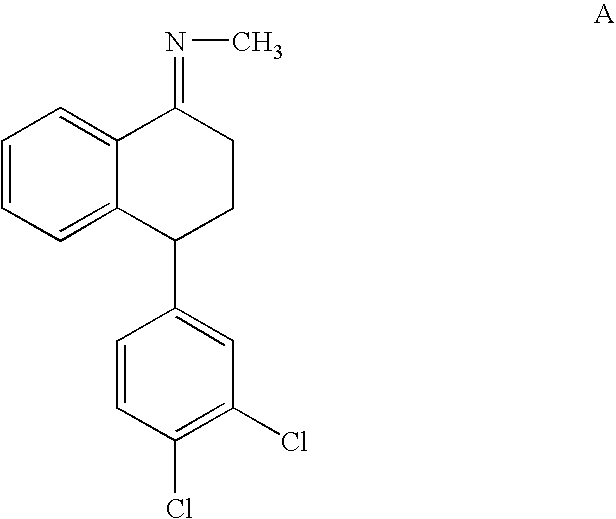
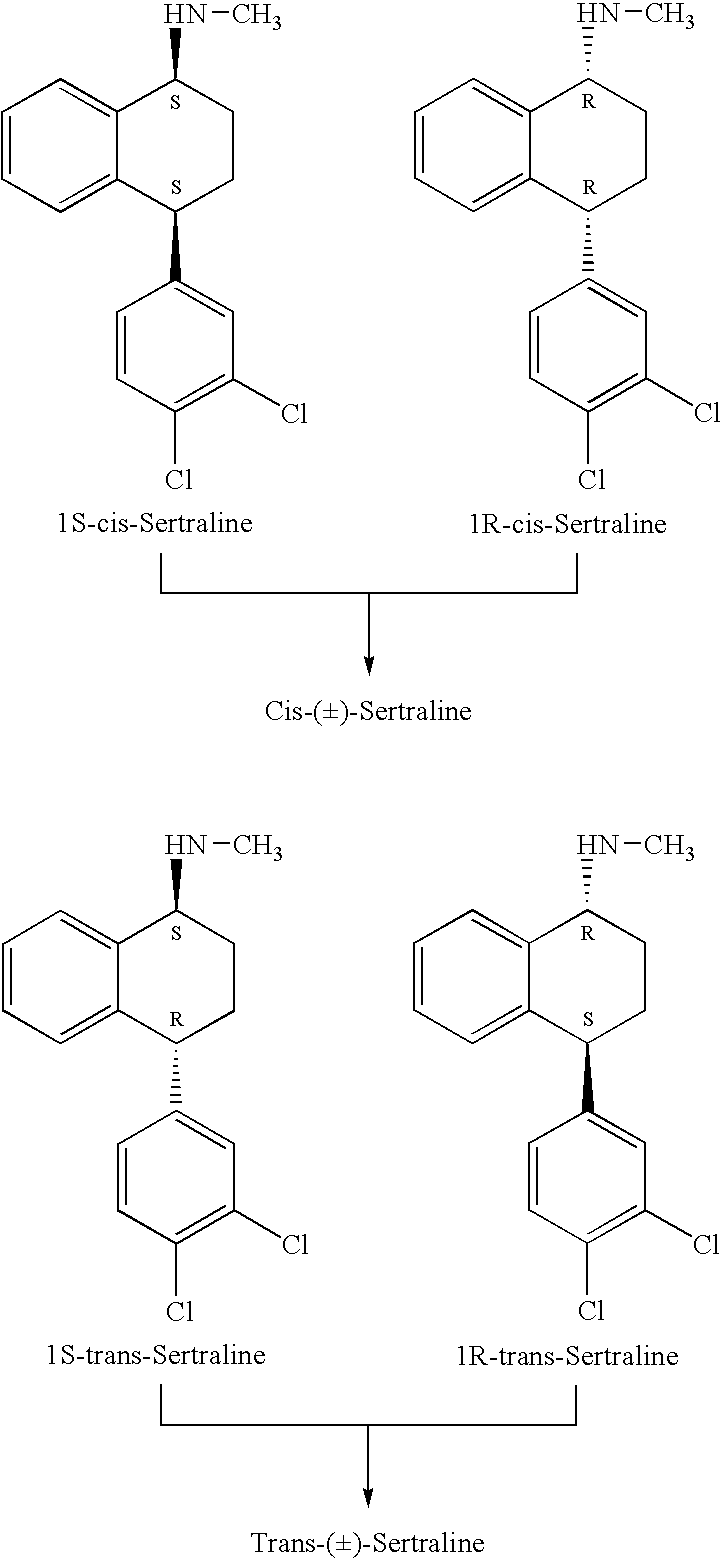
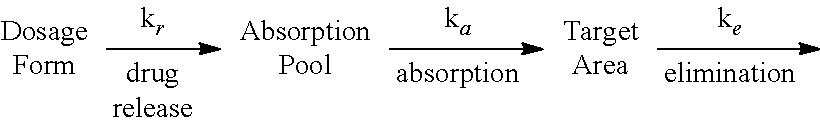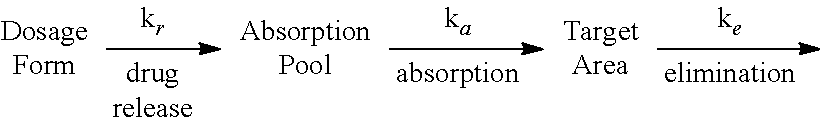Patents
Literature
51 results about "Sertraline" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
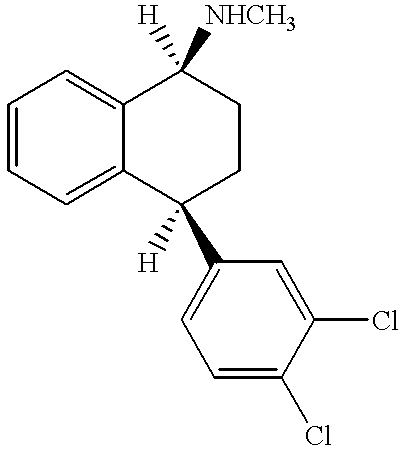
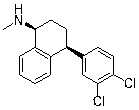
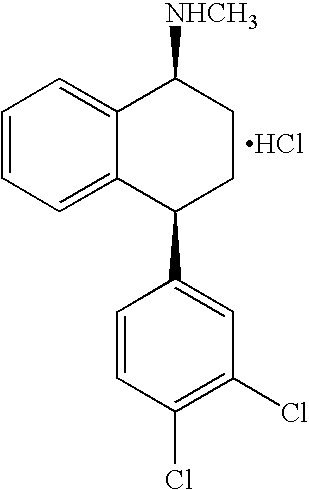
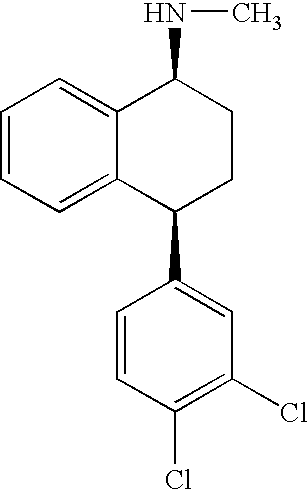
Sertraline is used to treat depression, panic attacks, obsessive compulsive disorder, post-traumatic stress disorder, social anxiety disorder (social phobia), and a severe form of premenstrual syndrome (premenstrual dysphoric disorder).
Taste-masked formulations containing sertraline and sulfoalkyl ether cyclodextrin
InactiveUS20050250738A1High photochemical stabilityReduce probabilityBiocideOrganic active ingredientsSertralineFruit juice
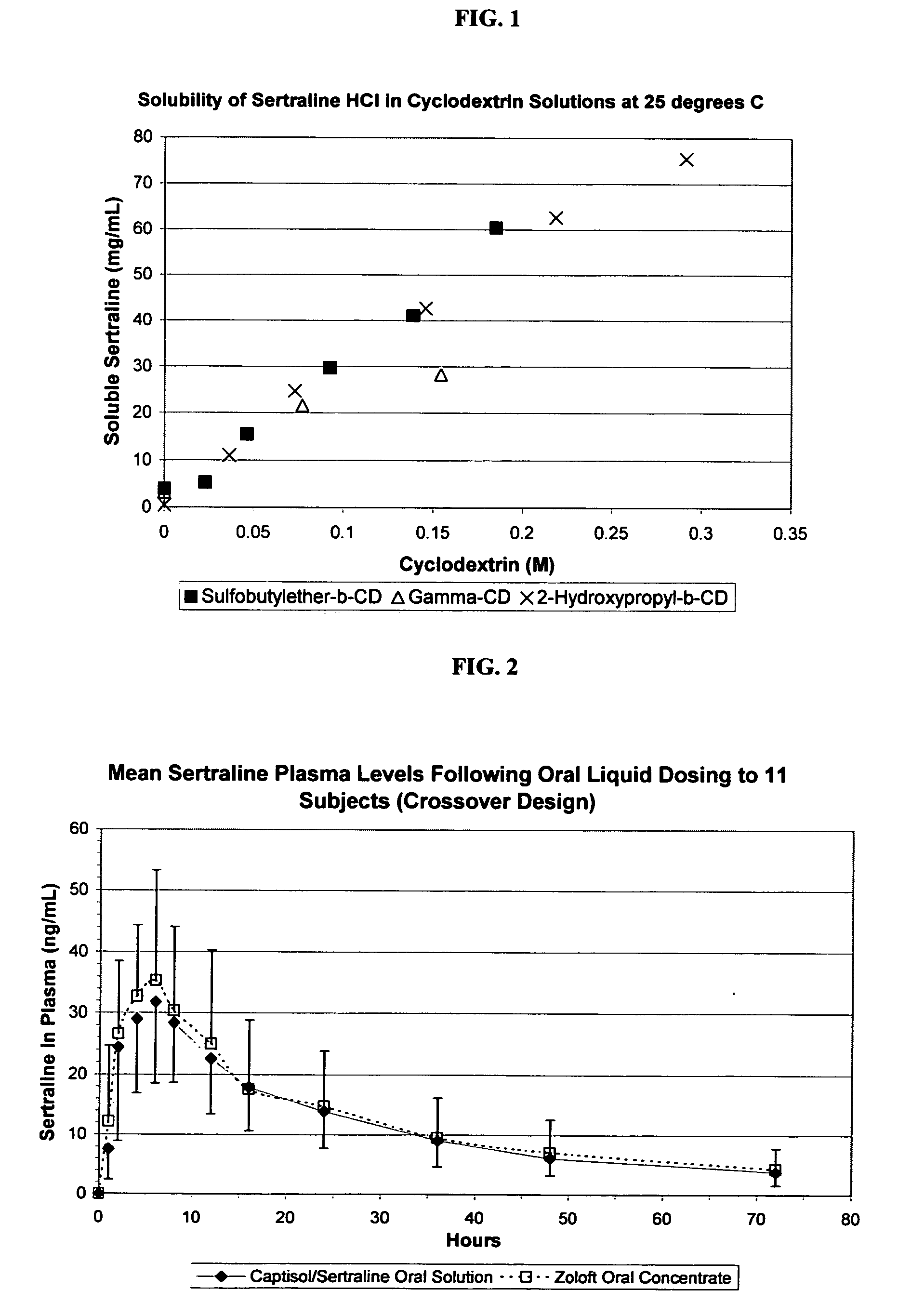
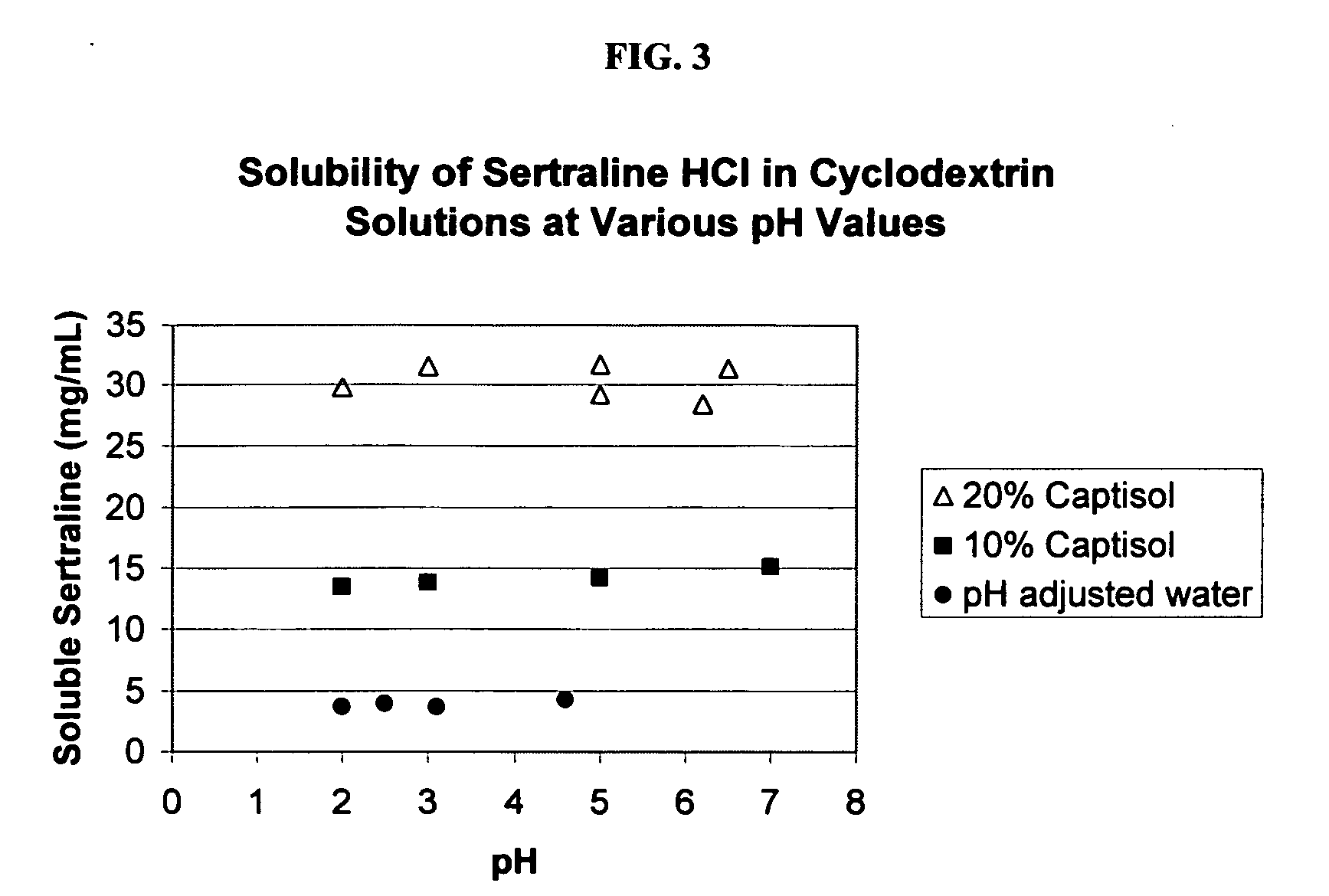
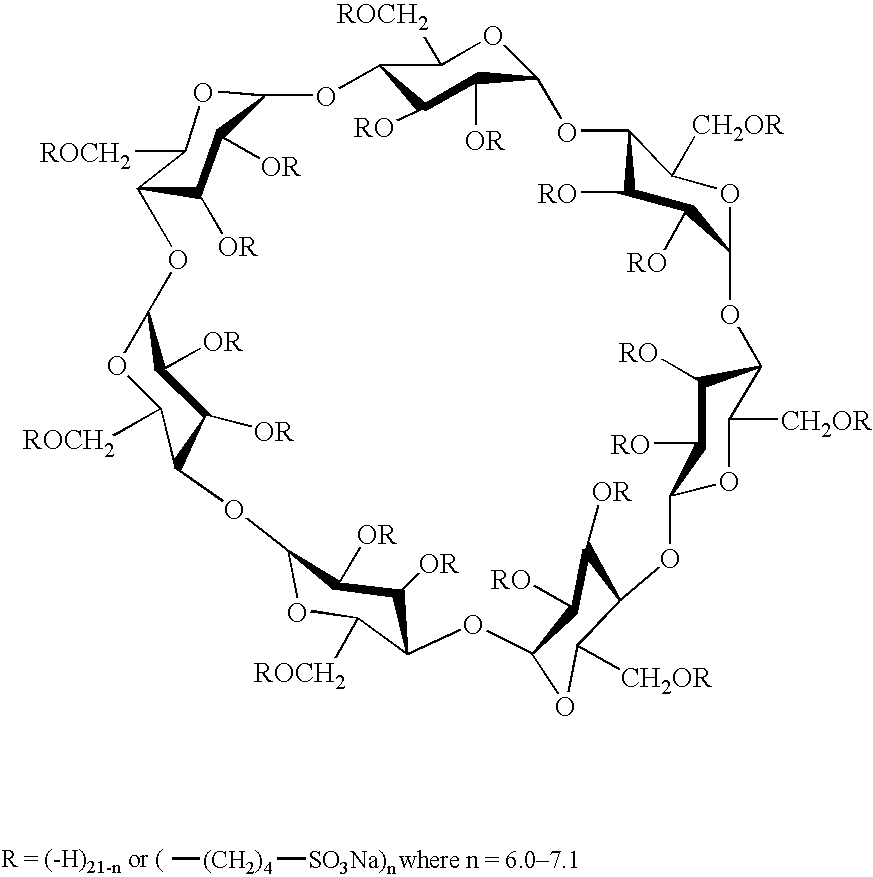
The present invention provides aqueous oral formulations containing sertraline, or a pharmaceutically acceptable salt thereof, and a sulfoalkyl ether cyclodextrin. The liquid formulations are pleasant tasting, convenient to