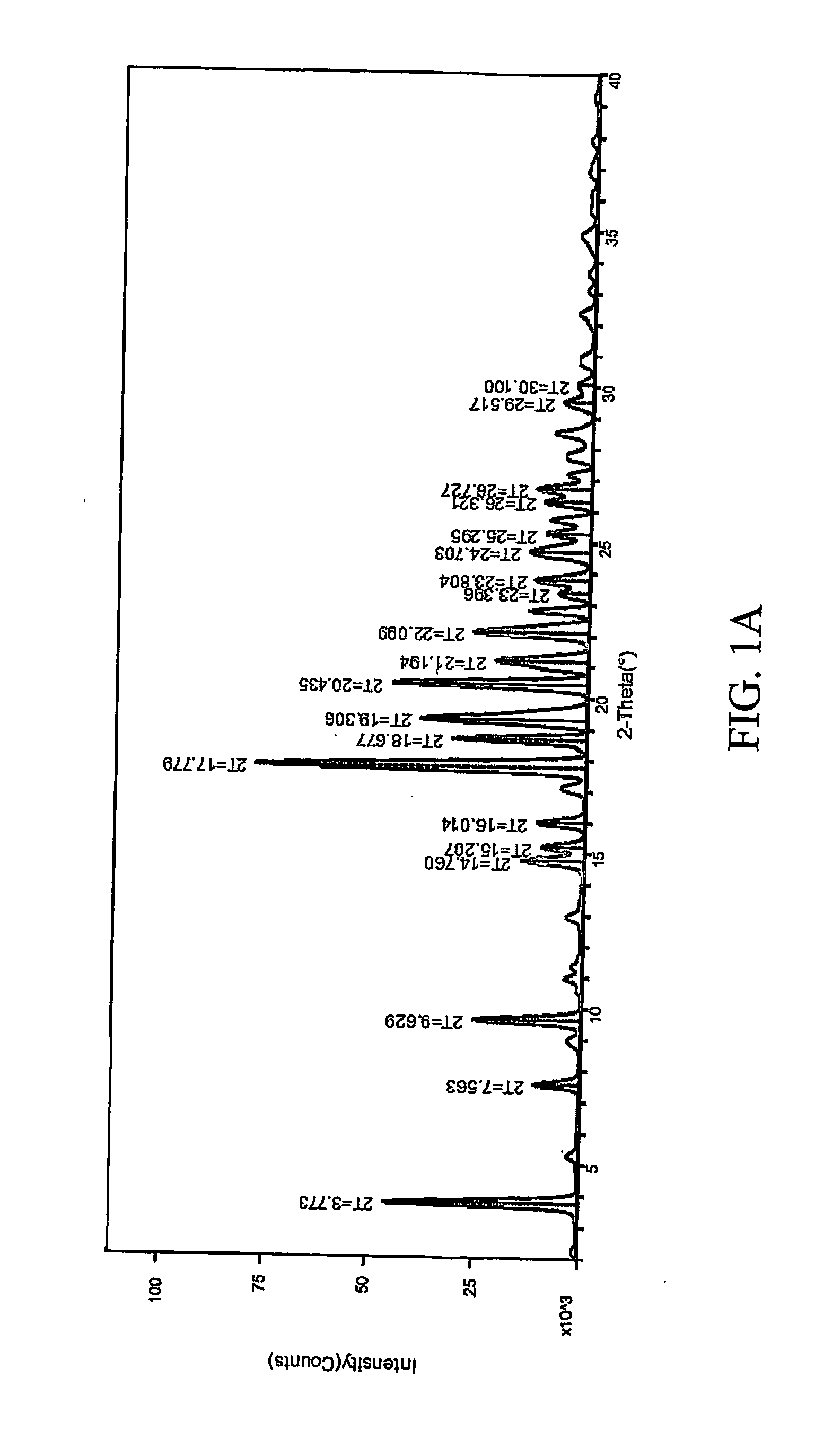
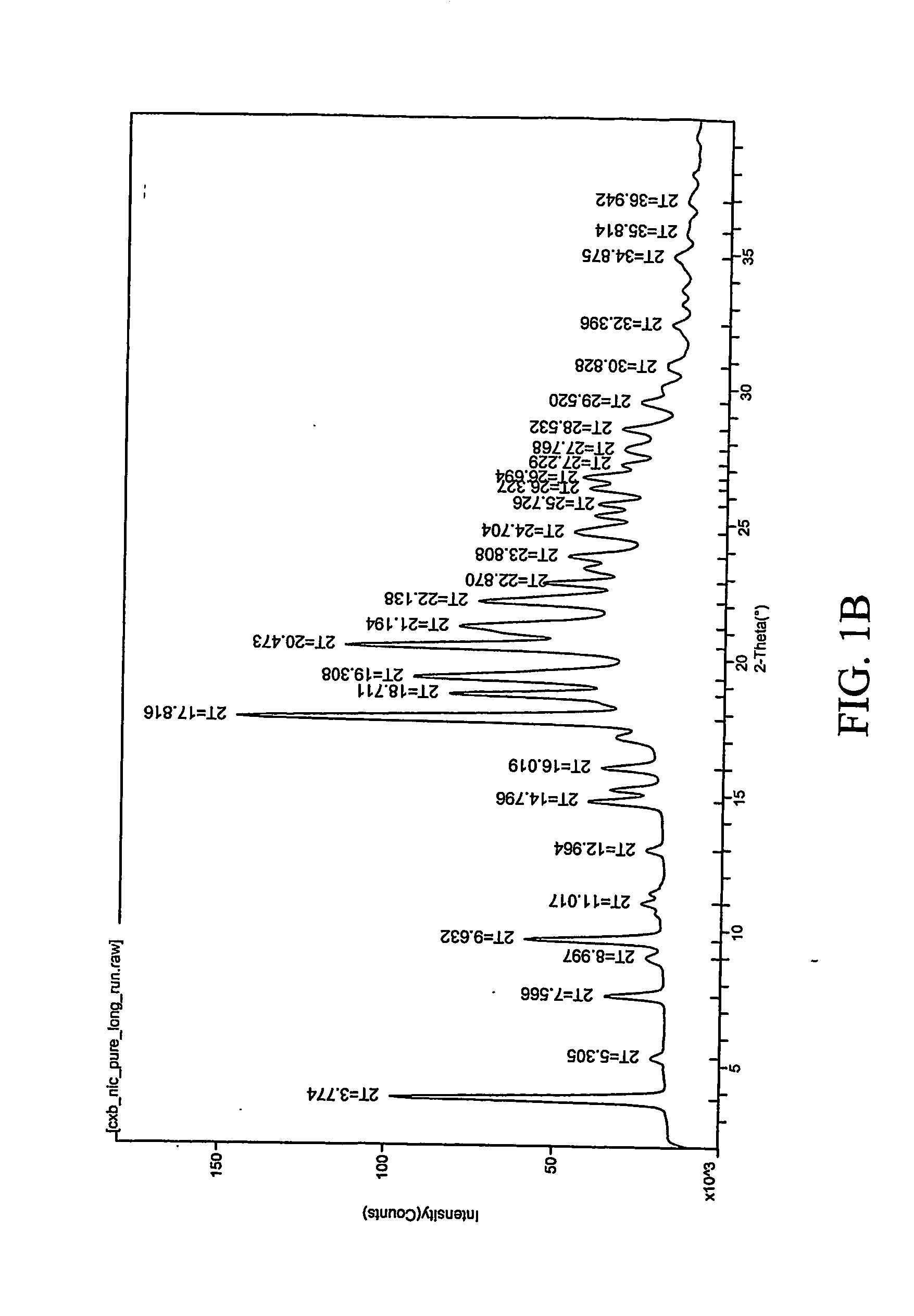
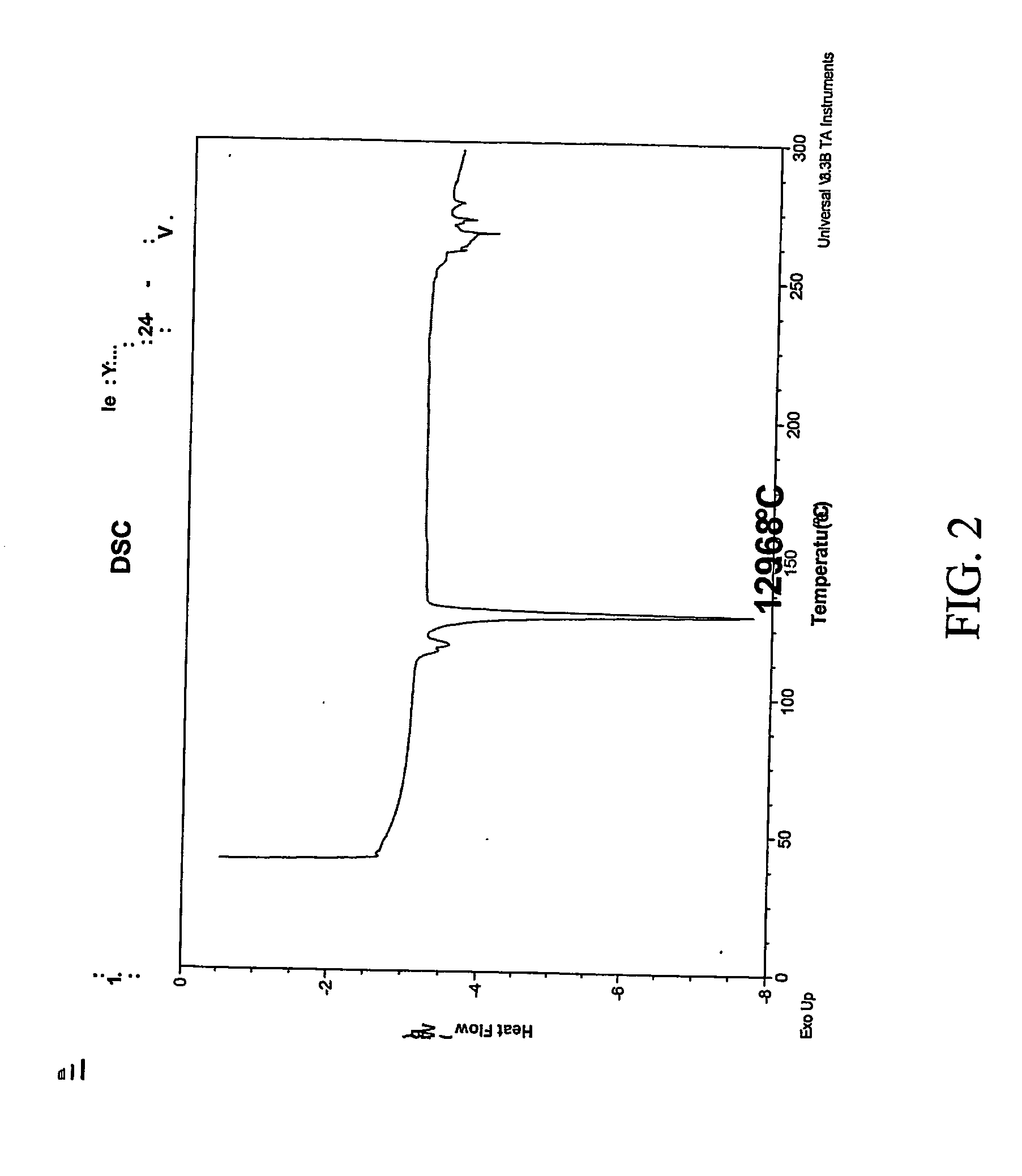
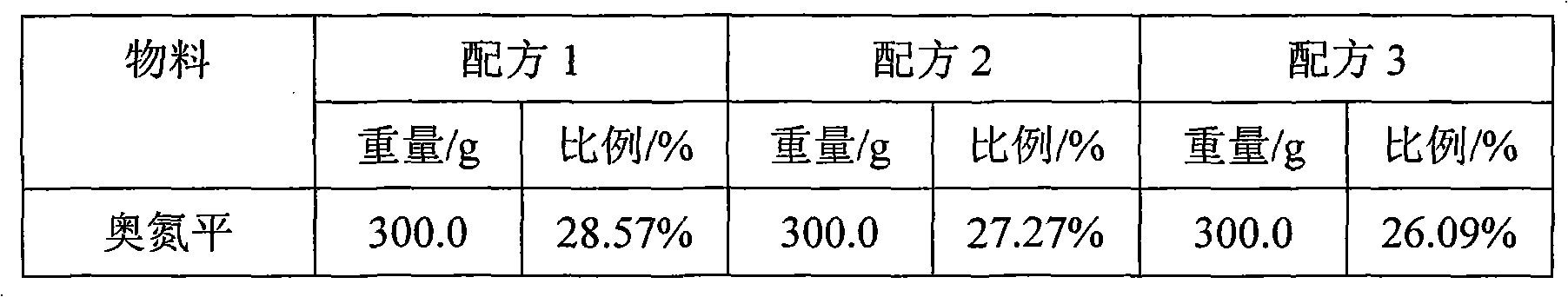
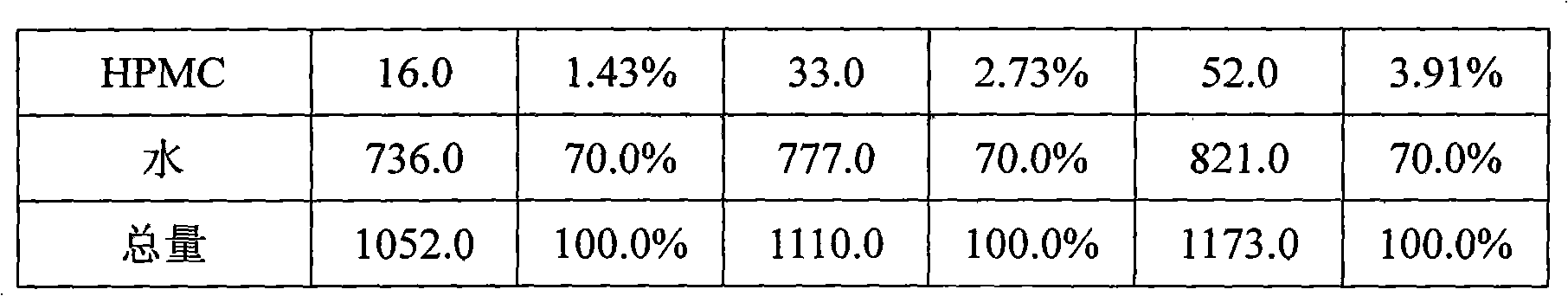
Patents
Literature
201 results about "Olanzapine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Olanzapine is used to treat certain mental/mood conditions (such as schizophrenia, bipolar disorder). It may also be used in combination with other medication to treat depression.
Pharmaceutical co-crystal compositions of drugs such as carbamazepine, celecoxib, olanzapine, itraconazole, topiramate, modafinil, 5-fluorouracil, hydrochlorothiazide, acetaminophen, aspirin, flurbiprofen, phenytoin and ibuprofen
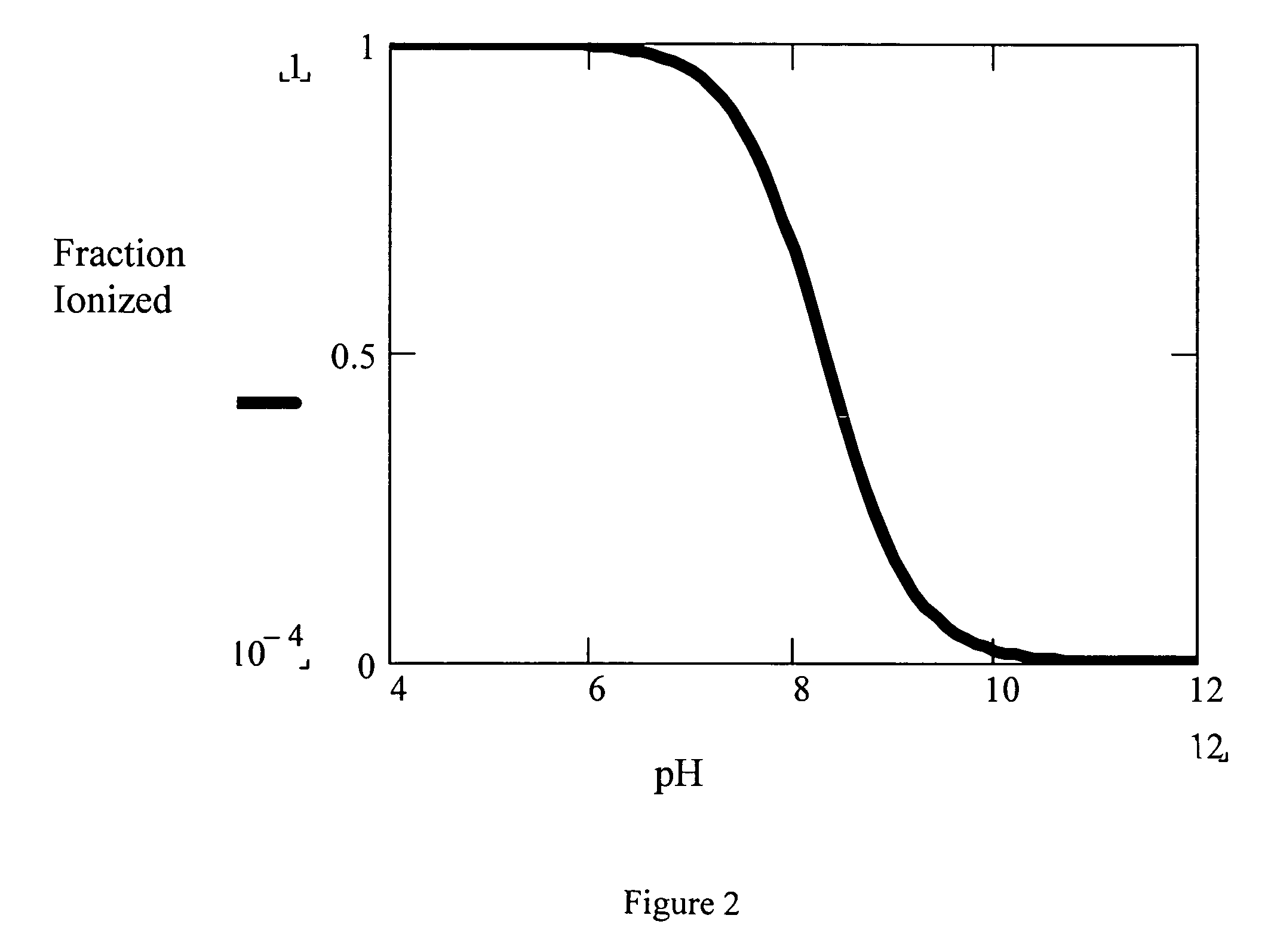
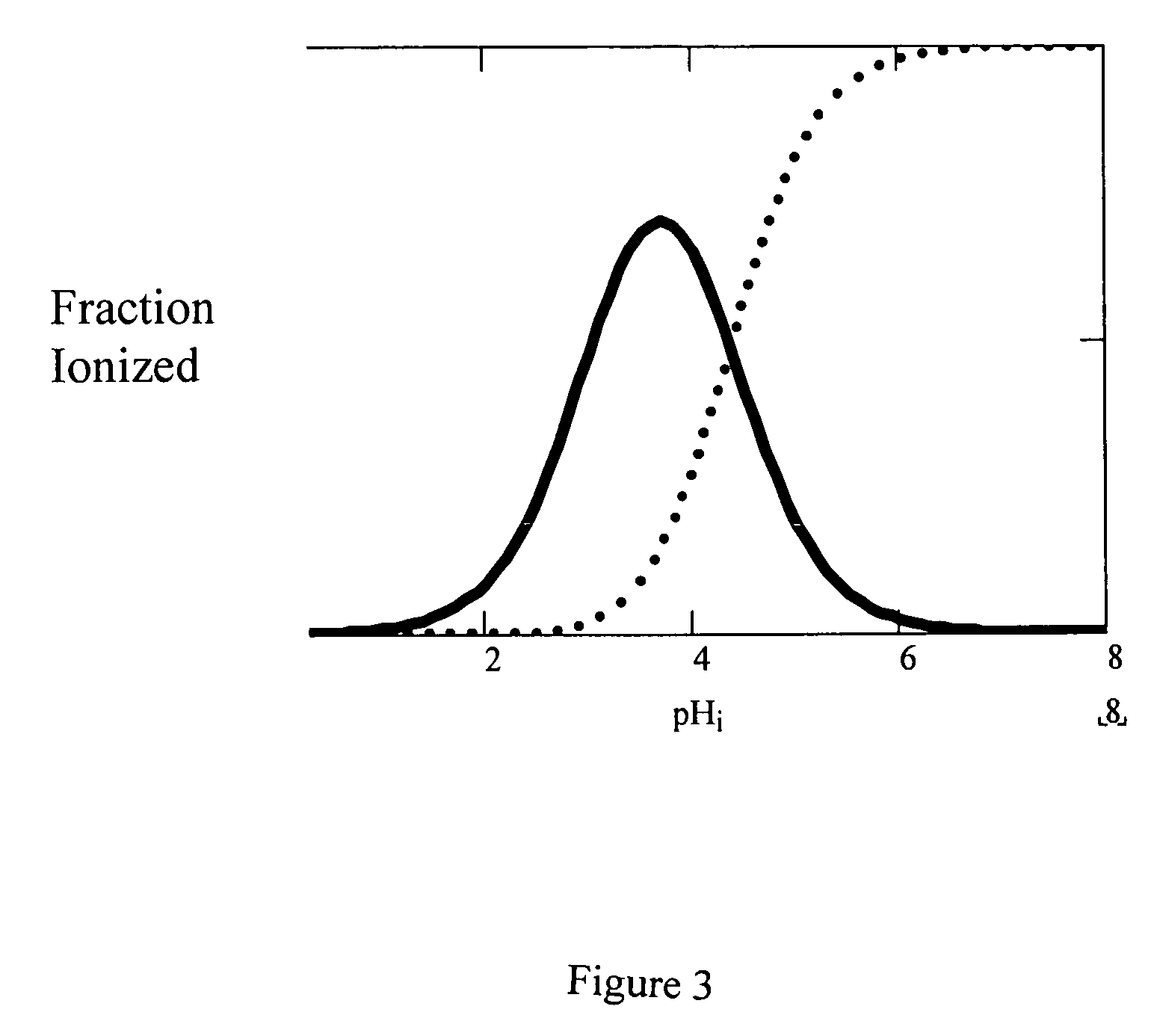
A pharmaceutical composition comprising a co-crystal of an API and a co-crystal former; wherein the API has at least one functional group selected from ether, thioether, alcohol, thiol, aldehyde, ketone, thioketone, nitrate ester, phosphate ester, thiophosphate ester, ester, thioester, sulfate ester, carboxylic acid, phosphinic acid, phosphonic acid, sulfonic acid, amide, primary amine, secondary amine, ammonia, tertiary amine, imine, thiocyanate, cyanamide, oxime, nitrile diazo, organohalide, nitro, S-heterocyclic ring, thiophene, N-heterocyclic ring, pyrrole, 0-heterocyclic ring, furan, epoxide, peroxide, hydroxamic acid, imidazole, pyridine and the co-crystal former has at least one functional group selected from amine, amide, pyridine, imidazole, indole, pyrrolidine, carbonyl, carboxyl, hydroxyl, phenol, sulfone, sulfonyl, mercapto and methyl thio, such that the API and co-crystal former are capable of co-crystallizing from a solution phase under crystallization conditions.
Owner:UNIV OF SOUTH FLORIDA +3
1,4-oxazepines as bace1 and/or bace2 inhibitors
InactiveUS20120238548A1Good pharmacological propertiesImprove propertiesBiocideOrganic active ingredientsDiseaseProphylactic treatment
The present invention relates to 1,4-Oxazepines of formula Ihaving BACE1 and / or BACE2 inhibitory activity, their manufacture, pharmaceutical compositions containing them and their use as therapeutically active substances. The active compounds of the present invention are useful in the therapeutic and / or prophylactic treatment of e.g. Alzheimer's disease and type 2 diabetes.
