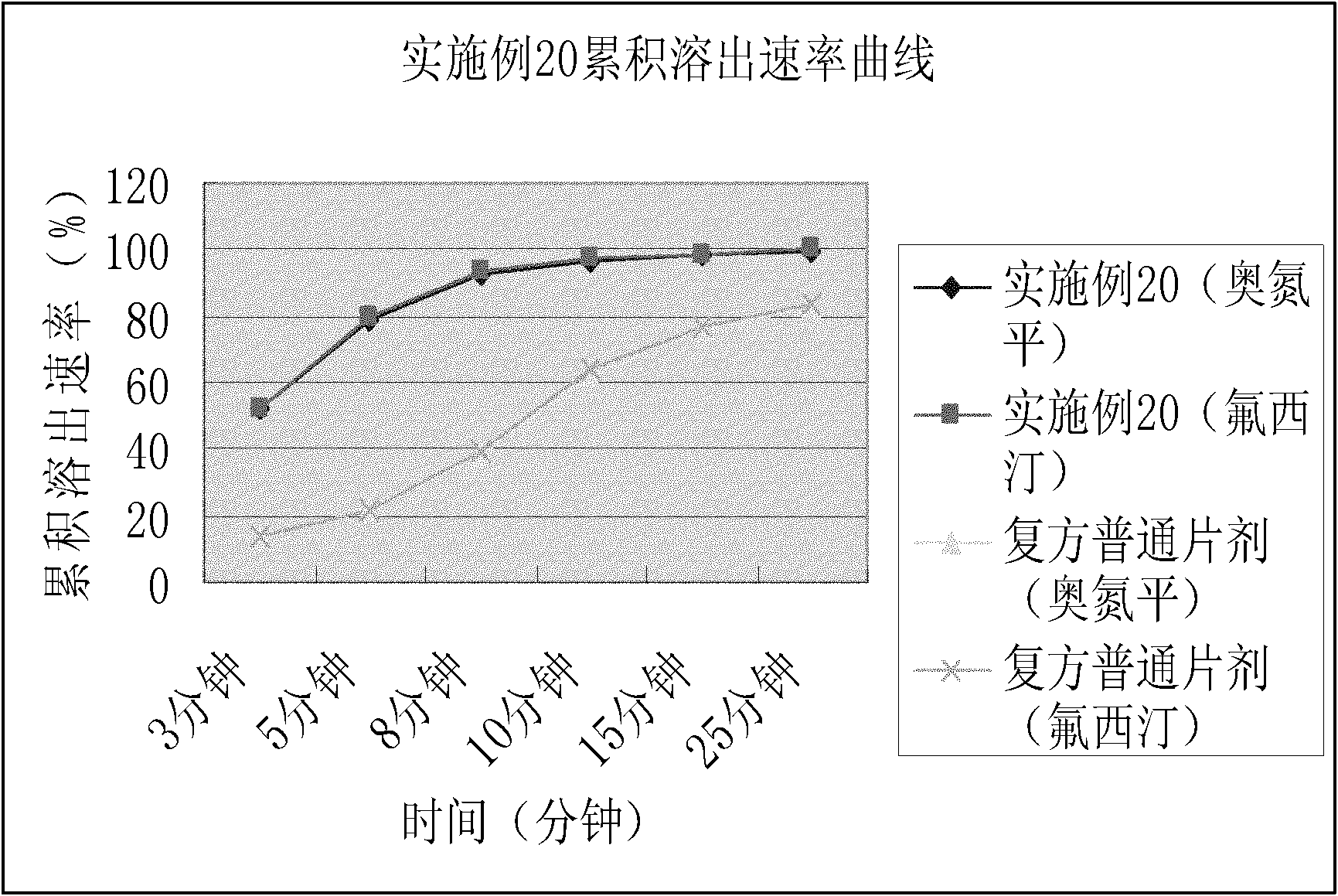
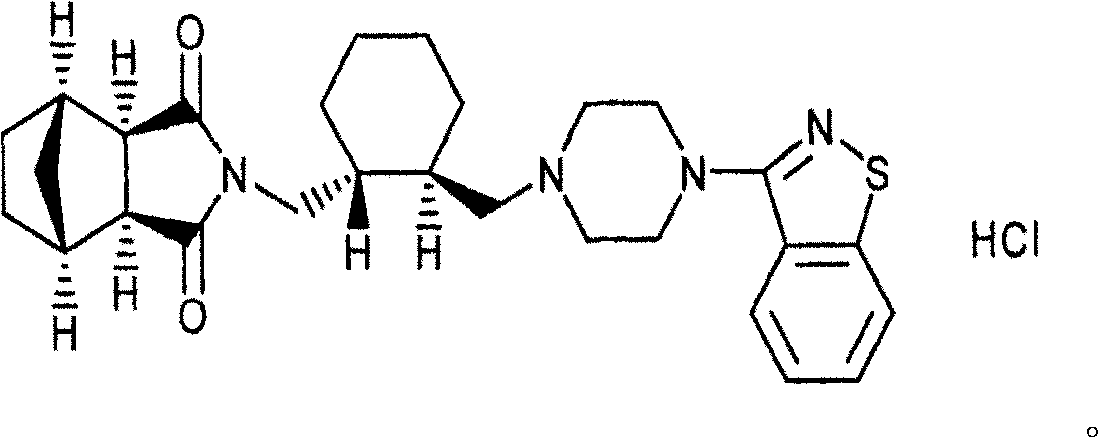
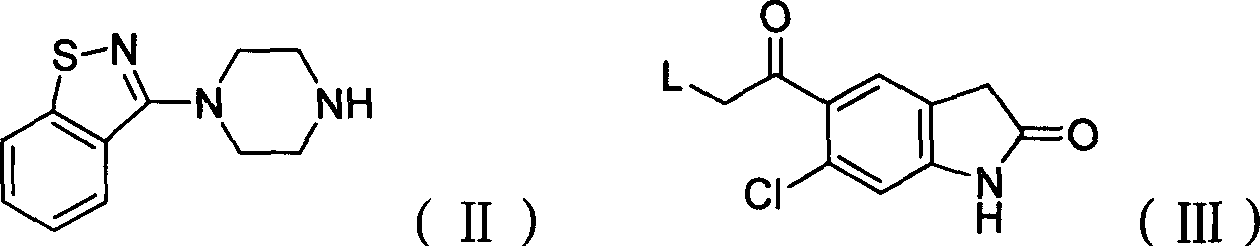Patents
Literature
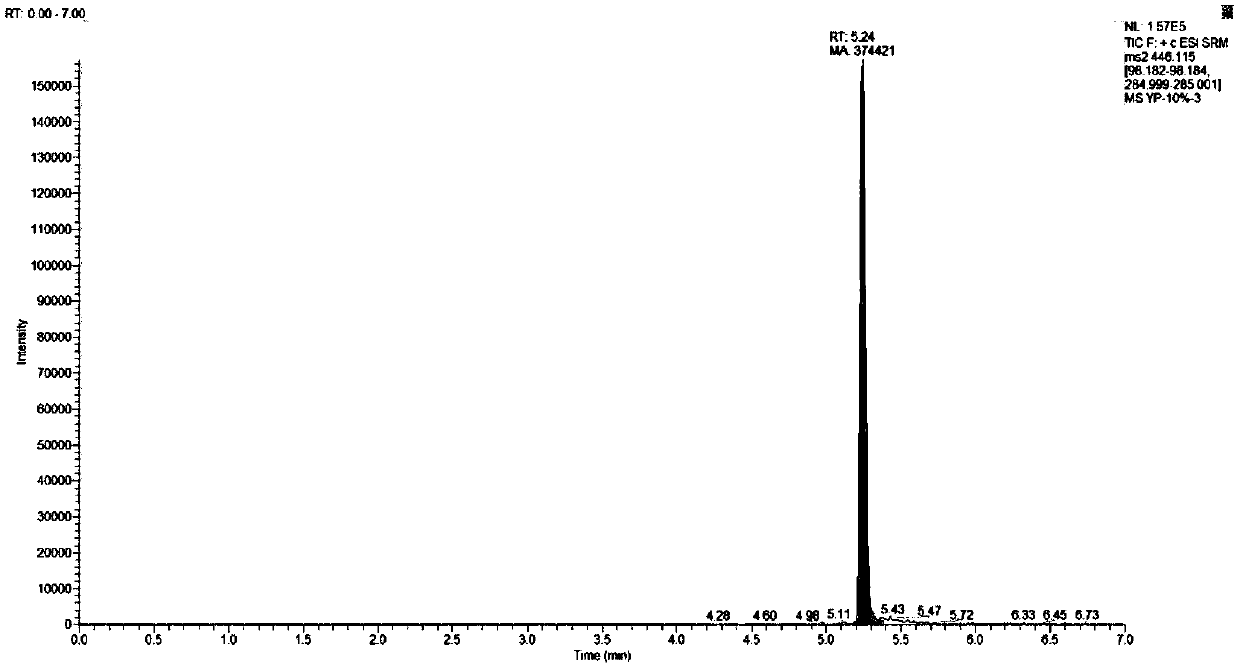
64 results about "Ziprasidone" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Ziprasidone injection is used to treat occurrences of severe agitation in patients with schizophrenia.
Methods for treating idiopathic hyperhidrosis and associated conditions
InactiveUS20040192754A1Reduced activityAlleviate and treat symptomBiocideAnimal repellantsAllosteric modulatorMianserin Hydrochloride
The subject invention provides methods for treating symptoms and / or conditions associated with idiopathic hyperhidrosis by using compounds that decrease the activity of serotonin 5-HT2C receptors. Compounds that can ameliorate symptoms of idiopathic hyperhidrosis and associated conditions include 5-HT2C receptor antagonists (i.e., ketanserin, ritanserin, mianserin, mesulergine, cyproheptadine, fluoxetine, mirtazapine, olanzapine, and ziprasidone) as well as 5-HT2C receptor modulators (i.e., inverse agonists, partial agonists, and allosteric modulators).
