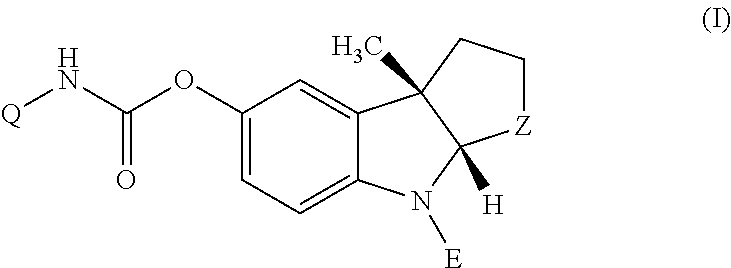
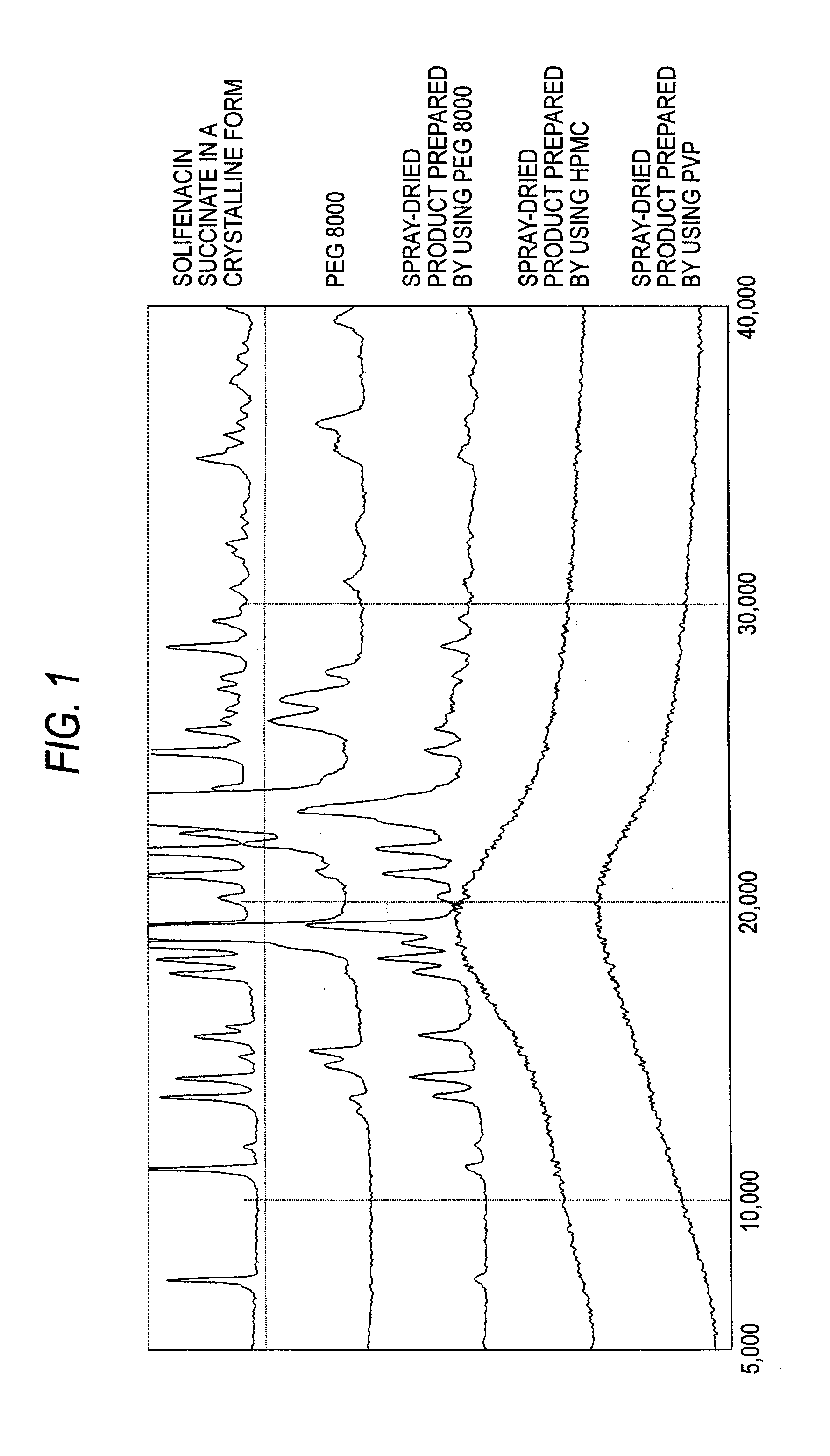
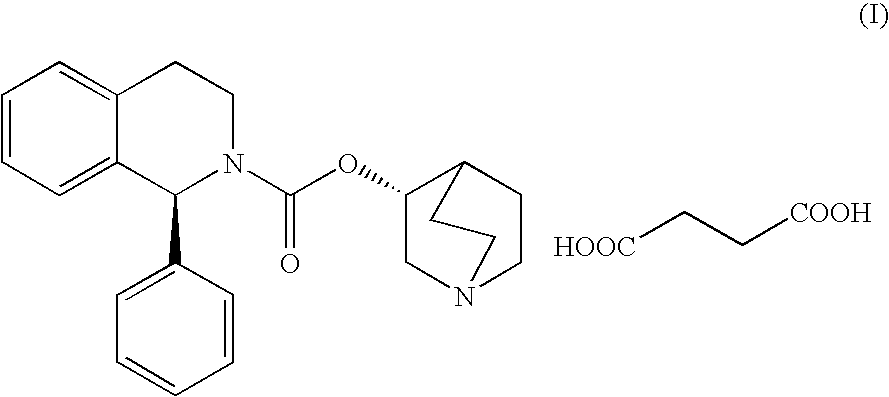
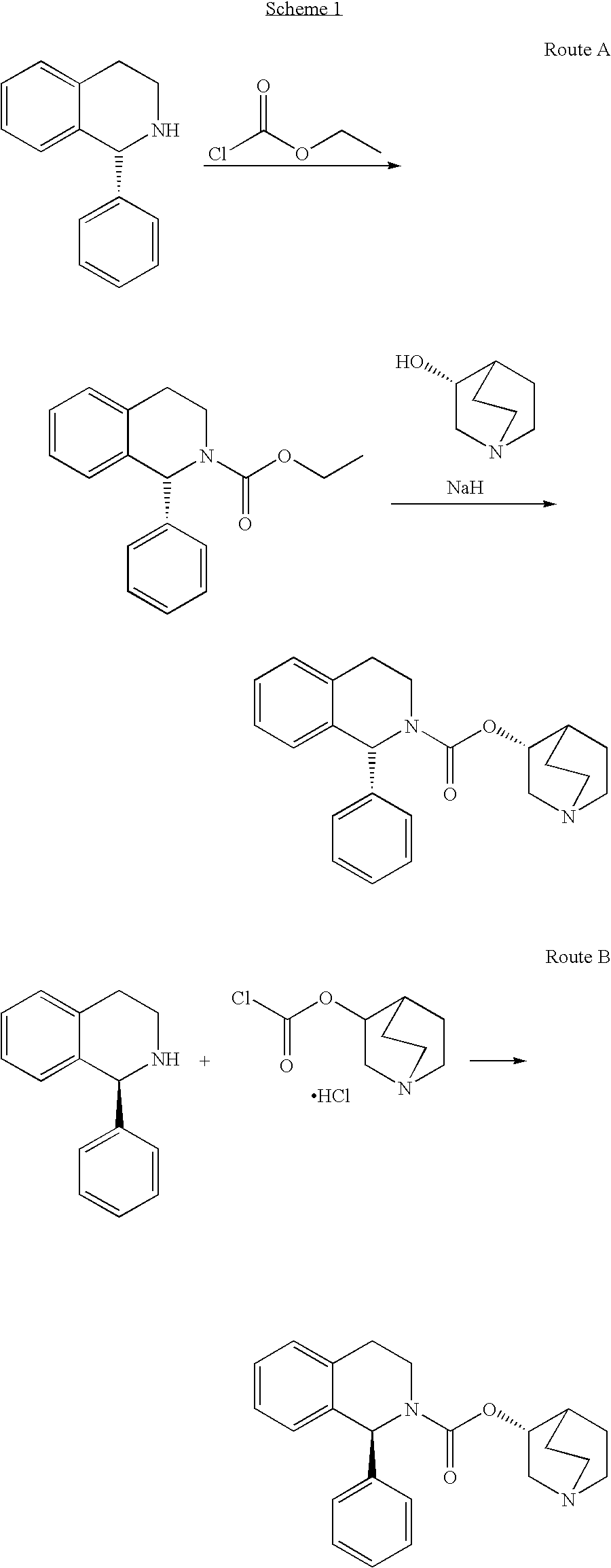
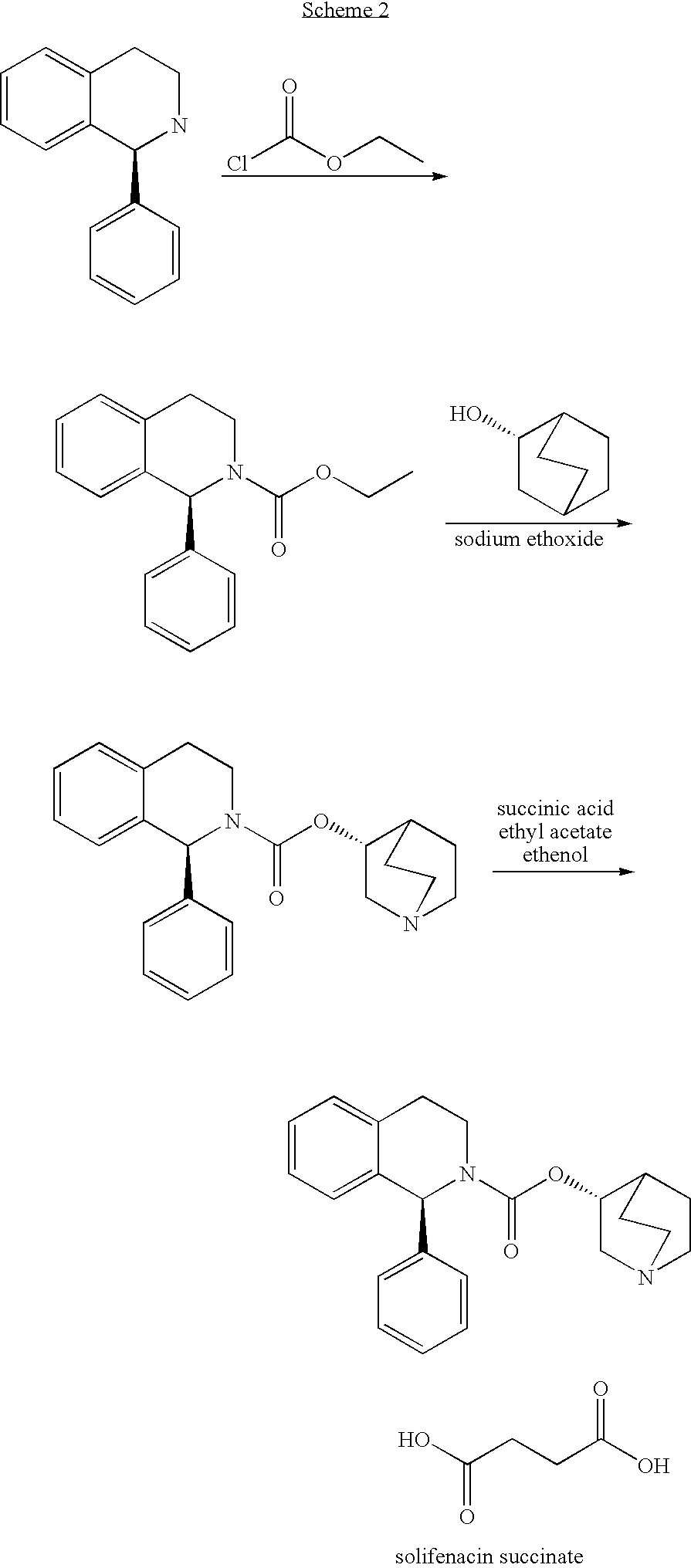
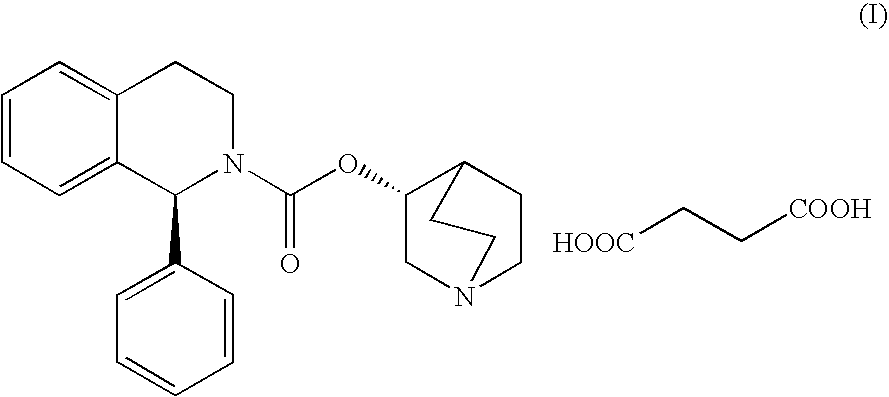
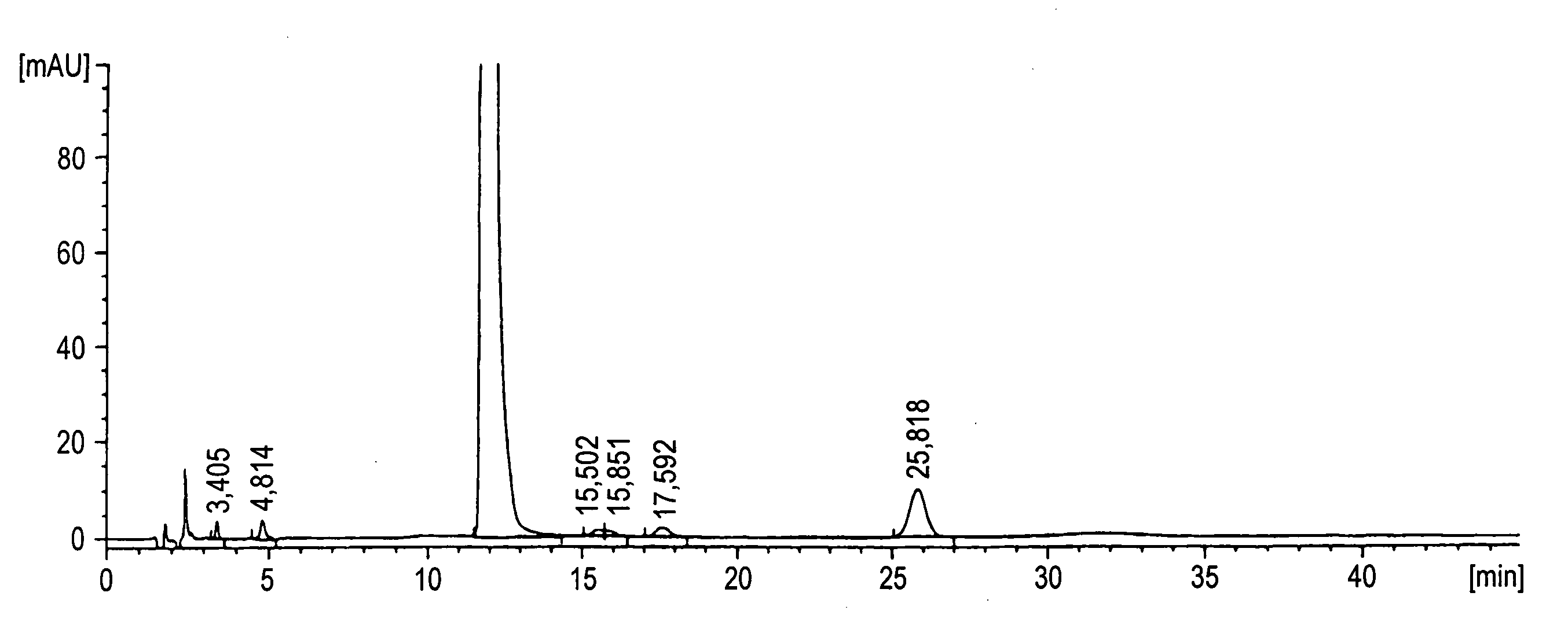
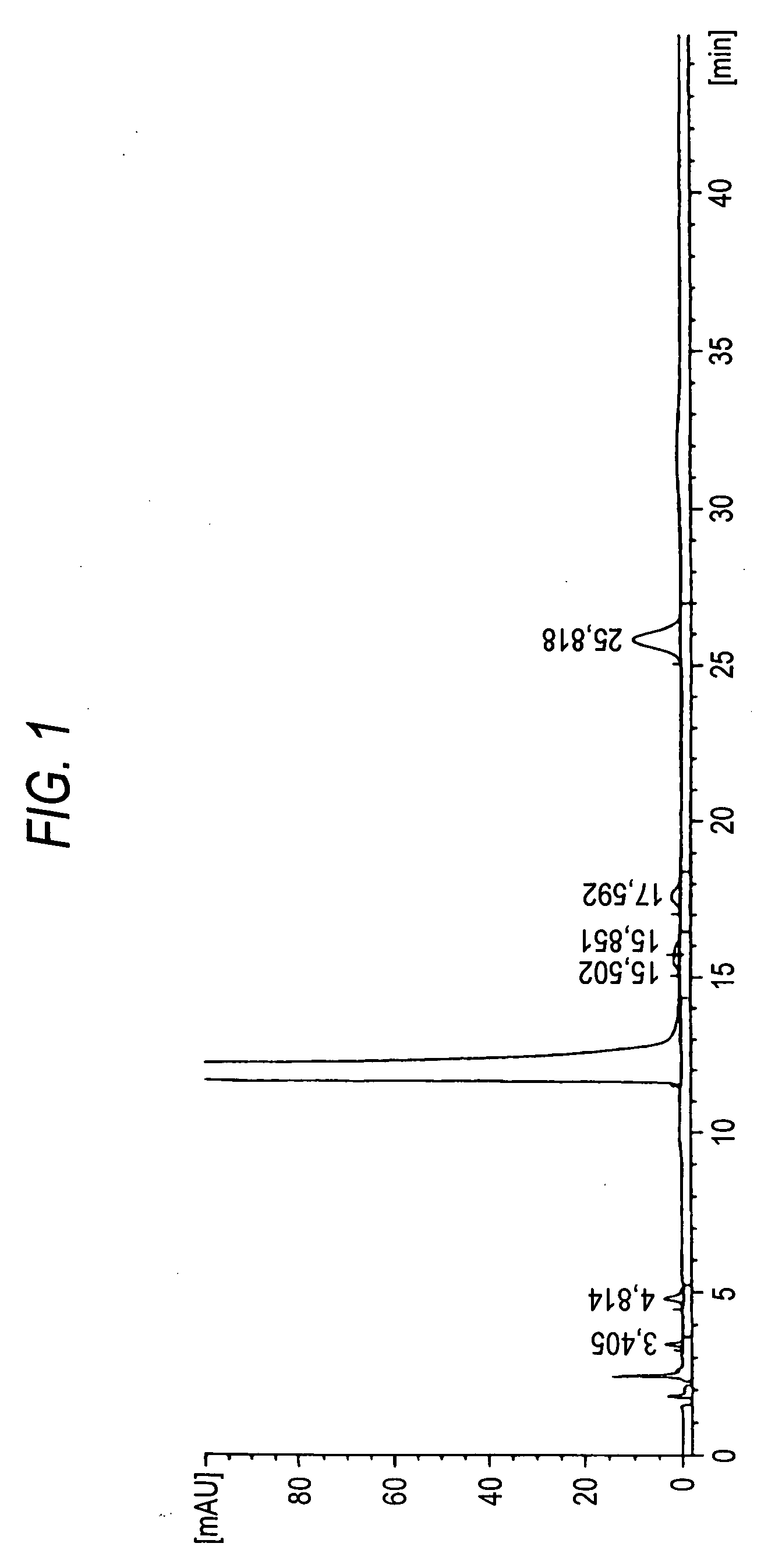
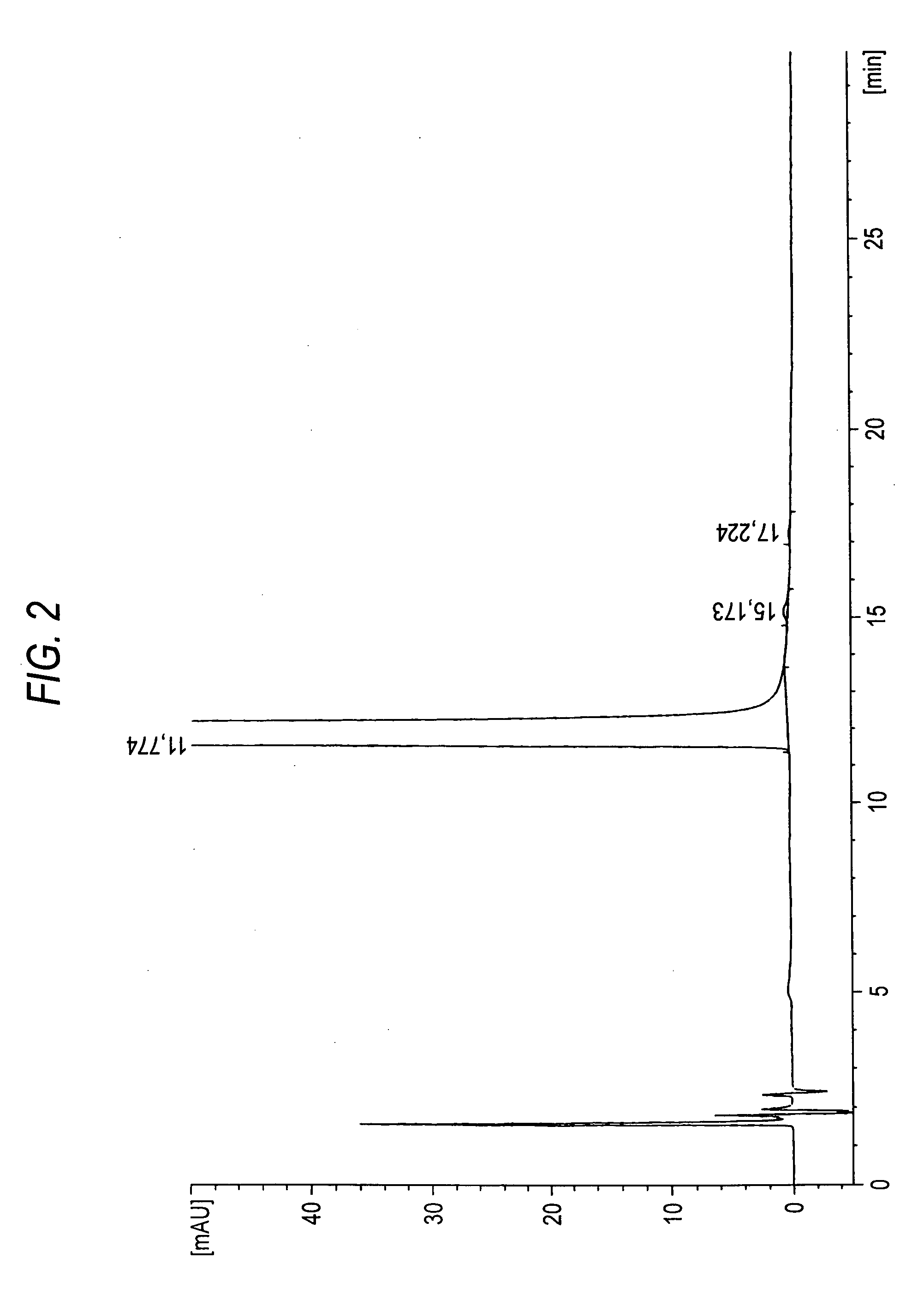
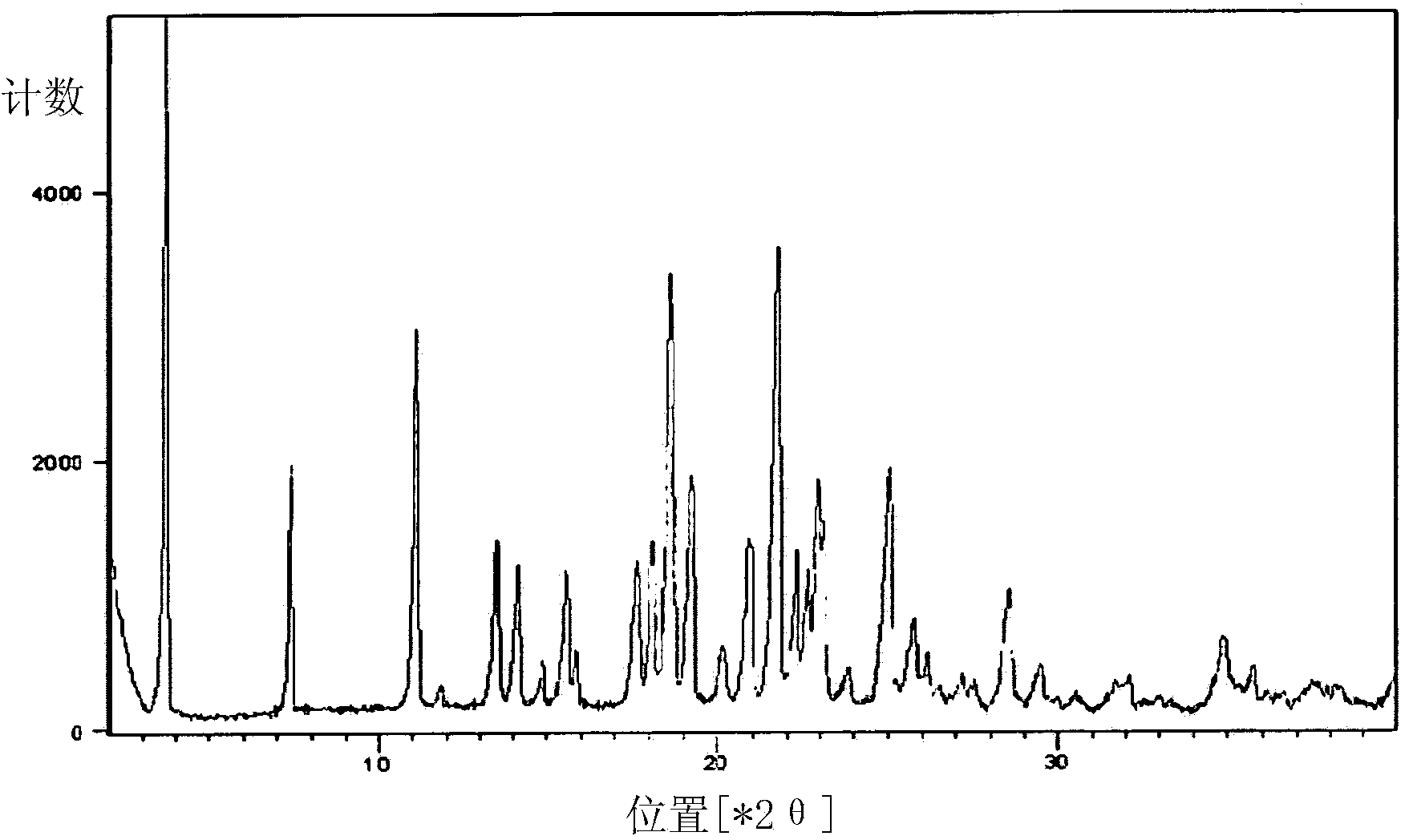
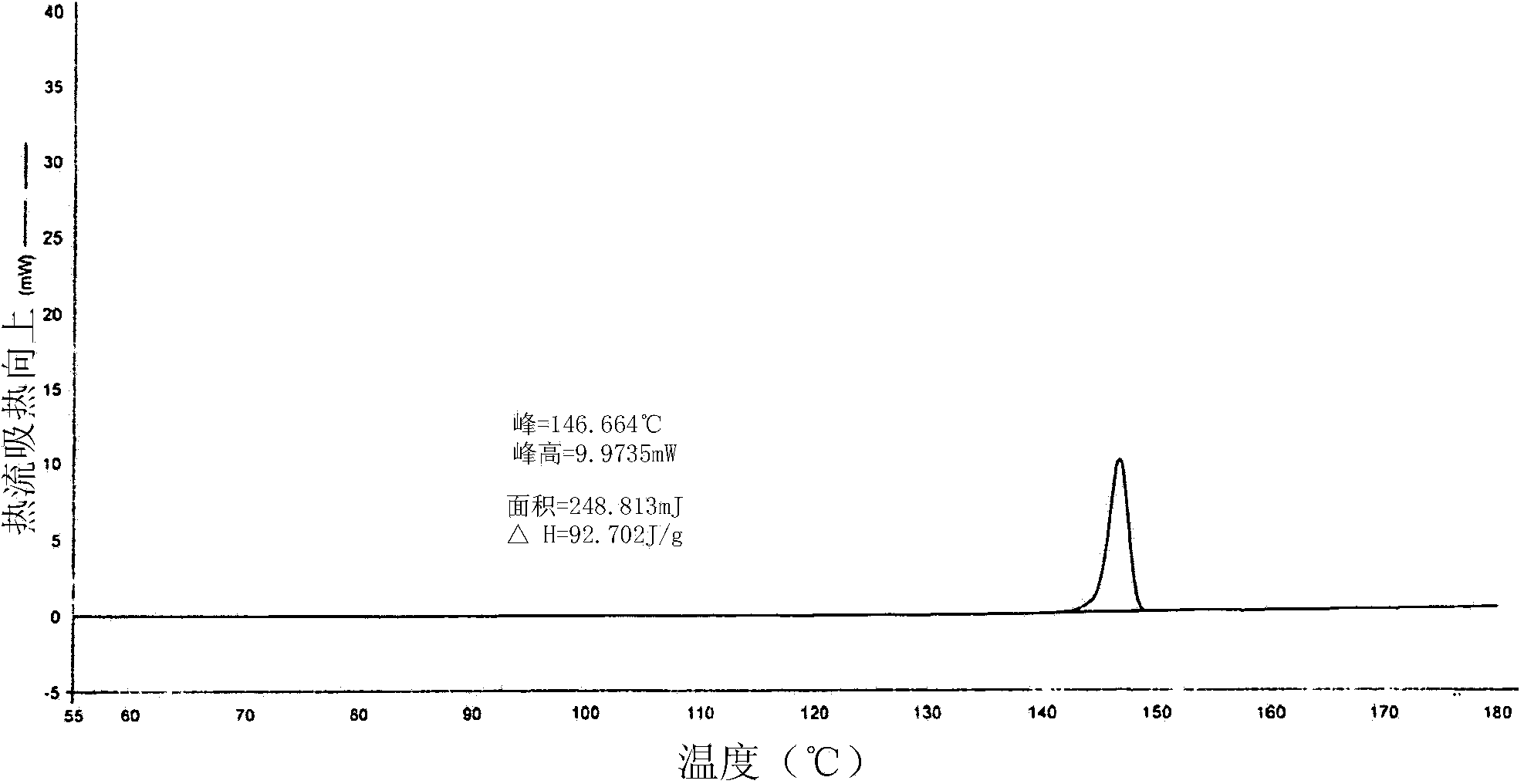
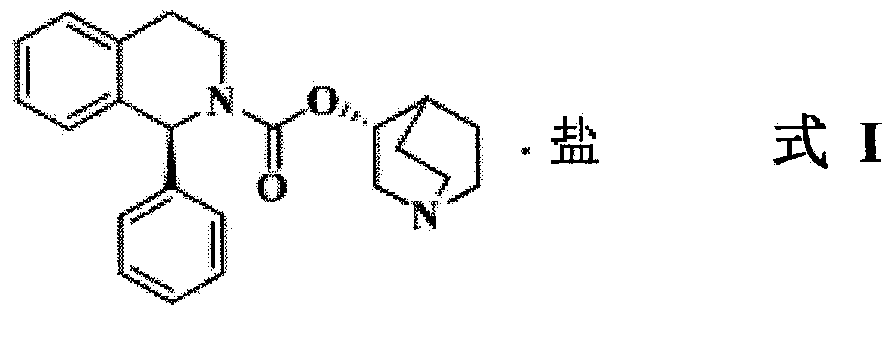
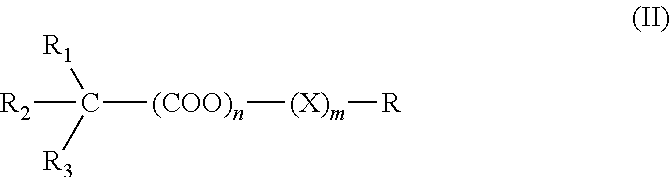Patents
Literature
65 results about "Solifenacin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
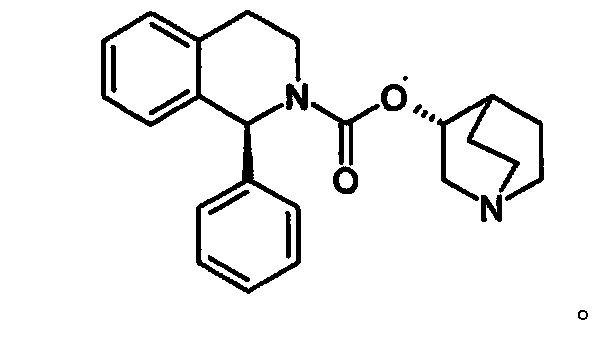
Solifenacin is used to treat an overactive bladder.
Solifenacin transdermal preparation and method for enhancing transdermal permeation thereof
InactiveUS20050181031A1Improves transdermal penetrationExceeds levelBiocideOrganic non-active ingredientsDiseaseSolifenacin
The transdermal permeable property of solifenacin or a salt thereof as a biologically active substance, is remarkably improved using a fatty acid ester, a terpene or the like as a selected transdermal permeable promoter. The difference in effect exceeded expectations by producing an enhancement in permeability that represents an increase of several hundredfold at most, and several tenfold for practical application, enabling the provision of extremely useful means for preventive and therapeutic agents for urologic diseases or respiratory diseases that use solifenacin.
