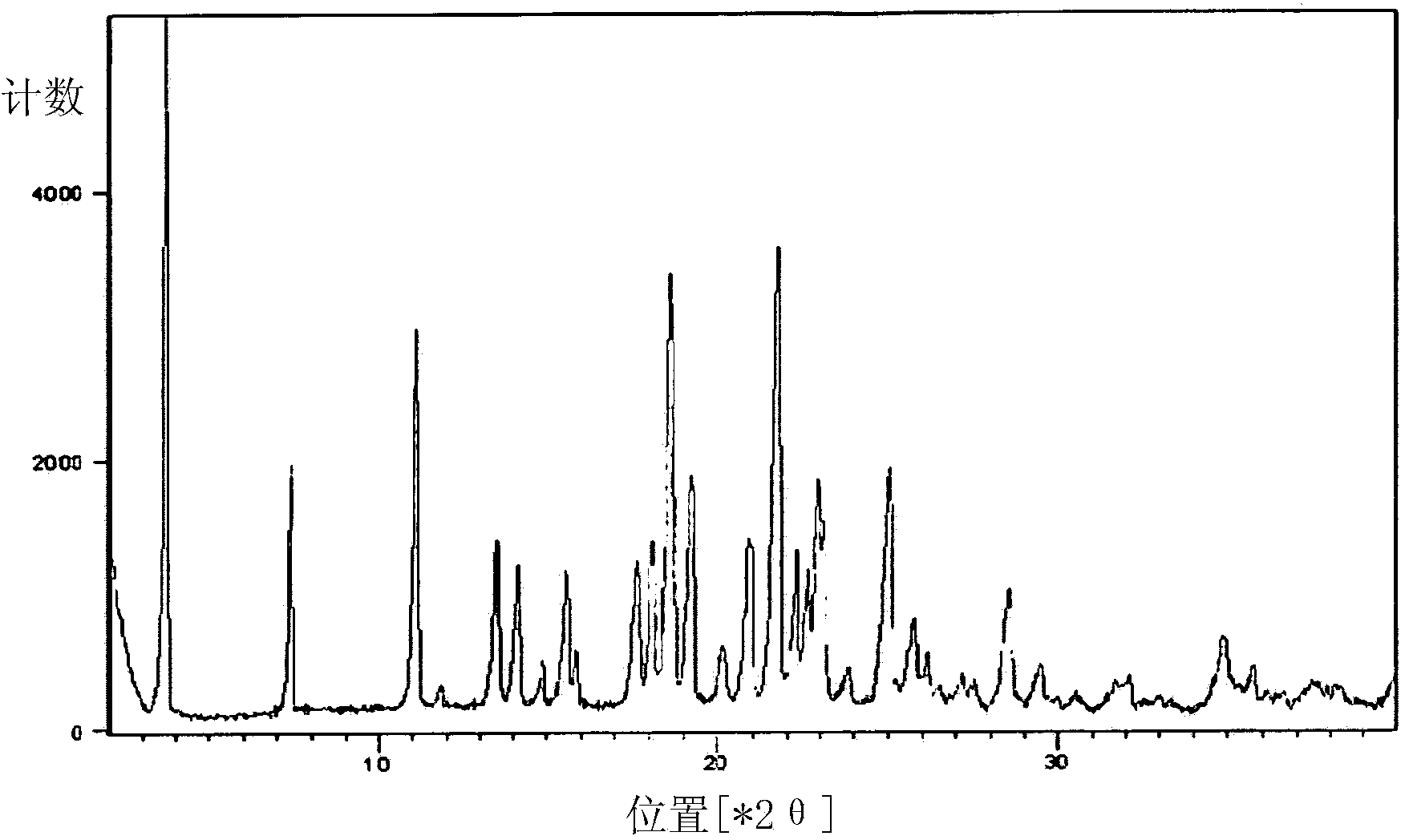
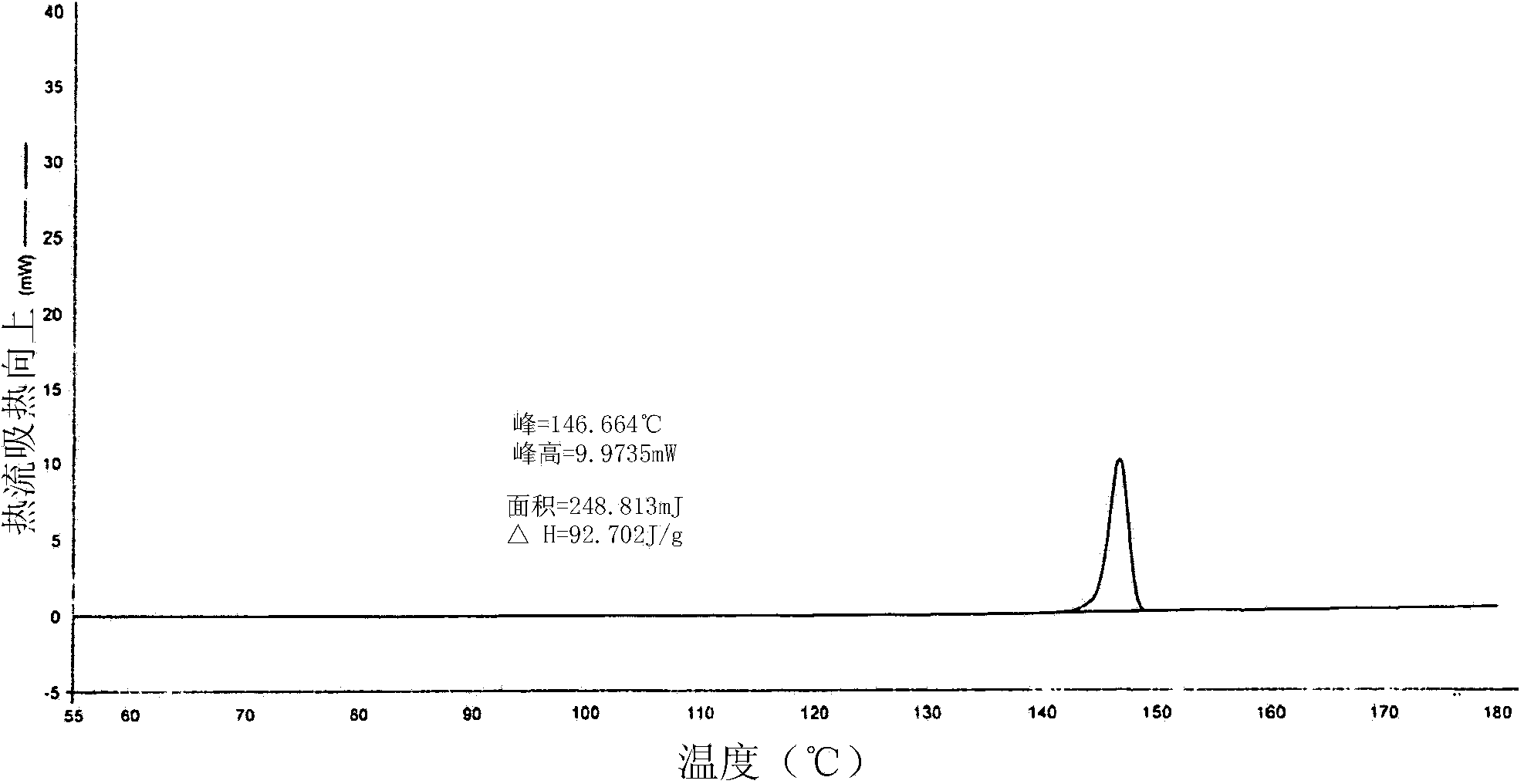
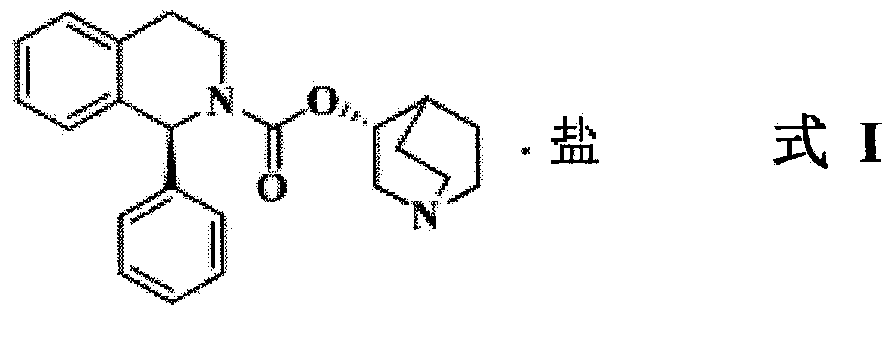
A process for the preparation of solifenacin or a salt thereof
A technology of solifenacin and its compound, which is applied in the field of preparing high-purity solifenacin or its salt, can solve the problems of long reaction time, harmfulness, increased production cost, etc., and achieve the effect of high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] Phase I) Preparation of Solifenacin Free Base
[0058] The reaction of a compound of formula II with a compound of formula III is carried out in a solvent, wherein R and R' are as defined above, selected from polar aprotic solvents such as dichloromethane, acetonitrile, dimethylformamide, tetrahydrofuran , ethyl acetate and a non-polar solvent such as toluene, cyclohexane or mixtures thereof, preferably acetonitrile. The reaction mixture is stirred at ambient temperature for about 3-6 hours, preferably for about 3-4 hours, more preferably until the reaction is complete. The completion of the reaction is monitored by any suitable technique such as thin layer chromatography (TLC) or