Owner:SIENA BIOTECH +1
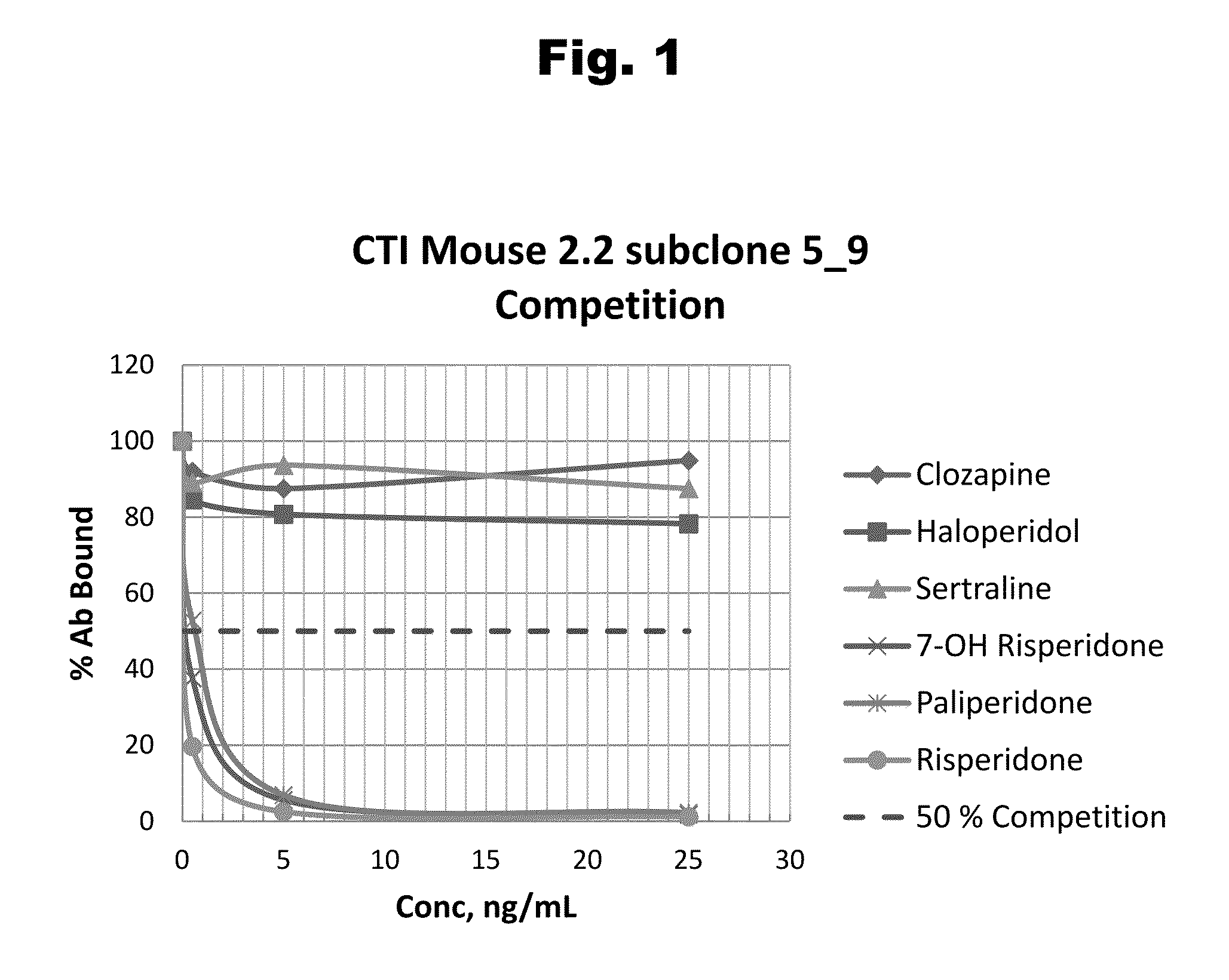
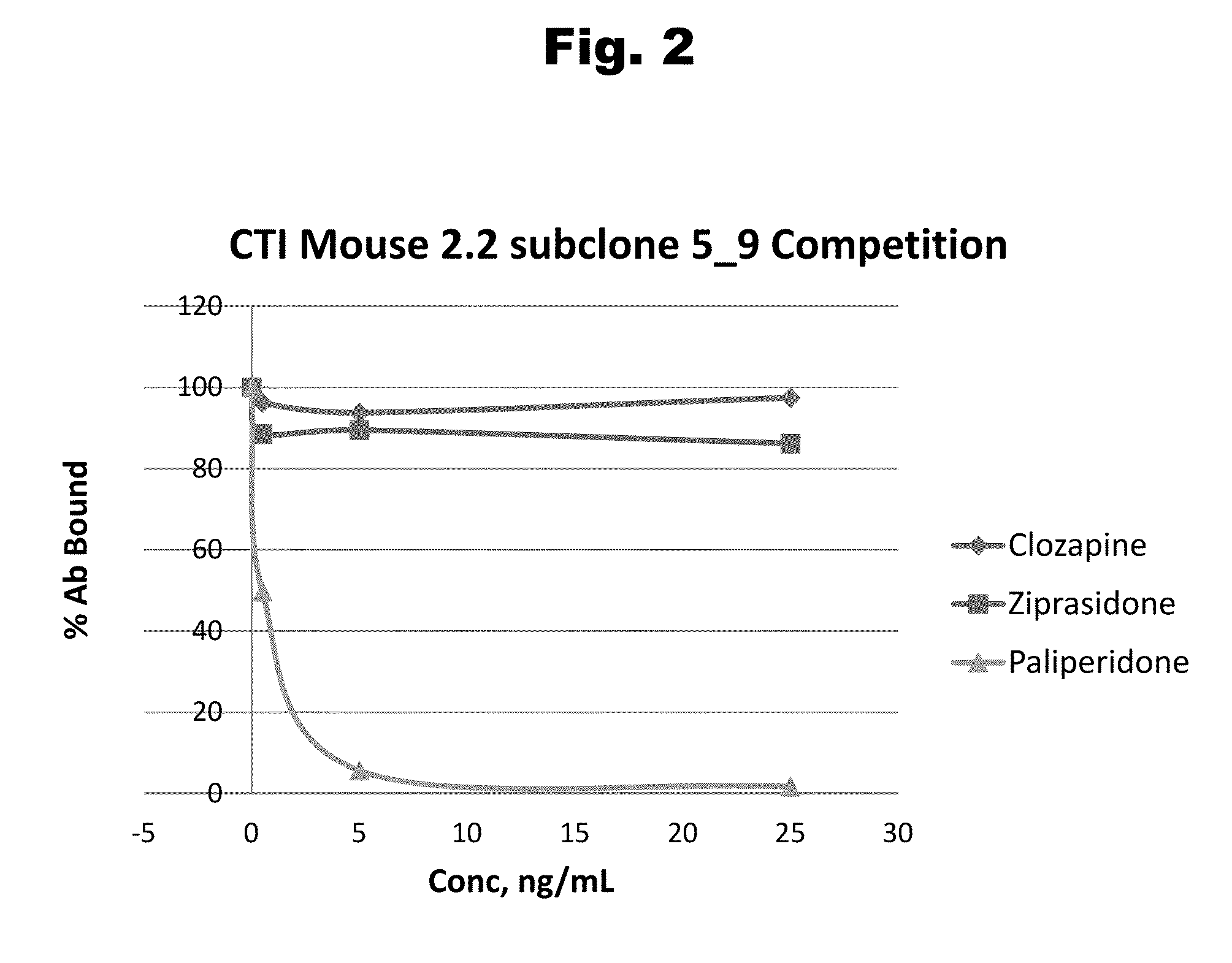
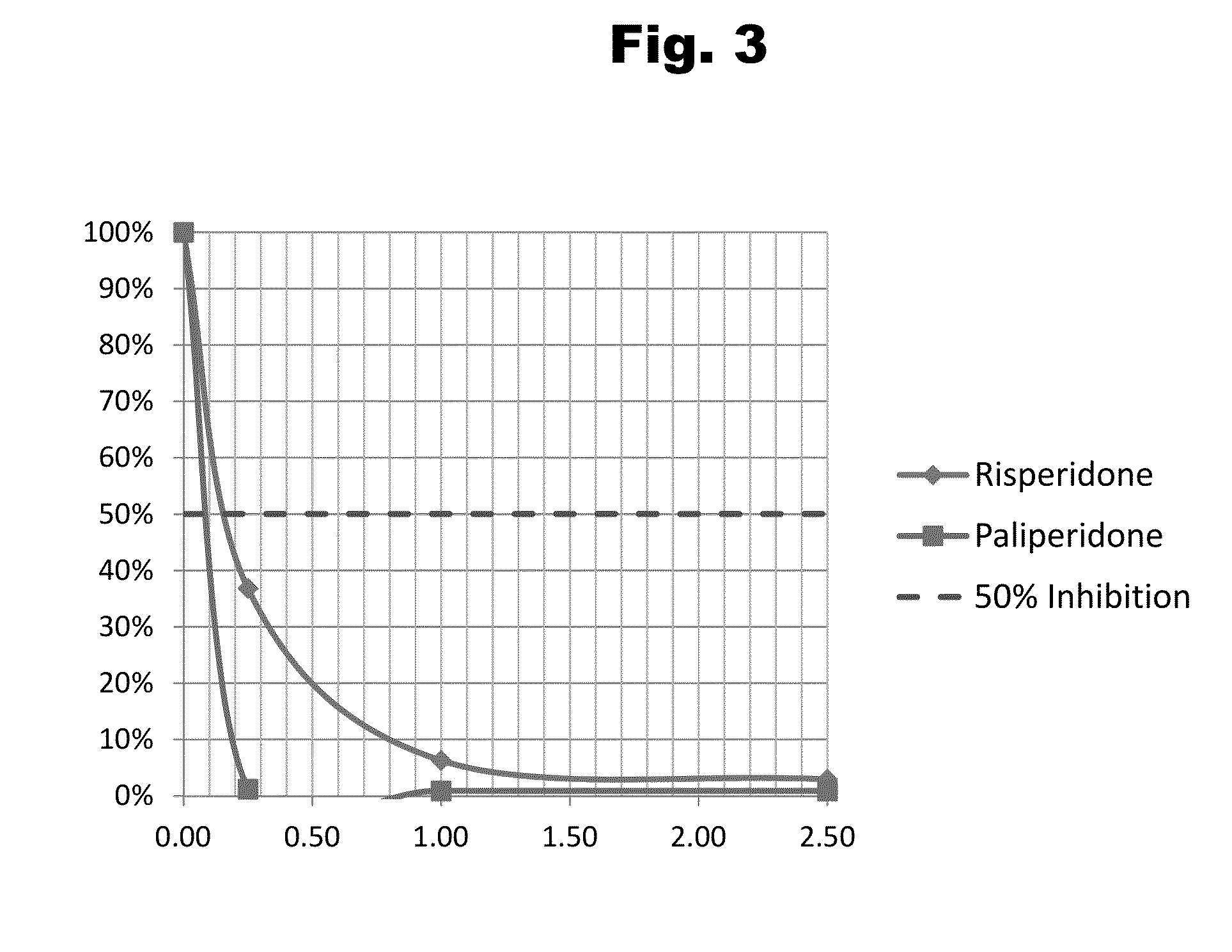
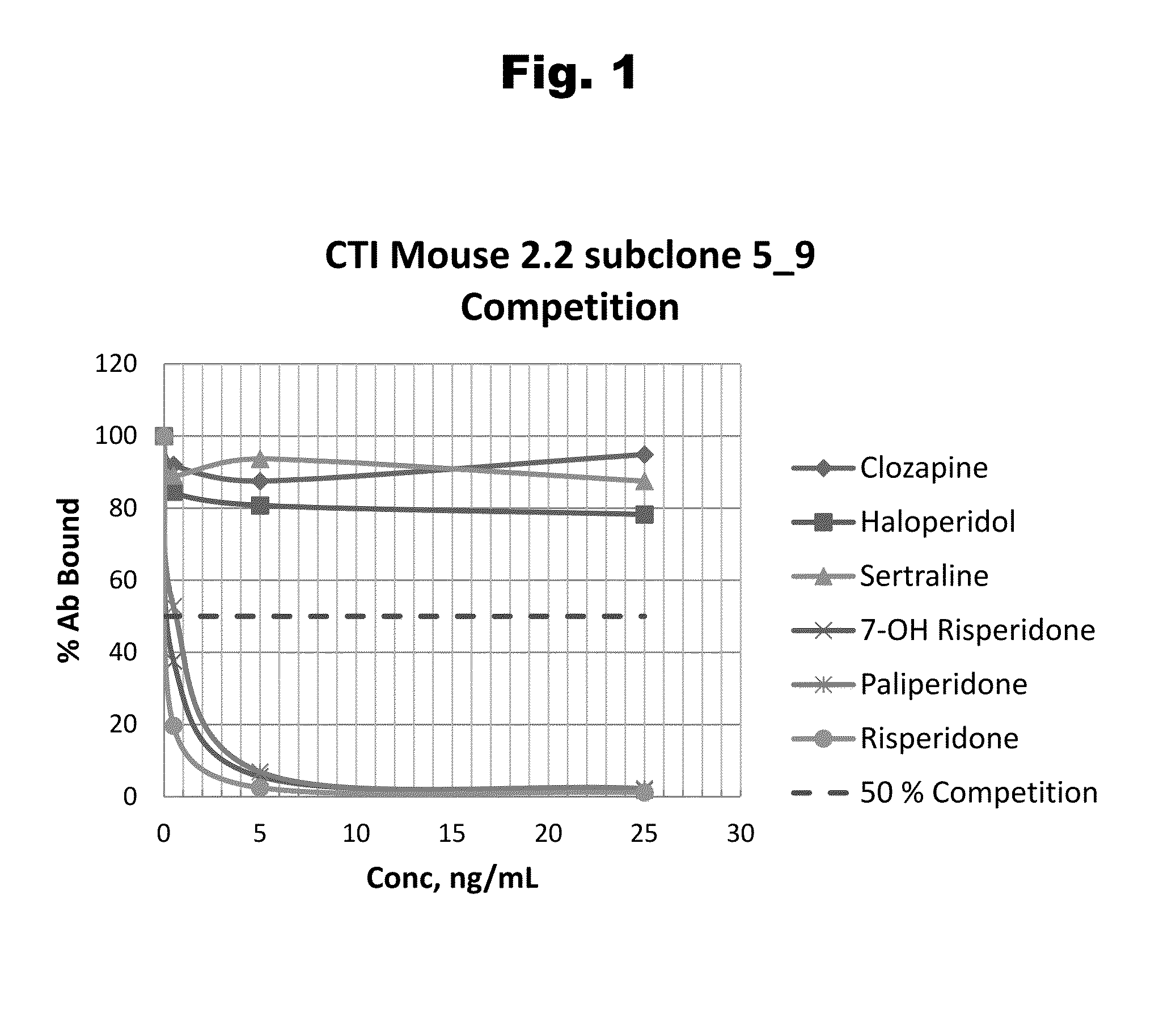
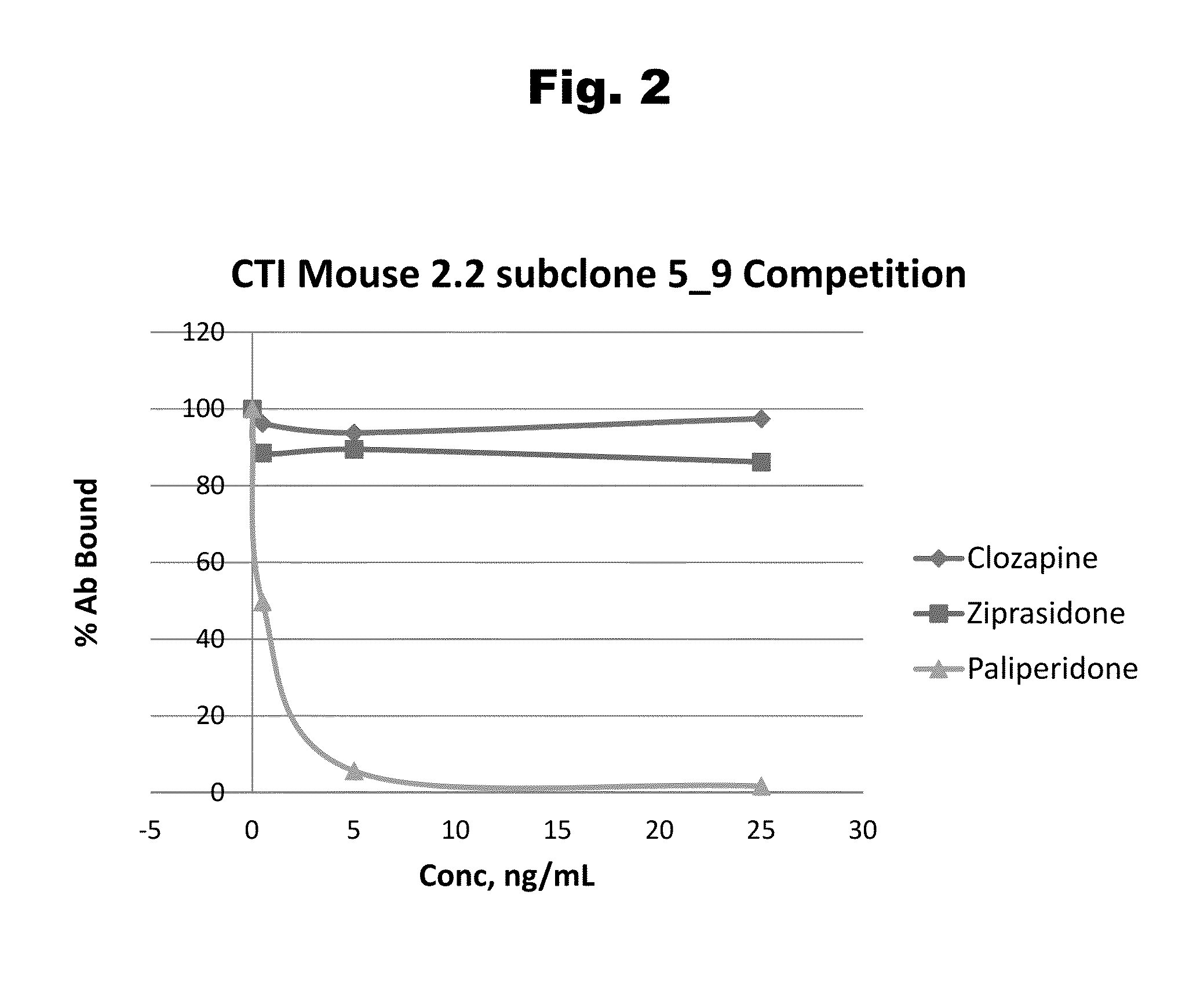
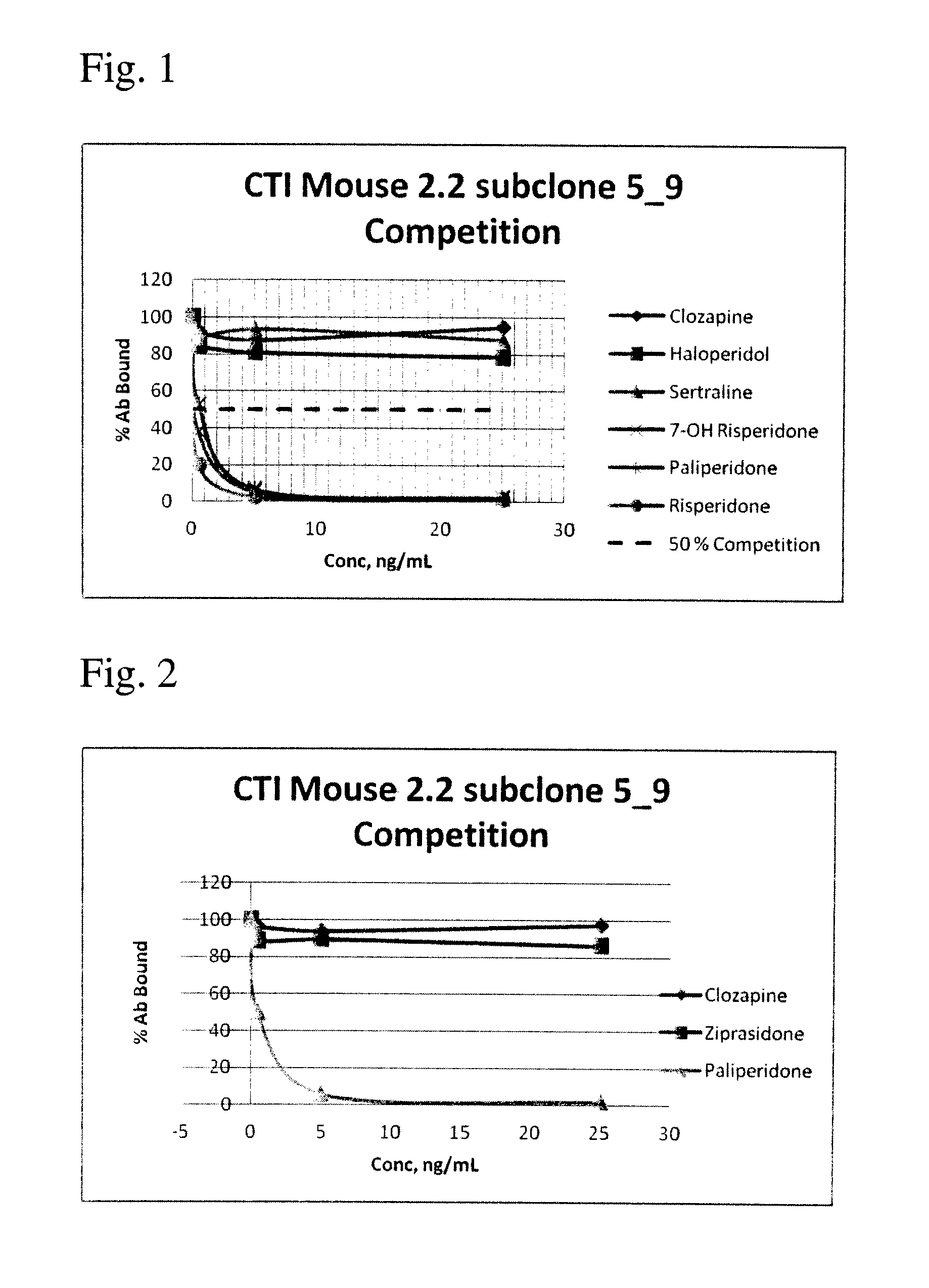
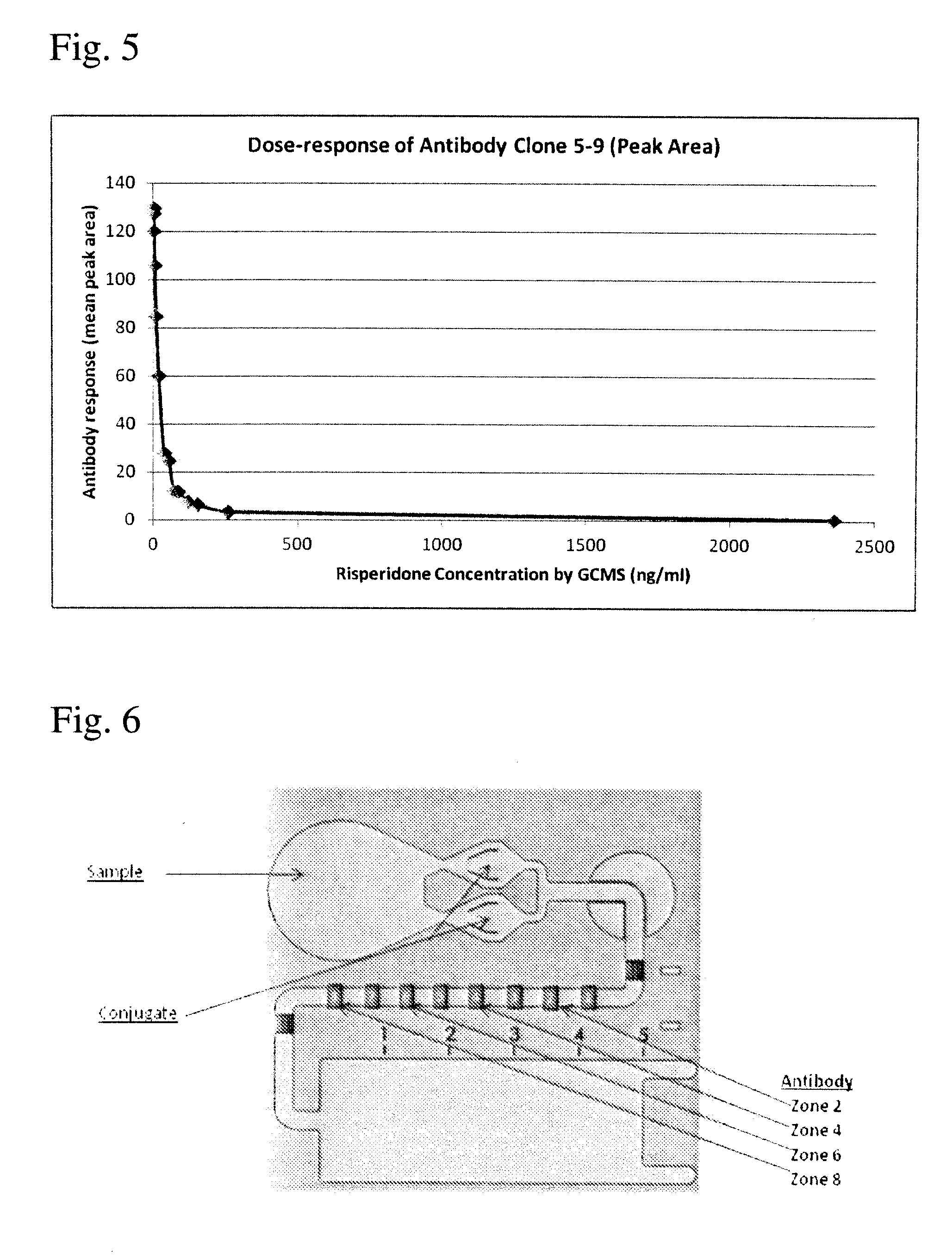
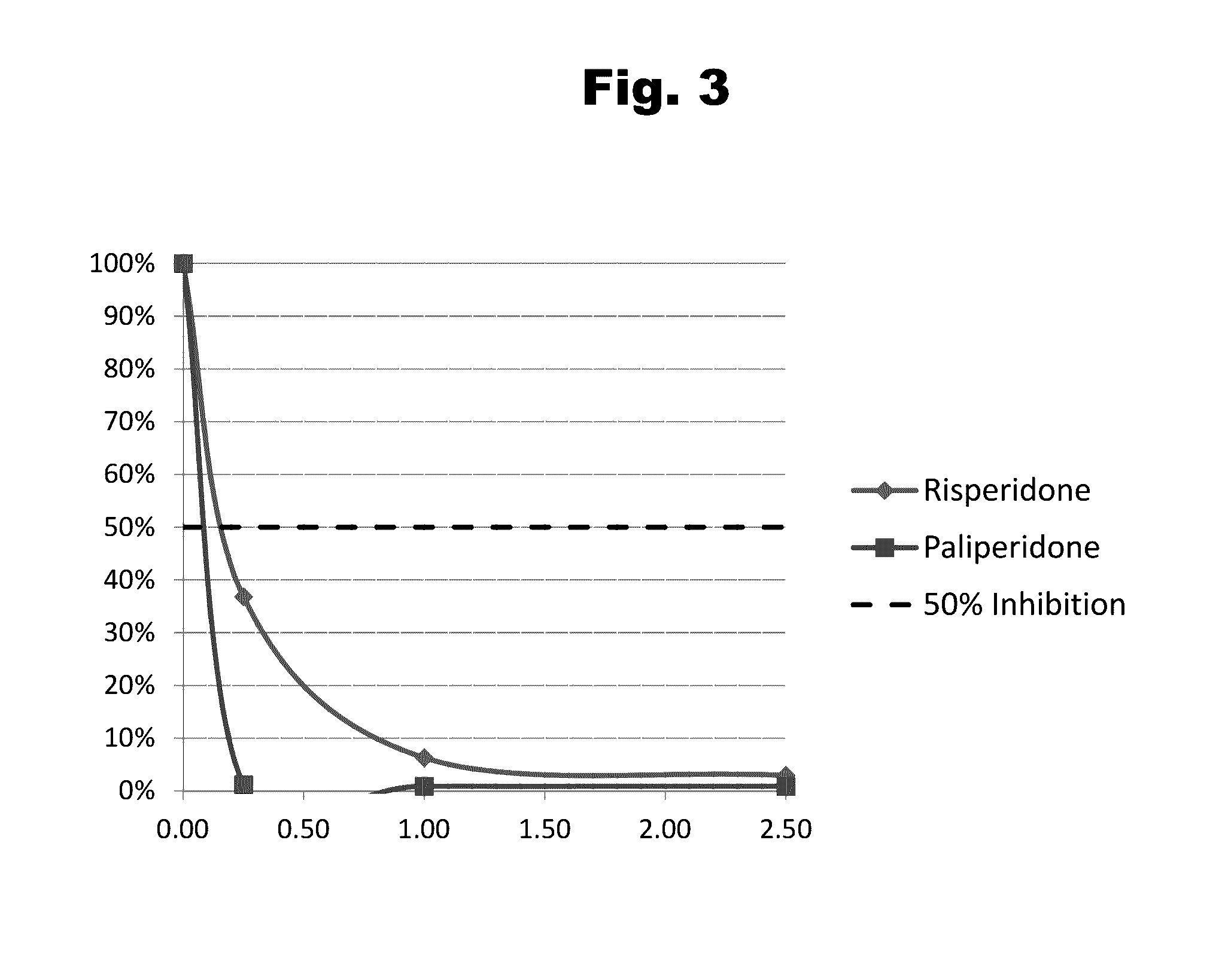
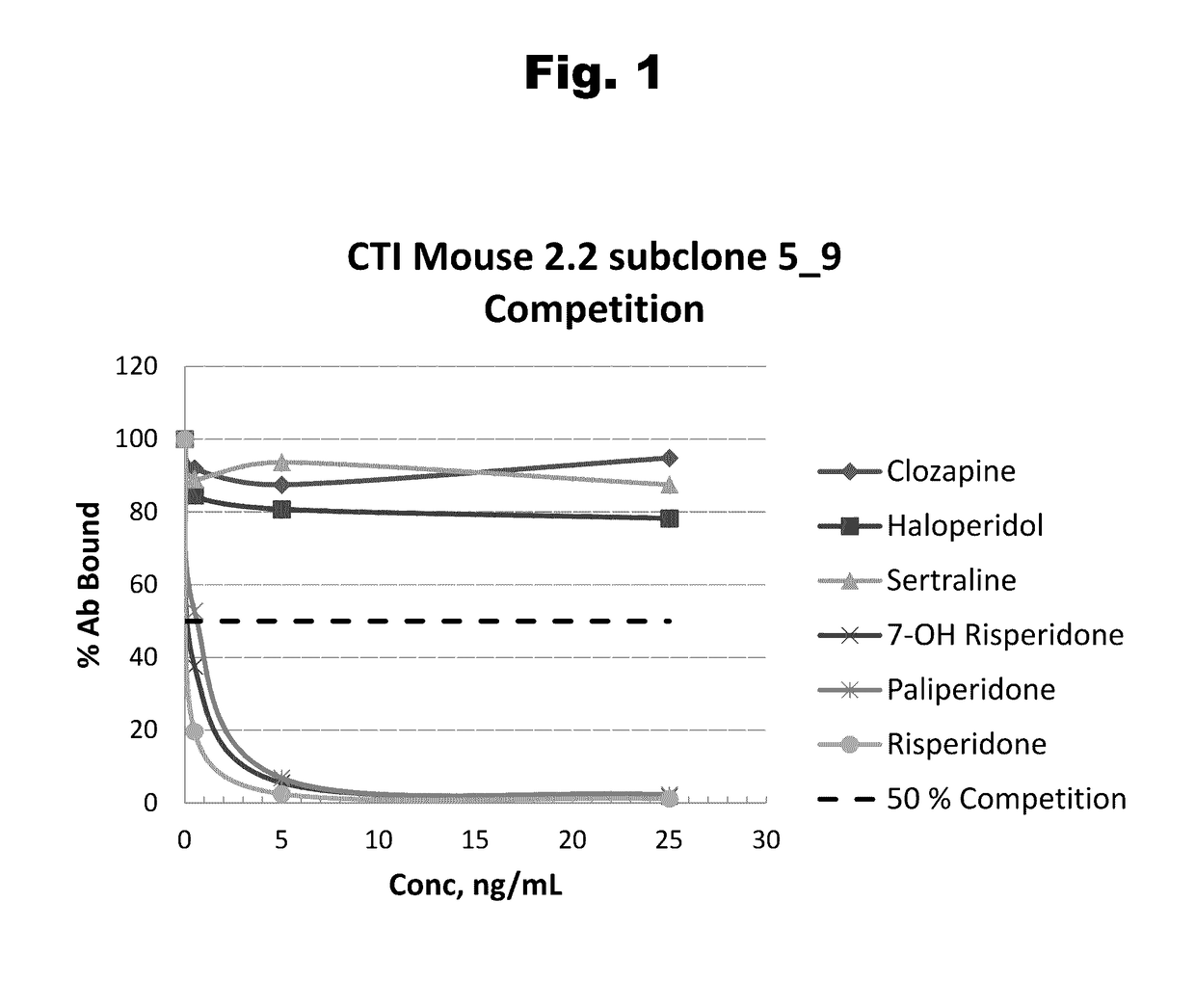
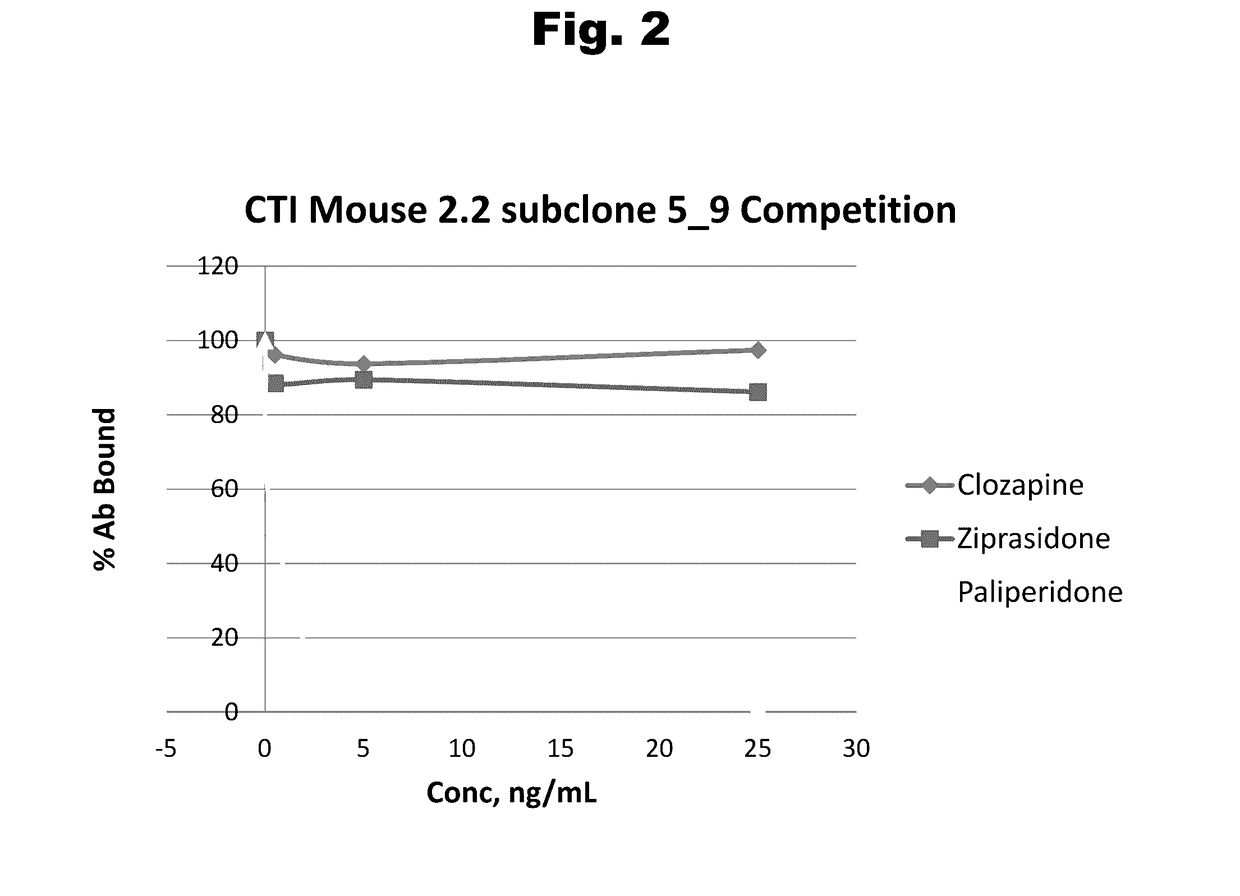
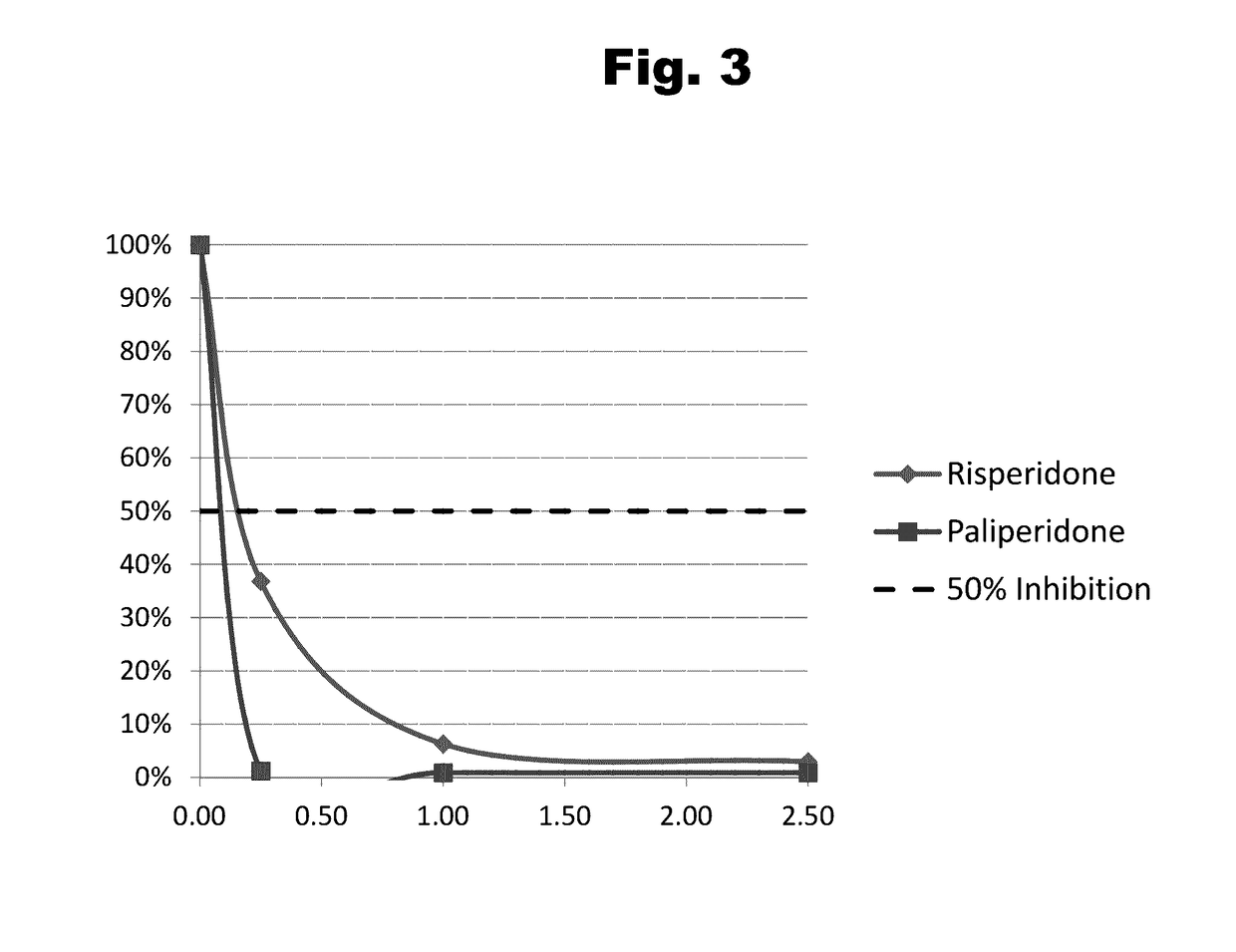
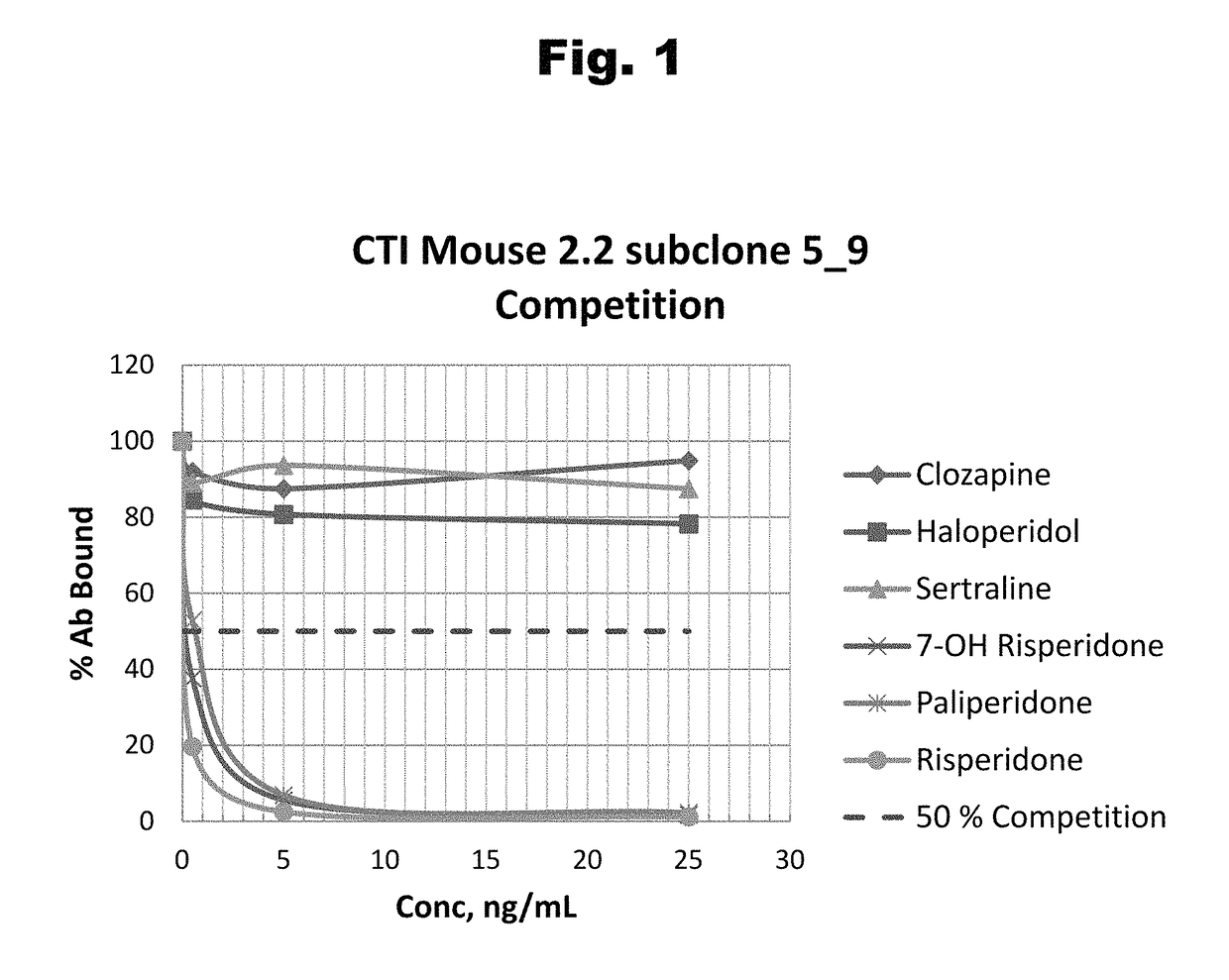
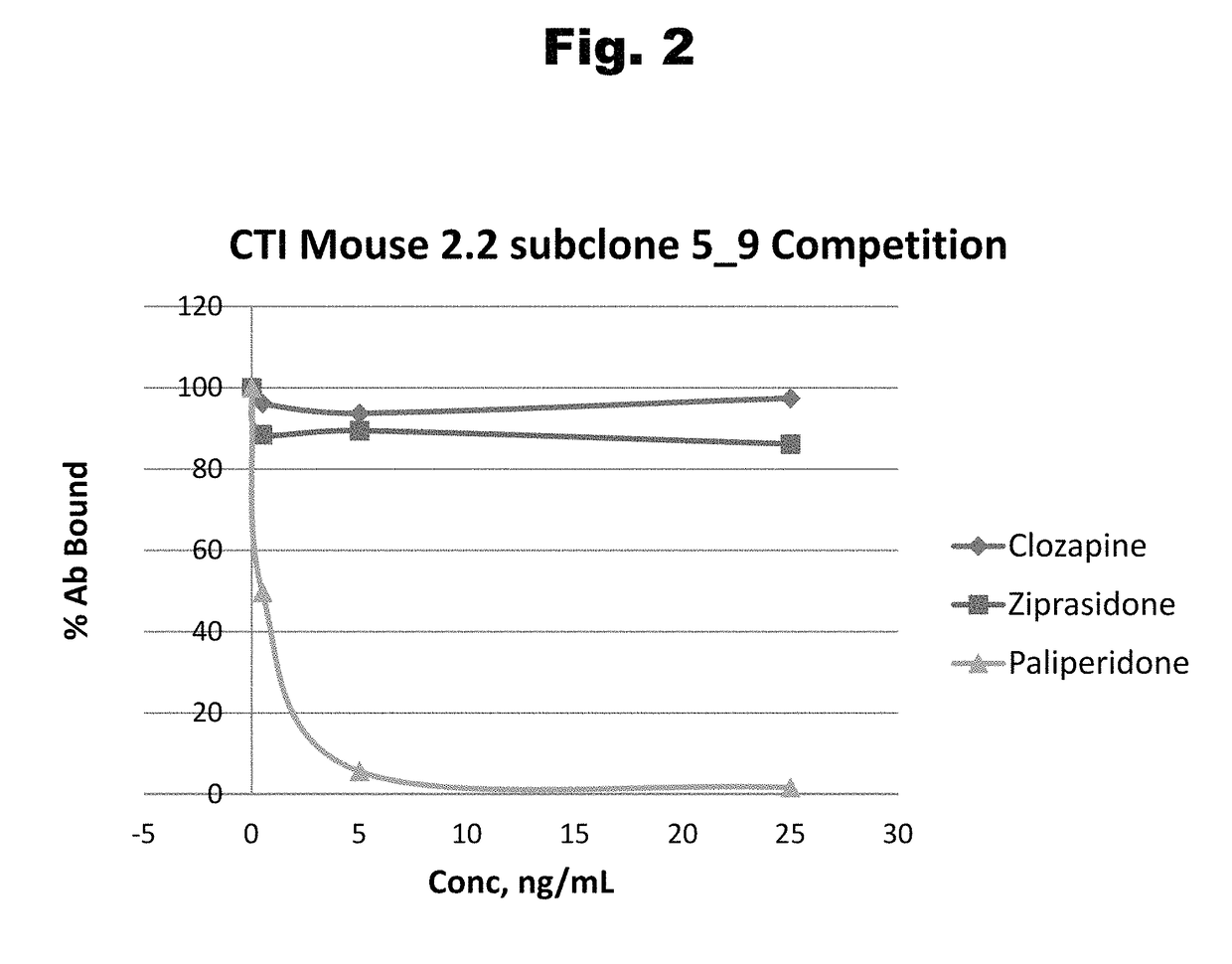