use, and chemically and physically stable. The liquid formulations can be administered directly or diluted before administration. Unlike the commercially available ZOLOFT™ formulation, the liquid formulations herein do not precipitate upon dilution with water, fruit juices, sodas or other pharmaceutically acceptable oral liquid carriers. The sulfoalkyl ether cyclodextrin-containing formulation provides significant advantages over the marketed non-aqueous formulation and other cyclodextrin-containing formulations of sertraline. The formulation can be self-preserved against microbial growth. The SAE-CD-containing formulation of sertraline can be provided in liquid form or as a reconstitutable powder. Both ready-to-use and concentrated liquid formulations can be prepared. The formulation is available as a clear solution or a suspension.
Owner:CYDEX PHARMACEUTICALS INC
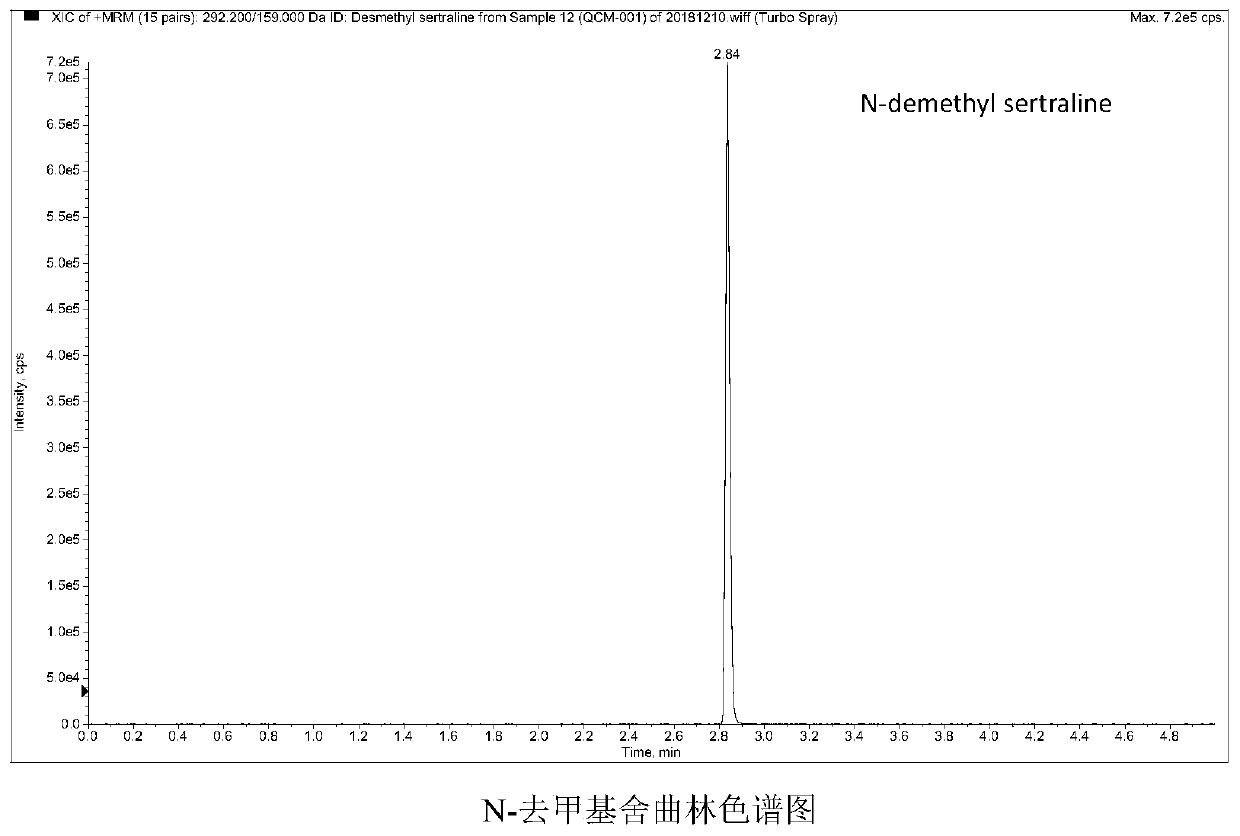
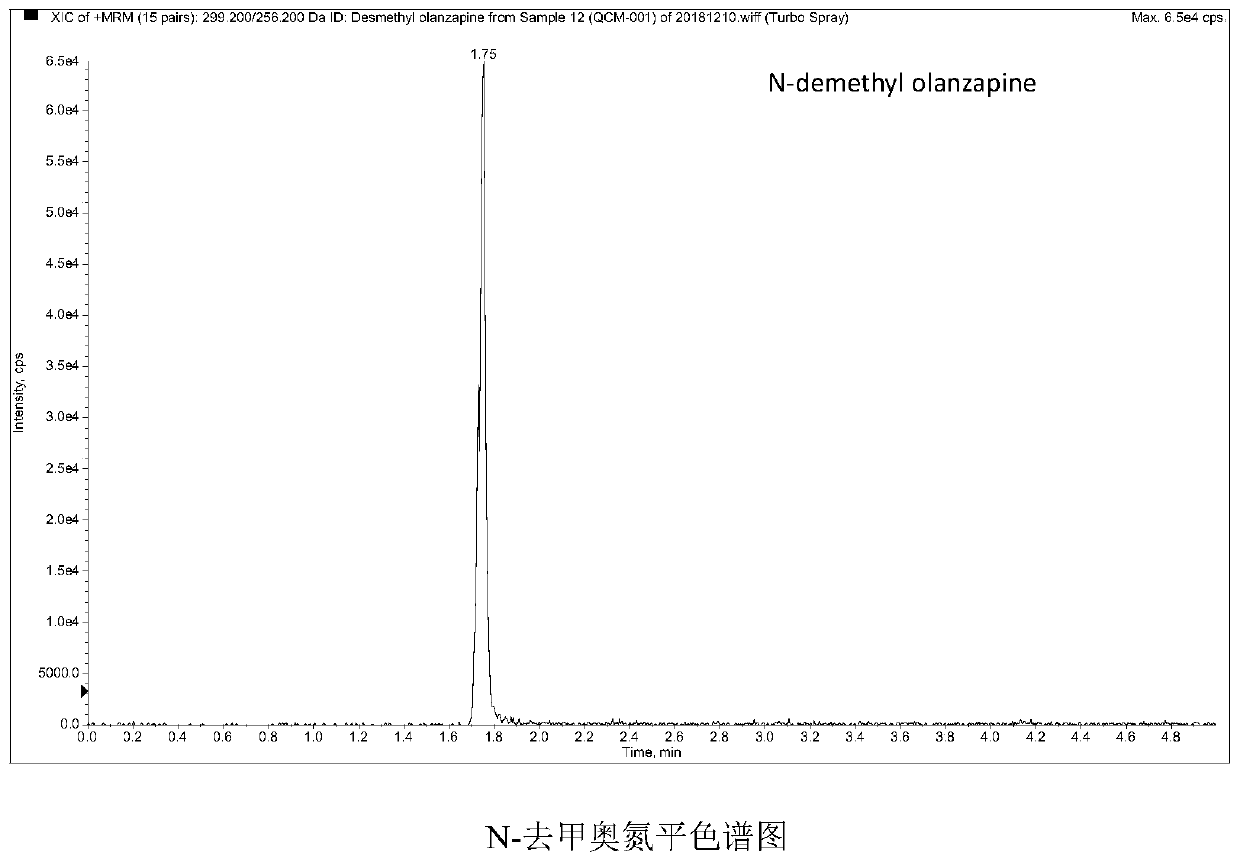
Method and kit for detecting five psychotropic drugs and main metabolites thereof in blood
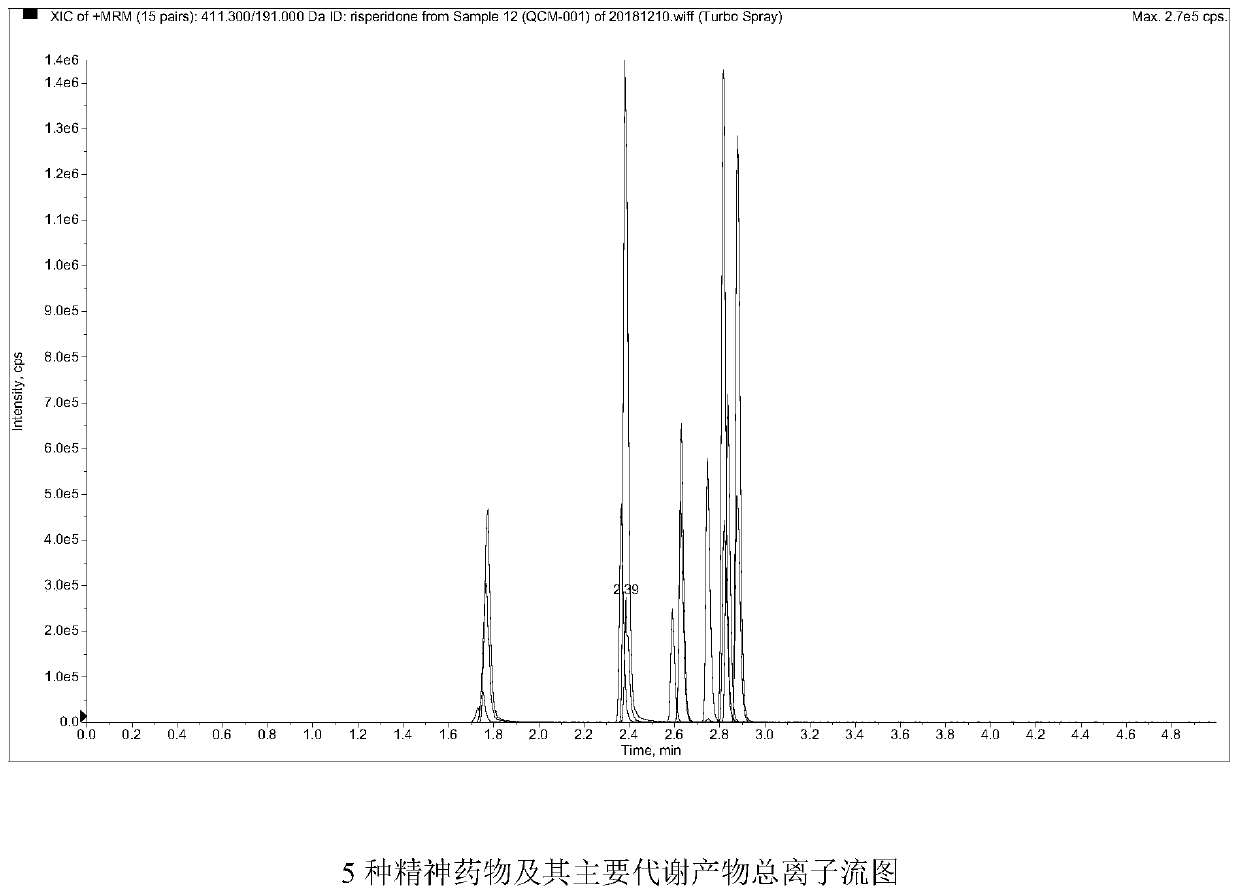
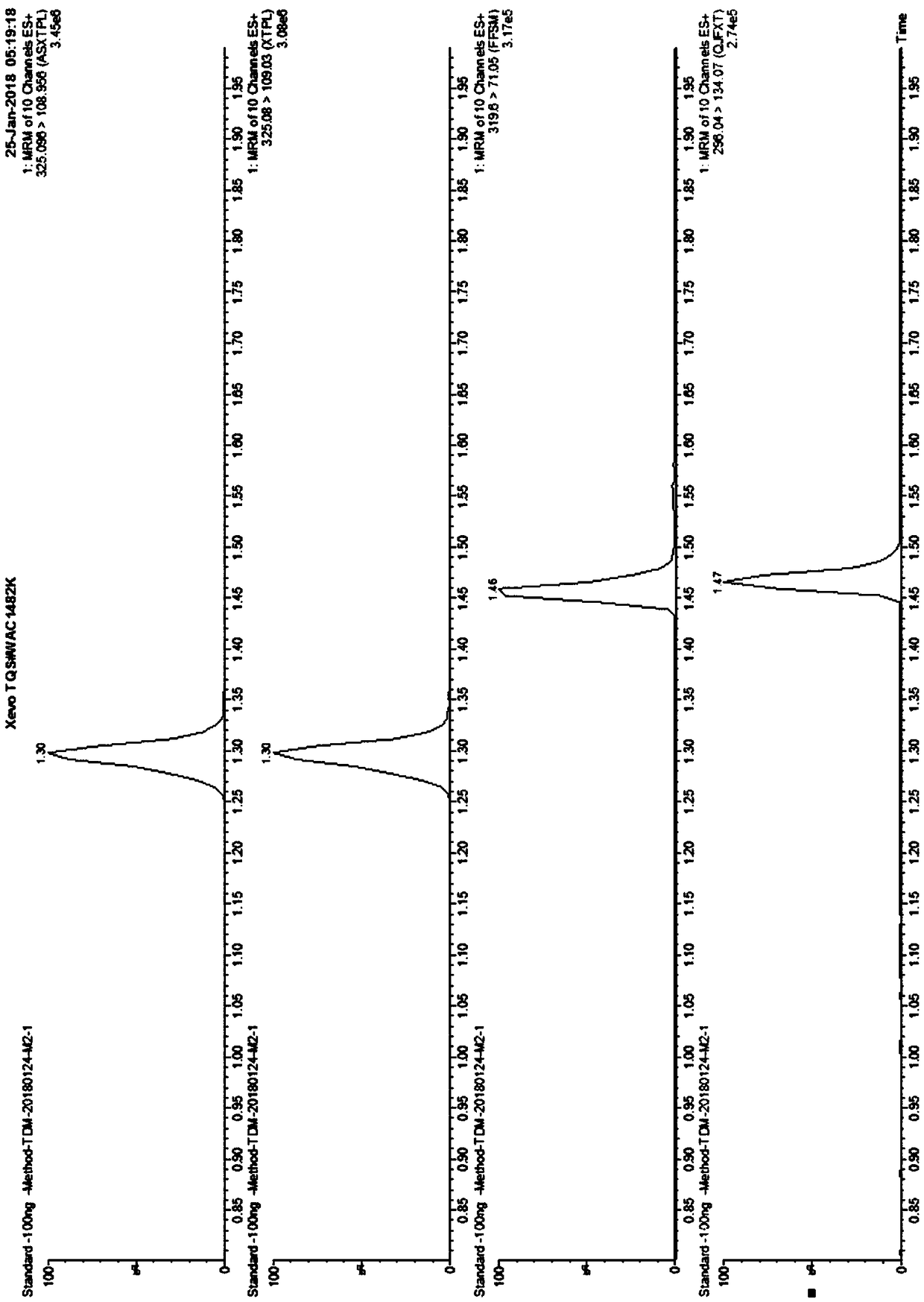
The invention belongs to the field of drug detection, and particularly relates to a method and a kit for detecting five psychotropic drugs and main metabolites thereof in blood. The five psychotropicdrugs and the main metabolites thereof comprise: olanzapine and demethyl olanzapine, risperidone and 9-hydroxy risperidone, aripiprazole and dehydrogenated aripiprazole, Escitalopram and demethyl citalopram, sertraline and N-demethyl sertraline. Accoridng to the method provided by the invention, a pair of quantitative ion pairs is respectively selected for each detection substance, a relative retention time thereof is used as a qualitative basis, and a standard curve is made by using a standard product for quantification; furthermore, the accuracy and effectiveness of the method are evaluatedfrom quality control of three low, middle and high levels, thereby avoiding distortion of the detection result; and meanwhile, an internal standard working solution is applied to correction, so that matrix effects can be avoided, and accurate quantification is realized. The method provided by the invention has the advantages of simple and rapid operation, high flux and low cost, and can be appliedto the therapeutic drug monitoring of the psychotropic drugs in the clinical work of the psychiatry department.