high performance liquid chromatography (HPLC) or gas chromatography. Compounds of formula A or compounds of formula B can optionally be isolated by conventional techniques such as acid-base treatment, solvent extraction, column chromatography and crystallization or used directly in the next...
Embodiment 1
[0084] N,N'-Disuccinimidyl carbonate (14.4mmol, 3.69g) was added to (R)-3-quinribol (11.5mmol, 1.46g) in acetonitrile (50ml) stirred at ambient temperature in solution. The resulting mixture was stirred at ambient temperature for 4 hours. (1S)-1-Phenyl-1,2,3,4-tetrahydroisoquinoline (9.6mmol, 2.0g, chromatographic purity: 99.93%, chiral purity: 98.65%) in acetonitrile (25ml) The solution was added to the reaction mass and then stirred overnight. The reaction mixture was concentrated under reduced pressure. The residue was then diluted with toluene (10ml) and 1N hydrochloric acid (20ml) was added thereto. The reaction mixture was stirred and the phases were separated. The separated aqueous layer was basified with 20% sodium carbonate (20ml) solution, followed by extraction with ethyl acetate (20ml). The aqueous layer was re-extracted with ethyl acetate (10ml). The organic layers were combined and washed with water (10 ml), and the solvent therein was distilled off. To th...
Embodiment 2
[0086](R)-3-Quinuclear alcohol (110 mmol, 14 g) was added to a stirred solution of N,N'-disuccinimidyl carbonate (143 mmol, 36.8 g) in acetonitrile (60 ml) at ambient temperature. The resulting mixture was stirred at ambient temperature for 4 hours. (1S)-1-Phenyl-1,2,3,4-tetrahydroisoquinoline (95.6mmol, 20g, chromatographic purity: 99.99%, chiral purity: 99.0%) was added to the reaction mass and heated at 25 Stir at -35°C until the reaction is complete. The reaction mass was concentrated under reduced pressure. The residue was then diluted with toluene (100ml) and 1N hydrochloric acid (200ml) was added thereto. The reaction mixture was stirred and the phases were separated. The separated aqueous layer was basified with potassium carbonate to pH 9-10, followed by extraction with ethyl acetate (100ml). The aqueous layer was re-extracted with ethyl acetate (40ml). The organic layers of ethyl acetate were combined, washed with water (60 ml) and the solvent was distilled off....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com