Owner:SHAPIRA NATHAN ANDREW +2
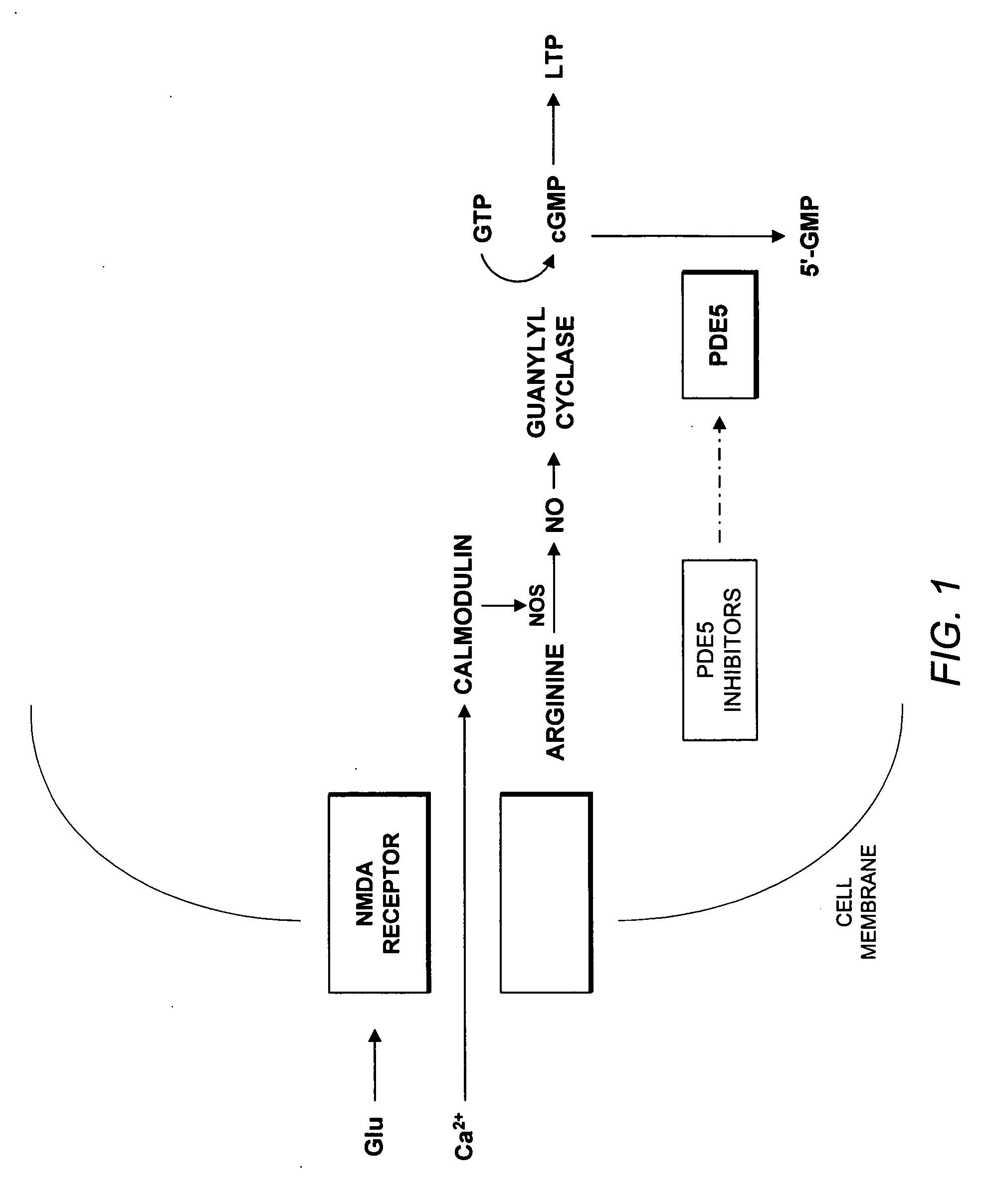
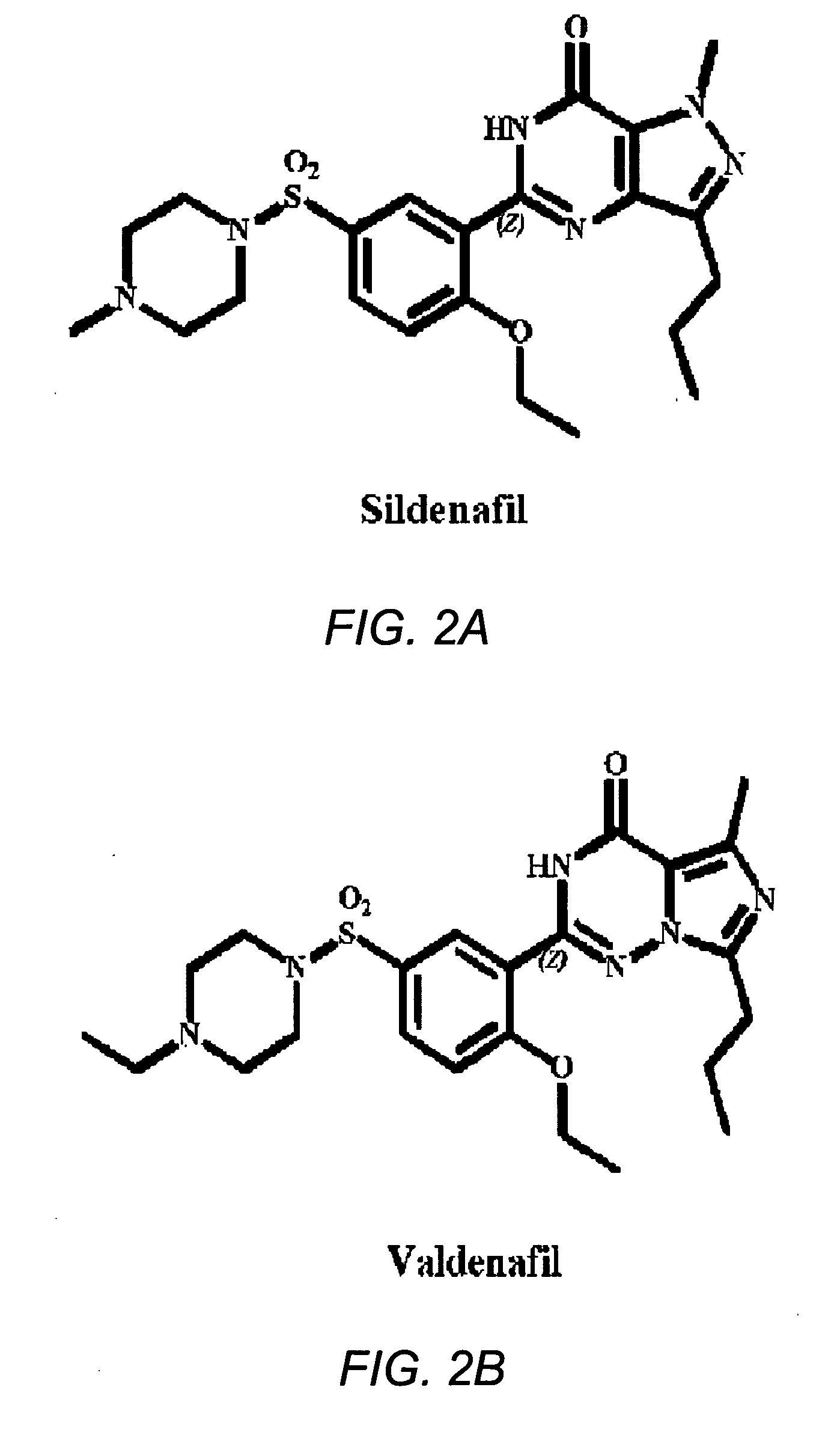
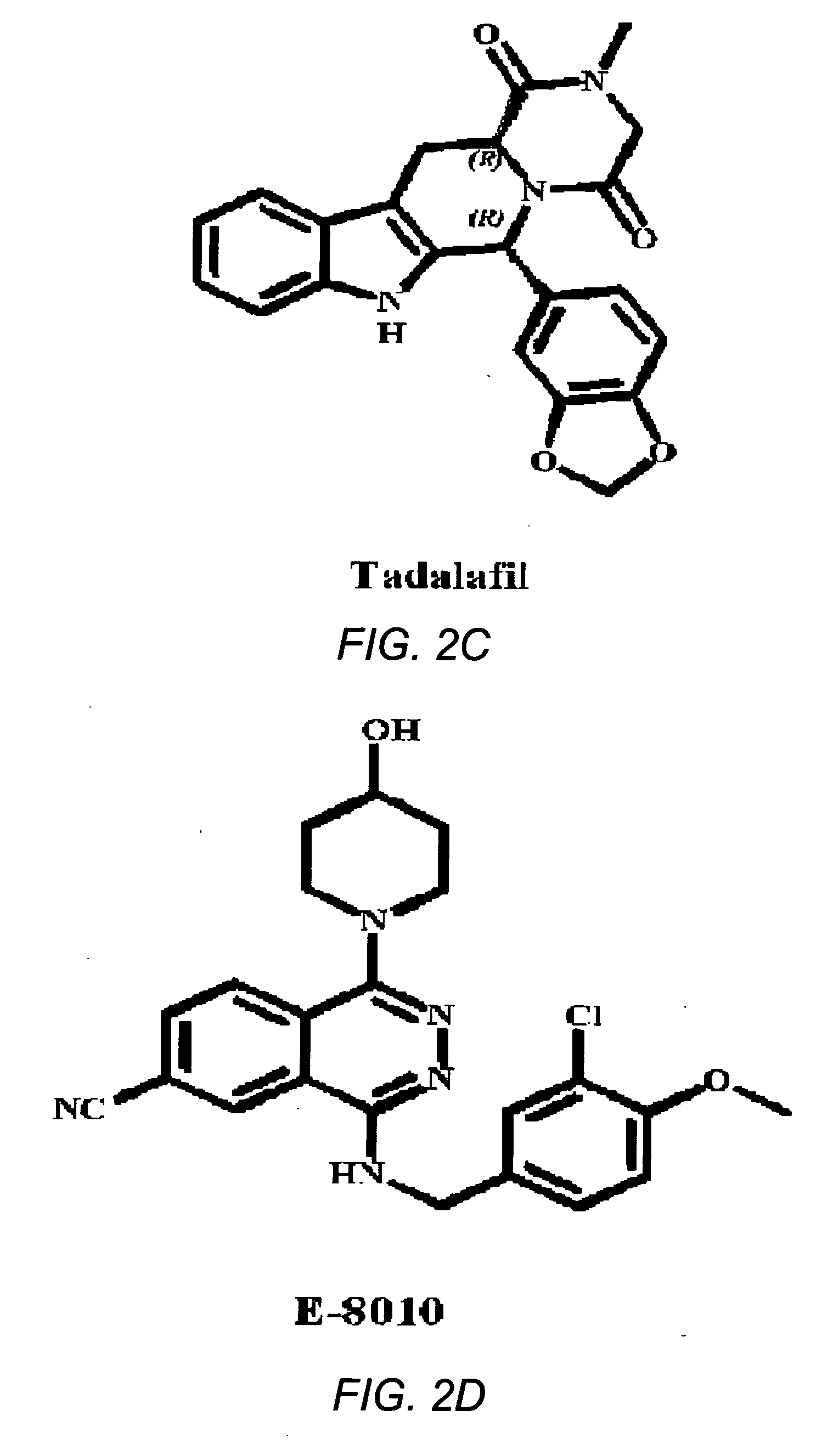
Use of phosphodiesterase 5 (PDE5) inhibitors in the treatment of schizophrenia
The use of phosphodiesterase 5 (PDE5) inhibitors for treatment of schizophrenia is described. Suitable PDE5 inhibitors for use for treatment of schizophrenia include sildenafil, vardenafil, tadalafil, E-8010, zaprinast, and E-4021. In one embodiment, for example, a method is described for treating schizophrenia in a patient which comprises treating the patient with an effective amount of a PDE5 inhibitor, or a pharmaceutically acceptable salt, solvate, or composition thereof. The PDE5 inhibitor may be administered orally. The PDE5 inhibitor may also be administered together with one or more conventional antipsychotic medications such as risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine, haloperidol, and fluphenazine.
Owner:SHARY CIRCLE
Treatment of psychosis associated with parkinson's disease and subcortical dementias using a combination of an atypical antipsychotic with a dopamine agonist
InactiveUS20070015763A1Opportunities decreaseSimple processBiocidePeptide/protein ingredientsAtypical antipsychoticZiprasidone
This invention relates to combinations of an atypical antipsychotic, for example ziprasidone, and a dopamine agonist, kits containing such combinations, pharmaceutical compositions comprising such combinations, and methods of using such combinations to treat patients suffering from psychosis and movement disorders associated with Parkinson's disease and subcortical dementias.
Owner:PFIZER INC +1
Dispersible tablet containing antipsychotic medicines and application thereof
The invention relates to a novel dispersible tablet which is prepared from a certain amount of antipsychotic medicines or pharmaceutically acceptable salt or ester thereof or mixture thereof, a certain amount of selective serotonin reuptake inhibitors (SSRIs) and at least one of pharmaceutically acceptable carriers, wherein the antipsychotic medicines are aripiprazole, fluvoxamine, escitalopram, olanzapine, mirtazapine, clozapine, ziprasidone, mianserin, agomelatine, lurasidone, iloperidone, blonanserin, moclobemide, timiperone, palipeddone, trimipramine, carpipramine, lofepramine or mosapramine. The novel dispersible tablet is used for preventing, delaying or treating depression or schizophrenia of patients. Compared with common tablets or capsules, the novel dispersible tablet has the characteristics of quick and uniform dispersion, short disintegration time, quick medicine absorption, high bioavailability and good stability, and is convenient to take.