Owner:BEIJING HUILONGGUAN HOSPITAL +1
Kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry and application thereof
InactiveCN109085264APharmacologically activeInterpret blood levelsComponent separationSertralineTandem mass spectrometry
The invention provides a kit for detecting anti-depressant drugs in serum and plasma by liquid chromatography tandem mass spectrometry. The kit comprises drug standard substances, drug internal standardization compounds, drug extraction compositions, negative plasma and a diluent. The drug standard substances comprise amfebutamone, oxybupropion, citalopram, Escitalopram, venlafaxine, O-desmethylvenlafaxine, duloxetine, fluoxetine, norfloxetine, fluvoxamine, mirtazapine, paroxetine, sertraline and trazodone. The drug internal standardization compounds comprise amfebutamone-d9, oxybupropion-d6,citalopram-d6, venlafaxine-d6, O-desmethylvenlafaxine-d6, duloxetine-d3, fluoxetine-d6, norfloxetine-d6, fluvoxamine-d4, mirtazapine-d3, paroxetine-d6, sertraline-d3 and trazodone-d6. The drug extraction compositions comprise, by volume, 60% of methanol solution, 20% of acetonitrile solution, 10% of isopropyl alcohol solution and 10% of purified water. The diluent comprises 50 % of methanol waterfluid. The kit can be used for simultaneous detection of the anti-depressant drugs and active metabolites, the detection time is short, and flux is high.
Owner:HANGZHOU BAICHEN MEDICAL INSTR CO LTD +1
Hydrogenation of imine intermediates of sertraline with catalysts
Provided are hydrogenation processes of sertraline imine intermediates with catalysts in various reactors.
Owner:TEVA PHARM USA INC
End-substituted homoallylic amine derivatives, a preparing method thereof and uses of the derivatives
ActiveCN107473982AHigh optical purityPromote conversionCarbamic acid derivatives preparationCarboxylic acid nitrile preparationIridiumSertraline
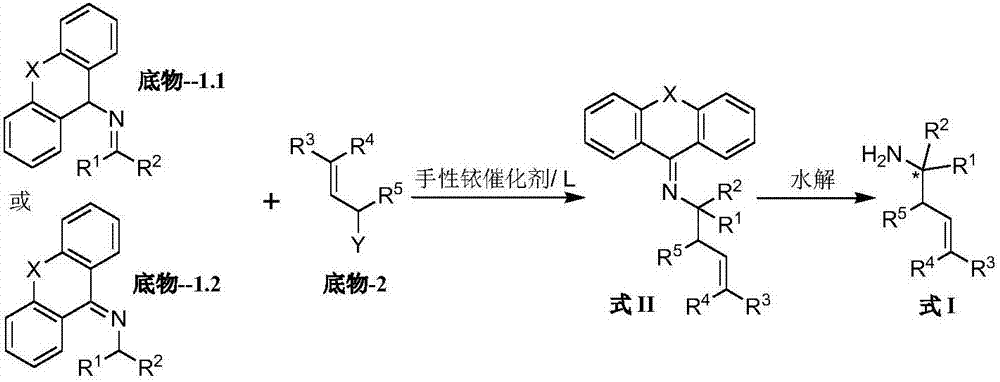
The invention belongs to the field of chemical medicines, and particularly relates to end-substituted homoallylic amine derivatives, a preparing method thereof and uses of the derivatives. In the method, the end-substituted homoallylic amine derivatives are prepared by allowing a 2-aza-allyl anion to participate, in a high regioselectivity manner, an allylation reaction catalyzed by iridium, and by an intramolecular 2-aza-Cope rearrangement reaction. The derivatives can be synthesized efficiently by the method. The method is simple, convenient and feasible. Prepared compounds have extremely high optical purity. The derivatives prepared by the method can be used for preparing Sertraline and Tametraline which are anti-depression medicines and some natural products.
Owner:SICHUAN UNIV
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Hydrogel-driven layered drug dosage form
InactiveUS6899896B2Promote absorptionImproved release profileOrganic active ingredientsBiocideSertralineWater insoluble
A controlled release dosage form for sertraline has a core comprising a sertraline-containing composition and a water-swellable composition wherein the water-swellable composition is in a separate region within the core. A coating around the core is water-permeable, water-insoluble, and has at least one delivery port therethrough. In one embodiment, the dosage form releases sertraline to the use environment at an average rate of 6 to 10 wt % per hour from the second to the twenth hour after introduction to a use environment and less than about 25 wt % for the first two hours and at least 70 wt % by the twelfth hour, where the percentages correspond to the mass of drug released from the tablet divided by the total mass of drug originally present in the tablet. In another embodiment, the dosage form releases less than about 25 wt % of sertraline to the use environment by the second hour after introduction of the dosage form to the use environment, and delivers at least 25 wt % from the eighth to the twenty-fourth hour.
Owner:BEND RES
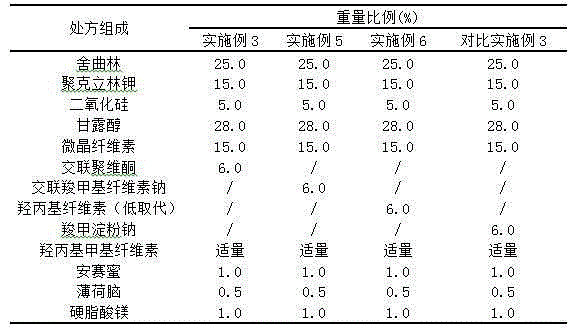
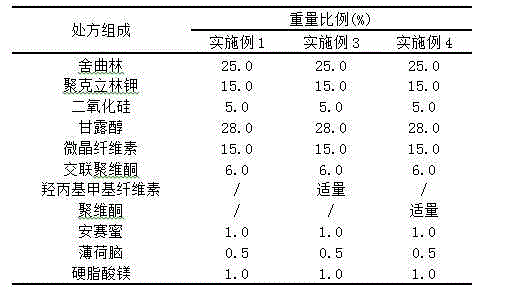
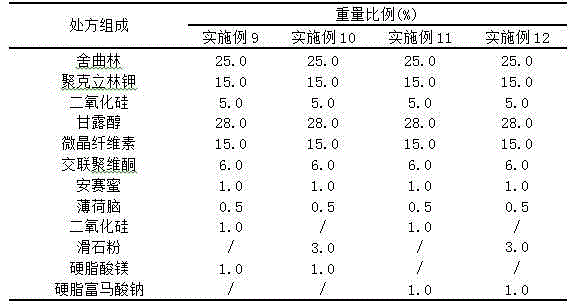
Orally disintegrating tablet containing sertraline and preparation method thereof
The invention discloses an orally disintegrating tablet containing sertraline and a preparation method thereof. The tablet contains a water-soluble filler, a binder, a disintegrating agent, a lubricant and an odor masking agent, and the taste of the tablet is improved through combined usage of solid dispersion technology. The orally disintegrating tablet has the characteristics of good taste, good oral compliance, rapid action, etc., and is applicable to treatment of patients with depression. The invention also provides the preparation method. The preparation method is simple, has low cost and belongs to the technical fields of medical science and pharmacy.
Owner:AVENTIS PHARMA HAINAN
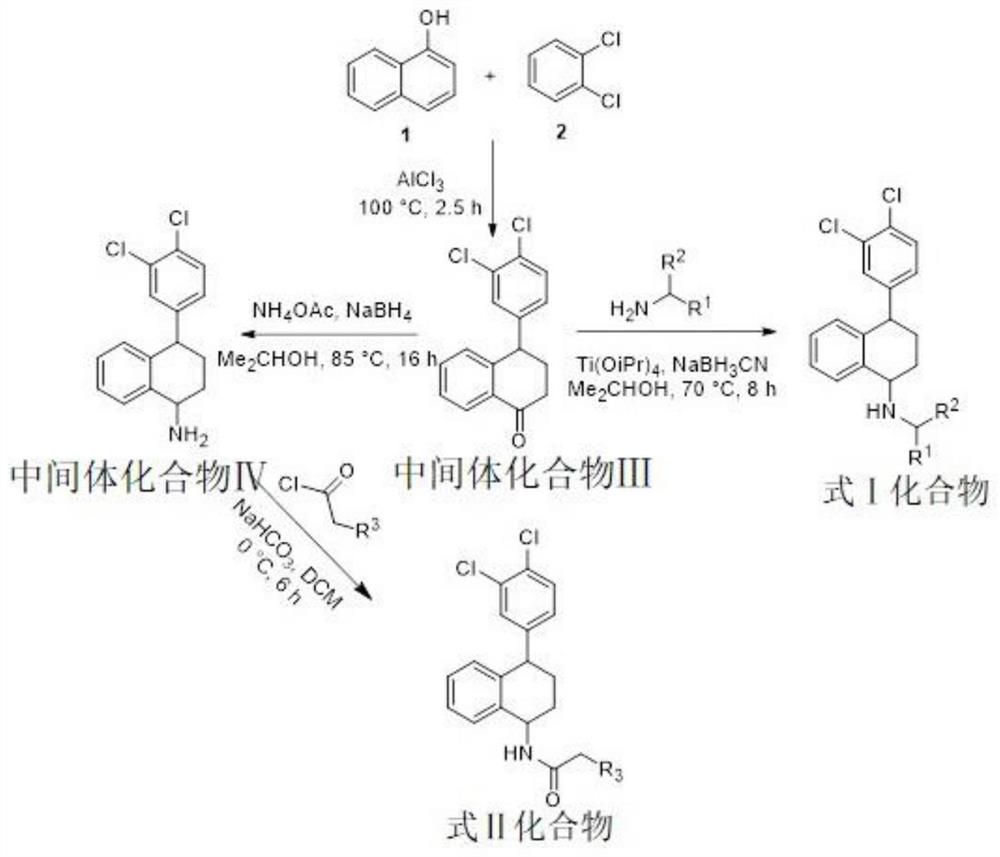
Preparation method and application of chiral amine compound
ActiveCN103923005AWill not polluteOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSezolamideSertraline
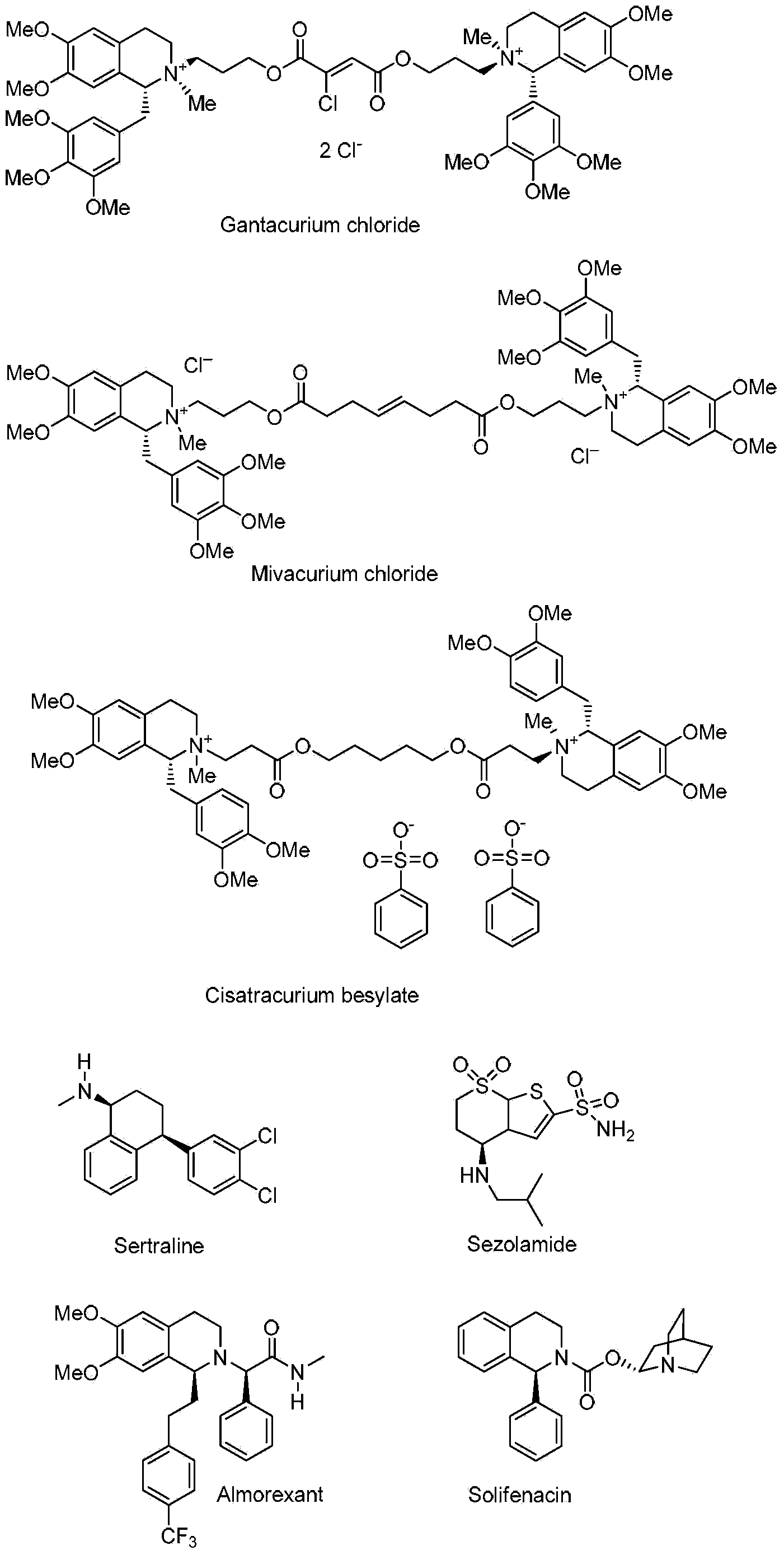
The invention discloses a preparation method of a chiral amine compound. In presence of a semi-sandwich type complex and an acid additive, an asymmetrization reaction is carried out on a prochiral amine compound in a hydrogen atmosphere, and postprocessing is carried out after the reaction is completely finished, so that the chiral amine compound is obtained. According to the preparation method of the chiral amine compound, a certain amount of appropriate acid is added in a certain sequence, so that activation of a C=N bond in a reduction reaction can be promoted; the prepared chiral amine compound can be taken as an intermediate and used for synthesizing the following medicines (including but not limited to the medicines) or precursors of the medicines: gantacurium, mivacurium chloride, atracurium besylate, sertraline, sezolamide, almorexant or solifenacin.