Owner:ASTELLAS PHARMA INC
Memantine combinations and use
ActiveUS20180116979A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
Stable Particular Pharmaceutical Composition of Solifenacin or Salt Thereof
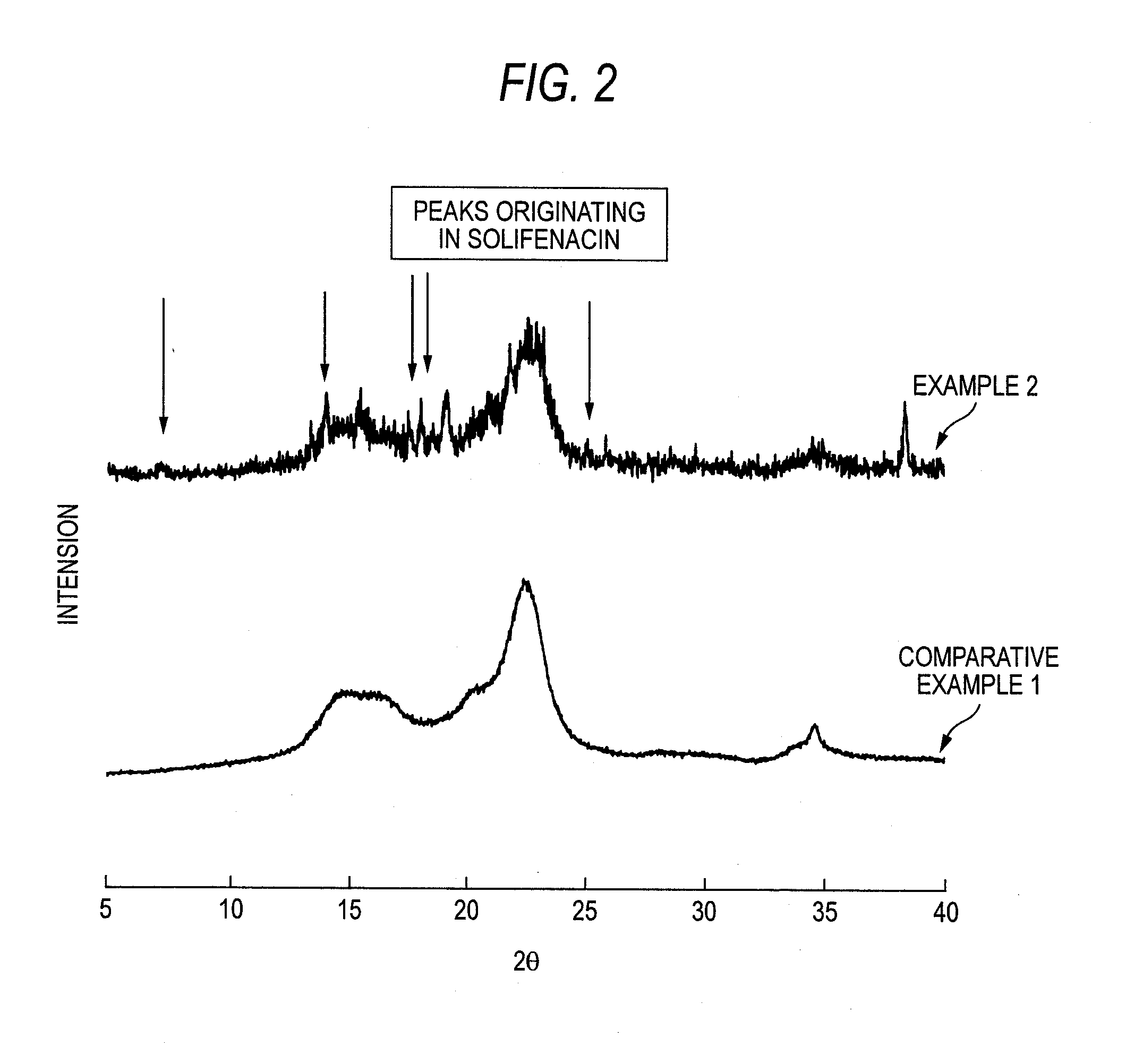
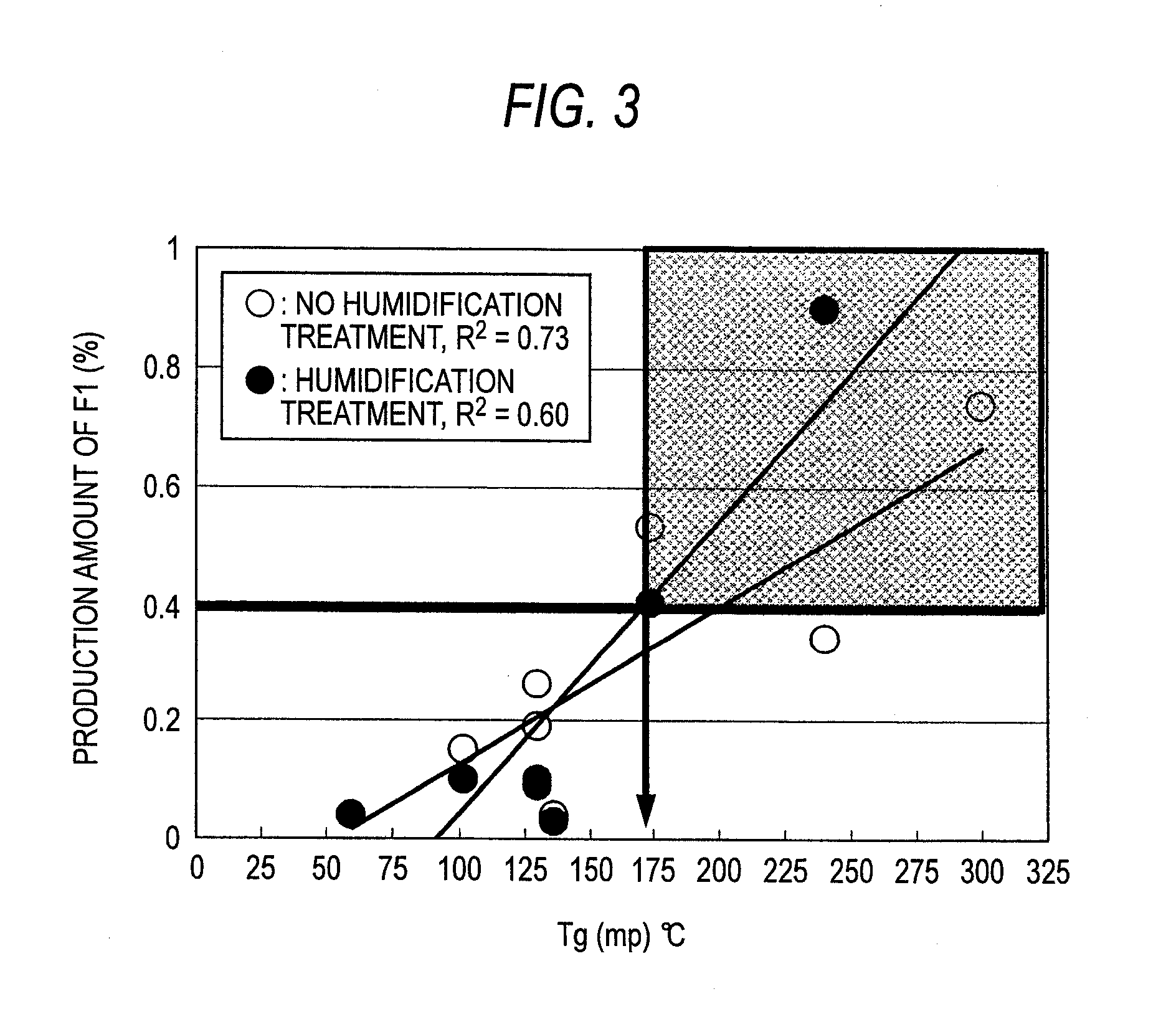
InactiveUS20080103171A1Improve stabilityEasy to getBiocidePharmaceutical non-active ingredientsParticulatesSolifenacin
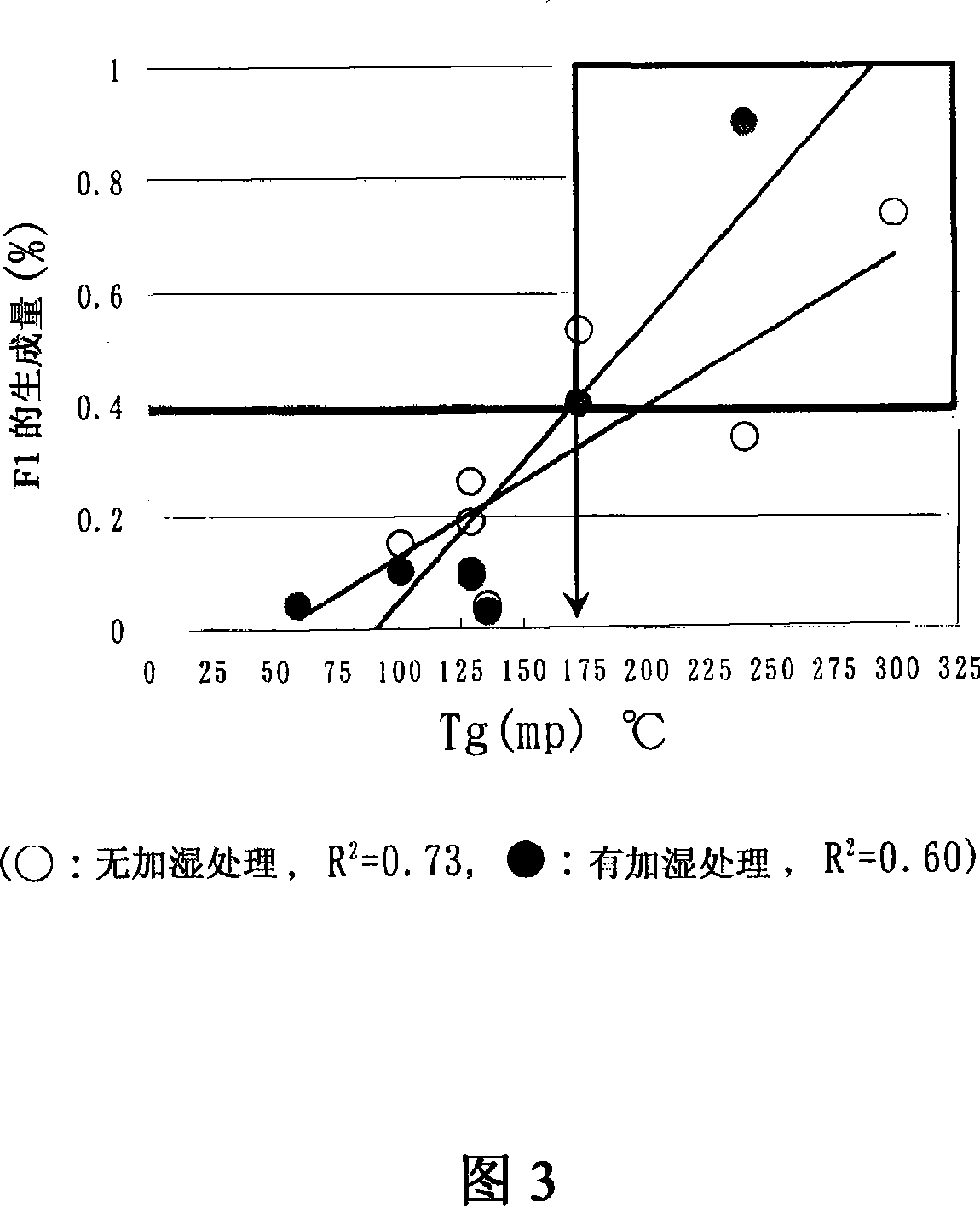
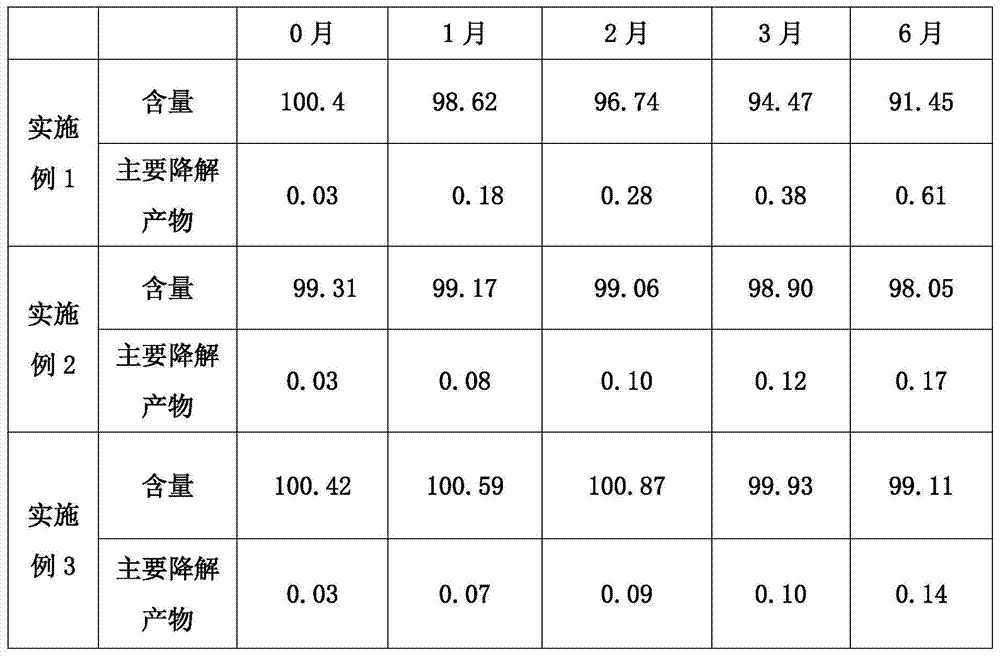
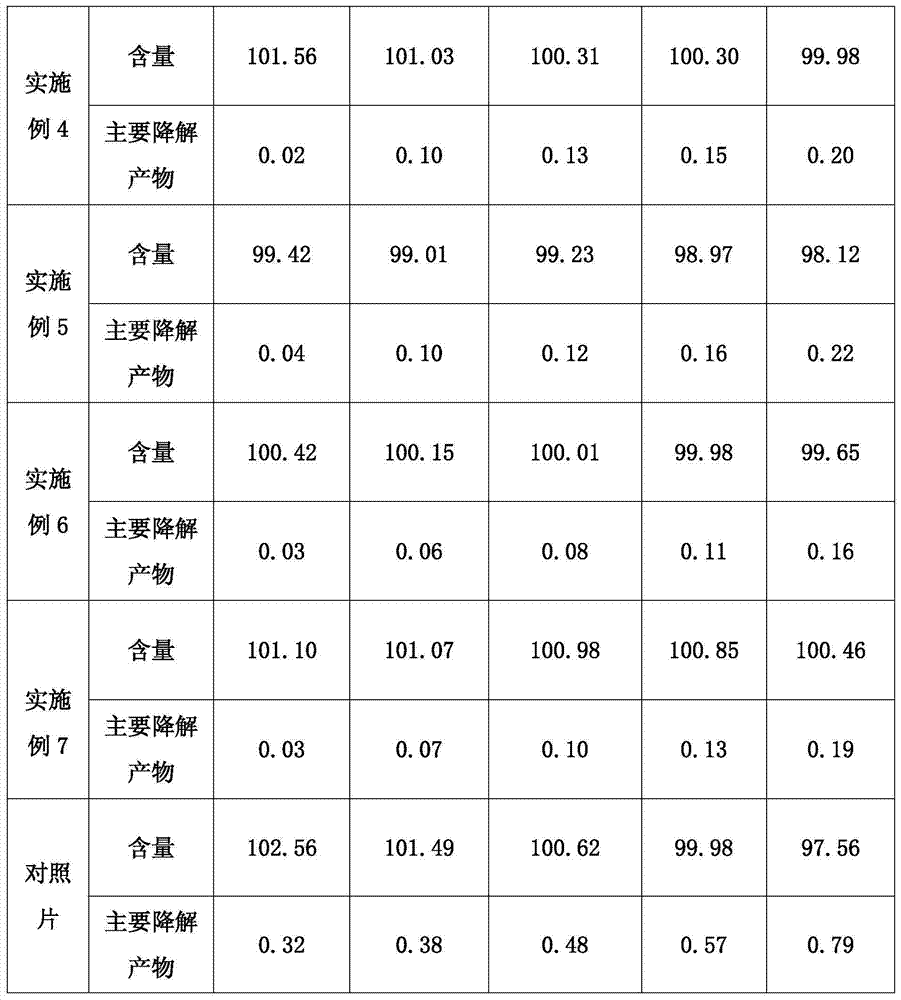
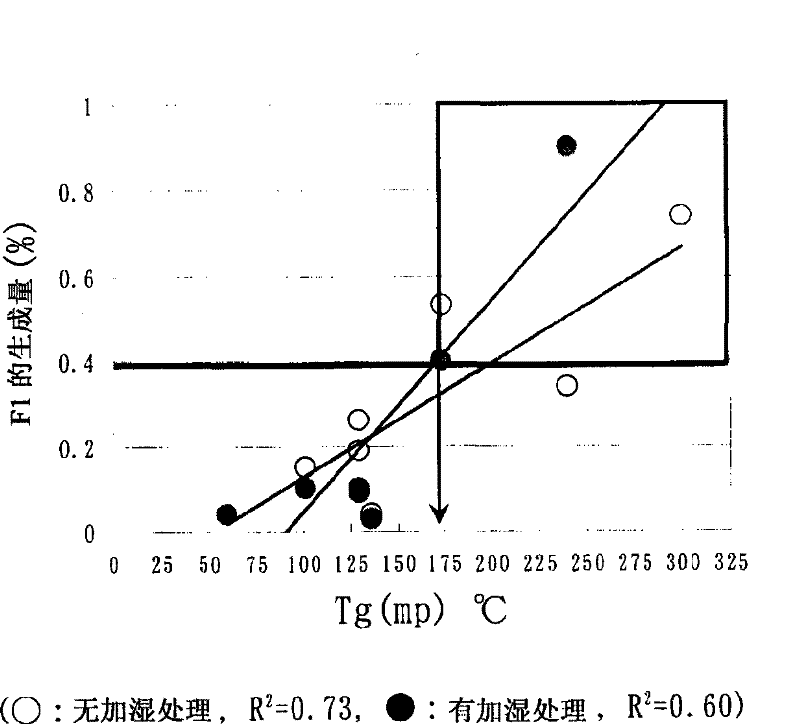
The present invention relates to the provision of a stable particulate pharmaceutical composition of solifenacin or a salt thereof, which is in a spherical shape suitable for coating and in which degradation with time can be inhibited when a pharmaceutical preparation of solifenacin or a salt thereof is supplied to clinical fields. More particularly, it relates to a particulate pharmaceutical composition that can be obtained by using a binder having a Tg or mp lower than 174C upon formulating a particulate composition of solifenacin into a pharmaceutical preparation. Further, by performing a crystallization-promoting treatment after the particulate pharmaceutical composition is produced, a more stable particulate composition of solifenacin or a salt thereof can be provided.