Owner:王定豪
Kit for detecting anti-psychosis drugs in serum and plasma by liquid chromatography tandem mass spectrometry method and application thereof
InactiveCN109085263AReduce matrix effectThe test result is accurateComponent separationPsychosis drug9-Hydroxyrisperidone
The invention provides a kit for detecting anti-psychosis drugs in a serum and plasma by a liquid chromatography tandem mass spectrometry method. The kit comprises drug standards: amsulpiride, aripiprazole, dehydroaripiprazole, chlorpromazine, clozapine, n-desmethylclozapine, risperidone, 9-hydroxyrisperidone, quetiapine, olanzapine, ziprasidone; drug internal standard compounds: amplepiride-d5, aripiprazole-d8, chlorpromazine-d3, clozapine-d8, risperidone-d4, 9-hydroxyrisperidone-d4, quetiapine-d8, olanzapine-d8, ziprasidone-d8; drug extraction compounds: methanol solution 60% (volume ratio),acetonitrile solution 20%, isopropanol solution 10%, purified water 10%; negative plasma; and a diluent: 50% aqueous methanol solution. The kit can be used to simultaneously detect anti-psychosis drugs and active metabolites thereof, the detection time is short and the flux is large.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
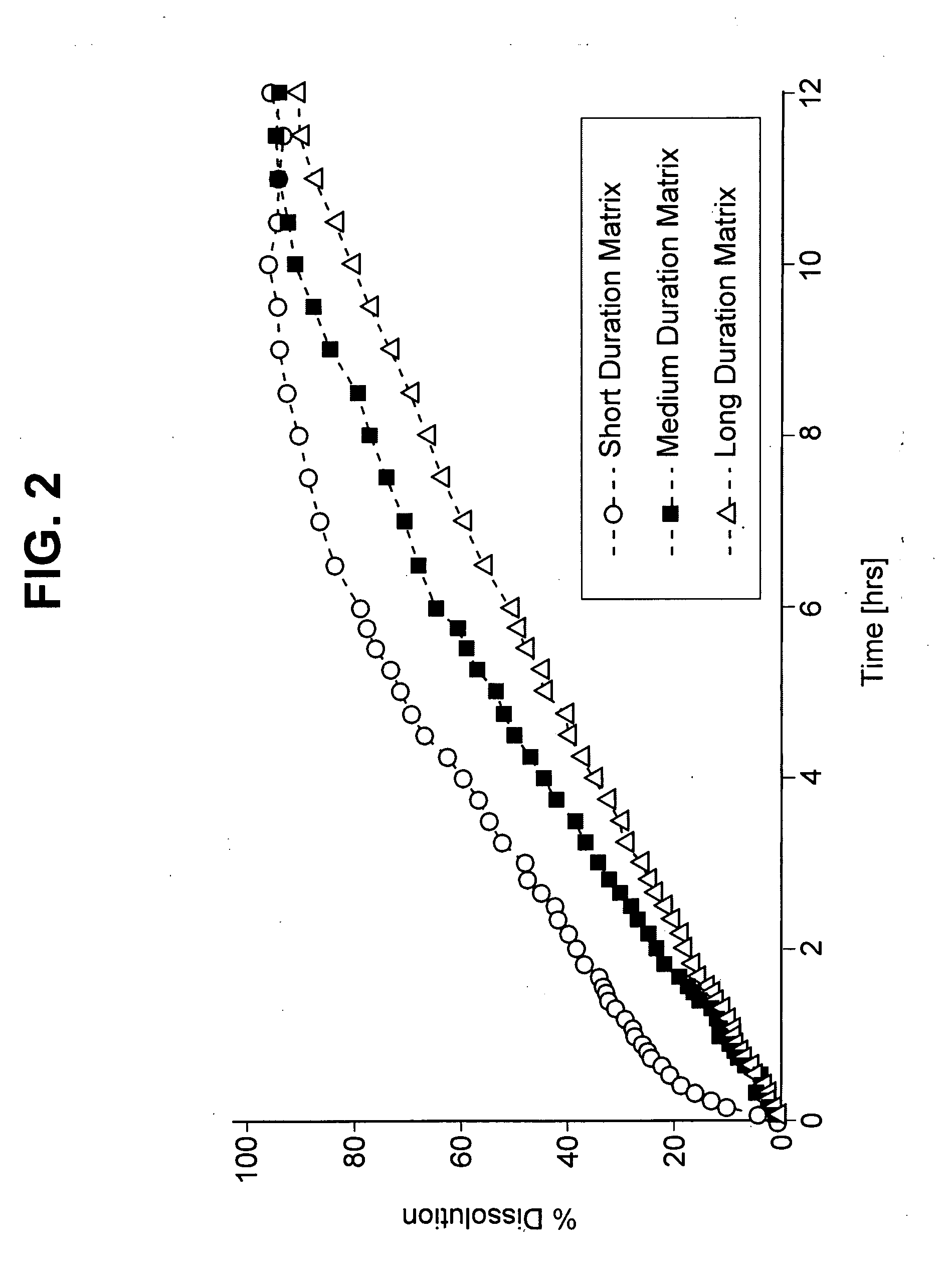
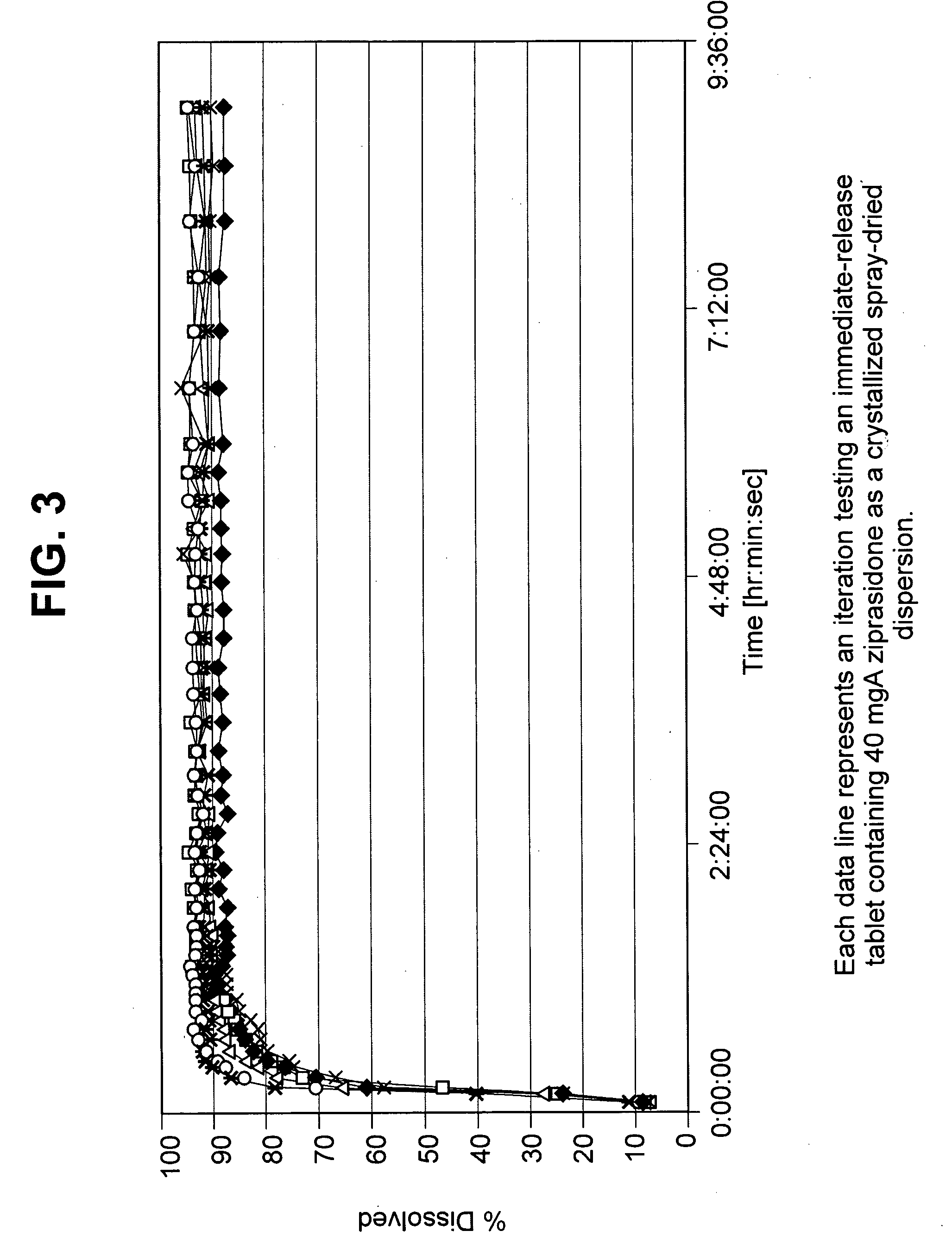
Ziprasidone formulations
Ziprasidone formulations, including controlled-release formulations, formulations containing ziprasidone dihydrochloride, and combinations of ziprasidone and an additional active agent are described.
Owner:ACTAVIS GRP PTC EHF
Method for synchronously detecting seventeen antipsychotics in blood sample
InactiveCN109668979ARealize detectionAchieving Simultaneous DetectionComponent separation9-HydroxyrisperidoneSolvent
The invention discloses a method for synchronously detecting seventeen antipsychotics in a blood sample. The method comprises the steps that the sample is extracted with a mixed solution of methanol and acetonitrile to obtain supernate, and after a solvent is removed from the supernate, the supernate is dissolved in a methanol aqueous solution and filtered to obtain a specimen; a high performanceliquid chromatography tandem mass spectrometry method is adopted to detect the specimen; the sample is serum or plasma, and the antipsychotics are amisulpride, aripiprazole, dehydro aripiprazole, chlorpromazine, clozapine, desmethylclozapine, fluphenazine, haloperidol, olanzapine, paliperidone, perphenazine, quetiapine, risperidone, 9-hydroxyrisperidone, sulpiride, ziprasidone and thioridazine; inthe high performance liquid chromatography, a mobile phase A is an aqueous solution of formic acid, and a mobile phase B is a methanol solution of the formic acid; and the mass spectrometry adopts amulti-ion reaction monitoring mode of positive ion electrospray ionization.
Owner:JINAN YING SHENG BIOTECH
Use of metformin to counteract weight gain associated with psychotropic medications
InactiveUS20060246131A1Minimized weight gainBiocideMetabolism disorderSide effectPsychotropic medication
A method for minimizing the weight gain side effect associated with ABILIFY® (aripiprazole) or GEODON® (ziprasidone) treatment is disclosed. In this method, metformin, a biguanide compound, is concurrently administered to a patient taking the ABILIFY® (aripiprazole) or GEODON® (ziprasidone) therapy. A pharmaceutical composition containing the combination of ABILIFY® (aripiprazole) or GEODON® (ziprasidone), together with metformin is also disclosed.
Owner:COTTLINGHAM ELIZABETH M
Ziprasidone formulations
InactiveUS20080286373A1Good water solubilityHigh dissolution rateOrganic active ingredientsPowder deliverySide effectPolyethylene glycol
A ziprasidone formulation containing at least (a) one ziprasidone compound and at least an excipient component (b) that includes at least one of(i) one or more of a mono-, di-, or tri-ester of C12-24fatty acids and glycerol, in which each fatty acid group is chosen independently of the others, or mixtures thereof; and / or (ii) one or more mono- or di-esters of C12-24fatty acids and polyC2-3alkyleglycol, in which each fatty acid group is chosen independently of the others, or mixtures thereof; and / or (iii) a TPGS (tocopherol-succinic acid-polyethyleneglycol); and where this component (b) may optionally include (iv) optionally free polyC2-3alkyleglycol; (v) optionally free glycerol; and (vi) optionally free fatty acids having 12-24 carbon atoms; and (vii) mixtures thereof;the formulation further comprising (c) at least one surfactant selected from anionic and non-ionionic surfactants and still further comprising (d) at least one hydroxylalkyl alkylcellulose in which each alkyl group and each hydroxyalkyl group independently has from 1 to 4 carbon atoms. The formulation achieves improved dissolution and bioavailability of the formulation. Reduction in side effect profile and increased efficacy and utility in additional indications are also disclosed.
Owner:SCIDOSE
Method for detecting liquid quality of antipsychotic drug in serum or plasma
The invention relates to a method for detecting the liquid quality of an antipsychotic drug in serum or plasma and pretreatment of a specific purification material of the antipsychotic drug. The method comprises the following steps: a serum or plasma sample is subjected to protein precipitation by a certain proportion of organic reagents; a supernatant obtained through centrifugal precipitation ispretreated with a specific purification material to obtain a purified sample solution, the purified sample solution is subjected to liquid chromatography-tandem mass spectrometry detection and analysis, and eight antipsychotic drugs including olanzapine, clozapine, quetiapine, aripiprazole, flupiperidinol, risperidone, pariperidone and ziprasidone in serum or plasma are detected at the same time.The pretreatment of the specific purification material is a mixed solid-phase extraction column taking silica gel as a matrix, and the blood is purified by the specific purification material, so thatthe interference of the matrix in the blood can be removed, the matrix effect of eight antipsychotic drugs can be improved, and the recovery rate of the antipsychotic drugs can be better ensured.