Owner:ZHEJIANG MENOVO PHARMA
Sertraline oral aqueous solution and preparation method
InactiveCN101632632AGreat tasteOvercome the defect of poor drug complianceOrganic active ingredientsNervous disorderSertralineOral medication
The invention relates to sertraline oral solution for treating depression. The invention provides the sertraline aqueous solution for oral administration and a preparation method thereof. The sertraline oral aqueous solution comprises sertraline, pharmacologically acceptable salt thereof, and a or more pharmacologically acceptable excipients. The invention adopts a method of adding the surfactant to prepare the sertraline oral aqueous solution, and overcomes the defects that the nonaqueous solution has no good taste and the patient compliance is not good. Moreover, the invention adopts unit dose package so as to have accurate dosage and convenient administration.
Owner:北京迈劲医药科技有限公司
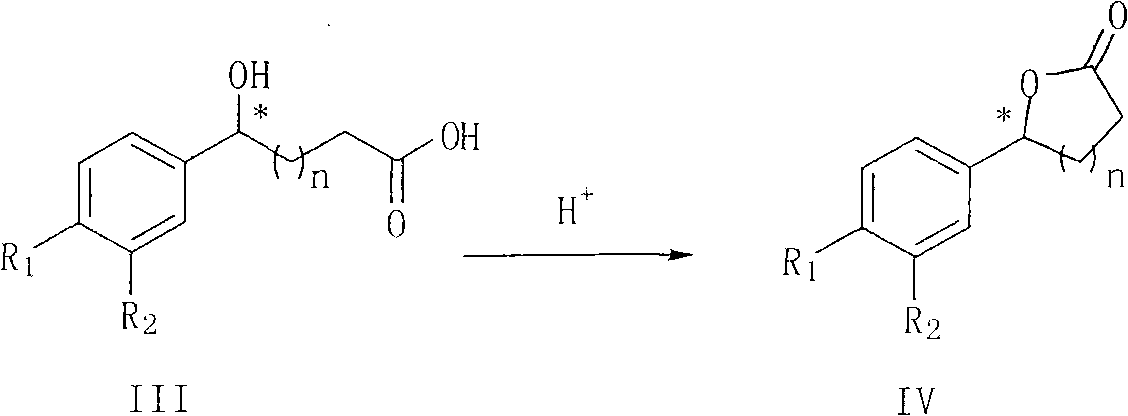
New synthesis method of optically pure lactone
InactiveCN102126954AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsSertralineHydrogen
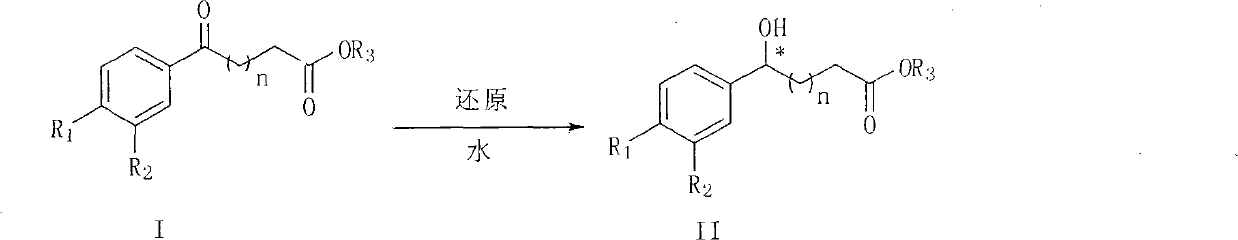
The invention provides a new preparation method of chiral hydroxy ester (II) and optically pure lactone (IV). The preparation method comprises the following steps: the ketonic ester shown in the general formula (I) performs aqueous-phase asymmetric reduction reaction and obtain chiral hydroxy ester (II), chiral hydroxy ester (II) is hydrolyzed and then cyclized in solvent in the presence of acid to obtain the chiral lactone compound shown in the general formula IV, wherein R1 and R2 are the same or different and do not represent hydrogen or halogen; and n is 1 or 2, and R3 is CH3, CH2CH3, or CH(CH3)2. When R1 is Cl, R2 is Cl and n is 1, the compound (IV) is the chiral intermediate of the antidepressant chiral medicine sertraline; and when R1 is F, R2 is H and n is 2, the compound (IV) is the chiral intermediate of the antilipemic chiral medicine ezetimibe.
Owner:CHENGDU ORGANIC CHEM CO LTD CHINESE ACAD OF SCI
Method and kit for detecting 19 drugs and metabolites thereof in blood by liquid chromatography-tandem mass spectrometry
The invention belongs to the technical field of drug detection, and particularly relates to a method and a kit for detecting 19 drugs and metabolites thereof in blood through liquid chromatography-tandem mass spectrometry. The substances to be detected comprise sulpiride, pentafluridol, mianserin, buspirone, tandospirone, hydroxyazine, diazepam, venlafaxine, moclobemide, imipramine, paroxetine, reboxetine, amitriptyline, sertraline, digoxin, clonazepam, clopidogrel, toluenesulfobutyl urea, glimepiride, 1-pyrimidinepiperazine, desmethylvenlafaxine, 6-hydroxy buspirone and normipramine, and the substances to be detected are selected from the group consisting of sulpiride, pentafluridol, mianserin, venlafaxine, metandospirone, metandospirone, hydroxazine, diazepam, venlafaxine, moclobemide, the pharmaceutical composition is prepared from noramitriptyline, nordiazepam and clopidogrel metabolite; the detection method comprises the following steps: calibrating a standard solution, treating a to-be-detected sample, and detecting the to-be-detected sample by adopting high performance liquid chromatography-mass spectrometry. The embodiment of the invention can quickly and accurately measure the content, and the sample treatment method is simple and easy to implement, high in sensitivity and accurate in quantification.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
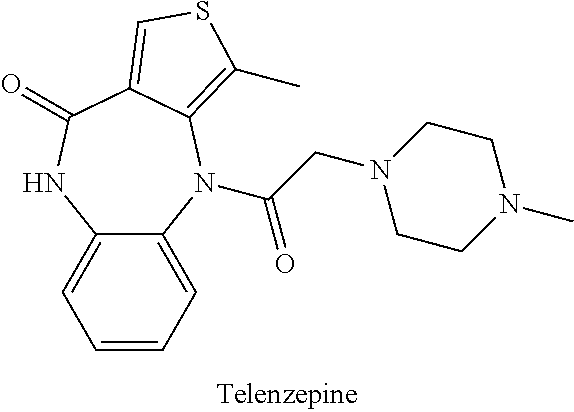
Combination therapy for the treatment of diabetes
InactiveUS8440655B2Relieve symptomsLower Level RequirementsBiocideMetabolism disorderDiabetes mellitusSertraline
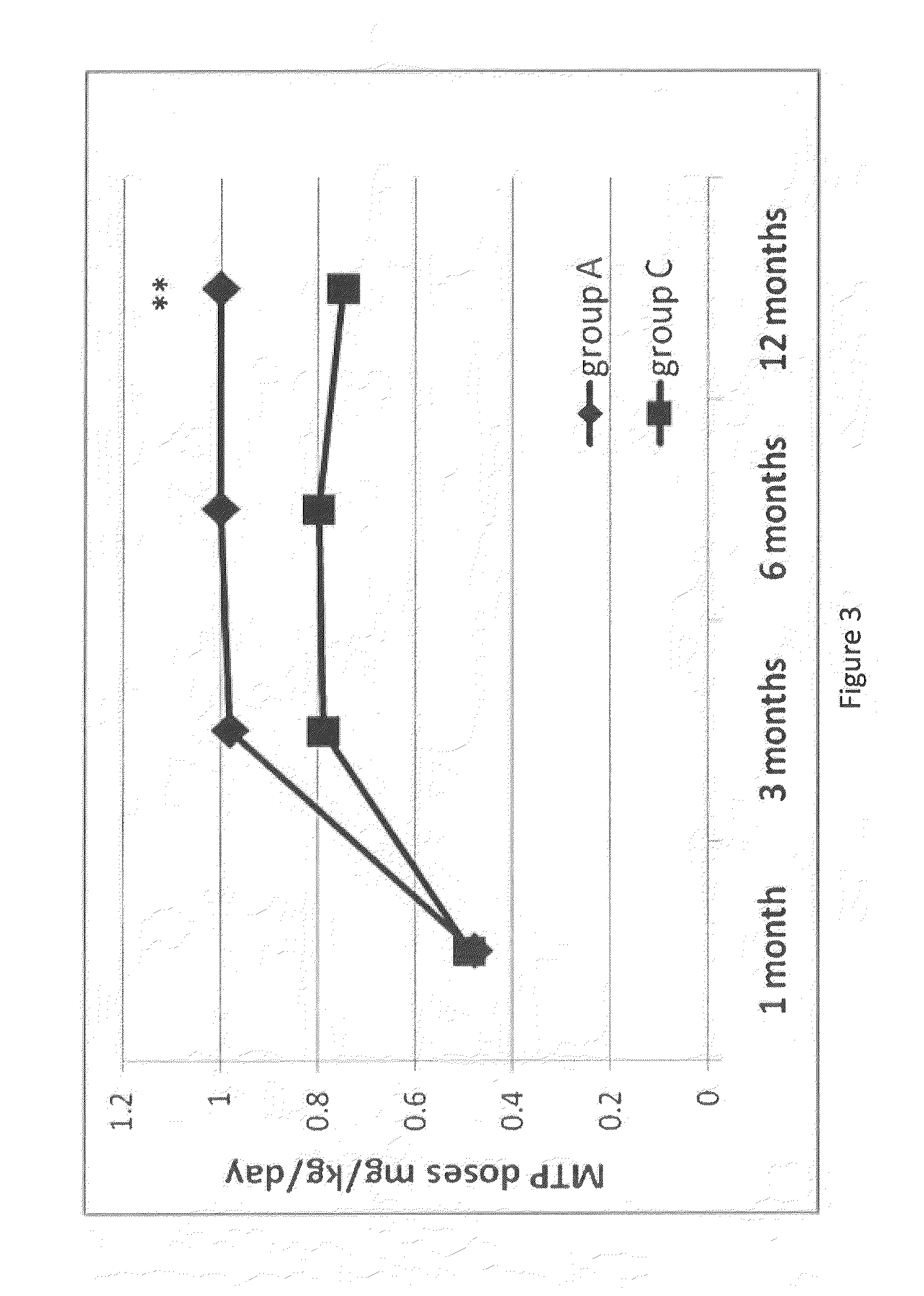
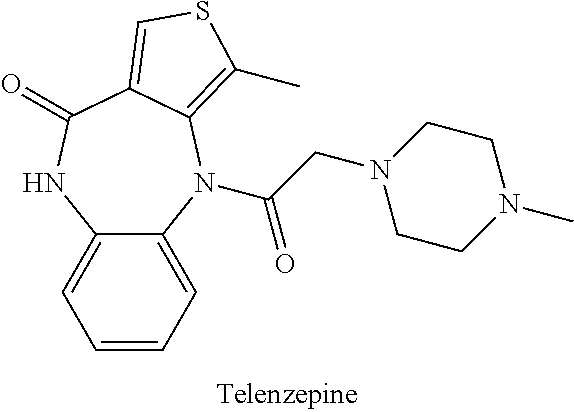
The present invention provides methods of treating, reducing, preventing, or inhibiting symptoms of diabetes and / or lowering plasma levels of HbA1c by co-administration of therapeutic or subtherapeutic doses of telenzepine and sertraline to a subject in need thereof.