Owner:ASTELLAS PHARMA INC
Combined Use of an Alpha-Adrenergic Receptor Antagonist and an Anti-Muscarinic Agent
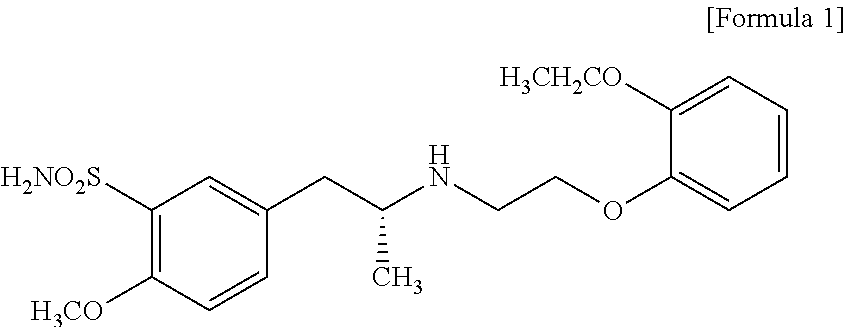
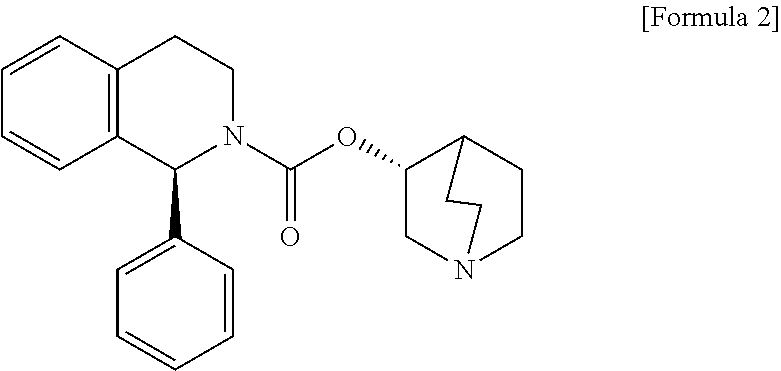
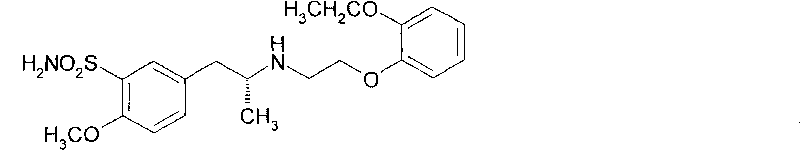
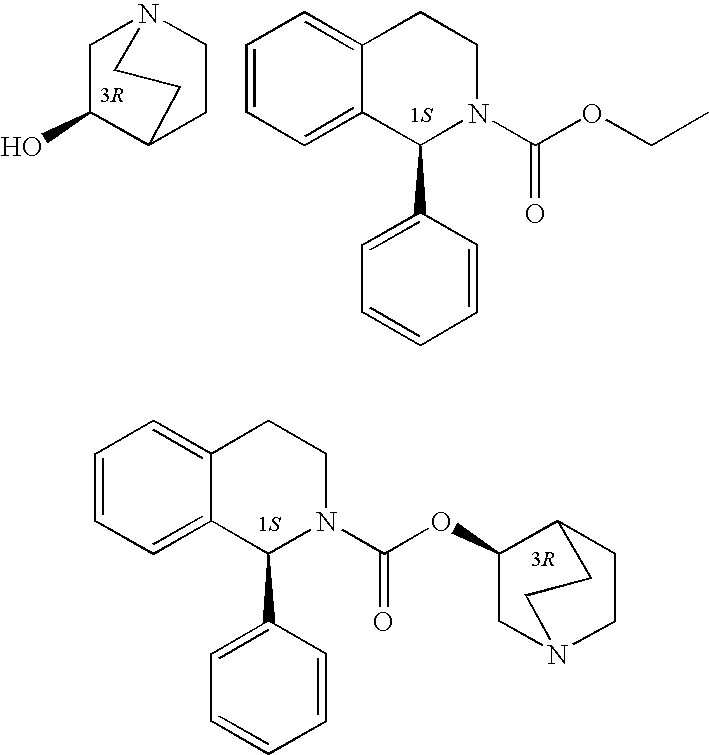
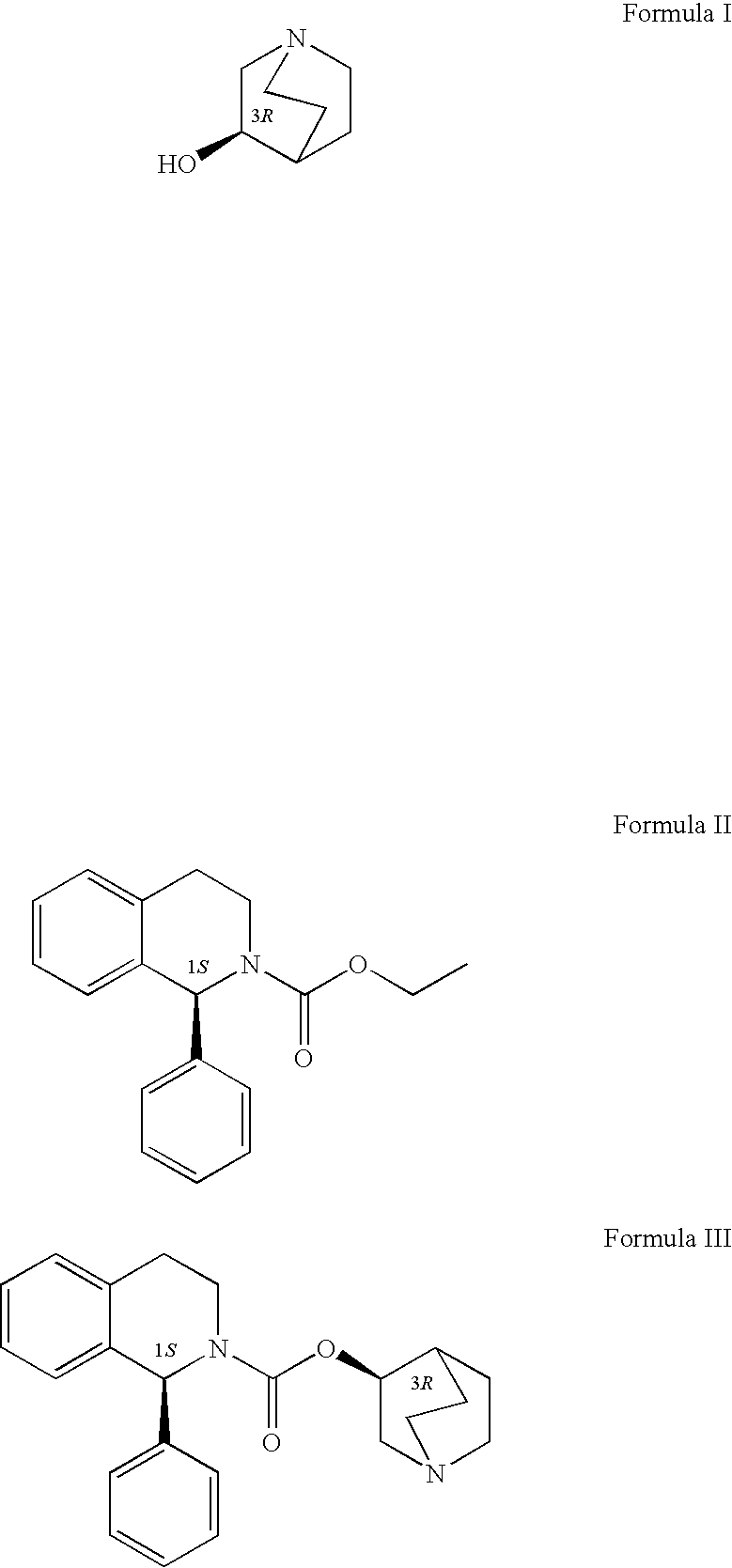
The combined use of (R)-5-(2-{[2-(2-ethoxyphenoxy)ethyl]amino}propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin), or its pharmaceutically acceptable salt, and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylic acid (3R)-quinuclidin-3-yl ester (solifenacin), or its pharmaceutically acceptable salt, for the preparation Of a medicament for the improvement of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS / BPH) with a substantial storage component is provided.
Owner:ASTELLAS IRELAND
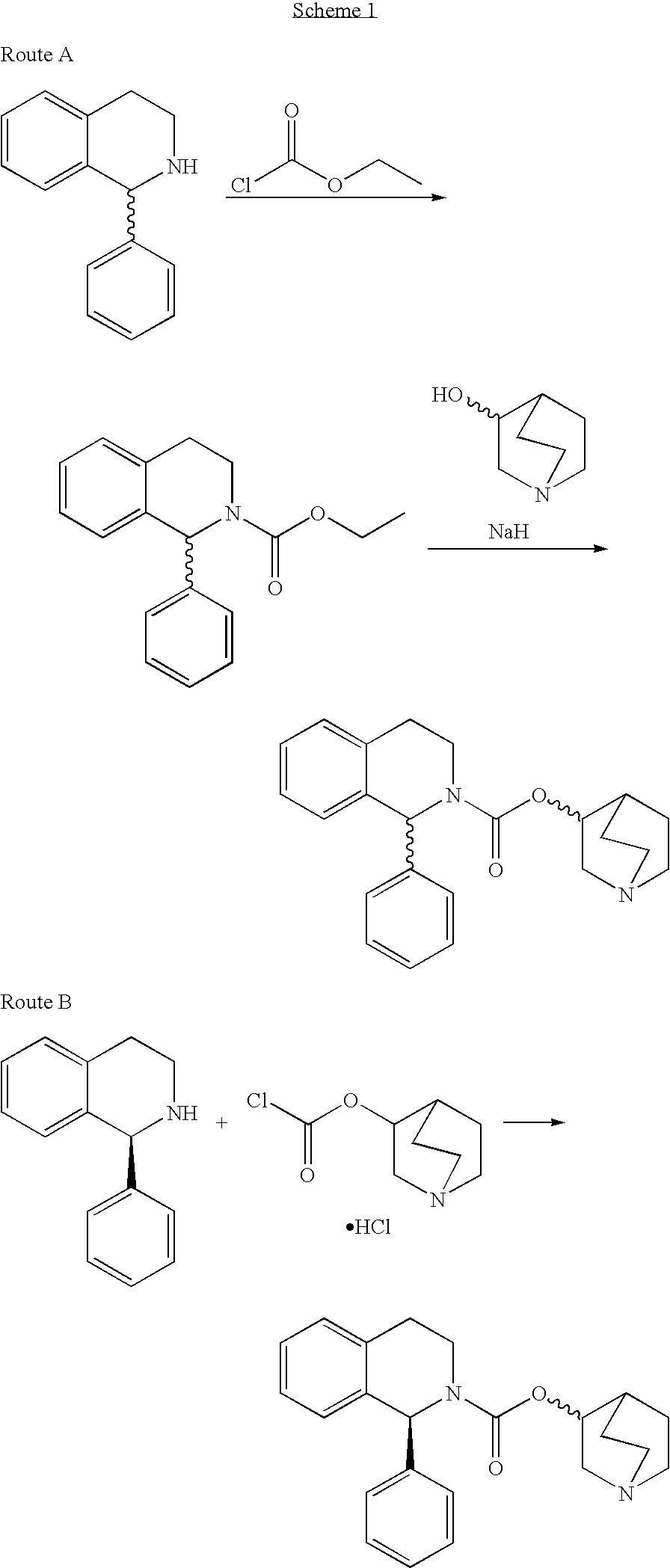
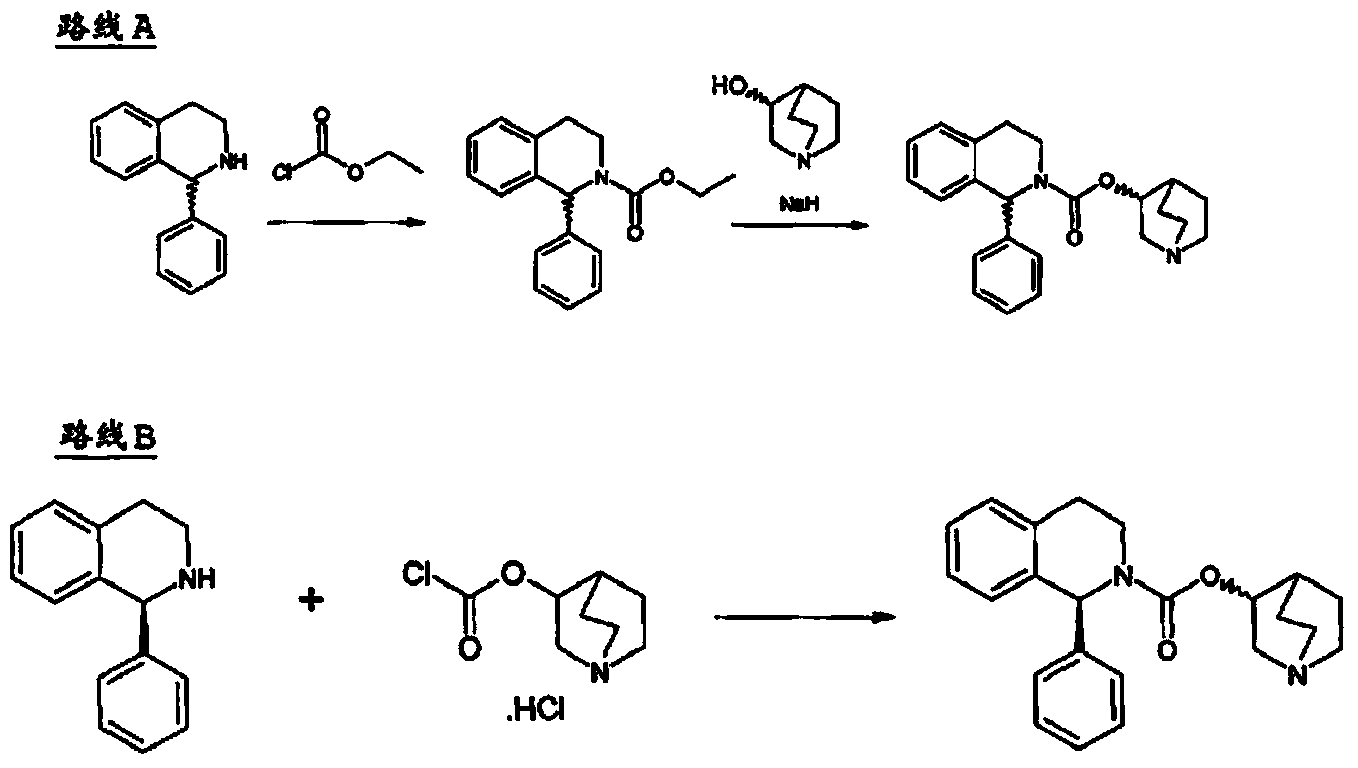
Process for the Synthesis of Solifenacin
This invention provides improved methods for making solifenacin and pharmaceutically acceptable salts thereof. The instant methods are unexpectedly advantageous in their simplicity and efficiency.
Owner:MEDICHEM
Process for the synthesis of solifenacin
InactiveUS20080242697A1Efficient and simplified wayHigh yieldBiocideOrganic chemistrySolifenacinCombinatorial chemistry
The present invention provides an improved synthetic strategy for the preparation of solifenacin and pharmaceutically acceptable salts thereof.
Owner:MEDICHEM
Solid pharmaceutical composition containing solifenacin amorphous form
The present invention relates to the provision of a stable solid pharmaceutical composition containing an amorphous form of solifenacin or a pharmaceutically acceptable salt thereof and capable of inhibiting decomposition accompanied by long-term storage, to provide the medical field with a formulation of solifenacin or a pharmaceutically acceptable salt thereof. More particularly, the present invention relates to a solid pharmaceutical composition, comprising an amorphous form of solifenacin or a pharmaceutically acceptable salt thereof, and one stabilizer for amorphous solifenacin or two or more stabilizers for amorphous solifenacin, selected from the group consisting of citric acid or a pharmaceutically acceptable salt (excluding a calcium salt) thereof, sodium pyrosulfite, and a pharmaceutically acceptable salt of ethylenediaminetetraacetic acid.