Owner:大连润生康泰医学检验实验室有限公司
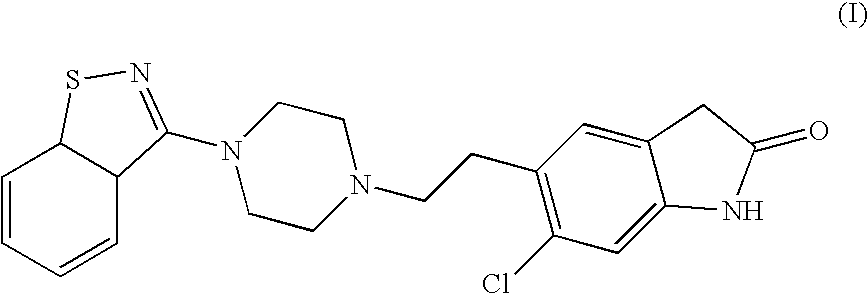
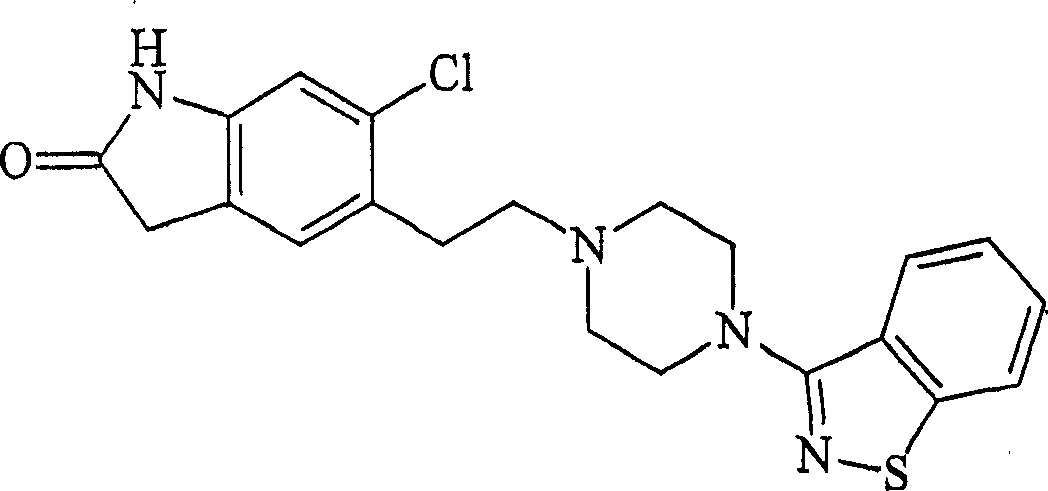
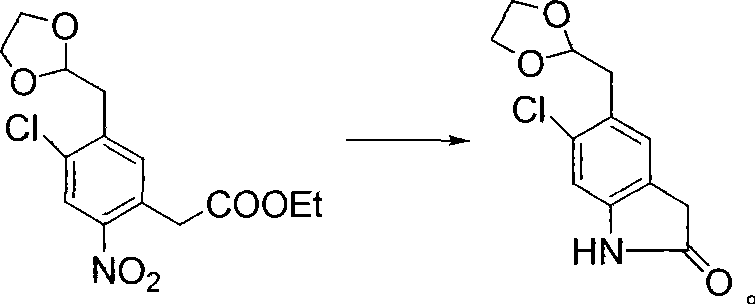
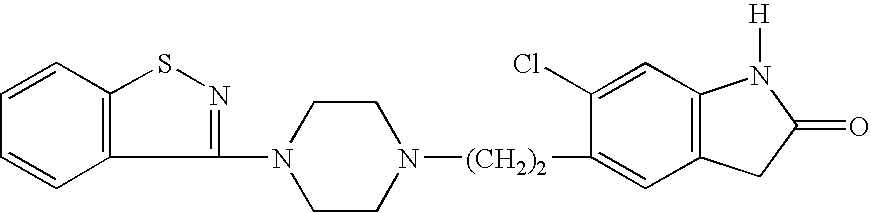
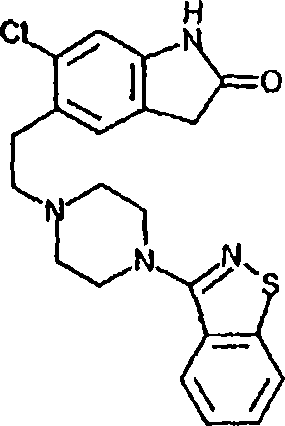
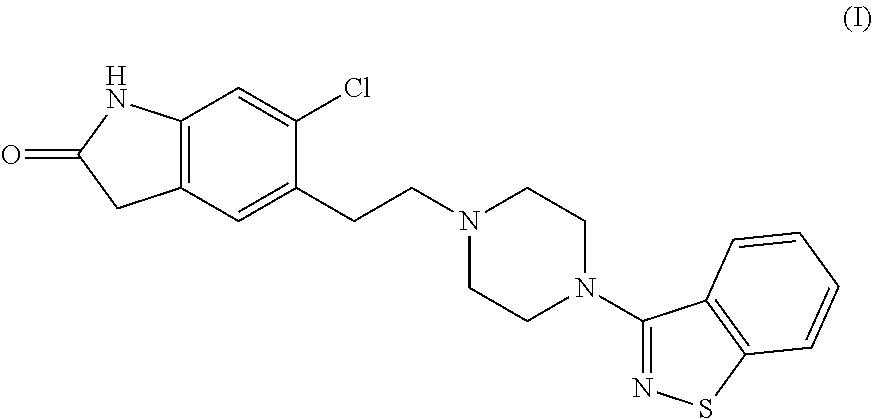
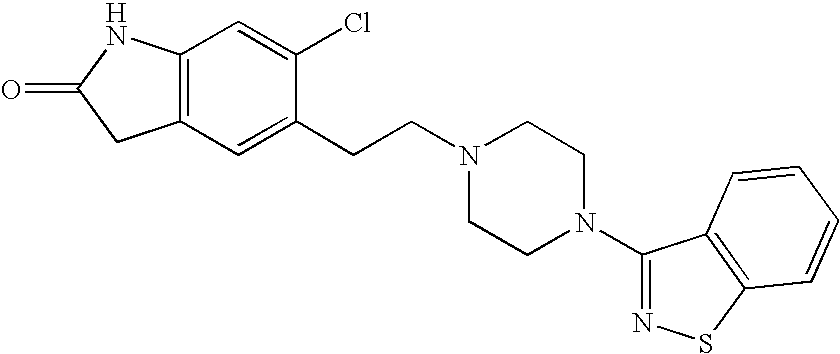
Preparation method of ziprasidone
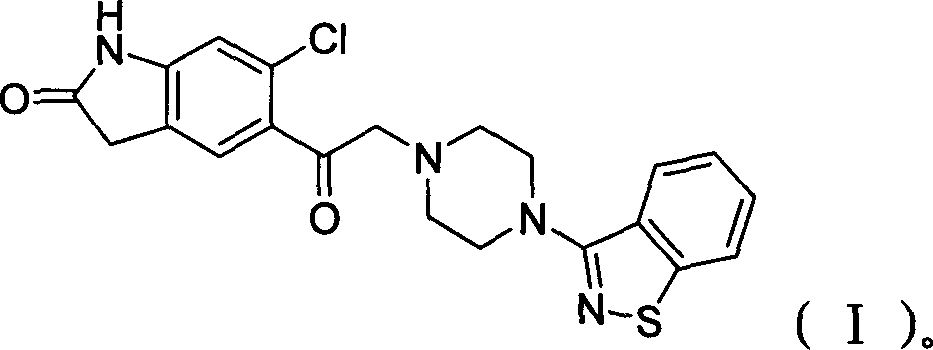
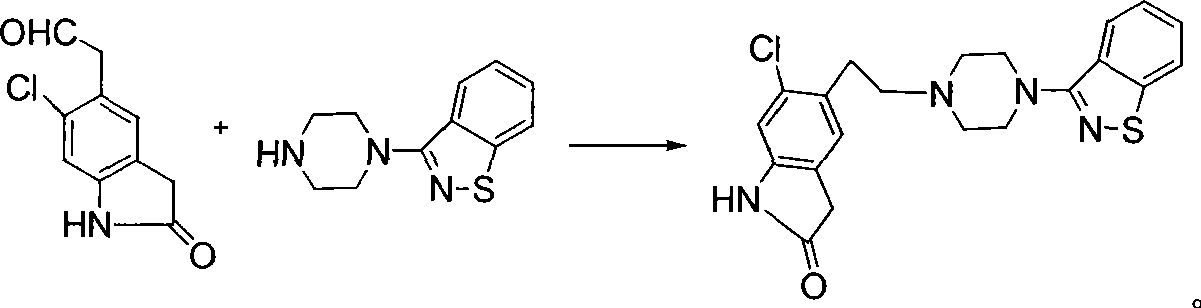
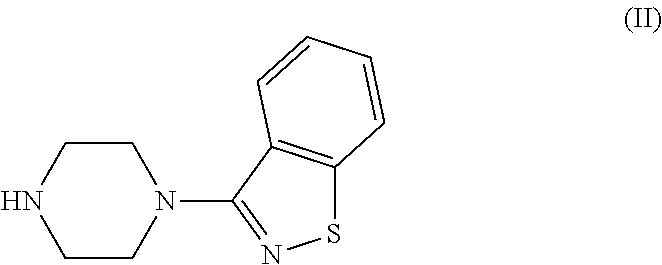
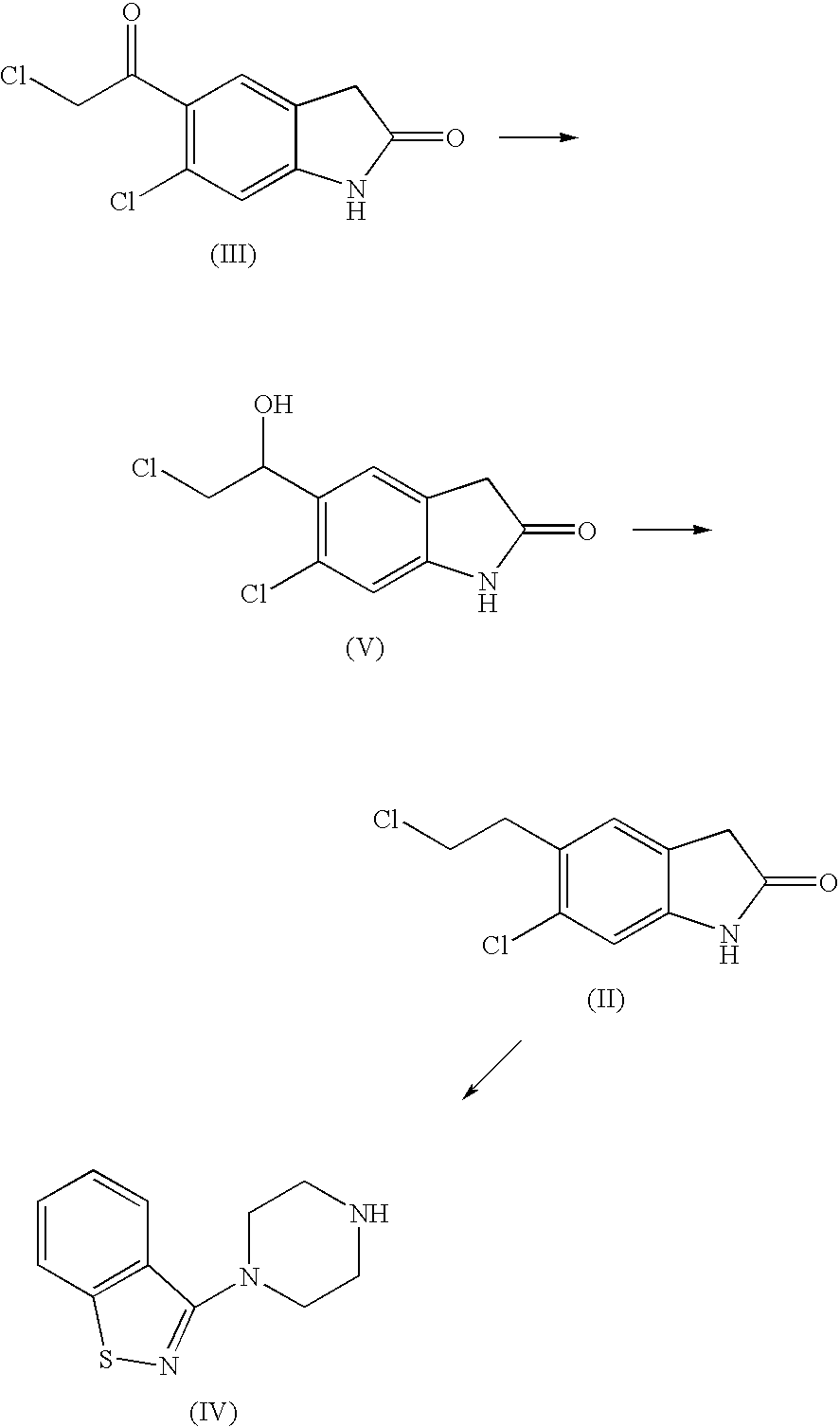
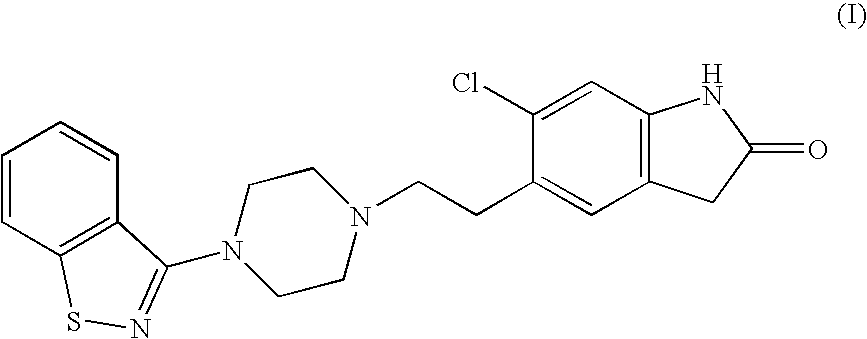
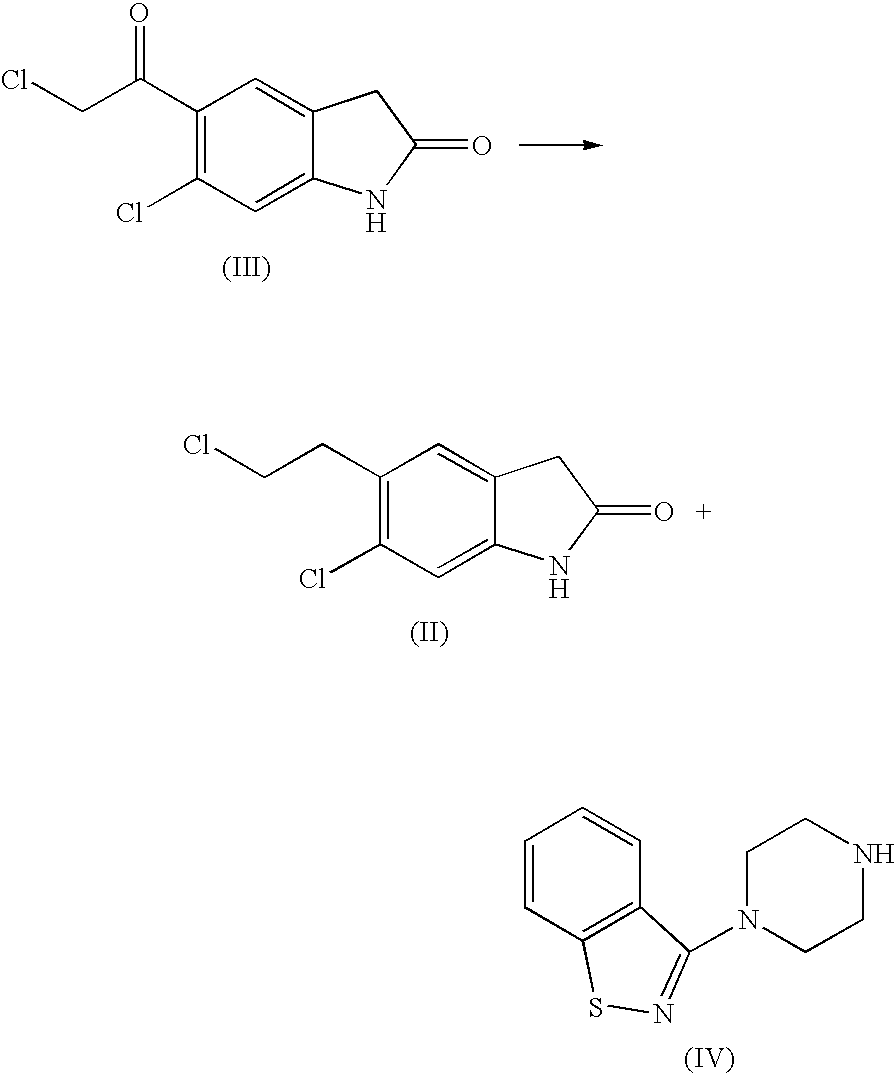
The invention discloses a making method of qilaxi ketone with structural formula as formula (I), which is characterized by the following: the chemical name of compound is 5-(2-(4-(1,2-benzo isothiazole-3-base)-piperazine) acetyl)-6-chloride-1, 3-dihydrogen-2H-indole-2-ketone, which is reduced in the organic acid solvent; the solvent is C1-C6 paraffin acid substituted by at least one halogenate atom with carboxyl function group.