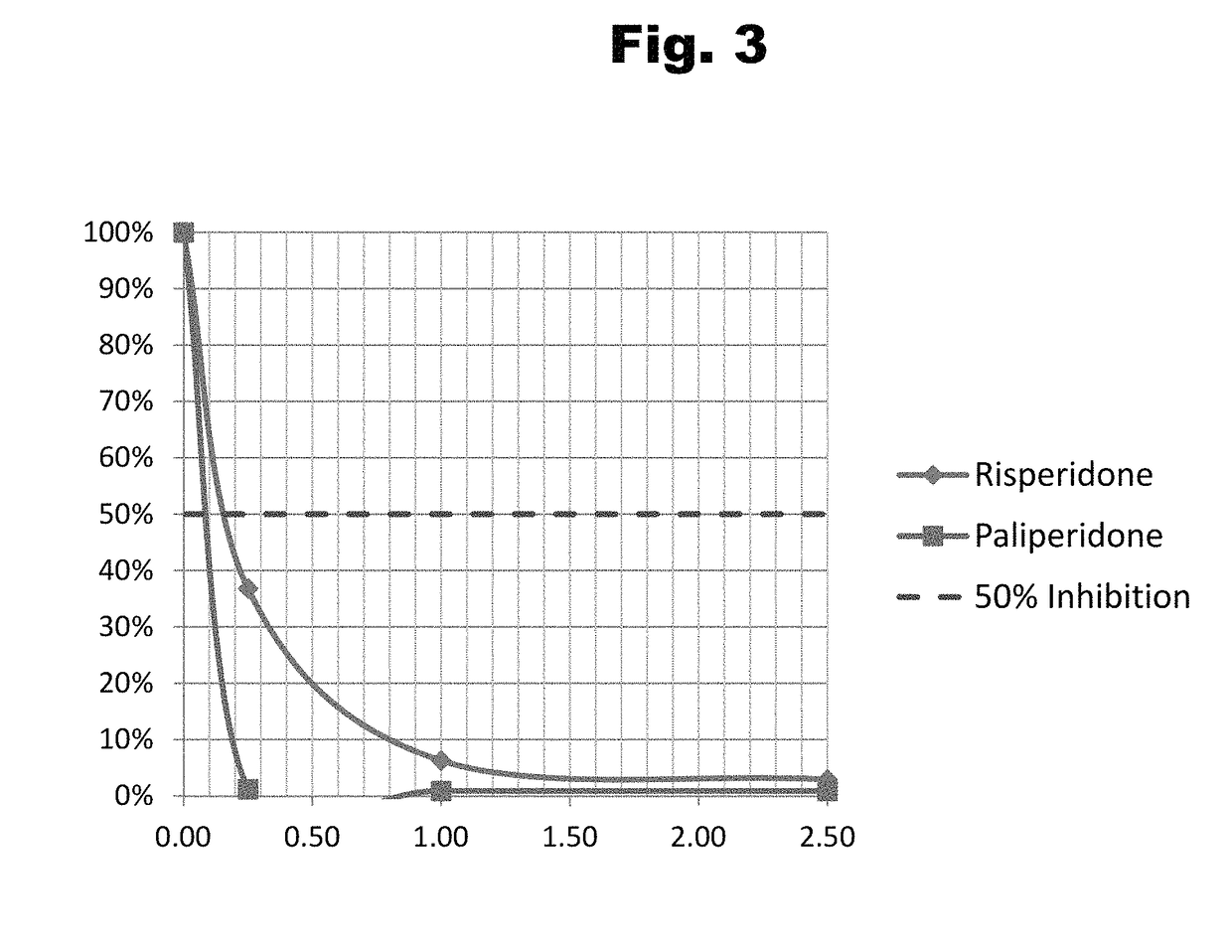
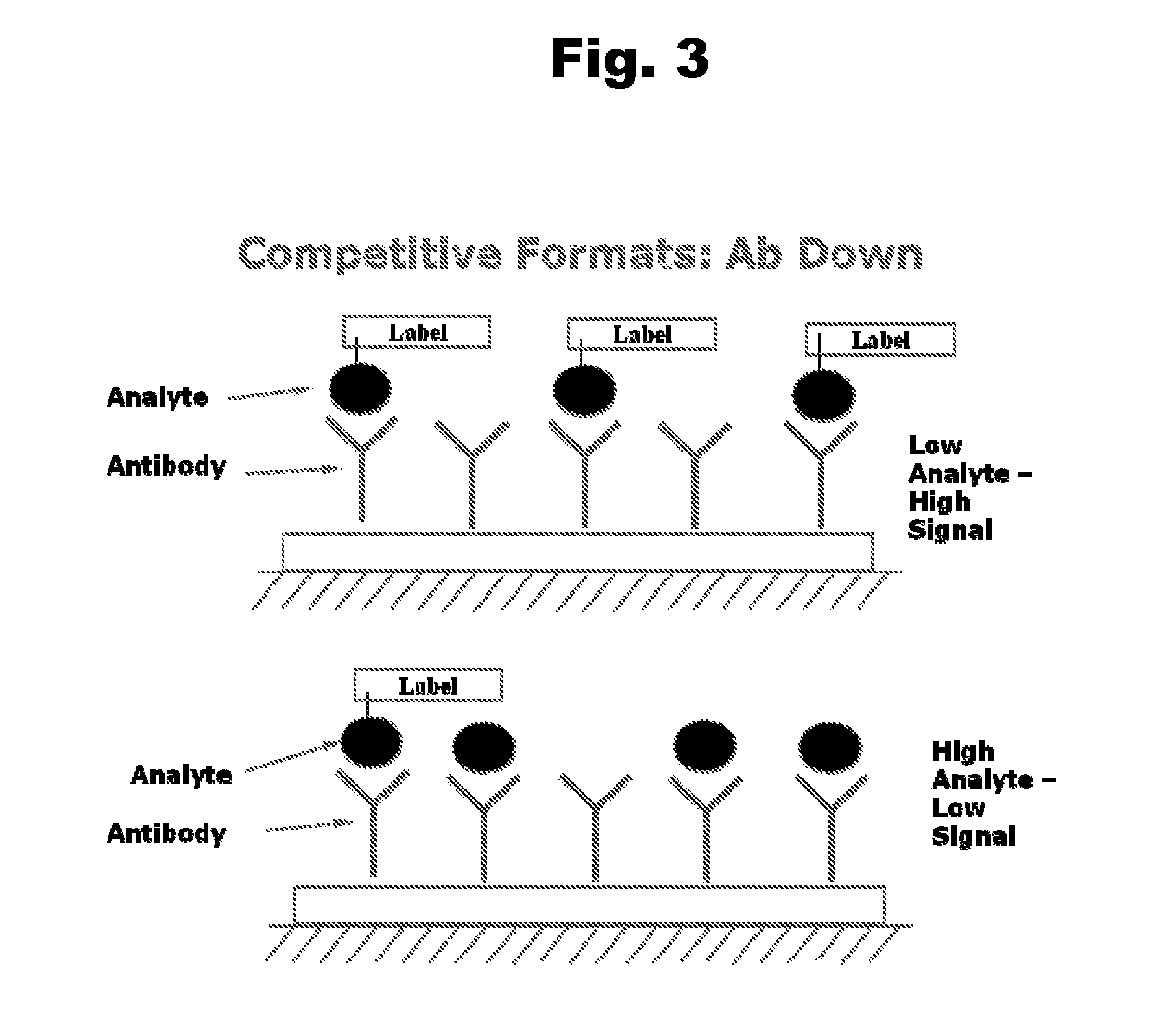
Antibodies to Risperidone Haptens and Use Thereof
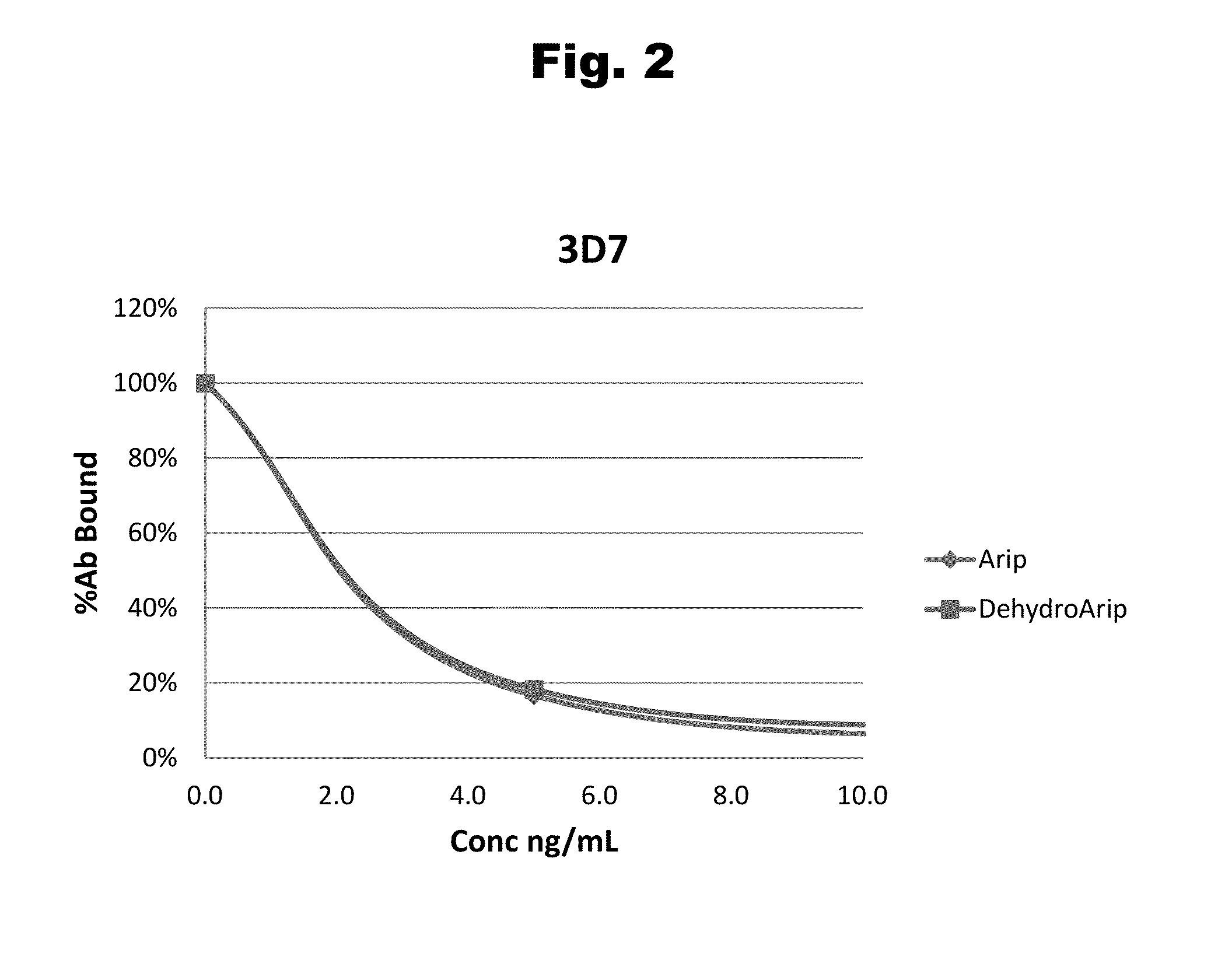
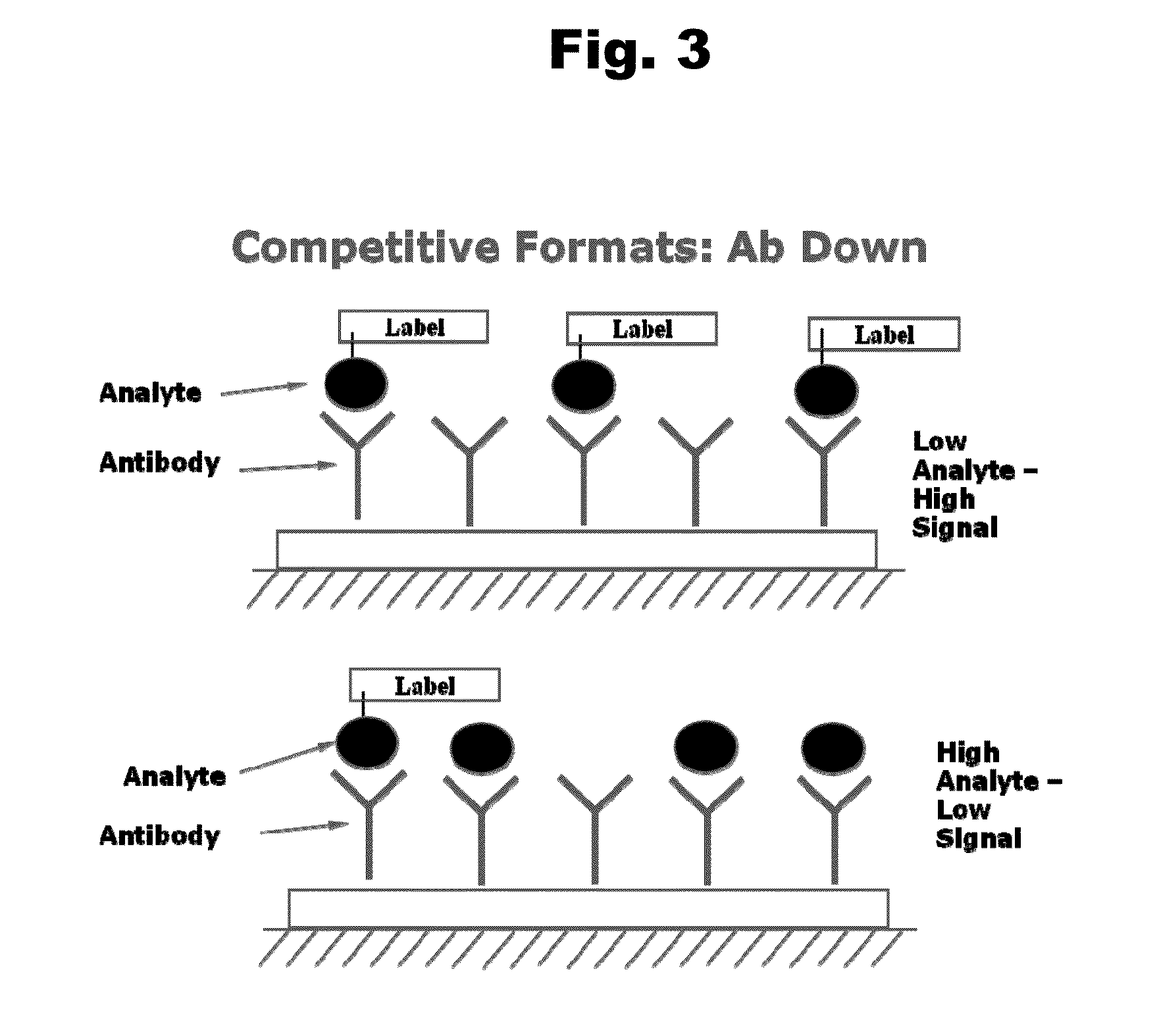
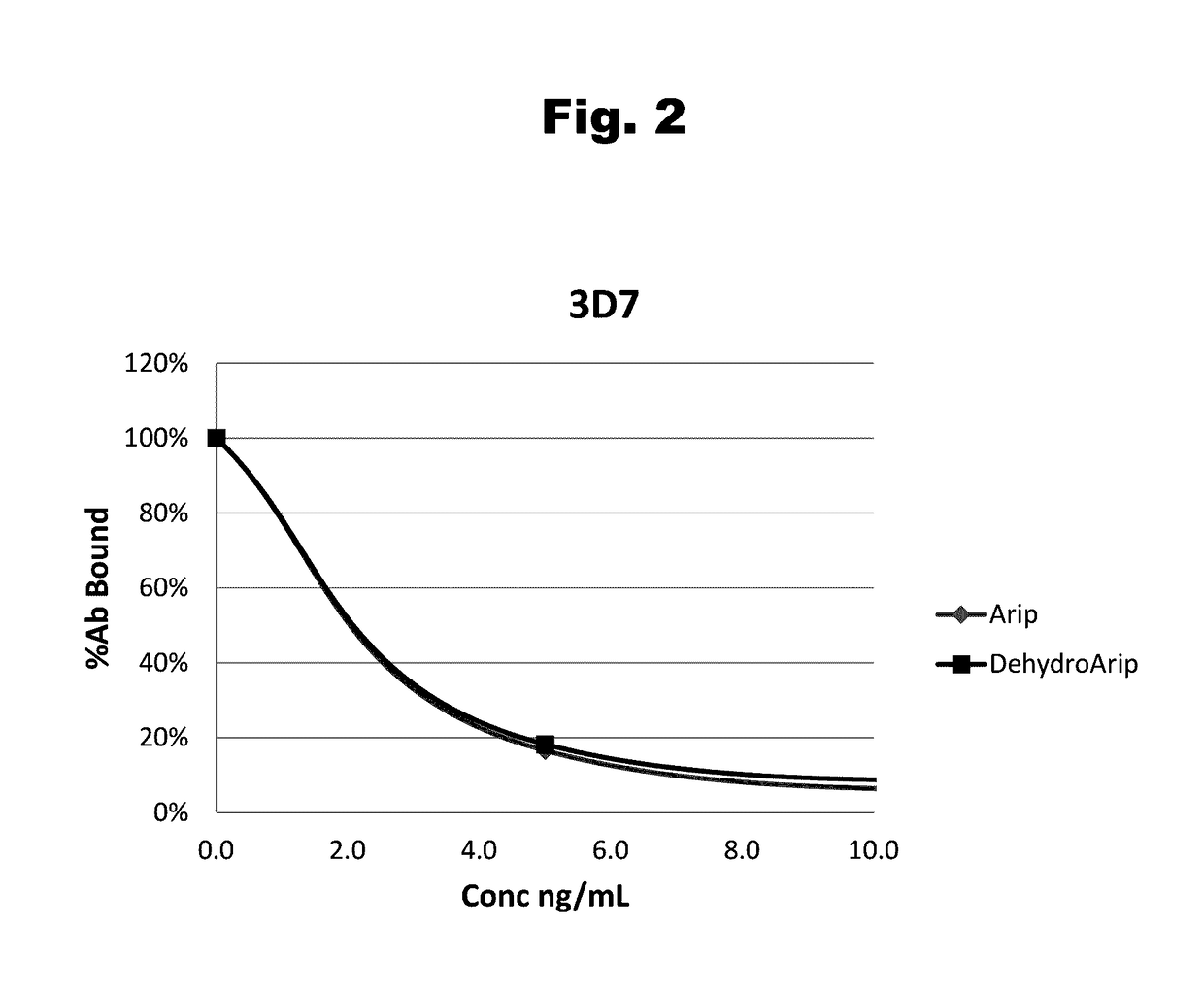
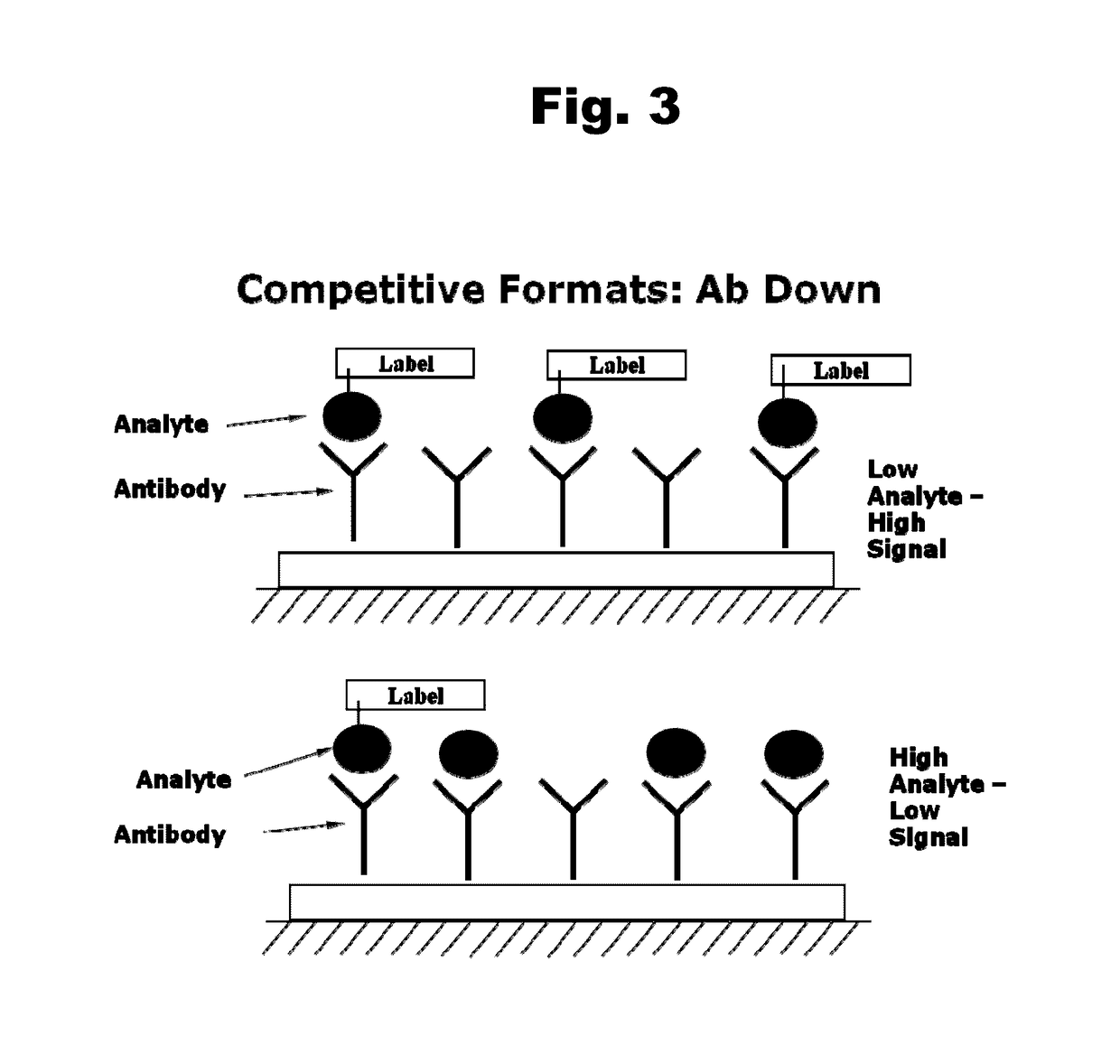
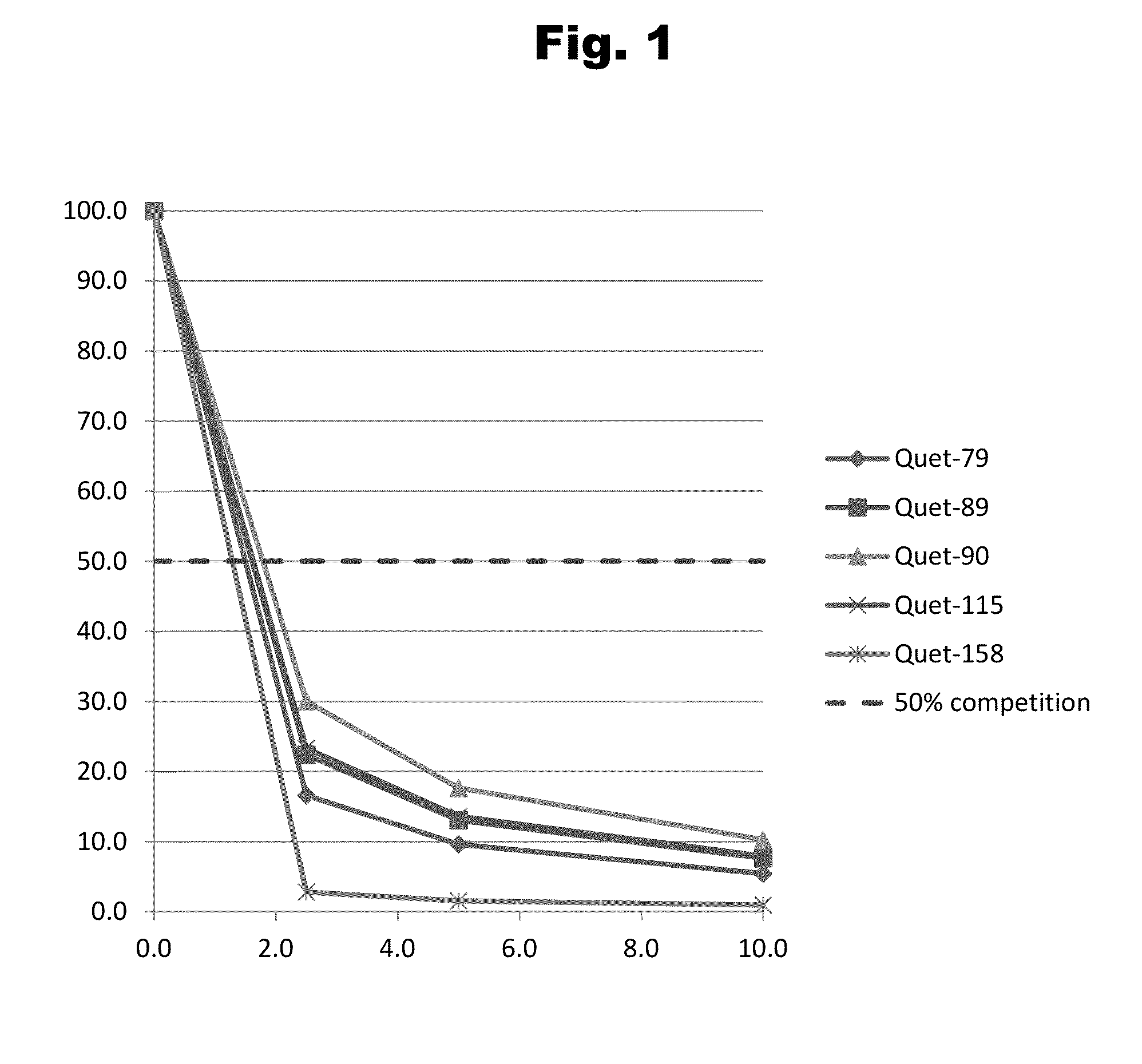
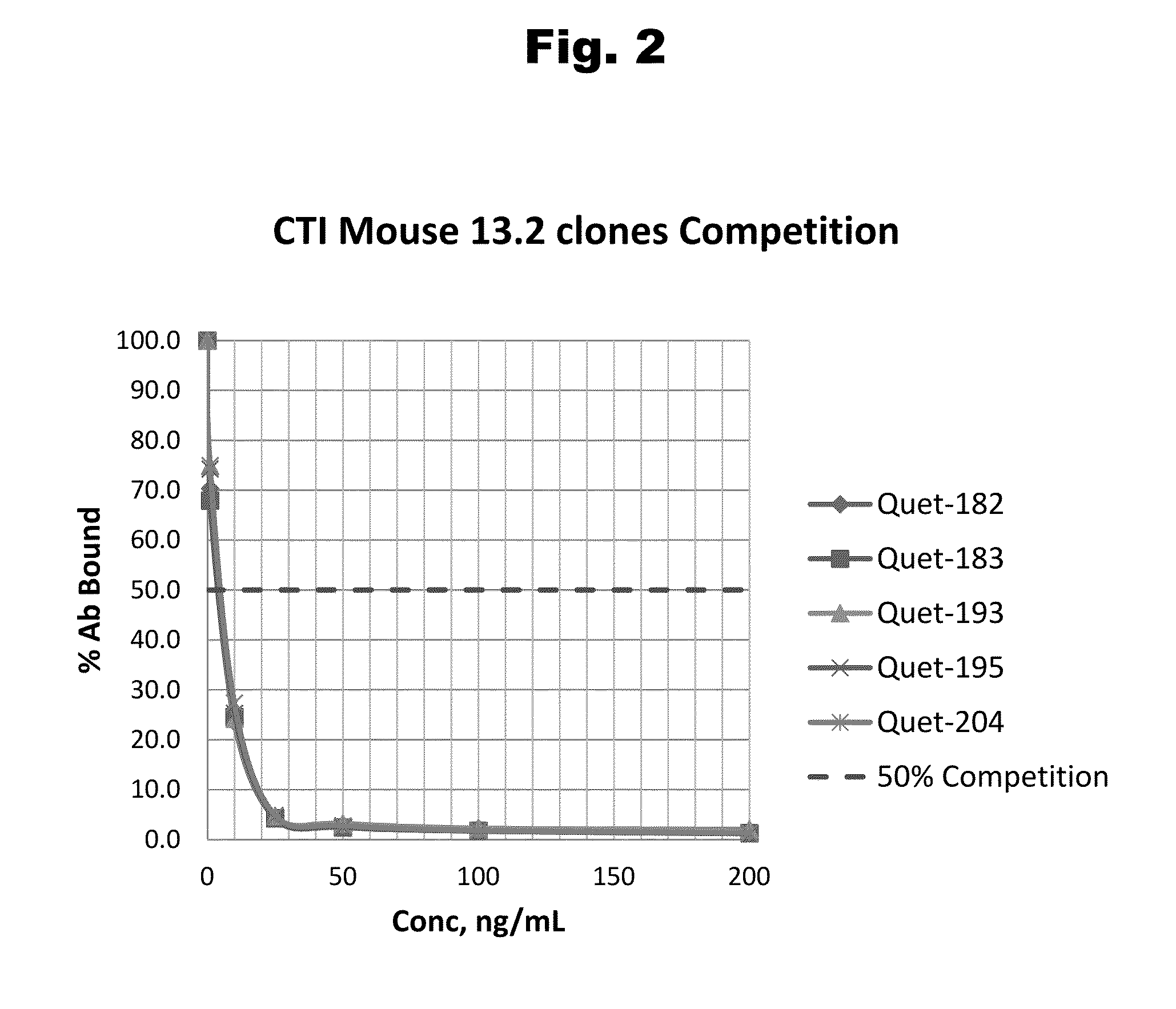
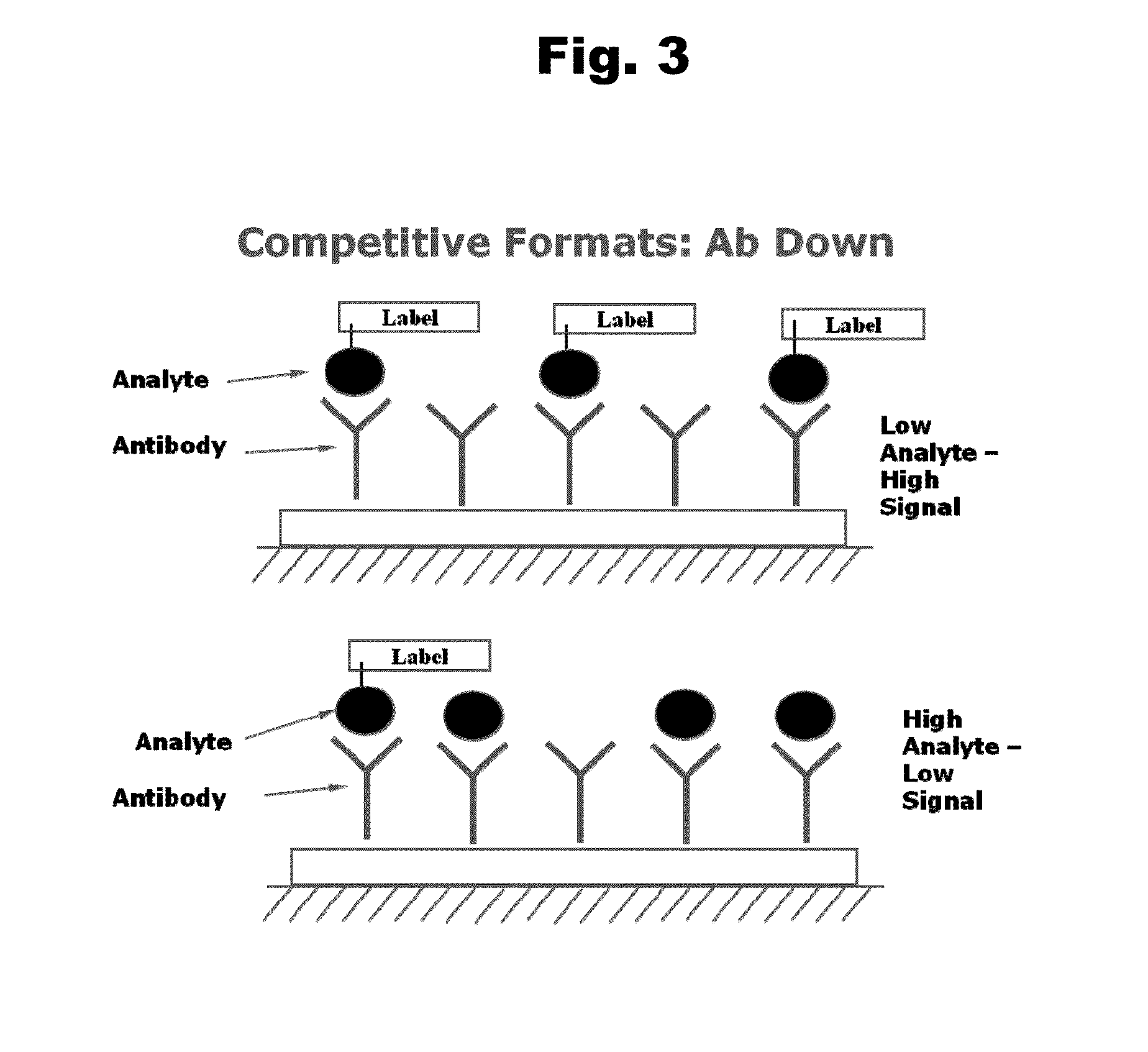
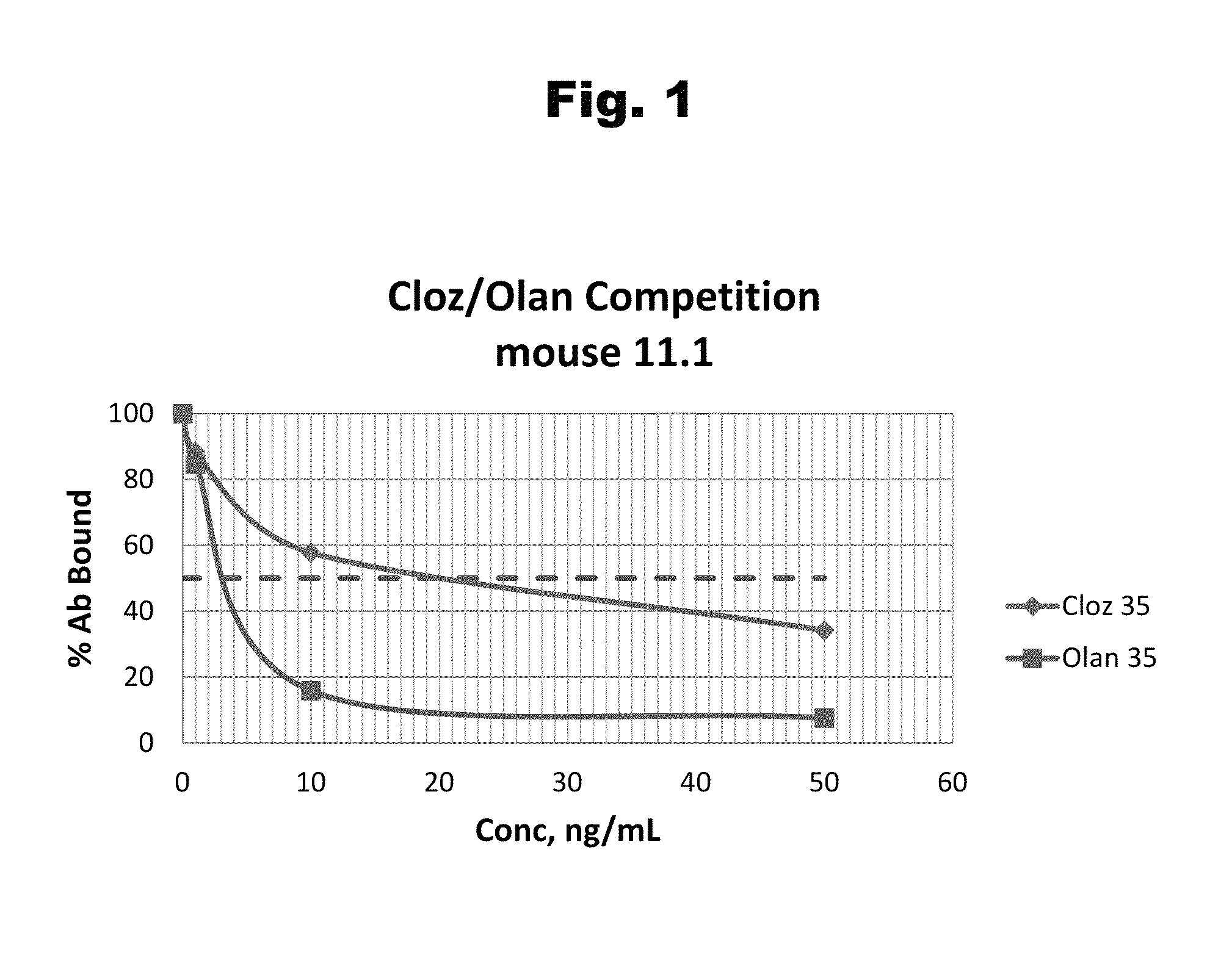
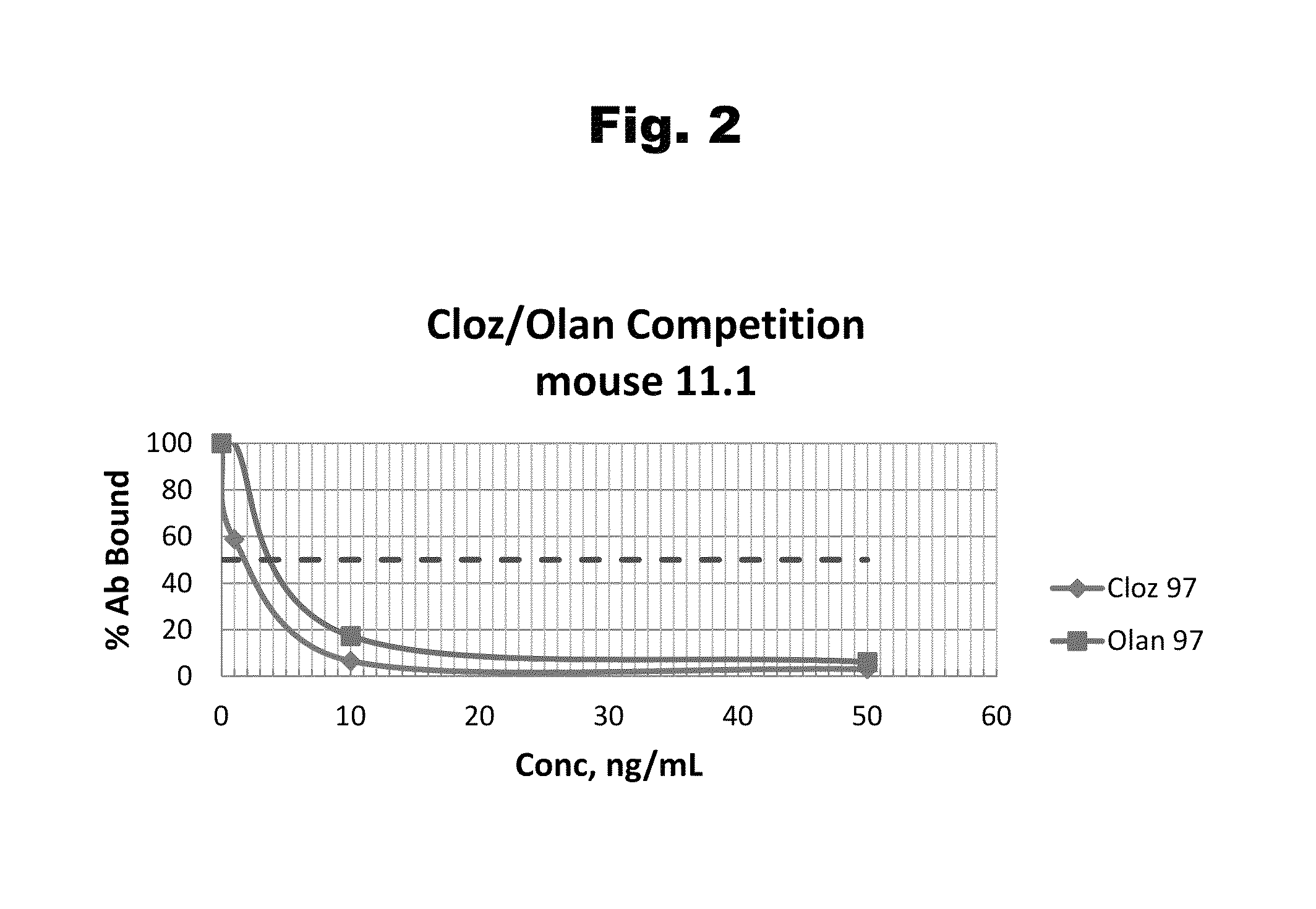
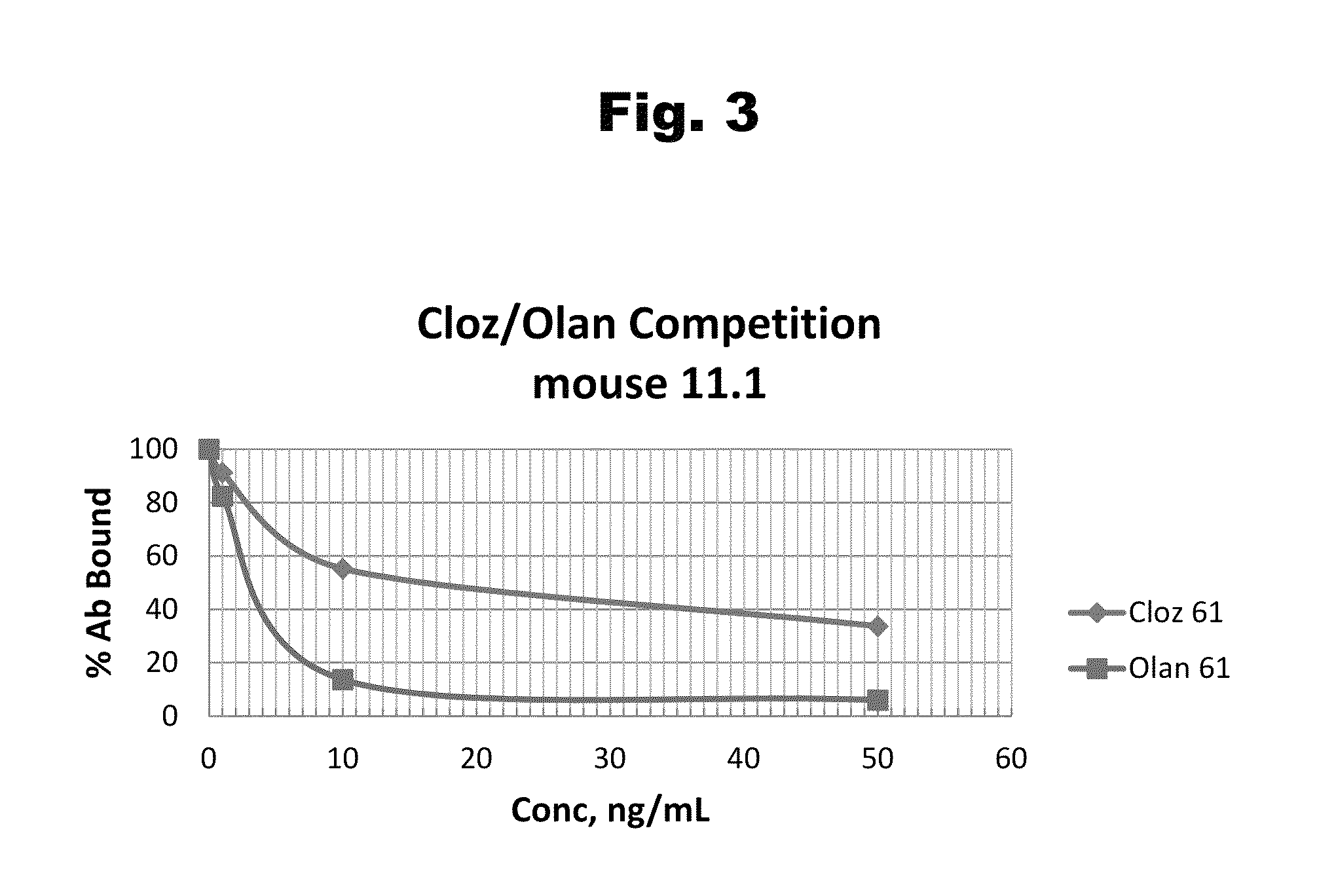
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Stable salts of olanzapine