Owner:THERAKOS INC
Oral disintegrated sertraline tablet and its prepn
InactiveCN1771920AReduce stimulationQuick effectOrganic active ingredientsNervous disorderSertralineOtic Agents
The present invention belongs to the field of medicine, and is especially one kind of oral disintegrated tablet of sertraline as depression treating medicine and its preparation process. The oral disintegrated tablet of sertraline includes sertraline as the effective medicine component and excipient mixture comprising disintegrating agent, stuffing, soluble polyol and penetrant. Specifically, the oral disintegrated tablet of sertraline contains sertraline 20-50 wt%, disintegrating agent 5-15 wt%, stuffing 10-30 wt%, soluble polyol 30-60 wt% and penetrant 1-5 wt%. Compared with other oral preparation forms, the present invention has wide covering of disintegrated medicine on gastrointestinal tract mucous membrane resulting in fast acting, no first pass effect, high bioavailability, less stimulation to gastrointestinal tract and easy taking.
Owner:范敏华
Hydrogenation of imine intermediates of sertraline with catalysts
Provided are hydrogenation processes of sertraline imine intermediates with catalysts in various reactors.
Owner:TEVA PHARM USA INC
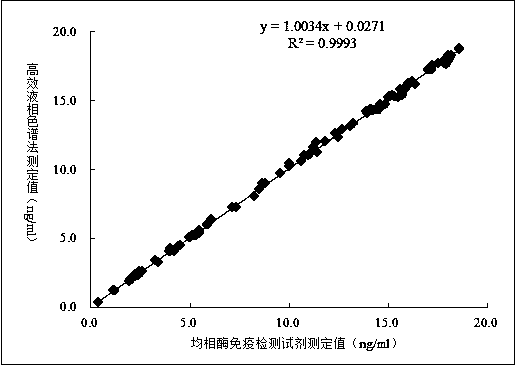
Sertraline detection reagent, and preparation and use methods thereof
ActiveCN110456087AStrong specificityImprove throughputOvalbuminSerum albuminSertralineVaccine Immunogenicity
The invention relates to a sertraline detection reagent, and preparation and use methods thereof. Through repeated experiments, a bran-new sertraline maleimide derivative is obtained; the sertraline maleimide derivative is used for preparing a sertraline immunogen with high immunogenicity; further, experiment animals are immunized to obtain a high-potency anti-sertraline specific antibody; and meanwhile, the sertraline maleimide derivative is used for preparing a sertraline enzyme conjugate. The automatic determination of the sertraline content on a full-automatic biochemical analyzer can be realized by the sertraline detection reagent containing the anti-sertraline specific antibody and the sertraline enzyme conjugate; the content of sertraline in a biological sample can be fast and precisely determined at high flux; the advantages of operation simplicity and convenience, high sensitivity, high specificity, high result accuracy and the like are realized; the sertraline detection costis effectively reduced; and the wide popularization and use in clinics are facilitated.
Owner:长沙博源医疗科技有限公司
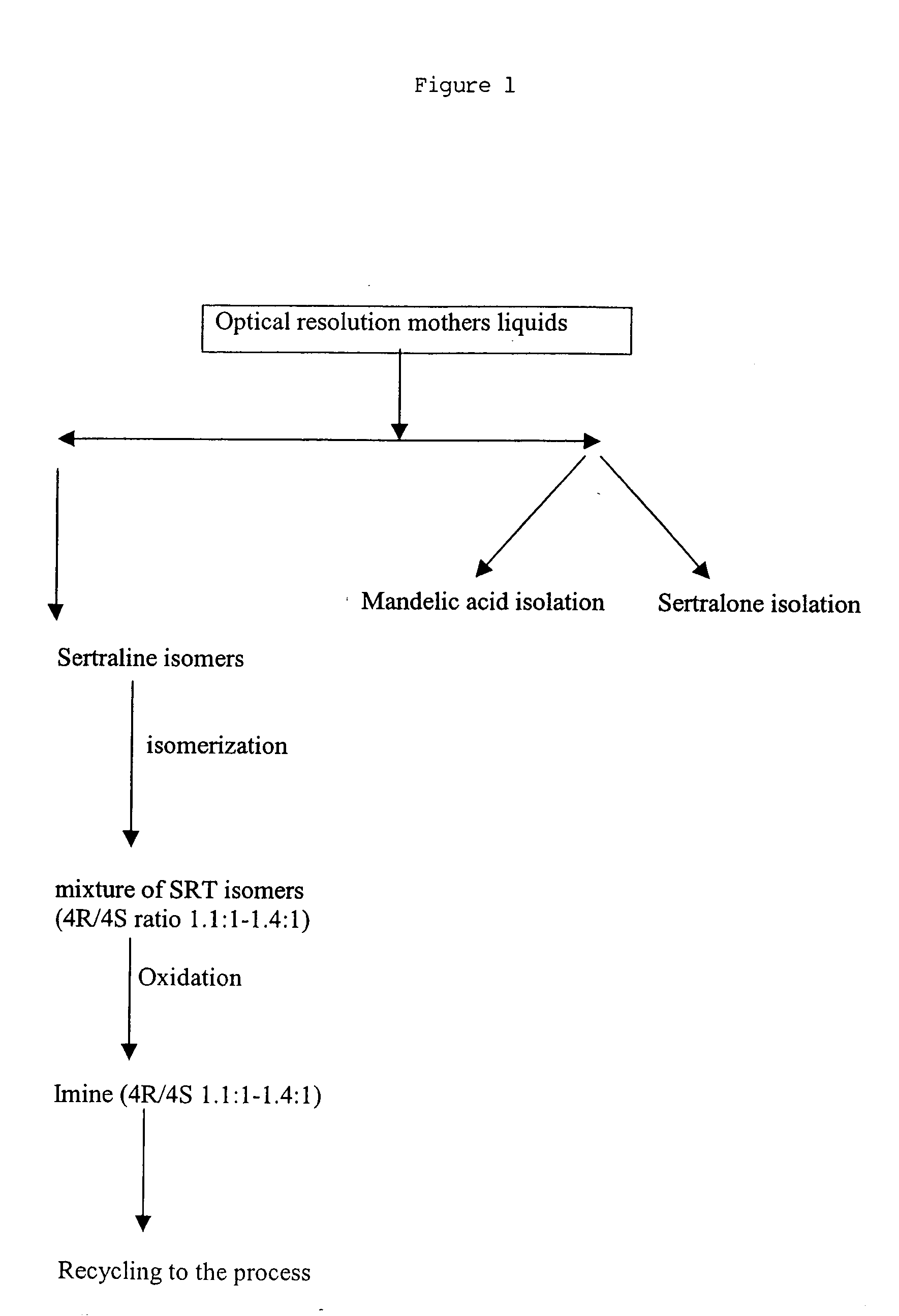
Recycling process for preparing sertraline
InactiveUS20070213562A1Amino compound purification/separationAmino preparation from aminesSertralineIndustrial scale
Provided is a recycling process for preparing sertraline, which may be carried out on an industrial scale.
Owner:TEVA PHARM USA INC
Method for treating neurasthenia or somatic form disorders and medicinal composition
ActiveCN1850271AOrganic active ingredientsSolution deliveryNorepinephrine reuptake inhibitorSertraline
The present invention relates to a method for curing and preventing neurosism or somatic formal disturbance and its medicine composition. Said invention belongs to the field of pharmacy. Said invention provides a medicine composition containing norepinephrine reuptake inhibitor with medicinal dose or its medicinal salt and selective 5-hydroxytryptamine reuptake inhibitor with medicinal dose or its medicinal salt for preventing and curing neurosism or somatic formal disturbance. The norepinephrine reuptake inhibitor is selected from reboxetine, tomoxetine, amfebutamone, nortriptyline, norimipramine, maprotiline and protiline, and the selective 5-hydroxytryptamine reuptake inhibitor is selected from sertraline, citalopram, s-citalopram, fluoroxeline, paroxetine and fluvoxamine.
Owner:SHENZHEN AUSA PHARM CO LTD +2
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
ActiveUS20140221388A1Effective treatmentNervous disorderAmine active ingredientsSertralineDuloxetine
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Recycling process for preparing sertraline
ActiveUS20050107636A1Increase SRaise the ratioAmino compound purification/separationAmino preparation from aminesSertralineBiochemical engineering
Provided is a recycling process for preparing sertraline, which may be carried out on an industrial scale.