Owner:ZHEJIANG MENOVO PHARMA
Methods, dosage forms and kits for administering ziprasidone without food
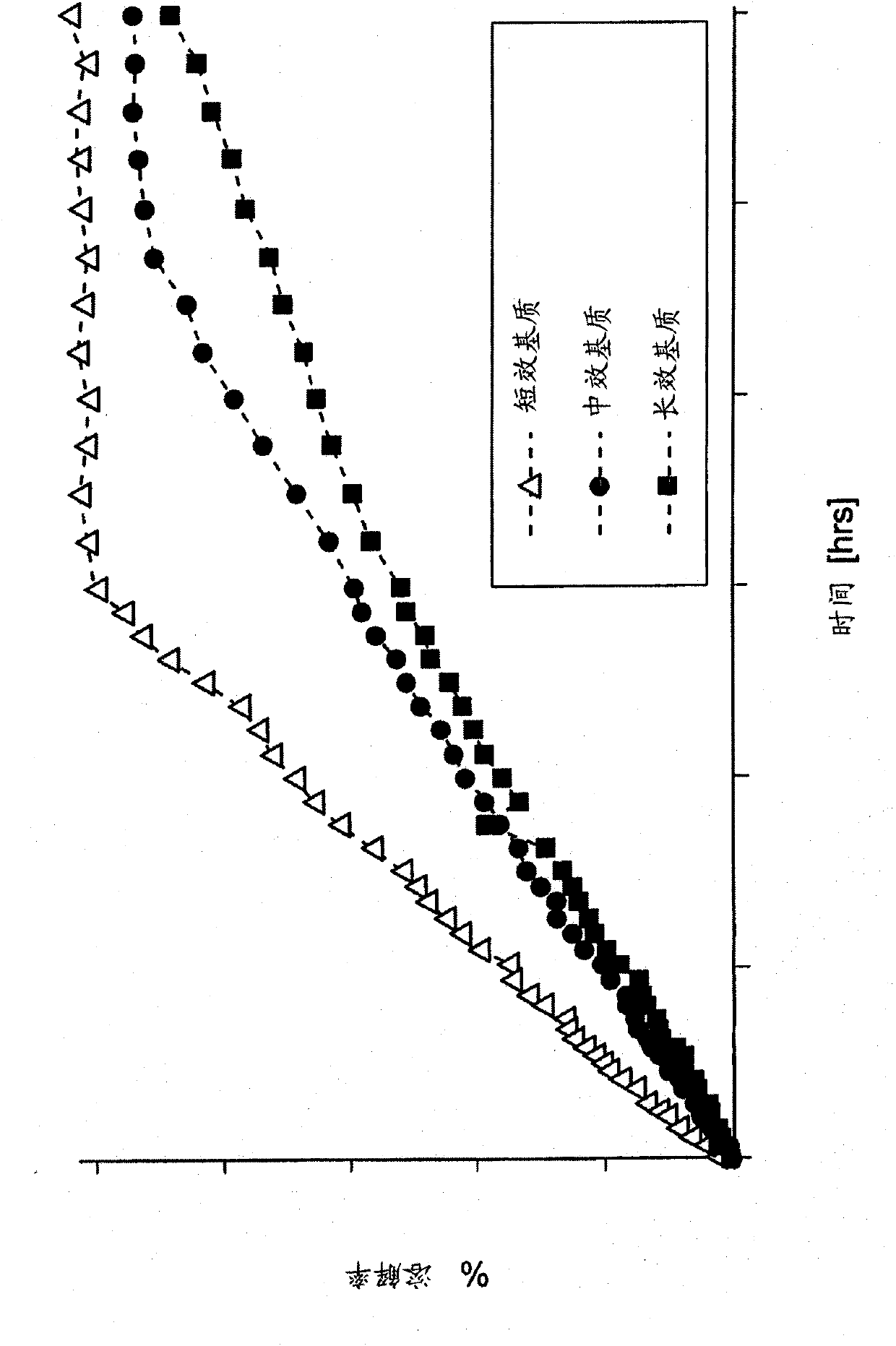
The present invention provides methods, dosage forms and kits for treating with an effective amount of ziprasidone a CNS disorder in a human when the human is in a fasted state. In one embodiment, the invention relates to a method for treating a CNS disorder in a human, which method comprises administering to the human in a fasted state, a solid oral dosage form comprising an amount of ziprasidone effective to treat said CNS disorder, wherein the area under the serum concentration versus time curve (AUC0-inf) of the ziprasidone in the human subsequent to said administering is from 70% to 140% of the mean area under the ziprasidone serum concentration versus time curve (AUC0-inf) resulting from administration of a control ziprasidone immediate release oral capsule containing the same amount of ziprasidone to a cohort of humans in a fed state.
Owner:PFIZER INC
Solid and semi-solid polymeric ionic conjugates
Aqueous solubility of drugs including insoluble or poorly soluble drugs such as ziprasidone is improved using a functional polymer to form an ionic conjugate with said drug.
Owner:PFIZER INC +1
Processes for preparation of ziprasidone
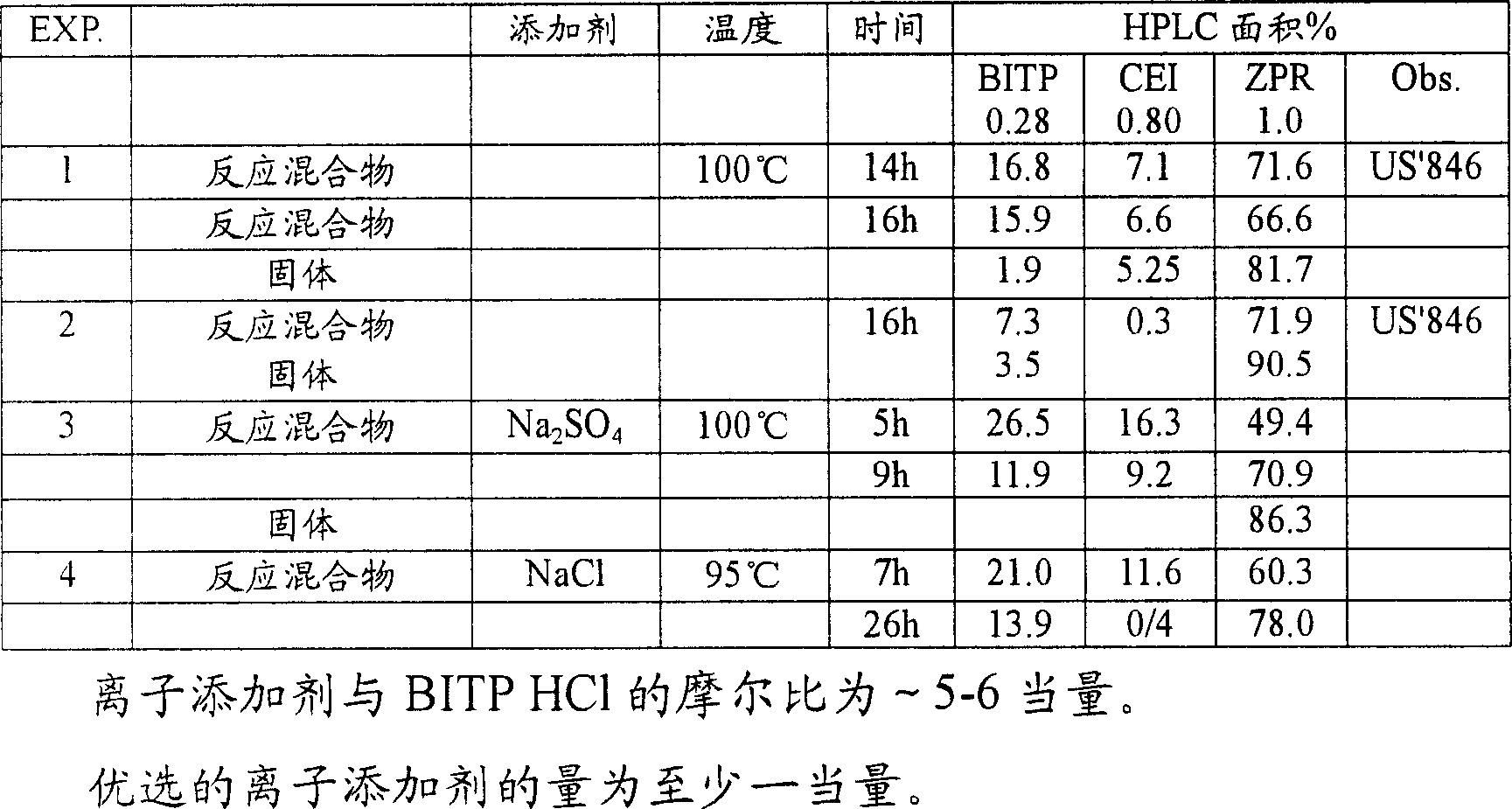
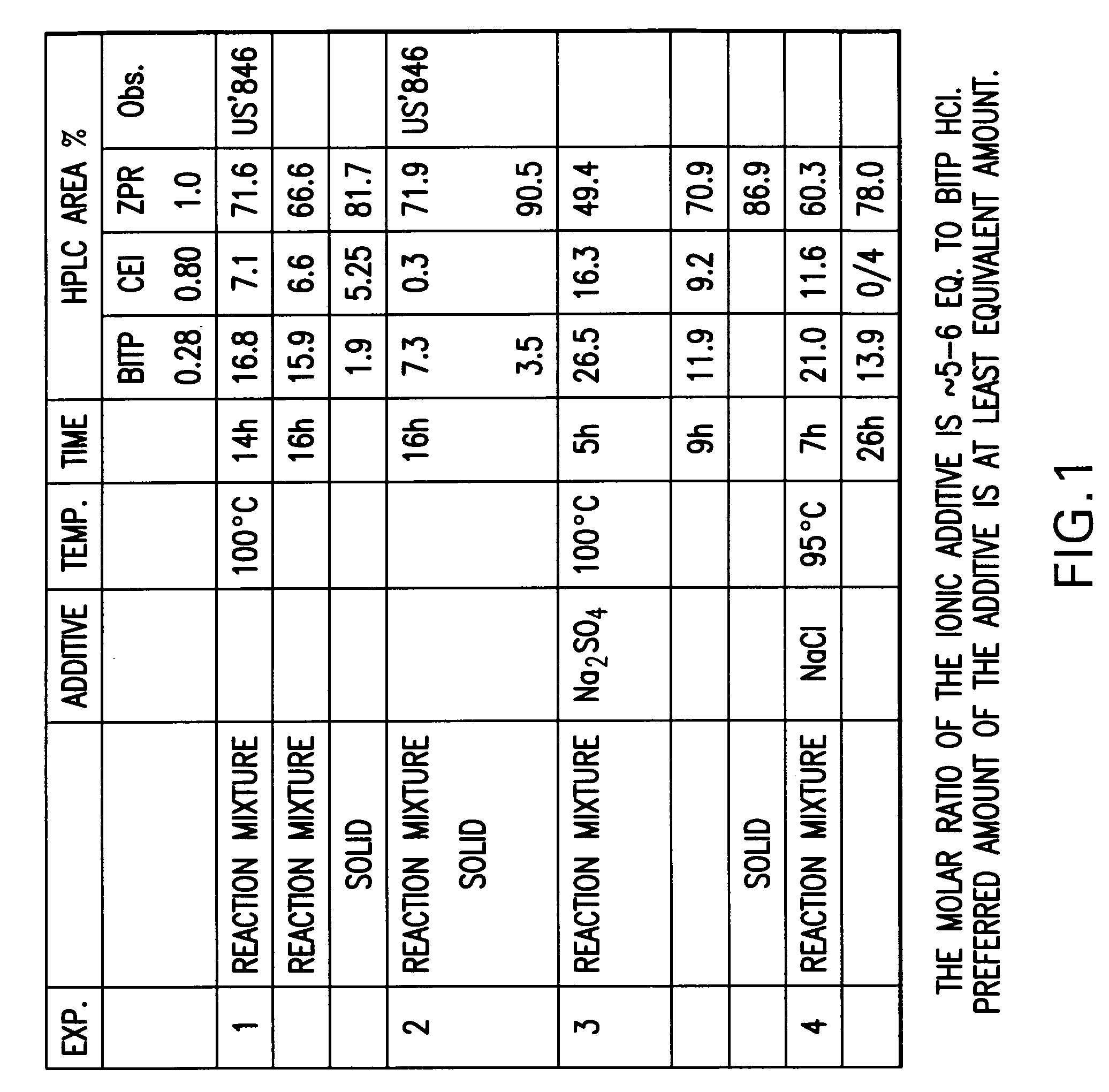
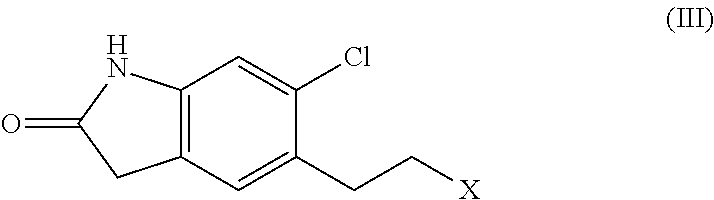
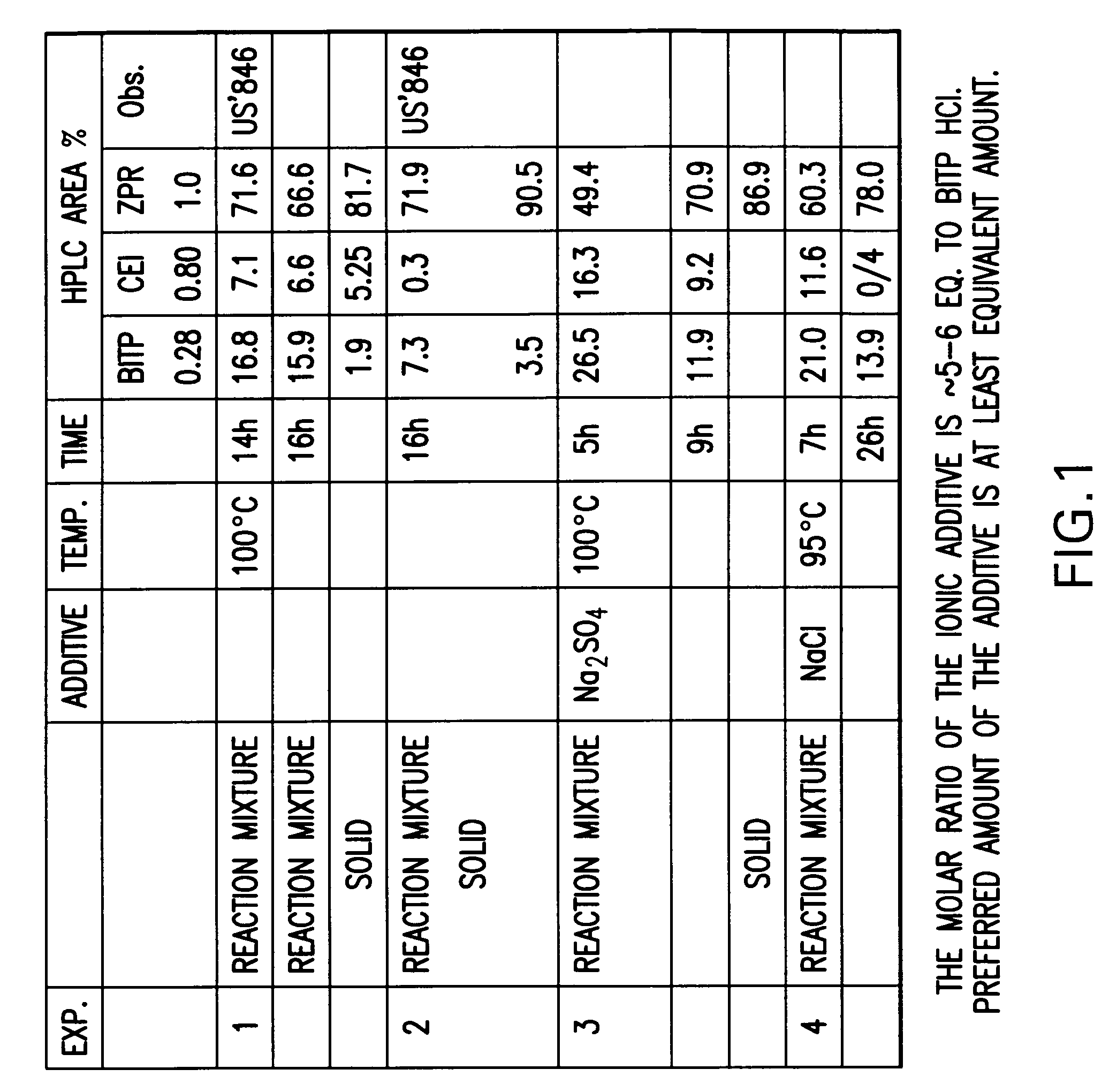
Provided are processes for preparing ziprasidone from 1, 2-benzothiazole-3-piperazinyl (BITP) and 5-(2-chloroethyl)-6-chloro-1, 3-dihydro-indole-2(2H) one (CEI) using various solvents, bases and promoters.