Owner:ASTELLAS PHARMA INC
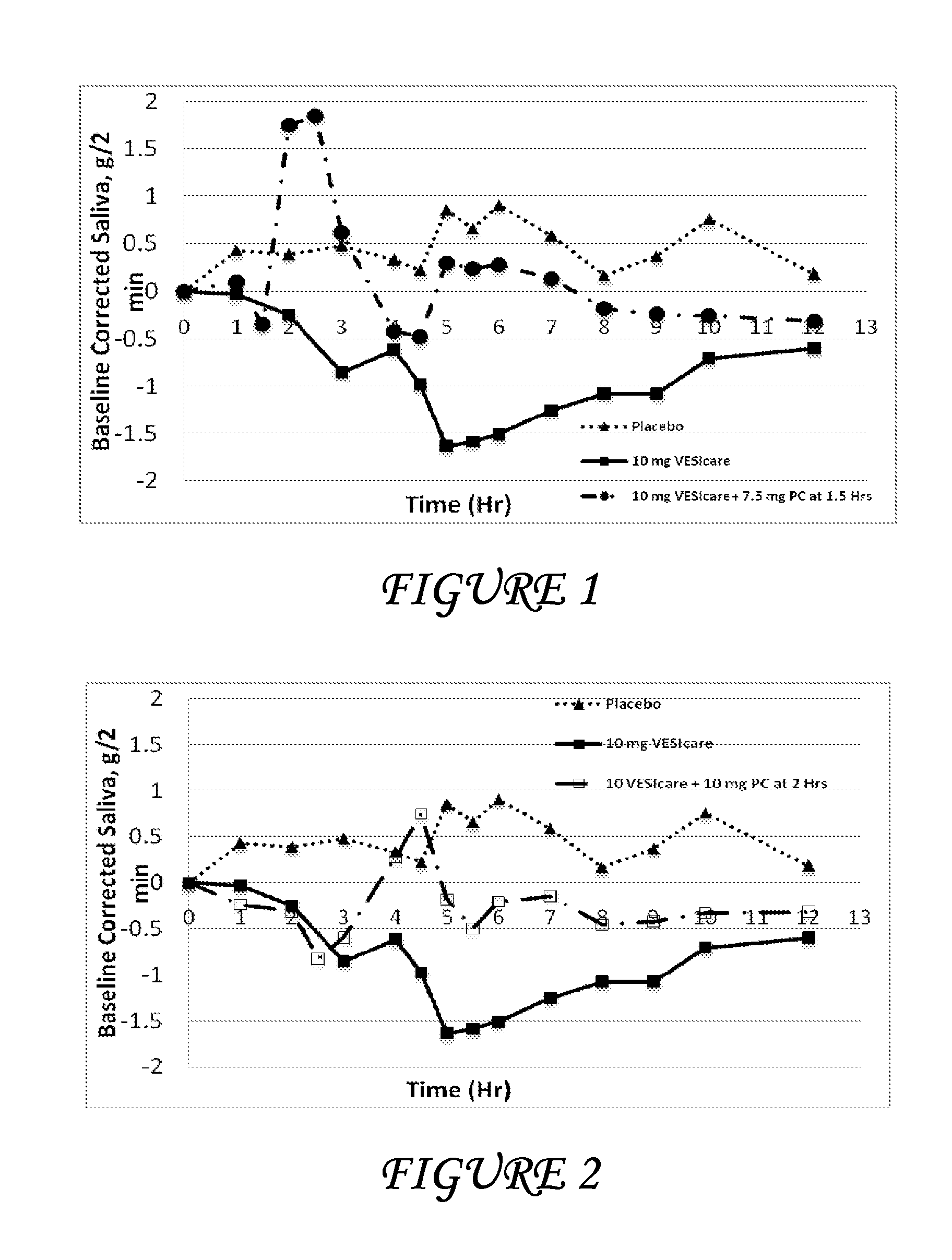
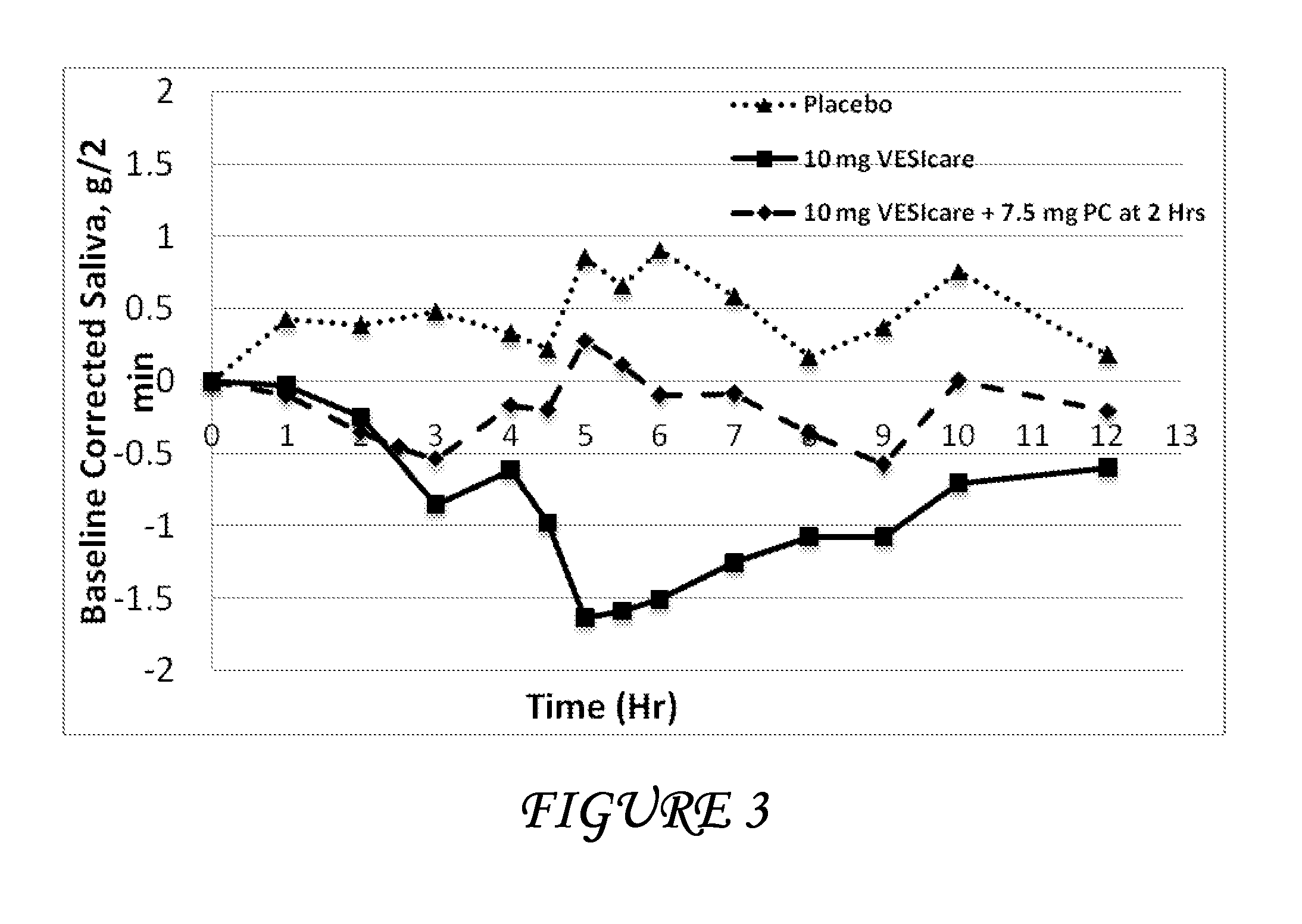
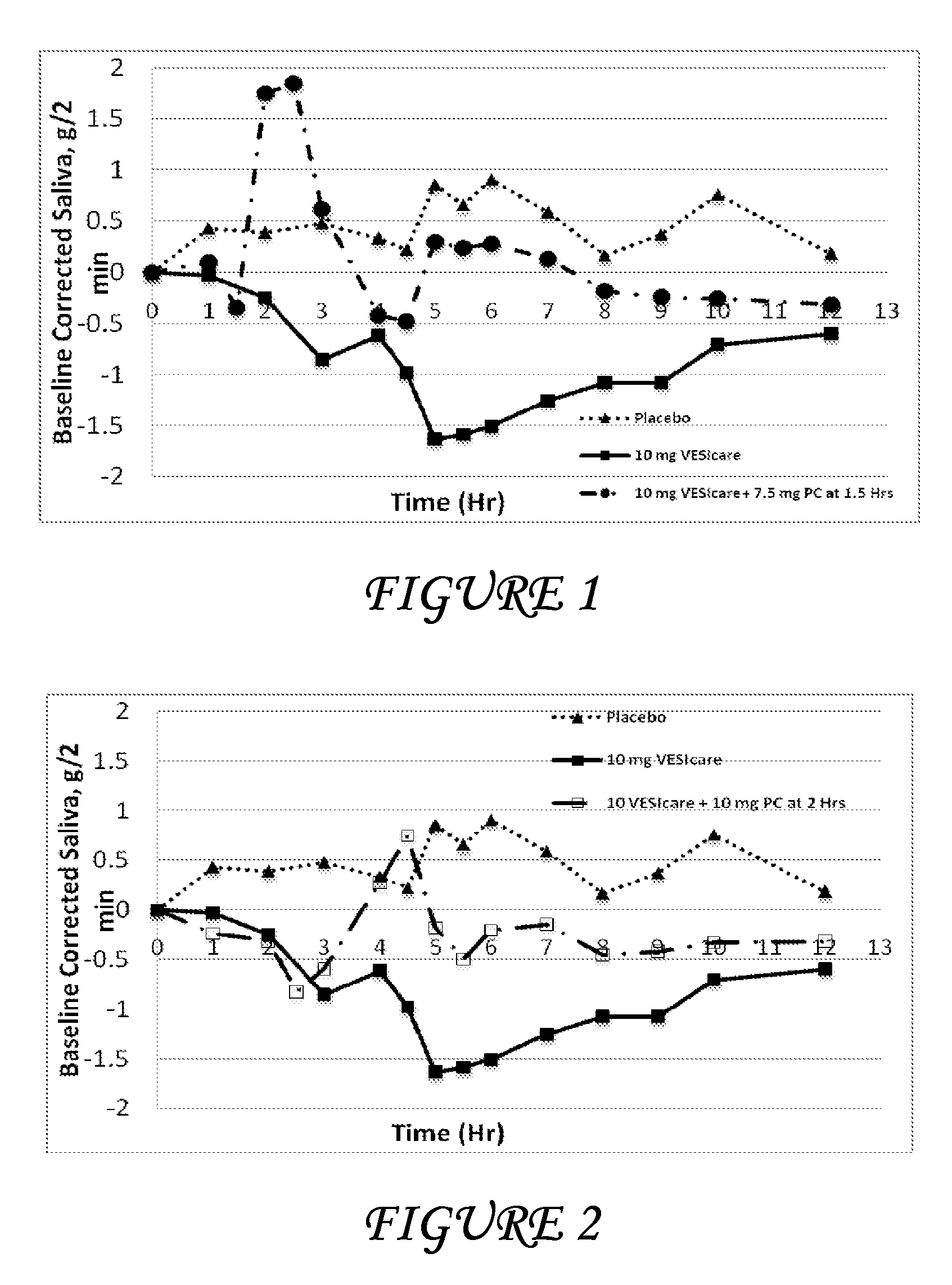
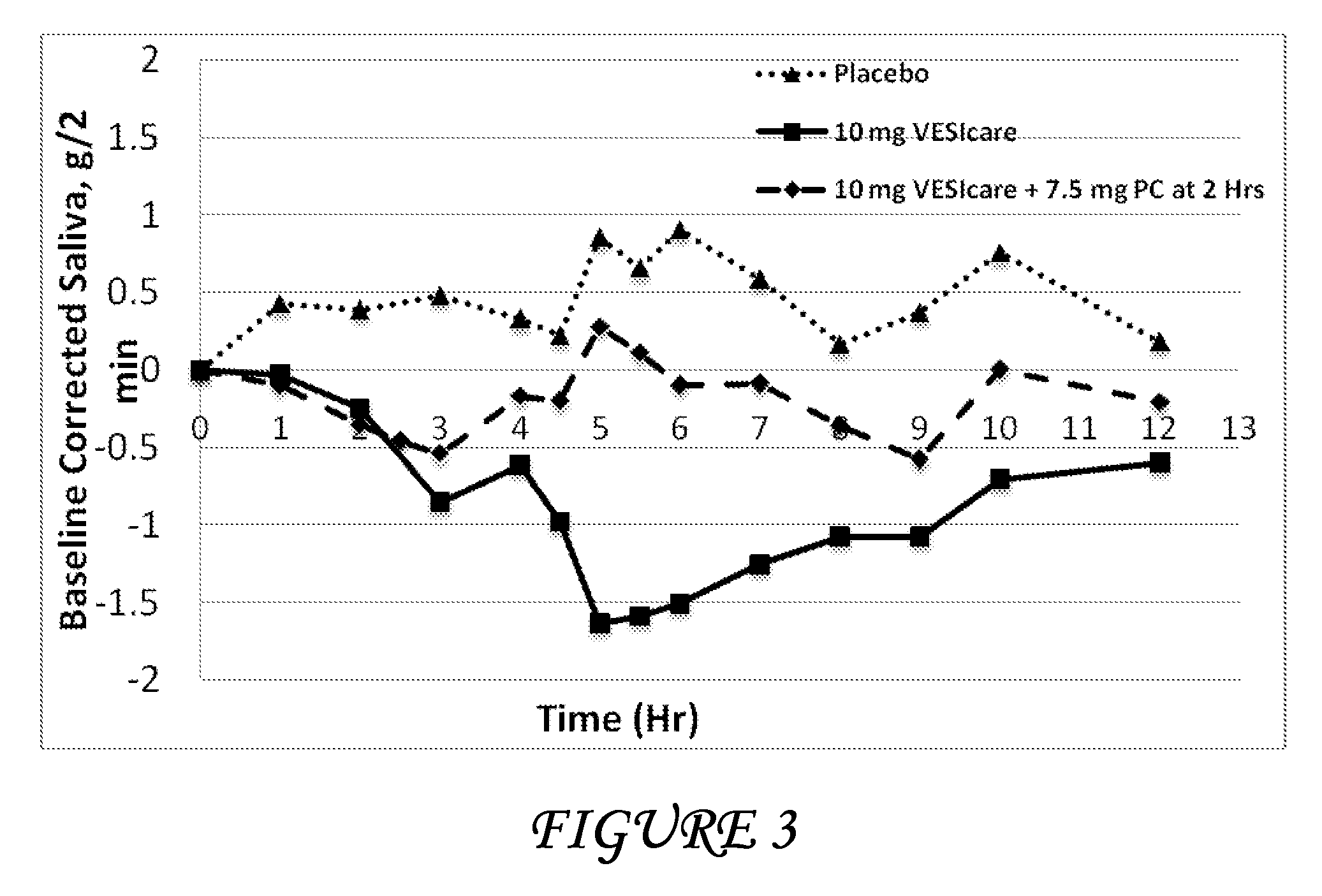
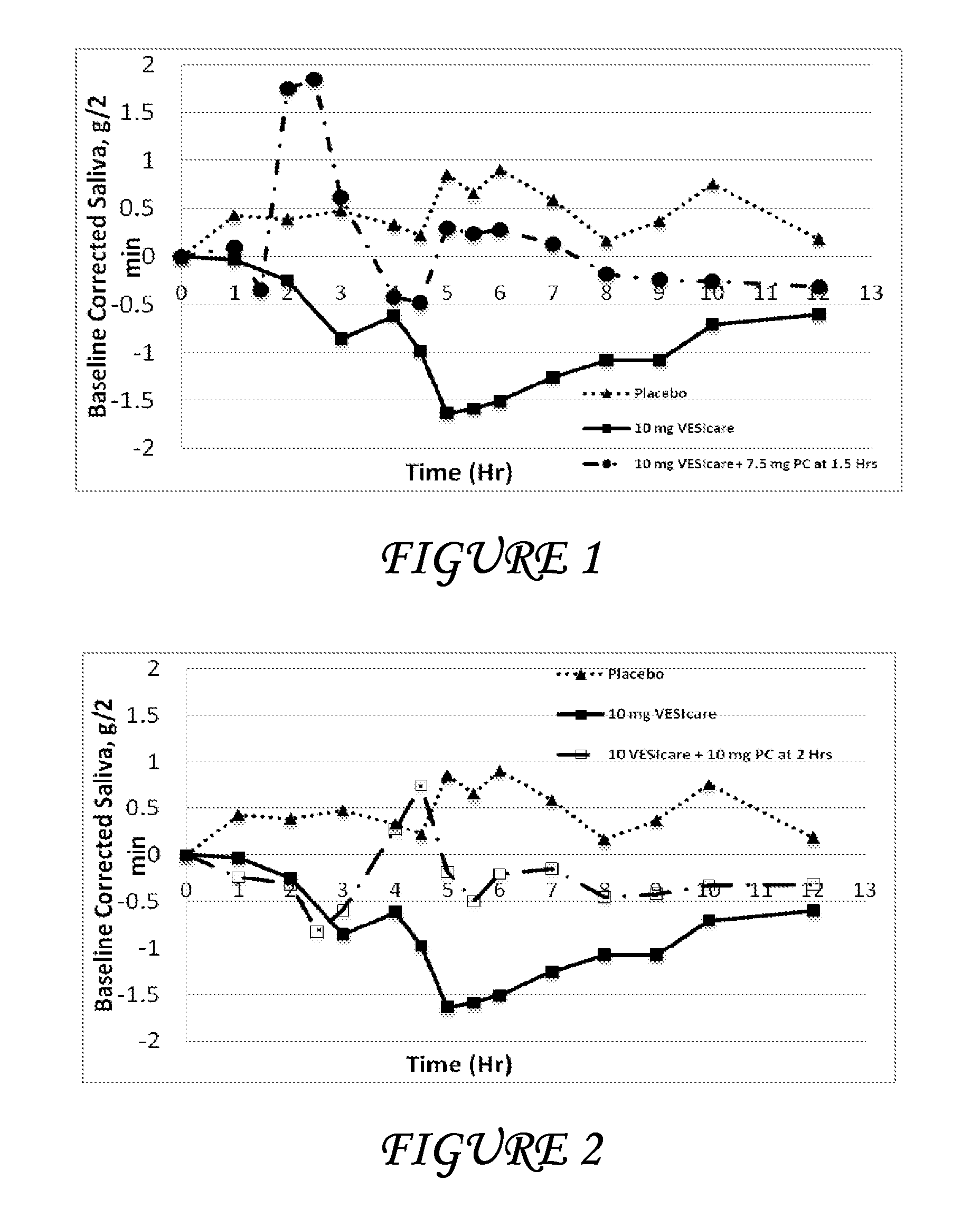
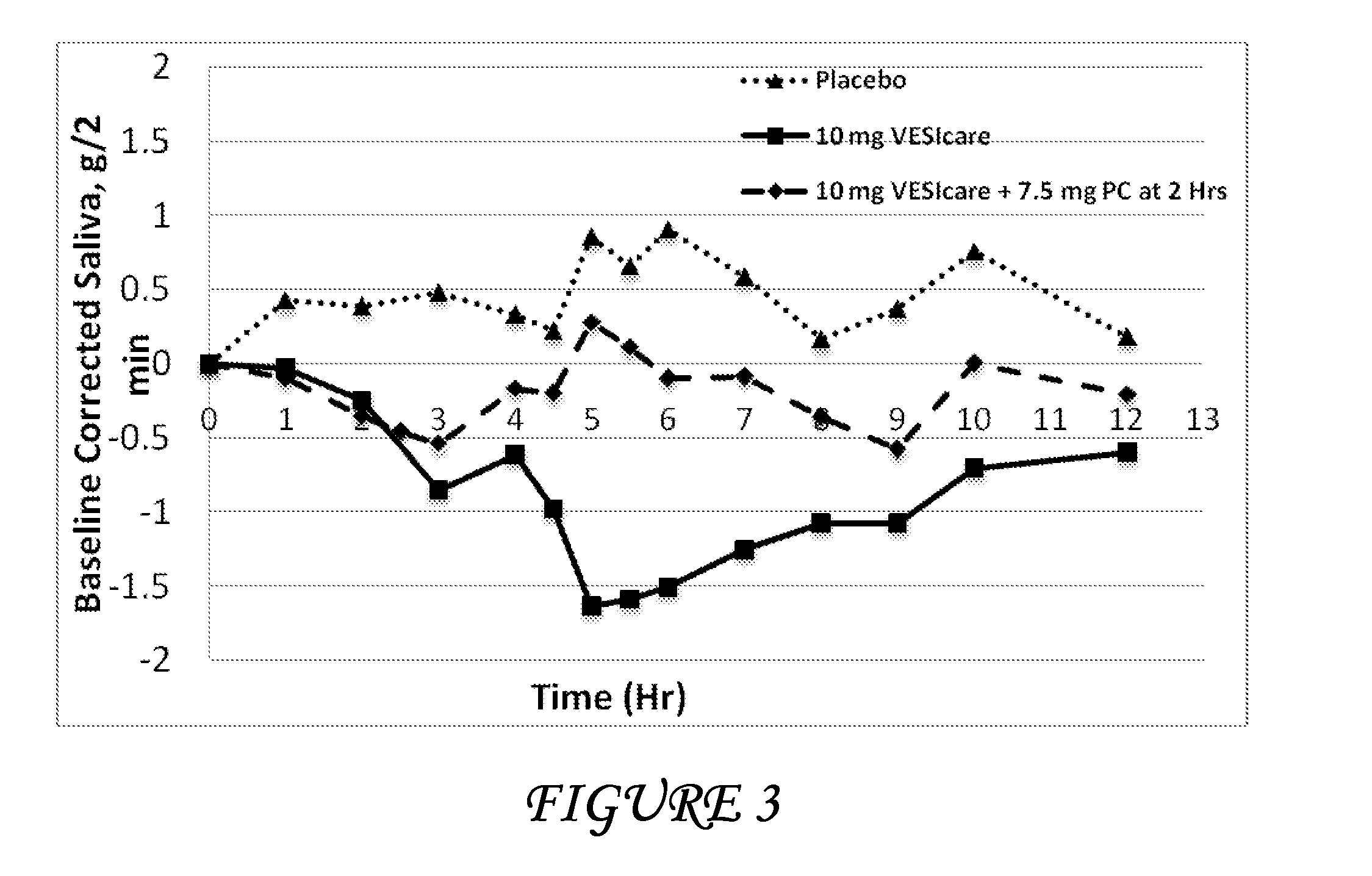
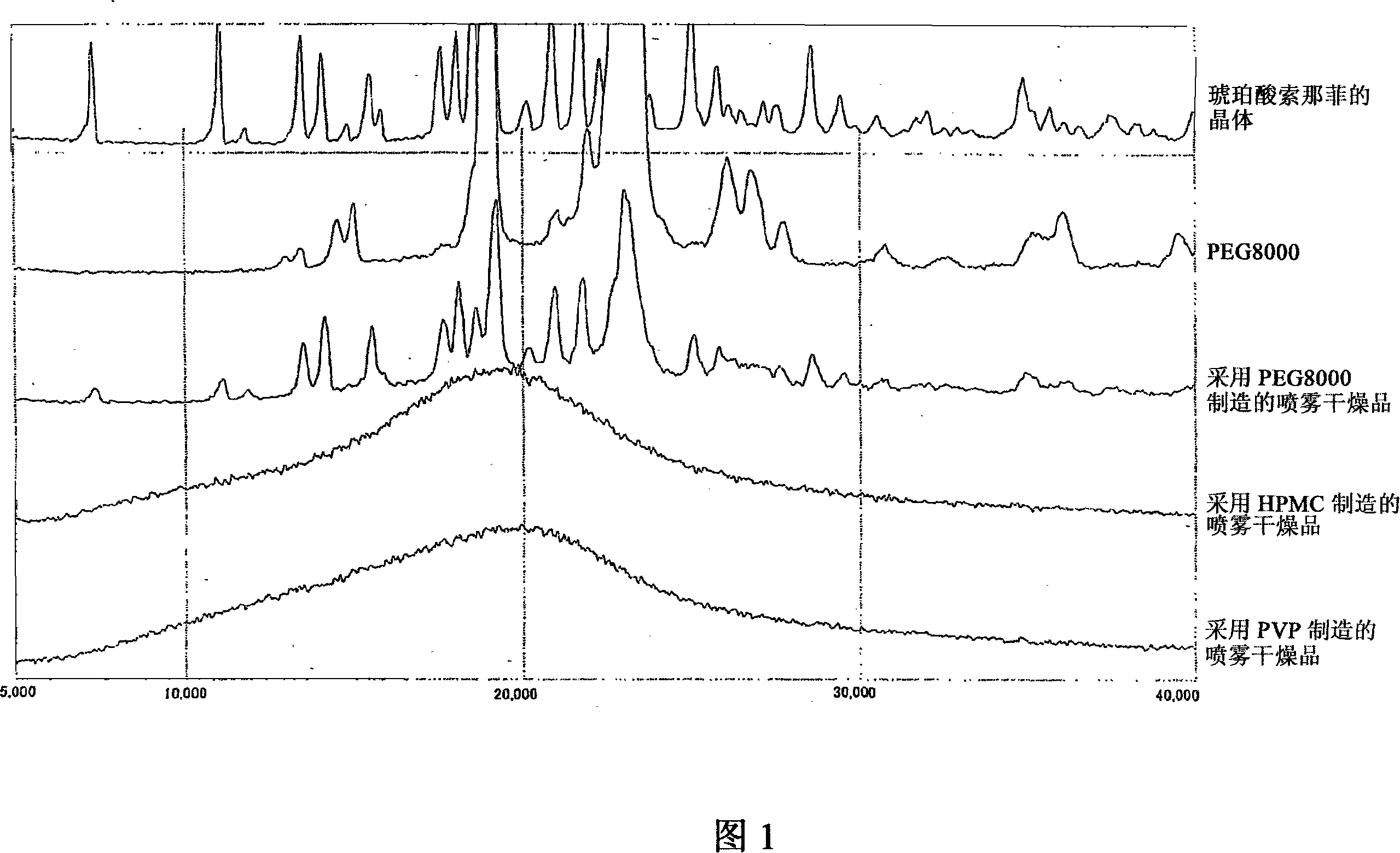
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Combinations of solifenacin and salivary stimulants for the treatment of overactive bladder
Disclosed herein are pharmaceutical compositions comprising a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of treating a patient suffering from overactive bladder, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof. Also disclosed herein are methods of alleviating a side effect of treatment for overactive bladder in a patient suffering therefrom, the method comprising identifying a patient in need thereof, and administering to the patient a therapeutically effective amount of extended release solifenacin, or a pharmaceutically acceptable salt thereof, and a therapeutically effective amount of pilocarpine, or a pharmaceutically acceptable salt thereof.
Owner:THERAVIDA INC
Stable particular pharmaceutical composition of solifenacin or salt thereof
The present invention relates to a granule of solifenacin or its salt that is suitable for coating when clinically providing solifenacin or its salt preparation, is spherical, and can inhibit decomposition over time pharmaceutical composition. More specifically, the present invention relates to a granular pharmaceutical composition obtained when solifenacin or its salt is formulated with a binder whose Tg or mp value is less than 174°C. Moreover, a more stable granular composition of solifenacin or a salt thereof can be provided by performing a crystallization-promoting treatment after manufacturing the granular pharmaceutical composition.
Owner:ASTELLAS PHARMA INC
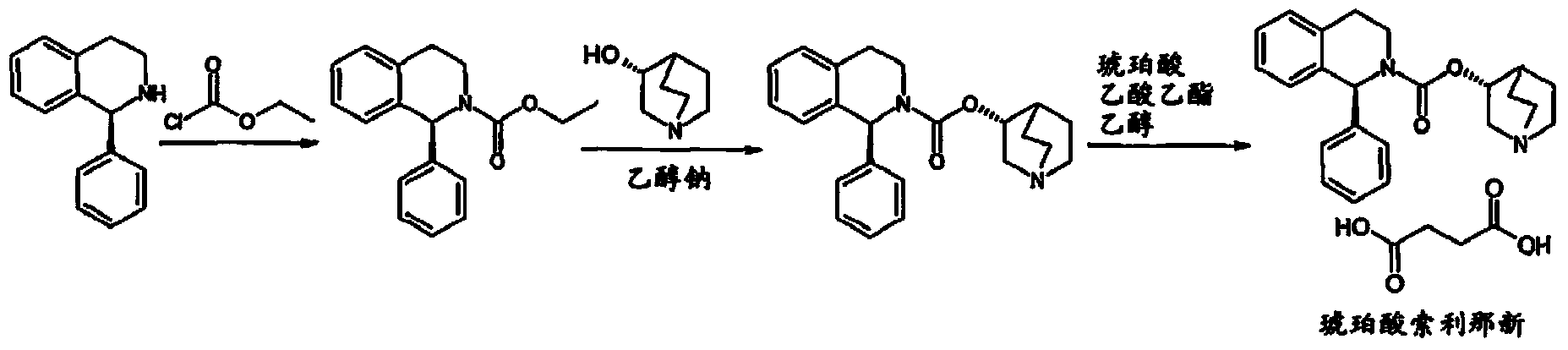
Method for preparing solifenacin intermediate
ActiveCN105541712AReduce manufacturing costAdvanced process routeOrganic chemistryDistillationNitromethane
The invention belongs to the field of medicine and particularly relates to a method for preparing a solifenacin intermediate. After 2-halogenated diphenyl ketone is used for carbonyl protection, n-butyllithium is used for removing bromine, then the formyl group is added; a condensation reaction with nitromethane and catalytic hydrogenation for reduction are carried out, and then acidification is conducted; later, cyclization is carried out, and a solifenacin intermediate compound I is obtained through alkaline hydrolysis after reduction and chiral resolution. The structural formula of the solifenacin intermediate is shown in the description. According to the method for preparing the solifenacin intermediate, the initial raw materials easy to obtain are utilized, and the production cost is reduced. The process route is advanced, the reaction conditions are mild, the reaction yield is high, less three wastes are caused, expensive and toxic reagents do not exist, reaction solvents can be used repeatedly after distillation, industrial production is facilitated, and implementation value and social, economic and environmental protection benefits are high.