InactiveUS20050272721A1Improve stabilityGood water solubilityBiocideNervous disorderMalonatePharmacology
Several salts of olanzapine, including olanzapine malonate, olanzapine glycolate, olanzapine maleate, and olanzapine benzoate, have been found to have favorable solid state characteristics.
Owner:SYNTHON IP
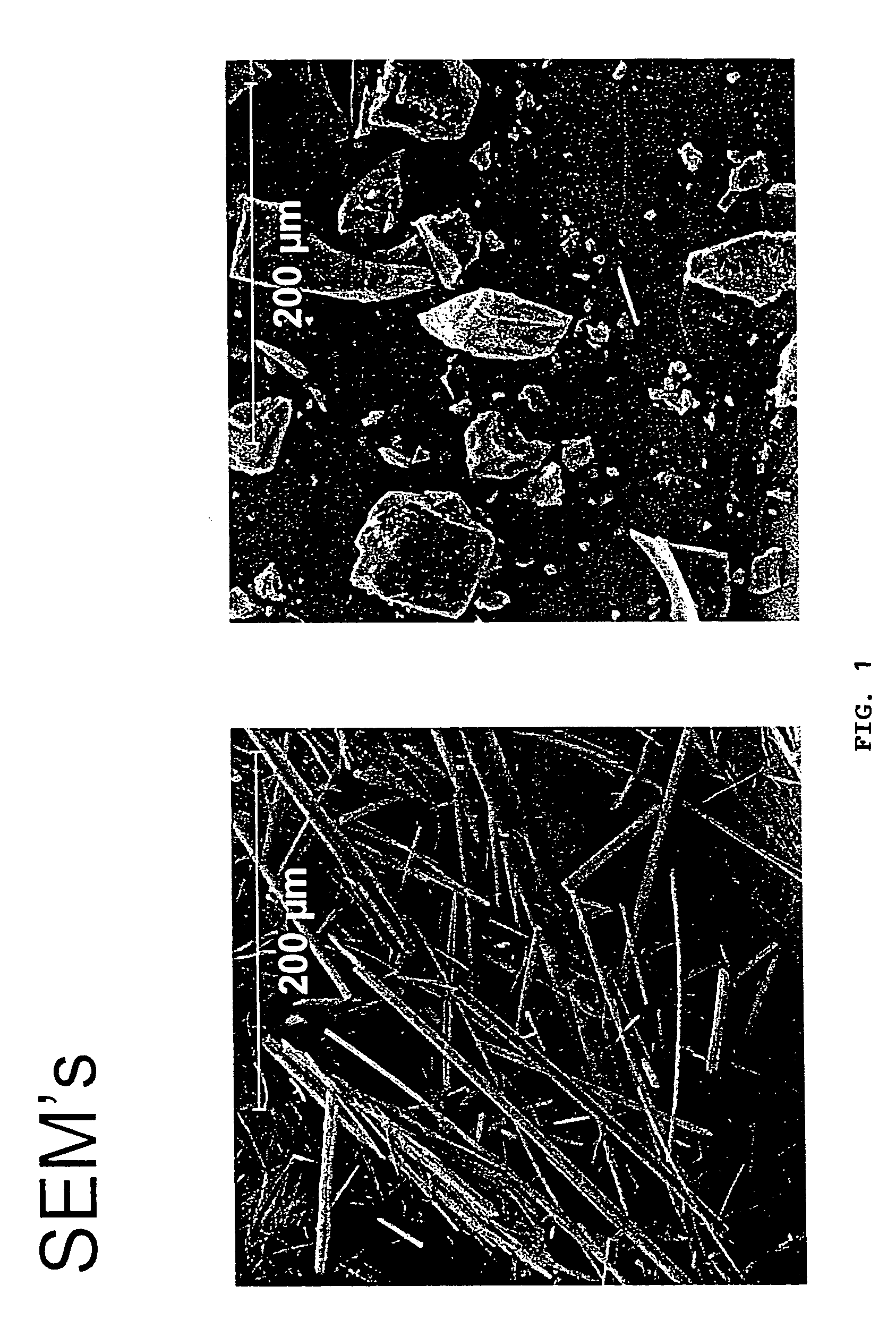
Aripiprazole, olanzapine and haloperidol pamoate salts
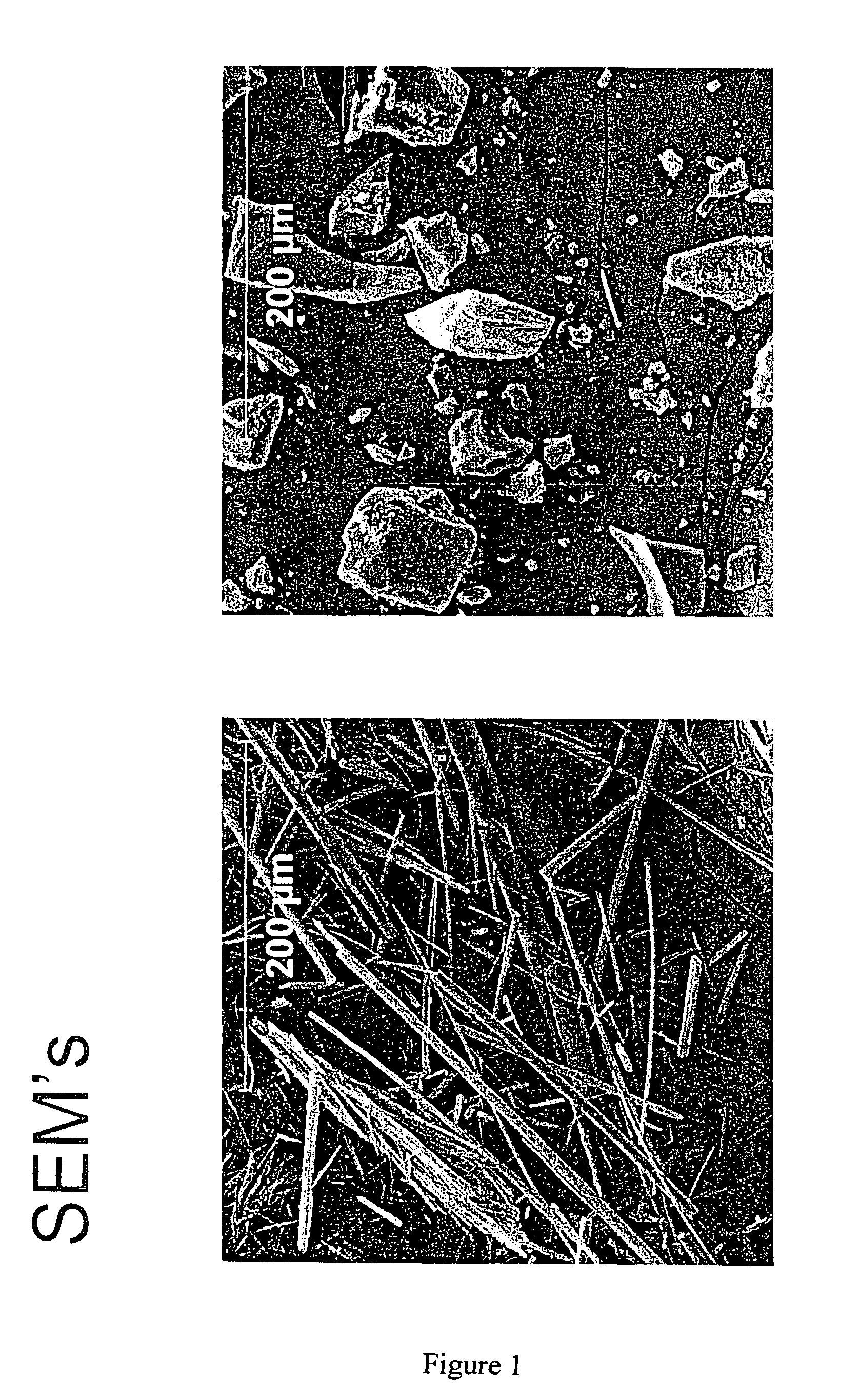
The invention relates to the discovery that pamoate salts of haloperidol and aripiprazole result in a good to superior long acting and / or extended release profile. Thus, in one aspect of the invention, the invention includes pamoate salts of haloperidol or aripiprazole. Preferably, the pamoate salt is characterized by a ratio of haloperidol to pamoate of 1:1 or 2:1. The pamoate salt can be crystalline, such as a needle or a dense crystal, such as described in the Figures.The invention further relates to methods of treating an individual in need thereof comprising administering a pharmaceutical composition comprising a pamoate salt of haloperidol and aripiprazole.