Owner:TEVA PHARMA IND LTD
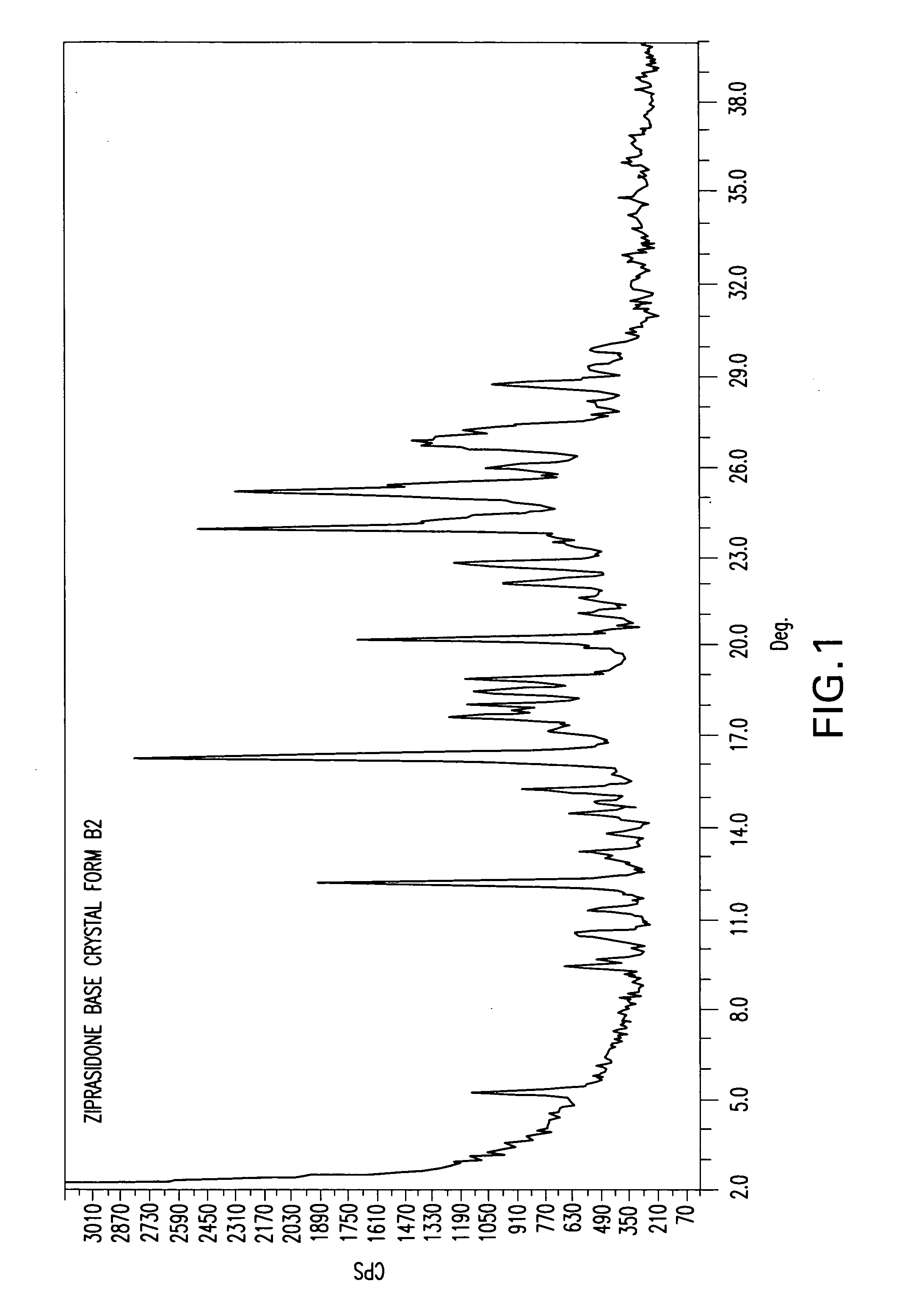
Polymorphic form B2 of ziprasidone base
Owner:TEVA PHARM USA INC
Processes for preparation of ziprasidone
InactiveUS20050143397A1Improve reaction speedImproved purity profileBiocideOrganic active ingredientsCombinatorial chemistryZiprasidone
Owner:TEVA PHARM USA INC
Method for preparing ziprasidone
The invention discloses a method for preparing ziprasidone, which comprises the following steps of: dissolving 5-(2-chloroacetyl)-6-chloro-1,3-dihydro-indol-2-(2H)-one and 3-piperazinyl-1,2-benzisothiazole hydrochloride in a mixed system of a water-soluble polar nonprotonic solvent and an aqueous solution of inorganic base, and reacting at the temperature of between 60 and 70DEG C for 3 to 6 hours; and after the reaction is finished, adding purified water, performing suction filtration, washing, and drying to obtain the ziprasidone. A reaction solvent is a mixed solution of the water-soluble polar nonprotonic solvent and the aqueous solution of the inorganic base, so that the dissolubility of reactants is increased, the acid binding effect is enhanced, and the reaction time is shortened; reaction conditions are concise, the operation is simple and the method is suitable for industrial production; and posttreatment is simple, the product yield is high, and impurity content is low.
Owner:QILU PHARMA HAINAN +1
Preparation method of ziprasidone intermediate
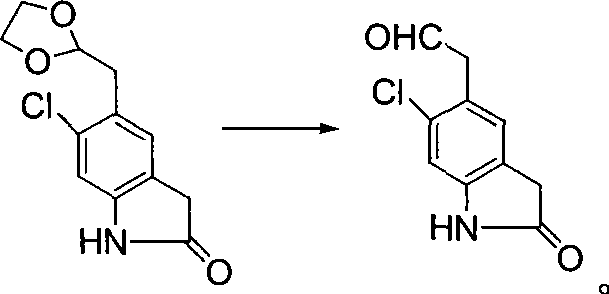
The invention relates to a preparation method of a ziprasidone intermediate.The preparation method is a method to prepare 6-chloro-2-indolone by one-pot process, an alkali liquid and phase-transfer catalyst low in price are used in the preparation process to substitute the prior art sodium hydride necessary to use and high in price, recyclable low-grade aliphatic ketone and low-grade fatty alcohol are used to substitute DMF (dimethyl formamide) and DMSO (dimethylsulfoxide) difficult to recycle, the cost is reduced greatly, the materials used herein are cheap and easy to obtain, the process is simple and feasible, posttreatment steps are simplified, particularly the step (1) requires no washing, extracting and purifying operation, one step may be performed directly, the whole operating step needs no use of column chromatography for purification, the whole preparation process is simplified, and the method is convenient to industrialize; in the synthetic process of formula III compound, a deacidifying process is mild and safe; in the synthesis of formula II compound, low-valence sulfur-containing compound is used in reduction, generation of mass waste acid liquid is avoided, production is safe and environment-friendly, and the preparation process is simple and easy.