Owner:TEVA PHARM USA INC
Novel method for Racemizing sertraline isomer
InactiveCN101284789ATake advantage ofMild reaction conditionsAmino compound purification/separationSertralineOrganic solvent
The invention discloses a method for the racemization of sertraline isomer. The prior method has the disadvantages that the product recovery rate is low, the cost is high, different processing methods are adopted for different isomers, and the steps are complex. In the method, an organic solvent and a strong base are added into the sertraline isomer, racemization processing is performed after the sertraline isomer is heated to backflow, heat preservation is performed, distillation is performed to remove the solvent, water is added, the pH value of the solution is adjusted by acid, extraction is performed, and then the extract is dried by distillation to obtain the sertraline. The method can perform racemization processing to all sertraline isomers and can be completed in one process, the sertraline isomer is fully utilized, and the content of obtained sertraline is high.
Owner:ZHEJIANG JINGXIN PHARMA
Novel omega-3 and omega-6 fatty acid compositions and uses thereof
InactiveUS20130295179A1Preventing functionsReducing secondary adverse eventsHeavy metal active ingredientsBiocideSertralineStimulant
Owner:TERREAUX CHRISTIAN +3
Combination therapy for the treatment of diabetes
InactiveUS20110312946A1Relieve symptomsLower Level RequirementsBiocideMetabolism disorderDiabetes mellitusSertraline
The present invention provides methods of treating, reducing, preventing, or inhibiting symptoms of diabetes and / or lowering plasma levels of HbA1c by co-administration of therapeutic or subtherapeutic doses of telenzepine and sertraline to a subject in need thereof.
Owner:THERAKOS INC
Sertraline side chain amino structure derivative as well as preparation method and application thereof
ActiveCN112661657AObvious sensitization effectEnhanced inhibitory effectOrganic compound preparationPreparation by reductive alkylationSertralineSide chain
The structural formula of the sertraline side chain amino structure derivative is shown as a formula I or a formula II, wherein R1 is H or cyclopropanyl, and R2 is selected from alkyl, naphthyl, alkylamine, cyclopropanyl, phenyl, or phenyl of which the No.3 position and / or the No.4 position is substituted by alkyl, methoxyalkyl, haloalkyl and halogen; and R3 is a hydrogen group or a 1-halogenated alkyl group. The invention also provides a preparation method of the sertraline derivative and application of the sertraline derivative in preparation of a medicine for treating gastric cancer. The sertraline derivative provided by the invention has an obvious sensitization effect on drug-resistant gastric cancer cells.
Owner:ARMY MEDICAL UNIV
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
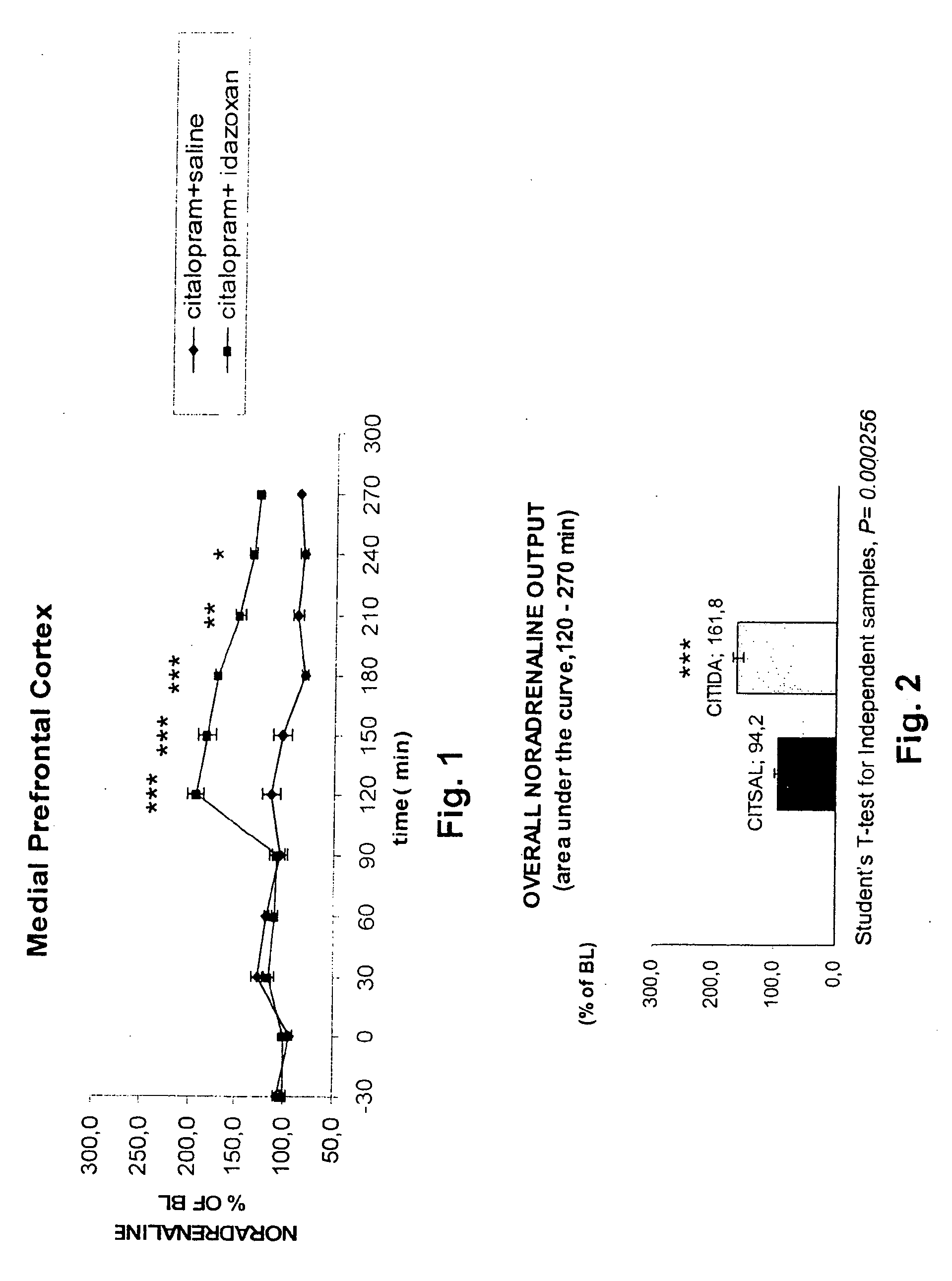
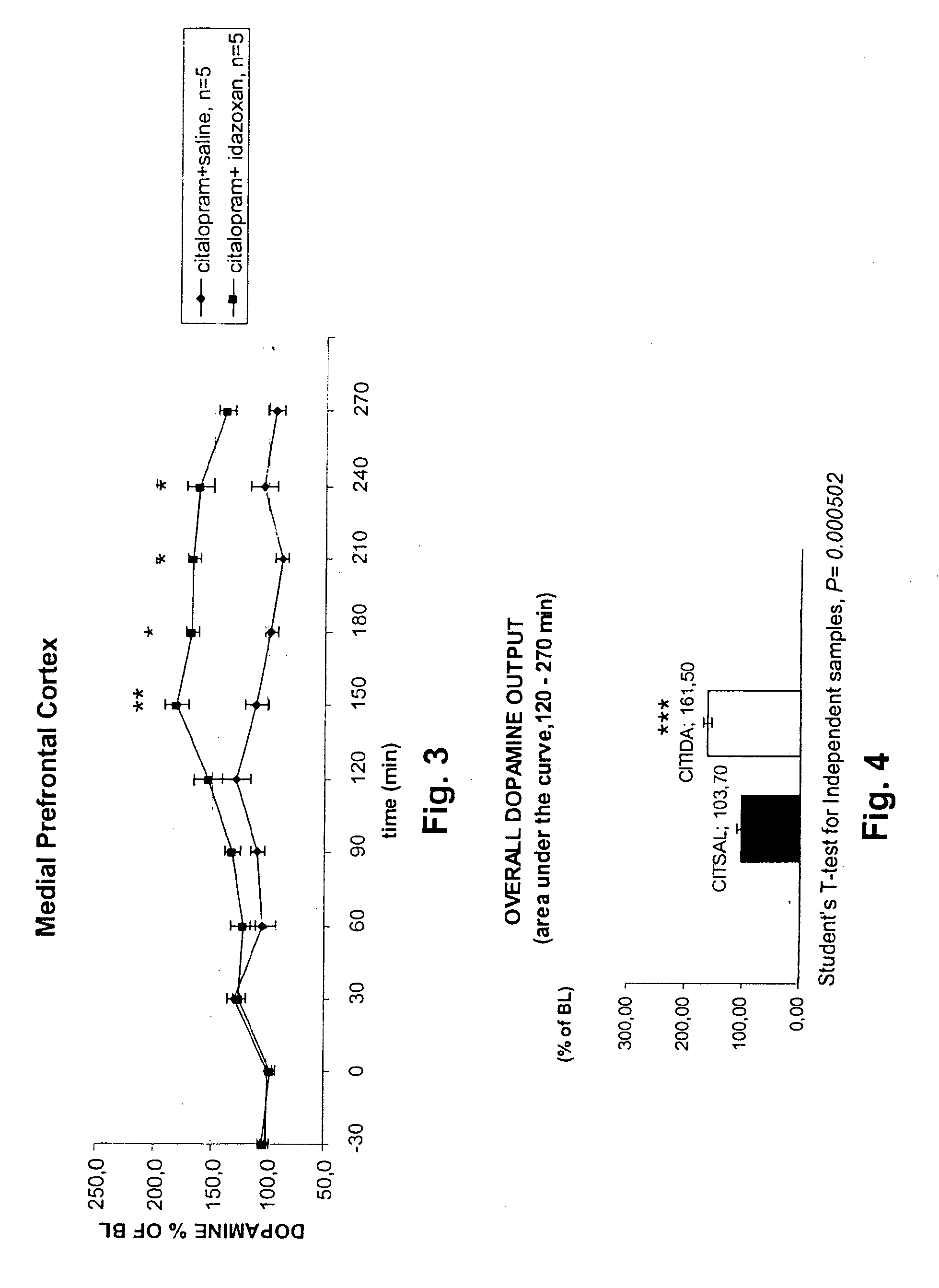
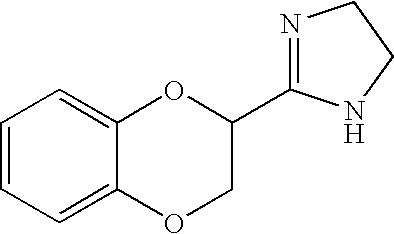
Antidepressant Medicament Comprising Idazoxan and a Selective Serotonin Reuptake Inhibitor
Use of Idazoxan or an active enantiomer thereof, and treatment of patients receiving therapy with selective serotonin uptake inhibitor(s), such as Fluoxetine, Citalopram, Paroxetine, Sertraline and Fluvoxamine, are described. The patients being treated are in particular those who are suffering from severe depression, anxiety-depression, PTSD (Post Traumatic Stress Disorder), or ADHD (Attention Deficit Hyperactive Disorder).
