discloses a method for detecting tadalafil and analogues thereof in health-care food by utilizing the kit. The provided kit for detecting tadalafil and analogues thereof employs an indirect competitive enzyme-linked immunosorbent analysis technology, possesses the maximum detection scope of 0.12-7.45 ng / mL, the half-inhibitory concentration of 0.933 ng / ml, the detection limit of 0.095 ng / mL and the recovery rate of 83.0-119.2%, is low in detection limit, high in sensitivity, good in stability and low in cost, is extremely suitable for primary screening on a large amount of samples, and possesses practical significance on rapid detection on illegally-added tadalafil in health-care food.
Owner:SOUTH CHINA AGRI UNIV
A method for preparing tadala amorphous form a
The invention discloses a method for preparing Tadalafil crystal form A. The method is characterized in that the crystallization solvent is a binary mixed solvent of a C1-C4 alcohol and acetic acid or a ternary mixed solvent of a C1-C4 alcohol, acetic acid and water. The method has the characteristics that: a mixed solvent system is used, thus the dosage of the crystallization solvent can be greatly reduced, the drying process can be simplified and the corrosion of acidic materials to the equipment can be reduced.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com