Owner:ZHEJIANG MENOVO PHARMA
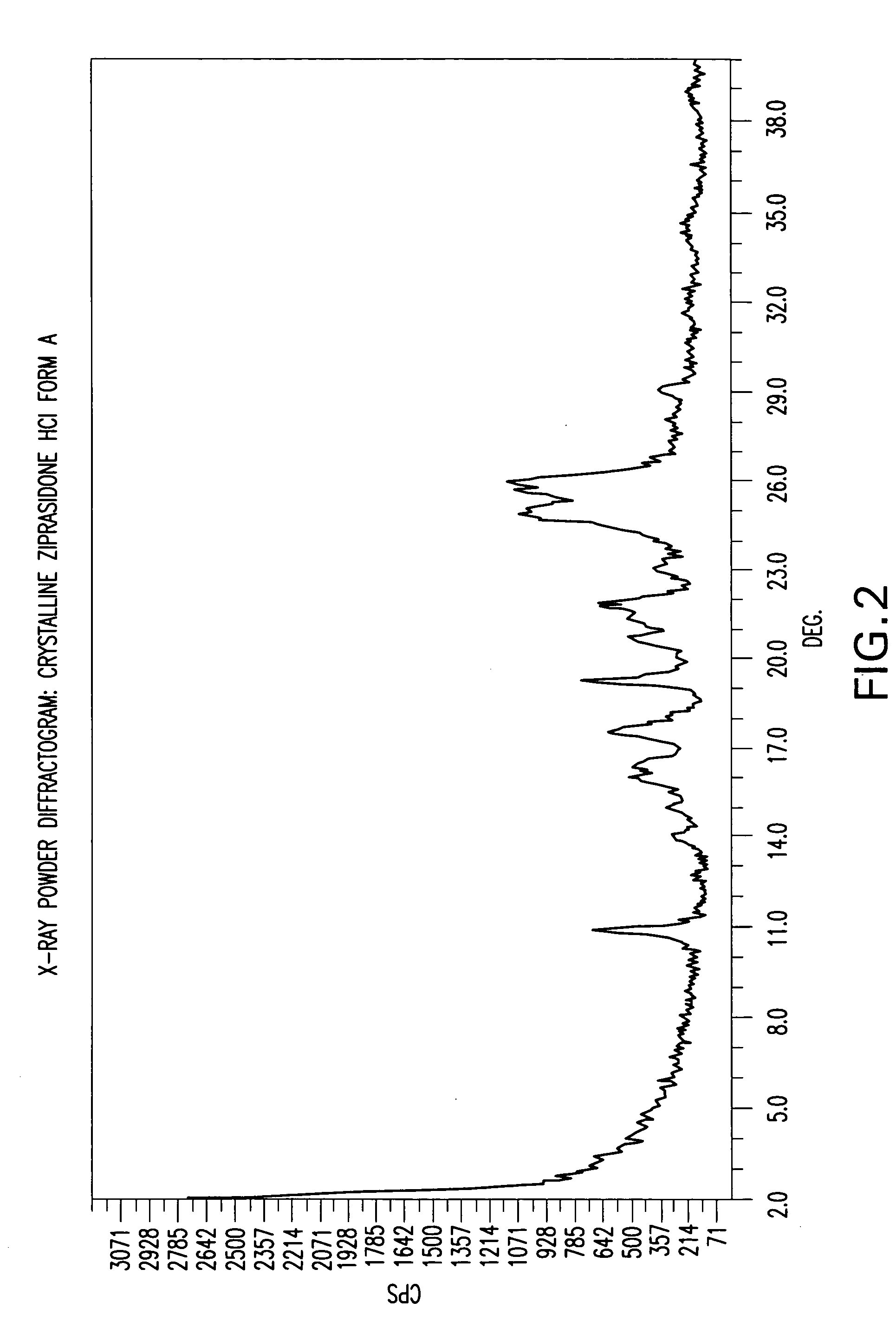
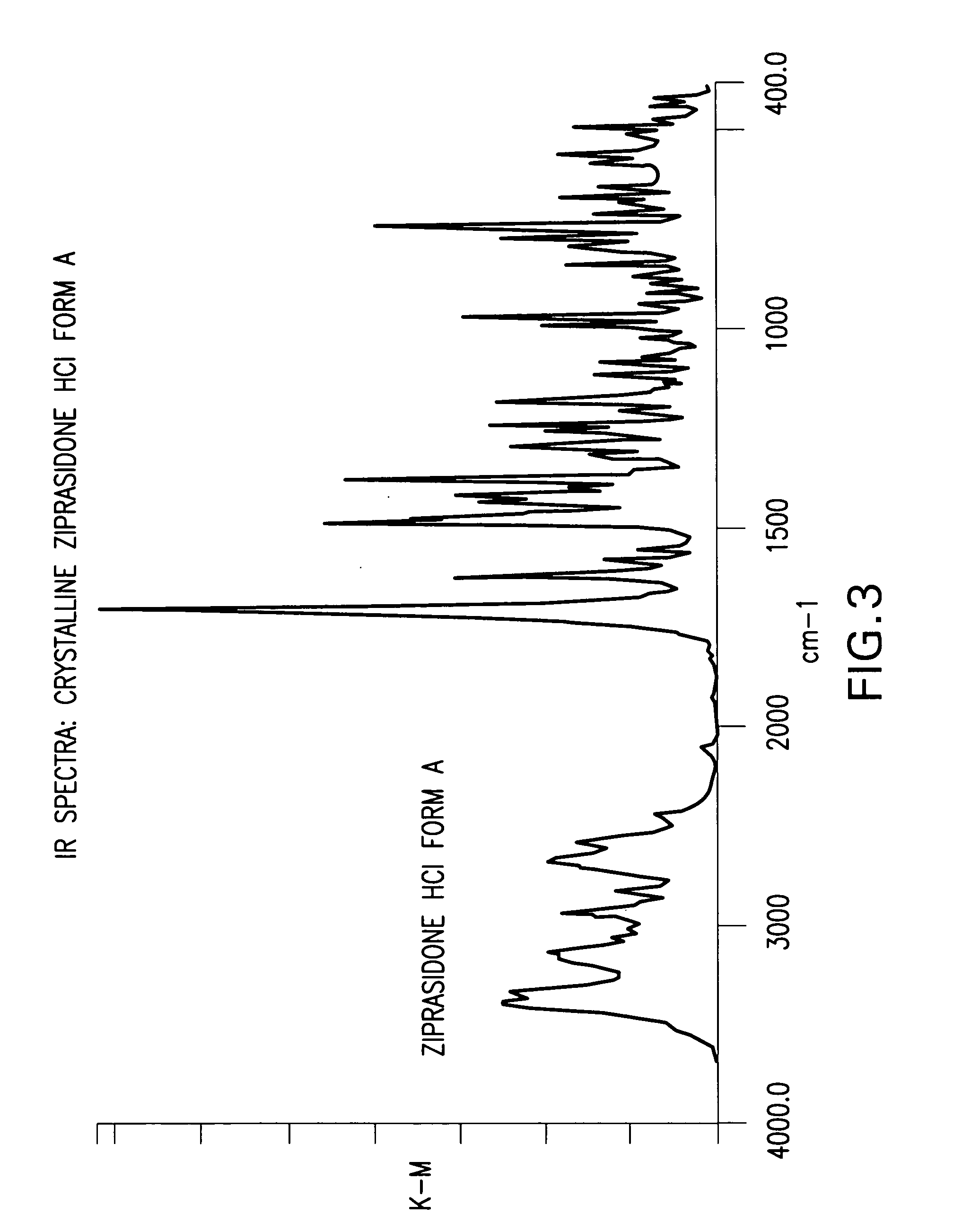
Ziprasidone free from colored impurities and a process for its preparation
InactiveUS20060211708A1Improve the immunityTherapy is simpleOrganic active ingredientsOrganic chemistryZiprasidoneImpurity
Ziprasidone base or a pharmaceutically acceptable salt thereof free from colored impurities, in particular those giving the product a “slightly pink to pink” coloration.
Owner:DIPHARMA FRANCIS +1
Injectable Deopot Formulations and Methods For Providing Sustained Release of Nanoparticle Compositions
InactiveUS20080193542A1Reduce risk of recurrenceReduce exposurePowder deliveryOrganic active ingredientsNanoparticlePharmaceutical formulation
Pharmaceutical formulations comprising: a compound selected from the group consisting of ziprasidone, having a maximum average particle size; a carrier; and preferably at least two surface stabilizers are disclosed. The present invention also comprises methods of treating psychosis with such a formulation and processes for making such a formulation.
Owner:PFIZER INC
Synthetic method of ziprasidone
The invention provides a new method for synthesizing ziprasidone. The method comprises the following steps: 2,5-dichlorotoluene is taken as a starting material to be condensed with N,N-dimethyldimethoxymethylamine after being nitrified; an obtained intermediate is converted to an acetal compound in the presence of oxalic acid and glycol; and the acetal compound is reacted with ethyl malonate and then is subjected to decarboxylation, reduction and cyclization to form a key intermediate 7; the compound 7 is subjected to deprotection under an acidic condition to from an aldehyde 8; and the aldehyde 8 is reacted with 3-piperazinyl-1,2-benzisothiazole in the presence of sodium triacetoxyborohydride to form the ziprasidone. The invention has the advantages of easy and simple control, readily available raw materials and convenient operation.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Ziprasidone suspension
InactiveUS7175855B1Improve wettabilityFacile re-suspendabilityPowder deliveryBiocideColloidSilicon dioxide
Compositions comprising ziprasidone free base or a difficult to wet pharmaceutically acceptable ziprasidone acid addition salt, a polysorbate, and colloidal silicon dioxide form good aqueous suspensions having a useful shelf life and are easily re-suspended if setting occurs.
Owner:PFIZER INC
Nanoparticulate and controlled release compositions comprising aryl-heterocyclic compounds
InactiveCN101242813AImprove convenienceImprove compliancePharmaceutical delivery mechanismArylControlled release
The present invention provides a composition comprising ziprasidone useful in the treatment and prevention of schizophrenia and similar psychiatric disorders. In one embodiment, the composition comprises nanoparticulate particles comprising ziprasidone and at least one surface stabilizer. The nanoparticulate particles have an effective average particle size of less than about 2000 nm. In another embodiment, the composition comprises a modified release composition that, upon administration to a patient, delivers ziprasidone in a bimodal, multimodal or continuous manner. The invention also relates to dosage forms containing such compositions, and to methods for the treatment and prevention of schizophrenia and similar psychiatric disorders.
Owner:ELAN PHRMA INT LTD
Process for the Preparation of Ziprasidone
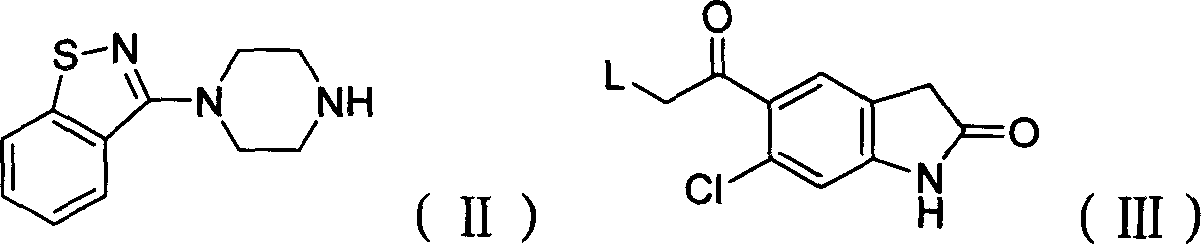
The present invention relates to a process for preparing Ziprasidone of formula I,or a pharmaceutically acceptable salt or a solvate or a hydrate thereof;comprising the steps of reacting 1-(1,2-benzisothiazol-3-yl) piperazine of formula II or its salt:with 5-(2-haloethyl)-6-chloro-oxindole of formula III:wherein X is leaving groups like fluoro, chloro, bromo, iodo or sulphonyl;in the presence of a dispersing agent and a base in a solvent to form ziprasidone of formula I; and optionally converting the ziprasidone formed into a pharmaceutically acceptable acid addition salts of ziprasidone; or a solvate or a hydrate thereof.
Owner:ALKEM LAB LTD
Process for the preparation of ziprasidone
InactiveUS20070117810A1Reduce the amount requiredReduce contentOrganic active ingredientsOrganic chemistryZiprasidoneOrganic chemistry
A process for the preparation of ziprasidone and a novel intermediate useful in its preparation. The process comprises the reduction of a compound (III) to give a compound (V) which is then reduced to compound (II). This is reacted with compound (IV) to give the desired compound.