Owner:SHANDONG JINCHENG PHARMACEUTICAL GROUP CO LTD
Orally administered medical composition
InactiveUS20150306090A1Improve drug dosing complianceReduce in quantityPowder deliveryBiocideImmediate releaseDissolution
In order to provide the medical field with a single formulation comprising a modified release portion containing mirabegron or a pharmaceutically acceptable salt thereof and an immediate release portion containing solifenacin or a pharmaceutically acceptable salt thereof, (1) a single formulation having dissolution rates of both drugs similar to those of the current single drug formulations is provided, and (2) a single formulation having maximum percentages of dissolution of both drugs of 90% or more, and having a bioavailability equivalent to those of the current single drug formulations. Further, in order to provide a single formulation, (3) a single formulation having good productivity whereby failures in tabletting are reduced, and having good storage stability whereby the coloration of the immediate release portion is suppressed is provided. The pharmaceutical composition for oral administration of the present invention contains (1) a modified release portion comprising mirabegron or a pharmaceutically acceptable salt thereof, and (2) an immediate release portion comprising solifenacin or a pharmaceutically acceptable salt thereof, and calcium stearate.
Owner:ASTELLAS PHARMA INC
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
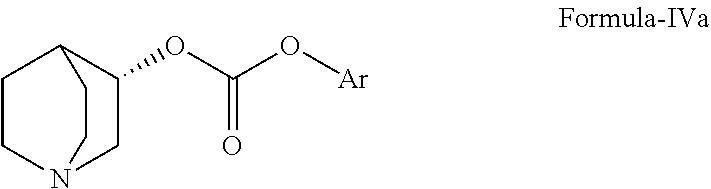
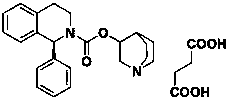
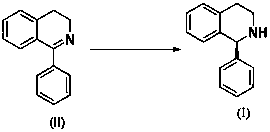
Method for the preparation of solifenacin and intermediate thereof
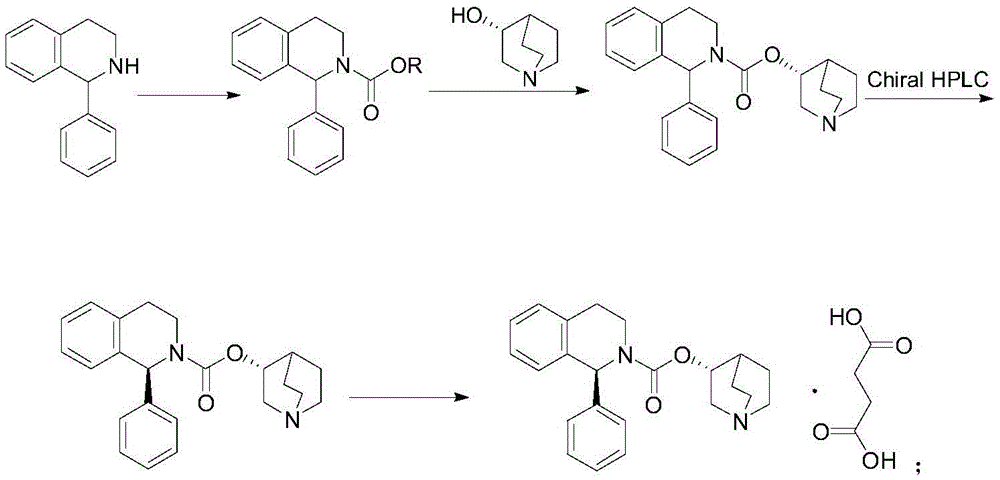
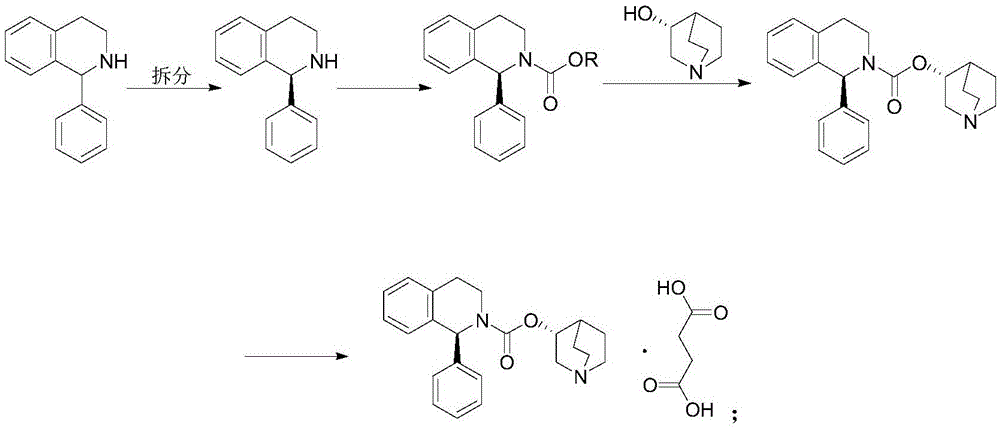
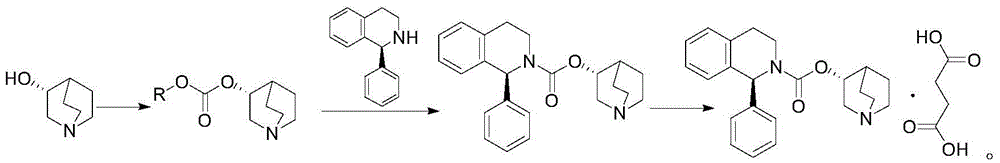
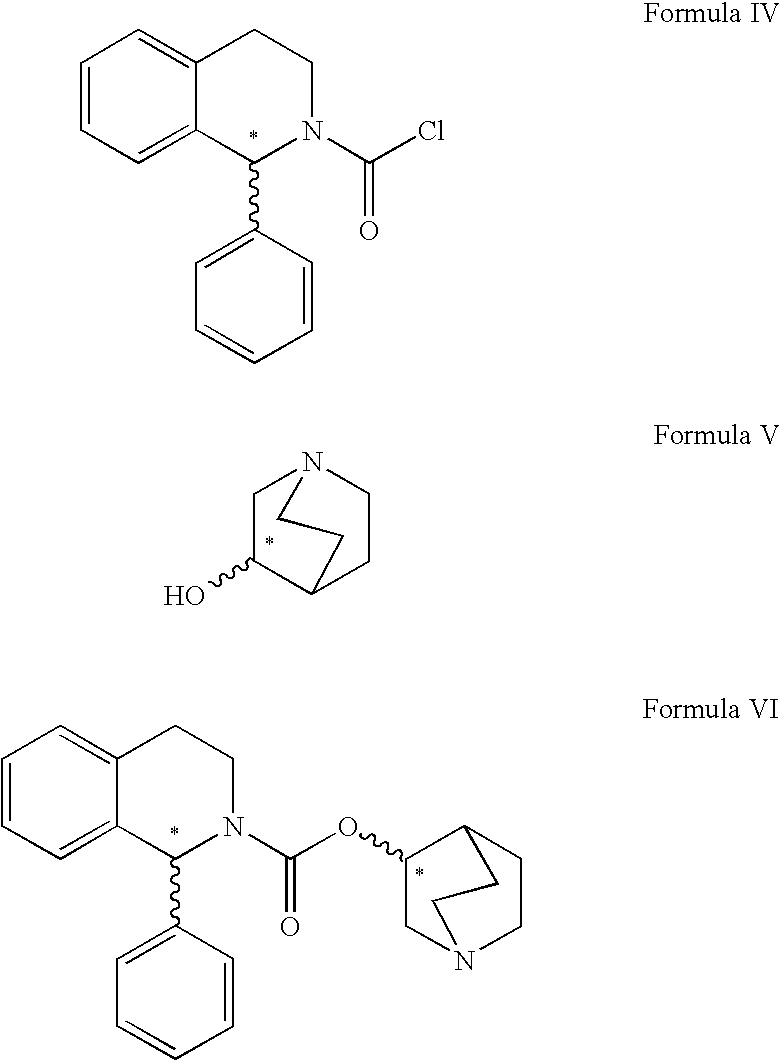
A method for the preparation of solifenacin by reacting quinuclidin-3-ol and bis(aryl) carbonate to form (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa); and treating (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa) with (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline of formula (V) in an inert atmosphere to form a Solifenacin base, which is converted into its pharmaceutically acceptable salts. The invention also provides a compound, (3R)-1-azabicyclo[2.2.2]oct-3-yl 4-aryl carbonate of formula (IVa), which is used as an intermediate for the preparation of Solifenacin base and a process for the preparation thereof.