Owner:OTSUKA PHARM CO LTD
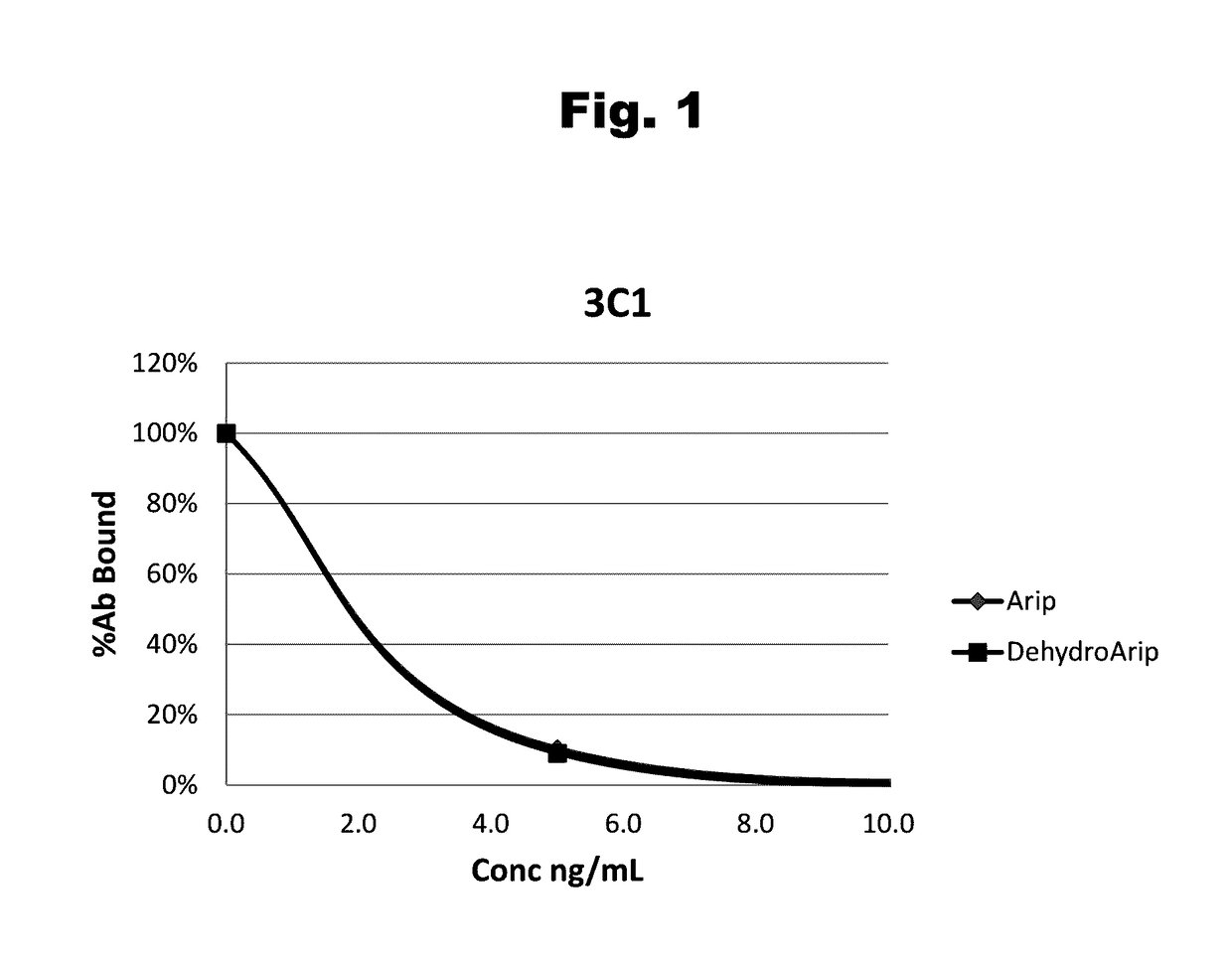
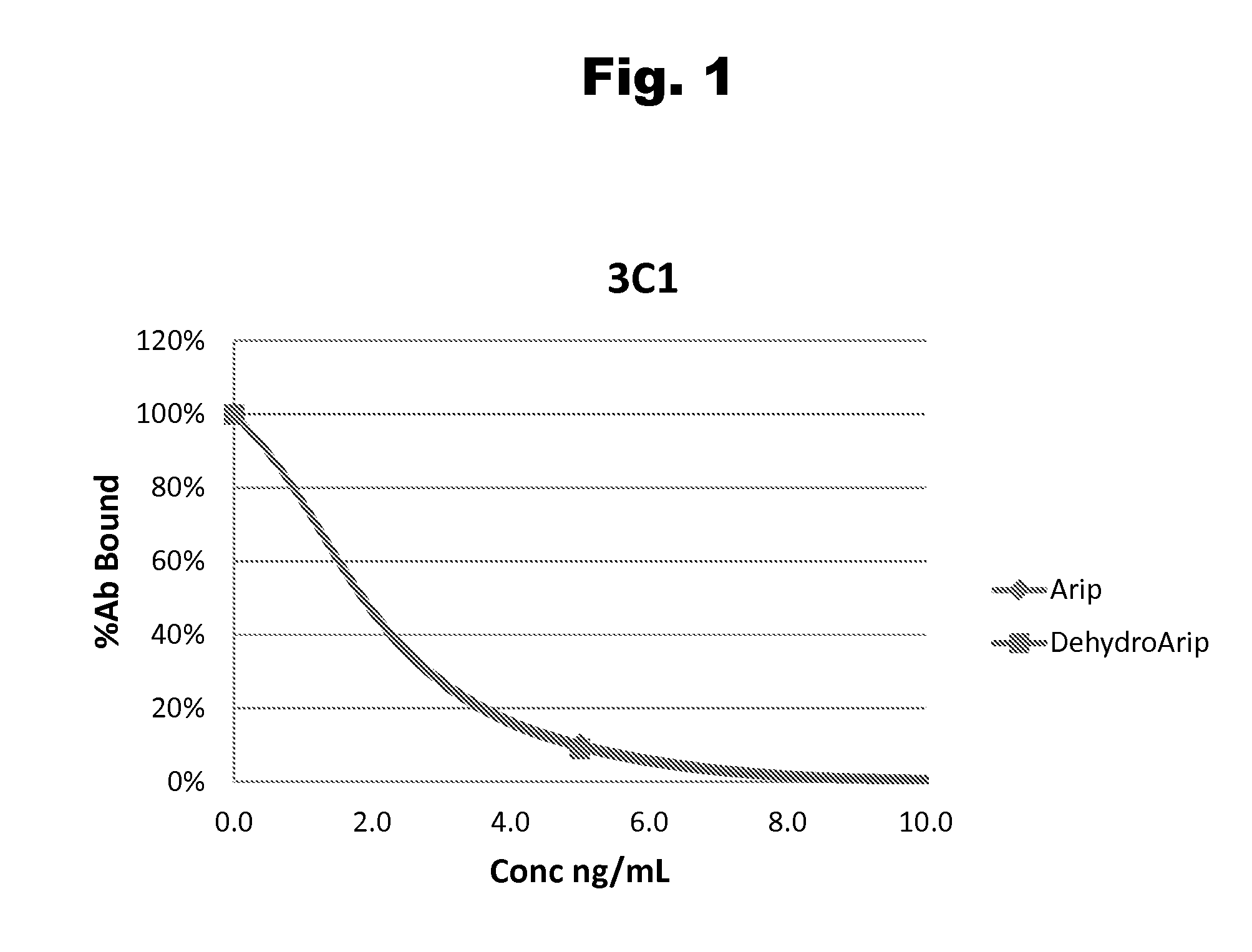
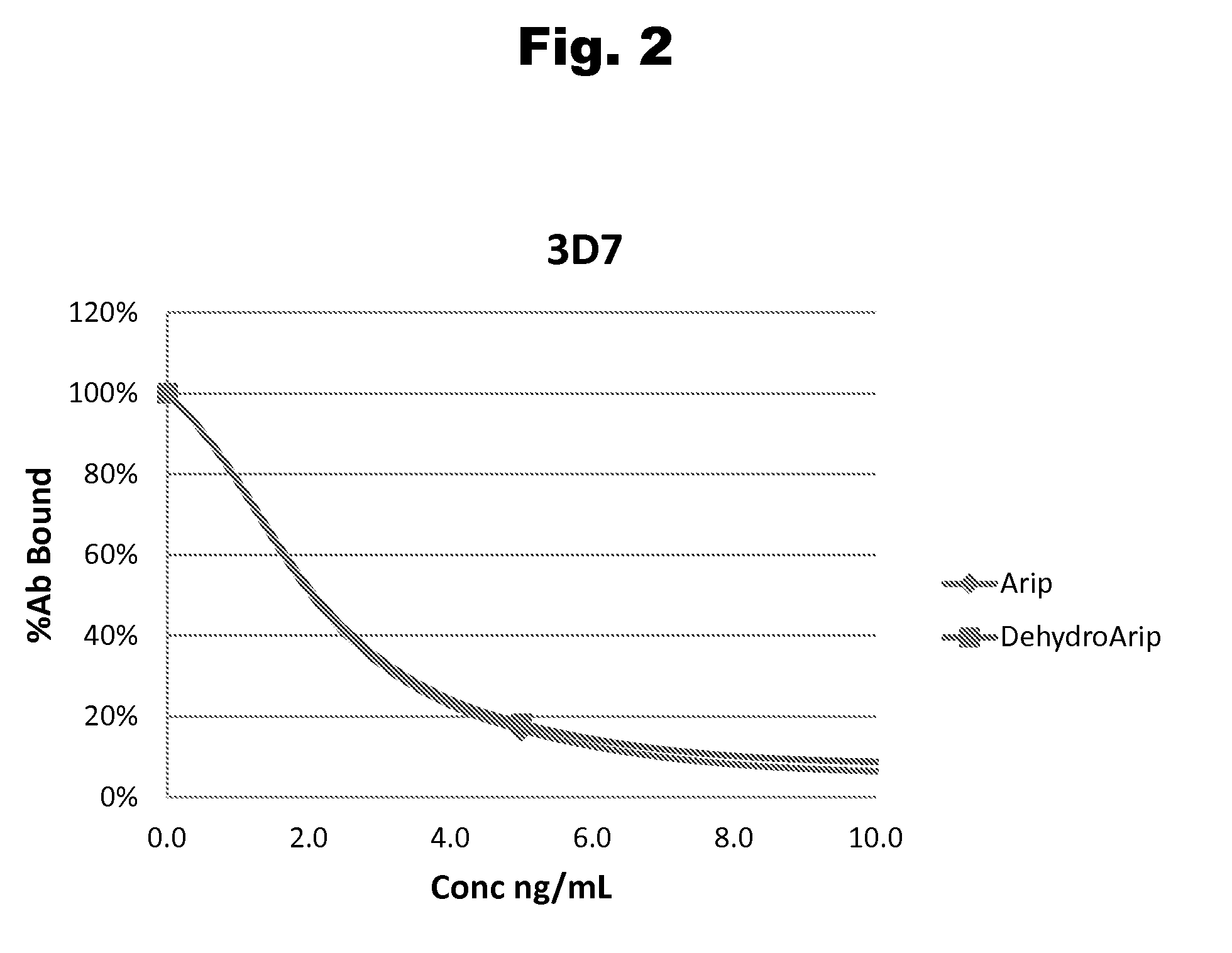
Antibodies to Aripiprazole Haptens and Use Thereof
ActiveUS20140057299A1Bioreactor/fermenter combinationsBiological substance pretreatmentsQuetiapineHapten
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
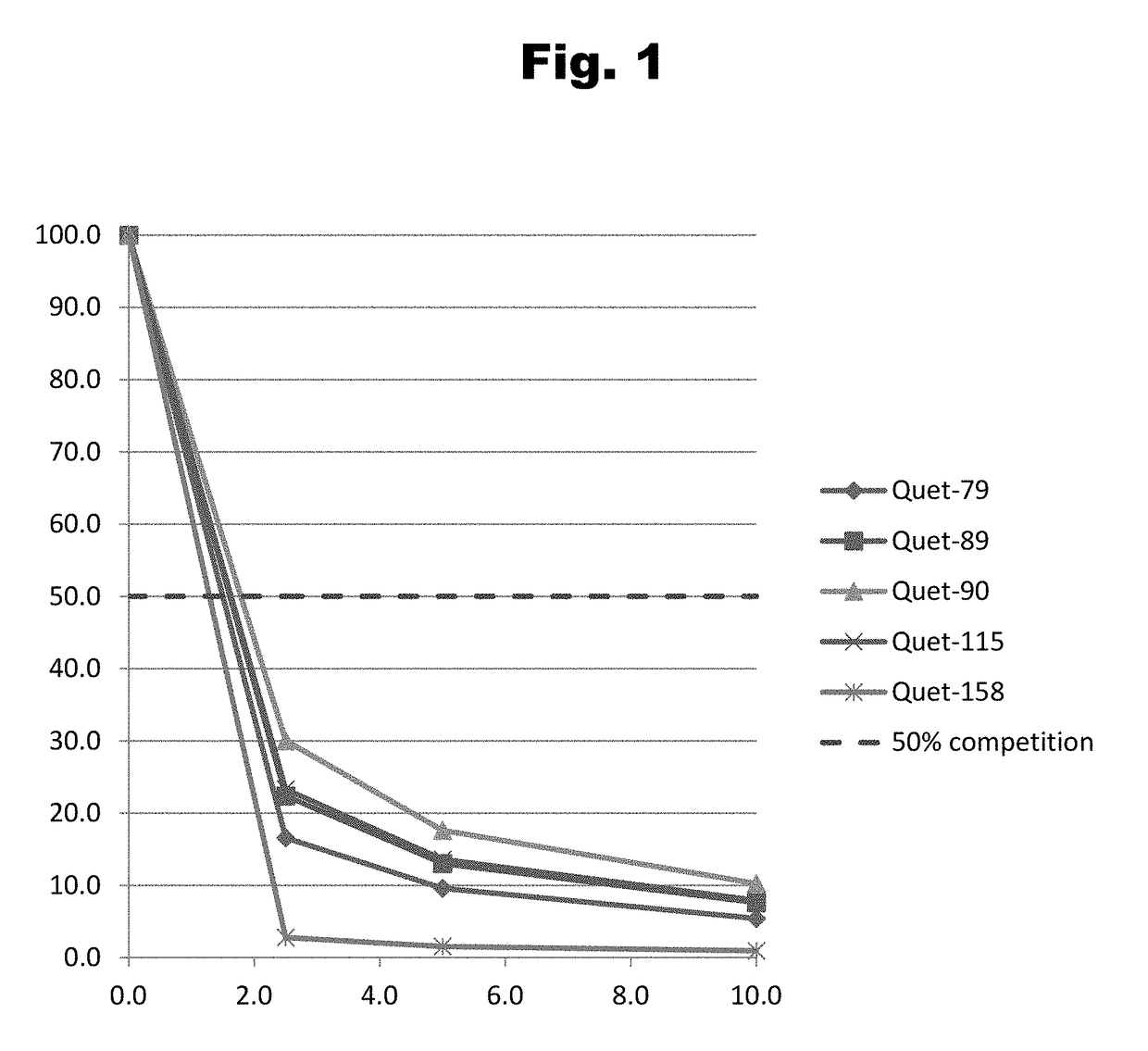
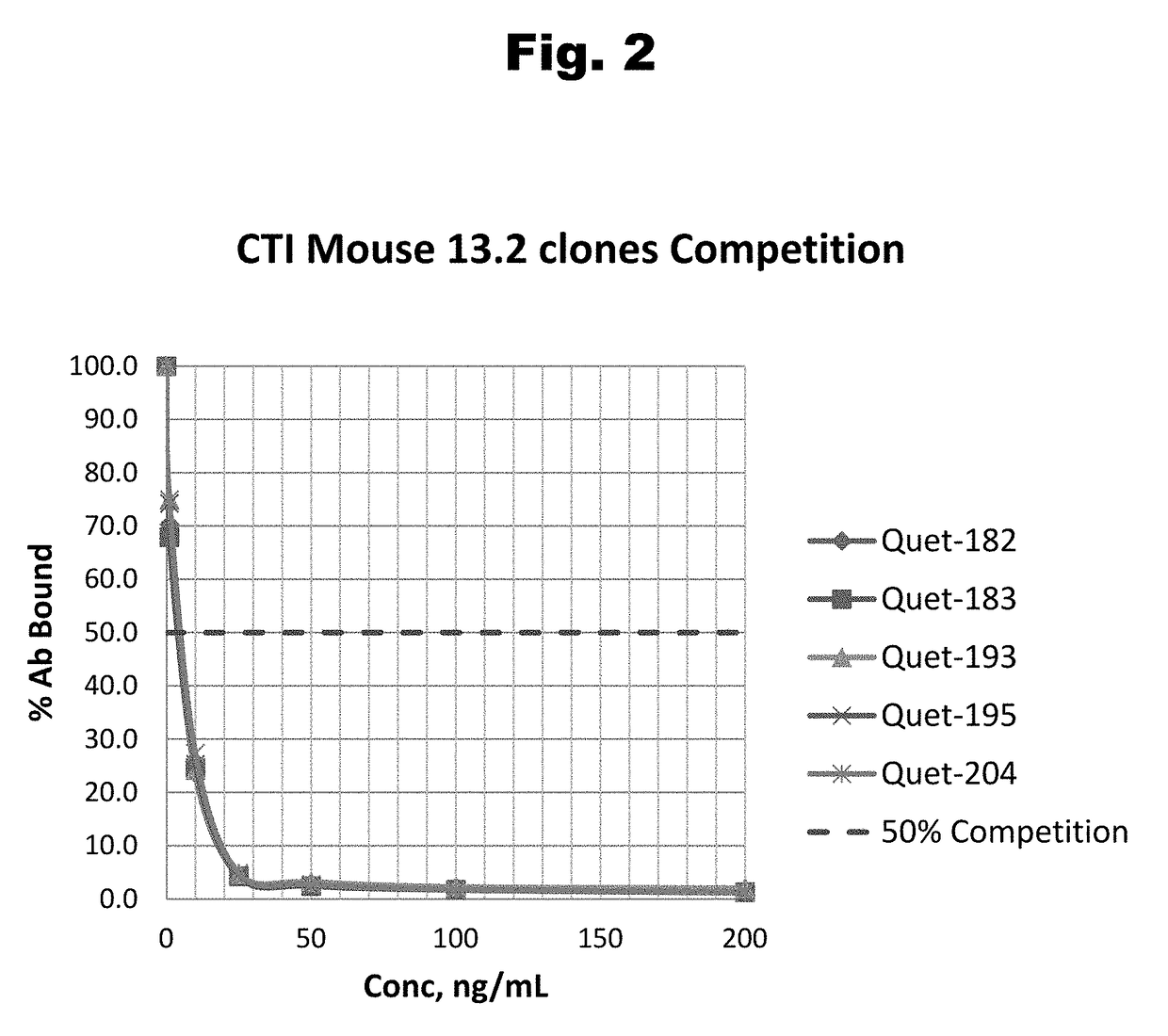
Antibodies to Quetiapine Haptens and Use Thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to Olanzapine and Use Thereof
Disclosed is an antibody which binds to olanzapine, which can be used to detect olanzapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of olanzapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Solid Pharmaceutical Dosage Form
InactiveUS20110028456A1High drug loadingEasy to manufacturePowder deliveryBiocideValsartanTrenbolone
A pharmaceutical composition comprising a solid unit dosage form comprising: one or more of pharmaceutically active ingredients selected from valacyclovir, olanzapine, voriconazole, topotecan, artesunate, amodiaquine, guggulosterone, ramipril, telmisartan, tibolone, atorvastatin, simvastatin, amlodipine, ezetimibe, fenofibrate, tacrolimus, valgancyclovir, valsartan, clopidrogel, estradiol, trenbolone, efavirenz, metformin, pseudoephedrine, verapamil, felodipine, valproic acid / sodium valproate, mesalamine, hydrochlorothiazide, levosulpiride, nelfinavir, cefixime and cefpodoxime proxetil in combination with a water insoluble polymer and / or a water soluble polymer. Methods for making the pharmaceutical composition are also disclosed.
Owner:CIPLA LTD
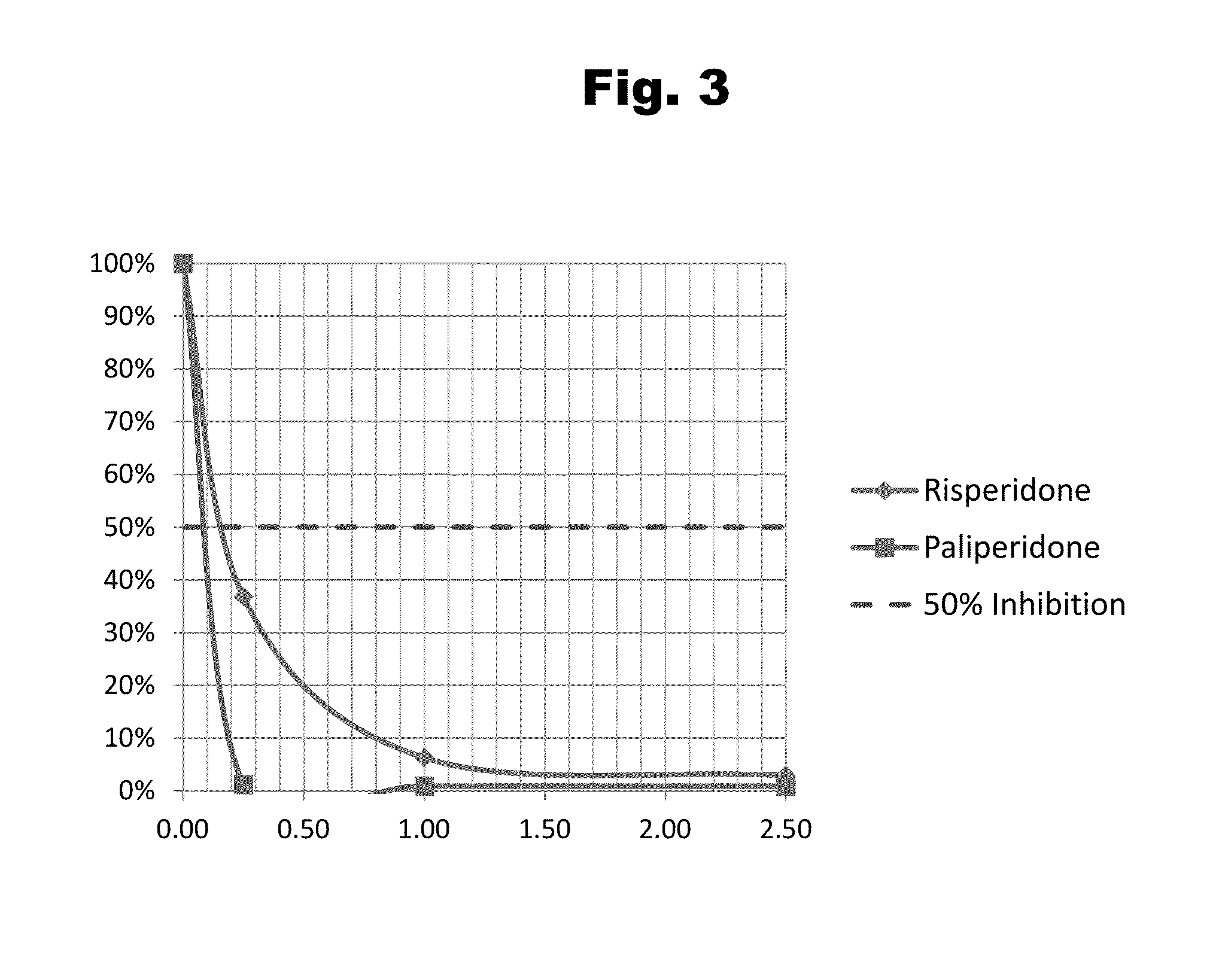
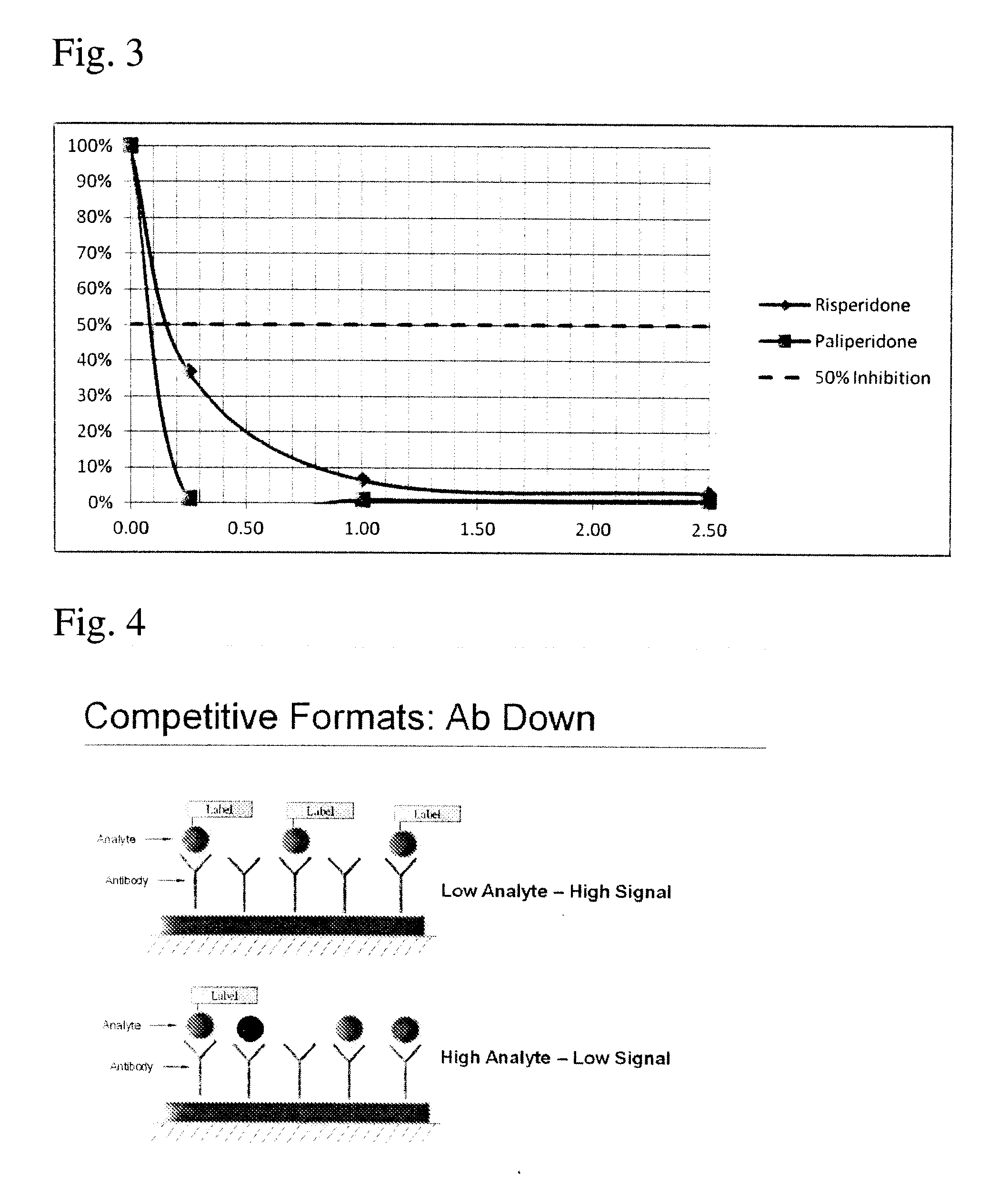
Antibodies to Paliperidone and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Compositions of 5-ht3 antagonists and dopamine d2 antagonists for treatment of dopamine-associated chronic conditions
InactiveUS20080004291A1Relieve distressPoor quality of lifeBiocideOrganic active ingredientsDisease5-HT3 antagonist
The present invention provides novel compositions comprising a combination of a 5-HT3 receptor antagonist and a selective dopamine D2 receptor antagonist for the treatment of obsessive, impulsive and compulsive behavioral activities and other dopamine pathway-associated disorders or conditions. Preferably, the pharmaceutical compositions of the present invention comprise amounts of the 5-HT3 receptor antagonist ondansetron and a selective dopamine D2 receptor antagonist, such as risperidone or olanzapine, that are sufficient to control a subjects obsessive, impulsive and compulsive behavioral activities. Kits comprising the combination of antagonists for the treatment of addictive disorders such as alcohol dependence are also provided.