Owner:DIPHARMA SPA +1
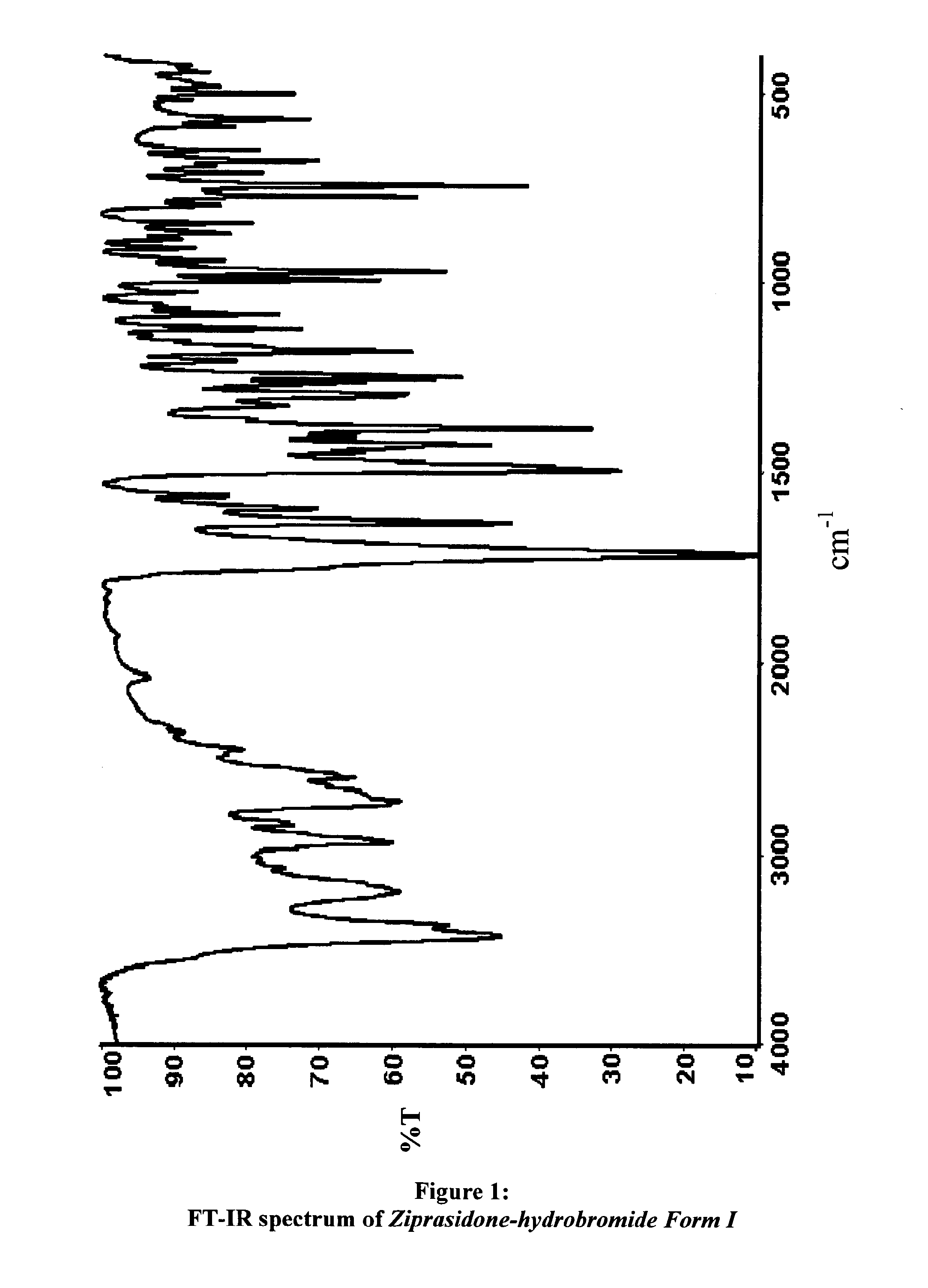
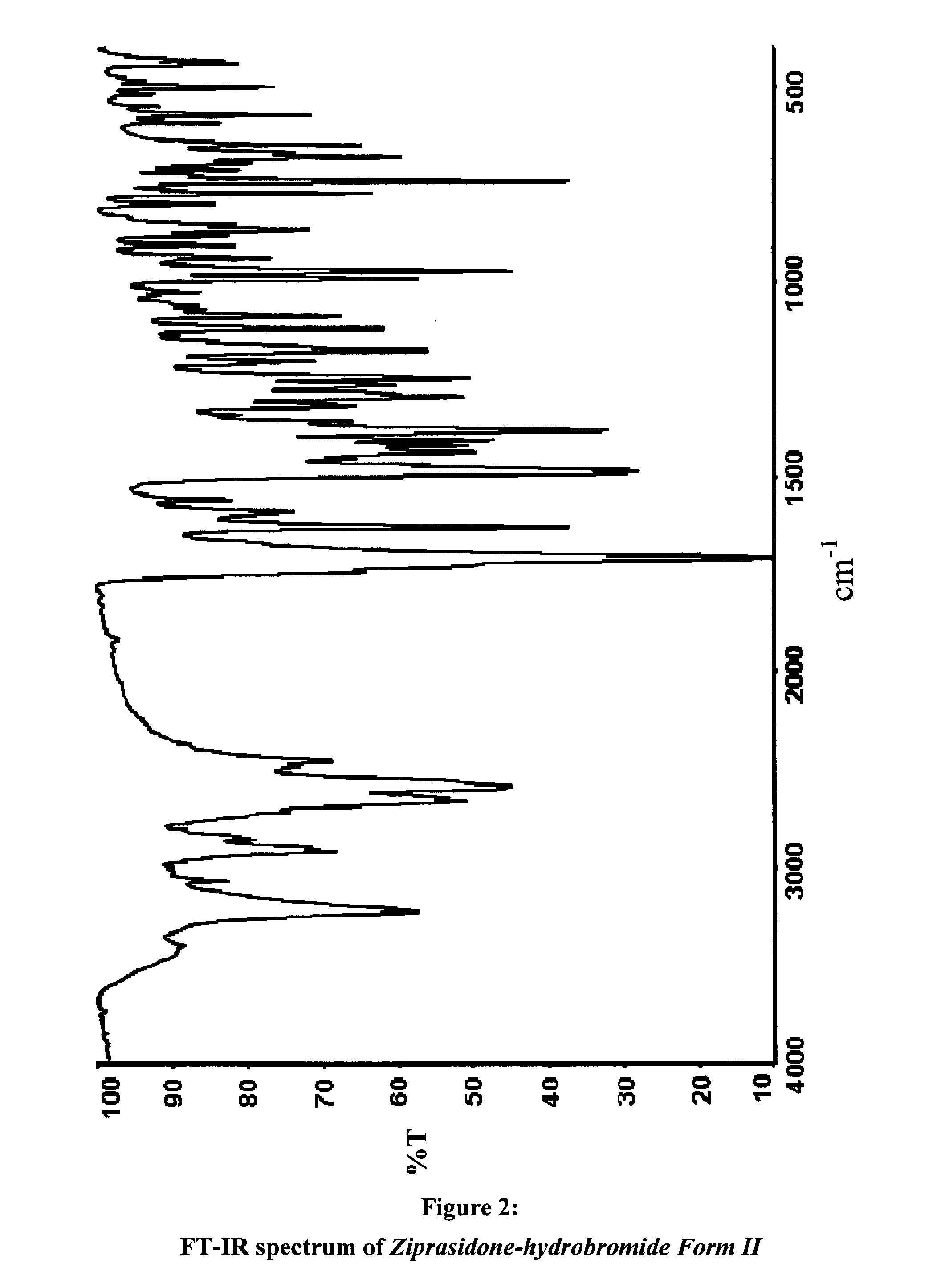
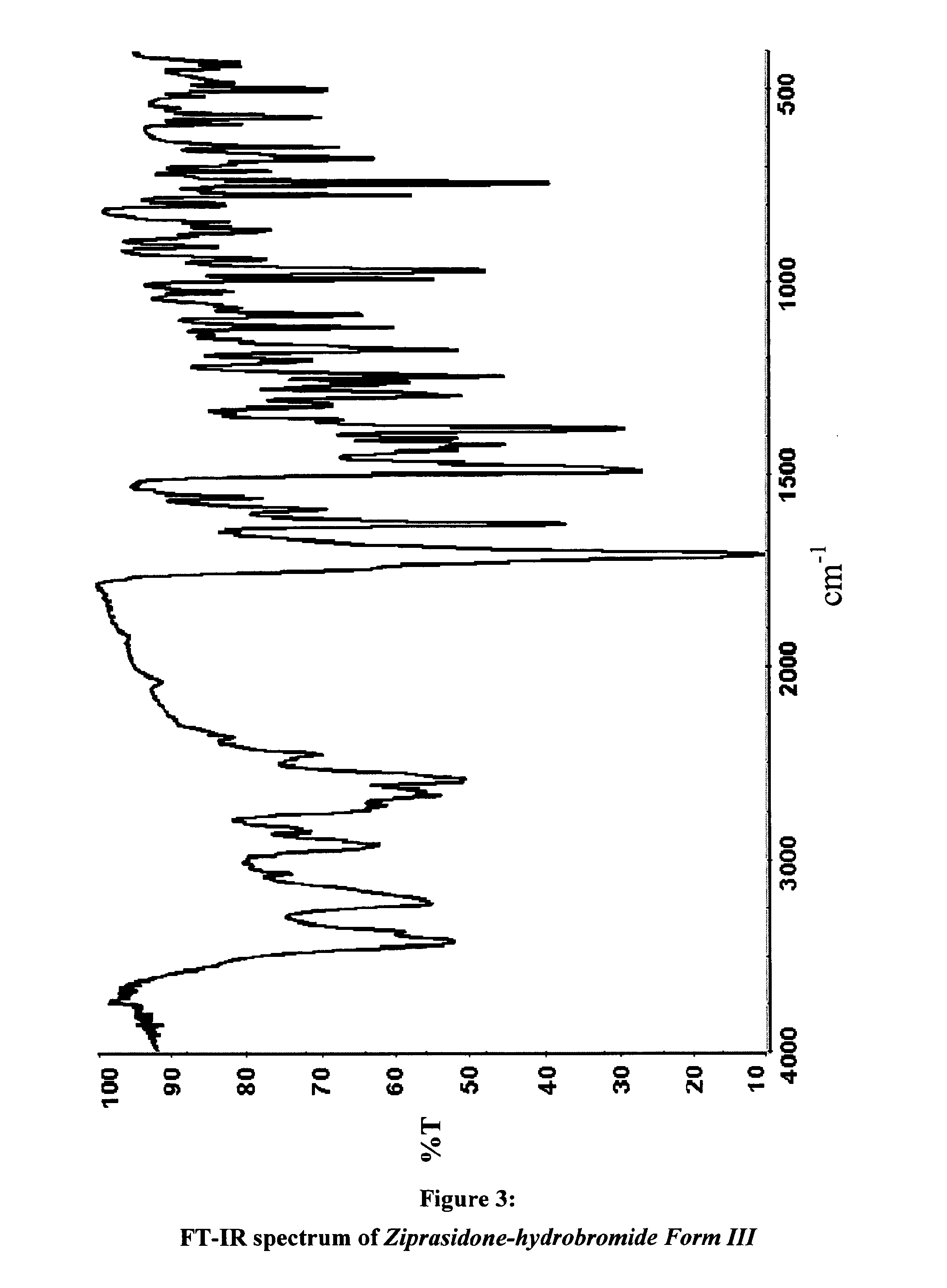
Polymorphs of 5--6-chloro-1,3-dihydro-2h-indol-2-one hydrobromide and processes for preparation thereof
InactiveUS20100081668A1Improve stabilityAdvantage in formulationOrganic active ingredientsNervous disorderHydrobromideMedicine
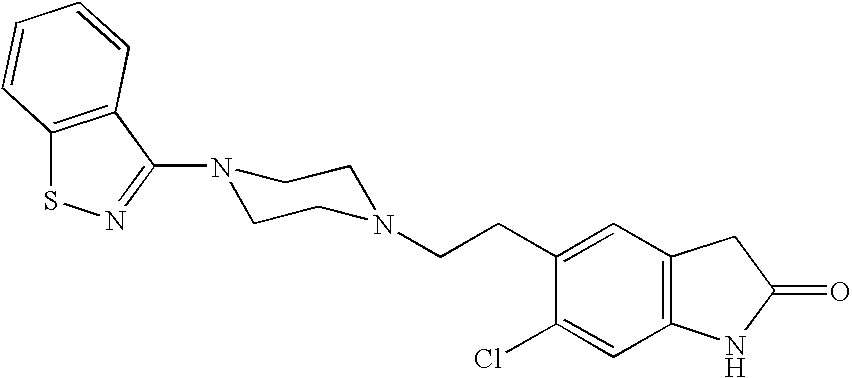
The present invention provides pharmaceutically applicable compounds and polymorphs belonging to the ziprasidone hydrobromide compound group with antipsychotic effect. The present invention provides hydrobromide polymorphs of 5-{-2-[4-(1,2-benzisothiazol-3-yl)-1-piperazinyl]-ethyl}-6-chloro-1,3-dihydro-2H-indol-2-one, ziprasidone of Formula (I) having neuroleptic activity.
Owner:RICHTER GEDEON NYRT
Processes for preparation of ziprasidone
InactiveUS7667037B2Rate of reactionImproved profileOrganic active ingredientsBiocideMedicinal chemistryZiprasidone
Owner:TEVA PHARM USA INC
Methods, dosage forms, and kits for administering ziprasidone without food
The present invention provides methods, dosage forms and kits for treating with an effective amount of ziprasidone a CNS disorder in a human when the human is in a fasted state. In one embodiment, the invention relates to a method for treating a CNS disorder in a human, which method comprises administering to the human in a fasted state, a solid oral dosage form comprising an amount of ziprasidone effective to treat said CNS disorder, wherein the area under the serum concentration versus time curve (AUC0-inf) of the ziprasidone in the human subsequent to said administering is from 70% to 140% of the mean area under the ziprasidone serum concentration versus time curve (AUC0-inf) resulting from administration of a control ziprasidone immediate release oral capsule containing the same amount of ziprasidone to a cohort of humans in a fed state.
Owner:PFIZER INC
Ziprasidone Dosage Form
InactiveUS20070237828A1Good treatment effectOrganic active ingredientsPowder deliveryPharmaceutical formulationExcipient
Pharmaceutical formulations of ziprasidone comprise ziprasidone or a salt thereof, in the form of particles having a mean particle size greater than about 90 μm and a pharmaceutically acceptable excipient.
Owner:DR REDDYS LAB LTD +1
Ziprasidone key intermediate preparation method
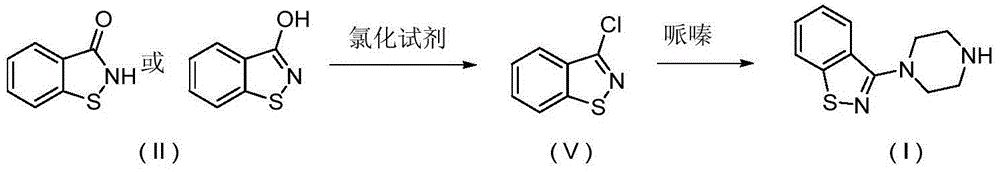
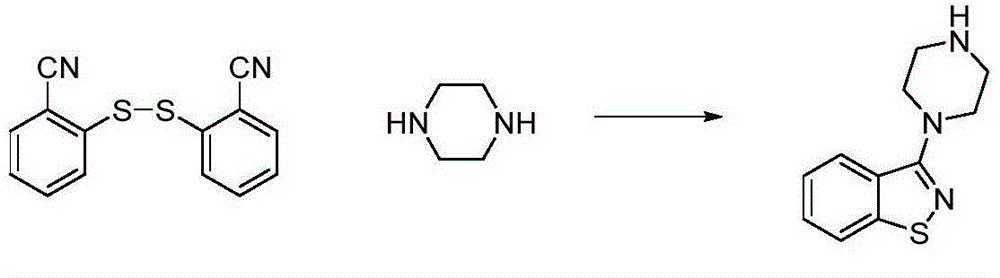
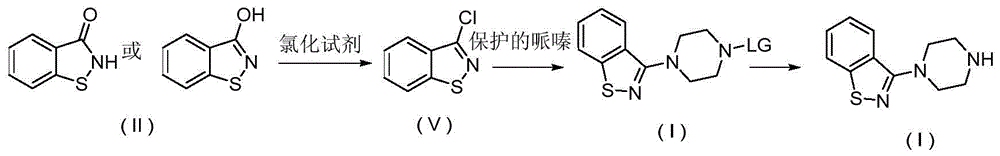
The present invention relates to a ziprasidone key intermediate preparation method, wherein benzo[d]isothiazole-3-ol (or one) is adopted as a raw material, and reacts with a substituted sulfonyl chloride or anhydride under an alkaline condition to obtain benzo[d]isothiazole-3-substituted sulfonate, and the benzo[d]isothiazole-3-substituted sulfonate reacts with piperazine to prepare the ziprasidone key intermediate 3-(1-piperazinyl)-1,2-benzoisothiazole. The method of the present invention has characteristics of simple operation, easily available raw materials, less byproducts, simple post-treatment, less industrial three-waste and the like, and is especially suitable for industrial production.
Owner:SHANGHAI TIANCI BIOLOGICAL VALLEY BIOLOGICAL ENG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com