Owner:MEGAFINE PHARMA (P) LTD
Preparation method and application of chiral amine compound
ActiveCN103923005AWill not polluteOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsSezolamideSertraline
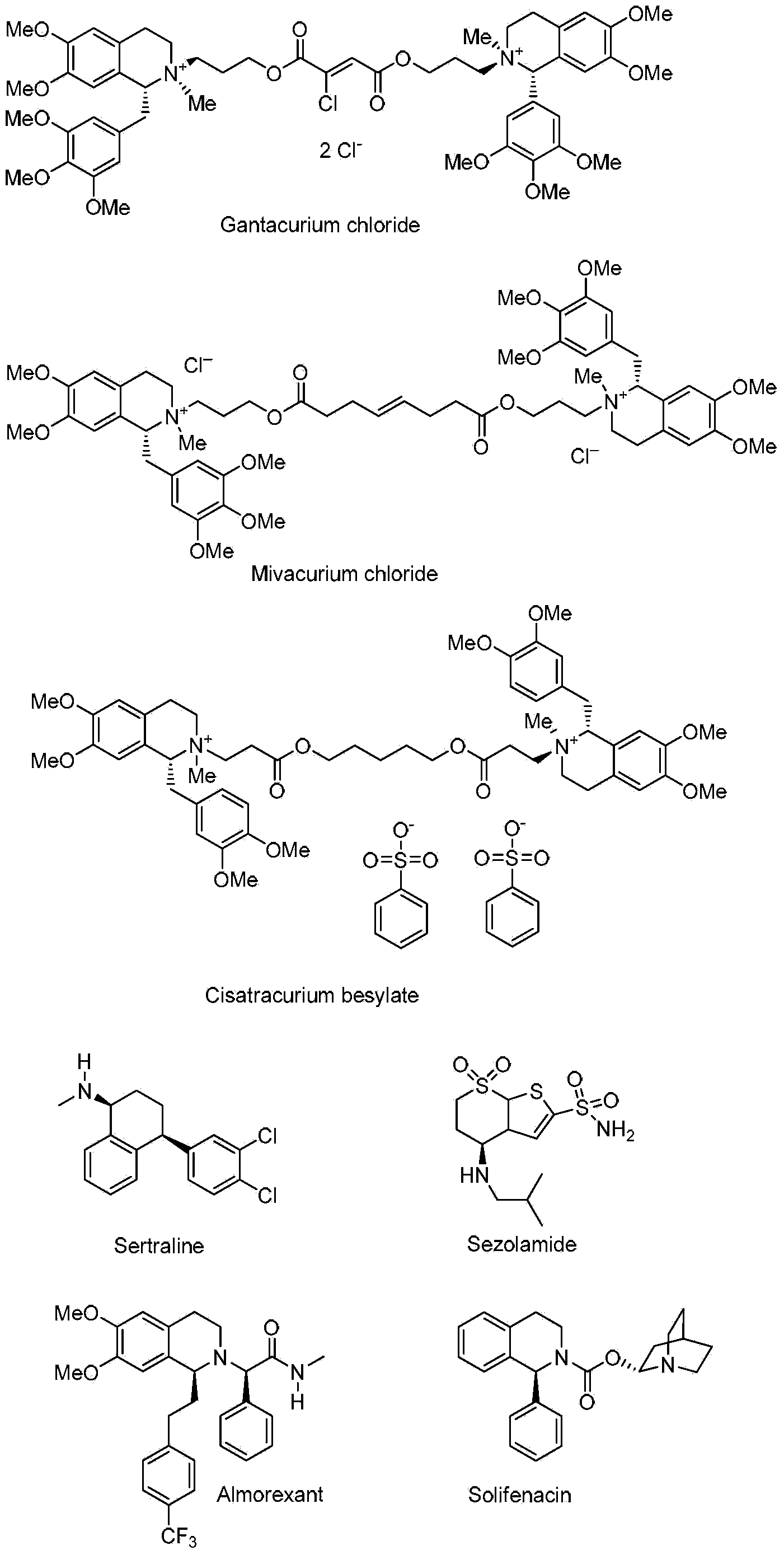
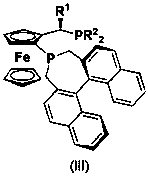
The invention discloses a preparation method of a chiral amine compound. In presence of a semi-sandwich type complex and an acid additive, an asymmetrization reaction is carried out on a prochiral amine compound in a hydrogen atmosphere, and postprocessing is carried out after the reaction is completely finished, so that the chiral amine compound is obtained. According to the preparation method of the chiral amine compound, a certain amount of appropriate acid is added in a certain sequence, so that activation of a C=N bond in a reduction reaction can be promoted; the prepared chiral amine compound can be taken as an intermediate and used for synthesizing the following medicines (including but not limited to the medicines) or precursors of the medicines: gantacurium, mivacurium chloride, atracurium besylate, sertraline, sezolamide, almorexant or solifenacin.
Owner:ZHEJIANG MENOVO PHARMA
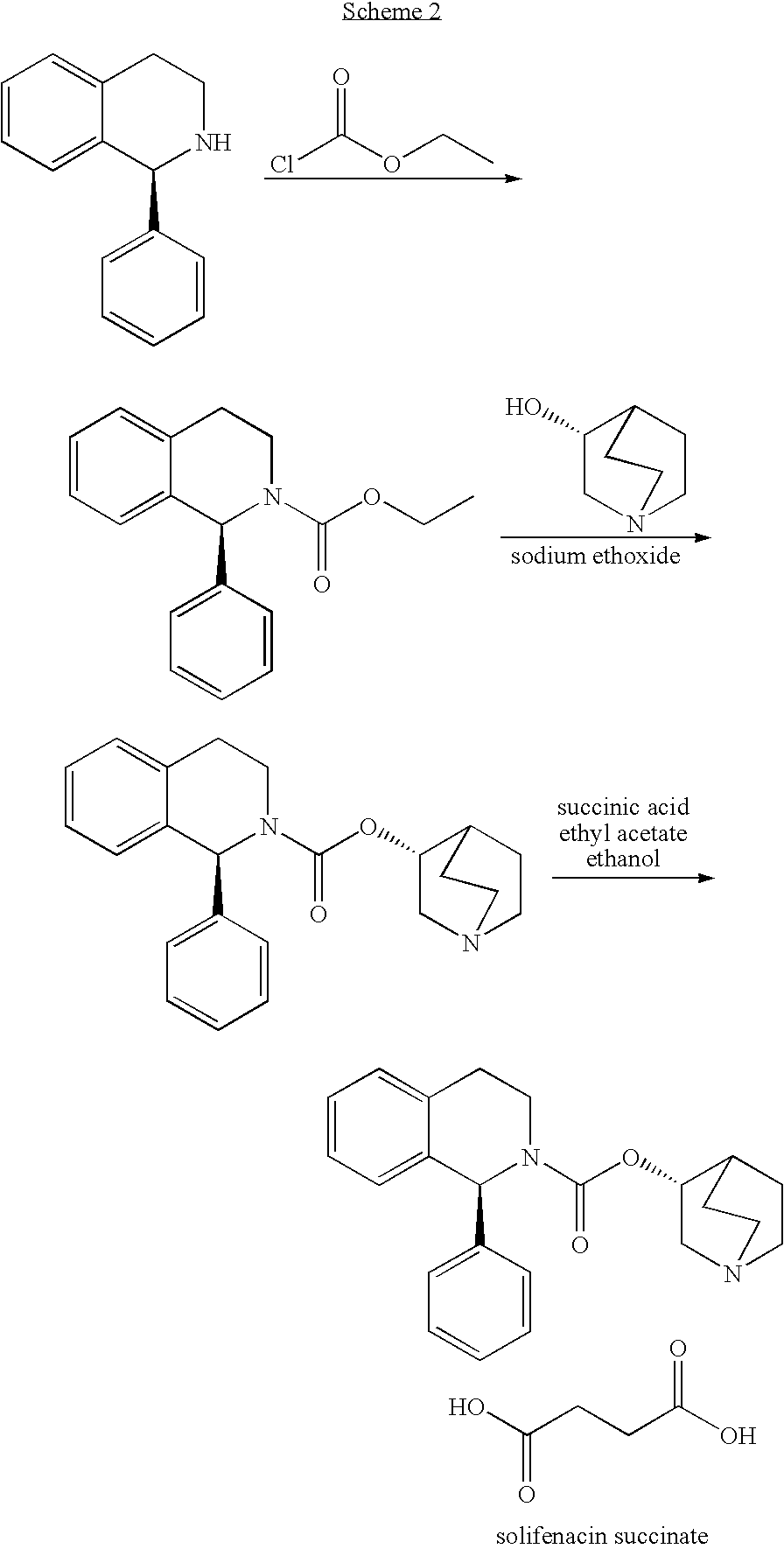
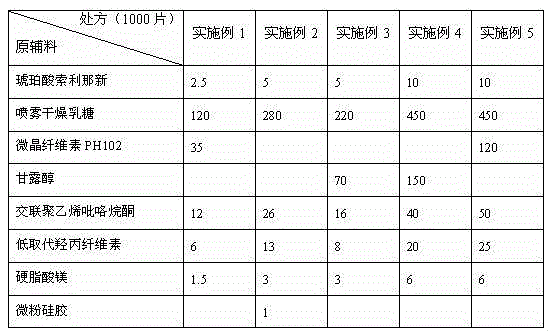
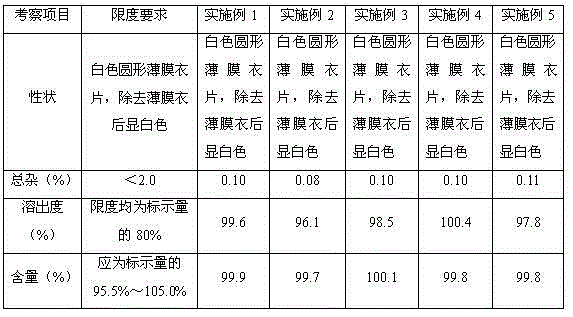
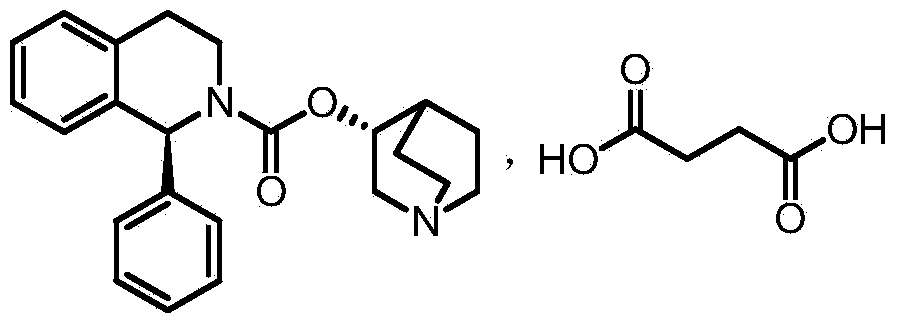
Succinic acid solifenacin tablet capable of achieving direct powder compression and preparation method of succinic acid solifenacin tablet
ActiveCN104523628AGuaranteed dissolution effectSimple preparation processOrganic active ingredientsUrinary disorderSolifenacinMedicine
The invention belongs to the technical field of medicine preparations, and particularly relates to a succinic acid solifenacin tablet capable of achieving direct powder compression and a preparation method of the succinic acid solifenacin tablet. The tablet comprises the following components in percentage by mass: 1%-10% of succinic acid solifenacin, 10%-90% of a filler, 3%-20% of a disintegrating agent, 0.5%-5% of a lubricant and 0-5% of a flow aid. According to the succinic acid solifenacin tablet, the defect that direct dry compression cannot be carried out in the prior art is solved; and the prescription and the process for direct powder compression of the succinic acid solifenacin tablet are achieved, so that the preparation process is relatively simple and suitable for large-scale production.
Owner:LEPU PHARMACEUTICAL CO LTD
Asymmetric synthesis method of Solifenacin intermediate
PendingCN110041255AImprove efficiencyEasy to prepareOrganic chemistry methodsOrganic acidSolifenacin
The invention discloses a preparation method of a Solifenacin intermediate. The Solifenacin intermediate is prepared by subjecting 1-phenyl-3,4-dihydroisoquinoline as a raw material, a chiral diphosphine ligand metal complex as a catalyst, and an organic acid or an inorganic acid as an additive to an asymmetric hydrogenation reaction; the chiral diphosphine ligand metal complex is a catalyst solution obtained by in-situ complexation of a chiral diphosphine ligand and a metal precursor. The preparation method of the invention is simple, efficient and practical, and the catalyst used is high inefficiency, and the method is suitable for industrial production.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Pharmaceutical composition containing solifenacin or salt thereof
ActiveCN104739789AImprove stabilityIncrease productivityPill deliveryUrinary disorderSolifenacinAntioxidant
The invention discloses a pharmaceutical composition containing solifenacin or salt thereof. The pharmaceutical composition mainly contains solifenacin or salt thereof and an antioxidant, and the weight ratio of the antioxidant to the solifenacin or salt thereof is at (1:70)-(1:20). According to the invention, the stability of the pharmaceutical composition containing the solifenacin or salt thereof is effectively improved, and the pharmaceutical composition is prepared by a conventional and simple preparation process; and the pharmaceutical composition has the advantages of simple process, convenience in operation and low cost.
Owner:ZHEJIANG JINGXIN PHARMA +1
Tablet composition with solifenacin and preparation method of tablet composition
InactiveCN105362245AGood treatment effectEasy route of administrationInorganic non-active ingredientsDrageesFormularyHyperactivity bladder
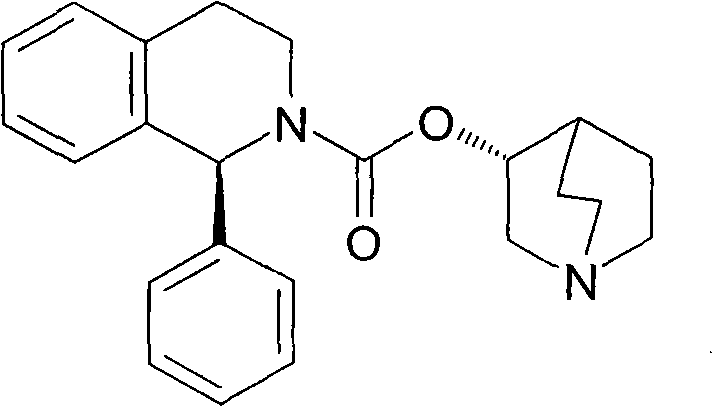
The invention discloses a formula of a medicine composition with solifenacin and a preparation method of the tablet composition. The active component of the medicine composition is (1S)-(3R)-1-azabicyalo[2.2.2]oct-3-yl3,4-dihydro-l-phenyl-2-(1H)-isoquinoline carboxylic acid succinate (solifenacin). The preparation method includes the steps of smashing the active component and corresponding auxiliaries respectively, conducting physical mixing, conducting direct tabletting on dry powder, wrapping film coatings on tablets, and obtaining the medicine composition. The tablets can be used for treating or improving symptoms of overactive bladder including frequent micturition, urgent urination, acute urinary incontinence and other urinary tract symptoms.