Owner:TRANSCEPT PHARMA
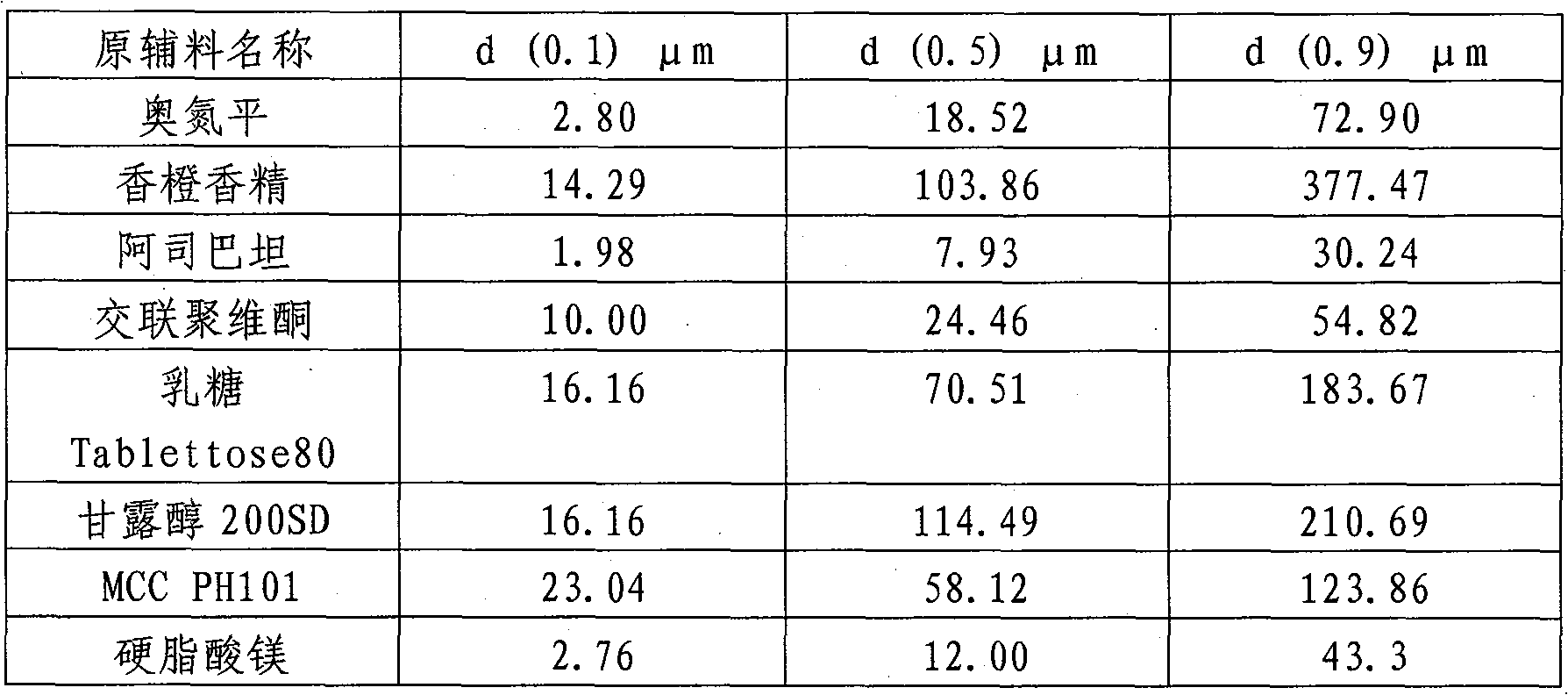
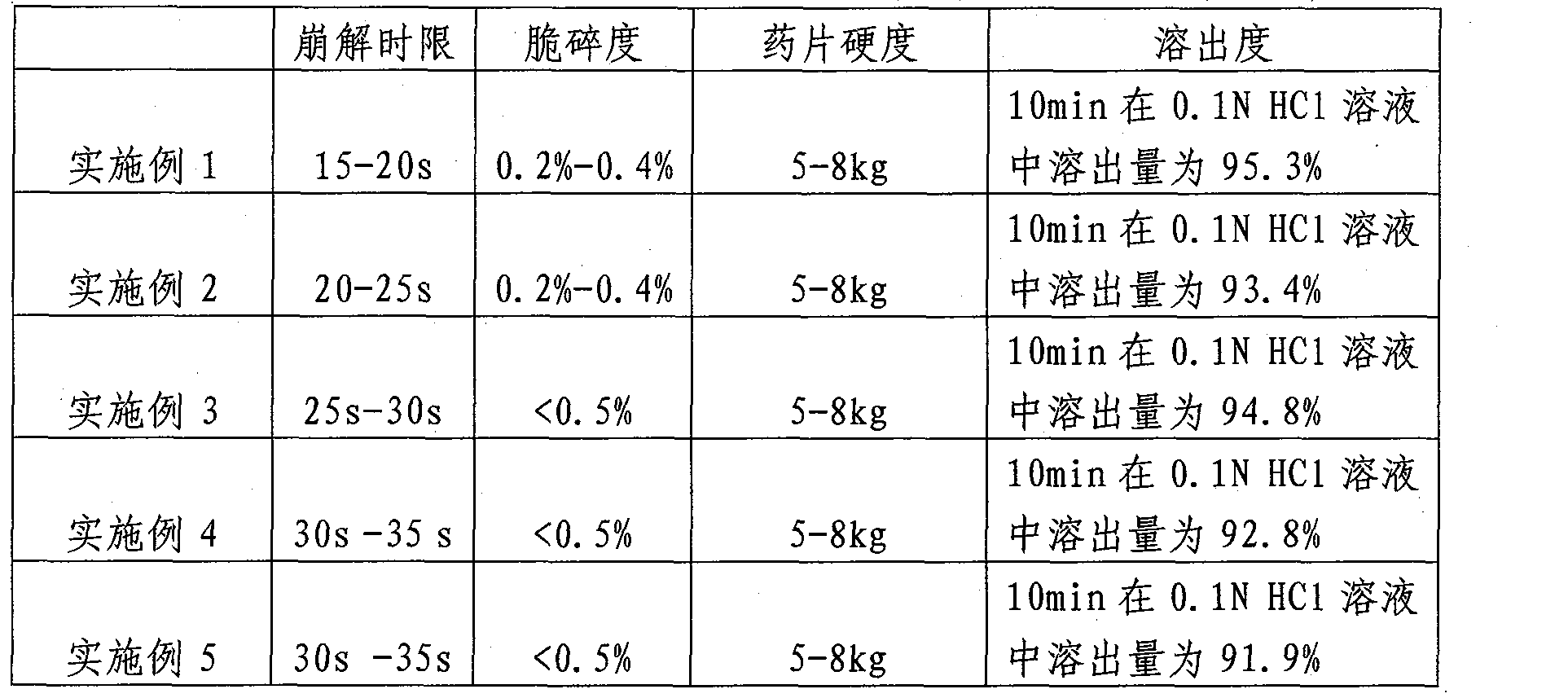
Olanzapine orally-disintegrating tablet preparation and preparation method thereof
ActiveCN101904824ASimple preparation processLow costOrganic active ingredientsNervous disorderMANNITOL/SORBITOLAdhesive
The invention belongs to the technical field of medicines and relates to an olanzapine orally-disintegrating tablet preparation and a preparation method thereof. The orally-disintegrating tablet comprises the following components in weight percentage: 2.5%-10% of olanzapine, 35%-74% of mannitol, 10%-40% of lactose, 0-10% of dry adhesives, 2.0%-10% of disintegrating agents, 0.1%-6% of flavoring agents and 0.4%-3% of lubricants. A dry-method direct tablet compressing technology and a dry-compressing particle tablet compressing technology can be adopted in the preparation process. The invention aims to provide an olanzapine orally-disintegrating tablet having simple preparation process, low cost, convenient taking and rapid effect taking. After being orally taken, the olanzapine orally-disintegrating tablet is rapidly disintegrated and dispersed into fine particles or powder and is especially suitable for patients with dysphagia and psychopaths; as the preparation exists in a fine particle or powder form before arriving at gastrointestinal tracts, medicines have quickened dissolution, large distribution area in the gastrointestinal tracts and more adsorption points; and for indissoluble medicines, such as the olanzapine, the bioavailability of the indissoluble medicines can be improved.
Owner:QILU PHARMA
Compositions of 5-HT3 antagonists and dopamine D2 antagonists for treatment of dopamine-associated chronic conditions
InactiveUS20080004260A1Relieve distressPoor quality of lifeBiocideNervous disorder5-HT3 antagonistDopamine
The present invention provides novel compositions comprising a combination of a 5-HT3 receptor antagonist and a selective dopamine D2 receptor antagonist for the treatment of alcohol dependence and other dopamine pathway-associated disorders or conditions. Preferably, the pharmaceutical compositions of the present invention comprise amounts of the 5-HT3 receptor antagonist ondansetron and the selective dopamine D2 receptor antagonist olanzapine that are sufficient to control a subject's craving for alcohol or other addictive substances. Kits comprising the combination of antagonists for the treatment of addictive disorders such as alcohol dependence are also provided.
Owner:TRANSCEPT PHARMA
Antibodies to Paliperidone Haptens and Use Thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, risperidone and paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
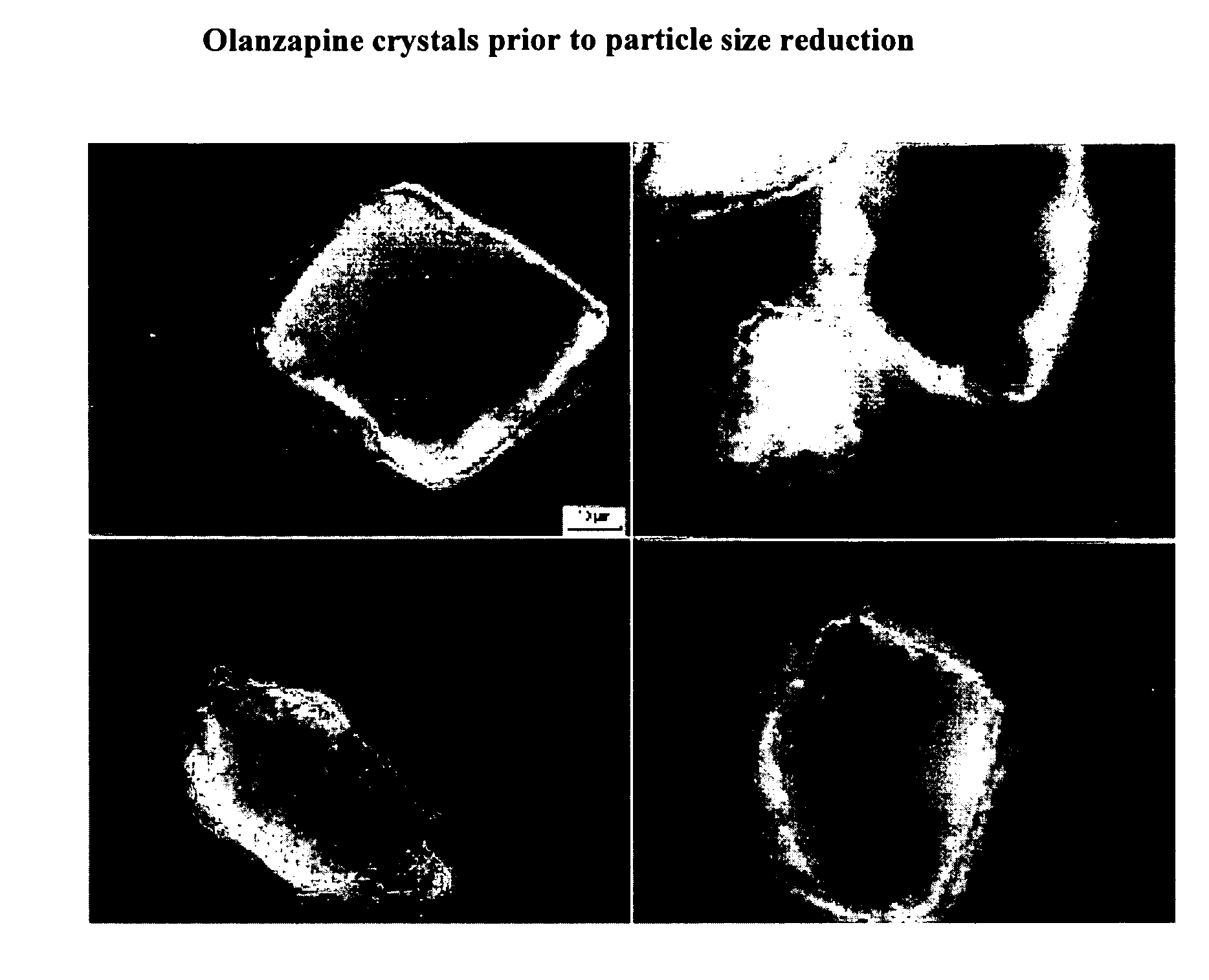
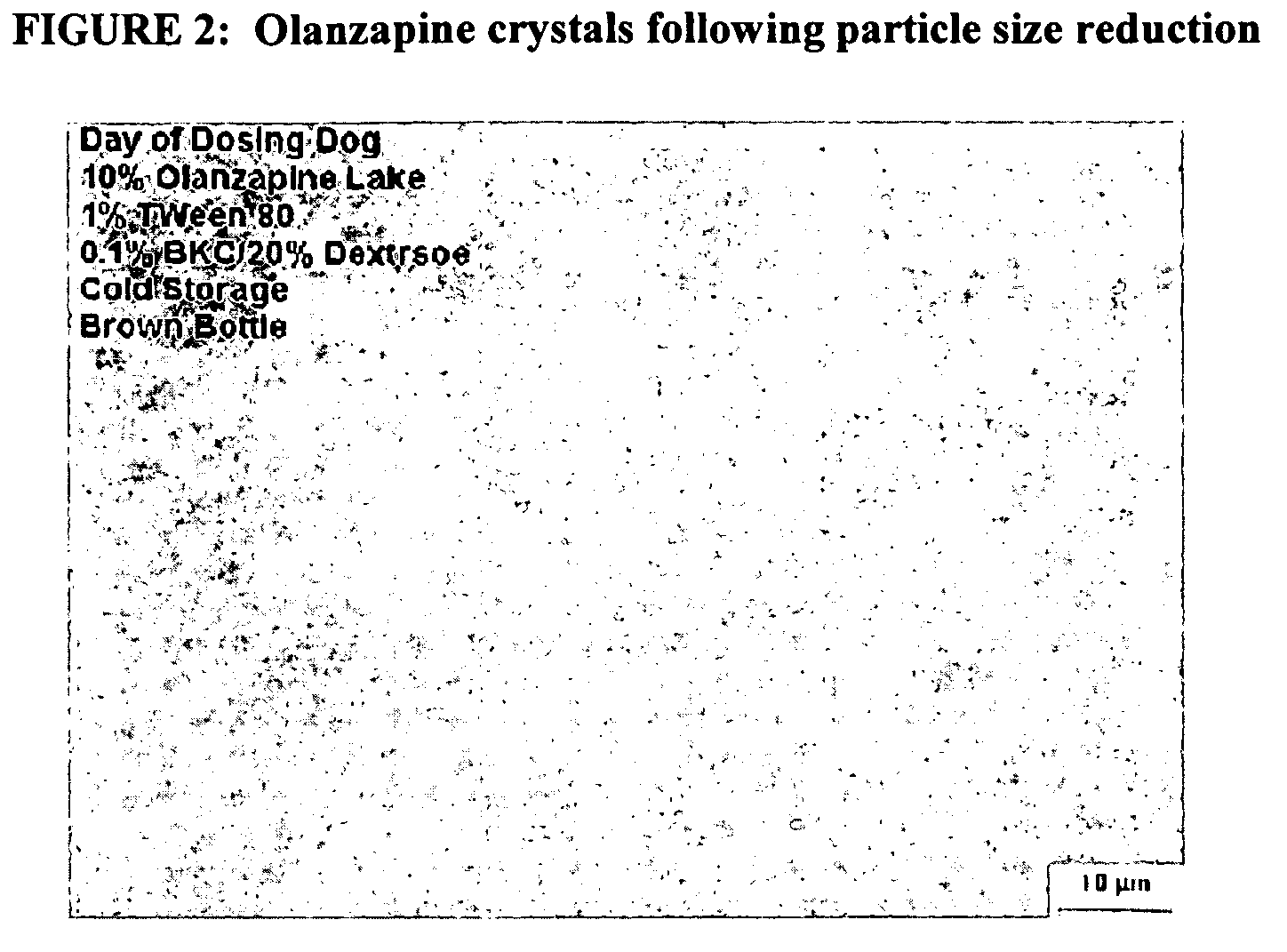
Injectable nanoparticulate olanzapine formulations
ActiveUS7910577B2Sufficient amountMaintain curative effectPowder deliveryBiocidePharmacologyOlanzapine
Described are injectable formulations of nanoparticulate olanzapine that produce a prolonged duration of action upon administration, and methods of making and using such formulations. The injectable formulations comprise nanoparticulate olanzapine.
Owner:ALKERMES PHARMA IRELAND LTD
Aripiprazole, olanzapine and haloperidol pamoate salts
The invention relates to the discovery that pamoate salts of haloperidol and aripiprazole result in a good to superior long acting and / or extended release profile. Thus, in one aspect of the invention, the invention includes pamoate salts of haloperidol or aripiprazole. Preferably, the pamoate salt is characterized by a ratio of haloperidol to pamoate of 1:1 or 2:1. The pamoate salt can be crystalline, such as a needle or a dense crystal, such as described in the Figures. The invention further relates to methods of treating an individual in need thereof comprising administering a pharmaceutical composition comprising a pamoate salt of haloperidol and aripiprazole.
Owner:ALKERMES INC
Antibodies to paliperidone and use thereof
Disclosed is an antibody which binds to paliperidone, which can be used to detect paliperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of paliperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone / paliperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Olanzapine containing transdermal drug delivery compositions
The invention features compositions for the transdermal administration of olanzapine. The compositions include olanzapine or a pharmaceutically acceptable salt thereof, a pressure sensitive adhesive, and an excipient, such as a permeation enhancer and / or a solubilizer of olanzapine. The compositions are useful for the treatment of certain psychiatric disorders, for example schizophrenia and bipolar mania.
Owner:3M INNOVATIVE PROPERTIES CO
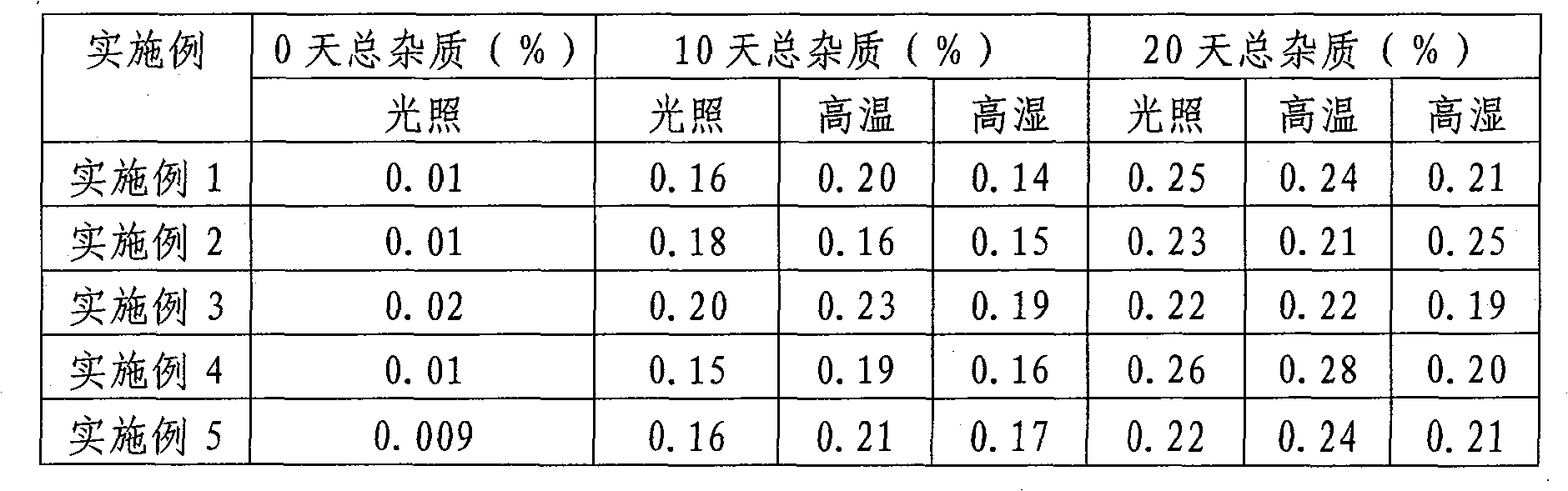
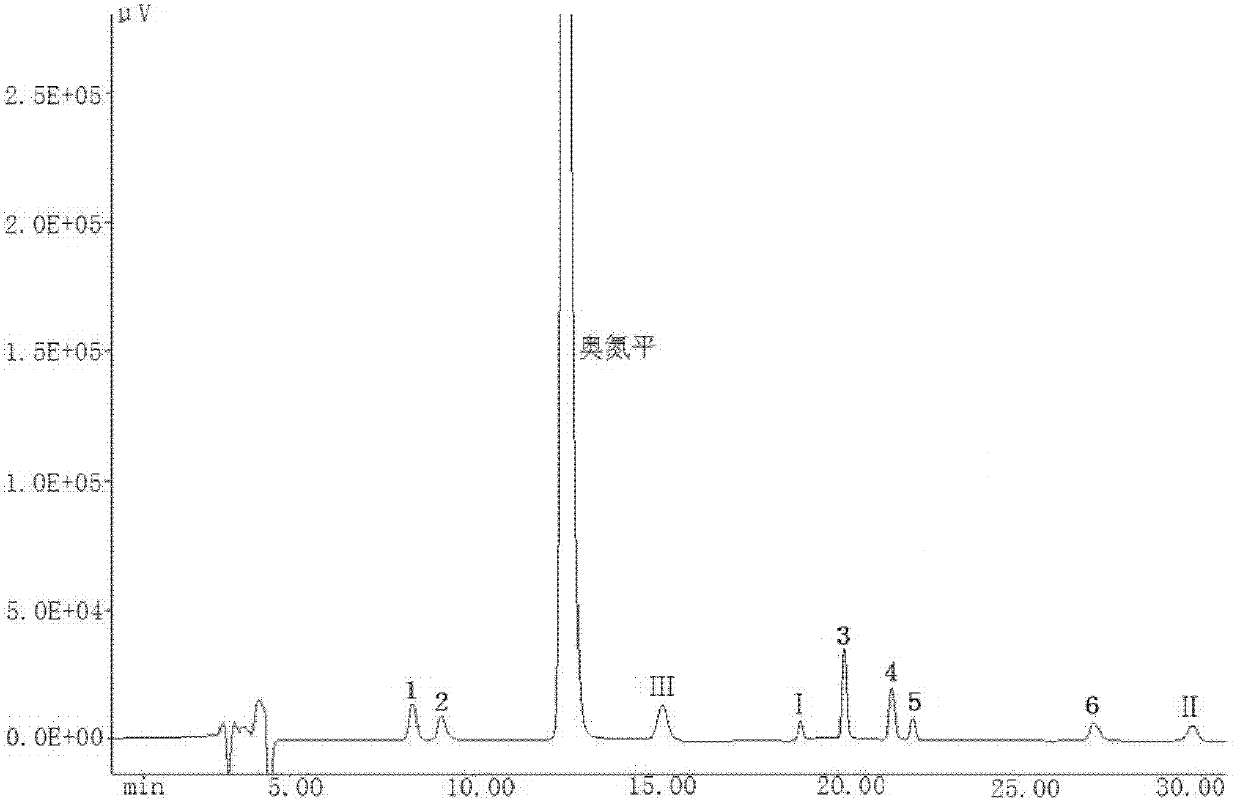
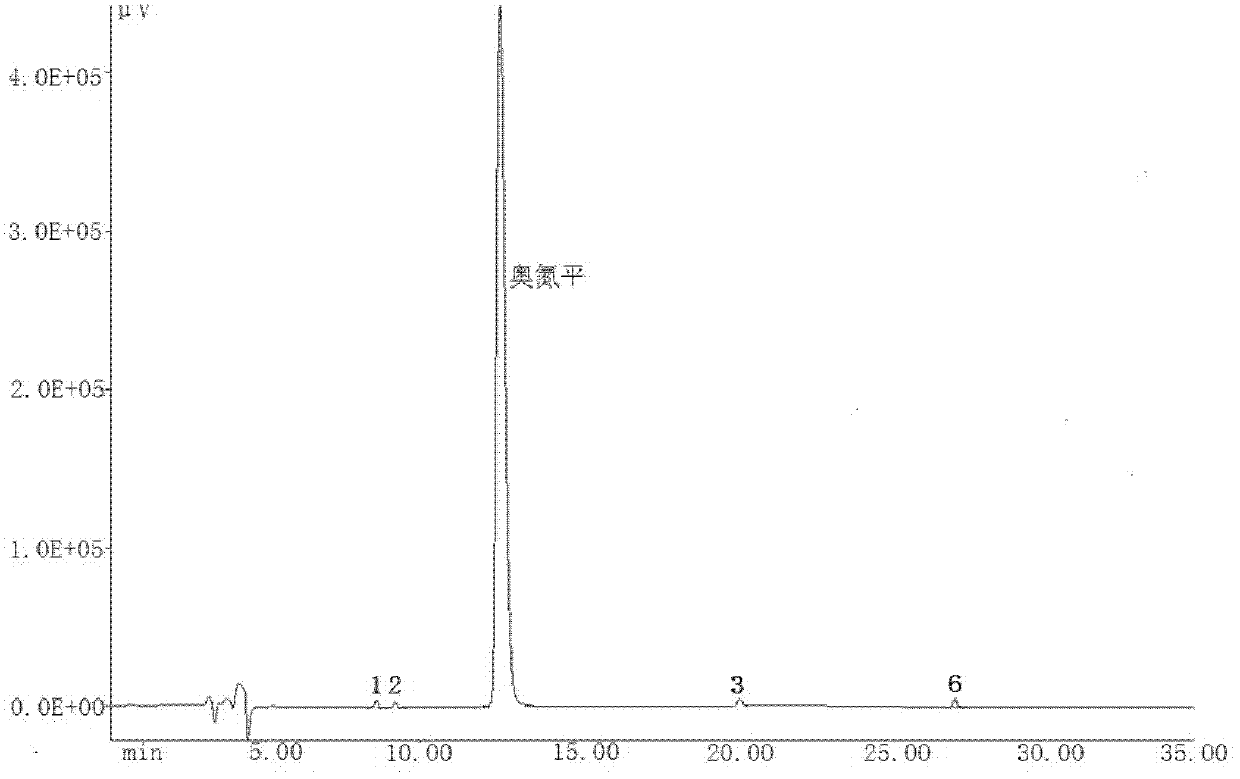
A kind of olanzapine related substance and its preparation method and high performance liquid chromatography analysis method
ActiveCN102276624ARich varietyHigh sensitivityOrganic chemistryComponent separationHplc methodGradient elution
The invention discloses an olanzapine related substance and a preparation method as well as a high-efficiency liquid-phase chromatographic analysis method thereof. The olanzapine related substance has a structural formula shown in the specification. The preparation method of the olanzapine related substance comprises the following steps of: concentrating olanzapine ethanol recrystallization mother liquor; and separating through silica gel column chromatography to prepare the olanzapine related substance. In addition, the invention provides the high-efficiency liquid-phase chromatographic analysis method of the olanzapine related substance. In the high-efficiency liquid-phase chromatographic analysis method, a reversed phase C18 chromatographic column is selected and used, the detection wavelength is 220-280nm, the flow velocity is 0.8-1.0ml / minute, the column temperature is 25-30 DEG C, acetonitrile and a 0.1-0.4-percent buffer solution of glacial acetic acid and triethylamine in equal proportion are used as a mobile phase to perform gradient elution; the olanzapine related substance and other eight related substances can be simultaneously detected; and thus, quality control of olanzapine and olanzapine-containing medicaments can be completely, scientifically, effectively and quickly realized.