Owner:ALPHA 2 PHARMA
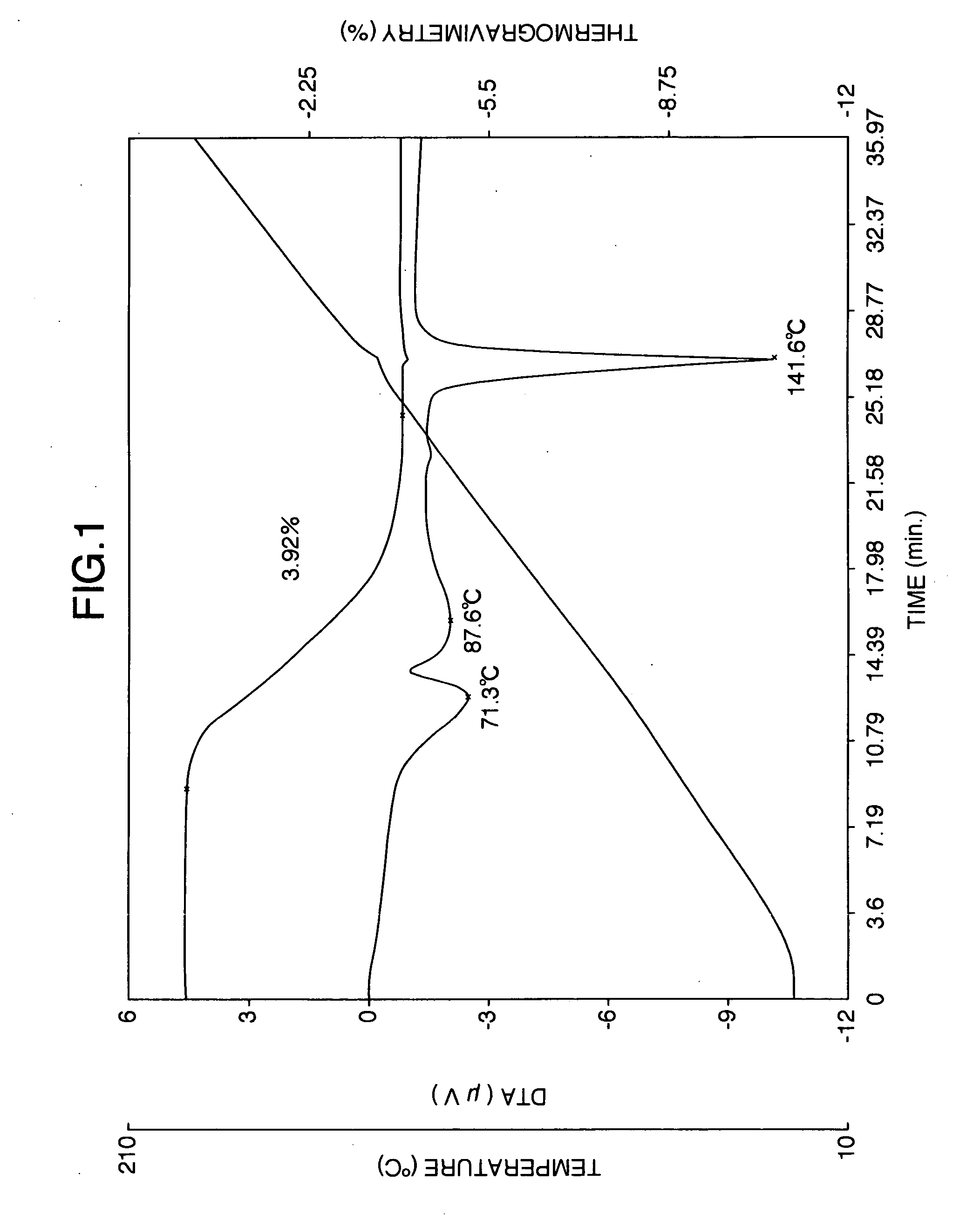
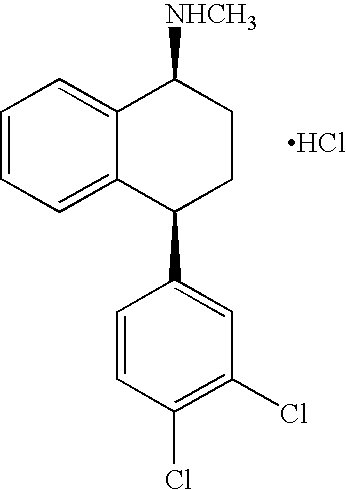
Two crystal forms of sertraline citrate, and preparation methods thereof
InactiveCN105061217AImprove performanceImprove bioavailabilityOrganic active ingredientsAmino compound purification/separationSertralineBiochemical engineering
The invention relates to the field of polymorphism researches of medicine synthesis, and concretely relates to a crystal form A and a crystal form B of sertraline citrate, and preparation methods thereof. The crystal form A and the crystal form B of sertraline citrate have the advantages of good medicinal performances, medicinal preparation requirement meeting, good dissolvability, high dissolubility, high stability and storage benefiting, so the requirement of medicine stability is met. The preparation methods have the advantages of simple operation, few steps, mild preparation conditions, small environmental protection pressure, and suitableness for industrial production.
Owner:徐静 +1
Solubilized sertraline compositions
Compositions of matter comprising sertraline and a solubilizing agent which increases the solubility of sertraline in aqueous chloride ion-containing use environments.
Owner:PFIZER PRODS ETAT DE CONNECTICUT
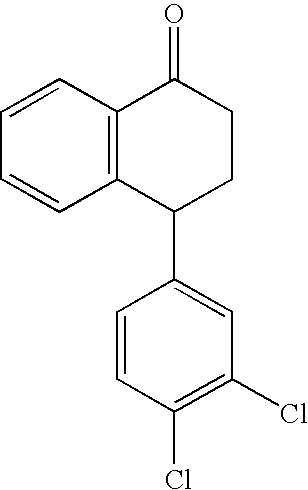
Highly Steroselective Synthesis of Sertraline
InactiveUS20070260090A1Organic compound preparationAmino compound preparationSertralineHydrogen pressure
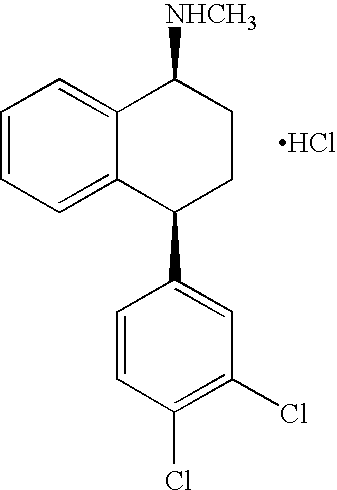
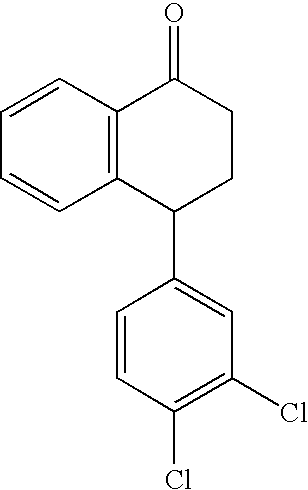
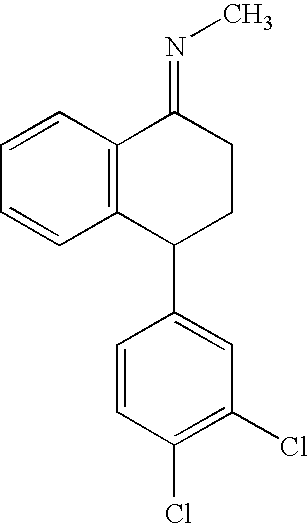
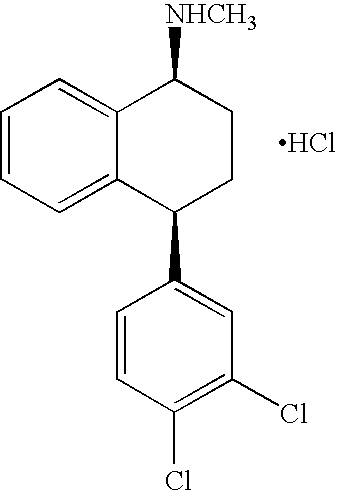
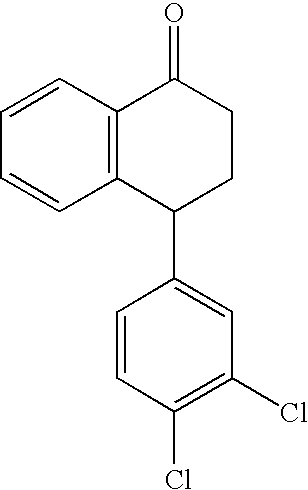
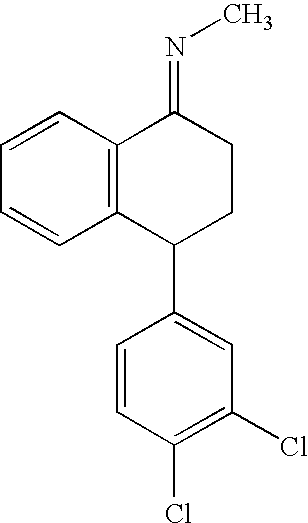
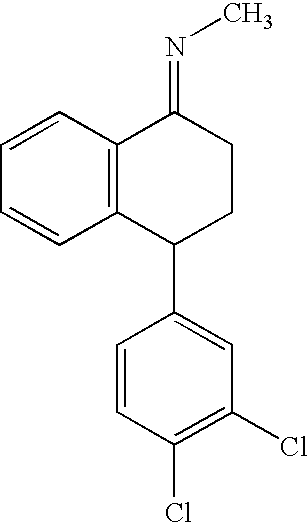
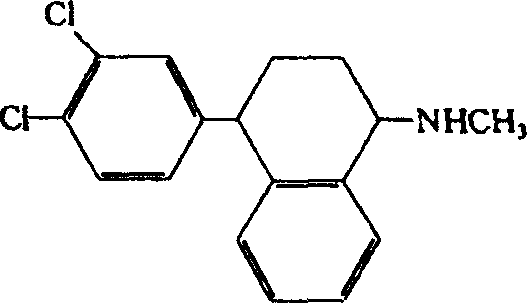
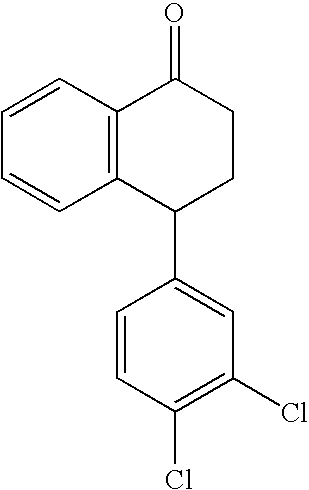
The present invention relates to a process for highly stereoselective synthesis of sertraline and sertraline intermediate. Thus, the mixture of 4-(3,4-Dichlorophenyl)-3,4-dihydro-N-methyl-1(2H)-naphthalenimine, 5% Pd / CaCO3, water and methanol is taken in a hydrogenation flask and then subjected to hydrogenation under a hydrogen pressure of 0.5 Kg at 20-35° C. for 3 hours 30 minutes. The catalyst is removed by filtration and the solvent is evaporated completely under vacuum to obtain cis-(±)-4-(3,4-dichlorophenyl)-1,2,3,4-tetrahydro-N-methyl-naphthalen amine. (trans-(±): 0.2).
Owner:HETERO DRUGS LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com