Owner:SHANDONG ZHONGTAI PHARMA
Pharmaceutical composition for amelioration of lower urinary tract symptom associated with prostatomegaly
Disclosed is a pharmaceutical composition comprising (R)-5-(2-¢¢2-(2-ethoxyphenoxy)ethyl!amino!propyl)-2-methoxybenzene-1-sulfonamide (tamsulosin) or a pharmaceutically acceptable salt thereof and (1S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline-2-carboxylate (3R)-quinuclidin-3-yl ester (solifenacin) or a pharmaceutically acceptable salt thereof as active ingredients, particularly a pharmaceutical composition for ameliorating a lower urinary tract symptom associated with prostatomegaly. Also disclosed is use of a combination of tamsulosin or a pharmaceutically acceptable salt thereof and solifenacin or a pharmaceutically acceptable salt thereof for the amelioration of a lower urinary tract symptom associated with prostatomegaly. Further disclosed is a combination therapy.
Owner:ASTELLAS PHARMA INC
Stable particular pharmaceutical composition of solifenacin or salt thereof
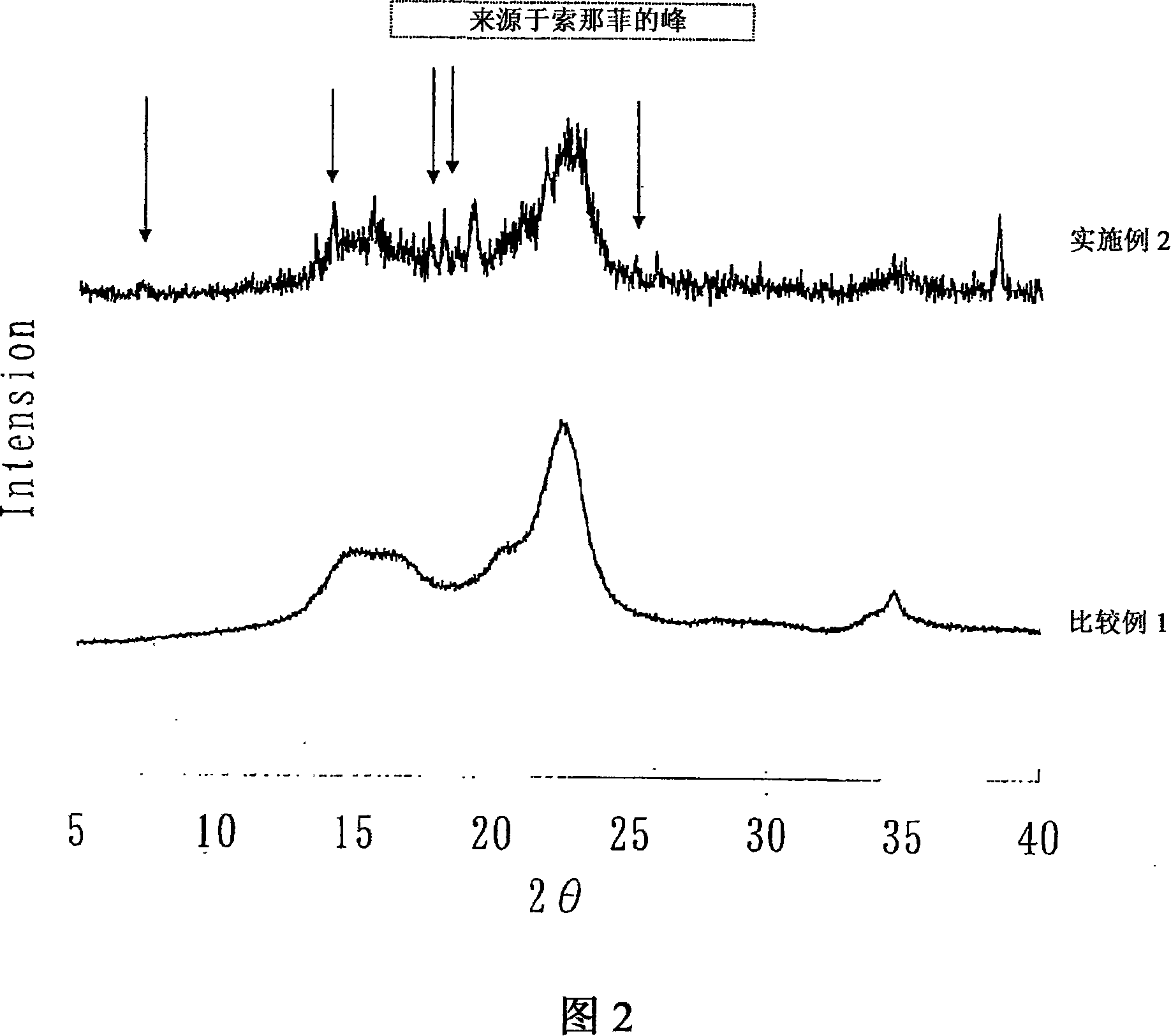
InactiveCN101141961BStable decompositionPowder deliveryOrganic active ingredientsSolifenacinPharmaceutical drug
A solid pharmaceutical preparation of solifenacin or a salt thereof, the preparation being stable and inhibited from decomposing with time when supplied to clinical fields. In a pharmaceutical preparation containing solifenacin or a salt thereof, the compound in an amorphous form was revealed to be causative of cardinal-drug decomposition with time. The composition for a solid pharmaceutical preparation of solifenacin or a salt thereof contains solifenacin or its salt each in a crystalline form, and the content of the compound in an amorphous form in the composition is within the range which exerts no influence on product stability. Also provided are: a process for producing the composition; and a medicinal composition for solid pharmaceutical preparations which contains solifenacin and an amorphization inhibitor.
Owner:ASTELLAS PHARMA INC
Process for the preparation of solifenacin
InactiveUS20090203914A1High degree of conversionReduce racemizationOrganic chemistryIsoquinolineHydrogen
A process for the preparation of (1S)-QR)-I -azabicyclo[2.2.2.]oct-3-yl 3,4-dihydro-1-phenyl- 2(1H)-isoquino-line carboxylate by reacting (1S)-alkyl 1-phenyl-1,2,3,4-tetrahydro-2-isoquinoline carboxylate with 3-(R)-quinuclidol in an inert solvent, where a primary alkyl ester of the carboxylate whose alkyl length is C1-C4 is used and the reaction is catalyzed by a non-nu-cleophilic base.
Owner:ZENTIVA AS
Process for Preparation Of Solifenacin and/or the Pharmaceutically Acceptable Salts Thereof of High Pharmaceutical Purity
InactiveUS20110065922A1High purityMore constantOrganic active ingredientsOrganic chemistryPhenyl groupPhosgene
A process for the preparation of solifenacin and / or the pharmaceutically acceptable salts thereof of high pharmaceutical purity is characterized in that 3-(R)-quinuclidinoloxy anion generated in situ from 3-(R)-quinuclidinol in a presence of strong base in polar organic solvent is subject to acylation with (S)-1-phenyl-1,2,3,4-tetrahydroisoquinolinecarbonyl chloride of chemical purity at least 98%, while maintaining constant anion excess in a reaction mixture, and after reaction completion solifenacin base is optionally transformed into solifenacin salt according to standard procedures. (S)-1-Phenyl-1,2,3,4-tetrahydroisoquinolinecarbonyl chloride of chemical purity at least 98% is obtained in a reaction of (S)-1-phenyl-1,2,3,4-tetrahydroisoquinoline and molar excess of phosgene in a presence of tertiary aromatic amine in aromatic hydrocarbon, and isolated in a crystalline form.
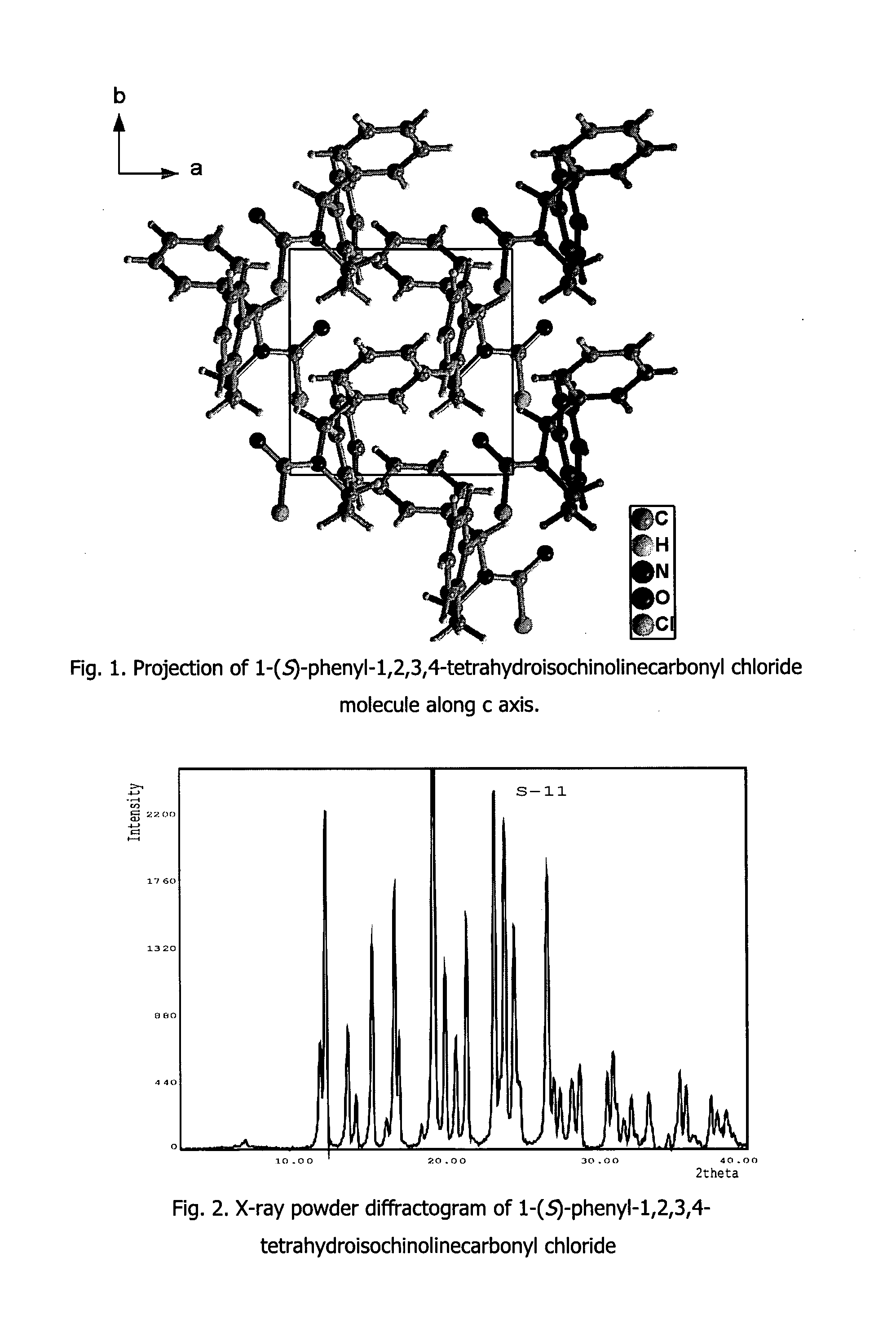
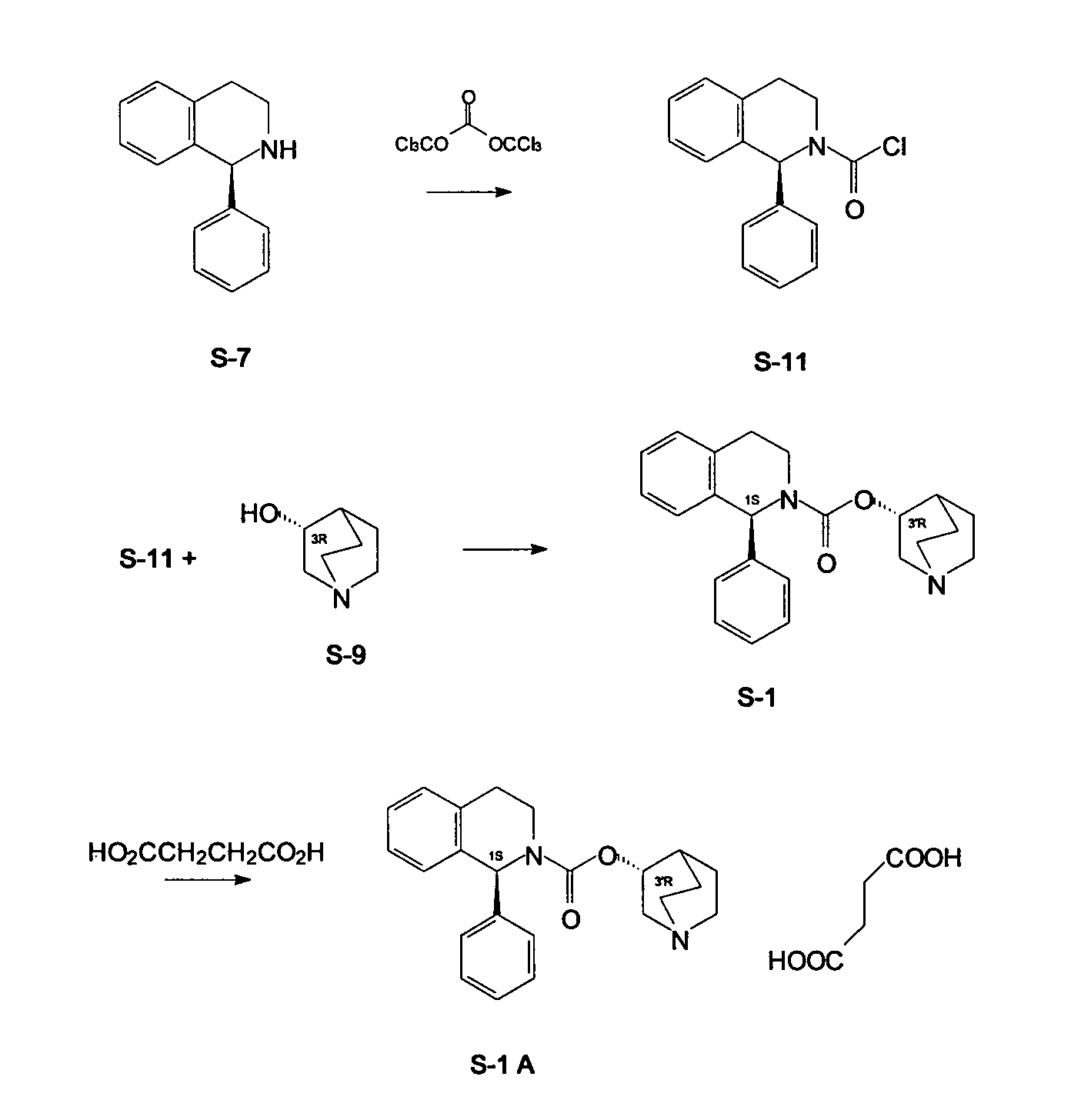
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
(1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-phenyl formate novel crystal form and preparation method thereof
The invention belongs to the technical field of pharmaceutical chemicals, and in provides to a crystal form of (1S)-1-phenyl-3,4-dihydro-1H-isoquinoline-2-phenyl formate and a preparation method thereof. The obtained crystal form A has the advantages of easy dissolution, mild reaction conditions and good stability in the preparation process of solifenacin, and does not need long-term high temperature reflow conditions, so as to increase industrial maneuverability and reduce energy consumption and production cost. The good stability of the crystal form A is conducive to impurity removal and intermediate preservation in the preparation process, thereby greatly improving the disadvantages caused by usage of an oil intermediate in the existing production process of solifenacin.
Owner:QILU PHARMA HAINAN +1
Process for the preparation of solifenacin and salts thereof
InactiveCN103702997AMild conditionsMild timeOrganic chemistryUrinary disorderBenzaldehydeCarboxylic acid
The invention provides a new process for the preparation of solifenacin or a pharmaceutically acceptable acid addition salt thereof, comprising reacting (R)-quinuclidin-3-yl phenethylcarbamate with benzaldehyde in the presence of an acid to obtain a diasteroisomeric mixture (S,R)-((R)-quinuclidin-3-yl)1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate of formula (IV) which can be resolved and the solifenacin or a pharmaceutically acceptable acid addition salt thereof recovered. The invention also provides the new key intermediate (R)-quinuclidin-3-yl phenethylcarbamate involved in the process. Further the invention provides a method for the transformation of (R)-((R)-quinuclidin-3-yl)1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate into a diasteroisomeric mixture (S,R)-((R)-quinuclidin-3-yl)1-phenyl-3,4-dihydroisoquinoline-2(1H)-carboxylate.
Owner:ISOCHEM SA
Solifenacin-containing composition
To provide a novel method for producing a composition comprising solifenacin or a salt thereof, and a composition comprising solifenacin or a salt thereof as produced by the method, wherein an optionally substituted lower alkyl is added to the 2-position of the quinuclidine of solifenacin. The composition of the present invention contains a highly pure solifenacin, while the unexpected compounds specific to the method in an extremely low content, so that it has very preferable properties as a bulk for pharmaceutical products.
Owner:ASTELLAS PHARMA INC
Memantine combinations and use
InactiveUS20190269627A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
A process for the preparation of solifenacin or a salt thereof
The present invention relates to an improved process for the preparation of Solifenacin or its salt of formula I, more particularly the present invention relates to an economically viable and industrially advantageous process for the preparation of highly pure Solifenacin or its salt of formula I.
Owner:PHARMATHEN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com