Owner:DALIAN UNIV OF TECH
Olanzapine oral instant membrane
ActiveCN102920683AImprove complianceRemove restrictions or completely preventOrganic active ingredientsNervous disorderPlasticizerDissolution
The present invention belongs to the field of pharmaceutical preparations, particularly relates to an olanzapine oral instant membrane agent, a preparation method and pharmaceutical uses thereof. The membrane agent comprises the following components, by weight, 1-30% of olanzapine, 40-90% of a polymer membrane forming material, 0-40% of a plasticizer, 0-30% of a flavoring agent, and 0-5% of otherauxiliary agents. The olanzapine oral instant membrane agent has the following characteristics that: no water is required during administration, the olanzapine oral instant membrane agent can rapidlymelt in mouth, phenomena of drug hiding in mouth and drug spitting of schizophrenia patients can be avoided, and patient medication compliance is easily improved. With the preparation method, the main drug of the olanzapine oral instant membrane agent has characteristics of good dispersing effect in a hydrophilic matrix glue liquid, good finished product appearance, rapid dissolution and good stability. In addition, characteristics of accurate product dose and no dust flying during a production process are provided, and problems of labor protection and environmental pollution can be solved.
Owner:JIANGSU HANSOH PHARMA CO LTD +1
Antibodies to risperidone and use thereof
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to aripiprazole haptens and use thereof
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to risperidone haptens and use thereof
Disclosed is an antibody which binds to risperidone, which can be used to detect risperidone in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of risperidone, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to aripiprazole and use thereof
Disclosed is an antibody which binds to aripiprazole, which can be used to detect aripiprazole in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of aripiprazole, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to quetiapine and use thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, quetiapine, olanzapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
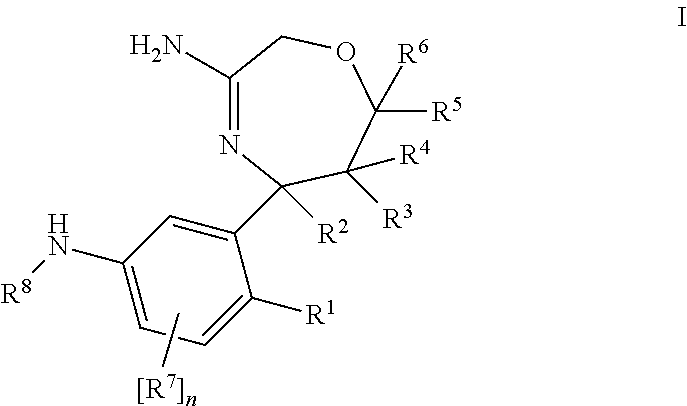
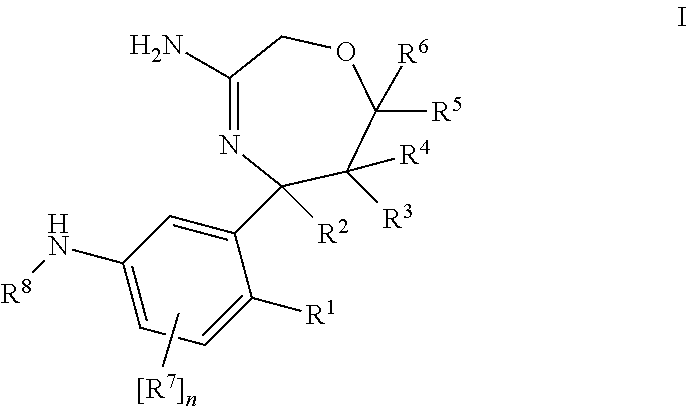
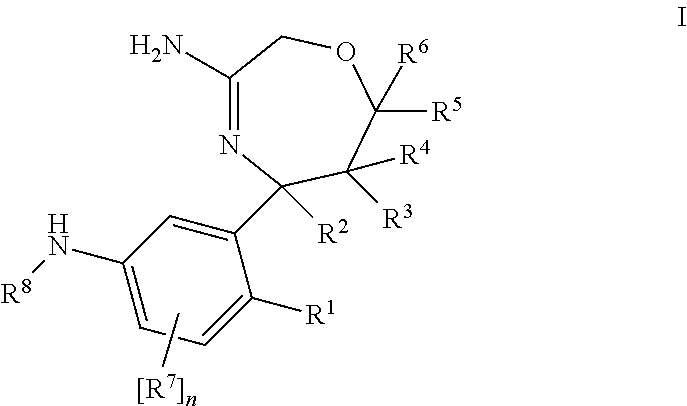
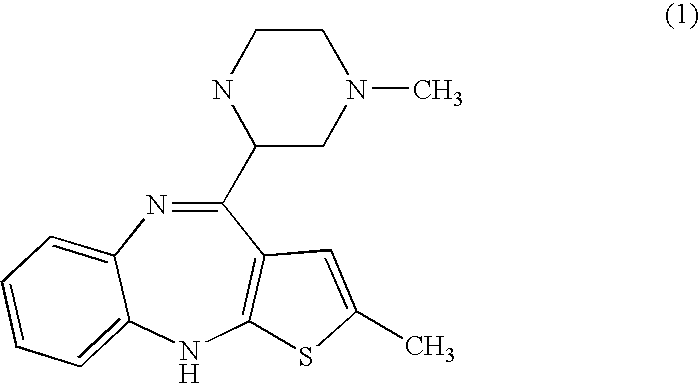
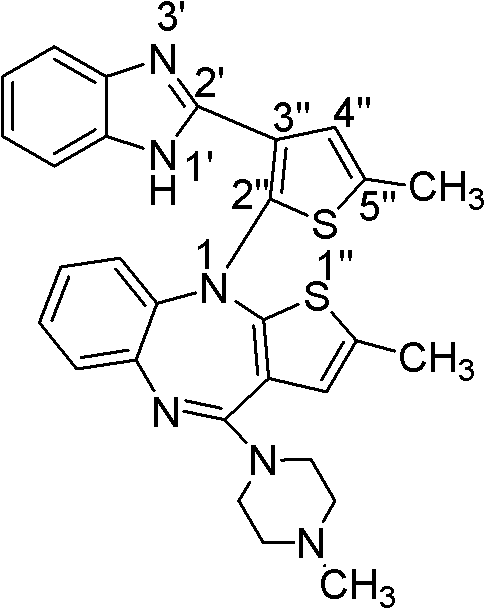
Haptens of olanzapine
The invention relates to compounds of Formula I, wherein R1, R2, and R3 are defined in the specification, useful for the synthesis of novel conjugates and immunogens derived from olanzapine. The invention also relates to conjugates of an olanzapine hapten and a protein.
Owner:JANSSEN PHARMA NV
Antibodies to quetiapine haptens and use thereof
Disclosed is an antibody which binds to quetiapine, which can be used to detect quetiapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of quetiapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
Antibodies to olanzapine and use thereof
Disclosed is an antibody which binds to olanzapine, which can be used to detect olanzapine in a sample such as in a competitive immunoassay method. The antibody can be used in a lateral flow assay device for point-of-care detection of olanzapine, including multiplex detection of aripiprazole, olanzapine, quetiapine, and risperidone in a single lateral flow assay device.
Owner:JANSSEN PHARMA NV
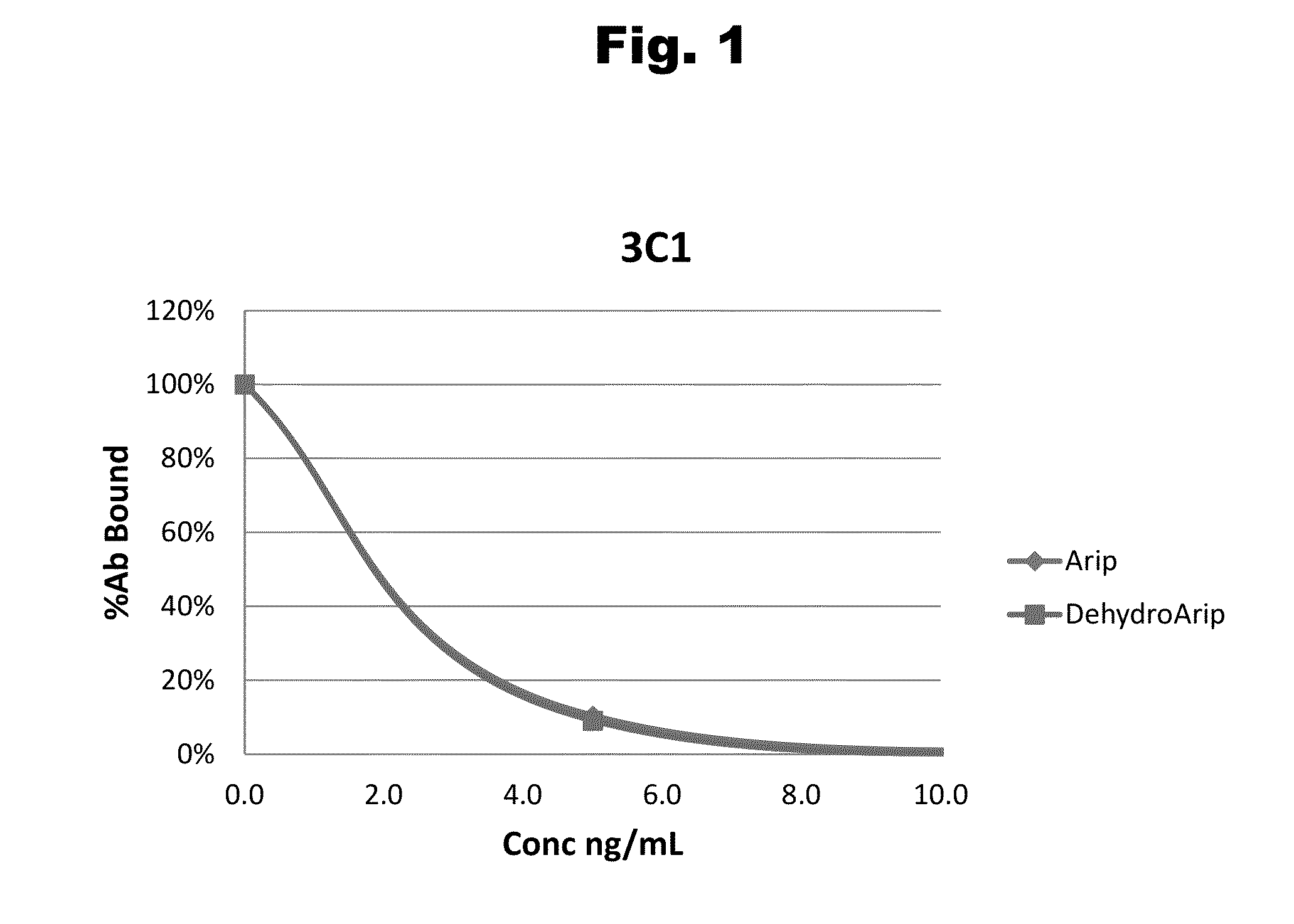
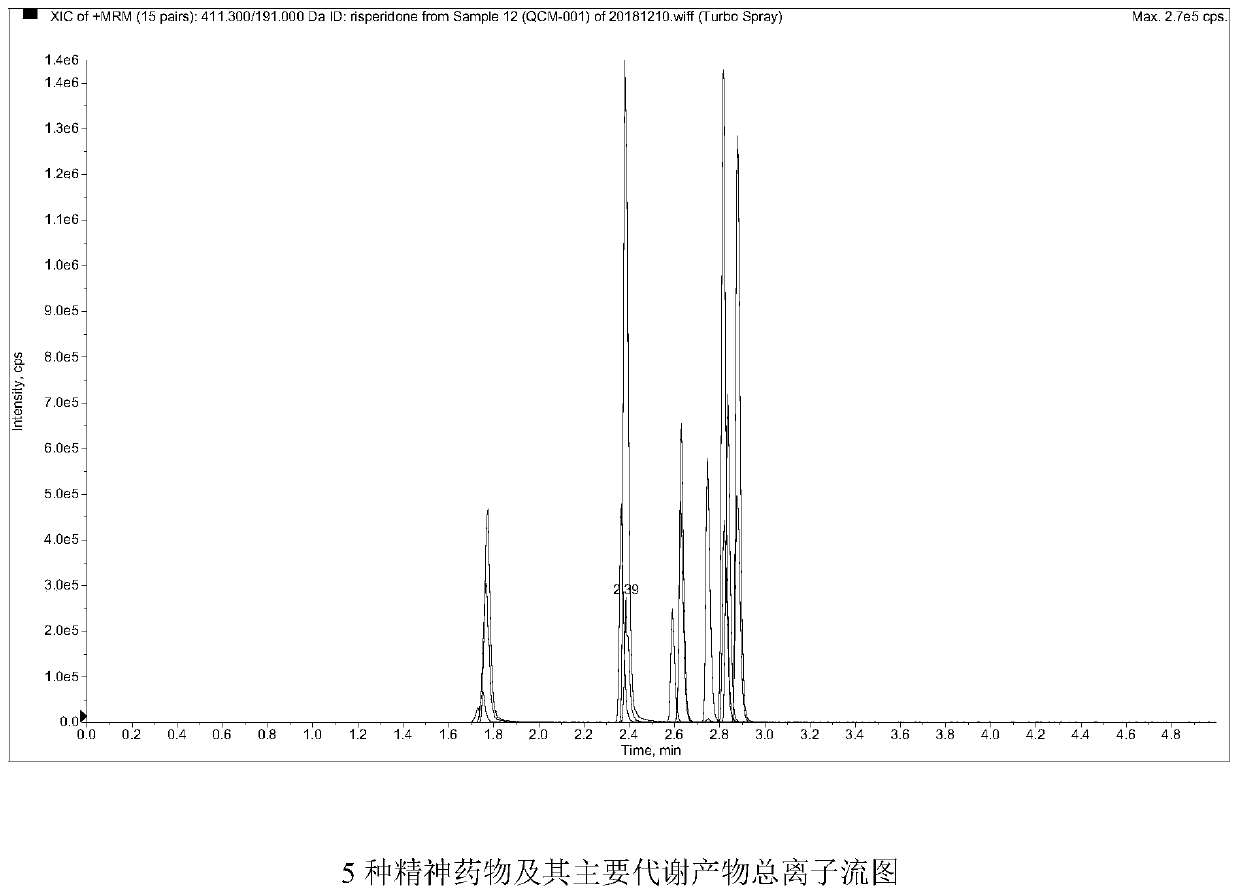
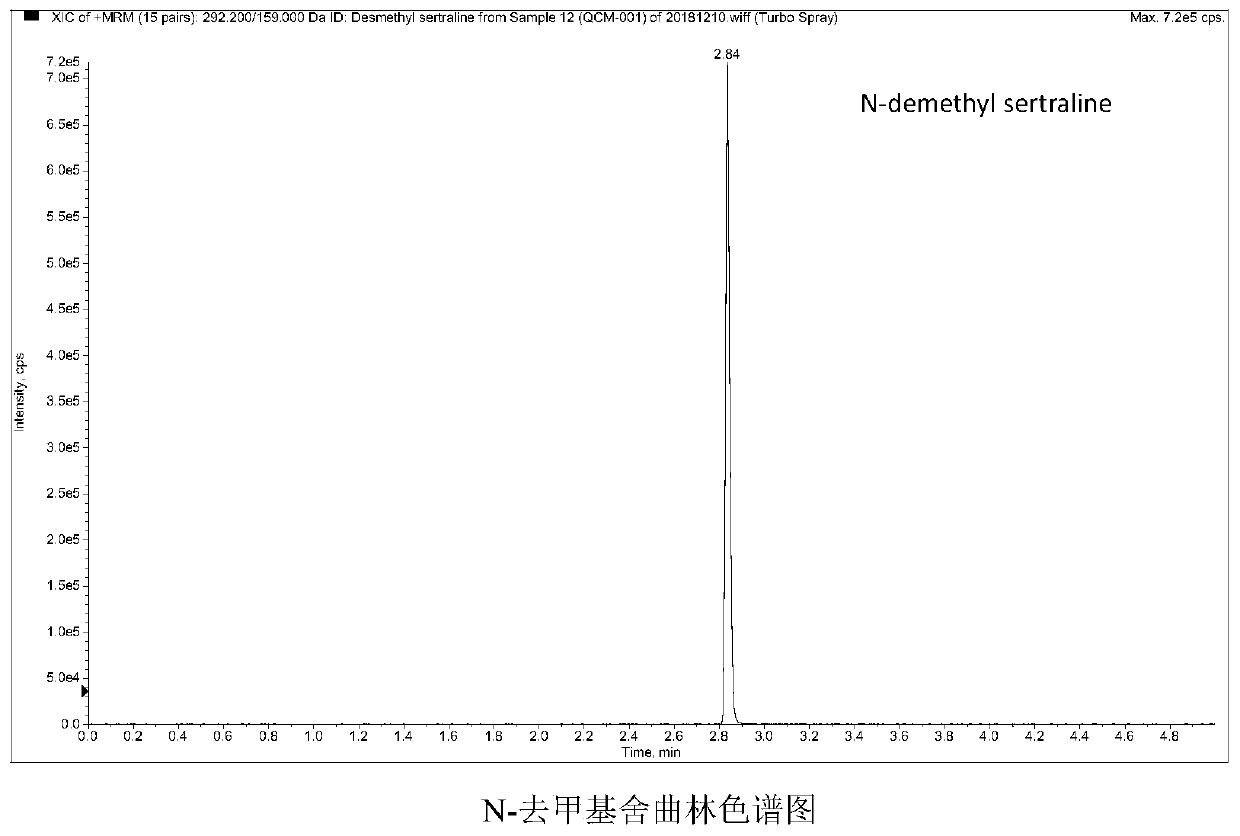
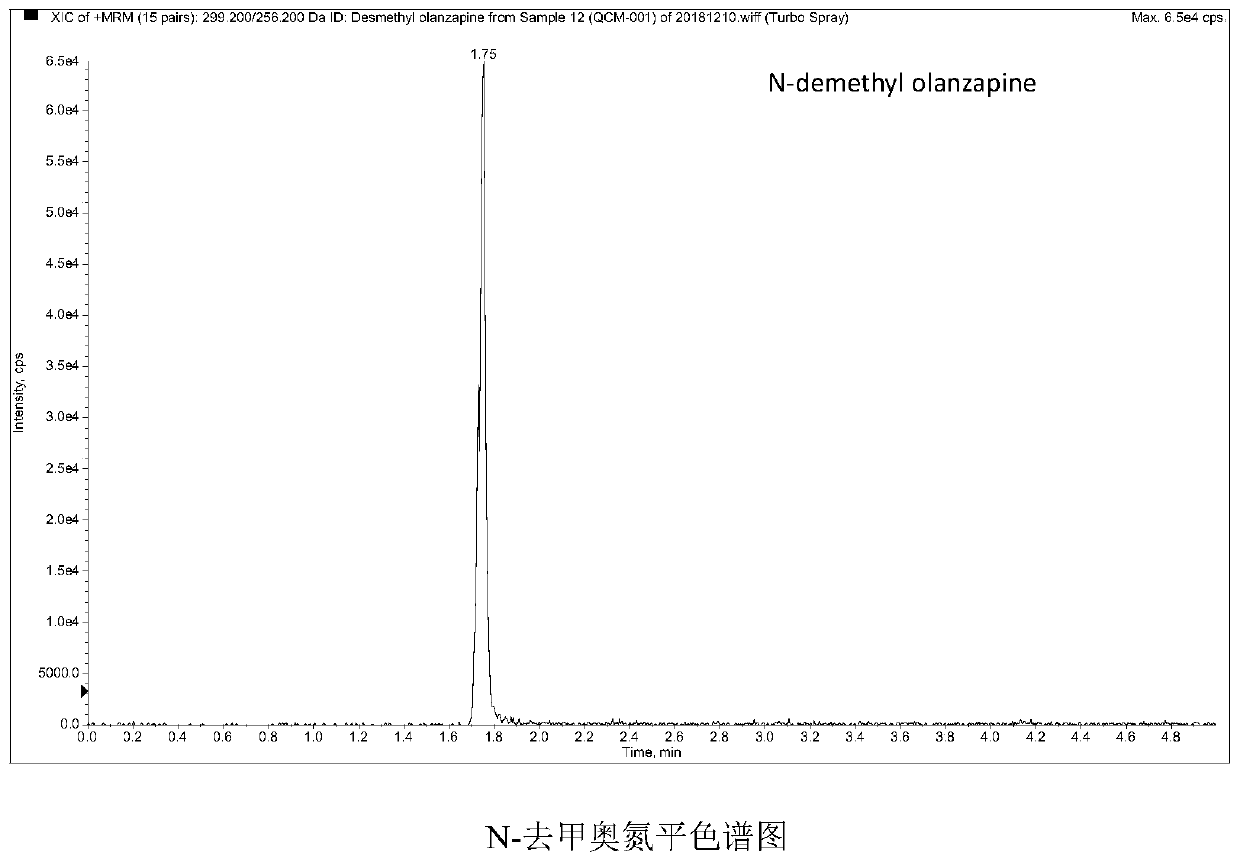
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Novel Olanzapine orally disintegrating tablet
ActiveCN102178657APrevent oxidationAvoid degradationOrganic active ingredientsNervous disorderParticulatesOrally disintegrating tablet
The invention relates to a novel Olanzapine orally disintegrating tablet. The tablet comprises (I) a stabilizing Olanzapine coating particulate and (II) an additive, wherein the stabilizing Olanzapine coating particulate contains: (i) a neutral core; (ii) an active layer surrounding the neutral core; and (iii) a protective layer surrounding the active layer. The invention also relates to a preparation method of the stabilizing Olanzapine coating particulate and the novel Olanzapine orally disintegrating tablet containing the stabilizing Olanzapine coating particulate.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com